- 1Centre for Sustainable Futures, The University of the South Pacific, Suva, Fiji
- 2School of Science, Technology and Engineering and Australian Centre for Pacific Islands Research, University of the Sunshine Coast, Maroochydore, QLD, Australia
Anthropogenic stressors, including those associated with water quality influence reef benthic communities. This study assesses how changes in water quality influence the benthic composition of an urban reef system in Fiji, by first characterizing reef substrate composition in Suva, assessing substrate composition change across a water quality gradient, and identifying key water quality parameters associated with shifts in benthic composition. Results reveal an urban reef stabilized at coral coverage of ca. 30%, below Fiji’s typical range (45%), but consistent with prior levels (22–33%) from 2006-2007. Predictive modelling identifies temperature as the most consistent predictor of benthic composition (appearing in 77.5% of top models), highlighting its role in structuring communities through physiological and nutrient-cycling effects. Turbidity and nutrients further drive substrate patterns, with turbidity likely promoting sediment accumulation, and elevated nutrients influencing phase shifts towards alternative regimes. Our results demonstrate how urbanization filters benthic communities, creating distinct configurations with varying resilience. Notably, sites with moderate anthropogenic stress levels are characterized by the coexistence of scleractinian coral, seagrass, and soft coral, differing from typical coral-to-algae dominance shifts. While Suva’s reefs currently persist in a degraded-but-stable state, sustained pressures risk further decline. We emphasize targeted strategies (e.g., reef crest protection, watershed management) and long-term monitoring to inform adaptive management. These insights are critical for Fiji and other Pacific Island nations facing similar urban reef stressors, offering a framework for balancing conservation with development.
1 Introduction
Globally, tropical coastal reef systems are recognized amongst the most biodiverse and economically valuable ecosystems on earth (Camp et al., 2018; Burt et al., 2020). These ecosystems are generally characterized by their dominance of scleractinian corals, which are the primary builders and calcifiers, forming complex, colorful three-dimensional structures (Graham and Nash, 2013; Guest et al., 2016). While aptly referred to as coral reefs, it is important to recognize that these systems are not monolithic structures made solely of scleractinian corals, but rather are a complex aggregation composed of numerous prominent biological assemblages and diverse substrate types (Tebbett et al., 2023a, b). Beyond the foundational scleractinian corals, these systems include other calcifiers such as crustose coralline algae (CCA) and hydrocorals, as well as habitat-forming organisms such as sponges and octocorals, all of which contribute to reef structural complexity (Graham and Nash, 2013; Tan et al., 2016; Tebbett et al., 2023a). Various fleshy algae, seagrass and other biotic constituents like bivalves and ascidians, as well as non-living components such as sand, sediment, and rubble, all contribute to the composition and complexity of the benthic substrate (Swierts and Vermeij, 2016; Abrego et al., 2021; Carturan et al., 2022). The interplay between these diverse biological and physical components creates an intricate network of niches and habitats that drive essential ecological processes and functioning of the reef (Graham and Nash, 2013; Carturan et al., 2022). Understanding the various benthic assemblages and substrate types is crucial for assessing the overall structure and resilience of the reef, as well as the ecosystem services it supports (Chong-Seng et al., 2012).
Numerous natural and anthropogenic pressures, operating at both local and global scales, are known to influence the composition of benthic assemblages and substrate types, often resulting in significant shifts in substrate structure. Local pressures are diverse, often including coastal encroachment, overfishing, sedimentation, nutrient loading, and pollution while global pressures are centered around climate change, ocean warming and acidification (Hughes et al., 2018; Tebbett et al., 2023a, b; Estradivari et al., 2025). Conventionally, it has been understood that such shifts favor a proliferation of fleshy algae (i.e., algal phase shift), whereby fleshy algae outcompete and replace scleractinian corals to become the dominant benthic structure (Ban et al., 2015; Cruz et al., 2018; Roth et al., 2018). However, several studies suggest a more nuanced transition involving multiple benthic regime types (Jouffray et al., 2015; Tebbett et al., 2023a, b). For example, Smith et al. (2016) found no consistent evidence of coral to algal phase shift across their dataset covering the central western Pacific (Mariana Archipelago, Hawaiian Archipelago, Line Islands, Phoenix Islands and American Samoa). Instead, they observed that reefs surrounding inhabited islands were predominantly composed of fleshy, non-reef-building organisms (particularly turf algae), while benthic communities on uninhabited islands were more variable, but generally supported a higher abundance of calcifiers and active reef builders. Similarly, a spatially extensive study from Pacific Island Countries and Territories (PICTs) found reefs subject to higher levels of human impacts had less hard coral and more turf algae, but not more fleshy algae, than reefs further removed from humans, and found that benthic community relationships to biophysical drivers became less predictable with higher human impacts (Williams et al., 2015; Ford et al., 2020; Kurihara et al., 2021; Kim et al., 2022). Despite these emerging insights, there remains a broad consensus that anthropogenic stressors, in particular overfishing and urbanization, are key local drivers of benthic change (Lai et al., 2015; Portugal et al., 2016; Tebbett et al., 2023b). This study focuses specifically on local pressures, particularly on nutrient and sediment loading from urban terrestrial runoff and coastal development.
Amongst the PICTs, coastal reefs are not only recognized as biodiversity hotspots but also serve as essential ecosystems that support livelihoods, food security, and cultural practices of local communities (Kitolelei et al., 2021; Thomas et al., 2021; Veitayaki, 2021). However, these ecosystems face persistent, acute stress from land-based pressures, particularly those driven by rapid urbanization (Hoegh-Guldberg et al., 2011; Dutra et al., 2021). Fiji’s Greater Suva Urban Area (GSUA) exemplifies these challenges. As the most heavily urbanized metropolitan area within the PICTs (outside of Papua New Guinea), the GSUA is home to approximately one third (~300,000) of Fiji’s population and exerts a mounting pressure on its adjacent coastal marine environment (Phillips and Keen, 2016; Shiiba et al., 2023; Dehm et al., 2024). Key stressors influencing nearshore water quality, and consequently benthic habitats, include urban runoff, wastewater discharge, and riverine inputs from domestic, agricultural, and industrial sources (Arikibe and Prasad, 2020; Pratap et al., 2020; Dehm et al., 2024). These anthropogenic activities create distinct spatial variability in exposure, ranging from heavily urban-impacted nearshore areas to less-impacted offshore reefs. For instance, Dehm et al. (2025) identified water quality clusters within the Suva coastal environment where nearshore areas, particularly around river mouths and the Kinoya Sewage Treatment Plant outfall, show elevated turbidity, total suspended solids (TSS), and high concentrations of nutrients including ammonia, nitrite, and dissolved inorganic phosphate (DIP). In contrast, offshore sites and outer reef areas are generally characterized by clear, oxygen-rich, and nutrient-poor waters due to oceanic influences.
While the general patterns of water quality degradation and its impacts on benthic substrates are well documented globally, there remains a gap in fine-scale spatial studies that comprehensively link water quality and benthic substrate composition across urban gradients, particularly in PICT contexts. Reefs in the Pacific region are diverse and strongly influenced by oceanic conditions, and although hard coral cover has remained relatively stable over the last three decades, the number of people living within 5km of reefs has increased by 28.7% since 2000 (Wicquart et al., 2025). By linking water quality gradients to benthic assemblages in an urban Pacific reef, this study contributes to the growing discourse on reef persistence under marginal environmental conditions. The findings will support more informed coastal management strategies in Fiji and other PICTs where urban migration and the urge for modernization is rapidly expanding urbanization within coastal marine systems, making the balance between development and reef conservation increasingly critical. Therefore, this study aims to assess how urbanization-driven changes in water quality influence benthic substrate composition on Pacific Island coral reefs, using Suva’s reef system (Fiji) as a case study. Specifically, we (i) characterize the benthic substrate composition of Suva’s urban reef system, (ii) compare substrate composition across a water quality gradient, and (iii) identify key water quality parameters associated with shifts in benthic composition.
2 Materials and methods
2.1 Study area
This study was centered along the southeastern coastline of Viti Levu, Fiji, specifically along the urban reef system of the GSUA (approximately 178.14°S, 178.45°E, Figure 1A). The region is characterized as a typical tropical island coastline with fringing and barrier reefs, a network of mangroves, and lagoons. Multiple urban stressors are present (Dehm et al., 2024), including untreated and treated wastewater discharge from inadequate or aging sewage infrastructure (Morrison et al., 2005), and urban stormwater runoff (Veitayaki, 2010). These pressures are compounded by freshwater inputs from the Rewa River and smaller tributaries, which transport sediment loads and terrestrial contaminants, such as nutrients and chemical pollutants into the coastal marine environment, subsequently influencing coral reef regime and diversity (Rodda, 2005; Tamata et al., 2010; Dehm et al., 2025).
