- 1Marine Biodiversity Research Group, Department of Biology, Faculty of Science, Ramkhamhaeng University, Bangkok, Thailand
- 2Maritime Security Division, Office of the National Security Council, Government House, Dusit, Bangkok, Thailand
- 3Chumphon Marine National Park Operation Center 1, Department of National Parks Wildlife and Plant Conservation, Chumphon, Thailand
- 4Marine and Coastal Resources Research and Development Center, Klaeng, Rayong, Thailand
This study investigates the occurrence, abundance, and characteristics of microplastics (MPs) in coral reef ecosystems at two reef sites, Ko Khang Khao and Ko Ngam Yai, in the Gulf of Thailand. Coral, seawater, and sediment samples were analyzed to evaluate the spatial variability of microplastics in terms of their abundance, size distribution, shape, polymer composition, and color. MPs were detected in all coral samples, with concentrations ranging from 0.24 to 2.60 particles g-¹ w.w. Ko Khang Khao exhibited higher MP concentrations across coral species, with Pavona decussata, Pocillopora acuta, and Galaxea fascicularis showing the greatest accumulation. Statistically significant inter-site differences in MP abundance were observed in four coral species (p < 0.05). Coral surface was the dominant compartment for MP accumulation (63.05% at Ko Khang Khao and 61.82% at Ko Ngam Yai), followed by skeleton and tissue. MP size distribution varied by coral compartment, with coral skeletons retaining larger particles (>3000 µm) and tissues favoring smaller MPs (25–1500 µm). Fiber-shaped MPs were predominant across all sites and compartments. Polymer analysis revealed polyethylene terephthalate (PET) and polyethylene (PE) as the most common polymers, while notable amounts of polypropylene (PP), nylon, and polystyrene (PS) were also identified, indicating diverse anthropogenic sources. Color analysis showed blue MPs as the most abundant, followed by black, white/yellow, red, and green particles. Green MPs were notably absent at Ko Ngam Yai. In seawater, MP concentrations ranged from 0.88 to 1.61 particles m-³, with higher levels at Ko Khang Khao. Fiber was again the dominant shape, and PE and PET were the most common polymers. Sediment samples exhibited MP abundances ranging from 164.80 to 295.20 particles kg-¹, with Ko Khang Khao having a greater prevalence of smaller particles (<600 µm) and more diverse polymer types. Across all matrices, Ko Khang Khao consistently showed higher MP concentrations and greater diversity in MP characteristics compared to Ko Ngam Yai, likely reflecting greater anthropogenic influence. These findings emphasize the spatial heterogeneity of microplastic pollution in coral reef systems and underscore the need for localized management strategies to mitigate microplastic inputs in nearshore marine environments.
Introduction
The escalating demand for plastics has led to a substantial surge in production, which attained 368 million tons in 2020. Worldwide plastic manufacturing is expected to surpass 1,100 million tons per year by 2050 (Bai et al., 2025). Unsustainable production and consumption of plastics, coupled with insufficient waste management practices, have contributed to rising global pollution by synthetic debris that ultimately degrades into microplastics (MPs) in ecosystems (Walker and Fequet, 2023; Piskuła et al., 2025). MPs are described as plastic particles or fragments with diameters less than 5 millimeters (GESAMP, 2015). They pose a significant global challenge in marine habitats (Hollerová et al., 2021; Sutthacheep et al., 2021), including those in the Gulf of Thailand (Chamchoy et al., 2018). Plastic waste reaches marine environments via multiple routes, originating from terrestrial and marine sources alike (Rellán et al., 2023). Terrestrial sources encompass river discharges, sewage outflows, wind-driven deposition, and direct pollution of the environment (Peng et al., 2020; Belioka and Achilias, 2023). Key marine sources involve fishing activities, which are estimated to contribute about 10–15% of all marine debris globally (Fabres et al., 2016; Wongnutpranont et al., 2021). The size of microplastics is viewed as a key determinant in evaluating their impacts on ecosystems and the environment (Zhang et al., 2023a). Extensive studies have indicated that the forms of microplastics, such as their dimensions, polymer types, and geometries, may negatively affect the immune systems and metabolic processes of marine life (Murano et al., 2020; Liu et al., 2022; Zu et al., 2023). Therefore, microplastics in marine habitats represent a substantial risk to ecosystem health overall, and smaller microplastic particles merit heightened attention (Zhang et al., 2025).
As passive suspension feeders, scleractinian corals capture plankton drifting across their tentacles but may inadvertently ingest microplastics present in surrounding seawater (Houlbrèque and Ferrier-Pagès, 2009; Allen et al., 2017; Savinelli et al., 2020; Soares et al., 2023). Experimental evidence demonstrates that corals ingest microplastics at rates comparable to natural prey consumption (Chamchoy et al, 2018; Chapron et al., 2018; Reichert et al., 2019; Wongnutpranont et al., 2021). In other marine invertebrates, plastic ingestion reduces energy budgets and compromises physiological performance and reproductive output (Wright et al., 2013; Sussarellu et al., 2016; Cole et al., 2011). Although calcification in corals relies mainly on photosynthesis (Berry et al., 2019), heterotrophic feeding becomes critical during periods of low light availability or thermal stress events such as bleaching (Ferrier-Pagès et al., 2011; Hughes et al., 2017; Zhang et al., 2023). Consequently, ingested microplastics may exacerbate energetic deficits by interfering with digestion and assimilation of natural food sources, particularly under stressful conditions (Grillo et al., 2021).
Coral reefs are increasingly recognized as hotspots for microplastic accumulation (Tetu et al., 2019; Huang et al., 2021). Their structural complexity promotes the retention of suspended particles, while proximity to urban coastlines, riverine discharges, and tourism activities further elevates exposure to plastic debris (Beckwith and Fuentes, 2018; de Smit et al., 2021; Biswas et al., 2024). Once deposited, microplastics adhere to coral surfaces, become embedded within mucus layers, and are ingested by coral polyps (Reichert et al., 2018; Savinelli et al., 2020). Such ingestion has been shown to reduce energy intake, suppress growth, disrupt symbiotic relationships with zooxanthellae, and increase susceptibility to disease (Lamb et al., 2018; Okubo et al., 2018; Grillo et al., 2021). These findings underscore the urgency of further research into the physiological impacts and long-term ecological risks that microplastics pose to coral reef health and resilience.
The surface waters of the global oceans constitute primary reservoirs for buoyant microplastics, which tend to accumulate in hydrodynamic convergence zones such as subtropical gyres (Eriksen et al., 2014). However, these particles are not confined solely to surface layers. Mechanisms including biofouling, aggregation with organic matter, and sedimentation facilitate their vertical transport into deeper water columns and seabed sediments, resulting in extensive spatial dispersal both horizontally and vertically (Long et al., 2015; Kane et al., 2020; Pabortsava and Lampitt, 2020). The widespread occurrence of microplastics in seawater presents significant ecological and toxicological threats. Diverse marine taxa, ranging from plankton and bivalves to fish and larger vertebrates, such as sharks have been documented to ingest these particles, either directly or indirectly through trophic transfer (Wright et al., 2013; Cole et al., 2015; Cutroneo et al., 2020; Matupang et al., 2023). Ingestion of microplastics can induce gastrointestinal obstruction, reduce feeding efficiency, impose energetic stress, and impair reproductive performance (Lusher et al., 2017; Bucci et al., 2020; Sheela and Dhinagaran, 2023). Furthermore, microplastics function as vectors for a range of contaminants, including persistent organic pollutants, heavy metals, antibiotic resistance genes, and pathogenic microorganisms, all of which can bioaccumulate and propagate through marine food webs (Rochman et al., 2013; Zettler et al., 2013; Kirstein et al., 2016; Pittura et al., 2018).
Microplastics commonly accumulate in marine sediments, particularly those found on beaches, in river mouths, and across the seafloor (Rohais et al., 2024). The gradual deposition of microplastics in sediments can reveal long-term trends of regional contamination (Eo et al., 2023). Sediments act as major sinks for microplastics, where particles accumulate over time due to their high residence periods and continuous input from terrestrial runoff, riverine discharge, and marine activities (Corami et al., 2020). The deposition of microplastics in reef-associated sediments is of particular concern because these materials can physically and chemically interact with benthic organisms, such as corals, sponges, and seagrasses, altering sediment characteristics and potentially introducing toxic additives or adsorbed contaminants (do Amparo et al., 2023). Recent studies have begun to quantify the spatial and temporal distribution of microplastics within coral reef sediments across multiple regions, revealing considerable variability in contamination levels and polymer types (Alves et al., 2023). Some research indicates that nearshore reefs adjacent to urbanized coastlines tends to have a higher microplastic loads compared to more remote reef sites (Reuning et al., 2025). Machendiranathan et al. (2024) showed sediment samples collected from the Xuwen Coral Reef National Nature Reserve in southern China contained microplastic abundances ranging from 0 to 1,340 particles per kilogram, with fibers accounting for over 70% of all particles. Similarly, Ulfah et al. (2025) found average concentrations of approximately 16.7 particles per kilogram in reef sediments along the Krueng Raya coast of Aceh, Indonesia, where fragments were the predominant form. These findings are consistent with broader regional assessments indicating that sediments in the Gulf of Thailand and adjacent areas act as major sinks for microplastics due to their capacity to trap and retain particles over extended time periods (Sarvajayakesavalu et al., 2023). Given the ecological significance of coral reefs and their vulnerability to multiple stressors, understanding the occurrence, distribution, and potential impacts of microplastics in reef sediments remains a critical research priority (Wang et al., 2021). Such evidence underscores the need for continued monitoring of microplastics in coral reef sediments, where they can pose risks to benthic organisms and potentially interfere with ecological processes critical to reef resilience.
In Thailand, microplastic accumulation in coral reef ecosystem is still limited. Only a few publications have been found. For example, Yeemin et al. (2018) assessed amount of microplastics in marine sediment at the coral reef hotspots of Mu Ko Similan National Park, Phang Nga Province in the Andaman Sea. Chamchoy et al. (2018) investigated microplastics in scleractinian corals from the upper Gulf of Thailand. Jandang et al. (2024) studied the accumulation patterns of microplastics in reef-building corals in the Gulf of Thailand. However, none of integrated studies quantifying all corals, seawater, and marine sediment was found. Due to their persistence, global distribution, and complex impacts, microplastics have become a critical environmental issue requiring urgent scientific attention to fill a large gap of knowledge. Addressing this challenge demands integrated research to clarify the accumulation patterns of MPs in relevant matrices in coral reef ecoystems to guide effective mitigation strategies. This study aimed to assess the abundance of microplastics in scleractinian corals, seawater, and marine sediment at Ko Khang Khao, Chonburi Province, the Upper Gulf of Thailand and Ko Ngam Yai, Chumphon Province, the Gulf of Thailand.
Materials and methods
Study areas
This study was conducted at Ko Khang Khao, which is a part of Mu Ko Sichang, Chonburi Province, located in the Upper Gulf of Thailand, and Ko Ngam Yai, Chumphon National Park, Chumphon Province, in the Western Gulf of Thailand. The two sampling sites (Ko Khang Khao and Ko Ngam Yai) were selected to represent the Upper Gulf of Thailand and the Western Gulf of Thailand, respectively. The Upper Gulf of Thailand is generally influenced by land-based pollution from five major rivers. Ko Khang Khao is located approximately 60 km, 40 km, and 12 km from the Chao Phraya River mouth, the Bang Pakong River mouth, and the mainland, respectively. In contrast, Ko Ngam Yai, located in the Western Gulf of Thailand about 20 km from both the Chumphon River mouth and the mainland, represents an area with lower influence from land-based pollution. Additionally, Ko Ngam Yai is in Mu Ko Chumphon National Park, which is a type of marine protected areas in Thailand (Table 1, Figure 1).
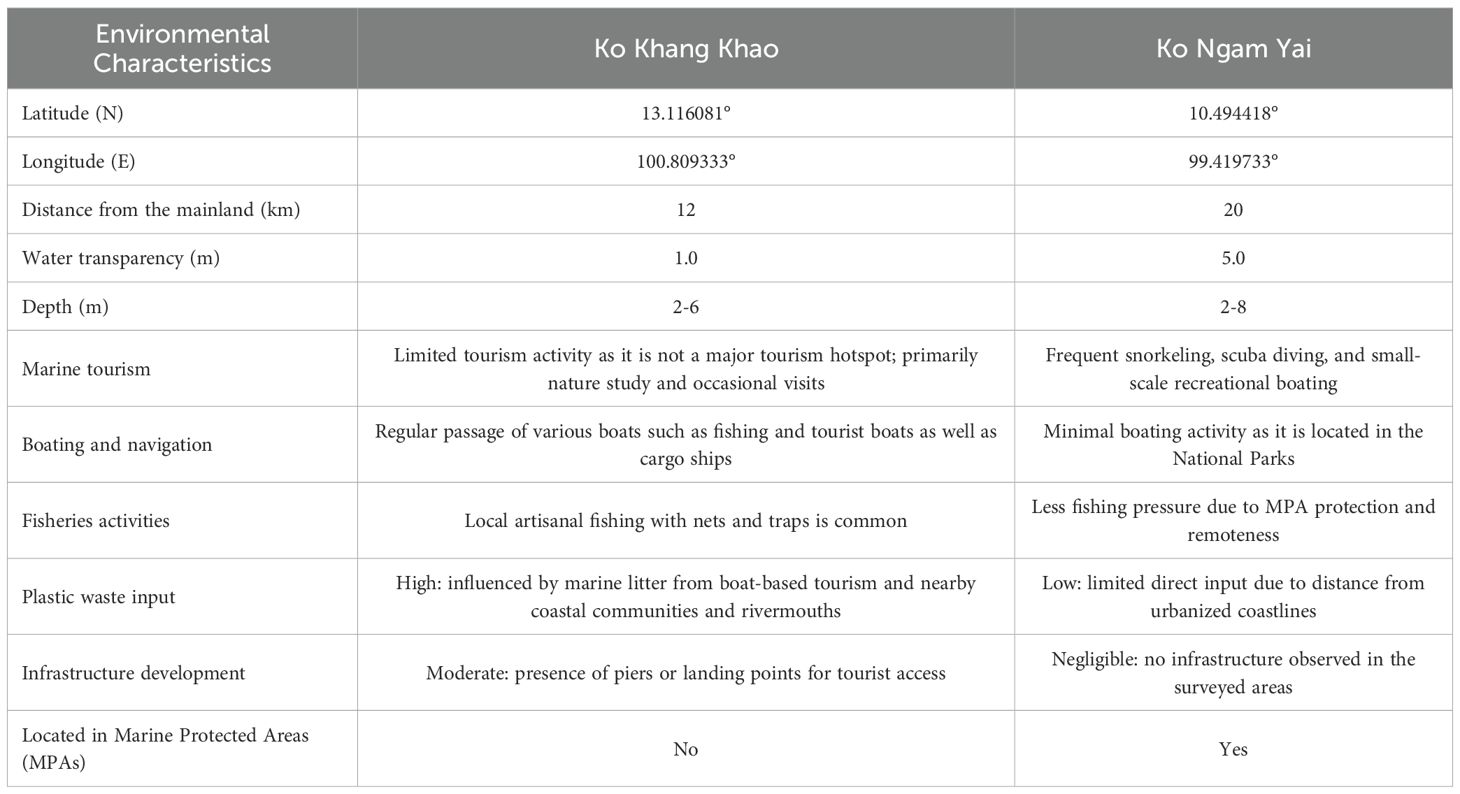
Table 1. Location and information of the study sites at Ko Khang Khao and Ko Ngam Yai in the Gulf of Thailand.
