Abstract
To combat the plastic problem in the marine environment, bioindicators are essential because they can provide insights into the extent and ecological impacts of plastic pollution. The ingestion and accumulation of microplastics (MPs) in the striped barnacle Amphibalanus amphitrite was studied by exposing them to MPs with or without biofilm. Three types (polyethylene, polystyrene/polyester), two sizes (27-32 µm and 90-106 µm) and two forms (microspheres and microfibers) of MPs at three concentrations (7.2, 72 and 720 P/mL) were investigated. The presence of biofilm did not affect the MP ingestion. The ingestion of MPs was concentration-dependent, irrespective of the size, form and type of the MPs. The numbers of microspheres and microfibers ingested by A. amphitrite were similar, and so were their numbers accumulated in the body. The results suggest a lack of both pre-ingestive and post-ingestive sorting and removal of MPs in A. amphitrite. The MP body burden, therefore, reflects levels of environmental contamination and the actual MPs composition in the water body. Considering the global distribution of A. amphitrite and its high abundance on rocky shores and man-made structures such as wharf piles, ease of finding and sampling, clear taxonomic status, small body size, high reproductive rate, specialized feeding mode, and well-known biology and life history, it has great potential to be considered as a member of a list of global marine bioindicators of MPs. Further investigations should focus on how seasonal changes in environmental factors and body conditions, such as reproductive cyclicity, influence the ingestion and accumulation of MPs, and the associated ecotoxicological effects.
1 Introduction
Since the first synthetic plastic, “Bakelite”, was invented in 1907, we have become addicted to plastics because they are chemically stable, easily moulded into different sizes and shapes, lightweight, good insulators, and have a low production cost. About 300 million tons of plastic waste are generated annually, with over 8 million tons ending up in the oceans (Fava, 2022). More than 1300 marine species have been found to ingest plastics (Santos et al., 2021). Microplastics (MPs) are plastic pieces with a diameter of < 5 mm. They can be created for specific purposes, such as microbeads used in personal care products, or by degrading larger plastic debris. The concentration of MPs in the five oceans ranged between 0.04 items/m3 in the Southern Ocean and 4.98 items/m3 in the Atlantic Ocean (Mutuku et al., 2024).
The impact of MPs on marine organisms has raised significant global concerns (Guzzetti et al., 2018). Due to their small size, MPs are ingested by numerous marine creatures and cause negative impacts such as inflammation, decreased calorie intake, and reduction in growth and reproductive output (Marmara et al., 2023; Jeong et al., 2024). The hydrophobicity and large surface area of MPs make them ideal for adsorbing persistent organic pollutants in the water, including PCBs and PAHs (Pastorino et al., 2021). MPs also contain additives for various purposes, such as reducing fire risks or improving malleability of the polymers (da Costa et al., 2023). After MPs are ingested by marine organisms, these chemicals can be leached from the MPs and absorbed in the intestine (Siri et al., 2021), causing toxicological effects (Jeong et al., 2024).
After MPs are released into the aquatic environment, they are colonized by microbial communities. First comers are the bacteria and viruses, followed by phytoplankton, such as diatoms and dinoflagellates (Ye et al., 2022). These microbes secrete EPSs (extracellular polymeric substances), which are high molecular weight natural polymers. They provide structural integrity and determine the physicochemical properties of biofilms. Since biofilms have changed the surface properties of MPs, which some filter feeders use as clues for particle sorting, the ingestion of MPs could potentially be altered. Some studies have shown that the ageing of MPs enhances their ingestion by marine zooplankton (Vroom et al., 2017), probably because these marine invertebrates are confused by the biofilm on the MPs and consider MPs as edible. However, many laboratory studies assessed the ingestion and accumulation of MPs and their associated health impact on experimental animals using virgin MPs. The ingestion rate and health impact of MPs, therefore, may be underestimated. Nevertheless, contrasting results were obtained in the larvae of the acorn barnacle Amphibalanus amphitrite in which the biofilm on MPs did not significantly affect their ingestion (Nousheen et al., 2022), indicating that either the barnacle larvae do not possess particle sorting ability, or the nature of the biofilm does not affect their particle selection.
Filter feeders, such as bivalves and barnacles, have plankton as their primary diet. In benthic environments, sediment resuspension can lower the food quality for filter feeders by increasing the inorganic materials in the water column. Therefore, collecting nutritious particles as food is challenging, especially in areas where resuspension of sediments commonly occurs. Particle sorting and selection can enhance the ingestion of organic matter. In bivalves, sorting of particles occurs on the gills and labial palps, based on the physical (e.g., wettability, density, shape, size), chemical, and nutritional properties of the particles (Ward et al., 1997; 1998). The selected particles are then transported to the mouth for ingestion. Unlike bivalves, which draw feeding water current into their body, barnacles create water movement by the rhythmic movement of the cirri (thoracic appendages) that they use to feed. These cirri have fine, hair-like structures known as setae, which act as filters. Extension of the cirri out of the body collect food particles, which are transferred to the mouth when cirri withdraw into the mantle cavity (Crisp and Southward, 1961). However, barnacles become passive filter feeders when water flow is high and unidirectional (Trager et al., 1990). The setae are organized in a pattern which allows barnacles to catch and filter particles effectively within their feeding apparatus. When exposed to a variety of particle sizes, barnacles preferentially retain and ingest particles within their preferred size range, while actively rejecting or expelling particles that are too large or too small (Wotton, 1994). The ability to discriminate between food items varied among barnacle species, with balanomorphan species having a very high diversity of setae adapted for more diversified feeding habits, in contrast to pedunculate barnacles, which possess simple serrulate types, indicating their inability to discriminate between food items (Chan et al., 2008).
The ingestion of MPs in many aquatic invertebrates is determined by their feeding modes and the concentration and form of MPs in the water (Scherer et al., 2017). MPs come in various forms, including fragments, fibers, and pellets, and each potentially affects ingestion differently. Studies have shown that mussels and oysters reject a much higher proportion of microspheres (diameter: 1000 μm) than long fibers (length: 1075 μm), when both were administered simultaneously (Ward et al., 2019b). The irregular shapes and larger surface areas of fibrous and fragmented MPs may enhance their capture efficiency during the filter-feeding process.
There are over 2116 barnacle species in the world, among them 67 species are found in Hong Kong (Chan et al., 2021), inhabiting coastal habitats, such as rocky and boulder shores, mangrove stems, and wharf piles. Acorn barnacles are dominant space occupiers on the middle and upper shores. Their high abundance and aggregated distribution made them ideal prey for predators such as starfish, ribbon worms and dog whelks (Connell, 1970; Menge, 1972). They are ecosystem engineers that modify the physical environment of the shore, hence affecting the abundance and activities of other species (Harley, 2006). Owing to their wide distribution ranges, high abundance, ease of collection and high tolerance to environmental stresses, they are commonly used as biomonitors for heavy metals and persistent organic pollutants (Vaezzadeh et al., 2021).
Various barnacle species have been found to ingest MPs. Goldstein and Goodwin (2013) examined the gastrointestinal tracts of the gooseneck barnacles (Lepas spp.) collected from the North Pacific Subtropical Gyre. Among them, 33.5% contained plastics, with the majority being polyethylene. Others included polystyrene and polypropylene. Zhang T. et al. (2022) showed high concentrations of MPs (14.09 ± 21.31 items/g) in Balanus albicostatus collected in the intertidal area of the Yellow Sea, and the spatial variations in the concentrations of MPs agreed with the pollution levels of respective sites. Amphibalanus amphitrite collected from Thailand (Thushari et al., 2017) and Indonesia (Raufanda et al., 2024) also contained microplastics with respective concentrations of 0.23–0.43 particles/g tissue and 5.37 particles/g tissue. In Hong Kong, the types and composition of MPs in four barnacle species (Tetraclita japonica japonica, Capitulum mitella, Amphibalanus amphitrite, Fistulobalanus albicostatus) collected at 30 sites were studied, and MPs were found in 84.8% of all the individuals, with the majority of the MPs being fibers (95.7%). The abundance of MPs in A. amphitrite was positively correlated with that in the sediment (Xu et al., 2020). The ingestion of microplastics was also reported in laboratory studies. Being exposed to either polypropylene fibers or fragments for 8 days, A. amphitrite accumulated MPs in their tissue in a concentration-dependent manner for both forms of MPs (Xu et al., 2023).
Amphibalanus amphitrite is a cosmopolitan species occurring in all major continents (Chen et al., 2014). In Hong Kong, it is a dominant species on rocky shores spanning from the eastern to western waters (Xu et al., 2020). The present study investigated whether A. amphitrite exhibited selective feeding in the presence of biofilm on the MPs. Three types (polyethylene, polystyrene/polyester), two sizes (27-32 µm and 90-106 µm) and two forms (microspheres and microfibers) of MPs at three concentrations (7.2, 72 and 720 P/mL) with or without biofilm were offered to the barnacles. The numbers of MPs ingested, egested and accumulated in the tissue were recorded. Since barnacles trap particles by setae, which form a filter net on the cirri, the efficiency of particle capture should vary with the shape and size of the particles. Therefore, we hypothesized that the presence of biofilm and the type of MPs should not affect the ingestion rate because these features are not used by barnacles in sorting particles for feeding. However, the ingestion rate of MPs was higher for larger sizes of MPs and also higher for microspheres than microfibers of the same length because of the larger surface area of the former. The results could provide insight into the ingestion and removal of MPs in barnacles and hence the relative potential risks of these MPs to the barnacles, and help assess the suitability of barnacles as bioindicators and biomonitors of MPs.
2 Materials and methods
2.1 Collection and maintenance of experimental animals
Individuals of Amphibalanus amphitrite on pebbles were collected near the Wu Kai Sha beach (22°25’44.0”N 114°14’06.4”E) during low tides. Ten individuals with a mean basal diameter of 4 ± 0.5 mm on each pebble were selected, and other barnacles and epifauna were removed by a pair of scissors with extra care to minimize damage to the animals. In the laboratory, barnacles were acclimated to laboratory conditions by being maintained in 60 L aquaria (10 pebbles in each aquarium). Each aquarium was equipped with a filtration system and air supply, and the seawater was maintained at 30 ppt, 22-24 °C and a 12/12 hr light/dark cycle. Every day, a 400 mL solution containing the log-phase growing diatom (Skeletonema costatum) was offered as food, and the feces was removed from each aquarium. The animals were acclimated for at least 7 days before experimentation.
2.2 Preparation of microplastics with biofilm
This was a full factorial experiment that investigated how the ingestion and accumulation of MPs in A. amphitrite varied with the form (microfiber, microsphere), type (polyethylene, polystyrene/polyester), size (length/diameter: ~ 30 and 100 μm; width of fiber: 42-48 μm), and concentration (7.2, 72 and 720 particles mL-1) of MPs, and the presence/absence of biofilm on MPs. Each treatment group had 10 pebbles (each with 10 barnacles) as replicates. Polyethylene and polystyrene microspheres were studied, but polyester fibers were used because polystyrene fibers were unavailable in the market. These polymers were chosen because they are the most abundant MPs found in the marine environment of Hong Kong (Zhang et al., 2024). The size classes of MPs used fall into the size range of prey captured by barnacles (Southward, 1955), and the range of particle concentrations selected was within the range obtained in Hong Kong waters (Tse et al., 2023; Zhang et al., 2024).