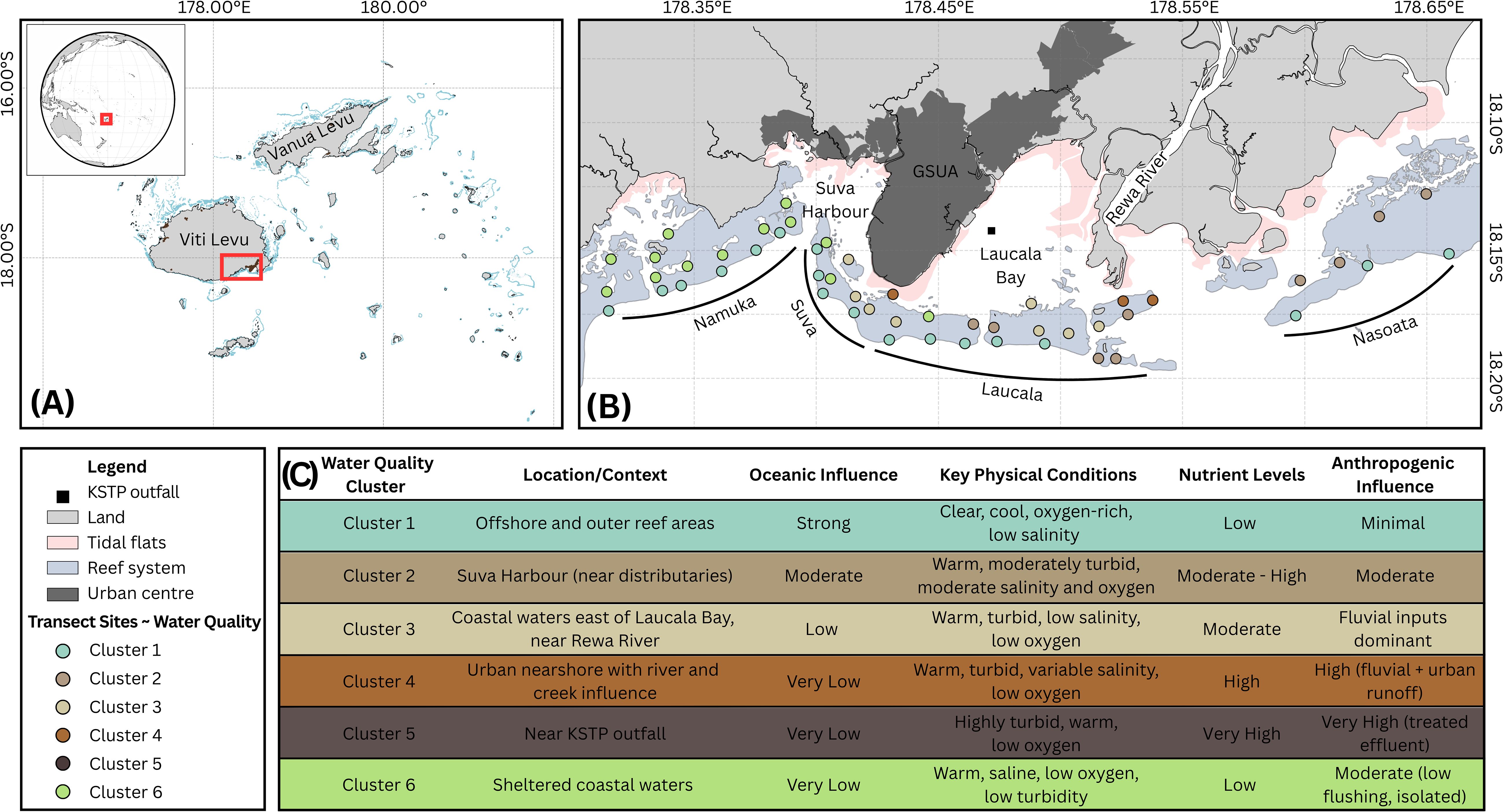
Figure 1. Study area and reef survey design in relation to the Greater Suva Urban Area (GSUA), Fiji. (A) Location of the GSUA on the island of Viti Levu, with inset map showing its position within Fiji and globally. (B) Reef survey sites along four major reef systems adjacent to the GSUA: Namuka Fringing Reef System, Suva Barrier Reef System, Laucala Barrier Reef System, and Nasoata Fringing Reef System. Sites are color-coded by water quality clusters, adapted from Dehm et al. (2025). Cluster 5 is not represented, as no transects were conducted near the Kinoya Sewage Treatment Plant (KSTP) outfall. (C) Tabulated summary of coastal water quality gradients across the GSUA, based on clusters of similar physiochemical properties. Adapted from Dehm et al. (2025).
2.2 Survey design
Field surveys to characterize coral reef substrate composition were conducted between May and July 2022 across 51 sites (Figure 1B). The sampling was designed to encompass the four major urban ref systems, including Namuka Fringing Reef, Suva Barrier Reef, Laucala Barrier Reef, and Nasoata Fringing Reef (Dehm et al., 2024). To ensure we captured potential spatial variation in reef substrate composition, the survey area was extended slightly beyond these core systems, to include part of Nasilai Reef (included in the Nasoata system to the east, 18.13°S, 178.68°E), and part of Naqara Reef (included in the Namuka system to the west; 18.167°S, 178.31°E).
Sampling followed methods adapted from (Hill and Wilkinson, 2004; Roelfsema and Phinn, 2008; Roth et al., 2018). In summary, at each of the sites, three 50 meter transects were laid parallel to the reef flat, spaced approximately 50 meters apart to ensure spatial independence. This resulted in a total of 153 transects (51 sites × 3 transects per site). Along each transect, a 1 × 1 meter photo quadrat (Hill and Wilkinson, 2004), was taken every 2 meters, resulting in 25 photographs per transect (75 per site, 3825 in total). Photographs were captured 1 meter above the substrate using a digital camera (Olympus TG6) mounted on a plumb line to ensure consistency in scale and perspective (Lerma and Cabrelles, 2007).
2.3 Image analysis and benthic substrate characterization
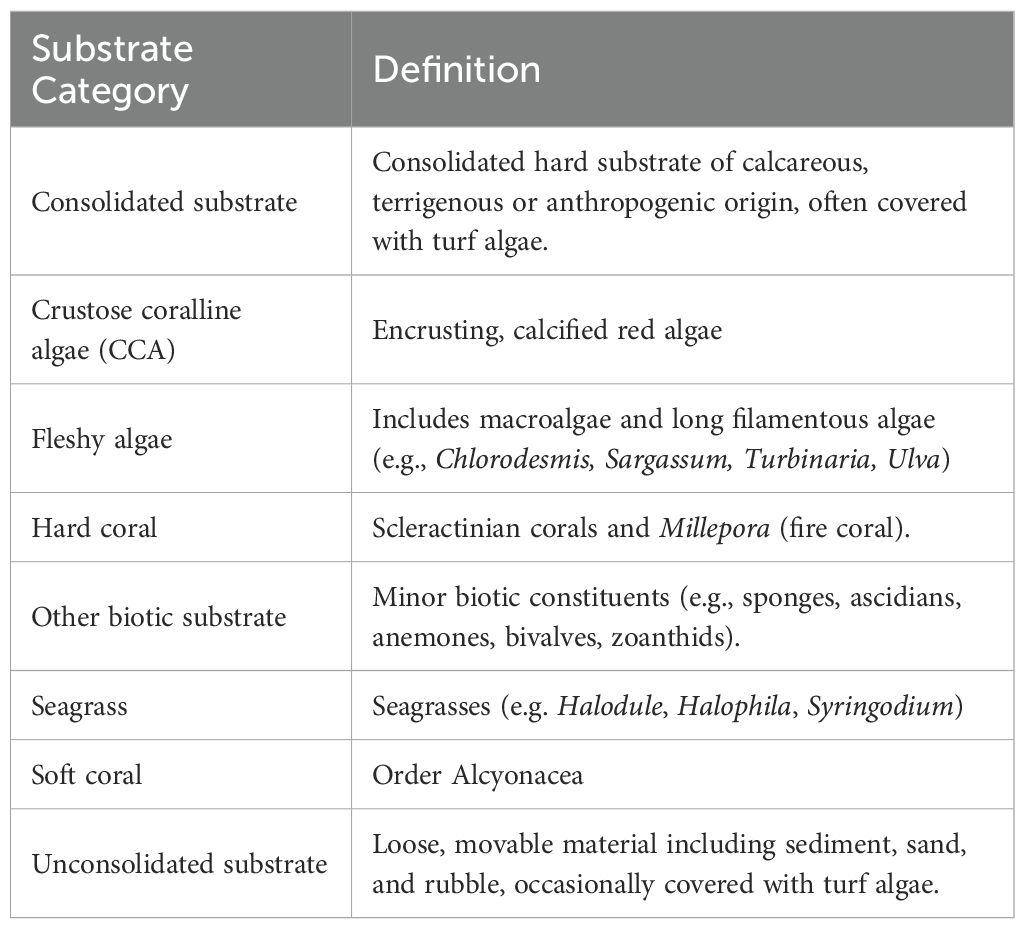
All photographs were manually analyzed using Coral Point Count with Excel Extensions (CPCe, v.4.1) (Kohler and Gill, 2006). Fifty randomly generated points per image were classified into substrate categories (Table 1) following standardized definitions adapted from Tebbett et al. (2023a) and Teichberg et al. (2018). We followed a coarse categorization scheme to maximize dataset compatibility, aligning with a recommended framework for global standardization (Tebbett et al., 2023a). Care was taken to ensure the ‘other’ biotic substrate category (including sponges, ascidians, anemones, bivalves and zoanthids) did not comprise dominant benthic components, although we acknowledge finer-resolution classifications could provide additional ecological insights. For each site, the percentage cover of each substrate category was calculated as the proportion of points assigned to that category, relative to the total number of points analyzed across all photographs captured for that site (objective 1; Figure 2). To assess spatial patterns, substrates were compared across predefined reef zones (Reef Crest, Reef Flat, Back Reef, Patch Reef), and to individual reef systems Kruskal-Wallis tests, followed by Dunn’s post hoc pairwise comparisons where significant differences were detected (p < 0.05). Substrate cover is presented as mean percent cover (mean ± SE%). All analyses were conducted in Python (v. 3.11) using pandas (v 2.2, McKinney, 2010), scipy (v. 1.15, Virtanen et al., 2020), seaborn (v. 0.13, Waskom, 2021), and scikit-posthocs (v. 0.11, Terpilowski, 2019).