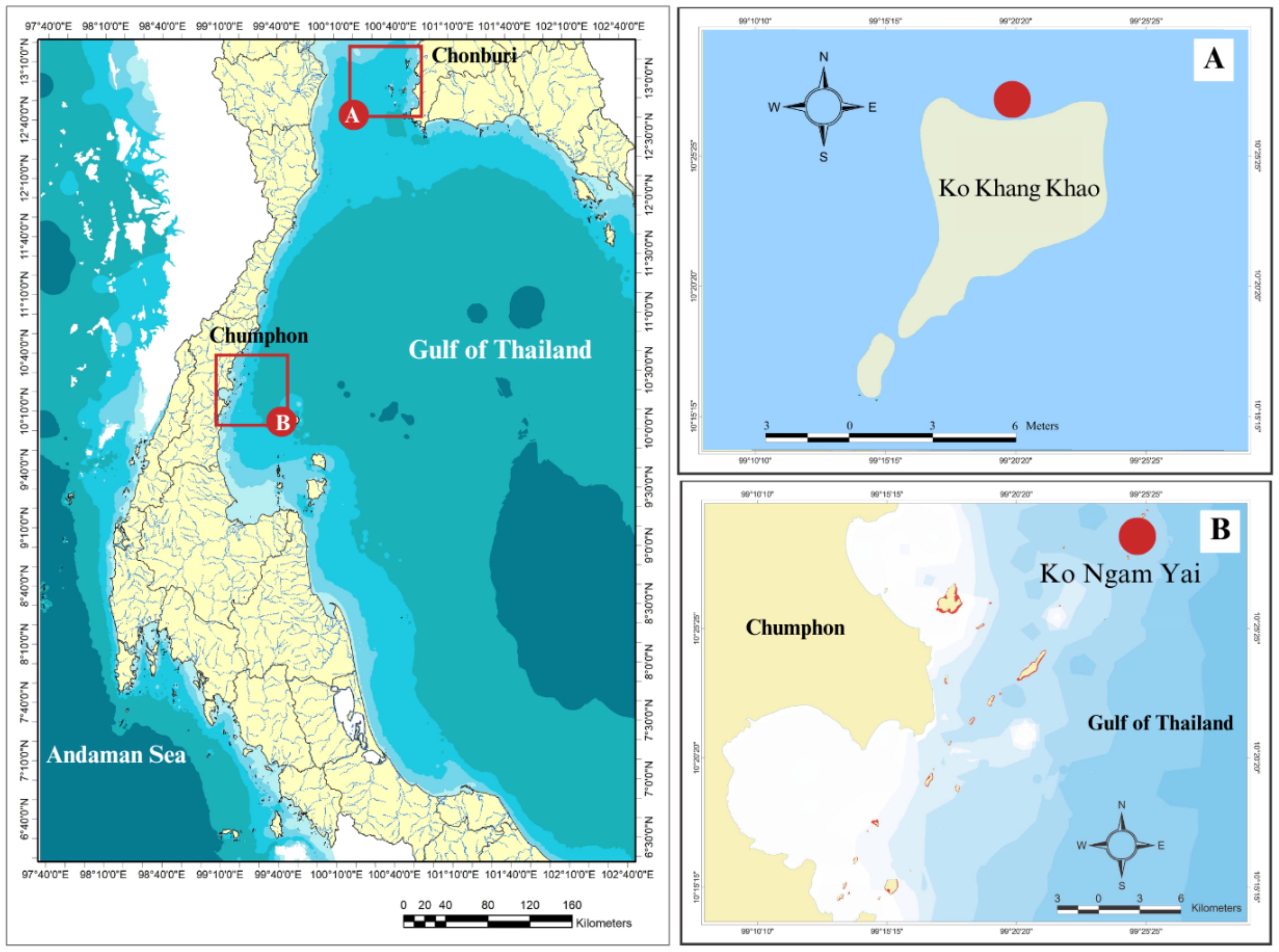
Figure 1. Map of sampling sites (A) Ko Khang Khao, the Upper Gulf of Thailand Ko Ngam Yai, the Western Gulf of Thailand.
Sample collection
Because the wet season occurs at different times in different regions of the Gulf of Thailand, sampling was conducted during the wet season at the two sites: in September for the Upper Gulf of Thailand and in February for the Western Gulf of Thailand.
Scleractinian corals
A total of 55 fragments from six coral species (Acropora muricata, Galaxea fascicularis, Pocillopora acuta, Porites lutea, Favites abdita, and Pavona decussata) were collected from two sites. From Ko Khang Khao, 28 fragments were sampled, comprising A. muricata (n = 5), G. fascicularis (n = 5), P. acuta (n = 5), P. lutea (n = 3), F. abdita (n = 5), and P. decussata (n = 5). From Ko Ngam Yai, 27 fragments were obtained, including A. muricata (n = 4), G. fascicularis (n = 5), P. acuta (n = 5), P. lutea (n = 3), F. abdita (n = 5), and P. decussata (n = 5). These species were selected due to their distinct morphologies and polyp sizes, and because they are dominant components of the local coral assemblages. Coral fragments approximately 3 cm in length were taken from branching corals, while pieces measuring 3 cm² in surface area were collected from massive coral species. All coral samples were preserved in 10% formalin mixed with seawater and subsequently transported to the laboratory.
Seawater
Seawater samples (n = 10; 5 from each sampling site) were collected using a neuston net measuring 2 meters in length with a mesh size of 315 µm. The net was carefully deployed and towed behind the research vessel at a steady speed of 1.5 knots for 15 minutes. A calibrated flowmeter was attached to record the volume of water filtered during each tow. After retrieval, the collected material was gently rinsed from the detachable cod-end with distilled water to ensure complete recovery of suspended particles. The rinsed sample was then transferred into clean glass bottles for storage and subsequent laboratory analysis. In addition, water quality parameters were measured at each sampling location to characterize the prevailing environmental conditions.
Marine sediment
Marine sediment samples (n = 16; 8 from each sampling site) were collected from coral reef areas using cylindrical stainless-steel core samplers measuring 3.5 cm in diameter and 10 cm in height to avoid contamination from plastic materials. At each sampling site, the corer was gently inserted vertically into the seafloor sediments by divers at water depths at the study sites. To ensure representative sampling and minimize disturbance, three replicate cores were obtained per site. Each core was carefully extracted, sealed in pre-cleaned aluminum foil or glass containers, and labeled with the sampling date, location, and water depth. Samples were immediately stored in cool conditions (approximately 4°C) and transported to the laboratory for further processing. All equipment was rinsed with filtered distilled water, and field blanks were collected to monitor potential contamination.
Extraction of MPs
Scleractinian corals
Coral surface and mucus layer were recovered by carefully handling coral fragments with sterilized tweezers and vigorously agitating them within the sampling container. The residual water containing detached particles was subsequently vacuum-filtered through 25 µm cellulose filters (Whatman Grade 4). To ensure complete recovery of particles, the container was rinsed with Milli-Q Type I water (final filtration pore size 0.22 µm). Following this procedure, the coral fragments were weighed using an analytical balance (Ohaus Pioneer; precision ± 0.01 g) and documented. The fragments were then thoroughly rinsed with filtered water to remove any potential contaminants introduced during handling and subsequently transferred into clean 600 ml glass beakers for further processing.
The coral tissue was digested in 7% sodium hypochlorite (NaClO) for 24 hours. The residual skeleton was thoroughly rinsed with distilled water and placed in a clean glass beaker. The NaClO solution, containing microplastics and residual organic material, was passed through a 50 µm stainless steel sieve (ISO 3310-1; Edinger Industrievertretung) to separate the liquid phase. The retained residues on the sieve were rinsed with filtered seawater and transferred into 50 ml Falcon tubes for further analysis. The filtered material was examined under a stereomicroscope at 45× magnification to detect any remaining microplastic particles. Identified microplastics were carefully retrieved using stainless steel tweezers, photographed with a digital camera, and documented for abundance and morphological characteristics, including size, color, and shape.
The coral skeleton, previously subjected to thorough tissue removal, was immersed in 35% hydrogen peroxide (H2O2) at a standardized ratio of 2 mL per gram of sample and incubated for 48 hours to ensure the oxidative degradation of any residual organic constituents. Following repeated rinsing with ultrapure water to eliminate reagent residues, the skeletal material was digested in 5.5% hydrochloric acid (HCl) at 5 mL per gram for up to 48 hours or until complete dissolution of the calcareous matrix was confirmed. The resulting solution was subsequently filtered through 0.6 µm glass fiber filter paper (Whatman), applying an identical filtration, recovery, and contamination control protocol as established for tissue-derived fractions (Chamchoy et al., 2018).
Seawater
In the laboratory, the samples were homogenized and pre-filtered again to remove coarse debris using a vacuum pump and Whatman No. 3 filter paper. For the digestion of organic matter, two complementary protocols were applied depending on the particle load: (1) treatment with 1 M sodium hydroxide at room temperature for 24 hours, followed by sequential heating at 60 °C for 2 hours and 100 °C for 1 hour (modified from Mathalon and Hill, 2014), and (2) oxidation with 30% hydrogen peroxide at 60 °C for 24 hours (Masura et al., 2015).
Following digestion, microplastics were isolated by density separation using a saturated sodium chloride solution (250 g/L). The suspension was allowed to settle undisturbed for 6 hours to enable buoyant particles to float to the surface. The supernatant was then carefully decanted and filtered under vacuum onto pre-weighed glass fiber filters (GF/F or GF/C, pore size 1.2 µm). Filters were subsequently dried at 40 °C.
Marine sediment
Marine sediment samples were initially suspended in pre-filtered seawater and subsequently filtered using a vacuum pump equipped with Whatman No. 3 filter paper. To remove organic matter, the retained material was treated with 1 M sodium hydroxide (NaOH) at room temperature for 24 hours. The mixture was then heated to 60 °C for two hours, followed by an increase to 100 °C for an additional hour to enhance digestion efficiency. The mixture was then heated to 60 °C for two hours, followed by an increase to 100 °C for an additional hour to enhance digestion efficiency. After digestion, microplastics were separated by density flotation in a saturated sodium chloride solution (250 g/L) for six hours. The overlying solution was then filtered again using a vacuum filtration unit and glass fiber filter papers (GF/C, 1.2 µm pore size). These procedures were adapted from the protocols described by Yeemin et al., 2018.
MPs identification
Visual identification based on morphological and physical features remains one of the most commonly applied approaches for microplastic detection, typically used in conjunction with complementary analytical techniques. In this study, optical examination of particles ranging from 25 µm to 3 mm in size, which were recovered from filters following density separation, was conducted using a stereomicroscope. Microplastics were classified and enumerated following the protocols described by Utami et al. (2021). Indicators of potential microplastic particles included unnatural colors, glossy surfaces, and distinctive artificial shapes or structures. Particles displaying likely cellular or organic textures, as well as translucent fibers lacking consistent thickness or three-dimensional curvature, were excluded from further analysis. All confirmed or suspected microplastic (MP) particles were categorized by shape, polymer type, and color, and documented photographically. Identification was carried out using Fourier-transform infrared (FT-IR) spectroscopy (Bruker ALPHA II Compact FT-IR Spectrometer, Germany). Spectra were collected over a range of 4000–400 cm-¹ with a resolution of 2 cm-¹, processed with OPUS software, and matched against a reference library of standard polymers. Particles were classified as MPs when their spectral profiles showed a similarity index of ≥70% to known polymers. Spectra falling below this threshold or corresponding to non-plastic materials were excluded, and total MP counts were recalculated accordingly.
Data analysis
The normality of the data was evaluated using the Shapiro–Wilk test to assess the assumption of normal distribution. Differences in microplastic (MP) abundance among study sites were analyzed using one-way analysis of variance (ANOVA). Where significant differences were detected (p < 0.05), post hoc pairwise comparisons were performed using Tukey’s Honestly Significant Difference (HSD) test to determine specific group differences of mean MP abundance among coral species and coral compartments. Independent samples t-tests were applied to compare mean MP abundance in corals, seawater and, marine sediments between the two sampling sites. All statistical analyses were conducted using R version 4.5.1. Unless otherwise stated, data are reported as means ± standard deviations (SD).
