Abstract
Introduction:
Ria de Aveiro, a coastal lagoon on the Atlantic coast of Portugal, was invaded by Arenicola spp. 15 years ago, with the new species successfully spreading throughout the system and replacing the native Diopatra species. With opposite bioturbation traits (Diopatra as sediment stabilizers vs Arenicola as sediment reworkers), the impacts of this replacement can spread across the entire ecosystem.
Methods:
In a 21 days microcosm study, we evaluated the effects of the incremental substitution of Diopatra by Arenicola species on relevant proxies of ecosystem functioning, such as sediment reworking depths and nutrient dynamics.
Results:
The results show a strong directional influence on most of the analyzed parameters as a response to higher densities of Arenicola. Specifically, Arenicola-dominated communities were characterized by deeper reworking depths and higher concentrations of ammonium and phosphate in the water column.
Discussion:
These results are discussed in the context of the available knowledge on the accompanying biological communities, which are typically fostered by these distinct functional groups. Therefore, there is strong evidence that the introduction of a novel species’ trait will have major consequences across several levels of the invaded system.
Introduction
Coastal lagoons are complex and dynamic socio-ecological systems, supporting highly productive biological communities (Barbier et al., 2011; Daborn and Redden, 2017; Rodrigues-Filho et al., 2023). Several ecosystem services provided by coastal systems are related to sediment reworking or bioturbation (the mixing of sediments by living organisms) (Volkenborn et al., 2007; Mermillod-Blondin, 2011; Kristensen et al., 2012). In fact, bioturbation - including bio-mixing (sediment particle mixing) and bioirrigation (water transport induced by fauna) – is an ecological processes that affect other processes such as decomposition and organic matter remineralization, nutrient balance, or sediment and pollutant resuspension (Mortimer et al., 1999; Michaud et al., 2010; Kristensen et al., 2012; Aller and Cochran, 2019; Bugnot et al., 2022). Also, bioirrigation, by transporting solutes from, to, and within the sediment matrix, alters several biogeochemical parameters and disrupts diffusional gradients within sediments (Kristensen et al., 2012). The rates of these processes can be affected directly, either by the mechanical remobilization of sediment or by effects on the physicochemical environment and on micro-, meio- and macrofaunal endobenthic communities. However, the impact of bioturbation depends on the species and on the context where they occur (i.e., influenced by biotic and abiotic variables), and, therefore, is fundamental to quantify the amount of sediment reworking mediated by species with different traits. In aquatic systems, the way that species interact with the sediment could be very diverse: while some species are sediment stabilizers, acting as ecosystem engineers that create semi-permanent structures that benefit other species (Berke, 2022; Bugnot et al., 2022), other species can have high rates of sediment remobilization, disturbing and raising unconsolidated sediments (Volkenborn et al., 2007; Kauppi et al., 2018; Lacoste et al., 2018).
Due to human use, coastal areas are under several stressors (Dolbeth et al., 2016; Rodrigues-Filho et al., 2023). Among these, the invasion by Invasive Alien Species (IAS) threatens the ecological functions of these ecosystems, compromising their provision of ecosystem services and incurring in the risk of being irreversibly compromised (Queirós et al., 2011; Gallardo et al., 2016; Roy et al., 2024). The introduction of IAS in an ecosystem might result in changes in the overall functioning if, for instance, the novel species also introduces a new trait to the system. This is the case of Ria de Aveiro, a shallow coastal lagoon located on the western Atlantic coast of Portugal (Sousa et al., 2016; Bueno-Pardo et al., 2018; Costa et al., 2022), and the replacement of annelid polychaetes from the genus Diopatra spp. by non-indigenous Arenicola spp. The events related with this replacement and the recent status of the species are included in Figure 1. Arenicola marina and A. defodiens (lugworms) were likely introduced via oyster seeds from France for oyster cultures at Mira Channel (Pires et al., 2015). Although no long-term quantitative data are available, information gathered through structured questionnaires with key stakeholders - particularly those who rely directly on the lagoon for their livelihoods - indicates that Diopatra neapolitana is perceived as having declined in the past and is expected to continue declining. In contrast, the IAS Arenicola spp. is perceived to be expanding its distribution in the future (Luís et al., 2025). Even though no additional quantitative data is currently available, in situ observation in the last five years confirm the increase of the Arenicola spp. density and distribution in other areas of the lagoon (authors’ personal observation).
Figure 1
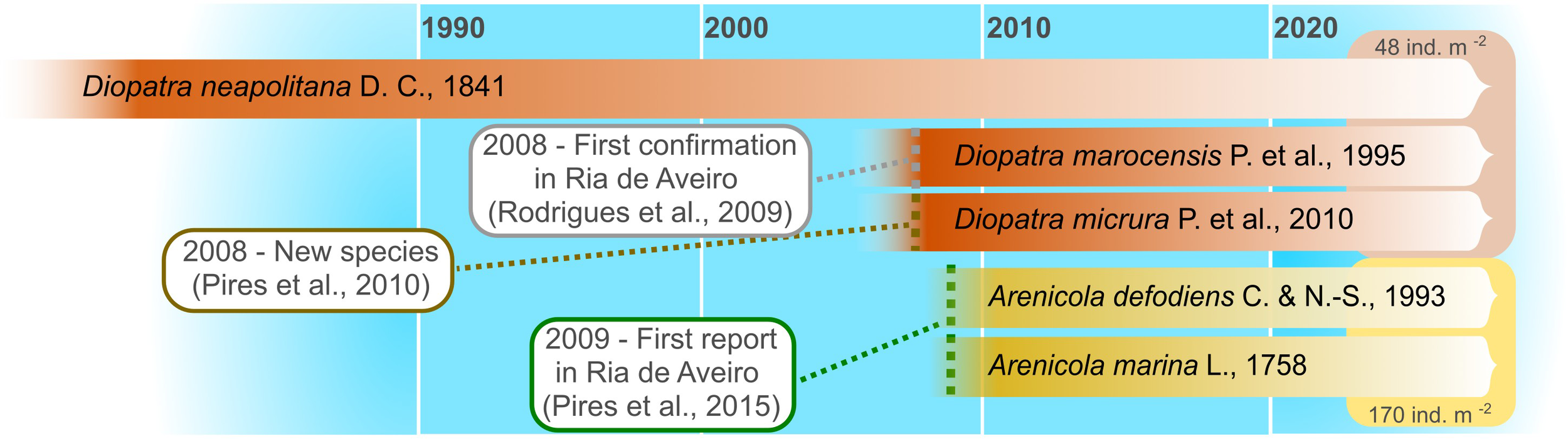
Timeline for the status of the species of the genus Diopatra and Arenicola in Ria de Aveiro, Portugal. Density values from BioPradaRia project (unpublished data).
The species from the genus Diopatra (hereinafter referred to as Diopatra) are considered omnivorous grazers (Jumars et al., 2015; Berke, 2022), and inhabit intertidal mud sediments or mixed mud-sand areas, living inside buried and emergent tubes made of a secreted layer and externally covered by sand particles and other fragments, like shells and algae (Fauvel, 1923; Dağli et al., 2005; Rodrigues et al., 2009). Arenicola species (hereinafter referred to as Arenicola) are deposit feeders, inhabiting U- or J-shaped burrows in subtidal and intertidal habitats (Reise, 1985; Andresen and Kristensen, 2002; Volkenborn and Reise, 2006). They ingest surface sediment through a feeding funnel and expel it in the form of fecal casts (Reise, 1985; Zebe and Schiedek, 1996; Wendelboe et al., 2013), with pervasive impacts on sediment stabilization and infaunal communities (Volkenborn and Reise, 2006; Wendelboe et al., 2013). Diopatra and Arenicola co-exist in many coastal areas worldwide (Berke et al., 2010). Both taxa are commonly used as bait (Cunha et al., 2005; Dağli et al., 2005; Escobar-Ortega et al., 2024), and can reach high densities in many habitats: e.g. Diopatra, 144 ind.m-2 in Ria de Vigo, Spain (Escobar-Ortega et al., 2024), or 198 ind.m-2, in Izmir Bay, Turkey (Dağli et al., 2005); Arenicola, 66 ind.m-2 in Oosterschelde, The Netherlands (Montserrat et al., 2011), or 70 ind.m.2 in Odense Fjord, Denmark (Delefosse et al., 2012). In Ria de Aveiro, Diopatra specimens are mainly found in the Mira channel, with densities reaching 48 ind.m-2 in 2020 (unpublished data - BioPradaRia project), while Arenicola specimens are widely spread all over the lagoon with higher abundances also found in the Mira channel (as high as 170 ind.m-2 in 2020) (unpublished data - BioPradaRia project). Amongst the most expensive marine bait sold on the global fisheries market (Watson et al., 2017), both taxa are highly sought after by bait diggers.
Introduced species can often constitute new functional components in the recipient community and produce shifts in the structure and functioning of ecosystems (Thomsen et al., 2014; Gallardo et al., 2016). Contrary to the sediment-stabilizer Diopatra (Berke, 2022), Arenicola species are important ecosystem engineers with high sediment reworking capacity (Volkenborn and Reise, 2006; Montserrat et al., 2011). In fact, Berke et al (2010) highlighted the opposite engineering abilities of both taxa, with different effects on the ecosystem functioning.
The replacement of a sediment stabilizer by a highly active bioturbator will increase sediment reworking and bioirrigation. This will be reflected on nutrients and gas fluxes across the sediment-water interface (Kauppi et al., 2018), sediment properties (Volkenborn et al., 2007), organic matter remobilization and mineralization (Michaud et al., 2010), displacement and/or bioaccumulation of chemical and organic contaminants and metals (Remaili et al., 2017), and benthic fauna community structure and diversity (Whitton et al., 2016). Benthic fauna behavior (Moyo et al., 2017), microbial community (Lei et al., 2010; Lacoste et al., 2018) and vegetation (e.g., seagrass) fragmentation and establishment (Philippart, 1994; Blackburn and Orth, 2013; Costa et al., 2022) will also be affected by this species replacement. Therefore, it is important to understand how the replacement of a native species, with strong ecosystem engineering capabilities, by an IAS, with opposite traits, affects a) sediment reworking and b) the flux of nutrients across the water-sediment interface. Microcosms are highly useful to assess ecosystem processes under controlled conditions (Benton et al., 2007): by manipulating the ratios of Diopatra and Arenicola under controlled microcosm conditions for 21 days, we evaluated the impact of this replacement, to test the null hypothesis that the native species substitution by IAS with opposed ecosystem engineering traits have no effect on sediment reworking and nutrient cycling. Hence, the objective of this work is to: a) quantify the sediment reworking effect of the different species ratios, traced with luminophores; b) quantify the sediment-water column nutrient flux promoted by the different species ratios; and c) discuss and conclude on the potential impact of native/invasive replacement among contrasting ecosystem engineers’ on overall ecosystem functions (sediment reworking) and processes (nutrient dynamics).
