- 1Interdisciplinary Centre of Marine and Environmental Research (CIIMAR) University of Porto, Matosinhos, Portugal
- 2ICBAS – Institute of Biomedical Sciences Abel Salazar, Porto University, Porto, Portugal
- 3School of Environmental and Life Sciences, University of Hull, Hull, United Kingdom
- 4International Estuarine & Coastal Specialists (IECS) Ltd., Leven, United Kingdom
- 5Faculty of Sciences, University of Porto, Porto, Portugal
Microplastics (MPs) ingestion in wild fish during the early stages remains a field with scarce information and contradictory findings in laboratory studies. This study evaluated whether MPs contamination of larval fish stages begins at the commencement of the exogenous feeding phase and whether different species and ontogenetic development stages exhibit different profiles of MPs contamination. We assessed, for the first time, the presence of MPs in the larval stages of two species: the European sardine (Sardina pilchardus), a marine migrant species, and the common goby (Pomatoschistus microps), an estuarine resident species, inhabiting the Douro Estuary (NW Portugal). In both species, MPs were found even in the yolk-sac stage, when fish larvae still have endogenous feeding and do not actively prey on other organisms. This illustrates that fish larvae are already contaminated at a stage where the mouth is still not fully open, further indicating that MPs were not actively ingested. MPs contamination did not vary between species or throughout the ontogenetic development stages, showing similar levels of contamination and MPs contamination profiles. This novel study provides relevant insights into MPs contamination processes, showing that MPs contamination can occur early in the life cycle of fishes, from hatching onwards. Furthermore, the presence of MPs in fish larvae appears to be more dependent on the MPs that are in higher abundance in the surrounding water than fish larvae preferences or ecological guild, physical characteristics, or even the ontogenetic developmental stage.
1 Introduction
Poor management of plastic waste has resulted in approximately 2 million tonnes of plastic debris entering aquatic environments annually (Defontaine et al., 2020; Oo et al., 2021; Plastics Europe, 2022). Once in aquatic systems, plastic exposed to abiotic factors such as UV radiation and mechanical abrasion starts to degrade and fragment, breaking down into progressively smaller particles (Oo et al., 2021; Ramos et al., 2024). The widespread presence of plastic, particularly microplastics (MPs; plastic particles < 5 mm), has raised significant concerns regarding environmental health impacts and potential harm to diverse aquatic organisms (Yap and Yasid, 2023).
The physical characteristics of MPs, particularly their size and colour, closely resemble natural food sources for several marine organisms, leading to active ingestion or accidental capture by species such as planktonic organisms (Figueiredo and Vianna, 2018; He et al., 2022). This is particularly concerning given that plankton encompasses a diverse and valuable group of organisms, including the early life stages of fish. Fish larvae have considerable potential economic value as well as an essential ecological role in marine food webs. Hence, their health and survival are fundamental to the sustainability of fish populations (Russell, 1976; Steer et al., 2017). In the early developmental stages of life, fish are still undergoing major morphological and physiological changes, including in their natatory capacity and feeding behaviour. Moreover, they are at their most sensitive stage, and several factors can affect their survival, including exposure to MPs (Steer et al., 2017; Botterell et al., 2019). Compromising the growth and survival of larval fish can affect the population structures and marine food webs. Hence, understanding the dynamics of MP contamination in fish larvae is crucial for assessing ecological impacts and developing mitigation strategies.
Previous studies on adult fish have shown that MPs ingestion can be species-specific, and the rate is influenced by different feeding behaviours and strategies (Siddique et al., 2024). In addition, characteristics such as mouth size and prey preferences can influence the amount and type of MPs ingested (Siddique et al., 2024). While the presence of MPs in adult fish has been widely documented, their presence and impact on early life stages remain poorly understood. Laboratory studies have demonstrated that MPs ingestion by fish larvae can cause multiple adverse effects, including impacts on feeding, growth, fecundity, oxidative stress, intestinal damage, and morphological deformation, and eventually lead to mortality (Rodrigues et al., 2021; Chouchene et al., 2023; Liu et al., 2024). However, currently, data on MPs’ presence in wild fish larvae is scarce, and existing related studies often fail to distinguish between species, specify the ontogenetic development stages, or even compare fish larvae characteristics such as ecological guilds, feeding behaviours, or physical characteristics (Rodrigues et al., 2021). Consequently, there is a significant gap in the literature regarding the mechanisms of MP contamination, particularly whether uptake occurs through active ingestion or accidental or passive contamination. Furthermore, it is still unclear when MPs contamination starts to occur and whether different ontogenetic development stages exhibit different preferences based on the colour and shape of the particles, as suggested by previous studies (Botterell et al., 2022; Sambolino et al., 2022).
The Douro estuary is a nursery for various fish species and exhibits significant MPs contamination (Ramos et al., 2015; Rodrigues et al., 2019). In the current study, two species of fish larvae (the common goby, Pomatoschistus microps, and the sardine, Sardina pilchardus), which have different life cycles and anatomical features, were used to address fundamental questions about MP bioaccumulation in fish larvae, including when the contamination starts to occur and to provide the MP contamination profile throughout the ontogenetic development stages and in different species. Hence, this study aimed to: i) evaluate at the ontogenetic development stage at which MPs contamination in fish larvae starts and in particular to determine if MPs were present before independent feeding occurred; ii) determine whether MPs contamination in the organism varied throughout the ontogenetic development of the fish larvae; iii) quantify and characterize MPs present in S. pilchardus and P. microps in different larval stages, and lastly, iv) assess whether different species have different MPs concentrations and MPs preferences.
2 Methods
2.1 Study area and sampling methodology
Fish larvae were collected from the Douro Estuary, a Portuguese urban estuary located in the northwest of Portugal (41°8’36.00”N 8°39’25.00”W). This estuary extends 21.6 km from the estuary mouth until its upstream limit, the Crestuma Lever Dam (Rodrigues et al., 2022 and references therein). It is highly impacted by intense urbanization and touristic activity, with a high density of boat traffic, several freshwater streams, as well as wastewater treatment plant (WWTP) effluents draining into the estuary (Azevedo et al., 2008; Ramos et al., 2015). Monthly sampling surveys were conducted from September 2021 to September 2022 at 11 sampling stations along the estuary (Supplementary Figure S1 in the Supplementary Material). At each sampling station, planktonic samples were collected using a conical plankton net with a 1 m diameter, 4 m length, and 500 μm mesh size. Subsurface (1–2 m depth) planktonic circular tows were performed during the slack phase of spring tides (i.e., 2 hours before high tide) at a constant velocity of approximately 1ms−1 for 5 min. The volume of water filtered was determined using a Hydro-Bios flowmeter attached to the net. Samples were immediately transferred to flasks and fixed and preserved in 70% ethanol until laboratory analysis. The number of individuals was counted for the entire sample, and using the volume of water filtered, it was standardized to the number of fish larvae per 100 m−3.
2.2 Fish larvae processing
Fish larvae were sorted and isolated from the collected samples under a stereomicroscope (Nikon SMZ800) and then identified to the highest possible taxonomic separation using specialized literature (Russell, 1976; Ré, 1999; Ré and Meneses, 2008; Rodriguez et al., 2017). The two most abundant and frequent larval fish species observed, P. microps and S. pilchardus (Figure 1), were selected for MPs quantification, due to their contrasting differences in terms of pelagic larval duration and their use of estuaries as habitats. The ontogenetic development stage of each fish larva was also recorded, namely: yolk-sac, preflexion, flexion, and postflexion. Fish larvae in the yolk-sac stage are newly hatched larvae and still have yolk-sac or remnants of yolk; they frequently have an unformed mouth, unpigmented eyes, and undeveloped pectoral fins. Then the subsequent stages are preflexion, flexion and postflexion, which are related to the flexion of the notochord during caudal fin development. The preflexion stage begins once complete absorption of the yolk sac has occurred and ends with the start of notochord flexion. The flexion stage occurs at the beginning of the dorsal bending of the notochord tip and ends when the notochord tip reaches its final position and the principal caudal-fin rays and supporting skeletal elements are in the adult longitudinal position. The postflexion stage begins after the conclusion of notochord flexion and ends at the beginning of metamorphosis (Russell, 1976; Ré and Meneses, 2008).