Fluorescent polyethylene and polystyrene microspheres of different colors were purchased from Cospheric LLC, and polyethylene and polyester fibers were prepared using a cryogenic microtome (Cole, 2016) (Supplementary Figures S1, S2). The MPs were characterized by the micro-FTIR. An FTIR spectrum was obtained for each piece of MP in the range of 650–4000 cm-1, and the spectrum was compared with the OMNIC reference spectra in the Hummel Polymer library. A matching percentage > 70% was accepted as the correct identification of the MPs.
Since MPs with different amounts of biofilm were offered to the barnacles simultaneously, different groups of MPs were prepared using different colors of MPs so that their ingestion by the barnacles could be differentiated and compared. In all the experiments, blue MPs were incubated for 14 days, green MPs for 3 days, and red MPs as a control group without incubation. In each set of experiments, the same weight of MPs was put into a nylon bag (10 ×10 cm) with a mesh size of 25 μm, and two lock straps were tightly wound around the mouth of the bag. Two groups of MPs were incubated in the sea at the Ma Liu Shui Public Pier for biofilm development. One group was kept for 3 days, and the other group for 14 days. The third group was virgin MPs without biofilm and served as the control. To understand how the duration of incubation of MPs in the sea affected the biofilm development, the biofilm formed on PE microspheres (90 – 106 μm) that had been incubated separately for 0 day, 3 days or 14 days was examined using SEM (Carson et al., 2013), flow cytometry and Crystal Violet Assay (see below).
2.3 Biofilm analysis
2.3.1 Scanning electron microscopy
The microspheres were gently rinsed onto a PET cell strainer (pore size: 70 μm), fixed for at least two hours with 2.5% glutaraldehyde in PBS, and then washed three times with PBS and twice with deionized water. After that, the microspheres were dehydrated by progressively treated for 10 minutes each time with 30%, 50%, 70%, 80%, 95%, and twice with 100% ethanol. The PE microspheres were physically dried using CO2 critical point drying after being dehydrated and then placed on carbon tape. After allowing the tape containing the microspheres to sputter coat with gold, SEM images (Quattro S, Thermo Fisher Scientific) were taken.
2.3.2 Flow cytometry
Flow cytometry can be used to determine the number and biomass of cells in biofilms because the main components of biofilms are microorganisms and extracellular polymeric substances (EPS) (Tu et al., 2020), the majority of which have cell sizes smaller than 30 μm, the largest cell size that can be analyzed using flow cytometry.
To minimize the unintentional detachment of biofilm from the MPs, a sieve (cell strainer, pore size: 40 μm) instead of filtration by vacuum was used to collect the MPs. Since the biofilm would be washed away by excess and rough washing, MPs were washed once only after being collected on the sieve before transferring to a conical tube with phosphate-buffered saline (PBS). Ultrasonication (Transsonic T460, Elma) was performed for 10 minutes for the biofilm detachment. To prevent the cell suspension from spilling out during ultrasonication, parafilm (Bemis) was used to seal the conical tube. After ultrasonication, the cell suspension was sieved through the cell strainer again to remove the MPs and large clusters to prevent blockage in the flow cytometer. 450 μL of cell suspensions were extracted and mixed with 50 μL of CountBright™ Absolute Count Beads (Invitrogen, diameter = 7 μm) for the flow cytometry analysis (BD FACSCalibur Flow Cytometer, BD Biosciences, US). To eliminate the bacterial contamination of the air within the treatment group, controls were established that included varying quantities of microbeads but did not involve biofilm incubation. On the day of analysis, microspheres were submerged in seawater and removed using the same protocol as the treatment groups. Additionally, all of the processes were carried out in a laminar flow cabinet (model: LFR-800 vertical laminar flow recirculatory, Labguard Corporation) to avoid bacterial contamination of the samples from the air (Jacobi et al., 2017).
Data were presented by the forward scatter height versus side scatter height (FSC-H vs SSC-H) plot and analyzed by the software, Cell Quest. The biomass of the biofilm was calculated by the following equation:
2.3.3 Crystal violet assay
To assess how the amount of biofilm changed with the duration of the MPs being incubated in the sea, a modified crystal violet (CV) biofilm assay was used to quantify the biomass of the biofilm indirectly (Xu Z. et al., 2016). The 90-106 μm microspheres were fully immersed in 1% aqueous C25H30CIN3 solution and placed on a cell strainer with pore sizes of 70 μm. The microspheres were washed until the filtrate was clear after 45 minutes at room temperature. After 45 minutes of drying, the microspheres were moved to fresh 50 mL polypropylene tubes filled with 95% ethanol and inverted. The absorbance was measured at 595 nm with 1.0 mL of the solution in a cuvette after 10 minutes (Biotek Powerwave xs and Molecular Devices ID5).
2.4 Ingestion of MPs of different colors
To investigate if A. amphitrite preferentially ingested MPs with biofilm, MPs of different colors (blue, green and red) were used in different treatment groups with or without biofilm. Therefore, confirmation was required to ensure that A. amphitrite exhibited no color preference in MP ingestion. An experiment was conducted by offering different colors of virgin microspheres to A. amphitrite simultaneously, and the ingestion of each type of microsphere was quantified and compared. The experimental details and results are shown in Supplementary Information S3 and Supplementary Figure S7.
2.5 Selective feeding experiments on MPs with biofilm
On the day of the experiment, the MPs cultured for 14 days and 3 days in nylon bags placed in the sea were retrieved. MPs of different colors were used for different incubation periods because the previous experiment showed that A. amphitrite exhibited no color preference during feeding (Supplementary Figure S7).
Each type of MPs was made as concentrated stock suspensions in glass bottles with Milli-Q water. Using the 90-106 μm fluorescent microspheres as an example, three different colors of microspheres of roughly the same weight were weighed and placed in separate 100 mL glass bottles to prepare stock solutions containing 100, 1000 and 10000 microspheres/mL. The concentration of each color of microspheres in each glass bottle was determined by extracting 1 mL of the stock solution using a filter paper and the number of microspheres was counted in triplicates. Prior to exposure, the MP stock solutions were sonicated (30 seconds, 40 kHz) and vigorously agitated to disrupt aggregates and ensure a homogeneous suspension. After 15 minutes, a pipette was used to extract different volumes of stock solutions containing different colors of microspheres so as to obtain equal amounts of microspheres of different colors. The solutions were then added to each experimental chamber containing barnacles and 500 mL of seawater to obtain three experimental concentrations of microspheres (7.2, 72 and 720 microspheres/mL).
The feeding selectivity of A. amphitrite was determined using an indirect method, which measured the decline in MP concentration in a closed system after 48 hours (Stuart and Klumpp, 1984). Microalga Skeletonema costatum at 5000 cells/mL was added to the beaker as food at the beginning of the experiment. After 15 minutes, ten individuals of A. amphitrite in each chamber were offered three types of MP (with different amounts of biofilm) in equal proportions. To facilitate the feeding process, a magnetic stirrer was used to maintain water circulation in the chamber. The water flow direction does not affect the food-collecting behavior of A. amphitrite because they always adjust their cirral fan to face the incoming flow, and their beating rate and duration are unaffected by the rostro-carinal axis deviation from the flow direction (Pasternak and Achituv, 2007). Weak aeration was supplied to each container. The concentration of each type of MP was measured by drawing 2 ml of the solution from the chamber and counting under a motorized fluorescence stereo-microscope (Leica M205 FA). The feces and body tissue of the barnacles were collected separately after 48 hours. After centrifugation at 4500 RPM for 10 minutes and digestion with 10% KOH at 40 °C for 48 hours, the digested solution was filtered onto a 47 mm MCE membrane filter paper (pore size: 0.45 µm). All the glassware and the filter setup were washed thoroughly with filtered deionized water. The filter papers containing MPs were stored in petri dishes and air dried before microscopic examination.
2.6 Quality assurance and quality control
The algal culture solution was bubbled with air through an aerating system equipped with a filter, and the lid was covered with aluminum foil to prevent airborne contamination. Since the fragments and fibers we had prepared were either blue, green or red in color, MPs in other colors, if any, were not counted. All equipment and glassware were cleaned with running water and rinsed with deionized water three times before use. Contamination by the detachment of synthetic fibers was further reduced by wearing cotton lab coats in the laboratory.
2.7 Statistical analysis
The ingestion of MPs in A. amphitrite was studied under various sizes, forms, concentrations, and types of MPs, both in the presence and absence of biofilm. The two-way mixed model repeated measures analysis of variance (ANOVA, GLM) was used to compare the number of MPs ingested (total number of MPs in the body and in the feces) using biofilm as a within-subject factor and the concentration and form of MPs as between-subject factors. The same models were run separately for different sizes and types of MPs.
Since the ingestion of MPs was independent of the biofilm (see the experimental results), the total number of MPs ingested was calculated as the sum of MPs with different amounts of biofilm ingested by the barnacles. The results were analyzed again using the two-way mixed model repeated measures analysis of ANOVA (ANOVA, GLM), with concentration and form of MPs as fixed factors. The same models were run separately for different sizes and types of MPs.
The comparisons between the ingestion of microspheres and microfibers, and between PE and PS were tested using paired t-test. Prior to the analyses, data were tested for normality and homoscedasticity, and transformed (square root) if required. Statistical analyses were performed using GraphPad Prism 9.5.0 and IBM SPSS Statistics 26 at an alpha level of 0.05.
3 Results
3.1 Biofilm incubated on MPs
3.1.1 Scanning electron microscopy
The morphology of biofilms incubated for 0, 3, and 14 days is shown in Supplementary Figure S4. Over the course of the incubation period, the biomass of the biofilm increased significantly, and SEM images show that MPs incubated for 14 days had denser microbial colonization than those for 3 days. The microbial communities on the surface of the MPs included agglomerated microorganisms, sphere-shaped bacterial cells, and rod-like bacterial cells. As the biofilm culture time increased, some diatoms and diatom assemblages also emerged.
3.1.2 Flow cytometry
The number of cells in the biofilm on MPs increased with the incubation period (Supplementary Figure S5), with the results obtained in different periods (0, 3 and 14 days) being significantly different from each other (3 days: 4.7 × 107 ± 1.3 × 107; 14 days: 8.2 ×107 ± 1.3 ×107).
3.1.3 Crystal violet assay
The quantity of biofilm increased with the incubation period, with the results obtained in different treatment groups being significantly different from each other (Supplementary Figure S6).
