- 1Sharklab-Malta, San Ġwann, Malta
- 2Go Shark Azores, Horta, Azores, Portugal
This study constitutes a novel quantitative assessment of Aetomylaeus bovinus distribution and demographics within the Maltese archipelago (MA, Central Mediterranean). According to the IUCN red list, A. bovinus is critically endangered, both globally and in the Mediterranean. Therefore, a comprehensive understanding of its ecological attributes and behaviors is necessary for adequate conservation measures. Despite their crucial role in providing ecosystem services, information on batoids around the MA remains limited, raising concerns over their increasing population decline and extinction risk. During this fourteen-year-long study (2011-2024), photo-identification data were collected by members of Sharklab-Malta in conjunction with the Fly With Bull Rays (FWBR) program in order to identify site fidelity, population structure and temporal-spatial behavioral patterns of local A. bovinus. Seasonal patterns and relative abundance were also ascertained. A total of 859 surveys were conducted around the MA, with a total of 407 sightings of A. bovinus and the identification of 135 individuals. Approximately 44.8% of identified individuals were re-sighted at least once following initial observations, with multiple individuals showing high re-sighting rates across lengthy temporal periods and indicating site fidelity. Certain key areas in the NW, NE and E of Malta represent the greatest abundance of A. bovinus in the MA. The population is dominated by younger individuals, defined by disc width, with 97.7% of sightings consisting of young of the year to sub-adults. These data, in tandem with site fidelity, higher abundances of juveniles in these key areas and consistent use of these areas over the fourteen-year study period suggest that areas of the MA support a nursery function for A. bovinus. Anthropogenic stressors such as high touristic pressure, unregulated fishing and climate change are likely to impact key areas, posing a need for targeted conservation measures.
1 Introduction
Elasmobranch fishes (sharks, rays and skates) are amongst some of the most endangered groups of animals, with 37% of known species at risk of extinction globally (Dulvy et al., 2021; IUCN, 2025). Threats are myriad, from pollution to climate change, with a particular emphasis on overfishing and habitat destruction (Sawyna et al., 2017; Pacoureau et al., 2021; Yan et al., 2021; Cerutti-Pereyra et al., 2024). These threats are often compounded by elasmobranch’s status as K-selected species, presenting low offspring productivity, late sexual maturity, long lifespans, and thus slow stock recovery in the face of multiple stressors (Stevens et al., 2000; Nejmeddine Bradai et al., 2023). The loss of elasmobranchs presents major ramifications; as predators, they help to regulate food webs, trophic structure, and the functioning of marine ecosystems (Myers et al., 2007; Ferretti et al., 2010; Hammerschlag et al., 2025). Their decline could therefore have serious implications, not just for the marine environment, but for communities which rely on the sea for sustenance, livelihoods and other ecosystem services (Nejmeddine Bradai et al., 2012).
These global trends and their impacts are reflected within the Mediterranean Sea, hereafter referred to as the Mediterranean. The Mediterranean is a biodiversity hotspot for elasmobranchs, and historically experienced a high biomass of these fishes (Serena et al., 2020; Nejmeddine Bradai et al., 2023). However, over the last century, it has seen a dramatic decline in these assemblages, resulting in over half of Mediterranean cartilaginous species at risk of extinction (Ferretti et al., 2008; Dulvy et al., 2016). Several stressors are particularly acute, including intense fishing pressure, the rapid impacts of climate change and widespread coastal development (Nejmeddine Bradai et al., 2012; Colloca et al., 2017; Piroddi et al., 2017; WWF, 2021; Ouled-Cheikh et al., 2022). While often not a targeted fishery, elasmobranchs in the Mediterranean are at considerable risk of bycatch, in addition to illegal and unregulated fisheries (La Mesa et al., 2016; O’Keefe et al., 2023; Tiralongo et al., 2023; IUCN, 2025). Although long-term datasets have been instrumental in advancing our understanding of elasmobranch population dynamics and threats, data coverage across the basin remains geographically uneven, with some areas still relatively under-surveyed (Koehler, 2025).
Within the global and regional context of widespread elasmobranch population declines, the identification, understanding and protection of nursery areas is particularly critical. The juveniles of many species have been known to rely on specific coastal habitats, accounting for the provision of abundant prey items, structural complexity and relative protection from potential predators (Simpfendorfer and Milward, 1993; Beck et al., 2001; Cerutti-Pereyra et al., 2014; Rangel et al., 2018; O’Shea et al., 2021). Even amongst highly mobile or migratory species, these habitats can serve as focal points and provide location permanence in early life stages (Knochel et al., 2022; Elston and Murray, 2024; Páez-Rosas et al., 2024). The quality and integrity of these areas can significantly influence local generational survival rates, and by extension, the broader demographic stability of populations (Heupel et al., 2018; Silva and Vianna, 2018; Yan et al., 2021; Pierce and Charles, 2022). Further, many elasmobranch species exhibit natal philopatry (Chapman et al., 2009; Feldheim et al., 2014), therefore the identification and protection of nursery habitats is an essential component of their conservation.
Heupel et al. (2007) formally defined these nursery areas through a tripartite classification: 1) juveniles are more commonly encountered in a specific area than elsewhere, 2) they remain or return for extended periods and 3) they repeatedly use the area or habitat across multiple years. This framework can also be applied to nursery areas for viviparous and ovoviviparous batoids, whose nursery dynamics are less well understood than those of Selachii (Martins et al., 2018). Nursery areas have been identified through a variety of means, including the utilization of local ecological knowledge (LEK) and citizen science methodologies (Tuya et al., 2020; Espino et al., 2022). The identification and safeguarding of these habitats in the Mediterranean is essential, where the cascade of pressures on coastal ecosystems are intensifying and few nursery areas have been formally designated or afforded effective protections (Colloca et al., 2015; Giménez et al., 2020; Barbato et al., 2023).
The broader Central Mediterranean region, including areas such as the Gulf of Gabes, Strait of Sicily and Pelagie Islands have been documented in regard to their reproductive importance to various elasmobranch species (Echwikhi et al., 2014; Scannella et al., 2020; Geraci et al., 2021; Falsone et al., 2022; Cattano et al., 2023; IUCN SSC Shark Specialist Group, 2023). However, in Malta no such areas have been formally designated or studied systematically, reflecting persistent gaps in both research and conservation management. This study addresses part of these data gaps by presenting a long-term photo identification dataset of bull rays, Aetomylaeus bovinus (Geoffroy Saint- Hilaire, 1817), in Maltese coastal waters, offering insights into a species of particular conservation concern both regionally and globally.
Malta is a small island state in the Central Mediterranean, with a land area of 316 km2 and a population in 2023 of 563,443, making the islands one of the most densely populated countries worldwide (Central Bank of Malta, 2025). The country experiences intense coastal development, in addition to a heavy tourism footprint and ongoing challenges related to the regulation and monitoring of fisheries and environmental activities, despite the existence of a seemingly strong policy framework (Briguglio and Bonello, 2018; Azzopardi et al., 2023; Koehler and Lowther, 2025; OECD, 2025).
While relatively small in area (approx. 4424 km2), the territorial waters of the Maltese archipelago support a diverse assemblage of elasmobranch species, including threatened taxa of both sharks and rays (Schembri et al., 2003; Borg et al., 2023; IUCN SSC Shark Specialist Group, 2023). Over the preceding two decades, there have been repeated recorded observations of juvenile batoids of several species in Maltese coastal waters, however no datasets existed documenting these observations (Greg Nowell, pers. comms. 2008-2025). As part of an ongoing fisheries verification study, there has also been the consistent commercial capture of gravid females and young juveniles of various elasmobranch species, namely Squalus blainville, Mustelus spp., Hexanchus griseus, Heptranchias perlo, Scyliorhinus spp., Raja clavata, and Raja radula (Sharklab-Malta, unpublished data; 2008-2025). These two factors indicated the potential presence of habitats utilized consistently during early life stages in Maltese territorial waters, but these observations do not appear in the scientific literature, indicating data gaps within the archipelago.
Aetomylaeus bovinus, also referred to as ‘bull rays’ or ‘duckbilled eagle rays’, are a demersal and semi-pelagic batoid species belonging to the Myliobatidae family. They are found from the surface down to 500 m but with an average depth preference of up to 30 m (Dulčić et al., 2008; Zogaris and Dussling, 2010; Başusta and Aslan, 2018; Gerovasileiou et al., 2020; Geraci et al., 2021; Mulas et al., 2021). Bull rays have a wide range, from Northwest Spain to Mozambique, including the Mediterranean, Madeira and the Canary Islands, and are assessed critically endangered both globally and in the Mediterranean by the IUCN Red List of Threatened Species, but were considered data deficient as recently as 2020 (Last et al., 2016; Ebert and Dando, 2020; IUCN, 2020; Serena et al., 2020; Moreno et al., 2022). Although known to occur across the Mediterranean basin, reports are sparse, with the species considered rare in the region (Capapé and Quignard, 1975; Capapé, 1977; Dulčić et al., 2008; Akyol et al., 2017; Geraci et al., 2021; Giovos et al., 2021; Mulas et al., 2021).
Aetomylaeus bovinus possesses many characteristics, both behavioral and biological, that makes the species especially vulnerable to anthropogenic pressures. Bull rays are large-bodied, displaying sexual dimorphism with the largest females reaching a reported maximum disc width (DW) of 222 cm and a mass of 116 kg (Dulčić et al., 2008; Lipej et al., 2009). They are slow-growing, reaching maturity between 80–100 cm DW with inferred generational lengths of 17 years (Capapé et al., 1995; Last et al., 2016; IUCN, 2020). Combined with a low reproductive output, with small litter sizes of 3–6 pups, further highlights the vulnerability of their populations to fishing pressures (Seck et al., 2002; Last et al., 2016). They have also been demonstrated to undertake migrations of up to 925 km along the South African coast, indicating a migratory nature and demonstrating seasonal philopatry (Elston et al., 2023, 2024). Their preference for shallow coastal habitats combined with these biological traits have resulted in estimated population declines of >80% across their range in the last 3 generations, underlining the need for urgent targeted conservation efforts (IUCN, 2020).
The first confirmed record of this species in Malta was reported in 2011, but has been observed consistently following the initial observation (Schembri et al., 2003; Katsanevakis et al., 2014). Subsequently to this initial sighting, the additional repeated observation of juveniles suggested the presence of a nursery area - a potentially critical habitat that warranted further investigation. The overarching goal of this study has been to better understand the species’ spatial ecology within the Maltese archipelago, and to ascertain if the presence of juveniles supported the hypothesis of the existence of a nursery area as defined by Heupel et al. (2007) and Martins et al. (2018). Specifically, we aimed to: (i) describe occurrence and encounter rates across a 14-year period (2011–2024); (ii) evaluate patterns of presence across different bays to determine which were most utilized; (iii) assess temporal patterns in encounter rates, particularly at the monthly scale to investigate possible seasonality; and (iv) examine individual resightings to assess potential site fidelity and recurring use of specific areas. By contributing to the understanding of their spatial ecology, this study aims to inform broader conservation initiatives aimed at preserving such vulnerable batoid species and their habitats in a data-poor area of the Mediterranean.
2 Methods
Data collection for this study has been ongoing since 2011, following the first confirmed sighting of A. bovinus in Golden Bay (NW Malta). During that first documented observation of the species, it was noted that the individual in question was very small compared to the known maximum disc width in Dulčić et al. (2008) (Greg Nowell, pers. obs. 2011). Data collection was performed by citizen scientists via the authorship team, as well as generations of trained volunteers. While initially collected as part of a citizen science initiative looking at coastal elasmobranch species in Malta, the photo identification methodology was retroactively applied following the proof of the stability and uniqueness of the dorsal patterns in Solleliet-Ferreira et al. (2018), and a clear methodology of data collection was set from that point on.
2.1 Study area
The Maltese Islands are a small archipelago (approx. 316 km2) in the Central Mediterranean (Lat: 36.10°N-35.70°N; Lon: 14.16°E-14.60°E). It consists of 3 main islands: Malta, Gozo and Comino, in descending order of size. The seabed around the islands presents a wide variety of geomorphological habitats and bathymetries. The North and East are characterized by low lying coastal areas and a shallow subsea plateau (approx. 100–200 m depth) connecting the islands to Sicily, with fine sand, seagrass meadows (Posidonia oceanica) and maërl beds as the principal nearshore habitats (UNEP/MAP-SPA/RAC, 2019; Prampolini et al., 2021). Conversely, the Western side is dominated by steep cliffs and deeper waters, eventually culminating in the Maltese Graben trench with depths in excess of 1000 m (Drago et al., 2003; Knittweis et al., 2019). Nearshore, the Western region is defined by fine sand, boulders and sporadic P.oceanica outcrops, outside of a handful of more sheltered bays toward the Northwest, and the Southern area surrounding Għar Lapsi to the islet of Filfla where seagrasses and algal reefs are more commonly encountered (UNEP/MAP-SPA/RAC, 2019; Prampolini et al., 2021; Darmanin et al., 2023).
The Maltese archipelago contains 18 marine protected areas (MPAs), covering 35% of its coastal and marine waters within 25 nautical miles (Environment and Resources Authority, 2020). Since 2008, supplementary MPAs have been implemented to protect 4 marine habitats, 3 aquatic species and 3 seabird species (Environment and Resources Authority, 2020). The MPAs are Special Areas of Conservation (SACs) and Special Protection Areas (SPAs), created under the Habitats Directive (92/43/EEC) and the Birds Directive (2009/147/EEC), and form part of the European Natura 2000 Network (European Commission Directorate General for the Environment, 2008). Elasmobranchs do not appear in the justifications for any of these MPAs, further highlighting a key data gap (Environment and Resources Authority, 2020).
2.2 Data collection
Data were collected through freediving and the Roving Diver Technique (RDT) underwater census method as per protocols clarified by Solleliet-Ferreira et al. (2018). This allowed for the survey of wide areas in reduced time periods, beneficial to the monitoring of species with low sighting rates (Ward-Paige and Lotze, 2011; Moreno et al., 2022). Encounters with A. bovinus were photographed with date, time, depth, behavior, sex and location of the sighting logged according to a combination of camera metadata, dive computers and personal observations.
