- Reefscapers, Male, Maldives
Coral reefs support a vast diversity of marine life but are increasingly threatened by anthropogenic activities. To assess changes in community composition and the recovery potential of coral reefs in a changing climate, documenting sexual reproduction and understanding its environmental drivers are essential. Here, we report the exact timing and reproductive strategies for 375 individual coral colonies across 28 species from 10 genera between February 2024 and April 2025 at a near-shore reef in Baa Atoll, Maldives. We identify two peak periods of multi-specific spawning, coinciding with the monsoonal transition seasons. We note variations in spawning patterns within and between taxa, documenting the occurrence of extended breeding via asynchronous spawning of colonies within a species and split-spawning of individual colonies across lunar months and bi-annual seasons. Our results show that larger spawning events in a given month are significantly correlated with lower wind speeds, higher sea surface temperatures, and higher levels of solar insolation. These findings highlight the influence of environmental cues on the timing and extent of coral reproduction and provide the first detailed spawning records for several non-Acropora genera in the Maldives, contributing a critical baseline for future research.
Introduction
Corals are autogenic ecosystem engineers; the complex structures they build support a vast diversity of marine life and provide key services such as fisheries, coastal protection, tourism and recreational activities (Grafeld et al., 2017; Graham and Nash, 2013; Woodhead et al., 2019). Globally, coral reefs are threatened by anthropogenic stressors such as ocean acidification, pollution and climatic changes (Emslie et al., 2024; Frölicher et al., 2018; Hoegh-Guldberg, 1999; Hughes et al., 2017, 2003; Kleypas et al., 1999; Spalding and Brown, 2015). Ocean warming events are a particularly prevalent threat to coral reefs, as elevated sea surface temperatures (SST) can disrupt the reproductive cycles and spawning synchrony of corals (Paxton et al., 2016; Shlesinger and Loya, 2019) and can cause bleaching-induced mortality on large scales (Baker et al., 2008; Bruno and Selig, 2007; Hughes et al., 2018; Perry and Morgan, 2017a). Whilst coral bleaching can reduce a colony’s immediate growth rates, reproductive output, and larval supply, studies have shown coral reefs have the capacity to recover from mass bleaching events (Cantin and Lough, 2014; Donati et al., 2025; Gilmour et al., 2013; Gold and Palumbi, 2018; Gouezo et al., 2019; Graham et al., 2024; Pisapia et al., 2016). Reef recovery and repopulation is driven by the growth and reproduction of surviving colonies and the successful settlement of recruits (Adjeroud et al., 2017; Gilmour et al., 2013; Johnston et al., 2020). Hence, understanding the reproductive patterns of coral at the colony level is key for assessing how communities restructure following disturbances and for evaluating impacts on ecosystem function.
Reef-building corals reproduce by brooding or, for most species, via broadcast spawning events in which gametes are released into the water column for external fertilisation at the surface (Harrison, 2011). The success of broadcast reproductive events is dependent on synchronous release of gametes between colonies to enable cross-fertilisation (Levitan, 2004; Oliver and Babcock, 1992; Willis et al., 1985). The timing and extent of synchronous broadcast spawning events can vary greatly within and between taxa and biogeographic regions (Baird et al., 2022, 2009; Gouezo et al., 2020; Harrison, 2011; Harrison and Wallace, 1990; Mangubhai and Harrison, 2008; Moritz et al., 2025; Sheridan et al., 2025). An expansion of research on coral spawning in recent years has revealed reproductive patterns ranging from the highly synchronised mass spawning of over 100 different coral species as documented on the Great Barrier Reef (Babcock et al., 1986; Harrison et al., 1984; Oliver et al., 1988; Willis et al., 1985), to temporal reproductive isolation of 24 coral species studied in the Gulf of Eliat (Shlesinger et al., 1998; Shlesinger and Loya, 1985). Between these two extremes are records of multi-specific spawning (Monfared et al., 2023), asynchronous spawning (Gouezo et al., 2020), and extended breeding seasons (Mangubhai and Harrison, 2008) in other regions (summarised in Harrison, 2011). It has been hypothesised that there is a breakdown of spawning synchrony at low latitudes due to weaker fluctuations in environmental conditions that enable protracted breeding seasons (Oliver et al., 1988), as supported by studies on Kenyan reefs (Mangubhai and Harrison, 2008). However, synchronous spawning has been recorded in other equatorial regions (Gilmour et al., 2016; Gouezo et al., 2020; Monfared et al., 2023; Sheridan et al., 2025; Sola et al., 2016; Wijayanti et al., 2019).
The timing of sexual reproduction of reef-building corals has been linked to various environmental factors such as sea temperature (Keith et al., 2016; Lin and Nozawa, 2023; Nozawa, 2012; Monfared et al., 2023; Sheridan et al., 2025), solar insolation (Penland et al., 2004; van Woesik et al., 2006), regional wind fields (van Woesik, 2010), moon phase, sunrise (Bronstein and Loya, 2011; Moritz et al., 2025), and sunset (Baird et al., 2022; Lin and Nozawa, 2017). Whilst knowledge of coral reproduction has expanded in recent years, fundamental background information on the timing of spawning events is limited in some regions. Elucidating our understanding of spawning patterns across vast geographic scales is key for assessing the reproductive phylogeny of scleractinian corals and hence the impacts of climatic changes and the potential for recovery of coral reef ecosystems.
The Maldivian archipelago is considered the world’s seventh largest reef system (MEE, 2015). With 80% of all land area lying less than 1 metre above sea level, the islands that comprise the Maldives rely heavily on the 2,041 distinct coral reefs identified for protection, and provision for the population (Dhunya et al., 2017; Dryden et al., 2020; Naseer and Hatcher, 2004). In the Maldives, observations of asynchronous coral spawning in diverse assemblages over multiple lunar phases have been reported (Harrison and Hakeem, 2007). Monfared et al. (2023) noted extended breeding seasons of 22 species of Acropora over an 8 month period, detailing peak multi-specific spawning events coinciding with the transitional monsoon period, and asynchronous spawning between Acropora species and between colonies of the same species over multiple lunar phases. Further, Sheridan et al. (2025) assessed the degree of spawning synchrony of Acropora and found that larger, multi-specific spawning events occur more frequently than single species spawning events. These studies reported exact timings of Acropora spawning and correlated earlier spawning events relative to the full moon with lower tide depths, wind speeds, daily precipitation and higher sea surface temperatures.
However, to date, knowledge of spawning patterns and exact spawning times of non-Acropora corals is comparatively limited. Increasingly, restoration initiatives are utilising sexual reproduction for reef replenishment, including larval propagation, assisted fertilisation and selective breeding (Dela Cruz and Harrison, 2020; Harrison, 2024a, 2024b; Humanes et al., 2021; Koch et al., 2022; Pollock et al., 2017; Randall et al., 2020). Such techniques rely on accurately predicting when corals will spawn; advancing knowledge on the months of spawning and exact time of spawning for each region is pivotal for success and optimization of these initiatives. With both asexual and sexual restoration methods practised widely across the Maldives, understanding reproductive patterns of corals at the assemblage level is key for managing reef recovery via restorative intervention and for predicting the extent of community shifts following bleaching events. This study provides key baseline data for restoration initiatives utilising sexual reproduction. Here, we document the exact timing and sexual reproductive strategies for 375 individual colonies of 10 different genera of corals between February 2024 and April 2025. Data collection occurred before, during, and following the fourth global bleaching event confirmed by the International Coral Reef Initiative (International Coral Reef Initiative, 2024). This study describes two peak seasons for multispecific spawning events in Baa Atoll [as first identified for Acropora by Monfared et al. (2023)], as well as taxon-specific differences in reproductive synchronicity, noting instances of annual and bi-annual spawning, split spawning of individual colonies of various genera over multiple days in a lunar month and over multiple lunar months in a year. To date, this study is the first to report exact spawning times of non-Acropora genera in the Maldives.
Materials and methods
Survey site
Coral spawning surveys were conducted from February 2024 to April 2025 on a near-shore reef on the southern side of Landaa Giraavaru, a resort island in Baa Atoll, Maldives (5.2862°N, 73.1121°E). The “House Reef” site, approximately 1,300m2 in area, was chosen for surveys based on accessibility from the shore and due to the higher diversity of coral genera present on this reef in comparison to other sites. The depth range of colonies surveyed was 1m-4m.
Reproductive maturity assessments
To inform for which lunar months nightly surveys would be undertaken, reproductive maturity of colonies at the survey site was assessed. For colonies of Acropora, an approximately 5cm fragment was removed from the colony base using scissors; for Galaxea, a single polyp was excised similarly to check for the presence or absence of gametes (Baird et al., 2002; Craggs, Personal Communication, August 26, 2024; Monfared et al., 2023). Maturity assessments of Acropora colonies coincided with ongoing coral restoration activities to reduce negative ecological impacts, and between four and seven randomly selected Galaxea colonies were surveyed in the week prior to the full moon each month.
Additional opportunistic reproductive maturity assessments were conducted on colonies of Astrea, Cyphastrea, Goniastrea and Leptoria at restoration sites. Small fragments from these colonies were removed using a hammer and a fine chisel, and examined for the presence of gametes using a microscope (Adonstar AD407 Digital Microscope) (Craggs, Personal Communication, August 26, 2024; Whiting, Personal Communication, June 21, 2024). Fragmentation of these genera for maturity assessments did not take place between March 2024 and June 2024 to avoid causing stress to colonies during the ongoing coral bleaching event (International Coral Reef Initiative, 2024; Reefscapers, 2024). From June 2024 onwards, only a proportion of colonies that remained healthy throughout the bleaching event were fragmented. Other genera later identified spawning were not fragmented to assess reproductive maturity due to time constraints, and as some had very small polyps that made positive gamete identification difficult.
Colonies with gametes identified were noted and mapped on a slate for observer reference, but not tagged at this stage; reproductive maturity data was used only to inform when field surveys should take place and was not included in analysis.
