Abstract
Aquaponics is a soilless farming approach that integrates aquaculture with hydroponics to produce food. In regions with limited arable land, aquaponics can help address food insecurity challenges. Both fish and plants are produced using aquaponic systems. The aquafeeds used to feed the fish in aquaponic systems are also the main source of nutrition for the plants. Currently, commercial aquafeeds such as fishmeal and fish oil are used in aquaponics, but they do not completely meet the nutritional requirements of plants. Additionally, commercial aquafeeds are expensive, and their production is unsustainable. This review focuses on the suitability of microalgae as a replacement for commercial aquafeeds and its role in meeting the nutritional requirements of plants growing in aquaponic systems. Microalgae production is sustainable and cost effective compared to commercial aquafeed production. Many studies have been conducted on the impact of microalgae-based feed on fish growth and its role as a biofertilizer and biostimulants for plant growth. However, using microalgae as aquafeed for the development of both fish and plants in aquaponic systems remains underexplored. This review aims to provide insights into the dual role of microalgae in aquaponics—enhancing fish nutrition while supplementing plant nutrient requirements. Although some micronutrient gaps may persist, further optimisation could help make aquaponic systems more efficient and sustainable.
Introduction
Aquaponics is an innovative and sustainable food production system that integrates recirculating aquaculture with hydroponics (König et al., 2016; Okomoda et al., 2023). Aquaponic plants perform comparably to hydroponic plants, despite lower nutrient concentrations (Sreekumar et al., 2023). This system offers numerous benefits, including water conservation, a reduced environmental impact, and the simultaneous production of fish and plants (Mishra et al., 2020). Aquaponics can be implemented in various settings, ranging from urban to rural areas, and at different scales, ranging from small-scale farms to industrial production units (König et al., 2016). This system is particularly valuable for addressing food security challenges as it can be utilised on non-arable land and in areas with limited water resources (Shreejana et al., 2022). Furthermore, as aquaponics is a closed-loop system that includes both hydroponic and aquaculture systems, it presents potential solutions for food production in the face of climate change-related challenges such as global warming, desertification, water scarcity, famine, and increased pests and diseases. Aquaponics is an eco-friendly cultivation system that has gained interest in various fields and industries including ecology, agriculture, and fisheries (Hao et al., 2020). Several areas of aquaponic systems need to be explored further to fully understand their potential. These areas include nitrogen cycling, nutrient recovery from fish waste, plant nutrition, plant pathogens, pest control strategies, and sustainable aquafeed (Goddek et al., 2019). This review focuses on microalgae as a sustainable alternative to commercial aquafeeds in aquaponic systems. Additionally, this review examines how microalgae can fulfil the nutritional requirements of both plants and fish in aquaponic systems., In aquaponic systems, residual fish feed and fish waste provide nutrients for plant growth. Current aquaponic systems that use commercial aquafeeds such as fish oil, fishmeal, and fish waste do not completely meet the nutritional requirements for plant growth (Eck et al., 2019; Yep and Zheng, 2019). Macronutrients such as potassium, phosphorus, sulphur, and calcium, along with micronutrients such as manganese, iron, zinc, copper, boron and molybdenum, often accumulate in inadequate amounts or disproportionate ratios in the water. Consequently, plants do not receive sufficient nutrients for their growth (Delaide et al., 2017; Suhl et al., 2016). Furthermore, commercial aquafeeds are expensive and unsustainable. I The water footprint of commercial aquafeeds was estimated to be between31–35 km3 in 2008, with the top five species alone accounting for 18.2 km3. For example, fisheries that provide commercial aquafeeds, such as fish oil and fishmeal, emitted 4.6 million tons of carbon dioxide-equivalent greenhouse gases in 2014 (Cashion et al., 2017). The carbon footprints of extruded and pelleted grass carp aquafeeds were 1334 and 1071 kg CO2 eq/t, respectively, with raw material production being the largest contributor. To overcome these challenges, sustainable sourcing of raw materials and the utilisation of renewable energy in aquafeed production can substantially reduce the environmental impact (Wang et al., 2022). Additionally, exploring alternative aquafeed sources, such as single-cell proteins and insects, can help address sustainability concerns (D’Abramo, 2021). Microalgae have emerged as promising alternative aquafeed ingredients because of their nutritional profile and sustainability benefits. They contain essential amino acids, fatty acids, vitamins, pigments, and bioactive compounds that enhance fish health, survival, and product quality (Nagappan et al., 2021; Sagaram et al., 2021). Compared to traditional feed sources, such as fishmeal and soymeal, microalgae offer a more diverse set of fatty acids, pigments, sterols, and vitamins (Dixit et al., 2022). Microalgae production has a lower environmental footprint than terrestrial crops in terms of water use and land requirements (Nagappan et al., 2021; Mahata et al., 2022). Additionally, microalgae can positively influence gut microbiota and immune responses in aquatic species (Sagaram et al., 2021). Microalgae, small but powerful photosynthetic organisms, are emerging as a sustainable solution to a range of global challenges—from clean energy and environmental protection to food security and human health. Rich in nutrients and bioactive compounds, microalgae are being explored for use in biofuels (Akhtar et al., 2023), functional foods (Andrade-Bustamante et al., 2025), and health supplements (Ayub et al., 2025), offer benefits like antioxidant, anti-inflammatory, and heart-protective effects. Their ability to treat wastewater, capture CO2, and support circular practices makes them valuable in aquaculture (Dasari et al., 2025) and environmental cleanup, including antibiotic pollution removal (Wani et al., 2024). They use minimal land and water, making them eco-friendly alternatives to traditional crops, and contribute significantly to achieving climate goals and UN Sustainable Development Goals (Ahmad and Ashraf, 2024). Despite challenges like high production costs, regulatory hurdles, and taste issues in food applications, advances in biotechnology, AI, and strain development are paving the way for large-scale, sustainable use of microalgae across industries. This review examines the potential of microalgae as sustainable components of aquafeed formulations. It also provides valuable insights into the utilisation of microalgae aquafeeds as replacements for commercial aquafeeds such as fish oil and fishmeal. Microalgae may provide complete nutrition for plant growth in aquaponic systems, and its use will enhance the efficiency and sustainability of these systems and contribute to global food security.
Aquaponics: an overview
Aquaponics is a climate-smart technology used for sustainable food production (Nishanth et al., 2024). Aquaponic systems use less than 90% of the water used in conventional fish and plant farming which support sustainable food production and facilitate complete biological processes between fish, plants, and microbes. Aquaponic systems are composed of three main components that work together: the growing bed (hydroponic unit) for plant growth, biofilter for microbes to perform nitrification, and aquaculture tank to rear fish. All three components must function in coordination to support fish and plant growth. Fish waste is the primary nutrient component for plant growth in aquaponics. Fish waste acts as a primary nutrient source undergoing microbial nitrification to convert ammonia into plant-available nitrates. Based on the designs of the hydroponic and aquaculture units, aquaponic systems are classified into coupled and decoupled systems. Common hydroponic designs include Nutrient Film Technique (NFT), floating raft or deep-water culture, and media-based grow beds. peat moss and perlite are used as plant growth media. Media-based systems use substrates like peat moss and perlite and are ideal for vegetables and fruits due to their capacity to support high root density. NFT systems are typically used for smaller vegetables, while floating raft systems are most common, allowing roots to freely absorb nutrients. To maintain a stable environment, it is essential to monitor water quality, pH, temperature, water-use efficiency, waste management, and nutrient cycling (Goddek et al., 2019).
Nutrient cycling in an aquaponic system has many advantages because it is a recirculating system combining hydroponics and aquaculture. Because no effluent is discharged, it prevents environmental pollution. Additionally, the nutrient-rich aquaculture water can be reused as an organic fertiliser for plants in the hydroponic units. Some studies have indicated that aquaponics produces plant growth and yields comparable to or even exceeding those of soil-grown plants (Yogev et al., 2016). Nutrient cycling in aquaponic systems is influenced by multiple factors such as the aquafeed type, fish species, fish density, plant type, and microbial community. The main nutrient sources in aquaponic systems are aquafeed and aquaculture water, which contribute essential elements such as magnesium, calcium, and sulphur (Delaide et al., 2017; Schmautz et al., 2016). The two main types of aquafeed are plant-based and fishmeal-based feeds. After being introduced into the system, aquafeed is partly consumed and excreted by the fish, while some residual feed remains in the tank. Fish excreta and uneaten feed dissolve in the water, releasing nutrients that are absorbed by plants. To support optimal plant growth, additional supplements like potassium and iron may be introduced—without harming the fish (Schmautz et al., 2016). Residual aquafeed, which accounts for less than 5%, and fish excreta also contribute to carbon dioxide and ammonia production, increasing the nutrient load of the water and influencing plant development (Yogev et al., 2016). Water quality and fish biomass are strongly influenced by aquafeed type, highlighting the importance of selecting feed that meets the nutritional needs of both fish and plants (Schmautz et al., 2016).
Microbial communities in aquaponic systems undergo many biological processes that convert fish waste and residual aquafeed into nutrient rich solutions for plant growth. One such process is solubilisation carried out by bacteria that break down complex organic compounds into ionic forms absorbable by plants. Heterotrophic bacteria such as Pseudomonas sp., Flavobacterium sp., Rhizobium sp., Aeromonas sp., and Sphingobacterium sp. are involved in this solubilisation process. Additionally, some γ-proteobacteria can solubilise phytates making phosphorus available to plants. The primary nitrogen source in aquaponic systems is the proteins present in aquafeeds. However, fish utilise only about30% of the nitrogen present in aquafeed, and the remaining is excreted in the form of ammonia (Ru et al., 2017; Wongkiew et al., 2017; Yavuzcan Yildiz et al., 2017). This ammonia is oxidised to nitrite by ammonia-oxidising bacteria such as Nitrosococcus, Nitrosospira, Nitrosomonas, Nitrosolobus, and Nitrosovibrio, and subsequently converted to nitrate by nitrite-oxidising bacteria such as Nitrobacter, Nitrococcus, Nitrospina, and Nitrospira (Wongkiew et al., 2017). Understanding the nutrient cycles is essential for the effective operation of aquaponic systems, as plants require different nutrients at various growth stages. Some of these nutrients can be supplemented either as foliar application or by adding nutrients directly to the water. Macronutrients, such as carbon, are supplied through the organic compounds in the aquafeed then metabolised by both fish and microbes, releasing carbon dioxide(CO2) as a byproduct. This CO2 is then absorbed by plants and used in photosynthesis via carbon fixation. Plants uptake nitrogen as nitrate or ammonium ions (Wongkiew et al., 2017), Phosphorus as orthophosphate (Resh, 2022), and potassium which is important for growth and accumulates especially in fruit (Schmautz et al., 2016). Other essential elements such as calcium, magnesium, and sulphur typically present in tap water, while micronutrients like manganese, iron, and zinc, are derived from aquafeed., Copper and boron are also present in tap water (Delaide et al., 2017). Overall, aquaponic systems foster a symbiotic relationship among fish, plants, and microbes in a recirculating sustainable food production process.
Nutrient imbalance and aquafeed unsustainability in aquaponics
In aquaponic systems, nutrients are transferred from fish waste to plants through biological processes; however, an imbalance often exists between the nutrient content in fish waste and the nutrient requirements for optimal plant growth. Factors such as fish tank size, biofilter capacity, and system design influence the nutrient availability Therefore, the nutrient composition of aquafeed, and the specific requirements of each plant species must be carefully considered (Resh, 2022). Monitoring nutrient availability is challenging, as nutrients originate primarily from fish waste and residual aquafeed. Processes such as fish waste removal, water renewal, and denitrification contribute to nutrient loss in the system. Research studies has shown that fish waste and residual aquafeed contain 86% manganese, 22% copper, 89% magnesium, 24% iron, 16% calcium, 6% potassium, 6% nitrogen, and 18% phosphorus. However, not all these nutrients are efficiently utilised by plants, particularly macronutrients like potassium, phosphorus, iron, manganese, and sulphur. Nitrogen released from fish protein metabolism enters nitrogen cycle and is transformed into usable forms usable by plants. Since aquafeed and fish waste are the main nutrient sources their selection and utilisation are crucial for supporting both fish and plant growth in integrated aquaponic systems (Zhanga et al., 2021).
Some studies have shown that, minerals added as supplements to aquafeed, can be utilised by plants in aquaponic systems. Soluble minerals are not absorbed by fish may be taken up by plants, enhancing nutrient recovery. However, the mineral requirements and metabolism in aquaculture species has not been extensively investigated. The addition of anions and their accompanying cations to aquafeed has been shown to improve nutrient availability for plants (Ng and Koh, 2017). Plant-based minerals in aquafeed may contain phosphorus in phytate form, which is not readily metabolised by plants. The exogenous addition of enzymes to aquafeed can help release phosphorus from improving bioavailability. However, this approach has some limitations, such as potential release of undesirable compounds that may affect fish health. Further research is needed to evaluate the safe and effective use of these enzymes. Moreover, adding supplements to aquafeed or directly into hydroponic systems is expensive. To reduce this cost it is essential to understand the amount of aquafeed required to meet both fish and plant nutritional requirements. In aquaponic systems, plant physiological processes such as photosynthesis, flowering, defence, and seed germination are regulated in a circadian rhythm pattern which ideally should work in coordination with circadian rhythm patterns of fish. However, when commercial aquafeed is used, this rhythm is not always well-coordinated and plants nutritional requirements may not be fully met. Some microalgae like Chlamydomonas reinhardtii has been widely studied for its role in research on photosynthesis, metabolism, and cilia function. Beyond its laboratory significance, it is increasingly recognised for its biotechnological potential due to its fast growth, metabolic flexibility, and low-cost cultivation. It has been applied in biofuel production, nutraceutical development, and wastewater treatment, where it contributes to contaminant removal and resource recovery. Recent studies have also explored the synergistic benefits of co-cultivating Chlamydomonas with bacteria to enhance detoxification and bioproduction processes. Although challenges such as genome editing remain, ongoing technological progress continues to expand the industrial and environmental applications of this versatile alga (Salomé and Merchant, 2019; Scranton et al., 2015; Bellido-Pedraza et al., 2024; Bellido-Pedraza, Torres and Llamas, 2024). In addition to its utility in biofuels and bioremediation, microalgae are increasingly explored as sustainable alternatives to commercial aquafeeds.