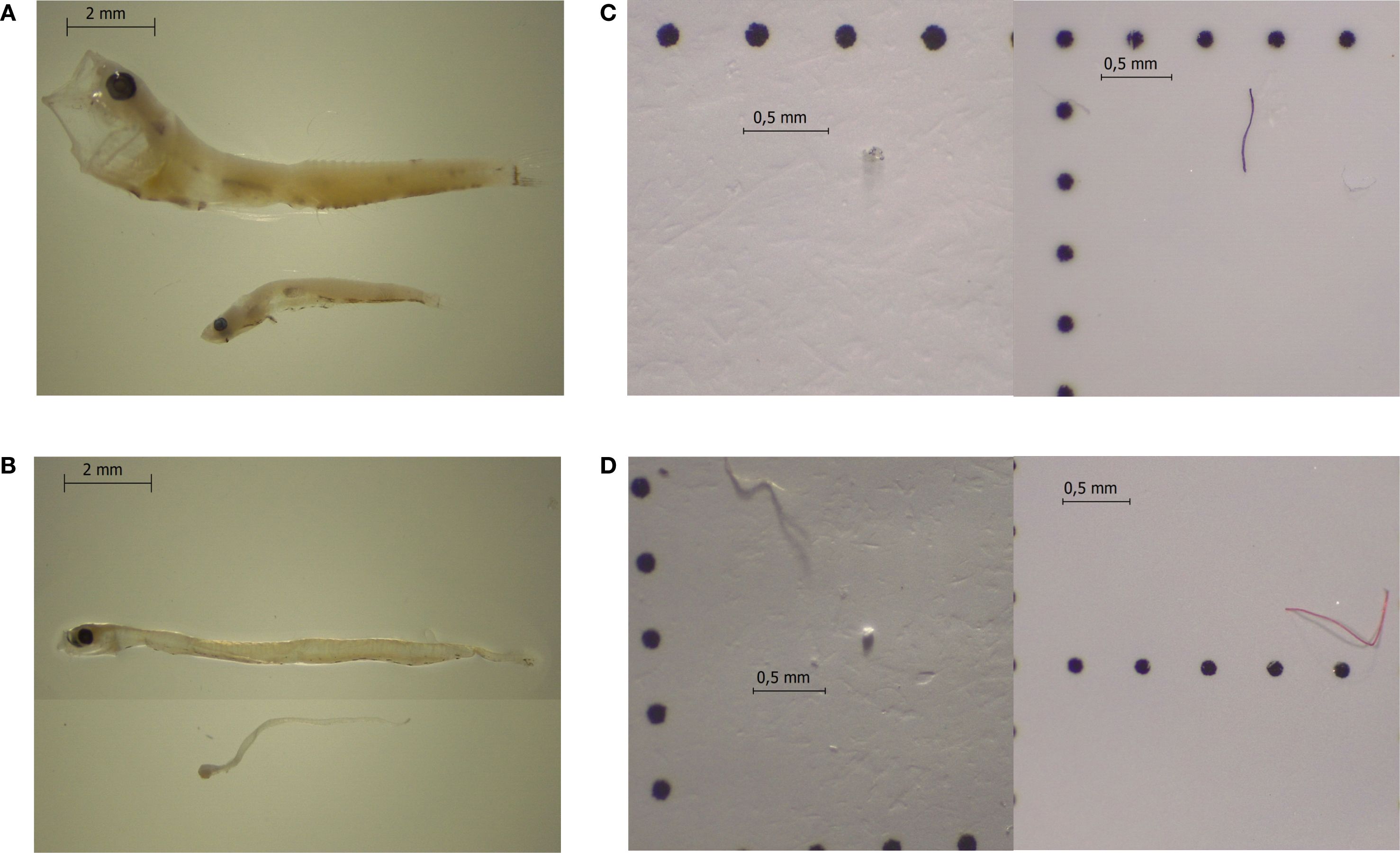
Figure 1. Examples of (A) Pomatoschistus microps and (B) Sardina pilchardus larvae in different ontogenetic development stages and examples of MPs recovered from (C) P. microps and (D) S. pilchardus larvae.
2.3 MPs quantification
The presence of MPs in planktonic species has been assessed in different ways, namely by the incidence of ingestion (by analysing organisms individually, e.g., Steer et al., 2017) or by encounter rate (by analysing a pool of organisms, e.g., Desforges et al., 2015; Sun et al., 2017). Here, the presence of MPs in the two selected species was quantified by analysing pools of organisms. All fish larvae belonging to the two species selected were isolated and grouped in pools according to the station and month where they were collected, the species, and the ontogenetic development stage, making a total of 99 samples (Supplementary Figure S1 in the Supplementary Material). The abundance of MPs per fish in each sample was obtained by dividing the total number of MPs obtained in each sample by the total number of fish larvae digested in that same sample. The average MPs abundance was calculated by dividing the total number of MPs by the total number of fish larvae. Those samples were subjected to a protocol previously optimized to quantify MPs in planktonic organisms, including fish larvae (Rodrigues et al., 2023). In brief, each fish larva was individually selected, thoroughly rinsed with deionized water, and checked for any external MPs using a stereomicroscope to ensure that no particles were attached/adsorbed to their body, and then transferred to the respective vial.
In each vial, 2 mL of 30% (v/v) H2O2 solution was added and placed in the oven at 65°C for a maximum of 7 hours. This protocol guarantees the complete digestion of the organism’s tissues while preserving the characteristics of MPs (e.g., shape, colour) present in the organism. After the digestion, the samples were filtered through a glass filtration system using a 0.45 μm pore size cellulose membrane and stored in a petri dish, which was left to dry at room temperature (20-25°C). Finally, the filters were visually inspected for the presence of MPs; due to equipment restrictions, only plastic particles between 5 mm and 0.01 mm were considered.
MPs recovered from each sample were observed, counted, and characterized under a stereomicroscope (Nikon SMZ800) and classified according to shape (fiber, fragment, film, sphere, and foam) and colour. Those with more than one colour were classified according to the predominant one. MPs were photographed and their precise size was obtained using the stereomicroscope Leica EZ04 software; in the results, MPs were assigned to size classes (0.01-0.05 mm, 0.06-0.5 mm, 0.6–1 mm, 1.1-1.5 mm, 1.6–2 mm, 2.1-2.5 mm and 2.6-3.0 mm).
A subsample of the most representative particles found was selected for polymer identification using a stereomicroscope for further FTIR (Fourier Transform Infrared Spectroscopy) analysis (45% of the total particles found); this follows the recommendation under the European Union Marine Strategy Framework Directive (MSFD) that recommends the examination of at least 10% of the sample (Galgani et al., 2023). Polymer spectra were registered in a PerkinElmer (Waltham, Massachusetts, USA) FTIR Spectrum 2 instrument, coupled with attenuated total reflectance (ATR). The resulting spectra were compared with reference library spectra, and matches with confidence levels of 65% were accepted. Of the particles analysed using FTIR, 85% were successfully confirmed as plastic polymers. The remaining 15% of the particles were not suitable for placement on the crystal surface of the FTIR, and their polymer type could not be confirmed; hence, they were not included in the results presented here. The fibers entangled in a bundle had different colours and may have had differing chemical compositions; however, their polymer was not confirmed using FTIR due to equipment restrictions.
2.3 Quality assurance/analytical quality control measures
To prevent any contamination, the following QA/AQC measures were taken: i) coloured cotton laboratory coats were worn during all laboratory procedures; ii) all the materials used were made of glass; only in a few exceptions plastic polymers materials were used (e.g., sieving of water samples to collect fish larvae – the plastic polymers of these material were confirmed by FTIR and MP contamination from these materials were not observed); iii) cleaning steps were applied before use including rinsing steps to all the material used in the field and laboratory; v) all the procedures in the laboratory were made inside a fume hood; iv) a petri dish with deionized water was exposed to the environment near the stereomicroscope as an air contamination control and inspected for MPs at the end of all the sorting processes (including fish larvae and MPs); and vi) blank samples with only 30% (v/v) H2O2 solution were included, in triplicate, each time the protocol for MPs quantification was performed (these blank samples were subjected to all the steps of the protocol together with the vials with samples). All blanks and petri dish controls were always inspected, and none of them had any type of MP contamination.
2.4 Data analysis
Larval length measurements for all specimens of both species were obtained using a stereomicroscope and Leica EZ04 software. The ontogenetic development stage of each fish larva was also recorded, namely: yolk-sac, preflexion, flexion, and postflexion (Russell, 1976; Ré and Meneses, 2008). The relationship between larval length and ontogenetic stage was assessed using linear regression analysis, with statistical significance set at α = 0.05. Two-way analysis of variance (ANOVA) was used to determine the effect of species and larval ontogenetic development stages on MPs concentration, and shape (i.e., number of different shapes observed in that sample), colour (i.e., number of different colours observed in that sample) and size (i.e., number of different sizes observed in that sample) diversity with species and larval ontogenetic development stages as fixed factors. A two-way analysis of variance (ANOVA) was used to ascertain the effect of month and sampling station on MPs concentration, with months and sampling stations as fixed factors. MPs concentration data were square root transformed to fulfil the ANOVA assumptions of homogeneous variance and normally distributed data (Zar, 1996). The normality and homogeneity of the data were verified using the Shapiro-Wilks and Levene’s tests. A significance level of 0.05 was applied to all analyses. The error term accompanying the mean values refers to the standard deviation; however, in some cases, the standard deviation is not presented since only one sample was analysed for that species/ontogenetic development stage/month/site. All tests were performed using TIBCO Statistica™ 14.0 software.
3 Results
3.1 Fish larvae collected
A total of 707 fish larvae were collected: 350 fish larvae of S. pilchardus and 357 fish larvae of P. microps (Figure 1). The two species presented different temporal patterns: S. pilchardus was collected mainly between September 2021 and May 2022, with its abundance peaking in October, January, and April, reaching an average of 8.0 ± 13.7 fish larvae per 100 m-3 in April. This species was not observed in July 2022 or September 2022. Spatially, S. pilchardus was more abundant between 0.5 and 5.5 km from the estuary mouth, corresponding to the lower and middle areas of the estuary, near the sea (Supplementary Figures S1, S2 in the Supplementary Material). P. microps was collected in all sampling months and sampling stations, with the highest abundance observed in June, reaching an average of 5.0 ± 12.1 fish larvae per 100 m-3. Throughout the estuary, P. microps was more abundant between the middle and upper areas of the estuary, at 5.5 and 10.5 km from the estuary mouth (Supplementary Figures S1, S2 in the Supplementary Material).
S. pilchardus larvae were identified in three different ontogenetic development stages: 81% at the yolk-sac stage, 15% at the preflexion and 5% in the flexion stage. No S. pilchardus larvae were found in the postflexion stage. P. microps larvae were identified in four stages: 0.3% at the yolk-sac stage (n = 1 fish larvae), 59% at the preflexion stage, 38% at the flexion stage, and 4% at the postflexion stage. A linear relationship between larva length and the ontogenetic stage was observed in both species. P. microps larvae had an average length of 3.8 ± 0.1 mm at the preflexion stage, 8.2 ± 1.1 mm at the flexion stage, and 11.1 ± 0.7 mm at the postflexion stage (r2 = 0.97, p < 0.001). S. pilchardus larvae had an average length of 4.7 ± 0.2 mm at the yolk-sac stage, 7.4 ± 0.7 mm, and 11.0 ± 0.8 mm at the flexion stage (r2 = 0.93, p < 0.001) (Supplementary Figure S3 in the Supplementary Material).
