- 1Institute of Urban Agriculture, Chinese Academy of Agricultural Sciences, Chengdu National Agricultural Science and Technology Center, Chengdu, China
- 2Department of Agricultural Economics and Engineering, Kizilsu Vocational Technical College, Atushi, China
- 3Nutrition and Bromatology Group, Department of Analytical Chemistry and Food Science, Faculty of Science, Universidade de Vigo, Ourense, Spain
- 4Zhengzhou Research Base, State Key Laboratory of Cotton Biology, School of Agricultural Sciences, Zhengzhou University, Zhengzhou, China
- 5Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, United Arab Emirates
In recent years, the impacts of global warming, including glacial melting, extreme weather events, food crises, and epidemics, have become increasingly severe, posing significant challenges to global sustainability. The primary driver of the current climate crisis is the substantial emission of greenhouse gases (GHGs), particularly carbon dioxide (CO2). Microalgae, as photoautotrophic microorganisms, offer a promising solution by utilizing CO2 for biosynthesis. Previous research indicates that microalgae can fix CO2 at rates exceeding 1.5 kg/m2/year under optimal conditions, and produce lipids with high content of unsaturated fatty acids. This review delves into recent advancements understanding the causes and effects of global warming, with a particular focus on agricultural GHG emissions. It critically examines the carbon sequestration mechanisms of microalgae and their potential as single-cell biofactories for carbon neutralization and biomanufacturing. The review highlights their ability to fix CO2 and produce high-value products such as biofuels, chemicals, pharmaceuticals, and foods. Among these species, the characteristics and value of seven edible microalgae are also described. We outline the technical and economic challenges associated with scaling up microalgae cultivation from laboratory to industrial scale, including the optimization of cultivation systems and the improvement of harvesting and processing techniques. This review serves as a useful and informative reference for the application of CO2 capture and high-value bioproducts by microalgae, aiming to provide a reference for the realization of carbon neutrality and the mitigation of climate change.
Highlights
● Increasing emission of GHGs mainly contribute to global warming.
● Microalgae are ideal single-cell biofactories for carbon sequestration.
● Microalgal biotechnology could boost production of energy, chemicals, pharmaceuticals, and food.
● Microalgae-based strategy has advantages and challenges on attaining carbon neutrality and mitigating climate change.
1 Introduction
Recently, global warming has increased the frequency of extreme weather events. Over the past five years, ambient temperatures and droughts that have swept across the globe have resulted in humans now facing the most severe event of the global climate crisis (Yin et al., 2022). Massive emissions of GHGs, such as carbon dioxide (CO2), nitrous oxide (N2O) and methane (CH4), are increasing dramatically (Williams, 2021). In particular, the emissions of GHGs from agriculture and livestock have surpassed the total GHG emissions from all transportation methods, a fact that is receiving increasing attention (Sun and Xu, 2022). How to reduce GHGs is vital for dealing with global warming.
GHG emission reductions can be achieved concurrently by implementing integrated mitigation strategies, including environmental conservation, ecosystem resilience maintenance, and carbon neutrality. Carbon capture and storage (CCS) technology is one of the key technologies and measures for reducing the concentration of CO2 in the atmosphere (Cherepovitsyn et al., 2020). Geological storage, afforestation and reforestation, seaweed and algae, and the application of biochar are reported to be effective for CCS. Geological storage involves capturing CO2 from emission sources, transporting it to a storage site, and burying it in a suitable underground geological formation. Some procedures for CO2 sequestration are useful, such as pre-combustion, post-combustion, and oxyfuel combustion, as well as the use of algae, biochar, and charcoal (Anwar et al., 2018). Currently, numerous efforts are still underway, including the use of biochar used for soil amendment and captured carbon injected into onshore or offshore reservoirs.
Carbon capture and utilization (CCU) is also regarded as a key solution for mitigating global climate change (Zhou et al., 2017). It could convert atmospheric CO2 into products with net-zero or negative emissions. Previous research has noted that emissions from steel mills and gasified waste biomass can be converted directly into monoethylene glycol via fermentation for various plastic products such as polyethylene terephthalate, fibers, resins, proteins, and bottles. Carbonic anhydrase is also reported to be an excellent candidate for novel biocatalytic processes based on CCU (Russo et al., 2022). Carbon capture, utilization, and storage (CCUS) is defined as the process of capturing and purifying high concentrations of CO2 from industrial tail gases or fuel combustion processes for subsequent storage or industrial use. The essential processes of CCUS include CO2 capture, transportation, storage, and utilization (Jiang et al., 2022b).
Microalgae, a diverse group of photosynthetic organisms found in both aquatic and terrestrial environments, possess the remarkable ability to utilize CO2 for self-biosynthesis (Zheng et al., 2022). They can produce about 280 tons of dry biomass per hectare per year by using 9% of the freely accessible solar energy. Microalgae-based CCS technology is crucial for addressing challenges associated with the utilization of industrial-emitted flue gases. The CO2 fixation rates of microalgae-based systems range from 80 mg L-1 day-1 to over 578 mg L-1 day-1, primarily influenced by physiochemical parameters and the composition of the flue gases (Padhi et al., 2025).
Microalgae can capture and utilize atmospheric CO2 to convert it into value-added products (Bhujade et al., 2017; Chia et al., 2022). Compared to terrestrial plants, the notable advantages of microalgae in CO2 removal are mainly reflected in the following areas: (1) Rapid growth: Microalgae have a much faster growth rate than traditional plants. Under optimal light and nutrient conditions, their biomass can increase rapidly in a short period (Chen et al., 2019). (2) Non-competition with crops for land: As microalgae can thrive in unproductive land, it has little competition with agricultural land for growing crops (Shareefdeen et al., 2023). Microalgae-based wastewater purification allows nutrient recovery while accumulating high-value-added biomass, making the operation more economically sustainable and viable. (3) Efficient cultivation technologies: The cultivation and harvesting processes of microalgae are relatively simple and can be efficiently carried out using advanced cultivation technologies such as photobioreactors (PBRs). Open raceways, biofilm PBRs, and flat panel PBRs, are the most commonly used systems for widespread microalgae cultivation (Nguyen et al., 2024). (4) High-value products: During biomass production, microalgae can simultaneously produce a range of high-value by-products, such as biofuels, proteins, polyunsaturated fatty acids (PUFAs), and carotenoids (Xu et al., 2024). (5) High photosynthetic efficiency and high capacity for CO2 sequestration: The remarkable photosynthetic efficiency of microalgae can be attributed to their pyrenoid-based CO2-concentrating mechanism (CCM). As carbon is a fundamental component of microalgal biomass, their growth demands a significant amount of CO2, with estimates suggesting that 1.3 to 2.4 tons of CO2 are required to produce just 1 ton of microalgal biomass. Moreover, microalgae demonstrate a CO2 sequestration efficiency that is 10 to 50 times greater than that of terrestrial plants (Wang et al., 2025).
The majority of current studies on microalgal CCS and CCU technologies have been limited to the laboratory scale, with only a few successes at pilot or industrial scale. This study presents a comprehensive review of the potential of microalgae-based strategies for achieving carbon neutrality and combating climate change. The causes and effects of global warming are systematically analyzed, with special attention given to the impact of agricultural production on GHG emissions. The potential of microalgae for achieving carbon neutrality is thoroughly evaluated, and the application and challenge of microalgal biotechnology is highlighted. A forward-looking perspective on microalgal biotechnology is also provided to contribute to carbon emission reduction.
2 Causes and effects of global warming
2.1 Increasing emissions of GHGs mainly contribute to global warming
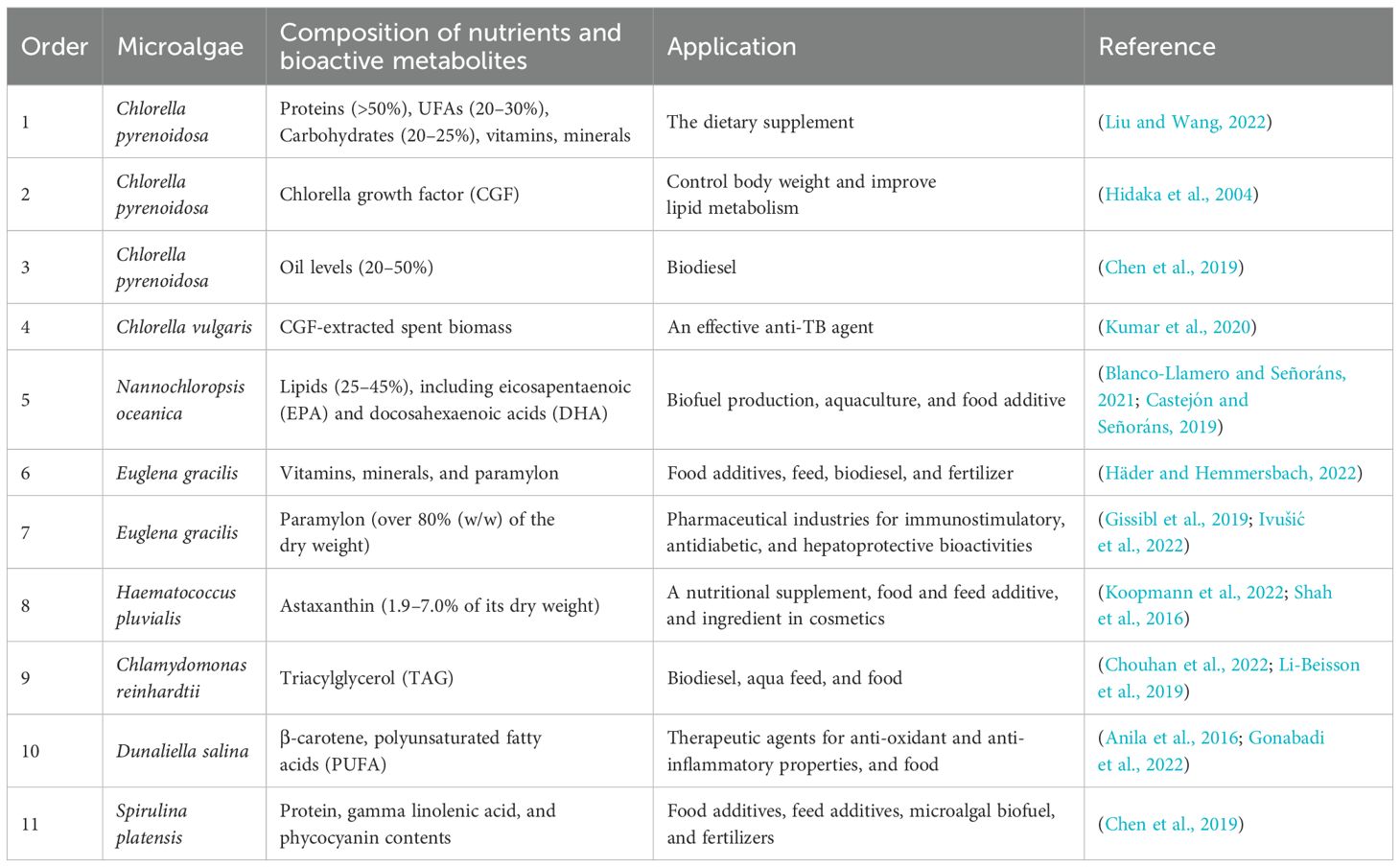
GHGs, including CO2, CH4, N2O, hydrofluorocarbons, perfluorocarbons, and sulfur hexafluoride, can absorb solar radiation, thereby increasing the Earth’s temperature and contribute to global warming (Harmsen et al., 2019) (Figure 1A). Among these gases, CO2 and CH4 are the most critical GHGs, with atmospheric CO2 and CH4 levels increasing from 350 ppm to 410 ppm and from 1100 ppb to 1875 ppb, respectively, since 1950 (Bačėninaitė et al., 2022). Human activities, such as agricultural production of crops and livestock, land disposal methods like landfills and composting, anaerobic wastewater treatment, natural gas extraction, and coal mining, have led to large emissions of CH4, accounting for over 60% of total CH4 emissions (La et al., 2018).
Figure 1. The greenhouse gas (GHG) emissions of the global world. (A) The composition of GHG; (B) The global GHG emissions (blue) and agricultural methane emissions (orange) from 1990 to 2019; (C) The percentage of total GHG emissions for different countries in 2019. The data are sourced from the World Bank Group’s database (2020).
According to the World Bank Group’s database (2020), annual global GHG emissions have risen over the past decade at an average rate of approximately 33 billion tons per year (Figure 1B). In 2019, global GHG emissions reached approximately 46.2 billion tons, which was approximately 3.1 times higher than that in 1965. Since 2007, China has surpassed the United States as the world’s largest emitter of CO2 and is expected to emit more than 12 billion tons of CO2 by 2025 (Figure 1C) (Zhang et al., 2022).
2.2 Agricultural production is the primary source of GHGs
Agricultural GHG emissions, a major part of human-induced GHG emissions, are rising due to the growing global population and the increasing demand for food production. The contribution of agriculture to GHG emissions indicates a level of 18% of total GHGs, mainly from carbon CO2, CH4, and N2O (Ozlu et al., 2022). Land is crucial in global GHG cycles, and land-use change (LUC) can either release or remove these gases from the atmosphere. Agricultural land conversion (ALC), a common form of LUC, involves transforming agricultural lands for other purposes. When ALC exceeds 8% of available land, it tends to increase GHG emissions during economic development (Huang et al., 2023). GHG emissions are associated with synthetic N fertilizer manufacture, transportation, and field use in agricultural systems. It has been reported that synthetic N fertilizer supply chain was responsible for estimated emissions of 1.13 GtCO2e in 2018, representing 10.6% of agricultural emissions and 2.1% of global GHG emissions (Menegat et al., 2022).
