Abstract
Astaxanthin, a potent ketocarotenoid of C40 family, has drawn significant attention in food, healthcare and personal care industries due to its exceptional anti-inflammatory, antioxidant, and anti-proliferative properties. Microalgae have recently gained recognition as one of the most promising natural sources for astaxanthin production. However, there are several technical barriers in both the upstream and downstream processes that result in microalgae-derived astaxanthin remaining costly. Hence, this review explores recent trends and advancements in astaxanthin production from microalgae. It provides an in-depth review of the biochemical processes that lead to the accumulation of astaxanthin in microalgal cells, highlighting significant factors that affect production, including environmental factors, strain selection, and bioreactor configuration. Different emerging extraction techniques such as physical, biological, and chemical methods are evaluated based on scalability and efficiency. The diverse applications of astaxanthin, particularly in the food, nutraceutical, and pharmaceutical sectors, are also discussed. Furthermore, the review identifies major scientific and technological challenges, such as low biomass yield and high production costs, which hinder large-scale commercialization. In conclusion, future research directions are proposed to overcome these limitations and promote the development of sustainable, cost-effective, and commercially viable microalgae-based astaxanthin production systems.
1 Introduction
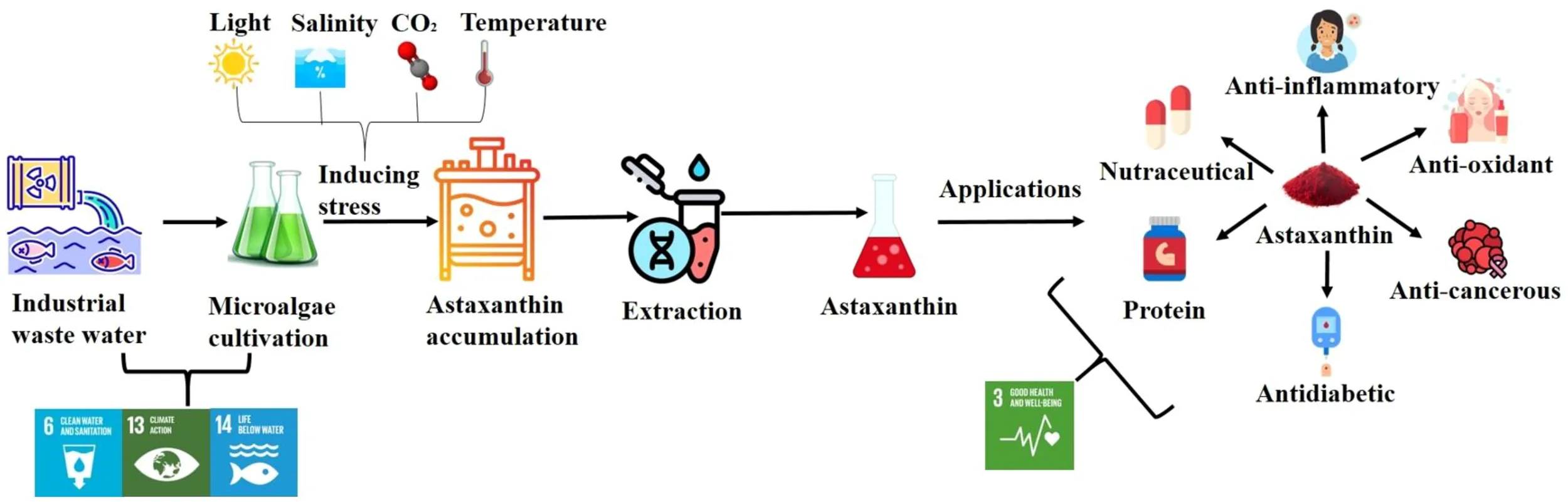
Astaxanthin is a naturally occurring antioxidant classified under the xanthophyll group within the carotenoid family. It exhibits superior anti-inflammatory, antioxidant, and anti-proliferative properties. It has also shown good potential as an antidiabetic agent, contributing to the efficacy of anti-diabetic medications, and has gained attention as a dietary supplement and cosmetic ingredient (Villaró et al., 2021; Zhuge et al., 2021). In addition, astaxanthin plays a crucial role to shield the skin from UV-induced photo oxidation, in anti-tumor treatments and to prevent and treat brain damage linked to age-associated disorders including macular degeneration, Parkinson’s, and Alzheimer’s (Panis and Carreon, 2016) (Guerin et al., 2003; Villaró et al., 2021). Astaxanthin is naturally present in various marine organisms such as shrimp, trout, and salmon (Ambati et al., 2014). However, vegetarians hesitate to consume it due to its animal-derived origin. While the benefits of this pigment have been known for decades but there has been resurgence of interest in plant based pigments. Microalgae is a highly sustainable and efficient alternative for the pigment production. Microalgae-derived astaxanthin offers an ideal solution for vegetarians and vegan population, providing the health benefits of this powerful antioxidant without compromising ethical or dietary values.
Microalgae are capable of synthesizing astaxanthin as a defense mechanism against environmental stressors such as intense sunlight and deprivation of essential nutrients (Debnath et al., 2024). Microalgae have superior photosynthetic efficiency compared to terrestrial plants, making them highly effective in carbon sequestration and playing a vital role in climate change mitigation (Sharma et al., 2021, 2022). Certain microalgae species can double their biomass within 24 hours. They have ability to grow in both fresh and the wastewater environments, including sewage, dairy, and fruit processing wastewater (Shuba and Kifle, 2018).
Currently, the high expenses of microalgae cultivation, astaxanthin extraction and purification techniques hinders its large-scale production. In the EU, especially in Greece, the production cost of natural astaxanthin from Haematococcus pluvialis is around €1500 per kilogram, whereas in the Netherlands, it can reach approximately €6400 per kilogram (Panis and Carreon, 2016; Villaró et al., 2021; Zhang and Lu, 2024). Additionally, synthetic astaxanthin derived from petrochemicals, also raises concerns about sustainability, pollution, and food safety. Therefore, the efforts are conducted to enhance cost-effective astaxanthin production from microalgae. Selection of suitable microalgae species, growth media, optimizing environmental stress conditions and development of cost-effective photo bioreactors/algae ponds are some crucial factors that significantly affect the microalgae based astaxanthin production (Nishshanka et al., 2021; Villaró et al., 2021). Among the various factors influencing astaxanthin production, the choice of growth nutrient media plays a pivotal role in improving yield. While microalgae are traditionally cultured using synthetic media, industrial effluents have garnered significant interest as a sustainable alternative. Using industrial effluents not only decreases the reliance on costly synthetic media but also improves the sustainability of the entire production process (Abd Karim et al, 2024; Yen et al., 2024; Zhang and Lu, 2024). Hence, this article presents a comprehensive review on advances in microalgal based astaxanthin production using dairy wastewater as a growth nutrient media. Furthermore, this review article also delves into the cellular mechanisms involved in astaxanthin biosynthesis and innovative technologies for astaxanthin extraction and purification. Factors influencing boosting up astaxanthin yield are also highlighted in the manuscript. Furthermore, the article also addresses its different industrial applications, and the challenges associated with scaling up production on a commercial scale.
2 Structure of astaxanthin
Astaxanthin is chemically a 40-carbon tetraprene (3,3’-dihydroxy- β, β’-carotene-4,4;-dione) having empirical formula C40H52O4 that composed of isoprene units like other carotenoids, having two β-rings at its terminal positions and a linear polyene chain (Guerin et al., 2003; Villaró et al., 2021). There are 11 double conjugated bonds which are responsible for its pink and red color along with the antioxidant potential. However, both terminal rings of astaxanthin have two functional groups which have polar nature i.e hydroxyl (-OH) and the keto group (=O) (Stachowiak and Szulc, 2021). The hydroxyl group is present at two asymmetric carbons C3 and C3 and the keto group at carbon C4. Conversely, the polar end rings stay near the surface of the membrane. Its characteristic red and pink color and antioxidant potential are attributed to its conjugated structure of eleven double bonds (Lorenz, 1999). Additionally, it has two polar function groups in its two terminal rings: keto (=O) at carbon C4 and hydroxyl (OH) at carbon C3 and C30.
Natural astaxanthin, predominantly sourced from microalgae, consists as the levorotatory (3S,3′S) enantiomer, which have all-trans configuration facilitating hydrophobic binding confined to lipid bilayers through ideally aligned hydrophilic ring moieties along with a lipophilic chain. This enhances the intramembranous antioxidant efficacy (Snell and Carberry, 2022). The synthetic astaxanthin which is derived from petrochemicals forms a racemic admixture corresponding to a 1:2:1 molar ratio of (3S,3S’): (3R,3S): (3R:3’R) stereoisomers, with the meso (3R,3’S) providing reduced thermal lability along with chiral specificity. This frequently leads towards the enrichment of cis isomer under the stress which limits bioavailability and diminishes its capability for mitochondrial sequestration (Villaró et al., 2021). These configurational disparities reveal the esterification characteristics which promotes mono and di ester conjugates in natural isolates for the improved solubility. Additionally, these disparities determine varied bio potencies. Wherein the enantiopure natural isoform prevail over its synthetic equivalent in the quenching of reactive oxygen species without altering the physiological redox signaling. Such structural distinctions highlight the necessity for stereospecific sourcing in therapeutic formulations, as validated by different production outputs and the regulatory approvals through jurisdictions (Muthuraman et al., 2021).
2.1 Stereoisomers
The molecular structure of astaxanthin is symmetrical. There is a presence of two chiral carbons at 3 and 3′ positions of each terminal β-ionone groups. This gives rise to the three possible optical isomers which are the stereoisomers i.e (3S,3′S), meso (3R,3′S), and (3R,3′R). The presence of nine double bonds in polyene moiety, can demonstrate 512 geometric isomers theoretically. Whereas the naturally occurring astaxanthin is present in all-trans configuration, 9-, 13-, and 15-cis isomers (Nishida et al., 2023). Natural astaxanthin exists in mixture of geometrical isomers which includes 9-cis, 13-cis, and 15-cis forms and all-trans form. According to a study, Raman spectra of astaxanthin isomers by density functional theory (DFT), authors confirmed the calculated Raman spectra theoretically and accurately and recorded Raman spectra of all-trans, 9-cis, and 13-cis isomers. Elaboration of theoretical studies towards the other isomers of Astaxanthin i.e 15-cis, 9,90 -cis, 9,13-cis, 9,130 -cis, 9,15-cis, 13,130 -cis, and 13,15-cis isomers was performed along with the attribution of vibrational modes. Stability of the isomers was demonstrated through the comparison with the predicted relative energies theoretically with the estimation of all-trans configuration as 70% approximately, whereas 9-cis and 13-cis isomers 10% approximately. The remaining isomers have than 2% configuration under thermal equilibrium conditions (Yao et al., 2022). Optical and major geometrical isomers of astaxanthin are represented in Figure 1.
