Abstract
Introduction:
Individuals of some marine species can modify their phenotype in response to environmental factors, allowing them to adapt to new conditions throughout their ontogeny. Echinoids represent an ecologically significant taxon that exhibit such plasticity throughout a biphasic life history in response to known biotic and abiotic factors. Preliminary lab-based observations have suggested that morphological traits, specifically pluteal arm length, may be influenced by physical processes such as hydrodynamic flow during planktotrophic larval development. This dynamic remains understudied despite potentially critical demographic implications.
Methods:
Here, we tested the effect of continuous exposure to different shear stress treatments on larval morphology and life history timing shifts in three co-occurring species: Lytechinus variegatus, Tripneustes ventricosus, and Diadema antillarum.
Results:
Both T. ventricosus and D. antillarum displayed significantly longer postoral arms and increased percent metamorphic competence in response to greater shear. Treatment effects were not observed for L. variegatus.
Discussion:
These findings represent the first observation of morphogenic plasticity in response to a hydrodynamic factor for larval echinoderms. Species-specific effects revealed a plasticity continuum which may be mediated by phylogeny, ecological niche, and/or functional morphology. This dynamic response offers insights into larval dispersal and recruitment potential, adult distribution, and the boom-and-bust cycles characteristic of ecologically relevant echinoid populations.
1 Introduction
Sea urchins are often referred to as ecosystem engineers due to their ability to alter benthic algal communities through herbivory (Precht and Precht, 2015). For this reason, they are well represented in foundational ecological studies (Leighton et al., 1966; Lang and Mann, 1976; Duggins, 1980). Urchin populations are subject to rapid ‘boom’ and ‘bust’ phases which can have cascading ecosystem effects. For example, overpopulation and intense grazing by Strongylocentrotus purpuratus contributed to large-scale kelp deforestation in the Pacific Ocean (Harris and Eddy, 2015). Similarly, over-grazing by Diadema africanum reduced benthic biodiversity within urchin barrens along temperate coastlines in the eastern Atlantic Canary Islands (Hernández et al., 2008). Conversely, mass mortality of Diadema antillarum in 1983–84 and resulting lack of herbivory resulted in algal proliferation and concomitant decline of hard coral on Caribbean reefs (Carpenter, 1990; Harborne et al., 2009; Lessios, 1988a). The ecological importance of sea urchin grazing justifies expanded understanding of factors, especially during early life history, influencing population dynamics.
Sea urchin demographics are tightly linked to their planktonic larval stage (Roughgarden et al., 1988; Pineda et al., 2010). Since benthic adults have limited mobility and narrow home ranges, species distribution is dependent on larval dispersal and post-larval recruitment patterns (Prado et al., 2012). Reproductive success, which can be highly variable, is therefore mediated by factors such as spawning behavior, gamete encounter rates and fertilization success, larval developmental mode and duration, and settlement behavior, all occurring and interacting within dynamic physical environments (Harris and Eddy, 2015). Water currents surrounding spawning aggregations, for example, likely influence gamete encounter rates, fertilization success, and dispersal direction and range (Feehan et al., 2016). This stochasticity has been likened to a ‘recruitment sweepstake’, where chance environmental conditions enact some degree of passive influence on the diversity and magnitude of parental contribution to future populations (Cowen et al., 2000; Flowers et al., 2002).
Hydrodynamic factors also directly influence larval biology. For example, the chaotic swirling motion of water, i.e., turbulence, can alter the swimming behavior and distribution of planktonic urchin larvae within the water column (Sameoto et al., 2010; Wheeler et al., 2016). The process of larval settlement is also sensitive to hydrodynamic exposure; in one field study, settlement rates for two Strongylocentrotus species correlated positively with turbulence and negatively with stratified water columns and low wind stress environments (Miller and Emlet, 1997). Later studies investigated this dynamic in the laboratory and broadly revealed an accelerated timeline to metamorphic competence and increased settlement success for some species in response to higher shear flow (Gaylord et al., 2013; Hodin et al., 2020). In these instances, turbulence was hypothesized to facilitate maturation and settlement in favorable nearshore habitat characterized by high wave energy. Species-specific responses further highlighted potential evolutionary differences. Despite evident impacts on the rate and timing of settlement, direct hydrodynamic effects on morphological traits during larval development are understudied.
Urchin larvae with a planktotrophic (i.e., feeding) mode can express different morphologies in response to certain abiotic environmental conditions (Byrne et al., 2008; Soars et al., 2009; McAlister and Miner, 2018). For example, some larvae adapt to food-limited conditions by elongating ciliated arms to increase food capture efficiency (Strathmann et al., 1992; Sewell et al., 2004; Byrne et al., 2008). Soars et al. (2009) further revealed that this plastic growth response is also species-specific and may depend on larval form. Typical echinopluteus larvae possess four pairs of arms (hereafter referred to as ‘typical’), whereas echinopluteus transversus larvae, such as those of Diadema antillarum, have a single dominant pair of elongated post-oral arms (hereafter referred to as ‘transversus’). Since hydrodynamic factors influence larval biology, and some larvae exhibit plastic growth responses to known environmental conditions, it is plausible that larvae may also adapt their morphology in response to hydrodynamic forces. Preliminary lab-based observations have indicated that morphological traits, specifically pluteal arm length, may be influenced by flow regime during planktonic larval development in an aquaculture setting. Further understanding of these adaptations relates to physical factors, such as hydrodynamic condition, is essential for predicting sea urchin demographics and ecosystem level impacts.
This study examined larval ontogeny under varying turbulent shear conditions to gain insights into how hydrodynamics influence larval functional morphology and potential phenotypic plasticity in two typical form species – Lytechinus variegatus and Tripneustes ventricosus – and one transversus form species – Diadema antillarum. All three Caribbean species occupy overlapping yet ecologically distinct niches (Randall et al., 1964; Cameron, 1986; Lessios, 1988b; Maciá and Lirman, 1999). This work provides a framework for understanding species-specific developmental strategies and their implications for recruitment success under climate change and other stressors on marine ecosystems.
2 Materials and methods
2.1 Broodstock and spawning
Lytechinus variegatus and T. ventricosus broodstock were collected by licensed marine life collectors in the Florida Keys, USA. Diadema antillarum broodstock were collected from the same region by the Florida Fish and Wildlife Conservation Commission under National Oceanic and Atmospheric Administration permit number FKNMS-0218-023. All bloodstock were maintained in separate greenhouse recirculating aquaculture systems (RAS) operated by The Florida Aquarium in Apollo Beach, Florida, at latitude N27° 46′ 43.81″, as described in Pilnick et al. (2021).
Lytechinus variegatus and T. ventricosus broodstock primarily fed on naturally derived benthic algal growth and sporadically received a commercially available herbivore diet ([34% crude protein, 8% crude fat, 8% crude fiber], Algaemax Wafers, New Life Spectrum, Homestead, FL, USA). Gametes from a serendipitous, un-induced broadcast spawning event were collected via pipette from one male and one female of each species on November 7, 2022. Salinity and temperature at the time of spawning were 37.1 ppt and 26.6°C, respectively. Egg and diluted sperm concentrations were then combined, and percent fertilization was recorded by quantifying the proportion of embryos displaying a fertilization envelope or initial cell division approximately two hours post-spawn in 1-ml Sedgewick rafter cell subsamples. Embryos were lightly agitated and continuously suspended for a three-day incubation period before experimentation began. Diadema antillarum broodstock were fed the same commercial diet and induced to spawn using thermal stress, as described in Pilnick et al. (2021). In the current study, a mixture of gametes from two females and four males was collected on December 14, 2023. Fertilization was confirmed using the same methods described above and embryos from this single cohort were similarly incubated for four days. Average diameter was calculated from 25 eggs for each species.
2.2 Larviculture
Experimental larviculture for all three species occurred within replicate 1-litre borosilicate glass bottles (100 mm diameter x 230 mm height) placed on rotating orbital shaker tables located in a temperature-controlled (24°C) lab. A natural photoperiod was provided. This method, as described in Wijers et al. (2023), allowed for easy manipulation of rotational speed, accommodated multiple replicates per table (Heathrow Scientific 120,460), and has been used to successfully suspend and rear difficult-to-culture species like D. antillarum. Following the incubation period, concentrated batches of 3–4 days post-fertilization (DPF) larvae were placed in a 10-mL Ward zooplankton counting wheel, enumerated, and transferred to individual bottles containing 600-mL of 35 ppt natural seawater at a target initial stocking density of 1 larva mL-1. Twice weekly, larvae from each bottle were carefully poured into a submerged sieve and replaced into new, disinfected bottles. For D. antillarum only, an additional 50% water change supplemented the twice-weekly 100% water change and bottle cleanings to account for heightened aquaculture difficulty (Bielmyer et al., 2005). Alkalinity, pH, and total ammonia nitrogen levels were monitored weekly to ensure water quality parameters matched acceptable values as reported in Pilnick et al. (2021).