Figure 2. Overview of the data analysis workflow used to address the three study objectives. The schematic outlines key methodological steps, including data processing, statistical analysis, and visualization techniques applied to each objective. Water quality clusters are color-coded consistently with Figure 1C, allowing for easy extrapolation between figures and interpretation of how each cluster corresponds to reef location, degree of oceanic influence, key physical conditions, nutrient levels, and anthropogenic pressures.
2.4 Substrate composition across water quality gradients
To contextualize substrate composition across water quality gradients (objective 2), this study utilized the spatial framework and clustering analysis of Dehm et al. (2025), who characterized coastal water quality along the GSUA over 12 months of sampling (97 stations and six campaigns spanning wet/dry seasons). Their study, employing conductivity–temperature–depth (CTD) profiling and colorimetric nutrient analysis, identified six distinct water quality clusters (Figure 1C), driven by oceanic processes, riverine inputs, and anthropogenic pressures. Key findings revealed severe nutrient enrichment near urban centers (e.g., NH3 >17.8 mg/L at Kinoya sewage outfall; NO3−/NO2− peaks of 0.24 ± 0.06 mg/L near rivers) and elevated turbidity and total suspended solids during wet seasons (up to 3.5 NTU, 14.7 mg/L), contrasting with oligotrophic offshore waters.
For our analysis, reef survey sites were assigned to five of the six clusters that overlapped with a reef system, enabling comparison of benthic community composition across water bodies of varying water quality. Following Shapiro-Wilk test for normality, substrate data was normalized using log(x-1) transformation and Levene’s test was conducted to assess homogeneity of variances. Based on the outcome of Levene’s test, Welch’s ANOVA was applied to hard coral, unconsolidated substrate, CCA, seagrass, consolidated substrate soft coral and fleshy algae, while standard one-way ANOVA was conducted on the ‘other’ category. For substrates exhibiting significant variation (p < 0.05), post-hoc pairwise comparisons were conducted using Games-Howell post-hoc tests to identify specific cluster differences. All analyses were performed in Python using pandas (v 2.2, McKinney, 2010), pingouin (v 0.5.5, Vallat, 2018), scipy (v. 1.15, Virtanen et al., 2020), statsmodels (v.0.14, Seabold and Perktold, 2010), and seaborn (v. 0.13, Waskom, 2021).
2.5 Environmental drivers of substrate composition
To identify the key water quality parameters associated with shifts in benthic composition (objective 3), we correlated yearly averaged water quality parameters extracted from Dehm et al. (2025) with substrate data, using an information-theoretic model selection approach by means of generalized linear models (GLMs) followed by automated model dredging (Bolker et al., 2009). Predictors included physical parameters (temperature, salinity, dissolved oxygen, turbidity, and total suspended solids) and nutrient metrics (nitrate, nitrite, ammonium, dissolved inorganic phosphorus, and chemical oxygen demand). All eight substrate categories were subjected as response variables.
For each substrate type, we fitted all possible additive GLMs, using up to a maximum of 3 predictors, (excluding interactions for parsimony) and ranked them using Akaike’s Information Criterion (AIC) (Salkind, 2007), with lower AIC values indicating better trade-offs between model fit and complexity. After ranking, the top five models per substrate were further evaluated using adjusted R² to assess explanatory power and to compare models with different numbers of predictors. While we acknowledge that interactions among environmental variables are ecologically plausible, especially in marine systems, we prioritized model interpretability and comparability across substrate types, both of which become increasingly difficult to maintain when including interaction terms. As such we used AIC to guide model selection and considered adjusted R² and predictor interpretability to support final choices. Model validation included residual diagnostics for homoscedasticity and normality, alongside variance inflation factor checks (≤ 5) for multicollinearity, following a conservative threshold used in prior studies (McClanahan et al., 2024), to reduce redundancy among predictors and preserve meaningful relationships. Analyses were conducted in Python using pandas (v.2.2, McKinney, 2010), statsmodels (v.0.14, Seabold and Perktold, 2010), and by adapting R’s MuMIn:dredge (v.1.48, Bartoń, 2010) methodology in using itertools (Python Software Foundation v 3.13, 2024) for systematic model comparison. This approach allowed systematic identification of key environmental predictors while accounting for model uncertainty.
3 Results
On average, hard coral cover had the highest mean coverage (30.68 ± 2.18%), followed by unconsolidated substrate (21.8 ± 2.07%), consolidated substrate (18.59 ± 1.33%) and fleshy algae (17.17 ± 1.24%) (Figure 3). Seagrass (5.78 ± 1.02%) and CCA (3.56 ± 0.45%) were minor contributors to the substrate type while other biotic substrate (1.26 ± 0.09%) and soft coral (1.16 ± 0.28%) show sporadic presence across the entirety of the reef systems studied.
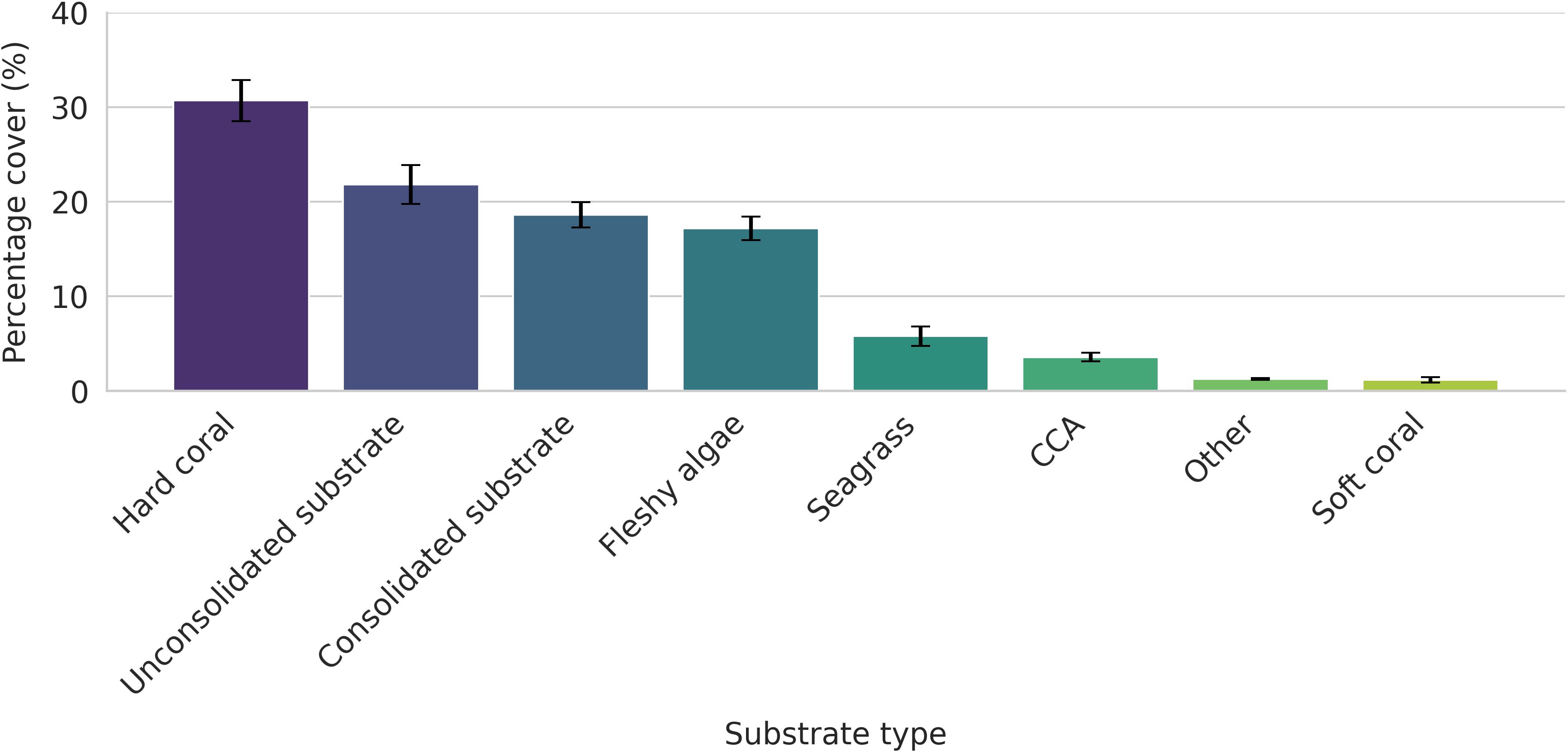
Figure 3. Proportions of substrate coverage throughout the four major reef systems of the Greater Suva Urban Area (GSUA).
Across all four reef systems, substrate composition was comparable, with no statistically significant differences noted among substrate categories (p > 0.05, Supplementary Table 1). However, across reef zones, significant differences (p < 0.05) in substrate composition were observed (Table 2, Figure 4). On reef crests, the dominant substrate type was hard corals (44.11 ± 2.41%), with significantly higher (p < 0.01) coverage than in other zones. This was followed by consolidated substrate (23.06 ± 2.16%) and CCA (5.76 ± 0.72%), both of which also had significantly greater cover compared to certain other zones. Specifically, CCA was significantly more (p < 0.01) abundant on reef crests than on reef flats (1.62 ± 0.36%) and patch reefs (0.28% ± 0.10), while consolidated substrate was significantly higher (p < 0.01) on crests than reef flats. On reef flats, the dominant substrate type was unconsolidated substrate (33.14 ± 2.30%), which was significantly higher (p < 0.01) than on crests (8.01 ± 1.49%) and patch reefs (27.82 ± 1.38%). Seagrass (11.48 ± 1.96%) and soft corals (3.22 ± 0.73%) were also notably more abundant on flats than in all other zones, with soft coral cover significantly higher (p < 0.01) than elsewhere. Along the back reefs, unconsolidated substrate again dominated (35.05 ± 2.72%), significantly exceeding (p < 0.01) cover on crests and being comparable to that on flats. Seagrass (9.87 ± 2.57%) was also relatively abundant, with significantly higher (p < 0.01) coverage than on crests. Patch reefs exhibited a distinct profile, with fleshy algae being the dominant cover type (30.86 ± 1.59%), significantly greater (p < 0.05) than on reef flats (14.25 ± 1.77%) and back reefs (15.73 ± 2.56%). These sites also had substantial consolidated substrate cover (20.93 ± 1.35%), though not significantly (p > 0.05) different from other zones. Other biotic substrates were generally limited across all zones but were significantly more (p=0.04) represented on reef crests (1.52 ± 0.17%) compared to back reefs (0.98 ± 0.16%).