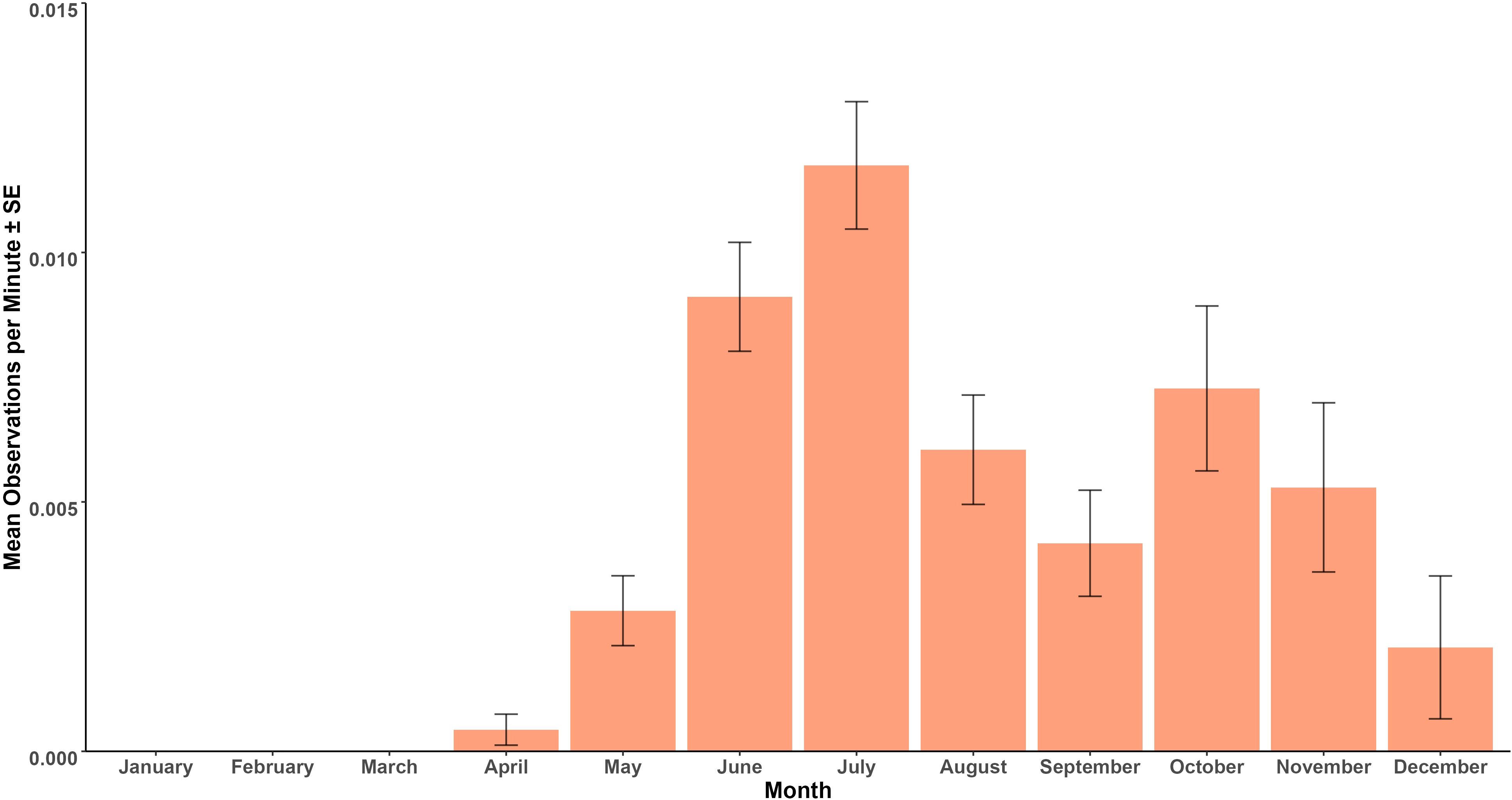
Site selection was based upon the identification of similar benthic compositions to Golden Bay (sand, seagrasses and algal reefs), eventually expanding to allow for full coverage of these habitats around the Maltese archipelago as seen in Figure 1 (Katsanevakis et al., 2014).
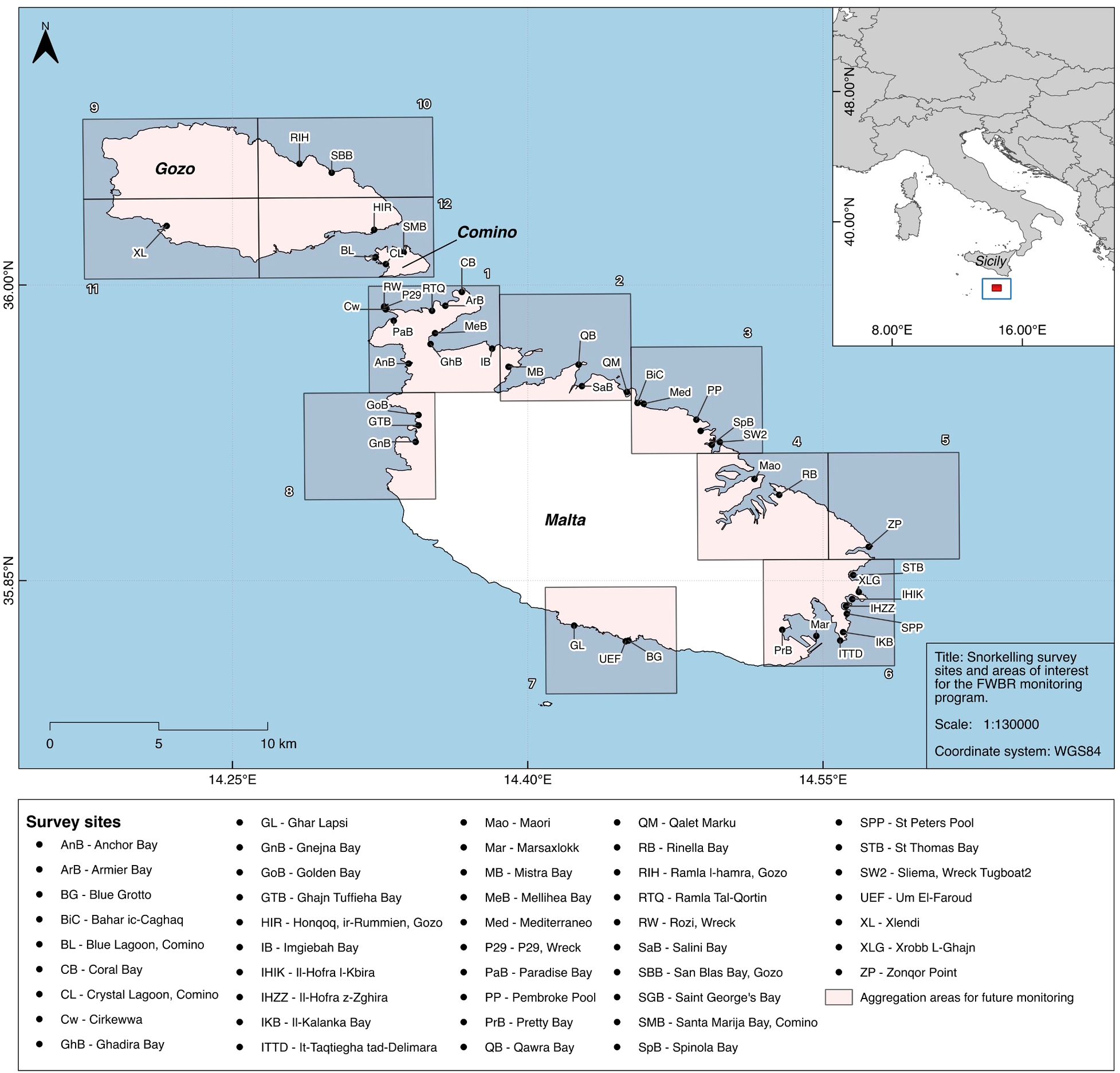
Figure 1. Map of survey sites around the Maltese archipelago, demonstrating their division into sub-regions for logistical purposes.
2.3 Photo-identification
Photo-identification is a widely used, non-invasive and robust methodology for the study of various species with distinctive markings including elasmobranch fishes (Speed et al., 2007; Marshall and Pierce, 2012; Tyson Moore et al., 2022). This technique has been used in elasmobranch research extensively and provides a cost-effective way of determining residency, population structure and other demographics, as well as areas of key life stages such as nurseries - all of which can aid in conservation efforts of threatened species (Marshall et al., 2011; Boggio-Pasqua et al., 2019; Knochel et al., 2022; Vossgaetter et al., 2024; Scott et al., 2025). The stability of distinctive dorsal stripes and patterns present on A. bovinus were analyzed by Solleliet-Ferreira et al. (2018) and proven to be both unique and consistent as the animal ages in captivity and in the wild. This entails the appropriate use of photo-identification as a non-invasive and effective means of studying these animals (Solleliet-Ferreira et al., 2018; Moreno et al., 2022). Examples of the dorsal patterns are illustrated in Figure 2.

Figure 2. Example images of A. bovinus dorsal patterns utilised for ID and captured survey data in clear water conditions. Bottom right demonstrates the use of the dual laser photogrammetry system.
Photographic data were captured using Olympus TG-5 cameras. Photos were taken directly perpendicular to the dorsal side of the ray. Aetomylaeus bovinus individuals were approached from above and behind, to maximize photographic success and minimize possible parallax errors. This technique is shown in Figure 3.

Figure 3. Example of correct approach procedure towards an A. bovinus individual by a volunteer researcher.
When approaching in this way, the researchers were able to get to a 1–2 m distance to the A. bovinus individual and secure clear images of the dorsal patterns without provoking a flight response. One of the cameras was configured to feature dual-laser photogrammetry, with 2 green lasers set at an interval of 20cm, to assist in the determination of DW as per protocols in Deakos (2010). These were frequently calibrated to maintain the consistency and accuracy of measurements. In the case of laser malfunction or unavailability, the demersal nature of many of the encounters with A. bovinus allowed nearby leaves of P.oceanica to be utilized as a proxy of 0.9cm for later size analysis. This width has been determined to be consistent throughout the Maltese Islands, with no significant difference between locations or bed types (Borg and Schembri, 1995; Borg et al., 2005).
2.4 Analysis
2.4.1 Individual identification
The identification of individual rays was performed manually which has been demonstrated as a reliable method of identification for a variety of elasmobranch species with limited data sets (Vossgaetter et al., 2024; Scott et al., 2025). New individuals were cross-referenced against an existing catalogue of known individuals based on dorsal patterns, scars or other injuries, size and sex. To reduce human error, all identifications were independently verified by a team of 4 researchers: Charlie Matthews, Christian Caruso, Sophie Babbs and Beth Ducker.
Prior to comparison against known individuals, photos where the dorsal patterns were unclear or difficult to determine were enhanced through a variety of methods to help to emphasize these patterns, such as editing aspects of the image (e.g. sharpness or contrast), creating negatives or the conversion of the image into black and white and subsequent processing through programs such as Adobe Lightroom, Adobe Photoshop and ImageJ (Figure 4).
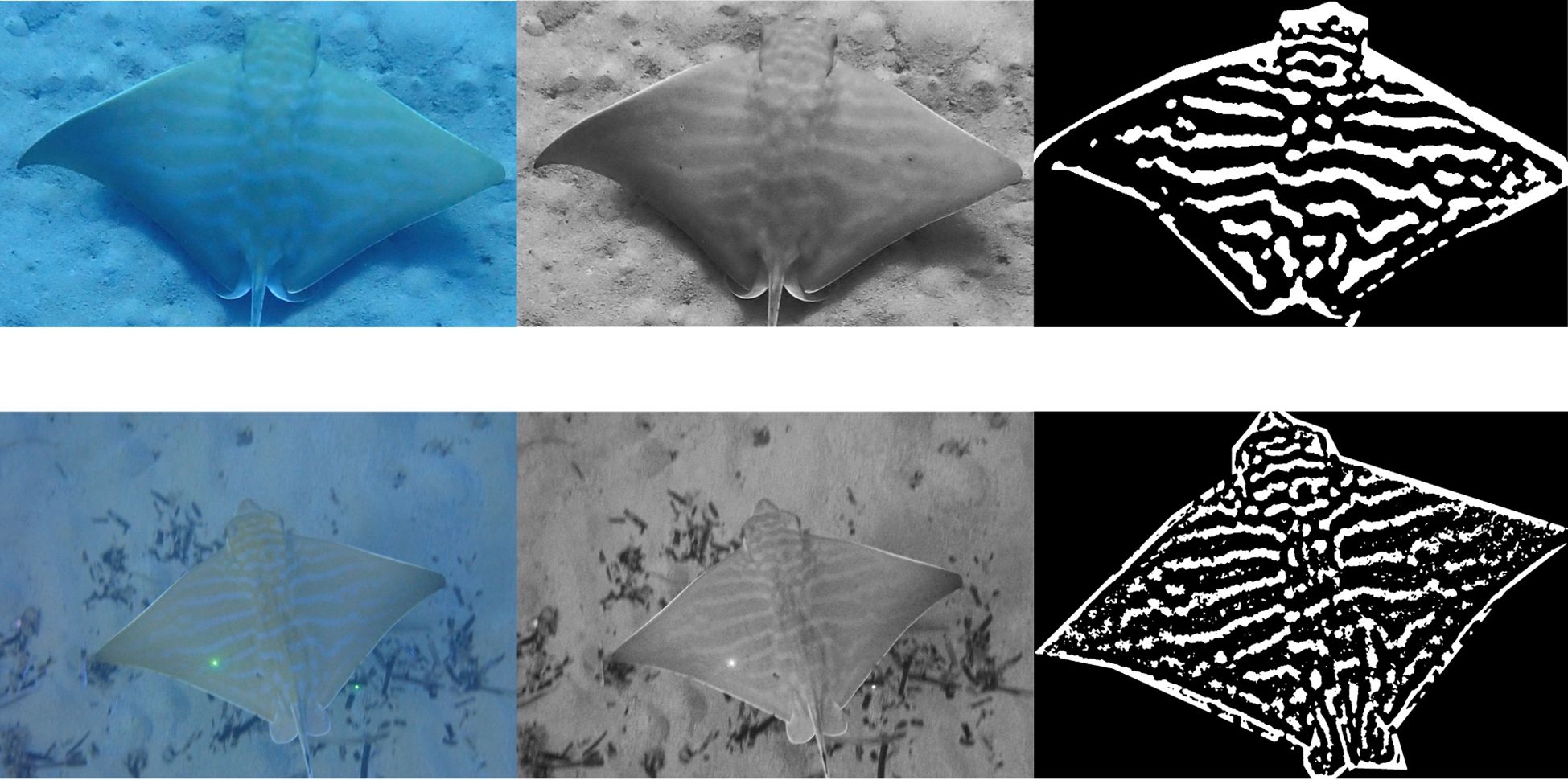
Figure 4. Examples of image enhancement or processing used to aid in the manual photo-ID methodology. Left to right - RAW file, Adobe Lightroom, ImageJ. Bottom image set features example of dual laser photogrammetry.
In addition to dorsal patterns, other features such as unique scars, damage to the wings, lack of a tail or other injuries were used as an extra individual identification tool. Sex was determined through the presence or absence of claspers when visible. When possible, the size (and thus maturity) of the individuals were extrapolated through ImageJ, using either dual-laser photogrammetry or the aforementioned P. oceanica proxy. Maturity was derived from available literature (Seck et al., 2002; Başusta and Aslan, 2018; Ebert and Dando, 2020). Individuals with a DW of<45 cm were considered young of the year (YOY), 46–70 cm were considered juvenile, 71–89 cm as sub-adult, and >90 cm as adults. As this study focused on dorsal patterns, the ventral side of the rays, through which umbilical scars could be identified were not seen owing to the demersal nature of the species. Thus, the designation of YOY was derived through DW from known literature (Seck et al., 2002; Başusta and Aslan, 2018; Ebert and Dando, 2020).
2.4.2 Statistical analysis
All statistical analyses were carried out using R (R Core Team, 2025). Data manipulation and transformation were undertaken using packages “tidyverse” and “dyplr” (Wickham et al., 2019, 2023). All visualizations were created using “ggplot2” (Wickham, 2016).
Due to variations in survey length and uneven number of visits to bays due to transport limitations, a standardized metric was calculated to assess sightings. Catch per unit effort (CPUE) is widely used in fisheries and ecological monitoring as a proxy for relative abundance (Shono, 2008; Chang et al., 2023). However, as our methodology was non-extractive, we adopted an analogous metric: Observations per unit effort (OPUE). This retains the core logic of CPUE, while reflecting our methods. OPUE was calculated as the number of A. bovinus sightings per minute of surveying. OPUE is a commonly used metric in ecology, and has been utilized for observational datasets on a variety of animals, including elasmobranchs (Bruns and Henderson, 2020; Putra et al., 2025). OPUE was calculated using the formula:
The years 2011, 2012, and 2013 were removed from OPUE calculations, as the survey methodology was unstandardized during this period, and thus only observations were catalogued, skewing the OPUE data for these years, this aspect changed from 2014. No surveys occurred during 2016 due to a lack of volunteers.
Shapiro-Wilk tests were performed, along with visualizations, to determine the normality of the data. Non-normal distributions were revealed; therefore, the non-parametric Kruskal-Wallis test was used to determine significant differences in OPUE across months.
