Abstract
Biological invasions are one of the major threats to ecosystem services and biodiversity. Thus, it is crucial to understand the mechanisms involved in the invasion success of alien species. In addition to generalist traits and high tolerance that enable persistence in novel environments, invasive species can use volatile chemical compounds from specialized metabolism [biogenic volatile organic compounds (BVOCs)] to compete with native species, a process known as allelopathy. These compounds could contribute to invasions in marine environments, and the associated mechanisms need to be deciphered. The aim of this study was to characterize the volatilome (i.e., all BVOCs produced by a species) of two Caribbean native seagrass species (Syringodium filiforme and Thalassia testudinum) and one invasive (Halophila stipulacea). For that purpose, leaf samples were collected, and BVOCs were trapped through headspace solid-phase microextraction followed by analyses in GC-MS. H. stipulacea’s volatilome was significantly different from the two native species, with the presence of compounds showing, in literature, allelochemical properties (e.g., geranyl acetone, 6-methyl, 5-hepten-2-one, and cyclohexane isothiocyanate). We hypothesized that these compounds could be “novel weapons” to enhance the invasion success of H. stipulacea, but it needs further investigations in the laboratory (e.g., mesocosms) as well as in situ.
Introduction
Biological invasions are considered as one of the major threats to native ecosystems’ integrity (Ehrenfeld, 2010). Invasive species, once settled, can affect ecological processes (Gandhi and Herms, 2010), engineer ecosystem structure (Crooks, 2002), or affect community dynamics (Yurkonis et al., 2005). Therefore, they can significantly alter ecosystem functioning that may result in a significant modification of ecosystem services provided. Moreover, this threat will increase with climate change (Hulme, 2017). Thus, it is crucial to understand underlying mechanisms to biological invasions to better counteract their consequences. However, most of the studies on biological invasions are performed on terrestrial environments, whereas marine environments are still poorly studied (Lowry et al., 2013; Mačić et al., 2018) despite the highlighting of numerous biological invasions (Chan and Briski, 2017; Rilov and Crooks, 2009).
Halophila stipulacea, a native seagrass from the Indian Ocean and the Red Sea (Mejia et al., 2016; Spalding et al., 2003), is considered a highly invasive species (Lowe et al., 2000; Winters et al., 2020). In the Mediterranean Sea, the first occurrences of H. stipulacea were recorded in 1894 near Greek coasts following the opening of the Suez Canal in 1869 (Winters et al., 2020). Even though this species is now found in many places, especially in eastern parts of the Mediterranean Sea (e.g., Greece, Tunisia, Libya, Syria, and Lebanon; Sghaier et al., 2011), observations point toward a relatively limited “invasion success” in this region (Nguyen et al., 2020). However, in the Caribbean Sea, where it was observed for the first time in 2002 near Grenada (Ruiz and Ballantine, 2004), H. stipulacea quickly colonized a large part of the Eastern Caribbean (e.g., Dominica, Martinique, and Virgin Islands) as well as the coasts of South America (e.g., Venezuela) in a decade (Winters et al., 2020). Moreover, it has been shown that H. stipulacea even displaces native seagrass species (e.g., Syringodium filiforme) by monopolizing their spaces (Willette and Ambrose, 2012), whereas in the Mediterranean Sea, H. stipulacea forms multispecies beds, especially with Cymodocea nodosa (Chiquillo et al., 2023). These differences in H. stipulacea invasion success between both areas is probably due to warmer conditions in the Caribbean Sea compared to the Mediterranean Sea. The actual tropicalization of the Mediterranean Sea could increase the invasiveness potential of H. stipulacea (Nguyen et al., 2020). For example, in Cap Monastir, a small patch of H. stipulacea (0.2 ha) covered more than 2 ha after only 4 years and displaced C. nodosa (Sghaier et al., 2014). Thus, this species starts to colonize northern and western parts of the Mediterranean Sea and could be an important problem in the future (Thibaut et al., 2022).
Numerous studies have highlighted what are the characteristics that make a non-native species a successful invader (Van Kleunen et al., 2010). In principle, they should present high reproductive capacity (sexual and/or asexual), wide phenotypic plasticity, high dispersal ability, and strong competitive ability. H. stipulacea presents those characteristics, namely a high tolerance to irradiance (35 to 450 μmol.m−2.s−1), to salinity levels (24 to 70 g.L−1), as well as to water temperatures (17°C–42°C, Winters et al., 2020). Moreover, this species also shows a very efficient asexual reproduction by fragmentation or vegetative rhizome growth (Smulders et al., 2017) and is more fecund than larger seagrass species (Malm, 2006). Furthermore, the receiving environment should, in theory, exhibit “invadable” characteristics such as an elevated level of disturbance for the native species, availability of empty niches, a low level of biotic resistance, and a high availability of resources (Olyarnik et al., 2009). It has been thought that H. stipulacea only colonized disturbed native ecosystems. However, new evidence in the field showed that H. stipulacea displaces native species in the Caribbean Sea and, to a lesser extent, in the Mediterranean sea (Sghaier et al., 2014; Willette and Ambrose, 2012). Chiquillo et al. (2023) have demonstrated that H. stipulacea had a negative impact on S. filiforme and C. nodosa growth, whereas the presence of native species facilitates H. stipulacea settlement in a laboratory experiment. These results strongly indicate that this species is a driver of its own invasion success, implying that H. stipulacea can invade intact native ecosystems.