3.2 Feeding selectivity on MPs in barnacles
Regardless of the size, form, type and concentration of the MPs, the number of MPs ingested by A. amphitrite did not vary with the amounts of biofilm on the MPs (Table 1, Supplementary Figure S8). Therefore, the number of MPs with different amounts of biofilm ingested by the barnacles was added up as the total number of MPs ingested, and the variations in the MPs ingestion with the concentration and form of MPs were tested again for each size group of PE and PS using two-way mixed model repeated measures ANOVA. The total number of MPs ingested is shown in Supplementary Table S9. Since there were significant interactions between the concentration and form of MPs for each size group of PE and PS, the effect of MPs concentration on the ingestion rate was determined separately for microspheres and microfibers. The higher the concentration of MPs, the higher was the number ingested by the barnacles, except for 100 µm and 30 µm PE microfibers, at which the number of MPs ingested was statistically insignificant between concentrations of 7.2 and 72 P/mL (Table 2, Supplementary Table S9). The mean number of MPs ingested by each group of barnacles (10 individuals) varied between 41 particles (or 4.1 particles/individual) for 100 µm PS microspheres at 7.2 P/mL and 956 particles (9.56 particles/individual) for 100 µm PE microspheres at 720 P/mL. There was no significant difference between the number of microspheres and microfibers ingested by A. amphitrite (Figure 1), except for both size groups (30 and 100 µm) of PS and 30 µm PE at 72 P/mL, at which more microspheres were ingested. For both microspheres and microfibers, the number of PE and PS MPs ingested by the barnacles at the lowest concentration (7.2 P/mL) was statistically insignificant. However, more PE MPs were ingested at higher concentrations (Figure 2).
Table 1
| MPs | Number ingested* (mean ± SD) | Significance | ||
|---|---|---|---|---|
| 0-day biofilm | 3-day biofilm | 14-day biofilm | ||
| PE microspheres (90-106 μm) | ||||
| 7.2 P/mL | 18.7 ± 11.2 | 20.0 ± 12.6 | 17.9 ± 11.3 | ns |
| 72 P/mL | 92.6 ± 15.9 | 89.3 ± 23.9 | 100.8 ± 22.3 | ns |
| 720 P/mL | 318.4 ± 115.2 | 326.7 ± 166.0 | 310.9 ± 115.2 | ns |
| PE microspheres (27-32 μm) | ||||
| 7.2 P/mL | 16.8 ± 9.0 | 18.0 ± 10.0 | 16.5 ± 5.5 | ns |
| 72 P/mL | 83.5 ± 29.2 | 81.5 ± 27.9 | 82.8 ± 22.8 | ns |
| 720 P/mL | 244.3 ± 88.5 | 246.3 ± 76.1 | 235.3 ± 75.4 | ns |
| Polystyrene microspheres (100 μm) | ||||
| 7.2 P/mL | 12.1 ± 6.9 | 14.1 ± 6.8 | 14.8 ± 8.0 | ns |
| 72 P/mL | 64.4 ± 21.2 | 74 ± 27.2 | 72.2 ± 19.9 | ns |
| 720 P/mL | 118.9 ± 32.5 | 125.1 ± 31.9 | 129.1 ± 43.7 | ns |
| Polystyrene microspheres (30 μm) | ||||
| 7.2 P/mL | 12.6 ± 5.4 | 15.1 ± 9.5 | 13.8 ± 7.1 | ns |
| 72 P/mL | 53.6 ± 22.8 | 54.1 ± 16.4 | 51.2 ± 12.7 | ns |
| 720 P/mL | 132.3 ± 56.4 | 132.0 ± 62.3 | 134.1 ± 56.8 | ns |
| PE microfibers (100 μm) | ||||
| 7.2 P/mL | 20.1 ± 8.2 | 19.9 ± 9.8 | 19 ± 10.1 | ns |
| 72 P/mL | 69.1 ± 19.8 | 70.8 ± 24.6 | 66.2 ± 15.6 | ns |
| 720 P/mL | 128.4 ± 42.0 | 140.3 ± 45.2 | 143.7 ± 60.8 | ns |
| PE microfibers (30 μm) | ||||
| 7.2 P/mL | 17.5 ± 10.6 | 16.7 ± 6.9 | 18.1 ± 9.0 | ns |
| 72 P/mL | 41.1 ± 16.7 | 44.3 ± 19.1 | 39.5 ± 16.8 | ns |
| 720 P/mL | 272.8 ± 47.5 | 275.5 ± 42.9 | 270.6 ± 47.0 | ns |
| Polyester microfibers (100 μm) | ||||
| 7.2 P/mL | 15.0 ± 7.0 | 15.5 ± 10.1 | 14.1 ± 9.1 | ns |
| 72 P/mL | 45.3 ± 12.3 | 45.0 ± 10.7 | 49.9 ± 11.9 | ns |
| 720 P/mL | 121.8 ± 28.8 | 124.2 ± 30.5 | 125.7 ± 32.6 | ns |
| Polyester microfibers (30 μm) | ||||
| 7.2 P/mL | 13.7 ± 6.0 | 14.1 ± 4.1 | 15.4 ± 4.7 | ns |
| 72 P/mL | 47.8 ± 9.1 | 47.8 ± 10.4 | 51.5 ± 13.0 | ns |
| 720 P/mL | 147.3 ± 48.6 | 143.8 ± 45.4 | 149.9 ± 40.4 | ns |
The analysis of the number of MPs ingested (number collected in body tissue and feces) by barnacles using two-way mixed model repeated measures analysis of variance (ANOVA, GLM) with biofilm being a within-subject factor and concentration, form and size of MPs as between-subject factors.
* The number of MPs ingested was calculated as the sum of MPs found in the feces and the body tissue in 10 barnacles. Data are means ± standard deviation; ns=not statistically significant with p > 0.05).
Table 2
| Type/Size of MPs | Form of MPs | |||||
|---|---|---|---|---|---|---|
| Microspheres | Microfibers | |||||
| Pairwise comparison | Significance | Pairwise comparison | Significance | |||
| PE (100 μm) | 7.2 P/mL | 72 P/mL | ** | 7.2 P/mL | 72 P/mL | ns |
| 72 P/mL | 720 P/mL | *** | 72 P/mL | 720 P/mL | * | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
| PE (30 μm) | 7.2 P/mL | 72 P/mL | ** | 7.2 P/mL | 72 P/mL | ns |
| 72 P/mL | 720 P/mL | *** | 72 P/mL | 720 P/mL | *** | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
| PS# (100 μm) | 7.2 P/mL | 72 P/mL | ** | 7.2 P/mL | 72 P/mL | *** |
| 72 P/mL | 720 P/mL | *** | 72 P/mL | 720 P/mL | *** | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
| PS# (30 μm) | 7.2 P/mL | 72 P/mL | * | 7.2 P/mL | 72 P/mL | * |
| 72 P/mL | 720 P/mL | *** | 72 P/mL | 720 P/mL | *** | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
Two-way mixed model repeated measures analysis of variance (ANOVA, GLM) was used to compare the total number of MPs ingested (number collected in the body and feces) using concentration and form of MPs as fixed factors and the number of MPs ingested as a dependent variable.
#PS refers to polystyrene in microspheres and polyester in microfibers. *P<0.05, **P<0.01, ***P<0.001, ns, not significant.
Figure 1
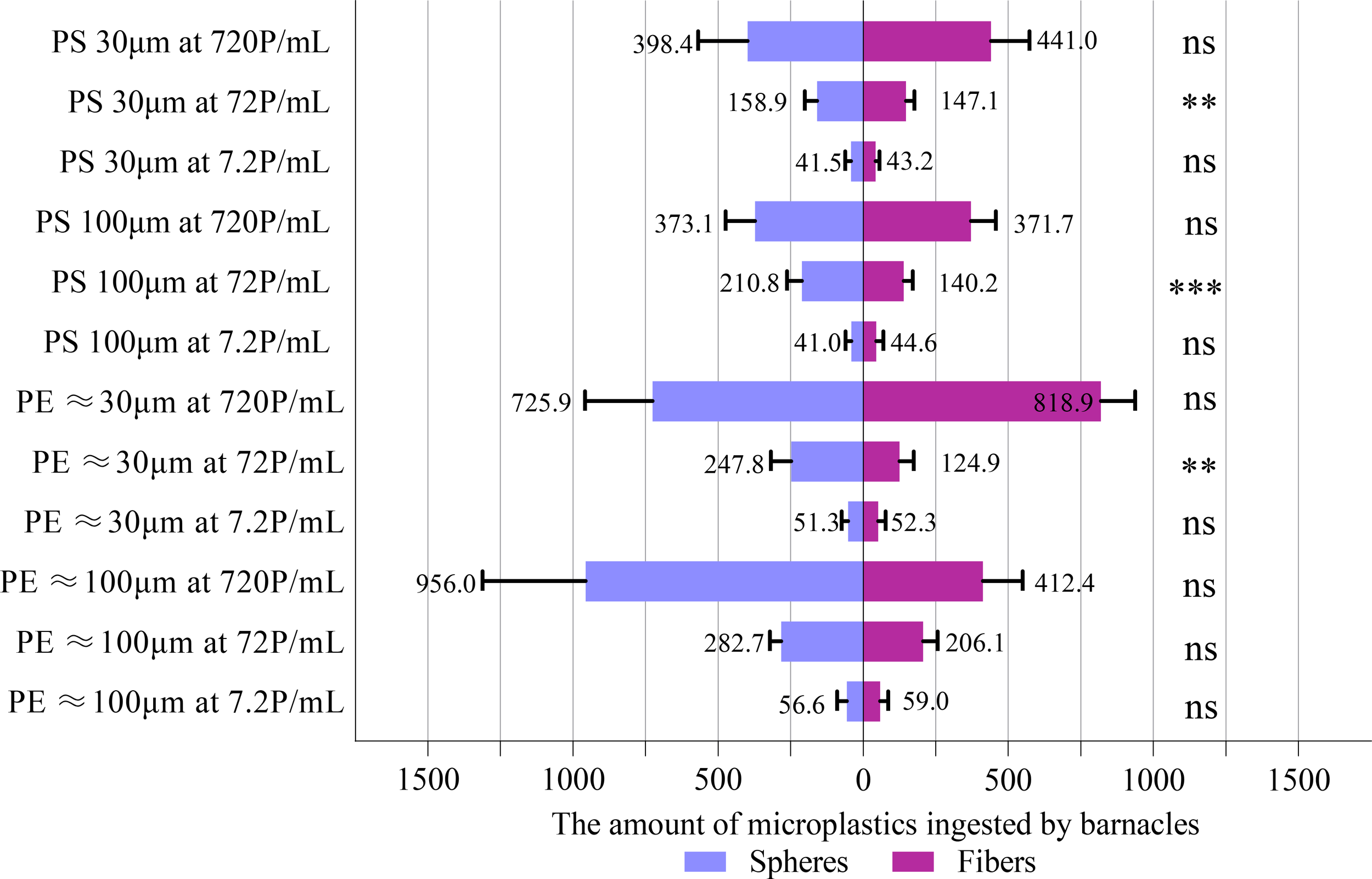
Comparisons between the ingestion (number of MPs collected in the body and feces) of microspheres and microfibers using paired t-test. Data are means (+ SD) of 10 replicates, and the number of MPs refers to the sum of 10 barnacles in the same experimental chamber. **P<0.01, ***P<0.001, ns, not significant.
