Abstract
Climate change and human activity pose increasing challenges to endangered sea turtles, which are key species in many marine ecosystems worldwide. Among these challenges are the flooding and erosion of nesting beaches. In this perspective, we argue that existing methods and tools from coastal science and management hold significant, yet underused, potential for sea turtle conservation. We introduce a stepwise framework for integrating sea turtle ecology and coastal management to address these coastal threats. The framework follows an Observe–Understand–Predict–Intervene cycle and links ecological thresholds, coastal processes, and management interventions across scales, from Regional Management Units (RMUs) to individual beaches. We illustrate how state-of-the-art monitoring, modeling, and nature-based solutions (NBS) can be embedded within this framework to inform when and how to intervene. Increased in-situ data collection and interdisciplinary collaboration will be critical to apply and refine this approach, thereby enhancing the long-term resilience of nesting habitats.
1 Introduction
Climate change and human activity pose various challenges to endangered sea turtles, who fulfill critical ecological roles in many marine ecosystems worldwide (Christianen et al., 2023; Heithaus, 2013; Patrício et al., 2021). Sea turtles rely on sandy beaches for nesting. Successful incubation requires a relatively narrow range of sand temperature and moisture conditions, which in turn depend on various factors, including sediment characteristics, beach elevation, hydrodynamic processes, and anthropogenic disturbances (e.g., Ackerman, 1997; Foley et al., 2006; Culver et al., 2020). Consequently, the long-term survival of sea turtles directly depends on the availability of suitable nesting beaches around the world.
Among the challenges they face are the flooding and erosion of nests during the breeding season and the loss of suitable nesting habitat due to long-term beach erosion and coastal development (Figures 1A, B). These threats occur globally, but their severity varies regionally—for instance, Mediterranean and North-West Atlantic nesting beaches face widespread coastal development (Biddiscombe et al., 2020; Hirsch et al., 2022); the Gulf of Mexico is projected to experience high relative sea level rise (Fox-Kemper et al., 2021); while Caribbean and Indo-Pacific rookeries are particularly exposed to tropical cyclones (Dewald and Pike, 2014). Although both flooding and erosion are recognized as significant threats to sea turtles (e.g., Gammon et al., 2023; Rivas et al., 2023; Van Houtan and Bass, 2007; Ware et al., 2021), they remain under-represented in conservation management and research, which are primarily focused on in-situ protection, fishery by-catch, human consumption, plastic pollution, and changing hatchling sex-ratios due to warming temperatures (Hays et al., 2025; Nel et al., 2014; Patrício et al., 2021; Fuentes et al., 2023; Wallace et al., 2025).
Figure 1
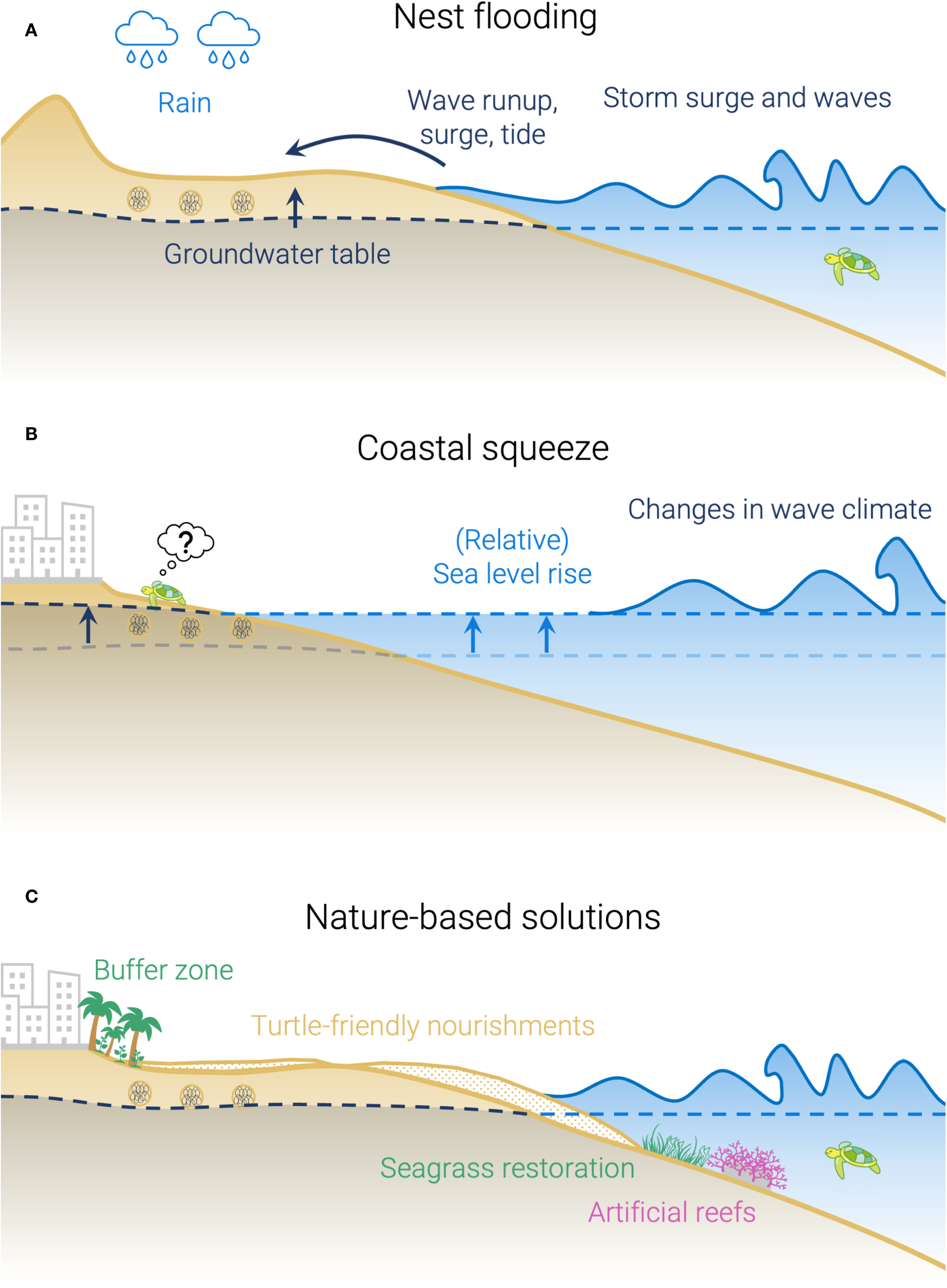
Schematic cross-shore profile of a nesting beach (not to scale), showing: (A) processes related to nest flooding and erosion; (B) processes related to coastal squeeze; and (C) examples of potential NBS that could help mitigate flooding and erosion on nesting beaches. Although a cross-shore schematic is shown here for simplicity, these processes also have alongshore drivers and variability.
The driving processes behind nest flooding and beach erosion are extensively studied and understood by coastal scientists and managers. Coastal management is increasingly shifting toward nature-based solutions (NBS; e.g., de Vriend et al., 2015; Masselink and Lazarus, 2019; Spalding et al., 2014), defined by the International Union for Conservation of Nature (IUCN) as actions to protect, sustainably manage, and restore natural or modified ecosystems, that address societal challenges effectively and adaptively, simultaneously providing human well-being and biodiversity benefits (Cohen-Shacham et al., 2016). Examples of NBS for nesting beaches could include turtle-friendly beach nourishments (e.g., Smithers and Dawson, 2023), artificial reefs, and seagrass restoration aimed at reducing erosion and flooding (Figure 1C; Barbier et al., 2011). However, effectively implementing such solutions requires a comprehensive understanding of the entire beach ecosystem, including biotic (e.g., nesting characteristics) and abiotic (e.g., longshore/cross-shore processes) factors at play (Slinger and Vreugdenhil, 2020). Despite the clear need for an interdisciplinary approach (Nel et al., 2014), collaboration between the sea turtle ecology and coastal engineering communities remains limited and, as a result, nesting habitats are not commonly considered in coastal engineering projects.
In this perspective, we argue that existing methods and tools used in coastal science and management hold significant potential for sea turtle conservation and should be leveraged more effectively. To guide this integration, we propose a stepwise framework (Figure 2) that connects ecological thresholds, coastal threats, and management interventions from regional to local scales. We outline how state-of-the-art coastal monitoring and modeling approaches can enable us to observe, understand, and predict coastal processes at nesting beaches, which is required to decide if and how to intervene with NBS. Finally, we call for increased interdisciplinary collaboration that bridges ecology, biology, and coastal management to enhance the future resilience of these critical habitats.