The Food and Agriculture Organization (FAO) has studied why animal farming generates massive GHG emissions worldwide. Farming livestock, including cattle, sheep, and pigs, is a significant source of global GHG emissions, producing large amounts of GHGs (Figure 2). GHGs from gastrointestinal fermentation and manure emissions from livestock farming account for over 41% of total GHG emissions from agricultural production, measured in CO2 equivalents. Dairy farming, primarily for milk production, is the largest on-farm GHG emission source, accounting for over 70% of all on-farm GHG emissions. CH4 emissions from the intestinal tract of animals contribute 35-55% of all on-farm emissions (Holtshausen et al., 2021). Pork accounts for approximately 35% of the global meat supply, with approximately 747 million tons of CO2e GHG emissions annually (Zhang et al., 2024).
Figure 2. Simplified schematic representation of the agricultural greenhouse gas (GHG) emissions mode. The left and right represent GHG from animal farming and rice cultivation, respectively. The bottom represents the GHG from agricultural soil.
Rice cultivation generates extensive CH4 emissions. The rhizosphere microbes of rice produce large amounts of CH4 when they lack oxygen. This CH4 is absorbed by rice roots and transported through leaves and stems, leading to atmospheric emissions. According to statistical data, annual CH4 emissions from rice fields range from 3.1 × 1010 to 11.2 × 1010 kg, accounting for 7%-17% of total atmospheric CH4 (Li et al., 2022; Saha et al., 2022).
N2O, a critical non-CO2 GHG, has received increasing attention due to its high global warming potential. N2O is the third-largest contributor to GHGs, after CO2 and CH4. The production of N2O has risen dramatically as the worldwide population has climbed and the demand for nitrogen fertilizer has grown. These emissions simultaneously deplete stratospheric ozone and and contribute to climate change (Tian et al., 2020). In 2019, China’s N2O emissions were 710,300 tons, with agricultural land use and livestock farming being the primary sources (Wang et al., 2022). The excessive use of nitrogen fertilizers and denitrification are leading causes of N2O emissions from agricultural land (Menegat et al., 2022).
2.3 Global warming and the global environmental crisis
Global warming can lead to decreased clean drinking water, increased ocean acidification, and more frequent natural disasters such as droughts, floods, heatwaves, storms, and dust storms. Furthermore, it can directly cause air pollution and respiratory diseases (Kutlu, 2020). Since the 1950s, the average surface temperature has increased by 0.6°C, and plain observations show that snow and ice now cover less surface area of the Earth. The impacts of rising global temperatures include soil degradation, agricultural productivity loss, desertification, biodiversity loss, ecosystem degradation, freshwater resource reduction, ocean acidification, and stratospheric ozone depletion, all of which have implications for human health (Rossati, 2017). With accelerating GHG emissions, species loss from warming and oxygen depletion alone becomes comparable to current direct human impacts within a century, culminating in a mass extinction rivaling those of Earth’s past. Biodiversity is thus under significant threat (Schipper et al., 2024).
In response to this extreme climate crisis, countries worldwide are actively taking measures to control and reduce emissions of GHGs. In 1992, the United Nations Framework Convention on Climate Change was established to stabilize GHG concentrations to avoid harmful impacts. The Kyoto Protocol, adopted in 1997, established the legal responsibility of developed countries for reducing GHG emissions. The Paris Agreement was adopted in 2015, replaced the Kyoto Protocol and set a global goal to limit GHG emissions (Kutlu, 2020; Nisbet et al., 2021). Major countries and regions worldwide have mapped out their key timelines for carbon peaking and neutrality.
3 The potential of microalgae for carbon neutrality
3.1 The role of microalgae in the ecology of the Earth
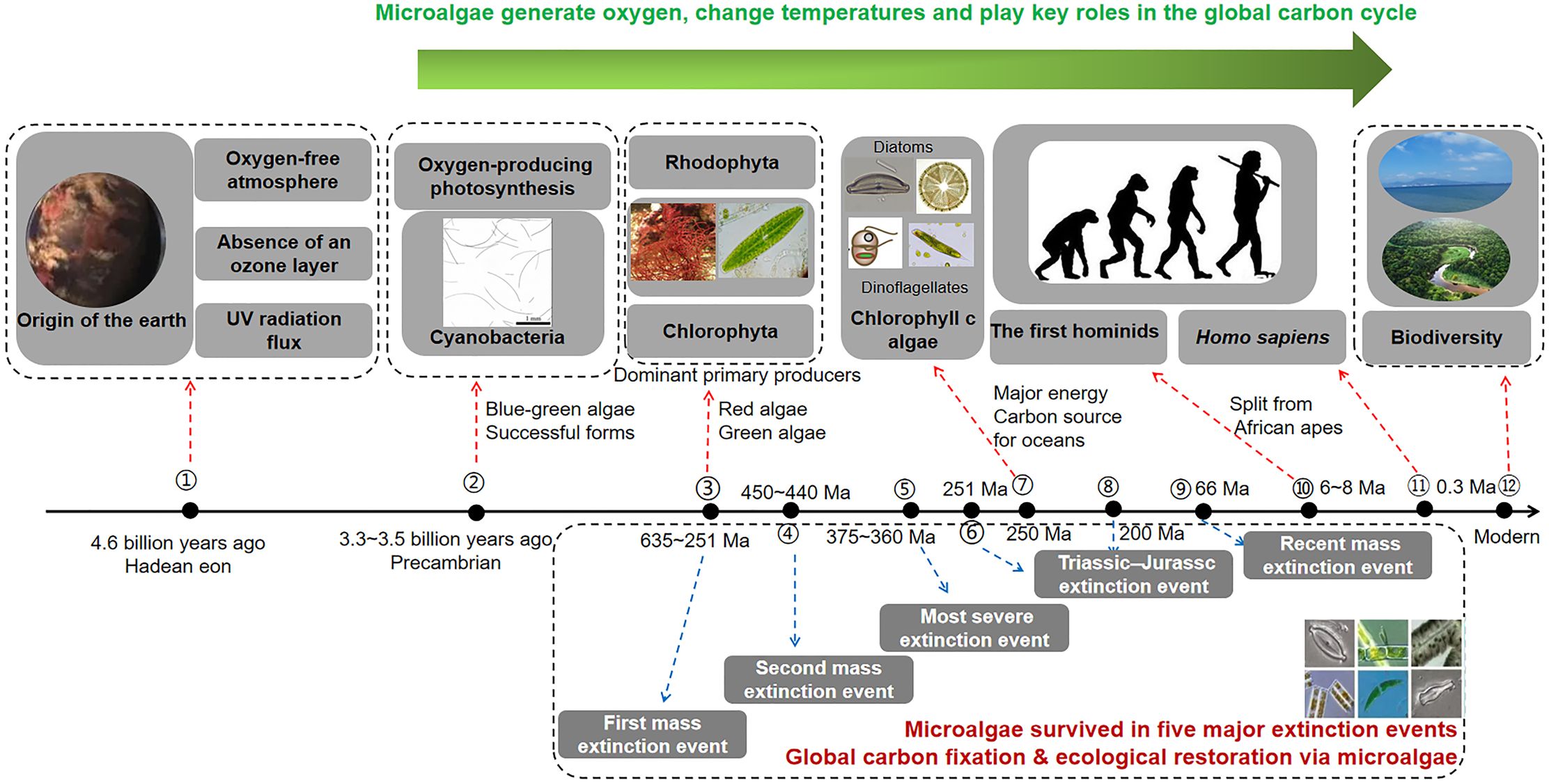
Algae comprise a large group of photosynthetic aquatic organisms, ranging from tiny, single-celled cyanobacteria to giant kelps that can reach tens of meters in length. They account for approximately half of the Earth’s total photosynthetic capacity and play a vital role in global biogeochemical and energy cycles (Zheng et al., 2022). Microalgae have played a significant role in shaping the Earth’s ecosystems and accelerating the production of biomass. Throughout the Earth’s over four-billion-year history, five major biological extinction events have occurred (Raven, 2022). Microalgae have survived due to their high adaptability (Mallén-Ponce et al., 2022) (Figure 3). Unicellular microalgae that appeared in ocean water enhanced atmospheric composition through CO2 consumption and day-night oxygen production, overcoming GHG barriers.
3.2 Carbon sequestration by microalgae
Microalgae capture atmospheric CO2 through their photosynthetic ability, thereby reducing GHG emissions (Barati et al., 2021). This capability is assessed by measuring the amount of CO2 absorbed per unit of microalgae biomass which not only showcases environmental benefits but also boosts biomass yield, as CO2 acts as a vital carbon source (Verma and Srivastava, 2018; Yadav et al., 2019; Isiramen et al., 2022). The absorption of CO2 by microalgae is crucial for mitigating climate change, with specific strains like Chlorella pyrenoidosa and Scenedesmus abundans displaying notable sequestration in batch PBR systems (Kargupta et al., 2015). Microalgae play a key role in achieving carbon neutrality by converting sequestered CO2 into biomass, thereby reducing atmospheric CO2 levels and addressing global warming threats. While plants generally reduce carbon emissions by only approximately 3%-6%, microalgae can be 50-fold more effective under ideal conditions. It has been estimated that 1 g of biomass produced by microalgae can fix 1.83 g CO2 (Li et al., 2023). On a broader scale, microalgae covering 100,000 km2 have the potential to sequester 2.35 Gt CO2 annually, averaging around 324.33 million tons (Zhao and Su, 2020). The effectiveness of carbon sequestration varies based on the microalgal strain and cultivation conditions. Specific cultivation systems and innovations enhance carbon sequestration rates; For example, the CO2 absorption and microalgae conversion (CAMC) system, particularly with Spirulina under mixotrophic conditions, demonstrates increased CO2 sequestration, highlighting the significance of growth conditions (Rame et al., 2023).
In terms of carbon fixation mechanisms of microalgae, three main systems are involved: (1) the inorganic carbon (Ci) transporter; (2) carbonic anhydrase (CA), which converts Ci to CO2; and (3) microcompartments containing rubisco for CO2 delivery. Microalgae convert Ci into organic carbon through rubisco to achieve carbon fixation (Prasad et al., 2021; Singh et al., 2016). When CO2 dissolves in water, it forms CO2, HCO3−, CO32−, and H2CO3, with CO2 and HCO3− being the primary species utilized by microalgal cells. Carbonic anhydrases help maintain the equilibrium between CO2 and HCO3−, facilitating their transport across membrane. Once Rubisco acts, CO2 is transformed into the carbohydrate precursors used for cell metabolism and growth (Morales et al., 2018). Most microalgae can assimilate a wide range of inorganic carbon including CO2, carbonate, and bicarbonate (Xu et al., 2023).
