Abstract
Marine organisms rely on stable seawater conditions and vary in taxa-specific tolerances to environmental change. The capacity for acclimatization in marine taxa is dependent on local adaptation. Our ability to generate accurate global predictions starts in identifying regional responses, informing facets that fit globally in a mosaic of response to environmental extremes. The northwestern Gulf of Mexico (nwGoM) has not previously been isolated as a region with significant multi-taxon level comparisons under geochemical extremes. Therefore, we aim to procure a nwGoM regional baseline via a literature search in all known marine taxa’s response to elevated CO2 partial pressure (pCO2) coupled with real-time ecosystem modeling of this region. The baseline carbonate chemistry conditions indicate that pH, aragonite saturation state (Ωarag), and pCO2 exhibit greater temporal and spatial variability within the upper 20 m of the water column, with nearshore waters showing more pronounced seasonal spatial variation than offshore waters. Of the taxon reported, 68.5% reported a negative response to increased pCO2, whereas 31.4% showed a neutral or mixed neutral response (positive or negative). Only 11.4% of reported taxa showed a positive response to elevated pCO2. Shown here is a holistic negative response to increased pCO2 through collating external studies. Data was only found on 1.0% of the total species we recorded in the nwGoM region, highlighting a significant gap in our understanding of regional ecosystem wide sensitivity. Of the species shown here, 83% have habitat ranges within the top 20 m of the water column, and with seasonal variability they may be exposed to several extremes, modeled here but overlooked when compared to global predictions. Continuing experimental work on the reported species here will inform regional predictions to fit the global mosaic predicting the state of our oceans to future conditions.
Introduction
The Gulf of Mexico (GoM) is an essential marine ecosystem that supports significant fishery activities (both commercial and recreational). There are two types of coral reefs in the northwestern GoM (nwGoM). In the area managed by Flower Garden Banks National Marine Sanctuary (FGBNMS), coral reefs grow on salt dome features located near the edge of the continental shelf, with some reaching as shallow as 18 meters below the sea surface (Schmahl et al., 2008). The FGBNMS manages only 3 out of ~130 similar reefs, banks, and topographic features in the nwGoM. The much larger expanse of similar environments has significant ecological benefits not only to fisheries but also to the ecosystem health of this region by providing a network of interconnected communities within and around the FGBNMS. In addition to these naturally-based features, decommissioned oil and gas structures can also provide a hard substrate for coral growth (Ajemian et al., 2015; Sammarco et al., 2013). In the deeper parts of the region, non-reef building corals also exist, forming deep water communities with similar biodiversity. It is worth noting that the nwGoM reef system, specifically the FGB area, received an official score of ‘good’, which indicates relatively healthy condition, from the NOAA Coral Reef Program (2020) based on coral reef ecosystem survey data from 2014 to 2018. Making this reef ecosystem the only U.S. jurisdiction Atlantic coral reef to achieve such a rating (Manzello et al., 2021). These studies may indicate a degree of stability and conservation within the FGB area.
Since the industrial revolution (ca. 1750), carbon dioxide (CO2) has been continuously added into the atmosphere through human activities, including fossil fuel combustion, deforestation, and cement production (IPCC, 2013; Le Quéré et al., 2018). Because of this global scale disturbance to the carbon cycle, CO2 concentration in the atmosphere has increased from the preindustrial level of ~280 ppm to the present ~474 ppm (NOAA Global Monitoring Laboratory). Over the past ~250 years, the ocean has taken up ~1/4 of anthropogenic CO2 (Le Quéré et al., 2018; Sabine et al., 2004). Because CO2 dissolved in seawater produces weak carbonic acid, surface ocean pH and carbonate saturation state (Ω) have been decreasing gradually, a process widely known as “ocean acidification” (OA) (Doney et al., 2009; Feely et al., 2004). Regions like the nwGoM with complex assemblages of biodiversity in near surface water may be threatened by OA in ways that global predictions fail to capture through generalized approaches.
Compared to the pelagic ocean, the coastal ocean experiences more complex impacting factors and lacks stable seasonality. Examples include coastal upwelling of deep “corrosive” CO2-rich and low pH water (Feely et al., 2010, 2008; Mathis et al., 2012), “basification” due to enhanced export of alkalinity via large rivers, which is caused by either agricultural practices (Raymond and Cole, 2003; Raymond et al., 2008; Regnier et al., 2013) or changing acidification conditions in river headwaters (Kaushal et al., 2013; Stets et al., 2014), and eutrophication induced acidification in shallow coastal waters subject to hypoxia (i.e., dissolved oxygen or DO concentration <2 mg L-1) (Cai et al., 2011; Mucci et al., 2011; Wallace et al., 2014).
While the Mississippi and Atchafalaya rivers influence the shelf water on the continental shelf, resulting in high pH (up to 8.8) and aragonite saturation state (Ωarag, up to 8) (Guo et al., 2012) in the river plume region and as low as 7.7 and 1.6 in hypoxic bottom waters, these parameters show much smaller spatial variations in the oligotrophic outer shelf waters (Wang et al., 2013; Wanninkhof et al., 2015). However, upwelling from deeper water could depress both pH and Ωarag. For example, in the shelf-slope break, subsurface waters have much lower pH and Ωarag, and pH is 7.9, and Ωarag is as low as 1.8 in ~200 m waters along the Galveston transect on the shelf-slope break (data from the 2017 Gulf of Mexico Ecosystems and Carbon Cruise, or GOMECC-3). Therefore, the northwestern Gulf of Mexico (nwGoM) and the organisms that inhabit this region are exposed to a wide range of geochemical extremes, independent of open ocean-based predictions. This region has not previously been isolated as a region with significant multi-taxon level comparisons under geochemical extremes. This study aims to procure a nwGoM regional baseline via a literature search in all known marine taxa’s response to elevated CO2 partial pressure (pCO2) coupled with real-time ecosystem modeling of this region.
Considering geochemical regimes, this paper outlines marine organismal sensitivities to acidification based on the synthesized baseline carbonate chemistry conditions in the nwGoM shelf. This study provides a contextual understanding of potential chemistry and biology shift from natural anthropogenic activities. This information may be helpful for future environmental monitoring or species sensitivities assessments.
Methods
Synthesis of baseline carbonate chemistry data
Water column carbonate chemistry data (typically total alkalinity or TA, total dissolved inorganic carbon or DIC) and hydrographic data collected during various research cruises from 2006 to 2019 were retrieved as detailed in Table 1. This study focused on the continental shelf region of the nwGoM, specifically between 27-30°N latitude and 90-98°W longitude (Figures 1, 2). Most sampling stations were located in areas with water depths less than 150 m (Figure 3), with the most significant recorded depth being 1250 m. The spatial distribution of stations across the study area was not uniform, with a higher density of sampling near the Louisiana coast (between 28-29°N, 90-93°W) and around FGBNMS (approximately 28.3°N, 94.5°W; Figure 1). The Texas coast featured more sparsely spaced stations, and the sampling usually did not cover the outer shelf. In addition, sampling dates were unequally distributed across seasons, with most of the data collected in the summer and fall months and much fewer spring and no winter data from the Texas coast by the time when this manuscript was written (Figures 1, 2).
Table 1
| Data name | Time | URL | Author/leading scientist |
|---|---|---|---|
| Northern Gulf of Mexico hypoxic zone data from shelf-wide cruises | 2010-2016 | 2010: doi.org/10.7266/N7Z899TR 2011: doi.org/10.7266/N7513WM8 2012: doi.org/10.7266/N78913VT 2013: doi.org/10.7266/N7000046 2014: doi.org/10.7266/N73R0QXQ 2015: doi.org/10.7266/N77H1GM0 2016: doi.org/10.7266/N7GF0S2N |
Xinping Hu Xinping Hu, Wei-Jun Cai Xinping Hu, Wei-Jun Cai Xinping Hu Xinping Hu Xinping Hu Xinping Hu |
| Northern Gulf of Mexico data from BCO-DMO (NSF) | 2017-2019 | https://www.bco-dmo.org/dataset/831523/data | Wei-Jun Cai |
| Water column carbonate chemistry data in Flower Garden Banks NMS | 2013-2016 | doi.org/10.7266/N7G15Z9M | Xinping Hu |
| South Texas Shelf cruise data | 2012 | unpublished | Xinping Hu |
| Gulf of Mexico and East Coast Carbon Cruise (GOMECC-1) | 2007 | https://www.aoml.noaa.gov/ocd/gcc/GOMECC1/data.php | Tsung-Hung Peng, Chris Langdon |
| Gulf of Mexico Ecosystems and Carbon Cruise (GOMECC-3) | 2017 | https://www.aoml.noaa.gov/ocd/gcc/GOMECC3/ | Leticia Barbero |
| Northern Gulf of Mexico summer bottom water data | 2006-2017 | https://www.bco-dmo.org/dataset/818773/data | Wei-Jun Cai |
Datasets used for carbonate chemistry baseline synthesis.
Figure 1
Geographic setting of the study area. The general boundaries of the sampling stations along the Texas and Louisiana coasts are indicated by black boxes. The Flower Garden Banks National Marine Sanctuary (FGBNMS) is highlighted in red.
Figure 2
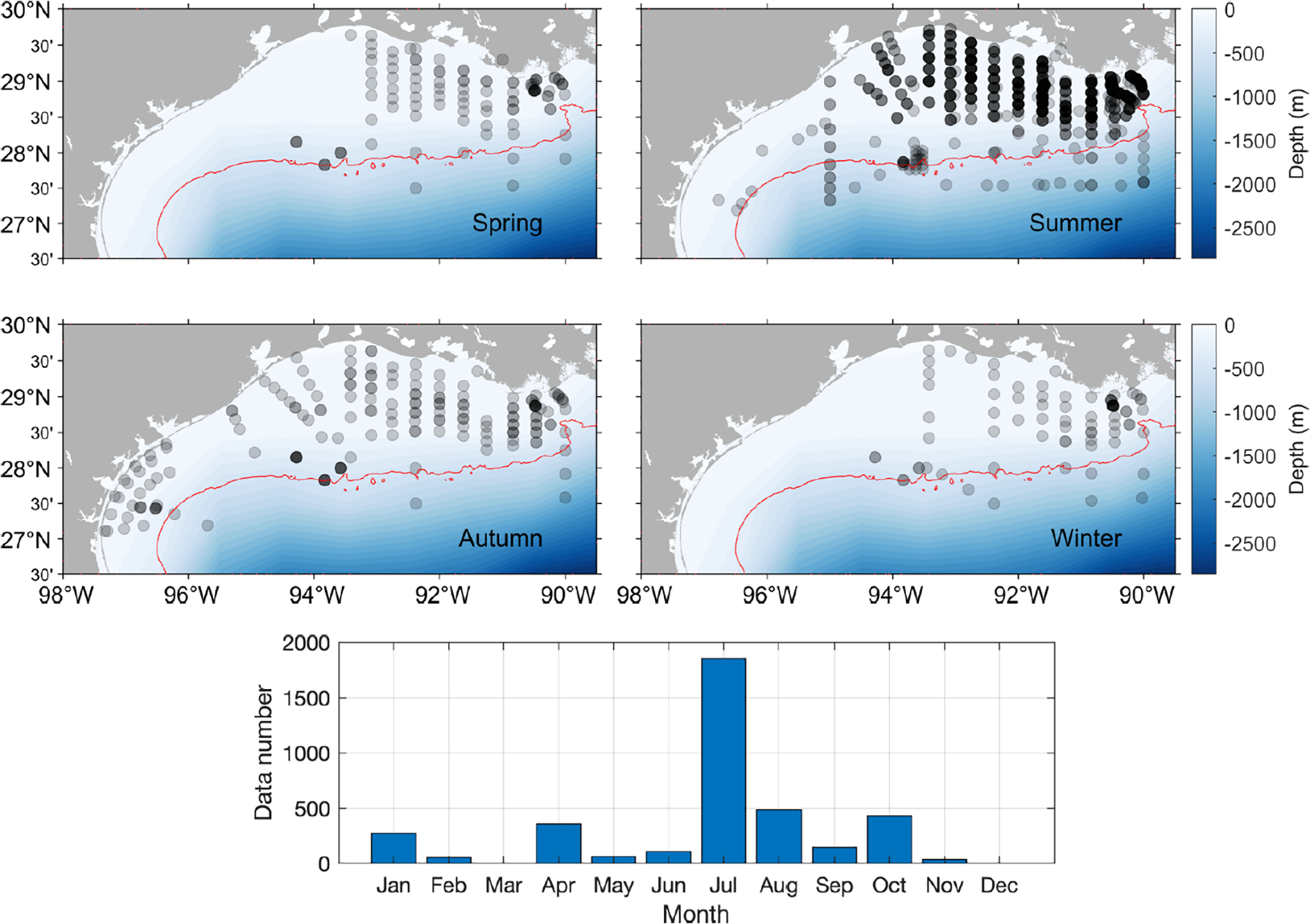
(Top panel)The spatial and seasonal distribution of sampling stations included in the synthesis, and (Bottom panel) the amount of data available in each month. All data are from the datasets listed in Table 1, with the analyses limited to stations located north of 27°N and west of 90°W. The denser color in the station map indicates a higher sampling density. The red contour line indicates 150 m isobath.
