Abstract
We investigated the estrogenic effects of 2-ethylhexyl 4-methoxycinnamate (EHMC) and benzophenone-3 (BP-3) on steroidogenesis in maturing oocytes of the longchin goby. Oocytes with 0.77-, 0.82-, and 0.87-mm diameters were incubated with EHMC (0.5–500 ng/mL) or BP-3 (0.5–500 ng/mL) for 24 h with [³H]17α-hydroxyprogesterone as a precursor. The major steroid metabolites were separated and identified as androstenedione, testosterone (T), estradiol-17β (E2), estrone, and 17,20β-dihydroxy-4-pregnen-3-one (17α20βP). The E2 metabolite was significantly increased at 5 and 0.5 ng/mL of EHMC in 0.82- and 0.87-mm-diameter oocytes, respectively. Furthermore, it was increased significantly at 5 ng/mL of BP-3 in the oocytes of 0.87 mm. In addition, 5, 50, and 500 ng/mL of EHMC increased the ratio of E2/17α20βP in the oocytes of 0.82 mm. 0.5 ng/mL of BP-3 increased the ratio of E2/T in the oocytes of 0.87 mm. These results suggest that EHMC and BP-3 have a potential estrogenic activity in the steroidogenic shift process, vitellogenic stage to final maturation stage.
1 Introduction
UV filters are a group of compounds invented to protect the skin from UV radiation and are used in sunscreens, a wide range of cosmetics and personal care products (Lebarone, 2022; Zhang et al., 2024). Additionally, it is used in plastics, cartons, and transparent packing materials to minimize UV-induced damage to products (Manová et al., 2013; Ramos et al., 2015). UV filters can be released into the aquatic environment via two pathways: either indirectly via wastewater treatment plant effluent or directly from swimming and other recreational activities, as well as industrial wastewater discharge (Pal et al., 2014; Ramos et al., 2015). The most common UV filter ingredients are 2-ethylhexyl-4-methoxycinnamate (EHMC) and 2-hydroxy-4-methoxybenzophenone (BP-3). According to previous research indicating their potential for bioaccumulation, EHMC and BP-3 have been observed in aquatic biota. BP-3 concentrations of up to 6.52 ng/g have been found in the muscles of lionfish (Pterois volitans) from Grenada, West Indies (Horricks et al., 2019). EHMC and BP-3 levels as high as 241.7 ng/g body weight (BW) and 24.3 ng/g BW, respectively, were detected in Andalusian barbel fish (Luciobarbus sclateri) collected from the Guadalquivir River, southern Spain (Gago-Ferrero et al., 2013).
In teleosts, oocyte maturation is regulated by sex steroid hormones synthesized from follicle cells, which are controlled by the hypothalamus–pituitary–gonad axis (Nagahama and Yamashita, 2008). In female fish, estradiol-17β (E2), as a major sex steroid, is transported to the liver and produces vitellogenin (VTG) during vitellogenesis. Following vitellogenesis, progestogens, including 17α,20β-dihydroxy-4-pregnen-3-one (17α20βP) and/or 17α,20β,21-trihydroxy-4-pregnen-3-one (17α20β21P), induce final oocyte maturation and result in ovulation (Patiño et al., 2001). Multiple in vitro and in vivo studies in teleosts have reported adverse effects of EHMC and BP-3 on VTG levels. EHMC increased plasma VTG levels in male fathead minnow, whereas VTG gene expression profiles showed antiestrogenic activity in fathead minnow and zebrafish (Christen et al., 2011; Zhou et al., 2019a). BP-3 induces VTG expression in zebrafish, rainbow trout, male medaka, and male California halibut (Coronado et al., 2008; Schlenk et al., 2005; Rodríguez-Fuentes et al., 2015). Although the effects of EHMC and BP-3 on the reproductive endocrine system have been investigated, their effects on steroidogenesis, including progestin production, during oocyte maturation have not been reported.
The longchin goby, Chasmichthys dolichognathus, is a small marine fish of the family Gobiidae that inhabits the coastal waters and tide pools of Korea and Japan (Kim et al., 1986). Gobiid fish are appropriate subjects for investigating the effects of various chemicals due to their small size, ease of handling through in vitro and in vivo study, and strong tolerance with environmental changes (Robinson et al., 2007). This species has been used in our previous studies to test reproductive endocrinology with different endocrine-disrupting chemicals (Baek et al., 2007; Hwang and Baek, 2011). Herein, we investigated the potential estrogenic effects of EHMC and BP-3 on in vitro steroid metabolism in maturing oocytes of the longchin goby. In particular, we focused on the effects of these chemicals during the critical period of the steroidogenic shift from estrogens to progestogens.
2 Materials and methods
2.1 Chemicals
EHMC (CAS number: 5466-77-3) and BP-3 (CAS number: 131-57-7) were purchased from Sigma–Aldrich. (St. Louis, MO, USA). They were dissolved in ethanol to obtain a concentrated stock solution. Steroid hormones, 17α-hydroxyprogesterone (17αP), androstenedione (A4), testosterone (T), E2, estrone (E1), 17,20α-dihydroxy-4-pregnen-3-one (17α20αP), 17α20βP, and 17α20β21P were purchased from Sigma-Aldrich or Steraloids (Wilton, NH, USA). The radioactive steroid, [³H]17αP, was obtained from Amersham Biosciences (London, England).
2.2 Experimental fish and histological analysis of ovarian follicles
We selected the oocytes of 0.75-0.90 mm in average diameter according to our previous studies (Baek et al., 2007; Kim et al., 2023); the developmental stage of these oocytes is from fully vitellogenic stage to final maturation stage. Female longchin gobies were collected from tide pools in Cheongsa-po, Busan, Korea, between February and March 2020. For histological analysis, fish were anesthetized, and ovarian fragments from each oocyte diameter, 0.77, 0.82, and 0.87 mm, were fixed in 10% neutral formalin for 24 h and subsequently embedded in paraffin. The paraffin-embedded tissues were prepared in 5–6-μm-thick sections. Sections were stained with Mayer’s hematoxylin and eosin and observed under a light microscope (BX50; Olympus, Tokyo, Japan).
2.3 In vitro oocyte incubation
Ovaries were isolated from seven fish. Individual oocytes were manually dissected from ovaries. The oocytes were immediately transferred into an ice-cold balanced salt solution (132.96 mM NaCl, 3.09 mM KCl, 0.28 mM MgSO4 7H2O, 0.98 mM MgCl2 6H2O, 3.40 mM CaCl2 6H2O, and 3.65 mM HEPES) under sterile conditions. Subsequently, the oocytes were separated in accordance with the average oocyte diameter of 0.77, 0.82, and 0.87 mm and were selected for the in vitro incubation. There were 20 oocytes of each diameter that were incubated in 24-well plates (conducted in triplicates) containing 1 mL Leibovitz L15 medium (Gibco, Grand Island, NY, USA).
To evaluate the estrogenic potency of EHMC and BP-3 on the steroidogenic metabolism of oocytes, we quantified steroid metabolites after EHMC and BP-3 exposure in the presence of a precursor. We incubated two oocyte groups of 0.77 and 0.87 mm with 0.5, 5, 50, and 500 ng/mL EHMC and BP-3 in the presence of [3H]-17αP (55 kBq) as a precursor in two separate experiments. Exposure concentrations were considered and selected with relevant concentrations from coastal waters, from sediments, and even in ecotoxicological research according to previous studies (Watkins and Sallach, 2021; Bordalo et al., 2025). Additionally, we incubated the oocytes of 0.82 mm with 5, 50, and 500 ng/mL EHMC in the presence of the precursor. Ethanol as a solvent for EHMC, BP-3, and [3H]-17αP was evaporated by nitrogen gas and re-dissolved in incubation medium.
Culture plates were incubated for 24 h at 18°C with gentle shaking. The media’s pH and osmolarity were adjusted to plasma values of 7.7 and 300 mOsm, respectively. At the end of the incubation, the medium and oocytes were collected and stored at −76°C until analyses.