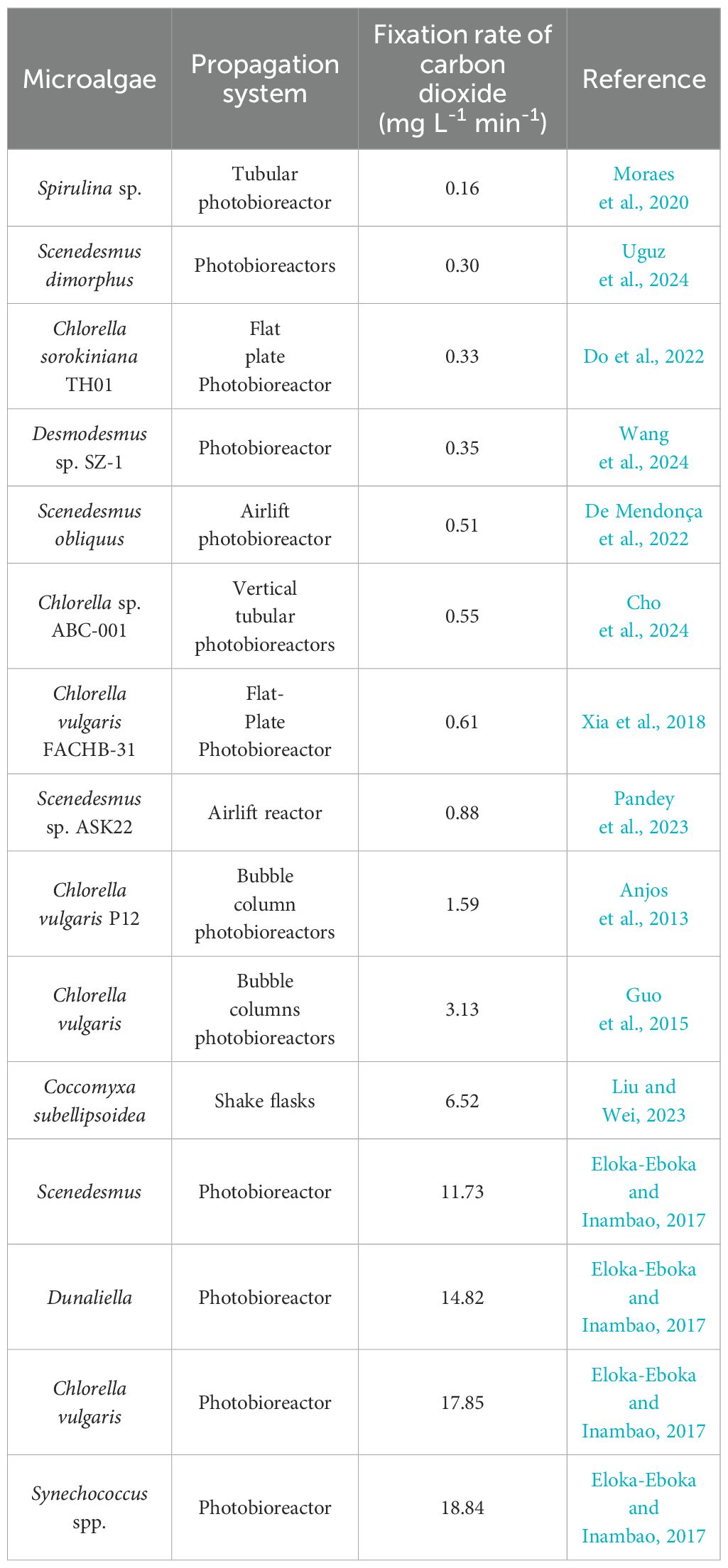
The capability of different microalgae to tolerate CO2 varies. As shown in Table 1, microalgal-based CO2 fixation rates range from 0.16 mg L-1 min-1 (Spirulina sp.) to over 18.84 mg L-1 min-1 (Synechococcus spp.). For example, the maximum CO2 removal rate for Chlorella vulgaris was reported to be 17.85 mg L-1min-1, which is lower than that of Synechococcus spp. (18.84 mg L-1 min-1). Dunaliella is also effective in CO2 sequestration, achieving a rate of 14.82 mg L-1 min-1 due to its high uptake of CO2 (Eloka-Eboka and Inambao, 2017). Even within the same microalgal species, there are differences in carbon sequestration capacity among different strains. The fixation rate of carbon dioxide C. vulgaris FACHB-31 is lower than that of C. vulgaris P12, which may be associated the strains and propagation system. Cultivation mode and reactor design can also affect scalability and efficiency. Generally, in terms of CO2 fixation, bubble columns PBRs are better than flat plate PBRs. Thus, photoautotrophic CO2 fixation capacities differ in strains and propagation system. Research has indicated that the optimal design of PBRs, particularly in terms of optimizing gas-liquid two-phase flow and enhancing the transfer of light, CO2, and nutrients, significantly impacts microalgal growth. PBRs should be designed based on the characteristics of gas-liquid flow and mass transfer (Fu et al., 2019). Relatively, C. vulgaris with bubble column PBRs may be suitable models for carbon fixation.
3.3 Advantages of microalgae-based carbon sequestration
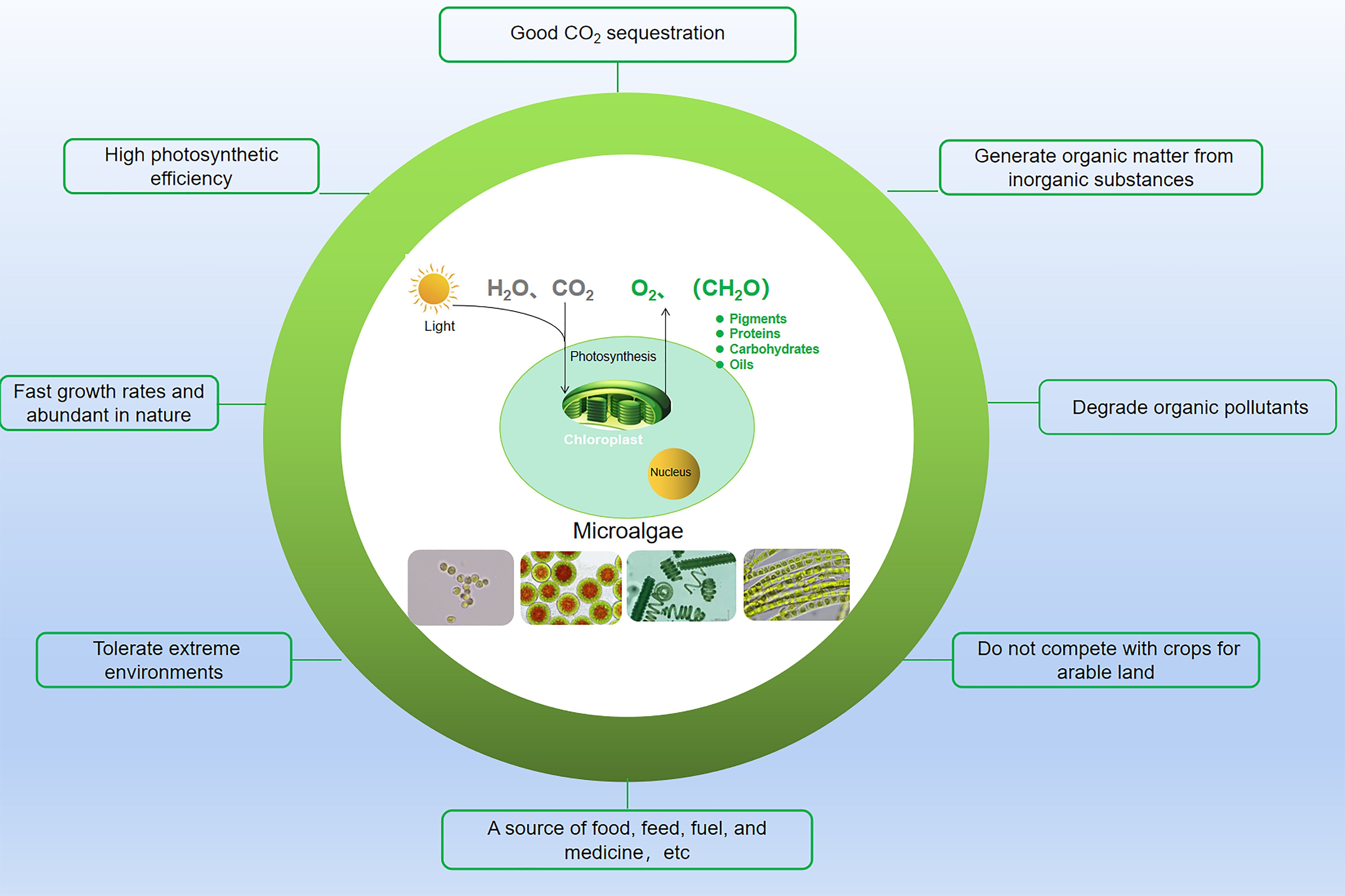
To date, approximately 50,000 species of microalgae have been discovered and classified into cyanobacteria, green algae, golden algae, and red algae. Compared to terrestrial carbon sinks, microalgae possess several advantageous characteristics: they do not occupy arable land, exhibit high biomass productivity, grow rapidly, and demonstrate strong adaptability (Figure 4) (Wang et al., 2020b). For instance, microalgae thrive in diverse habitats across temperate, tropical, and polar regions, not just in an aquatic environment but also in soil, deserts, oil fields, rocky terrains, and hot springs (Dhingra and Ahluwalia, 2007; Sidhu and Ahluwalia, 2011). As primitive plants (thallophytes), they lack roots, stems, and leaves. This structural simplicity enables efficient energy conversion and adaptability to various environmental conditions. Consequently, biological CO2 fixation by microalgae is considered a promising method of carbon capture and utilization (Zhou et al., 2017).
Carbon is the main component of microalgal cells, accounting for approximately 50% of their dry cell mass. It is estimated that the production of 100 tons of microalgal biomass equates to the fixation of 183 tons of CO2. As the main photosynthetic oxygen-producing microorganism, microalgae are responsible for approximately 50% of global CO2 fixation (Zhou et al., 2017). Microalgae, with their unique properties, serve as efficient photosynthetic cell factories for CO2 sequestration. Importantly, to ensure a sufficient supply of CO2 for the Calvin cycle during photosynthesis, microalgae have evolved CCM over billions of years. This mechanism can elevate the CO2 concentration at the Rubisco enzyme site to 1000 times higher than that of the surrounding environment. CCM primarily enhances the affinity of cells for inorganic carbon during photosynthesis by facilitating the transport of inorganic carbon. This process significantly increases the concentration of CO2 at the carboxylase active site of Rubisco. By doing so, it promotes Rubisco’s function as a carboxylase while simultaneously inhibiting its oxidase activity, thereby improving the overall efficiency of photosynthesis (Ma et al., 2022). Species like C. sorokiniana and Chlamydomonas reinhardtii, exhibit a faster regeneration of ribulose 1,5-bisphosphate. This accelerates carbon flux into the tricarboxylic acid cycle, amino acid synthesis, and lipid synthesis, facilitated by reduced glycolytic pathways and anaplerotic reactions. Consequently, these microalgae achieve higher photosynthetic efficiency compared to C3 and C4 plants (Treves et al., 2022). Moreover, microalgae also require lower light intensity, yet achieve higher aerial productivity, which markedly enhance their potential for carbon sequestration (Singh and Ahluwalia, 2013). Their metabolic versatility is equally compelling: They can be cultivated autotrophically, heterotrophically, or mixotrophically, offering unparalleled flexibility in production systems. Salinity tolerance further extends their geographic reach, allowing deployment in regions where freshwater is scarce (Rodolfi et al., 2009). Environmentally, microalgae outperform bacteria and fungi in degrading a wide spectrum of organic pollutants, conferring a decisive advantage in wastewater treatment. Within ecosystems, they act as foundational trophic links, supplying carbon and nutrients to bacteria and invertebrates (Subashchandrabose et al., 2013). Emerging research also positions microalgae as a next-generation protein source: Compared with conventional plant proteins, they deliver a denser nutrient profile; they could substantially curb GHG emissions, partially replacing livestock farming and agriculture (Liu et al., 2022).
In addition to their intrinsic capabilities, microalgae can form synergistic relationships with bacteria, creating consortia that enhance their carbon capture and utilization efficiency. These consortia leverage the complementary metabolic pathways of microalgae and bacteria to optimize CO2 fixation and biomass production (Hasnain et al., 2023). During photosynthesis, algae produce organic matter that is subsequently utilized by bacteria. As bacteria decompose this organic matter, they release nutrients and other compounds that, in turn, boost algal growth. Moreover, algae and bacteria can be employed to treat wastewater. Algae absorb nutrients and contaminants, while bacteria break down organic substances, thus effectively purifying the water. Thus microalgal-bacterial nexuses are valuable for atmospheric CO2 sequestration and the pollutants remediation. For example, engineered systems that integrate algal-bacterial symbiosis have been reported to achieve over 80% nutrient removal efficiency and a 22-35% increase in CO2 fixation efficiency compared to axenic algal systems. These findings highlight their dual role in climate mitigation and promoting a circular economy (Hu et al., 2025). The application of such consortia in bioreactors and other cultivation systems has shown promising results, suggesting that they could play a significant role in future carbon capture and utilization strategies.
Thus, in terms of techno-economic feasibility and scalability, microalgae-based carbon sequestration demonstrates more pronounced advantages and represents an ideal solution.
3.4 The potential of microalgae as carbon-neutralizing biofactories to provide high-value chemicals
Microalgae function as self-contained, carbon-neutralizing biofactories capable of synthesizing a sweeping portfolio of high-value commodities: human food, animal and aquaculture feeds, cosmetics, nutraceuticals, pharmaceuticals, fertilizers, bioactives, and advanced biofuels (Zhou et al., 2017). Microalgae are prolific biochemical foundries, generating an expansive suite of high-value metabolites: proteins, carbohydrates, lipids, and PUFAs (such as omega-3 fatty acids), polysaccharides, polyphenols, sterols, and pigments (chlorophylls, carotenoids, and algal bisphenol). Folic acid, pantothenic acid, dietary fibre, and trace elements required by humans also exist in microalgae ((Ścieszka and Klewicka, 2019). In addition to direct consumption, incorporation into food matrices, several of these algal-derived bioactives are commercially isolated and formulated as dietary supplements (Yu et al., 2022). Representative species and their corresponding high-value products are summarized in Table 2.
4 Microalgal biotechnology for carbon neutrality
4.1 Microalgal biotechnology
Synthetic biology is a cutting-edge, future-oriented discipline that leverages multidisciplinary approaches to quantify and reengineer biological processes. By designing, building, testing, and learning about standardized components and modules, it enables the modification or complete redesign of existing biological systems (Clarke and Kitney, 2020; Singh et al., 2020). This technology has been successfully applied to a variety of engineered cells, including Escherichia coli, yeast, animal cells, and microalgae, to produce a broad range of products. The products include chemicals, pharmaceuticals, proteins, probiotics, biosensors, fertilizers, textiles, and food items, with applications spanning environmental, agricultural, and health sectors (Meng and Ellis, 2020).