Figure 3
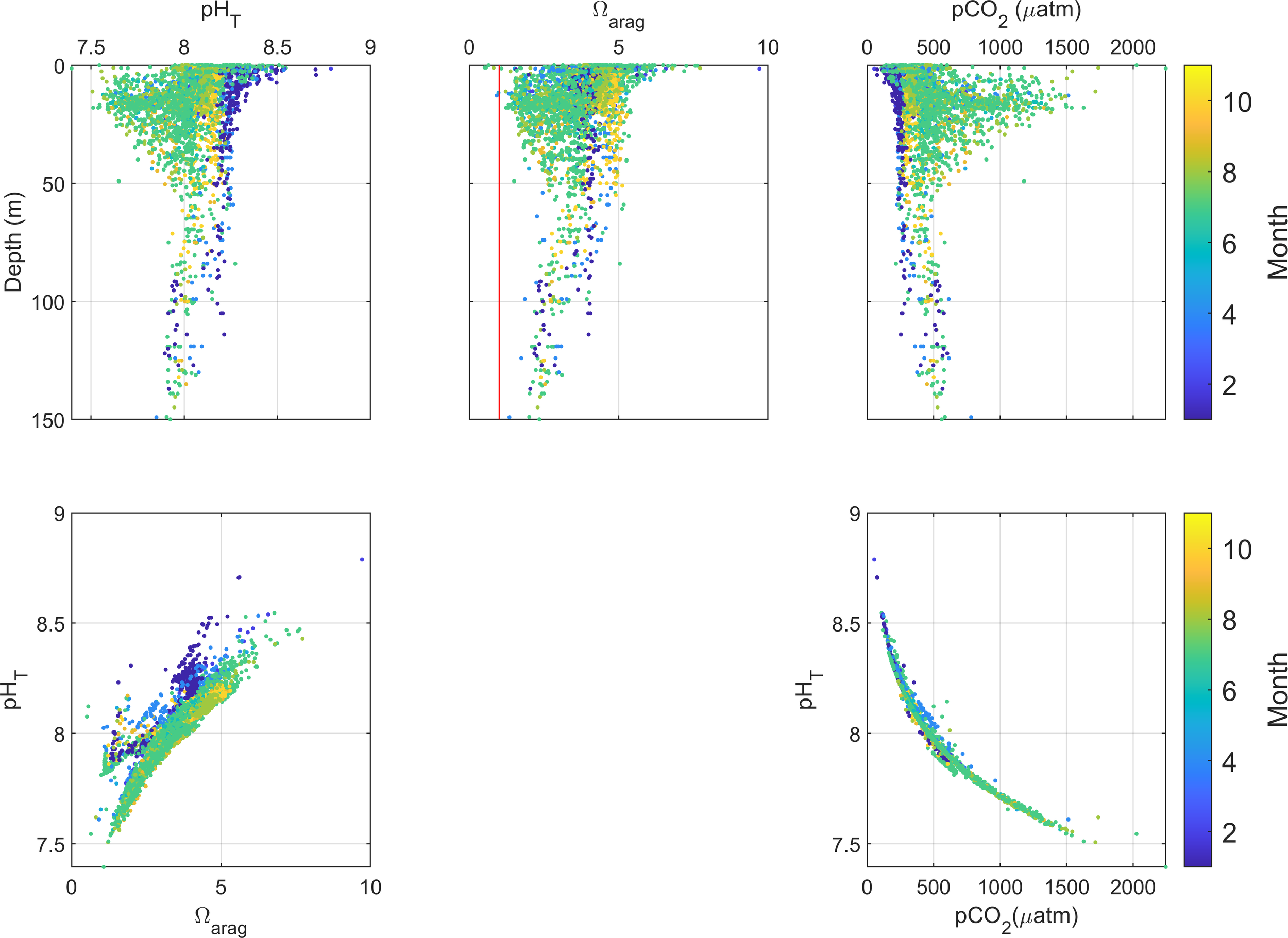
Depth distributions of pH, Ωarag, and pCO2 in the top 150 m of water at all stations. Also shown are the relationships between Ωarag and pH, and between pH and pCO2. The vertical red line indicates Ωarag =1.
Vulnerable species selection
The second part of the study aims to provide a comprehensive overview of nwGoM species potentially sensitive to CO2 changes with vulnerabilities supported by the literature. The selection and inclusion of these potentially vulnerable species were based on the following merits:
-
Locationally relevant species: We compiled over 3,480 species recorded within the habitat range of the nwGoM, ensuring appropriate and region-specific information.
-
Phyla selection: The choice of phyla with locational relevancy was determined by available literature conducting CO2 enrichment analyses on related species (Table 2).
-
Class refinement: Further subdivision into classes was carried out for cases where a particular phylum was large with many species.
Table 2
| Class | Genus/Species | Impact (%) | Biological Response | pCO2 Range | Exposure (d) | Direction | Habitat-Biology | Depth (m) | Location |
|---|---|---|---|---|---|---|---|---|---|
| Rhodophyceae | J. adhaerens | ↑50 | Calcification | 1180-1520 | 3 | o | epi, hsb | 0->10 | Entire |
| Florideophyceae | Neosiphonia spp. | NA | Photosynthesis & growth | 800 - 1500 | 14-24 | (+) | epi, hsb | 3->10 | Entire |
| Florideophyceae | Hydrolithon spp. | ↓7 | Calcification | 900* | 5 | (-) | epi, hsb | 3->10 | Entire |
| Bacillariophyceae | Coscinodiscus spp. | ↑30-40 | Growth & C fix. rate | 400 & 800 | 2 | (+) | ben, plk, bns, osp | 0->10 | Entire |
| Bacillariophyceae | T. pseudonana | ↓9 | Photosynthesis | 800-1400 | 0.2-0.3 | (-)/o | Unk | Unk | Entire |
| Actinopterygii | M. beryllina | ↓73 | Larval survival | 780 | 10 | (-) | bns, est | Near-shore | nw, ne, sw |
| Actinopterygii | S. ocellatus | ↓14 | Larval survival | 1300 | 3 | o | dem, crr, est | 0-27 | nw, ne, sw |
| Chondrichthyes | L. erinacea | ↑67 | Jaw mineralization | 1100 | 155-186 | (+) | dem, slp, end | 53-588 | Entire |
| Chondrichthyes | Scyliorhinus spp. | ↑15 | Absolute lateralization | 990 | 28 | (-) | slp, sft | 73-548 | Entire |
| Asteroidea | L. clathrata | ↑67 | Relative juvenile growth | 780 | 97 | o | snd | 0-175 | nw, ne |
| Malacostraca | Menippe spp. | ↓28-37 | Hatching & survivorship | 600 | 12 | (-) | ben, rbl, com, end | 0-51 | Entire |
| Malacostraca | Clibanarius spp. | NA | Larval survival | 110-1200 | 30 | (-) | ben, itd, bns | 0-22 | Entire |
| Malacostraca | Portunus spp. | ↓100 | (Adult) survival | 2200- 231280 | 3 | (-) | bplg, sft, shl, rbl | 0-640 | Entire |
| Malacostraca | C. sapidus | ↓23 | Larval survival | 4650 | 77 | (-) | bplg, eur, com | 0-90 | Entire |
| Copepoda | P. crassirostris | NA | Physiological pathways | 7.70-7.95 ** | NA | o | plk, cep | 0-50 | Entire |
| Copepoda | Calanus spp. | ↓50 | Scope for growth | 680-19,460 | 77-50 | (-)/o | plk, osp, ner | 0-200 | Entire |
| Copepoda | Arcatia sp. | NA | Size decrease | 880-1410 *** | 31 | (-) | plk, cep, ner, est | 0-200 | Entire |
| Cirripedia | Amphibalanus spp. | ↑39 | Base plate damage | 970-1600 | 77-84 | (-)/o | est, bns, hsb, epi | 0-62 | Entire |
| Bivalvia | Panopea spp. | ↓13 | Larval shell length | 2490 | 13-19 | (-) | ben, bur, est | 0-46 | nw, ne |
| Bivalvia | Mercenaria spp. | ↓80 | Larval survival | 620-1500 | 21-147 | (-)/o | ben, com, inf, sft | 0-60 | Entire |
| Bivalvia | Spisula sp. | NA | Energy costs | 570-2160 | 28-84 | (-) | ben, inf | 0-90 | nw, ne |
| Bivalvia | A. irradians | ↓97 | Larval survival | 509-1987 | 7-21 | (-) | ben, epi, mud, est | 0-26 | nw, sw, se |
| Bivalvia | Pinctada spp. | ↓102 | Calcification | 1420-2940 | 3 | (-) | byn, com, epi, hsb | 0-26 | Entire |
| Bivalvia | C. virginica | ↓22 | Total shell area | 800-3500 | 26-140 | (-) | com, epi, orf, ses | 0-79 | Entire |
| Bivalvia | Macoma spp. | ↓29-48 | Hatching success | 600-2130 | 17-19 | (-) | ben, est, inf | 0-183 | Entire |
| Cephalopoda | D. pealeii | ↓5 | Paralarvae size | 1300-2200* | 16-19 | (-) | cep, cts | 1-366 | Entire |
| Gastropoda | C. fornicata | ↓11 | Shell surface area | 1400 | 17 | (-) | ben, hsb, sgr, spf | 0-70 | Entire |
| Gastropoda | Haliotis spp. | NA | Shell growth | 750-1400 | 93 | (-) | ben, her, hsb | 36-366 | nw, ne, se |
| Gastropoda | Aplysia spp. | ↓30 | Metabolic rate | 2800 | 19 | o | ben, her, sft | 0-42 | Entire |
| Polyplacophora | Leptochitonid spp. | 0 | Shell strength | 2000 | 28 | o | ben, epi, swd | 300-912 | wnw, ese, nne |
| Scyphozoan | A. aurita | NA | Statolith growth | 800-4000 | 7-122 | (+)/o | est, ner, pth | 0-1250 | Entire |
| Scleractinia | A. palmata | ↓52-73 | Larval settlement | 560-800 | 11 | (-) | ben, zoo, hsb, crr | 0-30 | nw, se sw |
| Scleractinia | Madracis spp. | 0 | Calcification | 1480 | 9 | o | ben, azo, hsb, ocs | 2-1220 | nw, ne, se |
| Scleractinia | P. astreoides | ↓27-63 | Larval metabolism | 560 & 800 | NA | (-) | ben, zoo, hsb, crr | 1-70+ | Entire |
A northwest Gulf of Mexico (nwGoM) vulnerable species survey from supporting literature, showing class and genus or specific species names, relative % impact (clearly stated by the authors), biological responses, the pCO2 treatment range (µatm, * = ppm, ** = pH, *** = fCO2), exposure time in days, directionality (i.e. the generalized and nonspecific response determined by the authors), habitat biology (see footnotes), depth range which this species is found, and regionality within the GoM.
*All species present in this table used information from Felder and Camp (2009), and experimental response data can be found cited throughout this paper.
Ecological group descriptors: ben, benthic; plk, planktonic; epi, epiphytic or epizoic on soft tissue; par, parasite; or, oyster reef; est, estuary.
General depth/strata descriptors: bns, bay and nearshore; dr, drift; int, intertidal; oc, ocean; ofs, offshore; osp, oceanic surface and epipelagic;
shw, shallow subtidal (from 3 to 10 m depth); subt, sub- tidal (from deeper than 10 m depth).
Substrate descriptors: hsb, hard substratum; sft, soft substrata (mud, sands, clays).
Habitat-Biology Descriptors: bplg, benthopelagic; bab, bathyal; bsl, beach and shoreline; bur, burrower or borer; dem, demersal; end, endemic solely to Gulf of Mexico; epi, epibiotic;
est, estuarine; inf, infaunal; ins, interstitial; itd, intertidal to semiterrestrial; rbl, rubble; sym, symbiotic; dcrr, deep-reef; nid, non- indigenous to Gulf of Mexico; azo, azooxanthellate;
zoo, zooxanthellate; and tbi, tubicolous.
The bold values indicate emphasis on the northwestern region in the Gulf of Mexico.
All species were selected from the collection Felder and Camp (2009). Each section was divided by phyla, and phylum was assessed using a preliminary literature search. We scanned through 77 phyla and 15,419 species to apply the abovementioned constraints. Three thousand four hundred eighty species (3480) were recorded as locationally relevant. Each species within a given phylum with locational relevancy was searched in Google Scholar or Research Rabbit with the keywords “(genus) CO2” and “(genus) ocean acidification.” All species with peer-reviewed articles that experimentally addressed or reviewed direct exposure to elevated pCO2 on any biological function were pulled and used in this report. Of the 3480 locationally relevant species, this review found relevant data on a subset of 35 species. Those species with one or more reports of exposure to increased pCO2 were listed in Table 2. Sections within this survey were refined by class, as some phyla may have had locationally relevant classes with no relevant literature, and species identified can be found in the data provided. The literature search was conducted in January 2024.
Carbonate chemistry calculations
Carbonate chemistry parameters (pH, Ωarag, pCO2) were calculated using the CO2SYS program, with TA and DIC as input parameters, along with silicate and phosphate concentrations when available. In this study, carbonic acid dissociation constants, bisulfate dissociation constant, hydrofluoric acid dissociation constant, and borate-to-salinity ratio from Lueker et al. (2000); Dickson (1990); Perez and Fraga (1987), and Lee et al. (2010), respectively, were used for carbonate speciation calculations. All reported data are in situ temperature and pressure. The pH values are presented on the total scale.
Results and discussion
Geochemical baselines
Depth distributions of pH, Ωarag, and pCO2
The pH values of shelf water ranged from 7.54 to 8.8 across all stations in the nwGoM (Figure 3). However, significant variations in pH (up to 1.6 pH unit) were observed within the top 50 m of the water column. Conversely, a remarkably uniform pH range of approximately 7.9 to 8.3 prevailed in the depth interval between 50 and 150 m, irrespective of temporal and spatial variations. These distinct vertical variations suggest much more diverse biogeochemical, physical mixing, and temperature effects in the surface water. In most cases, Ωarag values were above 1, indicating that shelf waters were saturated with respect to aragonite. Similar to pH, the variation in Ωarag was also more pronounced within the top 20 meters of the water column, whereas values at greater depth until 150 m remained near Ωarag = 2. Surface water pCO2 (upper 20 m) exhibited substantial variability, ranging from <100 µatm to >2000 µatm, whereas values at greater depths were comparatively stable, typically between 200 and 500 µatm. Seasonally, pCO2 was generally elevated during summer relative to winter. When all the data were combined, there was a positive relationship between pH and Ωarag, and a negative relationship between pH and pCO2 was found (Figure 3).
Spatial and seasonal variabilities of carbonate chemistry in the surface and bottom waters
The spatial and seasonal variabilities of carbonate chemistry are demonstrated along the Louisiana coast (Figure 4). Both surface and bottom sampling stations covered the whole study region, and the seasonality represented the mean values of carbonate chemistry parameters across different cruises (interannual variability was averaged). pH levels were variable in both the surface and bottom layers. In the surface layer, there were high pH hotspots in the nearshore regions (pH up to 8.6, spring and summer). In contrast, bottom water pH was generally lower than surface water in each corresponding season (Figures 4, 5). In spring and summer, areas of lower pH in the bottom water generally corresponded with those that had higher surface pH (Figure 4), suggesting organic matter production through photosynthesis in the surface water sinking to the bottom and getting remineralized. Data were also analyzed based on their geological locations (nearshore locations = north of 28.5°N; offshore locations = south of 28.5°N) (Figure 5). Summer surface layer pH had the largest variations (up to 1.4 pH unit) across all the subsets, suggesting the complex biogeochemical and physical processes happening in the nearshore locations. More extreme values, identified as outliers in the boxplot, were observed in the nearshore surface water, and in general, the nearshore locations have a stronger variation in pH compared to the corresponding offshore data subset except for the surface layer in autumn (Figure 5). In contrast, low variations in pH (0.4-0.6 pH unit) were observed in offshore locations across seasons. The absence of significant terrestrial input and greater depth (inferring water column stability) could be the major reason for the low variabilities in pH in the offshore regions.