Allelochemicals mostly belong to specialized metabolism and act as defense compounds to suppress other plant competitors (Kong et al., 2019). Among those compounds, it has been shown that biogenic volatile organic compounds (BVOCs) are allelochemicals in terrestrial environments (Effah et al., 2019; Santonja et al., 2019; Xie et al., 2021). For instance, BVOCs emitted by Solanum lycopersicum foliage inhibited seed germination of the tropical species Amaranthus mangostanus (Kim and Kil, 2001). It has been hypothesized that BVOCs from invasive species could enhance their competitiveness since they could inhibit native species germination and/or growth, with the magnitude of the effect being regulated by co-evolution (Clavijo McCormick et al., 2023; Mollo et al., 2015). Thus, according to this hypothesis, species A would be more prepared for the biochemicals of species B if they evolved together compared to species C, which came from another biome (Callaway and Ridenour, 2004). Similar allelopathic processes, involving BVOCs, were also highlighted in marine environments (Allen et al., 2016; Sudatti et al., 2020), such as octanol showing an inhibitory effect on spore germination of Ulva prolifera (Zhang et al., 2014). Since BVOCs are lipophilic molecules, they are insoluble in water. When mediating marine species interactions, we consider that they are mainly involved in short-range or contact communication in marine environments (Mollo et al., 2014). However, they could also act on higher ranges since BVOCs can move in water through filaments over long distances (Webster and Weissburg, 2009) or their solubility can be punctually increased by water physico-chemical properties (Sander, 2023). To really decipher the action range of marine BVOCs, further investigations are required.
The aims of this study were to highlight the volatilome (i.e., all volatile compounds from a species) of the three main seagrass species from Martinique. In this area, H. stipulacea was detected in 2006 for the first time. Its presence was confirmed in 2010, and since then, the species has shown a wide distribution along the Martinique coasts at the expense of native species, mainly S. filiforme (Maréchal et al., 2013). Our hypothesis is that H. stipulacea could have a different volatilome compared to both native species, which might explain a part of its invasion success in the Caribbean area. Moreover, we expected to detect compounds from H. stipulacea having allelopathic properties, in comparison to literature, highlighted in terrestrial and marine species.
Materials and methods
Plant material
Three seagrass species were studied: two endemic species, namely, Thalassia testudinum and Syringodium filiforme, and the invasive species Halophila stipulacea. T. testudinum and H. stipulacea belongs to Hydrochataceae, whereas S. filiforme belongs to the Cymodoceaceae family. The three species were located on the same site, Grande Anse d’Arlet (N14°29’34’’O61°5’10’’). Sampling was done on the 27th of November 2024 and kept in seawater during the transportation to the laboratory.
Headspace solid-phase microextraction
BVOCs collection was performed according to methods used in Coquin et al. (2024). It was done within 24h after seagrass harvesting. Before collection, each sample was taken out of the tanks, and each leaf was gently scraped with a scalpel to remove epiphytes while taking care to prevent leaf damage. One gram of cut fresh leaves was placed into small pieces in 20-ml glass vials and hermetically sealed with PTFE/silicone septa. Vials were maintained in a water bath at 50°C for 10 min for equilibrium, and the headspace solid-phase microextraction collection took place for 1h. Collection of BVOCs from the headspace was carried out manually using an SPME fiber (DVB/CAR/PDMS, Supelco Co., Bellefonte, USA). Blanks were performed using the same vials without plant material. After sampling, the SPME fibers were stored at −20°C before injection in GC-MS. BVOCs collection was carried out in six replicates for each species.
Gas chromatography–mass spectrometry analyses
Analyses were performed according to methods used in Coquin et al. (2024) on a GC instrument (7890B GC, Agilent Technologies, Santa Clara, USA) equipped with an HP5-MS column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, Santa Clara, USA) coupled to an MS instrument (MSD5977A, Agilent Technologies, Santa Clara, USA). Thermal desorption of the fibers was directly carried out to the GC column through the injector for 15 min at 250°C in splitless mode. The gradient temperature was initially set at 70°C (2 min), then reached 200°C at 3°C/min−1 and finally reached 315°C at 15°C/min−1. This temperature was, then, held for 5 min. Helium was used as a carrier gas with a constant flow of 1 ml/min−1. The EI mode was operated at 70 eV, and the mass range was 40–450 amu. The identification of VOCs was based on the comparison of their retention indices (RIs), determined using the retention times of a series of alkanes (C8 to C40), and on a spectral match with the NIST20 mass spectral libraries. In parallel, blank samples, which consisted of sampling without plant material, were performed. Then, compounds detected in blanks were subtracted from samples.
Chemical profile
To highlight chemical profiles of each species, compounds were classed according to their chemical family/biosynthesis pathway (alcohol, alkane, alkene, aldehyde, benzenoid, terpene, sulfur/nitrogen compound, halogenated compound, and other). Then, relative abundances of groups, in percentage, were calculated. Moreover, a Venn diagram was built according to detected specific compounds per species through Venny 2.0.2 (https://bioinfogp.cnb.csic.es/tools/venny/index2.0.2.html).
Statistical analyses
All statistical analyses were performed on absolute abundances with R software (4.3.3) and metaboanalyst (https://www.metaboanalyst.ca/). Partial Least Squares–Discriminant Analysis (PLS-DA) was performed with the RVAideMemoire and pls packages on non-transformed and auto-scaled (mean 0, standard deviation 1) data, giving all compounds equal weight. To highlight volatilome differences according to species, PERMANOVA and pairwise tests were performed to test differences between groups (1,000 permutations for each). VIP scores (Variable Importance in Projection), obtained through PLS-DA, were used to highlight the ten most discriminant compounds. A VIP score summarizes the variable contribution to the model. A variable is considered relevant for the model when its VIP score is above 1. Then, Kruskal–Wallis tests followed by a Dunn post-hoc test were performed on these compounds between species after checking the normality and the homoscedasticity of the dataset.