All larvae were fed a 3:1 ratio of Rhodomonas salina and Chaetoceros muelleri, with incremental increases in live microalgal cell density based on development status for L. variegatus and T. ventricosus and cohort age for D. antillarum, as detailed here Table 1 and Pilnick et al. (2022), respectively. The feeding regime was adjusted upon observing ≥ 50% of larval population transition to a subsequent developmental stage.
Table 1
| Time period (D. antillarum) | Developmental stage (L. variegatus and T. ventricosus) | Feeding rate |
|---|---|---|
| 3–6 dpf | 2-arm pluteus | 1000 cells mL-1 |
| 6–12 dpf | 4-arm pluteus | 5000 cells mL-1 |
| 12–22 dpf | 6-arm pluteus | 10,000 cells mL-1 |
| 22 dpf–settlement | 8-arm pluteus | 20,000 cells mL-1 |
Feeding regime for Lytechinus variegatus, Tripneustes ventricosus, and Diadema antillarum larvae based on developmental stage or cohort age.
Dpf, days post-fertilization.
2.3 Experiment 1
An initial experiment concurrently examined the effects of shear flow on larval growth, survival, percent metamorphic competence, and percent settlement for two species: L. variegatus and T. ventricosus. Four orbital shaker tables, each containing four replicate bottles, were used to establish a 2x2 factorial design. Two tables were set to a high rotational speed (high-shear treatment) and two to a low rotational speed (low-shear treatment); no direct measurements of shear in natural habitats were used. Within each shear treatment, four bottles per species were distributed across two tables, resulting in four replicates per species per shear level (L. variegatus: n = 4 high-shear, n = 4 low-shear; T. ventricosus: n = 4 high-shear, n = 4 low-shear). The shaker tables’ capacity limited the experiment to two treatments per species. The centrifugal force generated within the bottles, which sustained larvae in suspension, is herein referred to as the turbulent shear force. Fifty rotations per minute (rpm) (0.6 Pa) was visually identified as the minimum rotation speed at which larvae remained suspended and therefore was deemed the minimum viable speed. A higher speed of 125 rpm (2.5 Pa) was based on established methods (Wijers et al., 2023). To mitigate potential error introduced by individual tables and table positioning, bottles and corresponding rotation speeds were interchanged between and among tables daily.
The following formula was used to estimate turbulent shear within bottles for each treatment, as outlined in Dardik et al. (2005):
where a is the orbital radius of rotation of the shaker (1.90 cm), ρ is the density of the medium (1.02 g ml-1), η is the viscosity of the medium (0.09 poise), and f is the rotation frequency (rotations s-1). The model assumes a Newtonian fluid with uniform viscosity and density and approximates the orbital-influenced fluid motion as isotropic shear. While the formula provides a useful estimate of turbulent shear, real flow dynamic within bottles may differ due to boundary effects, fluid stratification, and non-laminar conditions.
Larval sampling was conducted once weekly for all three species; however, experiment length varied according to species-specific larval durations (15 days, 22 days, and 45 days for L. variegatus, T. ventricosus, and D. antillarum, respectively). During sampling, all sieved larva were counted to determine survival. Fifteen intact, i.e., possessing all standard features, larva per bottle were also haphazardly selected, transferred to a 1 mL Sedgewick rafter cell, and photographed at 40x magnification using a Moticam 10-megapixel digital camera. Standard morphometric features including midline body length (MBL), body width (BW), post-oral arm length (PO), and stomach length (SL) were measured using ImageJ version 1.53o (Figure 1). The PO to MBL ratio was used to assess morphological plasticity, since MBL typically develops at consistent rates and can serve as a standardized metric for comparing relative growth rates of other features (Strathmann et al., 1992); this method is consistent with other studies (Hart and Strathmann, 1994; McAlister, 2008; Soars et al., 2009; Guete-Salazar et al., 2021). Metamorphic competence was determined for individual larvae by the presence of an imaginal rudiment and pedicellariae, following criteria described in Guete-Salazar et al. (2021).
Figure 1
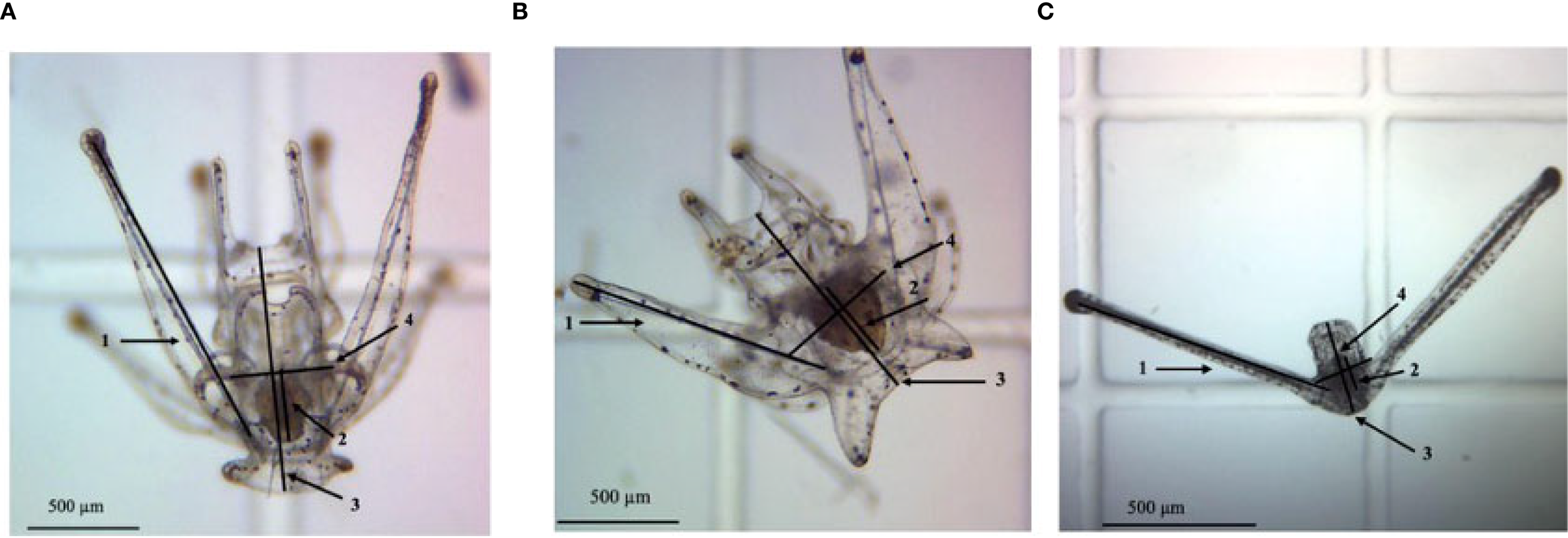
Ventral view of (A)Lytechinus variegatus larva (8-arm stage, 11 days post-fertilization [dpf]); (B)Tripneustes ventricosus larva (8-arm stage, 18 dpf); (C)Diadema antillarum larva (22 dpf). Numbers indicate the following morphometric features: post-oral arm (1), stomach length (2), midline body length (3), and body width (4).
Settlement assays were performed only for Experiment 1 with L. variegatus and T. ventricosus. Larvae were induced to settle after the population within each replicate bottle exceeded an estimated value of 75% competence. This approach aimed to understand the effect of shear treatment on both time-to-competence and settlement success of competent larvae and therefore did not reflect the maximum percent competence attained for each species and treatment level. Competent larvae from each replicate were distributed into six sterile petri dishes (100 mm x 15 mm) each containing 40 mL of filtered natural seawater and a single ceramic tile (7.5 mm × 7.5 mm × 5 mm) which was conditioned in a separate established greenhouse RAS for several months. Each dish contained a maximum of 15 larvae. Competent T. ventricosus and L. variegatus were exposed to tiles coated with crustose coralline algae (CCA) and naturally derived biofilm, respectively. These species-specific settlement cues were selected based on best-practice knowledge from prior studies (Nesbit and Hamdoun, 2020; Guete-Salazar et al., 2021). The number of settled urchins in each dish was counted multiple times daily until no new settlement was observed within a 24-hour period.
2.4 Experiment 2
A second experiment utilized similar methods, with modifications to accommodate interspecific differences in larval development, to examine the effects of shear flow on larval growth, survival, and percent metamorphic competence for D. antillarum. Six orbital shaker tables, each containing three replicate bottles and one blank bottle, were used to establish a three-level shear treatment design. Two tables were set to each rotational speed: high-shear, intermediate-shear, and low-shear, allowing all treatments to run concurrently. Within each shear level, six replicate bottles were distributed across two tables (n = 6 per treatment). During an initial pilot attempt, the same minimum rotation speed used for T. ventricosus and L. variegatus was insufficient to keep D. antillarum suspended and resulted in 0% survival by 14 dpf. Consequently, a new low-shear 93 rpm (1.6 Pa) treatment was established between 50 rpm (Exp. 1 low-shear;0.6 Pa) and 125 rpm (Exp. 1 high-shear;2.5 Pa). The intermediate-shear treatment for this experiment was established at 125 rpm (2.5 Pa). The same increment increase was then applied to 125rpm to establish a new high-shear 158 rpm (3.5 Pa) treatment, in effort to increase a detectable effect size.