Figure 2
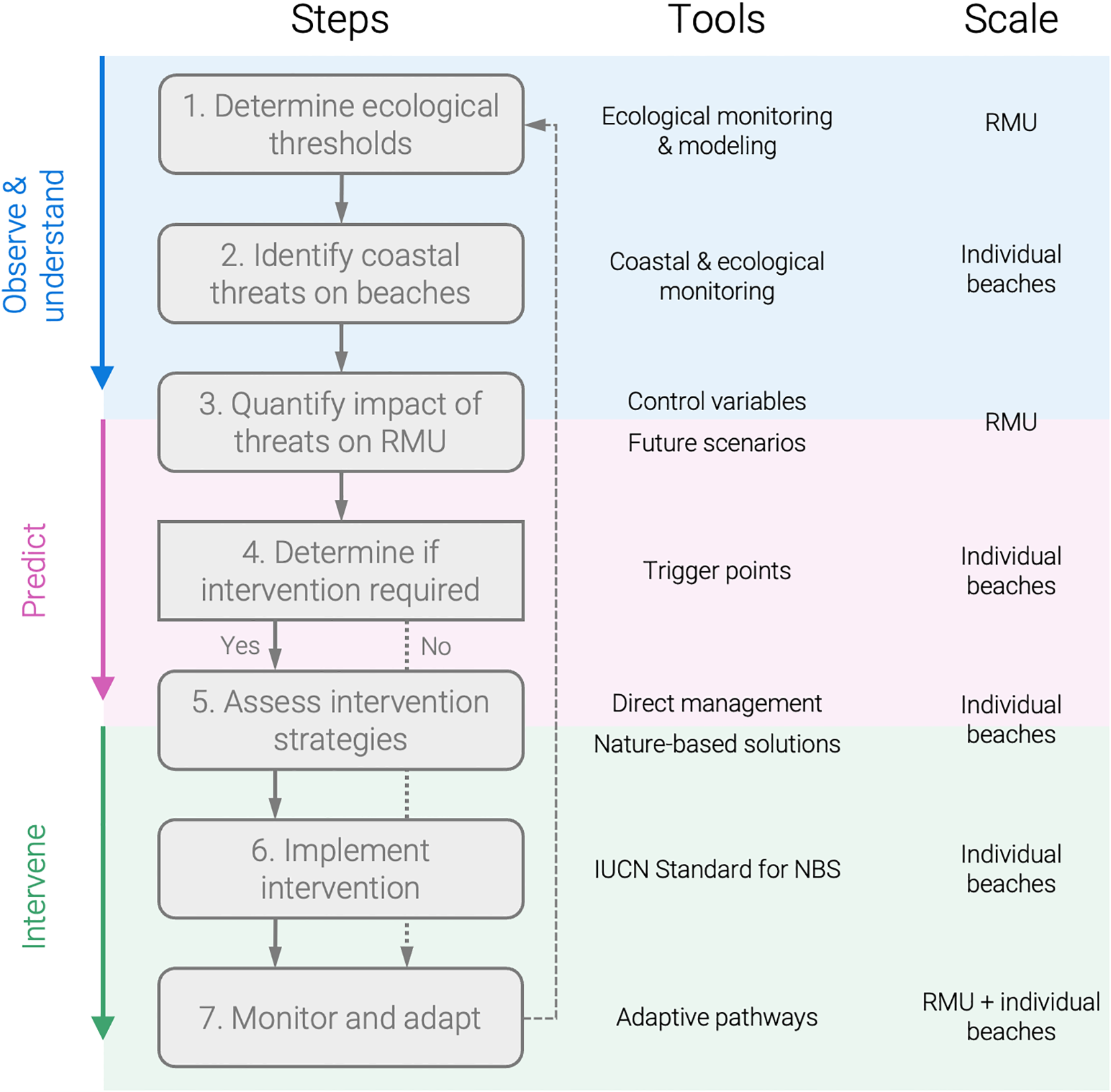
A stepwise framework for integrating sea turtle ecology and coastal management to tackle coastal threats to nesting beaches. The framework progresses through seven steps, following the Observe-Understand-Predict-Intervene phases (the first two are combined here). Each step is associated with specific tools and spatial scales, linking regional assessments (RMU) with local management of nesting beaches. Step 4 represents a key decision point on whether to intervene, while feedback loops emphasize the adaptive and iterative nature of the framework.
2 Nesting beaches under pressure
Nesting beaches are shaped by diverse coastal processes that operate across multiple temporal and spatial scales. An imminent threat is the inundation and erosion of nesting beaches during high wave and water level events (e.g., storms or tropical cyclones), which are increasing with climate change (Morim et al., 2025; Reguero et al., 2019). Such episodic events can flood or wash out incubating nests, significantly decreasing hatching success (Patrício et al., 2021; Van Houtan and Bass, 2007). Though flooding mainly occurs overland—through wave runup, storm surges, high tides, and rainfall—it effectively raises the beach groundwater table (GWT) in the nesting zone (Figure 1A). Since egg viability is sensitive to inundation duration (Limpus et al., 2021; Pike et al., 2015), the GWT response strongly influences the fate of individual nests (Christiaanse et al., 2025a; McGehee, 1990; Patino-Martinez et al., 2014). These flooding events are modulated by seasonal and interannual variability in storm activity (e.g., storm seasonality, El Niño/Southern Oscillation). Moreover, storm erosion can significantly alter beach morphology, which can impact nesting on a time-scale of multiple seasons (Long et al., 2011), especially after intense storms like tropical cyclones (Fuentes et al., 2019; Pike and Stiner, 2007).
Another significant threat is the long-term loss of nesting habitat due coastal squeeze, which arises from the combination of structural erosion and increasing pressure from the landward side (Figure 1B). Structural erosion can have several causes, including sea level rise (SLR), coastal subsidence, longshore sediment transport disruption, river damming, or climate-induced shifts in sediment supply. At the same time, urban development and population growth increasingly encroach from the landward side (Neumann et al., 2015). Coastal squeeze occurs when the eroding coastline cannot migrate landward due to a fixed barrier, either human-made or natural (e.g., cliff; Pontee, 2013). Even where migration space exists, it remains unclear whether sediment supply and beach morphology can keep up with accelerating SLR (Rosati et al., 2013; Vitousek et al., 2017). Erosion can also expose underlying bedrock, which may hinder nesting access or increase injury risk to turtles (Wildermann et al., 2024).
3 When to intervene through NBS?
The first criterion of the IUCN standard for NBS is that the NBS effectively addresses a societal challenge, which is clearly understood (IUCN, 2020). This aligns with the common approach toward NBS or Building with Nature (de Vriend et al., 2015) in coastal management, which we summarize here in three phases: (1) Observe and understand, (2) Predict, and (3) Intervene, if necessary (Figure 2). We first need to observe and understand the ecosystem, predict how the relevant natural processes might evolve, and then assess whether intervention is needed.
The goal is achieving and maintaining stable sea turtle habitats and populations rather than saving every turtle or nesting beach. Therefore, it becomes essential to identify ecological thresholds, like trigger points (when intervention becomes necessary) and tipping points (beyond which recovery is unlikely) in the sea turtle population (Figure 2, Step 1; de Bie et al., 2018; Lindenmayer et al., 2013; Botero et al., 2015). The widely used Regional Management Units (RMUs) framework in sea turtle conservation (Wallace et al., 2023) provides a practical scale for assessing such thresholds, which are likely to vary among regions and species. Thresholds should be quantified through long-term monitoring and modeling (e.g., population viability analyses) to provide concrete decision points for managers. For example, maintaining 70% hatching success is widely accepted as a minimum threshold for population stability (Mortimer, 1999)—crossing below this value would indicate a trigger point for intervention (Figure 2, Step 4), while sustained declines well below it may constitute a tipping point for population collapse. Determining robust species- and region-specific thresholds will require increased interdisciplinary research and closer integration of ecological and coastal management perspectives (Hilton et al., 2023; Slinger and Vreugdenhil, 2020).
When ecological thresholds have been determined, the impact of coastal threats on sea turtle populations should be assessed accordingly (Figure 2, Steps 2 and 3). For example, if a certain percentage of nests are likely to be flooded each year, will that push the hatching success beyond a trigger/tipping point? This can be done by defining and monitoring a set of control variables for each threat. A multitude of existing coastal tools can be leveraged to observe, understand, and predict these control variables at nesting beaches (Sections 4 and 5). Below we propose potential control variables for flooding and erosion of nesting beaches based on recent work, though these should be evaluated and refined through further interdisciplinary research.
For nest flooding, control variables should include the beach GWT (controls inundation duration) and a variable related to inundation frequency (e.g., water level exceedance). Important drivers of these control variables are the elevation of the nesting area, beach slope, sediment properties (e.g., grain size, permeability), and wave and water level climates. For example, in low-elevation, mild sloping coastal settings, the beach GWT drains slower, meaning longer inundation events negatively affect egg viability (e.g., on Galveston Island, USA and Raine Island, Australia; Christiaanse et al., 2025a; Guard et al., 2008). On steeper, coarser grained beaches, the faster drainage means egg viability may be more influenced by inundation frequency (e.g., from wave runup; Pike et al., 2015; Limpus et al., 2021; Caut et al., 2010).
Regarding beach loss, the shoreline position is a widely used indicator of coastal change (Splinter and Coco, 2021; Vitousek et al., 2023a), and may serve as a control variable to monitor nesting beach erosion (Christiaanse et al., 2025c). The advantage of using the shoreline position is that it can be monitored relatively easily at scale through remote sensing tools (e.g., CoastSat; Vos et al., 2019b). More sophisticated control variables may be required at more complex coastlines or for detailed assessments of individual nesting beaches (e.g., a beach resilience index as in Dong et al., 2018), though this requires more data and resources. Next to the shoreline position, the available migration space behind the beach is an important control variable, as it controls whether shoreline retreat will translate to beach loss (Christiaanse et al., 2025c). Migration space is determined by the geological properties of the backbeach and potential human infrastructure in the vicinity.
4 Monitoring and data
Monitoring nearshore hydrodynamics and beach groundwater can help understand the processes behind the repeated flooding of turtle nests on the beach (Christiaanse et al., 2025a; Foley et al., 2006; Ware and Fuentes, 2018). Previous studies on nest flooding have mainly focused on monitoring the high tide line and wave runup to identify flooded or exposed nests and compare their emergence success to non-flooded ones (e.g., Caut et al., 2010; Ware et al., 2019, Ware et al., 2021). Such approaches may provide similar accuracy in identifying at-risk nests as more time- and cost-intensive monitoring of the beach GWT (Ware and Fuentes, 2018). However, they are less useful for understanding and predicting the driving processes underlying nest flooding (Christiaanse et al., 2025a; Guard et al., 2008). Monitoring sediment characteristics (e.g., grain size) is also important, as they influence nesting suitability as well as beach morphology, slope, permeability, etc (Bujan et al., 2019; Mortimer, 1990; Yamamoto et al., 2012; Botterell et al., 2025). Hence, we argue that more in-situ data collections (e.g., Christiaanse et al., 2025b; Culver et al., 2020; Foley et al., 2006) will be required to understand the processes underlying coastal hazards to nesting beaches and design NBS that enable nesting.