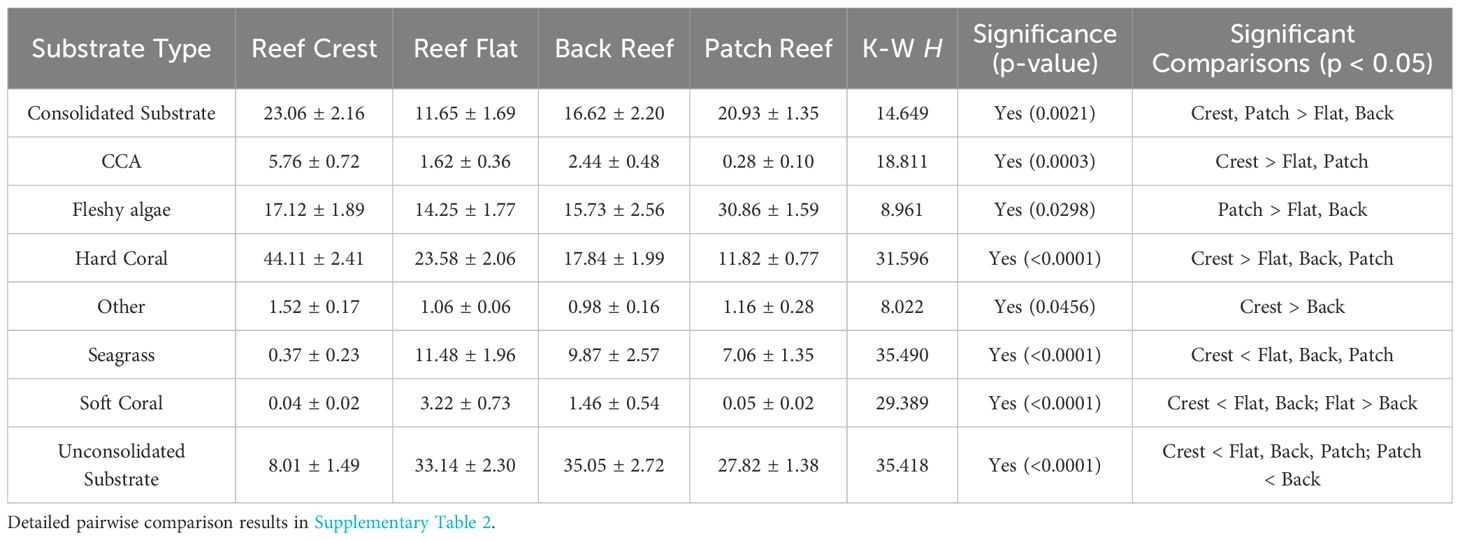
Table 2. Substrate composition across reef zones (mean percent cover ± SE) and statistical comparisons across the four major reef systems of the GSUA.

Figure 4. Proportions of substrate coverage between reef zones across the four major reef systems of the Greater Suva Urban Area (GSUA).
Five of the six distinct water quality clusters (Clusters 1, 2, 3, 4, and 6) identified by Dehm et al. (2025) overlapped with the benthic survey sampling sites, across which substrate composition varied significantly (Table 3). Cluster 1 stood out as markedly distinct, as it had the highest mean hard coral (47.66 ± 0.79%) and CCA (6.78 ± 0.56%) coverage, significantly higher (p < 1e-43) than all other clusters (Figure 5). In contrast, unconsolidated substrate was far less abundant in cluster 1 (6.77 ± 0.61%) compared to all other clusters (26.4–33.1%). Similarly, seagrass cover was minimal in cluster 1 (~0.04 ± 0.03%) but reached up to 11.98 ± 1.20% in cluster 6. Consolidated substrate coverage varied, with cluster 4 highest (30.10 ± 1.80%) and cluster 6 lowest (11.31 ± 0.84%) among all clusters, while soft coral coverage was significantly higher (p < 2e-8) within cluster 6 (3.29 ± 0.50%) than in cluster 1 (0.05 ± 0.03%). Fleshy algae coverage was significantly highest (p < 2e-7) in cluster 4 (25.61 ± 1.00%) compared to all other clusters. Other substrate types showed no significant (p=0.33) variation between water quality clusters.

Table 3. Statistical summary of substrate differences among clusters across the four major reef systems of the GSUA.

Figure 5. Substrate composition relative to water bodies of varying water quality clusters across the four major reef systems of the Greater Suva Urban Area (GSUA).
Predictive models, selected using AIC and evaluated with adjusted R², showed varying ability to resolve relationships between water quality parameters and substrate categories, with model fit values ranging from an R² of 0.08 to 0.61 (Table 4). For fleshy algae, the highest-ranking model (AIC=364.07, Adj R²=0.13) identified temperature, salinity, and dissolved oxygen as key predictors. Among these, salinity showed a moderately negative relationship (Slope=-1.6, p=0.072; Figure 6), while temperature and dissolved oxygen had weakly positive but non-significant associations (Slope=0.99 and 8.6, respectively; p > 0.4). For CCA, the best model (AIC=233.71, Adj R²=0.48), designated temperature and total suspended solids as having the highest influence, both showing significantly negative effects on cover (temperature: slope=-4.3, p=4.3e-06; TSS: slope=-0.81, p=2.2e-05). Hard coral models had the highest explanatory power overall, with the top model (AIC=379.94, Adj R²=0.61) identifying significantly highly negative relationships with temperature (slope=-21, p=2.4e-06) and turbidity (slope=-21, p=1.1e-07) as key drivers of cover. For consolidated substrate, the top model (AIC=356.21, Adj R²=0.35) featured a negative correlation to temperature (slope=-6.6, p=0.027), and a significantly strong relationship to nitrite (slope=37, p=0.017), and dissolved inorganic phosphorus (slope=22, p=0.0025); with other models including combinations of dissolved inorganic phosphorus, turbidity, and nitrate. Seagrass cover increased strongly with temperature (Slope=9.5, p=6.5e-06) and moderately with turbidity (Slope=4.3, p=0.04) (AIC=317.21, Adj R²=0.49). Soft coral showed significant reductions with higher dissolved oxygen (Slope=-9.8, p=0.00012) (AIC=184.26, Adj R²=0.50), while unconsolidated substrates expanded markedly with temperature (Slope=20, p=2.7e-06) and included chemical oxygen demand (Slope=0.19, p=0.17), and turbidity (Slope=13, p=0.00094) (AIC=391.92, Adj R²=0.46). Other substrates demonstrated minor but significant temperature-linked declines (Slope=-0.61, p=0.0029) insignificant decline relative to turbidity (Slope=-0.12, p=0.55), and displayed positive relationship with Salinity (Slope=0.07, p=0.25), (AIC=91.34, Adj R²=0.27).

Table 4. Top predictive models of substrate-water quality relationships across the four major reef systems of the GSUA.
