Abstract
Nitrite is one of the most common pollutants in high-density aquaculture systems and has a potentially serious impact on the health of fish. The gill, as the organ directly in contact with the environment, performs several vital physiological functions, including gas exchange, osmotic pressure regulation, and mucosal immunity. Using a series of nitrite concentration gradients, we investigated the impact of nitrite on tilapia gills, with a focus on its immune responses. The results indicated that nitrite exposure could significantly increase the number of mucus cells and change the subtypes of chloride cells. Nitrite exposure significantly elevated serum catalase (CAT) activity and decreased superoxide dismutase (SOD) activity. After nitrite treatment, the expression of ion channel protein genes was markedly affected, with a substantial increase in Na+/Cl− cotransporter (ncc) expression. Inflammatory gene expression in the gill was also significantly altered. Both in vitro and in vivo experiments revealed that key genes in the IL-17 signaling pathway were considerably altered after nitrite exposure. Mitochondrial damage and apoptosis in the gill tissues were also found after nitrite exposure. In conclusion, nitrite exposure altered immune function and ion transport, especially the activation of the IL-17 signaling pathway, damage to mitochondrial structure, and apoptosis in the gill. This study enhances our understanding of the mechanisms underlying nitrite-induced damage to the gill.
1 Introduction
Nitrite is an intermediate nitrogen compound in aquatic environments, produced during the nitrification of ammonia and the denitrification of nitrate (Svobodova et al., 2005). Normally, nitrite concentrations remain low (Madison and Wang, 2006). However, several factors can lead to nitrite accumulation, including 1) insufficient phytoplankton in the water, 2) an imbalance between ammonifying and nitrifying bacteria, and 3) low dissolved oxygen levels, which inhibit complete nitrification and promote denitrification (Jensen, 2003). Therefore, nitrite levels in complex aquatic environments often exceed regulatory standards. In poorly managed overfed aquaculture, nitrite concentrations can reach as high as 50 mg/L (Kroupova et al., 2005). In real-world aquaculture environments, water is exposed to direct sunlight, and the ultraviolet (UV) rays can induce the photodecomposition of water and the activation of nitrogen-containing groups (Qu et al., 2024). As a result, under the same nitrite concentration, natural aquaculture environments may exhibit greater toxicity. In waters with elevated nitrite concentrations, nitrite is enriched in different tissues of the fish, including the serum, gill, liver, and muscle (Kroupova et al., 2005).
Nitrite affects many physiological functions in fish. First, nitrite exposure damages the structural integrity of the gill. Acute nitrite exposure in Ctenopharyngodon idella leads to gill filament swelling, the fusion of second lamellae, and hyperplasia (Liu et al., 2022). Similarly, in acute nitrite exposure in Larimichthys crocea after 48 hours, secondary lamellae were distorted, slightly shrunken, and disorganized (Xu et al., 2022). Nitrite exposure induces pathological damage to gill tissue. Second, nitrite exposure disrupts the ion transport mechanisms in fish gills. In grass carp, nitrite significantly increases extracellular potassium ion concentrations (Jensen et al., 1987). Nitrite enters the gill by competing with chloride ions for the same ion channel proteins (Jensen et al., 2000) and subsequently converts functional hemoglobin into methemoglobin, reducing its oxygen-binding capacity. Similarly, in Cyprinus carpio, nitrite exposure causes arterial oxygen levels to drop to values matching the environmental oxygen content (Williams and Eddy, 1988), resulting in metabolic imbalance. Third, the gill is one of the most important mucosal immune tissues in fish. The mucosal epithelium activates a variety of innate and adaptive immune responses that function together to provide immune protection (Cabillon and Lazado, 2019). Innate immunity is supported by antimicrobial peptides, interleukins, and interferons, while adaptive immunity is mediated by B and T lymphocytes (Gomez et al., 2013). In L. crocea, nitrite stress significantly reduced lysozyme levels and increased immunoglobulin levels (Xu et al., 2022). Similarly, in Takifugu rubripes, nitrite exposure significantly increased the expression of tnf-α, il-6, and il-12 (Gao et al., 2022).
The interleukin-17 (IL-17) family is an ancient pro-inflammatory cytokine. It plays a critical role in host defense against pathogenic microbial infections and in mediating inflammatory responses. IL-17 cytokines activate key signaling molecules in the IL-17 signaling pathway, such as NF-κB, MAPK, and C/EBP. These molecules, in turn, initiate immune responses or promote cell proliferation (Gu et al., 2013; Zhong et al., 2023). In fish, the gills are one of the most important mucosal immune and barrier organs (Lazado and Caipang, 2014). Notably, IL-17 functions as a tissue-signaling cytokine that contributes to the protection of barrier organs such as the skin and lungs (Eyerich et al., 2017). The IL-17 signaling pathway plays a protective role in mucosal tissues by enhancing host defense against bacterial infections (Gaffen and Moutsopoulos, 2020). Thus, the IL-17 signaling pathway plays a central role in the immune responses of the gills. Studies have demonstrated that nitrite stress disrupts metabolic homeostasis (Yang et al., 2024; Ha et al., 2019) and induces oxidative damage in fish (Zhang et al., 2022; Guo et al., 2020). Prolonged exposure to harmful aquatic environmental conditions may lead to a state of chronic inflammation (Agostini et al., 2018). Meanwhile, the IL-17 signaling pathway has been identified as a key regulator of chronic inflammation and immune responses (Ritzmann et al., 2022). It also plays an important role in the regulation of cellular and systemic metabolic processes (Bechara et al., 2021). For example, transcriptomic and proteomic studies have shown elevated IL-17 signaling pathway gene and protein expression in the gills of Pseudobagrus fulvidraco exposed to ammonia (Zhong et al., 2023). In Scophthalmus maximus infected with Vibrio anguillarum, the mRNA expression levels of il-17c and its receptor were also significantly upregulated (Xue et al., 2021). Taken together, current evidence reveals a knowledge gap in understanding the role of the IL-17 signaling pathway in fish gills under nitrite stress.
Meanwhile, studies have reported that the toxicity of harmful environmental conditions is closely associated with mitochondrial damage and apoptosis. In Danio rerio, arsenic exposure induced mitochondrial autophagy, leading to mitochondrial damage (Zhang et al., 2023a). Similarly, abamectin exposure in grass carp caused structural damage and a decrease in membrane potential (Wu et al., 2023). The expression of the pro-apoptotic gene bax was significantly upregulated, while the anti-apoptotic gene bcl-2 was significantly downregulated (Xie et al., 2019). In the study of nitrite exposure in T. rubripes, the expression of apoptosis-related genes, such as caspase-3, caspase-8, caspase-9, and p53, was significantly upregulated. In contrast, the expression of Bcl-2 was considerably decreased (Gao et al., 2020a).
The gill is a vital organ for gas exchange and the regulation of osmotic pressure in fish. It is also in direct contact with aquaculture water, serving as a crucial chemoreceptor. However, research on the effects of nitrite stress on the gill and its underlying mechanisms remains limited. This study aims to investigate the effects of nitrite exposure on tissue damage, ion transport, immune functions, and apoptosis in the gill.
2 Materials and methods
2.1 Experimental animals
The Genetically Improved Farmed Tilapia (Oreochromis niloticus) used in the experiment, weighing 100 ± 10 g, was purchased from Huadu, Guangzhou, China. Following the acquisition, the fish were kept in water with a temperature maintained at approximately 28 °C, dissolved oxygen levels between 9 and 10 mg/L, and a pH range of 7.1–7.6. The fish were fed a commercial diet (crude protein 32%, crude fat 3.9%, starch 26.4%, and ash content 7.5%) at a rate of 1% of their body weight, twice daily. The experiments were conducted after a 2-week domestication period.
2.2 Experimental design
The domesticated tilapia were divided into two weight classes based on their body weight: Class 1 (90–100 g) and Class 2 (100–110 g). Within each class, the fish were assigned to eight experimental groups using a simple randomization method, with each group comprising 12 fish. Each group was assigned to a separate 120-L tank in a random order to avoid any potential bias related to tank placement. A pre-laboratory study was conducted to determine the lethal nitrite concentration for tilapia. In this study, tilapia were exposed to various nitrite concentrations (ranging from 0 to 90 mg/L) over 14 days. The results showed that tilapia began to die when the nitrite concentration exceeded 60 mg/L. Accordingly, eight nitrite treatment groups at concentrations of 0, 30, 40, 50, 60, 70, 80, and 90 mg/L were established by dissolving the corresponding amount of sodium nitrite (FuChen Chemistry, Tianjin) in 100 L of water. Two-thirds of the water in the tanks was replaced every 24 hours. The nitrite concentrations were corrected and measured using a nitrite kit (EasyBox, CircleCare Biologicals, Beijing), keeping the temperature and pH levels in the tank stabilized.
The study adhered to the ethical guidelines for experimental animals set forth by the Ethics Committee of Sun Yat-sen University (Approval No. SYSU-IACUC-2023-B0453) in June 2023.
2.3 Isolation and culture of gill cells
To perform cardiac perfusion using phosphate-buffered saline (PBS) solution, the connection between the heart and the vein was dissected by making a precise incision. Subsequently, a 1-mL syringe was inserted into the arterial bulb of the fish. PBS was then injected into the arterial bulb, which facilitated the displacement of blood from the gill. Gill filaments were excised using sharp scissors, and mucus was removed through multiple washes using PBS. Gill filaments were digested with 0.25% trypsin (Gibco, Massachusetts) following mechanical trituration using a tissue chopper (The Mickle Laboratory Engineering Co., Ltd., HongKong). The viability of gill cells isolated from fish was assessed using propidium iodide (PI; YuanYe, Shanghai) staining. In each experimental instance, the viability of the cells obtained was consistently above 95%. The cells were plated onto a 24-well plate ( NEST, Wuxi) pre-coated with poly-d-lysine (MCE, Shanghai). The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Cytiva, Hangzhou) supplemented with 2% penicillin/streptomycin/amphotericin (YuanYe) and 10% fetal bovine serum (Gibco). The culture was maintained in an incubator at 28 °C with a CO2 atmosphere.
