Abstract
Microplastic (MP) pollution is an emergent global threat with widespread implications for ecological integrity, food security, and public health. These particles, typically smaller than 5 mm, originate from diverse sources, including the breakdown of larger plastic debris and direct emissions from products and industrial processes. This review critically examines the current understanding of MP sources, environmental distribution, detection technologies, ecotoxicological impacts, and mitigation strategies. Incorporating recent advances—including AI-enhanced detection, microbe-mediated degradation, and circular economy policies—it provides a comprehensive, multidisciplinary synthesis and proposes a roadmap toward microplastic-free ecosystems. It highlights the complex journey of microplastics through various ecosystems, driven by processes such as photolysis, weathering, and microbial activity, and their subsequent transportation via water bodies, soil, and atmospheric deposition. The review emphasizes recent innovations in detection techniques, including hyperspectral imaging, machine learning algorithms, and laser-induced breakdown spectroscopy (LIBS), which have significantly enhanced the sensitivity and accuracy of microplastic identification across complex environmental matrices. The ecotoxicological impacts of microplastics, including their physical and chemical effects on aquatic organisms and potential for bioaccumulation and trophic transfer, are explored in depth, underscoring the urgency of addressing this global issue. The review discusses advanced mitigation strategies, such as biodegradable alternatives, circular economy approaches, and stringent regulatory measures, which are essential to reduce the environmental burden of microplastics. Integrating scientific innovation with robust policy frameworks is crucial to curb the widespread dispersion of microplastics and mitigate their long-term impacts on ecosystems and human health. This review advances our understanding of microplastic pollution and serves as a call to action for coordinated global efforts to address this pressing environmental challenge.
1 Introduction
Plastics, known for their durability, corrosion resistance, low density, and affordability, have become vital in many fields, such as agriculture, industry, and everyday life (Rochman and Hoellein, 2020). Once celebrated, this material now poses a significant environmental threat. By 2020, worldwide plastic production had reached an astonishing 9.0 × 109 tonnes and continues to grow (Pan et al., 2023; Xu et al., 2023). Alarmingly, only 78% of this waste is managed properly, leaving 22% to pollute our environment (OECD, 2022). The scale of plastic production and waste has sparked an unprecedented environmental crisis, with projections suggesting that by 2050, annual plastic output could exceed 34 billion tonnes (Geyer et al., 2017; Silva et al., 2020a). The dramatic rise since the 1950s is exemplified by the 359 million tonnes produced in 2018 alone (PlasticsEurope, 2019). This growth has led to a global crisis, with just 9% of plastic waste recycled, 12% incinerated, and 79% landfilled or released into nature (Geyer et al., 2017). Single-use plastics, which made up about 50% of global waste in 2015, have worsened pollution due to improper disposal (UNEP, 2018). Achieving the Sustainable Development Goals (SDGs) by 2030 is crucial to tackling this crisis, requiring united efforts from all countries (UN, 2015). Over the past 25 years, global plastic production has tripled (Feil and Pretz, 2020), with most of the estimated 8.3 billion tonnes of virgin plastics being single-use and often discarded into natural environments (Tables 1, 2). This has caused severe land impacts, including landfill accumulation, soil contamination, and higher greenhouse gas emissions. Recent studies highlight plastics’ harmful effects on soil microbiota activity and diversity, reproductive health in soil organisms (Lahive et al., 2019), and leaching effects in soil invertebrates (Selonen et al., 2020).
Table 1
| Country | Site | Sources | Types | Size | Reference |
|---|---|---|---|---|---|
| Antarctica | Between Marie Byrd Land and Victoria Land of Ross Sea Bay | The vicinity of wastewater treatment plants (WWTPs), ship traffic, coastal activities, transportation via sea chains, and other factors | Polyethylene, polypropylene, polyethylene styrene, polyacrylic acid, and polymethyl methacrylate | NA | Cincinelli et al., 2017 |
| Australia | Rivers in Victoria | Field of agriculture, close to the city | Rayon, PA, and polyethylene styrene | NA | Nan et al., 2020 |
| Bangladesh | Floating trash in the ocean | PS and other MP polystyrene | < 5 cm | Ryan, 2013 | |
| China | Dongting Lakes and Hong | Fishing operations, surface runoff, agriculture, air deposition, and waste and effluents from cities and sewage plants | PE and polypropylene | In the MP samples, <2 mm predominated; <300 μm, greater than 20% of all MPs gathered from the two lakes | Wang et al., 2018 |
| Inside Guangdong-Hong, Greater Kong-Macao Bay Area, the Maozhou River | Sources from the city and industry | PS, polyvinyl chloride, polypropylene, and PE | 100–1,000 μm | ||
| Tenregganu coastal waters, China | Boats, fishing nets, and water waste | Acrylic, EVA (ethylene-vinyl acetate), PP, polyethylene styrene, PE, and polyamide | NA | Md Amin et al., 2020 | |
| China Wei River | Industries, agriculture, human activity in the context of regional features, and domestic sewage | Polyethylene, polyvinyl chloride, and polyethylene styrene | 500 μm, 1,000 μm, 2,000 μm, 3,000 μm, 4,000 μm, 5,000 μm, and more than 5,000 μm | Ding et al., 2019 | |
| Fiji | Coastal waters of Viti Levu, South Pacific | Cloth washing, wastewater treatment | PET, polyethylene, PP, nylon, cellulose acetate, EVA, latex, nitrile, polycarbonate, polymethyl methacrylate, polyethylene styrene, polyurethane, polyvinyl acetate, and PVC | 0.5–0.9 mm, 1.0–1.4 mm totaling 48% of the samples | Dehm et al., 2020 |
| Suva coastal waters | Human activities on land and fishing | Polyethylene, Latex, Polypropylene, Nylon, PET, Polyethylene Styrene, EVA | NA | Ferreira et al., 2020 | |
| France | Gulf of Lyon ((NW) Mediterranean Sea) | Anthropogenic action, proximity to towns and cities, upstream populated regions and highly processed products | NA | Average of 1,480 μm (± 880 μm) | Schmidt et al., 2018 |
| Hawaii | Western Pacific Ocean | Fishing gear, including nets, clothes, and accessories, with an emphasis on moving MPs via the North Equatorial Counter Current | Polypropylene, polymethyl methacrylate, PE, and PET | 1,000–2,500 μm (35.1%); 300–500 μm (18.5%); 500–100,000 μm (28.5%) | Wang et al., 2020 |
| India | Southwest coastal waters | Offshore transportation, tourist activities, river flow, fisheries, and proximity to urban agglomeration | Polyethylene, polypropylene, alkyd, rayon, PS, cellulose, others | 300–600 μm | Enders et al., 2015 |
| Indonesia | Jatiluhur Reservoir | Human activity related to plastics (not specified) and fishing industries of the region | Polyethylene and polypropylene | 100–500 μm (13.21%), 501– 1,000 μm (37.18%), 1,001–5,000 μm (49.61%) | Ramadan and Sembiring, 2020 |
| Southern coast of Pramuka Island and Southern coast of Jakarta Bay | Disposal of textiles and utilization of fishing lines and nets | Fibers and fragments | NA | Priscilla et al., 2019 | |
| Italy | Coastal waters of Tuscany | NA | Polyethylene, polypropylene, ethylene-vinyl acetate, and styrene butadiene | <500 μm, 500–1,000 μm, 1,000–2,500 μm, and 2,500–5,000 μm, the largest quantity between 1,000 and 2,500 μm | Baini et al., 2018 |
| Subalpine lakes | Municipal disposal, sewage, urban runoff | Polyethylene, polypropylene, expanded polystyrene, polyethylene styrene, PET, PU, PVC, cellulose acetate, polyester, and acrylonitrile-butadiene-styrene | <1,000 μm, 1,000 < size <5,000 μm and >5,000 μm | Sighicelli et al., 2018 | |
| Kenya | Lake Naivasha | Human waste, because every year more people move into the area around the lake | Polyethylene, polyester, and polypropylene | NA | Migwi et al., 2020 |
| Kwazulu-Natal | South Africa Estuary | The port is used by industries that can dump waste into rivers and bays | Polyethylene styrene | 250–500 μm, 500–1,000 μm, 1,000–5,000 μm, >5,000 μm | Naidoo et al., 2015 |
| Netherland | Dutch riverine | Wastewater treatment plant discharges (WWTPs) | Polyethylene, polypropylene, diene monomer rubber, and ethylene propylene | 67.1% < 100 μm 26.3% < 25 μm 18.5% 25–50 μm 6.7% > 300 μm 1.1% > 1,000 μm | Mintenig et al., 2020 |
| Nigeria | Yenagoa (Ox-Bow Lake) | Airborne particles, industrial effluent, and sewage all contribute to this problem; aquaculture, farming, watering, and garbage from cities and towns | Polyethylene terephthalate, plasticized polyvinyl chloride | 20–500 μm (4.3%), 500–1,000 μm (6.7%), 1,000–3,000 μm (74.9%), 3,000–5,000 μm (14.1%) | Oni et al., 2020 |
| Northeast Greenland | Arctic Ocean | NA | Polyethylene, polypropylene, polyvinyl chloride, polyethylene styrene, and PA | 1,600 and <500 μm | Morgana et al., 2018 |
| Pakistan | Ravi River, Lahore city area | NA | Polyethylene, polypropylene, and polyethylene styrene | <300 to 5,000 μm | Irfan et al., 2020 |
| Patagonia | Patagonia Lakes | Urban garbage and fishing; the disposal of plastic fibers into the atmosphere | Polyethylene, polypropylene, and polyethylene styrene and Indigo Blue dye compound from textile fibers | 200 and <400 μm | Alfonso et al., 2020 |
| Qatar | Marine waters | Ship operations, such as hull losses and ballast water tanks, and oil rig installations are close nearby | Polypropylene, polyethylene styrene, PA, low-density polyethylene, PE, poly (methyl methacrylate), cellophane, and acrylonitrile-butadiene-styrene | 125 to 1820 μm (granular form) 50 to 15980 μm (fibrous form) | Castillo et al., 2016 |
| South Korea | Marine water | NA | MPs consisted of intact plastics, fragment, and styrofoam | NA | Lee et al., 2013 |
| Spain | Ebro River (NW Mediterranean) | Irrigation and drainage channels, wastewater treatment plants (WWTPs) | PA, polyethylene, polymethyl methacrylate, polyester, polypropylene, and polyacrylate | <50–>3,000 μm | Simon-Sánchez et al., 2019 |
| Sweden/Skagerrak | Gulf of Bothnia, Kattegat, Baltic Sea and | NA | Polyethylene, polypropylene | Drag manta: 30 μm; pump: ≥30 and 50 μm | Schönlau et al., 2020 |
| Turkey | Sea of Marmara | Disposal water | PVC, polystyrene, PP, and PE | There is a peak quantity between 2,000 and 4,000 μm | Tunçer et al., 2018 |
| UK | Southern North Sea | NA | PP, acrylates/polyurethane/varnish and polyamide | 86% <100 μm; 11–5,000 μm | Lorenz et al., 2019 |
| USA | Tampa Bay, Florida | Disposal water, beauty products, synthetic fibers | Fibers and beads, polyethylene, polypropylene, and PVC in smaller quantity | 63–630 μm, 630–5,000 μm | McEachern et al., 2019 |
| Charleston Harbor Estuary, North Carolina | Debris and wear on tires | Fragments most TWP-tire wear particle, fibers, polypropylene, and polyvinyl alcohol | NA | Morgana et al., 2018 |
Global aquatic distribution of microplastics.
NA, Not applicable.
Table 2
| Location/site | Source | Types | Reference |
|---|---|---|---|
| Andaman (Port Blair) and Nicobar Islands |
Sediment | Surlyn ionomer, PEI, acrylic, PPS, acrylonitrile, NY, EVA, PIP, PU, ethylene, vinyl alcohol PVC, Fiber, fragment, pellet |
Goswami et al., 2020 |
| Andaman (Port Blair) and Nicobar Islands |
Water | Surlyn ionomer, PEI, acrylic, PPS, acrylonitrile, NY, EVA, ethylene vinyl alcohol, PVC, Fiber, fragment, pellet, PIP, PU |
Goswami et al., 2020 |
| Andaman & Nicobar Archipelago |
Sediment | Irregular, filament, film, pellet, polyethylene, PVC, polypropylene, PS, NY, others |
Krishnakumar et al., 2020; Nobi et al., 2010 |
| Andaman Islands (South) | Sediment | Fragment, fiber, spherule poly dimer acid-coalkyl, polyamine, polypropylene, melamine, PVF, polyperfluoroethylene oxide, polysulfide, polybutadiene, Polybutadiene-acrylonitrile acrylic acid, PVB, PVC, nylon 6, epoxy epichlorohydrin, ABS |
Patchaiyappan et al., 2020; Sachithanandam et al., 2020 |
| Arunachal Pradesh (Brahmaputra River) |
Fibers, fragments, beads | Tsering et al., 2021 | |
| Chennai (Kosasthalaiyar River) | Fibers, fragments, films, pellets | Lechthaler et al., 2021 | |
| Chennai (Adyar River) | Fibers, fragments | Lechthaler et al., 2021 | |
| Goa (Keri, Vagator, Calangute, Colva, Mobor and Galgibaga beaches |
Sediment | Pellet, polyethylene, polypropylene | Veerasingam et al., 2016 |
| Goa (Palolem Beach) | Sediment | Fiber | Balasubramaniam and Phillott, 2016 |
| Goa (Vagator, Calangute, Colva) |
Sediment | Fragment, fiber, film, pellet polyethylene, polypropylene, others | Maharana et al., 2020 |
| Goa (South–Sal River) | Fibers, fragments, films | Ma et al., 2019 | |
| Gujarat (Alang-Sosiya ship-breaking yard) |
Sediment | Fragment PU, NY, PEST, PS | Reddy et al., 2006 |
| Gujrat (Sabarmati River) | Fibers | ||
| Haridwar (Ganga River) | Fragments, films, fibers | ||
| India and Bangladesh (Ganga River) | Fibers, fragments | Napper et al., 2021 | |
| Jabalpur City, Madhya Pradesh (Narmada River) | Fibers, fragments, films, beads | Tomar, 2022 | |
| Kanpur (Ganga River) | Fragments, fibers, films | ||
| Karnataka (Netravathi River) | Fibers, films, fragments | Amrutha and Warrier, 2020 | |
| Karnataka (Sharavathi River) | Fibers | Amrutha et al., 2022 | |
| Karnataka (Devbagh, Karwar, Kasarkod) |
Sediment | Fragment, fiber, film, pellet, polyethylene, polypropylene, others | Maharana et al., 2020 |
| Kerala (Kochi) | Sediment | Film, filament, foam, pellet, fiber, fragment | James et al., 2020; Joseph et al., 2019 |
| Kerala (Muthirappuzhayar River) | Fibers, fragments | Lechthaler et al., 2021 | |
| Kerala (Periyar River) | Fibers, fragments, film, foam, pellets, and round | Joshy et al., 2022 | |
| Kerala (Mahe, Koyilandy, Padinjarekkara, Munakkal, Azheekkal, Varkala, Veli, Poovar) |
Sediment | Fragment, fiber/line, foam polyethylene, polypropylene, PP, PA, PET, RY, PU, alkyd, CE, ABS, PVC, PVF | Robin et al., 2020 |
| Lakshadweep (Tinnakara) | Sediment | Pellet |
Mugilarasan et al., 2017, Thangaradjou et al., 2014 |
| Maharashtra (Aksa, Juhu, Dadar, Girgaon) |
Sediment | Fragment, fiber, film, pellet, PE, PP, others |
Maharana et al., 2020, Jayasiri et al., 2014 |
| Maharashtra (Girgaon, Mumbai) |
Sediment | Granule, fiber, film PE, PET, PS, PP, PVC, others | Tiwari et al., 2019, Ingole & Kadam, 2003 |
| Maharashtra (Mumbai), | Sediment | Pellet, polyethylene, polypropylene, others | Ogata et al., 2009 |
| Pondicherry (Puducherry) | Sediment | Fragment, fiber/line, pellet, film/sheet, foam, polyethylene, polypropylene, HDPE, LDPE, PS, PVC, CA, PVK, polypropylene, acrylic acid, polymer resin, polyvinyl behenate, acrylonitrile/styrene copolymer | Dowarah & Devipriya, 2019, Solai et al., 2013 |
| South India (Kaveri River) | Fibers, fragments, films, foams | Maheswaran et al., 2022 | |
| Tamil Nadu (Chennai) | Sediment | Pellet, polyethylene, polypropylene |
Mugilarasan et al., 2017, Tholkappian et al., 2018; Veerasingam et al., 2016, Suman et al., 2020 |
| Tamil Nadu (Dhanushkodi) | Sediment | Granule, fiber, film polyethylene, polypropylene, PET, PS, PVC, others |
Tiwari et al., 2019 |
| Tamil Nadu (Gulf of Mannar, Nallathani Island) |
Sediment | Polyethylene, polypropylene PVC, NY, others | Krishnakumar et al., 2018 |
| Tamil Nadu (Kanyakumari) | Sediment | Fiber, fragment | |
| Tamil Nadu (Marina Beach, Manapad, Kanyakumari, Thiruchendur, Tuticorin) |
Sediment | Fiber, fragment, foam, polyethylene, polypropylene, NY, PEST | Sathish et al., 2019 |
| Tamil Nadu (Rameswaram Island) |
Sediment | Fiber, fragment, film, foam, polyethylene, polypropylene, PET, PA, CP, PU, PEST, PS, PVA, PVC |
Jeyasanta et al., 2020a |
| Tamil Nadu (Rameswaram Island) |
Water | Fiber, fragment, film, foam, polyethylene, polypropylene, PET, PA, CP, PU, PEST, PS, PVA, PVC |
Jeyasanta et al., 2020a |
| Tamil Nadu (Silver Beach) | Sediment | Pellet, fiber, irregular PVC, polyethylene, NY | Vidyasakar et al., 2020, Krishnakumar et al., 2020b |
| Tamil Nadu (Tuticorin) | Water | Fiber, film, fragment, foam, polyethylene, polypropylene, PA, PEST, RY, PET, PVC, PVA, PS, blended PE-PP |
Sathish et al., 2020b, Rajaram et al., 2020 |
| Tamil Nadu (Tuticorin) | Sediment | Fiber, film, fragment, foam, polyethylene, polypropylene, PVC, PS, PET |
Jeyasanta et al., 2020b, Rajaram et al., 2020 |
| Tamil Nadu (Tuticorin & Vembar Coral Islands) |
Sediment | Fiber, fragment, film, foam, polyethylene, polypropylene, PA, PEST, PET, PVC, PVA, PEU, alkyd resin | Patterson et al., 2020 |
| Tamil Nadu (Tuticorin & Vembar Coral Islands) | Water | Fiber, fragment, film, foam, polyethylene, polypropylene, PA, PEST, PET, PVC, PVA, PU |
Patterson et al., 2020, Rajaram et al., 2020 |
| Uttarakhand (Alakananda River) | Fibers, fragments, films, pellets, foams | Chauhan et al., 2021 |
Indian scenario of microplastics in sediment and water (Vaid et al., 2021).
The widespread distribution of microplastics worldwide is now a serious concern, as these particles are found in various environments. They are present in urban, island, and beach ecosystems as well as in water bodies such as oceans, rivers, lakes, and reservoirs and even in the atmosphere (Auta et al., 2017; Li et al., 2018; Chen et al., 2020). The enormous quantity of plastic waste entering the oceans annually—estimated between 4.8 and 12 million tonnes—underscores the severity of this environmental issue (Jambeck et al., 2015). Marine sources of microplastics are diverse, including the breakdown of marine plastic debris, land-based transport via rivers, plastic waste from tourism, discarded fishing gear, and atmospheric deposition (Dong et al., 2020). In freshwater systems, key sources include urban runoff, wastewater discharge, fishing activities, and land-based plastic waste (Liu et al., 2019; Yan et al., 2019).
In 2014, Marcus Eriksen from the Five Gyres Institute highlighted the extent of marine plastic pollution, estimating over 5.25 trillion fragments totaling 269,000 tonnes spread across the oceans (Eriksen et al., 2019). Plastics degrade gradually through physical, chemical, and biological processes, creating fragments classified as macroplastics (>20 mm), mesoplastics (5–20 mm), microplastics (<5 mm), and nanoplastics (<0.0001 mm) (Olivatto et al., 2019; Thompson et al., 2009). Microplastics are most studied due to their widespread presence (Hendrickson et al., 2018; Tran et al., 2023). The first marine plastic debris was identified in 2004 by Plymouth University researchers, led by Thompson, who introduced the term “microplastics” (Thomson et al., 2011). These fragments vary by shape spheres, pellets, foams, fibers, fragments, and films and by color, polymer type, and origin. They come from primary plastics made for industry or secondary plastics from larger debris breakdown (Cole et al., 2011; Silva et al., 2018). Physical and chemical processes reduce polymers into smaller particles (Arthur et al., 2009; Potrykus et al., 2021). Detecting and monitoring microplastics in environments require sophisticated techniques to accurately quantify and characterize particles. Traditional methods like visual identification and density separation have been enhanced with FTIR, Raman spectroscopy, and Py-GC/MS, allowing the identification of polymer types and the detection of smaller particles (Prata et al., 2019; Shim et al., 2017). Recent innovations aim to improve sensitivity and accuracy, especially in complex matrices like soil and sediment—for example, hyperspectral imaging and machine learning automate identifying microplastics, reduce human error, and increase sample throughput. Non-invasive methods like laser-induced breakdown spectroscopy (LIBS) enable in situ, real-time monitoring of contamination levels in ecosystems (Hu et al., 2021).
Microplastics impact ecosystems profoundly across all levels. In aquatic environments, organisms from zooplankton to whales ingest microplastics, leading to adverse effects like impaired feeding, growth, reproduction, immunity, and genetic health, disrupting food webs and biodiversity (Rezania et al., 2018). They also carry toxins such as POPs, heavy metals, and additives, which bioaccumulate and biomagnify, threatening ecosystem and human health (Wang et al., 2016; Verla et al., 2019). Physically, microplastics can cause intestinal blockage and abrasion, compounded by chemical toxicity. In land ecosystems, microplastics alter soil structure, reduce microbial activity, and hinder plant growth, impacting crop yields and soil fertility (de Souza MaChado et al., 2018). They contaminate soils through sewage sludge and plastic mulching, raising concerns about long-term food system sustainability (Ng et al., 2018). Addressing microplastic pollution requires improving waste management, promoting biodegradable plastics, enforcing stricter regulations, and developing advanced filtration for wastewater plants, which are key pollution sources (Carr et al., 2016).
Recent advances reveal the widespread presence of microplastics (MPs) across ecosystems and their complex environmental and biological effects. Globally, policies—from circular economy to cleaning efforts—aim to reduce MP pollution, underlining its urgency (Alam and Rahman, 2025). Sustainable strategies addressing soil, water, and food contamination focus on biodegradation, phytoremediation, and policy coherence for remediation (Bhattacharjee and Roy, 2025). In wastewater, sewage sludge contains significant MPs, influenced by solid concentrations, leading to long-term terrestrial pollution as shown by a 25-year study (Casella et al., 2025). In freshwater, MPs threaten fish health through ingestion, inflammation, and oxidative stress (Ghosh et al., 2025). Terrestrial MPs originate from agrochemicals, biosolids, and atmospheric fallout, traveling through water and food webs. The fate of plastics in soils depends on additive leaching and ingestion, requiring impact assessments (Vázquez-Vázquez et al., 2025). Detection, tracing, and sustainable waste policies are vital for aquatic MP mitigation (Wu et al., 2025). On a molecular level, MPs affect marine biological pathways, linked to immunotoxicity and endocrine disruption (Yoganandham, 2025). Legislation struggles to regulate MPs, though evidence shows genotoxic and neurotoxic effects at the nanoscale (Casella et al., 2024; Casella and Ballaz, 2024). Plastic production hit 400.3 million tonnes in 2022, forecasting increased MP pollution unless stricter policies, technology, and sustainable practices are adopted.
This comprehensive review covers the multifaceted issue of microplastic (MP) pollution, including production, sources, distribution, ecological impacts, and removal strategies. It discusses their presence in aquatic ecosystems, accumulation in organisms, analytical techniques like microscopy and spectroscopy, and advanced extraction methods such as CPE and APLE. Mitigation strategies include ecolabeling, recycling, bans, clean-up efforts, behavioral changes, and various removal methods like adsorption and membrane separation. Degradation processes examined encompass physical (incineration, filtration), chemical (Fenton oxidation, coagulation), and biological (microbial, enzymatic) methods, with recent advances in biofilm and nanomaterial technologies. Challenges, research gaps, and future directions emphasize interdisciplinary collaboration, detection harmonization, and policy measures to reduce risks. The review highlights the importance of detection technologies, evaluates removal methods, and explores innovative biodegradation techniques, including genetic and enzyme-based approaches, calling for molecular cloning and pathway design to enhance degradation. It stresses the need for robust methodologies and scientometric analyses to develop effective countermeasures against environmental and health impacts of microplastics, citing studies by Waring et al. (2018); Garrido Gamarro et al. (2020), and Zhou et al. (2020). The structured review covers plastic classification, environmental occurrence, detection methods, health implications, and emerging mitigation technologies. It advocates scaling lab results to real-world solutions, proposing integrated degradation systems and emphasizing global policies like China’s plastic waste ban, circular economy, public engagement, and biotech solutions to combat pollution.
2 Production of plastics and emergence of microplastics
Over the past seven decades, the world’s plastic production has grown exponentially, from a small 1.5 million tonnes per year in the 1950s to over 359 million tonnes annually, with forecasts suggesting that it will soon hit 500 million tonnes (Bui et al., 2020; Huang et al., 2021). Asia remains the top contributor, especially China, which alone produces about 63 million tonnes each year. When combined with other Asian countries, the continent contributes over 114 million tonnes, followed by the European Union (50 million tonnes) and North America (49 million tonnes) (Ryan, 2015; Kumar et al., 2021) (Tables 1, 2). Meanwhile, nearly 37 million tonnes come from regions including the Middle East, Africa, the Commonwealth, and Latin America, which still make significant contributions to global plastic production. The challenges of managing plastic waste continue to be significant. A large portion of plastic waste is incinerated, landfilled, or released into the environment without control. In the United States, only about 10% of plastic waste is recycled (Cessi et al., 2014), and worldwide, more than 75% of marine debris is made of plastics. The Mediterranean Sea, once known for its rich biodiversity and clear waters, has now become one of the most microplastic-polluted areas in the world. Five countries—Turkey, Spain, Italy, Egypt, and France—are the main contributors, with Turkey alone releasing an estimated 144 tonnes of plastic waste into the sea each day (Sharma et al., 2021).
Particularly troubling is the increasing concern over microplastics, which are plastic particles smaller than 5 mm. Thousands of particles per cubic meter are currently found in coastal waters; if immediate action is not taken, this number is expected to quadruple in the coming years (Isobe et al., 2019) (Figures 1–4). Accurately measuring microplastics remains difficult due to the lack of standardized sampling protocols, leading to potential underestimations (Brandon et al., 2020). Microplastics not only persist environmentally because of their chemical stability but also serve as carriers of toxicants such as heavy metals and persistent organic pollutants, posing significant ecological risks (Van Emmerik et al., 2018).
Figure 1
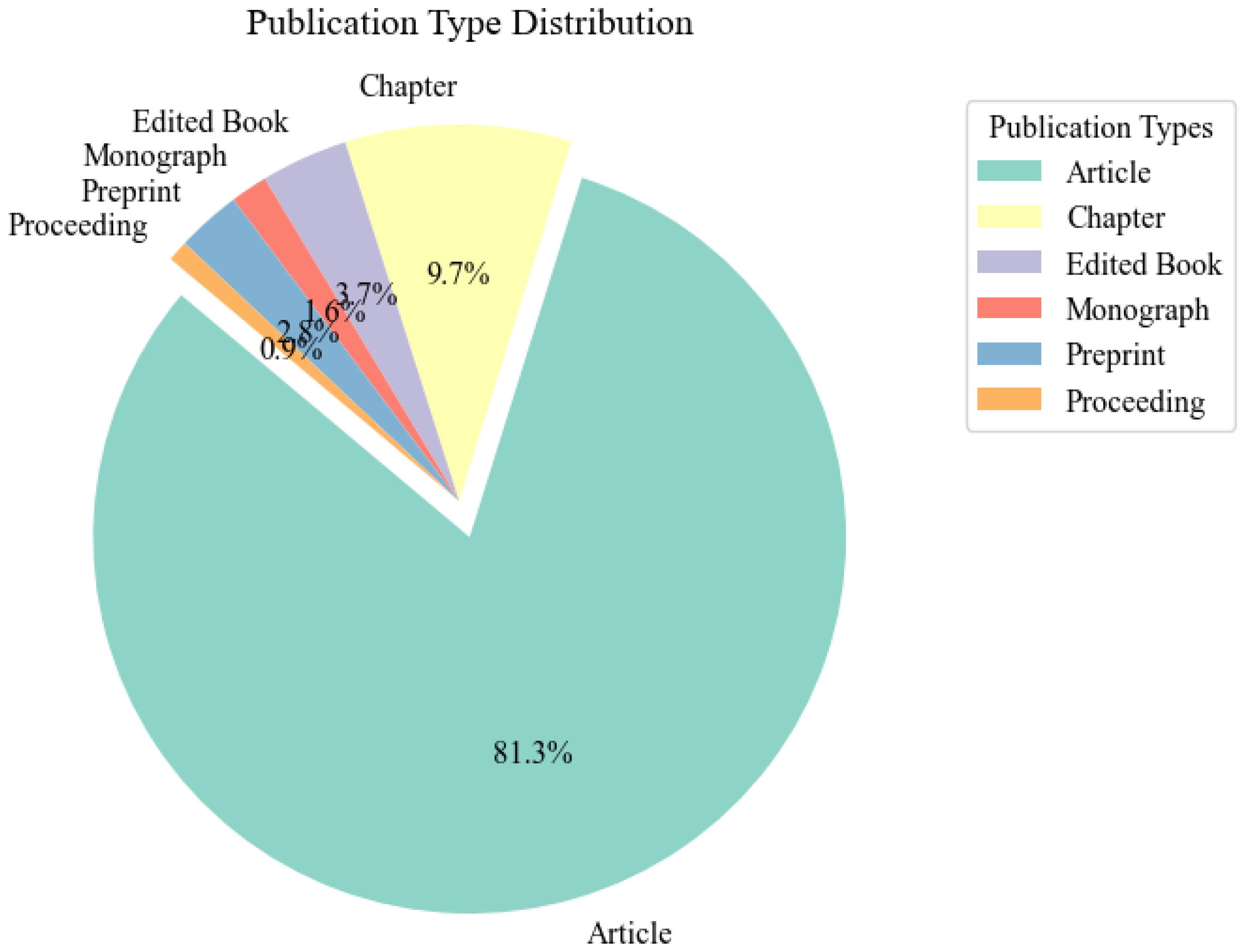
Representative graph illustrating the distribution and trends in publications on microplastic research from 1976 to August 2024, categorized by publication type (e.g., original research articles, reviews, meta-analyses). The graph highlights the evolving landscape of microplastic research, showcasing the percentage contributions of each publication type over the specified period.
Figure 2
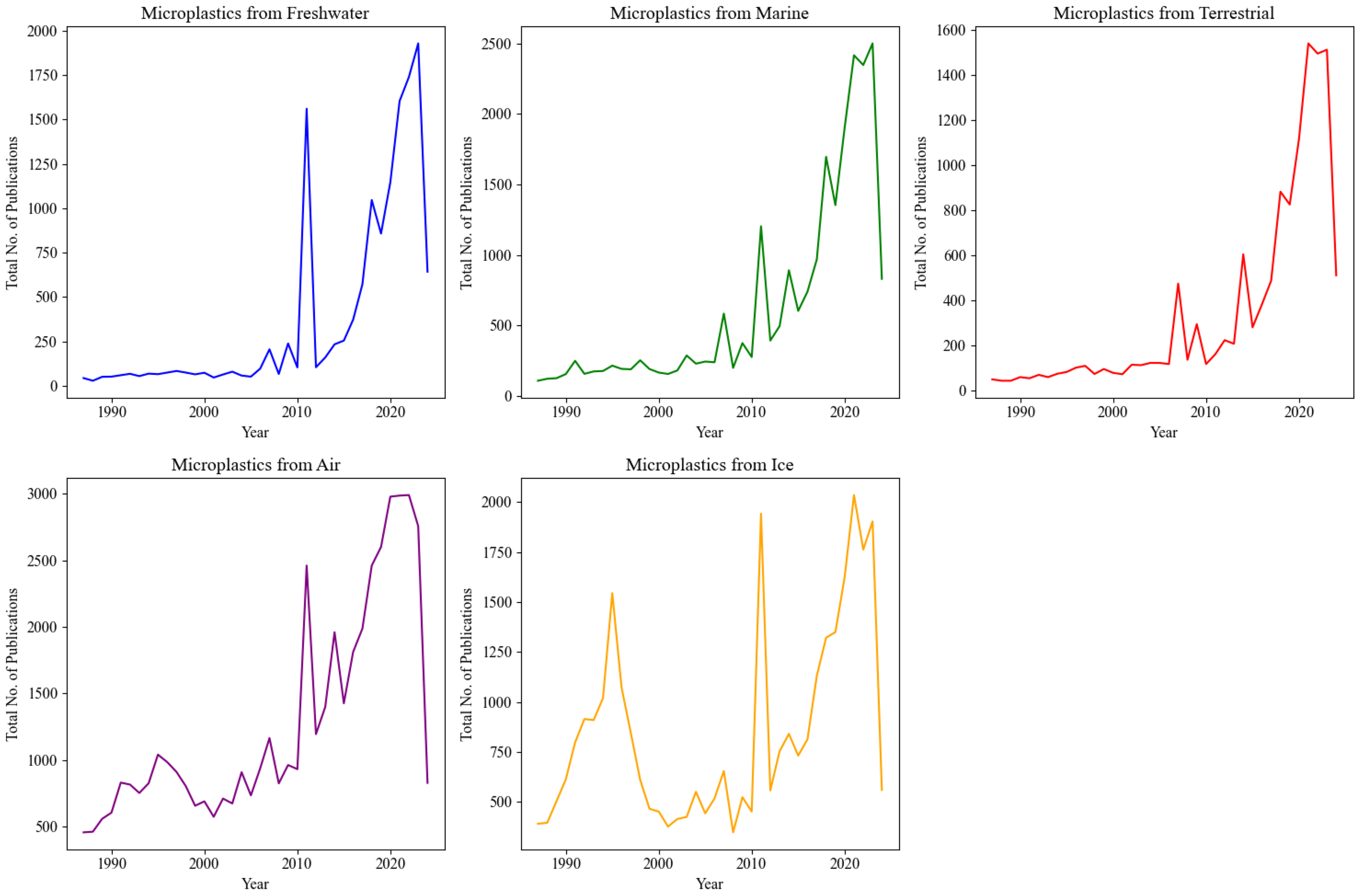
A representative graph illustrating the total number of publications on microplastic research across different habitats, including freshwater, marine, terrestrial, air, and ice, over the last decade.
Figure 3
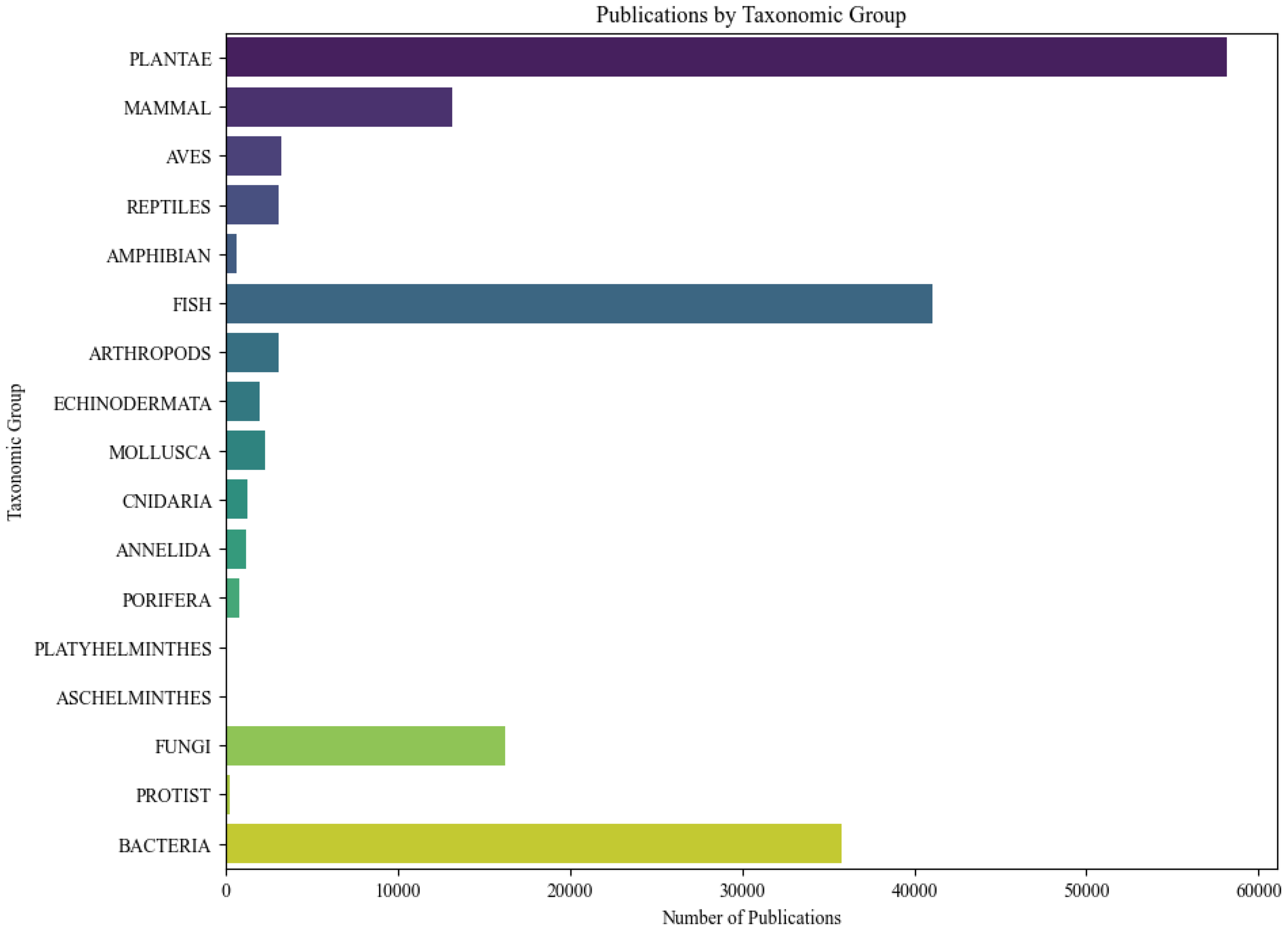
A representative graph depicting the number of publications on microplastic research across major taxonomic groups, including kingdoms plantae, animalia, fungi, protista, and bacteria. The graph highlights the distribution of research efforts among these biological kingdoms, reflecting the focus areas and interest in microplastics’ impact across different life forms.