Figure 2
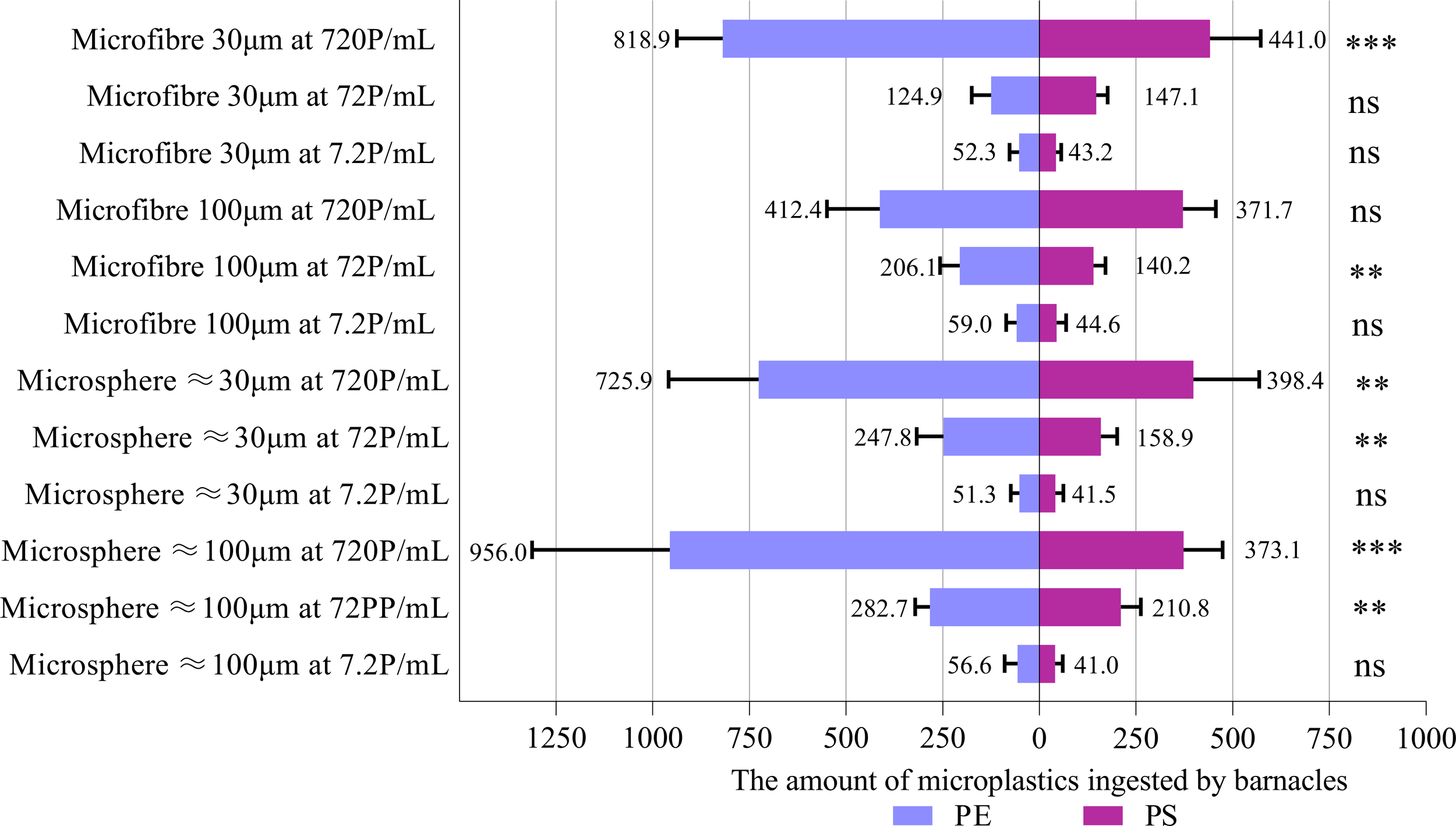
Comparisons between the ingestion (number of MPs collected in the body and feces) of PE MPs and PS MPs using paired t-test. Data are means (+ SD) of 10 replicates, and the number of MPs refers to the sum of 10 barnacles in the same experimental chamber. **P<0.01, ***P<0.001, ns, not significant.
The number of MPs accumulated in the body as a function of the concentration and form of MPs was determined for each type and size of MPs. Due to the interactive effect between the concentration and form of MPs, the effect of MPs concentration was studied separately for each form of MPs (Table 3). In most of the cases, the number of microspheres and microfibers accumulated by A. amphitrite was concentration-dependent for each size group of PE and PS. The number of both size groups of PS microspheres accumulated in the body, however, was not significantly different between two higher MP concentrations, i.e., 72 and 720 p/mL. The same was also observed for 30 µm polyester microfibers and 100 µm PE microfibers. The number of microfibers accumulated in the body was similar to that of microspheres, except for 30 µm and 100 µm of PS and 30 µm of PE, at which more microspheres were accumulated (Figure 3). Barnacles exposed to 30 µm PS microspheres at 7.2 P/mL accumulated the smallest number of MPs in the body (13.6 particles per group, or 1.36 particles per individual) while those exposed to 30 µm PE microfibers at 720 P/mL accumulated the highest number of MPs (441.8 particles per group, or 44.18 particles per individual). The number of PS and PE MPs accumulated in the body of barnacles was similar, except at the highest concentration at which more PE MPs were accumulated (Figure 4).This study utilized ten replicates per treatment group, a sample size consistent with established experimental designs in microplastic ingestion research (e.g., Browne et al., 2008; Cole et al., 2013). This replication level provides robust detection of moderate to large effect sizes, as demonstrated by statistically significant outcomes in key endpoints like ingestion rate. Based on effect sizes reported in comparable studies (e.g., Wright et al., 2013), our design had adequate power (≥ 0.8) to detect differences in ingestion rates exceeding 20%.
Table 3
| Type/Size of MPs | Form of MPs | |||||
|---|---|---|---|---|---|---|
| Microspheres | Microfibers | |||||
| Pairwise comparison | Significance | Pairwise comparison | Significance | |||
| PE (100 μm) | 7.2 P/mL | 72 P/mL | ns | 7.2 P/mL | 72 P/mL | ** |
| 72 P/mL | 720 P/mL | *** | 72 P/mL | 720 P/mL | ns | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
| PE (30 μm) | 7.2 P/mL | 72 P/mL | ns | 7.2 P/mL | 72 P/mL | ns |
| 72 P/mL | 720 P/mL | ** | 72 P/mL | 720 P/mL | *** | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
| PS (100 μm) | 7.2 P/mL | 72 P/mL | *** | 7.2 P/mL | 72 P/mL | * |
| 72 P/mL | 720 P/mL | ns | 72 P/mL | 720 P/mL | ** | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
| PS (30 μm) | 7.2 P/mL | 72 P/mL | ** | 7.2 P/mL | 72 P/mL | ns |
| 72 P/mL | 720 P/mL | ns | 72 P/mL | 720 P/mL | ns | |
| 7.2 P/mL | 720 P/mL | *** | 7.2 P/mL | 720 P/mL | *** | |
Two-way mixed model repeated measures analysis of variance (ANOVA, GLM) for comparing the number of MPs accumulated in barnacles’ bodies using concentration and form of MPs as fixed factors and the number of MPs accumulated in the body as a dependent variable.
*P<0.05, **P<0.01, ***P<0.001, ns, not significant.
Figure 3
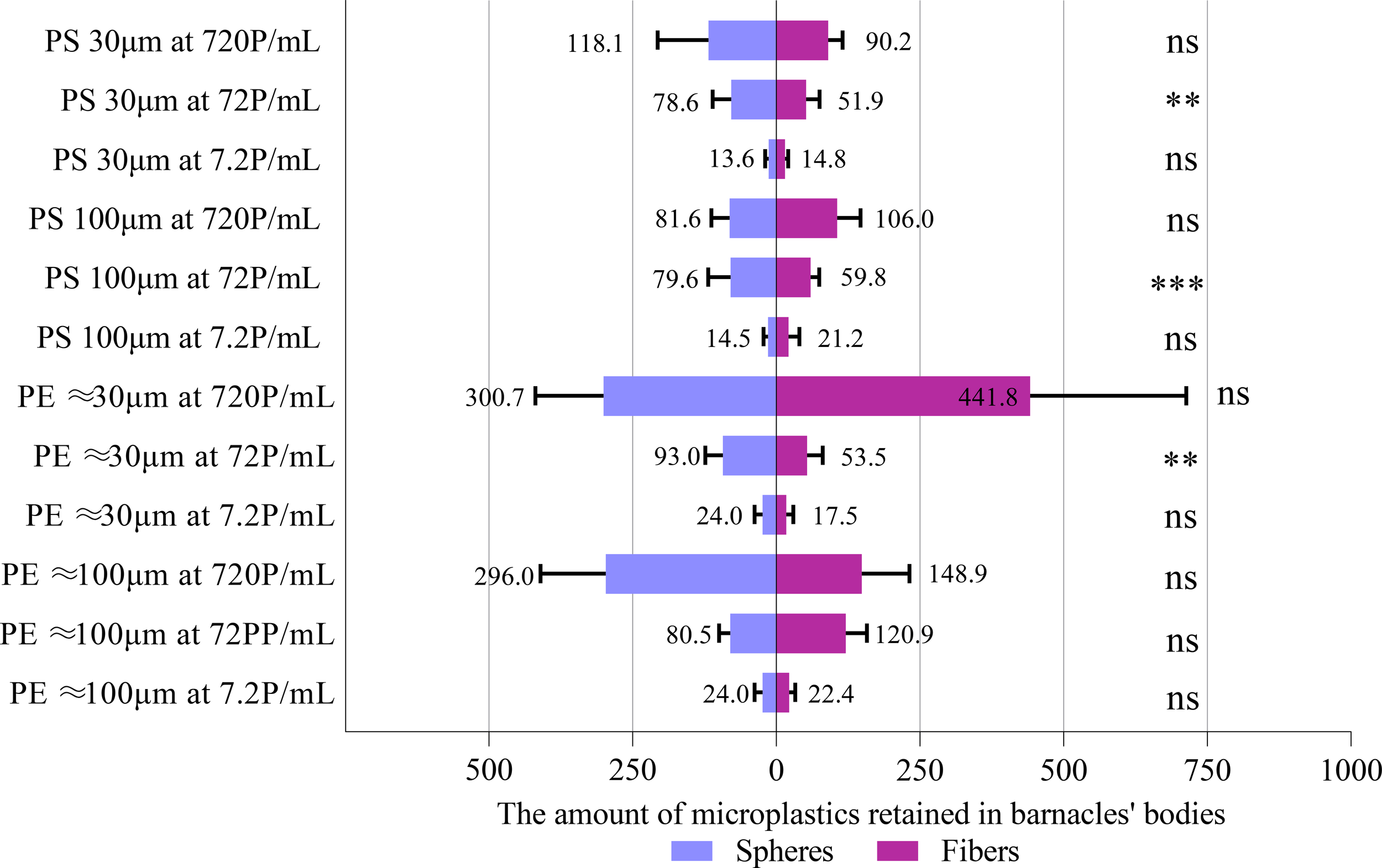
Comparisons between the number of microfibers and microspheres accumulated in the body of A. amphitrite using t-test. Data are means (+ SD) of 10 replicates, and the number of MPs refers to the sum of 10 barnacles in the same experimental chamber. **P<0.01, ***P<0.001, ns, not significant.