2.4 Analysis of the steroid metabolites
Steroid metabolites were analyzed as previously described (Hwang and Baek, 2023). Briefly, the metabolites were extracted, concentrated, and subjected to thin-layer chromatography (TLC). Each separated metabolite was eluted and identified using reverse-phase high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) (QP5050A, Shimadzu, Japan). The details are demonstrated in Supplementary Materials. The metabolic rate (%) of each isolated metabolite was calculated based on the percentage of the total steroid-recovered radioactivity from the initial precursor radioactivity.
2.5 Data and statistical analyses
Data were checked using Kolmogorov–Smirnov and Levene’s tests to verify whether the variance fulfilled the conditions of normality and homogeneity, respectively. Differences between groups were analyzed using the two-sample t-test or one-way analysis of variance (ANOVA), followed by Scheffe’s test. The Kruskal–Wallis test was used if data were not normally distributed, followed by the Mann–Whitney U test with Bonferroni correction. Results are indicated as the means ± standard error of the mean, and a value of p < 0.05 was considered statistically significant using SPSS ver. 21.0 (IBM, Armonk, NY, USA). The EHMC and BP-3 effects on each steroid metabolite were analyzed and visualized by heatmap analysis using the pheatmap package ver. 1.10.12 in R project ver. 4.0.2.
3 Results
3.1 Histological observations of the oocytes
Numerous yolk granules (Yg) were spread over the ooplasm, and the nucleus (N) was located in the vicinity of the center of oocytes in oocytes of 0.77 mm in average diameter (Figure 1A). Yg continued to accumulate in the ooplasm, and N began to migrate in oocytes of 0.82 mm in average diameter (Figure 1B). In oocytes of 0.87 mm in average diameter, N migration was clearly observed compared with 0.77 and 0.82 mm oocytes (Figure 1C).
Figure 1
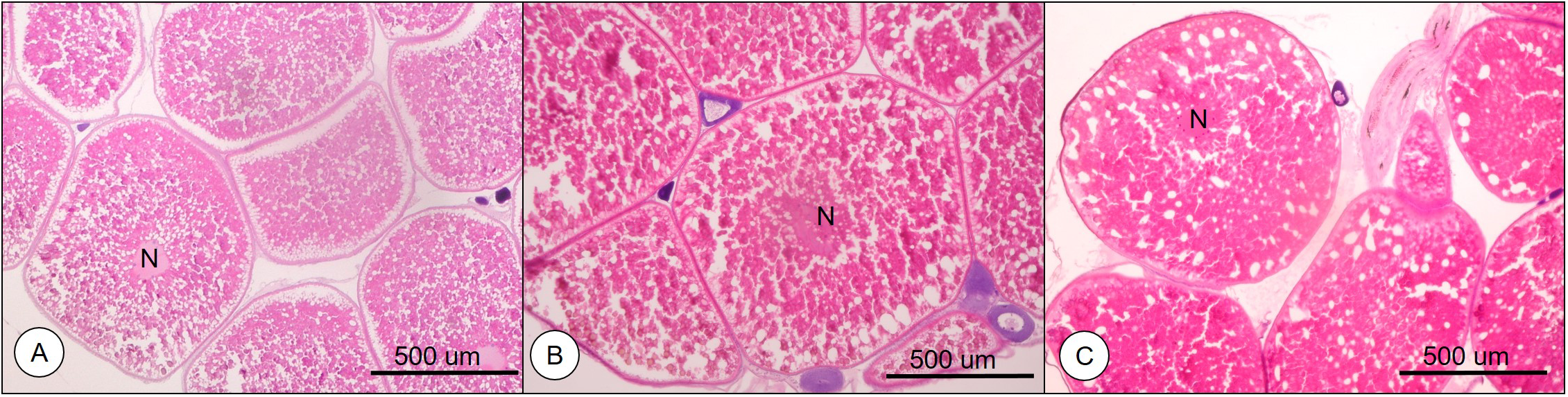
Histological observation of the oocytes from longchin goby. (A) oocytes of 0.77 mm in diameter; (B) oocytes of 0.82 mm in diameter; (C) oocytes of 0.87 mm in diameter. N, nucleus.
3.2 Identification of the major steroid metabolites
Steroid metabolites converted from [³H]17αP using the maturing oocytes (0.77, 0.82, and 0.87 mm average diameter) were observed via TLC (Figure 2). Estrogen metabolites (E1 and E2) (colorless spots developed by iodine vapor) were separated via HPLC and identified using GC/MS (Figure 3).
Figure 2
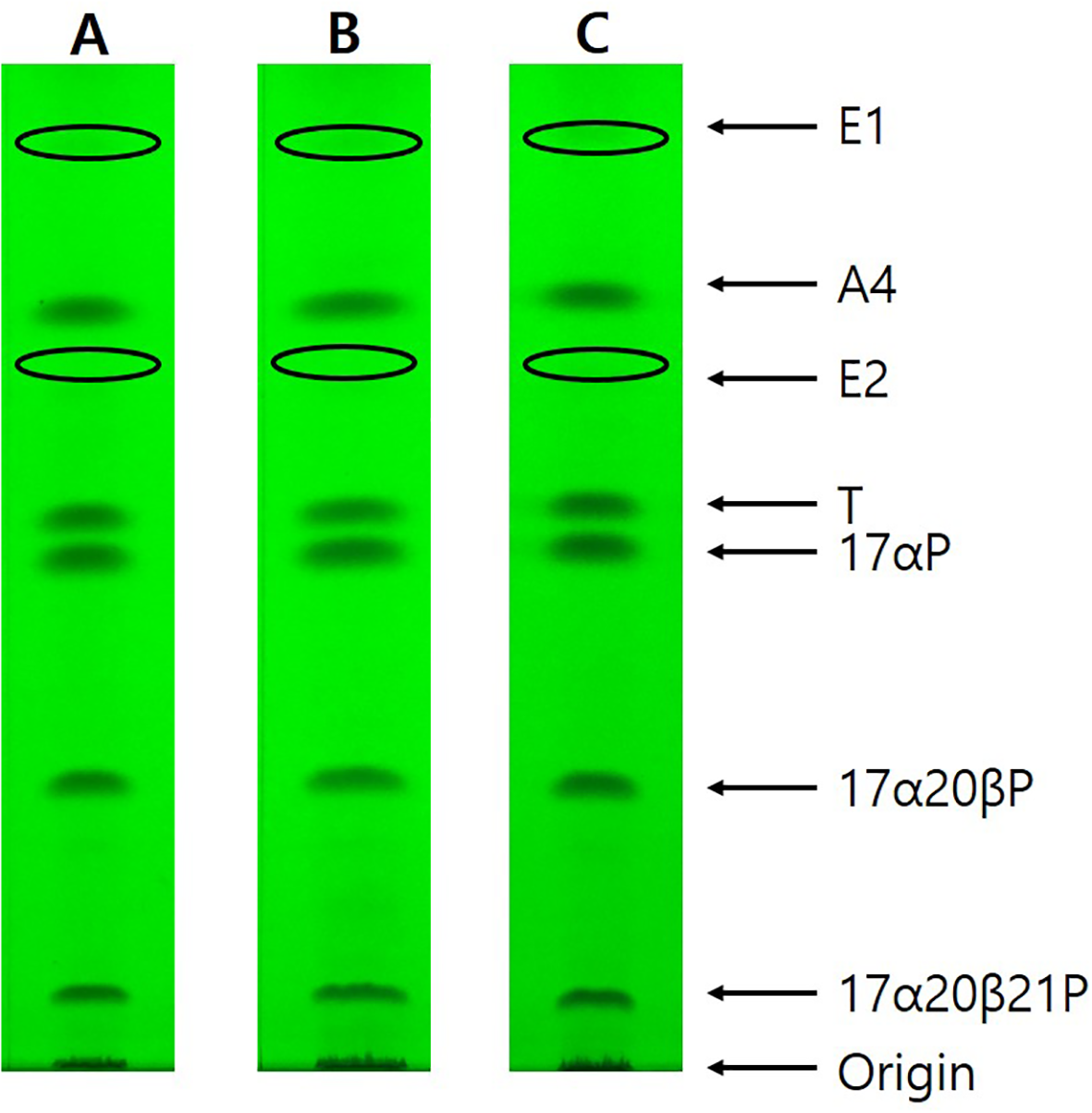
Thin layer chromatography under UV 254 nm of steroid metabolites incubated with [³H]17αP from longchin goby oocytes at two different diameters. Seven metabolites were separated by TLC developed with a benzene: acetone = 4: 1 (v:v) and benzene: ethyl acetate = 4: 1 (v:v) solvent system. E1 and E2 bands were observed after exposure to iodine vapors. (A) oocytes of 0.77-mm average diameter; (B) oocytes of 0.82 mm average diameter; (C) oocytes of 0.87 mm average diameter.