Materials and methods
Fauna and sediment preparation
Specimens of Diopatra and Arenicola complexes, as well as sediment, were collected in Ria de Aveiro, a shallow, mesotidal costal lagoon located on the northwestern coast of Portugal, between 40° 30`N and 40° 51`N (Vaz et al., 2005), with a longitudinal gradient of salinity from about 0 to 36. Extensive intertidal mud and sand flats, salt marshes and islands can be found along its main channels (Costa et al., 2022). Given the large number of specimens needed and the effort associated with their capture, Diopatra and Arenicola were obtained from a fishing store that receives the specimens collected by local fishermen in Ria de Aveiro. Sediment was collected in Mira Channel, one of the southern channels, where the presence of Arenicola has been reported (Pires et al., 2015; Costa et al., 2022). Sediment was homogenized and macrofauna removed by hand-piking for use in housing and microcosms experiments. Specimens were transported to the laboratory and placed in containers (370 x 270 x 230 mm, L x W x H) with 120 mm of sediment overlain with 100 mm of artificial seawater (salinity 28, prepared by mixing Red Sea Coral Pro Salt (Germany) and reverse osmosis water). The salinity level was established based on the distribution areas of both species in Ria de Aveiro (Rodrigues et al., 2011; Vargas et al., 2017). The specimens passed by a housing period of 12 days to ensure the complete regeneration of the posterior part and to ensure that all the individuals were healthy and feeding (Pires et al., 2012). During the housing period, continuous aeration was provided using air-stones and the containers were supplied with fish food (Supreme 50, Skretting) every day by placing a small grain next to the entrance of each tube. If the food was not dragged into the tube within 5 minutes, it was removed. Food was not provided on the day before the polychaete specimens were transferred to the microcosms. During the housing period, the average water temperature in the containers was of 17.9 ± 1.0°C, pH 8.2 ± 0.3, oxygen concentration 7.3 ± 0.8 mg L−1, and salinity 28.0 ± 0.1 (mean ± s.d.).
Microcosm setting
A microcosm set-up was designed to simulate different degrees of Diopatra (Dp) habitat invasion by Arenicola (Ar). Sediment was distributed along 30 vertical glass aquaria (135 × 135 × 500 mm, internal dimensions), creating a 150 mm column (~ 1/3 of the height of the aquarium, 2.7 L of sediment) and filled with artificial seawater (~ 2/3 of the height of the aquarium, 5.5 L of water). The detailed timeline of the microcosms’ experiment, related to water replacement, housing period, acclimation, luminophores introduction, and monitoring of bioturbation and water column nutrients’ concentration is provided in Supplementary Table S1. Each aquarium was gently aerated by releasing air bubbles through two capillary tubes (ø = 0.84 mm), connected by a silicon tube (ø = 4.00 mm) to an air pump (RESUN LP-100, 140 L min-1). Aquaria were randomly distributed across the bench and exposed to natural light conditions with a 10 h light:14 h dark photoperiod. Five different ratio of Diopatra: Arenicola, with an equivalent constant density of ~180 ind.m-2, plus Control (no organisms), were placed in the aquaria to be tested: 4 Diopatra (coded as 4Dp:0Ar), 3 Diopatra and 1 Arenicola (3Dp:1Ar), 2 Diopatra and 2 Arenicola (2Dp:2Ar), 1 Diopatra and 3 Arenicola (1Dp:3Ar), and 4 Arenicola (0Dp:4Ar) (5 replicates each, totaling 30 aquaria). Adult Diopatra individuals were introduced into the experimental units without their original tubes. However, by Day 0, prior to the introduction of luminophores and the start of the experiment, all individuals had already built new tubes (confirmed by direct observation). These newly constructed tubes were functional and appeared structurally stable throughout the experimental period. To minimize evaporation and prevent pronounced salinity shifts, all aquaria were covered with Parafilm® Sealing Film. The water in each aquarium was not renewed and neither sediment nor food were added during the acclimation and experimental period.
To avoid additional manipulative stress before implementing the experiment, the polychaetes were measured (total length) and weighted only at the end of the experiment. Organisms passed by a depuration period of 24 h to clear their gut before weighing. The average length and weight were 74.08 ± 10.63 mm and 2.12 ± 0.46 g for Diopatra, respectively; and 86.62 ± 22.23 mm and 3.78 ± 0.75 g for Arenicola (mean ± s.d.), respectively.
Bioturbation
Particle reworking was measured non-invasively using fluorescent sediment profile imaging (f-SPI; Solan et al., 2004; Lopes et al., 2018; Crespo et al., 2021), based on the visual detection of fluorescent luminophores on the sides of the aquaria. This profile imaging provides descriptors for faunal-mediated sediment reworking, which are surrogate measures for different aspects of infaunal organisms’ behavior (Teal et al., 2010; Hale et al., 2014). To avoid the strong initial activity associated with polychaetes establishment and burrowing, luminophores (40 g, 125 - 250 µm diameter, yellow color) were added on the top of the sediment, near the side of each aquarium which would be photographed later, at least 24 h after the introduction of organisms (D0). This method allows the deposition of a 1–2 cm layer of luminophores on the side that will be photographed and ensures the supply of luminophores throughout the entire experimental period.
The aquaria were photographed within a dark box, under Ultraviolet light, using a GoPro HERO6 camera (12 megapixels sensor, set for 1/8 seconds exposure, diaphragm aperture of f/2.8, and film speed (light sensitivity) equivalent to ISO 3199). Each image obtained was subsequently cropped according to the internal width of the aquarium (135 mm = 2050 pixels, effective resolution = 63.4 µm px-1), converted to a red-green-blue (RGB) stack and saved with JPEG compression (Joint Photographic Experts Group). Images were then analyzed using a custom-made plugin that runs within ImageJ (Version 1.48c), a java-based public domain program developed at the US National Institutes of Health (available at http://rsb.info.nih.gov/ij/index.html). The plugin output provides details on the mean (f-SPILmean, time-dependent indication of mixing), median (f-SPILmed, typical short-term depth of mixing), and maximum (f-SPILmax, maximum extent of mixing over the long-term) mixed depth of particle redistribution (Hale et al., 2014), for each aquarium, after the semi-automatic conversion of the images to a binary data matrix of the distribution luminophore pixels (0 = background sediment, 1 = luminophore). For each image, the vertical distance between the highest and lowest points of the water sediment boundary (surface boundary roughness – SBR) was manually assessed. SBR is a proxy for surficial activity and is particularly useful in the case of species with high accretion abilities, such as Arenicola, which creates noticeable faecal casts on the surface of the sediment (Volkenborn et al., 2007). As bioturbation processes are cumulative, only the final day (day 21) image data were considered, and the values found for the tested species ratio were considered against those found for the procedural control.
Sediment and water characteristics; dissolved nutrient content
Before the experiment inception, five sub-samples were analyzed to determine grain-size and organic matter content on the homogenized sediment. Sediment grain-size was analyzed by dry sieving standard method (Sutherland, 1998). Median and percentage of fines were used to classify the sediment, according to the Wentworth scale (Blott and Pye, 2001): very fine sand (median from 3 - 4 Φ; 63 - 125 µm); fine sand (2 - 3 Φ; 125 - 250 µm); medium sand (1 - 2 Φ; 250 - 500 µm); coarse sand (0 - 1 Φ; 500 - 1000 µm); very coarse sand (-1 - 0 Φ; 1000 - 2000 µm). The final classification adopted the description ‘clean’, ‘silty’ or ‘very silty’ when the % fines fraction of the total sediment, by dry weight, ranged from 0% to 5%, 5% to 25% and 25% to 50%, respectively (Blott and Pye, 2001). Samples with >50% fines content were classified as mud. Total organic matter content was obtained by loss on ignition as described by Kristensen and Andersen (1987).
At days 0, 2, 5, 8, 12, 16, and 21, 10 ml water aliquots (filtered through Whatman GF/C glass-fiber filters) were sampled on the top of the water column (0–5 cm), from each aquarium: this low volume ensured a loss lower than 10% on the microcosm total volume. Samples were stored at −20°C until analysis of dissolved inorganic nutrients (ammonium, NH4-N; oxidized form of dissolved inorganic nitrogen, NOx-N; phosphate, PO4-P). The determination of the concentrations of NH4-N and PO4-P was performed following standard methods described in Limnologisk Metodik (Københavns Universitet, 1992). The concentrations of NOx-N were determined using a flow injection system (FIAstar 5000 Analyzer, Höganäs, Sweden), following Strickland and Parsons (1972). Analytical quality control was ensured by calibration curves derived from standard solutions, run at the beginning of the analysis and in parallel with blanks and samples. Water temperature, pH, concentration of dissolved oxygen and salinity were measured in the mesocosm on every sampling day using a WTW – pH 330i/set equipped with SenTix® 41; a WTW – cond 3110/set 1 equipped with TetraCon® 325 and a WTW – Oxi 3210/set 2 equipped with CellOx® 325-3.
Statistical analyses
The dependent variables related to sediment reworking descriptors (SBR, f-SPILmean, f-SPILmed, and f-SPILmax) and dissolved nutrient concentrations ([NH4-N], [NOx-N], and [PO4-P]), on day 21, were analyzed using independent regression models with fauna ratios as the fixed term. The dependent variables were analyzed under the null hypothesis of no significant differences among the levels of the response variables across the different ratios of Diopatra (Dp) and Arenicola (Ar), and the Control (defaunated microcosms), and the statistical significance of their terms (p < 0.05) were tested. To deal with heteroscedasticity and avoid data transformation, the models were extended to include the appropriate variance covariate structure using a generalized least squares (GLS) estimation procedure (Zuur et al., 2009; Pinheiro et al., 2014). The variance covariate structure was determined using a restricted maximum likelihood (REML) estimation, supported by the Akaike Information Criteria (AIC) and visual comparisons of residuals plots, in contrast to the initial model. Pairwise comparisons of the treatments with different ratios of Dp and Ar were derived from each model’s t-table, re-expressed successively against each level as baseline.
Additionally, water nutrient concentrations of NH4-N, NOx-N and PO4-P were represented in ordination analyses, using a Principal Component Analysis (PCA) (Anderson et al., 2008). All statistical analyses were performed using R statistical and programming environment (R Development Core Team, 2020) and the packages nlme (Pinheiro et al., 2014) for the regression analysis, and vegan (Oksanen et al., 2020) with functions metaMDS and envfit, to overlay vectors in the ordination analysis. Graphical outputs were produced with R’s package ggplot2 (Wickham, 2016).
Results
Physical-chemical parameters and sediment characterization
The sediment sampled in Mira channel was classified as medium sand (125- 250 µm; φ = 2) with 8.00 ± 0.86% of fines (< 63 µm) and the total organic matter content was of 3.0 ± 0.5% (mean ± s.d.).
During the experimental period, the average water temperature in the aquaria was of 18.4 ± 1.2°C, pH 8.1 ± 0.2, oxygen concentration was 7.8 ± 0.7 mg L−1 and salinity was 28.0 ± 0.2 (mean ± s.d.).