Figure 4
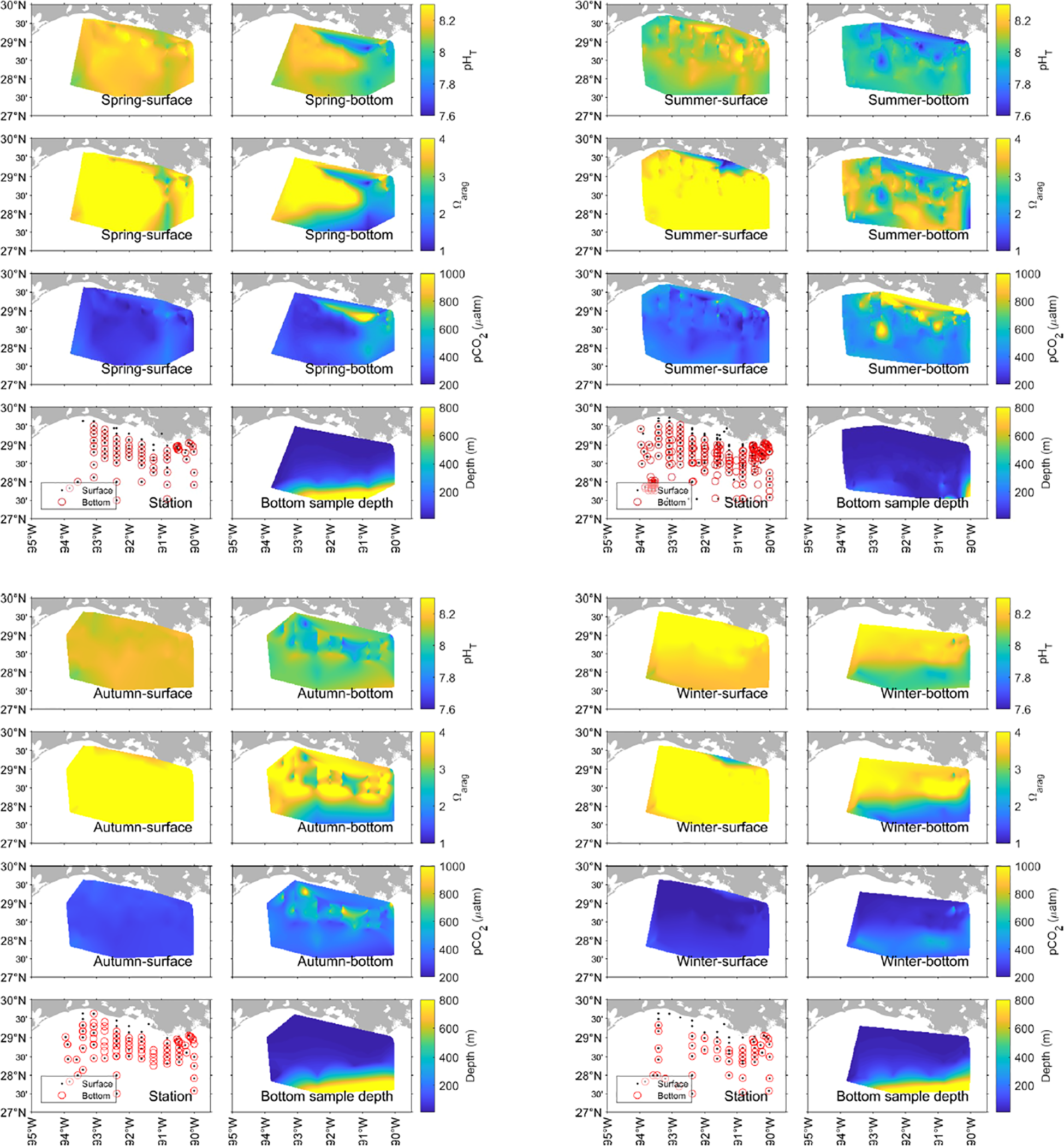
Seasonal arithmetic means of pH, Ωarag, and pCO2 in the surface and bottom layers along the Louisiana coast. The surface layer refers to the shallowest depth recorded at each station (<10 m), and the bottom layer is the deepest sampling depth, with a minimum depth of 10 m, across the studied area.
Figure 5
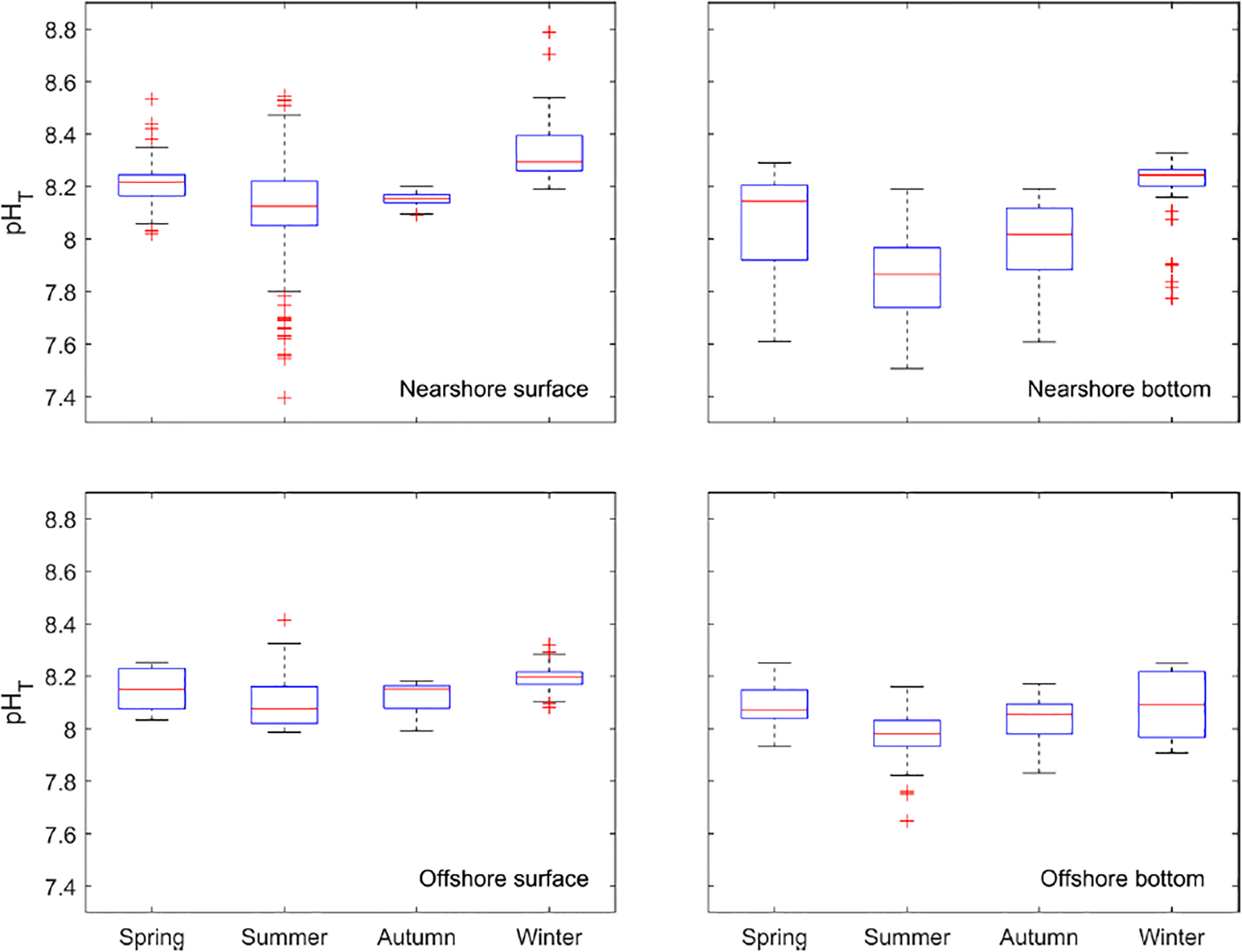
Boxplots of pH across different seasons at nearshore and offshore locations (nearshore locations = north of 28.5°N; offshore locations = south of 28.5°N).
Surface water Ωarag was generally higher than that of bottom water in each corresponding season (Figures 4, 6), even though spatial heterogeneity in Ωarag was observed across seasons and locations. Surface water Ωarag was more homogeneously distributed in autumn and winter (Figure 4), although low Ωarag (~3.5 in autumn and ~2 in winter) regions were observed in the nearshore Mississippi River mouth, suggesting the influence from terrestrial input (Figure 4). In the subset data analysis, similar to pH data, more extreme Ωarag values (outliers in the boxplot) were observed in the nearshore surface water subset. In the nearshore surface layer, Ωarag generally exhibited larger variability (up to 10 across seasons) than that of the offshore surface layer, but in the bottom layer, Ωarag ranged up to 5 regardless of season and location (Figures 6).
Figure 6
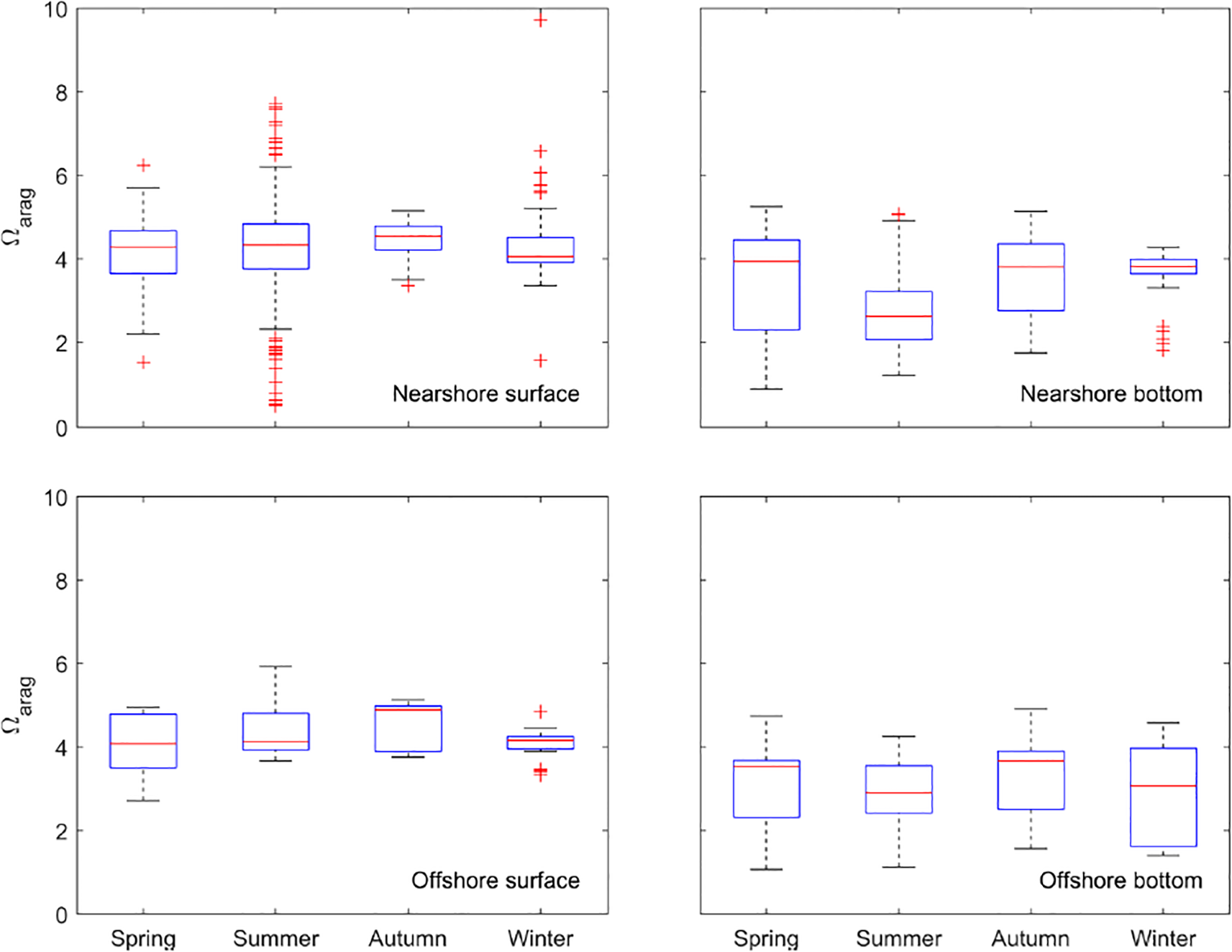
Boxplots of Ωarag across different seasons at nearshore and offshore locations (nearshore locations = north of 28.5°N; offshore locations = south of 28.5°N).
The spatial and seasonal distributions of pCO2 confirmed its negative relationship with pH (Figure 3, 4). Surface water pCO2 was generally lower than bottom-water values, with this vertical contrast being more pronounced in nearshore regions (Figure 4 and 7). Episodes of extreme pCO2 (>1000 µatm) were most frequently observed in nearshore waters, particularly in the summer surface layer. Consistent with the pH patterns, nearshore regions exhibited stronger variability in pCO2 relative to offshore waters, except for the surface layer in autumn (Figure 7). Collectively, these results emphasize the substantial spatiotemporal variability of carbonate chemistry in the northwestern Gulf of Mexico and highlight the dynamic nature of nearshore environments.