However, microbial chassis such as bacteria (e.g., E. coli) and yeast (e.g., Saccharomyces cerevisiae and Pichia pastoris) have notable limitations. These include restricted capacities for protein and lipid production, high production costs, and risks of contamination by endotoxins and pathogens (Moses et al., 2017; Tran and Prindle, 2021; Tsai et al., 2015). Microalgae serve as ideal microbial chassis for natural product biosynthesis, being unicellular organisms that undergo rapid cell division (for example, C. reinhardtii has a doubling time of 5-8 h). They can thrive in photosynthetic, heterotrophic, and mixotrophic lifestyles. Moreover, microalgae can fix CO2 through photosynthesis without requiring agricultural land (Moses et al., 2017). Interestingly, microalgae and diazotrophic organisms are capable to embrace different types of symbiotic associations. As a result, the utilization of microalgae and diazotrophic organisms (especially for various species of Azotobacter and Azospirillum) in consortia is garnering significant interest as a potential alternative for reducing production costs and increasing yields of microalgae biomass, as well as for producing derived products and serving biotechnological purposes (Llamas et al., 2023). Importantly, microalgae possess a suite of complex biosynthetic capabilities for natural product formation, and new genes have been identified that enable the synthesis of novel products (O’Neill et al., 2016). In recent years, gene editing technologies, such as clustered regularly interspaced short palindromic repeats (CRISPR) and ribonucleic acidinterference (RNAi), have propelled the development of microalgal synthetic biology (Chen et al., 2022b). Successful genetic engineering in microalgae has been reported for recombinant protein, oil, and hydrogen production, carotene synthesis, and CO2 fixation (Vazquez-Villegas et al., 2018). For instance, given that inefficient light utilization can limit biomass accumulation in high-density microalgal cultures, synthetic biology has been applied to enhance light-capture systems in these organisms. Additionally, genetic modifications of C. reinhardtii to introduce new functions, such as highly efficient nitrogen fixation, or to enable pharmaceutical and vaccine production, have also been demonstrated (Suarez et al., 2022). Genetic engineering, coupled with other technological innovations, can improve the photosynthetic efficiency of microalgae for enhanced CO2 biosequestration and biorefinery applications (Ma et al., 2022).
4.2 Prospects for the application of microalgae in biomanufacturing and carbon capture
Biofuelus are renewable, with biorthanol and biodiesel being particularly clean-burning. Amid the global energy crisis, microalgae have emerged as a promising alternative feedstock for biodiesel production (Jagadevan et al., 2018). Microalgae efficiently convert CO2 and solar energy into biomass, making them an excellent source of renewable biofuels (Thanigaivel et al., 2022). Unlike the first two generations of biodiesel feeds (i.e., food and non-food crops), microalgae, as the third generation of biodiesel feeds, can utilize sunlight more efficiently to produce oils and lipids. Biodiesel can be obtained from microalgae either by extracting algal oil with organic solvents or catalyzing microalgal lipids using acids or enzymes (Castillo López et al., 2015).
The unicellular industrial oil-producing microalgae N. oceanica stands out for its ability to accumulate large amounts of oil through the tricarboxylic acid cycle, making it suitable for large-scale industrial cultivation (Taleb et al., 2015). Saponifiable lipids (SLs) constitute approximately 12.1% of N. oceanica’s dry biomass. These SLs can be extracted as free fatty acids and subsequently catalyzed, esterified, and transformed into methyl esters (biodiesel) with up to 85% purity. N. oceanica is also a model organism for microalgal systems and synthetic biology research (Gong et al., 2020). Euglena, a unicellular eukaryote with both plant and animal characteristics, can potentially produce biodiesel when cultured in the presence of glucose and in the dark. This makes it a high-quality feedstock for producing short-chain aviation fuel (Chen et al., 2022a). In addition to biodiesel, hydrogen is another clean alternative to fossil fuels for power generation and transportation. The unicellular microalgae C. reinhardtii is capable of producing hydrogen, but its efficiency is limited by factors such as the presence of hydrogenase. Synthetic biology has been applied to enhance hydrogen production in C. reinhardtii by modifying photosystem II (PSII) or disrupting cyclic electron flow (King et al., 2022).
4.2.1 Chlorella pyrenoidosa: a versatile microalgae with high nutritional and environmental value
C. pyrenoidosa is a widely recongized green microalgae with a long history of use as a food additive globally. This microalgae is renowned for its high protein content, which exceeds 50%, positioning it as an exceptional protein source (Liu and Wang, 2022). Additionally, it contains 20%-30% unsaturated fatty acids, 20%-25% carbohydrates, and a comprehensive array of essential amino acids. It is also rich in a wide variety of vitamins and minerals, including potassium, zinc, calcium, and iron. Moreover, it contains growth factors that have been shown to enhance immune function, particularly in children and the elderly. As a result, it has gained significant attention as a health food, with industrial production primarily concentrated in Japan, China, France, Portugal, and South Korea (Hidaka et al., 2004; Kumar et al., 2020).
C. pyrenoidosa has emerged as a highly promising candidate for carbon capture. This is attributed to its elevated photosynthetic rate, robust environmental adaptability, and strong reproductive capacity (Wang et al., 2018). A novel filled sphere carrier reactor was reported to optimize biomass production and enhance the carbon sequestration rate of C. pyrenoidosa. Notably, the carbon sequestration rate achieved impressive levels of 9.98 g/L and 18.32 g/L/d within a single day, representing a substantial increase of 249.5 and 79.65 times, respectively. This innovative utilization of C. pyrenoidosa demonstrates remarkable efficiency in carbon capture, thereby contributing significantly to the advancement of environmentally sustainable practices (Wei et al., 2023).
4.2.2 Nannochloropsis oceanica: a promising lipid producer for sustainable carbon fixation
N. oceanica is highly regarded as a valuable lipid producer, particularly rich in triglycerides and polar lipids such as phospholipids and glycolipids. These lipids constitute approximately 25%-45% of the dry weight of the microalgal biomass (Blanco-Llamero and Señoráns, 2021; Castejón and Señoráns, 2019). Notably, N. oceanica can accumulate high levels of eicosapentaenoic (EPA), a key omega-3 PUFAs, making it a viable substitute for fish oil-derived omega-3 PUFAs in various applications.
In recent study, the halotolerant microalgae N. oceanica CCMP1779 was selected for its exceptional efficiency in carbon fixation. When cultivated with methanol and 15% CO2, the biomass yield and carbon fixation efficiency of N. oceanica were found to be 8.4 times and 78.3% higher, respectively, compared to those of N. oceanica grown solely with 15% CO2 gas (Wang et al., 2020a). This underscores the potential of N. oceanica as a sustainable and ideal resource for carbon fixation.
4.2.3 Euglena gracilis: a multifunctional microorganism with high value
E. gracilis is a unique microorganism characterized by a prominent red spot composed of carotenoid granules located near the base of its emergent flagellum (O’Neill et al., 2016). This species is nontoxic to humans and serve as a rich source of vitamins, minerals, essential amino acids, and unsaturated fatty acids. It possesses a range of health-promoting properties, including anticancer, anti-inflammatory, antioxidative, and anti-obesity effects. Consequently, E. gracilis has garnered attention for its potential applications in food additives, feed, biodiesel, medicine, and fertilisers (Häder and Hemmersbach, 2022).
A distinctive feature of E. gracilis is its ability to accumulate large reserves of the polysaccharide 1,3-glucan, also known as paramylon. This compound is structurally similar to starch and is highly crystalline (approximately 90%). The paramylon content often exceeds 50% of the dry cell weight, especially under heterotrophic growth conditions. Paramylon has been shown to enhance immune function, lower cholesterol levels, and exhibits antidiabetic, antihyperglycemic, and hepatoprotective properties. It also demonstrated therapeutic activity against colorectal and gastric cancer (Gissibl et al., 2019). Given its high value, paramylon is frequently utilized as a food additive in the pharmaceutical industry (Ivušić et al., 2022).
Recent research has focused on optimizing the cultivation conditions for E.gracilis to maximize its yield and functionality. A study investigated the influence of light intensity and hydraulic retention time (HRT) on the biomass production of E. gracilis. An advanced pilot-scale PBR with a larger working volume of 1000 liters was developed to enhance the production of E. gracilis (Chae et al., 2006). The results demonstrated the efficacy and cost-effectiveness of E. gracilis in sequestering CO2, highlighting its potential as a sustainable solution for carbon capture and utilization.
In summary, E. gracilis stands out as a high-value microorganism with diverse applications in health, nutrition, and environmental sustainability. Its ability to produce paramylon and sequester CO2 positions it as a promising candidate for both industrial and ecological applications.
4.2.4 Haematococcus pluvialis: a premier natural astaxanthin producer with environmental and industrial potential
H. pluvialis is renowned as the foremost natural astaxanthin producer of astaxanthin, a powerful antioxidant with a wide range of applications (Jiang et al., 2022a). Astaxanthin is utilized in nutritional supplements, food and feed additives, and cosmetics due to its high antioxidant activity (Chen et al., 2015; Sharma et al., 2018). Under optimal environmental conditions, H. pluvialis exists as flagellated motile cells. However, under stress, it transforms into spherical, non-motile cysts that accumulate significant amounts of astaxanthin, reaching 1.9-7% of the dry cell weight (Koopmann et al., 2022; Shah et al., 2016).
H. pluvialis has also been approved as a color additive in salmon feed and as a dietary supplement ingredient for humans in the United States, Japan, and several European countries (Li et al., 2011). It was also classified as a novel food resource by the National Health Commission of China in 2010. In 2019, the Korea Food and Drug Administration validated astaxanthin-enriched biomass as a safe poultry feed, noting its ability to enhance immunity without adverse effects (Hong et al., 2019).
A recent research evaluated the efficency of CO2 fixation using H. pluvialis in a sequentially operated tubular photobioreactor system. The sequential operation system, combined with H. pluvialis cultivation, significantly enhanced CO2 fixation efficiencies. Indoor CO2 fixation rates increased from 12.34% to 49.37%, while outdoor rates rose from 13.55% to 49.15%, compared to single bioreactor operation (Lee et al., 2015). This study underscores the potential of H. pluvialis in mitigating GHG emissions through photosynthetic CO2 sequestration, offering a promising solution to combat climate change.
In summary, H. pluvialis not only serves as a valuable source of astaxanthin for various industries but also demonstrates significant potential in environmental applications, particularly in carbon capture and mitigation of GHG emissions.
4.2.5 Chlamydomonas reinhardtii: a model organism for lipid metabolism and biofuel production
Lipid metabolism in microalgae is a key pathway for producing lipids that can serve as raw materials for biofuels. C. reinhardtii is widely recognized as a model organism for the production of neutral lipid triacylglycerol (TAG), which is a primary feedstock for biodiesel (Li-Beisson et al., 2015, 2019). This microalgae is particularly notable for its ability to accumulate TAG under specific conditions. Intense light exposure can induce reactive oxygen species (ROS) stress in C. reinhardtii, triggering cellular autophagy and leading to the accumulation of TAG and increased lipid content (Chouhan et al., 2022). This stress-induced lipid accumulation makes C. reinhardtii an attractive candidate for biodiesel production, as well as for applications in aquafeeds and human health products. Besides, C. reinhardtii is also regarded as a reference organism to study algal-microbial interactions. By means of their interaction with other microorganisms, several biotechnological processes like hydrogen can be increased in C. reinhardtii (Calatrava et al., 2023).
Recent research has focused on enhancing the lipid productivity of C. reinhardtii through genetic engineering and optimized culture conditions. One study demonstrated that engineered C. reinhardtii CC-400, harboring carbonic anhydrase, significantly increased carbon flux and biomass production. Under mixotrophic culture conditions with 5% CO2 in a PBR, the engineered strain achieved an optical density (OD680) of 4.56 (Lin et al., 2022).
The application of microalgae in bioremediation and bioproduct production has garnered substantial attention in recent literature. Several species, including C. pyrenoidosa and C. vulgaris, have been extensively tested for their potential to bioaccumulate and degrade both organic and inorganic pollutants present in wastewaters derived from a wide range of industrial and domestic sources. For example, the introduction of a cyanobacterial cyanase gene into C. reinhardtii has been demonstrated to effectively remediate cyanide pollutants in freshwater, positioning it as a promising eco-friendly bioremediator (Sobieh et al., 2022). Additionally, the biodegradation of phenol by alginate-immobilized C. reinhardtii cells and the potential of algal cells for silver nanoparticle (Ag-NPs) bioremediation have been documented (Nazos and Ghanotakis, 2021; Xu et al., 2022). Fluoroquinolones (FQs), a class of antimicrobial agents whose usage has surged in recent years, have also been targeted for biodegradation studies. Notably, C. reinhardtii achieved a maximum removal rate of 77.67% for moxifloxacin (MOX) at a concentration of 1 mg/L and 34.04% for gatifloxacin (GAT) at 20 mg/L, highlighting the microalgae’s capacity for FQ biodegradation (Wan et al., 2022). In the realm of bioproduct production, C. reinhardtii has been identified as a prolific producer of a wide array of valuable biomolecules, including polysaccharides, lipids, functional proteins, pigments, hormones, vaccines, and antibodies. These biomolecules, produced either spontaneously or under controlled conditions, have direct implications for human nutrition and diet (Masi et al., 2023). A particularly intriguing development is the establishment of a photosynthetic platform in C. reinhardtii for the production of pentalenene through the expression of a heterologous pentalenene synthase (penA). This innovation not only underscores the potential for producing other sesquiterpenes in microalgae but also offers a rational engineering strategy for their synthesis in other industrial microorganisms (Li et al., 2025).