Many nesting beaches lie in remote areas with low data availability. Moreover, most are located in the tropics and in developing countries (Mazaris et al., 2014), where access and capacity for in-situ data collection may be difficult. Nonetheless, the sea turtle community has succeeded to collect (long-term) nesting data at many (remote) beaches (e.g., Balazs and Chaloupka, 2004; Lasala et al., 2023; Restrepo et al., 2023; Willson et al., 2020). Most of these monitoring programs are ongoing and collect new data each nesting season. Including simple, cost-effective coastal monitoring in these campaigns could help fill some of the existing data gaps, without significantly increasing the workload. Key parameters would be beach elevation (e.g., weekly cross-shore GPS profiles or low-cost alternatives as in Andrade and Ferreira, 2006) and grain size. An emerging and promising way of collecting more coastal data worldwide are citizen science-based programs—e.g., CoastSnap for shoreline positions (Harley and Kinsela, 2022) or SandSnap for grain size estimation (McFall et al., 2024)—which could work particularly well on nesting beaches that are near local communities, have ongoing nest monitoring programs, and/or attract tourism.
In-situ data collection is often limited by cost and time constraints, making it difficult to scale up to large temporal and spatial scales. However, in recent years, remote sensing tools have transformed coastal monitoring by enabling the extraction of key geomorphic and environmental variables from satellite imagery. For example, satellite-derived shorelines allow for the quantification of beach width, slope, and erosion/accretion trends (e.g., Luijendijk et al., 2018; Vos et al., 2019a), which directly affect the availability of nesting habitat. While not well-established in sea turtle conservation yet, satellite imagery has previously been used to track nesting activity and distribution (Casale and Ceriani, 2019), quantify night lighting at nesting sites (Mazor et al., 2013), characterize developmental habitats (Hardy et al., 2018), and assess long-term morphological changes of nesting beaches (Maneja et al., 2021; Christiaanse et al., 2025c). We argue that the use of such tools in sea turtle conservation should be expanded, specifically to assess how nesting beaches around the world will respond to SLR in the coming decades (e.g., Christiaanse et al., 2025c). Moreover, the opportunities opening up through remote sensing are increasing at a fast pace, with new data now being collected on a near-daily scale, everywhere in the world, and may offer a much broader slate of data in the future (e.g., wave and water level climate, bathymetry, sediment characteristics; Bergsma et al., 2021; Vitousek et al., 2023a; Turner et al., 2021).
When there is no in-situ data and remote sensing tools cannot provide the required data or resolution (yet), global hindcast or reanalysis datasets offer an alternative for long-term time-series data of atmospheric and oceanic variables—e.g., ERA5 for waves, sea surface temperature, and many other variables (Hersbach et al., 2018), GTSM for water levels (Muis et al., 2020), and DeltaDTM for coastal topography (Pronk et al., 2024). Though such datasets have limited resolution and accuracy, they are often good enough for long-term statistics and exploratory modeling studies. Next to historical data, many global models and datasets offer future climate projections (e.g., the AR6 SLR projections; Garner et al., 2021). These can inform predictive models or be used to assess habitat suitability under various scenarios. For example, Christiaanse et al. (2024) combined global datasets with machine learning to identify patterns in the coastal characteristics of nesting regions and map new, potentially suitable nesting regions. Moreover, various types of coastal vulnerability indices have been used to provide a useful first estimate of the exposure of nesting beaches to coastal threats (de Vos et al., 2019; Gammon et al., 2023; Santana Garcon et al., 2010; Von Holle et al., 2019). These are often based on global or regional datasets making them effective at large spatial scales. Hence, they can be leveraged to identify high-risk beaches, to prioritize for in-situ data collections.
Ultimately, hydromorphological monitoring should be complemented by ecological monitoring. Most existing nest monitoring programs focus on nest counts, hatching success, and nesting turtle characteristics (e.g., Margaritoulis, 2005; Balazs and Chaloupka, 2004; Restrepo et al., 2023). While these data are invaluable, measuring nest positions (GPS, ideally including elevation) and nest depth could significantly improve assessments of erosion and flooding impacts on nesting populations. While some datasets include nest coordinates (e.g., Ware et al., 2021; Culver et al., 2020), elevation is not commonly recorded. We realize this may not always be feasible, especially at high density nesting beaches. In such cases, (horizontal) coordinates of a subset of nests would already help, particularly in combination with digital elevation models of the beach.
5 Modeling
Collected data can be used to force, calibrate, and train models to predict the potential impact of coastal threats and NBS on nesting beaches. There are countless models available, for different purposes and scopes, generally categorized into physics/process-based models, statistical/data-driven models, and hybrid models, combining both approaches. Physics/process-based models numerically solve physical equations (often combined with empirical formulations) to simulate natural processes like sediment transport or wave propagation. They range from 1D alongshore (e.g., ShorelineS; Roelvink et al., 2020) or cross-shore models (e.g., Unibest-TC; Walstra et al., 2012) over reduced-complexity shoreline models (e.g., COCOONED, CoSMoS-COAST; Antolínez et al., 2019; Vitousek et al., 2023b) to complex 2D/3D area models (e.g., Delft3D, XBeach; Lesser et al., 2004; Roelvink et al., 2009). Hybrid models use statistical tools to interpolate results from databases created from process-based models, significantly reducing computational cost (e.g., Antolínez et al., 2018; McCall et al., 2024). Finally, fully data-driven models use statistical and machine learning algorithms (e.g., neural networks) to learn from and extrapolate existing data (e.g., Gomez-de la Peña et al., 2023; Simmons and Splinter, 2025). Which model(s) to use depends on the objective (control variables), the location-specific boundary conditions, and the desired spatial/temporal scale and resolution.
To predict nest flooding, the driving hydrodynamic processes (waves and water levels) could be simulated with a 1/2-dimensional XBeach model. If the GWT is important (e.g., on mild-sloping, fine-grained beaches; Christiaanse et al., 2025a), XBeach has a groundwater module. However, this module was created for gravel beaches, and preliminary results on a mild-sloping nesting beach were poor (Galveston Island, TX; Taal, 2024). Coupling a tailored groundwater model (e.g., PFLOTRAN; Hammond et al., 2014) to the hydrodynamics from XBeach may provide better results. When data-availability is low, hybrid or surrogate models can be useful—for example, in many coral-lined coasts and islands, where models like HyCReWW (Rueda et al., 2019) or BEWARE-2 (McCall et al., 2024; Scott et al., 2020) can provide first estimates of nest flooding from wave runup (Dédina et al., 2025).
Predicting longer-term processes, like SLR and erosion vulnerability on nesting beaches is more difficult as the larger time-scales inherently introduce more uncertainty (Vitousek et al., 2017; Vitousek et al., 2024). Many studies have tried to quantify the loss of nesting area under various SLR scenarios, however, most use the simple bathtub approach—combining digital elevation models with SLR scenarios to derive inundation maps (e.g., Beber et al., 2024; Fish et al., 2005; Fuentes et al., 2010; Katselidis et al., 2014; Patrício et al., 2019; Rivas et al., 2023; Varela et al., 2019; Veelenturf et al., 2020). While time-efficient, this approach does not consider any morphological response of the system and therefore cannot provide actionable estimates of beach loss (Wolinsky and Murray, 2009; Christiaanse et al., 2025c). Others have applied the Bruun rule (Bruun, 1962) to estimate shoreline retreat on nesting beaches (e.g., Fish et al., 2008; Mazaris et al., 2009; Reece et al., 2013), though its validity is still debated (Cooper et al., 2020; Ranasinghe, 2020; Wolinsky and Murray, 2009). The relatively small amount of SLR over the past decades (our only dataset) means that the beach response is difficult to distinguish from more dominant modes of change, like seasonal and inter-annual signals (Vitousek et al., 2017). Hence, beach response to SLR remains a subject of ongoing debate and corresponding model predictions come with high uncertainty. Nonetheless, reduced complexity models combined with satellite-derived shorelines at least allow us to explore potential future shoreline evolution, even in remote, data-scarce environments (Christiaanse et al., 2025c; Vitousek et al., 2024).
Ultimately, no model is perfect and any prediction comes with uncertainty. There is already intrinsic uncertainty in using future projections to force these models (e.g., SLR rates or wave climate variability; Ruggiero et al., 2010; Le Cozannet et al., 2019; Vitousek et al., 2021). Model choice is often a balancing act between achieving reasonable accuracy and minimizing uncertainty and computational cost. Simpler models are faster to run, but introduce uncertainty by missing processes and detail (Kroon et al., 2019). As more natural processes are included, the model becomes more computationally intensive, requires more input data, and uncertainty is introduced through free model parameters (Kroon et al., 2025). For robust decision-making, it is crucial to quantify these uncertainties in the predictions.
6 Toward nature-based solutions that enable nesting
Once we have observed the system, understand the natural processes at play, and identified a need to intervene (Figure 2, Step 4), we can think of solutions (Step 5). In some cases, direct conservation or management measures may suffice. For instance, nests can be relocated to mitigate inundation risk (though risky and costly; Pintus et al., 2009) and establishing Marine Protected Areas (or other area-based protection instruments) can minimize some external influences on the ecosystem (Spalding et al., 2014). Beyond direct management measures, coastal NBS offer promising ways to improve the beach system for sea turtle nesting, by altering the driving processes behind the threats (Figure 3; Ostertag, 2025).
