Abstract
The population of pillar coral, Dendrogyra cylindrus, in Florida was decimated from 2013 to 2020, primarily by the emergence of stony coral tissue loss disease (SCTLD). Monitoring of survivors from 2021 to early 2025 showed that the population underwent an additional 96% decline in live tissue, a 78% loss in living colonies, and a 55% loss of genotypes. SCTLD continued to be the primary cause of these losses. Although some surviving tissue isolates exhibited small amounts of growth, the population remains extremely small, with only an estimated 9.6 m2 of tissue remaining on 23 colonies (15 genotypes). Additionally, the colonies are far too dispersed to successfully fertilize spawned gametes. Further declines in the population since 2020 highlight the instability of the remnant population and underscore the value of the pillar coral rescue program and ongoing propagation efforts. As of February 2025, eight different in situ and ex situ facilities were caring for rescued D. cylindrus. Experimental fragmentation at one in situ nursery identified variable, but continually improving, growth rates across multiple fragmentation events. Sexual propagation efforts at an ex situ nursery documented 105 different rescue fragments spawning over 5 years. The smallest fragment was 9 cm × 7 cm × 9 cm, suggesting a potential “minimum colony size” for this species to have reproductive capacity. During these spawning events, 82 juveniles were being raised ex situ in early 2025. Two of these sexually propagated juveniles spawned 6 years after settlement, thus establishing a potential minimum age for reproduction.
Introduction
The pillar coral, Dendrogyra cylindrus, is a historically uncommon but conspicuous coral species in the Caribbean region. It is listed as critically endangered on the IUCN Red List (Aronson et al., 2008) and as endangered under the U.S. Endangered Species Act. The Florida population of the species was extensively monitored from 2013 to 2020 (Jones et al., 2021; Neely et al., 2021a). During that time, the population suffered extensive decline, with minor losses from bleaching and white plague and catastrophic losses from stony coral tissue loss disease (SCTLD). Reported losses to the population through the end of 2020 included a 94% loss of tissue, a 93% loss of colonies, and an 86% loss of genotypes (Neely et al., 2021a).
This disastrous decline resulted in the functional extinction of the species in Florida, with only a handful of survivors scattered across the reef tract and essentially no chance for successful reproduction in the wild. From 2021 to 2025, we continued to monitor the remaining colonies for continued decline or potential recovery and also updated the Florida D. cylindrus database when previously unknown colonies (live or dead) were found.
The rapid decline of D. cylindrus in Florida also prompted a rescue program in which over 550 fragments of a presumed 128 wild genotypes were collected between 2015 and 2019 and brought to in situ and ex situ facilities for care (Neely et al., 2021b). These corals have grown substantially and reproduced successfully (O’Neil et al., 2021). Here, we provide updates on the sexual and asexual reproduction of the rescued individuals.
Methods
Newly discovered corals
Reports of D. cylindrus colonies came from scientific divers at government agencies, universities, environmental consulting groups, and non-profit organizations. We cross-referenced these with existing geographic coordinates and photos to determine whether their presence was already documented. If the coral was previously unknown, we obtained the following either through the report or via ground truthing: coral size (straight-line length, width, and height), percent coverage of live tissue/recent mortality/old mortality, and depth. For sites with multiple colonies, distances and bearings were taken between corals for differentiation and repeatable identification of individuals. Any reported live corals were added to regular targeted monitoring efforts.
Monitoring of survivors
Surviving D. cylindrus were monitored at least annually through targeted surveys of known colonies. Colonies in Dry Tortugas National Park were monitored triannually, those in southeast Florida were monitored monthly or triannually, and those in the Florida Keys National Marine Sanctuary were monitored opportunistically at least once a year, but sometimes more frequently. During each monitoring event, the corals were assessed for percent tissue coverage and percent recent mortality, with the cause(s) of any recent mortality recorded.
Calculating population change
The amount of live D. cylindrus tissue on Florida reefs was calculated using the same methodology as in the baseline study by Neely et al. (2021a). The live tissue area was determined by multiplying the estimated surface area of the entire colony [assumed and calculated as a half-ovoid using length (L), width (W), and height (H) measurements] by the proportion of living tissue remaining.
The amount of tissue remaining at the beginning of 2025 was compared to the amount remaining at the end of 2020 and to the amount of tissue present during the 2013–2014 baseline surveys. Any living D. cylindrus colonies found after the baseline surveys were assumed to have remained unchanged since 2013. Given that these corals very likely underwent tissue loss since 2013–2014 (as observed via long-term colony monitoring), our estimates were conservative, with the actual tissue loss probably being much higher.
We calculated the proportion of remaining colonies in 2025 by dividing the number of colonies alive in early 2025 by the number known to be alive at two previous time points: the end of 2020 and the 2013–2014 baseline. Any dead colonies first found after 2020 were assumed to have been dead since the baseline surveys, again making the reported losses conservative.