Quality assurance and quality control
All procedures were conducted with the utmost care to minimize contamination during sample analysis. Prior to use, all sampling tools and materials were thoroughly cleaned three times with distilled water filtered through GF/C glass microfiber filters and subsequently rinsed with acetone to reduce potential contaminants. Standard precautionary measures, including washing glassware, wearing cotton laboratory coats and nitrile gloves, and pre-filtering all solutions, were applied as recommended by Ding et al. (2019). Microplastic identification was performed in a closed laboratory environment, and the stereomicroscope was covered with a glass lid during particle verification to reduce airborne contamination. All filter papers were inspected under the microscope prior to experimentation to ensure cleanliness. To further minimize contamination, procedures were completed promptly, and beakers were covered with aluminum foil throughout the process. To prevent microfiber contamination, 100 mL of ultrapure water was included as a blank and subjected to the same MPs isolation procedure in parallel to evaluate potential background contamination. No MP particle was detected in the blanks, indicating the experiment process did not be contaminated. In addition, laboratory plastic particles of varying sizes, shapes, and colors were processed using the same MPs isolation protocol to assess recovery. The procedure achieved high recovery rates (75–90%) for MPs from both coral surfaces and tissues, confirming minimal particle loss and ensuring the study’s reliability. Five commercial reference polymers, including Nylon, polyethylene (PE), polyethylene terephthalate (PET), polypropylene (PP), and polystyrene (PS), were analyzed to validate FT-IR Spectrometer performance.
Results
Abundance of MPs in coral surface, coral tissue and skeleton
MPs were present in all 55 coral samples analyzed in this study. In total, 184 suspected MP particles were extracted from the coral fragments. The abundance of MPs in coral samples ranged from 0.24 to 2.60 particles g-¹ w.w. Pavona decussata showed the highest mean abundance (1.96 ± 1.24 particles g-¹ w.w.), while Porites lutea had the lowest (0.16 ± 0.17 particles g-¹ w.w.). Overall, MP abundances among coral species were not statistically different, except for the pairs of P. lutea vs P. decussata (ANOVA, p = 0.006) and P. lutea vs Pocillopora acuta (ANOVA, p = 0.009) (Figure 2A). Overall, Ko Khang Khao exhibited the highest MP concentrations. The greatest MP accumulations were observed in P. decussata, P. acuta, G. fascicularis, A. muricata, F. abdita, and P. lutea at Ko Khang Khao, respectively. Similarly, at Ko Ngam Yai, the highest concentrations were recorded in P. decussata, followed by P. acuta, G. fascicularis, F. abdita, A. muricata, and P. lutea. Statistically significant differences in MP accumulation between sites were found for P. decussata (t-test, p = 0.003), F. abdita (t-test, p = 0.050), G. fascicularis (t-test, p = 0.050), and A. muricata (t-test, p = 0.003) (Figure 2A).
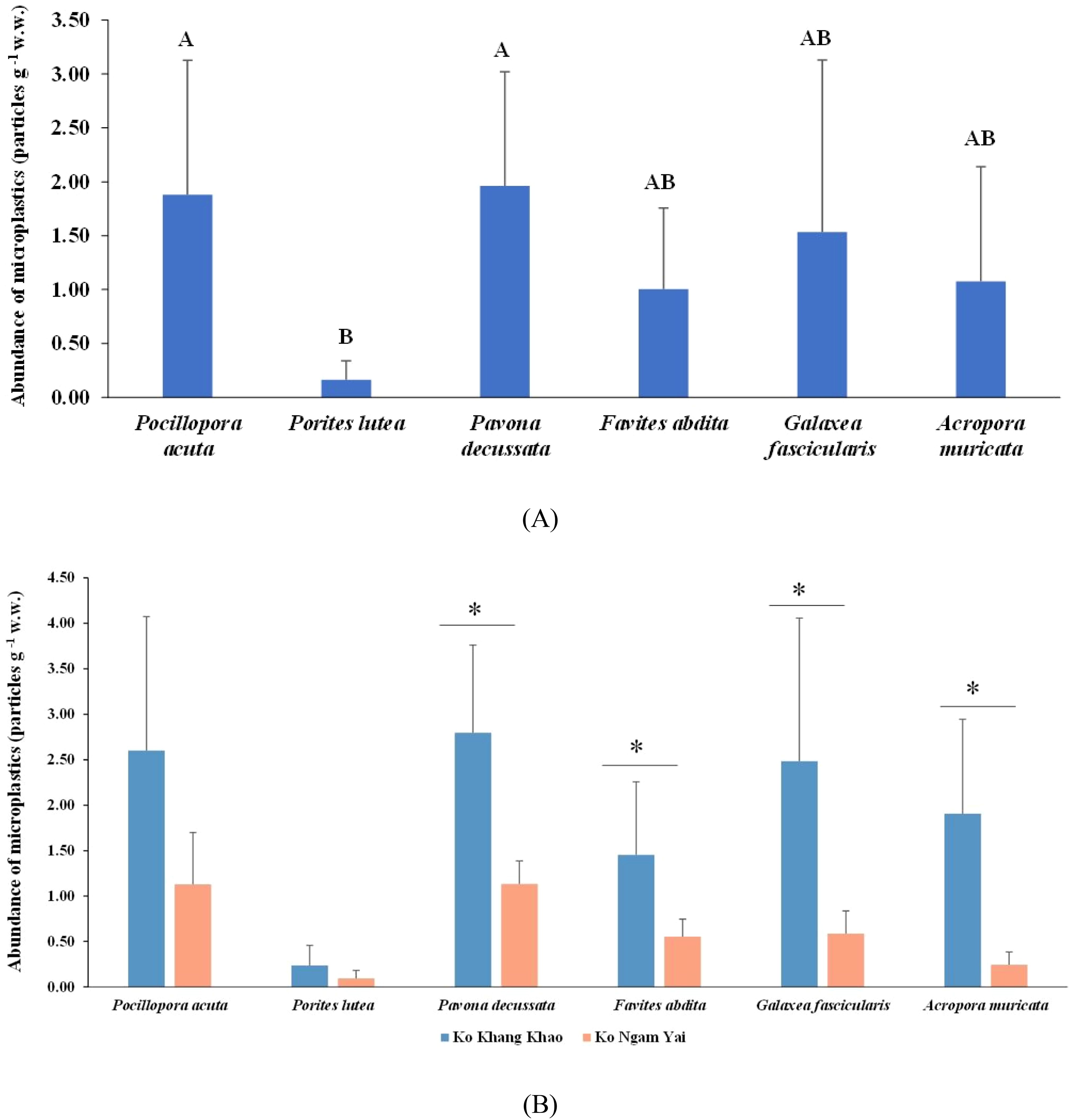
Figure 2. Abundance of microplastics (MPs) found in different coral genera (A) at Ko Khang Khao and Ko Ngam Yai (B). Shared letters denote no significant difference based on Tukey’s HSD. Asterisks indicate statistically significant differences based on an independent t-test.
The mean MP abundance in coral surface (9.55 ± 4.43 particles g-¹ w.w.) was significantly (ANOVA, p = 0.001) higher than that in coral tissue (2.36 ± 1.67 particles g-¹ w.w.) and skeleton (3.31 ± 2.03 particles g-¹ w.w.) (Figure 3). At both sites, the coral surface was the dominant compartment for MP accumulation, accounting for 63.05% at Ko Khang Khao and 61.82% at Ko Ngam Yai. The skeleton contributed 21.38% and 22.94% at Ko Khang Khao and Ko Ngam Yai, respectively. The lowest proportion of MPs was consistently found in coral tissue, representing 15.56% at Ko Khang Khao and 15.24% at Ko Ngam Yai (Figure 4A). The least number of MPs in coral surface, coral tissue, and skeleton was found in P. lutea at both study sites. MP accumulation in different coral species showed statistically significant differences (ANOVA, p = 0.001) (Figures 4B, C).
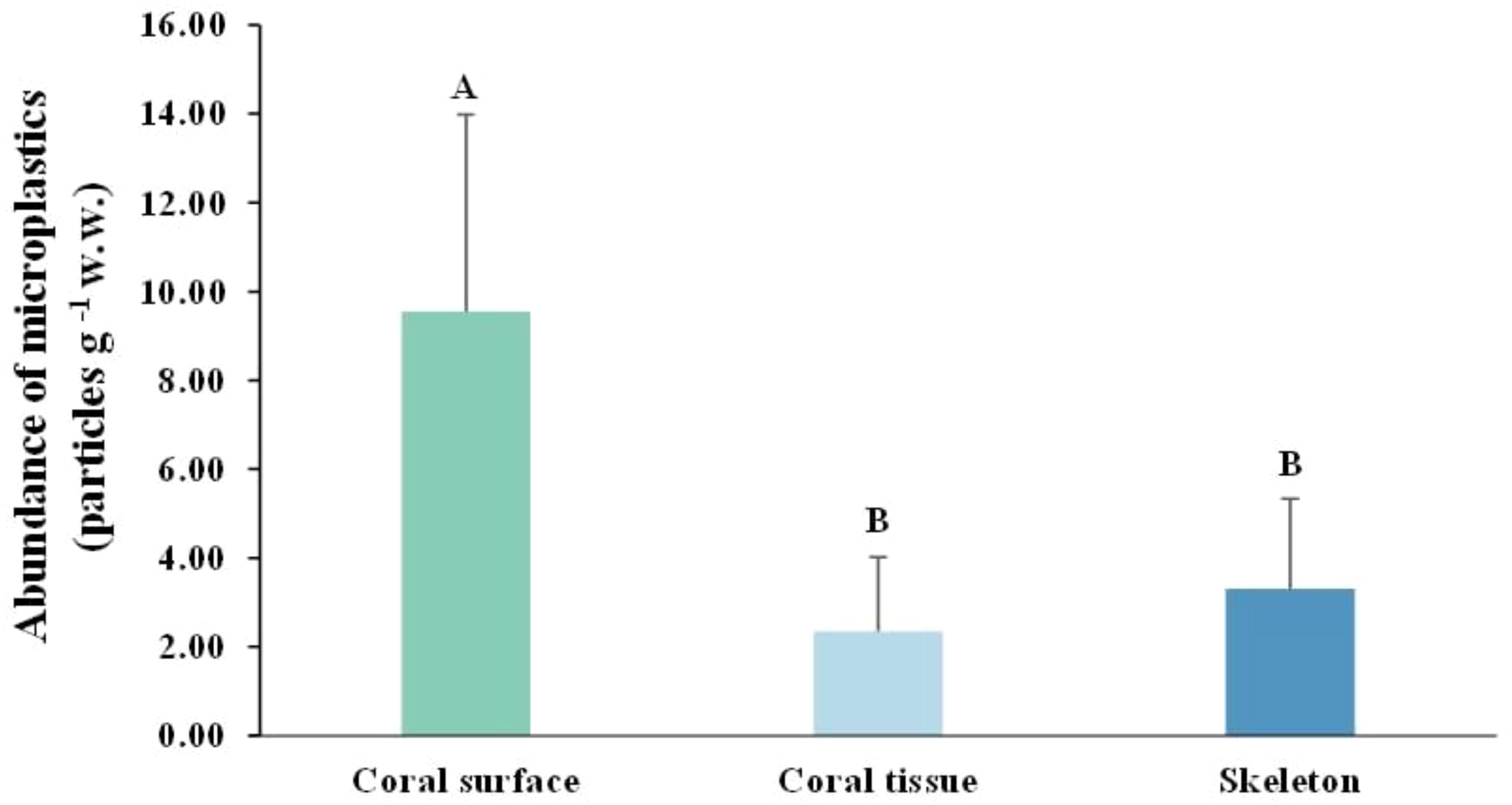
Figure 3. Distribution of microplastic (MP) on coral surface, coral tissue, and skeletonat each survey site in the Gulf of Thailand. Shared letters denote no significant difference based on Tukey’s HSD.
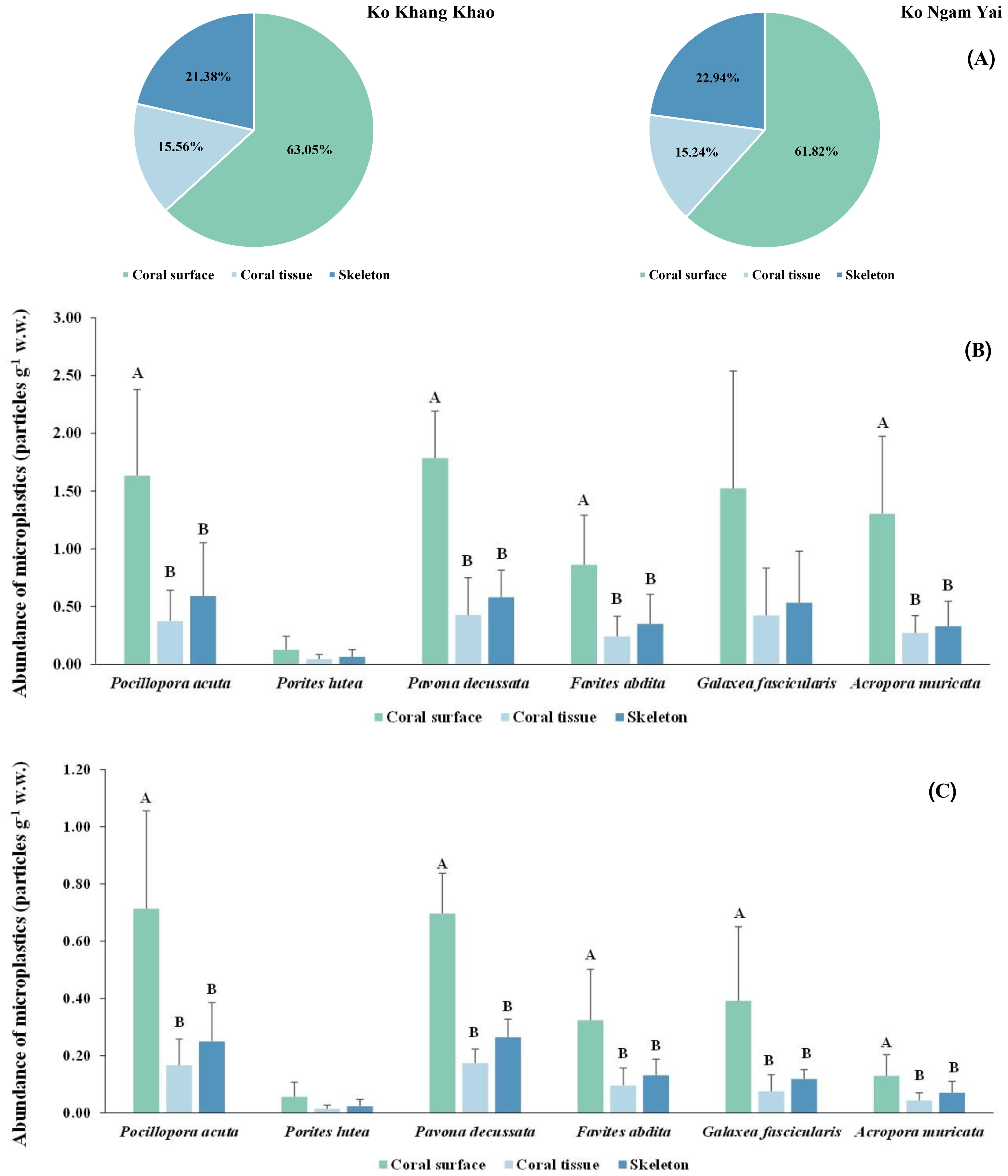
Figure 4. Distribution of microplastic (MP) on coral surface, coral tissue, and skeleton at each coral species in the Gulf of Thailand (A) all the coral species at the study site (B) each individual species at Ko Khang Khao and (C) each individual species at Ko Ngam Yai. Shared letters denote no significant difference based on Tukey’s HSD.