Figure 7
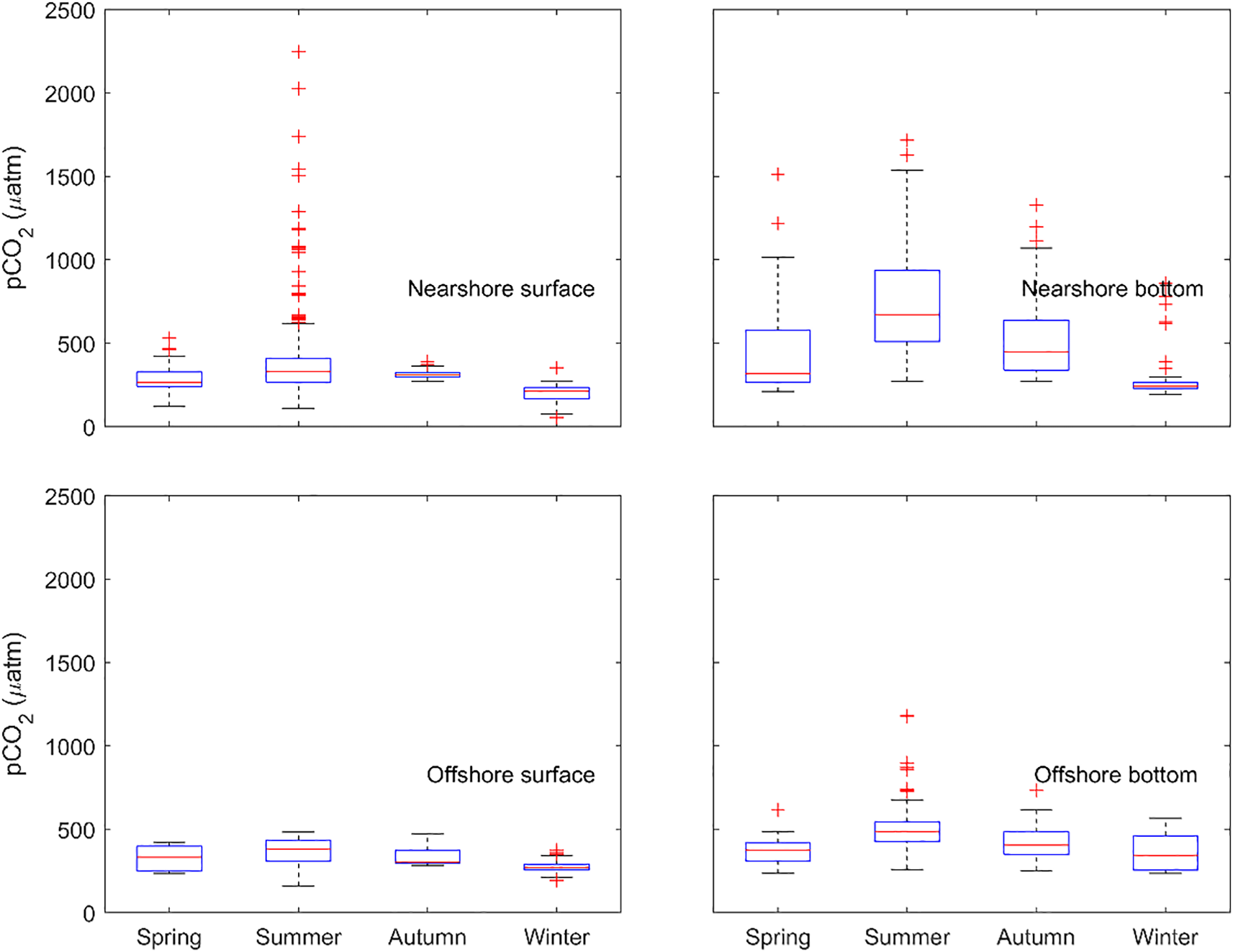
Boxplots of pCO2 across different seasons at nearshore and offshore locations (nearshore locations = north of 28.5°N; offshore locations = south of 28.5°N).
Collectively, these results emphasize the substantial spatiotemporal variability of carbonate chemistry in the northwestern Gulf of Mexico and highlight the dynamic nature of nearshore environments. Interannual variability in carbonate chemistry was not assessed in this review paper due to limited spatial coverage of the data (sampling stations differed among cruises and the shelf region exhibits large spatial heterogeneity) and the relatively short time span available for long-term analysis. Instead, based on more comprehensive underway pCO2 data, Kealoha et al. (2020) demonstrated that surface water pCO2 in the nwGoM has been increasing at a rate of approximately 3 µatm per year, primarily driven by the uptake of anthropogenically produced atmospheric CO2. Considering this trend, marine organisms are expected to experience the effects of ocean acidification in the near future, if they are not already doing so. The response of organisms to OA are reviewed below.
Vulnerable species survey
Fourteen classes and 35 species were identified in experimental conditions of CO2 enrichment (Table 2). Among these were vertebrates belonging to the classes Actinopterygii and Chondrichthyes, along with invertebrates such as Malacostraca, Copepoda, Cirrepedia, Bivalvia, Cephalopoda, Gastropoda, Polyplacophora, Scyphozoa, and Scleractinia. Rhodophycea, Florideophyceae, and Bacillariophyceae were also included. Biological responses ranged from calcification, survival (larval & adult), growth, settlement, and gut microbial communities (Table 2). Exposures to elevated CO2 level were also highly variable. Most attempted to provide an effect curve from future predictions of oceanic CO2 conditions resulting from climate change scenarios detailed in the Intergovernmental Panel on Climate Change (IPCC, 2013) report. This effect curve also extended much further than these predictions and simulated extreme values (+2000 µatm)—with one study reaching levels shown at hydrothermal vents (231,280 µatm). Exposure times also ranged from the lowest 0.2 days to the highest at 187 days (Table 2).
Of the taxa reported, 68.5% showed a negative directionality response to increased pCO2, whereas 31.4% showed a neutral or mixed neutral response (positive or negative). Only 11.4% of reported taxa showed a positive response to elevated pCO2. The taxa here only represent 1.0% of the total we recorded in the nwGoM region, and inconsistencies in experimental methods, pCO2 levels, and exposure times may be challenging for one-to-one comparisons. Also, these taxa are present in the nwGoM, but most studies in this report were done elsewhere, which could potentially introduce bias with regionally derived tolerances. We recommend a survey driven toward manipulation experiments under relevant controlled pCO2 and exposure times to these taxa regionally sourced from the nwGoM.
Coral reef builders
Rhodophyta or red algae are distinctly red due to phycoerythrin pigments–allowing for greater photosynthetic capacities in deeper waters (Fredericq et al., 2009). Red algae play a crucial role in reef cementation, accretion, and sedimentation, providing a settlement substrate for sessile invertebrates, including coral larvae (McCoy and Kamenos, 2015; McNicholl et al., 2020; Nelson, 2009). Elevated levels of pCO2 in seawater have been observed to directly affect certain rhodophytes or calcifying reef algae species, increasing the energetic costs associated with calcification. However, McNicholl et al. (2020) examined Jania adhaerens and found that daytime calcification was less impacted compared to nighttime dissolution, where dissolution was more pronounced in a high pCO2 (1881-1524 µatm) environment (Table 2). Indirectly, the impacts of elevated pCO2 have also been observed to increase environmental competition, with macroalgae outcompeting crustose coralline algae species for space (McCoy and Kamenos, 2015; Nelson, 2009).
Florideophyceae is a class of red algae containing most species found within the phylum Rhodophyta and is essential in both photic and benthic marine zones. Like the class described above, calcifying Florideophyceae provides a settlement substrate for benthic marine organisms (De Carvalho et al., 2022). Shown in Neosiphonia harveyi species, lower temperatures and elevated pCO2 (800-1500 µatm) positively impacted the coralline algae by increasing and supporting photosynthetic processes and growth rates (Olischläger and Wiencke, 2013). Porzio et al. (2011) showed that many species within Florideophyceae continue to photosynthesize and grow in conditions of increased pCO2 (pH 7.8). Approximately 95% of the algal species studied in the examination by Porzio et al. (2011) could tolerate and survive in conditions of 7.8 pH (Table 2). However, other work show elevated pCO2 acting in reducing tissue growth (McCoy and Kamenos, 2015). The Hydrolithon spp. will respond negatively to increased pCO2 (900 ppm) which has been shown to exhibit reduced calcification (Semesi et al., 2009).
The FGB sanctuary in the nwGoM is home to 24 specific species of Scleractinia corals, contributing to the rich coral diversity in the area. The coral cover within the West and East FGB has been noted as some of the most pristine in the region, with approximately 50% to 80% total coverage (Garavelli et al., 2018; Hickerson et al., 2012; Johnston et al., 2016). Coral calcification facilitates benthic complexity, supporting marine biodiversity and building large reef structures, but these communities could be threatened by ocean acidification with reduced calcification (Jokiel et al., 2014; Page et al., 2017; DeCarlo et al., 2017). Also, coral’s biphasic life history strategies present challenges in assessing tolerances due to fundamentally different physiologies dependent on life stage (Gleason and Hofmann, 2011).
Studies have examined the effects of pCO2 increases on carbonate-based organisms, including corals. Albright and Langdon (2011) found that increased pCO2 (560 & 800 µatm) significantly impacted larval metabolism, settlement, and post-larval growth in Porites astreoides (Table 2). Madracis mirabilis, forms dense clusters and provides shelter for marine microfauna and flora (Jury et al., 2010). In a study conducted by Jury et al. (2010), researchers demonstrated that Madracis auretenra (mirabilis) exhibited varying responses to ocean chemistries associated with increased pCO2 (1480 µatm), showing a nonlinear relationship between chemistry and resistance levels that is not yet fully understood. Additionally, Albright et al. (2010) observed a significant reduction in fertilization, larval settlement, and growth of Acropora palmata caused by increased pCO2 (560-800 µatm; Table 2), further emphasizing the potential negative impacts of increased pCO2 on coral reproduction and development.
Benthic invertebrates
Polyplacophorans or chitons are marine grazers characterized by a dorsal shell composed of eight articulating plates formed with aragonite (Sigwart and Carey, 2014). Increased environmental pCO2 could impact marine grazers by increased metabolism and subsequent feeding (Burnell et al., 2013; Sigwart and Carey, 2014). The exclusive formation of chiton shells from aragonite renders them susceptible to the effects of hypercapnia (i.e., increases in environmental pCO2; Sigwart and Carey, 2014). A study conducted by Sigwart et al. (2015) found no change in the fracture strength required to break chiton shells under hypercapnia (2000 µatm; Table 2). Suggesting that chitons may possess specific attributes that reduce or mitigate the effects of increased pCO2, even with predominantly aragonitic shell composition.
The class Gastropoda, is comprised of a diverse group of marine mollusks, and exhibit varying responses to hypercapnic scenarios. The genus Aplysia is a sea hare that is found in benthic habitats in the nwGoM (Moroz, 2011). Most sea hares possess an internal shell that protect internal organs composed primarily of aragonite (Carey et al., 2016). In a study by Carey et al. (2016), it was found that the cosmopolitan sea hare species Aplysia puncata could maintain normal calcification rates under increased pCO2 (2800 µatm), although their metabolic rates were significantly impacted. Studies on species within the genus Haliotis (abalone) by Auzoux-Bordenave et al. (2019) demonstrated that raised pCO2 environments (740-1400 µatm) significantly impacted shell growth and mineralization (Table 2). Furthermore, larval shell responses of the slipper limpet Crepidula fornicate were examined by Noisette et al. (2014), revealing an overall reduction in shell length under increased pCO2 conditions (1400 µatm; Table 2; Bogan et al., 2019; Kriefall et al., 2018; Reyes-Giler et al., 2021).
Bivalves are essential animals in marine food webs and contribute to benthic biodiversity. Typically, bivalves synthesize their shells out of calcite, a polymorph of calcium carbonate. These calcification processes, which are of significant concern in several other taxa (as discussed i.e., corals, snails, limpets, and chitons), could be affected by increased pCO2. Marine clams belonging to the genus Macoma were examined for their susceptibility to increased pCO2. The life history strategies of this species were impacted by elevated pCO2 at 1454 μatm (Van Colen et al., 2012), and it was found that pCO2 at > 500 μatm (600–1650 μatm) impacted larval settlement as well (Jansson et al., 2015). Crassostrea virginica, or the eastern oyster, shows variable responses to increased pCO2 (800-3500 μatm), some showing regulatory compensation and others showing decreased calcification (Beniash et al., 2010; Miller et al., 2020; Waldbusser et al., 2011). At levels ~3500 μatm, Beniash et al. (2010) reported decreased calcification, juvenile mortality, and soft body growth. Larval growth was slowed substantially in pCO2 manipulations at ~800 μatm, as Miller et al. (2020) showed. Also, pH manipulation by CO2 bubbling significantly decreased calcification with a reduction of ~0.5 units from Waldbusser et al. (2011).
The bivalve Pinctada spp. was examined, and significant upregulation of acid-based control in lower pH environments (7.5 & 7.8) resulted in higher metabolic demand (Li et al., 2016). Another study found weaker shells and deformations in outer growth patterns due to lower pH (7.8 & 7.6; Welladsen, 2010). A separate analysis found significant differences in calcium content and organization of nacre caused by decreased pH (7.4; Liu et al., 2017). Two studies examining Argopecten irradians found significantly reduced larval survival at increased [CO2] (650 ppm and 1987 μatm respectively; Talmage and Gobler, 2009; White et al., 2013). The reduction in larval survival was significant after one day of exposure (1987 μatm), showing pulse vulnerability to increased pCO2 (White et al., 2013).
The genus Spisula was investigated, and researchers found increased energy costs due to increased pCO2 exposure (2163 μatm; Pousse et al., 2020). The surf clam larvae showed decreased growth rates in high pCO2 of 1243 µatm but resistance to moderate levels of 821 µatm (Meseck et al., 2021). In Mercenaria spp., larvae exhibited extreme mortality and delayed growth due to increased concentration of CO2 ([CO2]) conditions (650 ppm; Talmage and Gobler, 2009). However, some studies suggest these species are relatively tolerant to moderate pCO2 levels of ~800 µatm (Dickinson et al., 2013; Matoo et al., 2013). The genus Panopea spp. larvae were investigated, and proteomic shifts were found due to exposure to decreased pH (7.1). Metabolism, cell cycle, and protein turnover rates were all impacted by this decreased pH level (Timmins-Schiffman et al., 2020).
Barnacles are ecologically important contributing significantly to marine food webs and intertidal coastal communities. Larvae are generally planktonic and are exposed to pelagic open water before being distributed intertidally via ocean currents. Amphibalanus spp., a common barnacle species, has been examined in several studies in the past decade (Eriander et al., 2016; McDonald et al., 2009; Nardone et al., 2018; Pansch et al., 2013). Increased pCO2 ranging from 970 to1600 µatm were found to have no effects on this barnacle genus’s larval or adult stages (Table 2). However, some explored the variability of these responses and showed variations between individuals (Pansch et al., 2013). There is also an increasing need for research in this field as there are very few experiments on barnacles exposed to elevated pCO2 (Eriander et al., 2016).