We calculated the proportion of genotypes remaining in 2025 by dividing the number of genotypes alive in early 2025 by the number of genotypes known to be alive at the end of 2020 and the number known to be alive at the baseline surveys. As described in Neely et al. (2021a), a genotype was defined as a colony or group of colonies that was specifically genotyped in Chan et al. (2019) or, when colonies were not genotyped, any colony that was at least 70 m from any other colony.
We also replicated the methods from Neely et al. (2021a) when estimating the distances between D. cylindrus genotypes to highlight the unlikelihood of successful sexual reproduction via unassisted spawning. We used ArcGIS Pro to calculate the nearest surviving genotype for each genotype.
Rescue population breeding and settler growth: land-based nursery
Induced spawning and propagation efforts for ex situ D. cylindrus have been ongoing at The Florida Aquarium Coral Conservation and Research Center (CCRC) since 2019. Parent fragments were housed in fully recirculating aquaria in either greenhouse systems receiving natural light or in induced spawning systems artificially replicating in situ temperature and light regimes (O’Neil et al., 2021). Across all years, we assessed the total number of distinct fragments observed spawning and identified the smallest spawning individuals.
To assess growth, we measured the diameter of each surviving settler from each year’s spawning cohort. Measurements were taken in February 2025 for surviving settlers from the 2024, 2022, 2021, and 2018 spawning events; the 2018 cohort was spawned from fragments held at the Keys Marine Laboratory (Neely et al., 2020), with the larvae transported to and settled at the CCRC.
Rescue population fragmentation and growth: field-based nursery
Dendrogyra cylindrus fragments historically collected from wild colonies were housed on “boulder tree” platforms—PVC trays with fixed mounts—suspended in the water column at the Coral Restoration Foundation (CRF) nursery in the upper Florida Keys. The fragments were asexually propagated with the goal of creating additional fragments for eventual outplanting and increasing growth rates. Asexual propagation was conducted across four time periods. In January 2021, fragments were created in situ using hand cutters and loppers or, for larger D. cylindrus pieces, they were temporarily moved ex situ and cut with a wet tile saw. In January 2022, the in situ fragments were cut the same way, while the ex situ fragments were cut with a wet band saw. During two additional fragmentation events—in November/December 2022 and February 2023—all fragments were cut in situ. All fragments were epoxied to PVC cards and returned to the boulder trees.
We assessed the growth rates of each fragment by comparing the initial fragmentation diameter to the most recent diameter measurement (taken 147–539 days after fragmentation) and dividing the number of days between the two assessments. The maximum diameter of each fragment from the January 2021 to January 2022 propagation events was measured using calipers. Fragments produced from the November 2022 to February 2023 events were measured using top-down photographs, which were assessed for total surface area using ImageJ, and then the diameter was recalculated based on the assumption of a circular area. Any corals for which partial or full mortality was observed, or for which the end maximum diameter was smaller than the initial diameter, were discarded from the dataset as non-representative of normal growth rates. We used multiple regression to assess correlations between the growth rates and 1) the maximum diameter of the newly cut fragments and 2) the date of fragmentation (with dummy values for each of the four fragmentation events).
Results
In situ colonies
The total number of known D. cylindrus colonies, live or dead, on Florida’s Coral Reef as of February 2025 was 876 (Figure 1). A total of 811 (93%) were alive upon discovery, and 61 of the 876 colonies, representing a presumed 13 genotypes, were discovered since the publication of Neely et al. (2021a). Of these new discoveries, 46 colonies (three new genotypes) were found in Dry Tortugas National Park as part of an unprecedented coral survey effort ahead of the arrival of SCTLD to the area. The Dry Tortugas colonies were all alive at first sighting. In contrast, of the 15 colonies found elsewhere on Florida's Coral Reef from 2021 to 2025, only 5 had live tissue. Two of these, both in Biscayne National Park, had all remaining tissue collected for ex situ rescue in 2023.
