Abstract
The ecology of the Greater Caribbean manatee (Trichechus manatus manatus) remains underexplored in southern Central America, particularly in Panama and Costa Rica. This study presents, for the first time, significant information about their local and regional movements, connectivity, and residence times in various wetlands. Since 2016, we have employed acoustic monitoring to track the manatee population, identifying individuals through their vocalizations. This method has been in use in Costa Rica since 2021. We identified 61 presumed individuals in Panama and 49 in Costa Rica, using calls that contained squeak, hi-squeak, and a combination of squeak and hi-squeak vocalizations. Their average residence time was 1,059 days in Panama and 292 days in Costa Rica, with some individuals remaining in the wetland complex for up to 3,026 and 1,160 days, respectively, occasionally venturing into the sea for short periods. Nine individuals exhibited regional movements, with an average of 340 days between detections in the two countries. The timing of this migration was analyzed using remote sensing data (air and sea temperatures, precipitation, and wave height) during the study period, which coincided with times of high rainfall and sea levels, as well as increased air and water temperatures. The observed connectivity and residence times suggest that manatees in this region of Central America rely on wetlands for both breeding and feeding. To support the long-term conservation of this area, we propose a binational corridor for manatees, approximately 984 km in length.
1 Introduction
The Greater Caribbean manatee (Trichechus manatus manatus), a subspecies of the American manatee formerly referred to as the Antillean manatee (Mignucci-Giannoni et al., 2024), is a large, endangered herbivore that inhabits the coastal and riverine brackish environments of the Caribbean, Mexico, Central America, and northern South America (O’Shea and ‘Lex’ Salisbury, 1991; Lefebvre et al., 2001; Lacommare et al., 2008; Self-Sullivan and Mignucci-Giannoni, 2012). As megaherbivores, manatees are considered “ecosystem engineers” in marine and aquatic plant-dominated environments, as they influence community dynamics and shape ecosystem characteristics in their feeding areas (Aragones et al., 2012; Bakker et al., 2016; Wirsing et al., 2022). Although historically widespread, their current distribution is variable, with several poorly studied populations existing within this geographical range (Lefebvre et al., 2001; Castelblanco-Martínez et al., 2012; Marsh, 2012). These populations are generally small, geographically isolated, and are increasingly threatened by human activities and habitat degradation. The subspecies is listed as endangered, with an estimated population of fewer than 2,500 mature individuals and a projected 20% population decline over the next two generations (Morales-Vela et al., 2024).
Central America, including Belize, Guatemala, Honduras, Nicaragua, Costa Rica, Panama, and parts of Mexico, serves as a vital stronghold for Greater Caribbean manatees. The diverse landscape of seagrass beds, mangrove-fringed lagoons, and numerous shallow coastal wetlands with abundant aquatic plants provides essential foraging and resting habitats, crucial for supporting both survival and reproduction (Mou et al., 1990; Lacommare et al., 2008; Self-Sullivan and Mignucci-Giannoni, 2012). Simultaneously, the dynamic nature of these environments requires manatees to adopt flexible movement patterns, ensuring access to optimal resources across various seasonal cycles. In this context, understanding how manatees navigate and maintain connectivity between isolated habitat patches is essential to ensure the long-term viability of their population (Deutsch et al., 2003, 2022a, 2022b).
Previous studies in Central America have revealed complex daily foraging movements interspersed with occasional long-distance migrations (Lacommare et al., 2008; Castelblanco-Martínez et al., 2012; Deutsch et al., 2022a, 2022b; Marmontel et al., 2012; Brady et al., 2023). These studies suggest that while some individuals show high site fidelity, often returning to familiar foraging and resting sites, others exhibit more nomadic behaviors, promoting genetic exchange across larger areas. Seasonal variability considerably influences movement patterns. During the dry season, as freshwater availability and seagrass productivity decrease, manatees often congregate in sheltered wetlands and near river mouths, protecting them from salinity and temperature extremes. In contrast, the wet season allows broader dispersal, with individuals traversing a network of interconnected habitats in search of more productive feeding grounds (Deutsch et al., 2003, 2022a). Such seasonal migration represents an essential survival strategy that balances the advantages of foraging in optimal areas with the need to avoid potentially hazardous conditions, including high-traffic zones or polluted waters (Castelblanco-Martínez et al., 2012; Deutsch et al., 2022a, 2022b).
The intertwined phenomena of connectivity, migration, residence time, and habitat use create a complex framework that underpins the spatial ecology of the Greater Caribbean manatees. This framework is crucial for understanding population dynamics, assessing conservation risks, and developing management strategies that address both local and regional threats (Marsh, 2012; Ordoñez-Nieto et al., 2024; Meirelles et al., 2024).
Passive acoustic monitoring has enabled long-term assessment of manatee populations through vocalization parameters and different analytical techniques (Merchan et al., 2019; Schneider et al., 2024). However, vocalization parameters cannot be compared across studies because recordings are obtained from captive or wild animals and under different instrumental settings. Average vocalization rates are one to two times per 5-minute period (Phillips et al., 2004). Manatees produce unique and complex vocalizations that are individually distinctive with considerable variations in various acoustic parameters, including fundamental frequency, emphasized bandwidth, frequency range, and call contour (Alicea-Pou, 2001; Sousa-Lima et al., 2002, 2008; Merchan et al., 2019; O’Shea and Poché, 2006). This distinctive acoustic characteristic, classified by Brady et al. (2020, 2022) into five wild-call categories (e.g., squeak, squeal, high squeak, chirp, and squeak-squeal) or by Schneider et al. (2024) into four captivity-specific call categories (e.g., squeak, squeal, high squeak, squeak-squeal, and mixed), is believed to support recognition, particularly in mother-calf bonding and maintaining broader social cohesion within social groups, as well as population size estimates (Sousa-Lima et al., 2002; Umeed et al., 2018; Brady et al., 2020, 2022; Schneider et al., 2024; Hodson, 2025). Research also suggests that slight variation in the frequencies of each manatee’s vocalization enables individual identification using noninvasive acoustic methods (Schneider et al., 2024), even considering age and sex differences in call categories and frequency (Williams, 2005). Furthermore, studies of visually identified wild Florida manatees have shown that some individuals’ vocalization parameters within individuals are not consistent or stable over time or may vary under conditions of alarm or noise (Williams, 2005; Miksis-Olds and Tyack, 2009). However, other acoustic features of manatees recorded over 1–3 years, 19 years, and 22 years remained stable across different time spans in 79-82%, 47%, and 33%, respectively, with some differences explained by sex and age group (Williams, 2005). Individuals recognize one another through sound, as females and their calves selectively respond to each other when reuniting with a group (O’Shea and Poché, 2006). Although age and sex may result in variations in class and rate of vocalizations (males vocalize less frequently), individual vocal patterns remain relatively stable over extended periods, sometimes lasting several years (Williams, 2005; Sousa-Lima et al., 2008; Umeed et al., 2018; Dietrich et al., 2022; Schneider et al., 2024). Acoustic monitoring represents a promising non-invasive methodology for identifying specific manatees, contributing to more accurate population estimates and home ranges (Merchan et al., 2019; Schneider et al., 2024).
The present study examined, for the first time, residence time and connectivity among a population of manatees in southern Central America (Panama and Costa Rica) using individual vocalizations of wild manatees without visual identification or captivity conditions (Williams, 2005; Dietrich et al., 2022; Schneider et al., 2024), which has been acoustically monitored since 2016 (Merchan et al., 2019, 2020, 2024). This is not an attempt to estimate population size (sensuSchneider et al., 2024). This novel approach provides a challenging quantitative alternative to long-term visual observations, which are often hindered by poor visibility and the brackish water conditions typical in tropical wetlands where manatees are year-round observed feeding and breeding (Guzman and Condit, 2017; Castelblanco-Martínez et al., 2018; Corona-Figueroa et al., 2021; Factheu et al., 2023; Merchan et al., 2024). Addressing these questions through an integrated, multidisciplinary approach provides valuable insights into the spatial strategies employed by the Greater Caribbean manatees in this region. More importantly, it highlights conservation priorities that are critical for preserving both the species and their habitats, as well as the overall integrity of Central America’s coastal ecosystems (Castelblanco-Martínez et al., 2012).
2 Materials and methods
2.1 Study area and climate conditions
The present study was conducted within four protected areas: San San Pon Sak (09°31’0.92” N; 82°30’27.10” W) and Damani-Guariviare (08°56’36.60” N; 81°43’10.53” W) in Panama, and Barra del Colorado (10°48’27.42’ N; 83°36’44.81” W) and Tortuguero (10°32’16.47” N; 83°30’21.10” W) in Costa Rica. This area covers approximately 305 km and was monitored through an acoustic network encompassing 18 protected areas, including four wetland Ramsar sites of international importance: Caribe-Norteste, Gandoca-Manzanillo, San San-Pond Sak, and Damani-Guariviare (Figures 1, S1). The Caribbean coast comprises natural wetlands that have historically been interconnected for various developmental purposes. Overall, both countries have similar wetland habitats that are suitable for the feeding and breeding of manatees, along with similar aquatic vegetation and brackish water conditions typical of Central American tropical wetlands. However, the physical geography of the regions is quite different and may influence the soundscape; habitats in Costa Rica are dominated by large rivers, broader linear channels, and sizable lagoons, whereas in Panama, rivers are winding, narrow, and contain fewer small lagoons.
Figure 1
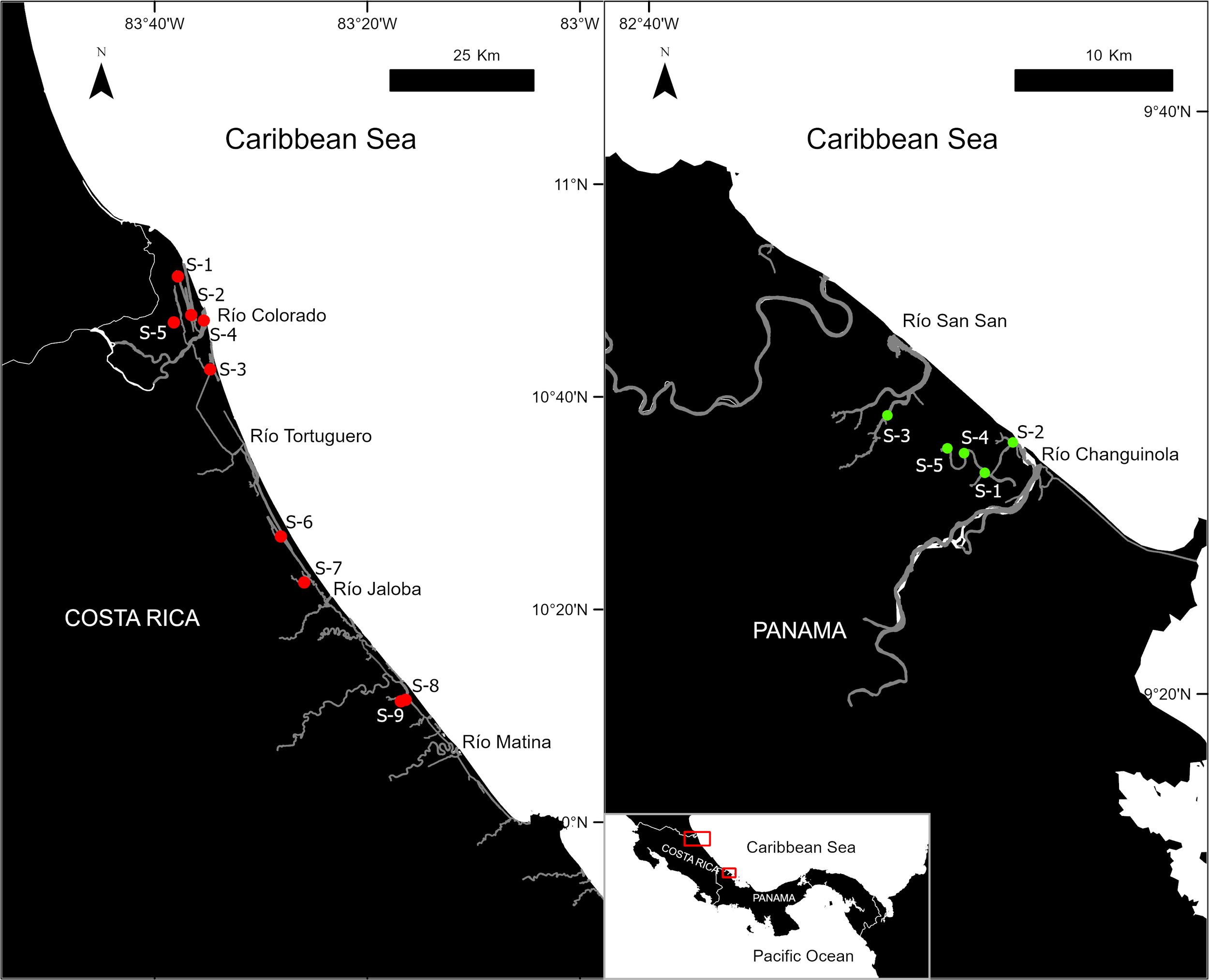
Acoustic monitoring network along 200 km of Costa Rica and Panama coastline. Colored dots indicate the locations of deployed hydrophones. The most important rivers are shown.
In Panama, the protected area consists of artificial channels constructed and dredged by a banana company in the early 1900s for navigation and plantation drainage (Stephens, 2002). These channels have been expanded since 1964 for river navigation, linking rivers, lagoons, and canals (Stephens, 2002). In Costa Rica, all rivers from Barra del Colorado to Moín are connected by a 112 km channel system, 50–150 m wide, considered one of the longest in Central America (Aguilar and Peytrequín Gómez, 2020). This entire binational network of “artificial” wetlands has evolved for decades into a natural ecosystem that today supports rich biodiversity. It consists of interconnected coastal and inland lagoons, channels, rivers, and streams, as well as palm swamps, marshes, and seagrass beds in the coastal areas. These are generally no deeper than 10 m and can be as shallow as 0.5 m in some areas during certain seasons.