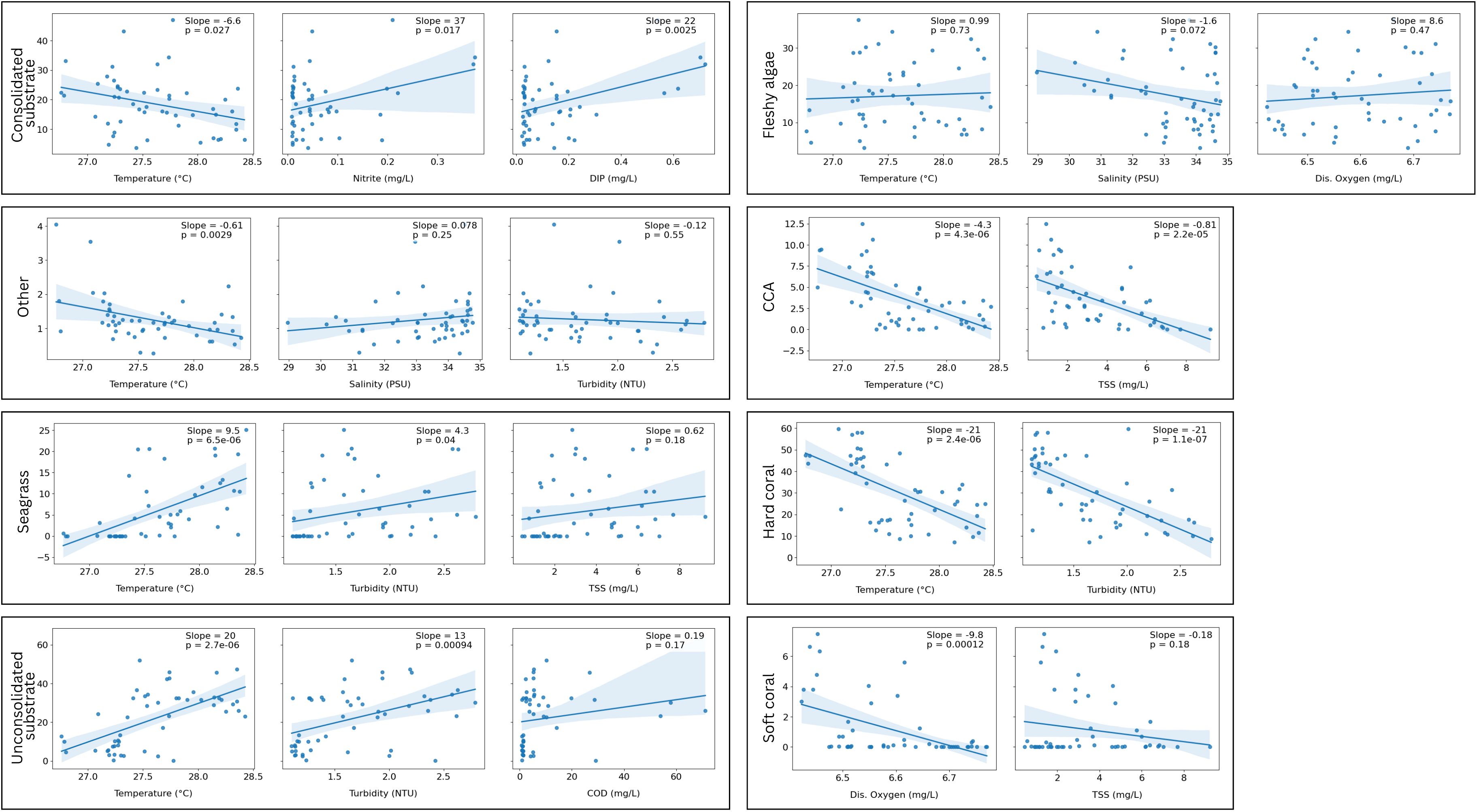
Figure 6. Regression plots along with the slope and p-value for each substrate type showing the relationship between substrate cover (%) and top ranked AIC-selected environmental predictors.
4 Discussion
Water quality is a key factor influencing substrate composition in reef ecosystems, with different conditions driving distinct shifts in benthic communities. Our analysis examines these patterns along an extensive system of interconnected reefs in Suva, Fiji where gradients of water quality, shaped by urbanization, form distinct clusters that reflect differential impacts on benthic communities. The results reveal several important trends regarding how water quality shapes reef composition and show a progressive decline in calcifying benthic taxa as water quality deteriorates. These patterns highlight the importance of managing water quality to protect key reef-building organisms and functional diversity, which are essential for maintaining ecosystem resilience reducing risk and sustaining coastal livelihoods (Raymundo et al., 2009; Huang et al., 2024).
The benthic assemblage of Suva’s urban reefs demonstrates patterns consistent with long-term urban degradation. Observed hard coral cover (~30%) remains lower than Fiji’s average (45%) range (Morris and Mackay, 2021), it falls within the 22–33% range documented in Suva during 2006–2007 surveys (Morris, 2007), suggesting stability over time in this urban setting. Surprisingly, this cover also exceeds average coral cover reported across Pacific reefs 2000 (Wicquart et al., 2025), indicating that despite chronic local stressors, Suva’s urban reefs may retain a degree of ecological resilience. In addition, substrate composition follows general patterns of marginal assemblages dominated by hard coral followed by unconsolidated sediment (21.8 ± 2.07%), consolidated substrate (18.59 ± 1.33%), and fleshy algae (17.17 ± 1.24%), with minor contributions from seagrass (5.78 ± 1.02%) and CCA (3.56 ± 0.45%). This depressed coral cover coupled with elevated cover of algae, unconsolidated sediment and consolidated substrate reflects the known impacts of urban stressors, consistent with Samperiz et al. (2025), whereby reduced coral growth was observed under turbidity and thermal stress in Fijian inshore reefs.
The GSUA study region maintains strong ecological zonation, with reef crests having the highest coral cover (44.11 ± 2.41%) while nearshore areas transition to algal and unconsolidated sediment dominance; a pattern mirroring other tropical nearshore reef systems. For example, studies along the Great Barrier Reef (Gordon et al., 2016; Tebbett et al., 2018), and along the Spermonde Archipelago (Teichberg et al., 2018; Syafruddin et al., 2025) have documented increasing sediment accumulation and turf algal dominance in inshore zones, where elevated sediment loads and greater algae cover persist. Similar patterns of zonation are also observed across other smaller PICTs such as Federated States of Micronesia, Guam, and Palau, where in addition to sediment runoff and associated contaminants, in addition to wave energy and prevailing currents have been linked to substrate distribution (Ferreira et al., 2023; Kim et al., 2022; Kurihara et al., 2021; Mills et al., 2023). While our study does not explicitly assess wave energy and consider sediment load and nutrient levels as proxies of human pressure, we acknowledge that other harmful contaminant, such as heavy metals, hydrocarbons, and plastics, were not considered. These stressors may be critical in shaping substrate composition across Suva’s reef systems (Dehm et al., 2020; Pratap et al., 2020; Varea et al., 2021), warranting further investigation. Of particular concern is the fleshy algal dominance on patch reefs, (30.86 ± 1.59%) and depressed CCA levels (0.28 ± 0.10%), highlighting vulnerable habitats needing targeted management. These substrate conditions, particularly the prevalence of unconsolidated sediment and fleshy algae, are likely to impede coral recruitment and thus recovery potential (Kuffner et al., 2006; Kenyon et al., 2023). In contrast, reef flats, with their moderate hard coral (23.58 ± 2.06%) and intermediate seagrass (11.48 ± 1.96%) coverages and comparatively high soft coral (3.22 ± 0.73%); may represent more resilient zones, as habitat heterogeneity and functional redundancy are known to support ecological stability and recovery (Brown et al., 2002; Carturan et al., 2022; Roth et al., 2018; Violette et al., 2024. Altogether, these findings reveal an urban reef ecosystem that has stabilized at lower coral cover levels, as coverage levels and types have remained consistent between observations by Morris (2007) and our findings.
Hard coral and CCA coverage decrease abruptly from the water quality clusters defined by oceanic conditions (cluster 1) to a cluster defined by high anthropogenic input (cluster 4, high fluvial and urban runoff); where noticeable increases in turbidity, temperature, and nutrients coupled with declines in oxygen availability and salinity are evident, suggesting these foundational reef organisms are particularly vulnerable to declining water quality conditions (Fabricius, 2005). Substrate within water quality cluster 4 shows minimal hard coral coverage and nearly absent CCA, instead being dominated by fleshy algae and consolidated substrate (covered by turf algae). Furthermore, an additional distinct community configuration, where moderate coral cover (~22.3%) and algae (~15.1%) coexists with the highest levels of seagrass (~12.0%) and soft coral (~3.3%), is noted within water clusters defined as having moderate anthropogenic influence (clusters 2 and 6), where flushing is low and isolated, and water is generally described as being warm, saline and low in oxygen and turbidity levels. This suggests that these conditions may foster an alternative reef state that differs from the typical coral dominated or algal dominated configurations seen elsewhere. This finding is in line with growing global consensus that reef ecosystems may respond to environmental pressures in more complex ways than a simple linear decline from coral to algal dominance (Tebbett et al., 2023a). For example, along Singapore’s urbanized coastline massive plating species persist in environments shaped by urbanization, Ng et al. (2021), and Smith et al. (2016) found that in the central-northern Pacific archipelagos, reefs show no correlation between coral and fleshy macroalgae but rather turf algae and CCA. Similarly, Roth et al. (2018) conceptualized mosaic reef dynamics in the Red Sea, whereby a network of patches exists alongside each other dominated by either algae or by calcifiers. These examples support the notion that urbanized or degraded reefs may not follow classical trajectories, but instead settle into novel, functionally distinct states that challenge conventional assumptions of reef phase shifts and underscore the importance of considering localized drivers for predicting reef community dynamics.
Predictive models of substrate-water quality relationships depict distinct water quality parameters that influence benthic substrate composition, with temperature identified as the most consistent predictor, appearing in 77.5% of the top 5 models, across all substrate types. This finding aligns with established literature demonstrating how temperature regulates metabolic processes. Although the observed range of yearly average temperature across our sites is within 2°C (26.7°C to 28.7°C), it is ecologically meaningful, especially in tropical reef systems and similar gradients have been shown in past studies to alter coral-algal dynamics, recruitment success and bleaching susceptibility (Roth et al., 2018; Abrego et al., 2021; Boonnam et al., 2022; Jiang et al., 2024). While temperature may also covary with other environmental factors, it consistently appeared in top performing models across all substrates (Table 4). This underscores its fundamental role in structuring benthic communities, likely through both direct physiological effects and indirect impacts on microbial and nutrient cycling.
Turbidity and nutrient dynamics emerged as secondary but critical drivers, with varying effects across substrates. Turbidity showed particularly strong associations with hard coral (negative) and unconsolidated substrate (positive) distributions, likely due to light limitation or smothering during coral recruitment and growth, while promoting sediment accumulation. This aligns with studies showing that elevated turbidity reduces light penetration, limiting photosynthesis and calcification of corals. Turbidity has also been shown to influence larval settlement by masking orientation queues and smothering settlement surfaces (e.g., CCA), or by directly smothering juvenile or adult corals directly (Adjeroud et al., 2010; Lal et al., 2018; Abrego et al., 2021). Nutrient variables exhibited substrate-specific relationships, i.e., dissolved inorganic phosphorus was most relevant for consolidated substrates, while nitrite/nitrate influenced fleshy algae and soft coral distributions. These patterns suggest phosphorus may mediate CCA recruitment on hard surfaces, whereas nitrogen availability favors fleshy algae and filter-feeding soft corals (Kumar and Bera, 2020; Nguyen et al., 2020).
