Abstract
Food waste is considered as a critical global issue in food production, leading to environmental and economic consequences. Repurposing food industry by-products for animal feed can reduce waste, lower feed costs, and improve sustainability in aquaculture. Solid-state fermentation (SSF) has emerged as a promising biotechnological approach to enhance the nutritional value of these by-products. SSF employs microorganisms such as fungi, yeasts, and bacteria to convert low-value agro-industrial residues into bioactive-rich, digestible feed ingredients. This process reduces anti-nutritional factors (ANFs) like phytic acid and tannins, commonly found in plant-based meals, while producing beneficial enzymes and organic acids that support gut health and nutrient utilization. Compared to conventional methods, SSF is energy-efficient, produces minimal wastewater, and aligns well with circular bioeconomy principles. Although fishmeal and soybean meal remain dominant protein sources in aquafeed, their sustainability issues call for alternatives. SSF derived ingredients improve feed efficiency, growth, and immunity in aquatic species, offering a viable substitute. However, challenges remain in optimizing microbial strains, fermentation conditions, and substrate safety. This review discusses SSF’s mechanisms, benefits, and applications in aquafeed, highlighting recent advances, current limitations, and future directions for establishing SSF as a sustainable solution in modern aquaculture feed development.
1 Introduction
Food waste presents a major challenge, with about 19% of consumer-available food (approx. 931 million tonnes) wasted in 2024, accounting for 8-10% of global greenhouse gas emissions, $1 trillion in economic losses, and heightened strain on global food systems (Food, Waste Index Report, 2024), contributing to environmental degradation, economic losses, and increased pressure on global food systems (EFFPA, 2018; Economou et al., 2024). Utilizing food industry by-products as alternative raw materials can decrease reliance on global markets, cut costs, and boost the aquaculture sector’s competitiveness, though enhancement of nutritional profiles of aquafeed for potential use in aquafarms. Currently, 5 million tons of raw food are redirected to animal feed, a figure expected to rise to 7 million tons by 2025 (EFFPA, 2018; Ibarruri et al., 2024). Solid-state fermentation (SSF) has gained grip for its remediation potential, transforming underutilized biomass into valuable products, addressing environmental issues from improper waste disposal (EFFPA, 2018). SSF is a heterogeneous process involving three phases, such as solid, liquid, and gas and presents numerous advantages for microbial cultivation in bioprocessing and product development. The resulting products contribute to secondary fermentation within the gastrointestinal tract of livestock, delivering residual bioactive compounds, such as enzymes, organic acids, and peptides, capable of hydrolysing the indigestible dietary components, including complex carbohydrates and fibers. Research has shown improved growth and feed efficiency in animals when SSF-derived products are included in modern aquafeed and poultry diets (Hooge et al., 2010; Hassaan et al., 2017).
Over the past two decades, SSF has attracted considerable interest for industrial applications, primarily because it requires less energy, delivers higher product yields, and generates minimal wastewater, all while reducing the likelihood of bacterial contamination. Moreover, it is environmentally friendly, as it typically employs solid agro-industrial residues as the primary carbon source (Thomas et al., 2013). SSF’s ability to valorize agricultural byproducts aligns with sustainability goals, reducing waste and reliance on costly fishmeal while enhancing the nutritional and functional quality of aquafeed (Thomas et al., 2013; Sadh et al., 2018a,b; Vieira et al., 2023; Ibarruri et al., 2024). This eco-friendly, cost-effective method holds immense potential for aquaculture, addressing nutritional challenges and supporting a more sustainable feed industry (Verduzco-Oliva and Gutierrez-Uribe, 2020). In the aquaculture feed industry, fishmeal and soybean meal remain the dominant protein sources due to their high digestibility and balanced amino acid profiles (Bowyer et al., 2020). However, their limited global availability and unsustainable production call for reduced usage in feeds (Watanabe, 2002; Ibarruri et al., 2024). Although alternatives like animal by-products, plant-based feeds (mainly soya), and single-cell proteins are used (Miles and Chapman, 2006; Yang et al., 2021), they often fall short in amino acid balance, digestibility, and palatability, and may contain antinutrients.
Despite a decent protein content, plant-based meals are constrained by ANFs like phytic acid, trypsin inhibitors, and non-starch polysaccharides (Mandal and Ghosh, 2013; Mandal and Ghosh, 2019). Conventional methods, such as heat treatment, soaking, and germination, often inadequately reduce ANFs but can also result in nutrient losses (Saha and Ray, 2011; Mandal and Ghosh, 2020). From an evolutionary perspective, gut microbiota in herbivorous fish helps to counteract the negative effects of plant-derived ANFs (Xu et al., 2021). Gut microbiota in other fish, incapable of breaking down cellulose, tannins, phytates, and xylans, whereas SSF helps to alleviate ANF impacts, by improving nutrient utilization (Soltani et al., 2019; Ringø et al., 2022). Recent studies have shown that the inclusion of SSFs in fish feed promotes beneficial gut microbiota in various fish species, enhancing host nutrition (Ray et al., 2012; Ringø et al., 2022). Though less studied in aquatic species, in vitro solid-state fermentation (SSF) is considered a promising method to decrease ANFs in plant feed, with microbial enzymes improving nutrient bioavailability in the processed aqua feed.
The global aquaculture industry faces increasing pressure to develop sustainable, cost-effective, and nutritionally optimized feed solutions to meet the rising demand for aquatic products. Solid-state fermentation (SSF) has emerged as a promising technology to address these challenges by enhancing the nutritional quality of aquafeed, reducing ANFs, and promoting environmental sustainability through the utilization of agro-industrial byproducts. By leveraging the enzymatic capabilities of microorganisms such as fungi, yeasts, and bacteria, SSF transforms low-value substrates into nutrient-rich feed ingredients, improving digestibility, gut health, and immune responses in aquatic species. Despite its potential, challenges remain in optimizing fermentation parameters, ensuring the safety of SSF-derived products, and exploring novel substrates and microbial strains for greater efficacy.
Therefore, this review article is targeted to addresses the critical research gaps, including the environmental sustainability of SSF, details its biochemical mechanisms, compares it with other processing methods, and evaluates its applications in aquafeed through case studies on nutritional benefits, growth performance, gut microbiota modulation, and immune system enhancement. This review also critically discusses the limitations of microbial applications in enhancing the nutrient profile of aquafeed ingredients, and highlights the current challenges and future research directions required to advance SSF technology for sustainable aquaculture feed production.
2 Solid-state fermentation
2.1 Principle
Solid-state fermentation (SSF) uses low-water substrates and could be an alternative to conventional aquaculture feed (Betchem et al., 2024). As a bio-process, SSF develops low-value agro-industrial byproducts into high-value feed nutrients while enhancing the nutritional value of aquafeeds by adding microbial growth, enzymatic activity, and bioactive components (Dawood and Koshio, 2020). The added advantage of microbial growth by SSF is that SSF conditions are more favorable than anything conceivable, providing preferential microbial growth that can exist naturally (Betchem et al., 2024). Microbial growth via SSF can improve digestibility, nutrient bioavailability, and feed efficiency in aquaculture (Bowyer et al., 2020). Solid-state fermentation cultivates microorganisms on solid substrates with a low moisture content, usually 40-60% (Bhargav et al., 2008). It is a mode of fermentation that mimics the natural conditions that microbes would encounter when they break down complex organic sources into simpler forms that aquatic organisms can take up (Singhania et al., 2018). Agricultural waste products, such as cassava peel, rice bran, soybean meal, and wheat straw, are typically used as substrates in SSF for aquaculture (Yafetto et al., 2023).
The fermentation process can be controlled by varying temperature, moisture, pH, and aeration parameters. Each parameter must be fine-tuned to ensure the growth of specific microorganisms (Singhania et al., 2017). SSF allows microorganisms to break down a plant material (with agricultural wastes typically having much higher nutrient content), and it enhances the nutritional profile of the substrate, including protein digestibility, and reduces anti-nutritional factors (Sadh et al., 2018a) as shown in Figure 1. In addition, SSF allows for the production of essential enzymes (e.g., proteases, lipases, cellulases, and amylases) that improve the bioavailability of nutrients in aquafeeds (Vieira et al., 2023). SSF also allows for the production of secondary metabolites, such as antimicrobial peptides and bioactive compounds, that could improve fish health and immune responses (Verduzco-Oliva and Gutierrez-Uribe, 2020).
Figure 1
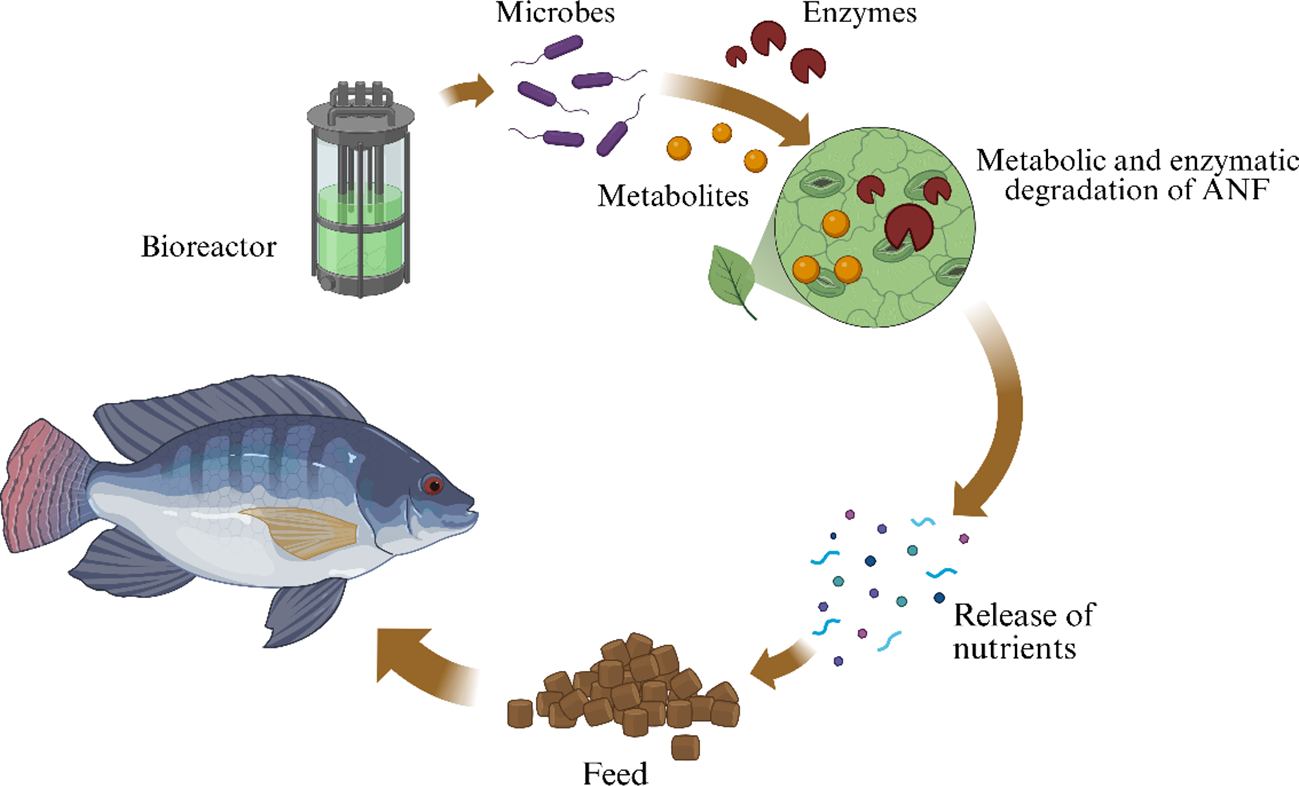
Principle of solid-state fermentation in aquafeed.
2.2 Microorganisms utilized in SSF
Microorganisms employed during SSF are carefully chosen for their enzymatic features, substrate specificity, and ability to grow under low-moisture conditions (El-Bakry et al., 2015). The microorganisms most widely used in SSF for feeding aquaculture include fungi (filamentous fungi and yeasts) and bacteria (Vandenberghe et al., 2021).
2.2.1 Filamentous fungi and yeasts
Common species of fungi employed in SSF, such as Aspergillus niger, Trichoderma reesei, Rhizopus oligosporus, and Pleurotus ostreatus, have been utilized because of the extensive array of enzymes they produce (Feng et al., 2024). As shown in Table 1, fungi produce a wide range of enzymes such as cellulase, xylanase, protease, and lipase, which decompose complex polysaccharides, proteins, and lipids in a variety of plant-based feed materials (El-Gendi et al., 2021). A. niger has been used in SSF for the production of enzyme proteases from aquaculture sludge (Kuan et al., 2024). The enzymatic action of fungi provides improved nutritional value, and their incorporation offers an indirect way to reduce anti-nutritional factors, such as phytate, tannins, and protease inhibitors, which seems to be a major problem in aquaculture systems (Onomu and Okuthe, 2024). Because the requirement utilized in SSF closely resembles the natural habitat of fungi, the growth of these microorganisms can significantly alter the substrate composition they inhabit. Fungi have been shown to enrich lignocellulosic materials with microbial proteins and enzymes. This bioconversion process reduces the crude fiber content while enhancing crude protein levels, protein solubility, and digestibility of both protein and fiber. As a result, the nutritional value of plant-based feedstuffs has improved, making them more suitable for use in aquaculture (Vieira et al., 2023).
Table 1
| Micro-organism | Major enzymes produced | Effects on aquafeed |
|---|---|---|
| Filamentous fungi | Amylase, Protease, Lipase, Cellulase, Phytase, Xylanase, Pectinase, and β-glucanase (Wösten, 2019). | Protease and Amylase: Protein and starch are broken down which increases digestibility. Lipase: Hydrolyses triglyceride ester linkages to produce glycerol and fatty acids, improves fat digestion, and lowers crude fat in fermented foods. Cellulase, xylanase, and β-glucanase: Enhance the digestibility of fiber by breaking down non-starch polysaccharides (NSPs) Phytase: Enhances mineral bioavailability in animal feed by hydrolyzing phytic acid and liberating phosphorus. Pectinase: Enhances the digestion of carbohydrates by breaking down pectin-rich fiber. Lacasses: Oxidate phenolic compounds and lignin. Invertase: also known as sucrase, increases the availability of sugar by converting sucrose to glucose and fructose. |
| Bacteria | Amylase, Protease, Lipase, Cellulase, Phytase, Xylanase, Laccases (Quax, 2013). | |
| Yeast | Amylase, Protease, Invertase (Sucrase), and β-glucanase (Amillano-Cisneros et al., 2025; Sultana et al., 2024) |
Key microorganisms (fungi, yeast, bacteria), their main enzymatic outputs, and their primary effects on aquafeed.
Jannathulla et al. (2017) and (2018) revealed that A. niger fermented guar meal and soybean meal fed to Penaeus vannamei shows fermented meals could be utilized successfully as a potent protein source than the untreated ingredients in the diet of shrimp. Similarly, Kim et al. (2009) and (2010) revealed that Aspergillus oryzae-fermented soybean meal was fed to Oplegnathus fasciatus, and Paralichthys olivaceus enhanced phosphorus absorption and non-specific immune mechanisms in fish. Other commercially available fungal species employed for the fermentation of soybean meal are Candida utilis (Zhou et al., 2011), and Aspergillus niger (Jannathulla et al., 2019), which have been observed to enhance the health status and growth performance of fish. In this regard, Amaral et al. (2023) and Vieira et al. (2023) recorded that A. niger has been used in SSF was fed to European seabass (Dicentrarchus labrax) juveniles showed improved growth performances with excellent survivability. Similarly, Aspergillus ibericus, when employed as an SSF product prepared using a red algae by-product, was fed to D. labrax and was recorded to increase the immune mechanism of fish (Ferreira et al., 2025). Likewise, Aspergillus carbonarius, A. ibericus, and A. uvarum were utilized in the SSF of corn distillers’ dried grains fed to E. labrax to improve its digestibility (Filipe et al., 2023).
In SSF, Saccharomyces cerevisiae, Candida utilis, and Kluyveromyces marxianus are some of the yeasts that can be used to produce single-cell proteins (SCP) that are rich in amino acids, vitamins, and minerals and contribute to aqua-feeding nutrition by supplying bioavailable protein and improving the feed intake of fish (Bilal et al., 2022). Yeast fermentation can also enhance feed stability during storage (Dai et al., 2020). Yeasts can also produce bioactive compounds that are immuno-supportive in aquatic species (Tadioto et al., 2023). Sharawy et al. (2016) noted that Saccharomyces cerevisiae employed in soybean meal was fed to Fenneropenaeus indicus, which shows a 50% replacement of fish meal protein with better growth performance. Similarly, Dossou et al. (2018) reported that S. cerevisiae fermented rapeseed meal fed to Pagrus major resulted in 56.25% fishmeal replacement with better feed utilization and growth performance. Likewise, Plaipetch and Yakupitiyage (2012) found that S. cerevisiae fermented canola meal fed to Lates calcarifer and Oreochromis niloticus revealed that 50% fish meal replacement resulted in excellent growth performance of fish. Wang et al. (2024) reported that common carp (Cyprinus carpio) fed products manufactured using SSF showed increased resistance against springviremia of carp virus. Other commercially available yeast species of Ganodermalucidum fermented mushroom bran hydrolysate and a proportion of 64-80% fishmeal were fed to Carrassius auratus gibelio, which revealed improved growth, digestive enzymes, and antioxidant status of fish (Zhang et al., 2017).