Figure 1
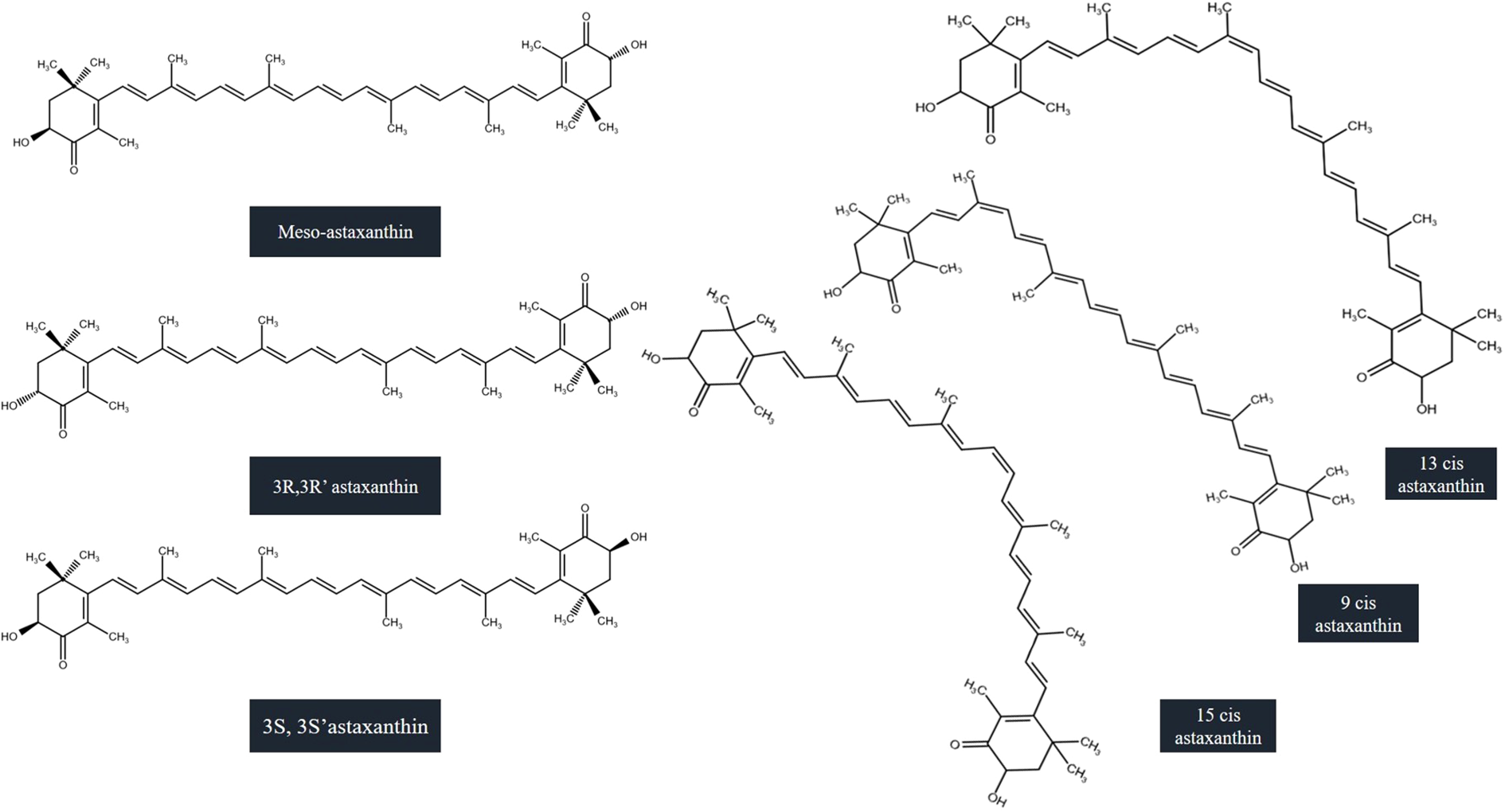
Optical and major geometrical isomers of astaxanthin (Nishida et al., 2023).
2.2 Fatty acid esters
Astaxanthin occurs in the free form in which no hydroxyl group modifications are present. However, it also exists naturally in a particular form where the modification of hydroxyl groups by fatty acid esters. This compound occurs in mono and di ester forms both. The ester moieties are composed of fatty acids (saturated) from C12 to C18 (Breithaupt, 2004; Miao et al., 2006). The astaxanthin derivatives which are esterified with high unsaturated fatty acids i.e eicosapentaenoic acid and docosahexaenoic acid. have also been reported in marine animals. During the conversion of all the esterified astaxanthin to its free form, is used as the astaxanthin concentration. Thus, for the accurate quantification of astaxanthin, the alkaline saponification of esterified astaxanthin is required with cholesterol esterase under anoxic conditions from Pseudomonas sp (Nishida et al., 2023).
3 Biosynthesis of astaxanthin in Microalgae
Microalgae, unicellular and photosynthetic organisms, have demonstrated significant potential as an alternative source for food and high-value products such as astaxanthin due to their rapid growth in both freshwater and wastewater, along with their resilience under stress conditions (Shah et al., 2016; Bratosin et al., 2021; Saadaoui et al., 2021). Microalgae species such as Chlorella vulgaris, Chlorella zofingiensis, Chlorococcum sp., Chlorella sorokiniana, and Haematococcus pluvialis have shown considerable potential for astaxanthin production (Debnath et al., 2024; Zhang and Lu, 2024). For example, high astaxanthin yields of 30.94 and 36.68 mg L-1 are found in H. lacustris and C. zofingiensis respectively (Zhang and Lu, 2024).
As shown in Figure 2, when stress conditions are employed to microalgae cultivation, it shifts from green phase to red phase accounting 80-99% astaxanthin of total. This may be due to the replacement of primary carotenoids from the green stage with secondary carotenoids in red phase (Dragoş et al., 2010). The synthesis of astaxanthin starts through canthaxanthin and this stress response is exceptional and mediated by the reactive oxygen species (ROS). This pigment is the byproduct of a defense mechanism not the defending substance itself (Boussiba, 2000). Initially, there is the formation of isopentenyl pyrophosphate (IPP), which can be produced by two distinct pathways: the methyl erythritol 4-phosphate (MEP) approach and the mevalonate (MVA) pathway (Lichtenthaler, 1987; Nair et al., 2023). There are three key mechanisms involved in the synthesis of astaxanthin: the biosynthesis of β-carotene, the synthesis of IPP and dimethylallyl pyrophosphate (DMAPP), and the synthesis of astaxanthin itself, which is produced by the methyl erythritol 4-phosphate (MEP) and mevalonate (MVA) pathways (Zhu et al., 2023). IPP is converted into β-carotene by several essential steps. The breakdown of DMAPP to form farnesyl diphosphate (FPP) and geranyl diphosphate (GPP) is catalyzed by farnesyl diphosphate synthase (FDPPS). Then, FPP is changed into geranylgeranyl pyrophosphate (GGPP) by geranylgeranyl diphosphate synthase (GGPS). Furthermore, farnesyl-diphosphate farnesyltransferase (FDFT1) can catalyze FPP into sterols and squalene, which may affect the carbon flux going into astaxanthin production (Du et al., 2019). The production of astaxanthin is thought to depend on GGPS. Phytone synthase (PSY), phytoene desaturase (PDS), zeta-carotene desaturase (ZDS), and lycopene cyclase are among the enzymes that convert GGPP into β-carotene. (LYC) (Zhu et al., 2023). Finally, β-carotene is transformed into astaxanthin using some specific enzymes. Two important enzymes, β-carotene hydroxylase (CrtZ) and β-carotene ketolase (CrtW), mediate this conversion. These enzymes produce a variety of intermediate molecules, including valuable metabolites like canthaxanthin and zeaxanthin (Johnson, 2014). β-carotene is transported from the chloroplast to the cytoplasm, where it is catabolized into astaxanthin by the enzyme Cyt P450-β-carotene hydroxylase (CRTR-b). This enzyme is specific to the cytoplasm and is regulated by the crtr-b gene, which also has an impact on ketolase activity.
Figure 2
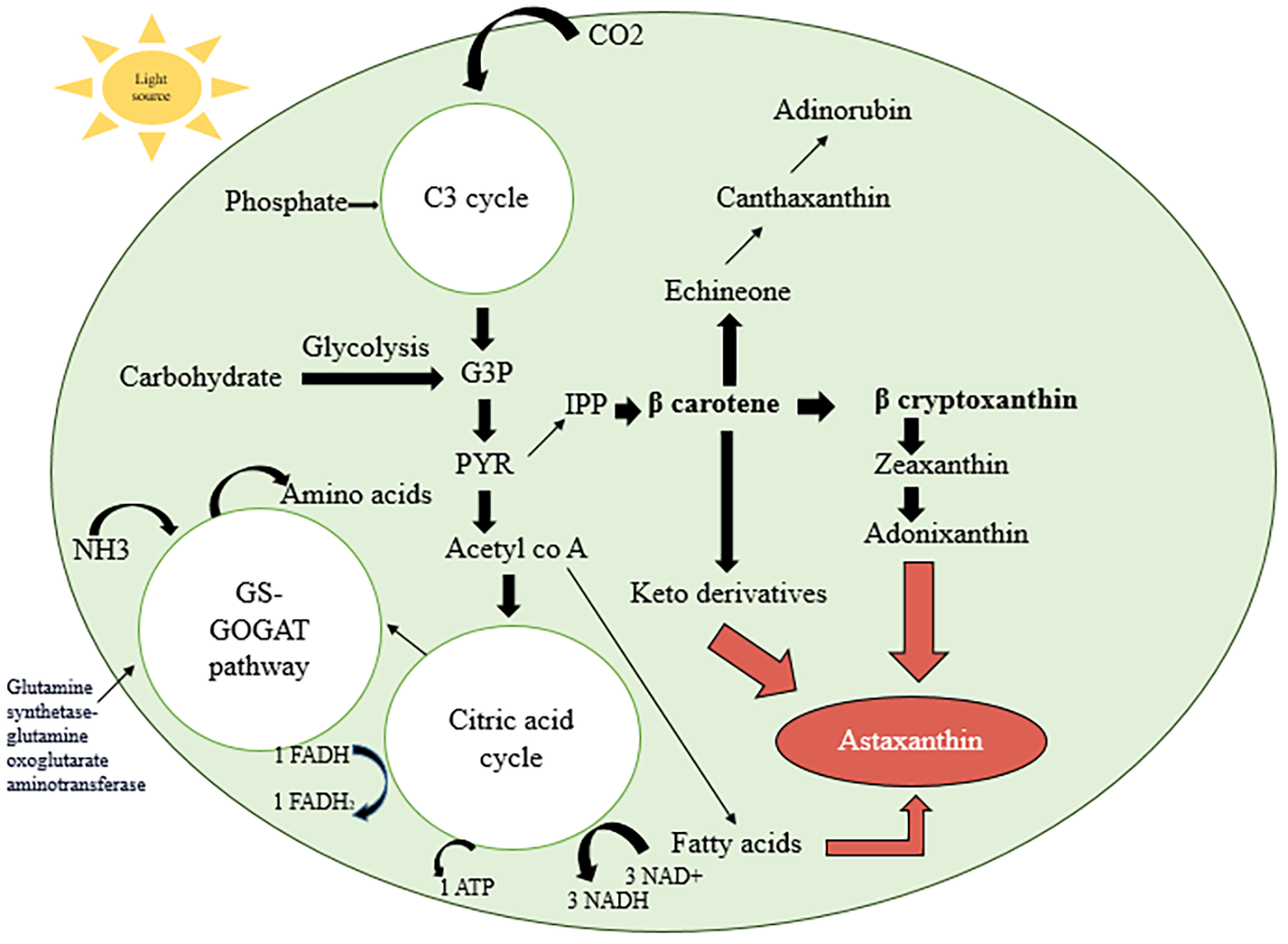
“Pathways involved in astaxanthin accumulation in microalgae.” Adapted with modification from (Lu and Lu, 2022), Journal of Chemical Technology & Biotechnology, 97: 3035–3048. © 2022 Wiley. Reprinted with permission. Microorganisms assimilate both organic and inorganic nutrients from the external environment and convert them into β-carotene, a key precursor in astaxanthin biosynthesis. GS-GOGAT, glutamine synthetase–glutamine oxoglutarate aminotransferase; G3P, glyceraldehyde 3-phosphate; PYR, pyruvate; IPP, isopentenyl pyrophosphate; acetyl-CoA, acetyl coenzyme A; α-KG, α-ketoglutarate; ATX, astaxanthin.
4 Recent advances in astaxanthin production from microalgae
Extensive research on microalgae has been conducted globally, owing to their exceptional capacity to generate a wide range of high-value bio products, such as bioenergy, bio fertilizers, algae-based feed, and dietary supplements like astaxanthin. However, despite the numerous benefits offered by microalgae, several challenges persist in their use as a feedstock for astaxanthin production. Optimizing key aspects is one of the key challenges to improve astaxanthin production in microalgae. Strain selection, cultivating conditions such as temperature, salinity pH, growth nutrients and carbon sources are some important upstream process factors which can play a significant role in increasing astaxanthin yield in microalgae.
4.1 Prerequisites influencing the bio synthesis of astaxanthin from microalgae
The efficiency and effectiveness of upstream processes significantly impact the quantity and quality of astaxanthin produced. It includes strain selection, microalgae cultivation mode, bio reactors, growth nutrients with several environmental factors like light intensity, temperature and oxygen saturation etc.