Moreover, commercial aquafeed production relies heavily on wild fisheries, making it environmentally unsustainable. Some studies have shown that plant-based alternatives like soybean meal and corn gluten meal commonly used to replace fishmeal, contain anti-nutritional factors that limit their effectiveness as aquafeeds (Gerile and Pirhonen, 2017). Additionally, most plant-based aquafeeds contain phosphorus in phytate form, which is unavailable to plants, necessitating the supplementation of nutrients like phosphorus and zinc in aquaponic systems. Selecting appropriate aquafeed is critical, and supplements should be added carefully to avoid harming both fish and plant health in aquaponics. While plant-based aquafeeds are often promoted as eco-friendly option, they are not fully sustainable due to their negative ecological impacts such as destruction of plant communities for feed production. Animal-based aquafeeds, such as animal proteins sourced from slaughterhouses that are free from anti-nutritional factors, can serve as viable fishmeal substitutes. Additionally, insect-based feeds such as those derived from black soldier flies, have emerged as promising alternatives due to their high protein content, low land and water requirements, reduced greenhouse gas emissions, and superior feed conversion efficiency. However, further research is necessary to evaluate the quality, efficacy, and safety of using insects as aquafeed in aquaponic systems. Recently, the use of microalgae such as Arthrospira platensis, Chlorella vulgaris, Schizochytrium sp., Nannochloropsis sp., Dunaliella salina., Haematococcus pluvialis., and Isochrysis galbana as a replacement for commercial aquafeeds has gained increasing attention because microalgae can produce higher biomass than plants. As shown in Figure 1, the global average water footprint varies significantly among different aquafeed ingredients, highlighting the need for more sustainable alternatives such as microalgae (Pugazhendhi et al., 2020).
Figure 1

Global average water footprint of different aquafeed ingredients. Adapted from Nagappan et al., 2021, licensed CC-BY-4.0.
Microalgae exhibit remarkable adaptability and rapid growth rates, which make them valuable for various applications. They can thrive in extreme conditions, such as highly alkaline environments, with growth rates of 1.10–1.30/d (Praveen et al., 2023). Thermally tolerant mutant species of Nitzschia inconspicua microalgae have shown 1.4- to 6.7-fold higher growth rates than wild types at different temperatures. Adaptive laboratory evolution has been used to enhance the growth rate, stress tolerance, and product yield of microalgae (LaPanse, 2024). These fast-growing organisms have diverse applications as functional foods and in biofuel production, greenhouse gas mitigation, and wastewater treatment. Their ability to efficiently remove carbon (70–80%) and other nutrients (80–90%) from wastewater demonstrates their potential in environmental remediation (Praveen et al., 2023). The high adaptability and rapid growth rate of microalgae make them promising candidates for sustainable biotechnology and industrial innovation across various sectors. Their diverse nutritional compositions makes them valuable in aquaculture, food and other industries. The protein, carbohydrate, and lipid contents of microalgae typically range from 18 to 52%, 18 to 46%, and 12 to 48%, respectively (Zhang et al., 2023; Tibbetts et al., 2017). Most species also contain abundant essential amino acids with high digestibility (>80%) (Tibbetts et al., 2017). Their fatty acid profiles vary, with marine species being rich in monounsaturated fatty acids and freshwater species being rich in polyunsaturated fatty acids. Microalgae are also source of various vitamins, particularly B2 and B3, and pigments such as chlorophyll-a and carotenoids (Zhang et al., 2023). Cultivation conditions, including irradiance and residence time, strongly influence the nutritional composition. Species such as Isochrysis galbana, Dunaliella tertiolecta, and Tetraselmis gracilis have shown promising nutritional profiles (Zhang et al., 2023)that meet the United Nations Food and Agriculture Organization nutritional requirements for adults and children, highlighting their potential for food applications.
Impact of microalgae on fish growth
Microalgae have great potential as sustainable aquafeed ingredients, offering high nutritional value and environmental benefits. Microalgal species, such as Nannochloropsis salina and Dunaliella salina can accumulate substantial lipid and protein contents while fixing carbon dioxide (CO2) (Chen et al., 2019). While microalgae are often highlighted for their potential to lower the carbon footprint of aquafeed production, current evidence remains limited. Specifically, comprehensive life-cycle assessment (LCA) studies comparing microalgae with conventional feed ingredients such as fishmeal and fish oil are scarce. Further system-level evaluations are needed to substantiate these environmental claims. Nonetheless, the ability of microalgae to fix CO2 and reduce reliance on fish stocks presents a promising opportunity for developing more sustainable aquafeed strategies. Microalgae are emerging as a sustainable and nutritious alternative to traditional aquafeed ingredients such as fishmeal and fish oil (Nagappan et al., 2021; Ma and Hu, 2024). They offer a high protein content, essential amino acids, omega-3 fatty acids, and bioactive compounds that enhance the growth, colouration, immunity, and survival rates of aquatic species (Dineshbabu et al., 2019; Idenyi et al., 2022). Microalgae play a crucial role in aquaculture by supplying essential nutrients that support the health and growth of fish and shellfish (Kapara, 2018). These microscopic organisms are rich in amino acids, long-chain polyunsaturated fatty acids, vitamins, proteins, and minerals, which are particularly important for enhancing the larval survival, growth, and overall well-being of aquatic species (Siddik et al., 2024). Microalgae are used as live feed for various growth stages of molluscs, crustaceans, and some fish species. Additionally, certain microalgae contain bioactive compounds with antioxidant, anti-inflammatory, and immunomodulatory properties that can improve immunity and disease resistance in farmed aquatic animals (Abdel-Latif et al., 2022). Although microalgae are typically cultivated in-house in hatcheries, commercial concentrates are becoming more widely used. However, the high cost of algal biomass limits its widespread use in commercial aquafeeds (Siddik et al., 2024). Co-cultivation of microalgae with nitrogen-fixing bacteria that release ammonium can significantly reduce the costs of algal biomass. Microalgae are sustainable sources of omega-3 fatty acids, particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are essential for human and animal nutrition (Norzagaray-Valenzuela et al., 2017). These fatty acids provide significant health benefits especially for cardiovascular health and brain function in humans. Since fish oil is the traditional source of these compounds concerns over depletion of global fish stocks has led to an increased interest in alternative sources (Topuz, 2016). Microalgae as primary producers of omega-3 fatty acids can be cultivated at industrial scale and processed into various food products and animal feeds (Norzagaray-Valenzuela et al., 2017). Recent advancements in microalgal biotechnology, such as metabolic engineering and selective breeding, have further enhanced the potential of omega-3 fatty acid production in autotrophic microalgae. As research progresses, microalgal oil is expected to become a viable replacement for fish oil (Topuz, 2016).Among microalgae, Nannochloropsis spp., show strong potential as sustainable aquafeed ingredients, capable of replacing fishmeal and fish oil. These species can accumulate high lipid (20–46%) and protein (30–57%) contents while also efficiently fixing carbon dioxide CO2 (Chen et al., 2020b). They also produce valuable EPA and can be cost-effectively cultivated in solar-powered open ponds (Li et al., 2020). Although Nannochloropsis spp. have lower digestibility than Isochrysis sp. in rainbow trout, they remain a promising fishmeal substitute (Sarker et al., 2020). Additionally, Schizochytrium have shown high digestibility of macronutrients, energy, and fatty acids, particularly DHA, in rainbow trout at both 8°C and 15°C, further supporting its potential as a fish oil substitute in aquafeeds (Bélanger et al., 2021). In Nile tilapia diets, the complete replacement of fish oil with Schizochytrium resulted in improved growth, increased feed efficiency, and a higher DHA content in fillets (Sarker et al., 2016).
Schizochytrium supplemented diets have demonstrated higher phosphorus digestibility and lower solid phosphorus discharge in tilapia, indicating potential environmental benefits (Gamble et al., 2021). The optimal inclusion level of Schizochytrium in fish feed varies by species, ranging from 20 to 80% fish oil replacement, and has been associated with improved growth, survival, and feed intake across various fish species (Pratiwi and Zidni, 2023). In addition to Schizochytrium, Tetraselmis spp. have also emerged as promising microalgal ingredients in aquafeeds. For example dietary supplements with Tetraselmis suecica has been shown to improve growth performance, feed utilisation, and gene expression in Pacific white shrimp (Litopenaeus vannamei) (Sharawy et al., 2020). Furthermore, cultivation of Tetraselmis striata has been optimised at both laboratory and pilot scales, yielding biomass rich in proteins, lipids, carbohydrates, pigments and notable high EPA content (Patrinou et al., 2023). Spirulina, a cyanobacterium also referred to as blue-green algae, has gained attention as a potential aquafeed ingredient due to its high nutritional value and sustainability (El-Sheekh et al., 2023). It offers a promising alternative to fishmeal, addressing the growing demand for aquaculture feed while reducing environmental impacts (Nagappan et al., 2021). Spirulina is rich in proteins, essential amino acids, fatty acids, vitamins, and minerals, making it suitable for use by various aquatic species (Ragaza et al., 2020). In addition, it enhances the innate immunity and disease resistance of fish and shrimp (Rakocy, 2012). Different species of microalgae, such as Nannochloropsis, Schizochytrium, and Isochrysis, have been incorporated into fish diets. In some studies, microalgae-based diets (e.g. Nannochloropsis spp. and Nanochloropsis salina) resulted in comparable or slightly lower weight gain than the reference diets but showed acceptable growth rates and feed efficiency. Notable exceptions include defatted Nannochloropsis oculata and Schizochytrium in juvenile Nile tilapia, where the microalgae-based diet resulted in a higher specific growth rate (SGR) and weight gain than the reference diet. Schizochytrium spp. fed to Atlantic salmon and Pacific white shrimp resulted in increased weight gain compared with the reference diets, highlighting the high lipid content of microalgae as an effective substitute for fish oil.
Schizochytrium resulted in a higher weight gain (426 g) than fish oil (326 g) in Atlantic salmon (Salmo salar) (Wei et al., 2021). Defatted N. oculata and Schizochytrium sp. diets outperformed the reference diet in terms of weight gain and SGR in juvenile Nile tilapia (Oreochromis niloticus) (Ju et al., 2017). Diets with microalgae such as Schizochytrium (Allen et al., 2019) have demonstrated competitive growth performance in shrimp (Litopenaeus vannamei). Microalgal diets generally maintain feed conversion ratio (FCR) values that are similar to or slightly higher than those of the reference diets. In some cases, a lower FCR (e.g. Schizochytrium spp.) for Atlantic salmon indicates efficient feed utilisation. Thus, microalgae show promise as sustainable feed ingredients with growth performance comparable to that of traditional feeds, especially for specific species. Variations in performance suggest that diet formulations need to be species specific and that some microalgae might not completely replace conventional ingredients without compromising efficiency. Data from previous studies support the viability of microalgae as a sustainable alternative to traditional aquafeed, provided that they are tailored to the nutritional needs of the target fish species in aquaponic systems. Table 1 shows the list of microalgae and its effect on fish growth.
Table 1
| Microalgae | Fish species | Effect | Reference |
|---|---|---|---|
| Schizochytrium sp. | Nile tilapia (Oreochromis niloticus) | Improved gut health | Souza et al., 2020 |
| Euglena sp. | Atlantic salmon | Immunostimulant | Kiron et al., 2016a, b; Montoya et al., 2017; Yamamoto et al., 2018 |
| Schizochytrium sp. | Atlantic salmon | Enhanced fillet firmness | Kousoulaki et al., 2016 |
| Spirulina sp. | Red tilapia, Koi, Striped jack, Black tiger prawn, and yellow catfish | Enhanced coloration | Ansarifard et al., 2018; Dineshbabu et al., 2019; Liu et al., 2021 |
| 2.5% Phaeodactylum tricornutum | Gilthead seabream | High fucoxanthin content | Ribeiro et al., 2017 |
| Arthrospira platensis | Freshwater prawns (Macrobrachium rosenbergii) | Enhanced growth performance | Radhakrishnan et al., 2016 |
| 5% Schizochytrium sp. oil | Atlantic salmon (Salmo salar L.) | Weight gain | Wei et al., 2021 |
| 0.75% Tetraselmis suecica | Post larvae pacific white shrimp (Litopenaeus vannamei) | 30% weight gain | Sharawy et al., 2020 |
| 15% Chlorella sp. | Nile tilapia (Oreochromis niloticus) | 30% reduction in FCR (feed conversion ratio) | Fadl et al., 2020 |
| Nannochloropsis gaditana | African catfish and Nile tilapia | Improved weight gain and FCR | Agboola et al., 2019 |
| Spirulina-based fish feed | Mozambique tilapia fingerlings (Oreochromis mossambicus) | Improved digestibility | Sharma et al., 2021 |
| Nannochloropsis sp.extruded feed | Gibel carp | Improved digestibility | Shi et al., 2016 |
| Pavlova sp., Chaetoceros sp., Nannochloropsis ocu lata, and Isochrysis sp., in feed, | Seahorses (Hippocampus reidi) and Oysters (Pinctada margaritifera) | Increased survivability | Martínez-Fernández and Southgate, 2007; Mélo et al., 2016 |
| 1-2% Dunaliella salina supplemented feed | Litopenaeus vannamei | Increased survival rate | Medina-Félix et al., 2014 |
| Tetraselmis suecica live cells | White shrimp (Fenneropenaeus indicus | Reduced gut pathogenic bacterial load | Regunathan and Wesley, 2004 |
| Microencapsulated Chaetoceros sp. | Pacific white shrimp (Lito penaeus vannamei) | Survivability at larval stage increased | Nimrat et al., 2011 |
| Paramylon in Euglena sp. cell wall | Atlantic salmon, mussels, red drum, and matrinxa | Immunostimulant | Bianchi et al., 2015; Kiron et al., 2016a, b; Montoya et al., 2017; Yamamoto et al., 2018 |
| 6-8% of Chlorella vulgaris | Post larvae of Macrobrachium rosenbergii | Improved immune response and survivability agianst Aeromonas hydrophila infection | Maliwat et al., 2017 |
| Tetraselmis chuii, Nanno chloropsis gaditana, and P. tricornutum | Gilthead seabream (Sparus. aurata) | Enhanced defence activity | Cerezuela et al., 2012 |
| Euglena viridis | Rohu fish (Labeo rohita) | Increased immunostimulatory effects | Das et al., 2009 |
| Dunaliella salina | Penaeus monodon | Increased antioxidant factors and survival rate | Madhumathi and Rengasamy, 2011 |
| Fish diet with Lactobacillus sakei and Navicula sp. | Pacific red snapper (Lutjanus peru) | Improved humoral response | Reyes-Becerril et al., 2013 |
| Feed with Prunus incisa | Guppy fish (Poecilia reticulata) | Increased survival rate | Nath et al., 2012 |
| 10% A. Platensis diet | Rainbow trout (Oncorhynchus mykiss) | Increased in total proteins level | Yeganeh et al., 2015 |
| 5% Schizochytrium sp. | Atlantic salmon | Improved fillets quality | Kousoulaki et al., 2016 |
| Schizochytrium limacinum | Atlantic salmon | Improved fillets taste and odour | Katerina et al., 2020 |
| Schizo chytrium sp. | Atlantic salmon | Rich in PUFA (polyunsaturated fatty acids) | Ren et al., 2010 |
| 4% defatted-Spirulina and 0.4% Spirulina-lipid-ex tract | Yellow catfish (Pelteobagrus fulvidraco) | Improved skin colour | Liu et al., 2021 |
| 7.5% Spirulina platensis | Showa koi | Improved pigmentation | Sun et al., 2012 |
Effect of microalgae on fish growth.