Figure 4
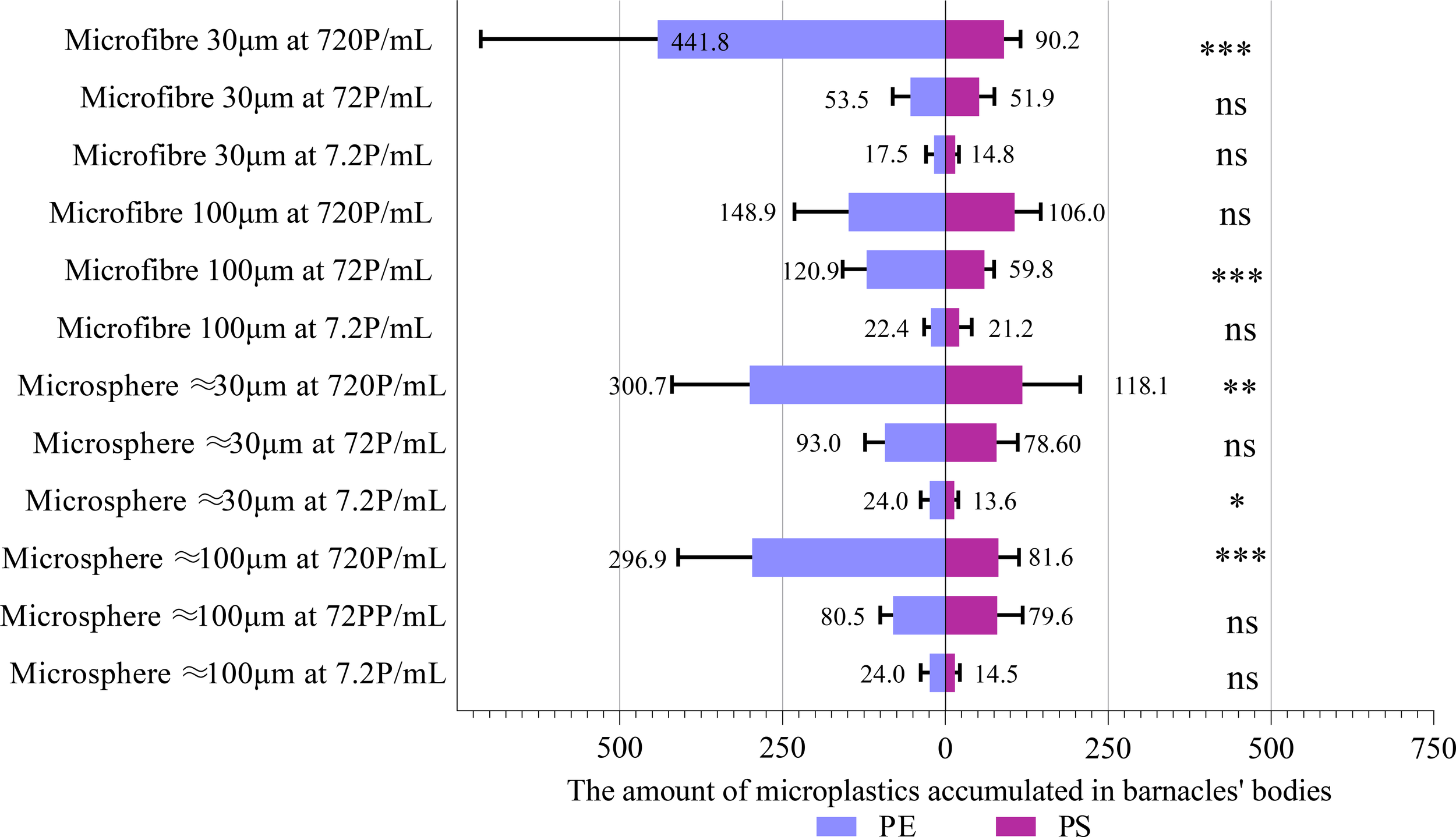
Comparisons between the number of PE MPs and PS MPs accumulated in the body of A. amphitrite using t-test. Data are means (+ SD) of 10 replicates, and the number of MPs refers to the sum of 10 barnacles in the same experimental chamber. *P < 0.05, **P<0.01, ***P<0.001, ns, not significant.
4 Discussion
Once discharged into the sea, MPs are rapidly colonized by a variety of microbes which secrete extracellular polymeric substances, forming a matrix called biofilm. Rod-shaped bacteria and bacterial assemblages appeared first on the biofilm and were later colonized by diatoms. The results were similar to those reported upon by Chan et al. (2003) in a successional study of biofilm on a Hong Kong rocky shore. Since the biofilm has changed the surface properties of the MPs and is nutrient-rich, the biofilm-coated MPs are preferentially ingested by marine invertebrates such as polychaetes (Unabia and Hadfield, 1999). In the oyster Ostrea edulis, the gills and mantle retained ten times more microbeads coated with biofilm containing Escherichia coli than virgin microbeads (Fabra et al., 2021). Zooplankton Acartia longiremis and Calanus finmarchicus preferentially ingested polystyrene beads aged in natural seawater over pristine ones (Vroom et al., 2017). Although the biofilm was not studied in Vroom’s study, the authors attributed the feeding preference to the formation of a biofilm. Unlike the above invertebrates, the ingestion of MPs in A. amphitrite did not vary with the presence of biofilm. To ingest biofilmed MPs preferentially implies that the organism is able to discriminate between MPs with or without biofilm. In addition to particle size, the particle selection in bivalves is chemical-mediated and determined by the hydrophobicity of the particles (Ward and Targett, 1989). Probably, this kind of particle selection mechanism does not exist in barnacles, in which particles collected by the cirri sweep net are scraped off by special setae on neighboring cirri closer to the head end and finally passed to the mouth (Riisgård, 2015). Such a feeding mechanism lacks a region for particle sorting similar to the gills and labial palps of bivalves, rendering particle sorting impossible. Our findings that biofilm did not affect ingestion contrast with studies on other taxa, such as mussels and polychaetes, where biofilm enhances palatability and selectivity via chemosensory cues. This discrepancy may arise from taxonomic differences in feeding ecology. As barnacles are passive, mechanical feeders, they are likely less sensitive to chemical signals than selective feeders like mussels. Or the biofilm composition in our study may have lacked strong cues or sufficient nutritional value to induce selectivity. High particle concentrations may also overwhelm selective feeding mechanisms. These results suggest biofilm effects on microplastic ingestion are taxon-specific. Regardless of the form, type and size of MPs, the ingestion of MPs in A. amphitrite was positively correlated with the MPs concentration in this study. The concentration- dependent ingestion of MPs is commonly observed in aquatic invertebrates. Scherer et al. (2017) offered five freshwater invertebrates (Chironomus riparius, Daphnia magna, Gammarus pulex, Lumbriculus variegatus, Physella acuta) fluorescent polystyrene spheres at concentrations between 3 and 3000 particles/mL, and all the species ingested microplastics in a concentration-dependent manner except L. variegatus. In the zebra mussel D. polymorpha, the MP concentration in the body increased when exposed to a 10‐fold increase in the ambient MP concentration (Weber et al., 2018). A previous study on A. amphitrite also found that the ingestion of MPs was concentration dependent between exposure concentrations of 10 and 1000 items/L for both fragments and fibers (Xu et al., 2023).
An increase in the ingestion rate of MPs implies that the number of MPs processed per unit time in the gut increased, resulting in an increase in feces production and an accumulation of MPs in the body because MPs are indigestible. An increase in the removal rate of MPs when exposed to higher ambient concentrations of MPs can be achieved by shortening the gut retention time. This strategy increases the removal efficiency of non-nutritious particles in mussels, hence enhancing energy intake (Galimany et al., 2013). In barnacle naupliar larvae, the gut retention time increased for smaller microbeads (Yu et al., 2021). They also found that the retention time was habitat dependent, with a shorter retention time being found in barnacle species living on muddy shores than those on rocky shores and coral reefs. Since inert microparticles, such as clay and kaolin, are more abundant on muddy shores, a shorter retention time is essential for barnacles to improve the efficiency of removing inorganic particles in turbid water. A. amphitrite is widely distributed in Hong Kong waters, spanning from the oceanic eastern waters characterized by high salinity and low turbidity to the estuarine western waters with low salinity and high turbidity because of the influence of Pearl River which has an average total suspended sediment concentration of 26.89 mg/L between 2016 and 2020 (Ji et al., 2025). Although the gut retention time for MPs was not determined for A. amphitrite in the present study, the ability of A. amphitrite to thrive in turbid water helps explain why it can remove MPs efficiently at high concentrations.
Since the number of MPs accumulated in the body increased with the ambient MP concentration, this indicates that the removal rate of MPs was unable to cope with the increase in the ingestion of MPs when ambient MP concentration increased. Unlike bivalves, which can reduce the ingestion of MPs through pre-ingestive particle sorting and pseudofeces production, barnacles are less efficient in removing unwanted particles. A. amphitrite collects particles by extending the cirral fan rhythmically. By withdrawing the fan into the mantle cavity, particles are transferred to the mouth for ingestion (Pasternak and Achituv, 2007). Therefore, A. amphitrite can only reduce the MP ingestion by lowering the beating frequency of the cirral fan. This process, however, also inevitably reduces food intake. Although the accumulation of MPs in A. amphitrite was concentration-dependent, one-third of the pairwise comparisons of the number of MPs in the body between different ambient concentrations of MPs were statistically insignificant (Table 3), regardless of the size, form and type of MPs. This indicates that the particle processing rate in A. amphitrite was at least partially regulated to reduce the impact of MPs intake. More MPs accumulated in the body impose greater biological impacts, including blockage and injury of the intestine (Lei et al., 2018), food dilution (Xu X. et al., 2016; Gardon et al., 2018), and toxicities caused by adhered pollutants or additives (Martín et al., 2022). Whether the accumulation of MPs has any biological impacts on A. amphitrite, such as growth, reproduction and survival, deserves further investigation.