3.2 MPs characterisation
3.2.1 Sardina pilchardus
A total of 359 MPs were recovered from the 350 S. pilchardus larvae analysed, with an average MPs contamination of 1.0 MPs per fish larva. As MPs presence was quantified using pools of organisms from the same species at the same larval stage, collected in the same station and month, it was not possible to determine the exact percentage of fish larvae that were contaminated. However, since some samples had an average MPs concentration < 1 MPs per fish larva, it was possible to conclude that not all fish larvae were contaminated with MPs, and MPs contamination varied between 0.1 and 12 MPs per fish larva (Figures 2 and Supplementary Figures S4 in the Supplementary Material). S. pilchardus larvae were contaminated with MPs of three different shapes (fiber, fragment and film) in eleven different colours (Table 1). Of the MPs recovered from this species, 32% were transparent nylon fibers of 0.5 mm, followed by 16% blue polyethylene fibers of 0.3 mm and 11% white polyethylene fragments of 0.1 mm (Table 1). In general, more than 50% of the MPs recovered from S. pilchardus were fibers (57%), followed by fragments (41%). Fiber bundles were observed in 3 samples. Transparent (46%) and blue (21%) colours represented more than 50% of the MPs recovered. MPs of white, red, black, yellow, green, pink, brown, and purple colours were also identified. All the MPs recovered were less than 3 mm, and MPs between 0.06-0.5 mm represented more than 70% of the MPs recovered (Table 1). Nylon, polyethylene (PE), and polypropylene (PP) were the main plastic polymers of the MPs recovered from fish larvae samples (Supplementary Figure S4 in the Supplementary Material).
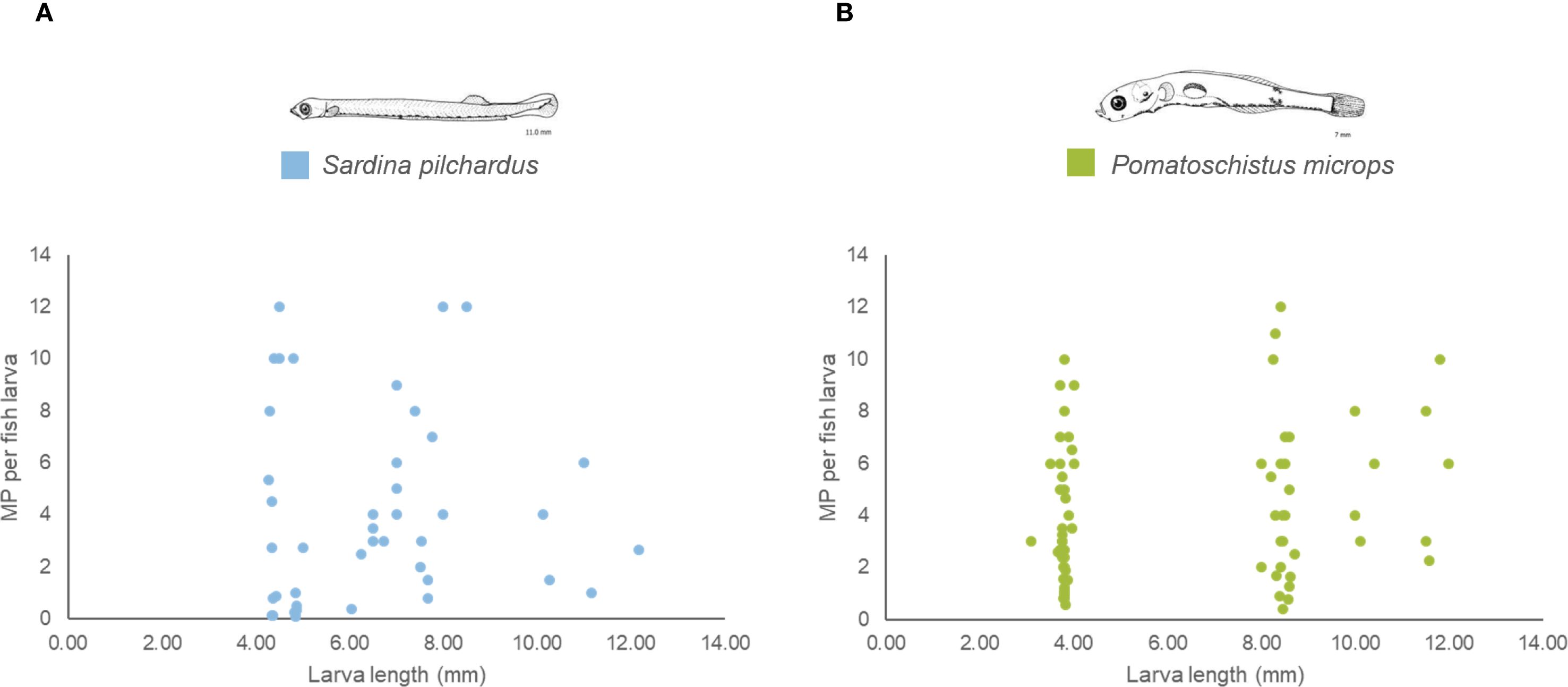
Figure 2. Average MPs concentration per fish larva along larva length (mm) for each species (A) Sardina pilchardus and (B) Pomatoschistus microps collected in the Douro Estuary between 2021 and 2022.
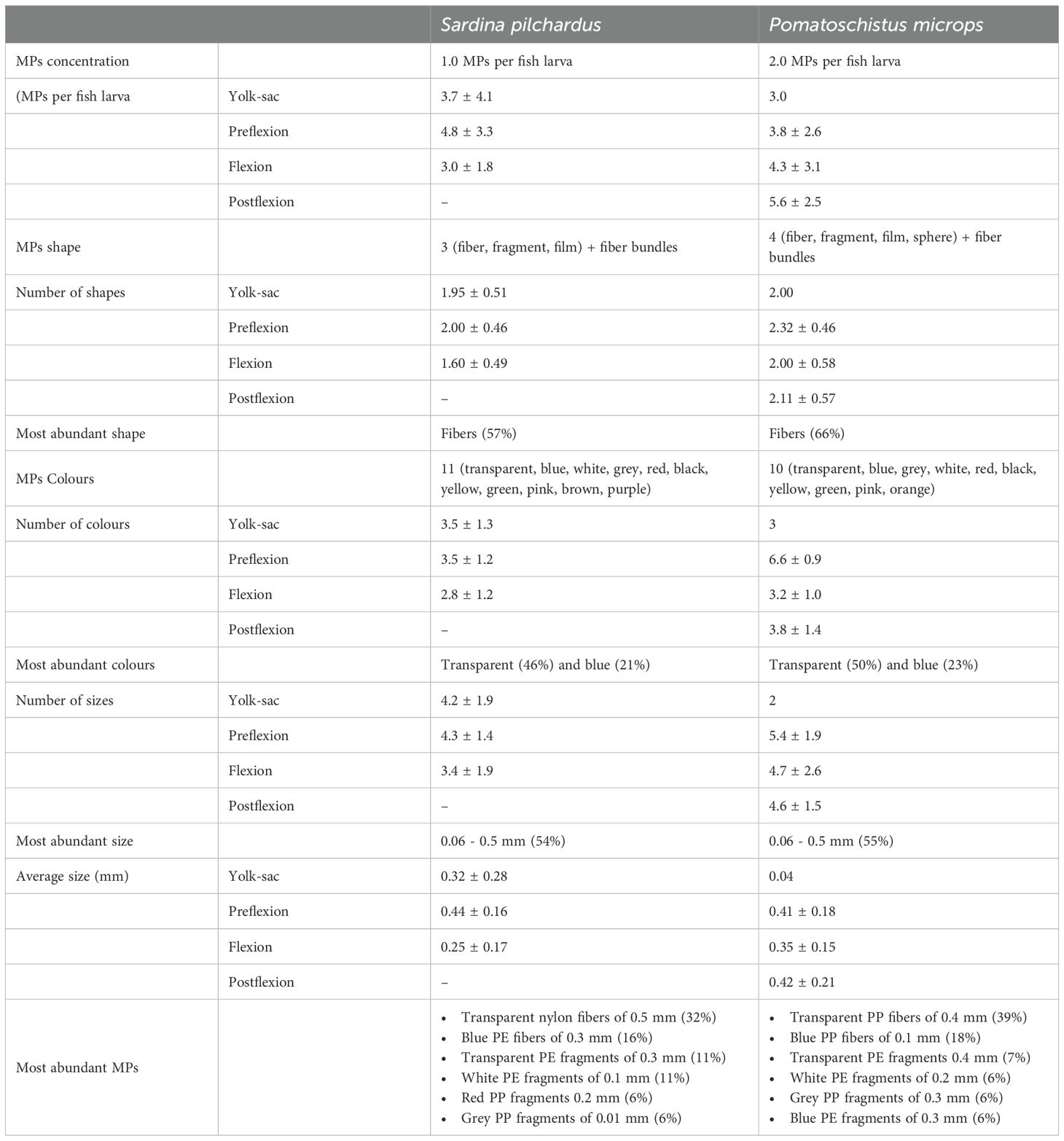
Table 1. Description of MPs recovered from Sardina pilchardus and Pomatochistus microps samples, namely concentrations (average ± standard deviation) and MPs characteristics.
MPs concentrations in S. pilchardus larvae varied significantly between seasons (ANOVA, DF = 9, F = 2.59, p < 0.05) with higher levels in months with lower larval fish abundance, namely in December 2021 and March and August 2022 (between 8.0 (only one sample analysed) and 11.0 ± 1.0 MPs per fish larva) (Supplementary Figure S5 in the Supplementary Material). Spatially, a higher MP concentration was observed between the middle and upper parts of the estuary, between 7.5 and 10.5 km from the estuary mouth sampling stations, with an average contamination of 9.7 ± 2.1 and 12.0 (only one sample analysed) MPs per fish larva, respectively (ANOVA, DF = 6, F = 2.47, p < 0.05) (Supplementary Figure S5 in the Supplementary Material).
3.2.2 Pomatoschistus microps
A total of 709 MPs were recovered from the 357 P. microps larvae analysed, with an average MPs contamination of 2.0 MPs per fish larva. As with S. pilchardus, it was not possible to quantify the percentage of larvae contaminated with MPs. However, since in some samples the average MP MPs average contamination was < 1, it was concluded that some larvae were not contaminated with MPs, and contamination varied between 0.41 and 12 MPs per fish larva (Figures 2; Supplementary Figures S4 in the Supplementary Material). P. microps larvae were contaminated with MPs of four different shapes and ten different colours (Table 1).