Figure 1
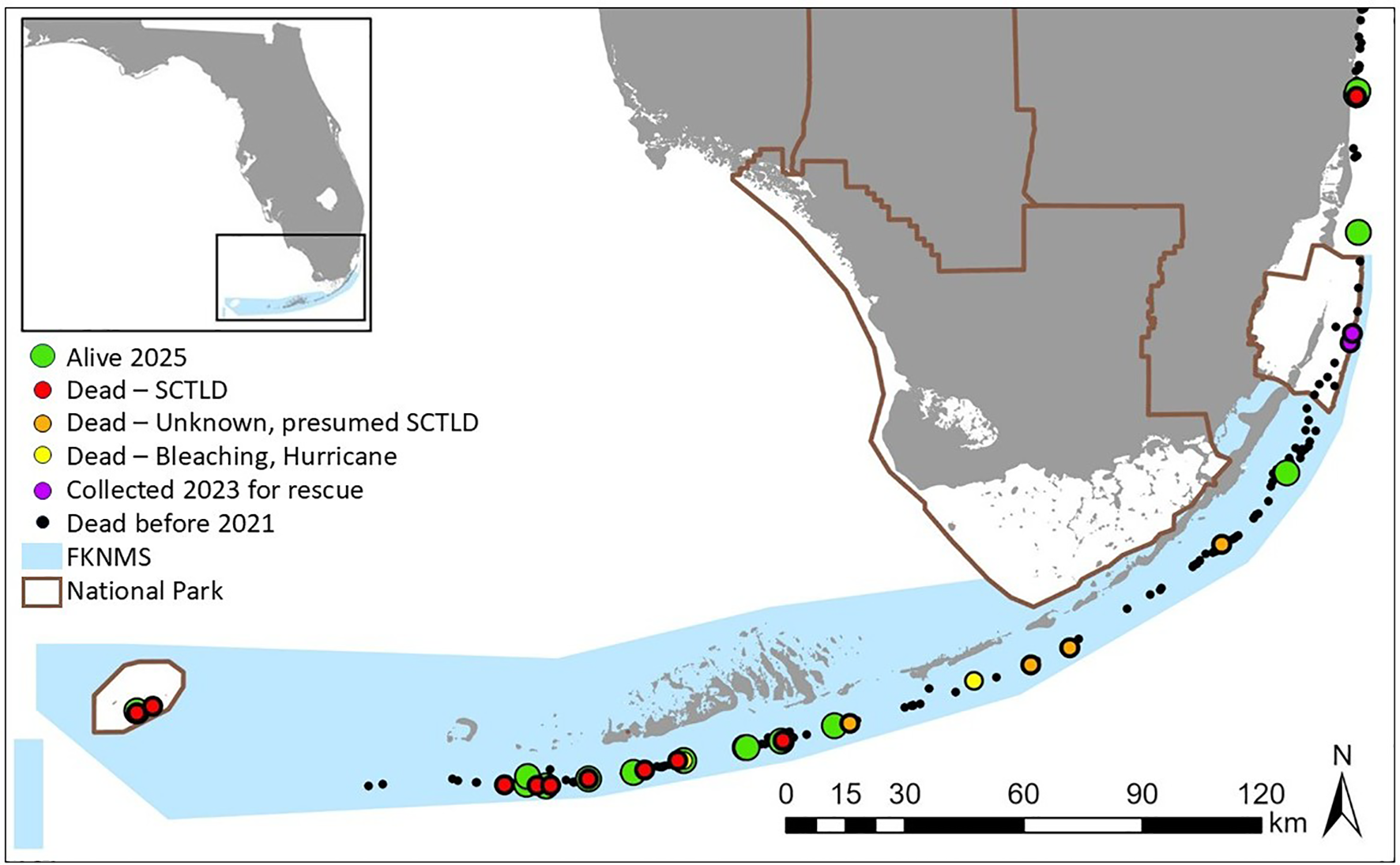
Distribution of Dendrogyra cylindrus genotypes on the Florida’s Coral Reef. The majority of colonies were dead before 2021 (black dots). Of the genotypes still alive at the end of 2020, by 2025, some retained live tissue (green dots), some died of stony coral tissue loss disease (red dots), some died of presumed SCTLD (orange dots), two died from other causes (one from bleaching, one from a hurricane; yellow dots), and two had their last remaining tissue collected for ex situ holding (purple dots). All corals lie within (from southwest to northeast) Dry Tortugas National Park, Florida Keys National Marine Sanctuary, Biscayne National Park, or the southeast Florida region.
Monitoring of survivors from 2021 to 2025 documented continued declines in coral tissue and the number of living colonies and genotypes (Figure 2A). From 2021 to 2025, the amount of living D. cylindrus tissue on Florida’s Coral Reef decreased by an additional 96%, for a total loss of at least 99.7% since the 2013 baseline surveys. The number of surviving colonies also continued to decline, with a 78% loss of colonies between 2021 and 2025, and a total loss of 97% since the baseline surveys. Similarly, the number of surviving genotypes declined by an additional 55% from 2021 to 2025 for a total loss of 92% since the baseline surveys.
