- 1Nippon Foundation Ocean Nexus/Department of Marine Affairs, The University of Rhode Island, Kingston, RI, United States
- 2Non Profit Organization (NPO) Satoumi Research Institute, Okayama, Japan
- 3Oregon State University, School of Public Policy, Corvallis, OR, United States
- 4Daniel J. Evans School of Public Policy and Governance, University of Washington, Seattle, WA, United States
- 5Graduate School of Environmental Science, Hokkaido University, Sapporo, Japan
- 6Association for shore Environmental Creation, Yokohama, Kanagawa, Japan
Ocean acidification (OA) poses significant threats to shellfish aquaculture. Although governments and organizations around the globe are taking actions to mitigate the impacts of OA, few studies directly report shellfish farmer perceptions of OA and corresponding responses. In this study, we document Japanese shellfish (oyster) commercial farmer perceptions of, and adaptive strategies for OA with respect to oyster aquaculture. We also review and compare our results with existing studies of shellfish commercial farmer perceptions of OA in three regions, including the United States (U.S.), the Mediterranean region and British Columbia, Canada. We found variation in the perceptions of OA around the globe; it is common among all shellfish farmers to have difficulty distinguishing OA from other environmental stressors. OA adaptive strategies from shellfish farmers were only reported for the U.S. (in the literature), and Japan (this study). Acknowledging the diverse geographical and cultural backgrounds, we discussed the similarity and difference of adaptive strategies between the U.S. (as a post-event case with documented OA-related shellfish mortality) and Japan (as a pre-event case) to cope with OA. For example, farmers from both countries suggest, or are already utilizing flexibility in farm management and applying knowledge through hands-on learning. While U.S. farmers rely on networking with different stakeholders to learn about OA knowledge and solutions while Japanese farmers do not. Learning from the strategies that U.S. farmers applied to adapt to OA events, several areas of policies and actions (e.g., financial support, collaboration with scientists and OA awareness enhancement) were identified to better support and empower Japanese shellfish farmers to adapt to future OA scenarios. However, future study on suitability and transferability of implementing policies and actions in Japan is required due to different geographical and cultural contexts.
1 Introduction
Known as “the other carbon dioxide (CO2) problem”, ocean acidification (OA) has been acknowledged as a serious environmental issue, caused by the ocean absorbing excess anthropogenic CO2 from fossil fuel and industrial emissions (Gattuso et al., 2015; Licker et al., 2019). Numerous studies have revealed the impacts of OA on the marine organisms (Bach et al., 2017; Xiao et al., 2021), ecosystems (Bach et al., 2017; Marshall et al., 2017; Mirasole et al., 2020) and human society (Schmutter et al., 2017; Doney et al., 2020). One of the most prominent effects of OA is the dissolution of shells or skeletons of organisms by the acidic water, especially in mollusks and corals (Green et al., 2009; Miller et al., 2009; Hettinger et al., 2012; Waldbusser and Salisbury, 2014, 2015). Comorbidities from OA on mollusks include increased larval mortality and abnormalities, reduced feeding rates, high susceptibility of predation, and lower body volume throughout different life stages (Lemasson et al., 2017; Sadler et al., 2018; Zhao et al., 2020). Furthermore, fluctuation in pH in the coastal waters where commercially important shellfish reside is considerably greater than that in the open ocean due to a variety of physio-biological factors, such as local metabolism, freshwater discharge and coastal upwelling (Cai et al., 2011; Wallace et al., 2014; Vargas et al., 2016, 2022).
Molluscan aquaculture1 is particularly susceptible to these negative impacts of OA. If high emissions continue or increase, global economic losses in capture and aquaculture of shellfish could be higher than US$100 billion by the end of this century due to OA (Narita et al., 2012). Specific economic losses range from juvenile mollusk mortality events (Barton et al., 2015), physical damage to shellfish and corresponding reduction in market price (Duarte et al., 2022), to decreases in demand by consumers for OA-affected shellfish (Martin et al., 2019). OA also impacts human livelihoods: shellfish larvae die-offs in the U.S. Pacific Northwest due to OA resulted in a loss of $270 million in revenue and 3,200 jobs per year in the aquaculture industry in Washington state alone (Washington State Blue Ribbon Panel on Ocean Acidification, 2012).
Global molluscan shellfish (hereafter, shellfish) aquaculture production grew 4.3% between 2000 and 2022 (FAO, 2024). China is the largest producer and consumer of shellfish, accounting for 84% of global production by quantity in 2022 (FAO, 2024). After China, top-producing countries (>100,000 ton production/year) include Chile, Vietnam, North Korea, Japan, U.S., Spain and France (FAO, 2024). Mussels are the dominant cultured species in Chile and Spain; oyster production dominates U.S. and North Korea shellfish production. Clams and oysters dominate Vietnam shellfish production (Nash et al., 2021); oysters and scallops, Japan (FAO, 2024); mussels and oysters, France (FAO, 2024). These countries all display overall high sensitivity, exposure and or vulnerabilities to climate change and OA due to various environmental, social or economical reasons (Stewart-Sinclair et al., 2020).
A variety of top-down and bottom-up adaptive strategies have been proposed to mitigate the impacts of OA on commercial shellfish farming as well as enhance the adaptive capacity2 of communities reliant on shellfish aquaculture (Turner et al., 2021). Although policies with the explicit task of addressing OA rarely exist (Harrould-Kolieb and Hoegh-Guldberg, 2019), there are top-down government-led marine policies that more broadly support OA adaptation of shellfish aquaculture. For example, in Scotland, policies that promote co-existence of aquaculture with other marine uses or flexibility in new license agreements help commercial operators diversify their products as insurance against OA-related impacts (Greenhill et al., 2020). Conventional top-down approaches have been shown to be ineffective in small scale fisheries management (Hauzer et al., 2013; Jentoft, 2005), while building local adaptive capacity and information sharing in the fisheries community have shown potential to facilitate climate adaptation measures (Obregón et al., 2020; Rubio et al., 2021).
Shellfish farmers are on the frontline of detecting OA impacts; thus OA adaptive strategies initiated by, or developed in partnership with farmers are not only an effective way to mitigate direct OA impacts on shellfish farming, but also build OA adaptive capacity more broadly (Whitefield et al., 2021). For example, collaborations between the shellfish industry and researchers to identify OA problems and remediate water quality successfully resumed operations in the Whisky Creek Shellfish Hatchery (Oregon, US) after a dramatic crash in production due to larval shellfish mortality in 2008 (Barton et al., 2015). Yet to date, only a few studies have explored the perceptions of OA among shellfish farmers and corresponding adaptive actions (Advani and Satterfield, 2024; Green et al., 2023; Mabardy et al., 2015; Rodrigues et al., 2015; Ward et al., 2022). Despite the volume of shellfish production in Asian countries (China, Vietnam, South Korea and Japan), these studies have all focused on perceptions of North American and European farmers. Thus, there is a lack of both geographical and cultural variation represented in shellfish farmer perceptions of OA in the literature. This is important because there is no one-size-fits-all solution when it comes to climate adaptation (Berrang-Ford et al., 2015). Thus, through recognizing and learning from geographical and cultural variation in adaptations of farmers to climate challenges, tailored adaptive strategies can be designed and implemented by local communities according to their own contexts. For example, family units can strengthen climate adaptive capacity among small-scale local fisheries communities through knowledge sharing and preservation of traditions (Suh and Nyiawung, 2023; El-Shayeb et al., 2025).
Within Asia, Japan exhibits the highest exposure to climate change and OA and overall high vulnerability (Steward-Sinclair et al., 2020), primarily attributable to the prominence of in-situ aquaculture and the acidifying Japanese coastal waters (Fujii et al., 2023; Pang et al., 2024). However, information about how Japanese shellfish (oyster) farmers perceive the effects of OA on their livelihoods remains unknown. Therefore, in this study, we first conduct a case study in Japan through questionnaires and interviews, to understand how oyster farmers perceive OA impacts on farmed oysters and farmers-derived strategies for adapting to OA. Then we compare these results with an overview of OA perceptions and adaptive strategies extracted from a literature review of global shellfish farmers. Finally, we provide Japan-specific information for managers and policymakers accordingly.
Japanese Case Study Location
Within our Japanese case study, we focus specifically on Hinase, in the Okayama prefecture (Figure 1), a world famous community for oyster production and coastal restoration (Yanagi, 2012; Tsurita, 2022). Hinase accounts for 50% of the Okayama Prefecture oyster aquaculture production (Ministry of Agriculture, Forest and Fisheries, 2023), and a total of 94 oyster farmers live in Hinase. Hinase has been regarded as a model of “Satoumi”, a Japanese term initially defined as “a coastal area with high productivity and biodiversity due to human interaction” (Yanagi, 2006). Hinase is predicted to have high exposure to OA by the end of this century (Fujii et al., 2023), and thus is an exemplary case to understand shellfish farmer perceptions of, and adaptation to, OA. See Appendix 1 for more information about Hinase and oyster farming.