2.4 Histological and ultrastructure studies
After 7 days of nitrite exposure, gill filaments were collected from four to five randomly selected fish per group. The tissues were fixed, dehydrated, and sectioned at 5-μm thickness using a microtome (Leica, Wetzlar). The gill section was stained with hematoxylin and eosin (H&E). Three representative sections from each group were randomly selected and examined under a Nikon orthoptic microscope.
Additionally, gill filaments from three randomly selected fish per group were dissected, and 1-mm3 samples of filament ends were fixed in 3% glutaraldehyde. The gill filaments were dehydrated through a graded ethanol and subsequently observed using a focused ion and electron dual-beam scanning electron microscope (Carl Zeiss, Oberkochen).
2.5 Serum collection and oxidative stress parameter analysis
Twelve serum samples were collected from each group. Tilapia were anesthetized by immersion in an ice-water mixture for 10 seconds. Blood was drawn from the vein. A 1-mL blood sample was incubated at room temperature for 2 hours and subsequently centrifuged at 7,500 rpm for 10 min to separate the serum. The serum was used to assess the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) using commercial assay kits (Beyotime, Shanghai).
2.6 Gene expression analysis
Twelve samples were collected from each group for quantitative real-time PCR (qPCR) analysis. Total RNA was extracted using TRIzol reagent (Omega, Cambridge), and its concentration and purity were measured using a Nanodrop 2000 spectrophotometer (Bio-Rad, California). Reverse transcription was performed using the MLV Reverse Transcriptase Kit (Thermo Fisher, Massachusetts). qPCR was conducted using the SYBR green kit (Accurate Biology, Changsha) on a Roche real-time PCR system with a reaction volume of 10 μL.
All the primers used in this study were designed using the NCBI Primer Design tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primer sequences for the internal control gene (β-actin) and genes related to inflammation and immunity (tnf-α, il-1β, and ifn-γ), ion transportation (ncc, nhe, and nka1), and the IL-17 signaling pathway (mapk, fosb, traf4a, il-1β, il-8, and il17ra) are provided in the Supplementary Material (Supplementary Table S1). All primers were verified for specificity using a melting curve analysis in qPCR. The real-time PCR system was 10 μL, and the reaction group was divided into 5 μL 2× SYBR green mix (Accurate Biology), 0.3 μL each of the primers (10 μM stock), 0.5 μL of the template (100 ng/μL), and 3.4 μL of ultrapure water. There was one technical replicate for each reaction. The reaction conditions were initial denaturation at 95 °C for 1 min and amplification at 95°C for 15 s, 58°C for 15 s, and 72°C for 30 s, 40 cycles. The relative expression levels of the target genes were calculated using the formula 2−ΔΔCT.
2.7 Analysis of mitochondria in gill tissues
After exposure to 60 mg/L of nitrite for 7 days, gill filaments were collected from tilapia for transmission electron microscopy (TEM), with three fish sampled per group. The gill filaments were initially fixed in 3% glutaraldehyde for 24 hours. They were dehydrated through a gradient of ethanol concentrations. Sectioning was performed at the electron microscopy platform of the School of Life Sciences, Sun Yat-sen University. Images were captured using a 120-kV transmission electron microscope (Jeol Ltd, Tokyo).
2.8 Analysis of apoptosis in gill tissues
After 7 days of exposure to 60 mg/L nitrite, gill filaments were randomly sampled from three fish in each group. The fish were euthanized using eugenol, and tissues were fixed in 4% paraformaldehyde for 24 hours. The samples were then dehydrated through gradient concentrations of ethanol and xylene, embedded in paraffin, and sectioned into 5-μm slices using an automatic microtome (Leica). The sections were incubated overnight at 37°C and subjected to apoptosis staining using a TUNEL Apoptosis Assay Kit (Beyotime).
2.9 Statistical analyses
Statistical analyses were performed using SPSS version 27.0 (SPSS Inc., Chicago, IL, USA), and data visualization was conducted using GraphPad Prism (GraphPad Software, USA). Data were first tested for normality using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. Statistical comparisons between two groups were performed using Student’s t-test or a non-parametric test, depending on the result of the normality test. For datasets involving more than three groups, statistical analyses were conducted based on the distribution characteristics and variance homogeneity of the data. Specifically, when the data were confirmed to follow a normal distribution and exhibit homogeneity of variance, one-way ANOVA was employed to examine the differences among groups. Subsequently, Dunnett’s test was applied for post-hoc comparisons to assess differences between the control group and each experimental group (p < 0.05). In cases where the data did not meet the assumptions of normality or homogeneity of variance, the Kruskal–Wallis test was used. Post-hoc comparisons were adjusted using the Bonferroni correction (p < 0.05).
3 Results
3.1 Nitrite exposure caused histological damage to the gill filament
A significant number of mucus cells (as shown in the black arrow in Figure 1) were observed at the end of the gill filaments after nitrite stress. The most significant increase in mucus cells was observed in the 60 mg/L and 90 mg/L treatment groups.
Figure 1

Hematoxylin and eosin (H&E) staining of gills under different concentrations of nitrite treatment. Black arrows indicate mucus cells. The magnification of the microscope was ×60. Number of tissue vacuoles in filaments following exposure to different concentrations of nitrite. Values indicate the mean ± SEM (n = 3). *p < 0.05; **p < 0.01.
3.2 Change in chloride cell subtypes and ion transport function
Chloride cells were observed using scanning electron microscopy (Figure 2), with the majority in the control group exhibiting a deep-hole morphology. However, the number of shallow-basin and wave-convex chloride cells increased after exposure to nitrite concentrations of 60 and 90 mg/L.
Figure 2
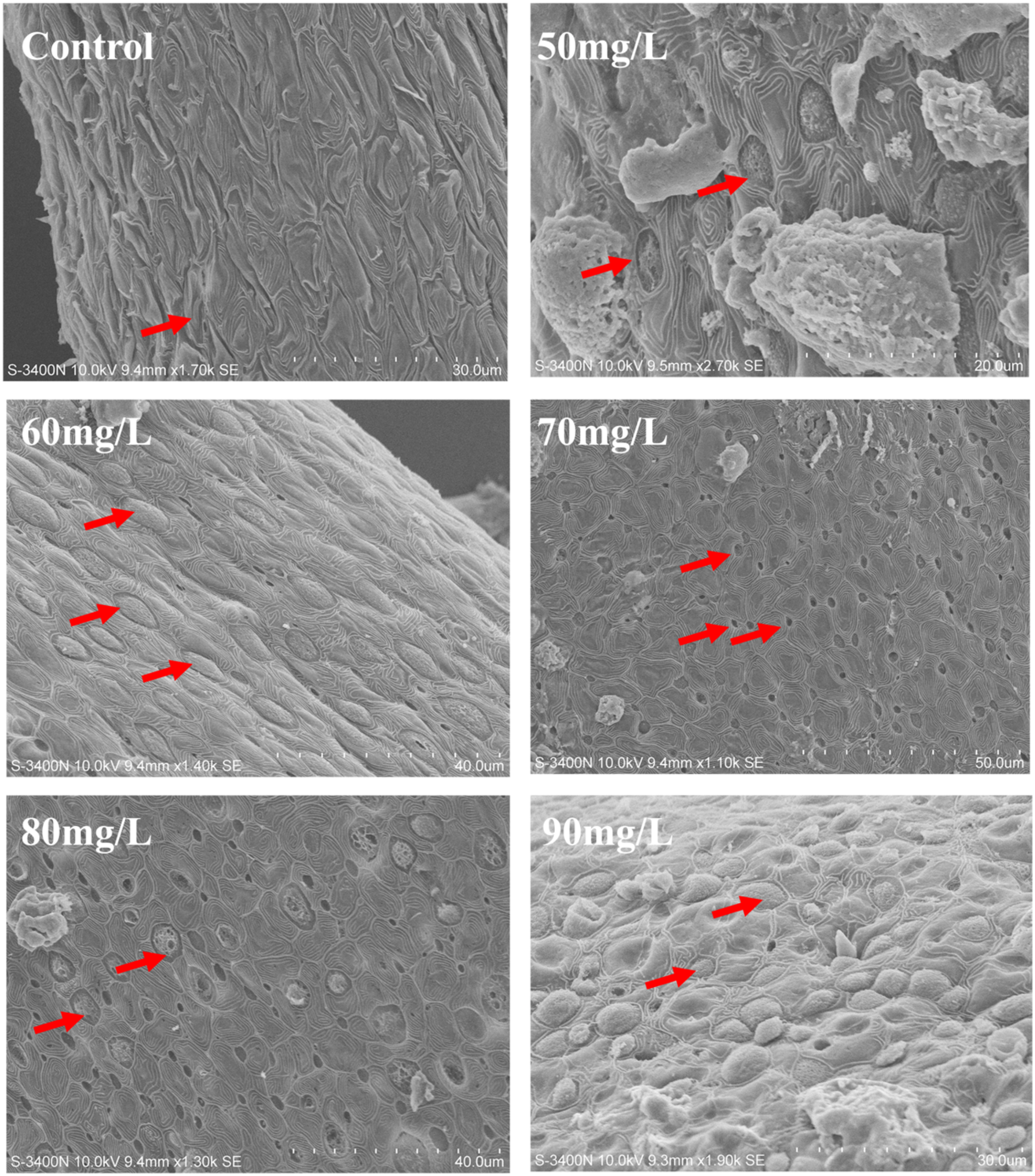
Scanning electron micrographs of the number and morphological changes of chlorine cells on gill filaments under different concentrations of nitrite treatment. The red arrows are the chlorine cells.
Nitrite treatment also induced an increasing trend of ncc expression in the gill, with significant increases observed at concentrations of 70, 80, and 90 mg/L (Figure 3A). Nitrite exposure significantly reduced nhe expression in the gill, with a significant decrease at 50, 60, 70, and 80 mg/L (Figure 3B). Similarly, the expression of nka1 was significantly reduced at 50, 60, 70, and 90 mg/L (Figure 3C).