2.2.2 Bacteria
Bacteria, such as Bacillus subtilis, Lactobacillus spp., and Streptomyces, play an essential role during SSF because they produce extracellular enzymes (Table 1) and bioactive compounds, which can promote digestion of feed in fish gut health by increasing beneficial microbiota and producing antimicrobial peptides by reducing pathogenic load (De Villa et al., 2023). Refstie et al. (2005) revealed that when Lactobacillus brevis fermented soybean white flakes were fed Salmo salar, it improved the growth performance of fish, as lactic acid fermentation enhanced the nutritional value of soybean white flakes. Similarly, Yamamoto et al. (2010) reported that Bacillus spp. fermented soybean meal fed Oncorhynchus mykiss enhanced feed digestibility and fish growth performance. Likewise, Wang et al. (2016) observed that when Lactobacillus plantarum PB fermented soybean meal was fed to Scophthalmus maximus L shows 45% fish meal replacement with better feed digestibility. Similarly, C. carpio fed with 0.3 and 0.4 g/kg SSF product of Bacillus velezensis has an improved inflammatory response to gut health and modulates the gut microbiota of species (Chen et al., 2025). Other species of bacteria (Bacillus substilis E20; Shiu et al., 2015, Bacillus subtilis; Lee et al., 2016a, Bacillus subtilis U304; Moniruzzaman et al., 2018, Lactobacillus spp.; Lin and Mui, 2017) were employed to ferment soybean meal shown to improve growth performances of fishes by enhancing health status.
2.3 Substrates used in SSF
The growth of microorganisms and yield of the end product in SSF are significantly influenced by substrates from different sources. Different organisms used in the fermentation mechanism belong to various species, each capable of producing distinct metabolites, such as lactic acid, ethanol, or acetic acid, depending on the substrate employed (Siddik et al., 2024). According to this hypothesis, Lactobacillus species are known to produce lactic acid and citric acid, whereas yeasts primarily generate ethanol and carbon dioxide. Therefore, the substrate was selected to provide a developing culture with nutritional and physical support (Bhargav et al., 2008). The most commonly used solid substrates are cereal grains, such as corn and wheat, and a variety of components and byproducts from plants and animals (fishery byproducts, poultry, and legume seeds) (Šelo et al., 2021). A 25% mixture of rapeseed, soybean, rice bran, and sunflower seed meal has been used as a substrate for SSF feeding of D. labrax (Vieira et al., 2023). In addition, corn starch and soybean protein concentrate were incorporated as substrates in the SSF process for feeding Nile tilapia (O. niloticus) (Bowyer et al., 2020). Fisheries byproducts such as prawn shell powder have also been used in the SSF process for aquaculture effluent treatment (Kuan et al., 2024). The final products of fermentation vary depending on the type and composition of the substrate. It is also noted that the lower water content within the SSF process boosts the process with lower wastewater levels (Chilakamarry et al., 2022). In this regard, many agro-industrial byproducts with high water content, such as pomaces, can be used when no additional water is needed for the substrate. Using natural and agro-based substrates to encourage pigment creation by microorganisms has become a crucial research approach in microbial biotechnology (Venil et al., 2020). For example, in submerged fermentation, various agro-waste substrates have been tested against different bacteria (Sadh et al., 2018b).
3 Biochemical mechanisms behind nutrient enrichment in SSF
SSF enhances the nutritional profile of plant-based ingredients through microbial action. During SSF, the selected microorganisms grow on the surface of the solid substrate and secrete enzymes through breaking down the complex compounds, reduce ANFs, and produce the essential nutrients. Despite increase in the nutritional profile, SSF also produces microbial metabolites in an environment local to their habitats, contributing to overall feed functionality and digestibility (Ulmer et al., 1981).
3.1 Metabolites production
Microbial metabolites in solid-state fermentation (SSF) are primarily the result of metabolic activities by microorganisms, such as fungi, bacteria, and yeasts, which grow on solid substrates with minimal free water. These microorganisms enzymatically degrade complex polymers, such as cellulose, starch, and lignin, which serve as both carbon and nutrient sources, into simpler compounds as shown in Figure 2. This process involved several key steps. First, the substrate was selected and inoculated with the desired microorganism, followed by microbial growth either on the surface or within the matrix of the substrate, depending on the organism and substrate porosity. Following colonization, complex macromolecules are hydrolyzed into simple sugars or nutrients, which are then utilized by microbes for growth and maintenance. As microbial activity progresses, especially under controlled SSF conditions (e.g., moisture, temperature, pH, and oxygen), secondary metabolites such as enzymes, organic acids, antibiotics, and other bioactive compounds are synthesized. The efficiency and yield of these valuable metabolites are highly dependent on the optimization of these physicochemical parameters, making SSF a fine-tuned and environmentally sustainable bioprocess (Robinson et al., 2001).
Figure 2
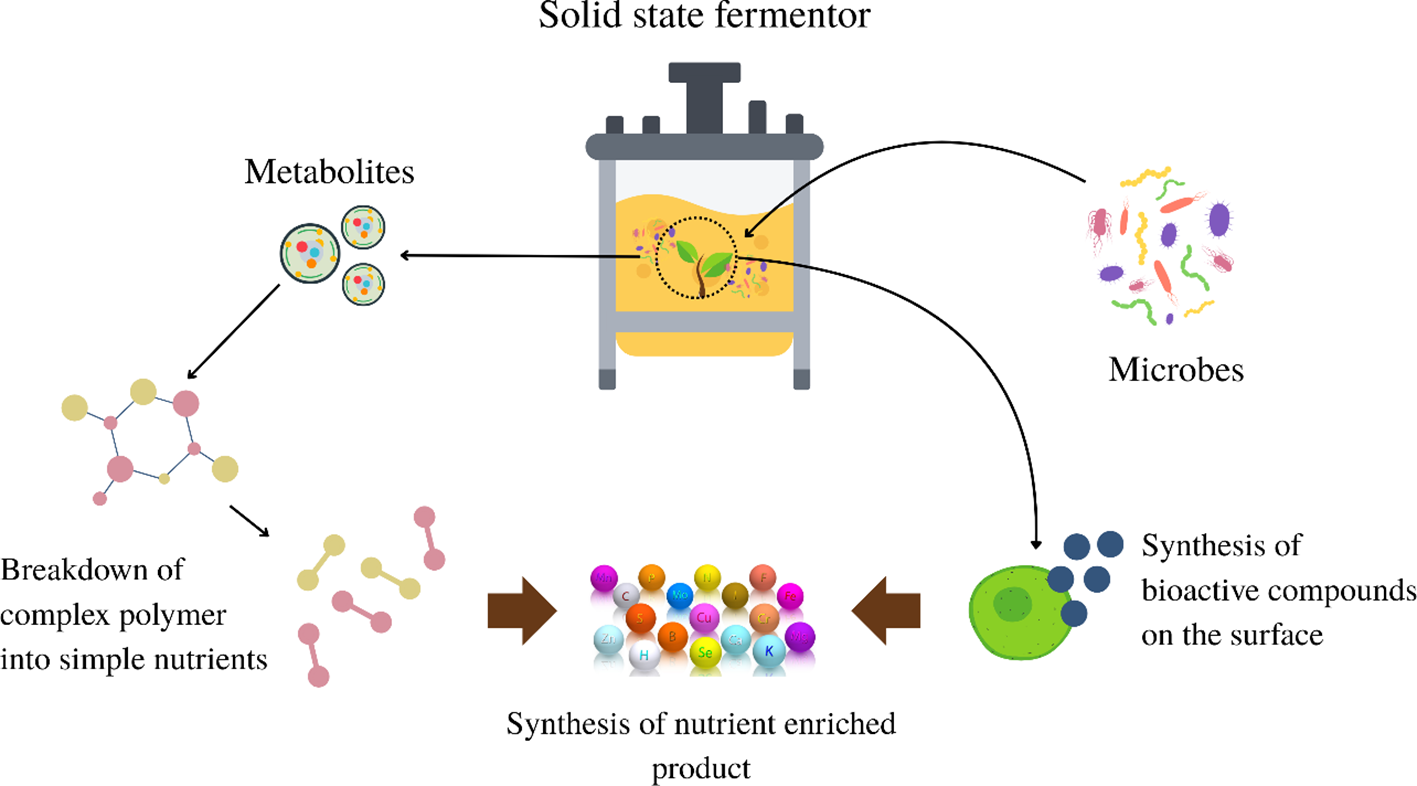
Mechanistic pathways behind the bacterial degradation in Solid-state fermentation.
3.2 Enzyme production
In solid-state fermentation, extracellular enzymes secreted by microorganisms degrade complex insoluble substrates into soluble simpler fragments. Enzymes such as cellulases, amylases, proteases, and ligninases degrade polymers such as cellulose, starch, proteins, and lignin into smaller molecules that can be absorbed and exploited by microbes for growth and metabolism (Graminha et al., 2008). In this process, there are accumulated cellular secreted hydrolytic enzymes that must diffuse through a pore structure to catalyze the hydrolytic degradation of a polymeric substrate into very small water-soluble fragments that diffuse back to the vicinity of the cells for further metabolism. The factors affecting the efficiency of enzymatic degradation are: porosity of the substrates; crystallinity of solid-state substrates; and chemical composition of solid-state substrates, which govern ‘activity’ and the access to enzymes. When the pore structure allows for activity inside the structure, the time to convert polymers into controlled nutrients for cellular grazing by microbes is significantly reduced by providing channels, diffusion paths, and surface area. If the pore structure does not permit the distribution of enzymes, degradation will not occur, and activity will only occur on the surface of the substrate and, in some cases, fall potentially below acceptable levels. Therefore, rapid hydrolysis will not occur as solids remain inaccessible to enzymatic degradation by microbial hydrolytic enzymes secreted into the solid substrate. Accordingly, it is essential to understand how SSF processes allow microorganisms and their secreted enzymes to convert complex insoluble polymers to assimilable soluble nutrients for microbial growth and product formation. As an example of enzymatic degradation that may occur during solid-state fermentation, fungi and bacteria produce cellulases that degrade cellulose (Pandey et al., 1999) as shown in Figure 2.
3.3 Reduction in ANFs
SSF is considered as an effective biotechnological approach for reducing ANFs in plant-derived ingredients, thereby improving their nutritional value for food and feed applications. The reduction in ANFs through SSF occurs through multiple mechanisms. First, enzymatic degradation plays a central role, where specific microbes such as Aspergillus spp., Bacillus subtilis, and Rhizopus oryzae produce enzymes such as proteases, phytases, and tannases that break down ANFs, such as trypsin inhibitors, phytic acid, and tannins. For instance, Aspergillus oryzae-mediated SSF of soybean meal eliminated the protein bands associated with trypsin inhibitors, confirming their breakdown (Hong et al., 2004). Second, microbial metabolism contributes to ANF reduction because microbes utilize ANFs as nutrient sources. A significant reduction in phytate and trypsin inhibitor activities was observed in de-oiled rice bran fermented with Rhizopus oryzae, suggesting that the fungus actively metabolizes these compounds (Ranjan et al., 2019). Third, the synergistic effects of co-culture further enhanced ANF degradation. Co-fermentation using Aspergillus niger, Candida utilis, and Bacillus subtilis in Moringa oleifera leaf meal led to a greater reduction in tannins and phytic acid than single-microbe fermentation (Shi et al., 2020). Finally, SSF reduced ANFs and improved nutrient bioavailability. For example, fermentation of corn-soybean meal with Bacillus subtilis and Enterococcus faecium lowered allergenic proteins, such as glycinin and β-conglycinin, thereby enhancing the nutritional quality of the feed (Shi et al., 2017). Collectively, these mechanisms demonstrate the potential of SSF to transform plant-based materials into more digestible and nutritious forms for sustainable animal nutrition.
3.4 Enhancing nutrient content
SSF technology was also reported to enhance the nutritional profile of plant-based materials through various biological mechanisms. First, the microbial biomass directly contributes to protein enrichment. As microbial communities, primarily bacteria and fungi, proliferate on the substrate during fermentation, their biomass increases crude protein content. For instance, the fermentation of oats with Monascus purpureus increased the protein content from 12.64% to 24.91% (Yu et al., 2025). Second, enzyme-mediated degradation plays a key role. Microbes produce enzymes that break down complex carbohydrates, fibers, and antinutritional components into simpler, more digestible forms. This enzymatic activity improves nutrient availability by reducing molecular barriers that hinder absorption (Jeyakumar and Lawrence, 2022). Third, SSF leads to the synthesis of beneficial bioactive compounds, such as vitamins, organic acids, and antioxidants, which contribute not only to enhanced nutritional value but also to health-promoting properties (Dey et al., 2016; Vandenberghe et al., 2018). Fourth, SSF reduces anti-nutritional factors, such as tannins and phytates, which are known to interfere with mineral and amino acid absorption. This reduction enhances the bioavailability of the essential nutrients (Olukomaiya et al., 2020). Improved digestibility is another vital benefit. Microbial degradation of structural polysaccharides and fibers leads to improved nutrient access. For example, juvenile European seabass (Dicentrarchus labrax) exhibited enhanced digestibility when fed dried distillers’ grains fermented by SSF (Filipe et al., 2023). Together, these mechanisms underscore SSF’s potential of SSF as a sustainable tool for upgrading the feed quality and nutritional efficiency in aquaculture and animal nutrition.
3.5 High nutrient digestibility
The significant improvement in digestibility caused by SSF is one of its key advantages for both food and feed applications. This enhancement is primarily attributed to microbial enzymatic activity, breakdown of structural barriers, and reduction in ANFs.
-
i. Enzymatic Hydrolysis of Macromolecules: Microorganisms used in SSF secrete a variety of enzymes that mimic gastrointestinal digestion. Enzymes, such as amylases, break down starch into simple sugars, proteases hydrolyze proteins into peptides and amino acids, and cellulases and hemicellulases degrade plant cell wall components, releasing entrapped nutrients as shown in Figure 3. This pre-digestion effect increases nutrient bio accessibility, similar to the natural digestive processes of the stomach and intestine (Wang et al., 2024).
-
ii. Reduction of ANFs: SSF also reduces ANFs, such as trypsin inhibitors, tannins, and phytic acid, which are known to impair nutrient absorption. Microbial enzymes, such as tannase and phytase, degrade these compounds, enhancing the bioavailability of key minerals (e.g., calcium, iron, and zinc) and improving the digestibility of proteins and carbohydrates (Adebo et al., 2022).
-
iii. Disruption of Plant Cell Walls: Fungal fermentation is particularly effective in breaking down fibrous plant materials, thereby weakening structural barriers and facilitating the release of intracellular nutrients. This is especially beneficial for fibrous feedstocks, such as oilseed meals, bran, and legumes (Verduzco-Oliva and Gutierrez-Uribe, 2020).
-
iv. Increase in Amino Acid Content: SSF can enhance the amino acid profile of substrates through microbial metabolism. For instance, fermenting soybean meal with Bacillus coagulans for 48 h significantly increased the essential amino acid lysine by 93%, tryptophan by 42%, and valine, isoleucine, and leucine by smaller but notable margins (Imelda et al., 2008).
Figure 3
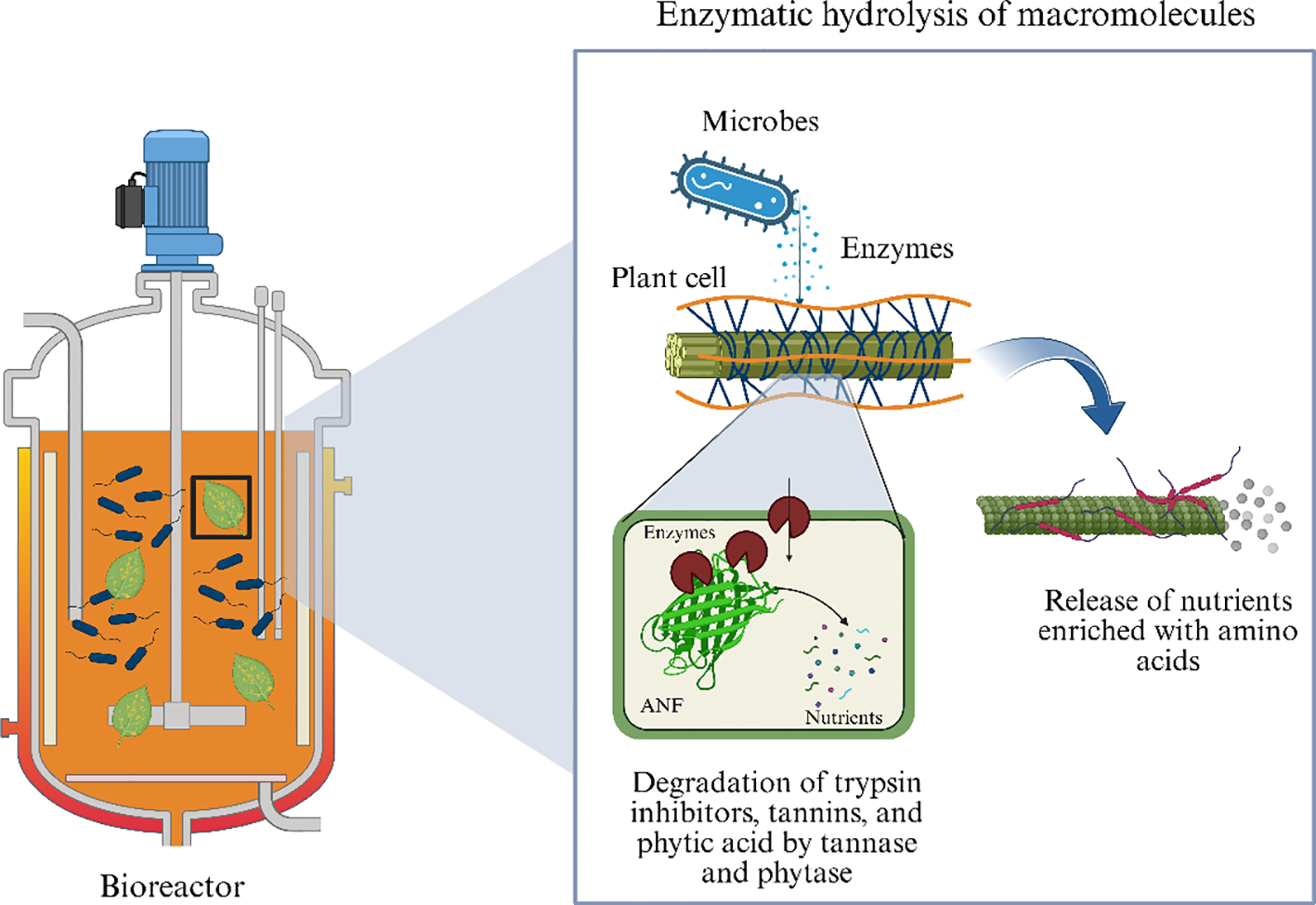
Microbial enzymatic hydrolysis of plant macromolecules.