The phylum Echinodermata has 512 species in the nwGoM; Asteroidea comprises around 7% of the global echinoderm diversity (Felder and Camp, 2009; Table 3). Echinoderms play a significant role in marine food webs due to their grazing habits and interactions with other species. Their skeletons are composed of magnesium-rich calcite, particularly vulnerable to increased pCO2 (Schram et al., 2011). In the sea star Luidia clathrata, a common species in the nwGoM, decreased pH and increased pCO2 (780 µatm) had little to no effect on the species’ regenerative capacity or chemical constitution (Schram et al., 2011). However, various studies observed that increased temperature significantly impacted Asteroidea species more than reduced pH or increased pCO2 did on physiological responses (Carey et al., 2016; Curtis et al., 2021; Parajuli, 2023).
Table 3
| Species/Genus | Global Distribution |
|---|---|
| J. adhaerens | Fl to NC, Pac Mex, CA, Carib, GMx, Eur, Atl Isls, Brazil, Ecuador, Afr, Indian Oc Isls, SW & SE Asia, China, Japan, Korea, Taiwan, N & W Aust, trop Pac Isls |
| Neosiphonia spp. | Carib, GMx, CA, N Afr, Mauritius, Philippines, S Eur, Atl Isls, Chile, W & N Afr, Maldives, N & W Aust, trop Pac Isls, Vietnam, Fl to NC, Uruguay |
| Hydrolithon spp. | FL to VA, Carib, GMx, W Afr, Japan, Eur, Atl Isls, Brazil, Afr, Chile, SW & SE Asia, China, Russia, New Zeal, Aust, trop Pac Isls, S Aust |
| Coscinodiscus spp. | Cos |
| T. pseudonana | Cos |
| M. beryllina | MA to GoM |
| S. ocellatus | MA to GoM |
| L. erinacea | Fla Keys, E & SW GoM, Cuba |
| Scyliorhinus spp. | NC to S FL, Massachusetts to Nicaragua, Bahamas, Cuba |
| L. clathrata | VA to Brazil, Bermuda |
| Menippe spp. | GoM, NW FL-Tams Mx |
| Clibanarius spp. | VA to GoM, Brazil |
| Portunus spp. | Ber, NC to GoM, Antil to Brazil, ASI, S Carib to Brazil, MA to GoM, N Scotia to GoM, nS Am, mid to E Atl, Cuba to S Antil, Antil to nS, Am & Brazil, NJ to GoM, GA to GoM |
| C. sapidus | Ber, N Scotia to Arg, E Atl, Med, Japan |
| P. crassirostris | Atlantic, Pacific, Indian |
| Calanus spp. | FL Keys, GoM, RI to Brazil, Antil, NC to N Yucatan |
| Acartia sp. | Atl, Pac, Indian |
| Amphibalanus spp. | Cos in Trop & warm Temp, N Temp Atl, W Temp Atl |
| Panopea spp. | NC to TX |
| Mercenaria spp. | NJ to QR |
| Spisula sp. | NC to TX |
| A. irradians | TX to Mex, Colombia |
| Pinctada spp. | SC to QR, SE GoM, WI, Brazil, Ber |
| C. virginica | GS to YU, Cuba, WI |
| Macoma spp. | GoM, Cuba, PR, JM, Ber, AL to TX, Brazil, GP, FL Keys, DT, QR, GE, MA to CB, WI |
| D. pealeii | W Atl, N Scotia to VE, GoM, Carib |
| C. fornicata | N Scotia to TX, E Atlantic |
| Haliotis spp. | NC to VE |
| Tegula spp. | E FL, to Brazil |
| Aplysia spp. | SC to Brazil, Ber to Brazil, MA to Brazil, RI to TX |
| Leptochiton spp. | GMx, W, S Carib |
| A. aurita | Cos |
| A. palmata | SE Fl, Ba, Carib |
| Madracis spp. | E Atl, Carib, ESA, Ber, SE Fl, Ba |
| P. astreoides | E Atl, Ber, SE Fl, Ba, Carib, ESA |
The global distribution of species or genera identified, see footnotes for information on abbreviations used.
*All species present in this table used information from Felder and Camp (2009).
Geographic descriptors: Afr, Africa; Aust, Australia; Arg, Argentina; Antil, Antilles; ASI, Ascension Island; Atl, Atlantic; AL, Alabama; Ber, Bermuda; Ba, Bahamas; Carib, Caribbean Sea; CA, California; CB, Campeche Bank; Cos, Cosmopolitan; DE, Dry Tortugas; E, East; Eur, Europe; Fl, Atlantic Florida; Fla Keys, Florida Keys; GA, Georgia; GMx, Gulf of Mexico; GS, Gulf of St. Laurence; GP, Galapagos Islands; GE, Greater Antilles; Isls, Islands; JM, Jamaica; MA, Massachusetts; Med, Mediterranean Mex, Mexico; NJ, New Jersey; NC, North Carolina; N, North; N Scotia, Nova Scotia; nS Am, Northen South America; Oc, Ocean; Pac, Pacific; PR, Puerto Rico; QR, Quintana Roo; RI, Rhode Island; ESA, Eastern South America SE, Southeast; S, South; SC, South Carolina; trop, Tropical; temp, Temperate; Tams Mx, Tamaulipas Mexico; TX, Texas; VA, Vriginia; VE, Venzuela; W, West; West Indies; YU, Yucatan.
The blue crab Callinectes sapidus has been subjected to many experimental trials regarding the increase in pCO2 due to its abundance and ecological/economic importance (Giltz and Taylor, 2017; Longmire et al., 2022). Researchers have found decreased claw pinch force (pH 7.0) and decreased larval survival (pH 7.8) due to exposure to increased pCO2 (Giltz and Taylor, 2017; Longmire et al., 2022). One study on the Portunus spp. looked at biomarkers following acute exposure (24 hrs) to extreme levels of pCO2 like those found at hydrothermal vents, and found all pCO2 ranges above normal levels to be stressful environments (2203-231287 µatm), with complete mortality in 72 hours when pH was less than 5.5 (Jeeva Priya et al., 2017), although this condition is not expected for the nwGoM coastal waters.
Smaller crustaceans like the hermit crab Clibanarius spp. have been shown to decrease larval survival due to exposure to acidified seawater through in situ measurements of pH swings (Tomatsuri and Kon, 2019). The commercially viable stone crab Menippe spp. has also been examined when exposed to increased pCO2 (596 µatm), and researchers found geotactic differences in larval swimming patterns due to decreased pH, which could be detrimental to overall distribution and larval supply (Gravinese et al., 2019). Lower seawater pH (7.5) also showed delayed larval embryonic development in Menippe spp. by over 24% of the original time (Gravinese, 2018).
Planktonic primary producers and consumers
Diatoms are ecologically significant primary producers contributing nearly 25% to the world’s carbon fixation rate (Felder and Camp, 2009). Marine diatoms support higher trophic levels and represent the base of marine food webs (Qu et al., 2018). Sobrino et al. (2014) found that elevated pCO2 (1050 ppm) decreased multiple physiological mechanisms such as esterase (metabolic) activity, radical oxidative stress (ROS), cell death, and DNA damage (Table 2). This study also noted that high pCO2 conditions increase phytoplankton sensitivity to solar irradiance, carbon fixation, and photosynthesis rate. These changes in physiological responses indicate that elevated pCO2 affects the photosynthetic structure’s downregulation and carbon concentrating mechanisms (CCMs) in marine diatoms (Sobrino et al., 2014).
Contrary to the findings of Sobrino et al. (2014), other studies have found that elevated pCO2 (1050 ppm) does not play a significant role in diatom composition, cell size, or physiological function (Table 2). Qu et al. (2018) found that instead of CO2 concentration, temperature and nitrate availability played more significant roles in the growth, carbon export, and physiology of larger diatoms such as Coscinodiscus spp. In experimental groups that combined elevated pCO2 (400 & 800 µatm) and warmer temperatures, carbon fixation rates were enhanced, suggesting that OA may provide negative feedback to increasing atmospheric CO2 concentrations (Qu et al., 2018). For experimental treatments that combined elevated pCO2 with optimal temperatures of 30°C and higher, it was found that the increased pCO2 (800-1400 µatm) depressed growth rates in the diatom species Thalassiosira pseudonana (Laws et al., 2020). In addition, Goldman et al. (2017) mention that enriched pCO2 effects on diatom photosynthesis, growth, and respiration will likely be minor for the end-of-the-century predictions of pCO2 concentrations ranging from 800-1400 µatm.
Marine copepods are primary consumers and play an integral role in marine food webs (Habibi et al., 2023; Wang et al., 2018). Almost 70% of the ocean’s metazoan biomass is allocated to marine copepods and zooplankton (Habibi et al., 2023). Copepods are challenging to assess because of stage-dependent and species-dependent responses to increased pCO2 (Wang et al., 2018). A species of zooplankton Acartia located in the nwGoM was tested in mesocosm experiments to identify plasticity across generations and found that female size decreased with a possible threshold of pCO2 (884 – 1413 µatm) for these species (Vehmaa et al., 2016). Calanus spp. were tested with low to high pCO2 (320-1700 µatm) levels, and there were no effects on developmental rate, dry weight, C and N mass, and oxygen consumption rate, indicating possible tolerance (Bailey et al., 2017). Some conflicting research has shown population differences in physiological tolerances for Calanus spp (Thor et al., 2018). Transcriptomic analysis of differentially expressed genes revealed in Parvocalanus crassirostris that decreased pH (7.9-7.7) affected several biological pathways related to cellular processes (Habibi et al., 2023). In this study, an increase in pCO2 resulted in 17 upregulated and 31 downregulated physiological pathways, suggesting physiological stress responses to increased pCO2 (Habibi et al., 2023).
Nektonic grazers and consumers
Aurelia aurita, a well-studied species of Scyphozoan, is found worldwide in various environments (Algueró-Muñiz et al., 2016; Baumann and Schernewski, 2012; Falkenhaug, 2014; Luparello et al., 2020). This species response to pCO2 increases (800-4000 µatm) has been well studied, with findings suggesting both larval and adult stages of Aurelia spp. exhibit some level of resistance to acidification (Algueró-Muñiz et al., 2016; Hall-Spencer and Allen, 2015; Winans and Purcell, 2010). However, Winans and Purcell (2010) observed a decrease in statolith sizes in adult medusae of Aurelia spp. under low pH (7.9-7.2) conditions. This reduction in statolith size could potentially impact the ability of adult medusae to orient themselves in the water column. It is debated whether Scyphozoans, including Aurelia spp., will benefit from increased pCO2 by exploiting open niches and potentially becoming top trophic predators (Richardson and Gibbons, 2008).
Cephalopods are essential for marine ecosystems as both predator and prey of several taxa. Cephalopods act as prey sources for many commercially important fish species like red drum while supporting fisheries directly being fished by local fishermen for food or bait (Kaplan et al., 2013). Recent investigations concerning the cephalopod species Doryteuthis pealeii, commonly found along the nwGoM reef shelf, have revealed the detrimental impacts of elevated pCO2 (2200 µatm) on larvae rearing (Kaplan et al., 2013). Kaplan et al. (2013) evaluated various biological responses, such as the time duration to hatching, aragonite statolith sizes, and overall body size in this species, and found negative developmental/physiological responses to CO2 enrichment (2200 µatm). Additional studies analyzed the disparities observed in the egg-laying behavior of D. pealeii, which primarily occurred around a depth of 50 m (Zakroff et al., 2019), emphasizing the importance of understanding taxa-specific reproductive ecology and vulnerability linked to [CO2] concentrations (1300 ppm; Table 2). The effects on larvae were contingent upon the clutch and were typically only observed when [CO2] surpassed 2200 ppm (Zakroff and Mooney, 2020).
Fishes
Chondrichthyes are significant in almost all marine ecosystems and maintain ecosystem balance through predation. Elasmobranchs within Chondrichthyes have effective acid-base buffering capacities, which may allow them to have higher tolerances to elevated pCO2 levels (Rummer et al., 2020). In near-future elevated pCO2 conditions, shark embryo development was largely unaffected, although there were clear adverse effects on behavioral lateralization (turning left or right), hunting ability, growth, aerobic potential, and prey detection (Rosa et al., 2017). Odor tracking, swimming ability, hunting behavior, and other similar abilities were also negatively impacted by increased pCO2 (~1000 µatm) and a decrease or total loss of lateralization (Zemah-Shamir et al., 2022). Contrary to these findings, Green and Jutfelt (2014) found that the small-spotted catshark, Scyliorhinus canicula, did not have adverse effects in growth, aerobic scope, or metabolic rate when exposed to 990 µatm pCO2 for four weeks (Table 2). This species displayed increased lateralization when exposed to pCO2 rather than decreased or complete loss, as shown in other studies (Green and Jutfelt, 2014). An increase in lateralization is not necessarily a positive effect, though, as most changes from the “natural” state of fish physiology likely mean it is compensation for other adverse effects it is experiencing.
Similar to the findings of Zemah-Shamir et al. (2022) and Rosa et al. (2017); Pistevos et al. (2015) found that elevated pCO2 and temperature had adverse, synergistic effects on metabolic efficiency, growth rates, and olfactory mechanisms in sharks. A reduction in the olfaction mechanisms in sharks can result in various adverse consequences, as smell is the primary sense that sharks utilize to find prey. Di Santo (2019) conducted a study that exposed Leucoraja erinacea embryos to an increased temperature of 20°C and pCO2 conditions of 1100 µatm (Table 2). This research showed that in the elevated pCO2 conditions, mineralization increased in the jaws and cartilage of the crura in L. erinacea (Di Santo, 2019). Proper mineralization in elasmobranchs is essential because it can impact feeding and swimming abilities, directly affecting several skeletal elements’ strength and stiffness (Di Santo, 2019). Additionally, the stress that resulted from high-temperature conditions on the development and formation of embryos was intensified by elevated pCO2 (Di Santo, 2019; Rosa et al., 2017).
The class Actinopterygii within the phylum Vertebrata consists of ray-finned bony fishes that comprise a large part of marine food webs and are crucial to the health and maintenance of marine ecosystems. As predator and prey, bony fishes contribute to ecosystem balance with bottom-up or top-down control of the abundance of organisms in the marine environment. It was documented that increased pCO2 will likely have impacts on behavioral and sensory mechanisms in Actinopterygii as well as potentially interfere with the metabolic rate in marine fishes (Wang et al., 2022). It has been shown that elevated [CO2] (~1000 ppm) can increase reproduction in fish through indirect effects on fish reproductive tissues (Nagelkerken et al., 2021). Other indirect effects from increased [CO2] (~1000 ppm) were observed, such as the alteration of territory defenses shown by mature males, investment into gonads in both males and females and the altering of the abundance of parental males in areas with naturally increased [CO2] levels (Nagelkerken et al., 2021).
