Abstract
The discovery of Physalia mikazuki sp. nov. from the temperate waters of Gamo Beach, Sendai Bay (Miyagi Prefecture) in the Tohoku (northeast) region of Japan, represents a significant addition to the taxonomic and ecological understanding of this genus. Morphological analysis reveals key diagnostic traits, distinguishing it from all known Physalia species. Phylogenetic analyses of the 16S rRNA gene and COI (cytochrome c oxidase subunit 1) regions further confirm its classification as a distinct species, forming a well-supported monophyletic clade separate from other Physalia species. Oceanographic data and Lagrangian particle trajectory simulations suggest that P. mikazuki may have dispersed northward via the recent 100 km northward extension of the Kuroshio Current (KE) in tandem with record-breaking sea surface temperature changes (SST) of more than 2°C in the Tohoku region between 2022 and 2024. Long-term monitoring confirmed no previous reports of Physalia at the type locality of Gamo Beach, Sendai City (Tohoku) prior to 2023, indicating a likely recent introduction. Molecular barcode sequences matching samples from both Pakistan and Mexico indicate a broad Indo-Pacific connectivity for the new species. The occurrence of P. mikazuki sp. nov. in the Tohoku region poses potential ecological and public health concerns, particularly due to its predation on fish larvae and risk of envenomation during beach recreation. This study underscores the importance of integrative experimental design combining taxonomy, molecular data, and oceanographic modeling to understand species range shifts and cryptic diversity in a changing ocean.
1 Introduction
The genus Physalia, commonly known as the Portuguese man-of-war, is a neustonic organism that has evolved innovative gas-filled structures allowing it to float freely at the ocean’s surface (Anthony et al., 2024). Unlike most gelatinous zooplankton, Physalia is among a few jellyfish taxa whose habitat and trophodynamics are primarily associated with the air-water interface (Prieto et al., 2015; Martins, 2022; Anthony et al., 2024). Its gas-filled pneumatophore enables passive drifting with wind and surface currents, resulting in broad distribution patterns that are generally shaped by regional oceanography (Church et al., 2025). However, fine-scale drift trajectories can vary depending on sail orientation, colony morphology, and local wind conditions (Bourg et al., 2024). Historically, putative species of Physalia were illustrated or described from aggregations of blue-tinged floats stranded along beaches around the globe from the Northern to Southern hemispheres (Duperrey, 1830). Recent studies suggest that variability in oceanic and coastal currents, influenced by changing wind and temperature patterns, can result in aggregations of jellyfishes, including Physalia, along coastlines where they have not been commonly reported (Purcell, 2005; Prieto et al., 2015; Bourg et al., 2022; Torres-Conde and Rodríguez-Martínez, 2024).
Physalia is the sole genus in the family Physaliidae of the Siphonophorae suborder Cystonectae (subphylum Medusozoa; class Hydrozoa) (Bardi and Marques, 2007). Being colonial hydroids, each Physalia colony consists of multiple highly specialized individuals, or zooids, that together function as one organism. These zooids are connected along a central stem beneath a gas-filled float called the pneumatophore (Totton and Mackie, 1960; Munro et al., 2019). Within each colony, gastrozooids facilitate feeding, tentacular palpons (tentacle-bearing polyp, specialized in nematocyst production) provide defense and capture prey, gonodendra (compound reproductive structure) manage reproduction, and the single pneumatophore acts as a buoyancy mechanism that allows the organism to float at the surface. This functional specialization of zooids within the Physalia colony supports the colony’s survival at the water surface, where it passively drifts with wind and current (Mapstone, 2014; Munro et al., 2018; Munro et al., 2019). The tentacles of Physalia aid in the capture of prey such as tiny fish and zooplankton (Purcell, 1984; Purcell and Anderson, 1995) but they also pose an incidental envenomation risk to humans upon contact (Burnett et al., 1994; Fenner, 1998; Cegolon et al., 2013). Historic records describe Physalia as widely distributed across both southern and northern oceanic latitudes, floating on calm seas during sunny days, or stranded on beaches following persistent winds and surface currents (Duperrey, 1830). Incidents of Physalia stings have been reported worldwide, resulting in several hospitalizations and three fatal cases apparently from P. physalis (Linnaeus, 1758) raising considerable public health concerns (Stein et al., 1989; Burnett et al., 1994; Purcell and Anderson, 1995; Cegolon et al., 2013; Kajfasz, 2015; Guevara et al., 2017; Mulyadi and Sianturi, 2021). Understanding the mode of distribution of these colonies, which can occur in significant numbers, is crucial for safeguarding beachgoers, fishers and marine enthusiasts.
The original species assigned to the genus PhysaliaLamarck, 1801 was P. physalis (Linnaeus, 1758) while the most recently described species is P. minuta (Church et al., 2025). The taxonomy of this genus has a long and complicated history, beginning with its original designation as Holothuria physalis by Linnaeus in 1758 (Lamarck, 1801; Totton and Mackie, 1960). Over the past two centuries, numerous species names have been proposed, and many later synonymized, due to inconsistent morphological interpretations, the lack of type material, and the cryptic nature of variation within the genus. Although P. physalis was historically treated as the only valid species, recent genomic studies have revealed the existence of multiple genetically distinct lineages across global ocean basins, supporting the hypothesis of hidden species diversity (Mapstone, 2014; Pugh, 2019; Church et al., 2025). The absence of designated type specimens and precise locality data for P. physalis and other early Physalia taxa further complicates efforts to resolve species boundaries (Schneider, 1898). In the early 19th century, Duperrey (1930) provided a comparative description of what he believed to be five distinct Physalia species based on having witnessed thousands of individuals in their respective geographic locations and commissioning detailed illustrations by Rénaud. He stated that P. physalis (as Physalia atlantica) is the most distinct and easiest to identify (Figure 1) (Duperrey, 1830) (pp. 36-43). Additionally, local vernaculars such as galères (French), moucieu (Brazilian Portuguese), and man-of-war (English) reflect the widespread familiarity with Physalia appearances across geographic regions. Similarly, Sloane referred to the organism as carvell, drawing on terminology from wind-sailing ships (Sloane, 1707; Duperrey, 1830). Despite broad acceptance of P. physalis as a globally distributed species, divergent interpretations persist, particularly in the Pacific. In Australia and New Zealand, stranded colonies are commonly referred to as Physalia utriculus (Gmelin, 1788) or “bluebottles” (Yanagihara et al., 2002; Pontin and Cruickshank, 2012; Bourg et al., 2022), though a formal species designation is lacking. Literature sources from Japan, such as Kawamura (1910) and Moser (1925) reported Physalia in the Kanto area as katsuo no eboshi; also referenced in Pugh (2019) (Pugh, 2019) who notes that both authors concluded that only P. physalis occurs in the Japanese Pacific waters and any apparent morphological similarity with P. utriculus was due to developmental variability. Recently, molecular phylogenetic studies have identified distinct genetic lineages in the southwest Pacific, including around New Zealand, suggesting the existence of multiple undescribed Physalia species (Pontin, 2009).
Figure 1
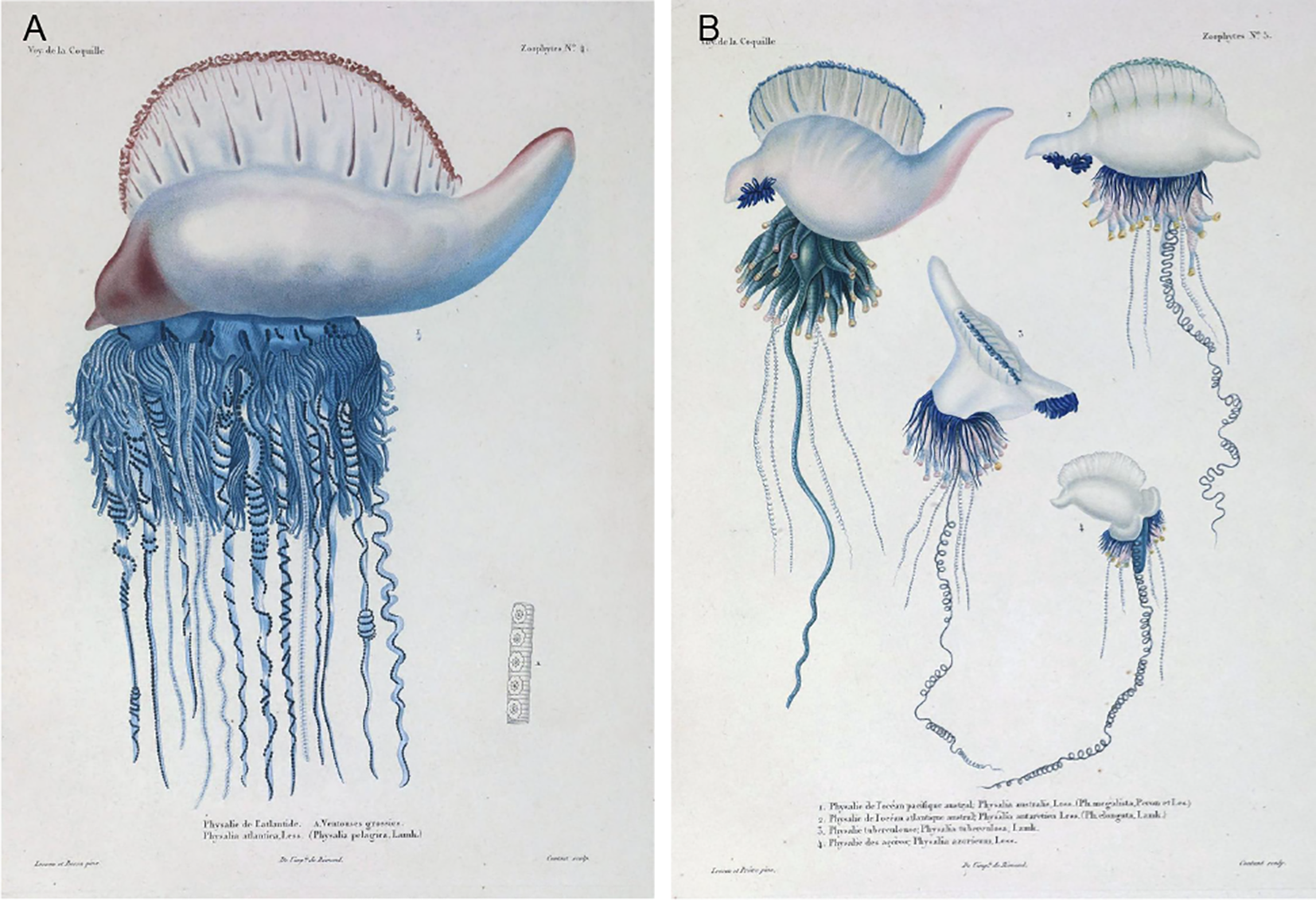
Plates reproduced from Duperrey (1830) in the “Zoophytes” section of the French-language manuscript written by Lesson, depicting five named Physalia species at the time of its publication; illustrations commissioned by Renaud. (A) Zoophytes Plate 4. Physalia atlantica, referred to as “Physalie de l’Atlantique” (Physalia of the Atlantic; synonymized as Physalia pelagicaLamarck, 1801, originally Holothuria physalisLinnaeus, 1758, later renamed Physalia physalis by Schneider in 1898 (Schneider, 1898)). Reported from the tropical North Atlantic Ocean (7°N, 23°W) (Duperrey, 1830). Insert “A” shows “Ventouse grossies” or “enlarged suckers”. Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): Zoophytes Plate 4, page 36: https://www.biodiversitylibrary.org/item/119446#page/71/mode/1up Published in France. All figures are in the public domain. (B) Zoophytes Plate 5 illustrates (Anthony et al., 2024): P. australis, referred to as “Physalie de l’océan Pacifique” (Physalia of the Pacific Ocean; synonymized as P. megalista Péron & Lesueur, in (Delandmeter and Van Sebille, 2019; Kehl et al., 2023; Church et al., 2025)). Reported from the South Pacific Ocean, specifically at the entrance of Port Jackson Bay, New South Wales, Australia (Duperrey, 1830) (Martins, 2022). P. antarctica, referred to as “Physalie de l’océan Atlantique austral” (Physalia of the southern Atlantic Ocean; synonymized as P. elongata (Duperrey, 1830)). Reported from the South Atlantic Ocean (1°S, 25°W) (Duperrey, 1830) (Church et al., 2025). P. tuberculosaLamarck, 1801, referred to as “Physalie tuberculeuse”. Reported from the South Atlantic Ocean (7°S, along the coast of the Americas), potentially a variant of P. antarctica (Duperrey, 1830) (Bourg et al., 2024). P. azoricum, referred to as “Physalie des Açores – de l’hémisphère boréal”. Reported near the Canary Islands and Azores in the North Atlantic Ocean (26°N, 20°W) (Duperrey, 1830). Information translated from pages 351–381 of the original (Duperrey, 1830). Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): Zoophytes Plate 5 38-41: https://www.biodiversitylibrary.org/item/119446#page/73/mode/1up Published in France. All figures are in the public domain.
A recent multi-genomic and population structure analysis using high-quality SNPs of 141 mitochondrial genomes of Physalia specimens collected globally revealed five well-supported reciprocally monophyletic lineages almost entirely lacking mixture (albeit a minor proportion of mixing documented between C1 and C2) (Church et al., 2024). The findings of the study revealed that genomic differentiation between subpopulations was generally small (based on average reciprocal fixation index (FST)) and equivalent for geographically overlapping lineages, and that the presence of regionally endemic subpopulations as well as long-distance dispersal events was indicative of the effects of both local and major ocean currents and wind patterns (Church et al., 2024; Church et al., 2025), reported that all five lineages were sufficiently supported in additional PCA (principal component analysis) and Iso-Seq transcriptome reference mapping conducted with the high-quality datasets (Church et al., 2024). Of the five lineages to which were assigned alpha-numeric clusters (Church et al., 2024), three were in common use in the literature as P. physalis (A), P. utriculus (B1), and P. megalista (C1) though lacking type vouchers, a fourth (cluster C2) was subsequently described as a new species P. minuta Church & Dunn 2025 (Church et al., 2025), and a fifth lineage was tentatively left as “cluster B2” due to limited molecular samples and absence of photo or morphological voucher for the sequenced specimens (Church et al., 2024; Church et al., 2025). The formal description of P. minuta by and identification of four other molecular lineages (Church et al., 2025), underscores the need for continued integrative efforts to stabilize species names in common usage that lack proper descriptions to properly characterize both the genetic and morphological diversity of the genus.
This study reports the discovery of Physalia mikazuki sp. nov. from the Tohoku (northeast) region of Japan, at Gamo Beach in Sendai Bay, Miyagi Prefecture. Analyses of multiple specimens reveal that P. mikazuki sp. nov. is cluster B2 (sensu (Church et al., 2024; Church et al., 2025) and distinguishable as a new species based on unique morphological features, findings of multi-genome and structural analyses (see (Church et al., 2024; Church et al., 2025), and molecular phylogenetic analyses of additional samples using two mitochondrial gene markers. This represents the first formal description of a Physalia species from Japan and the first recorded occurrence of Physalia in the Tohoku region, 2000 km north of Okinawa (Fenner, 1998) and 300 km north of Kanagawa Bay where previous reports of Physalia have long occurred (Oguchi et al., 2024).
To clarify its taxonomic status and explore the potential environmental drivers of the apparently recent introduction, we conducted an integrative study combining morphological and molecular analyses with an oceanographic modeling approach. Our analysis included Physalia specimens collected from Okinawa, which we showed is P. utriculus based on common usage of this name in the literature and results of phylogenetic analyses conducted herein, and in the literature (Church et al., 2024; Church et al., 2025). This multidisciplinary approach contributes to broader efforts to document marine biodiversity and assess biogeographic significance to understand species distributions under rapidly changing oceanographic conditions, particularly in the Tohoku region.
2 Materials and methods
2.1 Sample collection and morphological study
Approximately 30 Physalia floats bearing zooid colonies were discovered stranded along a 1.5 km span of Gamo Beach with coordinates 38.259110° N, 141.018898° E, near the mouth of Nanakita River, Sendai Bay, Sendai City, Miyagi Prefecture, Japan on 11 and 12 June 2024 (Figures 2A–J). Of these, six Physalia specimens were collected, and placed into a 2 L PET bottle (smaller individuals) and a wide-mouth container (larger individuals) filled with seawater and then transported to the Graduate School of Agricultural Science, Tohoku University (Aobayama Campus) for further observation and analyses. Approximately 1 cm of tissue was excised from each colony and placed in separate 1.5 mL tubes containing 99.5% ethanol for molecular analysis. Extensive photo-documentation (iPhone and Huawei P30) was conducted on live specimens that were then fixed in 10% buffered formalin solution for morphological observations using a dissecting microscope (Nikon SMZ745T). Holotype and paratype specimens were deposited into the Tohoku University Museum (Accession numbers 112960–112965 respectively). Two additional Physalia colonies collected from Okinawa Prefecture on 28 January 2025 (Figure 2N), were included for molecular and morphological comparison. Specimen vouchers were also deposited in the Tohoku University Museum as P. utriculus (Accession numbers 112966 and 112967).