4 Solid-state fermentation vs. submerged fermentation
Generally, SSF utilizes solid substrates, like bran, bagasse, and paper pulp. The main advantage of using these substrates is that nutrient-rich waste materials can be easily recycled as substrates. In this fermentation technique, the substrates are utilized very slowly and steadily, so the same substrate can be used for long fermentation periods (Umrao et al., 2024). Hence, this technique supports controlled release of nutrients. SSF is best suited for fermentation techniques involving fungi and microorganisms that require less moisture content. However, it cannot be used in fermentation processes involving organisms that require high aw (water activity), such as bacteria (Dawood and Koshio, 2020). On the other hand, SMF is typically performed using free-flowing liquid substrates such as molasses, wet distillers’ grains, and broths to produce fermented liquid feeds (Sugiharto and Ranjitkar, 2019). The substrates are utilized quite rapidly; hence need to be constantly replaced/supplemented with nutrients. This fermentation technique is best suited for microorganisms such as bacteria that require high moisture content (Subramaniyam and Vimala, 2012). SMF is primarily used in the extraction of secondary metabolites that need to be used in liquid form.
4.1 Nutritional enrichment
SSF is a highly effective technique to enhance the nutritional value of animal feed, particularly in aquaculture (Sun et al., 2023). It significantly improves protein content, both in quantity and quality, by promoting the activity of filamentous fungi, such as Aspergillus spp., which produce proteolytic enzymes that break down complex proteins into more digestible forms (Dai et al., 2020). SSF also enhances amino acid profiles and increases nutrient availability through the action of enzymes such as cellulases, phytases, and proteases (El-Bakry et al., 2015). Additionally, it reduces crude fiber and indigestible polysaccharides, degrades anti-nutritional factors such as phytic acid and tannins, and suppresses harmful pathogens, such as Salmonella, through the production of organic acids and antimicrobial compounds (De Villa et al., 2023). This not only boosts the nutritional and antioxidant properties of feed ingredients, particularly those derived from cereals and legumes, but also improves feed digestibility, fish growth performance, and feed conversion ratios (FCR). SSF is commonly performed using a single type of agro-industrial substrate, which simplifies process control and ensures consistent quality before the fermented product is incorporated into the final feed formulations (Dai et al., 2020). The low-moisture environment of SSF also reduces contamination risks and results in products with a longer shelf life (Krishna, 2005). Nutrient enrichment, particularly protein and fiber breakdown, is less efficient in SMF, although it can be enhanced using specific microbial strains (Cao et al., 2024). However, SMF excels in producing probiotics and bioactive compounds that can support fish immunity and gut health when included in feed (Sørensen, 2022). However, the high moisture content increases the risk of microbial contamination and necessitates further processing steps, such as drying, in addition to operational costs.
4.2 Enzyme production
Fermentation is a fundamental method for producing a wide range of enzymes, and both fungi and bacteria are capable of generating valuable enzymes when cultivated on suitable substrates. Enzymatic production can be performed using either SSF or SMF. SMF is typically used for bacterial enzyme production because of its higher water requirement, whereas SSF is more suitable for fungal enzymes, as fungi thrive in low-moisture environments. The metabolism exhibited by microorganisms is different in SSF and SMF, and the influx of nutrients and efflux of waste materials must be carried out based on these metabolic parameters (Manpreet et al., 2005). Any deviation from optimal parameters can lead to reduced product quality. Notably, enzymes produced via SSF tend to exhibit higher resistance to substrate inhibition and maintain greater stability over a broad range of temperatures and pH levels (Barrios-González, 2012). Traditionally, bacterial enzymes such as amylase, xylanase, L-asparaginase, and cellulase have been produced using SMF. However, emerging research suggests that SSF is more effective in bacterial enzyme production. This shift is largely due to the accumulation of intermediate metabolites in SMF, which can inhibit enzyme activity and reduce the overall productivity. SSF provides a more favorable environment for microbial metabolism, leading to enhanced enzyme yield and activity.
4.3 Bioactive compounds
Fermentation has been widely employed to extract various bioactive compounds, including antibiotics, pigments, enzymes, hypocholesterolemic agents, antioxidants, antihypertensive agents, antitumor compounds, biosurfactants, and bioactive peptides (Sadh et al., 2018a; Chai et al., 2020). Numerous studies have demonstrated the successful production of these compounds through microbial fermentation. Despite this, there is limited research comparing the efficiency and effectiveness of different fermentation methods, specifically solid-state and submerged fermentation, for the production of such bioactive substances. Antibiotics are one of the most significant categories of bioactive compounds derived from microorganisms via fermentation. The first commercially produced antibiotic, penicillin, was extracted from Penicillium notatum as early as the 1940s using both SSF and SMF (Arumugam et al., 2013). Since then, a wide range of antibiotics, including cyclosporins, tetracyclines, surfactins, streptomycin, and cephalosporins, have been successfully produced using fermentation techniques (Subramaniyam and Vimala, 2012).
Initially, the SMF was the predominant method used for antibiotic production. However, with advancements in substrate development, SSF has gained increasing popularity because of its advantages in terms of yield and compound stability. Recent studies have indicated that SSF often results in higher antibiotic production and improved product stability compared to SMF, primarily because of the reduced accumulation of inhibitory intermediate metabolites (Barrios-González and Miranda, 2012; Barrios-González, 2012). Despite these benefits, the choice between SSF and SMF largely depends on the specific microbial strain involved, as some microorganisms perform better in one system than in the other. In addition, the efficiency of SSF is heavily influenced by the physical and chemical properties of the substrate, which can limit its application (Pandey et al., 2000; Thomas et al., 2013). Therefore, it is essential to evaluate a broad range of substrate materials during the development phase to optimize the fermentation process and maximize antibiotic yield.
4.4 Economic feasibility and resource efficiency
The overall economic viability of a fermentation process is influenced by several key factors including the availability and cost of substrates, scalability of the process, energy requirements, and complexity of downstream processing. SSF is often considered more cost-effective because it utilizes low-cost agricultural residues, requires minimal water and energy inputs, and generates less wastewater (Karimi et al., 2021). However, maintaining optimal environmental conditions and ensuring consistent substrate quality pose operational challenges. In contrast, SMF is easier to automate and scale for industrial production but typically involves higher operational expenses because of its greater demand for water, energy, and more intensive waste management (Holker and Lenz, 2005; Thomas et al., 2013). Consequently, choosing the most economically feasible method depends on balancing the production efficiency with resource use and sustainability.
In recent decades, SSF has attracted considerable attention as an alternative to SMF, largely because of its cost-effectiveness and its ability to replicate the natural environment of many microorganisms. SSF offers several key advantages over SMF, including the use of minimal moisture, reduced risk of bacterial contamination, improved oxygen flow, simpler fermentation media, lower capital investment, higher productivity, and decreased energy consumption (Holker and Lenz, 2005; Pandey et al., 2000; Olukomaiya et al., 2019). Additionally, SSF typically does not require strict control of fermentation conditions and involves less effort in downstream processing (Olukomaiya et al., 2019). Due to its low-tech equipment requirements and cost-effectiveness, SSF is considered a more suitable and widely applicable method for the feed industry. This technique has been extensively applied in various sectors for the production of enzymes, biofuels, food, animal feed, and secondary metabolites such as antibodies and immunological drugs. However, one of the main limitations of SSF is the difficulty in controlling certain operational parameters, particularly agitation, which restricts its broader industrial use. Despite this, recent advances in bioreactor design show promise in overcoming these challenges, paving the way for improved agitation control and scalability in industrial applications.
4.5 Environmental sustainability
The environmental benefits of SSF stem from its operation without free-flowing water, leading to minimal water usage and low wastewater generation (Pandey et al., 2000). This eliminates the need for antifoaming agents and allows some SSF processes to be carried out under semi-sterile conditions (Hernandez et al., 1992). Since SSF occurs at water activity levels below 1, the risk of contamination by bacteria and yeasts is significantly reduced, potentially removing the need for energy-intensive sterilization procedures (Thomas et al., 2013). Moreover, SSF is eco-friendly because it often utilizes agricultural waste as a source of carbon and energy (Vandenberghe et al., 1999; Pandey et al., 2000). This is especially common in the production of enzymes and organic acids, where plant residues are used as substrates and inducers. Additional advantages of SSF include decreased water usage, reduced wastewater output, the potential for greater volumetric productivity and higher product concentrations, enhanced consistency in results, and more space-efficient operations. This is largely due to the lower moisture content in SSF, which enables greater substrate loading in smaller, more compact fermentation units. Furthermore, contamination control is easier, and the fermentation media are typically simpler (Gowthaman et al., 2001; Durand, 2003; Thomas et al., 2013).
In contrast, SMF has a higher environmental burden due to its reliance on large volumes of water and energy-intensive operations. SMF systems require continuous agitation, aeration, and temperature control, all of which contribute to increased electricity consumption (Holker and Lenz, 2005; Thomas et al., 2013). The production process also generates significant quantities of liquid waste, which must be treated before disposal, thereby adding to environmental management costs. Moreover, SMF often uses refined substrates, which may involve upstream resource-intensive processing. Although SMF offers advantages in process scalability and control, its environmental sustainability is lower than that of SSF unless integrated with efficient waste treatment and energy recovery systems (Pandey et al., 2000; Thomas et al., 2013). Therefore, from an ecological standpoint, SSF holds a clear advantage as a low-impact, eco-efficient method for fermentation-based feed enhancement.
5 Advantages of solid-state fermentation over radiation and chemical methods
SSF offers several key advantages over radiation and chemical methods in the enhancement of aqua feed. Unlike radiation techniques, which are often energy-intensive and carry safety risks due to exposure, SSF utilizes natural microbial processes that are environmentally friendly and free from hazardous residues. In contrast to chemical fermentation, where synthetic additives or harsh reagents can leave residues potentially harmful to aquatic animals and ecosystems, SSF employs beneficial microorganisms to degrade complex substrates and improve nutrient profiles, offering a safer and more sustainable alternative. According to Karimi et al. (2021), SSF provides considerable economic and environmental benefits in the conversion of agro-industrial waste into valuable products like bioethanol and animal feed, outperforming chemical and irradiation methods that require costly materials and infrastructure. Moreover, the mild operational conditions of SSF help preserve thermolabile bioactive components such as antioxidants, vitamins, and enzymes, which are often diminished during chemical or radiation-based processing (Pandey et al., 2000). Despite growing evidence of SSF’s effectiveness in enhancing feed quality, direct comparative studies assessing its superiority over radiation and chemical methods remain limited and warrant further investigation.
6 SSF technology in fish feed
As mentioned above, SSF enhances the nutritional quality of feed by increasing enzyme activity and improving the bioavailability of proteins and carbohydrates, while simultaneously reducing anti-nutritional factors such as phytic acid and tannins (Figure 4). This leads to better digestibility and nutrient absorption, resulting in improved growth performance, including higher weight gain and more efficient feed conversion. SSF also enriches feeds with beneficial microbial metabolites and immunostimulants like β-glucans, which boost the fish’s non-specific immune response and enhance disease resistance. Moreover, it promotes a healthier gut microbiota and increases antioxidant enzyme activity, helping fish withstand environmental and physiological stress (Siddik et al., 2024). Overall, SSF-based diets not only support better health and survival in fish but also offer a sustainable, cost-effective alternative to traditional feed ingredients. However, there are limited studies despite the multiple benefits of SSF in fish feeds. Hence, in the following section we will emphasize on exploring more about the fermented ingredients incorporated diets to provide deeper insight into the potential of SSF.
Figure 4
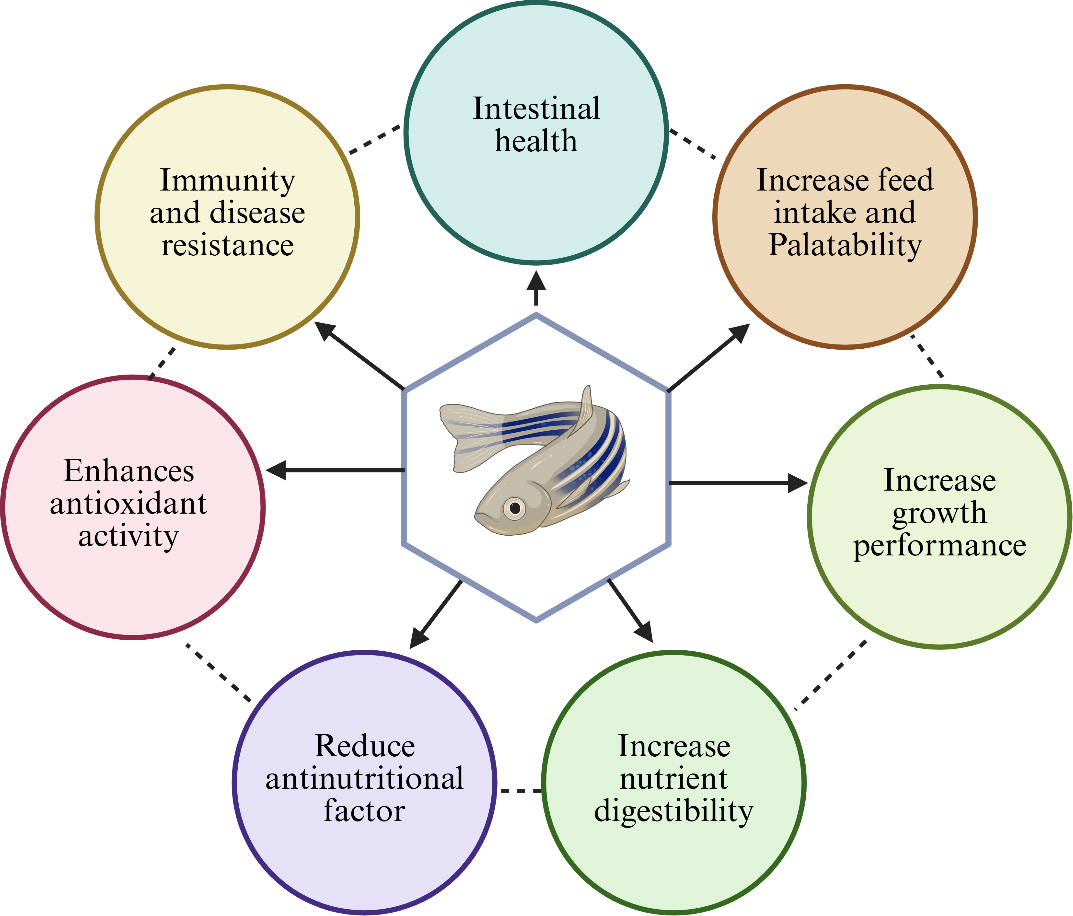
Advantages of incorporation of solid-state fermented ingredients in aquafeeds.
6.1 Feed intake and palatability
Feed intake is a critical parameter for assessing the effectiveness of fermented ingredients in aquaculture. A deficiency in essential amino acids, particularly common in alternative protein sources, such as plant-based proteins, has been identified as a potential cause of reduced feed intake (Gómez-Requeni et al., 2004; Kader and Koshio, 2012). Supplementing fermented soybean meal-based diets deficient in methionine (an essential amino acid) and taurine (an essential nutrient) has been shown to improve the amino acid balance and increase feed intake (Lee et al., 2016a). Overall, feed intake in fish is primarily governed by the energy content of the diet, as fish tend to eat until their energy requirements are fulfilled (Cho, 1992). Azarm and Lee (2014) found an inverse relationship between the daily feed intake and digestible energy content of formulated diets in juvenile black sea bream. Other studies have also reported decreased feed intake in response to diets with higher digestible energy levels (Van Vo et al., 2020a,b). Feed intake can be modulated by nutritional management practices, including feeding frequency and regimen, which are often species-specific in their effectiveness and response (Gilannejad et al., 2019; Sirakov et al., 2023). Furthermore, hybrid striped bass consuming diets formulated with either fermented or traditional soybean meal exhibited a significant decrease in feed consumption relative to fish fed a control diet containing 30% fishmeal (Rombenso et al., 2013). This improved response is likely linked to the removal of feed intake inhibitors, especially oligosaccharides, which are substantially reduced through fermentation (Rombenso et al., 2013).