The time to first settlement was 46 dpf. Competence for D. antillarum was defined by the presence of internal or external tube feet and rudiment tissue that constituted 50% or more of the larva’s body size, as outlined in Pilnick et al. (2023). Settlement was systematically attempted for all shear treatments once larvae reached competence. Ceramic tiles naturally conditioned with crustose coralline algae (CCA) and biofilm were used as the settlement substrate. Petri dish assays were deemed insufficient for standardized comparison because settlement rates were variable, and the high-shear (3.5 Pa) treatment exhibited such low larval survival that meaningful comparisons across treatments were not possible. Despite D. antillarum being a challenging species to rear, the shaker table method has been documented to produce settled urchins (Wijers et al., 2023), and multiple juveniles were successfully produced from this cohort.
2.5 Statistical analyses
Statistical analyses were conducted using R statistical software, version 4.4.1 (R Core Team, 2024). Linear mixed-effects models (‘lmer’ function, R package ‘lme4’) were used to evaluate the effect of shear treatment on individual larval morphometric traits and on the PO: MBL ratio for each species. For time series data, fixed effects included treatment, timepoint, and their interaction. These terms were included not to evaluate biological effects of time per se, but to enable post hoc comparisons between treatments at each individual timepoint. For end-point analyses conduced at the final pre-settlement timepoint, only treatment was included as a fixed effect. In all models, replicate bottle was included as a random intercept to account for non-independence of larvae nested within bottles. Trait values were log-transformed where necessary to meet model assumptions, which were verified through residual plots, Q-Q plots, and Shapiro-Wilk tests. Model significance was assessed using Type II analysis of deviance tables with chi-square tests. Post hoc pairwise comparisons were conducted (‘emmeans’ function, R package ‘emmeans’), with Tukey-adjusted p-values for multiple comparisons. The significance threshold was set at α = 0.05.
Larval survival was calculated as the proportion of live larvae remaining at each time point relative to the initial stocking. Competence was assessed only at the final pre-settlement timepoint (13 dpf for L. variegatus, 21 dpf for T. ventricosus, 45 dpf for D. antillarum) and defined as the proportion of competent individuals. Both metrics were modeled using generalized linear mixed-effects models (‘glmer’ function, R package ‘lme4’) with binomial distributions, treatment and time (for survival) or treatment alone (for competence) as fixed effects and bottle as a random effect. Model fit and post hoc comparisons were conducted as described above.
Due to heterogeneous variances and the zero-inflated nature of the data, proportional settlement in Experiment 1 was analyzed using a two-way permutational analysis of variance (‘adonis’ function, R package ‘vegan’) with treatment and species as factors (Mos et al., 2011).
3 Results
3.1 Experiment 1
The average egg diameter for T. ventricosus and L. variegatus was 86 ± 3.5 µm and 88 ± 3.5 µm, respectively. The overall effect of shear flow treatment on proportional survival was non-significant for both T. ventricosus (XX2 = 1.008, df = 1, p = 0.315) and L. variegatus (X2 = 2.332, df = 1, p = 0.127), and no post hoc contrasts were conducted (Figure 2). Treatment effects on several morphometric traits were observed but depended on species (Supplementary Table 1). For T. ventricosus, both arm length and body length significantly increased in response to high shear (PO: X² = 0.785, df = 1, p = 0.020; MBL: X² = 0.817, df = 1, p < 0.001) at 22 dpf, but the increase in arm length was proportionally greater. A significant effect on the PO: MBL ratio was observed (X² = 16.10, df = 1, p < 0.001), which indicated a plastic growth response to shear (Figure 3). Similar survival indicated that these differences were likely not influenced by a confounding variable, i.e., density-dependent growth. In contrast, treatment did not significantly affect morphometric traits or the PO: MBL ratio for L. variegatus at 15 dpf (Figure 3). For this species, both arm length and body length remained consistent between treatments (Supplementary Table 1) which indicated lack of a plastic growth response to shear.
Figure 2
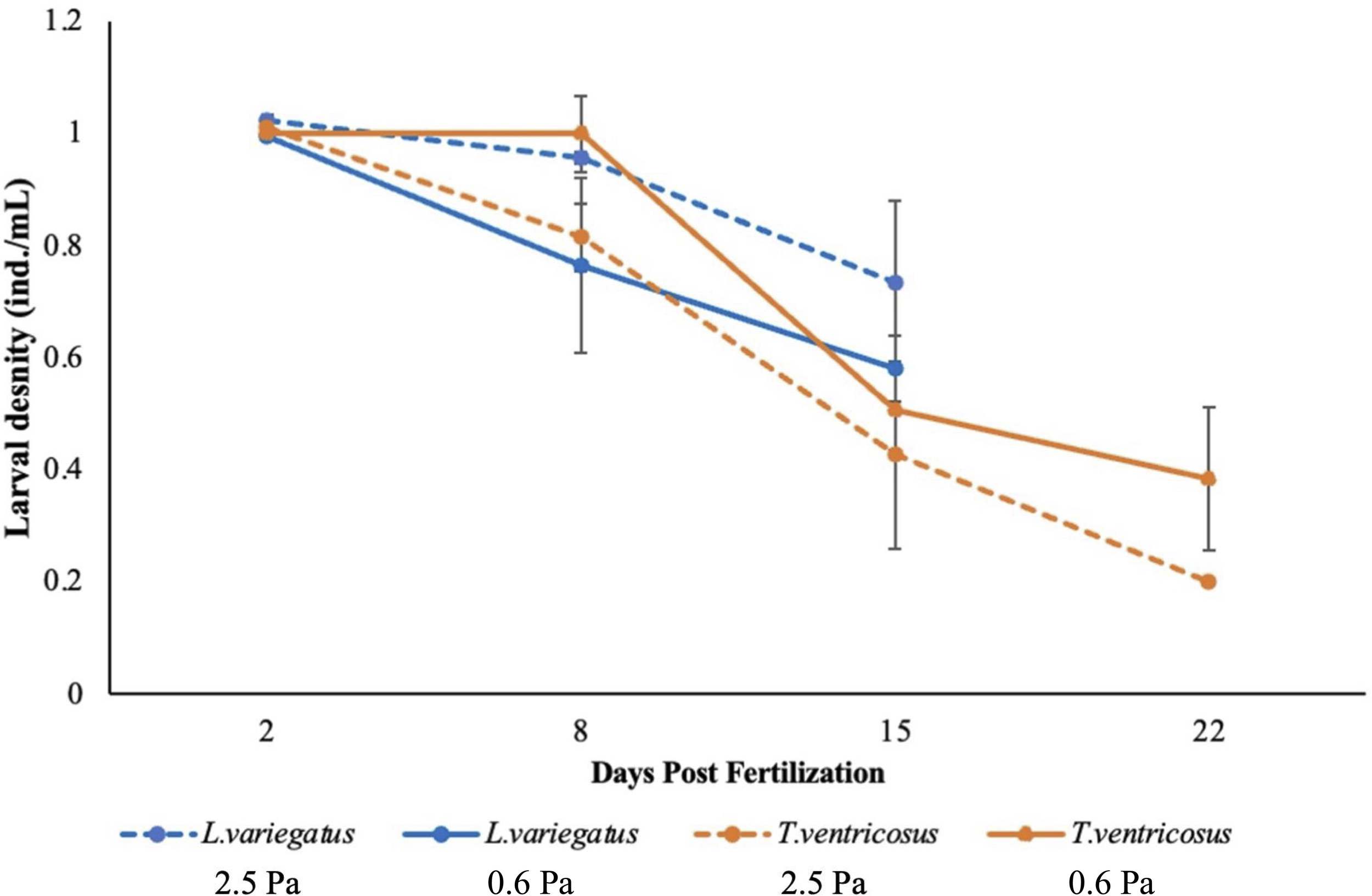
The density of surviving Lytechinus variegatus and Tripneustes ventricosus larvae exposed to high-shear (2.5 Pa) and low-shear (0.6 Pa) treatments over time. There was no significant difference in survival between treatments within species. Bars represent mean values ± standard error.