Impact of microalgae on plant growth
Studies have shown that the integration of microalgae into aquaponic systems can improve the physicochemical properties of aquaculture water (Addy et al., 2017; Tejido-Nuñez, 2020). Residual fish feed and fish excreta that accumulate in aquaculture water can be used by microalgae to support its growth and biomass production (Delrue et al., 2016). Microalgae cultivation in aquaponics helps to improve water quality by decreasing the pH. Microalgae interactions with bacteria could be the reason for the conversion of fish waste into nutrients, thereby increasing water quality. However, studies on the mechanism of fish waste-to-nutrient conversion through interactions between algae and bacteria are scarce. It has been hypothesised that microalgae facilitate the proliferation of beneficial bacteria and reduce the risk of pathogenic bacteria that could otherwise cause diseases in fish and plants in aquaponic systems. A study evaluating the effects of three microalgal species (Chlorella vulgaris, Scenedesmus spp., and Spirulina platensis), cultivated in an aquaponic system along with Nile tilapia and garlic plants, showed growth similar to that of the control in terms of plant biomass, leaf number, and shoot length. Water quality parameters such as dissolved oxygen, pH, temperature, ammonia, nitrate, and nitrite were maintained at ideal levels for aquaponic systems (Addy et al., 2017; Tejido-Nuñez et al., 2020; Chen et al., 2020a).
A previous study revealed that diverse populations of beneficial microorganisms were significantly higher in fish tanks and biofilters when microalgae were co-cultivated in an aquaponic system. This was a positive outcome owing to the mutual interaction between microalgae and bacteria, which could play an important role in nutrient cycling. Studies have shown that microalgae play a crucial role in atmospheric nitrogen recycling and soil fertility. They can fix atmospheric nitrogen into bioavailable forms like ammonia, particularly through specialized cells called heterocysts in cyanobacteria (Singh, 2021). Many studies have highlighted that bacterial richness is higher in the presence of Chlorella vulgaris, which helps in the removal of nitrogen and phosphorus. A stable association between C. vulgaris and specific bacterial species such as Flavobacterium sp., Terrimonas sp., Sphingobacterium sp., Rhizobium sp., and Hyphomonas sp. has been observed (Ramanan et al., 2016; Han et al., 2019). In aquaponic systems, compared with fish tanks, biofilters had a higher bacterial population because they act as a growth substrate for microalgae and bacteria that form biofilm known as a ‘phycosphere’. Diverse beneficial bacterial species are attracted to this phycosphere, and this algae–bacteria interaction plays a major role in regulating water quality in aquatic environments. Nitrogen cycling in aquaponic systems occurs because of the presence of the phyla Proteobacteria and Bacteroidetes, which are indicators of the good health status of the system (Schmautz et al., 2017; Wongkiew et al., 2018). Bacteroidetes convert nitrates into various nitrogen compounds that are essential for degrading complex organic matter (Wongkiew et al., 2018). In one study, it was found that Bacteroidetes were more abundant when microalgae were present. Bacteroidetes play a vital role in nutrient cycling and support optimal plant growth in aquaponics (Kasozi et al., 2021). In addition, fish fed microalgae showed resistance to bacterial infection. In a previous study, Nile tilapia fish fed Spirulina platensis in an aquaponic system showed lower mortality than the control group. Some studies have shown that microalgae such as Chlorella vulgaris can produce the antibacterial compound “chlorellin”. Another study showed that when Spirulina platensis was given as a feed supplement, the antibacterial compound “phycocyanin” it produces decreased the mortality of Nile tilapia. Nannochloropsis oculata, Schizochytrium sp., and Spirulina sp., the microalgal mix used in Nile tilapia feed, increased immunity against Vibrio and Staphylococcus bacterial species and enhanced its antioxidant enzyme activity (Falaise et al., 2016). Studies have indicated that purple sulphur bacteria such as those from the genus Thiobaca play a key role in the sulphur cycle and were observed to be more abundant in aquaponics water treated with Chlorella vulgaris. Studies on iron-reducing bacterial species belonging to the genus Geothrix have shown that they oxidise organic compounds by reducing iron (III) to iron (III) oxide, manganese (IV) oxide, and nitrate, thereby preventing the production of environmentally harmful compounds in aquaponic systems. Another study revealed that the bacterial genus Fusibacter contributes to the reduction of elemental sulphur, or thiosulfate, to sulphides during the sulphur cycle. Previous studies on the bacterial genus Treponema showed that it plays a crucial role in scavenging nutrients through fermentation processes (Buyuktimkin et al., 2019). These biological processes are important for converting fish waste into nutrient solutions in aquaponic systems. In aquaponics, the co-cultivation of microalgae (e.g. C. vulgaris, Scenedesmus sp., and Spirulina platensis) showed better performance of the bacterial genera Thiobaca, Geothrix, Fusibacter, and Treponema in terms of nutrient cycling. Further studies on microalgae–bacteria interactions will provide insights into the effects on fish growth and plant development in aquaponics (Schmautz et al., 2021; Kasozi et al., 2021; Bartelme et al., 2019). Microalgae enhance plant growth by acting as biostimulant, biofertilizers, and biopesticides. These properties are due to the presence of bioactive compounds such as phenols, phytohormones, amino acids, polysaccharides, and terpenoids (Lee and Ryu, 2021). Although microalgae play an important role in nutrient uptake and cycling, it’s the cyanobacteria often referred as blue green algae that are responsible for nitrogen fixation. Microalgae can mobilize nutrients like phosphate, potassium, and copper Win et al., 2018; Gonçalves, 2021). Another essential micronutrient molybdenum (MO) is a key cofactor for two enzymes: nitrogenase (in nitrogen-fixing microbes like diazotrophs or cyanobacteria) and nitrate reductase (in microalgae and plants). These enzymes are essential for converting atmospheric nitrogen (N2) into ammonium and nitrate (NO3⁻) into usable nitrogen forms, respectively. In integrated aquaponics systems where microalgae or nitrogen-fixing bacteria are involved, the presence of trace levels of Mo ensures these microbes can effectively perform biological nitrogen fixation and nitrate assimilation (Glass et al., 2012).
Microalgae, particularly cyanobacteria, have a specific mechanism for fixing nitrogen. Cyanobacteria, such as Cyanothece spp., Lyngbya spp., and Trichodesmium spp., colonise the leaves and roots of plants, penetrate cell tissues, and colonise internally with plant host specificity. Microalgae produce enzymes such as alkaline phosphatases, 5’nucleotidases, phytases, and phosphodiesterases that help to release bound phosphorus from organic sources such as phytate. Some species such as Tetraselmis suecia, Nannochloropsis gaditana, and Nanochloropsis oceania adopt a luxury uptake mechanism to store excess or relocate phosphorus by remodelling polar lipids (Cañavate et al., 2017). Microalgal species, such as Spirulina platensis, Chlorella spp., Scenedesmus spp., Acutodesmus spp., Calothrix elenkini, and Dunaliella spp (Ronga et al., 2019; Colla and Rouphael, 2020), enhance crop production by improving nutrient uptake, enhancing resistance to both abiotic and biotic stress, and maintaining essential functions such as respiration, photosynthesis, nucleic acid synthesis, and iron uptake (Lee and Ryu, 2021; Kumar et al., 2022).
Microalgae synthesise phytohormones that play important roles in shoot and root development, plant tissue differentiation, aging, and defence against biotic and abiotic stressors. Studies on microalgal species, such as Coenochloris spp., Chlorella spp., Scenedesmus spp., Chlorococcum spp., and Acutodesmus spp., have shown that they can synthesise auxin hormones, such as indol-3-acetamide and indole 3-acetic acid, which play roles in the formation and elongation of plant roots (Kapoore et al., 2021). Microalgae that synthesise auxins form colonies with cyanobacteria, which has been observed in wheat and rice plants (Hussain et al., 2017). Recent studies have revealed that the green alga Chlamydomonas reinhardtii can synthesize auxin (indole-3-acetic acid, IAA) through an extracellular L-amino acid oxidase (LAO1) under nitrogen-limited conditions (Calatrava et al., 2022). This auxin production plays a role in algal-bacterial mutualism, particularly with Methylobacterium species. Nannochloropsis spp. synthesise cytokinin phytohormones that enhance resistance to nitrogen and water stress in tomato plants. Studies on Chlorella vulgaris extracts containing gibberellic acid phytohormones suggest that the extract could mitigate the harmful effects of heavy metals, such as lead and cadmium, on plant growth. Microalgae belonging to the genera Chlorella, Chlamydomonas, and Scenedesmus, and cyanobacteria including Anabaena spp., Synechococcus spp., Calothrix spp., Nostoc spp., Cylindrospermum spp., and Scytonema spp., have been reported to synthesise ethylene phytohormones that regulate cell division, fruit ripening, aging, and biotic and abiotic stress tolerance (Han et al., 2018). Microalgae can synthesise signalling molecules, such as jasmonic acid, polyamines, brassinosteroids, and salicylic acid, which are associated with stress-tolerance mechanisms that enhance enzymatic and non-enzymatic defence responses in plants (Kapoore et al., 2021; Lee and Ryu, 2021). Some studies have reported that Spirulina can produce polyamines which promote the growth of lettuce seedlings (Mógor et al., 2018). Studies on microalgae, such as Chlorella stigmatophora, Chlorella vulgaris, Tetraselmis spp., Dunaliella salina, and Porphyridium cruentum, have shown that microalgae can synthesise exopolysaccharides (EPS) which stimulate plant growth and metabolism (Chanda et al., 2019). El Arroussi et al. (2018) reported that EPS from Dunaliella saline microalgal species enhanced salinity stress tolerance in tomato plants. Studies on protein-rich extracts of Spirulina platensis have revealed increased flower number, freshness, and dry weight in Petunia x hybrida plants (Plaza et al., 2018). Green algal extracts rich in amino acids enhanced the total solid and organic contents of three hot pepper varieties (Zamljen et al., 2021). Some microalgal species also synthesise phenolic compounds and carotenoids, which support photoprotection and defence responses in plants (Vidyashankar et al., 2017; Cezare-Gomes et al., 2019; Del Mondo et al., 2021). Some microalgae species are a good source of micronutrients, such as calcium,, iron, zinc, a and magnesium (Sandgruber et al., 2021). Tetraselmis chuii is rich in total calcium and phosphorus, Chlorella has high phosphorus and iron contents, and Spirulina is rich in potassium. Microalgae synthesize vitamins that act as plant growth promoting factors. Vitamin C and nicotinic acid are abundant in Tetraselmis suecia. Freshwater microalgae, such as Spirulina platensis and Chlorella spp., are rich in vitamins such as niacin, riboflavin, cyanocobalamin, and folic acid (Edelmann et al., 2019). Studies have shown that plants like soybean, barley, and spinach absorb vitamin B complex when microalgal biomass is applied as biofertilizer. In addition to vitamins and minerals, microalgae produce terpenoids, betaines, humic substances, and peptides that function as biopesticides (Kapoore et al., 2021). Table 2 shows the list of microalgae and its effect on plant growth.