Figure 3
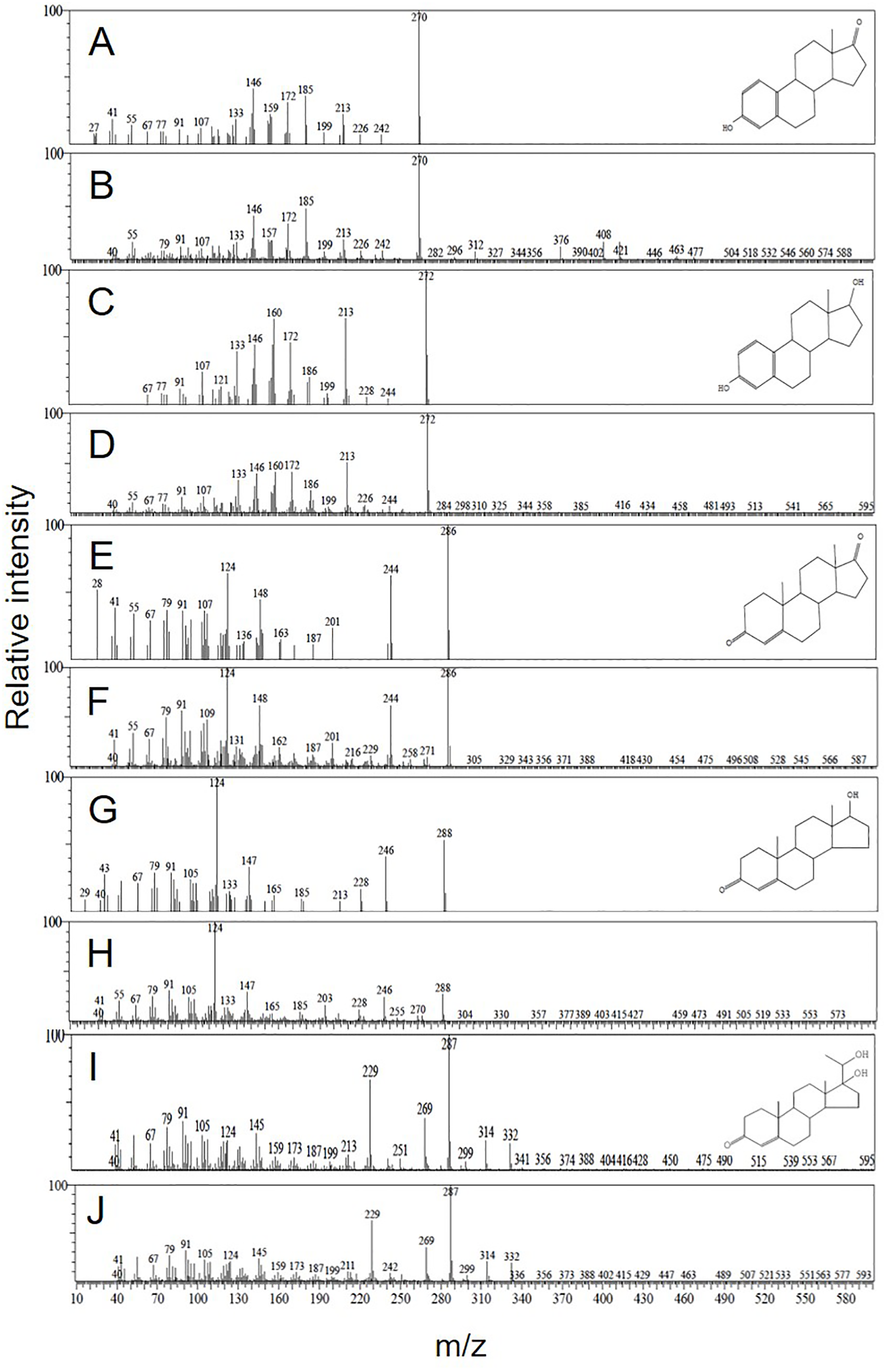
Mass spectra of steroid metabolites identified in longchin goby oocytes. (A) authentic E1; (B) metabolized E1; (C) authentic E2; (D) metabolized E2; (E) authentic A4; (F) metabolized A4; (G) authentic T; (H) metabolized T; (I) authentic 17α20βP; (J) metabolized 17α20βP.
3.3 Effects of EHMC on steroid metabolites during the oocyte maturation process
In the oocytes of 0.77-mm diameter, EHMC did not substantially modulate any metabolites (Table 1). In the oocytes of 0.82- and 0.87-mm diameters, 5 and 0.5 ng/mL of EHMC increased the E2 metabolites compared with the controls, respectively (P < 0.05). The levels of the other metabolites did not substantially increase or decrease. The heatmap analysis of each metabolite also supported these findings (Figure 4). Additionally, the alterations in the 17α20βP metabolite stood out from the other metabolites in the oocytes of 0.87 mm from the heatmap analysis, although the differences were insignificant.
Table 1
| Oocyte diameter (mm) | EHMC (ng/mL) | Metabolic rate (%) | ||||
|---|---|---|---|---|---|---|
| A4 | T | E1 | E2 | 17α20βP | ||
| 0.77 | 0 | 1.07 ± 0.17 | 1.57 ± 0.08 | 10.06 ± 2.78 | 14.99 ± 2.20 | 2.48 ± 0.44 |
| 0.5 | 1.16 ± 0.07 | 1.77 ± 0.01 | 8.04 ± 1.75 | 18.17 ± 3.46 | 2.83 ± 0.32 | |
| 5 | 1.04 ± 0.13 | 1.56 ± 0.78 | 9.37± 1.96 | 16.73 ± 3.73 | 2.65 ± 0.60 | |
| 50 | 1.19 ± 0.28 | 1.65 ± 0.51 | 8.81± 2.58 | 17.43 ± 3.33 | 2.87 ± 0.74 | |
| 500 | 1.21 ± 0.08 | 1.68 ± 0.04 | 8.63± 1.24 | 17.75 ± 6.63 | 2.77 ± 0.78 | |
| 0.82 | 0 | 1.34 ± 0.39 | 2.57 ± 0.19 | 4.81 ± 0.57 | 10.55 ± 0.66 | 3.23 ± 0.34 |
| 5 | 1.96 ± 0.09 | 2.89 ± 0.33 | 7.44 ± 0.41 | 13.99 ± 0.79* | 3.11 ± 0.23 | |
| 50 | 2.07 ± 0.71 | 3.16 ± 0.21 | 6.93 ± 0.45 | 11.26 ± 0.53 | 2.75 ± 0.22 | |
| 500 | 1.84 ± 0.62 | 2.75 ± 0.23 | 6.99 ± 0.50 | 12.52 ± 0.37 | 2.93 ± 0.20 | |
| 0.87 | 0 | 1.72 ± 0.01 | 1.85 ± 0.13 | 7.21± 0.78 | 10.93 ± 0.91 | 3.85 ± 0.51 |
| 0.5 | 1.22 ± 0.06 | 1.94 ± 0.28 | 9.43 ± 0.89 | 14.77 ± 0.54* | 3.13 ± 0.70 | |
| 5 | 1.57 ± 0.14 | 2.16 ± 0.22 | 7.94 ± 2.16 | 12.97 ± 0.41 | 3.18 ± 0.35 | |
| 50 | 1.12 ± 0.01 | 1.95 ± 0.14 | 7.60 ± 1.03 | 10.34 ± 0.15 | 2.84 ± 0.06 | |
| 500 | 1.34 ± 0.99 | 1.78 ± 0.03 | 8.54 ± 1.54 | 11.40 ± 0.25 | 2.94 ± 0.44 | |
Effects of EHMC on steroid metabolism rate from [³H]17αP of longchin goby oocytes (average diameter= 0.77, 0.82, and 0.87 mm) after 24 h of incubation.