From the MPs recovered in P. microps, 39% were transparent polypropylene fibers of size 0.4 mm, followed by 18% blue polypropylene fibers of 0.1 mm; 7% were transparent polyethylene fragments of 0.4 mm, 6% white polyethylene fragments of 0.2 mm, 6% grey polypropylene fragments of 0.3 mm, and 6% blue polyethylene fragments of 0.3 mm. Fibers represented most of the recovered MPs, accounting for 66% of the total recovered. Fifty percent of the MPs were transparent, followed by blue (23%) and white (9%). A similar distribution of MPs sizes was found in both species, with MPs between 0.06-0.5 mm representing more than 75% of the MPs recovered. Polypropylene (PP) and PE were the main plastic polymers of the MPs recovered from the P. microps fish larvae samples (Supplementary Figure S4 in the Supplementary Material).
The average MPs concentration in P. microps larvae ranged from 2.3 ± 0.3 to 9.0 (only one sample analysed) MPs per fish larva between September 2021 and September 2022 (Figure 2). From December to March, a significantly higher MPs concentration, between 5.1 ± 4.0 and 9.0 (only one sample analysed) MPs per fish larva was observed (ANOVA, DF = 10, F = 2.74, p < 0.05), MPs concentration in P. microps also showed significant differences between sampling stations (ANOVA, DF = 12, F = 2.24, p < 0.01): at the end of the lower and beginning of middle areas of the estuary, between 2.5 and 3.5 km from the estuary mouth, a significantly higher MP concentration of 5.0 ± 2.2 and 6.0 ± 3.1 MPs per fish larva, respectively, was observed. A peak in MPs concentration in fish larvae was also observed in the upper part of the estuary (10.5 km and between 14.5 and 20.5 km from the estuary mouth), with values ranging from 5.3 ± 3.4 to 7.0 ± 3.7 MPs per fish larva (Supplementary Figure S5 in the Supplementary Material). MPs concentrations in the two species did not differ significantly (ANOVA, DF = 1, F = 0.11, p ≥ 0.05). Both species also presented a similar number of colours in each larva (ANOVA, DF = 1, F = 0.29, p ≥ 0.05) (Figures 3, 4). However, there was a significantly higher diversity of MPs shapes (ANOVA, DF = 1, F = 6.87, p < 0.001) and sizes (ANOVA, DF = 1, F = 4.65, p < 0.05) in P. microps larvae than in S. pilchardus larvae (Figures 3, 4).
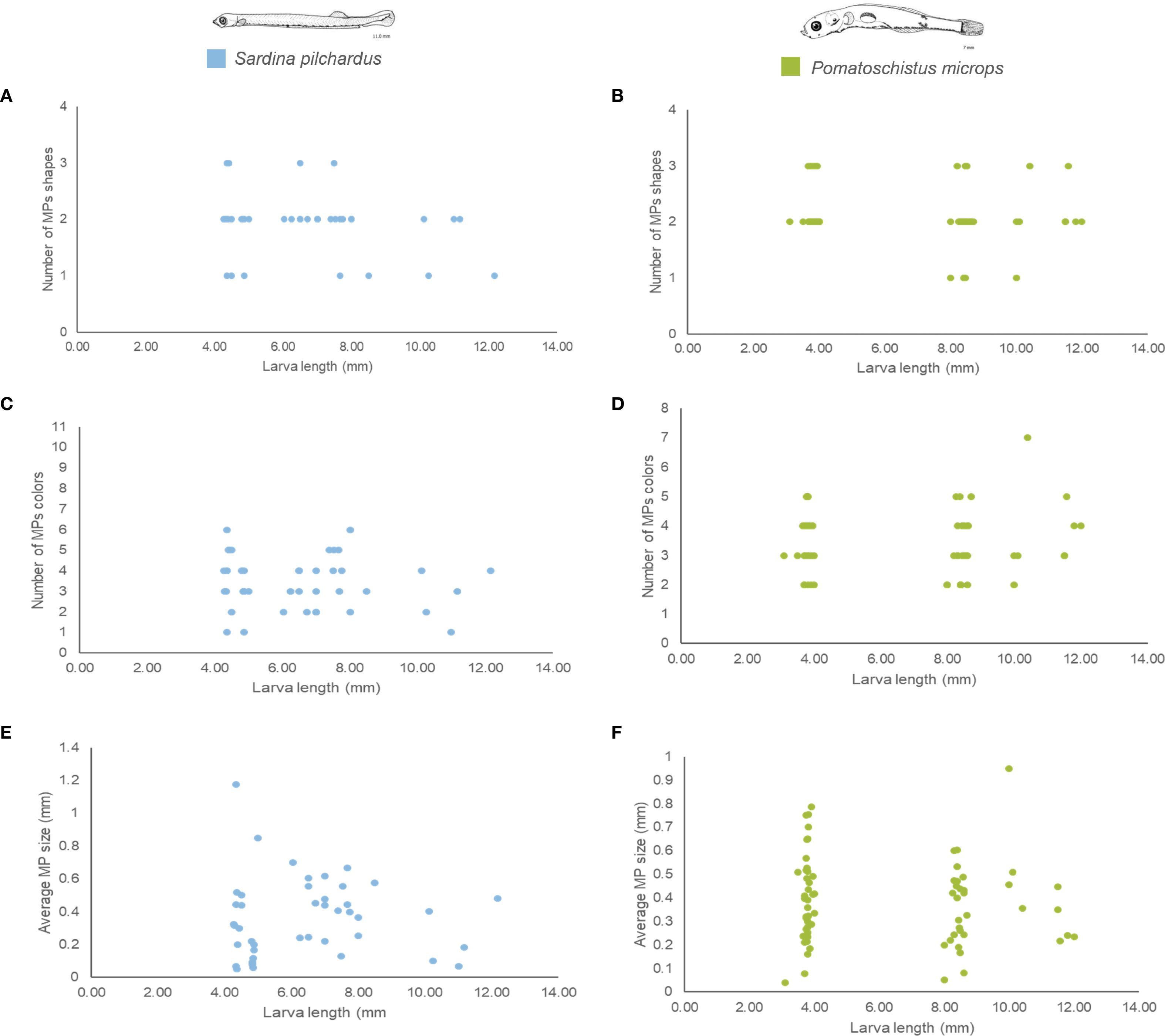
Figure 3. Diversity of MPs collected in each larva along the larva length for Sardina pilchardus and Pomatoschistus microps collected in the Douro Estuary between 2021 and 2022. (A, B) number of MPs shapes in Sardina pilchardus and Pomatoschistus microps; (C, D) number of MPs colours in Sardina pilchardus and Pomatoschistus microps and (E, F) average MPs size in Sardina pilchardus and Pomatoschistus microps.
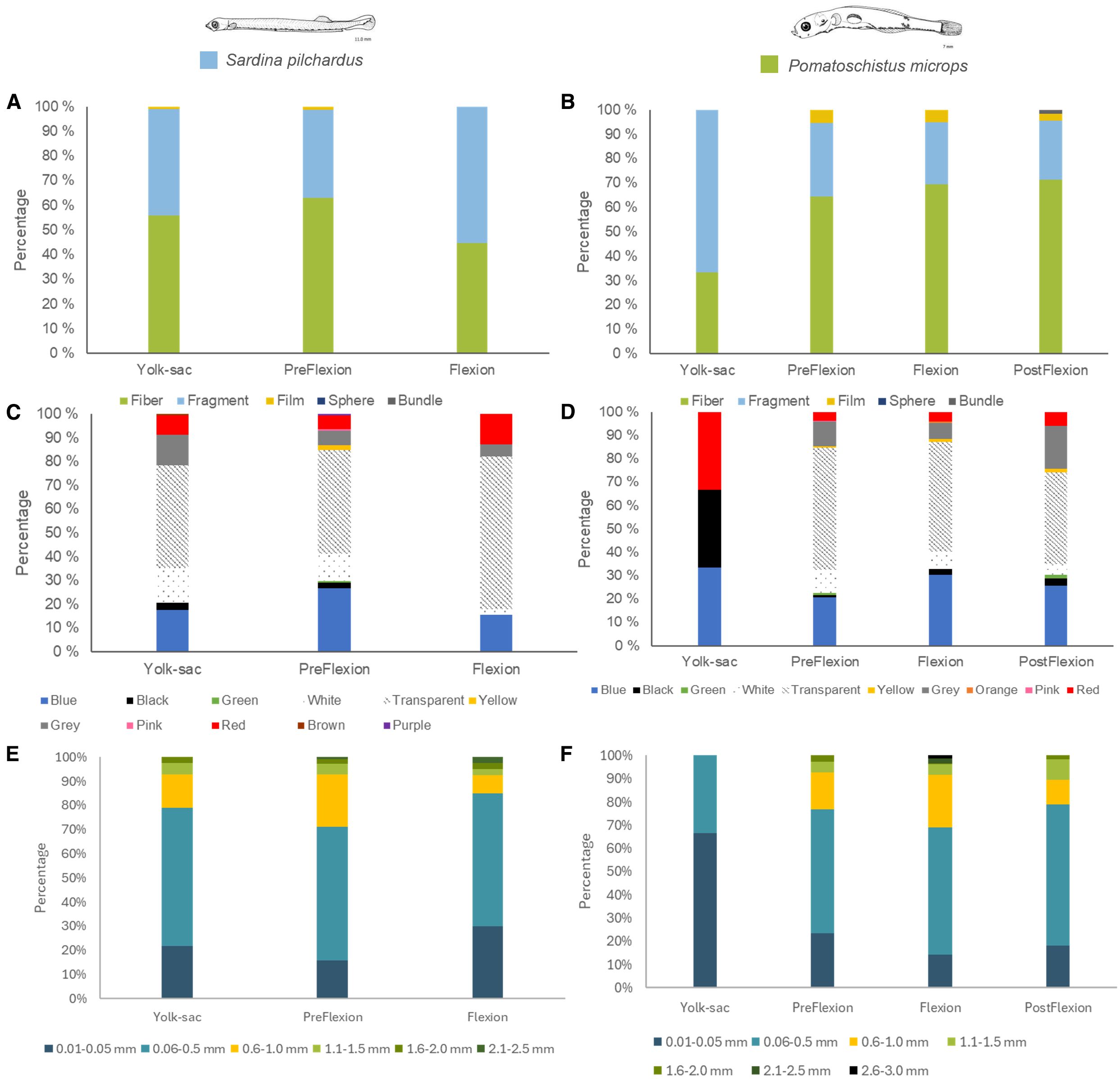
Figure 4. MPs distribution by type, colour and size of the microplastics recovered in different ontogenetic development stages (yolk-sac, preflexion, flexion and postflexion) of Sardina pilchardus (A, C, E) and Pomatoschistus microps (B, D, F) collected in the Douro Estuary between 2021 and 2022.