Spawning surveys
As spawning events are typically documented in accordance with the lunar cycle (Babcock et al., 1994; Komoto et al., 2023), nightly surveys to check for signs of coral spawning were conducted around the full and new moons. Upon observation of mature gametes in any species, nightly surveys would take place from two days prior to the full moon (FM) (FM-2) until seven days after the full moon (FM + 7), and from two days prior to the new moon (NM) (NM-2), until two days after the new moon (NM + 2) to check for signs of coral spawning. Therefore, resulting surveys occurred in March, April, October, November, and December 2024, and in March and April 2025. As spawning has been observed over multiple lunar phases (Mangubhai and Harrison, 2008; Gouezo et al., 2020), opportunistic surveys (where weather and staffing constraints allowed) in months where no mature gametes had been observed took place. Where possible, “full surveys” from FM-2 until FM + 7 and from NM-2 until NM + 2 took place, but if not possible “short surveys” from FM-1 until FM + 4 took place. Such opportunistic “full surveys” occurred in February, May, and September 2024. Opportunistic “short surveys” took place in June 2024 and in January 2025.
Nightly surveys were conducted by a minimum of two observers via SCUBA or freediving from 19:00-20:30 local time, to encompass known spawning periods of Acropora (Monfared et al., 2023) and anticipated spawning times of massive and encrusting genera (Baird et al., 2021). Observers would swim along a course routinely taken on the reef and check colonies in which mature gametes had been noted, as well as other corals from various genera for “setting” (mature gametes visible in the mouth of the polyp prior to release) or spawning (Monfared et al., 2023). If observed, divers would remain in the water until the release of gametes was complete for all colonies. Accurate time of spawning onset was obtained by placing surveyors in a specified area of the routinely taken course and ensuring setting colonies were frequently checked to document the moment of gamete release. In the event that a colony was observed setting but spawning onset was not recorded, the colony was fragmented the following day. If a loss of gametes was recorded then we would deduce that the colony had spawned (Monfared et al., 2023).
During nightly surveys, spawning colonies were temporarily tagged with numbered cloth ties (tied on to nearby stable substrate) for ease of identification the following day. The following data was collected for each colony in-situ using dive slates: cloth tie number, colony ID (if a permanent identification tag was already present on the spawning colony), time at which setting was first observed, exact time of spawning onset, depth, genus, species (if known), location on the reef relative to coral frames planted for restoration activities (each with GPS coordinates noted), and reproduction type (hermaphroditic/gonochoric, specifying egg/sperm release where known). Where time constraints allowed, pictures of spawning colonies were also taken with a digital camera for reference (Olympus Tough TG-6 digital camera) (Figure 1).

Figure 1. Images to show setting and spawning in various coral species. (A) Spawning in Goniastrea retiformis. Photograph by Amelia JF Errington. (B) Setting in Astrea curta. Photograph by Amelia JF Errington. (C) A bundle ready for release in Goniastrea pectinata. Photograph by Amelia JF Errington. (D) Spawning in Cyphastrea micropthalma. Photograph by Amelia JF Errington. (E) Spawning in Dipsastrea matthai. Photograph by Amelia JF Errington. (F) Bundles of eggs ready for release in Galaxea fascicularis. Photograph by Amelia JF Errington. (G) Setting and onset of spawning in Montipora tuberculosa. Photograph by Kate P Moody. (H) Spawning in Echinopora spp. Photograph by Amelia JF Errington.
Spawning and environmental condition database
The following day, colonies spawning the night prior were located and temporary cloth ties were removed. If a colony already had a permanent identification (ID) tag, no further information would be collected. Colonies recorded spawning for the first time were given a permanent ID tag with a unique colony ID for future monitoring of spawning events at the individual colony level. Five high-quality pictures of each colony would be taken: ID tag in-shot with the colony, and a wide-shot, mid-shot, close-up and scaled-shot of the colony for positive identification to genus level (and species where possible) using the Indo-Pacific Coral Finder (Kelley, 2022) and Corals of the World (Veron et al., 2025). These images were stored in a photo ID database for reference. Genetic testing would be required to confirm species identification but was not done for this study.
The unique colony ID was input into a spawning database along with the data collected from the night of spawning so reproductive patterns of individual colonies could be followed over time. Additional data input included the date of spawning, the day of spawning relative to the nearest full moon, and the lunar month of spawning (eg the March 2024 full moon (FM) was on 25th March, but spawning was recorded on April 1st (FM + 7); for these colonies, lunar month of spawning was recorded as March). The environmental factors solar radiation and wind speed were obtained using a weather station located on Landaa Giraavaru (Gill Instruments GMX 500 Maximet). Moon phase (Moon Giant, 2025), average daily sea surface temperature (SST) (Sea Temperatures, 2025), sunset time, and depth of the low tide closest to sunset (Tideschart, 2025) on days where spawning occurred were gathered for Baa Atoll. The day of spawning relative to the full moon was recorded and spawning time in minutes after sunset for each colony was calculated. Average monthly SST, wind speed and solar insolation were calculated across all days of each calendar month for the duration of the study.
Statistical analysis
To assess whether environmental factors such as sea surface temperature (SST), wind speed, and solar insolation influence the occurrence of coral spawning, a generalized linear model (GLM) with a binomial distribution and logit link was fitted. Spawning occurrence was used as a binary response variable, coded as 1 if spawning of any species/genus occurred in a given month and 0 if it did not. Univariate models were fitted separately for each predictor variable: average SST, average wind speed, and average solar insolation, each measured during the month preceding the spawning event. Model fit was assessed using residual deviance, Akaike Information Criterion (AIC), and pseudo-R² statistics.
To examine whether environmental variables influence the proportion of colonies spawning each month, beta regression was fitted. The monthly proportion was calculated as the number of colonies of any genus/species to spawn in a given month divided by the total number of spawning events recorded throughout the study period of all species/genera combined. Three univariate beta regressions were conducted using average SST, wind speed, and solar insolation (per lunar month) as explanatory variables. Model diagnostics, including Pearson residual plots, Q-Q plots, and pseudo-R², indicated that beta regression assumptions were adequately met. Multicollinearity among predictors was assessed, and a strong negative correlation between SST and wind speed (r = -0.71) was identified, which prevented multivariable modeling. Final model selection was based on AIC values.
Results
A total of 449 observations of spawning from 375 individual colonies of 10 different genera (Acropora (N = 162), Astrea (N = 9), Astreopora (N = 16), Cyphastrea (N = 21), Dipsastrea (N = 20), Echinopora (N = 1), Galaxea (N = 60), Goniastrea (N = 134), Montipora (N = 25) and Porites (N = 2)) were recorded at Landaa Giraavaru, Baa Atoll, between February 2024 and April 2025 (Table 1). The majority of studied coral species (99.3% of colonies observed) released gametes in multi-specific spawning events during two peak seasons; in March and April (N = 326) and between October and December (N = 120) (Figure 2).
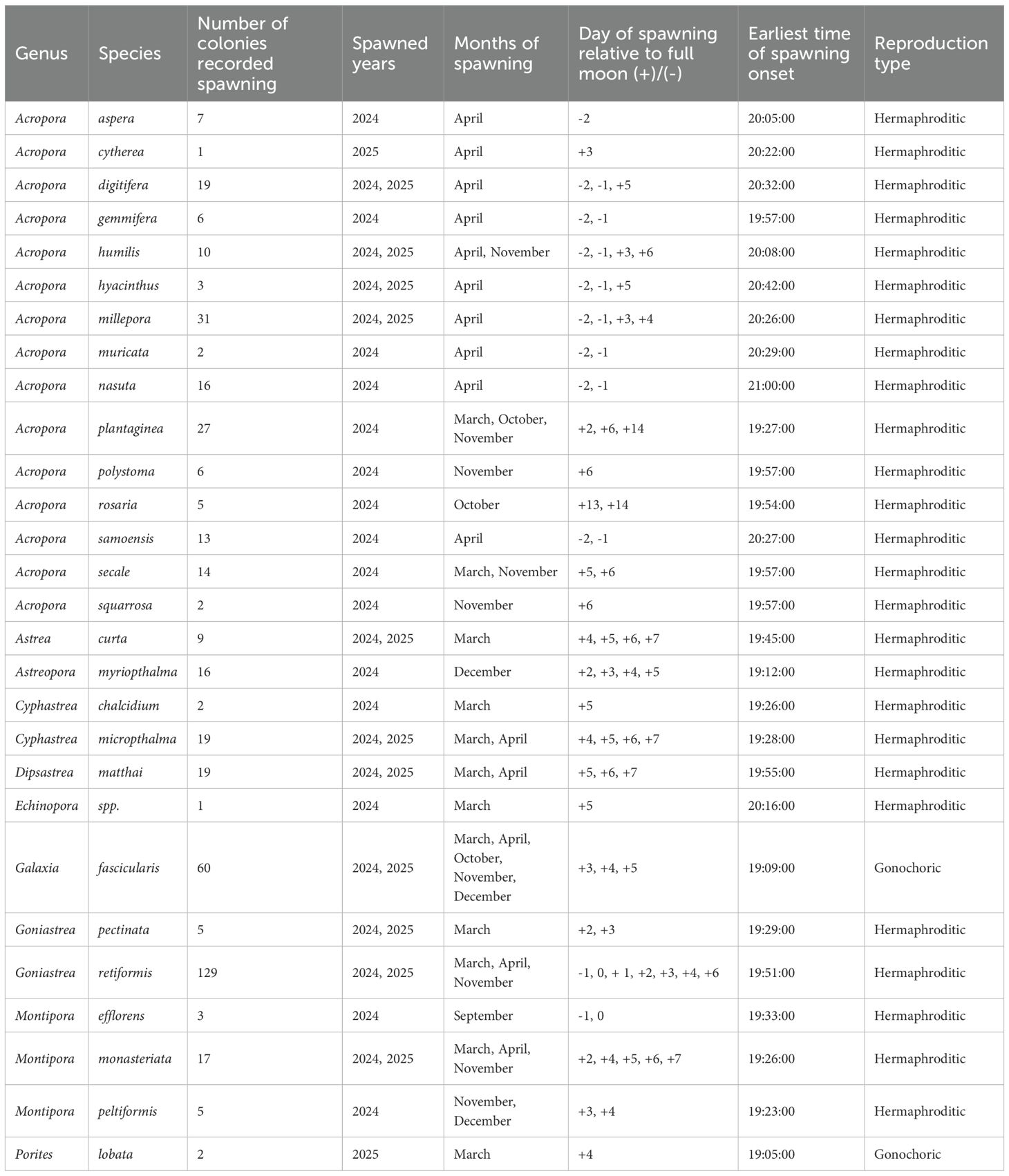
Table 1. A summary of spawning records from Baa Atoll, showing the number of colonies, years and month of spawning, day of spawning relative to full moon and earliest time of spawning onset for each species.