Microfibers are removed more efficiently than microspheres in bivalves because preingestive particle sorting occurs on the gills or labial palps, and the more elongated microfibers are mostly rejected in pseudofeces (Ward et al., 2019a). Although preingestive particle sorting is not found in crustaceans, some species, such as the Atlantic ditch shrimp Palaemon varians, do not egest microfibers through the gut but remove them through the esophagus, a process known as regurgitation (Saborowski et al., 2019). Regurgitation of unwanted substances is also reported in crustaceans, echinoderms and gastropods (Saborowski et al., 2019). Unlike bivalves, barnacles do not have preingestive particle sorting, and there is no report of regurgitation in barnacles. Therefore, whether a particle is ingested is determined by the particle capture efficiency of the cirri. Barnacles can capture food within a size range of between 2 µm and 1 mm (Southward, 1955). A “cirri sweep net” formed by the long cirri is mainly used in feeding on larger particles, e.g., zooplankton and large phytoplankton cells. Small phytoplankton cells (1 or 2 µm) are not trapped by the cirral net, but filtered off by the short cirri guarding the entrance to the mantle cavity (Riisgård, 2015). There was no significant difference between the number of microspheres and microfibers ingested by A. amphitrite in this study. Since the minimum width of the spaces in the sweep net formed by the long cirri was at least 33 µm (Southward, 1955), the MPs used in this study were mostly captured by the net rather than by the filter formed by the short cirri. Although elongated microfibers had a higher chance of passing through the cirral net, they could be filtered off by the short cirri and ingested. The indiscriminate ingestion of particles collected by the cirri was also reported in the pelagic gooseneck barnacle Lepas (Lepas) anatifera, with different sizes of plastic fibers and fragments, as well as shell fragments of bivalves and ostracods being found in the digestive tract (Scotti et al., 2023).
Similar to the ingestion of MPs, the number of MPs accumulated in the body did not vary with the form of MPs. This indicates that the rate of removal of microspheres and microfibers was similar. Xu et al. (2023) also showed no significant difference between the abundance of fibers and fragments in A. amphitrite for all the exposure concentrations and durations. By contrast, the blue mussel Mytilus edulis preferentially retain smaller particles with an elongated shape (Zhao et al., 2018), because these particles are transported to digestive glands for intra-cellular digestion, whereas larger and longer particles are rejected through pseudofeces. This strategy enhances the rejection of non-nutritous particles, hence increasing energy intake (Xu Z. et al., 2016). Nevertheless, the ingestion of MPs varies with species and the size, type, shape and concentration of polymers. Therefore, direct comparisons between studies are difficult and contradictory results are commonly found (Yu et al., 2021).
The present study showed that more PE MPs were ingested and accumulated than PS MPs at high particle concentrations (72 and 720 P/mL), regardless of the form of MPs. The density of PE microspheres used in the present study ranged between 1.09 and 1.13 g/cm3, and that of PS microspheres ranged between 1.05 and 1.10 g/cm3. For microfibers, the density was 0.97-0.98 g/cm3 for PE and 1.36-1.49 g/cm3 for PS (polyester). The density of PS and PE microspheres was similar, but PS microfibers were denser than PE microfibers. Since higher ingestion and accumulation rates were observed for PE MPs, regardless of their forms (microspheres and microfibers), such differences in the rates were not caused by density differences between the polymers. Whether a particle is ingested or rejected in bivalves is determined by factors such as charge, wettability, hydrophobicity and surface characteristics of the particle (Rosa et al., 2018; Ward and Shumway, 2004). For example, larvae of the hard clam, Mercenaria mercenaria, capture negatively charged PS microspheres more efficiently than those without charge (Solow and Gallager, 1990). In the blue mussel, Mytilus edulis, whether the PS microspheres were ingested depends on the type of neoglycoprotein attached to the microspheres. Particles which are more hydrophobic or negatively charged are preferentially ingested by M. edulis, and oysters, Crassostrea virginica (Rosa et al., 2013). However, the type of coating has no effect on the ingestion of microspheres in the bay scallop, Argopecten irradians (Rosa et al., 2017). Compared with studies on bivalves, how the surface properties of MPs affect their ingestion by barnacles is totally unknown. An explanation for a higher preference toward PE in A. amphitrite is probably due to its extremely high hydrophobicity (Zhang Y. et al., 2022) and softness, which facilitates biofilm formation, in contrast to polystyrene with a hard and smooth surface. Such differences may affect the chance that the particles can be trapped by the cirri. Future mechanistic studies are deserved to understand such differences.
Hilty and Merenlender (2000) reviewed criteria for selecting faunal indicators to monitor ecosystem health. These criteria fall into four categories: baseline information, locational information, niche and life history characteristics, and others. They suggested that selected indicators should provide early warning while minimizing unpredictable fluctuations in populations. Therefore, indicator species should have a clear taxonomic status, with their biology, life history, and tolerance levels to human impact being studied. They are preferred to have a cosmopolitan distribution with limited mobility, small body size, specialized feeding mode at low or medium trophic levels, and are easy to find. Having said that, not one indicator taxon can satisfy all the criteria, hence, a suite of indicators from different taxa complementary to each other should be selected in order to satisfy multiple criteria. The mangrove barnacle B. albicostatus was suggested as an ecological indicator of MPs by Zhang T. et al., (2022) because the body burden of MPs was correlated with the degree of MP pollution at different intertidal sites in the Yellow Sea. Through studying populations of A. amphitrite at eight locations with different degrees of MP pollution, Xu et al. (2020) demonstrated a positive correlation between the abundance of MPs in sediments and that in A. amphitrite. Raufanda et al. (2024) also considered A. amphitrite as a potential MP bioindicator because their study revealed similarities in the forms, colors, and types of MPs found in barnacles, water, and sediment. The cosmopolitan distribution of A. amphitrite also supports this species as a global MP bioindicator (WoRMS, 2025). The present study showed that the accumulation of MPs in A. amphitrite was concentration-dependent across a wide range of concentrations (7.2–720 P/mL) and did not vary with the presence/absence of biofilm and the form and type of MPs. The results provide strong justifications for A. amphitrite being a bioindicator of MPs pollution when compared with other filter feeders, such as mussels and oysters, which selectively consume some MPs over others. As demonstrated by Ward et al. (2019b), mussels and oysters rejected a higher proportion (98%) of larger microspheres than smaller ones (10-30%). A higher proportion of microspheres was also rejected than microfibers of similar length. As a result, the number and types of MP found in the body of these bivalves will depend upon the physical characteristics of the particles. Therefore, bivalves are considered poor bioindicators of MP pollution by Ward et al. (2019b). The study of microplastics in green mussels and their epibionts, mostly barnacles, found that the concentration of microplastics in epibionts was higher than that in green mussels, indicating barnacles are probably better at accumulating microplastics, hence better MP bioindicators (Yaqin et al., 2023). In contrast, Li et al. (2019) argued that marine mussels are suitable global bioindicators for MP pollution because of their wide range of distribution, significant ecological roles, consumption by humans as a source of protein, and concentration-dependent intake of MPs in both field and laboratory studies. Their filter feeding mode of nutrition also facilitates them to accumulate MPs in the body more rapidly than invertebrates with other feeding modes, such as predation or grazing.
The MP concentration in Hong Kong waters varied between 0.05 to 29.52 particles/m3 (Zhang et al., 2024). In another study, the average abundance of MPs in marine surface waters at twelve sampling locations in Hong Kong was found to vary between 27 and 104 particles/L for the size range of 50 μm – 5 mm. However, very high concentrations (43,675–387,901 particles/L) of small MPs (1–50 μm) were reported (Tse et al., 2023). The range of exposure concentrations used in the present experiment was either comparable to or higher than the environmentally relevant concentrations. Using high MP concentrations in laboratory exposure experiments is not uncommon because environmentally relevant concentrations are often too low to cause any adverse effects (Koelmans et al., 2017). For example, the intertidal amphipod Echinogammarus marinus exposed to microbeads (8 μm) in concentrations of ∼0.9, 9 and 99 items per gram of seaweed for 35 days did not show any significant effects on food consumption and growth because of the high egestion rate of the microbeads (Bruck and Ford, 2018). Another freshwater amphipod, Gammarus pulex, also showed no effects on survival, molting, metabolism (glycogen, lipid storage) and feeding activity following chronic exposure to PET fragments for 48 days in concentrations of 0.8–4,000 items/mL (Weber et al., 2018). Similar results were obtained in the Mediterranean mussel (Mytilus galloprovincialis) exposed to 10 and 1000 items/mL polystyrene microbeads for 21 days. No significant damage to gut epithelia was found. Lipid peroxidation and Glutathione S-transferase activity also indicated the absence of significant oxidative stress (Gonçalves et al., 2019). Other studies used similar MP concentrations to those in the present study. Lobster (Homarus americanus) larvae were exposed to microfibers at 0, 1, 10 and 25 items/mL, and survival of the early larval stages and oxygen consumption rates in later larval stages were reduced only at the highest microfiber concentration (Woods et al., 2020). The filtration rate of the blue mussel (Mytilus edulis) was reduced at the microfiber concentration of 30 items/mL but not at 3 items/mL (Woods et al., 2018). Given that the production and disposal of plastic will continue to increase, higher than present concentrations of MPs will become relevant in assessing future risks (Koelmans et al., 2017).
There are several limitations in this study. The 48 hour exposure experiments are too short to reveal chronic and sublethal effects, which commonly occur in nature because the exposure to microplastics in the marine environment is continuous and the effects are cumulative. For example, in a 60 day chronic exposure study, juvenile sea cucumbers of Holothuria scabra exposed to polymethylmethacrylate MPs showed a reduction in the mean weight, percentage weight gain, and specific growth rate, as compared with the control. Histopathological examination also revealed an inflammatory response and impaired integrity of the epithelial barrier in the gastrointestinal tract of H. scabra in the treatment groups that may affect their digestion and absorption of nutrients (Shukhairi et al., 2025). Unlike laboratory experiments, which are conducted in relatively stable conditions, field populations experience temporal and spatial variations in environmental factors such as temperature, water velocity, food availability, and the presence of predator cues, resulting in changes in physiological responses, including feeding, respiration, growth and reproduction, hence affecting the body burden and toxicity of MPs. For example, the growth of many filter-feeding invertebrates in response to water velocity and temperature is either positive, negative or unimodal (Nishizaki and Carrington, 2015). Seasonal changes in body burden of MPs were found in crayfishes with increased levels of MPs in the spring for the native Cambarus appalachiensis due to warmer temperatures, but decreased levels in the non-native Faxonius cristavarius (Gray et al., 2024) The toxicity of MPs is temperature-dependent in the freshwater zooplankton Daphnia pulex which suffered from higher mortality when exposed to high MP concentrations at warmer temperatures (20 °C and 24°C), but not at 12°C, although there was no effect of MPs on time to first reproduction or average growth rate at any temperature (Klasios et al., 2024). Since static laboratory conditions do not replicate the complexity of natural environments, mesocosm studies incorporating factors such as tidal flows, variable food and sediment conditions, and the presence of predator cues, providing a more natural setting, should be encouraged, and the results should be more realistic and ecologically relevant. The present study only focused on the ingestion and accumulation of MPs, without considering their physiological effects, such as tissue damage, feeding inhibition, and changes in enzyme activities. Future directions should include functional biomarkers or sublethal impact assessments.
While this study identifies Amphibalanus amphitrite as a promising bioindicator candidate due to its non-selective feeding strategy and global distribution, we emphasize that these laboratory findings require validation under natural conditions. Specifically, future research should prioritize field studies across diverse environmental gradients—including variations in hydrology, temperature, and pollution regimes—to assess the robustness of this species as a bioindicator. Furthermore, investigations into seasonal variability and physiological states (e.g., reproductive condition, age) is necessary to account for potential confounding factors. Only through such comprehensive field validation can the global applicability of A. amphitrite as a reliable bioindicator for microplastics be confidently established.