Results
A total of 104 compounds were detected (Supplementary Files S1), with 90, 89, and 85 compounds present in H. stipulacea, S. filiforme, and T. testudinum, respectively (Figure 1A). The three species shared numerous compounds but also presented specific compounds (6 for H. stipulacea, 8 for S. filiforme, and 3 for T. testudinum). Volatilome profiles between species were quite different, with alcohol (e.g., 3-hexen-1-ol) being the major component of S. filiforme and T. testudinum (24.6 and 74.5%, respectively, Figure 1B). Then, S. filiforme volatilome included alkene (16.9%, e.g., 1-pentadecene) and aldehyde (9.6%, e.g., decanal). Concerning T. testudinum, the second major components were aldehyde (5.8%) followed by alkane (5.6%). By contrast, for H. stipulacea, major compounds of its volatilome were alkanes, representing 35% (e.g., tridecane), followed by aldehyde (15.8%) and alcohol (9.6%).
Figure 1
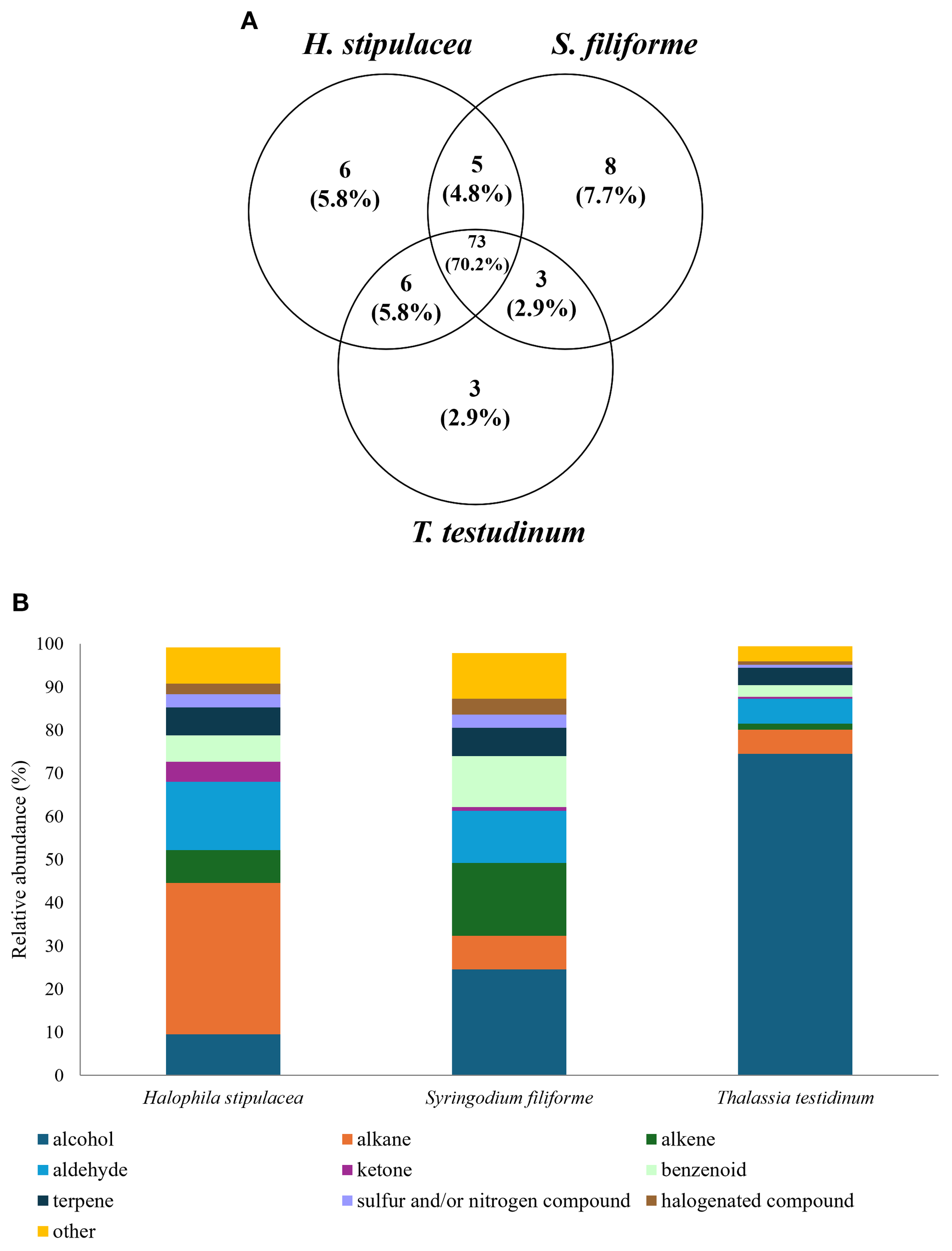
(A) Venn diagram based on detected compounds for each species and (B) Volatilome profiles according to chemical families (alcohol, alkane, alkene, aldehyde, ketone, benzenoid, terpene, sulfur and/or nitrogen compound, halogenated compound, and other) in relative abundance (%) according to the species with n=6.