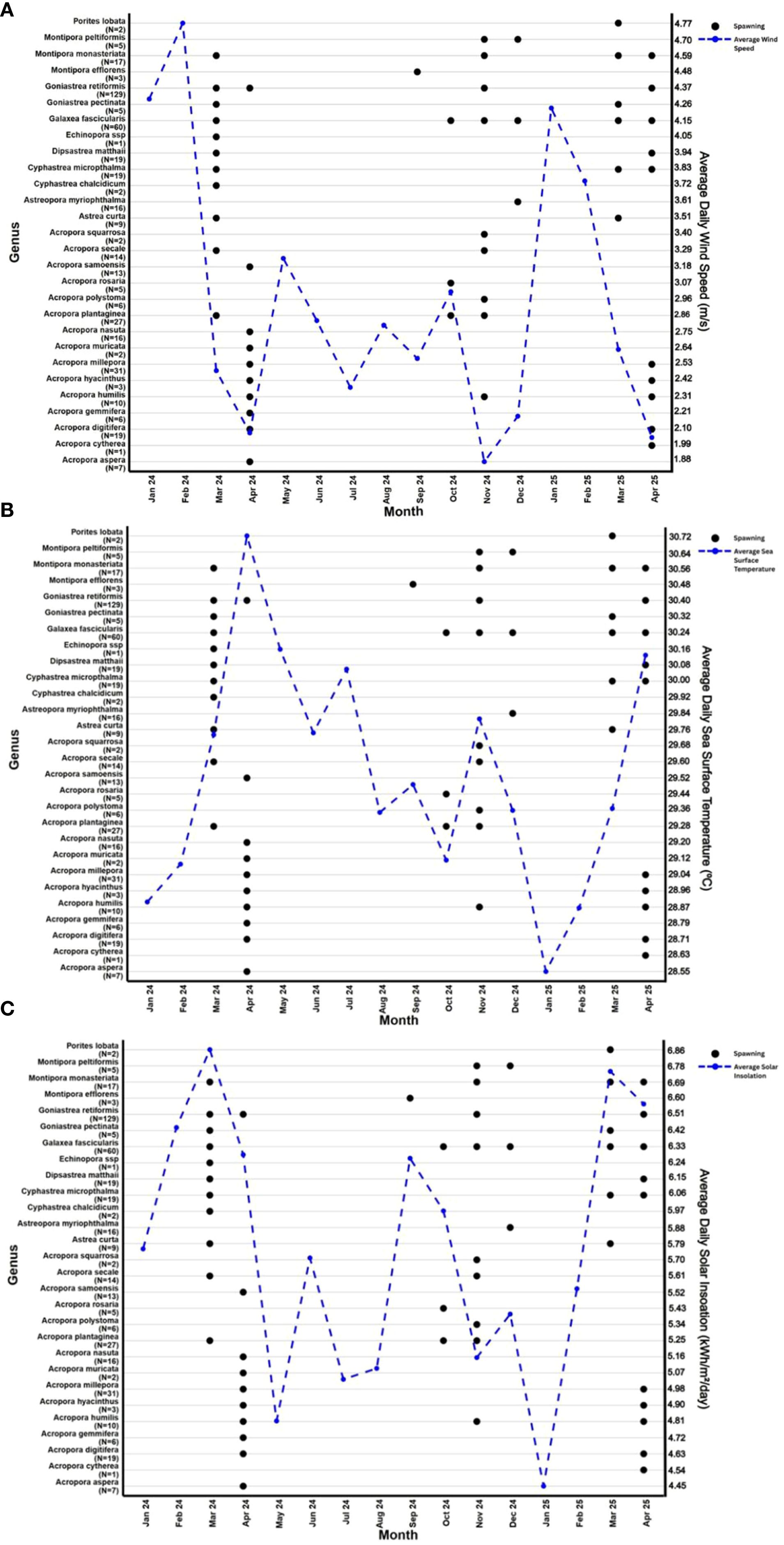
Figure 2. Monthly coral spawning observations by genus overlaid with daily averages of environmental variables. Back circles refer to months in which spawning occurred for each genus. Blue circles indicate the monthly average values of environmental factors, wind speed (m/s) (A), sea surface temperature (°C) (B) and solar insolation levels (kWh/m²/day) (C). The number of spawning observations for each genus is shown on the y-axis as N= X.
All colonies recorded spawning released gametes approximately one to three hours after sunset (Figure 3) within a few days of the full moon (Figure 4). Depending on the taxa, spawning occurred as a single event or extended spawning patterns (via asynchronous spawning amongst colonies of a species and/or split spawning of individual colonies) was observed.
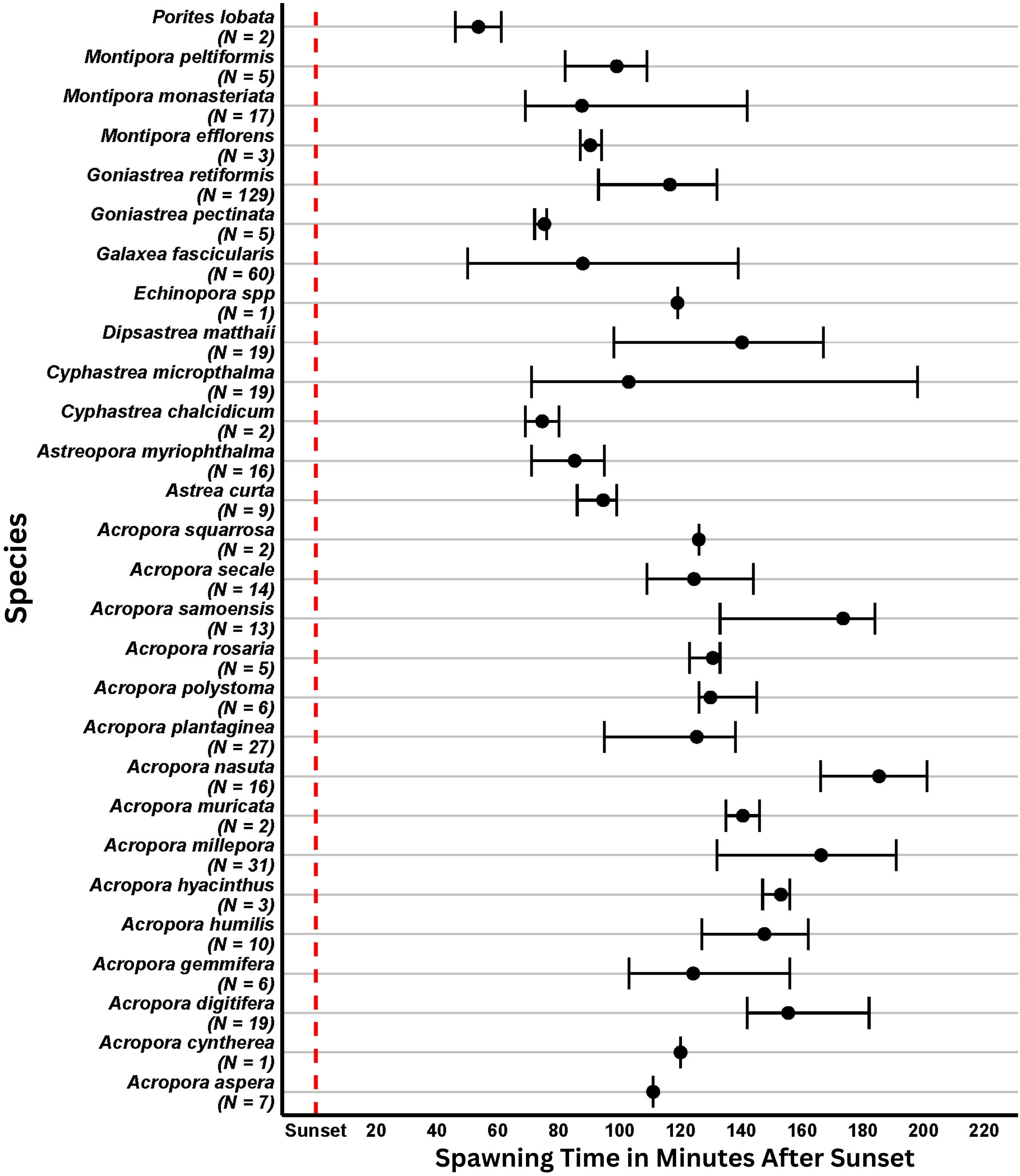
Figure 3. The spawning time relative to sunset for various coral species. The black circles represent the mean observed spawning time for each species recorded. The associated error bars display the range of time spawning was observed across all individual colonies of that species. The number of spawning colonies for each species was totaled and placed under the y-axis labels, in the form of (N=X).