Overall, the Caribbean coasts of Costa Rica and Panama have humid tropical climates, characterized by consistently high temperatures and significant rainfall throughout the year, with no distinct dry season. The climate is influenced by trade winds and moisture transport from the Caribbean Sea, resulting in consistent precipitation patterns (Orozco-Montoya and Penalba, 2022). Still, some unique conditions are specific to each country along the coastal areas. On Costa Rica’s Caribbean coast, particularly in the Moist Tropical Caribbean Region (MTCR), rainfall occurs throughout the year, peaking from June to August (JJA) and from December to February (DJF), accounting for over 70% of the annual precipitation. In contrast, March–April and September–October are relatively drier months, with rainfall dropping below 100 mm each month. The Caribbean Low-Level Jet (CLLJ) plays a crucial role in moisture transport, particularly during El Niño events, which typically result in increased rainfall in most months (Quesada and Waylen, 2020; WBG, 2021; Orozco-Montoya and Penalba, 2022). Herrera (1986) characterized northern Costa Rica up to the Matina River (see Figure 1) as hot and extremely humid, with the absence of a distinct dry season. In contrast, the southern region is warm and humid, experiencing a short dry season. Vargas (2001) reported a total annual rainfall of 5,420 mm (ranging from 285 mm in March to 635 mm in December) for northern Costa Rica (Barra del Colorado), 3,915 mm (ranging from 205 mm to 445 mm) for southern Costa Rica (Limon), while Herrera (2016) reported slightly values of 3,000 to 6,000 mm and 1,800 to 3,500 mm for northern and southern Costa Rica, respectively. The Caribbean coast of Panama experiences similar climatic conditions, characterized by high humidity and frequent rainfall, similar to southern Costa Rica. The topography of the region, including coastal plains and mountain ranges, influences local precipitation variability. The El Niño-Southern Oscillation (ENSO) substantially impacts rainfall distribution, with El Niño events often leading to increased precipitation owing to intensified moisture convergence (Kusunoki et al., 2019; WBG, 2021a, 2021b; Orozco-Montoya and Penalba, 2022). Vargas (2001) reported a total annual rainfall of 5,335 mm (ranging from 135 mm to 510 mm) for western Panama (Changuinola). Both Costa Rica and Panama are highly vulnerable to climate change, particularly rising temperatures, altered precipitation patterns, and extreme weather events (WBG, 2021a).
2.2 Acquisition of manatee vocalizations
Starting in September 2015 in Panama and November 2020 in Costa Rica, hydrophones have been installed at multiple locations along the Caribbean coast of both countries, establishing an acoustic monitoring network that includes up to 15 instruments primarily located in protected areas (Figure 1, Supplementary Table S1). The site selection was based on previous surveys where manatee common foraging areas were identified through feeding marks. The average distance between hydrophones in Barra del Colorado, Costa Rica (S1, S2, S3, S4) was 12.73 km. In Tortuguero, Costa Rica (S6 and S7), the average distance between hydrophones was 9.82 km, while in Pacuare, Costa Rica (S8 and S9), the distance was 0.98 km. In Changuinola, Panama, the average distance between hydrophones (S1, S2, S4, and S5) was 4.40 km. Hydrophone models SM3M, manufactured by Wildlife Acoustics (Maynard, MA), and SoundTrap STD-600, manufactured by Ocean Instruments New Zealand (Auckland, NZ), were installed at depths of up to 3 m along the river and canal margins. Regarding the sensitivity of the recording systems, the SM3M hydrophone has a nominal sensitivity of –165 dB re 1 V/µPa, whereas the ST600, when operated in its high-gain configuration, exhibits an end-to-end system sensitivity of –176 dB re 1 µV/µPa. The term high gain refers specifically to one of the two selectable configuration modes available in the ST600 (high gain and low gain).
The hydrophones’ duty cycle was programmed to record 2-minute audio clips at intervals of every 8 or 10 minutes, with a sampling frequency set at 96 kHz, during over a 4-month period of continuous deployment, with batteries and memory cards serviced quarterly. Supplementary Table S2 shows the initial deployment, redeployment, and retrieval dates for maintenance at each recording station during the study period. Equipment loss prevented some stations from operating year-round during the study period; some were replenished or relocated as needed.
2.3 Acoustic data processing
Recordings were processed following the general framework of Merchan et al. (2019, 2020, 2024), structured initially into four stages: detection, denoising, classification, and clustering. In the present study, this workflow was modified in two ways: (i) an initial denoising stage was applied prior to detection to enhance the robustness and accuracy of vocalization detection, and (ii) an additional denoising step was incorporated as a preprocessing stage for clustering, depending on the noise levels of the recordings. The first three stages (detection, denoising, and classification) were designed to identify and extract manatee vocalizations, producing a curated dataset suitable for subsequent clustering based on acoustic similarity.
Analyses were carried out on two computational platforms: (i) a server (Intel Xeon, 128 cores, 256 GB RAM) used for denoising, detection, and classification, and (ii) a workstation (AMD Ryzen 5950X 16-core processor, 128 GB RAM, NVIDIA GeForce RTX 3080 GPU with 8 GB VRAM, running WSL2 with Ubuntu 22.04.1) which was used for denoising, detection, classification, and clustering. In total, 1,130,407 two-minute audio files from Panama (37,680.23 hours, equivalent to 1,570 days of recordings) and 800,672 files from Costa Rica (26,689.07 hours, equivalent to 1,112 days) were analyzed. A detailed breakdown of the sampling distribution by recording station and year is provided in Supplementary Table S2.
2.3.1 Denoising
All recordings were first processed with a 2 kHz high-pass filter to remove low-frequency noise. Depending on the noise levels present in each sample, additional denoising was applied using Wiener filtering or spectral subtraction (Xie et al., 2021). This denoising stage was carried out prior to detection in order to improve its accuracy and robustness.
2.3.2 Detection
Candidate vocalizations were detected using a simplified version of the ACF-RMS method (Merchan et al., 2019). In this approach, ACF-RMS refers to the use of the autocorrelation function (ACF) combined with the calculation of root mean square (RMS) values over the autocorrelation curve. Harmonic or periodic signals, typical of manatee calls, exhibit slower autocorrelation decay than noise-like signals, allowing them to be flagged as potential vocalizations. In the original implementation, which included subband wavelet analysis and heuristic rules, true positive rates (TPR) of up to 0.74 and false discovery rates (FDR) as low as 0.20 were reported, depending on dataset conditions and detection rules. In the present study, the detector was simplified to prioritize sensitivity by omitting the subband and rule-based stages, but it retained the same analysis window of 2000 samples and the lag range of 20–200 used for RMS calculation. Targeted filtering was also applied using two sub-bands—2–6 kHz and above 10 kHz—explicitly configured to minimize interference from broadband noise of undetermined origin reported in Panama recordings (Merchan et al., 2019), particularly within the 6–10 kHz range, which can otherwise lead to false positives.
2.3.3 Classification
The denoised ACF-RMS outputs identified as possible vocalizations were then fed into a convolutional neural network (CNN). This two-phase process (ACF-RMS detection followed by CNN classification) reduces computational time by filtering candidate segments before spectrogram analysis. In Merchan et al. (2020), pyramidal CNN architectures achieved accuracies between 92–98% under different database variants (rivers, noise conditions, and call types). Building on that framework, in the present study, we adopted a more efficient MobileNet architecture with transfer learning (Howard et al., 2017). For each detected signal, a spectrogram was computed using an FFT of 512 points with 50% overlap and zero-padding, yielding matrices of 257×150 pixels. These spectrograms were subsequently binarized, resized, and stacked to generate 224×224×3 image representations compatible with MobileNet input requirements. The model was fine-tuned using k-fold cross-validation (80/20 split, no data augmentation) in TensorFlow 2.9, and was trained on a more diverse and comprehensive audio dataset that included a wider variety of environmental sounds. All outputs underwent manual curation, consisting of visual inspection of spectrograms to discard false positives such as bird calls with manatee-like spectral features.
2.3.4 Clustering and parameter configuration
This stage builds upon the clustering methodology described in Merchan et al. (2024), which was validated under both simulated and empirical conditions. In that study, recordings were combined to emulate different numbers of individuals and vocalization counts, demonstrating the robustness of the approach. The algorithm achieved a mean estimation error of 14.05% in predicting the number of individuals and an assignment accuracy of 83.75% in mapping vocalizations to their source. When applied to the dataset of 23 captured manatees, the model estimated 24 individuals, showing strong agreement between predicted and actual groupings. These results established the baseline performance that the present work adopts and extends.
Consistent with the structure of Merchan et al. (2024), the clustering pipeline was organized into five stages: preprocessing, feature extraction, dimensionality reduction, clustering, and validation, with additional refinements incorporated at the preprocessing step to improve spectral definition and cluster separability.
-
Preprocessing – Signal Subspace and spectral refinement: Noisy recordings were processed with a Signal Subspace Denoising algorithm (Jensen et al., 2005). A hybrid post-processing step then combined the Medial Axis Transform with the Canny edge detector (threshold = 25) to refine spectral representations.
-
Feature extraction – Scattering Wavelet Transform (SWT): SWT was applied to capture stable time–frequency representations of vocalizations. Parameters were set to Q = 128 (number of wavelets per octave, determining frequency resolution) and J = 7 (number of scattering scales, corresponding to the depth of the multiresolution decomposition). In this implementation, complex Morlet wavelets were used as the filter bank.
-
Dimensionality reduction – PaCMAP: Features were embedded into a five-dimensional space (output dimension = 5) using PaCMAP, with the number of nearest neighbors determined automatically.
-
Clustering – HDBSCAN: Clustering was performed with HDBSCAN using minimum cluster size = 7, 8, 10 and minimum samples = 7, 8, 10 (tuned according to dataset size and noise level). Euclidean distance was used as the metric, and the “leaf” cluster selection algorithm was applied.
-
Validation – CDbw index: Cluster validity was assessed with the CDbw index, which considers both compactness and separation. Solutions with CDbw ≥ 10 were retained.
For the core algorithms of the clustering methodology presented in Merchan et al. (2024), we utilized the following open-source repositories: PaCMAP (Wang et al., 2021), HDBSCAN (McInnes et al., 2017), and Kymatio (Andreux et al., 2020a). Examples of clustering outcomes obtained with this pipeline are shown in Figure 2, illustrating results from both the controlled dataset of Merchan et al. (2024) and the present study. The complete set of parameter configurations applied in the stages of the acoustic processing workflow is summarized in Supplementary Table S3.
Figure 2
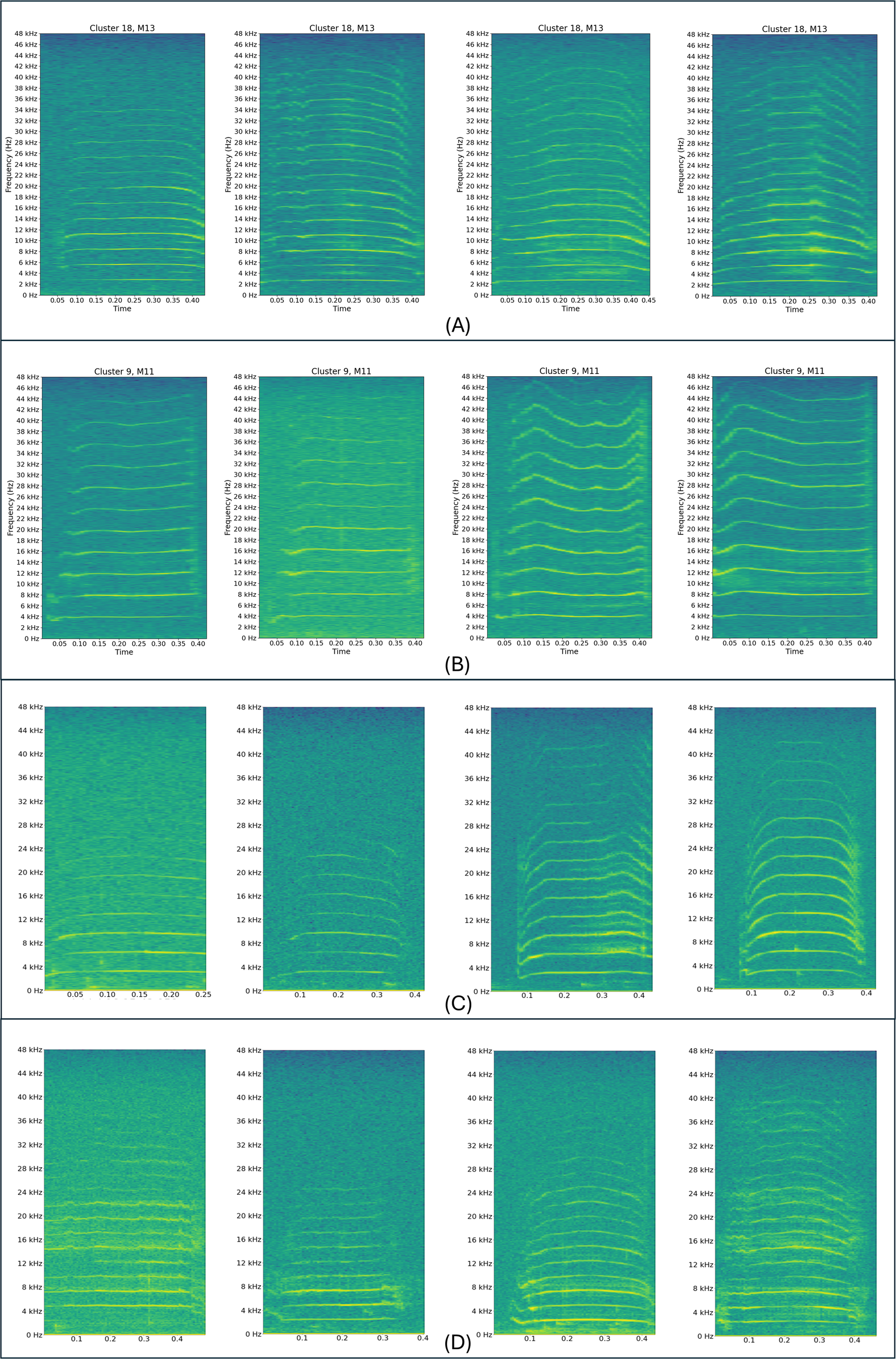
Panels (A) and (B) display spectrograms of manatee vocalizations from the clustering analysis conducted in Merchan et al. (2024). Panel (A) shows spectrograms corresponding to vocalization classes from manatee M13 (cluster 18), and Panel (B) from manatee M11 (cluster 9). In both cases, the vocalizations exhibit variability in contour and structure yet are consistently grouped by the clustering algorithm. Panels (C) and (D) present spectrograms of vocalizations from the current residency study in Panama. Panel (C) corresponds to vocalizations labeled as manatee P63, and Panel (D) to manatee P15, as determined by the clustering stage. The method effectively groups vocalization classes with both flat-like contours ("squeaks") and hill-shaped contours ("hi-squeaks"), based on similarity and correlation in their harmonic components.
2.3.5 Cluster fusion and revision
To address the over-segmentation observed in preliminary results (Merchan et al., 2024), a cluster fusion procedure was implemented as a post-processing step. For each cluster, a mean SWT representation was computed, and cosine distances between cluster means were calculated. Clusters with distances below the 10th percentile of this distribution were proposed for merging. These candidates were then visually inspected, confirming merges only when spectral contours were highly similar and fundamental frequency values, estimated with the YIN algorithm (De Cheveigné and Kawahara, 2002), closely matched. A global manual revision was subsequently conducted to ensure overall consistency and signal quality. During this process, clusters were discarded if they contained ambiguous acoustic content, broadband noise that obscured harmonic structures, or bird sounds with spectral characteristics resembling manatee calls. After this step, the number of clusters was reduced from 82 to 63 in Costa Rica and from 141 to 88 in Panama, retaining 533 and 1,332 vocalizations, respectively. This additional curation reduced cluster fragmentation and increased dataset reliability by retaining only those clusters with clear, structured, and biologically interpretable vocal patterns.