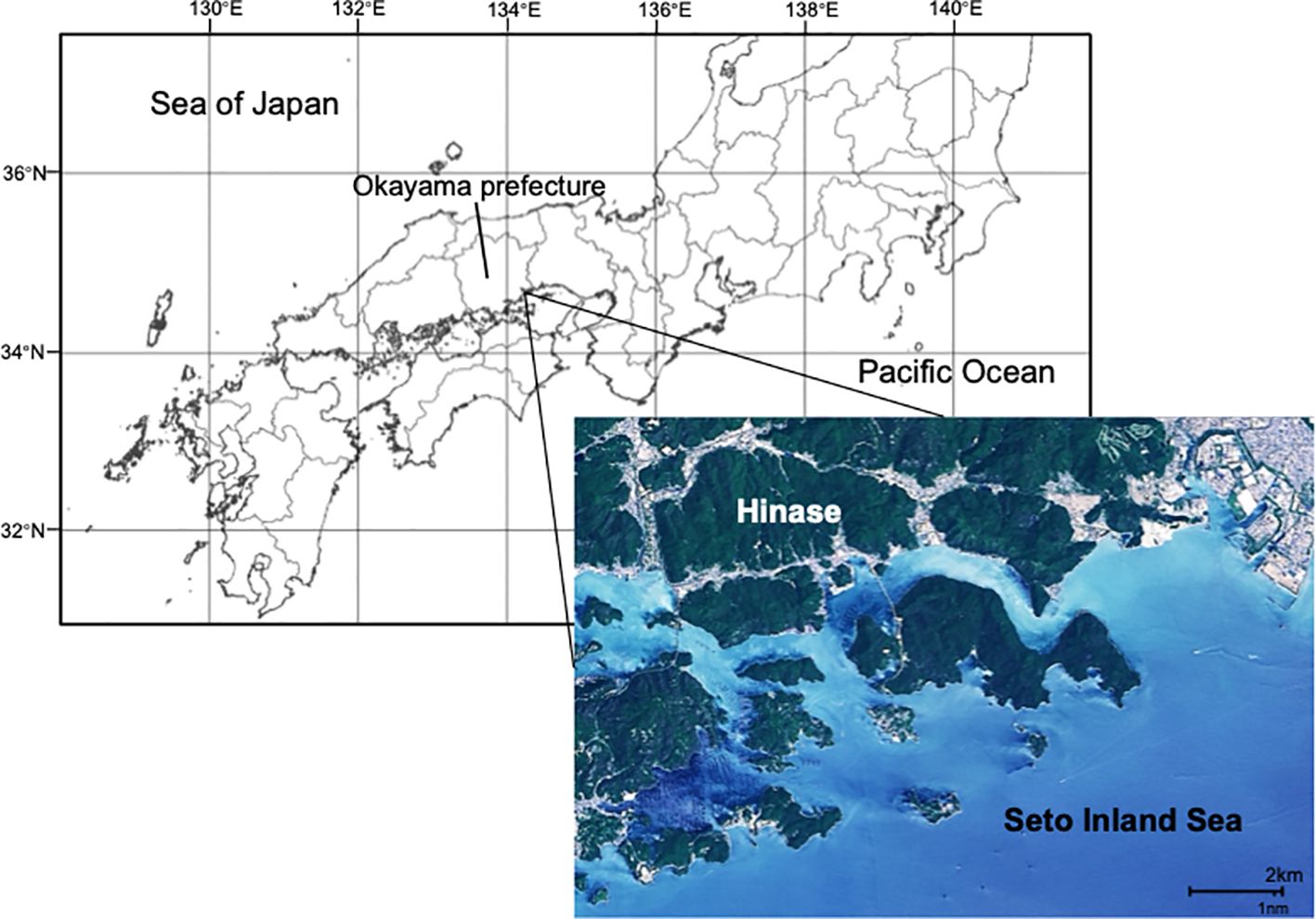
Figure 1. Map of Japan highlighting Okayama Prefecture, and an inset shows a detailed satellite view of Hinase situated in the Seto Inland Sea.
2 Methods
Our approach consisted of three steps: (1) focused survey of oyster farmers in Hinase, Okayama, Japan to investigate farmer perceptions and their OA-adaptive strategies through questionnaires and unstructured interviews, (2) a systematic review and analysis of the literature to identify global case studies that document shellfish farmer perceptions of OA and other environmental stressors as well as farmers-derived adaptive strategies to OA, and (3) based on the interview transcripts, adaptive strategies were grouped into three categories including farm management, policy and networking, and science.
2.1 Survey of oyster farmers in Hinase, Okayama, Japan
We surveyed oyster farmers in Hinase, Okayama, Japan in November 2020 using a questionnaire designed as part of the Inclusive Ocean Data 4 Decision Making (Inclusive OD4D)3. The questionnaire included a mix of 26 closed-ended, and two open-ended questions, to allow respondents to select from predetermined answers as well as write their own answers (Roopa and Rani, 2012). Closed-ended questions included multiple choice responses; farmers were asked to select one or more answers that described: 1) the background and history of oyster farming operations; and 2) their perceptions about OA and other environmental stressors (Appendix 2). There was also an opportunity for farmers to write down their own answers to close-ended questions if no given choice fit. The questionnaire closed with two open-ended questions: 1) current and proposed adaptive strategies to respond to OA, and 2) any other thoughts they wanted to share about oyster farming or OA adaptation.
Between November 2020 to October 2021, 94 oyster farmers were contacted through local fisheries cooperation and presented with questionnaires to fill out. Short (~30 min) in-person, unstructured interviews were offered to farmers following the written questionnaire. These interviews repeated similar themes from the questionnaire, such as stressors that affect oyster farming, and strategies to adapt to OA, but also inquired about farmer visions of, and desire to, practice oyster farming in the future, and their participation in the aquaculture management process.
Responses to unstructured interviews with Hinase oyster farmers were transcribed by hand (not verbatim) by the co-author R.H., and interpreted by the lead author. The lead author categorized the interview themes surrounding environmental stressors and OA adaptive strategies. Questionnaire and interview data were also included as part of the Global literature review and analysis (Section 2.2) and were the source of the Adaptive strategies of Japanese farmers (Section 2.3). Ethical approval for this research study was granted by the Institutional Review Boards at University of Washington (#00009522).
2.2 Global literature review and analysis
We searched the literature for studies that reported the perceptions, experiences of environmental stressors, and adaptive strategies in response to OA of global shellfish industry members (growers, farmers, and farm owners; hereafter, farmers) towards OA. This study focused on farmer OA perceptions and their adaptive strategies, thus only studies involving interviews or questionnaires with shellfish farmers were searched. Literature searches were conducted in English using Google Scholar with the following search string: (“ocean acidification” AND “shellfish aquaculture” AND (“interview” OR “questionnaire”) AND (“perception” OR “adapt*”). This search resulted in 137 publications (including government reports, theses/dissertations, and other non-peer-reviewed studies); these were further filtered to exclude studies that did not interview or survey shellfish farmers directly (e.g., public opinion surveys). A total of seven peer-reviewed publications, and our Japanese case study were included for final analysis.
From five of the eight studies, we extracted farmer perceptions of OA awareness, experience, concerns, understanding/knowledge, and information sources. We further compared shellfish farmer rankings of OA relative to other environmental stressors across all eight studies.
2.3 Adaptive strategies of Japanese farmers
We identified and enumerated OA-specific adaptive strategies of Japanese farmers from questionnaires (results from the open-ended questions) and interviews, illustrating these with exemplary quotes. We applied the structure from Green et al. (2023) and Ward et al. (2022), where adaptive strategies were organized into themes (e.g., spatial flexibility, shellfish health knowledge) and then grouped into three larger categories (farm management, policy and networking, and science). New adaptive themes were added if none of the existing themes were applicable.