Figure 4
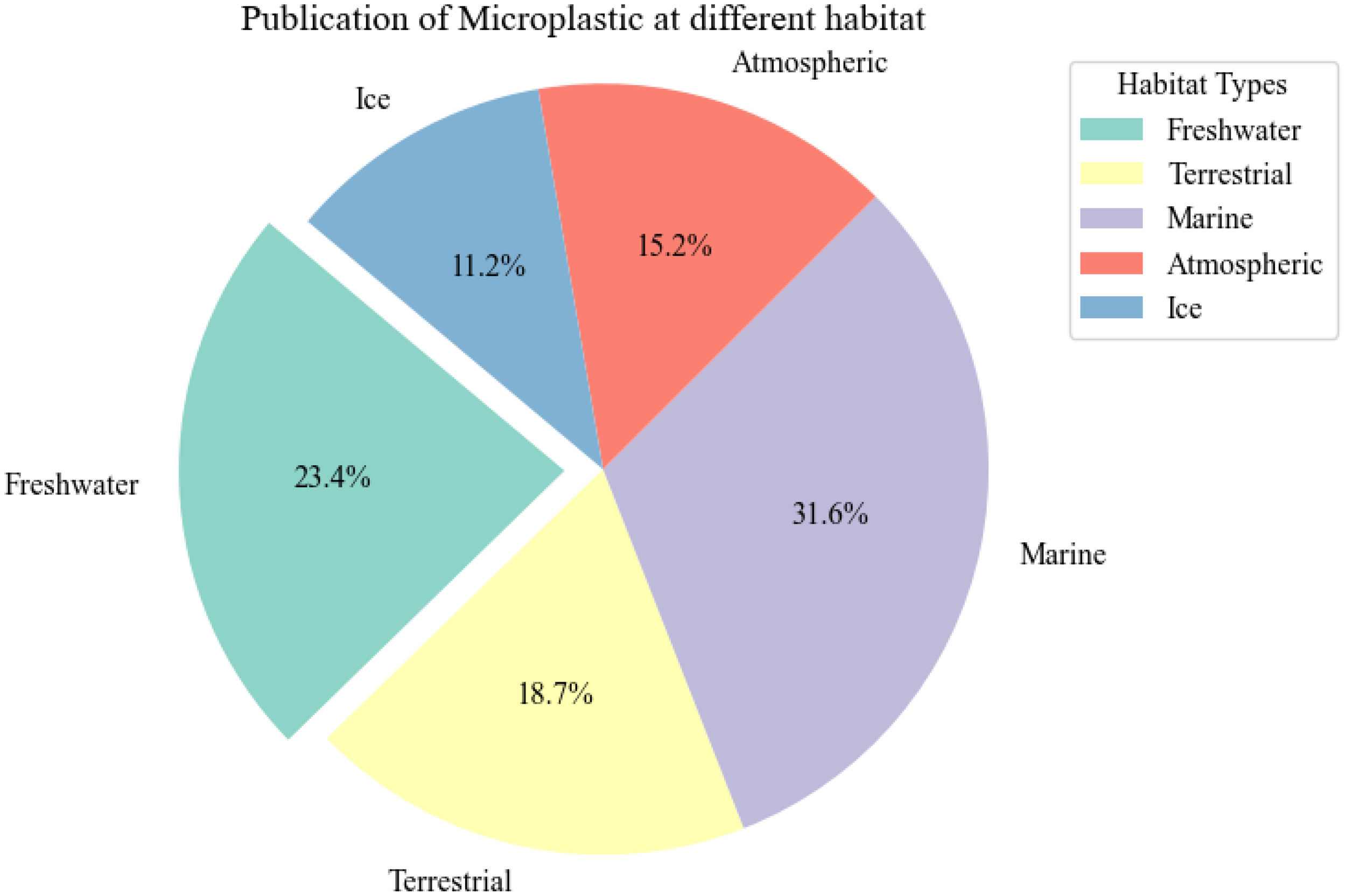
A representative graph illustrating the total number of publications on microplastics research across different habitats—freshwater, marine, terrestrial, air, and ice—for the past almost five decades.
3 Sources and pathways of microplastic pollution
Understanding the complex sources of microplastics (MPs) is essential to tackle their spread in the environment and ecological effects. MPs come from either primary particles (such as microbeads and pellets) or secondary fragments (broken down from larger plastics). They are dispersed through runoff, air deposition, sewage sludge, and direct waste dumping. Land sources like agriculture and urban infrastructure also play a major role alongside marine litter. Common polymers making up plastic debris that lead to MPs include polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET), polycarbonate (PC), and polymethyl methacrylate (PMMA). Construction materials, agricultural films (PE, EVA), medical devices (PVC, PE, PS, PTFE), and environmental stressors such as photolysis, hydrolysis, mechanical wear, and microbiological breakdown are significant contributors (Liu et al., 2021) (Table 3). Land-based sources account for 80%–90% of aquatic microplastic pollution (Duis and Coors, 2016), originating from various sectors including industrial processes, household activities, and urban infrastructure (Wei et al., 2023). Major pathways include fiber shedding during laundry—especially from synthetic textiles like PET, PA, PAN, and PU (Zhou et al., 2023; Zhuang & Wang, 2023)—tire wear particles from vehicles (Kole et al., 2017), and microbeads from personal care products (Bostan et al., 2023). The household environment, often overlooked, also significantly contributes via clothing drying and abrasion, with natural drying increasing fiber release (Dris et al., 2015). Industrial activities such as plastic incineration release MPs through ash and particulate residues (Yang et al., 2021). Sewage sludge and wastewater treatment plants (WWTPs) serve as both reservoirs and pathways for MPs, which often bypass filtration and contaminate rivers, lakes, and oceans (Rolsky et al., 2020; Hale et al., 2020). Atmospheric deposition and surface runoff further enhance their environmental transport (Yin et al., 2021b; Klingelhofer et al., 2020) (Figure 2). While ocean-based sources contribute 10%–20% of total microplastics, their ecological impact is much greater. These sources include abandoned fishing gear, shipping waste, and offshore petrochemical discharges (Naji et al., 2017; Calero et al., 2021). Alarmingly, over 600,000 tonnes of plastic fishing gear are discarded each year, increasing entanglement risks and disrupting ecosystems (Good et al., 2010) (Figure 3). Overall, the widespread and lasting presence of MPs requires a comprehensive approach involving scientific innovation, public awareness, and strict policy measures to reduce further releases and protect ecological health.
Table 3
| Source | Composition and structure | Shape | Size | Location | Reference |
|---|---|---|---|---|---|
| Shower gels | Polyethylene | Irregular shapes | 422 ± 185 μm | Beijing, China supermarkets | Lei et al. (2017) |
| Facial cleansers | Polyethylene | Spherical and irregular shapes | >0.5 mm | New Zealand supermarkets | Fendall and Sewell (2009) |
| Car tires | Polypropylene/acrylic/nylon/rubber | Fragment/fiber | >500 μm | Queensland’s Gold Coast | |
| Beverage products | Polyamide/acrylonitrile–butadiene–styrene/poly(esteramide)/poly(ethylene terephthalate) | Fibers/fragments | 0.1–3 mm | Supermarket (Walmart) of Mexico City, Mexico | Zhou et al. (2021) |
| Facial scrubs | Polyethylene/polyvinyl chloride | Spherical/irregular/granular | 85 to 186 μm | Mainland China | Cheung and Fok (2017) |
| Textile industrial area | Polyester | Fiber | 0.1–1 mm | Shaoxing city, China | Deng et al. (2020a) |
| Cosmetic products | Polyethylene | Irregular/granular/spherical | 54–115 μm | United Arab Emirates | Habib et al. (2020) |
| Plastic mulch | Polyester, polypropylene | Fiber/fragment/foam/film | >500 μm | Qinghai–Tibet plateau, west of China | Feng et al. (2021) |
| Industrial sources | Polyethylene/nylon/polypropylene | Films/fragments/lines/granules/sheets/lines | 0.5–1.0 mm | Northwestern Pacific Ocean | Hou et al. (2021) |
| Mariculture activities | Polyester/polypropylene/polyethylene/polyamide (nylon)/polystyrene/polyoxymethylene/polyetherurethane/polybutylene terephthalate | Fragments/flakes/fiber/foam | <0.25 mm | Maowei Sea, China | Anderson et al. (2017) |
| Fishing and shipping activities | Ionomer surlyn/acrylic (acryl fiber)/polyetherimide/polyphenylene sulphide/ethylene vinyl alcohol/acrylonitrile/nylon/polyisoprene/polyvinyl chloride/ethylene–vinyl acetate/polyurethane | Fiber/pellet/fragment | 1,489 ± 1,017 μm | Port Blair Bay, Andaman Islands | He et al. (2022) |
| Anthropogenic activity | Polystyrene/polyethylene/polypropylene | Fiber/styrofoam/fragment/film/pellet | < 0.5 mm | Three Gorges Reservoir, China | Bui et al. (2020) |
| Personal care products/facial cleansers/sewage sludge | Polystyrene/polyester/amino thermoset plastic/polyallyl di glycol carbonate | Fragment/pellet/foam/film/line | 0.355–0.999 mm | The Laurentian Great Lakes of the USA | Huang et al. (2021a) |
| Urban sewage | Polyethylene/polystyrene/polypropylene | Fragment/lines/foam/film | 1–4.75 mm | The Southern Caspian Sea Coasts | Ryan (2015) |
| Industrial areas | Polyester/nylon | Fiber/foam/fragment | 50 to 2,000 μm | Ciwalengke River, Indonesia | Wang et al. (2020) |
| Fishery activities and human domestic sewage/building industry | Polyvinylchloride/polyethylene/polyamide | Fibers/pellets/films/fragments | <0.5 mm | Nanxun Reef in Nansha Islands, South China Sea | |
| Urbanization | Polyethylene/polypropylene/nylon | Fibers/fragments | 0.1–5 mm | Northern shores of the United Arab Emirates | Sharma et al. (2021) |
| Industrial activities | Polyethylene/polyethylene terephthalate/polyester/poly(vinyl stearate)/polypropylene/cellulose | Fragment/fiber/pellet | 1,001–2,000 mm | The Karasu River Erzurum, Turkey | Brandon et al. (2020) |
| Tertiary industry | Polyethylene/polypropylene/polyacrylonitrile/polyethylene terephthalate | Fragment/fiber/film | 500 μm to 5 mm | Tourist city in China | Van Emmerik et al. (2018) |
| Sludge and wastewater treatment plants | Polyamide (i.e., nylon)/polyethylene/polypropylene | Fragment/fiber/film/granule | 0.003–0.05 mm | The Persian Gulf | Xiang et al. (2022) |
| Anthropogenic activity | Polypropylene/polyethylene terephthalate/polyamide (nylon)/polystyrene/polyethylene | Fiber/film/pellet/granular | <2 mm | Wuhan, China | Matsuguma et al. (2017); Hipfner et al. (2018); Caron et al. (2018) |
| Local inputs/ocean transport | Polypropylene/polyester/polyethylene | Fiber/flake/film/granule | 2.0–2.5 mm | Antarctic seawater | Schymanski et al. (2018) |
| Artificial ecosystems | Polyethylene/rayon/polypropylene | Fiber/flake/film/granule | <1 mm | Southwestern China | Čulin and Bielić (2016) |
| Domestic, agriculture effluent, industry, upstream inflow, and airborne settlement | Polyethylene terephthalate/polyethylene/polypropylene/polystyrene/polycarbonate/polyvinyl chloride/cellulose propionate/polyamide/ethylene–vinyl acetate copolymer | Pellets/fragments | 0.05–5 mm | Xiangjiang river, China | Alomar et al. (2016) |
| Plastic industries | Polypropylene/polyester/nylon/polystyrene | Fiber/line/spherule/fragment/granule/film | <0.5 mm | South Yellow Sea, China | Rochman (2018) |
| Commercial fish species | Polyethylene terephthalate/polyethylene/polypropylene/polyamide/phthalocyanine | Fibers/fragments | >215 μm | Seri Kembangan, Malaysia | Karbalaei et al. (2019) |
| Anthropogenic activities | Polyethylene terephthalate/cellulose acetate/polyvinyl chloride/polypropylene/polyethylene | Fibers/spheres/fragments | ≥1 to <10 μm | Drinking water treatment plants, the Úhlava River (Czech Republic) | Naji et al. (2017) |
Sources, composition, shape, size, and location of commonly used microplastics.
4 Forms of microplastics and their derivatives
Microplastics (MPs), classified into primary and secondary types, originate from both intentional manufacturing and environmental breakdown of larger plastic items (Ali et al., 2023). Primary MPs are intentionally created particles used in commercial products such as personal care items, detergents, pharmaceuticals, and pesticides (Figure 5). Due to their small size, these particles can easily enter aquatic systems through surface runoff and wastewater discharge, often traveling long distances from their original sources (Gregory, 1996; Fendall and Sewell, 2009; Cole et al., 2011). In contrast, secondary MPs result from various physical, chemical, and biological degradation processes that gradually break down larger plastic debris like containers, fishing nets, and packaging materials (Gregory and Andrady, 2003; Browne et al., 2011). Environmental factors such as UV radiation and mechanical abrasion on beaches speed up these breakdown processes, producing MPs through microcrack formation and oxidative degradation (Shaw and Day, 1994; Cunliffe & Davis, 1982). Microplastics display a wide range of physicochemical properties, including size, shape, density, crystallinity, and surface morphology—factors that influence their interactions with the environment (Crawford and Quinn, 2017)—for example, particle size and surface area directly affect their bioavailability and sorption capacity, which, in turn, influence their sinking potential and distribution within water columns (Kowalski et al., 2016; Hüffer et al., 2018). Fragmentation causes surface erosion, changing chemical reactivity, and interactions with contaminants. Additionally, the level of crystallinity, often increased through oxidative aging, impacts their environmental durability and ecological risk (Rouillon et al., 2016; Ter Halle et al., 2017). Microplastics also come in a variety of colors and shapes, with filamentous forms (1–5 mm) being common in many aquatic environments. A study in the Arabian Gulf found that 75% of microplastics were blue, with black (9%), red (6.3%), green (4.4%), and gray (2.2%) particles following (Giani et al., 2019). This morphological variety makes monitoring and mitigation more challenging, highlighting the need for advanced detection methods (Table 2).
Figure 5
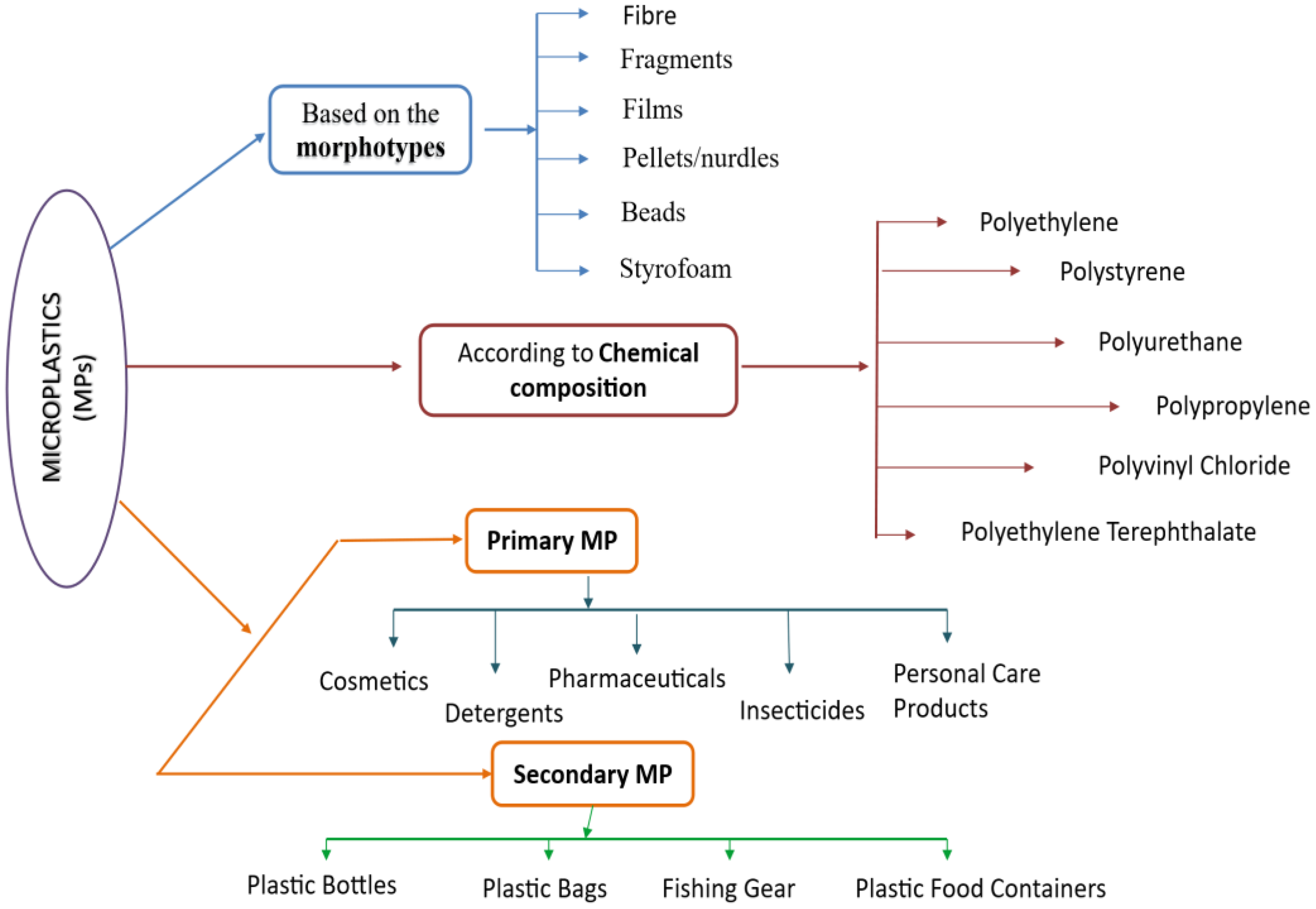
Different forms of plastic’s presence in the environment.
MPs and their environmental derivatives, like microplastic-derived dissolved organic matter (MP-DOM), display complex behavior due to interactions with co-contaminants. Photoaging and microbial degradation produce environmentally persistent free radicals (EPFRs) on MP surfaces, promoting reactive oxygen species (ROS) formation and changing contaminant dynamics (Zhu et al., 2019; Jiang et al., 2023). UV-driven photooxidation also damages MP structure, releasing monomers, oligomers, and additives such as bisphenol A (BPA) and DEHP into the water (Lee et al., 2020; Ouyang et al., 2023). These compounds affect biogeochemical cycles, contaminant bioavailability, and aquatic toxicity. Notably, biodegradable plastics contribute disproportionately to the formation of MP-DOM, further complicating their ecological impacts (Taghavi et al., 2021; Luo et al., 2019). In conclusion, understanding the diverse forms, behaviors, and degradation processes of MPs is crucial to develop effective mitigation strategies. Ongoing advances in analytical methods will be vital to clarify MPs’ environmental fate and assess their long-term effects (Figure 6; Table 3).
Figure 6
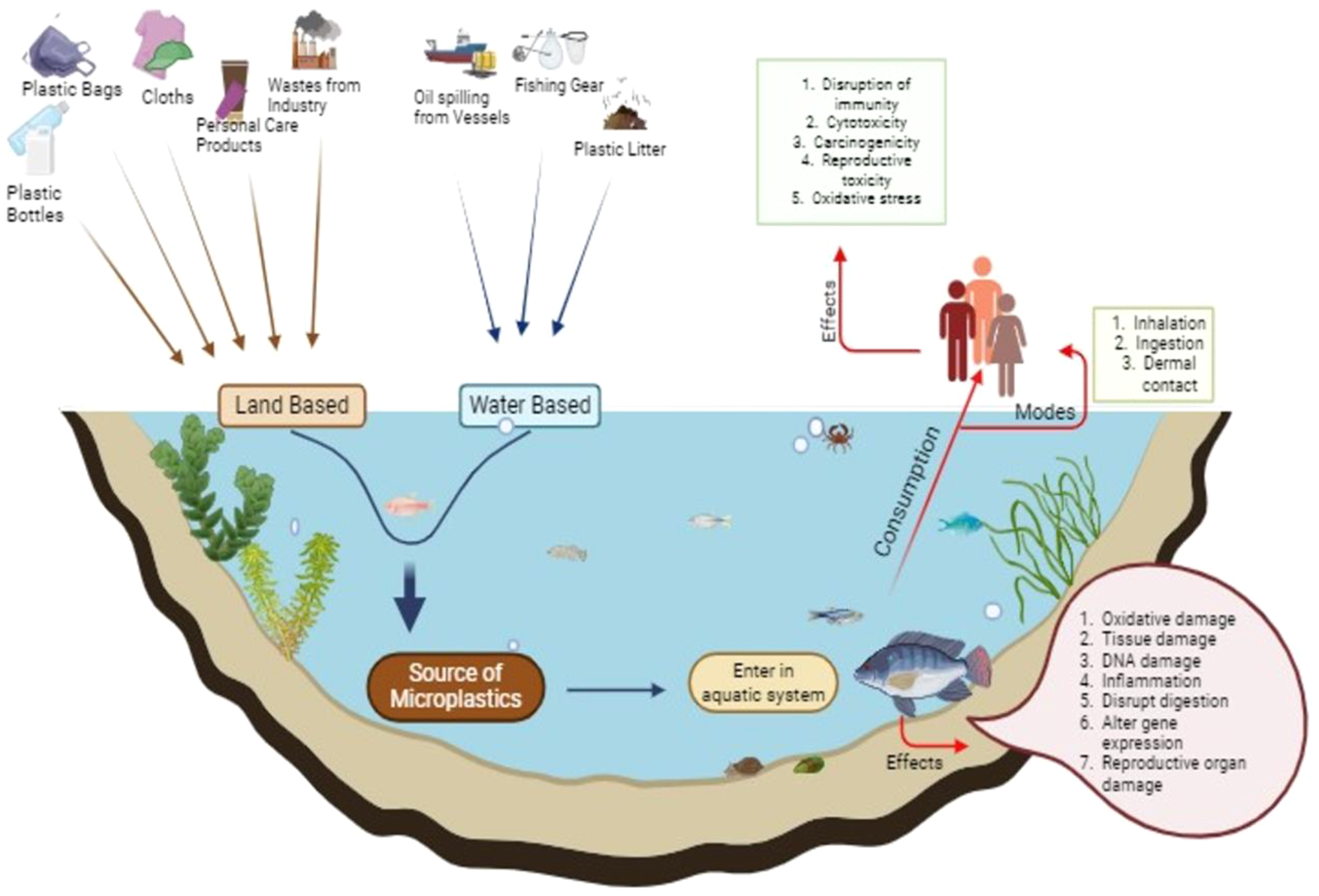
Destiny of plastics in aquatic environments.
5 Microplastics in aquatic ecosystems: distribution and ecological ramifications
The distribution of microplastics (MPs) in aquatic ecosystems results from complex interactions between particle properties (e.g., density, shape, surface chemistry) and dynamic environmental processes like hydrological turbulence, biofouling, and biotic interactions. Low-density polymers such as polypropylene (PP) and polyethylene (PE) usually stay suspended or float on the water surface, while denser particles like polyvinyl chloride (PVC) or fouled plastics tend to settle in sediments (Molazadeh et al., 2023). These particles are not fixed but are constantly exchanged between water, sediments, and living organisms through ingestion, bioturbation, and excretion, creating a dynamic flow of contamination (Besseling et al., 2017). Figure 2 shows the environmental cycling of MPs in aquatic systems.
Freshwater inflows, especially from urban catchments, further speed up MP dispersal. Modeling studies have shown that river flow plays a significant role in carrying MPs into marine environments (Besseling et al., 2017). MPs, due to their small size, are more bioavailable than macroplastics and can penetrate cellular barriers, raising serious ecological concerns (Ali et al., 2021). Notably, MPs have been found in drinking water, posing severe human health risks—ranging from inflammation to cancer and genetic damage—especially in areas without effective filtration systems (Ali et al., 2021). MPs are classified as primary (such as microbeads in cosmetics) or secondary, produced when larger plastic debris breaks down through environmental processes like oxidation, mechanical abrasion, and UV-driven photodegradation (Fotopoulou and Karapanagioti, 2019). The main sources and pathways of MPs are listed in Table 1. Aquatic species, from top predators to invertebrates, can ingest MPs, potentially leading to trophic transfer and bioaccumulation of both the plastics and the pollutants adsorbed on them (Ding et al., 2022). The extent of toxicant transfer and its physiological effects remain under discussion (Negrete-Bolagay et al., 2021). MP contamination is now widespread in freshwater systems worldwide—including rivers, lakes, and estuaries across Europe, Africa, Asia, and North America (Cera et al., 2020; Galafassi et al., 2021; Onoja et al., 2022). Although freshwater and marine MPs share similar transport mechanisms, their physical and chemical properties often differ due to variations in pollution sources and land use (Blettler et al., 2017). Alarmingly, MPs can move from aquatic to terrestrial ecosystems through water use, bioaccumulation, or food chains, posing unexpected risks to biodiversity and human health.
5.1 In freshwater
According to Iyare et al. (2020), microplastics enter rivers through wastewater treatment plants and urban drainage systems. The main sources of microplastics include synthetic fibers, personal hygiene products, and intentionally created micro-sized plastics used in scrubs. Secondary sources result from the weathering-related breakdown of larger plastic items (Horton et al., 2024; Prata et al., 2019). Sampling in rivers is more difficult than in the atmosphere or on land due to complex water circulation patterns influenced by tidal currents (Crew et al., 2020; Skalska et al., 2020). These patterns influence how microplastics spread in rivers. Microplastics enter urban and semi-urban river systems from multiple sources: airborne transport, surface runoff, leachates from landfills and farms, wastewater from industrial operations, sewer systems, and urban runoff. These sources (Bruge et al., 2018; Kapp and Yeatman, 2018; Tramoy et al., 2019; Dris et al., 2018; Brahney et al., 2021) contribute to the microplastic levels in river ecosystems. When microplastics reach river channels during non-flooded conditions, they either settle on the riverbed or travel downstream into estuarine and marine environments (Horton et al., 2017; Hurley et al., 2018; Pojar et al., 2021). Particle properties, such as density, shape, and flow conditions, determine how far a particle disperses (Schwarz et al., 2019). Sarkar et al. (2021a) reported significant amounts of microplastics (63 μm–5 mm) in the sediments (2,124.84. to 6,886.76 items/kg) and surface water (7.87 to 20.39 items/L) of treatment ponds in the East Kolkata Wetland (EKW). In the related wastewater canals (WWC), microplastics in surface water ranged from 30.46 to 137.72 items/L, and in sediment from 1,108.78 to 34,612.87 items/kg. Additionally, Sarkar et al. (2021a) found 17.88 items/L of fibers, films, and fragments, including polyethylene terephthalate and polyethylene, in raw water from Ganga River. In the three Gorges Reservoirs in China, Di and Wang (2018) reported 16−126 items/L (48 µm–5 mm in diameter) of microplastics. Su et al. (2016) found 3.4–25.8 items/L (100–1,000 µm) of microplastics in Taihu Lake, China. Leslie et al. (2017) also found 48−187 items/L (10 µm–5 mm) in Amsterdam canal water. Therefore, analyzing suspended microplastics in surface water is essential, especially considering the potential for freshwater sources to produce drinking water (Sarkar et al., 2021b).
5.2 In marine water
Disposable, hygienic instruments are used in the medical field to maintain high standards of hygiene. Plastic waste from these instruments eventually makes its way into the world’s oceans. Global coastal communities dispose of between 0.4 and 12.7 million tonnes of land-based plastic waste into the ocean each year (Auta et al., 2017; Jambeck et al., 2015; Lebreton et al., 2017; Schmidt et al., 2017). Microplastics enter marine environments through various channels, such as rivers, stormwater systems, and wastewater treatment plant effluents (Yin et al., 2021b). These tiny plastic particles have a significant impact on marine habitats.
Marine plastic pollution also stems from activities like fishing, aquaculture, and shipping, which dump trash onto beaches and into oceans (Lusher and Welden, 2020). According to Sagawa et al. (2018), the size distribution of microplastics varies across beach and bottom sediments, emphasizing the need for thorough monitoring. Ono et al. (2023) reported that yearly microplastic emissions in Tokyo Bay included 10.2 ± 1.6 tons from personal care products (PCPs), 38 ± 22 tons from clothing fibers, and 1,500–1,800 tons from tire wear particles (TWPs).
Interestingly, after washing clothes, plastics like polyester, polystyrene, and polyamide materials denser than seawater tend to accumulate in sediments and sink more quickly, affecting deposit feeders and bottom-dwelling fish (Wang et al., 2019). In Mumbai, large amounts of plastic waste are dumped into coastal waters from sewage, fishing, aqua tourism, industrial discharges, and untreated household wastewater (Takar et al., 2020). Mumbai’s coastal waters receive over 2,200 million liters of waste daily (Jelil and Jain, 2014).
Rabari et al. (2023) identified seven different types of plastic polymers in muddy beach samples from the Gulf of Khambhat, India. These polymers were present in the following order of abundance: polypropylene (32.46%), polyurethane (32.16%), polystyrene (9.62%), acrylonitrile butadiene styrene (14.93%), polyethylene terephthalate (4.61%), polyethylene (3.71%), and polyvinyl chloride (2.51%). The coastal area of Cape Town, South Africa, faces microplastic pollution due to stormwater runoff. The ingestion of plastic polymers by various pelagic and demersal species is greatly influenced by their density (Sathish et al., 2020b)—for instance, fecal pellets, secondary ingestion, and biofouling can cause polyethylene, which is less dense than seawater, to sink (Kane and Clare, 2019).
5.3 Microplastics in wastewater treatment plants
Wastewater treatment plants (WWTPs) act as pathways for microplastics, allowing them to enter aquatic ecosystems. Common polymers such as polyethylene (PE), polyester (PES), polypropylene (PP), and polyamide (PA) are frequently found at different stages of WWTPs, lakes, and sludge. According to Lares et al. (2018), PE and polyethylene terephthalate (PET) particles are abundant in discharges from many WWTPs. PES is often found in final effluents, with high concentrations at Scottish WWTPs (28%) and Australian WWTPs (67%). As summarized by Lv et al. (2019), dominant microplastic types identified in WWTPs in Wuxi, Jiangsu, include PP (15%), PE (18%), PS (20%), and PET (47%), analyzed using Fourier transform infrared spectroscopy (FTIR). The FTIR spectra reveal information about the main types of microplastics and their forms, such as fragments, fibers, films, and foams, observed in the wastewater at Wuxi WWTP. This widespread presence of microplastics underscores the important role of WWTPs in reducing plastic pollution in aquatic environments. While primary wastewater treatment effectively removes larger particles, secondary treatments often do not sufficiently remove microplastics due to the absence of dedicated removal processes (Sheriff et al., 2023). This shortcoming has led researchers to highlight wastewater treatment plants as major sources of environmental microplastic pollution (Liao et al., 2023).
6 The life cycle of microplastics in aquatic systems
Microplastics’ (MPs’) life cycle in aquatic systems is a complex and widespread process that significantly impacts human and environmental health. MPs’ journey begins when they are released into terrestrial and aquatic environments, where they may start as primary particles or form as a result of larger polymers breaking down. After entering water systems, MPs undergo various physical, chemical, and biological processes that contribute to their accumulation and dispersion. MPs enter the aquatic food chain during the crucial bioaccumulation phase when they are consumed by zooplankton, small fish, and larger marine species. These particles build up in the tissues of aquatic organisms as they move through the food chain, causing serious physiological damage. Studies have documented adverse effects on a range of organisms, including sea turtles, mussels, and fish, manifesting as compromised digestive and immune systems and, in severe cases, death (Huang et al., 2021; Miller et al., 2020). The implications of MPs extending into the food chain pose serious consequences for human health. Humans may ingest MPs by consuming contaminated seafood, potentially leading to cytotoxic effects on human cells, including those in the brain (Shi et al., 2022). The large surface area of MPs allows them to adsorb hazardous compounds, such as antibiotics and other pollutants, worsening the contamination issue (Joo et al., 2021). MPs can be excreted by humans or released through the disposal of personal care products, continuing their environmental cycle. Recent studies have detected MPs in drinking and mineral water bottles, highlighting their persistence and widespread presence in items consumed by humans (Gambino et al., 2022; Menon et al., 2023). Conventional water treatment methods often fail to effectively remove these tiny particles, emphasizing the urgent need for innovative and advanced remediation technologies (Elgarahy et al., 2021). The increasing prevalence of MPs calls for greater focus on research and development to create solutions that address both the environmental and health impacts of these pollutants.
7 Fate of plastics in the aquatic environment
The fate of plastics in aquatic ecosystems is influenced by a complex combination of environmental processes, such as photodegradation, mechanical weathering, and biological interactions. Over time, plastic debris breaks into smaller pieces, becoming macroplastics (>5 mm), mesoplastics (1–25 mm), and ultimately microplastics (<5 mm). Microplastics (MPs) are particularly concerning due to their longevity, mobility, and ability to be taken up by organisms (Peters and Bratton, 2016; van Weert et al., 2019). MPs can originate as primary MPs—materials used directly in industry—or as secondary MPs formed from the breakdown of larger plastic debris, both significantly adding to marine plastic pollution (Mvovo, 2021; van Wijnen et al., 2019; Andrady, 2011; Martín et al., 2022; Yin et al., 2019). Rivers play a vital role by transporting land-based plastic waste into oceans (Lebreton et al., 2017; Meijer et al., 2021). Once in the water, MPs are carried over long distances by wind, tides, and surface currents, eventually accumulating in coastal and estuarine areas (Li et al., 2019). MPs come in various shapes (e.g., fragments, fibers, films, microbeads), sizes (1 µm–5 mm), and polymer types (e.g., polyethylene, polystyrene), which affect how they behave environmentally and how they interact with living organisms. Seasonal changes affect microplastic presence, with white fiber-like MPs being more common in winter and autumn—likely because of increased stormwater runoff and less photodegradation. In contrast, spring and summer tend to have fewer MPs, possibly due to dilution from higher fishing activity (Ariefdien et al., 2024; Jiang et al., 2022). MPs also serve as surfaces for microbial growth, forming biofilms made up of bacteria, fungi, algae, and archaea, creating what is known as the plastisphere (Amaral-Zettler et al., 2020; Wang et al., 2021; Fabra et al., 2021; Kirstein et al., 2019). These biofilms help gather diverse microbes, including cyanobacteria, choanoflagellates, and diatoms, especially on polyethylene MPs (Castano-Ortiz et al., 2024). Because MPs are common across food webs, drinking water supplies, and aquatic environments, their long-lasting presence in ecosystems—particularly in the plastisphere—raises serious ecological concerns (Nelis et al., 2023). Understanding the complex interactions between plastics and microbes is crucial to assess long-term effects on aquatic biodiversity and ecosystem services.