2.3.6 Joint clustering
Running the full SWT–PaCMAP–HDBSCAN pipeline on the combined dataset (>30,000 vocalizations) exceeded the capacity of a workstation equipped with an AMD Ryzen 5950X CPU, 128 GB RAM, and an NVIDIA RTX 3080 GPU (Ubuntu 22.04, Python/TensorFlow 2.9), resulting in processing failures and excessively long runtimes. In contrast, the pipeline could be applied successfully to each country’s dataset independently (26,787 vocalizations from Panama; 4,141 from Costa Rica), and the resulting clusters were subsequently compared and manually matched across countries. During manual validation, clusters with high internal variability, low signal power, or unreliable spectral structure were excluded. In cases where visual inspection revealed uncertainties—such as differences in power levels or inconsistencies in spectral features—the cluster was conservatively discarded. This approach retained only clusters with high internal consistency, strong signal power, and well-defined harmonic contours, primarily corresponding to squeaks and hi-squeaks. Representative examples of clusters conservatively excluded during this stage are provided in Supplementary Figure S2 of the Supplementary Material.
2.3.7 Cluster categorization and selection for analysis
After clustering, all resulting clusters were visually inspected using spectrograms to verify the predominant vocalization classes present. Each cluster was then labeled into one of several categories: clusters dominated by squeaks, clusters dominated by hi-squeaks, clusters containing both squeaks and hi-squeaks (i.e., clusters in which the two call types co-occur, not a single hybrid vocalization), clusters dominated by squeals, and clusters with other combinations. For the residence time analysis, only clusters categorized as squeaks, hi-squeaks, or squeak/hi-squeak mixes were retained, as these categories provide the most reliable basis for individual-level identification (Supplementary Table S4). This filtering resulted in 49 clusters out of 63 in Costa Rica and 61 clusters out of 88 in Panama being included in subsequent analyses. The rationale for this selection, and its implications compared to previous approaches (e.g., Schneider et al., 2024), is further addressed in the Discussion.
2.4 Environmental data processing
To analyze the environmental conditions related to manatee movement patterns between Panama and Costa Rica, we focused on periods of migration and non-migration. We defined migration periods as times when manatees moved between Panama and Costa Rica, or were detected in both countries, and non-migration periods as times when they remained resident within a single country. In particular, we examined the precipitation patterns, air temperature anomalies, sea surface temperature (SST), and sea level anomaly during these periods. All environmental datasets were obtained from the NASA Earthdata portal via the Giovanni online data system, as mosaics corresponding to the study area. The spatial extent for environmental data extraction was defined by a polygon encompassing the locations of the hydrophones deployed along the Caribbean coasts of Panama and Costa Rica (see Section 2.2 and Figure 1), within the acoustic monitoring network used in this study.
Precipitation data were obtained from the Global Precipitation Measurement (GPM) Integrated Multi-satellite Retrievals for GPM (IMERG) Final Run Version 07 dataset (GPM_3IMERGDF v07), which provides daily mean precipitation estimates with a spatial resolution of 0.1° × 0.1°, spanning the years 2020–2024. These data were aggregated into biweekly (15-day) intervals to correspond with the temporal scale of the observed manatee movement. Air temperature anomalies were evaluated using the Heatwave Magnitude dataset (M2SMNXEDI v2), which reports average 2-meter temperature anomalies. In this dataset, anomalies are defined relative to the 1991–2020 climatological baseline, with daily percentiles computed using a ±7-day moving window and calculated as the difference between daily temperature and its corresponding climatology. This dataset provides monthly global data at a resolution of 0.625° × 0.5°from 2020 to 2024. Sea surface temperature data were obtained from the MODIS Aqua Level 3 SST Thermal IR Monthly 9 km Daytime Version 2019.0 (MODISA_L3m_NSST_Monthly_9km vR2019.0), which provides monthly SST data at a spatial resolution of 0.083° × 0.083°. Finally, sea level anomaly data were obtained from the Global Ocean Gridded L4 Sea Surface Heights and Derived Variables Reprocessed dataset (SEALEVEL_GLO_PHY_L4_MY_008_047), which provided daily SSH data at a resolution of 0.125° × 0.125°.
All the spatial data were processed using ArcGIS Pro (version 3.5). The datasets were re-projected onto a standard coordinate system to ensure spatial consistency. For each variable, temporal subsets corresponding to periods of manatee migration and non-migration were generated. The “Raster to Point” tool was utilized to extract pixel values at predefined sampling sites for each period and environmental layer, facilitating direct comparison across locations and timeframes. Subsequently, the environmental data extracted at each sampling location were analyzed using R (version 4.4.2). Boxplots were generated using the “ggplot2” package (Wickham, 2016) to visualize differences in ecological conditions between the migration and non-migration periods. Before hypothesis testing, the assumptions of normality and homoscedasticity were assessed using the Shapiro-Wilk test for normality and Levene’s test for equality of variances. No variables met the assumptions of normality or homoscedasticity. Consequently, the distribution of each environmental variable between the migration states was compared using Wilcoxon rank-sum tests. This statistical framework enabled a robust, non-parametric comparison of the environmental conditions associated with manatee movement.
To further investigate the spatial patterns of manatee space use, Kernel Density Estimation (KDE) was employed to analyze the distribution of vocalization events recorded by hydrophones across the river systems of Costa Rica and Panama. The analysis was performed using ArcGIS Pro (version 3.4), with vocalization events as the input data. These events were first converted into point features based on their geographic coordinates. KDE was conducted using the planar method, with a fixed search radius of 0.05 and an output cell size of 0.0001. Population fields were not used for the estimation. The resulting density surfaces provide a continuous spatial representation of vocalization intensity, highlighting areas of recurrent use and allowing the identification of potential core areas within each country’s monitored river systems.
3 Results
Clustering was conducted separately for the two datasets: Costa Rica, with 4,141 vocalizations, and Panama, with 26,787 vocalizations. The initial results yielded 82 clusters for Costa Rica and 141 for Panama. After merging overlapping groups and performing a global manual revision, the totals were reduced to 63 and 88 clusters, respectively. These clusters were then examined to determine the predominant call types they represented (Supplementary Table S4). In line with the criteria for residence time analyses, only those groups characterized by squeaks, hi-squeaks, or a mixture of both were retained. The latter category does not represent a hybrid vocalization but rather clusters in which both call types co-occur. On this basis, we identified 49 presumed individuals in Costa Rica (343 vocalizations) and 61 presumed individuals in Panama (1,012 vocalizations). The reduction in vocalization count primarily reflects the conservative nature of the HDBSCAN algorithm, which excludes outliers that do not form dense clusters, thereby enhancing the reliability of individual-level assignments.
As a result of the joint analysis and the application of the validation criteria, nine clusters were identified that potentially correspond to presumed individual manatees vocalizing in both Panama and Costa Rica (Supplementary Table S5). These findings provide novel insights into the potential for transboundary movements and support the hypothesis of regional population connectivity among West Indian manatees in this part of Central America.
Consequently, a total of 61 manatees were recorded along the Caribbean coast of Panama between December 25, 2015, and August 4, 2024, while 49 individuals were acoustically detected in Costa Rica between May 12, 2021, and August 28, 2024. Among these, nine presumed individuals were identified as cross-border animals and were detected in both countries after traveling approximately 200 km at different times. To facilitate cross-site comparisons, these individuals were labeled B1 through B9, each representing a match between a detection in Costa Rica (CR ID) and the corresponding detection in Panama (P ID). The details of all detected individuals, including the matched IDs for cross-border cases, are provided in Supplementary Table S5.
3.1 Manatee local and large-scale movement
Manatee vocalization detections showed significant spatiotemporal variations across both Panama and Costa Rica during the monitoring period. In Costa Rica, manatees were consistently detected throughout the study, with peaks in vocalization frequency in mid-2021, early 2022, and again in 2024. Stations S1 and S2 had the highest number of vocalizations over several months, with more than 12 identified individuals (Figure 3A). In contrast, detection patterns in Panama were sporadic. After the initial vocalizations in 2016 and 2018, there was a considerable gap in activity until December 2019, primarily attributed to data loss during that period. The highest number of vocalizations was noted in late 2016, late 2017, early 2018, early 2021, and early 2022. Notably, 17 individuals were identified in April 2021. Remarkably, the number of individuals detected often varied independently of the total number of vocalizations, suggesting variable residence times or differing movement dynamics at each site (Figure 3B).
Figure 3
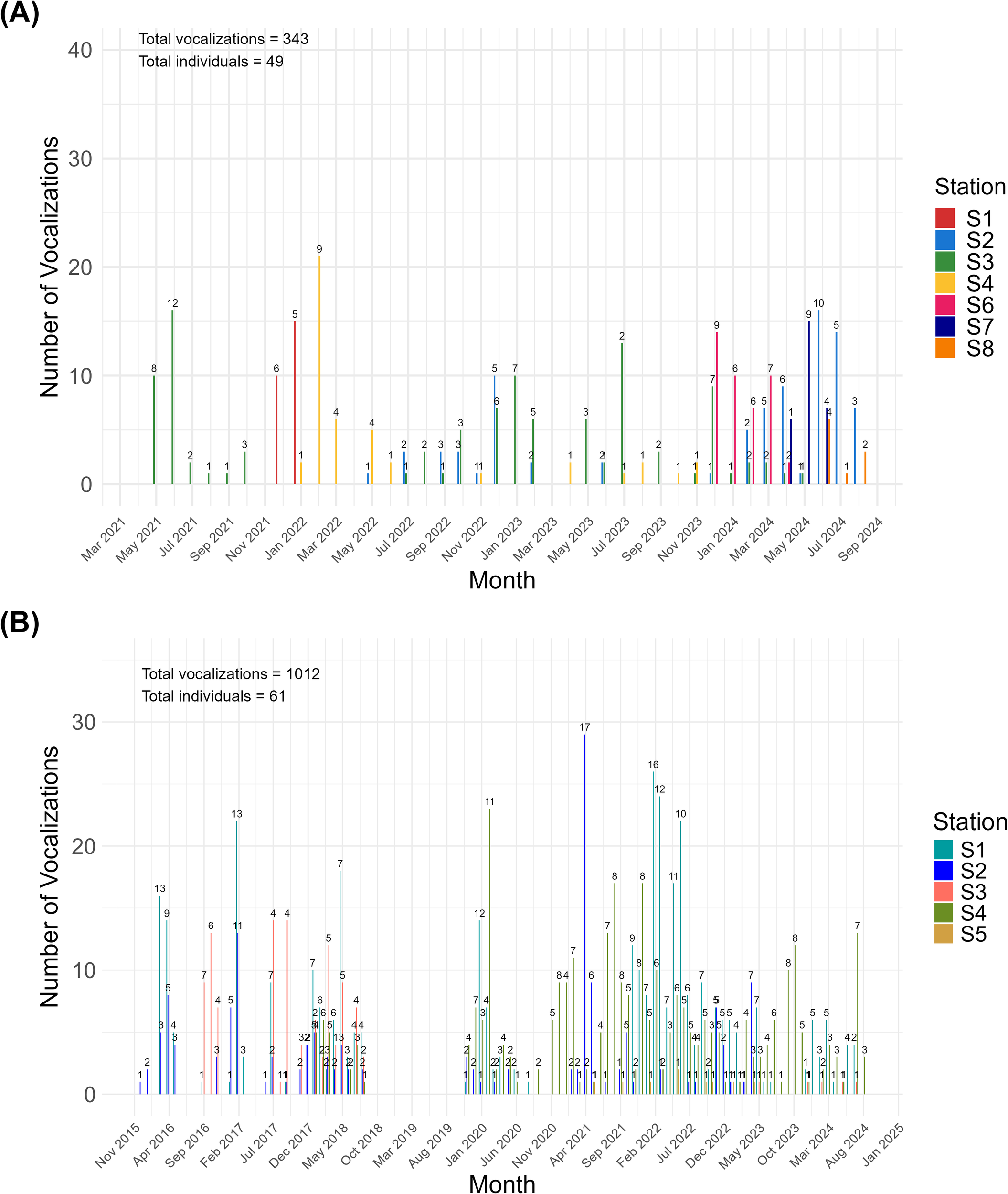
Temporal distribution of manatee vocalizations retained after clustering at acoustic monitoring stations in (A) Costa Rica and (B) Panama, based on vocalization classes Squeaks, Hi-squeaks, Mix of Squeaks and Hi-squeaks. The numbers above each line indicate the number of presumed individual manatees detected at each detection event.
The nine presumed individual manatees detected in both countries exhibited multiple spatiotemporal recurrences spanning several years and seasons (Figure 4). For example, individual B2 was repeatedly recorded from 2016 to 2024, initially in Panama, before migrating 200 km toward Costa Rica. Similar cross-border detection patterns were observed for B1, B3, B5, B6, B8, and B9, indicating long-term site fidelity and regional connectivity. In contrast, individual B7 was detected over a shorter period but still showed movement across national boundaries. The geospatial visualization (Figure 4) highlights the vocalization locations across the hydrophone stations, further reinforcing the connection between Panama and Costa Rica. The trajectories indicate repeated use of a shared corridor or habitat patches along the Caribbean coast, rather than isolated events. Notably, this is the first acoustic evidence of long-range connectivity in the Greater Caribbean manatees between these two nations.
Figure 4
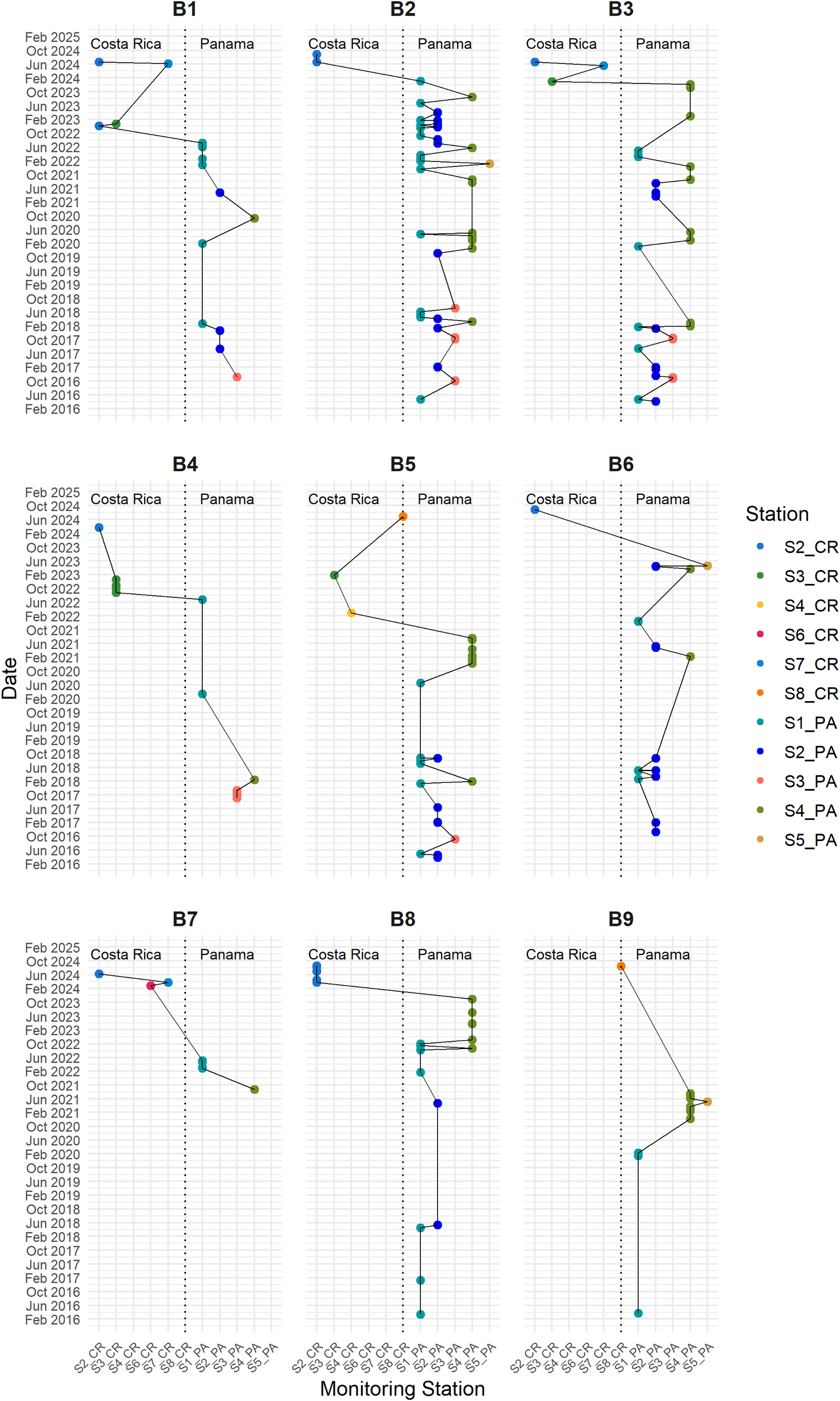
Spatial distribution of nine matched manatee (B1-B9) vocalizations along the Caribbean coasts of Costa Rica and Panama, based on vocalization classes Squeaks, Hi-squeaks, Mix of Squeaks and Hi-squeaks. Each panel represents an individual manatee.