Figure 2
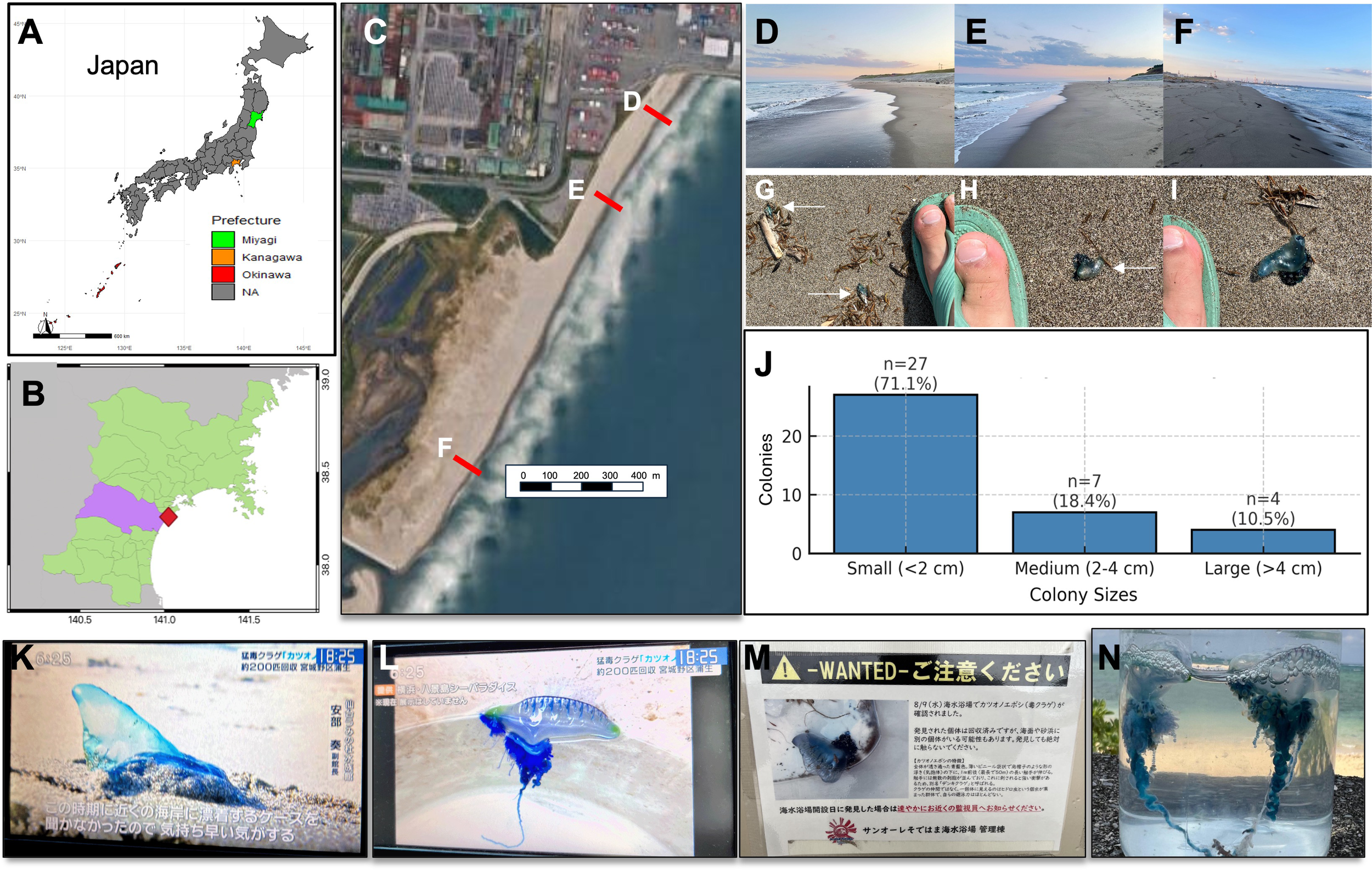
Sightings and public reports of Physalia spp. in Japan. (A) Map of Japan showing the location of Miyagi Prefecture (highlighted in green); (B) Map of Miyagi Prefecture with Sendai Bay marked in red (lavender fill indicates prefecture boundaries) where Physalia mikazuki sp. nov. individuals were collected at Gamo Beach; (C) Satellite image of Gamo Beach, Sendai Bay, illustrating the stretch of sandy coastline between the Nanakita River (north) and the Sendai Minami Breakwater (south), where P. mikazuki sp. nov. individuals were found stranded on Gamo Beach (sampling points marked (D–F). Maps generated using QGIS-LTR Prizren version 3.34.; (D–F) Photos of Gamo Beach at each sampling point for P. mikazuki sp. nov. on June 11, 2024 (12:30–13:00) at the south end of the beach, and on June 12, 2024 (13:00–14:30) along the entire beach; (D) The north end of Gamo Beach with some large debris; (E) The central portion of Gamo Beach near a popular surf zone with some debris; (F) The south end with minimal debris; (G–I) Photographs of P. mikazuki sp. nov. stranded along the beach (arrows), including size comparison with foot for scale; (J) A bar graph of size distribution of collected colonies (n = 38), grouped arbitrarily into three size categories: small (<2 cm), medium (2–4 cm), and large (>4 cm). (K) Tohoku regional evening news story aired on the television 14 June 2024, headline: “Venomous man-of-war jellyfish, about 200 individuals collected from Gamo, Miyagino-ku Ward [Sendai City]” the type locality of Physalia mikazuki sp. nov. The screenshot shows a stranded specimen with a prominent sail-like float. The on-screen caption quotes Mr. Abe, Deputy Director of Sendai Umi no Mori Aquarium: “During this season I have never heard of them being stranded on a beach so close by ….”; (L) During the same television story as (K) a different Physalia individual is shown on display at Hakkeijima Sea Paradise, Yokohama (Kanto region) that is morphologically distinct from P. mikazuki sp. nov. and likely corresponds to P. utriculus; (M) A sign posted at Sun Ole Recreation Beach, headline : “Wanted – Please be on the alert! … man-of-war jellyfish confirmed on 9 Aug 2024.” in Shizugawa Bay, Minamisanriku, more than 90 km north of Gamo Beach ; (N) A specimen collected in this study from Okinawa in January 2025, differing from P. mikazuki sp. nov. by having only one prominent primary tentacle, a distinct cormidia arrangement, and unique gastrozooid morphology.
Shortly after these Physalia collections efforts at Gamo Beach, Sendai City, on 14 June 2024 a Tohoku regional television station aired warnings about unprecedented mass strandings of Physalia along Gamo Beach, Sendai Bay (Figure 2K); the broadcast also discussed Physalia sightings in the Kanto region, whose morphology on the television screen suggests it is P. utriculus (Figure 2L). In the same summer, signs were posted at San Ole Beach in Shizugawa Bay, Minamisanriku, more than 90 km north of Gamo Beach, alerting bathers of “dangerous man-of-war jellyfish sightings” there on 9 Aug 2024 (Figure 2M).
In this study, morphological examination was conducted of specimens collected from Tohoku and Okinawa, Japan. Morphometric parameters of the different zooids and general aspects of the colony were described with terminology used by (Totton and Mackie, 1960) (Bardi and Marques, 2007), and (Munro et al., 2019) to refer to P. physalis from the Atlantic Ocean. Additional systematic morphometric analysis of the Tohoku specimens included: float length and width; number of wrinkles on the crest of the pneumatophore; tentacle length; gastrozooid and tentacular palpon length and diameter; gonopalpon length; and gonophores, nectophores, and gonodendron diameters. Terminology follows current standards, with “posterior zone” and “principal tentacle” used in place of outdated terms such as “oral zone” and “primary tentacle” sensu (Munro et al., 2019). Measurements were taken directly using a DN150 SCITOOLS digital caliper (resolution 0.1 mm/0.01 inch, accuracy ±0.2 mm/0.01 inch), and from high-resolution images using ImageJ v1.54f (NIH, USA). The cnidome (repertoire of nematocyst types) was described according to Weill’s (1934) classification system (Weill, 1934) and subsequent modifications by (Östman, 2000). A comparative analysis of key character traits among the two species collected from Japan showed the Okinawa colonies mainly differ from P. mikazuki sp. nov. in having a single prominent principal tentacle, a distinct arrangement of the zooid cluster (formerly termed “cormidia”), and differences in gastrozooid morphology (Figures 2N, 3), discussed further in Section 3.
Figure 3
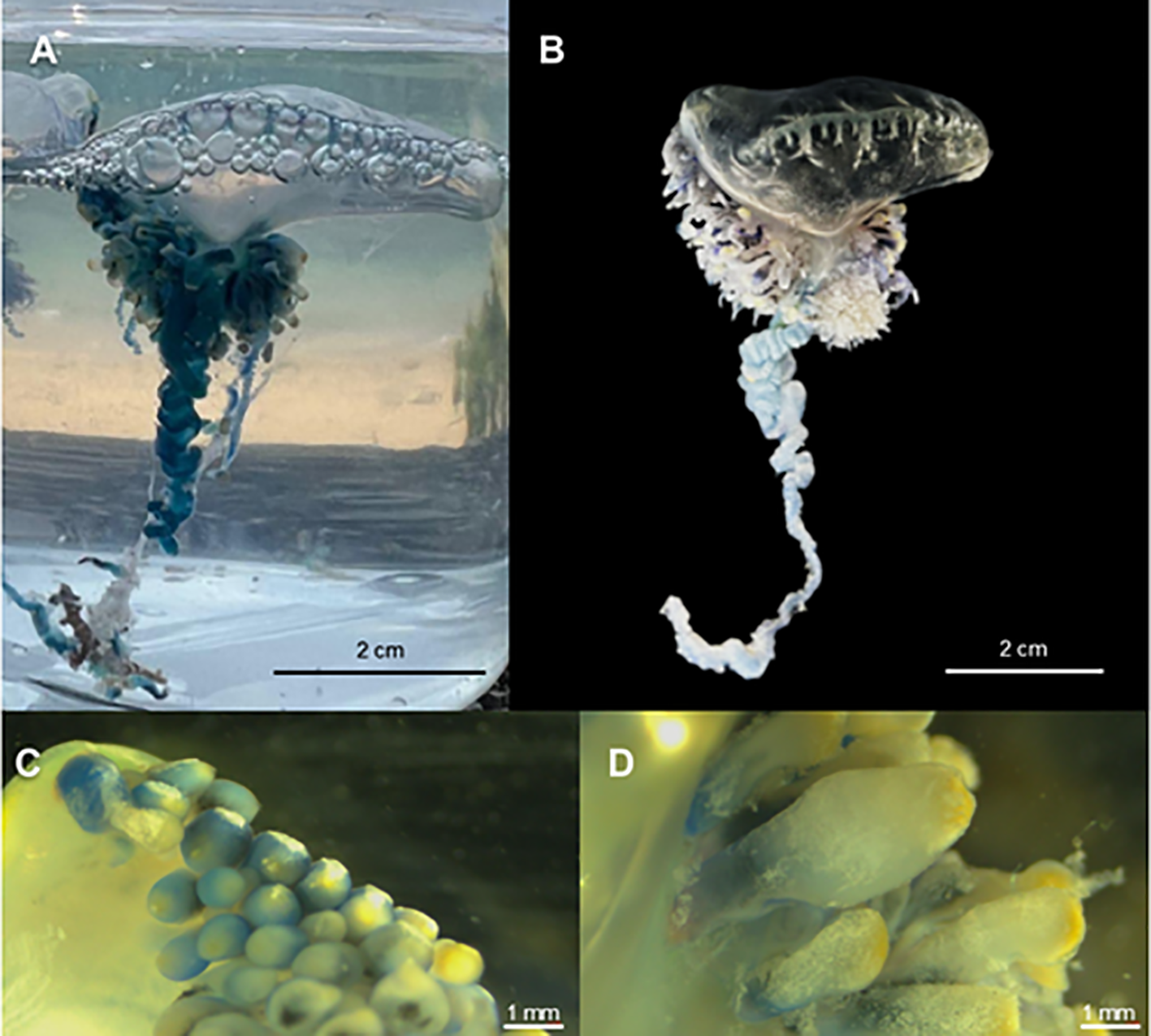
Physalia utriculus specimen from Okinawa, Japan. (A) Live individual photographed in seawater shortly after collection. (B) Preserved colony following fixation in 10% buffered formalin, showing contracted pneumatophore and shortened tentacles. (C) Gastrozooid buds from the posterior zone with bulbous morphology and yellow apex. (D) Main zone gastrozooids showing flask-shaped structure with thick texture and diffused yellow pigmentation.
2.2 DNA extraction and phylogenetic analysis
DNA was extracted from the ethanol-preserved secondary tentacle tissue subsampled from Physalia individuals (n=4) using the QIAGEN DNeasy Blood and Tissue Kit following the developer’s protocol. Two mitochondrial gene regions, a ~550 bp fragment of the 16S ribosomal RNA gene (16S rRNA) and a ~800 bp fragment of the cytochrome c oxidase subunit I (COI) fragment were targeted for amplification via polymerase chain reaction. The 16S rRNA region was amplified using the primers med-rnl-F 5’- GACTGTTTACCAAAGACATAGC-3’ and med-rnl-R 5’-AAGATAGAAACCTTCC TGTC-3’ (Lawley et al., 2016), while the COI region was amplified using the primers Jellyfish_CO1_F 5’-KKTCACAAAYCATAAAGATATWGG -3’ and Jellyfish_CO1_R2 5’-GGAACTGCTATWATCATWGTWGC-3’ (Minamoto et al., 2017). PCR protocol for 16S were: initial denaturation (94°C for 3 mins), then 38 cycles of amplification comprising denaturation (94°C for 30s), annealing (54°C for 30s), extension (72°C for 45s), and final extension (72°C for 7 mins); in COI amplification protocol was modified during initial denaturation (95°C for 5 mins), denaturation (96°C for 30s), annealing (50°C for 40s), and extension (72°C for 55s).
The PCR product was visualized on 1.5% agarose gel and used for Sanger sequencing at Onagawa Field Centre (Graduate School of Agricultural Science, Tohoku University) following the BigDye™ Terminator v3.1 Cycle Sequencing Kit protocols (Applied Biosystems, Thermofisher Scientific, Japan). Samples were cleaned using Agencourt AMPure XP beads (Beckman Coulter, Inc., CA, USA) prior to sequencing. Resulting nucleotide sequences were checked and edited in Geneious Prime (ver. 2025.1, https://www.geneious.com), and consensus sequences (n=6) were subsequently generated from forward and reverse strands.
All available 16S and COI sequences for Physalia spp., including two outgroup species from the sister family Rhizophysidae (order Siphonophorae, suborder Cystonectae), were retrieved from NCBI GenBank (accessed on 13 March 2025). Additionally, as none of the sequences generated by Church et al., 2025, for their 16S or COI phylogenetic analyses was accessioned into GenBank we obtained alignments for 16S (https://github.com/shchurch/Physalia_population_genomics/blob/main/results/iqtree/alignments/16S.aln.fasta) and COI (https://github.com/shchurch/Physalia_population_genomics/blob/main/results/iqtree/alignments/CO1.aln.fasta)for multiple Physalia samples (from the Pacific, Atlantic, and Indian Oceans) from the GitHub link referenced in the supplementary material. In total, 129 sequences were analyzed for 16S and 198 for COI. Accession numbers, sample identifiers, and localities are listed in Supplementary Table S1. All sequences were aligned using the G-INS-I algorithm in MAFFT (ver. 7.520, https://mafft.cbrc.jp/alignment/software/) (Katoh and Standley, 2013). Maximum likelihood (ML) phylogenetic trees were reconstructed using IQ-TREE v2.2.2 (Trifinopoulos et al., 2016). The best-fit substitution models were selected using the lowest Akaike Information Criterion (AIC) scores via the integrated model selection function in IQ-TREE (16S: TIM3+F+G4; COI: GTR+F+G4+I). Node support was evaluated using the Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT), and the approximate Bayes test (aBayes) (Anisimova et al., 2011) and 1000 ultrafast bootstrap replicates (UFBoot2) (Hoang et al., 2018). To compare and validate tree topology, additional ML analyses were performed in MEGA version 12.0 using the GTR+G4 model (Kumar et al., 2024). Final trees were visualized and annotated using FigTree v1.4.4 (Rambaut, 2009), Interactive Tree of Life (iTOL) (Letunic and Bork, 2024) and Inkscape software (Inkscape, 2024).
2.3 Environmental data and processing
The sampling location map for Physalia individuals (Figures 2A–C) was generated using QGIS-LTR Prizren version 3.34, a robust, free, and open-source geographic information system (GIS) software widely utilized for geospatial data analysis and mapping (all software documentation available at https://qgis.org/). City-level administrative boundary data for Japan was sourced from the Global Administrative Areas (GADM) database accessible at https://gadm.org/download_country.html (accessed on 12 September 2024). To enhance the map’s contextual accuracy and provide a realistic background for the sampling locations, a hybrid map layer from Google Maps was imported as XYZ tiles. This was achieved through the QGIS “Add Layer” plugin, which facilitated seamless integration of satellite imagery and map layers, thereby offering an intuitive and spatially relevant overview of the study area.