7.1 Accumulation of microplastic in the aquatic organisms
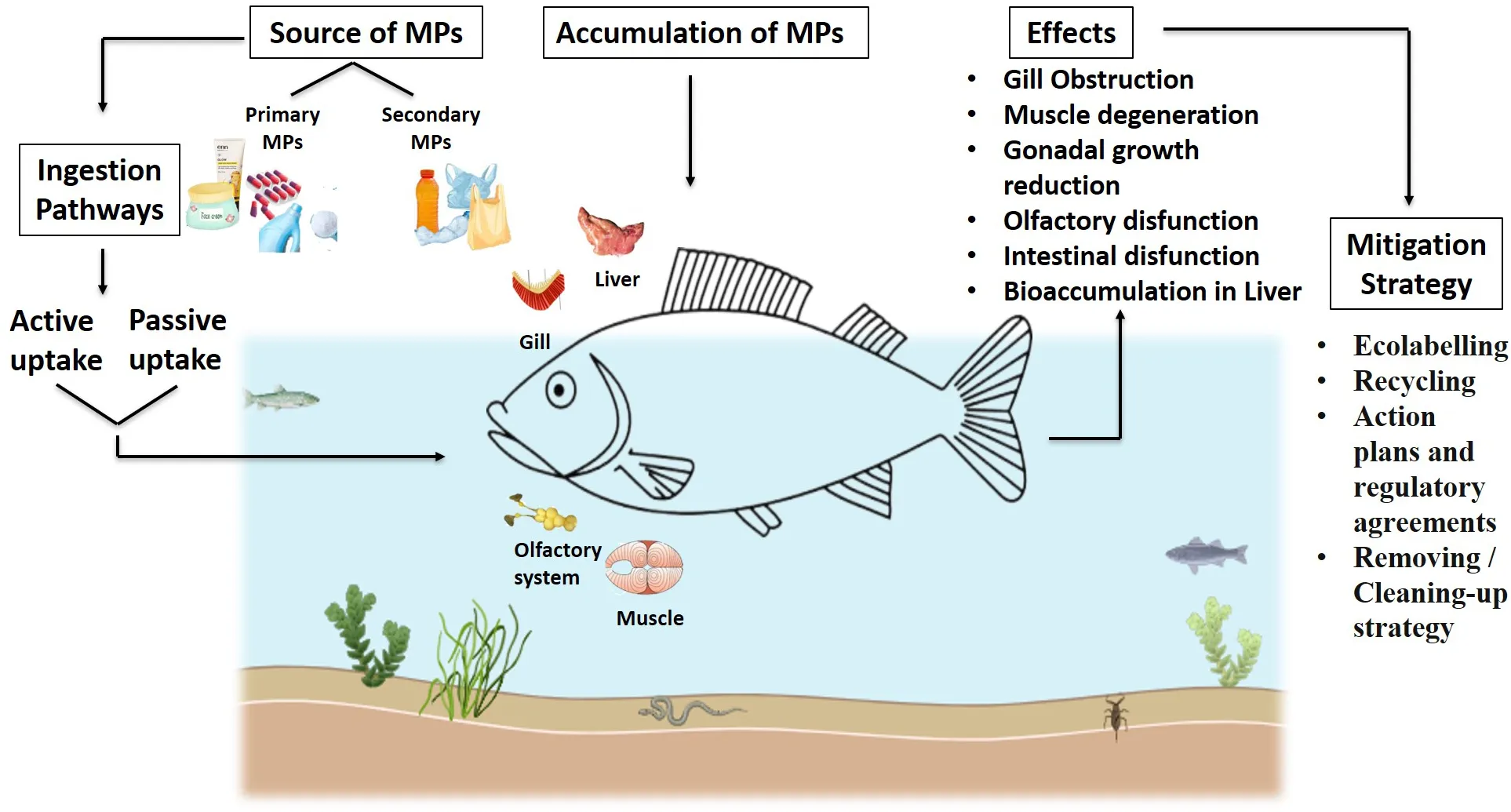
Numerous research projects have studied the impacts of microplastics (MPs) on various aquatic creatures, such as fish, bivalves, and macroinvertebrates (Windsor et al., 2019). Many marine species, including shrimp, fish, zooplankton, cetaceans, and birds, accidentally ingest these tiny plastic particles (Cole et al., 2016; Lusher et al., 2013, Lusher et al., 2015; Ferreira et al., 2016; Gurjar et al., 2021). Microplastics transfer from lower to higher organisms in the food chain by moving between trophic levels (Farrell and Nelson, 2013; Bouwmeester et al., 2015). These originate from larger polymers (macro- and mesoplastics) that gradually break down over time (Ugwu et al., 2021). Fish may consume microplastics directly or indirectly. They can absorb MP particles directly through feeding or indirectly via trophic transfer—by eating other creatures that carry MP particles (Figure 7) (Benjamin et al., 2014; Nelms et al., 2018; Cartes et al., 2016; Walkinshaw et al., 2020). Ingesting microplastic particles can cause intestinal blockage in smaller fish (Carpenter and Smith, 1972). Larger fish are known to eat plastic material due to their feeding habits, but how often or how much they consume remains unclear. Many predatory fish with large mouths can ingest significant amounts of plastic. Though they cannot digest it, the plastic may become lodged in their intestines or cause ulcers (Hoss and Settle, 1990; Limonta et al., 2019). Organs associated with digestion—such as the stomach and intestines—and organs related to breathing, like the gills, are more prone to accumulate plastic particles. A global review found that 427 fish species have ingested plastic (Azevedo-Santos et al., 2019). Among these, freshwater fish made up 17.1%, while marine fish constituted the majority at 54.6%, followed by estuarine–marine at 5.6% and estuarine–freshwater at 0.2%. The most common trophic group was carnivores (54.8%), followed by herbivores (3.5%), detritivores (0.7%), omnivores (23.2%), and other herbivores (3.5%). Approximately 17.1% of the species did not have a specified trophic group (Azevedo-Santos et al., 2019). Studies show that large fish eat plastic, but details about how often or how much they consume are still unknown. Microplastic bioaccumulation impacts many animals, from tiny zooplankton to large whales (Chatterjee and Sharma, 2019). Organisms like plankton (Desforges et al., 2015), cnidarians, echinoderms, annelids, bivalves (van Cauwenberghe and Janssen, 2014), fish (Alomar et al., 2017), seabirds, marine reptiles (Vélez-Rubio et al., 2018), and mammals (Nelms et al., 2018) are frequently studied groups. These organisms often ingest microplastics from both the substrate and the water column without intention. Microplastics can cause physical harm after ingestion (Bellas et al., 2016; Jabeen et al., 2018). In aquatic species, microplastics may reduce feeding, reproductive capacity, growth, and survival (Cole et al., 2016). Santillo et al. (2017) have expressed concern about the bioaccumulation of microplastics in fish, which could lead to biomagnification of plastic-associated pollutants in humans. Trophic transfer allows for indirect ingestion of microplastics when fish eat contaminated prey, leading to MP build-up in their digestive systems (Farrell and Nelson, 2013; Balkhuyur et al., 2018; Bessa et al., 2018; Pozo et al., 2019). Previous studies have examined microplastics in fish digestive systems (Sathish et al., 2020b; Koongolla et al., 2020), including in the gastrointestinal tracts of fish (Zhang et al., 2019), sharks (Maes et al., 2020; Mancia et al., 2020), amphibians (Kolenda et al., 2020), birds (Masia et al., 2019; Weitzel et al., 2021), and mammals (Zantis et al., 2021; Meaza et al., 2021).
Figure 7
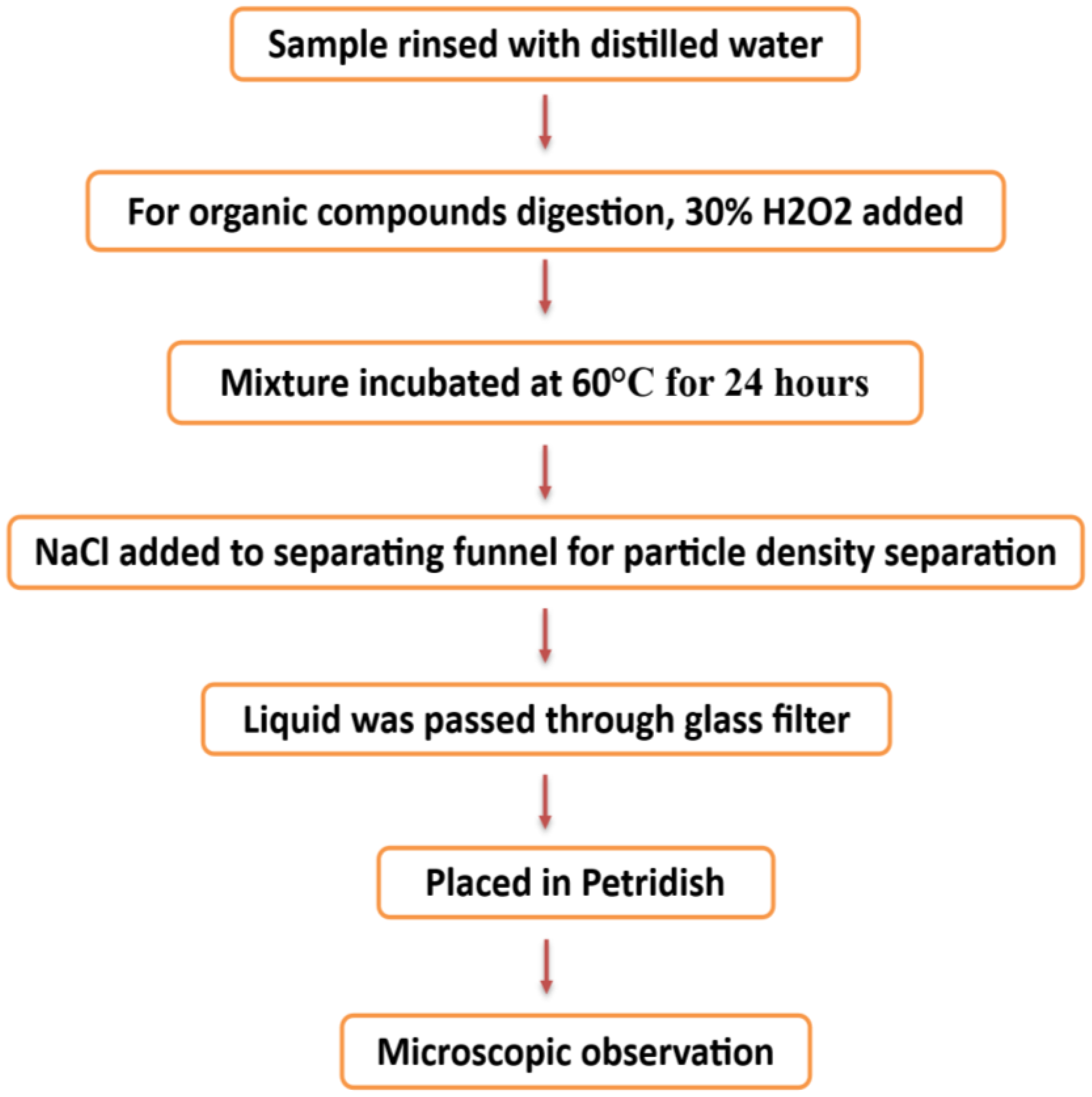
Schematic diagram for the extraction of microplastics from water samples (Chau et al., 2023; Mai et al., 2019; Zhao et al., 2019).
In 2023, Onay and colleagues identified 335 microplastics, including six distinct polymers, within the digestive tracts of 120 red mullet fish collected from Turkey’s Southeast Black Sea Region. Because of their feeding habits, bivalve filter-feeders such as mussels that live around rocky shorelines are prone to consuming microplastic particles (Barkhau et al., 2022). According to Sussarellu et al. (2016), Pacific oysters exposed to polystyrene microspheres (2–6 µm; 0.023 mg/L) displayed accelerated feeding rates, disrupted reproductive systems, and decreased offspring development. Roy et al. (2023) reported that the freshwater snail Filopaludina bengalensis readily accumulated microplastics, reaching up to 82 ± 6.02 particles per individual at 5-ppm levels of polystyrene microspheres (~30 µm) on the 27th day, without any mortality. Abbasi et al. (2018) revealed that several fish species have microplastics in their stomachs—for example, in the Musa estuary of the Persian Gulf, Platycephalus indicus, Saurida tumbil, Cynoglossus abbreviatus, and Sillago sihama were found to contain varying levels of microplastic particles.
Black-colored microplastics predominated in demersal fish from the Tyrrhenian Sea (Capillo et al., 2020). Dicentrarchus labrax, caught in the Northeast Atlantic Ocean, had 1.3 ± 2.5 microplastic particles per specimen in its gastrointestinal system, according to Barboza et al. (2020). In China’s Yangtze Estuary and Hangzhou Bay, 13 commercial fish species were studied. The guts of these fish contained microplastic particles, with individual particle sizes ranging from 0.3 to 5.3 mm (Su et al., 2019). Zebrafish gills continuously filter microplastic particles measuring between 1 and 5 mm and up to 20 mm in size, which superficially attach to fish filaments (Batel et al., 2018). However, research on microplastic contamination in fish gills remains limited (Su et al., 2019; Zhang et al., 2019). An average of 2.6 ± 1.6 microplastic objects per individual was found in the gills of spiny-head croaker from the Yangtze Estuary and Hangzhou Bay in China (Su et al., 2019). Further investigation is needed to understand the physiological interactions of microplastics in fish gills. At least 267 fish species have data on microplastic concentrations recorded (Bongaarts, 2019; Lopez-Martínez et al., 2021). A study in South China’s Pearl River Estuary found that each fish’s GI tract and gills contained between 0.17 and 0.17 microplastic particles. According to Lin et al. (2020), polyethylene terephthalate (38.2%) was the most common polymer, and black was the most common color. Found that the average number of microplastics specifically polyethylene terephthalate, or PET in the gills ranged from 0.03 to 3.0 particles per gill across various fish collected from the Zhoushan fishing area in China. Fourier transform infrared (FTIR) analysis indicated that PET and polypropylene (PP) made up most of the microplastics identified, with fibers being the predominant morphology. Juvenile Dicentrarchus labrax exposed to microplastics in their gills (collected from the North East Atlantic Ocean) experienced oxidative stress and tissue damage, along with increased mercury bioconcentration (Barboza et al., 2020). When coastal crabs, Carcinus maenas, inhaled microplastics into their gill chambers, it affected their oxygen consumption (Watts et al., 2016).
The gastrointestinal tracts of economically important fish caught between Chennai and Nagapattinam in the Bay of Bengal contained microplastics and mesoplastics. Karuppasamya et al. identified three types of plastic polymers in these particles: polyamide (PA), polyethylene (PE), and polyethylene terephthalate (PT), with polyethylene being the most prevalent. Recent studies in the coastal districts of southwest India found that fish ingested 21.4% of microplastics, primarily polyethylene (Robin et al., 2020). Debbarma et al. (2022) examined microplastic pollution in demersal fish, specifically croaker (Johnius dussumieri), near Mumbai, India. They found 6.6 ± 1.7 microplastic objects in the gastrointestinal system and 6.2 ± 1.7 in the gills. During the post-monsoon season, blue to black microplastics, mostly in bead form and smaller than 100 µm, were the most common. These findings highlight the potential for microplastics to bioaccumulate in fish tissues, posing risks to consumers and other higher-trophic-level organisms.
8 Toxicity of ingested microplastics
The ingestion of microplastics poses serious ecological risks across aquatic ecosystems, impacting a wide range of organisms from planktonic species to higher trophic levels. Microplastics, particles smaller than 5 mm, are common in marine and freshwater environments mainly due to the breaking apart of larger plastic debris and direct sources like urban runoff and industrial discharges (Cole et al., 2011; Jambeck et al., 2015). Ingested microplastics can lead to various harmful effects caused by both their physical and chemical traits.
8.1 Physical effects
The physical effects of microplastics on aquatic life are a major concern related to their presence. Marine species may encounter blockages, internal injuries, and shifts in feeding behavior owing to microplastic build-up in their gastrointestinal tracts (Wright et al., 2013; Farrell and Nelson, 2013). For filter-feeding organisms like bivalves and planktonic species, the accumulation of microplastics in their digestive systems can impede nutrient absorption and energy intake, ultimately impacting growth, reproduction, and survival (Browne et al., 2008; Cole et al., 2013). The physical abrasion from microplastics can also damage biological tissues, making organisms more vulnerable to infections and other stressors (Galloway et al., 2017).
8.2 Chemical effects
Beyond causing physical harm, microplastics can serve as carriers for toxic chemicals that stick to their surfaces or are absorbed into their polymer structure. Because plastic surfaces are hydrophobic, persistent organic pollutants (POPs), including pesticides, polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), have a strong attraction to them (Teuten et al., 2007; Rochman, 2018). Hotspots of contamination can form when these pollutants accumulate on microplastics’ surfaces at much higher concentrations than in the surrounding water (Rios and Moore, 2007; Andrady, 2011). When ingested, these chemicals can leach from the microplastics into the digestive systems of organisms, causing systemic exposure and potential toxicity (Endo et al., 2005).
8.3 Toxicological impacts on different organisms
The toxic effects of ingested microplastics differ among various aquatic organisms, influenced by factors such as particle size, shape, chemical makeup, and the physiological traits of the species. Phytoplankton and zooplankton, which form the foundation of aquatic food webs, can consume microplastics either directly or indirectly through filter feeding (Cole et al., 2013). While microplastics themselves may not be inherently toxic, their ingestion can cause secondary toxic effects due to associated POPs and additives (Table 4)—for instance, studies have indicated that exposure to microplastics with leached additives can disrupt cellular functions, induce oxidative stress, and impair reproductive and immune responses in aquatic invertebrates and fish (Wright and Kelly, 2017). The impact of MPs on plant and animal life is becoming more apparent, affecting catalase activity, oxidative stress responses, immune regulation, and reproductive health (Chang et al., 2023). Humans, at the top of the food chain, unknowingly ingest and breathe in MPs, exposing themselves to toxic chemicals like phthalates and polybrominated diphenyl ethers, which can cause inflammation and interfere with cellular functions (Haque and Fan, 2023; Ng and Todd, 2023). The persistence of MPs in the environment, their multiple sources, and their biological effects emphasize the urgent need for comprehensive strategies to reduce their release and build-up. Future research should focus on understanding MPs’ physicochemical properties, their environmental half-lives, and developing effective removal technologies to protect ecosystems and human health. Using advanced analytical methods and interdisciplinary strategies will be vital in expanding our understanding and tackling the complex issues caused by microplastic pollution. Bivalves and other filter-feeding species are especially vulnerable to microplastic ingestion because they are constantly exposed to suspended particles in water (Galloway et al., 2017). The build-up of microplastics in their tissues can lead to physiological changes such as decreased feeding rates, altered energy distribution, and higher mortality rates (Browne et al., 2008; Sussarellu et al., 2016). In marine mammals and seabirds, ingesting larger plastic pieces, which can break down into microplastics, has been linked to gastrointestinal blockages, reduced food consumption, and impaired nutrient absorption (Laist, 1997; Avery-Gomm et al., 2012). Microplastics (MPs) have become a serious environmental threat, causing severe negative effects on many organisms, including humans. Their persistent toxicity has been extensively documented across different species in aquatic environments, with important consequences for individual health and overall ecological stability. MPs enter the food web, often accumulating in key organs such as the liver, gastrointestinal tract, and gills of marine animals, as seen in red tilapia (Huang et al., 2021). The toxic effects of MPs are complex, leading to oxidative stress, cell toxicity, slowed growth, immune system suppression, and even changes in gene expression (Meaza et al., 2021). Long-term exposure to MPs can disrupt the gut health of marine species, evidenced in adult zebrafish that show microbiota imbalances, damage to gut lining, and villi fractures, ultimately resulting in microbiota imbalance and metabolic issues (Qiao et al., 2019).
Table 4
| Aquatic organisms | Body part | Types of MP | Toxicological effects | Reference | |
|---|---|---|---|---|---|
| Phytoplankton | Chlamydomonas reinhardtii | Polyethylene, polypropylene | Disruption of cell surface polysaccharide synthesis and detoxification system, inhibit the expression of genes related to growth | Anbumani and Kakkar, 2018 | |
| Pseudokirchneriella subcapitata | Polystyrene | Light and air flow are blocked, photosynthesis and respiration rates of organisms are affected, and growth is inhibited | Nolte et al., 2017 | ||
| Zooplankton | Copepod, Chaetognath, jellyfish larvae, shrimp larvae, fish larvae |
Tissue | Fragment | Detrimental | Goswami et al., 2020 |
| Lumbriculus variegatus | Gut | PE | Induced depletion of their energy reserves | Silva and de Sousa, 2021 | |
| Daphnia magna | Full body | Polystyrene | NA | Kim et al., 2017 | |
| Paracyclopina nana | Full body | Polystyrene | NA | Jeong et al., 2017 | |
| Brachyura larva | Fibers, pellets, and fragments |
NA | Sun et al., 2018 | ||
| Decapod larva | Polystyrene | NA | Vroom et al., 2017 | ||
| Crepidula onyx | Polystyrene | Slower growth, | Lo and Chan, 2018 | ||
| Paracentrotus lividus | NA | Altered body shape | Messinetti et al., 2017 | ||
| Fish larvae, Stomatopoda larva |
Fibers, pellets, and fragments |
Sun et al., 2018 | |||
| Arthropoda | Shrimp Fenneropenaeus Indicus Metapenaeus dobsoni, Portunus pelagicus, Uroteuthis duvaucelii |
Tissue with gut | Fiber, PEST, PA, PE, PP, fragment | Improper feeding behavior, transparent carapace, affects digestion, excretion | Daniel et al., 2020, Daniel et al., 2021, Mohan and Raja, 2024 |
| Mollusca | Meretrix | Tissue | Fragments, polyester urethane, plasticized polyvinyl chloride, polyester, PVCA copolymer, ABS, styrene butadiene, copolymer, PVK, PET, PVC, PEVA | NA | Dowarah et al., 2020 |
|
Amarilladesma
mactroides |
Fibers, polyamides, and polyacrylates | NA | Truchet et al., 2021 | ||
|
Batillaria
multiformis |
Polyethylene terephthalate and polyamide | NA | Xu et al., 2020 | ||
| Barbatia sp. | Polyethylene terephthalate and polyamide | NA | Xu et al., 2020 | ||
| Blue mussel | Polyethylene, PS | NA | Katija et al., 2017 | ||
| Pacific oyster | PE, PP | NA | Katija et al., 2017 | ||
| Batillaria zonalis | Polyethylene terephthalate and polyamide | NA | Xu et al., 2020 | ||
| Perna viridis | Fragments, polyester urethane PET, PVC |
NA | Naidu, 2019, Phuong et al., 2018, Fang et al., 2019; Joshy et al., 2022 | ||
| Pinctada sp. | Fibers | NA | Tahir et al., 2019 | ||
|
Ruditapes
decussatus |
Polyethylene and polypropylene | NA | Abidli et al., 2019 | ||
| Pirenella alata | Fibers | NA | Xu et al., 2020 | ||
| Mytilus edulis | HDPE and PLA particles | NA | Green et al., 2019 | ||
|
Spondylus
spinosus |
Polyethylene, PET | NA | Kazour et al., 2019 | ||
| Sepia officinalis | Fibers | NA | Oliveira et al., 2020 | ||
| Siliqua patula | Polyethylene | NA | Baechler et al., 2020 | ||
|
Tapes
philippinarum |
Polyethylene, polypropylene, polystyrene, and polyester | NA | Cho et al., 2019 | ||
| Phorcus lineatus | PE, polyester, PET, PP, nylon, PS, PVB, and acrylic fibers | NA | Janssens and Garcia-Vazquez, 2021 | ||
| Crepidula onyx | Full body | Polystyrene | Growth hampers | Lo and Chan, 2018 | |
| Mytilus galloprovincialis | Tissue | PP, PE | NA | Gedik and Eryaşar, 2020 | |
| Annelida | Arenicola marina | Full body | Fiber, particles | NA | Thompson et al., 2004, Voparil et al., 2004, Teuten et al., 2007 |
| Nereis virens | Tissue | PBDEs and PCBs | Bioaccumulation | Klosterhaus et al., 2011 | |
| Eisenia Andrei | Tissue | Polyethylene | causes histopathological damage and immune response, and increases the content of proteins, lipids, and polysaccharides in the body | Rodriguez-Seijo et al., 2016 | |
| Hediste diversicolour | Tissue | PS, PE, PEVA, LDPE, HDPE, PP, PA | Behavior neurotoxicity, oxidative stress biomarkers, energy reserves, metabolic activity | Silva et al., 2020b, Silva et al., 2020a; Missawi et al., 2021 | |
| Perinereis aibuhitensis | Tissue | PS | Survivorship | Leung and Chan, 2018 | |
| Echinodermata |
Holothuria fieldana
Holothuria grisea Cucumaria frondose Thyonella gemmata |
Full body | Mesoplastic | NA | Graham and Thompson, 2009 |
| Fish | Pomatoshistos microps | Tissue | PE | AChE activity decrease | Vaid et al., 2021 |
| Solea | Tissue | PVC, PP, PE, PES and PA | bioaccumulation | Cole et al., 2016 | |
| Acanthochromis polyacanatvhus | Full body | PET | growth decrease | Vaid et al., 2021 | |
| Carangoides malabaricus | Gut | Fiber, PEI, acrylic, PPS, ethylene vinyl alcohol, NY, EVA, PIP, PU, polyvinyl chloride | Starvation, stomach fullness | Goswami et al., 2020 | |
| Bagre bagre | Gut | Not mentioned | Effects on GI tract | Schmid et al., 2018 | |
| Cynoglossus lida | Gut | Surlyn ionomer, polyester imide, acrylic, polyphenylene sulphide, ethylene vinyl alcohol, NY, EVA, poly isoprene, PU, polyvinyl chloride | NA | Goswami et al., 2020 | |
| Penacus indicus | Gut | Fiber, PEI, acrylic, PPS, ethylene vinyl alcohol, NY, EVA, PIP, PU, PVC | Part blocking in digestive system | Goswami et al., 2020 | |
| Batrachoides surinamensis | Gut | Not mentioned | NA | Schmid et al., 2018 | |
| Dictrarchus labrax | Full body | PE, PVC, polymer | mortality increase, inflammation, swimming speed decrease |
Vaid et al., 2021 | |
| Pomacentrids | Full body | PS | Resulting in higher mortality rates | McCormick et al., 2020 | |
|
Sardinella
Longiceps |
Gut | Fragment, polyethylene, polypropylene | Starvation | James et al., 2020 | |
| Chaetodipterus faber | Gut | Not mentioned | Stomach illness | Schmid et al., 2018 | |
| Sardinella gibbosa | Gut | Polyethylene, polypropylene, fragments | NA | James et al., 2020, Hossain et al., 2019 | |
| Oryzias Lapites | Reproductive organ | Polyethylene | Abnormal proliferation of sperm cells in male | Vaid et al., 2021 | |
| Katsuwonus pelamis | Gut | Fiber, polyethylene, polyethylene terephthalate, PA, PS, polypropylene, acrylic | Impaired satiation signal leads to starvation | Sathish et al., 2020b | |
| Carassius carassius | Full body | PS | Vitality decrease | Vaid et al., 2021 | |
| Stolephorus indicus | Gut | Fragments, polyethylene, PP | James et al., 2020 | ||
| Rastrelliger kanagurta | Gut | polyethylene, polyethylene terephthalate, PA, PS, polypropylene, acrylic | Stomach fullness | Sathish et al., 2020b | |
| Rastrelliger kanagurta | Gut | Fragment, polyethylene, PP | NA | James et al., 2020 | |
|
Chirocentrus
dorab |
Gut | Fibers, polythene, PEST, PA, PS, polypropylene, acrylic | NA | Sathish et al., 2020b | |
| Danio rerio | Full body | Polyethylene, polypropylene, PA, PVC | Body length decrease, intestinal injury, oxidative stress, protein levels altered |
Vaid et al., 2021 | |
| Cynoglossus macrostomus | Gut | Fragment, polyethylene, polypropylene | Internal ulcer, blockage | James et al., 2020 | |
|
Piaractus
brachypomus |
Gut | Fiber, polyethylene, nylon 6, polypropylene, PBT, PET | Devi et al., 2020 | ||
| Harpadon nehereus | Gut | Polyethylene, PEST, PA, P, fiber, PP, acrylic | Stomach fullness, affects to buoyancy control | Sathish et al., 2020b | |
| Sardinella albella | Gut | Fiber, PE, PEST, PA, PS, polypropylene, acrylic | Internal ulceration | Sathish et al., 2020b | |
|
Istiophorus
platypterus |
Gut | Fiber, PE, PEST, PA, PS, PP, acrylic | Partial blockage of GI tract | Sathish et al., 2020b | |
| Acipenser transmontanus | Full body | NA | Changes in eating habits and protein levels | Rochman et al., 2017 | |
| Ambassis dussumieri | Full body | Fiber, PP | Growth, survivability, and physical state all declined | Naidoo and Glassom, 2019 | |
| Barbodes gonionotus, | Full body | NA | Elevated protein levels and thickening of the epithelium | Romano et al., 2018 | |
| Carassius auratus | Full body | PP, PE | Weight loss; harm to the mouth and digestive system GIT impairment, oxidative stress, and changed activity or growth |
Yang et al., 2020, Jabeen et al., 2018 | |
| Clarias gariepinus | Full body | Fiber, PE | GIT degradation; changed blood chemistry and protein levels, modified protein levels, damage to the liver, and oxidative stress |
Iheanacho and Odo, 2020 | |
| Cyprinus carpio | Full body | Fiber | Deficiencies in development, gastrointestinal injury, altered protein levels, oxidative stress, blood chemistry, immunological function, and blood chemistry | Xia et al., 2020, Banaee et al., 2019, Hatami et al., 2019 | |
| Amphibia | Microhyla ornata | Tissue | Polyester fibers and polypropylene fibers and fragments | Growth directly varied with microplastics ingestion | Hu et al., 2018 |
| Pelophylax nigromaculatus | Tissue | Fragments | Maturation affects | Hu et al., 2018 | |
| Rana limnochari | Tissue | Polyester fibers and polypropylene fibers and fragments | Growth decreases | Hu et al., 2018 | |
| Bufo gargarizans | Tissue | Polyester | Harmful for proper growth | Hu et al., 2018 | |
| Reptiles | Emys orbicularis | Tissue | PE | Liver and kidney disfunction | Banaee et al., 2020 |
| C. caretta | Tissue | PE, PP, PS | Gastrointestinal impairment and an important level of contamination in tissues | Di Renzo et al., 2021, Eastman et al., 2020 | |
| C. mydas | Gut | Polyethene | Gastrointestinal tract obstructions | Colferai et al., 2017 | |
| Crocodilians | NA | PP, PE | Immobilization of the crocodile, stomach flushing disfunction | Gonzalez-Jauregui et al., 2019 | |
| Sea turtle | NA | PE, polyester, PET, PP | Normal lifestyle obstruction | ||
| Python molurus | NA | Polyethylene | Death of snakes entangled in fishing nets | Sindha et al., 2020 | |
Toxicological effects of MPs on aquatic organisms.
NA, Not applicable.
The reproductive and developmental effects of MPs are equally concerning. In aquatic species, MPs have been associated with lower hatching rates and shorter larval lengths in eggs, as well as abnormal behaviors caused by gastrointestinal blockages (Wu et al., 2021; Yin et al., 2021b). In mammals, including humans, the risks go further, with MPs found in tissues like meconium, stool, and even the placenta, indicating possible long-term health effects (Braun et al., 2021) (Tables 5, 6). Aquatic mammals, in particular, face immediate and long-term toxicity from ingesting various polymers such as polyethylene (PE), polypropylene (PP), polyester, and nylon through multiple routes—including dermal, subcutaneous, intraperitoneal, oral, and intravenous exposure (Du et al., 2020). The ecological consequences are just as serious, with research showing MPs can damage the immune and detoxification systems of coral species like Pocillopora damicornis after acute exposure (Tang et al., 2018). Similarly, sea urchin larvae show stunted growth proportional to MP exposure, and zebrafish experience oxidative stress caused by changes in glutathione levels and increased superoxide dismutase activity in their intestinal tissues (Oliviero et al., 2019; Qiao et al., 2018). The negative effects of MPs also extend to invertebrates and algae, with slowed growth seen in species like Skeletonema costatum, Chlorella pyrenoidosa, and Tetraselmis chuii (Deng et al., 2019). Furthermore, prolonged exposure results in reproductive toxicity in Daphnia, highlighting the widespread impact of MPs across multiple levels of marine ecosystems (Jaikumar et al., 2019).
Table 5
| Animals | Found in | Types of MP | Toxicological effects | Reference |
|---|---|---|---|---|
| Earthworms | Full body | PS | Oxidative stress, severe DNA damage in coelomocytes | Xu et al., 2021 |
| Female Wistar rats | Gonad | PS | Ovarian fibrosis and granulosa cells apoptosis due to activation of Wnt/b-catenin signaling pathway and oxidative stress | An et al., 2021 |
| Lumbricus terrestris | Full body | PE | Increased uptake and mortality rate, weight loss, and lower growth rates | Huerta Lwanga et al., 2016 |
| Male Wistar rats | Testis | PS | Histological lesions in testis tissue, DNA damage, sperm abnormalities, alterations in productive hormones and gene expression patterns | Amereh et al., 2020 |
| Achatina fulica | Full body | PET fibers | Severe villi damage in the gastrointestinal walls, inhibited feeding and excretion, oxidative stress | Song et al., 2019 |
| Human | Lung epithelial cells, adenocarcinoma cell lines, dermal fibroblasts, peripheral blood mononuclear cells | Cytotoxicity, immune response, oxidative stress, barrier attributes, genotoxicity induced by microplastics | Danopoulos et al., 2021, Dong et al., 2020; Wang et al., 2020, Hwang et al., 2020 |
Toxicological effects of MPs on terrestrial organisms.
Table 6
| Organism/model | Exposure details | Main findings | Reference |
|---|---|---|---|
| Human cells | Various types of human cells including lung epithelial cells, adenocarcinoma cell lines, dermal fibroblasts, peripheral blood mononuclear cells | Cytotoxicity, immune response, oxidative stress, barrier attributes, genotoxicity induced by microplastics | Danopoulos et al. (2021); Dong et al. (2020); Wang et al. (2020); Hwang et al. (2020) |
| Marine organisms | Various marine species exposed via intravenous, subcutaneous, intraperitoneal, oral, and skin routes | Accumulation, gastrointestinal tract effects, immune system depression, oxidative stress, cytotoxicity, gene expression alterations | Jin et al. (2018); Akhbarizadeh et al. (2018); Oliviero et al. (2019); Mateos-Cárdenas et al. (2019) |
| Rodents | In vivo studies on rodents including mice | Tissue accumulation, neurological effects, reproductive toxicity | Li et al. (2020b); Santana et al. (2018); Deng et al. (2017); Zhu et al. (2018) |
| Coral and sea urchins | Coral species (e.g., Pocillopora damicornis) and sea urchins exposed to acute microplastic exposure | Stress response activation, immune suppression, developmental inhibition | Oliviero et al. (2019); Qiao et al. (2019) |
| Amphipods | Invertebrates (e.g., amphipods) exposed to chronic microplastic exposure in marine environments | Growth inhibition, reproductive toxicity | Deng et al. (2017); Jaikumar et al. (2019); Davarpanah and Guilhermino (2019) |
| Freshwater organisms | Freshwater algae (e.g., Chlorella pyrenoidosa), Daphnia magna, Daphnia pulex, and Ceriodaphnia dubia exposed to microplastics | Growth inhibition, reproductive toxicity | Davarpanah and Guilhermino (2019); Jaikumar et al. (2019 |
| Nematodes | Nematodes exposed to microplastics of varying sizes and concentrations | Gene expression downregulation, neuronal damage | Zhu et al. (2018) |
Toxicological profiles of microplastic exposure.
MNPs can cross biological barriers in fish, accumulating in gonadal tissues and causing reproductive toxicity. A key concern is transgenerational harm, where offspring not directly exposed still show toxic effects. Due to their large surface areas and hydrophobic surfaces, MNPs easily adsorb and concentrate other environmental pollutants, which may worsen reproductive and transgenerational toxicity (Table 7) (Yi et al., 2024). Similarly, Wu et al. (2024) demonstrated that microplastics and nanoplastics in aquatic environments are a major challenge affecting the behavior and reproductive health of aquatic organisms while posing potential risks to human health and ecosystems.
Table 7
| Model | Parental impact | Offspring/transgenerational effect |
|---|---|---|
| Zebrafish (MPs) | Steroid gene disruption (no visible defects) | Offspring development largely unaffected |
| Zebrafish (PSNPs) | Decreased fertility, oxidative damage | Increased offspring malformation; partially mitigated by melatonin |
| Zebrafish (MPs + ACT) | Reproductive, endocrine disruption | Offspring growth and thyroid impairment; exacerbated by nanoplastics |
| Daphnia magna | Reduced survival, reproduction | Persisting defects across generations; slow recovery |
| C. elegans | Neurotoxicity, oxidative stress | Lasted through F1–F2; oxidative genes upregulated |
| Fathead minnow | DNA methylation changes | Epigenetic patterns transmitted to F1 juveniles |
| Mice (mammalian) | Reproductive dysfunction (PCOS-like phenotypes) | F1 male sperm damage, epigenetic alteration; some F2 effects |
Transgenerational toxicity of microplastics (MPs) and nanoplastics (NPs).
