- 1School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand
- 2National Institute of Water & Atmospheric Research, Wellington, New Zealand
- 3Univ. Brest, Ifremer, Biologie et écologie des écosystèmes marins profonds (BEEP), Plouzané, France
Habitat heterogeneity is known to influence faunal community structure, but its influence on deep-sea benthic communities remains understudied, particularly for polymetallic nodule environments in abyssal waters. As nodules are currently of interest for mining, understanding the potential impact of this disturbance on habitat heterogeneity, and the subsequent effect on faunal communities, becomes critical for developing environmental management plans. Although some aspects of the influence of habitat heterogeneity on the nodule-associated fauna have been studied, the influence on multiple size components of the benthic community across varying spatial scales has not yet been fully assessed, and the current metrics by which habitat heterogeneity is measured may be insufficient. This review synthesizes existing research regarding habitat heterogeneity, the influence of disturbance on habitat heterogeneity, and the influence of this heterogeneity on metazoan fauna (megafauna, macrofauna, and meiofauna) in polymetallic nodule environments across spatial scales. Current gaps in knowledge and the implications of this knowledge for the management of proposed deep-seabed mining are also discussed.
1 Introduction
Habitat heterogeneity is generally defined as variety in habitat types and has several synonymous terms, including habitat diversity, habitat complexity, and habitat structure (Carvalho and Barros, 2017). Extensive research has examined the influence of habitat heterogeneity on the structure of communities (e.g., Watson, 1964; Bazzaz, 1975; Jumars, 1975a, Jumars, 1975b; Heck and Wetstone, 1977; Abele and Walters, 1979; Rigby and Lawton, 1981; Sardà et al., 1994; Kaiser et al., 1999; Tews et al., 2004; Bulling et al., 2008; McClain and Barry, 2010; Godbold et al., 2011; Zeppilli et al., 2016), including ecological succession (Cordes et al., 2010; Meyer et al., 2016; Aguzzi et al., 2018), and community resilience to disturbance (Kaiser et al., 1999; Boyero, 2003; Rees et al., 2009; Godbold et al., 2011; Clark et al., 2016; Sweetman et al., 2017). However, due to its inaccessibility, much of the deep sea (> 200 m water depth) remains understudied and therefore the influence of habitat heterogeneity on benthic communities in this environment remains poorly understood (Baco et al., 2016; Amon et al., 2022; Radziejewska et al., 2022). This lack of knowledge is particularly true for polymetallic nodule environments, which are commonly located in the most remote parts of the ocean (typically at abyssal depths, 3000–5000 m deep; Simon-Lledó et al., 2019b; Amon et al., 2022; Radziejewska et al., 2022).
In the deep sea, habitat heterogeneity comes in many forms, and varies considerably across spatial and temporal scales (Figure 1). On large (i.e., regional) scales, habitat heterogeneity generally takes the form of broad-scale environmental variability. For example, depth gradients and proximity to shore influence the presence of physiological stressors (e.g., pressure, salinity) and critical resources (e.g., oxygen, food, calcium carbonate, etc.), which together exert a considerable influence on the diversity, composition, and abundance of deep-sea faunal communities over large spatial scales (Sanders and Hessler, 1969; Thiel, 1979; Etter and Grassle, 1992; Rex et al., 2006; Rex and Etter, 2010; Priede et al., 2013). Temporal scales may also influence deep-sea habitat heterogeneity through seasonal (Billett et al., 2001; Ruhl and Smith, 2004; Moran et al., 2005; Sun et al., 2006) or climatic cycles (e.g., climate oscillations; Billett et al., 2001; Levin et al., 2001; Ruhl and Smith, 2004; Arntz et al., 2006; Hooff and Peterson, 2006) that influence the flux of surface-derived carbon to the seafloor. However, the extent of the relationship between temporal cycles and benthic community structure remains ambiguous (Thurston et al., 1998; Radziejewska, 2002; Gambi et al., 2014; Woolley et al., 2016), particularly in remote and understudied abyssal environments (Lutz et al., 2007; Kahn et al., 2012). At intermediate scales, habitat heterogeneity in the form of habitat type (e.g., methane seep, seamounts) and substrate type (e.g., soft or hard substrate, biogenic structures) remains a significant factor influencing community structure (Levin et al., 1986; Cordes et al., 2010; Danovaro et al., 2014; Meyer et al., 2016; Gooday et al., 2021; Kazanidis et al., 2021), with higher heterogeneity generally correlated with higher diversity and higher abundance of fauna (Samadi et al., 2006; Levin et al., 2010; Huvenne et al., 2011; Zeppilli et al., 2011, Zeppilli et al., 2012; Robert et al., 2015; Lacharité and Metaxas, 2017). However, variation in environmental variables (e.g., currents/flows, hypoxic conditions) can overshadow these effects at certain locations (Gooday et al., 2010; Pereira et al., 2022). At intermediate to small scales, food flux can enhance habitat heterogeneity through the creation of food patches, which can be characterized by low (e.g., diffuse patches of POC flux or marine snow; McClain et al., 2011; Danovaro et al., 2013; Gambi et al., 2014; Lacharité and Metaxas, 2017) or high organic carbon enrichment (e.g., an organic fall; Lundsten et al., 2010; Laurent et al., 2013; Smith et al., 2015; Silva et al., 2021). Low-enrichment food patches often support higher faunal diversity (McClain et al., 2011; Danovaro et al., 2013; Lacharité and Metaxas, 2017), while high-enrichment food patches (i.e., organic falls) consistently increase faunal abundance (Smith et al., 2015; Webb et al., 2017; Young et al., 2022). Although these high-enrichment patches often also support higher species richness, dominance by organic fall specialists generally results in lower evenness than background communities (Lundsten et al., 2010; Cunha et al., 2013; Young et al., 2022). At smaller spatial scales, the influence of substrate heterogeneity tends to be stronger than the influence of broader scale environmental heterogeneity. For example, across ocean basins, environmental conditions, and broader habitat type, high sediment grain size diversity consistently correlates with higher infauna species diversity in the deep sea (Etter and Grassle, 1992; Parry et al., 2003; Rex and Etter, 2010). Habitat heterogeneity can also be influenced by geological factors in the deep sea, which can operate at a variety of scales, including mineral composition (e.g., heavy metals or minerals grains decrease faunal diversity, as in Cerrano et al., 1999), seafloor bathymetry (e.g., heterogenous seafloor morphology supports higher faunal abundance or diversity as in Durden et al., 2015 and Zeppilli et al., 2016, respectively), and hydrodynamics (e.g., structures that alter hydrodynamics influence community composition, as in Levin et al., 1986; Zajac et al., 2000; Alt et al., 2013).
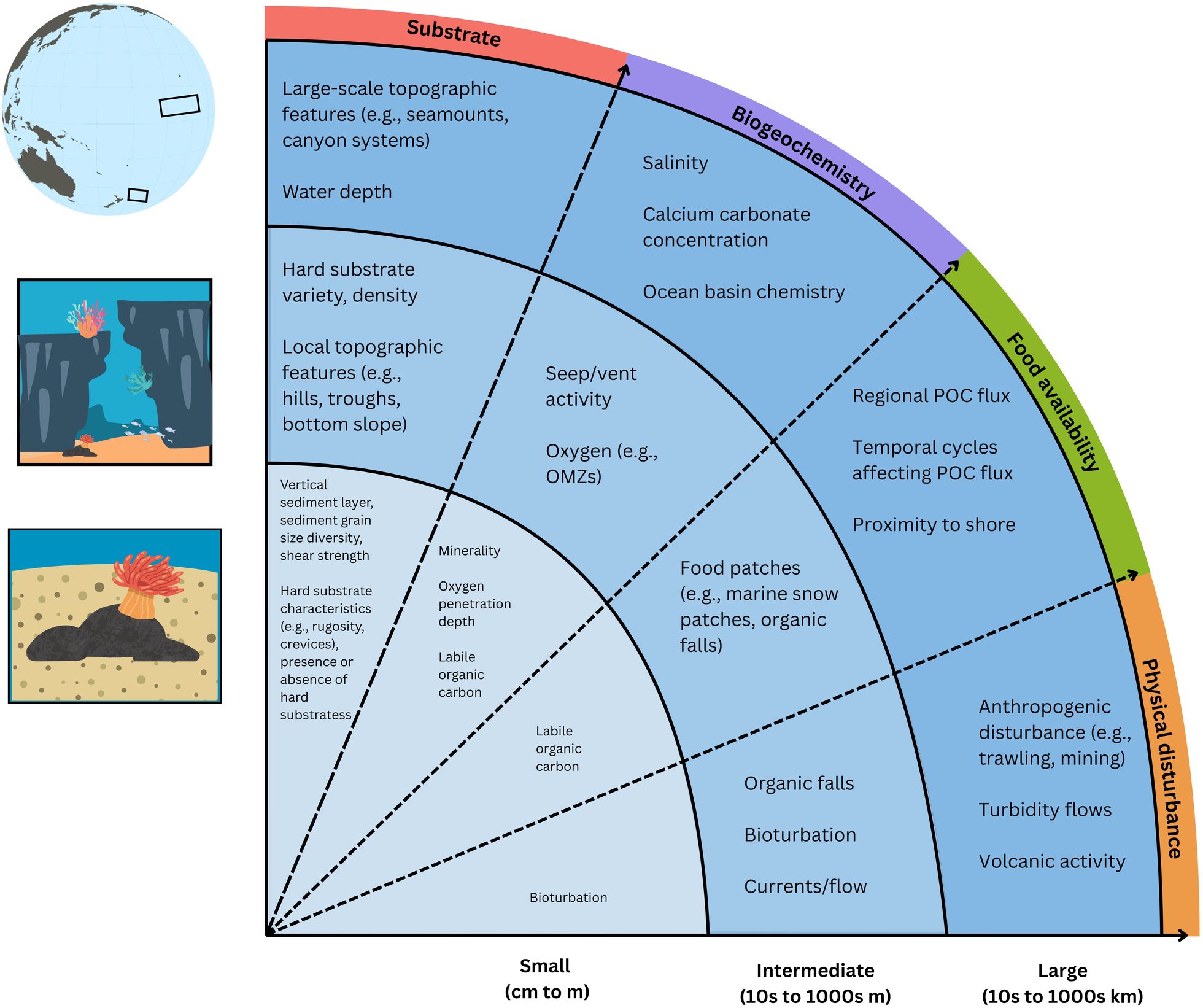
Figure 1. Summary of factors influencing habitat heterogeneity in the deep sea, according to the spatial scale of focus. Arrows indicate direction of increasing scale and delineate different types of factors. Dotted line indicates interaction or overlap between types of factors. Modified from Rosli et al., 2017.
Habitat heterogeneity is also frequently influenced by disturbance, which creates habitat patches that may increase or decrease local habitat heterogeneity depending on the size and scale of the disturbance and the spatial scale of focus (Grassle and Morse-Porteous, 1987; Gallucci et al., 2008; Willig and Presley, 2018). The kinds of natural disturbance influencing habitat heterogeneity in the deep ocean can range from small disturbances created by burrowing fauna or biogenic structures (e.g., burrows, tests, etc.; Kukert and Smith, 1992; Levin et al., 2003; Jones et al., 2007) to strong bottom currents (Levin et al., 1994; Harris, 2014; Liao et al., 2017; Tung et al., 2023) to underwater landslides and turbidity currents (Glover et al., 2010; Harris, 2014; Heijnen et al., 2022; Bigham et al., 2023) to episodic influxes of food from the surface (e.g., organic falls, strong seasonal pulses of phytodetritus; Thurston et al., 1998; Smith et al., 2008, Smith et al., 2015). Often, one of these disturbances (e.g., turbidity currents) can lead to another (e.g., food flux; Harris, 2014; Heijnen et al., 2022). The smallest of these disturbances (e.g., bioturbation) increase small-scale patchiness and local habitat heterogeneity to promote overall species diversity for larger faunal size classes (Jumars, 1975b; Thistle, 1979; Levin et al., 1986; McClain et al., 2011). However, meiofauna bioturbation has been observed to homogenize surface sediments in some cases, which may reduce surface patchiness (Cullen, 1973). Larger disturbances that significantly alter baseline habitat structure or food availability (e.g., an underwater landslide, a whale or wood fall), can create significant patchiness that results in colonization by faunal communities in a series of distinct successional stages (Bienhold et al., 2013; Harris, 2014; Smith et al., 2015).
The deep sea is also subjected to disturbance from human activities (Ramirez-Llodra et al., 2011; Clark et al., 2016; Chiba et al., 2018; Jamieson et al., 2022; Santibañez-Aguascalientes et al., 2023). This includes large quantities of pollution and litter (Tyler, 2003a; Ramirez-Llodra et al., 2011; Chiba et al., 2018; Abel et al., 2023), nuclear and chemical waste disposal (Looser et al., 2000; Tyler, 2003a; Kivenson et al., 2019), sunken infrastructure (Tyler, 2003a; Macreadie et al., 2011; Ramirez-Llodra et al., 2011), drilling and blasting (Coleman and Koenig, 2010; Montagna et al., 2013; Fisher et al., 2014; Nakajima et al., 2015; Gates et al., 2017), resource extraction (Roberts, 2002; Benn et al., 2010; Coleman and Koenig, 2010; Ramirez-Llodra et al., 2011; Bakke et al., 2013; Clark et al., 2016), and disturbance resulting from climate change (Company et al., 2008; Levin and Le Bris, 2015; Sweetman et al., 2017). In the future, the deep sea at abyssal depths (3000–6000 m) is likely to be subjected to disturbance from deep-seabed mining (Amon et al., 2022; Leduc et al., 2024a; Pickens et al., 2024). Proposed forms of mining would alter habitat heterogeneity by removing hard substrates (e.g., polymetallic nodules) and removing and mixing the top layers of sediment (Amon et al., 2016; Vanreusel et al., 2016; Simon-Lledó et al., 2019c; Uhlenkott et al., 2023b; Pickens et al., 2024). As a result, consideration of the effect of habitat heterogeneity on benthic faunal communities of these environments has become critical for developing management and conservation plans for regions targeted for mining. Most recent research on polymetallic nodule ecosystems has focused on establishing ecological baselines and cataloguing ecological communities in mining license areas in the Clarion-Clipperton Zone (CCZ) in the Central Pacific Ocean, with the goal of informing the management of deep-seabed mining under the jurisdiction of the International Seabed Authority (Durden et al., 2015; Amon et al., 2016; Vanreusel et al., 2016; Gooday et al., 2017; Simon-Lledó et al., 2019c; Weaver and Billett, 2019; Washburn et al., 2021a; Kaiser et al., 2023). Though earlier research focused on the impact of potential mining disturbances on nodule communities (Bluhm, 1994; Bluhm et al., 1995; Borowski and Thiel, 1998; Tkatchenko and Radziejewska, 1998; Fukushima et al., 2000; Radziejewska, 2002; Ingole et al., 2005) and on the physical environment (Jankowski et al., 1996; Koschinsky et al., 2001; Sharma et al., 2001; Khadge, 2005; Khripounoff et al., 2006), the potential alteration of habitat heterogeneity from mining has only recently begun to attract research interest (Simon-Lledó et al., 2019b; Cuvelier et al., 2020; Amon et al., 2022; Uhlenkott et al., 2023b).
Previous reviews that have included a focus on the influence of habitat heterogeneity on one or more size classes of benthic communities in the deep sea have considered continental margins (Levin and Sibuet, 2012), reducing ecosystems (Bernardino et al., 2012), and submarine canyons (De Leo and Puig, 2018). Although some reviews have explored abyssal polymetallic nodule ecosystems, these have focused solely on the CCZ (e.g., Gooday et al., 2021; Kaiser et al., 2023) or on disturbance experiments (e.g., Jones et al., 2017) without a dedicated focus on habitat heterogeneity, or have been part of a larger comprehensive review across habitats (e.g., Vanreusel et al., 2010).
This review covers three main size classes of benthic metazoans: megafauna, macrofauna, and meiofauna. Megafauna has been defined variably throughout the scientific literature, but generally refers to “animals readily visible in photographs” (Grassle et al., 1975) or collected on mesh sizes of 1–3 cm (as in Haedrich and Rowe, 1977), and are generally easy to see with the naked eye (e.g., holothuroids, ophiuroids, decapods). Macrofauna is the next-largest faunal size class, and generally refers to fauna collected in the deep sea on a 300-micron screen, though some European research institutes use a 250-micron screen with similar results (Gage et al., 2002). Macrofauna may be just visible, but not identifiable, with the naked eye (e.g., polychaetes, amphipods, tanaids). Meiofauna is the smallest faunal size class relevant to this review and is dominated by nematodes, but also includes other invertebrates such as harpacticoid copepods, tardigrades, and ostracods (Hakenkamp and Palmer, 2000). Sieve sizes used to separate meiofauna vary within a range of 20–64 microns, with 20–45 microns considered best practice in the deep sea (Leduc et al., 2014; Jones et al., 2017; Neira et al., 2018; dos Santos et al., 2020; Uhlenkott et al., 2020).
This review evaluates existing knowledge about habitat heterogeneity, the influence of disturbance on habitat heterogeneity, and the influence of this heterogeneity on benthic metazoan fauna in polymetallic nodule environments across faunal size classes and spatial scales. Current gaps in knowledge and the implications of this knowledge for the management of proposed deep-seabed mining are also discussed.
2 Polymetallic nodules
Polymetallic nodules, also referred to as manganese nodules, are small rocks found on the ocean floor in various parts of the world's oceans. While they commonly measure between 3 and 10 centimeters in diameter and exhibit a spherical or oblong shape (Kuhn et al., 2020), larger nodules exceeding 20 centimeters and displaying irregular shapes or structures are not uncommon (Joseph, 2017). Nodules originate from substrates such as rocks or shark's teeth, onto which minerals gradually precipitate and bind over geological timescales, growing at a rate of a few millimeters per million years (Cronan, 2019). Though nodules can occur at other depths, they are most abundant in the abyssal ocean basins of the Pacific, Indian, and Atlantic oceans between 4000–6500 m (Figure 2). Economically viable nodules are primarily iron or manganese-based (approximately 6 to 30%) and contain smaller concentrations of valuable minerals such as nickel, cobalt, copper, and rare earth elements (0.25-3%; Joseph, 2017).
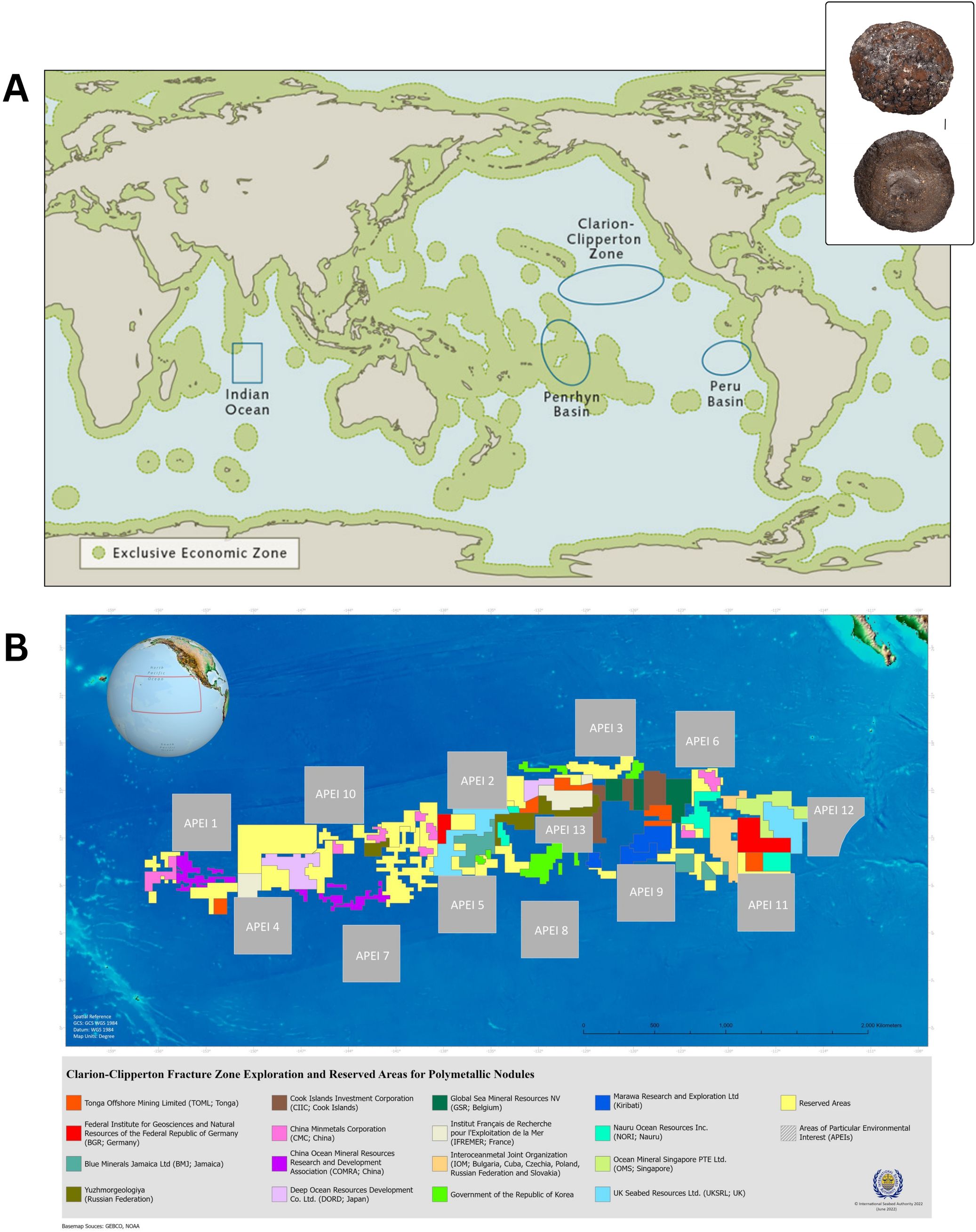
Figure 2. (A) Map of polymetallic nodule environments of interest for mining (outlined in blue). Inset figure shows a whole polymetallic nodule and cross-section (1 cm scale bar). Map from World Ocean Review; inset photos from Earth Sciences New Zealand. (B) Map of the exploration and reserved areas for polymetallic nodules in the Clarion-Clipperton Zone. Map by the International Seabed Authority (https://www.isa.org.jm/).
As a geological feature, polymetallic nodules have been known to science since the Challenger Expedition of 1873-1876 (Murray and Renard, 1891). While this and other early oceanographic expeditions revealed a fair amount about the chemistry and geology of polymetallic nodules (Summerhayes, 1967; Glasby, 1976; Kerr, 1984), little was known about their associated fauna until technological developments facilitated better exploration of abyssal depths in the late 1900s (Danovaro et al., 2014). Recent studies into polymetallic nodule ecosystems—particularly those in proposed mining areas (e.g., the CCZ)—have provided greater knowledge about faunal communities associated with polymetallic nodules in the central Pacific (e.g., Simon-Lledó et al., 2020; Uhlenkott et al., 2021, Uhlenkott et al., 2023a; Washburn et al., 2021b). However, these environments remain understudied, particularly outside of the CCZ, and baselines for their associated communities are still being established (e.g., Simon-Lledó et al., 2019c, Simon-Lledó et al., 2019d).
