Abstract
Plastic and microplastic debris (MP) constitute the most important pollutants of the solid litter with a high risk of sediment accumulation. Posidonia oceanica (L.) Delile is the main marine seagrass of the Mediterranean Sea which forms immense underwater meadows and deposits of seagrass beds covering the facades of sandy beaches. They are formed by roots and rhizome fragments gathered in fibrous marine balls, called aegagropiles (EGs), having the ability to trap several pollutants from the beaches and mainly microplastic. The present study aims at evaluating microplastic contamination in aegagropiles collected from four locations along the Tunisian coast in the southern Mediterranean basin (two northern sites (S1 and S2) and two southern-central sites (S3 and S4). Microscopic analysis revealed that red and blue microplastics dominated at all sites, with black fibers and fragments being the most prevalent forms and yellow (S3) and transparent particles in S1, S2, and S4. Polymer identification conducted using nuclear magnetic resonance (NMR) and Fourier-transform infrared spectroscopy (FTIR) detected microplastic types with contamination levels and microplastic accumulation variation among the four sites including polystyrene (PS) at sites S1, S3, and S4; ethyl vinyl acetate (EVA) at S1 and S3; polyethylene terephthalate (PET) at S1 and S2; and polyvinyl chloride (PVC) at S2 and S3. Our results highlight close relationships between anthropogenic activities, extensive plastic use, and elevated microplastic pollution in marine ecosystems, particularly in seagrass beds. These findings emphasize the importance of monitoring microplastic contamination to preserve the health of Mediterranean coastal environments.
Introduction
Waste management is becoming a major issue with the increase in human population density; indeed, recent studies have highlighted a serious issue of marine litter (De Luca et al., 2025; Sarkar et al., 2025). It is well documented that the human-produced waste which accumulates in marine environments consists essentially of large quantities of microplastics in water like rivers, lakes, seas and oceans (Fraissinet et al., 2025; Gurjar et al., 2023; Jamsek et al., 2024). Research has demonstrated that UV light and low temperature facilitate the breakdown of conventional plastics into smaller fragments, commonly known as microplastics. These microplastics are subsequently transported into marine environments through runoff (Nguyen et al., 2025). In fact, the plastic material size ranging from 1 μM to 5 mm has been classified as microplastic (Cole et al., 2011). Microplastics are divided into primary and secondary microplastics (Bhuyan et al., 2021). The main source of primary plastic involves cosmetic products, personal healthcare products, and children’s products (Hartmann et al., 2019). However, the major sources of secondary plastics are fragmented products produced via the physical fragmentation as well as the biological and chemical degradation of large-sized plastic material (Sait et al., 2021; Yuan et al., 2022). It is important to mention that most current studies on microplastic toxicity are increasingly focused on elucidating the underlying mechanisms responsible for their toxicity (Zhang et al., 2022). The latter area of microplastics research is important considering that some of the chemicals associated with plastic contamination are able to disrupt the endocrine system in vertebrates, including fish and mammals (Gugliandolo et al., 2020; Folbert et al., 2022; Corti et al., 2023; Sharma et al., 2024). Furthermore, microplastics are readily assimilated by plankton, which can serve as a transfer route to secondary and tertiary consumers in the marine food chain, potentially leading to consequences for humans, the final consumers (Gunaalan et al., 2023). It should be noted that the smaller the size of microplastics, the more the toxicological consequences (Markic et al., 2020; Tang, 2024).
On the other hand, Posidonia oceanica (PO) is known as marine seagrass and an endemic species to Mediterranean Sea (De Luca et al., 2025). The shoots of P. oceanica constitute structurally complex ecosystems, providing adequate living conditions and ecological niches for a significant number of organisms (Boudouresque et al., 2016). On the beaches of the Mediterranean Sea, one often finds ball-shaped clumps of plant debris. These natural formations are called “aegagropiles (EG),” and they are usually made from fibers of the seagrass Posidonia oceanica, whose size diverges from millimeters to centimeters (Verhille and Le Gal, 2018). These balls occupied large areas, notably after storms. Research revealed that EG are formed by hydrodynamic flows and composed of different plant fibrous elements (PO) and sand grains (Matheson et al., 2017). The leaf cells of Posidonia are distinguished by their thin and lignified walls, and thus the fibers offer the rigidity necessary to form EG (Piñeiro-Juncal et al., 2021).
PO plays an important ecological role by acting as a sink for contaminants, particularly by storing them in its roots and shoots, thereby helping in reducing their availability in the marine environment (Tahir et al., 2019; Sghaier et al., 2025). It actively removes certain pollutants, sequestering a portion within its tissues and thereby limiting their transfer through trophic networks. Moreover, several studies highlight the role of P. oceanica as an effective bioindicator of marine pollution, as well as a long-term archive of contaminants through accumulation in the matte (the dense, long-lasting underground structure formed by intertwined rhizomes, roots, and trapped sediments), which serves as an environmental memory of pollutant inputs (Telesca et al., 2015; Sghaier et al., 2025).
In this context, it is essential to identify effective strategies to mitigate the impact of chemical pollution on seagrass beds. Although various studies have investigated the distribution of microplastics in specific geographical regions and others have examined their effects on certain marine organisms, global knowledge remains limited due to the relatively small number of in-depth studies focusing on natural bioaggregates such as aegagropiles (EGs) and their role in trapping and transferring microplastics. Therefore, the present study aims to assess the accumulation of microplastics in aegagropiles and to identify the most abundant polymers present in the marine environment.
Material and methods
Sampling
P. oceanica aegagropiles were sampled at four different sites, four from the North of Tunisia including Bizerte (latitude 37°17′45.02″N, longitude 9°52′23.22″E) (S1), and Hammamet (longitude 10°32′29.84″E, latitude 36°22′13.76″N) (S2), and from the east-central Tunisia at two different sites: Mahdia (latitude 35°30′56.39″N, longitude 11° 2′56.66″E) (S3) and Chebba (latitude 35°26′56.96″N, longitude 11° 0′16.78″E) (S4) (Figure 1). These sites are characterized by tourism and fishing activities. Aegagropiles were manually collected in triplicate from seagrass banquettes (dense accumulations of dead seagrass leaves along the shoreline), as illustrated in Figure 2. The samples were placed in sterile sample bags and transported to the Laboratory of Marine Ecotoxicology at National Institute of Marine sciences and Technology for subsequent laboratory analysis.
Figure 1
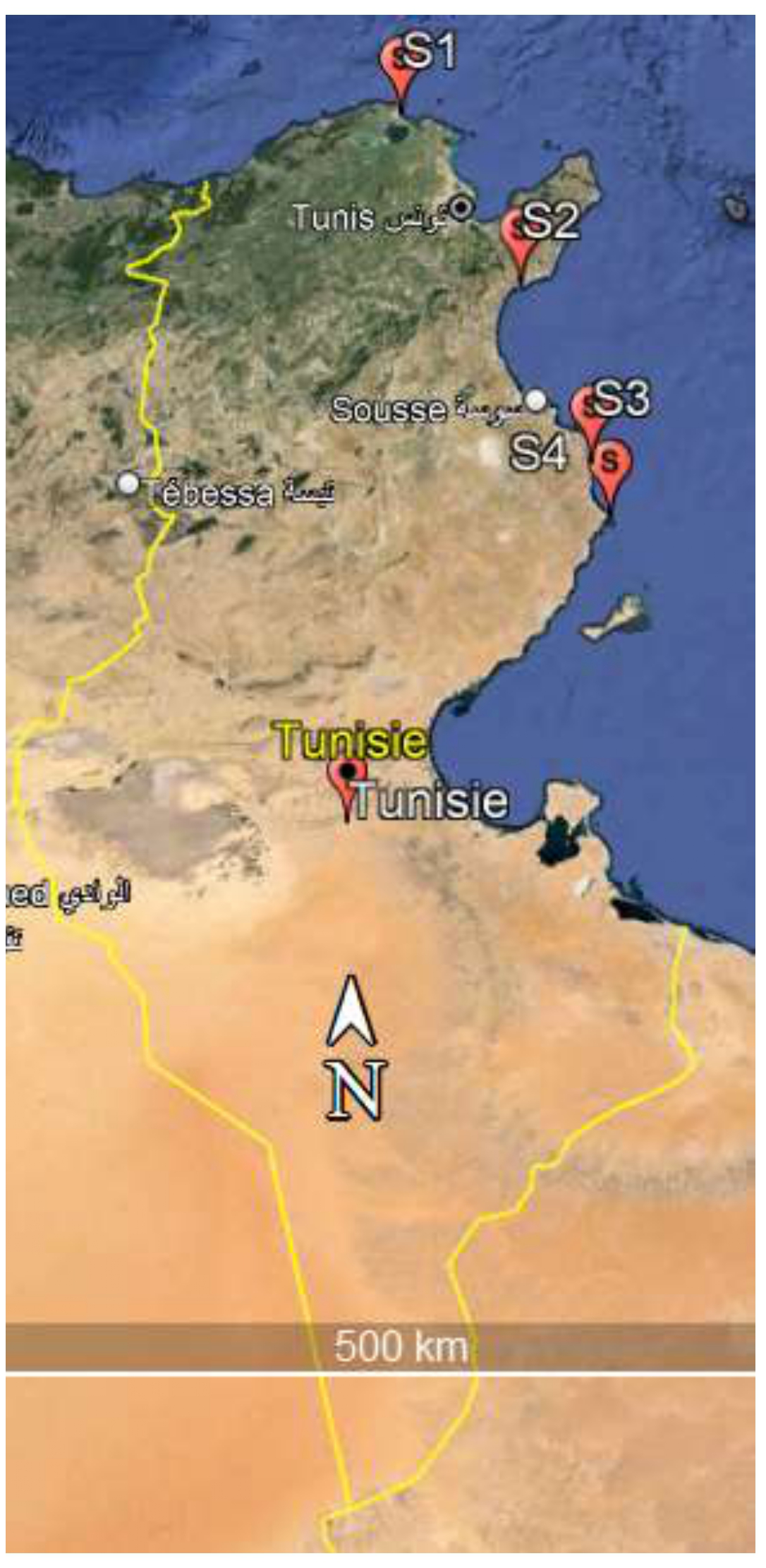
Map showing the four sampling areas sites along the coast of Tunisia. (S1) Bizerte, (S2) Hammamet, (S3) Mahdia, (S4) Chebba.