The high explanatory power of models for foundational taxa like hard corals and CCA (adj. R² up to 0.61) contrasts with weaker fits for algae dominated substrates, suggesting abiotic factors primarily constrain calcifying taxa, while algal communities are also strongly influenced by unmeasured biotic interactions. For example, studies regularly show that herbivory by both fish and invertebrates regulate growth and expansion of algal beds (Houk et al., 2010; McAndrews et al., 2019; Estradivari et al., 2025), while other studies depict competitive interactions as a regulator, whereby turf and macroalgae rapidly occupy space and release allelopathic compounds that chemically inhibit coral and CCA growth (Swierts and Vermeij, 2016; Altman-Kurosaki et al., 2024). This supports prioritizing water quality management, particularly terrestrial runoff (turbidity and nutrient) to maintain reef-building taxa, while complementary measures like herbivore protection may be needed to halt/slow algal phase shifts. Further to this, our results contrast with previous work showing low model predictability in human-impacted reefs. For example, Ford et al. (2020) found live coral cover near populated areas to be largely unpredictable, and Williams et al. (2015) reported that models explained 72% of variation on uninhabited reefs but only 14.7% on reefs near human populations. These differences may reflect the value of in situ water quality measurements, which directly captured local abiotic conditions rather than relying on proxies or remotely sensed data.
Taken together, our results demonstrate how urbanization reshapes reef ecosystems through environmental filtering, producing distinct benthic configurations. While Suva’s reefs currently persist in a stable state, exemplifying the ‘urban reef paradox’, continued urban pressures may drive phase shifts, potentially reducing coral cover and triggering cascading effects on overall ecosystem function. This emphasizes the need for targeted management strategies and long-term monitoring. Zone specific approaches are recommended, for example, protection of reef crest zones should be prioritized to maintain coral cover and preserve recovery potential, while within nearshore systems where algal cover is highest, runoff mitigation through improved sewage treatment and watershed management may be prioritized. However, given the system’s current stability and the complexity of urban stressors, we emphasize the importance of establishing long term monitoring programs, through permanent transects and fine-scale surveys, to track benthic changes and pollution levels. Such efforts will enable adaptive management, allowing interventions to be refined as the system responds to ongoing urbanization and climate change. Ultimately, sustaining reef function in Suva will depend on both addressing immediate land based pressures and implementing proactive monitoring to anticipate future state shifts.
Our findings highlight the importance of managing water quality to protect reef building organisms and functional diversity of reefs, which are essential for maintaining reef resilience, mitigating ecosystem phase shifts and for supporting livelihoods in coastal systems. Additionally, the findings in this study have broader relevance for PICTs facing similar urban pressures, particularly atoll systems like Tarawa and Funafuti where limited flushing may exacerbate pollution impacts. While this study provides valuable insights into the spatial patterns of benthic composition and water quality across Suva’s urban reef systems, several limitations must be acknowledged, including the lack of seasonal variation in water quality and fine scale substrate dynamics that may mask important ecological transitions (e.g., emerging cyanobacterial mats as degradation indicators) (Wenger et al., 2024). Furthermore, our study prioritized model interpretability and comparability across substrate types and did not account for cumulative and interactive effects. While we recognize that such interactions are ecologically important and can shape benthic trajectories in complex ways (McCarthy et al., 2025), incorporating them would have substantially increased model complexity and reduced the clarity of predictor-specific interpretations. Looking forward, we recommend establishing long term monitoring combining permanent transects with high resolution surveys to track benthic shifts, alongside experimental studies to disentangle local versus global climate stressors and consequent cumulative interactions. Future research should incorporate long-term monitoring frameworks that include both ecological (e.g., coral growth, recruitment, herbivore biomass) and biogeochemical indicators to better understand the trajectories of urban reef ecosystems. Such advances will be critical for developing adaptative management strategies that are responsive to the complex, chronic stressors facing urban coral reef systems, while providing scalable solution for other vulnerable PICT communities that depend on these systems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
JD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. AF: Methodology, Writing – review & editing. AS: Conceptualization, Supervision, Writing – review & editing, Funding acquisition. ML: Visualization, Writing – review & editing, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. J.D. is supported by the "Allocations de recherche pour une thèse au Sud (ARTS-IRD)" fellowship, under the guidance of Christophe Menkes, and receives additional funding through a research student grant from the School of Agriculture, Geography, Environment, Oceans & Natural Sciences at the University of the South Pacific (F3354 FST15-71502-001). Financial support towards the publication fee was received from the Pacific Ocean Climate Crisis Assessment (POCCA) project and the Centre for Sustainable Futures (CSF), funded by the New Zealand Ministry of Foreign Affairs and Trade (MFAT).
Acknowledgments
The authors gratefully acknowledge the Ministry of iTaukei Affairs for endorsing this study (Reference: MTA-42/2-3). We extend our sincere appreciation to the Rewa and Tailevu Provincial Council Offices, as well as to the traditional custodians of the iQoliqoli spanning Namuka, Navakavu, Suva, Laucala, Nasoata, and Nasilai, for granting research access to their waters and reefs. We are especially thankful to Merelesita Fong, Alicia Emberson, Renee Hill-Lewenilovo, and Taine Reiher for their invaluable assistance in the field, and to the Coffee Bure for discussion that helped shape the development of this study. This research forms part of the doctoral work of J.D., supervised by A.S.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used ChatGPT/OpenAI in order to refine sentence structure and improve grammar. The authors reviewed, edited and verified the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1659324/full#supplementary-material
References
Abrego D., Howells E. J., Smith S. D. A., Madin J. S., Sommer B., Schmidt-Roach S., et al. (2021). Factors limiting the range extension of corals into high-latitude reef regions. Diversity (Basel) 13(12), 632. doi: 10.3390/d13120632
Adjeroud M., Fernandez J. M., Carroll A. G., Harrison P. L., and Penin L.. (2010). Spatial patterns and recruitment processes of coral assemblages among contrastingenvironmental conditions in the southwestern lagoon of New Caledonia. Mar. Pollut. Bull. 61, 375–386. doi: 10.1016/j.marpolbul.2010.06.015, PMID: 20621316
Altman-Kurosaki N. T., Pratte Z. A., Stewart F. J., and Hay M. E. (2024). Coral–algal competition: allelopathy, temporal variance, and effects on coral microbiomes. Coral Reefs 44, 49–62. doi: 10.1007/S00338-024-02585-7
Arikibe J. E. and Prasad S. (2020). Determination and comparison of selected heavy metal concentrations in seawater and sediment samples in the coastal area of Suva, Fiji. Mar. pollut. Bull. 157, 111157. doi: 10.1016/j.marpolbul.2020.111157, PMID: 32658659
Ban S. S., Pressey R. L., and Graham N. A. J. (2015). Assessing the effectiveness of local management of coral reefs using expert opinion and spatial Bayesian modeling. PloS One 10 (8), 1–16. doi: 10.1371/journal.pone.0135465, PMID: 26284372
Bartoń K. (2010). MuMIn: Multi-Model Inference (CRAN: Contributed Packages). doi: 10.32614/CRAN.package.MuMIn
Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/J.TREE.2008.10.008, PMID: 19185386
Boonnam N., Udomchaipitak T., Puttinaovarat S., Chaichana T., Boonjing V., and Muangprathub J. (2022). Coral reef bleaching under climate change: prediction modeling and machine learning. Sustainability (Switzerland) 14 (10), 6161. doi: 10.3390/su14106161
Brown B. E., Clarke K. R., and Warwick R. M. (2002). Serial patterns of biodiversity change in corals across shallow reef flats in KO Phuket, Thailand, due to the effects of local (sedimentation) and regional (climatic) perturbations. Mar. Biol. 141, 21–29. doi: 10.1007/S00227-002-0810-0
Burt J. A., Camp E. F., Enochs I. C., Johansen J. L., Morgan K. M., Riegl B., et al. (2020). Insights from extreme coral reefs in a changing world. Coral Reefs 39, 495–507. doi: 10.1007/S00338-020-01966-Y
Camp E. F., Schoepf V., Mumby P. J., Hardtke L. A., Rodolfo-Metalpa R., Smith D. J., et al. (2018). The future of coral reefs subject to rapid climate change: Lessons from natural extreme environments. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00004
Carturan B. S., Parrott L., and Pither J. (2022). Functional richness and resilience in coral reef communities. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.780406
Chong-Seng K. M., Mannering T. D., Pratchett M. S., Bellwood D. R., and Graham N. A. J. (2012). The influence of coral reef benthic condition on associated fish assemblages. PloS One 7, e42167. doi: 10.1371/journal.pone.0042167, PMID: 22870294
Cruz I. C. S., Waters L. G., Kikuchi R. K. P., Leão Z. M. A. N., and Turra A. (2018). Marginal coral reefs show high susceptibility to phase shift. Mar. pollut. Bull. 135, 551–561. doi: 10.1016/j.marpolbul.2018.07.043, PMID: 30301073
Dehm J., Le Gendre R., Lal M., Menkes C., and Singh A. (2025). Water quality within the greater Suva urban marine environment through spatial analysis of nutrients and water properties. Mar. pollut. Bull. 213, 117601. doi: 10.1016/j.marpolbul.2025.117601, PMID: 39892061
Dehm J., Singh S., Ferreira M., and Piovano S. (2020). Microplastics in subsurface coastal waters along the southern coast of Viti Levu in Fiji, South Pacific. Mar. pollut. Bull. 156, 111239. doi: 10.1016/j.marpolbul.2020.111239, PMID: 32510383
Dehm J., Singh A., Le Gendre R., and Menkes C. (2024). Suva Urban Reefs: An ecological history of the coral reefs of the Greater Suva Urban Area, Fiji. Reg. Stud. Mar. Sci. 80, 103894. doi: 10.1016/j.rsma.2024.103894
Dutra L. X. C., Haywood M. D. E., Singh S., Ferreira M., Johnson J. E., Veitayaki J., et al. (2021). Synergies between local and climate-driven impacts on coral reefs in the Tropical Pacific: A review of issues and adaptation opportunities. Mar. pollut. Bull. 164, 111922. doi: 10.1016/j.marpolbul.2020.111922, PMID: 33632532
Estradivari, Pratama A. M. A., Syafruddin G., Kanna P. L., Stuhr M., Torres A. F., et al. (2025). Coastal urbanization-related stressors affect fish herbivory in the Spermonde Archipelago, Indonesia. Front. Mar. Sci. 12. doi: 10.3389/fmars.2025.1359139
Fabricius K. E. (2005). Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. pollut. Bull. 50, 125–146. doi: 10.1016/j.marpolbul.2004.11.028, PMID: 15737355
Ferreira S. B., Burns J. H. R., Pascoe K. H., Kapono C. A., Reyes A. J., and Fukunaga A. (2023). Prediction of habitat complexity using a trait-based approach on coral reefs in Guam. Sci. Rep. 13, 1–11. doi: 10.1038/S41598-023-38138-1, PMID: 37422484
Ford A. K., Jouffray J. B., Norström A. V., Moore B. R., Nugues M. M., Williams G. J., et al. (2020). Local human impacts disrupt relationships between benthic reef assemblages and environmental predictors. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.571115
Gordon S. E., Goatley C. H. R., and Bellwood D. R. (2016). Composition and temporal stability of turf sediments on inner-shelf coral reefs. Mar. pollut. Bull. 111, 178–183. doi: 10.1016/j.marpolbul.2016.07.013, PMID: 27427199
Graham N. A. J. and Nash K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326. doi: 10.1007/S00338-012-0984-Y
Guest J. R., Tun K., Low J., Vergés A., Marzinelli E. M., Campbell A. H., et al. (2016). 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Sci. Rep. 6, 36260. doi: 10.1038/SREP36260, PMID: 27824083
Hill J. and Wilkinson C. R. (2004). Methods for ecological monitoring of coral reefs : a resource for managers (Townsville, Australia: Australian Institute of Marine Science).