Bioturbation
In the Control microcosms, luminophores were concentrated in the superficial layer (<2.0 cm) (Figure 2A), corresponding to the initial layer deposited on the top of the sediment. In the different treatments, the number of luminophores in the superficial layer tend to decrease, increasing in deeper layers with the increasing number of Arenicola (Figures 2A–F). All bioturbation parameters responded to the increasing density of Arenicola (Figures 3A–D; Table 1).
Figure 2
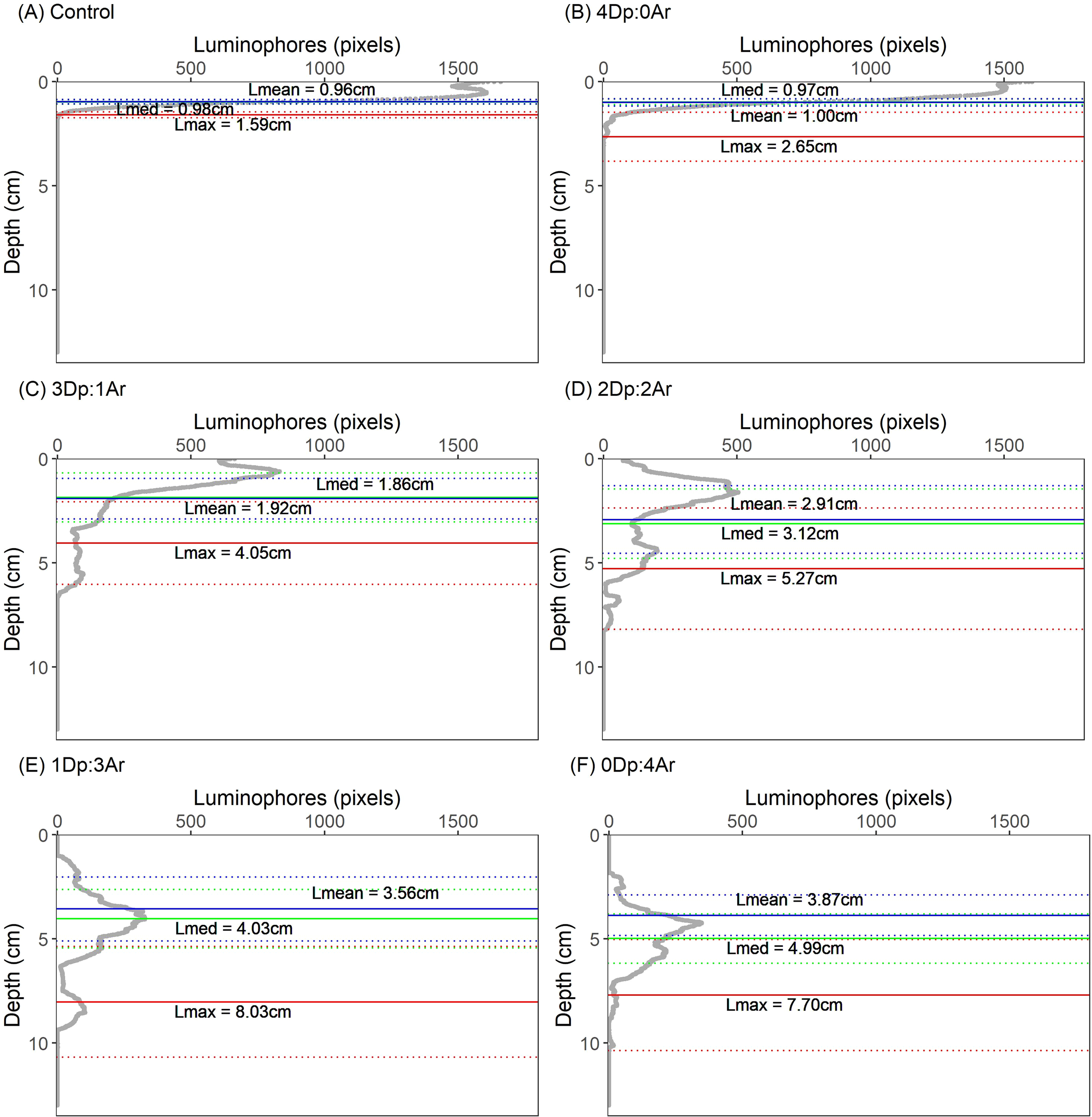
Averaged (n=5) vertical profile of luminophores in the sediment after 21 days of bioturbation associated to different ratios of Arenicola (Ar): Diopatra (Dp): (A) Control (no fauna); (B) 4Dp:0Ar; (C) 3Dp:1Ar; (D) 2Dp:2Ar; (E) 1Dp:3Ar; (F) 0Dp:4Ar. Solid horizontal lines represent mean f-SPILmean (blue), f-SPILmed (green) and f-SPILmax (red) mixed depths of luminophores’ redistribution, and dashed lines their respective standard deviation limits.
Figure 3
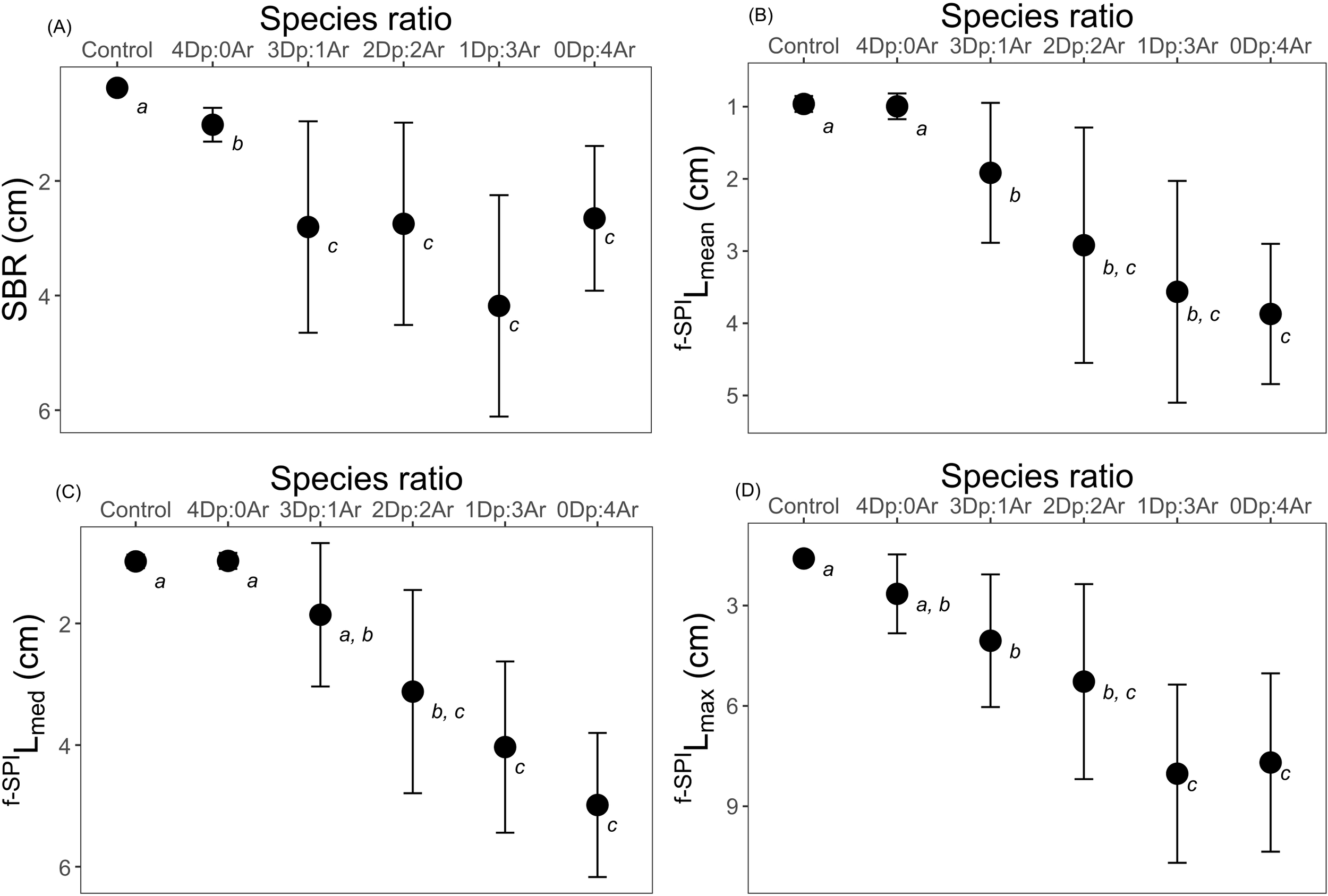
The effects of different species ratio [Arenicola (Ar): Diopatra (Dp)] and Control (no organisms) on the sediment reworking (cm, mean ± s.d.; n=5) in the final day (day 21): (A) Surface boundary roughness (SBR); (B) mean mixed depth of luminophores’ redistribution (f-SPILmean); (C) median mixed depth of luminophores’ redistribution (f-SPILmed); (D) maximum mixed depth of luminophores’ redistribution (f-SPILmaxn). Italicized letters identify statistical similarities.
Table 1
| Dependent variable | Num. d.f. | F-value | P-value | Residuals S.E. |
|---|---|---|---|---|
| Sediment reworking variables | ||||
| SBR | 5 | 14.654 | <.0001 | 1.845 |
| f-SPILmean | 5 | 13.857 | <.0001 | 0.969 |
| f-SPILmed | 5 | 18.128 | <.0001 | 1.179 |
| f-SPILmax | 5 | 14.723 | <.0001 | 1.983 |
| Nutrients | ||||
| [NH4-N] | 5 | 145.105 | <.0001 | 0.077 |
| [NOx-N] | 5 | 136.971 | <.0001 | 0.251 |
| [PO4-P] | 5 | 110.530 | <.0001 | 0.026 |
Summary of significant terms for the generalized least squares (GLS) models analysis of the sediment particle redistribution parameters and nutrient concentrations, against species ratio as explanatory variable and as variance co-variate.
(Denom. d.f.: 24).
Surface boundary roughness (SBR) ranged between 0.26 cm (Control) and 6.48 cm (1Dp:3Ar). The highest mean SBR value was recorded in the 1Dp:3Ar treatment (4.18 ± 1.93 cm, mean ± s.d., Figure 3A), and no significant differences in the mean SBR were found whenever Arenicola was present. Nevertheless, the monospecific treatment 4Dp:0Ar was significantly different from all the other treatments (Figure 3A; Table 1; Supplementary Table S2).
The mean mixed depth of particle redistribution (f-SPILmean) for each aquarium ranged between 0.81 cm (4Dp:0Ar) and 5.86 cm (1Dp:3Ar), with the highest value of 3.87 ± 0.97 cm (mean ± s.d.), for the 0Dp:4Ar treatment (Figure 3B). There were no statistical differences between the Control and the 4Dp:0Ar ratio, between the 3Dp:1Ar, 2Dp:2Ar and 1Dp:3Ar ratios, and finally when comparing 2Dp:2Ar, 1Dp:3Ar and 0Dp:4Ar ratios (Figure 3B; Table 1; Supplementary Table S3).
