Abstract
Acropora cervicornis historically exists in large, dense thickets that provide a functionally unique habitat. However, populations in the Caribbean have declined by up to 98%, frequently isolating extant colonies. Remnant thickets are valuable research areas as they provide opportunities to assess community dynamics, resilience, succession, and the response to disturbances. BCA (Broward County Acropora) is a 10,000-m2A. cervicornis thicket located offshore Broward County in the Southeast Florida Coral Reef Ecosystem Conservation Area (Coral ECA), which has been monitored since 2003. The objective of this study was to analyze temporal changes in the thicket, specifically assessing the impact of disturbances and community dynamics that corresponded with fluctuations in the ratio between living and dead A. cervicornis. Photographic data were collected along 12 permanent transects to assess temporal changes in the percent benthic cover from 2003 to 2022. Demographic data on non-A. cervicornis corals were collected along four belt transects from 2012 to 2022. Live A. cervicornis cover declined from 34.8% ± 2% SE in 2003 to 2.4% ± 0.6% SE in 2022. The most severe declines corresponded with heat stress events and two hurricanes in 2005; a cold stress event in 2010; and heat stress, disease, and predation outbreaks between 2014 and 2016. As A. cervicornis died, other taxa utilized the remaining dead structure, with increases in the encrusting, weedy coral species Agaricia agaricites and the macroalgal and crustose coralline algal cover. However, the structural decline in later years suggests complete loss of this unique and complex habitat in the coming years. Thicket recovery will likely require active restoration and a reduction in local and global stressors.
1 Introduction
The Atlantic staghorn coral, Acropora cervicornis, has experienced severe declines within the last several decades (Aronson and Precht, 2001; Goergen et al., 2020) and is currently listed as “threatened” under the US Endangered Species Act and “critically endangered” on the International Union for Conservation of Nature (IUCN) Red List (Hogarth, 2006). Once dominant along forereefs throughout the Caribbean, populations have experienced declines of 80%–90%, with some areas experiencing losses of up to 98% (Aronson and Precht, 2001; Bruckner, 2002; Miller et al., 2002). These declines are due to a variety of disturbances and stressors, including disease (Davis, 1982; Aronson and Precht, 2001; Miller et al., 2002), predation (Lirman et al., 2010; Goergen et al., 2020), thermal stress (Bruno et al., 2007; Lirman et al., 2011; Jones et al., 2020), and tropical storms and hurricanes (Knowlton et al., 1990; Gardner et al., 2005; Brandt et al., 2013; Goergen et al., 2019). Many of these disturbances can interact, causing further declines (Knowlton et al., 1990; Wilkinson and Souter, 2008; Goergen et al., 2019, 2020; Renzi et al., 2022). As a result, there are very few areas where the species is thriving today (Greer et al., 2020). According to the fossil record, A. cervicornis historically survived through many disturbances until the 1980s, and thus the recent declines can be considered unusual (Aronson et al., 2002; Wapnick et al., 2004; Greer et al., 2009).
A. cervicornis has a fast growth rate, prolific branching morphology, and can reproduce through asexual fragmentation. These characteristics allow A. cervicornis to propagate quickly (Tunnicliffe, 1981; Neigel and Avise, 1983; Vargas-Angel et al., 2006) and form large patches or thickets (Lirman et al., 2010; Larson et al., 2014; Huntington et al., 2017; Walker, 2017). Much like general A. cervicornis population trends, the abundance, distribution, and extent of these thickets have declined, and although rare, large and dense thickets still exist (Vargas-Angel et al., 2003; Lirman et al., 2010; Walker, 2017). The complex framework created by A. cervicornis thickets are unique in the Caribbean and serve a critical and irreplaceable ecological role (Goergen et al., 2019). Principally, these thickets provide an important habitat for a variety of reef fish and invertebrates, particularly enhancing the diversity and the abundance of herbivores and fishery-targeted species (Hernández-Delgado et al., 2025), as well as being significant contributors to reef framework (Precht et al., 2002). The ecological role that A. cervicornis thickets play extends beyond, although with some alterations, the loss of living tissue. The remnant dead skeletons can provide substrate for coral settlement (Bozec et al., 2015), as well as the colonization of macroalgae, zoanthids, tunicates, sponges, and other benthic taxa (Greenstein et al., 1998; Aronson and Precht, 2001; Tkachenko et al., 2007; Norström et al., 2009; Caterham et al., 2019; Jones and Gilliam, 2020; Gilliam et al., 2021). These other benthic taxa may contribute significantly to the benthic community structure (Hughes et al., 2010). Although studies have documented the settlement and growth of benthic taxa on dead A. cervicornis structure (Caterham et al., 2019; García-Urueña and Garzón-MaChado, 2020), no studies have been conducted to document long-term changes in the benthic community structure in response to fluctuations in the ratio of dead and live A. cervicornis and the subsequent changes associated with skeletal erosion if not replaced by live A. cervicornis.
Goergen et al. (2019) investigated the A. cervicornis population dynamics of two large thickets in the northernmost region of Florida’s Coral Reef (FCR), called the Southeast Florida Coral Reef Ecosystem Conservation Area (Coral ECA), from 2008 to 2016. They found cycles of mortality following acute disturbances, particularly in dense areas, and recovery during the inter-disturbance periods. The objective of this study was to describe 20 years of population dynamics within the largest A. cervicornis thicket, the Broward County Acropora (BCA), specifically examining whether the thicket recovered or suffered further mortality and assessing changes in the benthic community structure that accompanied the changes in live A. cervicornis. We analyzed the annual benthic community cover and the stony coral assemblage structure along permanent transects at BCA, addressing three questions: 1) how has the A. cervicornis population changed over time; 2) have disturbance events contributed to these population changes; and 3) how have these changes impacted the benthic community structure?
2 Materials and methods
2.1 Study site
BCA is a naturally occurring A. cervicornis thicket with a total area that was once greater than 10,000 m2 and is located approximately 400 m offshore Broward County, Florida, USA, on the nearshore ridge habitat in 6 m of water (center coordinates: 26°08.985′ N, 80°05.810′ W) (Figure 1). It was first described by Vargas-Angel et al. (2003), and various aspects of its extent and condition have been documented in Walker et al. (2012); Goergen et al. (2019), and Jones and Gilliam (2020).
Figure 1
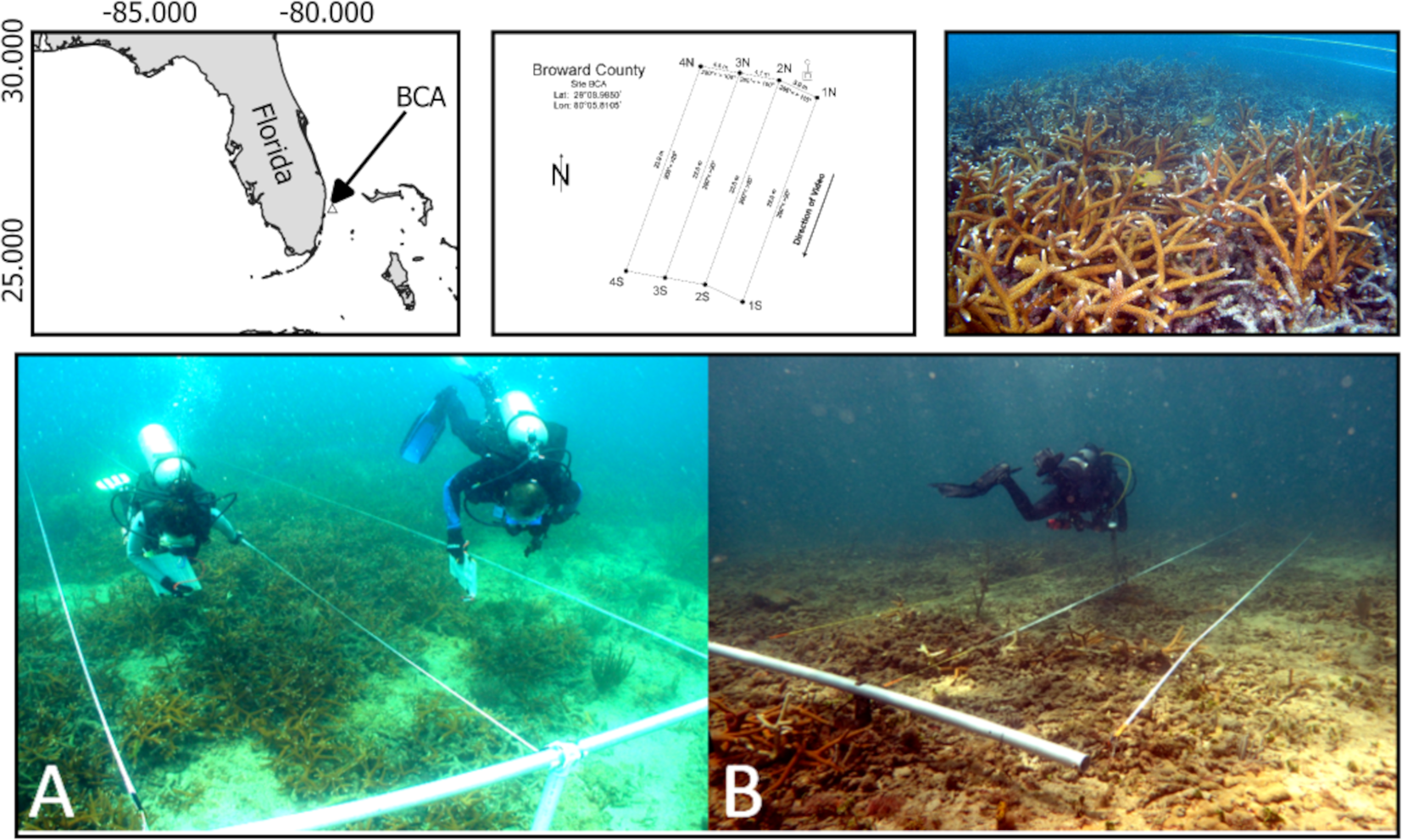
The larger image shows a map of the four stations used and where they are located within the Broward County Acropora (BCA). The perimeter of BCA used in this image is from 2012. The stations were placed in the denser areas of BCA in 2003. The smaller images above (from left to right) show where BCA is located in Florida, how the stations were placed relative to each other, and an image of Acropora cervicornis at BCA in 2010 looking horizontally toward the transects. Images on the bottom provide a top-down view of BCA in 2003 (A) and 2021 (B).