Figure 3
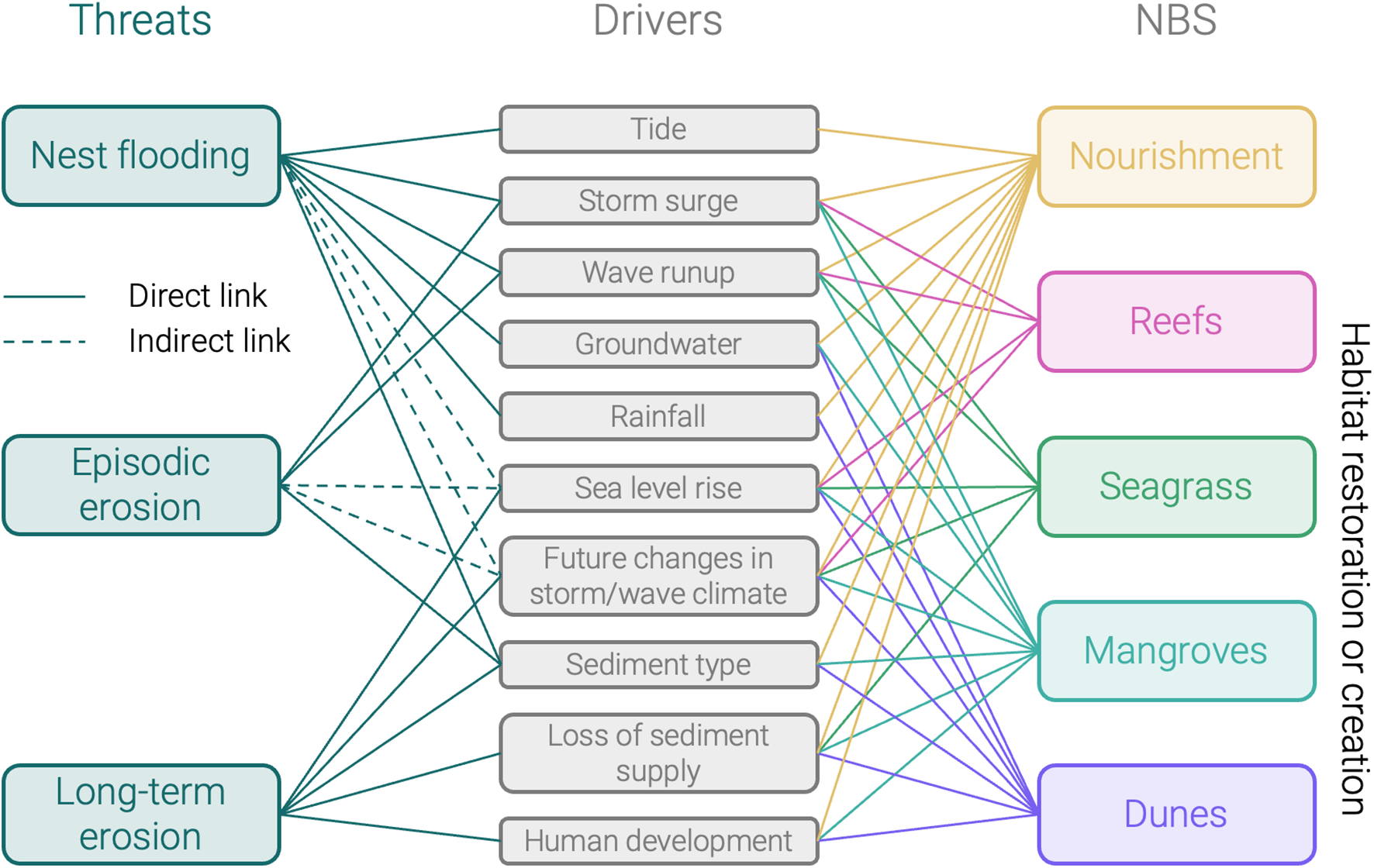
Threat-Driver-Solution matrix (non-exhaustive) showing coastal threats to sea turtle nesting beaches on the left, their driving processes in the middle, and potential NBS building blocks that could mitigate some of these threats. The lines show links between the three: NBS solutions affect one or more of the driving processes, through which they can mitigate threats.
Likely the most widely applied NBS for beaches is the use of nourishments to replenish/re-profile the beach with sand or even create new beaches (de Schipper et al., 2020). At nesting beaches, nourishments can elevate the nesting zone to protect it from flooding, or restore nesting area lost to erosion (Figure 1C; Limpus et al., 2021; Montague, 1993). The first beach re-profiling designed entirely for sea turtle nesting was implemented recently at Raine Island, Australia, which hosts the largest green turtle rookery worldwide (Smithers and Dawson, 2023). Early results indicate that it has successfully boosted hatchling production. However, several studies have also reported negative effects of nourishments on turtle nesting, especially shortly after implementation and when specific characteristics like grain size, beach slope, or compaction changed (e.g., Brock et al., 2009; Grain et al., 1995; Rumbold et al., 2001; Steinitz et al., 1998). Fortunately, nourishment designs increasingly include societal and ecosystem services (de Schipper et al., 2020). As a NBS for sea turtle nesting beaches, nourishments must be carefully designed to effectively enhance nesting, ideally over multiple years. Further research should therefore focus on specific design requirements for nourishments that generate positive outcomes for sea turtles.
Additional NBS building blocks may include the restoration or creation of ecosystem services that mitigate flooding and erosion (Figure 3; Spalding et al., 2014). For example, (coral) reefs are known to dissipate wave and surge energy and can therefore reduce flooding of the nesting area (Lowe et al., 2005; Borsje et al., 2011). Similarly, seagrass beds can attenuate waves and currents, provide erosion control by stabilizing the foreshore, and stimulate accretion through sediment trapping (Gacia et al., 1999; Bradley and Houser, 2009; James et al., 2019). Moreover, they function as foraging habitats for green turtles (Christianen et al., 2023) and provide an added climate benefit through carbon sequestration (Duarte et al., 2013). Mangroves also have similar benefits of reducing flooding and erosion (Gedan et al., 2011; Thampanya et al., 2006). Although their presence near the shoreline may reduce beach access for turtles, eastern-pacific hawksbill turtles are known to nest in mangrove estuaries (Gaos et al., 2016; Mast et al., 2025). Dunes may also offer benefits for sea turtle nesting, by providing sediment reserves, storage capacity for groundwater, and a buffer from human development (Barbier et al., 2011; Spalding et al., 2014). These ecosystem services can be leveraged through NBS by either restoring degraded habitats or creating new ones (e.g., through artificial reefs; Duarte et al., 2020; Reguero et al., 2018).
What type of NBS to implement (Figure 2, Step 6) depends on the environmental and societal challenges and the existing ecosystem (e.g., developed vs. undeveloped coastline), and design should follow a standardized design framework (e.g., the IUCN Global Standard for NBS; IUCN, 2020). Specific NBS that enable sea turtle nesting have not yet been tested at scale, thus to better understand their effect on sea turtle nesting and the ecosystem as a whole, they need to be implemented and evaluated in the field. The above listed ecosystem services (non-exhaustive) may function as building blocks to design tailored solutions, depending on the identified threats and their driving processes (Figure 3). These building blocks can be combined, which may generate positive synergies. For instance, coral or shellfish reefs can create favorable conditions for seagrass and mangroves to develop (Barbier et al., 2011; Smith et al., 2009). There can also be drawbacks to these solutions—for example, while reefs, seagrass, and mangroves can locally trap sediment and reduce erosion, this can potentially disrupt longshore transport, causing erosion downstream. Ultimately, the effectiveness of any NBS is dependent on a healthy and stable ecosystem (Spalding et al., 2014). Potential side-effects and maintenance costs should, therefore, also be taken into account: Are there detrimental effects on other ecosystem services? How long will the NBS last without intervening again (e.g., re-nourishment period)? In that regard, NBS should be managed adaptively and based on evidence (criterion 7 of the IUCN Standard; IUCN, 2020)—i.e., the ecosystem should be continuously monitored after implementation, to evaluate if the NBS has the desired effects and, if not, plan adjustment strategies (Figure 2, Step 7).
7 Conclusion & outlook
In this perspective, we argue that a stronger connection between coastal management and sea turtle conservation is needed to advance the research, design, and implementation of NBS that enable sea turtle nesting on sandy beaches. We support this perspective through a stepwise framework (Figure 2), which provides a conceptual roadmap for linking ecological thresholds, coastal processes, and management interventions across scales, from RMUs to individual beaches. We highlight how monitoring and modeling tools can be used to observe and understand coastal threats to nesting beaches, predict their impacts on sea turtle populations, and, where appropriate, intervene through adaptive NBS.