Then, to go further, PLS-DA were performed to take into account the volatilome profile as well as the compounds abundance (Figure 2). The Classification Error Rate (CER) equals to 0.14, indicated that this analyse was quite robust. PLS-DA showed that volatilomes were graphically separated from each other, suggesting that the three species had different volatilomes (in terms of compounds and abundance). This result was confirmed by PERMANOVA, indicating a significant effect of species (p-value<0.01). Then, pairwise test was performed to highlight differences between species and clearly showed that H. stipulacea volatilome was different from the two other species (p-value<0.01) whereas native species did not show any difference in their volatilome. According to VIP scores extracted from PLS-DA (1.5<VIP scores, Supplementary Files S2), 10 compounds were highlighted as the most discriminant between the three species (Table 1).
Figure 2
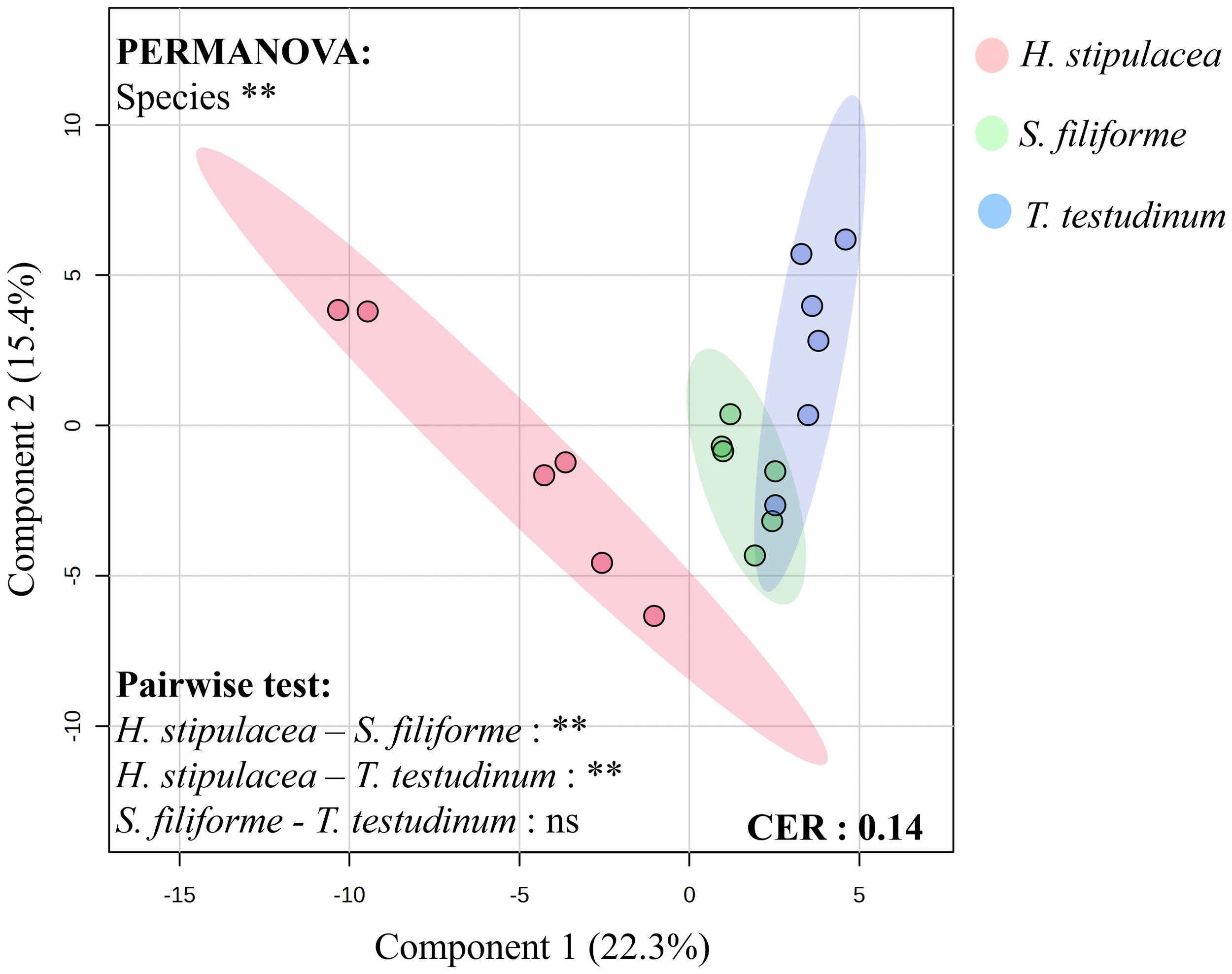
PLS-DA performed on peak intensities of all detected compounds according to species. PERMANOVA and pairwise tests were performed with 1,000 permutations, n=6 and ns=p > 0.05 et **=0.001<p<0.01.
Table 1
| Compounds | H. stipulacea | S. filiforme | T. testudinum |
|---|---|---|---|
| pentadecane | 291139734 ± 32968046 a | 12518137 ± 2639858 b | 19473312 ± 3890254 b |
| geranyl acetone | 11892425 ± 1297379 a | 5339537 ± 1226886 b | 3367621 ± 544173 b |
| tetradecane | 10529653 ± 930601 a | 1168082 ± 337676 b | 2437330 ± 370721 b |
| 3-hexen-1-ol | n.d. c | 4930033 ± 3690997 bc | 1587266248 ± 322438591 a |
| dodecanal | 13125210 ± 1726599 a | 2074243 ± 675814 b | 3133752 ± 390225 b |
| 6-methyl, 5-hepten-2-one | 34805172 ± 8800795 a | 546438 ± 546438 b | n.d. b |
| benzaldehyde | 27021028 ± 8618983 a | 7818287 ± 3554188 ab | n.d. b |
| undecanal | 3188719 ± 930280 a | 596118 ± 390743 b | 188084 ± 188084 b |
| cyclohexane, isothiocyanate | 25580448 ± 6938908 a | 1960997 ± 899917 b | 3386687 ± 1319280 b |
| 1-dodecanol | 12630120 ± 2095409 a | 3120520 ± 1107447 b | 4112568 ± 1332406 b |
Peak intensities of the ten most discriminant compounds (based on VIP scores from PLS-DA) according to the species.
n.d. means not detected.