Figure 2

Aegagropiles collected from Tunisian beaches: (A) Bizerte, (B) Mahdia, (C) close-up image of an aegagropile.
Microplastic extraction
In the laboratory, the samples underwent a thorough cleaning process with distilled water to remove any sediment, followed by air drying at room temperature (25 °C) and low humidity for several days. The aegagropiles of P. oceanica were weighed using an analytical balance with a sensitivity of 0.01 mg. To dissolve and digest the organic matter, two methods were applied following Sanchez-Vidal et al. (2021), with some modifications. In the first method, a solution containing 10% HCl and 30 mL of 30% H2O2 was introduced. After a reaction period of 48 h, a KOH solution (10%) was added to promote chemical digestion. The samples were dried in the oven at 50 °C for 1 week. Then, the resulting mixture containing solid residues and MP was sieved. The contents of the sieves were transferred to a glass jar for decanting. The supernatant was filtered under vacuum through a Whatman glass fiber filter with a diameter of 47 mm (GF/D, with a particle retention size of 2.7 µm). The filter cake was completely rinsed with deionized water and then dried in an oven at 60°C, and the membrane was stored in a glass Petri dish for air drying at room temperature.
With the second method, the EGs were carefully disentangled, and the fibers were sieved at 5, 0.36, and 0.2 mm using a stainless-steel sieve. The contents retained on the sieves were treated with 4–6 mL of hydrogen peroxide (H2O2, 30%), followed by 10% hydrochloric acid (HCl) to remove most of the organic matter and calcium carbonate. The samples were then dried in an oven at 50°C for more than 24 h.
Quality control
During the sampling, no plastic tools and materials were used. Additionally, the laboratory contamination was assessed by placing a moist filter over an opened Petri dish. The operators were required to wear cotton coats to further reduce the risk of contamination. Prior to use, the filters were meticulously inspected under a microscope to ensure they were free from any airborne microplastic particles. When handling samples, stainless-steel forceps were used to maintain the integrity of the samples.
Microplastics identifications
All extracted plastic particles were picked out with metal tweezers into a 90-mm Petri dish containing a black and white background that enabled high contrast with plastic colors and types, which was photographed. The Petri dishes were inspected for plastic debris under a Leica M60 stereomicroscope (Leica Microsystems AG, Glattbrugg, Switzerland) equipped with a CMOS microscope camera and a 6:1 zoom system, offering a continuous magnification range from 2× to 5×, with engageable click stops for precise settings (see Hassen et al., 2023).
1H-NMR characterization
Using proton nuclear magnetic resonance spectroscopy (1H-NMR) characterization, MP particles were dissolved in a suitable deuterated solvent. Deuterated dimethyl sulfoxide (DMSO-d6) (99.8 atom %D) and deuterated chloroform (CDCl3) (99.8 atom %D, stab. with Ag) from Deutero, Germany, were used.
The NMR measurements were performed using a Bruker Avance III 300 MHz spectrometer. Data were recorded at room temperature, at a spinning rate of 14 kHz and with a pulse length of 90° and 3.25 μs with a 5-s interval between scans (see Peez and Imhof, 2020).
FT-IR characterization
The Fourier-transform infrared (FTIR) measurements were carried out on a Perkin Elmer Spectrum BX FTIR device (Perkin Elmer, USA), utilizing a Golden Gate single reflection diamond ATR system (SpecacLda, USA). All spectra were obtained using 64 scans each and a resolution of 4 cm−1, within the 4,000–450-cm−1 range (see Blindheim and Ruwoldt, 2023).
Results
Microscopy identification
An examination of the colors and types of microplastics trapped in the EGs (Figure 3) revealed that the plastic items mainly consisted of fragments and threads. These were of various sizes and colors and were found to be intertwined within the examined EGs.
Figure 3
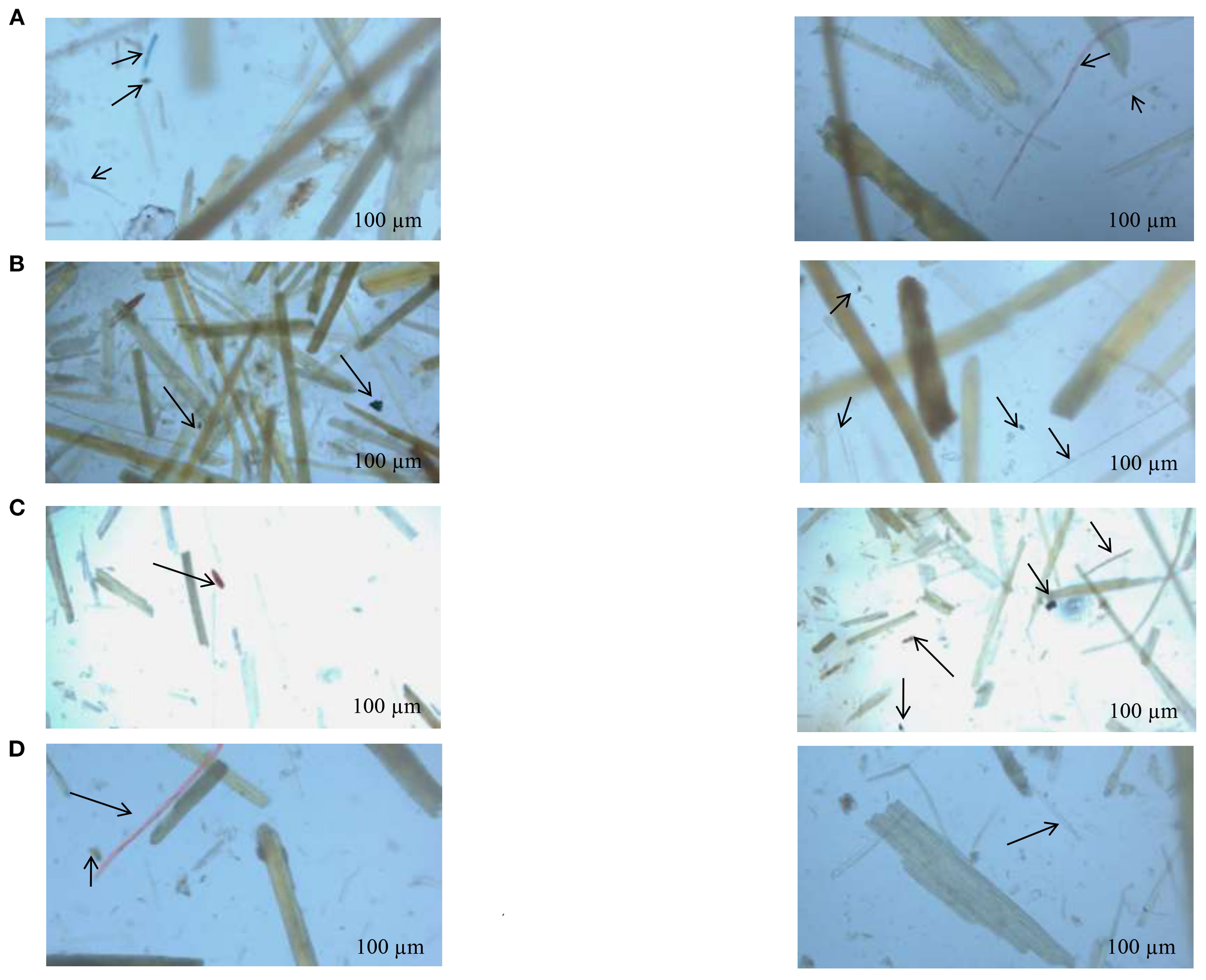
Microplastic particles found in EG extract samples under a stereomicroscope. (A) Bizerte, (B) Hammamet, (C) Chebba, (D) Mahdia.