Figure 2
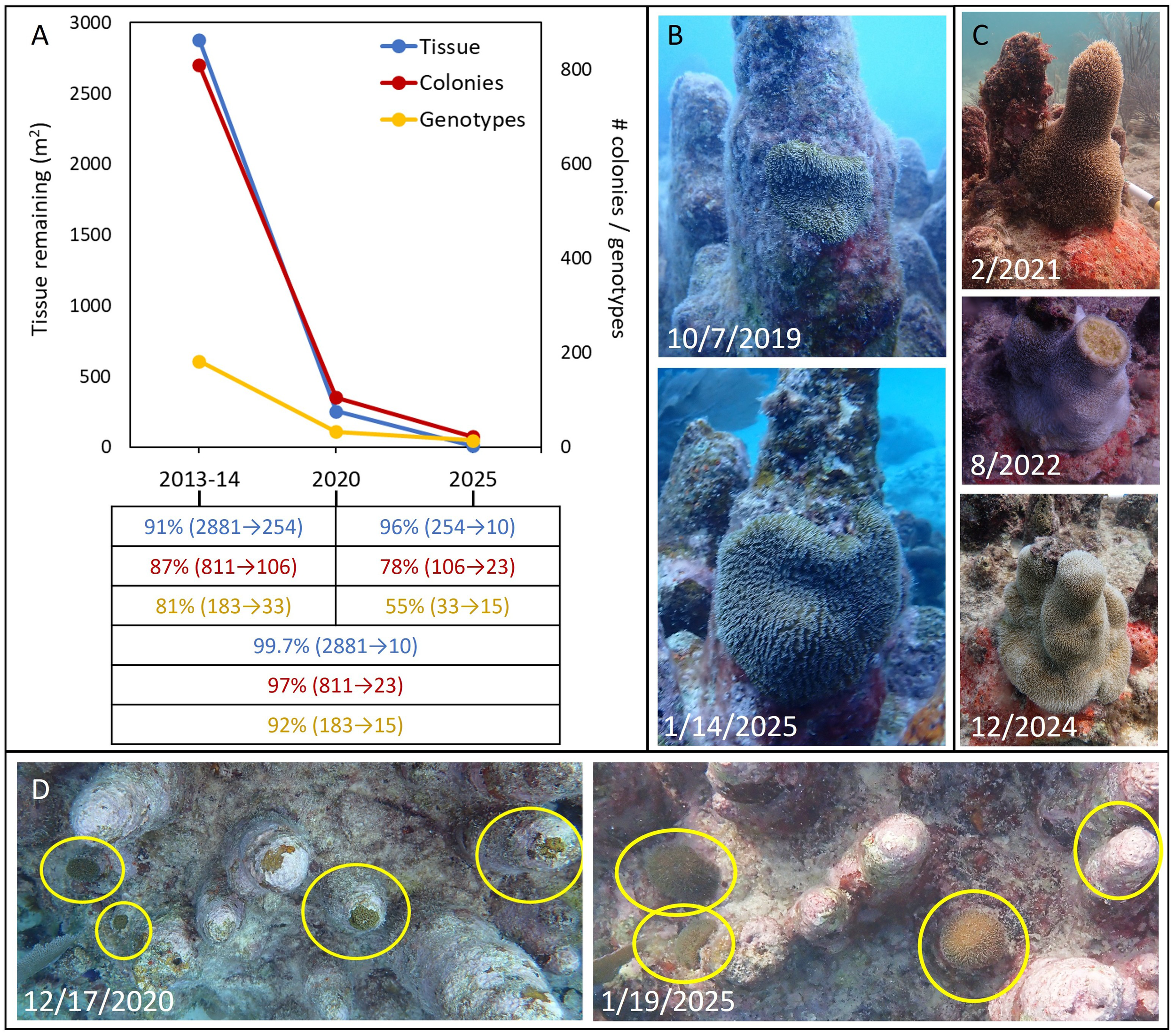
(A) Decline of the Florida Dendrogyra cylindrus population as measured by square meters of live tissue (blue), number of living colonies (red), and number of surviving genotypes (yellow). The values each represent the sum of each direct measurement (no variance) and show the change in each metric from the 2013–2014 baseline surveys to the end of 2020, from the beginning of 2021 to early 2025, and from the baseline surveys to early 2025. Values from baseline to 2020 differ slightly from Neely et al. (2021a) because the newly found colonies were included in these updated numbers. (B) Growth of the last remaining tissue isolate of a lower Keys genotype. (C) Changes to a tissue isolate in the southeast Florida region, showing the loss of the pillar tip to an unknown cause in 2022, followed by growth of the isolate. (D) Four remaining isolates (yellow circles) of a lower Keys genotype, showing growth in three of them, but death in the fourth.
By the beginning of 2025, we measured only 9.6 m2 of living D. cylindrus tissue on Florida’s reefs, spread among 23 colonies representing 15 genotypes. Of this tissue, 26% was in Dry Tortugas National Park (8 colonies, 1 genotype), 24% was in the Florida Keys National Marine Sanctuary (11 colonies, 11 genotypes), and 49% was on southeastern Florida reefs north of Biscayne National Park (4 colonies, 3 genotypes). All surviving colonies underwent substantial tissue loss over the years. Among the survivors, the percentage of tissue remaining by 2025 ranged from 0.1% to 25%. With only three exceptions (10%, 15%, and 25%—all of which were in the southeastern Florida region), all survivors had only 5% or less of their tissue remaining.
For genotypes remaining in 2025, the median distance between survivors was 8.0 km. Only two genotypes resided within less than 1 km (two at 402 m) of a conspecific. The most remote genotype was the sole surviving genotype in Dry Tortugas National Park, which was 91.4 km from its nearest neighbor.
Losses to the D. cylindrus population from 2021 to 2025 continued to be predominantly caused by SCTLD. Of the 18 genotypes lost during that time, 12 were observed to perish completely from SCTLD (multifocal, non-linear, acute lesions), and an additional 4 were presumed to do so based on appearance and the absence of other stressors. One genotype was lost to a hurricane that knocked off the last remaining piece of tissue. Another genotype, which had 12 individual colonies with varying degrees of live tissue in the early summer of 2023, experienced total loss of live tissue by November 2023 due to bleaching-related mortality. All other D. cylindrus genotypes in Florida bleached completely during this hyperthermal event but had no resulting tissue loss.