The environmental indicators for modeling, including daily surface current and temperature data were obtained from HYCOM (Hybrid Coordinate Ocean Model), specifically the GLBy0.08 dataset, which provides global ocean data at a 1/12° spatial resolution in NetCDF format. This study focused on the coastal area encompassing Sagami Bay to the Pacific coastal area of the Tohoku region (34° – 42° N and 138° – 146° E), and particularly around the Sendai Bay area (37.4° – 38.6° N and 140.6° – 142.2° E). To examine recent marine environmental trends, oceanographic data from 1 January 2018, to 31 August 2024, were obtained from the HYCOM Data Server (https://www.hycom.org/dataserver; accessed on 3 September 2024) and, subsequently, processed using the Python programming language. The details for each indicator are as follows:
2.3.1 Surface current
Surface current data is available for both Eastward Water Velocity (water_u) and Northward Water Velocity (water_v), measured in meters per second (m/s). The current intensity and direction were calculated using the following formula:
To analyze the temporal and spatial variations in surface currents between 2018 and 2024, the daily data were aggregated into monthly averages. Subsequently, annual averages were calculated for each year to capture the broader trends in surface current behavior. As a possible proxy for tracking Physalia mikazuki sp. nov. meandering along the northeast coast of Japan, the daily surface current data served as input for OceanParcels (Probably A Really Computationally Efficient Lagrangian Simulator) (Delandmeter and Van Sebille, 2019; Kehl et al., 2023), a Python package used to simulate particle tracking. This allowed for the modeling of P. mikazuki’s transtemporal floating, providing insight into potential trajectories of colonies based on ocean current dynamics.
2.3.2 Surface temperature
The monthly averages of surface temperature data (SST°C) calculated from daily observations were then used to calculate annual averages to ascertain the general temporal and spatial variation in surface temperature spanning 2018 to 2024.
3 Results
3.1 Systematic account
Phylum Cnidaria Verrill, 1865
Subphylum Medusozoa Peterson, 1979
Class Hydrozoa Owen, 1843
Subclass Hydroidolina Collins, 2000
Order Siphonophorae Eschscholtz, 1829
Family Physaliidae Brandt, 1835
Genus PhysaliaLamarck, 1801
Physalia mikazuki sp. nov. Yongstar, Ochiai & Lewis Ames (Figures 4–6)
Figure 4
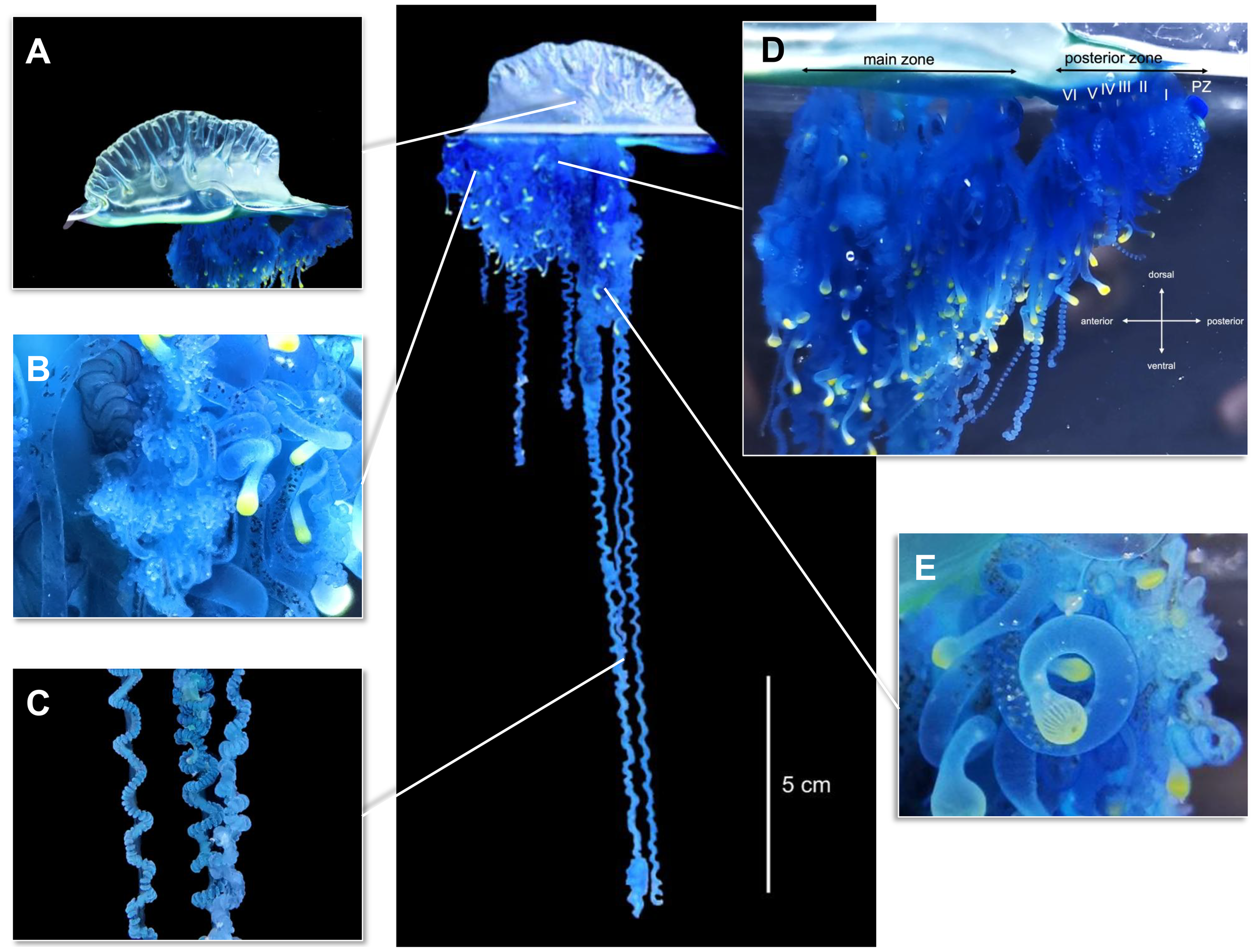
Morphological characteristics of Physalia mikazuki sp. nov. collected from Gamo Beach, Sendai City, Miyagi Prefecture, Japan. Type 112960 (Tohoku University Museum).; Central image: Entire colony displaying the gas-filled pneumatophore and trailing tentacles (scale bar = 5 cm).; (A) Lateral view of the pneumatophore with a well-defined wrinkled crest and transparent, sail-shaped float.; (B) Close-up of the dorsal surface beneath the pneumatophore, highlighting clusters of gonodendra, tentacular palpons, and gastrozooids with yellow-tipped oral regions.; (C) Multiple principal tentacles exhibiting characteristic coiled morphology.; (D) Zooid clusters divided into the posterior zone (right), containing six zooid clusters (I–VI) and a protozooid (PZ), and the main zone (left), with densely packed tripartite zooid groups extending aborally. Colony orientation is indicated (anterior, posterior, dorsal, ventral).; (E) Close-up view of the gastrozooids elongate with distally swollen, balloon-like yellow tips, highlighting their feeding structures. Photographs taken of live specimens under natural and aquarium lighting to preserve color and morphology.
Figure 5
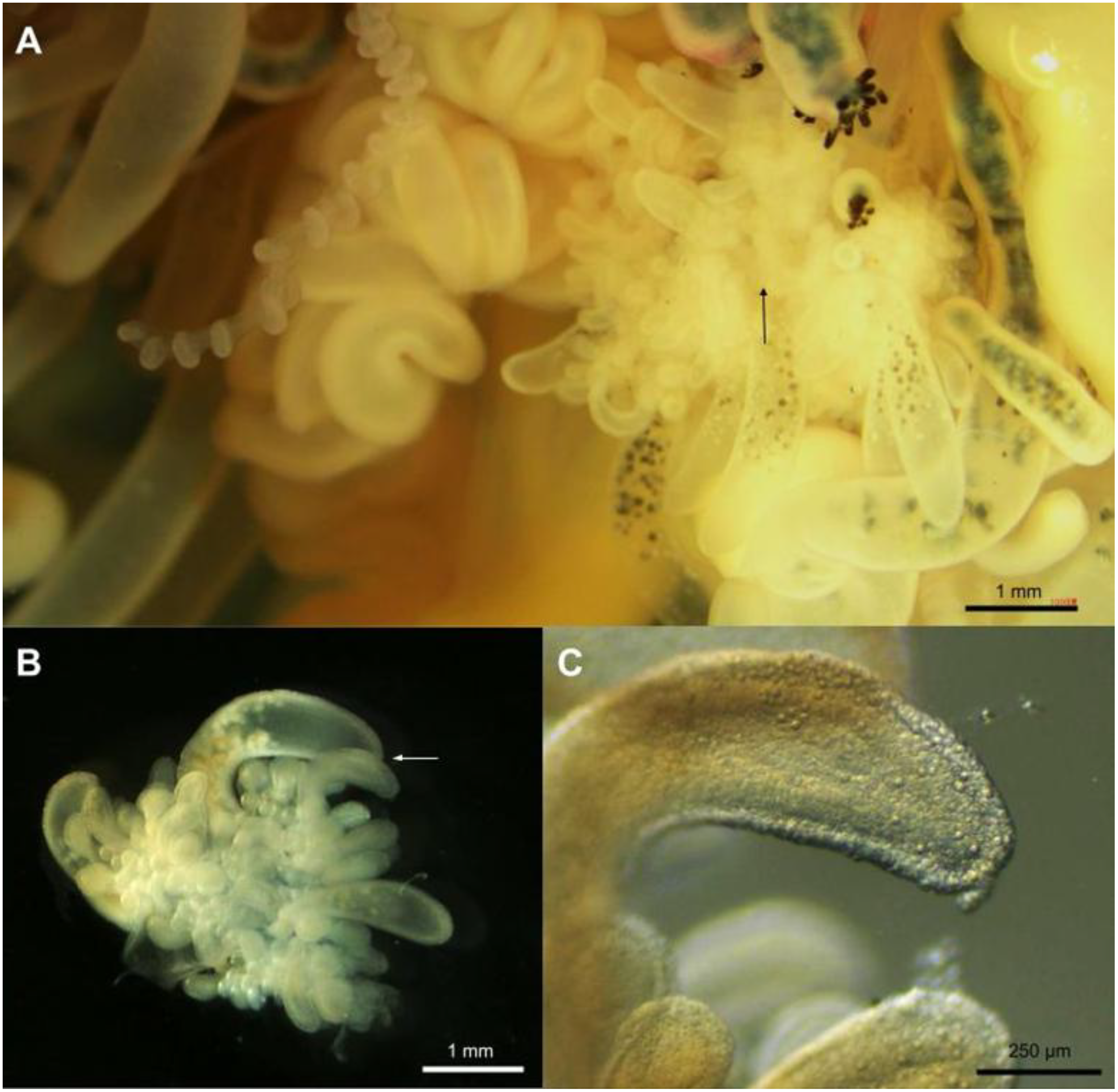
Gonodendron morphology of Physalia mikazuki sp. nov.; (A) Branched gonodendron showing a complex reproductive structure bearing gonophores palpons, jelly polyps, and nectophores (scale bar = 1 mm); (B) Isolated branchlet within gonodendron: gonophore (Go), nectophore (N), palpon (P), and jelly polyp (Jp) along the branchlet; the arrow indicates the position magnified in panel (C) (scale bar = 1 mm).; (C) High-magnification view of stenotele nematocysts on the palpon, demonstrating their structural details (scale bar = 250 µm); see also Figure 11C for complementary imaging.
Figure 6

Close-up views of the posterior zone of Physalia mikazuki sp. nov.; (A) Posterior region showing a mature gastrozooid (G) and a budding gastrozooid (BG). The gastrozooid displays a distinct elongate, banana-like shape (scale bar = 1 mm).; (B) Close-up of the posterior portion of the colony highlighting the protozooid (PZ) along with adjacent gastrozooids (G) (scale bar = 1 mm).
Genus description: Original French, “Corps libre, gélatineux, ovale, comprimé sur les côtés, et ayant sur le dos une crête élevée, rayonnée et membraneuse. Des tentacules nombreuses, filiformes, articulées, placées sous le ventre, et qui paraissent être des suçoirs.” (Lamarck, 1801); English translation: “Free body, gelatinous, oval, compressed on the sides, and having on the back a high, radiated and membranous crest. Numerous tentacles, filiform, articulated, from under the ventral side, which appear to be suckers.” (Lamarck, 1801)
Type species: Physalia physalis (Linnaeus, 1758) Figures 1, 7. Original description reproduced herein along with the translation of the original Latin description as Figure 7B. More than 50 synonymized species names exist (Pugh, 2019) for this type species many of which are insufficiently known to be considered valid. Originally called Holothuria physalis (Linnaeus, 1758), it took 140 years before the name Physalia physalis was established by (Schneider, 1898). No type specimen voucher exists. However, an illustration in (Sloane, 1707) to which Linnaeus referred in his species description has been reproduced herein as Figure 7A.
Figure 7

Historical illustrations and original species description of Physalia physalis. (A) Reproduction of Tab. IV, Figure 5 from Sir Hans Sloane’s Natural History of Jamaica (1707) Volume 1 (Sloane, 1707), depicting an early detailed rendering of Physalia physalis (Sloane, 1707). Sloane described the specimen with the Latin polynomial Urtica Marina, soluta, purpurea, oblonga, cirrhis longissimis p.7 – A Carvell, later formalized as P. physalis by Linnaeus in 1758. The image was engraved by Michael van der Gucht based on a watercolor by John White (1585–1593), now preserved in the British Museum (Museum number: 1906,0509.1.45). All other figures on the same plate are unrelated to Physalia. Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/11242#page/172/mode/1up Published in the UK. All figures are in the public domain.; (B) Reproduction of Page 657 of Systema Naturae (10th edition, Linnaeus 1758) (Linné and Caroli a Linné, 1758), presenting the original Latin species description under the name Holothuria physalis, placed within VERMES. MOLLUSCA. Holothuria. No type specimen or accompanying illustration was established. Junior synonyms listed include H cirris difformibus, Urtica Marina, soluta, purpurea, oblonga, cirrhis longissimis (Sloane), Arethrusa crista subrubella venosa (Brown), and Physalia pelagica (Ost.).; The translated Latin description reads: “Body oval, somewhat triangular, glassy. Back sharp, dark green, from which many nerves extend; reddish anteriorly. Beak spiral, reddish, with a thickened apex. Tentacles under the thick apex are numerous and unequal: the shorter ones cylindrical and thick; the middle ones hair-like, with wide-globose tips; the rest are long and threadlike, with one intermediate tentacle being thicker and twice as long.” Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/10277#page/679/mode/1up Published in Netherlands. All figures are in the public domain.
Valid species: Physalia physalis (Linnaeus, 1758); Physalia minuta Church & Dunn, 2025 (Church et al., 2025); Physalia mikazuki sp. nov. described herein. Though P. utriculus (Gmelin, 1788), P. megalistaLesueur and Petit, 1807 are among those synonymized as junior synonyms of P. physalis due to lack of type reference vouchers, they likely are valid species based on distinct morphological descriptions and illustrations previously published (Figures 1, 7–9 and a summary is provided in Table 1), a claim recently corroborated by a multi-genomic analysis (see (Church et al., 2024); (Church et al., 2025)), and distribution evidence in the literature and citizen science efforts, but, nonetheless lack formal species descriptions.
Figure 8

Historical illustrations and descriptions related to the species Physalia utriculus. Reproduced from de La Martinière (1787) Tome 31 (de La Martinière, 1787). (A) Plate II (page 365), depicting detailed illustrations labeled Figure 13 and “Figure 14” that correspond to specimens later named Physalia utriculus by Gmelin (1788) (Gmelin, 1788). This plate also includes drawings “Figure 15” and “Figure 16,” which appear to represent blue sea dragon nudibranchs (Glaucus spp.), possibly the earliest recorded observation of this kleptoparasitic relationship. “Figures 1–3” on this plate are unrelated to Physalia. Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/29344#page/425/mode/1up Published in France. All figures are in the public domain.; (B) Reproduced from de La Martinière (1787) Tome 31 (de La Martinière, 1787), Page 365. The original French description for Physalia utriculus in which he refrains from describing the animal as a new species though clearly documents the unique morphology. An English translation is provided. Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/29344#page/387/mode/1up Published in France. All figures are in the public domain. (C)Gmelin (1788) (Gmelin, 1788),Tome I Pars 6, Pages 3155–3156. Here the species P. utriculus is formally described under the name Medusa utriculus within the taxonomic group Vermes Mollusca, genus Medusa. The brief Latin description reads: “M. utriculosa, subtus centri tentaculo longissimo granulosos, margine tentaculis numerosis caeruleis apice flavi cantibus.” This refers to a bladder-like organism with a very long central tentacle and numerous smaller blue tentacles tipped in yellow—features that differentiate it from P. physalis (Holothuria physalisLinnaeus 1758). Neither de La Martinière nor Gmelin designated a type specimen or illustration as a voucher. Consequently, Physalia utriculus was later regarded as a nomen nudum and synonymized with P. physalis by Leloup (1934) (Leloup, 1934).; Reported from the North Pacific Ocean (20°N, 179°E). Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/83098#page/141/mode/1up Published in the Netherlands. All figures are in the public domain.