The general characteristics of microplastics, such as type, size, color, and form, were the main focus of most research. According to reports (Ziccardi et al., 2016; Caruso, 2019), microplastics may also contain additional hazardous substances like heavy metals, polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and others. Microplastics have been shown to harm various organisms (Lei et al., 2018a, Lei et al., 2018b). The spatiotemporal dynamics of riverine microplastics and the harm they pose to freshwater fish are complex, as highlighted by Sulaiman et al. (2023). The physical and chemical properties of microplastics, river geomorphology, and fluvial processes influence their distribution and assemblages. The distribution of microplastics both vertically and horizontally, bioavailability, fish foraging habits, and the potential for trophic transfer all affect the risks. How microplastics impact fish depends on several factors, such as how long they are retained, how much accumulates, the extent to which they penetrate distant organs and tissues, and the chemical and physical properties of the particles. These tiny particles may transport pathogens and xenobiotic contaminants. Most research on the toxicological effects of microplastics has been conducted at the cellular to organismal level, usually with short-lived animals. Nonetheless, studies are essential to understand how riverine fish populations and larger ecosystems are affected by microplastic pollution. According to Roy et al. (2023), the profile of enzyme antioxidants catalase and SOD (superoxide dismutase) showed little fluctuation and remained steady as microplastic dose and exposure time increased. Conversely, the nonenzymatic antioxidant profile demonstrated clear variability, with the ferric reducing antioxidant potential (FRAP) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) activity gradually declining across all dose ranges and the complete cessation of DPPH (2,2-diphenyl-1-picrylhydrazyl) activity on the 27th day at a 5-ppm microplastic dose. Even the acetylcholinesterase (AChE) activity decreased with higher exposure levels. For the first time, they showed how microplastic pollution directly affects Filopaludina bengalensis, a widespread freshwater snail found throughout the Indian subcontinent. This suggests that microplastic pollution could eventually cause havoc in the Ganga River ecosystem. Microplastic contamination may also impact oyster reef resilience. Changes in fatty acid content and inflammatory pathways were observed in a proteomics study on oysters that ingested microplastics made of polyethylene and polyethylene terephthalate with irregular shapes (Teng et al., 2021). Zebrafish briefly exposed to polystyrene microspheres exhibited inflammation and disruptions in their metabolic functions. Prolonged exposure resulted in gut microbial dysbiosis, skin and gill inflammation, and decreased male fertility. When medaka were exposed to polystyrene particles, their metabolic pathways changed, and their livers began accumulating fatty acids and esters (Ye et al., 2021).
In recent years, several works have been conducted on the toxicological mechanisms of nanoplastics in animal models—for instance, Cheng et al. (2025) demonstrate that the size of microplastic particles critically influences toxicity mechanisms, with ferroptosis being more prevalent for smaller particles and YAP-mediated metabolic disruption for larger ones. This suggests potential therapeutic targets for reducing microplastic-induced intestinal damage. Du et al. (2024) examined the molecular mechanisms behind the effects of Cd pollution and NPs combined with Cd pollutants on HSA. They investigated the differences in HSA toxicity between Cd alone and NPs–Cd exposure. Similarly, Li et al. (2025) highlight the impact and mechanisms of NPs on the immunotoxicity of Cd2+, providing key data and strategies for accurate assessment of the environmental behavior and health risks of NPs. Huang et al. (2024) summarize recent research on the potential hazards that MNPs may pose to the urinary system, highlighting the mechanisms of toxicity and the current state of knowledge. Studies have shown that MNPs enter the human body through drinking water, the food chain, inhalation, and skin contact. They may penetrate the bloodstream via the digestive, respiratory, and skin systems, subsequently dispersing to various organs, including the urinary system. Overall, these findings highlight the widespread and far-reaching adverse effects of MPs, not only on marine organisms but also on human health and the environment. The ongoing build-up of MPs in aquatic systems calls for urgent research and intervention strategies to reduce their impacts and protect ecosystem health.
9 Estimation of microplastics
9.1 Sampling methods from the aquatic environment for MP estimation
The detection and analysis of MPs in aquatic environments presents a significant challenge due to their small size, diverse shapes, and high mobility. MPs, particularly polyethylene (PE) and polypropylene (PP), are commonly found in water bodies, often in the sub-millimeter size range, making them difficult to detect using traditional methods (Wu et al., 2025). The methods for sampling water, sediment, and aquatic organisms, as well as the sample sizes and procedures for isolating and identifying microplastics, vary significantly among researchers. A notable negative correlation has been observed in some studies between the amount of microplastics detected and the number of samples collected. There is currently no systematic review of the study characteristics and techniques used, so the data provided cannot be compared directly. Efforts are ongoing to standardize monitoring methods for mapping MP contamination in marine environments worldwide. Some commonly used methodologies for estimating MPs, as employed by scientists across different countries and regions, are summarized below.
9.1.1 Sampling of water
Numerous factors can influence the vertical movement of plastic in water, including hydrodynamics and physicochemical properties like density, shape, size, chemical adsorption, depth, and location. These elements affect both the quantity and quality of microplastic (MP) samples collected during sampling. Researchers use nets such as plankton, phytoplankton, and neuston nets to collect MPs from water (Govender et al., 2020; Wicaksono et al., 2021; Jong et al., 2022). This method allows for quick filtering of large water volumes, providing concentrated samples in a short time. It also yields samples that represent a large water body well (Prata et al., 2019). Mesh sizes for these nets vary based on research goals, ranging from tens of microns to several millimeters (Sajjad et al., 2025). Unfortunately, using different mesh sizes complicates direct comparison of results across regions. Although various MP sampling and analysis techniques exist, the lack of standardized procedures remains a challenge (Table 8). These include methods like tramp blankets (Rose and Webber, 2019), steel water testers (Jiao et al., 2022; Zhu et al., 2021; Li et al., 2020), bottle sampling (Kumkar et al., 2021), glass containers, and metal gallon containers for surface water collection (Celis-Hernández et al., 2021). Reaching consensus on these sampling methods, whether traditional or modern, is crucial. Keep in mind that MP counts can vary significantly depending on the collection method used (Hale et al., 2022). The sampling approach greatly influences the effectiveness of microplastic collection and the identification of different plastic types, which impacts the reliability of results. In dynamic river systems, the distribution and concentration of MPs can fluctuate due to changes in tidal flow over time, making the sampling schedule even more critical. To address this, researchers have employed various techniques, such as collecting water from intertidal zones using metal buckets.
Table 8
| Sampling method | Process description | Filtration and preservation | Storage conditions | Reference |
|---|---|---|---|---|
| Surface water collection (60 L) | Surface water samples were collected from designated sampling locations using sterile containers. | The collected samples were sieved through filters of appropriate mesh size to remove debris and large particulates. | Samples were securely stored in sterile containers until further processing. | Das Sarkar et al., 2023 |
| Intertidal zone sampling (100 L) | Water samples (depth 0–0.5 m) were collected from the intertidal zone using metal buckets to capture suspended and free-floating particles. | Samples were filtered through a 250 µm mesh sieve to concentrate particulate material. | Filtered water was stored at −20°C to preserve until analysis. | Ariefdien et al., 2024 |
| Shallow subsurface collection (100 L) | Water samples were collected using a 2 L stainless steel jug at depths between 0 and 25 cm below the surface. Sampling containers were rinsed three times with site water prior to collection to prevent contamination. |
The collected water was passed through a stainless-steel sieve (30 cm diameter; 50 µm mesh size) to retain fine particulate matter. | Processed samples were stored at 4°C to minimize degradation before extraction. | Kumkar et al., 2021 |
Sampling methods of water for microplastic analysis.
For example, to prevent contamination, Ariefdien et al. (2024) used a metal bucket to collect 100 L of surface water from the intertidal zone, ensuring that the depth was between 0 and 50 m downwind. The water was then passed through a 250-μm mesh to remove any remaining particles. Afterward, a 50-mL Falcon tube was pre-cleaned and kept at -20°C until analysis. A good rule of thumb is to collect samples within an hour of the flood or ebb tide’s lowest point when water flow is least active. To obtain a 10–100-L bulk sample, a clean polycarbonate container can be inserted vertically into the river. Before filling, the bottle should be cleaned three times with water from the site. To avoid airborne contamination, it is recommended to take samples from the upper 0.3–0.5 m of surface water and quickly cover them with a lid. Finally, samples should be transported to the laboratory and stored at 4°C prior to analysis. Sarkar et al. (2021b) state that 50 lL of surface water were collected from each East Kolkata Wetland (EKW) wastewater canal and treatment pond (at 0–40-cm depth) using a steel bucket. The water was immediately filtered through a 63-μm mesh and then stored in airtight containers. The samples were then separated into two portions: 63–850 μm and 850 μm–5 mm. A process involving counting and weighing is used alongside visual inspection to separate larger plastic particles (5–10 and >10 mm) (METTLER TOLEDO, Switzerland; New Classic MS, Model MS30002SE/A01).
9.1.2 Samplings of sediment
Because of their individual properties and environmental variables such as currents, winds, tides, and the specific collecting area, microplastics (MPs) do not disperse uniformly in sediments. This means that sampling depth and location (such as intertidal zones or transects) can significantly affect MP analysis results. Some regions may have higher concentrations of MPs than others (Hanvey et al., 2017). When collecting mangrove sediment samples for MP analysis, researchers typically collect from the top layer of sediment at depths between 1 and 5 cm during ebb tides. Researchers use square structures made of metal or wood, known as quadrats, along with steel spoons or shovels to extract sediment. In addition, samples from lakes, rivers, and oceans are gathered using tools such as cores, metal boxes, and cylindrical tube samplers. Metal drills are valued for their durability and ease of use when obtaining undisturbed sediment cores. Numerous studies highlight their benefits (Govender et al., 2020; Wicaksono et al., 2021; Pradit et al., 2024). Van Veen or Ekman samplers, sometimes called dredgers, can be used to collect bottom sediment samples in lakes, rivers, and coastal areas—for example, Ariefdien et al. (2024) collected silt samples at 5-meter intervals along the strandline. They used a metal spoon to gather the top 5 cm of sediment within a 0.25-m by 0.25-m quadrat (Table 9) and stored the samples in Ziploc bags. A wet sediment sample weighing 2 to 3 kg was taken from three different sites in EKW. These sites were spaced 60–90 m apart and at least 80 m from the wastewater entry point. The wet sediments were dried in an oven at 65°C for 36 h until reaching a constant weight. The dried sediments were then sieved through mesh sieves of various sizes (10 mm, 5 mm, 850 μm, and 63 μm) as described by Sarkar et al. (2019).
Table 9
| Sampling procedure | Description | Reference |
|---|---|---|
| Surface water collection (60 L) | Surface water samples (60 L) were collected from designated sampling stations using sterilized containers to avoid contamination. | Das Sarkar et al., 2023 |
| Filtration | The collected samples were sieved through a filter of the required mesh size to retain particulate matter and microplastic debris. | |
| Storage | Processed samples were immediately sealed and stored under controlled conditions to prevent degradation prior to analysis. | |
| Intertidal zone water collection (100 L) | Water samples (100 L) were collected using a metal bucket from the intertidal zone at depths ranging from 0 to 0.5 m. | Ariefdien et al., 2024 |
| Filtration and preservation | Samples were filtered through a 250 μm mesh sieve and subsequently stored at −20°C until laboratory analysis. | |
| Surface sampling with stainless steel jug | 100 L of water was collected using a 2 L stainless steel jug at a depth of 0–25 cm below the surface. Containers were rinsed thrice with site water before actual sampling to avoid contamination. | Kumkar et al., 2021 |
| Secondary filtration | Samples were filtered through a 30 cm diameter stainless steel sieve (50 μm mesh size) to separate suspended solids and microplastic fragments. | |
| Storage condition | Filtered water samples were preserved at 4°C until further processing for microplastic extraction and analysis. |
Sampling methods of sediment for microplastic analysis.
9.1.3 Sampling of aquatic organisms
Collecting microplastics (MPs) from organisms presents significant challenges. Different techniques are used depending on the type of organism. Planktonic organisms, including zooplankton and phytoplankton, can be collected using plankton nets with various mesh sizes (Table 10). These nets have been widely used in numerous studies (Celis-Hernández et al., 2021; Maghsodian et al., 2021; Huang et al., 2020). Methods for collecting nektonic organisms such as fish and prawns include cast nets, trawl nets, bag nets, and direct hand collection, as shown in studies. Fish samples are also obtained through bottom trawling. Before analysis, the samples are stored at -20°C, wrapped in aluminum foil. Patria et al. (2020) and Addo et al. (2022) all state that bottom dredging with Van Veen or Ekman samplers is the preferred method for collecting benthic organisms like gastropods, bivalves, polychaetes, and echinoderms. Both intertidal and subtidal zones are used to collect mussels, sea urchins, and whelks. Subtidal samples can be collected by snorkeling up to a depth of 3 m. Intertidal samples are gathered from rock coastlines. All samples are stored in pre-cleaned Ziploc bags and kept at -20°C until analysis. Sarkar et al. (2021a) used a cast net with a mesh size of 10–15 mm to collect macroinvertebrates and large and small fin fishes, which were preserved in a sealed container with ice for later analysis.
Table 10
| Type of organisms | Collection methodology | Reference |
|---|---|---|
| Planktonic (Zooplankton and Phytoplankton) | Collected using plankton nets of varying mesh sizes, depending on the target taxa. Samples are carefully transferred into sterile containers to prevent cross-contamination and stored at −20°C until further processing. | Celis-Hernández et al., 2021; Maghsodian et al., 2021; Huang et al., 2020 |
| Nektonic (Fish, Shrimp) | Sampling performed using cast nets, trawl nets, and bag nets, complemented by direct hand collection in shallow waters. Collected specimens or water samples containing organismal traces are preserved at −20°C for molecular analysis. | |
| Benthic (Gastropods, Bivalves, Polychaetes, and Echinoderms) | Samples are collected via bottom dredging using Van Veen or Ekman grab samplers and immediately placed in sterile Ziploc bags at −20°C. | Patria et al., 2020; Addo et al., 2022; Kumkar et al., 2021 |
| Mussels, sea urchins, and whelks are collected from both intertidal and subtidal zones. Subtidal samples are obtained by snorkeling at depths between 1 and 3 m, ensuring minimal disturbance to the substrate. | ||
| Intertidal samples are hand-picked from rocky shores and stored in pre-cleaned Ziploc bags at −20°C until extraction and analysis. |
Sampling methods of aquatic organisms for microplastic analysis.
9.2 Analytical processes
Addressing the research gaps related to micro- and nanoplastics (MNP) pollution requires overcoming several analytical challenges. These include identifying and characterizing the NP (nano-plastic) fraction, creating standardized reference MNP particles with well-defined weathering conditions, developing gentle extraction methods to study the plastisphere (microbial communities on plastic surfaces) and leaching profiles in complex matrices, improving spectrometric imaging techniques to assess how weathering impacts MNP structure (such as shape, porosity, polymer degradation, and plastic additive leaching), designing cost-effective, high-throughput methods for evaluating MNP pollution in different matrices (like biota, water, and soils), and establishing analytical pipelines to identify and quantify plastic additives and pollutants. The methods outlined by GESAMP (2019) are commonly used to extract MPs from various samples, including water, sediment, and biota. Recent research has also explored new analytical procedures to detect MNPs in these aquatic organism samples.
9.2.1 Extraction of MPs from water and sediment
After making a few minor adjustments, Chau et al. (2023) employed procedures that have been previously described (Mai et al., 2019; Zhao et al., 2019). First, a nylon sieve with a 20-μm pore size was used to reduce the volume of the riverine water samples. To remove any salts, filtered distilled water was used to rinse the samples. To facilitate the digestion of organic compounds, the residues were treated with a 30%-v/v hydrogen peroxide solution and then incubated in an oven at 60°C for 24 h. Sodium chloride (NaCl) solution (1.2 g/mL) was added to a separating funnel to achieve particle density separation (Figure 8). Before examination, the liquid was filtered through a 1-μm glass fiber filter and placed in a petri dish. It was then prepared for spectroscopic and microscopic analysis. GESAMP also developed a method to remove MPs from sediment and water (2019). In this process, water samples in falcon tubes were thawed and transferred to glass jars that had been thoroughly washed with ultrapure MilliQ water. All storage containers underwent rigorous rinsing to ensure that all samples were thoroughly cleaned. Each sample was digested by adding 10% KOH at a ratio of 1:2 and then incubated in an oven at 50°C for 24 h to remove organic material. After digestion, the samples were filtered using a Buchner funnel and a vacuum pump. The samples were filtered through a pre-cleaned 20-μm nylon mesh and then dried in pre-cleaned petri dishes before analysis.
Figure 8
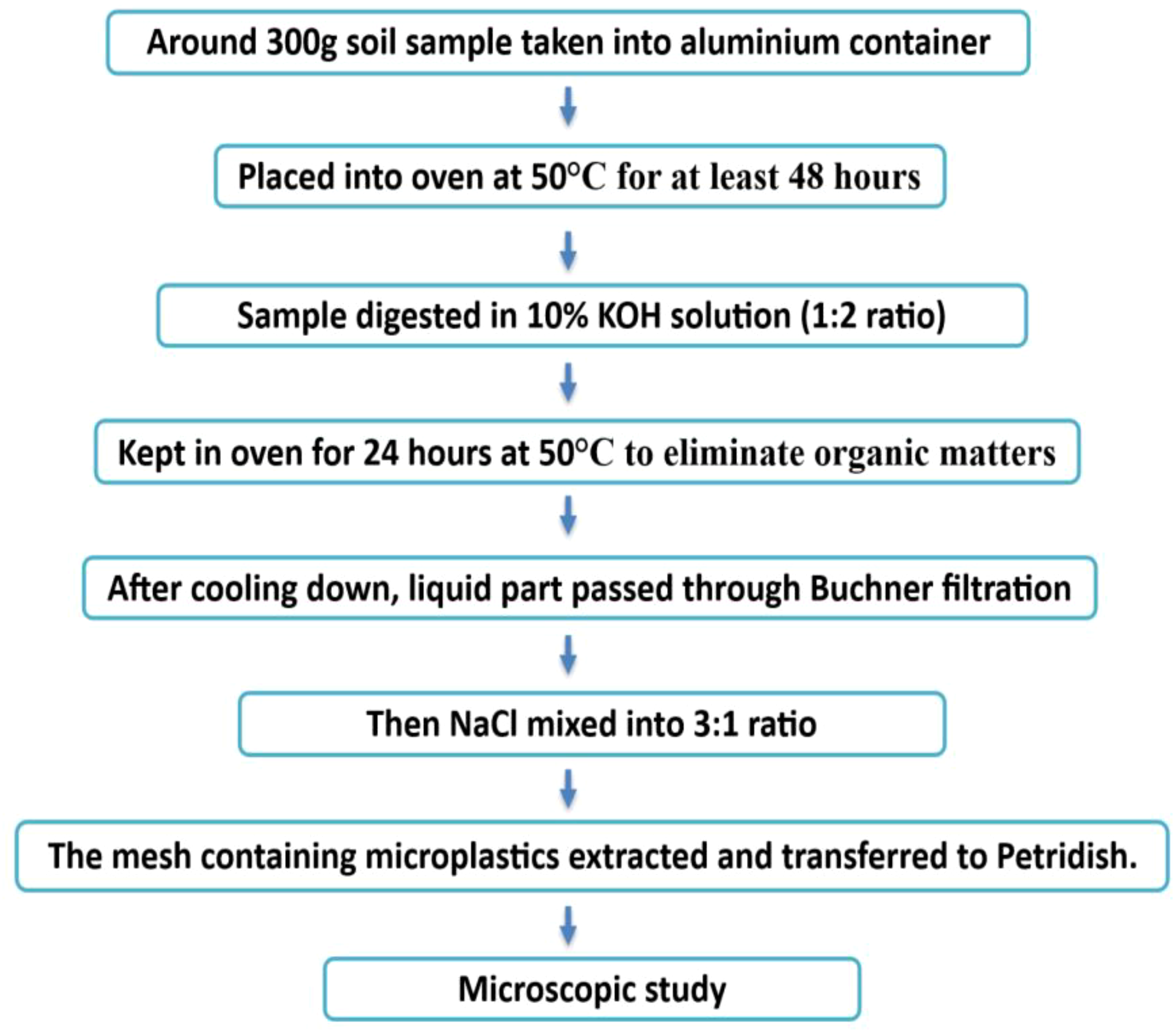
Schematic diagram for the extraction of microplastics from sediment samples (GESAMP, 2019).
For sediment samples, at least 300 g of sediment was carefully extracted from Ziploc bags, then placed into aluminum containers, and baked in an oven at 50°C for a minimum of 48 h or until a consistent weight was achieved. A 200-g sample was digested in 10% KOH solution (ratio of 1:2) at 50°C for 24 h to remove organic matter. Once cooled, the liquid was filtered through a Buchner funnel with a 20-μm nylon mesh. A highly concentrated saline solution (NaCl 360 g/L), filtered through a 10-μm mesh, was mixed with the sediment residue at a ratio of 3:1. The mixture was gently stirred for about 2 min and left undisturbed for 15 min. The liquid was then filtered again with the Buchner system. This process was repeated three times, using the same filtered saline solution each time (Figure 9). For microscopic and spectroscopic analysis, the microplastic material was carefully transferred to petri dishes.
Figure 9
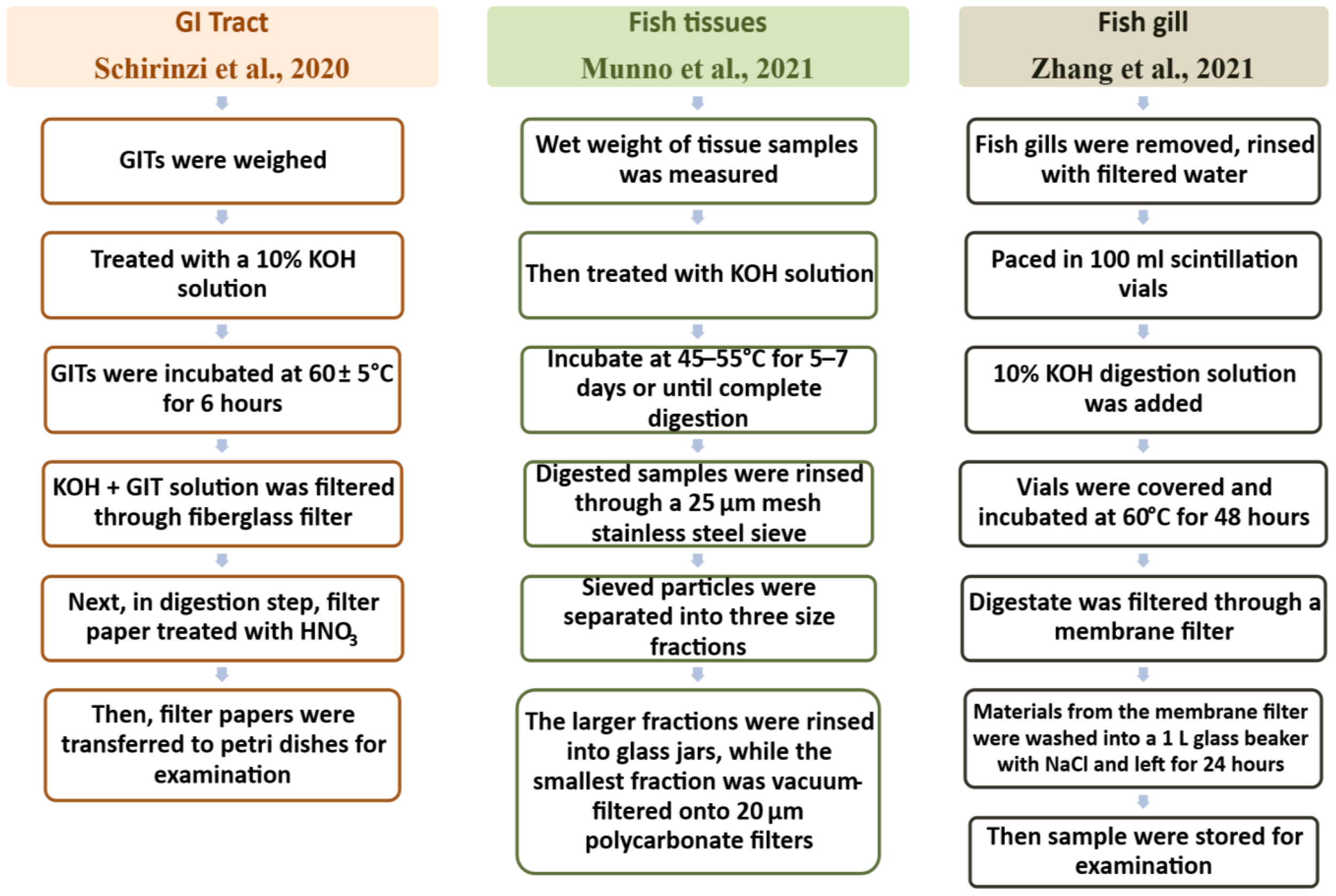
Schematic diagram for the extraction of microplastics from fish tissues.
Studies by Sarkar et al. (2019) and Tien et al. (2020) described methods to remove MPs from sediment and water. Initially, microplastics from the sieved sediment and water samples (850 μm–5 mm and 63–850 μm) were separated using density separation with a standard ZnCl2 solution (1.80 g/cm−3). In brief, a saturated solution of ZnCl2 from Thermo Fisher Scientific Inc. was added to the samples at a ratio of 10:1 (v/w). The mixture was stirred for 15–20 min and left undisturbed overnight. The floating particles were carefully separated using precise techniques and collected on filter paper with very small pores to ensure effective filtration. The process involved a specialized setup with a vacuum pump. The floating debris was rinsed thoroughly with deionized water to remove any remaining salt. It was then treated with 30% hydrogen peroxide for 3 h to fully digest any biological material. The remaining plastic debris was washed thoroughly with deionized water using vacuum filtration, specifically with a glass microfiber filter with a 0.7-μm pore size. The density separation and digestion steps were repeated a second time to isolate the plastics, which were then dried for 36 h in a vacuum desiccator.
9.2.2 Extraction of MPs from tissues
Using the procedures outlined by GESAMP (2019), all organisms were allowed to thaw (Figure 10). The wet weight of soft tissue was measured for each organism. Soft tissue from each sample was stored in a glass jar. A 10% KOH solution was used to aid digestion. The samples were heated to 50°C and left to incubate for 24 h. The samples underwent Buchner filtration with a 20-μm nylon mesh. Carefully, the mesh was removed and placed into a thoroughly cleaned petri dish for further examination. Schirinzi et al. (2017) developed a different method for extracting MPs from the gastrointestinal tract. In this approach, gastrointestinal tracts (GITs) were weighed using a precision balance to achieve the desired size. The tissue samples were treated with a 10% potassium hydroxide (KOH) solution (1/3 w/v). GITs were incubated for 6 h at 60°C ± 5°C and then allowed to cool at room temperature overnight. The KOH + GIT solution was filtered through a 1.6-μm pore fiberglass filter (GF/A Whatman) using a vacuum system. During the second digestion step, filter papers were treated with 40 mL of 20% nitric acid (HNO3) for 1 h to analyze plastics selectively. Cleaned filter papers were transferred to petri dishes for examination under a stereo-microscope.
Figure 10
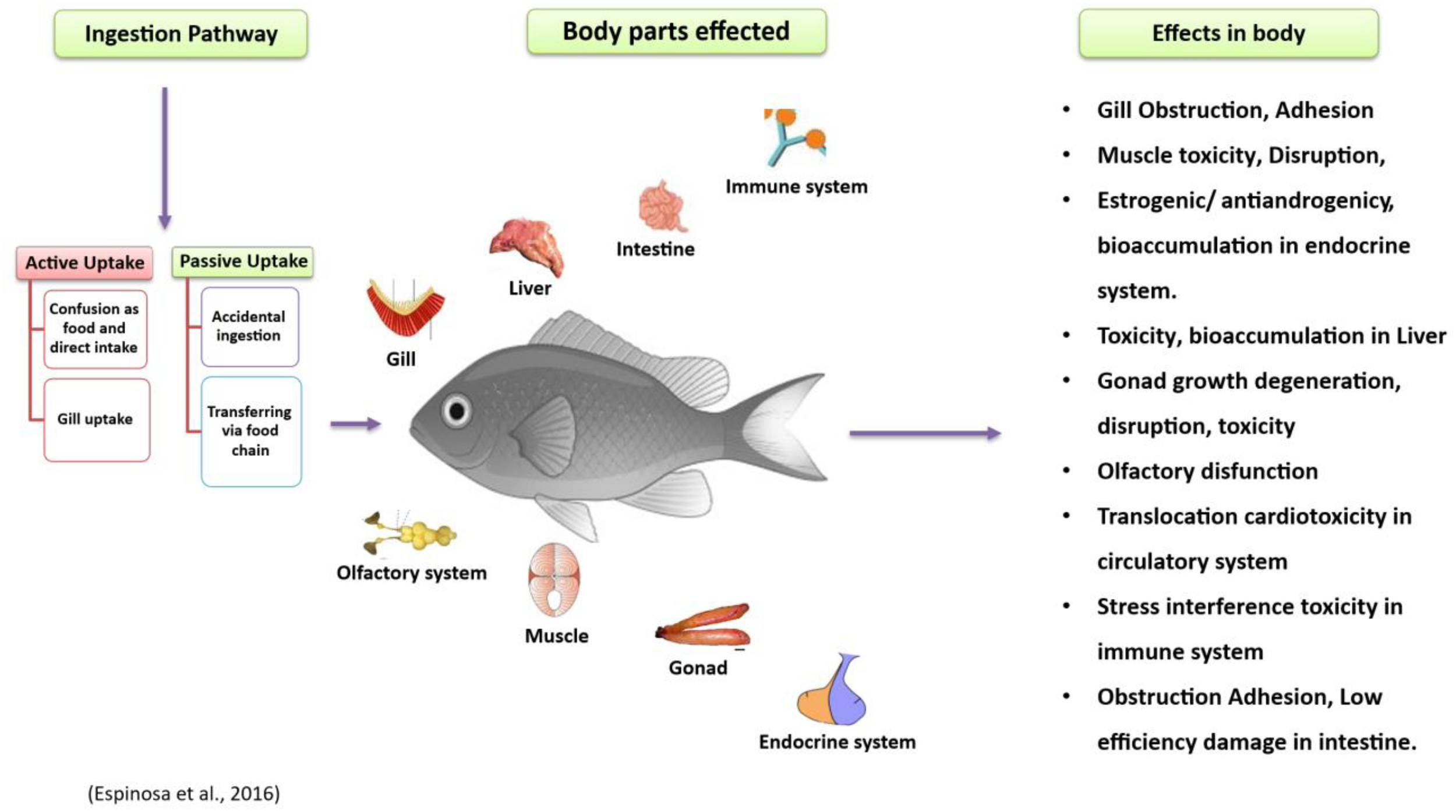
Accumulation of microplastics in a fish’s body.
The alkaline digestion method for fish GI tracts was proposed by Karuppasamya et al. (2020). In this method, alcoholic potassium hydroxide (C2H5OH + KOH) was used to remove organic substances (Figure 9). The alkoxide ions (C2H5O−) produced in this process are more basic than hydrated hydroxide ions, which speeds up digestion. Munno et al. developed a rigorous method to estimate MPs in fish tissues. Using a precision balance, the moist weight of tissue samples was measured. The tissue was fully submerged in 20% potassium hydroxide (KOH) solution filtered through a 1-μm filter. Samples were incubated for 5 to 7 days at 45°C–55°C or until complete digestion. The digested samples were rinsed through a 25-μm pore stainless steel sieve. An additional 24 h of rinsing was done with liquid detergent. Three size fractions were separated from the sieved particles: >355, 125–355, and 45–125 μm. The larger fractions (125–355 and >355 μm) were rinsed in glass canisters, while the smallest fraction (45–125 μm) was filtered onto 20-μm polycarbonate filters using a vacuum.
Zhang et al. (2021) proposed an additional approach to extract MPs from fish respiratory tissues. After removal and rinsing with filtered water, fish gills were transferred to 100-mL scintillation vials. A 10% KOH (w/v) digestion solution was added to each vial (Zhang et al., 2019). The containers were sealed and incubated at 60°C in a constant-temperature shaker for 48 h or until the gills were fully digested. The digested content was filtered through a membrane filter. The residue on the filter was washed with 1 L of water in a glass beaker containing a flotation agent (NaCl), and the solution was left undisturbed for 24 h. The surface layer was then filtered again through a membrane filter. After drying in a desiccator for 24 h, the filtered materials were collected for MP analysis. To isolate microplastics from fish gastrointestinal (GI) and gill tissues, Debbarma et al. (2022) used a rigorous method that enabled complete digestion and efficient separation for further analysis. The GI and gill samples were placed in a 60-mL borosilicate glass tube and immersed in 10% KOH for 72 h to remove organic matter and extract microplastics (MPs). During this period, the samples were stirred intermittently to ensure proper decomposition of tissues. After 72 h, the mixture was filtered using a 0.45-μm nitrocellulose filter membrane via vacuum filtration and then transferred into a 50-mL lab vial. To this, 25 mL of 4.4 M NaI solution was added. The solution was then agitated for 5 min on an orbital shaker and sonicated at 50 Hz for 5 min. The samples were centrifuged for 3 min at 1,000 RPM. The filtered samples were then dried at room temperature in individual petri dishes before further analysis. The technique by Hove et al. (2023) streamlines and improves the microplastic analysis process for fish tissues. Fish tissue samples, including oily and lean fillets, livers, and oils, were collected using this method. Scalpels and forceps were used to carefully remove bones and scales, and the tissue was then sectioned into approximately 1-cm³ segments. All procedures were performed within laminar flow cabinets to reduce the risk of airborne microplastic contamination. This protocol relies on surfactant-assisted alkaline digestion using potassium hydroxide (KOH). NaTT was added to a homogenized 100 g sample as a NaCl-surfactant solution (Tween®20 + Triton™X100). After stirring in 60 g of 4.2 M KOH, a 1.4-M KOH solution was obtained. The mixture was incubated at 40°C for 16–24 h. After digestion, the solution was cooled on ice and neutralized with 1.0 M citric acid to reach the desired pH. Vacuum filtration was used to separate the digested material. Compared to other methods, this protocol significantly reduces the time and steps required. This allows for more samples to be analyzed for MPs, which is crucial for monitoring and surveillance. The digestion efficiency (DE%) for most matrices exceeded 99.9%. In a recent study, Sarkar et al. (2021a) developed a method to extract MPs from tissue samples. After thorough rinsing with distilled water, fish were dissected to remove the entire gastrointestinal (GI) system, including the esophagus and cloaca. To ensure complete cleaning, the specimens were digested with 30% H2O2 at 70°C, which effectively degraded the remaining organic materials (Gbogbo et al., 2020). For smaller fish under 10 g and snails, their digestive tracts and entire bodies were combined and processed as described. After filtration, the samples were prepared for further analysis. Microplastics (MPs) have been extracted from fish tissues using various filtration methods and digesting solutions. Common approaches include using potassium hydroxide (KOH) for tissue digestion, often followed by filtration and incubation, as detailed by Munno et al. (2021), Schirinzi et al. (2017), and GESAMP (2019). More recent methods incorporate surfactant-assisted alkaline digestion and other improvements to increase efficiency and reduce contamination, as demonstrated in protocols by Hove et al. (2023) and Sarkar et al. (2021).
10 Characterization of microplastic
One of the main methods used to identify microplastics (MPs) is visual examination under a microscope—for example, GESAMP (2019) used a stereo microscope (Zeiss Stemi DV4) at 40X magnification to identify MPs in tissue samples held on nylon mesh. This technique allowed to identify MPs based on shape, such as fibers, fragments, spheres, and filaments, as well as color and size, with particles up to 5,000 μm. Fourier transform infrared spectrometry (FTIR) was used to analyze the polymer makeup of the MPs, with a minimum size limit of 500 μm for FTIR analysis (Sparks et al., 2021). Schirinzi et al. (2017) improved MP characterization by classifying them by size, color, and physical features using a stereomicroscope. They used FTIR spectroscopy, specifically in the Attenuated Total Reflection (ATR) mode, to examine the polymer structure of plastics, achieving a 70% spectrum matching rate across the 650–4,000-cm⁻¹ range (Table 11). Expanded on these methods by studying MPs in tissue samples with a dissecting microscope (Olympus SZ61 stereomicroscope). Suspected human-made particles were visually counted and described based on color and shape, including fibers, fiber bundles, fragments, films, foams, spheres, pellets, and rubber. To confirm the accuracy of visual ID, chemical analysis was performed using ATR-FTIR and Raman spectroscopy, providing insights into MP composition. Zhang et al. used advanced stereomicroscopy (Leica with built-in Leica Application Suite X software) to carefully analyze and measure suspected MPs. The detailed images captured attributes like color, shape, and polymer type, while μ-FTIR analysis in transmittance mode was done with the Nicolet iN10 instrument from Thermo Fisher, USA. This comprehensive approach gave valuable insights into the characteristics and makeup of MPs. Gao et al. (2022) studied MPs in water samples. Using a stereomicroscope at about ×40 magnification, they counted MPs by measuring the number of particles per volume of water (particles/L). The MPs were categorized by color and sorted into five shapes: line/fiber, film/sheet, pellet, foam, and fragment. For polymer identification, they used an attenuated total reflectance FTIR spectrometer (Thermo Nicolet iS10), comparing the spectra to standard references in open-access databases to ensure accurate identification with a 70% matching threshold. Debbarma et al. (2022) used an Olympus stereo zoom microscope (SZX16 Model, India) to visually examine materials on filter papers after filtration. They focused on MP shape, color, and size, identifying four MP shapes: fragments, films, fibers, and pellets/beads. Sizes ranged from 1,000 μm to less than 100 μm, and eight colors were identified: translucent, white, black, blue, brown, red, green, and yellow. Laser Raman spectroscopy confirmed the presence of polymer functional groups, verifying the particles were plastics. In a detailed study, Hove et al. (2023) characterized MPs from digested samples using double filtration with cellulose nitrate (CN) filters and stainless-steel filters. A digital microscope with magnification from ×20 to ×220 was used for observation, and polymer composition was analyzed with both FTIR (micro-FTIR) and py-GC–MS (pyrolysis-gas chromatography–mass spectrometry). These techniques provided deep insights into the properties of the MP particles.