Characteristics of microplastics in corals
Microplastic (MP) size distribution varied across coral compartments and sites. At Ko Khang Khao, MPs on coral surfaces showed a wide size range, with dominant sizes at 25–300 μm, 1201–1500 μm, and 2001–3000 μm. Coral tissues primarily retained smaller MPs (25–300 μm and 1201–1500 μm), particularly in P. acuta and F. abdita. Skeletons at this site contained larger MPs, including >3000 μm, especially in G. fascicularis (Figure 5A). At Ko Ngam Yai, MPs were more concentrated in smaller size classes (25–600 μm) across all coral parts. Larger MPs (1501–3000 μm) were still present in G. fascicularis and P. decussata skeletons. Tissues at Ko Ngam Yai mostly retained MPs in the 25–1500 μm range (Figure 5B). P. lutea consistently showed narrow MP size ranges in both sites, dominated by 601–1200 μm. Overall, coral skeletons served as passive traps for large MPs, while tissues favored smaller particles. Surface MPs showed the highest size diversity, likely influenced by coral morphology and external adhesion.
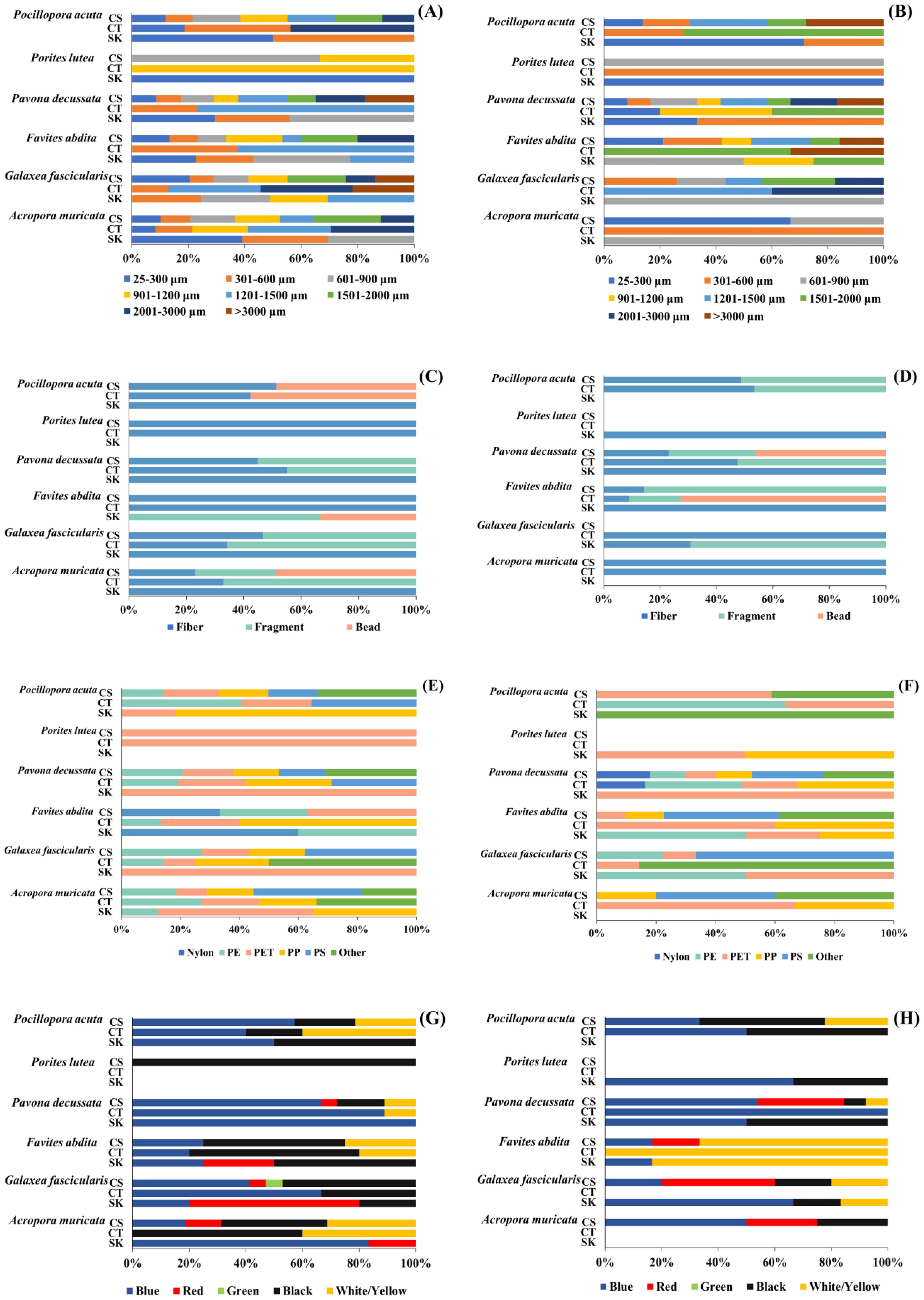
Figure 5. Relative proportions of different microplastic size [(A): Ko Khang Khao, (B): Ko Ngam Yai], shapes [(C): Ko Khang Khao, (D): Ko Ngam Yai], polymer types [(E): Ko Khang Khao, (F): Ko Ngam Yai], and colors [(G): Ko Khang Khao, (H): Ko Ngam Yai], obtained on the coral surface, coral tissue, and skeleton of different coral genera.
Microplastic (MP) shape distributions varied among coral compartments and between the two reef sites. Across all coral species and tissue layers at both Ko Khang Khao and Ko Ngam Yai, MPs with fibrous morphology were consistently the most abundant. At Ko Khang Khao, a relatively high proportion of bead-shaped MPs was detected in P. acuta and F. abdita, particularly in coral tissue (CT) and skeleton (SK), while moderate levels of fragments were also observed in P. decussata and G. fascicularis (Figure 5C). At Ko Ngam Yai, fragment-shaped MPs were more frequently encountered, especially in P. decussata and G. fascicularis (CT and CS). In contrast, F. abdita exhibited a high proportion of beads in both tissue and skeletal compartments (Figure 5D). Although some variation in shape composition was noted between sites and species, fibrous MPs remained the dominant form overall, suggesting that textile-related sources such as synthetic fibers from clothing and fishing gear are the primary contributors. The presence of bead-shaped MPs in certain coral species may indicate localized contamination from personal care products or industrial microbeads.
Polymer composition of microplastics (MPs) varied among coral species, tissue compartments, and reef sites. At Ko Khang Khao, PET was the dominant polymer, comprising 66.67% in the coral surface (CS) of P. lutea and 55.56% in F. abdita. PE was abundant in the coral tissue (CT) of P. acuta (57.14%) and the CS of G. fascicularis (71.43%). The skeleton (SK) of F. abdita displayed a diverse polymer mix, 50% nylon, 33.33% PE, and 16.67% PET, while P. decussata exhibited high levels of PP (55%), PS (50%), and other polymers (100%) in the CS (Figure 5E). At Ko Ngam Yai, PET dominated in P. lutea (100% in SK), whereas G. fascicularis showed a balanced composition of PE and PET (66.67% each in SK). Nylon was prominent in P. decussata (75% in CS) and F. abdita (100% in CS). A. muricata presented high PS levels (50% in CT; 100% in CS) (Figure 5E). Overall, PET and PE were the most prevalent polymers, reflecting their widespread use in consumer plastics. The presence of nylon (50–100%) and PS in several species suggests localized contamination from various sources such as fishing gear, textiles, and foam packaging, etc. These findings highlight the complexity of microplastic pollution in coral reef ecosystems, influenced by species-specific and site-dependent factors.
The proportional distribution of microplastic (MP) colors detected in the coral surface (CS), tissue (CT), and skeleton (SK) of six scleractinian coral species from Ko Khang Khao (Figure 5G) and Ko Ngam Yai (Figure 5H). Across both sites, blue MPs were the most abundant color across nearly all coral species and compartments, particularly in P. acuta, P. decussata, and A. muricata. Black and white/yellow particles were also frequently observed, especially in P. lutea, F. abdita, and G. fascicularis. Notably, red and green MPs occurred in lower proportions but were more evident in Ko Khang Khao, particularly within the tissue and skeleton of G. fascicularis and A. muricata. In contrast, Ko Ngam Yai showed a relatively more uniform distribution dominated by blue and white/yellow MPs, with fewer instances of red particles. Green-colored microplastic particles were not observed in any coral samples collected from Ko Ngam Yai. Color variability was greater in Ko Khang Khao, suggesting potentially more diverse MP sources or environmental dynamics influencing color deposition.
Abundance of MPs in seawater and marine sediment
Analyzing 10 seawater samples revealed that MP abundance varied from 0.88 to 1.61 particles m-³. MP concentrations in seawater were measured at 1.61 particles m-3 at Ko Khang Khao and 0.88 items/m³ at Ko Ngam Yai. Differences in MP size distribution, shape, polymer composition, and color were observed between the two sites. At Ko Khang Khao, MPs were predominantly found in the 1201–1500 μm (27.45%) and 301–600 μm (19.61%) size classes, with considerable proportions also in the 901–1200 μm (13.73%) and >3000 μm (9.80%) ranges. The dominant shape was fibers (96.08%), while fragments accounted for only 3.92%. The most abundant polymer types were polyethylene (PE; 45.10%), polyethylene terephthalate (PET; 27.45%), and nylon (9.80%). The predominant color was blue (74.51%), followed by red and green (each 7.84%). At Ko Ngam Yai, MPs were generally smaller, with the highest proportions in the 1201–1500 μm (25.00%) and 2001–3000 μm (21.43%) size classes. Similar to Ko Khang Khao, fibers were the dominant shape (92.86%), although a slightly higher proportion of fragments was observed (7.14%). PE remained the most abundant polymer (46.43%), followed by polypropylene (PP; 14.29%), nylon (17.86%), and PET (10.71%). Blue-colored MPs were most prevalent (71.43%), with red and black particles each accounting for 10.71%. Overall, microplastic contamination at both sites was characterized by the predominance of fiber-shaped particles, with polyethylene (PE) and blue-colored MPs representing the most prevalent types. These results indicate the presence of common anthropogenic sources, most likely associated with textile-derived fibers and plastic packaging materials discharged into the surrounding coastal marine environment.
Based on the analysis of 16 sediment samples, the abundance ranged between 164.80 and 295.20 particles/kg, highest at Ko Khang Khao and lowest at Ko Ngam Yai. The characteristics of microplastic (MP) particles, including size, shape, polymer type, and color, revealed notable differences between Ko Ngam Yai and Ko Khang Khao. In terms of size distribution, MPs in Ko Khang Khao were predominantly small, with the majority falling within the 25–300 µm (45.76%) and 301–600 µm (33.90%) ranges. In contrast, Ko Ngam Yai exhibited a more diverse size profile, with greater proportions of particles in the 601–900 µm (24.24%), 901–1200 µm (15.15%), and 2001–3000 µm (18.18%) categories, suggesting potential differences in fragmentation processes or sources. Particles larger than 3000 µm were uncommon at both sites. Regarding particle morphology, fibers overwhelmingly dominated at both locations, accounting for 90.91% of MPs at Ko Ngam Yai and 88.14% at Ko Khang Khao. Only minor proportions of fragments and beads were observed in both areas. The polymer composition also differed between the sites. At Ko Ngam Yai, polyethylene terephthalate (PET) was the most common polymer type (45.45%), followed by polypropylene (PP; 27.27%), polystyrene (PS; 6.06%), polyethylene (PE; 3.03%), and others (9.09%). In comparison, MPs at Ko Khang Khao were dominated by PET at an even higher proportion (64.41%) and exhibited a more diverse polymer profile, including PP (10.17%), PE (6.78%), PS (6.39%), nylon (5.08%), and others (7.17%). Color analysis showed that blue particles were the most abundant at both sites, comprising 72.73% at Ko Ngam Yai and 62.71% at Ko Khang Khao. Black particles were more frequent at Ko Ngam Yai (21.21%) than at Ko Khang Khao (13.56%), while red, green, and white/yellow particles were observed at lower frequencies. Notably, green microplastics were absent in samples from Ko Ngam Yai, whereas they were present in small amounts (1.69%) at Ko Khang Khao.
Overall, microplastic (MP) abundance was highest in sediments, followed by corals and seawater. Ko Khang Khao clearly showed higher MP abundance than Ko Ngam Yai across all matrices (corals, seawater, and sediments). In particular, the MP abundance in corals at Ko Khang Khao was approximately 10 times higher than that at Ko Ngam Yai (Figures 6, 7).