Figure 9
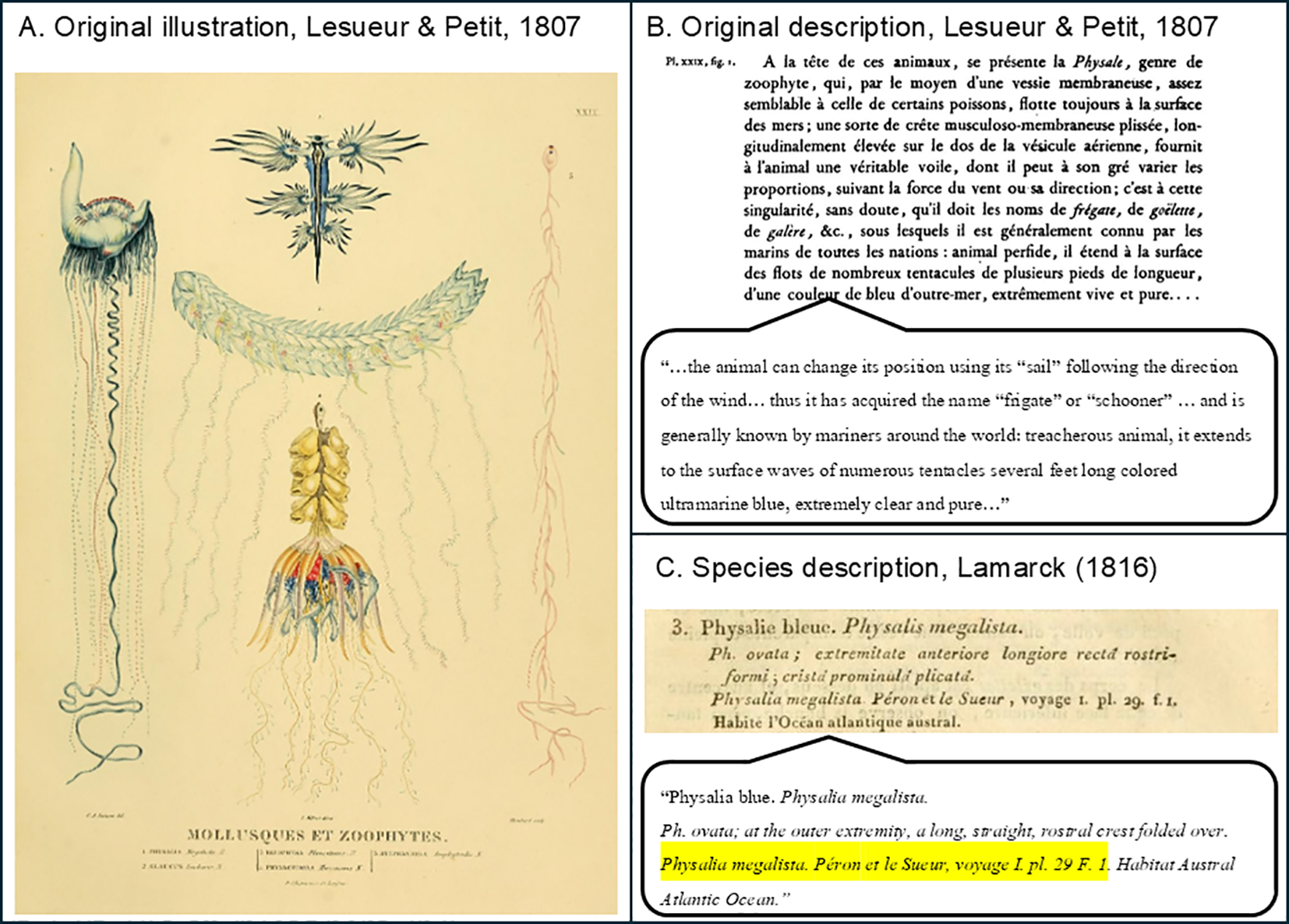
Historical illustrations and early taxonomic descriptions of Physalia megalista; (A) Plate XXIX from Voyage de découvertes aux terres australes, Atlas by Lesueur and Petit, 1807). Published in France. This plate is illustrated by Lesueur and Petit, showing the original depiction of Physalia megalista as “Figure 1” on the left (Lesueur and Petit, 1807). “Figure 2” on the same plate illustrates blue sea dragon nudibranchs (Glaucus spp.), highlighting the kleptoparasitic relationship between these organisms and Physalia. Note: Figures 3–5 on the same plate are unrelated to Physalia. Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/96197#page/67/mode/1up; (B) French-language description of “Figure 1” in Lesueur and Petit, 1807 Page 42–43 of Peron, 1807 (Péron, 1807). English translation provided. Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/262514#page/68/mode/1up Published in France. All figures are in the public domain.; (C) Page 481 of Lamarck’s Histoire naturelle des animaux sans vertèbres (1816), in which P. megalista is given a brief but formal species description based on the illustration by Lesueur & Petit. In the preceding pages (478–480), Lamarck describes the biology and ecological context of Physalia, noting, “On assure que l’apparition des physalies vers les côtes, est le présage d’une tempête prochaine” (One can be sure that the appearance of Physalia on the coasts is an omen of a coming storm) (Péron, 1807). Accessed April 14, 2025, on the Biodiversity Heritage Library (BHL): https://www.biodiversitylibrary.org/item/47698#page/491/mode/1up Published in France. All figures are in the public domain.
Table 1
| Species name in current usage | Documented Geographic location(s) | Molecular data | Type specimen voucher | Original illustration(s) or image(s) | Original Description | Other images, photos, vouchers |
|---|---|---|---|---|---|---|
| Physalia mikazuki sp. nov. Yongstar, Ochiai & Lewis Ames | NW Pacific (Japan, Tohoku region. Type locality, Sendai City) and Kanto region, Mexico, Pakistan | NCBI GenBank | The Tohoku University Museum 112960-112965 | This study | This study | None |
| 16S: PV469480, PV469481, PV469482, PV469483; | Figure 4 | |||||
| COI: PV452818, PV452819, PV452820, PV452821 | ||||||
| (This study) | ||||||
| Church et al., 2025 | ||||||
| Figure 2, Clade B2 | ||||||
| Physalia minuta Church & Dunn, 2025 | Wellington, New Zealand | Church et al., 2025 | NIWA 173305; YPM IZ 111207, YPM IZ 111208, YPM IZ 111209, and YPM IZ 111210. | Church et al., 2025, Figure 8 | Church et al., 2025 | None |
| Zealand, catalog number | Figure 2, Clade C2 | |||||
| P. physalis (Linnaeus, 1758) | Atlantic Ocean |
Church et al., 2025
Figure 2, Clade A |
None |
Sloane (1707) Tab IV, Figure 8 Reproduced herein as Figure 7 |
Linnaeus (1758) | Church et al., 2025, Figure 2 Cluster A, Figure 4 |
|
Duperrey (1830) Zoophytes Plate 4 Reproduced herein as Figure 1 |
||||||
| P. utriculus (Gmelin, 1788) | Pacific Ocean, including Japan (Okinawa, Sagami Bay), Indian Ocean | Church et al., 2025, | None |
de La Martinière (1787) Plate II, Figure 13 & 14 Reproduced herein as Figure 8 |
Gmelin (1788) | Church et al., 2025, Figure 2 Cluster B1, Figure 4 |
| Figure 2, Clade B1 | ||||||
| P. megalista (Péron, 1807) | Southern part of Pacific, Indian and Atlantic Oceans | Church et al., 2025 | None | Lesueur & Petit in Péron (1807) Plate XXIX Reproduced herein as Figure 9 |
Lesueur & Petit in Péron (1807) | Church et al., 2025, Figure 2 Cluster C1, Figure 4 |
| Figure 2, Clade C1 |
Comparative summary of Physalia species, including the newly described Physalia mikazuki sp. nov., with details on their documented geographic locations, molecular data, type specimen vouchers, original illustrations, formal descriptions, and additional specimen records.
Molecular clades and clusters are sensu (Church et al., 2025).
Physalia utriculus (Gmelin, 1788). An illustration in (de La Martinière, 1787) to which Gmelin (1788) referred in his species description (Gmelin, 1791; de La Martinière, 1787); both have been reproduced herein as Figures 8A, B, and described in detail in the corresponding figure header.
Physalia megalista Lesueur and Petit, 1807. An illustration by Lesueur & Petit in Péron (1807) (de La Martinière, 1787; Lesueur and Petit, 1807; Péron, 1807) used for their initial description and then later to which Lamarck (1816) (Lamarck, 1816) referred in his attempt to redescribe the species (Figure 9C), however, the authorship remains as P. megalista (Lesueur and Petit, 1807); the species description and illustration have been reproduced herein as Figures 9A, B, and described in detail in the corresponding figure header.
A recent study of global Physalia populations by (Church et al., 2025) used a genomic approach that combined morphological data (museum vouchers and iNaturalist images) to resolve multiple species that corresponded to original descriptions for P. physalis (as Cluster A), P. utriculus (as Cluster B1), P. megalista (as Cluster C1). Additionally in the study by (Church et al., 2025), two potentially new species of Physalia were identified – one as Cluster C2 (sensu (Church et al., 2024) (Church et al., 2025)) and described as Physalia minuta Church & Dunn, 2025, present in the Tasman Sea, and the other as Cluster B2 present in Sagami Bay (overlapping with B1 sensu (Church et al., 2024) (Church et al., 2025)) with matches to GenBank sequences for Physalia from Pakistan and Mexico but with insufficient morphological data for species comparison therein.
Physalia mikazuki sp. nov. is the first species in the genus to be described in Japan. Previous works named P. physalis as the sole Physalia species in Japan (Pugh, 2019), but recently (Oguchi et al., 2024) described the development of P. utriculus from Kanagawa Prefecture (Japan) without an explanation for the use of nomenclature. However, given that the photo and drawing depicted therein bear a prominent principal tentacle it is likely P. utriculus (“Cluster B1” (Church et al., 2024; Church et al., 2025)).
Abbreviations: PL: Pneumatophore Length - measured from the anterior end of the main zone to end of the posterior zone (previously oral zone) of pneumatophore (float); PW: Pneumatophore Width - measured from the apex to the basal region of pneumatophore; PD: Pneumatophore Diameter - measured at the widest point of pneumatophore; NPW: Number of pneumatophore wrinkles; NPT: Maximum number of principal tentacles - the main tentacle, develops from the first tripartite group of a zooid cluster in the main zone (much larger and thicker at its base compared to the secondary tentacles); TUM (Tohoku University Museum).
Distribution: Tohoku and Kanto regions of Japan; Pakistan and Mexico.
Type locality: Gamo Beach, Sendai City, Miyagi Prefecture, Japan.
Material examined: Holotype: 112960 (TUM), mature, female, 67 mm PL, 29 mm PW, 19 NPW, 3 NPT, Gamo Beach (Sendai Bay), Sendai City, Miyagi Prefecture, Japan, 12 June 2024.; Paratypes: 112961 (TUM), mature, 73 mm PL, 30 mm PW, 20 NPW, 3 NPT; 112962, mature, 61 mm PL, 23 mm PW, 12 NPW, 3 NPT; 112963, 49 mm PL, 19.5 mm PW, 9 NPW, 1 NPT; 112964, juvenile, 21 mm PL, 9 mm PW, 5 NPW, 1 NPT; 112965, juvenile, 24 mm PL, 11 mm PW, 5 NPW, 1 NPT, all from Gamo Beach (Sendai Bay), Sendai City, Miyagi Prefecture, Japan, 12 June 2024.
Etymology: The Japanese word “mikazuki” refers to the “crescent moon” shape of the warrior helmet worn by Samurai Masamune Date (1567 – 1636) of the Tohoku region who founded Sendai City. Vernacular “mikazuki no eboshi” (Japanese), “crescent helmet man-of-war” (English).
Diagnosis: Physalia mikazuki sp. nov. Yongstar, Ochiai & Lewis Ames is distinguished from other members of the genus Physalia by a combination of morphological traits: Pneumatophore length range 9.25–72.4 mm, with maximum known size smaller than that reported for P. physalis (8.1–134 mm) but overlapping with P. utriculus. Coloration of the crest is bluish with deep blue to purple hues and membrane is a translucent bluish-green (vs. dark green/carmine in P. physalis, blue and clear-glassy in P. utriculus, transparent with green patch at anterior apex in P. minuta). Up to six zooid clusters are present in the posterior zone (vs. five in P. physalis). Three primary zooid cluster groups are found in the main zone (vs. up to seven in P. physalis and one in P. utriculus). Posterior zone gastrozooids are cylindrical, elongate and banana-shaped (vs. bulbous, rounded with yellow apex in P. utriculus). In the main zone, P. mikazuki has gastrozooids that are elongated with distally swollen balloon-like yellow tips (vs. shorter, flask-shaped gastrozooids in P. utriculus, and elongated, blue at base and pale at tip in P. minuta). More than two principal tentacular palpons are present (vs. 1–2 in P. utriculus and P. megalista), each arising from a separate zooid cluster. Tentacle axial tissue is significantly widened with an axial width ratio of approximately 2:1. Trait comparison among P. mikazuki and other Physalia species is summarized in Table 2.
Table 2
| Character | P. mikazuki sp. nov. | P. minuta | P. physalis | P. utriculus |
|---|---|---|---|---|
| Pneumatophore length (mm) | 9.24–72.4 | 2–3 | 8.1–134 | unknown |
| Crest color | deep blue to purple | unknown | carmine | blue |
| Pneumatophore membrane color | transparent blue-green | transparent with green patch at anterior apex | pink, purple, or blue | blue, clear-glassy |
| Posterior zooid clusters | up to 6 | unknown | up to 5 | unknown |
| Main zone cluster groups | up to 3 | unknown | up to 7 | 1 |
| Posterior gastrozooid shape | elongate, | elongate, | unknown | bulbous with |
| banana-like shape | blue at base and pale at tip | yellow apex | ||
| Main zone gastrozooid shape | elongated with balloon-like yellow tip | unknown | unknown | flask-shaped structure with diffuse yellow pigmentation tip |
| Principal tentacles per colony | >2 | >2 | >2 | 1–2 |
| Tentacle axial tissue | 2:1 | unknown | unknown | uniform |
| (width ratio) | ||||
| Reference | This study (Figures 4–6, 10) | Church et al. (2025) | (Totton and Mackie, 1960) (Bardi and Marques, 2007) and (Munro et al., 2019) | (Pontin and Cruickshank, 2012) and this study (Figure 3) |
Diagnostic morphological comparisons between Physalia mikazuki sp. nov. and related species.
unknown = inconclusive in literature or this study given insufficient specimen number.
Description: Physalia mikazuki sp. nov. Yongstar, Ochiai & Lewis Ames. Colonies with an asymmetrical triangular sail-shaped, gas-filled pneumatophore with bluish coloration and deep bluish to purple hues in the upper region. Translucent structure with transparent bluish-green membrane (Figures 4A). Size ranging from 9.25–72.40 mm (n=37) in total pneumatophore length, 8.90–35.20 mm in pneumatophore width (n=6) and 8.10–27.50 mm in pneumatophore diameter (n=6), with a longitudinal wrinkled crest showing 5–20 wrinkles (Figure 4A).
Description of zooid arrangement follows the general pattern described for Physalia physalis by (Totton and Mackie, 1960) (Bardi and Marques, 2007), and (Munro et al., 2019). Zooids arranged ventrally in clusters budding from the basal region of the pneumatophore. Colony divided into two zones: posterior zone and main zone, separated by a basal internode (a gap devoid of polyps) (Figure 4D) seen also in P. utriculus, but least demarcated in P. physalis.
Posterior zone contains up to six zooid clusters and a protozooid (PZ), the first gastrozooid. Clusters I, II, and III form the reduced group, composed solely of gastrozooids with yellow-tipped oral parts that increase in length from cluster I (shortest). Clusters IV, V, and VI constitute the tripartite group, each comprising a gastrozooid, a tentacular palpon, and a gonodendron (Figure 4D).
Main zone, situated aboral to the posterior zone, is more developed and organized into three primary zooid cluster groups (Figure 4D), each containing up to two or three zooid clusters showing varied composition and developmental stages; an initial reduced group with a single gastrozooid and a gonodendron at its base; a primary tripartite group with a gastrozooid, a tentacle with ampulla or tentacular palpon [refers to a zooid type found in P. physalis, this zooid is responsible for bearing the tentacles (Munro et al., 2019)], and a gonodendron; a sequence of lateral tripartite groups branching from the primary tripartite group; and secondary basal buds groups as trifid structures, with additional trifid secondary basal buds emerging from the base of each zooid group.