2.2 Data collection
The data collection and methods were from the Southeast Florida Coral Reef Evaluation and Monitoring Project (SECREMP) (Gilliam et al., 2021), a long-term monitoring project designed to track changes along the Coral ECA. The A. cervicornis population and the benthic community structure were surveyed in June/July annually on four 22-m × 1-m permanent stations from 2003 to 2022 within the densest area of the largest A. cervicornis thicket in the Coral ECA. Linearly along three transects per station, approximately 60 abutting images (40 m × 30 cm) were taken at a fixed distance from the substrate. Each transect covered approximately 8.8 m2 of substrate, with a total station area of 26.4 m2 and a total site area of 105.6 m2. Percent benthic cover was calculated from 15 randomly generated points on each image using Coral Point Count with Excel Extensions (CPCe) (Kohler and Gill, 2006). The benthic taxon categories included living A. cervicornis, non-acroporid scleractinian (stony) corals (pooled together due to the low cover of individual species), macroalgae, sponges, crustose coralline algae (CCA), encrusting and branching gorgonians, zoanthids, other living biota (e.g., hydroids and anemones, among others), and substrate. The substrate category was split into dead A. cervicornis (standing dead branches and rubble) and hardbottom substrate (any substrate that was not dead A. cervicornis). Cyanobacteria were grouped with the macroalgae due to identification challenges, particularly from images taken in earlier years. During image analysis, the substrate that a benthic taxon was growing on (dead A. cervicornis or hardbottom) was noted.
Stony coral demographic data were collected in situ on one 22-m × 1-m belt transect per station from 2012 to 2022. Stony coral demographic data were not part of the SECREMP sampling protocol prior to 2012. Stony coral colonies ≥4 cm in diameter were identified to the species level, measured, and their health conditions (i.e., presence of bleaching, disease, and/or predation) recorded. In situ data did not differentiate between stony corals growing on substrate versus dead A. cervicornis. The stony coral demographic data did not include A. cervicornis as individual colonies could not be identified in a thicket due to their interconnected, complex branching morphology.
2.3 Disturbances
An examination of the published literature was conducted to identify the disturbance events that may have impacted BCA during the study period (Table 1). These included heat stress and cold stress events, when bleaching/mortality was recorded in the area, and disease or predation outbreaks when >50% of the A. cervicornis plots surveyed annually had incidences of disease (white band or rapid tissue loss) or incidences of predation (Hermodice carunculata or Coralliophila erosa, formerly Coralliophila abbreviata), as per Goergen et al. (2019).
Table 1
| Disturbance | Dates | Reference |
|---|---|---|
| Heat stress | May–October 2005 May–November 2005 |
Wilkinson and Souter, 2008 Eakin et al., 2010 |
| Heat stress | May–October 2009 | Goergen et al., 2019 |
| Cold stress | January–March 2010 | Lirman et al., 2011 Jones et al., 2020 |
| Disease outbreak | Summer/Fall 2012 | Goergen et al., 2019 |
| Predation outbreak | Summer/Fall 2012 | Goergen et al., 2019 |
| Heat stress | Summer 2014 | Goergen et al., 2019 |
| Disease outbreak | Summer 2014 | Goergen et al., 2019 |
| Predation outbreak | Summer 2014 | Goergen et al., 2019 |
| Heat stress | Summer 2015 | Goergen et al., 2019 Jones et al., 2020 |
| Disease outbreak | Summer 2015 | Goergen et al., 2019 |
| Predation outbreak | Summer 2015 | Goergen et al., 2019 |
Disturbances affecting the Broward County Acropora (BCA) area as identified in the literature or as part of published studies.
To examine the impact of tropical storms and hurricanes on BCA, wind data were gathered from the National Hurricane Center and Central Pacific Hurricane Center website managed by the National Oceanographic and Atmospheric Administration (NOAA; nhc.noaa.gov) (Table 2). NOAA defines a tropical storm as a tropical cyclone where the maximum sustained surface wind speed, the highest 1-min average wind (measured at a height of 10 m with unobstructed exposure) associated with the system at a given moment in time, reaches 34–63 kt. Hurricanes are defined as tropical cyclones where the maximum sustained wind speed exceeds 63 kt. Based on this criterion, storms that reached a maximum sustained wind speed of at least 34 kt, as recorded from land stations or data buoys located within 11 km of BCA, were included in this study (Table 2). These stations or buoys included Fort Lauderdale Executive Airport (KFXE), Dania Pier (XDAN), Port Everglades (XPEG), South Port Everglades (PEGF1), Port Everglades Channel (PVGF1), and Fort Lauderdale International Airport (KFLL). Although the wind strength measured during these storms may not directly have impacts on BCA, the maximum sustained surface wind speed serves as a proxy for times of increased wave and surge energy that may have physically impacted BCA. Nearly all selected events occurred after the BCA data were collected that year; therefore, the effects of those events would have been documented during the next monitoring year. Only one storm, Hurricane Elsa, was an exception. This storm made landfall on July 7, 2021, in northern Florida, 2 weeks before SECREMP monitoring occurred on July 22, 2021.
Table 2
| Storm | Date | Station | Max. sustained surface wind (kt) | Max. surface gusts (kt) |
|---|---|---|---|---|
| Frances | September 5, 2004 | KFXE | 36 | 48 |
| Jeanne | September 26, 2004 | KFLL | 35 | 49 |
| Katrina | August 25, 2005 | KFLL | 52 | 71 |
| Wilma | October 24, 2005 | KFLL | 61 | 86 |
| Isaac | August 27, 2012 | XPEG | 42 | 58 |
| Sandy | October 25, 2012 | PVGF1 | 36 | 46 |
| Irma | September 10, 2017 | XPEG | 61 | 74 |
| Gordon | September 3, 2018 | PEGF1 | 42 | 49 |
| Sally | September 12, 2020 | XDAN | 34 | 42 |
| Eta | November 9, 2020 | XPEG | 51 | 59 |
| Elsa | July 5, 2021 | XDAN | 35 | 41 |
Tropical cyclone wind strength data (nhc.noaa.gov) for each of the selected tropical storm or hurricane events.
In cases where multiple stations/buoys gathered wind data, the values for maximum sustained surface wind and maximum sustained gusts were selected from the station/buoy that recorded the highest maximum sustained surface wind.
KFXE, Fort Lauderdale Executive Airport; KFLL, Fort Lauderdale International Airport; XPEG, Port Everglades; PVGF1, Port Everglades Channel; PEGF1, South Port Everglades; XDAN, Dania Pier.
2.4 Statistical analyses
2.4.1 Acropora cervicornis dynamics
Generalized linear mixed models (GLMMs) were used to statistically analyze temporal changes in the percent benthic cover of living A. cervicornis, dead A. cervicornis, and hardbottom from 2003 to 2022 using R software (R Core Team, 2022). For each response variable, a single binomial GLMM was created with station per year as a replicate (n = 80). Percent cover was fitted as a continuous dependent variable, year was fitted as a categorical fixed effect, and station was fitted as a categorical random effect. The number of points used to calculate the percent cover per station was fitted as weights. Fitted models were validated using the package “DHARMa” (Hartig, 2022), with residual diagnostics conducted to detect overdispersion, zero inflation, variance homogeneity, and temporal autocorrelation. Model validation detected significant overdispersion, and a beta-binomial distribution was fitted (Harrison, 2015). Model validation of the beta-binomial GLMM indicated no issues. Post-hoc pairwise comparisons of the year-to-year changes in the response variable were conducted using the package “emmeans” and Tukey’s method (Lenth, 2023). Estimated marginal means (emmeans) linear contrasts were used to assess significant variations in the levels of a fixed effect against the mean value.
2.4.2 Benthic community dynamics
Multivariate analyses were performed to assess temporal changes in the benthic community structure and the non-A. cervicornis stony coral assemblage structure using Primer 7 (Clarke and Gorley, 2006). The benthic community structure was assessed as the percent benthic cover per station per year from photographic data. The non-A. cervicornis stony coral assemblage structure was assessed as the abundance of each species per station per year using in situ survey data. Before analysis, each dataset was square root-transformed to reduce the influence of abundant taxa and enable rarer taxa to contribute to the similarity calculation. Thereafter, a Bray–Curtis similarity matrix was generated. To analyze significant between-year differences in the benthic and coral community structure, an analysis of similarity (ANOSIM) test was performed with station per year as a replicate (9,999 permutations). To assess significant temporal grouping structure within the benthic and coral communities, the mean benthic and coral community structure per year was analyzed using similarity profile (SIMPROF) analysis (9,999 permutations, 95% similarity between samples). A similarity percentage (SIMPER) analysis was performed to identify which species, taxa, or substrate types were driving the differences between the SIMPROF groups and years. From the SIMPER analysis, the taxa that cumulatively explained 50% of the dissimilarity between groups were selected. Temporal variation in the benthic or coral community structure was visualized using a non-metric multidimensional scaling (nMDS) plot, where each sample represents the mean community structure per year. Vectors were overlaid to visualize the origin of the differences between samples. SIMPROF groups were overlaid onto each nMDS plot for visualization. After initially generating the nMDS plot, it was determined that a single large Pseudodiploria clivosa colony that was growing on the hardbottom adjacent to the A. cervicornis patch had an overweighted effect on the nMDS ordination (Supplementary Figure S1). It was removed from the data to more clearly show the transition from the 2012–2016 coral community to 2017–2022. Statistical analysis showed the same significant temporal changes in the coral community in the ANOSIM and SIMPROF models with and without P. clivosa.
Temporal variations in substrate utilization by the non-A. cervicornis taxa that drove variability in the benthic community and the macroalgal and CCA cover were further analyzed using binomial GLMMs. The CCA cover on hardbottom was fitted into a binomial distribution with a zero-inflation parameter. The CCA and the macroalgal cover on dead A. cervicornis, as well as the macroalgal cover on hardbottom, were fitted with a beta-binomial model. Model validation and post-hoc analysis were conducted as previously described.
Due to their greater abundance and density, the Agaricia agaricites data were separated from all other non-A. cervicornis stony coral data. Temporal variations in the A. agaricites density and all other non-A. cervicornis stony coral density from 2012 to 2022 were analyzed using Poisson GLMMs. The A. agaricites and all other non-A. cervicornis abundance data were fitted as a continuous dependent variable, year was fitted as a categorical fixed effect, station was fitted as a categorical random effect, and the area of each transect was used as an offset term to account for unit effort. Model validation and post-hoc analysis were conducted as previously described.
3 Results
3.1 Acropora cervicornis dynamics
The mean (±SE) live A. cervicornis cover in BCA was highest in 2004 (41.4% ± 1.3%) and showed a general decline to 2.4% ± 0.6% by 2022 (Figure 2). Notably, significant periods of live cover decline generally coincided with acute disturbances (Tables 1; 2). These included declines from 2004 (41.4% ± 1.3%) to 2006 (22.8% ± 1.6%), from 2008 (28.4% ± 0.93%) to 2011 (13.7% ± 1.2%), and from 2012 (15.05% ± 1.65%) to 2016 (4.4% ± 0.97%). Significant consecutive annual declines occurred across the 20-year duration, from 2005–2006 (GLMM, Tukey’s pairwise comparisons, p < 0.001), 2010–2011 (GLMM, Tukey’s pairwise comparisons, p = 0.044), and 2015–2016 (GLMM, Tukey’s pairwise comparisons, p = 0.0038). The only period with a significant interannual increase in live cover was from 2018 to 2020, with cover increasing from 1.8% ± 0.4% to 5.1% ± 1.2% (GLMM, Tukey’s pairwise comparisons, p = 0.049).