2.5 Ethical considerations
No ethical approval or permits were required for this study as A. bovinus was not a protected species under Maltese or international legislation during the study period, and the research involved no capture, handling, or disturbance of the animals. No living specimens were obtained, and all investigations occurred in-situ. Data collection was entirely non-invasive, conducted through underwater visual surveys and photogrammetry during freediving, in full accordance with established ethical standards for wildlife research and observational fieldwork, as outlined in the ARRIVE guidelines (Percie Du Sert et al., 2020) and the ASAB Guidelines for the Treatment of Animals in Behavioural Research and Teaching (ASAB Ethical Committee/ABS Animal Care Committee, 2023).
3 Results
3.1 Overall
For this paper, data from 2011–2024 have been analyzed. A total of 407 sightings of A. bovinus were recorded across the Maltese Islands, over a span of 859 surveys (totaling 65852 mins). The records range from August 18, 2011, to August 30, 2024. A. bovinus were observed in survey sites around the Maltese archipelago every year in which survey efforts occurred following the initial observation of the species in Golden Bay in 2011. The number of A. bovinus sightings in a single survey ranged from 0 to 6. Of these observations, all occurred at sites off the coast of the main island of Malta, with no sightings at the islands of Gozo or Comino. A total of 135 individuals of A. bovinus were identified and catalogued.
3.2 Size distribution and population demographics
The DW of 171 observed specimens (42.0% of total observations) were estimated. The normality of these data was assessed using a Shapiro-Wilk test, which revealed a deviation from normality (W = 0.95, p< 0.001). DW ranged from 17.8 cm to 107.4 cm, with a mean DW of 52.3 cm (SD ± 15.2 cm; median = 49.5 cm and IQR = 18.6 cm). 97.7% of measurable specimens were determined to be either young of the year (36.3%, n=62), juveniles (52.0%, n=89) or sub-adults (9.36%, n=16). During the study period, only 4 adults (>90 cm DW) were observed, all between 90 cm and 107.4 cm. Distribution of age categories based on DW is presented in Figure 5.
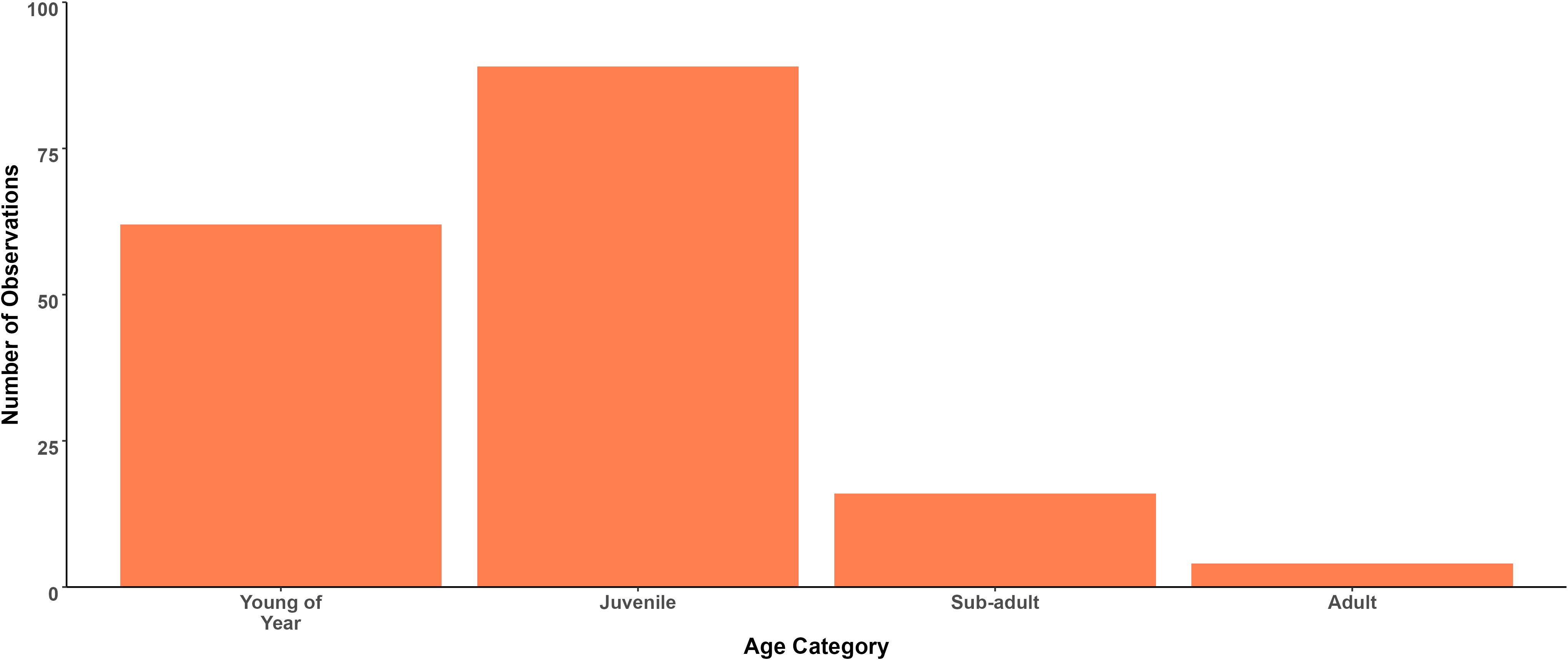
Figure 5. Calculated DW of A. bovinus individuals,<45 cm - YOY, 46–70 cm - Juvenile, 71–89 cm - Sub-adult, and >90 cm - Adult.
3.3 Spatial distribution
During the study period, 47 bays were surveyed across the Maltese archipelago, 40 in Malta, 4 in Gozo and 3 in Comino. A total of 23 bays were excluded from the calculation of OPUE due to a low number of survey repetitions (<5), which resulted from logistical limitations surrounding transport availability and the natural accessibility of these sites. The removal of these 23 bays from OPUE calculations accounted for 6 sightings or 1.47% of total sightings.
The average OPUE of the Maltese archipelago was 0.00604 (± 0.01) sightings per survey minute. There was a prevalence of 0 values in the data (70.7%, n=567), likely owing to the species’ rarity. Additional information on the calculated OPUE values can be found in the Supplementary Materials. Figure 6 shows that the highest OPUE occurred at Baħar iċ-Ċagħaq, Golden Bay, Għadira Bay, Għajn Tuffieħa, Paradise Bay, and Mediterraneo, with their average OPUE values above the wider archipelago average.
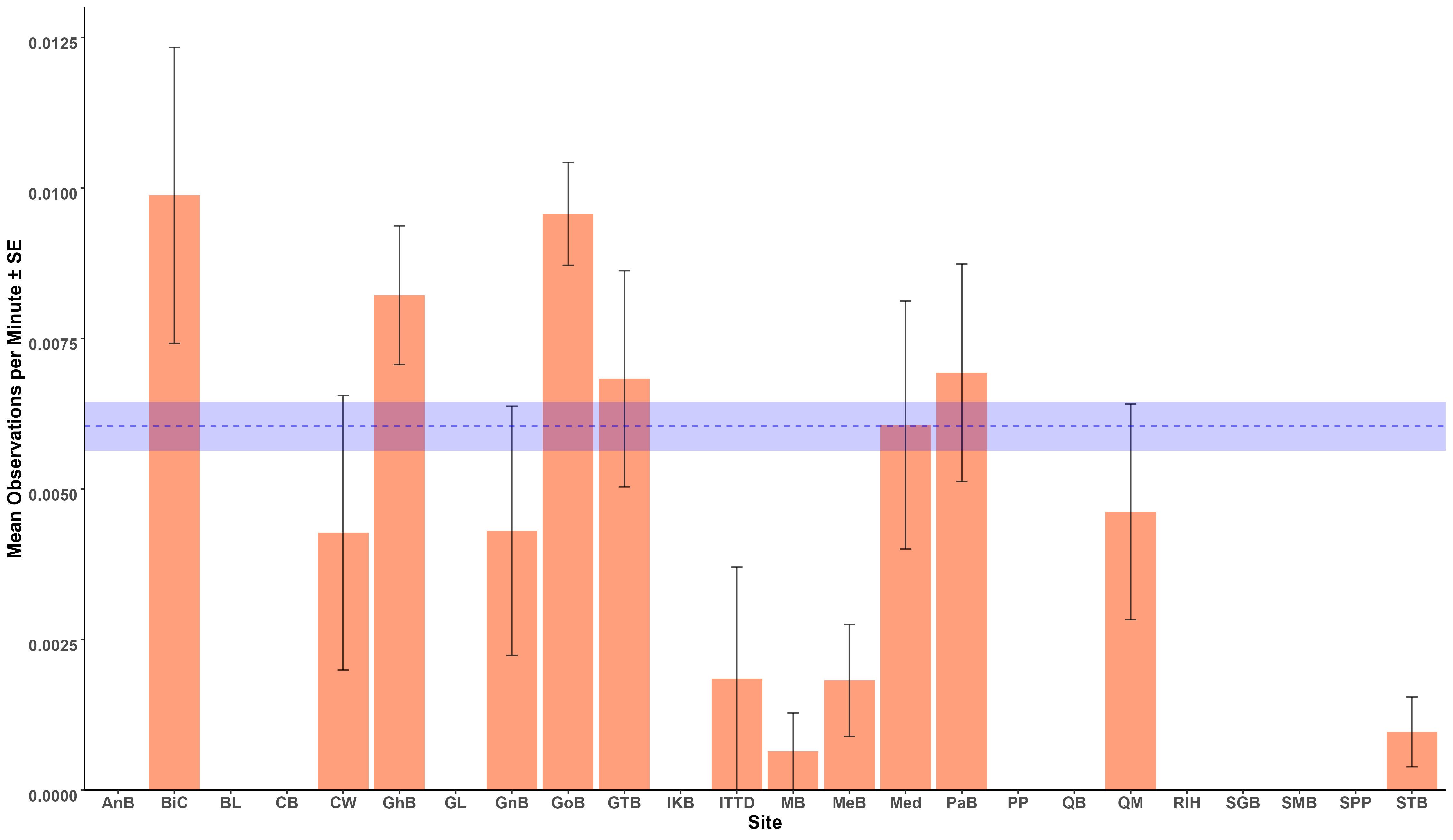
Figure 6. Mean observations per minute ± standard error of 24 bays across the Maltese archipelago. The blue line represents the average across the archipelago.
3.4 Seasonality
Aetomylaeus bovinus were observed from April to December, with a peak in observations during the month of July (Figure 7). No sightings were observed between January and March during the standardized study period of 2014-2024. A Kruskal-Wallis test indicated statistically significant differences in OPUE between months (X2(11) = 116.28, p< 0.001).
3.5 Resightings
‘Resightings’ were defined as the observation of an A. bovinus individual subsequent to its initial encounter following the identification and cataloging process. Resightings were split into calendar years, such as: if an A. bovinus individual was observed in May, and subsequently in October, this would entail a resighting within the same calendar year. Alternatively, if this same A. bovinus individual was observed in May and then subsequently in the next April, this would entail a resighting over a second calendar year, following the observed period of absence during Winter months as per Figure 7.
A total of 135 individual A. bovinus were identified and catalogued. Of those 135 catalogued, 55.2% (n=74) were observed only once, and were not encountered again, and 44.8% (n=61) were observed or resighted 1 or more times. A total of 34 individuals were resighted within 1 calendar year, 17 across 2 calendar years, 7 across 3 calendar years, 2 across 4 calendar years, and 1 across more than 5 calendar years. A specific female A. bovinus individual named ‘Beatroot’ displayed the longest resighting period in the Maltese archipelago (>5 years), example observations over time are illustrated in Figure 8. Of the 61 individuals that were resighted, 77% (n=47) were resighted within the same survey site they were originally seen. The remaining 23% were resighted outside of the survey site they were originally encountered. 13% (n=8) were observed in immediately adjacent sites. The remaining 10% (n=6) were resighted in non-adjacent areas, with 2 particular individuals, ‘Sinbad’ and ‘Ray-Mora’, being resighted in survey sites on opposing sides of Malta.

Figure 8. Selection of images over time of A. bovinus individual ‘Beatroot’. (A) Initial observation on 28/9/2019 at Golden Bay. (B) Observation on 18/7/2021 at Għajn Tuffieħa. (C) Observation on 4/8/2024 at Ċirkewwa.
4 Discussion
4.1 Evidence of a possible nursery area
The repeated presence of A. bovinus YOY and juveniles in the same shallow coastal sites across multiple years provide strong evidence that certain areas in North of Malta may function as nursery habitats. Using the widely accepted tripartite definition from Heupel et al. (2007), these sites satisfy all three core criteria. Demographically, the assemblages observed were overwhelmingly immature, with 97.7% of individuals encountered being YOY to sub-adults.
Aetomylaeus bovinus were consistently observed from April to December, and while adverse sea conditions limited survey efforts during Winter months, A. bovinus were still not observed even when conditions allowed surveys to continue over this season. A significant portion of identified individuals (44.8%) were resighted at least once, over a period of time across weeks, months and years. This suggests that the areas in question are likely not only selected for pupping but also utilized for extended periods during key life stages such as early development. Resighting data indicated that a significant majority of observed individuals (77%) exhibit strong site fidelity, being observed only within the survey site in which they were first encountered. Accounting for movement between immediately adjacent sites, defined based on logistical survey divisions rather than ecological boundaries, such as between Golden Bay and Għajn Tuffieħa or between Qalet Marku, Baħar iċ-Ċagħaq and Mediterraneo, this value rises to 90%. In the cases of resightings across years, this could indicate seasonal philopatry, a behavior seen in other elasmobranch fishes including A. bovinus elsewhere in their range (Chapman et al., 2009; Feldheim et al., 2014; Flowers et al., 2016; Elston et al., 2023)
While occurrences were limited, the utilization of both Western and Eastern sites by some individuals (n=2) raises further questions as to the possible connectivity of habitats around the Maltese archipelago. The movements of A. bovinus individual ‘Ray-Mora’ were particularly notable, with the juvenile (DW = 52.9 cm) moving approx. 12km in the space of 13 days between Golden Bay (5/6/2021), Qalet Marku (15/6/2021) and back to Għajn Tuffieħa (18/6/2021).
The A. bovinus individual ‘Beatroot’ displayed the longest resighting period. Judging by her DW (>100 cm) and associated age (approx. 6 years), we believe that ‘Beatroot’ could be the first documented example of a YOY A. bovinus of Maltese origin growing to maturity according to known relationships between DW and maturity observed elsewhere in the Mediterranean (Başusta and Aslan, 2018). ‘Beatroot’s consistent resighting could suggest the existence of natal philopatry, but further documentation of this phenomenon through a more rigorous methodology such as acoustic or satellite tagging would be required to establish to what extent, if at all, this behavior occurs. Seasonal philopatry has however been documented in A. bovinus in South Africa by acoustic telemetry (Elston et al., 2023).
Additionally, while this study has focused specifically on A. bovinus, the co-occurrence of other juvenile batoid species utilizing the same areas was observed and reported: Dasyatis spp., R. radula, Torpedo marmorata and Myliobatis aquila. Example observations of these species are illustrated in Figure 9 (Charlie Matthews and Sharklab-Malta, pers. obs 2019-2024; Sophie Babbs, pers. comms. 2024; Henry Copperstone, pers. comms. 2024). Data were not collected on these species, other than photographic evidence, as the focus of the study was A. bovinus, however this could be an avenue for future examination. The shared use of the same habitats may be indicative of suitable environmental conditions for a multispecies nursery such as prey availability or protection from predation, both essential for the early development of multiple batoid species (Martins et al., 2018).