Gonodendra, branched, composed of complex reproductive structures (Figure 5A), including male or female gonophores, bearing nectophores, jelly polyps, and palpons (as in P. physalis) (Figure 5B). Subterminal branch end with elongated vestigial nectophore and palpon (Figure 5B). Terminal branch end with jelly polyp and palpon (Figure 5B). Palpons concentrated at distal ends of subterminal and terminal branches (Figure 5B) bearing stenotele nematocysts (Figure 5C).
P. mikazuki gastrozooids in posterior zone are elongated and banana-shaped (Figure 6) with length range of 0.92-13.79 mm (n=69). In the main zone, gastrozooids are elongated with distally swollen, balloon-like yellow tips (Figure 4E) with length ranging from 0.883-24.871 mm (n=236).
Tentacular palpon of P. mikazuki includes principal tentacles and secondary tentacles. Principal tentacle developed from the first tripartite group of a zooid cluster in the main zone. This tentacle is much larger and thicker at ampulla (1.709 mm) compared to the secondary tentacles (0.879 mm) with wider tentacle axial tissue (the region formed by epitheliomuscular cells longitudinally arranged along mesoglea lamellae adhering to both sides of tentacular axis): about twice the thickness of secondary tentacles (Figure 10). Tentacles bear prominent nematocyst batteries arranged along their length, which are spirally arranged as button-like structures along the tentacles (Figure 10).
Figure 10

Tentacular palpon morphology of Physalia mikazuki sp. nov. from Gamo Beach, Sendai City, Miyagi Prefecture, Japan. Comparison of principal (right) and secondary (left) tentacle axial tissues, showing that the principal axial tissue is approximately twice the width of the secondary (scale bar = 1 mm).
Despite the limited number of specific traits reported in the literature for P. utriculus, colonies from Okinawa observed in this study were clearly morphologically distinct from P. mikazuki sp. nov. in having a prominent single principal tentacle (Figures 3A,B), a distinct zooid cluster arrangement, and gastrozooid of distinct morphology (Figures 3C, D). In contrast to P. mikazuki, P. utriculus exhibits main zone gastrozooids that are shorter, flask-shaped, and opaquer in texture, with less distinctly defined yellow tips in a more irregular, tightly clustered arrangement (Figure 3D). Posterior zone gastrozooid buds of P. utriculus are bulbous and rounded, with a swollen body and a yellow apical region (Figure 3C).
Cnidome: Two main types of nematocysts observed in P. mikazuki: isorhiza (large and small size classes) and stenoteles (one size class). Isorhizas, spherical, mean length of 11.012 ± 0.20 μm for small size class and 22.511 ± 0.41 μm for large class size; both present in principal tentacles (Figure 11A), secondary tentacles (Figures 11B) and gastrozooids. Stenoteles, spherical, mean length of 18.07 ± 0.33 μm, present on gonopalpons (Figure 11C). All measurements were made after preservation in 10% formalin. The cnidome of P. mikazuki sp. nov. is identical to that of P. physalis, as reported by (Bardi and Marques, 2007) and thus, not diagnostic at the species level in this case.
Figure 11
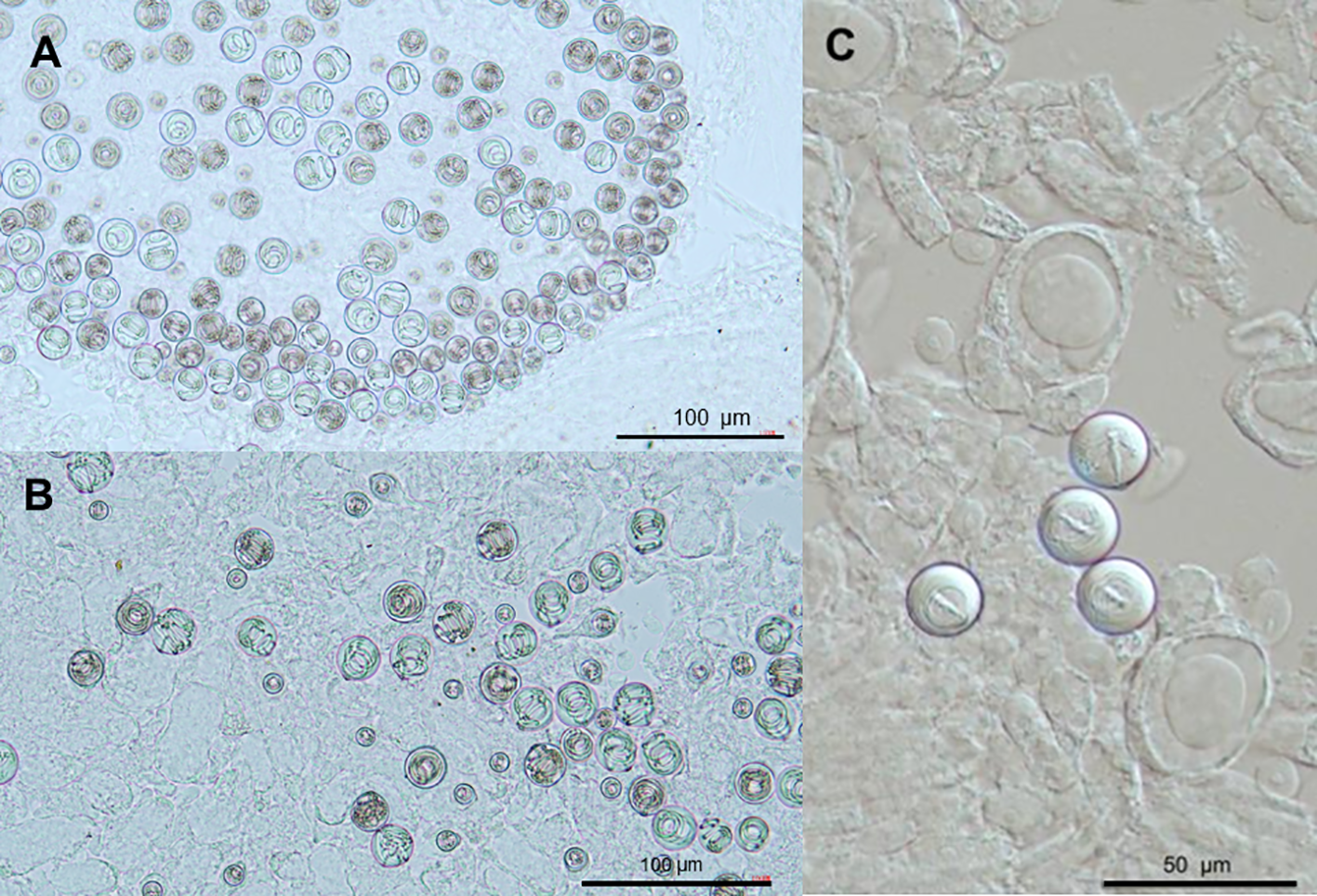
Nematocyst types observed in different regions of the Physalia mikazuki sp. nov. colony; (A) Two distinct size classes of isorhiza nematocysts isolated from principal tentacles; (B) Two distinct size classes of isorhiza nematocysts isolated from secondary tentacles; (C) Stenotele nematocysts located in the palpon region of the gonodendron.
3.2 Molecular analysis
Phylogenetic analyses of the mitochondrial 16S rRNA region revealed the distinctiveness of the Physalia specimens collected from Gamo Beach, Japan (Figure 12A). The four 16S sequences generated in this study (GenBank accession numbers PV469480–PV469483) formed a strongly supported monophyletic clade, with SH-aLRT/aBayes/ultrafast bootstrap values of 95.3/1.00/90, respectively. This clade includes two specimens from Japan (YPM-IZ-110931 and YPM-IZ-110943) and one from Mexico (YPM-IZ-110878), previously reported by (Church et al., 2025). Notably, this group is phylogenetically distinct from other known Physalia species, including P. physalis, P. utriculus, and P. megalista. Analysis of the COI region yielded a matching pattern. The four COI sequences from this study (PV452818–PV452821) also formed a monophyletic clade, this time with full support (SH-aLRT/aBayes/ultrafast bootstrap = 99.3/1.00/100), clustering with sequences from Mexico and Pakistan. The clade is Cluster B2 of (Church et al., 2024; Church et al., 2025), which was not formally described as a new species.
Figure 12
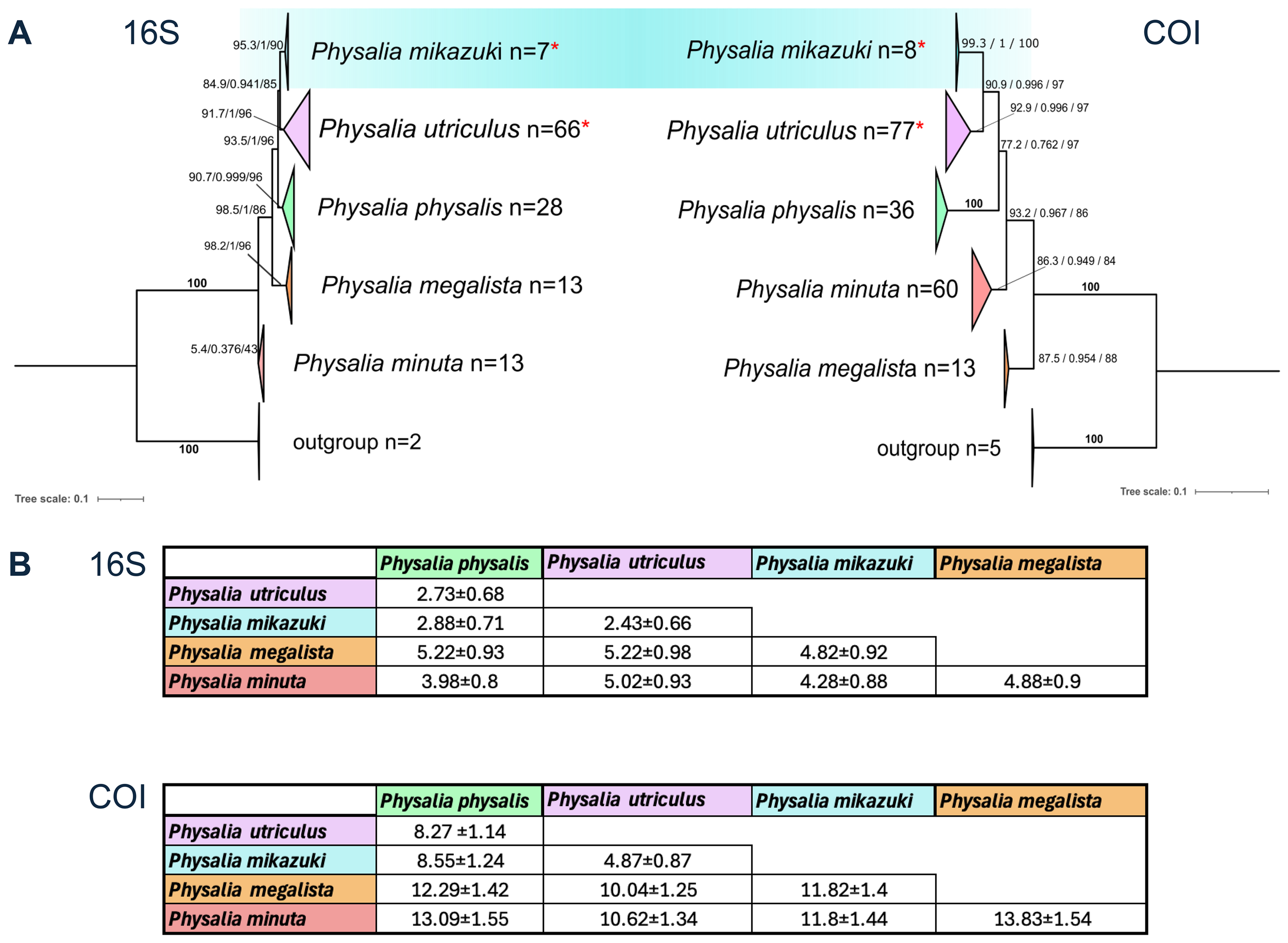
Maximum likelihood (ML) phylogenetic trees and pairwise genetic divergence of Physalia species based on mitochondrial markers. (A) ML trees were constructed using 16S rRNA (127 Physalia + 2 Rhizophysa outgroups) and COI (193 Physalia + 5 Rhizophysa) sequences. Clades are collapsed and color-coded by species hypothesis: P. mikazuki sp. nov. (blue), P. utriculus (purple), P. physalis (green), P. megalista (orange), and Physalia sp. (red). The number of sequences per clade is shown (n = number of sequences). Trees were inferred using IQ-TREE, and node support is shown as SH-aLRT/aBayes/ultrafast bootstrap values. Bold “100” indicates full support across all three measures. Specimens from Sendai Bay formed a distinct clade with strong support (16S: 95.3/1/90; COI: 99.3/1/100), clustering with sequences from Pakistan (MK084614), Japan, and Mexico (Church et al., 2025) and are herein described as P. mikazuki sp. nov. Samples from Okinawa are grouped in a neighboring Pacific clade of P. utriculus. Asterisks indicate clades containing sequences generated in this study, including four P. mikazuki and two P. utriculus samples in both the 16S and COI datasets. (refer to Supplementary Figures S1 and S2 for full sequence-level resolution) (B) Estimates of evolutionary divergence between clades based on the Kimura 2-parameter model (Kimura, 1980), shown as mean ± SE. The 16S dataset included 124 sequences (654 positions), and COI included 191 sequences (1,048 positions), both analyzed in MEGA12 (Kumar et al., 2024, Stecher et al., 2020) with pairwise deletion of ambiguous sites. These values support significant genetic separation of P. mikazuki from other Physalia lineages. Details of all samples provided in Supplementary Table S1.
To validate the topology inferred by IQ-TREE, maximum likelihood (ML) trees were reconstructed in MEGA11 for both markers. These reconstructions yielded comparable topologies, with bootstrap support of 87.6% (Std. Dev. = 1.06348) for 16S and 97.9% (Std. Dev. = 0.58944) for COI. Pairwise evolutionary divergence estimates between clades were calculated using the Kimura 2-parameter (K2P) model in MEGA (Figure 12B). For the 16S marker (noting its slower substitution rate), the clade here proposed as Physalia mikazuki sp. nov. diverged from P. physalis by 2.88%, from P. utriculus by 2.43%, and from P. megalista by 4.28%. For the COI marker, divergence from these same species were more pronounced, at 8.55%, 4.87%, and 11.82%, respectively.
Based on consistent molecular distinctiveness across two mitochondrial loci, high nodal support from multiple phylogenetic inference methods, and distinguishing morphological traits described in Section 3.1, we designate the name Physalia mikazuki sp. nov. for this genetically and morphologically distinct species and Gamo Beach as the type locality. The description of a new Physalia species herein is also the first verified record of the genus in the Tohoku region of Japan. Overall, these findings enhance current understanding of species diversity within Physalia and emphasize the value of integrating molecular and morphological evidence for resolving the taxonomy and biogeographic patterns of pelagic hydrozoans.
Remarks. Together the morphological and molecular analyses of Physalia specimens collected at Gamo Beach show it is a distinct species that we herein designate Physalia mikazuki sp. nov. Diagnostic traits include a smaller pneumatophore (9.25–72.4 mm) than P. physalis, a crest with bluish coloration and deep blue to purple hues, a translucent greenish membrane, and up to six posterior zone zooid clusters (vs. five in P. physalis). The main zone contains three primary zooid cluster groups (vs. seven in P. physalis, one in P. utriculus). Posterior gastrozooids are a distinctly elongated, banana-like shape (vs. bulbous with yellow apex in P. utriculus), and gastrozooids in the main zone are elongated with distally swollen, balloon-like yellow tips. Each colony possesses more than two principal tentacular palpons with tentacle axial tissue approximately twice as wide as that of secondary tentacles. These features, summarized in Table 2, support the recognition of P. mikazuki as a novel species. The taxonomy of the genus Physalia has long been contested (Totton and Mackie, 1960). synonymized historical names under P. physalis, arguing for a single globally distributed species and highlighting the dimorphic characteristic of left- versus right-handed “sails” (floats) as a possible mode of drift in either wind direction. In contrast, much earlier Chun (1897) and Schneider (1898) proposed species delineation between the so-called Atlantic P. physalis and the Indo-Pacific P. utriculus based on morphological differences (Garstang, 1946). Recent reports, particularly those from Australian waters, have further challenged the current taxonomic constraints, suggesting the presence of multiple Physalia species with distinct morphological traits (Pugh, 2019). The long-held belief that P. physalis is the sole species in the genus stemmed from a lack of reliable morphological characters to unequivocally delineate potential taxa. In this study, Tohoku specimens exhibited consistent morphological differences from P. physalis, P. minuta, P. utriculus, and what is illustrated in the literature as P. megalista (Figures 1, 7–9), including variation in pneumatophore size, coloration, gastrozooid structure, and tentacle morphology. In particular, the shape of gastrozooids and the number and structure of principal tentacles provide strong distinguishing features for P. mikazuki. Twenty years ago, Collins et al., 2005 revealed the utility of rnl (16S rRNAgene) for broad-scale species delineation among hydrozoans, one of the four classes of the subphylum Metazoa (jellyfishes) (Collins et al., 2005). Later, a study by Ortman et al., 2010 designed to delineate population boundaries of Medusozoa, showed that the mitochondrial COI target was an appropriate DNA barcode for estimating species-level distinction within the Medusozoa using Kimura 2-parameter genetic distances (K2P) (Ortman et al., 2010). Though no solid cut-off exists for species delimitation, as sequence divergence values are notable variable between hydrozoan species, guidelines for certain clades (Schuchert, 2005; Collins et al., 2008; Schuchert and Collins, 2021) are reported using difference models (but see (Srivathsan and Meier, 2012)). Furthermore, Zhen et al. (2014) showed that K2P values differ between the mtCOI and 16S rRNA genes, with the former being a faster evolving region (higher substitution rate) among Medusozoa species (Zheng et al., 2014). That study concluded that while 16S might be more efficient for validation at the family level and below, hydrozoan taxa are identified at the species level using either barcode marker alone. Thus, given that proven efficacy of the 16S rRNA and mtCOI genes in medusozoan phylogenetic reconstruction studies over the past 20 years, we are confident in the capacity for these two standard mitochondrial markers, analyzed in tandem with morphological data, to delineate P. mikazuki sp nov. as the newest species of Physalia.