Figure 2
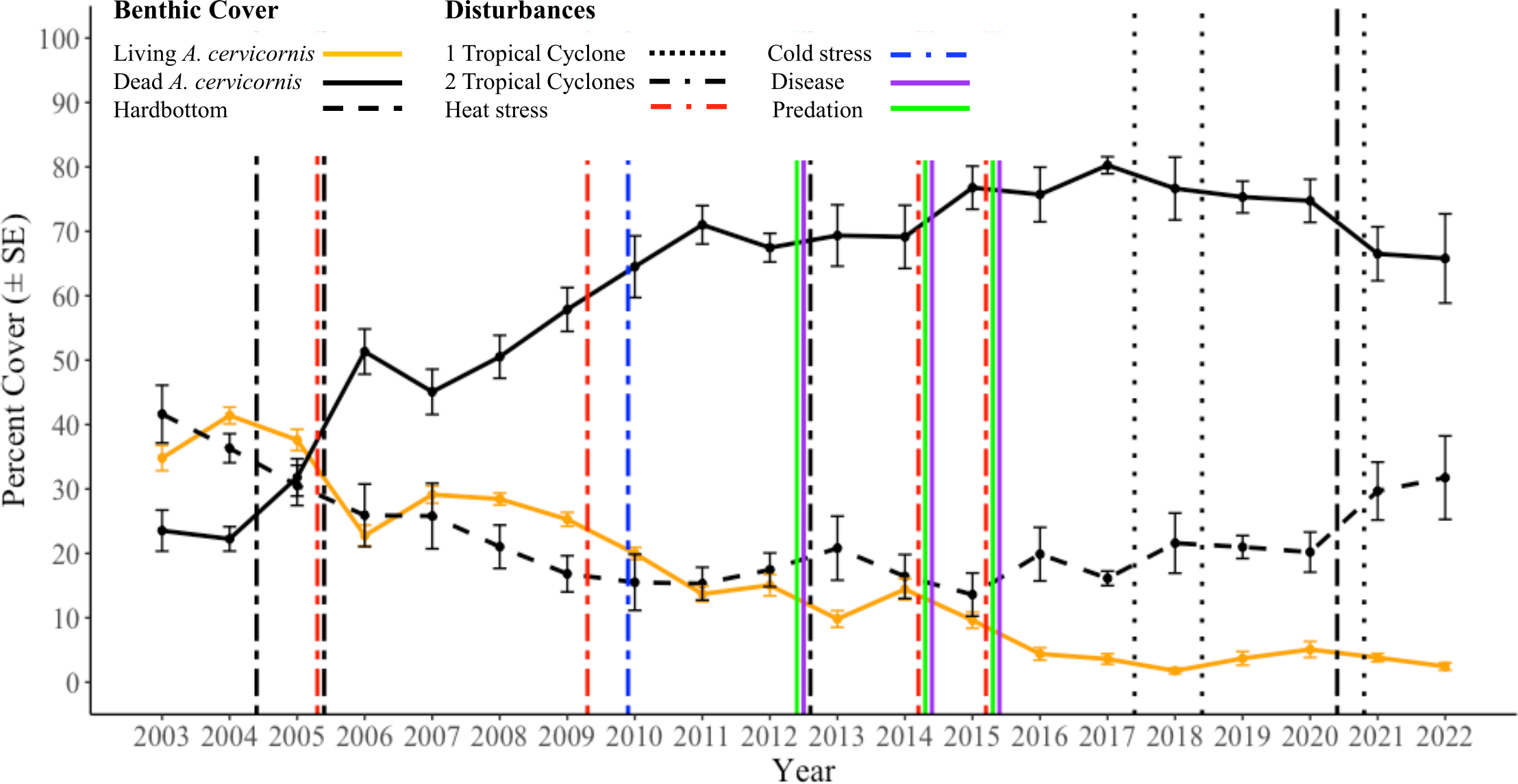
Annual mean (±SE) percent cover of living Acropora cervicornis, dead A. cervicornis, and hardbottom (horizontal lines). The x-axis values correspond with the monitoring dates (usually in June). Disturbance events are indicated by vertical lines, with color representing the disturbance type. Tropical cyclone information was taken from nhc.noaa.gov.
Dead A. cervicornis cover increased from 23.5% ± 3.2% in 2003 to 80.3% ± 1.3% in 2017, but declined significantly to 65.8% ± 6.9% from 2017 to 2022 (Tukey’s pairwise comparisons, p = 0.046). The greatest consecutive year-to-year increase in dead A. cervicornis cover occurred from 2005 (31.8% ± 2.9%) to 2006 (51.3% ± 3.5%) (GLMM, Tukey’s pairwise comparisons, p = 0.0025).
Temporal changes in the hardbottom cover mirrored the changes in the dead A. cervicornis cover (Figure 2). The mean (±SE) hardbottom cover was greatest in 2003 (41.6% ± 4.5%), which was significantly greater than the second lowest cover year (2017; GLMM, Tukey’s pairwise comparisons, p < 0.001). In 2015, the lowest cover of hardbottom was reported (13.6% ± 3.3%), which was significantly less than that in 2022 (GLMM, Tukey’s pairwise comparisons, p = 0.012).
3.2 Benthic community dynamics
The benthic community structure changed from 2003 to 2022, coinciding with the declines in the live A. cervicornis cover and the changes in substrate availability (Figure 3). Three periods of significant change in the benthic community structure were identified: from 2005 to 2006, from 2008 to 2010, and from 2019 to 2021 (ANOSIM, p < 0.05). These changes were predominantly due to fluctuations in the live A. cervicornis, dead A. cervicornis, hardbottom, and macroalgal and/or CCA cover. SIMPROF analysis identified five year groups that varied significantly (SIMPROF, p = 0.001) (Figure 3, green circles). The period 2003–2005 had higher live A. cervicornis and hardbottom cover, but lower dead A. cervicornis cover, compared with 2006–2008 (SIMPER, cumulative percent contribution = 56.75%). The period 2006–2008 had higher live A. cervicornis and hardbottom cover than 2009–2015, which had higher dead A. cervicornis and macroalgal cover on dead A. cervicornis (SIMPER, cumulative percent contribution = 54.77%). The variability between the SIMPROF groups 2009–2015 and 2016–2020 was driven by a further decline in A. cervicornis and increases in hardbottom, as well as significant increases in the macroalgal cover on dead A. cervicornis and CCA cover on dead A. cervicornis (SIMPER, cumulative percent contribution = 55.46%). The variability between the SIMPROF groups 2016–2020 and 2021–2022 was driven by a decline in dead A. cervicornis, increases in the hardbottom cover, and significant changes in the macroalgal cover on dead A. cervicornis and macroalgal cover on hardbottom (SIMPER, cumulative percent contribution = 59.86%).
Figure 3
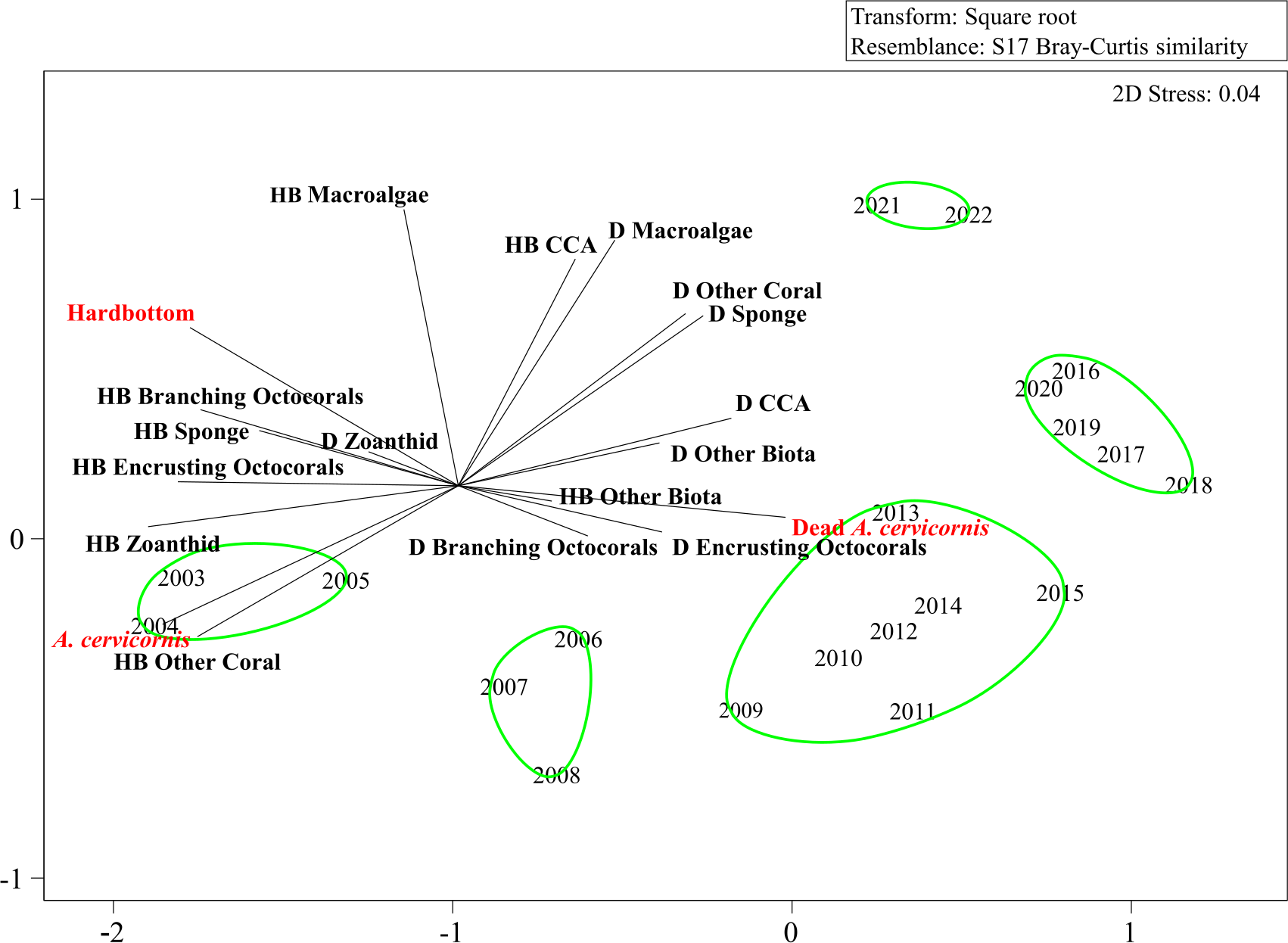
Non-metric multidimensional scaling (nMDS) plot of the benthic community structure based on the percent benthic cover over time. Vectors represent the benthic community taxa separated by the type of substrate they were recorded growing on. The “D” in front of the taxon labels denotes “percent cover of this taxon growing on dead Acropora cervicornis,” while the “HB” denotes “percent cover of this taxon growing on hardbottom.” The two different substrates observed with nothing but turf algal growth are also displayed (dead A. cervicornis and hardbottom). Green circles identify significant groups from the similarity profile (SIMPROF) analysis.