Figure 3
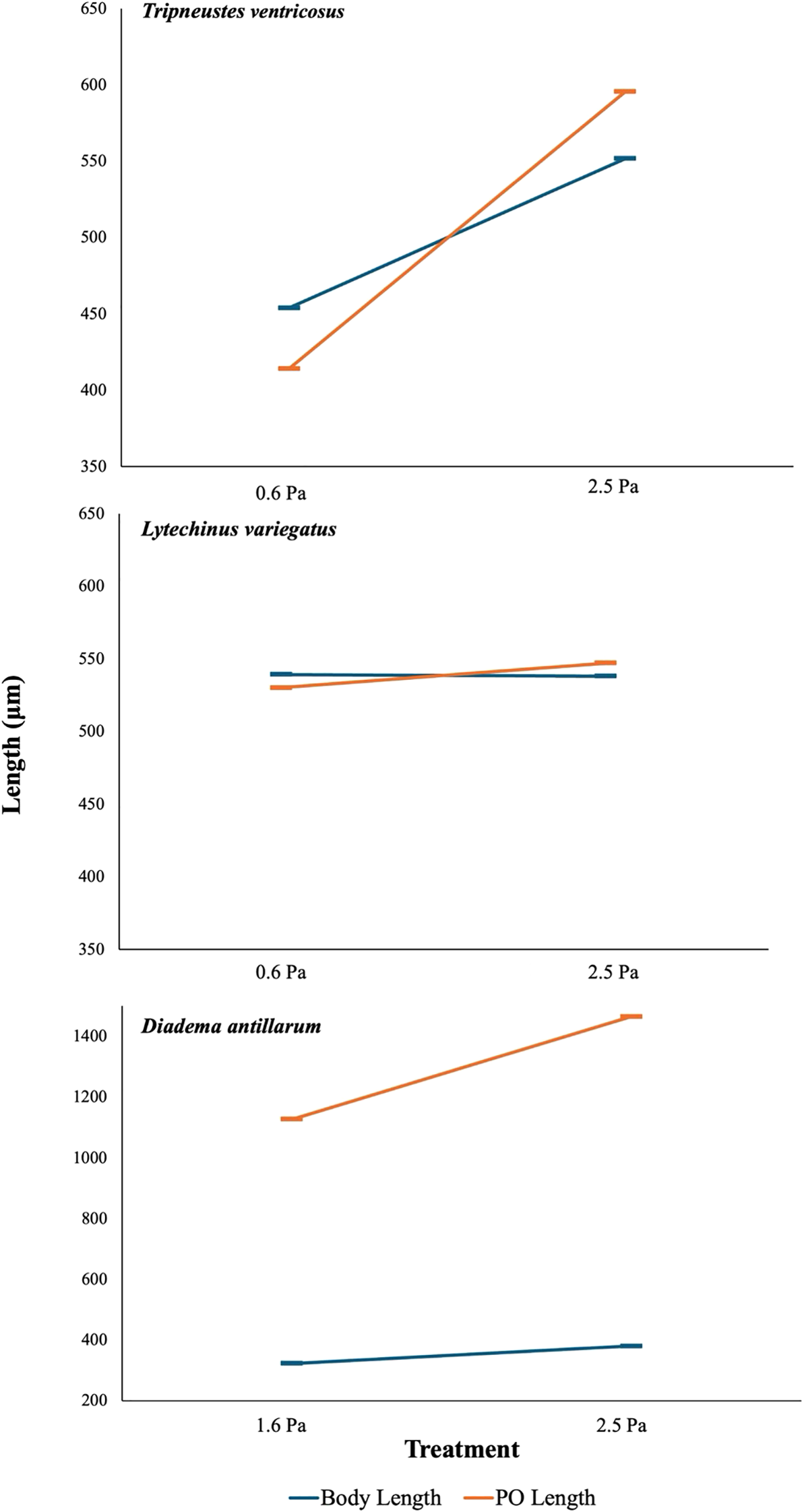
Average larval post-oral arm length (PO) to midline body length (MBL) ratios visualized in response to low shear flow (1.6 Pa) and high shear flow (2.5 Pa) treatments for Tripneustes ventricosus at 22 dpf (0.91–1.08; X² = 16.10, df = 1, P < 0.001), Lytechinus variegatus at 15 dpf (0.98–1.02; X² = 0.57, df = 1, P = 0.94), and Diadema antillarum at 45 dpf (3.49–3.86; X² = 202.85, df = 1, P < 0.001). The highest shear treatment (3.5 Pa) is not included due to high mortality. Bars represent mean ± standard error.
For L. variegatus, there was no significant effect of shear on the proportion of metamorphically competent larvae at 14 dpf (X2 = 2.845, df = 1, p = 0.092). In contrast, for T. ventricosus, a significant difference was observed at 22 dpf (X2 = 13.292, df = 1, p = 0.0003), with the 2.5 Pa treatment resulting in more competent larvae (Figure 4). The time to first settlement was 15 and 22 dpf for L. variegatus and T. ventricosus, respectively. Settlement was compared between species as the maximum, or peak percent settlement observed in competent larvae only. Thus, the methods are not appropriate for comparing time to settlement. No significant difference in settlement was detected between treatments or species (PERMANOVA: F = 0.676, df = 1, p = 0.38). However, both species exhibited a trend where the 2.5 Pa treatment resulted in numerically higher percent settlement: 81.9% vs. 73.6% for L. variegatus and 48.1% vs. 35.2% for T. ventricosus.
Figure 4
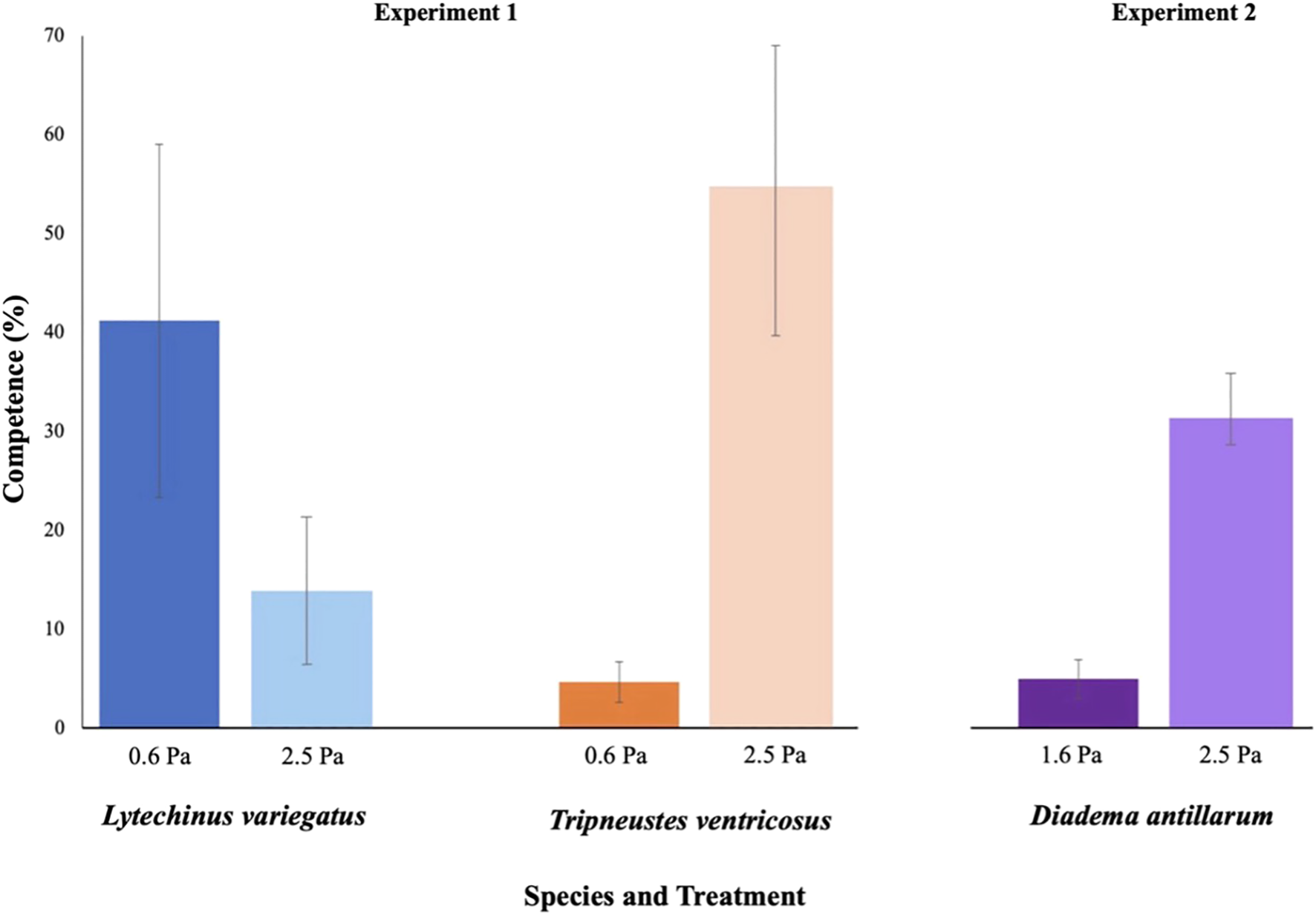
Percent competence for each urchin species on the final day before first settlement (dpf = 13 for L. variegatus, dpf = 21 for T. ventricosus, dpf = 45 for D. antillarum) across respective turbulent shear treatments. Due to different rates of attaining competence for each species, data here do not represent maximum competence for any of the urchin species. Bars represent mean values ± standard error.