3 Metrics of habitat heterogeneity used to describe polymetallic nodule environments
Though few studies focus explicitly on habitat heterogeneity, most studies in nodule environments include metrics that characterize heterogeneity in the form of environmental factors (standard for most ecological studies) and/or nodule characteristics (relevant both for its ecological influence and its focus for mining interests). At the smallest spatial scales, habitat heterogeneity in nodule environments is generally measured in the form of grain size heterogeneity for nearby sediments (Mewes et al., 2014; De Smet et al., 2017; Lefaible et al., 2023), or—in some cases—by nodule type or “facies” (a qualitative description of nodule surface texture and some aspects of nodule density or seafloor topography; Wright et al., 2005; Veillette et al., 2007a; Fleming et al., 2025). Intermediate spatial scales of heterogeneity are generally focused on nodule abundance or percent cover (Mewes et al., 2014; De Smet et al., 2017; Simon-Lledó et al., 2019c, Simon-Lledó et al., 2020; Durden et al., 2021), and may include other aspects of seafloor heterogeneity such as bottom topography (Simon-Lledó et al., 2019b, Simon-Lledó et al., 2020; Durden et al., 2021) or the occurrence of non-nodule hard substrates (Mejía-Saenz et al., 2023; Uhlenkott et al., 2023b). At larger spatial scales, heterogeneity is generally measured based on study area and its associated environmental conditions (e.g., depth, POC flux, etc.; Jones et al., 2021; Washburn et al., 2021a). However, since most studies in nodule environments focus on one or two spatial scales, the most prevalent metrics used to characterize substrate heterogeneity are nodule abundance (kg/m2 or nodules/m2; e.g., Amon et al., 2016) or nodule cover (percent cover; e.g., Simon-Lledó et al., 2019b). Though other metrics have been used across a range of studies (e.g., nodule size, facies, volume), it is common for studies to use just one metric (typically nodule cover), which can cause the other aspects of habitat heterogeneity (e.g., nodule distribution, nodule patch size, bottom topography, sediment heterogeneity) to be underestimated or overlooked.
4 Habitat heterogeneity in polymetallic nodule environments
Habitat heterogeneity in polymetallic nodule environments depends on the spatial scale of focus (Figure 3). The smallest scale (millimeters to centimeters) depends on variability of the nodule itself, such as the presence or absence thereof, and—if present—differences in their minerality, rugosity, size, and shape, as well as the surrounding sediments (e.g., vertical resource gradients and grain size heterogeneity). At the patch-scale (centimeters to meters) heterogeneity is driven by differences in nodule density and arrangement. At the field-scale (10s to 1000s of meters), heterogeneity across a nodule field may take the form of nodule patch density and arrangement, differences in bottom topography, or in the occurrence of non-nodule hard substrates such as rock outcrops or seamounts. Finally, at the regional scale (10s to 1000s of kilometers), heterogeneity generally takes the form of broad environmental differences (e.g., resource availability such as organic matter flux, geomorphological structures, geological variability). Due to the different “windows of perception” of different faunal size classes (Kotliar and Wiens, 1990; Attrill et al., 2000), taxa naturally interact with these spatial scales differently (Figure 3). As a result, a deeper, more comprehensive assessment of habitat heterogeneity across these spatial scales is critical for understanding nodule faunal community structure and resilience to potential disturbance from seabed mining.
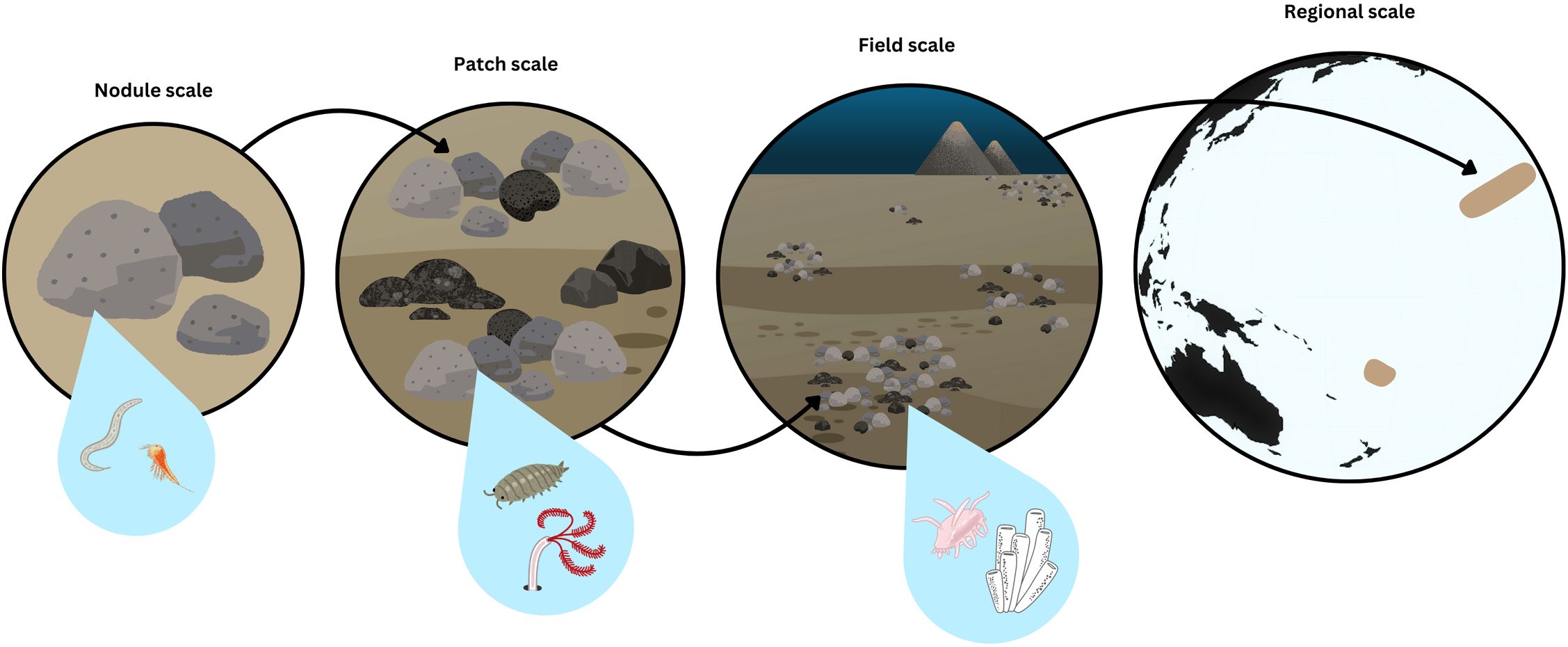
Figure 3. Habitat heterogeneity at different spatial scales of interest in polymetallic nodule environments from smallest (left) to largest (right). The variability at each spatial scale will hold different relevance for different size classes of fauna. For example, variability at the nodule scale (e.g., number and size of crevices) will be most relevant for meiofauna; while variability at the patch scale (e.g., density of nodules blocking sediment habitats or providing attachment surfaces for sessile fauna) may be more relevant for macrofauna; variability at the field scale (e.g., available sediment patches for mobile scavengers and density of nodule patches for connectivity between hard substrate communities) may be most relevant for megafauna.
4.1 Habitat heterogeneity among nodules and within sediments
At small spatial scales, there is substantial habitat heterogeneity within sediments, in both nodule-free and nodule-rich areas. Sediment depth is one of the most important drivers of infaunal communities (particularly meiofauna) due to heterogeneity in resource availability (namely organic matter content). As organic matter content decreases with depth, infaunal abundance often mirrors this decline, and community composition shifts (Thiel, 1983; Soetaert et al., 2002; Ingels and Vanreusel, 2013; Rosli et al., 2018). While infaunal abundance is generally higher at shallower sediment depths, peaks may occur in subsurface sediment layers (e.g., 1–2 or 2–3 cm deep) due to environmental conditions (e.g., strong currents, coarser sediments; Zeppilli et al., 2012, Zeppilli et al., 2014). In nodule-free areas, the effect of the vertical gradient through sediment layers is relatively consistent across habitat type (Ingels and Vanreusel, 2013; Rosli et al., 2018). In nodule environments, it remains unknown whether nodules may block the penetration of organic matter into the sediments beneath them, leading to the concentration of organic matter in exposed sediments between the nodule, creating coarser sediments through nodule fragments, or altering the flux of other nutrients (e.g., oxygen). However, it is possible that their presence in otherwise sediment-dominated environments may influence sediment depth-related patterns in community structure by contributing to greater resource patchiness.
Among nodules within a patch, variations in habitat heterogeneity are largely structural and geochemical. Polymetallic nodules can vary in size, shape, and composition (Joseph, 2017; Mizell et al., 2022). This heterogeneity not only influences the size of the hard substrate habitat provided by nodules (e.g., as an attachment surface for mega- and/or macrofaunal sessile fauna; Meyer et al., 2016; Simon-Lledó et al., 2019b), but also the rugosity (roughness) of the nodule. Higher rugosity nodules can provide additional habitat beyond acting as an attachment surface, as their pores and crevices often support distinct faunal communities dominated by smaller-sized meiofauna (namely small nematodes; Thiel et al., 1993; Singh et al., 2019). Though nodule crevices tend to be small, and therefore support fewer individuals and species than nearby sediments (Thiel et al., 1993; Singh et al., 2019; Pape et al., 2021), this heterogeneity within the nodule could have implications for nodule-specific faunal communities. Nodule-to-nodule faunal community comparisons that focus on this small-scale heterogeneity are not common, but a positive relationship between nodule dimensions and crevice meiofaunal abundance has been observed (Pape et al., 2021). Based on observations of distinct crevice-faunal communities on nodules (Thiel et al., 1993; Singh et al., 2019), it is likely that substantial differences in rugosity among nodules would influence the structure of meiofaunal nodule communities. The varied geochemical composition of nodules can also contribute to this small-scale habitat heterogeneity, as different minerals support different bacterial communities (Blöthe et al., 2015), which may influence metazoan communities through short-term carbon cycling and resource availability (Sweetman et al., 2019; Stratmann et al., 2021; Stratmann, 2023). For example, laboratory experiments have indicated that higher diversity of bacterial communities directly influences nematode community composition by supporting higher nematode abundance and reducing competitive exclusion amongst nematode species (Derycke et al., 2016; Guden et al., 2021).
4.2 Habitat heterogeneity among nodule patches
The next largest spatial scale of interest is among nodule patches within a field, namely through differences in the density and arrangement of nodules among patches. Broadly, the occurrence of nodules (i.e., compared to sediments within a field without nodules) has been shown to increase megafaunal density (Amon et al., 2016; Vanreusel et al., 2016; Cuvelier et al., 2020; Simon-Lledó et al., 2020; Durden et al., 2021; Uhlenkott et al., 2023b), diversity (Uhlenkott et al., 2023b), and exhibit different community composition (Simon-Lledó et al., 2019c; Cuvelier et al., 2020; Simon-Lledó et al., 2020; Durden et al., 2021). Megafaunal abundance largely correlates with nodule abundance across the CCZ, particularly for suspension feeders (Tilot, 2006; Amon et al., 2016; Vanreusel et al., 2016; Simon-Lledó et al., 2019b). The relationship between nodule abundance and diversity, though positive, may be limited: the habitat heterogeneity provided by nodules enhances megafaunal species richness and diversity, but this effect exhibited diminishing returns above a certain level of nodule abundance in the eastern CCZ (UK-1; Amon et al., 2016). Certain macrofaunal taxa (e.g., polychaetes, tanaids, and isopods) in the eastern CCZ (GSR) have been observed to covary with nodule abundance, but sampling design may account for much of this trend (De Smet et al., 2017). Studies in both the Indian (CIOB) and Pacific Oceans (multiple areas in the CCZ) have indicated a positive relationship between nodule density and macrofaunal diversity (Parulekar et al., 1982; Yu et al., 2018) and/or abundance (Parulekar et al., 1982; Mullineaux, 1987; Tilot, 2006), while others indicate a unimodal relationship between nodule density and macrofaunal abundance (GSR, eastern CCZ; De Smet et al., 2017), or no discernible relationship at all (GSR, eastern CCZ; Pasotti et al., 2021). These varying relationships indicate that macrofaunal communities may be influenced by additional factors found at each study site. For example, in the southwest Pacific at around 400 m, macrofaunal community structure was found to correlate with phosphorite nodule abundance, but mesoscale (10-100s of m) topographical variability (e.g., uneven areas, seafloor depressions) drove diversity patterns at smaller spatial scales (< 1 m; Leduc et al., 2015). However, across study locations in the CCZ, macrofaunal community composition largely varied according to polymetallic nodule density, likely due to substrate preference (e.g., higher percent composition of sessile fauna at nodule-rich sites vs. higher percent composition of infauna at nodule-poor sites; Mullineaux, 1987; Tilot, 2006). Though the presence of nodules (and therefore nodule crevice fauna) may marginally increase local meiofaunal diversity at the centimeter-scale (Pape et al., 2021), nodules also occupy the first several centimeters of sediment that could otherwise serve as habitat for sediment infauna, often resulting in lower abundance of sediment meiofauna at nodule-rich sites compared to nodule-free areas in both the Indian and Pacific Oceans (Tilot, 2006; Mahatma, 2009; Miljutina et al., 2010; Singh et al., 2016; Hauquier et al., 2019; Pape et al., 2021).
4.3 Habitat heterogeneity among nodule fields
At larger spatial scales, habitat heterogeneity among nodule fields within a region may also influence benthic communities. In particular, among-field differences in local topography that occur on scales of 10s to 1000s of meters can influence mega- and macrofaunal communities at smaller spatial scales (< 1 m to 5 m) by providing additional habitat heterogeneity in the form of depressions, non-nodule hard substrates, or hills/seamounts (Durden et al., 2015, Durden et al., 2020; Leduc et al., 2015; Cuvelier et al., 2020; Leitner et al., 2021; Mejía-Saenz et al., 2023). Topographical variability has been observed to support higher mega- and macrofaunal diversity in deep-sea sediment communities, including in nodule regions, likely due to increased sediment heterogeneity and the alteration of small-scale environmental factors (e.g., the accumulation of POC in seafloor depressions; Durden et al., 2015, Durden et al., 2020; Leduc et al., 2015). The presence of non-nodule hard substrates (e.g., rocks, seamounts) has also been observed to strongly influence megafaunal community structure, with higher diversity and densities than sediment-dominated areas in the eastern CCZ (BGR, GSR, APEI-3, and APEI-6; Cuvelier et al., 2020; Mejía-Saenz et al., 2023; Uhlenkott et al., 2023b). The communities found on these substrates are distinct from nodule communities (Cuvelier et al., 2020; Laroche et al., 2020; Mejía-Saenz et al., 2023; Uhlenkott et al., 2023b), indicating that nodules provide unique habitat that may not be easily substituted by other hard substrates. Heterogeneity in the form of the distribution of nodule fields within a region could also have implications for the biodiversity of faunal communities. For example, higher density of nodule fields could enhance population connectivity and successful larval dispersion within regions, particularly for fauna dependent on hard substrates (Taboada et al., 2018). Smaller habitat patches acting as “stepping stones” that connect larger populations of fauna have been observed in other deep-sea ecosystems (e.g., organic falls connecting vents or seeps; Bienhold et al., 2013; Cunha et al., 2013). Scattered fields of nodules could similarly act as “stepping stones” that connect nodule fauna populations across sediment-dominated areas. However, connectivity and larval dispersion in the abyss remain understudied, particularly in nodule environments (Kersten et al., 2017), and no study has yet explored connectivity and larval dispersion with regard to habitat heterogeneity in nodule environments.
4.4 Habitat heterogeneity within and among regions
At the largest spatial scales (among nodule fields within and among regions), environmental variability (e.g., resource availability, geomorphological structures) influences faunal community structure both directly and through its influence on habitat heterogeneity.
4.4.1 Food flux variability
At within-region scales (100s of meters to 100s of kilometers), the influence of environmental variability—particularly food availability—on polymetallic nodule communities becomes more pronounced. For example, POC flux to the seafloor varies substantially (> 2x) across the CCZ, generally declining from east (closer to nutrient inputs from land) to west (Tyler, 2003b; Washburn et al., 2021a). Mega-, macro-, and meiofaunal community structure has been observed to vary accordingly across this gradient, with higher POC flux generally correlating with both higher faunal abundance and diversity (Hannides and Smith, 2003; Smith and Demopoulos, 2003; De Smet et al., 2017; Christodoulou et al., 2020; Laroche et al., 2020; Nomaki et al., 2021; Washburn et al., 2021a; Tong et al., 2022). However, when sites within a region exhibit substantial differences in both local habitat heterogeneity (e.g., nodule abundance) and POC flux, the influence of POC flux on community structure may be diminished or masked (Durden et al., 2021; Washburn et al., 2021b). For example, an area of particular environmental interest (APEI) in the CCZ with 73% higher POC flux but lower habitat heterogeneity (i.e., dominated by soft sediments; APEI-7) exhibited lower megafaunal densities than in sites with lower POC flux but high nodule abundance (APEI-1; Durden et al., 2021). Variability in these two ecological factors also influenced megafaunal community composition, as fauna with certain functional traits (e.g., sessile suspension feeders vs mobile scavengers) correlated with higher availability of related resources (e.g., hard substrates for attachment; Durden et al., 2021). However, related environmental factors (e.g., oxygen content), may have also played a role in this trend, as APEI-7 has lower dissolved oxygen content (3.84 ± 0.02 ml/L) than APEI-1 (4.12 ± 0.05 ml/L; (Washburn et al., 2021a).
POC flux to the seafloor also varies among regions (1000s of kilometers or more), including among ocean basins that contain large nodule reserves (e.g., the central Indian Ocean, the central Pacific, the southwest Pacific; Jahnke, 1996). Though the waters over polymetallic nodule environments tend to be oligotrophic (Mizell et al., 2022), POC flux to the seafloor does vary between ocean basins (Xie et al., 2019), which can contribute to differences in nodule fauna among regions (Washburn et al., 2021b). Equatorial ocean regions (e.g., the central Pacific) tend to exhibit higher POC flux to the seafloor than tropical and sub-tropical regions due to heightened surface productivity fed by equatorial upwelling (Honjo et al., 2008). The Indian Ocean basin, due to its proximity to large landmasses and the equator, tends to exhibit higher POC flux than the north or south Pacific Ocean basins (Jahnke, 1996; Rixen et al., 2019). As a result, in addition to among-basin variability in habitat heterogeneity that may influence faunal community patterns (e.g., presence of nodules, seamounts, rock outcrops), POC flux can create regional differences in faunal communities directly (e.g., higher abyssal benthic standing stock in the Pacific than the Indian Ocean; Neyman et al., 1973; Parulekar et al., 1982; Tyler, 2003b). Ultimately, the complexities of the relationship between mega-, macro-, and meiofaunal community structure and POC flux remain poorly understood and merit further investigation in nodule environments at different sites, in different regions, and on longer timescales. However, existing research indicates that neither the presence of certain habitat features (e.g., nodules) nor the level of certain environmental conditions (e.g., POC flux) can serve as a perfect proxy for faunal diversity or abundance. Though preliminary efforts to model polymetallic nodule meio- and megafauna communities in the eastern CCZ (BGR) have shown promise (Uhlenkott et al., 2020, Uhlenkott et al., 2021, Uhlenkott et al., 2022), the explanatory and predictive capabilities of these models remain constrained by limited baseline data.
4.4.2 Temporal variability
Environmental conditions in abyssal regions can also vary considerably on temporal scales due to seasonality and climate oscillations affecting surface productivity, and thereby POC flux to the seafloor (Billett et al., 2001; Kuhnz et al., 2014; Taylor et al., 2017). Though evidence of temporal variability in food flux has been observed in nodule environments (Kaufmann and Smith, 1997; Hannides and Smith, 2003; Miljutin et al., 2015; Hoving et al., 2023), the results of the few studies monitoring temporal changes in nodule faunal communities have been mixed. Across the CCZ, isopod diversity exhibited strong temporal variation (Kaiser et al., 2023), and macrofaunal densities overall were higher during El Niño years, though low sample sizes limited statistical testing and inconsistencies in sampling methodology have contributed substantially to the observed results (Kaiser et al., 2024). Also in the CCZ (eastern CCZ; IOM and Ifremer areas), certain meiofauna taxa were found to exhibit a strong shift in community structure following natural episodes of phytodetritus input (Radziejewska et al., 2001; Radziejewska, 2002; Miljutin et al., 2015). However, a different study in the eastern CCZ (GSR area) found that meiofauna showed no significant temporal variation in abundance, diversity, or community composition (Pape et al., 2017), though this difference may be due to geographical variation among the contract areas, which span hundreds of kilometers. Additional research on megafaunal communities has been largely limited to disturbance response studies (see below), in which the effects of anthropogenic (experimental) disturbances and the effect of spatiotemporal cycles can be difficult to parse.
Among ocean basins, temporal variation can differ further. For example, the Indian Ocean is influenced by monsoons, which create seasonal nutrient inputs that boost surface productivity and therefore POC flux to the seafloor (Lutz et al., 2007). Due to its size, the Pacific Ocean does not have universal seasonal variation. However, the east Pacific Ocean experiences seasonal coastal upwelling that can have a cascading influence on POC flux to the deep sea (Lutz et al., 2007). The Pacific is also strongly influenced by the El Niño-Southern Oscillation (ENSO), which creates substantial temporal variation in POC flux to the seafloor on cycles of several years, and whose influence extends to the Indian and Southern Oceans (Wang et al., 2017; Xie et al., 2019). The effects of ENSO in the central and eastern equatorial Pacific (including around the CCZ) are particularly acute, with decreased POC flux during El Niño events (due to weakened equatorial upwelling) and increased during La Niña events (due to enhanced upwelling; Smith et al., 2006; Xie et al., 2019). The temporal variability in the southwestern Pacific tends to be less dramatic and less consistent, as it is further removed from both seasonal and climactic shifts in equatorial upwelling (Lutz et al., 2007; Wang et al., 2017). However, ENSO still influences regional ocean circulation and temperatures in the southwestern Pacific, which likely influences surface productivity and therefore POC flux to the seafloor (Chiswell et al., 2015). Though no consistent trends in temporal faunal variability among regions have yet emerged, food flux to the seafloor remains a strong environmental influence on abyssal communities. Due to the strong but variable influence of climatic cycles (e.g., ENSO) and seasonality on POC flux among regions, it remains likely that these temporal cycles have a strong influence on regional nodule faunal communities that could be revealed by future research (Hannides and Smith, 2003; Ruhl and Smith, 2004). However, to date, the paucity of long-term sampling and ocean monitoring programs in remote polymetallic nodule regions have prevented a robust assessment of the influence of large-scale temporal cycles on nodule-associated fauna.
4.4.3 Bathymetric and topographic variability
At within- and among-region scales, bathymetry becomes more influential, both through depth gradients and through the presence of large geomorphological structures. Though depth remains relatively consistent in most nodule environments, generally between 4000 and 5000 m, community structure can still be strongly influenced by this variability. For example, in the CCZ, the carbon compensation depth lies between 4,300 and 4,800 m (Berger et al., 1976). Across this threshold, megafaunal community composition shifts significantly as animals relying on calcium carbonate body parts (e.g., corals, shelled mollusks) are replaced by soft-bodied organisms (e.g., anemones, sea cucumbers; Simon-Lledó et al., 2023). Despite this dramatic shift in phylum-level community composition, megafaunal species richness across this threshold is maintained (Simon-Lledó et al., 2023). As in most deep-sea environments, food availability also declines with depth, and abyssal environments with lower POC flux often exhibit lower mega-, macro-, and meiofaunal densities (Thurston et al., 1998; Veillette et al., 2007b; Schmidt and Martínez Arbizu, 2015; Wilson, 2017; Błażewicz et al., 2019) and diversities (Glover et al., 2002; Veillette et al., 2007b; Wilson, 2017; Błażewicz et al., 2019; Laroche et al., 2020), though this can vary depending on the amount of substrate heterogeneity present (i.e., nodules vs. no nodules; Veillette et al., 2007a; Vanreusel et al., 2016) and across taxonomic groups (Wilson, 2017; Simon-Lledó et al., 2020).