3 Results
3.1 Global literature review of shellfish farmer perceptions of OA
3.1.1 Global perceptions of OA among shellfish farmers
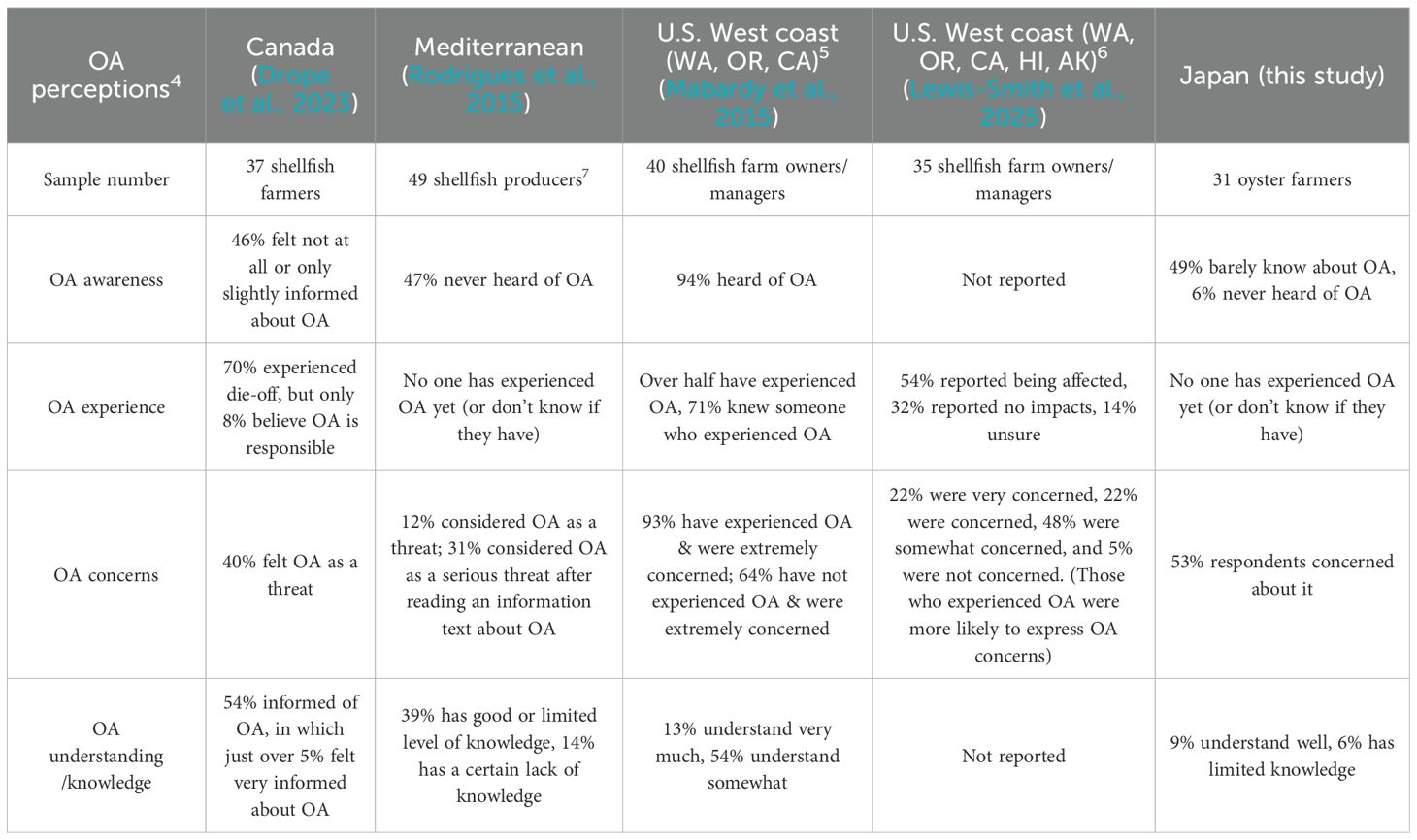
Studies reporting global perceptions of OA from shellfish farmers were available from Canada (Advani and Satterfield, 2024; Drope et al., 2023), the U.S (Mabardy et al., 2015; Lewis-Smith et al., 2025), the Mediterranean (Rodriguez et al., 2015), and Hinase, Japan (this study). Supplementary Table 1 lists the shellfish species cultured in each study. Extremely high awareness of OA (94% of the respondents) was found in U.S. shellfish farmers (Mabardy et al., 2015); half of these farmers had experienced OA in their own businesses in both survey years of 2013 and 2023-2024 (Table 1). In contrast, 46-55% of the interviewed shellfish farmers in Canada, Japan and Mediterranean areas had either never heard of, or barely knew about OA, and few farmers in these regions reported shellfish mortality directly associated with OA (Table 1). Almost all U.S. shellfish farmers (93%) were extremely concerned about OA in 2013 (Mabardy et al., 2015), yet this declined 22% in 2023-2024 (Lewis-Smith et al., 2025). Despite lower awareness/experience of OA in non-U.S. countries, a relatively high percentage (40–53%) of these farmers expressed concerns about OA as a threat. Overall, 67% of U.S. farmers reported understanding OA “very well” or “somewhat” in 2013 (Mabardy et al., 2015), versus 54% (Canadian farmers), 39% (Mediterranean farmers), and 15% (Japanese farmers).
3.1.2 Ranking of OA as an environmental stressor for global shellfish farmers
We compared farmer rankings of OA relative to other environmental stressors among regions (Table 2). While Oregon and California shellfish farmers rankings of OA high relative to other stressors (Table 2), OA was ranked as the least important environmental stressor for shellfish farmers in Canada and the Mediterranean (11th and 9th stressor, respectively), seventh of eight stressors in Hinase, Japan and fifth of eight environmental stressors in total in the U.S West coast (~70% Washington respondents). Increased water temperature was identified as the top environmental stressor among shellfish farmers in Japan, Mediterranean and the U.S. West coast, and “Mollusc disease/pathogens” consistently in the top four stressors for all regions except Oregon, U.S.
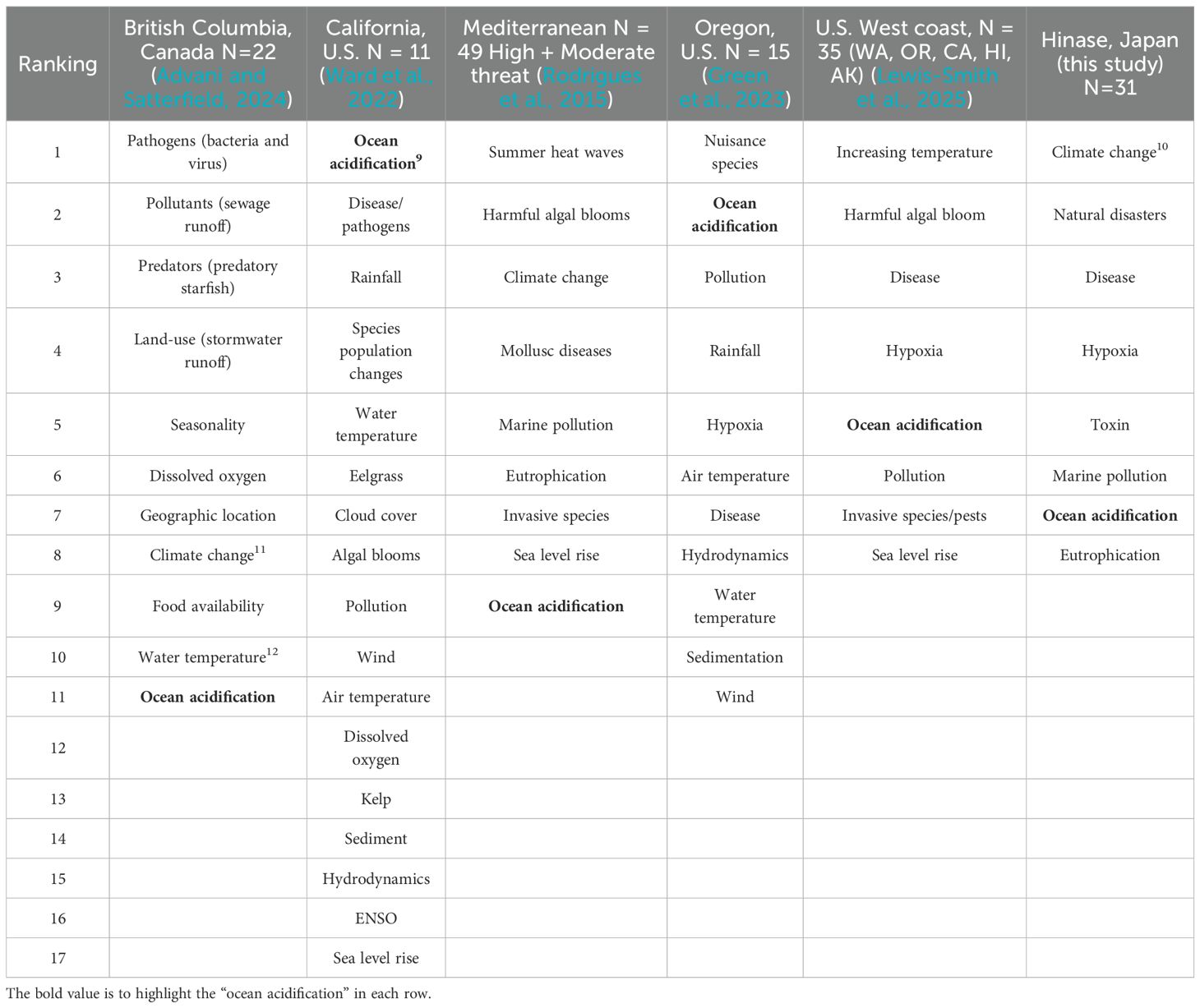
Table 2. Comparison of environmental stressors ranked by shellfish farmers8 among different regions.