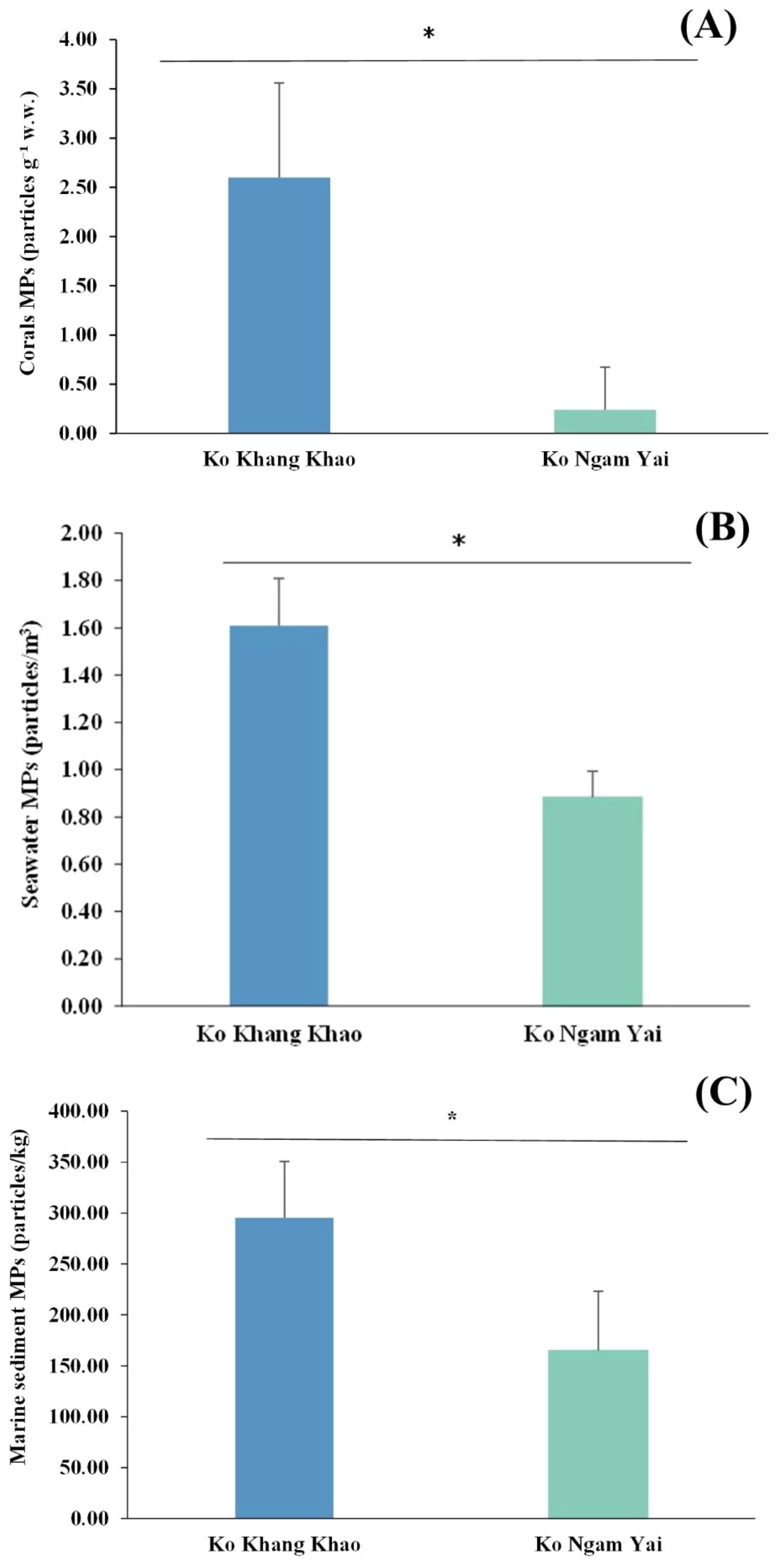
Figure 6. Differences of microplastic (MP) abundance in corals, seawater and, marine sediments between the two sampling sites. Asterisks indicate statistically significant differences based on an independent t-test. (A) microplastic (MP) in corals. (B) microplastic (MP) in seawater. (C) microplastic (MP) in marine sediment.
Figure 7. Characteristics of the microplastics (MPs) identified at coral, seawater, and marine sediment.
Discussion
Microplastics in scleractinian corals
The detection of microplastics (MPs) in all 55 coral samples analyzed in this study confirms the pervasive contamination of coral reef environments in both Ko Khang Khao and Ko Ngam Yai. A total of 184 suspected MP particles were extracted, with concentrations ranging from 0.24 to 2.60 particles g-¹ w.w., consistent with previous reports of microplastic presence in coral tissues from tropical reef ecosystems (Huang et al., 2021). These findings also align with previous research from the Gulf of Thailand, which reported significant interspecific variation in MP accumulation among four coral species, with Lobophyllia sp. and Platygyra sinensis exhibiting lower concentrations compared to Pocillopora cf. damicornis and Porites lutea. Notably, Pocillopora cf. damicornis showed the highest accumulation rate at 2.28 particles g-¹ (Jandang et al., 2024). Collectively, both studies confirm that MP accumulation is highly species-dependent, driven by traits such as polyp size, skeletal morphology, feeding strategies, and microhabitat conditions.
Spatial comparison revealed a significantly higher abundance of MPs in corals collected from Ko Khang Khao compared to Ko Ngam Yai. Notably, P. decussata, A. muricata, G. fascicularis, and F. abdita exhibited significantly greater MP accumulation at Ko Khang Khao, indicating potential site-specific factors influencing MP retention. These differences may be attributed to variations in environmental exposure, oceanographic conditions, and anthropogenic pressures. Ko Khang Khao is subject to more exposure to impacts from the mainland, which likely contributes to increased plastic debris input and subsequent microplastic fragmentation in situ (Liu et al., 2021). Conversely, Ko Ngam Yai, located farther offshore and subjected to lower human activity, showed comparatively reduced levels of microplastic contamination across most coral species. This pattern aligns with studies reporting lower MP loads in remote or less disturbed reef systems (Lin et al., 2024). The similarity in species-specific accumulation trends at both sites-particularly the consistently high MP burden observed in P. decussata and P. acuta, suggests that coral morphology and feeding strategies may also play a role in MP uptake. Encrusting and massive corals with high mucus production and sediment-trapping capacity, such as P. decussata, are more likely to retain microplastics from surrounding waters and sediments (Bejarano et al., 2022; Islam et al., 2023). The significant spatial differences observed in this study underscore the importance of local environmental factors and human activities in shaping microplastic pollution patterns in coral reef ecosystems. These findings contribute to a growing body of evidence highlighting the vulnerability of reef-building corals to microplastic contamination and emphasize the need for spatially resolved monitoring and targeted mitigation strategies in high-risk coastal zones.
The present study demonstrates significant variation in microplastic (MP) accumulation across different anatomical compartments of scleractinian corals, with the coral surface acting as the primary site for MP retention. The higher MP abundance on the coral surface than in the tissue and skeleton was consistently observed across both study sites, where the surface compartment accounted for over 60% of the total MP load-63.05% at Ko Khang Khao and 61.82% at Ko Ngam Yai. These findings align with previous reports indicating that coral surfaces serve as effective traps for MPs through passive deposition and mucus-mediated adhesion mechanisms (Bejarano et al., 2022; Reichert et al., 2022). Coral surfaces, particularly in species with intricate morphologies or high mucus production, have been shown to facilitate the adherence of MPs, often driven by weak van der Waals forces and electrical double-layer interactions (Sharifi and Khavarian-Garmsir, 2020). The coral skeletons, although more internal, contributed a moderate proportion of the total MP burden (21.38–22.94%). This suggests that MPs may infiltrate into the porous calcareous structure, potentially becoming entrapped in bioerosion cavities or skeletal interstices-a phenomenon also observed in studies by Islam et al. (2023). The lowest levels of MP accumulation were found in coral tissues (15.24–15.56%), possibly reflecting the physiological barrier posed by live tissue or the active egestion of foreign particles by coral polyps (Tang et al., 2021). Species-specific analysis further revealed significant differences in MP accumulation, with P. lutea consistently exhibiting the lowest MP levels in all anatomical compartments at both sites. This may be attributed to the dense, massive morphology of P. lutea, which presents fewer microhabitats for MP entrapment compared to branching or encrusting forms such as P. decussata or G. fascicularis. These interspecific differences support previous findings that coral morphology and surface complexity influence MP retention capacity (Huang et al., 2021). These results underscore that MP accumulation in corals is strongly compartmentalized, with surface exposure being the dominant driver of retention but modulated by species-specific traits. The differential accumulation also suggests that certain coral species may serve as more reliable indicators of MP contamination in reef monitoring efforts. Future research should explore the biological implications of MP retention in different compartments, including potential impacts on tissue integrity, calcification, and microbial symbiosis.
MP characteristics including size, shape, and polymer composition were found to differ significantly between coral compartments (surface, tissue, skeleton), coral species, and reef sites, underscoring the influence of both biological traits and localized pollution inputs. Such compartmental differentiation in MP accumulation patterns highlights the dynamic interaction between coral morphology, feeding mechanisms, skeletal porosity, and surrounding hydrodynamic conditions, which collectively modulate MP retention and exposure (Ding et al., 2019; Huang et al., 2021; Bian et al., 2024).The variation in MP shape across coral compartments and reef sites reflects complex interactions between MPs and coral biology. Fibrous MPs were consistently dominant across all coral species and compartments, aligning with global trends linked to textile fibers used in fishing gear (Vered and Shenkar, 2023; He et al., 2024). Their prevalence indicates high mobility and entanglement potential, posing risks of abrasion and sublethal stress (Cordova et al., 2018; Rahman et al., 2023; Axworthy et al., 2024). Bead-shaped MPs were notably abundant in P. acuta and F. abdita, likely from localized sources such as personal care products and industrial microbeads (Wu et al., 2018; Xu et al., 2022, 2023). These smooth, spherical MPs are easily entrapped in coral mucus or skeletal pores. Fragment-shaped MPs were more common in P. decussata and G. fascicularis, especially at Ko Ngam Yai, where coastal activities may accelerate plastic fragmentation and deposition (John et al., 2022; Zhang et al., 2023b). The morphological diversity observed emphasizes the role of coral traits and hydrodynamic conditions in MP retention. Understanding MP shape is critical for source attribution and impact prediction. Future studies should combine shape, polymer, and chemical profiles for effective mitigation strategies.
The variation in polymer composition across coral species and compartments reflects the diversity of local microplastic (MP) sources and coral morphology. PET and PE were the dominant polymers, consistent with their widespread use in packaging, textiles, and fishing gear (Ding et al., 2019; Oldenburg et al., 2021). Coral tissues and surfaces retained higher proportions of PET and PE, whereas coral skeletons, particularly in F. abdita and P. decussata showed high levels of nylon and PS, indicating input from fishing lines and foam products (Tan et al., 2020; Lo Patterson et al., 2022). Species-specific differences, such as the exclusive presence of nylon in F. abdita at Ko Ngam Yai, suggest localized contamination patterns. The physical complexity and porosity of coral skeletons likely facilitate passive entrapment of denser MPs (Mouchi et al., 2019). The presence of multiple polymer types in a single colony highlights the potential risk to corals. Ecologically, polymers like PS and nylon may cause oxidative stress, inflammation, and bleaching (Reichert et al., 2022; Yen et al., 2024). These results emphasize the need for site-specific mitigation, particularly in areas of intense coastal activity.
The color distribution of microplastics (MPs) observed in coral samples from Ko Khang Khao and Ko Ngam Yai revealed distinct patterns that may reflect both environmental conditions and sources of plastic input. Across both locations, blue MPs were the most dominant color found across nearly all coral species and anatomical compartments, particularly in P. acuta, P. decussata, and A. muricata. The prevalence of blue particles has been consistently reported in marine microplastic studies and is commonly associated with synthetic fibers originating from textiles and fishing gear (Lin et al., 2019; Vassallo et al., 2020). Given the high proportion of blue MPs observed on coral surfaces and in internal compartments, it is plausible that these particles originated from both local artisanal fishing activities and land-based sources, which are known contributors to synthetic fiber discharge into coastal waters (Zhang et al., 2019). Black and white/yellow MPs were also frequently detected, especially in P. lutea, F. abdita, and G. fascicularis. These color categories have been linked to degraded packaging materials, rubber products, and low-density polyethylene, which often fragment into small pigmented particles under UV exposure and physical abrasion (Frias and Nash, 2019; Saliu et al., 2019). The consistent occurrence of black and yellow/white MPs across species and compartments may reflect long-term accumulation and persistence of weathered plastic debris in the reef environment. Interestingly, red and green MPs were observed in lower proportions and were largely restricted to samples from Ko Khang Khao-particularly within the tissue and skeleton of G. fascicularis and A. muricata. The absence of green MPs in all coral samples from Ko Ngam Yai and the overall reduced color diversity at that site suggest that Ko Khang Khao receives a broader and more variable array of MP inputs. This may be attributed to higher anthropogenic activity, including to mainland waste outflows, which increase the diversity of plastic debris entering the reef system (Wang, 2025). In contrast, Ko Ngam Yai exhibited a more uniform color distribution dominated by blue and white/yellow MPs, consistent with lower disturbance levels and fewer direct pollution sources. Color diversity is increasingly recognized as a proxy for identifying microplastic sources and degradation states (Büks and Kaupenjohann, 2021; Su et al., 2024). For instance, brightly colored MPs such as red and green are often derived from single-use packaging and consumer goods, which degrade more rapidly and are often less persistent than industrially pigmented plastics (Primpke et al., 2018). The detection of such colors in coral tissue and skeleton at Ko Khang Khao may indicate more recent or ongoing plastic deposition, as well as enhanced particle retention within certain coral morphologies that promote microhabitat formation and sediment entrapment (Reichert et al., 2022). Overall, the observed spatial variation in MP color composition reflects the combined influence of coral species traits, hydrodynamic conditions, and site-specific anthropogenic inputs. Ko Khang Khao, characterized by higher human presence and greater color variability, appears to be more vulnerable to diverse sources of plastic pollution, whereas Ko Ngam Yai, with lower diversity and absence of certain pigments (e.g., green), represents a comparatively less impacted reef system. These findings reinforce the need for spatially explicit monitoring of MP characteristics as indicators of pollution sources and reef health.