A study on animal-derived proteins found that freshwater carp (Labeo bata) readily consumed aquafeeds containing fermented fish offal (Mondal et al., 2011).The increased feed intake suggests that fermentation enhances the palatability of the diet. In contrast, partially replacing fishmeal with a fermented mulberry leaf and fish offal blend in L. rohita diets has no significant effect on feed intake (Kaviraj et al., 2013). Espe et al. (1992) reported that, the Atlantic salmon also consumes high level of fish silage containing diets. In contrast, the European sea bass had lower affinity towards the feed intake of fish silage prepared by fermentation using apple pomase, molasses, formic acid and Lactobacillus plantarum (Davies et al., 2020). The nutritional quality of fish silage may be compromised by inadequate drying procedures, which can degrade key nutrients and reduces the palatability to fish. Additionally, different variables such as source and makeup of ingredients, changes occurring during processing, microorganisms used, and conditions under which fermentation takes place affect the palatability of feed and reduces the dietary intake of fish. Subsequently, the feed intake also gets affected by the feed ingredients quality, formulation, fish species and water quality. Although diets vary across studies on substrates, microbial communities, and moisture levels, the fermentation process consistently breaks down nutrients and food particles, enhancing their digestibility compared with unfermented alternatives. However, fermentation elevates free amino acid and small peptide content, improves feed taste, and promotes better nutrient uptake in fish.
6.2 Growth performance
In recent years, different research has provided newer insights in aquatic species receiving feeds formulated with fermented components compared with those given non-fermented alternatives (Meng et al., 2023). The observed increase in growth may stem from fermentation-induced enhancements in protein quality and amino acid balance coupled with the breakdown of anti-nutritional compounds, and carbohydrates (Olukomaiya et al., 2019). The probiotics used in the fermentation process synthesizes different metabolites which breakdown the antinutritional compounds into simple sugars and nutrients (Nagarajan et al., 2022). These metabolites support gut health and enhance digestive efficiency, and the quality of the ingredients chosen for the feed formulation. Additionally, the microbes synthesizes different enzymes such as amylase, cellulase and protease to catabolize the complex mixture into bioactive compounds (Tamang et al., 2016). Altering feed components through fermentation can improve nutrient absorption, and in turn, promote better growth in fish. However, studies on plant-based proteins have suggested that fermented variants can only be included in fish diets up to a certain limit, beyond which growth performance may decline.
Zhou et al. (2011) reported that substituting up to 20% of fishmeal protein with fermented soybean meal in juvenile black sea bream (Sparus macrocephalus) diets did not adversely affect the growth performance. However, substituting more than 20% of fishmeal protein with fermented soybean meal resulted in a decreased feed efficiency and hindered growth. Similarly, Lee et al. (2016a) observed that replacing up to 20% of fishmeal protein with fermented soybean meal in juvenile rockfish (Sebastes sp.) diets did not negatively affect growth performance. However, feeding black sea bream with 24% fermented cottonseed meal resulted in a significant decline in its growth performance, which is due to the reduction in the lysine bioavailability by the high level of fermented cottonseed meal in the diet. Similarly, the tilapia fed with 16% fermented cottonseed meal resulted in the reduction in its growth performance (Lim and Lee, 2011). Subsequently, a higher level of inclusion of fermented soybean up to 30-40% in the diet of black seabream often associated with poor growth performance (Zhou et al., 2011). Similar findings were reported in the study of Rinchard et al. (2003) in Onchorynchus mykiss and Oreochromis niloticus by El-Saidy and Saad (2011). Consequently, direct comparisons among these studies are challenging owing to diet formulations with fermented cotton seed meal.
A few studies reported that, the partial replacement of fish meal with unfermented ingredients resulted in improved growth performance without causing negative effects. For example, fishmeal replacement levels of up to 60% in sharp snout sea bream, 50% in red sea bream (ranging from 5% to 50%), and 50% in gilthead sea bream (ranging from 20% to 50%) have been shown to have no adverse effects on growth performance (Martínez-Llorens et al., 2007). Similarly, evidence suggests that raw soybean protein can serve as a viable alternative to fishmeal in rainbow trout diets, supporting both nutrient absorption and growth without adverse effects (Luo et al., 2006). Numerous studies have used formulated feeds containing synthetic amino acids, most notably lysine and methionine, which likely contributed to a more balanced nutrient composition and permitted a higher degree of fishmeal replacement (Chaklader et al., 2020; Lim and Lee, 2009). For instance, when taurine, along with essential amino acids such as lysine and methionine, is included, fermented soybean protein can substitute up to 40% of fish-derived protein in young black sea bream diets without negatively affecting growth outcomes (Azarm and Lee, 2014).
6.3 Nutrient digestibility and anti-nutritional factor reduction
The fish fed with fermented plant ingredients have shown better digestibility and improved growth performance compared to unfermented plant diets. This is owing to changes in the proximate composition of the diet after fermentation, which improved the nutritional value of plant ingredients. The European seabass fed with corn distellers grain fermented with Aspergillus carbonarius, A. ibericus, and A. uvarum improved the level of soluble proteins, reduced the fiber content and enhances the production of lignocellulolytic enzymes (Filipe et al., 2023). According to Ngandzali et al. (2011), incorporating soybean protein concentrate into the diet of black sea bream improves the efficiency of protein digestion. This improvement may be partly attributed to the addition of phytase to soybean meal, which helps mitigate the adverse effects of phytic acid, a known anti-nutritional compound (Ngandzali et al., 2011). Nonetheless, decreased nutrient digestibility has been reported at elevated inclusion rates of fermented plant ingredients in the diets of juvenile black sea bream (Sun et al., 2015) and rainbow trout (Luo et al., 2006). Additionally, the higher-level inclusion of fermented soybean meal up to 30% in the diet of black sea bream significantly affected the apparent digestibility of the nutritional value of the ingredients (Zhou et al., 2011). Similar results were obtained in the study of Nguyen et al. (2015), where the soybean-derived compounds (oligosaccharides and lectins), affected the digestibility of lipids and absorption by inhibiting the release of pancreatic lipase and bile acids which aid in digestion and the fermented cotton seed meal affected the digestibility of proteins and lipids in the black sea bream by lowering its apparent digestibility (Sun et al., 2015). Reduced protein digestibility in fermented cottonseed meal may be due to antinutritional factors, such as free gossypol and phytic acid, as well as an imbalanced amino acid profile (Zhou and Yue, 2012). Fish species differ in their ability to utilize plant proteins in their diets, which is influenced by their varying tolerances to anti-nutritional factors present in these ingredients (Francis et al., 2001).Moreover, smaller fish generally tolerate lower inclusion levels of plant proteins than larger fish, which tends to be less sensitive to the antinutritional factors found in plant ingredients (Martínez-Llorens et al., 2007). Lim et al. (2004) found that larger juvenile rockfish could replace up to 30% of the dietary fishmeal with dehulled soybean meal without negatively affecting growth performance. However, exceeding the maximum dietary inclusion level of soybean meal leads to decreased feed utilization efficiency, which can be attributed to factors such as imbalanced amino acid profiles, presence of antinutritional factors, poor protein digestibility, higher levels of indigestible carbohydrates, and reduced feed palatability (Francis et al., 2001). Improving fish performance can be achieved by minimizing anti-nutritional factors such as phytic acid and tannins, decreasing crude fiber levels, and enhancing the availability of low-molecular-weight peptides and fatty acids (Ramachandran and Ray, 2007).
6.4 Antioxidant activity
The fish experience oxidative stress due to internal metabolism and external mediators, hence the antioxidative enzymes plays a main role in maintaining the homeostasis (Ding et al., 2015). Antioxidant enzymes, such as catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD), are well-established biomarkers of antioxidant status and are commonly used to assess the impact of different dietary protein sources on fish health (Siddik et al., 2022). Dietary inclusion of 160 g/kg fermented soybean meal in juvenile black sea bream results in elevated liver glutathione peroxidase (GPx) and SOD activities (Azarm and Lee, 2014). Studies have shown that fermenting soybean meal with A. oryzae enhances the bioavailability of key antioxidant compounds, such as isoflavones and flavones, thereby improving the antioxidant activity in fish (Kim et al., 2010). Lee et al. (2016a) reported that high levels of fermented soybean meal in rockfish diets did not negatively affect feeding behavior or overall health, indicating that these inclusion levels may boost antioxidant enzyme activity and protect against oxidative stress induced by increased concentrations of antinutritional factors in plant-based proteins (Zheng et al., 2017). The dietary incorporation of dried fermented soybeans (meju) in olive flounder between 3 to 6% increased the SOD activity. At higher level, it enhances the nitro-blue tetrazolium (NBT) reduction activity and liver SOD activity. This is due to the higher bioavailability of bioactive polyphenol compounds in the diet (Kim et al., 2010). Aspergillus oryzae serves as the key microorganism in fermenting commercial meju, contributing to its enhanced antimutagenic and antioxidative effects (Lin et al., 2006).
However, the antioxidant properties of the diets is exhibited by the microbes or by the metabolites or the enzymes such as α-amylase, cellulose-degrading enzymes, phytase, and carboxypeptidase is remains unclear. Soybean meal, which is rich in isoflavones and flavones, is well known for its positive effects on antioxidant activity and immune function in organisms. The bioavailability of isoflavones and flavones is influenced by their chemical structure, vulnerability to microbial breakdown, and hydrophobicity (Birt et al., 2001). Soy isoflavones in their glycoside-conjugated form are not readily utilized by fish, as they require hydrolysis by enzymes, such as glucuronidase or sulfatase, to become bioavailable. Usually, the enzymes such as Bacillus subtilis and Aspergillus oryzae had the properties to convert the unsoluble isoflavone glycosides to readily soluble isoflavone aglycones. In addition, it enhances the flavonoids and polyphenol compounds in the meju which attributed to the elevation in the liver SOD activity of olive flounder (Li et al., 2019; Kim et al., 2010).
6.5 Immunity and disease resistance
The fermentation process usually involves the external addition of microbes to improve the nutritional value of the ingredients. Lactic acid bacteria (LAB), a common beneficial microbes used in the process, however feeding the fish with fermented feed ingredients formulated diets enhances the proliferation of LAB population in the gut, which determines the health of intestinal flora and also plays a key role in digestion and absorption. In addition, the LAB population enhance the immune function of the host (Zhang et al., 2020). However, the underlying mechanism in elevating the immune response is still remains unclear. It is believed that, LAB activates immune cells and promote cytokine production, both of which are essential for initiating and regulating the immune response. Lutful Kabir (2009) reported that lactic acid bacteria, particularly Lactobacillus species, enhance the production of Th2 cytokines such as interleukins IL-4 and IL-10, which support B cell development and immunoglobulin class switching, both critical for antibody generation. Fermented feed boosts systemic antibody production and mucosal immune responses in fishes (Dossou et al., 2018). In recent studies, metabolites produced during the fermentation process is involved in elevating the immunoglobulin (Ig) levels in fish (Tang et al., 2012; Zhuo et al., 2021). Additionally, the fermented plant based ingredients were known to strength the non-specific immunity, which is an essential defense mechanism against infections and diseases (Lee et al., 2016b, 2013). However, the results are inconsistent; while some studies on fermented soybean meal show no significant impact on innate immunity (Ding et al., 2015; Katya et al., 2014), others have reported that it stimulates or enhances nonspecific immune responses, thereby improving fish disease resistance (Siddik et al., 2019b; Abdul Kader et al., 2012). Lysozyme activity is widely regarded as a key indicator of nonspecific immunity and is an essential part of the immune system, playing a crucial role in defending the body against microbial invasion (Katya et al., 2014). Lysozyme, a key enzyme modulates the immune response in causing disease resistance against the pathogens (Katya et al., 2014). It is evident in the study of Siddik et al. (2019a), where the Asian seabass juvenile fed with fermented poultry by-product meal added with fish hydrolysate enhanced the lysozyme activity to defend against the Vibrio harveyi infection. These studies provided newer insights of the fermentation process associated with immune modulation in the fishes.
6.6 Gastrointestinal morphology
Proper intestinal development is vital, because intestinal function is strongly associated with fish growth performance and overall health (Siddik et al., 2019a, 2020). The intestinal mucosa is essential for nutrient digestion and absorption, and its structural morphology provides a reliable measure of fish health status (Siddik et al., 2020). Longer mucosal folds and taller villi are indicators of good health and enhanced nutrient absorption, whereas shorter folds and reduced villus height suggest impaired nutrient uptake and decreased fish growth performance (Siddik et al., 2018; Dimitroglou et al., 2011). A study by Siddik et al. (2019b) revealed the effect of changes in the intestinal morphology of the juvenile Asian seabass fed with fermented poultry by-product meal added with fish hydrolysate. It is noted that complete replacement of fish meal with fermented poultry by-product meal added with fish hydrolysate increased the fold length and villus height in the distal intestine. Similarly, the fold length and lamina propria height were remain unaffected with the dietary supplementation of corn fermented protein-soluble meals in the diet of Atlantic salmon (Hossain et al., 2023). These findings suggest that fermented diets may stimulate the proliferation of intestinal epithelial cells, expand absorptive surface area, and enhance the overall efficiency of nutrient utilization in fish.
The peptides produced during fermentation process also modulated the morphology of the intestine and also provided beneficial effects (Van Vo et al., 2020b; Tang et al., 2012). Studies on non-fish species have demonstrated that fermented diets enhance intestinal structure and promote better health (Xu et al., 2012), largely because of (1) the close relationship between gut microbiota and the digestive process, which facilitates improved nutrient absorption. (2) An increased population of LAB can suppress harmful pathogens that damage gut tissue and structure, (3) fermentation breaks down complex polysaccharides into smaller peptides, and (4) fermentation reduces ANFs, improving overall gut health. The dietary supplementation of fermented ingredients not only served as an alternative protein rich sources but also added an advantage over enhancing the performance and the intestine health, as it is serve as a vital organ in regulating the absorption of vital nutrients, growth and immune response against the harmful pathogens. Numerous studies have provided insights into the fermentation process associated bacteria as it contribute to greater tolerance of environmental factors in the fish gastrointestinal tract by supporting optimal oxygen levels as well as stabilizing pH and temperature (Dawood and Koshio, 2020). Such favorable conditions promote the proliferation of beneficial bacteria while inhibiting the growth of potential pathogens, thereby creating an environment that supports improved fish health (Dawood and Koshio, 2020).
Fermented diets can promote the proliferation of beneficial bacteria, leading to higher colonization of probiotic microbes on mucous membranes, which helps prevent pathogens from adhering to the intestinal lining. Furthermore, probiotic bacteria generate antimicrobial peptides, such as bacteriocins, which help suppress the growth of harmful pathogens in the intestine. Few studies specified that the intestinal microbes facilitate the movement of different solutes and compounds by regulating the proteins at the juncture of the epithelial cell membrane, which shielded from the effect of infectious microbes (Gareau et al., 2010). Additionally, probiotic bacteria can stimulate intestinal epithelial cells to release cytokines that regulate immune cells, including dendritic, T, and B cells, and enhance the capacity of lipopolysaccharides to trigger TNF-α gene transcription in animal models (Chiang et al., 2009).
6.7 Intestinal microflora
Gut microbiota is crucial for various physiological functions in fish, including supporting digestion, lowering intestinal pH, maintaining the integrity of the mucosal barrier, limiting enterobacterial colonization, interacting with the immune system, and enhancing disease resistance (Dimitroglou et al., 2011; Romero et al., 2014). Fermented diets may help to maintain a healthy gastrointestinal environment in fish because of their low pH, abundant lactobacilli, high lactic acid content, and reduced levels of enterobacteria (Catalán et al., 2018). The host fish intestine serves as the substrate for the proliferation of the beneficial microbes, which modulates the absorption of the dietary compounds (Ringø et al., 2006). These beneficial microbes produce metabolites and outcompetes the growth of pathogenic microbes in the intestine (Meng et al., 2023). Juvenile turbot fed Enterococcus faecium-fermented soybean meal showed a significant increase in beneficial bacteria, such as Lactobacillus and the anti-inflammatory Faecalibaculum, while the presence of Vibrio was reduced compared to those fed unfermented soybean meal (Li et al., 2020). This is mainly attributed to the properties fermented meals in creating acidic environment in the gut, which favors the growth of beneficial microbes and reduces the harmful pathogenic microbes especially Vibrio sp (Li et al., 2020). Additionally, the proliferation of Fusobacteriota and Cetobacterium in the gut of zebra fish were improved by feeding the fish with fermented rice bran and soybean meal using Bacillus subtilis (Wang et al., 2022). Similarly, the Asian seabass diets incorporated with the fermented poultry by-product meals improved the proliferation of LAB in the gut (Siddik et al., 2020). Hence, these studies have underscored the insights of fermentation process in improving the beneficial microbes’ population and also in modulating the immune response and enhancing the growth performance of the fishes.