Hoegh-Guldberg O., Andréfouët S., Fabricius K. E., Diaz-Pulido G., Lough J. M., Marshall P. A., et al. (2011). “Vulnerability of coral reefs in the tropical Pacific to climate change,” in Vulnerability of tropical pacific fisheries and aquaculture to climate change. Eds. Bell J. D., Johnson J. E., and Hobday A. J. (Secretariat of the Pacific Community, Noumea), 647–732. Available online at: http://www.spc.int/climate-change/fisheries/assessment (Accessed June 15, 2025).
Houk P., Musburger C., and Wiles P. (2010). Water quality and herbivory interactively drive coral-reef recovery patterns in american Samoa. PloS One 5 (11), 1–9. doi: 10.1371/journal.pone.0013913, PMID: 21085715
Huang L., McWilliam M., Liu C., Yu X., Jiang L., Zhang C., et al. (2024). Loss of coral trait diversity and impacts on reef fish assemblages on recovering reefs. Ecol. Evol. 14, 11. doi: 10.1002/ece3.70510, PMID: 39493612
Hughes T. P., Kerry J. T., Baird A. H., Connolly S. R., Dietzel A., Eakin C. M., et al. (2018). Global warming transforms coral reef assemblages. Nature 556, 492–496. doi: 10.1038/S41586-018-0041-2, PMID: 29670282
Jiang S., Wang J., Fan W., Chen L., Chen J., and Li B. (2024). Decadal variation and temporal stability of the macrobenthic community in the Bohai Sea, China. Mar. pollut. Bull. 207, 116904. doi: 10.1016/j.marpolbul.2024.116904, PMID: 39226821
Jouffray J. B., Nyström M., Norström A. V., Williams I. D., Wedding L. M., Kittinger J. N., et al. (2015). Identifying multiple coral reef regimes and their drivers across the hawaiian archipelago. Philos. Trans. R. Soc. B: Biol. Sci. 370, 1–8. doi: 10.1098/rstb.2013.0268
Kenyon T. M., Doropoulos C., Wolfe K., Webb G. E., Dove S., Harris D., et al. (2023). Coral rubble dynamics in the Anthropocene and implications for reef recovery. Limnol. Oceanogr. 68, 110–147. doi: 10.1002/lno.12254
Kim T., Lee D. W., Kim H. J., Jung Y. H., Choi Y. U., Oh J. H., et al. (2022). Estimation of the benthic habitat zonation by photo-quadrat image analysis along the fringing reef of Weno island, Chuuk, Micronesia. J. Mar. Sci. Eng. 10, 1643. doi: 10.3390/JMSE10111643/S1
Kitolelei S., Thaman R., Veitayaki J., Breckwoldt A., and Piovano S. (2021). Na vuku makawa ni qoli: indigenous fishing knowledge (IFK) in Fiji and the pacific. Front. Mar. Sci. 8. doi: 10.3389/FMARS.2021.684303
Kohler K. E. and Gill S. M. (2006). Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269. doi: 10.1016/J.CAGEO.2005.11.009
Kuffner I. B., Walters L. J., Becerro M. A., Paul V. J., Ritson-Williams R., and Beach K. S. (2006). Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar. Ecol. Prog. Ser. 323, 107–117. doi: 10.3354/MEPS323107
Kumar A. and Bera S. (2020). Revisiting nitrogen utilization in algae: A review on the process of regulation and assimilation. Bioresour. Technol. Rep. 12, 100584. doi: 10.1016/J.BITEB.2020.100584
Kurihara H., Watanabe A., Tsugi A., Mimura I., Hongo C., Kawai T., et al. (2021). Potential local adaptation of corals at acidified and warmed Nikko Bay, Palau. Sci. Rep. 11, 1–10. doi: 10.1038/S41598-021-90614-8, PMID: 34045589
Lai S., Loke L. H. L., Hilton M. J., Bouma T. J., and Todd P. A. (2015). The effects of urbanisation on coastal habitats and the potential for ecological engineering: A Singapore case study. Ocean Coast. Manag. 103, 78–85. doi: 10.1016/J.OCECOAMAN.2014.11.006
Lal R., Kininmonth S., N’Yeurt A. D. R., Riley R. H., and Rico C. (2018). The effects of a stressed inshore urban reef on coral recruitment in Suva Harbour, Fiji. Ecol. Evol. 8, 11842–11856. doi: 10.1002/ECE3.4641, PMID: 30598781
Lerma J. L. and Cabrelles M. (2007). A review and analyses of plumb-line calibration. Photogrammetric Rec. 22, 135–150. doi: 10.1111/J.1477-9730.2007.00412.X
McAndrews R. S., Eich A., Ford A. K., Bejarano S., Lal R. R., and Ferse S. C. A. (2019). Algae sediment dynamics are mediated by herbivorous fishes on a nearshore coral reef. Coral Reefs 38, 431–441. doi: 10.1007/s00338-019-01780-1
McCarthy O. S., Kelly E. L. A., Akiona A. K., Clements S. M., Martinez T., Pedersen N. E., et al. (2025). Using community composition and successional theory to guide Site-Specific coral reef management. Global Change Biol. 31 (1), e70050. doi: 10.1111/gcb.70050, PMID: 39873121
McClanahan T. R., Azali M. K., Muthiga N. A., Porter S. N., Schleyer M. H., and Guillaume M. M. M. (2024). Complex multivariate model predictions for coral diversity with climatic change. Ecosphere 15, 12. doi: 10.1002/ecs2.70057
McKinney W. (2010). Data structures for statistical computing in python. Science Conference, 56–61. doi: 10.25080/Majora-92bf1922-00a
Mills M. S., Schils T., Olds A. D., and Leon J. X. (2023). Structural complexity of coral reefs in Guam, Mariana islands. Remote Sens (Basel) 15, 5558. doi: 10.3390/RS15235558/S1
Morris C. (2007). South-West Pacific status of coral reefs report 2007. Available online at: www.crisponline.net (Accessed June 1, 2025).
Morris C. and Mackay K. (2021). Status of the coral reefs in the Southwest Pacific: Fiji, New Caledonia, Samoa, Solomon Islands, Tuvalu and Vanuatu. Available online at: https://pacific-data.sprep.org/dataset/status-coral-reefs-south-west-pacific-Fiji-new-caledonia-Samoa-solomon-islands-Tuvalu-and (Accessed June 19, 2025).
Morrison R., Gangaiya P., Singh S., Maata M., and Chandra A. (2005). “Contamination of Suva Lagoo,” in At the Crossroads: Science and Management of Suva Lagoon. Eds. Morrison R. and Aalbesberg W. (Suva, Fiji: University of the South Pacific, Institute of Applied Sciences), 146–155.