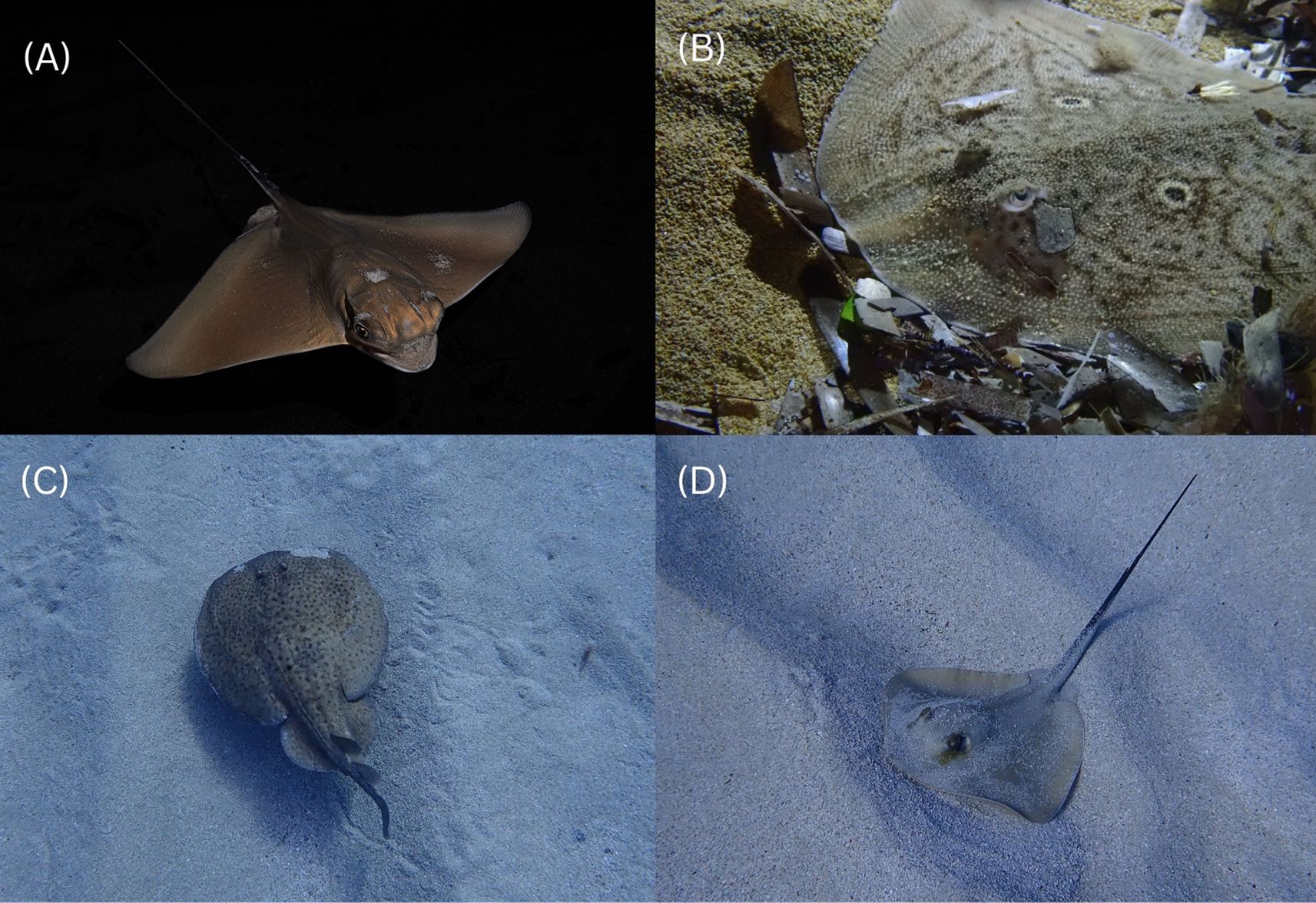
Figure 9. Example images of co-occuring juvenile batoids observed in survey areas. (A) Mylobatis aquila approx. 20cm DW, 2m depth, Għadira Bay, 3/10/2024, photo credit: Henry Copperstone. (B) R. radula approx. 15cm DW, 5m depth, Gnejna Bay, 9/10/2024, photo credit: Sophie Babbs. (C) Torpedo mamorata approx. 10cm DW, 4m depth, Paradise Bay, 14/1/2020, photo credit: Sharklab-Malta. (D) Dasyatis spp. approx. 15cm DW, 5m depth, Qalet Marku, 28/11/2019, photo credit: Sharklab-Malta.
While a comprehensive exploration of all of the ecological drivers behind site selection is outside the scope of this study, several factors may play a role. Shallow coastal areas, which characterize the identified sites, typically possess a wide variety of beneficial attributes for young batoids - such as reduced predator pressure and prey availability (Heupel et al., 2007, 2018; Martins et al., 2018). We did not observe any predation attempts on A. bovinus during this study, and there was generally an absence of larger predatory animals capable of predation on the juvenile batoids such as groupers (e.g Epinephelus marginatus), barracuda (e.g Sphyraena sphyraena) or other bigger elasmobranchs in these areas (Buchholz-Sørensen and Vella, 2016; pers. obs. 2020-2024). If individuals of these species were present, they were small and likely also juvenile, thus unable to engage in predatory attempts on A. bovinus. Rather, despite their immature nature, juvenile A. bovinus were usually the largest animal encountered during surveys. However, successful predation was observed of juvenile D. pastinaca by the invasive Portunus segnis (pers. obs. 2021; Deidun and Sciberras, 2016). While the devastating ecological impact of P. segnis invasion has been assessed in regard to small-scale fisheries, this observation raises questions as to their potential impact in coastal areas that could be utilized by young elasmobranchs (Castriota et al., 2022; Marchessaux et al., 2023).
A comprehensive benthic analysis is outside the scope of this study, but the presence of seagrass meadows and adjacent sand flats at all of the surveyed sites likely enhanced habitat complexity and the provision of ample prey items, helping to support growth and survival. While studies on the diet of A. bovinus are rare, the analysis of morphologically similar species and the examination of some stomach contents indicate a diet of crustaceans, benthic mollusks, and marine worms such as sipunculids and polychaetes (Jardas et al., 2004; Mulas et al., 2021). Observations of A. bovinus in the survey areas often featured foraging behaviors, indicating possible prey provision. Footage has also been shared with the researchers of aggregations of A. bovinus and other batoid species feeding upon deceased Dicentrarchus labrax and Sparus aurata underneath local sea cage fish farms, which has been observed in similar circumstances in Türkiye (Anonymous, pers. comms. 2021-2024; Akyol et al., 2022). The impacts of this provisioning are unknown, but the presence of similar sea cages in Türkiye and in Għadira bay, one of the key areas identified, raises questions for further enquiries. Attempts to contact the relevant aquaculture companies for additional information have so far proved unsuccessful.
Interestingly, other published evidence for A. bovinus nursery areas in South Africa indicated the importance of estuarine systems (Elston et al., 2023; Elston and Murray, 2024). Whilst Malta lacks riverine input and estuarine environments, the areas that are most frequently associated with juvenile A. bovinus sightings share a common feature: they receive unimpeded rainwater runoff from inland catchments (Environment and Resources Authority, 2015, 2023; Ministry for Public Works and Planning, 2022; Environment and Resources Authority, 2023). Unlike other parts of the coastline characterized by steep limestone cliffs or dense urban development, these areas could allow for more natural freshwater and nutrient input following winter rainfall. Although speculative, it is plausible that this runoff could contribute with elevated levels of organic material, helping to fuel provision of standard benthic prey items of bull rays (Dafforn et al., 2013; Ehrnsten et al., 2020; Martins and Barros, 2022). In this sense, these areas may represent a functional analogue to estuarine systems found elsewhere. Further research would be required to quantify prey availability and identify the role that rainwater runoff-driven productivity could serve in Maltese coastal environments.
Despite these insights into the spatial ecology of A. bovinus juveniles, the movement patterns of adults around the Maltese archipelago remain unknown. Sightings of adult specimens (DW >90 cm) are exceptionally rare, with only 4 being directly observed during surveys in the study period of 2011-2024 (max. DW observed = 107 cm). The observed adults were also significantly smaller than the documented maximum DW of 222 cm (Dulčić et al., 2008), potentially indicating a young age profile of observed adult individuals. The demonstrated capacity of the species to undertake significant migrations compounds the current lack of information on the spatial ecology of mature individuals in the Central Mediterranean, illustrating that future studies are required on this matter. Nonetheless, the consistent use of these sites by juvenile A. bovinus for more than 14 years points to their critical importance for the species’ early life stages.
4.2 Threat identification
The identification of nursery areas along the northern coasts of Malta underscores the importance of safeguarding these critical habitats. However, these same shallow, accessible coastal sites are under significant and growing pressures from anthropogenic activities. We identified several possible threats to these areas: tourism and coastal development, unregulated fishing and climate change, but no studies or data exist directly quantifying the exact magnitude of any of these possible stressors on A. bovinus, presenting a needed avenue for future study.
Anthropogenic pressures from tourism and its associated impacts are established to be a major driver of disturbance in coastal biodiversity around the globe and in the Mediterranean (Nunes et al., 2020; Fuentes et al., 2023; IUCN & ETC-UMA, 2024). Malta has been identified by the IUCN Centre for Mediterranean Cooperation (IUCN-Med) as a high pressure region in regards to cumulative tourism pressure on vulnerable coastal and marine ecosystems (IUCN & ETC-UMA, 2024). During 2023, the country welcomed nearly 3 million tourists to the islands, and the period of January-August 2024 demonstrated a 21.1% increase on the same timeframe a year prior (Central Bank of Malta, 2024). Government reports indicate a goal of 4.5–5 million tourists per year by 2050 (Government of Malta, 2025), and increased tourism has also proven to be a major driver behind coastal development in the islands (Ministry for Tourism and Consumer Protection - Malta Tourism Authority, 2021; Malta Tourism Observatory, 2022). The key sites in which juveniles were consistently identified, are also the areas under some of the heaviest touristic pressure in the country, such as Golden Bay, Għajn Tuffieħa (Riviera) and Għadira Bay. These sites experience dramatic increases in seasonal footfall, boat traffic, noise and pollution during the summer months in which juvenile bull rays were frequently observed in this study. Increasing boat traffic has been demonstrated to be a major threat to some elasmobranch megafauna such as Rhincodon typus (Rowat et al., 2021), however few studies have evaluated the impact of boats on demersal species such as A. bovinus. Alternatively, the impact of noise from boats, and the destruction of seagrass habitats from anchoring are likely to impact demersal species (Rider et al., 2021; Bockel et al., 2024). These tourism-based stressors can also contribute to other forms of disturbance for biodiversity, such as habitat degradation and reduction in water quality and could therefore also be impacting juvenile A. bovinus (Nunes et al., 2020; Beeharry et al., 2021; Kurniawan et al., 2023). However, further research would be required to directly quantify the impacts of tourism on local biodiversity in these key high-pressure areas.
In addition to the impacts of growing tourism and associated coastal development, some identified nursery areas face direct threats from proposed land reclamation projects - specifically, the Qalet Marku and Baħar iċ-Ċagħaq sites, which have been highlighted as possible locations for land reclamation (Times of Malta, 2025), and which could result in the complete loss of critical shallow habitats. Large-scale land reclamation has been identified as a “Key Potential Macro-Initiative” by 2050 in the Maltese Government’s ‘ENVISION2050’ report (Government of Malta, 2025), and could present a major threat to some key nearshore habitats and their inhabitants, including A. bovinus.
Globally, fishing is attributed to be the major driving force in the decline of elasmobranch species (Dulvy et al., 2021; Yan et al., 2021). However, in regard to A. bovinus, we have observed limited local commercial catches of this species as part of the ongoing separate fisheries verification study conducted by Sharklab-Malta, with only 4 specimens passing through the central fisheries market in Valletta and subsequently in Marsa since 2008 (unpublished data, 2008-2025). Nevertheless, it is unclear what proportion of total fishing efforts pass through these locations owing to an estimated prevalence of approx. 25% of catches being unreported (Khalfallah et al., 2015). Additionally, any data collected as part of this separate fisheries verification study would also exclude Gozo, which does not have a similar central market reporting system as the main island of Malta (Khalfallah et al., 2015), further illustrating the challenge of assessing the reality of the species’ commercial catch in the archipelago. Despite the lack of commercial landings, across all survey sites juvenile ray individuals of A. bovinus have been observed with their tails clipped, an indicator of prior capture but not retention, weakening their self-defense capabilities (Greg Nowell, pers. comms. 2011-2025; Last et al., 2016).
The opacity of data surrounding fishing efforts also extends outside of commercial endeavors, reaching recreational fishing and spearfishing. Recreational fishing, including with spearguns, is estimated to account for 18% of all total catches in Malta between 1950-2010 - however, there are no official statistics regarding the true extent of this activity (Khalfallah et al., 2015; Department of Fisheries and Aquaculture, 2023). Empirical data on the extent of spearfishing in particular around Malta is nonexistent, following deregulation and the removal of harpoon (speargun) licenses in 2013 (Government of Malta, 2013). Prior to this removal, the obtaining of a harpoon license required both a police vetting and a minimum age requirement of 18 years old, and could be revoked in cases of illegal activities, helping to act as a possible deterrent and management mechanism (Government of Malta, 2005; Times of Malta, 2014). Furthermore, the decentralized and unlicensed nature of modern spearfishing around the Maltese islands makes it particularly difficult to quantify, regulate or address through traditional legislative frameworks. Unlike hunting, which is more politically documented and organizationally visible through the national lobbying organization Federazzjoni Kaċċaturi Nassaba Konservazzjonisti (FKNK), spearfishing operates in a socio-legal blind spot, with no union or regulatory structure to cite and with participants who are heterogeneous and invisible to formal oversight.
Spearfishing is a practice that could be considered sustainable, as it is highly selective, with the potential to avoid illegal undersized individuals and mitigate bycatch. Nevertheless, this high selectivity implies a larger impact upon certain taxa (particularly large-bodied species and top or meso predators) which has a cascading negative impact on local fish assemblages (Lloret et al., 2008; Rocklin et al., 2011; Barbosa et al., 2021). LEK from the diving community across the Maltese archipelago indicates significant unregulated spearfishing pressure particularly on E. marginatus or Octopus vulgaris. Instructors routinely caution both staff and customers against publishing sighting locations as such information often results in immediate targeted exploitation. Despite its illegal status, spearfishing at night with lights, or utilizing SCUBA equipment (European Commission, 2016), including in protected areas, is not uncommon, with enforcement extremely sparse (Weinman, 2023; Malta Ranger Unit, pers. comms. 2024; Times of Malta, 2024b, 2024a; DivingInfo.mt, 2025).
Elasmobranchs are known to be targeted opportunistically by elements of the spearfishing community in Malta, including species such as Prionace glauca (Times of Malta, 2011). These activities ultimately extend to A. bovinus, with the authors having directly observed the spearfishing of this species twice during surveys in 2020 and 2021 (pers. obvs. 2020, 2021). Figure 10 displays an example of a direct observation of the spearfishing of the species captured by researchers (2020). We also continue to observe this activity indirectly through social media posts, such as an image shared in a private online group displaying an individual displaying 3 small A. bovinus caught via this method in a single morning in a northern survey site (Anonymous, pers. comms. 2023).