3.3 MPs contamination by larval ontogenetic development stage
3.3.1 Sardina pilchardus
Only 3 ontogenetic development stages were observed in S. pilchardus. The average MPs concentration in this species varied from 3.7 ± 4.1 MPs per fish larva at the yolk-sac stage to 4.7 ± 3.3 MPs per fish larva at the preflexion stage and decreased to 3.0 ± 1.8 MPs per fish larva at the flexion stage (Table 1; Figure 2). However, MPs concentration did not vary significantly throughout the three development stages (ANOVA, DF = 2, F = 1.31, p ≥ 0.05). Regarding the diversity of MPs present in S. pilchardus larvae, a similar number of shapes (ANOVA, DF = 2, F = 1.27, p ≥ 0.05) and colours (ANOVA, DF = 2, F = 0.68, p ≥ 0.05) were observed in the three development stages (Figure 3). There was no difference in the number of sizes (ANOVA, DF = 2, F = 0.55, p ≥ 0.05) or in the average size of MPs (ANOVA, DF = 2, F = 1.95, p ≥ 0.05) between the three ontogenetic development stages in S. pilchardus larvae (Figure 3). Fibers represented more than 50% of the MPs recovered in the first two stages (yolk-sac and preflexion stages) and slightly decreased in the flexion stage (Figure 4). During the preflexion stage, there was a slightly higher concentration of MPs colours (Figure 4).
3.3.2 Pomatoschistus microps
P. microps larvae were identified in 4 different stages of development: yolk-sac, preflexion, flexion, and postflexion. Although MPs concentration did not vary significantly between the four different larval stages (ANOVA, DF = 3, F = 1.07, p ≥ 0.05), there was a tendency for an increase in MPs contamination from 3.8 ± 2.6 MPs per fish larva at the preflexion stage to 5.6 ± 2.5 MPs per fish larva at the postflexion stage (Table 1, Figure 2). It is of note that only one fish larva in the yolk-sac stage was collected and analysed and therefore, the remaining results did not include that stage.
Regarding the diversity of MPs present in P. microps larvae, a similar number of shapes (ANOVA, DF = 3, F = 1.84, p ≥ 0.05), colours (ANOVA, DF = 3, F = 0.99, p ≥ 0.05), and sizes (ANOVA, DF = 3, F = 1.72, p ≥ 0.05) were observed throughout the ontogenetic development stages; in addition, the average size of MPs did not vary with the larval phase of P. microps (ANOVA, DF = 3, F = 1.94, p ≥ 0.05) (Figure 3). Throughout the ontogenetic development stages, there was a tendency for an increase in fiber presence as the larvae developed (from 65% at the preflexion stage to 71% at the postflexion stage); consequently, a decrease in fragments was also observed (from 30% at the yolk-sac stage to 24% at the postflexion stage) (Figure 4). Regarding colours, between the preflexion to postflexion stages, there was an increase of darker colours such as blue, black, grey and red, and a decrease in translucent colours such as transparent and white (Figure 4). More than 50% of the MPs recovered were always in the size range of 0.06 and 0.5 mm; however, throughout the larval stages, there was an increase in the number of MPs between 0.6 and 1.5 mm (Figure 4).
4 Discussion
4.1 MPs contamination since yolk-sac stage
This novel MPs research provides much-needed field evidence on MPs contamination in early life stages of fish, as it makes a direct comparison between MPs contamination in two larval fish species with different ecological guilds. Importantly, it is the first study to investigate whether MPs contamination varies throughout the ontogenetic development stages in wild fish larvae. Consequently, we evaluated when MPs contamination of wild larval stages of fish starts. A previous laboratory study reported that MPs contamination in fish larvae starts with the beginning of exogenous feeding, when the yolk reserves are depleted, and the fish larvae need to start predating other organisms (Cousin et al., 2020). However, our results showed that fish larvae in the yolk-sac stage, which were still endogenously feeding, were already contaminated with MPs. At this stage, fish larvae still have their mouths closed, are not yet feeding on prey, and are still being fed endogenously via the yolk-sac. In the case of S. pilchardus, approximately 80% of the fish larvae analysed were at the yolk-sac stage, and an average of 3.7 ± 4.1 MPs per fish larva was observed. S. pilchardus larvae at the yolk-sac stage had between 0.1 and 12 MPs per fish larva, meaning that not all the fish larvae had MPs. However, some of these young larvae had up to 12 MPs per larva, a concentration similar to that reported for adult stages of S. pilchardus (e.g., Lopes et al., 2020; Bouzekry et al., 2023; Trani et al., 2023). Hence, our results importantly demonstrate that MP contamination occurs much earlier in fish development than previously understood, which can have important implications. Finding MPs in this early stage means a higher lifetime exposure to this contaminant than just accumulating them through feeding as adults. Cousin et al. (2020) concluded that this might potentially have a larger impact on fish than exposure only during adulthood, since fish in their early life stages are at their most sensitive stage and typically have less developed detoxification mechanisms and are more sensitive to contaminants. Moreover, MPs found at these stages indicate greater ecosystem contamination than surface-level ingestion, which could ultimately affect the entire marine food web structure, as contaminated larvae serve as prey for numerous species and carry this contamination load throughout their lives.
Taking into consideration previous laboratory studies on zooplankton and fish, the presence of MPs in the yolk-sac fish larvae, a development stage when larvae are not yet feeding on prey, can occur through transfer to offspring from the mother (Dai et al., 2025). Martins and Guilhermino (2018) reported the transfer of MPs (around 0.005 mm) to the F1 generation of Daphnia magna and that exposure to MPs caused reduced fertility and increased mortality among the offspring. Moreover, the offspring growth rate decreased, indicating a reduction in population fitness within just one generation. Pitt et al. (2018) observed the transfer of plastic particles to fish offspring, and the presence of plastic particles (around 42 nm) in the yolk-sac of F1 embryos of the zebrafish Danio rerio. Also, Teng et al. (2022) observed maternal transfer of plastic particles (around 44 nm) when F0 D. rerio were exposed to these particles, which were subsequently detected in the intestine, liver, and pancreas of F1 D. rerio. Malafaia et al. (2022) showed that plastic particles (around 26 nm) were transferred to embryos after gestational exposure of the guppy Poecilia reticulata females. Hence, these studies demonstrated not only the transfer of plastic particles to offspring but also their impacts on the offspring. Even more concerning, species may require several generations to recover completely from developmental and reproductive negative effects induced by this contaminant (Martins and Guilhermino, 2018). Nonetheless, these laboratory studies used particles several orders of magnitude smaller than the MPs recovered in this study, and the mechanisms underlying maternal transfer of plastic particles remain unclear. Some of those studies reported that plastic particles were able to penetrate the chorion of the egg (Pitt et al., 2018) while others suggested accumulation in the maternal gametes through binding to lipids and vitellogenin, which facilitates transfer to oocytes and subsequently to the embryonic yolk sac. Given the above, it is essential to include the analysis of eggs in future studies on MPs contamination and to evaluate other contamination pathways in subsequent research. These results demonstrate the complexity and magnitude of the impacts caused by plastic particles on fish larvae, highlighting the need for new investigations, not only to understand the mechanisms of uptake of plastic particles but also the impacts of this transfer on fish growth and development. It is of note that the larval stages of fish are their most sensitive stage, during which they use estuaries as nursery areas to maximize survivorship (Ramos et al., 2010, and references therein). However, as those areas tend to be highly contaminated by MPs (Ramos et al., 2024), the estuarine nursery function, which is associated with enhanced growth and survival during the initial development stages of fish, can be compromised by these contaminants.
4.2 MPs contamination along the ontogenetic development stages
The understanding of whether MPs contamination profile changes throughout the ontogenetic development stages was another fundamental question approached in this study. P. microps and S. pilchardus have a pelagic larval duration of 10 and 40 days, respectively. In that period since hatching, the newly hatched larvae already had MPs, with an increase of 47% from preflexion to postflexion in the case of P. microps and reaching an average of 5.6 ± 2.5 MPs per P. microps larva in the stage of postflexion and 3.0 ± 1.8 MPs per S. pilchardus larva in the flexion stage. However, MPs’ concentration did not vary significantly throughout the development stages of each species. Moreover, throughout ontogenetic development, MP diversity remained similar across all stages, in terms of number of shapes, colours and sizes. A similar diversity of MPs was observed in the stage of endogenous feeding and well as in the stages of exogenous feeding. Even in the stages where fish larvae start to prey more actively, there was no change in the diversity of MPs towards a specific MP type of interest.
S. pilchardus has a pelagic larval duration of 40 days, while that of P. microps is of 10 days, which implies important differences in terms of morphological and physiological development (Russell, 1976). For example, S. pilchardus newly hatched larvae are very rudimentary and the mouth is not open, and the feeding is internal and based on yolk reserves. In contrast, P. microps hatched larvae are more developed, with eyes that are already pigmented and have reduced yolk reserves, needing external feeding in an earlier stage (Russell, 1976). Concerning the use of estuaries as habitats, these species belong to two different ecological guilds according to their estuarine use pattern, P. microps being as an estuarine species (ES) and S. pilchardus a marine migrant (MM; a species that spawn at sea and regularly enter estuaries in large numbers, using estuaries as nursery grounds) (Franco et al., 2008). Fish larvae at the yolk-sac stage still have yolk-sac or remnants of yolk, and they frequently an unformed mouth, unpigmented eyes, and undeveloped pectoral fins (Russell, 1976; Ré and Meneses, 2008). Although these two species present different morphological and physiological developments and periods of ontogenetic development, our results indicate that, in that early stage of fish life, MPs contamination appears to occur independently of species-specific traits.