Table 11
| Characterization method | Study | Reference |
|---|---|---|
| Stereo microscope (Zeiss Stemi DV4) | Shape (including fibers, fragments, spheres, and filaments), color (ranging from white and transparent to red, yellow, black, and blue), and size (up to 5,000 μm) | GESAMP, 2019 |
| Stereo microscope (Leica M165 FC, Germany with built-in Leica Application Suite X software) | Including color, shape, and polymer type based on the morphology of the particles | |
| Olympus stereo zoom microscope (SZX16 Model, India) | Shape, color, and size | Debbarma et al., 2022 |
| FTIR (micro-Fourier transform infrared spectroscopy) and py-GC–MS | Polymer composition | Hove et al., 2023 |
| Fluorescence microscope (Zeiss AXIO, Scope. A1 fitted with camera, Zeiss AxioCamICc 5) | Color change | Shim et al., 2016 |
| Raman spectroscopy | Mass and particle count | Park and Park, 2021; Abimbola et al., 2024 |
| Two-dimensional correlation spectroscopy (2D-COS) | MP behaviors, their aging steps, and their relationships with naturally occurring organic matter (NOM) | Peng et al., 2023 |
| GC/MS | Polymer composition | Park and Park, 2021 |
Characterization of microplastics in the aquatic environment.
Sarkar et al. (2021a) classified MPs into macroplastics (>10 mm), mesoplastics (5–10 mm), and microplastics (<5 mm), further dividing MPs into fractions from 850 μm to 5 mm and 63 μm to 850 μm. Macro- and mesoplastic fractions were identified visually, while MPs were distinguished under a microscope (Nikon Eclipse Ci with Nikon DS-Fi2 camera) based on their shape—film, fiber, pieces, foam, and beads. The fractions were accurately counted and weighed using a Denver Instrument SI-234 precision balance. For MPs from biological samples, larger MPs (850 μm to 5 mm) were observable with an optical microscope, while smaller MPs (63 μm to 850 μm) were detected using a fluorescence microscope after staining with Nile Red (NR), a hydrophobic fluorescent dye, following methods documented by Shim et al. (2016) and Erni-Cassola et al. (2017). In this approach, the digested gut content was mixed with NR solution and left at room temperature to allow the dye to adhere to the microplastic surface. The stained samples were then examined under a fluorescence microscope (Zeiss AXIO Scope. A1) to detect color changes, with excitation and emission wavelengths optimized for highlighting MP particles. ATR FT-IR (Spectrum 100 FTIR Spectrometer, PerkinElmer) was subsequently used to analyze the chemical composition of sorted MPs by comparing the spectra to reference spectra for common plastics such as polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), and polystyrene (PS) (Sarkar et al., 2019; Cowger et al., 2021; Amrutha and Warrier, 2020). Current MP characterization methods are varied and increasingly advanced. Visual discrimination remains the most basic technique but often lacks precision. Microscopic techniques, including standard optical microscopy, offer a more detailed examination of MPs but have limitations in resolving finer details. More comprehensive characterization is enabled by high-resolution imaging methods such as atomic force microscopy (AFM) and scanning electron microscopy (SEM), which provide enhanced insights into the surface morphology and structural features of MPs.
Raman and Fourier transform infrared (FT-IR) spectroscopy are now essential methods for determining the chemical makeup of MPs. FT-IR spectroscopy analyzes the infrared light absorbed by MPs, producing a spectral fingerprint unique to specific polymers. Raman spectroscopy, by contrast, uses laser light to measure molecular vibrations, providing complementary data to FT-IR and offering important insights into the types of polymers and additives present in MPs (Cowger et al., 2021). Another advanced technique, pyrolysis analysis, involves the thermal decomposition of MPs in the absence of oxygen, breaking down polymers into smaller molecules. These by-products are then analyzed using techniques like gas chromatography–mass spectrometry (GC-MS), which helps identify complex additives and contaminants within MPs. Pyrolysis analysis is particularly effective for understanding the chemical composition of MPs, though it is a destructive method that does not preserve the original shape of the particles. Despite these advancements, each method has limitations. Visual and microscopic techniques can have difficulty distinguishing MPs from other particles in complex environmental samples. While SEM and AFM provide detailed analysis, they are time-consuming and require specialized equipment. FT-IR and Raman spectroscopy, although powerful, can be limited by the physical state of the sample and the presence of interfering substances. Pyrolysis analysis, although informative, destroys the sample and cannot reveal the original form of MPs. Future research should aim to develop integrated approaches that combine multiple characterization techniques to address these individual limitations. Advances in machine learning and artificial intelligence could improve the accuracy and efficiency of MP identification and measurement. Additionally, standardizing protocols and methods across studies will be crucial for generating comparable and reproducible data, leading to a better understanding of MPs in different environmental settings.
10.1 Visual discrimination for microplastic identification: advantages and challenges
Visual discrimination is a key way to identify larger microplastics, especially those measuring 1 to 5 mm, often found in coastal areas. This method involves directly separating and identifying particles with tools like tweezers and trays (McDermid et al., 2004). However, it faces challenges because many organic and inorganic substances look very similar to plastics in size and appearance, making accurate identification difficult. Sometimes, smaller but brightly colored plastics can also be seen visually (Li et al., 2016). An important observation is that while synthetic fibers often show vivid colors, natural fibers tend to create white and clear microplastics (González-Pleiter et al., 2021). The visual approach is simple and easy to use, but because plastics and other materials look alike, it can lead to many errors. De Witte et al. (2014) proposed a method that uses a heated needle tip to improve visual accuracy. They determined if the material was plastic by touching it with the needle and checking if it melted or curled. Despite its innovative idea, this technique has some limitations. If the needle’s temperature is not high enough, some plastics may not change in appearance. Knowing the exact properties of the plastic beforehand helps improve this method. In summary, visual discrimination is a straightforward way to detect microplastics, but it has many flaws that can cause serious mistakes. The reliability of this method is reduced because plastics and other substances look similar, and even advanced techniques like the heated needle test are not without limitations.
10.1.1 Microscopic discrimination in microplastic identification
According to Wang et al., traditional optical microscopy has long been a key method for identifying microplastics, especially those that are several hundred microns in size. By enlarging the image, this method provides detailed information about surface texture and structural features of particles, making it easier to distinguish plastics from visually similar materials. While this technique is effective at detecting smaller microplastics, it struggles to accurately identify colorless and amorphous particles smaller than 100 μm (Song et al., 2015). Studies have revealed significant discrepancies in plastic classification using microscopy, with misidentification rates reaching up to 20%. Notably, transparent particles account for 70% of these errors, a finding supported by spectral analysis (Eriksen et al., 2013). This highlights the inherent limitations of optical microscopy in reliably identifying certain microplastics and underscores the need for additional analytical techniques to improve accuracy. Despite these challenges, optical microscopy remains a useful tool for the initial assessment and identification of microplastics, providing essential baseline data for more detailed, precise analyses (Table 12).
Table 12
| Microalgae used | MP size | Instrument used | Analysis | Mechanism | Process variable | Result | Reference |
|---|---|---|---|---|---|---|---|
| Scenedesmus abundans | 1. Red fluorescent polystyrene (PS) (diameter: 2 μm) 2. Red fluorescent poly methyl methacrylate (PMMA) (diameter: 2 μm) 3. Red fluorescent polylactide (PLA) (diameter: 2 μm) |
1. Flow cytometry was used to identify concentrations of microalgae, free suspended microplastics, and aggregations | 1. SEM 2. Zeta potential analysis 3. Statistical analysis |
1. Extracellular polymeric substances (EPS) 2. Hetero-aggregation between microalgae and MPs |
Plastic characteristics: 1. Plastic size 2. Plastic density 3. Hydrophobicity Algae species: 1. Cell morphology 2. EPS amount |
It is observed from this paper that: 1. PMMA had the highest overall removal efficiency among the three types of MPs (η = 98%), while PS and PLA had the maximum removal efficiencies (84% and 87%, respectively) 2. Larger percentage of MPs removed by hetero-aggregations between microalgae’s EPS and MPs |
(Cheng and Wang, 2022) |
| Freshwater algae (Microcystis panniformis and Scenedesmus sp.) Marine algae (Tetraselmis sp. and Gloeocapsa sp.) |
Fluorescent PMMA (purple and green) and fluorescent PS (blue and yellow). Green particles of PMMA and yellow PS size 106–250 mm fraction and the purple particles of PMMA and blue PS size <106 mm fraction | 1. Fluorescent microscopy 2. Scanning electron microscopy (SEM) |
1. SEM 2. Statistical analysis |
Hetero-aggregation between microalgae EPS and MP. | 1. MPs particle type, size and density 2. Production of EPS 3. Heteroaggregate potential |
The obtained result shows that: 1. The influence of MPs on microalgae growth varies depending on the size of the MPs and the characteristics of the microalgae species (cell wall and mobility) 2. There is an interrelation between the size, yield, and stability of the microalgae and the size or type of MPs when defining aggregation 3. Among all microalgae studied, Gloeocapsa sp. represents the most suitable microalgae for EPS production as well as consequent MP (various types, densities, and size fractions) aggregation |
(Cunha et al., 2019) |
| Phormidium lucidum and Oscillatoria subbrevis | Polyethylene (PE) sheets—20-μm thickness | 1. Optical microscopy 2. NMR spectroscopy 3. FTIR spectroscopy |
1. SEM analysis 2. FTIR analysis 3. CHN analysis 4. TGA-DSC analysis 5. Enzyme activity analysis 6. Tensile property analysis |
Polyethylene (PE) biodegradation by cyanobacteria or bluegreen algae Phormidium lucium and Oscillatoria subbrevis | 1. Sunlight 2. Oxygen 3. Crystallinity 4. Surface treatment 5. Additives 6. Molecular weight 7. Surfactants 8. Extracellular and intracellular enzymes |
It is clearly investigated that: 1. Polyethylene was biodegraded approximately 30% after 42 days by Phormidium lucium and Oscillatoria subbrevis 2. These species on the polyethylene surface grew quickly, which suggested that the microalgae were still obtaining energy from polyethylene 3. These cyanobacterial species could be able to decompose polyethylene rather more effectively in their natural environment, providing an alternative polyethylene waste management solution |
(Sarmah and Rout, 2018) |
| Spirulina sp. | PE and PP microplastics (0.5–1 mm2) | 1. Spectrophotometer (OPTIMA SP-300)—optical density was measured 2. Krisbow ultrasonic cleaner- extraction of phycocyanin |
1. Phycocyanin analysis 2. FTIR analysis 3. SEM 4. Statistical analysis |
Polyethylene (PE) and polypropylene (PP) microplastics were biodegraded by Spirulina sp. microalgae | 1. Algal cell surface 2. Penetrating EPS |
1. MPs were decomposed by microalgae Spirulina sp. 2. MPs also can enhance the growth of Spirulina sp. |
(Hadiyanto et al., 2021) |
| Chlamydomas reinhardtii | PS and HDPE microparticles—<400 μm | 1. Optic microscopy 2. Infrared spectroscopy 3. Scanning electron microscopy |
1. SEM analysis | Heteroaggregation between by microalgae and microplastic | 1. Polymer type 2. Cellular density |
This study reported that: 1. High-density polyethylene (HDPE) microplastics were unable to create hetero-aggregations with microalgae despite long-term interaction 2. PP microplastics are quickly aggregated by microalgae |
(Lagarde et al., 2016) |
Removal of MPs using microalgae to date.
10.1.2 Scanning electron microscopy in microplastic characterization
Using a strong electron beam to illuminate samples, scanning electron microscopy (SEM) generates secondary electrons that reveal fine morphological details. By providing high-resolution, enlarged images of plastic particles, this method allows for accurate differentiation between tiny microplastics and organic particles (Fries et al., 2013). Cooper et al., for example, used SEM to carefully analyze the morphological features of various plastic pieces from coastal habitats. Their research confirmed that plastic surfaces develop cracks, grooves, and notches due to both chemical and mechanical weathering processes on shorelines, which eventually lead to increased fragmentation (Cooper et al., 2010). However, SEM has several limitations, including the need for samples to be analyzed in a vacuum, which restricts the types of specimens that can be examined; the fact that SEM only produces two-dimensional images without height or directional data, making it challenging to fully understand the three-dimensional structure of samples; and the inability to observe liquid samples in particular (Qiu et al., 2016).
10.1.3 Atomic force microscopy in microplastic characterization
Because it can detect particles as small as a few microns and provide a realistic three-dimensional depiction of surface topography, atomic force microscopy (AFM) has become an essential technique for studying microplastics (MPs) (Nolte et al., 2017). This method does not require special sample preparation and works well in liquid environments and under normal pressure. Demir-Yilmaz et al. (2022) used AFM to investigate the biophysical characteristics of MPs, revealing their hydrophobic, uneven, and rough nanostructures. The integration of AFM with microfluidics, as demonstrated by Meister et al. (2009), allows for precise assessment of interactions between microalgae and MPs, as well as accurate determination of their hydrophobic properties. Despite its benefits, AFM has some drawbacks, such as a limited imaging range, slow imaging speed, and susceptibility to probe interference (Karami et al., 2017). Nonetheless, AFM remains a crucial method for thoroughly characterizing MPs, as it provides valuable information about their surface morphology and interactions in aquatic environments.
10.1.4 Fourier transform infrared spectroscopy in microplastic characterization
The non-invasive nature, straightforward sample preparation, and high qualitative accuracy make Fourier transform infrared spectroscopy (FT-IR) a preferred technique for analyzing material structures (He et al., 2018; Fan et al., 2021). FT-IR uses spectral analysis to reduce misidentification of MPs lacking distinct color or material features and to prevent false positives when MPs are absent. It employs infrared radiation to detect molecular vibration frequencies and specific functional groups, enabling the assessment of MPs’ weathering degree through oxygen-containing bonds. Interference from organic contaminants and water can hinder the detection of oxidation functional groups and particles smaller than 20 µm (Lin et al., 2022). Principal component analysis (PCA) combined with FT-IR was used by Wander et al. (2020) to enhance MP recognition accuracy by reducing data complexity and visually representing particle similarities. FT-IR not only identifies sample composition but also facilitates quantitative analysis of MP quantities (Renner et al., 2017). The combination of statistical techniques with FT-IR significantly boosts the accuracy and effectiveness of MP detection and characterization, highlighting the method’s strong analytical capabilities in environmental research studies.
10.1.5 Raman spectrometry in microplastic characterization
Using the fascinating phenomena of inelastic light scattering, Raman spectrometry—a sophisticated vibrational spectroscopy technique—provides precise vibrational spectra. The accurate analysis of small particles is a common application for this powerful method (Araujo et al., 2018). Raman analysis supplies essential information on the composition of the samples, in addition to identifying these particles. The sensitivity of the Raman spectrometer is impressive; it can detect particles as small as 1 μm (Becucci et al., 2021). Because it is non-contact, it preserves the structural integrity of materials, increasing sensitivity and allowing for further analysis (Cole et al., 2013). Compared to Fourier transform infrared (FT-IR) spectroscopy, Raman spectroscopy is better at detecting matrix polymers, coatings, and both organic and inorganic additives. Identifying additives and coatings can be challenging due to increased scattering from these materials, which may mask the Raman signal from the matrix. The main hurdle for Raman detection is fluorescence in samples. Conversely, infrared spectroscopy, especially for fluorescent samples, is more effective at identifying coatings and additives. Despite its limitations, the effectiveness of Raman spectroscopy in characterizing materials depends heavily on the specific requirements of the samples analyzed. This technique, with its non-destructive and highly sensitive nature, remains an invaluable tool in the detailed study of complex materials.
10.1.6 Thermal cleavage in microplastic characterization
A novel spectroscopic method for detecting microplastics (MPs), thermal cleavage analysis, utilizes samples’ thermal stability to identify changes in their physicochemical properties (Vilakati et al., 2021). Polyethylene (PE) and polypropylene (PP) can be differentiated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) (Majewsky et al., 2016). Combining TGA with solid-phase extraction (SPE) and thermal desorption gas chromatography–mass spectrometry (TDS-GC–MS) enhances the detection capabilities of both techniques, revealing detailed features with high resolution (Dumichen et al., 2015). This technology offers a relatively simple and fast way to analyze data by directly identifying samples and mixed polymers. However, the inherently destructive nature of this method limits its application to chemical analysis alone and prevents gathering essential data on the size, shape, and quantity of microplastics (Liu et al., 2023).
11 Advanced extraction techniques for microplastic analysis
11.1 Cloud-point extraction
Cloud-point extraction (CPE) is an innovative and effective method for separating microplastics (MPs) from environmental samples. Zhou et al. (2019) examined this technique, focusing on isolating polystyrene (PS) and poly(methyl methacrylate) (PMMA) particles from water samples. Using Triton X-45, a non-ionic surfactant, CPE relies on temperature-induced micelle formation to trap MPs. This process is based on the principle that when the surfactant concentration exceeds the critical micelle concentration (CMC), a cloudy phase appears. This phase, called the “cloud point,” occurs when surfactant molecules gather around MP particles, effectively capturing them inside micellar structures (Li et al., 2022; Zhou et al., 2023). CPE’s method involves forming these micelles, which enclose MPs and facilitate their removal from the sample matrix. This temperature-driven phase separation works especially well in liquid samples, making it a powerful technique for extracting MPs from various environmental contexts. Researchers often pair this method with pyrolysis-gas chromatography–mass spectrometry (Py-GC–MS) to accurately analyze the chemical makeup of the plastic particles.
The advantages of CPE are numerous. It performs well even when complex sample matrices interfere, ensuring high sensitivity and specificity for MP detection. Moreover, the process is user-friendly and suitable for routine laboratory work. CPE’s capacity to selectively extract MPs from complex samples highlights its potential for evaluating MP pollution across different ecosystems. It is also cost-effective and environmentally friendly, aligning with sustainable scientific practices. Its effectiveness in isolating MPs from real-world environmental samples underscores its value. The robustness of this technique, along with its high sensitivity, makes it a valuable addition to MP analysis methods. By enabling precise and consistent MP extraction, CPE significantly enhances our understanding of MP distribution and impact in the environment. Overall, cloud-point extraction stands out as a sophisticated and efficient technique for MP analysis. Its ability to handle complex matrices, combined with its affordability and low environmental impact, makes it a vital tool in ongoing efforts to monitor and reduce MP pollution. As research progresses, methods like CPE will be essential in providing the detailed, accurate data needed to address the widespread presence of microplastics issue of microplastic contamination (Zhou et al., 2019; Li et al., 2022; Zhou et al., 2023).
11.2 Automated pressurized liquid extraction for microplastics
Automated Pressurized Liquid Extraction (APLE), also known as Accelerated Solvent Extraction (ASE), is an advanced technique for extracting microplastics (MPs) from environmental samples. As described by Dierkes et al., APLE enables the extraction and subsequent analysis of various MPs, including polyethylene (PE), polystyrene (PS), and polypropylene (PP), from different environmental matrices. This method combines MP enrichment and matrix removal into a single, fully automated process, providing significant advantages in efficiency and accuracy. The APLE process is notable for its reproducibility, automation, high extraction yields, and versatility across various sample types. These features make it a valuable tool in MP research. One key benefit of APLE is its eco-friendly nature, reflected in reduced solvent use, aligning with sustainable laboratory practices. This method efficiently extracts MPs while minimizing environmental impact, which is especially important given the growing emphasis on sustainable scientific methods. Additionally, APLE’s automated operation reduces human error and improves result consistency. Its parameters can be adjusted to optimize MP extraction for specific samples, offering both versatility and standardization in MP analysis. Kamp et al. (2023) note that adjustable parameters help researchers fine-tune the extraction process, ensuring maximum recovery of MPs from various environmental sources. Although APLE offers many advantages, it also has limitations, such as the high initial cost of equipment and the need for specialized training to operate it effectively. Despite these challenges, APLE has been standardized, establishing it as a reliable and useful tool for studying MP pollution. Its automation and efficiency streamline the extraction process, making it suitable for routine use in laboratories focused on environmental monitoring and pollution assessment. In summary, APLE is a sophisticated, efficient, and eco-friendly method for extracting MPs from environmental samples. Its automation, high extraction yields, and adaptability across different sample types make it a valuable resource in environmental science. As research on MP pollution expands, methods like APLE will play a crucial role in advancing our understanding and efforts to mitigate this widespread environmental issue.
Recent developments have enhanced detection sensitivity and throughput:
-
Machine learning and hyperspectral imaging: Enhance accuracy in distinguishing MPs from organic debris. The incorporation of artificial intelligence (AI) has revolutionized microplastic detection. AI algorithms, particularly machine learning and deep learning techniques, have been applied to image processing, Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and hyperspectral imaging (HSI). These methods have significantly improved the efficiency and accuracy of microplastic identification, enabling real-time monitoring and pollution hotspot detection. Innovative solutions, such as the fluorescence imaging microplastic analysis platform (FIMAP), have been developed. FIMAP uses Nile Red staining combined with multispectral imaging to detect and classify microplastics with high accuracy, effectively excluding natural organic matter and reducing false positives. Machine learning (ML) and hyperspectral imaging (HSI) are increasingly used for microplastic detection, addressing the urgent need for efficient environmental monitoring. These technologies enhance the identification and quantification of different plastic types, offering rapid, non-destructive methods that surpass traditional techniques. The following sections highlight key contributions of these technologies to microplastic detection. HSI captures the reflectance spectra of materials, enabling differentiation of MPs based on spectral signatures (Tamin et al., 2023). It effectively identifies various plastic types, including polyethylene and polypropylene, through advanced preprocessing and feature extraction (Xu et al., 2023). However, HSIs struggle with black plastics due to their light absorption properties, which limit detection capabilities (Tamin et al., 2023). ML algorithms, such as support vector machines (SVM) and convolutional neural networks (CNN), are used to classify and quantify MPs from hyperspectral data (Xu et al., 2023). Studies demonstrate high accuracy rates (over 94%) in identifying MPs, showcasing ML’s potential to improve detection efficiency (Gong et al., 2023). Combining ML with HSI allows for better feature extraction and classification, resulting in improved detection outcomes (Daranagama & Liyanage, 2024).
-
Laser-induced breakdown spectroscopy (LIBS): Enables real-time, in situ monitoring. Advances in quantum cascade laser direct infrared (QCL-LDIR) imaging provide high-throughput analysis of microplastics. This advanced technique offers rapid and accurate characterization, overcoming challenges related to particle size and spectral range. LIBS has become a promising method for detecting and analyzing microplastics in various environments, including drinking water and air samples. Often combined with other techniques like Raman spectroscopy, this approach allows for quick identification of microplastic types and their contaminants, improving environmental monitoring. LIBS has successfully identified microplastics in bottled drinking water, detecting polymers such as polyethylene (PE), polyethylene terephthalate (PET), polystyrene (PS), polypropylene (PP), and polyvinyl chloride (PVC). The study found PE to be the most common polymer, present in 35.71% of samples, highlighting the widespread contamination potential in consumer products. In atmospheric research, LIBS has been combined with machine learning to classify microplastics, achieving high accuracy in identifying different types (Chen et al., 2025). This method not only improves detection efficiency but also addresses the environmental risks of airborne microplastics, which can impact human health and ecosystems (Chen et al., 2025). The performance of LIBS has been compared to traditional techniques like ATR-FTIR and SEM-EDS, showing comparable or better results in speed and sample preparation (Vasudeva et al., 2024). Combining LIBS with Raman spectroscopy has also proven effective for detecting microplastics in human tissues, demonstrating its versatility across various sample types.
-
Automated pressurized liquid extraction (APLE): APLE is emerging as an important method for detecting microplastics (MPs) in various environmental samples. This technique improves the efficiency and accuracy of measuring microplastics, especially in complex samples like sediments and sewage sludge. Combining APLE with advanced analytical methods, such as pyrolysis-gas chromatography–mass spectrometry (GC–MS), enables the detection of low levels of common synthetic polymers, with limits of quantification as low as 0.007 mg/g. APLE involves a pre-extraction step using methanol, followed by pressurized extraction with tetrahydrofuran, which efficiently separates MPs from solid matrices. The method has shown recoveries above 80% for solid samples, although variability in MP distribution can lead to statistical uncertainties. APLE has been successfully used to analyze sediments, suspended matter, and sewage sludge, revealing significant amounts of polyethylene and polypropylene. A specialized extraction device has been developed to improve MP separation from marine sediments, using air pumps and filtration systems (Wang et al., 2024). While APLE offers a strong approach for microplastic detection, challenges remain in standardizing methods across different environmental settings, which can impact the comparability and reproducibility of results (Oh, 2022).
-
AI-powered microfluidics and nanosensors are emerging as key technologies for detecting microplastics, tackling challenges related to their small size and diverse properties. These innovative methods utilize artificial intelligence (AI) to improve the sensitivity and accuracy of detection, enabling the identification and analysis of microplastics in different environments. The combination of AI with plasmonic probes, such as estrogen receptor-functionalized surfaces, has shown 90.3% accuracy in identifying various types of microplastics based on size and composition (Seggio et al., 2024). Using liquid–solid triboelectric nanogenerators (LS-TENG) combined with deep learning allows for quantitative microplastic detection, achieving high recognition rates through voltage signal analysis. Microfluidic systems with surface nanodroplets can effectively isolate and analyze microplastics as small as 10 μm. This technique enables both physical and chemical characterization through optical and Raman spectroscopy (Faramarzi et al., 2024). AI-driven robotics are being explored for automated collection and analysis of microplastics, streamlining the detection process and improving data accuracy (Guo et al., 2024).
12 Mitigation of microplastic pollution in the aquatic environment
The treatment and extraction of MPs from various aquatic environments require careful consideration of the sample source (water layer, sediment, or organism) and the characteristics of the MPs, including size, density, and composition. Contreras-Llin and Diaz-Cruz (2024) noted that optimizing reactive barriers can enhance their ability to retain microplastics (MPs) and reduce their environmental impact. Reactive barriers represent a promising approach for managed aquifer recharge (MAR) systems to tackle MPs in wastewater. Their effectiveness depends on the barrier materials and MAR system conditions. Different types of reactive barriers exist. Among them, compost- and woodchip-based materials have shown effectiveness in removing various contaminants. The performance of zero-valent iron/aluminum mixtures shows promise for MP removal from aquatic environments. Red mud–loess mixtures are another barrier used for removing MPs from wastewater, though their MP removal efficiency is less well documented (Lu et al., 2022; Valhondo et al., 2020). Sarkar et al. (2021b) proposed pulse clarification and sand filtration methods to remove MPs from water, achieving 63% and 85% removal efficiencies of microplastics from raw water, respectively. The study also observed higher microplastic abundance on the sand filter bed due to the screening effect (Table 13).
Table 13
| Source | Potential mitigation |
|---|---|
| Microplastics in additives | Eliminating them from the products. Substitute with benign alternatives |
| Mismanaged preproduction pellets | Implement measures to control pellet handling. The operation aims to clean and remove any debris or unwanted items thoroughly |
| Industrial erosive | Enhance the ability to confine and retrieve, and mandate substitute options |
| Laundromat exhaust | Improved separation |
| Agriculture-degraded film, pots, and Pipes |
Advanced recovery, biodegradable plastics |
| Tire dust | Technological advances, road surface |
| Littering of small plastic items (cigarette filters, torn corners of packaging, small film wrappers, etc.) |
The implementation of penalties for littering, educating consumers, and implementing Extended Producer Responsibility (EPR) in product design |
| Domestic laundry, wastewater effluent | Utilize top-load machines for washing, employ wastewater containment systems, use single-filter woven fabrics, and apply textile coatings |
| Fragmentation caused by automobiles traversing uncollected garbage. | Improved waste management |
| UV and chemically degraded terrestrial plastic waste |
Improved waste management |
| Sewage effluent (synthetic fibers) | Laundry filtration, textile industry Innovation |
| Combined sewage overflow (large items) | Infrastructure improvement |
| The process of mechanically shredding roadside debris is carried out as part of the routine vegetation cutting (mostly grass) | Enhanced legislation and enforcement of laws; optimization of waste products |
Potential mitigation strategies of microplastics with source (adapted from Park and Park, 2021).
Addressing the various sources and entry points of microplastic pollution into aquatic habitats requires a multipronged approach. Improving waste management systems, developing alternatives to plastic, and enforcing laws to reduce plastic use and disposal are all effective strategies (Andrady, 2011; Horton et al., 2017; Nizzetto et al., 2016). A modern perspective highlights the importance of advanced monitoring methods, public awareness campaigns, and international cooperation in tackling this global issue. The primary aim of preventive measures is to stop debris from being created or prevent it from entering the ocean. These efforts include reducing source generation, reusing, recycling, and composting waste, converting waste into energy, keeping debris out of water bodies at points of entry, and implementing various land-based waste management practices (Ogunola et al., 2018; Bergmann et al., 2015). Additional strategies involve removing microplastics from consumer products, promoting biodegradable alternatives such as polyhydroxyalkanoates, and advancing recycling technologies (Wu et al., 2017; Calero et al., 2021).
12.1 Ecolabeling
Ecolabeling is a voluntary, globally implemented system for certifying and labeling a product’s or service’s environmental performance. Attributes scientifically shown to be environmentally beneficial are marked with an ecolabel (https://globalecolabelling.net). Ecolabels act as visual tools for businesses to communicate the environmentally friendly features of their products to consumers, thereby aiding environmental management (Thøgersen et al., 2010). The Agenda 21 report significantly enhanced environmental labeling programs to promote sustainable consumer behavior. It is recommended that labels be used to support cleaner production across multiple market sectors (UNCED, 1992). Ecolabeling also plays a vital role in fighting marine plastic pollution by encouraging environmentally friendly products and informing consumers about their environmental impact. This labeling system, increasingly adopted by governments and businesses, aims to reduce harmful environmental effects, promote responsible consumer choices, and better resource management. Examples like the Nordic Swan Ecolabel demonstrate its effectiveness in advancing sustainability and cutting waste (Ogunola et al., 2018).
12.2 Recycling
Gathering and processing discarded items and materials to create reusable materials. Recycling plays a crucial role in reducing the environmental impact of plastic waste worldwide, although current rates remain low. Thermoplastics like PET, PE, and PP have excellent mechanical recycling capabilities. Mechanical recycling of solid plastic waste is an eco-friendly and resource-efficient solution to indiscriminate disposal (Lazarevic et al., 2010; Wäger and Hischier, 2015). There are two main methods for recycling segregated MPs. First, the separated MPs are processed further. Pyrolysis produces hydrogen, value-added liquid fuels, carbon nanomaterials, and composite catalysts. Additionally, isolated MPs are used directly to produce plastic products, such as foam and flame-retardant materials. Governments have adopted strategies like color-coded sorting systems to support recycling efforts. Despite challenges such as high operational costs, recycling offers potential environmental and economic benefits, leading to increased public awareness and participation, especially in developed countries (Ogunola et al., 2018).
12.3 Bans and imposed fees
Governments worldwide are introducing bans and fees to cut down on plastic waste, focusing on lightweight bags and microplastics. Over 30 countries have banned plastic bags, with some also limiting microplastic use. Charging fees on plastic bags has been effective in lowering their use, with notable drops in places like Wales, Ireland, and Scotland. These actions aim to change consumer habits and reduce plastic pollution, although the success depends on how strict the regulations are and how well they are enforced (Ogunola et al., 2018).
12.4 Action plans and regulatory agreements
According to Ogunola et al. (2018), international agreements such as the 1992 Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR Convention) and the 1992 Helsinki or HELCOM Convention for the Protection of the Baltic Sea are designed to address plastic pollution from both land-based and marine sources. These agreements involve collective actions and regional plans to reduce marine litter, focusing on education, outreach, and regulatory measures. Despite challenges in implementation and enforcement, these efforts are an essential step toward reducing the environmental impacts of plastic waste on marine ecosystems.
12.5 Removing/cleaning-up strategy
Ogunola et al. (2018) explained that beaches function as complex socio-ecological systems that are vulnerable to plastic pollution from various sources. Community-based beach clean-ups involving volunteers have proven effective in removing plastic debris and reducing threats to marine ecosystems. These efforts, organized globally, show a shared commitment to keeping beaches clean and fighting plastic pollution, though they face challenges such as high costs and the need for consistent, coordinated actions. According to Nohara et al. (2024), several countries have started designing laws to regulate the use and disposal of single-use plastics. The European Union, for example, issued the Single-Use Plastic Directive (EU) 2019/904 to lessen the environmental impact of certain products.
12.6 Behavioral change strategy
Educational outreach and public awareness campaigns are essential for encouraging behavioral change and decreasing plastic pollution. Specific efforts, like school programs and community workshops, have effectively shifted perceptions and actions regarding plastic use and waste management, emphasizing the value of grassroots involvement and cultural context in environmental efforts. Social media and engaging children and youth are powerful methods for raising awareness and fostering a sense of responsibility for marine conservation (Ogunola et al., 2018).
12.7 Biotechnology
Biotechnology offers a promising way to combat plastic pollution by developing biodegradable bioplastics. These eco-friendly alternatives, produced from renewable resources and microbial processes, have the potential to reduce environmental damage caused by traditional plastics. However, challenges such as high production costs, limited scalability, and uncertainties about their natural degradation still pose significant barriers to widespread adoption. Further research is needed to improve manufacturing methods and assess long-term environmental impacts (Ogunola et al., 2018). According to Park and Park (2021), microplastic removal can be categorized into four technologies: wastewater treatment plants (WWTP), physical removal technologies, chemical removal technologies, and biological removal technologies, as shown in Table 9. Among these, physical and chemical methods are more effective, removing approximately 85%–99% of microplastics. Continued research is crucial to developing better barriers for eliminating microplastics from aquatic environments. In summary, reducing MP pollution in water requires multiple strategies, including optimizing reactive barriers, improving recycling efforts, implementing bans and fees, and promoting ecolabeling. Preventive measures, action plans, clean-up initiatives, and biotechnology are also essential, but more research is necessary to enhance their effectiveness.
13 Current trends of MP degradation
Microplastic (MP) pollution, which results from the breakdown of larger plastics, poses serious threats to ecosystems due to its increased bioavailability and longevity (Chamas et al., 2020). Removing MPs from aquatic environments is challenging because of their inert nature and small size, allowing them to bypass traditional water filtration systems (Tofa et al., 2019b). Although wastewater treatment plants (WWTPs) can eliminate most MPs through primary and secondary treatments, smaller particles often escape these processes, contaminating municipal waters and rivers or remaining in biosolids. These MPs remain stable under standard physical treatments like coagulation, sedimentation, screening, and flotation, which call for improved removal strategies (Lin et al., 2020). Advanced treatment technologies have shown promise, such as membrane bioreactors (MBRs), which Talvitie et al. (2017) demonstrated could remove 99.9% of MPs larger than 20 µm from secondary effluents. Foam and air flotation techniques are also effective for extracting microplastics from sediments. Dissolved air flotation, combined with coagulation, can enhance sewage filtration (Mohammadi et al., 2020). Sand filtration has emerged as a quick, feasible technology for removing microplastics, although it can be costly due to the multiple application steps and is particularly effective for particles larger than 200 μm (Dayal et al., 2024). Combining various treatment strategies can improve microplastic removal efficiency. However, the persistence of these particles in the environment continues to pose ecological risks. Physicochemical methods, such as ultraviolet (UV) irradiation, can break down MPs, with reactive oxygen species (ROS) playing a key role in causing morphological changes and chemical chain scission (Wang et al., 2013; Guo and Wang, 2019; Zhu et al., 2019). Despite this, UV treatments are often slow due to low energy intensity and may not fully prevent the formation of toxic intermediates and volatile organic compounds (VOCs), which can harm air quality and water safety (La Nasa et al., 2020). Chemical recycling has gained attention as a promising approach for reducing MP pollution by converting MPs into useful products like chemicals or fuels (Miao et al., 2020). This process offers a partial solution by transforming MPs into reusable forms, thus lowering environmental impact. However, degradation can generate toxic by-products, and the mechanisms behind these reactions are not yet well understood. MP degradation occurs via two main mechanisms: physicochemical (abiotic) and biodegradation (biotic). Abiotic degradation involves physical factors such as sunlight, temperature variations, and mechanical forces, which weaken molecular bonds and break down plastics over time (Varlamov et al., 2018; Li et al., 2016; Gobbi et al., 2017). Biodegradation depends on microbial enzymes like lipases and esterases to enzymatically cleave polymer chains into simpler compounds. This biological process is influenced by microbial diversity, environmental conditions, and the chemical makeup of the plastic substrate (Li et al., 2016).
14 Methods of MP removal
The widespread presence of microplastics (MPs) in aquatic environments has led to the development of various clean-up strategies. To reduce the environmental impact of microplastics (MPs), substantial research focuses on their removal from wastewater. Current approaches include electrocoagulation, membrane separation, activated sludge, coagulation precipitation, photocatalytic degradation, and biodegradation with green algae (Xu et al., 2020; Egea-Corbacho et al., 2023). These methods encompass physical, biological, and chemical techniques (Table 14). Physical methods often transfer MPs to sludge, increasing the burden on sludge treatment. Biological methods depend on specific environmental conditions and have limited applicability. Both physical adsorption and biological methods face challenges, prompting the need to explore alternative options. Among these, chemical methods have shown promise for removing microplastics from water, focusing mainly on degrading, adsorbing, and coagulating MPs through advanced chemical processes and materials.