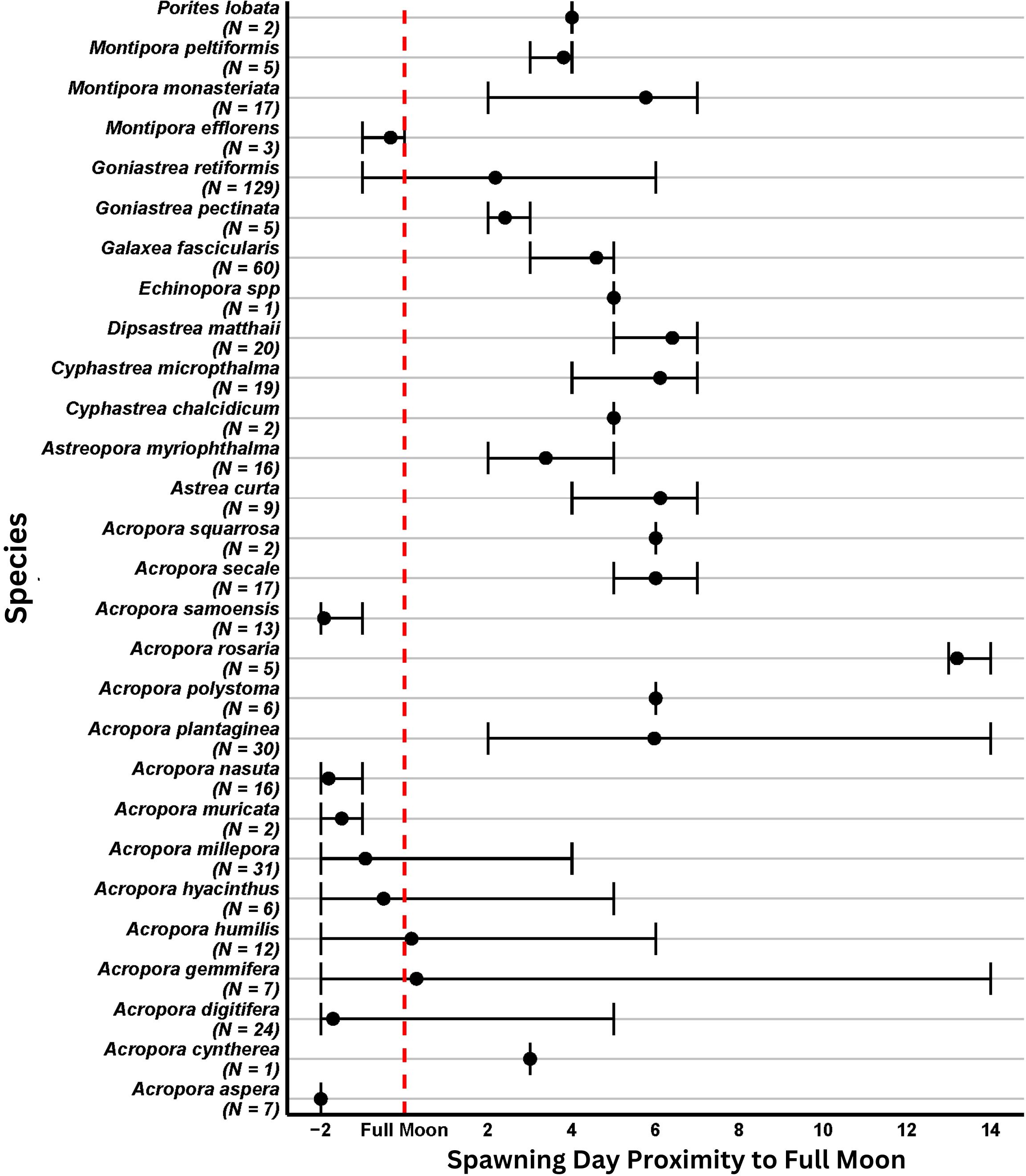
Figure 4. The spawning day relative to the closest full moon. The black circles represent the mean observed day that spawning occurred per species. The associated error bars indicate the range of days in which colonies of each species spawned relative to the nearest full moon. The number of colonies recorded spawning for the duration of this study is noted in the y axis (N=x).
Neither SST (p = 0.200) nor wind speed (p = 0.071) were statistically significant predictors of the probability of spawning occurrence in the binomial GLM (Table 2). However, solar insolation was a significant predictor (p = 0.047), with a positive association (β = 2.079 ± 1.046), indicating that higher solar insolation increased the likelihood of spawning occurring in a given month.
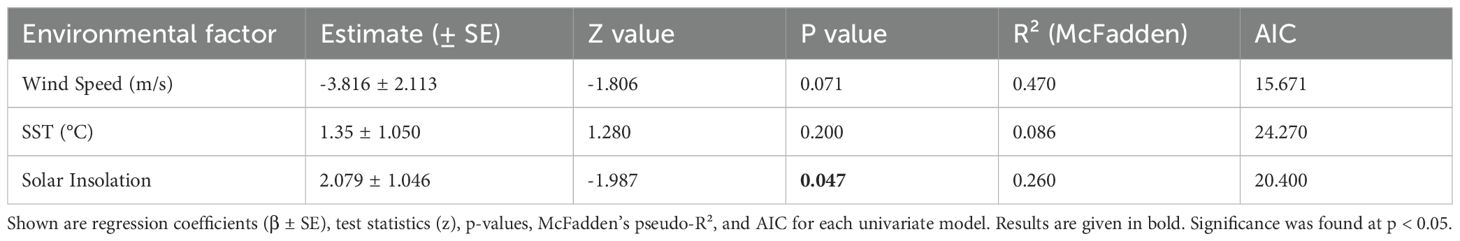
Table 2. Results of binomial generalised linear models to explore the influence of environmental conditions (sea surface temperature, wind speed and solar insolation) on the occurrence of coral spawning.
In the beta regression analysis, all three environmental variables (average SST, wind speed, and solar insolation) were found to significantly influence the proportion of colonies spawning per month (Figure 5). Average wind speed had the strongest effect (β = –0.709 ± 0.219, p = 0.001), with a negative association, suggesting that higher wind speeds were associated with a lower proportion of colonies spawning (Table 3). SST also showed a significant positive effect (β = 0.701 ± 0.304, p = 0.021), indicating increased spawning with higher SST. Solar insolation was similarly positively associated with the proportion of spawning events (β = 0.585 ± 0.250, p = 0.019).
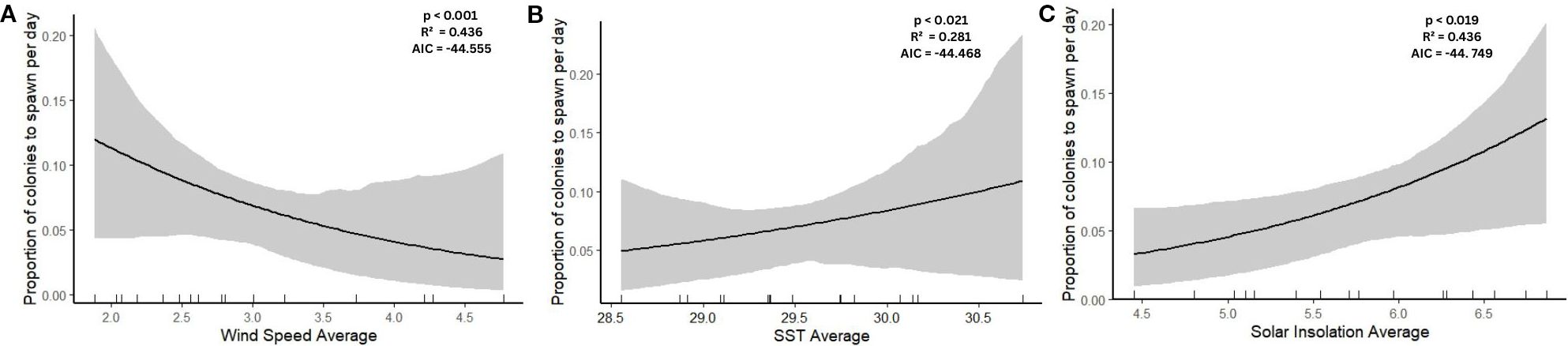
Figure 5. Effect of environmental conditions on the proportion of colonies to spawn per month based on the results of beta regression model. The explanatory variables included in the model are average monthly (A) wind speed (m/s), (B) sea surface temperature (SST) (°C) and (C) daily solar insolation (kWh/m²/day). The black line indicates the relationship between the environmental variables and proportion of colonies to spawn. The grey shading indicates the 95% confidence interval. Rug marks along the x-axis displays the distribution of observed data points. P-values, AIC and R2 are exhibited within each panel.
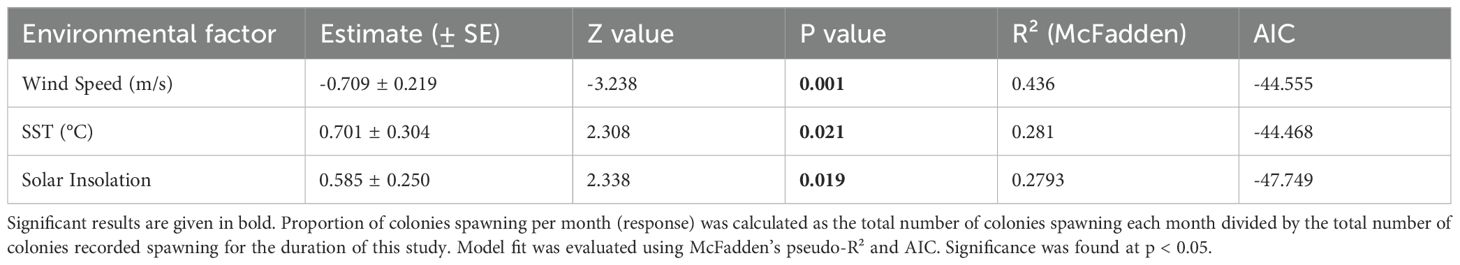
Table 3. Results of beta regression models testing the effects of environmental conditions (sea surface temperature, wind speed and solar insolation) on the proportion of colonies to spawn each month.
Species-specific spawning patterns observed
Spawning patterns differed within and between species of Acropora. Some species spawned in both peak seasons (A.gemmifera, A.humilis, A.plantaginea and A.secale) via asynchronous spawning of different colonies or via individual colonies undergoing multiple gametogenic cycles in a year. Some species spawned only in March/April (A.aspera, A.digitifera, A.hyacinthus, A.millepora, A.muricata, A.nasuta and A.samoensis) and some species spawned only between October and December (A.polystoma, A.rosaria and A.squarrosa).