Each element of the framework points to concrete priorities for research and practice. Defining robust ecological thresholds and control variables requires increased collaboration between coastal scientists, managers, and sea turtle eco-/biologists. Improved in-situ monitoring and integration of cost-effective coastal measurements into ongoing nest monitoring programs will be essential for understanding flooding and erosion dynamics at nesting beaches. Combined with recent advances in remote sensing and machine learning, these data can feed into a growing suite of coastal models to simulate nesting beach evolution under various climate and management scenarios. Finally, interventions should be tested and implemented adaptively, guided by the IUCN Global Standard for NBS (IUCN, 2020) and evaluated through continuous monitoring and refinement.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JC: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. AR: Supervision, Writing – review & editing. SA: Supervision, Writing – review & editing. EO: Investigation, Writing – review & editing. RN: Writing – review & editing. CD: Writing – review & editing. JA: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. JC and JA were partly funded by TKI Deltatechnologie project TURTLE (TU11), with support from Boskalis, Deltares, Texas A&M University, KAUST, University of Exeter, and Universidad de Costa Rica. Boskalis was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We would like to thank Prof. dr. Brendan Godley from the University of Exeter for the many inspiring conversations and Dr. Vanesa Chalastani for early discussions leading up to this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ackerman R. A. (1997). “ The nest environment and the embryonic development of sea turtles,” in The biology of sea turtles, Eds. LutzP. L.MusickJ. A. (Boca Raton in Florida, USA: CRC Press), 1, 83–10.
2
Andrade F. Ferreira M. A. (2006). A simple method of measuring beach profiles. J. Coast. Res.22, 995–999. doi: 10.2112/04-0387.1
3
Antolínez J. A. A. Méndez F. J. Anderson D. Ruggiero P. Kaminsky G. M. (2019). Predicting climate-driven coastlines with a simple and efficient multiscale model. J. Geophysical Research: Earth Surface124, 1596–1624. doi: 10.1029/2018JF004790
4
Antolínez J. A. A. Murray A. B. Méndez F. J. Moore L. J. Farley G. Wood J. (2018). Downscaling changing coastlines in a changing climate: the hybrid approach. J. Geophysical Research: Earth Surface123, 229–251. doi: 10.1002/2017JF004367
5
Balazs G. H. Chaloupka M. (2004). Thirty-year recovery trend in the once depleted Hawaiian green sea turtle stock. Biol. Conserv.117, 491–498. doi: 10.1016/j.biocon.2003.08.008
6
Barbier E. B. Hacker S. D. Kennedy C. Koch E. W. Stier A. C. Silliman B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr.81, 169–193. doi: 10.1890/10-1510.1
7
Beber I. Sellés-Ríos B. Whitworth A. (2024). Future sea-level rise impacts to Olive Ridley (Lepidochelys olivacea) and Green Sea Turtle (Chelonia mydas) nesting habitat on the Osa Peninsula, Costa Rica. Climate Change Ecol.7, 100085. doi: 10.1016/j.ecochg.2024.100085
8
Bergsma E. W. Almar R. Rolland A. Binet R. Brodie K. L. Bak A. S. (2021). Coastal morphology from space: A showcase of monitoring the topography-bathymetry continuum. Remote Sens. Environ.261, 112469. doi: 10.1016/j.rse.2021.112469
9
Biddiscombe S. J. Smith E. A. Hawkes L. A. (2020). A global analysis of anthropogenic development of marine turtle nesting beaches. Remote Sens.12, 1492. doi: 10.3390/rs12091492
10
Borsje B. W. van Wesenbeeck B. K. Dekker F. Paalvast P. Bouma T. J. van Katwijk M. M. et al . (2011). How ecological engineering can serve in coastal protection. Ecol. Eng.37, 113–122. doi: 10.1016/j.ecoleng.2010.11.027
11
Botero C. A. Weissing F. J. Wright J. Rubenstein D. R. (2015). Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl. Acad. Sci.112, 184–189. doi: 10.1073/pnas.1408589111
12
Botterell Z. L. Ardren J. Dove E. McArthur E. Addison D. S. Adegbile O. M. et al . (2025). A global assessment of microplastic abundance and characteristics on marine turtle nesting beaches. Mar. pollut. Bull.215, 117768. doi: 10.1016/j.marpolbul.2025.117768
13
Bradley K. Houser C. (2009). Relative velocity of seagrass blades: Implications for wave attenuation in low-energy environments. J. Geophysical Research: Earth Surface114 (F01004). doi: 10.1029/2007JF000951
14
Brock K. A. Reece J. S. Ehrhart L. M. (2009). The effects of artificial beach nourishment on marine turtles: differences between loggerhead and green turtles. Restor. Ecol.17, 297–307. doi: 10.1111/j.1526-100X.2007.00337.x
15
Bruun P. (1962). Sea-level rise as a cause of shore erosion. J. Waterways Harbors Division88, 117–130. doi: 10.1061/JWHEAU.0000252
16
Bujan N. Cox R. Masselink G. (2019). From fine sand to boulders: Examining the relationship between beach-face slope and sediment size. Mar. Geology417, 106012. doi: 10.1016/j.margeo.2019
17
Casale P. Ceriani S. A. (2019). Satellite surveys: a novel approach for assessing sea turtle nesting activity and distribution. Mar. Biol.166, 47. doi: 10.1007/s00227-019-3494-4
18
Caut S. Guirlet E. Girondot M. (2010). Effect of tidal overwash on the embryonic development of leatherback turtles in French Guiana. Mar. Environ. Res.69, 254–261. doi: 10.1016/j.marenvres.2009.11.004
19
Christiaanse J. C. Antolínez J. A. A. Luijendijk A. P. Athanasiou P. Duarte C. M. Aarninkhof S. (2024). Distribution of global sea turtle nesting explained from regional-scale coastal characteristics. Sci. Rep.14, 752. doi: 10.1038/s41598-023-50239-5
20
Christiaanse J. C. Antolínez J. A. Marshall C. D. Figlus J. Dellapenna T. M. Reniers A. J. (2025a). Beach groundwater response to ocean processes and rain on a mild-sloping barrier island: Implications for sea turtle nest flooding. Coast. Eng.201, 104795. doi: 10.1016/j.coastaleng.2025.104795
21
Christiaanse J. C. Antolínez J. A. A. van der Grinten M. J. Taal F. Figlus J. Dellapenna T. M. et al . (2025b). Measurements of groundwater, hydrodynamics, and sand characteristics at a dissipative sea turtle nesting beach. Sci. Data12, 123. doi: 10.1038/s41597-025-04455-5
22
Christiaanse J. C. Vitousek S. Reniers A. J. H. M. Antolínez J. A. A. (2025c). Vulnerability of key sea turtle nesting beaches to future erosion and sea level rise (in review).
23
Christianen M. J. A. Smulders F. O. H. Vonk J. A. Becking L. E. Bouma T. J. Engel S. M. et al . (2023). Seagrass ecosystem multifunctionality under the rise of a flagship marine megaherbivore. Global Change Biol.29, 215–230. doi: 10.1111/gcb.16464
24
Cohen-Shacham E. Walters G. Janzen C. Maginnis S. (2016). Nature-based solutions to address global societal challenges (Gland: Switzerland: IUCN). doi: 10.2305/IUCN.CH.2016.13.en
25
Cooper J. A. G. Masselink G. Coco G. Short A. D. Castelle B. Rogers K. et al . (2020). Sandy beaches can survive sea-level rise. Nat. Climate Change10, 993–995. doi: 10.1038/s41558-020-00934-2
26
Culver M. Gibeaut J. C. Shaver D. J. Tissot P. Starek M. (2020). Using lidar data to assess the relationship between beach geomorphology and kemp’s ridley (Lepidochelys kempii) nest site selection along padre island, TX, United States. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00214
27
de Bie K. Addison P. F. E. Cook C. N. (2018). Integrating decision triggers into conservation management practice. J. Appl. Ecol.55, 494–502. doi: 10.1111/1365-2664.13042
28
Dédina D. Christiaanse J. C. Roelvink F. Elshinnawy A. I. A. Mccall R. Reniers A. et al . (2025). “ Runup modeling in low-data coral reef environments: implications for nesting sea turtles,” in Proceedings of the 10th International Coastal Dynamics Conference.