A study published by Cattano et al. (2020) found that elevated pCO2 (402 to 952 µatm) can cause shifts in benthic habitats, significantly impacting fish communities (Table 2). A reduction in benthic complexity (structural rugosity) in coral reefs was observed in reefs subjected to these increased pCO2, resulting in a diminution of corresponding food webs and habitats (Cattano et al., 2020). The simplification of marine habitats due to increased pCO2 may adversely affect fish communities by forcing them to relocate or risk their survival in a changing ecosystem. As discussed above, the increased impact of pCO2 on marine benthic organisms could significantly affect indirectly reliant species.
Several studies have noted that increased pCO2 (ranging from 600 to 1900 µatm) directly affects various fish species stress response, behavior, metabolic rate, and neurosensory mechanisms (Esbaugh, 2018; Heuer and Grosell, 2014; Servili et al., 2023). The alteration of any of these physiological processes can decrease fitness, reducing species population abundance in the long term. Studies have shown that otolith growth has increased in many species of marine fish exposed to high pCO2 conditions (reviewed by: Heuer and Grosell, 2014; ~1600 µatm; Kwan and Tresguerres, 2022). Otoliths are calcium carbonate structures found in the inner ear of teleost fishes that aid in sensory detection and balance, and the overgrowth of these concretions can lead to hearing impairments, balance issues, and reduced survival success (Kwan and Tresguerres, 2022). The increase in otolith growth is believed to stem from hypercapnia in fish, which can result in increased CO32- levels in the endolymph that may lead to enlarged otoliths (Kwan and Tresguerres, 2022).
Enriched seawater CO2 effects can also lead to disturbances in the acid-base regulation in marine fish. Maintaining a stable pH in the blood, intracellular, and extracellular fluids is critical for maintaining homeostasis and proper physiological functions in marine vertebrates. Acid-base regulation is done mainly through the respiratory and circulatory systems in fish, and an internal increase in pCO2 due to externally elevated pCO2 levels (from 600 to 1900 µatm) can lead to the acidification of internal fluids (Heuer and Grosell, 2014). To combat the fluctuation in pH and pCO2 levels internally, an increase in HCO3- in the blood plasma can help buffer additional acidity that is present and help maintain homeostasis (Brauner and Baker, 2009; Heuer and Grosell, 2014). Even with this acid-base regulation, fish exposed to elevated pCO2 (from 600 to 1900 µatm) still show increased HCO3- and pCO2 in their extracellular fluids, which can lead to various complications in physiological processes (Heuer and Grosell, 2014).
Sciaenops ocellatus is an extremely valuable species in the nwGoM ecologically, recreationally, and commercially. At pCO2 levels of 1000 µatm in S. ocellatus was shown to result in consistent hyperventilation that can alter the acid-base regulation functionality and lead to a reduction in plasma HCO3- retention requirement by nearly 40% (Esbaugh, 2018). This reduction in necessary HCO3- retention means that S. ocellatus could effectively remove some excess pCO2 from the body via the respiratory system. However, this energy allocation to hyperventilation likely means a tradeoff is occurring downstream for another physiological process (Esbaugh, 2018). Furthermore, Menidia beryllina, a significant food source for many larger fish in the nwGoM, showed a 73% reduction in 10-day survival after exposure to 780 µatm of pCO2 (Esbaugh, 2018). In larvae reared at 1800-4200 µatm pCO2, wide-spread tissue damage and other developmental delays were seen (Esbaugh, 2018). The species-specific studies showed differences in survival rates and maintenance of physiological mechanisms between species, life stages, and individuals within the same species.
Marine parasites Trematoda, Cestoda, and Dicyemida
According to a report from 2007, the GoM is home to nearly 1000 species of parasitic organisms belonging to Trematoda, Cestoda, and Dicyemida (Felder and Camp, 2009). Trematodes have approximately 3575 potential host species, including Fishes, Aves, Reptiles, Mammals, and Marine Mammals. Conversely, Cestodes have roughly 9774 likely host species, including Chondrichthyes, Actinopterygii, Marine Mammals, Bivalves, Gastropods, and Scyphozoans.
It is worth noting that parasites and relationships with pCO2 increases have been highly understudied, with no identified articles linking the two before 2011 (Poulin et al., 2016). This knowledge gap presents an intriguing area of interest for marine ecologists, as the effects of increased pCO2 on host-parasite interactions remain largely unknown (MacLeod and Poulin, 2012; Poulin et al., 2016). The infectivity rates of parasites and their responses to decreases in oceanic pH levels are also widely unknown.
Synthesis of response
From our survey of 3,480 locationally relevant species, only 35 (1.0%) had published data on responses to elevated pCO2. Of these, 68.5% showed negative physiological or developmental responses, 31.4% exhibited neutral or mixed responses, and just 11.4% responded positively (Figure 8). Interspecific interactions may be disrupted substantially by the decline of ecologically important taxonomic groups shown here (Figure 8). Moreover, 83% of species shown here occupy the top 20 m of the water column, placing them within the depth range of the highest seasonal variability we observed. These findings suggest that many nwGoM taxa may already experience several events of geochemical extremes annually—patterns that are largely absent from global-scale projections.
Figure 8
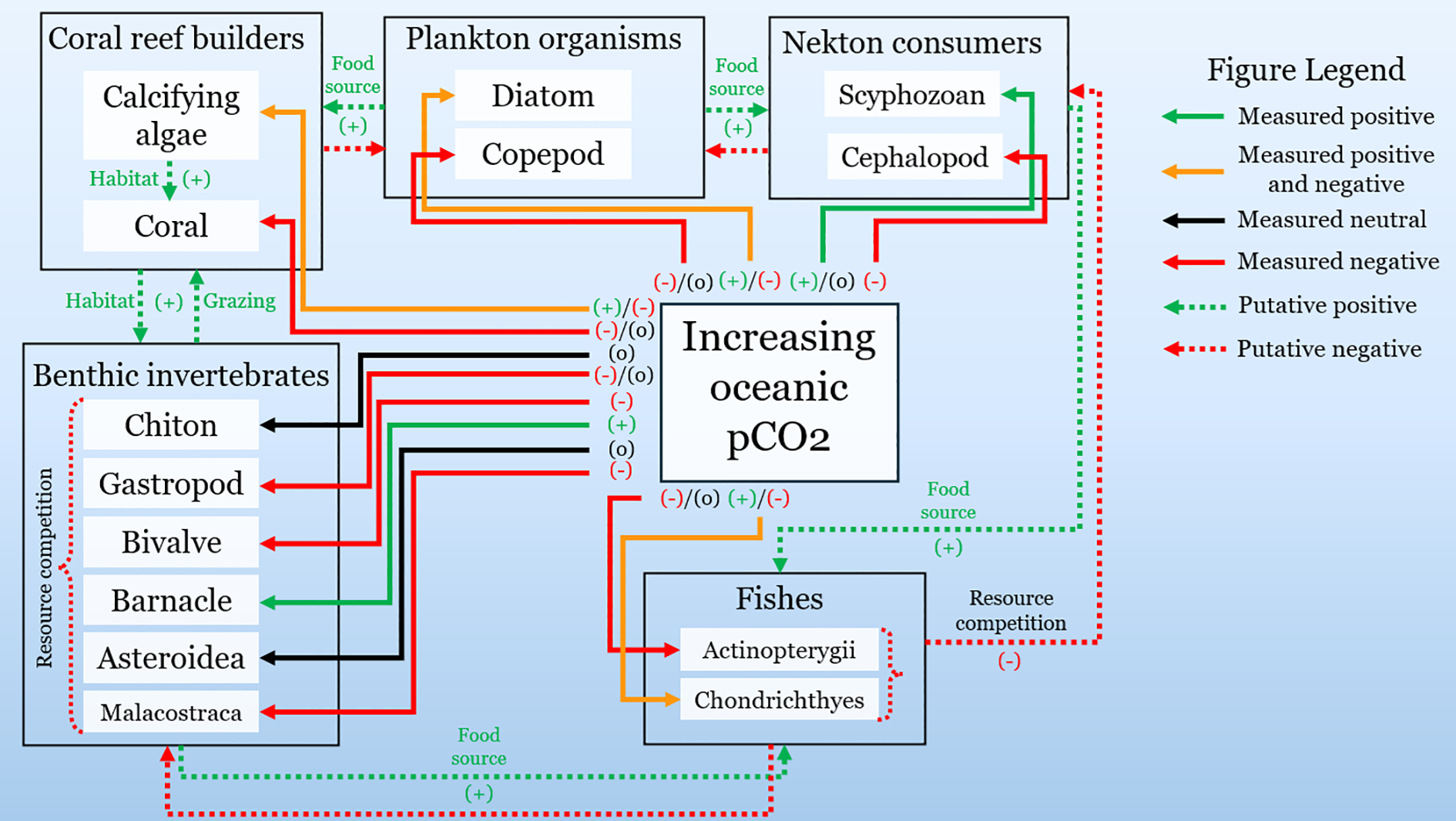
Conceptual model of taxonomic group responses to increased oceanic pCO2. Arrow color represents a specific response directionality, and line type (solid or dashed) represents direct measures or conceptual (putative) predictions.
Conclusions
This study establishes the first integrated carbonate chemistry baseline for the nwGoM, synthesizing data from research cruises spanning from 2006 to 2019. Our analysis showed greater spatial and seasonal variability in pH, pCO2, and Ωarag within the upper 20 m of the water column, with nearshore waters showing more extreme and variable conditions than offshore sites. Stratification-driven benthic remineralization further reduced pH and Ωarag as well as elevated pCO2 in bottom waters during productive summer months. These patterns highlight that nwGoM carbonate chemistry is shaped by local biogeochemical and hydrological drivers that differ substantially from open-ocean trends, underscoring the need for region-specific baselines in vulnerability assessments.
This work subsequently builds from Osborne et al. (2022), with respect of an analysis of carbonate chemistry and reporting of known species responses. Our findings coincide with responses shown by Osborne et al. (2022) of oysters, bay scallops, hard clams, and corals. By highlighting the nwGoM region we focused on specific biodiversity hotspots like the FGBNMS. Further work should continue to divide the GoM, and alongside Osborne et al., 2022, determine the holistic response this region may have to elevated CO2.
By linking detailed carbonate chemistry baselines with species-specific sensitivity data, this work provides a framework for integrating the nwGoM into the global mosaic of ocean acidification responses. Furthermore, our findings suggest that the vast majority of nwGoM species remain unstudied under elevated pCO2, yet most tested taxa already show negative responses—often within the shallow depth range experiencing the greatest seasonal extremes. Without region-specific experiments, current global projections risk underestimating vulnerability in this high-value ecosystem. Ground truthing and filling the gaps discussed here is essential to safeguard the biodiversity, fisheries, and multiple reef habitats that support the ecological and economic health of the nwGoM.
Statements
Author contributions
DA: Investigation, Data curation, Methodology, Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization, Visualization. HY: Formal analysis, Writing – original draft, Writing – review & editing, Methodology, Visualization, Data curation, Conceptualization, Investigation. AH: Writing – original draft, Investigation. XH: Resources, Supervision, Writing – review & editing, Project administration, Investigation, Conceptualization, Methodology, Funding acquisition. KM: Project administration, Conceptualization, Supervision, Writing – review & editing, Resources, Investigation. KB: Resources, Investigation, Methodology, Validation, Funding acquisition, Writing – review & editing, Supervision, Project administration, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. ExxonMobil Biomedical Sciences, Inc. LAW-2022-0601. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank ExxonMobil Biomedical Sciences, Inc. for supporting this synthesis under the contract LAW-2022-0601. We thank the scientists who were involved in the carbonate chemistry and vulnerable species data collection in the Gulf of Mexico, as well as Trent A. Key, for providing valuable comments on this report.
Conflict of interest
Author KM was employed by the company ExxonMobil Biomedical Sciences Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ajemian M. J. Wetz J. J. Shipley-Lozano B. Dale Shively J. Stunz G. W. (2015). An analysis of artificial reef fish community structure along the Northwestern Gulf of Mexico shelf: potential impacts of “Rigs-to-Reefs” Programs. PloS One10, 5. doi: 10.1371/journal.pone.0126354
2
Albright R. Langdon C. (2011). Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides: Ocean acidification impacts coral recruitment. Global Change Biol.17, 7. doi: 10.1111/j.1365-2486.2011.02404.x
3
Albright R. Mason B. Miller M. Langdon C. (2010). Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl. Acad. Sci.107, 47. doi: 10.1073/pnas.1007273107
4
Algueró-Muñiz M. Meunier C. L. Holst S. Alvarez-Fernandez S. Boersma M. (2016). Withstanding multiple stressors: ephyrae of the moon jellyfish (Aurelia aurita, Scyphozoa) in a high-temperature, high-CO2 and low-oxygen environment. Mar. Biol.163. doi: 10.1007/s00227-016-2958-z
5
Auzoux-Bordenave S. Wessel N. Badou A. Martin S. M’Zoudi S. Avignon S. et al . (2019). Ocean acidification impacts growth and shell mineralization in juvenile abalone (Haliotis tuberculata). Mar. Biol.167, 1. doi: 10.1007/s00227-019-3623-0
6
Bailey A. Thor P. Browman H. Fields D. Runge J. Vermont A. et al . (2017). Early life stages of the Arctic copepod Calanus glacialis are unaffected by increased seawater pCO2. ICES J. Mar. Sci.74, 4. doi: 10.1093/icesjms/fsw066
7
Baumann S. Schernewski G. (2012). Occurrence and public perception of jellyfish along the German Baltic coastline. J. Coast. Conserv.16, 4. doi: 10.1007/s11852-012-0199-y
8
Beniash E. Ivanina A. Lieb N. S. Kurochkin I. Sokolova I. M. (2010). Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar. Ecol. Prog. Ser.419, 95–108. doi: 10.3354/meps08841
9
Bogan S. N. McMahon J. B. Pechenik J. A. Pires A. (2019). Legacy of multiple stressors: responses of gastropod larvae and juveniles to ocean acidification and nutrition. Biol. Bull.236, 3. doi: 10.1086/702993
10
Brauner C. J. Baker D. W. (2009). “ Patterns of acid–base regulation during exposure to hypercarbia in fishes,” in Cardio-Respiratory Control in Vertebrates. Eds. GlassM. L.WoodS. C. ( Springer Berlin Heidelberg, Berlin, Heidelberg), 43–63.