Colonies of Galaxea fascicularis (N = 60) exhibited varied spawning patterns. We commonly noted individual colonies spawning over consecutive days and months in a year. Asynchronous spawning within the population was also documented, as some colonies spawned only in March/April (29%) and some only between October and December (39%). Bi-annual spawning was also recorded as some colonies (32%) spawned in both peak seasons.This species exhibits pseudo-gynodioecious reproduction; we noted that, for the duration of this study, colonies first recorded spawning one gender would spawn the same gender in future reproductive events.
Colonies of Goniastrea retiformis were observed spawning in both peak seasons. In contrast to many other genera, spawning was noted in only one month of each peak season; in March 2024 (N = 55), November 2024 (N = 11), and April 2025 (N = 68). No split-spawning over consecutive lunar months was noted, and release of gametes only once per year was recorded for each colony. In some colonies, spawning would occur over consecutive days over the full moon period.
Cyphastrea micropthalma, Cyphastrea chalcidium, Dipsastrea matthai, Echinopora spp., Goniastrea pectinata and Porites lobata released gametes in multi-specific spawning events in March/April. Spawning of Astreopora myriopthalma colonies occurred as a multi-species event following the December full moon.
Montipora species were observed spawning in multiple months of the year, with inter-specific spawning asynchrony noted but synchronised spawning within species. Colonies of M. monasteriata (N = 17) spawned predominantly in March/April, with only one colony recorded spawning in November. Colonies of M.peltiformis (N = 5) spawned in November and December. Colonies of Montipora efflorens (N = 3) released gametes outside of the identified peak spawning seasons, spawning in September 2024 as a singular event.
Multi-specific spawning
Multispecific spawning events coincided with sharp seasonal declines in average wind speed (Figure 2A), which occurred consistently each year in March and November. Multispecific spawning events also occurred in April during periods of declining wind speeds (2.50 to 2.08 m/s). A smaller multispecies spawning event was noted in December during a period of increasing wind speed (1.88 to 2.19 m/s).
Solar insolation levels revealed two main peaks a year; in March and a smaller peak in September. Multi-specific spawning events occurred during these peaks and were additionally observed during the subsequent months, occurring during periods where solar insolation declined (Figure 2C). There was no coral spawning observed during the months containing the lowest solar insolation levels or during periods when solar insolation was increasing towards that of dual maxima.
SST rises and falls in a biannual cycle, with distinct peaks in April and November (Figure 2B). The largest multispecific spawning events coincided with sharp rises and/or peaks in SST in March, April and November. Spawning was also observed in the months surrounding these maxima, extending from October through to December.
Discussion
The results of this study expand on previous observations of Acropora coral spawning in the Maldives (Monfared et al., 2023; Sheridan et al., 2025) and provide new information about the exact time of spawning onset and months of spawning for 13 species of 12 genera of non-Acropora corals in this region. We support the identification of two peak spawning seasons in the Maldives by Monfared et al. (2023) in March and April and between October and December, in which large, multispecific spawning events occur. We note variations in spawning patterns within and between taxa, documenting the occurrence of extended breeding via asynchronous spawning of colonies within a species and split-spawning of individual colonies across lunar months. We also note the occurrence of spawning as an annual or bi-annual event for individual colonies, demonstrating that the number of annual reproductive cycles and degree of spawning synchrony varies among taxa in this region. Our data suggests varied and complex patterns of reproduction and provides crucial foundational observations of coral spawning in the Maldives, a data-deficient region where little is known about the reproduction of non-Acropora corals.
Varied spawning patterns were observed between taxa in this study. Goniastrea retiformis colonies spawned in only one peak season per year. Temporal segregation between March/April and November spawning cohorts, coupled with a single gametogenic cycle annually may reduce gamete encounter rates and the chance of successful fertilisation (Shlesinger and Loya, 2019). Conspecific coral populations that spawn in different seasons may become reproductively isolated and experience limited gene flow, which over time can lead to genetic divergence (Rosser, 2015; Rosser et al., 2020). While our single-year dataset limits long-term inferences, these findings warrant further investigation into the genetic structure of G. retiformis populations in the Maldives. Region-wide data collected over extended time periods is needed to assess the degree of temporal segregation and potential for genetic divergence in this population.
Contrastingly, spawning of individual colonies over bi-annual spawning seasons was noted in Galaxea fascicularis and some colonies of Acropora humilis, A.plantaginea and A.secale. Split-spawning, where coral colonies release gametes over consecutive lunar months, was commonly noted in colonies G.fascicularis, for some species of Acropora, and for Cyphastrea micropthalma. This reproductive strategy has been widely documented across multiple taxa and regions (Baird et al., 2022, 2009; Bastidas et al., 2005; Bauman et al., 2011; Foster et al., 2018; Hock et al., 2019; Kongjandtre et al., 2010; Moritz et al., 2025; Willis et al., 1985). Split-spawning may lead to multiple smaller or staggered spawning events, reducing reproductive synchrony and gamete encounter rates, and increasing the vulnerability of gametes released to predation (Oliver and Babcock, 1992). However, whilst large spawning events maximise gamete encounter rates, at high gamete densities polyspermy can reduce fertilisation success (Levitan, 2004); as such, split-spawning may increase local fertilisation success by preventing gamete saturation. Further, by releasing gametes over multiple days following the full moon and in consecutive lunar months, the risk of total reproductive failure in a single event is reduced (Babcock et al., 1986; Harrison et al., 1984). Split-spawning can enable corals to realign reproductive timing with favourable environmental conditions, particularly when lunar cycles may cause spawning to occur outside optimal windows (Foster et al., 2018; Hock et al., 2019). Split-spawning is often associated with suboptimal environmental conditions in the lead-up to the full moon, or when thermal or photic thresholds are not met synchronously across coral populations (Baird et al., 2009; Harrison et al., 1984; Keith et al., 2016; Nozawa et al., 2015; Sweeney et al., 2011). Foster et al. (2018) noted that years with slower seasonal rises in SST showed increased incidences of split spawning, suggesting strong environmental cues may drive more synchronised spawning in a single lunar cycle. As such, corals in tropical regions where seasonal changes are less distinct may show less synchronised spawning. Split-spawning has been documented on the Great Barrier Reef (GBR) and in Western Australia most commonly when the full moon falls in the first half of the month (Baird et al., 2011; Gilmour et al., 2009; Willis et al., 1985), but can also occur when the full moon falls in the second half of the month (Babcock et al., 1986; Foster et al., 2015). Our study shows that split-spawning occurs in G.fascicularis and Acropora when the full moon falls in both the first and second half of the month, supporting observations of no consistent relationship between split-spawning and full moon timing. Long-term studies have shown that split-spawning is not an annual feature but rather occurs periodically (Foster et al., 2018), but recent research on daytime spawning corals has documented individual colonies of Porites rus spawning over five consecutive months each year (Moritz et al., 2025); as such, more data collected over extended time periods would be needed to identify the extent, environmental drivers, and impact on successful fertilisation of split-spawning in this region.
It has been hypothesised that equatorial reefs exhibit extended breeding seasons due to weaker environmental seasonality enabling multiple spawning events in a year (Oliver et al., 1988). Whilst the results of some studies support this hypothesis (Mangubhai and Harrison, 2008), others at similar latitudes have countered it (Baird et al., 2002; Chelliah et al., 2015; Guest et al., 2005b; Monfared et al., 2023; Gouezo et al., 2020; Novriansyah et al., 2023; Penland et al., 2004; Sola et al., 2016; Wijayanti et al., 2019). Bouwmeester et al. (2021) used data from 90 sites across the Indo-Pacific and found no correlation between latitude and reproductive synchrony, indicating that other factors drive reproductive synchronicity. In the Maldives, Monfared et al. (2023) noted that Acropora corals spawn over an extended period of eight months of the year. Further analysis of the degree of spawning synchrony found that Acropora corals in the Maldives spawn most frequently and in greater numbers during multispecific spawning events than they do in single species events, with 95.1% of colonies in Baa Atoll and 96.7% of colonies in North Male Atoll spawning during multispecific events (Sheridan et al., 2025). Our study concurs with these findings and reveals that previously unstudied genera at the same site in Baa Atoll also follow similar patterns, indicating similar environmental drivers of spawning.
The timing of peak spawning seasons in the Maldives coincide with the transition between the southwest and northeast monsoon, characterised by a reversal in wind direction and current (Aleem, 2013; Litster, 2016; Schott et al., 2009; Su et al., 2021). Similar patterns have been noted on other equatorial reefs (Wijayanti et al., 2019) and a global analysis spanning seven regions by van Woesik (2010) revealed that regional periods of low wind speeds were often tightly coupled with mass spawning events. Transitional periods of reduced wind intensity offer evolutionary advantages for coral reproduction by increasing successful fertilisation (Babcock et al., 1994) and enhancing larval retention, ultimately supporting local recruitment and population resilience (Elmhirst et al., 2009). However, low current (induced or not by wind) may limit the dispersal of larvae to other reef systems, reducing larval exchange between reef systems and genetic mixing, impacting metapopulation resilience (Cowen et al., 2000; Jones et al., 2009). As an archipelago comprising approximately 1,192 islands spread across 26 geographic atolls (Maldives National Statistical Bureau, 2020), more research from different regions is needed to assess the extent of larval dispersal and genetic connectivity in the Maldives.
In this study, while wind speed was not a significant predictor of the occurrence of spawning in a given month, it had the strongest influence on the proportion of colonies spawning each month. The largest multispecific spawning events observed occurred in March, April and November (annually); periods that displayed abrupt drops in average wind speed. Notably, these months coincided with the transitional periods between monsoon seasons in the Maldives. The Hulhangu (Southwest) monsoon occurs from May to September, while the Iruvai (Northeast) monsoon occurs from December to February (Aleem, 2013). These results are consistent with equatorial studies conducted in Palau (Penland et al., 2004), the Karimunjawa Archipelago in Indonesia (Wijayanti et al., 2019), Singapore (Guest et al., 2005a) and in Baa and North Malè Atolls in the Maldives (Monfared et al., 2023; Sheridan et al., 2025), all of which documented large multispecific coral spawning during the inter-monsoonal periods. These transition phases mark distinct and pronounced environmental shifts that may act as major spawning cues for gamete release in regions where wind conditions are rarely calm (van Woesik, 2010), and where changes in SST and solar insolation are less pronounced than in reefs at higher latitudes.