Regional environmental variability in the form of larger topographical variations (e.g., seamounts, troughs) can also influence communities as bottom currents and hard substrate availability in nodule environments influence resource availability (e.g., food, habitat). For example, the occurrence of seamounts near polymetallic nodule fields in the eastern CCZ provides increased habitat heterogeneity that supports high faunal diversity and abundance (Cuvelier et al., 2020; Mejía-Saenz et al., 2023; Uhlenkott et al., 2023b). In the Bounty Trough of the southwest Pacific (1500–4800 m depth), bottom currents are strong enough to create ripples in sedimented areas (Daniel Leduc, personal communication), which may influence communities both directly and through the creation of greater habitat heterogeneity that can alter hydrodynamic conditions and influence sediment grain heterogeneity, larval settlement, and food availability (Leduc et al., 2012, Leduc et al., 2015; Durden et al., 2015). In the abyssal sediments of the Peru Basin (equatorial eastern Pacific), high-walled troughs created by an experimental disturbance collected pyrosomes (megafauna) at significantly higher concentrations (4-76x) than in the flat (undisturbed) sediments nearby (Hoving et al., 2023). Though the benthic community response to this influx of food was not assessed at this site, other studies of pyrosomes as a food source in the deep sea indicate that this substantial input of organic matter would likely have influenced the community structure (Lebrato and Jones, 2009; Smith et al., 2014). Additionally, topographic features such as trenches have been documented to share genera with nearby nodule environments, indicating that connectivity within regions is likely not impeded by these features (Vanreusel et al., 2010; Horacek et al., 2022).
Seafloor topographic complexity (e.g., seamounts, steppes, troughs) can also vary substantially among regions. The Pacific Ocean is estimated to contain significantly more seamounts (9,000-16,000/106 km2 seamounts) than the Indian Ocean (500-1600/106 km2 seamounts; Das et al., 2007). However, in polymetallic nodule environments specifically, the abundance of seamounts is relatively similar between the Indian and Pacific Oceans, despite the CCZ being almost 40x larger than the surveyed area of the Central Indian Ocean Basin (CIOB; Das et al., 2007; Vineesh et al., 2009; Leitner et al., 2021, and references therein). More seafloor features (e.g., steps, troughs, hills, rises) have been observed in the CCZ than the CIOB (Parianos and Madureira, 2021), but this could be a product of the different sizes of the two regions and/or of the differing research efforts to characterize them. Seafloor topographic complexity also varies among Pacific basins. The southern Pacific Ocean contains slightly more seamounts per square kilometer than the central Pacific, and nodule regions of the southwest Pacific can be topographically complex. For example, the Cook Islands (southwest Pacific) Exclusive Economic Zone (EEZ) contains over 50 large seamounts, many of which are connected through chains of smaller volcanic knolls and the majority of which lie within the nodule-rich areas of the country’s EEZ (Browne et al., 2023). Though recent research has found that seamounts provide additional habitat heterogeneity that supports distinct megafaunal communities (Cuvelier et al., 2020; Leitner et al., 2021; Uhlenkott et al., 2023b), there have not yet been any studies assessing the possible regional differences in these communities by comparing seamounts in different ocean basins.
4.4.4 Geological and geochemical variability
Finally, polymetallic nodules themselves can vary considerably in shape, size, and density at among-region scales. For nodules to form, environmental conditions must be relatively stable. Sedimentation rates, in particular, must be low (< 10 mm per thousand years) to prevent the burial of developing nodules (Kuhn et al., 2017; Hein et al., 2020; Mizell et al., 2022). As a result, nodules generally form in areas with relatively flat seafloor topography and in regions with lower rates of surface productivity (Kuhn et al., 2017; Hein et al., 2020; Mizell et al., 2022), due to their associated low sedimentation rates (Mewes et al., 2014; Mizell et al., 2022). Though polymetallic nodules are found globally, their mineral makeup and structure vary based on the environmental conditions and process under which they form (Hein and Mizell, 2022). There are several varieties of polymetallic nodules (Cronan, 2019), but the ones of greatest commercial interest are hydrogenetic nodules (formed by mineral precipitates from seawater; e.g., west and southwest Pacific nodules), diagenetic nodules (formed by mineral precipitates from sediment pore waters; e.g., Peru Basin nodules), and mixed hydrogenetic-diagenetic nodules (formed from both seawater and sediment pore waters; e.g., CCZ and CIOB nodules; Mizell et al., 2022). Hydrogenetic nodules are most common in regions with low surface productivity, while diagenetic nodules form in areas with moderate surface productivity, which provides the organic matter (and resulting suboxic sediment conditions) needed for diagenetic reactions in sediment pore waters (Mewes et al., 2014; Mizell et al., 2022). Based on these different processes—and differing mineral concentrations in seawater and sediment pore water among ocean basins—the mineral makeups of nodules vary according to both nodule type and location (Hein and Mizell, 2022; Mizell et al., 2022). This variability in both geology and biogeochemistry may also contribute directly to regional differences in faunal communities. Nodules of different varieties and/or from different regions have been shown to harbor distinct microbial communities and exhibit different local biogeochemistry (Blöthe et al., 2015; Wear et al., 2021; Bergo et al., 2022). Microbes play a critical role in nutrient cycling, metal sequestration, and abyssobenthic food webs (de Jonge et al., 2020; Orcutt et al., 2020), and biogeochemical conditions (e.g., high concentrations of certain metals, low oxygen penetration depths; Paul et al., 2018; Haffert et al., 2020) can favor or preclude certain fauna. As a result, it is likely that these regional differences in nodule composition or geochemistry may have a direct influence on regional faunal community structure.
The same broad-scale environmental conditions that influence the formation of nodule environments also directly influence the communities living in them. Due to the stability of nodule environments, nodule fauna are generally slow growing and many are sessile, particularly at larger faunal size classes (Veillette et al., 2007b; De Smet et al., 2017). Reproduction in polymetallic nodule communities remains poorly understood for all faunal size classes, but existing research indicates that they exhibit significantly lower larval abundance and flux (vertical movement of larvae to the seafloor over time; ind. d−1 m−2) and higher retention of larvae near the benthos than in other deep-sea habitats (Kersten et al., 2017, Kersten et al., 2019). This may contribute to slow recovery rates (Miljutin et al., 2011; Jones et al., 2017; Simon-Lledó et al., 2019a), as larvae retained near the seafloor would be more vulnerable to disturbances that create adverse conditions near the benthos (e.g., sediment plumes).
5 Disturbance in polymetallic nodule environments
Disturbance in polymetallic nodule environments can influence fauna both directly and through the disturbance’s impact on habitat heterogeneity. Though many forms of disturbance occur naturally in polymetallic nodule environments, large-scale natural disturbances are uncommon. Polymetallic nodule mining would effectively act as a large-scale disturbance that would alter habitat heterogeneity through the removal of hard substrates, the creation of troughs or tracks in the sediment, and the deposition of sediment that may smother fauna and homogenize the texture of the seafloor (Figure 4). While existing research has offered valuable insights about disturbance in nodule environments, particularly at smaller spatial scales, the influence of disturbance on nodule communities—including through its impact on habitat heterogeneity—remains poorly understood.
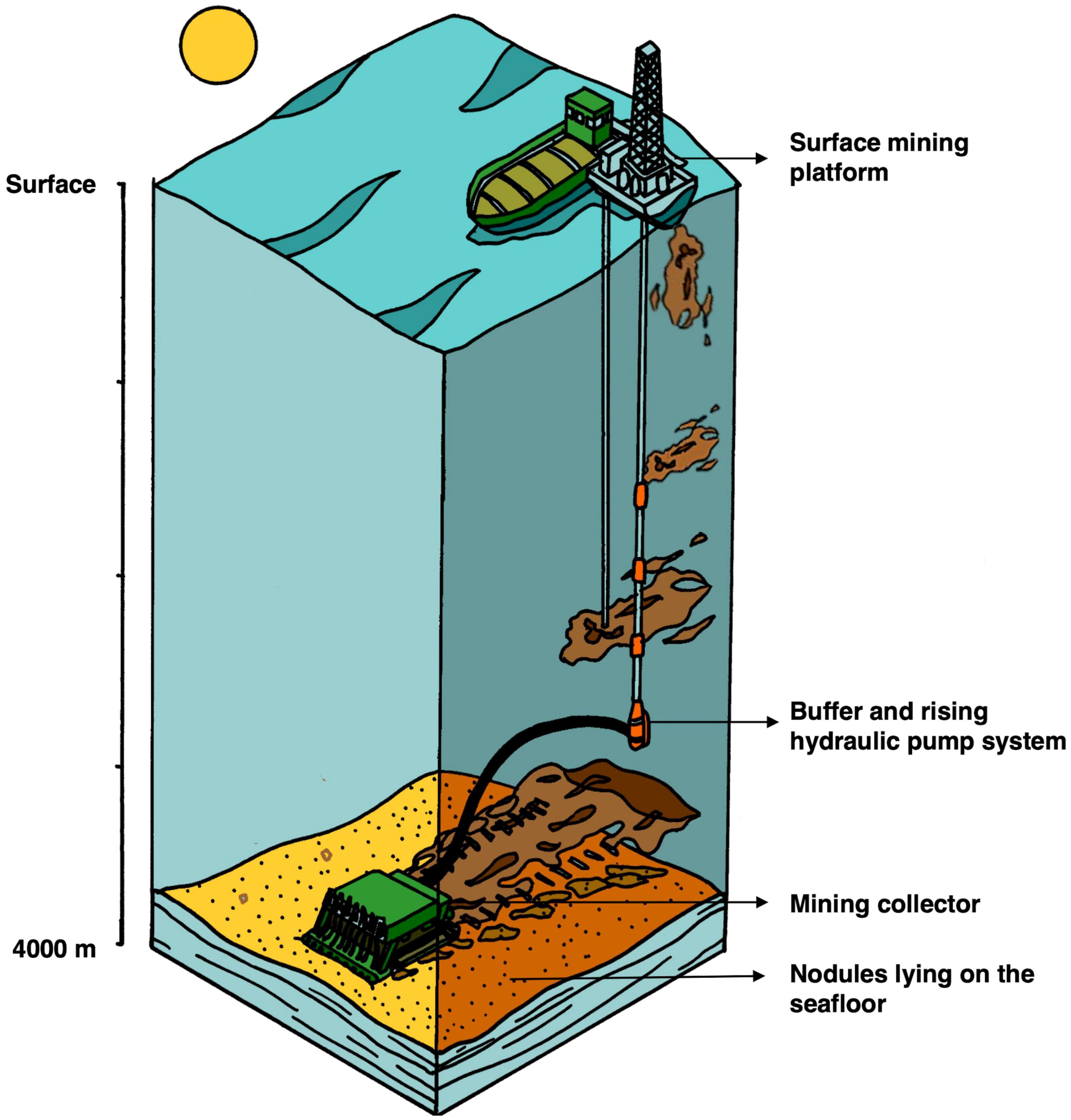
Figure 4. Schematic of conceptual deep-sea polymetallic nodule mining system (figure adapted from Figure 2 in Gillard et al., 2022).
5.1 Natural disturbance
Due to most nodule fields’ locations beneath abyssal waters, naturally reoccurring disturbance in nodule ecosystems generally takes the form of biogenic disturbance (e.g., bioturbation; Volz et al., 2020) or food flux (e.g., Amon et al., 2017). Regular small-scale disturbances in the form of bioturbation and biogenic structures (i.e., Lebensspuren) generally correlate with higher faunal diversity and density throughout the deep sea (Kukert and Smith, 1992; Meadows and Meadows, 1994; Meadows et al., 2012), including at abyssal depths (Ruhl and Smith, 2004; Bell et al., 2016; Rosli et al., 2018). Bioturbation lowers sediment shear strength, which may provide more microhabitats that support higher infaunal diversity or abundance (Tong et al., 2022) and helps to transport critical resources (namely food and oxygen) into deeper sediments where they support life ranging from microbes to megafauna (Rosli et al., 2018; Bonaglia et al., 2020; Haffert et al., 2020; Tong et al., 2022). The openings to biogenic structures also increase the texture of the seafloor. This texture creates greater habitat heterogeneity and promotes the resuspension of materials at the sediment-water interface (Huettel et al., 1996), which facilitates nutrient cycling and supports local faunal communities through the resuspension of organic matter (Levinton, 1995; Ford et al., 1999).
As in other ecosystems in the food-limited deep sea, food flux can also act as a disturbance agent in polymetallic nodule environments. Though thought to be less common than on continental margins, organic falls do occur in nodule environments (Amon et al., 2017), and exhibit similar faunal communities to continental margin organic falls (Bienhold et al., 2013; Cunha et al., 2013; Amon et al., 2017). Organic falls observed throughout the CCZ hosted common organic fall specialists (e.g., Xylophagaidae mollusks, mobile scavengers), along with several other species not observed elsewhere in the CCZ (Amon et al., 2017). While temporal variability in the influx of food (e.g., phytodetritus, pyrosome carcasses) has been observed in some nodule environments, the extent to which these food fluxes may be cyclical or act as a disturbance remains poorly understood (Durden et al., 2021; Uhlenkott et al., 2021). However, based on the influence of large food fluxes in other abyssal environments, it remains likely that these events may temporarily restructure local faunal communities (Thurston et al., 1998; Ruhl and Smith, 2004; Bailey et al., 2006; Woolley et al., 2016).
Large natural disturbances are not uncommon in abyssal environments, which can be impacted by gravity currents such as turbidity flows that reach abyssal plains through canyons and channels on the continental rise (Bigham et al., 2021) or from benthic storms (Miguez-Salas et al., 2020). The CCZ is subject to energetic mesoscale eddies (Aleynik et al., 2017), which may cause episodic environmental stress from strengthened bottom currents and sediment resuspension. However, the characteristics that facilitate the formation of nodules over long time frames indicate that nodule environments are generally relatively stable, with flat topography and low sedimentation rates. As a result, catastrophic large-scale disturbances are unlikely to occur naturally in polymetallic nodule environments.
5.2 Anthropogenic disturbance
Understanding the potential impact that deep-seabed mining may have on nodule communities has necessitated in-situ experimentation or the study of proxies in similar environments, though these have remained limited in scope and scale (Gollner et al., 2017; Jones et al., 2017; Cuvelier et al., 2018). The study of proxies, in particular, has yielded little information about the possible reaction of nodule communities due either to the scale of the proxy disturbance (e.g., Jamieson et al., 2022) or its location (e.g., at bathyal depths, close to shore, at hydrothermal vents; Gollner et al., 2017; Bigham et al., 2024; Leduc et al., 2024a; Murray et al., 2024). As a result, in-situ experimentation remains the most promising avenue for estimating the possible impacts of polymetallic nodule mining on faunal communities.
5.2.1 Historic disturbance experiments
Experiments conducted in the late 1980s and 1990s throughout nodule areas of the Central and Eastern Pacific provided the first glimpses of ecological responses to disturbances meant to mimic the effects of mining in the CCZ (Brockett and Richards, 1994; Trueblood and Ozturgut, 1997; Borowski and Thiel, 1998; Tkatchenko and Radziejewska, 1998) and in the Peru Basin (Thiel and Schriever, 1990; Borowski and Thiel, 1998; Bluhm, 2001; Borowski, 2001; Thiel et al., 2001; Radziejewska, 2014)(Table 1). The footprint of these experiments varied, with most experiments disturbing at the patch or field scale, using either a series of unidirectional tracks (generally 2–4 km each in length) or disturbed plots (up to 11 km2; Jones et al., 2017). Across all the experiments, the disturbances left physical marks (troughs or scars generally 2–8 m wide and 2–4 km long) on the seafloor that remained visible throughout the timeframes of the experiments (1–26 years) (Figure 5). All experiments resulted in a decrease in both faunal abundance and diversity immediately following the disturbances (Bluhm et al., 1995; Schriever et al., 1997; Borowski and Thiel, 1998; Ahnert and Schriever, 2001; Borowski, 2001; Radziejewska, 2014). Most experiments resulted in long-term reductions of both faunal density and diversity, though some faunal groups (e.g., meiofauna in the Indian Ocean, mobile deposit feeders in the CCZ) showed partial recovery towards pre-disturbance densities on the timescale of months to years, depending on the site (Jones et al., 2017, Jones et al., 2025). The only studies investigating fauna living directly associated with the nodules saw a shift in megafauna community composition from mixed sessile and mobile fauna to solely mobile fauna (e.g., holothurians, ophiuroids) at study sites where nodules were removed, and recovery by sessile fauna (e.g., gorgonians, sponges, crinoids) was not observed after 26 years—likely due to the lack of hard substrates for attachment (Bluhm et al., 1995; Bluhm, 2001; Miljutin et al., 2011; Amon et al., 2016; Jones et al., 2025).
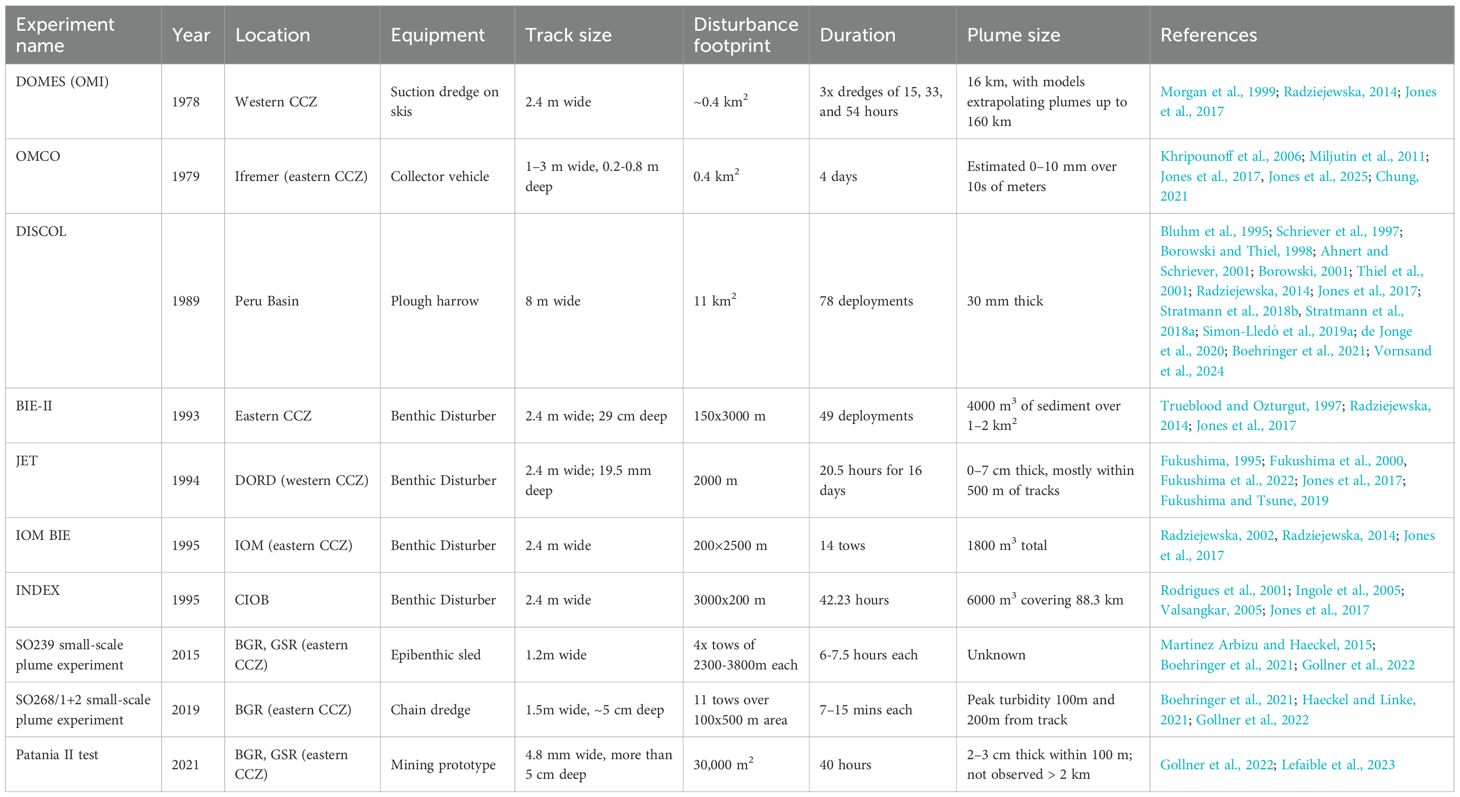
Table 1. Historic and recent disturbance experiments conducted to simulate the effects of deep-seabed mining, including the equipment used and the scale of the disturbance, along with an inexhaustive list of relevant references.

Figure 5. Example of plough-disturbed seafloor from a disturbance experiment (top) and undisturbed seafloor from a nearby site (bottom) in the Peru Basin. Photographs from Figure 1 of Simon-Lledó et al., 2019d.
These first long-term studies of mining-like disturbances in polymetallic nodule environments provided important early observations of the resilience of nodule communities. However, the studies were limited in scope, scale, and sampling integrity (Radziejewska, 2014; Gollner et al., 2017; Jones et al., 2017). Each of the experiments used different methodologies and sampling devices, and the wider applicability of the findings in many cases suffered from technological limitations and low replicate numbers (Fukushima, 1995; Fukushima et al., 2000; Gollner et al., 2017; Jones et al., 2017; Fukushima and Tsune, 2019). The devices used to create the disturbances also varied between studies (e.g., nodules removed vs displaced or buried; different depths of sediment disturbance; different sediment plume scales; Jones et al., 2017), which may have contributed to the different results observed across sites (e.g., increased sediment heterogeneity supporting increased nematode diversity at some experiment sites in the CCZ; Jones et al., 2017; Fukushima et al., 2022). This research also focused on soft sediment communities in areas with lower nodule densities, and, with the exception of megafauna studies, did not investigate the impact of disturbance on nodule-specific fauna, despite their vulnerability to nodule removal (Jones et al., 2017). Since the scale of the experiments was small (a maximum of tens of square kilometers over a few days), it remains difficult to extrapolate their results to the scale of proposed commercial mining (tens of thousands of square kilometers over years), which will influence habitat heterogeneity with greater totality (i.e., complete removal of nodules over large spatial and temporal scales), and therefore benthic communities are likely to exhibit longer recovery times (Borowski and Thiel, 1998; Borowski, 2001; Jones et al., 2017).
5.2.2 Recent disturbance experiments
Given the limitations of previous research, further experimentation has begun in the CCZ and the Peru Basin to fill in the gaps of earlier research (e.g., Gollner et al., 2022; Muñoz-Royo et al., 2022; Stenvers et al., 2023; Lefaible et al., 2024; Vornsand et al., 2024)(Table 1). However, the timescales of these experiments currently remain short, as many of these studies began only within the past several years (e.g., Lefaible et al., 2024) and/or rely on the limited data collected in the aforementioned studies to serve as baselines (e.g., Vornsand et al., 2024; Jones et al., 2025). Furthermore, while many recent disturbance experiments use nodule collector prototypes that closely imitate proposed mining (e.g., nodule removal, movement via caterpillar tracks), the spatial scales of these experiments (<0.05 km2) remain small compared to commercial exploitation (10–100 km2; Muñoz-Royo et al., 2022; Lefaible et al., 2024; Vornsand et al., 2024).