Table 2
| Microalgae | Plant species | Effect | Reference |
|---|---|---|---|
| Spirulina platensis | Raphanus sativus | Enhanced germination rate and seedling vigour | Godlewska et al., 2019 |
| Chlorella vulgaris | Solanum lycopersicum L., Cucumus sativus | Improved root parameters, increased biomass yield | Bumandalai and Tserennadmid, 2019 |
| Scenedesmus quadricauda, Chlorella vulgaris, Arthrospira spp. | Beta vulgaris L. | Improved root parameters, enhanced biomass and nutritional quality | Barone et al., 2018; |
| Navicula spp. | Solanum lycopersicum L., Capsicum annuum L., Solanum melongena | Enhanced biomass | Alshehrei et al., 2021 |
| Oscillatoria agardhii | Triticum spp. | Drought tolerance | Haggag et al., 2018 |
| Chlorella vulgaris, Nannochloropsis salina | Moringa oleifera | Salinity tolerance | Al Dayel and El Sherif, 2021 |
| Chlorella vulgaris | Vigna mungo L. | Enhanced growth (acts as a biostimulant) | Dineshkumar et al., 2019 |
| Spirulina extract | Triticum aestivum, Hordeum vulgare | Enhanced germination and biomass yield | Akgül, 2019 |
| Spirulina platensis extract | Calotropis procera Ait | Improved root growth and germination rate | Bahmani Jafarlou et al., 2021 |
| Spirulina platensis extract | Vigna mungo L. | Enhanced germination, nutritional content, root growth, biomass and stress tolerance | Thinh, 2021 |
| Spirulina platensis Phycocyanin extract | Solanum lycopersicum L. | Increased biomass, nutritional content, and germination | Metwally et al., 2022 |
| Chlorella spp. Cell suspension | Triticum aestivum, Hordeum vulgare | Enhanced root development, biomass and germination rate | Odgerel and Tserendulam, 2016 |
| Nostoc commune aqueous extracts | Oryza sativa L. | Enhanced root development, biomass and germination rate | Abedi Firoozjaei et al., 2021 |
| Scenedesmus quadricauda and Chlorella vulagaris extract | Beta vulgaris | Improved seed vigour and root growth | Puglisi et al., 2020 |
| Consortia of Chlorococcum spp. Micractinium spp. Scenedesmus spp. Chlorella spp. |
Spinacia oleraceae | Enhanced biomass, nutritional content and germination rate | Rupawalla et al., 2022 |
| Scenedesmus subspicatus | Allium cepa L | Improved root development | Gemin et al., 2022 |
| Chlorella vulgaris biomass with cow dung | Solanum lycopersicum L. | Enhanced root growth, leaf phytochemical content, soil, enzyme activity and stress tolerance | Suchithra et al., 2022 |
| Chlorella vulgaris extract | Lactuca sativa | Increased crop yield, leaf pigment content, fruits, flowers numbers and nutritional quality | La Bella et al., 2021 |
| Chlorella vulgaris | Brassica oleracea var. italica | Enhanced leaf pigments, stress tolerance, enzymatic activity, early flowering | Kusvuran, 2021 |
| Chlorella vulgaris extract | Latuca sativa L. | Increased enzymatic activity, early flowering, nutritional quality | Puglisi et al., 2022 |
| Cell lysates of Chlamydomonas reinhardtii CC124 Chlorella sp. MACC360 |
Solanum lycopersicum L. | Improved crop yield, enzymatic activity, number of fruits, early flowering | Gitau et al., 2022 |
| Polysaccharides extract of Dunaliella salina MS002 and MS067 Phaeodactylum tricornotum MS023 Porphyridium spp.MS081, Desmodesmus spp. Spirulina platensis MS001 |
Solanum lycopersicum L. | Enhanced nutritional quality and stress tolerance | Rachidi et al., 2021 |
| Extracts of microalgae consortium Chlorella spp., Scenedesmus spp., Spirulina spp., Synechocystis spp |
Solanum lycopersicum L. | Increased biomass, leaf pigment content, nutritional quality | Hans et al., 2020 |
| Chlorella vulgaris | Cyamopsis tetragonoloba (L.) Taub. | Early flowering, improved nutritional quality and stress tolerance | Kusvuran and Can, 2020 |
| Polysaccharide extracts of Chlorella vulgaris, Chlorella Sorokiniana | Solanum lycopersicum L. | Early flowering, increased enzymatic activity and stress tolerance | Farid et al., 2019 |
| Scenedesmus spp.extract, Arthrospira platensis cell hydrolysate | Petunia x hybrida | Enhanced crop yield, nutritional quality | Plaza et al., 2018 |
| Scenedesmus obliquus Chlorella vulgaris and Anabaena oryzae biomass | Musa spp. | Improved root growth, leaf phytochemical content, soil quality, stress tolerance | Hamouda and El-Ansary, 2017 |
| Chlorella fusca | Cucumis sativus Arabidopsis thaliana | Stress tolerance | Kim et al., 2018; Lee et al., 2020 |
Effect of microalgae on plant growth.
Integration of microalgae in aquaponics
Microalgae serve as excellent nutrient sources for aquatic organisms, providing proteins, omega-3 fatty acids, vitamins, and minerals. They also play crucial roles in water quality management, larviculture, and Integrated Multi-Trophic Aquaculture (IMTA) systems (Hashmi et al., 2023). Algaeponics is a recent innovation in the field of aquaponics (Nair et al., 2025) and it is a novel extension of conventional aquaponics that incorporates microalgae as an integral biological component within the system. Unlike standard aquaponics—where fish waste provides nutrients for higher plants—algaeponics uses microalgae to recycle nutrients, improve water quality, and serve as a supplementary or primary feed source for fish (Zhang et al., 2022). Microalgae enhance aquaponic systems by supporting nutrient removal, improving water quality, and serving as feed for fish like tilapia (Edwards et al., 1981; Kinh et al., 2024). Factors such as fish density, food-to-microorganism ratio (F/M), and hydraulic retention time (HRT) influence algal integration and system stability (Medina and Neis, 2007). While species like Chlorella sp. aid in ammonia control and pH balance, their growth may be limited in systems optimized for fish and plant productivity (Addy et al., 2017). Microalgae can interact with nitrogen fixing bacteria called diazotrophs that could possess combined biotechnological applications in a sustainable production system. In aquaponics systems, integrating microalgae with nitrogen-fixing bacteria (diazotrophs) offers a promising, sustainable way to enhance nutrient cycling, water quality, and productivity. While microalgae contribute to carbon fixation, oxygenation, and biomass production, diazotrophs help convert atmospheric nitrogen into plant-available forms like ammonium. Together, they can naturally supplement nitrogen when fish waste is insufficient, reduce the need for synthetic inputs, and support plant growth through biofertilization. Additionally, the protein-rich algal biomass can be harvested and reused as fish feed, creating a closed-loop system that improves efficiency, reduces operational costs, and boosts environmental resilience (Llamas et al., 2023).
Microalgae added to aquaponic systems in the form of aquafeeds is shown in Figure 2, as they can act as an essential food source for fish, but in-depth research is necessary to determine their potential benefits for plant growth in aquaponic systems. Microalgae can effectively remediate aquaculture water acting as nutrient recyclers while producing valuable biomass (Dourou et al., 2020; Han et al., 2019). This integration reduces environmental impacts, improves water quality, and provides a sustainable source of aquafeed (Han et al., 2019). Upscaling the production of microalgae can lead to improved resource efficiency and a reduced carbon footprint. Recent advances in recirculating aquaculture systems (RAS) have focused on incorporating microalgae to close the system loop, thereby enhancing performance and deriving value from waste streams. Microalgae in RAS facilitate oxygenation, carbon dioxide sequestration, and nutrient recovery (Ende et al., 2024). Various cultivation systems, harvesting technologies, and species selection strategies have been explored to optimise microalgae-assisted aquaculture (Han et al., 2019). Microalgal biomass production has a water footprint of 2857 L/kg when using freshwater. This footprint can be reduced considerably by employing wastewater or seawater or recycling growth media, with recycling potentially lowering the footprint by 90% (Pugazhendhi et al., 2020). Compared with plant and insect production, microalgae production has a lower water footprint. In open cultivation systems, evaporation is a major contributor to water loss, with evaporation rates reaching up to 2 cm/d in 20 cm deep raceway ponds (Das et al., 2016). Cultivating microalgae using wastewater for human consumption raises legitimate food safety concerns. These concerns stem from the potential accumulation of harmful substances, including heavy metals, pathogens, and emerging contaminants (Markou et al., 2018; Álvarez-González et al., 2023). While treatment processes such as anaerobic digestion can significantly reduce biological and chemical risks, the persistence of certain xenobiotics remains a challenge. Currently, the legal frameworks in most regions—including the European Union—do not support the use of such biomass in food products. However, some studies indicate that treated microalgal biomass may be suitable for non-food applications, such as fertilizers and aquafeed, although elements like cadmium can still exceed allowable limits (Álvarez-González et al., 2023). Continued research and clearer regulatory guidance are essential as the industry evolves (de Oliveira and Bragotto, 2022; Salehipour-Bavarsad et al., 2024).
Figure 2
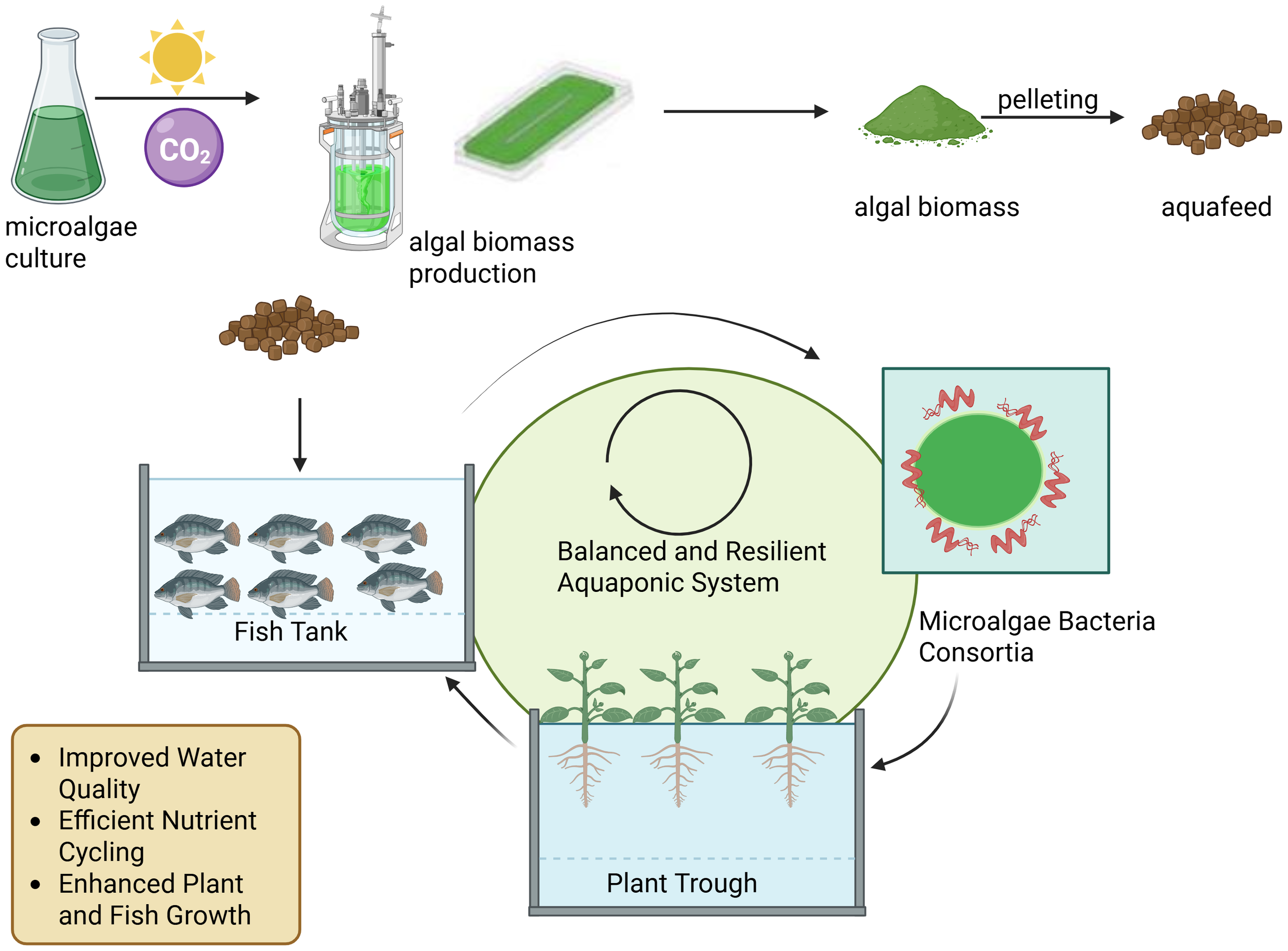
Schematic representation of microalgae contributions to Sustainable Aquaponics: From Biomass Cultivation to Aquafeed.
Major challenges in microalgae cultivation is its biomass productivity which is highly variable due to numerous cultivation factors such as light intensity and spectrum, nutrient availability, temperature, and strain-specific physiological differences. Light is a key determinant, with suboptimal intensity, poor spectral quality, and inefficient distribution significantly reducing photosynthetic efficiency, especially in dense cultures and closed photobioreactors (Ooms et al., 2016; Nwoba et al., 2019). Nutrient limitations, particularly of nitrogen and phosphorus, can both constrain growth and stimulate desired metabolite accumulation, but must be precisely managed to balance productivity and product quality (Chu, 2017). Additionally, different microalgal strains respond uniquely to environmental conditions, making strain selection critical for consistent biomass yield and target compound production (Štěrbová et al., 2023). Innovations such as spectral conversion, temperature control strategies, genetically modified strains, and advanced photobioreactor designs aim to mitigate these inconsistencies and improve biomass uniformity and scalability (Nwoba et al., 2019; Zhang et al., 2024).Freshwater is often required to counteract evaporation and maintain salinity for marine microalgae. However, certain halotolerant microalgal strains (e.g. Dunaliella sp., Tetraselmis sp., and Picochlorum sp.) can adapt to salinity changes, thereby reducing freshwater use and lowering the overall water footprint (Das et al., 2019). Microalgae show potential as sustainable alternatives to fish-based aquafeed in addressing the growing demand for high-quality proteins (Tham et al., 2023; Yarnold et al., 2019). Integrating microalgae cultivation with aquaculture, agriculture, aquaponics, and livestock farming could create a circular bioeconomy based on recycling nutrients and wastewater. This approach offers environmental benefits, resource recovery, and potential socioeconomic improvements in rural areas. However, challenges remain, including developing large-scale production methods and addressing energy-intensive harvesting and processing methods. Some studies have shown that freshwater microalgae like spirulina might contain contaminants like microcystins (MCs) which have raised increasing concern due to their potential health risks. Spirulina, a cyanobacterial supplement—has been examined for safety, especially in France where over 180 small-scale farms contribute to local production. A review of data from 95 producers between 2013 and 2021, showed that MCs levels generally remained within safe limits. These findings support the relative safety of French spirulina and other microalgae while emphasizing the importance of refining cultivation practices to prevent contamination (Scoglio, 2018; Pinchart et al., 2023). Further research on life cycle assessment and pilot-scale demonstrations is needed to establish the feasibility and sustainability (Figure 3) of integrating algae-based systems into aquaculture, aquaponics, and related sectors (Vishwakarma et al., 2022).