4.1.1 Strain selection
The selection of optimal strains plays a crucial role in effective astaxanthin production. The strain should be robust and efficient to adapt to environmental conditions easily. Research has shown that species such as Haematococcus pluvialis and Chlorella zofingiensis are the primary producers of astaxanthin (Shah et al., 2016; Patel et al., 2022). However, other microalgae, including Tetraselmis sp., Scenedesmus sp., and Chlorella sorokiniana, also shown good potential under different stress conditions for astaxanthin production (Patel et al., 2022). Additionally, strain improvement using genetic and metabolic engineering are some emerging advance techniques to improve astaxanthin accumulation in microalgae (Debnath et al., 2024).
4.1.2 Bioreactor design
This is another important factor which significantly affects cost-effective astaxanthin production from microalgae. These include open ponds, photo bioreactor, and hybrid system. Among them, open raceway ponds are considered low-cost cultivation technology for large scale production, but it leads to low biomass production and high risk of contamination. On the other hand, photo bioreactors result in higher biomass productivity with lower risk of contamination and water loss. However, photo bioreactors are expensive, and the research is still going to make cost effective photo bioreactors. Polythene bag-based photo bioreactors are emerging as most promising cultivation option due to lower maintenance, easy to operate, good light permeability, and lower material cost. Some researchers are also focusing hybrid system that combine of both approaches and providing more flexibility and scalability to meet varying production demands (Tambat et al., 2023).
4.1.3 Environmental factors
In addition, the production of astaxanthin is significantly modulated by surrounding conditions, notably temperature, the intensity of illumination, and light spectrum characteristics (Khoo et al., 2019; Kou et al., 2020; Debnath et al., 2024). Adding LED colored light sources and temperature regulation systems to advance photo bioreactors results in stimulating stress environmental conditions that promote astaxanthin productivity. Furthermore, the accumulation of astaxanthin in microalgae is highly influenced by different carbon sources, nitrogen deficient conditions, and salinity (Shah et al., 2016; Khoo et al., 2019; Butler and Golan, 2020; Park et al., 2020; Nair et al., 2023). For instance, H. pluvialis microalgae can accumulate up to 3-5% of dry weight under stress conditions (Oslan et al., 2021). Similarly, another study found that Microcystis aeruginosa can accumulate more astaxanthin under NaCl/KCl stress conditions (Huang et al., 2024). Some studies conducted on astaxanthin production from different types of microalgae species are represented in Table 1.
Table 1
| Sl.No | Microalgae | Culture Media | Culture conditions (Additional) | Astaxanthin yield (g/L) | References |
|---|---|---|---|---|---|
| 1. | Chlorella zofingiensis | Kuhl medium | 5 g/L glucose, Light: 120 μmol/m2/s | 0.2388 | (Ma et al., 2025) |
| 2. | Haematococcus pluvialis | BG-11 medium | 2 g/L NaHCO3 | 0.01295 | (Nguyen et al., 2024) |
| 3. | Chromochloris zofingiensis | Kuhl medium | 0 mM NaCl | ~10.5 | (Kou et al., 2020) |
| 4. | Chromochloris zofingiensis | Kuhl medium | 0 mM NaCl | ~10.5 | (Kou et al., 2020) |
| 5. | Haematococcus pluvialis | BG-11 medium | 0 mM CaCl2 0.9 g L− 1 glycerin |
~0.66 0.07828 |
(Cui et al., 2020) (Azizi et al., 2019) |
| 6. | Haematococcus pluvialis | Bold’s Basal medium | 17.1 mM NaCl | 0.00858 | (Liu et al., 2018) |
| 7. | Chlorella zofingiensis | Kuhl medium | 30 g/L industrial waste cane molasses | 0.0138 | (Liu et al., 2012) |
| 8. | Chlorella sorokiniana | Proteose medium | 0.14 g/L nitrate | 0.001–0.0012 | (Raman and Mohamad, 2012) |
| 9. | Tetraselmis sp. | F/2 medium | 0.14 g/L nitrate | 0.0021–0.0022 | (Raman and Mohamad, 2012) |
Astaxanthin production from microalgae under various culture conditions.
Providing carbon-rich substrates, including glucose enhances significantly both biomass and astaxanthin yield. For example, a study was conducted using Chlorella zofingiensis with supply of 50 g/L glucose under heterotrophic mode and they achieved 10.29 mg/L of astaxanthin yield (Ip and Chen, 2005).
In addition, astaxanthin levels are significantly raised by nitrogen shortage. The astaxanthin synthesis pathway is triggered in algal cells exposed to a nitrogen-deficient environment, which causes the cells to transition from a growth phase to a resting state. This technique is widely utilized since it is economical and doesn’t call for the addition of chemicals or specialist equipment to stimulate the synthesis of astaxanthin in algal cells. Moraes et al., 2024 cultivated Haematococcus pluvialis under various nitrogen sources, employing nitrogen feeding and depletion conditions (De Moraes et al., 2024). They discovered that nitrogen-deficient conditions yielded a higher astaxanthin concentration of 23 mg/L. In one another study, Nguyen et al. optimized a two-stage mixotrophic bioprocess from Chlorella zofingiensis, yielding astaxanthin and biomass of 16.7 mg/g and 3.3 g/L, respectively. Three times the concentration of micronutrients and a gradual increase in light intensity (4–8 Klux) were the crucial factors in optimizing the biomass production of 2.5 g/L throughout the course of the 15-day stage I. Furthermore, at stage II, astaxanthin output was increased by stress conditions such as high CO2, light, salt, etc. For optimum astaxanthin production, the ideal values were 20k lux light, 3x nutrients, and 5% CO2 (Tambat et al., 2023). Figure 3 explains both one stage and two stage astaxanthin production under nutrient deficient condition.
Figure 3
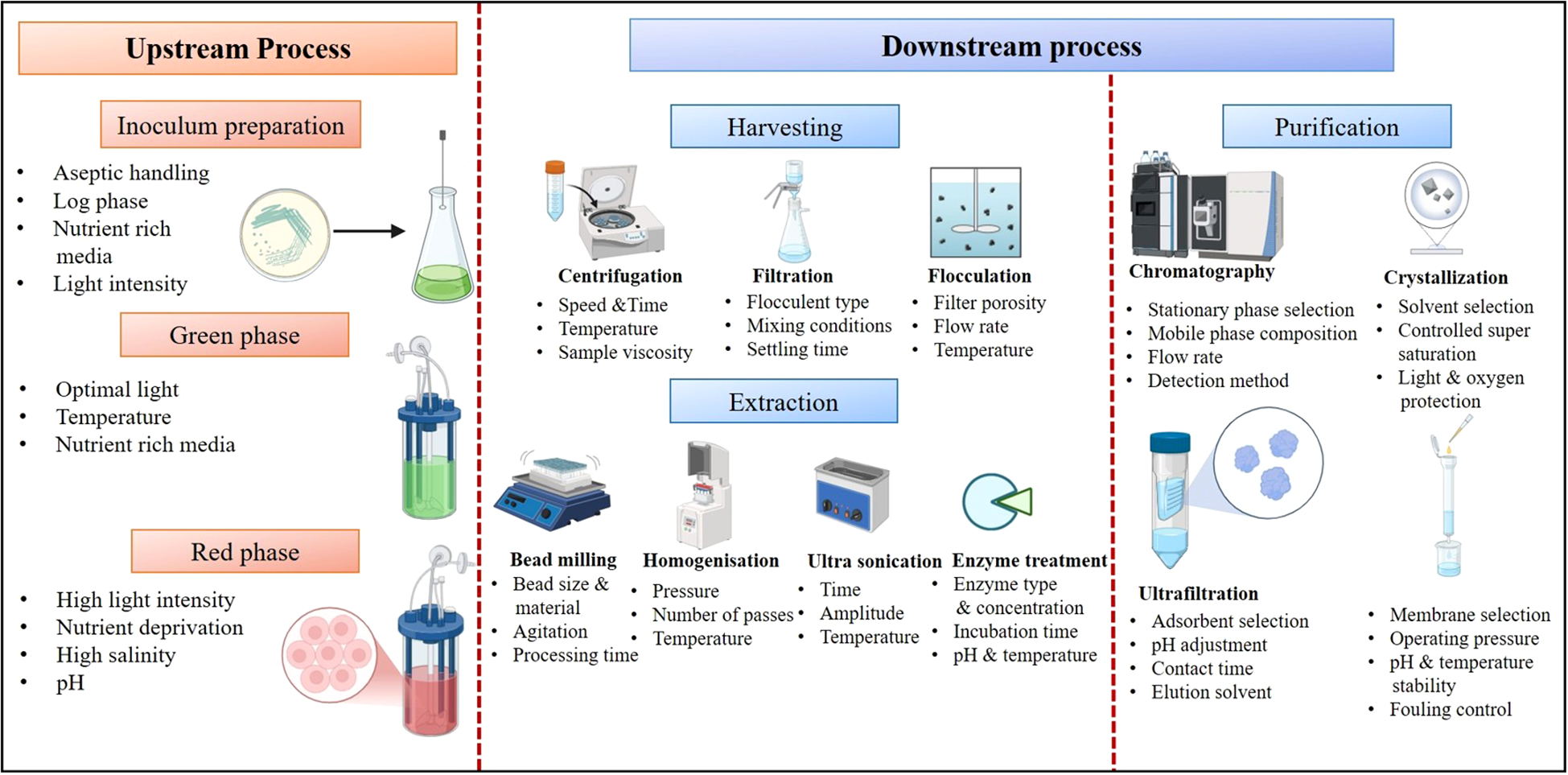
Current strategies for production and purification of astaxanthin.
4.1.4 Cultivation strategies
Microalgae require a high-water content in addition to a variety of macronutrients (like nitrogen, phosphorus, and carbon) and micronutrients (such as cobalt, manganese, molybdenum, and copper) to support cell growth and metabolite synthesis, especially for the accumulation of valuable compounds like astaxanthin. While microalgae are efficient producers of bioactive compounds, the cultivation process can become resource-intensive and costly due to the high demand for water and nutrients (Sharma et al., 2015; Ganesan et al., 2020). To address this issue, wastewater can serve as an alternative culture medium, as it contains most of the essential nutrients required for microalgal growth (Nagarajan et al., 2020). Various types of industrial wastewater, including dairy effluent, agricultural runoff, fruit juice processing wastewater, and effluent from the palm oil industry, have been extensively explored for their potential to support microalgae growth. These waste streams not only supply essential nutrients for biomass production but also enable the bioconversion of microalgae into value-added products such as biofuels, biopolymers, and bioactive compounds, as summarized in Table 2.
Table 2
| Sl. No | Wastewater | Microalgae | Cultivation setup | Cultivation scale | Culture Conditions | Pollutant Removal | Purpose | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Dairy Wastewater | Chlorella | 1L photo bioreactor | Lab | Temperature: 25 ± 1°C, Agitation: 200 rpm, Light intensity: 140 μmol m− 2 s − 1 | TN:77.6%, TP:87.1%, COD:70.4% | Lipid extraction | (Kusmayadi et al., 2022) |
| Sorokiniana | ||||||||
| 2 | Milk processing wastewater |
Chlorella
vulgaris |
2L clear glass bottle with a screw cap | Lab | Temperature: 25 ± 2°C, Light intensity: 50-55μmol/m2s−1, Agitation: 120 rpm |
TN: 45.82-69.18%, Ammonical nitrogen:92.94-94.54%, SO4: 85.13-87.34% TP:75.09-78.78%, BOD:89.53-92.40% |
Lipid profiling | (Verma et al., 2022) |
| 3 | Dairy Wastewater | Coelastrella sp. | 190 L Raceway pond |
Lab | Temperature:28 ± 2°C Light: Dark i.e 16h:8h |
NH4-N:90.38%, NO3-N:90.24%, TP:66.75%, BOD:67.15% COD:69.44%, TOC:83.51% |
Electricity production | (Raja et al., 2022) |
| 4 | Municipal wastewater |
Chlorella vulgaris
Chlorella sp. |
500ml Erlenmeyer flask | Lab | pH: 7.1 ± 0.1, Temperature: 25 ± 2°C Light intensity: 1200 lx. |
COD: 94%, Ammonium nitrogen: 94% |
Biodiesel production | (Leong et al., 2022) |
| 5 | Municipal Wastewater | Scenedesmus obliquus | 2000 mL cylindrical flasks | Lab | Temperature: 23.2 ± 2 °C Light intensity: 2500-3000 lx Agitation: 120 rpm |
TN: 99.8%, TP: 83.1% (primary settling tank), TN: 98.9%, TP:97.6% (secondary settling tank) |
Biodiesel production | (Álvarez-Díaz et al., 2015) |
| 6 | Domestic wastewater | Chlorella vulgaris | 1.8 liters Erlenmeyer flasks | Lab | Temperature: 25 ± 1°C Humidity: 65% |
TN: 36% TP: 23% |
Biodiesel | (Ali et al., 2021) |
| 7 | Dairy wastewater | Scenedesmus quadricauda | 1 L glass flasks | Lab | Temperature: 25°C, Light: 90μmol/m-2 s −1 |
TN: 92.15%, TP: 100%, TOC: 76.77% |
Biochemical analysis | (Daneshvar et al., 2019) |
| 8 | Dairy wastewater | Tetraselmis suecica | 1 L glass flasks | Lab | Temperature: 25°C, Light: 90μmol/m-2 s −1 |
TN:83.17%, TP:100% TOC: 38.82% |
Biochemical analysis | (Daneshvar et al., 2019) |
| 9 | Dairy wastewater | Chlorella vulgaris | 250 mL Erlenmeyer flasks | Lab | pH: 7.3 ± 0.4, Temperature: 28-32°C, Dark: Light i.e 8h:16h Agitation: 80rpm |
TN: 85.47%, BOD: 85.61%, COD: 80.62%, TP: 65.96% |
Biodiesel production | (Choi, 2016) |
Bioremediation potential of different microalgae grown in various types of industrial wastewater.