3.2 Experiment 2
The average egg diameter for D. antillarum was 80 ± 3.2 µm. A significant overall main effect of shear treatment on survival was observed (X2 = 178.04, df = 2, p < 0.001); post-hoc analysis revealed significantly reduced survival in the high shear (3.5 Pa) treatment relative to both the intermediate (2.5 Pa) and low (1.6 Pa) shear treatments (Figure 5). Regarding individual morphometrics, multiple differences were found between the high shear treatment and the two lower-shear treatments (Supplementary Table 2). Larvae in the 3.5 Pa treatment exhibited shorter arms (X2 = 153.53, df = 2, p < 0.001), longer body lengths (X2 = 56.325, df = 2, p < 0.001), larger body widths (X2 = 214.16, df = 2, p < 0.001), and longer stomach lengths (X2 = 34.172, df = 2, p < 0.001) compared to those in the 2.5 Pa and 1.5 Pa treatments. The intermediate shear (2.5 Pa) treatment resulted in significantly longer arms compared to the other treatments (X2 = 153.53, df = 2, p < 0.001). No other significant differences in morphometrics features were observed between the 2.5 and 1.6 Pa treatments (Supplementary Table 2). Diadema antillarum larvae in the high shear (3.5 Pa) treatment were excluded from consideration when analyzing the PO: MBL ratio due to considerable mechanical damage including missing or truncated arms and low survival rates, which confounded detection of a natural plastic response. When considering the low and intermediate shear treatments, a significant treatment effect on this growth ratio was observed at 45 dpf (X2 = 202.85, df = 12, p < 0.001), which indicated a plastic growth response (Figure 3).
Figure 5
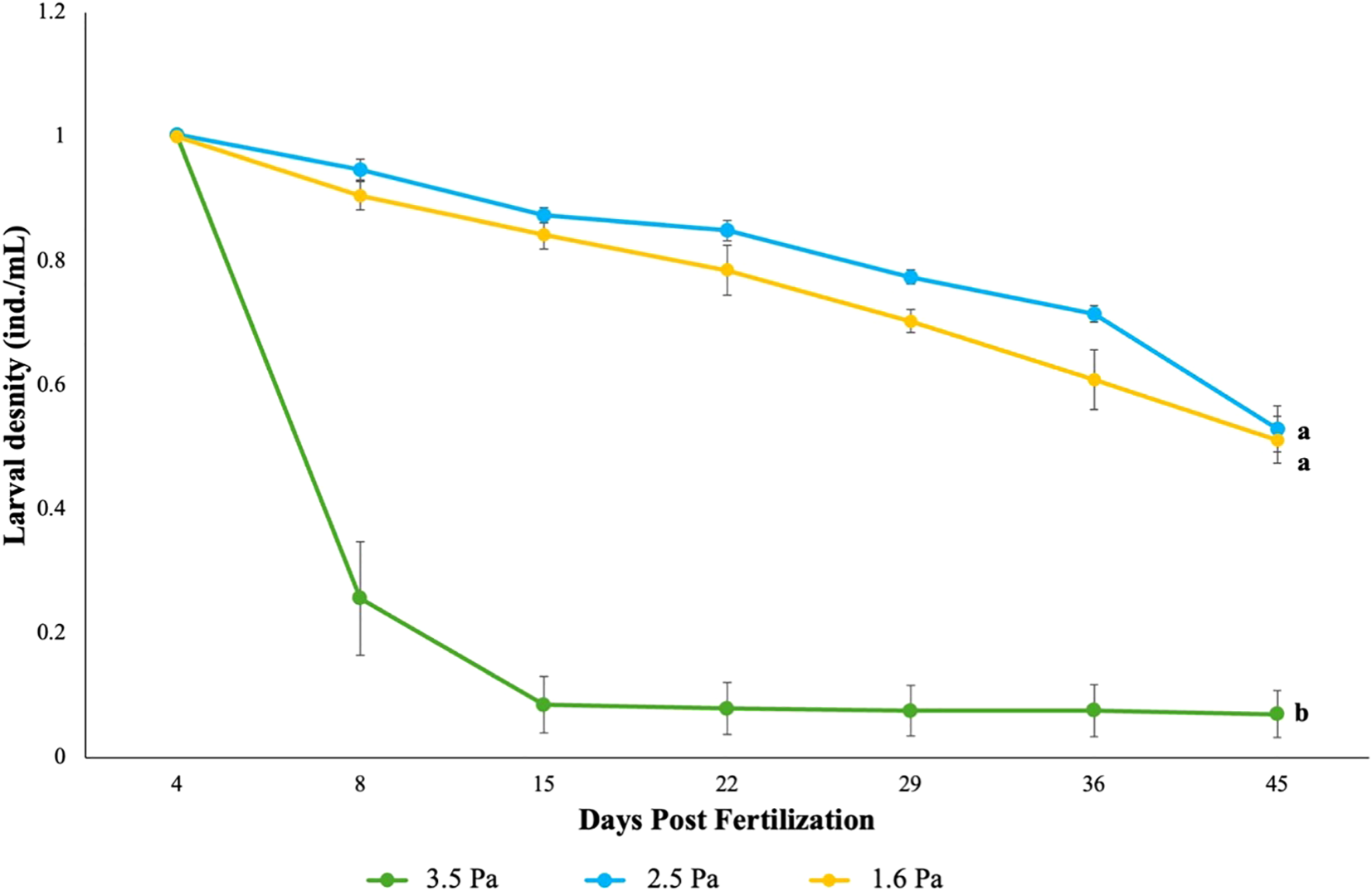
The density of surviving Diadema antillarum larvae exposed to low-shear (1.6 Pa), intermediate-shear (2.5 Pa), and high-shear (3.5 Pa) treatments over time. Letters indicate a significant difference between treatments. Bars represent mean values ± standard error.
On day 45, significant differences in proportional competence were observed among treatments (X2 = 60.61, df = 2, p < 0.0001) (Figure 4). The intermediate shear (2.5 Pa) treatment resulted in significantly higher proportional competence than observed in the low shear (1.6 Pa) treatment (p < 0.0001). The high shear (3.5 Pa) treatment resulted in dramatically lower survival rates and was consequently excluded from this analysis.
4 Discussion
Our data provide the first evidence of a plastic growth response to hydrodynamic flow in larval echinoderms, with multiple species displaying increased arm length when developing in higher shear environments. Given that our larvae were fed to satiation, in consensus with other larviculture studies (see Soars et al., 2009; Byrne et al., 2008), and the apparent lack of density-dependent growth effects from relevant shear treatments, we believe that standardized changes in T. ventricosus and D. antillarum postoral arm length were driven by flow. Food encounter rates in all treatments were assumed sufficient based on comparable survivorship across treatments. Although food clearance rates were not directly quantified, algal particles were visualized in the larval stomachs for each species and treatment across the entire study. Notably, the timing of developmental staging for L. variegatus and T. ventricosus, and body length values for D. antillarum, were similar between relevant high surviving treatments. Altogether, these results strongly suggests that echinoid arm-length plasticity was driven by hydrodynamics rather than food limitation. The significant differences in arm length to body length ratio were detected at species-specific time points that correspond to the final stages of larval development prior to metamorphic competence. A plastic growth response was most likely to be detected at these endpoints, after prolonged exposure to shear conditions. This strategy enabled standardized inter-specific comparisons while capturing the effects of differential shear on functional morphology. We observed a continuum of responsiveness across three species, with D. antillarum exhibiting the greatest plasticity, T. ventricosus exhibiting moderate plasticity, and L. variegatus exhibiting no plasticity, i.e., resistance to shear stress. These findings suggest species-specific adaptations to flow and possibly other physical oceanographic factors.
Echinoid larvae use cilia, arranged in bands along their arms, as a primary means of feeding and locomotion (Hart, 1991; Yaguchi et al., 2022). The skeletal structure of the post-oral arms also facilitates a larvae’s ability to maintain an upright (arms-up) orientation in the water (Pennington and Strathmann, 1990; Wheeler et al., 2016). This reliance on post-oral arms for stability and orientation may be more pronounced in the two urchin species showing a plastic arm growth response: T. ventricosus and D. antillarum. In larvae with a transversus morphology, such as D. antillarum, this dependence on post-oral arms is likely amplified, as they rely primarily on a single dominant pair of arms for both feeding and locomotion (Hernández et al., 2020). In contrast, typical morphologies distribute these tasks across multiple appendages (Randall et al., 1964; Soars et al., 2009; Rendleman and Pace, 2018). This specialization of the transversus form limits the potential for morphological adaptation to the single pair of post-oral arms and possibly contributes to D. antillarum’s pronounced arm length extension response compared to T. ventricosus and L. variegatus (Soars et al., 2009). It must be noted that the larvae of T. ventricosus and L. variegatus in this study were derived from single full siblingships. As such, our findings should be interpreted with the understanding that they may be influenced by genetic factors specific to these siblingships. Additionally, as the D. antillarum experiment was conducted one year apart from the L. variegatus and T. ventricosus experiment, temporal variation should be considered in interpreting patterns in inter-specific differences.