The frequent colors of microplastics were mainly blue and red. In site S1, the found microplastic elements included blue, black, and transparent fragments and filaments with red and yellow colors (Figure 3A).
At the S2 site (Figure 3B), red, blue, and transparent filaments were detected with the presence of black and blue fragments. Concerning site S3 (Figure 3C), black and yellow fragments are essentially observed with the presence of transparent filaments. For site S4, black and red fragments are identified (Figure 3D).
FTIR results
According to the literature (Table 1), the results obtained by FTIR showed intense absorptions at 3,250 (hydrogen bonded N–H stretch), 2,917-2,840 cm−1 (ns CH2), 1,722-1,529 (amide I), 1,354, 1,249 (amide III), 1,152-1,003 (C–C str), 800-700 (CH2 rocking), and 694-500 (Aromatic CH out-of-plane bending) cm−1.
Table 1
| POLYMER | Characteristic peaks (cm-1 ) | Assignement | References |
|---|---|---|---|
| Low density polyethylene (LDPE) | 2915 | C–H stretching | Nishikida and Coates, 2003; Noda et al., 2007; Asensio et al., 2009; Mecozzi et al., 2016; Jung et al., 2018 |
| 2845 | C–H stretching | ||
| 1467 | CH2 bending | ||
| 1462 | CH2 bending | ||
| 1377 | CH2 bending | ||
| 730 | CH2 rocking | ||
| 717 | CH2 rocking | ||
| Polyethylene terephthalate (PET) | 1713 | C=O stretching | Verleye et al., 2001; Noda et al., 2007; Asensio et al., 2009; Mecozzi et al., 2016; Jung et al., 2018 |
| 1241 | C–O stretching | ||
| 1094 | C–O stretching Aromatic | ||
| 720 | CH out-of plane bending | ||
| Polypropylene (PP) | 2950 | C–H stretching | Verleye et al., 2001; Noda et al., 2007; Asensio et al., 2009; Mecozzi et al., 2016; Jung et al., 2018 |
| 2838 | C–H stretching | ||
| 2915 | C–H stretching | ||
| 1455 | CH2 bending | ||
| 1377 | CH3 bending | ||
| 1166 | CH bending, CH3 rocking, C–C stretching | ||
| 997 | CH3 rocking, CH3 bending, CH bending | ||
| 972 | CH3 rocking, C–C stretching | ||
| 840 | CH2 rocking, C–CH3 stretching | ||
| 808 | CH2 rocking, C–C stretching, C–CH stretching Aromatic | ||
| Polystyrene (PS) | 3024 | C–H stretching | Verleye et al., 2001; Noda et al., 2007; Asensio et al., 2009; Mecozzi et al., 2016; Jung et al., 2018 |
| 2847 | C–H stretching | ||
| 1601 | Aromatic ring stretching | ||
| 1492 | Aromatic ring stretching | ||
| 1451 | CH2 bending | ||
| 1027 | Aromatic CH bending | ||
| 694 | Aromatic CH out-of plane bending | ||
| 537 | Aromatic ring out-of plane bending | Verleye et al., 2001; Noda et al., 2007; Jung et al., 2018 | |
| Polyvinyl chloride (PVC) | 1427 | CH2 bending | |
| 1331 | CH bending | ||
| 1255 | CH bending | ||
| 1099 | C–C stretching | ||
| 966 | CH2 rocking | ||
| 616 | C–Cl stretching | ||
| Ethylene vinyl acetate (EVA) | 2917 | C–H stretching | Verleye et al., 2001; Asensio et al., 2009; Jung et al., 2018 |
| 2848 | C–H stretching | ||
| 1740 | C = O stretching | ||
| 1469 | CH2 bending, CH3 bending | ||
| 1241 | C (=O) O stretching | ||
| 1020 | C–O stretching | ||
| 720 | CH2 rocking | ||
| Nylon (all polyamides) | 3298 | N–H stretching | Verleye et al., 2001; Noda et al., 2007; Mecozzi et al., 2016; Jung et al., 2018 |
| 2932 | CH stretching | ||
| 2858 | CH stretching | ||
| 1634 | C =O stretching | ||
| 1538 | NH bending, C–N stretching | ||
| 1464 | CH2 bending | ||
| 1372 | CH2 bending | ||
| 1274 | NH bending, C–N stretching | ||
| 1199 | CH2 bending | ||
| 687 | NH bending, C=O bending |
FTIR characteristic peak assignments in cm-1for various types of MPs.
Figure 4 shows different types of polymers determined in each site. In site S1 (Figure 4A), bands at 3,300, 2,992, 1,700, 1,500, 1,250, 1,050, 750, and 550 cm−1 are related to polysterene (PS), polyethylene terephthalate (PET), and ethylene-vinyl acetate (EVA). Similarly, in site S2 (Figure 4B), the collected spectra revealed that bands at 2,875, 1,750, 1,650, 1,500, 1,250, 1,000, 750, 550, and 500 cm−1 demonstrated the existence of polyvinylchloride (PVC), propylene (PP), and PET.
Figure 4
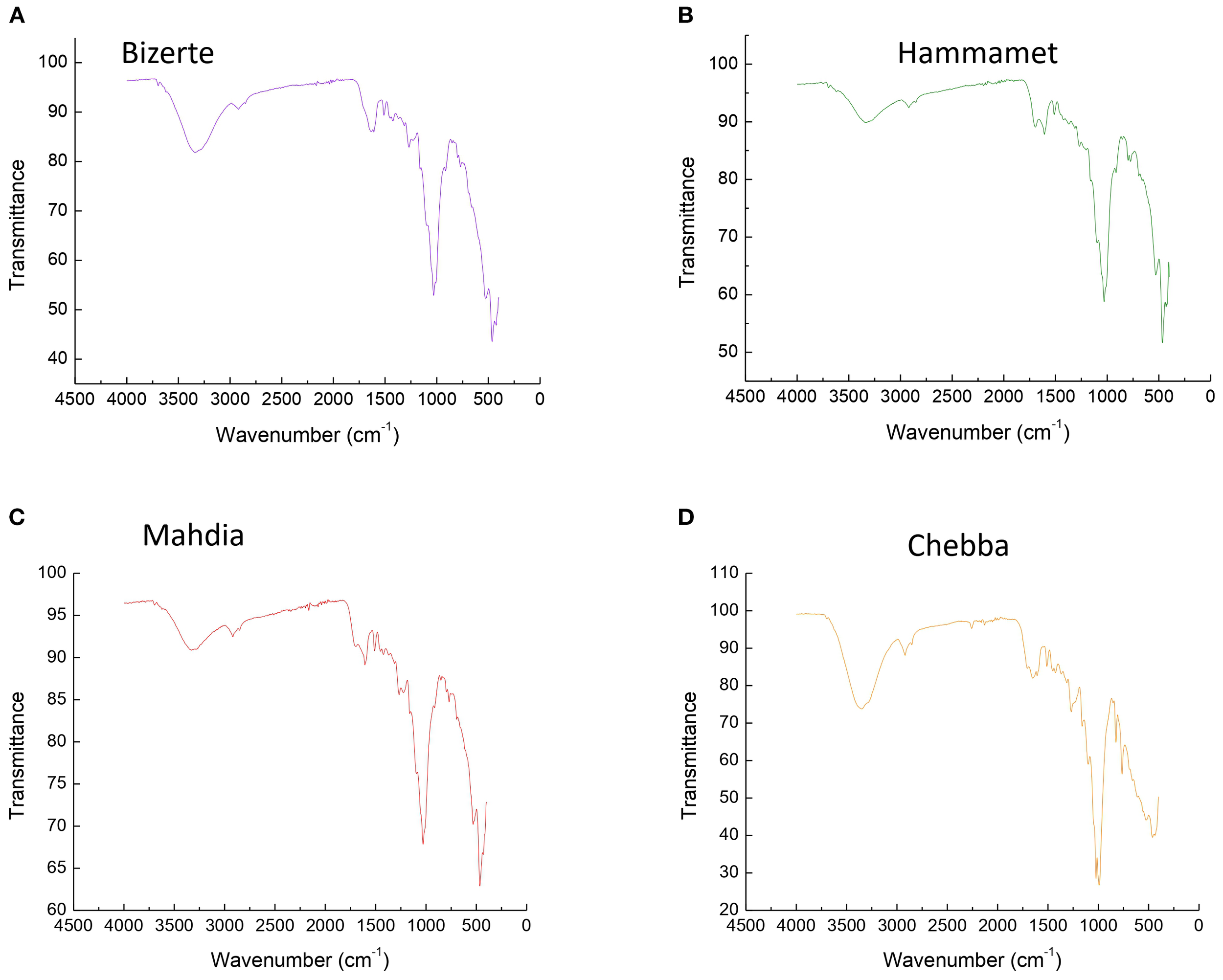
Polymers of microplastic obtained from FTIR spectroscopy reads found in (A) Bizerte, (B) Hammamet, (C) Chebba, and (D) Mahdia (assigned characteristic peaks in cm−1).