In the recent times, one industrial wastewater i.e., dairy wastewater is shown a great potential for microalgae growth and astaxanthin production due to being rich in carbon content along with other valuable micro and macro nutrients. The dairy industry generates significant wastewater, ranging from 2.5 to 10 liters per litter of product, primarily due to material losses (0.1%–1.9%) and processes such as rinsing, cooling, and sanitizing. The Food and Agriculture Organization (FAO) report states that global milk production was 852 million tons in 2019 and is projected to grow at an annual pace of 1.6% to hit 997 million tons by 2029 (Stasinakis et al., 2022). In 2022-23, India produced over 230.58 million and was the largest milk producer accounting 25% of the world’s total (Ye and Li, 2023). Dairy wastewater (DW) is characterized by high levels of total nitrogen (TN): 14–830 mg/L, total phosphorus (TP): 9–280 mg/L, biochemical oxygen demand (BOD): 40,000–48,000 mg/L, and a pH range of 4.7–11. The biochemical composition shows that it contains lactose, milk solids, lipids, sanitizing agents, detergents, and minerals. As shown in Table 2, several studies have been carried out to investigate dairy wastewater for reducing the cost of microalgae cultivation and bioremediation. For example, Kiani et al. (2024) investigated Nannochloropsis oceanica microalgae cultivation in nano-filtered whey permeate, a dairy industry waste for cultivation and fatty acid production (Kiani et al., 2024). In another study, Sudhanthiran and Perumalsamy (2024) explored the impacts of dairy industry wastewater on the biomass productivity of Chlorella vulgaris using 100% (pure dw), 75% dw, 50% dw, and 25% dw (Sudhanthiran and Perumalsamy, 2024). The study demonstrated that when Chlorella vulgaris was cultured in dairy wastewater, it achieved a maximum biomass productivity of 225 g/L/day and a peak biomass concentration of 2.43 g/L, reflecting promising growth potential. In terms of bioremediation, the process exhibited notable efficiency, with a chemical oxygen demand (COD) removal rate of 81.48%, total nitrogen (TN) reduction of 87.70%, and total phosphorus (TP) removal of 93.5%. Kiani et al. (2023) cultivated freshwater Chlorella vulgaris, Tetradesmus obliquus, and N. ocenica microalgae using dairy wastewater and found good biomass productivity with effective bioremediation of nitrogen and phosphate (Kiani et al., 2023). Singh et al. (2023) cultivated Monoraphidium sp. KMC4 using dairy wastewater under mixotrophic condition for bioenergy production (Singh et al, 2023). Most of the studies are focused on dairy wastewater treatment using microalgae for bioremediation and bioenergy production, highlighting the ability of microalgae to reduce pollutants. However, the production of high-value compounds like astaxanthin from microalgae grown in dairy effluent remains in the research and development (R&D) phase. However, the nutrient-rich nature of dairy wastewater holds promise for supporting astaxanthin synthesis, further investigation is needed to optimize cultivation conditions, stress factors, and extraction techniques to achieve commercially viable yields. Despite the potential, scaling up astaxanthin production from dairy wastewater is still being explored in pilot studies and experimental trials.
5 Extraction techniques for astaxanthin from microalgae biomass
The extraction and purification of astaxanthin is essential to regulate for efficient and cost effective astaxanthin production. Microalgae harvesting methods can be energy-intensive, while conventional solvent extraction techniques for astaxanthin pose environmental and health concerns. Though advancements in green extraction technologies and encapsulation methods are promising, scaling up these processes while maintaining cost-effectiveness and sustainability remains a significant challenge. Several current strategies for production and purification of astaxanthin are demonstrated in Figure 3.
5.1 Physical methods
The astaxanthin is extracted from microalgae using different physical cell disruption techniques such ultrasonic treatment, bead milling, High-pressure homogenization (HPH), pulse electric field treatment and supercritical fluid extraction depending on various energy input ranges such as shear forces, electrical pulses and the heat waves (Lee et al., 2017). Generally, these methods are employed with conjunction of other methods for the reduction of consumption of energy and the cell destruction efficiency (Kim et al., 2022).
Bead milling is a procedure of the disruption of cells from an algal broth. In this technique, cells are employed to a combination of compression and shear forces using rapidly moving beads within a specially designed chamber equipped with grids. This process splits, breaks, and abrades the algal cells, making the intracellular target product(s) accessible to organic solvents. Ethanol and acetone, which are recognized as safe solvents, can be used in this method as part of pre-treatment processes to extract astaxanthin for use in food and feed applications. One of the studies shown that the astaxanthin recovery was significantly increased by 92 ± 5% by adding a step of bead milling before the supercritical CO2 extraction (Valderrama et al., 2003; Nobre et al., 2006) (Kusmayadi et al., 2022; Verma et al., 2022; Raja et al., 2022; Leong et al., 2022; Álvarez-Díaz et al., 2015; Ali et al., 2021; Daneshvar et al., 2019, 2019; Monteiro Dos Santos et al., 2024; Das et al., 2017; Choi, 2016). In another study, astaxanthin extraction was boosted up to 15 mg/g using acetone and ethanol and Retch MM400® ball milling method. On the other hand, strong milling favors negative impacts on the astaxanthin levels since it is easily harmed by heat and physical stress (like hitting a ball). Furthermore, it resulted in a large amount of cake development on milling vessel’s surface, which made this process challenging. HPH is another most important method which is carried out by using high pressure to force the suspension of algae with the help of a narrow valve. There are several categories of mechanical stress provided in HPH for cell wall disruption which are shown in Figure 4. The characteristics of the algal cell and the specifications of equipment and operational parameters which are as pressure for loading, cycling with the design of valve determine HPH effectiveness (Kim et al., 2022).
Figure 4
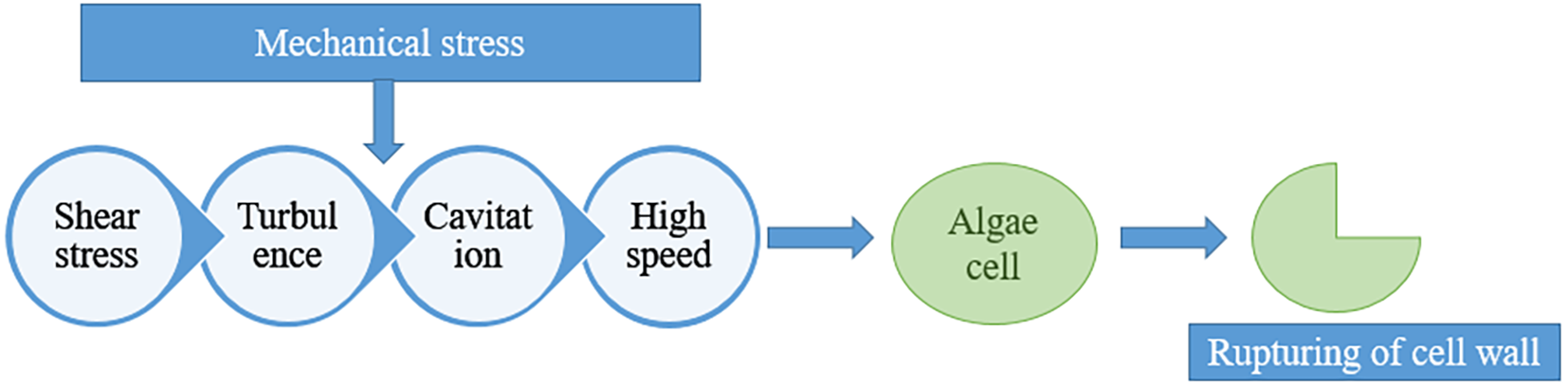
Different types of mechanical stress provided in HPH for cell wall disruption.
Ultra sonication is also one of the impressive techniques to pretreat and enhance astaxanthin recovery. In this technique, sound waves higher than 20 kHz are commonly used to create acoustic cavitation in liquid media. This enhances the mass transfer of the employed solvent and deactivates and ruptures microbial cells (Lee et al., 2017). However, astaxanthin and other antioxidants may be severely biodegraded and oxidized because of excessive ultrasonic treatment due to the production of free radicals. Additionally, to prevent thermal damage to the target biomolecules, the cell solution needs to be appropriately chilled or sonicated (Saini and Keum, 2018).
5.2 Chemical methods
Extraction of astaxanthin is performed by a range of organic solvents i.e. acetone, hexane, dichloromethane (DCM), methanol, and ethyl acetate. Although ethanol and acetone are green solvents and advised for environmental health and safety reasons (Molino et al., 2018b; Park et al., 2020). However, these technologies have exhibited poor extraction of astaxanthin as they are unable in disrupting the rigid cell wall structure of H. pluvialis cysts, which is generally composed of algaenan (Saini and Keum, 2018). According to one study, lyophilized H. pluvialis treated with acetone for 24 hours had a very poor 14% astaxanthin extraction efficiency. Some scientist tried a combination of ethanol and acetone to extract carotenoid from wet H. pluvialis biomass, but the performance was poor yielding with 6.2–7.0 mg g-1 cell (Sarada et al., 2006). Hence, it is essential to use some physical cell disruption method such as HPH (Park et al., 2020) or mild bead milling (Irshad et al., 2019) with chemical solvent extraction methods. In a study, astaxanthin extraction was achieved by up to 96.7% using acetone from the dried H. pluvialis biomass, which was carefully ground manually before employed for solvent extraction (Kim et al., 2018). Ssolvent-based extraction techniques are highly dependent on solvent selection. In one study, it was observed that ethyl acetate was used by adding H2O and CO2, the astaxanthin extraction efficiency increased significantly. In a further experiment, the extraction efficiency of astaxanthin increased up to 93.1%, when a mixture of methanol and N-ethyl-n-butyl amine employed along with the same solvent recovery procedure (Liu et al., 2020). Therefore, even small losses of the exceedingly hazardous SHS need to be handled carefully. In another study, using a liquid biphasic flotation approach with gas bubbles, astaxanthin was efficiently and efficiently recovered by solvent extraction without the need for further purification steps from mechanically disrupted dry H. pluvialis powder (Khoo et al., 2019). The partition coefficient and astaxanthin recovery rate using food-grade ethanol and 2-propanol were 385 and 95.1%, respectively, which were both relatively high numbers. The same treatment was completed with a comparable result using a non-bubble ultrasound approach (Khoo et al., 2020). Compared to traditional organic solvent extraction techniques, accelerated solvent extraction procedures employ comparatively greater pressures and/or temperatures. In algal bio refineries, these procedures can drastically aid in the time reduction of processing and dosage of solvent (Saini and Keum, 2018). The high pressure promotes intramolecular interactions and increases the solvent molecules’ cell permeability, which improves the chemical breakdown of components of cell wall. Elevated temperatures have the dual impact of increasing internal pressure through the heating effect and reducing solvent viscosity, which allows the solvent to diffuse into the cell matrix. When combined, these impacts cause both chemical and physical disruption of algal cells. As a result, these methods fall within the categories of chemical and physical cell disruption methods (Lee et al., 2017; Nitsos et al., 2020). In a study, it was discovered that the extraction efficiency of astaxanthin wet H. pluvialis was significantly higher using accelerated solvent extraction in comparison to dimethyl ether extraction (Boonnoun et al., 2014). Despite considerable process improvement, astaxanthin yield of about 15 mg g-1 cell was achieved utilizing acetone and ethanol at about 100°C and about 100 bars. The astaxanthin extractability was shown to be enhanced by pressure increases (5 to 10 MPa), but temperature increases (50 to 100 ◦C) had the reverse effect. This is needed to maintain an inert atmosphere and precisely regulate temperature, pressure, and residence duration to prevent astaxanthin oxidation. Also, it might be a crucial component in the use of this method in algal bio refineries (Kim et al., 2022).