Figure 3
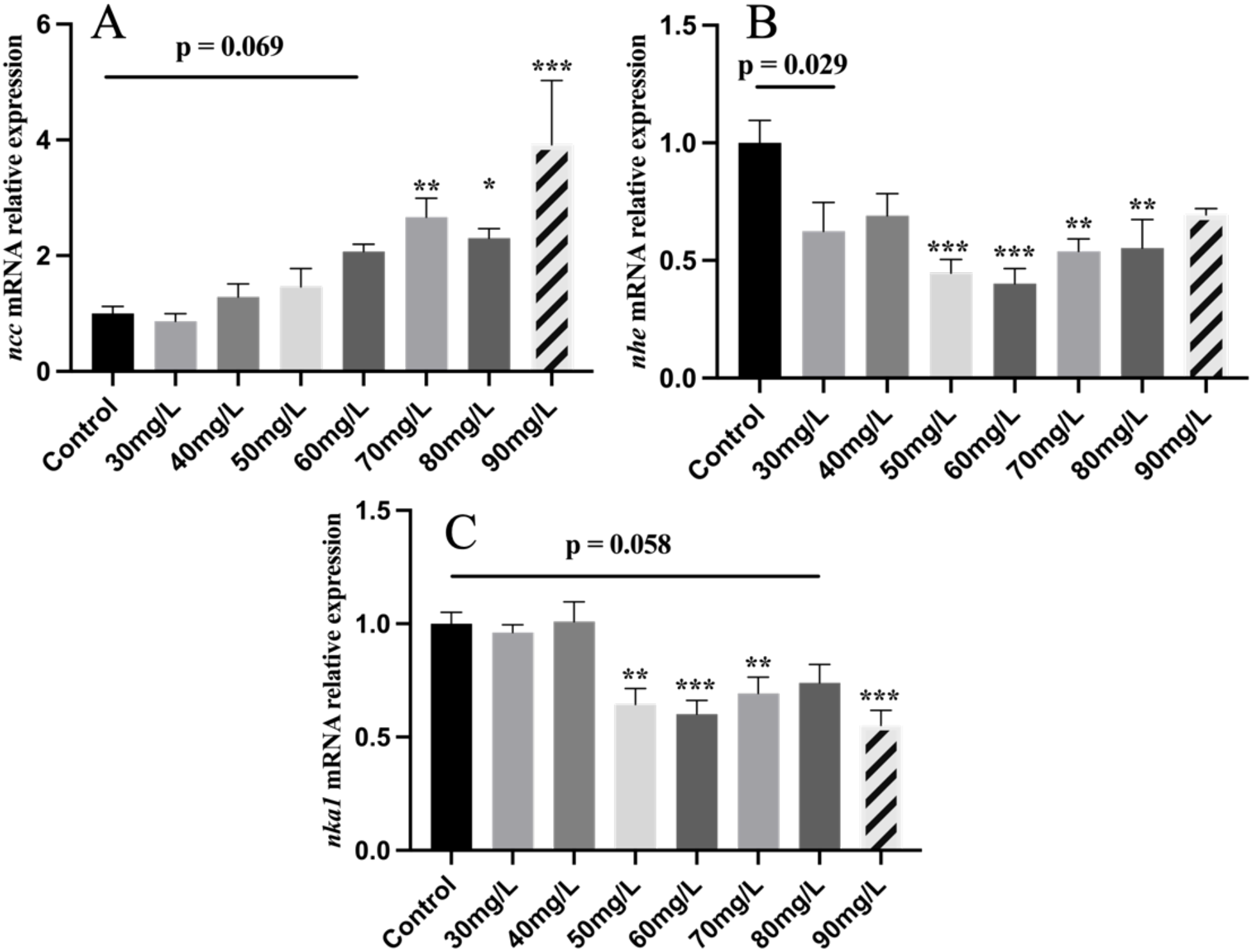
The mRNA levels of ion transportation genes in Nile tilapia treated with different concentrations of NO2−, including ncc (A), nhe (B), nka1 (C). Values indicate the mean ± SEM (n = 6-12). *p < 0.05; **p < 0.01; ***p < 0.001.
3.3 Nitrite exposure caused oxidative stress in fish
As shown in Figure 4, CAT activity in tilapia serum was significantly increased in the 40, 50, 70, and 90 mg/L nitrite-treated groups. In contrast, SOD activity was significantly reduced in the 30, 50, and 80 mg/L nitrite-treated groups, while no significant change was observed in the level of GSH-Px.
Figure 4
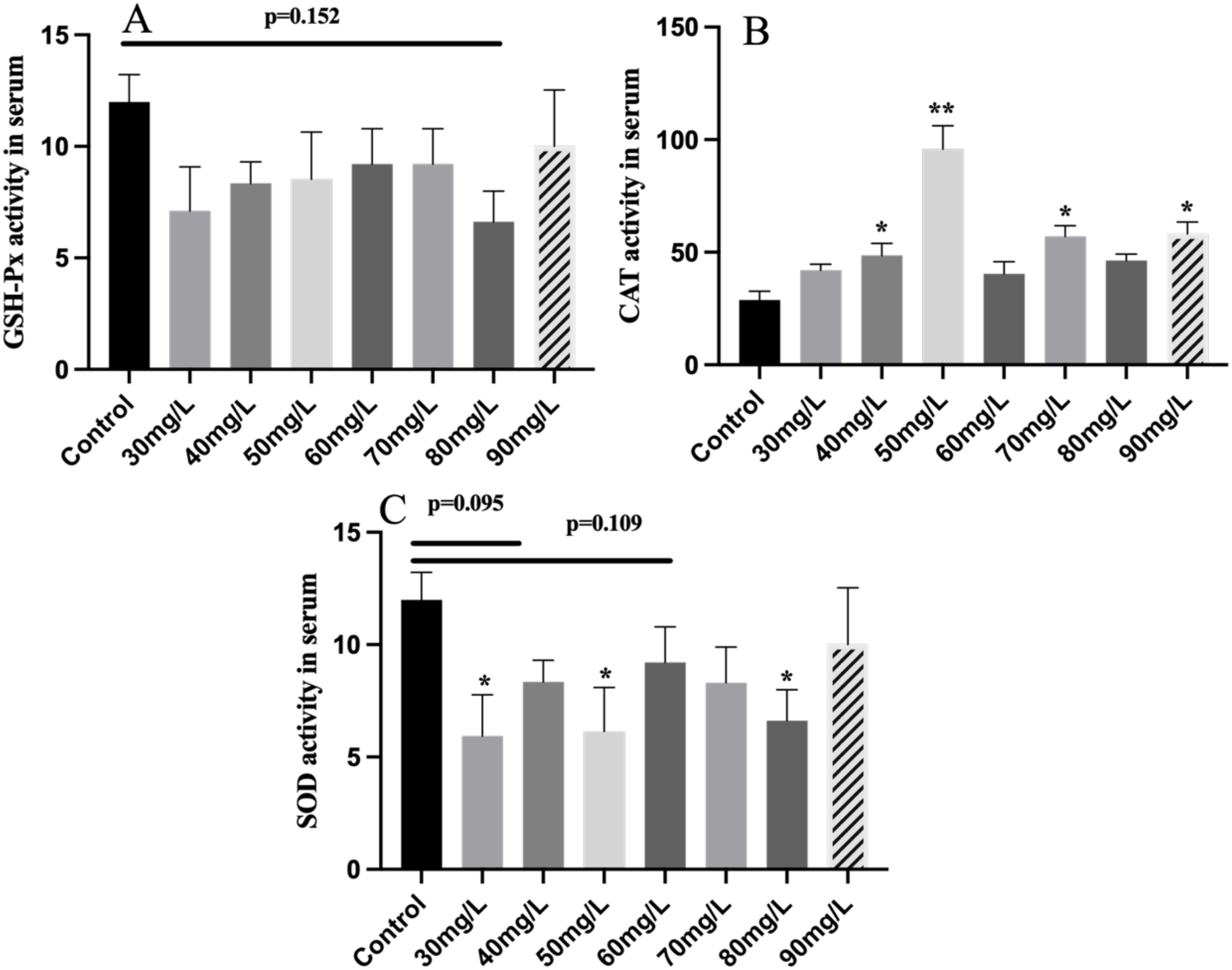
The antioxidant enzyme activity of serum in Nile tilapia treated with different concentrations of NO2−, including GSH-Px (A), SOD (B), and CAT (C). Values indicate the mean ± SEM (n = 6–12). *p < 0.05; **p < 0.01. GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase.
3.4 Effects of nitrite exposure on the gill immune gene
As shown in Figure 5, ifn-γ expression exhibited a decreasing trend following nitrite stress, with significant downregulation at 40 and 90 mg/L concentrations (Figure 5C). The expression of tnf-α showed an increasing then decreasing trend, showing a significant increase at 60 mg/L (Figure 5A). After nitrite exposure, il-1β expression significantly increased only at 90 mg/L, with no notable changes at other concentrations (Figure 5B). The expression of hsp70 tended to increase after nitrite exposure, with significant increases observed at the 60, 70, 80, and 90 mg/L treatment groups (Figure 5D).