Temporal trends in macroalgae and CCA were further analyzed using GLMMs due to their influence on benthic community changes (Figure 4). The macroalgal cover on dead A. cervicornis significantly increased from 2008 to 2013 (GLMM, Tukey’s pairwise comparisons, p = 0.017), with 2013 having the highest cover within the interval (GLMM, emmeans linear contrasts, p = 0.0202). The CCA cover on dead A. cervicornis was significantly higher than the study mean (2.19%) in 2015 and that between 2017 and 2019 (GLMM, emmeans linear contrasts, p < 0.05). The macroalgal cover on hardbottom increased significantly from 2017 to 2022 (GLMM, Tukey’s pairwise comparisons, p = 0.023), as well as being significantly higher than the mean values in 2018, 2021, and 2022 (GLMM, emmeans linear contrasts, p < 0.05). The macroalgal cover on dead A. cervicornis significantly increased from 2018 to 2021 (GLMM, Tukey’s pairwise comparisons, p = 0.0005) and was significantly higher than the mean values in 2017, 2020, 2021, and 2022 (GLMM, emmeans linear contrasts, p < 0.05).
Figure 4
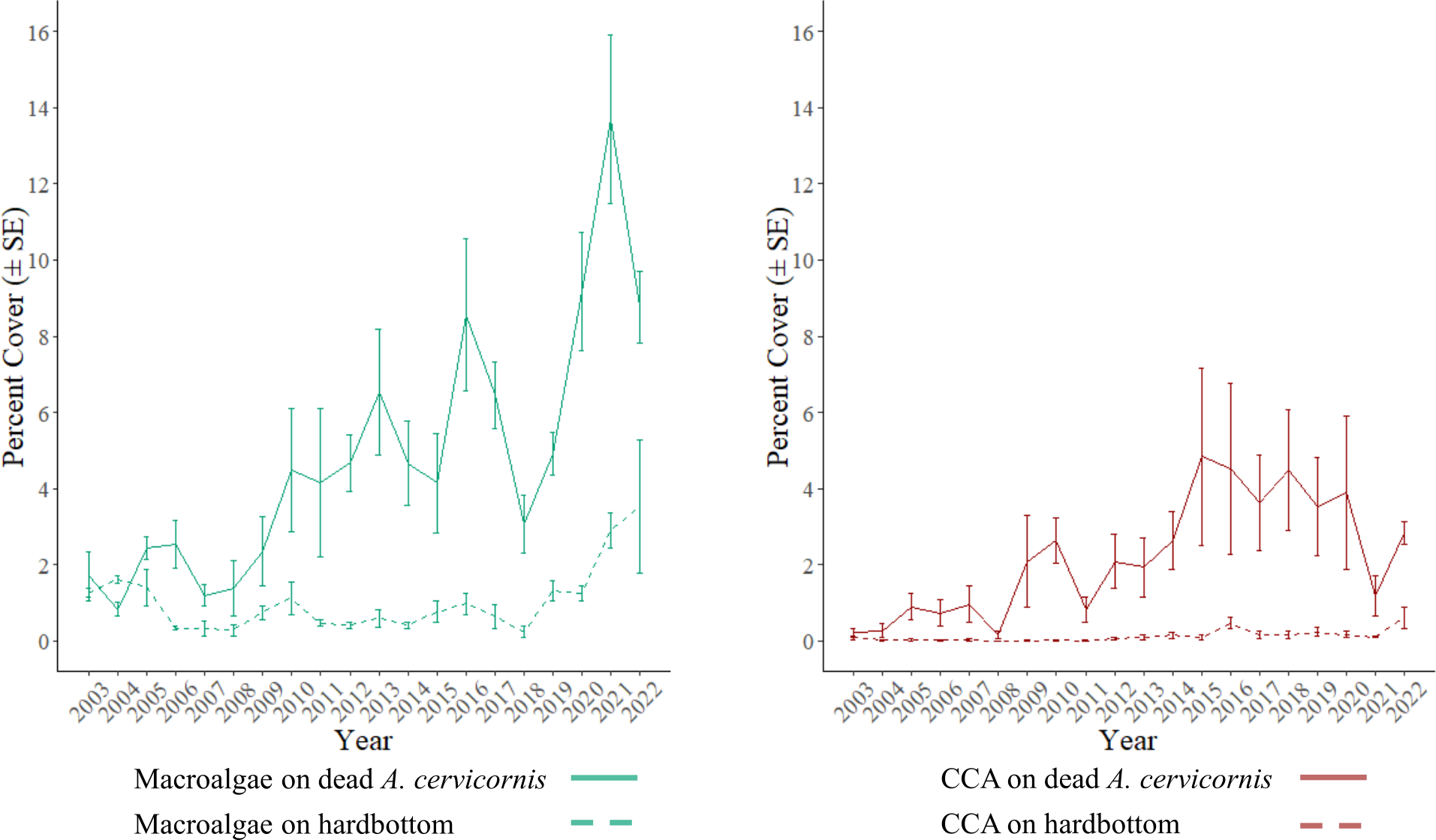
Temporal change in the percent cover of macroalgae and crustose coralline algae (CCA) from 2003 to 2022 by the substrate type they were recorded on. The macroalgal and CCA cover explained much of the temporal variability in the benthic community structure identified by analysis of similarity (ANOSIM) and similarity profile (SIMPROF) analysis.
Across the duration of the project, there was a significant change in the non-A. cervicornis stony coral assemblage (Figure 5). The A. agaricites colony density significantly increased from 2012 to 2020 from 0.33 colonies/m2 to 4.49 colonies/m2, with three significant annual changes (2014–2015, 2016–2017, and 2019–2020; Tukey’s pairwise comparisons, p < 0.008) (Figure 6). Two stony coral assemblage SIMPROF groups were identified, 2012–2016 and 2017–2022 (Figure 5), driven by increases in A. agaricites (SIMPER, cumulative percent contribution = 59.24%). A. agaricites did experience a significant decrease in density from 2020 to 2021 (GLMM, Tukey’s pairwise comparisons, p < 0.001). The density of other non-A. cervicornis stony coral increased over the study, with a mean density significantly above those in 2020, 2021, and 2022 (GLMM, Tukey’s pairwise analysis, p < 0.05). This was mostly driven by increases in the density of Porites porites and Porites astreoides. P. porites had increased from 0.01 colonies/m2 in 2012 to 0.11 colonies/m2 in 2020, 0.13 colonies/m2 in 2021, and 0.09 colonies/m2 in 2022, while P. astreoides had increased from 0.16 colonies/m2 in 2012 to 0.31 colonies/m2 in 2020 and 2021, and 0.32 colonies/m2 in 2022.
Figure 5
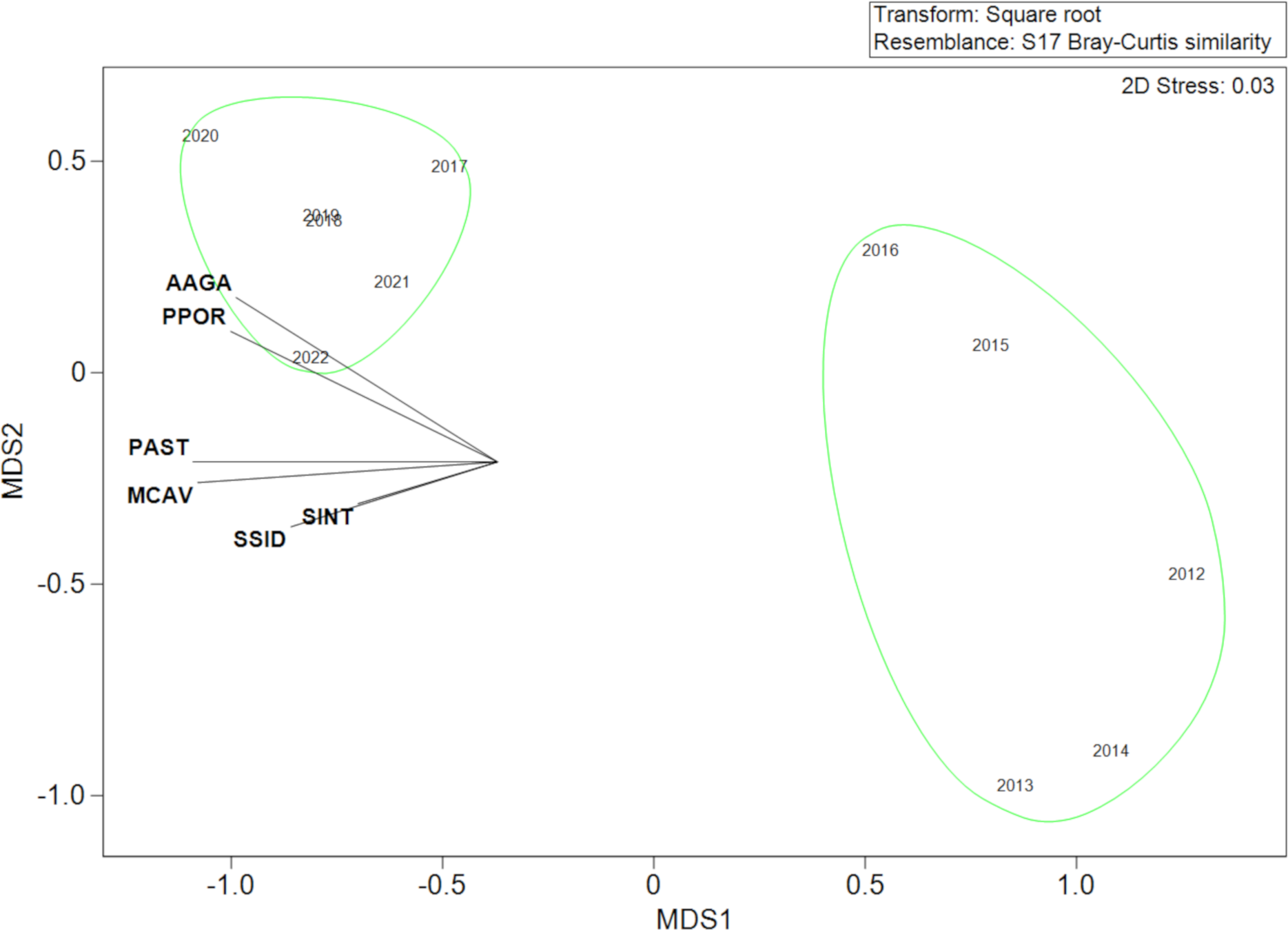
Non-metric multidimensional scaling (nMDS) plot comparing the mean density of the non-Acropora cervicornis stony coral assemblage over time. Green circles identify significant groups from the similarity profile (SIMPROF) analysis. Names correspond to the first letter of the genus and the first three letters of the species name, e.g., AAGA, Agaricia agaricites and PPOR, Porites porites.