A study of a sediment track in the Peru Basin (near the 1989 experiment site) exhibited 50% lower abundance of Lebensspuren (track, trails and other visible signs of benthic epi- and in-fauna activity) six months after a disturbance event compared to pre-disturbance levels, potentially due to reduced labile carbon availability in the sediments (Vornsand et al., 2024). This speculation is supported by a recent study in the CCZ, which found significantly lower total organic carbon in sediments along the path of a mining prototype and lower food availability in nearby sediments covered by settling sediment plumes (Lefaible et al., 2024). This disturbance and its associated reduction in organic carbon may effectively homogenize sediments and resource availability, therefore reducing patch-scale habitat heterogeneity that supports biodiversity. Additionally, the deposition of sediments may smother sediment fauna and disrupt natural processes of nutrient flux along sediment depths (Miljutin et al., 2011; Gollner et al., 2017). An ongoing study in the CCZ is currently investigating the potential for restoration through the provision of artificial hard substrates, but the feasibility of this possible restoration method will not be known until the completion of the decades-long experiment (expected to end in approximately 30 years; Gollner et al., 2022). The initial observation of colonization after eight weeks of deployment found only one mobile polychaete on the deployment apparatus and no sessile fauna on the ceramic “nodules” (Gollner et al., 2022), which aligns with the slow colonization and recovery rates known to characterize nodule ecosystems. Though still in the early stages of long-term monitoring (and still limited in scale compared to proposed commercial mining), the CCZ experiments using nodule collector prototypes will likely provide a more robust estimation of the potential effects of industrial mining than historic experiments, including on habitat heterogeneity, as the devices use similar collection and locomotory mechanisms to devices proposed for industrial-scale operations.
5.3 Climate change
In addition to the impact of direct anthropogenic disturbance, polymetallic nodule environments are also increasingly influenced by climate change. Climate change is expected to impact primary productivity throughout the ocean, including through the disruption of regular large-scale climate oscillations (e.g., ENSO), which will have cascading impacts on food flux to the deep sea and therefore on faunal communities (Jones et al., 2014; Yasuhara and Danovaro, 2016; Sweetman et al., 2017; Intergovernmental Panel on Climate Change, 2022). Though the influence of large-scale climatic variations on nodule communities remains understudied, climate oscillations have been observed to influence abyssal fauna, including nodule fauna, through their influence on regional habitat heterogeneity via their effect on resource availability (Billett et al., 2001; Smith et al., 2006; Kaiser et al., 2024). The deep sea is also vulnerable to other climate impacts, including ocean acidification and deoxygenation, whose impacts are only beginning to be understood in abyssal environments (Hennige et al., 2015; Levin and Le Bris, 2015; Sweetman et al., 2017), and which could degrade biogenic structures that provide patch- and field-scale substrate heterogeneity, or reduce regional habitat heterogeneity through the homogenization of environmental conditions, respectively. As the impacts of climate change intensify and accelerate, these disruptions to the environmental conditions of the deep sea are likely to compound with direct anthropogenic disturbances such as deep-seabed mining (Sweetman et al., 2017; Levin et al., 2020; Smith et al., 2020). Proponents of deep-seabed mining have presented nodules as essential for addressing climate change by providing minerals for green energy technologies. However, new discoveries (e.g., the possible production of “dark oxygen” in nodule environments; Sweetman et al., 2024) indicate that knowledge gaps about nodule environments remain, including unknown ecological or environmental phenomena that may interact with known climate impacts. It is therefore critical that these effects are studied further and considered in concert with ongoing mining disturbance studies and other baseline research to inform the development of environmental management plans for the mining of polymetallic nodules.
6 Knowledge gaps and management implications
Among polymetallic nodule environments, studies of habitat heterogeneity (e.g., Simon-Lledó et al., 2019b; Mejía-Saenz et al., 2023; Uhlenkott et al., 2023b) remain relatively rare, and are often limited in size or scope (e.g., covering only one faunal size class or spatial scale). While existing studies represent a crucial advancement on the subject, it is critical to include considerations of habitat heterogeneity in a greater breadth of future studies spanning faunal size classes, spatial scales, and sites.
Examinations of multiple faunal size classes in the same study ensures the most holistic approach to investigating benthic faunal communities, however, this is rarely achieved in deep-sea studies. Size differences naturally influence the interaction between faunal communities and different spatial scales of habitat heterogeneity (Gee and Warwick, 1994a; McClain and Barry, 2010; van der Grient and Rogers, 2019), as organisms of different sizes have different windows of perception (e.g., a sessile macrofaunal polychaete will interact with its environment at a smaller spatial scale than a mobile megafaunal scavenger will; Kotliar and Wiens, 1990; Attrill et al., 2000). Therefore, while a nodule-rich area of only a few square meters could be enough to support macrofaunal diversity and connectivity, megafauna communities may require a larger area that encompasses multiple spatial scales of habitat heterogeneity, which could have substantial implications for the designation of areas protected from mining. Furthermore, fauna of different size classes generally exhibit different life cycles and other biological traits which influence their response to disturbance (Gage and Tyler, 1991; Warwick and Clarke, 1996; Rex and Etter, 2010; Zeppilli et al., 2015), including any disturbance affecting habitat heterogeneity. If mining regulators decided that mining operators needed to leave patches of untouched nodule habitat between mining tracks to facilitate recolonization and recovery, information about the complex interactions between faunal size class, community structure, and nodule density and distribution would be crucial to determine the most effective patch size and arrangement to leave undisturbed. Though multiple studies of nodule fauna have included multiple size classes (e.g., Veillette et al., 2007a; Jones et al., 2021; Stratmann et al., 2021), no studies have yet done so with a specific focus on the habitat heterogeneity-community structure relationship. Future studies should therefore include an assessment of the influence of habitat heterogeneity on multiple faunal size classes together within the same study to increase comparability, help elucidate this habitat heterogeneity-community structure relationship, and inform relevant management decisions.
In conjunction with understanding the influence of habitat heterogeneity across faunal size classes, its influence must be further investigated across spatial scales to help determine at what scale habitat heterogeneity most profoundly affects faunal communities (e.g., what metric of habitat heterogeneity supports the highest biodiversity, and at what magnitude). Though a growing body of research has compared faunal communities among nodule fields (e.g., sampling sites; Simon-Lledó et al., 2019c; Chuar et al., 2020; Pasotti et al., 2021) and regions (Vanreusel et al., 2016; Hauquier et al., 2019; Cuvelier et al., 2020; Simon-Lledó et al., 2020), little research has compared communities at the nodule or patch scales (Veillette et al., 2007a; Singh et al., 2019; Pape et al., 2021). Furthermore, though studies have investigated the influence of habitat heterogeneity at more than one spatial scale, no research has yet examined its influence comprehensively across a broad range of spatial scales, from among nodules to among regions. Due to the size of mining contract areas and the variability in sampling methodology, comparing results at different scales from different studies can be difficult. As a result, conducting these scale-dependent assessments comprehensively within one research endeavor could ensure more reliable results about how the influence of habitat heterogeneity changes with the spatial scale of focus. Information about how habitat heterogeneity supports faunal diversity, density, or rarity can inform which areas should be prioritized for protection (e.g., via an Area of Particular Environmental Interest or other marine protected area) and can ensure that rare or endemic species are protected from extinction (e.g., Reed et al., 2006; Lowry et al., 2011). Additionally, this information can be used to protect areas of higher faunal diversity or abundance on spatial scales that preserve population connectivity and facilitate the recolonization of areas disturbed by mining. More knowledge about the habitat heterogeneity-biodiversity relationship will ultimately be crucial for managing nodule mining.
Multiple studies have explored and developed management frameworks to assess the environmental impact of proposed mining activities and avoid “serious harm” as required by ISA regulations (Levin et al., 2016; Durden et al., 2017; Ellis et al., 2017; Christiansen and Bräger, 2023; Leduc et al., 2024a). These frameworks use an assessment of both the mining disturbance (e.g., duration, size, frequency, etc.) and the structural components of the ecosystem being impacted (e.g., rarity/endemism, productivity, growth rates, etc.; Leduc et al., 2024b). An understanding of the relationship between habitat heterogeneity and community structure—particularly biodiversity—could be a critical contribution to the assessment of these structural ecosystem components and could therefore help inform the designation of thresholds that trigger management or mitigation requirements under these frameworks. Figure 6 illustrates the different theoretical habitat heterogeneity-biodiversity relationships that could exist at the field scale (10s to 1000s of meters) in a polymetallic nodule contract area, and the associated levels of caution with which management decisions should be made for seabed mining. For example, if a positive linear relationship exists between habitat heterogeneity and biodiversity in a mining contract area (as has been found in other deep-sea habitats; Etter and Grassle, 1992), serious harm to benthic communities in areas with high levels of heterogeneity may impact more fauna than in areas with low levels of heterogeneity if mining occurs there. As a result, mining regulator authorities may decide to exercise increased caution when determining if and how mining may occur in these high heterogeneity areas (Figure 6A). If a unimodal relationship between habitat heterogeneity and biodiversity is observed (as suggested for nodule environments in Amon et al., 2016), this may increase the level of caution with which management occurs for areas with moderate levels of habitat heterogeneity, where biodiversity is highest and therefore serious harm from mining more likely to occur to more fauna (Figure 6B). Alternatively, an asymptotic relationship may be discovered or predicted between habitat heterogeneity and biodiversity in a contract area, in which habitat heterogeneity only supports high diversity up to a certain threshold after which there are diminishing returns (as suggested for cold water coral reefs in Rowden et al., 2020), mining regulators could exercise less caution in the bottom half of the curve, but may take a more conservative management approach to avoid serious harm for areas at or near the maximum point in the biodiversity-habitat heterogeneity curve (Figure 6C). In this case, the required level of caution would likely vary on a case-by-case basis, depending on the scale of proposed mining, the habitats or communities of interest, and the overall management goals. Ultimately, the biodiversity-habitat heterogeneity relationship will be just one of many factors informing the management of claim areas under a future mining scenario. However, the nature of the habitat heterogeneity-biodiversity relationship can be used to help inform managers which areas in the claim area may be vulnerable to serious harm from seabed mining, and thus where mitigation measures or a more precautionary approach are likely to be required (Figure 6).
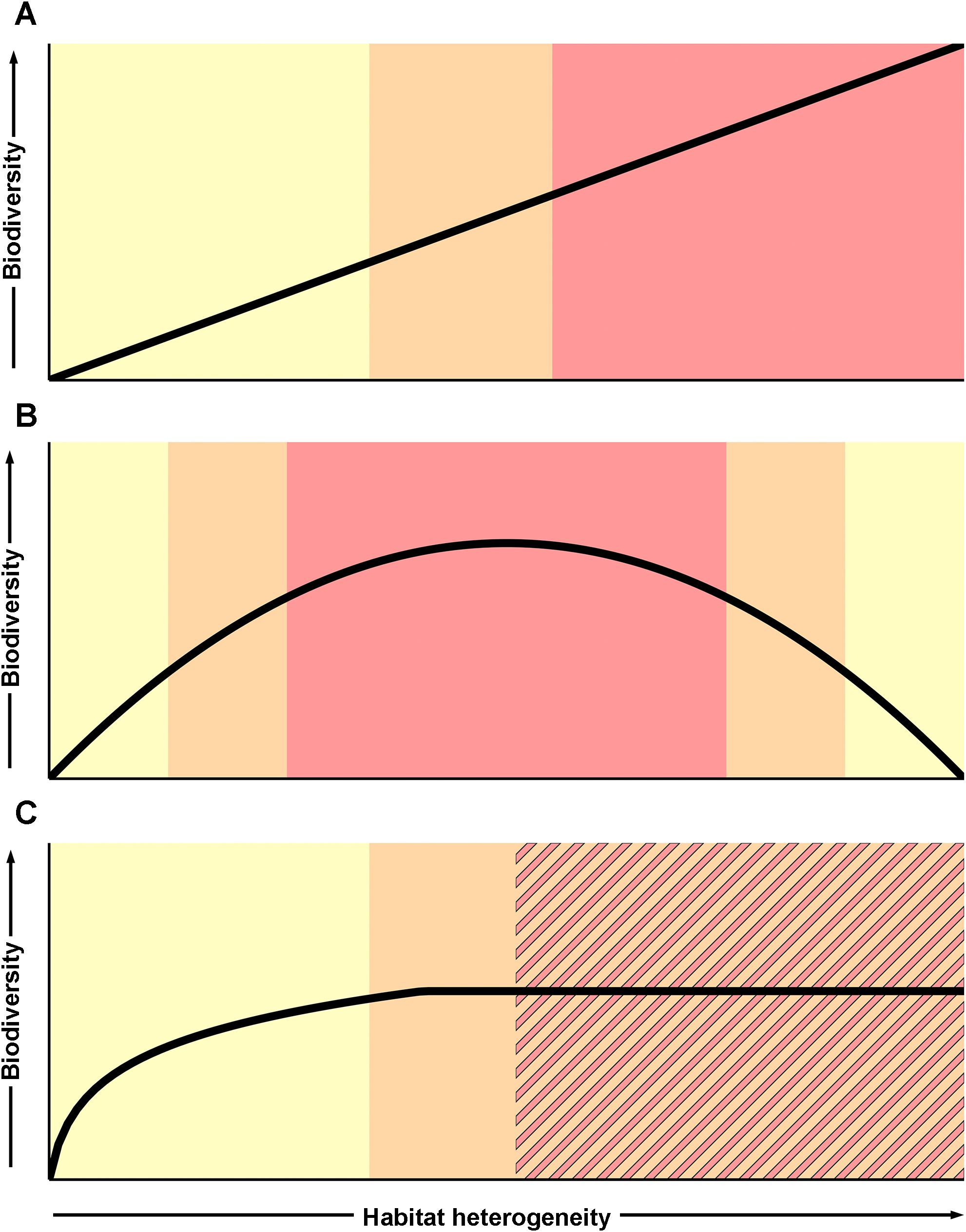
Figure 6. Different theoretical relationships between biodiversity and habitat heterogeneity in nodule environments at the field scale (solid lines) and potential associated levels of caution (shaded colors) recommended for determining management strategies for nodule mining license claim areas: (A) linear, (B) unimodal, and (C) asymptotic. Shaded colors indicate lower (yellow), intermediate (orange), and high (red) levels of caution, based on the hypothetical ranges at which disturbance to habitat heterogeneity would be more likely to cause serious harm. Levels of caution could be associated with different impact assessment or mitigation requirements for mining contractors, with higher levels of caution necessitating adherence to more stringent requirements.
Another substantial knowledge gap regarding habitat heterogeneity is a lack of research across nodule-rich geographic locations. Understandably, the recent surge of research activity on the benthic fauna associated with polymetallic nodules has been focused in the CCZ, where proposed mining is under the most immediate consideration. However, even the best-studied nodule environments in the CCZ remain poorly understood compared to other deep-sea environments, and areas considered for nodule mining within countries’ EEZs (e.g., the Cook Islands nodule fields in the Penrhyn Basin) have never been the subject of any formal ecological research. In the deep sea, habitat heterogeneity and broad-scale environmental variability are often deeply intertwined. As a result, both habitat heterogeneity and its influence on benthic fauna may vary substantially between different geographical regions, making area-specific research both necessary and urgent. Without understanding the composition of different nodule communities and how the habitat heterogeneity-biodiversity relationship varies across geographic regions, management practices suitable to one area may be applied universally with ineffective results.
In addition to the above knowledge gaps, current methodology and metrics for studying habitat heterogeneity in nodule ecosystems may be insufficient to thoroughly describe it and its associated fauna. The metrics used to describe habitat heterogeneity in nodule environments vary considerably (Table 2), but most studies focus on just one metric (generally related to nodule abundance) at one spatial scale. Many of these metrics only measure habitat area or volume, simplifying patch- and field-scale habitat complexity into the presence or absence of hard substrates and overlooking heterogeneity at the smallest scale (millimeters to centimeters) entirely. Quantifying habitat heterogeneity is notoriously difficult, as the irregularity and complexity of natural heterogeneity makes simple equations difficult to create and apply broadly (Loke and Chisholm, 2022). Furthermore, measuring the relationship between habitat heterogeneity and biodiversity can be confounded by the species-area relationship (Arrhenius, 1921) when using simply habitat area or volume (or metrics based on area or volume) as a metric (Steinmann et al., 2011). However, metrics like rugosity or fractal dimension, which allow a quantitative assessment of surface complexity along a continuous scale, have successfully quantified habitat heterogeneity in other studies (e.g., on coral reefs, macroalgae; Gee and Warwick, 1994b; McAbendroth et al., 2005; Walker et al., 2009), and show promise as a method for nodule environments, particularly at the nodule scale. Rugosity is calculated as the ratio of a surface’s actual 3D area to its planar (projected) area, providing a measure of surface roughness relative to flatness. Fractal dimension quantifies surface complexity by evaluating how detail or irregularity scales with measurement resolution (e.g., using box-counting or other multi-scale methods), making it particularly successful at describing fine-scale habitat heterogeneity. However, fractal dimension requires habitats or objects to be fractal (or nearly fractal), which is done by testing its proximity to fractal at 2–3 orders of magnitude (Gonzato et al., 1998), making it difficult to apply to real-world heterogeneity, and particularly to objects as small as nodules. Vector dispersion, which describes the heterogeneity of surface angles at a specific resolution (with higher values indicating greater roughness), has also showed promise in some heterogeneity studies (e.g., on coral reefs; (Carleton and Sammarco, 1987; McCormick, 1994; Young et al., 2017), but has not been as widely tested and, like many metrics (Table 2), can be confounded with area (Loke and Chisholm, 2022). There are also metrics of habitat heterogeneity that can be borrowed from studies of landscape ecology which can be usefully applied at the patch and field scale in nodule environments. Metrics such as spatial congruence and nearest neighbor distance have been used for many years to examine the habitat heterogeneity-biodiversity relationship in terrestrial environments (e.g., Watling et al., 2011; Huntington and Lirman, 2012; Xu et al., 2019), and can provide information about the uniformity or irregularity of patches and their spatial arrangement, respectively. The application of metrics like these, and those indicated for examining the nodule scale, could more robustly characterize habitat heterogeneity—and therefore its influence-in nodule environments, and do so in a more standardized way. Although there are some commonalities, sampling devices vary in both design and size, which can complicate comparing these metrics of habitat heterogeneity across studies (Washburn et al., 2021b; Kaiser et al., 2023). Semi-quantitative, information-based metrics (e.g., defining a metric for nodule patch density) also show promise at intermediate spatial scales (Loke and Chisholm, 2022), but would similarly require standardization to ensure the metric is comparable across study designs and sampling devices.
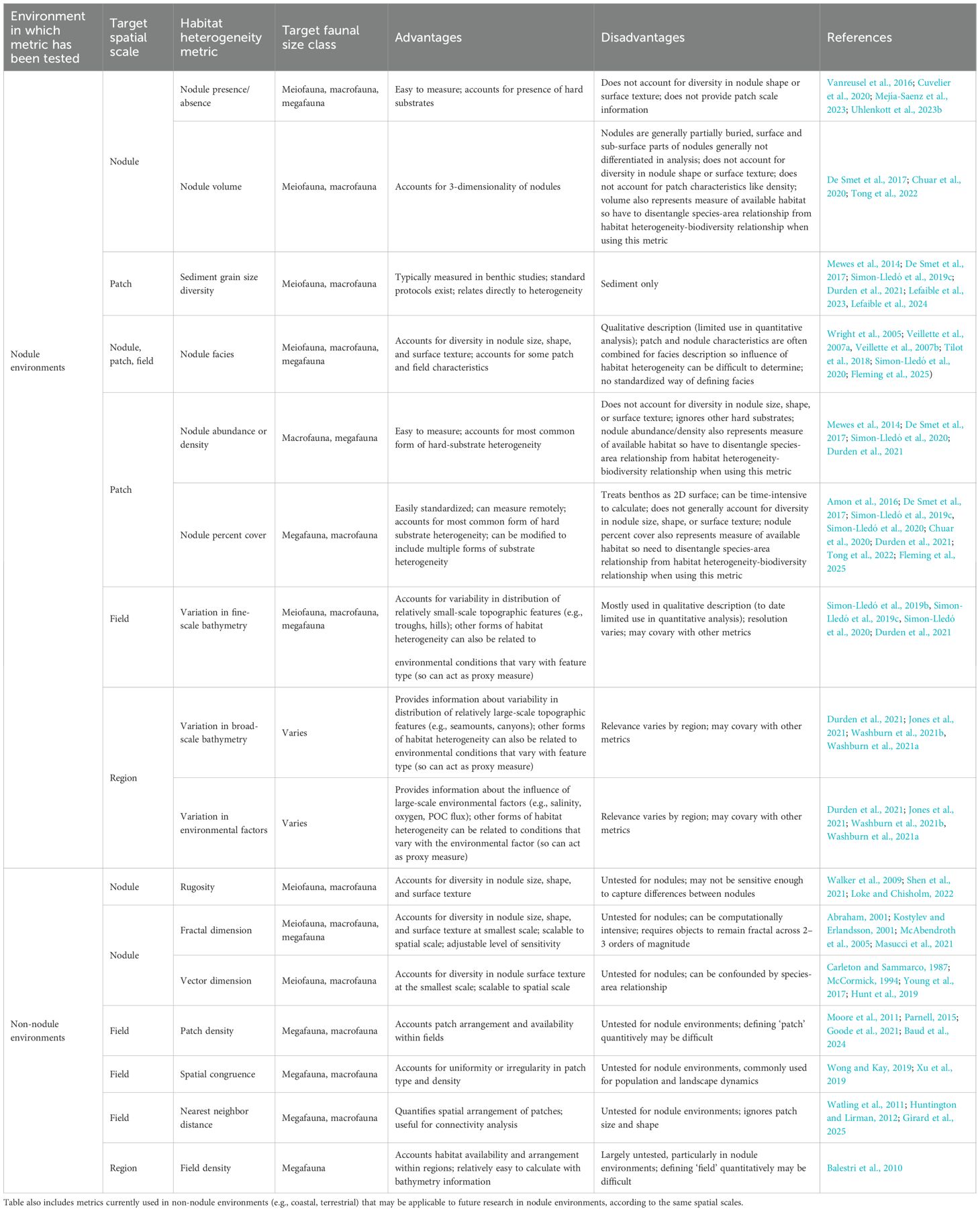
Table 2. Metrics for measuring habitat heterogeneity in nodule environments and their associated advantages and disadvantages, according to spatial scale and faunal size class of focus.
Finally, the monetary and temporal costs of more focused sampling and analysis remain hurdles to closing the knowledge gaps around the influence of habitat heterogeneity and disturbance on benthic community structure. However, closing these gaps remains critical to informing the successful management and conservation of polymetallic nodule ecosystems.