7 Limitations of microbial applications in enhancing the nutrient profile of aquafeed ingredients
Although SSF has various benefits in enhancing the nutritional quality of feed by increasing enzyme activity and improving the bioavailability of proteins and carbohydrates, along with reducing anti-nutritional factors such as phytic acid and tannins, several studies have reported the nutrient loss during fermentation due to microbial utilization. However, the mechanistic role behind the nutrient utilization by various microbes during fermentation was limited. A concise overview of how microbial fermentation can negatively influences the nutrient profile of various feed ingredients is presented below:
7.1 Amino acid
While fermentation often improves protein quality, some microbial strains can decrease amino acid levels in aquafeed ingredients Lactobacillus spp. fermentation sometimes led to reduced levels of specific amino acids such as phenylalanine, lysine, and leucine, suggesting active microbial metabolism of these substrates during fermentation (Refstie et al., 2005). Similarly, studies using Bacillus spp. or A. oryzae have reported losses in sulfur-containing amino acids like cysteine and methionine under certain fermentation conditions (Song et al., 2008). Soybean meal fermented with S. cerevisiae showed significant reduction in crude protein (Sharawy et al., 2016). Additionally, Shi et al. (2022) found that SSF of Moringa oleifera leaf meal with mixed strains of A. Niger, C. utilis and B. subtilis led to major reductions in amino acids. Thus, the influence of microbial fermentation on the amino acid profile of plant proteins largely depends on factors such as substrate composition, type of microorganism, incubation temperature, pH, moisture level, and fermentation duration (Lim and Lee, 2011).
7.2 Lipid and fatty acids
Refstie et al. (2005) observed a decrease in the lipid content of soybean meal following fermentation with L. acidophilus. Also, Gao et al. (2020) reported that SSF of rapeseed cake with Pichia pastoris led to a reduction in saturated (SFAs) and unsaturated fatty acids (UFAs), along with an increase in polyunsaturated fatty acids (PUFAs), compared to the unfermented counterpart Similarly, Siddik et al. (2019a) reported that fermentation of poultry by-product meal with S. cerevisiae and L. casei resulted in an increase in PUFAs, accompanied by a reduction in SFAs and UFAs. Conversely, fermentation of fish silage with L. plantarum and Streptococcus thermophilus has been reported to reduce PUFA levels compared to raw poultry by-products (Özyurt et al., 2016). Also, it has been reported that Shewanella spp. fermentation of soybean meal increased crude protein but caused a significant decrease in crude lipid (Li et al., 2019), indicating possible metabolic use of other nutrients. The variation in raw materials, microbial strains, and fermentation conditions may account for these conflicting findings.
7.3 Crude fiber and carbohydrate
Soybean meal subjected to fermentation with S. cerevisiae exhibited a significant decrease in fiber content compared to its commercial counterpart (Sharawy et al., 2016). They suggested that the secretion of various fiber-degrading enzymes during fermentation may be responsible for the reduced fiber content in fermented soybean meal. In another study, fermentation of grass pea (Lathyrus sativus) seeds with Bacillus spp. resulted in a significant reduction in crude fiber content (Ramachandran et al., 2005). Similar reductions were also reported by Zhou et al. (2011) in C. utilis-fermented soybean meal and by Kim et al. (2016) in Bacillus-fermented soybean meal. Ahmed et al. (2014) and Hassaan et al. (2015) found that Canola meal upon fermentation with L. salivarius and sunflower meal with S. cerevisiae, B. subtilis revealed reduction in crude fiber content. In another study involving Moringa oleifera leaf flour upon fermentation (SSF) with A. niger, C. utilis and B. subtilis led to 70% reduction in crude fiber, 30% decrease in fat content, as well as marked reductions in total reducing sugars (12-2% decrease) indicating microbial consumption of energy substrates rather than enhancing feed value (Shih et al., 2021). Additionally, soybean meal fermented with L. plantarum led to reduction of non-digestible carbohydrates such as stachyose, raffinose and sucrose (Wang et al., 2016).
7.4 Minerals
Controlled SSF experiments have shown decline in mineral content with increasing fermentation time and under conditions that expose substrates to oxygen or heat. In fenugreek seed SSF with A. awamori, mineral concentrations (Fe, Zn, Ca, Cu, Na) and antioxidant markers rose during early fermentation but declined after extended incubation (after day 5) indicating a clear time dependence where prolonged fermentation can reverse initial mineral gains (Dhull et al., 2021). However, there is limited information available on the reduction of minerals in aquafeed ingredients during microbial fermentation, as most studies primarily focus on improving nutrient bioavailability and reducing antinutritional factors rather than assessing potential nutrient losses.
8 Challenges and limitations
Although SSF supports important bioprocessing activities, setting precise control of moisture, temperature, pH, and airflow is still very challenging. A lack of moisture in fermenters causes solid materials to resist heat flow, which may result in the accumulation of heat, inhibiting both microbial growth and other product reactions (Manan and Webb, 2017). An inappropriate climate may lead to substrate moisture reduction, disruption of microbial life, food mold, and allow nutrients to escape (Alp and Bulantekin, 2021). Because different parts of a solid substrate may have differing amounts of pH, nutrients, and oxygen, it is challenging to stay throughout the fermentation process (Raimbault, 1998). Because there are not enough real-time monitoring systems for solid-state fermentation, SSF requires more effort and is less reliable than SMF (Jin et al., 2024). Ensuring the safety of SSF-derived products is critical, especially when using agro-industrial by-products as substrates that may introduce pathogens or mycotoxins if not properly managed. The use of unsuitable microbes or poor growth conditions may lead to mycotoxin development by filamentous fungi, which are hazardous to both livestock and humans (Egbuta et al., 2017). The regular removal of ANFs is necessary to maintain unsafe amounts of residual compounds that harm nutrient absorption (Abu Hafsa et al., 2022). Because safety standards are not standardized, commercial growth in the industry is restrained, underlining the importance of rigorous post-fermentation studies (Sabahi et al., 2023). If good optimization is not used in fermentation, important nutrients, mainly vitamins and amino acids, might be lost from production (Chavan et al., 1989). Heat problems, longer fermentation periods, and high moisture in the grain can degrade critical nutrients, making finished animal feed less valuable (Mukherjee et al., 2015). Remaining stable through storage is a major issue, as continuing growth by microbes can lead to the gradual loss of nutrients and quality in the food over time (Leistner and Gould, 2002). Scaling up SSF from the laboratory to the industrial level is logistically complex (Mitchell et al., 2006). Designing a good bioreactor is necessary to provide proper heat and mass transfer, sufficient aeration, and complete mixing of the substrate while supporting the growth of microbes (Mitchell et al., 2000). SSF often requires the use of tray or rotary drum bioreactors, which are costly and challenging to operate (Singhania et al., 2018). As agro-industrial waste materials vary greatly, it is difficult to achieve equal product results and maintain consistency in the process (Hoque and Devi, 2025). Furthermore, it is difficult to monitor and control SSF because its substrate is naturally heterogeneous. Because advanced sensors are lacking, it is more difficult to control the behavior of the growing bacteria (Molin and Givskov, 1999). As fermentation progresses, it becomes more difficult to measure and control the pH, nutrients, and metabolite levels (Chai et al., 2022). Although not using sterile procedures may be cheaper, it makes it much easier for contamination to occur and for the process to vary, which affects how much is produced and how reproducible it is (Kumar, 1998).
A key problem with most static bed SSF systems is that there is not enough oxygen transferred unless air is forced. As the particle size of the system increases, the availability of oxygen at the surface becomes more important (Raghavarao et al., 2003). Therefore, effective heat management is a key issue. The energy produced during fermentation in solid substrates is difficult to remove, which may cause the substrate to dry out and slow the growth of microbes. Large systems have lower heat release efficiency through conduction and convection (Casciatori and Thoméo, 2018). Mixing in SSF systems can be complicated because the process tends to use a large amount of energy and may damage sensitive microbial structures. The presence of surfaces for internal heat transfer may lead to weaker mixing, and this tendency increases with increasing size, causing uneven temperature and nutrient zones (Zhang et al., 2018). It is also necessary to maintain the water activity at the proper level because high evaporation for refrigeration can drastically reduce water activity, preventing microbes from multiplying. Consequently, growers need precise control of aeration and humidity throughout the process (Krishna, 2005). Scaling up SSF does not always run smoothly. Only identical geometries fail to maintain balanced local conditions, because longer transfer paths become less effective. In addition, the lack of sufficient pressure drops, air access, and efficient ways to handle solids negatively affects large-scale SSSF reactors (Mitchell et al., 2006).
9 Future prospects and research directions
The exploration of novel substrates, including agro-industrial byproducts and food waste, is a promising direction for sustainable SSF. Rice bran, soybean meal, brewery spent grain, seaweed, fruit pomace, and vegetable residues supply a variety of nutrients that can be easily found (Šelo et al., 2021). Merging ingredients and finding efficient pretreatment processes (mechanical or enzymatic) can improve microbial activity and nutrition, which supports the aims of a circular economy (El-Bakry et al., 2015). Improvements in nutrient conversion and the reduction of substances harmful to nutrition during SSF heavily depend on the proper selection and engineering of microbes. Aspergillus, Trichoderma, and certain Bacillus and Lactobacillus species have been used over the years because of their strong enzyme-producing abilities (Raimbault, 1998). New findings have also brought attention to Streptomyces species that produce strong and durable enzymes, and Debaryomyces hansenii, which ferments high-salt agro-industrial materials (Martin et al., 2010; Jain et al., 2021). Furthermore, non-traditional eukaryotic organisms, such as protists and microalgae, are gaining attention for their potential in the synthesis of bioactive compounds and degradation of complex feedstock components (Burleson, 2012). Combining genetic engineering with synthetic biology allows scientists to develop custom microbial strains that enhance their enzymes, improve the use of different materials, and minimize the risk of toxin production (Boukid et al., 2023). Technically integrating these microorganisms with SSF results in higher process reliability, more secure products, and an environmentally conscious approach, mainly in the production of aquafeeds and various other bioproducts. In addition, examining probiotic traits and beneficial metabolites in SSF-based diets supports the production of good nutritional feeds.
Innovations in bioreactor design and process monitoring are essential for overcoming scalability and control challenges. The use of sensors for parameters such as pH, temperature, and moisture in automated systems allows precise control and a substantial decrease in manual tasks (Bellon-Maurel et al., 2003). New bioreactor methods, such as Reusable Immobilized Temporary Immersion (RITA), have increased mass transfer in cultures and help them to remain stable by putting less strain on microbes, leading to greater performance and consistency in production (Kaya et al., 2018). In addition, combining AI and machine learning in fermentation provides strong capabilities to improve the fermentation process. These methods can be used to predict the outcomes of SSF, ensure accuracy in repeated experiments, and increase the overall SSF dependability (Vinestock et al., 2024). Developing standardized protocols for safety and nutritional quality assessments is a research priority. The production of safe and high-quality SSF-derived products depends on the development of guidelines for selecting strains, pre-processing substrates, and testing after fermentation. Such guidelines are intended to confirm that pathogens, toxins, and residual antinutritional factors are not present in food (Alhomodi, 2022). With the help of advanced analytical tools, it is easier to observe how microbial communities perform and which metabolites they produce, providing essential information about the safety and function of the process (Singh and Shyu, 2024).
To encourage wider use of SSF technologies in business, strong regulations must be developed. They need to be created through teamwork between academic groups and industry members, helping spur innovation and ensuring that new products remain safe and meet standards. A combination of SSF with enzymatic hydrolysis and probiotic use promises to improve the efficiency and application of the final products (Deng et al., 2025). The use of enzymes to modify substrates makes it easier for microbes to use, which can lead to better fermentation. To promote health in aquaculture diets, probiotics can be added to the feed even after fermentation is complete (Mishra et al., 2024). Ongoing studies of combined approaches will help to make SSF more effective and useful in improving aquafeeds. More research is needed to assess how SSF systems benefit both the environment and economy, which would encourage their use in aquaculture (Henry et al., 2024). Life cycle assessments make it easier to assess the major aspects of sustainability, including carbon emissions, using water, and waste. In addition, analyzing the economics of SSF with cheaper agro-industrial inputs helps to confirm its viability for industrial use (Bruno et al., 2023; Shih et al., 2021). By carrying out such detailed examinations, we will make aquafeeds more sustainable and raise the chances for SSF technologies to be considered by those making investment decisions for aquaculture.
10 Conclusion
To summarize, this review highlighted the pivotal role of solid-state fermentation (SSF) in revolutionizing aquaculture feed production by addressing key issues such as nutritional enhancement, waste reduction, and sustainability. SSF effectively transforms agro-industrial byproducts into nutrient-rich feed ingredients, boosting protein quality, digestibility, and the reduction of ANFs like phytic acid and tannins. By harnessing microbial activity, SSF upgrades the nutritional value of alternative feed sources, reducing ANFs, enhancing digestibility, and enriching diets with bioactive compounds that support fish growth, gut health, and disease resistance. Moreover, SSF’s low environmental footprint, marked by reduced water and energy consumption and minimal waste, positions it as a greener alternative to traditional methods, supporting the shift toward sustainable aquaculture practices.
The future of SSF in aquaculture is promising, with potential to drive innovation through the exploration of novel substrates, such as food waste and seaweed, and the development of tailored microbial strains for enhanced nutrient conversion. Advances in bioreactor technology and real-time monitoring systems will improve process control, ensuring product safety and consistency. By integrating SSF with complementary approaches like probiotic supplementation and enzymatic pre-treatments, the industry can further optimize feed quality. This review lays the groundwork for future research to overcome challenges like scalability and nutrient retention, fostering the widespread adoption of SSF to deliver sustainable, high-quality aquafeed for a growing global demand. Overall, this review article clearly indicates that solid-state fermentation (SSF) improves palatability in aquafeeds by reducing anti-nutritional compounds. However, outcomes depend on the substrate, fish species, and inclusion levels, highlighting SSF’s potential as a sustainable technology for enhancing feed quality. This process enhances nutrient bioavailability, making it an eco-friendly approach to optimize feed efficiency for future aquaculture feed production.
Statements
Author contributions
PK: Conceptualization, Writing – review & editing. ND: Conceptualization, Writing – original draft. MD: Writing – original draft. AD: Writing – original draft. KA: Conceptualization, Visualization, Writing – original draft. PD: Writing – original draft. SD: Writing – original draft. DV: Writing – original draft. DR: Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to their respective institutions for the support provided during the preparation of this manuscript. We acknowledge the collaborative efforts and intellectual contributions of all co-authors, which significantly enriched the quality and scope of this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdul Kader M. Koshio S. Ishikawa M. Yokoyama S. Bulbul M. Nguyen B. T. et al . (2012). Can fermented soybean meal and squid by-product blend be used as fishmeal replacements for Japanese flounder (Paralichthys olivaceus)? Aquac. Res.43, 1427–1438. doi: 10.1111/j.1365-2109.2011.02945.x
2
Abu Hafsa S. H. Hassan A. A. Elghandour M. M. M. Y. Barbabosa-Pliego A. Mellado M. Salem A. Z. M. (2022). Dietary anti-nutritional factors and their roles in livestock nutrition. Sustain. Agric. Rev.57, 131–174. doi: 10.1007/978-3-031-07496-7_4
3
Adebo J. A. Njobeh P. B. Gbashi S. Oyedeji A. B. Ogundele O. M. Oyeyinka S. A. et al . (2022). Fermentation of cereals and legumes: Impact on nutritional constituents and nutrient bioavailability. Fermentation8, 63. doi: 10.3390/fermentation8020063
4
Ahmed A. Zulkifli I. Farjam A. S. Abdullah N. Liang J. B. Awad E. A. (2014). Effect of solid state fermentation on nutrient content and ileal amino acids digestibility of canola meal in broiler chickens. Ital. J. Anim. Sci.13, 3293. doi: 10.4081/ijas.2014.3293
5
Alhomodi A. F. N. (2022). Exploring bioprocessing technologies for diverse industrial application of canola. (PhD thesis). South Dakota State University, Brookings, South Dakota, United States.
6
Alp D. Bulantekin Ö. (2021). The microbiological quality of various foods dried by applying different drying methods: a review. Eur. Food Res. Technol.247, 1333–1343. doi: 10.1007/s00217-021-03731-z
7
Amaral D. Filipe D. M. Cavalheri T. F. Vieira L. Magalhães R. P. Belo I. et al . (2023). Solid-state fermentation of plant feedstuff mixture affected the physiological responses of European Seabass (Dicentrarchus labrax) reared at different temperatures and subjected to salinity oscillation. Animals13, 393. doi: 10.3390/ani13030393
8
Amillano-Cisneros J. M. Fuentes-Valencia M. A. Leyva-Morales J. B. Savín-Amador M. Márquez-Pacheco H. Bastidas-Bastidas P. D. J. et al . (2025). Effects of microorganisms in fish aquaculture from a sustainable approach: a review. Microorganisms13, 485. doi: 10.3390/microorganisms13030485
9
Arumugam G. K. Selvaraj V. Gopal D. Ramalingam K. (2013). “ Solid-state fermentation of agricultural residues for the production of antibiotics,” in biotransformation of waste biomass into high value biochemicals ( Springer New York, New York, NY), 139–162. doi: 10.1007/978-1-4614-8005-1_7
10
Azarm H. M. Lee S. M. (2014). Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquac. Res.45, 994–1003. doi: 10.1111/are.12040
11
Barrios-González J. (2012). Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Proc. Biochem.47, 175–185. doi: 10.1016/j.procbio.2011.11.016
12
Barrios-González J. Miranda R. U. (2012). Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol.94, 555–566. doi: 10.1007/s00253-009-2239-6
13
Bellon-Maurel V. Orliac O. Christen P. (2003). Sensors and measurements in solid state fermentation: a review. Process Biochem.38, 881–896. doi: 10.1016/S0032-9592(02)00093-6
14
Betchem G. Monto A. R. Lu F. Billong L. F. Ma H. (2024). Prospects and application of solid-state fermentation in animal feed production–a review. Ann. Anim. Sci.24, 1123–1137. doi: 10.2478/aoas-2024-0029
15
Bhargav S. Panda B. P. Ali M. Javed S. (2008). Solid-state fermentation: an overview. Chem. Biochem. Eng. Q.22, 49–70.