Ng C. S. L., Chan Y. K. S., Nguyen N. T. H., Kikuzawa Y. P., Sam S. Q., Toh T. C., et al. (2021). Coral community composition and carbonate production in an urbanized seascape. Mar. Environ. Res. 168, 105322. doi: 10.1016/J.MARENVRES.2021.105322, PMID: 33857701
Nguyen H. T. T., Pritchard D. W., and Hepburn C. D. (2020). Nitrogen and phosphorus ecophysiology of coralline algae. J. Appl. Phycol. 32, 2583–2597. doi: 10.1007/S10811-019-02019-W
Phillips T. and Keen M. (2016). “Sharing the City : Urban Growth and Governance in Suva, Fiji,” in SSGM DISCUSSION PAPER, vol. 6. (Australian National University, Canberra, Australia). doi: 10.25911/5f2004d9403d2
Portugal A. B., Carvalho F. L., de Macedo Carneiro P. B., Rossi S., and de Oliveira Soares M. (2016). Increased anthropogenic pressure decreases species richness in tropical intertidal reefs. Mar. Environ. Res. 120, 44–54. doi: 10.1016/j.marenvres.2016.07.005, PMID: 27428738
Pratap A., Mani F. S., and Prasad S. (2020). Heavy metals contamination and risk assessment in sediments of Laucala Bay, Suva, Fiji. Mar. pollut. Bull. 156, 111238. doi: 10.1016/j.marpolbul.2020.111238, PMID: 32510382
Raymundo L. J., Halford A. R., Maypa A. P., and Kerr A. M. (2009). Functionally diverse reef-fish communities ameliorate coral disease. Proc. Natl. Acad. Sci. 106, 17067–17070. doi: 10.1073/pnas.0900365106, PMID: 19805081
Rodda P. (2005). “Geomorphology and geology of the Suva Lagoon catchment,” in At the Crossroads: Science and Management of Suva Lagoon. Eds. Morrison R. J. and Aalbesberg W. (Suva, Fiji: The University of the South Pacific, Institute of Applied Sciences), 15–32.
Roelfsema C. and Phinn S. (2008). Evaluating eight field and remote sensing approaches for mapping the benthos of three different coral reef environments in Fiji. Remote Sens. Inland Coastal Oceanic Waters 7150, 95–108. doi: 10.1117/12.804806
Roth F., Saalmann F., Thomson T., Coker D. J., Villalobos R., Jones B. H., et al. (2018). Coral reef degradation affects the potential for reef recovery after disturbance. Mar. Environ. Res. 142, 48–58. doi: 10.1016/j.marenvres.2018.09.022, PMID: 30274715
Salkind N. (2007). Akaike information criterion. Encyclopedia Measurement Stat 1, 16–17. doi: 10.4135/9781412952644
Samperiz A., Sosdian S., Hendy E., Johnson K., John E. H., Jupiter S. D., et al. (2025). Coastal seawater turbidity and thermal stress control growth of reef-building Porites spp. corals in Fiji. Sci. Rep. 15, 1–15. doi: 10.1038/S41598-025-02283-6, PMID: 40382443
Seabold S. and Perktold J. (2010). Statsmodels: econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference. van der Walt S. and Millman J. (Eds.), 92–96. doi: 10.25080/Majora-92bf1922-011
Shiiba N., Singh P., Charan D., Raj K., Stuart J., Pratap A., et al. (2023). Climate change and coastal resiliency of Suva, Fiji: a holistic approach for measuring climate risk using the climate and ocean risk vulnerability index (CORVI). Mitig Adapt Strateg. Glob. Chang. 28, 1–31. doi: 10.1007/S11027-022-10043-4, PMID: 36685809
Smith J. E., Brainard R., Carter A., Grillo S., Edwards C., Harris J., et al. (2016). Re-evaluating the health of coral reef communities: Baselines and evidence for human impacts across the central pacific. Proc. R. Soc. B: Biol. Sci. 283 (1822), 20151985. doi: 10.1098/RSPB.2015.1985, PMID: 26740615
Swierts T. and Vermeij M. J. A. (2016). Competitive interactions between corals and turf algae depend on coral colony form. PeerJ 2016, e1984. doi: 10.7717/peerj.1984, PMID: 27190707
Syafruddin G., Estradivari, Pratama A. M. A., Yasir I., Ferse S. C. A., and Ambo-Rappe R. (2025). Sediment, substrate, and structure: Factors shaping algal turf dynamics in urban Indonesian reefs. Reg. Stud. Mar. Sci. 86, 104172. doi: 10.1016/J.RSMA.2025.104172
Tamata B., Tuiwawa M., Meo S., Vave R., Comley J., Fong S., et al. (2010). Rewa River Dredging Project Final Eia Report, Suva, Fiji: Institute of Applied Sciences, The University of the South Pacific. Vol. 201.
Tan K. S., Acerbi E., and Lauro F. M. (2016). Marine habitats and biodiversity of Singapore’s coastal waters: A review. Reg. Stud. Mar. Sci. 8, 340–352. doi: 10.1016/J.RSMA.2016.01.008
Tebbett S. B., Connolly S. R., and Bellwood D. R. (2023a). Benthic composition changes on coral reefs at global scales. Nat. Ecol. Evol. 7, 71–81. doi: 10.1038/s41559%2D022%2D01937%2D2
Tebbett S. B., Crisp S. K., Evans R. D., Fulton C. J., Pessarrodona A., Wernberg T., et al. (2023b). On the challenges of identifying benthic dominance on anthropocene coral reefs. Bioscience 73, 220–228. doi: 10.1093/BIOSCI/BIAD008, PMID: 36936383
Tebbett S. B., Goatley C. H. R., and Bellwood D. R. (2018). Algal turf sediments across the Great Barrier Reef: Putting coastal reefs in perspective. Mar. pollut. Bull. 137, 518–525. doi: 10.1016/J.MARPOLBUL.2018.10.056, PMID: 30503463
Teichberg M., Wild C., Bednarz V. N., Kegler H. F., Lukman M., Gärdes A. A., et al. (2018). Spatio-temporal patterns in coral reef communities of the Spermonde Archipelago 2012-2014, I: Comprehensive reef monitoring of water and benthic indicators reflect changes in reef health. Front. Mar. Sci. 5. doi: 10.3389/FMARS.2018.00033
Terpilowski M. A. (2019). scikit-posthocs: Pairwise multiple comparison tests in Python. J. Open Source Software. 4 (36), 1169. doi: 10.21105/joss.01169
Thomas A., Mangubhai S., Fox M., Meo S., Miller K., Naisilisili W., et al. (2021). Why they must be counted: Significant contributions of Fijian women fishers to food security and livelihoods. Ocean Coast. Manag. 205, 105571. doi: 10.1016/j.ocecoaman.2021.105571
Vallat R. (2018). Pingouin: statistics in python. J. Open Source Softw. 3, 1026. doi: 10.21105/joss.01026
Varea R., Paris A., Ferreira M., and Piovano S. (2021). Multibiomarker responses to polycyclic aromatic hydrocarbons and microplastics in thumbprint emperor Lethrinus harak from a South Pacific locally managed marine area. Sci. Rep. 11, 1–10. doi: 10.1038/S41598-021-97448-4, PMID: 34504212
Veitayaki J. (2010). “Pacific Islands drowning in their waste: Waste management issues that threaten sustainability,” in International Seminar on Islands and Oceans (Ocean Policy Research Foundation, Tokyo, Japan).
Veitayaki J. (2021). Securing coastal fisheries in the Pacific: Critical resources for food, livelihood and community security Vol. 56 (Canberra, Australia: Development Bulletin). Available online at: https://pacificsecurity.net/wp-content/uploads/2021/10/DB82_Part11.pdf.
Violette C., Adjeroud M., Payri C., Purkis S. J., and Andréfouët S. (2024). Changes of Tiahura (Moorea Island) reef flat habitats using 67 years of remote sensing observations. Coral Reefs 43, 1775–1792. doi: 10.1007/S00338-024-02576-8
Virtanen P., Gommers R., Oliphant T. E., Haberland M., Reddy T., Cournapeau D., et al. (2020). SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272. doi: 10.1038/s41592-019-0686-2, PMID: 32015543
Waskom M. (2021). seaborn: statistical data visualization. J. Open Source Softw. 6, 3021. doi: 10.21105/joss.03021
Wenger A. S., Gómez Juárez E. A., Falinski K., Amaya T., Corbin C., Cramer K., et al. (2024). A guide for pollution assessment and monitoring in coastal ecosystems (New York, USA: Wildlife Conservation Society). doi: 10.19121/2024.REPORT.50065.P1
Wicquart J., Towle E. K., Dallison T., Staub F., and Planes S. (2025). Status and Trends of Coral Reefs of the Pacific: 1980-2023. Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI). doi: 10.59387/WIUJ2936
Keywords: urban coral reefs, anthropogenic reefs, Pacific Island Countries and Territories, reef resilience, reef substrate, phase shifts
Citation: Dehm J, Ford AK, Singh A and Lal M (2025) Influence of urbanization-driven water quality on reef substrate composition along Suva, Fiji, one of the Pacific Islands most urbanized reefs. Front. Mar. Sci. 12:1659324. doi: 10.3389/fmars.2025.1659324
Received: 03 July 2025; Accepted: 21 August 2025;
Published: 04 September 2025.
Edited by:
Tamara Cibic, National Institute of Oceanography and Applied Geophysics, ItalyReviewed by:
Robert H. Richmond, University of Hawaii at Manoa, United StatesCaitie Kuempel, Griffith University, Australia
Copyright © 2025 Dehm, Ford, Singh and Lal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jasha Dehm, czExMDY0NzU2QHN0dWRlbnQudXNwLmFjLmZq
‡ORCID: Jasha Dehm, orcid.org/0000-0002-9325-2826
Amanda K. Ford, orcid.org/0000-0002-4971-048X
Awnesh Singh, orcid.org/0000-0002-5341-0703
Monal Lal, orcid.org/0000-0002-8545-2887
 Jasha Dehm
Jasha Dehm Amanda K. Ford
Amanda K. Ford Awnesh Singh
Awnesh Singh Monal Lal
Monal Lal