The median mixed depth of particle redistribution (f-SPILmed) had its lowest value in the 4Dp:0Ar treatment, 0.83 cm, while the highest value was recorded in the 0Dp:4Ar treatment, with a f-SPILmed of 6.01 cm. Collectively, the ratio 0Dp:4Ar had the highest mean f-SPILmed, with 4.99 ± 1.18 cm (mean ± s.d.) (Figure 3C). Pairwise comparisons revealed no significant differences among Control, 4Dp:0Ar, and 3Dp:1Ar, among 3Dp:1Ar and 2Dp:2Ar, and among 2Dp:2Ar, 1Dp:3Ar and 0Dp:4Ar (Figure 3C; Table 1; Supplementary Table S4).
Finally, the maximum mixed depth of particle redistribution (f-SPILmax) ranged from 1.36 cm (4Dp:0Ar) to 11.26 cm (0Dp:4Ar). The lowest mean f-SPILmax was found in the Control (1.59 ± 0.15 cm, mean ± s.d.), followed by the 4Dp:0Ar treatment (2.65 ± 1.18 cm, mean ± s.d.) while the highest mean of 8.03 ± 2.66 cm (mean ± s.d.), was recorded in the 1Dp:3Ar treatment (Figure 3D). When comparing the different species ratios, no significant differences were detected between the Control and the 4Dp:0Ar ratio, between the 4Dp:0Ar, 3Dp:1Ar and 2Dp:2Ar, and between 2Dp:2Ar, 1Dp:3Ar 0Dp:4Ar ratios (Figure 3D; Table 1; Supplementary Table S5).
Water nutrients’ concentration
The statistical model indicates that the concentration of all analyzed nutrients in the water column in the end of the experiment (21 days later) was influenced by species ratio (Table 1). During the experimental period, the concentration of NH4-N increased with the increasing number of Arenicola in the aquaria (2Dp:2Ar < 1Dp:3Ar < 0Dp:4Ar) (Figure 4A). However, the concentration of NH4-N in the lower Arenicola densities (Control, 4Dp:0Ar, 3Dp:1Ar) have peaked around day 5, while in the intermediate levels (2Dp:2Ar and 1Dp:3Ar) the concentration of NH4-N has peaked around day 16. The monospecific Arenicola treatment (0Dp:4Ar) showed an increase in the concentration of NH4-N until day 21 (Figure 4A). At the end of the experiment, in the presence of fauna, the highest and lowest mean concentrations of NH4-N were registered in the monospecific scenarios ([NH4-N]0Dp:4Ar = 8.07 ± 0.6, [NH4-N]4Dp:0Ar = 1.49 ± 0.04 mg L−1, mean ± s.d.). In the Control, the mean concentration of NH4-N was 1.42 ± 0.03 mg L−1 (mean ± s.d.). The statistical analysis revealed significant differences between all comparisons, except between 2Dp:2Ar and 1Dp:3Ar (Figure 4A; Table 1; Supplementary Table S6).
Figure 4
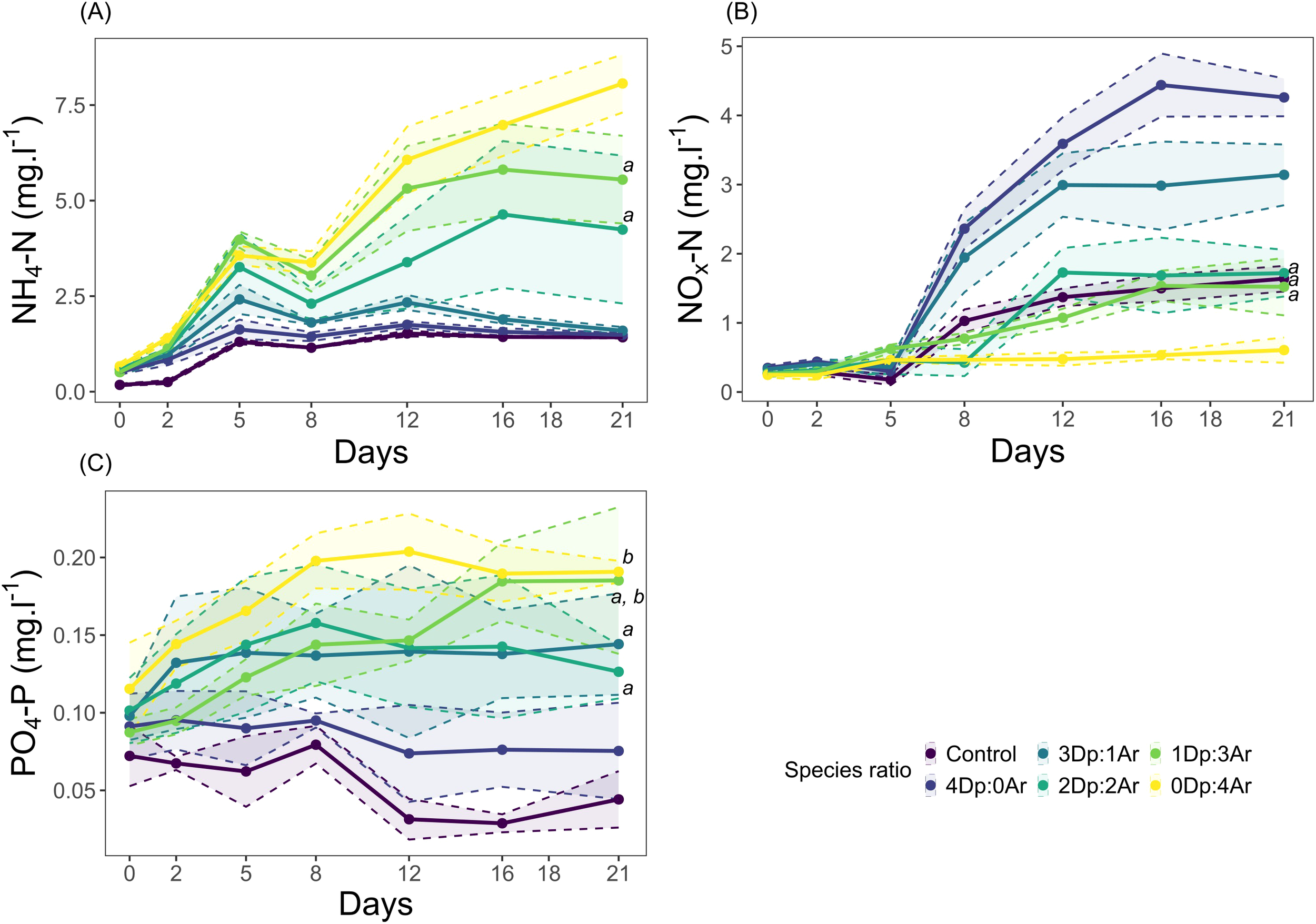
The effects of different species ratio [Arenicola (Ar): Diopatra (Dp)] and Control (no organisms) on the inorganic dissolved nutrient content of the water column along time (mg L-1, mean; n=5; shaded bands represent the 95% confidence interval): (A) ammonium (NH4-N); (B) oxidized form of dissolved inorganic nitrogen (NOx-N); (C) phosphate (PO4-P). Italicized letters identify statistical similarities (p-value > 0.05) in the final day (day 21).
During the experimental period, the average concentration of NOx-N showed an opposite trend comparatively with NH4-N, i.e., it increased in the aquaria with higher densities of Diopatra. The maximum concentration of NOx-N was 4.44 mg L−1, measured at day 16, in the monospecific treatment 4Dp:0Ar. In most treatments, NOx-N concentrations started to increase after the 5th day. At the end of the experiment, the lowest concentration of NOx-N was 0.61 ± 0.15 mg L−1 (mean ± s.d.), found in the 0Dp:4Ar treatment, while the highest, 4.26 ± 0.22 mg L−1 (mean ± s.d.), was found in the 4Dp:0Ar treatment. For day 21, the concentration of NOx-N in the Control was 1.64 ± 0.15 mg L−1 (mean ± s.d., Figure 4B). Contrarily to what was observed for NH4-N, significant differences were observed in the mean concentration of NOx-N at day 21, between the Control and the aquaria with higher ratios of Diopatra (4Dp:0Ar, 3Dp:1Ar) and also between the aquaria with 0Dp:4Ar. No differences were found the concentration of NOx-N between the Control, 2Dp:2Ar and 1Dp:3Ar treatments (Figure 4B; Table 1; Supplementary Table S7).
For PO4-P, the Control showed a reduction from day 0 to day 21, while the monospecific 4Dp:0Ar treatment had only a marginal decrease. The remaining treatments showed an increase in the concentration of PO4-P from day 0 to day 21 (Figure 4C). The lowest concentration of PO4-P was 0.02 mg L−1 (day 12, Control), while the highest was 0.24 mg L−1 (day 21, 1Dp:3Ar). The mean concentration of PO4-P tended to increase with the increasing ratio of Arenicola, achieving a maximum concentration of 0.20 ± 0.02 mg L−1 (mean ± s.d.) for the monospecific Arenicola treatment (0Dp:4Ar) in day 12 (Figure 4C). The statistical analysis for the concentration of PO4-P at day 21 showed no differences between 3Dp:1Ar, 2Dp:2Ar, and 1Dp:3Ar ratios (respectively 0.14 ± 0.03, 0.13 ± 0.10. and 0.19 ± 0.04 mg L-1, mean ± s.d.), and between 1Dp:3Ar and 0Dp:4Ar (0.19 ± 0.01 mg L-1, mean ± s.d.) ratios, (Figure 4C; Table 1; Supplementary Table S8).
The principal components ordination clearly shows the evolution of the mesocosms water concentration along time, clustering the samples from days 0, 2 and 5 along Axis 1, which explains almost 60% of the total variance (Figure 5). The nutrients concentration vectors are spread also along Axis 2 and showed that the Control, the monospecific scenario 4Dp:0Ar and the ratio 3Dp:1Ar were characterized by higher concentrations of NOx-N, while the other treatments (2Dp:2Ar, 1Dp:3Ar, 0D:4Ar) were defined by higher concentrations of NH4-N and PO4-P, particularly for the dates after day 8 (Figure 5).