The percentage of radioactivity associated with each isolated steroid was calculated to the percentage of total steroid recovered from initial TLC. Values are mean ± SEM of the rate of each steroid in two or three replicate wells with 20 oocytes/well. Asterisks indicate significant difference compared with controls (p < 0.05).
The bold values were for emphasizing the statistically significant difference with asterisk.
Figure 4
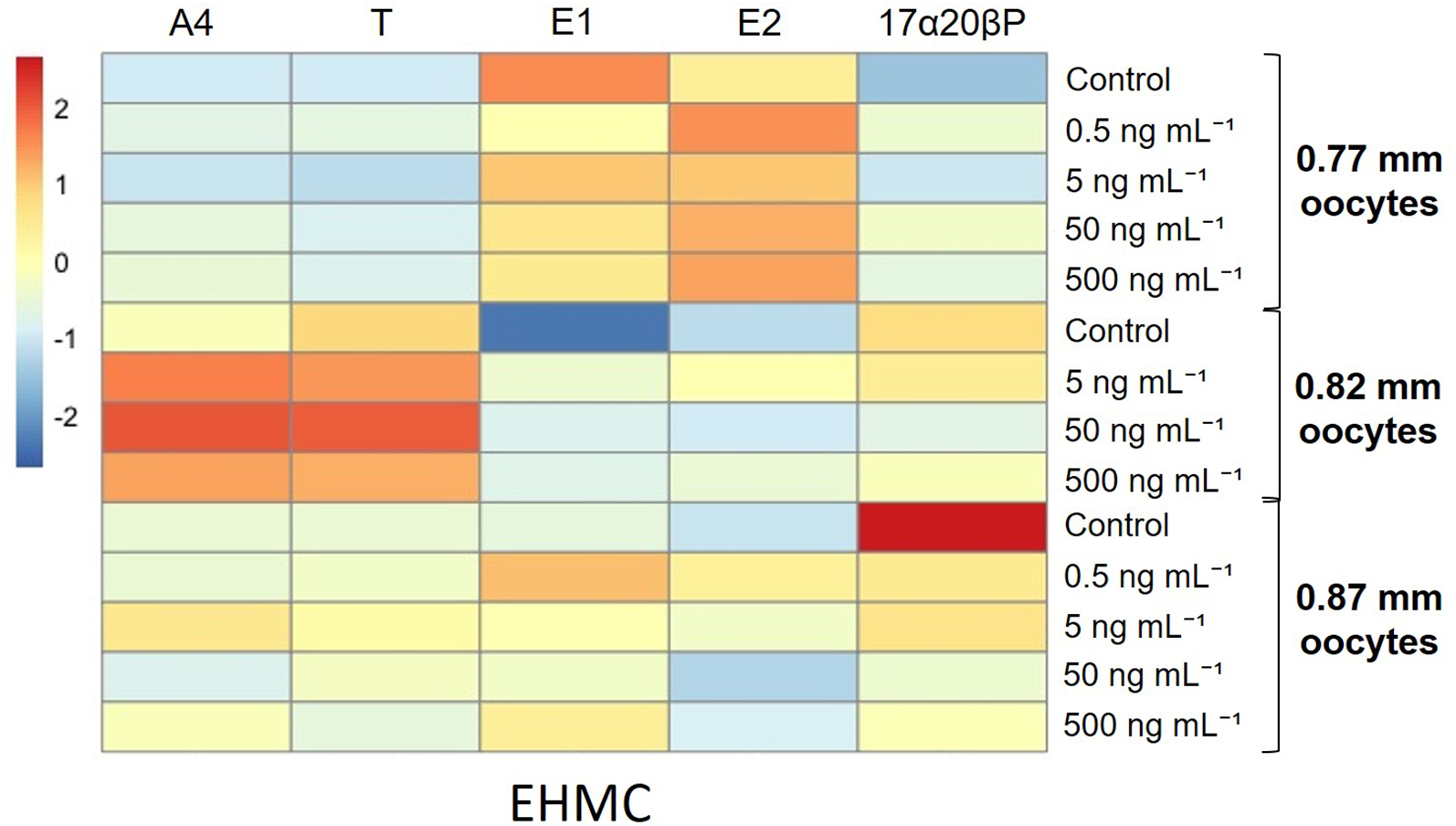
Heatmap analysis representing different levels of steroid metabolic rates of longchin goby oocytes exposed to EHMC. The lower relative rates are represented by blue color, and the higher relative rates are represented by red color. EHMC dosages were represented in the columns. Each steroid metabolite was represented in the row and scaled in the row direction (Z-scores).
In the E2/T and E2/17α20βP ratios, as sensitive biomarkers of estrogenicity (Bevans et al., 1996; Folmar et al., 1996), 5, 50, and 500 ng/mL EHMC significantly increased the E2/17α20βP ratio compared with the controls in the oocytes of 0.82 mm diameter (P < 0.05, Figure 5). In the oocytes of 0.87 mm diameter, no significant effects were observed on either ratio, although these ratios peaked at the lowest dose.
Figure 5
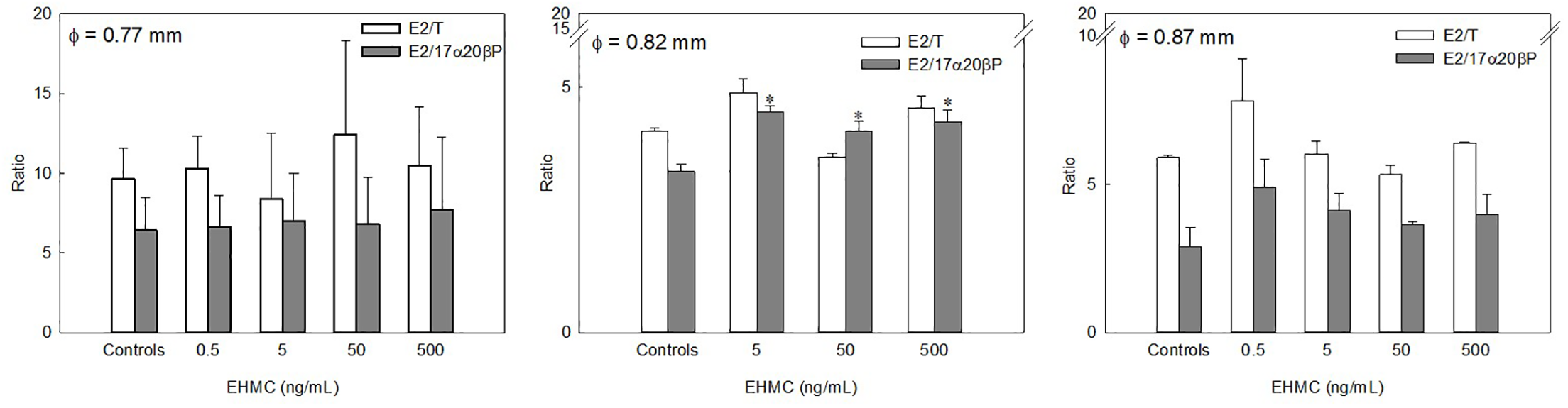
Effects of EHMC on the ratio of E2/T and E2/17α20βP calculated from each steroid metabolite from [³H]17αP of longchin goby oocytes (average diameter= 0.77, 0.82, and 0.87 mm) after 24 h of incubation. Values are mean ± SEM in two or three replicate wells with 20 oocytes/well. Asterisks indicate the significant differences between each treatment and controls (P < 0.05).