The majority of binational manatees (e.g., B1, B2, B3, B5, B6, and B9) exhibited periods of activity (i.e., vocalization number) in both countries (Figure 5). While some individuals displayed long intervals of non-vocalization or localized movement patterns (e.g., B4 and B7), the dataset underscores strong habitat connectivity across the international border.
Figure 5
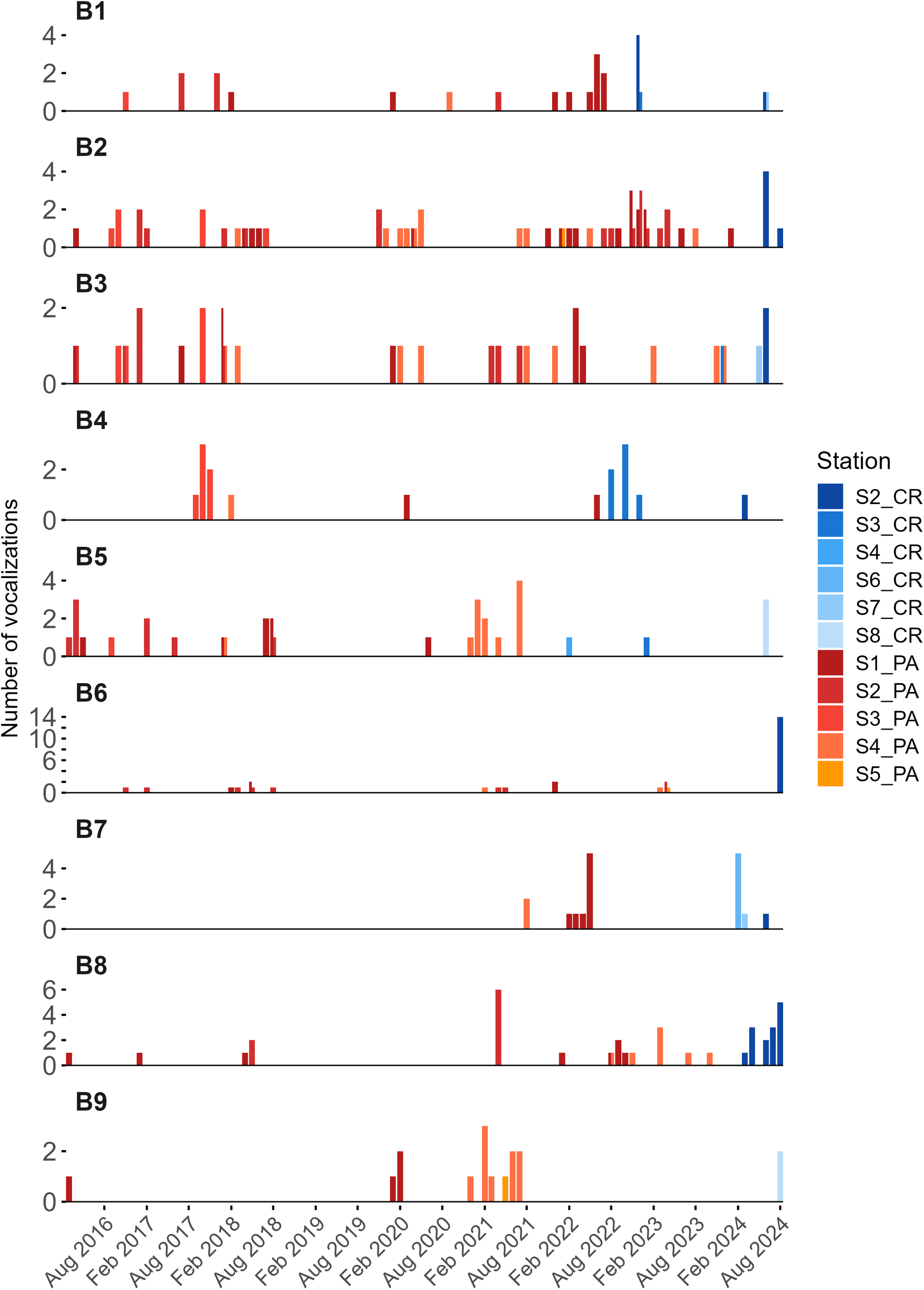
Timeline of acoustic vocalizations of nine Greater Caribbean manatees identified in Panamanian wetlands between 2016 and 2024 and later in Costa Rica, based on vocalization classes Squeaks, Hi-squeaks, Mix of Squeaks and Hi-squeaks. Each plot represents an individual (B1–B9), with bar height indicating the number of vocalizations per period and color denoting the country of detection (red orange for Panama, blue for Costa Rica).
Our results reveal distinct movement routes for manatees from Panama to Costa Rica (Figure 5). All manatees left Panama exclusively through stations in the Changuinola river system (S1, S2, S4, S5; Figure 1), highlighting a very marked directional movement pattern. In Costa Rica, most manatees entered through stations in the Barra del Colorado River system (S2, S3, S4), and only two of them used stations in Tortuguero-Pacuare (S6, S8; Figure 1). Despite some variations in entry points within Costa Rica, the Changuinola River appears to be the main and likely only exit corridor from Panama.
Furthermore, the results revealed inter-individual variation in movement range. For instance, individuals B2, B3, B5, and B6 were detected across multiple hydrophone stations more than others, such as B4 and B7, indicating broader spatial utilization. All manatees were observed to depart from Panama via the Changuinola River toward Costa Rica. In Costa Rica, several individuals likely entered the northern sector near Barra del Colorado (e.g., B1, B2, B3, B4, B5, B6, and B8). In contrast, others (e.g., B7 and B9) may have accessed the Costa Rican waters further south via the Tortuguero–Pacuare region, suggesting individual variability in movement routes and potential directional preferences along the Caribbean coastline.
3.2 Residence time and home range
The analysis of residence time revealed substantial variation across sites and countries, underscoring the dynamic habitat use of individual manatees within the transboundary region (Supplementary Figures S3, S4). The overall country average was 292.10 days and 1,059.12 days for Costa Rica and Panama, respectively (Table 1). Manatees tracked in Costa Rica between 2021 and 2024 had an average residence time of 546.50 days at the northern sites of Barra del Colorado. In contrast, much shorter and more variable residence times were observed in the southern Tortuguero-Pacuare area, with an average of 37.71 days (Table 1). In Panama, high average values were recorded for Changuinola (1,926.31 days), indicating long-term site fidelity. In comparison, residence times in San San were more variable and generally shorter (191.93 days), suggesting a more transient use of the area (Table 1). Since monitoring began in 2016 and continued through 2024 in this area, individuals demonstrated consistently longer residence times.
Table 1
| Country | Site | Residence time (days) |
|---|---|---|
| Costa Rica | Barra del Colorado | 546.50 (± 363.86) |
| Tortuguero-Pacuare | 37.71 (± 53.54) | |
| Total (Costa Rica) | 292.10 (± 363.76) | |
| Panama | Changuinola | 1,926.31 (± 944.45) |
| San San | 191.93 (± 328.86) | |
| Total (Panama) | 1,059.12 (± 1119.90) | |
| Binational | Panama | 2,202.22 (± 855.75) |
| Costa Rica | 277.05 (± 307.83) | |
| Movement time | 339.92 ( ± 361.15) |
Average residence time (median ± SD) of Greater Caribbean manatees (Trichechus manatus manatus) across monitored sites in Panama (from 2016 to 2024), Costa Rica (from 2021 to 2024), and binationally detected individuals.
Movement time corresponds to the duration between the last detection of an individual in Panama and the first detection in Costa Rica.
Binational cases, where comparative analyses were conducted using both national datasets, revealed that some individual manatees exhibited a substantial average residence time in both countries, especially in Panamanian waters (2,202.22 days), before the last detection and potential migration to Costa Rica (277.05 days). The average migration (referring to the number of days between the last detection in one country and the first detection in the subsequent country) or movement between the two countries was 339.92 days, with a maximum of 1128.82 days and a minimum of 25.24 days (Table 1). This highlights the ecological connectivity of coastal corridors and the importance of coordinating conservation efforts across borders (Table 1). A detailed breakdown of the residence time for each individual is provided in Supplementary Table S5.
Home range analyses using KDE reflect the density of vocalization events recorded by fixed hydrophone locations along the Caribbean coasts of Costa Rica and Panama (Figure 6), rather than direct animal movement patterns. In Costa Rica, the highest densities were concentrated near Barra del Colorado, particularly around sites S2–S5, with additional hotspots detected near Tortuguero (S6 and S7) and Pacuare (S8 and S9). The core areas indicated zones of recurrent vocalization that were likely associated with essential resources or preferred habitat features.
Figure 6
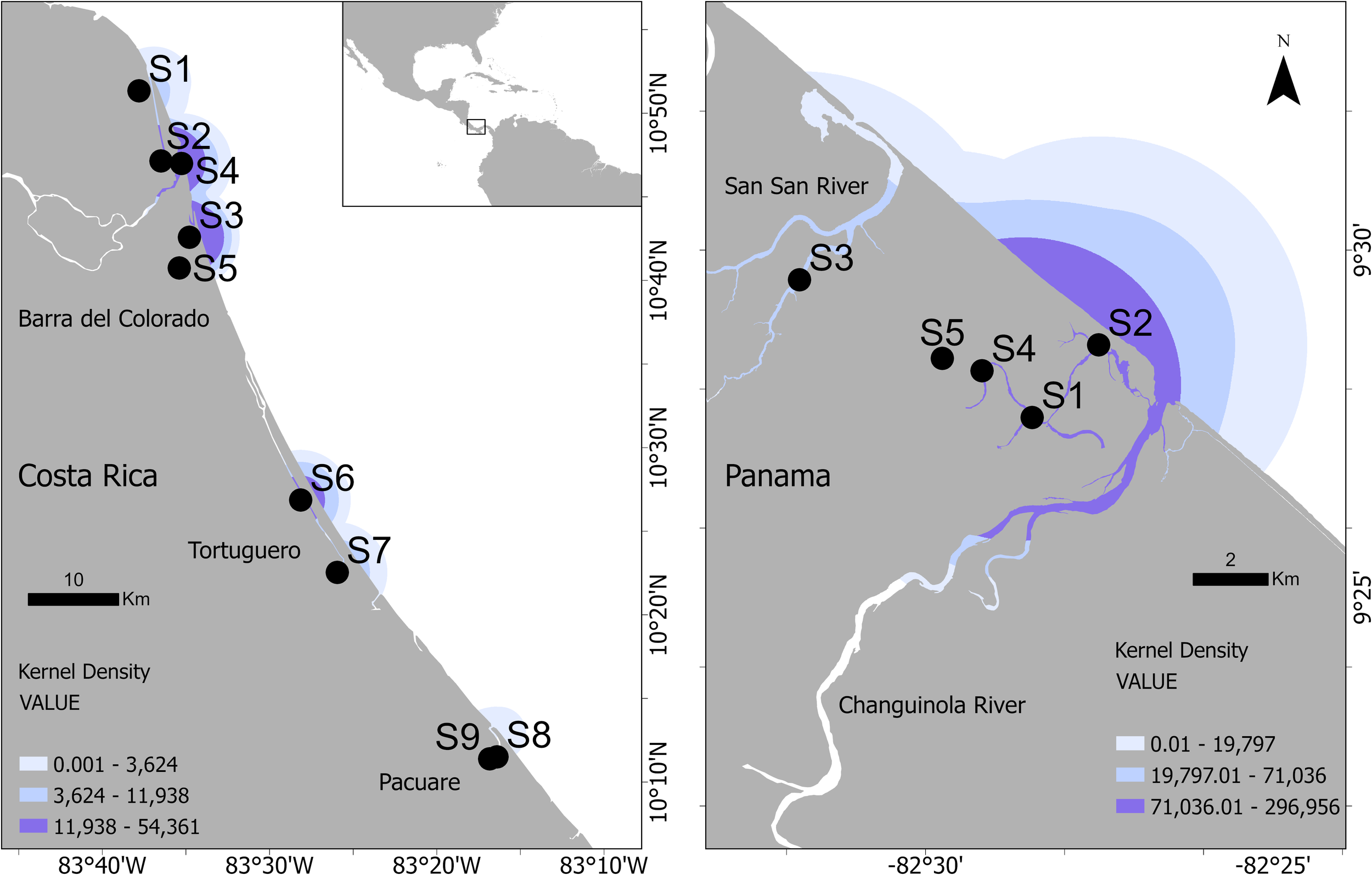
Kernel density estimation of vocalization events based on fixed hydrophone locations in coastal regions of Costa Rica (left) and Panama (right), based on vocalization classes Squeaks, Hi-squeaks, Mix of Squeaks and Hi-squeaks. Darker shades indicate areas with higher vocalization frequencies, reflecting a greater concentration of manatee acoustic vocalizations. Density values were classified into three quantiles for visual comparison.
In Panama, the largest and most intense vocalizations centers were located near the Changuinola River and adjacent coastal areas, especially around sites S1 and S2. The extent and intensity of the Panamanian vocalization ranges were notably higher than those in Costa Rica, which is consistent with previous findings of longer residence times in Panamanian waters.