Figure 3
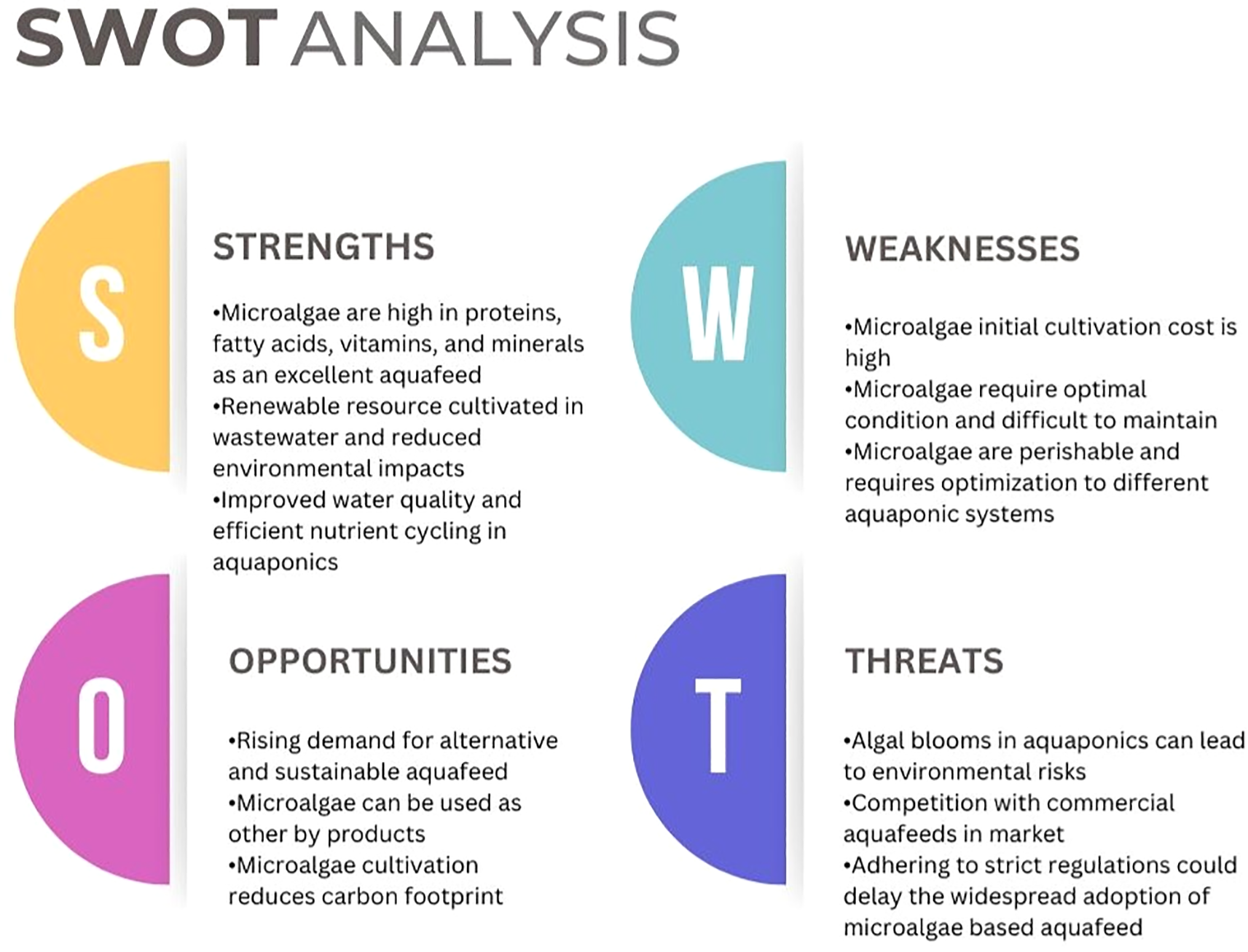
SWOT analysis of microalgae-based aquafeed in aquaponic systems.
Conclusion
Aquaponics holds immense potential to address global food and nutrition security challenges by integrating fish and plant production in a sustainable manner. However, one of the key limitations of current systems lies in the inefficient conversion of fish effluent into complete nutrient solutions for plant growth, often necessitating external fertiliser inputs. Recent studies suggest that microalgae could offer a promising solution to this bottleneck by serving dual roles as functional aquafeed for fish and as biostimulants or biofertilizers for plants. Certain species, such as Spirulina and Chlorella, have demonstrated benefits in nutrient recycling, water purification, and enhancement of fish health and plant biomass. While some microalgae species needs to be optimised for its application as aquafeed. Despite these promising insights, the research on microalgae as sustainable aquafeed in aquaponics system remains fragmented and limited in scope. Most existing studies are either species-specific or focused on isolated benefits rather than on integrated system-wide performance. Additionally, the long-term stability, scalability, and economic viability of incorporating microalgae in aquaponics remain underexplored. Future research should aim to systematically evaluate a broader range of microalgal species in aquaponic settings, including their interactions with microbial communities, effects on nutrient dynamics, and their contribution to overall system productivity and resilience. Moreover, multidisciplinary approaches combining aquaculture, plant science, and microbial ecology are needed to optimise microalgae integration. By addressing these knowledge gaps, aquaponics can evolve into a more self-sustaining, circular food production system capable of meeting future global demands.
Statements
Author contributions
RM: Conceptualization, Writing – original draft, Visualization. CS: Data curation, Writing – review & editing. DN: Data curation, Writing – review & editing. RS: Writing – review & editing. ZA:Writing – review & editing. LR: Writing – review & editing. X-LX: Writing – review & editing. M-ZR: Writing – review & editing. AJ: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research received funding from UAEU in the form of Strategic Research Fund (Grant Code: 12R292) addressing the SDG 2: Zero Hunger, SDG 13: Climate Action and SDG 14: Life below Water.
Acknowledgments
The authors would like to thank all the co-authors and corresponding authors for their valuable support and contributions during the preparation of this review. We are grateful to United Arab Emirates University for providing the financial assistance necessary for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdel-Latif H. M. El-Ashram S. Yilmaz S. Naiel M. A. Kari Z. A. Hamid N. K. A. et al . (2022). The effectiveness of Arthrospira platensis and microalgae in relieving stressful conditions affecting finfish and shellfish species: An overview. Aquaculture Rep.24, 101135. doi: 10.1016/j.aqrep.2022.101135
2
Abedi Firoozjaei M. H. Hassani S. B. Nazifi E. Keypour S. (2021). Study the effect of the terrestrial cyanobacterium nostoc commune aqueous extract on seed germination and seedling growth of rice. Plant Algae Environ.5, 642–653. doi: 10.48308/jpr.2021.223334.1008
3
Addy M. M. Kabir F. Zhang R. Lu Q. Deng X. Current D. et al . (2017). Co-cultivation of microalgae in aquaponic systems. Bioresource Technol.245, 27–34. doi: 10.1016/j.biortech.2017.02.020
4
Agboola J. O. Teuling E. Wierenga P. A. Gruppen H. Schrama J. W. (2019). Cell wall disruption: an effective strategy to improve the nutritive quality of microalgae in African catfish (Clarias gariepinus). Aquaculture Nutr.25, 783–797. doi: 10.1111/anu.12896
5
Ahmad A. Ashraf S. S. (2024). Harnessing microalgae: Innovations for achieving UN Sustainable Development Goals and climate resilience. J. Water Process Eng.68, 106506. doi: 10.1016/j.jwpe.2024.106506
6
Akgül F. (2019). Effect of Spirulina platensis (Gomont) geitler extract on seed germination of wheat and barley. Alinteri J. Agric. Sci.34, 148–153. doi: 10.28955/alinterizbd.639000
7
Akhtar N. Wani A. K. Singh R. Chopra C. Mulla S. I. Sher F. et al . (2023). “Bioprospecting microalgae for biofuel synthesis: a gateway to sustainable energy,” in Green approach to alternative fuel for a sustainable future (Amsterdam, Netherlands: Elsevier), 453–462. doi: 10.1016/B978-0-12-824318-3.00008-4
8
Al Dayel M. F. El Sherif F. (2021). Evaluation of the effects of Chlorella vulgaris, Nannochloropsis salina, and Enterobacter cloacae on growth, yield, and active compound compositions of Moringa oleifera under salinity stress. Saudi J. Biol. Sci.28, 1687–1696. doi: 10.1016/j.sjbs.2020.10.020
9
Allen K. M. Habte-Tsion H. M. Thompson K. R. Filer K. Tidwell J. H. Kumar V. (2019). Freshwater microalgae (Schizochytrium sp.) as a substitute to fish oil for shrimp feed. Sci. Rep.9, 6178. doi: 10.1038/s41598-019-42529-2
10
Alshehrei F. Al-Enazi N. M. Ameen F. (2021). Vermicomposting amended with microalgal biomass and biochar produce phytopathogen-resistant seedbeds for vegetables. Biomass Conversion Biorefinery15, 1–8. doi: 10.1007/s13399-021-01639-2
11
Álvarez-González A. Uggetti E. Serrano L. Gorchs G. Casas M. E. Matamoros V. et al . (2023). The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. pollut.324, 121399. doi: 10.1016/j.envpol.2023.121399
12
Andrade-Bustamante G. Martínez-Ruiz F. E. Ortega-García J. Renganathan P. Gaysina L. A. Mahendhiran M. et al . (2025). Microalgae-based functional foods: A blue-green revolution in sustainable nutrition and health. Appl. Microbiol.5, 39. doi: 10.3390/applmicrobiol5020039
13
Ansarifard F. Rajabi Islami H. Shamsaie Mehrjan M. Soltani M. (2018). Effects of Arthrospira platensis on growth, skin color, and digestive enzymes of Koi, Cyprinus carpio. Iranian J. Fisheries Sci.17, 381–393. doi: 10.22092/ijfs.2018.115878
14
Ayub A. Rahayu F. Khamidah A. Antarlina S. S. Iswari K. Supriyadi K. et al . (2025). Harnessing microalgae as a bioresource for nutraceuticals: advancing bioactive compound exploration and shaping the future of health and functional food innovation. Discover Appl. Sci.7, 389. doi: 10.1007/s42452-025-06916-3
15
Bahmani Jafarlou M. Pilehvar B. Modarresi M. Mohammadi M. (2021). Performance of algae extracts priming for enhancing seed germination indices and salt tolerance in Calotropis procera (Aiton) WT. Iranian J. Sci. Technology Trans. A: Sci.45, 493–502. doi: 10.1007/s40995-021-01071-x
16
Barone V. Baglieri A. Stevanato P. Broccanello C. Bertoldo G. Bertaggia M. et al . (2018). Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycology30, 1061–1071. doi: 10.1007/s10811-018-1411-4
17
Bartelme R. P. Smith M. C. Sepulveda-Villet O. J. Newton R. J. (2019). Component microenvironments and system biogeography structure microorganism distributions in recirculating aquaculture and aquaponic systems. mSphere4, e00112–e00119. doi: 10.1128/mSphere.00112-19
18
Bélanger A. Sarker P. K. Bureau D. P. Chouinard Y. Vandenberg G. W. (2021). Apparent digestibility of macronutrients and fatty acids from microalgae (Schizochytrium sp.) fed to rainbow trout (Oncorhynchus mykiss): A potential candidate for fish oil substitution. Animals11, 456. doi: 10.3390/ani11020456
19
Bellido-Pedraza C. M. Torres M. J. Llamas A. (2024). The microalgae Chlamydomonas for bioremediation and bioproduct production. Cells13, 1137. doi: 10.3390/cells13131137
20
Bianchi V. A. Castro J. M. Rocchetta I. Nahabedian D. E. Conforti V. Luquet C. M. (2015). Long-term feeding with Euglena gracilis cells modulates immune responses, oxidative balance and metabolic condition in Diplodon Chilensis (Mollusca, Bivalvia, Hyriidae) exposed to living Escherichia coli. Fish shellfish Immunol.42, 367–378. doi: 10.1016/j.fsi.2014.11.022
21
Bumandalai O. Tserennadmid R. (2019). Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int. J. Aquat. Biol.7, 95–99. doi: 10.22034/ijab.2019.676455
22
Buyuktimkin B. Zafar H. Saier M. H. Jr. (2019). Comparative genomics of the transportome of ten Treponema species. Microbial Pathogenesis132, 87–99. doi: 10.1016/j.micpath.2019.04.015
23
Calatrava V. Hom E. F. Llamas A. Fernández E. Galván A. (2022). Auxin production in the green alga Chlamydomonas involves an extracellular L-amino acid oxidase and supports algal-bacterial mutualism with methylobacteria. bioRxiv, 2022–2010. doi: 10.1101/2022.10.02.510520
24
Cañavate J. P. Armada I. Hachero-Cruzado I. (2017). Interspecific variability in phosphorus-induced lipid remodelling among marine eukaryotic phytoplankton. New Phytol.213, 700–713. doi: 10.1111/nph.14256
25
Cashion T. Tyedmers P. Parker R. W. (2017). Global reduction fisheries and their products in the context of sustainable limits. Fish Fisheries18, 1026–1037. doi: 10.1111/faf.12200
26
Cerezuela R. Guardiola F. A. Meseguer J. Esteban M. A. (2012). Enrichment of gilthead seabream (Sparus aurata L.) diet with microalgae: effects on the immune system. Fish Physiol. Biochem.38, 1729–1739. doi: 10.1007/s10695-012-9670-9
27
Cezare-Gomes E. A. Mejia-da-Silva L. D. C. Pérez-Mora L. S. Matsudo M. C. Ferreira-Camargo L. S. Singh A. K. et al . (2019). Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol.188, 602–634. doi: 10.1007/s12010-019-03053-9
28
Chanda M. J. Merghoub N. El Arroussi H. (2019). Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol.35, 177. doi: 10.1007/s11274-019-2652-0
29
Chen W. Wang Y. Han D. Zhu X. Xie S. Han D. et al . (2019). Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio). Aquaculture Nutr.25, 1145–1155. doi: 10.1111/anu.12993
30
Chen Y. Wang C. Xu C. (2020a). Nutritional evaluation of two marine microalgae as feedstock for aquafeed. Aquaculture Res.51, 946–956. doi: 10.1111/are.14585
31
Chen F. Xiao Y. Wu X. Zhong Y. Lu Q. Zhou W. (2020b). Replacement of feed by fresh microalgae as a novel technology to alleviate water deterioration in aquaculture. RSC Adv.10, 20794–20800. doi: 10.1039/D0RA03416D
32
Chu W. L. (2017). Strategies to enhance production of microalgal biomass and lipids for biofuel feedstock. Eur. J. Phycology52, 419–437. doi: 10.1080/09670262.2017.1379100
33
Colla G. Rouphael Y. (2020). Microalgae: New source of plant biostimulants. Agronomy10, 1240. doi: 10.3390/agronomy10091240
34
D’Abramo L. R. (2021). Sustainable aquafeed and aquaculture production systems as impacted by challenges of global food security and climate change. J. World Aquaculture Soc.52, 1162–1167. doi: 10.1111/jwas.12859
35
Das B. K. Pradhan J. Sahu S. (2009). The effect of Euglena viridis on immune response of rohu, Labeo rohita (Ham.). Fish Shellfish Immunol.26, 871–876. doi: 10.1016/j.fsi.2009.03.016
36
Das P. Thaher M. AbdulQuadir M. Khan S. Chaudhary A. Al-Jabri H. (2019). Long-term semi-continuous cultivation of a halo-tolerant Tetraselmis sp. using recycled growth media. Bioresource Technol.276, 35–41. doi: 10.1016/j.biortech.2018.12.108
37
Das P. Thaher M. I. Hakim M. A. Q. M. A. Al-Jabri H. M. S. Alghasal G. S. H. (2016). A comparative study of the growth of Tetraselmis sp. in large scale fixed depth and decreasing depth raceway ponds. Bioresource Technol.216, 114–120. doi: 10.1016/j.biortech.2016.05.058
38