Figure 5
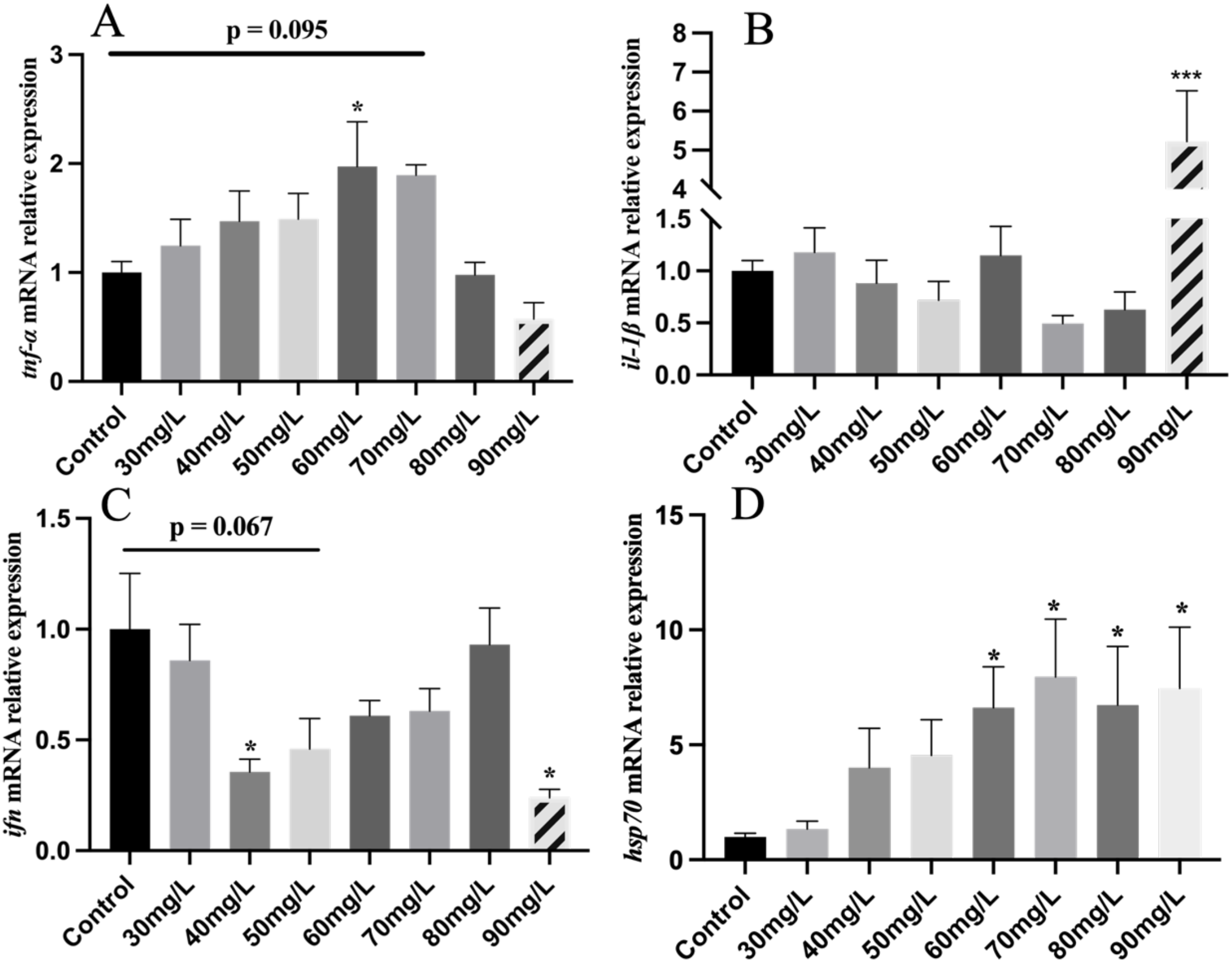
The mRNA levels of inflammation and immunity genes in Nile tilapia treated with different concentrations of NO2−, including tnf-α (A), il-1β (B), ifn (C), hsp70 (D). Values indicate the mean ± SD (n = 6-12). *p < 0.05; ***p < 0.001.
3.5 Nitrite exposure activated the IL-17 signaling pathway
After exposing gill cells to 60 mg/L nitrite for 3 and 6 hours, the expression of key genes in the IL-17 signaling pathway was assessed (Figure 6). The expression of mapk was significantly increased, while fosb mRNA abundance was significantly downregulated after 6 hours of 60 mg/L nitrite treatment. However, traf4a expression showed an increasing trend. In addition, exposure to 30 and 60 mg/L nitrite significantly upregulated il-1β expression, while il-8 expression also tended to increase. The expression of il17ra, the receptor gene of IL-17, was significantly increased after 6 hours of 60 mg/L exposure.
Figure 6
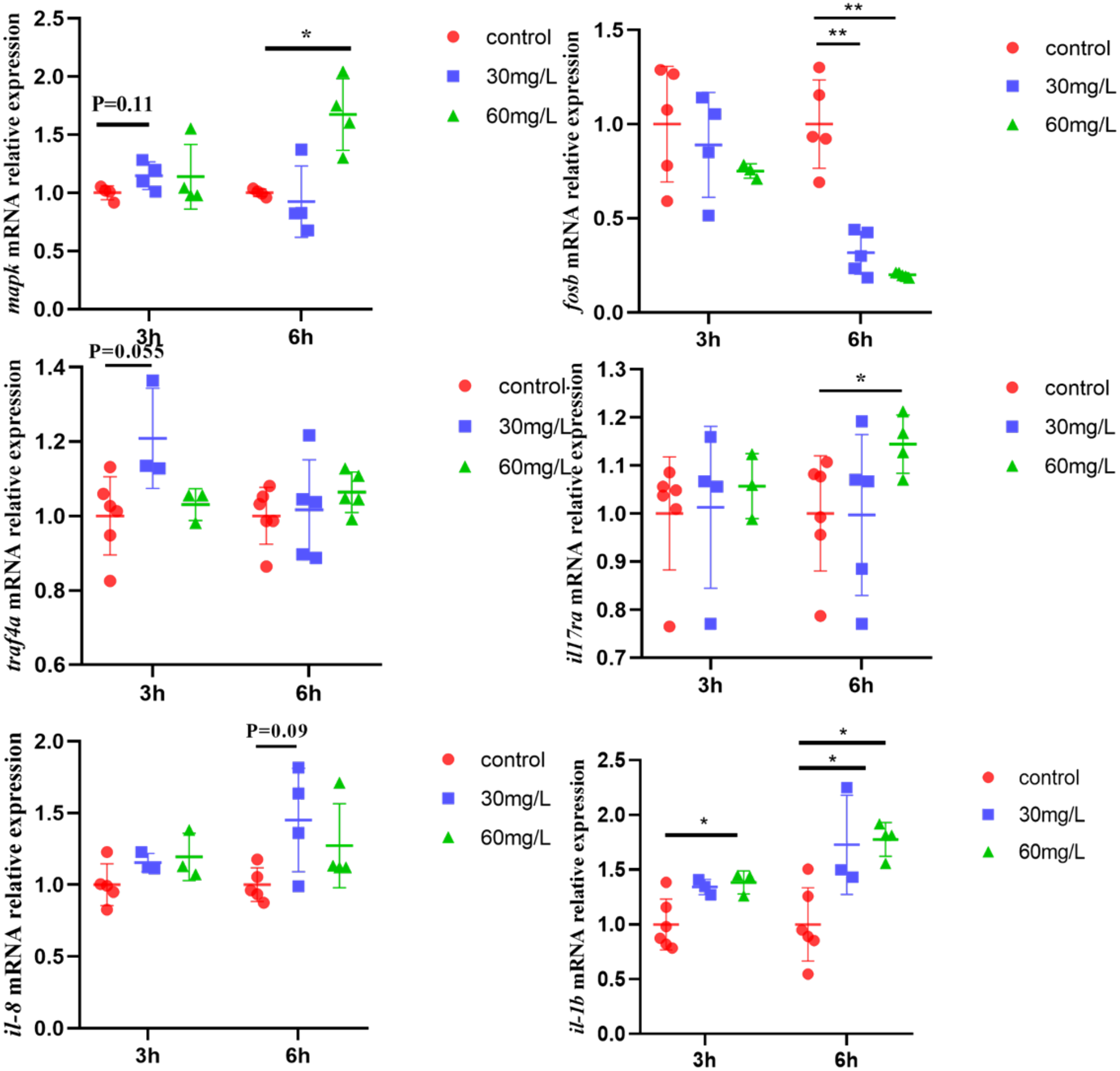
The mRNA levels of IL-17 signaling pathway key genes in primary gill cells treated with NO2−. Values indicate the mean ± SEM (n = 4–6). *p < 0.05; **p < 0.01.
In a parallel experiment, tilapia were exposed to 60 mg/L nitrite for 7 days to examine the in vivo expression of IL-17 pathway-related genes (Figure 7). The results showed that the expression of traf4a and mapk was significantly upregulated, while that of fosb was significantly downregulated. il-1β expression showed an upward trend, and il17ra was significantly upregulated.