Thus, C. reinhardtii emerges as a model organism for studying lipid metabolism and biofuel production. Its capacity to accumulate TAG under stress conditions, coupled with advancements in genetic engineering and optimized culture strategies, renders it a highly promising candidate for sustainable biofuel production. It not only holds significant potential for industrial applications but also contributes to environmental sustainability.
4.2.6 Dunaliella salina: a halophilic microalgae with high value in nutrition and environmental sustainability
D. salina is a single-celled marine phytoplankton known for its high carotenoids content, particularly beta-carotene, and its ability to thrive under saline conditions. This microalgae is widely used in food and medicine due to its antioxidant and anti-inflammatory properties (Anila et al., 2016; Spolaore et al., 2006). Notably, D. salina has been reported to mitigate intestinal mucositis damage caused by gamma radiation (Khayyal et al., 2019). Additionally, it produces PUFAs, lipids, and other valuable metabolites (Gonabadi et al., 2022).
A recent study investigated the capacity of eukaryotic green algae to utilize sodium bicarbonate (NaHCO3) as their primary carbon source. D. salina, a halophilic green microalgae commonly found in marine habitats, was chosen as a representative species (Kim et al., 2017). The study found that adding 5 g/L of sodium bicarbonate significantly enhanced the growth of D. salina, resulting in a 2.84-fold higher specific growth rate compared to a bicarbonate-free control. This bicarbonate-based cultivation method achieved biomass productivity comparable to that of CO2-based systems, provided that pH was carefully controlled.
4.2.7 Spirulina platensis: a high-value microalga for CO2 sequestration and industrial applications
S. platensis is a nutrient-dense microalgae, rich in protein, gamma-linolenic acid, and phycocyanin, and has been extensively developed as a food additive. China is currently the world’s largest producer of S. platensis (Chen et al., 2019). A research study investigated the potential of S. platensis for CO2 concentrations (0.5% to 10%), temperatures (10°C to 40°C), and light intensities (60 μmol s−1 m−2 to 200 μmol s−1 m−2). The results demonstrated exceptional CO2 sequestration efficiency, achieving up to 99.9% (Ramírez-Pérez and Janes, 2009). Further research and development could unlock its full potential in addressing global environmental challenges while supporting economic growth.
4.3 Prospects for the application of microalgae in pharmaceuticals
As photosynthetic organisms, microalgae can efficiently convert solar energy into biomass, making them promising platforms for low-cost and environment-friendly production of pharmaceuticals, recombinant proteins, and other high-value products (Yan et al., 2016). For instance, Phaeodactylum tricornutum has been used as a chassis to synthesize a monoclonal human immunoglobulin G (IgG) antibody against the hepatitis B surface protein and related antigens. The resulting product was fully assembled and functional, accumulating up to 8.7% of the total soluble protein, equating to 21 mg of antibody per gram of algal dry weight (Hempel et al., 2011). Another example is the construction of an expression system using Thalassiosira pseudonana to produce immunoglobulin-binding protein A (IbpA) DR2, a vaccine against bovine respiratory disease.The system elicited immune responses in a mouse immunization model (Davis et al., 2017). Compared to microbial, mammalian, and plant systems, microalgal chloroplasts offer several advantages in the biosynthesis of therapeutic proteins. Products synthesized in microalgal chloroplasts can be efficiently folded and accumulated without contamination, making them suitable for manufacturing complex immunogens and low-cost oral vaccines. This reduces downstream processing requirements. Vaccines such as CTB-VP1and E2 have been successfully synthesized in the chloroplasts of C. reinhardtii (Dyo and Purton, 2018).
The use of microalgae for oral vaccine production is highly promising. Vaccine antigens can accumulate and fold correctly in microalgae, enabling the effective oral administration of certain antigenically enhanced fusion proteins (Yan et al., 2016). Marine antimicrobial peptides (AMPs) are gaining attention as potential feed additives for fish and novel drugs against fish intestinal pathogens, enhancing fish immunity in aquaculture. For example, N. oceanica has been used to biosynthesize various AMPs, including bovine lactoferrin. Medaka (Oryzias latipes) acquired immunity to Vibrio parahaemolyticus after being fed algal powder that produced vaccine, demonstrating the feasibility of synthesizing edible vaccines using microalgae as a chassis cell (De Grahl and Reumann, 2021).
Since the COVID-19 pandemic began in 2019, microalgae has been explored for vaccine development. C. sorokiniana and C. reinhardtii have been used to synthesize the SARS-CoV-2 receptor-binding domain (RBD) vaccine. The RBD produced by these microalgae was found to be antigenic (Govea-Alonso et al., 2022; Malla et al., 2021). These studies indicated that microalgae have the potential to produce high-value pharmaceuticals, including vaccines for global health concerns.
The commercial-scale production of microalgal biomass for valuable bioproducts faces challenges due to limitations in production yields, regulatory hurdles, and high downstream processing costs (Kaur et al., 2023). Innovations in closed-system lighting and control conditions, along with scale-up technologies, are crucial for improving cost-effectiveness. The downstream processes of isolation, harvesting, extraction, and purification are complex and costly. Bioactive metabolites may be insufficient, fragile, or altered during extraction, complicating their analysis and commercial-scale production. Moreover, achieving sufficient annual biomass production under sterile conditions and developing high-throughput screening methods for microalgae culture are essential. Medical safety is a critical aspect that must be rigorously examined during the development and advancement of microalgae technology. Therefore, the establishment of comprehensive regulations for the production, exploitation, and consumption of microalgae-based bioactive compounds is imperative.
In summary, microalgae offer a versatile and sustainable platform for the production of pharmaceuticals, recombinant proteins, and vaccines. Their ability to efficiently convert solar energy into biomass, combined with their capacity for low-cost and environmentally friendly production, positions them as a valuable resource for the biopharmaceutical industry. Future research and development should focus on optimizing microalgal expression systems and scaling up production to meet global demands for high-value pharmaceuticals.
4.4 The challenge for microalgal biotechnology
While microalgal biotechnology holds significant promise for addressing global challenges such as climate change, food security, and sustainable energy production, the limitations, technical challenges, economic feasibility, and scalability are existed.
Microalgal biomass production can be more expensive than crop cultivation due to various reasons such as special maintenance requirements of specific microalgal species. The cultivation may require precise control of environmental conditions such as light intensity, temperature, pH, and nutrient availability. While PBRs offer controlled environments, they are expensive to build and operate. Open pond systems, on the other hand, are more cost-effective but are susceptible to contamination and less efficient in terms of biomass productivity (Singh et al., 2023). Genetic engineering has proven highly effective in enhancing the productivity and stress tolerance of microalgae under laboratory conditions. However, its application in industrial settings has achieved only limited success thus far (Grama et al., 2022). While genetically modified (GM) microalgal strains may offer economic advantages, they also pose potential environmental risks. These strains, despite their promise, face significant regulatory hurdles and challenges in gaining public acceptance. Moreover, the use of genetically modified organisms (GMOs) in food and environmental applications often encounters skepticism, which can significantly hinder their widespread commercial adoption. Scaling up from laboratory to industrial scale is not straightforward. It often necessitates substantial adjustments in cultivation conditions, process optimization, and infrastructure. These changes can be both technically challenging and economically demanding. For instance, despite progress of microalgae harvesting technologies has been made in laboratory studies, large-scale harvesting for microalgae is still severely impeded by the high cost (Liu et al., 2023).
Future research and development efforts should prioritize the optimization of cultivation systems, the improvement of harvesting and processing techniques, and the creation of cost-effective and scalable solutions. Additionally, engaging stakeholders, including policymakers, industry leaders, and the public, is crucial for navigating regulatory landscapes and fostering widespread acceptance of microalgae-based technologies.
5 Conclusion and future perspectives
Microalgae represent a highly promising solution for achieving carbon neutrality and addressing the global climate crisis. Their inherent advantages, such as rapid growth rates, minimal nutritional requirements, and the ability to thrive on non-arable land, making them an attractive and sustainable resource. Additionally, microalgae can be fully used as food without complex food preparation, reducing waste production and generating valuable by-products. Advances in microalgal biotechnology further enhance their potential by improving photosynthetic efficiency, carbon fixation capacity, and lower toxin content, thereby increasing biomass yield and overall productivity.
The potential applications of microalgal biotechnology in mitigating the global climate crisis and achieving carbon neutrality are illustrated in Figure 5. By fixing CO2 through photosynthesis, microalgae can significantly reduce GHG emissions, contribute to carbon peaking and neutrality, and help address the extreme global climate crisis (Figures 5A, B). Beyond carbon sequestration, microalgae are rich sources of high-quality proteins, PUFAs, astaxanthin, polysaccharides, and minerals. These attributes make them an excellent food source for humans and a valuable supplement due to their high nutritional value (Figure 5C). Additionally, microalgal biomass is poised to become a green energy source, offering a sustainable alternative to fossil fuels (Figure 5D). Microalgae also serve as ideal cell factories for the green bio-manufacturing of proteins, oils, and biopharmaceuticals through synthetic biology (Figure 5E). The microalgal biotechnology applications may play a pivotal role in fostering a circular economy by integrating waste streams into productive cycles. For instance, microalgae can be cultivated using waste gas and wastewater, thereby remediating pollutants while generating valuable biomass. This biomass can then be processed into high-value products such as functional food, biofuels, and pharmaceuticals, thus minimizing waste and maximizing resource efficiency. Moreover, microalgae-based biotechnology may treat gaseous effluents and simultaneously capture carbon sources for further biomass valorization. Within the concept of circular economy, bioenergy products and products in the agri-food industry as well as in the field of human health can be obtained from microalgae biomass.

Figure 5. An overview of the potential of microalgal biotechnology in dealing with the global climate crisis. (A) Microaglae can absorb the greenhouse gas (GHG), wasted water and light to improve environment (Gray dashed box); (B) The role of microalgae in reducing GHG emissions (Green dashed box); (C) The microalgae are rich for proteins, polyunsaturated fatty acids, carotenoids, polysaccharides and minerals, which can be used in food supplementary (orange dashed box); (D) The biomass and cell factories of microalgae with biological synthesis can be used in green energy (Light green dashed box); (E) Microalgae serve as ideal cell factories for the green bio-manufacturing of biopharmaceuticals (Purple dashed box).
Despite these promising prospects, microalgae-based carbon sequestration faces several challenges. These include infrastructure limitations, legal and regulatory issues, fairness concerns, pollution control, economic factors, social and cultural barriers, and the availability of energy resources. It requires further research to enhance microalgal carbon fixation, optimize cost-effective high-density cultivation methods, and improve the biosynthesis of valuable products. By addressing the identified challenges and advancing key research areas, microalgae can be positioned as a cornerstone in the global effort to achieve carbon neutrality and mitigate climate change (Figure 6). Hence, further research may focus on the following aspects: (1) optimizing genetic engineering and biosynthetic pathways to enhance the high-value biomass productivity, stress tolerance, and CO2 fixation ability of microalgae; (2) integrating microalgae technology with other technologies such as wastewater remediation and CCUS to offers multiple benefits; (3) conducting life cycle assessment and techno-economic analysis to identify the most sustainable and cost-effective approaches for microalgae-based carbon sequestration; (4) developing innovative cultivation techniques including designing economic PBRs and optimizing culture and processing systems; (5) establishing safety assessment of microalgae-derived products and evaluating the environmental risk of microalgae technology.