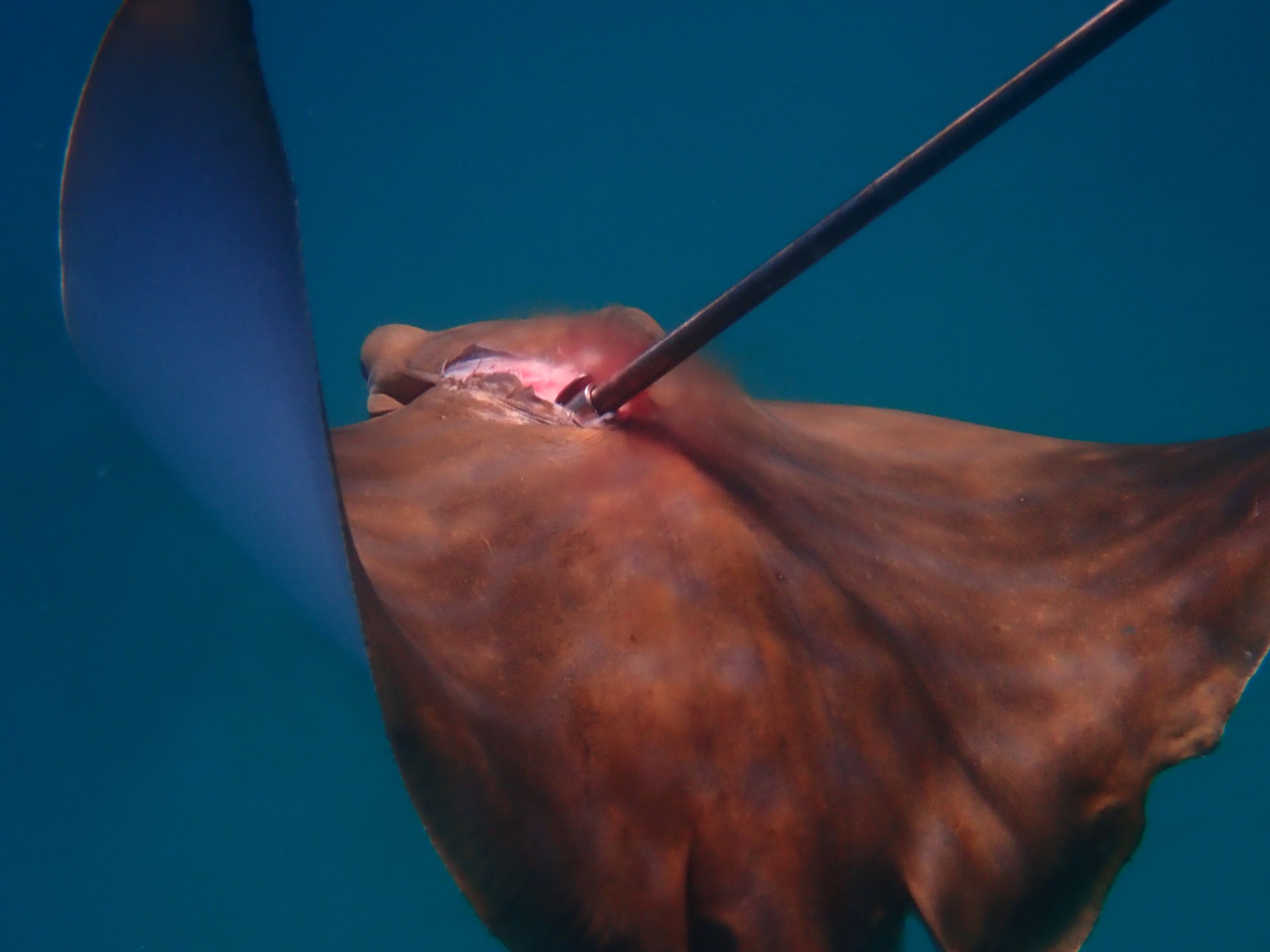
Figure 10. Deceased A. bovinus shot by a speargun, directly observed by researchers in Għadira Bay (2020).
Particularly during the early portion of the season (April-June), researchers noted that small A. bovinus display a relatively low flight response and do not flee from approaching divers as quickly as older individuals (pers. obvs. 2020-2024). This could potentially make them particularly easy prey for spearfishers. Similar behavior has been observed in the Mediterranean for Diplodus sargus, with younger individuals more likely to approach speargun range (≤4 m) than older, more mature animals (Sbragaglia et al., 2024). The approach likelihood also decreased directly with increasing body size (Sbragaglia et al., 2024). This low flight response behavior amongst young A. bovinus combined with their preference for shallow coastal waters, site fidelity and small litter sizes of this species ensure that risk to spearfishing is high, although the true extent and ecological consequences of local captures via this method are unknown, indicating that further scientific enquires are required.
Compounding these local pressures is the broader threat of climate change. The Mediterranean is one of the fastest-warming regions globally, with a warming rate about 20% faster than the global average (Lionello and Scarascia, 2018; Pastor et al., 2020; López García, 2021; Lazoglou et al., 2024). This has significant implications for marine ecosystems and biodiversity (MedECC et al., 2020). While data on thermal sensitivity for A. bovinus are lacking, studies in other regions have identified coastal batoids as particularly vulnerable to climate-driven changes in temperature, salinity and prey dynamics (Cerutti-Pereyra et al., 2024). This includes Aetomylaeus asperrimus, a related species which shares many similar ecological characteristics with A. bovinus, such as a shallow habitat (0–50 m) and possible prey preferences (crustaceans, benthic mollusks, and marine worms), and is considered amongst the most vulnerable of assessed species in the eastern tropical Pacific to climate change impacts (Last et al., 2016; Chávez et al., 2022; Cerutti-Pereyra et al., 2024). Although the Mediterranean and the Tropical Eastern Pacific differ ecologically, the parallels between some of the A. asperrimus and A. bovinus ecological characteristics in tandem with a rapidly warming Mediterranean suggest that Maltese nursery habitats and their inhabitants may be similarly at risk as their Pacific relatives.
4.3 Protection measures and possible mitigation strategies
In recent years, bull rays have gained increasing recognition within international and regional conservation frameworks, reflecting their dramatic population declines, precarious conservation status and the overall growing concern over the state of Mediterranean batoids more generally. The species is now listed under Appendix II of the Convention of the Conservation of Migratory Species of Wild Animals (CMS), in addition to Annex II of the Specially Protected Areas and Biological Diversity Protocol (SPA/BD) of the Barcelona Convention (CMS, 2024; UNEP/MAP, 2024). These listings mandate increased protection and monitoring efforts by signatories and underscore the urgency of improving the species’ data profile, particularly in regions such as the central Mediterranean, where information regarding occurrence, habitat use, and life history traits can be limited or anecdotal. In parallel, the Important Shark and Rays Areas (ISRA) initiative by the IUCN Species Survival Commission Shark Specialist Group (IUCN SSC Shark Specialist Group) has designated the Strait of Sicily and Tunisian Plateau (including Malta) as an ISRA, highlighting the ecological significance of the wider region for elasmobranchs (IUCN SSC Shark Specialist Group, 2023). However, local data regarding A. bovinus were scarce in this initiative, as well as entirely lacking from Maltese NGOs, emphasizing the data-poor nature of the archipelago. The efficacy of these frameworks depends on the availability of robust, location-specific data, and this study directly contributes to these efforts while supporting national compliance with emerging international obligations.
Although A. bovinus is now formally protected under several international and regional instruments (CMS, 2024; UNEP/MAP, 2024), at the time of submission these have not yet been translated into the relevant Maltese legislation (Government of Malta, 2006). However, updates to this legislation are reportedly in progress, which would significantly strengthen legal frameworks for their local conservation in the near future and elevate A. bovinus to the same level of local protection as other iconic elasmobranch species such as Isurus oxyrinchus, Carcharodon carcharias and Mobula mobular (Government of Malta, 2006; Environment and Resources Authority, pers. comms. 2025). For now, potential enforcement and protection measures remain limited until the relevant legislation is updated. The identification of nursery habitats adds an important dimension to these incoming legislative changes, underscoring the need to safeguard not just the species, but also the specific areas which they depend on during some of their most vulnerable life stages.
Nevertheless, while formal protections such as the abovementioned are a critical first step for the species’ conservation in Malta, their effectiveness depends on the implementation of robust enforcement mechanisms by numerous stakeholders. The areas identified in this study are subject to a kaleidoscope of anthropogenic pressures that could threaten their long-term suitability as nursery habitats. All identified sites face varying degrees of pressure from direct human activity such as increased boat traffic, wayward anchoring and litter accumulation, especially during peak tourist season. Despite these mounting threats and several of the bays falling within the boundaries of marine protected areas (Environment and Resources Authority, 2020), enforcement is often weak or non-existent, especially in regard to in-water activities (Malta Ranger Unit, pers. comms. 2023-2025).
The overlap between these critical habitats and areas of high human activity creates an urgent need for integrated, site-specific conservation measures. Without spatial protections that reflect ecological importance rather than political or market convenience, serious questions remain regarding the long-term viability of these habitats. Recreational fishing (including with spearguns) in these areas in particular needs to be better regulated and monitored, as well as forthcoming protections enforced.
Currently, there is an absence of LEK regarding A. bovinus, potentially indicating a prior lack of encounters or its rarity in the wider region (Mulas et al., 2021). This is represented anecdotally by a lack of consensus regarding the name of the species in Maltese, with variations of ‘għasfur’ (pigeon or small bird) and ‘hamiema’ (generic term for batoid) often cited colloquially. Localized signage indicating the presence of A. bovinus and an explanation of their rarity and importance for increased public awareness could therefore also provide an extra level towards effective mitigation and conservation strategies. However, increased awareness without proper legislative measures and enforcement could have a detrimental impact, either through targeted fishing or increased tourism pressure. The creation of ‘best practices’ or ‘code of conduct’ for safe and respectable observation could help to mitigate elements of pressure from tourism interactions, as had been advocated or implemented for other elasmobranch species elsewhere in the broader region such as for Carcharhinus plumbeus around Lampione (Italy) (Cattano et al., 2021) or for Squatina spp. in the Canary Islands and Wales (Angel Shark Project: Canary Islands, 2017; Angel Shark Project: Wales, 2020). Nevertheless, other small-scale interventions, such as increased stakeholder engagement with fishers, dive and tour operators, or the integration of this study’s data into environmental impact assessments for future developments and marine spatial planning may offer feasible first steps towards conservation of these areas and their fragile inhabitants.
4.4 Study limitations
As with many long-term monitoring efforts conducted outside formal institutional frameworks, this study was subject to several logistical and methodological constraints, such as limited research staffing availability and lack of financial resources. These largely reflect the reality of a wholly volunteer-driven program with extremely limited resources.
4.4.1 Sampling bias and survey consistency
Survey effort varied across both seasons and sites, largely driven by access constraints. The entirety of the data collection was facilitated by public transport, an unavoidable limitation for a project reliant on the most cost-effective methods. Despite Malta’s small size, its aforementioned population density entails a well-documented difficulty in traversing the archipelago efficiently through any means (Bajada, 2017; Briguglio and Bonello, 2018; Camilleri, 2020; Central Bank of Malta, 2025). Similarly, those same financial and logistical constraints impacted the regularity of surveys of Gozo and Comino, requiring ferry and private boat transport respectively. Separately, Malta’s topography as presented in section 2.1 entailed that some areas of coastline were inaccessible by any means and thus were not considered for survey sites. These constraints introduced an element of sampling bias in temporal and spatial coverage, but efforts have been and continue to be made to widen the resolution of survey efforts to encompass the whole of the Maltese coastline.
Seasonal variations in weather and sea conditions impacted survey efforts during the course of the year, particularly unsafe sea conditions, such as large waves (wind force >4 Beaufort scale), currents, storms, and very poor water visibility. Occasionally the presence of harmful marine species such as blooms of Pelagia noctiluca (Tanti and Deidun, 2014; Gatt et al., 2018) also impacted survey efforts. Nevertheless, surveys did continue to take place throughout the whole year accounting for these limiting factors, and ultimately the safety of the researchers took priority. Conclusions were also not drawn on abiotic factors such as wind speed/direction to reflect this limitation.
During this study, data were not collected on the benthic habitat in which A. bovinus individuals were sighted, and the combination of dynamism in coastal habitats, unquantified anthropogenic pressures and lack of quantifiable baseline habitat mapping ensured that any conclusions based upon retrospective photogrammetry would bear false conclusions. However, this presents ample avenue for future study.
Surveys did not occur during 2016 owing to a lack of volunteers, and while regretful, this is often the reality of small island NGOs with limited resources.
4.4.2 Methodological variability
The determination of size evolved over the years as resources allowed. The laser photogrammetry equipment only became available to the project from 2017–2018 onwards, meaning that prior encounters relied on the approximation of DW from the P.oceanica proxy of 0.9cm (Borg and Schembri, 1995; Borg et al., 2005). While these earlier surveys may lack the precision of later methods, they still offer valuable insight into the presence of the species over time.
Survey depth was limited to a maximum of 15–20 m depth owing to freediving capabilities of researchers and other volunteers and thus carries limitations regarding depth and the individual ability of each volunteer on a given survey. However this methodology was selected for its cost-effective nature, scalability to volunteers and lack of stress upon the animals being studied.
4.4.3 Threats and qualitative data gaps
While this study focused primarily on the occurrence and spatial ecology of A. bovinus, qualitative data on anthropogenic threats in Malta remain incredibly sparse. In many cases, data simply do not exist such as in the case of informal or unreported activities e.g spearfishing. While other qualitative datasets, such as the declining quality of bathing water or variations in sewage discharges in response to the changing demographics of the island were deemed outside the scope of this study.
4.4.4 Statistical and modelling constraints
While important to reflect ecological realities of a critically engendered batoid species, the prevalence of zero values in the survey sightings data (70.7%) made analytics challenging. When combined with aforementioned logistical constraints, this entailed significant challenges to statistical interpretation given the abnormality of the collected data. These limitations do not detract from the present study’s value, but rather highlight areas that future papers could address, especially within the application of more complex statistical techniques and advanced modelling.
4.4.5 Scalability
The selection of the photogrammetry methodology in tandem with freediving was chosen to broaden wider scalability. However, manual image processing and ID work remain labor intensive and could limit this future scalability. Attempts were made to utilize programs and resources such as Wildbook via MantaMatcher in order to assist image processing and thus speed up the identification process, and part of this study focused on the training of MantaMatcher’s algorithm for this purpose. However, this endeavor was unsuccessful owing to the small size of our data set, which was insufficient to train these systems effectively. Such tools seemed better suited for other species with larger and more developed data sets, such as R. typus or Mobula spp (Arzoumanian et al., 2005; Town et al., 2013; Moskvyak et al., 2019). Recent ongoing developments in machine learning and automated image recognition could offer promising tools for long-term improvement. The groundwork laid by our dataset could nevertheless prove invaluable for training such models, if and when resourcing allows.
4.5 Summary, next steps and conclusions
A total of 135 A. bovinus individuals were identified and documented over a 14-year period. 44.8% of these individuals were resighted at least once, indicating elements of site fidelity. The survey sites of Baħar iċ-Ċagħaq, Golden Bay, Għadira Bay, Għajn Tuffieħa, Paradise Bay, and Mediterraneo showed a higher OPUE than other sites, indicating the Northwest, Northeast and East of Malta as key areas for the species. Demographically, 97.7% of observations were of YOY, juvenile and sub-adult individuals, demonstrating a dominance of immature age classes.
These findings indicate that these areas of the Maltese archipelago serve as a nursery area for A. bovinus. Baħar iċ-Ċagħaq, Golden Bay, Għadira Bay, Għajn Tuffieħa, Paradise Bay, and Mediterraneo in particular satisfy the tripartite definition for a nursery area as per Heupel et al. (2007). This adds evidence to the potential importance of Malta’s coastal habitats for the species’ early life stages around the Central Mediterranean.
This study fills a major data gap in the Central Mediterranean region for a critically endangered species, supports the inclusion of Malta in regional conservation initiatives such as the ISRA project, and assists Malta’s policy obligations under both CMS and SPA/BD protocols.
While outside the scope of this study, we have identified several anthropogenic activities (e.g habitat loss, unregulated spearfishing and climate change) which may pose plausible threats to these nursery areas of a critically endangered species. This demonstrates an urgent need for the recognition and protection of these suspected sites under obligations to CMS and SPA/BD protocols. These possible stressors, and others need to be quantitively examined in order to best inform policy and conservation strategies for these areas. Currently, suggestions for potential site-based management actions include incorporation into marine spatial planning, fisheries restrictions and enforcement or monitoring programs.