Figure 5
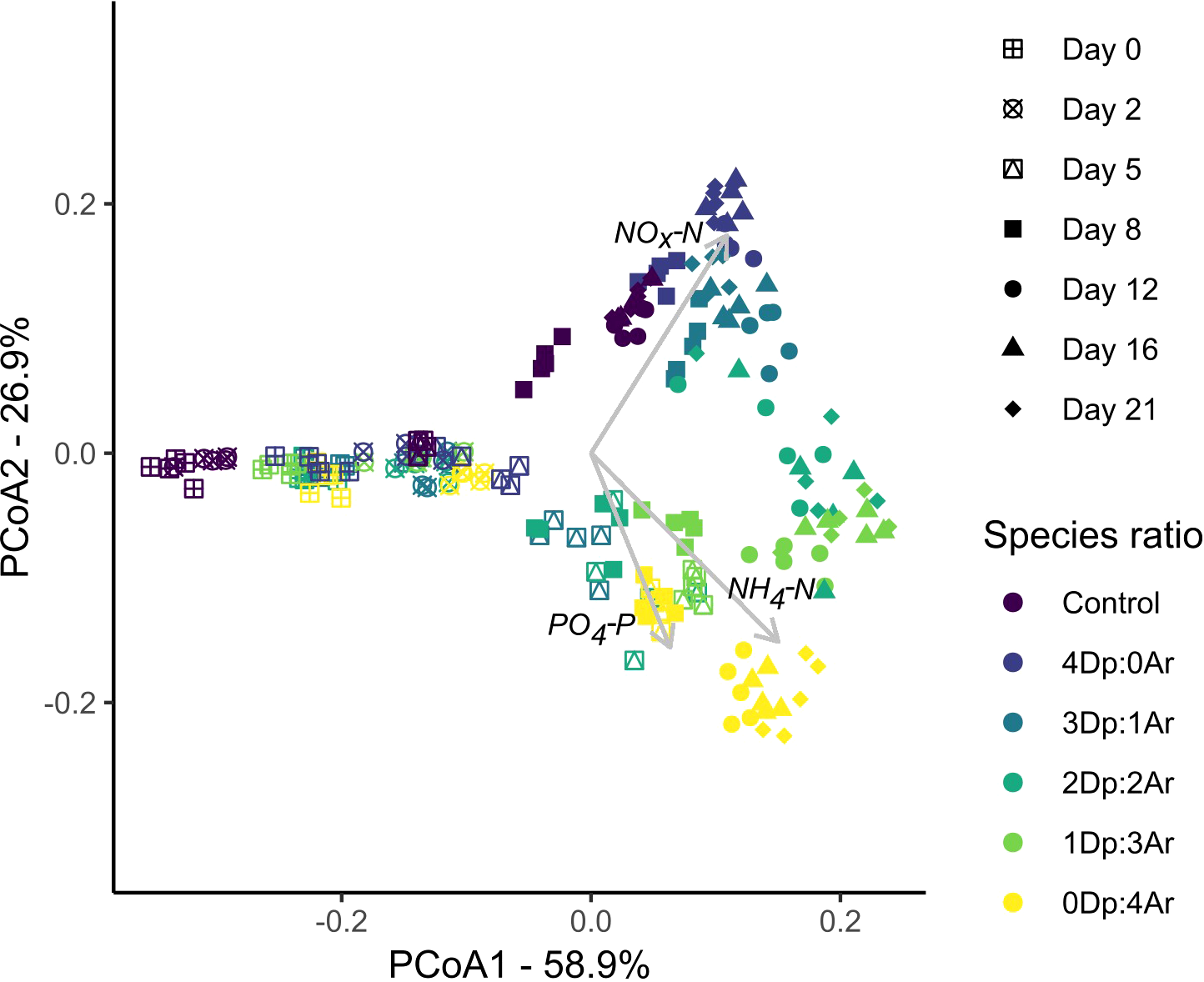
Principal component analysis based on the concentrations (mg L-1) of ammonium (NH4-N), oxidized form of dissolved inorganic nitrogen (NOx-N) and phosphate (PO4-P) in the Control (C) and different treatments of Diopatra (Dp) and Arenicola (Ar) considering seven sampling times (Days 0, 2, 5, 8, 12 and 21) during the experimental period.
Discussion
In the context of the worldwide spread of species across different habitats and ecosystems, it is fundamental to understand the outcomes of species substitution by functionally distinct counterparts. Measuring the amount of change that these substitutions will produce can help predict the ecological consequences in invaded habitats, as different species might also show different traits and non-linear responses (Strayer and Hillebrand, 2012; Strayer, 2020). The present study confirms that the replacement of native sediment stabilizer species of the Diopatra (solitary tube worm) genus by highly active bioturbator Arenicola (lugworm) species will have consequences on important ecological processes, which can resonate across the functioning of the overall ecosystem. The results of the experiment showed a strong response to the increasing density of Arenicola individuals in the microcosms: higher densities on Arenicola exhibited higher values in almost all analyzed parameters, both for bioturbation and nutrients concentration.
Ecological effects of functionally distinct species
Sediment reworking
Sediments and infaunal organisms in aquatic systems have severe limitations in accessing oxygen (Jansen et al., 2009; Kristensen et al., 2012). Bioturbation in aquatic systems is a process that facilitates the transport of fluids within the sediment matrix and across the sediment-water interface through ventilation, including oxygen from the overlying water into sediment interstices (Maire et al., 2010; Kristensen et al., 2012). Bioturbation also includes particle reworking (i.e., the transport of particles within the sediment matrix), which enables the relocation of several forms of organic matter from and into the sediment (Kristensen et al., 2012; Aller and Cochran, 2019). This means that bioturbation is fundamental to the proper ecological functioning of benthic communities (Mermillod-Blondin, 2011; Aller and Cochran, 2019). However, species differ widely in the way they bioturbate (Volkenborn et al., 2009; Mermillod-Blondin, 2011; Kristensen et al., 2012) and, therefore, different bioturbation mechanisms will mediate different ecological responses. Diopatra species are known as proficient ecosystem engineers, that create robust and permanent tubes that extends deep into the sediment (Berke, 2022; Arias et al., 2023). These tubes provide structural complexity and heterogeneity, benefiting other endo- or epibenthonic species (Bailey-Brock, 1984; Thomsen et al., 2011; Santos and Aviz, 2019). Additionally, the stabilizing effect of Diopatra tubes is important in highly hydrodynamic systems, by reducing the resuspension of sediments (Bailey-Brock, 1984; Luckenbach, 1986; Berke, 2022). On the other hand, Arenicola species are known to be upward conveyors, with a highly intense reworking activity (Riisgård and Banta, 1998; Wethey and Woodin, 2022), reducing the sediment heterogeneity, and with the potential to increase sediment erosion under strong currents (Wendelboe et al., 2013). As evidenced by our results, shifting from the monospecific Diopatra to Arenicola treatments led to a 3 to 4-fold increase in the reworking depths, reflecting the different mechanisms on which each genera uses to rework the sediment. Although Diopatra tubes can reach high depths within the sediment, the sediment matrix is kept relatively undisturbed, which is supported by the low particle redistribution depth observed in Diopatra dominated treatments. Particularly, the monospecific Diopatra treatment was statistically indistinct from the controls in all bioturbation parameters, with luminophores concentrated on the uppermost 2 cm. By contrast, in Arenicola dominated microcosms there was an effective homogenization of the sediment along the uppermost 6 cm. Luminophores’ particles redistribution could have been evaluated on all sides of the aquaria, increasing the probabilities to detect movement and providing more robust data. This is particularly true for Diopatra which presented very low mobility, and which random distribution along the sediment could have impaired the detection of their eventual sediment reworking. However, the results were consistent across replicates, even when using data from only one side of the aquaria as a proxy for bioturbation potential for the full sediment column.
We could not statistically differentiate the reworking depths when there were two or more Arenicola individuals in each microcosm, suggesting that even if communities show some equitability, there is a disproportionate effect of Arenicola on sediment reworking: even lower densities show strong responses on the sediment reworking depths. Our Arenicola specimens were able to bury as deep as 11 cm in the highest ratio treatment, perhaps as an intraspecific avoidance behavior. De Cubber (2020) mentions that these species avoid intra-specific competition by migrating down the shore, reflecting a behavioral tendency for avoidance. Confined within the volume of our microcosms, the only way to avoid each other is to go deeper. However, the average maximum luminophores mixing depth, of ~8 cm, was very similar in 1Dp:3Ar and 0Dp:4Ar ratios, which could indicate an asymptotic threshold dependent on the specimens’ size. The depths of reworking in Arenicola species have been reported to vary with organisms’ size/age: while the species could live in burrows up to 30 cm in depth, Wethey and Woodin (2022) (after Beukema and De Vlas, 1979) mention that this depth can be reduced as function of body size. Based on De Cubber et al. (2020), for the average size of our Arenicola specimens (~8.7 cm length) the burrows could extend as deep as ~22 cm. This means that our specimens did not attain the full reworking potential that was expected for their size, which could be related with the size of the aquaria. However, in no circumstance during our experiment the sediment reworking reached the maximum sediment depth of our microcosms, which means that some other drivers have limited these movements. It seems that under starvation or other stress sources the species reduce their reworking activity and burrow size (Wethey and Woodin, 2022), which can explain the difference between our maximum reworking depths and the predicted size of the burrows. Additionally, as an intertidal species, low tide might trigger deeper burrowing. However, tides were not considered in our experimental setup which held a constant water level throughout the all period. The Surface Boundary Roughness (SBR) values were statistically indistinguishable across treatments containing Arenicola, regardless of the number of individuals. These values were a consequence of the faecal cast deposition on the top of the sediment, due to the highly intensive feeding activity, based on the non-selective, conveyer-belt, ingestion of large amounts of sediment (Riisgård and Banta, 1998; Volkenborn and Reise, 2006). In this case, SBR offers information on the height of the faecal casts (the distance between the highest and the lowest points on the water-sediment interface), which could be an asymptoticly limited, i.e. there should be a limit on the amount of sediment that can be accumulated vertically on these casts, before they start to collapse. Even if it seems contra-intuitive, it was expected that this measure would reach an asymptotic threshold, and, in this case, this limit was reached in the treatment with the lowest densities of Arenicola.