16
Bilal M. Ji L. Xu Y. Xu S. Lin Y. Iqbal H. M. et al . (2022). Bioprospecting Kluyveromyces marxianus as a robust host for industrial biotechnology. Front. Bioeng. Biotechnol.10. doi: 10.3389/fbioe.2022.851768
17
Birt D. F. Hendrich S. Wang W. (2001). Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Ther.90, 157–177. doi: 10.1016/S0163-7258(01)00137-1
18
Boukid F. Ganeshan S. Wang Y. Tülbek M.Ç. Nickerson M. T. (2023). Bioengineered enzymes and precision fermentation in the food industry. Int. J. Mol. Sci.24, 10156. doi: 10.3390/ijms241210156
19
Bowyer P. H. El-Haroun E. R. Salim H. S. Davies S. J. (2020). Benefits of a commercial solid-state fermentation (SSF) product on growth performance, feed efficiency and gut morphology of juvenile Nile tilapia (Oreochromis niloticus) fed different UK lupin meal cultivars. Aquaculture523, 735192. doi: 10.1016/j.aquaculture.2020.735192
20
Bruno M. Marchi M. Ermini N. Niccolucci V. Pulselli F. M. (2023). Life cycle assessment and cost–benefit analysis as combined economic–environmental assessment tools: application to an anaerobic digestion plant. Energies16, 3686. doi: 10.3390/en16093686
21
Burleson C. (2012). Production of bioactive secondary metabolites by Florida harmful bloom dinoflagellates Karenia brevis and Pyrodinium bahamense. Florida Institute of Technology, Melbourne, Florida.
22
Cao Y. Xu M. Lu J. Cai G. (2024). Simultaneous microbial fermentation and enzymolysis: A biotechnology strategy to improve the nutritional and functional quality of soybean meal. Food Rev. Int.40, 1296–1311. doi: 10.1080/87559129.2023.2212048
23
Casciatori F. P. Thoméo J. C. (2018). Heat transfer in packed-beds of agricultural waste with low rates of air flow applicable to solid-state fermentation. Chem. Eng. Sci.188, 97–111. doi: 10.1016/j.ces.2018.05.024
24
Catalán N. Villasante A. Wacyk J. Ramírez C. Romero J. (2018). Fermented soybean meal increases lactic acid bacteria in gut microbiota of Atlantic salmon (Salmo salar). Probiotics Antimicrob. Proteins10, 566–576. doi: 10.1007/s12602-017-9366-7
25
Chai W. Y. Teo K. T. K. Tan M. K. Tham H. J. (2022). Fermentation process control and optimization. Chem. Eng. Technol.45, 1731–1747. doi: 10.1002/ceat.202200029
26
Chai K. F. Voo A. Y. H. Chen W. N. (2020). Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf.19, 3825–3885. doi: 10.1111/1541-4337.12651
27
Chaklader M. R. Siddik M. A. Fotedar R. (2020). Total replacement of fishmeal with poultry by-product meal affected the growth, muscle quality, histological structure, antioxidant capacity and immune response of juvenile barramundi, Lates calcarifer. PloS One15, e0242079. doi: 10.1371/journal.pone.0242079
28
Chavan J. K. Kadam S. S. Beuchat L. R. (1989). Nutritional improvement of cereals by fermentation. Crit. Rev. Food Sci. Nutr.28, 349–400. doi: 10.1080/10408398909527507
29
Chen X. Liu S. Teame T. Luo J. Liu Y. Zhou Q. et al . (2025). Effect of Bacillus velezensis T23 solid-state fermentation product on growth, gut and liver health, and gut microbiota of common carp (Cyprinus carpio). Aquaculture596, 741733. doi: 10.1016/j.aquaculture.2024.741733
30
Chiang G. Lu W. Q. Piao X. S. Hu J. K. Gong L. M. Thacker P. A. (2009). Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas J. Anim. Sci.23, 263–271. doi: 10.5713/ajas.2010.90145
31
Chilakamarry C. R. Sakinah A. M. Zularisam A. W. Sirohi R. Khilji I. A. Ahmad N. et al . (2022). Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol.343, 126065. doi: 10.1016/j.biortech.2021.126065
32
Cho C. Y. (1992). Feeding systems for rainbow trout and other salmonids with reference to current estimates of energy and protein requirements. Aquaculture100, 107–123. doi: 10.1016/0044-8486(92)90353-M
33
Dai Z. Cui L. Li J. Wang B. Guo L. Wu Z. et al . (2020). “ Fermentation techniques in feed production,” in Animal agriculture (Cambridge, UK: Academic press), 407–429. doi: 10.1016/B978-0-12-817052-6.00024-0
34
Davies S. J. Guroyd G. Hassaan M. El-Ajnaf S. M. El-Haroun E. (2020). Nutritional evaluation of a novel co-fermented apple-pomace, molasses and formic acid generated sardine based fish silages as ingredients in diets for juvenile European sea bass (Dicentrachus labrax). Aquaculture521, 735087. doi: 10.1016/j.aquaculture.2020.735087
35
Dawood M. A. Koshio S. (2020). Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac.12, 987–1002. doi: 10.1111/raq.12368
36
Deng X. Chen K. Jiang D. Lu L. (2025). Advancements in synergistic fermentation of probiotics and enzymes for non-grain feed raw materials. Anim. Res. One Health3, 31–42. doi: 10.1002/aro2.90
37
De Villa R. Roasa J. Mine Y. Tsao R. (2023). Impact of solid-state fermentation on factors and mechanisms influencing the bioactive compounds of grains and processing by-products. Crit. Rev. Food Sci. Nutr.63, 5388–5413. doi: 10.1080/10408398.2021.2018989
38
Dey T. B. Chakraborty S. Jain K. K. Sharma A. Kuhad R. C. (2016). Antioxidant phenolics and their microbial production by submerged and solid-state fermentation process: A review. Trends Food Sci. Technol.53, 60–74. doi: 10.1016/j.tifs.2016.04.007
39
Dhull S. B. Punia S. Kumar R. Kumar M. Nain K. B. Jangra K. et al . (2021). Solid state fermentation of fenugreek (Trigonella foenum-graecum): Implications on bioactive compounds, mineral content and in vitro bioavailability. J. Food Sci. Technol.58, 1927–1936. doi: 10.1007/s13197-020-04704-y
40
Dimitroglou A. Merrifield D. L. Carnevali O. Picchietti S. Avella M. Daniels C. et al . (2011). Microbial manipulations to improve fish health and production–a Mediterranean perspective. Fish Shellfish Immunol.30, 1–16. doi: 10.1016/j.fsi.2010.08.009
41
Ding Z. Zhang Y. Ye J. Du Z. Kong Y. (2015). An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense: Growth, nonspecific immunity, and resistance to Aeromonas hydrophila. Fish Shellfish Immunol.44, 295–301. doi: 10.1016/j.fsi.2015.02.024
42
Dossou S. Koshio S. Ishikawa M. Yokoyama S. Dawood M. A. El Basuini M. F. et al . (2018). Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture490, 228–235. doi: 10.1016/j.aquaculture.2018.02.010
43
Durand A. (2003). Bioreactor designs for solid state fermentation. Biochem. Eng. J.13, 113–125. doi: 10.1016/S1369-703X(02)00124-9
44
Economou F. Chatziparaskeva G. Papamichael I. Loizia P. Voukkali I. Navarro-Pedreño J. et al . (2024). The concept of food waste and food loss prevention and measuring tools. Waste Manage. Res.42, 651–669. doi: 10.1177/0734242X241237187
45
EFFPA (2018). Reducing food waste. Available online at: https://www.effpa.eu/reducing-food-waste/ (Accessed 05 June 2025).
46
Egbuta M. A. Mwanza M. Babalola O. O. (2017). Health risks associated with exposure to filamentous fungi. Int. J. Environ. Res. Public Health14, 719. doi: 10.3390/ijerph14070719
47
El-Bakry M. Abraham J. Cerda A. Barrena R. Ponsá S. Gea T. et al . (2015). From wastes to high value added products: novel aspects of SSF in the production of enzymes. Crit. Rev. Environ. Sci. Technol.45, 1999–2042. doi: 10.1080/10643389.2015.1010423
48
El-Gendi H. Saleh A. K. Badierah R. Redwan E. M. El-Maradny Y. A. El-Fakharany E. M. (2021). A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi8, 23. doi: 10.3390/jof8010023
49
El-Saidy D. M. Saad A. S. (2011). Effects of partial and complete replacement of soybean meal with cottonseed meal on growth, feed utilization and haematological indexes for mono-sex male Nile tilapia, Oreochromis niloticus (L.) fingerlings. Aquac. Res.42, 351–359. doi: 10.1111/j.1365-2109.2010.02629.x
50
Espe M. Haaland H. Njaa L. R. (1992). Autolysed fish silage as a feed ingredient for Atlantic salmon (Salmo salar). Comp. Biochem. Physiol.103, 369–372. doi: 10.1016/0300-9629(92)90596-I
51
Feng X. Ng K. Ajlouni S. Zhang P. Fang Z. (2024). Effect of solid-state fermentation on plant-sourced proteins: A review. Food Rev. Int.40, 2580–2617. doi: 10.1080/87559129.2023.2274490
52
Ferreira M. Ramos-Oliveira C. Magalhães R. Martins N. Serra C. R. Salgado J. M. et al . (2025). Effects of solid-state fermentation of Gelidium corneum byproduct on immune status and gut microbiota in European seabass. Anim. Feed Sci. Technol.324, 116332. doi: 10.1016/j.anifeedsci.2025.116332
53
Filipe D. Dias M. Magalhães R. Fernandes H. Salgado J. Belo I. et al . (2023). Solid-state fermentation of Distiller’s dried grains with Solubles improves digestibility for European seabass (Dicentrarchus labrax) juveniles. Fishes8, 90. doi: 10.3390/fishes8020090
54
Food Waste Index Report . (2024). Think eat save –tracking progress to halve global food waste. ( United Nations Environment Programme (UNEP)). Available online at: https://knowledge4policy.ec.europa.eu/publication/food-waste-index-report-2024-think-eat-save-tracking-progress-halve-global-food-waste_en. (Accessed August 30, 2025).
55
Francis G. Makkar H. P. Becker K. (2001). Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture199, 197–227. doi: 10.1016/S0044-8486(01)00526-9
56
Gao M. Cieślak A. Kierończyk B. Huang H. Yanza Y. R. Zaworska-Zakrzewska A. et al . (2020). Effects of raw and fermented rapeseed cake on growth performance, methane production, and breast meat fatty acid composition in broiler chickens. Animals10, 2250. doi: 10.3390/ani10122250
57
Gareau M. G. Sherman P. M. Walker W. A. (2010). Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol.7, 503–514. doi: 10.1038/nrgastro.2010.117
58
Gilannejad N. Silva T. Martínez-Rodríguez G. Yúfera M. (2019). Effect of feeding time and frequency on gut transit and feed digestibility in two fish species with different feeding behaviours, gilthead seabream and Senegalese sole. Aquaculture513, 734438. doi: 10.1016/j.aquaculture.2019.734438
59
Gómez-Requeni P. Mingarro M. Calduch-Giner J. A. Médale F. Martin S. A. M. Houlihan D. F. et al . (2004). Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture232, 493–510. doi: 10.1016/S0044-8486(03)00532-5
60
Gowthaman M. K. Krishna C. Moo-Young M. (2001). Fungal solidstate fermentation—an overview. Appl. Mycol. Biotechnol.1, 305–352. doi: 10.1016/S1874-5334(01)80014-9
61
Graminha E. B. N. Gonçalves A. Z. L. Pirota R. D. P. B. Balsalobre M. A. A. Da Silva R. Gomes E. (2008). Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol.144, 1–22. doi: 10.1016/j.anifeedsci.2007.09.029
62
Hassaan M. Goda A. S. Kumar V. (2017). Evaluation of nutritive value of fermented de-oiled physic nut, Jatropha curcas, seed meal for Nile tilapia, Oreochromis niloticus fingerlings. Aquac. Nutr.23, 571–584. doi: 10.1111/anu.12422
63
Hassaan M. S. Soltan M. A. Abdel-Moez A. M. (2015). Nutritive value of soybean meal after solid state fermentation with Saccharomyces cerevisiae for Nile tilapia, Oreochromis niloticus. Anim. Feed Sci. Technol.201, 89–98. doi: 10.1016/j.anifeedsci.2015.01.007
64
Henry S. Dhital S. Sumer H. Butardo V. Jr. (2024). Solid-state fermentation of cereal waste improves the bioavailability and yield of bacterial cellulose production by a Novacetimonas sp. isolate. Foods13, 3052. doi: 10.3390/foods13193052
65
Hernandez M. T. Raimbault M. Roussos S. Lonsane B. K. (1992). Potential of solid state fermentation for production of ergot alkaloids. Lett. Appl. Microbiol.15, 156–159. doi: 10.1111/j.1472-765X.1992.tb00751.x
66
Holker U. Lenz J. (2005). Solid-state fermentation—are there any biotechnological advantages? Curr. Opin. Microbiol.8, 301–306. doi: 10.1016/j.mib.2005.04.006
67
Hong K. J. Lee C. H. Kim S. W. (2004). Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food7, 430–435. doi: 10.1089/jmf.2004.7.430
68
Hooge D. M. Pierce J. I. McBride K. W. Rigolin P. J. (2010). Meta-analysis of broiler chicken trials using diets with or without Allzyme® SSF enzyme complex. Int. J. Poult. Sci.9, 819–823. doi: 10.3923/ijps.2010.819.823
69
Hoque M. Devi K. T. R. (2025). Agro-industrial waste management: solid-state fermentation for biomass conversion and valorisation to value-added products. Syst. Microbiol. Biomanuf.5, 1–10. doi: 10.1007/s43393-025-00371-2
70
Hossain M. S. Zhang Y. Small B. C. (2023). Evaluation of a corn fermented protein with solubles (CFPS) as a complete soybean meal replacer in practical diets for Atlantic salmon (Salmo salar). Aquaculture566, 739198. doi: 10.1016/j.aquaculture.2022.739198
71
Ibarruri J. Manso M. López I. F. Cabello-Gómez J. F. Costas C. Cebrián M. (2024). Solid-state fermentation of fruit and vegetable discards: Production of nutritionally enriched ingredients and potential bioactive extracts for aquaculture sector. Biomass Conv. Bioref.1–15. doi: 10.1007/s13399-024-06414-3
72
Imelda J. Paulraj R. Bhatnagar D. (2008). Effect of solid state fermentation on nutrient composition of selected feed ingredients. Indian J. Fish.55, 327–332.
73
Jain S. Choudhary D. K. Varma A. (2021). “ Ecological perspectives of halophilic fungi and their role in bioremediation,” in Soil bioremediation. Eds. ParrayJ. A.Abd Elkhalek MahmoudA. H.SayyedR. (Hokoben, NJ: Wiley), 175–192. doi: 10.1002/9781119547976.ch8
74
Jannathulla R. Dayal J. S. Ambasankar K. Muralidhar M. (2018). Effect of Aspergillus Niger fermented soybean meal and sunflower oil cake on growth, carcass composition and haemolymph indices in Penaeus vannamei Boon. Aquaculture486, 1–8. doi: 10.1016/j.aquaculture.2017.12.005
75
Jannathulla R. Dayal J. S. Vasanthakumar D. Ambasankar K. Muralidhar M. (2017). Effect of fermentation methods on amino acids, fiber fractions and anti-nutritional factors in different plant protein sources and essential amino acid index for Penaeus vannamei Boon. Indian J. Fish.64, 40–47. doi: 10.21077/ijf.2017.64.2.60341-07
76
Jannathulla R. Dayal J. S. Vasanthakumar D. Ambasankar K. Panigrahi A. Muralidhar M. (2019). Apparent digestibility coefficients of fungal fermented plant proteins in two different penaeid shrimps – A comparative study. Aquac. Res.50, 1491–1500. doi: 10.1111/are.14024
77
Jeyakumar E. Lawrence R. (2022). Microbial fermentation for reduction of antinutritional factors. Curr. Dev. Biotechnol. Bioeng, 239–260. doi: 10.1016/B978-0-12-823506-5.00012-6
78
Jin G. Zhao Y. Xin S. Li T. Xu Y. (2024). Solid-state fermentation engineering of traditional Chinese fermented food. Foods13, 3003. doi: 10.3390/foods13183003
79
Kader M. A. Koshio S. (2012). Effect of composite mixture of seafood by-products and soybean proteins in replacement of fishmeal on the performance of red sea bream, Pagrus major. Aquaculture368, 95–102. doi: 10.1016/j.aquaculture.2012.09.014
80
Karimi F. Mazaheri D. Saei Moghaddam M. Mataei Moghaddam A. Sanati A. L. Orooji Y. (2021). Solid-state fermentation as an alternative technology for cost-effective production of bioethanol as useful renewable energy: a review. Biomass Conv. Bioref, 1–17. doi: 10.1007/s13399-021-01875-2
81
Katya K. Yun Y. H. Park G. Lee J. Y. Yoo G. Bai S. C. (2014). Evaluation of the efficacy of fermented by-product of mushroom, Pleurotus ostreatus, as a fish meal replacer in juvenile Amur catfish, Silurus asotus: effects on growth, serological characteristics and immune responses. Asian-Australa J. Anim. Sci.27, 1478. doi: 10.5713/ajas.2014.14038
82
Kaviraj A. Mondal K. Mukhopadhyay P. K. Turchini G. M. (2013). “ Impact of fermented mulberry leaf and fish offal in diet formulation of Indian major carp (Labeo rohita),” in Proceedings of the zoological society (Berlin: Springer-Verlag, Berlin Heidelberg), 64–73. doi: 10.1007/s12595-012-0052-1
83
Kaya E. Galatalı S. Güldağ S. Öztürk B. Ceylan M. Çelik O. et al . (2018). Mass production of medicinal plants for obtaining secondary metabolite using liquid mediums via bioreactor systems: SETIS™ and RITA®. Turk Bilimsel Derlem Derg11, 5–10.