The distribution of the density zones showed marked variations in terms of the area used within each site. In Barra del Colorado, high-, medium-vocalization and low-vocalization zones represented 24.53%, 12.33% and 11.49% of the total vocalization range, respectively, indicating a relatively concentrated use of space. In Tortuguero, vocalizations were more evenly distributed, with 5.79% of the area classified as high-vocalization, 21.11% and 18.8% falling into low- and medium-vocalization categories, respectively. In Pacuare, low-vocalization zones were dominant (5.95%). Panama exhibited the broadest distribution, with low-vocalization areas comprising 39.69% of the range, followed by medium- (30.72%) and high-density areas (29.59%).
3.3 Daily activity and co-occurrence
Demographically, the monitoring data included 61 manatees in Panama and 49 in Costa Rica. In Panamá, one manatee (P4) was detected at two different stations on the same day, suggesting potential site-switching behavior within a 24-hour period. This individual was first recorded at station S1 and subsequently at station S2, which was located approximately 3 km away. Manatee P4 was detected at S2 14.32 hours after its first detection at S1. Although this behavior was observed only in one individual, it is possible that site switching occurs more frequently but remains undetected due to limitations in the detection of vocalizations by hydrophones. In contrast, in Costa Rica, no manatees were recorded at more than one station on the same day. This pattern suggests that individuals remained within a single site throughout their daily activity period, with no evidence of short-term site switching in this dataset.
Manatees exhibited notable social behavior in Panama, as evidenced by 92 co-occurrence events in which two or more individuals were recorded at the same monitoring station within one hour of each other. These short-term co-occurrence intervals were distributed across the four monitored sites, with the highest number recorded at S1 (n = 38), followed by S4 (n = 27), S2 (n = 17), and S3 (n = 10). On average, 2.12 to 2.30 manatees were detected together per co-occurrence interval, with up to four individuals recorded simultaneously at specific sites (Table 2).
Table 2
| Country | Monitoring stations | Average number of manatees | Maximum number of manatees | Number of co-occurrence events |
|---|---|---|---|---|
| Panama | S1 | 2.13 | 3 | 38 |
| S2 | 2.12 | 3 | 17 | |
| S3 | 2.30 | 4 | 10 | |
| S4 | 2.11 | 4 | 27 | |
| Costa Rica | S1 | 2 | 2 | 3 |
| S2 | 2.57 | 4 | 7 | |
| S3 | 2.18 | 3 | 11 | |
| S4 | 2 | 2 | 3 | |
| S6 | 2.20 | 3 | 5 | |
| S7 | 2.33 | 4 | 6 | |
| S8 | 2 | 2 | 2 |
Recorded co-occurrence intervals (≤1 hour) with detections of two or more manatees at the same monitoring station in Panamá and Costa Rica. No co-occurrence events detected in station S5 of both countries.
Manatees also displayed social tendencies in Costa Rica, with a total of 37 co-occurrence events detected. These short-term co-occurrence intervals were distributed across seven monitored sites, with the highest number recorded at S2 (n = 7), followed by S3 (n = 11), S6 (n = 5), S7 (n = 6), S4 (n = 3), S1 (n = 3), and S8 (n = 2). The average number of individuals per co-occurrence interval ranged from 2.0 to 2.57, and up to five manatees were simultaneously detected at certain locations (Table 2).
3.4 Environmental data and migration
Different environmental variables were compared to assess their effects on the migration of manatees from Panama to Costa Rica.
In the southern zone, where migration originated, precipitation was significantly higher during migration (12.3 ± 2.14 mm) than during periods without migration (8.3 ± 1.67 mm; V = 4095, p < 0.0001, Table 3 and Figure 7). Meanwhile, in the destination area of the migration (in the north), the average precipitation during migration periods was lower (9.76 mm ± 5.95 mm), while during non-migration periods it was higher (9.90 mm ± 6.12). Air temperature anomalies also showed a significant increase during migration periods (0.97 ± 0.04 °C, Table 3 and Figure 7) compared to non-migration (0.80 ± 0.05 °C; V = 136, p < 0.0001). In contrast, SST did not differ significantly between the two behavioral states (V = 897, p = 0.8975), with very similar mean values (29.5 ± 0.46 °C during migration vs. 29.5 ± 0.55 °C during non-migration, Table 3 and Figure 8). Sea level anomaly was significantly lower during migration (0.087 ± 0.008 m, Table 3 and Figure 8) compared to non-migration (0.115 ± 0.004 m; V = 0, p < 0.0001). The Wilcoxon signed-rank test yielded a statistic of V = 0, indicating that all paired differences were negative (i.e., sea level height anomalies were consistently lower during migration periods compared to non-migration). This confirms that sea level height anomalies tend to be reduced when manatees migrate. Overall, these results suggest that migration tends to occur under specific environmental conditions, particularly those characterized by higher precipitation, warmer air anomalies, and lower sea level anomalies. The distributions of environmental values further support this during migration, which show apparent shifts compared to non-migration periods, consistent with the statistical outputs (Figure 9).
Table 3
| Variable | Migration | Non-Migration | p-value |
|---|---|---|---|
| Sea surface temperature (°C) | 29.5±0.46 | 29.5±0.55 | 0.8975 |
| Precipitation (mm) | 12.30 ± 2.14 | 8.28 ± 1.67 | < 0.0001 |
| Air temperature anomaly (°C) | 0.972 ± 0.038 | 0.804 ± 0.047 | < 0.0001 |
| Sea level height anomaly (m) | 0.087 ± 0.008 | 0.115 ± 0.004 | < 0.0001 |
Summary statistics (mean ± SD) of environmental variables during migratory and non-migratory periods, with p-values from Wilcoxon rank-sum tests.
Figure 7
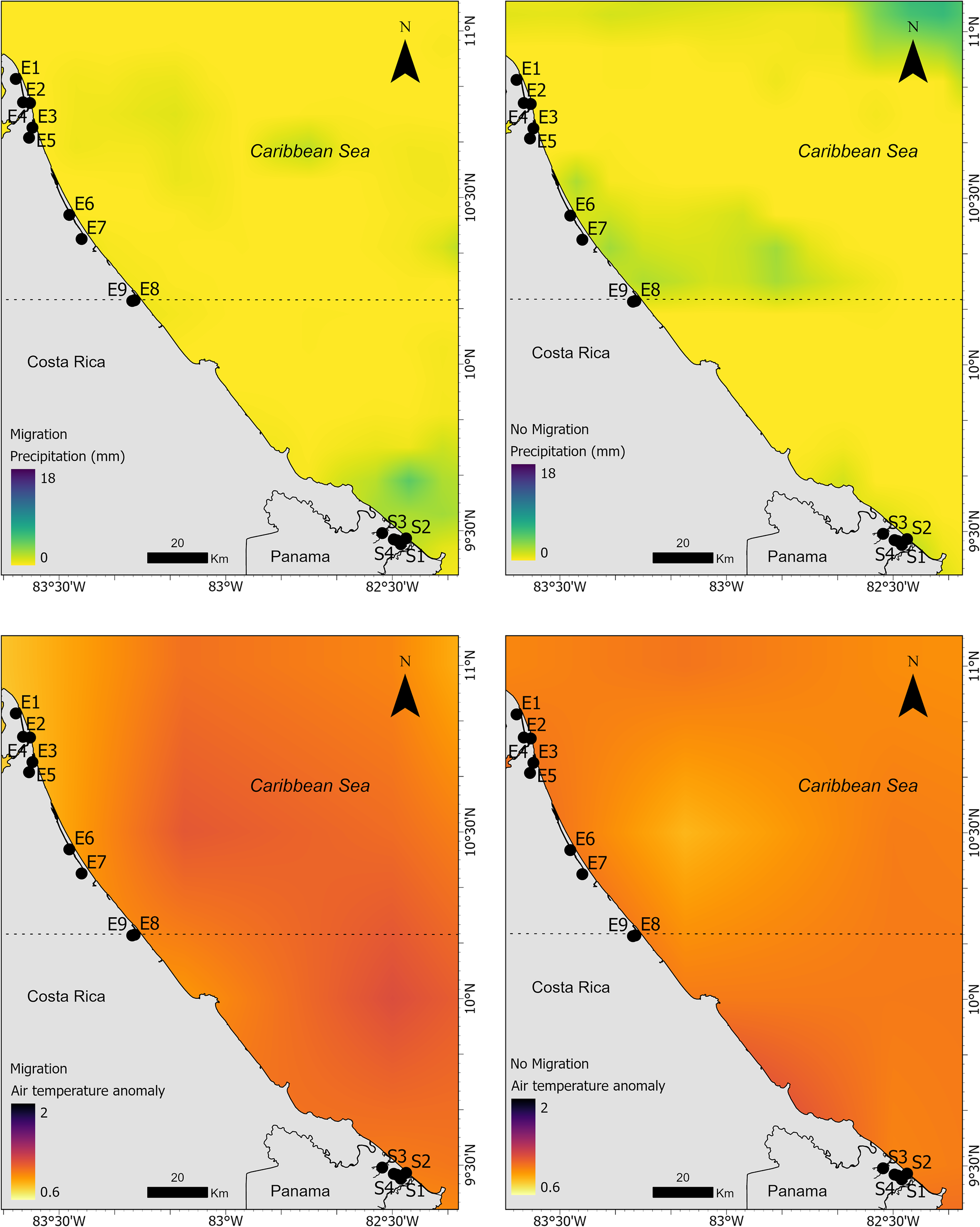
Spatial distribution of precipitation (top panels) and air temperature anomalies (bottom panels) in the Changuinola region (Panama) during periods of manatee migration (left panels) and non-migration (right panels). Black points indicate monitoring stations.
Figure 8
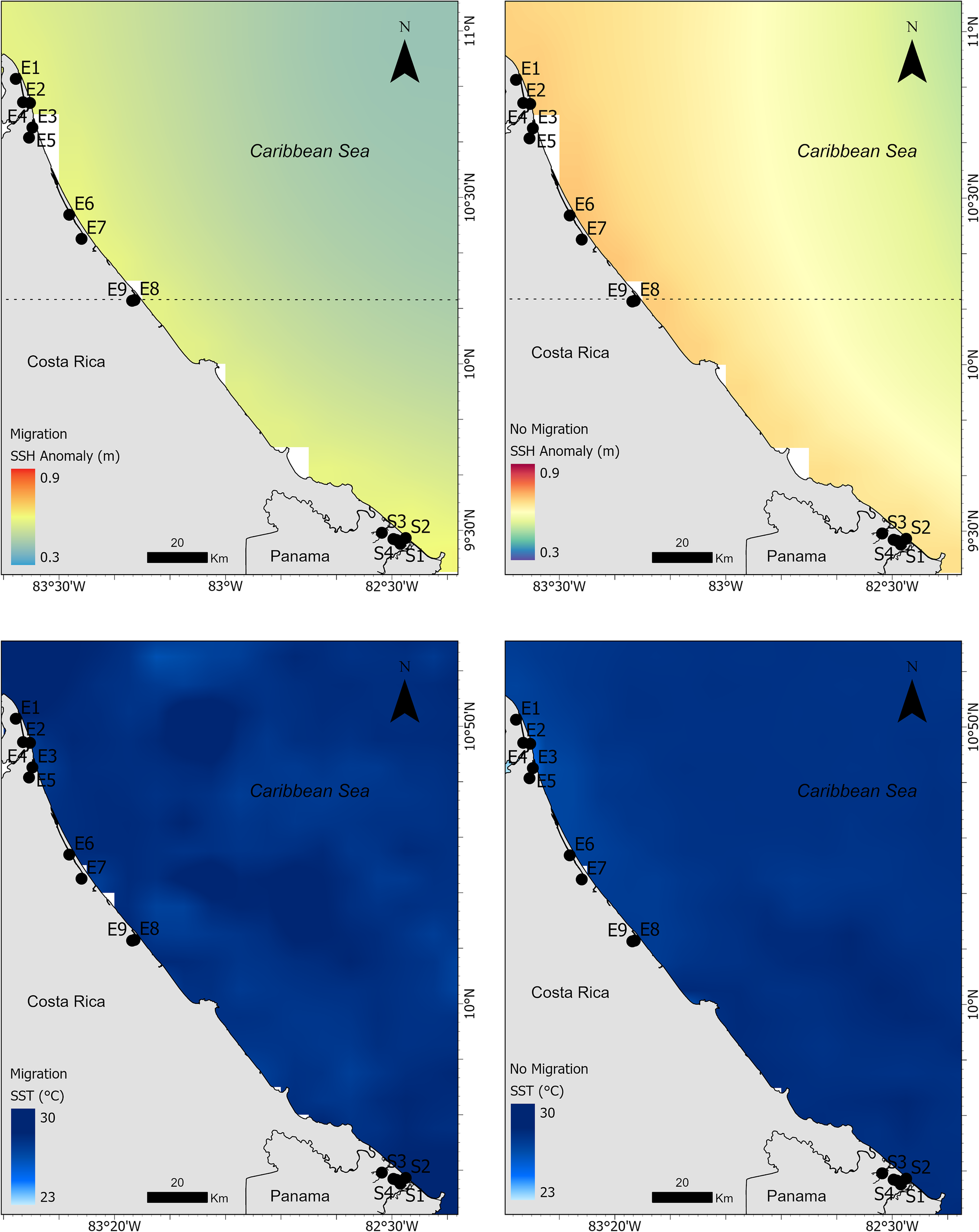
Spatial distribution of sea level anomaly (top panels) and sea surface temperature (bottom panels) in Costa Rica and Panama during periods of manatee migration (left panels) and non-migration (right panels). Black points indicate monitoring stations.