Dasari D. Dong C. D. Singhania R. R. Tambat V. S. Piechota G. Patel A. K. (2025). Harnessing microalgae for sustainable aquaculture and mariculture: Marine pollution mitigation and circular economy strategies. Mar. pollut. Bull.219, 118292. doi: 10.1016/j.marpolbul.2025.118292
39
Delaide B. Delhaye G. Dermience M. Gott J. Soyeurt H. Jijakli M. H. (2017). Plant and fish production performance, nutrient mass balances, energy and water use of the PAFF box, a small-scale aquaponic system. Aquacultural Eng.78, 130–139. doi: 10.1016/j.aquaeng.2017.02.002
40
Del Mondo A. Smerilli A. Ambrosino L. Albini A. Noonan D. M. Sansone C. et al . (2021). Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol.41, 155–171. doi: 10.1080/07388551.2020.1831407
41
Delrue F. Álvarez-Díaz P. D. Fon-Sing S. Fleury G. Sassi J. F. (2016). The environmental biorefinery: Using microalgae to remediate wastewater, a win-win paradigm. Energies9, 132. doi: 10.3390/en9030132
42
de Oliveira A. P. F. Bragotto A. P. A. (2022). Microalgae-based products: Food and public health. Future Foods6, 100157. doi: 10.1016/j.fufo.2022.100157
43
Dineshbabu G. Goswami G. Kumar R. Sinha A. Das D. (2019). Microalgae–nutritious, sustainable aqua- and animal feed source. J. Funct. Foods62, 103545. doi: 10.1016/j.jff.2019.103545
44
Dineshkumar R. Subramanian J. Gopalsamy J. Jayasingam P. Arumugam A. Kannadasan S. et al . (2019). The impact of using microalgae as biofertilizer in maize (Zea mays L.). Waste Biomass Valorization10, 1101–1110. doi: 10.1007/s12649-018-0199-5
45
Dixit R. B. Sagaram U. S. Gocher C. Krishna Kumar G. R. Dasgupta S. (2022). Biomolecular characterisation of marine microalga in comparison to fishmeal and soymeal as an alternative feed ingredient. Phytochemical Anal.33, 365–372. doi: 10.1002/pca.3144
46
Dourou M. Dritsas P. Baeshen M. N. Elazzazy A. Al-Farga A. Aggelis G. (2020). High-added value products from microalgae and prospects of aquaculture wastewaters as microalgae growth media. FEMS Microbiol. Lett.367, fnaa081. doi: 10.1093/femsle/fnaa081
47
Eck M. Körner O. Jijakli M. H. (2019). “Nutrient cycling in aquaponics systems,” in Aquaponics food production systems: Combined aquaculture and hydroponic production technologies for the future. Eds. GoddekS.JoyceA.KotzenB.BurnellG. M. (Cham, Switzerland: Springer Nature), 231–246.
48
Edelmann M. Aalto S. Chamlagain B. Kariluoto S. Piironen V. (2019). Riboflavin, niacin, folate, and vitamin B12 in commercial microalgae powders. J. Food Composition Anal.82, 103226. doi: 10.1016/j.jfca.2019.103226
49
Edwards P. Sinchumpasak O. A. Tabucanon M. (1981). The harvest of microalgae from the effluent of a sewage fed high rate stabilization pond by Tilapia nilotica: Part 2: studies of the fish ponds. Aquaculture23, 107–147. doi: 10.1016/0044-8486(81)90010-7
50
El Arroussi H. Benhima R. Elbaouchi A. Sijilmassi B. El Mernissi N. Aafsar A. et al . (2018). Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycology30, 2929–2941. doi: 10.1007/s10811-018-1474-5
51
El-Sheekh M. M. Rashad S. El-Chaghaby G. A. (2023). The blue-green microalga (Spirulina) in the fishery: A mini review. Egyptian J. Aquat. Biol. Fisheries27, 49–61. doi: 10.21608/ejabf.2023.307318
52
Ende S. Henjes J. Spiller M. Elshobary M. Hanelt D. Abomohra A. (2024). Recent advances in recirculating aquaculture systems and role of microalgae to close system loop. Bioresource Technol.131, 107. doi: 10.1016/j.biortech.2024.131107
53
Fadl S. E. Elsadany A. Y. El-Shenawy A. M. Sakr O. A. El Gammal G. A. Gad D. M. et al . (2020). Efficacy of cyanobacterium Anabaena sp. as a feed supplement on productive performance and immune status in cultured Nile tilapia. Aquaculture Rep.17, 100406. doi: 10.1016/j.aqrep.2020.100406
54
Falaise C. François C. Travers M. A. Morga B. Haure J. Tremblay R. et al . (2016). Antimicrobial compounds from eukaryotic microalgae against human pathogens and diseases in aquaculture. Mar. Drugs14, 159. doi: 10.3390/md14090159
55
Farid R. Mutale-Joan C. Redouane B. Mernissi Najib E. L. Abderahime A. Laila S. et al . (2019). Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum. Appl. Biochem. Biotechnol.188, 225–240. doi: 10.1007/s12010-018-2916-y
56
Gamble M. M. Sarker P. K. Kapuscinski A. R. Kelson S. Fitzgerald D. S. Schelling B. et al . (2021). Toward environmentally sustainable aquafeeds: Managing phosphorus discharge from Nile tilapia (Oreochromis niloticus) aquaculture with microalgae-supplemented diets. Elementa: Sci. Anthropocene9, 170. doi: 10.1525/elementa.00170
57
Gemin L. G. Mógor Á.F. Amatussi J. D. O. De Lara G. B. Mógor G. (2022). Organic onion growth, yield and storage improved by foliar sprays of microalgae and fulvic acid as a natural biofertilizer. Bioscience J.38, e38045. doi: 10.14393/BJ-v38n0a2022-58854
58
Gerile S. Pirhonen J. (2017). Replacement of fishmeal with corn gluten meal in feeds for juvenile rainbow trout (Oncorhynchus mykiss) does not affect oxygen consumption during forced swimming. Aquaculture479, 616–618. doi: 10.1016/j.aquaeng.2017.05.002
59
Gitau M. M. Farkas A. Ördög V. Maróti G. (2022). Evaluation of the biostimulant effects of two Chlorophyta microalgae on tomato (Solanum lycopersicum). J. Cleaner Production364, 132689. doi: 10.1016/j.jclepro.2022.132689
60
Glass J. B. Axler R. P. Chandra S. Goldman C. R. (2012). Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures. Front. Microbiol.3, 331. doi: 10.3389/fmicb.2012.00331
61
Goddek S. Joyce A. Kotzen B. Burnell G. M. (2019). Aquaponics food production systems: Combined aquaculture and hydroponic production technologies for the future (Cham, Switzerland: Springer Nature), 619.
62
Godlewska K. Michalak I. Pacyga P. Baśladyńska S. Chojnacka K. (2019). Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol.35, 1–18. doi: 10.1007/s11274-018-2583-0
63
Gonçalves A. L. (2021). The use of microalgae and cyanobacteria in the improvement of agricultural practices: A review on their biofertilising, biostimulating and biopesticide roles. Appl. Sci.11, 871. doi: 10.3390/app11020871
64
Haggag W. M. Hoballah M. M. E. Ali R. R. (2018). Applications of nano biotechnological microalgae product for improve wheat productivity in semi arid areas. International Journal of Agricultural Technology14 (5), 675–692.
65
Hamouda R. A. El-Ansary M. S. M. (2017). Potential of plant-parasitic nematode control in banana plants by microalgae as a new approach towards resistance. Egyptian J. Biol. Pest Control27, 1–9. doi: 10.1186/s42269-020-00463-0
66
Han P. Lu Q. Fan L. Zhou W. (2019). A review on the use of microalgae for sustainable aquaculture. Appl. Sci.9, 2377. doi: 10.3390/app9112377
67
Han X. Zeng H. Bartocci P. Fantozzi F. Yan Y. (2018). Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation4, 25. doi: 10.3390/fermentation4020025\
68
Hans A. L. Mishra R. Singh A. Roy S. Mishra S. Baskar R. et al . (2020). Microalgal Extracts for Tomatoes: Seed treatment or foliar spray? Curr. Sci. (00113891)119. doi: 10.1016/j.indcrop.2020.112453
69
Hao Y. Ding K. Xu Y. Tang Y. Liu D. Li G. (2020). States, trends, and future of aquaponics research. Sustainability12, 7783. doi: 10.3390/su12187783
70
Hashmi Z. Abbas S. H. Osama S. M. Muhammad A. Usman M. T. Jatoi A. S. et al . (2023). Microalgae technology in aquaculture applications: a comprehensive literature review. AMPLITUDO: J. Sci. Technol. Innovation2, 61–69. doi: 10.56566/amplitudo.v2i2.88
71
Hussain F. Shah S. Z. Zhou W. Iqbal M. (2017). Microalgae screening under CO2 stress: Growth and micro-nutrients removal efficiency. J. Photochem. Photobiol. B: Biol.170, 91–98. doi: 10.1016/j.jphotobiol.2017.02.016
72
Idenyi J. N. Eya J. C. Nwankwegu A. S. Nwoba E. G. (2022). Aquaculture sustainability through alternative dietary ingredients: Microalgal value-added products. Eng. Microbiol.2, 100049. doi: 10.1016/j.engmicro.2022.100049
73
Ju Z. Y. Davis S. Ramm K. Steck M. Soller F. Fox B. K. (2017). Effects of microalgae‐added diets on growth performance and meat composition of tilapia (Oreochromis mossambicus). Aquaculture Research, 48(9), pp.5053-5061. doi: 10.1111/are.13322
74
Kapara J. (2018). Application of microalgae in aquaculture. Phykos48, 21–26.
75
Kapoore R. V. Wood E. E. Llewellyn C. A. (2021). Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv.49, 107754. doi: 10.1016/j.bioteChadv.2021.107754
76
Kasozi N. Abraham B. Kaiser H. Wilhelmi B. (2021). The complex microbiome in aquaponics: Significance of the bacterial ecosystem. Ann. Microbiol.71, 1–13. doi: 10.1007/s13213-021-01472-w
77
Katerina K. Berge G. M. Turid M. Aleksei K. Grete B. Trine Y. et al . (2020). Microalgal Schizochytrium limacinum biomass improves growth and filet quality when used long-term as a replacement for fish oil, in modern salmon diets. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00057
78
Kim S. J. Ko E. J. Hong J. K. Jeun Y. C. (2018). Ultrastructures of Colletotrichum orbiculare in cucumber leaves expressing systemic acquired resistance mediated by Chlorella fusca. Plant Pathol. J.34, 113. doi: 10.5423/PPJ.OA.09.2017.0204
79
Kinh C. T. Nho N. T. Hien V. T. D. Huy L. Q. Van Tung T. Manh V. Q. et al . (2024). Effects of fish stocking density on water quality and the growth of red tilapia and vegetables in microalgae-aquaponic systems. J. Sci. Technol.7, 62–71. doi: 10.55401/7k3bpd04
80
Kiron V. Kulkarni A. Dahle D. Lokesh J. Elvebo O. (2016a). Recognition of purified beta 1, 3/1, 6 glucan and molecular signalling in the intestine of Atlantic salmon. Dev. Comp. Immunol.56, 57–66. doi: 10.1016/j.dci.2015.11.003
81
Kiron V. Kulkarni A. Dahle D. Lokesh J. Elvebo O. (2016b). Recognition of purified beta 1, 3/1, 6 glucan and molecular signalling in the intestine of Atlantic salmon. Developmental & Comparative Immunology56, 57–66. doi: 10.1016/j.dci.2015.11.007
82
König B. Junge R. Bittsanszky A. Villarroel M. Kőmíves T. (2016). On the sustainability of aquaponics. Ecocycles2, 26–32. doi: 10.19040/ecocycles.v2i1.50
83
Kousoulaki K. Mørkøre T. Nengas I. Berge R. K. Sweetman J. (2016). Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.). Aquaculture451, 47–57. doi: 10.1016/j.aquaculture.2015.09.042
84
Kumar S. Korra T. Singh U. B. Singh S. Bisen K. (2022). “Microalgal based biostimulants as alleviator of biotic and abiotic stresses in crop plants,” in New and future developments in microbial biotechnology and bioengineering (Amsterdam, Netherlands: Elsevier), 195–216. doi: 10.1016/B978-0-323-85949-5.00013-6
85
Kusvuran S. (2021). Microalgae (Chlorella vulgaris Beijerinck) alleviates drought stress of broccoli plants by improving nutrient uptake, secondary metabolites, and antioxidative defense system. Hortic. Plant J.7, 221–231. doi: 10.1016/j.hpj.2021.03.007
86
Kusvuran A. Can A. G. (2020). Effects of microalga (Chlorella vulgaris beijerinck) on seconder metabolites and antioxidative defense system improve plant growth and salt tolerance in guar [Cyamopsis tetragonoloba (L.) taub. Legume Research-An Int. J.43, 56–60. doi: 10.18805/LR-492
87
La Bella E. Baglieri A. Rovetto E. I. Stevanato P. Puglisi I. (2021). Foliar spray application of Chlorella vulgaris extract: Effect on the growth of lettuce seedlings. Agronomy11, 308. doi: 10.3390/agronomy11020308
88
LaPanse A. J. (2024). Industrial microalgal characterization and enhancement for a sustainable future. Doctoral dissertation, Colorado School of Mines.