Further study is required into many aspects of A. bovinus’ behavioral and spatial ecology around Malta and the Central Mediterranean. The dynamic nature of coastal ecosystems entails a variability in benthic composition over time, but a comprehensive mapping initiative of survey areas could provide a needed baseline with which to compare future changes. Separately, molecular and tagging studies may help to address key data gaps, especially for adult individuals, whose movements remain unknown. Expansion of photo-ID monitoring to more remote sites, including the islands of Gozo and Comino could also help to provide a more accurate picture of residency, additional nursery areas or movement patterns around the archipelago. There is also a need to increase public and stakeholder awareness as to the presence, vulnerability and unique nature of A. bovinus in Maltese coastal waters.
Ultimately, this study highlights the critical conservation value of Malta’s shallow coastal zones for A. bovinus and helps to underscore the value of long-term, NGO-driven monitoring efforts for shaping marine conservation strategies in data poor areas.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
Author contributions
CM: Data curation, Writing – review & editing, Supervision, Conceptualization, Project administration, Investigation, Writing – original draft, Funding acquisition, Visualization. CC: Investigation, Data curation, Visualization, Writing – original draft, Writing – review & editing. CK: Writing – original draft, Writing – review & editing, Investigation, Data curation, Formal analysis, Visualization. SB: Investigation, Data curation, Writing – review & editing, Visualization, Writing – original draft. TP: Investigation, Writing – original draft, Writing – review & editing. BD: Investigation, Writing – review & editing, Writing – original draft. GN: Funding acquisition, Supervision, Writing – review & editing, Writing – original draft. SS-F: Methodology, Writing – original draft, Investigation, Supervision, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Oceanário de Lisboa (Lisbon, Portugal) (2017-2022) under the Fly With Bull Rays (FWBR) project, and the National Geographic Society (Washington, DC, USA) under grant number #WW-098ER-179817 from the young National Geographic Explorer fund granted to Silvio Solleliet-Ferreira (June 2017 -June 2019). Additional funding for publication costs and public engagement workshops related to this work was provided by the Malta Environment Foundation.
Acknowledgments
We thank all past and present contributors to this project, in particular Silvio Solleliet-Ferreira for building the foundations of this work in Malta and beyond. Although the project spans over a decade, with contributions from many individuals, we acknowledge that not all contributors could be contacted or confirmed at the time of writing. We are especially grateful to the many generations of volunteers and students who supported data collection and engaged the public through workshops and educational events. This project and publication would not be possible without you. We also thank Henry Copperstone for his beautiful photo of Mylobatis aquila. Special thanks to the Malta Environment Foundation and Malta National Aquarium for supporting ongoing science communication efforts related to this work.
Conflict of interest
Author SS-F is the founder of Go Shark Azores, a commercial ecotourism company, which had no involvement in the research presented here.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1653284/full#supplementary-material
References
Akyol O., Aydın I., El Kamel-Moutalibi O., and Capapé C. (2017). Bull ray, Aetomylaeus bovinus (Geoffroy Saint-Hilaire 1817) (Myliobatidae) in the Mediterranean Sea and captures of juveniles from Izmir Bay (Aegean Sea, Turkey). J. Appl. Ichthyol 33, 1200–1203. doi: 10.1111/jai.13420
Akyol O., Şen H., and Capapé C. (2022). Occurrence of a shoal of bull ray aetomylaeus bovinus (Myliobatidae) around a sea-cage farm in iskenderun bay (Türkiye, NE mediterranean sea). Çanakkale Onsekiz Mart Univ. J. Mar. Sci. Fisheries 5, 199–202. doi: 10.46384/jmsf.1185563
Angel Shark Project: Canary Islands (2017). Code of Conduct for Diving with Angelsharks. Available online at: https://angelsharknetwork.com/wp-content/uploads/sites/16/2021/11/ZSL00173-Angel-Shark-Code-of-Conduct-A2-Poster_LRv3.pdf (Accessed May 21, 2025).
Angel Shark Project: Wales (2020). Angel Shark Code of Conduct for SCUBA and Snorkel. Available online at: https://angelsharknetwork.com/wp-content/uploads/sites/16/2018/08/Code-of-Conduct-English.pdf (Accessed May 21, 2025).
Arzoumanian Z., Holmberg J., and Norman B. (2005). An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus. J. Appl. Ecol. 42, 999–1011. doi: 10.1111/j.1365-2664.2005.01117.x
ASAB Ethical Committee/ABS Animal Care Committee (2023). Guidelines for the ethical treatment of nonhuman animals in behavioural research and teaching. Anim. Behav. 195, I–XI. doi: 10.1016/j.anbehav.2022.09.006
Azzopardi A., Vella M. G., Vella G., Cuff A., and Farrugia M. (2023). The Environment’s contribution for Neighbourhood Liveability and Wellbeing in the Maltese Islands (Msida, Malta: Faulty for Social Wellbeing - University of Malta, Environment and Resources Authority). Available online at: https://era.org.mt/wp-content/uploads/2024/03/Improving-Wellbeing-in-Maltas-Towns-and-Villages.pdf.
Bajada T. (2017). The Impact of Bus Reform on Behaviour and Policy: The Case of Malta (London: University College London). Available online at: https://discovery.ucl.ac.uk/id/eprint/10047134/1/Bajada_ID_thesis.pdf.
Barbato M., Zampieri C., D’Acunto S., Pennino M. G., Barausse A., and Mazzoldi C. (2023). Too young to die: Mapping nursery areas for early juveniles of the endangered sandbar shark (Carcharhinus plumbeus) to inform conservation in the Mediterranean Sea. J. Appl. Ecol. 60, 2223–2234. doi: 10.1111/1365-2664.14494
Barbosa M. C., Luiz O. J., Cordeiro C. A. M. M., Giglio V. J., and Ferreira C. E. L. (2021). Fish and spearfisher traits contributing to catch composition. Fisheries Res. 241, 105988. doi: 10.1016/j.fishres.2021.105988
Başusta N. and Aslan E. (2018). Age and growth of bull ray Aetomylaeus bovinus (Chondrichthyes: Myliobatidae) from the northeastern Mediterranean coast of Turkey. Roscoff, France: Cahiers de Biologie Marine, Station Biologique de Roscoff, Place Georges Teissier. doi: 10.21411/CBM.A.5F77152E
Beck M. W., Heck K. L., Able K. W., Childers D. L., Eggleston D. B., Gillanders B. M., et al. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51, 633. doi: 10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Beeharry Y., Bekaroo G., Bussoopun D., Bokhoree C., and Phillips M. R. (2021). Perspectives of leisure operators and tourists on the environmental impacts of coastal tourism activities: a case study of Mauritius. Environ. Dev. Sustain 23, 10702–10726. doi: 10.1007/s10668-020-01080-7
Bockel T., Bossut N., Mouquet N., Mouillot D., Fontaine Q., and Deter J. (2024). Quantifying the impact of small boats on Posidonia seagrass meadows: Methods and path for future efficient management of anchoring pressure. Ocean Coast. Manage. 259, 107454. doi: 10.1016/j.ocecoaman.2024.107454
Boggio-Pasqua A., Flam A. L., and Marshall A. D. (2019). Spotting the “small eyes”: using photo-ID methodology to study a wild population of smalleye stingrays (Megatrygon microps) in southern Mozambique. PeerJ 7, e7110. doi: 10.7717/peerj.7110
Borg J. A., Attrill M. J., Rowden A. A., Schembri P. J., and Jones M. B. (2005). Architectural characteristics of two bed types of the seagrass Posidonia oceanica over different spatial scales. Estuarine Coast. Shelf Sci. 62, 667–678. doi: 10.1016/j.ecss.2004.10.003
Borg J. A., Dandria D., Evans J., Knittweis L., and Schembri P. J. (2023). A critical checklist of the marine fishes of Malta and surrounding waters. Diversity 15, 35. doi: 10.3390/d15020225
Borg J. A. and Schembri P. J. (1995). The state of Posidonia oceanica (L.) Delile meadow in the Maltese Islands (Central Mediterranean). Available online at: https://www.researchgate.net/publication/234053662_The_state_of_Posidonia_oceanica_L_Delile_meadow_in_the_Maltese_Islands_Central_Mediterranean. (Accessed May 05, 2025).
Briguglio M. and Bonello S. (2018). NO MAN’S LAND: people, place & pollution. (Ħamrun, Malta: S.l.: KITE GROUP LTD).
Bruns S. and Henderson A. C. (2020). A baited remote underwater video system (BRUVS) assessment of elasmobranch diversity and abundance on the eastern Caicos Bank (Turks and Caicos Islands); an environment in transition. Environ. Biol. Fish 103, 1001–1012. doi: 10.1007/s10641-020-01004-4
Buchholz-Sørensen M. and Vella A. (2016). Population structure, genetic diversity, effective population size, demographic history and regional connectivity patterns of the endangered dusky grouper, Epinephelus marginatus (Teleostei: Serranidae), within Malta’s Fisheries Management Zone. PLoS ONE 11 (7), e0159864. doi: 10.1371/journal.pone.0159864
Camilleri K. (2020). Research traffic congestion in Malta: an analysis of the attributes, attitudes and solutions. MCAST J. Of Appl. Res. Pract. 4, 31. doi: 10.5604/01.3001.0014.4396
Capapé C. (1977). Etude du regime alimentaire de la Mourine vachette, Pteromylaeus bovinus (Geoffroy Saint-Hilaire 1817) (Pisces, Myliobatidae) des cotes tunisiennes. ICES J. Mar. Sci. 37, 214–220. doi: 10.1093/icesjms/37.3.214
Capapé C., N’dao M., and Diop M. (1995). Observations sur la biologie de la reproduction de quatorze espèces de Sélaciens batoïdes capturés dans la région marine de Dakar-Ouakam (Sénegal, Atlantique oriental tropical) Vol. 48A (Dakar: Bulletin de I’Institut Fondamental d”Afrique Noire Cheikh Anta Diop), 89–102.
Capapé C. and Quignard J.-P. (1975). Contribution à la systématique et à la biologie de Pteromylaeus bovinus (Geoffroy Saint-Hilaire 1817) (Pices Myliobalidae) des côtes tunisiennes. Bull. du Muséum Natl. d’histoire naturelle 338, 1329–1347. doi: 10.5962/p.281398
Castriota L., Falautano M., Maggio T., and Perzia P. (2022). The blue swimming crab portunus segnis in the mediterranean sea: invasion paths, impacts and management measures. Biology 11, 1473. doi: 10.3390/biology11101473
Cattano C., Gambardella C., Grancagnolo D., Principato E., Aglieri G., Turco G., et al. (2023). Multiple interannual records of young-of-the-year identify an important area for the protection of the shortfin mako, Isurus oxyrinchus. Mar. Environ. Res. 192, 106217. doi: 10.1016/j.marenvres.2023.106217
Cattano C., Turco G., Di Lorenzo M., Gristina M., Visconti G., and Milazzo M. (2021). Sandbar shark aggregation in the central Mediterranean Sea and potential effects of tourism. Aquat. Conservation: Mar. Freshw. Ecosyst. 31, 1420–1428. doi: 10.1002/aqc.3517
Central Bank of Malta (2024). Developments in Malta’s tourism industry and hotel sector Vol. 4 (CBM Business Dialogue), 4.
Central Bank of Malta (2025). The Native Maltese Population: Projections And Implications On The Labour Supply (Valletta, Malta: Central Bank of Malta). Available online at: https://www.centralbankmalta.org/site/Publications/Economic%20Research/2025/Policy-Note-1-25.pdf?revcount=9087.
Cerutti-Pereyra F., Drenkard E. J., Espinoza M., Finucci B., Galván-Magaña F., Hacohen-Domené A., et al. (2024). Vulnerability of Eastern Tropical Pacific chondrichthyan fish to climate change. Global Change Biol. 30, e17373. doi: 10.1111/gcb.17373
Cerutti-Pereyra F., Thums M., Austin C. M., Bradshaw C. J. A., Stevens J. D., Babcock R. C., et al. (2014). Restricted movements of juvenile rays in the lagoon of Ningaloo Reef, Western Australia – evidence for the existence of a nursery. Environ. Biol. Fish 97, 371–383. doi: 10.1007/s10641-013-0158-y
Chang H.-Y., Sun M., Rokosz K., and Chen Y. (2023). Evaluating effects of changing sampling protocol for a long-term ichthyoplankton monitoring program. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1237549
Chapman D. D., Babcock E. A., Gruber S. H., Dibattista J. D., Franks B. R., Kessel S. A., et al. (2009). Long-term natal site-fidelity by immature lemon sharks (Negaprion brevirostris) at a subtropical island. Mol. Ecol. 18, 3500–3507. doi: 10.1111/j.1365-294X.2009.04289.x
Chávez E. J., Heidemeyer M., Arauz R., Arauz-Naranjo D., Mora-Vargas R., Molina-Quirós J. L., et al. (2022). Presencia de la raya águila de piel áspera Aetomylaeus asperrimus (Chondrichthyes: Myliobatidae) en la costa del Pacífico Norte de Costa Rica. Rev. Cien. Mar. Cost. 14(1), 73–82. doi: 10.15359/revmar.14-1.5
CMS (2024). Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS) (Bonn, Germany: Convention on the Conservation of Migratory Species of Wild Animals). Available online at: https://www.cms.int/en/species/appendix-ii.
Colloca F., Garofalo G., Bitetto I., Facchini M. T., Grati F., Martiradonna A., et al. (2015). The seascape of demersal fish nursery areas in the North Mediterranean Sea, a first step towards the implementation of spatial planning for trawl fisheries. PLoS One 10, e0119590. doi: 10.1371/journal.pone.0119590
Colloca F., Scarcella G., and Libralato S. (2017). Recent trends and impacts of fisheries exploitation on mediterranean stocks and ecosystems. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00244
Dafforn K. A., Kelaher B. P., Simpson S. L., Coleman M. A., Hutchings P. A., Clark G. F., et al. (2013). Polychaete richness and abundance enhanced in anthropogenically modified estuaries despite high concentrations of toxic contaminants. PLoS One 8, e77018. doi: 10.1371/journal.pone.0077018
Darmanin G., Gauci A., Deidun A., Galone L., and D’Amico S. (2023). Satellite-derived bathymetry for selected shallow maltese coastal zones. Appl. Sci. 13, 5238. doi: 10.3390/app13095238
Deakos M. (2010). Paired-laser photogrammetry as a simple and accurate system for measuring the body size of free-ranging manta rays Manta alfredi. Aquat. Biol. 10, 1–10. doi: 10.3354/ab00258
Deidun A. and Sciberras A. (2016). A further record of the blue swimmer crab Portunus segnis Forskal 1775 (Decapoda: Brachyura: Portunidae) from the Maltese Islands (Central Mediterranean). BIR 5, 43–46. doi: 10.3391/bir.2016.5.1.08
Department of Fisheries and Aquaculture (2023). Annual Report on efforts to achieve a sustainable balance between fishing capacity and fishing opportunities for the year 2022 (Malta: Government of Malta, Ministry for Agriculture, Fisheries and Animal Rights). Available online at: https://oceans-and-fisheries.ec.europa.eu/document/download/253d0656-a918-4324-ab2a-03b2f621fe03_en?filename=2022-fleet-capacity-report-malta_en.pdf&prefLang=es.
DivingInfo.mt (2025). Spearfisherman with scuba gear caught red-handed (Malta: divinginfo.mt). Available online at: https://www.divinginfo.mt/spearfisherman-with-scuba-gear-caught-red-handed/.
Drago A. F., Sorgente R., and Ribotti A. (2003). A high resolution hydrodynamic 3-D model simulation of the Malta shelf area. Ann. Geophys. 21, 323–344. doi: 10.5194/angeo-21-323-2003
Dulčić J., Lipej L., Orlando-Bonaca M., Jenko R., Grbec B., Guélorget O., et al. (2008). The bull ray, Pteromylaeus bovinus (Myliobatidae) (northern Adriatic Sea). doi: 10.26028/CYBIUM/2008-322-004
Dulvy N. K., Allen D. J., Ralph G. M., and Walls R. H. L. (2016). The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea (Malaga, Spain: IUCN). Available online at: https://portals.iucn.org/library/sites/library/files/documents/Rep-2016-024.pdf. (Accessed April 26, 2025).