11
Burnell O. Russell B. Irving A. Connell S. (2013). Eutrophication offsets increased sea urchin grazing on seagrass caused by ocean warming and acidification. Mar. Ecol. Prog. Ser.485, 37–46. doi: 10.3354/meps10323
12
Cai W. J. Xinping H. Wei-Jen H. Murrell M. C. Lehrter J. C. Lohrenz S. E. et al . (2011). Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci.4. doi: 10.1038/ngeo1297
13
Carey N. Dupont S. Sigwart J. D. (2016). Sea Hare Aplysia punctata (Mollusca: Gastropoda) Can Maintain Shell Calcification under Extreme Ocean Acidification. Biol. Bull.231, 2. doi: 10.1086/690094
14
Cattano C. Agostini S. Harvey B. P. Wada S. Quattrocchi F. Turco G. et al . (2020). Changes in fish communities due to benthic habitat shifts under ocean acidification conditions. Sci. Total Environ.725, 138501. doi: 10.1016/j.scitotenv.2020.138501
15
Curtis M. D. Morrow C. D. McClintock J. B. (2021). Impacts of near−future ocean warming on microbial community composition of the stomach of the soft−bottom sea star Luidia clathrata (Say) (Echinodermata: Asteroidea). doi: 10.21203/rs.3.rs-306104/v1. In Review.
16
DeCarlo T. M. Cohen A. L. Wong G. T. F. Shiah F. K. Lentz S. J. Davis K. A. et al . (2017), Community production modulates coral reef pH and the sensitivity of ecosystem calcification to ocean acidification, J. Geophys. Res. Oceans, 122, 745–761, doi: 10.1002/2016JC012326
17
De Carvalho R. T. Wendt C. H. C. Willemes M. J. Bahia R. G. Farina M. Salgado L. T. et al . (2022). Ontogeny and early steps of the calcification process in coralline algae Lithophyllum corallinae (Florideophyceae, Rhodophyta). Front. Mar. Sci.9. doi: 10.3389/fmars.2022.900607
18
Dickinson G. H. Matoo O. B. Tourek R. T. Sokolova I. M. Beniash E. (2013). Environmental salinity modulates the effects of elevated CO2 levels on juvenile hard-shell clams, Mercenaria mercenaria. J. Exp. Biol.216, 14. doi: 10.1242/jeb.082909
19
Dickson A. G. (1990). Standard potential of the reaction: AgCl (s)+ 12H2 (g)= Ag (s)+ HCl (aq), and the standard acidity constant of the ion HSO4– in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn.22, 2. doi: 10.1016/0021-9614(90)90074-Z
20
Di Santo V. (2019). Ocean acidification and warming affect skeletal mineralization in a marine fish. Proc. R. Soc. B286, 1894. doi: 10.1098/rspb.2018.2187
21
Doney S. C. Fabry V. J. Feely R. A. Kleypas J. A. (2009). Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci.1, 1. doi: 10.1146/annurev.marine.010908.163834
22
Eriander L. Wrange A. L. Havenhand J. N. (2016). Simulated diurnal pH fluctuations radically increase variance in—but not the mean of—growth in the barnacle Balanus improvisus. ICES J. Mar. Sci.73, 3. doi: 10.1093/icesjms/fsv214
23
Esbaugh A. J. (2018). Physiological implications of ocean acidification for marine fish: emerging patterns and new insights. J. Comp. Physiol. B188, 1. doi: 10.1007/s00360-017-1105-6
24
Falkenhaug T. (2014). Review of jellyfish blooms in the Mediterranean and Black Sea. Mar. Biol. Res.10, 10. doi: 10.1080/17451000.2014.880790
25
Feely R. A. Alin S. R. Newton J. Sabine C. L. Warner M. Devol A. et al . (2010). The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci.88, 4. doi: 10.1016/j.ecss.2010.05.004
26
Feely R. A. Sabine C. L. Hernandez-Ayon J. M. Ianson D. Hales B. (2008). Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science320, 5882. doi: 10.1126/science.1155676
27
Feely R. A. Sabine C. L. Lee K. Berelson W. Kleypas J. Fabry V. J. et al . (2004). Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science305, 362–366. doi: 10.1126/science.1097329
28
Felder D. L. Camp D. K. (2009). Gulf of Mexico origin, waters, and biota: Biodiversity, 1 (College Station, TX: Texas A&M University Press).
29
Fredericq S. Cho T. Earle S. Gurgel C. Krayesky D. Mateo-Cid L. et al . (2009). “ Seaweeds of the Gulf of Mexico,” in Gulf of Mexico Origin, Waters, and Biota, vol. 1. , 187–259.
30
Garavelli L. Studivan M. S. Voss J. D. Kuba A. Figueiredo J. Chérubin L. M. (2018). Assessment of mesophotic coral ecosystem connectivity for proposed expansion of a marine sanctuary in the northwest Gulf of Mexico: Larval Dynamics. Front. Mar. Sci.5. doi: 10.3389/fmars.2018.00174
31
Giltz S. M. Taylor C. M. (2017). Reduced growth and survival in the larval blue crab Callinectes sapidus under predicted ocean acidification. J. Shellfish Res.36, 2. doi: 10.2983/035.036.0219
32
Gleason D. F. Hofmann D. K. (2011). Coral larvae: From gametes to recruits. J. Exp. Mar. Biol. Ecol.408, 1. doi: 10.1016/j.jembe.2011.07.025
33
Goldman J. A. L. Bender M. L. Morel F. M. M. (2017). The effects of pH and pCO2 on photosynthesis and respiration in the diatom Thalassiosira weissflogii. Photosynth. Res.132, 1. doi: 10.1007/s11120-016-0330-2
34
Gravinese P. M. (2018). Ocean acidification impacts the embryonic development and hatching success of the Florida stone crab, Menippe mercenaria. J. Exp. Mar. Biol. Ecol.500. doi: 10.1016/j.jembe.2017.09.001
35
Gravinese P. M. Enochs I. C. Manzello D. P. van Woesik R. (2019). Ocean acidification changes the vertical movement of stone crab larvae. Biol. Lett.15, 12. doi: 10.1098/rsbl.2019.0414
36
Green L. Jutfelt F. (2014). Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol. Lett.10, 9. doi: 10.1098/rsbl.2014.0538
37
Guo X. Wei-Jun C. Wei-Jen H. Yongchen W. Feizhou C. Murrell M. C. et al . (2012). CO2 dynamics and community metabolism in the Mississippi River plume. Limnol. Oceanogr.57. doi: 10.4319/lo.2012.57.1.0001
38
Habibi N. Uddin S. Behbehani M. Khan M. W. Razzack N. A. Shirshikhar F. (2023). The transcriptome profile of the marine Calanoid copepod Parvocalanus crassirostris isolated from Kuwait territorial waters and generations cultured under different ocean acidification scenarios. Regional Stud. Mar. Sci.67, 103231. doi: 10.1016/j.rsma.2023.103231
39
Hall-Spencer J. Allen R. (2015). The impact of CO2 emissions on nuisance marine species. Res. Rep. Biodivers. Stud.33, 33–46. doi: 10.2147/RRBS.S70357
40
Heuer R. M. Grosell M. (2014). Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integr. Comp. Physiol.307, 9. doi: 10.1152/ajpregu.00064.2014
41
Hickerson E. L. Schmahl G. P. Johnston M. A. Nuttall M. F. Eckert R. J. (2012). “ Flower garden banks – A refuge in the Gulf of Mexico?,” in Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, Cairns, Australia: 12th International Coral Reef Symposium9–13 July 2012.
42
IPCC (2013). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press).
43
Jansson A. Lischka S. Boxhammer T. Schulz K. Norkko J. (2015). Larval development and settling of Macoma balthica in a large-scale mesocosm experiment at different fCO2 levels. Biogeosciences12, 3377–3385. doi: 10.5194/bg-13-3377-2016
44
Jeeva Priya R. Anand M. Maruthupandy M. Hameedha Beevi A. (2017). Biomarker response of climate change-induced ocean acidification and hypercapnia studies on brachyurian crab Portunus pelagicus. Global J. Environ. Sci. Manage.3, 2. doi: 10.22034/gjesm.2017.03.02.005
45
Johnston M. A. Embesi J. A. Eckert R. J. Nuttall M. F. Hickerson E. L. Schmahl G. P. (2016). Persistence of coral assemblages at East and West Flower Garden Banks, Gulf of Mexico. Coral Reefs35, 3. doi: 10.1007/s00338-016-1452-x
46
Jokiel P. L. Jury C. P. Rodgers K. S. (2014). Coral-algae metabolism and diurnal changes in the CO2-carbonate system of bulk sea water. PeerJ2. doi: 10.7717/peerj.378
47
Jury C. P. Whitehead R. F. Szmant A. M. (2010). Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (= Madracis mirabilis sensu Wells 1973): bicarbonate concentrations best predict calcification rates. Global Change Biol.16, 5. doi: 10.1111/j.1365-2486.2009.02057.x
48
Kaplan M. B. Mooney T. A. McCorkle D. C. Cohen A. L. (2013). Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PloS One8, 5. doi: 10.1371/journal.pone.0063714
49
Kaushal S. S. Likens G. E. Utz R. M. Pace M. L. Grese M. Yepsen M. (2013). Increased river alkalinization in the Eastern U.S. Environ. Sci. Technol.47, 18. doi: 10.1021/es401046s
50
Kealoha A. K. Shamberger K. E. F. DiMarco S. F. Thyng K. M. Hetland R. D . (2020). Surface Water CO2 variability in the Gulf of Mexico (1996–2017). Sci Rep10, 12279. doi: 10.1038/s41598-020-68924-0
51
Kriefall N. G. Pechenik J. A. Pires A. Davies S. W. (2018). Resilience of Atlantic slippersnail Crepidula fornicata larvae in the face of severe coastal acidification. Front. Mar. Sci.5. doi: 10.3389/fmars.2018.00312
52
Kwan G. T. Tresguerres M. (2022). Elucidating the acid-base mechanisms underlying otolith overgrowth in fish exposed to ocean acidification. Sci. Total Environ.823, 153690. doi: 10.1016/j.scitotenv.2022.153690
53
Laws E. A. McClellan S. A. Passow U. (2020). Interactive effects of CO2, temperature, irradiance, and nutrient limitation on the growth and physiology of the marine diatom Thalassiosira pseudonana (Coscinodiscophyceae). J. Phycol.56, 6. doi: 10.1111/jpy.13048
54
Lee K. Kim T. W. Byrne R. H. Millero F. J. Feely R. A. Liu Y. M. (2010). The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochim. Cosmochim. Acta76, 6. doi: 10.1016/j.gca.2009.12.027
55
Le Quéré C. Andrew R. M. Friedlingstein P. Sitch S. Pongratz J. Manning A. C. et al . (2018). Global carbon budget 2017. Earth Syst. Sci. Data10, 1. doi: 10.5194/essd-10-405-2018
56
Li S. Liu C. Huang J. Liu Y. Zhang S. Zheng G. et al . (2016). Transcriptome and biomineralization responses of the pearl oyster Pinctada fucata to elevated CO2 and temperature. Sci. Rep.6, 18943. doi: 10.1038/srep18943
57
Liu W. Yu Z. Huang X. Shi Y. Lin J. Zhang H. et al . (2017). Effect of ocean acidification on growth, calcification, and gene expression in the pearl oyster, Pinctada fucata. Mar. Environ. Res.130, 174–180. doi: 10.1016/j.marenvres.2017.07.013
58
Longmire K. Seitz R. Seebo M. Brill R. Lipcius R. (2022). Biological responses of the predatory blue crab and its hard clam prey to ocean acidification and low salinity. Mar. Ecol. Prog. Ser.701, 67–81. doi: 10.3354/meps14198
59
Lueker T. J. Dickson A. G. Keeling C. D. (2000). Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem.70, 1–3. doi: 10.1016/S0304-4203(00)00022-0
60
Luparello C. Mauro M. Lazzara V. Vazzana M. (2020). Collective locomotion of human cells, wound healing and their control by extracts and isolated compounds from marine invertebrates. Molecules25, 11. doi: 10.3390/molecules25112471
61
MacLeod C. D. Poulin R. (2012). Host–parasite interactions: a litmus test for ocean acidification? Trends Parasitol.28, 9. doi: 10.1016/j.pt.2012.06.007
62
Manzello D. P. Kolodziej G. Kirkland A. Besemer N. Enochs I. C. (2021). Increasing coral calcification in Orbicella faveolata and Pseudodiploria strigosa at Flower Garden Banks, Gulf of Mexico. Coral Reefs40, 4. doi: 10.1007/s00338-021-02108-8
63
Mathis J. T. Pickart. R. S. Byrne R. H. McNeil C. L. Moore G. W. K. Juranek L. W. et al . (2012). Storm-induced upwelling of high pCO2 waters onto the continental shelf of the western Arctic Ocean and implications for carbonate mineral saturation states. Geophys. Res. Lett.39, 7. doi: 10.1029/2012GL051574
64
Matoo O. B. Ivanina A. V. Ullstad C. Beniash E. Sokolova I. M. (2013). Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Physiol. A Mol. Integr. Physiol.164, 4. doi: 10.1016/j.cbpa.2012.12.025
65
McCoy S. J. Kamenos N. A. (2015). Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. J. Phycol.51, 1. doi: 10.1111/jpy.12262
66
McDonald M. McClintock J. B. Amsler C. D. Rittschof D. Angus R. A. Orihuela B. et al . (2009). Effects of ocean acidification over the life history of the barnacle Amphibalanus amphitrite. Mar. Ecol. Prog. Ser.385, 179–187. doi: 10.3354/meps08099
67
McNicholl C. Koch M. S. Swarzenski P. W. Oberhaensli F. R. Taylor A. Gómez Batista M. et al . (2020). Ocean acidification effects on calcification and dissolution in tropical reef macroalgae. Coral Reefs39, 6. doi: 10.1007/s00338-020-01991-x
68
Meseck S. L. Mercaldo-Allen R. Clark P. Kuropat C. Redman D. Veilleux D. et al . (2021). Effects of ocean acidification on larval Atlantic surfclam (Spisula solidissima) from Long Island Sound in Connecticut. Fishery Bull.119, 1. doi: 10.7755/FB.119.1.8
69
Miller A. W. Reynolds A. Minton M. S. Smith R. (2020). Evidence for stage-based larval vulnerability and resilience to acidification in Crassostrea virginica. J. Molluscan Stud.86, 4. doi: 10.1093/mollus/eyaa022
70
Moroz L. L. (2011). Aplysia. Curr. Biol.: CB21, 2. doi: 10.1016/j.cub.2010.11.028
71
Mucci A. Starr M. Gilbert D. Sundby B. (2011). Acidification of lower St. Lawrence Estuary bottom waters. Atmosphere-Ocean49, 3. doi: 10.1080/07055900.2011.599265
72
Nagelkerken I. Alemany T. Anquetin J. M. Ferreira C. M. Ludwig K. E. Minami S. et al . (2021). Ocean acidification boosts reproduction in fish via indirect effects. PloS Biol.19, 1. doi: 10.1371/journal.pbio.3001033
73
Nardone J. A. Patel S. Siegel K. R. Tedesco D. McNicholl C. G. O’Malley J. et al . (2018). Assessing the impacts of ocean acidification on adhesion and shell formation in the barnacle Amphibalanus amphitrite. Front. Mar. Sci.5. doi: 10.3389/fmars.2018.00369
74
Nelson W. A. (2009). Calcified macroalgae - critical to coastal ecosystems and vulnerable to change: a review. Mar. Freshw. Res.60, 8. doi: 10.1071/MF08335
75
NOAA Coral Program (2020). National Coral Reef Monitoring Program - Status Reports. Available online at: https://www.coris.noaa.gov/monitoring/status_report/. (Accessed October 15, 2023).