It is of note that the most abundant MPs present in fish larvae in this study, namely in shape and colour, tend to be the predominant ones reported in water in different types of the aquatic environments worldwide, including estuaries (e.g. Ramos et al., 2024; Botterell et al., 2022; Tang et al., 2023), suggesting that MPs contamination in fish larvae may happen incidentally. For example, fibers, which are one of the most abundant types of MPs reported in estuarine waters (Ramos et al., 2024), represented more than 50% of the MPs recovered, except in the flexion stage of S. pilchardus. Moreover, fibers presented a higher diversity of sizes and the bigger MPs recovered from fish larvae in this study were fibers. Previous research has shown that fibers can be more easily mistaken for natural prey and have a shape that facilitates ingestion, even accidentally (Tang et al., 2023). Additionally, fibers tend to become entangled and often cluster together in bundles within the aquatic environment. These factors, combined with fibers being the most abundant microplastic shape in water, likely explain their high concentration across all developmental stages of fish larvae, including the yolk-sac stage, and their diverse size ranges (Tang et al., 2023). Other studies have reported colour-selective ingestion by certain organisms due to resemblance to natural prey (e.g., Wright et al., 2013). Hence, the colour of MPs seems to be an important characteristic since colours as green, transparent and blue could potentially resemble fish larvae prey, increasing the active uptake of MPs. Nonetheless, throughout the ontogenetic development stages of the two species studied here, a similar diversity of colours was observed and the MPs’ colours in higher abundance were the same as the ones in water, again reinforcing that contamination occurs randomly and is environment-dependent rather than driven by species-specific characteristics as prey preferences. Regarding the size, Ding et al. (2023) observed an exponential relationship between MP concentration and the size ingested and the adult wild fish age. MPs size diversity and the average size were not different among the different ontogenetic development stages in our study, indicating that MPs size is similar in all stages and there was no relationship between the size and the ontogenetic development stages, possibly suggesting that environmental availability rather than selective feeding drives contamination patterns. These results increase the much-needed understanding of the contamination mechanisms in wild organisms, suggesting that fish larvae contamination happens randomly and is environment-dependent rather than species-specific traits. Hence, environmental management needs to be redirected from focusing on species-specific mitigation strategies towards prioritizing the reduction of overall MP pollution in aquatic environments.
4.3 MPs ecological impact on different larval fish species
Another key objective of this study was to determine whether the species-specific MPs contamination profiles documented in adult fish extend to the early larval stages. Our results revealed that both species exhibited similar MPs concentrations and MPs types, despite their distinctly different ecological characteristics. Although P. microps had a higher diversity of MP shapes and sizes compared to S. pilchardus, this difference possibly reflects their contrasting habitat exposure rather than selective ingestion. P. microps is an estuarine species that spends its entire life in estuaries; they are always exposed to the highly contaminated estuarine waters of the Douro estuary (Rodrigues et al., 2019), possibly explaining the higher diversity of MPs shapes and sizes in their larvae. In fact, the predominance of transparent and blue fibers in both species,which mirrors the most abundant types of MPs reported in estuarine environments (Ramos et al., 2024), suggests a more passive contamination mechanism. Both Steer et al. (2017) and Tang et al. (2023) also reported a similarity between MPs in water and those ingested by fish larvae and zooplankton, respectively. The similarity between MPs in water and fish larvae may indicate that MP presence in fish larvae represents primarily accidental ingestion driven by environmental availability rather than active selection based on ecological guild or any physical characteristics. Hence, while Siddique et al. (2024) previously demonstrated that adult fish feeding behaviour and physical characteristics such as mouth size significantly influence MP ingestion, our study reveals a different scenario in the early life stages, since larval fish contamination appears to be independent of species-specific traits or ecological guilds.
Despite this, the average MPs concentration in this study (1.0 MPs in the case of S. pilchardus and 2.0 MPs in the case of P. microps) was much higher than in other studies focused on fish larvae, namely 0.005 MPs (Zavala-Alarcón et al., 2023) and 0.57 MPs per fish larva (Rashid et al., 2021) (Table 2). In contrast, two other studies reported higher MPs concentrations, namely 5.2 MPs (Steer et al., 2017) and 7.5 MPs per fish larva (Carrillo-Barragan et al., 2024) (Table 2). Despite this, the concentration reported in this study is of concern since it is higher or in the same range as the contamination values reported in other Portuguese estuaries for adult fish (1.67 MPs per adult fish in species as Dicentrarchus labrax, Diplodus vulgaris, and Platichthys flesus collected in the Mondego estuary – Bessa et al., 2018, and between 2 and 8 MPs per adult fish in species as Cyprinus carpio, Mugil cephalus and P. flesus collected in the Minho Estuary – Guilhermino et al., 2021). However, it is important to emphasize that these comparisons should be treated with caution due to possible differences in the MPs methodologies used in each study.
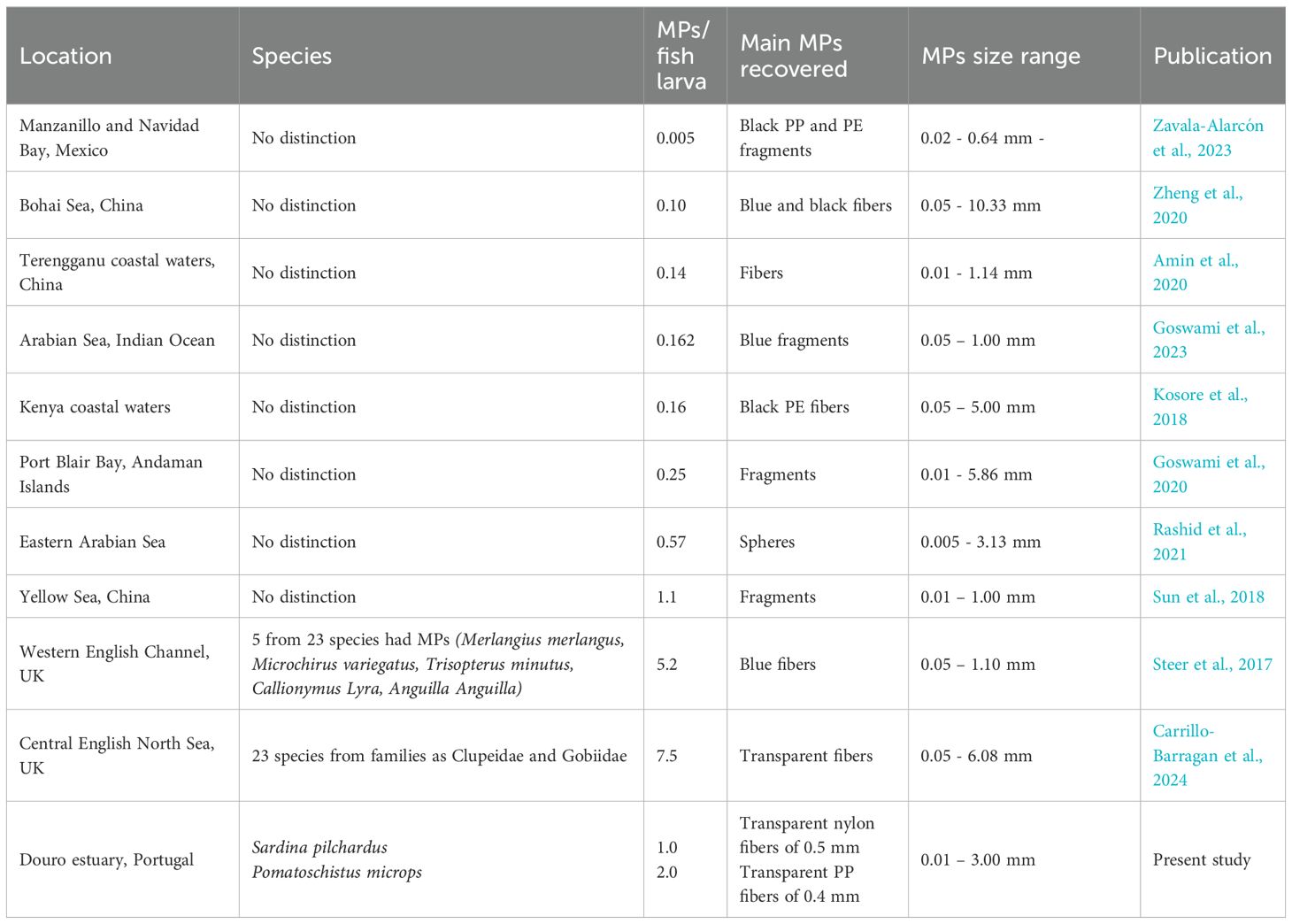
Table 2. Summary of field studies reporting MPs presence in fish larvae, namely sampling location, species analysed, MPs concentration (MPs per fish larvae ± standard deviation) and main MPs type recovered from fish larvae.
S. pilchardus is a marine migrant, using estuaries occasionally and migrating into the estuary if the environmental conditions, such as salinity, temperature, and river flow, are favourable (Morais et al., 2009; Ramos et al., 2009). The present results support this behaviour since S. pilchardus was only observed in part of the year and areas close to the sea. This species showed a higher MPs ingestion in December, March and August. It is important to highlight that in some S. pilchardus larvae, contamination reached 12 MPs per larva, a concentration higher than reported for adult S. pilchardus in other studies on Portugal (0.16 MPs per fish – Lopes et al., 2020), Moroccan Mediterranean coast (9.6 MPs per fish - Bouzekry et al., 2023), Adriatic Sea (4.6 MPs per fish - Trani et al., 2023) and Italy (6.2 MPs per fish - Trani et al., 2023). S. pilchardus has a very high socio-economic importance as a major food-fish species and also plays a vital role in marine ecosystems via predation by larger fishes and seabirds (Whitfield et al., 2022). There is a risk to survival and healthy growth since early stages can impact the adult stocks and ultimately their role as one of the main consumed fish by humans (Bouzekry et al., 2023). P. microps, an estuarine species, was collected all year round and in every area of the estuary and the peak of MPs ingestion was observed for a longer period (between December and May). It is important to note that the pelagic larval duration for P. microps is only 10 days; however, in that small period, fish larvae of this species already had an average of 2.0 MPs per fish larva and reached until 12 MPs per fish larva. This species is a central species in estuarine food webs as it is preyed upon by crustaceans, larger fishes and shorebirds (Whitfield et al., 2022) and, hence, any contamination is passed through that food chain.