By early 2025, surviving corals generally had only one or a few small isolates of live tissue remaining. While the majority of colonies continued to lose tissue between 2021 and 2025, we know of at least three colonies where isolates exhibited moderate growth (Figures 2B–D).
Rescue population breeding and settler growth: land-based nursery
Over 5 years of propagation efforts, spawning was observed from 105 D. cylindrus fragments rescued from wild colonies and held at the CCRC. The smallest ex situ fragment observed spawning was 9 cm × 7 cm × 9 cm (L × W × H). In 2024, spawning efforts focused on fragments greater than or equal to that size, and 75% of those successfully spawned.
In February 2025, sexual recruits that had settled 6 months prior (the 2024 cohort) measured between 1.5 and 4 mm in maximum diameter (Figures 3A, B). Recruits from 42 and 54 months earlier (the 2020 and 2021 cohorts) ranged from 2 to 11 cm, and those spawned 78 months earlier (the 2018 cohort, whose larvae spawned at the Keys Marine Lab and transported to the CCRC) ranged from 10 to 21 cm. Assuming a linear growth rate, the maximum diameter of sexually spawned individuals increased by 1.8 mm/month from settlement to 6.5 years of age. Three settlers from the 2018 sexually produced cohort spawned as males in 2024 (sizes at spawning: 9.5 cm × 8 cm × 8 cm, 10 cm × 10 cm × 5.5 cm, and 14.5 cm × 13 cm × 5 cm). Rescue project fragments collected in situ and held at the CCRC also exhibited notable growth (Figures 3C, D).
Figure 3
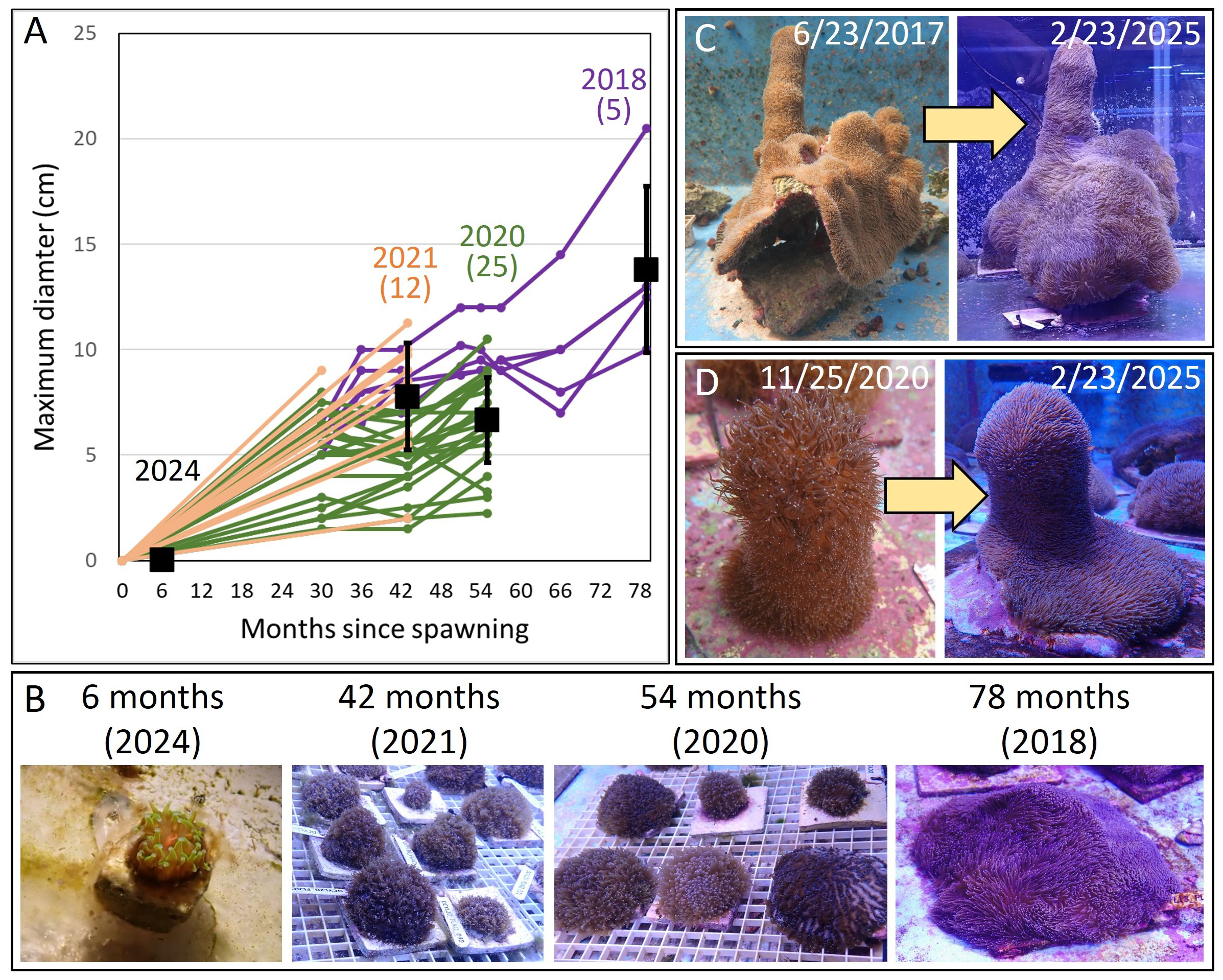
(A) Size of sexually produced juveniles from four cohorts (2018, 2020, 2021, and 2024). Individual lines represent the growth measurements of specific juveniles, while black boxes represent the mean maximum diameters (± SD) of corals from each cohort, as measured in February 2025. (B) Images taken in February 2025 of sexually propagated juveniles from the four different year cohorts. (C, D) Examples of multiyear growth of two Dendrogyra cylindrus fragments collected from the wild and held in ex situ raceways.