3.4 Effects of BP-3 on steroid metabolites during the oocyte maturation process
In the oocytes of 0.77 mm diameter, BP-3 did not markedly modulate any metabolites (Table 2). In the oocytes of 0.87 mm diameter, 5 ng/mL BP-3 increased the E2 metabolites compared with the controls (P < 0.05). The heatmap analysis supported these findings for each metabolite (Figure 6). The alterations in the 17α20βP metabolite were obvious in the oocytes of 0.87-mm diameter as observed in the heatmap analysis, although the difference was insignificant.
Figure 6
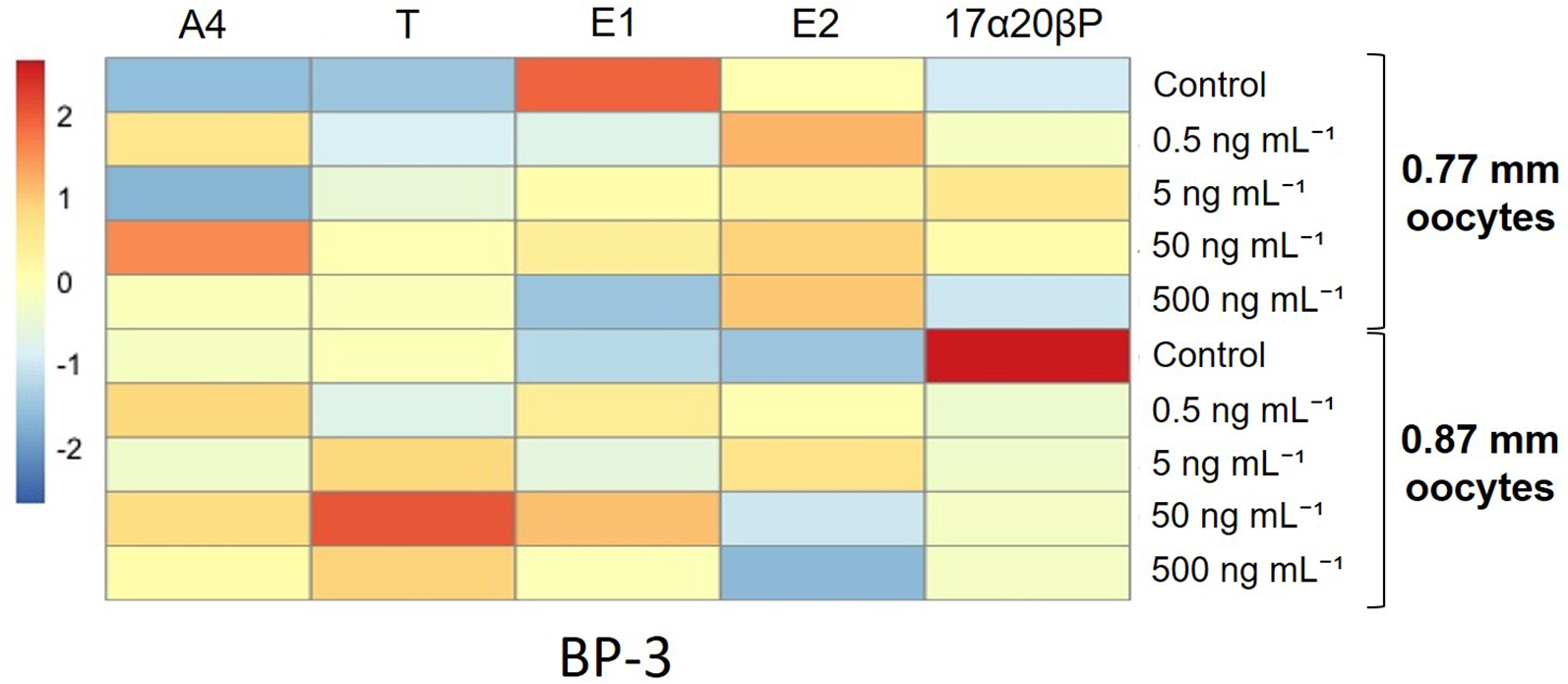
Heatmap analysis representing different levels of steroid metabolic rates of longchin goby oocytes exposed to BP-3. The lower relative rates are represented by blue color, and the higher relative rates are represented by red color. BP-3 dosages were represented in the columns. Each steroid metabolite was represented in the row and scaled in row direction (Z-scores).
Table 2
| Oocyte diameter (mm) | BP-3 (ng/mL) | Metabolic rate (%) | ||||
|---|---|---|---|---|---|---|
| A4 | T | E1 | E2 | 17α20βP | ||
| 0.77 | 0 | 1.07 ± 0.17 | 1.57 ± 0.08 | 10.06 ± 2.78 | 14.99 ± 2.20 | 2.48 ± 0.44 |
| 0.5 | 1.33 ± 0.02 | 1.84 ± 0.02 | 7.66 ± 2.40 | 18.55 ± 2.89 | 2.76 ± 0.15 | |
| 5 | 1.06 ± 0.20 | 1.86 ± 0.05 | 8.38 ± 2.73 | 15.63 ± 2.15 | 3.04 ± 0.45 | |
| 50 | 1.45 ± 0.21 | 1.76 ± 0.18 | 8.68 ± 161 | 17.87 ± 2.99 | 2.88 ± 0.22 | |
| 500 | 1.25 ± 0.32 | 1.69 ± 0.04 | 7.01 ± 1.62 | 18.15 ± 4.80 | 2.45 ± 0.05 | |
| 0.87 | 0 | 1.72 ± 0.01 | 1.85 ± 0.13 | 7.21± 0.78 | 10.93 ± 0.91 | 3.85 ± 0.51 |
| 0.5 | 1.37 ± 0.10 | 1.72 ± 0.01 | 8.74 ± 1.11 | 15.25 ± 0.57 | 2.67 ± 1.08 | |
| 5 | 1.22 ± 0.03 | 2.05 ± 1.03 | 7.83 ± 0.86 | 17.08 ± 1.07* | 2.69 ± 0.58 | |
| 50 | 1.35 ± 0.02 | 2.29 ± 0.24 | 9.31 ± 0.75 | 12.33 ± 1.30 | 2.73 ± 0.38 | |
| 500 | 1.28 ± 0.02 | 2.06 ± 0.43 | 8.26 ± 2.03 | 10.51 ± 1.50 | 2.74 ± 0.41 | |
Effects of BP-3 on in vitro steroid metabolism rate from [³H]17αP of longchin goby oocytes (average diameter= 0.77 and 0.87 mm) after 24 h of incubation.
The percentage of radioactivity associated with each isolated steroid was calculated to the percentage of total steroid recovered from initial TLC. Values are mean ± SEM of the rate of each steroid in two or three replicate wells with 20 oocytes/well. Asterisk indicates significant difference compared with controls (p < 0.05).
The bold values were for emphasizing the statistically significant difference with asterisk.
In the oocytes of 0.77-mm diameter, no obvious effect of BP-3 on the E2/T or E2/17α20βP ratios was observed (Figure 7). However, 0.5 ng/mL BP-3 significantly increased the E2/T ratio compared with the controls in the oocytes of 0.87-mm diameter (P < 0.05). Additionally, the lowest BP-3 dose increased the E2/17α20βP ratio, although that peak was not considerably different.