89
Lee S. M. Kim S. K. Lee N. Ahn C. Y. Ryu C. M. (2020). D-lactic acid secreted by Chlorella fusca primes pattern-triggered immunity against Pseudomonas syringae in Arabidopsis. Plant J.102, 761–778. doi: 10.1111/tpj.14661
90
Lee S. M. Ryu C. M. (2021). Algae as new kids in the beneficial plant microbiome. Front. Plant Sci.12. doi: 10.3389/fpls.2021.599742
91
Li T. Chen Z. Wu J. Wu H. Yang B. Dai L. et al . (2020). The potential productivity of the microalga, Nannochloropsis oceanica SCS-1981, in a solar powered outdoor open pond as an aquaculture feed. Algal Res.46, 101793. doi: 10.1016/j.algal.2019.101793
92
Liu C. Li Y. Chen Z. Yuan L. Liu H. Han D. et al . (2021). Effects of dietary whole and defatted Arthrospira platensis (Cyanobacterium) on growth, body composition, and pigmentation of the yellow catfish Pelteobagrus fulvidraco. J. Appl. Phycology33, 2251–2259. doi: 10.1007/s10811-021-02425-0
93
Llamas A. Leon-Miranda E. Tejada-Jimenez M. (2023). Microalgal and nitrogen-fixing bacterial consortia: From interaction to biotechnological potential. Plants12, 2476. doi: 10.3390/plants12132476
94
Ma M. Hu Q. (2024). Microalgae as feed sources and feed additives for sustainable aquaculture: Prospects and challenges. Rev. Aquaculture16, 818–835. doi: 10.1111/raq.13016
95
Madhumathi M. Rengasamy R. (2011). Antioxidant status of Penaeus monodon fed with Dunaliella salina supplemented diet and resistance against WSSV. Int. J. Eng. Sci. Technol.3, 7249–7259.
96
Mahata C. Das P. Khan S. Thaher M. I. Abdul Quadir M. Annamalai S. N. et al . (2022). The potential of marine microalgae for the production of food, feed, and fuel (3F). Fermentation8, 316. doi: 10.3390/fermentation8070316
97
Maliwat G. C. Velasquez S. Robil J. L. Chan M. Traifalgar R. F. Tayamen M. et al . (2017). Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii (De Man) postlarvae fed diets containing Chlorella vulgaris (Beijerinck). Aquaculture Res.48, 1666–1676. doi: 10.1111/are.13004
98
Markou G. Wang L. Ye J. Unc A. (2018). Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv.36, 1238–1254. doi: 10.1016/j.bioteChadv.2018.04.003
99
Martínez-Fernández E. Southgate P. C. (2007). Use of tropical microalgae as food for larvae of the black-lip pearl oyster Pinctada margaritifera. Aquaculture263, 220–226. doi: 10.1016/j.aquaculture.2006.09.040
100
Medina M. Neis U. (2007). Symbiotic algal bacterial wastewater treatment: effect of food to microorganism ratio and hydraulic retention time on the process performance. Water Sci. Technol.55, 165–171. doi: 10.2166/WST.2007.351
101
Medina-Félix D. López-Elías J. A. Martínez-Córdova L. R. López-Torres M. A. Hernández-López J. Rivas-Vega M. E. et al . (2014). Evaluation of the productive and physiological responses of Litopenaeus vannamei infected with WSSV and fed diets enriched with Dunaliella sp. J. invertebrate Pathol.117, 9–12. doi: 10.1016/j.jip.2013.12.004
102
Mélo R. C. S. Santos L. P. D. S. Brito A. P. M. Gouveia A. D. A. Marçal C. Cavalli R. O. (2016). Use of the microalga Nannochloropsis occulata in the rearing of newborn longsnout seahorse Hippocampus reidi (Syngnathidae) juveniles. Aquaculture Res.47, 3934–3941. doi: 10.1111/are.12843
103
Metwally R. A. Abdelhameed R. E. Soliman S. A. Al-Badwy A. H. (2022). Potential use of beneficial fungal microorganisms and C-phycocyanin extract for enhancing seed germination, seedling growth and biochemical traits of Solanum lycopersicum L. BMC Microbiol.22, 108. doi: 10.1186/s12866-022-02509-x
104
Mishra R. P. Das S. K. Kumar N. Mishra J. (2020). Sustainable aquaponics system and its challenges: A review. Int. J. Curr. Microbiol. Appl. Sci.9, 577–588. doi: 10.20546/ijcmas.2020.905.065
105
Mógor Á.F. de Oliveira Amatussi J. Mógor G. de Lara G. B. (2018). Bioactivity of cyanobacterial biomass related to amino acids induces growth and metabolic changes on seedlings and yield gains of organic red beet. Am. J. Plant Sci.9, 966–978. doi: 10.4236/ajps.2018.95067
106
Montoya L. N. F. Martins T. P. Gimbo R. Y. Zanuzzo F. S. Urbinati E. C. (2017). β-Glucan-induced cortisol levels improve the early immune response in matrinxã (Brycon amazonicus). Fish shellfish Immunol.60, 197–204. doi: 10.1016/j.fsi.2016.11.055
107
Nagappan S. Das P. AbdulQuadir M. Thaher M. Khan S. Mahata C. et al . (2021). Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol.341, 1–20. doi: 10.1016/j.jbiotec.2021.04.013
108
Nair C. S. Manoharan R. Nishanth D. Subramanian R. Neumann E. Jaleel A. (2025). Recent advancements in aquaponics with special emphasis on its sustainability. J. World Aquaculture Soc.56, e13116. doi: 10.1111/jwas.13116
109
Nath P. R. Khozin-Goldberg I. Cohen Z. Boussiba S. Zilberg D. (2012). Dietary supplementation with the microalgae Parietochloris incisa increases survival and stress resistance in guppy (Poecilia reticulata) fry. Aquaculture Nutr.18, 167–180. doi: 10.1111/j.1365-2095.2011.00885.x
110
Ng W. K. Koh C. B. (2017). The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquaculture9, 342–368. doi: 10.1111/raq.12118
111
Nimrat S. Boonthai T. Vuthiphandchai V. (2011). Effects of probiotic forms, compositions of and mode of probiotic administration on rearing of Pacific white shrimp (Litopenaeus vannamei) larvae and postlarvae. Anim. feed Sci. Technol.169, 244–258. doi: 10.1016/j.anifeedsci.2011.07.003
112
Nishanth D. Alshamsi M. H. Alkaabi A. M. AlKaabi A. H. Alnuaimi S. H. Nair C. S. et al . (2024). Aquaponics as a climate-smart technology for sustainable food production: A comparison with conventional production system in United Arab Emirates. J. World Aquaculture Soc.55, e13049. doi: 10.1111/jwas.13049
113
Norzagaray-Valenzuela C. D. Valdez-Ortiz A. Shelton L. M. Jiménez-Edeza M. Rivera-López J. Valdez-Flores M. A. et al . (2017). Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J. Appl. Phycology29, 189–198. doi: 10.1007/s10811-016-1007-x
114
Nwoba E. G. Parlevliet D. A. Laird D. W. Alameh K. Moheimani N. R. (2019). Light management technologies for increasing algal photobioreactor efficiency. Algal Res.39, 101433. doi: 10.1016/j.algal.2019.101433
115
Odgerel B. Tserendulam D. (2016). Effect of Chlorella as a biofertilizer on germination of wheat and barley grains. Proc. Mongolian Acad. Sci., 26–31. doi: 10.5564/pmas.v56i4.839
116
Okomoda V. T. Oladimeji S. A. Solomon S. G. Olufeagba S. O. Ogah S. I. Ikhwanuddin M. (2023). Aquaponics production system: A review of historical perspective, opportunities, and challenges of its adoption. Food Sci. Nutr.11, 1157–1165. doi: 10.1002/fsn3.3154
117
Ooms M. D. Dinh C. T. Sargent E. H. Sinton D. (2016). Photon management for augmented photosynthesis. Nat. Commun.7, 12699. doi: 10.1038/ncomms12699
118
Patrinou V. Patsialou S. Daskalaki A. Economou C. N. Aggelis G. Vayenas D. V. et al . (2023). Laboratory- and pilot-scale cultivation of Tetraselmis striata to produce valuable metabolic compounds. Life13, 480. doi: 10.3390/life13020480
119
Pinchart P. E. Leruste A. Pasqualini V. Mastroleo F. (2023). Microcystins and cyanobacterial contaminants in the french small-scale productions of spirulina (Limnospira sp.). Toxins15, 354. doi: 10.3390/toxins15060354
120
Plaza B. M. Gómez-Serrano C. Acién-Fernández F. G. Jimenez-Becker S. (2018). Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J. Appl. Phycology30, 2359–2365. doi: 10.1007/s10811-018-1427-0
121
Pratiwi D. Y. Zidni I. (2023). The effect of Schizochytrium sp. on the growth and health of fish. Asian J. Res. Zoology6, 1–6. doi: 10.9734/ajriz/2023/v6i1101
122
Praveen K. Abinandan S. Venkateswarlu K. Megharaj M. (2023). Harnessing extremophilic trait and metabolic flexibility of microalgal strains for the treatment of highly alkaline winery wastewater. ACS ES&T Eng.4, 455–465. doi: 10.1021/acsestengg.2c00394
123
Pugazhendhi A. Nagappan S. Bhosale R. R. Tsai P. C. Natarajan S. Devendran S. et al . (2020). Various potential techniques to reduce the water footprint of microalgal biomass production for biofuel—a review. Sci. Total Environ.749, 142218. doi: 10.1016/j.scitotenv.2020.142218
124
Puglisi I. Barone V. Fragalà F. Stevanato P. Baglieri A. Vitale A. (2020). Effect of microalgal extracts from Chlorella vulgaris and Scenedesmus quadricauda on germination of Beta vulgaris seeds. Plants9, 675. doi: 10.3390/plants9060675
125
Puglisi I. La Bella E. Rovetto E. I. Stevanato P. Fascella G. Baglieri A. (2022). Morpho-biometric and biochemical responses in lettuce seedlings treated by different application methods of Chlorella vulgaris extract: foliar spray or root drench? J. Appl. Phycology34, 889–901. doi: 10.1007/s10811-021-02671-1
126
Rachidi F. Benhima R. Kasmi Y. Sbabou L. Arroussi H. E. (2021). Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep.11, 930. doi: 10.1038/s41598-020-78820-2
127
Radhakrishnan S. Belal I. E. Seenivasan C. Muralisankar T. Bhavan P. S. (2016). Impact of fishmeal replacement with Arthrospira platensis on growth performance, body composition, and digestive enzyme activities of the freshwater prawn, Macrobrachium rosenbergii. Aquaculture Rep.3, 35–44. doi: 10.1016/j.aqrep.2016.08.001
128
Ragaza J. A. Hossain M. S. Meiler K. A. Velasquez S. F. Kumar V. (2020). A review on Spirulina: Alternative media for cultivation and nutritive value as an aquafeed. Rev. Aquaculture12, 2371–2395. doi: 10.1111/raq.12378
129
Rakocy J. E. (2012). “Aquaponics—integrating fish and plant culture,” in Aquaculture production systems (Hoboken, NJ, USA: Wiley-Blackwell), 344–386.
130
Ramanan R. Kim B. H. Cho D. H. Oh H. M. Kim H. S. (2016). Algae–bacteria interactions: Evolution, ecology, and emerging applications. Biotechnol. Adv.34, 14–29. doi: 10.1016/j.bioteChadv.2015.12.003
131
Regunathan C. Wesley S. G. (2004). Control of Vibrio spp. in shrimp hatcheries using the green algae Tetraselmis suecica. Asian Fisheries Sci.17, 147–158. doi: 10.33997/j.afs.2004.17.2.006
132
Ren L. J. Ji X. J. Huang H. Qu L. Feng Y. Tong Q. Q. et al . (2010). Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp. Appl. Microbiol. Biotechnol.87, 1649–1656. doi: 10.1007/s00253-010-2639-7
133
Resh H. M. (2022). Hydroponic food production: A definitive guidebook for the advanced home gardener and the commercial hydroponic grower (Boca Raton, FL, USA: CRC Press).