Figure 6
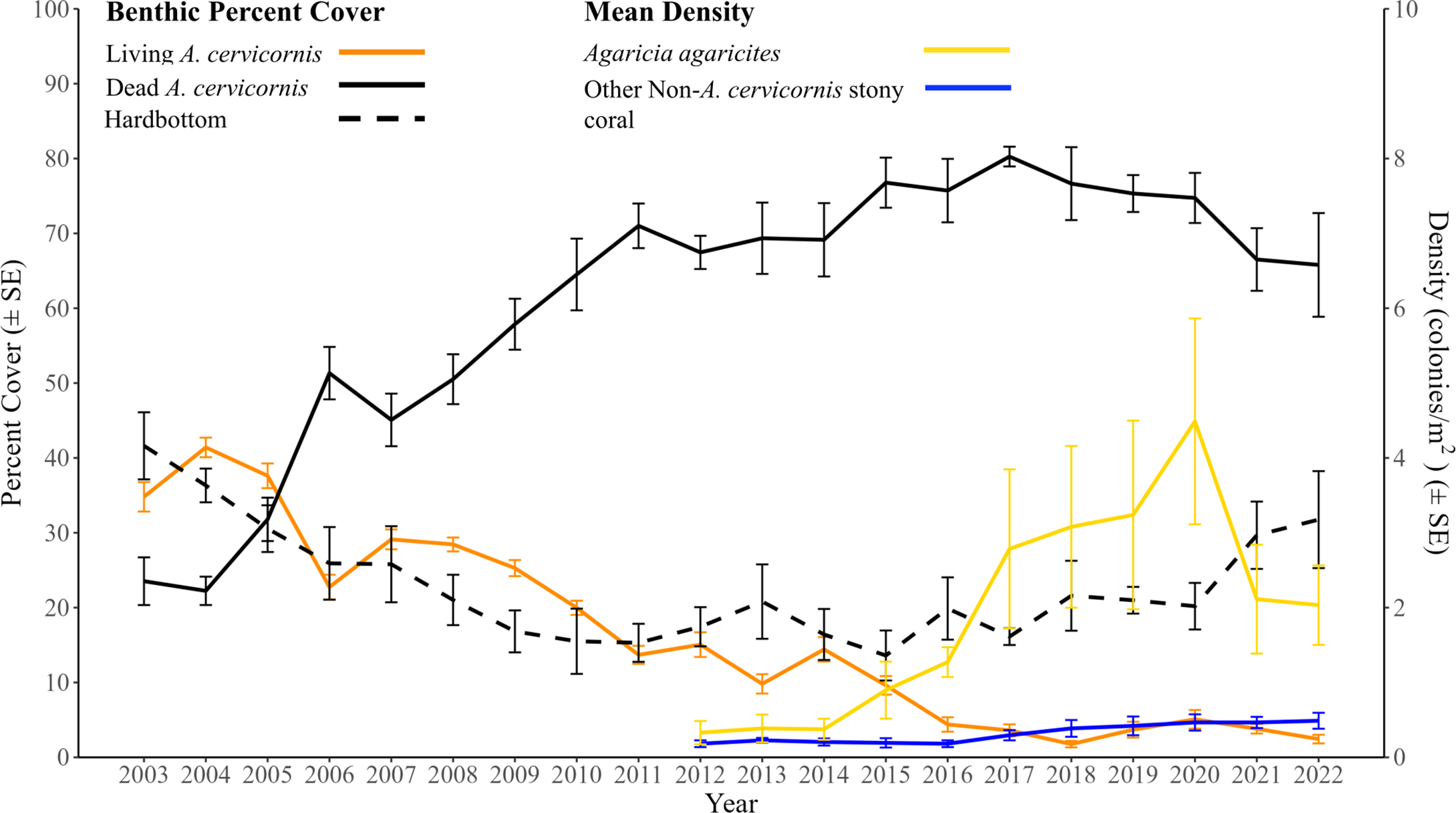
Annual mean (±SE) percent benthic cover of living Acropora cervicornis, dead A. cervicornis, and hardbottom from Figure 2, with the mean density (±SE) of A. agaricites and other non-A. cervicornis stony corals excluding A. agaricites from 2012 to 2022 superimposed over it for comparison.
4 Discussion
The living A. cervicornis cover in the densest section of BCA declined by 93% from 2003 to 2022, primarily due to multiple acute disturbances, such as repeated incidences of heat stress, disease outbreaks, predation outbreaks, and tropical cyclones. The high disturbance frequency provided limited opportunities for recovery (Jones et al., 2022), resulting in the prolonged decline of the A. cervicornis cover and significant shifts in the benthic community structure. Despite the continuous loss of living A. cervicornis in the thicket, standing dead A. cervicornis branches continued to provide available substrate. This substrate promoted successional changes in the benthic community structure, primarily increases in the macroalgal and CCA cover, and a substantial increase in the density of the encrusting, brooding, stony coral species A. agaricites. While these taxa occupy space, they contribute little to the structural integrity of the thicket. Hence, without replacement by living A. cervicornis, ongoing disturbances and the mechanical and biological degradation of dead branches will result in the gradual loss of the unique functional services provided by BCA, ultimately transforming the environment into a relatively flat hardbottom-dominated habitat.
A total of 11 tropical cyclones, four heat stress events, one cold stress event, three disease outbreaks, and three predation outbreaks impacted BCA over 20 years, which often preceded significant declines in live A. cervicornis and concomitant increases in dead A. cervicornis, and/or hardbottom benthic cover. Declines in the live cover were mostly gradual, except for the acute declines observed following the 2005 hurricane season, the 2010–2011 cold stress event, and the 2014–2016 heat stress events, which were exacerbated by disease and predation outbreaks. Tropical cyclones have been known to cause immediate mechanical damage to coral reefs after impact (Gardner et al., 2005; Eakin et al., 2010), and several documented here impacted other habitats along the FCR. Hurricanes Wilma and Katrina (in 2005) were associated with sediment resuspension and cold water upwelling that affected FCR (Collier et al., 2008), and Hurricane Irma in 2017 contributed to the dislodgment of many A. cervicornis colonies (Walker, 2018).
The major declines in the A. cervicornis cover followed the multi-disturbance years with intense heat stress (Jones et al., 2022). Acroporids are known to be particularly susceptible to thermal stress (Schopmeyer et al., 2012; Hughes et al., 2018; Riegl et al., 2018; Jones et al., 2020), even when compared against other reef-building coral species, such as Orbicella faveolata (Langdon et al., 2018). Both heat and cold stress can result in acroporid mortality. For example, cold stress in the Dry Tortugas caused 96% of the A. cervicornis colonies to die from 1976 to 1977 (Porter et al., 1982), heat stress caused up to 95% Acropora sp. mortality on the Great Barrier Reef following the 1998 marine heat wave (Berkelmans and Oliver, 1999), and the A. cervicornis cover declined significantly following heat stress in 2014–2015 along the FCR (Jones et al., 2020), which were the hottest years on record during the study period (Manzello, 2015).
Thermal stress can also increase the susceptibility of A. cervicornis to disease outbreaks (Muller et al., 2018). Major disease outbreaks followed the 2014 and 2015 heat stress events, leading to further mortality. Disease outbreaks have greatly affected A. cervicornis and are a principal driver of mortality in many areas of the Caribbean (Aronson and Precht, 2001), including Florida, especially during the summer months (Goergen et al., 2020). The causative agent of the disease outbreaks seen here is unknown, but disease is often exacerbated by environmental stress, particularly temperature (e.g., Rogers et al., 2009; Randall and van Woesik, 2015), water quality (Voss and Richardson, 2006), and tropical storms and hurricanes (Knowlton et al., 1981; Goergen et al., 2019) elsewhere. The 2014–2015 A. cervicornis disease outbreaks at BCA coincided with the initial stony coral tissue loss disease (SCTLD) outbreak in southeast Florida (Precht et al., 2016), adding to suggestions that environmental conditions reduced coral immunity and/or exacerbated the pathogen activity in the region. All three disease outbreaks (2012, 2014, and 2015) coincided with the predation outbreaks, which have been linked with increased disease prevalence (Renzi et al., 2022) and can directly cause extensive mortality. Predation played a major role in the A. cervicornis decline in Jamaica after the passing of Hurricane Allen (Knowlton et al., 1990), and at BCA, the H. carunculata predation outbreaks in 2012, 2014, and 2015 likely also contributed substantially to the declines in the A. cervicornis cover (Goergen et al., 2019). Our data suggest that the frequency and the variety of disturbances that have impacted BCA have caused the dramatic decline observed in the A. cervicornis cover over the last 20 years.
Not all disturbances led to significant changes in the A. cervicornis cover. While this may represent resistance to some disturbances, annual surveys can undoubtedly mask intra-annual (i.e., seasonal) changes. For instance, high disease prevalence was detected at BCA 2 weeks after the passing of Hurricane Isaac in 2012, which resulted in substantial morality (Goergen et al., 2019). While we detected a slight decline in the A. cervicornis cover the following summer, 2013, it was not significant, likely due to some recovery directly before our 2013 survey, when Goergen et al. (2019) reported the largest increase in the percent live cover of A. cervicornis. In other cases, lower wind speeds and heterogeneity in wave action may have prevented extensive mortality (Gardner et al., 2005; Mallin and Corbett, 2006). For instance, Hurricanes Francis and Jeanne in 2004 both passed through South Florida, but caused a non-significant decline in the A. cervicornis cover. Although they met the threshold of this study, Hurricanes Francis and Jeanne had weaker wind speeds recorded (maximum sustained surface wind speeds of 36 and 35, respectively) than Hurricanes Wilma and Katrina (maximum sustained surface wind speeds of 61 and 52, respectively). Distance from the storm may have played a role. Hurricanes Jeanne and Francis had almost identical paths making landfall in North Palm Beach County (Collier et al., 2008) approximately 80 km north of BCA. Hurricane Wilma made landfall on the Gulf Coast (Collier et al., 2008) and passed approximately 70 km north of BCA when reaching the East Coast. Hurricane Katrina made landfall on the East Coast (Collier et al., 2008) approximately 20 km south of BCA, the closest of the four, but was a weaker category 1 storm at the time. Furthermore, after 2016, the lack of a significant decline in cover following disturbance was at least in part because there was little live Acropora left to die.