At site S3 in the Tunisian east-center, as depicted in Figure 4C, bands observed at 2,900, 1,800, 1,650, 1,300, 1,150, and 1,000 cm−1 indicated the presence of PS and EVA. However, in site S4 (Figure 4D), the spectrum revealed the existence of PVC and PS, characterized by bands at 2,900, 1,800, 1,750, 1,600, 1,400, 1,100, 950, and 600 cm−1.
1H-NMR spectroscopy results
The spectra obtained for each compound separately were determined according to previous studies related to microplastic identifications (Table 2). According to the literature, in site S1, three types of microplastics are determined, namely, PS, PA, and PVC (Figure 5A). In fact, two signals can be clearly assigned to PVC, one of which with a chemical shift of 4.6-4.2 ppm (H1) corresponds to the α-Cl H atoms and the other in the range of 2.5-2.1 ppm (H2) matches with the β-Cl H atom. With respect to PS, four signals were assigned: 1.87 ppm (H1) 1.46 ppm (H2), 6.4-6.8 ppm (H3), and 7.11 ppm (H4, 5). It is worthwhile to note that PA signals at high field with chemical shifts between 3.2 and 1.1 ppm can be assigned to protons H1-H5. The signal with the chemical shift of 3.2-3.0 ppm (H1) aligns with the α-NH H atoms and the signal in the range of 2.3-2.15 ppm (H2) matches the protons H2 of the α-CO group. Signals in the range of 1.65-1.1 ppm represent CH2 groups H3-H5 of the polymer chain.
Table 2
| Polymer | ppm | Assignement | References |
|---|---|---|---|
| PET | 8.119 | H1 | Papini et al., 2022 |
| 4.713 | H2 | ||
| 1.33 | H2 | Peez et al., 2019 | |
| PE | 0.93 | H1 | |
| PS | 1.878 | H1 | Papini et al., 2022 |
| 1.463 | H2 | ||
| 6.400-6.800 | H3 | ||
| 7.112 | H4,5 | ||
| 3.2-3.0 | H1 | Peez and Imhof, 2020 | |
| PA | 2.3-2.15 | H2 | |
| 1.65-1.1 | H3-H5 | ||
| 7.31 | (amines) | ||
| 0.74 | H1 | Hero and Kali, 2020 | |
| PP | 1.19 | H2 | |
| 1.48 | H3 | ||
| PVC | 2.000-2.500 | H2 | Papini et al., 2022 |
| 4.250-4.670 | H1 | ||
| 0.86 | H1 | Ren et al., 2019 | |
| 1.25 | H2 | ||
| EVA | 1.74 | H3 | |
| 2.01 | H4 | ||
| 4.85 | H5 |
1H- NMR characteristic signal assignments in ppm for various types of MPs.
Figure 5
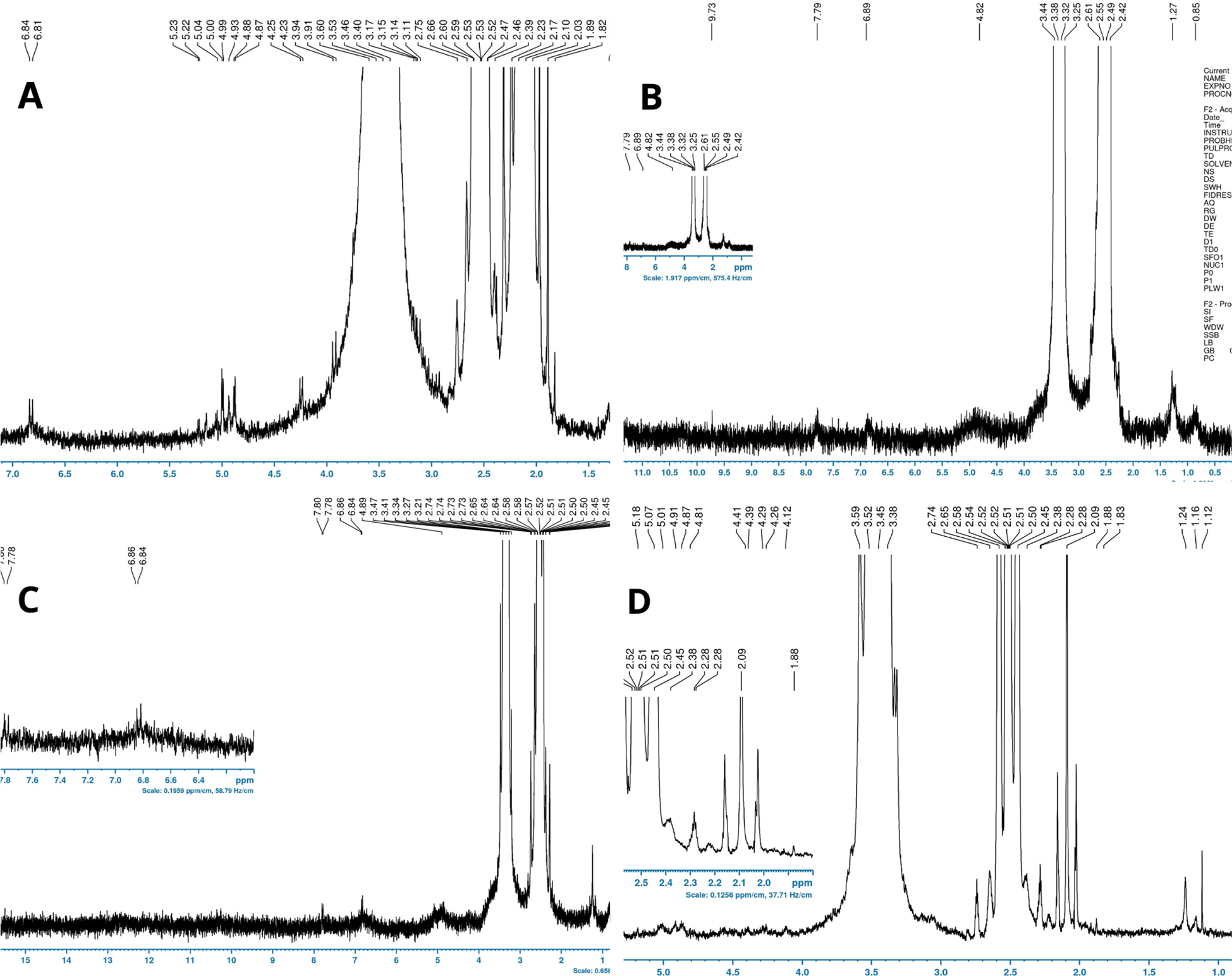
1H-NMR spectra of plastic polymers found in the Tunisian coastlines of the four different sites (A) Bizerte, (B) Hammamet, (C) Chebba, and (D) Mahdia (assigned chemical shifts in ppm).
In site S2 (Figure 5B), EVA, PP, and PE can be observed. As regards EVA, five signals were assigned at 0.86 to H1, 1.25 (H2), 1.74 (H3), 2.01 (H4), and 4.85 to (H5). The PE can be assigned to two signals. The signal at 1.33 ppm (H2) corresponds to the protons of the CH2 groups and the one at 0.93 ppm (H1) corresponds to the protons of the CH3 group. Three signals defined PP and can be seen in the NMR spectrum at 0.74, 1.19, and 1.48 ppm, belonging to the methyl, methylene, and methine groups of oligo/polymeric propylene.