Laboratory studies suggest that MPs uptake and effects will depend on MPs characteristics (e.g., Pannetier et al., 2020; Kim et al., 2022; Yap and Yasid, 2023). A great variety of shapes and sizes were reported in this study, which can potentially cause different effects with different levels of harm to different species, namely as deposition of particles in the liver, gill and gut in the case of fibers or, damage to the intestine and morphological deformations in the case of fragments (Chouchene et al., 2023). Nonetheless, other studies reported no significant effects or minimal impact on fish larvae (e.g., Karami et al., 2017; Sleight et al., 2017). It is of note that all the fish larvae collected in this study were alive when collected, implying that they were not carrying a lethal load of MPs, which may be higher than that encountered here. However, this study did not assess the health status of the fish larvae or the toxicity of MPs ingested. It will be important in future research to investigate the health status of fish larvae contaminated with MPs, together with the potential sublethal effects of these contaminants. Our findings reveal a widespread occurrence of MPs in field fish larvae, emphasizing bioavailable transfer through food chains by MPs pollution.
5 Conclusions
MPs presence in S. pilchardus and P. microps and as well as the variation throughout the ontogenetic development stages of the fish larvae, was assessed in this study. Both species showed a similar MPs contamination load, 1.0 MPs in the case of S. pilchardus and 2.0 MPs in the case of P. microps, and similar MPs characteristics, sometimes reaching contamination levels higher than those reported for adults. It is of note that MPs contamination in the yolk-sac stage was observed, which is the first development stage after hatching, where fish larvae are still not feeding on prey yet (endogenous feeding). These results importantly demonstrate that MP contamination occurs much earlier in fish development than previously considered. The larval stages of fish are their most sensitive stage and during that period, especially as they tend to use estuaries as nursery areas to maximize their survivorship. However, if those areas tend to be more contaminated by MPs, their growth and survival are at greater risk than previously presumed. Hence, new investigations are needed not only to understand the mechanisms of uptake of plastic particles and the transfer mechanism from the female during egg production, but also the impacts of these transfer mechanisms on the growth and development of fish.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SMR: Data curation, Methodology, Conceptualization, Writing – original draft, Formal Analysis, Writing – review & editing. FE: Methodology, Writing – review & editing, Data curation. ME: Conceptualization, Writing – review & editing, Supervision. CA: Funding acquisition, Writing – review & editing, Formal Analysis, Conceptualization, Project administration, Supervision. SR: Methodology, Supervision, Conceptualization, Writing – review & editing, Formal Analysis, Funding acquisition, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was partially supported by ATLANTIDA (NORTE-01–0145-FEDER-000040) project, Ocean3R (NORTE-01-0145-FEDER-39 000064) project and Free LitterAT (EAPA_0009/2022) co-financed by the European Union (EU) and the European Regional Development Fund through the Interreg Atlantic Area Programme. The views expressed herein should not be taken, in any way, to reflect the official opinion of the EU, and the Commission is not responsible for any use that may be made of the information it contains. Financial support from FCT - Foundation for Science and Technology, Portugal was also provided within the scope of UIDB/04423/2020 and UIDP/04423/2020 and through the grant awarded to SMR (SFRH/BD/145736/2019) and a research contract to SR (DL57/2016/CP1344/CT0020).
Acknowledgments
The authors would like to thank the three reviewers for their insightful and substantial comments on earlier versions of the paper.
Conflict of interest
Author ME was employed by the company International Estuarine & Coastal Specialists IECS Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1645179/full#supplementary-material
References
Amin R., Sohaimi E. S., Tuan Anuar S., and Bachok Z. (2020). Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea. Mar. pollut. Bull. 150, 110616. doi: 10.1016/j.marpolbul.2019.110616
Azevedo I. C., Duarte P. M., and Bordalo A. A. (2008). Understanding spatial and temporal dynamics of key environmental characteristics in a mesotidal Atlantic estuary (Douro, NW Portugal). Estuar. Coast. Shelf Sci. 76, 620–633. doi: 10.1016/j.ecss.2007.07.034
Bessa F., Barría P., Neto J. M., Frias J. P. G. L., Otero V., Sobral P., et al. (2018). Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. pollut. Bull. 128, 575–584. doi: 10.1016/j.marpolbul.2018.01.044
Botterell Z. L., Beaumont N., Dorrington T., Steinke M., Thompson R. C., and Lindeque P. K. (2019). Bioavailability and effects of microplastics on marine zooplankton: a review. Environ. pollut. 245, 98–110. doi: 10.1016/j.envpol.2018.10.065
Botterell Z. L. R., Bergmann M., Hildebrandt N., Krumpen T., Steinke M., Thompson R. C., et al. (2022). Microplastic ingestion in zooplankton from the Fram Strait in the Arctic. Sci Total Environ. 831, 154886. doi: 10.1016/j.scitotenv.2022.154886
Bouzekry A., Mghili B., Bouadil O., Mancuso M., Ben-Haddad M., Bottari T., et al. (2023). First report of microplastic ingestion in edible fish along moroccan mediterranean coasts. Sustainability 15, 16313. doi: 10.3390/su152316313
Carrillo-Barragan P., Fitzsimmons C., Lloyd-Hartley H., Tinlin-Mackenzie A., Scott C., and Sugden H. (2024). Fifty-year study of microplastics ingested by brachyuran and fish larvae in the central English North Sea. Environ. pollut. 342, 123060. doi: 10.1016/j.envpol.2023.123060
Chouchene K., Pinto da Costa J., Chamkha M., Ksibi M., and Sayadi S. (2023). Effects of microplastics’ physical and chemical properties on aquatic organisms: State-of-the-art and future research trends. TrAC Trends Analytical Chem. 166, 117192. doi: 10.1016/j.trac.2023.117192
Cousin X., Batel A., Bringer A., Hess S., Bégout M. L., and Braunbeck T. (2020). Microplastics and sorbed contaminants - Trophic exposure in fish sensitive early life stages. Mar. Environ. Res. 161, 105126. doi: 10.1016/j.marenvres.2020.105126
Dai Y., Han R., Yao Z., Yan H., Liu Z., Liu X., et al. (2025). Intergenerational transfer of micro(nano)plastics in different organisms. J. Hazardous Materials 488, 137404. doi: 10.1016/j.jhazmat.2025.13740
Defontaine S., Sous D., Tesan J., Monperrus M., Lenoble V., and Lanceleur L. (2020). Microplastics in a salt-wedge estuary: Vertical structure and tidal dynamics. Mar. pollut. Bull. 160, 111688. doi: 10.1016/j.marpolbul.2020.111688
Desforges J. P. W., Galbraith M., and Ross P. S. (2015). Ingestion of microplastics by zooplankton in the northeast pacific ocean. Arch. Environ. Contam Toxicol. 69, 320–330. doi: 10.1007/s00244-015-0172-5
Ding R., Ma Y., Li T., Sun M., Sun Z., and Duan J. (2023). The detrimental effects of micro-and nano-plastics on digestive system: An overview of oxidative stress-related adverse outcome pathway. Sci Total Environ. 878, 163144. doi: 10.1016/j.scitotenv.2023.163144
Figueiredo G. M. and Vianna T. M. P. (2018). Suspended microplastics in a highly polluted bay: Abundance, size, and availability for mesozooplankton. Mar. pollut. Bull. 135, 256–265. doi: 10.1016/j.marpolbul.2018.07.020
Franco A., Franzoi P., and Torricelli P. (2008). Structure and functioning of Mediterranean lagoon fish assemblages: a key for identification of water body types. Estuar. Coast. Shelf Sci. 79, 549–558. doi: 10.1016/j.ecss.2008.05.011
Galgani F., Ruiz-Orejon L. F., Ronchi F., Tallec K., Fischer E., Matiddi M., et al. (2023). Guidance on the monitoring of marine litter in European seas (European Commission, Joint Research Centre, Guidance on the Monitoring of Marine Litter in European Seas).
Goswami P., Selvakumar N., Verma P., Saha M., Suneel V., Vinithkumar N. V., et al. (2023). Microplastic intrusion into the zooplankton, the base of the marine food chain: Evidence from the Arabian Sea, Indian Ocean. Sci. Total Environ. 864, 160876. doi: 10.1016/j.scitotenv.2022.160876
Goswami P., Vinithkumar N. V., and Dharani G. (2020). First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay, Andaman Islands. Mar. pollut. Bull. 155, 111163. doi: 10.1016/j.marpolbul.2020.111163
Guilhermino L., Martins A., Lopes C., Raimundo J., Vieira L., Barboza L., et al. (2021). Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Mar. Pollut. Bull. 173, 113008.
He M., Yan M., Chen X., Wang X., Gong H., Wang W., et al. (2022). Bioavailability and toxicity of microplastics to zooplankton. Gondwana Res. 108, 120–126. doi: 10.1016/j.gr.2021.07.021
Karami A., Groman D. B., Wilson S. P., Ismail P., and Neela V. K. (2017). Biomarker responses in zebrafish (Danio rerio) larvae exposed to pristine low-density polyethylene fragments. Environ. pollut. 223, 466–475. doi: 10.1016/j.envpol.2017.01.047
Kim S., Kim L., Kim T. H., and An Y. J. (2022). Assessing the size-dependent effects of microplastics on zebrafish larvae through fish lateral line system and gut damage. Mar. Pollut. Bull. 185. 114279. doi: 10.1016/j.marpolbul.2022.114279
Kosore C., Ojwang L., Maghanga J., Kamau J., Kimeli A., Omukoto J., et al. (2018). Occurrence and ingestion of microplastics by zooplankton in Kenya’s marine environment: first documented evidence. Afr. J. Mar. Sci 40, 225–234. doi: 10.2989/1814232X.2018.1492969
Liu W., Liao H., Wei M., Junaid M., Chen G., and Wang J. (2024). Biological uptake, distribution and toxicity of micro(nano)plastics in the aquatic biota: A special emphasis on size-dependent impacts. TrAC Trends Analytical Chem. 170, 117477. doi: 10.1016/j.trac.2023.117477
Lopes C., Raimundo J., Caetano M., and Garrido S. (2020). Microplastic ingestion and diet composition of planktivorous fish. Limnol. Oceanogr. Lett. 5. doi: 10.1002/lol2.10144
Malafaia G., Nóbrega R. H., da Luz T. M., and Araújo A. P. (2022). Shedding light on the impacts of gestational exposure to polystyrene nanoplastics on the reproductive performance of Poecilia reticulata female and on the biochemical response of embryos. J. Hazardous Materials 427, 127873. doi: 10.1016/j.jhazmat.2021.127873
Martins A. and Guilhermino L. (2018). Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci. Total Environment 631–632, 421–428. doi: 10.1016/j.scitotenv.2018.03.054
Morais P., Faria A., Chicharo M. A., and Chicharo L. M. Z. (2009). The unexpected occurrence of late Sardina pilchardus (Walbau) (Osteichthyes: Clupeidae) larvae in a temperate estuary. Cahiers Biologie Mar. 50, 79–89. Available online at: http://hdl.handle.net/10400.1/2404.