Figure 7
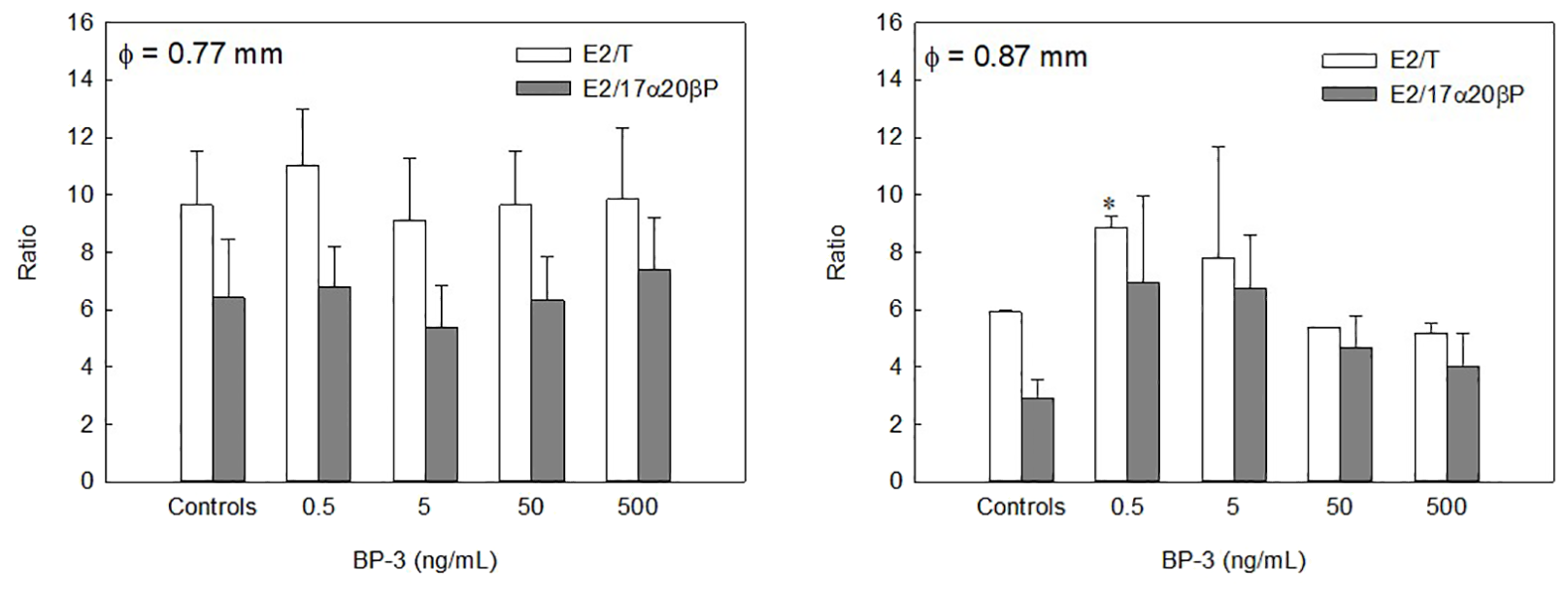
Effects of BP-3 on the ratio of E2/T and E2/17α20βP calculated from each steroid metabolite from [³H]17αP of longchin goby oocytes (average diameter= 0.77 and 0.87 mm) after 24 h of incubation. Values are mean ± SEM in two or three replicate wells with 20 oocytes/well. Asterisk indicates the significant differences between each treatment and controls (P < 0.05).
4 Discussion
The endocrine-disrupting profiles of UV filters, including EHMC and BP-3, have been reported in mammals, amphibians, and freshwater fish (Wang et al., 2016). Although EHMC and BP-3 are widespread in aquatic biota, information on their effects on sex steroid production, especially in marine fish species, is scarce. Herein, we aimed to show the potential estrogenic effects of EHMC and BP-3 on the in vitro oocyte maturation process in marine fish species during steroidogenic shifts from estrogens to progestogens.
In steroid metabolites, E2 metabolic rates decreased and 17α20βP metabolic rates increased with an increase in the oocyte diameter. In teleosts, E2 is produced in the oocyte follicle cell layer and transported to the liver during the vitellogenic phase. The liver subsequently produces vitellogenin, the yolk protein precursor. After vitellogenesis, E2 decreases, and progestogens such as 17α20βP increase, inducing final maturation (Patiño et al., 2001). This is in good agreement with the histological finding of the present study that oocytes with average diameters of 0.77 mm during the fully vitellogenic stage, and 0.82 mm and 0.87 mm during the germinal vesicle migration stage, were transitioning from vitellogenesis to maturation.
Our results indicate that EHMC and BP-3 demonstrate estrogenic potency in steroidogenesis during oocyte maturation. In particular, both chemicals exhibited a weak estrogenic effect on E2 metabolites in fully vitellogenic oocytes (0.77 mm in oocyte diameter), although the E2 metabolite values increased. In the oocytes of 0.82- and 0.87-mm diameters, EHMC or BP-3 substantially increased E2 metabolite and weakly decreased 17α20βP metabolite, although the difference was insignificant. These findings suggest that EHMC and BP-3 have potential estrogenic effects on oocyte maturation following vitellogenesis. In a previous study, EHMC showed estrogenic effects that increased vitellogenin levels and gene expression, although its antiestrogenic effects have also been reported (Inui et al., 2003; Christen et al., 2011; Zucchi et al., 2011; Lee et al., 2019; Zhou et al., 2019a, b). Additionally, EHMC demonstrated androgenic effects with estrogenic effect inhibition including a decrease in the expression of E2, VTG, estrogen receptor (ER), progesterone receptor, aromatase, and 17β-HSD (Zhou et al., 2019b).
In a previous study, BP-3 demonstrated estrogenic effects with ER agonism and the VTG gene upregulation, although adverse or unclear effects have also been reported (Coronado et al., 2008; Molina-Molina et al., 2008; Kerdivel et al., 2013: Rodríguez-Fuentes et al., 2015; Mustieles et al., 2023). Moreover, Kunz and Fent (2006) reported the estrogenic and anti-estrogenic effects of EHMC and BP-3 as multiple steroidal responses. Additionally, they reported on whether multiple hormonal activities are the general characteristics of certain chemicals is unclear, although most endocrine-disrupting chemicals have not been completely analyzed. The reasons for these contradictory results remain elusive. A possible hypothesis is that the sensitivity of the specimens differs depending on the chemical. This includes various fish species, exposure methods, and oocyte developmental stages (Baek et al., 2007; Lee et al., 2019). Another hypothesis is restricted VTG responses, a representative index of estrogenicity in the endocrine activity of fish. A recent study reported that VTG responses do not always detect endocrine activity and that it is rare for substances without endocrine activity in vitro to cause a concentration-dependent VTG response in fish (Brown et al., 2023). In this regard, we comparatively analyzed vitellogenic oocytes and mature oocytes for consecutive steroid hormonal shifts during the maturation process induced by EHMC and BP-3 exposure.
As mentioned above, 17α20βP is known as a maturation-inducing steroid synthesized and secreted from the ovarian follicle layers and plays a crucial role in the final maturation and ovulation process of oocytes (Nagahama and Yamashita, 2008). The decrease in 17α20βP in the matured oocytes by EHMC or BP-3 exposure was intuitive on the results of heatmap analysis; however, no significant difference was observed compared with the controls. Contrastingly, in the oocytes of 0.82-mm diameter, EHMC markedly increased the E2/17α20βP ratio, which is thought to have been combined with the results of the decrease in 17α20βP along with the increase in E2. Additionally, BP-3 markedly increased the E2/T ratio, which was the combined result of the decrease in T along with an increase in E2. We were unable to obtain sufficient amounts of 0.82-mm oocytes with limitations for collecting the appropriate developmental stage of oocytes from matured females; therefore, we could not conduct BP-3 exposure experiments with them. Despite the insufficient data, we carefully presume that BP-3 may act analogously to EHMC in 0.82-mm oocytes since the results of BP-3 exposure in the other two oocyte groups were analogous to the results of EHMC exposure. In the oocytes of 0.87-mm diameters showing that the metabolic rate of 17α20βP was the highest among three groups of oocytes, BP-3 increased significantly the E2 metabolite and E2/T ratio. In general, progestogen acts as a maturation-inducing steroid after vitellogenesis (Lubzens et al., 2010) and increased estrogenic effects by BP-3 in that stage would be mentioned as an estrogenic potency of BP-3. This finding is consistent with a recent review by Mustieles et al. (2023) that 20 references demonstrated estrogenic effects of BP-3.