Kruskall-Walis tests followed by a Dunn post hoc test were performed to highlight significant differences between species with a > b > c. Mean ± SE and n=6.
Nine of the 10 most discriminant compounds were present in higher quantities in H. stipulacea by 2 to 63-fold more. Only 3-hexen-1-ol abundance were higher in T. testudinum compared to both other species.
Discussion
Our results showed, for the first time in the Caribbean area, that the invasive seagrass H. stipulacea had a volatilome significantly different from both native species, S. filiforme and T. testudinum (both showing similar volatilomes). This suggests that volatilomes do not seem to be driven by phylogeny since both species belonging to the same family showed different volatilomes (T. testudinum and H. stipulacea from the Hydrochataceae family). Similar findings have been highlighted on bryophytes (Yáñez-Serrano et al., 2024). Moreover, our results revealed the presence of several compounds in H. stipulacea volatilome, highlighted as allelochemicals in literature. For instance, within the most discriminant compounds, apocarotenoids (geranyl acetone and 6-methyl, 5-hepten-2-one also known as sulcatone) and isothiocyanates (cyclohexane, isothiocyanate) have shown strong allelopathic effects in terrestrial and marine species, as discussed below (Moreno et al., 2021; Motmainna et al., 2021).
Regarding the apocarotenoids, Jüttner (1979) showed an inhibition of cyanobacterial growth when exposed to 6-methyl, 5-hepten-2-one and even a lethal effect for geranyl acetone. Similar inhibitory effects on growth were highlighted on a green microalga, Auxenochlorella pyrenoidosa, until a concentration threshold (10mg.ml−1) where no growth was observed (Ikawa et al., 2001). That could result in disturbances of both glucose uptake and respiration and/or on pigment production as shown on freshwater isolates of Chromobacterium lividum and Arthrobacter sp (Reichardt, 1981). Another type of allelochemical has been highlighted in our study, namely cyclohexane isothiocyanate. This compound belongs to isothiocyanates, well known to be produced through the degradation of glucosinolates by the enzyme myrosinase (Kliebenstein et al., 2005). It has been shown that Brassicaceae extracts, the main terrestrial plant family producing isothiocyanates (Ramirez et al., 2020), reduced germination and growth in several legume species such as Phaseolaris vulagri, Medicago sativa, and Cuscuta campestris (Almhemed and Ustuner, 2023; Choesin and Boerner, 1991; Smith, 2000). It has to be noted that other compounds detected in H. stipulacea present also allelopathic impacts (e.g., DMS, dihydroactinidiolide, and terpenoids), although they are less abundant than the previously discussed compounds. All together, these compounds could help H. stipulacea in its invasion success and to settle into a new habitat and replace native species, but this needs further investigation.
Study limitations
Observations from previous works showed that H. stipulacea affected native species growth under control and field conditions in both Caribbean and Mediterranean seas and even, replaced them in some specific locations (Chiquillo et al., 2023; Sghaier et al., 2014; Willette and Ambrose, 2012). This invasion success can be explained by its high tolerance to wide ranges of environmental conditions implying a better capacity to compete with native species (Winters et al., 2020). In addition, allelopathic effects from H. stipulacea could potentially enhance its invasion success but this is not investigated yet. Our work brings evidence on the presence of several compounds with such properties present in higher abundances in the invasive species (e.g., isothiocyanates and apocarotenoids). Even though allelopathic effects of these compounds were not tested in this study, these compounds have shown allelopathic effects in literature. Moreover, the H. stipulacea exotic volatilome, compared to native species, could be an advantage for its invasion success in this region (Winters et al., 2020). According to the theory, the strength of allelopathic effects is conditioned by evolutionary history shared by community species (Callaway and Aschehoug, 2000; Hierro and Callaway, 2021). Therefore, the introduction of an alien species in a native environment can alter habitats by releasing specialized metabolites (volatile and non-volatile) and can be considered as a « novel weapon » for the successful invasion of an alien species (Callaway and Ridenour, 2004). It has been already demonstrated, in terrestrial environments, with greater allelopathic effects of the invasive weed Chromolaena odorata, originating from South America, on Chinese native species compared to another South American species (Hu and Zhang, 2013). For H. stipulacea, all these hypotheses still need to be tested, especially because in some cases, neutral or even positive allelopathic effects can also be observed (Orr et al., 2005). To answer these questions, it would be necessary to perform growth experiment on native species exposed to BVOCs from both invasive and native species under laboratory and field conditions (Gross, 2023; Lv et al., 2021). It would be also necessary to determine the range of action of these compounds (short vs. long distances) with diffusion tests and concentration assessments directly into water. Moreover, it is well known that phenolic compounds, another type of specialized metabolites that are non-volatile, have allelopathic effects (Li et al., 2010). Since it has been shown that H. stipulacea produce this type of compounds (Chebaro et al., 2024, highlighted only for their bioactive properties and not their ecological roles), it is possible that H. stipulacea allelopathic effects on native species could also results from those non-volatile compounds. Thus, it would be interesting to evaluate allelopathic potential of combined volatile and non-volatile metabolites to have the full picture of H. stipulacea allelopathic potential.