Sediment-water column nutrient dynamics
The concentrations of water nutrients can have different sources: 1) the feeding and metabolic activity of the polychaetes; and 2) the microbiome and meiofauna (considering that the sediment was defaunated, and no other macroinvertebrates are present, besides the introduced polychaetes). Regarding the first source, the feeding and metabolic activity of the worms, it is possible that the highly active Arenicola is responsible for the larger production of ammonium, which is accumulated in the water column. The initial values (day 0) were very similar between treatments, but soon the concentration of NH4-N started to become distinct in the different treatments. However, in the final day, the Diopatra dominated treatments (4Dp:0Ar; 3Dp:1Ar) showed a reduction in the NH4-N values, similar to those found in the control, after peaking on day 5. This suggests that the microbial community fostered by Diopatra could be intercepting this nutrient, even if is being generated by the polychaetes in the microcosm. The trends observed for NOx-N were different, with the highest values recorded in the monospecific Diopatra treatment, while the lowest values occurred in the monospecific Arenicola treatment, which showed lower values than the control, after day 8. This indicates that, under the influence of Arenicola, there is a consumption of NOx-N. However, the prominent increase in the Diopatra dominated treatments, after day 5 could indicate that Diopatra might be stimulating the nitrifying community (Voss et al., 2013), responsible for the oxidation of NH4-N to NOx-N. This is reinforced by the fact that the NOx-N values in the Diopatra dominated treatments are significantly higher than those in the Control. If, indeed, there is an increase in photosynthetic organisms biomass promoted by Diopatra, as described in the literature (Berke et al., 2010; Fuirst et al., 2021; Bugnot et al., 2023), the oxygen present in the water and in the interstitial water of the top layers of the sediment can alter the redox conditions, influencing the nitrification-denitrification processes (Voss et al., 2013; Høgslund et al., 2023). This higher oxygen availability also affects the phosphate dynamics, which is consumed by the microphytobenthos to build biomass, while also causing the oxidation of iron compounds in the sediment which improves P adsorption and sequestration in the sediment (Høgslund et al., 2023). The phosphate concentrations were very fast to reach the equilibrium, with most treatments reaching the peak after day 2. This fast response could indicate that the concentration of PO4-P was chemically controlled, more than biologically mediated, i.e., independent from the microbiome community establishment. The microcosms dominated by Arenicola were characterized by high levels of NH4-N and PO4-P, while the Control and Diopatra microcosms were dominated by high levels of NOx-N. However, this distinction was only evident in samples collected after day 8. Without following the microbiome dynamics, it is only possible to speculate regarding the detailed mechanisms involving the nutrient generation and consumption, as they depend on the relative equilibrium between the elements C, N, and P (Voss et al., 2013) and on the biological interactions that take place (Mallin and Cahoon, 2020). Also, the Arenicola species are not only strong biomixers, but also highly active advective bioirrigators, causing a dramatic effect on the porewater flow, and able to flush significant levels of solutes (Kristensen et al., 2012) which can include NH4-N and PO4-P. Nevertheless, without assessing the rates of critical biogeochemical processes such as remineralization, nitrification, and denitrification, or bioirrigation, it is unclear if the detected nutrient differences are a consequence of increased production, intense flushing, or decreased removal, or due to a combination of the these processes. Yet, our results demonstrate that species occupying similar niches may not share similar traits and their impacts on the ecosystem will be profoundly distinct.
While other factors may influence the composition of aquatic communities (Menegotto et al., 2019; Kennish, 2021; Guan et al., 2024), primary production, which is highly dependent on nutrient availability, is the main responsible for the biomass build-up (Philippart et al., 2003; Beukema and Dekker, 2020). However, the effects of the increase in the nutrient loads can lead to more eutrophic aquatic systems and more frequent anoxic events, magnified by global climate warming and the raise in the average water temperature. Consequently, widespread mortalities may occur more frequently in these systems (Rabalais et al., 2009; Antón et al., 2011) (Rabalais et al., 2009; Antón et al., 2011) when dominated by Arenicola, and associated with low water circulation and long retention periods.
Other potential effects of species substitution
Beside the ecological effects shown by our results, the replacement of Diopatra by Arenicola in Ria de Aveiro will have consequences on the accompanying biota. Several studies have reported that Arenicola species exclude other macroinvertebrates from their habitats (Pires et al., 2015) leading to communities with lower macroinvertebrates diversity, particularly among tube building amphipods and polychaetes, and small bivalves (Riisgård and Banta, 1998; Volkenborn et al., 2009). Arenicolidae species are also responsible for the uprooting of macrophytes (Philippart, 1994; Costa et al., 2022). Among the meiofauna, nematodes suffer negative effects (Riisgård and Banta, 1998). Nevertheless, some species can benefit from the presence of Arenicola, such as amphipods, copepods, or subsurface deposit feeding worms such as Scoloplos sp (Volkenborn and Reise, 2007), along with various groups of microorganisms (Lei et al., 2010; Chennu et al., 2015). Diopatra tubes provide substantial benefits to meiofaunal communities, which explore the tube caps and capitalize food availability (Bell and Coen, 1982). These tubes also serve as hotspots for microbiome diversity (Fuirst et al., 2021). Bugnot et al (2022) reported an increase in species richness following the introduction of Diopatra aciculata in a restoration experiment. Even under small densities, Diopatra cuprea seems to enhance the community diversity in macrotidal sandy beaches along the Brazilian Amazon Coast (Santos and Aviz, 2019). The replacement of Diopatra species by Arenicola will likely drive profound changes in the accompanying community (Berke, 2022), with predominantly negative consequences outweighing potential benefits.
Finally, it should be noted that while both genera are economically relevant as bait species (Pires et al., 2012; Watson et al., 2017; Cabral et al., 2019; Berke, 2022; Arias et al., 2023), Diopatra has historically been the most sought species in the Ria de Aveiro (Cunha et al., 2005; Cabral et al., 2019). Understanding whether bait diggers and fishermen are willing to adapt their long acquired habits is crucial, as bait digging represents an important supplementary income for local communities.
Conclusion
Prominent shifts in species range are expected to become more frequent, as consequence of human-mediated dispersion with changes in environmental parameters of receiving habitats due to modification and climate change impacts. Therefore, it is crucial to clarify the context that frames these events, as consequences that arise from those are often difficult to predict if based only on generalized assumptions. Yet, our results demonstrate a directional forcing on relevant ecological proxies, such as bioturbation and nutrient concentrations, elicited by the shift in equitability towards Arenicola dominated communities. The present work demonstrates that the substitution of native polychaete species by IAS can result in significant impacts in fundamental ecological processes and functions (bioturbation and nutrient availability), particularly if the new species has contrasting traits.
Moving forward, it will be important to disclose which are the accompanying microbial communities and simulate scenarios which include the natural environmental variability in transitional aquatic systems, such as tidal effects on salinity, temperature, and oxygen availability.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
ML: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. DC: Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. VC: Methodology, Writing – review & editing. PR: Methodology, Writing – review & editing. AS: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing. AL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was financially supported by the project BioPradaRia - Restoration, management and conservation of biodiversity and biological resources associated with Ria de Aveiro seagrass ecosystems (MAR-01.04.02-FEAMP-0020), funded by the Operational Program MAR2020, EMFF-European Maritime and Fisheries Fund, European Union, Portugal2020. ML, VC and PR were funded by BioPradaRia post-doc and research grants (BPD/UI88/8302/2019, BDP/UI88/5474/2018, BI/CESAM/0070/MAR-01.04.02-FEAMP-0020, respectively). This work is funded by national funds through FCT – Fundação para a Ciência e a Tecnologia I.P., under the project/grant UID/50006 + LA/P/0094/2020 (doi.org/10.54499/LA/P/0094/2020). AI Sousa was funded by national funds through the FCT-Foundation for Science and Technology, I.P., under the research contract CEECIND/00962/2017/CP1459/CT0008 (doi.org/10.54499/CEECIND/00962/2017/CP1459/CT0008). The work was also supported by the project A AAGORA - Blueprint for Atlantic-Arctic Agora on cross-sectoral cooperation for restoration of marine and coastal ecosystems and increased climate resilience through transformative innovation. This Project has received funding from the European Union’s Horizon Europe research and innovation program under grant agreement 101093956. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the granting authority. Neither the European Union nor the granting authority can be held responsible for them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1641983/full#supplementary-material
References
1
Aller R. C. Cochran J. K. (2019). The critical role of bioturbation for particle dynamics, priming potential, and organic C remineralization in marine sediments: Local and basin scales. Front. Earth Sci. (Lausanne)7. doi: 10.3389/feart.2019.00157
2
Anderson M. J. Gorley R. N. Clarke K. R. (2008). PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E Ltd214.
3
Andresen M. Kristensen E. (2002). The importance of bacteria and microalgae in the diet of the deposit-feeding polychaete Arenicola marina. Ophelia56, 179–196. doi: 10.1080/00785236.2002.10409498
4
Antón A. Cebrian J. Heck K. L. Duarte C. M. Sheehan K. L. Miller M.-E. C. et al . (2011). Decoupled effects (positive to negative) of nutrient enrichment on ecosystem services. Ecol. Appl.21, 991–1009. doi: 10.1890/09-0841.1
5
Arias A. Woodin S. A. Paxton H. (2023). An introduction to Diopatra, the amazing ecosystem engineering polychaete. Biol. (Basel)12, 1027. doi: 10.3390/biology12071027
6
Bailey-Brock J. H. (1984). Ecology of the tube-building polychaete Diopatra leuckarti Kinber(Onuphidae) in Hawaii: community structure, and sediment stabilizing properties. Zool J. Linn Soc.80, 191–199. doi: 10.1111/j.1096-3642.1984.tb01972.x
7
Barbier E. Hacker S. Kennedy C. Koch E. W. Stier A. C. Silliman B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr.81, 169–193. doi: 10.1890/10-1510.1\nhttp://www.esajournals.org/doi/abs/10.1890/10-1510.1