Microplastics in seawater and marine sediment
The concentrations and characteristics of microplastics (MPs) in surface seawater at Ko Khang Khao and Ko Ngam Yai reflect spatial heterogeneity shaped by local anthropogenic activities and environmental dynamics in the Gulf of Thailand. The overwhelming dominance of fiber-shaped MPs exceeding 90% at both sites corroborates global observations that synthetic fibers are the most prevalent microplastic type in marine environments. These fibers are commonly shed from household laundry, wastewater effluent, and degraded fishing gear, particularly in coastal areas with significant human activity (Pan et al., 2023; Feng et al., 2024). Polyethylene (PE) was the most frequently detected polymer, which is consistent with its widespread use in consumer packaging and fishing-related products. Its low density and high environmental persistence contribute to its prevalence in surface waters (Bhore and Kamble, 2022; Chen et al., 2024). The predominance of blue-colored MPs at both sites typically associated with fishing ropes and nets further supports the hypothesis of marine-based sources, particularly from small-scale fisheries common in this region (Belioka and Achilias, 2024; Zhu et al., 2024). Interestingly, Ko Ngam Yai exhibited a slightly different MP profile, characterized by a greater proportion of fragments, higher concentrations of polypropylene (PP), and a tendency toward smaller particle sizes. This variation may be indicative of more intense plastic degradation processes, possibly driven by boat traffic, wave action, or nearshore runoff that accelerates the breakdown of macroplastics (Taha et al., 2021; Lin et al., 2022; Xue et al., 2024). The higher proportion of nylon in this area also suggests closer proximity to fishing grounds or aquaculture infrastructure, which are known sources of nylon ropes and nets. Overall, the observed differences in MP assemblages between Ko Khang Khao and Ko Ngam Yai underscore the pivotal role of localized hydrodynamics, land–sea interactions, and pollutant inputs in shaping contamination patterns. In the Gulf of Thailand, monsoon-driven circulation regulates water transport, vertical exchange, and particle retention (Sojisuporn et al., 2010), thereby driving seasonal variability in MP distribution. During the southwest monsoon (May–September), counterclockwise circulation, mesoscale eddies, and localized upwelling along the western margin enhance resuspension and land-derived inputs, leading to elevated MP retention in western reef habitats such as Ko Khang Khao. By contrast, the northeast monsoon (November–February) promotes clockwise circulation, intensified coastal currents, and widespread downwelling, facilitating offshore export of buoyant plastics and reducing accumulation at sites such as Ko Ngam Yai.
The presence of MPs in sediment samples from both Ko Khang Khao and Ko Ngam Yai highlights the widespread distribution of plastic contaminants in reef-associated benthic environments. A spatial variation in microplastic abundance was evident, ranging from 164.80 to 295.20 particles kg-¹, with Ko Khang Khao showing markedly higher concentrations than Ko Ngam Yai. These findings are consistent with previous studies indicating that nearshore environments with greater anthropogenic activity, such as tourism and small-scale fishing, may accumulate more MPs in sediments due to increased input from terrestrial runoff, vessel traffic, and on-site littering (Bergmann et al., 2019; Zhou et al., 2020; Zhang et al., 2025). Distinct differences in MP characteristics between the two sites further suggest variability in sources and environmental dynamics. Ko Khang Khao sediments were dominated by smaller particles (25–600 µm), indicating either more advanced fragmentation or continual abrasion processes that break down larger plastics into micro-sized debris. Conversely, Ko Ngam Yai exhibited a broader size distribution, including larger particles up to 3000 µm, suggesting either newer inputs or reduced degradation pressures, which may be attributed to its relatively isolated location and lower human impact. Similar spatial variations in MP size profiles have been reported in remote coral reef sediments across the Indo-Pacific and Caribbean, where particle size is closely linked to proximity to human activity and exposure to hydrodynamic forces (Kane and Clare, 2019; Kazour et al., 2019). Fibers overwhelmingly dominated MP morphology at both sites, accounting for over 88% of all particles. This is consistent with global findings that fibers, derived primarily from synthetic textiles and fishing gear, are the most prevalent MP form in marine sediments (Jang et al., 2018; Xu et al., 2020). The relatively low proportions of fragments and beads further suggest that the primary sources of MPs at both sites are fibrous materials, likely originating from wastewater effluent and degraded nets and ropes used in artisanal fisheries. Polymer analysis revealed that polyethylene terephthalate (PET) was the dominant polymer type at both sites, with particularly high representation at Ko Khang Khao (64.41%). PET is commonly used in beverage containers, textiles, and packaging and is frequently detected in coastal sediments due to its high production volume and resistance to environmental degradation (Primpke et al., 2018). The broader polymer diversity at Ko Khang Khao, including PP, PE, PS, and nylon, suggests more heterogeneous sources of plastic waste, potentially linked to nearby land-based activities, sea-based activities, and pollution.
Color analysis also reflected spatial variation in MP sources and environmental exposure. Blue particles were the most dominant at both sites, commonly associated with synthetic fibers (Frias and Nash, 2019; Ding et al., 2022). Black MPs, possibly from tire wear or degraded industrial plastics, were more abundant at Ko Ngam Yai, while red, green, and yellow/white MPs occurred in low proportions overall. Notably, green MPs were entirely absent at Ko Ngam Yai, which may reflect either a lack of certain source materials or differential degradation dynamics. Such color variability has been linked to environmental factors such as UV exposure, abrasion, and polymer aging (Li et al., 2024b). These results suggest that Ko Khang Khao is subjected to more diverse and intense anthropogenic pressures, leading to higher MP concentrations and broader variation in size, polymer, and color profiles. In contrast, Ko Ngam Yai, with its lower MP load and narrower material diversity, likely reflects a system under comparatively lower human influence. The observed spatial variation highlights the value of assessing both the quantity and traits of microplastics in sediments to trace pollution sources and guide effective interventions. Integrating these findings with MP abundance patterns suggests that Ko Khang Khao experiences more diverse and intense anthropogenic pressures, likely driven by its proximity to river mouths, coastal settlements, and active navigation routes. This setting contributes to not only higher MP concentrations but also greater diversity in particle size, polymer composition, and color profiles, which is essential for tracing pollution sources and informing targeted management interventions in marine ecosystems.
In this study, we found that polyethylene terephthalate (PET) was mostly found in marine sediment, while polypropylene (PP) was mostly found in seawater. This could be explained by the characteristics of microplastics, particularly their intrinsic density, which governs diffusion dynamics, buoyancy-driven behavior, and eventual accumulation patterns. The present findings, in line with earlier studies, reinforce that polymer-specific density exerts a primary control on microplastic mobility across environmental compartments. Denser polymers, such as polyethylene terephthalate (PET) and polyvinyl chloride (PVC), consistently display reduced transport capacity and greater propensity for deposition within porous substrates, whereas lighter polymers, including polyethylene (PE), polypropylene (PP) and polymethyl methacrylate (PMMA), exhibit comparatively higher mobility under equivalent physicochemical conditions (Li et al., 2024a; Cao et al., 2023). In aquatic environments, the same density-driven patterns remain evident. MPs with densities below that of water (e.g., PP) often persist in suspension or at the air–water interface, enhancing their potential for long-distance dispersal via surface currents and facilitating bioavailability to pelagic organisms. Conversely, high-density polymers are more susceptible to gravitational settling, particularly under conditions of reduced hydrodynamic energy, resulting in enhanced sedimentation rates and accumulation hotspots in benthic habitats (Sheela et al., 2022; Stride et al., 2024).
In conclusion, this study investigates the occurrence, abundance, and characteristics of microplastics (MPs) in coral reef ecosystems at two reef sites, Ko Khang Khao and Ko Ngam Yai, in the Gulf of Thailand. MPs were detected in all coral samples, with concentrations ranging from 0.24 to 2.60 particles g-¹ w.w. Ko Khang Khao exhibited higher MP concentrations across coral species, with Pavona decussata, Pocillopora acuta, and Galaxea fascicularis showing the greatest accumulation. Coral surface was the dominant compartment for MP accumulation, followed by skeleton and tissue. MP size distribution varied by coral compartment, with coral skeletons retaining larger particles (>3000 µm) and tissues favoring smaller MPs (25–1500 µm). Fiber-shaped MPs were predominant across all sites and compartments. Polymer analysis revealed polyethylene terephthalate (PET) and polyethylene (PE) as the most common polymers, mostly found in marine sediments, and sea water, respectively. Color analysis showed blue MPs as the most abundant, followed by black, white/yellow, red, and green particles. Across all matrices (corals, seawater, and sediment), Ko Khang Khao consistently showed higher MP concentrations and greater diversity in MP characteristics compared to Ko Ngam Yai, likely reflecting greater anthropogenic influence, particularly land-based pollution from mainland and major rivers, making coral reef systems less resilience.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. WS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. AW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. LJ: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. WA: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. SP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft. WK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft. MJ: Investigation, Methodology, Resources, Writing – original draft. LS: Investigation, Methodology, Resources, Writing – original draft. TY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by Thailand Science Research and Innovation (TSRI), the National Science, Research and Innovation Fund (NSRF) by the Program Management Unit Competitiveness (PMUC), National Research Council of Thailand (NRCT) and Ramkhamhaeng University (RU).
Acknowledgments
We are most grateful to the staffs of Department of Marine and Coastal Resources, Department of National Parks Wildlife and Plant Conservation and Marine Biodiversity Research Group, Department of Biology, Faculty of Science, Ramkhamhaeng University, for their support and assistance in the field.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to assist in refining the language, improving clarity, and formatting text in accordance with journal guidelines. All scientific content, data interpretation, and conclusions were conceived, written, and verified by the authors. The use of AI was limited to editorial support, and the authors affirm that no confidential, personal, or proprietary data were input into the AI system.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allen A. S., Seymour A. C., and Rittschof D. (2017). Chemoreception drives plastic consumption in a hard coral. Mar. pollut. Bull. 124, 198–205. doi: 10.1016/j.marpolbul.2017.07.030
Alves F. L., Pinheiro L. M., Bueno C., Agostini V. O., Perez L., Fernandes E. H. L., et al. (2023). The use of microplastics as a reliable chronological marker of the Anthropocene onset in Southeastern South America. Sci. Total Environ. 857, 159633. doi: 10.1016/j.scitotenv.2022.159633
Axworthy J. B., Lasdin K. S., and Padilla-Gamiño J. L. (2024). Low incidence of microplastics in coral reefs of Kāne'ohe Bay, Hawai'i, USA. Mar. pollut. Bull. 208, 116996. doi: 10.1016/j.marpolbul.2024.116996
Bai H., Liu B., Jiang Y., Zhang J., Zhang M., Zhang H., et al. (2025). Adsorption-desorption behavior of malachite green on aged microplastics in seawater environment. Separation Purification Technol. 354, 128991. doi: 10.1016/j.seppur.2024.128991
Beckwith S. T. and Fuentes M. M. P. B. (2018). Microplastic ingestion by marine megafauna. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00379
Bejarano S., Diemel V., Feuring A., Ghilardi M., and Harder T. (2022). No short-term effect of sinking microplastics on heterotrophy or sediment clearing in the tropical coral Stylophora pistillata. Sci. Rep. 12, 1468. doi: 10.1038/s41598-022-05420-7
Belioka M. P. and Achilias D. S. (2023). Microplastic pollution and monitoring in seawater and harbor environments: A meta-analysis and review. Sustainability 15 (11), 9079. doi: 10.3390/su15119079
Belioka M. P. and Achilias D. S. (2024). The effect of weathering conditions in combination with natural phenomena/disasters on microplastics’ transport from aquatic environments to agricultural soils. Microplastics 3, 518–538. doi: 10.3390/microplastics3030033
Bergmann M., Gutow L., and Klages M. (2019). Marine anthropogenic litter (Berlin: Springer). doi: 10.1007/978-3-319-71279-6
Berry K. L., Epstein H. E., Lewis P. J., Hall N. M., and Negri A. P. (2019). Microplastic contamination has limited effects on coral fertilisation and larvae. Diversity 11, 228. doi: 10.3390/d11120228
Bhore R. K. and Kamble S. B. (2022). Nano adsorptive extraction of diverse microplastics from the potable and seawater using organo-polyoxometalate magnetic nanotricomposites. J. Environ. Chem. Eng. 10, 108720. doi: 10.1016/j.jece.2022.108720
Bian W., Zeng Y., Li Y., Na G., Mu J., Lv S., et al. (2024). Microplastic pollution in tropical coral reef ecosystems from the coastal South China Sea and their impacts on corals in situ. J. Hazardous Materials 480, 135898. doi: 10.1016/j.jhazmat.2024.135898
Biswas T., Pal S. C., Saha A., Ruidas D., Shit M., Islam A. R. M. T., et al. (2024). Microplastics in the coral ecosystems: a threat which needs more global attention. Ocean Coast. Manage. 249, 107012. doi: 10.1016/j.ocecoaman.2023.107012
Bucci K., Tulio M., and Rochman C. M. (2020). What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 30, e02044. doi: 10.1002/eap.2044
Büks F. and Kaupenjohann M. (2021). The impact of microplastic weathering on interactions with the soil environment: A review. Soil Discussions 2021, 1–22. doi: 10.5194/soil-8-373-2022
Cao S., Wang L., Suakollie E. B., Wu H., and Yu Y. (2023). Transport of polypropylene, polyvinyl chloride, polyethylene terephthalate and polymethyl methacrylate microplastics in porous media under gradient ionic strength. Environ. Pollutants Bioavailability. 35, 2269315. doi: 10.1080/26395940.2023.2269315
Chamchoy C., Sutthacheep M., Sangiamdee D., Musig W., Boonmewisate S., Sangsawang L., et al. (2018). Microplastics in scleractinian corals from the upper Gulf of Thailand. Ramkhamhaeng Int. J. Sci. Technol. 1, 1–8.