Figure 7
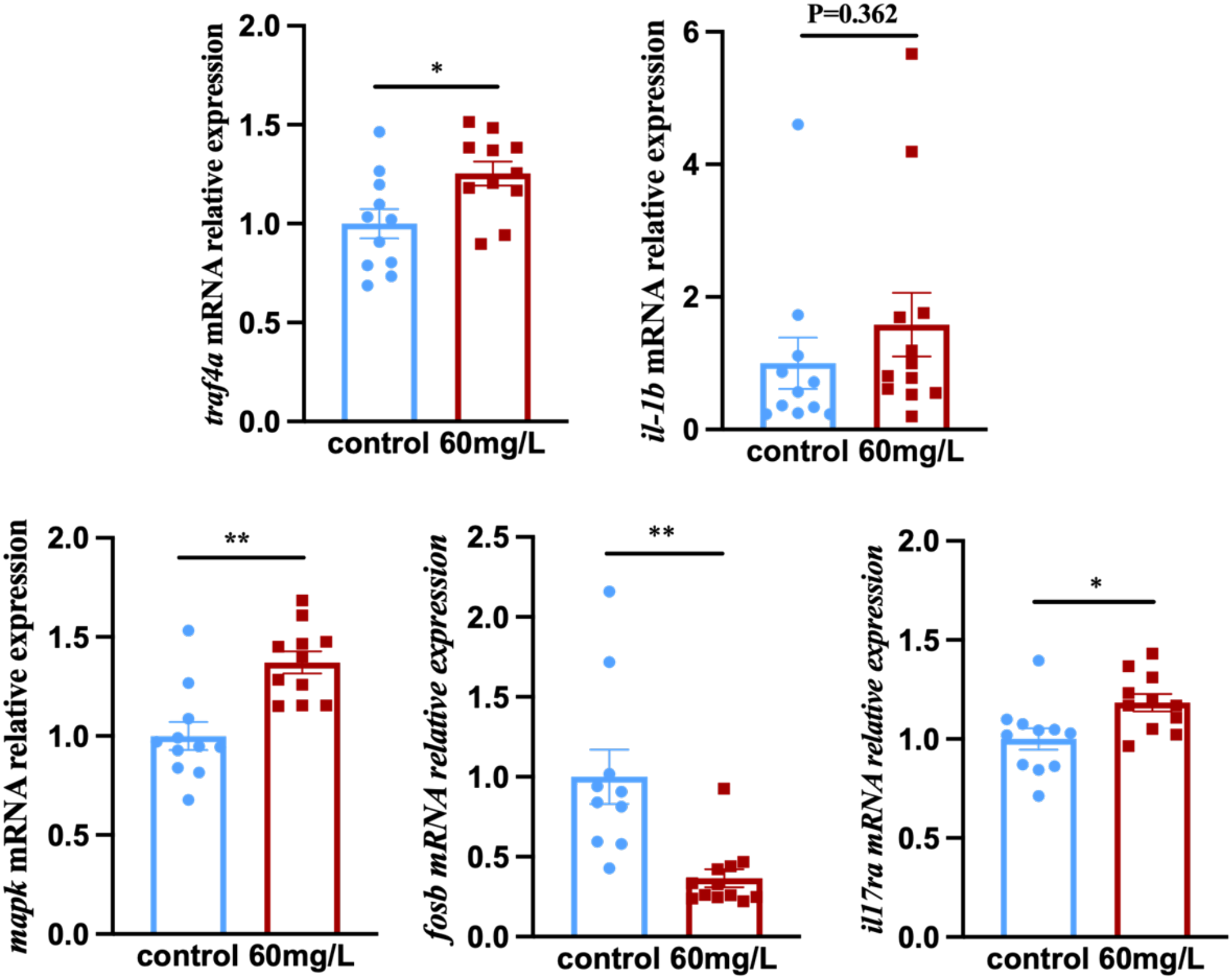
The mRNA levels of IL-17 signaling pathway key genes in tilapia treated with NO2−. Values indicate the mean ± SEM (n = 10–12). *p < 0.05; **p < 0.01.
3.6 Nitrite exposure led to mitochondrial structural damage and apoptosis
Transmission electron microscopy showed clear differences between the control and nitrite-treated groups (Figure 8E). In the control group, the gill mitochondria had long and well-organized cristae (Figures 8A, B). In contrast, the nitrite-treated group showed fragmented, punctate, and disorganized cristae (indicated by red arrows in Figures 8C, D), indicating mitochondrial damage.
Figure 8
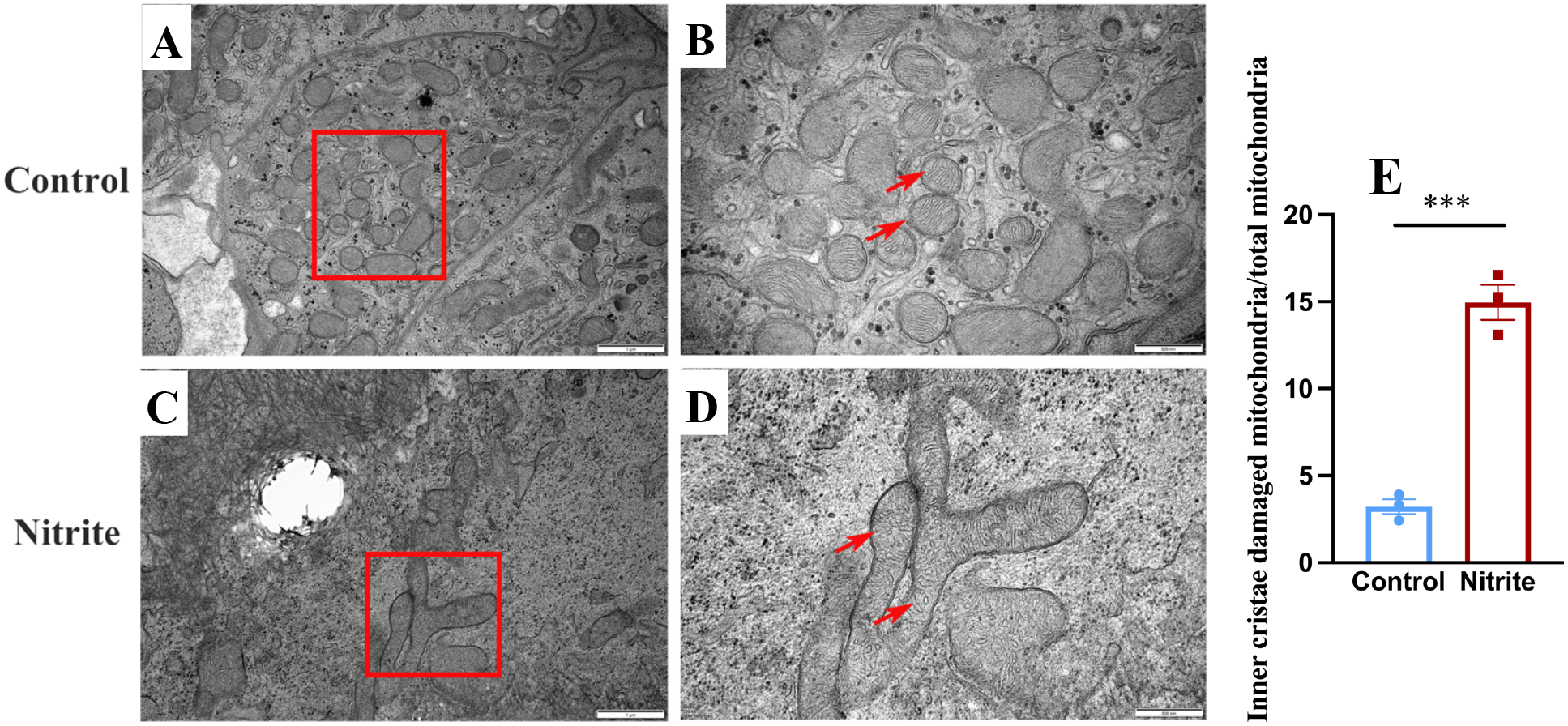
Transmission electron micrographs of gill filaments exposed to 60 mg/L nitrite for 7 days. Control group (A, B) and nitrite group (C, D). The red arrows are the inner cristae of mitochondria. (E) Proportion of mitochondria with damaged inner cristae before and after nitrite exposure. Values indicate the mean ± SEM (n = 3). ***p < 0.001.
A TUNEL apoptosis assay was performed after 7 days of nitrite exposure. As shown in Figures 9D–F, numerous Fluorescein isothiocyanate (FITC) positive green fluorescent cells were detected in the interlamellar at the end of gill filaments, in contrast to the control group (Figures 9A–C), indicating the presence of apoptotic cells in this region (Figure 9G).
Figure 9
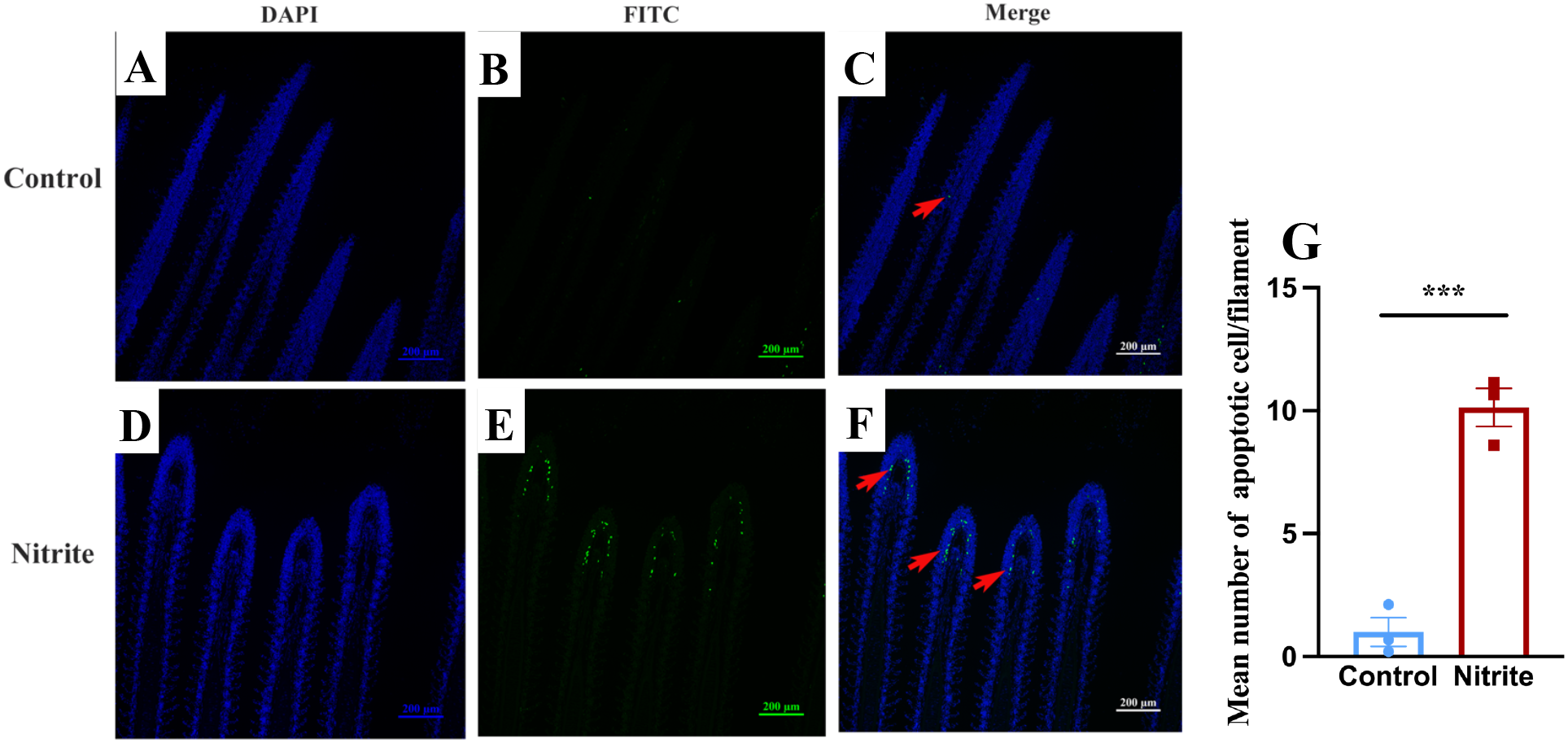
TUNEL apoptotic staining was performed on the gill filaments of tilapia exposed to 60 mg/L nitrite for 7 days: (A, D) 4'6-Diamidino-2'-phenylindole (DAPI) staining, (B, E) FITC staining, and (C, F) Merge. (G) Average number of apoptotic cells per filament. Values indicate the mean ± SEM (n = 3). ***p < 0.001.