3.2 OA adaptive strategies proposed by Japanese farmers
Between November 2020 and October 2021, 33 percent (31 of 94) of Hinase oyster farmers responded to the written questionnaires in person; 20 percent (19 of 94) agreed to interview on-site immediately after the questionnaire. All 31 respondents were males, with an average 21.5 years of experience in oyster farming (range 4–45 years). In addition to oyster farming, 57 percent of respondents also conducted other fishing activities, such as bottom trawl (21%), set net (11%) and others (25%, seine net). All farmers used oyster rafts for culturing, operating 8–12 rafts on average per operation. 22 percent of Hinase oyster farmers held a college degree, 19 percent went to a fishery specialist school and 50 percent graduated from high school.
We recorded six adaptive strategies for Farm Management, and three strategies for Science; Japanese farmers did not report strategies for Policy and Networking. We discuss the details of these strategies below.
3.2.1 Farm management
Current and/or proposed OA adaptive strategies from Hinase farmers were divided into four themes (1) increasing resilience through farming multiple oyster life stages (Multiple life stages); (2) increasing spatial flexibility by farming in other regions in the country (Spatial flexibility); (3) increasing the diversity of farmed species (Species diversification in aquaculture and fishery); and (4) employing OA mitigation methods through traditional practices passed down through generations (Methods/gear) (Table 3).
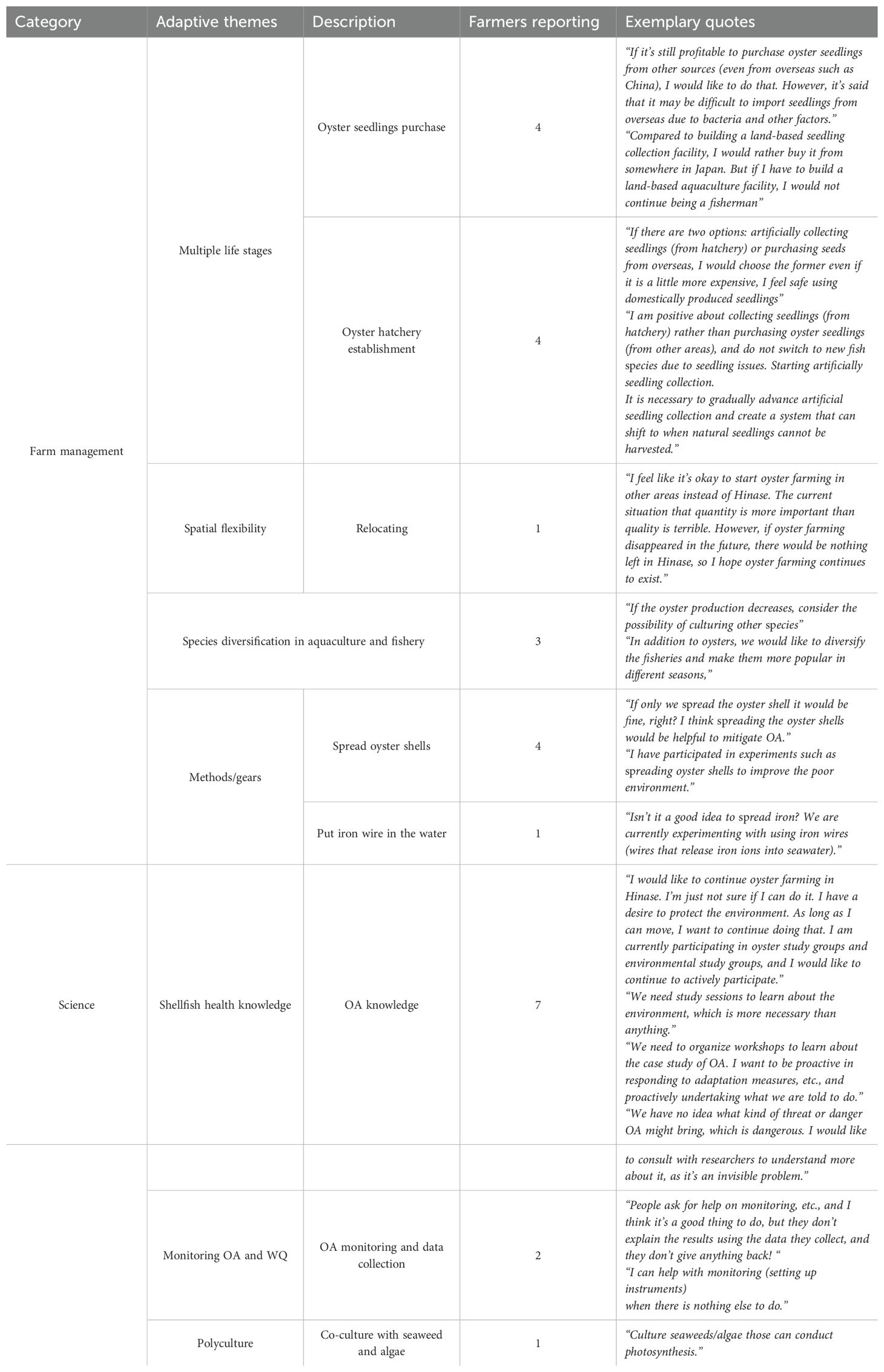
Table 3. Categories, adaptive themes and description, frequency (the number of interviewees describing a proposed/current adaptive strategy), and exemplary quotes. A total of 31 respondents were analyzed.
Farmers described the desire to increase resilience through farming multiple life stages of oysters, for example, four farmers suggested oyster seedlings overseas or from other regions in Japan when supply of natural seedlings was insufficient due to the OA impacts on oyster early life stage (Table 3). These farmers believed purchasing seedlings would be more economically efficient than initiating their own on-land hatchery. However, a few farmers raised concerns with nonlocal seedlings such as disease or differences in growth. Four farmers wanted to initiate a local hatchery despite the expense (Table 3), preferring to use locally grown seedlings.
One farmer proposed increasing spatial flexibility through moving/relocating to other regions in Japan (Table 3), where OA is less of a concern. Specifically, he described practicing oyster farming through joining the local FCA in other prefectures.
Another set of proposed solutions involved increasing the diversification of cultured species in the hopes of maintaining production with other aquaculture species or expanding to finfish fisheries as an alternative livelihood option under increasing OA (Table 3). For example, in years when oyster mortality is high due to OA, farmers can rely on other species for income.
Lastly, four farmers described employing methods to mitigate OA such as spreading crushed oyster shells in the bay to buffer changes in pH as well as provide nursery habitat for invertebrates and fishes recruitment. One farmer proposed deploying iron wire in the bay, suggesting that high iron concentrations could accelerate phytoplankton growth; mitigating OA impacts through absorbing CO2 by photosynthesis (Table 3). Farmers described learning these methods from other farmers, or their families.
3.2.2 Science
Current and/or proposed OA adaptive strategies under science were divided into three subcategories: (1) increasing shellfish and OA knowledge through working groups and seminars (Shellfish Health Knowledge); (2) improving OA detection through monitoring (Monitoring OA and WQ); and (3) mitigating OA impacts through co-culturing oysters with algae/marine plants (Polyculture) (Table 3). Seven farmers expressed the urgent need to understand OA to create OA-specific solutions. These farmers suggested the establishment of OA-specific working groups and seminars where scientists could explain OA impacts on shellfish culture. Two farmers described the need to increase long-term water quality monitoring to detect and monitor OA; one of these farmers further described the need for scientists to explain these monitoring results to the farmers. However, one farmer expressed that monitoring shouldn’t be the responsibility of the farmers, citing a lack of time to invest in monitoring (Table 3). One farmer mentioned co-culturing oysters with algae/marine plants, as a strategy to improve resilience to OA through adjusting pH in its ambient environment by photosynthesis (Table 3).