Figure 9
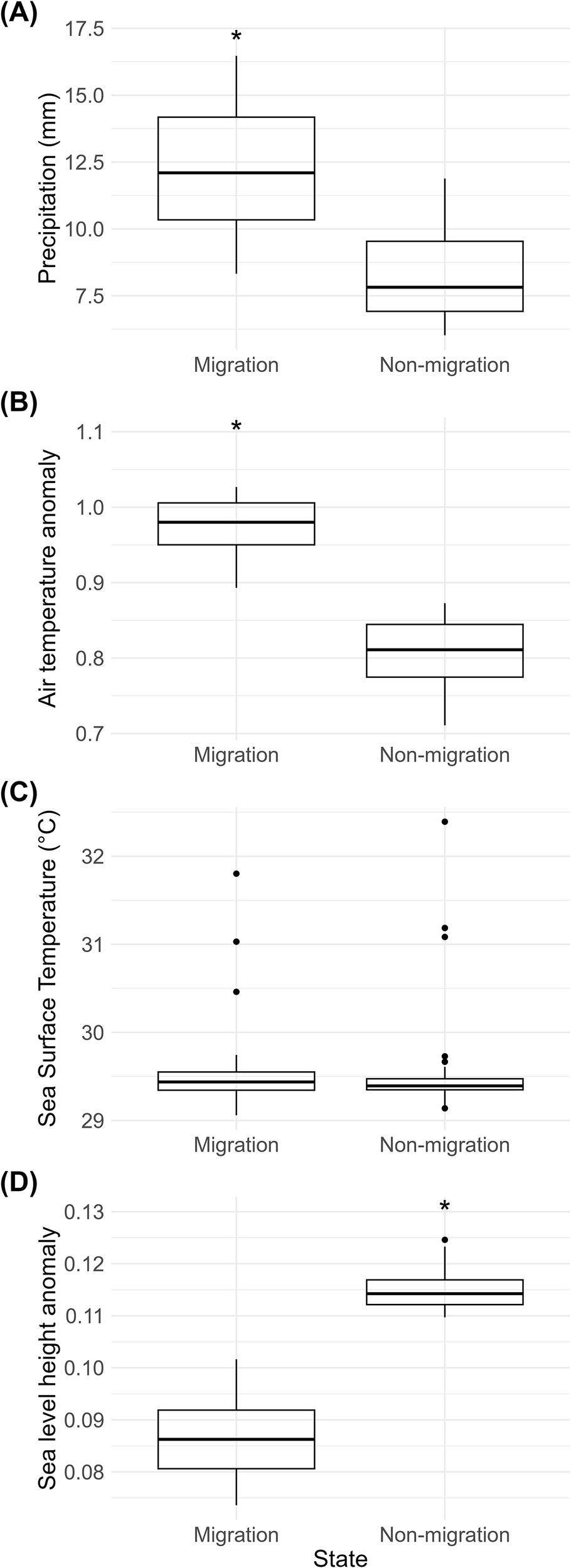
Variation of environmental variables in the Changuinola wetlands (Panama) during migration and non-migration period. Panels show monthly averages (± SD) of precipitation (A), air temperature anomaly (B), sea surface temperature (C), and sea level anomaly (D). Asterisks represent significant differences (Wilcoxon rank-sum tests).
4 Discussion
Accurately grouping manatee vocalizations poses a major challenge due to natural variability in call structure within and across individuals. To address this, we applied the clustering framework developed in Merchan et al. (2024), which had previously been validated on a dataset of 23 individuals recorded in the San San River under semi-controlled conditions. That validation showed the method’s capacity to handle intra-individual variability, particularly for squeaks and hi-squeaks, making it well-suited for application to the broader datasets analyzed here.
During the validation phase, each manatee was temporarily held in a floating cage for approximately eight hours, while free-ranging conspecifics often vocalized outside the cage (Merchan et al., 2024). This enriched the dataset by incorporating calls influenced by natural acoustic and social interactions. Although such recordings may not capture the complete vocal repertoire of each individual, the algorithm effectively grouped vocalizations—such as squeaks and hi-squeaks—that exhibited moderate variation in spectral contour when produced by the same animal. Examples of these intra-individual variations, successfully grouped by the algorithm, are shown in Figure 2. This performance stems from the combination of SWT representations, edge-based image descriptors, and the non-linear mapping provided by PaCMAP, which together preserve within-individual variation while enhancing between-individual separability. HDBSCAN further refined the results by discarding outliers and enforcing density-based clustering, yielding more conservative and reliable estimates.
Given the demonstrated ability of the Merchan et al. (2024) method to group acoustically diverse vocalizations produced by the same presumed individual, all call types were preliminarily included in the clustering process. Examination of the resulting clusters confirmed the method’s capacity to group calls with moderate acoustic variation. As illustrated in Figure 2, some clusters contained both squeaks and hi-squeaks that shared correlated contour shapes and occurred in close temporal proximity, suggesting they were likely emitted by the same individual.
For the residency analysis, however, we adopted a more conservative selection strategy. Following visual inspection, only clusters predominantly composed of squeaks, hi-squeaks, or a combination of both were retained. This decision contrasts with the approach of Schneider et al. (2024), who restricted their analysis exclusively to squeaks on account of their abundance and acoustic consistency. While such a conservative restriction reduces variability, it also omits hi-squeaks, which have been consistently reported as types of individual vocal signatures in manatees (Williams, 2005; Dietrich et al., 2022; O’Shea and Poché, 2006) and are particularly frequent among calves and juveniles (Brady et al., 2020; Umeed et al., 2023). By retaining both squeaks and hi-squeaks, our analysis preserved a wider set of biologically relevant signals, enhancing the potential for individual identification across diverse acoustic contexts. Nonetheless, occasional clustering errors remain possible—for instance, duplicate clusters for a single individual or merges of acoustically similar calls from different individuals—highlighting the need for cautious interpretation.
Despite these strengths, we recognize the inherent challenges in applying clustering methods to complex bioacoustic datasets derived from long-term recordings of wild manatees. Subtle intra-individual variability in spectral components and contour shapes can lead the same animal to produce acoustically diverse calls, while individuals with similar demographic or environmental characteristics may exhibit overlapping acoustic features. Such overlap increases the risk of misclassifications, including merges of distinct individuals’ vocalizations or duplications of clusters representing the same animal. The key challenge for clustering algorithms is to balance intra-individual variability with inter-individual separation. Non-linear dimensionality reduction techniques such as PaCMAP, when applied to SWT-based time–frequency representations, have shown promise in enhancing this separation (Merchan et al., 2024). Nonetheless, occasional duplications or merges remain possible, and these limitations must be carefully considered when interpreting individual identification results obtained through unsupervised clustering.
Individual identification algorithms (Stowell et al., 2019; Brady et al., 2022; Knight et al., 2024; Merchan et al., 2024) have considerably enhanced our understanding of the movement patterns and social behaviors of the Greater Caribbean manatees in this subregion. The initial detection records based on unclassified call vocalizations indicated remarkable regional presence and connectivity among manatees. In Panama, 61 individuals were recorded between December 2023 and August 2024, whereas 49 individuals were identified in Costa Rica between May 2021 and August 2024. Individual numbers used for residence time and migration assessments dropped by almost one-third when considering the three most crucial call vocalizations (e.g., squeak, hi-squeak, and a mix of squeak and hi-squeak). Notably, nine individuals were acoustically detected in both regions after a short migration of nearly 200 km, highlighting the cross-border movement and potential connectivity between these populations. Detailed individual matches (Supplementary Table S5) lay the foundation for understanding movement dynamics and can inform future research aimed at identifying factors that facilitate such connectivity. The detection of these cross-border individuals (B1–B9) suggests that manatees are likely to respond to shared ecological drivers or resource distribution patterns that transcend national boundaries. Herein, we reveal notable spatiotemporal variations in manatee detection across Costa Rica and Panama.
In Costa Rica, consistent detection throughout the monitoring period, with peaks in mid-2021, early 2022 and 2024, indicates stable habitat use, particularly at Stations S1 and S2, which recorded months with more than 12 individuals. Such consistent patterns suggest that these areas offer favorable environmental conditions or vital resources for resident manatees. Rainfall and flooding patterns in northern Costa Rica occur year-round (non-seasonal), unlike in southern Costa Rica and Panama, which experience more seasonal patterns. The region features a three-lagoonal inner system at the northwestern limit of the Barra del Colorado protected area, offering a suitable habitat for manatees year-round with minimal disturbance from transiting boats or human activities (Miksis-Olds et al., 2007; Brady et al., 2023; Slone et al., 2023). Conversely, the detection patterns in Panama were sporadic. Early detection in 2015 and 2018, interrupted by a data loss gap until December 2019, was followed by intermittent peaks, including a remarkable count of 17 individuals in April 2021. The lack of a consistent correlation between the number of detections and distinct individuals suggests variable residency and movement dynamics, again highlighting potential differences in local habitat use or transient activity with no clear response to seasonal drivers (Deutsch et al., 2022a; Factheu et al., 2023). The results indicate that all recorded manatees departed from Panama via the Changuinola River before entering Costa Rican waters. Notably, while several individuals likely accessed Costa Rica through the northern sector near Barra del Colorado, others appeared to have entered further south via the Tortuguero–Pacuare region. This pattern of diverging entry routes suggests marked individual variability and potential directional preferences along the Caribbean coastline, possibly reflecting differences in habitat suitability, resource availability, and environmental conditions as previously reported (Castelblanco-Martínez et al., 2009, 2013a, 2013b; Deutsch et al., 2022a, 2022b; Brady et al., 2023).
Analysis of residence times revealed considerable spatial variation in manatee habitat use across the transboundary region and in interannual fidelity. In Costa Rica, manatees remained at the northern sites of Barra del Colorado for an average of 546.50 days. In contrast, the southern Tortuguero-Pacuare area recorded much shorter and more variable stays (37.71 days). In Panama, the Changuinola region exhibited long-term site fidelity, with an average residence time of 1,926.31 days, in contrast to the more transient use observed in San San (191.93 days). Our data initially suggested high interannual fidelity for several individuals (Deutsch et al., 2022a). Binational comparisons revealed that specific individuals accumulated substantial residence times, averaging 2,202.22 days in Panamanian waters, before migrating to Costa Rica, where they stayed for an average of 277.05 days, with an intermediate migration period of 340 days.
KDE revealed distinct spatial patterns of vocalization density along the coasts of Costa Rica and Panama. In Costa Rica, high-density areas were concentrated near Barra del Colorado, and additional hotspots near Tortuguero and Pacuare suggest that these regions provide essential resources and preferred habitat conditions. Meanwhile, Panama exhibited broader and more intense activity centers near the Changuinola River than near the San San River. Both scenarios aligned with prior manatee habitat assessments, corroborating earlier observations of extended residency and site fidelity in this region (Castelblanco-Martínez et al., 2013a, 2013b; Deutsch et al., 2022a, 2022b). Additionally, within-site analyses revealed localized variations in habitat use. In Barra del Colorado, high- and medium-density zones accounted for 24.53% and 12.33% of the home range, respectively, indicating a concentrated spatial use pattern probably linked to the interconnected lagoonal habitats. Tortuguero presented a more uniform distribution, probably related to the lineal orography of the wetlands, whereas Pacuare was characterized by a predominantly low-density vocalization (5.95% of the area). In Panama, the distribution was notably extensive, with low-, medium-, and high-density zones comprising 39.69%, 30.72%, and 29.59% of the home range, respectively, underscoring the complex spatial utilization patterns previously documented in coastal manatee populations in Puerto Rico, Belize, and Mexico (Deutsch et al., 2003, 2022a, 2022b). Therefore, the KDE results highlight recurrent vocalization hotspots that may indicate manatees in this region of Central America are capable of long-distance travel over short periods and exhibit sedentary or high-site fidelity behaviors similar to those reported for manatees with satellite tags and photo IDs (Deutsch et al., 2022a).
This study examined the environmental factors influencing manatee migration in tropical coastal regions, with a focus on both migratory and non-migratory periods. Migration has been described as a generally synchronized, directional movement of individuals between distinct environments (Cooke et al., 2024), often recurring and not solely driven by immediate resource availability (Dingle and Drake, 2007). Similarly, Kennedy’s classic definition, later expanded by Morais and Daverat (2016), emphasizes persistent and directed movements that differ from local displacements or dispersal. In our study system, manatees exhibit what can be considered partial or seasonal migrations, as they repeatedly move across the Costa Rica–Panama boundary in response to hydrological and climatic drivers, while also maintaining long-term site fidelity in specific lagoons and river systems. Sea surface temperature (SST) remained relatively constant throughout both periods (migratory and non-migratory periods), indicating that thermal surface conditions are unlikely to be the primary migratory trigger in these ecosystems (Deutsch et al., 2022a). However, regional differences exist, as subtropical manatee populations have been shown to retreat to warmer waters in response to temperature changes, while Costa Rican manatees prefer warmer habitats in inner lagoon systems and may spend extended periods in deeper, stratified waters (Jimenez, 2005; Marsh, 2012). Precipitation levels and air temperature anomalies were significantly higher during migration periods, suggesting a possible atmospheric cue or consequence tied to broader climatic shifts. Low precipitation and runoff may impede migration by reducing waterway accessibility, as observed in parts of Costa Rica and Panama. Sea level anomalies were consistently lower during migration, which may reflect hydrodynamic changes or freshwater influxes associated with seasonal flooding, cold fronts, or wind-driven surges (Kjerfve, 1981; Lizano, 2006; Torres and Tsimplis, 2014). Extreme wave events, particularly during the dry season or storm periods, further highlight the role of meteorological forces (Morales-Marquez et al., 2023). Although two manatees initiated migration during the dry season, the majority migrated during the rainy season, pointing to a systemic environmental driver. These findings suggest that subregional atmospheric and hydrological variables—more than SST—are strongly associated with migratory behavior. Additional research into other factors such as wind regimes, ocean currents, and storm frequency is necessary to understand how climate variability and change will continue to shape migratory patterns (Deutsch et al., 2022a; WBG, 2021a, 2021b).
In summary, this pattern of movement reinforces the need for coordinated transboundary conservation strategies, as effective management must account for the use of multiple jurisdictions by species. Conservation of the Greater Caribbean manatee, widely regarded as a sentinel species for the health of coastal ecosystems, depends on our ability to understand and protect the intricate connections between habitat use, migratory behavior, and residency patterns. This study builds on previous regional research (Mou et al., 1990; Lacommare et al., 2008; Díaz-Ferguson et al., 2017; Castelblanco-Martínez et al., 2012) and extends it by incorporating new data sources and analytical frameworks (Merchan et al., 2024) to elucidate how manatees traverse an increasingly fragmented seascape. We conclude that manatees remain in residence over the years and follow “partial migrations” by utilizing coastal areas during the rainy season, and that “manatees inhabiting flood-pulse river systems closer to the coast may exhibit seasonal movements” (Deutsch et al., 2022a). A deeper understanding of these dynamics in biodiverse and rapidly changing regions, such as Central America, is a key to developing robust, science-based conservation strategies that ensure the persistence of this endangered species. The measurement of the residence time has practical implications for conservation management. By identifying hotspots of extended manatee occupancy, resource managers can implement targeted protective measures more effectively and establish marine protected areas (MPAs) or impose temporal restrictions on human activities during periods of critical use. These approaches are crucial in areas where competing uses, such as tourism development and commercial boating, intersect with key manatee habitats. Slone et al. (2023) reported that “manatee decisions were consistent with avoiding human interactions,” underscoring the need for minimizing human interference.
Furthermore, movement ecology can elucidate local and regional patterns, particularly using satellite telemetry and acoustic tracking, which have greatly enhanced our understanding of manatee spatial behavior across various temporal scales (Cooke, 2008). Notably, these movement patterns are not uniform across the entire population. Behavioral differences have been observed among sexes, age classes, and even individual animals (Deutsch et al., 2022a, 2022b; Hodson, 2025). For instance, some studies have documented that females, particularly those with calves, tend to spend extended periods in safe, sheltered areas. In contrast, males may travel longer distances in search of new resources or mating opportunities (Lacommare et al., 2008; Deutsch et al., 2022a, 2022b). Consequently, residence time—the duration for which an individual remains within a specific habitat—is a valuable metric for inferring habitat quality and ecological stability. Extended occupancy is generally associated with locations that offer abundant resources, minimal disturbances, and favorable physical conditions. For Greater Caribbean manatees, habitats such as seagrass meadows and sheltered wetland lagoons not only provide critical foraging opportunities but also serve as refuges from human-induced disturbances and predators (Lacommare et al., 2008; Marmontel et al., 2012; Deutsch et al., 2022a, 2022b; Hodson, 2025).