84
Kim S. S. Galaz G. B. Pham M. A. Jang J. W. Oh D. H. Yeo I. K. et al . (2009). Effects of dietary supplementation of a meju, fermented soybean meal, and Aspergillus oryzae for juvenile parrot fish (Oplegnathus fasciatus). Asian-Australas. J. Anim. Sci.22, 849–856. doi: 10.5713/ajas.2009.80648
85
Kim S. K. Kim T. H. Lee S. K. Chang K. H. Cho S. J. Lee K. W. et al . (2016). The use of fermented soybean meals during early phase affects subsequent growth and physiological response in broiler chicks. Asian-Australasian J. Anim. Sci.29, 1287. doi: 10.5713/ajas.15.0653
86
Kim S. S. Pham M. A. Kim K. W. Son M. H. Lee K. J. (2010). Effects of microbial fermentation of soybean on growth performances, phosphorus availability, and antioxidant activity in diets for juvenile olive flounder (Paralichthys olivaceus). Food Sci. Biotech.19, 1605–1610. doi: 10.1007/s10068-010-0227-3
87
Krishna C. (2005). Solid-state fermentation systems—an overview. Crit. Rev. Biotechnol.25, 1–30. doi: 10.1080/07388550590925383
88
Kuan O. C. Rasit N. Abdullah W. R. W. Hamzah S. Harun M. H. C. Siddique M. N. I. (2024). “ Aquaculture sludge treatment: Leveraging Aspergillus Niger for protease production through solid-state fermentation,” in Sustainable Approaches to Environmental Design, Materials Science, and Engineering Technologies. (Singapore: Springer), 141–152. doi: 10.1007/978-3-031-76025-9_13
89
Kumar H. D. (1998). Modern concept of biotechnology (Uttar Pradesh, India: Vikas Publishing House).
90
Lee S. M. Azarm H. M. Chang K. H. (2016a). Effects of dietary inclusion of fermented soybean meal on growth, body composition, antioxidant enzyme activity and disease resistance of rockfish (Sebastes schlegeli). Aquaculture459, 110–116. doi: 10.1016/j.aquaculture.2016.03.036
91
Lee Y. D. Hong Y. F. Jeon B. Jung B. J. Chung D. K. Kim H. (2016b). Differential cytokine regulatory effect of three Lactobacillus strains isolated from fermented foods. J. Microbiol. Biotechnol.26, 1517–1526. doi: 10.4014/jmb.1601.01044
92
Lee B. J. Kim S. S. Song J. W. Oh D. H. Cha J. H. Jeong J. B. et al . (2013). Effects of dietary supplementation of citrus by-products fermented with a probiotic microbe on growth performance, innate immunity and disease resistance against Edwardsiella tarda in juvenile olive flounder, Paralichthys olivaceus (Temminck & Schlegel). J. Fish Dis.36, 617–628. doi: 10.1111/jfd.12035
93
Leistner L. Gould G. W. (2002). Hurdle technologies: combination treatments for food stability, safety and quality (Heidelberg, Germany: Springer Science & Business Media).
94
Li C. Zhang B. Liu C. Zhou H. Wang X. Mai K. et al . (2020). Effects of dietary raw or Enterococcus faecium fermented soybean meal on growth, antioxidant status, intestinal microbiota, morphology, and inflammatory responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol.100, 261–271. doi: 10.1016/j.fsi.2020.02.070
95
Li C. Zhang B. Zhou H. Wang X. Pi X. Wang X. et al . (2019). Beneficial influences of dietary Aspergillus awamori fermented soybean meal on oxidative homoeostasis and inflammatory response in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol.93, 8–16. doi: 10.1016/j.fsi.2019.07.037
96
Lim S. R. Choi S. M. Wang X. J. Kim K. W. Shin I. S. Min T. S. et al . (2004). Effects of dehulled soybean meal as a fish meal replacer in diets for fingerling and growing Korean rockfish Sebastes schlegeli. Aquaculture231, 457–468. doi: 10.1016/j.aquaculture.2003.09.008
97
Lim S. J. Lee K. J. (2009). Partial replacement of fish meal by cottonseed meal and soybean meal with iron and phytase supplementation for parrot fish Oplegnathus fasciatus. Aquaculture290, 283–289. doi: 10.1016/j.aquaculture.2009.02.018
98
Lim S. J. Lee K. J. (2011). A microbial fermentation of soybean and cottonseed meal increases antioxidant activity and gossypol detoxification in diets for Nile tilapia, Oreochromis niloticus. J. World Aquac. Soc42, 494–503. doi: 10.1111/j.1749-7345.2011.00491.x
99
Lin Y. H. Mui J. J. (2017). Comparison of dietary inclusion of commercial and fermented soybean meal on oxidative status and non-specific immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol.63, 208–212. doi: 10.1016/j.fsi.2017.02.011
100
Lin C. H. Wei Y. T. Chou C. C. (2006). Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol.23, 628–633. doi: 10.1016/j.fm.2005.12.004
101
Luo L. Xue M. Wu X. Cai X. Cao H. Liang Y. (2006). Partial or total replacement of fishmeal by solvent-extracted cottonseed meal in diets for juvenile rainbow trout (Oncorhynchus mykiss). Aquac. Nutr.12, 418–424. doi: 10.1111/j.1365-2095.2006.00443.x
102
Lutful Kabir S. M. (2009). The role of probiotics in the poultry industry. Int. J. Mol. Sci.10, 3531–3546. doi: 10.3390/ijms10083531
103
Manan M. A. Webb C. (2017). Design aspects of solid state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng.4, 511–532. doi: 10.15406/jabb.2017.04.00094
104
Mandal S. Ghosh K. (2013). Optimization of tannase production and improvement of nutritional quality of two potential low-priced plant feedstuffs under solid state fermentation by Pichia kudriavzevii isolated from fish gut. Food Biotechnol.27, 86–103. doi: 10.1080/08905436.2012.755929
105
Mandal S. Ghosh K. (2019). Utilization of fermented Pistia leaves in the diet of Rohu, Labeo rohita (Hamilton): Effects on growth, digestibility and whole body composition. Waste Biomass Valor.10, 3331–3342. doi: 10.1007/s12649-018-0336-4
106
Mandal S. Ghosh K. (2020). Effect of different processing techniques on nutrient and anti-nutrient compositions of plant feedstuffs for their probable use as aqua-feed ingredients. J. Inland Fish. Soc India52, 173–182. doi: 10.47780/jifsi.52.2.2020.108383
107
Manpreet S. Sawraj S. Sachin D. Pankaj S. Banerjee U. C. (2005). Influence of process parameters on the production of metabolites in solid-state fermentation. Malays. J. Microbiol.2, 1–9. doi: 10.21161/mjm.120501
108
Martin N. Guez M. A. U. Sette L. D. Da Silva R. Gomes E. (2010). Pectinase production by a Brazilian thermophilic fungus Thermomucor indicae-seudaticae N31 in solid-state and submerged fermentation. Microbiology79, 306–313. doi: 10.1134/S0026261710030057
109
Martínez-Llorens S. Moñino A. V. Tomás Vidal A. Salvador V. J. M. Pla Torres M. Jover Cerdá M. (2007). Soybean meal as a protein source in gilthead sea bream (Sparus aurata L.) diets: effects on growth and nutrient utilization. Aquac. Res.38, 82–90. doi: 10.1111/j.1365-2109.2006.01637.x
110
Meng X. Cai H. Li H. You F. Jiang A. Hu W. et al . (2023). Clostridium butyricum-fermented Chinese herbal medicine enhances the immunity by modulating the intestinal microflora of largemouth bass (Micropterus salmoides). Aquaculture562, 738768. doi: 10.1016/j.aquaculture.2022.738768
111
Miles R. D. Chapman F. A. (2006). The benefits of fish meal in aquaculture diets. Available online at: https://edis.ifas.ufl.edu/publication/FA122 (Accessed 05 June 2025).
112
Mishra B. Mishra A. K. Mohanta Y. K. Yadavalli R. Agrawal D. C. Reddy H. P. et al . (2024). Postbiotics: the new horizons of microbial functional bioactive compounds in food preservation and security. Food Prod. Process Nutr.6, 28. doi: 10.1186/s43014-023-00200-w
113
Mitchell D. A. Krieger N. Stuart D. M. Pandey A. (2000). New developments in solid-state fermentation: II. Rational approaches to the design, operation and scale-up of bioreactors. Process Biochem.35, 1211–1225. doi: 10.1016/S0032-9592(00)00157-6
114
Mitchell D. A. Von Meien O. F. Luz L. F. L. Berovič M. (2006). “ The scale-up challenge for SSF bioreactors,” in Solid-state fermentation bioreactors. Eds. MitchellD. A.BerovičM.KriegerN. ( Springer, Berlin), 57–64. doi: 10.1007/3-540-31286-2_5
115
Molin S. Givskov M. (1999). Application of molecular tools for in situ monitoring of bacterial growth activity. Environ. Microbiol.1, 383–391. doi: 10.1046/j.1462-2920.1999.00056.x
116
Mondal K. Kaviraj A. Mukhopadhyay P. K. (2011). Partial replacement of fishmeal by fermented fish-offal meal in the formulation of diet for Indian minor carp Labeo bata. J. Appl. Aquac.23, 41–50. doi: 10.1080/10454438.2011.549783
117
Moniruzzaman M. Bae J. H. Won S. H. Cho S. J. Chang K. H. Bai S. C. (2018). Evaluation of solid-state fermented protein concentrates as a fish meal replacer in the diets of juvenile rainbow trout, Oncorhynchus mykiss. Aquac. Nutr.24, 1198–1212. doi: 10.1111/anu.12658
118
Mukherjee R. Chakraborty R. Dutta A. (2015). Role of fermentation in improving nutritional quality of soybean meal—a review. Asian-Australas J. Anim. Sci.29, 1523. doi: 10.5713/ajas.15.0627
119
Nagarajan M. Rajasekaran B. Venkatachalam K. (2022). Microbial metabolites in fermented food products and their potential benefits. Int. Food Res. J.29, 466–486. doi: 10.47836/ifrj.29.3.01
120
Ngandzali B. O. Zhou F. Xiong W. Shao Q. J. Xu J. Z. (2011). Effect of dietary replacement of fish meal by soybean protein concentrate on growth performance and phosphorus discharging of juvenile black sea bream, Acanthopagrus schlegelii. Aquac. Nutr.17, 526–535. doi: 10.1111/j.1365-2095.2010.00835.x
121
Nguyen H. P. Khaoian P. Fukada H. Suzuki N. Masumoto T. (2015). Feeding fermented soybean meal diet supplemented with taurine to yellowtail Seriola quinqueradiata affects growth performance and lipid digestion. Aquac. Res.46, 1101–1110. doi: 10.1111/are.12267
122
Olukomaiya O. O. Adiamo O. Q. Fernando W. C. Mereddy R. Li X. Sultanbawa Y. (2020). Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem.315, 126238. doi: 10.1016/j.foodchem.2020.126238
123
Olukomaiya O. Fernando C. Mereddy R. Li X. Sultanbawa Y. (2019). Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr.5, 319–330. doi: 10.1016/j.aninu.2019.05.005
124
Onomu A. J. Okuthe G. E. (2024). The application of fungi and their secondary metabolites in aquaculture. J. Fungi10, 711. doi: 10.3390/jof10100711
125
Özyurt G. Gökdoğan S. Şimşek A. Yuvka I. Ergüven M. Kuley Boga E. (2016). Fatty acid composition and biogenic amines in acidified and fermented fish silage: a comparison study. Archiv Anim. Nutr.70, 72–86. doi: 10.1080/1745039X.2015.1117696
126
Pandey A. Selvakumar P. Soccol C. R. Nigam P. (1999). Solid state fermentation for the production of industrial enzymes. Curr. Sci.77, 149–162.