76
Noisette F. Comtet T. Legrand E. Bordeyne F. Davoult D. Martin S. (2014). Does encapsulation protect embryos from the effects of ocean acidification? The example of Crepidula fornicata. PloS One9, 3. doi: 10.1371/journal.pone.0093021
77
Olischläger M. Wiencke C. (2013). Ocean acidification alleviates low-temperature effects on growth and photosynthesis of the red alga Neosiphonia harveyi (Rhodophyta). J. Exp. Bot.64, 18. doi: 10.1093/jxb/ert329
78
Osborne E. Hu X. Hall R. E. Yates K. Vreeland-Dawson J. Shamberger K. et al . (2022). Ocean acidification in the Gulf of Mexico: Drivers, impacts, and unknowns. Prog. Oceanogr.209. doi: 10.1016/j.pocean.2022.102882
79
Page H. N. Courtney T. A. Collins A. De Carlo E. H. Andersson A. J. (2017). Net community metabolism and seawater carbonate chemistry scale non-intuitively with coral cover. Front. Mar. Sci.4. doi: 10.3389/fmars.2017.00161
80
Pansch C. Schlegel P. Havenhand J. (2013). Larval development of the barnacle Amphibalanus improvisus responds variably but robustly to near-future ocean acidification. ICES J. Mar. Sci.70, 4. doi: 10.1093/icesjms/fst092
81
Parajuli K. (2023). Sea star, Luidia clathrata, responses to physical and thermal stress. West Lafayette, IN: Purdue University Graduate School. doi: 10.25394/PGS.22932248.v1
82
Perez F. F. Fraga F. (1987). Association constant of fluoride and hydrogen ions in seawater. Mar. Chem.21, 2. doi: 10.1016/0304-4203(87)90036-3
83
Pistevos J. C. A. Nagelkerken I. Rossi T. Olmos M. Connell S. D. (2015). Ocean acidification and global warming impair shark hunting behaviour and growth. Sci. Rep.5, 1. doi: 10.1038/srep16293
84
Porzio L. Buia M. C. Hall-Spencer J. M. (2011). Effects of ocean acidification on macroalgal communities. J. Exp. Mar. Biol. Ecol.400, 1–2. doi: 10.1016/j.jembe.2011.02.011
85
Poulin R. Blasco-Costa I. Randhawa H. S. (2016). Integrating parasitology and marine ecology: Seven challenges towards greater synergy. J. Sea Res.113, 3–10. doi: 10.1016/j.seares.2014.10.019
86
Pousse E. Poach M. E. Redman D. H. Sennefelder G. White L. E. Lindsay J. M. et al . (2020). Energetic response of Atlantic surfclam Spisula solidissima to ocean acidification. Mar. pollut. Bull.161, 111740. doi: 10.1016/j.marpolbul.2020.111740
87
Qu P. Fu F. Hutchins D. A. (2018). Responses of the large centric diatom Coscinodiscus sp. to interactions between warming, elevated CO2, and nitrate availability. Limnol. Oceanogr.63, 3. doi: 10.1002/lno.10781
88
Raymond P. A. Cole J. J. (2003). Increase in the export of alkalinity from North America’s largest river. Science371, 5629. doi: 10.1126/science.1083788
89
Raymond P. A. Oh N.-H. Turner R. E. Broussard W. (2008). Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nature451, 7177. doi: 10.1038/nature06505
90
Regnier P. Friedlingstein P. Ciais P. Mackenzie F. T. Gruber N. Janssens I. A. et al . (2013). Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat. Geosci.6, 597–607. doi: 10.1038/ngeo1830
91
Reyes-Giler C. L. Benson B. E. Levy M. Chen X. Pires A. Pechenik J. A. et al . (2021). The marine gastropod Crepidula fornicata remains resilient to ocean acidification across two life history stages. Front. Physiol.12. doi: 10.3389/fphys.2021.702864
92
Richardson A. J. Gibbons M. J. (2008). Are jellyfish increasing in response to ocean acidification? Limnol. Oceanogr.53, 5. doi: 10.4319/lo.2008.53.5.2040
93
Rosa R. Rummer J. L. Munday P. L. (2017). Biological responses of sharks to ocean acidification. Biol. Lett.13, 3. doi: 10.1098/rsbl.2016.0796
94
Rummer J. L. Bouyoucos I. A. Mourier J. Nakamura N. Planes S. (2020). Responses of a coral reef shark acutely exposed to ocean acidification conditions. Coral Reefs39, 5. doi: 10.1007/s00338-020-01972-0
95
Sabine C. L. Feely R. A. Gruber N. Key R. M. Lee K. Bullister J. L. et al . (2004). The oceanic sink for anthropogenic CO2. Science305, 5682. doi: 10.1126/science.1097403
96
Sammarco P. W. Lirette A. Tung Y. F. Boland G. S. Genazzio M. Sinclair J. et al . (2013). Coral communities on artificial reefs in the Gulf of Mexico: standing vs. toppled oil platforms. ICES J. Mar. Sci.71, 2. doi: 10.1093/icesjms/fst140
97
Schmahl G. P. Hickerson E. Precht W. (2008). “ Biology and ecology of coral reefs and coral communities in the flower garden banks region, northwestern Gulf of Mexico,” in Coral Reefs of the USA. Eds. RieglB.DodgeR. E. (Springer, Dordrecht: Springer Netherlands), 221–261.
98
Schram J. B. McClintock J. B. Angus R. A. Lawrence J. M. (2011). Regenerative capacity and biochemical composition of the sea star Luidia clathrata (Say) (Echinodermata: Asteroidea) under conditions of near-future ocean acidification. J. Exp. Mar. Biol. Ecol.407, 2. doi: 10.1016/j.jembe.2011.06.024
99
Semesi I. S. Kangwe J. Björk M. (2009). Alterations in seawater pH and CO2 affect calcification and photosynthesis in the tropical coralline alga, Hydrolithon sp. (Rhodophyta). estuarine. Coast. Shelf Sci.84, 3. doi: 10.1016/j.ecss.2009.03.038
100
Servili A. Lévêque E. Mouchel O. Devergne J. Lebigre C. Roussel S. et al . (2023). Ocean acidification alters the acute stress response of a marine fish. Sci. Total Environ.858, 159804. doi: 10.1016/j.scitotenv.2022.159804
101
Sigwart J. D. Carey N. (2014). Grazing under experimental hypercapnia and elevated temperature does not affect the radula of a chiton (Mollusca, Polyplacophora, Lepidopleurida). Mar. Environ. Res.102, 73–77. doi: 10.1016/j.marenvres.2014.05.004
102
Sigwart J. D. Green P. A. Crofts S. B. (2015). Functional morphology in chitons (Mollusca, Polyplacophora): influences of environment and ocean acidification. Mar. Biol.162, 11. doi: 10.1007/s00227-015-2761-2
103
Sobrino C. Segovia M. Neale P. J. Mercado J. M. García-Gómez C. Kulk G. et al . (2014). Effect of CO2, nutrients and light on coastal plankton. IV. Physiological responses. Aquat. Biol.22, 77–93. doi: 10.3354/ab00590
104
Stets E. G. Kelly V. J. Crawford C. G. (2014). Long-term trends in alkalinity in large rivers of the conterminous U.S. in relation to acidification, agriculture, and hydrologic modification. Sci. Total Environ.488(2014), 280–289. doi: 10.1016/j.scitotenv.2014.04.054
105
Talmage S. C. Gobler C. J. (2009). The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnol. Oceanogr.54, 6. doi: 10.4319/lo.2009.54.6.2072
106
Thor P. Bailey A. Dupont S. Calosi P. Søreide J. E. De Wit P. et al . (2018). Contrasting physiological responses to future ocean acidification among Arctic copepod populations. Global Change Biol.24, 1. doi: 10.1111/gcb.13870
107
Timmins-Schiffman E. Guzmánet J. M. Thompson R. E. Vadopalas B. Eudeline B. Roberts S. B. (2020). Dynamic response in the larval geoduck (Panopea generosa) proteome to elevated pCO2. Ecol. Evol.10, 1. doi: 10.1002/ece3.5885
108
Tomatsuri M. Kon K. (2019). Impacts of ocean acidification on hermit crab communities through contrasting responses of Pagurus filholi (de Man 1887) and Clibanarius virescens (Krauss 1843). Aquat. Ecol.53, 4. doi: 10.1007/s10452-019-09709-0
109
Van Colen C. Debusschere E. Braeckman U. Van Gansbeke D. Vincx M. (2012). The early life history of the clam Macoma balthica in a high CO2 world. PloS One7, 9. doi: 10.1371/journal.pone.0044655
110
Vehmaa A. Almén A.-K. Brutemark A. Paul A. Riebesell U. Furuhagen S. et al . (2016). Ocean acidification challenges copepod phenotypic plasticity. Biogeosciences13, 22. doi: 10.5194/bg-13-6171-2016
111
Waldbusser G. G. Voigt E. P. Bergschneider H. Green M. A. Newell R. I. E. (2011). Biocalcification in the Eastern Oyster (Crassostrea virginica) in Relation to Long-term Trends in Chesapeake Bay pH. Estuar. Coasts34, 2. doi: 10.1007/s12237-010-9307-0
112
Wallace R. B. Baumann H. Grear J. S. Aller R. C. Gobler C. J. (2014). Coastal ocean acidification: The other eutrophication problem. Estuar. Coast. Shelf Sci.148, 1–13. doi: 10.1016/j.ecss.2014.05.027
113
Wang M. Jeong C.-B. Lee Y. H. Lee J.-S. (2018). Effects of ocean acidification on copepods. Aquat. Toxicol.196, 17–24. doi: 10.1016/j.aquatox.2018.01.004
114
Wang Z. A. Wanninkhof R. Cai W.-J. Byrne R. H. Hu X. Peng T.-H. et al . (2013). The marine inorganic carbon system along the Gulf of Mexico and Atlantic coasts of the United States: Insights from a transregional coastal carbon study. Limnol. Oceanogr.58, 1. doi: 10.4319/lo.2013.58.1.0325
115
Wang X. J. Xie J. L. Yuan Y. X. (2022). Response of fish to ocean warming and acidification. Acta Ecol. Sin.42, 2. doi: 10.1016/j.aquatox.2018.01.004
116
Wanninkhof R. Barbero L. Byrne R. Cai W.-J. Huang W.-J. Zhang J.-Z. et al . (2015). Ocean acidification along the Gulf Coast and East Coast of the USA. Cont. Shelf Res.98, 0. doi: 10.1016/j.csr.2015.02.008
117
Welladsen H. M. (2010). The effects of exposure to near-future levels of ocean acidification on shell characteristics of Pinctada fucata (Bivalvia: Pteriidae). Molluscan Res.30, 125–130. doi: 10.11646/mr.30.3.2
118
White M. M. McCorkle D. C. Mullineaux L. S. Cohen A. L. (2013). Early exposure of bay scallops (Argopecten irradians) to high CO2 causes a decrease in larval shell growth. PloS One8, 4. doi: 10.1371/journal.pone.0061065
119
Winans A. K. Purcell J. E. (2010). Effects of pH on asexual reproduction and statolith formation of the scyphozoan, Aurelia labiata. Hydrobiologia645, 1. doi: 10.1007/s10750-010-0224-9
120
Zakroff C. J. Mooney T. A. (2020). Antagonistic interactions and clutch-dependent sensitivity induce variable responses to ocean acidification and warming in squid (Doryteuthis pealeii) embryos and paralarvae. Front. Physiol.11. doi: 10.3389/fphys.2020.00501
121
Zakroff C. Mooney T. A. Berumen M. L. (2019). Dose-dependence and small-scale variability in responses to ocean acidification during squid, Doryteuthis pealeii, development. Mar. Biol.166, 5. doi: 10.1007/s00227-019-3510-8
122
Zemah-Shamir Z. Zemah-Shamir S. Scheinin A. Tchernov D. Lazebnik T. Gal G. (2022). A systematic review of the behavioural changes and physiological adjustments of elasmobranchs and teleost’s to ocean acidification with a focus on sharks. Fishes7, 2. doi: 10.3390/fishes7020056
Summary
Keywords
ocean acidification, carbonate chemistry, aragonite saturation state (Ω), organismal physiology, climate change
Citation
Armstrong DA, Yin H, Hampton AR, Hu X, McFarlin KM and Bahr KD (2025) Interspecific vulnerabilities to elevated pCO2 in the northwestern Gulf of Mexico, a baseline of sensitivity and geochemical regimes. Front. Mar. Sci. 12:1644030. doi: 10.3389/fmars.2025.1644030
Received
17 June 2025
Accepted
08 September 2025
Published
02 October 2025
Volume
12 - 2025
Edited by
Effi Helmy Ariffin, University of Malaysia Terengganu, Malaysia
Reviewed by
Wenjian Li, Chinese Academy of Sciences (CAS), China; Jameer Ahammad Shaik, National Aquaculture Group, Saudi Arabia
Updates
Copyright
© 2025 Armstrong, Yin, Hampton, Hu, McFarlin and Bahr.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David A. Armstrong, david.alexander.armstrong04@gmail.com; Hang Yin, hangyin.phd@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.