7 Conclusion
There is a growing body of knowledge regarding the community structure and disturbance resilience of benthic communities associated with polymetallic nodule environments. However, better ecological baselines and other knowledge, particularly in understanding the relationship between habitat heterogeneity and benthic community structure, will be required to accurately predict—and therefore manage—the impact that mining may have on faunal communities (Gollner et al., 2017; Jones et al., 2017; Haffert et al., 2020). Current research indicates that nodules play an important role in structuring communities (Veillette et al., 2007a, Veillette et al., 2007b; Simon-Lledó et al., 2019c; Chuar et al., 2020; Cuvelier et al., 2020; Leitner et al., 2021). Nodules have been consistently found to support higher diversity and abundance in megafauna (Amon et al., 2016; Vanreusel et al., 2016; Tilot et al., 2018; Mejía-Saenz et al., 2023; Uhlenkott et al., 2023b, Uhlenkott et al., 2023a) and macrofauna (De Smet et al., 2017; Yu et al., 2018; Chuar et al., 2020) compared to nodule-free sediments. Though some studies have indicated an inverse relationship between meiofauna abundance and nodule abundance (Hauquier et al., 2019; Pape et al., 2021), nodules have also been found to support slightly higher meiofaunal diversity in others (Singh et al., 2016; Pape et al., 2021). Other forms of habitat heterogeneity in nodule environments (e.g., seamounts, rocks, topographical variations) have been shown to support distinct communities with higher diversity than flat, soft sediments (Cuvelier et al., 2020; Leitner et al., 2021; Mejía-Saenz et al., 2023; Uhlenkott et al., 2023b). However, the full extent of the relationship between habitat heterogeneity and faunal community structure remains ambiguous in polymetallic nodule environments, particularly across different spatial scales and faunal size classes, and due in part to the limited metrics currently used to measure it. Without the development of robust knowledge about the habitat heterogeneity-community structure relationship in polymetallic nodule environments, extractive industries may irreversibly alter the habitat heterogeneity that nodules provide before its importance is fully understood.
Author contributions
AU: Investigation, Writing – original draft, Visualization, Writing – review & editing, Conceptualization. AR: Supervision, Writing – original draft, Writing – review & editing. DL: Writing – review & editing, Writing – original draft, Supervision. DZ: Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. DZ was supported by the Ifremer Marine Mineral Resources project (REMIMA project) and by the French National Research Agency under France 2030 (reference ANR-22-MAFM-0001). DZ was also supported by the project “Massive mEIOfauna DiscoverY of new Species of our oceans and SEAs (MEIODYSSEA) funded by the Ocean Shot Research Grant Program of the Sasakawa Peace Foundation, supported by the Nippon Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abel S. M., Wu F., Primpke S., Gerdts G., and Brandt A. (2023). Journey to the deep: plastic pollution in the hadal of deep-sea trenches. Environ. pollut. 333, 122078. doi: 10.1016/j.envpol.2023.122078
Abele L. G. and Walters K. (1979). Marine benthic diversity: A critique and alternative explanation on JSTOR. J. Biogeogr. 6, 115–126. doi: 10.2307/3038047
Abraham E. R. (2001). The fractal branching of an arborescent sponge. Mar. Biol. 138, 503–510. doi: 10.1007/s002270000479
Aguzzi J., Fanelli E., Ciuffardi T., Schirone A., De Leo F. C., Doya C., et al. (2018). Faunal activity rhythms influencing early community succession of an implanted whale carcass offshore Sagami Bay, Japan. Sci. Rep. 8, 11163. doi: 10.1038/s41598-018-29431-5
Ahnert A. and Schriever G. (2001). Response of abyssal Copepoda Harpacticoida (Crustacea) and other meiobenthos to an artificial disturbance and its bearing on future mining for polymetallic nodules. Deep Sea Res. Part II Top. Stud. Oceanogr 48, 3779–3794. doi: 10.1016/S0967-0645(01)00067-4
Aleynik D., Inall M. E., Dale A., and Vink A. (2017). Impact of remotely generated eddies on plume dispersion at abyssal mining sites in the Pacific. Sci. Rep. 7, 16959. doi: 10.1038/s41598-017-16912-2
Alt C. H. S., Rogacheva A., Boorman B., Alan Hughes J., Billett D. S. M., Gooday A. J., et al. (2013). Trawled megafaunal invertebrate assemblages from bathyal depth of the Mid-Atlantic Ridge (48°–54°N). Deep Sea Res. Part II Top. Stud. Oceanogr 98, 326–340. doi: 10.1016/j.dsr2.2013.02.003
Amon D. J., Gollner S., Morato T., Smith C. R., Chen C., Christiansen S., et al. (2022). Assessment of scientific gaps related to the effective environmental management of deep-seabed mining. Mar. Policy 138, 105006. doi: 10.1016/j.marpol.2022.105006
Amon D. J., Hilario A., Arbizu P. M., and Smith C. R. (2017). Observations of organic falls from the abyssal Clarion-Clipperton Zone in the tropical eastern Pacific Ocean. Mar. Biodivers 47, 311–321. doi: 10.1007/s12526-016-0572-4
Amon D. J., Ziegler A. F., Dahlgren T. G., Glover A. G., Goineau A., Gooday A. J., et al. (2016). Insights into the abundance and diversity of abyssal megafauna in a polymetallic-nodule region in the eastern Clarion-Clipperton Zone. Sci. Rep. 6, 30492. doi: 10.1038/srep30492
Arntz W. E., Gallardo V. A., Gutiérrez D., Isla E., Levin L. A., Mendo J., et al. (2006). El Niño and similar perturbation effects on the benthos of the Humboldt, California, and Benguela Current upwelling ecosystems. Adv. Geosci 6, 243–265. doi: 10.5194/adgeo-6-243-2006
Attrill M. J., Strong J. A., and Rowden A. A. (2000). Are macroinvertebrate communities influenced by seagrass structural complexity? Ecography 23, 114–121. doi: 10.1111/j.1600-0587.2000.tb00266.x
Baco A. R., Etter R. J., Ribeiro P. A., von der Heyden S., Beerli P., and Kinlan B. P. (2016). A synthesis of genetic connectivity in deep-sea fauna and implications for marine reserve design. Mol. Ecol. 25, 3276–3298. doi: 10.1111/mec.13689
Bailey D. M., Ruhl H. A., and Smith J. K.L. (2006). Long-term change in benthopelagic fish abundance in the abyssal northeast pacific ocean. Ecology 87, 549–555. doi: 10.1890/04-1832
Bakke T., Klungsøyr J., and Sanni S. (2013). Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 92, 154–169. doi: 10.1016/j.marenvres.2013.09.012
Balestri E., Vallerini F., and Lardicci C. (2010). Effect of seed density and sediment nutrient heterogeneity on recruitment and early patch growth in the seagrass Cymodocea nodosa. Mar. Ecol. Prog. Ser. 417, 63–72. doi: 10.3354/meps08783
Baud M., Macpherson E., Pérez M., Romero J., and Ricart A. M. (2024). Multiple scale assessment of habitat, landscape, and geographic-specific attributes driving decapod assemblages in Posidonia oceanica seagrass meadows. Mar. Environ. Res. 197, 106464. doi: 10.1016/j.marenvres.2024.106464
Bazzaz F. A. (1975). Plant species diversity in old-field successional ecosystems in southern illinois. Ecology 56, 485–488. doi: 10.2307/1934981
Bell J. B., Alt C. H. S., and Jones D. O. B. (2016). Benthic megafauna on steep slopes at the Northern Mid-Atlantic Ridge. Mar. Ecol. 37, 1290–1302. doi: 10.1111/maec.12319
Benn A. R., Weaver P. P., Billet D. S. M., Hove S.v. d., Murdock A. P., Doneghan G. B., et al. (2010). Human activities on the deep seafloor in the north east atlantic: an assessment of spatial extent. PloS One 5, e12730. doi: 10.1371/journal.pone.0012730
Berger W. H., Adelseck C. G. Jr., and Mayer L. A. (1976). Distribution of carbonate in surface sediments of the Pacific Ocean. J. Geophys. Res. 1896-1977 81, 2617–2627. doi: 10.1029/JC081i015p02617
Bergo N. M., Torres-Ballesteros A., Signori C. N., Benites M., Jovane L., Murton B. J., et al. (2022). Spatial patterns of microbial diversity, composition and community structure in Fe-Mn deposits and associated sediments in the Atlantic and Pacific oceans. Sci. Total Environ. 837, 155792. doi: 10.1016/j.scitotenv.2022.155792
Bernardino A. F., Levin L. A., Thurber A. R., and Smith C. R. (2012). Comparative composition, diversity and trophic ecology of sediment macrofauna at vents, seeps and organic falls. PloS One 7, e33515. doi: 10.1371/journal.pone.0033515
Bienhold C., Ristova P. P., Wenzhöfer F., Dittmar T., and Boetius A. (2013). How deep-sea wood falls sustain chemosynthetic life. PloS One 8, e53590. doi: 10.1371/journal.pone.0053590
Bigham K. T., Leduc D., Rowden A. A., Bowden D. A., Nodder S. D., and Orpin A. R. (2024). Recovery of deep-sea meiofauna community in Kaikōura Canyon following an earthquake-triggered turbidity flow. PeerJ 12, e17367. doi: 10.7717/peerj.17367
Bigham K. T., Rowden A. A., Bowden D. A., Leduc D., Pallentin A., Chin C., et al. (2023). Deep-sea benthic megafauna hotspot shows indication of resilience to impact from massive turbidity flow. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1180334
Bigham K. T., Rowden A. A., Leduc D., and Bowden D. A. (2021). Review and syntheses: Turbidity flows – evidence for effects on deep-sea benthic community productivity is ambiguous but the influence on diversity is clearer. Biogeosciences 18, 1893–1908. doi: 10.5194/bg-18-1893-2021
Billett D. S. M., Bett B. J., Rice A. L., Thurston M. H., Galéron J., Sibuet M., et al. (2001). Long-term change in the megabenthos of the Porcupine Abyssal Plain (NE Atlantic). Prog. Oceanogr. 50, 325–348. doi: 10.1016/S0079-6611(01)00060-X
Błażewicz M., Jóźwiak P., Menot L., and Pabis K. (2019). High species richness and unique composition of the tanaidacean communities associated with five areas in the Pacific polymetallic nodule fields. Prog. Oceanogr. 176, 102141. doi: 10.1016/j.pocean.2019.102141
Blöthe M., Wegorzewski A., Müller C., Simon F., Kuhn T., and Schippers A. (2015). Manganese-cycling microbial communities inside deep-sea manganese nodules. Environ. Sci. Technol. 49, 7692–7700. doi: 10.1021/es504930v
Bluhm H. (1994). Monitoring megabenthic communities in abyssal manganese nodule sites of the East Pacific Ocean in association with commercial deep-sea mining. Aquat. Conserv. Mar. Freshw. Ecosyst. 4, 187–201. doi: 10.1002/aqc.3270040302
Bluhm H. (2001). Re-establishment of an abyssal megabenthic community after experimental physical disturbance of the seafloor. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 3841–3868. doi: 10.1016/S0967-0645(01)00070-4
Bluhm H., Schriever G., and Thiel H. (1995). Megabenthic recolonization in an experimentally disturbed abyssal manganese nodule area 1. Mar. Georesources Geotechnol. 13, 393–416. doi: 10.1080/10641199509388295
Boehringer L., Ramalho S. P., Marcon Y., Boetius A., Cuvelier D., and Purser A. (2021). Recovery of Paleodictyon patterns after simulated mining activity on Pacific nodule fields. Mar. Biodivers. 51, 97. doi: 10.1007/s12526-021-01237-1
Bonaglia S., Hedberg J., Marzocchi U., Iburg S., Glud R. N., and Nascimento F. J. A. (2020). Meiofauna improve oxygenation and accelerate sulfide removal in the seasonally hypoxic seabed. Mar. Environ. Res. 159, 104968. doi: 10.1016/j.marenvres.2020.104968
Borowski C. (2001). Physically disturbed deep-sea macrofauna in the Peru Basin, southeast Pacific, revisited 7 years after the experimental impact. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 3809–3839. doi: 10.1016/S0967-0645(01)00069-8
Borowski C. and Thiel H. (1998). Deep-sea macrofaunal impacts of a large-scale physical disturbance experiment in the Southeast Pacific. Deep Sea Res. Part II Top. Stud. Oceanogr. 45, 55–81. doi: 10.1016/S0967-0645(97)00073-8
Boyero L. (2003). The quantification of local substrate heterogeneity in streams and its significance for macroinvertebrate assemblages. Hydrobiologia 499, 161–168. doi: 10.1023/A:1026321331092
Brockett T. and Richards C. Z. (1994). Deepsea mining simulator for environmental impact studies:’Benthic disturber’Designed to assist multinational team assess impact of ocean nodule mining. Sea Technol. 35, 77–82.
Browne R., Parianos J., and Murphy A. (2023). Geomorphology of the Cook Islands, tropical south pacific ocean. J. Maps 19, 2169889. doi: 10.1080/17445647.2023.2169889
Bulling M. T., Solan M., Dyson K. E., Hernandez-Milian G., Luque P., Pierce G. J., et al. (2008). Species effects on ecosystem processes are modified by faunal responses to habitat composition. Oecologia 158, 511–520. doi: 10.1007/s00442-008-1160-5
Carleton J. H. and Sammarco P. W. (1987). Effects of Substratum Irregularity on Success of Coral Settlement: Quantification by Comparative Geomorphological Techniques. Bull. Mar. Sci. 40, 85–98.
Carvalho L. R. S. and Barros F. (2017). Physical habitat structure in marine ecosystems: the meaning of complexity and heterogeneity. Hydrobiologia 797, 1–9. doi: 10.1007/s10750-017-3160-0
Cerrano C., Arillo A., Bavestrello G., Benatti U., Calcinai B., Cattaneo-Vietti R., et al. (1999). Organism–quartz interactions in structuring benthic communities: towards a marine bio-mineralogy? Ecol. Lett. 2, 1–3. doi: 10.1046/j.1461-0248.1999.00041.x
Chiba S., Saito H., Fletcher R., Yogi T., Kayo M., Miyagi S., et al. (2018). Human footprint in the abyss: 30 year records of deep-sea plastic debris. Mar. Policy 96, 204–212. doi: 10.1016/j.marpol.2018.03.022
Chiswell S. M., Bostock H. C., Sutton P. J., and Williams M. J. (2015). Physical oceanography of the deep seas around New Zealand: a review. N. Z. J. Mar. Freshw. Res. 49, 286–317. doi: 10.1080/00288330.2014.992918
Christiansen S. and Bräger S. (2023). Developing best environmental practice for polymetallic nodule mining - a review of scientific recommendations. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1243252
Christodoulou M., O’Hara T., Hugall A. F., Khodami S., Rodrigues C. F., Hilario A., et al. (2020). Unexpected high abyssal ophiuroid diversity in polymetallic nodule fields of the northeast Pacific Ocean and implications for conservation. Biogeosciences 17, 1845–1876. doi: 10.5194/bg-17-1845-2020
Chuar C. H., Tong S. J. W., Chim C. K., Wong H. P. S., and Tan K. S. (2020). Abyssal macrofaunal community structure in the polymetallic nodule exploration area at the easternmost region of the Clarion-Clipperton Fracture Zone, Pacific Ocean. Deep Sea Res. Part Oceanogr. Res. Pap. 161, 103284. doi: 10.1016/j.dsr.2020.103284
Chung J. S. (2021). Manganese nodule miners on 18,000-ft deep seabed: touchdown, track-keeping control and disturbed seabed track history. Int. J. Offshore Polar Eng. 31, 385–394. doi: 10.17736/ijope.2021.jc858
Clark M. R., Althaus F., Schlacher T. A., Williams A., Bowden D. A., and Rowden A. A. (2016). The impacts of deep-sea fisheries on benthic communities: a review. ICES J. Mar. Sci. 73, i51–i69. doi: 10.1093/icesjms/fsv123
Coleman F. C. and Koenig C. C. (2010). The effects of fishing, climate change, and other anthropogenic disturbances on red grouper and other reef fishes in the gulf of Mexico. Integr. Comp. Biol. 50, 201–212. doi: 10.1093/icb/icq072
Company J. B., Puig P., Sardà F., Palanques A., Latasa M., and Scharek R. (2008). Climate influence on deep sea populations. PloS One 3, e1431. doi: 10.1371/journal.pone.0001431
Cordes E. E., Cunha M. R., Galéron J., Mora C., Olu-Le Roy K., Sibuet M., et al. (2010). The influence of geological, geochemical, and biogenic habitat heterogeneity on seep biodiversity. Mar. Ecol. 31, 51–65. doi: 10.1111/j.1439-0485.2009.00334.x
Cronan D. S. (2019). “Manganese nodules,” in Encyclopedia of ocean sciences, 3rd ed.Eds. Cochran J. K., Bokuniewicz H. J., and Yager P. L. (Academic Press, Oxford), 607–614. doi: 10.1016/B978-0-12-409548-9.11383-1
Cullen D. J. (1973). Bioturbation of superficial marine sediments by interstitial meiobenthos. Nature 242, 323–324. doi: 10.1038/242323a0
Cunha M. R., Matos F. L., Génio L., Hilário A., Moura C. J., Ravara A., et al. (2013). Are organic falls bridging reduced environments in the deep sea? - results from colonization experiments in the gulf of cádiz. PloS One 8, e76688. doi: 10.1371/journal.pone.0076688
Cuvelier D., Gollner S., Jones D. O. B., Kaiser S., Arbizu P. M., Menzel L., et al. (2018). Potential mitigation and restoration actions in ecosystems impacted by seabed mining. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00467
Cuvelier D., Ribeiro P. A., Ramalho S. P., Kersken D., Martinez Arbizu P., and Colaço A. (2020). Are seamounts refuge areas for fauna from polymetallic nodule fields? Biogeosciences 17, 2657–2680. doi: 10.5194/bg-17-2657-2020
Danovaro R., Carugati L., Corinaldesi C., Gambi C., Guilini K., Pusceddu A., et al. (2013). Multiple spatial scale analyses provide new clues on patterns and drivers of deep-sea nematode diversity. Deep Sea Res. Part II Top. Stud. Oceanogr. 92, 97–106. doi: 10.1016/j.dsr2.2013.03.035
Danovaro R., Snelgrove P. V. R., and Tyler P. (2014). Challenging the paradigms of deep-sea ecology. Trends Ecol. Evol. 29, 465–475. doi: 10.1016/j.tree.2014.06.002
Das P., Iyer S. D., and Kodagali V. N. (2007). Morphological characteristics and emplacement mechanism of the seamounts in the Central Indian Ocean Basin. Tectonophysics 443, 1–18. doi: 10.1016/j.tecto.2007.08.002
de Jonge D. S. W., Stratmann T., Lins L., Vanreusel A., Purser A., Marcon Y., et al. (2020). Abyssal food-web model indicates faunal carbon flow recovery and impaired microbial loop 26 years after a sediment disturbance experiment. Prog. Oceanogr. 189, 102446. doi: 10.1016/j.pocean.2020.102446
De Leo F. C. and Puig P. (2018). Bridging the gap between the shallow and deep oceans: The key role of submarine canyons. Prog. Oceanogr. 169, 1–5. doi: 10.1016/j.pocean.2018.08.006
Derycke S., De Meester N., Rigaux A., Creer S., Bik H., Thomas W. K., et al. (2016). Coexisting cryptic species of the Litoditis marina complex (Nematoda) show differential resource use and have distinct microbiomes with high intraspecific variability. Mol. Ecol. 25, 2093–2110. doi: 10.1111/mec.13597
De Smet B., Pape E., Riehl T., Bonifácio P., Colson L., and Vanreusel A. (2017). The community structure of deep-sea macrofauna associated with polymetallic nodules in the eastern part of the clarion-clipperton fracture zone. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00103
dos Santos G. A. P., Silva A. C., Esteves A. M., Ribeiro-Ferreira V. P., Neres P. F., Valdes Y., et al. (2020). Testing bathymetric and regional patterns in the southwest atlantic deep sea using infaunal diversity, structure, and function. Diversity 12, 485. doi: 10.3390/d12120485
Durden J. M., Bett B. J., Jones D. O. B., Huvenne V. A. I., and Ruhl H. A. (2015). Abyssal hills – hidden source of increased habitat heterogeneity, benthic megafaunal biomass and diversity in the deep sea. Prog. Oceanogr. 137, 209–218. doi: 10.1016/j.pocean.2015.06.006
Durden J. M., Bett B. J., and Ruhl H. A. (2020). Subtle variation in abyssal terrain induces significant change in benthic megafaunal abundance, diversity, and community structure. Prog. Oceanogr. 186, 102395. doi: 10.1016/j.pocean.2020.102395
Durden J. M., Murphy K., Jaeckel A., Van Dover C. L., Christiansen S., Gjerde K., et al. (2017). A procedural framework for robust environmental management of deep-sea mining projects using a conceptual model. Mar. Policy 84, 193–201. doi: 10.1016/j.marpol.2017.07.002
Durden J. M., Putts M., Bingo S., Leitner A. B., Drazen J. C., Gooday A. J., et al. (2021). Megafaunal ecology of the western clarion clipperton zone. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.671062
Ellis J., Clark M., Rouse H., and Lamarche G. (2017). Environmental management frameworks for offshore mining: the New Zealand approach. Mar. Policy 84, 178–192. doi: 10.1016/j.marpol.2017.07.004
Etter R. J. and Grassle J. F. (1992). Patterns of species diversity in the deep sea as a function of sediment particle size diversity. Nature 360, 576–578. doi: 10.1038/360576a0
Fisher C. R., Hsing P.-Y., Kaiser C. L., Yoerger D. R., Roberts H. H., Shedd W. W., et al. (2014). Footprint of Deepwater Horizon blowout impact to deep-water coral communities. Proc. Natl. Acad. Sci. - PNAS 111, 11744–11749. doi: 10.1073/pnas.1403492111
Fleming B. F. M., Simon-Lledó E., Benoist N., O’Malley B., and Jones D. O. B. (2025). Influence of seabed heterogeneity on benthic megafaunal community patterns in abyssal nodule fields. Elem. Sci. Anthr. 13, 49. doi: 10.1525/elementa.2024.00049
Ford P. W., Bird F. L., and Hancock G. J. (1999). Effect of burrowing macrobenthos on the flux of dissolved substances across the water–sediment interface. Mar. Freshw. Res. 50, 523–532. doi: 10.1071/mf98059
Fukushima T. (1995). OverviewJapan deep-sea impact experiment = JET (Tsukuba, Japan: OnePetro). Available online at: https://dx.doi.org/.