Rescue population fragmentation and growth: field-based nursery
The maximum diameter growth rate of D. cylindrus fragments at the CRF nursery varied across fragments from 0 to 3.58 mm/month (Figure 4), with an average of 0.83 (± 0.68 SD) mm/month. Multiple linear regression analysis (R2 = 0.31) found that the maximum diameter of a newly-cut fragment was not a significant predictor of subsequent growth rates (p = 0.16). Growth rates did, however, increase with each subsequent fragmentation date (January 2021: 0.44 ± 0.33; January 2022: 0.65 ± 0.43; November/December 2022: 1.04 ± 0.68; February 2023: 1.45 ± 0.88 mm/month), and the multiple regression identified fragmentation date as a significant factor in determining growth rates (January 2021: p < 0.001; January 2022: p < 0.001; November/December 2022: p = 0.02).
Figure 4
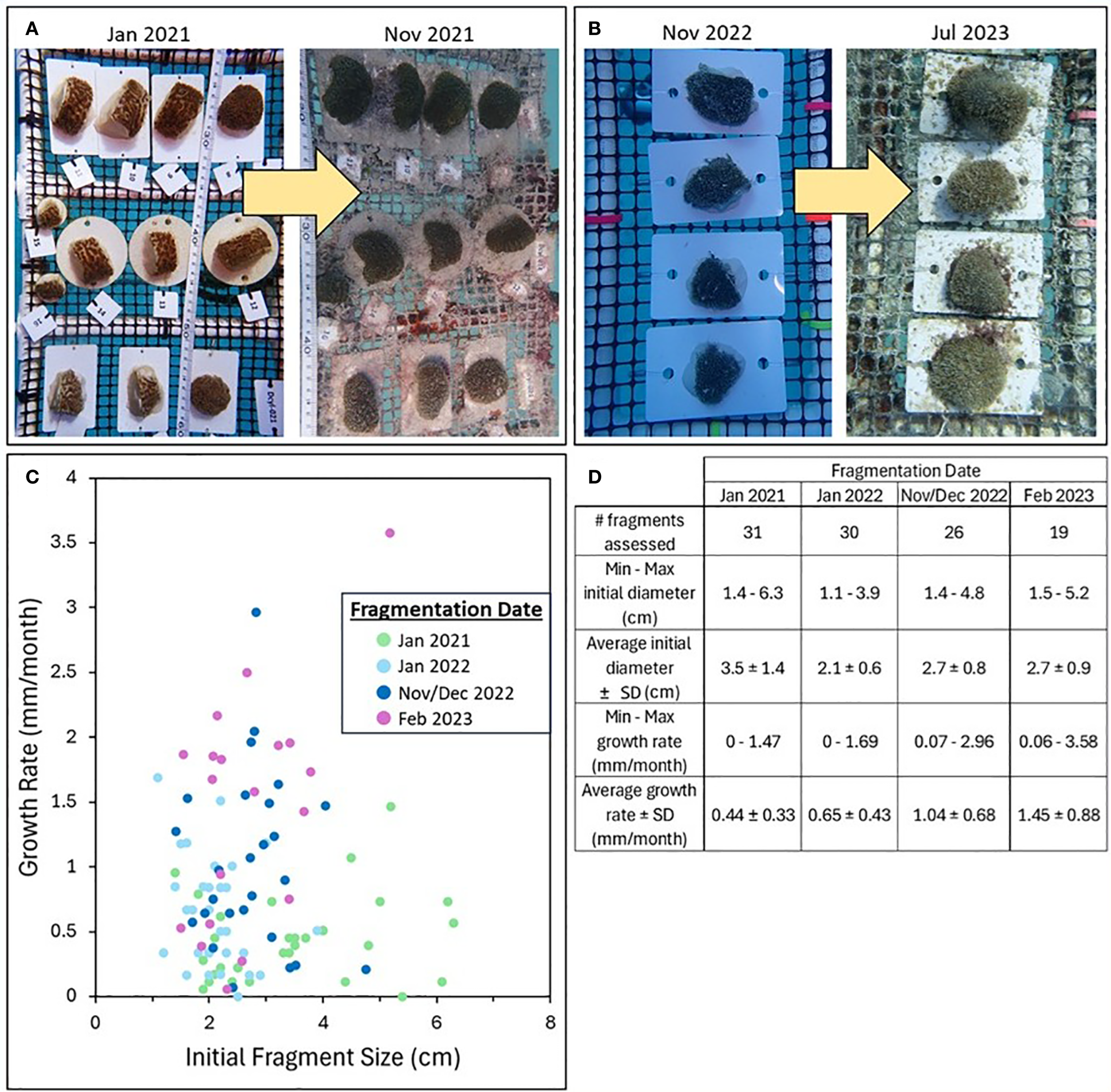
(A, B) Examples of Dendrogyra cylindrus fragment growth between the initial fragmentation dates and subsequent monitoring dates. (C) Growth rates plotted against initial fragment size and color-coded by fragmentation event; fragment size did not significantly impact growth rate, but fragmentation date did. (D) Table of fragment sizes and growth rates across each fragmentation event (SD, standard deviation).