5 Conclusions
The ingestion and accumulation of MPs in the striped barnacle A. amphitrite was dependent on the exposure concentration but independent of the presence of biofilm and the form, type and size of MPs. The results suggest a lack of both pre-ingestive and post-ingestive sorting and removal of MPs in A. amphitrite. The MP body burden, therefore, reflects levels of environmental contamination and an unbiased MPs composition in the water body. Considering the global distribution of A. amphitrite and its high abundance on rocky shores and man-made structures such as wharf piles, ease of finding and sampling, clear taxonomic status, small body size, high reproductive rate, specialized feeding mode, and well known biology and life history, it has great potential to be considered as a member of a list of global marine bioindicators of MPs. Further investigations should focus on how seasonal changes in environmental factors and body conditions, such as reproductive cyclicity, influence the ingestion and accumulation of MPs, and the associated ecotoxicological effects.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by Animal Research Ethics Committee City University of Hong Kong for the studies involving animals because no prior approval is needed for studies on invertebrates. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
YW: Investigation, Formal analysis, Methodology, Writing – original draft. AW: Funding acquisition, Writing – review & editing, Supervision, Resources, Conceptualization. JC: Project administration, Funding acquisition, Supervision, Writing – review & editing, Resources, Conceptualization. SC: Supervision, Project administration, Conceptualization, Methodology, Resources, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work described in this paper was fully supported by GRF - RGC General Research Fund (CityU 11100722).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1646294/full#supplementary-material
References
1
Browne M. A. Dissanayake A. Galloway T. S. Lowe D. M. Thompson R. C. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol.42, 5026–5031. doi: 10.1021/es800249a
2
Bruck S. Ford A. T. (2018). Chronic ingestion of polystyrene microparticles in low doses has no effect on food consumption and growth to the intertidal amphipod Echinogammarus marinus? Environ. pollut.233, 1125–1130. doi: 10.1016/j.envpol.2017.10.015
3
Carson H. S. Nerheim M. S. Carroll K. A. Eriksen M. (2013). The plastic-associated microorganisms of the North Pacific Gyre. Mar. pollut. Bull.75, 126–132. doi: 10.1080/08927010310001645229
4
Chan B. K. K. Chan W. K. Walker G . (2003). Patterns of biofilm succession on a sheltered rocky shore in Hong Kong. Biofouling19, 371–80. doi: 10.1080/08927010310001645229
5
Chan B. K. K. Dreyer N. Gale A. S. Glenner H. Ewers-Saucedo C. Pérez-Losada M. et al . (2021). The evolutionary diversity of barnacles, with an updated classification of fossil and living forms. Zool. J. Linn. Soc193, 789–846. doi: 10.1093/zoolinnean/zlaa160
6
Chan B. K. K. Garm A. Høeg J. T. (2008). Setal morphology and cirral setation of thoracican barnacle cirri: adaptations and implications for thoracican evolution. J. Zool.275, 294–306. doi: 10.1111/j.1469-7998.2008.00441.x
7
Chen H. N. Tsang L. M. Chong V. C. Chan B. K. (2014). Worldwide genetic differentiation in the common fouling barnacle, Amphibalanus amphitrite. Biofouling30, 1067–1078. doi: 10.1080/08927014.2014.967232
8
Cole M. Lindeque P. Fileman E. Halsband C. Goodhead R. Moger J. et al . (2013). Microplastic ingestion by zooplankton. Environ. Sci. Technol. 47, 6646–6655. doi: 10.1021/es400663f
9
Cole M. (2016). A novel method for preparing MP fibers. Sci. Rep.6, 34519. doi: 10.1021/es400663f
10
Connell J. H. (1970). A predator-prey system in the marine intertidal region. I. Balanus glandula and several predatory species of Thais. Ecol. Monogr.40, 49–78. doi: 10.2307/1942441
11
Crisp D. J. Southward A. J. (1961). Different types of cirral activity of barnacles. Philos. Trans. R. Soc Lond. B. Biol. Sci.243, 271–307. doi: 10.1098/rstb.1961.0003
12
da Costa J. P. Avellan A. Mouneyrac C. Duarte A. Rocha-Santos T. (2023). Plastic additives and microplastics as emerging contaminants: Mechanisms and analytical assessment. TrAC. Trends Anal. Chem.158, 116898. doi: 10.1016/j.trac.2022.116898
13
Fabra M. Williams L. Watts J. E. M. Hale M. S. Couceiro F. Preston J. (2021). The plastic Trojan horse: Biofilms increase microplastic uptake in marine filter feeders impacting microbial transfer and organism health. Sci. Total Environ.797, 149217. doi: 10.1016/j.scitotenv.2021.149217
14
Fava M . (2022). Ocean plastic pollution an overview: data and statistics. Intergovernmental Oceanographic Commission of UNESCO. Avialable online at: https://oceanliteracy.unesco.org/plastic-pollution-ocean/ (Accessed August 9, 2024).
15
Galimany E. Rose J. M. Dixon M. S. Wikfors G. H. (2013). Quantifying feeding behavior of ribbed mussels (Geukensia demissa) in two urban sites (Long Island Sound, USA) with different seston characteristics. Estuar. Coast.36, 1265–1273. doi: 10.1007/s12237-013-9633-0
16
Gardon T. Reisser C. Soyez C. VQuillien V. Le Moullac G. (2018). Microplastics affect energy balance and gametogenesis in the Pearl Oyster Pinctada margaritifera. Environ. Sci. Technol.52, 5277–5286. doi: 10.1021/acs.est.8b00168
17
Goldstein M. C. Goodwin D. S. (2013). Gooseneck barnacles (Lepas spp.) ingest microplastic debris in the North Pacific Subtropical Gyre. PeerJ1, e184. doi: 10.7717/peerj.184
18
Gonçalves C. Martins M. Sobral P. Costa P. M. Costa M. H. (2019). An assessment of the ability to ingest and excrete microplastics by filter-feeders: A case study with the Mediterranean mussel. Environ. pollut.245, 600–606. doi: 10.1016/j.envpol.2018.11.038
19
Gray A. Mayer K. Gore B. Gaesser M. Nathan Ferguson N. (2024). Microplastic burden in native (Cambarus appalachiensis) and non-native (Faxonius cristavarius) crayfish along semi-rural and urban streams in southwest Virginia, USA. Environ. Res.258, 119494. doi: 10.1016/j.envres.2024.119494
20
Guzzetti E. Sureda A. Tejada S. Faggio C. (2018). Microplastics in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol.64, 164–171. doi: 10.1016/j.etap.2018.10.009
21
Harley C. D. G. (2006). Effects of physical ecosystem engineering and herbivory on intertidal community structure. Mar. Ecol. Prog. Ser.317, 29–39. doi: 10.3354/meps317029
22
Hilty J. Merenlender A. (2000). Faunal indicator taxa selection for monitoring ecosystem health. Biol. Conserv.92, 185–197. doi: 10.1016/S0006-3207(99)00052-X
23
Jacobi Y. Yahel G. Shenkar N. (2017). Efficient filtration of micron and submicron particles by ascidians from oligotrophic waters. Limnol. Ocenogr.63, S267–S279. doi: 10.1002/lno.10736
24
Jeong E. Lee J.-Y. Redwan M. (2024). Animal exposure to microplastics and health effects: A review. Emerg. Contam.10, 100369. doi: 10.1016/j.emcon.2024.100369
25
Ji C. Zhang Y. Nejstgaard J. C. Ogashawara I. (2025). Assessment of the sediment load in the Pearl River estuary based on land use and land cover changes. Catena250, 108726. doi: 10.1016/j.catena.2025.108726
26
Klasios N. Birch A. Murillo A. M. Tseng M. (2024). Warming temperatures exacerbate effects of microplastics in a widespread zooplankton species. Environ. pollut.349, 123918. doi: 10.1016/j.envpol.2024.123918
27
Koelmans A. A. Besseling E. Foekema E. Kooi M. Mintenig S. Ossendorp B. C. et al . (2017). Risks of plastic debris: Unravelling fact, opinion, perception, and belief. Environ. Sci. Technol.51, 11513–11519. doi: 10.1021/acs.est.7b02219
28
Lei L. Wu S. Lu S. Liu M. Song Y. Fu Z. et al . (2018). Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ., 619-620: 1–8. doi: 10.1016/j.scitotenv.2017.11.103
29
Li J. Lusher A. L. Rotchell J. M. Deudero S. Turra A. Bråte I. L. N. et al . (2019). Using mussel as a global bioindicator of coastal microplastic pollution. Environ. pollut.244, 522–533. doi: 10.1016/j.envpol.2018.10.032
30
Marmara D. Katsanevakis S. Brundo M.-V. Tiralongo F. Ignoto S. Krasakopoulou E. (2023). Microplastics ingestion by marine fauna with a particular focus on commercial species: a systematic review. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1240969
31
Martín J. Santos J. L. Aparicio I. Alonso E. (2022). Microplastics and associated emerging contaminants in the environment: Analysis, sorption mechanisms and effects of co-exposure. Trends Environ. Anal. Chem.35, e00170. doi: 10.1016/j.teac.2022.e00170
32
Menge B. A. (1972). Competition for food between two intertidal starfish species and its effect on body size and feeding. Ecology53, 635–644. doi: 10.2307/1934777
33
Mutuku J. Yanotti M. Tocock M. MacDonald D. H. (2024). The abundance of microplastics in the world’s oceans: A systematic review. Oceans5, 398–428. doi: 10.3390/oceans5030024
34
Nishizaki M. T. Carrington E. (2015). The effect of water temperature and velocity on barnacle growth: Quantifying the impact of multiple environmental stressors. J. Therm. Biol.54, 37–46. doi: 10.1016/j.jtherbio.2015.02.002
35
Nousheen R. Rittschof D. Hashmi I. (2022). Toxic effects of pristine and aged polystyrene microplastics on selective and continuous larval culture of acorn barnacle Amphibalanus amphitrite. Environ. Toxicol. Pharmacol.94, 103912. doi: 10.1016/j.etap.2022.103912
36
Pasternak Z. Achituv Y. (2007). Feeding behavior of shallow-water barnacles from the Mediterranean and Red Seas. J. Crustacean Biol.27, 543–547. doi: 10.1651/S-2785.1
37
Pastorino P. Nocita A. Ciccotelli V. Zaccaroni A. Anselmi S. Giugliano R. et al . (2021). Health risk assessment of potentially toxic elements, persistence of NDL-PCB, PAHs, and microplastics in the translocated edible freshwater Sinotaia quadrata (Gasteropoda, Viviparidae): a case study from the Arno River Basin (Central Italy). Expo. Health13, 583–596. doi: 10.1007/s12403-021-00404-w
38
Raufanda M. S. Aunurohim A. Prabowo R. E. (2024). Barnacle analysis as a microplastic pollution bioindicator on the East Coast of Surabaya. PeerJ12, e17548. doi: 10.7717/peerj.17548
39
Riisgård H. U. (2015). “ Filter-feeding mechanisms in crustaceans,” in Natural History of Crustaceans Vol II: Lifestyles and Feeding Biology, Chapter 15: Life Styles and Feeding Biology. Eds. ThielM.WatlingL. ( Oxford University Press, Oxford), 418–463.