Perspectives
Detection of potential allelochemical is just the first step to highlight allelopathic interactions in marine invasive species such H. stipulacea. Further investigations are required to understand if and how these compounds impact competition between marine native and invasive species. That would help to better understand H. stipulacea dynamics and evaluate its invasion success under current and future environmental conditions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AS: Formal Analysis, Writing – review & editing, Conceptualization, Investigation, Writing – original draft. SC: Formal Analysis, Writing – review & editing, Conceptualization, Investigation, Writing – original draft. LH: Investigation, Writing – review & editing, Writing – original draft. CO: Writing – original draft, Investigation, Writing – review & editing. BdM: Writing – original draft, Writing – review & editing, Investigation. CL: Investigation, Writing – review & editing, Writing – original draft. EO: Writing – review & editing, Writing – original draft. CF: Conceptualization, Investigation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by an internal funding (AOI SUD) and A*MIDEX “2022 White Research” program through the Benthic-VOC project (AMX-22-RE-AB-081) and the CNRS through the MITI interdisciplinary programs within the GDR OMER (CNRS) for PhD funding.
Acknowledgments
Data used in this study were partly produced through the technical facilities of the “ Metabolomics and Natural Products Chemistry service “ (IMBE, Marseille). We also thank Aquasearch for their help on the field.
Conflict of interest
Authors CO and BdM were employed by AQUASEARCH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1641139/full#supplementary-material
References
1
Allen J. L. Ten-Hage L. Leflaive J. (2016). Allelopathic interactions involving benthic phototrophic microorganisms. Environ. Microbiol. Rep.8, 752−762. doi: 10.1111/1758-2229.12436
2
Almhemed K. Ustuner T. (2023). Assessment of allelopathic influence of some cruciferous species on germination indicators of field dodder seeds. Adv. Weed Sci.41, e020230048. doi: 10.51694/AdvWeedSci/2023;41:00029
3
Callaway R. M. Aschehoug E. T. (2000). Invasive plants versus their new and old neighbors : A mechanism for exotic invasion. Science290, 521−523. doi: 10.1126/science.290.5491.521
4
Callaway R. M. Ridenour W. M. (2004). Novel weapons : Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ.2, 436−443. doi: 10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
5
Chan F. T. Briski E. (2017). An overview of recent research in marine biological invasions. Mar. Biol.164, 121. doi: 10.1007/s00227-017-3155-4
6
Chebaro Z. Mesmar J. E. Badran A. Al-Sawalmih A. Maresca M. Baydoun E. (2024). Halophila stipulacea : A comprehensive review of its phytochemical composition and pharmacological activities. Biomolecules14, 991. doi: 10.3390/biom14080991
7
Chiquillo K. L. Barber P. H. Vasquez M. I. Cruz-Rivera E. Willette D. A. Winters G. et al . (2023). An invasive seagrass drives its own success in two invaded seas by both negatively affecting native seagrasses and benefiting from those costs. Oikos2023, e09403. doi: 10.1111/oik.09403
8
Choesin D. N. Boerner R. E. (1991). Allyl isothiocyanate release and the allelopathic potential of Brassica napus (Brassicaceae). Am. J. Bot.78, 1083−1090. doi: 10.1002/j.1537-2197.1991.tb14516.x
9
Clavijo McCormick A. Effah E. Najar-Rodriguez A. (2023). Ecological aspects of volatile organic compounds emitted by exotic invasive plants. Front. Ecol. Evol.11, 1059125. doi: 10.3389/fevo.2023.1059125
10
Coquin S. Ormeno E. Pasqualini V. Monnier B. Culioli G. Lecareux C. et al . (2024). Chemical diversity of mediterranean seagrasses volatilome. Metabolites14, 705. doi: 10.3390/metabo14120705
11
Crooks J. A. (2002). Characterizing ecosystem-level consequences of biological invasions : The role of ecosystem engineers. Oikos97, 153−166. doi: 10.1034/j.1600-0706.2002.970201.x
12
Effah E. Holopainen J. K. McCormick A. C. (2019). Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Systematics38, 58−63. doi: 10.1016/j.ppees.2019.04.003
13
Ehrenfeld J. G. (2010). Ecosystem consequences of biological invasions. Annu. Rev. ecol. evolution systematics41, 59−80. doi: 10.1146/annurev-ecolsys-102209-144650
14
Gandhi K. J. Herms D. A. (2010). Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions12, 389−405. doi: 10.1007/s10530-009-9627-9
15
Gross E. M. (2023). “ Allelopathy in benthic and littoral areas : Case studies on allelochemicals from benthic cyanobacteria and submersed macrophytes,” in Principles and practices in plant ecology ( CRC Press), 179−199.
16
Hierro J. L. Callaway R. M. (2021). The ecological importance of allelopathy. Annu. Rev. Ecol. Evolution Systematics52, 25−45. doi: 10.1146/annurev-ecolsys-051120-030619
17
Hu G. Zhang Z. (2013). Allelopathic effects of Chromolaena odorata on native and non-native invasive herbs. J. Food Agric. Environ.11, 878−882.