8
Bell S. S. Coen L. D. (1982). Investigations on epibenthic meiofauna I. Abundances on and repopulation of the tube-caps of Diopatra cuprea (Polychaeta: onuphidae) in a subtropical system. Mar. Biol.67, 303–309. doi: 10.1007/BF00397671
9
Benton T. G. Solan M. Travis J. M. J. Sait S. M. (2007). Microcosm experiments can inform global ecological problems. Trends Ecol. Evol.22, 516–521. doi: 10.1016/j.tree.2007.08.003
10
Berke S. K. (2022). A review of Diopatra ecology: current knowledge, open questions, and future threats for an ecosystem engineering polychaete. Biol. (Basel)11, 1485. doi: 10.3390/biology11101485
11
Berke S. K. Mahon A. R. Lima F. P. Halanych K. M. Wethey D. S. Woodin S. A. (2010). Range shifts and species diversity in marine ecosystem engineers: patterns and predictions for European sedimentary habitats. Global Ecol. Biogeography19, 223–232. doi: 10.1111/j.1466-8238.2009.00509.x
12
Beukema J. J. Dekker R. (2020). Half a century of monitoring macrobenthic animals on tidal flats in the Dutch Wadden Sea. Mar. Ecol. Prog. Ser.656, 1–18. doi: 10.3354/meps13555
13
Beukema J. J. De Vlas J. (1979). Population parameters of the lugworm, Arenicola marina, living on tidal flats in the Dutch Wadden Sea. Netherlands J. Sea Res.13, 331–353. doi: 10.1016/0077-7579(79)90010-3
14
Blackburn N. Orth R. (2013). Seed burial in eelgrass Zostera marina: the role of infauna. Mar. Ecol. Prog. Ser.474, 135–145. doi: 10.3354/meps10103
15
Blott S. J. Pye K. (2001). GRADISTAT: A grain size distribution and statistic package for the analysis of unconsolidated sediments. Earth Surf Process Landf26, 1237–1248. doi: 10.1002/esp.261
16
Bueno-Pardo J. García-Seoane E. Sousa A. I. Coelho J. P. Morgado M. Frankenbach S. et al . (2018). Trophic web structure and ecosystem attributes of a temperate coastal lagoon (Ria de Aveiro, Portugal). Ecol. Modell378, 13–25. doi: 10.1016/j.ecolmodel.2018.03.009
17
Bugnot A. B. Dafforn K. A. Erickson K. McGrath A. O’Connor W. A. Gribben P. E. (2023). Reintroducing a keystone bioturbator can facilitate microbial bioremediation in urban polluted sediments. Environ. pollut.324, 121419. doi: 10.1016/j.envpol.2023.121419
18
Bugnot A. B. Gribben P. E. O’Connor W. A. Erickson K. Coleman R. A. Dafforn K. A. (2022). Below-ground ecosystem engineers enhance biodiversity and function in a polluted ecosystem. J. Appl. Ecol.59, 2094–2105. doi: 10.1111/1365-2664.14221
19
Cabral S. Alves A. S. Castro N. Chainho P. Sá E. Cancela da Fonseca L. et al . (2019). Polychaete annelids as live bait in Portugal: Harvesting activity in brackish water systems. Ocean Coast. Manag181, 104890. doi: 10.1016/j.ocecoaman.2019.104890
20
Chennu A. Volkenborn N. de Beer D. Wethey D. S. Woodin S. A. Polerecky L. (2015). Effects of bioadvection by arenicola marina on microphytobenthos in permeable sediments. PloS One10, e0134236. doi: 10.1371/journal.pone.0134236
21
Costa V. Flindt M. R. Lopes M. Coelho J. P. Costa A. F. Lillebø A. I. et al . (2022). Enhancing the resilience of Zostera noltei seagrass meadows against Arenicola marina spp. bio-invasion: A decision-making approach. J. Environ. Manage302, 113969. doi: 10.1016/j.jenvman.2021.113969
22
Crespo D. Leston S. Rato L. D. Martinho F. Novais S. C. Pardal M. A. et al . (2021). Does an invasive bivalve outperform its native congener in a heat wave scenario? A laboratory study case with Ruditapes decussatus and R. philippinarum. Biol. (Basel)10, 1284. doi: 10.3390/biology10121284
23
Cunha T. Hall A. Queiroga H. (2005). Estimation of the Diopatra neapolitana annual harvest resulting from digging activity in Canal de Mira, Ria de Aveiro. Fish Res.76, 56–66. doi: 10.1016/j.fishres.2005.05.008
24
Daborn G. R. Redden A. M. (2017). “Estuaries,” in The wetland book. Eds. DabornG. R.ReddenA. M. (Springer, Dordrecht), 1–19. doi: 10.1007/978-94-007-6173-5
25
Dağli E. Ergen Z. Çinar M. E. (2005). One-year observation on the population structure of Diopatra neapolitana Delle Chiaje (Polychaeta: Onuphidae) in Izmir Bay (Aegean Sea, eastern Mediterranean). Mar. Ecol.26, 265–272. doi: 10.1111/j.1439-0485.2005.00055.x
26
De Cubber L. Lefebvre S. Lancelot T. Duong G. Gaudron S. M. (2020). Investigating down-shore migration effects on individual growth and reproduction of the ecosystem engineer Arenicola marina. J. Mar. Syst.211, 103420. doi: 10.1016/j.jmarsys.2020.103420
27
Delefosse M. Banta G. Canal-Vergés P. Penha-Lopes G. Quintana C. Valdemarsen T. et al . (2012). Macrobenthic community response to the Marenzelleria viridis (Polychaeta) invasion of a Danish estuary. Mar. Ecol. Prog. Ser.461, 83–94. doi: 10.3354/meps09821
28
Dolbeth M. Stålnacke P. Alves F. L. Sousa L. P. Gooch G. D. Khokhlov V. et al . (2016). An integrated Pan-European perspective on coastal Lagoons management through a mosaic-DPSIR approach. Sci. Rep.6, 19400. doi: 10.1038/srep19400
29
Escobar-Ortega D. Fernández N. Casal G. Muíño R. Couceiro L. (2024). Understanding the density patterns of the polychaete Diopatra neapolitana and its relationship with environmental factors in the NW Spain. Estuar. Coast. Shelf Sci.296, 108590. doi: 10.1016/j.ecss.2023.108590
30
Fauvel P. (1923). Faune de France, Polychètes errantes Vol. 5. Ed. le Chevalier.F.F. V. (Paris: Fédération Française des Sociétés de Sciences Naturelles).
31
Fuirst M. Ward C. S. Schwaner C. Diana Z. Schultz T. F. Rittschof D. (2021). Compositional and functional microbiome variation between tubes of an intertidal polychaete and surrounding marine sediment. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.656506
32
Gallardo B. Clavero M. Sánchez M. I. Vilà M. (2016). Global ecological impacts of invasive species in aquatic ecosystems. Glob Chang Biol.22, 151–163. doi: 10.1111/gcb.13004
33
Guan Q. Kang Y. He F. Li Z. Xue Z. Wu H. (2024). Diversity patterns and community assembly of aquatic macroinvertebrates in permanent and temporary inland saline-alkaline wetlands. Biodivers Conserv34, 571–588. doi: 10.1007/s10531-024-02987-7
34
Hale R. Mavrogordato M. N. Tolhurst T. J. Solan M. (2014). Characterizations of how species mediate ecosystem properties require more comprehensive functional effect descriptors. Sci. Rep.4, 6463. doi: 10.1038/srep06463
35
Høgslund S. Fossing H. Carstensen J. (2023). Microphytobenthic impact on benthic pelagic nutrient exchange in temperate shallow estuaries. Estuar. Coast. Shelf Sci.292, 108475. doi: 10.1016/j.ecss.2023.108475
36
Jansen S. Walpersdorf E. Werner U. Billerbeck M. Böttcher M. E. de Beer D. (2009). Functioning of intertidal flats inferred from temporal and spatial dynamics of O2, H2S and pH in their surface sediment. Ocean Dyn59, 317–332. doi: 10.1007/s10236-009-0179-4
37
Jumars P. A. Dorgan K. M. Lindsay S. M. (2015). Diet of worms emended: an update of polychaete feeding guilds. Ann. Rev. Mar. Sci.7, 497–520. doi: 10.1146/annurev-marine-010814-020007
38
Kauppi L. Bernard G. Bastrop R. Norkko A. Norkko J. (2018). Increasing densities of an invasive polychaete enhance bioturbation with variable effects on solute fluxes. Sci. Rep.8, 7619. doi: 10.1038/s41598-018-25989-2
39
Kennish M. J. (2021). Drivers of change in estuarine and coastal marine environments: an overview. Open J. Ecol.11, 224–239. doi: 10.4236/oje.2021.113017
40
Københavns Universitet (1992). Limnologisk metodik., universite (København: Ferskvandsbiologisk Laboratorium, Akademisk Forlag).
41
Kristensen E. Andersen F.Ø. (1987). Determination of organic carbon in marine sediments: a comparison of two CHN-analyzer methods. J. Exp. Mar. Biol. Ecol.109, 15–23. doi: 10.1016/0022-0981(87)90182-1
42
Kristensen E. Penha-Lopes G. Delefosse M. Valdemarsen T. Quintana C. O. Banta G. T. (2012). What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser.446, 285–302. doi: 10.3354/meps09506
43
Lacoste É. Piot A. Archambault P. McKindsey C. W. Nozais C. (2018). Bioturbation activity of three macrofaunal species and the presence of meiofauna affect the abundance and composition of benthic bacterial communities. Mar. Environ. Res.136, 62–70. doi: 10.1016/j.marenvres.2018.02.024
44
Lei Y. Stumm K. Volkenborn N. Wickham S. A. Berninger U.-G. (2010). Impact of Arenicola marina (Polychaeta) on the microbial assemblages and meiobenthos in a marine intertidal flat. Mar. Biol.157, 1271–1282. doi: 10.1007/s00227-010-1407-7
45
Lopes M. L. Rodrigues J. P. Crespo D. Dolbeth M. Calado R. Lillebø A. I. (2018). Functional traits of a native and an invasive clam of the genus Ruditapes occurring in sympatry in a coastal lagoon. Sci. Rep.8, 16901. doi: 10.1038/s41598-018-34556-8
46
Luckenbach M. W. (1986). Sediment stability around animal tubes: The roles of hydrodynamic processes and biotic activity. Limnol Oceanogr31, 779–787. doi: 10.4319/lo.1986.31.4.0779
47
Luís S. Pinho M. Lopes M. Sousa A. I. Lillebø A. I. (2025). Are native species of Ria de Aveiro under invasion? The relations between local activities and environmental perceptions on marine biological resources. Front. Mar. Sci.12. doi: 10.3389/fmars.2025.1603724
48
Maire O. Lecroart P. Meysman F. Rosenberg R. Duchêne J. Grémare A. (2010). Quantification of sediment reworking rates in bioturbation research: a review. Aquat Biol.2, 219–238. doi: 10.3354/ab00053
49
Mallin M. A. Cahoon L. B. (2020). The Hidden Impacts of Phosphorus Pollution to Streams and Rivers. Bioscience70, 315–329. doi: 10.1093/biosci/biaa001
50
Menegotto A. Dambros C. S. Netto S. A. (2019). The scale-dependent effect of environmental filters on species turnover and nestedness in an estuarine benthic community. Ecology100, e02721. doi: 10.1002/ecy.2721
51
Mermillod-Blondin F. (2011). The functional significance of bioturbation and biodeposition on biogeochemical processes at the water–sediment interface in freshwater and marine ecosystems. J. North Am. Benthol Soc.30, 770–778. doi: 10.1899/10-121.1
52
Michaud E. Aller R. C. Stora G. (2010). Sedimentary organic matter distributions, burrowing activity, and biogeochemical cycling: Natural patterns and experimental artifacts. Estuar. Coast. Shelf Sci.90, 21–34. doi: 10.1016/j.ecss.2010.08.005
53
Montserrat F. Suykerbuyk W. Al-Busaidi R. Bouma T. J. van der Wal D. Herman P. M. J. (2011). Effects of mud sedimentation on lugworm ecosystem engineering. J. Sea Res.65, 170–181. doi: 10.1016/j.seares.2010.09.003
54
Mortimer R. J. G. Davey J. T. Krom M. D. Watson P. G. Frickers P. E. Clifton R. J. (1999). The effect of macrofauna on porewater profiles and nutrient fluxes in the intertidal zone of the Humber Estuary. Estuar. Coast. Shelf Sci.48, 683–699. doi: 10.1006/ecss.1999.0479
55
Moyo R. Pillay D. Baeza J. A. (2017). Symbiont-mediated shifts in sandprawn behaviour: Implications for ecosystem functioning in marine soft-sediment ecosystems. J. Exp. Mar. Biol. Ecol.486, 296–304. doi: 10.1016/j.jembe.2016.10.022
56
Oksanen J. Blanchet F. G. Friendly M. Kindt R. Legendre P. McGlinn D. et al . (2020). vegan: community ecology package. Available online at: https://CRAN.R-project.org/package=vegan (Accessed April 19, 2025).
57
Philippart C. J. M. (1994). Interactions between Arenicola marina and Zostera noltii on a tidal flat in the Wadden Sea. Mar. Ecol. Prog. Ser.111, 251–257. doi: 10.3354/meps111251
58
Philippart C. J. M. Aken H. M. Beukema J. J. Bos O. G. Cadée G. C. Dekker R. (2016). Climate-related changes in recruitment of the bivalve Macoma balthica. Climat Limnol Oceanogr48, 2171–2185. doi: 10.4319/lo.2003.48.6.2171
59
Pinheiro J. Bates D. DebRoy S. Sarkar D. R Core Team (2014). “nlme: linear and nonlinear mixed effects models,” in R package version 3, R Core Team1–107. Available online at: http://cran.r-project.org/package=nlme (Accessed April 11, 2025).