While PVC, EVA, and PP were determined in site S3 (Figure 5C), PA and PP were detected in site S4 (Figure 5D).
Discussion
Microplastics (MPs) are composed of various polymers, and their distribution in the environment depends on both morphological and chemical characteristics (Giaganini et al., 2023). Understanding these characteristics is important because the shape and size of MPs can influence their transport and accumulation in different environments. For instance, morphological traits may help infer how MPs are distributed across coastal areas (horizontally), whereas polymer type can affect their behavior in the water column (vertically). Although our study does not directly assess horizontal or vertical distribution, we adopted a similar approach by characterizing MPs in eagagropiles (EGs) based on morphology (e.g., fragments, threads) and polymer composition, using microscopy and spectroscopy. This characterization helps to understand the types and potential sources of MPs accumulating along the Tunisian coasts. Previous studies have similarly emphasized the importance of such classification (e.g., Anderson et al., 2017; Veerasingam et al., 2020). Microscopy identification of microplastics (Figure 3) showed that fibers particularly red (100%), transparent (100%), and blue (75%) were the most commonly observed in all samples. While the black fragment was revealed in two sites, the yellow fragment was identified in one site (Figure 3). However, Ben Ismail et al. (2022) reported different MP forms in water samples collected in the Gulf of Gabes, with fragments being the most abundant plastic form, whereas fibers, pellets, films, and foams were detected in only a small fraction. This observation was confirmed by Zayen et al. (2020), and five different categories of MP forms, fragments, films, filaments, pellets, and foam, were found in the water of the same site. The color of MP items was white in the majority, whereas blue, black, green, and red items accounted on average for 9%, 5%, 5%, and 3% of the total MPs, respectively.
In other studies, other identified colors, including yellow, orange, gray, and purple, were also present within MPs (4%; 344 of total MPs) (Zhang et al., 2022). Dahl et al. (2021) examined soil collected in PO meadows at three locations along the Spanish Mediterranean coast and reported that the most common particle colors were transparent (30%), white (18%), and green (15%), and the dominant forms were irregular (58%) and flat (30%). In addition, according to several studies, transparent particles of MPs represented approximately 20% to 70% of the total plastic-like particles, initially revealed by stereomicroscopy and afterward classified by other methods (He et al., 2025; Song et al., 2015; Mariano et al., 2021). Likewise, it is difficult to distinguish between natural and synthetic fibers when using a stereomicroscope, which are prevalent constituents in water, soil, and biota (Lusher et al., 2013; de los Santos et al., 2021; Mariano et al., 2021). This allows for the rapid identification of the shape, size, and color of the particles before subsequent characterization by other techniques. The use of microscopy combined with additional methods, such as spectroscopy, enhances the accuracy and comprehensiveness of microplastic analysis (Mariano et al., 2021).
Within the same vein, to determine the type of microplastics accumulated in EGs, FTIR and NMR analyses were carried out (Figures 4, 5). Using two spectroscopy methods allowed for defining the polymers identified in the samples. Different types of microplastics are identified, principally polysterene, propylene, ethyl-vinyl acetate, polyamide, and polyvinyl chloride which are mainly the most found.
The dominance of polymers such as polysterene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), and ethyl-vinyl acetate (EVA) reflects their widespread use in everyday items ranging from packaging, textiles, to construction materials commonly found in the region (Gurjar et al., 2023; Jamsek et al., 2024). Coastal tourism, which plays a vital role in the local economy, often brings seasonal surges of plastic waste, whereas urban runoff, wastewater discharge, and river inputs transport land-based sources into the marine ecosystem (Gurjar et al., 2023; Jamsek et al., 2024).
Several previous studies have confirmed similar polymer distributions in the Mediterranean and other regions (Pourebrahimi and Pirooz, 2023). According to Zayen et al. (2020), the common MP items were attributed to polyolefins, basically polyethylene and reformulated polyethylene, as well as polypropylene (PP) and ethylene–propylene copolymers, from water samples collected in near-surface waters of the Gulf of Gabes. Based on water samples taken from the Gulf of Gabes, Ben Ismail et al. (2022) characterized 11 different polymer typologies determined as PE, constituting the majority of MPs, followed by PP. Less frequent polymers included (<6%) PS, polyvinyl alcohol (PVA), polyamides (PA), acrylic (Acr), ethylene-vinyl acetate (EVA), polyvinyl chloride (PVC), ethylene propylene diene monomer (EPDM), and polyesters (mainly PET). In addition, Ben Ismail et al. (2022) admitted that from biota samples, a mixture of PE and EVA was determined. Nevertheless, Pietrelli et al. (2017) reported that PE and PP were found in significantly higher amounts in sand, whereas PE, nylon, polyester, and microfibers (as pills) were more frequently detected in EGs from samples collected along the central coast of Italy.
In this context, in Tarragona coastal regions, PP and PE fragments were the prevalent MPs on beaches, whereas polyester fibers were dominated in the bottom sediments and saltwater (Expósito et al., 2021). The abundance of fiber balls coupled with bottom sediments, organic materials, and plankton hid the true fibers present in each reservoir (Expósito et al., 2021). Nevertheless, other plastic polymers have been detected from marine sediment from the German coast showing the presence of PP in each sample (Lorenz, 2014). PS was also present throughout the samples, although it occurred less frequently than other polymer types. Denser polymers, such as PVC and PVA, have been detected alongside low-density polymers like PE. In other studies, for example, in the Venice lagoon and in PO meadows adjacent to agricultural hinterland in Spain, PE and PP were revealed as the most abundant materials in seawater and sediments. Additionally, fragments and filaments were the most common forms determined, as signaled by Grego et al. (2022).
Generally, PP, PE, and PET were admitted as common polymer types in the marine environment (Zhou et al., 2021). More deeply, PE and PP are frequently detected materials due to their low density (Pedrotti et al., 2016; Yao et al., 2024), which permits their floating and immersion. These polymers are widely used as polymers in various commodities like packaging, household plastic waste, and numerous personal care and cosmetic products (Cole et al., 2011; Plastic Europe, 2020).
Beyond the Mediterranean, studies such as Ballent et al. (2016) reported the presence of various polymers including PE, PS, PU, PVC, and PSS in high abundance in Canadian Lake Ontario, with other polymers like PET, nylon, and ABS found in smaller quantities. Although our study focuses on the Tunisian coast, these findings support the global ubiquity and diversity of microplastic polymer types, aligning with the polymeric variety we observed in the EGs.
The identified microplastics differ from one site to another, depending on the specific activities in these regions, such as tourism, agriculture, fishing, and industry. In fact, it is difficult to determine their origin because a single polymer may come from several products and have a huge range of sources (Ballent et al., 2016). PE, PP, PS, PA, PVC, EVA, and nylon are the most dominating forms of microplastic distributed in marine, freshwater, and estuarine environments (Zhang et al., 2022). PE and its derivatives are generally used in fishing gears (Wang et al., 2021), food packaging, agricultural film, and plastic bottles and plastic bags (Zhang et al., 2022). However, PP is used in plastic containers, food packaging, carpets, and pipes (Zhang et al., 2022). While PS is used in food containers, rubber tires, and boat hulls, surfboards, bathtubs, and shower enclosures, EVA is used as padding in the equipment of various sports such as ski boots, bicycle saddles, waterski boots, fishing rods, and fishing-reel handles (Zhang et al., 2022).
Anthropogenic pressures, combined with insufficient waste management and densely populated coastlines typical of the Mediterranean basin, contribute significantly to the observed microplastic contamination patterns in Posidonia oceanica seagrass aegagropiles (EGs). Therefore, seagrass meadows constitute an area of microplastic accumulation, which accords well with the findings obtained by Cozzolino et al. (2020), who reported higher macroplastic presence in vegetated coastal areas compared with unvegetated ones. Such variation in microplastic distribution within vegetated zones is influenced by multiple factors, including anthropogenic activities, local physical drivers, and seagrass leaf morphology and spatial distribution (Unsworth et al., 2021).
Although the capacity of microplastic retention generally increases with seagrass canopy density and particle abundance, it tends to decrease with higher flow velocities, with likely varied relationships among particle types (Boshoff et al., 2023). For instance, Zostera capensis leaves are thinner and more flexible than the tougher leaves of Zostera marina, affecting their ability to trap litter (Kreitsberg et al., 2021). The significant accumulation of plastic fragments intertwined within P. oceanica seagrass beds washed ashore on Mediterranean beaches supports the idea that seagrass meadows act as effective litter traps (Sanchez-Vidal et al., 2021; Sghaier et al., 2025). This accumulation is likely enhanced in sheltered coastal areas and influenced by episodic events such as storms or increased runoff during heavy precipitation (Huang et al., 2020).