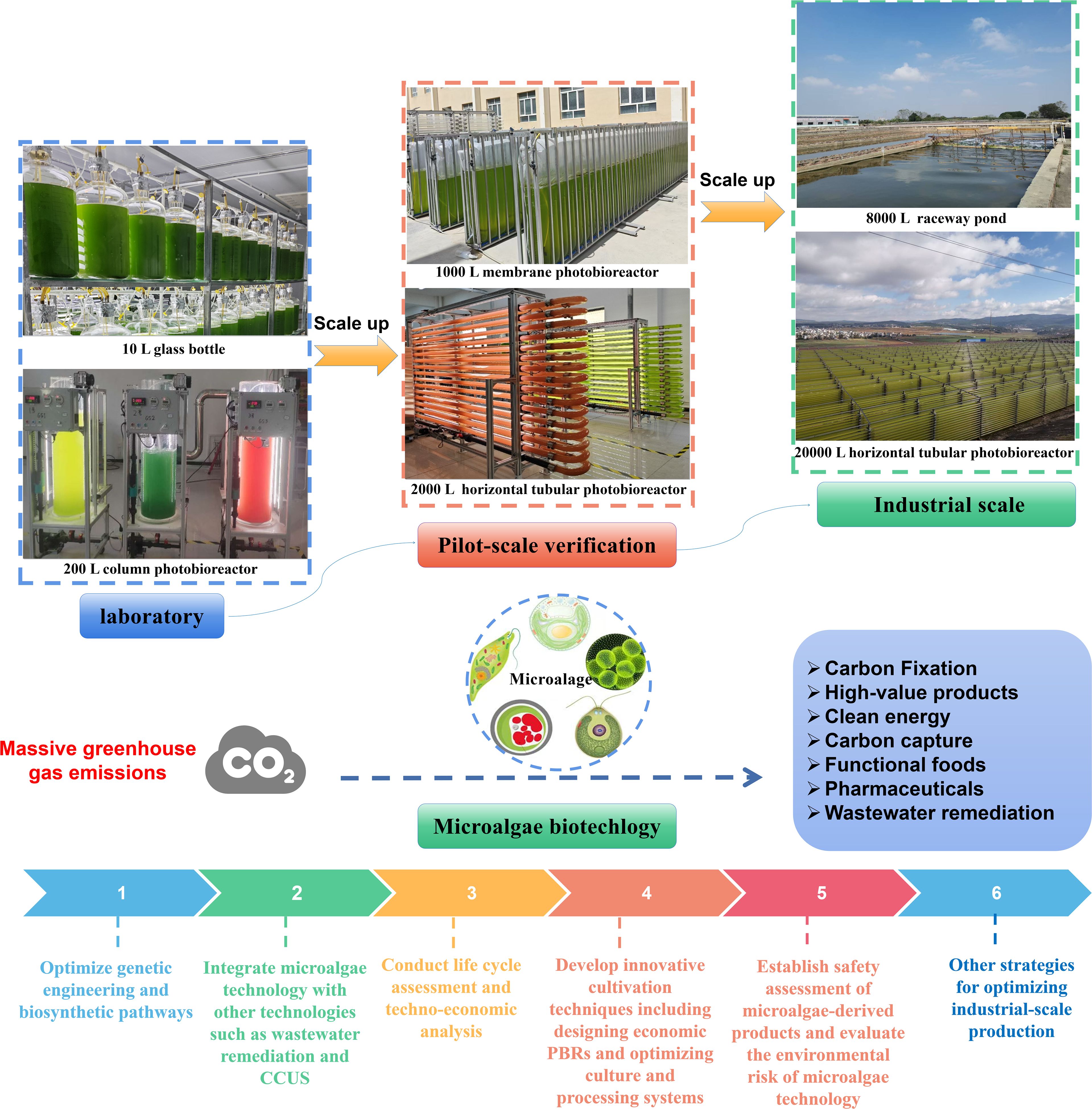
Figure 6. Schematic roadmap for scaling up microalgal strategies from laboratory to industrial scale.
Author contributions
HC: Data curation, Writing – original draft, Resources. YL: Data curation, Resources, Writing – original draft. ZD: Data curation, Writing – original draft, Resources. CY: Writing – review & editing, Resources, Data curation. XX: Resources, Writing – review & editing, Data curation. HB: Data curation, Resources, Writing – review & editing. WX: Writing – review & editing, Resources, Data curation. HZ: Writing – review & editing, Data curation, Resources. JG: Writing – review & editing, Resources, Data curation. ZQ: Writing – review & editing, Resources, Data curation. AJ: Supervision, Writing – review & editing, Funding acquisition. MR: Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Chinese Academy of Agricultural Sciences (grant number: 34-IUA-02), the Sichuan Science and Technology Department (grant number: 2024NSFC1261), the Xinjiang Science and Technology department (grant number: ZYYD2024CG09 and ZYYD2025CG10), and the National Agricultural Science and Technology Centre, Chengdu (Grant numbers: NASC2022KR06 and NASC2024KY22).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anila N., Simon D. P., Chandrashekar A., Ravishankar G. A., and Sarada R. (2016). Metabolic engineering of Dunaliella salina for production of ketocarotenoids. Photosynth. Res. 127, 321–333. doi: 10.1007/s11120-015-0188-8
Anjos M., Fernandes B. D., Vicente A. A., Teixeira J. A., and Dragone G. (2013). Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 139, 149–154. doi: 10.1016/j.biortech.2013.04.032
Anwar M. N., Fayyaz A., Sohail N. F., Khokhar M. F., Baqar M., Khan W. D., et al (2018). CO2 capture and storage: A way forward for sustainable environment. J. Environ. Manage. 226, 131–144. doi: 10.1016/j.jenvman.2018.08.009
Bačėninaitė D., Džermeikaitė K., and Antanaitis R. (2022). Global warming and dairy cattle: how to control and reduce methane emission. Anim. (Basel). 12, 2687. doi: 10.3390/ani12192687
Barati B., Zeng K., Baeyens J., Wang S., and Abomohra E. F. (2021). Recent progress in genetically modified microalgae for enhanced carbon dioxide sequestration. Biomass Bioenergy. 145, 105927. doi: 10.1016/j.biombioe.2020.105927
Bhujade R., Chidambaram M., Kumar A., and Sapre A. (2017). Algae to economically viable low-carbon-footprint oil. Annu. Rev. Chem. Biomol. Eng. 8, 335–357. doi: 10.1146/annurev-chembioeng-060816-101630
Blanco-Llamero C. and Señoráns F. J. (2021). Biobased solvents for pressurized liquid extraction of Nannochloropsis gaditana omega-3 lipids. Mar. Drugs 19, 107. doi: 10.3390/md19020107
Calatrava V., Tejada-Jimenez M., Sanz-Luque E., Fernandez E., Galvan A., and Llamas A. (2023). Chlamydomonas reinhardtii, a reference organism to study algal-microbial interactions: Why can’t they be friends? Plants (Basel). 12, 788. doi: 10.3390/plants12040788
Castejón N. and Señoráns F. J. (2019). Simultaneous extraction and fractionation of omega-3 acylglycerols and glycolipids from wet microalgal biomass of Nannochloropsis gaditana using pressurized liquids. Algal Res. 37, 74–82. doi: 10.1016/j.algal.2018.11.003
Castillo López B., Cerdán L. E., Robles Medina A., Navarro López E., Martín Valverde L., Hita Peña E., et al. (2015). Production of biodiesel from vegetable oil and microalgae by fatty acid extraction and enzymatic esterification. J. Biosci. Bioeng. 119, 706–711. doi: 10.1016/j.jbiosc.2014.11.002
Chae S. R., Hwang E. J., and Shin H. S. (2006). Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour. Technol. 97, 322–329. doi: 10.1016/j.biortech.2005.02.037
Chen Z., Chen Y., Zhang H., Qin H., He J., Zheng Z., et al. (2022a). Evaluation of Euglena gracilis 815 as a new candidate for biodiesel production. Front. Bioeng. Biotechnol. 10. doi: 10.3389/fbioe.2022.827513
Chen Y. Y., Lee P. C., Wu Y. L., and Liu L. Y. (2015). In vivo effects of free form astaxanthin powder on anti-oxidation and lipid metabolism with high-cholesterol diet. PloS One 10, 134733. doi: 10.1371/journal.pone.0134733
Chen H., Li T., and Wang Q. (2019). Ten years of algal biofuel and bioproducts: gains and pains. Planta 249, 195–219. doi: 10.1007/s00425-018-3066-8
Chen Z., Zhu J., Du M., Chen Z., Liu Q., Zhu H., et al. (2022b). A synthetic biology perspective on the bioengineering tools for an industrial microalga: Euglena gracilis. Front. Bioeng. Biotechnol. 10. doi: 10.3389/fbioe.2022.882391
Cherepovitsyn A., Chvileva T., and Fedoseev S. (2020). Popularization of carbon capture and storage technology in society: principles and methods. Int. J. Environ. Res. Public Health 17 (22), 8368. doi: 10.3390/ijerph17228368
Chia S. R., Nomanbhay S. B. H. M., Chew K. W., Munawaroh H. S. H., Shamsuddin A. H., and Show P. L. (2022). Algae as potential feedstock for various bioenergy production. Chemosphere 287, 131944. doi: 10.1016/j.chemosphere.2021.131944
Cho J. M., Oh Y. K., Lee J., Chang Y. K., and Park W. K. (2024). Development of dual strain microalgae cultivation system for the direct carbon dioxide utilization of power plant flue gas. Bioresour. Technol. 393, 130051. doi: 10.1016/j.biortech.2023.130051
Chouhan N., Devadasu E., Yadav R. M., and Subramanyam R. (2022). Autophagy induced accumulation of lipids in pgrl1 and pgr5 of Chlamydomonas reinhardtii under high light. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.752634
Clarke L. and Kitney R. (2020). Developing synthetic biology for industrial biotechnology applications. Biochem. Soc Trans. 48, 113–122. doi: 10.1042/BST20190349
Davis A., Crum L. T., Corbeil L. B., and Hildebrand M. (2017). Expression of Histophilus somni IbpA DR2 protective antigen in the diatom Thalassiosira pseudonana. Appl. Microbiol. Biotechnol. 101, 5313–5324. doi: 10.1007/s00253-017-8267-8
De Grahl I. and Reumann S. (2021). Stramenopile microalgae as “green biofactories” for recombinant protein production. World J. Microbiol. Biotechnol. 37, 163. doi: 10.1007/s11274-021-03126-y
De Mendonça H. V., Otenio M. H., Marchão L., Lomeu A., de Souza D. S., and Reis A. (2022). Biofuel recovery from microalgae biomass grown in dairy wastewater treated with activated sludge: The next step in sustainable production. Sci. Total Environ. 824, 153838. doi: 10.1016/j.scitotenv.2022.153838
Dhingra R. and Ahluwalia A. S. (2007). Genus Phormidium kutzing ex Gomont (Cyanoprokaryote) from diverse habitat of Punjab. J. Indian Bot. Soc 86, 86–94.
Do C. V. T., Dinh C. T., Dang M. T., Dang Tran T., and Giang Le T. (2022). A novel flat-panel photobioreactor for simultaneous production of lutein and carbon sequestration by Chlorella sorokiniana TH01. Bioresour. Technol. 345, 126552. doi: 10.1016/j.biortech.2021.126552
Dyo Y. M. and Purton S. (2018). The algal chloroplast as a synthetic biology platform for production of therapeutic proteins. Microbiol. (Reading). 164, 113–121. doi: 10.1099/mic.0.000599
Eloka-Eboka A. C. and Inambao F. L. (2017). Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Appl. Energy 195, 1100–1111. doi: 10.1016/j.apenergy.2017.03.071
Fu J., Huang Y., Liao Q., Xia A., Fu Q., and Zhu X. (2019). Photo-bioreactor design for microalgae: A review from the aspect of CO2 transfer and conversion. Bioresour Technol. 292, 121947. doi: 10.1016/j.biortech.2019.121947
Gissibl A., Sun A., Care A., Nevalainen H., and Sunna A. (2019). Bioproducts from Euglena gracilis: synthesis and applications. Front. Bioeng. Biotechnol. 7. doi: 10.3389/fbioe.2019.00108
Gonabadi E., Samadlouie H. R., and Shafafi Zenoozian M. (2022). Optimization of culture conditions for enhanced Dunaliella salina productions in mixotrophic culture. Prep. Biochem. Biotechnol. 52, 154–162. doi: 10.1080/10826068.2021.1922917
Gong Y., Kang N. K., Kim Y. U., Wang Z., Wei L., Xin Y., et al. (2020). The NanDeSyn database for Nannochloropsis systems and synthetic biology. Plant J. 104, 1736–1745. doi: 10.1111/tpj.15025
Govea-Alonso D. O., Malla A., Bolaños-Martínez O. C., Vimolmangkang S., and Rosales-Mendoza S. (2022). An algae-made RBD from SARS-CoV-2 is immunogenic in mice. Pharm. (Basel). 15, 1298. doi: 10.3390/ph15101298
Grama S. B., Liu Z., and Li J. (2022). Emerging trends in genetic engineering of microalgae for commercial applications. Mar. Drugs 20, 285. doi: 10.3390/md20050285
Guo X., Yao L., and Huang Q. (2015). Aeration and mass transfer optimization in a rectangular airlift loop photobioreactor for the production of microalgae. Bioresour. Technol. 90, 189–195. doi: 10.1016/j.biortech.2015.04.077
Häder D. P. and Hemmersbach R. (2022). Euglena, a gravitactic flagellate of multiple usages. Life 12, 1522. doi: 10.3390/life12101522
Harmsen M. J. H. M., van Vuuren D. P., Nayak D. R., Hof A. F., Höglund-Isaksson L., Lucas P. L., et al. (2019). Data for long-term marginal abatement cost curves of non-CO2 greenhouse gases. Data Brief. 25, 104334. doi: 10.1016/j.dib.2019.104334
Hasnain M., Zainab R., Ali F., Abideen Z., Yong J. W. H., El-Keblawy A., et al. (2023). Utilization of microalgal-bacterial energy nexus improves CO2 sequestration and remediation of wastewater pollutants for beneficial environmental services. Ecotoxicol Environ. Saf. 267, 115646. doi: 10.1016/j.ecoenv.2023.115646
Hempel F., Lau J., Klingl A., and Maier U. G. (2011). Algae as protein factories: expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PloS One 6, 28424. doi: 10.1371/journal.pone.0028424
Hidaka S., Okamoto Y., and Arita M. (2004). A hot water extract of Chlorella pyrenoidosa reduces body weight and serum lipids in ovariectomized rats. Phytother. Res. 18, 164–168. doi: 10.1002/ptr.1178
Holtshausen L., Benchaar C., Kröbel R., and Beauchemin K. A. (2021). Canola meal versus soybean meal as protein supplements in the diets of lactating dairy cows affects the greenhouse gas intensity of milk. Anim. (Basel). 11, 1636. doi: 10.3390/ani11061636
Hong M. E., Chang W. S., Patel A. K., Oh M. S., Lee J. J., and Sim S. J. (2019). Microalgal-based carbon sequestration by converting LNG-fired waste CO2 into red gold astaxanthin: the potential applicability. Energ. 12, 1718. doi: 10.3390/energies12091718
Hu L., Ye Y., Li Y., Tan X., Liu X., Zhang T., et al. (2025). Bacteria-algae synergy in carbon sequestration: Molecular mechanisms, ecological dynamics, and biotechnological innovations. Biotechnol. Adv. 83, 108655. doi: 10.1016/j.bioteChadv.2025.108655
Huang S., Ghazali S., Azadi H., Movahhed M. S., Viira A. H., Janečková K., et al. (2023). Contribution of agricultural land conversion to global GHG emissions: A meta-analysis. Sci. Total Environ. 876, 162269. doi: 10.1016/j.scitotenv.2023.162269
Isiramen O. E., Bahri P. A., Moheimani N. R., Vadiveloo A., Shayesteh H., and Parlevliet D. A. (2022). Improving pH control and carbon dioxide utilisation efficiency in microalgae cultivation systems with the use of a Proportional-integral+ dead-zone control strategy. Bioresour. Technol. Rep. 17, 100917. doi: 10.1016/j.biteb.2022.100917