Table 14
| Treatment type | Removal technologies | MP removal rate | Reference |
|---|---|---|---|
| Wastewater treatment plant (WWTP) | Primary: screening, grit removal, primary sedimentation | 76.5%–82% | Park and Park, 2021 |
| Secondary: membrane bioreactor, biofiltration, A2O process | 72.1%–99.3% | ||
| Tertiary: membrane bioreactor, denitrification, and UF | 41.6%–95% | ||
| Physical removal technology | Membrane bioreactor (MF) | 75%–79% | Bayo et al., 2020 |
| Dynamic membrane (UF) | 94% | Pizzichetti et al., 2021 | |
| Membrane bioreactor (UF) | 98%–99% | Lares et al., 2018 | |
| Glass membrane | 90% | Li et al., 2021 | |
| Rapid sand filtration (RSF) | Varies | Hu et al., 2019 | |
| Granular activated carbon filtration (GAC) | Varies | Kim and Park, 2021 | |
| RO membrane | 85%–90% | Schuhen et al., 2019 | |
| MF membrane | 98% | Yahyanezhad et al., 2021 | |
| Disk filter | 89%–90% | Kim and Park, 2021 | |
| Chemical removal technology | Alum and PAM coagulant | 80-88% | Lapointe et al., 2020; Zhou et al., 2024 |
| Electrocoagulation | 99% | Perren et al., 2018 | |
| Agglomeration | 92.8% | Zhou et al., 2024 | |
| Fe- and Al-salt coagulation with plant-derived tannic acid | 95% | Park et al., 2021 | |
| AlCl3 coagulation with an without PAM |
46% | Ma et al., 2019 | |
| Ozonation | Varies | Zahmatkesh et al., 2023 | |
| FeCl3 with PAM coagulation | 99% | Kim and Park, 2021 | |
| Biological removal technology | Activated sludge treatment, aerobic and anaerobic digestion, lagoons, and septic tanks | Approximately 47% | Park and Park, 2021 |
Various treatment technologies for microplastic removal (adapted from Park and Park, 2021).
14.1 Adsorption techniques
Adsorption uses materials with high surface area and affinity for microplastics to capture and remove them from water. Activated carbon, biochar, and innovative nanomaterials like graphene oxide have proven effective in adsorbing MPs from water. These materials work through physical adsorption, where MPs stick to the surface, and chemisorption, which involves stronger chemical bonds. Recent research emphasizes the potential of functionalized adsorbents, designed to improve their interaction with specific types of MPs (Mou et al., 2020; Li et al., 2021).
14.2 Coagulation–flocculation
The coagulation–flocculation method is widely used in water treatment and involves adding coagulants to gather microplastic particles into larger flocs, which can then be easily removed through sedimentation or filtration. Coagulants such as aluminum sulfate (alum), ferric chloride, and natural polymers like chitosan have been studied for their effectiveness in aggregating MPs. This approach is favored because of its simplicity and efficiency in treating large volumes of water (Rajala et al., 2018; Ding et al., 2019).
14.3 Chemical reduction and hydrolysis
Chemical reduction and hydrolysis are techniques that chemically change the structure of microplastics to help remove them. Reducing agents like sodium borohydride (NaBH4) can break down specific types of MPs, while hydrolysis involves breaking polymer chains by adding water or acids. These methods can convert MPs into smaller molecules or monomers, making them easier to remove or degrade further through biological processes (Luo et al., 2020).
14.4 Membrane separation processes
Membrane separation processes are crucial for effectively removing microplastics from water sources by selectively allowing passage based on size and properties. Thin-film semipermeable membranes, vital for these processes, ensure optimal performance through traits like chemical inertness, insolubility in water, and resistance to degradation, which are essential for durability and efficiency over long periods. Reverse osmosis (RO) and nanofiltration (NF) membranes are prominent in microplastic removal, with RO applying pressure to push water through a semipermeable membrane to trap microplastics (Zhang and Gao, 2022). NF performs well in lower-concentration environments, blocking larger microplastics through size exclusion and electrostatic interactions (Zhang and Gao, 2022). Ultrafiltration (UF) membranes, with larger pores than NF, concentrate microplastics from dilute solutions and work together with other treatments. Donnan dialysis and electrodialysis serve specialized roles; the former separates microplastics by ion exchange, while the latter uses electric fields to facilitate their removal via ion transport (Zhang and Gao, 2022). Advances in membrane materials, like nanocomposites and surface modifications, improve selectivity and efficiency, which are key for sustained operation in tough conditions. Combining these technologies with biological and chemical methods creates comprehensive solutions to fight microplastic pollution in aquatic environments, requiring ongoing research for optimization and wider adoption.
14.5 Emerging chemical methods
New chemical methods are continually developed to improve the efficiency and feasibility of microplastic removal—for example, the use of green solvents and catalysts that reduce environmental impact while effectively breaking down MPs is an active research area. Additionally, combining chemical methods with other treatment technologies, like photocatalysis and biological degradation, shows promising potential for comprehensive MP clean-up (Chen et al., 2022; Zhang et al., 2023).
15 Methods of microplastic degradation
Advanced oxidation processes (AOPs) and biological degradation are two key strategies currently used for breaking down microplastics (MPs). These methods sever the chemical bonds in polymer MPs, turning them into smaller molecules that can either be converted into useful products or fully mineralized into CO2 and H2O (Silva et al., 2018). The uneven breaking of polymer chains can happen at any monomer unit, leading to MPs decomposing into organic or inorganic substances (Asandei et al., 2006). The degradation process and final products differ depending on the type of polymer and are heavily affected by environmental factors. MP degradation mainly occurs through physicochemical (abiotic) and biodegradation (biotic) mechanisms. Over time, abiotic processes weaken molecular bonds and fragment plastics via physical means such as photodegradation, temperature changes, and mechanical forces (Varlamov et al., 2018; Li et al., 2016; Gobbi et al., 2017). Meanwhile, enzymes like lipases and esterases are crucial for biotic degradation, as they enzymatically break down polymer chains (Li et al., 2016). Understanding these mechanisms is essential to develop effective solutions to reduce MP pollution.
15.1 Advanced oxidation processes
Advanced oxidation processes (AOPs) have recently become an effective method for degrading persistent organic contaminants in water by generating reactive oxygen species (ROS), such as hydroxyl radicals (•OH, E0 = 2.7 V vs. NHE) in Fenton reactions and sulfate radicals (SO4•-, E0 = 3.1 V vs. NHE) (Liu et al., 2020). Studies show that sulfate radical-based AOPs (SR-AOPs) are highly effective at degrading stubborn organic pollutants in complex water environments (Wang et al., 2017a), while Fenton treatment efficiently converts plastic waste into useful chemicals (Wang et al., 2017b). SR-AOPs also demonstrate superior catalytic breakdown of cosmetic microplastics, mainly polyethylene. The high redox potentials of these species boost the oxidation process, causing chain scission, formation of valuable products, or complete mineralization of microplastics.
15.2 Photochemical oxidation process
The breakdown of polymers largely depends on photochemical oxidation, particularly through photodegradation processes (Gewert et al., 2015; Liu et al., 2019). The formation of environmentally reactive free radicals, oxygen addition, hydrogen abstraction, and the breaking or cross-linking of chemical chains in microplastics (MPs) can all result from prolonged exposure to sunlight, especially UV light (Zhu et al., 2019). This process also causes significant morphological changes, such as flakes and cracks (Cai et al., 2018), with UV light being the main driving factor. Natural photodegradation of MPs is unpredictable, making it crucial to understand how MPs’ aging properties relate to the degree of aging. Under lab-accelerated photodegradation conditions, Song et al. (2017) demonstrated that MPs’ surfaces develop oxygen-containing groups and fissures. However, limited information is available on how reactive oxygen species (ROS) influence the aging process of MPs due to their low concentration in aquatic environments. Photodegradation in nature proceeds very slowly, especially for polystyrene microplastics (PS-MPs), which are common in aquatic settings. Zhu et al. (2020) studied the aging of PS-MPs in aquatic environments under simulated sunlight (295 to 2,500 nm) for up to 150 days. They found ROS such as singlet oxygen (¹O2), hydroxyl radicals (•OH), hydrogen peroxide (H2O2), and superoxide radicals (O2•-) in the PS-MPs suspension caused by light exposure, shedding light on the mechanisms of ROS formation and MPs photodegradation under these conditions. The study did not evaluate the extent of MPs’ photoaging or the types of intermediate products created during photochemical reactions. Additionally, long-term simulated light exposure presents challenges like high energy use and potential light pollution. While traditional Fenton processes are ineffective for degrading polystyrene materials, Feng et al. (2011) investigated breaking down polystyrene microspheres using a photo-assisted Fenton process. Most other research have focused on large plastic films rather than MPs or NPs (Zan et al., 2006). Due to the scavenging effect of H2O2 radicals, the Fenton reaction is limited by the oxidant, and its practical use is hindered by secondary pollution from iron ion leaching and substantial sludge production. Recently, Kang et al. studied the breakdown of cosmetic MPs through catalytic activation of peroxymonosulfate, generating active radicals with strong carbon nanosprings. Although this advanced oxidation process (AOP) did not directly turn cosmetic MPs into value-added products, microorganisms can metabolize non-toxic organic by-products from MPs breakdown into useful substances like proteins, biofuels, and sugars. This bioconversion process fosters an environmentally friendly and sustainable carbon cycle. Building on these findings, the next section will explore the photocatalytic degradation of MPs using catalysts.
15.3 Photocatalytic oxidation process
Photocatalysis is recognized as an advanced oxidation process (AOP) for removing contaminants, including microplastics (Klavarioti et al., 2009). This mature green technology utilizes the infinite and free energy of solar radiation, showing promising potential as an eco-friendly and cost-effective treatment option. Photocatalytic breakdown of organic pollutants mainly depends on semiconductor materials. When the energy of the absorbed photon exceeds the band gap energy of the semiconductor (E ≥ Eg), electrons in the valence band are excited to the conduction band, creating positive holes in the valence band (Nakata and Fujishima, 2012). These electron–hole pairs react with OH-, O2, or H2O to produce highly reactive oxygen species (ROS), initiating the degradation of plastics and organic contaminants (Tofa et al., 2019a).
According to earlier research, ROS is effective at breaking down large plastic particles and films (Jiang et al., 2020). A comprehensive overview of plastic photodegradation mechanisms was provided by Zhang et al. (2020a), who also included detailed illustrations of the process. Despite these advancements, most plastic films and particles only partially degrade when exposed to UV light, indicating that the ROS generated by visible light is insufficient for complete chain cleavage and subsequent oxidation events. This limitation highlights the need for further research into the use of photocatalysis to break down microplastics. Using semiconductor materials as photocatalysts, many studies have explored the photodegradation of microplastics in aquatic environments (Table 2). Titanium dioxide (TiO2)-based nanomaterials have been widely used as model photocatalysts because of their high oxidation potential for organic pollutants (Yuan et al., 2017). Additionally, zinc oxide (ZnO)-based materials have also been employed for degrading microplastics and plastics due to their strong catalytic activity and high redox potential. Despite these promising results, most photocatalytic systems have only achieved partial degradation and have not extensively analyzed the final degradation products using techniques like liquid chromatography–mass spectrometry (LC–MS). Moreover, these processes may release volatile organic compounds (VOCs), creating additional environmental concerns. A significant challenge with current photocatalytic systems is the difficulty of recycling suspended catalysts in water, which can lead to secondary pollution. This issue could be addressed by immobilizing the catalyst on conductive substrates, improving the practicality and sustainability of photocatalytic microplastic breakdown. By deepening our understanding of photocatalytic oxidation processes and overcoming these challenges, we can develop more efficient and environmentally friendly methods to combat microplastic pollution.
15.4 Electrochemical oxidation process
The two main types of electrochemical oxidation are anodic oxidation (AO) and indirect cathode oxidation. AO is the more common form. In AO, organic pollutants are either directly oxidized on the anode surface through charge transfer or indirectly through hydroxyl radicals (•OH) and other reagents such as hydrogen peroxide (H2O2), ozone (O3), active chlorine species, and peroxymonosulfate in aqueous solutions. In contrast, indirect cathode oxidation is mainly linked to electro-Fenton (EF) technology, which produces •OH radicals and other reactive oxygen species from H2O2 decomposition catalyzed by Fe²+ ions via the Fenton reaction. These free radicals play a key role in the oxidation of organic pollutants, making EF technology a promising approach for treating stubborn organic compounds. Kang et al. (2019) demonstrated the effectiveness of sulfate radical-based advanced oxidation processes (SR-AOPs) in breaking down microplastics (MPs) mainly made of polyethylene. This was achieved through catalytically activating peroxymonosulfate to generate active radicals, and toxicity tests showed that the resulting organic products were harmless to aquatic microorganisms. The intermediate products, however, were not identified. EF-like processes, enhanced by high-efficiency heterogeneous catalysts, have gained attention for producing strong oxidizing •OH radicals to eliminate organic contaminants because of their controllability, ease of operation, and low risk of secondary pollution (Chen et al., 2020). The type of cathode material is crucial, as the overall degradation efficiency in EF-like systems depends on cathodic processes. Therefore, choosing highly effective catalyst materials is essential for optimal results. Although electrochemical methods have great potential for MP degradation, their use in aquatic environments remains limited. A notable study from the previous year explored the breakdown of PVC MPs using an EF-like system with a TiO2/graphite cathode (Miao et al., 2020). This system showed significant activity in decomposing PVC via •OH oxidation and cathodic reduction dechlorination, producing desirable and useful products. It is still uncertain whether this system is effective for other types of MPs. Nevertheless, this research offers a promising direction for developing efficient electrochemical oxidation techniques for MP breakdown into valuable substances. The field of electrochemical oxidation for MP degradation is continually evolving and holds great promise. Future research should aim to understand the intermediate products, improve catalyst materials, and expand the applicability of these methods to different types of MPs. By advancing these techniques, we can create sustainable solutions to reduce microplastic pollution, protect aquatic ecosystems, and promote environmental health.
15.5 Biodegradation
Researchers have discovered that although microplastics (MPs) remain stable in natural environments for long periods, specific microorganisms can effectively decompose them (Krueger et al., 2015). Microorganisms, thanks to their remarkable adaptability, can survive nearly everywhere and break down various organic pollutants, including MPs (Brooks et al., 2011). The process of MP degradation (Zurier and Goddard, 2021) involves MPs serving as substrates for biofilm growth. As the biofilm develops, it causes pitting and cracking, which weakens the structure of the MPs. Bacterial enzymes then target these weakened MP fragments, attacking them both specifically and non-specifically during bio-deterioration, which is the rate-limiting step. Once the weight of MP fragments drops below 600 kDa, bacteria in the biofilm can easily ingest them during assimilation. These fragments are further broken down by enzymes into smaller molecules such as CO2, N2, CH4, H2O, and H2S, which microorganisms use as energy sources, eventually releasing back into the atmosphere and completing the transformation of small molecules into useful products. To enhance the biodegradation of MPs and make it a practical solution for addressing MP pollution, it is essential to overcome bottlenecks at each step of the process—for example, biodegradation by a single bacterial culture often produces toxic products that inhibit microbial growth. Additionally, secreted enzymes may not be well-adapted to the plastic substrate, complicating MP degradation. The performance of MP biodegradation can be improved by utilizing specific microbes, offering an eco-friendly and promising approach to increase natural MP breakdown with minimal ecological impact (Yuan et al., 2020). Consequently, alongside advanced oxidation processes (AOPs), bioremediation is viewed as a highly desirable method for removing MP pollution. Several studies have explored using bacteria to decompose MPs. These focus mainly on pure bacterial cultures under laboratory conditions, primarily sourced from wastewater, sludge, and sediment. Auta et al. (2018) examined two pure bacterial cultures from mangrove sediment for degrading polypropylene (PP) MPs. Bacillus sp. strain 27 and Rhodococcus sp. strain 36 increased the weight loss of PP by 4.0% and 6.4%, respectively, after 40 days. Additionally, various irregularities appeared on the PP surface, suggesting that PP could be adhered to, colonized, and damaged by pure bacterial cultures isolated from the environment. Fungi, along with bacteria, can also degrade MPs (Table 15). In recent decades, new fungi with enhanced MP decomposition activity have been identified. Reports on fungal-assisted MP decomposition are still limited, indicating challenges in isolating fungal strains with superior degradation capabilities through ectopic screening (Yuan et al., 2020). The biodegradation of MPs by fungi under different environmental conditions remains an active research area with some progress achieved. The effective use of fungi to degrade MPs and other pollutants is gradually attracting more attention.
Table 15
| Microorganism | Type of plastic | Method of degradation | References |
|---|---|---|---|
| Bacteria | |||
| Acinetobacter calcoaceticus | Polyurethane | Urea bond hydrolysis | Mohanan et al., 2020 |
| Corynebacterium sp. | Polyurethane | Ester bonds hydrolysis | Puiggené et al., 2022 |
| Bacillus subtilis | Polyurethane | Ester bonds hydrolysis | Puiggené et al., 2022 |
| Nocardia sp. sp. | Polyurethane | Enzymatic degradation | Venkatesh et al., 2021 |
| Comamonas acidovorans | Polyurethane | Enzymatic degradation using esterase | Puiggené et al., 2022 |
| Acinetobacter sp. sp. | Polyethylene tetraphthalate | De-polymerization and mineralization | |
| Thermomonospora curvata | Polyethylene tetraphthalate | Enzymatic degradation | Mohanan et al., 2020 |
| Thermobifida alba | Polyethylene tetraphthalate | Enzymatic degradation using carboxylesterase | Kour et al., 2023 |
| Clostridium thermocellum | Polyethylene tetraphthalate | Enzymatic degradation by thermophilic cutinase | |
| Pseudomonas chloraphis | Polyethylene tetraphthalate | Enzymatic degradation | Kumar Sen and Raut, 2015 |
| Ideonella sakaiensis | Polyethylene tetraphthalate | Enzymatic degradation using PETase and MHETase | Palm et al., 2019 |
| Bacillus cereus | Polystyrene | Forming biofilms, oxidizing the polystyrene | Udochukwu et al., 2022 |
| Pseudomonas aeruginosa | Polystyrene | Enzymatic degradation | Kim et al., 2021 |
| Exiguobacterium sp. sp. | Polystyrene | Enzyme sequence, depolymerization and epoxidation, aromatic ring attack biofilm formation | Chauhan et al., 2018 |
| Rhodococcus sp. | Polypropylene | Biofilm formation | Auta et al., 2018 |
| Rhodococcus ruber | Polystyrene | Biofilm formation | |
| Pseudomonas citronellolis | Poly vinyl chloride | Biofilm formation | Giacomucci et al., 2019 |
| Bacillus flexus | Poly vinyl chloride | Biofilm formation | Giacomucci et al., 2019 |
| Acinetobacter sp. sp. | Poly vinyl chloride | Biofilm formation and enzymatic degradation | |
| Bacillus cereus | Polypropylene, polyethylene | Enzymatic degradation using lipase or dehydrogenase | Mouafo Tamnou et al., 2021, Auta et al., 2018 |
| Yokenella regensburgei | Polypropylene | Enzymatic degradation | Temporiti et al., 2022 |
| Lysinibacillus sp. sp. | Polypropylene | Manganese peroxidase degradation | Jeon et al., 2021 |
| Pseudomonas sp. sp. | Polypropylene | Hydrolytic enzyme degradation | Jeon et al., 2021 |
| Vibrio sp. sp. | Polypropylene | Enzymatic degradation | Viel et al., 2023 |
| Brevibacillus borstelensis | Polyethylene | Enzymatic degradation using hydroxylases, peroxidases | Mouafo Tamnou et al., 2021 |
| Acinetobacter pitti | Polyethylene | Enzymatic degradation | Montazer et al., 2018 |
| Micrococcus luteous | Polyethylene | Enzymatic degradation | Montazer et al., 2018 |
| Pseudomonas aeruginosa | Polyethylene | Enzymatic degradation | Kyaw et al., 2012 |
| Rhodococcus rhodochrous | Polyethylene | Enzymatic oxidation using dehydrolyases and esterases | Rose et al., 2020 |
| Bacillus sp. sp. | Polyethylene, polypropylene | Enzymatic degradation using esterases, lipases, depolymerase | Rani et al., 2022, Auta et al., 2018, Park and Kim, 2019, Huerta Lwanga et al., 2018 |
| Bacillus gottheilii | Polyethylene, polypropylene, polyethylene tetraphthalate, polystyrene | Enzymatic degradation | Auta et al., 2017 |
| Paenibacillus sp. sp. | Polypropylene | Enzymatic degradation | Park and Kim, 2019 |
| Bacillus simplex | Polyethylene | Enzymatic degradation | Huerta Lwanga et al., 2018 |
| Fungi | |||
| Aspergillus sp. sp. | Polyurethane | Enzymatic degradation using esterase and protease, Biofilm formation | Puiggené et al., 2022 |
| Fusarium solanii | Polyethylene tetraphthalate | Surface hydrophilization using cutinase | Ahmaditabatabaei et al., 2021 |
| Curvularia sp. sp. | Polystyrene | Enzymatic degradation | |
| Phanerochaete chrysoporium | Poly vinyl chloride | Enzymatic degradation using laccases and peroxidases | Bautista-Zamudio et al., 2023 |
| Trichoderma hematum | Poly vinyl chloride | Enzymatic degradation | Bautista-Zamudio et al., 2023 |
| Aspergillus fumigatus | Poly vinyl chloride | Enzymatic degradation by depolymerase | El-Dash et al., 2023 |
| Aspergillus niger | Polypropylene | Hydrolase and oxidoreductase, cutinases | Viel et al., 2023 |
| Cladosporium cladosporioides | Poly ethylene | Enzymatic degradation using laccase enzyme | Puliga et al., 2023 |
| Chaetomium sp. sp | Poly ethylene | Enzymatic degradation by laccase and manganese peroxidase | Sowmya et al., 2014 |
| Aspergillus flavus | Polyethylene | Enzymatic degradation | Zhang et al., 2020 |
Microplastic degradation by microorganisms.
16 Physical treatments of microplastics
16.1 Incineration
Incineration is a prominent physical method for managing plastic waste, with widespread adoption dating back to the 1980s (Ncube et al., 2021). Often seen as a definitive solution for plastic waste disposal, incineration aims to convert polymers into CO2 and mineral residues (Yang et al., 2021). Recent studies have highlighted significant challenges in the effectiveness of incineration for removing microplastics from the waste stream. Research by Yang et al. (2021) examined microplastic content in bottom ash from multiple incineration sites across China, revealing that incineration does not fully eliminate microplastics and may even release them into the environment via bottom ash residues. Further investigations by Shen et al. (2021) emphasized the complexities of managing microplastics through incineration. Their findings, using scanning electron microscopy (SEM), demonstrated that microplastics in incinerator residues have surface irregularities and tend to adsorb heavy metals like Cu, Cr, Pb, and Zn, which may originate from plastic polymers or external sources such as discarded batteries mixed with municipal solid waste. While incineration offers advantages like saving space compared to landfilling and potential energy recovery, it also presents environmental risks. The combustion process releases greenhouse gases, toxic chemicals, and air pollutants, affecting air quality and potentially threatening human and animal health (Webb et al., 2013). Moreover, the formation of microplastics and their association with heavy metals in incinerator residues pose ongoing challenges for environmental stewardship and warrant careful consideration in waste management strategies (Table 16). Therefore, although incineration is a feasible method for managing plastic waste, its effects on microplastic contamination and environmental health require continuous research and strict regulatory measures to reduce harmful impacts.
Table 16
| Methods | Principle of technique | Efficiency | Type of MPs | Remarks | Reference | |
|---|---|---|---|---|---|---|
| Physical | ||||||
| Magnetic polyoxometalate-supported ionic liquid phases (magPOM-SILPs) | Physisorption | Over 90% | PS | a. High efficiency b. It can detect and remove organic, inorganic, and MPs pollutants from water |
a. Only applicable for PS microplastics of 1–10 μm in size | (Misra et al., 2020) |
| Biochar adsorbents | Physisorption | 100% (polyethylene particles) | All MPs | a. High adsorption capacity b. High efficiency c. Less maintenance d. Low cost |
a. Inefficient for reducing MPs with micrometer size | (Siipola et al., 2020) |
| Zirconium metalorganic frame-work based foams | Filtration | 95.5 ± 1.2% | All MPs | a. High efficiency MPs removal in water or marine water conditions b. Applicable for all types of MPs removal with various concentrations of MPs suspension c. Automatic filtration system done by solar power d. Recyclable foam |
a. Typically only performed in laboratories, large-scale filtration experiments are crucial for the practical applications | (Chen et al., 2020) |
| Rapid sand filter | Filtration | 97% | All MPs | a. Applicable for each type of MPs b. Low cost c. Easy procedure |
a. Only effective on >20 μm size of MPs | (Talvitie et al., 2017) |
| Disc filter | Retention | 89% | All MPs | a. High efficiency | a. High maintenance b. Not applicable for small-sized MPs |
(Talvitie et al., 2017) |
| Dissolved air flotation | Floatation | 95% | All MPs | a. High efficiency | a. Only applicable for low-density particles | (Talvitie et al., 2017) |
| Magnetic carbon nanotubes | Physisorption | 100% | All MPs | a. High efficiency | a. Efficiency decreases after more times of used | (Tang et al., 2021) |
| Coagulative colloidal gas aphrons | Physisorption | 94% | Carboxyl-modified poly-(methyl methacrylate) (PMMA) and nonsurface-coated polystyrene (PS) | a. High efficiency b. Salinity does not affect the efficiency | a. Size-dependent efficiency | |
| Non-fluorinated superhydrophobic aluminum surface | Physisorption | 99% | Polypropylene (PP) | a. Higher efficiency b. Can be applied in natural conditions |
a. Efficiency only examined with 262-μm-sized MPs | (Rius-Ayra and Llorca-Isern, 2021) |
| Sponge made of graphene oxide and chitin | Physisorption | 89.8%, 88.9%, and 72.4% for neat polystyrene, amine modified polystyrene, and carboxylate modified polystyrene, respectively | Polystyrene, amine-modified polystyrene and carboxylate-modified polystyrene | a. Reusability, biodegradability, and biocompatibility of the sponge increase its suitability for MP removal | a. Complicated to scale up | (Sun et al., 2020) |
| Magnetic microsubmarines | 70% | All MPs | a. Cooperative behavior b. High adsorption c. Good efficiency d. Eco-friendly e. High environmental adaptability f. Chemicals not required |
a. Less efficient than other methods b. Expensive |
(Sun et al., 2020a) | |
| Microplastics retention in a huge secondary wastewater treatment | 91.7% | All MPs | a. Low cost. b. Harmful reagents not required c. Good efficiency |
a. Less effective for small MPs | (Gies et al., 2018) | |
| Chemical alum coagulant and alum combined with cationic polyamine-coated sand | Coagulation and flocculation | 70.7%–92.7% | Polyethylene | a. The MP removal efficiency increases with increasing alum doses (up to 30 mg/L) b. Good efficiency |
a. Inefficient for lower-sized MPs (10–30 μm) | (Shahi et al., 2020) |
| Influence of linear and branched alkyltrichlorosilanes | Chemisorption, agglomeration, and filtration | 98.3 ± 1.0% | Low-density polyethylene (LDPE), high-density polyethylene (HDPE) and polypropylene (PP)-based MPs | a. Alkyl group increases the reaction rate b. They affect the adhesion to the microplastics as well as the kinetics of hydrolysis and condensation in water |
a. Long alkyl groups of more carbon atoms and short-chain methyl groups are unsuitable for this reaction. These chain decreases the MP removal efficiencies. | (Sturm et al., 2020) |
| Granular activated carbon | Filtration | 56.8%–60.9% | All MPs | a. Remove chemicals, specifically organic chemicals, MPs from water | a. It is capable of microbial contamination in the carbon bed | (Wang et al., 2020) |
| Coagulation combined with sedimentation | Coagulation and settling | >99% | All MPs | a. Higher efficiency b. Applicable for >10 μm size of MPs |
a. Removal efficiency decreases for sizes <10 μm of MPs |
(Wang et al., 2020) |
| Coagulation/flocculation with Fe, Al, and polyamine-based chemicals | Coagulation and flocculation | 95% for 1-μm MPs and >76% for 6.3-μm MPs | Polystyrene spheres | a. High efficiency. | a. Removal efficiency varies with the size of MPs | |
| Ozone | Chemical degradation | 89.9% | All MPs | a. High efficiency. | a. The cost of this process is high b. Harmful and toxic for our environment |
(Hidayaturrahman and Lee, 2019) |
| Photocatalysis | Visible light induced heterogeneous photocatalysis activated by zinc oxide nanorods | 30% | Low-density polyethylene | a. Low cost b. Eco-friendly c. No secondary pollution d. Requires a simple reactor e. Chemical–physical stability |
a. Low efficiency b. Lack of solar sensitivity |
(Tofa et al., 2019b) |
| Electrocoagulation | Flocculation and settling | 90%–100% | Polyethylene microbeads | a. Less energy requirements b. Cost effective c. Efficiency is high d. Minimum sludge e. No secondary pollution |
a. pH dependent b. Continuous electricity supply requirement c. High concentration of Cl- ions affects the removal efficiency. |
(Perren et al., 2018) |
| Alkoxy-silyl induced agglomeration | Sol–gel agglomeration | >70% | All MPs | b. Cost effective method c. Controllable operational conditions |
a. More research needed for various types of MPs b. Recovery of materials |
(Herbort et al., 2018) |
| Biological membrane bioreactor (MBR) | Filtration | >99% | All MPs | a. Good efficiency b. Easy method c. Environmentally friendly process d. High effluent quality |
a. Expensive b. A new membrane must be developed to reduce fouling brought on by MPs c. High energy requirement |
(Lares et al., 2018) |
| Dynamic membranes | Filtration | >90% | a. Low cost b. Low energy consumption c. Low filtration resistance d. Easy method e. Pumps and chemicals are not necessary |
a. Sludge accumulation b. Control of excessive membrane fouling is necessary |
||
| Microalgal-based biopolymer | Aggregation and flocculation | Potential to removal of nano or microplastics | Polystyrene nano- and microplastics | a. High growth rate b. High yield of products c. Produces from renewable sources |
a. Production cost is high b. Complex production process |
(Cunha et al., 2020) |
| Anaerobic–anoxic–oxic (A2O) | Microbial biodegradation | 93.7% | All MPs | a. Good efficiency b. Simple configuration c. Short hydraulic retention time d. No energy required e. Cheap investment cost |
a. Require disinfection chemicals b. Footprint required is large |
(Edo et al., 2020) |
| Oxidation ditch | Microbial biodegradation | 97% | Polyethylene terephthalate (PET), polyethylene (PE), polystyrene (PS), polypropylene (PP) | a. High efficiency b. Easily maintained c. Forms little sludge d. Less energy is required |
a. Expensive b. Large land area is required c. Suspended solids abundance of wastewater are higher |
(Lv et al., 2019) |
| Conventional activated sludge | Microbial biodegradation | 98.3% | All MPs | a. Low installation cost b. Easily maintained c. Good quality effluent. d. Self-sustaining system. e. Small area requirement. |
a. Large areas are required b. Larger sludge volumes c. Higher disposal cost d. Take large hydraulic retention time |
(Lares et al., 2018) |
| Wastewater treatment plants | Coagulation, flocculation, sedimentation | >95 | All MPs | a. Low maintenance cost b. Easy operation c. Improve efficiency d. Prevent waterborne pollution |
a. Sludge aggregation b. Not applicable for small-sized MPs c. Large mechanical devices |
|
| Bioremediation | Microbial biodegradation (uses microorganisms like bacteria or fungi or algae) | Mainly above 20%, but depends on the type of microorganism | All MPs | a. Low cost b. Low energy demand c. Environmentally friendly d. Uses of bacteria in bioremediation process is highly specific, chances of forming toxic by-products is lower |
a. Time-consuming process b. Contamination problem c. Pollutants are many times removed incompletely d. Products more harmful than original pollutants can release during bioremediation |
|
| Constructed wetlands (CWs) | 88% | All MPs | a. Natural technology b. Low cost c. Efficient tertiary treatment |
a. Few studies on constructed wetlands removal of MPs | (Wang et al., 2020) | |
Physical, chemical, and biological methods for microplastic removal.
16.2 Ultraviolet-radiation-induced degradation
Exposure to ultraviolet (UV) radiation causes significant degradation in synthetic polymers like PET and PA, a process called photooxidative degradation (Yousif and Haddad, 2013). A recent research by Sørensen et al. (2021) examined the effects of UV exposure on PET and PA microfibers over 56 days in seawater under simulated sunlight. The study showed that UV radiation led to surface morphological changes, including holes and pits on both PET and PA surfaces. PA showed more noticeable surface changes and broke into smaller fiber pieces more than PET. This degradation process promotes the formation of microplastics, worsening environmental pollution with potentially harmful ecological effects. Understanding UV-induced degradation mechanisms is essential for developing strategies to reduce microplastic contamination in aquatic ecosystems.
16.3 Microplastic by photocatalyst treatment
Photocatalyst treatment offers a promising way to break down microplastics by using visible light to activate semiconductor materials like zinc oxide (ZnO NRs), as shown by Uheida et al. (2020). This method uses glass fiber substrates coated with ZnO nanorods to trap and degrade polypropylene (PP) microplastic particles, a major pollutant in aquatic environments. Under continuous visible light exposure, the ZnO photocatalyst effectively reduced PP microplastics by 65% over 2 weeks. The process works by generating reactive species that break down microplastics into harmless substances like CO2 and H2O. Eco-friendly and cost-effective, visible light photocatalysis shows promise for reducing microplastic pollution while harnessing solar energy sustainably. This innovative approach highlights the potential of semiconductor photocatalysts in environmental clean-up efforts worldwide.
16.4 Filtration method
In the early stages of microplastic (MP) separation, filtration became a popular technique because it was easy to use and quick (Liu et al., 2021; Sol et al., 2020)—for example, Tadsuwan et al. used a series of filters with sizes ranging from 5 mm to 0.05 mm to remove MPs from wastewater from Thai municipal treatment plants, which resulted in a removal rate of 33.33% (Tadsuwan et al., 2021). MPs with a diameter of 10 μm were removed by Wang et al. using a charcoal filter, demonstrating an impressive removal efficiency of over 95% (Wang et al., 2020). Although filtration technology has intrinsic limitations despite its widespread use, it is effective at capturing microplastics but does not completely eliminate them; it instead often produces smaller MPs that are more difficult to remove during subsequent processing. This approach also imposes strict constraints on the types of filters that can be used. Large-scale applications tend to be prohibitively expensive due to the high investments needed for processing large volumes. Consequently, while the filtration method is common, it requires improvements to boost its efficiency and cost-effectiveness. Future research should aim to develop advanced filters that can increase throughput while lowering treatment costs. Innovations in filter materials and designs could lead to more efficient and sustainable solutions for MP removal. Additionally, combining filtration with other complementary methods could create a more comprehensive approach to managing MP pollution. By overcoming current limitations and enhancing filtration systems, researchers can deploy more effective strategies to reduce the environmental impact of MPs, contributing to cleaner water bodies and a healthier ecosystem.
16.5 Adsorption method
The adsorption technique uses a porous solid adsorbent to trap various adsorbates from water samples, then employs solvent extraction, heating, or blowing methods to release and separate the adsorbate. Known for its effectiveness in removing water pollutants, adsorption has become important in the removal of microplastics (MPs) (Bu et al., 2020a, 2020b, 2021). Wang et al. created a natural biodegradable sponge crosslinked with plant protein that shows excellent mechanical properties. Using a polystyrene MP solution in deionized water, the adsorbent removed 38% of MPs in 10 s and maintained an 81.2% removal rate in simulated wastewater even after 20 cycles (Wang et al., 2021). Particle diffusion and hydrophobic interactions were the main mechanisms involved (Wang et al., 2021). In another study, Sun et al. developed a solid, compressible sponge made from chitin and graphene oxide (ChGO). ChGO’s adsorption rates for pure polystyrene, carboxylic acid-modified polystyrene, and amine-modified polystyrene were 89.8%, 72.4%, and 88.9%, respectively, in tests with deionized water-based MP solutions. This material has excellent activation and regeneration properties and primarily interacts with MPs through electrostatic and π- π interactions (Sun et al., 2020). Yuan et al. identified strong π– π interactions as the key adsorption mechanism when studying three- dimensional reduced graphene oxide as an adsorbent for polystyrene MPs. They demonstrated impressive regeneration capabilities and achieved a maximum adsorption capacity of 617.28 mg/g in deionized water (Yuan et al., 2020). The adsorption method is valued for its simplicity, low equipment requirements, and high effectiveness. However, its widespread use is limited by concerns about cost, structural stability, and adsorbent selectivity. Although effective at removing MPs from water, additional approaches are needed for complete MP clean-up. Future research should focus on designing adsorbents that perform well in microplastics management, offering high efficiency, strong recyclability, and easy reuse. Advances in material science and adsorption technology will be key to developing sustainable solutions to reduce the environmental and health impacts of microplastics on aquatic ecosystems. The ongoing development of adsorption for microplastics removal highlights the need for interdisciplinary collaboration, technological innovation, and policy support to protect our water resources and promote environmental responsibility in a rapidly changing world.