4 Discussion
4.1 Global perceptions of OA relative to other stressors for shellfish farmers
More than 10,000 scientific studies on OA have been published in 2013-2023 (Sam Dupont, personal communication in OA Week 2023), yet we found only seven studies that focus specifically on commercial shellfish farmer perceptions of OA. In the papers we reviewed, we found great variability in farmer awareness and understanding of OA globally. Compared to U.S. shellfish farmers surveyed from Mabardy et al., 2015, farmers from the other regions of the world reported low awareness of OA. The capacity of the shellfish industry to adapt to OA hinges on their recognition of OA as an environmental hazard (O’Brien et al., 2006; Adger et al., 2009). Differentiating between the complex interplay of environmental stressors that influence shellfish survival rate is challenging (Advani and Satterfield, 2024; Lewis-Smith et al., 2025). These stressors both overlap with, and worsen OA (e.g., increasing water temperature) (Lacoue-Labarthe et al., 2016). Thus, this lack of OA awareness is likely due to the inability of farmers to correctly identify OA. Specifically, farmers are experiencing, yet not recognizing, the direct effects of OA on shellfish cultivation. For example, in British Columbia, news media highlights the scientist voices about decreasing pH in the Salish Sea, but in a follow-up survey, few shellfish farmers linked these die-off events to OA (Drope et al., 2023). Likewise, in a Japan 2022 national survey on recent environmental and oyster changes occurred in oyster aquaculture, farmers recognized the influence of “increasing water temperature in summer” (81% of the respondents), more significant than OA-associated “freshwater runoffs” (13%) and “thinner shells” (9%) (unpublished data). The incapability of identifying the impacts of OA might lead to the high level of concerns among shellfish farmers (Mabardy et al., 2015; this study).
Interestingly, “extreme concern” about OA among 93% of U.S. West coast farmers (WA, OR, and CA) occurred a decade ago (Mabardy et al., 2015), while in a more recent study, only 22% of U.S. West coast (AK, WA, OR, CA and HI) farmers reported being “very concerned” about OA. Although the follow up survey included additional U.S. states, both surveys were dominated by WA farmers (~70% of respondents). This change in perceptions may in part be due to the extensive scientific research on OA and growing partnerships between scientists and shellfish farmers, which have enabled farmers to learn more knowledge about OA and enact adaptive strategies against OA, particularly in land-based hatcheries where water chemistry can be controlled to largely mitigate/avoid acidification effects on larvae (Barton et al., 2015; Lewis-Smith et al., 2025). Thus, the increase of OA understanding/knowledge could improve the capability of shellfish growers to identify OA and address their concerns about the impacts of OA on their operation.
In actuality, farmers may not be able to discern, and therefore rank OA higher than other environmental stressors. For U.S. West coast farmers, OA was described as an unknown stress multiplier in California (Ward et al., 2022), and an issue for oyster larvae (but not adult animals) in Oregon (Green et al., 2023). Washington farmers, however, ranked OA in the middle of other stressors, which might be attributed to an increased application of adaptive strategies over the past decade, such as pH monitoring and buffering, increased hatchery production and selective breeding (Lewis-Smith et al., 2025). Thus, appropriate water quality monitoring should be applied to help farmers around the world to discern OA impacts.
4.2 Geographical and cultural variation in OA adaptive strategies between U.S. and Japan
Farmer-derived adaptive strategies for OA were only documented in the U.S. West coast (Ward et al., 2022; Green et al., 2023) and Japan (this study). The U.S. West coast has a unique oceanographical system where shellfish larvae are naturally exposed to upwelled deep water and highly affected by OA (Feely et al., 2008). On the other side, shellfish larvae are threatened by acidifying coastal waters in Hinase, Japan due to summer heavy rainfalls (Fujii et al., 2023; Pang et al., 2024). With the increase of anthropogenic CO2 emission and progressive OA events, adaptive measures are necessary to prevent/mitigate OA-related damage to the Japanese oyster industry to adapt to future OA scenarios. To learn from the successful example with developed monitoring systems and adaptive strategies (Barton et al., 2015; Ekstrom et al., 2015), we compared adaptive strategies derived from farmers in Hinase, Japan as a pre-OA event case, with U.S. West coast farmer-derived adaptive strategies as the post-OA event case (Supplementary Table 2). Despite the geographical and culture difference between these two regions, we found several commonalities between Japanese and U.S. West coast farmers. One commonality we found across Japanese and U.S. West coast farmers was the use of flexibility in farm management, an adaptive capacity domain observed across communities and geographies (Cinner et al., 2018), to adapt to OA. We found OA-specific flexible adaptive strategies such as increasing the diversity of species cultivated to reduce vulnerability of farmers from OA events on a single species, or using seeds from a hatchery in addition to wild harvest of oyster seeds to reduce strong dependence on natural seeds suggested by Japanese farmers. U.S. West coast farmers utilized similar strategies of diversifying cultured species and spatial flexibility in planting oysters (e.g. planting oysters father apart to decrease hypoxia related mortality), for example, allowing a U-pick mussel farm to increase diversity of cultured species (Green et al., 2023). Japanese farmers also suggested spatial flexibility for relocating oyster operations to an area with improved water quality for OA in the event of unfavorable water conditions. This mirrors tactics from the Pacific Northwest where shellfish hatchery industries are expanding their operations towards Hawaii (where OA is less prominent) to enhance shellfish survivorship at the early life stage (Barton et al., 2015; Cooley et al., 2017). However, flexibility in farm management might be able to be implemented over a technical level but not in the context of local culture. For example, Hinase people regard locally farmed oysters as an important component of “Satoumi” culture (Yanagi, 2012), and many farmers would rather quit the business than rely on imported seeds. Likewise, some Washington farmers raise inferior native species due to their cultural and historical significance instead of pursuing higher income (Lewis-Smith et al., 2025).
Other overlapping adaptive strategies among U.S. and Japanese farmers were the use of local or traditional ecological knowledge or hands-on learning to develop OA-specific strategies. Japanese farmers suggested spreading oyster shells and depositing iron wire in the water to mitigate OA as adaptive measures based on hands-on learning and generational traditions. Applying oyster shells for eelgrass substrate has been performed successfully in the Hinase area (Guidelines for utilization of oyster shells, Okayama Prefecture, 2022), which was found to be the most effective way of creating bottom substrate for eelgrass restoration by local people. These traditions, developed by Hinase farmers through generations, have contributed greatly to local “Satoumi” culture. Likewise, U.S. West coast farmers described strategies based on hands-on learning such as planting oysters on-bottom farther apart to avoid mortality caused by hypoxia (Green et al., 2023). These strategies, which differ by geographical region and cultural history, reflect the importance of local and community knowledge and can be used in conjunction with academic knowledge to adapt to OA.
Increasing shellfish health knowledge was advocated by shellfish farmers globally, but we found variations in the information sources that farmers utilize. U.S. West coast farmers relied on networking to share scientific knowledge about OA impacts, and solutions with other farmers, leading to the overall high OA awareness of farmers. For example, Oregon farmers communicate within the same bay, and even across state lines to discuss best practices for farming (Green et al., 2023). More formally, the state of Oregon coordinates an OAH network, where researchers, fishermen, and management agencies and coastal communities focused on building capacity and developing solutions for OA (Whitefield et al., 2021). In contrast, while Japanese farmers relied on their local Fisheries Cooperative Associations (FCAs) for information, the local culture of disseminating top-down information from the FCAs to the farmers discouraged communication and collaboration among farmers. Furthermore, Hinase farmers reported that when their opinions were not valued within the local FCA, they felt unmotivated to participate in the decision-making process. Other studies have shown that networking with scientists facilitates the interpretation of scientific findings and knowledge of ocean changes to farmers (Cross et al., 2019; Keil et al., 2021; Ward et al., 2022). Increasing networking opportunities in Japan between farmers, scientists and government officials could increase meaningful participation in decision-making and thus adaptive action.
4.3 Translating adaptive strategies into policies and actions for Japanese oyster aquaculture
Based on our survey of Japanese farmers, we identified several areas in which farmers could be better supported and empowered through policy actions to adapt to OA going forward. For example, Hinase farmers expressed concerns about the financial risk of implementing adaptive strategies: “I am not opposed to adaptive measures, but I am concerned about whether the business will be profitable after taking these measures. I am afraid I do not have the financial capability”. The Japanese government could offer subsidies or grants to mitigate the financial uncertainty that comes with investing in new adaptive strategies for OA resilience. For example, a government-subsidized on-land hatchery could provide a reliable, long-term supply of oyster seeds to supplement wild oyster seeds, and avoid reliance on international oyster seed imports. However, government-subsidized hatcheries can bring about negative impacts, such as unfair competition and restricted sources of seeds, which ultimately leads to low resilience in the oyster industry. Likewise, grants that allow farmers to experiment culturing other shellfish species could diversify shellfish products with little or no economic risk. Co-culturing experimental programs have been successful in other areas, such as adding quahogs as a diversification strategy in the Gulf of Maine oyster farms. This provides economic resilience as well as a novel seafood product (Mcmahan, 2021). Sea cucumbers (A. japonicus) is an example of species that can be potentially co-cultured with Pacific oysters (Yokohama, 2015), which could generate new avenues for additional income for Hinase farmers. Moreover, financial support could be offered to Hinase oyster farmers relocating to other regions/prefectures for better oyster production performance where OA threats are less severe, for example, the Miyagi Prefecture (Fujii et al., 2023). However, such support should be considered within the context of “Satoumi” culture in Hinase, Japan (Yanagi, 2012), and care should be given to respect local farming and the cultural and site-specific knowledge that could potentially be displaced with farmers relocating. Thus, financial support in shellfish farmer operations for OA adaptation should consider these non-pecuniary cultural factors greatly influencing the preferences and decisions of farmers. Future study is required to examine the feasibility and appropriateness of the implementation of financial policies and actions in the Japanese oyster industry.