5.3 Biological methods
These methods are considered more ecofriendly than chemical methods. There are several biological methods which have been established in literature for the extraction of astaxanthin which are enzyme treatment, germination and milking.
5.3.1 Enzyme treatment
This technique facilitates the extraction of astaxanthin by weakening the structural integrity of algal cell walls using hydrolytic enzymes, allowing internal chemicals to extracted more rapidly using solvents. A study examining the pre-treatment effects for extracting astaxanthin from dried microalgae using dichloromethane (DCM) investigated three distinct enzyme formulations. when β-1,3-glucanase and protease collectively employed, 79.3% astaxanthin yield achieved under optimal conditions (pH 4.5, 55°C, and 30 minutes (Machado et al., 2016). In another study, H. pluvialis cells were treated with proteinase and cellulase at the same time to produce cell wall-free protoplasts (Cheng et al., 2018). However, only around 40% of the protoplasts were produced, which is a relatively poor yield. It is possible that this is because these enzymes did not break down the second layer of cell walls. Astaxanthin was extracted from dry powders of H. pluvialis using ethyl acetate by utilizing the hydrolytic activity of different combinations of cellulase and pectinase (Zhao et al., 2019). Despite strict optimization of pH, temperature, and hydrolysis duration, only a moderate astaxanthin extraction yield of 67% was achieved. Enzyme-based extraction has a lower environmental impact when compared to other physical and chemical treatment techniques.
5.3.2 Milking
It is a prominent approach for the reduction of costs in the downstream processing, to yield the substances such as lipids from microalgae in a cost-efficient way. As in this process there is the release of substantial amount of hydrocarbons (long chain) in the extracellular matrix (Kleinert and Griehl, 2022). In this technique, the microalgae suspension culture was extracted from the process of cultivation directly. Ultimately, there is the reduction in cost and energy. These are considered as the highest effort taking steps in the extraction, removal of water and the cell wall rupturing. This exhibits the 50-80% of the average expense of the conventional processes (Acién et al., 2012; Khoo et al., 2020).
5.3.3 Germination
This is another efficient way to break down the rigid cell walls of microalgae cysts in an energy-efficient way. As a result of the cysts, three-layered cell wall structure breaking down during germination, the cysts divide and create new zooids with weaker cell walls. Astaxanthin and other important substances can be extracted more easily because of this mechanism, which leaves the zooids extremely vulnerable to physical and chemical stress (Praveenkumar et al., 2015). According to a study, when samples of H. pluvialis were homogenized for 30 seconds while the germination process was carried out outside, the concentration of astaxanthin was 58% higher than that of non-germinated cells in a significant amount of the differentiated zooids (Choi et al., 2015). Different extraction and pretreatments techniques for the recovery of astaxanthin from microalgae have been demonstrated in Table 3.
Table 3
| Sl.No. | Pre-Treatment method | Cultivation scale | Operating conditions | Astaxanthin Yield (%) | References |
|---|---|---|---|---|---|
| 1. | Bead Beating | Lab | 4.4 M/S in 2 ml Eppendorf tube, 300 mg of 0.5 mm zirconium beads, 3 min | 35–37 | (Tambat et al., 2023) |
| 2. | Acid Treatment | Lab | 4 N HCl, 70 °C, 10 min | 99 | (Vechio et al., 2021) |
| 3. | Milking | Lab | Cyclohexane for 1 h | 20.6 | (Samorì et al., 2019) |
| 4. | Bead Beating | Lab | Zirconia balls 30 min at 200 rpm |
>99 | (Irshad et al., 2019) |
| 5. | Pulsed electric field | Lab | 30 kV/cm in methanol,30 min | 6.5 | (Liu et al., 2018) |
| 6. | Supercritical fluid extraction | Lab | CO2 and ethanol at 55 MPa and 65°C | ~92 | (Molino et al., 2018a) |
| 7. | High-pressure homogenization | Lab | 14,500 psi and three passes | >80 | (Liu et al., 2018) |
| 8. | Ultra sonication | Lab | 750 W, 2 N NaOH, 35 min | 81 | (Haque et al., 2016) |
| 9. | Enzyme treatment | Lab | β-1,3-glucanase and protease pH 4.5, 55 °C, 30 min |
79.3 | (Machado et al., 2016) |
Extraction techniques for astaxanthin with several operating conditions from microalgae.
6 Application of astaxanthin in food and health industries
Based on its present advantages, astaxanthin has potential uses in human treatment due to its anti-aging, anti-diabetic, and anti-inflammatory qualities. It also offers benefits for the food and feed aquaculture industry.
6.1 Food industry
Development of foods containing algal components, especially astaxanthin, has garnered substantial interest in functional food ingredient research. The food and feed sectors also often use astaxanthin as dietary supplements. The EFSA Panel on Nutrition, Novel Foods, and Food Allergens concluded in 2020 that 8 mg of astaxanthin consumed by adults through food supplements is safe (EFSA Panel on Nutrition et al., 2020). Moreover, one of the main causes of beef products’ declining food quality in the food industry is oxidation. Sausage producers frequently use artificial antioxidants, like Butylated Hydroxyl Toluene (BHT), to stop oxidation without shortening the product’s shelf life or nutritional value. Conversely, synthetic antioxidants may include carcinogens even if they aid in the oxidation process. Oxidation stability increased when natural astaxanthin was substituted, and BHT prevented malondialdehyde production to the same extent over storage days (Seo et al., 2021).
Astaxanthin can also be added to the food in addition to its antioxidant properties to improve growth performance in aquaculture (Lim et al., 2019; Xie et al., 2020). Astaxanthin has been given for juvenile largemouth bass (Micropterus salmoides) at doses of 75 and 150 mg kg-1 on a diet containing high fat. This has an impact on fishery growth, lipid metabolism, and immune response. Fish supplemented with astaxanthin demonstrated significant improvements in superoxide dismutase activity, lower levels of malondialdehyde, and a notable reduction in oxidative stress, highlighting the benefits of this powerful antioxidant (Xie et al., 2020). A study found that feeding fish a range of diets high in astaxanthin significantly improved their hematology and indices (white blood cell count, red blood cell count, and hemoglobin). This carotenoid pigment is also widely known for being a necessary aquaculture feed additive that gives salmon flesh, trout, ornamental fish, shrimp, lobsters, and crabs a pinkish-red color, enhancing their quality and raising market acceptance (Lim et al., 2019). Current study suggests that supplementing broiler chicks with astaxanthin (40 or 80 mg kg-1) is a practical strategy to increase the overall carotenoid content of the chickens’ breast, liver, and thighs. Additionally, the study discovered that while broiler chickens’ flesh had less malondialdehyde (MDA) and had higher redness or yellowness, their astaxanthin intake boosted the superoxide dismutase activity (Ao and Kim, 2019).
6.2 Health and medical industry
6.2.1 Anti inflammatory
Astaxanthin can prevent the formation of inflammation in biological systems. It has been shown that astaxanthin, a naturally occurring powerful antioxidant xanthophyll carotenoid, has anti-inflammatory properties and may be protective against acute lung injury (Cai et al., 2019). Both in vitro and in vivo, astaxanthin lessens lung damage brought on by LPS by blocking the TLR4/MyD88 and MAPK/NF-B signaling pathways. Additionally, it guards against lipopolysaccharide-induced sepsis and acute lung damage in mice. In cardiovascular disorders, the antioxidant astaxanthin is thought to be a critical regulator of inflammatory reactions because of its ability to control redox equilibrium. In addition to its favorable effects as an antioxidant on the cardiovascular system, it also helps prevent diseases including atherosclerosis, dyslipidemia, and arterial hypertension (Pereira et al., 2021). Research carried out in vitro has demonstrated that astaxanthin elongates LDL oxidation in a dose-dependent manner. Moreover, it works better than tocopherol and lutein. Subsequently, blood samples from those who took supplements every day were examined, and it was shown that a dose of 14.4 mg astaxanthin for 14 days produced the most benefit—a significant delay in LDL oxidation (Iwamoto et al., 2000).
6.2.2 Anti diabetic
Astaxanthin has been thoroughly investigated for use in anti-diabetic medications as an agent against diabetes. This can regulate and stop diet-induced hepatic steatosis and insulin resistance in rats. In one experiment, the male wistar rats have given a high-energy diet containing 15–50 mg kg-1 of astaxanthin to test the compound’s anti-diabetic effects. The rats were then given a low dose of streptozotocin (STZ) at a rate of 40 mg kg-1 to mimic diabetes (Zhuge et al., 2021). The results demonstrate that by upregulating the expression of genes associated with insulin sensitivity (adiponectin, adipoR1, and adipoR2), astaxanthin therapy dramatically lowers STZ-induced diabetes in vivo. No adverse effects were observed in rats given high doses of ASX (up to 1240 mg kg-1day-1) for an extended period (90 days) (Vega et al., 2015). In rats fed a high-fat fructose diet (HFFD), another study indicated that 6 mg kg-1 day-1 for 45 days dramatically decreased insulin and plasma glucose levels and improved insulin sensitivity (Arunkumar et al., 2012). Furthermore, when taken at doses ranging from 8 to 45 mg daily for 4 to 12 weeks, astaxanthin has minimal adverse effects on humans. The astaxanthin mechanism was evaluated in animal models of type 1 and type 2 diabetes by either oral or parenteral treatment (Landon et al., 2020). In addition, for lowering insulin resistance and secretion and preventing retinopathy, nephropathy, and neuropathy, astaxanthin also lowers hyperglycemia. Moreover, astaxanthin demonstrated promise in improving glucose and lipid metabolism in a randomized, placebo-controlled clinical investigation. Astaxanthin (8 mg/day for 8 weeks) supplementation was found to significantly raise serum adiponectin (4714 g/mL) as compared to baseline and placebo (4513 and 3615 g/mL, respectively) (Mashhadi et al., 2018).
6.2.3 Anti-Ageing
A carotenoid with potent anti-inflammatory and antioxidant qualities is astaxanthin. Two main processes are known to be responsible for age-related skin changes: ageing and UV radiation exposure (photo aging). Photo aging results in wrinkles with pigmentation, and a breakdown of texture of skin because it destroys collagen and elastin, which are components of the extracellular matrix (Davinelli et al., 2018). Also, the reactive oxygen species are produced by the skin as a response to UV light. By virtue of its anti-inflammatory properties, astaxanthin supplementation has the potential to halt skin deterioration and maintenance of skin conditions caused by environmental exposure. Therefore, reducing oxidative stress through the inhibition of inflammatory cytokines is essential to avert age-related skin deterioration (Oslan et al., 2021). Additionally, when a human model received 4 mg daily dose of astaxanthin, the levels of malondialdehyde, a recognized indication of systemic oxidative stress, decrease (by 11.2% on day 15 and by 21.7% on day 29 (Chalyk et al., 2017). Furthermore, it has been discovered that astaxanthin as a dietary supplement helps in the inhibiting of the phosphorylation of extracellular signal regulated kinases, which is caused by the generation of reactive oxygen species (ROS) and prevents the skin fibroblastic autophagic cell death caused by bisphenol A (BPA) in normal human dermal fibroblasts (NHDF). BPA significantly boosted autophagy and apoptotic cell death in NHDF. Efficient restoration of cell death by autophagy caused by BPA was achieved by the suppression of intracellular ROS generation by astaxanthin (Lim et al., 2021).
7 Scientific obstacles and future prospects of astaxanthin production from microalgae grown in dairy wastewater
There are various scientific obstacles in the study of using dairy wastewater for microalgae-based astaxanthin production. The challenges and future perspectives of astaxanthin production are represented through a flowchart in Figure 5.