4 Discussion
Nitrite is a common pollutant in aquaculture environments and is toxic to aquatic organisms, impairing various physiological functions. In this study, we exposed tilapia to graded concentrations of nitrite for 7 days to investigate the effects of nitrite on gill structure and immune function. We investigated the histological and ultrastructural changes of the gill after nitrite treatment. H&E staining of the sections revealed a significant increase in the number of mucus cells after nitrite treatment. These findings are in line with those of other studies on nitrite exposure (Dos Santos Silva et al., 2018). Increased mucus production is also considered a biomarker of harmful environmental conditions affecting the gills (Valdenegro-Vega et al., 2014; Muthukumaravel et al., 2023). In our study, high levels of nitrite stress significantly increased the number of mucus cells, suggesting that the mucosal immune function of the gills was “challenged”, damaging the gill histology.
Chloride cells with different morphologies of apical surfaces—deep-hole, shallow-basin, and wavy-convex—were observed on the gill filament. The wavy-convex subtypes express the ncc ion channel protein (Dymowska et al., 2012). The wavy-convex and shallow-basin subtypes are responsible mainly for the uptake of Cl− and Ca2+, respectively, in freshwater fish (Chang et al., 2001). Scanning electron microscopy revealed an increase in the number of wave-convex-subtype chloride cells after nitrite exposure. A similar change was observed under chloride-deficient conditions (Lin and Hwang, 2004). Previous research has shown that nitrite and chloride ions competitively inhibit each other’s entry into the gills (Williams and Eddy, 1986), both entering the fish through ncc ion channel proteins (Chang et al., 2001). This suggests that increased nitrite levels impair chloride uptake, resulting in a shift in chloride cell subtypes, and that nitrite exposure may alter the ion transport function of fish gills. Meanwhile, nitrite at concentrations of 70, 80, and 90 mg/L significantly increased the expression of the ncc in the gill. Notably, ncc is the most important pathway for chloride ion uptake in tilapia (Horng et al., 2009). Na+/H+ exchanger (NHE) plays a key role in sodium ion uptake (Edwards et al., 2001). It has been found that nitrite and chloride ions competitively inhibit each other’s entry into the gills through ncc ion channel proteins. These findings suggest that elevated nitrite levels reduce chloride ion availability in fish, thereby inducing the upregulation of ncc expression in the gill. This is consistent with the results of scanning electron microscopy and further demonstrates that nitrite stress reduces chloride ion uptake and alters ion transport function in fish. Meanwhile, nitrite exposure at concentrations of 50, 60, 70, and 80 mg/L significantly downregulated nhe expression in the gill. The significant downregulation of nhe may be attributed to the addition of sodium nitrite to the water. Increased sodium ion levels, in turn, induce a compensatory reduction in nhe expression. Furthermore, significant reductions in nka1 mRNA levels were observed in the 50, 60, and 90 mg/L nitrite-treated groups. The downregulation of nka1 expression likely reflects an adaptive regulatory response to the elevated sodium ion concentration in the water. A limitation of our study is that changes in ion levels and osmolality in fish serum were not assessed. However, this did not affect the validity of our conclusions. Taken together, the alterations in ion transport-related gene expression observed after nitrite stress likely represent compensatory responses. These changes suggest a shift in ion transport patterns and capacity, reflecting the gill’s adaptive mechanisms to cope with nitrite stress.
SOD, CAT, and GSH-Px constitute the primary antioxidant defense system in fish, helping to mitigate oxidative stress. SOD catalyzes the conversion of superoxide radicals into hydrogen peroxide (Korkmaz et al., 2023). In this study, SOD activity was significantly reduced under nitrite concentrations of 30, 50, and 80 mg/L. CAT plays a crucial role in removing hydrogen peroxide and minimizing oxidative damage (Wang et al., 2022). The activity of CAT was significantly increased in the 30, 50, 70, and 90 mg/L nitrite treatments. This response is in line with findings from a study on acute nitrite stress in L. crocea, where similar trends in CAT and SOD activities were observed (Xu et al., 2022; Sun et al., 2020). The reduction of SOD activity may be attributed to the gills’ limited ability to neutralize external oxidative stimuli, thereby impairing enzymatic function. In contrast, the increased CAT activity indicates that the fish activated compensatory antioxidant responses to mitigate nitrite-induced oxidative stress. GSH-Px activity showed no significant change, suggesting that it may play a less prominent role in the antioxidant response to nitrite stress compared to SOD and CAT.
IL-1β is induced by bacterial lipopolysaccharide (LPS) and acts as a key mediator in the immune response to microbial infections and tissue injuries. It activates lymphocytes and stimulates the release of other pro-inflammatory cytokines (Evavold et al., 2018). IFN-γ stimulates the synthesis of antiviral factors that play a crucial role in defending against viral infections (Sieger et al., 2009). Compared to the control group, the relative expression of il-1β significantly increased after 90 mg/L nitrite treatment. Similarly, ifn-γ expression also declined, with significant reductions observed at 40 mg/L. Similar trends in il-1β expression have been reported in studies examining the effects of glyphosate stress in tilapia, suggesting a comparable impact to nitrite stress (Zheng et al., 2021). The decreased levels of il-1β in the gills may suggest a weakening of the immune response. The sharp increase at 90 mg/L may indicate a dysregulated immune response resulting from excessive nitrite-induced stress. The significant upregulation of ifn-γ expression suggests that nitrite stress may enhance the gill’s susceptibility to microbial infection in the aquatic environment. TNF-α is a pro-inflammatory cytokine that works in synergy with IL-1β to mediate a range of inflammatory responses (Sonmez Kaplan et al., 2023). Compared to that in the control group, the expression of tnf-α was significantly increased at 60 mg/L nitrite treatments. Acute nitrite exposure also significantly upregulated the expression of tnf-α and hsp70 in T. rubripes (Gao et al., 2020b). High levels of nitrite treatment groups significantly increased hsp70 expression. Similarly, the expression of hsp70 was significantly upregulated in the gill during acute nitrite stress in juvenile S. maximus (Jia et al., 2016). Changes in hsp70 expression levels reflect the stability of the fish’s physiological state. The upregulation of hsp70 in response to external stressors suggests that the fish are experiencing chronic stress. In addition, the expression of tnf-α was not consistent with that of il-1β, implying that a tnf-α-specific immune regulatory pathway may be involved in the response to nitrite stress.
The gill is a critical mucosal immune organ in fish and serves as a primary barrier between the fish and the external environment (Zaccone, 2022). IL-17 is a common cytokine secreted by immune cells and plays an essential role in the body’s defense against external pathogens. IL-17 is involved in regulating several crucial immune pathways (Saco et al., 2021). In our preliminary experiment, 7-day nitrite exposure significantly increased il-17 expression. This prompted us to investigate whether the IL-17 signaling pathway plays a critical immune role in the gill.
The IL-17 signaling pathway plays an important role in the body’s defense against external pathogens and autoimmune responses (Blauvelt and Chiricozzi, 2018). In this study, we examined changes in key genes of the IL-17 signaling pathway after nitrite exposure both in vitro and in vivo. The results from both in vivo and in vitro experiments were generally consistent. The expression of key genes in the IL-17 signaling pathway was significantly upregulated in both settings. Interestingly, only fosb expression was significantly downregulated. In a study of S. maximus infected with V. anguillarum, a significant increase in the expression of both il-17c and its receptor was found in the skin (Xue et al., 2021; Ni et al., 2023). Our study focused on the expression of the IL-17 gene and receptors and further examined changes in key downstream genes within the IL-17 signaling pathway. IL-17 primarily transmits signals through IL-17RA and IL-17RC heterodimers (Huangfu et al., 2023). In this study, il17ra was selected for analysis because it is more broadly expressed in all tissues, while il17rc is mainly expressed in non-hematopoietic epithelial cells and mesenchymal cells. Notably, fosb expression was significantly downregulated, in contrast to the upregulation observed for other genes in the IL-17 signaling pathway (Veldhoen, 2017). The protein encoded by fosb can dimerize with JUN family proteins to form the AP-1 transcription factor complex, a key component of the IL-17 signaling pathway. Meanwhile, the expression of traf4a, a gene crucial for inhibiting IL-17 signaling, was significantly upregulated (Gu et al., 2013). Ammonia stress has been shown to significantly upregulate fosb mRNA expression in the gills (Zhong et al., 2023). The difference in fosb expression may be attributed to a feedback regulation mechanism, in which increased AP-1 activity induced by 7-day nitrite stress suppresses fosb expression. Meanwhile, the increased expression of traf4a, a negative regulator of the IL-17 signaling pathway, may serve to prevent excessive inflammatory responses. Nevertheless, il-1β, as an inflammatory cytokine downstream of the IL-17 signaling pathway, significantly increased after nitrite exposure, suggesting the induction of an inflammatory response in the gill. Although we further investigated changes in the transcriptional levels of mapk within the IL-17 signaling pathway, we were unable to assess transient MAPK phosphorylation due to the limited availability of fish-specific antibodies. In general, the altered expression of key genes within the IL-17 signaling pathway indicates that the pathway is activated under nitrite-induced stress.