60
Pires A. Freitas R. Quintino V. Rodrigues A. M. (2012). Can Diopatra neapolitana (Annelida: Onuphidae) regenerate body damage caused by bait digging or predation? Estuar. Coast. Shelf Sci.110, 36–42. doi: 10.1016/j.ecss.2011.12.039
61
Pires A. Martins R. Magalhes L. Soares A. M. V. M. Figueira E. Quintino V. et al . (2015). Expansion of lugworms towards southern European habitats and their identification using combined ecological, morphological and genetic approaches. Mar. Ecol. Prog. Ser.533, 177–190. doi: 10.3354/meps11315
62
Queirós A. M. Hiddink J. G. Johnson G. Cabral H. N. Kaiser M. J. (2011). Context dependence of marine ecosystem engineer invasion impacts on benthic ecosystem functioning. Biol. Invasions13, 1059–1075. doi: 10.1007/s10530-011-9948-3
63
Rabalais N. N. Turner R. E. Díaz R. J. Justić D. (2009). Global change and eutrophication of coastal waters. ICES J. Mar. Sci.66, 1528–1537. doi: 10.1093/icesjms/fsp047
64
R Development Core Team (2020). “R: A language and environment for statistical computing,” in R foundation for statistical computing. R Core Team. Available online at: http://www.r-project.org (Accessed February 28, 2025).
65
Reise K. (1985). “Macrofauna promotes meiofauna,” in Tidal flat ecology: an experimental approach to species interactions (Springer Berlin Heidelberg, Berlin, Heidelberg), 119–145. doi: 10.1007/978-3-642-70495-6_11
66
Remaili T. M. Simpson S. L. Jolley D. F. (2017). Effects of enhanced bioturbation intensities on the toxicity assessment of legacy-contaminated sediments. Environ. pollut.226, 335–345. doi: 10.1016/j.envpol.2016.11.038
67
Riisgård H. U. Banta G. T. (1998). Irrigation and deposit feeding by the lugworm Arenicola marina, characteristics and secondary effects on the environment. A review of current knowledge. Vie Milieu48, 243–257.
68
Rodrigues A. M. Pires A. Mendo S. Quintino V. (2009). Diopatra neapolitana and Diopatra marocensis from the Portuguese coast: Morphological and genetic comparison. Estuar. Coast. Shelf Sci.85, 609–617. doi: 10.1016/j.ecss.2009.10.004
69
Rodrigues A. M. Quintino V. Sampaio L. Freitas R. Neves R. (2011). Benthic biodiversity patterns in Ria de Aveiro, Western Portugal: Environmental-biological relationships. Estuar. Coast. Shelf Sci.95, 338–348. doi: 10.1016/j.ecss.2011.05.019
70
Rodrigues-Filho J. L. Macêdo R. L. Sarmento H. Pimenta V. R. A. Alonso C. Teixeira C. R. et al . (2023). From ecological functions to ecosystem services: linking coastal lagoons biodiversity with human well-being. Hydrobiologia850, 2611–2653. doi: 10.1007/s10750-023-05171-0
71
Roy H. E. Pauchard A. Stoett P. J. Renard Truong T. Meyerson L. A. Bacher S. et al . (2024). Curbing the major and growing threats from invasive alien species is urgent and achievable. Nat. Ecol. Evol.8, 1216–1223. doi: 10.1038/s41559-024-02412-w
72
Santos T. M. T. Aviz D. (2019). Macrobenthic fauna associated with Diopatra cuprea (Onuphidae: Polychaeta) tubes on a macrotidal sandy beach of the Brazilian Amazon Coast. J. Mar. Biol. Assoc. United Kingdom99, 751–759. doi: 10.1017/S0025315418000711
73
Solan M. Wigham B. Hudson I. Kennedy R. Coulon C. Norling K. et al . (2004). In situ quantification of bioturbation using time-lapse fluorescent sediment profile imaging (f-SPI), luminophore tracers and model simulation. Mar. Ecol. Prog. Ser.271, 1–12. doi: 10.3354/meps271001
74
Sousa L. P. Sousa A. I. Alves F. L. Lillebø A. I. (2016). Ecosystem services provided by a complex coastal region: Challenges of classification and mapping. Sci. Rep.6, 22782. doi: 10.1038/srep22782
75
Strayer D. L. (2020). Non-native species have multiple abundance–impact curves. Ecol. Evol.10 (13), 6833–6843. doi: 10.1002/ece3.6364
76
Strayer D. L. Hillebrand H. (2012). Eight questions about invasions and ecosystem functioning. Ecol. Lett.15, 1199–1210. doi: 10.1111/j.1461-0248.2012.01817.x
77
Strickland J. D. H. Parsons T. R. (1972). A practical handbook of seawater analysis (Ottawa: Fisheries Research Board of Canada). doi: 10.1002/iroh.19700550118
78
Sutherland R. A. (1998). Loss-on-ignition estimates of organic matter and relationships to organic carbon in fluvial bed sediments. Hydrobiologia389, 153–167. doi: 10.1023/A:1003570219018
79
Teal L. Parker E. Solan M. (2010). Sediment mixed layer as a proxy for benthic ecosystem process and function. Mar. Ecol. Prog. Ser.414, 27–40. doi: 10.3354/meps08736
80
Thomsen M. S. Byers J. E. Schiel D. R. Bruno J. F. Olden J. D. Wernberg T. et al . (2014). Impacts of marine invaders on biodiversity depend on trophic position and functional similarity. Mar. Ecol. Prog. Ser.495, 39–47. doi: 10.3354/meps10566
81
Thomsen M. S. Muth M. F. McGlathery K. J. (2011). Tube-forming polychaetes enhance invertebrate diversity and abundance in sandy sediments of Mozambique, Africa. Afr J. Mar. Sci.33, 327–332. doi: 10.2989/1814232X.2011.600433
82
Vargas C. I. C. Vaz N. Dias J. M. (2017). An evaluation of climate change effects in estuarine salinity patterns: Application to Ria de Aveiro shallow water system. Estuar. Coast. Shelf Sci.189, 33–45. doi: 10.1016/j.ecss.2017.03.001
83
Vaz N. Dias J. M. Leitão P. Martins I. (2005). Horizontal patterns of water temperature and salinity in an estuarine tidal channel: Ria de Aveiro. Ocean Dyn55, 416–429. doi: 10.1007/s10236-005-0015-4
84
Volkenborn N. Hedtkamp S. I. C. van Beusekom J. E. E. Reise K. (2007). Effects of bioturbation and bioirrigation by lugworms (Arenicola marina) on physical and chemical sediment properties and implications for intertidal habitat succession. Estuar. Coast. Shelf Sci.74, 331–343. doi: 10.1016/j.ecss.2007.05.001
85
Volkenborn N. Reise K. (2006). Lugworm exclusion experiment: Responses by deposit feeding worms to biogenic habitat transformations. J. Exp. Mar. Biol. Ecol.330, 169–179. doi: 10.1016/j.jembe.2005.12.025
86
Volkenborn N. Reise K. (2007). Effects of Arenicola marina on polychaete functional diversity revealed by large-scale experimental lugworm exclusion. J. Sea Res.57, 78–88. doi: 10.1016/j.seares.2006.08.002
87
Volkenborn N. Robertson D. M. Reise K. (2009). Sediment destabilizing and stabilizing bio-engineers on tidal flats: cascading effects of experimental exclusion. Helgol Mar. Res.63, 27–35. doi: 10.1007/s10152-008-0140-9
88
Voss M. Bange H. W. Dippner J. W. Middelburg J. J. Montoya J. P. Ward B. (2013). The marine nitrogen cycle: recent discoveries, uncertainties and the potential relevance of climate change. Philos. Trans. R. Soc. B: Biol. Sci.368, 20130121. doi: 10.1098/rstb.2013.0121
89
Watson G. J. Murray J. M. Schaefer M. Bonner A. (2017). Bait worms: a valuable and important fishery with implications for fisheries and conservation management. Fish Fisheries18, 374–388. doi: 10.1111/faf.12178
90
Wendelboe K. Egelund J. T. Flindt M. R. Valdemarsen T. (2013). Impact of lugworms (Arenicola marina) on mobilization and transport of fine particles and organic matter in marine sediments. J. Sea Res.76, 31–38. doi: 10.1016/j.seares.2012.10.013
91
Wethey D. S. Woodin S. A. (2022). Climate change and Arenicola marina: Heat waves and the southern limit of an ecosystem engineer. Estuar. Coast. Shelf Sci.276, 108015. doi: 10.1016/j.ecss.2022.108015
92
Whitton T. A. Jenkins S. R. Richardson C. A. Hiddink J. G. (2016). The effect of macrofaunal disturbance on Cerastoderma edule post-larvae. J. Sea Res.112, 23–31. doi: 10.1016/j.seares.2016.03.002
93
Wickham H. (2016). ggplot2: elegant graphics for data analysis (New York: Springer-Verlag). doi: 10.18637/jss.v035.b01
94
Zebe E. Schiedek D. (1996). The lugworm Arenicola marina: A model of physiological adaptation to life in intertidal sediments. Helgoländer Meeresuntersuchungen50, 37–68. doi: 10.1007/BF02367136
95
Zuur A. Ieno E. Walker N. Saveliev A. Smith G. (2009). Mixed effects models and extensions in ecology with R., Springer (New York: Springer). Available online at: http://books.google.com/books?hl=en&lr=&id=vQUNprFZKHsC&oi=fnd&pg=PA1&dq=Mixed+Effects+Models+and+Extensions+in+Ecology+with+R&ots=kbqJtW_FYu&sig=tDGrGN2py5_VkfntCqZKlrpC-Pw.
Summary
Keywords
species replacement, biological invasions, ecological processes, microcosm, bioturbation, Ria de Aveiro
Citation
Lopes ML, Crespo D, Costa V, Rainha P, Sousa AI and Lillebø AI (2025) Effects of the native-invasive-alien substitution of ecosystem engineers on sediment reworking and nutrient cycling. Front. Mar. Sci. 12:1641983. doi: 10.3389/fmars.2025.1641983
Received
05 June 2025
Accepted
29 July 2025
Published
01 September 2025
Volume
12 - 2025
Edited by
Daniele Brigolin, Università Iuav di Venezia, Italy
Reviewed by
Olmo Miguez-Salas, University of Barcelona, Spain
Guillaume Bernard, Laboratoire Environnement Ressources d’Arcachon (IFREMER), France
Updates
Copyright
© 2025 Lopes, Crespo, Costa, Rainha, Sousa and Lillebø.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Crespo, daniel.crespo@ua.pt; Ana I. Lillebø, lillebo@ua.pt
‡These authors have contributed equally to this work
†Present address: Valentina Costa, Department of Integrative Marine Ecology, Stazione Zoologica Anton Dohrn, CRIMAC – Calabria Marine Centre, Amendolara, Italy
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.