Furthermore, seagrasses directly influence sediment dynamics by reducing water velocity, which promotes sedimentation and vertical accretion (Jones et al., 2020). This sediment trapping is governed by the availability of terrestrial and marine sediments, as well as by wave and tidal energy (Boshoff et al., 2023). Vegetated coastal systems thus provide the important ecosystem service of capturing and storing environmental contaminants and organic matter, including microplastics (Celis-Hernandez et al., 2020; Veettil et al., 2020; Navarrete-Fernández et al., 2022). Microplastic deposition may be further enhanced by changes in relative density caused by salinity fluctuations, biofouling, and the reduced water flow within dense seagrass canopies (Pinheiro et al., 2021; Cesarini et al., 2023).
Aegagropiles (EGs) with fibrous aggregates of Posidonia oceanica debris and trapped sediments play a crucial ecological role as bioindicators of plastic pollution in coastal marine environments (Sanchez-Vidal et al., 2021; Alomar et al., 2024). Formed and deposited on shorelines, especially following storm events, EGs efficiently capture and retain microplastics and other anthropogenic pollutants, integrating contaminants over time and space (Verhille et al., 2017; Sanchez-Vidal et al., 2021; Rigatou et al., 2025). Their lignocellulosic matrix (a natural structure composed of cellulose, hemicellulose, and lignin) facilitates pollutant retention, making EGs reliable tools for detecting microplastic presence and accumulation. Variations in microplastic concentration and composition across different sites reflect local human pressures and pollution sources (Sanchez-Vidal et al., 2021; Alomar et al., 2024; Rigatou et al., 2025).
Beyond trapping pollutants, EGs provide important insights into the transport and dispersal of plastic debris within marine habitats, as they are moved by waves and currents. Their visible, persistent presence on beaches offers a practical and cost-effective method for monitoring coastal plastic pollution. Utilizing EGs as indicators supports the identification of pollution hotspots, source attribution, and evaluation of waste management effectiveness (Porcino et al., 2023; Restaino et al., 2023).
Conclusion
This study demonstrated that the aegagropiles (EGs), formed from the lignocellulosic debris of Posidonia oceanica meadows, can serve as effective indicators of microplastic pollution along the Tunisian coasts. Through morphological and chemical characterization of the microplastics trapped in the EGs, we found a wide diversity of plastic types of plastics often entangled with the fibrous structure of the EGs. Our findings suggest that EGs contribute to both the accumulation and transport of microplastics from shallow seagrass habitats to coastal areas, in particular during storms that facilitate their movement ashore. These results highlight the ecological role of EGs in reflecting local pollution pressures and underscore the urgent need for targeted strategies to reduce plastic input into marine environments. In particular, the high occurrence of certain polymer types points to potential land-based and maritime sources. Therefore, future research should prioritize tracing the origins of these polymers as such knowledge is critical for designing effective mitigation policies and pollution control efforts.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study did not take place on any private or protected areas. No specific permissions were required for corresponding locations.
Author contributions
DS: Writing – review & editing. IC: Formal Analysis, Methodology, Writing – original draft. TB-S: Funding acquisition, Validation, Writing – review & editing. NZ: Formal Analysis, Methodology, Validation, Writing – review & editing. ME: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alomar C. Compa M. Fagiano V. Concato M. Deudero S . (2024). Posidonia oceanica egagropiles: Good indicators for plastic pollution in coastal areas? Reg. Stud. Mar. Sci.77, 103653. doi: 10.1016/j.rsma.2024.103653
2
Anderson P. J. Warrack S. Langen V. Challis J. K. Hanson M. L. Rennie M. D. et al . (2017). Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut.225, 223–231. doi: 10.1016/j.envpol.2017.02.072
3
Asensio R. C. Moya M. S. A. de la Roja J. M. Gomez M. (2009). Analytical character isation of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal. Bioanal. Chem.395, 2081–2096.
4
Ballent A. Corcoran P. L. Madden O. Helm P. A. Longstaffe F. J. (2016). Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull.110, 383–395. doi: 10.1016/j.marpolbul.2016.06.037
5
Ben Ismail S. Costa E. HeLa J. Morgana S. Boukthir M. Ben Ismail M. A. et al . (2022). Evolution of the distribution and dynamic of microplastic in water and biota: a study case from the Gulf of Gabes (southern Mediterranean Sea). Front. Mar. Sci.385. doi: 10.3389/fmars.2022.786026
6
Bhuyan M. S. Venkatramanan S. Selvam S. Szabo S. Hossain M. M. Rashed-Un-Nabi M. et al . (2021). Plastics in marine ecosystem: A review of their sources and pollution conduits. Reg. Stud. Mar. Sci.41, 101539. doi: 10.1016/j.rsma.2020.101539
7
Blindheim F. H. Ruwoldt J. (2023). The effect of sample preparation techniques on lignin fourier transform infrared spectroscopy. Polymers15, 2901. doi: 10.3390/polym15132901
8
Boshoff B. J. Robinson T. B. von der Heyden S. (2023). The role of seagrass meadows in the accumulation of microplastics: Insights from a South African estuary. Mar. pollut. Bull.186, 114403. doi: 10.1016/j.marpolbul.2022.114403
9
Boudouresque C. F. Pergent G. Pergent-Martini C. Ruitton S. Thibaut T. Verlaque M. et al . (2016). The necromass of the Posidonia oceanica seagrass meadow: fate, role, ecosystem services and vulnerability. Hydrobiologia781, 25–42. doi: 10.1007/s10750-015-2333-y
10
Celis-Hernandez O. Giron-Garcia P. M. Ontiveros-Cuadras J. F. Canales-Delgadillo J. C. Pérez-Ceballos R. Y. Ward R. D. et al . (2020). Environmental risk of heavy metals in mangrove ecosystems: an assessment of natural vs oil and urban inputs. Sci. Total Environ.730, 138643. doi: 10.1016/j.scitotenv.2020.138643
11
Cesarini G. Crosti R. Secco S. Gallitelli L. Scalici M . (2023). From city to sea: Spatiotemporal dynamics of floating macrolitter in the Tiber River. Sci. Total Environ.857, 59713. doi: 10.1016/j.scitotenv.2022.159713
12
Cole M. Lindeque P. Halsband C. Galloway T. S. (2011). Microplastics as contaminants in the marine environment: A review. Mar. Polut Bull. 62, 2588–2597. doi: 10.1016/j.marpolbul.2011.09.025
13
Corti A. La Nasa J. Biale Ceccarini G. A. Manariti A. Petri F . (2023). Microplastic pollution in the sediments of interconnected lakebed, seabed, and seashore aquatic environments: polymer-specific total mass through the multianalytical “PISA” procedure. Anal. Bioanal Chem.415, 2921–2936. doi: 10.1007/s00216-023-04664-0
14
Cozzolino L. Nicastro K. R. Zardi G. de los Santos C. B. (2020). Species-specific plastic accumulation in the sediment and canopy of coastal vegetated habitats. Sci. Total Environ.723, 138018. doi: 10.1016/j.scitotenv.2020.138018
15
Dahl M. Bergman S. Bjork M. Diaz-Almela E. Granberg M. Gullstrom M. et al . (2021). A termporal record of microplastic pollution in Mediterranean seagrass soils. Environ. pollut.273, 116451. doi: 10.1016/j.envpol.2021.116451
16
de los Santos C. B. Krång A. S. Infantes E. (2021). Microplastic retention by marine vegetated canopies: Simulations with seagrass meadows in a hydraulic flum. Environ. pollut.269, 1–10. doi: 10.1016/j.envpol.2020.116050