18
Hulme P. E. (2017). Climate change and biological invasions : Evidence, expectations, and response options. Biol. Rev.92, 1297−1313. doi: 10.1111/brv.12282
19
Ikawa M. Sasner J. J. Haney J. F. (2001). Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth. Hydrobiologia443, 19−22. doi: 10.1023/A:1017535801766
20
Jüttner F. (1979). Nor-carotenoids as the major volatile excretion products of Cyanidium. Z. für Naturforschung C34, 186−191. doi: 10.1515/znc-1979-3-405
21
Kim Y. S. Kil B.-S. (2001). Allelopathic effects of some volatile substances from the tomato plant. J. Crop production4, 313−321. doi: 10.1300/J144v04n02_13
22
Kliebenstein D. J. Kroymann J. Mitchell-Olds T. (2005). The glucosinolate–myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol.8, 264−271. doi: 10.1016/j.pbi.2005.03.002
23
Kong C.-H. Xuan T. D. Khanh T. D. Tran H.-D. Trung N. T. (2019). Allelochemicals and signaling chemicals in plants. Molecules24, 2737. doi: 10.3390/molecules24152737
24
Li Z.-H. Wang Q. Ruan X. Pan C.-D. Jiang D.-A. (2010). Phenolics and plant allelopathy. Molecules15, 8933−8952. doi: 10.3390/molecules15128933
25
Lowe S. Browne M. Boudjelas S. De Poorter M. (2000). 100 of the world’s worst invasive alien species : A selection from the global invasive species database Vol. 12 ( Invasive Species Specialist Group Auckland).
26
Lowry E. Rollinson E. J. Laybourn A. J. Scott T. E. Aiello-Lammens M. E. Gray S. M. et al . (2013). Biological invasions : A field synopsis, systematic review, and database of the literature. Ecol. Evol.3, 182−196. doi: 10.1002/ece3.431
27
Lv M. Yuan M. Wang Y. Tang X. Zhao Y. (2021). Allelopathic effects of Ulva linza on marine phytoplankton and identification of the allelochemicals. Environ. Sci. pollut. Res.28, 45714−45723. doi: 10.1007/s11356-021-13734-8
28
Mačić V. Albano P. G. Almpanidou V. Claudet J. Corrales X. Essl F. et al . (2018). Biological invasions in conservation planning : A global systematic review. Front. Mar. Sci.5, 178. doi: 10.3389/fmars.2018.00178
29
Malm T. (2006). Reproduction and recruitment of the seagrass Halophila stipulacea. Aquat. Bot.85, 345−349. doi: 10.1016/j.aquabot.2006.05.008
30
Maréchal J. Meesters E. Vedie F. Hellio C. (2013). Occurrence of the alien seagrass Halophila stipulacea in Martinique (French West Indies). Mar. Biodiversity Records6, el27. doi: 10.1017/S1755267213000961
31
Mejia A. Y. Rotini A. Lacasella F. Bookman R. Thaller M. C. Shem-Tov R. et al . (2016). Assessing the ecological status of seagrasses using morphology, biochemical descriptors and microbial community analyses. A study Halophila stipulacea (Forsk.) Aschers meadows northern Red Sea. Ecol. Indic.60, 1150−1163. doi: 10.1016/j.ecolind.2015.09.014
32
Mollo E. Cimino G. Ghiselin M. T. (2015). Alien biomolecules : A new challenge for natural product chemists. Biol. Invasions17, 941−950. doi: 10.1007/s10530-014-0835-6
33
Mollo E. Fontana A. Roussis V. Polese G. Amodeo P. Ghiselin M. T. (2014). Sensing marine biomolecules : Smell, taste, and the evolutionary transition from aquatic to terrestrial life. Front. Chem.2, 92. doi: 10.3389/fchem.2014.00092
34
Moreno J. C. Mi J. Alagoz Y. Al-Babili S. (2021). Plant apocarotenoids : From retrograde signaling to interspecific communication. Plant J.105, 351−375. doi: 10.1111/tpj.15102
35
Motmainna M. Juraimi A. Uddin M. K. Asib N. B. Islam A. Hasan M. (2021). Assessment of allelopathic compounds to develop new natural herbicides : A review. Allelopathy J.52, 21−40. doi: 10.26651/allelo.j/2021-52-1-1305
36
Nguyen H. M. Yadav N. S. Barak S. Lima F. P. Sapir Y. Winters G. (2020). Responses of invasive and native populations of the seagrass Halophila stipulacea to simulated climate change. Front. Mar. Sci.6, 812. doi: 10.3389/fmars.2019.00812
37
Olyarnik S. V. Bracken M. E. Byrnes J. E. Hughes A. R. Hultgren K. M. Stachowicz J. J. (2009). “ Ecological factors affecting community invasibility,” in Biological invasions in marine ecosystems : Ecological, management, and geographic perspectives ( Springer), 215−238.
38
Orr S. P. Rudgers J. A. Clay K. (2005). Invasive plants can inhibit native tree seedlings : Testing potential allelopathic mechanisms. Plant Ecol.181, 153−165. doi: 10.1007/s11258-005-5698-6
39
Ramirez D. Abellán-Victorio A. Beretta V. Camargo A. Moreno D. A. (2020). Functional ingredients from Brassicaceae species : Overview and perspectives. Int. J. Mol. Sci.21, 1998. doi: 10.3390/ijms21061998
40
Reichardt W. (1981). Influence of methylheptenone and related phytoplankton norcarotenoids on heterotrophic aquatic bacteria. Can. J. Microbiol.27, 144−147. doi: 10.1139/m81-023
41
Rilov G. Crooks J. A. (2009). Biological invasions in marine ecosystems. Ecological Manage. Geographic Perspectives ( Springer), XXVI, 641.