3.3 Environmental description and particle tracking simulation
Species distribution ranges are often influenced by changes in environmental conditions (Hoegh-Guldberg and Bruno, 2010). In this study, we conducted a temporal and spatial analysis of oceanographic data of ocean currents and temperature from 2018 to 2024, to examine potential shifts that could affect marine ecosystems in Japan’s northern coastal waters. Our aim was to assess whether Physalia “Clade B2” (as documented by (Church et al., 2024; Church et al., 2025)) sampled from Sagami Bay in the Kanto region of Japan where P. utriculus [“Clade B1” sensu (Church et al., 2024; Church et al., 2025)] is also reported (Okada, 1932) could, in fact, be moving northward to the Tohoku region.
Data processing revealed that variation in oceanographic parameters from 2018 to 2022 was relatively consistent, prompting us to consolidate these years for separate comparisons with more recent data from 2023 and 2024, respectively (Figure 13). The comparative analysis indicated notable differences between the earlier period (2018–2022) and more recent years (2023 and 2024), particularly in terms of changes in ocean currents and SST (sea surface temperature) (Figure 13).
Figure 13
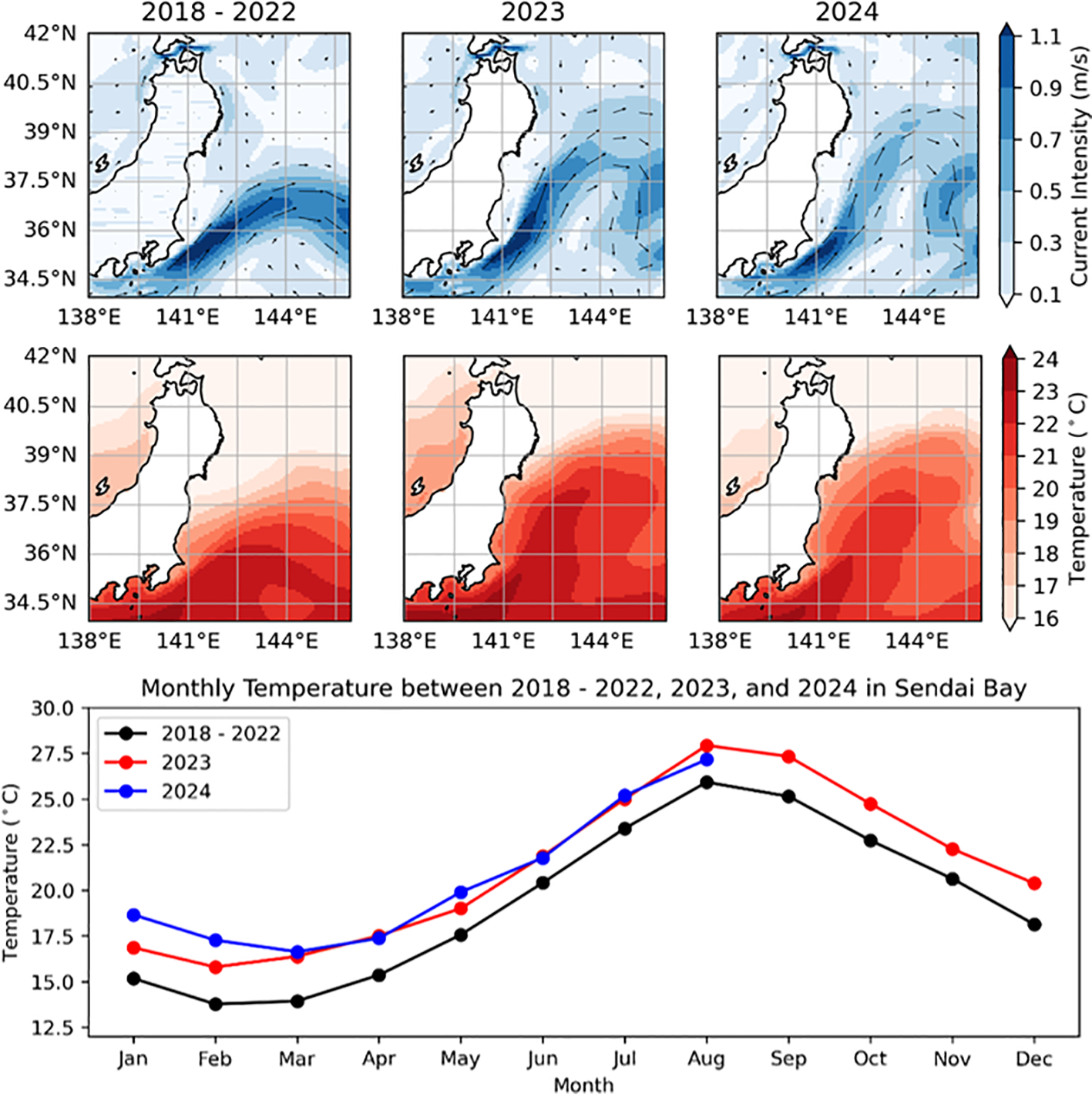
Annual variations of currents and temperature in study area and monthly trends of temperature variations in Sendai Bay from 2018 to 2024. Top Panels: Surface Current (in m/s) maps for the periods 2018–2024 indicating northward shift of the Kuroshio current in 2023 and 2024. Middle Panel: Sea surface temperature (in°C) maps for the periods 2018-2024, indicating a warming condition, especially in the northern regions near our sampling location in Sendai Bay. Bottom Panel: Line graph comparing monthly average temperatures for 2018-2022 (black), 2023 (red), and 2024 (blue) focused on the northern regions near our sampling location in Sendai Bay. A distinct seasonal cycle with temperature peaks in August is evident, but an overall temperature increase is observed in 2023 and 2024 compared to the 2018–2022 baseline throughout the year.
Recent oceanographic observations reveal a notable shift in the Kuroshio Current’s intensity and trajectory, with its path moving approximately 2° latitude northward in 2023 and 2024 compared to earlier periods (Figure 13, top panel). This northward shift may have facilitated the transport of marine organisms, including P. mikazuki, to higher latitudes due to altered current pathways. Concurrently, our analysis of the spatial distribution of average temperatures along the Pacific coastal area of Japan, from Sagami Bay to the northern region (Figure 13, middle panel), reveals generally warmer conditions. In particular, the monthly average temperatures near the sampling location in Sendai Bay consistently recorded approximately 2-4°C higher throughout 2023 and 2024 compared to the period from 2018 to 2022 (Figure 13, bottom panel). This indicates that the northern region, particularly near Sendai Bay, experienced relatively warmer conditions compared to previous years. This SST jump is considered to have occurred globally and is dubbed a “record-shattering” extreme event by ocean modelers (Terhaar et al., 2025).
Previously, Ferrer & Gonzales (2021) used an improved version of the Lagrangian particle tracking model SOFT (Sediment, Oil spill and Fish Tracking model) to show wind (drift) as the main mechanism controlling circulation on the sea-air interface along the Basque coast (Bay of Biscay) where thousands of Physalia individuals converge, with the majority being right-handed; thus hypothesizing drift from the Sargasso sea to American and European shores (Ferrer and Gonzalez, 2021). To test for additional factors controlling circulation of Physalia mikazuki sp. nov., we conducted a particle trajectory simulation using OceanParcels in Python “A highly customizable Lagrangian simulation framework” (https://oceanparcels.org/) based on the daily surface current data as another approach to investigate the possibility of P. mikazuki being transported northward by ocean currents. The simulation was initiated from Sagami Bay, Kanagawa region where the presence of Cluster B2 was initially reported (Kumar et al., 2024). The trajectory simulation included the period from May to August 2024, as P. mikazuki was discovered in Sendai, Miyagi in mid-June (this study) and in Hachinohe, Aomori in mid-August (from an iNaturalist report https://www.inaturalist.org/observations/245526594). Based on the particle simulation results, particles representing potential drifting Physalia individuals could reach Sendai within approximately 30 days and Aomori within 45 days (Figure 14).Visualization of the complex movement patterns and mapped distribution of particles, shaped by variability in current velocities, particularly the influence of the Kuroshio Current (Figure 15) provides insights into the mechanisms driving both biological and physical dispersal processes in the region.
Figure 14
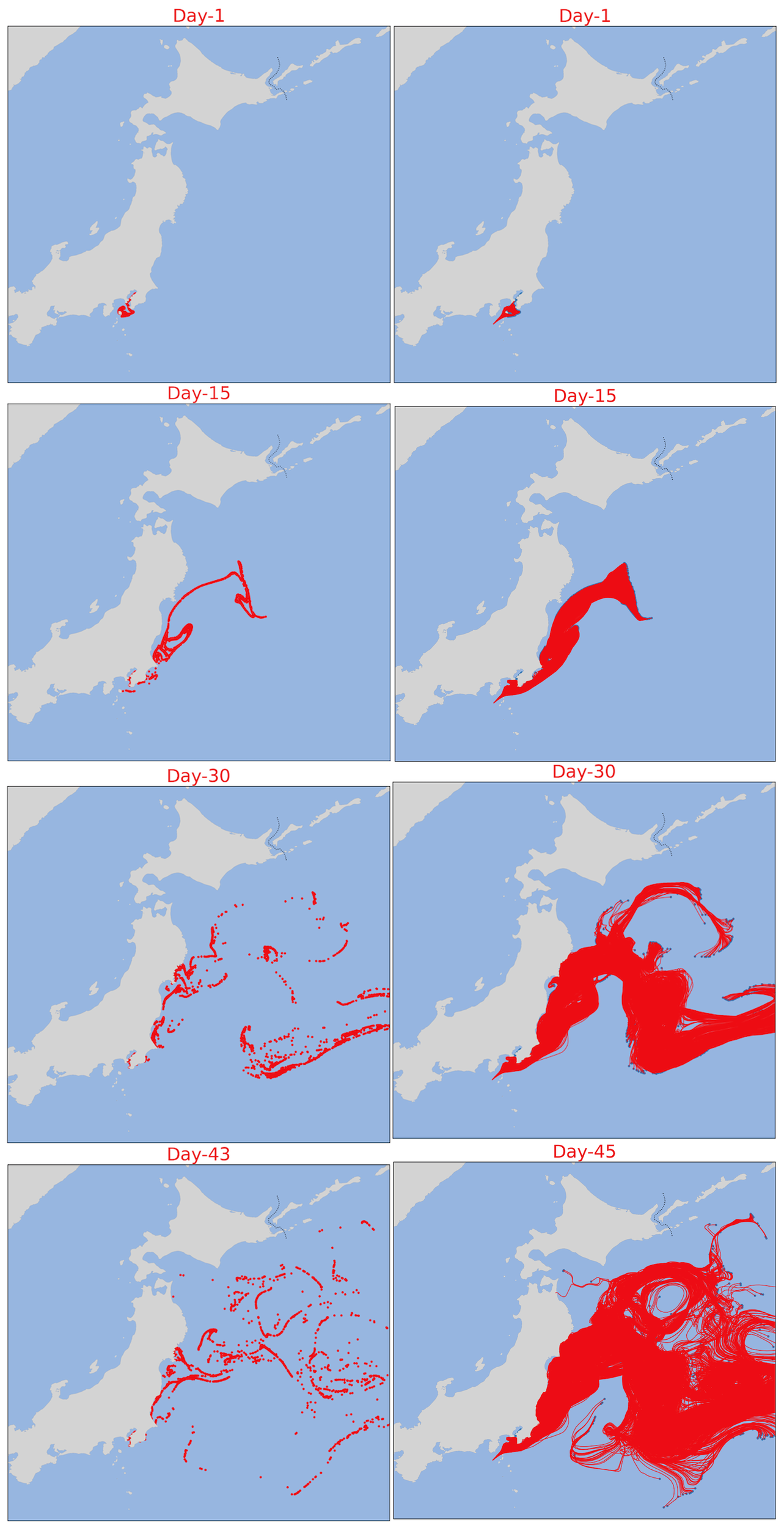
Dispersion patterns over time in coastal waters of Japan based on the OceanParcels Simulation from May 12, 2024, as Day 1. Left Column: Sequential maps showing the dispersion pattern of particles (in red) in the coastal waters of Japan at Day 1, Day 15, Day 30, and Day 45. These maps illustrate the gradual spread and movement of the particle over a 45-day period. Right Column: Corresponding maps for dispersion pattern of particles with recorded path.
Figure 15
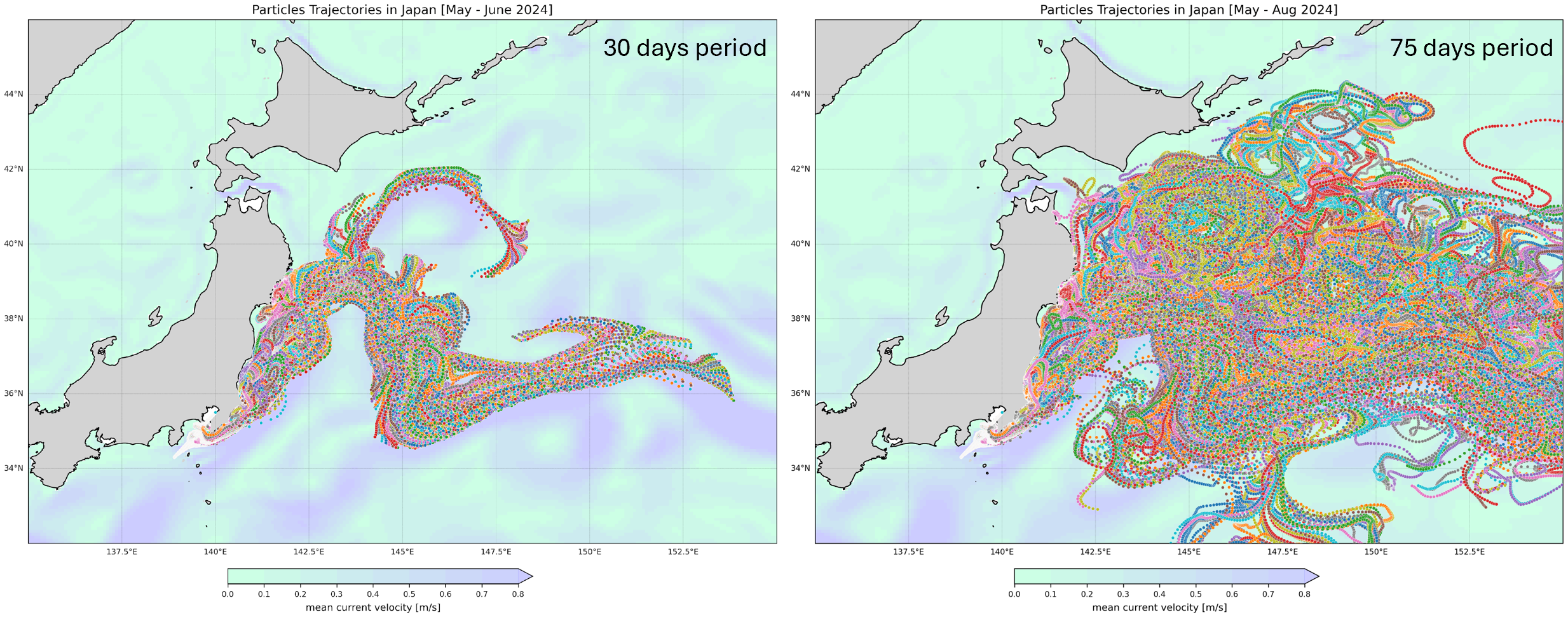
Particle trajectories in coastal waters of Japan (MayA-August 2024) based on the OceanParcels simulation. This map illustrates the trajectories of particles in the coastal waters of Japan over a 30-day period (left) and 75-day period (right). The colored lines represent the paths taken by particles under the influence of ocean currents, with the background color indicating mean current velocity (in m/s).