While A. cervicornis is among the fastest growing species in the Caribbean and acroporids are frequently associated with coral reef recovery (e.g., Pratchett et al., 2020), no periods of sustained substantial recovery were recorded at BCA over the 20 years; however, Goergen et al. (2019) reported increases in the live cover of A. cervicornis at BCA during the summer periods when minimal disturbances were observed. The only period of significant increase was during the inter-disturbance period from 2018 to 2020, when the live A. cervicornis cover increased by 3.3%. This recovery period was followed by a significant A. cervicornis cover loss of 2.6% from 2020 to 2022, coinciding with three tropical cyclones. Recovery may be facilitated by A. cervicornis colony growth, successful reattachment and growth of loose fragments, and recruitment. However, the reattachment rates of loose fragments have been found to be low in southeast Florida (Goergen et al., 2019) and did not compensate for the losses associated with the disturbance events in BCA. Furthermore, while a high connectivity of the A. cervicornis populations across the FCR has been modeled (King et al., 2023), the larval recruitment in this area is very low (Vargas-Angel et al., 2006), with no records of recruits during long-term monitoring studies (Hayes et al., 2023). These findings corroborate the limited stony coral recovery seen throughout the FCR, with high disturbance frequency and chronic pressures (Jones et al., 2022) limiting the potential for significant recovery of this thicket.
This research provides new insights into the changing substrate dynamics at BCA over the study period. The decline in the living A. cervicornis cover, coupled with repeated tropical cyclones, caused temporal fluctuations in the substrate type, which prevent long-term recovery or community development. At the start of the study in 2003, 41.6% of the substrate was hardbottom, 34.8% live A. cervicornis, and 23.5% dead A. cervicornis. After the passing of Hurricanes Wilma and Katrina in 2005, two of the more powerful storms documented during the study period, the dead A. cervicornis cover exceeded both the living A. cervicornis cover and the hardbottom cover for the first time. Although this study did not differentiate between rubble and standing dead A. cervicornis, in many years, the loss of living A. cervicornis was not proportional to the increase in the dead, with many branches fragmenting, remaining in the thicket on the substrate as rubble and removing substrate suitable for community development (Kenyon et al., 2023). The constant movement of rubble prevents attachment and binding, creating an unstable environment and making it difficult for benthic taxa to grow on both the rubble itself and the hardbottom underneath (Kenyon et al., 2023). At BCA, the utilization of stable dead A. cervicornis structure increased as the living A. cervicornis died and rubble covered the hardbottom. Only when Hurricane Irma passed in 2017 did the rubble begin to be removed, the dead A. cervicornis cover declined, and hardbottom for colonization began to increase again. From this point onward, physical disturbance from multiple tropical cyclones likely continued to remove rubble and, coupled with bio-erosional processes, contributed to the breakdown of the dead A. cervicornis structure, resulting in increased hardbottom cover.
The three-dimensional structure created by A. cervicornis thickets such as the BCA provides unique functional services. In thickets, structure is primarily maintained by the growth of the existing or the establishment of new A. cervicornis colonies; however, despite the continued loss of live cover, the dead structure here remained for years, creating a habitat for the settlement and growth of other benthic taxa. The loss of live A. cervicornis cover created available substrate, leading to increases in the macroalgal and CCA cover, which in turn prevented A. cervicornis from regrowing over the dead framework. Macroalgal increases have been reported over the last few decades throughout the Caribbean (Aronson and Precht, 2001; Hughes et al., 2010; García-Urueña and Garzón-MaChado, 2020; Jones et al., 2022; but see Bruno et al., 2009) and have often been linked to a lack of stony coral recovery (e.g., Jones et al., 2022) by limiting recruitment success and causing mortality by abrasion (Box and Mumby, 2007). García-Urueña and Garzón-MaChado (2020) specifically noted that macroalgae reduced the recovery of A. cervicornis. In the Coral ECA, the macroalgal cover has historically been represented as a dominant component of the benthic community along the hardbottom nearshore ridge complex (Banks et al., 2008), and its cover is expected to increase under climate change (Jones et al., 2020). Without A. cervicornis growth driving thicket recovery, and with limited sexual recruitment, benthic taxa such as macroalgae will continue to increase in cover, and these unique sites will soon be homogeneous to the rest of the nearshore hardbottom habitat found throughout the Coral ECA.
The non-A. cervicornis stony coral density increased during the study period, but this was predominantly due to increases in the small, encrusting species A. agaricites that do not provide the same functional services as A. cervicornis (Alvarez-Filip et al., 2013). The density of A. agaricites significantly increased from 2014 to 2020, peaking at 4.5 colonies/m2, and represented 91% of the non-A. cervicornis stony coral assemblage in 2020. Despite the increased density, it contributed little to the benthic cover (<1% cover). A. agaricites is known to colonize shaded regions of the reef, including within the understory of the A. cervicornis dead structure (Orrell, 1981), and, as a brooding species, can colonize disturbed reef environments quickly (Robbart et al., 2004). Agaricia species have been documented as increasing in other areas that are dominated by A. cervicornis in the Caribbean, such as in Roatan (Riegl et al., 2009) and Belize (Aronson et al., 2002; Caterham et al., 2019). While it has always been part of the stony coral assemblage within BCA, its population explosion coincided with the live A. cervicornis cover declining below 10% in 2014. Other brooding species, such as P. astreoides and P. porites, also increased in abundance after this time, but not to the same extent as A. agaricites. Although all three species are considered “weedy,” as they have a brooding mode of reproduction and a low dispersal rate (Darling et al., 2012; Cramer et al., 2021), the ability of A. agaricites to conform to the shape and structure of A. cervicornis due to its more encrusting nature and its ability to perform well in shaded areas may be a contributing factor why it has proliferated while other species have not. Similarly to A. agaricites, both species provide little structural or skeletal support for the dead A. cervicornis branches (Alvarez-Filip et al., 2013). The lack of A. cervicornis recovery and support for the dead structure will lead to reef flattening, or the loss of complex architectural structure, a phenomenon documented in the wider Caribbean (Alvarez-Filip et al., 2009). When these habitats are lost, there are fewer niches for organisms, which reduces functional services and decreases biodiversity. This can lead to biotic homogenization, where species assemblages become more similar at different locations over time, as has been noted elsewhere on the FCR (Burman et al., 2012). The long-term persistence of A. cervicornis thickets, including the dead structure, defines their irreplaceable role in providing essential functional services in the Coral ECA and the urgent need for conservation and restoration efforts to prevent further loss of these unique habitats.
The annual nature of the data collection and the specific disturbances analyzed may not fully explain the changes in the benthic community structure observed in this study. Water quality (De'ath and Fabricius, 2010; Jones and Gilliam, 2024), the effects of pollution (Finkl and Charlier, 2003), and the presence/absence of herbivores (Hughes et al., 2010) may be additional drivers of change. Some acute disturbances may not have been captured during the monitoring or literature review: for example, there were reports of a cyanobacteria (Lyngbya spp.) bloom at BCA in 2004 (Gilliam et al., 2008). More frequent monitoring, similar to the tri-annual monitoring utilized by Goergen et al. (2019), could aid in the identification of the direct causes of the benthic community change. Another limitation in this study is its analysis of only a portion of BCA instead of the entire thicket area. These results represent data from the densest area of the thicket, in the northeast corner of BCA. Through time, A. cervicornis thickets can move, but previous research on BCA has documented similar declines in A. cervicornis cover in different sections of the thicket (Walker, 2017). The data utilized from SECREMP represent an invaluable source of long-term data spanning 20 years, which was the main reason for the use of this dataset.
This study brings new insights into the BCA thicket, in particular following Goergen et al. (2019), by assessing changes to the community structure that follow the A. cervicornis population decline. While not captured here, the data hint at the complete collapse of BCA in the coming years. Stable dead structure remained from many years as living A. cervicornis cover continued to decline, but as frequent disturbances interact with BCA, the proportion of stable dead structure has begun to fall, leading to unstable rubble and, eventually, increases in hardbottom cover.
Frequent acute disturbances have significantly impacted BCA and drastically reduced the recovery potential of the large A. cervicornis thicket, which provides unique ecosystem services in the Coral ECA. Over the next few years, it is likely that continued monitoring will capture the complete collapse of this critical habitat by documenting increases in hardbottom. The importance of A. cervicornis is widely recognized, and thus, it is a frequent component of restoration efforts, in which many practitioners have had success in propagating and outplanting the species (Schopmeyer et al., 2017). Despite this, our results suggest that it will prove difficult to consistently promote growth and maintain large thickets such as BCA under the current environmental conditions, particularly in areas as impacted by increasing disturbances as southeast Florida. Previous research in southeast Florida found that outplanting A. cervicornis at a high density increased disease, predation, and colony fragmentation (Goergen and Gilliam, 2018). This may make restoring large A. cervicornis thickets such as BCA difficult. Mitigation of local pressures and active restoration may slow the decline, but sweeping actions regarding climate change are needed to improve environmental conditions that stimulate thicket recovery (Miller et al., 2016; Goergen and Gilliam, 2018; Goergen et al., 2019). The likely loss of the BCA thicket offshore highly urbanized South Florida may be a harbinger for the fate of the remaining thickets throughout the Caribbean that are impacted by growing local human population and climate change. Management efforts that incorporate active restoration, reduction of local pressures, and, most importantly, global pressures, are required to promote the recovery of A. cervicornis populations and the growth of extant and future thickets that provide valuable ecological services.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
DP: Formal Analysis, Supervision, Methodology, Data curation, Software, Project administration, Writing – review & editing, Writing – original draft, Conceptualization, Visualization, Investigation, Validation. NJ: Supervision, Formal Analysis, Methodology, Software, Investigation, Data curation, Validation, Writing – review & editing. EG: Methodology, Writing – review & editing, Validation. DG: Conceptualization, Funding acquisition, Resources, Validation, Investigation, Writing – review & editing, Project administration, Supervision, Methodology, Visualization, Software.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded in part from contracts and agreements from the Florida Fish and Wildlife Conservation Commission (multiple contracts), the Florida Department of Environmental Protection, Office of Coastal and Aquatic Managed Areas, as amended, through National Oceanic and Atmospheric Administration Awards (multiple awards), and the National Coral Reef Institute.