In addition, improving science-policy coordination and raising public awareness are essential to facilitating policy implementation for addressing ocean acidification (Kelly et al., 2011; Breitburg et al., 2015). For example, facilitating collaborations between scientists and Japanese farmers to conduct water monitoring at the oyster aquaculture sites can help farmers better understand changes in their water environment, but also help scientists make more reliable forecasts for OA events. However, information about OA needs to be put into context and terms that oyster farmers can both understand and be empowered to use. As one Japanese farmer underscored, “People ask for help on monitoring. I think it’s a good thing to do, but they don’t explain the results using the data they collect, and they don’t give anything back!”. An increased knowledge of fundamental mechanisms of OA would help farmers to better distinguish OA impacts (Lewis-Smith et al., 2025). Various sources of OA-related information were found among the U.S. West coast farmers, such as conferences, industry publications, NGOs, government agencies and news (Lewis-Smith et al., 2025); while 60% of Hinase, Japan farmers sought OA related information and solution development from local FCA (this study). As 91 percent of Hinase oyster farmers obtained at least high-school level education, OA pamphlets and OA/shellfish-related information seminars could be produced and distributed by local fishery extension officers to raise OA awareness of Japanese farmers. For example, seminar topics might include how to identify OA-related drivers (heavy rainfall, freshwater influx etc.) and related effects on oysters such as deformed oyster larvae, poor growth or even massive mortality, so oyster farmers can recognize and respond to environmental stressors at the early stage. With the increase of knowledge within the local communities and connections between scientists and shellfish farmers, a strong “Knowledge-to-Action” network can be formed and strengthened to build resilience to OA impacts (Cross et al., 2019).
4.4 Study limitations and future directions for research
Our global synthesis was necessarily limited by the studies available on shellfish farmer perceptions of OA. Not all the available studies covered all the information we were assessing, thus leaving gaps in our overall synthesis. This snapshot of the current state of OA perceptions among farmers could be strengthened by additional studies on OA perceptions in other top shellfish-producing countries, namely China, Chile, South Korea, France, and Spain.
Furthermore, the case study portion of our research is limited to Hinase, Japan. Future research could expand the regional survey results to a national scale to have a more comprehensive view of the OA perceptions and adaptive strategies in Japan.
Finally, even though we compare the geographical and cultural differences in farmer-derived adaptive strategies between the U.S. and Japan, we were not able to explore the underlying causes of these differences. Also, our suggested policies and regulations based on the U.S. West coast farmer experiences require more considerations on suitability and transferability when it comes to implementation in Japan. Future survey/interview should identify farmer primary concerns and inquire about farmer opinions on the pros and cons of adaptive strategies before implementation, so that gaps of OA perceptions among farmers can be effectively addressed and locality of adaptation can be fully embraced.
5 Conclusion
Through illustrating the diversity within farmer perceptions worldwide, this study not only fills in the gap of farmer perceptions of OA in one of the top shellfish production countries in Asia, but also reflects the importance of implementing OA adaptive strategies tailored to different geographical regions and cultures. Perceptions of OA impacts were complicated by overlapping environmental stressors, some of which create greater concerns for farmers globally than OA, such as marine heatwaves and pathogens. Although adaptive strategies utilized or proposed by Japanese farmers were based on their limited understanding of OA, we found some similarities with the strategies U.S. West coast farmers applied, specifically spatial flexibility in farm management and strategies based on traditional knowledge or hands-on learning. However, while U.S. farmers benefit from networking with different actors (e.g. government, academia, organizations and industry) in the shellfish industry to mitigate OA impacts, Japanese farmers did not report networking. Increasing equity among farmers within local fisheries cooperatives and providing networking opportunities with government, industry and academia, could improve knowledge sharing and enhance the adaptive capacity of Japanese shellfish farmers to OA.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval for this research study was granted by the Institutional Review Boards at University of Washington (#00009522). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YP: Data curation, Conceptualization, Investigation, Writing – review & editing, Methodology, Writing – original draft, Formal Analysis. KG: Formal Analysis, Conceptualization, Writing – review & editing, Supervision, Methodology. YK: Formal Analysis, Writing – review & editing, Methodology. RH: Investigation, Writing – review & editing. KF: Investigation, Writing – review & editing. TT: Supervision, Methodology, Investigation, Writing – review & editing, Funding acquisition. YO: Conceptualization, Supervision, Funding acquisition, Writing – review & editing, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. We acknowledge the Nippon Foundation for providing the funding of this research work.
Acknowledgments
We are deeply grateful to the kind cooperation of local oyster farmers and staff in the local fish coop in Hinase, Japan, to provide with their insight and experience and contribute to this research. We thank Chris Rothschild for helping with designing the questionnaire used in this study and editing the methodology of this paper. We also thank Nippon Foundation Ocean Nexus Center and Washington Ocean Acidification Center for kind support and strong connections throughout this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1648960/full#supplementary-material
Supplementary Table 1 | Cultured species of the interviewed shellfish farmers from each survey region reported by six peer-reviewed publications and an NOAA report.
Supplementary Table 2 | Comparison of the category, adaptive items, description and frequency of adaptive strategies appeared among farmers from Oregon (Green et al., 2023), California, U. S (Ward et al., 2022). and Hinase, Japan (this study).
Footnotes
- ^ In this study, we refer specifically to non-cephalopod molluscan shellfish, (clams, oysters, scallops and mussels, abalone), which constitute the majority of molluscan aquaculture (FAO, 2022).
- ^ Adaptive capacity is the “ability or capacity of a system to modify or change its characteristics or behavior so as to cope better with existing or anticipated external stresses'' (Adger 2004:28)
- ^ Inclusive OD4D is a method of data planning, capturing, assessing, and application for decision-making as well as a method for enhancing community participation, acknowledge local knowledge and allow knowledge diversity (https://oceannexus.org/specialreports/inclusive-ocean-data-for-decision-making/).
- ^ In the category of OA perceptions, we define OA awareness as farmers having heard the term“OA”. OA experience describe farmer experiences of negative OA impacts and associated economic loss. OA understanding/knowledge refers to farmer understanding/knowledge about OA-related carbonate chemistry. OA concern reflects farmer concerns about the OA-induced economic loss in their business based on their understanding or experience. OA information sources focus on the sources where shellfish farmers obtained OA information from (Marbardy et al., 2015).
- ^ Washington (WA) represented 73% of the total respondents, Oregon (OR) and California (CA) represented 12% and 15%, respectively.
- ^ WA respondents made up 71% of the total survey respondents, followed by CA (8%), OR and Alaska (AK) (5%) respectively, and Hawaii (HI) (2%).
- ^ The term shellfish “producer” was used in Rodrigues et al., 2015, which in practice is equivalent to shellfish farmer.
- ^ A list of choices of environmental stressors was given in the closed ended survey conducted in the research of Rodrigues et al., 2015 (9 choices) and this study (8 choices), with option to add additional stressors in the category of “Others”. Environmental stressors were derived from open-ended questions during the interviews conducted in the research of Advani and Satterfield, 2024; Ward et al., 2022; Green et al., 2023 and Lewis-Smith et al., 2025.
- ^ Also includes other carbonate chemistry factors such as pH, pCO2, Ωarag, dissolved inorganic carbon (Ward et al., 2022).
- ^ Rising water temperature due to climate change
- ^ Elevated and unseasonal water temperatures affecting marine food webs and the metabolism of oysters.
- ^ Temperature above 19 °C, in particular.