Larval post-oral arm length also has significant effects on metamorphic development. Arm extension in conjunction with skeletal growth enhances nutritional resources available for metamorphosis (Gustafson and Wolpert, 1963; Burke, 1981, 1983). During metamorphosis, epidermal cells of the arms and other ephemeral larval structures are phagocytosed, which supports the transition from larva to juvenile (Burke, 1983). Longer arms observed in T. ventricosus and D. antillarum thus could have provided a more favorable energy budget for the demanding transition from larvae to juvenile. Variations in arm length among echinoid larvae reflect evolutionary strategies that balance resource acquisition with development time (Strathmann et al., 1992; Hart and Strathmann, 1994). The transversus form morphology (longer arms, extended planktonic larval duration) likely carries a trade-off between dispersal potential and developmental rate (Grünbaum and Strathmann, 2003). This morphology theoretically increases horizontal swimming speed and dispersal capacity, but may also reduce growth dynamics (Grünbaum and Strathmann, 2003)— D. antillarum developed over 46 days, while T. ventricosus and L. variegatus developed over 22 and 15 days, respectively. In contrast, typical echinoplutei like L. variegatus and T. ventricosus are associated with faster progression to juvenile stages, likely due to higher assimilation efficiencies (energy absorbed relative to energy ingested) (Rendleman and Pace, 2018), but these larvae have shorter planktonic stages, potentially limiting their dispersal ranges (Hart and Strathmann, 1994).
Larvae of some echinoid species extend their post-oral arms under food-limited conditions, a process that may increase food capture by up to 20% (Hart and Strathmann, 1994). The theory behind food-driven arm length plasticity suggests that temporal variation in food availability drives morphological adaptations to enhance survival and dispersal (Baythavong, 2011). Soars et al. (2009) examined this plastic response in contrasting larval forms, documenting the expected response (longer post-oral arms) in Heliocidaris turberculata (typical), but no plastic response in Centrostephanus rodgersii (transversus) when exposed to varying food concentrations. The authors proposed that transversus-form larvae, having only two primary arms for feeding and swimming, are adapted for long-distance dispersal and maintaining arm length regardless of food conditions (Soars et al., 2009). Biologically, these plastic responses are often mediated by stress pathways, such as stress-induced modulation of skeletal growth and calcite deposition, as observed in the stunted growth of Arbacia lixula larvae exposed to suboptimal temperature and pH conditions (Visconti et al., 2017). Our findings reveal a strong plastic response to hydrodynamic variability in transversus-type larvae, while this response was absent in one of the two typical form species. This suggests that arm length plasticity is not universal across form types and is influenced by a combination of phylogeny, ecological niche, and larval body form. Further, responses to food availability and flow conditions may not be the only types of phenotypic plasticity exhibited by these species.
The variability in larval forms may also reflect broader trends in population connectivity among echinoids. Diadema antillarum, with its long larval duration and substantial dispersive potential, exhibits high population connectivity, facilitating gene flow across large distances (Chandler et al., 2017). In contrast, L. variegatus has a more localized dispersal range and lower genetic connectivity (Nunez, 2017), aligning with its observed resistance to arm length plasticity. Little is known about T. ventricosus population genetics (Godinho et al., 2016), but other species in the genus (e.g. T. gratilla) are documented to have high genetic connectivity between populations (Casilagan et al., 2013). The role of larval form in population connectivity warrants further research to understand how genetics, dispersal potential, and environmental conditions interact in these species. The comparison of plasticity among these species highlights how evolutionary pressures in oligotrophic environments (e.g., reef systems inhabited by D. antillarum and T. ventricosus) may necessitate dispersal mechanisms to locate more favorable habitats. Variations in plasticity and connectivity likely stem from a combination of species-specific factors, including habitat stability, larval duration, and ecological niche.
The plastic arm length response in echinoid larvae, particularly those with extended planktonic phases like D. antillarum, may offer several advantages. In the context of this study, arm length and number of arms could represent a trade-off between optimizing swimming efficiency and maintaining stability in turbulent environments. Longer post-oral arms, supported by rigid calcite skeletons, may improve larval orientation and vertical stability, thereby reducing the energy required to maintain position in turbulent waters (Emlet, 1982, 1983; Pennington and Strathmann, 1990). Grünbaum and Strathmann (2003) developed a hydromechanical model that suggested larvae with fewer, lower-angled arms may swim faster and have greater ballast weight carrying capacity, while those with more numerous, higher-angled arms exhibit greater stability in turbulent conditions. This model suggests that larvae in turbulent environments, like those of D. antillarum, might prioritize stability over speed by developing longer arms and increasing skeletal ballast weight, aiding in vertical orientation in turbulent shear (Grünbaum and Strathmann, 2003). Additionally, as larvae grow, body size increases alongside post-oral arm length, which enhances positional stability. However, the swimming ability of the three species in this study has not been directly measured. Based on theoretical models, it is plausible that the benefits of increased ballast and stability might outweigh the energetic costs associated with reduced swimming capacity, particularly in environments where turbulence disrupts larval orientation and feeding, such as in the high shear treatment with D. antillarum (Maldonado, 2009; Sameoto et al., 2010). In such conditions, larvae may allocate more energy towards growth and metamorphic development rather than maintaining vertical position and orientation. A comparative empirical study examining the relationships between echinoid arm morphology, swimming performance, and ballast weight across various hydrodynamic conditions would provide valuable insights into these trade-offs.
The notable differences in tolerance and morphology exhibited by these three species reflect their divergent developmental traits and ecological niches. Diadema antillarum demonstrated a narrow tolerance for hydrodynamic conditions, with 100% mortality observed in the piloted slow 0.6 Pa treatment and less than 10% survival in the fast 3.5 Pa treatment. Additionally, D. antillarum displayed a more pronounced response to turbulent shear than T. ventricosus. This heightened sensitivity, along with differences in larval forms (with D. antillarum exhibiting transversus morphology and T. ventricosus and L. variegatus displaying typical forms), may reflect broad evolutionary differences between the species. It should be noted, however that the low- and high-shear treatments for D. antillarum (1.6 Pa and 3.5 Pa, respectively) were not equivalent to the shear levels applied to L. variegatus and T. ventricosus (0.6 Pa and 2.0 Pa, respectively), as lower shear caused complete mortality in D. antillarum. Consequently, interspecific comparisons are limited to the subset of shear levels that were survivable for all species, and this asymmetry should be considered when interpreting differences in larval responses across species.
In this study, measured egg diameters were 80 µm for D. antillarum, 86 µm for T. ventricosus, and 88 µm for L. variegatus, indicating relative similarity (e.g., T. ventricosus eggs were ~7.5% larger than those of D. antillarum). However, prior work by Lessios (1988c) reported more substantial differences in egg volume and lipid content, with L. variegatus showing significantly greater maternal provisioning than both T. ventricosus and D. antillarum. These discrepancies could reflect inter-population variability, methodological differences in how egg volume was measured (diameter vs. volume vs. lipid content), or seasonal and environmental effects on maternal investment. These patterns are consistent with broader developmental strategies among echinoids, where larval energy allocation, arm growth, and timing of rudiment development are all flexible responses to maternal investment and environmental conditions (Strathmann et al., 1992; Bertram and Strathmann, 1998; McEdward and Miner, 2001; Miner, 2003).
Our study found that higher shear conditions accelerated the onset of competence in T. ventricosus and D. antillarum. Despite intraspecific variations in competence across treatments, the ontogenetic timing (i.e., the development of paired arm appendages) remained consistent. This finding aligns with Gaylord et al. (2013), who used a Taylor-Couette cell to expose competent and precompetent S. purpuratus (typical morphology) larvae to high rotational speeds (450 rpm) at short durations (3 minutes). They discovered that while turbulent shear did not directly induce settlement in competent larvae, elevated turbulence accelerated the transition to competence. This suggests turbulence may play a functional role in habitat selection, a hypothesis supported by comparisons to hydrodynamic sensing used by zooplankton for predator avoidance (Gaylord et al., 2013). Hodin et al. (2020) corroborated these results by showing that species typically settling in high-energy environments exhibited increased settlement responses to turbulent shear. The shear conditions applied in this study were performed in a controlled laboratory setting to explore the potential influence of turbulent shear on larval development. However, the hydrodynamic conditions in experimental setups such as glass bottles, Taylor-Couette cells, and other laboratory chambers differ significantly from those in natural oceanic environments. The magnitude, distribution, and consequences of natural shear in the ocean are likely to be more complex and variable than the controlled conditions we simulated in the laboratory, and this distinction should be considered when extrapolating the results to natural settings. While the experimental shear levels used in this study were not based on field measurements of hydrodynamic conditions, they represent a sufficient range to maintain larval suspension and may provide insight into potential larval responses to shear in natural habitats, although exact flow rates likely differ.