Ivušić F., Rezić T., and Šantek B. (2022). Heterotrophic cultivation of Euglena gracilis in stirred tank bioreactor: a promising bioprocess for sustainable paramylon production. Molecules 27, 5866. doi: 10.3390/molecules27185866
Jagadevan S., Banerjee A., Banerjee C., Guria C., Tiwari R., Baweja M., et al. (2018). Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol. Biofuels. 11, 185. doi: 10.1186/s13068-018-1181-1
Jiang K., Ashworth P., Zhang S., and Hu G. (2022b). Print media representations of carbon capture utilization and storage (CCUS) technology in China. Renew. Sustain. Energy Rev. 155, 111938. doi: 10.1016/j.rser.2021.111938
Jiang D., Wang L., Liu Y., Huo X., Lin J., and Li L. (2022a). Simple and rapid separation of Haematococcus pluvialis and ciliate based on the dean-coupled inertial microfluidics. J. Sep. Sci. 45, 3900–3908. doi: 10.1002/jssc.202200308
Kargupta W., Ganesh A., and Mukherji S. (2015). Estimation of carbon dioxide sequestration potential of microalgae grown in a batch photobioreactor. Bioresource Technology. 180, 370–375. doi: 10.1016/j.biortech.2015.01.017
Kaur M., Bhatia S., Gupta U., Decker E., Tak Y., Bali M., et al. (2023). Microalgal bioactive metabolites as promising implements in nutraceuticals and pharmaceuticals: inspiring therapy for health benefits. Phytochem. Rev. 14, 1–31. doi: 10.1007/s11101-022-09848-7
Khayyal M. T., El-Baz F. K., Meselhy M. R., Ali G. H., and El-Hazek R. M. (2019). Intestinal injury can be effectively prevented by Dunaliella salina in gamma irradiated rats. Heliyon 5, e01814. doi: 10.1016/j.heliyon.2019.e01814
Kim G. Y., Heo J., Kim H. S., and Han J. I. (2017). Bicarbonate-based cultivation of Dunaliella salina for enhancing carbon utilization efficiency. Bioresource Technology. 237, 72–77. doi: 10.1016/j.biortech.2017.04.009
King S. J., Jerkovic A., Brown L. J., Petroll K., and Willows R. D. (2022). Synthetic biology for improved hydrogen production in. Chlamydomonas reinhardtii. Microb. Biotechnol. 15, 1946–1965. doi: 10.1111/1751-7915.14024
Koopmann I. K., Möller S., Elle C., Hindersin S., Kramer A., and Labes A. (2022). Optimization of astaxanthin recovery in the downstream process of Haematococcus pluvialis. Foods 11, 1352. doi: 10.3390/foods11091352
Kumar T. S., Josephine A., Sreelatha T., Azger Dusthackeer V. N., Mahizhaveni B., and Dharani G. (2020). Fatty acids-carotenoid complex: an effective anti-TB agent from the chlorella growth factor-extracted spent biomass of Chlorella vulgaris. J. Ethnopharmacol. 249, 112392. doi: 10.1016/j.jep.2019.112392
Kutlu L. (2020). Greenhouse gas emission efficiencies of world countries. Int. J. Environ. Res. Public Health 17, 8771. doi: 10.3390/ijerph17238771
La H., Hettiaratchi J. P. A., Achari G., and Dunfield P. F. (2018). Biofiltration of methane. Bioresour. Technol. 268, 759–772. doi: 10.1016/j.biortech.2018.07.043
Lee J. Y., Hong M., Chang W. S., and Sim S. (2015). Enhanced carbon dioxide fixation of Haematococcus pluvialis using sequential operating system in tubular photobioreactors. Process Biochem. 50, 1091–1096. doi: 10.1016/j.procbio.2015.03.021
Li C., Ji Q., Fu X., Yu X., Ye Z., Zhang M., et al. (2022). Low-cost detection of methane gas in rice cultivation by gas chromatography-flame ionization detector based on manual injection and split pattern. Molecules 27, 3968. doi: 10.3390/molecules27133968
Li M., Ma S., Zhao J., Dou X., Liu K., Yin G., et al. (2025). Efficient photoproduction of a high-value sesquiterpene pentalenene from the green microalga Chlamydomonas reinhardtii. Plant J. 123, e70354. doi: 10.1111/tpj.70354
Li G., Xiao W., Yang T., and Lyu T. (2023). Optimization and process effect for microalgae carbon dioxide fixation technology applications based on carbon capture: a comprehensive review. C 9, 35. doi: 10.3390/c9010035
Li J., Zhu D., Niu J., Shen S., and Wang G. (2011). An economic assessment of astaxanthin production by large-scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 29, 568–574. doi: 10.1016/j.bioteChadv.2011.04.001
Li-Beisson Y., Beisson F., and Riekhof W. (2015). Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J. 82, 504–522. doi: 10.1111/tpj.12787
Li-Beisson Y., Thelen J. J., Fedosejevs E., and Harwood J. L. (2019). The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 74, 31–68. doi: 10.1016/j.plipres.2019.01.003
Lin J., Effendi S., and Ng I. (2022). Enhanced carbon capture and utilization (CCU) using heterologous carbonic anhydrase in Chlamydomonas reinhardtii for lutein and lipid production. Bioresour. Technol. 351, 127009. doi: 10.1016/j.biortech.2022.127009
Liu Z., Hao N., Hou Y., Wang Q., Liu Q., Yan S., et al. (2023). Technologies for harvesting the microalgae for industrial applications: Current trends and perspectives. Bioresour Technol. 387, 129631. doi: 10.1016/j.biortech.2023.129631
Liu Y. Q. Y. and Wang Q. (2022). Antioxidative effect of Chlorella pyrenoidosa protein hydrolysates and their application in krill oil-in-water emulsions. Mar. Drugs 20, 345. doi: 10.3390/md20060345
Liu Y. and Wei D. (2023). Enhancing carbon dioxide fixation and co-production of protein and lutein in oleaginous Coccomyxa subellipsoidea by a stepwise light intensity and nutrients feeding strategy. Bioresour. Technol. 376, 128885. doi: 10.1016/j.biortech.2023.128885
Liu Y., Wei D., and Chen W. (2022). Oleaginous microalga Coccomyxa subellipsoidea as a highly effective cell factory for CO2 fixation and high-protein biomass production by optimal supply of inorganic carbon and nitrogen. Front. Bioeng. Biotechnol. 10. doi: 10.3389/fbioe.2022.921024
Llamas A., Leon-Miranda E., and Tejada-Jimenez M. (2023). Microalgal and nitrogen-fixing bacterial consortia: from interaction to biotechnological potential. Plants (Basel). 12, 2476. doi: 10.3390/plants12132476
Ma Z., Cheah W. Y., Ng I. S., Chang J. S., Zhao M., and Show P. L. (2022). Microalgae-based biotechnological sequestration of carbon dioxide for net zero emissions. Trends Biotechnol. 40, 1439–1453. doi: 10.1016/j.tibtech.2022.09.002
Malla A., Rosales-Mendoza S., Phoolcharoen W., and Vimolmangkang S. (2021). Efficient transient expression of recombinant proteins using DNA viral vectors in freshwater microalgal species. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.650820
Mallén-Ponce M. J., Pérez-Pérez M. E., and Crespo J. L. (2022). Deciphering the function and evolution of the target of rapamycin signaling pathway in microalgae. J. Exp. Bot. 73, 6993–7005. doi: 10.1093/jxb/erac264
Masi A., Leonelli F., Scognamiglio V., Gasperuzzo G., Antonacci A., and Terzidis M. A. (2023). Chlamydomonas reinhardtii: a factory of nutraceutical and food supplements for human health. Molecules 28 (3), 1185. doi: 10.3390/molecules28031185
Menegat S., Ledo A., and Tirado R. (2022). Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 12, 14490. doi: 10.1038/s41598-022-18773-w
Meng F. and Ellis T. (2020). The second decade of synthetic biology: 2010-2020. Nat. Commun. 11, 5174. doi: 10.1038/s41467-020-19092-2
Moraes L., da Rosa G. M., Santos L. O., and Costa J. A. V. (2020). Innovative development of membrane sparger for carbon dioxide supply in microalgae cultures. Biotechnol. Prog. 36, e2987. doi: 10.1002/btpr.2987
Morales M., Sánchez L., and Revah S. (2016). The impact of environmental factors on carbon dioxide fixation by microalgae. FEMS Microbiol. Lett. 365 (3). doi: 10.1093/femsle/fnx262
Moses T., Mehrshahi P., Smith A. G., and Goossens A. (2017). Synthetic biology approaches for the production of plant metabolites in unicellular organisms. J. Exp. Bot. 68, 4057–4074. doi: 10.1093/jxb/erx119
Nazos T. T. and Ghanotakis D. F. (2021). Biodegradation of phenol by alginate immobilized Chlamydomonas reinhardtii cells. Arch. Microbiol. 203 (9), 5805–5816. doi: 10.1007/s00203-021-02570-6
Nisbet E. G., Dlugokencky E. J., Fisher R. E., France J. L., Lowry D., Manning M. R., et al. (2021). Atmospheric methane and nitrous oxide: challenges along the path to Net Zero. Philos. Trans. A Math. Phys. Eng. Sci. 379, 20200457. doi: 10.1098/rsta.2020.0457
Nguyen D. T., Johir M. A. H., Mahlia T. M. I., Silitonga A. S., Zhang X., Liu Q., et al (2024). Microalgae-derived biolubricants: Challenges and opportunities. Sci. Total Environ. 954, 176759. doi: 10.1016/j.scitotenv.2024.176759
O’Neill E. C., Saalbach G., and Field R. A. (2016). Gene discovery for synthetic biology: exploring the novel natural product biosynthetic capacity of eukaryotic microalgae. Methods Enzymol. 576, 99–120. doi: 10.1016/bs.mie.2016.03.005
Ozlu E., Arriaga F., Bilen S., Gozukara G., and Babur E. (2022). Carbon footprint management by agricultural practices. Biol. (Basel). 11, 1453. doi: 10.3390/biology11101453
Padhi D., Kashyap S., Mohapatra R. K., Dineshkumar R., and Nayak M. (2025). Microalgae-based flue gas CO2 sequestration for cleaner environment and biofuel feedstock production: a review. Environ. Sci. pollut. Res. Int. 32, 13539–13565. doi: 10.1007/s11356-025-35958-8
Pandey A., Srivastava S., and Kumar S. (2023). Carbon dioxide fixation and lipid storage of Scenedesmus sp. ASK22: A sustainable approach for biofuel production and waste remediation. J. Environ. Manage. 332, 117350. doi: 10.1016/j.jenvman.2023.117350
Prasad R., Gupta S. K., Shabnam N., Oliveira C. Y. B., Nema A. K., Ansari F. A., et al (2021). Role of microalgae in global CO2 sequestration: physiological mechanism, recent development, challenges, and culture prospective. Sustainability 13 (23), 13061. doi: 10.3390/su132313061
Rame R., Purwanto P., and Sudarno S. (2023). Sustainable energy harnessing: Microalgae as a potential biofuel source and carbon sequestration solution. Renew. Energy Focus. 47, 100498. doi: 10.1016/j.ref.2023.100498
Ramírez-Pérez J. C. and Janes H. (2009). Carbon dioxide sequestration by Spirulina platensis in photo-bioreactors. Habitation 12, 65–77. doi: 10.3727/154296610X12686999887328
Raven P. H. (2022). How the living world evolved and where it’s headed now. Philos. Trans. R. Soc Lond. Ser. B: Biol. Sci. 377, 20210377. doi: 10.1098/rstb.2021.0377
Rodolfi L., Chini Zittelli G., Bassi N., Padovani G., Biondi N., Bonini G., et al. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112. doi: 10.1002/bit.22033
Rossati A. (2017). Global warming and its health impact. Int. J. Occup. Environ. Med. 8, 7–20. doi: 10.15171/ijoem.2017.963
Russo M. E., Capasso C., Marzocchella A., and Salatino P. (2022). Immobilization of carbonic anhydrase for CO2 capture and utilization. Appl. Microbiol. Biotechnol. 106 (9-10), 3419–3430. doi: 10.1007/s00253-022-11937-8
Saha M. K., Mia S., Abdul Ahad Biswas A., Sattar M. A., Kader M. A., and Jiang Z. (2022). Potential methane emission reduction strategies from rice cultivation systems in Bangladesh: a critical synthesis with global meta-data. J. Environ. Manage. 310, 114755. doi: 10.1016/j.jenvman.2022.114755