The IL-17 signaling pathway may play an important physiological role in the gill’s response to nitrite-induced stress, particularly in modulating immune and inflammatory functions. The activation of the IL-17 signaling pathway can indirectly promote mucosal integrity (Song et al., 2015) and facilitate the differentiation of T cells into T helper 17 (Th17) cells (Miossec and Kolls, 2012). Analysis of the in vitro results revealed that nitrite stress activates the IL-17 signaling pathway within 3–6 hours, suggesting a direct regulatory response. However, it is also possible that the activation of the IL-17 signaling pathway in the in vivo experiments is an indirect response to tissue damage. After nitrite stress, the activation of this pathway leads to the increased expression of downstream inflammatory genes, such as il-1β, thereby enhancing mucosal immune protection in the gills. In parallel, Th17 cell differentiation promotes the upregulation of pro-inflammatory cytokines, including tnf-α, which helps defend against pathogens and microbial infections in the aquatic environment. Meanwhile, the IL-17 signaling pathway is modulated by negative regulatory factors to prevent excessive inflammatory responses and maintain immune homeostasis.
In this study, we preliminarily investigated mitochondrial damage and apoptosis in fish gills following nitrite exposure. Previous research has shown that arsenic disrupts mitochondrial function in zebrafish, leading to the fragmentation and loss of inner mitochondrial cristae (Zhang et al., 2023b). Similarly, our results revealed that nitrite stress disrupted the inner mitochondrial cristae in tilapia gills, suggesting that nitrite may impair mitochondrial function. This mitochondrial damage is likely associated with oxidative stress. Under the same nitrite exposure conditions, we also observed apoptosis in the gill tissue. Consistent with our findings, a previous study on acute nitrite stress in T. rubripes reported significant upregulation of apoptosis-related genes (Gao et al., 2020c). A limitation of this study is the lack of investigation into the relationship between mitochondrial damage and apoptosis. The underlying molecular mechanisms can be further investigated in-depth in future studies.
5 Conclusion
In summary, we found that fish gills respond to nitrite stress by altering ion transport patterns, accompanied by corresponding changes in chloride cell subtypes. The activation of the IL-17 signaling pathway in the gills under nitrite exposure was first observed, and its downstream cytokines may enhance inflammatory responses in the gills. The molecular mechanisms by which the IL-17 signaling pathway regulates immune responses in the gill warrant further investigation. Furthermore, nitrite stress induces both apoptosis and mitochondrial damage. The potential correlation between these two phenomena requires further investigation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Animal housing, use and experimentation were approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University (Approval No: SYSU-IACUC-2023-B0453) in June 2023. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CS: Data curation, Validation, Conceptualization, Project administration, Investigation, Methodology, Writing – review & editing, Resources, Writing – original draft, Formal analysis, Software. TZ: Methodology, Investigation, Writing – review & editing, Supervision. RC: Writing – review & editing, Investigation, Conceptualization, Validation. YY: Investigation, Data curation, Writing – review & editing. ZL: Supervision, Writing – review & editing, Methodology. JY: Supervision, Writing – review & editing, Investigation. YL: Writing – review & editing, Investigation, Supervision. JL: Conceptualization, Software, Writing – review & editing. WL: Supervision, Writing – review & editing, Investigation, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Key R&D Program of China 2022YFF1000301, China Agriculture Research System of MOF and MARA (CARS-46) to Dr. Wensheng Li. National Natural Science Foundation of China (32072968, 32373102, and 32102761) also provided important assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1655930/full#supplementary-material
References
1
Agostini J. F. Toe D. Vieira K. M. Cruz B. C . (2018). Cholinergic system and oxidative stress changes in the brain of a zebrafish model chronically exposed to ethanol. Neurot. Res.33, 749–758. doi: 10.1007/s12640-017-9816-8
2
Bechara R. McGeachy M. J. Gaffen S. L. (2021). The metabolism-modulating activity of IL-17 signaling in health and disease. J. Exp. Med.218. doi: 10.1084/jem.20202191
3
Blauvelt A. Chiricozzi A. (2018). The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allerg. Immunol.55, 379–390. doi: 10.1007/s12016-018-8702-3
4
Cabillon N. Lazado C. (2019). Mucosal barrier functions of fish under changing environmental conditions. Fishes4, 2. doi: 10.3390/fishes4010002
5
Chang I. Lee T. Yang C. Wei Y. Chou F. Hwang P. et al . (2001). Morphology and function of gill mitochondria-rich cells in fish acclimated to different environments. Physiol. Biochem. Zool.74, 111–119. doi: 10.1086/319304
6
Dos Santos Silva M. J. Da Costa F. F. B. Leme F. P. Takata R. Costa D. C. Mattioli C. C. et al . (2018). Biological responses of Neotropical freshwater fish Lophiosilurus alexandri exposed to ammonia and nitrite. Sci Tot. Environ.616–617, 1566–1575. doi: 10.1016/j.scitotenv.2017.10.157
7
Dymowska A. K. Hwang P.-P. Goss G. G. (2012). Structure and function of ionocytes in the freshwater fish gill. Respir. Physiol. Neurobiol.184, 282–292. doi: 10.1016/j.resp.2012.08.025
8
Edwards S. L. Claiborne J. B. Morrison-Shetlar A. I. Toop T. (2001). Expression of Na+/H+ exchanger mRNA in the gills of the Atlantic hagfish (Myxine glutinosa) in response to metabolic acidosis. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol.130, 81–91. doi: 10.1016/S1095-6433(01)00367-1
9
Evavold C. L. Ruan J. Tan Y. Xia S. Wu H. Kagan J. C. et al . (2018). The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity48, 35–44.e6. doi: 10.1016/j.immuni.2017.11.013
10
Eyerich K. Dimartino V. Cavani A. (2017). IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol.47, 607–614. doi: 10.1002/eji.201646723
11
Gaffen S. L. Moutsopoulos N. M. (2020). Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci. Immunol.5(43), 3. doi: 10.1126/sciimmunol.aau4594
12
Gao X. Q. Fei F. Huo H. H. Huang B. Meng X. S. Zhang T. et al . (2020a). Effect of acute exposure to nitrite on physiological parameters, oxidative stress, and apoptosis in Takifugu rubripes. Ecotoxicol Environ. Saf.188, 109878. doi: 10.1016/j.ecoenv.2019.109878
13
Gao X. Q. Fei F. Huo H. H. Huang B. Meng X. S. Zhang T. et al . (2020b). Effect of acute exposure to nitrite on physiological parameters, oxidative stress, and apoptosis in Takifugu rubripes. Ecotoxicol Environ. Saf.188, 109878. doi: 10.1016/j.ecoenv.2019.109878
14
Gao X. Q. Fei F. Huo H. H. Huang B. Meng X. S. Zhang T. et al . (2020c). Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol.218, 105362. doi: 10.1016/j.aquatox.2019.105362
15
Gomez D. Sunyer J. Salinas I. . (2013). The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immun35, 1729–1739. doi: 10.1016/j.fsi.2013.09.032
16
Gu C. Wu L. Li X. (2013). IL-17 family: Cytokines, receptors and signaling. Cytokine64, 477–485. doi: 10.1016/j.cyto.2013.07.022
17
Guo R. Fan F. Wang L . (2020). The effect of nitrite and sulfide on the antioxidant capacity and microbial composition of the intestines of red swamp crayfish, Procambarus clarkii. Fish Shellfish Immunol.96, 290–296. doi: 10.1016/j.fsi.2019.11.052
18
Ha N. T. K. Huong D. T. T. Phuong N. T. Bayley M. Jensen F. B. (2019). Impact and tissue metabolism of nitrite at two acclimation temperatures in striped catfish (Pangasianodon hypophthalmus). Aquat. Toxicol.212, 154–161. doi: 10.1016/j.aquatox.2019.05.008
19
Horng J. L. Hwang P. P. Shih T. H. Wen Z. H. Lin C. S. Lin L. Y. et al . (2009). Chloride transport in mitochondrion-rich cells of euryhaline tilapia ( Oreochromis mossambicus ) larvae. Am. J. Physiol-Cell Physiol.297, C845–C854. doi: 10.1152/ajpcell.00218.2009
20
Huangfu L. Li R. Huang Y. Wang S. (2023). The IL-17 family in diseases: from bench to bedside. Sig. Transduct. Targ. Ther.8, 402. doi: 10.1038/s41392-023-01620-3
21
Jensen F. B. (2003). Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol.135, 9–24. doi: 10.1016/S1095-6433(02)00323-9
22
Jensen F. B. Andersen N. A. Heisler N. (1987). Effects of nitrite exposure on blood respiratory properties, acid-base and electrolyte regulation in the carp (Cyprinus carpio). J. Comp. Physiol. B.157, 533–541. doi: 10.1007/BF00700972
23