The duration of the gamete release is understood to be correlated with sustained periods of low wind speeds (van Woesik, 2010). A study on Kenyan reefs at a similar latitude to this study (4°S) found no evidence of a main spawning period to be during the inter-monsoon season; instead, a protracted breeding season of approximately 8 months was observed (Mangubhai and Harrison, 2008). This may be linked to Kenya’s less sharply defined seasonal wind transitions, with spawning occurring predominantly during the Northeast monsoon (December to March), when conditions are relatively calm and stable. Whilst our study concurs with findings of two peak spawning seasons in the Maldives coinciding with monsoonal transitions, we consider that extensive surveys of spawning in Acropora in this region revealed asynchronous spawning occurring outside of these peaks during the Northeast monsoon (Monfared et al., 2023; Sheridan et al., 2025). These patterns suggest that where there is no abrupt drop in wind speed, the persistence of calm conditions over a sustained period of time may too serve as a cue for coral spawning.
Monsoonal transitions are typically associated with rapid increases in sea surface temperature (SST), which have been identified as a key driver of coral spawning across various temporal and vast geographic scales (Keith et al., 2016; Novriansyah et al., 2023). SST has been shown to influence both gametogenic development and the timing of gamete release, with several studies correlating earlier or more synchronous spawning events with higher SSTs (Howells et al., 2014; Lin and Nozawa, 2023; Moritz et al., 2025; Nozawa, 2012; Monfared et al., 2023; Sun et al., 2024). Moritz et al. (2025) noted that at both large scales and at the individual colony level, increases in temperature induced earlier spawning. The role of SST as a spawning cue appears to vary by region and species. As such, peak spawning months could be inferred from rapid rises in SST, but not months where few colonies spawned. Sheridan et al. (2025) noted that while change in SST was a significant predictor of the number of colonies to spawn within a month, it did not significantly predict the month in which spawning occurred. Our study suggests that SST influences the intensity of spawning rather than the timing, as a higher proportion of colonies spawning in a given month were significantly correlated with higher average monthly SSTs. Sheridan et al. (2025) also found that Acropora species differed in their response to SST changes indicating that thermal cues alone do not universally regulate spawning patterns. In Palau, Gouezo et al. (2020) recorded spawning over an extended period of nine months with no consistent correlation between SST and the timing of reproductive events, suggesting that temperature alone may not serve as a universal proximate cue. These studies underscore the complexity of coral reproductive cues and highlight the need for further research to understand the interplay of various environmental factors influencing coral spawning.
Solar insolation cycles play an important role in entraining the endogenous reproductive rhythms of corals on broad seasonal scales (Mangubhai and Harrison, 2008; Gouezo et al., 2020; van Woesik et al., 2006). Studies have shown that in some equatorial regions the rate of change of solar insolation is a reliable predictor of coral spawning, with gamete release often occurring after the peak of solar insolation maxima, during the subsequent fall (Mangubhai and Harrison, 2008; Gouezo et al., 2020; van Woesik et al., 2006; Penland et al., 2004). Our study found that solar insolation significantly influenced both the likelihood of spawning occurrence and the proportion of colonies spawning in a given month. This dual influence underscores its importance as a key environmental cue. Studies have demonstrated that a combination of increased solar irradiance and high SST can result in oxidative stress to coral tissues (Yakovleva et al., 2009; Hoegh-Guldberg and Jones, 1999). As such, timing spawning to occur after peak solar insolation may serve as an evolutionary advantage to improve larval viability and reduce post-spawning stress on parent colonies. These studies highlight the complex interplay of multiple environmental factors influencing scleractinian coral spawning.
Conclusion
This study provides new insights into the timing, synchrony, and species-specific patterns of coral spawning in the Maldives, offering the first detailed records for several non-Acropora genera in this region. We found that larger spawning events in a given month were significantly correlated with lower wind speeds, higher sea surface temperatures, and increased solar insolation, highlighting the influence of environmental conditions on both the occurrence and extent of coral reproduction. Because more frequent and severe bleaching events can damage the reproductive fitness of corals, studying the successful reproduction and recruitment of surviving colonies is central to maintaining coral populations and restoring reef structure and function. Understanding when and how corals reproduce is critical for evaluating the potential of coral populations to recover following disturbance. With restoration initiatives in the Maldives increasingly utilising sexual reproduction (e.g. larval propagation and assisted fertilisation), these findings serve as an important baseline for reef managers, researchers, and restoration practitioners. However, as this study is based on a single year of observations from one atoll, caution must be taken in extrapolating trends across space and time. Long-term, region-wide monitoring is needed to identify the environmental drivers of coral spawning and assess how spawning patterns may shift under future climate scenarios.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
AE: Data curation, Methodology, Writing – review & editing, Supervision, Conceptualization, Writing – original draft, Investigation, Software, Project administration, Funding acquisition, Visualization, Resources, Formal analysis, Validation. KM: Writing – review & editing, Validation, Conceptualization, Methodology, Supervision, Investigation, Funding acquisition, Software, Formal analysis, Resources, Writing – original draft, Visualization, Data curation, Project administration. TL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
This research took place at Four Seasons Landaa Giraavaru, Baa Atoll. We would like to thank this property for their support throughout this study, and the wider Reefscapers team who assisted during data collection, with particular thanks to Laura Alonso Diaz and Akbar Ahmed. We thank Dr. Phil Hosegood, University of Plymouth, who installed and operates the meteorological station on Landaa Giravaaru for the provision of weather data. We thank Margaux Monfared for her valuable supervision and expertise throughout this study.
Conflict of interest
Authors AE, KM, and TL were employed by Reefscapers.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1646721/full#supplementary-material
References
Adjeroud M., Kayal M., and Penin L. (2017). “Importance of recruitment processes in the dynamics and resilience of coral reef assemblages,” in Marine animal forests (Springer, Cham), 549–569.
Aleem A. (2013). Environmental management plan: for the construction of an access jetty in Hibalhidhoo Island, Baa Atoll, Maldives (Male, Maldives: Maldives National University).
Babcock R. C., Bull G. D., Harrison P. L., Heyward A. J., Oliver J. K., Wallace C. C., et al. (1986). Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394. doi: 10.1007/BF00428562
Babcock R. C., Wills B. L., and Simpson C. J. (1994). Mass spawning of corals on a high latitude coral reef. Coral Reefs 13(3), 161–169.
Baird A. H., Blakeway D. R., Hurley T. J., and Stoddart J. A. (2011). Seasonality of coral reproduction in the Dampier Archipelago, northern Western Australia. Mar. Biol. 158, 275–285. doi: 10.1007/s00227-010-1557-7
Baird A. H., Edwards A. J., Guest J. R., Harii S., Hatta M., Lachs L., et al. (2022). A coral spawning calendar for Sesoko Station, Okinawa, Japan. Galaxea J. Coral Reef Stud. 24, 41–49. doi: 10.3755/galaxea.G2021_S10O
Baird A. H., Guest J. R., Edwards A. J., Bauman A. G., Bouwmeester J., Mera H., et al. (2021). An Indo-Pacific coral spawning database. Sci. Data 8, 35. doi: 10.1038/s41597-020-00793-8
Baird A. H., Guest J. R., and Willis B. L. (2009). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. ecology evolution systematics 40, 551–571. doi: 10.1146/annurev.ecolsys.110308.120220
Baird A. H., Marshall P. A., and Wolstenholme J. (2002). “Latitudinal variation in the reproduction of Acropora in the Coral Sea,” In Proceedings of the 9th International Coral Reef Symposium, Vol. 1. 385–389.
Baker A. C., Glynn P. W., and Riegl B. (2008). Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuarine Coast. shelf Sci. 80, 435–471. doi: 10.1016/j.ecss.2008.09.003
Bastidas C., Cróquer A., Zubillaga A. L., Ramos R., Kortnik V., Weinberger C., et al. (2005). Coral mass-and split-spawning at a coastal and an offshore Venezuelan reefs, southern Caribbean. Hydrobiologia 541, 101–106. doi: 10.1007/s10750-004-4672-y
Bauman A. G., Baird A. H., and Cavalcante G. H. (2011). Coral reproduction in the world’s warmest reefs: southern Persian Gulf (Dubai, United Arab Emirates). Coral Reefs 30, 405–413. doi: 10.1007/s00338-010-0711-5
Bouwmeester J., Edwards A. J., Guest J. R., Bauman A. G., Berumen M. L., and Baird A. H. (2021). Latitudinal variation in monthly-scale reproductive synchrony among Acropora coral assemblages in the Indo-Pacific. Coral Reefs 40, 1411–1418. doi: 10.1007/s00338-021-02129-3
Bronstein O. and Loya Y. (2011). Daytime spawning of Porites rus on the coral reefs of Chumbe Island in Zanzibar, Western Indian Ocean (WIO). Coral Reefs 30, 441–441. doi: 10.1007/s00338-011-0733-7
Bruno J. F. and Selig E. R. (2007). Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PloS One 2, e711. doi: 10.1371/journal.pone.0000711
Cantin N. E. and Lough J. M. (2014). Surviving coral bleaching events: Porites growth anomalies on the Great Barrier Reef. PloS One 9, e88720. doi: 10.1371/journal.pone.0088720
Chelliah A., Amar H. B., Hyde J., Yewdall K., Steinberg P. D., and Guest J. R. (2015). First record of multi-species synchronous coral spawning from Malaysia. PeerJ 3, e777. doi: 10.7717/peerj.777
Cowen R. K., Lwiza K. M., Sponaugle S., Paris C. B., and Olson D. B. (2000). Connectivity of marine populations: open or closed? Science 287, 857–859. doi: 10.1126/science.287.5454.857
Dela Cruz D. W. and Harrison P. L. (2020). Enhancing coral recruitment through assisted mass settlement of cultured coral larvae. PloS One 15, e0242847. doi: 10.1371/journal.pone.0242847
Dhunya A., Huang Q., and Aslam A. (2017). Coastal habitats of Maldives: status, trends, threats, and potential conservation strategies. International Journal of Scientific and Engineering Research 8, 47–62.