Fukushima T., Shirayama Y., and Kuboki E. (2000). The characteristics of deep-sea epifaunal megabenthos community two years after an artificial rapid deposition event. Publ. SETO Mar. Biol. Lab. 39, 17–27. doi: 10.5134/176293
Fukushima T. and Tsune A. (2019). “Long-term monitoring of environmental conditions of benthic impact experiment,” in Environmental issues of deep-sea mining: impacts, consequences and policy perspectives. Ed. Sharma R. (Springer International Publishing, Cham), 191–211. doi: 10.1007/978-3-030-12696-4_7
Fukushima T., Tsune A., and Sugishima H. (2022). “Comprehensive understanding of seafloor disturbance and environmental impact scenarios,” in in perspectives on deep-sea mining: sustainability, technology, environmental policy and management. Ed. Sharma R. (Springer International Publishing, Cham), 313–337. doi: 10.1007/978-3-030-87982-2_12
Gage J. D., Hughes D. J., and Vecino J. L. G. (2002). Sieve size influence in estimating biomass, abundance and diversity in samples of deep-sea macrobenthos. Mar. Ecol. Prog. Ser. 225, 97–107. doi: 10.3354/meps225097
Gage J. D. and Tyler P. A. (Eds.) (1991). “Smaller animals,” in in deep-sea biology: A natural history of organisms at the deep-sea floor (Cambridge University Press, Cambridge). doi: 10.1017/CBO9781139163637.009
Gallucci F., Moens T., Vanreusel A., and Fonseca G. (2008). Active colonisation of disturbed sediments by deep-sea nematodes: evidence for the patch mosaic model. Mar. Ecol. Prog. Ser. 367, 173–183. doi: 10.3354/meps07537
Gambi C., Pusceddu A., Benedetti-Cecchi L., and Danovaro R. (2014). Species richness, species turnover and functional diversity in nematodes of the deep Mediterranean Sea: searching for drivers at different spatial scales. Glob. Ecol. Biogeogr. 23, 24–39. doi: 10.1111/geb.12094
Gates A. R., Benfield M. C., Booth D. J., Fowler A. M., Skropeta D., and Jones D. O. B. (2017). Deep-sea observations at hydrocarbon drilling locations: Contributions from the SERPENT Project after 120 field visits. Deep Sea Res. Part II Top. Stud. Oceanogr. 137, 463–479. doi: 10.1016/j.dsr2.2016.07.011
Gee J. M. and Warwick R. M. (1994a). Body-size distribution in a marine metazoan community and the fractal dimensions of macroalgae. J. Exp. Mar. Biol. Ecol. 178, 247–259. doi: 10.1016/0022-0981(94)90039-6
Gee J. M. and Warwick R. M. (1994b). Metazoan community structure in relation to the fractal dimensions of marine macroalgae. Mar. Ecol. Prog. Ser. 103, 141–150. doi: 10.3354/meps103141
Gillard B., Harbour R. P., Nowald N., Thomsen L., and Iversen M. H. (2022). Vertical distribution of particulate matter in the clarion clipperton zone (German sector)—Potential impacts from deep-sea mining discharge in the water column. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.820947
Girard F., Caress D. W., Paduan J. B., Kuhnz L. A., Litvin S. Y., Flattery E., et al. (2025). Habitat heterogeneity over multiple scales supports dense and diverse megafaunal communities on a northeast Pacific ridge. Limnol. Oceanogr. 70, 377–392. doi: 10.1002/lno.12766
Glasby G. P. (1976). Surface densities of manganese nodules in the southern sector of the South Pacific. N. Z. J. Geol. Geophys. 19, 771–790. doi: 10.1080/00288306.1976.10420739
Glover A. G., Gooday A. J., Bailey D. M., Billett D. S. M., Chevaldonné P., Colaço A., et al. (2010). “Chapter one - temporal change in deep-sea benthic ecosystems: A review of the evidence from recent time-series studies,” in in advances in marine biology. Ed. Lesser M. (Amsterdam, Netherlands: Academic Press), 1–95. doi: 10.1016/B978-0-12-381015-1.00001-0
Glover A. G., Smith C. R., Paterson G. L. J., Wilson G. D. F., Hawkins L., and Sheader M. (2002). Polychaete species diversity in the central Pacific abyss: local and regional patterns, and relationships with productivity. Mar. Ecol. Prog. Ser. 240, 157–170. doi: 10.3354/meps240157
Godbold J. A., Bulling M. T., and Solan M. (2011). Habitat structure mediates biodiversity effects on ecosystem properties. Proc. R. Soc B Biol. Sci. 278, 2510–2518. doi: 10.1098/rspb.2010.2414
Gollner S., Haeckel M., Janssen F., Lefaible N., Molari M., Papadopoulou S., et al. (2022). Restoration experiments in polymetallic nodule areas. Integr. Environ. Assess. Manage. 18, 682–696. doi: 10.1002/ieam.4541
Gollner S., Kaiser S., Menzel L., Jones D. O. B., Brown A., Mestre N. C., et al. (2017). Resilience of benthic deep-sea fauna to mining activities. Mar. Environ. Res. 129, 76–101. doi: 10.1016/j.marenvres.2017.04.010
Gonzato G., Mulargia F., and Marzocchi W. (1998). Practical application of fractal analysis: problems and solutions. Geophys. J. Int. 132, 275–282. doi: 10.1046/j.1365-246x.1998.00461.x
Gooday A. J., Bett B. J., Escobar E., Ingole B., Levin L. A., Neira C., et al. (2010). Habitat heterogeneity and its influence on benthic biodiversity in oxygen minimum zones. Mar. Ecol. 31, 125–147. doi: 10.1111/j.1439-0485.2009.00348.x
Gooday A. J., Holzmann M., Caulle C., Goineau A., Kamenskaya O., Weber A. A.-T., et al. (2017). Giant protists (xenophyophores, Foraminifera) are exceptionally diverse in parts of the abyssal eastern Pacific licensed for polymetallic nodule exploration. Biol. Conserv. 207, 106–116. doi: 10.1016/j.biocon.2017.01.006
Gooday A. J., Lejzerowicz F., Goineau A., Holzmann M., Kamenskaya O., Kitazato H., et al. (2021). The biodiversity and distribution of abyssal benthic foraminifera and their possible ecological roles: A synthesis across the clarion-clipperton zone. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.634726
Goode S. L., Rowden A. A., Bowden D. A., Clark M. R., and Stephenson F. (2021). Fine-scale mapping of mega-epibenthic communities and their patch Characteristics on two New Zealand seamounts. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.765407
Grassle J. F. and Morse-Porteous L. S. (1987). Macrofaunal colonization of disturbed deep-sea environments and the structure of deep-sea benthic communities. Deep Sea Res. Part Oceanogr. Res. Pap. 34, 1911–1950. doi: 10.1016/0198-0149(87)90091-4
Grassle J. F., Sanders H. L., Hessler R. R., Rowe G. T., and McLellan T. (1975). Pattern and zonation: a study of the bathyal megafauna using the research submersible Alvin. Deep Sea Res. Oceanogr. Abstr. 22, 457–481. doi: 10.1016/0011-7471(75)90020-0
Guden R. M., Derycke S., and Moens T. (2021). A multi-faceted approach to understand how resource diversity can mediate the coexistence of cryptic marine nematode species. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.777425
Haeckel M. and Linke P. (2021). RV SONNE fahrtbericht/cruise report SO268 - assessing the impacts of nodule mining on the deep-sea environment: noduleMonitoring, manzanillo (Mexico) – vancouver (Canada) (Kiel, Germany: GEOMAR Helmholtz-Zentrum für Ozeanforschung Kiel), 17.02.–27.05.2019. doi: 10.3289/GEOMAR_REP_NS_59_20
Haedrich R. L. and Rowe G. T. (1977). Megafaunal biomass in the deep sea. Nature 269, 141–142. doi: 10.1038/269141a0
Haffert L., Haeckel M., De Stigter H., and Janßen F. (2019). DISCOL experiment revisited: Assessing the temporal scale of deep-sea mining impacts on sediment biogeochemistry. doi: 10.5194/bg-2019-361
Haffert L., Haeckel M., de Stigter H., and Janssen F. (2020). Assessing the temporal scale of deep-sea mining impacts on sediment biogeochemistry. Biogeosciences 17, 2767–2789. doi: 10.5194/bg-17-2767-2020
Hakenkamp C. C. and Palmer M. A. (2000). “13 - the ecology of hyporheic meiofauna,” in Streams and ground waters. Eds. Jones J. B. and Mulholland P. J. (Academic Press, San Diego), 307–336. doi: 10.1016/B978-012389845-6/50014-4
Hannides A. K. and Smith C. R. (2003). “The northeastern Pacific abyssal plain,” in Biogeochemistry of marine systems (Oxford, UK: Blackwell).
Harris P. T. (2014). Shelf and deep-sea sedimentary environments and physical benthic disturbance regimes: A review and synthesis. Mar. Geol. 353, 169–184. doi: 10.1016/j.margeo.2014.03.023
Hauquier F., Macheriotou L., Bezerra T. N., Egho G., Martínez Arbizu P., and Vanreusel A. (2019). Distribution of free-living marine nematodes in the Clarion–Clipperton Zone: implications for future deep-sea mining scenarios. Biogeosciences 16, 3475–3489. doi: 10.5194/bg-16-3475-2019
Heck K. L. and Wetstone G. S. (1977). Habitat complexity and invertebrate species richness and abundance in tropical seagrass meadows. J. Biogeogr. 4, 135–142. doi: 10.2307/3038158
Heijnen M. S., Clare M. A., Cartigny M. J. B., Talling P. J., Hage S., Pope E. L., et al. (2022). Fill, flush or shuffle: How is sediment carried through submarine channels to build lobes? Earth Planet. Sci. Lett. 584, 117481. doi: 10.1016/j.epsl.2022.117481
Hein J. R., Koschinsky A., and Kuhn T. (2020). Deep-ocean polymetallic nodules as a resource for critical materials. Nat. Rev. Earth Environ. 1, 158–169. doi: 10.1038/s43017-020-0027-0
Hein J. R. and Mizell K. (2022). “Deep-ocean polymetallic nodules and cobalt-rich ferromanganese crusts in the global ocean: new sources for critical metals,” in in the united nations convention on the law of the sea, part XI regime and the international seabed authority: A twenty-five year journey (Leiden, Netherlands: Brill Nijhoff), 177–197. doi: 10.1163/9789004507388_013
Hennige S. J., Wicks L. C., Kamenos N. A., Perna G., Findlay H. S., and Roberts J. M. (2015). Hidden impacts of ocean acidification to live and dead coral framework. Proc. R. Soc B Biol. Sci. 282, 20150990. doi: 10.1098/rspb.2015.0990
Honjo S., Manganini S. J., Krishfield R. A., and Francois R. (2008). Particulate organic carbon fluxes to the ocean interior and factors controlling the biological pump: A synthesis of global sediment trap programs since 1983. Prog. Oceanogr. 76, 217–285. doi: 10.1016/j.pocean.2007.11.003
Hooff R. C. and Peterson W. T. (2006). Copepod biodiversity as an indicator of changes in ocean and climate conditions of the northern California current ecosystem. Limnol. Oceanogr. 51, 2607–2620. doi: 10.4319/lo.2006.51.6.2607
Horacek H. J. III, Soto E. H., Quiroga E., and Ingels J. (2022). Meiofaunal nematode abundance, composition, and diversity at bathyal to hadal depths in the Southeast Pacific Ocean. Deep Sea Res. Part Oceanogr. Res. Pap. 188, 103837. doi: 10.1016/j.dsr.2022.103837
Hoving H.-J., Boetius A., Dunlop K., Greinert J., Haeckel M., Jones D. O. B., et al. (2023). Major fine-scale spatial heterogeneity in accumulation of gelatinous carbon fluxes on the deep seabed. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1192242
Huettel M., Ziebis W., and Forster S. (1996). Flow-induced uptake of particulate matter in permeable sediments. Limnol. Oceanogr. 41, 309–322. doi: 10.4319/lo.1996.41.2.0309
Hunt C. L., Kelly G. R., Windmill H., Curtis-Quick J., Conlon H., Bodmer M. D. V., et al. (2019). Aggregating behaviour in invasive Caribbean lionfish is driven by habitat complexity. Sci. Rep. 9, 783. doi: 10.1038/s41598-018-37459-w
Huntington B. E. and Lirman D. (2012). Species-area relationships in coral communities: evaluating mechanisms for a commonly observed pattern. Coral Reefs 31, 929–938. doi: 10.1007/s00338-012-0917-9
Huvenne V. A. I., Tyler P. A., Masson D. G., Fisher E. H., Hauton C., Hühnerbach V., et al. (2011). A picture on the wall: innovative mapping reveals cold-water coral refuge in submarine canyon. PloS One 6, e28755. doi: 10.1371/journal.pone.0028755
Ingels J. and Vanreusel A. (2013). The importance of different spatial scales in determining structural and functional characteristics of deep-sea infauna communities. Biogeosciences 10, 4547–4563. doi: 10.5194/bg-10-4547-2013
Ingole B. S., Pavithran S., and Ansari Z. A. (2005). Restoration of deep-sea macrofauna after simulated benthic disturbance in the central Indian basin. Mar. Georesources Geotechnol. 23, 267–288. doi: 10.1080/10641190500446573
Intergovernmental Panel on Climate Change (2022). The ocean and cryosphere in a changing climate: special report of the intergovernmental panel on climate change (Cambridge: Cambridge University Press). doi: 10.1017/9781009157964
Jahnke R. A. (1996). The global ocean flux of particulate organic carbon: Areal distribution and magnitude. Glob. Biogeochem. Cycles 10, 71–88. doi: 10.1029/95GB03525
Jamieson A. J., Bond T., and Vescovo V. (2022). No recovery of a large-scale anthropogenic sediment disturbance on the Pacific seafloor after 77 years at 6460 m depth. Mar. pollut. Bull. 175, 113374. doi: 10.1016/j.marpolbul.2022.113374
Jankowski J. A., Malcherek A., and Zielke W. (1996). Numerical modeling of suspended sediment due to deep-sea mining. J. Geophys. Res. Oceans 101, 3545–3560. doi: 10.1029/95JC03564
Jones D. O. B., Arias M. B., Van Audenhaege L., Blackbird S., Boolukos C., Bribiesca-Contreras G., et al. (2025). Long-term impact and biological recovery in a deep-sea mining track. Nature 642, 1–3. doi: 10.1038/s41586-025-08921-3
Jones D. O. B., Bett B. J., and Tyler P. A. (2007). Megabenthic ecology of the deep Faroe–Shetland channel: A photographic study. Deep Sea Res. Part Oceanogr. Res. Pap. 54, 1111–1128. doi: 10.1016/j.dsr.2007.04.001
Jones D. O. B., Kaiser S., Sweetman A. K., Smith C. R., Menot L., Vink A., et al. (2017). Biological responses to disturbance from simulated deep-sea polymetallic nodule mining. PloS One 12, e0171750. doi: 10.1371/journal.pone.0171750
Jones D. O. B., Simon-Lledó E., Amon D. J., Bett B. J., Caulle C., Clément L., et al. (2021). Environment, ecology, and potential effectiveness of an area protected from deep-sea mining (Clarion Clipperton Zone, abyssal Pacific). Prog. Oceanogr. 197, 102653. doi: 10.1016/j.pocean.2021.102653
Jones D. O. B., Yool A., Wei C.-L., Henson S. A., Ruhl H. A., Watson R. A., et al. (2014). Global reductions in seafloor biomass in response to climate change. Glob. Change Biol. 20, 1861–1872. doi: 10.1111/gcb.12480
Joseph A. (2017). “Chapter 2 - secrets of Bermuda triangle and formation of polymetallic nodules,” in Investigating seafloors and oceans. Ed. Joseph A. (Amsterdam, Netherlands: Elsevier), 81–138. doi: 10.1016/B978-0-12-809357-3.00002-3
Jumars P. A. (1975a). Environmental grain and polychaete species’ diversity in a bathyal benthic community. Mar. Biol. 30, 253–266. doi: 10.1007/BF00390748
Jumars P. A. (1975b). Methods for measurement of community structure in deep-sea macrobenthos. Mar. Biol. 30, 245–252. doi: 10.1007/BF00390747
Kahn A. S., Ruhl H. A., and Smith K. L. (2012). Temporal changes in deep-sea sponge populations are correlated to changes in surface climate and food supply. Deep Sea Res. Part Oceanogr. Res. Pap. 70, 36–41. doi: 10.1016/j.dsr.2012.08.001
Kaiser S., Bonifácio P., Kihara T. C., Menot L., Vink A., Wessels A.-K., et al. (2024). Effects of environmental and climatic drivers on abyssal macrobenthic infaunal communities from the NE Pacific nodule province. Mar. Biodivers. 54, 35. doi: 10.1007/s12526-024-01427-7
Kaiser S., Christodoulou M., Janssen A., Kihara T. C., Mohrbeck I., Pasotti F., et al. (2023). Diversity, distribution and composition of abyssal benthic Isopoda in a region proposed for deep-seafloor mining of polymetallic nodules: a synthesis. Mar. Biodivers. 53, 30. doi: 10.1007/s12526-023-01335-2
Kaiser M. J., Rogers S. I., and Ellis J. R. (1999). Importance of benthic habitat complexity for demersal fish assemblages. Am. Fish. Soc Symp. 22, 212–223.
Kaufmann R. S. and Smith K. L. (1997). Activity patterns of mobile epibenthic megafauna at an abyssal site in the eastern North Pacific: results from a 17-month time-lapse photographic study. Deep Sea Res. Part Oceanogr. Res. Pap. 44, 559–579. doi: 10.1016/S0967-0637(97)00005-8
Kazanidis G., Henry L.-A., and Roberts J. M. (2021). Hidden structural heterogeneity enhances marine hotspots’ biodiversity. Coral Reefs 40, 1615–1630. doi: 10.1007/s00338-021-02114-w
Kerr R. A. (1984). Manganese nodules grow by rain from above. Science 223, 576–577. doi: 10.1126/science.223.4636.576
Kersten O., Smith C. R., and Vetter E. W. (2017). Abyssal near-bottom dispersal stages of benthic invertebrates in the Clarion-Clipperton polymetallic nodule province. Deep Sea Res. Part Oceanogr. Res. Pap. 127, 31–40. doi: 10.1016/j.dsr.2017.07.001
Kersten O., Vetter E. W., Jungbluth M. J., Smith C. R., and Goetze E. (2019). Larval assemblages over the abyssal plain in the Pacific are highly diverse and spatially patchy. PeerJ 7, e7691. doi: 10.7717/peerj.7691
Khadge N. H. (2005). Changes in geotechnical properties of sediments from the central Indian basin induced by disturbance experiment. Mar. Georesources Geotechnol. 23, 401–417. doi: 10.1080/10641190500446797
Khripounoff A., Caprais J.-C., Crassous P., and Etoubleau J. (2006). Geochemical and biological recovery of the disturbed seafloor in polymetallic nodule fields of the Clipperton-Clarion Fracture Zone (CCFZ) at 5,000-m depth. Limnol. Oceanogr. 51, 2033–2041. doi: 10.4319/lo.2006.51.5.2033
Kivenson V., Lemkau K. L., Pizarro O., Yoerger D. R., Kaiser C., Nelson R. K., et al. (2019). Ocean dumping of containerized DDT waste was a sloppy process. Environ. Sci. Technol. 53, 2971–2980. doi: 10.1021/acs.est.8b05859
Koschinsky A., Gaye-Haake B., Arndt C., Maue G., Spitzy A., Winkler A., et al. (2001). Experiments on the influence of sediment disturbances on the biogeochemistry of the deep-sea environment. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 3629–3651. doi: 10.1016/S0967-0645(01)00060-1
Kostylev V. and Erlandsson J. (2001). A fractal approach for detecting spatial hierarchy and structure on mussel beds. Mar. Biol. 139, 497–506. doi: 10.1007/s002270100597
Kotliar N. B. and Wiens J. A. (1990). Multiple scales of patchiness and patch structure: A hierarchical framework for the study of heterogeneity. Oikos 59, 253–260. doi: 10.2307/3545542
Kuhn T., Uhlenkott K., Vink A., Rühlemann C., and Martinez Arbizu P. (2020). “Chapter 58 - Manganese nodule fields from the Northeast Pacific as benthic habitats,” in Seafloor geomorphology as benthic habitat, 2nd ed.Eds. Harris P. T. and Baker E. (Chantilly, France: Elsevier), 933–947. doi: 10.1016/B978-0-12-814960-7.00058-0
Kuhn T., Wegorzewski A., Rühlemann C., and Vink A. (2017). “Composition, formation, and occurrence of polymetallic nodules,” in Deep-sea mining: resource potential, technical and environmental considerations. Ed. Sharma R. (Springer International Publishing, Cham), 23–63. doi: 10.1007/978-3-319-52557-0_2
Kuhnz L. A., Ruhl H. A., Huffard C. L., and Smith K. L. (2014). Rapid changes and long-term cycles in the benthic megafaunal community observed over 24 years in the abyssal northeast Pacific. Prog. Oceanogr. 124, 1–11. doi: 10.1016/j.pocean.2014.04.007
Kukert H. and Smith C. R. (1992). Disturbance, colonization and succession in a deep-sea sediment community: artificial-mound experiments. Deep Sea Res. Part Oceanogr. Res. Pap. 39, 1349–1371. doi: 10.1016/0198-0149(92)90073-3
Lacharité M. and Metaxas A. (2017). Hard substrate in the deep ocean: How sediment features influence epibenthic megafauna on the eastern Canadian margin. Deep Sea Res. Part Oceanogr. Res. Pap. 126, 50–61. doi: 10.1016/j.dsr.2017.05.013
Laroche O., Kersten O., Smith C. R., and Goetze E. (2020). Environmental DNA surveys detect distinct metazoan communities across abyssal plains and seamounts in the western Clarion Clipperton Zone. Mol. Ecol. 29, 4588–4604. doi: 10.1111/mec.15484
Laurent M. C. Z., Le Bris N., Gaill F., and Gros O. (2013). Dynamics of wood fall colonization in relation to sulfide concentration in a mangrove swamp. Mar. Environ. Res. 87–88, 85–95. doi: 10.1016/j.marenvres.2013.03.007
Lebrato M. and Jones D. O. B. (2009). Mass deposition event of Pyrosoma atlanticum carcasses off Ivory Coast (West Africa). Limnol. Oceanogr. 54, 1197–1209. doi: 10.4319/lo.2009.54.4.1197
Leduc D., Clark M. R., Rowden A. A., Hyman J., Dambacher J. M., Dunstan P. K., et al. (2024a). Moving towards an operational framework for defining serious harm for management of seabed mining. Ocean Coast. Manage. 255, 107252. doi: 10.1016/j.ocecoaman.2024.107252
Leduc D., Murray C., Rowden A. A., Nodder S. D., Hale R., and Clark M. R. (2024b). Experimental seabed disturbance effects on Chatham Rise deep-sea meiofaunal communities, Southwest Pacific. N. Z. J. Mar. Freshw. Res. 59, 1–34. doi: 10.1080/00288330.2024.2347623
Leduc D., Rowden A. A., Bowden D. A., Nodder S. D., Probert P. K., Pilditch C. A., et al. (2012). Nematode beta diversity on the continental slope of New Zealand: spatial patterns and environmental drivers. Mar. Ecol. Prog. Ser. 454, 37–52. doi: 10.3354/meps09690
Leduc D., Rowden A. A., Nodder S. D., Berkenbusch K., Probert P. K., and Hadfield M. G. (2014). Unusually high food availability in Kaikoura Canyon linked to distinct deep-sea nematode community. Deep Sea Res. Part II Top. Stud. Oceanogr. 104, 310–318. doi: 10.1016/j.dsr2.2013.06.003
Leduc D., Rowden A. A., Torres L. G., Nodder S. D., and Pallentin A. (2015). Distribution of macro-infaunal communities in phosphorite nodule deposits on Chatham Rise, Southwest Pacific: Implications for management of seabed mining. Deep Sea Res. Part Oceanogr. Res. Pap. 99, 105–118. doi: 10.1016/j.dsr.2015.01.006
Lefaible N., Macheriotou L., Pape E., Molari M., Haeckel M., Zeppilli D., et al. (2024). Industrial mining trial for polymetallic nodules in the Clarion-Clipperton Zone indicates complex and variable disturbances of meiofaunal communities. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1380530
Lefaible N., Macheriotou L., Purkiani K., Haeckel M., Zeppilli D., Pape E., et al. (2023). Digging deep: lessons learned from meiofaunal responses to a disturbance experiment in the Clarion-Clipperton Zone. Mar. Biodivers. 53, 48. doi: 10.1007/s12526-023-01353-0
Leitner A. B., Drazen J. C., and Smith C. R. (2021). Testing the seamount refuge hypothesis for predators and scavengers in the western clarion-clipperton zone. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.636305
Levin L. A., DeMaster D. J., McCann L. D., and Thomas C. L. (1986). Effects of giant protozoans (class: Xenophyophorea) on deep-seamount benthos. Mar. Ecol. Prog. Ser. 29, 99–104. doi: 10.3354/meps029099
Levin L. A., Etter R. J., Rex M. A., Gooday A. J., Smith C. R., Pineda J., et al. (2001). Environmental influences on regional deep-sea species diversity. Annu. Rev. Ecol. Syst. 32, 51–93. doi: 10.1146/annurev.ecolsys.32.081501.114002
Levin L. A. and Le Bris N. (2015). The deep ocean under climate change. Science 350, 766–768. doi: 10.1126/science.aad0126
Levin L. A., Leithold E. L., Gross T. F., Huggett C. L., and DiBacco C. (1994). Contrasting effects of substrate mobility on infaunal assemblages inhabiting two high-energy settings on Fieberling Guyot. J. Mar. Res. 52, 489–522. doi: 10.1357/0022240943077028
Levin L. A., Mengerink K., Gjerde K. M., Rowden A. A., Van Dover C. L., Clark M. R., et al. (2016). Defining “serious harm“ to the marine environment in the context of deep-seabed mining. Mar. Policy 74, 245–259. doi: 10.1016/j.marpol.2016.09.032
Levin L. A., Rathburn A. E., Gutiérrez D., Muñoz P., and Shankle A. (2003). Bioturbation by symbiont-bearing annelids in near-anoxic sediments: Implications for biofacies models and paleo-oxygen assessments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 199, 129–140. doi: 10.1016/S0031-0182(03)00500-5
Levin L. A. and Sibuet M. (2012). Understanding continental margin biodiversity: A new imperative. Annu. Rev. Mar. Sci. 4, 79–112. doi: 10.1146/annurev-marine-120709-142714
Levin L. A., Sibuet M., Gooday A. J., Smith C. R., and Vanreusel A. (2010). The roles of habitat heterogeneity in generating and maintaining biodiversity on continental margins: an introduction. Mar. Ecol. 31, 1–5. doi: 10.1111/j.1439-0485.2009.00358.x
Levin L. A., Wei C.-L., Dunn D. C., Amon D. J., Ashford O. S., Cheung W. W. L., et al. (2020). Climate change considerations are fundamental to management of deep-sea resource extraction. Glob. Change Biol. 26, 4664–4678. doi: 10.1111/gcb.15223
Levinton J. (1995). “Bioturbators as ecosystem engineers: control of the sediment fabric, inter-individual interactions, and material fluxes,” in Linking species & Ecosystems. Eds. Jones C. G. and Lawton J. H. (Springer US, Boston, MA), 29–36. doi: 10.1007/978-1-4615-1773-3_3
Liao J.-X., Chen G.-M., Chiou M.-D., Jan S., and Wei C.-L. (2017). Internal tides affect benthic community structure in an energetic submarine canyon off SW Taiwan. Deep Sea Res. Part Oceanogr. Res. Pap. 125, 147–160. doi: 10.1016/j.dsr.2017.05.014
Loke L. H. L. and Chisholm R. A. (2022). Measuring habitat complexity and spatial heterogeneity in ecology. Ecol. Lett. 25, 2269–2288. doi: 10.1111/ele.14084
Looser R., Froescheis O., Cailliet G. M., Jarman W. M., and Ballschmiter K. (2000). The deep-sea as a final global sink of semivolatile persistent organic pollutants? Part II: organochlorine pesticides in surface and deep-sea dwelling fish of the North and South Atlantic and the Monterey Bay Canyon (California). Chemosphere 40, 661–670. doi: 10.1016/S0045-6535(99)00462-2
Lowry L., Laist D., Gilmartin W., and Antonelis G. (2011). Recovery of the hawaiian monk seal (Monachus schauinslandi): A review of conservation efforts 1972 to 2010, and thoughts for the future. Aquat. Mamm. 37, 397–419. doi: 10.1578/AM.37.3.2011.397
Lundsten L., Schlining K. L., Frasier K., Johnson S. B., Kuhnz L. A., Harvey J. B. J., et al. (2010). Time-series analysis of six whale-fall communities in Monterey Canyon, California, USA. Deep Sea Res. Part Oceanogr. Res. Pap. 57, 1573–1584, doi: 10.1016/j.dsr.2010.09.003
Lutz M. J., Caldeira K., Dunbar R. B., and Behrenfeld M. J. (2007). Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean. J. Geophys. Res. Oceans 112, 1–26. doi: 10.1029/2006JC003706
Macreadie P. I., Fowler A. M., and Booth D. J. (2011). Rigs-to-reefs: will the deep sea benefit from artificial habitat? Front. Ecol. Environ. 9, 455–461. doi: 10.1890/100112
Mahatma R. (2009). Meiofauna communities of the Pacific Nodule Province: abundance, diversity and community structure (Oldenburg, Germany: Universität Oldenburg). Available online at: http://oops.uni-oldenburg.de/1009/.