Jensen F. B. Koldkjær P. Bach A. (2000). Anion uptake and acid-base and ionic effects during isolated and combined exposure to hypercapnia and nitrite in the freshwater crayfish, Astacus astacus. J. Comp. Physiol. B.: Biochem. Syst. Environ. Physiol.170, 489–495. doi: 10.1007/s003600000126
24
Jia R. Liu B.-L. Han C. Huang B. Lei J.-L. (2016). The physiological performance and immune response of juvenile turbot (Scophthalmus maximus) to nitrite exposure. Comp. Biochem. Physiol. Part C.: Toxicol. Pharmacol.181–182, 40–46. doi: 10.1016/j.cbpc.2016.01.002
25
Korkmaz N. Uğurer O. Örün İ. (2023). Toxic effects of the synthetic pyrethroid permethrin on the hematological parameters and antioxidant enzyme systems of the freshwater fish Cyprinus carpio L. Ecotoxicology32, 646–655. doi: 10.1007/s10646-023-02675-2
26
Kroupova H. Machova J. Svobodova Z. (2005). Nitrite influence on fish: a review. Vet. Med.50, 461–471. doi: 10.17221/5650-VETMED
27
Lazado C. C. Caipang C. M. A. (2014). Mucosal immunity and probiotics in fish. Fish Shellfish Immunol.39, 78–89. doi: 10.1016/j.fsi.2014.04.015
28
Lin L.-Y. Hwang P.-P. (2004). Mitochondria-rich cell activity in the yolk-sac membrane of tilapia( Oreochromis mossambicus ) larvae acclimatized to different ambient chloride levels. J. Exp. Biol.207, 1335–1344. doi: 10.1242/jeb.00869
29
Liu H. J. Dong M. Jiang W. D. Wu P. Liu Y. Jin X. W. et al . (2022). Acute nitrite exposure-induced oxidative damage, endoplasmic reticulum stress, autophagy and apoptosis caused gill tissue damage of grass carp (Ctenopharyngodon idella): Relieved by dietary protein. Ecotoxicol Environ. Saf.243, 113994. doi: 10.1016/j.ecoenv.2022.113994
30
Madison B. N. Wang Y. S. (2006). Haematological responses of acute nitrite exposure in walleye (Sander vitreus). Aquat. Toxicol.79, 16–23. doi: 10.1016/j.aquatox.2006.04.011
31
Miossec P. Kolls J. K. (2012). Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov.11, 763–776. doi: 10.1038/nrd3794
32
Muthukumaravel K. Kanagavalli V. Pradhoshini K. P. Vasanthi N. Santhanabharathi B. Alam L. et al . (2023). Potential biomarker of phenol toxicity in freshwater fish C. mrigala: Serum cortisol, enzyme acetylcholine esterase and survival organ gill. Comp. Biochem. Physiol. Part C.: Toxicol. Pharmacol.263, 109492. doi: 10.1016/j.cbpc.2022.109492
33
Ni Q. Liu J. L. Huang X. Ge H. Dong Z. G. Peng Y. X. et al . (2023). Effects of chronic ammonia nitrogen stress on hydrolases and interleukin 17-3 (IL-17-3) in clam Cyclina sinensis. Aquacult. Int.31, 2339–2354. doi: 10.1007/s10499-023-01090-y
34
Qu Z. P. Zhou R. Sun R. W. Gao T. Y. Li Z. Zhang T. Q. et al . (2024). Plasma-assisted sustainable nitrogen-to-ammonia fixation: mixed-phase, synergistic processes and mechanisms. ChemSusChem17. doi: 10.1002/cssc.202300783
35
Ritzmann F. Lunding L. P. Bals R. Wegmann M. Beisswenger C. (2022). IL-17 cytokines and chronic lung diseases. Cells11, 2132. doi: 10.3390/cells11142132
36
Saco A. Rey-Campos M. Rosani U. Novoa B. Figueras A. (2021). The evolution and diversity of interleukin-17 highlight an expansion in marine invertebrates and its conserved role in mucosal immunity. Front. Immunol.12, 692997. doi: 10.3389/fimmu.2021.692997
37
Sieger D. Stein C. Neifer D. van der Sar A. M. Leptin M. (2009). The role of gamma interferon in innate immunity in the zebrafish embryo. Dis. Models Mech.2, 571–581. doi: 10.1242/dmm.003509
38
Song X. Dai D. He X. Zhu S. Yao S. Gao H. C. et al . (2015). Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity43, 488–501. doi: 10.1016/j.immuni.2015.06.024
39
Sonmez Kaplan S. Sazak Ovecoglu H. Genc D. Akkoc T. (2023). TNF-α, IL-1B and IL-6 affect the differentiation ability of dental pulp stem cells. BMC Oral. Health23. doi: 10.1186/s12903-023-03288-1
40
Sun J. L. Zhao L. L. Liao L. Tang X. H. Cui C. Liu Q. et al . (2020). Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish Immunol.98, 923–936. doi: 10.1016/j.fsi.2019.11.056
41
Svobodova Z. Machova J. Drastichova J. Groch L. Luskova V. Poleszczuk G. et al . (2005). Haematological and biochemical profiles of carp blood following nitrite exposure at different concentrations of chloride. Aquacult. Res.36, 1177–1184. doi: 10.1111/j.1365-2109.2005.01334.x
42
Valdenegro-Vega V. A. Crosibe P. Bridle A. Leef M. Wilson R. Nowak B. et al . (2014). Differentially expressed proteins in gill and skin mucus of Atlantic salmon (Salmo salar) affected by amoebic gill disease. Fish Shellfish Immunol.40, 69–77. doi: 10.1016/j.fsi.2014.06.025
43
Veldhoen M. (2017). Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol.18, 612–621. doi: 10.1038/ni.3742
44
Wang X. Jian S. Zhang S. Wu D. Wang J. Gao M. et al . (2022). Enrichment of polystyrene microplastics induces histological damage, oxidative stress, Keap1-Nrf2 signaling pathway-related gene expression in loach juveniles (Paramisgurnus dabryanus). Ecotoxicol Environ. Saf.237, 113540. doi: 10.1016/j.ecoenv.2022.113540
45
Williams E. M. Eddy F. B. (1986). Chloride uptake in freshwater teleosts and its relationship to nitrite uptake and toxicity. J. Comp. Physiol. B.156, 867–872. doi: 10.1007/BF00694263
46
Williams E. Eddy F. (1988). Anion transport, chloride cell number and nitrite-induced methaemoglobinaemia in rainbow trout (Salmo gairdneri) and carp (Cyprinus carpio). Aquat. Toxicol.13, 29–42. doi: 10.1016/0166-445X(88)90070-7
47
Wu X. Ma Y. Li X. He N. Zhang T. Liu F. et al . (2023). Molecular mechanism of kidney damage caused by abamectin in carp: Oxidative stress, inflammation, mitochondrial damage, and apoptosis. Toxicology494. doi: 10.1016/j.tox.2023.153599
48
Xie L. Chen S. Yao C. Li D. Li L. Tang R. et al . (2019). Nitrite induces endoplasmic reticulum stress and associates apoptosis of liver cells in grass carp (Ctenopharyngodon idella). Aquaculture507, 275–281. doi: 10.1016/j.aquaculture.2019.04.016
49
Xu Z. Zhang H. Guo M. Fang D. Mei J. Xie J. et al . (2022). Analysis of acute nitrite exposure on physiological stress response, oxidative stress, gill tissue morphology and immune response of large yellow croaker (Larimichthys crocea). Animals12, 1791. doi: 10.3390/ani12141791
50
Xue T. Liu Y. Cao M. Zhang X. Fu Q. Yang N. et al . (2021). Genome-wide identification of interleukin-17 (IL-17) / interleukin-17 receptor (IL-17R) in turbot (Scophthalmus maximus) and expression pattern analysis after Vibrio Anguillarum infection. Dev. Comp. Immunol.121. doi: 10.1016/j.dci.2021.104070
51
Yang H. Yang K. O. He Y. Wang X. Y. Wang L. M. Yang Q. et al . (2024). Nitrite induces hepatic glucose and lipid metabolism disorders in zebrafish through mitochondrial dysfunction and ERs response. Aquat. Toxicol.273, 107015. doi: 10.1016/j.aquatox.2024.107015
52
Zaccone G. (2022). Immunity and neuroimmune interactions at the mucosal barriers in fish. Fishes7. doi: 10.3390/fishes7060381
53
Zhang T. T. Ma P. Yin X. Y. Yang D. Y. Li P. D. Tang R. et al . (2022). Acute nitrite exposure induces dysfunction and oxidative damage in grass carp isolated hemocytes. J. Aquat. Anim. Health34, 58–68. doi: 10.1002/aah.10149
54
Zhang C. Li Y. Yu H. Ye L. Li T. Zhang X. et al . (2023a). Nanoplastics promote arsenic-induced ROS accumulation, mitochondrial damage and disturbances in neurotransmitter metabolism of zebrafish (Danio rerio). Sci Tot. Environ.863. doi: 10.1016/j.scitotenv.2022.161005
55
Zhang C. Li Y. Yu H. Ye L. Li T. Zhang X. et al . (2023b). Nanoplastics promote arsenic-induced ROS accumulation, mitochondrial damage and disturbances in neurotransmitter metabolism of zebrafish (Danio rerio). Sci Tot. Environ.863. doi: 10.1016/j.scitotenv.2022.161005
56
Zheng T. Jia R. Cao L. Du J. Gu Z. He Q. et al . (2021). Effects of chronic glyphosate exposure on antioxidative status, metabolism and immune response in tilapia (GIFT, Oreochromis niloticus). Comp. Biochem. Physiol. Part C.: Toxicol. Pharmacol.239, 108878. doi: 10.1016/j.cbpc.2020.108878
57
Zhong L. Liu S. Zuo F. Geng Y. Ouyang P. Chen D. et al . (2023). The IL17 signaling pathway: A potential signaling pathway mediating gill hyperplasia and inflammation under ammonia nitrogen stress was identified by multi-omics analysis. Sci Tot. Environ.867. doi: 10.1016/j.scitotenv.2023.161581
Summary
Keywords
gill, nitrite, aquaculture pollution, ion transport, IL-17 signaling pathway
Citation
Song C, Zhu T, Cai R, Yu Y, Liu Z, Yan J, Lian Y, Li J and Li W (2025) Nitrite exposure on gills of Oreochromis niloticus: structure change, immune response, and apoptosis. Front. Mar. Sci. 12:1655930. doi: 10.3389/fmars.2025.1655930
Received
29 June 2025
Accepted
30 October 2025
Published
20 November 2025
Volume
12 - 2025
Edited by
Haitham Abo-Al-Ela, Suez University, Egypt
Reviewed by
Francesca Falco, National Research Council (CNR), Italy
D. K. Meena, Central Inland Fisheries Research Institute (ICAR), India
Updates
Copyright
© 2025 Song, Zhu, Cai, Yu, Liu, Yan, Lian, Li and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wensheng Li, lsslws@mail.sysu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.