Oo P. Z., Boontanon S. K., Boontanon N., Tanaka S., and Fuji S. (2021). Horizontal variation of microplastics with tidal fluctuation in the Chao Phraya River Estuary, Thailand. Mar. pollut. Bulletin, Part A 173, 112933. doi: 10.1016/j.marpolbul.2021.112933
Pannetier P., Morin B., Le Bihanic F., Dubreil L., Clérandeau C., Chouvellon F., et al. (2020). Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 134, 105047. doi: 10.1016/j.envint.2019.105047
Pitt J. A., Trevisan R., Massarsky A., Kozal J. S., Levin E. D., and Di Giulio R. T. (2018). Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci Total Environ. 643, 324–334. doi: 10.1016/j.scitotenv.2018.06.186
Plastics Europe (2022). Plastics - the facts 2022. Available online at: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (Accessed January 24, 2025).
Rashid C. P., Jyothibabu R., Arunpandi N., Abhijith V. T., Josna M. P., Vidhya V., et al. (2021). Microplastics in zooplankton in the eastern Arabian Sea: The threats they pose to fish and corals favoured by coastal currents. Mar. Pollut. Bull. 173(Part B), 113042. doi: 10.1016/j.marpolbul.2021.113042
Ramos S., Cabral H., and Elliott M. (2015). Do fish larvae have advantages over adults and other components for assessing estuarine ecological quality? Ecol. Indic. 55, 74–85. doi: 10.1016/j.ecolind.2015.03.005
Ramos S., Re P., and Bordalo A. (2009). New insights into the early life ecology of Sardina pilchardus (Walbau) in the northern Iberian Atlantic. Scientia Marina. 73, 449–459. doi: 10.3989/scimar.2009.73n3449
Ramos S., Re P., and Bordalo A. (2010). Recruitment of flatfish species to an estuarine nursery habitat (Lima estuary, NW Iberian Peninsula). J. Sea Res. 64, 473–486. doi: 10.1016/j.seares.2010.01.010
Ramos S., Rodrigues S. M., Pereira R., Silva D., and Almeida C. M. R. (2024). “Floata bles and plastic debris in estuarine and coastal marine environments,” in Treatise on estuarine and coastal science, 2nd Edition, vol. 6 . Eds. Baird D. and Elliott M., (Amsterdam: Elsevier), 467–511. doi: 10.1016/B978-0-323-90798-9.00111-6
Ré P. (1999). Ictioplâncton estuarino da Península Ibérica: guia de identificação dos ovos e estados larvares planctónicos (Cascais: Câmara Municipal de Cascais).
Ré P. and Meneses I. (2008). Early stages of marine fishes occurring in the iberian peninsula (IPIMAR/IMAR), 282, ISBN-978-972-9372-34-6.
Rodrigues S. M., Almeida C. M. R., Silva D., Cunha J., Antunes C., Freitas V., et al. (2019). Microplastic contamination in an urban estuary: Abundance and distribution of microplastics and fish larvae in the Douro estuary. Sci Total Environ. 659, 1071–1108. doi: 10.1016/j.scitotenv.2018.12.273
Rodrigues S. M., Elliott M., Almeida C. M. R., and Ramos S. (2021). Microplastics and plankton: knowledge from laboratory and field studies to distinguish contamination from pollution. J. Hazard. Mater. 417, 126057. doi: 10.1016/j.jhazmat.2021.126057
Rodrigues S. M., Espincho F., Elliott M., Almeida C. M. R., and Ramos S. (2023). Methodology optimization to quantify microplastic presence in planktonic copepods, chaetognaths and fish larvae. Methods X Volume 11, 102466. doi: 10.1016/j.mex.2023.102466
Rodrigues S. M., Silva D., Cunha J., Pereira R., Freitas V., and Ramos S. (2022). Environmental influences, particularly river flow alteration, on larval fish assemblages in the Douro Estuary, Portugal. Regional Stud. Mar. Sci 56, 102617. doi: 10.1016/j.rsma.2022.102617
Rodriguez J. M., Alemany F., and Garcia A. (2017). A guide to the eggs and larvae of 100 common western mediterranean sea bony fish species (Rome, Italy: FAO), 256.
Russell F. S. (1976). The eggs and planktonic stages of british marine fishes (London: Academic Press).
Sambolino A., Herrera I., Álvarez S., Rosa A., Alves F., Canning-Clode J., et al. (2022). Seasonal variation in microplastics and zooplankton abundances and characteristics: The ecological vulnerability of an oceanic island system. Mar. pollut. Bull. 181, 113906. doi: 10.1016/j.marpolbul.2022.113906
Siddique M. A. M., Shazada N. E., Ritu J. A., Turjo K. E. Z., and Das K. (2024). Does the mouth size influence microplastic ingestion in fishes? Mar. pollut. Bull. 198, 115861. doi: 10.1016/j.marpolbul.2023.115861
Sleight V. A., Bakir A., Thompson R. C., and Henry T. B. (2017). Assessment of microplastic sorbed contaminant bioavailability through analysis of biomarker gene expression in larval zebrafish. Mar. pollut. Bull. 116, 291–297. doi: 10.1016/j.marpolbul.2016.12.055
Steer M., Cole M., Thompson R. C., and Lindeque P. K. (2017). Microplastic ingestion in fish larvae in the western English Channel. Environ. pollut. 226, 250–259. doi: 10.1016/j.envpol.2017.03.062
Sun X., Li Q., Zhu M., Liang J., Zheng S., and Zhao Y. (2017). Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar. pollut. Bull. 15 115, 217–224. doi: 10.1016/j.marpolbul.2016.12.004
Sun X., Liu T., Zhu M., Liang J., Zhao Y., and Zhang B. (2018). Retention and characteristics of microplastics in natural zooplankton taxa from the East China Sea. Sci. Total Environ. 640, 232–242. doi: 10.1016/j.scitotenv.2018.05.308
Tang C. N., Kuwahara V. S., Leong S. C. Y., Moh P. Y., and Yoshida T. (2023). Effect of monsoon on microplastic bioavailability and ingestion by zooplankton in tropical coastal waters of Sabah. Mar. pollut. Bull. 193, 115182. doi: 10.1016/j.marpolbul.2023.115182
Teng M., Zhao X., Wu F., Wang C., Wang C., White J., et al. (2022).Charge-specific adverse effects of polystyrene nanoplastics on zebrafish (Danio rerio) development and behavior. Environment International. 163. 107154. doi: 10.1016/j.envint.2022.107154
Trani A., Mezzapesa G., Piscitelli L., Mondelli D., Nardelli L., Belmonte G., et al. (2023). Microplastics in water surface and in the gastrointestinal tract of target marine organisms in Salento coastal seas (Italy, Southern Puglia). Environ. pollut. 316, 120702. doi: 10.1016/j.envpol.2022.120702
Whitfield A. K., Able K. W., Blaber S. J. M., and Elliott M. (2022). Fish and fisheries in estuaries – A global perspective (ed.), vols. 1 and 2 (Oxford, UK: John Wiley & Sons), 1056, ISBN: ISBN 9781444336672.
Wright S. L., Thompson R. C., and Galloway T. S. (2013). The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 178, 483–492. doi: 10.1016/j.envpol.2013.02.031
Yap H. S. and Yasid N. (2023). The microplastics in the marine environment: origin, hazardous effects and possible biological solutions: A review. J. Biochemistry Microbiol. Biotechnol. 11, 41–50. doi: 10.54987/jobimb.v11i2.852
Zavala-Alarcón F., Huchin-Mian J., González-Muñoz M., and Kozak E (2023). In situ microplastic ingestion by neritic zooplankton of the central Mexican Pacific. Environ. Pollut. 120994. doi: 10.1016/j.envpol.2022.120994
Keywords: microplastics, fish larvae, ontogenetic development stages, Sardina pilchardus, Pomatoschistus microps
Citation: Rodrigues SM, Espincho F, Elliott M, Almeida CMR and Ramos S (2025) Are fish larvae contaminated before they start eating? First evidence of microplastic contamination in the yolk-sac of wild fish larvae. Front. Mar. Sci. 12:1645179. doi: 10.3389/fmars.2025.1645179
Received: 11 June 2025; Accepted: 08 September 2025;
Published: 24 September 2025.
Edited by:
Sonja M Ehlers, Leibniz Institute for Baltic Sea Research (LG), GermanyReviewed by:
Felipe Amezcua, National Autonomous University of Mexico, MexicoLukas Miksch, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), Germany
Trishan Naidoo, Rhodes University, South Africa
Copyright © 2025 Rodrigues, Espincho, Elliott, Almeida and Ramos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabrina M. Rodrigues, c21hZ2FsaGFlc0BjaWltYXIudXAucHQ=
 Sabrina M. Rodrigues
Sabrina M. Rodrigues Francisca Espincho1
Francisca Espincho1 Michael Elliott
Michael Elliott C. Marisa R. Almeida
C. Marisa R. Almeida Sandra Ramos
Sandra Ramos