Acknowledgments
This research in part was previously available online as Daniel Perez’s master’s thesis (Perez, 2023). We would like to thank Dr. Brian Walker and Dr. Abigail Renegar for their comments on early drafts of the work. We would also like to thank members of the Coral Reef Restoration, Assessment, Monitoring at NSU, past and present, for collecting SECREMP data. Data for SECREMP can be found through this website: https://myfwc.com/research/habitat/coral/cremp/data/.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views, statements, findings, conclusions, and recommendations expressed herein are those of the author(s) and do not necessarily reflect the views of the State of Florida or the Department of Commerce, NOAA or any of its sub agencies.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1641098/full#supplementary-material
Supplementary Figure 1nMDS plot comparing the mean density of the non-A. cervicornis stony coral assemblage over time, with P. clivosa included. Green circles identify significant groups from SIMPROF analysis. Names correspond to first letter of the genus and first three letters of the species name. e.g., AAGA, Agaricia agaricites; PPOR, Porites porites.
References
1
Alvarez-Filip L. Carricart-Ganivet J. P. Horta-Puga G. Iglesias-Prieto R. (2013). Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep.3, 1–5. doi: 10.1038/srep03486
2
Alvarez-Filip L. Dulvy N. K. Gill J. A. Côté I. M. Watkinson A. R. (2009). Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. B: Biol. Sci.276, 3019–3025. doi: 10.1098/rsbp.2009.0339
3
Aronson R. B. Macintyre I. G. Precht W. F. Murdoch T. J. Wapnick C. M. (2002). The expanding scale of species turnover events on coral reefs in Belize. Ecol. Monogr.72, 233–249. doi: 10.1890/0012-9615(2002)072[0233:TESOST]2.0.CO;2
4
Aronson R. B. Precht W. F. (2001). White-band disease and the changing face of Caribbean coral reefs. Ecol. Etiology Newly Emerging Mar. Dis.460, 25–38.
5
(1997). National hurricane center and central pacific hurricane center. Available online at: https://www.nhc.noaa.gov/ (Accessed 11 Jan 2023).
6
Banks K. W. Riegl B. M. Richards V. P. Walker B. K. Helmle K. P. Jordan L. K. et al . (2008). The reef tract of continental southeast Florida (Miami-Dade, Broward and Palm Beach counties, USA). Coral Reefs U.S.A., 175–220.
7
Berkelmans R. Oliver J. K. (1999). Large-scale bleaching of corals on the Great Barrier Reef. Coral reefs18, 55–60. doi: 10.1007/s003380050154
8
Box S. J. Mumby P. J. (2007). Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser.342, 139–149. doi: 10.3354/meps342139
9
Bozec Y. Alvarez-Filip L. Mumby P. J. (2015). The dynamics of architectural complexity on coral reefs under climate change. Global Change Biol.21, 223–235. doi: 10.1111/gcb.12698
10
Brandt M. E. Smith T. B. Correa A. M. Vega-Thurber R. (2013). Disturbance driven colony fragmentation as a driver of a coral disease outbreak. PloS One8, e57164. doi: 10.1371/journal.pone.0057164
11
Bruckner A. W. (2002). Proceedings of the CaribbeanAcropora Workshop: Potential application of the US Endangered Species Act as a conservation strategy. NOAA Technical Memorandum NMFS-OPR-24 (Silver Spring, MD), 199.
12
Bruno J. F. Selig E. R. Casey K. S. Page C. A. Willis B. L. Harvell C. D. et al . (2007). Thermal stress and coral cover as drivers of coral disease outbreaks. PloS Biol.5, e124. doi: 10.1371/journal.pbio.0050124
13
Bruno J.F. Sweatman H. Precht W.F. Selig E.R. Schutte V.G. (2009). Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology90 (6), 1478–1484.
14
Burman S. G. Aronson R. B. van Woesik R. (2012). Biotic homogenization of coral assemblages along the Florida reef tract. Mar. Ecol. Prog. Ser.467, 89–96. doi: 10.3354/meps09950
15
Caterham C. R. An N. Mabaka A. Johnson G. Wirth K. R. Greer L. (2019). “The response of algae and herbivores to acropora cervicornis coral decline: A case study in coral gardens, Belize,” in AGU fall meeting abstracts (San Francisco, CA: AGU (American Geophysical Union)).
16
Clarke K. R. Gorley R. N. (2006). Primer. PRIMER-e. Plymouth866.
17
Collier C. Ruzicka R. Banks K. Barbieri L. Beal J. Bingham D. et al . (2008). The state of coral reef ecosystems of southeast Florida. Available online at: https://nsuworks.nova.edu/occ_facreports/31 (Accessed March 02, 2023).
18
Cramer K. L. Donovan M. K. Jackson J. B. Greenstein B. J. Korpanty C. A. Cook G. M. et al . (2021). The transformation of Caribbean coral communities since humans. Ecol. Evol.11, 10098–10118. doi: 10.1002/ece3.7808
19
Darling E. S. Alvarez-Filip L. Oliver T. A. McClanahan T. R. Côté I. M. (2012). Evaluating life-history strategies of reef corals from species traits. Ecol. Lett.15, 1378–1386. doi: 10.1111/j.1461-0248.2012.01861.x
20
Davis G. E. (1982). A century of natural change in coral distribution at the Dry Tortugas: a comparison of reef maps from 1881 and 1976. Bull. Mar. Sci.32, 608–623.
21
De'ath G. Fabricius K. (2010). Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl.20, 840–850. doi: 10.1890/08-2023.1
22
Eakin C. M. Morgan J. A. Heron S. F. Smith T. B. Liu G. Alvarez-Filip L. et al . (2010). Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PloS One5, e13969. doi: 10.1371/journal.pone.0013969
23
Finkl C. W. Charlier R. H. (2003). Sustainability of subtropical coastal zones in southeastern Florida: challenges for urbanized coastal environments threatened by development, pollution, water supply, and storm hazards. J. Coast. Res.19, 934–943.
24
García-Urueña R. Garzón-MaChado M. A. (2020). Current status of Acropora palmata and Acropora cervicornis in the Colombian Caribbean: demography, coral cover and condition assessment. Hydrobiologia847, 2141–2153. doi: 10.1007/s10750-020-04238-6
25
Gardner T. A. Côté I. M. Gill J. A. Grant A. Watkinson A. R. (2005). Hurricanes and Caribbean coral reefs: impacts, recovery patterns, and role in long-term decline. Ecology86, 174–184. doi: 10.1890/04-0141
26
Gilliam D. S. Hayes N. K. Ruzicka R. Colella M. (2021). Southeast florida coral reef evaluation and monitoring project 2020 year 18 final report (Miami Beach FL: Florida DEP & FWC), 82.
27
Gilliam D. S. Wheaton J. Callahan M. Beal J. Collier C. Herren L. et al . (2008). Southeast florida coral reef evaluation and monitoring project 2007 year 5 final report (Miami: Florida DEP). Available online at: https://floridadep.gov/rcp/coral/documents/secremp-year-5-final-report-2007.
28
Goergen E. A. Gilliam D. S. (2018). Outplanting technique, host genotype, and site affect the initial success of outplanted Acropora cervicornis. PeerJ6, e4433. doi: 10.7717/peerj.443
29
Goergen E. A. Lunz K. S. Gilliam D. S. (2020). Spatial and temporal differences in Acropora cervicornis colony size and health. Adv. Mar. Biol. Population Dynamics Reef Crisis87, 83–114. doi: 10.1016/bs.amb.2020.08.004
30
Goergen E. A. Moulding A. L. Walker B. K. Gilliam D. S. (2019). Identifying causes of temporal changes in acropora cervicornis populations and the potential for recovery. Front. Mar. Sci.6. doi: 10.3389/fmars.2019.00036
31
Greenstein B. J. Curran H. A. Pandolfi J. M. (1998). Shifting ecological baselines and the demise of Acropora cervicornis in the western North Atlantic and Caribbean Province: A Pleistocene perspective. Coral Reefs17, 249–261. doi: 10.1007/s003380050125
32
Greer L. Clark T. Waggoner T. Busch J. Guilderson T. P. Wirth K. et al . (2020). Coral Gardens Reef, Belize: A refugium in the face of Caribbean-wide Acropora spp. coral decline. PloS One15, e0239267. doi: 10.1371/journal.pone.0239267
33
Greer L. Jackson J. E. Curran H. A. Guilderson T. Teneva L. (2009). How vulnerable is Acropora cervicornis to environmental change? Lessons early to middle Holocene. Geology37, 263–266. doi: 10.1130/G25479A.1
34
Harrison X. A. (2015). A comparison of observation-level random effect and Beta-Binomial models for modelling overdispersion in Binomial data in ecology & evolution. PeerJ3, e1114. doi: 10.7717/peerj.1114
35
Hartig F. (2022). DHARMa: residual diagnostics for hierarchical (Multi-level / mixed) regression models (R package version 0.4.7). Available online at: http://florianhartig.github.io/DHARMa/.
36
Hayes N. K. Gilliam D. S. Ruzicka R. Colella M. (2023). Southeast florida coral reef evaluation and monitoring project 2022 year 20 final report (Miami Beach, FL: Florida DEP & FWC), 75.
37
Hernández-Delgado E. A. Fonseca-Miranda J. S. Mercado-Molina A. E. Suleimán-Ramos S. E. (2025). Integrating 3D-printed and natural staghorn coral (Acropora cervicornis) restoration enhances fish assemblages and their ecological functions. Diversity17, 445. doi: 10.3390/d17070445
38
Hogarth W. T. (2006). Endangered and threatened species: final listing determinations for elkhorn coral and staghorn coral. Federal Register71, 26852–26861.
39
Hughes T. P. Graham N. A. Jackson J. B. Mumby P. J. Steneck R. S. (2010). Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol.25, 633–642. doi: 10.1016/j.tree.2010.07.011
40
Hughes T.P. Kerry J.T. Baird A.H. Connolly S.R. Dietzel A. Eakin C.M. et al . (2018). Global warming transforms coral reef assemblages. Nature556 (7702), 492–496.
41
Huntington B. E. Miller M. W. Pausch R. Richter L. (2017). Facilitation in Caribbean coral reefs: High densities of staghorn coral foster greater coral condition and reef fish composition. Oecologia184, 247–257. doi: 10.1007/s00442-017-3859-7
42
Jones N. P. Figueiredo J. Gilliam D. S. (2020). Thermal stress-related spatiotemporal variations in high-latitude coral reef benthic communities. Coral Reefs39, 1661–1673. doi: 10.1007/s00338-020-01994-8
43
Jones N. P. Gilliam D. S. (2020). Marine biological monitoring in broward county, florida: year 20 (2019) annual report (Broward County: Broward County Board of County Commissioners).