40
Rosa M. Ward J. E. Frink A. Shumway S. E. (2017). Effects of surface properties on particle capture by two species of suspension-feeding bivalve molluscs. Am. Malacol. Bull.35, 181–188. doi: 10.4003/006.035.0212
41
Rosa M. Ward J. E. Shumway S. E. (2018). Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: A review. J. Shellfish Res.37, 727–746. doi: 10.2983/035.037.0405
42
Rosa M. Ward J. E. Shumway S. E. Wikfors G. H. Pales-Espinosa E. Allam B. (2013). Effects of particle surface properties on feeding selectivity in the eastern oyster Crassostrea virginica and the blue mussel Mytilus edulis. J. Exp. Mar. Biol. Ecol.446, 320–327. doi: 10.1016/j.jembe.2013.05.011
43
Saborowski R. Paulischkis E. Gutow L. (2019). How to get rid of ingested microplastic fibers? A straightforward approach of the Atlantic ditch shrimp Palaemon varians. Environ. pollut.254, 113068. doi: 10.1016/j.envpol.2019.113068
44
Santos R. G. Machovsky-Capuska G. E. Andrades R. (2021). Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science373, 56–60. doi: 10.1126/science.abh0945
45
Scherer C. Brennholt N. Reifferscheid G. Wagner M. (2017). Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci. Rep.7, 17006. doi: 10.1038/s41598-017-17191-7
46
Scotti G. D’Alessandro M. Esposito V. Vivona P. Panti C. (2023). Anthropogenic fibers and microplastics in the pelagic gooseneck barnacle Lepas (Lepas) anatifera in Capo Milazzo Marine Protected Area (Tyrrhenian Sea): A first characterization. Ecol. Indic.152, 110368. doi: 10.1016/j.ecolind.2023.110368
47
Shukhairi S. S. Mazlan N. Abd Rahman N. N. Nazahuddin M. N. A. Shawel A. S. Kumar V. S et al . (2025). The effect of chronic microplastic exposure on the growth, biochemical responses, and histological changes of the juvenile sea cucumber Holothuria scabra. Environ. Sci. pollut. Res.32, 14980–14992. doi: 10.1007/s11356-025-36559-1
48
Siri C. Liu Y. Masset T. Dudefoi W. Oldham D. Minghetti M. et al . (2021). Adsorption of progesterone onto microplastics and its desorption in simulated gastric and intestinal fluids. Environ. Sci.: Process. Impacts23, 1566–1577. doi: 10.1039/D1EM00226K
49
Solow A. R. Gallager S. M. (1990). Analysis of capture efficiency in suspension feeding: Application of nonparametric binary regression. Mar. Biol.107, 341–344. doi: 10.1007/BF01319834
50
Southward A. J. (1955). Feeding of barnacles. Nature175, 1124–1125. doi: 10.1038/1751124b0
51
Stuart V. Klumpp D. W. (1984). Evidence for food-resource partitioning by kelp-bed filter feeders. Mar. Ecol. Prog. Ser.16, 27–37. doi: 10.3354/meps016027
52
Thushari G. G. N. Duminda J. Senevirathna M. Yakupitiyage A. Chavanich S. (2017). Effects of microplastics on sessile invertebrates in the eastern coast of Thailand: An approach to coastal zone conservation. Mar. pollut. Bull.124, 349–355. doi: 10.1016/j.marpolbul.2017.06.010
53
Trager G. C. Hwang J. S. Strickler J. R. (1990). Barnacle suspension-feeding in variable flow. Mar. Biol.105, 117–127. doi: 10.1007/BF01344277
54
Tse Y.-T. Lo H.-S. Tsang C.-W. Han J. Fang J. K.-H. Chan S. M.-N. (2023). Quantitative analysis and risk assessment to full-size microplastics pollution in the coastal marine waters of Hong Kong. Sci. Total Environ.879, 163006. doi: 10.1016/j.scitotenv.2023.163006
55
Tu C. Chen T. Zhou Q. Liu Y. Wei J. Waniek J. J. et al . (2020). Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci. Total Environ.734, 139237. doi: 10.1016/j.scitotenv.2020.139237
56
Unabia C. R. C. Hadfield M. G. (1999). Role of bacteria in larval settlement and meta morphosis of the polychaete Hydroides elegans. Mar. Biol.133, 55–64. doi: 10.1007/s002270050442
57
Vaezzadeh V. Thomes M. W. Kunisue T. Tue N. M. Zhang G. Zakaria M. P. et al . (2021). Examination of barnacles’ potential to be used as bioindicators of persistent organic pollutants in coastal ecosystem: A Malaysia case study. Chemosphere263, 1–12. doi: 10.1016/j.chemosphere.2020.128272
58
Vroom R. J. Koelmans A. A. Besseling E. Halsband C. (2017). Aging of microplastics promotes their ingestion by marine zooplankton. Environ. pollut.231, 987–996. doi: 10.1016/j.envpol.2017.08.088
59
Ward J. E. Levinton J. Shumway S. E. Cucci T. (1997). Site of particle selection in a bivalve molluscs. Nature390, 31–132. doi: 10.1038/36481
60
Ward J. Levinton J. Shumway S. Cucci T. (1998). Particle sorting in bivalves: in vivo determination of the pallial organs of selection. Mar. Biol.131, 283–292. doi: 10.1007/s002270050321
61
Ward J. E. Rosa M. Shumway S. E. (2019a). Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: a 40-year history. Anthr. Coasts2, 39–49. doi: 10.1139/anc-2018-0027
62
Ward J. E. Shumway S. E. (2004). Separating the grain from the chaff: Particle selection in suspension- and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol.300, 83–130. doi: 10.1016/j.jembe.2004.03.002
63
Ward J. E. Targett N. M. (1989). Influence of marine microalgal metabolites on the feeding behavior of the blue mussel Mytilus edulis. Mar. Biol.101, 313–321. doi: 10.1007/BF00428127
64
Ward J. E. Zhao S. Holohan B. A. Mladinich K. M. Griffin T. W. Wozniak J. et al . (2019b). Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): implications for using bivalves as bioindicators of microplastic pollution. Environ. Sci. Technol.53, 8776–8784. doi: 10.1021/acs.est.9b02073
65
Weber A. Scherer C. Brennholt N. Reifferscheid G. Wagner M. (2018). PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ. pollut.234, 181–189. doi: 10.1016/j.envpol.2017.11.014
66
Woods M. N. Hong T. J. Baughman D. Andrews G. Fields D. M. Matrai P. A. (2020). Accumulation and effects of microplastic fibers in American lobster larvae (Homarus americanus). Mar. pollut. Bull.157, 111280. doi: 10.1016/j.marpolbul.2020.111280
67
Woods M. Stack M. Fields D. Shaw S. Matrai P. (2018). Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. pollut. Bull.137, 638–645. doi: 10.1016/j.marpolbul.2018.10.061
68
Wotton R. S. (1994). “ The biology of particles in aquatic systems,”, 2nd ed. ( CRC Press, Boca Raton).
69
Wright S. L. Rowe D. Thompson R. C. Galloway T. S. (2013). Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol.23, R1031–R1033. doi: 10.1016/j.cub.2013.10.068
70
Xu X.-Y. Fang J. K.-H. Wong C.-Y. Cheung S.-G. (2023). Experimental accumulation of microplastics in acorn barnacle Amphibalanus amphitrite and its use in estimating microplastic concentration in coastal waters. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1081329
71
Xu X. Lee W. Chan A. Lo H. Shin P. Cheung S. G. (2016). Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar. pollut. Bull.124, 798–802. doi: 10.1016/j.marpolbul.2016.12.027
72
Xu Z. Liang Y. Lin S. Chen D. Li B. Li L. et al . (2016). Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr. Microbiol.73, 474–482. doi: 10.1007/s00284-016-1081-1
73
Xu X. Y. Wong C. Y. Tam N. F. Y. Liu H. M. Cheung S. G. (2020). Barnacles as potential bioindicator of microplastic pollution in Hong Kong. Mar. pollut. Bull.154, 111081. doi: 10.1016/j.marpolbul.2020.111081
74
Yaqin K. Hudriyah Rukminasari N. (2023). Microplastic concentrations in green mussel epibiont from Lae-Lae Island Makassar. JSSM18, 110–123. doi: 10.46754/jssm.2023.05.008
75
Ye T. Yang A. Wang Y. Song N. Wang P. Xu H. (2022). Changes of the physicochemical properties of extracellular polymeric substances (EPS) from Microcystis aeruginosa in response to microplastics. Environ. pollut.315, 120354. doi: 10.1016/j.envpol.2022.120354
76
Yu S. P. Nakaoka M. Chan B. K. K. (2021). The gut retention time of microplastics in barnacle naupliar larvae from different climatic zones and marine habitats. Environ. pollut.268, 115865. doi: 10.1016/j.envpol.2020.115865
77
Zhang K. Cheng M. C. Y. Liu M. Xu S. Ma Y. Chau H. S. et al . (2024). Microplastics in Hong Kong’s marine waters: Impact of rainfall and Pearl River discharge. Mar. pollut. Bull.205, 116635. doi: 10.1016/j.marpolbul.2024.116635
78
Zhang Y. Pedersen J. N. Eser B. E. Guo Z. (2022). Biodegradation of polyethylene and polystyrene: From microbial deterioration to enzyme discovery. Biotechnol. Adv.60, 107991. doi: 10.1016/j.bioteChadv.2022.107991
79
Zhang T. Song K. Meng L. Tang R. Song T. Huang W. et al . (2022). Distribution and characteristics of microplastics in barnacles and wild bivalves on the coast of the Yellow Sea, China. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.789615
80
Zhao S. Ward J. E. Danley M. Mincer T. J. (2018). Field-based evidence for microplastic in marine aggregates and mussels: Implications for trophic transfer. Environ. Sci. Technol.52, 11038–11048. doi: 10.1021/acs.est.8b03467
Summary
Keywords
biofilm, microplastic pollution, barnacles, bioindicator, microplastic ingestion
Citation
Wang Y, Wong ACY, Chiu JMY and Cheung SG (2025) Non-selective feeding on microplastics in the acorn barnacle Amphibalanus amphitrite: the implications in assessing barnacles as global microplastics bioindicators. Front. Mar. Sci. 12:1646294. doi: 10.3389/fmars.2025.1646294
Received
13 June 2025
Accepted
29 September 2025
Published
15 October 2025
Volume
12 - 2025
Edited by
Julius A. Ellrich, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), Germany
Reviewed by
Imran Hashmi, Namibia University of Science and Technology, Namibia; Khusnul Yaqin, Hasanuddin University, Indonesia
Updates
Copyright
© 2025 Wang, Wong, Chiu and Cheung.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siu Gin Cheung, bhsgche@cityu.edu.hk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.