Schipper C. A., Hielkema T. W., and Ziemba A. (2024). Impact of climate change on biodiversity and implications for nature-based solutions. Climate 12(11), 179. doi: 10.3390/cli12110179
Scieszka S. and Klewicka E. (2019). Algae in food: a general review. Crit. Rev. Food Sci. Nutr. 59, 3538–3547. doi: 10.1080/10408398.2018.1496319
Shah M. M. R., Liang Y., Cheng J. J., and Daroch M. (2016). Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00531
Shareefdeen Z., Elkamel A., and Babar Z. B. (2023). Recent developments on the performance of algal bioreactors for CO2 removal: focusing on the light intensity and photoperiods. Biotech. (Basel). 12, 10. doi: 10.3390/biotech12010010
Sharma K., Sharma D., Sharma M., Sharma N., Bidve P., Prajapati N., et al. (2018). Astaxanthin ameliorates behavioral and biochemical alterations in in-vitro and in-vivo model of neuropathic pain. Neurosci. Lett. 674, 162–170. doi: 10.1016/j.neulet.2018.03.030
Sidhu M. A. and Ahluwalia A. S. (2011). Water quality and cyanobacterial diversity in lower western Himachal lakes. Vegetos 24, 165–170. doi: 10.1111/j.1365-3040.2011.02397.x
Singh U. B. and Ahluwalia A. S. (2013). Microalgae: a promising tool for carbon sequestration. Mitig. Adapt. Strategies Glob. Change. 18, 73–95. doi: 10.1007/s11027-012-9391-8
Singh S. K., Sundaram S., Sinha S., Rahman M. A., and Kapur S. (2016). Recent advances in CO2 uptake and fixation mechanism of cyanobacteria and microalgae. Crit. Rev. Environ. Sci. Technol. 46, 1297–1323. doi: 10.1080/10643389.2016.1217911
Singh A., Walker K. T., Ledesma-Amaro R., and Ellis T. (2020). Engineering bacterial cellulose by synthetic biology. Int. J. Mol. Sci. 21, 9185. doi: 10.3390/ijms21239185
Singh K., Ansari F. A., Ingle K. N., Gupta S. K., Ahirwal J., Dhyani S., et al. (2023). Microalgae from wastewaters to wastelands: Leveraging microalgal research conducive to achieve the UN Sustainable Development Goals. Renew Sust Energ Rev. 188, 113773. doi: 10.1016/j.rser.2023.113773
Sobieh S. S., El-Gammal Abed R., El-Kheir W. S. A., El-Sheimy A. A., Said A. A., and El-Ayouty Y. M. (2022). Heterologous expression of Cyanobacterial Cyanase Gene (CYN) in microalga Chlamydomonas reinhardtii for bioremediation of cyanide pollution. Biol. (Basel). 11, 1420. doi: 10.3390/biology11101420
Spolaore P., Joannis-Cassan C., Duran E., and Isambert A. (2006). Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87–96. doi: 10.1263/jbb.101.87
Suarez J. V., Mudd E. A., and Day A. (2022). A chloroplast-localised fluorescent protein enhances the photosynthetic action spectrum in green algae. Microorganisms 10, 1770. doi: 10.3390/microorganisms10091770
Subashchandrabose S. R., Ramakrishnan B., Megharaj M., Venkateswarlu K., and Naidu R. (2013). Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 51, 59–72. doi: 10.1016/j.envint.2012.10.007
Sun B. and Xu X. (2022). Spatial-temporal evolution of the relationship between agricultural material inputs and agricultural greenhouse gas emissions: experience from China 2003-2018. Environ. Sci. pollut. Res. Int. 29, 46600–46611. doi: 10.1007/s11356-022-19195-x
Taleb A., Pruvost J., Legrand J., Marec H., Le-Gouic B., Mirabella B., et al. (2015). Development and validation of a screening procedure of microalgae for biodiesel production: application to the genus of marine microalgae Nannochloropsis. Bioresour. Technol. 177, 224–232. doi: 10.1016/j.biortech.2014.11.068
Thanigaivel S., Vickram S., Manikandan S., Deena S. R., Subbaiya R., Karmegam N., et al. (2022). Sustainability and carbon neutralization trends in microalgae bioenergy production from wastewater treatment: a review. Bioresour. Technol. 364, 128057. doi: 10.1016/j.biortech.2022.128057
Tian H., Xu R., Canadell J. G., Thompson R. L., Winiwarter W., Suntharalingam P., et al. (2020). A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586, 248–256. doi: 10.1038/s41586-020-2780-0
Tran P. and Prindle A. (2021). Synthetic biology in biofilms: tools, challenges, and opportunities. Biotechnol. Prog. 37, 3123. doi: 10.1002/btpr.3123
Treves H., Küken A., Arrivault S., Ishihara H., Hoppe I., Erban A., et al. (2022). Carbon flux through photosynthesis and central carbon metabolism show distinct patterns between algae, C3 and C4 plants. Nat. Plants. 8, 78–91. doi: 10.1038/s41477-021-01042-5
Tsai C., Kwak S., Turner T. L., and Jin Y. (2015). Yeast synthetic biology toolbox and applications for biofuel production. FEMS Yeast Res. 15, 1–15. doi: 10.1111/1567-1364.12206
Uguz S., Anderson G., Yang X., Simsek E., Osabutey A., Yilmaz M., et al. (2024). Microalgae cultivation using ammonia and carbon dioxide concentrations typical of pig barns. Environ. Technol. 45, 5899–5911. doi: 10.1080/09593330.2024.2311082
Vazquez-Villegas P., Torres-Acosta M. A., Garcia-Echauri S. A., Aguilar-Yanez J. M., Rito-Palomares M., and Ruiz-Ruiz F. (2018). Genetic manipulation of microalgae for the production of bioproducts. Front. Biosci. (Elite Ed) 10, 254–275. doi: 10.2741/e821
Verma R. and Srivastava A. (2018). Carbon dioxide sequestration and its enhanced utilization by photoautotroph microalgae. Environ. Dev. 27, 95–106. doi: 10.1016/j.envdev.2018.07.004
Wan L., Wu Y., Zhang Y., and Zhang W. (2022). Toxicity, biodegradation of moxifloxacin and gatifloxacin on Chlamydomonas reinhardtii and their metabolic fate. Ecotoxicol Environ. Saf. 240, 113711. doi: 10.1016/j.ecoenv.2022.113711
Wang Z., Cheng J., Li K., Zhu Y., Liu J., Yang W., et al. (2020a). Comparison of photosynthetic carbon fixation of Nannochloropsis oceanica cultivated with carbon suppliers: CO2, NaHCO3 and CH3OH. J. CO2 Util. 41, 101235. doi: 10.1016/j.jcou.2020.101235
Wang Y., Liu A., Amanze C., Clive Ontita N., and Zeng W. (2024). Isolation and whole-genome analysis of Desmodesmus sp. SZ-1: Novel acid-tolerant carbon-fixing microalga. Bioresour. Technol. 414, 131572. doi: 10.1016/j.biortech.2024.131572
Wang G., Liu P., Hu J., and Zhang F. (2022). Agriculture-induced N2O emissions and reduction strategies in China. Int. J. Environ. Res. Public Health 19, 12193. doi: 10.3390/ijerph191912193
Wang R., Wang X., and Zhu T. (2025). Research progress and application of carbon sequestration in industrial flue gas by microalgae: A review. J Environ Sci (China) 152, 14–28. doi: 10.1016/j.jes.2024.04.018
Wang Y., Tibbetts S. M., Berrue F., McGinn P. J., MacQuarrie S. P., Puttaswamy A., et al. (2020b). A rat study to evaluate the protein quality of three green microalgal species and the impact of mechanical cell wall disruption. Foods 9, 1531. doi: 10.3390/foods9111531
Wang Z., Wen X., Xu Y., Ding Y., Geng Y., and Li Y. (2018). Maximizing CO2 biofixation and lipid productivity of oleaginous microalga Graesiella sp. WBG-1 via CO2-regulated pH in indoor and outdoor open reactors. Sci. Total Environ. 619-620, 827–833. doi: 10.1016/j.scitotenv.2017.11.196
Wei S., Li F., Zhu N., Wei X., Wu P., and Dang Z. (2023). Biomass production of Chlorella pyrenoidosa by filled sphere carrier reactor: Performance and mechanism. Bioresour. Technol. 383, 129195. doi: 10.1016/j.biortech.2023.129195
Williams M. L. (2021). Global warming, heat-related illnesses, and the dermatologist. Int. J. Women’s Dermatol. 7, 70–84. doi: 10.1016/j.ijwd.2020.08.007
Xia A., Hu Z., Liao Q., Huang Y., Zhu X., Ye W., et al. (2018). Enhancement of CO2 transfer and microalgae growth by perforated inverted arc trough internals in a flat-plate photobioreactor. Bioresour. Technol. 269, 292–299. doi: 10.1016/j.biortech.2018.08.110
Xu P., Li J., Qian J., Wang B., Liu J., Xu R., et al. (2023). Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality. Chemosphere 319, 137987. doi: 10.1016/j.chemosphere.2023.137987
Xu P., Shao S., Qian J., Li J., Xu R., Liu J., et al. (2024). Scale-up of microalgal systems for decarbonization and bioproducts: Challenges and opportunities. Bioresour Technol. 398, 130528. doi: 10.1016/j.biortech.2024.130528
Xu L., Zhao Z., Yan Z., Zhou G., Zhang W., Wang Y., et al. (2022). Defense pathways of Chlamydomonas reinhardtii under silver nanoparticle stress: extracellular biosorption, internalization and antioxidant genes. Chemosphere 291, 132764. doi: 10.1016/j.chemosphere.2021.132764
Yadav G., Dash S. K., and Sen R. (2019). A biorefinery for valorization of industrial waste-water and flue gas by microalgae for waste mitigation, carbon-dioxide sequestration and algal biomass production. Sci. Total Environ. 688, 129–135. doi: 10.1016/j.scitotenv.2019.05.023
Yan N., Fan C., Chen Y., and Hu Z. (2016). The potential for microalgae as bioreactors to produce pharmaceuticals. Int. J. Mol. Sci. 17, 962. doi: 10.3390/ijms17060962
Yin H., Wang Z., Li H., Zhang Y., Yang M., Cui G., et al. (2022). MsTHI1 overexpression improves drought tolerance in transgenic alfalfa (Medicago sativa L.). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.992024
Yu Z., Hong Y., Xie K., and Fan Q. (2022). Research progresses on the physiological and pharmacological benefits of microalgae-derived biomolecules. Foods 11, 2806. doi: 10.3390/foods11182806
Zhang Z., Cao L., Dong H., Cai B., Geng Y., and Tang Y. (2022). Allocating China’s 2025 CO2 emission burden shares to 340 prefecture cities: methods and findings. Environ. Sci. pollut. Res. 29, 90671–90685. doi: 10.1007/s11356-022-22052-6
Zhang L., Mao Y., Chen Z., Hu X., Wang C., Lu C., et al. (2024). A systematic review of life-cycle GHG emissions from intensive pig farming: Accounting and mitigation. Sci. Total Environ. 907, 168112. doi: 10.1016/j.scitotenv.2023.168112
Zhao B. and Su Y. (2020). Macro assessment of microalgae-based CO2 sequestration: Environmental and energy effects. Algal Res. 51, 102066. doi: 10.1016/j.algal.2020.102066
Zheng S., Guo J., Cheng F., Gao Z., Du L., Meng C., et al. (2022). Cytochrome P450s in algae: Bioactive natural product biosynthesis and light-driven bioproduction. Acta Pharm. Sin. B. 12, 2832–2844. doi: 10.1016/j.apsb.2022.01.013
Keywords: global warming, microalgal biotechnology, carbon neutrality, application, challenge
Citation: Cheng H, Liu Y, Deng Z, Yang C, Xie X, Baloch H, Xu W, Zhang H, Gao J, Qin Z, Jaleel A and Ren M (2025) The potential microalgae-based strategy for attaining carbon neutrality and mitigating climate change: a critical review. Front. Mar. Sci. 12:1644390. doi: 10.3389/fmars.2025.1644390
Received: 16 June 2025; Accepted: 06 August 2025;
Published: 05 September 2025.
Edited by:
Christophe Brunet, Anton Dohrn Zoological Station Naples, ItalyCopyright © 2025 Cheng, Liu, Deng, Yang, Xie, Baloch, Xu, Zhang, Gao, Qin, Jaleel and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanke Qin, cWtfMDEyQDE2My5jb20=; Abdul Jaleel, YWJkdWwuamFsZWVsQHVhZXUuYWMuYWU=; Maozhi Ren, cmVubWFvemhpMDFAY2Fhcy5jbg==
†The authors have contributed equally to this work
 Hao Cheng
Hao Cheng Yi Liu
Yi Liu Ziai Deng1,2†
Ziai Deng1,2† Xiulan Xie
Xiulan Xie Abdul Jaleel
Abdul Jaleel Maozhi Ren
Maozhi Ren