29
de Schipper M. A. Ludka B. C. Raubenheimer B. Luijendijk A. P. Schlacher T. A. (2020). Beach nourishment has complex implications for the future of sandy shores. Nat. Rev. Earth Environ.2, 70–84. doi: 10.1038/s43017-020-00109-9
30
de Vos D. Nel R. Schoeman D. Harris L. R. du Preez D. (2019). Effect of introduced Casuarina trees on the vulnerability of sea turtle nesting beaches to erosion. Estuarine Coast. Shelf Sci.223, 147–158. doi: 10.1016/j.ecss.2019.03.015
31
de Vriend H. J. van Koningsveld M. Aarninkhof S. G. de Vries M. B. Baptist M. J. (2015). Sustainable hydraulic engineering through building with nature. J. Hydro-Environment Res.9, 159–171. doi: 10.1016/j.jher.2014.06.004
32
Dewald J. R. Pike D. A. (2014). Geographical variation in hurricane impacts among sea turtle populations. J. Biogeography41, 307–316. doi: 10.1111/jbi.12197
33
Dong Z. Elko N. Robertson Q. Rosati J. (2018). Quantifying beach and dune resilience using the coastal resilience index. Coast. Eng. Proc.1, 30. doi: 10.9753/icce.v36.papers.30
34
Duarte C. M. Agusti S. Barbier E. Britten G. L. Castilla J. C. Gattuso J.-P. et al . (2020). Rebuilding marine life. Nature580, 39–51. doi: 10.1038/s41586-020-2146-7
35
Duarte C. M. Losada I. J. Hendriks I. E. Mazarrasa I. Marba,` N. (2013). The role of coastal plant communities for climate change mitigation and adaptation. Nat. Climate Change3, 961–968. doi: 10.1038/nclimate1970
36
Fish M. R. Côte I. M. Gill J. A. Jones A. P. Renshoff S. Watkinson A. R. (2005). Predicting the impact of sea-level rise on caribbean sea turtle nesting habitat. Conserv. Biol.19, 482–491. doi: 10.1111/j.1523-1739.2005.00146.x
37
Fish M. R. Côté I. M. Horrocks J. A. Mulligan B. Watkinson A. R. Jones A. P. (2008). Construction setback regulations and sea-level rise: Mitigating sea turtle nesting beach loss. Ocean Coast. Manage.51, 330–341. doi: 10.1016/j.ocecoaman.2007.09.002
38
Foley A. M. Peck S. A. Harman G. R. (2006). Effects of sand characteristics and inundation on the hatching success of loggerhead sea turtle (Caretta caretta) clutches on low-relief mangrove islands in southwest florida. Chelonian Res. Foundation Turtle Conservancy5, 32–41. doi: 10.2744/1071-8443(2006)5[32:EOSCAI]2.0.CO;2
39
Fox-Kemper B. Hewitt H. T. Xiao C. Aalgeirsdóttir G. Drijfhout S. S. Edwards T. L. et al . (2021). “ Ocean, cryosphere and sea level change,” in Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change, vol. 9 . Eds. Masson-DelmotteV.ZhaiP.PiraniA.ConnorsS. L.PéanC.BergerS.CaudN.ChenY.GoldfarbL.GomisM. I.HuangM.LeitzellK.LonnoyE.MatthewsJ. B. R.MaycockT. K.WaterfieldT.YelekcO.YuR.ZhouB. (Cambridge, UK and New York, NY, USA: Cambridge University Press), 1211–1362. doi: 10.1017/9781009157896.011
40
Fuentes M. M. P. B. Godfrey M. H. Shaver D. Ceriani S. Gredzens C. Boettcher R. et al . (2019). Exposure of marine turtle nesting grounds to named storms along the continental USA. Remote Sens.11, 2996. doi: 10.3390/rs11242996
41
Fuentes M. M. P. B. Limpus C. J. Hamann M. Dawson J. (2010). Potential impacts of projected sea-level rise on sea turtle rookeries. Aquat. Conservation: Mar. Freshw. Ecosyst.20, 132–139. doi: 10.1002/aqc.1088
42
Fuentes M. McMichael E. Kot C. Silver-Gorges I. Wallace B. Godley B. et al . (2023). Key issues in assessing threats to sea turtles: knowledge gaps and future directions. Endangered Species Res.52, 303–341. doi: 10.3354/esr01278
43
Gacia E. Granata T. C. Duarte C. M. (1999). An approach to measurement of particle flux and sediment retention within seagrass (Posidonia oceanica) meadows. Aquat. Bot.65, 255–268. doi: 10.1016/S0304-3770(99)00044-3
44
Gammon M. Whiting S. Fossette S. (2023). Vulnerability of sea turtle nesting sites to erosion and inundation: A decision support framework to maximize conservation. Ecosphere14, e4529. doi: 10.1002/ecs2.4529
45
Gaos A. R. Lewison R. L. Liles M. J. Gadea V. Altamirano E. Henríquez A. V. et al . (2016). Hawksbill turtle terra incognita: conservation genetics of eastern Pacific rookeries. Ecol. Evol.6, 1251–1264. doi: 10.1002/ece3.1897
46
Garner G. G. Hermans T. Kopp R. E. Slangen A. B. A. Edwards T. L. Levermann A. et al . (2021). IPCC AR6 sea level projections. Version 20210809. doi: 10.5281/zenodo.5914709
47
Gedan K. B. Kirwan M. L. Wolanski E. Barbier E. B. Silliman B. R. (2011). The present and future role of coastal wetland vegetation in protecting shorelines: answering recent challenges to the paradigm. Climatic Change106, 7–29. doi: 10.1007/s10584-010-0003-7
48
Gomez-de la Peña E. Coco G. Whittaker C. Montaño J. (2023). On the use of convolutional deep learning to predict shoreline change. Earth Surface Dynamics11, 1145–1160. doi: 10.5194/esurf-11-1145-2023
49
Grain D. A. Bolten A. B. Bjorndal K. A. (1995). Effects of beach nourishment on sea turtles: review and esearch initiatives. Restor. Ecol.3, 95–104. doi: 10.1111/j.1526-100X.1995.tb00082.x
50
Guard P. McPherson K. Mohoupt J. (2008). A field investigation into the groundwater dynamics of raine island. Tech. Rep. Brisbane, Australia. doi: 10.14264/131499
51
Hammond G. E. Lichtner P. C. Mills R. T. (2014). Evaluating the performance of parallel subsurface simulators: An illustrative example with PFLOTRAN. Water Resour. Res.50, 208–228. doi: 10.1002/2012WR013483
52
Hardy R. F. Hu C. Witherington B. Lapointe B. Meylan A. Peebles E. et al . (2018). Characterizing a sea turtle developmental habitat using landsat observations of surface-pelagic drift communities in the eastern gulf of Mexico. IEEE J. Selected Topics Appl. Earth Observations Remote Sens.11, 3646–3659. doi: 10.1109/JSTARS.2018.2863194
53
Harley M. D. Kinsela M. A. (2022). CoastSnap: A global citizen science program to monitor changing coastlines. Continental Shelf Res.245, 104796. doi: 10.1016/j.csr.2022.104796
54
Hays G. C. Laloö J.-O. Seminoff J. A. (2025). Status, trends and conservation of global sea turtle populations. Nat. Rev. Biodiversity. 1, 119–133. doi: 10.1038/s44358-024-00011-y
55
Heithaus M. R. (2013). Chapter 10: predators, prey, and the ecological roles of sea turtles. Biol. Sea Turtles (Boca Raton, USA) 3, 249–273.
56
Hersbach H. Bell B. Berrisford P. Biavati G. Horányi A. Muñoz Sabater J. et al . (2018). ERA5 hourly data on single levels from 1979 to present. doi: 10.24381/cds.adbb2d47
57
Hilton M. Walsh J. C. Maloney R. F. Hansen N. A. Cook C. N. (2023). The value of capturing diverse perspectives when setting decision triggers for threatened species management. J. Appl. Ecol.60, 2267–2281. doi: 10.1111/1365-2664.14477
58
Hirsch S. E. Toonder M. Reilly J. D. Hoover S. R. Perrault J. R. (2022). Responses of three nesting sea turtle species to hard-armoring structures. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.980715
59
IUCN (2020). “ Global standard for nature-based solutions,” in A user-friendly framework for the verification, design and scaling up of NbS, 1 edn (Gland, Switzerland: IUCN). doi: 10.2305/IUCN.CH.2020.08.en
60
James R. K. Silva R. van Tussenbroek B. I. Escudero-Castillo M. Mariño-Tapia I. Dijkstra H. A. et al . (2019). Maintaining tropical beaches with seagrass and algae: A promising alternative to engineering solutions. BioScience69, 136–142. doi: 10.1093/biosci/biy154
61
Katselidis K. A. Schofield G. Stamou G. Dimopoulos P. Pantis J. D. (2014). Employing sea-level rise scenarios to strategically select sea turtle nesting habitat important for long-term management at a temperate breeding area. J. Exp. Mar. Biol. Ecol.450, 47–54. doi: 10.1016/j.jembe.2013.10.017
62
Kroon A. Christiaanse J. C. Luijendijk A. P. Schipper M. A. Ranasinghe R. (2025). Parameter uncertainty in medium-term coastal morphodynamic modeling. Sci. Rep.15, 18471. doi: 10.1038/s41598-025-02300-8
63
Kroon A. de Schipper M. van Gelder P. Aarninkhof S. (2019). “ Quantification of model uncertainty in lifetime predictions of nourishments,” in Coastal sediments 2019. Eds. WangP.RosatiJ. D.ValleeM. ( World Scientific). doi: 10.1142/9789811204487{\}0032
64
Lasala J. A. Macksey M. C. Mazzarella K. T. Main K. L. Foote J. J. Tucker A. D. (2023). Forty years of monitoring increasing sea turtle relative abundance in the Gulf of Mexico. Sci. Rep.13, 17213. doi: 10.1038/s41598-023-43651-4
65
Le Cozannet G. Bulteau T. Castelle B. Ranasinghe R. Wöppelmann G. Rohmer J. et al . (2019). Quantifying uncertainties of sandy shoreline change projections as sea level rises. Sci. Rep.9, 42. doi: 10.1038/s41598-018-37017-4
66
Lesser G. R. Roelvink J. A. van Kester J. A. Stelling G. S. (2004). Development and validation of a three-dimensional morphological model. Coast. Eng.51, 883–915. doi: 10.1016/j.coastaleng.2004.07.014
67
Limpus C. J. Miller J. D. Pfaller J. B. (2021). Flooding-induced mortality of loggerhead sea turtle eggs. Wildlife Res.48, 142. doi: 10.1071/WR20080
68
Lindenmayer D. B. Piggott M. P. Wintle B. A. (2013). Counting the books while the library burns: why conservation monitoring programs need a plan for action. Front. Ecol. Environ.11, 549–555. doi: 10.1890/120220
69
Long T. M. Angelo J. Weishampel J. F. (2011). LiDAR-derived measures of hurricane- and restoration-generated beach morphodynamics in relation to sea turtle nesting behaviour. Int. J. Remote Sens.32, 231–241. doi: 10.1080/01431160903439973
70
Lowe R. J. Falter J. L. Bandet M. D. Pawlak G. Atkinson M. J. Monismith S. G. et al . (2005). Spectral wave dissipation over a barrier reef. J. Geophysical Research: Oceans110, 1–16. doi: 10.1029/2004JC002711
71
Luijendijk A. Hagenaars G. Ranasinghe R. Baart F. Donchyts G. Aarninkhof S. (2018). The state of the world’s beaches. Sci. Rep.8, 6641. doi: 10.1038/s41598-018-24630-6
72
Maneja R. H. Miller J. D. Li W. Thomas R. El-Askary H. Perera S. et al . (2021). Multidecadal analysis of beach loss at the major offshore sea turtle nesting islands in the northern Arabian Gulf. Ecol. Indic.121, 107146. doi: 10.1016/j.ecolind.2020.107146
73
Margaritoulis D. (2005). Nesting Activity and Reproductive Output of Loggerhead Sea Turtles, Caretta caretta, Over 19 Season-2002) at Laganas Bay, Zakynthos, Greece: The Largest Rookery in the Mediterranean. Chelonian Conserv. Biol.4, 916–929.