Donati M., Bianchi C. N., Morri C., and Montefalcone M. (2025). Idiosyncratic recovery patterns in coral reefs of the Maldives following climate disturbance. Mar. Ecol. 46, e70009. doi: 10.1111/maec.70009
Dryden C., Basheer A., Grimsditch G., Musthaq A., Newman S., and Shan A. (2020). A rapid assessment of natural environments in the Maldives (Gland, Switzerland: IUCN and Government of Maldives).
Elmhirst T., Connolly S. R., and Hughes T. P. (2009). Connectivity, regime shifts and the resilience of coral reefs. Coral Reefs. 28, 949–957. doi: 10.1007/s00338-009-0530-8
Emslie M. J., Logan M., Bray P., Ceccarelli D. M., Cheal A. J., Hughes T. P., et al. (2024). Increasing disturbance frequency undermines coral reef recovery. Ecol. Monogr. 94, e1619. doi: 10.1002/ecm.1619
Foster T., Gilmour J. P., Chua C. M., Falter J. L., and McCulloch M. T. (2015). Effect of ocean warming and acidification on the early life stages of subtropical Acropora spicifera. Coral Reefs 34, 1217–1226. doi: 10.1007/s00338-015-1342-7
Foster T., Heyward A. J., and Gilmour J. P. (2018). Split spawning realigns coral reproduction with optimal environmental windows. Nat. Commun. 9, 718. doi: 10.1038/s41467-018-03175-2
Frölicher T. L., Fischer E. M., and Gruber N. (2018). Marine heatwaves under global warming. Nature 560, pp.360–pp.364. doi: 10.1038/s41586-018-0383-9
Gilmour J. P., Smith L. D., and Brinkman R. M. (2009). Biannual spawning, rapid larval development and evidence of self-seeding for scleractinian corals at an isolated system of reefs. Mar. Biol. 156, 1297–1309. doi: 10.1007/s00227-009-1171-8
Gilmour J. P., Smith L. D., Heyward A. J., Baird A. H., and Pratchett M. S. (2013). Recovery of an isolated coral reef system following severe disturbance. Science 340, 69–71. doi: 10.1126/science.1232310
Gilmour J., Speed C. W., and Babcock R. (2016). Coral reproduction in Western Australia. PeerJ 4, e2010. doi: 10.7717/peerj.2010
Gold Z. and Palumbi S. R. (2018). Long-term growth rates and effects of bleaching in Acropora hyacinthus. Coral Reefs 37, pp.267–pp.277. doi: 10.1007/s00338-018-1656-3
Gouezo M., Doropoulos C., Fabricius K., Olsudong D., Nestor V., Kurihara H., et al. (2020). Multispecific coral spawning events and extended breeding periods on an equatorial reef. Coral Reefs 39, 1107–1123. doi: 10.1007/s00338-020-01941-7
Gouezo M., Golbuu Y., Fabricius K., Olsudong D., Mereb G., Nestor V., et al. (2019). Drivers of recovery and reassembly of coral reef communities. Proc. R. Soc. B 286, 20182908. doi: 10.1098/rspb.2018.2908
Grafeld S., Oleson K. L., Teneva L., and Kittinger J. N. (2017). Follow that fish: Uncovering the hidden blue economy in coral reef fisheries. PloS One 12, e0182104. doi: 10.1371/journal.pone.0182104
Graham N. A. and Nash K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral reefs 32, 315–326. doi: 10.1007/s00338-012-0984-y
Graham N. A., Wilson S. K., Benkwitt C. E., Bonne R., Govinden R., and Robinson J. P. (2024). Increased resilience and a regime shift reversal through repeat mass coral bleaching. Ecol. Lett. 27, e14454. doi: 10.1111/ele.14454
Guest J. R., Baird A. H., Goh B. P. L., and Chou L. M. (2005a). Seasonal reproduction in equatorial reef corals. Invertebrate Reprod. Dev. 48, 207–218. doi: 10.1080/07924259.2005.9652186
Guest J. R., Baird A. H., Goh B. P. L., and Chou L. M. (2005b). Reproductive seasonality in an equatorial assemblage of scleractinian corals. Coral Reefs 24, 112–116. doi: 10.1007/s00338-004-0433-7
Harrison P. L. (2011). “Sexual reproduction of scleractinian corals,” in Coral reefs: an ecosystem in transition. Dordrecht, The Netherlands: Springer, 59–85.
Harrison P. L. (2024a). “Sexual reproduction of reef corals and application to coral restoration,” in Oceanographic Processes of Coral Reefs (Boca Raton, FL, USA: CRC Press), 419–437.
Harrison P. L. (2024b). Reef-based mass coral larval culture and restoration methods (Lismore, Australia: Southern Cross University). doi: 10.25918/report.434
Harrison P. L., Babcock R. C., Bull G. D., Oliver J. K., Wallace C. C., and Willis B. L. (1984). Mass spawning in tropical reef corals. Science 223, 1186–1189. doi: 10.1126/science.223.4641.1186
Harrison P. L. and Hakeem A. (2007). “Asynchronous and pulsed multispecific reef coral spawning patterns on equatorial reefs in the Maldives Archipelago,” in Australian Coral Reef Society National Conference, Perth.
Harrison P. L. and Wallace C. C. (1990). “Reproduction, dispersal and recruitment of scleractinian corals,” in Coral reefs, vol. 25. (Dordrecht, The Netherlands: Elsevier), 133–207.
Hock K., Doropoulos C., Gorton R., Condie S. A., and Mumby P. J. (2019). Split spawning increases robustness of coral larval supply and inter-reef connectivity. Nat. Commun. 10, p.3463. doi: 10.1038/s41467-019-11367-7
Hoegh-Guldberg O. (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 50, 839–866. doi: 10.1071/MF99078
Hoegh-Guldberg O. and Jones R. J. (1999). Photoinhibition and photoprotection in symbiotic dinoflagellates from reef-building corals. Mar. Ecol. Prog. Ser. 183, 73–86. doi: 10.3354/meps183073
Howells E. J., Abrego D., Vaughan G. O., and Burt J. A. (2014). Coral spawning in the Gulf of Oman and relationship to latitudinal variation in spawning season in the northwest Indian Ocean. Sci. Rep. 4, 7484. doi: 10.1038/srep07484
Hughes T. P., Baird A. H., Bellwood D. R., Card M., Connolly S. R., Folke C., et al. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. doi: 10.1126/science.1085046
Hughes T. P., Kerry J. T., Álvarez-Noriega M., Álvarez-Romero J. G., Anderson K. D., Baird A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Hughes T. P., Kerry J. T., and Simpson T. (2018). Large-scale bleaching of corals on the Great Barrier Reef. Ecology 99 (2), p. 501. doi: 10.1002/ecy.2092
Humanes A., Beauchamp E. A., Bythell J. C., Carl M. K., Craggs J. R., Edwards A. J., et al. (2021). An experimental framework for selectively breeding corals for assisted evolution. Front. Mar. Sci. 8, 669995. doi: 10.3389/fmars.2021.669995
International Coral Reef Initiative (2024). The Fourth Global Coral Bleaching Event. Available online at: https://icriforum.org/4gbe/ (Accessed 20.02.2025).
Johnston E. C., Counsell C. W., Sale T. L., Burgess S. C., and Toonen R. J. (2020). The legacy of stress: Coral bleaching impacts reproduction years later. Funct. Ecol. 34, 2315–2325. doi: 10.1111/1365-2435.13653
Jones G.P., Almany G.R., Russ G.R., Sale P.F., Steneck R.S., van Oppen M.J.H, et al. (2009). Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges.. Coral Reefs 28 (2), 307–325. doi: 10.1007/s00338‑009‑0469‑9
Keith S. A., Maynard J. A., Edwards A. J., Guest J. R., Bauman A. G., Van Hooidonk R., et al. (2016). Coral mass spawning predicted by rapid seasonal rise in ocean temperature. Proc. R. Soc. B: Biol. Sci. 283, 20160011. doi: 10.1098/rspb.2016.0011
Kelley R. (2022). “Coral Finder 2022: Indo-pacific hard corals,” in Australian Coral Reef Society (Townsville, Australia: BYO Guides).
Kleypas J. A., Buddemeier R. W., Archer D., Gattuso J. P., Langdon C., and Opdyke B. N. (1999). Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284, 118–120. doi: 10.1126/science.284.5411.118
Koch H. R., Matthews B., Leto C., Engelsma C., and Bartels E. (2022). Assisted sexual reproduction of Acropora cervicornis for active restoration on Florida’s Coral Reef. Front. Mar. Sci. 9, 959520. doi: 10.3389/fmars.2022.959520
Komoto H., Lin C. H., Nozawa Y., and Satake A. (2023). An external coincidence model for the lunar cycle reveals circadian phase-dependent moonlight effects on coral spawning. J. Biol. Rhythms 38, pp.148–pp.158. doi: 10.1177/07487304221135916
Kongjandtre N., Ridgway T., Ward S., and Hoegh-Guldberg O. (2010). Broadcast spawning patterns of Favia species on the inshore reefs of Thailand. Coral Reefs 29, 227–234. doi: 10.1007/s00338-009-0551-3
Levitan D. R. (2004). Density-dependent sexual selection in external fertilizers: variances in male and female fertilization success along the continuum from sperm limitation to sexual conflict in the sea urchin Strongylocentrotus franciscanus. Am. Nat. 164, 298–309. doi: 10.1086/423150
Lin C. H. and Nozawa Y. (2017). Variability of spawning time (lunar day) in Acropora versus merulinid corals: a 7-yr record of in situ coral spawning in Taiwan. Coral Reefs 36, 1269–1278. doi: 10.1007/s00338-017-1622-5
Lin C. H. and Nozawa Y. (2023). The influence of seawater temperature on the timing of coral spawning. Coral Reefs 42, 417–426. doi: 10.1007/s00338-023-02349-9
Litster M. (2016). Cowry shell money and monsoon trade: the Maldives in past globalizations. Unpublished PhD thesis. The Australian National University. Available at: https://openresearch-repository.anu.edu.au/items/1b477fbd-6bb5-48a6-b514-cfa2aff7297c.