42
Ruiz H. Ballantine D. L. (2004). Occurrence of the seagrass Halophila stipulacea in the tropical west Atlantic. Bull. Mar. Sci.75, 131−135.
43
Sander R. (2023). Compilation of Henry’s law constants (version 5.0. 0) for water as solvent. Atmospheric Chem. Phys.23, 10901–12440. doi: 10.5194/acp-23-10901-2023
44
Santonja M. Bousquet-Mélou A. Greff S. Ormeño E. Fernandez C. (2019). Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol.9, 8201−8213. doi: 10.1002/ece3.5390
45
Sghaier Y. R. Zakhama-Sraieb R. Benamer I. Charfi-Cheikhrouha F. (2011). Occurrence of the seagrass Halophila stipulacea (Hydrocharitaceae) in the southern Mediterranean Sea. Bot. Mar. 54, 575–582. doi: 10.1515/BOT.2011.061
46
Sghaier Y. R. Zakhama-Sraieb R. Charfi-Cheikhrouha F. (2014). “ Effects of the invasive seagrass Halophila stipulacea on the native seagrass Cymodocea nodosa”. In Proceedings of the 5th Mediterranean symposium on marine vegetation, 167−171.
47
Smith V. (2000). Reduction in snap bean emergence by seed treatment with dried canola residue. HortScience35, 92–94. doi: 10.21273/HORTSCI.35.1.92
48
Smulders F. O. Vonk J. A. Engel M. S. Christianen M. J. (2017). Expansion and fragment settlement of the non-native seagrass Halophila stipulacea in a Caribbean bay. Mar. Biol. Res.13, 967−974. doi: 10.1080/17451000.2017.1333620
49
Spalding M. Taylor M. Ravilious C. Short F. Green E. (2003). “ The distribution and status of seagrasses,” in World atlas of seagrasses ( University of California press), 5−26.
50
Sudatti D. B. Duarte H. M. Soares A. R. Salgado L. T. Pereira R. C. (2020). New ecological role of seaweed secondary metabolites as autotoxic and allelopathic. Front. Plant Sci.11, 347. doi: 10.3389/fpls.2020.00347
51
Thibaut T. Blanfuné A. Boudouresque C. F. Holon F. Agel N. Descamps P. et al . (2022). Distribution of the seagrass Halophila stipulacea : A big jump to the northwestern Mediterranean Sea. Aquat. Bot.176, 103465. doi: 10.1016/j.aquabot.2021.103465
52
Van Kleunen M. Weber E. Fischer M. (2010). A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett.13, 235−245. doi: 10.1111/j.1461-0248.2009.01418.x
53
Webster D. Weissburg M. (2009). The hydrodynamics of chemical cues among aquatic organisms. Annu. Rev. Fluid Mechanics41, 73−90. doi: 10.1146/annurev.fluid.010908.165240
54
Willette D. A. Ambrose R. F. (2012). Effects of the invasive seagrass Halophila stipulacea on the native seagrass, Syringodium filiforme, and associated fish and epibiota communities in the Eastern Caribbean. Aquat. Bot.103, 74−82. doi: 10.1016/j.aquabot.2012.06.007
55
Winters G. Beer S. Willette D. A. Viana I. G. Chiquillo K. L. Beca-Carretero P. et al . (2020). The tropical seagrass Halophila stipulacea : Reviewing what we know from its native and invasive habitats, alongside identifying knowledge gaps. Front. Mar. Sci.7, 300. doi: 10.3389/fmars.2020.00300
56
Xie Y. Tian L. Han X. Yang Y. (2021). Research advances in allelopathy of volatile organic compounds (VOCs) of plants. Horticulturae7, 278. doi: 10.3390/horticulturae7090278
57
Yáñez-Serrano A. M. Corbera J. Portillo-Estrada M. Janssens I. Llusia J. Filella I. et al . (2024). Drivers of biogenic volatile organic compound emissions in hygrophytic bryophytes. Sci. Total Environ.946, 174293. doi: 10.1016/j.scitotenv.2024.174293
58
Yurkonis K. A. Meiners S. J. Wachholder B. E. (2005). Invasion impacts diversity through altered community dynamics. J. Ecol.93 (6), 1053−1061. doi: 10.1111/j.1365-2745.2005.01029.x
59
Zhang M. Li R.-X. Hu C.-M. Yang L.-E. Tang J. Lu Q.-Q. et al . (2014). The metabolism of 8-heptadecene in pyropia (Bangiaceae, rhodophyta). J. Appl. phycol.26, 1181−1187. doi: 10.1007/s10811-013-0205-2
Summary
Keywords
BVOCs, seagrass, biological invasions, allelopathy, chemical weapon
Citation
Saunier A, Coquin S, Hannibal L, Ortole C, de Montgolfier B, Lecareux C, Ormeno E and Fernandez C (2025) Volatilome differences between native and invasive seagrass species in the Caribbean area. Front. Mar. Sci. 12:1641139. doi: 10.3389/fmars.2025.1641139
Received
04 June 2025
Accepted
05 September 2025
Published
26 September 2025
Volume
12 - 2025
Edited by
Chenhong Li, Shanghai Ocean University, China
Reviewed by
Jennifer Li Ruesink, University of Washington, United States; Seung Hyeon Kim, Gyeongsang National University, Republic of Korea
Updates
Copyright
© 2025 Saunier, Coquin, Hannibal, Ortole, de Montgolfier, Lecareux, Ormeno and Fernandez.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amélie Saunier, amelie.saunier@imbe.fr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.