17 Extraction method
Extraction techniques have gained significant attention for treating industrial wastewater contaminated with phenols, nitrogen heterocycles, dyes, heavy metals, and other pollutants (Cao et al., 2021; Warrag et al., 2020). To address microplastic (MP) pollution, researchers worldwide have focused on developing and applying extraction methods in recent years (Wagner et al., 2014; Hurley et al., 2018). To efficiently separate and extract various biodegradable plastics (such as polybutylene succinate, poly (adipic acid) butylene terephthalate, and polylactic acid) and non-degradable MPs (including low-density polyethylene, polystyrene, polypropylene, and polyvinyl chloride), Li et al. created a custom separation and extraction device. Its high recovery rates, ranging from 92% to 99.6%, proved its accuracy and reliability (Li et al., 2021). Nuelle et al. used a different approach by employing a two-step extraction process to recover MPs from sediment samples, achieving high efficiencies between 91% and 99% for polymers like polyethylene, polypropylene, polyvinyl chloride, poly (ethylene terephthalate), polystyrene, and polyurethane (Nuelle et al., 2014). Similarly, Han et al. enhanced the flotation process and solutions to successfully extract and separate six common MP types from soil and sediment samples, with efficiencies from 80% to 100%. Wang et al. extracted styrene MP spheres of various sizes (0.05 to 100 μm) from soil and biosolids. Smaller particles had extraction efficiencies from 5% to 80%, while larger particles were completely removed (Wang et al., 2018). This approach offers benefits such as excellent operational safety, automated control, and simple equipment. However, high operational costs and the challenge of effectively separating dissolved solutes in the extraction solvent remain issues. To improve the sustainability of MP treatment techniques, researchers should explore more affordable and environmentally friendly extraction solvents in the future. Developing advanced extraction technologies that can efficiently recover MPs from different environmental matrices remains essential. Such innovations will help mitigate microplastic pollution, protect aquatic ecosystems, and support sustainable resource management. The progress of extraction methods for microplastics highlights the importance of interdisciplinary collaboration and technological innovation to effectively address complex environmental problems. By refining these techniques and exploring new materials, scientists can help create a cleaner, healthier environment for future generations.
17.1 Magnetic separation
Magnetic separation technology, which uses magnetic fields to manipulate substances, has become a key tool in water treatment and is increasingly used to isolate microplastics (MPs) (Li et al., 2022). With impressive results, Tang et al. were the first to use hydrophobic iron nanoparticles for the magnetic separation and removal of MPs. According to their research, over 90% of MPs in seawater that were larger than 1 mm and between 10 and 20 μm were removed. Additionally, the nanoparticles successfully removed 84% and 78% of MPs from freshwater and sediment, respectively, that measured between 200 μm and 1 mm (Tang et al., 2021).
In a parallel study, Tang and colleagues developed magnetic carbon nanotubes aimed at using their magnetic properties to remove MPs from water solutions. At a concentration of 5 g/L, these magnetic carbon nanotubes completely removed MPs after 300 min and maintained 80% efficiency after four cycles. The ability of magnetic carbon nanotubes to effectively eliminate MPs was confirmed with scanning electron microscopy (SEM) images, which showed MPs attached to their surface (Tang et al., 2021). The magnetic separation technique offers numerous benefits, including long-range magnetic enhancement, low waste sludge production, and suitability for high-volume treatment. However, challenges such as the tendency of magnetic seeds, MPs, and other lipophilic/oleophobic substances to cluster on surfaces remain. Future research should aim to develop adaptable magnetic separation methods suited for different types of MPs to improve their effectiveness across various environmental matrices. Overcoming these challenges will help promote wider use of magnetic separation technologies in fighting microplastic pollution, protecting aquatic ecosystems, and supporting sustainable water management. The progress of magnetic separation methods highlights the importance of innovation and interdisciplinary teamwork in advancing environmental solutions. By improving magnetic materials and exploring new applications, researchers can help reduce the widespread impact of microplastics on global water resources, creating a cleaner and healthier environment for future generations.
17.2 Oil film separation
Oil film separation, a hydrophobic and density-independent technique, has become a prominent method for microplastic (MP) removal (Scopetani et al., 2020). Crichton et al. developed an innovative and cost-effective oil film approach for MP removal, achieving high removal efficiencies. Their study reported removal rates of 96.1% ± 7.4% for total MPs, 92.7% ± 4.3% for fibers, and 99% ± 1.4% for particles, demonstrating the effectiveness of this method in removing MPs from various environments (Crichton et al., 2017). Similarly, Mani et al. used castor oil membranes to separate MPs from water samples, showing high removal rates averaging up to 99%. Notably, this method was effective with a removal efficiency of 74% ± 13% in the Rhine River, highlighting its environmental compatibility and operational success (Mani et al., 2019). The technique is valued for being density independent, cost-effective, and environmentally friendly. However, challenges such as equipment blockage during separation have been noted (Thanh Truc et al., 2019). Addressing these technical issues is essential for improving the efficiency and scalability of oil film separation for MP treatment.
18 Chemical treatments of microplastic degradation
Chemical treatments provide a targeted method to break down plastic polymers by using additives that help disassemble polymer chains. Moharir and Kumar (2019) emphasize the role of chemical additives in starting disintegration processes, which can weaken the structural integrity of plastics—for example, Bomfim et al. (2019) studied the use of sodium hydroxide (NaOH) and sulfuric acid (H2SO4) on polypropylene (PP) coffee capsules, noting minimal surface effects with NaOH and adhesive deterioration with H2SO4. In another study, Hussein et al. (2018) examined the chemical breakdown of polyethylene terephthalate (PET) using ethylene glycol (EG) and nano-magnesium oxide (MgO), showing the effective conversion of PET into a powder through glycolysis. Amaro et al. (2011) expanded on this by using diethylene glycol (DEG) and (Ca/Zn) stearate as catalysts to degrade PET, turning the polymer into a molten state suitable for secondary use.
Although effective in controlled environments, chemical treatment faces challenges for large-scale use due to the complexity and environmental impact of chemical additives. The process needs careful oversight to avoid negative effects on human health and ecosystems. Chemical treatments still play an important initial role in polymer degradation pathways, working alongside biological processes and offering potential as secondary plasticizers for recycled materials.
18.1 Fenton oxidation and advanced oxidation technology
Applications of the Fenton oxidation technique in water treatment are widespread (Yang et al., 2022). Recent research has focused on using chemical oxidation methods to treat wastewater containing microplastics (MPs) (Liu et al., 2020). Liu et al. used the Fenton oxidation process and heat-activated K2S2O8 oxidation to treat MPs. Under Fenton-like and thermal activation, K2S2O8 generates a significant amount of sulfate and hydroxyl radicals, which help oxidize and break down MPs. Scanning electron microscopy (SEM) images showed surface deformation in polystyrene (PS) and polyethylene (PE), indicating some level of degradation of these MP materials (Liu et al., 2019).
For the breakdown of MP, advanced oxidation technologies (AOTs) such as photocatalytic oxidation, persulfate advanced oxidation, and electrochemical oxidation have also been studied (Lin et al., 2020; Wang et al., 2019). After irradiating polyethylene MPs with a 350-W metal halide lamp for 5 h, Venkataramana et al. observed a 12.5% weight loss, indicating partial degradation through photocatalytic processes (Venkataramana et al., 2021). To remove polypropylene MPs from water, Uheida et al. proposed a sustainable photocatalytic method using visible light. After 2 weeks of exposure, the average particle volume decreased by 65%. Degradation by-products such as hydroxypropyl, butyraldehyde, acetone, acrolein (propenal), ethynyloxy/acetyl radicals, and the pentyl group were detected via gas chromatography–mass spectrometry (GC/MS) analysis (Uheida et al., 2021). Electrochemical oxidation is another common method for wastewater treatment (Bensalah et al., 2021; De Vidales et al., 2020). Kiendrebeogo et al. used electrochemical oxidation to treat synthetic polystyrene MPs in wastewater. The process produced hydroxyl and sulfate radicals with strong oxidizing abilities, leading to the mineralization of polystyrene MPs into CO2. The removal efficiency reached 89% ± 8% within 6 h using a Na2SO4 dosage of 0.06 M. SEM analysis confirmed that the degradation did not fragment the MPs but instead converted them directly into gaseous products (Kiendrebeogo et al., 2021).
Polyvinyl chloride (PVC) MPs were broken down by Miao et al. using a two-dimensional electrocatalytic oxidation technique. They achieved 75% dechlorination efficiency and 56% weight reduction for PVC after 6 h of electrocatalytic oxidation. GC/MS and high-performance liquid chromatography (HPLC) analyses showed degradation by-products such as alkenes, alcohols, monocarboxylic acids, dicarboxylic acids, and esters. The mechanism involved direct electron transfer from the TiO2/C cathode to PVC, leading to dechlorination and oxidation by hydroxyl radicals, which formed oxygen-containing groups like C=O and O-H, and partially mineralized the substances into CO2 and H2O (Miao et al., 2020). Numerous benefits are offered by Fenton oxidation and advanced oxidation technologies, including cost-effectiveness, simple equipment, consistent results, high removal efficiency, and ease of operation and maintenance. However, these technologies also have drawbacks such as limited treatment effectiveness, high costs, potential secondary contamination, and strict process requirements. A complex water treatment system combining three-dimensional electrocatalytic oxidation with persulfate advanced oxidation was developed based on the investigation of Li et al. (2023) into electrochemical oxidation and persulfate advanced oxidation. Over 90 min, this system demonstrated the highest sulfadiazine elimination efficiency (99.95%) and mineralization efficiency (90.16%) (Bu et al., 2022). During the same period, the combined system removed 99.56% of sulfamethazine and mineralized 88.63% of it (Bu et al., 2022). Our self-assembled three-dimensional electrocatalytic oxidation reactor enabled us to break down sulfonamide with 88.958% mineralization and 99.845% elimination. Bromobenzonitrile was completely removed using a spherical bimetallic clay catalyst. These degradation processes are highly recyclable and generate substantial amounts of highly reactive oxygen species (Wan et al., 2022). It was found that chloride ions increased the degradation efficiency. Combining persulfate-accelerated oxidation with three-dimensional electrocatalytic oxidation is a promising method for MP degradation. Future research should focus on elucidating the treatment mechanisms to reduce the environmental impact of degradation intermediates and optimize the process for broader application.
18.2 Coagulation method
The coagulation method is a common technique used in wastewater treatment, where coagulants are added to destabilize and clump together organic pollutants into larger particles, often called flocs. These flocs, usually made of alum, can range from hundreds of microns to millimeters in size. The aggregated pollutants can then be removed through gravity sedimentation or other solid–liquid separation processes (Liu et al., 2019). Recently, coagulation has been successfully applied to treat wastewater containing microplastics (MPs) (Xu et al., 2021; Lapointe et al., 2020).
Poly-aluminium chloride (PAC) and FeCl3 were used as coagulants by Zhou et al. in a noteworthy study to remove polystyrene (PS) and polyethylene (PE) MPs. This interaction neutralizes the charge between MPs and the coagulant. MPs significantly aggregated and adhered to the coagulants, as shown by SEM images, and new chemical bonds formed during the coagulation process, as indicated by Fourier transform infrared (FTIR) spectra. Additionally, the effective removal of PS and PE MPs was confirmed by zeta potential changes before and after adsorption (Zhou et al., 2021). Similarly, Ma et al. examined the efficiency of iron-based (FeCl3·6H2O) and aluminum-based (AlCl3·6H2O) coagulants in removing polyethylene MPs. Their results showed that aluminium salts performed better than iron salts, especially as the size of the polyethylene particles decreased. The highest average removal rate was only 36. 36.89%, even with high doses of aluminum-based salt (15 mM) (Ma et al., 2019). To remove MPs from wastewater in drinking water treatment facilities, Shahi et al. used alum and a composite cationic polyamine-coated sand coagulant. The composite coagulant achieved a 26. 8% improvement over alum alone, highlighting the importance of MPs’ surface properties, shape, and particle size in the coagulation process (Shahi et al., 2020). Electrocoagulation is another method used for pollutant removal, involving the application of pulsed high voltage to drive electrochemical reactions. This technique has shown promise in eliminating MP pollutants—for example, Perren et al. used electrocoagulation to treat synthetic wastewater containing various concentrations of polyethylene MP spheres. According to Perren et al. (2018), electrocoagulation efficiency could exceed 90% across a pH range of 3 to 10, reaching an impressive 99.24% elimination at pH 7.5. The benefits of coagulation include its ease of use, low equipment requirements, and quick treatment times. However, it is important to remember that pH greatly influences coagulation effectiveness, and many coagulants can cause discoloration and reduce effectiveness. Overdosing can lead to decreased removal rates and increased chromaticity. Future research should focus on optimizing coagulation parameters to improve MP removal, including exploring different coagulant types, dosages, and environmental conditions.
18.3 Foam flotation method
A well- known method for separating minerals from contaminants is foam flotation, which exploits how chemicals selectively interact with desired minerals to alter their surface properties. By agitating the raw ore powder with water and chemicals and then adding air, targeted minerals rise to the surface and form a froth. Microplastics (MPs) can be removed from various habitats using this technique, which has gained recent attention (Bayo et al., 2020; Zhang et al., 2020)—for example, a foam-flotation-based method for MP separation was developed by Imhof et al. and achieved a removal efficiency rate of 55% (Imhof et al., 2012). The presence of uncertain factors that may hinder the separation process was highlighted by Nguyen et al., emphasizing the need for further technique improvement (Nguyen et al., 2019). Talvitie et al. demonstrated the effectiveness of an air flotation method with an impressive removal efficiency of up to 95%, reducing MP concentrations in aqueous solutions from 2 MP/L to 0. 0.1 MP/L (Talvitie et al., 2017). Additionally, Enfrin et al. and Sun et al. promote foam flotation as a practical approach to MP treatment due to its simplicity, affordability, and ability to decrease MP discharge into sewage systems (Enfrin et al., 2019; Sun et al., 2019). Jiang et al. used froth flotation to remove MPs from lake and beach sediments, employing sodium oleate to restore the hydrophobicity of MPs, making them easier to extract from sediments (Jiang et al., 2022). The benefits of the foam flotation approach include low cost and straightforward equipment. However, a major challenge is the reproducibility of experiments, which is influenced by various factors, including temperature. Unfortunately, there has been limited research on the inconsistent performance of foam flotation in scientific literature, highlighting an important area for future investigation. Future studies should aim to optimize conditions to enhance the reliability and effectiveness of foam flotation for MP removal, including examining the impacts of different reagents, temperatures, and other environmental factors. Additionally, developing standardized protocols could improve reproducibility and support broader application in environmental clean-up efforts.
19 Biodegradation treatment of microplastics
Biodegradation is a promising way to reduce microplastic pollution through natural processes driven by microorganisms (Table 14). Priyanka and Archana (2011) describe the three stages of polymer biodegradation: biodeterioration, where microorganisms change the physical and chemical properties of polymers; bio-fragmentation, which involves breaking down polymers into smaller oligomers and monomers; and assimilation, where microorganisms use these fragments as energy and nutrients, ultimately turning them into CO2, biomass, and water (Emadian et al., 2017). This biological process provides a sustainable way to decrease microplastic build-up in the environment, using microbial activity to break down synthetic polymers into harmless substances. Ongoing research continues to examine microbial diversity, environmental factors, and enzymatic mechanisms to improve biodegradation methods, emphasizing its potential as a natural and effective approach for managing plastic waste.
19.1 Microplastic in oxidation ditch and membrane bioreactor
Microplastics have become significant contaminants in wastewater treatment plants (WWTPs), prompting extensive research into effective removal strategies using various treatment technologies such as oxidation ditches (ODs) and membrane bioreactors (MBRs). Lv et al. (2019) studied the performance of OD and MBR systems at a full-scale WWTP in Wuxi, focusing on how well they removed polyethylene (PE), polystyrene (PS), polypropylene (PP), and polyethylene terephthalate (PET) microplastics. Their results showed that the MBR system performed better than the OD, achieving a microplastic removal rate of 82.1% compared to 53.6% in the OD. This better performance is due to the MBR’s microfiltration membrane, which has pores smaller than 0. 0.1 mm, effectively preventing microplastics from passing into the effluent. However, some microplastics still escaped through the MBR effluent because of membrane degradation over time, pipeline leaks, or airborne pollution. In contrast, Lares et al. (2018) examined the effectiveness of microplastic removal in WWTPs using conventional activated sludge (CAS) and advanced MBR technologies at the Kenkaveronniemi WWTP. They found that the MBR with a membrane pore size of 0.4 μm achieved an impressive 98.3% removal efficiency for microplastics. This study showed that most microplastics were removed early in the treatment process, before the activated sludge stage. The MBR consistently produced effluents with much lower microplastic concentrations (average 0. 4 MP/L) compared to CAS-treated effluents (average 1. 0 MP/L). Despite these improvements, the challenge remains in preventing microplastics from accumulating in sludge, which can become a secondary pollution source. The build-up of microplastics in sludge highlights a critical issue in WWTPs. Although these facilities are effective at removing a large percentage of microplastics from wastewater, managing microplastic-laden sludge remains inadequate. Once captured in sludge, microplastics pose risks of re-entering the environment through pathways like soil amendment and landfill disposal. Therefore, comprehensive strategies are necessary to treat and dispose of microplastic-contaminated sludge responsibly. The differences in efficiency between the OD and MBR systems reflect the evolving landscape of microplastic treatment technologies in WWTPs. While MBRs show superior microplastic removal capabilities thanks to advanced filtration, the sustainability of this approach depends on addressing operational challenges that compromise membrane integrity over time. Additionally, both studies highlight the importance of adopting strong monitoring and maintenance protocols to ensure consistent microplastic removal in wastewater treatment processes.
19.2 Microplastic degradation in activated sludge process
The activated sludge process, a key part of secondary wastewater treatment in WWTPs, plays a vital role in removing various pollutants, including microplastics, from wastewater. Microplastics enter WWTPs in different forms—films, fibers, microbeads, and debris—each presenting unique challenges for removal. A research by Zhang et al. (2020) highlights that the activated sludge process can effectively remove between 3.6% and 42.9% of microplastics, depending on their type and size—for example, films, which are similar in size to suspended solids (<20 μm), are efficiently captured during treatment. Fibers are easily adsorbed by extracellular polymeric substances (EPS) produced by microorganisms in the sludge, aiding their settlement during secondary sedimentation. The main mechanisms for microplastic removal in activated sludge include adsorption, entrapment within EPS matrices, and aggregation with sludge flocs (Zhang et al., 2020). However, it is important to note that while microplastics can be physically removed through these processes, there is no significant biological degradation occurring within the activated sludge system. Once trapped in the sludge, microplastics are typically disposed of via sludge management processes, such as incineration or landfilling, which can lead to their re-entry into the environment. Despite its effectiveness at physical removal, the activated sludge process is not designed to degrade microplastics. Their persistence in sludge poses a significant challenge for WWTPs, emphasizing the need for improved sludge management strategies to prevent microplastics from being released back into the environment. Future research and technological innovations should aim to increase the microplastic removal efficiency in WWTPs and develop sustainable methods for managing sludge containing microplastics to reduce environmental impact effectively. Addressing these issues will allow WWTPs to better mitigate microplastic pollution and protect water resources globally. Moving forward, combining technological advancements with rigorous environmental management practices will be essential to improve microplastic removal from wastewater. This includes optimizing filtration systems, developing durable membrane materials, and enforcing strict regulations to limit microplastic discharge into aquatic ecosystems. By tackling these challenges comprehensively, WWTPs can greatly contribute to reducing the environmental impact of microplastics and safeguarding water quality for future generations.
19.2.1 Anaerobic–anoxic–aerobic activated sludge method
By combining anaerobic, anoxic, and aerobic zones with different sludge return procedures, the anaerobic–anoxic–aerobic (AAO) activated sludge process is a widely used method for removing organic contaminants from water. The main goal of this technique is to eliminate biochemical oxygen demand (BOD) (Mirghorayshi et al., 2021). Recently, the issue of microplastics (MPs) in wastewater treatment has been addressed by adapting the AAO process (Carr et al., 2016; Hidayaturrahman et al., 2019)—for example, Yang et al. treated actual MPs collected from a Beijing sewage treatment plant using a technique based on the AAO activated sludge process, achieving a removal rate of 54.47% (Yang et al., 2019). Similarly, Jia et al. developed an AAO technique to treat real MPs in wastewater from a Shanghai treatment plant, with a removal rate of 26.01% (Jia et al., 2019). In another study, Jiang et al. treated MPs in wastewater from northern Chinese treatment plants using the AAO process, attaining a removal rate of 16.9% (Jiang et al., 2020). Liu et al. likewise used the AAO process to remove MPs from wastewater at a sewage treatment plant in a specific area of China, achieving a removal rate of 16.6% (Liu et al., 2019). The AAO method’s low cost, simple process flow, and short hydraulic retention time are some of its key benefits. However, despite these advantages, the method also has several notable drawbacks. It produces a large amount of sludge, requires a long treatment time, exhibits low efficiency in removing MPs, and is at risk of bacterial death. The low removal rates observed in multiple tests indicate that the AAO process needs further optimization to enhance its effectiveness in removing MPs.
To address these challenges, future research should focus on screening and domestication of high-quality bacterial communities capable of effectively degrading MPs in various environments. Developing such bacterial communities could greatly enhance the removal efficiency of MPs through the AAO process. Additionally, understanding how MPs interact with different microbial species in the activated sludge system can provide valuable insights for optimizing process conditions and improving MP degradation. Beyond microbial optimization, examining the effects of operational parameters like temperature, pH, and sludge retention time on MP removal efficiency is essential. Adjusting these parameters could lead to substantial improvements in the overall performance of the AAO process. Combining the AAO process with other advanced treatments, such as membrane bioreactors or advanced oxidation processes, could create a synergistic approach for achieving higher MP removal rates. Overall, while the AAO activated sludge process shows promise for MP removal in wastewater treatment, it still needs further refinement to overcome current limitations. By focusing on microbial refinement, operational adjustments, and potential integration with other technologies, researchers can develop a more robust and effective AAO process to combat MP pollution. This is crucial for reducing the environmental impact of MPs and protecting aquatic ecosystems from the harmful effects of plastic contamination.
19.3 Enzymatic degradation of microplastics
A developing field, the enzymatic breakdown of microplastics (MPs) in mild environments has garnered significant interest due to its potential in situ applications (Fecker et al., 2018; Joo et al., 2018). With this approach, MPs are enzymatically degraded or metabolized by native or introduced microbes, transforming them into harmless by-products. According to Han et al. (2017), the optimal enzyme shows remarkable efficiency in MP degradation, and the core concept of biocatalysis emphasizes environmental friendliness. In 2016, Yoshida’s research team discovered the bacterium Ideonella sakaiensis, which uses two enzymes, PETase and MHETase, to efficiently break down polyethylene terephthalate (PET) plastic as its primary carbon source at moderate temperatures. Although these enzymes hold great potential, their practical use in biodegradation processes has been limited by their inherent instability (Yoshida et al., 2016). Son et al. later utilized a thermally stable form of PETase to degrade PET MPs. However, this enzyme was not very durable; it lost much of its activity at 37°C within just 24 h (Son et al., 2019). Since then, other research teams have made significant advances in understanding PETase (Palm et al., 2019; Austin et al., 2018; Liu et al., 2018). Recently, Cui et al. introduced a novel computational design strategy called the Greedy Accumulated Strategy for Protein Engineering (GRAPE). This approach resulted in DuraPETase, a catalytic enzyme demonstrating strong stability and effectiveness in degrading PET MPs. Figures 9A–C illustrate the process, showing PET degradation into smaller, non-toxic molecules, noticeable surface changes on PET MPs observed through scanning electron microscopy (SEM), and confirmation of effective degradation via high-performance liquid chromatography analysis (Cui et al., 2021). Using advanced computational protein design methods, PETase stability was enhanced, leading to a redesigned enzyme with exceptional robustness. This breakthrough opens new possibilities for biodegradable polymers by addressing long-standing issues related to enzyme fragility and instability. However, the high cost and complexity of enzyme preparation still limit widespread use of enzymatic degradation (Liu et al., 2019). Despite these challenges, research continues to advance. Scientists are exploring various strategies to improve enzyme efficiency and stability—for example, recent studies have focused on the structural optimization of PETase and related enzymes to enhance their interaction with MP substrates. By employing techniques such as directed evolution and rational design, researchers aim to develop enzymes that perform effectively across wider environmental conditions and maintain activity over longer periods.
20 Recent strategies for microplastic remediation
In recent years, interest has grown in using advanced methods to effectively remove microplastics (MPs) from contaminated environments. These methods include a variety of technologies such as membrane bioreactors (MBRs), synthetic biology, organosilane-based techniques, biofilm-mediated MP remediation, and strategies involving nanomaterials. To enhance degradation efficiency and environmental sustainability, recent approaches to MP clean-up focus on combining cutting-edge technological methods with biological processes. Advanced oxidation processes (AOPs), such as photocatalytic and electrochemical oxidation, produce reactive oxygen species (ROS) like hydroxyl and sulfate radicals. These processes have shown promise in breaking down MPs into smaller, less harmful compounds (Liu et al., 2020). Photocatalysis, using semiconductors like TiO2 and ZnO, harnesses solar energy to generate ROS, which speeds up the breakdown of MPs in water environments (Nakata and Fujishima, 2012). Similarly, biological methods utilize specialized microbes and fungi to naturally decompose MPs. These organisms form biofilms on MPs, resulting in structural weakening and eventual breakdown into non-toxic substances (Yuan et al., 2020). Combining these approaches offers significant potential for developing effective, eco-friendly MP remediation technologies, advancing sustainable environmental management and pollution control.
20.1 Biofilm-mediated microplastic remediation
Microplastics (MPs) are quickly colonized by microorganisms in aquatic environments, resulting in the formation of durable biofilms on their surfaces. According to Rummel et al. (2017), these biofilms are crucial for breaking down organic pollutants and allowing pollutants to adhere to MPs. Significantly, interactions between MPs and biofilms can alter the physical and chemical properties of the polymer surface, which encourages biological deterioration. Polyethylene (PE) can be colonized and degraded by various bacteria that form biofilms, including Rhodococcus ruber, which can reduce the polymer’s average molecular weight by as much as 21% (Hadad et al., 2005). This indicates that biofilm-mediated biodegradation could improve MP clean-up efforts. Studies show that biofilm formation on MPs can cause considerable surface deterioration, especially in environments with high methane concentrations that promote bacterial aggregation (Faheem et al., 2020). The degradation process produces harmless by-products, such as CO2 and H2O, which are safe for the environment (Sutkar et al., 2023). Using glucose as an external carbon source has been found to speed up MP degradation compared to natural biofilms (Niu et al., 2023). Additionally, biofilms can help MPs attach to environmental pollutants, increasing the risk of MPs in ecosystems (He et al., 2022). To address this, biofilms can be cultivated in controlled conditions before being exposed to MPs. Incorporating biofilm degradation technology into freshwater MP remediation or in situ clean-up efforts can absorb more environmental pollutants as MPs degrade in water. Factors like pH, salinity, temperature, and UV radiation influence biofilm growth on MP surfaces, with a maximum degradation rate of 20% observed under ideal conditions (Faheem et al., 2020). Although biofilm-mediated degradation shows promise, its current progress is limited because microorganisms need time to alter MP properties. MPs are broken down by bacteria producing biofilms through extracellular oxidases and hydrolases, which convert large polymers into smaller oligomers and monomers that are eventually mineralized into CO2 and H2O.
The biofilm degradation process involves four main steps: initial bacterial colonization and changes in MPs’ composition, breakdown of additives and monomers, enzymatic or radical-mediated degradation of MPs, and the final microbial disintegration of the polymer matrix. The second step, which focuses on degrading the polymer additives, is especially important because these compounds hinder the overall degradation process. Microbial degradation of these additives promotes biofilm formation and bacterial adhesion, helping to accelerate further degradation (Sun , 2023). Identifying and cultivating microorganisms that significantly impact MP breakdown can improve biofilm-mediated remediation. Researchers have isolated specific bacteria from different environments, like wastewater, sludge, and sediment, that can degrade MPs—for example, Bacillus sp. strain 27 and Rhodococcus sp. strain 36 from mangrove sediment have demonstrated the ability to degrade polypropylene (PP) MPs, with weight loss and surface irregularities indicating bacterial colonization and damage (Auta et al., 2018). Fungi also play a role in MP degradation, although reports of fungal-mediated breakdown are less common. Recent advances highlight fungi’s potential to degrade MPs under diverse environmental conditions, garnering increasing research interest (Yuan et al., 2020). Despite progress, achieving substantial MP degradation remains difficult. Biofilm-mediated degradation is a promising approach, but more research is needed to address current limitations. By deepening our understanding of microbial interactions with MPs and improving biofilm formation, we can develop more effective and sustainable strategies for MP remediation. This will help reduce the environmental impact of MPs, protecting ecosystems and human health.
20.2 Synthetic biology and organosilane-based techniques
Synthetic biology has become a key tool in exploring the complex relationships between microorganisms and their environment, especially in the area of polymer degradation. Researchers frequently use advanced “omic” techniques to investigate these interactions (Zhou et al., 2023; Ali et al., 2022). A major focus in synthetic biology is engineering metabolic pathways to improve the breakdown of petroleum-based waste (Mukherjee et al., 2022). Challenges remain in fully understanding the wide variety of bacteria that can degrade synthetic polymers and the specific enzymes involved. Future research should focus on unraveling these complexities, aiming to identify resilient microorganisms and understand their enzymatic processes. Progress in environmental microbiology, biotechnology, gene engineering, and protein engineering is vital for overcoming these challenges. Combining approaches such as metabolic engineering, bioinformatics, molecular biology, genetics, and systems biology offers potential for ground-breaking advances in plastic biodegradation.
Conversely, the innovative use of organosilanes introduces a new method that combines physical agglomeration with a water-triggered chemical fixation process, creating strong particles and durable clumps (Dhiman et al., 2023). This technique allows for modifying organic groups to customize surface chemistries for different polymer types and water conditions (Collinson et al., 2017). The versatility and potential uses of organosilanes in water treatment and microplastic removal highlight their promising role in environmental clean-up strategies.
20.3 Membrane bioreactor
The use of membrane bioreactor (MBR) technology has proven highly effective in removing over 90% of microplastics (MPs) from wastewater, surpassing traditional wastewater treatment methods. It is important to recognize that implementing MBRs can be expensive and prone to rapid fouling. Nonetheless, bioreactor technologies for MPs removal ensure high purity by removing contaminants during initial treatment stages, offering potential for re-evaluating and recovering these plastic particles. Recent advances in membrane technologies, especially in nano-filtration membranes, have resulted in hybrid systems combining reverse osmosis and ultrafiltration, which show promising ability to effectively reduce MPs while reducing issues like membrane fouling (Khan et al., 2024; Najmi et al., 2020). MBRs are complex systems that integrate biological catalysts such as bacteria or enzymes within a partitioned structure, operating through a film-based mechanism (Son et al., 2019). These systems have played a key role in tackling water contamination by MPs. The efficiency of MBRs in degrading and removing MPs largely depends on the physical and chemical properties of the pollutants and operational factors such as feed rate and hydraulic retention time (Najmi et al., 2020). Notably, the discovery of Ideonella sakaiensis and its ability to metabolize polyethylene terephthalate (PET) into non-toxic by-products marks a major advancement with immediate implications for MBR use (Najmi et al., 2020). This bacterium uses specific enzymes to break down PET, producing terephthalate and ethylene glycol. In-depth studies of enzymatic pathways in species like Euphrasia superba have clarified their role in reducing MP size, paving the way for incorporating these enzymes into MBRs to improve PET-MPs biodegradation. Therefore, MBR technology shows great promise as a practical approach for bioremediation of water contaminated with MPs.
20.4 Nanomaterial-enabled strategies for microplastic remediation
Recent advances in nanomaterial research have greatly enhanced their role in wastewater treatment (WWT), especially in microplastic (MP) remediation. Nanomaterials provide energy-efficient options for treatment, improving overall effectiveness. New photocatalyst structures have been developed to maximize light absorption by reducing charge-carrier recombination and increasing light-active sites, enabling photocatalytic activity under visible light instead of just UV light (Jiang et al., 2022; Zhang et al., 2023). These improvements result from nanoscale design changes or doping photocatalysts with various nanomaterials, expanding their use (Ch-Th et al., 2021; You et al., 2021). In addition to photocatalysis, nanomaterials are successfully used in magnetism-based separation methods for MP removal, offering a simple and cost-effective solution (Shi et al., 2022). This process involves magnetizing MPs with nanoparticles like magnetite (Fe3O4), making it easier to extract them from water via magnetic recovery. Magnetized MPs of different types and sizes—including polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyethylene terephthalate (PET)—have been effectively removed from various water sources, demonstrating the versatility and efficiency of Fe3O4-based nanomaterials in MP clean-up.
The effectiveness of Fe3O4 nanoparticles in removing MPs depends on factors like particle concentration and the physicochemical properties of the MPs and surrounding medium—for example, electrostatic interactions between positively charged Fe3O4 nanoparticles and negatively charged MPs in seawater boost adsorption and magnetization, thereby enhancing overall removal efficiency (Shi et al., 2022). Notably, while magnetization is effective across various MP types, PET has relatively lower removal rates due to its weaker hydrophobic nature compared to other plastics (Sajid et al., 2023). The use of nanomaterials extends beyond magnetism-based methods and photocatalysis to include hybrid approaches and advanced membrane technologies. Hybrid nanomaterials with hierarchical designs, combining top-down and bottom-up approaches, synergistically improve MP removal compared to bulk nanomaterials used alone (Brakat et al., 2021). These designs leverage the high surface area and unique structural properties of nanomaterials to maximize uptake capacity, enabling larger-scale treatment before material saturation occurs. Although there have been significant advances in applying nanomaterials for MP clean-up, challenges such as membrane fouling in filtration processes remain. High-performance membranes incorporating nanomaterials offer promise for overcoming these challenges and increasing throughput in MP removal from wastewater (Kusworo et al., 2022). Photocatalytic membranes, a new type that combines physical separation with chemical breakdown functions, exemplify this progress. These membranes effectively capture and degrade MPs within a single device, reducing secondary pollution risks linked to traditional membrane filtration methods (Gokulakrishnan et al., 2021).
The development of photocatalytic membranes marks a significant shift in WWT, providing dual-action capabilities to target a wide range of water pollutants beyond MPs. Their effective use in treating emerging chemicals and micropollutants highlights their potential in comprehensive water clean-up strategies (Chabalala et al., 2021). Incorporating photocatalysts into membrane structures not only boosts MP removal efficiency but also expands the range of applications to effectively address various environmental contaminants.
20.5 Biodegradation and emerging remediation strategies
The build-up of persistent plastics like polyethylene (PE), polypropylene (PP), and polystyrene (PS) in the environment calls for innovative biodegradation and clean-up strategies. Current research targets both naturally occurring and engineered microbes, including Bacillus and Pseudomonas, for breaking down plastics. These microbes have enzymes capable of degrading complex polymers—for example, Pseudomonas species are known to break down various hydrocarbons and xenobiotic compounds, which paves the way for their use in plastic waste breakdown (Kopecká et al., 2022). Several Bacillus strains from different environments have likewise shown promising plastic degradation abilities in normal conditions (Ramos et al., 2024), (Kyaw et al., 2012). Algae such as Spirulina and Chlamydomonas are also being studied for their roles in bioplastic production and potential direct involvement in breaking down plastics (Iyer et al., 2023). Alongside natural microbial degraders, synthetic biology provides a powerful way to create custom microbial communities. This method allows for the design of microbes with improved features like higher enzyme activity and stability, essential for efficient polymer breakdown. Engineered communities can combine natural strains and genetically modified ones to optimize pathways for plastic degradation, as shown by studies on novel community groups producing valuable degradation products (Schaerer et al., 2023; Cao et al., 2022). Additionally, synthetic biology tools enable precise control of enzyme production, metabolic flow, and communication between species, creating strong, adaptable communities that can survive environmental stresses common in biodegradation (Salinas et al., 2023). With genetic tools, scientists can direct the evolution and enhancement of microbial groups to effectively break down resistant plastics (Lee et al., 2020). Hybrid treatment systems also show promise for plastic waste clean-up, combining microbial methods with chemical oxidation to improve degradation and scale-up. Chemical oxidation—using oxidants or UV light—prepares the plastic by adding reactive groups to the polymer chains, making them more vulnerable to microbial enzymes (Kyaw et al., 2012). This helps break large plastics like PE, PP, and PS into smaller, more manageable pieces that microbes can further decompose (Ramos et al., 2024). These hybrid systems combine quick chemical processing with eco-friendly biological treatment, reducing secondary pollution and boosting degradation results (Cao et al., 2022). Their scalability is especially promising for industrial use, where large amounts of plastic waste need fast, effective treatment under changing environmental conditions.
20.5.1 Bacterial-based plastic degradation: an emerging solution for mitigating plastic pollution
The global issue of plastic pollution has driven the search for innovative strategies to reduce its environmental impact. Among these, bacterial-driven plastic degradation has become a prominent approach, utilizing bacteria’s natural ability to break down plastics through enzymatic processes. Bacteria’s capacity to produce extracellular polysaccharides and form biofilms on plastic surfaces has been well documented, especially in wastewater treatment where these biofilms help facilitate plastic breakdown. This section offers a detailed review of recent developments in bacterial plastic degradation, emphasizing the potential of specific bacterial species, the mechanisms behind their ability to degrade plastics, and the implications for environmental biotechnology and waste management. Bacterial degradation of microplastics (MPs) is an increasingly studied area, particularly concerning specialized enzymes that target plastic polymers. Notably, species such as Vibrio, Campylobacter, and Arcobacter have been widely researched for their capacity to degrade MPs using enzymes like PET hydrolase (PETase) and mono-(2-hydroxyethyl) terephthalate hydrolase (MHETase). The effectiveness of these bacteria in breaking down plastics is enhanced by the large surface-to-volume ratio of plastic particles, which promotes bacterial colonization and enzymatic action. This process is further sped up by biofilm formation, which not only helps bacteria attach but also creates microenvironments that support Enzymatic activity accelerates plastic degradation. A key development in this area is identifying specific bacterial strains capable of breaking down various plastics—for example, Stenotrophomonas panacihumi has shown the ability to enzymatically decompose polypropylene (PP) into both low- and high-molecular-weight fractions over 90 days, highlighting its potential for practical waste management applications (Ru et al., 2020). Similarly, Aneurinibacillus spp. and Brevibacillus spp. have demonstrated effectiveness in degrading polyethylene (PE) and PP, achieving significant weight reductions between 37.2% and 45.7% after 140 days of incubation (Skariyachan et al., 2016). These results emphasize the potential of bacterial enzymes to reduce plastic pollution and underscore the importance of understanding the kinetics of bacterial degradation. Beyond PP and PE, other plastics such as low-density polyethylene (LDPE) have also been targeted. Research indicates that Pantoea sp. and Enterobacter sp. can notably decrease the weight of LDPE strips and pellets by 81% and 38%, respectively, after a 120-day incubation (Skariyachan et al., 2016). Further studies have identified Bacillus amyloliquefaciens strains BSM-1 and BSM-2 as effective in degrading plastic wrappers (PW), which aid in breaking down LDPE (Das et al., 2015). The degradation process of PW involves measuring methane (CH4) and carbon dioxide (CO2) emissions during anaerobic digestion and mineralization, highlighting the role of organic compounds and heteroatoms like nitrogen, oxygen, and sulfur in boosting enzymatic and hydrolytic activities (Kida et al., 2022; Mammo et al., 2020).