44
Jones N. P. Gilliam D. S. (2024). Temperature and local anthropogenic pressures limit stony coral assemblage viability in southeast Florida. Mar. pollut. Bull.200, 116098. doi: 10.1016/j.marpolbul.2024.116098
45
Jones N. P. Ruzicka R. R. Colella M. A. Pratchett M. S. Gilliam D. S. (2022). Frequent disturbances and chronic pressures constrain stony coral recovery on Florida’s Coral Reef. Coral Reefs41, 1665–1679. doi: 10.1007/s00338-022-02313-z
46
Kenyon T. M. Doropoulos C. Wolfe K. Webb G. E. Dove S. Harris D. et al . (2023). Coral rubble dynamics in the Anthropocene and implications for reef recovery. Limnology Oceanography68, 110–147. doi: 10.1002/lno.12254
47
King S. Saint-Amand A. Walker B. K. Hanert E. Figueiredo J. (2023). Larval dispersal patterns and connectivity of Acropora on Florida’s Coral Reef and its implications for restoration. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1038463
48
Knowlton N. Lang J. C. Christine Rooney M. Clifford P. (1981). Evidence for delayed mortality in hurricane-damaged Jamaican staghorn corals. Nature294, 251–252. doi: 10.1038/294251a0
49
Knowlton N. C. Lang J. D. Keller B. U. (1990). Case study of natural population collapse: Post-hurricane predation on Jamaican staghorn corals. Smithsonian Contributions to Mar. Sci. 31, 1–25. doi: 10.5479/si.01960768.31.1
50
Kohler K. E. Gill S. M. (2006). Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosciences32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
51
Langdon C. Albright R. Baker A. C. Jones P. (2018). Two threatened Caribbean coral species have contrasting responses to combined temperature and acidification stress. Limnology Oceanography63, 2450–2464. doi: 10.1002/lno.10952
52
Larson E. A. Gilliam D. S. Lopez Padierna M. Walker B. K. (2014). Possible recovery of Acropora palmata (Scleractinia: Acroporidae) within the Veracruz Reef System, Gulf of Mexico: A survey of 24 reefs to assess the benthic communities. Rev. Biología Trop.62, 299–308.
53
Lenth R. (2023). emmeans: Estimated Marginal Means, aka Least-Squares Means (R package version 1.8.4-1). Available online at: https://CRAN.R-project.org/package=emmeans.
54
Lirman D. Bowden-Kerby A. Schopmeyer S. Huntington B. Thyberg T. Gough M. et al . (2010). A window to the past: Documenting the status of one of the last remaining ‘megapopulations’ of the threatened staghorn coral Acropora cervicornis in the Dominican Republic. Aquat. Conserv: Mar. Freshw. Ecosyst.20, 773–781. doi: 10.1002/aqc.1146
55
Lirman D. Schopmeyer S. Manzello D. Gramer L. J. Precht W. F. Muller-Karger F. et al . (2011). Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida reef tract and reversed previous survivorship patterns. PloS One6, e23047. doi: 10.1371/journal.pone.0023047
56
Mallin M. A. Corbett C. A. (2006). How hurricane attributes determine the extent of environmental effects: multiple hurricanes and different coastal systems. Estuaries Coasts29, 1046–1061. doi: 10.1007/BF02798667
57
Manzello D. P. (2015). Rapid recent warming of coral reefs in the Florida Keys. Sci. Rep.5, 1–10. doi: 10.1073/pnas.0701194104
58
Miller M. Bourque A. Bohnsack J. (2002). An analysis of the loss of acroporid corals at Looe Key, Florida, USA: 1983–2000. Coral Reefs21, 179–182. doi: 10.1007/s00338-002-0028-7
59
Miller M. W. Kerr K. Williams D. E. (2016). Reef-scale trends in Florida Acropora spp. abundance and the effects of population enhancement. PeerJ4, e2523. doi: 10.7717/peerj.2523
60
Muller E. M. Bartels E. Baums I. B. (2018). Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. Elife7, e35066. doi: 10.7554/eLife.35066.028
61
Neigel J. E. Avise J. C. (1983). Clonal diversity and population structure in a reef-building coral, acropora cervicornis: self-recognition analysis and demographic interpretation. Evolution37, 437.
62
Norström A. V. Nyström M. Lokrantz J. Folke C. (2009). Alternative states on coral reefs: beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser.376, 295–306. doi: 10.3354/meps07815
63
Orrell S. A. (1981). Dynamic analysis of deformation lamellae occurring in vein calcite. AAPG Bull.65, 1015–1015.
64
Perez D. M. (2023). Twenty Years of Change in a Southeast Florida Acropora Cervicornis Thicket. [master's thesis] (Dania Beach (FL: Nova Southeastern University), 131. Available online at: https://nsuworks.nova.edu/hcas_etd_all/131 (Accessed April 20, 2023).
65
Porter J. W. Battey J. F. Smith G. J. (1982). Perturbation and change in coral reef communities. Proc. Natl. Acad. Sci.79, 1678–1681. doi: 10.1073/pnas.79.5.1678
66
Pratchett M. S. McWilliam M. J. Riegl B. (2020). Contrasting shifts in coral assemblages with increasing disturbances. Coral Reefs39, 783–793. doi: 10.1007/s00338-020-01936-4
67
Precht W. Bruckner A. Aronson R. Bruckner R. (2002). Endangered acroporid corals of the caribbean. Coral Reefs1, 41–42. doi: 10.1007/s00338-001-0209-2
68
Precht W. F. Gintert B. E. Robbart M. L. Fura R. Van Woesik R. (2016). Unprecedented disease-related coral mortality in Southeastern Florida. Sci. Rep.6, 31374. doi: 10.1038/srep31374
69
Randall C. J. van Woesik R. (2015). Contemporary white-band disease in Caribbean corals driven by climate change. Nat. Climate Change5, 375–379. doi: 10.1038/nclimate2530
70
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing (Vienna, Austria). Available online at: https://www.R-project.org/.
71
Renzi J. J. Shaver E. C. Burkepile D. E. Silliman B. R. (2022). The role of predators in coral disease dynamics. Coral Reefs41, 405–422. doi: 10.1007/s00338-022-02219-w
72
Riegl B. Purkis S. J. Keck J. Rowlands G. P. (2009). Monitored and modeled coral population dynamics and the refuge concept. Mar. pollut. Bull.58, 24–38. doi: 10.1016/j.marpolbul.2008.10.019
73
Riegl B. Johnston M. Purkis S. Howells E. Burt J. Steiner S.C. Sheppard C.R.C. Bauman A. (2018). Population collapse dynamics in Acropora downingi, an Arabian/Persian Gulf ecosystem‐engineering coral, linked to rising temperature. Glob. Chang. Biol.24 (6), 2447–2462. doi: 10.1111/gcb.14114
74
Robbart M. Peckol P. Scordilis S. Curran H. Brown-Saracino J. (2004). Population recovery and differential heat shock protein expression for the corals Agaricia agaricites and A. tenuifolia in Belize. Mar. Ecol. Prog. Ser.283, 151–160. doi: 10.3354/meps283151
75
Rogers C. S. Muller E. Spitzack T. Miller J. (2009). Extensive coral mortality in the US Virgin Islands in 2005/2006: A review of the evidence for synergy among thermal stress, coral bleaching and disease. Caribbean J. Sci.45, 204–214. doi: 10.18475/cjos.v45i2.a8
76
Schopmeyer S.A. Lirman D. Bartels E. Byrne J. Gilliam D.S. Hunt J. et al . (2012). In situ coral nurseries serve as genetic repositories for coral reef restoration after an extreme cold-water event. Restor. Ecol.20 (6), 696–703. doi: 10.1111/j.1526-100X.2011.00836.x
77
Schopmeyer S. A. Lirman D. Bartels E. Gilliam D. S. Goergen E. A. Griffin S. P. et al . (2017). Regional restoration benchmarks for Acropora cervicornis. Coral reefs36, 1047–1057. doi: 10.1007/s00338-017-1596-3
78
Tkachenko K. S. Wu B. J. Fang L. S. Fan T. Y. (2007). Dynamics of a coral reef community after mass mortality of branching Acropora corals and an outbreak of anemones. Mar. Biol.151, 185–194. doi: 10.1007/s00227-006-0467-1
79
Tunnicliffe V. (1981). Breakage and propagation of the stony coral Acropora cervicornis. Proc. Natl. Acad. Sci.78, 2427–2431. doi: 10.1073/pnas.78.4.2427
80
Vargas-Angel B. D. Colley S. B. Hoke S. M. Thomas J. D. (2006). The reproductive seasonality and gametogenic cycle of Acropora cervicornis off Broward County, Florida, USA. Coral Reefs25, 110–122. doi: 10.1007/s00338-005-0070-9
81
Vargas-Angel B. D. Thomas J. M. Hoke S. M. (2003). High-latitude Acropora cervicornis thickets off Fort Lauderdale, Florida, USA. Coral Reefs22, 465–473. doi: 10.1007/s00338-003-0336-z
82
Voss J. D. Richardson L. L. (2006). Nutrient enrichment enhances black band disease progression in corals. Coral Reefs25, 569–576. doi: 10.1007/s00338-006-0131-8
83
Walker B. K. (2017). Characterize the condition of previously known and newly identified large dense Acropora cervicornis patches in southeast Florida (Miami: Florida DEP). Available online at: https://floridadep.gov/rcp/coral/documents/acropora-dense-patch-condition-report-2017final.
84
Walker B. K. (2018). Southeast Florida reef-wide Post-Irma coral disease surveys (Miami: Florida DEP). Available online at: https://floridadep.gov/rcp/coral/documents/southeast-florida-reef-wide-post-irma-coral-disease-surveys.
85
Walker B. K. Larson E. A. Moulding A. L. Gilliam D. S. (2012). Small-scale mapping of indeterminate arborescent acroporid coral (Acropora cervicornis) patches. Coral Reefs31, 885–894. doi: 10.1007/s00338-012-0910-3
86
Wapnick C. M. Precht W. F. Aronson R. B. (2004). Millennial-scale dynamics of staghorn coral in Discovery Bay, Jamaica. Ecol. Lett.7, 354–361. doi: 10.1111/j.1461-0248.2004.00586.x
87
Wilkinson C. R. Souter D. (2008). Status of Caribbean coral reefs after bleaching and hurricanes in 2005 (Townsville, QLD: Global Coral Reef Monitoring network and Reef and Rainforest Research Centre), 152.
Summary
Keywords
benthic community dynamics, long-term coral reef monitoring, disturbances, endangered coral species, coral reef ecological services
Citation
Perez DM, Jones NP, Goergen EA and Gilliam DS (2025) Twenty years of change in a southeast Florida Acropora cervicornis thicket. Front. Mar. Sci. 12:1641098. doi: 10.3389/fmars.2025.1641098
Received
04 June 2025
Accepted
29 August 2025
Published
18 September 2025
Volume
12 - 2025
Edited by
Jesús Ernesto Arias González, Center for Research and Advanced Studies, National Polytechnic Institute of Mexico (CINVESTAV), Mexico
Reviewed by
Lauren T. Toth, United States Department of the Interior, United States
Erica K. Towle, NOAA Coral Reef Conservation Program, United States
Kathleen Lunz, US Department of Interior, United States
Updates
Copyright
© 2025 Perez, Jones, Goergen and Gilliam.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David S. Gilliam, gilliam@nova.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.