References
Adger W. N., Dessai S., Goulden M., Hulme M., Lorenzoni I., Nelson D. R., et al. (2009). Are there social limits to adaptation to climate change? Clim. Change 93, 335–354. doi: 10.1007/s10584-008-9520-z
Advani S. and Satterfield T. (2024). Attributions of cause of oyster mortality on the British Columbia coast: Oyster growers’ and scientists’ perspectives. Ocean. Coastal. Manage. 251, 107066. doi: 10.1016/j.ocecoaman.2024.107066
Bach L. T., Alvarez-Fernandez S., Hornick T., Stuhr A., and Riebesell U. (2017). Simulated ocean acidification reveals winners and losers in coastal phytoplankton. PloS One 12, e0188198. doi: 10.1371/journal.pone.0188198
Barton A., Waldbusser G. G., Feely R. A., Weisberg S. B., Newton J. A., Hales B., et al. (2015). Impacts of coastal acidification on the Pacific Northwest shellfish industry and adaptation strategies implemented in response. Oceanography 28, 146–159. doi: 10.5670/oceanog.2015.38
Berrang-Ford L., Pearce T., and Ford J. D. (2015). Systematic review approaches for climate change adaptation research. Reg. Environ. Change 15 (5), 755–769. doi: 10.1007/s10113-014-0708-7
Breitburg D. L., Salisbury J., Bernhard J. M., Cai W. J., Dupont S., Doney S. C., et al. (2015). And on top of all that … Coping with ocean acidification in the midst of many stressors. Oceanography 28, 48–61. doi: 10.5670/oceanog.2015.31
Cai W. J., Hu X., Huang W. J., Murrell M. C., Lehrter J. C., Lohrenz S. E., et al. (2011). Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 4, 766–770. doi: 10.1038/ngeo1297
Cinner J. E., Adger W. N., Allison E. H., Barnes M. L., Brown K., Cohen P. J., et al. (2018). Building adaptive capacity to climate change in tropical coastal communities. Nat. Clim. Change 8, 117–123. doi: 10.1038/s41558-017-0065-x
Cooley S. R., Cheney J. E., Kelly R. P., and Allison E. H. (2017). Ocean acidification and Pacific oyster larval failures in the Pacific Northwest United States. Glob. Change Mar. Syst. pp, 40–53).
Cross J. N., Turner J. A., Cooley S. R., Newton J. A., Azetsu-Scott K., Chambers R. C., et al. (2019). Building the knowledge-to-action pipeline in North America: Connecting ocean acidification research and actionable decision support. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00356
Doney S. C., Busch D. S., Cooley S. R., and Kroeker K. J. (2020). The impacts of ocean acidification on marine ecosystems and reliant human communities. Annu. Rev. Environ. Resour. 45, 83–112. doi: 10.1146/annurev-environ-012320-083019
Drope N., Morin E., Kohfeld K., Ianson D., and Silver J. J. (2023). Media representations and farmer perceptions: A case study of reporting on ocean acidification and the shellfish farming sector in british columbia, Canada. Environ. Commun. 18 (4), 406–417. doi: 10.1080/17524032.2023.2280873
Duarte B., Repolho T., Paula J. R., Caçador I., Matos A. R., and Rosa R. (2022). Ocean acidification alleviates Dwarf Eelgrass (Zostera noltii) lipid landscape remodeling under warming stress. Biology 11, 780. doi: 10.3390/biology11050780
Ekstrom J. A., Suatoni L., Cooley S. R., Pendleton L. H., Waldbusser G. G., Cinner J. E., et al. (2015). Vulnerability and adaptation of US shellfisheries to ocean acidification. Nat. Clim. Change 5, 207–214. doi: 10.1038/nclimate2508
El-Shayeb F., Pittman J., Jorge-Romero G., Gianelli I., and Defeo O. (2025). The role of family in shaping adaptation and adaptive capacity in small-scale fishing communities: The yellow clam fishers in Uruguay. J. Rural Stud. 116, 103601. doi: 10.1016/j.jrurstud.2025.103601
FAO (2022). “The state of world fisheries and aquaculture,” in Towards blue transformation (FAO, Rome). doi: 10.4060/cc0461en
FAO (2024). Global aquaculture production Quantity, (1950-2022) [Data set]. Available online at: https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculture_quantity (Accessed 8 May 2024).
Feely R. A., Sabine C. L., Hernandez-Ayon J. M., Ianson D., and Hales B. (2008). Evidence for upwelling of corrosive “acidified. Water onto. continental. shelf. Science 320, 1490–1492. doi: 10.1126/science.1155676
Fujii M., Hamanoue R., Bernardo L. P. C., Ono T., Dazai A., Oomoto S., et al. (2023). Assessing impacts of coastal warming, acidification, and deoxygenation on Pacific oyster (Crassostrea gigas) farming: a case study in the Hinase area, Okayama Prefecture, and Shizugawa Bay, Miyagi Prefecture, Japan. Biogeosciences 20, 4527–4549. doi: 10.5194/bg-20-4527-2023
Gattuso J. P., Magnan A., Billé R., Cheung W. W., Howes E. L., Joos F., et al. (2015). Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349, aac4722. doi: 10.1126/science.aac4722
Green K. M., Spalding A. K., Ward M., Levine A., Wolters E. A., Hamilton S. L., et al. (2023). Oregon shellfish farmers: Perceptions of stressors, adaptive strategies, and policy linkages. Ocean. Coastal. Manage. 234, 106475. doi: 10.1016/j.ocecoaman.2022.106475
Green M. A., Waldbusser G. G., Reilly S. L., Emerson K., and O’Donnell S. (2009). Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnol. Oceanogr. 54, 1037–1047. doi: 10.4319/lo.2009.54.4.1037
Greenhill L., Kenter J. O., and Dannevig H. (2020). Adaptation to climate change–related ocean acidification: An adaptive governance approach. Ocean. Coastal. Manage. 191, 105176. doi: 10.1016/j.ocecoaman.2020.105176
Harrould-Kolieb E. R. and Hoegh-Guldberg O. (2019). A governing framework for international ocean acidification policy. Mar. Policy 102, 10–20. doi: 10.1016/j.marpol.2019.02.004
Hauzer M., Dearden P., and Murray G. (2013). The effectiveness of community-based governance of small-scale fisheries, Ngazidja island, Comoros. Mar. Policy. 38, 346–354. doi: 10.1016/j.marpol.2012.06.012
Hettinger A., Sanford E., Hill T. M., Russell A. D., Sato K. N., Hoey J., et al. (2012). Persistent carry-over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology 93, 2758–2768. doi: 10.1890/12-0567.1
Jentoft S. (2005). Fisheries co-management as empowerment. Mar. Policy 29 , 1–7. doi: 10.1016/j.marpol.2004.01.003
Keil K. E., Feifel K. M., and Russell N. B. (2021). Understanding and advancing natural resource management in the context of changing ocean conditions. Coast. Manage. 49, 458–486. doi: 10.1080/08920753.2021.1947127
Kelly R. P., Foley M., Fisher W., Feely R., Halpern B., Waldbusser G., et al. (2011). Mitigating local causes of ocean acidification with existing laws. Science 332, 1036–1037. doi: 10.1126/science.1203815
Lacoue-Labarthe T., Nunes P. A., Ziveri P., Cinar M., Gazeau F., Hall-Spencer J. M., et al. (2016). Impacts of ocean acidification in a warming Mediterranean Sea: An overview. Reg. Stud. Mar. Sci. 5, 1–11. doi: 10.1016/j.rsma.2015.12.005
Lemasson A. J., Fletcher S., Hall-Spencer J. M., and Knights A. M. (2017). Linking the biological impacts of ocean acidification on oysters to changes in ecosystem services: a review. J. Exp. Mar. Biol. Ecol. 492, 49–62. doi: 10.1016/j.jembe.2017.01.019
Lewis-Smith C., Norman K., Root L., Crim R., Roberts S., and Gavery M. (2025). Navigating ocean acidification in shellfish aquaculture: Stakeholder perspectives of developing strategies in the US Pacific Region. Aquac. Rep. 42, 102858. doi: 10.1016/j.aqrep.2025.102858
Licker R., Ekwurzel B., Doney S. C., Cooley S. R., Lima I. D., Heede R., et al. (2019). Attributing ocean acidification to major carbon producers. Environ. Res. Lett. 14, 124060. doi: 10.1088/1748-9326/ab5abc
Mabardy R. A., Waldbusser G. G., Conway F., and Olsen C. S. (2015). Perception and response of the US west coast shellfish industry to ocean acidification: the voice of the canaries in the coal mine. J. Shellfish. Res. 34, 565–572. doi: 10.2983/035.034.0241
Marshall K. N., Kaplan I. C., Hodgson E. E., Hermann A., Busch D. S., McElhany P., et al. (2017). Risks of ocean acidification in the California Current food web and fisheries: ecosystem model projections. Glob. Change Biol. 23, 1525–1539. doi: 10.1111/gcb.13594
Martin V. A. S., Gelcich S., Vásquez Lavín F., Ponce Oliva R. D., Hernández J. I., Lagos N. A., et al. (2019). Linking social preferences and ocean acidification impacts in mussel aquaculture. Sci. Rep. 9, 4719. doi: 10.1038/s41598-019-41104-5
Mcmahan M. (2021). Expanding Quahog And Oyster Polyculture In Maine. NOAA Report. Available online at: https://repository.library.noaa.gov/view/noaa/48191
Miller A. W., Reynolds A. C., Sobrino C., and Riedel G. F. (2009). Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PloS One 4, e5661. doi: 10.1371/journal.pone.0005661
Ministry of Agriculture, Forestry and Fisheries (2023). Annual statistics of census of fisheries of 2022 (in Japanese). Available online at: https://www.maff.go.jp/j/tokei/kouhyou/kaimen_gyosei/index.html (Accessed May 15, 2024).