Discussion
The discovery of a small number of previously unknown D. cylindrus genotypes from 2020 to 2024 indicates the possibility of other survivors in Florida. However, the slow rate of discovery, despite the extensive surveys conducted by various monitoring and coral restoration programs in Florida each year, combined with the small number of colonies found alive, indicates that the vast majority of the corals of this species in Florida have already been found. It is highly unlikely that there is an unknown remnant population.
The known remaining individuals are incapable of rebuilding the population. As of February 2025, only 9 of the surviving 23 individuals had tissue isolates that met the minimum observed spawning size. Additionally, the median distance between surviving genotypes increased from the already large 1.0 km in 2020 to over 8.0 km in 2025. The two closest remaining genotypes (403 m apart) both contain only a few square centimeters of remaining tissue.
SCTLD remains the biggest threat to this species in Florida, where in 2025 the disease was largely considered endemic. Since 2020, 89% of genotype losses were due to SCTLD. All surviving colonies in the Dry Tortugas and two of those within the FKNMS were kept alive through amoxicillin treatments on SCTLD lesions. These treatments are no longer authorized within the FKNMS, so further losses are expected. Water temperatures and cumulative thermal stress in 2023 far exceeded those of any prior year [reviewed in Neely et al. (2024)]. Despite all surviving D. cylindrus colonies fully bleaching during that time, only a single genotype was lost, indicating that the remaining colonies are capable of handling temperature extremes and are unlikely to face mortality from this particular threat unless new thermal stress records are set or other synergistic factors occur simultaneously.
Despite the dire situation of the wild population, husbandry, fragmentation, and propagation efforts have demonstrated that ex situ corals can grow and reproduce. Fragmentation of rescued individuals identified highly variable growth rates, with highly variable growth rates, with growth rates during the 2023 fragmentation event being over three times greater, on average, than those from the 2021 one. This variability may have resulted from changing environmental factors over time within the nursery, minor changes in handling during fragmentation, the improved resilience of pre-fragmented colonies with longer pre-fragmentation nursery times, or other unknown factors. Further experimentation into optimizing growth rates based on these factors is needed to improve the efficiency of propagation efforts. Dendrogyra cylindrus fragments at the CRF nursery were generally cut to approximately 2.5 cm in diameter. With an average growth rate of 0.83 mm/month, we estimate that fragments would need to be held in the nursery for an average of 6.5 years to reach the minimum spawning size (9 cm) observed at the CCRC. Nurseries may need to plan for extended durations for fragments of this species to reach maturity.
Ex situ sexual propagation has resulted in successful larval production and settlement. Juvenile rearing requires extensive husbandry, yet successful spawning at age 6 by sexually produced juveniles indicates the potential for multigenerational ex situ production. The genetic material for this population, including next-generation individuals, remains extant, and it is hoped that one day, these genotypes and their offspring may be returned to Florida’s Coral Reef.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
KN: Data curation, Writing – review & editing, Writing – original draft, Conceptualization, Methodology, Visualization, Formal Analysis, Validation, Project administration. RM: Data curation, Writing – review & editing, Methodology, Investigation, Resources. KO’N: Resources, Project administration, Investigation, Supervision, Writing – review & editing. NC: Data curation, Investigation, Methodology, Writing – review & editing, Project administration. DG: Investigation, Writing – review & editing, Data curation, Methodology. MM: Writing – review & editing, Investigation, Methodology, Data curation. BW: Writing – review & editing, Funding acquisition, Supervision, Methodology. AZ: Methodology, Writing – review & editing, Investigation, Data curation. CH: Methodology, Data curation, Investigation, Writing – review & editing. AE: Methodology, Investigation, Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Assessments of the Magic Castles site in Dry Tortugas National Park were funded by National Park Service agreements P16AC00991 and P23AC01375. Assessments within the Florida Keys National Marine Sanctuary were done pro bono by KLN. Assessments in the SEFL region were funded by the Florida Department of Environmental Protection awards B2A150, B48140, B46AD7, B3C3AD, B558F2, B7B6F3, B96800, C00BAE, C20F00, C3D4C8, C2003, and C229F7. Funding for the Coral Restoration Foundation’s pilot study was provided by NFWF awards (2020: #302.20.068646; 2022: #74785) and a Disney Grant (2021, Pillar Corals: Zero to Hero). Coral holding and spawning at the CCRC were supported by the NOAA CRCP Award #NA18NOS4820206, the NOAA Office of Protected Resources Award NA15NMF4720280, the Paul G. Allen Family Foundation, and the CORDAP Coral Accelerator Program award CAP-2022-1193. The views, statements, findings, conclusions, and recommendations expressed herein are those of the authors and do not necessarily reflect the views of the State of Florida or any of its sub-agencies.