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 31, 4773–4787.e8. doi: 10.1016/j.cub.2021.08.062
Ebert D. A. and Dando M. (2020). Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean. (Princeton, New Jersey, United States: Princeton University Press). doi: 10.2307/j.ctv12sdwkk
Echwikhi K., Saidi B., and Bradai M. N. (2014). Elasmobranchs longline fisheries in the Gulf of Gabès (southern Tunisia). J. Mar. Biol. Ass. 94, 203–210. doi: 10.1017/S0025315413000726
Ehrnsten E., Sun X., Humborg C., Norkko A., Savchuk O. P., Slomp C. P., et al. (2020). Understanding environmental changes in temperate coastal seas: linking models of benthic fauna to carbon and nutrient fluxes. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00450
Elston C. and Murray T. (2024). Multi-method approach identifies a South African estuary as an important elasmobranch habitat and potential nursery ground. Afr. J. Mar. Sci. 46, 91–102. doi: 10.2989/1814232X.2024.2326814
Elston C., Murray T. S., Parkinson M. C., Filmalter J. D., and Cowley P. D. (2023). Female Diamond Rays Gymnura natalensis and Bull Rays Aetomylaeus bovinus Display Seasonal Philopatry to South African Estuaries. Estuaries Coasts 46, 1880–1894. doi: 10.1007/s12237-023-01239-1
Elston C., Murray T. S., Rogers T., Parkinson M. C., Mann B. Q., Daly R., et al. (2024). Diamond Gymnura natalensis and duckbill Aetomylaeus bovinus rays undertake nationwide coastal migrations. J. Fish Biol. 16. doi: 10.1111/jfb.15728
Environment and Resources Authority (2015). The 2nd Water Catchment Management Plan for the Malta Water Catchment District 2015 - 2021 (Marsa, Malta: Sustainable Energy and Water Conservation Unit, Environment and Resources Authority). Available online at: https://ampeid.org/static/998b9dcf37d3d5249b33617d298234e0/mlt2nd_Water_Catchment_Management_Plan-Malta_Water_in_Maltese_Islands-1.pdf.
Environment and Resources Authority (2020). Marine Protected Areas. Available online at: https://era.org.mt/topic/marine-protected-areas-2/ (Accessed November 14, 2022).
Environment and Resources Authority (2023). 3rd River Basin Management Plan: MALTA. 3. Pressures and Impacts (Marsa, Malta: The Water & Energy Agency, Environment and Resources Authority). Available online at: https://era.org.mt/wp-content/uploads/2023/09/3rd-River-Basin-Management-Plan-MALTA-Chapter-3-Pressures-and-Impacts-Final.pdf.
Espino F., González J. A., Bosch N. E., Otero-Ferrer F. J., Haroun R., and Tuya F. (2022). Distribution and population structure of the smooth-hound shark, Mustelus mustelus (Linnaeus 1758), across an oceanic archipelago: Combining several data sources to promote conservation. Ecol. Evol. 12, 14. doi: 10.1002/ece3.9098
European Commission (2016). Proposal for a Regulation of the European Parliament and of the Council establishing a multiannual plan for the stocks of cod, herring and sprat in the Baltic Sea and the fisheries exploiting those stocks, amending Council Regulation (EC) No 2187/2005 and repealing Council Regulation (EC) No 1098/2007 (Brussels, Belgium: European Commission). Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52016PC0134.
European Commission Directorate General for the Environment (2008). Natura 2000: protecting Europe’s biodiversity. (Bruxelles/Brussels, Belgium: LU: Publications Office). Available online at: https://data.europa.eu/doi/10.2779/45963.
Falsone F., Gancitano V., Geraci M. L., Sardo G., Scannella D., Serena F., et al. (2022). Assessing the stock dynamics of elasmobranchii off the southern coast of sicily by using trawl survey data. Fishes 7, 136. doi: 10.3390/fishes7030136
Feldheim K. A., Gruber S. H., DiBattista J. D., Babcock E. A., Kessel S. T., Hendry A. P., et al. (2014). Two decades of genetic profiling yields first evidence of natal philopatry and long-term fidelity to parturition sites in sharks. Mol. Ecol. 23, 110–117. doi: 10.1111/mec.12583
Ferretti F., Myers R. A., Serena F., and Lotze H. K. (2008). Loss of large predatory sharks from the mediterranean sea. Conserv. Biol. 22, 952–964. doi: 10.1111/j.1523-1739.2008.00938.x
Ferretti F., Worm B., Britten G. L., Heithaus M. R., and Lotze H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x
Flowers K., Ajemian M., Bassos-Hull K., Feldheim K., Hueter R., Papastamatiou Y., et al. (2016). A review of batoid philopatry, with implications for future research and population management. Mar. Ecol. Prog. Ser. 562, 251–261. doi: 10.3354/meps11963
Fuentes S. N., Díaz Andrade M. C., Awruch C. A., Moya A. C., and Arias A. H. (2023). Impacts of water pollutants on chondrichthyans species from South America: A review. Chemosphere 324, 138262. doi: 10.1016/j.chemosphere.2023.138262
Gatt M. P., Deidun A., Galea A., and Gauci A. (2018). Is citizen science a valid tool to monitor the occurrence of jellyfish? The spot the jellyfish case study from the Maltese Islands. J. Coast. Res. 85(1), 316–320. doi: 10.2112/SI85-064.1
Geraci M. L., Ragonese S., Scannella D., Falsone F., Gancitano V., Mifsud J., et al. (2021). Batoid abundances, spatial distribution, and life history traits in the strait of sicily (Central mediterranean sea): bridging a knowledge gap through three decades of survey. Animals 11, 2189. doi: 10.3390/ani11082189
Gerovasileiou V., Akyol O., Al-Hosne Z., Alshikh Rasheed R., Ataç E., Bello G., et al. (2020). New records of rare species in the Mediterranean Sea 2020. Medit. Mar. Sci. doi: 10.12681/mms.22148
Giménez J., Cardador L., Mazor T., Kark S., Bellido J. M., Coll M., et al. (2020). Marine protected areas for demersal elasmobranchs in highly exploited Mediterranean ecosystems. Mar. Environ. Res. 160, 105033. doi: 10.1016/j.marenvres.2020.105033
Giovos I., Serena F., Katsada D., Anastasiadis A., Barash A., Charilaou C., et al. (2021). Integrating literature, biodiversity databases, and citizen-science to reconstruct the checklist of chondrichthyans in Cyprus (Eastern Mediterranean sea). Fishes 6, 24. doi: 10.3390/fishes6030024
Government of Malta (2005). ACT No. XIV of 2005 (CAP. 480). (Valletta, Malta) Available online at: https://legislation.mt/eli/act/2005/14/eng/pdf. (Accessed May 20, 2025).
Government of Malta (2006). Flora, Fauna and Natural Habitats Protection Regulations (S.L. 549.44). Available online at: https://legislation.mt/eli/sl/549.44/eng/pdf (Accessed April 20, 2025).
Government of Malta (2013). Legal Notice 75 of 2013 - Arms Licensing (Amendment) Regulations 2013 (S.L.480.02). Available online at: https://legislation.mt/eli/ln/2013/75/eng/pdf. (Accessed May 20, 2025).
Government of Malta (2025). ENVISION2050: Malta Vision 2050 Executive Summary (Office of the Prime Minister, Ministry for the Economy, Enterprise and Strategic Projects, Government of Malta). Available online at: https://cdn-others.timesofmalta.com/915071a58855edbbef2593b51d417acc0ff17cd3.pdf.
Hammerschlag N., Herskowitz Y., Fallows C., and Couto T. B. A. (2025). Evidence of cascading ecosystem effects following the loss of white sharks from False Bay, South Africa. Front. Mar. Sci. 12. doi: 10.3389/fmars.2025.1530362
Heupel M., Carlson J., and Simpfendorfer C. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Heupel M. R., Kanno S., Martins A. P. B., and Simpfendorfer C. A. (2018). Advances in understanding the roles and benefits of nursery areas for elasmobranch populations. Mar. Freshw. Res. 70, 897. doi: 10.1071/MF18081
IUCN (2020). “Aetomylaeus bovinus,” in The IUCN Red List of Threatened Species 2021: e.T60127A124441812. Eds. Jabado R. W., Chartrain E., Cliff G., Derrick D., Dia M., Diop M., Doherty P., Dossa J., Leurs G. H. L., Metcalfe K., Porriños G., Seidu I., Soares A., Tamo A., VanderWright W. J., and Williams A. B. doi: 10.2305/IUCN.UK.2021-1.RLTS.T60127A124441812.en
IUCN (2025). IUCN Red List of Threatened Species 2025. (Cambridge, United Kingdom: IUCN Red List of Threatened Species). Available online at: https://www.iucnredlist.org/ (Accessed April 26, 2025).
IUCN & ETC-UMA (2024). Mapping Blue Tourism: Vulnerability Assessment of Mediterranean Coastal and Marine Ecosystems (Malaga, Spain: European Topic Centre for Spatial Analysis and Synthesis – University of Malaga: Blue Tourism Initiative). Available online at: https://bluetourisminitiative.org/wp-content/uploads/2024/09/Mapping-the-Impact-of-Blue-Tourism-in-the-Mediterranean_HD.pdf.
IUCN SSC Shark Specialist Group (2023). Strait of Sicily and Tunisian Plateau ISRA Factsheet (Gland, Switzerland: IUCN SSC Shark Specialist Group).
Jardas I., Šantić M., and Pallaoro A. (2004). Diet composition of the eagle ray, Myliobatis aquila (Chondrichthyes: Myliobatidae), in the Eastern Adriatic Sea. Cybium 28, 372–374.
Katsanevakis S., Acar Ü., Ammar I., Balci B. A., Bekas P., Belmonte M., et al. (2014). New mediterranean biodiversity records (October 2014). Medit. Mar. Sci. 15, 675. doi: 10.12681/mms.1123
Khalfallah M., Dimech M., Ulman A., Zeller D., and Pauly D. (2015). Reconstruction of marine fisheries catches for the Republic of Malta, (1950-2010) (British Columbia, Canada: University of British Columbia: Fisheries Centre, University of British Columbia). Available online at: https://www.seaaroundus.org/doc/publications/wp/2015/Khalfallah-et-al-Malta.pdf.
Knittweis L., Evans J., Aguilar R., Álvarez H., Borg J. A., García S., et al. (2019). “Recent discoveries of extensive cold-water coral assemblages in maltese waters,” in Mediterranean Cold-Water Corals: Past, Present and Future. Eds. Orejas C. and Jiménez C. (Springer International Publishing, Cham), 253–255. doi: 10.1007/978-3-319-91608-8_22
Knochel A. M., Cochran J. E. M., Kattan A., Stevens G. M. W., Bojanowksi E., and Berumen M. L. (2022). Crowdsourced data reveal multinational connectivity, population demographics, and possible nursery ground of endangered oceanic manta rays in the Red Sea. Aquat. Conserv. 32, 1774–1786. doi: 10.1002/aqc.3883
Koehler L. (2025). Elasmobranch research in the Mediterranean Sea: What is known, what is not, how it changed, and where it needs to go. Fisheries Res. 285, 107328. doi: 10.1016/j.fishres.2025.107328
Koehler L. and Lowther J. (2025). Tracking implementation of shark-related measures and actions in the Mediterranean region in the context of international law. Biol. Conserv. 302, 110930. doi: 10.1016/j.biocon.2024.110930
Kurniawan F., Adrianto L., Bengen D. G., and Prasetyo L. B. (2023). Hypothetical effects assessment of tourism on coastal water quality in the Marine Tourism Park of the Gili Matra Islands, Indonesia. Environ. Dev. Sustain 25, 7959–7985. doi: 10.1007/s10668-022-02382-8
La Mesa G., Annunziatellis A., Filidei E., and Fortuna C. M. (2016). Bycatch of myliobatid rays in the central mediterranean sea: the influence of spatiotemporal, environmental, and operational factors as determined by generalized additive modeling. Mar. Coast. Fisheries 8, 382–394. doi: 10.1080/19425120.2016.1167795
Last P., Seret B., Stehmann M., Naylor G., White W., Carvalho M., et al. (2016). Rays of the World (Clayton, Victoria, Australia: CSIRO Publishing).
Lazoglou G., Papadopoulos-Zachos A., Georgiades P., Zittis G., Velikou K., Manios E. M., et al. (2024). Identification of climate change hotspots in the Mediterranean. Sci. Rep. 14, 29817. doi: 10.1038/s41598-024-80139-1
Lionello P. and Scarascia L. (2018). The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Change 18, 1481–1493. doi: 10.1007/s10113-018-1290-1
Lipej L., Mavrič B., and Dulčić J. (2009). Size of the bull ray, Pteromylaeus bovinus (Geoffroy Saint-Hilaire 1817), from the northern Adriatic. J. Appl. Ichthyology 25, 103–105. doi: 10.1111/j.1439-0426.2008.01077.x
Lloret J., Zaragoza N., Caballero D., Font T., Casadevall M., and Riera V. (2008). Spearfishing pressure on fish communities in rocky coastal habitats in a Mediterranean marine protected area. Fisheries Res. 94, 84–91. doi: 10.1016/j.fishres.2008.07.002
López García M. J. (2021). ¿Cuánto se está calentando el Mediterráneo? Treinta y cinco años de observaciones desde satélite. Metode SSJ, 11, 193–199. doi: 10.7203/metode.11.16693
Malta Tourism Observatory (2022). Environment, Climate Change & Tourism Product (Kalkara, Malta: mtobservatory). Available online at: https://www.mtobservatory.org/environment.
Marchessaux G., Mangano M. C., Bizzarri S., M’Rabet C., Principato E., Lago N., et al. (2023). Invasive blue crabs and small-scale fisheries in the Mediterranean sea: Local ecological knowledge, impacts and future management. Mar. Policy 148, 105461. doi: 10.1016/j.marpol.2022.105461
Marshall A. D., Dudgeon C. L., and Bennett M. B. (2011). Size and structure of a photographically identified population of manta rays Manta alfredi in southern Mozambique. Mar. Biol. 158, 1111–1124. doi: 10.1007/s00227-011-1634-6
Marshall A. D. and Pierce S. J. (2012). The use and abuse of photographic identification in sharks and rays. J. Fish Biol. 80, 1361–1379. doi: 10.1111/j.1095-8649.2012.03244.x
Martins A. D. and Barros F. (2022). Ecological functions of polychaetes along estuarine gradients. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.780318
Martins A., Heupel M., Chin A., and Simpfendorfer C. (2018). Batoid nurseries: definition, use and importance. Mar. Ecol. Prog. Ser. 595, 253–267. doi: 10.3354/meps12545
MedECC, Cramer W., Guiot J., and Marini K. (2020). Climate and Environmental Change in the Mediterranean Basin – Current Situation and Risks for the Future. First Mediterranean Assessment Report. MedECC Reports (Marseille, France: MedECC Secretariat). doi: 10.5281/ZENODO.7224821
Ministry for Public Works and Planning (2022). Green stormwater infrastructure guidance manual (Floriana, Malta: Life 16 IPE/MT/000008 – “Optimising the Implementation of the 2nd RBMP in the Maltese RIver Basin District.”). Available online at: https://publicworksdepartment.gov.mt/wp-content/uploads/2024/07/Green-Stormwater-Infrastructure-Guidance-Manual-1.pdf.
Ministry for Tourism and Consumer Protection - Malta Tourism Authority (2021). recover. rethink. revitalise. Malta Tourism Strategy 2021-2030 (Malta: Minsitry for Tourism and Consumer Protection - Malta Tourism Authority). Available online at: https://tourism.gov.mt/wp-content/uploads/2023/04/National-Tourism-Strategy-2021-2030.pdf.