134
Reyes-Becerril M. Guardiola F. Rojas M. Ascencio-Valle F. Esteban M.Á. (2013). Dietary administration of microalgae Navicula sp. affects immune status and gene expression of gilthead seabream (Sparus aurata). Fish shellfish Immunol.35, 883–889. doi: 10.1016/j.fsi.2013.06.026
135
Ribeiro A. R. Gonçalves A. Barbeiro M. Bandarra N. Nunes M. L. Carvalho M. L. et al . (2017). Phaeodactylum tricornutum in finishing diets for gilthead seabream: Effects on skin pigmentation, sensory properties, and nutritional value. J. Appl. Phycology29, 1945–1956. doi: 10.1007/s10811-017-1083-2
136
Ronga D. Biazzi E. Parati K. Carminati D. Carminati E. Tava A. (2019). Microalgal biostimulants and biofertilisers in crop productions. Agronomy9, 192. doi: 10.3390/agronomy9040192
137
Ru D. Liu J. Hu Z. Zou Y. Jiang L. Cheng X. et al . (2017). Improvement of aquaponic performance through micro- and macro-nutrient addition. Environ. Sci. pollut. Res.24, 16328–16335. doi: 10.1007/s11356-017-8804-3
138
Rupawalla Z. Shaw L. Ross I. L. Schmidt S. Hankamer B. Wolf J. (2022). Germination screen for microalgae-generated plant growth biostimulants. Algal Res.66, 102784. doi: 10.1016/j.algal.2022.102784
139
Sagaram U. S. Gaikwad M. S. Nandru R. Dasgupta S. (2021). Microalgae as feed ingredients: Recent developments on their role in immunomodulation and gut microbiota of aquaculture species. FEMS Microbiol. Lett.368, fnab071. doi: 10.1093/femsle/fnab071
140
Salehipour-Bavarsad F. Nematollahi M. A. Pistocchi R. Pezzolesi L. (2024). Algal food safety: Possible contaminations, challenges of harmonized quality assessments, and suggested recommendations for the nascent industry of microalgae-based products. Algal Res.81, 103579. doi: 10.1016/j.algal.2024.103579
141
Salomé P. A. Merchant S. S. (2019). A series of fortunate events: introducing Chlamydomonas as a reference organism. Plant Cell31, 1682–1707. doi: 10.1105/tpc.18.00952
142
Sandgruber F. Gielsdorf A. Baur A. C. Schenz B. Müller S. M. Schwerdtle T. et al . (2021). Variability in macro- and micronutrients of 15 commercially available microalgae powders. Mar. Drugs19, 310. doi: 10.3390/md19060310
143
Sarker P. K. Kapuscinski A. R. Lanois A. J. Livesey E. D. Bernhard K. P. Coley M. L. (2016). Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus). PLoS One11, e0156684. doi: 10.1371/journal.pone.0156684
144
Sarker P. K. Kapuscinski A. R. Vandenberg G. W. Proulx E. Sitek A. J. (2020). Towards sustainable and ocean-friendly aquafeeds: Evaluating a fish-free feed for rainbow trout (Oncorhynchus mykiss) using three marine microalgae species. Elementa: Sci. Anthropocene8, 5. doi: 10.1525/elementa.457
145
Schmautz Z. Espinal C. A. Bohny A. M. Rezzonico F. Junge R. Frossard E. et al . (2021). Environmental parameters and microbial community profiles as indication towards microbial activities and diversity in aquaponic system compartments. BMC Microbiol.21, 1–11. doi: 10.1186/s12866-021-02059-4
146
Schmautz Z. Graber A. Jaenicke S. Goesmann A. Junge R. Smits T. H. M. (2017). Microbial diversity in different compartments of an aquaponics system. Arch. Microbiol.199, 613–620. doi: 10.1007/s00203-017-1341-3
147
Schmautz Z. Loeu F. Liebisch F. Graber A. Mathis A. Griessler Bulc T. et al . (2016). Tomato productivity and quality in aquaponics: Comparison of three hydroponic methods. Water8, 533. doi: 10.3390/w8110533
148
Scoglio S. (2018). Microcystins in water and in microalgae: do microcystins as microalgae contaminants warrant the current public alarm? Toxicol. Rep.5, 785–792. doi: 10.1016/j.toxrep.2018.07.002
149
Scranton M. A. Ostrand J. T. Fields F. J. Mayfield S. P. (2015). Chlamydomonas as a model for biofuels and bio-products production. Plant J.82, 523–531. doi: 10.1111/tpj.12780
150
Sharawy Z. Z. Ashour M. Abbas E. Ashry O. Helal M. Nazmi H. et al . (2020). Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers, and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquaculture Res.51, 2216–2228. doi: 10.1111/are.14552
151
Sharma S. A. Surveswaran S. Arulraj J. Velayudhannair K. (2021). Bromelain enhances digestibility of Spirulina-based fish feed. J. Appl. Phycology33, 967–977. doi: 10.1007/s10811-020-02337-4
152
Shi Z. Li X. Q. Chowdhury M. K. Chen J. N. Leng X. J. (2016). Effects of protease supplementation in low fish meal pelleted and extruded diets on growth, nutrient retention and digestibility of gibel carp, Carassius auratus gibelio. Aquaculture460, 37–44. doi: 10.1016/j.aquaculture.2016.03.049
153
Shreejana K. C. Thapa R. Lamsal A. Ghimire S. Kurunju K. Shrestha P. (2022). Aquaponics: A modern approach for integrated farming and wise utilization of components for sustainability of food security: A review. Arch. Agric. Environ. Sci.7, 121–126. doi: 10.26832/24566632.2022.0701017
154
Siddik M. A. Sørensen M. Islam S. M. Saha N. Rahman M. A. Francis D. S. (2024). Expanded utilisation of microalgae in global aquafeeds. Rev. Aquaculture16, 6–33. doi: 10.1111/raq.12978
155
Singh A. (2021). Role of Algae in soil nitrogen fixation. In Soil nitrogen ecology (Cham: Springer International Publishing), 483–496. doi: 10.1007/978-3-030-71206-8_24
156
Souza F. P. D. Lima E. C. S. D. Urrea-Rojas A. M. Suphoronski S. A. Facimoto C. T. Bezerra Júnior J. D. S. et al . (2020). Effects of dietary supplementation with a microalga (Schizochytrium sp.) on the hemato-immunological, and intestinal histological parameters and gut microbiota of Nile tilapia in net cages. PLoS One15, e0226977. doi: 10.1371/journal.pone.0226977
157
Sreekumar A. Pudhuvai B. Sundaram P. Das R. (2023). Aquaponics: A sustainable food production alternative. J. Inland Fisheries Soc. India56, 155–171. doi: 10.56093/jifsi.v56i2.2024.163404
158
Štěrbová K. Manoel J. C. Lakatos G. E. Grivalský T. Masojídek J. (2023). Microalgae as an aquaculture feed produced in a short light-path annular column photobioreactor. J. Appl. Phycology35, 603–611. doi: 10.1007/s10811-023-02928-x
159
Suchithra M. R. Muniswami D. M. Sri M. S. Usha R. Rasheeq A. A. Preethi B. A. et al . (2022). Effectiveness of green microalgae as biostimulants and biofertilizer through foliar spray and soil drench method for tomato cultivation. South Afr. J. Bot.146, 740–750. doi: 10.1016/j.sajb.2021.12.022
160
Suhl J. Dannehl D. Kloas W. Baganz D. Jobs S. Scheibe G. et al . (2016). Advanced aquaponics: Evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agric. Water Manage.178, 335–344. doi: 10.1016/j.agwat.2016.09.007
161
Sun X. Chang Y. Ye Y. Ma Z. Liang Y. Li T. et al . (2012). The effect of dietary pigments on the coloration of Japanese ornamental carp (koi, Cyprinus carpio L.). Aquaculture342, 62–68. doi: 10.1016/j.aquaculture.2012.02.019
162
Tejido-Núñez Y. (2020). Microalgae-based wastewater treatment processes: Implementation in aquaculture sector and WWTP.
163
Tejido-Nuñez Y. Aymerich E. Sancho L. Refardt D. (2020). Co-cultivation of microalgae in aquaculture water: Interactions, growth, and nutrient removal efficiency at laboratory-and pilot-scale. Algal Res.49, 101940. doi: 10.1016/j.algal.2020.101940
164
Tham P. E. Lim H. R. Khoo K. S. Chew K. W. Yap Y. J. Munawaroh H. S. H. et al . (2023). Insights of microalgae-based aquaculture feed: A review on circular bioeconomy and perspectives. Algal Res.74, 103186. doi: 10.1016/j.algal.2023.103186
165
Thinh N. Q. (2021). Influences of seed priming with Spirulina platensis extract on seed quality properties in black gram (Vigna mungo L.). Vietnam J. Science Technol. Eng.63, 36–41. doi: 10.31276/VJSTE.63(1).36-41
166
Tibbetts S. M. Yasumaru F. Lemos D. (2017). In vitro prediction of digestible protein content of marine microalgae (Nannochloropsis granulata) meals for Pacific white shrimp (Litopenaeus vannamei) and rainbow trout (Oncorhynchus mykiss). Algal Res.21, 76–80. doi: 10.1016/j.algal.2017.01.007
167
Topuz O. K. (2016). Algal oil: A novel source of omega-3 fatty acids for human nutrition. Sci. Bull. Ser. F. Biotechnologies20, 1–6.
168
Vidyashankar S. Daris P. S. Mallikarjuna K. G. Sarada R. (2017). “Microalgae as a source of nutritional and therapeutic metabolites,” in Plant secondary metabolites (Oakville, ON, Canada: Apple Academic Press), 21–82.
169
Vishwakarma R. Dhaka V. Ariyadasa T. U. Malik A. (2022). Exploring algal technologies for a circular bio-based economy in rural sector. J. Cleaner Production354, 131653. doi: 10.1016/j.jclepro.2022.131653
170
Wang S. Cheng L. Liu X. (2022). Comparative study on the carbon footprints of extruded and pelleted feed and their potential for carbon reduction: A case study of grass carp feed. J. Cleaner Production381, 135192. doi: 10.1016/j.jclepro.2022.135192
171
Wani A. K. Ul Gani Mir T. Akhtar N. Chopra C. Bashir S. M. Hassan S. et al . (2024). Algae-mediated removal of prevalent genotoxic antibiotics: molecular perspective on algae-bacteria consortia and bioreactor-based strategies. Curr. Microbiol.81, 112. doi: 10.1007/s00284-024-03631-x
172
Wei M. Parrish C. C. Guerra N. I. Armenta R. E. Colombo S. M. (2021). Extracted microbial oil from a novel Schizochytrium sp.(T18) as a sustainable high DHA source for Atlantic salmon feed: Impacts on growth and tissue lipids. Aquaculture534, 736249. doi: 10.1016/j.aquaculture.2020.736249
173
Win T. T. Barone G. D. Secundo F. Fu P. (2018). Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol.14, 203–211. doi: 10.1089/ind.2018.0002
174
Wongkiew S. Hu Z. Chandran K. Lee J. W. Khanal S. K. (2017). Nitrogen transformations in aquaponic systems: A review. Aquacultural Eng.76, 9–19. doi: 10.1016/j.aquaeng.2017.05.002
175
Wongkiew S. Park M. R. Chandran K. Khanal S. K. (2018). Aquaponic systems for sustainable resource recovery: Linking nitrogen transformations to microbial communities. Environ. Sci. Technol.52, 12728–12739. doi: 10.1021/acs.est.8b03643
176
Yamamoto F. Y. Yin F. Rossi W. Jr. Hume M. Gatlin D. M. III (2018). β-1, 3 glucan derived from Euglena gracilis and Algamune™ enhances innate immune responses of red drum (Sciaenops ocellatus L.). Fish Shellfish Immunol.77, 273–279. doi: 10.1016/j.fsi.2018.04.003
177
Yarnold J. Karan H. Oey M. Hankamer B. (2019). Microalgal aquafeeds as part of a circular bioeconomy. Trends Plant Sci.24, 959–970. doi: 10.1016/j.tplants.2019.06.005
178
Yavuzcan Yildiz H. Robaina L. Pirhonen J. Mente E. Domínguez D. Parisi G. (2017). Fish welfare in aquaponic systems: Its relation to water quality with an emphasis on feed and faeces—a review. Water9, 13. doi: 10.3390/w9010013
179
Yeganeh S. Teimouri M. Amirkolaie A. K. (2015). Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Veterinary Sci.101, 84–88. doi: 10.1016/j.rvsc.2015.06.002
180
Yep B. Zheng Y. (2019). Aquaponic trends and challenges – A review. J. Cleaner Production228, 1586–1599. doi: 10.1016/j.jclepro.2019.04.271
181
Yogev U. Barnes A. Gross A. (2016). Nutrients and energy balance analysis for a conceptual model of a three loops off grid, aquaponics. Water8, 589. doi: 10.3390/w8120589
182
Zamljen T. Hudina M. Veberič R. Slatnar A. (2021). Biostimulative effect of amino acids and green algae extract on capsaicinoid and other metabolite contents in fruits of Capsicum spp. Chem. Biol. Technol. Agric.8, 1–12. doi: 10.1186/s40538-021-00172-7
183
Zhang Q. Guan Y. Zhang Z. Dong S. Yuan T. Ruan Z. et al . (2024). “Sustainable microalgae cultivation: A comprehensive review of open and enclosed systems for biofuel and high value compound production,” in E3S web of conferences, vol. 577. (Les Ulis, France: EDP Sciences), 01008. doi: 10.1051/e3sconf/202457701008
184
Zhang J. X. Ran Z. S. Xie H. X. Kong F. Zhang M. Q. Zhou Y. et al . (2023). A systematic analysis and evaluation of nutritional composition of 23 strains of marine microalgae commonly used in aquaculture. Algal Res.72, 103122. doi: 10.1016/j.algal.2023.103122
185
Zhang Y. Zhang Y. K. Li Z. (2022). A new and improved aquaponics system model for food production patterns for urban architecture. J. Cleaner Production342, 130867. doi: 10.1016/j.jclepro.2022.130867
186
Zhanga H. Gaoa Y. Liua J. Lina Z. Leeb C. T. Hashimb H. et al . (2021). Recovery of nutrients from fish sludge as liquid fertilizer to enhance sustainability of aquaponics: A review. Chem. Eng.83, 55–60. doi: 10.3303/CET2183010
Summary
Keywords
microalgae, aquafeed, nutrient cycle, plant nutrition, sustainability, aquaponics
Citation
Manoharan R, Somanathan Nair C, Nishanth D, Subramanian R, Ahmed Z, Rastrelli L, Xie X-L, Ren M-Z and Jaleel A (2025) Harnessing microalgae for sustainable nutrition and ecosystem services in aquaponic systems: a blue–green approach to ecosystem health. Front. Mar. Sci. 12:1661042. doi: 10.3389/fmars.2025.1661042
Received
07 July 2025
Accepted
07 August 2025
Published
01 September 2025
Volume
12 - 2025
Edited by
Devendra Singh, Central Arid Zone Research Institute (ICAR), India
Reviewed by
Angel Llamas, University of Cordoba, Spain
Atif Khurshid Wani, Lovely Professional University, India
Updates
Copyright
© 2025 Manoharan, Somanathan Nair, Nishanth, Subramanian, Ahmed, Rastrelli, Xie, Ren and Jaleel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Jaleel, abdul.jaleel@uaeu.ac.ae
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.