17
De Luca M. Piazzi L. Guala I. Cinti M. F. Marras P. Pansini A. et al . (2025). Restoration of posidonia oceanica meadowUsingCuttingsfromanArea impactedbyHarborExtensionProject. J. Mar. Sci. Eng.13, 3. doi: 10.3390/jmse13010003
18
Expósito N. Rovira J. Sierra J. Folch J. Schuhmacher M . (2021). Microplastics levels, size, morphology and composition in marine water, sediments and sand beaches. Case study of the Tarragona Coast (western Mediterranean). Sci. Total Environ.786, 147–453. doi: 10.1016/j.scitotenv.2021.147453
19
Folbert M. E. Corbin C. Löhr A. J. (2022). Sources and leakages of microplastics in cruise ship wastewater. Front. Mar. Sci.9, 900047. doi: 10.3389/fmars.2022.900047
20
Fraissinet S. Mancini E. Funiati C. Martino C. De Benedetto G. E. Girelli C. R. et al . (2025). New plastitar record for the mediterranean sea: characterization of plastics and tar from the salento peninsula (Ionian sea). Toxics13, 13. doi: 10.3390/toxics13010013
21
Giaganini G. Cifelli M. Biagini D. Ghimenti S. Corti A. Castelvetro V. et al . (2023). Multi-analytical approach to characterize the degradation of different types of microplastics: identification and quantification of released organic compounds. Molecules28, 1382. doi: 10.3390/molecules28031382
22
Grego M. Viršek M. K. Bajt O. (2022). Microplastics in seawater and sediments-distribution and transport. Plast. pollut. Mar. Conserv. Acad. Press, 31–73. doi: 10.1016/B978-0-12-822471-7.00002-X
23
Gugliandolo E. Licata P. Crupi R. Albergamo A. Jebara A. Lo Turco V. et al . (2020). Plasticizers as microplastics tracers in Tunisian marine environment. Front. Mar. Sci.7, 589398. doi: 10.3389/fmars.2020.589398
24
Gunaalan K. Nielsen T. G. Rodríguez Torres R. Lorenz C. Vianello A. Andersen C. A. et al . (2023). Is zooplankton an entry point of microplastic s into the marine food web? Environ. Sci. Technol.57, 11643–11655. doi: 10.1021/acs.est.3c02575
25
Gurjar U. R. Xavier K. A. M. Shukla S. P. Takar S. Jaiswar A. K. Deshmukhe G. et al . (2023). Seasonal distribution and abundance of microplastics in the coastal sediments of north eastern Arabian Sea. Mar. pollut. Bull.187, 114545. doi: 10.1016/j.marpolbul.2022.114545
26
Hartmann N. B. Huffer T. Thompson R. C. Hassellöv M. Verschoor A. Daugaard A. E. et al . (2019). Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 53, 1039–1047. doi: 10.1021/acs.est.8b05297
27
Hassen B. Sghaier D. B. Matmati E. Mraouna R. El Bour M . (2023). Detection and quantification of microplastics in Posidonia oceanica banquettes in the Gulf of Gabes, Tunisia. Environ. Sci. pollut. Res. 1, 57196–57203. doi: 10.1007/s11356-023-30798-w
28
He H. Yu T. Chen X. Yang Y. Li F. Gan H. et al . (2025). Observation, distribution, and characteristics of microplastics in sediments from the estuary and river network areas of Pearl River Delta, China. Reg. Stud. Mar. Sci.81, 103977. doi: 10.1016/j.rsma.2024.103977
29
Hero D. Kali G. (2020). New, aqueous radical (Co) polymerization of olefins at low temperature and pressure. Processes8, 688. doi: 10.3390/pr8060688
30
Huang Y. Xiao X. Xu C. Perianen Y. D. Hu J. Holmer M. et al . (2020). Seagrass beds acting as a trap of microplastics - emerging hotspot in the coastal region? Environ. pollut.257, 113450. doi: 10.1016/j.envpol.2019.113450
31
Jamsek J. Prosen H. Bajt O. (2024). Differences in distribution and characteristics of microplastics in sediments of the southeastern part of the Gulf of Trieste. Front. Mar. Sci.11. doi: 10.3389/fmars.2024.1436565
32
Jones K. L. Hartl M. G. J. Bell M. C. Capper A. (2020). Microplastic accumulation in a Zostera marina L. bed at Deerness Sound, Orkney, Scotland. Mar. pollut. Bull.152, 110883. doi: 10.1016/j.marpolbul.2020.110883
33
Jung M. R. Horgen F. D. Orski S. V. Rodriguez V. C. Beers K. L. Balaz G. H. et al . (2018). Validation of ATR FTIR to identify polymers of plastic marine deb ris, including those ingested by marine organisms. Mar. Pollut. Bull.127, 704–716.
34
Kreitsberg R. Raudna-Kristoffersen M. Heinlaan M. Ward R. Visnapuu M. Kisand V. et al . (2021). Seagrass beds reveal high abundance of microplastic in sediments: A case study in the Baltic Sea. Mar. pollut. Bull.168, 112417. doi: 10.1016/j.marpolbul.2021.112417
35
Lorenz C. (2014). Detection of microplastics in marine sediments of the German Coast via FT-IR spectroscopy ( Diss. Universität Rostock). Available online at: https://epic.awi.de/id/eprint/35957/ (Accessed May 22, 2014).
36
Lusher A. L. McHugh M. Thompson R. C . (2013). Occurrence of MPs in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull.67, 94–99. doi: 10.1016/j.employable.2012.11.028
37
Mariano S. Tacconi S. Fidaleo M. Rossi M. Dini L . (2021). Micro and nanoplastics identification: classic methods and innovative detection techniques. Front. Toxicol.3, 636–640. doi: 10.3389/ftox.2021.636640
38
Markic A. Gaertner J. C. Gaertner-Mazouni N. Koelmans A. A. (2020). Plastic ingestion by marine fish in the wild. Crit. Rev. Environ. Sci. Technol.50, 657–697. doi: 10.1080/10643389.2019.1631990
39
Matheson F. E. Reed J. Dos Santos V. M. Mackay G. Cummings V. J . (2017). Seagrass rehabilitation: successful transplants and evaluation of methods at different spatial scales. N Z J Mar Freshwater Res. 51, 96–109. doi: 10.1080/00288330.2016.1265993
40
Mecozzi M. Pietroletti M. Monakhova Y. B. (2016). FTIR spectroscopy supported by statistical techniques for the structural characterisation of plastic debris in the marine environment: Application to monitoring studies. Mar. Pollut. Bull.106, 155–161.
41
Navarrete-Fernández T. Bermejo R. Hernández I. Deidun A. Andreu-Cazenave M. Cózar A. et al . (2022). The role of seagrass meadows in the coastal trapping of litter. Mar. pollut. Bull.174, 113299. doi: 10.1016/j.marpolbul.2021.113299
42
Nishikida K. Coates J . (2003). Infrared and Raman analysis of polymer. In LoboH.BonillaJ. V. (Eds), Handbook of plastics analysisMarcel Dekker, Inc.186–316.
43
Noda I. Dowrey A. E. Haynes J. L. Marcott C . (2007). Group frequency assignments for major infrared bands observed in common synthetic polymers. In MarkJ. E. (Ed), Physical properties of polymers handbookSpringer395–406.
44
Nguyen M. K. Rakib M. R. J. Hwangbo M. Kim J. (2025). Microplastic accumulation in soils: Unlocking the mechanism and biodegradation pathway. J. Hazard Mater Adv.17, 100629. doi: 10.1016/j.hazadv.2025.100629
45
Papini G. Petrella G. Cicero D. O. Boglione C. Rakaj A . (2024). Identification and quantification of olystyrene microplastics in marine sediments facing a river mouth through NMR spectroscopy. Mar. Pollut. Bull. 198, 115–784.
46
Pedrotti M. L. Petit S. Elineau A. Bruzaud S. Crebassa J. C. Dumontet B. et al . (2016). Changes in the Floating Plastic Pollution of the Mediterranean Sea in relation to the distance to land. PloS One11, e0161581. doi: 10.1371/journal.pone.0161581
47
Peez N. Imhof W. (2020). Quantitative 1H-NMR spectroscopy as an efficient method for identification and quantification of PVC ABS and PA microparticles. Analyst145, 5363–5371. doi: 10.1039/D0AN00879F
48
Peez N. Becker J. Ehlers S. M. Fritz M. Fischer C. B. Koop J. H. E. et al . (2019). Quantitative analysis of PET microplastics in environmental model samples using quantitative 1H-NMR spectroscopy: Validation of an optimized and consistent sample clean-up method. Anal. Bioanal. Chem.411, 7409–7418.