Martínez Arbizu P. and Haeckel M. (2015). RV SONNE fahrtbericht / cruise report SO239: ecoResponse assessing the ecology, connectivity and resilience of polymetallic nodule field systems, balboa (Panama) – manzanillo (Mexico) (Kiel, Germany: GEOMAR Helmholtz-Zentrum für Ozeanforschung). doi: 10.3289/GEOMAR_REP_NS_25_2015
Masucci G. D., Biondi P., and Reimer J. D. (2021). A comparison of size, shape, and fractal diversity between coral rubble sampled from natural and artificial coastlines around okinawa island, Japan. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.703698
McAbendroth L., Ramsay P. M., Foggo A., Rundle S. D., and Bilton D. T. (2005). Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos 111, 279–290. doi: 10.1111/j.0030-1299.2005.13804.x
McClain C. R. and Barry J. P. (2010). Habitat heterogeneity, disturbance, and productivity work in concert to regulate biodiversity in deep submarine canyons. Ecology 91, 964–976. doi: 10.1890/09-0087.1
McClain C. R., Nekola J. C., Kuhnz L., and Barry J. P. (2011). Local-scale faunal turnover on the deep Pacific seafloor. Mar. Ecol. Prog. Ser. 422, 193–200. doi: 10.3354/meps08924
McCormick M. I. (1994). Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar. Ecol. Prog. Ser. 112, 87–96.
Meadows A. and Meadows P. S. (1994). Bioturbation in deep sea Pacific sediments. J. Geol. Soc 151, 361–375. doi: 10.1144/gsjgs.151.2.0361
Meadows P. S., Meadows A., and Murray J. M. H. (2012). Biological modifiers of marine benthic seascapes: Their role as ecosystem engineers. Geomorphology 157–158, 31–48. doi: 10.1016/j.geomorph.2011.07.007
Mejía-Saenz A., Simon-Lledó E., Partridge L. S., Xavier J. R., and Jones D. O. B. (2023). Rock outcrops enhance abyssal benthic biodiversity. Deep Sea Res. Part Oceanogr. Res. Pap. 195, 103999. doi: 10.1016/j.dsr.2023.103999
Mewes K., Mogollón J. M., Picard A., Rühlemann C., Kuhn T., Nöthen K., et al. (2014). Impact of depositional and biogeochemical processes on small scale variations in nodule abundance in the Clarion-Clipperton Fracture Zone. Deep Sea Res. Part Oceanogr. Res. Pap. 91, 125–141. doi: 10.1016/j.dsr.2014.06.001
Meyer K. S., Young C. M., Sweetman A. K., Taylor J., Soltwedel T., and Bergmann M. (2016). Rocky islands in a sea of mud: biotic and abiotic factors structuring deep-sea dropstone communities. Mar. Ecol. Prog. Ser. 556, 45–57. doi: 10.3354/meps11822
Miguez-Salas O., Huffard C. L., Smith K. L., McGill P. R., and Rodríguez-Tovar F. J. (2020). Faunal assemblage changes, bioturbation and benthic storms at an abyssal station in the northeastern Pacific. Deep Sea Res. Part Oceanogr. Res. Pap. 160, 103277. doi: 10.1016/j.dsr.2020.103277
Miljutin D. M., Miljutina M. A., Arbizu P. M., and Galéron J. (2011). Deep-sea nematode assemblage has not recovered 26 years after experimental mining of polymetallic nodules (Clarion-Clipperton Fracture Zone, Tropical Eastern Pacific). Deep Sea Res. Part Oceanogr. Res. Pap. 58, 885–897. doi: 10.1016/j.dsr.2011.06.003
Miljutin D., Miljutina M., and Messié M. (2015). Changes in abundance and community structure of nematodes from the abyssal polymetallic nodule field, Tropical Northeast Pacific. Deep Sea Res. Part Oceanogr. Res. Pap. 106, 126–135. doi: 10.1016/j.dsr.2015.10.009
Miljutina M. A., Miljutin D. M., Mahatma R., and Galéron J. (2010). Deep-sea nematode assemblages of the Clarion-Clipperton Nodule Province (Tropical North-Eastern Pacific). Mar. Biodivers. 40, 1–15. doi: 10.1007/s12526-009-0029-0
Mizell K., Hein J. R., Au M., and Gartman A. (2022). “Estimates of metals contained in abyssal manganese nodules and ferromanganese crusts in the global ocean based on regional variations and genetic types of nodules,” in Perspectives on deep-sea mining: sustainability, technology, environmental policy and management. Ed. Sharma R. (Springer International Publishing, Cham), 53–80. doi: 10.1007/978-3-030-87982-2_3
Montagna P. A., Baguley J. G., Cooksey C., Hartwell I., Hyde L. J., Hyland J. L., et al. (2013). Deep-sea benthic footprint of the deepwater horizon blowout. PloS One 8, e70540. doi: 10.1371/journal.pone.0070540
Moore C. H., Van Niel K., and Harvey E. S. (2011). The effect of landscape composition and configuration on the spatial distribution of temperate demersal fish. Ecography 34, 425–435. doi: 10.1111/j.1600-0587.2010.06436.x
Moran S. B., Kelly R. P., Hagstrom K., Smith J. N., Grebmeier J. M., Cooper L. W., et al. (2005). Seasonal changes in POC export flux in the Chukchi Sea and implications for water column-benthic coupling in Arctic shelves. Deep Sea Res. Part II Top. Stud. Oceanogr. 52, 3427–3451. doi: 10.1016/j.dsr2.2005.09.011
Morgan C. L., Odunton N. A., and Jones A. T. (1999). Synthesis of environmental impacts of deep seabed mining. Mar. Georesources Geotechnol. 17, 307–356. doi: 10.1080/106411999273666
Mullineaux L. S. (1987). Organisms living on manganese nodules and crusts: distribution and abundance at three North Pacific sites. Deep Sea Res. Part Oceanogr. Res. Pap. 34, 165–184. doi: 10.1016/0198-0149(87)90080-X
Muñoz-Royo C., Ouillon R., El Mousadik S., Alford M. H., and Peacock T. (2022). An in situ study of abyssal turbidity-current sediment plumes generated by a deep seabed polymetallic nodule mining preprototype collector vehicle. Sci. Adv. 8, eabn1219. doi: 10.1126/sciadv.abn1219
Murray J. and Renard A. F. (1891). Report on deep-sea deposits based on the specimens collected during the voyage of HMS Challenger in the years 1872 to 1876 (Edinburgh, UK: HM Stationery Office).
Murray C., Rowden A. A., Leduc D., Nodder S. D., Hale R., Halliday J., et al. (2024). Effects of experimental in situ seabed disturbance on deep-sea macrofaunal communities of Chatham Rise, Southwest Pacific. N. Z. J. Mar. Freshw. Res. 59, 1–38. doi: 10.1080/00288330.2024.2404501
Nakajima R., Yamamoto H., Kawagucci S., Takaya Y., Nozaki T., Chen C., et al. (2015). Post-drilling changes in seabed landscape and megabenthos in a deep-sea hydrothermal system, the iheya north field, okinawa trough. PloS One 10, e0123095. doi: 10.1371/journal.pone.0123095
Neira C., Ingels J., Mendoza G., Hernandez-Lopez E., and Levin L. A. (2018). Distribution of meiofauna in bathyal sediments influenced by the oxygen minimum zone off Costa Rica. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00448
Neyman A. A., Sokolova M. N., Vinogradova N. G., and Pasternak F. A. (1973). “Some patterns of the distribution of bottom fauna in the Indian ocean,” in The biology of the Indian ocean. Eds. Zeitzschel B. and Gerlach S. A. (Springer, Berlin, Heidelberg), 467–473. doi: 10.1007/978-3-642-65468-8_42
Nomaki H., Rastelli E., Alves A., Suga H., Ramos S., Kitahashi T., et al. (2021). Abyssal fauna, benthic microbes, and organic matter quality across a range of trophic conditions in the western Pacific ocean. Prog. Oceanogr. 195, 102591. doi: 10.1016/j.pocean.2021.102591
Orcutt B. N., Bradley J. A., Brazelton W. J., Estes E. R., Goordial J. M., Huber J. A., et al. (2020). Impacts of deep-sea mining on microbial ecosystem services. Limnol. Oceanogr. 65, 1489–1510. doi: 10.1002/lno.11403
Pape E., Bezerra T. N., Gheerardyn H., Buydens M., Kieswetter A., and Vanreusel A. (2021). Potential impacts of polymetallic nodule removal on deep-sea meiofauna. Sci. Rep. 11, 19996. doi: 10.1038/s41598-021-99441-3
Pape E., Bezerra T. N., Hauquier F., and Vanreusel A. (2017). Limited Spatial and Temporal Variability in Meiofauna and Nematode Communities at Distant but Environmentally Similar Sites in an Area of Interest for Deep-Sea Mining. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00205
Parianos J. and Madureira P. (2021). Geomorphology of the clarion clipperton zone, tropical north pacific ocean. J. Maps 17, 760–768. doi: 10.1080/17445647.2021.2001387
Parnell P. E. (2015). The effects of seascape pattern on algal patch structure, sea urchin barrens, and ecological processes. J. Exp. Mar. Biol. Ecol. 465, 64–76. doi: 10.1016/j.jembe.2015.01.010
Parry D. M., Kendall M. A., Pilgrim D. A., and Jones M. B. (2003). Identification of patch structure within marine benthic landscapes using a remotely operated vehicle. J. Exp. Mar. Biol. Ecol. 285–286, 497–511. doi: 10.1016/S0022-0981(02)00546-4
Parulekar A. H., Harkantra S. N., Ansari Z. A., and Matondkar S. G. P. (1982). Abyssal benthos of the central Indian Ocean. Deep Sea Res. Part Oceanogr. Res. Pap. 29, 1531–1537. doi: 10.1016/0198-0149(82)90041-3
Pasotti F., Mevenkamp L., Pape E., Błażewicz M., Bonifácio P., Riehl T., et al. (2021). A local scale analysis of manganese nodules influence on the Clarion-Clipperton Fracture Zone macrobenthos. Deep Sea Res. Part Oceanogr. Res. Pap. 168, 103449. doi: 10.1016/j.dsr.2020.103449
Paul S. A. L., Gaye B., Haeckel M., Kasten S., and Koschinsky A. (2018). Biogeochemical regeneration of a nodule mining disturbance site: trace metals, DOC and amino acids in deep-sea sediments and pore waters. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00117
Pereira O. S., Gonzalez J., Mendoza G., Le J., McNeill M., Ontiveros J., et al. (2022). Does substrate matter in the deep sea? A comparison of bone, wood, and carbonate rock colonizers. PloS One 17, e0271635. doi: 10.1371/journal.pone.0271635
Pickens C., Lily H., Harrould-Kolieb E., Blanchard C., and Chakraborty A. (2024). From what-if to what-now: Status of the deep-sea mining regulations and underlying drivers for outstanding issues. Mar. Policy 169, 105967. doi: 10.1016/j.marpol.2023.105967
Priede I. G., Bergstad O. A., Miller P. I., Vecchione M., Gebruk A., Falkenhaug T., et al. (2013). Does presence of a mid-ocean ridge enhance biomass and biodiversity? PloS One 8, 1–10. doi: 10.1371/journal.pone.0061550
Radziejewska T. (2002). Responses of deep-sea meiobenthic communities to sediment disturbance simulating effects of polymetallic nodule mining. Int. Rev. Hydrobiol. 87, 457–477. doi: 10.1002/1522-2632(200207)87:4%3C457::AID-IROH457%3E3.0.CO;2-3
Radziejewska T. (2014). “Meiobenthos as a component of anthropogenic disturbance assessment in the abyssal pacific environment,” in Meiobenthos in the sub-equatorial pacific abyss: A proxy in anthropogenic impact evaluation. Ed. Radziejewska T. (Springer, Berlin, Heidelberg), 67–99. doi: 10.1007/978-3-642-41458-9_4
Radziejewska T., Drzycimski I., Galtsova V. V., Kulangieva L. V., and Stoyanova V. (2001). Changes in genus-level diversity of meiobenthic free-living nematodes (Nematoda) and harpacticoids (Copepoda harpacticoida) at an abyssal site following experimental sediment disturbance.
Radziejewska T., Mianowicz K., and Abramowski T. (2022). “Natural variability versus anthropogenic impacts on deep-sea ecosystems of importance for deep-sea mining,” in Perspectives on deep-sea mining: sustainability, technology, environmental policy and management. Ed. Sharma R. (Springer International Publishing, Cham), 281–311. doi: 10.1007/978-3-030-87982-2_11
Ramirez-Llodra E., Tyler P. A., Baker M. C., Bergstad O. A., Clark M. R., Escobar E., et al. (2011). Man and the last great wilderness: human impact on the deep sea. PloS One 6, e22588. doi: 10.1371/journal.pone.0022588
Reed J. K., Weaver D. C., and Pomponi S. A. (2006). Habitat and fauna of deep-water Lophelia pertusa coral reefs off the southeastern U.S.: Blake plateau, Straits of Florida, and Gulf of Mexico. Bull. Mar. Sci. 78, 343–375.
Rees H. L., Ellis J. R., Hiscock K., Boyd S. E., and Schratzberger M. (2009). “Benthic communities, ecosystems and fisheries,” in Advances in fisheries science: 50 years on from beverton and holt (Oxford, UK: John Wiley & Sons), 358–398.
Rex M. A. and Etter R. J. (2010). Deep-sea biodiversity: pattern and scale (Cambridge, MA, USA: Harvard University Press).
Rex M. A., Etter R. J., Morris J. S., Crouse J., McClain C. R., Johnson N. A., et al. (2006). Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar. Ecol. Prog. Ser. 317, 1–8. doi: 10.3354/meps317001
Rigby C. and Lawton J. H. (1981). Species-area relationships of arthropods on host plants: herbivores on bracken. J. Biogeogr. 8, 125–133. doi: 10.2307/2844555
Rixen T., Gaye B., and Emeis K.-C. (2019). The monsoon, carbon fluxes, and the organic carbon pump in the northern Indian Ocean. Prog. Oceanogr. 175, 24–39. doi: 10.1016/j.pocean.2019.03.001
Robert K., Jones D. O. B., Tyler P. A., Van Rooij D., and Huvenne V. A. I. (2015). Finding the hotspots within a biodiversity hotspot: fine-scale biological predictions within a submarine canyon using high-resolution acoustic mapping techniques. Mar. Ecol. 36, 1256–1276. doi: 10.1111/maec.12228
Roberts C. M. (2002). Deep impact: the rising toll of fishing in the deep sea. Trends Ecol. Evol. 17, 242–245. doi: 10.1016/S0169-5347(02)02492-8
Rodrigues N., Sharma R., and Nagender Nath B. (2001). Impact of benthic disturbance on megafauna in Central Indian Basin. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 3411–3426. doi: 10.1016/S0967-0645(01)00049-2
Rosli N., Leduc D., Rowden A. A., and Probert P. K. (2017). Review of recent trends in ecological studies of deep-sea meiofauna, with focus on patterns and processes at small to regional spatial scales. Mar. Biodivers. 48, 13–34. doi: 10.1007/s12526-017-0801-5
Rosli N., Leduc D., Rowden A. A., Probert P. K., and Clark M. R. (2018). Regional and sediment depth differences in nematode community structure greater than between habitats on the New Zealand margin: Implications for vulnerability to anthropogenic disturbance. Prog. Oceanogr. 160, 26–52. doi: 10.1016/j.pocean.2017.11.006
Rowden A. A., Pearman T. R. R., Bowden D. A., Anderson O. F., and Clark M. R. (2020). Determining coral density thresholds for identifying structurally complex vulnerable marine ecosystems in the deep sea. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00095
Ruhl H. A. and Smith K. L. (2004). Shifts in deep-sea community structure linked to climate and food supply. Science 305, 513–515. doi: 10.1126/science.1099759
Samadi S., Bottan L., Macpherson E., De Forges B. R., and Boisselier M.-C. (2006). Seamount endemism questioned by the geographic distribution and population genetic structure of marine invertebrates. Mar. Biol. 149, 1463–1475. doi: 10.1007/s00227-006-0306-4
Sanders H. L. and Hessler R. R. (1969). Ecology of the Deep-Sea Benthos: More detailed recent sampling has altered our concepts about the animals living on the deep-ocean floor. Science 163, 1419–1424. doi: 10.1126/science.163.3874.1419
Santibañez-Aguascalientes N. A., Borja Á., and Ardisson P.-L. (2023). Assessing the large-scale and long-term changes in the southern Gulf of Mexico benthic ecological status under natural and human-induced disturbances. Estuar. Coast. Shelf Sci. 283, 108282. doi: 10.1016/j.ecss.2023.108282
Sardà F., Cartes J. E., and Company J. B. (1994). Spatio-temporal variations in megabenthos abundance in three different habitats of the Catalan deep-sea (Western Mediterranean). Mar. Biol. 120, 211–219. doi: 10.1007/BF00349681
Schmidt C. and Martínez Arbizu P. (2015). Unexpectedly higher metazoan meiofauna abundances in the Kuril–Kamchatka Trench compared to the adjacent abyssal plains. Deep Sea Res. Part II Top. Stud. Oceanogr. 111, 60–75. doi: 10.1016/j.dsr2.2014.08.019
Schriever C., Ahnert A., Bluhm H., Borowski C., and Thiel H. (1997). Results of the large scale deep-sea environmental impact study DISCOL during eight years of investigation (Honolulu, HI, USA: OnePetro). Available online at: https://dx.doi.org/.