Figure 5
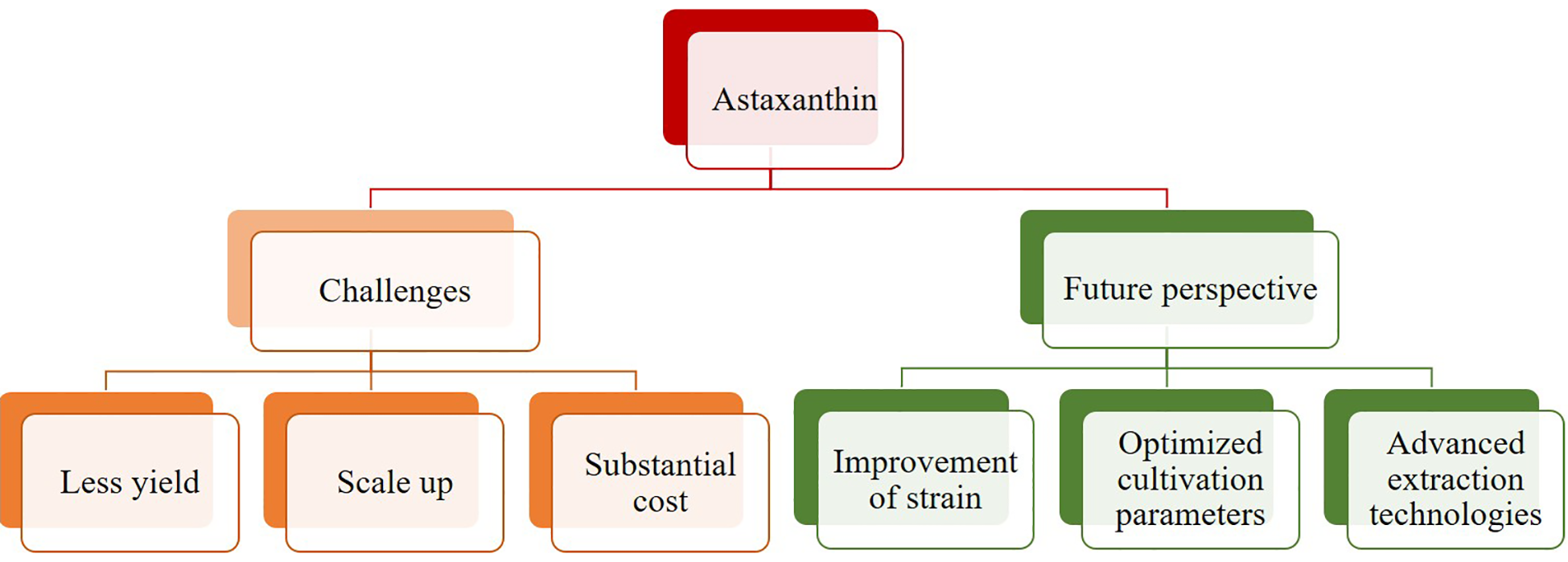
Challenges and future perspectives of astaxanthin production.
While significant advances have been employed in optimizing growth conditions for microalgae along with astaxanthin production, challenges remain unresolved. Especially with scaling up these processes for industrial applications. Crucial factors like temperature, nutrient availability, and light intensity are challenging to reliably regulate on a large scale, but they have a big impact on astaxanthin yield and quality.
The high organic load along with the turbidity including variable composition can be a greatest challenge. Some factors such as light penetration issue and residual chemicals which can lead towards the growth inhibition of microalgae. Due to the imbalances in nutrients, microbial contamination risk, pre-treatment and pre filtration adds to the complexity of the process. However, development of scaling up the systems for food grade astaxanthin have regulatory, quality, and safety concerns. Developing economical techniques to extract astaxanthin and harvest microalgae biomass is another significant problem. For large-scale operations, the current technologies are not economically viable due to their energy-intensive nature. Effective rupture of cell walls is another enduring problem; although several approaches have been investigated, they still these are expensive, ineffective, and frequently have adverse effects on the environment. Contamination risks also pose a big problem, especially considering the growing need for astaxanthin in the nutraceutical and pharmaceutical sectors. Although helpful, the use of solvents in the extraction process raises the risk of contamination, which is a significant issue for producing high purity astaxanthin that humans can consume. These difficulties highlight the need for further research to develop scalable, commercially feasible, and environmentally acceptable methods for generating astaxanthin from microalgae cultivated in dairy wastewater.
The European Algae Biomass Association (EABA) 2023 conducted a workshop on industrial technologies based on natural astaxanthin. Several technological barriers in the production of astaxanthin were highlighted. These barriers included high cost of production because of reliability on precise raw materials with the cultivation systems which are complex, susceptibility towards contamination during biomass production on large scale. These factors can affect the quality and yield. The feasibility of current production techniques is limited. There are some concerns related to environment i.e the high carbon emission during the production of synthetic astaxanthin as compared with the natural production. The workshop focused on the demand for innovative approaches to overcome the limitations and efficiency improvement, commercial feasibility, sustainability for the production of astaxanthin.
As the demand for naturally produced astaxanthin is increasing, especially in the nutraceutical and cosmetic industries, this is leading to the requirement of new methods for the large-scale production with the quality assurance. For the enhancement of astaxanthin yield, modification in genetics along with synthetic biology can play a significant role. Genetic modification of microalgae strains resulting in the improved metabolic pathways can increase the yield of astaxanthin. In spite of being recognized as the most prominent source of natural astaxanthin, there are several issues regarding the cultivation of Haematococcus pluvialis comes across several constraints, which comprises including a slow growth rate with confined biomass. There are a number of microorganisms i.e fungi, bacteria, and algae which contains exorbitant carotenoids, demonstrate the capability to produce have the astaxanthin (Nishshanka et al., 2022). With the development of new and more efficient extraction methods with the advanced photo bioreactor having optimized light distribution and nutrient providing can make this more efficient. This is less energy consuming and more productively efficient. The challenges and future prospects for the astaxanthin are shown in Figure 5. The techno economic modelling along with the life cycle analysis can aid in the commercial feasibility of production. Substantial research towards the reduction of costs during production and the improvement in the sustainability approach with the using of wastewater and capturing of carbon dioxide is important (Bauer and Minceva, 2021). These methods can decrease the aftereffects of the harmful solvents resulting in an environmentally sustainable method. Utilization of the dairy wastewater as a media alternative or a nutrient source for microalgae can be a promising option for the sustainable cultivation with the low-cost input. But if the astaxanthin production is integrated with the bio economic models for a wider bio economic framework in which the valorization of waste streams can be done and this could increase the economic feasibility.
8 Conclusion
Microalgae are represented as a significantly sustainable and natural source of natural astaxanthin. This offers an eco-friendly approach towards synthetic pigments. This review has summarized the recent advancements in the strategies of cultivation, stress factors along with the downstream processing. These factors are responsible in the contribution for improvement of astaxanthin yield. However, the utilization of dairy industry wastewater when used as a growth media represents prominent potential towards a sustainable approach which is even cost effective. This reduces the dependence on the synthetic culture media. Additionally, this review represents the innovations in emerging extraction and cell disruption technologies for the representation of recovery enhancement and shifting towards large scale production more. Apart from these findings, there are substantial industrial implications. Microalgae based astaxanthin represents significant promise for several applications in terms of pharmaceuticals, food, cosmetics, and nutraceuticals. Future research could be focused on the cultivation systems scale up, integration of growth strategies which are mixotrophic, and genetic engineering in microalgae for increased productivity. The establishment of standardized protocols, and the ensuring of regulatory compliances can be proven as a leading future prospect. Therefore, these are the directions which demonstrates a practical roadmap for the transformation of microalgae based astaxanthin into an impactful and reliable industrial product.
Statements
Author contributions
SJ: Writing – original draft, Conceptualization. AS: Writing – review & editing. BY: Writing – review & editing. AP: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. AP also wishes to acknowledge Norrlandsnavet, LTU for funding the project “Thriving in Uncertain Times: Sustainable and Resilient Microalgae-based Feed for SMEs in Northern Sweden (NutriFarm) LTU-490-2024.
Acknowledgments
The authors would like to express their gratitude to UPES Dehradun (R&D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abd Karim F. F. Mohamad S. E. Iwamoto K. (2024). Production of Omega-3 Fatty Acids and Astaxanthin from Chlorella vulgaris and Haematococcus pluvialis Cultivated in Chicken Manure Medium. J. Adv. Res. Micro Nano Engieering19, 78–85. doi: 10.37934/armne.19.1.7885
2
Acién F. G. Fernández J. M. Magán J. J. Molina E. (2012). Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv.30, 1344–1353. doi: 10.1016/j.biotechadv.2012.02.005
3
Ali M. Masood A. Saleem M. (2021). Microalgae cultivation in wastewater for simultaneous nutrients removal and biomass production. Int. J. Energy Environ. Eng.12, 475–485. doi: 10.1007/s40095-021-00383-3
4
Álvarez-Díaz P. D. Ruiz J. Arbib Z. Barragán J. Garrido-Pérez M. C. Perales J. A. (2015). Wastewater treatment and biodiesel production by Scenedesmus obliquus in a two-stage cultivation process. Bioresource Technol.181, 90–96. doi: 10.1016/j.biortech.2015.01.018
5
Ambati R. R. Moi P. S. Ravi S. Aswathanarayana R. G. (2014). Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs12, 128–152. doi: 10.3390/MD12010128
6
Ao X. Kim I. H. (2019). Effects of astaxanthin produced by Phaffia rhodozyma on growth performance, antioxidant activities, and meat quality in Pekin ducks. Poultry Sci.98, 4954–4960. doi: 10.3382/ps/pez256
7
Arunkumar E. Bhuvaneswari S. Anuradha C. V. (2012). An intervention study in obese mice with astaxanthin, a marine carotenoid–effects on insulin signaling and pro-inflammatory cytokines. Food Funct.3, 120–126. doi: 10.1039/C1FO10161G
8
Azizi M. Hejazi M. A. Hashemi M. (2019). Supplementation with polyalcohols and sequential mixotrophy dilution photoinduction strategy boost the accumulation of astaxanthin by Haematococcus pluvialis. Aquaculture511, 734225. doi: 10.1016/j.aquaculture.2019.734225
9
Bauer A. Minceva M. (2021). Techno-economic analysis of a new downstream process for the production of astaxanthin from the microalgae Haematococcus pluvialis. Bioresources Bioprocess.8, 1–18. doi: 10.1186/s40643-021-00463-6
10
Boonnoun P. Kurita Y. Kamo Y. Machmudah S. Okita Y. Ohashi E. et al . (2014). Wet extraction of lipids and astaxanthin from Haematococcus pluvialis by liquefied dimethyl ether. J. Nutr. Food Sci.4, 305. doi: 10.4172/2155-9600.1000305
11
Boussiba S. (2000). Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant.108, 111–117. doi: 10.1034/J.1399-3054.2000.108002111.X
12
Bratosin B. C. Darjan S. Vodnar D. C. (2021). Single cell protein: A potential substitute in human and animal nutrition. Sustainability13, 9284. doi: 10.3390/su13169284
13
Breithaupt D. E. (2004). Identification and Quantification of Astaxanthin Esters in Shrimp (Pandalus borealis) and in a Microalga (Haematococcus pluvialis) by Liquid Chromatography–Mass Spectrometry Using Negative Ion Atmospheric Pressure Chemical Ionization. J. Agric. Food Chem.52, 3870–3875. doi: 10.1021/JF049780B
14
Butler T. Golan Y. (2020). “ Astaxanthin production from microalgae,” in Microalgae Biotechnology for Food, Health and High Value Products, Singapore: Springer Singapore175–242.
15
Cai X. Chen Y. Xie X. Yao D. Ding C. Chen M. (2019). Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-κB. Am. J. Trans. Res.11, 1884.