49
Pietrelli L. Di Gennaro A. Menegoni P. Lecce F. Poeta G. Acosta A. T. R. et al . (2017). Pervasive plastisphere: First record of plastics in egagropiles (Posidonia spheroids). Environ. pollut.229, 1032–1036. doi: 10.1016/j.envpol.2017.07.098
50
Piñeiro-Juncal N. Díaz-Almela E. Leiva-Dueñas C. Deulofeu O. Frigola J. Soler M. et al . (2021). Processes driving seagrass soils composition along the western Mediterranean: The case of the southeast Iberian Peninsula. Sci. Total Environ.768, 144352. doi: 10.1016/j.scitotenv.2020.144352
51
Pinheiro L. M. Agostini V. O. Lima A. R. A. Ward R. D. Pinho G. L. L . (2021). The fate of plastic litter within estuarine compartments: An overview of current knowledge for the transboundary issue to guide future assessments. Environ. pollut., 116908. doi: 10.1016/j.envpol.2021.116908
52
Plastic Europe . (2020). Plastics-the Facts 2020 An Analysis of European plastics production, demand and waste data. Vienna: Plastic Europe. Available at: https://www.Plasticseurope.org/en/resources/publications/1804-plastics-facts-2019
53
Porcino N. Bottari T. Falco F. Natale S. Mancuso M . (2023). Posidonia spheroids intercepting plastic litter: implications for beach clean-ups. Sustainability15, 15740. doi: 10.3390/su152215740
54
Pourebrahimi S. Pirooz M. (2023). Microplastic pollution in the marine environment: A review. J. Hazard Mater10, 100327. doi: 10.1016/j.hazadv.2023.100327
55
Ren L. Men L. Zhang Z. Guan F. Tian J. Wang B. et al . (2019). Biodegradation of polyethylene by Enterobacter sp. D1 from the Guts of wax moth Galleria mellonella. Int. J. Environ. Res. Public Health16, 1941. doi: 10.3390/ijerph16111941
56
Restaino O. F. Giosafatto C. V. L. Mirpoor S. F. Mirpoor S. F. Cammarota M. Hejazi S. et al . (2023). Sustainable exploitation of posidonia oceanica sea balls (Egagropili): A review. Int. J. Mol. Sci.24, 7301. doi: 10.3390/ijms24087301
57
Rigatou D. Gerakaris V. Digka N. Adamopoulou A. Patsiou D. Hatzonikolakis Y. et al . (2025). The role of seagrass meadows (Posidonia oceanica) as microplastics sink and vector to benthic food webs. Mar. pollut. Bull.211, 117420. doi: 10.1016/j.marpolbul.2024.117420
58
Sait S. T. L. Sørensen L. Kubowicz S. Vike-Jonas K. Gonzalez S. V. Asimakopoulos A. G. et al . (2021). Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content. Environ Pollut. 268, 115745. doi: 10.1016/j.envpol.2020.115745
59
Sanchez-Vidal A. Canals M. de Haan W. P. Romero J. Veny M . (2021). Seagrasses provide a novel ecosystem service by trapping marine plastics. Sci. Rep.11, 254. doi: 10.1038/s41598-020-79370-3
60
Sarkar P. Xavier K. A. M. Shukla S. P. Bhuvaneswari G. R. (2025). Nanoplastic exposure inhibits growth, photosynthetic pigment synthesis and oxidative enzymes in microalgae: A new threat to primary producers in aquatic environment. J. Hazard Mater Adv.17, 100613. doi: 10.1016/j.hazadv.2025.100613
61
Sghaier D. B. Chniti I. Hassen Barhoumi‑Slimi B. T. El Bour M . (2025). Spectroscopic characterization and accumulation of microplastics in the banquette of Posidonia oceanica in the Tunisian coasts. Euro-Mediterr J. Environ. Integr.10, 2619–2631. doi: 10.1007/s41207-025-00853-8
62
Sharma S. Bhardwaj A. Thakur M. Saini A . (2024). Understanding microplastic pollution of marine ecosystem: a review. Environ. Sci. Pollut. Res. doi: 10.1007/s11356-023-28314-1
63
Song Y. K. Hong S. H. Jang M. Han G. M. Rani M. Lee J. et al . (2015). A comparison of microscopic and spectroscopic identification methods for analysis of MPs in environmental samples. Mar. Pollut. Bull. 93, 202–209. doi: 10.1016/j.employable.2015.01.015
64
Tahir A. Samawi M. F. Sari K. Hidayat R. Nimzet R. Wicaksono E. A. et al . (2019). Studies on microplastic contamination in seagrass beds at Spermonde Archipelago of Makassar strait, Indonesia. J. Phys. Conf Ser.1341, 1341. doi: 10.1088/1742-6596/1341/2/022008
65
Tang K. H. D. (2024). A review of the toxic effects of microplastics based on studies on mammals and mammalian cell lines. Environ. Sci. Adv.3, 1669–1678. doi: 10.1039/D4VA00227J
66
Telesca L. Belluscio A. Criscoli Ardizzone A. G. Apostolaki E. T. Fraschetti S . (2015). Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep.5, 12505. doi: 10.1038/srep12505
67
Unsworth R. K. F. Higgs A. Walter B. Unsworth L. C. C. Inman I. Jones B. L. et al . (2021). Canopy accumulation: are seagrass meadows a sink of microplastics? Oceans2, 162–176. doi: 10.3390/oceans2010010
68
Veerasingam S. Ranjani M. Venkatachalapathy R. Bagaevc A. Mukhanovd V. Litvinyuk D. et al . (2020). Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 51, 2681–2743. doi: 10.1080/10643389.2020.1807450
69
Veettil B. Ward R. D. Lima M. Stankovic M. Hoai P. N. Quang N. X. et al . (2020). Opportunities for seagrass research derived from remote sensing: a review of current methods. Ecol. Indic.117, 106560. doi: 10.1016/j.ecolind.2020.106560
70
Verhille G. Le Gal P. (2018). “ Aggregation of fibers by waves,” in Nonlinear Waves and Pattern Dynamics. Eds. AbchaN.PelinovskyE.MutabaziI. ( Springer, Cham). doi: 10.1007/978-3-319-78193-8_8
71
Verhille G. Moulinet S. Vandenberghe N. Le Gal P . (2017). Structure and mechanics of aegagropilae fiber network. Proc. Natl. Acad. Sci. U S A.114, 4607–4612. doi: 10.1073/pnas.1620688114
72
Verleye G. A. Roeges N. P. De Moor M. O . (2001). Easy identification of plastics and rubbers. Rapra Technology Limited pp. 174.
73
Wang G. Huang D. Ji J. Volker C. Wurm F. R. (2021). Seawater-degradable polymers—fighting the marine plastic pollution. Adv Sci. 8, 2001121. doi: 10.1002/advs.202001121
74
Yao J. Li J. Qi J. Wan M. Tang L. Han H. et al . (2024). Distribution patterns and environmental risk assessments of microplastics in the lake waters and sediments from eight typical wetland parks in Changsha city, China. Front. Public Health12. doi: 10.3389/fpubh.2024.1365906
75
Yuan C. Almuhtaram H. McKie M. J. Andrews R. C . (2022). Assessment of microplastic sampling and extraction methods for drinking waters. Chemosphere286, 131881, doi: 10.1016/j.chemosphere.2021.131881
76
Zayen A. Sayadi S. Chevalierm C. Boukthir M. Ismail S. B. Tedetti M. (2020). Microplastics in surface waters of the Gulf of Gabes, southern Mediterranean Sea: distribution, composition and influence of hydrodynamics. Estuar. Coast Shelf Sci. 242, 106, 832. doi: 10.1016/j.ecss.2020.106832
77
Zhang M. Liu S. Bo J. Zheng R. Hong F. Gao F. et al . (2022). First evidence of microplastic contamination in antarctic fish (Actinopterygii, perciformes). Water14, 3070. doi: 10.3390/w14193070
78
Zhou Q. Tu C. Yang J. Fu C. Li Y. Waniek J. J. et al . (2021). Trapping of microplastics in halocline and turbidity layers of the semi-enclosed baltic sea. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.761566
Summary
Keywords
Posidonia oceanica , aegagropile, microplastics, acid digestion, NMR spectroscopy, FTIR, stereomicroscopy
Citation
Sghaier DB, Chniti I, Barhoumi-Slimi T, Zaaboub N and EL Bour M (2025) The trapping of microplastics in the Posidonia oceanica aegagropiles in Tunisian coastal areas—Southern Mediterranean. Front. Mar. Sci. 12:1663783. doi: 10.3389/fmars.2025.1663783
Received
10 July 2025
Accepted
17 September 2025
Published
14 October 2025
Volume
12 - 2025
Edited by
Julius A. Ellrich, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), Germany
Reviewed by
Mario De Luca, University of Sassari, Italy; Paolo Marras, University of Cagliari, Italy
Updates
Copyright
© 2025 Sghaier, Chniti, Barhoumi-Slimi, Zaaboub and EL Bour.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dhouha Belhaj Sghaier, dhouhasghaier@hotmail.fr
ORCID: Dhouha Belhaj Sghaier, orcid.org/0000-0001-5733-8377
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.