Sharma R., Nagender Nath B., Parthiban G., and Jai Sankar S. (2001). Sediment redistribution during simulated benthic disturbance and its implications on deep seabed mining. Deep Sea Res. Part II Top. Stud. Oceanogr 48, 3363–3380. doi: 10.1016/S0967-0645(01)00046-7
Shen C., Lu B., Li Z., Zhang R., Chen W., Xu P., et al. (2021). Community structure of benthic megafauna on a seamount with cobalt-rich ferromanganese crusts in the northwestern Pacific Ocean. Deep Sea Res. Part Oceanogr. Res. Pap. 178, 103661. doi: 10.1016/j.dsr.2021.103661
Silva A. P., Colaço A., Ravara A., Jakobsen J., Jakobsen K., and Cuvelier D. (2021). The first whale fall on the Mid-Atlantic Ridge: Monitoring a year of succession. Deep Sea Res. Part Oceanogr. Res. Pap. 178, 103662. doi: 10.1016/j.dsr.2021.103662
Simon-Lledó E., Amon D. J., Bribiesca-Contreras G., Cuvelier D., Durden J. M., Ramalho S. P., et al. (2023). Carbonate compensation depth drives abyssal biogeography in the northeast Pacific. Nat. Ecol. Evol. 7, 1388–1397. doi: 10.1038/s41559-023-02122-9
Simon-Lledó E., Bett B. J., Huvenne V. A. I., Köser K., Schoening T., Greinert J., et al. (2019a). Biological effects 26 years after simulated deep-sea mining. Sci. Rep. 9, 8040. doi: 10.1038/s41598-019-44492-w
Simon-Lledó E., Bett B. J., Huvenne V. A. I., Schoening T., Benoist N. M. A., Jeffreys R. M., et al. (2019b). Megafaunal variation in the abyssal landscape of the Clarion Clipperton Zone. Prog. Oceanogr. 170, 119–133. doi: 10.1016/j.pocean.2018.11.003
Simon-Lledó E., Bett B. J., Huvenne V. A. I., Schoening T., Benoist N. M. A., and Jones D. O. B. (2019c). Ecology of a polymetallic nodule occurrence gradient: Implications for deep-sea mining. Limnol. Oceanogr. 64, 1883–1894. doi: 10.1002/lno.11157
Simon-Lledó E., Pomee C., Ahokava A., Drazen J. C., Leitner A. B., Flynn A., et al. (2020). Multi-scale variations in invertebrate and fish megafauna in the mid-eastern Clarion Clipperton Zone. Prog. Oceanogr. 187, 102405. doi: 10.1016/j.pocean.2020.102405
Simon-Lledó E., Thompson S., Yool A., Flynn A., Pomee C., Parianos J., et al. (2019d). Preliminary observations of the abyssal megafauna of Kiribati. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00605
Singh R., Miljutin D. M., Vanreusel A., Radziejewska T., Miljutina M. M., Tchesunov A., et al. (2016). Nematode communities inhabiting the soft deep-sea sediment in polymetallic nodule fields: do they differ from those in the nodule-free abyssal areas? Mar. Biol. Res. 12, 345–359. doi: 10.1080/17451000.2016.1148822
Singh R., Sautya S., and Ingole B. S. (2019). The community structure of the deep-sea nematode community associated with polymetallic nodules in the Central Indian Ocean Basin. Deep Sea Res. Part II Top. Stud. Oceanogr. 161, 16–28. doi: 10.1016/j.dsr2.2018.07.009
Smith K. L. Jr., Baldwin R. J., Ruhl H. A., Kahru M., Mitchell B. G., and Kaufmann R. S. (2006). Climate effect on food supply to depths greater than 4,000 meters in the northeast Pacific. Limnol. Oceanogr. 51, 166–176. doi: 10.4319/lo.2006.51.1.0166
Smith C. R., De Leo F. C., Bernardino A. F., Sweetman A. K., and Arbizu P. M. (2008). Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 23, 518–528. doi: 10.1016/j.tree.2008.05.002
Smith C. R., Glover A. G., Treude T., Higgs N. D., and Amon D. J. (2015). Whale-fall ecosystems: recent insights into ecology, paleoecology, and evolution. Annu. Rev. Mar. Sci. 7, 571–596. doi: 10.1146/annurev-marine-010213-135144
Smith K. L. Jr., Sherman A. D., Huffard C. L., McGill P. R., Henthorn R., Von Thun S., et al. (2014). Large salp bloom export from the upper ocean and benthic community response in the abyssal northeast Pacific: Day to week resolution. Limnol. Oceanogr. 59, 745–757. doi: 10.4319/lo.2014.59.3.0745
Smith C. R., Tunnicliffe V., Colaço A., Drazen J. C., Gollner S., Levin L. A., et al. (2020). Deep-sea misconceptions cause underestimation of seabed-mining impacts. Trends Ecol. Evol. 35, 853–857. doi: 10.1016/j.tree.2020.07.002
Soetaert K., Muthumbi A., and Heip C. (2002). Size and shape of ocean margin nematodes: morphological diversity and depth-related patterns. Mar. Ecol. Prog. Ser. 242, 179–193. doi: 10.3354/meps242179
Steinmann K., Eggenberg S., Wohlgemuth T., Linder H. P., and Zimmermann N. E. (2011). Niches and noise—Disentangling habitat diversity and area effect on species diversity. Ecol. Complex. 8, 313–319. doi: 10.1016/j.ecocom.2011.06.004
Stenvers V. I., Hauss H., Bayer T., Havermans C., Hentschel U., Schmittmann L., et al. (2023). Experimental mining plumes and ocean warming trigger stress in a deep pelagic jellyfish. Nat. Commun. 14, 7352. doi: 10.1038/s41467-023-43023-6
Stratmann T. (2023). Role of polymetallic-nodule dependent fauna on carbon cycling in the eastern Clarion-Clipperton Fracture Zone (Pacific). Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1151442
Stratmann T., Mevenkamp L., Sweetman A. K., Vanreusel A., and van Oevelen D. (2018a). Has phytodetritus processing by an abyssal soft-sediment community recovered 26 years after an experimental disturbance? Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00059
Stratmann T., Soetaert K., Kersken D., and van Oevelen D. (2021). Polymetallic nodules are essential for food-web integrity of a prospective deep-seabed mining area in Pacific abyssal plains. Sci. Rep. 11, 12238. doi: 10.1038/s41598-021-91703-4
Stratmann T., Voorsmit I., Gebruk A., Brown A., Purser A., Marcon Y., et al. (2018b). Recovery of Holothuroidea population density, community composition, and respiration activity after a deep-sea disturbance experiment. Limnol. Oceanogr. 63, 2140–2153. doi: 10.1002/lno.10929
Summerhayes C. P. (1967). Manganese nodules from the south-western pacific. N. Z. J. Geol. Geophys. 10, 1372–1381. doi: 10.1080/00288306.1967.10423222
Sun X., Corliss B. H., Brown C. W., and Showers W. J. (2006). The effect of primary productivity and seasonality on the distribution of deep-sea benthic foraminifera in the North Atlantic. Deep Sea Res. Part Oceanogr. Res. Pap. 53, 28–47. doi: 10.1016/j.dsr.2005.07.003
Sweetman A. K., Smith A. J., de Jonge D. S. W., Hahn T., Schroedl P., Silverstein M., et al. (2024). Evidence of dark oxygen production at the abyssal seafloor. Nat. Geosci. 17, 737–739. doi: 10.1038/s41561-024-01480-8
Sweetman A. K., Smith C. R., Shulse C. N., Maillot B., Lindh M., Church M. J., et al. (2019). Key role of bacteria in the short-term cycling of carbon at the abyssal seafloor in a low particulate organic carbon flux region of the eastern Pacific Ocean. Limnol. Oceanogr. 64, 694–713. doi: 10.1002/lno.11069
Sweetman A. K., Thurber A. R., Smith C. R., Levin L. A., Mora C., Wei C.-L., et al. (2017). Major impacts of climate change on deep-sea benthic ecosystems. Elem. Sci. Anthr. 5, 4. doi: 10.1525/elementa.203
Taboada S., Riesgo A., Wiklund H., Paterson G. L. J., Koutsouveli V., Santodomingo N., et al. (2018). Implications of population connectivity studies for the design of marine protected areas in the deep sea: An example of a demosponge from the Clarion-Clipperton Zone. Mol. Ecol. 27, 4657–4679. doi: 10.1111/mec.14888
Taylor J., Krumpen T., Soltwedel T., Gutt J., and Bergmann M. (2017). Dynamic benthic megafaunal communities: Assessing temporal variations in structure, composition and diversity at the Arctic deep-sea observatory HAUSGARTEN between 2004 and 2015. Deep Sea Res. Part Oceanogr. Res. Pap. 122, 81–94. doi: 10.1016/j.dsr.2017.02.008
Tews J., Brose U., Grimm V., Tielbörger K., Wichmann M. C., Schwager M., et al. (2004). Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeogr. 31, 79–92. doi: 10.1046/j.0305-0270.2003.00994.x
Thiel H. and Schriever G. (1990). Deep-sea mining, environmental impact and the DISCOL project. Ambio 19, 245–250.
Thiel H., Schriever G., Ahnert A., Bluhm H., Borowski C., and Vopel K. (2001). The large-scale environmental impact experiment DISCOL—reflection and foresight. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 3869–3882. doi: 10.1016/S0967-0645(01)00071-6
Thiel H., Schriever G., Bussau C., and Borowski C. (1993). Manganese nodule crevice fauna. Deep Sea Res. Part Oceanogr. Res. Pap. 40, 419–423. doi: 10.1016/0967-0637(93)90012-R
Thistle D. (1979). “Harpacticoid copepods and biogenic structures: implications for deep-sea diversity maintenance,” in Ecological processes in coastal and marine systems. Ed. Livingston R. J. (Springer US, Boston, MA), 217–231. doi: 10.1007/978-1-4615-9146-7_11
Thurston M. H., Rice A. L., and Bett B. J. (1998). Latitudinal variation in invertebrate megafaunal abundance and biomass in the North Atlantic Ocean Abyss. Deep Sea Res. Part II Top. Stud. Oceanogr. 45, 203–224. doi: 10.1016/S0967-0645(97)00077-5
Tilot V. (2006). “Biodiversity and distribution of the megafauna,” in The polymetallic nodule ecosystem of the Eastern Equatorial Pacific Ocean, vol. 1. (Paris, France: UNESCO).
Tilot V., Ormond R., Moreno Navas J., and Catalá T. S. (2018). The benthic megafaunal assemblages of the CCZ (Eastern pacific) and an approach to their management in the face of threatened anthropogenic impacts. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00007
Tkatchenko G. G. and Radziejewska T. (1998). “Recovery and Recolonisation Processes in the Area Disturbed by a Polymetallic Nodule Collector Simulator.,” in The Proceedings of the Eighth (1998) International Offshore and Polar Engineering Conference (Montreal, Canada: International Society of Offshore and POlar Engineers), 282–286. Available online at: https://dx.doi.org/.
Tong S. J. W., Gan B. Q., and Tan K. S. (2022). Community structure of deep-sea benthic metazoan meiofauna in the polymetallic nodule fields in the eastern Clarion-Clipperton Fracture Zone, Pacific Ocean. Deep Sea Res. Part Oceanogr. Res. Pap. 188, 103847. doi: 10.1016/j.dsr.2022.103847
Trueblood D. D. and Ozturgut E. (1997). The benthic impact experiment: A study of the ecological impacts of deep seabed mining on abyssal benthic communities (Honolulu, HI, USA: OnePetro). Available online at: https://dx.doi.org/.
Tung C.-C., Chen Y.-T., Liao J.-X., and Wei C.-L. (2023). Response of the benthic biomass-size structure to a high-energy submarine canyon. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1122143
Tyler P. A. (2003a). Disposal in the deep sea: analogue of nature or faux ami? Environ. Conserv. 30, 26–39. doi: 10.1017/S037689290300002X
Uhlenkott K., Meyn K., Vink A., and Martínez Arbizu P. (2023a). A review of megafauna diversity and abundance in an exploration area for polymetallic nodules in the eastern part of the Clarion Clipperton Fracture Zone (North East Pacific), and implications for potential future deep-sea mining in this area. Mar. Biodivers. 53, 22. doi: 10.1007/s12526-022-01326-9
Uhlenkott K., Simon-Lledó E., Vink A., and Martínez Arbizu P. (2022). Investigating the benthic megafauna in the eastern Clarion Clipperton Fracture Zone (north-east Pacific) based on distribution models predicted with random forest. Sci. Rep. 12, 8229. doi: 10.1038/s41598-022-12323-0
Uhlenkott K., Simon-Lledó E., Vink A., and Martínez Arbizu P. (2023b). Habitat heterogeneity enhances megafaunal biodiversity at bathymetric elevations in the Clarion Clipperton Fracture Zone. Mar. Biodivers. 53, 55. doi: 10.1007/s12526-023-01346-z
Uhlenkott K., Vink A., Kuhn T., Gillard B., and Martínez Arbizu P. (2021). Meiofauna in a potential deep-sea mining area—Influence of temporal and spatial variability on small-scale abundance models. Diversity 13, 3. doi: 10.3390/d13010003
Uhlenkott K., Vink A., Kuhn T., and Martínez Arbizu P. (2020). Predicting meiofauna abundance to define preservation and impact zones in a deep-sea mining context using random forest modelling. J. Appl. Ecol. 57, 1210–1221. doi: 10.1111/1365-2664.13621
Valsangkar A. B. (2005). First phase monitoring studies of simulated benthic disturbance delineating movement of fine particles in the central Indian basin. Mar. Georesources Geotechnol. 23, 357–371. doi: 10.1080/10641190500446771
van der Grient J. M. A. and Rogers A. D. (2019). Habitat structure as an alternative explanation for body-size patterns in the deep sea. Ecosphere 10, e02900. doi: 10.1002/ecs2.2900
Vanreusel A., Fonseca G., Danovaro R., Da Silva M. C., Esteves A. M., Ferrero T., et al. (2010). The contribution of deep-sea macrohabitat heterogeneity to global nematode diversity. Mar. Ecol. 31, 6–20. doi: 10.1111/j.1439-0485.2009.00352.x
Vanreusel A., Hilario A., Ribeiro P. A., Menot L., and Arbizu P. M. (2016). Threatened by mining, polymetallic nodules are required to preserve abyssal epifauna. Sci. Rep. 6, 26808. doi: 10.1038/srep26808
Veillette J., Juniper S. K., Gooday A. J., and Sarrazin J. (2007a). Influence of surface texture and microhabitat heterogeneity in structuring nodule faunal communities. Deep Sea Res. Part Oceanogr. Res. Pap. 54, 1936–1943. doi: 10.1016/j.dsr.2007.06.012
Veillette J., Sarrazin J., Gooday A. J., Galéron J., Caprais J.-C., Vangriesheim A., et al. (2007b). Ferromanganese nodule fauna in the Tropical North Pacific Ocean: Species richness, faunal cover and spatial distribution. Deep Sea Res. Part Oceanogr. Res. Pap. 54, 1912–1935. doi: 10.1016/j.dsr.2007.06.011
Vineesh T. C., Nagender Nath B., Banerjee R., Jaisankar S., and Lekshmi V. (2009). Manganese nodule morphology as indicators for oceanic processes in the Central Indian Basin. Int. Geol. Rev. 51, 27–44. doi: 10.1080/00206810802622773
Volz J. B., Haffert L., Haeckel M., Koschinsky A., and Kasten S. (2020). Impact of small-scale disturbances on geochemical conditions, biogeochemical processes and element fluxes in surface sediments of the eastern Clarion–Clipperton Zone, Pacific Ocean. Biogeosciences 17, 1113–1131. doi: 10.5194/bg-17-1113-2020
Vornsand I., Boehringer L., Thomsen L., and Purser A. (2024). Short and decadal impacts of seafloor physical perturbation on the abundances of Lebensspuren ‘traces of life’ in the Peru Basin manganese nodule province. Mar. Biodivers. 54, 11. doi: 10.1007/s12526-024-01405-z
Walker B. K., Jordan L. K. B., and Spieler R. E. (2009). Relationship of reef fish assemblages and topographic complexity on southeastern florida coral reef habitats. J. Coast. Res. 25, 39–48. doi: 10.2112/SI53-005.1
Wang C., Deser C., Yu J.-Y., DiNezio P., and Clement A. (2017). “El niño and southern oscillation (ENSO): A review,” in Coral reefs of the eastern tropical pacific: persistence and loss in a dynamic environment. Eds. Glynn P. W., Manzello D. P., and Enochs I. C. (Springer Netherlands, Dordrecht), 85–106. doi: 10.1007/978-94-017-7499-4_4
Warwick R. M. and Clarke K. R. (1996). Relationships between body-size, species abundance and diversity in marine benthic assemblages: facts or artefacts? J. Exp. Mar. Biol. Ecol. 202, 63–71. doi: 10.1016/0022-0981(96)00031-7
Washburn T. W., Jones D. O. B., Wei C.-L., and Smith C. R. (2021a).Environmental heterogeneity throughout the clarion-clipperton zone and the potential representativity of the APEI network.
Washburn T. W., Menot L., Bonifácio P., Pape E., Błażewicz M., Bribiesca-Contreras G., et al. (2021b). Patterns of macrofaunal biodiversity across the clarion-clipperton zone: an area targeted for seabed mining. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.626571
Watling J. I., Nowakowski A. J., Donnelly M. A., and Orrock J. L. (2011). Meta-analysis reveals the importance of matrix composition for animals in fragmented habitat. Glob. Ecol. Biogeogr. 20, 91–112. doi: 10.1111/j.1466-8238.2010.00586.x
Watson G. E. (1964). Ecology and evolution of passerine birds in the islands of the aegean sea (United States – Connecticut: Yale University). Available online at: https://www.proquest.com/docview/302111008/citation/2F41AC664C5C496FPQ/1.
Wear E. K., Church M. J., Orcutt B. N., Shulse C. N., Lindh M. V., and Smith C. R. (2021). Bacterial and archaeal communities in polymetallic nodules, sediments, and bottom waters of the abyssal clarion-clipperton zone: emerging patterns and future monitoring considerations. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.634803
Weaver P. P. E. and Billett D. (2019). “Environmental impacts of nodule, crust and sulphide mining: an overview,” in Environmental issues of deep-sea mining: impacts, consequences and policy perspectives. Ed. Sharma R. (Springer International Publishing, Cham), 27–62. doi: 10.1007/978-3-030-12696-4_3
Webb T. J., Barry J. P., and McClain C. R. (2017). Abundance–occupancy relationships in deep sea wood fall communities. Ecography 40, 1339–1347. doi: 10.1111/ecog.02618
Willig M. R. and Presley S. J. (2018). Biodiversity and disturbance. Encycl. Anthr. 3, 45–51. doi: 10.1016/B978-0-12-809665-9.09813-X
Wilson G. D. F. (2017). Macrofauna abundance, species diversity and turnover at three sites in the Clipperton-Clarion Fracture Zone. Mar. Biodivers. 47, 323–347. doi: 10.1007/s12526-016-0609-8
Wong M. C. and Kay L. M. (2019). Partial congruence in habitat patterns for taxonomic and functional diversity of fish assemblages in seagrass ecosystems. Mar. Biol. 166, 46. doi: 10.1007/s00227-019-3488-2
Woolley S. N. C., Tittensor D. P., Dunstan P. K., Guillera-Arroita G., Lahoz-Monfort J. J., Wintle B. A., et al. (2016). Deep-sea diversity patterns are shaped by energy availability. Nature 533, 393–396. doi: 10.1038/nature17937
Wright I. C., Graham I. J., Chang S. W., Choi H., and Lee S. R. (2005). Occurrence and physical setting of ferromanganese nodules beneath the Deep Western Boundary Current, Southwest Pacific Ocean. N. Z. J. Geol. Geophys. 48, 27–41. doi: 10.1080/00288306.2005.9515096
Xie F., Tao Z., Zhou X., Lv T., and Wang J. (2019). Spatial and temporal variations of particulate organic carbon sinking flux in global ocean from 2003 to 2018. Remote Sens. 11, 2941. doi: 10.3390/rs11242941
Xu J., García Molinos J., Su G., Matsuzaki S. S., Akasaka M., Zhang H., et al. (2019). Cross-taxon congruence of multiple diversity facets of freshwater assemblages is determined by large-scale processes across China. Freshw. Biol. 64, 1492–1503. doi: 10.1111/fwb.13322
Yasuhara M. and Danovaro R. (2016). Temperature impacts on deep-sea biodiversity. Biol. Rev. 91, 275–287. doi: 10.1111/brv.12169
Young G. C., Dey S., Rogers A. D., and Exton D. (2017). Cost and time-effective method for multi-scale measures of rugosity, fractal dimension, and vector dispersion from coral reef 3D models. PLOS ONE 12, e0175341. doi: 10.1371/journal.pone.0175341
Young E. L., Halanych K. M., Amon D. J., Altamira I., Voight J. R., Higgs N. D., et al. (2022). Depth and substrate type influence community structure and diversity of wood and whale-bone habitats on the deep NE Pacific margin. Mar. Ecol. Prog. Ser. 687, 23–42. doi: 10.3354/meps14005
Yu O. H., Lee H.-G., Kim D., Wi J. H., Kim K. H., and Yoo C. M. (2018). Characterization of deep-sea macrofauna in the Korean exploration claim area in the Clarion-Clipperton Fracture Zone, northeastern Pacific Ocean. Ocean Sci. J. 53, 301–314. doi: 10.1007/s12601-018-0029-8
Zajac R. N., Lewis R. S., Poppe L. J., Twichell D. C., Vozarik J., and DiGiacomo-Cohen M. L. (2000). Relationships among Sea-Floor Structure and Benthic Communities in Long Island Sound at Regional and Benthoscape Scales. J. Coast. Res. 16, 627–640.
Zeppilli D., Bongiorni L., Santos R. S., and Vanreusel A. (2014). Changes in nematode communities in different physiographic sites of the condor seamount (North-east atlantic ocean) and adjacent sediments. PloS One 9, e115601. doi: 10.1371/journal.pone.0115601
Zeppilli D., Canals M., and Danovaro R. (2012). Pockmarks enhance deep-sea benthic biodiversity: a case study in the western Mediterranean Sea. Divers. Distrib. 18, 832–846. doi: 10.1111/j.1472-4642.2011.00859.x
Zeppilli D., Mea M., Corinaldesi C., and Danovaro R. (2011). Mud volcanoes in the Mediterranean Sea are hot spots of exclusive meiobenthic species. Prog. Oceanogr. 91, 260–272. doi: 10.1016/j.pocean.2011.01.001
Zeppilli D., Pusceddu A., Trincardi F., and Danovaro R. (2016). Seafloor heterogeneity influences the biodiversity–ecosystem functioning relationships in the deep sea. Sci. Rep. 6, 26352. doi: 10.1038/srep26352
Keywords: habitat heterogeneity, polymetallic nodules, deep sea, deep-sea mining, community structure, disturbance, biodiversity, spatial scales
Citation: Ullmann A, Rowden AA, Leduc D and Zeppilli D (2025) The influence of habitat heterogeneity and disturbance on benthic community structure in deep-sea polymetallic nodule environments and management implications for seabed mining. Front. Mar. Sci. 12:1650660. doi: 10.3389/fmars.2025.1650660
Received: 20 June 2025; Accepted: 30 September 2025;
Published: 17 October 2025.
Edited by:
Ana Colaço, University of the Azores, PortugalReviewed by:
Lara Macheriotou, Ghent University, BelgiumDaphne Cuvelier, University of the Azores, Portugal
Alessandra Asioli, National Research Council (CNR), Italy
Copyright © 2025 Ullmann, Rowden, Leduc and Zeppilli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ailish Ullmann, YWlsaXNoLnVsbG1hbm5AZ21haWwuY29t
 Ailish Ullmann
Ailish Ullmann Ashley A. Rowden
Ashley A. Rowden Daniel Leduc
Daniel Leduc Daniela Zeppilli
Daniela Zeppilli