16
Chalyk N. E. Klochkov V. A. Bandaletova T. Y. Kyle N. H. Petyaev I. M. (2017). Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res.48, 40–48. doi: 10.1016/j.nutres.2017.10.006
17
Cheng T. Xu X. Zhang W. Chen L. Liu T. (2018). Protoplast preparation from enriched flagellates and resting cells of Haematococcus pluvialis. J. Appl. Microbiol.124, 469–479. doi: 10.1111/jam.13643
18
Choi H.-J. (2016). Dairy wastewater treatment using microalgae for potential biodiesel application. Environ. Eng. Res.21, 393–400. doi: 10.4491/eer.2015.151
19
Choi Y. Y. Hong M.-E. Sim S. J. (2015). Enhanced astaxanthin extraction efficiency from Haematococcus pluvialis via the cyst germination in outdoor culture systems. Process Biochem.50, 2275–2280. doi: 10.1016/j.procbio.2015.09.008
20
Cui J. Yu C. Zhong D.-B. Zhao Y. Yu X. (2020). Melatonin and calcium act synergistically to enhance the coproduction of astaxanthin and lipids in Haematococcus pluvialis under nitrogen deficiency and high light conditions. Bioresource Technol.305, 123069. doi: 10.1016/j.biortech.2020.123069
21
Daneshvar E. Zarrinmehr M. J. Koutra E. Kornaros M. Farhadian O. Bhatnagar A. (2019). Sequential cultivation of microalgae in raw and recycled dairy wastewater: microalgal growth, wastewater treatment and biochemical composition. Bioresource Technol.273, 556–564. doi: 10.1016/j.biortech.2018.11.059
22
Das C. Naseera K. Ram A. Meena R. M. Ramaiah N. (2017). Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris. J. Appl. Phycol.29, 235–243. doi: 10.1007/s10811-016-0910-8
23
Davinelli S. Nielsen M. E. Scapagnini G. (2018). Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients10, 522. doi: 10.3390/nu10040522
24
Debnath T. Bandyopadhyay T. K. Vanitha K. Bobby Md. N. Nath Tiwari O. Bhunia B. et al . (2024). Astaxanthin from microalgae: A review on structure, biosynthesis, production strategies and application. Food Res. Int.176, 113841. doi: 10.1016/j.foodres.2023.113841
25
de Moraes L. B. S. Mota G. C. P. dos Santos E. P. Campos C. V. F. da S. da Silva B. A. B. Olivera Gálvez A. et al . (2024). Haematococcus pluvialis cultivation and astaxanthin production using different nitrogen sources with pulse feeding strategy. Biomass Conversion Biorefinery14, 16231–16243. doi: 10.1007/s13399-023-03824-7
26
Dragoş N. Bercea V. Bica A. Druga B. Nicoará A. Coman C. (2010). Astaxanthin production from a new strain of haematococcus pluvialis grown in batch culture. Ann. Romanian Soc. Cell Biol.15, 353–361.
27
Du H. Liao X. Gao Z. Li Y. Lei Y. Chen W. et al . (2019). Effects of methanol on carotenoids as well as biomass and fatty acid biosynthesis in Schizochytrium limacinum B4D1. Appl. Environ. Microbiol.85, e01243–e01219. doi: 10.1128/AEM.01243-19
28
EFSA Panel on Nutrition, N.F. and F.A. (NDA) Turck D. Castenmiller J. de Henauw S. Hirsch-Ernst K. I. Kearney J. et al . (2020). Safety of astaxanthin for its use as a novel food in food supplements. EFSA J.18, e05993. doi: 10.2903/j.efsa.2020.5993
29
Ganesan R. Manigandan S. Samuel M. S. Shanmuganathan R. Brindhadevi K. Lan Chi N. T. et al . (2020). A review on prospective production of biofuel from microalgae. Biotechnol. Rep.27, e00509. doi: 10.1016/j.btre.2020.e00509
30
Guerin M. Huntley M. E. Olaizola M. (2003). Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol.21, 210–216. doi: 10.1016/S0167-7799(03)00078-7
31
Haque F. Dutta A. Thimmanagari M. Chiang Y. W. (2016). Intensified green production of astaxanthin from Haematococcus pluvialis. Food Bioproducts Process.99, 1–11. doi: 10.1016/j.fbp.2016.03.002
32
Huang L. Du X. Jin Z. Ma J. Zuo Z. (2024). Accumulation of astaxanthin in Microcystis aeruginosa under NaCl and KCl stresses. Bioresource Technol.403, 130898. doi: 10.1016/j.biortech.2024.130898
33
Ip P.-F. Chen F. (2005). Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem.40, 733–738. doi: 10.1016/j.procbio.2004.01.039
34
Irshad M. Myint A. A. Hong M. E. Kim J. Sim S. J. (2019). One-pot, simultaneous cell wall disruption and complete extraction of astaxanthin from Haematococcus pluvialis at room temperature. ACS Sustain. Chem. Eng.7, 13898–13910. doi: 10.1021/acssuschemeng.9b02089
35
Iwamoto T. Hosoda K. Hirano R. Kurata H. Matsumoto A. Miki W. et al . (2000). Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscl. Thromb.7, 216–222. doi: 10.5551/jat1994.7.216
36
Johnson E. J. (2014). Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev.72, 605–612. doi: 10.1111/nure.12133
37
Khoo K. S. Lee S. Y. Ooi C. W. Fu X. Miao X. Ling T. C. et al . (2019). Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresource Technol.288, 121606. doi: 10.1016/j.biortech.2019.121606
38
Khoo K. S. Chew K. W. Yew G. Y. Manickam S. Ooi C. W. Show P. L. (2020). Integrated ultrasound-assisted liquid biphasic flotation for efficient extraction of astaxanthin from Haematococcus pluvialis. Ultrasonics Sonochem.67, 105052. doi: 10.1016/j.ultsonch.2020.105052
39
Kiani H. Azimi Y. Li Y. Mousavi M. Cara F. Mulcahy S. et al . (2023). Nitrogen and phosphate removal from dairy processing side-streams by monocultures or consortium of microalgae. J. Biotechnol.361, 1–11. doi: 10.1016/j.jbiotec.2022.11.011
40
Kiani H. Ma Q. Xiao M. Li Y. Brooke F. J. Mulcahy S. et al . (2024). Growth and fatty acid profile of Nannochloropsis oceanica cultivated on nano-filtered whey permeate. J. Appl. Phycol.36, 2503–2516. doi: 10.1007/s10811-024-03287-x
41
Kim B. Youn Lee S. Lakshmi Narasimhan A. Kim S. Oh Y.-K. (2022). Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresource Technol.343, 126124. doi: 10.1016/j.biortech.2021.126124
42
Kim S. Y. Oh Y.-K. Ha S. H. (2018). Recovery of astaxanthin from microalgae using simple and energy-efficient method. Korean Chem. Eng. Res.56, 376–380. doi: 10.9713/kcer.2018.56.3.376
43
Kleinert C. Griehl C. (2022). In situ extraction (milking) of the two promising Botryococcus braunii strains Showa and Bot22 under optimized extraction time. J. Appl. Phycol.34, 269–283. doi: 10.1007/s10811-021-02633-7
44
Kou Y. Liu M. Sun P. Dong Z. Liu J. (2020). High light boosts salinity stress-induced biosynthesis of astaxanthin and lipids in the green alga Chromochloris zofingiensis. Algal Res.50, 101976. doi: 10.1016/j.algal.2020.101976
45
Kusmayadi A. Lu P.-H. Huang C.-Y. Leong Y. K. Yen H.-W. Chang J.-S. (2022). Integrating anaerobic digestion and microalgae cultivation for dairy wastewater treatment and potential biochemicals production from the harvested microalgal biomass. Chemosphere291, 133057. doi: 10.1016/j.chemosphere.2021.133057
46
Landon R. Gueguen V. Petite H. Letourneur D. Pavon-Djavid G. Anagnostou F. (2020). Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs18, 357. doi: 10.3390/md18070357
47
Lee S. Y. Cho J. M. Chang Y. K. Oh Y.-K. (2017). Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresource Technol.244, 1317–1328. doi: 10.1016/j.biortech.2017.06.038
48
Leong W. H. Saman N. A. M. Kiatkittipong W. Assabumrungrat S. Najdanovic-Visak V. Wang J. et al . (2022). Photoperiod-induced mixotrophic metabolism in Chlorella vulgaris for high biomass and lipid to biodiesel productions using municipal wastewater medium. Fuel313, 123052. doi: 10.1016/j.fuel.2021.123052
49
Lichtenthaler H. K. (1987). 34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol.148, 350–382. doi: 10.1016/0076-6879(87)48036-1
50
Lim K. C. Yusoff F. M. Shariff M. Kamarudin M. S. Nagao N. (2019). Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch 1790). Aquaculture512, 734339. doi: 10.1016/j.aquaculture.2019.734339
51
Lim S.-R. Kim D.-W. Sung J. Kim T. H. Choi C.-H. Lee S.-J. (2021). Astaxanthin inhibits autophagic cell death induced by bisphenol a in human dermal fibroblasts. Antioxidants10, 1273. doi: 10.3390/antiox10081273
52
Liu J. Huang J. Jiang Y. Chen F. (2012). Molasses-based growth and production of oil and astaxanthin by Chlorella zofingiensis. Bioresource Technol.107, 393–398. doi: 10.1016/j.biortech.2011.12.047
53
Liu Z. Zeng X. Cheng J. Liu D. Aadil R. M. (2018). The efficiency and comparison of novel techniques for cell wall disruption in astaxanthin extraction from Haematococcus pluvialis. Int. J. Food Sci. Technol.53, 2212–2219. doi: 10.1111/ijfs.13810
54
Liu H. Huang W.-C. Guo N. Mao X. (2020). Application of secondary amine switchable hydrophilicity solvents for astaxanthin extraction from wet Haematococcus pluvialis. Algal Res.48, 101892. doi: 10.1016/j.algal.2020.101892
55
Lorenz R. T. (1999). A technical review of Haematococcus algae. NatuRoseTM Tech. Bull.60, 1–12.