74
Masselink G. Lazarus E. (2019). Defining coastal resilience. Water11, 2587. doi: 10.3390/w11122587
75
Mast R. B. Hutchinson B. J. Villegas P. E. Bandimere A. (2025). SWOT report. 20. Available online at: https://www.seaturtlestatus.org/swot-report-vol-20.
76
Mazaris A. D. Almpanidou V. Wallace B. P. Pantis J. D. Schofield G. (2014). A global gap analysis of sea turtle protection coverage. Biol. Conserv.173, 17–23. doi: 10.1016/j.biocon.2014.03.005
77
Mazaris A. D. Matsinos G. Pantis J. D. (2009). Evaluating the impacts of coastal squeeze on sea turtle nesting. Ocean Coast. Manage.52, 139–145. doi: 10.1016/j.ocecoaman.2008.10.005
78
Mazor T. Levin N. Possingham H. P. Levy Y. Rocchini D. Richardson A. J. et al . (2013). Can satellite-based night lights be used for conservation? The case of nesting sea turtles in the Mediterranean. Biol. Conserv.159, 63–72. doi: 10.1016/j.biocon.2012.11.004
79
McCall R. Storlazzi C. Roelvink F. Pearson S. G. de Goede R. Antolínez J. A. (2024). Rapid simulation of wave runup on morphologically diverse, reef-lined coasts with the BEWARE-2 (Broad-range Estimator of Wave Attack in Reef Environments) meta-process model. Natural Hazards Earth System Sci.24, 3597–3625. doi: 10.5194/nhess-24-3597-2024
80
McFall B. C. Young D. L. Whitmeyer S. J. Buscombe D. Cohn N. Stasiewicz J. B. et al . (2024). SandSnap: Measuring and mapping beach grain size using crowd-sourced smartphone images. Coast. Eng.192, 104554. doi: 10.1016/j.coastaleng.2024.104554
81
McGehee A. M. (1990). Effects of moisture on eggs and hatchlings of loggerhead sea turtles (Caretta caretta). Herpetologica46, 251–258. Available online at: https://www.jstor.org/stable/3892967.
82
Montague C. L. (1993). Ecological engineering of inlets in southeastern florida: design criteria for sea turtle nesting beaches. J. Coast. Res.18, 267–276. Available online at: https://www.jstor.org/stable/25735685.
83
Morim J. Wahl T. Rasmussen D. J. Calafat F. M. Vitousek S. Dangendorf S. et al . (2025). Observations reveal changing coastal storm extremes around the United States. Nat. Climate Change15, 538–545. doi: 10.1038/s41558-025-02315-z
84
Mortimer J. A. (1990). The influence of beach sand characteristics on the nesting behavior and clutch survival of green turtles (Chelonia mydas). Copeia1990, 802. doi: 10.2307/1446446
85
Mortimer J. (1999). “ Reducing threats to eggs and hatchlings: hatcheries,” in Research and management techniques for the conservation of sea turtlesEds. EckertK. L.BjorndalK. A.Abreu-GroboisF. A.DonnellyM. ( IUCN/SSC Marine Turtle Specialist Group Publication).
86
Muis S. Irazoqui Apecechea M. Dullaart J. de Lima Rego J. Madsen K. S. Su J. et al . (2020). A high-resolution global dataset of extreme sea levels, tides, and storm surges, including future projections. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00263
87
Nel R. Campbell E. E. Harris L. Hauser L. Schoeman D. S. McLachlan A. et al . (2014). The status of sandy beach science: Past trends, progress, and possible futures. Estuarine Coast. Shelf Sci.150, 1–10. doi: 10.1016/j.ecss.2014.07.016
88
Neumann B. Vafeidis A. T. Zimmermann J. Nicholls R. J. (2015). Future coastal population growth and exposure to sea-level rise and coastal flooding - A global assessment. PloS One10, e0118571. doi: 10.1371/journal.pone.0118571
89
Ostertag E. F. (2025). Exploring nature-based solutions to mitigate climate change impacts on sea turtle nesting beaches (MSc thesis). Aachen, Germany.
90
Patino-Martinez J. Marco A. Quiñones L. Hawkes L. A. (2014). The potential future influence of sea level rise on leatherback turtle nests. J. Exp. Mar. Biol. Ecol.461, 116–123. doi: 10.1016/j.jembe.2014.07.021
91
Patrício A. R. Hawkes L. A. Monsinjon J. R. Godley B. J. Fuentes M. M. P. B. (2021). Climate change and marine turtles: recent advances and future directions. Endangered Species Res.44, 363–395. doi: 10.3354/esr01110
92
Patrício A. R. Varela M. R. Barbosa C. Broderick A. C. Catry P. Hawkes L. A. et al . (2019). Climate change resilience of a globally important sea turtle nesting population. Global Change Biol.25, 522–535. doi: 10.1111/gcb.14520
93
Pike D. A. Roznik E. A. Bell I. (2015). Nest inundation from sea-level rise threatens sea turtle population viability. R. Soc. Open Sci.2, 150127. doi: 10.1098/rsos.150127
94
Pike D. A. Stiner J. C. (2007). Sea turtle species vary in their susceptibility to tropical cyclones. Oecologia153, 471–478. doi: 10.1007/s00442-007-0732-0
95
Pintus K. J. Godley B. J. McGowan A. Broderick A. C. (2009). Impact of clutch relocation on green turtle offspring. J. Wildlife Manage.73, 1151–1157. doi: 10.2193/2008-103
96
Pontee N. (2013). Defining coastal squeeze: A discussion. Ocean Coast. Manage.84, 204–207. doi: 10.1016/j.ocecoaman.2013.07.010
97
Pronk M. Hooijer A. Eilander D. Haag A. de Jong T. Vousdoukas M. et al . (2024). DeltaDTM: A global coastal digital terrain model. Sci. Data11, 273. doi: 10.1038/s41597-024-03091-9
98
Ranasinghe R. (2020). On the need for a new generation of coastal change models for the 21st century. Sci. Rep.10 (2010). doi: 10.1038/s41598-020-58376-x
99
Reece J. Passeri D. Ehrhart L. Hagen S. Hays A. Long C. et al . (2013). Sea level rise, land use, and climate change influence the distribution of loggerhead turtle nests at the largest USA rookery (Melbourne Beach, Florida). Mar. Ecol. Prog. Ser.493, 259–274. doi: 10.3354/meps10531
100
Reguero B. G. Beck M. W. Agostini V. N. Kramer P. Hancock B. (2018). Coral reefs for coastal protection: A new methodological approach and engineering case study in Grenada. J. Environ. Manage.210, 146–161. doi: 10.1016/j.jenvman.2018.01.024
101
Reguero B. G. Losada I. J. Méndez F. J. (2019). A recent increase in global wave power as a consequence of oceanic warming. Nat. Commun.10, 205. doi: 10.1038/s41467-018-08066-0
102
Restrepo J. Webster E. Ramos I. Valverde R. (2023). Recent decline of green turtle Chelonia mydas nesting trend at Tortuguero, Costa Rica. Endangered Species Res.51, 59–72. doi: 10.3354/esr01237
103
Rivas M. L. Rodríguez-Caballero E. Esteban N. Carpio A. J. Barrera-Vilarmau B. Fuentes M. M. P. B. et al . (2023). Uncertain future for global sea turtle populations in face of sea level rise. Sci. Rep.13, 5277. doi: 10.1038/s41598-023-31467-1
104
Roelvink J. A. Huisman B. Elghandour A. Ghonim M. Reyns J. (2020). Efficient modeling of complex sandy coastal evolution at monthly to century time scales. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00535
105
Roelvink D. Reniers A. van Dongeren A. van Thiel de Vries J. McCall R. Lescinski J. (2009). Modelling storm impacts on beaches, dunes and barrier islands. Coast. Eng.56, 1133–1152. doi: 10.1016/j.coastaleng.2009.08.006
106
Rosati J. Dean R. Walton T. (2013). The modified Bruun Rule extended for landward transport. Mar. Geology340, 71–81. doi: 10.1016/j.margeo.2013.04.018
107
Rueda A. Cagigal L. Pearson S. Antolínez J. A. Storlazzi C. van Dongeren A. et al . (2019). HyCReWW: A Hybrid Coral Reef Wave and Water level metamodel. Comput. Geosciences127, 85–90. doi: 10.1016/j.cageo.2019.03.004
108
Ruggiero P. Buijsman M. Kaminsky G. M. Gelfenbaum G. (2010). Modeling the effects of wave climate and sediment supply variability on large-scale shoreline change. Mar. Geology273, 127–140. doi: 10.1016/j.margeo.2010.02.008
109
Rumbold D. G. Davis P. W. Perretta C. (2001). Estimating the effect of beach nourishment on caretta caretta (Loggerhead sea turtle) nesting. Restor. Ecol.9, 304–310. doi: 10.1046/j.526-100x.2001.009003304.x
110
Santana Garcon J. Grech A. Moloney J. Hamann M. (2010). Relative Exposure Index: an important factor in sea turtle nesting distribution. Aquat. Conservation: Mar. Freshw. Ecosyst.20, 140–149. doi: 10.1002/aqc.1057
111
Scott F. Antolinez J. A. A. McCall R. Storlazzi C. Reniers A. Pearson S. (2020). Hydro-morphological characterization of coral reefs for wave runup prediction. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00361
112
Simmons J. A. Splinter K. D. (2025). Data-driven shoreline modelling at timescales of days to years. Coast. Eng.197, 104685. doi: 10.1016/j.coastaleng.2024.104685
113
Slinger J. H. Vreugdenhil H. S. I. (2020). Coastal engineers embrace nature: characterizing the metamorphosis in hydraulic engineering in terms of four continua. Water12, 2504. doi: 10.3390/w12092504
114
Smith K. A. North E. W. Shi F. Chen S.-N. Hood R. R. Koch E. W. et al . (2009). Modeling the effects of oyster reefs and breakwaters on seagrass growth. Estuaries Coasts32, 748–757. doi: 10.1007/s12237-009-9170-z
115
Smithers S. G. Dawson J. L. (2023). Beach reprofiling to improve reproductive output at the world’s largest remaining green turtle rookery: Raine Island, northern Great Barrier Reef. Ocean Coast. Manage.231, 106385. doi: 10.1016/j.ocecoaman.2022.106385
116
Spalding M. D. Ruffo S. Lacambra C. Meliane I. Hale L. Z. Shepard C. C. et al . (2014). The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean Coast. Manage.90, 50–57. doi: 10.1016/j.ocecoaman.2013.09.007
117
Splinter K. D. Coco G. (2021). Challenges and opportunities in coastal shoreline prediction. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.788657
118
Steinitz M. J. Salmon M. Wyneken J. (1998). Beach renourishment and loggerhead turtle reproduction: A seven year study at jupiter island. J. Coast. Res.14, 1000–1013. Available online at: https://www.jstor.org/stable/4298852.