Chapron L., Peru E., Engler A., Ghiglione J. F., Meistertzheim A. L., Laroche J., et al. (2018). Macro- and microplastics affect cold-water corals growth, feeding and behaviour. Sci. Rep. 8, 15299. doi: 10.1038/s41598-018-33683-6
Chen D., Wang P., Liu S., Wang R., Wu Y., Zhu A. X., et al. (2024). Global patterns of lake microplastic pollution: Insights from regional human development levels. Sci. Total Environ. 954, 176620. doi: 10.1016/j.scitotenv.2024.176620
Cole M., Lindeque P., Halsband C., and Galloway T. S. (2011). Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62 (12), 2588–2597. doi: 10.1016/j.marpolbul.2011.09.025
Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., et al. (2015). Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ. Sci. Technol. 50, 3239–3246. doi: 10.1021/acs.est.5b05905
Corami F., Rosso B., Bravo B., Gambaro A., and Barbante C. (2020). A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR. Chemosphere 238, 124564. doi: 10.1016/j.chemosphere.2019.124564
Cordova M. R., Hadi T. A., and Prayudha B. (2018). Occurrence and abundance of microplastics in coral reef sediment: a case study in Sekotong, Lombok-Indonesia. AES Bioflux 10, 23–29. doi: 10.5281/zenodo.1297719
Cutroneo L., Reboa A., Besio G., Borgogno F., Canesi L., Canuto S., et al. (2020). Microplastics in seawater: sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ. Sci. pollut. Res. 27, 8938–8952. doi: 10.1007/s11356-020-07783-8
de Smit J. C., Nyström M., Hansson L. A., and Ahlgren G. (2021). Anthropogenic stressors interacting with climate change to affect coral reefs. Ambio 50, 1589–1600. doi: 10.1016/j.scitotenv.2021.145520
Ding J., Jiang F., Li J., Wang Z., Sun C., Wang Z., et al. (2019). Microplastics in the coral reef systems from Xisha Islands of South China Sea. Environ. Sci. Technol. 53, 8036–8046. doi: 10.1021/acs.est.9b01452
Ding Y., Zou X., Chen H., Yuan F., Liao Q., Feng Z., et al. (2022). Distribution pattern and influencing factors for the microplastics in continental shelf, slope, and deep-sea surface sediments from the South China Sea. Environ. pollut. 309, 119824. doi: 10.1016/j.envpol.2022.119824
do Amparo S. Z., Carvalho L. D. O., Silva G. G., and Viana M. M. (2023). Microplastics as contaminants in the Brazilian environment: an updated review. Environ. Monit. Assess. 195, 1414. doi: 10.1007/s10661-023-12011-0
Eo S., Hong S. H., Cho Y., Song Y. K., Han G. M., and Shim W. J. (2023). Spatial distribution and historical trend of microplastic pollution in sediments from enclosed bays of South Korea. Mar. pollut. Bull. 193, 115121. doi: 10.1016/j.marpolbul.2023.115121
Eriksen M., Lebreton L. C. M., Carson H. S., Thiel M., Moore C. J., Borerro J. C., et al. (2014). Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PloS One 9, e111913. doi: 10.1371/journal.pone.0111913
Fabres J., Savelli H., Schoolmeester T., Rucevska I., and Baker E. (2016). Marine litter vital graphics. United Nations Environ. Programme GRID-Arendal Nairobi Arendal. 60. doi: 10.13140/RG.2.2.20593.89442
Feng Q., Chen Z., Huang G., An C., Yang X., and Wang Z. (2024). Prolonged drying impedes the detachment of microplastics in unsaturated substrate: Role of flow regimes. Water Res. 252, 121246. doi: 10.1016/j.watres.2024.121246
Ferrier-Pagès C., Hoogenboom M., Houlbrèque F., Reynaud S., and Tambutté E. (2011). The role of plankton in coral trophodynamics. Coral Reefs 30, 689–701. doi: 10.1007/978-94-007-0114-4_15
Frias J. P. and Nash R. (2019). Microplastics: Finding a consensus on the definition. Mar. pollut. Bull. 138, 145–147. doi: 10.1016/j.marpolbul.2018.11.022
GESAMP (2015). “Sources, fate and effects of microplastics in the marine environment: A global assessment,” in Joint group of experts on the scientific aspects of marine environmental protection, rep. Stud., vol. 90 . Ed. Kershaw P. J. (London: GESAMP).
Grillo J. A., Silva C. V., Marques M. R. C., and da Gama B. A. P. (2021). Microplastics can act as a vector of coral pathogens. Mar. pollut. Bull. 171, 112743. doi: 10.1016/j.marpolbul.2021.112743
He J., Ma C., Zhao Z., Nie Y., Liu X., Xu L., et al. (2024). Record of microplastic deposition revealed by ornithogenic soil and sediment profiles from Ross Island, Antarctica. Environ. Res. 262, 119971. doi: 10.1016/j.envres.2024.119971
Hollerová A., Hodkovicová N., Blahová J., Faldyna M., Maršálek P., and Svobodová Z. (2021). Microplastics as a potential risk for aquatic environment organisms – a review. Acta Vet. Brno 90, 99–107. doi: 10.2754/avb202190010099
Houlbr`eque F. and Ferrier-Pag`es C. (2009). Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17. doi: 10.1111/j.1469-185X.2008.00058.x
Huang W., Chen M., Song B., Deng J., Shen M., Chen Q., et al. (2021). Microplastics in the coral reefs and their potential impacts on corals: a mini-review. Sci. Total Environ. 762, 143112. doi: 10.1016/j.scitotenv.2020.143112
Hughes T. P., Kerry J. T., Álvarez-Noriega M., Álvarez-Romero J. G., Anderson K. D., Baird A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Islam M. S., Islam A. R. M. T., Ismail Z., Ahmed M. K., Ali M. M., Kabir M. H., et al. (2023). Effects of microplastic and heavy metals on coral reefs: A new window for analytical research. Heliyon 9, e22692. doi: 10.1016/j.heliyon.2023.e22692
Jandang S., Alfonso M. B., Nakano H., Phinchan N., Darumas U., Viyakarn V., et al. (2024). Possible sink of missing ocean plastic: Accumulation patterns in reef-building corals in the Gulf of Thailand. Sci. Total Environ. 954, 176210. doi: 10.1016/j.scitotenv.2024.176210
Jang M., Shim W. J., Han G. M., Song Y. K., and Hong S. H. (2018). Formation of microplastics by polychaetes (Marphysa sanGuinea) inhabiting expanded polystyrene marine debris. Mar. pollut. Bull. 131, 365–369. doi: 10.1016/j.marpolbul.2018.04.017
John J., Nandhini A. R., Velayudhaperumal Chellam P., and Sillanpää M. (2022). Microplastics in mangroves and coral reef ecosystems: a review. Environ. Chem. Lett. 20, 397–416. doi: 10.1007/s10311-021-01326-4
Kane I. A. and Clare M. A. (2019). Dispersion, accumulation, and the ultimate fate of microplastics in deep-marine environments: a review and future directions. Front. Earth Sci. 7. doi: 10.3389/feart.2019.00080
Kane I. A., Clare M. A., Miramontes E., Wogelius R., Rothwell J. J., Garreau P., et al. (2020). Seafloor microplastic hotspots controlled by deep-sea circulation. Science 368, 1140–1145. doi: 10.1126/science.aba5899
Kazour M., Jemaa S., Issa C., Khalaf G., and Amara R. (2019). Microplastics pollution along the Lebanese coast (Eastern Mediterranean Basin): Occurrence in surface water, sediments and biota samples. Sci. Total Environ. 696, 133933. doi: 10.1016/j.scitotenv.2019.133933
Kirstein I. V., Kirmizi S., Wichels A., Garin-Fernandez A., Erler R., Löder M., et al. (2016). Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 120, 1–8. doi: 10.1016/j.marenvres.2016.07.004
Lamb J. B., Willis B. L., Fiorenza E. A., Couch C. S., Howard R., Rader D. N., et al. (2018). Plastic waste associated with disease on coral reefs. Sci 359, 460–462. doi: 10.1126/science.aar3320
Li W., Brunetti G., Bolshakova A., and Stumpp C. (2024a). Effect of particle density on microplastics transport in artificial and natural porous media. Sci. Total Environ. 935, 173429. doi: 10.1016/j.scitotenv.2024.173429
Li Z., Zheng Y., Maimaiti Z., Fu J., Yang F., Li Z.-Y., et al. (2024b). Identification and analysis of microplastics in human lower limb joints. J. Hazardous Materials 461, 132640. doi: 10.1016/j.jhazmat.2023.132640
Lin L., Li H., Hong H., Yuan B., Sun X., He L., et al. (2022). Enhanced heavy metal adsorption on microplastics by incorporating flame retardant hexabromocyclododecanes: Mechanisms and potential migration risks. Water Res. 225, 119144. doi: 10.1016/j.watres.2022.119144
Lin X., Lin S., Peng L., Chen M., Cheng X., Xie S., et al. (2024). Effects of polypropylene microplastics on carbon dioxide dynamics in intertidal mangrove sediments. Environ. pollut. 346, 123682. doi: 10.1016/j.envpol.2024.123682
Lin Y., Zheng L., Zheng Z., Wu Y., Hu Z., Yan C., et al. (2019). Improving person re-identification by attribute and identity learning. Pattern recognition 95, 151–161. doi: 10.1016/j.patcog.2019.06.006
Liu J., Liu Q., An L., Wang M., Yang Q., Zhu B., et al. (2022). Microfiber pollution in the earth system. Rev. Environ. Contam. Toxicol. 260, 13. doi: 10.1007/s44169-022-00015-9
Liu Y., Liu W., Yang X., Wang J., Lin H., and Yang Y. (2021). Microplastics are a hotspot for antibiotic resistance genes: Progress and perspective. Sci. Total Environ. 773, 145643. doi: 10.1016/j.scitotenv.2021.145643
Long M., Moriceau B., Gallinari M., Lambert C., Huvet A., Raffray J., et al. (2015). Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar. Chem. 175, 39–46. doi: 10.1016/j.marchem.2015.04.003
Lusher A. L., Hollman P. C. H., and Mendoza-Hill J. J. (2017). “Microplastics in fisheries and aquaculture: status of knowledge on their occurrence and implications for aquatic organisms and food safety,” in FAO fish. Aquac. Tech. Pap. No. 615 (FAO, Rome).
Machendiranathan M., Jin G., Huang H., Liang T., Lin Z., Lin H., et al. (2024). Vertical distribution of microplastics in coastal sediments of Xuwen Coral Reef National Nature Reserve, China. J. Oceanol. Limnol. 43, 422–432. doi: 10.1007/s00343-024-3292-9
Masura J., Baker J., Foster G., and Arthur C. (2015). Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments. NOAA Tech. Memorandum NOS-OR&R-48 Silver Spring, MD, United States, 31. doi: 10.25607/OBP-604
Mathalon A. and Hill P. (2014). Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 81 (1), 69–79. doi: 10.1016/j.marpolbul.2014.02.018
Matupang D. M., Zulkifli H. I., Arnold J., Lazim A. M., Ghaffar M. A., and Musa S. M. (2023). Tropical sharks feasting on and swimming through microplastics: first evidence from Malaysia. Mar. pollut. Bull. 189, 114762. doi: 10.1016/j.marpolbul.2023.114762
Mouchi V., Chapron L., Peru E., Pruski A. M., Meistertzheim A. L., Vétion G., et al. (2019). Long-term aquaria study suggests species-specific responses of two cold-water corals to macro- and microplastics exposure. Environ. pollut. 253, 322–329. doi: 10.1016/j.envpol.2019.07.024
Murano C., Agnisola C., Caramiello D., Castellano I., Casotti R., Corsi I., et al. (2020). How sea urchins face microplastics: uptake, tissue distribution and immune system response. Environ. pollut. 264, 114685. doi: 10.1016/j.envpol.2020.114685
Okubo N., Takahashi S., and Nakano Y. (2018). Microplastics disturb the anthozoan–dinoflagellate symbiotic relationship. Mar. pollut. Bull. 135, 83–89. doi: 10.1016/j.marpolbul.2018.07.016
Oldenburg K. S., Urban-Rich J., Castillo K. D., and Baumann J. H. (2021). Microfiber abundance associated with coral tissue varies geographically on the Belize Mesoamerican Barrier Reef System. Mar. pollut. Bull. 163, 111938. doi: 10.1016/j.marpolbul.2020.111938
Pabortsava K. and Lampitt R. S. (2020). High concentrations of plastic hidden beneath the surface of the Atlantic. Ocean. Nat. Commun. 11, 4073. doi: 10.1038/s41467-020-17932-9
Pan T., Liao H., Yang F., Sun F., Guo Y., Yang H., et al. (2023). Review of microplastics in lakes: sources, distribution characteristics, and environmental effects. Carbon Res. 2, 25. doi: 10.1007/s44246-023-00057-1
Patterson J., Jeyasanta K. I., Laju R. L., Booth A. M., Sathish N., and Edward J. P. (2022). Microplastic in the coral reef environments of the Gulf of Mannar, India—Characteristics, distributions, sources and ecological risks. Environ. pollut. 298, 118848. doi: 10.1016/j.envpol.2022.118848
Peng L., Fu D., Qi H., Lan C. Q., Yu H., and Ge C. (2020). Micro- and nano-plastics in marine environment: source, distribution and threats—A review. Sci. Total Environ. 698, 134254. doi: 10.1016/j.scitotenv.2019.134254
Piskuła P., Astel A., and Pawlik M. (2025). Microplastics in seawater and fish acquired from the corresponding fishing zones of the Baltic Sea. Mar. pollut. Bull. 211, 117485. doi: 10.1016/j.marpolbul.2024.117485
Pittura L., Avio C. G., Giuliani M. E., D’Errico G., Keiter S. H., and Regoli F. (2018). Microplastics as vehicles of environmental PAHs to marine organisms. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00103
Primpke S., Wirth M., Lorenz C., and Gerdts G. (2018). Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Analytical bioanalytical Chem. 410, 5131–5141. doi: 10.1007/s00216-018-1156-x
Rahman M. N., Shozib S. H., Akter M. Y., Islam A. R. M. T., Islam M. S., Sohel M. S., et al. (2023). Microplastic as an invisible threat to the coral reefs: sources, toxicity mechanisms, policy intervention, and the way forward. J. Hazard. Mater. 454, 131522. doi: 10.1016/j.jhazmat.2023.131522
Reichert J., Arnold A. L., Hammer N., Miller I. B., Rades M., Schubert P., et al. (2022). Reef-building corals act as long-term sink for microplastic. Global Change Biol. 28, 33–45. doi: 10.1111/gcb.15920
Reichert J., Arnold A. L., Hoogenboom M. O., and Schubert P. R. (2019). Impacts of microplastics on growth and health of hermatypic corals are species-specific. Environ. pollut. 254, 113074. doi: 10.1016/j.envpol.2019.113074
Reichert J., Schellenberg J., Schubert P., and Wilke T. (2018). Responses of reef building corals to microplastic exposure. Environ. pollut. 237, 955–960. doi: 10.1016/j.envpol.2017.11.006
Rellán A. G., Ares D. V., Brea C. V., López A. F., and Bugallo P. M. B. (2023). Sources, sinks and transformations of plastics in our oceans: review, management strategies and modelling. Sci. Total Environ. 854, 158745. doi: 10.1016/j.scitotenv.2022.158745
Reuning L., Hildebrandt L., Kersting D. K., and Pröfrock D. (2025). High levels of microplastics and microrubber pollution in a remote, protected Mediterranean Cladocora caespitosa coral bed. Mar. pollut. Bull. 217, 118070. doi: 10.1016/j.marpolbul.2025.118070
Rochman C. M., Hoh E., Hentschel B. T., and Kaye S. (2013). Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ. Sci. Technol. 47, 1646–1654. doi: 10.1021/es303700s
Rohais S., Armitage J. J., Romero-Sarmiento M. F., Pierson J. L., Teles V., Bauer D., et al. (2024). A source-to-sink perspective of an anthropogenic marker: a first assessment of microplastics concentration, pathways, and accumulation across the environment. Earth-Sci. Rev. 254, 104822. doi: 10.1016/j.earscirev.2024.104822
Saliu F., Montano S., Leoni B., Lasagni M., and Galli P. (2019). Microplastics as a threat to coral reef environments: detection of phthalate esters in neuston and scleractinian corals from the Faafu Atoll, Maldives. Mar. pollut. Bull. 142, 234–241. doi: 10.1016/j.marpolbul.2019.03.043
Sarvajayakesavalu S., Chareonsudjai P., Mongkonthum W., and Mathaiah R. (2023). A perspective study on occurrence, impacts and sources of microplastics in the marine environment of south China Sea and Gulf of Thailand. Res. J. Chem. Environ. 27, 6. doi: 10.25303/2706rjce1120125
Savinelli B., Vega Fernández T., Galasso N. M., D’Anna G., Pipitone C., Prada F., et al. (2020). Microplastics impair the feeding performance of a Mediterranean habitat-forming coral. Mar. Environ. Res. 155, 104887. doi: 10.1016/j.marenvres.2020.104887
Sharifi A. and Khavarian-Garmsir A. R. (2020). The COVID-19 pandemic: impacts on cities and major lessons for urban planning, design, and management. Sci. Total Environ. 749, 142391. doi: 10.1016/j.scitotenv.2020.142391
Sheela A. M., Manimekalai B., and Dhinagaran G. (2022). Review on the distribution of microplastics in the oceans and its impacts: need for modeling-based approach to investigate the transport and risk of microplastic pollution. Environ. Eng. Res. 27, e2021.243. doi: 10.4491/eer.2021.243
Sheela A. and Dhinagaran G. (2023). Prevalence of microplastics, antibiotic resistant genes and microplastic associated biofilms in estuary — A review. Environ. Eng. Res. 28 (4). doi: 10.4491/eer.2022.325
Soares M. O., Rizzo L., Neto A. R. X., Barros Y., Martinelli Filho J. E., Giarrizzo T., et al. (2023). Do coral reefs act as sinks for microplastics? Environ. pollut. 337, 122509. doi: 10.1016/j.envpol.2023.122509
Sojisuporn P., Morimoto A., and Yanagi T. (2010). Seasonal variation of sea surface current in the Gulf of Thailand, Coast. Mar. Sci. 34, 91–102. Available online at: https://www.researchgate.net/publication/285650066.