This study provides the first documentation of arm-length plasticity in echinoid larvae in response to hydrodynamics, revealing distinct responses across species. Interspecific differences were evident: D. antillarum and T. ventricosus larvae exposed to higher shear developed longer arms and exhibited accelerated metamorphic development, traits that may aid dispersal and settlement in energetic environments. Conversely, L. variegatus demonstrated lower responsiveness to turbulent flow and broader hydrodynamic tolerance. The ability to adjust arm length to maintain orientation is potentially advantageous for environments with varying turbulence levels. The narrow hydrodynamic tolerance observed in D. antillarum suggests that shifts in flow regimes, such as those associated with climate-driven storms or reef degradation, could disproportionately impact larval survival and recruitment, with potential consequences for reef restoration and population resilience. While we suggest a connection between plastic arm growth and accelerated competence, further research is needed to confirm this link. These findings highlight the significant role of hydrodynamics in the entirety of larval development and emphasize the broader ecological implications of flow dynamics in marine systems.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study. Lytechinus variegatus and T. ventricosus broodstock were collected by licensed marine life collectors in the Florida Keys, USA. Diadema antillarum broodstock were collected from the same region by the Florida Fish and Wildlife Conservation Commission under National Oceanic and Atmospheric Administration permit number FKNMS-0218-023.
Author contributions
MD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JP: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. APe: Investigation, Writing – review & editing. JS: Investigation, Writing – review & editing. APi: Conceptualization, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This material is partially based upon work supported by the Defense Advanced Research Projects Agency under the Reefense Program, BAA HR001121S0012. The views, opinions and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government. The authors also acknowledge financial assistance provided by the Florida Department of Environmental Protection’s Coral Protection and Restoration Program through agreement C061D7.
Acknowledgments
We would like to express our sincere gratitude to Casey Hudspeth and Lucas Haus for their invaluable assistance in data collection, including taking photographs and counting larvae. Their support was essential to the success of this project. Additionally, I would like to extend my thanks to The Florida Aquarium for their help with urchin broodstock care and spawning, which greatly contributed to the outcomes of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1671120/full#supplementary-material
References
1
Baythavong B. S. (2011). Linking the spatial scale of environmental variation and the evolution of phenotypic plasticity: Selection favors adaptive plasticity in fine-grained environments. Am. Nat.178, 75–87. doi: 10.1086/660281
2
Bertram D. F. Strathmann R. R. (1998). Effects of maternal and larval nutrition on growth and form of planktotrophic larvae. Ecology79, 315–327. doi: 10.2307/176885
3
Bielmyer G. K. Brix K. V. Capo T. R. Grosell M. (2005). The effects of metals on embryo-larval and adult life stages of the sea urchin, Diadema antillarum. Aquat. Toxicol.74, 254–263. doi: 10.1016/j.aquatox.2005.05.016
4
Burke R. D. (1981). Structure of the digestive tract of the pluteus larva of Dendraster excentricus (Echinodermata: Echinoida). Zoomorphology98, 209–225. doi: 10.1007/bf00312050
5
Burke R. D. (1983). The structure of the larval nervous system of Pisaster ochraceus (Echinodermata: Asteroidea). J. Morphol.178, 23–35. doi: 10.1002/jmor.1051780103
6
Byrne M. Sewell M. A. Prowse T. A. A. (2008). Nutritional ecology of sea urchin larvae: influence of endogenous and exogenous nutrition on echinopluteal growth and phenotypic plasticity in Tripneustes gratilla. Funct. Ecol.22, 643–648. doi: 10.1111/j.1365-2435.2008.01427.x
7
Cameron R. A. (1986). Reproduction, larval occurrence and recruitment in Caribbean sea urchins. Bull. Mar. Sci39, 332–346.
8
Carpenter R. C. (1990). Mass mortality of Diadema antillarum: I. Long-term effects on sea urchin population-dynamics and coral reef algal communities. Mar. Biol.104, 67–77. doi: 10.1007/bf01313159
9
Casilagan I. L. N. Juinio-Meñez M. A. Crandall E. D. (2013). Genetic diversity, population structure, and demographic history of exploited sea urchin populations (Tripneustes gratilla) in the Philippines. J. Exp. Mar. Biol. Ecol.449, 284–293. doi: 10.1016/j.jembe.2013.09.012
10
Chandler L. M. Walters L. J. Sharp W. C. Hoffman E. A. (2017). Genetic structure of natural and broodstock populations of the long-spined sea urchin, Diadema antillarum, throughout the Florida Keys. Bull. Mar. Sci93, 881–889. doi: 10.5343/bms.216.1101
11
Cowen R. K. Lwiza K. M. Sponaugle S. Paris C. B. Olson D. B. (2000). Connectivity of marine populations: open or closed? Science287, 857–859. doi: 10.1126/science.287.5454.857
12
Dardik A. Chen L. Frattini J. Asada H. Aziz F. Kudo F. A. et al . (2005). Differential effects of orbital and laminar shear stress on endothelial cells. J. Vasc. Surg41, 869–880. doi: 10.1016/j.jvs.2005.01.020
13
Duggins D. O. (1980). Kelp beds and sea otters: an experimental approach. Ecology61, 447–453. doi: 10.2307/1937405
14
Emlet R. B. (1982). Echinoderm calcite: a mechanical analysis from larval spicules. Biol. Bull.163, 264–275. doi: 10.2307/1541265
15
Emlet R. B. (1983). Locomotion, drag, and the rigid skeleton of larval echinoderms. Biol. Bull.164, 433–445. doi: 10.2307/1541253
16
Feehan C. J. Brown M. S. Sharp W. C. Lauzon-Guay J. Adams D. K. (2016). Fertilization limitation of Diadema antillarum on coral reefs in the Florida keys. Ecology97, 1897–1904. doi: 10.1002/ecy.1461
17
Flowers J. M. Schroeter S. C. Burton R. S. (2002). The recruitment sweepstakes has many winners: genetic evidence from the sea urchin Strongylocentrotus purpuratus. Evolution56, 1445–1453. doi: 10.1111/j.0014-3820.2002.tb01456.x
18
Gaylord B. Hodin J. Ferner M. C. (2013). Turbulent shear spurs settlement in larval sea urchins. Proc. Natl. Acad. Sci.110, 6901–6906. doi: 10.1073/pnas.1220680110
19
Godinho W. O. Maggioni R. Lacerda A. L. Lotufo T. M. (2016). Haplotype distribution and connectivity of the white sea urchin Tripneustes ventricosus across the Brazilian biogeographic province (No. e2529v1). PeerJ. Preprints. doi: 10.7287/peerj.preprints.2529v1
20
Grünbaum D. Strathmann R. R. (2003). Form, performance and trade-offs in swimming and stability of armed larvae. J. Mar. Res.61, 659–691. doi: 10.1357/002224003771815990
21
Guete-Salazar C. Barros J. Velasco L. A. (2021). Spawning, larval culture, settlement and juvenile production of the west Indian Sea egg, Tripneustes ventricosus (Lamarck 1816), under hatchery conditions. Aquaculture544, 737059. doi: 10.1016/j.aquaculture.2021.737059
22
Gustafson T. Wolpert L. (1963). The cellular basis of morphogenesis and sea urchin development. Int. Rev. Cytol.15, 139–214. doi: 10.1016/s0074-7696(08)61117-1
23
Harborne A. R. Renaud P. G. Tyler E. H. M. Mumby P. J. (2009). Reduced density of the herbivorous urchin Diadema antillarum inside a Caribbean marine reserve linked to increased predation pressure by fishes. Coral. Reefs.28, 783–791. doi: 10.1007/s00338-009-0516-6
24
Harris L. G. Eddy S. D. (2015). Sea urchin ecology and biology. Echinoderm. Aquacult., 1–24. doi: 10.1002/9781119005810.ch1
25
Hart M. W. (1991). Particle captures and the method of suspension feeding by echinoderm larvae. Biol. Bull.180, 12–27. doi: 10.2307/1542425
26
Hart M. W. Strathmann R. R. (1994). Functional consequences of phenotypic plasticity in echinoid larvae. Biol. Bull.186, 291–299. doi: 10.2307/1542275
27
Hernández J. C. Clemente S. García E. McAlister J. S. (2020). Planktonic stages of the ecologically important sea urchin, Diadema africanum: larval performance under near future ocean conditions. J. Plankton. Res.42, 286–304. doi: 10.1093/plankt/fbaa016
28
Hernández J. C. Clemente S. Sangil C. Brito A. (2008). The key role of the sea urchin Diadema aff. antillarum in controlling macroalgae assemblages throughout the Canary Islands (eastern subtropical Atlantic): an spatio-temporal approach. Mar. Environ. Res.66, 259–270. doi: 10.1016/j.marenvres.2008.03.002
29
Hodin J. Ferner M. C. Gaylord B. (2020). Choosing the right home: settlement responses by larvae of six sea urchin species align with hydrodynamic traits of their contrasting adult habitats. Zool. J. Linn. Soc.190, 737–756. doi: 10.1093/zoolinnean/zlz149
30
Lang C. Mann K. H. (1976). Changes in sea urchin populations after the destruction of kelp beds. Mar. Biol.36, 321–326. doi: 10.1007/bf00389193
31
Leighton D. L. Jones L. G. North W. J. (1966). “Ecological relationships between giant kelp and sea urchins in southern California,” in Proceedings of the Fifth International Seaweed Symposium, Halifax, August 25–28, 1965. 141–153, Pergamon.