119
Taal F. (2024). Modelling hydrodynamic and groundwater processes on a sea turtle nesting beach (MSc thesis). Delft, Netherlands.
120
Thampanya U. Vermaat J. Sinsakul S. Panapitukkul N. (2006). Coastal erosion and mangrove progradation of Southern Thailand. Estuarine Coast. Shelf Sci.68, 75–85. doi: 10.1016/j.ecss.2006.01.011
121
Turner I. L. Harley M. D. Almar R. Bergsma E. W. (2021). Satellite optical imagery in Coastal Engineering. Coast. Eng.167, 103919. doi: 10.1016/j.coastaleng.2021.103919
122
Van Houtan K. S. Bass O. L. (2007). Stormy oceans are associated with declines in sea turtle hatching. Curr. Biol.17, R590–R591. doi: 10.1016/j.cub.2007.06.021
123
Varela M. R. Patrício A. R. Anderson K. Broderick A. C. DeBell L. Hawkes L. A. et al . (2019). Assessing climate change associated sea-level rise impacts on sea turtle nesting beaches using drones, photogrammetry and a novel GPS system. Global Change Biol.25, 753–762. doi: 10.1111/gcb.14526
124
Veelenturf C. A. Sinclair E. M. Paladino F. V. Honarvar S. (2020). Predicting the impacts of sea level rise in sea turtle nesting habitat on Bioko Island, Equatorial Guinea. PloS One15 (7), e0222251. doi: 10.1371/journal.pone.0222251
125
Vitousek S. Barnard P. L. Limber P. (2017). Can beaches survive climate change? J. Geophysical Research: Earth Surface122, 1060–1067. doi: 10.1002/2017JF004308
126
Vitousek S. Buscombe D. Vos K. Barnard P. L. Ritchie A. C. Warrick J. A. (2023a). The future of coastal monitoring through satellite remote sensing. Cambridge Prisms: Coast. Futures1, e10. doi: 10.1017/cft.2022.4
127
Vitousek S. Cagigal L. Montaño J. Rueda A. Mendez F. Coco G. et al . (2021). The application of ensemble wave forcing to quantify uncertainty of shoreline change predictions. J. Geophysical Research: Earth Surface126, e2019JF005506. doi: 10.1029/2019JF005506
128
Vitousek S. Vos K. Splinter K. D. Erikson L. Barnard P. L. (2023b). A model integrating satellite-derived shoreline observations for predicting fine-scale shoreline response to waves and sea-level rise across large coastal regions. J. Geophysical Research: Earth Surface128 (e2022JF006936). doi: 10.1029/2022JF006936
129
Vitousek S. Vos K. Splinter K. D. Parker K. O’Neill A. Foxgrover A. C. et al . (2024). Scalable, data-assimilated models predict large-scale shoreline response to waves and sea-level rise. Sci. Rep.14, 28029. doi: 10.1038/s41598-024-77030-4
130
Von Holle B. Irish J. L. Spivy A. Weishampel J. F. Meylan A. Godfrey M. H. et al . (2019). Effects of future sea level rise on coastal habitat. J. Wildlife Manage.83, 694–704. doi: 10.1002/jwmg.21633
131
Vos K. Harley M. D. Splinter K. D. Simmons J. A. Turner I. L. (2019a). Sub-annual to multi-decadal shoreline variability from publicly available satellite imagery. Coast. Eng.150, 160–174. doi: 10.1016/j.coastaleng.2019.04.004
132
Vos K. Splinter K. D. Harley M. D. Simmons J. A. Turner I. L. (2019b). CoastSat: A Google Earth Engine-enabled Python toolkit to extract shorelines from publicly available satellite imagery. Environ. Model. Software122, 104528. doi: 10.1016/j.envsoft.2019.104528
133
Wallace B. P. Bandimere A. N. Abreu-Grobois F. Acosta H. Akiti J. Akomedi M. et al . (2025). Updated global conservation status and priorities for marine turtles. Endangered Species Res.56, 247–276. doi: 10.3354/esr01385
134
Wallace B. P. Posnik Z. A. Hurley B. J. DiMatteo A. D. Bandimere A. Rodriguez I. et al . (2023). Marine turtle regional management units 2.0: an updated framework for conservation and research of wide-ranging megafauna species. Endangered Species Res.52, 209–223. doi: 10.3354/esr01243
135
Walstra D. Reniers A. Ranasinghe R. Roelvink J. Ruessink B. (2012). On bar growth and decay during interannual net offshore migration. Coast. Eng.60, 190–200. doi: 10.1016/j.coastaleng.2011.10.002
136
Ware M. Ceriani S. Long J. Fuentes M. M. P. B. (2021). Exposure of loggerhead sea turtle nests to waves in the florida panhandle. Remote Sens.13, 2654. doi: 10.3390/rs13142654
137
Ware M. Fuentes M. M. (2018). comparison of methods used to monitor groundwater inundation of sea turtle nests. A J. Exp. Mar. Biol. Ecol.503, 1–7. doi: 10.1016/j.jembe.2018.02.001
138
Ware M. Long J. W. Fuentes M. M. P. B. (2019). Using wave runup modeling to inform coastal species management: An example application for sea turtle nest relocation. Ocean Coast. Manage.173, 17–25. doi: 10.1016/j.ocecoaman.2019.02.011
139
Wildermann N. E. Barrios-Garrido H. Jabby K. Hardenstine R. S. Shimada T. Williams I. D. et al . (2024). An emerging hazard to nesting sea turtles in the face of sea-level rise. Global Ecol. Conserv.56, e03334. doi. doi: 10.1016/j.gecco.2024.e03334
140
Willson A. Witherington B. Baldwin R. Tiwari M. Al Sariri T. Al Kiyumi A. et al . (2020). Evaluating the long-term trend and management of a globally important loggerhead population nesting on masirah island, sultanate of Oman. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00666
141
Wolinsky M. A. Murray A. B. (2009). A unifying framework for shoreline migration: 2. Application to wave-dominated coasts. J. Geophysical Research: Earth Surface114 (F01009). doi: 10.1029/2007JF000856
142
Yamamoto K. H. Powell R. L. Anderson S. Sutton P. C. (2012). Using LiDAR to quantify topographic and bathymetric details for sea turtle nesting beaches in Florida. Remote Sens. Environ.125, 125–133. doi: 10.1016/j.rse.2012.07.016
Summary
Keywords
sea turtle conservation, sea turtle beaches, coastal management, nature-based solutions, monitoring and modeling, remote sensing, sandy beaches, interdisciplinarity
Citation
Christiaanse JC, Reniers AJHM, Aarninkhof SGJ, Ostertag EF, Nel R, Duarte CM and Antolínez JAA (2025) Aiding sea turtle conservation through coastal management. Front. Mar. Sci. 12:1669885. doi: 10.3389/fmars.2025.1669885
Received
20 July 2025
Accepted
29 August 2025
Published
19 September 2025
Corrected
29 September 2025
Volume
12 - 2025
Edited by
Simone Bonamano, University of Tuscia, Italy
Reviewed by
Carla Cherubini, Politecnico di Bari, Italy; Daniele Piazzolla, Foundation Euro-Mediterranean Center on Climate Change (CMCC), Italy
Updates
Copyright
© 2025 Christiaanse, Reniers, Aarninkhof, Ostertag, Nel, Duarte and Antolínez.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jakob C. Christiaanse, j.c.christiaanse@tudelft.nl
ORCID: Jakob C. Christiaanse, orcid.org/0009-0007-4089-3578; Ad J. H. M. Reniers, orcid.org/0000-0001-8732-6748; Stefan G. J. Aarninkhof, orcid.org/0000-0002-4591-0257; Carlos M. Duarte, orcid.org/0000-0002-1213-1361; José A. A. Antolínez, orcid.org/0000-0002-0694-4817
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.