To date, only a few studies have investigated the effects of these chemicals on oocyte maturation. Schreurs et al. (2005) reported that EHMC acts as a weak ER agonist and potent progesterone receptor antagonist. BP-3 inhibits gonad maturation in females and males and induces feminization in zebrafish. Recently, Jin et al. (2021) showed BP-3 to inhibit the release of the first polar body and disrupt spindle assembly during oocyte maturation in mice. The authors did not mention BP-3’s estrogenic activity, and the specific link between estrogenic activity and BP-3 during oocyte meiosis should be studied in future research. In the progestogen profiling disruption by EHMC or BP-3 exposure, EHMC and BP-3 showed antiprogestogenic activity in the CALUX assay; however, BP-3 did not exhibit antiprogestogenic activity in the gonads of the common carp (Amankwah et al., 2024). Additionally, the authors reported that EHMC was not detected in the fish tissues from the collection site and suggested that the values indicating no effect of specific chemicals should be established. We strongly agree with this suggestion and hope that our findings will help designate a relevant index for progestogen evaluation by these chemicals.
Disruption of the oocyte maturation process by these chemicals may pose ecological risks in aquatic ecosystems because reproduction is directly associated with progeny production. Recently, Xu et al. (2021) reported possible transgenerational effects of BP-3 in zebrafish, where BP-3 exposure induced parental feminization and upregulated the expression of estrogen-related genes, such as ER and VTG. Moreover, the progeny hatching rate from the exposed adults decreased by half. Previous reports are in agreement with this finding. EHMC and BP-3 reduced egg production in exposed adult medaka and zebrafish, although the hatching rates of the progeny were not affected (Kim et al., 2014; Lee et al., 2019). Additionally, the thyroid hormone levels, which play a major role in growth in the juvenile stage, decreased in the progeny of EHMC-exposed adult medaka; however, survival did not decrease (Lee et al., 2019). Contrastingly, although the hatching rates of both F0 and F1 decreased, EHMC showed anti-estrogenic effects in the exposed adults (Zhou et al., 2019b). Furthermore, the progeny exhibited stronger biochemical responses and oxidative damage by EHMC than their parents. Comprehensively, future studies with in vivo exposure to extend the production of F1 generation from exposed F0 will enhance our understanding of the endocrine disruption mechanism despite our findings showing that EHMC and BP-3 demonstrated estrogenic potency, followed by progestogen inhibition during the oocyte maturation stage.
5 Conclusion
EHMC and BP-3 showed estrogenic potency during the oocyte maturation process, with a steroidal shift in the longchin goby. Additionally, our study underscores the progestogen metabolism inhibition and estrogen metabolism induction in the maturing oocytes. Despite the absence of reliable indices for precisely assessing estrogenicity or anti-progestogenicity, as mentioned by Amankwah et al. (2024), our findings of in vitro altered steroid production by EHMC and BP-3 in marine fish species would provide fundamental information for understanding the impact of these chemicals on marine fish reproduction. Future research on the gene expression of estrogen and/or progestogen receptors and in vivo experiments, including the influences of parental transfer of these chemicals, should be performed.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Animal Ethics Committee of Pukyong National University (PKNU; Regulation No. 554). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HW: Visualization, Formal analysis, Investigation, Software, Writing – original draft. HB: Supervision, Conceptualization, Writing – review & editing, Project administration, Funding acquisition. IH: Funding acquisition, Writing – original draft, Formal analysis, Visualization, Validation, Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Basic Science Research Program through the National Research Foundation (2020R111A3072395) and National Institute of Fisheries Science, Ministry of Oceans and Fisheries, Korea (R2025029).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1642233/full#supplementary-material
References
1
Amankwah B. K. Sauer P. Grabicova K. von der Ohe P. G. Ayikol N. S. Kroupova H. K. (2024). Organic UV filters: Occurrence, risks and (anti-)progestogenic activities in samples from the Czech aquatic environment and their bioaccumulation in fish. J. Harzard. Mat.471, 134338. doi: 10.1016/j.jhazmat.2024.134338
2
Baek H. J. Hwang I. J. Kim K. S. Lee Y. D. Kim H. B. Yoo M. S. (2007). Effects of BPA and DES on longchin goby (Chasmichthys dolichognathus) in vitro during the oocyte maturation. Mar. Environ. Res.64, 79–86. doi: 10.1016/j.marenvres.2007.01.006
3
Bevans H. E. Goodbred S. L. Miesner J. F. Watkins S. A. Gross T. S. Denslow N. D. et al . (1996). Synthetic organic compounds and carp endocrinology and histology in Las Vegas Wash and Las Vegas and Callville Bays of Lake Mead, Nevada 1992 and 1995. Water-Resour. Investig. Rep., 96–4266. doi: 10.3133/wri964266
4
Bordalo D. Soares A. M. V. M. Sokolova I. Pretti C. Freitas R. (2025). 2-Ethylhexyl-4-methoxycinnamate on marine and coastal environments: A comprehensive review of its environmental significance and biological impact. Mar. Pollut. Bull.211, 117340. doi: 10.1016/j.marpolbul.2024.117340
5
Brown R. J. Panter G. H. Burden N. Salinas E. R. Weltje L. Wheeler J. R. et al . (2023). Are changes in vitellogenin concentrations in fish reliable indicators of chemical-induced endocrine activity? Ecotoxicol. Environ. Saf.266, 115563. doi: 10.1016/j.ecoenv.2023.115563
6
Christen V. Zucchi S. Fent K. (2011). Effects of the UV-filter 2-ethyl-hexyl-4-trimethoxycinnamate (EHMC) on expression of genes involved in hormonal pathways in fathead minnows (Pimephales promelas) and link to vitellogenin induction and histology. Aquat. Toxicol.102, 167–176. doi: 10.1016/j.aquatox.2011.01.013
7
Coronado M. De Haro H. Deng X. Rempel M. A. Lavado R. Schlenk D. (2008). Estrogenic activity and reproductive effects of the UV-filter oxybenzone (2-hydroxy-4-methoxyphenyl-methanone) in fish. Aquat. Toxicol.90, 182–187. doi: 10.1016/j.aquatox.2008.08.018
8
Folmar L. C. Denslow N. D. Rao V. Chow M. Crain D. A. Enblom J. (1996). Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ. Health Perspect.104, 1096–1101. doi: 10.1289/ehp.961041096
9
Gago-Ferrero P. Demeestere K. Silvia Díaz-Cruz M. Barceló D. (2013). Ozonation and peroxone oxidation of benzophenone-3 in water: Effect of operational parameters and identification of intermediate products. Sci. Total. Environ.443, 209–217. doi: 10.1016/j.scitotenv.2012.10.006
10
Horricks R. A. Tabin S. K. Edwards J. J. Lumsden J. S. Marancik D. P. (2019). Organic ultraviolet filters in nearshore waters and in the invasive lionfish (Pterois volitans) in Grenada, West Indies. PLoS One14, e0220280. doi: 10.1371/journal.pone.0220280
11
Hwang I. J. Baek H. J. (2011). Assessment of in vitro oocyte maturation in two gobiid fish species, Chasmichthys dolichognathus and Tridentiger trigonocephalus after exposure to benzo [a]pyrene. Dev. Reprod.15, 223–230.