Maldives National Statistical Bureau (2020). The Maldives: Facts and Figures. Available online at: https://statisticsMaldives.gov.mv/ (Accessed 16 August 2025).
Mangubhai S. and Harrison P. L. (2008). Asynchronous coral spawning patterns on equatorial reefs in Kenya. Mar. Ecol. Prog. Ser. 360, 85–96. doi: 10.3354/meps07385
MEE (2015). Fifth National Report of Maldives to the Convention on Biological Diversity (Maldives: Ministry of Environment and Energy), 3–6.
Monfared M. A., Sheridan K., Dixon S. P., Gledhill M., and Le Berre T. (2023). Coral spawning patterns of Acropora across two Maldivian reef ecosystems. PeerJ 11, e16315. doi: 10.7717/peerj.16315
Moon Giant (2025). Available online at: https://www.moongiant.com/ (Accessed 10 March).
Moritz C., Andréfouët S., Azam C. S., Berthe C., Fourrière M., Goyaud A., et al. (2025). Shining a light on daytime coral spawning synchrony across oceans. Global Ecology and Biogeography 34 (7), e70072.
Naseer A. and Hatcher B. G. (2004). Inventory of the Maldives’ coral reefs using morphometrics generated from Landsat ETM+ imagery. Coral Reefs 23, 161–168. doi: 10.1007/s00338-003-0366-6
Novriansyah A., Huhn M., Wicaksono H., Senen B., Subhan B., Fenner D., et al. (2023). First observations of coral spawning at the Banda Islands, Maluku, Indonesia. Biodiversitas J. Biol. Diversity 24, 6082–6091. doi: 10.13057/biodiv/d241129
Nozawa Y. (2012). Annual variation in the timing of coral spawning in a high-latitude environment: influence of temperature. Biol. Bull. 222, 192–202. doi: 10.1086/BBLv222n3p192
Nozawa Y., Isomura N., and Fukami H. (2015). Influence of sperm dilution and gamete contact time on the fertilization rate of scleractinian corals. Coral Reefs 34, 1199–1206. doi: 10.1007/s00338-015-1338-3
Oliver J. and Babcock R. (1992). Aspects of the fertilization ecology of broadcast spawning corals: sperm dilution effects and in situ measurements of fertilization. Biol. Bull. 183, 409–417. doi: 10.2307/1542017
Oliver J. K., Babcock R. C., Harrison P. L., and Willis B. L. (1988). Geographic extent of mass coral spawning: clues to ultimate causal factors. Proc. 6th Int. Coral Reef Symposium 2, 803–810.
Paxton C. W., Baria M. V. B., Weis V. M., and Harii S. (2016). Effect of elevated temperature on fecundity and reproductive timing in the coral Acropora digitifera. Zygote 24, 511–516. doi: 10.1017/S0967199415000477
Penland L., KlouleChad J., Idip D., and Van Woesik R. (2004). Coral spawning in the western Pacific Ocean is related to solar insolation: evidence of multiple spawning events in Palau. Coral Reefs 23, 133–140. doi: 10.1007/s00338-003-0362-x
Perry C. T. and Morgan K. M. (2017a). Bleaching drives collapse in reef carbonate budgets and reef growth potential on Southern Maldives reefs. Sci. Rep. 7, 40581. doi: 10.1038/srep40581
Pisapia C., Burn D., Yoosuf R., Najeeb A., Anderson K. D., and Pratchett M. S. (2016). Coral recovery in the central Maldives archipelago since the last major mass-bleaching, in 1998. Sci. Rep. 6, 34720. doi: 10.1038/srep34720
Pollock F. J., Katz S. M., van de Water J. A., Davies S. W., Hein M., Torda G., et al. (2017). Coral larvae for restoration and research: a large-scale method for rearing Acropora millepora larvae, inducing settlement, and establishing symbiosis. PeerJ 5, e3732. doi: 10.7717/peerj.3732
Randall C. J., Negri A. P., Quigley K. M., Foster T., Ricardo G. F., Webster N. S., et al. (2020). Sexual production of corals for reef restoration in the Anthropocene. Mar. Ecol. Prog. Ser. 635, 203–232. doi: 10.3354/meps13206
Reefscapers. (2024). Unpublished observations of coral bleaching from Baa and North Malé Atolls. Reefscapers Maldives.
Rosser N. L. (2015). Asynchronous spawning in sympatric populations of a hard coral reveals cryptic species and ancient genetic lineages. Mol. Ecol. 24, 5006–5019. doi: 10.1111/mec.13372
Rosser N. L., Edyvane K., Malina A. C., Underwood J. N., and Johnson M. S. (2020). Geography and spawning season drive genetic divergence among populations of the hard coral Acropora tenuis from Indonesia and Western Australia. Coral Reefs 39, 989–999. doi: 10.1007/s00338-020-01923-9
Schott F. A., Xie S. P., and McCreary J. P. Jr. (2009). Indian Ocean circulation and climate variability. Rev. Geophysics 47, RG1002. doi: 10.1029/2007RG000245
Sea Temperatures (2025). Baa Atoll Sea Temperature. Available online at: https://seatemperatures.net/asia/Maldives/baa-atoll/ (Accessed 10 March 2025).
Sheridan K., Monfared M. A., Dixon S. P., Errington A. J., and Le Berre T. (2025). Synchrony on the reef: how environmental factors shape coral spawning patterns in Acropora corals in the Maldives. PeerJ 13, e19447. doi: 10.7717/peerj.19447
Shlesinger Y., Goulet T. L., and Loya Y. (1998). Reproductive patterns of scleractinian corals in the northern Red Sea. Mar. Biol. 132, 691–701. doi: 10.1007/s002270050433
Shlesinger Y. and Loya Y. (1985). Coral community reproductive patterns: red sea versus the great barrier reef. Science 228, 1333–1335. doi: 10.1126/science.228.4705.1333
Shlesinger T. and Loya Y. (2019). Breakdown in spawning synchrony: A silent threat to coral persistence. Science 365, 1002–1007. doi: 10.1126/science.aax0110
Sola E., Marques da Silva I., and Glassom D. (2016). Reproductive synchrony in a diverse Acropora assemblage, Vamizi Island, Mozambique. Mar. Ecol. 37, 1373–1385. doi: 10.1111/maec.12348
Spalding M. D. and Brown B. E. (2015). Warm-water coral reefs and climate change. Science 350, 769–771. doi: 10.1126/science.aad0349
Su D., Wijeratne S., and Pattiaratchi C. B. (2021). Monsoon influence on the island mass effect around the Maldives and Sri Lanka. Front. Mar. Sci. 8, 645672. doi: 10.3389/fmars.2021.645672
Sun Y., Zhang Y., Jiang L., Yu X., Huang L., Yuan T., et al. (2024). Coral spawning patterns on the Luhuitou fringing reef in Hainan Island of the northern South China Sea. Front. Mar. Sci. 11, 1418942. doi: 10.3389/fmars.2024.1418942
Sweeney A. M., Boch C. A., Johnsen S., and Morse D. E. (2011). Twilight spectral dynamics and the coral reef invertebrate spawning response. J. Exp. Biol. 214, 770–777. doi: 10.1242/jeb.043406
Tideschart (2025). Available online at: https://www.tideschart.com/Maldives/Baa-Atholhu/ (Accessed 10 March 2025).
van Woesik R. (2010). Calm before the spawn: global coral spawning patterns are explained by regional wind fields. Proc. R. Soc. B: Biol. Sci. 277, 715–722. doi: 10.1098/rspb.2009.1524
van Woesik R., Lacharmoise F., and Köksal S. (2006). Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol. Lett. 9, 390–398. doi: 10.1111/j.1461-0248.2006.00886.x
Veron J. E. N., Stafford-Smith M. G., Turak E., and DeVantier L. M. (2025). Corals of the World. Available online at: https://www.coralsoftheworld.org/page/home/ (Accessed 23 February 2025).
Wijayanti D. P., Indrayanti E., Wirasatriya A., Haryanto A., Haryanti D., Sembiring A., et al. (2019). Reproductive seasonality of coral assemblages in the Karimunjawa Archipelago, Indonesia. Front. Mar. Sci. 6, 195. doi: 10.3389/fmars.2019.00195
Willis B. L., Babcock R. C., Harrison P. L., Oliver J. K., and Wallace C. C. (1985). Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proceedings of the Fifth International Coral Reef Congress. 4, 343–348.
Woodhead A. J., Hicks C. C., Norström A. V., Williams G. J., and Graham N. A. (2019). Coral reef ecosystem services in the Anthropocene. Funct. Ecol. 33, 1023–1034. doi: 10.1111/1365-2435.13331
Keywords: coral, spawning, monsoon, reproduction, coral reef, synchrony
Citation: Errington AJF, Moody K and Le Berre T (2025) Multi-specific coral spawning and monsoonal transitions: assemblage-level observations from Baa Atoll, Maldives. Front. Mar. Sci. 12:1646721. doi: 10.3389/fmars.2025.1646721
Received: 13 June 2025; Accepted: 02 September 2025;
Published: 25 September 2025.
Edited by:
Joshua Patterson, University of Florida, United StatesReviewed by:
Antonella Lavorato, Universidad de Guadalajara - Centro Universitario de la Costa Puerto Vallarta Biblioteca, MexicoCharlotte Moritz, CMOANA Consulting, French Polynesia
Copyright © 2025 Errington, Moody and Le Berre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amelia J. F. Errington, bWVsaTI0MzJAaG90bWFpbC5jby51aw==
 Amelia J. F. Errington
Amelia J. F. Errington Kate Moody
Kate Moody Thomas Le Berre
Thomas Le Berre