In addition, integrating genetic data with movement ecology offers a promising method for quantifying and conserving the ecological corridors necessary to sustain Greater Caribbean manatee populations (Castelblanco-Martínez et al., 2012, 2019; Parr et al., 2012; Tucker et al., 2012; Díaz-Ferguson et al., 2017). Growing research evidence suggests that even geographically separated populations can remain functionally connected through intermittent gene flow and migratory movements. Genetic studies in Panama have identified shared mitochondrial haplotypes across distinct local groups, suggesting that connectivity barriers may be less absolute than previously assumed (Díaz-Ferguson et al., 2017). This connectivity is critical for maintaining genetic diversity (Vianna et al., 2006; Quintana-Rizzo and Reynolds, 2008; Hunter et al., 2010; Nourisson et al., 2011; Luna et al., 2021), which buffers populations against the harmful effects of inbreeding and small effective population sizes. In Central America, where coastal habitats are undergoing rapid environmental changes owing to urbanization and tourism, maintaining genetic corridors remains a cornerstone of long-term conservation strategies (Lefebvre et al., 2001; Flamm et al., 2005; Castleblanco-Martinez et al., 2013a; Marsh, 2012; Deutsch et al., 2022a).
Finally, we propose establishing a binational corridor to protect manatees along a transboundary area of approximately 984 km of coastline (220 km in Costa Rica and 764 km in Panama) and covering 2,631 km² (526 km² in Costa Rica and 2,015 km² in Panama), which includes coastal marine and littoral wetland ecosystems within their jurisdictional waters (Supplementary Figure S3). The coastal and littoral zones of this region serve as a feeding and breeding habitat for recently listed vulnerable species (Morales-Vela et al., 2024), with over 57 rivers extending up to the 20-meter isobath (Supplementary Figure S3). The corridor was designed to preserve functional ecological connectivity for this threatened species across both countries and between marine and coastal protected lands under various management categories, including 18 existing protected areas (eight in Costa Rica and ten in Panama) and four existing Ramsar sites: Humedal Caribe Noreste and Gandoca-Manzanillo in Costa Rica, and San San-Pond Sak and Damani-Guariviara in Panama. In Central America, where manatees inhabit a mosaic of habitats within rapidly changing wetlands, integrating multiple sources of evidence, including genetic connectivity studies, satellite telemetry, and direct habitat assessments, is crucial, to develop a comprehensive understanding of how these animals navigate their seascape. This corridor meets the requirements for long-term survival, considering the regional residence and movement patterns of the species (Flamm et al., 2005; Deutsch et al., 2022b).
Our research continues, and we have started a project using satellite telemetry (sensu Deutsch et al., 2022a) to improve our understanding of habitat use and large-scale movement patterns at a high spatial resolution in Central America. Additionally, we are analyzing our dataset to estimate the manatee population size in this area, considering the different call categories (sensu Schneider et al., 2024), and employing acoustic capture-recapture models.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Smithsonian Tropical Research Institute Animal and Care Committee (approval numbers 2019-0704-2022, SI-23044). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HG: Conceptualization, Writing – review & editing, Methodology, Funding acquisition, Data curation, Project administration, Writing – original draft, Visualization. RE: Software, Investigation, Writing – review & editing, Formal Analysis, Visualization, Methodology. KC: Writing – review & editing, Formal Analysis, Software, Methodology, Data curation. HP: Software, Methodology, Writing – review & editing. JS-G: Software, Writing – review & editing, Methodology. FM: Project administration, Data curation, Methodology, Writing – review & editing, Funding acquisition, Conceptualization, Software.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Secretaría Nacional de Ciencia Tecnología e Innovación (SENACYT-Panama) through grants FID18-76, FID21-90, and FID23-106; the MarViva Foundation, Costa Rica; the Blue Marine Foundation; and the Smithsonian Tropical Research Institute (STRI). The Sistema Nacional de Investigación (SNI-SENACYT) supported research activities by FM, HP, JS-G, and HG.
Acknowledgments
We thank the governments of Panama and Costa Rica for providing the research permits (SE/A-114-15, SE/A-79–2019 and ARG-004–2023 for Panama; SINAC-ACTo-DIR-RES-097-2021, SINAC-ACTo-DIR-RES-030-2023, and R-SINAC-SE-DT-PI-010–2023 for Costa Rica). We thank Sofia Pastor, Jossio Guillen, Carlos Guevara, Manuel Hernandez Robles, Eduardo Perez, Alfredo Caballero, and Alexis (Meme) Montenegro for their unconditional field assistance and continuous logistical support. The Costa Rica Wildlife Foundation provided initial logistical support. We thank the board of the COOBANA R.L. Banana Company in Panama, particularly Chito Quintero, Diomedes Rodriguez, and Dinora Beitia, for generously providing bananas at no cost for over two years. We also thank the board of AAMVECONA for granting rental access to the pier and electricity for observing the manatees. The authors acknowledge the administrative support provided by CEMCIT-AIP, the Universidad Tecnológica de Panamá (UTP), and the Smithsonian Tropical Research Institute (STRI). We thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. After using this tool/service, the authors reviewed and edited the content as required and took full responsibility for the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1661294/full#supplementary-material
Supplementary Figure 1The proposed binational corridor aims to protect manatees along 984 kilometers of coastline, covering approximately 2,631 km². This area includes coastal marine and littoral wetland ecosystems within jurisdictional waters that extend up to the 20-meter isobath (gray-shaded area). Additionally, 57 rivers were identified, some of which provide access to feeding and breeding wetlands (indicated by color dots).
Supplementary Figure 2Representative examples of clusters conservatively excluded during the joint clustering stage. Panels A, B, and C each show two vocalizations from Panama (left two columns) and two from Costa Rica (right two columns), presented as examples of the calls within clusters of individuals that were initially matched during joint clustering stage. Despite temporal and structural similarities, differences in power and spectral content between the Panama and Costa Rica calls, or generally low signal power, led to their exclusion during manual validation. This conservative procedure ensured that only clusters with high internal consistency and biologically reliable acoustic features were retained for subsequent analyses.
Supplementary Figure 3Tracks of manatees in Panamanian wetlands (Changuinola) based on vocalization classes Squeaks, Hi-squeaks, Mix of Squeaks and Hi-squeaks.
Supplementary Figure 4Tracks of manatees in Costa Rica wetlands based on vocalization classes Squeaks, Hi-squeaks, Mix of Squeaks and Hi-squeaks.
Supplementary Table 1Deployment and retrieval dates of passive acoustic monitoring hydrophones in Costa Rica and Panama. Dates are given as day–month–year.
Supplementary Table 2Summary of passive acoustic monitoring effort by country, station, and year. For each station, the number of audio files and the total recording hours are reported.
Supplementary Table 3Summary of parameter configurations used across algorithmic stages (detection, classification, clustering, and validation) in the acoustic data processing workflow.
Supplementary Table 4Comparison of manatee vocalization clusters from Costa Rica and Panama. Vocalization class abbreviations: SK = Squeaks, HS = Hi-squeaks, SK/HS = Mix of Squeaks and Hi-squeaks, SL = Squeals, SKL/SL = Mix of Squeaks-squeals and Squeals, ND = Not well defined (Mix of Squeals, Squeaks-squeals, Squeaks, Hi-squeaks and/or Chirps), SK-ND= Squeaks with broadband noise. The Binational column indicates clusters that include vocalizations recorded in both countries (CR = Costa Rica; P = Panama).
Supplementary Table 5Residence time (in days) of individual manatees recorded in three categories: Costa Rica, Panama, and binational individuals identified in both countries, based on vocalization classes Squeaks, Hi-squeaks, Mix of Squeaks and Hi-squeaks. For Costa Rica, times are shown for Barra del Colorado (B) and Tortuguero–Pacuare (T–P); for Panama, times are shown for Changuinola (C) and San San (S). Binational individuals include matched IDs and residence time in each country. CR = Costa Rica; P = Panama.
References
1
Aguilar B. M. Peytrequín Gómez J. (2020). Between timbers, channels and turtles. Archaeological approach to tortugueros’s industrial processes, Costa Rica, (1871-1950) (Heredia, Costa Rica: Universidad Nacional).
2
Alicea-Pou J. A. (2001). Vocalizations and behavior of the Antillean and Florida manatee (Trichechus manatus): Individual variability and geographical comparison. [master’s thesis]. California, EEUU: Moss Landing Marine Laboratories and San Francisco State University.
3
Andreux M. Exarchakis G. Leonarduzzi R. F. Angles T. (2020a). Kymatio: wavelet scattering transforms in Python with GPU acceleration. J. Mach. Learn. Res.21, 1–6. Available online at: https://github.com/kymatio/kymatio (Accessed October 9, 2025).
4
Aragones L. V. Lawler I. Marsh H. Domning D. Hodgson A. Marsh H. (2012). “ “The role of sirenians in aquatic ecosystems,”,” in Sirenian conservation. Eds. HinesE. M.ReynoldsJ. E.AragonesL. V.Mignucci-GiannoniA. A.MarmontelM. (Berlín, Alemania: University Press of Florida), 4–11.
5
Bakker E. S. Pagès J. F. Arthur R. Alcoverro T. (2016). Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography39, 162–179. doi: 10.1111/ecog.01651
6
Brady B. Hedwig D. Trygonis V. Gerstein E. (2020). Classification of Florida manatee (Trichechus manatus latirostris) vocalizations. J. Acoust. Soc Am.147, 1597–1606. doi: 10.1121/10.0000849
7
Brady B. Moore J. Love K. (2022). Behavior related vocalizations of the Florida manatee (Trichechus manatus latirostris). Mar. Mamm. Sci.38, 975–989. doi: 10.1111/mms.12904
8
Brady B. Sarbacker C. Lasala J. A. Maust-Mohl M. Collom K. A. Searle L. et al . (2023). Manatees display diel trends in acoustic activity at two microhabitats in Belize. PloS One18, 0294600. doi: 10.1371/journal.pone.0294600
9
Castelblanco-Martínez D. Blanco-Parra M. Charruau P. Prezas B. Zamora-Vilchis I. Niño-Torres C. (2019). Detecting, counting and following the giants of the sea: a review of monitoring methods for aquatic megavertebrates in the Caribbean. Wildl. Res.46, 545–556. doi: 10.1071/WR19008
10
Castelblanco-Martínez D. N. Morales-Vela B. Hernández-Arana H. A. Padilla-Saldivar J. (2009). Diet of the manatees (Trichechus manatus manatus) in Chetumal Bay, Mexico. LAJAM7, 39–46. doi: 10.5597/lajam00132
11
Castelblanco-Martínez D. N. Nourisson C. Quintana-Rizzo E. Padilla-Saldivar J. Schmitter-Soto J. J. (2012). Potential effects of human pressure and habitat fragmentation on population viability of the Antillean manatee Trichechus manatus manatus: a predictive model. Endanger. Species Res.18, 129–145. doi: 10.3354/esr00439
12
Castelblanco-Martínez D. N. Padilla-Saldívar J. Hernández-Arana H. A. Slone D. H. Reid J. P. Morales-Vela B. (2013a). Movement patterns of Antillean manatees in Chetumal Bay (Mexico) and coastal Belize: A challenge for regional conservation. Mar. Mamm. Sci.29, E166–E182. doi: 10.1111/j.1748-7692.2012.00602.x
13
Castelblanco-Martínez N. Powell J. Galves J. Auil Gomez N. (2013b). Preliminary information from first tagged manatees in Turneffe Atoll (Belize) reveal regular travel patterns to the mainland. Sirenews60, 12–13.
14
Castelblanco-Martínez D. N. Reis V. de Thoisy B. (2018). How to detect an elusive aquatic mammal in complex environments? A study of the Endangered Antillean manatee Trichechus manatus manatus in French Guiana. Oryx52, 382–392. doi: 10.1017/S0030605316000922
15
Cooke S. J. (2008). Biotelemetry and biologging in endangered species research and animal conservation: relevance to regional, national, and IUCN Red List threat assessments. Endange. Species Res.4, 165–185. doi: 10.3354/esr00063
16
Cooke S. J. Piczak M. L. Singh N. J. Åkesson S. Ford A. T. Chowdhury S. et al . (2024). Animal migration in the Anthropocene: threats and mitigation options. Biol. Rev.99, 1242–1260. doi: 10.1111/brv.13066
17
Corona-Figueroa M. F. Ríos N. Castelblanco-Martínez D. N. Vilchez-Mendoza S. Delgado-Rodríguez D. Niño-Torres C. A. (2021). Searching for manatees in the dark waters of a transboundary river between Mexico and Belize: a predictive distribution model. Aquat. Ecol.55, 59–74. doi: 10.1007/s10452-020-09810-9
18
De Cheveigné A. Kawahara H. (2002). YIN, a fundamental frequency estimator for speech and music. J. Acoust. Soc Am.111, 1917–1930. doi: 10.1121/1.1458024
19
Deutsch C. J. Castelblanco-Martínez D. N. Cleguer C. Groom R. (2022a). “ Movement behavior of manatees and dugongs: II. Small-scale movements reflect adaptations to dynamic aquatic environments,” in Ethology and behavioral ecology of sirenia. Ed. MarshH. ( Springer International Publishing, Cham) 151, 233–298. doi: 10.1007/978-3-030-90742-6_6
20
Deutsch C. J. Castelblanco-Martínez D. N. Groom R. Cleguer C. (2022b). “ Movement behavior of manatees and dugongs: I. Environmental challenges drive diversity in migratory patterns and other large-scale movements,” in Ethology and behavioral ecology of sirenia. Ed. MarshH. ( Springer International Publishing, Cham), 155–231. doi: 10.1007/978-3-030-90742-6_5
21
Deutsch C. J. Reid J. P. Bonde R. K. Easton D. E. Kochman H. I. O’Shea T. J. (2003). Seasonal movements, migratory behavior, and site fidelity of west Indian manatees along the atlantic coast of the United States. Wildl. Monogr., 81–77.
22
Díaz-Ferguson E. Hunter M. Guzmán H. M. (2017). Genetic Composition and Connectivity of the Antillean Manatee (Trichechus manatus manatus) in Panama. Aquat. Mamm.43, 378–386. doi: 10.1578/AM.43.4.2017.378
23
Dietrich A. von Fersen L. Hammerschmidt K. (2022). Signature calls in West Indian manatee (Trichechus manatus manatus)? Aquat. Mamm.48, 349–354. doi: 10.1578/AM.48.4.2022.349
24
Dingle H. Drake V. A. (2007). What is migration? Bioscience57, 113–121. doi: 10.1641/B570206
25
Factheu C. Rycyk A. M. Kekeunou S. Keith-Diagne L. W. Ramos E. A. Kikuchi M. et al . (2023). Acoustic methods improve the detection of the endangered African manatee. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1032464
26
Flamm R. O. Weigle B. L. Wright I. E. Ross M. Aglietti S. (2005). Estimation of manatee (Trichechus manatus latirostris) places and movement corridors using telemetry data. Ecol. Appl.15, 1415–1426. doi: 10.1890/04-1096
27
Guzman H. M. Condit R. (2017). Abundance of manatees in Panama estimated from side-scan sonar. Wildl. Soc Bull.41, 556–565. doi: 10.1002/wsb.793
28
Herrera W. (1986). “ Clima de Costa Rica,” in Vegetación y Clima de Costa Rica 2. Ed. GomezL. D. (San Jose: Editorial Universidad Estatal a Distancia (EUNED)), 118. 1-118.