Moreno J., Solleliet-Ferreira S. E., and Riera R. (2022). Distribution and abundance of coastal elasmobranchs in tenerife (Canary islands, NE atlantic ocean) with emphasis on the bull ray, aetomylaeus bovinus. Thalassas 38, 723–731. doi: 10.1007/s41208-021-00316-1
Moskvyak O., Maire F., Armstrong A. O., Dayoub F., and Baktashmotlagh M. (2019). Robust Re-identification of Manta Rays from Natural Markings by Learning Pose Invariant Embeddings. (New York, USA: Cornell University, New York, USA: Cornell University) doi: 10.48550/ARXIV.1902.10847
Mulas A., Bellodi A., Carbonara P., Cau A., Marongiu M. F., Pesci P., et al. (2021). Bio-ecological features update on eleven rare cartilaginous fish in the central-western mediterranean sea as a contribution for their conservation. Life 11, 871. doi: 10.3390/life11090871
Myers R. A., Baum J. K., Shepherd T. D., Powers S. P., and Peterson C. H. (2007). Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850. doi: 10.1126/science.1138657
Nejmeddine Bradai M., Enajjar S., and Saidi B. (2023). ““Sharks’ Status in the mediterranean sea urgent awareness is needed,” in Sharks - Past, Present and Future. Eds. Nejmeddine Bradai M., Enajjar S., and Saidi B. (London, United Kingdom: IntechOpen). doi: 10.5772/intechopen.108162
Nejmeddine Bradai M., Saidi B., and Enajjar S. (2012). Elasmobranchs Of The Mediterranean And Black Sea: Status, Ecology And Biology Bibliographic Analysis. (Rome: FAO).
Nunes L. J. R., Raposo M. A. M., and Gomes C. J. P. (2020). The Impact of Tourism Activity on Coastal Biodiversity: A Case Study at Praia da Cova Redonda (Algarve—Portugal). Environments 7, 88. doi: 10.3390/environments7100088
O’Keefe M., Bengil E. G. T., Palmer J. L., Beton D., Çağlar Ç., Godley B. J., et al. (2023). Diversity and distribution of elasmobranchs in the coastal waters of Cyprus: using bycatch data to inform management and conservation. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1181437
O’Shea O., Van Leeuwen T., O’Brien D., Arrowsmith L., McCalman R., Griffiths M., et al. (2021). Evidence and description of a nursery habitat for the recently reclassified stingray Styracura schmardae from The Bahamas. Mar. Ecol. Prog. Ser. 660, 141–151. doi: 10.3354/meps13589
OECD (2025). Strengthening the evidence base for a sustainable tourism future in Malta (Paris: OECD).
Ouled-Cheikh J., Coll M., Cardona L., Steenbeek J., and Ramírez F. (2022). Fisheries-enhanced pressure on Mediterranean regions and pelagic species already impacted by climate change. Elementa: Sci. Anthropocene 10, 28. doi: 10.1525/elementa.2022.00028
Pacoureau N., Rigby C. L., Kyne P. M., Sherley R. B., Winker H., Carlson J. K., et al. (2021). Half a century of global decline in oceanic sharks and rays. Nature 589, 567–571. doi: 10.1038/s41586-020-03173-9
Páez-Rosas D., Suarez-Moncada J., Arnés-Urgellés C., Espinoza E., Robles Y., and Salinas-De-León P. (2024). Assessment of nursery areas for the scalloped hammerhead shark (Sphyrna lewini) across the Eastern Tropical Pacific using a stable isotopes approach. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1288770
Pastor F., Valiente J. A., and Khodayar S. (2020). A warming mediterranean: 38 years of increasing sea surface temperature. Remote Sens. 12, 2687. doi: 10.3390/rs12172687
Percie Du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M. T., Baker M., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 16, 242. doi: 10.1186/s12917-020-02451-y
Pierce S. J. and Charles R. (2022). Humans Impacts on Sharks and Rays. (Bonn, Germany: CMS). Available online at: https://www.cms.int/lions/sites/default/files/publication/cms_sharks-mos4_inf.7_human%20impacts%20on%20sharks%20and%20rays_e_0.pdf.
Piroddi C., Coll M., Liquete C., Macias D., Greer K., Buszowski J., et al. (2017). Historical changes of the Mediterranean Sea ecosystem: modelling the role and impact of primary productivity and fisheries changes over time. Sci. Rep. 7, 44491. doi: 10.1038/srep44491
Prampolini M., Coratza P., Rossi S., Parenti C., Galea C., Caruana A., et al. (2021). Geomorphology of the seafloor north east of the Maltese Islands, Central Mediterranean. J. Maps 17, 465–475. doi: 10.1080/17445647.2021.1957034
Putra M. I. H., Malaiholo Y., Sahri A., Setyawan E., Herandarudewi S. M. C., Hasan A. W., et al. (2025). Insights into cetacean sightings, abundance, and feeding associations: observations from the boat lift net fishery in the Kaimana important marine mammal area, Indonesia. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1431209
Rangel B. S., Rodrigues A., and Moreira R. G. (2018). Use of a nursery area by cownose rays (Rhinopteridae) in southeastern Brazil. Neotrop. ichthyol. 16, 8. doi: 10.1590/1982-0224-20170089
R Core Team (2025). R: A Language and Environment for Statistical Computing. Available online at: https://www.R-project.org. (Accessed May 27, 2025).
Rider M. J., Kirsebom O. S., Gallagher A. J., Staaterman E., Ault J. S., Sasso C. R., et al. (2021). Space use patterns of sharks in relation to boat activity in an urbanized coastal waterway. Mar. Environ. Res. 172, 105489. doi: 10.1016/j.marenvres.2021.105489
Rocklin D., Tomasini J.-A., Culioli J.-M., Pelletier D., and Mouillot D. (2011). Spearfishing regulation benefits artisanal fisheries: the reGS indicator and its application to a multiple-use mediterranean marine protected area. PLoS One 6, e23820. doi: 10.1371/journal.pone.0023820
Rowat D., Womersley F. C., Norman B. M., and Pierce S. J. (2021). “Global threats to whale sharks,” in Whale Shark Biology, Ecology, and Conservation, United Kingdom: CRC Press, Oxfordshire 239–265.
Sawyna J. M., Spivia W. R., Radecki K., Fraser D. A., and Lowe C. G. (2017). Association between chronic organochlorine exposure and immunotoxicity in the round stingray (Urobatis halleri). Environ. pollut. 223, 42–50. doi: 10.1016/j.envpol.2016.12.019
Sbragaglia V., Cecapolli E., Morroni L., and Januchowski-Hartley F. A. (2024). Advancing the understanding of spearfisher-fish behavioural interactions and its management implications. J. Appl. Ecol. 61, 2594–2604. doi: 10.1111/1365-2664.14796
Scannella D., Vitale S., Di Maio F., Serena F., Zava B., Colloca F., et al. (2020). A new record of a great white shark, Carcharodon carcharias(Chondrichthyes: Lamnidae) in the Strait of Sicily, Central Mediterranean Sea. Acta Adriat. (Online) 61, 231–238. doi: 10.32582/aa.61.2.13
Schembri T., Fergusson I. K., and Schembri P. J. (2003). Revison of the records of sharks and ray species from the Maltese Islands (Chordata: Chondrichthyes). Cent. Mediterr. Nat. 4, 71–104.
Scott M., Campbell A., Scheeler L., Verbeck D., Marcoux S., Marcoux T., et al. (2025). Using photo identification to assess demographics and fishery interactions of oceanic whitetip sharks (Carcharhinus longimanus) in the main Hawaiian Islands. Mar. Ecol. Prog. Ser. 759:51–69. doi: 10.3354/meps14835
Seck A., Diatta Y., Gueye-Ndiaye A., and Capapé C. (2002). Observations on the reproductive biology of the Bull Ray, Pteromylaeus bovinus (E. GEOFFROY SAINT-HILAIRE 1817) (Chondrichthyes: Myliobatidae) from the coast of Senegal (Eastern tropical Atlantic). (Croatia: Acta Adriatica, Split)
Serena F., Abella A. J., Bargnesi F., Barone M., Colloca F., Ferretti F., et al. (2020). Species diversity, taxonomy and distribution of Chondrichthyes in the Mediterranean and Black Sea. Eur. Zoological J. 87, 497–536. doi: 10.1080/24750263.2020.1805518
Shono H. (2008). Application of the Tweedie distribution to zero-catch data in CPUE analysis. Fisheries Res. 93, 154–162. doi: 10.1016/j.fishres.2008.03.006
Silva F. G. and Vianna M. (2018). Diet and reproductive aspects of the endangered butterfly ray Gymnura altavela raising the discussion of a possible nursery area in a highly impacted environment. Braz. J. oceanogr. 66, 315–324. doi: 10.1590/s1679-8759201801906603
Simpfendorfer C. A. and Milward N. E. (1993). Utilisation of a tropical bay as a nursery area by sharks of the families Carcharhinidae and Sphyrnidae. Environ. Biol. Fish 37, 337–345. doi: 10.1007/BF00005200
Solleliet-Ferreira S., Fontes J., Boys R. M., Das D., Rodrigues N. V., and Afonso P. (2018). Photo-identification of Aetomylaeus bovinus (Geoffroy St. Hilaire 1817). The forgotten giants of the shallow. S. Solleliet-Ferreira et al, (2018) EEA conference, Unpublished. doi: 10.13140/RG.2.2.24662.91204
Speed C. W., Meekan M. G., and Bradshaw C. J. (2007). Spot the match – wildlife photo-identification using information theory. Front. Zool 4, 2. doi: 10.1186/1742-9994-4-2
Stevens J., Bonfil R., Dulvy N. K., and Walker P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494. doi: 10.1006/jmsc.2000.0724
Tanti G. and Deidun A. (2014). Assessing The Socio-Economic Impact Of Jellyfish Blooms On Tourists At Various Beaches/Coastal Locations Around The Maltese Islands (Msida, Malta: IOI-MOC, University of Malta).
Times of Malta (2011). Young blue shark caught off Salina (Birkakara, Malta: Times of Malta). Available online at: https://timesofmalta.com/article/Young-blue-shark-caught-off-Salina.384165.
Times of Malta (2014). ‘Children now using spearguns’ after licence withdrawn (Birkakara, Malta: Times of Malta). Available online at: https://timesofmalta.com/article/-Children-now-using-spearguns-after-licence-withdrawn.531969.
Times of Malta (2024a). Netted - Spear fishermen caught by Rangers and Fisheries Department officers (Birkakara, Malta: Times of Malta). Available online at: https://timesofmalta.com/article/netted-spear-fishermen-caught-rangers-fisheries-department-offcers.1094023.
Times of Malta (2024b). Spearfishermen attempt cliffside escape after illegal night fishing (Birkakara, Malta: Times of Malta). Available online at: https://timesofmalta.com/article/spearfishermen-attempt-cliffside-escape-illegal-night-fishing.1096027.
Times of Malta (2025). Six potential sites identified for land reclamation projects (Birkakara, Malta: Times of Malta). Available online at: https://timesofmalta.com/article/six-potential-sites-identified-land-reclamation-projects.1109505.
Tiralongo F., Lombardo B. M., Messina G., Tibullo D., and Barría C. (2023). Editorial: Elasmobranch - fisheries interactions in the Mediterranean. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1341136
Town C., Marshall A., and Sethasathien N. (2013). Manta Matcher: automated photographic identification of manta rays using keypoint features. Ecol. Evol. 3, 1902–1914. doi: 10.1002/ece3.587
Tuya F., Asensio M., and Navarro A. (2020). Urbanite” rays and sharks: Presence, habitat use and population structure in an urban semi-enclosed lagoon. Regional Stud. Mar. Sci. 37, 101342. doi: 10.1016/j.rsma.2020.101342
Tyson Moore R. B., Urian K. W., Allen J. B., Cush C., Parham J. R., Blount D., et al. (2022). Rise of the machines: best practices and experimental evaluation of computer-assisted dorsal fin image matching systems for bottlenose dolphins. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.849813
UNEP/MAP (2024). Amendments to Annexes II and III to the Protocol concerning Specially Protected Areas and Biological Diversity in the Mediterranean (Athens, Greece: UNEP/MAP–Barcelona Convention). Available online at: https://spa-rac.org/en/decision/download/1680/amendments-to-annexes-ii-and-iii-to-the-protocol-concerning-specially-protected-areas-and-biological-diversity-in-the-mediterranean.
UNEP/MAP-SPA/RAC (2019). Mapping of marine key habitats and assessing their vulnerability to fishing activities in Malta: available knowledge and gap analysis (Tunis Cedex, Tunisia: UNEP/MAP-SPA/RAC). Available online at: https://www.rac-spa.org/sites/default/files/doc_medkey2/mpantz_malta_en.pdf.
Vossgaetter L., Dudeck T., Crouch J., Cope M., Ivanova T., Siyan I., et al. (2024). Non-invasive methods characterise the world’s largest tiger shark aggregation in Fuvahmulah, Maldives. Sci. Rep. 14, 21998. doi: 10.1038/s41598-024-73079-3
Ward-Paige C. A. and Lotze H. K. (2011). Assessing the value of recreational divers for censusing elasmobranchs. PLoS One 6, e25609. doi: 10.1371/journal.pone.0025609
Weinman S. (2023). Malta: Speardiver nabbed + authorities slammed for scuba inertia (London, United Kingdom: Divernet). Available online at: https://divernet.com/scuba-news/conservation/malta-speardiver-nabbed-authorities-slammed-for-scuba-inertia/.
Wickham H. (2016). ggplot2: Elegant Graphics for Data Analysis. (Vienna, Austria). Available online at: https://ggplot2.tidyverse.org. (Accessed May 27, 2025).
Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., et al. (2019). Welcome to the tidyverse. JOSS 4, 1686. doi: 10.21105/joss.01686
Wickham H., François R., Henry L., Müller K., and Vaughan D. (2023). dplyr: A Grammar of Data Manipulation. doi: 10.32614/CRAN.package.dplyr
WWF (2021). “The climate change effect in the Mediterranean,” in Six stories from an overheating sea (World Wildlife Fund (WWF, Rome, Italy).
Yan H. F., Kyne P. M., Jabado R. W., Leeney R. H., Davidson L. N. K., Derrick D. H., et al. (2021). Overfishing and habitat loss drive range contraction of iconic marine fishes to near extinction. Sci. Adv. 7, eabb6026. doi: 10.1126/sciadv.abb6026
Keywords: Aetomylaeus bovinus, bull ray, batoids, Mediterranean, Malta, photoidentification, nursery areas
Citation: Matthews C, Caruso C, Kell C, Babbs S, Parreira do Amaral T, Ducker B, Nowell G and Solleliet-Ferreira S (2025) “Nursery bays and hidden rays”: First insights into long-term monitoring of Bull Rays (Aetomylaeus bovinus) within the Maltese Archipelago. Front. Mar. Sci. 12:1653284. doi: 10.3389/fmars.2025.1653284
Received: 24 June 2025; Accepted: 02 September 2025;
Published: 23 September 2025.
Edited by:
Todd Atwood, U.S. Geological Survey, United StatesReviewed by:
Fernando Tuya, University of Las Palmas de Gran Canaria, SpainPeter Gausmann, Ruhr University Bochum, Germany
Copyright © 2025 Matthews, Caruso, Kell, Babbs, Parreira do Amaral, Ducker, Nowell and Solleliet-Ferreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlie Matthews, bWF0dGhld3MuY2hhcmxlcy5jYUBnbWFpbC5jb20=
 Charlie Matthews
Charlie Matthews Christian Caruso
Christian Caruso Charlotte Kell
Charlotte Kell Sophie Babbs
Sophie Babbs Thaís Parreira do Amaral
Thaís Parreira do Amaral Beth Ducker
Beth Ducker Greg Nowell1
Greg Nowell1