4 Discussion
This study documents the discovery and description of Physalia mikazuki sp. nov., the first Physalia species formally described from Japan, from the Tohoku region. Phylogenetic analyses based on the 16S rRNA gene and COI (cytochrome c oxidase subunit 1) regions show its classification as a distinct species. The formation of a well-supported monophyletic clade distinct from P. physalis, P. minuta, P. utriculus, and other Physalia lineages indicates significant genetic divergence. These genetic findings align with morphological observations, including a pneumatophore that is smaller than P. physalis but bigger than P. utriculus, bluish with deep blue to purple coloration of crest, translucent bluish-green color of float, the arrangement of zooid clusters and the distinct shape of gastrozooids. This is the first record of Physalia in Tohoku, Japan, a region historically outside the genus’s known range (Prieto et al., 2015; Karunarathne and De Croos, 2022; Martins, 2022). With Physalia reports previously limited to the warmer temperate waters of Sagami Bay (Oguchi et al., 2024) and subtropical Okinawa (Fenner, 1998), the emergence of P. mikazuki in Sendai Bay highlights a significant biogeographical shift, raising important questions about the ecological implications.
One potential factor contributing to the northward transport of P. mikazuki is the recently observed shift in the Kuroshio Current (Figure 13). During the period from 2023 to 2024, the Kuroshio Current moved approximately 2° latitude northward, altering the flow dynamics of the coastal Pacific waters of Japan. This shift in current pathways may have facilitated the transport of this neustonic organism, P. mikazuki, to higher latitudes. In addition, oceanographic data revealed higher sea surface temperatures in northern areas of Japan, particularly near Sendai Bay, during the same period. The combination of warmer conditions and altered currents may create favorable environments that support the transport and potential establishment of P. mikazuki populations at higher latitudes.
Historical absence data further support this hypothesis. Despite regular field studies conducted by a coauthor on this work (Y. Ochiai) and long-term monitoring by local institutions (since 1996) such as the National Institute for Environmental Studies, until its discovery in June 2024 Physalia was never observed at Gamo Beach (G. Kanaya and T. Yuhara pers comm). While additional sightings were documented in nearby Yuriage and Sen-nan (Sendai Bay) in 2023, no records exist from prior decades. These findings, in combination with satellite-derived temperature data and current simulations, suggest that recent extreme oceanographic events, particularly the northward shift of the Kuroshio Current and warming trends of 2–4°C (this study), have facilitated the dispersal of P. mikazuki into northern temperate habitats.
Particle trajectory simulations using OceanParcels suggest that individuals of P. mikazuki could have been transported via the Kuroshio Current from southern waters, where their distribution overlapped with the northernmost distribution of P. utriculus and, thus, were undetected as a new species until reaching Sendai Bay within 30 days and extending as far north as Aomori within 45 days (supported by concurrent iNaturalist reports from Aomori (iNaturalist, 2024)). These findings highlight the dynamic nature of marine ecosystems and emphasize the influence of oceanographic changes on species distribution patterns (Martins, 2022; Bourg et al., 2024).
4.1 Speciation mechanisms and biogeographic isolation
The potential divergence of Physalia mikazuki sp. nov. in the temperate waters of Northeast Japan likely reflects complex interactions between environmental, oceanographic, and evolutionary processes. Ocean currents such as the Kuroshio and Oyashio not only influence the physical transport of neustonic organisms but also generate dynamic thermal fronts and eddies that can act as semi-permeable barriers to gene flow (Pelc et al., 2009; Treml et al., 2015). These thermal barriers, marked by steep temperature gradients and seasonal variability, may limit the dispersal of early developmental stages, especially in colonial cnidarians with environmentally sensitive larval forms (Bowen et al., 2013). Moreover, the bifurcation of these current systems along the Tohoku coastline results in regional water mass separation, potentially reducing interbreeding opportunities among Physalia populations. Over time, such physical and thermal discontinuities can lead to reproductive isolation, especially if reinforced by local adaptation or phenological shifts (Palumbi, 1994). These conditions, combined with environmental stochasticity and localized retention, may have contributed to the emergence of P. mikazuki sp. nov. as a distinct lineage. Similar processes have been proposed for other pelagic and neustonic taxa, highlighting the role of oceanographic complexity in driving cryptic speciation (Knowlton, 2000; Church et al., 2025). Our findings add to this growing body of evidence, suggesting that even wind-drifted surface organisms like Physalia are subject to fine-scale biogeographic structuring driven by both hydrographic and thermal constraints (Munro et al., 2019).
4.2 Genomic approaches and future directions
K2P (Kimura 2-parameter model (Kimura, 1980)) is a numeric value representing an estimate of average genetic divergence between DNA sequences (Kimura, 1980). K2P distances generated in this study for both the 16S and COI datasets for the 124 sequences, all previously used in the phylogenetic analysis by (Church et al., 2025), except those generated in this study for P. mikazuki from Sendai and P. utriculus from Okinawa, support significant genetic separation of P. mikazuki from the other Physalia lineages (Figure 12B) corroborating preliminary findings by Church et al., 2025, who suggested their unidentified “clade B2” was a new Physalia species. Genetic divergence calculated (mean K2P) among Physalia lineages for both the 16S rRNA gene (2.7% - 4.8%) and mtCOI (8.2% – 13.8%) are comparable to respective divergence distances reported among species of hydrozoan clades (as reviewed in (Church et al., 2025). As reported two decades ago by (Schuchert, 2005), in Hydrozoa both inter- and intraspecific variability is common. Ultimately, a combination of molecular and morphological analyses is needed especially when sympatric speciation occurs, as is likely the case with P. mikazuki and P. utriculus in Japan, whose morphological and molecular characteristics clearly differ, despite overlapping in the Kanto region (Church et al., 2024; Church et al., 2025),used a multi-genomics study to validate the presence of five separate Physalia species, one corresponding to P. mikazuki, the new species we described herein, thereby uncovering hidden diversity in this genus.
The introduction of P. mikazuki into the Tohoku region raises important ecological and public health concerns. As a new predator, it has the potential to disrupt local food webs by preying on fish eggs, larvae, and small planktonic organisms, potentially triggering cascading effects on native species and altering ecosystem dynamics (Purcell, 1984; Purcell, 1985). Competition with native predators could further impact fish populations of commercial and ecological importance (Brodeur et al., 1987; Haddad et al., 2002). Additionally, the presence of P. mikazuki sp. nov. in northeast waters poses public health risks, as stings from Physalia tentacles are known to cause severe pain and systemic reactions (Williamson et al., 1996; Burnett, 2001; Haddad et al., 2002; Yanagihara et al., 2002). These stings present a threat to beachgoers, swimmers, and fishers. Public awareness campaigns, monitoring systems, and emergency response protocols will be essential for safeguarding human activities in affected regions. The cnidome of Physalia physalis (previously considered a cosmopolitan species) has been described as comprising two sizes of holotrichous isorhizas and stenoteles, with fine details varying across time and geographic space, such as holotrichous isorhizas noted instead of atrichous isorhizas and stenoteles reported rather than euryteles (reviewed in (Yanagihara et al., 2002)). However, Yanagihara et al., 2002 reported electron microscopy evidence for the presence of anisorhizas (heteronemes) rather than isorhizas (haplonemes) in the fishing tentacles of P. utriculus of Hawaii, which further underscores the need for a comparative analysis of the cnidome among all species of this genus (Yanagihara et al., 2002).
Historical records from global expeditions conducted during the period spanning the 1700s to 1900s report envenomation symptoms following human contact with a Physalia in the water or stranded on the beach: Sloane (1707) mentioned that P. physalis in Jamaica (as Urtica Marina, soluta, purpurea, oblonga, cirrhis longissimus – Figure 7A) caused stronger burning sensation than those farther north (Sloane, 1707), and Lesueur and Petit, 1807 referred to P. utriculus as a “treacherous animal” (Figure 8A) (Lesueur and Petit, 1807). Duperrey (1830, Chapter XV, pg. 17-35) discussed at length the natural history, behavior, and prey items of different species of Physalia around the world (Figures 1A, B) and the infliction of their venom on humans and animals (based on both natural and experimental envenomation) which include: “excruciating pain causing convulsions throughout the body”, “prolonged contact resulting in raised lesions, fever, syncope (loss of consciousness) and delirium”, “the pain of the sting to the armpit spread to the heart causing a fainting spell”, “indigenous people desiccated and pulverized the tissue into an active poisonous powder mixed into food (e.g., added to chocolate by the Spanish)”, “ants, and fly larvae are known to eat them, but when a dog was fed one it responded with pain that went away in a few hours.” (Duperrey, 1830). Duperrey, 1830 provided detailed accounts of Physalia behavior, ecology, and severe symptoms from envenomation, highlighting the species’ potent venom capable of causing convulsions, delirium, and syncope (Duperrey, 1830). Indigenous practices included using Physalia tissues as toxic additives in food, further emphasizing the severe biological impacts of these organisms.
Current Physalia systematics remain minimalistic due to historical lack of any type specimens, except for P. minuta and P. mikazuki sp. nov. Resolving these taxonomic uncertainties requires further investigation. Thus, herein we provide type vouchers for our new species in the Tohoku Natural History Museum to stabilize the name. Several Physalia binomials are in current usage including P. megalista (Bourg et al., 2024) despite being synonymized with P. australis (Duperrey, 1830) which likely corresponds to a separate valid species although authorship is unclear. Additional names in use, such as P. antarctica Lesson 1826, P. tuberculosaLamarck, 1801, and P. azoricum Lesson 1826 are among species that may be junior synonyms of other Atlantic species, but much work is needed to test this hypothesis (Bigelow, 1911). Figure 8 in this study shows a plate reproduced from de La Martinière (1787) of which drawings (A) & (B) were referred to when describing the new species P. utriculus (as Medusa utriculus) from the North Pacific Ocean (20°N 179°E) (de La Martinière, 1787). Described as a wine-sac shaped organism bearing a large central tentacle and associated with blue sea dragon nudibranchs of the genus Glaucus (C) & (D) which may be the first representation of this kleptoparasitic relationship in which the small blue sea dragon floats on Physalia while nibbling and storing stolen nematocysts in its cnidosacs for personal protection and predation (Yamamoto et al., 2025). Although P. utriculus has been synonymized with P. physalis, its distinct central tentacle clearly detailed in the original description (Totton and Mackie, 1960) (Figures 1A, B, herein from the original report) distinguishes it from P. physalis which has multiple central tentacles. Although no type specimen has been designated for P. utriculus, the name has been used in several works (Yanagihara et al., 2002; Pontin and Cruickshank, 2012; Guevara et al., 2017; Pugh, 2019; Oguchi et al., 2024; Church et al., 2025). Based on the detailed illustration provided in Plate II, Figures 13 & 14 from de La Martinière P. utriculus resembles P. cf. australis (in Figure 1 Plate 5,1 (Duperrey, 1830)) suggesting Hawaii and Australia may share this species. According to our findings and those of Church et al., 2025, specimens from Okinawa belong to Cluster B1, which includes individuals from Hawaii, Guam, and the Indian Ocean, and is referred to as P. utriculus (Church et al., 2025). They also report a second lineage, Cluster B2 (Honshu), comprising specimens from Japan, Pakistan, and Mexico, for which no species name was assigned due to a lack of morphological vouchers (Church et al., 2025). Cluster B2 is P. mikazuki sp. nov. described herein.
This study validates Physalia mikazuki sp. nov. from Gamo Beach, Sendai City, Miyagi Prefecture, Tohoku region as a species new to science. It is distinguishable based on unique morphological features, and the findings of a recently published multi-genomic study and population structure analyses that supported reciprocal monophyly of five Physalia lineages (see (Church et al., 2024; Church et al., 2025)), corroborated by our robust molecular phylogenetic analyses using two mitochondrial gene markers and additional Physalia samples from Okinawa and Tohoku regions.
In this study we established specimen type and paratype vouchers for P. mikazuki sp. nov. and museum vouchers for P. utriculus from Okinawa. Additionally, we presented replicated illustrations and translated original foreign-language descriptions corresponding to P. physalis, P. utriculus, and P. megalista in the absence of museum type specimens to provide a clear reference to assist the scientific community in establishing neotypes. Going forward, establishing high-quality type material verified with molecular and morphological methods, and photographs for all Physalia taxa is essential for stabilizing the taxonomy of the genus.
Statements
Data availability statement
Supplementary data, including full-resolution phylogenetic trees (16S and COI), original tree files in Newick (.nwk) format, and multiple sequence alignments used in the analyses, are available at Zenodo: https://doi.org/10.5281/zenodo.15195677
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
CA: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YO: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. MN: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. KT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AT: Formal Analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. WS-O: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by World Premier International Research Center Initiative (WPI) AIMEC, MEXT, Japan (CA). CY was funded by the Japanese Government (MEXT) Scholarship Program. MN was funded by a grant from The Japan Science and Technology Agency (JST SPRING; No. JPMJSP2114). KT was funded by the K. Matsushita Foundation (KMMF) Scholarship.
Acknowledgments
We thank Drs. Nemoto and Kano, as well as Director Takashima of the Tohoku University Museum, for their kind assistance in accessioning the museum vouchers. We are grateful to Drs Gen Kanaya and Takeshi Yuhara (National Institute for Environmental Studies, NIES) for providing valuable insight on Physalia occurrence in the Tohoku region, including records from Gamo Beach, Sen-nan, and Yuriage. We also thank Bryson Torgovitsky and Sang Bobbit Hanna for their important contributions in reporting Physalia strandings in Okinawa. Special thanks to Non chan (Nori Suzuki) for generously sharing his knowledge of Japanese regional cultural heritage and members of the 19th Annual NCB meeting for tips on refining the Japanese vernacular, and to An chan for copy edit assistance. We are grateful to the two reviewers whose expertise and guidance facilitated the improvement of this manuscript and to the editor for his careful handling of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1653958/full#supplementary-material
References
1
Anisimova M. Gil M. Dufayard J.-F. Dessimoz C. Gascuel O. (2011). Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Systematic Biol.60, 685–699. doi: 10.1093/sysbio/syr041
2
Anthony C. J. Bentlage B. Helm R. R. (2024). Animal evolution at the ocean’s water-air interface. Curr. Biol.34, 196–203. doi: 10.1016/j.cub.2023.11.013
3
Bardi J. Marques A. C. (2007). Taxonomic redescription of the Portuguese man-of-war, Physalia physalis (Cnidaria, Hydrozoa, Siphonophorae, Cystonectae) from Brazil. Iheringia Série Zoologia.97, 425–433. doi: 10.1590/S0073-47212007000400011
4
Bigelow HB. XIII. (1911). Biscayan plankton. Part XIII.—The siphonophora. Transactions of the linnean society of london 2nd series. Zoology.10, 337–358. doi: 10.1111/j.1096-3642.1911.tb00485.x
5
Bourg N. Schaeffer A. Cetina-Heredia P. Lawes J. C. Lee D. (2022). Driving the blue fleet: Temporal variability and drivers behind bluebottle (Physalia physalis) beachings off Sydney, Australia. PloS One17, e0265593. doi: 10.1371/journal.pone.0265593
6
Bourg N. Schaeffer A. Molcard A. Luneau C. Hewitt D. E. Chemin R. (2024). Ocean wanderers: A lab-based investigation into the effect of wind and morphology on the drift of Physalia spp. Mar. pollut. Bulletin.207, 116856. doi: 10.1016/j.marpolbul.2024.116856
7
Bowen B. W. Rocha L. A. Toonen R. J. Karl S. A. (2013). The origins of tropical marine biodiversity. Trends Ecol. evolution.28, 359–366. doi: 10.1016/j.tree.2013.01.018
8
Brodeur R. D. Lorz H. V. Pearcy W. G. (1987). Food habits and dietary variability of pelagic nekton off Oregon and Washington. NOAA Technical Report NMFS57, 1979–1984.
9
Burnett J. W. (2001). Medical aspects of jellyfish envenomation: pathogenesis, case reporting and therapy. Hydrobiologia.451, 1–9. doi: 10.1023/A:1011883019506
10
Burnett J. W. Fenner P. J. Kokelj F. Williamson J. A. (1994). Serious Physalia (Portuguese man o’war) stings: implications for scuba divers. J. Wilderness Med.5, 71–76. doi: 10.1580/0953-9859-5.1.71
11
Cegolon L. Heymann W. C. Lange J. H. Mastrangelo G. (2013). Jellyfish stings and their management: a review. Mar. Drugs11, 523–550. doi: 10.3390/md11020523
12
Church S. H. Abedon R. B. Ahuja N. Anthony C. J. Destanović D. Ramirez D. A. et al . (2025). Population genomics of a sailing siphonophore reveals genetic structure in the open ocean. Curr. Biol. 35 (15), 3556–3569.e6. doi: 10.1016/j.cub.2025.05.066
13
Church S. H. Abedon R. B. Ahuja N. Anthony C. J. Ramirez D. A. Rojas L. M. et al . (2024). Global genomics of the man-o’-war (Physalia) reveals biodiversity at the ocean surface. bioRxiv.2024, 07.