29
Herrera W. (2016). “ Climate of Costa Rica,” in Costa rican ecosystems. Ed. KappelleM. (Houston, Texas, EE. UU: University of Chicago Press), 775. 19-29.
30
Hodson P. S. (2025). Behavioral response of Wild Greater Caribbean Manatees (Trichechus manatus manatus) to playbacks of ponspecifics in Maya Beach, Belize. MSc Thesis (Londres, Reino Unido: Antioch University New England).
31
Howard A. G. Zhu M. Chen B. Kalenichenko D. Wang W. Weyand T. et al . (2017). MobileNets: Efficient convolutional neural networks for mobile vision applications. arXiv. doi: 10.48550/arXiv.1704.04861
32
Hunter M. E. Auil-Gomez N. E. Tucker K. P. Bonde R. K. Powell J. McGuire P. M. (2010). Low genetic variation and evidence of limited dispersal in the regionally important Belize manatee. Anim. Conserv.13, 592–602. doi: 10.1111/j.1469-1795.2010.00383.x
33
Jensen J. Hendriks R. C. Heusdens R. Jensen S. H. (2005). “ Smoothed subspace based noise suppression with application to speech enhancement,” in Proceedings of the 13th european signal processing conference, 1–4.
34
Jiménez I. (2005). Development of predictive models to explain the distribution of the West Indian manatee Trichechus manatus in tropical watercourses. Biol. Conserv.125, 491–503. doi: 10.1016/j.biocon.2005.04.012
35
Kjerfve B. (1981). Tides of the caribbean sea. J. Geophy. Res.: Oceans86, 4243–4247. doi: 10.1029/JC086iC05p04243
36
Knight E. Rhinehart T. de Zwaan D. R. Weldy M. J. Cartwright M. Hawley S. H. et al . (2024). Individual identification in acoustic recordings. Trends Ecol. Evol.39, 947–960. doi: 10.1016/j.tree.2024.05.007
37
Kusunoki S. Nakaegawa T. Pinzón R. Sanchez-Galan J. E. Fábrega J. R. (2019). Future precipitation changes over Panama projected with the atmospheric global model MRI-AGCM3. 2. Clim. Dyn.53, 5019–5034. doi: 10.1007/s00382-019-04842-w
38
Lacommare K. Self Sullivan C. Brault S. (2008). Distribution and Habitat Use of Antillean Manatees (Trichechus manatus manatus) in the Drowned Cayes Area of Belize, Central America. Aquat. Mamm.34, 35–43. doi: 10.1578/AM.34.1.2008.35
39
Lefebvre L. W. Marmontel M. Reid J. P. Rathbun G. B. Domning D. P. (2001). “ Status and biogeography of the West Indian manatee,” in Biogeography of the west indies (Berlín, Alemania: CRC press), 425–474.
40
Lizano O. G. (2006). Algunas características de las mareas en la costa Pacífica y Caribe de Centroamérica. Rev. Cienc. Tecnol.24, 51–64.
41
Luna F. de O. Beaver C. E. Nourisson C. Bonde R. K. Attademo F. L. et al . (2021). Genetic connectivity of the West Indian manatee in the southern range and limited evidence of hybridization with Amazonian manatees. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.574455
42
Marmontel M. Reid J. Sheppard J. K. Morales-Vela B. (2012). “ Tagging and movement of sirenians,” in Sirenian conservation: Issues and strategies in developing countries. Eds. HinesE. M.ReynoldsJ. E.IIIAragonesL.Mignucci-GiannoniA. A.MarmontelM. ( University Press of Florida, Gainesville, FL), 116–125. Available online at: https://pubs.usgs.gov/publication/70038541 (Accessed October 9, 2025).
43
Marsh H. (2012). Sirenian conservation: issues and strategies in developing countries (Nueva York, EE. UU: University Press of Florida). Available online at: https://www.jstor.org/stable/j.ctvx079z0.
44
McInnes L. Healy J. Astels S. (2017). HDBSCAN: Hierarchical density based clustering. J. Open Source Software2, 205. doi: 10.21105/joss.00205
45
Meirelles A. C. O. Castelblanco-Martinez N. Borges J. C. G. Amaral R. (2024). Manatees across borders: Introduction to the special issue on the biology and conservation of manatees. LAJAM19, 1–6. doi: 10.5597/lajam00316
46
Merchan F. Contreras K. Poveda H. Guzman H. M. Sanchez-Galan J. E. (2024). Unsupervised identification of Greater Caribbean manatees using Scattering Wavelet Transform and Hierarchical Density Clustering from underwater bioacoustics recordings. Front. Mar. Sc.11, 1416247. doi: 10.3389/fmars.2024.1416247
47
Merchan F. Echevers G. Poveda H. Sanchez-Galan J. E. Guzman H. M. (2019). Detection and identification of manatee individual vocalizations in Panamanian wetlands using spectrogram clustering. J. Acoust. Soc Am.146, 1745–1757. doi: 10.1121/1.5126504
48
Merchan F. Guerra A. Poveda H. Guzmán H. M. Sanchez-Galan J. E. (2020). Bioacoustic classification of antillean manatee vocalization spectrograms using deep convolutional neural networks. Appl. Sci.10, 3286. doi: 10.3390/app10093286
49
Mignucci-Giannoni A. Gonzalez-Socoloske D. Alvarez Aleman A. Aquino J. Caicedo-Herrera D. Castelblanco-Martínez N. et al . (2024). What’s in a Name? Standardization of vernacular names for Trichechus manatus. Caribb. Nat.98, 1–17.
50
Miksis-Olds J. L. Donaghay P. L. Miller J. H. Tyack P. L. Nystuen J. A. (2007). Noise level correlates with manatee use of foraging habitats. J. Acoust. Soc Am.121, 3011–3020. doi: 10.1121/1.2713555
51
Miksis-Olds J. L. Tyack P. L. (2009). Manatee (Trichechus manatus) vocalization usage in relation to environmental noise levels. J. Acoust. Soc Am.125, 1806–1815. doi: 10.1121/1.3068455
52
Morais P. Daverat F. (2016). Definitions and concepts related to fish migration. In An Introduction to Fish Migration (pp. 14–19). CRC Press.
53
Morales-Márquez V. Cáceres-Euse A. Hernández-Carrasco I. Molcard A. Orfila A. (2023). Extreme waves in the Caribbean Sea: spatial regionalization and long-term analysis. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1294189
54
Morales-Vela B. Quintana-Rizzo E. Mignucci-Giannoni A. (2024). “ Trichechus manatus ssp. manatus,” in The IUCN red list of threatened species 2024 : International Union for Conservation of Nature (IUCN), Gland, Switzerland, e.T22105A43793924. doi: 10.2305/IUCN.UK.2024-2.RLTS.T22105A43793924.en
55
Mou S. L. Chen D. Bonde R. O’Shea T. (1990). Distribution and status of manatees (Trichechus manatus) in Panama. Mar. Mamm. Sci.6, 234–241. doi: 10.1111/j.1748-7692.1990.tb00247.x
56
Nourisson C. Morales-Vela B. Padilla-Saldívar J. Tucker K. P. Clark A. Olivera-Gómez L. D. et al . (2011). Evidence of two genetic clusters of manatees with low genetic diversity in Mexico and implications for their conservation. Genetica139, 833–842. doi: 10.1007/s10709-011-9583-z
57
O’Shea T. J. ‘Lex’ Salisbury C. A. (1991). Belize—a last stronghold for manatees in the Caribbean. Oryx25, 156–164. doi: 10.1017/S0030605300034189
58
O’Shea T. J. Poché Jr L.B. (2006). Aspects of underwater sound communication in Florida manatees (Trichechus manatus latirostris). J. Mammalogy.87, 1061–1071. doi: 10.1644/06-MAMM-A-066R1.1
59
Ordoñez-Nieto M. M. Castelblanco-Martinez N. Jotty-Arroyo K. (2024). Not everyone likes manatees: fishers’ Perceptions unveil opportunities and challenges for manatee conservation in the swamp complex of ayapel, Colombia. Aquat. Conser.: Mar. Freshw.34, e4258. doi: 10.1002/aqc.4258
60
Orozco-Montoya R. A. Penalba O. C. (2022). Spatial and temporal rainfall variability in critical periods for banana plantations in the caribbean coast of Costa Rica. FIHM22, 247–202. doi: 10.1007/s00704-022-04342-8
61
Parr L. Santos F. R. Waycott M. Vianna J. A. McDonald B. Caballero S. et al . (2012). “ Sirenian genetics and demography,” in Sirenian conservation. Eds. HinesE. M.ReynoldsJ. E.AragonesL. V.Mignucci-GiannoniA. A.MarmontelM. (Oxford University Press: University Press of Florida), 168–178. Available online at: http://www.jstor.org.ezproxy.uniandes.edu.co/stable/j.ctvx079z0.25.
62
Phillips R. Niezrecki C. Beusse D. O. (2004). Determination of West Indian manatee vocalization levels and rate. J. Acoust. Soc Am.115, 422–428. doi: 10.1121/1.1635839
63
Quesada M. E. Waylen P. (2020). Variability of daily precipitation on the caribbean coast of Costa Rica. Rev. Climatol.20, 61–74.
64
Quintana-Rizzo E. Reynolds J. (2008). Regional management plan for the West Indian manatee (Trichechus manatus). Caribbean Environ. Programme United Nations Environ. Programme.
65
Schneider S. von Fersen L. Dierkes P. W. (2024). Acoustic estimation of the manatee population and classification of call categories using artificial intelligence. Front. Conserv. Sci.5. doi: 10.3389/fcosc.2024.1405243
66
Self-Sullivan C. Mignucci-Giannoni A. A. (2012). West Indian manatees (Trichechus manatus) in the wider Caribbean regionGainesville, FL, USA: University Press of Florida.
67
Slone D. H. Butler S. M. Reid J. P. Kleen J. Palmer J. (2023). How do ambient conditions and management actions affect manatee movements and habitat use? J. Wildl Manage.87, e22411. doi: 10.1002/jwmg.22411
68
Sousa-Lima R. S. Paglia A. P. Da Fonseca G. A. (2002). Signature information and individual recognition in the isolation calls of Amazonian manatees, Trichechus inunguis (Mammalia: Sirenia). Anim. Behav.63, 301–310. doi: 10.1006/anbe.2001.1873
69
Sousa-Lima R. S. Paglia A. P. da Fonseca G. A. (2008). Gender, age, and identity in the isolation calls of Antillean manatees (Trichechus manatus manatus). Aquat. Mamm.34, 109–122. doi: 10.1578/AM.34.1.2008.109
70
Stephens C. S. (2002). Banana people: true stories from the tropics (Ann Harbor, Miichigan: Dollar Bill Books), 333.
71
Stowell D. Petrusková T. Šálek M. Linhart P. (2019). Automatic acoustic identification of individuals in multiple species: improving identification across recording conditions. J. R. Soc Interface16, 20180940. doi: 10.1098/rsif.2018.0940
72
Torres R. R. Tsimplis M. N. (2014). Sea level extremes in the Caribbean Sea. J. Geophys. Res.: Oceans119, 4714–4731. doi: 10.1002/2014JC009929
73
Tucker K. P. Hunter M. E. Bonde R. K. Austin J. D. Clark A. M. Beck C. A. et al . (2012). Low genetic diversity and minimal population substructure in the endangered Florida manatee: implications for conservation. J. Mammal.93, 1504–1511. doi: 10.1644/12-MAMM-A-048.1
74
Umeed R. Attademo F. L. N. Bezerra B. (2018). The influence of age and sex on the vocal repertoire of the Antillean manatee (Trichechus manatus manatus) and their responses to call playback. Mar. Mamm. Sci.34, 577–594. doi: 10.1111/mms.12467
75
Umeed R. Lucchini K. Coutinho P. D. F. dos Santos P. J. P. Borges J. C. G. Normade I. et al . (2023). Acoustic interactions between free-living mother–calf Antillean manatees, Trichechus manatus manatus. J. Ethol.41(3), 243–251.
76
Vargas G. U. (2001). Las lluvias en América Central: Una climatología geográfica. Anuario Estudios Centroamericanos, 47(5), 7–19.
77
Vianna J. A. Bonde R. K. Caballero S. Giraldo J. P. Lima R. P. Clark A. et al . (2006). Phylogeography, phylogeny and hybridization in trichechid sirenians: implications for manatee conservation. Mol. Ecol.15, 433–447. doi: 10.1111/j.1365-294X.2005.02771.x
78
Wang Y. Rudin C. Shaposhnik Y. (2021). PaCMAP: Large-scale dimension reduction technique preserving both global and local structure. J. Mach. Learn. Res.22, 1–73. Available online at: https://github.com/YingfanWang/PaCMAP (Accessed October 9, 2025).
79
WBG-World Bank Group (2021a). Climate risk profile: Costa Rica (Washington, D.C., USA: World Bank Group). 36.
80
WBG-World Bank Group (2021b). Climate risk profile: Panama (Washington, D.C., USA: World Bank Group), Vol. 40.
81
Wickham H. (2016). ggplot2: elegant graphics for data analysis (New York: Springer, Use R! Series), 213.
82
Williams L. E. (2005). Individual distinctiveness, short- and long-term comparisons, and context specific rates of Florida manatee vocalizations. [master’s thesis] (Nueva York, EE. UU: University of North Carolina Wilmington).
83
Wirsing A. J. Kiszka J. J. Allen A.-C. Heithaus M. R. (2022). Ecological roles and importance of sea cows (Order: Sirenia): a review and prospectus. Mar. Ecol. Prog. Ser.689, 191–215. doi: 10.3354/meps14031
84
Xie J. Colonna J. G. Zhang J. (2021). Bioacoustic signal denoising: a review. Artif. Intell. Rev.54, 3575–3597. doi: 10.1007/s10462-020-09906-1
Summary
Keywords
Trichechus manatus , Antillean manatee, bioacoustics, acoustic monitoring, home range, migration, conservation corridor, Central America wetlands
Citation
Guzman HM, Estévez RM, Contreras K, Poveda H, Sanchez-Galan JE and Merchan F (2025) Year-round residency and movement behavior of Greater Caribbean manatees (Trichechus manatus manatus) in Panama and Costa Rica. Front. Mar. Sci. 12:1661294. doi: 10.3389/fmars.2025.1661294
Received
07 July 2025
Accepted
02 October 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Sabrina Lo Brutto, University of Palermo, Italy
Reviewed by
Roger Lyons Reep, University of Florida, United States; Athena Rycyk, New College of Florida, United States; Camila Carvalho, Instituto de Desenvolvimento Sustentável Mamirauá, Brazil
Updates
Copyright
© 2025 Guzman, Estévez, Contreras, Poveda, Sanchez-Galan and Merchan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rocío M. Estévez, estevezr@si.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.