56
Lu Q. Lu Y. (2022). Microalga- and yeast-based astaxanthin production via nutrient recovery from wastewater for aquaculture practice: an emerging technology for sustainable development. J. Chem. Technol. Biotechnol.97, 3035–3048. doi: 10.1002/jctb.7164
57
Ma R. Ma X. Qiao Y. Wang B. Ho S. H. Chen J. et al . (2025). Improved production of astaxanthin in heterotrophic Chromochloris zofingiensis through optimized culture conditions incorporating an efficient fed-batch strategy. Algal Res.86, 103895. doi: 10.1016/J.ALGAL.2025.103895
58
Machado F. R. S. Jr Trevisol T. C. Boschetto D. L. Burkert J. F. M. Ferreira S. R. S. Oliveira J. V. et al . (2016). Technological process for cell disruption, extraction and encapsulation of astaxanthin from Haematococcus pluvialis. J. Biotechnol.218, 108–114. doi: 10.1016/j.jbiotec.2015.12.004
59
Mashhadi N. S. Zakerkish M. Mohammadiasl J. Zarei M. Mohammadshahi M. Haghighizadeh M. H. (2018). Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pacific J. Clin. Nutr.27, 341–346. doi: 10.6133/apjcn.052017.11
60
Miao F. Lu D. Li Y. Zeng M. (2006). Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. Analyt. Biochem.352, 176–181. doi: 10.1016/J.AB.2006.03.006
61
Molino A. Mehariya S. Iovine A. Larocca V. Di Sanzo G. Martino M. et al . (2018a). Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar. Drugs16, 432. doi: 10.3390/md16110432
62
Molino A. Rimauro J. Casella P. Cerbone A. Larocca V. Chianese S. et al . (2018b). Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol.283, 51–61. doi: 10.1016/j.jbiotec.2018.07.010
63
Monteiro Dos Santos L. Barbosa da Silva J. C. de Farias Silva C. E. Villar da Gama B. M. Almeida Medeiros J. Markou G. et al . (2024). Co-Cultivation between the Microalga Tetradesmus obliquus and Filamentous Fungus Cunninghamella echinulata Improves Tertiary Treatment of Cheese Whey Effluent in Semicontinuous Mode. Processes12, 1573. doi: 10.3390/pr12081573
64
Muthuraman A. Shaikh S. A. Ramesh M. Sikarwar M. S. (2021). The structure–activity relationship of marine products for neuroinflammatory disorders. Stud. Natural Prod. Chem.70, 151–194. doi: 10.1016/B978-0-12-819489-8.00013-2
65
Nagarajan D. Lee D. J. Chen C. Y. Chang J. S. (2020). Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresource Technol.302, 122817. doi: 10.1016/j.biortech.2020.122817
66
Nair A. Ahirwar A. Singh S. Lodhi R. Lodhi A. Rai A. et al . (2023). Astaxanthin as a king of ketocarotenoids: structure, synthesis, accumulation, bioavailability and antioxidant properties. Mar. Drugs21, 176. doi: 10.3390/md21030176
67
Nguyen H. T. T. Jadhav D. A. Eisa T. Nguyen H. Y. Le G. T. H. Le T. T. Q. et al . (2024). Sustainable conversion of carbon dioxide to high-value antioxidant astaxanthin through microbial electrosynthesis-assisted microalgae cultivation. Process Saf. Environ. Prot.190, 212–225. doi: 10.1016/J.PSEP.2024.07.030
68
Nishida Y. Berg P. C. Shakersain B. Hecht K. Takikawa A. Tao R. et al . (2023). Astaxanthin: past, present, and future. Mar. Drugs21, 514. doi: 10.3390/MD21100514/S1
69
Nishshanka G. K. S. H. Liyanaarachchi V. C. Nimarshana P. H. V. Ariyadasa T. U. Chang J.-S. (2021). Wastewater-based microalgal biorefineries for the production of astaxanthin and co-products: Current status, challenges and future perspectives. Bioresource Technol.342, 126018. doi: 10.1016/j.biortech.2021.126018
70
Nishshanka G. K. S. H. Liyanaarachchi V. C. Premaratne M. Nimarshana P. H. V. Ariyadasa T. U. Kornaros M. (2022). Haematococcus pluvialis: a potential feedstock for multiple-product biorefining. J. Cleaner Prod.344, 131103. doi: 10.1016/j.jclepro.2022.131103
71
Nitsos C. Filali R. Taidi B. Lemaire J. (2020). Current and novel approaches to downstream processing of microalgae: A review. Biotechnol. Adv.45, 107650. doi: 10.1016/j.biotechadv.2020.107650
72
Nobre B. Marcelo F. Passos R. Beirão L. Palavra A. Gouveia L. et al . (2006). Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol.223, 787–790. doi: 10.1007/s00217-006-0270-8
73
Oslan S. N. H. Tan J. S. Oslan S. N. Matanjun P. Mokhtar R. A. M. Shapawi R. et al . (2021). Haematococcus pluvialis as a potential source of astaxanthin with diverse applications in industrial sectors: current research and future directions. Molecules26, 6470. doi: 10.3390/molecules26216470
74
Panis G. Carreon J. R. (2016). Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res.18, 175–190. doi: 10.1016/j.algal.2016.06.007
75
Park J.-Y. Oh Y.-K. Choi S.-A. Kim M.-C. (2020). Recovery of astaxanthin-containing oil from Haematococcus pluvialis by nano-dispersion and oil partitioning. Appl. Biochem. Biotechnol.190, 1304–1318. doi: 10.1007/s12010-019-03167-y
76
Patel A. K. Tambat V. S. Chen C.-W. Chauhan A. S. Kumar P. Vadrale A. P. et al . (2022). Recent advancements in astaxanthin production from microalgae: A review. Bioresource Technol.364, 128030. doi: 10.1016/j.biortech.2022.128030
77
Pereira C. P. M. Souza A. C. R. Vasconcelos A. R. Prado P. S. (2021). Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases. Int. J. Mol. Med.47, 37–48. doi: 10.3892/ijmm.2020.4783
78
Praveenkumar R. et al . (2015). Breaking dormancy: an energy-efficient means of recovering astaxanthin from microalgae. Green Chem.17, 1226–1234. doi: 10.1039/C4GC01413H
79
Raja S. W. Thanuja K. G. Karthikeyan S. Marimuthu S. (2022). Exploring the concurrent use of microalgae Coelastrella sp. for electricity generation and dairy wastewater treatment. Bioresource Technol. Rep.17, 100889. doi: 10.1016/j.biteb.2021.100889
80
Raman R. Mohamad S. E. (2012). Astaxanthin production by freshwater microalgae Chlorella sorokiniana and marine microalgae Tetraselmis sp. Pak J. Biol. Sci.15, 1182–1186. doi: 10.3923/pjbs.2012.1182.1186
81
Saadaoui I. Rasheed R. Aguilar A. Cherif M. Al Jabri H. Sayadi S. et al . (2021). Microalgal-based feed: promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol.12, 76. doi: 10.1186/s40104-021-00593-z
82
Saini R. K. Keum Y.-S. (2018). Carotenoid extraction methods: A review of recent developments. Food Chem.240, 90–103. doi: 10.1016/j.foodchem.2017.07.099
83
Samorì C. Pezzolesi L. Galletti P. Semeraro M. Tagliavini E. (2019). Extraction and milking of astaxanthin from Haematococcus pluvialis cultures. Green Chem.21, 3621–3628. doi: 10.1039/C9GC01273G
84
Sarada R. Vidhyavathi R. Usha D. Ravishankar G. A. (2006). An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agric. Food Chem.54, 7585–7588. doi: 10.1021/jf060737t
85
Seo J.-K. Parvin R. Park J. Yang H.-S. (2021). Utilization of astaxanthin as a synthetic antioxidant replacement for emulsified sausages. Antioxidants10, 407. doi: 10.3390/antiox10030407
86
Shah M. M. R. Liang Y. Cheng J. J. Daroch M. (2016). Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front. Plant Sci.7, 531. doi: 10.3389/fpls.2016.00531
87
Sharma A. K. Sharma A. Singh Y. Chen W.-H. (2021). Production of a sustainable fuel from microalgae Chlorella minutissima grown in a 1500 L open raceway ponds. Biomass Bioenergy149, 106073. doi: 10.1016/j.biombioe.2021.106073
88
Sharma A. K. Ghodke P. Sharma P. K. Manna S. Pugazhendhi A. Matsakas L. et al . (2022). Holistic utilization of Chlorella pyrenoidosa microalgae for extraction of renewable fuels and value-added biochar through in situ transesterification and pyrolysis reaction process. Biomass Conversion Biorefinery14, 5261–5274. doi: 10.1007/s13399-022-02713-9
89
Sharma A. K. Sahoo P. K. Singhal S. (2015). Feasibility of biodiesel production from chlorella vulgarisgrown in flat plate photobioreactor under outdoor conditions. Int. J. ChemTech. Res.8, 671–678.
90
Shuba E. S. Kifle D. (2018). Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renewable Sustain. Energy Rev.81, 743–755. doi: 10.1016/j.rser.2017.08.042
91
Singh P. Venkata Mohan S. Mohanty K. (2023). Dairy wastewater treatment using Monoraphidium sp. KMC4 and its potential as hydrothermal liquefaction feedstock. Bioresource Technol.376, 128877. doi: 10.1016/j.biortech.2023.128877
92
Snell T. W. Carberry J. (2022). Astaxanthin bioactivity is determined by stereoisomer composition and extraction method. Nutrients14, 1522. doi: 10.3390/NU14071522
93
Stachowiak B. Szulc P. (2021). Astaxanthin for the food industry. Molecules26, 2666. doi: 10.3390/MOLECULES26092666
94
Stasinakis A. S. Charalambous P. Vyrides I. (2022). Dairy wastewater management in EU: Produced amounts, existing legislation, applied treatment processes and future challenges. J. Environ. Manage.303, 114152. doi: 10.1016/j.jenvman.2021.114152
95
Sudhanthiran C. M. Perumalsamy M. (2024). Bioremediation of dairy industry wastewater and assessment of nutrient removal potential of Chlorella vulgaris. Biomass Conversion Biorefinery14, 10335–10346. doi: 10.1007/s13399-022-03068-x
96
Tambat V. S. Patel A. K. Singhania R. R. Vadrale A. P. Tiwari A. Chen C.-W. et al . (2023). Sustainable mixotrophic microalgae refinery of astaxanthin and lipid from Chlorella zofingiensis. Bioresource Technol.387, 129635. doi: 10.1016/j.biortech.2023.129635
97
Valderrama J. O. Perrut M. Majewski W. (2003). Extraction of astaxantine and phycocyanine from microalgae with supercritical carbon dioxide. J. Chem. Eng. Data48, 827–830. doi: 10.1021/je020128r
98
Vechio H. Mariano A. B. Vieira R. B. (2021). A new approach on astaxanthin extraction via acid hydrolysis of wet Haematococcus pluvialis biomass. J. Appl. Phycol.33, 2957–2966. doi: 10.1007/s10811-021-02495-z
99
Vega K. Edwards J. Beilstein P. (2015). Subchronic (13-week) toxicity and prenatal developmental toxicity studies of dietary astaxanthin in rats. Regul. Toxicol. Pharmacol.73, 819–828. doi: 10.1016/j.yrtph.2015.10.013
100
Verma R. Suthar S. Chand N. Mutiyar P. K. (2022). Phycoremediation of milk processing wastewater and lipid-rich biomass production using Chlorella vulgaris under continuous batch system. Sci. Total Environ.833, 155110. doi: 10.1016/j.scitotenv.2022.155110
101
Villaró S. Ciardi M. Morillas-España A. et al . (2021). Microalgae derived astaxanthin: research and consumer trends and industrial use as food. Foods10, 2303. doi: 10.3390/foods10102303
102
Xie S. Yin P. Tian L. Yu Y. Liu Y. Niu J. (2020). Dietary supplementation of astaxanthin improved the growth performance, antioxidant ability and immune response of juvenile largemouth bass (Micropterus salmoides) fed high-fat diet. Mar. Drugs18, 642. doi: 10.3390/md18120642
103
Yao G. Muhammad M. Zhao J. Liu J. Huang Q. (2022). DFT-based Raman spectral study of astaxanthin geometrical isomers. Food Chem.: Mol. Sci.4, 100103. doi: 10.1016/J.FOCHMS.2022.100103
104
Ye M. Li Y.-Y. (2023). Methanogenic treatment of dairy wastewater: A review of current obstacles and new technological perspectives. Sci. Total Environ.866, 161447. doi: 10.1016/j.scitotenv.2023.161447
105
Yen S.-W. Nagarajan D. Chen W.-H. Chang J.-S. (2024). Enhanced astaxanthin production by Aurantiochytrium sp. CJ6 using sorghum distillery residue (SDR)-based growth medium and SDR-derived biochar carrier. Biochem. Eng. J.203, 109185. doi: 10.1016/j.bej.2023.109185
106
Zhang X. Lu Q. (2024). Cultivation of microalgae in food processing effluent for pollution attenuation and astaxanthin production: a review of technological innovation and downstream application. Front. Bioeng. Biotechnol.12. doi: 10.3389/fbioe.2024.1365514
107
Zhao X. Zhang X. Liu H. Zhu H. Zhu Y. (2019). Enzyme-assisted extraction of astaxanthin from Haematococcus pluvialis and its stability and antioxidant activity. Food Sci. Biotechnol.28, 1637–1647. doi: 10.1007/s10068-019-00608-6
108
Zhu X. Meng C. Sun F. Wei Z. Chen L. Chen W. et al . (2023). Sustainable production of astaxanthin in microorganisms: The past, present, and future. Crit. Rev. Food Sci. Nutr.63, 10239–10255. doi: 10.1080/10408398.2022.2080176
109
Zhuge F. Ni Y. Wan C. Liu F. Fu Z. (2021). Anti-diabetic effects of astaxanthin on an STZ-induced diabetic model in rats. Endocr. J.68, 451–459. doi: 10.1507/endocrj.EJ20-0699
Summary
Keywords
microalgae, wastewater, upstream process, downstream process, bioremediation, astaxanthin
Citation
Jaryal S, Sharma AK, Lamba BY and Patel A (2025) A comprehensive review on microalgae based astaxanthin: bioprocess optimization, technological barriers, industrial applications and future roadmap. Front. Mar. Sci. 12:1644644. doi: 10.3389/fmars.2025.1644644
Received
10 June 2025
Accepted
20 October 2025
Published
07 November 2025
Volume
12 - 2025
Edited by
Arun Kumar Mishra, Banaras Hindu University, India
Reviewed by
R. Anburaj, Thanthai Hans Roever College, India
Maria Victoria Martin, CONICET Instituto de Investigaciones en Biodiversidad y Biotecnología (INBIOTEC), Argentina
Updates
Copyright
© 2025 Jaryal, Sharma, Lamba and Patel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amit Kumar Sharma, amitsharma@ddn.upes.ac.in; Alok Patel, alok.kumar.patel@ltu.se
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.