Acknowledgments
We are grateful to the past and present divers of the following Nova Southeastern University labs: Disease Intervention, Coral Reef Restoration, Assessment & Monitoring (CRRAM), and GIS/Spatial Ecology. In particular, we thank Michelle Dobler, Kevin Macaulay, Arelys Chaparro, Kathryn Toth, and Reagan Sharkey. We also thank Amelia Moura and Phanor Montoya-Maya, in addition to the staff and interns involved in the pilot study at the Coral Restoration Foundation. We thank the current and past staff of The Florida Aquarium’s Coral Conservation Program, with special thanks to Emily Williams, Jenny Lee, Austyn Bushman, and Paula Holmes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Aronson R. Bruckner A. Moore J. A. Precht B. Weil E. (2008). Dendrogyra cylindrus. IUCN Red List Threatened Species. doi: 10.2305/IUCN.UK.2008.RLTS.T133124A3582471.en
2
Chan A. N. Lewis C. L. Neely K. L. Baums I. B. (2019). Fallen pillars: The past, present, and future population dynamics of a rare, specialist coral–algal symbiosis. Front. Mar. Sci6, 218. doi: 10.3389/fmars.2019.00218
3
Jones N. P. Kabay L. Semon Lunz K. Gilliam D. S. (2021). Temperature stress and disease drives the extirpation of the threatened pillar coral, Dendrogyra cylindrus, in southeast Florida. Sci. Rep.11, 14113. doi: 10.1038/s41598-021-93111-0
4
Neely K. L. Lewis C. L. Lunz K. S. Kabay L. (2021a). Rapid population decline of the pillar coral Dendrogyra cylindrus along the Florida Reef Tract. Front. Mar. Sci8, 656515. doi: 10.3389/fmars.2021.656515
5
Neely K. L. Lewis C. L. Macaulay K. A. (2020). Disparities in spawning times between in situ and ex situ pillar corals. Front. Mar. Sci7, 643. doi: 10.3389/fmars.2020.00643
6
Neely K. L. Lewis C. L. O’Neil K. Woodley C. M. Moore J. Ransom Z. et al . (2021b). Saving the last unicorns: the genetic rescue of Florida’s pillar corals. Front. Mar. Sci8, 657429. doi: 10.3389/fmars.2021.657429
7
Neely K. L. Nowicki R. J. Dobler M. A. Chaparro A. A. Miller S. M. Toth K. A. (2024). Too hot to handle? The impact of the 2023 marine heatwave on Florida Keys coral. Front. Mar. Sci11, 1489273. doi: 10.3389/fmars.2024.1489273
8
O’Neil K. L. Serafin R. M. Patterson J. T. Craggs J. R. K. (2021). Repeated ex situ Spawning in Two Highly Disease Susceptible Corals in the Family Meandrinidae. Front. Mar. Sci8. doi: 10.3389/fmars.2021.669976
Summary
Keywords
SCTLD, bleaching, population decline, pillar coral, propagation, spawning
Citation
Neely KL, Morgan RM, O’Neil KL, Cox N, Gilliam DS, Mair MB, Walker BK, Zummo A, Harrell C and Ellis A (2025) Status of Florida’s pillar coral population: in situ declines and ex situ successes. Front. Mar. Sci. 12:1650422. doi: 10.3389/fmars.2025.1650422
Received
19 June 2025
Accepted
03 September 2025
Published
18 September 2025
Volume
12 - 2025
Edited by
Hajime Kayanne, The University of Tokyo, Japan
Reviewed by
Adán Guillermo Jordán-Garza, Universidad Veracruzana, Mexico
Erik H. Meesters, Wageningen University and Research, Netherlands
Erica K. Towle, NOAA Coral Reef Conservation Program, United States
Updates
Copyright
© 2025 Neely, Morgan, O’Neil, Cox, Gilliam, Mair, Walker, Zummo, Harrell and Ellis.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen L. Neely, kneely0@nova.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.