127
Pandey A. Soccol C. R. Mitchell D. (2000). New developments in solid state fermentation: I-bioprocesses and products. Process Biochem.35, 1153–1169. doi: 10.1016/S0032-9592(00)00152-7
128
Plaipetch P. Yakupitiyage A. (2012). Use of yeast-fermented canola meal to replace fishmeal in the diet of Asian sea bass Lates calcarifer (Bloch 1970). J. Aquac. Res. Dev.3, 1–5. doi: 10.4172/2155-9546.1000125
129
Quax W. J. (2013). “ Bacterial enzymes,” in The prokaryotes ( Springer, Berlin, Heidelberg), 193–211. doi: 10.1007/0-387-30741-9_22
130
Raghavarao K. Ranganathan T. V. Karanth N. G. (2003). Some engineering aspects of solid-state fermentation. Biochem. Eng. J.13, 127–135. doi: 10.1016/S1369-703X(02)00125-0
131
Raimbault M. (1998). General and microbiological aspects of solid substrate fermentation. Electron J. Biotechnol.1, 26–27. doi: 10.4067/S0717-34581998000300007
132
Ramachandran S. Bairagi A. Ray A. K. (2005). Improvement of nutritive value of grass pea (Lathyrus sativus) seed meal in the formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after fermentation with a fish gut bacterium. Biores. Technol.96, 1465–1472. doi: 10.1016/j.biortech.2004.12.002
133
Ramachandran S. Ray A. K. (2007). Nutritional evaluation of fermented black gram (Phaseolus mungo) seed meal in compound diets for rohu, Labeo rohita (Hamilton), fingerlings. J. Appl. Ichthyol.23, 74–79. doi: 10.1111/j.1439-0426.2006.00772.x
134
Ranjan A. Sahu N. P. Deo A. D. Kumar S. (2019). Solid state fermentation of de-oiled rice bran: Effect on in vitro protein digestibility, fatty acid profile and anti-nutritional factors. Food Res. Int.119, 1–5. doi: 10.1016/j.foodres.2019.01.054
135
Ray A. K. Ghosh K. Ringø E. (2012). Enzyme-producing bacteria isolated from fish gut: A review. Aquac. Nutr.18, 465–492. doi: 10.1111/j.1365-2095.2012.00943.x
136
Refstie S. Sahlstrom S. Bråthen E. Baeverfjord G. Krogedal P. (2005). Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture246, 331–345. doi: 10.1016/j.aquaculture.2005.01.001
137
Rinchard J. Lee K. J. Dabrowski K. Ciereszko A. Blom J. H. Ottobre J. S. (2003). Influence of gossypol from dietary cottonseed meal on haematology, reproductive steroids and tissue gossypol enantiomer concentrations in male rainbow trout (Oncorhynchus mykiss). Aquac. Nutr.9, 275–282. doi: 10.1046/j.1365-2095.2003.00253.x
138
Ringø E. Ramasamy H. Soltani M. Ghosh K. (2022). The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals12, 3016. doi: 10.3390/ani12213016
139
Ringø E. Sperstad S. Myklebust R. Refstie S. Krogdahl Å. (2006). Characterisation of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.): the effect of fish meal, standard soybean meal and a bioprocessed soybean meal. Aquaculture261, 829–841. doi: 10.1016/j.aquaculture.2006.06.030
140
Robinson T. Singh D. Nigam P. (2001). Solid-state fermentation: a promising microbial technology for secondary metabolite production. Appl. Microbiol. Biotechnol.55, 284–289. doi: 10.1007/s002530000565
141
Rombenso A. Crouse C. Trushenski J. (2013). Comparison of traditional and fermented soybean meals as alternatives to fish meal in hybrid striped bass feeds. North Ame. J. Aquac.75, 197–204. doi: 10.1080/15222055.2012.756440
142
Romero J. Ringø E. Merrifield D. L. (2014). “ The gut microbiota of fish,” in Aquac nutr: gut health probiotics prebiotics. Eds. MerrifieldD. L.RingøE. ( Wiley-Blackwell, Oxford), 75–100. doi: 10.1002/9781118897263.ch4
143
Sabahi S. Homayouni Rad A. Aghebati-Maleki L. Sangtarash N. Ozma M. A. Karimi A. et al . (2023). Postbiotics as the new frontier in food and pharmaceutical research. Crit. Rev. Food Sci. Nutr.63, 8375–8402. doi: 10.1080/10408398.2022.2056727
144
Sadh P. K. Duhan S. Duhan J. S. (2018b). Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour. Bioprocess.5, 1–15. doi: 10.1186/s40643-017-0187-z
145
Sadh P. K. Kumar S. Chawla P. Duhan J. S. (2018a). Fermentation: a boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules23, 2560. doi: 10.3390/molecules23102560
146
Saha S. Ray A. K. (2011). Evaluation of nutritive value of water hyacinth (Eichhornia crassipes) leaf meal in compound diets for rohu, Labeo rohita (Hamilton 1822) fingerlings after fermentation with two bacterial strains isolated from fish gut. Turk. J. Fish. Aquat. Sci.11, 199–209. doi: 10.4194/trjfas.2011.0204
147
Šelo G. Planinić M. Tišma M. Tomas S. Koceva Komlenić D. Bucić-Kojić A. (2021). A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods10, 927. doi: 10.3390/foods10050927
148
Sharawy Z. Goda A. M. S. Hassaan M. S. (2016). Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, postlarvae. Anim. Feed Sci. Technol.212, 90–99. doi: 10.1016/j.anifeedsci.2015.12.009
149
Shi H. Su B. Chen X. Pian R. (2020). Solid state fermentation of Moringa oleifera leaf meal by mixed strains for the protein enrichment and the improvement of nutritional value. PeerJ8, e10358. doi: 10.7717/peerj.10358
150
Shi H. Yang E. Yang H. Huang X. Zheng M. Chen X. et al . (2022). Dynamic changes in the chemical composition and metabolite profiles of drumstick (Moringa oleifera Lam.) leaf flour during fermentation. LWT155, 112973. doi: 10.1016/j.lwt.2021.112973
151
Shi C. Zhang Y. Lu Z. Wang Y. (2017). Solid-state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J. Anim. Sci. Biotechnol.8, 1–9. doi: 10.1186/s40104-017-0184-2
152
Shih M. F. Lay C. H. Lin C. Y. Chang S. H. (2021). Exploring the environmental and economic potential for biogas production from swine manure wastewater by life cycle assessment. Clean Techn. Environ. Policy25, 451–464. doi: 10.1007/s10098-021-02157-1
153
Shiu Y. L. Wong S. L. Guei W. C. Shin Y. C. Liu C. H. (2015). Increase in the plant protein ratio in the diet of white shrimp, Litopenaeus vannamei (Boone), using Bacillus subtilis E20-fermented soybean meal as a replacement. Aquac. Res.46, 382–394. doi: 10.1111/are.12186
154
Siddik M. A. Howieson J. Partridge G. J. Fotedar R. Gholipourkanani H . (2018). Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates calcarifer. Sci Rep. 8 (1), 15942. doi: 10.1038/s41598-018-34182-4
155
Siddik M. A. Chungu P. Fotedar R. Howieson J. (2019a). Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch). PloS One14, e0215025. doi: 10.1371/journal.pone.0215025
156
Siddik M. A. Fotedar R. Chaklader M. R. Foysal M. J. Nahar A. Howieson J. (2020). Fermented animal source protein as substitution of fishmeal on intestinal microbiota, immune-related cytokines and resistance to Vibrio mimicus in freshwater crayfish (Cherax cainii). Front. Physiol.10. doi: 10.3389/fphys.2019.01635
157
Siddik M. A. Howieson J. Fotedar R. (2019b). Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health and disease resistance to Vibrio harveyi in juvenile barramundi, Lates calcarifer. Fish Shellfish Immunol.89, 61–70. doi: 10.1016/j.fsi.2019.03.042
158
Siddik M. A. Julien B. B. Islam S. M. Francis D. S. (2024). Fermentation in aquafeed processing: Achieving sustainability in feeds for global aquaculture production. Rev. Aquac.16, 1244–1265. doi: 10.1111/raq.12894
159
Siddik M. A. Vatsos I. N. Rahman M. A. Pham H. D. (2022). Selenium-enriched spirulina (SeE-SP) enhance antioxidant response, immunity, and disease resistance in juvenile Asian seabass, Lates calcarifer. Antioxidants11, 1572. doi: 10.3390/antiox11081572
160
Singh S. Shyu D. J. (2024). Metagenomics insight into microbial community analysis during pesticide degradation: state of the art, success stories, challenges, and future outlook. doi: 10.1039/BK9781837673131-00481
161
Singhania R. R. Patel A. K. Gottumukkala L. D. Rajasree K. Soccol C. R. Pandey A. (2018). “ Solid-state fermentation: current trends and future prospects,” in Fermentation microbiology and biotechnology, fourth edition (Boca Raton, Florida, United States: CRC Press), 243–254.
162
Singhania R. R. Patel A. K. Thomas L. Pandey A. (2017). “ Solid-state fermentation,” in Industrial Biotechnology: Products and Processes. (Hoboken, New Jersey, USA: Wiley), 187–204. doi: 10.1002/9783527807833.ch6
163
Sirakov I. Velichkova K. Dinev T. Slavcheva-Sirakova D. Valkova E. Yorgov D. et al . (2023). Detection of fungal diseases in lettuce by VIR-NIR spectroscopy in aquaponics. Microorganisms11, 2348. doi: 10.3390/microorganisms11092348
164
Soltani M. Ghosh K. Hoseinifar S. M. Kumar V. Lymbery A. J. Roy S. et al . (2019). Genus Bacillus, promising probiotics in aquaculture: Aquatic animal origin, bioactive components, bioremediation and efficacy in fish and shellfish. Rev. Fish. Sci. Aquac.27, 331–379. doi: 10.1080/23308249.2019.1597010
165
Song Y. S. Frías J. Martínez-Villaluenga C. Vidal-Valdeverde C. De Mejia E. G. (2008). Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem.108, 571–581. doi: 10.1016/j.foodchem.2007.11.013
166
Sørensen S. L. (2022). Influence of feed ingredients and additives on mucosal health with focus on the intestine of Atlantic salmon (Salmo salar) (PhD Thesis). Nord University, Bodo, Norway.
167
Subramaniyam R. Vimala R. (2012). Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int. J. Sci. Nat.3, 480–486.
168
Sugiharto S. Ranjitkar S. (2019). Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr.5, 1–10. doi: 10.1016/j.aninu.2018.11.001
169
Sultana S. Biró J. Kucska B. Hancz C. (2024). Factors affecting yeast digestibility and Immunostimulation in aquatic animals. Animals14, 2851. doi: 10.3390/ani14192851
170
Sun H. Tang J. W. Yao X. H. Wu Y. F. Wang X. Liu Y. et al . (2015). Partial substitution of fish meal with fermented cottonseed meal in juvenile black sea bream (Acanthopagrus schlegelii) diets. Aquaculture446, 30–36. doi: 10.1016/j.aquaculture.2015.04.020
171
Sun X. Urriola P. E. Shurson G. Tiffany D. Hu B. (2023). Enhancing feeding value of corn distiller’s grains with solubles via fungal co-cultured solid-state fermentation for monogastric animal nutrition. Anim. Feed Sci. Technol.303, 115673. doi: 10.1016/j.anifeedsci.2023.115673
172
Tadioto V. Giehl A. Cadamuro R. D. Guterres I. Z. dos Santos A. A. Bressan S. K. et al . (2023). Bioactive compounds from and against yeasts in the One Health context: A comprehensive review. Fermentation9, 363. doi: 10.3390/fermentation9040363
173
Tamang J. P. Shin D. H. Jung S. J. Chae S. W. (2016). Functional properties of microorganisms in fermented foods. Front. Microbiol.7. doi: 10.3389/fmicb.2016.00578
174
Tang J. W. Sun H. Yao X. H. Wu Y. F. Wang X. Feng J. (2012). Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian-Australas J. Anim. Sci.25, 393. doi: 10.5713/ajas.2011.11381
175
Thomas L. Larroche C. Pandey A. (2013). Current developments in solid-state fermentation. Biochem. Eng. J.81, 146–161. doi: 10.1016/j.bej.2013.10.013
176
Ulmer D. Tengerdy R. Murphy V. G. (1981). “ Solid-state fermentation of steam-treated feedlot waste fibers with Chaetomium cellulolyticum,” in Biotechnol Bioeng Symp, United States, 11 [CONF-810554-]. Colorado State University, Fort Collins, CO.
177
Umrao D. Singh S. Singh D. P. Maddirala S. Sevda S. Krishania M. (2024). “ Treatment of solid food waste using solid-state fermentation: its current use and future perspectives,” in Solid waste management (Boca Raton, Florida, United States: CRC Press), 161–180.
178
Vandenberghe L. P. Karp S. G. de Oliveira P. Z. de Carvalho J. C. Rodrigues C. Soccol C. R. (2018). Solid-state fermentation for the production of organic acids. Curr. Dev. Biotechnol. Bioeng, 415–434. doi: 10.1016/B978-0-444-63990-5.00018-9
179
Vandenberghe L. P. Pandey A. Carvalho J. C. Letti L. A. Woiciechowski A. L. Karp S. G. et al . (2021). Solid-state fermentation technology and innovation for the production of agricultural and animal feed bioproducts. Syst. Microbiol. Biomanuf.1, 142–165. doi: 10.1007/s43393-020-00015-7
180
Vandenberghe L. P. Soccol C. R. Pandey A. Lebeault J. M. (1999). Microbial production of citric acid. Braz. Arch. Biol. Technol.42, 263–276. doi: 10.1590/S1516-89131999000300001
181
Van Vo B. Siddik M. A. Fotedar R. Chaklader M. R. Foysal M. J. Pham H. D. (2020a). Digestibility and water quality investigations on the processed peanut (Arachis hypogaea) meal fed barramundi (Lates calcarifer) at various inclusion levels. Aquac. Rep.18, 100474. doi: 10.1016/j.aqrep.2020.100474
182
Van Vo B. Siddik M. A. Fotedar R. Chaklader M. R. Hanif M. A. Foysal M. J. et al . (2020b). Progressive replacement of fishmeal by raw and enzyme-treated alga, Spirulina platensis influences growth, intestinal micromorphology and stress response in juvenile barramundi, Lates calcarifer. Aquaculture529, 735741. doi: 10.1016/j.aquaculture.2020.735741
183
Venil C. K. Devi P. R. Ahmad W. A. (2020). “ Agro-industrial waste as substrates for the production of bacterial pigment,” in Valorization of agro-industrial residues–volume I: biological approaches, (Cham, Switzerland: Springer), 149–162. doi: 10.1007/978-3-030-39137-9_7
184
Verduzco-Oliva R. Gutierrez-Uribe J. A. (2020). Beyond enzyme production: Solid state fermentation (SSF) as an alternative approach to produce antioxidant polysaccharides. Sustainability12, 495. doi: 10.3390/su12020495
185
Vieira L. Filipe D. Amaral D. Magalhães R. Martins N. Ferreira M. et al . (2023). Solid-state fermentation as green technology to improve the use of plant feedstuffs as ingredients in diets for European sea bass (Dicentrarchus labrax) juveniles. Animals13, 2692. doi: 10.3390/ani13172692
186
Vinestock T. Short M. Ward K. Guo M. (2024). Computer-aided chemical engineering research advances in precision fermentation. Curr. Opin. Food Sci.101196. doi: 10.1016/j.cofs.2024.101196
187
Wang A. Meng D. Hao Q. Xia R. Zhang Q. Ran C. et al . (2022). Effect of supplementation of solid-state fermentation product of Bacillus subtilis HGcc-1 to high-fat diet on growth, hepatic lipid metabolism, epidermal mucus, gut and liver health and gut microbiota of zebrafish. Aquaculture560, 738542. doi: 10.1016/j.aquaculture.2022.738542
188
Wang Q. Qi Z. Fu W. Pan M. Ren X. Zhang X. et al . (2024). Research and prospects of enzymatic hydrolysis and microbial fermentation technologies in protein raw materials for aquatic feed. Fermentation10, 648. doi: 10.3390/fermentation10120648
189
Wang L. Zhou H. He R. Xu W. Mai K. He G. (2016). Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture464, 87–94. doi: 10.1016/j.aquaculture.2016.06.026
190
Watanabe T. (2002). Strategies for further development of aquatic feeds. Fish. Sci.68, 242–252. doi: 10.1046/j.1444-2906.2002.00418.x
191
Wösten H. A. (2019). Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol.59, 65–70. doi: 10.1016/j.copbio.2019.02.010
192
Xu Q. Qiao Q. Gao Y. Hou J. Hu M. Du Y. et al . (2021). Gut microbiota and their role in health and metabolic disease of dairy cow. Front. Nutr.8. doi: 10.3389/fnut.2021.701511
193
Xu F. Z. Zeng X. G. Ding X. L. (2012). Effects of replacing soybean meal with fermented rapeseed meal on performance, serum biochemical variables and intestinal morphology of broilers. Asian-Australa J. Anim. Sci.25, 1734. doi: 10.5713/ajas.2012.12249
194
Yafetto L. Odamtten G. T. Wiafe-Kwagyan M. (2023). Valorization of agro-industrial wastes into animal feed through microbial fermentation: A review of the global and Ghanaian case. Heliyon9, e14814. doi: 10.1016/j.heliyon.2023.e14814
195
Yamamoto T. Iwashita Y. Matsunari H. Sugita T. Furuita H. Akimoto A. et al . (2010). Influence of fermentation conditions for soybean meal in a non-fish meal diet on the growth performance and physiological condition of rainbow trout Oncorhynchus mykiss. Aquaculture309, 173–180. doi: 10.1016/j.aquaculture.2010.09.021
196
Yang G. Yu R. Geng S. Xiong L. Yan Q. Kumar V. et al . (2021). Apple polyphenols modulate the antioxidant defense and attenuate inflammatory response concurrent with hepatoprotective effect on grass carp (Ctenopharyngodon idellus) fed low fish meal diet. Aquaculture534, 736284. doi: 10.1016/j.aquaculture.2020.736284
197
Yu Y. Li Y. Zhang J. Wang J. (2025). Nutritional value improvement of oats by solid-state fermentation with Monascus purpureus. Foods14, 1703. doi: 10.3390/foods14101703
198
Zhang X. Sun Z. Cai J. Wang J. Wang G. Zhu Z. et al . (2020). Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish Immunol.102, 430–439. doi: 10.1016/j.fsi.2020.04.051
199
Zhang D. Zhang Y. Liu B. Jiang Y. Zhou Q. Wang J. et al . (2017). Effect of replacing fish meal with fermented mushroom bran hydrolysate on the growth, digestive enzyme activity, and antioxidant capacity of allogynogenetic crucian carp (Carassius auratus gibelio). Turk. J. Fish. Aquat. Sci.17, 1039–1048. doi: 10.4194/1303-2712-v17_5_20
200
Zhang L. Zhang B. Zhu X. Chang H. Ou S. Wang H. (2018). “ Role of bioreactors in microbial biomass and energy conversion,” in Bioreactors for microbial biomass and energy conversion. Green energy and technology. Eds. LiaoQ.ChangJ.HerrmannC.XiaA. ( Springer, Singapore), 39–78. doi: 10.1007/978-981-10-7677-0_2
201
Zheng L. Li D. Li Z. L. Kang L. N. Jiang Y. Y. Liu X. Y. et al . (2017). Effects of Bacillus fermentation on the protein microstructure and anti-nutritional factors of soybean meal. Lett. Appl. Microbiol.65, 520–526. doi: 10.1111/lam.12806
202
Zhou F. Song W. Shao Q. Peng X. Xiao J. Hua Y. et al . (2011). Partial replacement of fish meal by fermented soybean meal in diets for black sea bream, Acanthopagrus schlegelii, juveniles. J. World Aquac. Soc42, 184–197. doi: 10.1111/j.1749-7345.2011.00455.x
203
Zhou Q. C. Yue Y. R. (2012). Apparent digestibility coefficients of selected feed ingredients for juvenile hybrid tilapia, Oreochromis niloticus × Oreochromis aureus. Aquac. Res.43, 806–814. doi: 10.1111/j.1365-2109.2011.02892.x
204
Zhuo L. C. Chen C. F. Lin Y. H. (2021). Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to Photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol.117, 248–252. doi: 10.1016/j.fsi.2021.08.015
Summary
Keywords
fermentation, anti-nutritional factors, aquafeed, bioactive compounds, sustainability
Citation
Kalaiselvan P, Devi NC, Deepti M, Devi AA, Akamad K, Dheeran P, Debbarma S, Vadivel D and Rajesh D (2025) Solid-state fermentation—a sustainable future technology in aquafeeds?. Front. Mar. Sci. 12:1669719. doi: 10.3389/fmars.2025.1669719
Received
20 July 2025
Accepted
29 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Benjamin Costas, University of Porto, Portugal
Reviewed by
Jiajie Xu, Ningbo University, China; Rakhi Kumari, Central Institute of Freshwater Aquaculture (ICAR), India
Updates
Copyright
© 2025 Kalaiselvan, Devi, Deepti, Devi, Akamad, Dheeran, Debbarma, Vadivel and Rajesh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pandi Kalaiselvan, kalaiprs1641@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.