12
Hwang I. J. Baek H. J. (2023). Effects of 3,3’,4,4’,5-pentachlorobiphenyl on in vitro oocyte maturation in dusky tripletooth goby, Tridentiger obscurus: an implication of estrogenic potency. Front. Mar. Sci.10, 1159560. doi: 10.3389/fmars.2023.1159560
13
Inui M. Adachi T. Takenaka S. Inui H. Nakazawa M. Ueda M. et al . (2003). Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes). Toxicology194, 43–50. doi: 10.1016/S0300-483X(03)00340-8
14
Jin L. Zhu H. Y. Kang X. J. Lin L. P. Zhang P. Y. Tan T. et al . (2021). Melatonin protects against oxybenzone-induced deterioration of mouse oocytes during maturation. Aging13, 2727–2749. doi: 10.18632/aging.202323
15
Kerdivel G. Guevel R. L. Habauzit D. Brion F. Ait-Aissa S. Pakdel F. (2013). Estrogenic potency of benzophenone UV filters in breast cancer cells: Proliferative and transcriptional activity substantiated by docking analysis. PLoS One8, e60567. doi: 10.1371/journal.pone.0060567
16
Kim D. G. Hwang I. J. Baek H. J. (2023). Potent influence of exogenous melatonin on in vitro oocyte maturation in the longchin goby. Chaenogobius annularis. Dev. Reprod. 27, 127–135. doi: 10.12717/DR.2023.27.3.127
17
Kim S. Jung D. Kho Y. Choi K. (2014). Effects of benzophenone-3 exposure on endocrine disruption and reproduction of Japanese medaka (Oryzias latipes) - A two generation exposure study. Aquat. Toxicol.155, 244–252. doi: 10.1016/j.aquatox.2014.07.004
18
Kim I. S. Lee Y. J. Kim Y. U. (1986). Synopsis of the Family Gobiidae (Pisces, Perciformes) front Korea. Kor. J. Fish. Aquat. Sci.19, 387–408.
19
Kunz P. Y. Fent K. (2006). Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat. Toxicol.79, 305–324. doi: 10.1016/j.aquatox.2006.06.016
20
Lebarone P. (2022). UV filters and their impact on marine life: state of the science, data gaps, and next steps. J. Eur. Acad.36, 22–28. doi: 10.1111/jdy.18198
21
Lee I. Lee J. Jung D. Kim S. Choi K. (2019). Two-generation exposure to 2-ethylhexyl 4-methoxycinnamate (EHMC) in Japanese medaka (Oryzias latipes) and its reproduction and endocrine related effects. Chemosphere228, 478–484. doi: 10.1016/j.chemosphere.2019.04.123
22
Lubzens E. Young G. Bobe J. Cerda J. (2010). Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol.165, 367–389. doi: 10.1016/j.ygcen.2009.05.022
23
Manová E. von Goetz N. Hauri U. Bogdal C. Hungerbühler K. (2013). Organic UV filters in personal care products in Switzerland: A survey of occurrence and concentrations. Int. J. Hyg. Environ. Health216, 508–514. doi: 10.1016/j.ijheh.2012.08.003
24
Molina-Molina J. M. Escande A. Pillon A. Gomez E. Pakdel F. Cavaillès V. et al . (2008). Profiling of benzophenone derivatives using fish and human estrogen receptor-specific in vitro bioassays. Toxicol. Appl. Pharmacol.232, 384–395. doi: 10.1016/j.taap.2008.07.017
25
Mustieles V. Balogh R. K. Axelstad M. Montazeri P. Marquez S. Vrijheid M. et al . (2023). Benzophenone-3: Comprehensive review of the toxicological and human evidence with meta-analysis of human biomonitoring studies. Environ. Inter.173, 107739. doi: 10.1016/j.envint.2023.107739
26
Nagahama Y. Yamashita M. (2008). Regulation of oocyte maturation in fish. Dev. Growth Differ.50, S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x
27
Pal A. He Y. Jekel M. Reinhard M. Gin K. Y. H. (2014). Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int.71, 46–62. doi: 10.1016/j.envint.2014.05.025
28
Patiño R. Yoshizaki G. Thomas P. Kagawa H. (2001). Gonadotropic control of ovarian follicle maturation: The two-stage concept and its mechanisms. Comp. Biochem. Physiol.-B Biochem. Mol. Biol.129, 427–439. doi: 10.1016/S1096-4959(01)00344-X
29
Ramos S. Homem V. Alves A. Santos L. (2015). Advances in analytical methods and occurrence of organic UV-filters in the environment - A review. Sci. Total. Environ.526, 278–311. doi: 10.1016/j.scitotenv.2015.04.055
30
Robinson C. D. Brown E. John A. C. Davies I. M. Megginson C. Miller C. et al . (2007). Bioindicators and reproductive effects of prolonged 17β-oestradiol exposure in a marine fish, the sand goby (Pomatoschistus minutus). Aquat. Toxicol.81, 397–408. doi: 10.1016/j.aquatox.2006.12.020
31
Rodríguez-Fuentes G. Sandoval-Gío J. J. Arroyo-Silva A. Noreña-Barroso E. Escalante-Herrera K. S. Olvera-Espinosa F. (2015). Evaluation of the estrogenic and oxidative stress effects of the UV filter 3-benzophenone in zebrafish (Danio rerio) eleuthero-embryos. Ecotoxicol. Environ. Saf.115, 14–18. doi: 10.1016/j.ecoenv.2015.01.033
32
Schlenk D. Sapozhnikova Y. Irwin A. M. Xie L. Hwang W. Reddy S. et al . (2005). In vivo bioassay-guided fractionation of marine sediment extracts from the Southern California Bight, USA, for estrogenic activity. Environ. Toxicol. Chem.24, 2820–2826. doi: 10.1897/05-116R.1
33
Schreurs R. H. M. M. Sonneveld E. Jansen J. H. J. Seinen W. van der Burg B. (2005). Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol. Sci.83, 264–272. doi: 10.1093/toxsci/kfi035
34
Wang J. Pan L. Wu S. Lu L. Xu Y. Zhu Y. et al . (2016). Recent advances on endocrine disrupting effects of UV filters. Int. J. Environ. Res. Public Health13, 782. doi: 10.3390/ijerph13080782
35
Watkins Y. S. D. Sallach J. B. (2021). Investigating the exposure and impact of chemical UV filters on coral reef ecosystems: Review and research gap prioritization. Integr. Environ. Assess. Manage.17, 967–981. doi: 10.1002/ieam.4411
36
Xu M. Zheng D. Gong S. (2021). Effects of low concentration benzophenone−3 exposure on the sex ratio and offspring development of zebrafish (Danio rerio). Bullet. Environ. Contam. Toxicol.106, 740–746. doi: 10.1007/s00128-021-03166-y
37
Zhang Y. Tu L. T. Chen J. Zhou L. (2024). Interference mechanisms of endocrine system and other systems of endocrine-disrupting chemicals in cosmetics - In vitro studies. Int. J. Endocrinol., 2564389. doi: 10.1155/ije/2564389
38
Zhou R. Lu G. Yan Z. Bao X. Zhang P. Jiang R. (2019a). Bioaccumulation and biochemical effects of ethylhexyl methoxy cinamate and its main transformation products in zebrafish. Aquat. Toxicol.214, 105241. doi: 10.1016/j.aquatox.2019.105241
39
Zhou R. Lu G. Yan Z. Jiang R. Shen J. Bao X. (2019b). Parental transfer of ethylhexyl methoxy cinnamate and induced biochemical responses in zebrafish. Aquat. Toxicol.206, 24–32. doi: 10.1016/j.aquatox.2018.11.001
40
Zucchi S. Oggier D. M. Fent K. (2011). Global gene expression profile induced by the UV-filter 2-ethyl-hexyl-4-trimethoxycinnamate (EHMC) in zebrafish (Danio rerio). Environ. Pollut.159, 3086–3096. doi: 10.1016/j.envpol.2011.04.013
Summary
Keywords
benzophenone-3, estrogenic effects, longchin goby, maturing oocytes, steroidogenesis, 2-ethylhexyl 4-methoxycinnamate
Citation
Woo H, Baek HJ and Hwang IJ (2025) Effects of UV filters 2-ethylhexyl 4-methoxycinnamate and benzophenone-3 on in vitro steroidogenesis during oocyte maturation of the longchin goby, Chasmichthys dolichognathus. Front. Mar. Sci. 12:1642233. doi: 10.3389/fmars.2025.1642233
Received
06 June 2025
Accepted
04 August 2025
Published
01 September 2025
Volume
12 - 2025
Edited by
Lu Cai, Ministry of Water Resources and Chinese Academy of Sciences, China
Reviewed by
Siyag Dhere, Central Institute of Fisheries Education (ICAR), India
Yankun Zhang, Hainan Normal University, China
Updates
Copyright
© 2025 Woo, Baek and Hwang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: In Joon Hwang, astraroth@korea.kr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.