Mirasole A., Signa G., Gianguzza P., Bonaviri C., Mazzola A., and Vizzini S. (2020). Fish assemblages cope with ocean acidification in a shallow volcanic CO2 vent benefiting from an adjacent recovery area. Mar. Environ. Res. 157, 104851. doi: 10.1016/j.marenvres.2019.104851
Narita D., Rehdanz K., and Tol R. S. (2012). Economic costs of ocean acidification: a look into the impacts on global shellfish production. Clim. Change 113, 1049–1063. doi: 10.1007/s10584-011-0383-3
Nash R., Shibaev S., Besenyei L., Deegan M., Nguyen Hoang N. K., Nguyễn Văn T., et al. (2021). Aquaculture with focus on Vietnam and Thailand. Available online at: https://opus.ostfalia.de/frontdoor/deliver/index/docId/1261/file/Nash_Shibaev_Petkam_2021_AQUACULTURE.PDF (Accessed April 21, 2024).
O’Brien G., O’keefe P., Rose J., and Wisner B. (2006). Climate change and disaster management. Disasters 30, 64–80. doi: 10.1111/j.1467-9523.2006.00307.x
Obregón C., Admiraal R., Van Putten I., Hughes M., Tweedley J. R., and Loneragan N. R. (2020). Who you speak to matters: Information sharing and the management of a small-scale fishery. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.578014
Okayama Prefecture (2022). Guidelines for utilization of oyster shells (Updated version), in Japanese. Available online at: https://www.pref.okayama.jp/uploaded/life/820701_7739509_misc.pdf.
Pang Y., Ono T., and Tanaka T. (2024). Environmental effects on growth performance of Pacific oyster Crassostrea gigas cultured in the Seto Inland Sea, Japan, from 1990 to 2021. Fish. Oceanogr. 33 (6), e12686. doi: 10.1111/fog.12686
Rodrigues L. C., Van den Bergh J. C., Massa F., Theodorou J. A., Ziveri P., and Gazeau F. (2015). Sensitivity of Mediterranean bivalve mollusc aquaculture to climate change, ocean acidification, and other environmental pressures: findings from a producer survey. J. Shellfish. Res. 34, 1161–1176. doi: 10.2983/035.034.0341
Roopa S. and Rani M. S. (2012). Questionnaire designing for a survey. J. Indian Orthod. Soc. 46, 273–277. doi: 10.1177/0974909820120509S
Rubio Benito Del Valle I., Hileman J., and Ojea Fernandez Colmeiro E. (2021). Social connectivity and adaptive capacity strategies in large-scale fisheries. Ecol. Soc.
Sadler D. E., Lemasson A. J., and Knights A. M. (2018). The effects of elevated CO2 on shell properties and susceptibility to predation in mussels Mytilus edulis. Mar. Environ. Res. 139, 162–168. doi: 10.1016/j.marenvres.2018.05.017
Schmutter K., Nash M., and Dovey L. (2017). Ocean acidification: assessing the vulnerability of socioeconomic systems in Small Island Developing States. Reg. Environ. Change. 17, 973–987. doi: 10.1007/s10113-016-0949-8
Stewart-Sinclair P. J., Last K. S., Payne B. L., and Wilding T. A. (2020). A global assessment of the vulnerability of shellfish aquaculture to climate change and ocean acidification. Ecol. Evol. 10, 3518–3534. doi: 10.1002/ece3.6149
Suh N. N. and Nyiawung R. A. (2023). Climate change dynamics and youth participation decisions in aquatic food systems: Case of the oyster sector in The Gambia, West Africa. Mar. Policy 156, 105804. doi: 10.1016/j.marpol.2023.105804
Tsurita I. (2022). “. History of hinase,” in Ethnographic study of marine conservation: eelgrass restoration in hinase, Japan (Springer Nature Singapore, Singapore), 11–40.
Turner J., Gassett P., Dohrn C., Miller H., Boylan C., and Laschever E. (2021). Opportunities for U.S. State Governments and in-Region Partners to Address Ocean Acidification through Management and Policy Frameworks. Coast. Manage. 49, 436–457. doi: 10.1080/08920753.2021.1947126
Vargas C. A., Contreras P. Y., Pérez C. A., Sobarzo M., Saldías G. S., and Salisbury J. (2016). Influences of riverine and upwelling waters on the coastal carbonate system off Central Chile and their ocean acidification implications. J. Geophys. Res. G: Biogeosciences. 121, 1468–1483. doi: 10.1002/2015JG003213
Vargas C. A., Cuevas L. A., Broitman B. R., San Martin V. A., Lagos N. A., Gaitán-Espitia J. D., et al. (2022). Upper environmental pCO2 drives sensitivity to ocean acidification in marine invertebrates. Nat. Clim. Change 12, 200–207. doi: 10.1038/s41558-021-01269-2
Waldbusser G. G., Hales B., Langdon C. J., Haley B. A., Schrader P., Brunner E. L., et al. (2015). Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat. Clim. Change 5, 273–280. doi: 10.1038/nclimate2479
Waldbusser G. G. and Salisbury J. E. (2014). Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats. Annu. Rev. Mar. Sci. 6, 221–247. doi: 10.1146/annurev-marine-121211-172238
Wallace R. B., Baumann H., Grear J. S., Aller R. C., and Gobler C. J. (2014). Coastal ocean acidification: The other eutrophication problem. Estuar. Coast. Shelf. Sci. 148, 1–13. doi: 10.1016/j.ecss.2014.05.027
Ward M., Spalding A. K., Levine A., and Wolters E. A. (2022). California shellfish farmers: Perceptions of changing ocean conditions and strategies for adaptive capacity. Ocean. Coast. Manage. 225, 106155. doi: 10.1016/j.ocecoaman.2022.106155
Washington State Blue Ribbon Panel on Ocean Acidification (2012). Ocean Acidification: From Knowledge to Action, Washington State’s Strategic Response. eds. Adelsman H. and Whitely Binder L. (Olympia, Washington: Washington Department of Ecology). Publication no. 12-01-015.
Whitefield C. R., Braby C. E., and Barth J. A. (2021). Capacity building to address ocean change: organizing across communities of place, practice and governance to achieve ocean acidification and hypoxia resilience in oregon. Coast. Manage. 49, 532–546. doi: 10.1080/08920753.2021.1947133
Xiao X., Agustí S., Yu Y., Huang Y., Chen W., Hu J., et al. (2021). Seaweed farms provide refugia from ocean acidification. Sci. Total. Environ. 776, 145192. doi: 10.1016/j.scitotenv.2021.145192
Yanagi T. (2012). Japanese commons in the coastal seas: how the Satoumi concept harmonizes human activity in coastal seas with high productivity and diversity (Springer Japan, Tokyo: Springer Science & Business Media).
Yokoyama H. (2015). Suspended culture of the sea cucumber Apostichopus japonicus below a Pacific oyster raft–potential for integrated multi-trophic aquaculture. Aquac. Res. 46, 825–832. doi: 10.1111/are.12234
Keywords: ocean acidification, shellfish aquaculture, farmer perceptions, shellfish farmers, adaptive strategies
Citation: Pang Y, Green KM, Kim Y, Hamanoue R, Furukawa K, Tanaka T and Ota Y (2025) Japan shellfish farmer perceptions of ocean acidification, adaptive strategies and comparison with global shellfish farmers. Front. Mar. Sci. 12:1648960. doi: 10.3389/fmars.2025.1648960
Received: 17 June 2025; Accepted: 24 September 2025;
Published: 21 October 2025.
Edited by:
Roger A. Rulifson, East Carolina University, United StatesReviewed by:
Ercüment Genç, Ankara University, TürkiyeJames Morris, National Centers for Coastal Ocean Science (NOAA), United States
Copyright © 2025 Pang, Green, Kim, Hamanoue, Furukawa, Tanaka and Ota. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumeng Pang, eXVtZW5nLnBhY2lmaWNhQGdtYWlsLmNvbQ==
 Yumeng Pang
Yumeng Pang Kristen Marie Green3
Kristen Marie Green3 Yulan Kim
Yulan Kim Keita Furukawa
Keita Furukawa