14
Collins A. G. Bentlage B. Lindner A. Lindsay D. Haddock S. H. Jarms G. et al . (2008). Phylogenetics of Trachylina (Cnidaria: Hydrozoa) with new insights on the evolution of some problematical taxa. J. Mar. Biol. Assoc. United Kingdom.88, 1673–1685. doi: 10.1017/S0025315408001732
15
Collins A. G. Winkelmann S. Hadrys H. Schierwater B. (2005). Phylogeny of Capitata and Corynidae (Cnidaria, Hydrozoa) in light of mitochondrial 16S rDNA data. Zoologica Scripta.34, 91–99. doi: 10.1111/j.1463-6409.2005.00172.x
16
de La Martinière J. (1787). Mémoire sur quelques insectes [Memoir on some insects]. (Paris: Observations et Mémoires sur la Physique, sur l'Histoire Naturelle et sur les Arts et Métiers), 31, 207–366. Available online at: https://www.biodiversitylibrary.org/bibliography/6349.
17
Delandmeter P. Van Sebille E. (2019). The Parcels v2. 0 Lagrangian framework: new field interpolation schemes. Geoscientific Model. Dev.12, 3571–3584. doi: 10.5194/gmd-12-3571-2019
18
Duperrey L. I. (1830). Voyage autour du monde: exécuté par ordre du roi, sur la corvette de Sa Majesté, la Coquille, pendant les années 1822, 1823, 1824 et 1825: A. Bertrand. (Paris, France: Arthus Bertrand).
19
Fenner P. J. (1998). Dangers in the ocean: the traveler and marine envenomation. I. Jellyfish. J. Travel Med.5, 135. doi: 10.1111/j.1708-8305.1998.tb00487.x
20
Ferrer L. Gonzalez M. (2021). Relationship between dimorphism and drift in the Portuguese man-of-war. Continental Shelf Res.212, 104269. doi: 10.1016/j.csr.2020.104269
21
Garstang W. (1946). The morphology and relations of the Siphonophora. J. Cell Science.2, 103–193. doi: 10.1242/jcs.s2-87.346.103
22
Gmelin J. F. (1791). “ Vermes,” in Caroli a linnaei systema naturae per regna tria naturae, Ed. 13. Tome 1(6). Systema Naturae. Linneaeus (ed.). Ed. 13. 1: pars. 6. (Lipsiae [Leipzig]: G.E. Beer). . Ed. GmelinJ. F., 3021–3910.
23
Gmelin J. F. (1788). “ Vermes,” in Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. I, 13th ed. Ed. LinnéC. V. ( Georg. Emanuel. Beer, Lipsiae).
24
Guevara B. Dayrit J. Haddad V. (2017). Delayed allergic dermatitis presenting as a keloid-like reaction caused by sting from an Indo-Pacific Portuguese man-o’-war (Physalia utriculus). Clin. Exp. Dermatol.42, 182–184. doi: 10.1111/ced.13003
25
Haddad V. da Silveira F. L. Cardoso J. L. C. Morandini A. C. (2002). A report of 49 cases of cnidarian envenoming from southeastern Brazilian coastal waters. Toxicon.40, 1445–1450. doi: 10.1016/S0041-0101(02)00162-9
26
Hoang D. T. Chernomor O. Von Haeseler A. Minh B. Q. Vinh L. S. (2018). UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. evolution.35, 518–522. doi: 10.1093/molbev/msx281
27
Hoegh-Guldberg O. Bruno J. F. (2010). The impact of climate change on the world’s marine ecosystems. Science.328, 1523–1528. doi: 10.1126/science.1189930
28
iNaturalist. Observations of genus Physalia from Japan . Available online at: https://www.inaturalist.org/observations?nelat=47.23074&nelng=155.0957938&subview=map&swlat=19.7696372&swlng=119.2119354&taxon_id=117305 (Accessed October 5, 2024).
29
Inkscape P. (2024). Inkscape. 1.3 ed (Raleigh, North Carolina, USA: Inkscape Project).
30
Kajfasz P. (2015). A case of severe stinging caused by venomous marine animal,”Portuguese man of war”(Physalia species) in all probability. Int. Maritime Health66, 84–86. doi: 10.5603/IMH.2015.0020
31
Karunarathne K. De Croos M. (2022). Pleustonic colonies of cnidarians (Physalia physalis, Porpita porpita and Velella velella) found along the coastal belt of Sri Lanka. Indian J. Geo-Marine Sci. (IJMS).51, 45–55. doi: 10.56042/ijms.v51i01.34636
32
Katoh K. Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. evolution.30, 772–780. doi: 10.1093/molbev/mst010
33
Kehl C. Nooteboom P. D. Kaandorp M. L. van Sebille E. (2023). Efficiently simulating Lagrangian particles in large-scale ocean flows—Data structures and their impact on geophysical applications. Comput. Geosciences.175, 105322. doi: 10.1016/j.cageo.2023.105322
34
Kimura M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. evolution.16, 111–120. doi: 10.1007/BF01731581
35
Knowlton N. (2000). Molecular genetic analyses of species boundaries in the sea. Mar. Genet.420, 73–90. doi: 10.1007/978-94-017-2184-4_8
36
Kumar S. Stecher G. Suleski M. Sanderford M. Sharma S. Tamura K. (2024). MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol.41, msae263. doi: 10.1093/molbev/msae263
37
Lamarck J.-B. (1801). Systeême des animaux sans vertèbres, ou, Tableau geíneíral des classes, des ordres et des genres de ces animaux: preísentant leurs caractères essentiels et leur distribution, d’après la consideíration de leurs rapports naturels et de leur organisation, et suivant l’arrangement eítabli dans les galeries du Museíum d’hist. naturelle, parmi leurs deípouilles conserveíes: preíceídeí du discours d’ouverture du cours de zoologie, donneí dans le Museíum national d’histoire naturelle l’an 8 de la Reípublique. (Paris, France).
38
Lamarck J.-B. (1816). Histoire naturelle des animaux sans vertèbres … précédée d'une introduction offrant la détermination des caractères essentiels de l'animal, sa distinction du végétal et des autres corps naturels, enfin, l'exposition des principes fondamentaux de la zoologie (Paris: Verdière).
39
Lawley J. W. Ames C. L. Bentlage B. Yanagihara A. Goodwill R. Kayal E. et al . (2016). Box jellyfish Alatina alata has a circumtropical distribution. Biol. Bulletin.231, 152–169. doi: 10.1086/690095
40
Leloup E. (1934). Siphonophores de madras (Indes anglaises). Bull. Mus Hist Nat. Belg.10, 1–5.
41
Lesueur C.-A. Petit N.-M. (1807). Voyage de découvertes aux terres australes (Paris: Atlas Imprimerie Imperiale).
42
Letunic I. Bork P. (2024). Interactive Tree of Life (iTOL) v6: recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res.52, W78–W82. doi: 10.1093/nar/gkae268
43
Linné C. V. Caroli a Linné S. L. (1758). Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Holmiae: Impensis Direct (Holmiae (Stockholm): Laurentii Salvii).
44
Linnaeus C. (1758). Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. [The system of nature through the three kingdoms of nature, according to classes, orders, genera, species, with characters, differences, synonyms, places.]. Impensis Direct. Laurentii Salvii, Holmiae [Stockholm]. 1 (10), [iii], 824. Available online at: https://biodiversitylibrary.org/page/726886.
45
Mapstone G. M. (2014). Global diversity and review of Siphonophorae (Cnidaria: Hydrozoa). PloS One9, e87737. doi: 10.1371/journal.pone.0087737
46
Martins L. C. (2022). Modelling the occurrence of Physalia physalis in the North Atlantic Ocean at different spatial and temporal scales. (master's thesis). Universidade do Algarve, Portugal.
47
Minamoto T. Fukuda M. Katsuhara K. R. Fujiwara A. Hidaka S. Yamamoto S. et al . (2017). Environmental DNA reflects spatial and temporal jellyfish distribution. PloS One12, e0173073. doi: 10.1371/journal.pone.0173073
48
Mulyadi H. Sianturi O. (2021). “ The occurrence of harmful jellyfish outbreaks and human stung reported at recreational beaches in special region of Yogyakarta, Indonesia,” in IOP Conference Series: Earth and Environmental Science (Bristol, United: IOP Publishing) 789 (1).
49
Munro C. Siebert S. Zapata F. Howison M. Damian-Serrano A. Church S. H. et al . (2018). Improved phylogenetic resolution within Siphonophora (Cnidaria) with implications for trait evolution. Mol. Phylogenet Evol.127, 823–833. doi: 10.1016/j.ympev.2018.06.030
50
Munro C. Vue Z. Behringer R. R. Dunn C. W. (2019). Morphology and development of the Portuguese man of war, Physalia physalis. Sci. Rep.9, 15522. doi: 10.1038/s41598-019-51842-1
51
Oguchi K. Yamamoto G. Kohtsuka H. Dunn C. W. (2024). Physalia gonodendra are not yet sexually mature when released. Sci. Rep.14, 23011. doi: 10.1038/s41598-024-73611-5
52
Okada Y. K. (1932). Développement post-embryonnaire de la Physalie Pacifique Vol. 8 (Kyoto, Japan: Memoirs of the College of Science, Kyoto Imperial University Ser B), 1–26.
53
Ortman B. D. Bucklin A. Pages F. Youngbluth M. (2010). DNA barcoding the Medusozoa using mtCOI. Deep Sea Res. Part II: Topical Stud. Oceanography57, 2148–2156. doi: 10.1016/j.dsr2.2010.09.017
54
Östman C. (2000). A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Scientia Marina64, 31–46. doi: 10.3989/scimar.2000.64s131
55
Palumbi S. R. (1994). Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. systematics25, 547–572. doi: 10.1146/annurev.es.25.110194.002555
56
Pelc R. Warner R. Gaines S. (2009). Geographical patterns of genetic structure in marine species with contrasting life histories. J. Biogeography.36, 1881–1890. doi: 10.1111/j.1365-2699.2009.02138.x
57
Péron F. (1807). Voyage de découvertes aux terres australes: exécuté par ordre de Sa Majesté l’Empereur et Roi, sur les corvettes Le Géographe, Le Naturaliste, et la goélette Le Casuarina, pendant les années 1800, 1801, 1802, 1803 et 1804 (Paris: Imprimerie Impériale).
58
Pontin D. R. (2009). Factors influencing the occurrence of stinging jellyfish (Physalia spp.) at New Zealand beaches (Christchurch, New Zealand: Lincoln University).
59
Pontin D. Cruickshank R. (2012). Molecular phylogenetics of the genus Physalia (Cnidaria: Siphonophora) in New Zealand coastal waters reveals cryptic diversity. Hydrobiologia.686, 91–105. doi: 10.1007/s10750-011-0994-8
60
Prieto L. Macías D. Peliz A. Ruiz J. (2015). Portuguese Man-of-War (Physalia physalis) in the Mediterranean: A permanent invasion or a casual appearance? Sci. Rep.5, 11545. doi: 10.1038/srep11545
61
Pugh P. R. (2019). A history of the sub-order Cystonectae (Hydrozoa: Siphonophorae). Zootaxa4669, 1–91. doi: 10.11646/zootaxa.4669.1.1
62
Purcell J. E. (1984). Predation on fish larvae by Physalia physalis, the Portuguese man of war. Mar. Ecol. Prog. Series.19, 189–191. doi: 10.3354/meps019189
63
Purcell J. E. (1985). Predation on fish eggs and larvae by pelagic cnidarians and ctenophores. Bull. Mar. Science.37, 739–755.
64
Purcell J. E. (2005). Climate effects on formation of jellyfish and ctenophore blooms: a review. J. Mar. Biol. Assoc. United kingdom.85, 461–476. doi: 10.1017/S0025315405011409
65
Purcell J. Anderson P. (1995). Electrical responses to water-soluble components of fish mucus recorded from the cnidocytes of a fish predator, Physalia physalis. Mar. Freshw. Behav. Phy26, 149–162. doi: 10.1080/10236249509378936
66
Rambaut A. (2009). FigTree. Tree figure drawing tool. Available online at: http://treebioedacuk/software/figtree/ (Accessed May 14, 2025).
67
Schneider C. (1898). Mittheilungen über Siphonophoren. III. Systematische und andere Bemerkungen. Zoologischer Anzeiger21, 190.
68
Schuchert P. (2005). Species boundaries in the hydrozoan genus Coryne. Mol. Phylogenet. Evol.36, 194–199. doi: 10.1016/j.ympev.2005.03.021
69
Schuchert P. Collins R. (2021). Hydromedusae observed during night dives in the Gulf Stream. Rev. suisse Zoologie.128, 237–356. doi: 10.35929/RSZ.0049
70
Sloane H. (1707). A voyage to the islands Madera. Eds. BarbadosN.ChristophersS.. (London: B. M.)
71
Srivathsan A. Meier R. (2012). On the inappropriate use of Kimura-2-parameter (K2P) divergences in the DNA-barcoding literature. Cladistics.28, 190–194. doi: 10.1111/j.1096-0031.2011.00370.x
72
Stein M. R. Marraccini J. V. Rothschild N. E. Burnett J. W. (1989). Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann. Emergency Med.18, 312–315. doi: 10.1016/S0196-0644(89)80421-4
73
Terhaar J. Burger F. A. Vogt L. Frölicher T. L. Stocker T. F. (2025). Record sea surface temperature jump in 2023–2024 unlikely but not unexpected. Nature639, 942–946. doi: 10.1038/s41586-025-08674-z
74
Torres-Conde E. G. Rodríguez-Martínez R. E. (2024). Massive stranding of Physalia physalis (Hydrozoa: Physaliidae) on the Northwestern coast of Cuba. Revista de Investigaciones Marinas44, 5–11. doi: 10.5281/zenodo.11000091
75
Totton A. K. Mackie G. O. (1960). Studies on physalia physalis (L.). Part 1. Natural history and morphology & Part 2. Behavior and histology. Natural history morphology Discov. Rep.30, 301–368.
76
Treml E. A. Roberts J. Halpin P. N. Possingham H. P. Riginos C. (2015). The emergent geography of biophysical dispersal barriers across the Indo-West Pacific. Diversity Distributions.21, 465–476. doi: 10.1111/ddi.12307
77
Trifinopoulos J. Nguyen L.-T. von Haeseler A. Minh B. Q. (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res.44, W232–W2W5. doi: 10.1093/nar/gkw256
78
Weill R. (1934). Contribution à l'étude des cnidaires et de leurs nématocystes ((Faculté des sciences de Paris) Paris, France: Laboratoire d'évolution des êtres organisés).
79
Williamson J. A. Fenner P. J. Burnett J. W. Rifkin J. F. (1996). Venomous and poisonous marine animals: a medical and biological handbook (Sydney, Australia: UNSW Press).
80
Yamamoto G. Kanai N. Miura T. Oguchi K. (2025). Blue angels have devil hands: Predatory behavior using cerata in Glaucus atlanticus. Ecology.106, e70062. doi: 10.1002/ecy.70062
81
Yanagihara A. A. Kuroiwa J. M. Oliver L. M. Kunkel D. D. (2002). The ultrastructure of nematocysts from the fishing tentacle of the Hawaiian bluebottle, Physalia utriculus (Cnidaria, Hydrozoa, Siphonophora). Hydrobiologia.489, 139–150. doi: 10.1023/A:1023272519668
82
Zheng L. He J. Lin Y. Cao W. Zhang W. (2014). 16S rRNA is a better choice than COI for DNA barcoding hydrozoans in the coastal waters of China. Acta Oceanologica Sinica.33, 55–76. doi: 10.1007/s13131-014-0415-8
Summary
Keywords
Portuguese man-of-war, new species, ecosystem change, Kuroshio Extension (KE), distribution range
Citation
Yongstar C, Ochiai Y, Nugraha MI, Tan KC, Totsu A, Sato-Okoshi W and Ames CL (2025) Physalia mikazuki sp. nov. (Phylum Cnidaria; class Hydrozoa) blown into Japan’s northeast (Tohoku) at the whim of marine ecosystem change. Front. Mar. Sci. 12:1653958. doi: 10.3389/fmars.2025.1653958
Received
25 June 2025
Accepted
06 October 2025
Published
30 October 2025
Volume
12 - 2025
Edited by
Andrew Stanley Mount, Clemson University, United States
Reviewed by
Ramadoss Dineshram, Council of Scientific and Industrial Research (CSIR), India; Khaled Mohammed Geba, Menoufia University, Egypt
Updates
Copyright
© 2025 Yongstar, Ochiai, Nugraha, Tan, Totsu, Sato-Okoshi and Ames.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheryl Lewis Ames, ames.cheryl.lynn.a1@tohoku.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.