Stride B., Abolfathi S., Bending G. D., and Pearson J. (2024). Quantifying microplastic dispersion due to density effects. J. Hazardous Materials 466, 133440. doi: 10.1016/j.jhazmat.2024.133440
Su Y., Yang C., Peng Y., Yang C., Wang Y., Wang Y., et al. (2024). Lensless shadow microscopy-based shortcut analysis strategy for fast quantification of microplastic fibers released to water. Water Res. 258, 121758. doi: 10.1016/j.watres.2024.121758
Sussarellu R., Suquet M., Thomas Y., Lambert C., Fabioux C., Pernet M. E.J., et al. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. U.S.A. 113, 2430–2435. doi: 10.1073/pnas.1519019113
Sutthacheep M., Phoaduang S., Chamchoy C., Klinthong W., Salakphet C., Silparasarn A., et al. (2021). The particles of microplastics in shrimp paste from the Gulf of Thailand and the Andaman Sea. Ramkhamhaeng Int. J. Sci. Technol. 4, 27–34.
Taha Z. D., Amin R. M., Anuar S. T., Nasser A. A. A., and Sohaimi E. S. (2021). Microplastics in seawater and zooplankton: a case study from Terengganu estuary and offshore waters, Malaysia. Sci. Total Environ. 786, 147466. doi: 10.1016/j.scitotenv.2021.147466
Tan F., Yang H., Xu X., Fang Z., Xu H., Shi Q., et al. (2020). Microplastic pollution around remote uninhabited coral reefs of Nansha Islands, South China Sea. Sci. Total Environ. 725, 138383. doi: 10.1016/j.scitotenv.2020.138383
Tang J., Wu Z., Wan L., Cai W., Chen S., Wang X., et al. (2021). Differential enrichment and physiological impacts of ingested microplastics in scleractinian corals in situ. J. Hazard. Mater. 404, 124205. doi: 10.1016/j.jhazmat.2020.124205
Tetu S. G., Sarker I., Schrameyer V., Pickford R., Elbourne L. D. H., Moore L. R., et al. (2019). Plastic leachates impair growth and oxygen production in Prochlorococcus, the ocean’s most abundant photosynthetic bacteria. Commun. Biol. 2, 184. doi: 10.1038/s42003-019-0410-x
Ulfah M., Juliani R., Agustina S., Purnawan S., Malayana I., Muliari M., et al. (2025). The abundance of microplastics in coral reef ecosystems in the waters of Krueng Raya, Aceh Besar. Bio Web Conf. 156, 2003. doi: 10.1051/bioconf/202515602003
Utami D. A., Reuning L., Konechnaya O., and Schwarzbauer J. (2021). Microplastics as a sedimentary component in reef systems: a case study from the Java Sea. Sedimentology 68, 2270–2292. doi: 10.1111/sed.12879
Vassallo P., Paoli C., Aliani S., Cocito S., Morri C., and Bianchi C. N. (2020). Benthic diversity patterns and predictors: a study case with inferences for conservation. Mar. pollut. Bull. 150, 110748. doi: 10.1016/j.marpolbul.2019.110748
Vered G. and Shenkar N. (2023). Plastic pollution in a coral reef climate refuge: occurrence of anthropogenic debris, microplastics, and plasticizers in the Gulf of Aqaba. Sci. Total Environ. 905, 167791. doi: 10.1016/j.scitotenv.2023.167791
Walker T. R. and Fequet L. (2023). Current trends of unsustainable plastic production and micro (nano) plastic pollution. TrAC Trends Anal. Chem. 160, 116984. doi: 10.1016/j.trac.2023.116984
Wang J. (2025). The interplay between coastal tourism, plastic pollution, and harmful algal blooms: a case study of Florida and East China Sea. Doctoral dissertation, Johns Hopkins University, Baltimore, MD, United States.
Wang Y., Zheng X., He X., Lü Q., Qian X., Xiao Q., et al. (2021). Effects of Pseudomonas TCd-1 on rice (Oryza sativa) cadmium uptake, rhizosphere soils enzyme activities and cadmium bioavailability under cadmium contamination. Ecotoxicol. Environ. Saf. 218, 112249. doi: 10.1016/j.ecoenv.2021.112249
Wongnutpranont A., Suebpala W., Sutthacheep M., and Pengsakun S. (2021). Derelict fishing gears and other marine debris from coral communities on underwater pinnacles in Chumphon Province, Thailand. Ramkhamhaeng Int. J. Sci. Technol. 4, 28–37.
Wright S. L., Thompson R. C., and Galloway T. S. (2013). The physical impacts of microplastics on marine organisms: a review. Environ. pollut. 178, 483–492. doi: 10.1016/j.envpol.2013.02.031
Wu L., Liu X., Fang Y., Hou S., Xu L., Wang X., et al. (2018). Nitrogen cycling in the soil–plant system along a series of coral islands affected by seabirds in the South China Sea. Sci. Total Environ. 627, 166–175. doi: 10.1016/j.scitotenv.2018.01.213
Xu Q., Xing R., Sun M., Gao Y., and An L. (2020). Microplastics in sediments from an interconnected river-estuary region. Sci. Total Environ. 729, 139025. doi: 10.1016/j.scitotenv.2020.139025
Xu L., Han L., Li J., Zhang H., Jones K., and Xu E. G. (2022). Missing relationship between meso-and microplastics in adjacent soils and sediments. J. Hazardous Materials 424, 127234. doi: 10.1016/j.jhazmat.2021.127234
Xu D. S., Yan Y. L., Wang X., and Qin Y. (2023). Numerical modelling of the physical response of coral reef sandy island to sea level rise by considering seasonal patterns. Ocean Coast. Manage. 245, 106860. doi: 10.1016/j.ocecoaman.2023.106860
Xue J., Xu Z., Hu X., Lu Y., Zhao Y., and Zhang H. (2024). Microplastics in maternal amniotic fluid and their associations with gestational age. Sci. Total Environ. 920, 171044. doi: 10.1016/j.scitotenv.2024.171044
Yeemin T., Sutthacheep M., Plangngan P., Klomjit A., Aunkhongthong W., and Jungrak L. (2018). Microplastics in marine sediment at the coral reef hotspots of Mu Ko Similan National Park, Phang Nga Province. Ramkhamhaeng Int. J. Sci. Technol. 1, 22–29.
Yen L. P., Yong C. L. X., and Todd P. A. (2024). The effect of coral colony morphology, coral surface condition, particle size, and seeding point on the trapping and deposition of microplastics. Sci. Total Environ. 921, 171077. doi: 10.1016/j.scitotenv.2024.171077
Zettler E. R., Mincer T. J., and Amaral-Zettler L. A. (2013). Life in the plastisphere: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7146. doi: 10.1021/es401288x
Zhang B., Wu Q., Gao S., Ruan Y., Qi G., Guo K., et al. (2023). Distribution and removal mechanism of microplastics in urban wastewater plants systems via different processes. Environ. Pollut. 320, 121076. doi: 10.1016/j.envpol.2023.121076
Zhang L., Liu X., and Zhang C. (2023a). Effect of PET microplastics on the growth, digestive enzymes, and intestinal flora of the sea cucumber Apostichopus japonicus. Mar. Environ. Res. 190, 106125. doi: 10.1016/j.marenvres.2023.106125
Zhang H., Nie Y., Zhao S., Wu L., Xi X., Xu L., et al. (2025). Distribution characteristics and transport pathways of soil microplastics in coral reef islands with different developmental stages and human activities. Mar. pollut. Bull. 215, 117848. doi: 10.1016/j.marpolbul.2025.117848
Zhang W., Ok Y. S., Bank M. S., and Sonne C. (2023b). Macro- and microplastics as complex threats to coral reef ecosystems. Environ. Int. 174, 107914. doi: 10.1016/j.envint.2023.107914
Zhang B., Wu D., Yang X., Teng J., Liu Y., Zhang C., et al. (2019). Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China. Mar. pollut. Bull. 141, 9–15. doi: 10.1016/j.marpolbul.2019.02.021
Zhou Q., Tu C., Fu C., Li Y., Zhang H., Xiong K., et al. (2020). Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci. Total Environ. 703, 134807. doi: 10.1016/j.scitotenv.2019.134807
Zhu L., Ma M., Sun X., Wu Z., Yu Y., Kang Y., et al. (2024). Microplastics entry into the blood by infusion therapy: few but a direct pathway. Environ. Sci. Technol. Lett. 11, 67–72. doi: 10.1021/acs.estlett.3c00905
Keywords: microplastics, coral reef ecosystem, spatial variability, polymer composition, Gulf of Thailand
Citation: Sutthacheep M, Chamchoy C, Suebpala W, Wongnutpranont A, Jungrak L, Aunkhongthong W, Pengsakun S, Klinthong W, Jowantha M, Sangsawang L and Yeemin T (2025) Assessment of microplastic pollution in corals, seawater, and marine sediments in the Gulf of Thailand. Front. Mar. Sci. 12:1669901. doi: 10.3389/fmars.2025.1669901
Received: 20 July 2025; Accepted: 06 October 2025;
Published: 31 October 2025.
Edited by:
Jessica Reichert, University of Hawaii at Manoa, United StatesReviewed by:
Suchai Worachananant, Kasetsart University, ThailandJitraporn Phaksopa, Kasetsart University, Thailand
Copyright © 2025 Sutthacheep, Chamchoy, Suebpala, Wongnutpranont, Jungrak, Aunkhongthong, Pengsakun, Klinthong, Jowantha, Sangsawang and Yeemin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thamasak Yeemin, dGhhbWFzYWt5ZWVtaW5AaG90bWFpbC5jb20=
 Makamas Sutthacheep
Makamas Sutthacheep Charernmee Chamchoy
Charernmee Chamchoy Wichin Suebpala
Wichin Suebpala Arirush Wongnutpranont
Arirush Wongnutpranont Laongdow Jungrak
Laongdow Jungrak Wiphawan Aunkhongthong
Wiphawan Aunkhongthong Sittiporn Pengsakun
Sittiporn Pengsakun Wanlaya Klinthong
Wanlaya Klinthong Morakot Jowantha
Morakot Jowantha Laddawan Sangsawang
Laddawan Sangsawang Thamasak Yeemin
Thamasak Yeemin