32
Lessios H. A. (1988a). Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annu. Rev. Ecol. Syst.19, 371–393. doi: 10.1146/annurev.ecolsys.19.1.371
33
Lessios H. A. (1988b). Population dynamics of Diadema antillarum (Echinodermata: Echinoidea) following mass mortality in Panama. Mar. Biol.99, 515–526. doi: 10.1007/bf00392559
34
Lessios H. A. (1988c). Temporal and spatial variation in egg size of 13 Panamanian echinoids. J. Exp. Mar. Biol. Ecol.114, 217–239. doi: 10.1016/0022-0981(88)90139-6
35
Maciá S. Lirman D. (1999). Destruction of Florida Bay seagrasses by a grazing front of sea urchins. Bull. Mar. Sci65, 593–601.
36
Maldonado E. M. (2009). Biological-physical interactions in marine plankton: the effects of small-scale turbulence on grazing, growth, and swimming of sea urchin larvae (UC San Diego).
37
McAlister J. S. (2008). Evolutionary responses to environmental heterogeneity in Central American echinoid larvae: plastic versus constant phenotypes. Evolution62, 1358–1372. doi: 10.1111/j.1558-5646.2008.00368.x
38
McAlister J. S. Miner B. G. (2018). Phenotypic plasticity of feeding structures in marine invertebrate larvae (Oxford, UK: Oxford University Press), 103–123.
39
McEdward L. R. Miner B. G. (2001). Echinoid larval ecology. Develop. Aquacult. Fishe. Sci32, 59–78. doi: 10.1016/s0167-9309(01)80006-5
40
Miller B. A. Emlet R. B. (1997). Influence of nearshore hydrodynamics on larval abundance and settlement of sea urchins Strongylocentrotus franciscanus and S. purpuratus in the Oregon upwelling zone. Mar. Ecol. Prog. Ser.148, 83–94. doi: 10.3354/meps148083
41
Miner B. G. (2003). Evolution of phenotypic plasticity: Insights from Echinoid larvae. (Publication No. 312674) (Gainesville, FL, USA: University of Florida). ProQuest Dissertations & Theses Global.
42
Mos B. Cowden K. L. Nielsen S. J. Dworjanyn S. A. (2011). Do cues matter? Highly inductive settlement cues don’t ensure high post-settlement survival in sea urchin aquaculture. PloS One6, e28054. doi: 10.1371/journal.pone.0028054
43
Nesbit K. T. Hamdoun A. (2020). Embryo, larval, and juvenile staging of Lytechinus pictus from fertilization through sexual maturation. Dev. Dynamics.249, 1334–1346. doi: 10.1002/dvdy.223
44
Nunez J. A. M. (2017). Assortative Mating in the Tropical Sea Urchin Lytechinus Variegatus (Master's thesis Florida State University).
45
Pennington J. T. Strathmann R. R. (1990). Consequences of the calcite skeletons of planktonic echinoderm larvae for orientation, swimming, and shape. Biol. Bull.179, 121–133. doi: 10.2307/1541746
46
Pilnick A. R. O’Neil K. L. DiMaggio M. A. Patterson J. T. (2022). Development of larviculture protocols for the long-spined sea urchin (Diadema antillarum) and enhanced performance with diets containing the cryptophyte Rhodomonas lens. Aquacult. Int.30, 3017–3034. doi: 10.1007/s10499-022-00945-0
47
Pilnick A. R. O’Neil K. L. Moe M. Patterson J. T. (2021). A novel system for intensive Diadema antillarum propagation as a step towards population enhancement. Sci. Rep.11, 11244. doi: 10.1038/s41598-021-90564-1
48
Pilnick A. R. Petrosino A. Hassan M. M. Patterson J. T. (2023). Cue selection and ontogeny reveal larval settlement dynamics of the long-spined sea urchin Diadema antillarum, a keystone coral reef herbivore. Mar. Biol.170, 139. doi: 10.1007/s00227-023-04290-5
49
Pineda J. Porri F. Starczak V. Blythe J. (2010). Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. J. Exp. Mar. Biol. Ecol.392, 9–21. doi: 10.1016/j.jembe.2010.04.008
50
Prado P. Tomas F. Pinna S. Farina S. Roca G. Ceccherelli G. et al . (2012). Habitat and scale shape the demographic fate of the Keystone Sea Urchin Paracentrotus lividus in Mediterranean macrophyte communities. PloS One7, e35170. doi: 10.1371/journal.pone.0035170
51
Precht L. L. Precht W. F. (2015). The sea urchin Diadema antillarum–keystone herbivore or redundant species? PeerJ. PrePrints.3, e1565v1. doi: 10.7287/peerj.preprints.1565v2
52
R Core Team (2024). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org.
53
Randall J. E. Schroeder R. E. Starck W. A. (1964). Notes on the biology of the echinoid Diadema antillarum. Caribbean. J. Sci4, 421–433.
54
Rendleman A. J. Pace D. A. (2018). Physiology of growth in typical and transversus echinopluteus larvae. Invertebrate. Biol.137, 289–307. doi: 10.1111/ivb.12227
55
Roughgarden J. Gaines S. Possingham H. (1988). Recruitment dynamics in complex life cycles. Science241, 1460–1466. doi: 10.1126/science.11538249
56
Sameoto J. A. Ross T. Metaxas A. (2010). The effect of flow on larval vertical distribution of the sea urchin, Strongylocentrotus droebachiensis. J. Exp. Mar. Biol. Ecol.383, 156–163. doi: 10.1016/j.jembe.2009.11.014
57
Sewell M. A. Cameron M. J. McArdle B. H. (2004). Developmental plasticity in larval development in the echinometrid sea urchin Evechinus chloroticus with varying food ration. J. Exp. Mar. Biol. Ecol.309, 219–237. doi: 10.1016/j.jembe.2004.03.016
58
Soars N. A. Prowse T. A. A. Byrne M. (2009). Overview of phenotypic plasticity in echinoid larvae, ‘echinopluteus transversus’ type vs. typical echinoplutei. Mar. Ecol. Prog. Ser.383, 113–125. doi: 10.3354/meps07848
59
Strathmann R. R. Fenaux L. Strathmann M. F. (1992). Heterochronic developmental plasticity in larval sea urchins and its implications for evolution of nonfeeding larvae. Evolution46, 972–986. doi: 10.1111/j.1558-5646.1992.tb00613.x
60
Visconti G. Gianguzza F. Butera E. Costa V. Vizzini S. Byrne M. et al . (2017). Morphological response of the larvae of Arbacia lixula to near-future ocean warming and acidification. ICES. J. Mar. Sci74, 1180–1190. doi: 10.1093/icesjms/fsx037
61
Wheeler J. D. Chan K. Y. K. Anderson E. J. Mullineaux L. S. (2016). Ontogenetic changes in larval swimming and orientation of pre-competent sea urchin Arbacia punctulata in turbulence. J. Exp. Biol.219, 1303–1310. doi: 10.1242/jeb.129502
62
Wijers T. Hylkema A. Pilnick A. R. Murk A. J. Patterson J. T. (2023). Novel shaker bottle cultivation method for the long spined sea urchin (Diadema antillarum; Philippi 1845) results in high larval survival and settlement rates. Aquaculture562, 738855. doi: 10.1016/j.aquaculture.2022.738855
63
Yaguchi S. Taniguchi Y. Suzuki H. Kamata M. Yaguchi J. (2022). Planktonic sea urchin larvae change their swimming direction in response to strong photoirradiation. PloS Genet.18, e1010033. doi: 10.1371/journal.pgen.1010033
Summary
Keywords
developmental plasticity, larva, echinoid, competence, turbulence
Citation
Dakin M, Patterson J, Petrosino A, Smith J and Pilnick A (2025) Plasticity under pressure: the influence of shear stress on larval echinoid morphogenesis. Front. Mar. Sci. 12:1671120. doi: 10.3389/fmars.2025.1671120
Received
22 July 2025
Accepted
10 September 2025
Published
24 September 2025
Volume
12 - 2025
Edited by
Francesca Porri, South African Institute for Aquatic Biodiversity, South Africa
Reviewed by
Adriana Giangrande, University of Salento, Italy
Mirko Mutalipassi, Stazione Zoologica Anton Dohrn, Italy
Updates
Copyright
© 2025 Dakin, Patterson, Petrosino, Smith and Pilnick.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joshua Patterson, joshpatterson@ufl.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.