Bacterial degradation of microplastics (MPs) has become a promising biotechnological method to fight plastic pollution, focusing on biofilm-forming bacteria that can attach to plastic surfaces and improve enzymatic breakdown. Species like Erythrobacter sp. and Alcanivorax borkumensis have shown strong potential in breaking down low-density polyethylene (LDPE), helping to remove MPs from aquatic environments (Yang et al., 2020). Biofilms not only help microbes stick but also create special microenvironments that boost enzyme activity, speeding up degradation. Practical use is often limited by strict environmental needs, complex growth conditions, and long degradation times (Park et al., 2021). Advances in genetic engineering and synthetic biology help by improving the adaptability and efficiency of bacteria. Engineered microbes such as Stenotrophomonas panacihumi have shown promising results in breaking down polypropylene (PP) over 90 days (Ru et al., 2020), while Aneurinibacillus spp. and Brevibacillus spp. have effectively reduced the weights of polyethylene (PE) and polypropylene (PP) (Skariyachan et al., 2016). Bacteria from MP-rich habitats—including Rhodococcus 36, Bacillus 27, and Enterobacter asburiae YT1— likewise show notable degradation abilities (Auta et al., 2017; Yang et al., 2014). Despite these advances, ensuring biosafety, simplifying cultivation, and scaling up bioreactor systems remain key challenges. Combining microbiology, environmental science, and engineering is needed to develop efficient, safe, and scalable bacterial degradation technologies (Denaro et al., 2020; Janssen et al., 2002). Moreover, raising public awareness, gaining regulatory support, and reducing plastic use are critical to making bacterial degradation a mainstream part of broader plastic pollution solutions. Ultimately, improving microbial teams and using genetic tools could lead to a game-changing, nature-inspired solution to microplastic pollution.
20.5.2 Fungal-based plastic degradation: a promising approach to mitigating plastic pollution
Plastic pollution, especially from polyethylene (PE), is a widespread environmental issue affecting both land and water ecosystems. The presence of plastic waste in various water sources, such as streams, rainwater, surface waters, and oceans, underscores the urgent need for effective degradation methods. Among emerging solutions, fungal-based biodegradation shows significant promise in tackling this environmental problem. Certain fungi, like Fusarium sp., Aspergillus japonicus, and A. flavus, have proven capable of degrading low-density polyethylene (LDPE), achieving plastic weight reduction of 30% to 36% when used as a carbon source (Jyoti et al.). These fungi utilize a range of microbial enzymes that help break down plastic polymers, offering an eco-friendly way to reduce plastic waste. The fungi’s enzymatic toolkit includes cutinases, lipases, and esterases, which are essential for hydrolyzing polyethylene adipate and polycaprolactone—key steps in plastic degradation. These enzymes can cleave ester bonds within plastic polymers, such as polyethylene wrappers (PW), initiating degradation processes that gradually diminish plastic mass. Various fungal species, including Achromobacter sp., Rhizopus arrhizus, R. delemar, and Candida cylindracea, have been identified for their production of lipases and esterases, enzymes vital for PW biodegradation (Iram et al., 2019). These enzymes not only start the breakdown but also help transform complex polymers into simpler, more biodegradable substances. Beyond enzymatic pathways, fungi also produce lignin-degrading enzymes like laccases and peroxidases, which are crucial for breaking down LDPE in both aerobic and anaerobic conditions (Ali et al., 2023). These enzymes catalyze the oxidative degradation of plastic polymers, resulting in simpler compounds such as methane (CH4), carbon dioxide (CO2), and water (H2O). This process reduces plastic waste mass and channels the degradation products back into the natural carbon cycle, supporting environmental sustainability.
Fungal-based biodegradation presents a promising opportunity for reducing microplastics (MP), utilizing the enzymatic and metabolic diversity of naturally occurring fungal communities. Notably, fungi isolated from termite guts—including Meyerozyma caribbica, M. guilliermondii, and Sterigmatomyces halophilus—have demonstrated the ability to break down low-density polyethylene (LDPE), producing metabolites such as alcohols, alkanes, aldehydes, and fatty acids. Pestalotiopsis microspora can likewise degrade polyurethane even under anaerobic conditions, thanks to its invasive mycelium and secretion of polymer-degrading enzymes. These discoveries highlight fungi’s potential in environmental biotechnology, waste valorization, and circular economy initiatives. Improving fungal degradation requires a deeper understanding of enzyme activity, environmental adaptability, and genetic enhancement. Employing synthetic biology could create engineered “super-degrader” strains suited for various types of polymers. Additionally, developing bioreactor systems to scale fungal-based plastic degradation provides a practical approach for large-scale use. As fungal biotechnology aligns with sustainable waste management, it offers an innovative, scalable, and eco-friendly solution to the global MP pollution crisis.
20.5.3 Algal-based plastic degradation: a promising avenue for sustainable waste management
The increasing concern over plastic pollution, especially in aquatic environments, has driven interest in new biodegradation methods, with algal-based approaches emerging as a highly promising option. Although research in this area is still developing, several algae species, such as Chlorella and Scenedesmus, have shown significant potential in breaking down polyethylene (PE) and polyethylene terephthalate (PET) waste. These studies highlight the potential of algae as eco-friendly agents for plastic degradation, providing a sustainable solution to the growing environmental crisis caused by plastic waste (Kumar et al., 2017; Moog et al., 2019; Sarmah et al., 2019; Khoironi et al., 2019; Sanniyasi et al., 2021). The process of algae colonizing plastic surfaces is aided by water, sunlight, and essential nutrients, creating ideal conditions for biofilm formation (Table 12). This biofilm acts as the foundation for subsequent degradation, where algae interact with the plastic and break it down into simpler compounds. Microalgae’s ability to perform photoautotrophic respiration—relying only on sunlight and inorganic nutrients—further boosts their appeal for biotechnological uses. Unlike bacteria and fungi, which might need organic carbon sources and could release toxins, microalgae present a safer and more sustainable option for plastic degradation (Abdelfattah et al., 2013; Abdelfattah et al., 2022; Mastropetros et al., 2022; Ali et al., 2016).
Certain non-toxic algal species, such as those thriving in polluted water environments, have shown a significant ability to stick to plastic surfaces. These species, through the production of exopolysaccharides, support strong biofilm formation, which is essential for the subsequent enzymatic breakdown of plastic. Enzymes like lipases, esterases, and cellulases, produced by these algae, interact with plastic polymers, starting the process of breaking down and shredding the material. This enzymatic activity is especially effective in degrading plastics like PE and PET, which are otherwise resistant to standard degradation methods (Hossain et al., 2023). One of the most hopeful discoveries in this field is that microalgae can colonize PET surfaces and secrete PETase, an enzyme specifically involved in PET degradation. The release of PETase by microalgae not only speeds up PET breakdown but also highlights the potential of algae in broader plastic waste management strategies. Additionally, advances in genetic engineering have further improved the ability of algae to break down plastics—for example, Chlamydomonas reinhardtii has been genetically modified to produce PETase, demonstrating its effectiveness in PET degradation studies. This genetically enhanced algae strain marks a major step forward in developing algal-based solutions for plastic pollution.
Surface degradation of plastics by algae is shown by the formation of pits and cavities, clearly indicating enzymatic action and polymer breakdown. The enzymes produced by microalgae are crucial in this process, breaking down complex polymer structures into smaller, more manageable components. Despite these promising findings, much remains to be understood about the biochemical pathways involved in algal plastic degradation. Further research is necessary to uncover these pathways, improve degradation processes, and turn laboratory results into practical applications (Kumar et al., 2017). Although the potential for algal-based plastic degradation is evident, the field faces several challenges that need to be addressed to realize its full benefits. One major concern is the efficiency of degradation in natural environments, where factors like temperature, light, and nutrient availability can greatly affect the process. Additionally, the economic feasibility of scaling up these processes for large-scale use is still uncertain. The ecological impacts of introducing genetically modified algae into natural ecosystems also need careful consideration and further investigation.
Algal-based plastic degradation is quickly emerging as a transformative approach to reduce microplastic (MP) pollution, combining ecological resilience with biotechnological innovation. Modern viewpoints highlight the combined potential of omics technologies—genomics, proteomics, and metabolomics—in understanding the complex metabolic and enzymatic networks involved in plastic breakdown in algae (Ali et al., 2016). These insights help in engineering algal strains with improved efficiency and environmental adaptability through synthetic biology. The natural enzymes and diverse metabolic pathways of algae make them excellent candidates for sustainable MP clean-up, especially when used in groups or with other microbes working together. Beyond laboratory success, algal degradation complements circular economy principles by turning plastic waste into bioresources while reducing ecological harm. This approach shifts from passive waste disposal to active ecological reuse. While still early in development, ongoing research into algal degradation pathways offers great potential for scalable, eco-friendly MP reduction, paving the way toward cleaner aquatic environments and innovative biotech solutions.
21 Strain breeding technology
Among the many strategies for reducing microplastic (MP) pollution, biological degradation has become a sustainable and environmentally friendly alternative to traditional physical and chemical methods, which often produce harmful by-products or residual toxicity (Geyer et al., 2017; Uheida et al., 2021; Kaur Brar et al., 2023). Key to this biological approach is the development of microbial strain-breeding technology, which improves naturally occurring microorganisms for better MP biodegradation. MP biodegradation occurs in three main stages: physical and chemical changes of the polymer surface, enzymatic breakdown into smaller molecules like oligomers and monomers, and microbial uptake of these substances into biomass, releasing carbon dioxide and water (Emadian et al., 2017). The natural process is slow and inconsistent, so stronger strains with better degradation abilities are needed. Strain-breeding techniques—from natural selection under environmental stress to induced mutagenesis and advanced genetic engineering—allow precise editing of microbial genomes to increase enzyme production and degradation pathways (Park et al., 2021). Genetically engineered Pseudomonas and Bacillus strains, for example, have shown significantly faster degradation rates across various MP types. Additionally, synthetic biology now makes it possible to design microbial communities with combined degradation functions, enabling the breakdown of complex MP mixtures (Kasmuri et al., 2022). Despite obstacles related to environmental adaptation, biosafety, and large-scale implementation, microbial strain-breeding offers great potential for real-world MP clean-up. Future efforts should focus on improving hybrid breeding techniques, adding synthetic metabolic pathways, and meeting regulatory standards for field use. As a ground-breaking mix of biotechnology and environmental science, strain-breeding technology demonstrates the potential to turn MP management from a global crisis into a solvable problem.
21.1 Natural breeding in MP-degrading microorganisms
In the pursuit of sustainable solutions to microplastic (MP) pollution, natural strain-breeding of microorganisms has become a powerful biotechnological method, based on evolutionary principles and ecological adaptability. Natural breeding depends on spontaneous genetic mutations and the environmental selection of microbial strains capable of using MPs as a carbon source, often isolated from MP-contaminated environments (Adachi et al., 2022). Several studies have successfully identified such strains from marine, terrestrial, and insect gut habitats—for example, Bacillus subtilis H1584 from the Arabian Sea achieved 1.75% degradation of polyethylene (PE) over 30 days (Sarkhel et al., 2019), while Exiguobacterium a-1 from Bohai Bay showed 9.20% degradation of polypropylene (PP) in 80 days (Sun, 2023). Notably, Vibrio sp. PD6 and Aspergillus sp., both isolated from saline water, degraded plastic bottle polymers by 35% and 22%, respectively, in 6 weeks (Sarkhel et al., 2019).
Terrestrial microbes also show significant potential. Auta et al. (2017) isolated Bacillus cereus and Bacillus gottheilii from soil, which are effective against PE, PET, and PS, while Bacillus brevis achieved 19.80% PE degradation in 35 days (Tiwari et al., 2023). Fungal species such as Streptomyces (Soleimani et al., 2021) and Pseudomonas aeruginosa V1 (Pathak & Navneet, 2023) also demonstrate superior MP-degrading capabilities. Insect guts have become unique microbial reservoirs, where Ehommaechei LG3 degraded PS under both anaerobic and aerobic conditions (Kang et al., 2023), and Aspergillus flavus PEDX3 and Bacillus sp. YP1 from wax moth and meal moth guts showed degradation of HDPE and PE, respectively (Zhang et al., 2023; Yang et al., 2014). Natural selection has limitations due to low mutation rates, DNA repair mechanisms, and lengthy screening processes (Xiang et al., 2023). To address this, targeted strategies such as using MPs as the sole carbon source in minimal media can improve screening efficiency. Additionally, mutagenesis and genetic engineering provide methods to speed up strain development and optimize degradation traits. Engineered Bacillus and Pseudomonas strains, along with synthetic microbial consortia, show promise for increasing MP degradation efficiency.
21.2 Genetic engineering breeding for MP degradation
Genetic engineering, defined as the manipulation of an organism’s genome using biotechnology and modern molecular techniques (Kuzma et al., 2016), has great potential for microbial strain breeding aimed at improving MP degradation. This technology involves creating genetically engineered bacteria by selectively adding the necessary genetic information into microbial cells to enable targeted breeding of microorganisms. Unlike traditional methods, genetic engineering can precisely modify specific DNA sequences in microorganisms. By altering these sequences, it is possible to insert, delete, or replace nucleotides in particular genes, resulting in desired traits (Viana et al., 2019).
Genetic engineering techniques in microbial breeding usually involve two approaches. The first involves inserting new genes or sequences into microbial cells to achieve specific expression traits. The second approach, targeted mutagenesis, replaces particular receptor regions with effectively mutated gene segments. This process can greatly enhance certain traits in the desired strain by using wild-type copies of bacterial genes. The main goal of genetic engineering in this context is to identify, modify, and replicate genes involved in MP breakdown. Techniques such as antisense RNA technology, polymerase chain reaction (PCR), site-directed mutagenesis, and employing suitable hosts like Escherichia coli are essential to reach this goal (Lim et al., 2022)—for example, Yoon et al. (2012) reported that Pseudomonas sp. E4 could degrade polyethylene (PE). By expressing its alkane hydroxylase gene in E. coli, the host cells gained the ability to mineralize low-molecular-weight PE, resulting in the recombinant E. coli mineralizing 19.3% of the carbon to CO2 over 80 days.
Bollinger et al. (2020) cloned a gene for polyesterhydrolase (a PET hydrolase) from Pseudomonas aestuansigri and inserted it into E. coli to produce the enzyme. This enzyme could only degrade amorphous PET film at 30°C and not commercial PET bottle films. Through site-directed mutagenesis, a variant, PE-H (Y250S), was developed, showing increased activity and capable of hydrolyzing PET from commercial bottles. Similarly, Ribitsch et al. (2015) discovered that cutinase produced by Thermobifida cellulosilytica (Thc_Cut1) could degrade PET. By fusing this enzyme with a hydrophobic enzyme, they boosted the PET hydrolysis rate more than 16-fold. In another study, Austin et al. (2018) obtained a PETase from Ideonella sakaiensis 201-F6. After protein engineering, PETase’s degradation performance improved significantly. Ma et al. (2018) further improved PETase efficiency by targeting six key residues near the substrate-binding groove, creating mutants with higher activity. The most active mutant showed a PET film weight loss rate of 22.5 mg/μmol L⁻¹ PETase per day. These advances highlight the potential of genetic engineering to enhance MP-degrading abilities. Despite notable laboratory successes, practical applications still face challenges. The efficiency seen in controlled settings does not always translate to real-world conditions. Additionally, genetic engineering raises environmental and ecological safety concerns, including risks of gene pollution, gene loss, and impacts on biodiversity. Legal policies further limit the use of genetically modified microorganisms in natural environments, creating hurdles for applying this technology to MP management.
21.3 Mutation breeding for enhanced microplastic degradation
Microbial mutation breeding uses physical or chemical agents to alter the genetic material of microorganisms, inducing mutations in their genes through artificial means. This process aims to modify their genetic structure and function, helping identify mutant strains with desirable traits from a diverse pool. Mutations are rare, reversible, and often recessive, making them the primary source of genetic diversity in any organism. Mutation breeding is a simple, rapid, selective, and versatile technique, and it is the most common method with the highest success rate in strain cultivation. Despite success in various fields, its use in selecting and breeding MP-degrading bacteria remains limited. This gap presents an opportunity to utilize mutagenesis techniques for developing efficient MP-degrading strains. By exposing microorganisms to mutagens such as radiation or chemical agents, researchers can induce a wide range of genetic variations. These variations can be screened to find strains with improved MP degradation capabilities—for example, using ultraviolet (UV) radiation or chemical mutagens like ethyl methanesulfonate (EMS), scientists can induce mutations that create novel enzymes or improve existing metabolic pathways involved in MP breakdown. These mutant strains can be further refined through cycles of mutagenesis and selection, gradually boosting their efficiency and resilience in degrading MPs.
Furthermore, advances in high-throughput screening methods and molecular biology tools can significantly speed up the identification and analysis of beneficial mutations. Techniques such as whole-genome sequencing and transcriptomic analysis enable researchers to identify specific genetic changes responsible for enhanced MP degradation. This knowledge can guide further genetic modifications, either through additional mutagenesis or by employing genetic engineering to directly introduce or strengthen key genes. In summary, mutation breeding provides a promising approach to develop highly efficient MP-degrading bacteria. By harnessing the genetic diversity generated through mutagenesis and applying advanced screening and molecular techniques, researchers can discover and optimize strains with superior MP-degrading abilities. When combined with other biotechnological advancements, this method has great potential for addressing the global challenge of MP pollution in a sustainable and effective way.
21.4 UV mutagenesis for microplastic-degrading bacteria
UV mutagenesis was among the earliest techniques used for biological mutagenesis. The UV spectrum aligns with the absorption spectrum of nucleic acids in cells, with DNA absorbing UV light most effectively at around 250 nm. When the purines and pyrimidines in DNA and RNA absorb UV light, it leads to the formation of pyrimidine dimers (Chatterjee et al., 2015). These dimers cause distortion of the DNA double-helix, disrupting normal base pairing. This disruption can cause errors during DNA replication and transcription, resulting in mutations or even cell death. Additionally, the formation of dimers can block the unwinding of the double-helix, further affecting DNA replication and transcription.
UV mutagenesis can induce various genetic changes in bacteria, including base substitutions, transversions, frameshift mutations, or deletions, thereby causing mutagenesis. In strain-breeding technology, Watanabe et al. (2015) successfully produced a mutant strain of C. flavus GB-1 DMC1 via UV mutagenesis, which showed a degradation ability for biodegradable plastics that was more than 2.5 times higher than that of the parental strain GB-1. This highlights the potential of UV mutagenesis to boost bacteria’s ability to degrade polymers. However, UV mutagenesis also has notable drawbacks. One major issue is photoreactivation, where cells can repair UV-induced damage when exposed to visible light, reversing mutations. Furthermore, UV-induced mutations can cause genetic instability, complicating consistent application of the technique. Because of these issues, UV mutagenesis is less commonly used than other mutagenesis methods. Despite its limitations, UV mutagenesis remains a useful tool for microbial strain improvement. It induces a broad spectrum of genetic variations, providing a basis for selecting strains with enhanced properties. Advances in molecular biology and high-throughput screening can further improve the detection and application of beneficial mutations caused by UV light. Therefore, while UV mutagenesis may not be the first choice for all applications, it still plays a role in developing microorganisms capable of tackling environmental challenges, such as microplastic degradation.
21.5 Laser radiation mutagenesis for microplastic-degrading bacteria
Laser radiation mutagenesis is an advanced mutagenesis technique distinguished by its high energy density, specific concentration, monochromaticity, and excellent directionality. When organisms are exposed to a certain amount of laser light, the energy can be directly or indirectly deposited onto their DNA, inducing genetic mutations. This process involves the photodissociation, decomposition, and free radical reactions of biomacromolecules, leading to distortions in DNA molecules or chromosomes and promoting the development of mutant traits (Feng et al., 2023). By applying heat, light, pressure, and electromagnetic fields through laser radiation, this technique stimulates DNA, RNA, and proteins, resulting in the formation of various substances (Lu et al., 2022; Zhu et al. (2020)).
Laser radiation mutagenesis has been used in microorganism breeding to improve their functions—for example, Lotfabad et al. (2010) showed that the mutant strain MR01-C, created by γ-irradiating the native strain Pseudomonas aeruginosa MR01, produced more rhamnolipids with higher activity. Rhamnolipids greatly enhance polystyrene (PS) degradation, showing this mutagenesis method can help find strains with better microplastic breakdown abilities. The precise and targeted nature of laser radiation mutagenesis makes it a promising tool for improving microbial strains. It creates a variety of mutants with potential for better degradation, helping to identify and grow highly effective microplastic-degrading bacteria. This is especially important given the rising problem of microplastic pollution. However, like all mutagenesis techniques, laser radiation mutagenesis needs careful adjustment and screening to ensure the mutations are stable and effective. Advances in molecular biology and high-throughput screening will boost this method’s use, leading to stronger and more effective microbial strains for real-world environmental solutions.
21.6 Microwave mutagenesis for enhancing microplastic-degrading bacteria
Microwave mutagenesis is a new approach in microbial breeding, known for its simplicity, safety, ease of use, and lack of toxic by-products (Woo et al., 2000). Unlike traditional methods like UV or chemical mutagenesis, microwave mutagenesis provides benefits such as quickly producing many mutants at low cost, making it highly useful for large-scale microbial strain improvement. Microwaves are high-frequency electromagnetic waves that cause rapid vibration of polar molecules like water, proteins, nucleic acids, fats, and carbohydrates (Kirschvink et al., 1996; Chen et al., 2006). This vibration creates intense friction within the cell, especially around DNA, breaking hydrogen bonds and other chemical forces that hold DNA together. As a result, microwave radiation can cause chromosomal changes and mutations, leading to variants with different genetic traits. The mutagenic mechanism of microwave radiation involves stimulating polar molecules to oscillate, which damages DNA and causes mutations that can be beneficial for specific traits (Woo et al., 2000). This method has strong potential in microbial breeding because it can overcome the limitations of UV mutagenesis’s photo-repair and avoid the toxicity of chemical mutagens. Although promising, microwave mutagenesis has not yet been widely studied for selecting and breeding microplastic-degrading bacteria. Because it effectively creates genetic diversity and has a simple setup, microwave mutagenesis still offers a practical way to generate mutants capable of breaking down microplastics efficiently. Future research can explore this technology to improve the biodegradation abilities of microbial strains, aiding sustainable solutions to reduce microplastic pollution in various environments.
21.7 Atmospheric room temperature plasma mutagenesis: a modern tool for microbial breeding
Atmospheric room temperature plasma (ARTP) mutagenesis has become a cutting-edge technique in microbial breeding, utilizing atmospheric room temperature plasma to induce genetic mutations. Plasma generated in ARTP systems contains many chemically active particles capable of significantly impacting cellular structures and genetic material (Hua et al., 2010). These particles cause DNA damage, prompting cells to activate repair mechanisms that can lead to stable genetic mutations (Ottenheim et al., 2018). This method is known for its low cost, ease of use, high mutation rate, and genetic stability, making it a versatile tool for mutagenesis across various microorganisms, including bacteria, fungi, and microalgae (Xiang et al., 2023). The application of ARTP mutagenesis in microbial breeding has mainly focused on improving traits like metabolic efficiency, stress tolerance, and product synthesis in industrial microorganisms. However, its potential for selecting strains capable of degrading microplastics (MPs) remains largely unexplored. Given ARTP’s effectiveness in inducing genetic diversity and its straightforward experimental setup, researchers are encouraged to explore its use in increasing the efficiency of MP degradation by microbial strains. Currently, there is a significant lack of research using ARTP mutagenesis specifically for developing MP-degrading strains. This gap presents an exciting opportunity for future studies aimed at harnessing ARTP’s mutagenic capabilities to create microbial variants with enhanced ability to break down and metabolize MPs. By exposing microbial populations to ARTP treatment and then screening for variants proficient in MP degradation, researchers could accelerate the development of environmentally beneficial microbial strains.
In conclusion, genetic mutation is central to microbial diversity, and artificial mutagenesis techniques like ARTP provide a quick and effective way to induce beneficial genetic changes in microorganisms. The random nature of mutagenesis highlights the need for thorough screening processes to identify mutants with desired traits while removing those with harmful characteristics. Looking ahead, combining ARTP mutagenesis with targeted screening methods offers promise for advancing microbial breeding, especially in developing sustainable solutions for MP pollution reduction. By leveraging the strengths of ARTP mutagenesis—such as its high mutation rate, simplicity, and affordability—researchers can work toward creating new microbial strains to tackle urgent environmental issues like microplastic contamination. As research progresses, optimizing ARTP protocols and expanding its applications will be key steps in unlocking its full potential in microbial biotechnology.
22 Mechanisms of plastic degradation
Microplastics (MPs) originate from the breakdown of larger plastics and are carried through wastewater discharge, eventually entering freshwater, marine systems, or municipal effluents (Barchiesi et al., 2021; Wu et al., 2019). Due to their small size, MPs evade conventional filtration systems, posing risks to both aquatic and terrestrial organisms. Secondary MPs result from environmental stress, biological degradation, and UV exposure, which break down larger plastics (Ali et al., 2023). Coastal waves further facilitate this process through abrasion. Abiotic degradation of plastics involves UV radiation, oxidation, thermal impacts, hydrolysis, and wave action (Dimassi et al., 2022). In aquatic environments, biodegradation rates are minimal, especially at the benthic level, due to lower microbial populations. Shallow waters host diverse microbial communities crucial for biodegradation (Niu et al., 2021).
“Co-metabolism” describes a microbiological process where microorganisms degrade organic compounds using carbon and energy from different substrates, increasing microbial enzyme activity and improving substrate breakdown efficiency (Raza et al., 2023). The biodegradation process of plastic waste (PW) is affected by factors such as polymer type, functional groups, molecular weight, chemical additives, production method, and environmental conditions like temperature, pH, oxygen concentration, and salinity. The stages of PW biodegradation include biodeterioration, biofragmentation, assimilation, and mineralization. Extracellular enzymes play a crucial role in enhancing contaminant accumulation on plastic surfaces, promoting microbial growth and speeding up biodeterioration (Ali et al., 2021)—for example, fungi secrete laccase, which aids in the oxidative breakdown of high-density polyethylene (HDPE) structures (Othman et al., 2021). Laccase-producing Cochliobolus sp. has been shown to alter the physical and chemical properties of PVC, leading to erosion and the incorporation of carbonyl groups on the plastic surface (Sumathi et al., 2016).
Depolymerases and hydrolases are extracellular enzymes secreted by microorganisms that break down complex molecules into simpler components. Certain chemoorganotrophic microorganisms produce organic acids and other chemicals that greatly influence the entire biodeterioration process (Lepcha et al., 2023). Microbial cells can absorb the resulting monomers into their structure, promoting growth and mineralization, which leads to the production of CH4, CO2, and H2O under anaerobic conditions, or CO2 and H2O in aerobic environments. Fungal enzymes, such as chitinase from Rhizopus oryzae, play a significant role in degrading PE and PET materials. Fungi attach to plastic surfaces with proteins and polysaccharides, penetrate, and modify the polymer structure (Seenivasagan et al., 2022; Temporiti et al., 2022). Adding supplementary carbon sources to culture media can increase biodegradation efficiency—for instance, Aspergillus flavus PEDX3, isolated from the gut of Galleria mellonella, facilitated PE-MP breakdown via laccase-like multicopper oxidases, decreasing molecular weight after 28 days (Zhang et al., 2020). This highlights the potential of microbial communities and specific conditions to greatly enhance plastic degradation processes.
23 Challenges, future research, and research limitations
This paper critically analyzes previous research on identifying and managing microplastics (MPs). Despite significant progress, these methods still have notable limitations. Their limited applicability makes it hard to adequately describe and treat MPs with a single approach. As waste output and environmental contamination increase, detecting and disposing of MPs will become even more challenging. To effectively tackle these issues, a deeper focus on MP sources, transfer pathways, degradation intermediates, toxicity, and other key factors is essential.
23.1 Challenges and suggestions
Microplastic (MP) pollution presents complex challenges to environmental sustainability due to its diverse origins, environmental persistence, and unclear fate. Despite progress in detection and removal methods, upstream mitigation remains insufficiently emphasized. Proactive strategies in plastic production—such as redesigning polymers to reduce fragmentation and using alternative biodegradable materials—are crucial to prevent MP formation at the source. The cycling of MPs through air, water, and soil extends their ecological impact, requiring intervention at key transition points like wastewater treatment plants and stormwater outfalls. Current treatment methods may inadvertently produce toxic by-products, including persistent organic pollutants, emphasizing the urgent need for greener, non-toxic alternatives. Concerns also exist about the physicochemical changes of MPs during treatment, which could produce more harmful nano-sized fragments. A standardized framework to evaluate the effectiveness and safety of MP removal technologies is urgently needed. Although biodegradable MPs are promoted as sustainable, they can still fragment and release additives under different environmental conditions, with unknown effects on microbiota and food webs. A genuinely sustainable MP management approach must integrate prevention, eco-friendly removal, transformation research, and interdisciplinary collaboration to ensure mitigation efforts do not unintentionally cause further ecological harm.
23.2 Policy measures and approaches for mitigating microplastic pollution
Mitigating microplastic pollution requires a multifaceted approach that combines various policy tools and strategies. Establishing international agreements and conventions can set common goals and standards for reducing microplastic pollution (Nikpay and Roodsari, 2024). Promoting the exchange of information and best practices among countries can help develop effective mitigation strategies. Raising public awareness about the sources and impacts of microplastic pollution can encourage behavior change and support policy efforts (Nikpay and Roodsari, 2024). Engaging communities in clean-up efforts and other initiatives can foster a sense of responsibility and encourage participation in mitigation efforts. The need to reduce plastic pollution calls for a shift toward circular economy models, which focus on reducing virgin plastic production, improving recycling efficiency, and promoting eco-design principles to minimize environmental impact. Addressing the escalating plastic pollution crisis requires a fundamental change in how we design, produce, consume, and dispose of plastic products at the end of their lifecycle (Munhoz et al., 2022). Current linear models of “take–make–dispose” significantly contribute to plastic waste accumulation in landfills and natural environments, highlighting the urgent need for circular approaches that emphasize resource efficiency and waste reduction (Reis et al., 2019). A circular economy aims to minimize waste and maximize resource use by promoting practices such as reducing material consumption, reusing products, designing for recyclability, and recovering energy from non-recyclable plastics (Macheca et al., 2024; The Future of Plastic: From Pollution to Solution, 2018). Transitioning to a circular economy requires a comprehensive approach involving stakeholders across the entire value chain, including manufacturers, consumers, policymakers, and waste management facilities (Vanapalli et al., 2020). Extended producer responsibility (EPR) schemes hold producers accountable for the end-of-life management of their products, motivating them to design for recyclability and reduce plastic use (Nikiema and Asiedu, 2022). Producers may also be required to contribute financially to recycling programs and waste management initiatives, creating incentives to minimize plastic waste. Improving waste collection and sorting infrastructure can prevent plastic waste from contaminating the environment (Policies to Reduce Microplastics Pollution in Water, 2021). This involves investing in advanced sorting technologies and expanding collection services to underserved areas. Increasing investment in recycling infrastructure, including mechanical and chemical recycling facilities, can enhance recycling rates and lower the amount of plastic waste sent to landfills (Mitigation and Abatement of Microplastics, 2023). Waste-to-energy technologies, such as incineration with energy recovery, can reduce plastic waste volume while producing energy (Nikiema and Asiedu, 2022). Advanced wastewater treatment technologies can remove microplastics from water before discharge into waterways (Policies to Reduce Microplastics Pollution in Water, 2021). Stormwater management practices, such as green infrastructure and retention ponds, can capture microplastics and other pollutants before they reach water bodies. Requiring or incentivizing microfiber filters in washing machines can reduce microfiber release from textiles (Policies to Reduce Microplastics Pollution in Water, 2021). Developing textiles that shed fewer microfibers can further reduce their release during washing. Since microplastics are not universally regulated pollutants, there is a need for stronger international frameworks (Munhoz et al., 2022). EPR encourages industry accountability for the management of post-consumer plastics (Nikiema and Asiedu, 2022). Policy measures should be evaluated for cost-effectiveness, considering both implementation costs and the benefits of reducing microplastic pollution (Nikiema and Asiedu, 2022). Policies should also be flexible and adaptable to incorporate new scientific discoveries and technological advances (Policies to Reduce Microplastics Pollution in Water, 2021). Engaging stakeholders across the entire value chain, including industry, consumers, and policymakers, is essential for developing effective and sustainable solutions (Iroegbu et al., 2021).
23.3 Research limitations
Despite the rapid increase in microplastic (MP) research, significant knowledge gaps and methodological limitations still exist, impeding the application of findings to real-world solutions. Many current studies depend on isolated techniques within controlled laboratory settings that do not capture the complex, multifactorial nature of natural ecosystems. This reductionist approach neglects the potential benefits of integrated, multi-technique frameworks for MP detection and clean-up. Moreover, the interactions of MPs with environmental matrices—soil, water, and air—and co-contaminants are still poorly understood, which hampers accurate risk assessments and effective mitigation strategies. The absence of standardized evaluation protocols also makes it difficult to compare studies and limits the scalability of proposed solutions. Additionally, the lack of comprehensive toxicity assessments raises concerns about potential secondary ecological harm from untested treatment methods. To address these issues, interdisciplinary collaboration among material scientists, toxicologists, engineers, and ecologists is essential, promoting innovation in biodegradable materials, advanced detection systems, and environmentally friendly clean-up technologies. Policy measures and public engagement are equally important for enforcing regulations and encouraging behavioral change. Ultimately, the way forward requires a holistic, flexible, and science-based approach that combines prevention, innovation, and governance to effectively confront the complex and evolving problem of microplastic pollution.
23.4 Limitations of microplastic control strategies
Despite increasing global attention to microplastic pollution, current control strategies face significant limitations that impede effective mitigation. Reducing plastic production and consumption—though essential—presents economic and logistical challenges, especially in industrial sectors dependent on plastic-based infrastructure. Behavioral changes toward sustainable consumption require ongoing education, policy incentives, and cultural shifts, often lagging behind environmental urgency. While biodegradable plastics are promoted as a solution, they often require specific degradation conditions rarely found in natural environments, and their environmental benefits are inconsistent. Additionally, high production costs and limited functionality hinder their widespread adoption. Recycling, another core element of waste management, is challenged by energy needs, economic inefficiencies, and difficulties in processing mixed plastic waste streams. The COVID-19 pandemic further revealed vulnerabilities in recycling systems, particularly with the increase in non-recyclable medical plastics. These systemic barriers highlight the need for interdisciplinary innovation—improving material science, developing better recycling technologies (e.g., chemical recycling, AI-powered sorting), and establishing policies that support a circular economy (Table 9). Tackling microplastic pollution demands a comprehensive, resilient approach that combines technological advancements, sustainable alternatives, public participation, and strong governance—creating a foundation for long-term ecological health and planetary well-being.
23.5 Future recommendations and prospects in microplastic degradation
To effectively combat the complex and far-reaching threat of microplastic (MP) pollution, an integrative, science-driven approach is essential. Future research should focus on combining advanced characterization tools with multifaceted treatment technologies, enabling precise detection, targeted degradation, and efficient removal across aquatic, terrestrial, and atmospheric systems. The synergistic use of hybrid methods offers a more comprehensive mitigation strategy, capable of addressing the heterogeneous nature of MPs in various environmental matrices. Equally important is advancing toxicity profiling, especially concerning degradation intermediates and by-products, which may present unforeseen ecological risks. Developing standardized frameworks for assessing treatment efficiency and environmental safety will ensure consistency, comparability, and sustainability across emerging technologies. Addressing MPs in soil and air remains a critical frontier, requiring new interventions to understand their complex interactions and environmental fate. At the source, improving plastic waste classification, promoting recyclable alternatives, and refining recycling infrastructure are key steps to reducing MP emissions. These efforts must be embedded within circular economy principles to cut dependence on virgin plastics and prevent environmental leakage. Overall, a multi-pronged, harmonized strategy will create a resilient and adaptive framework to reduce microplastic pollution and protect ecosystem and human health in the Anthropocene.
The future of microplastic (MP) degradation depends on the combination of microbial biotechnology, synthetic biology, and environmental policy, offering a ground-breaking approach to sustainable clean-up. Recent advances in microbial strain development—especially the creation of MP-degrading bacteria through natural selection, mutagenesis, and genetic engineering—have greatly improved our ability to address MP pollution (Xiang et al., 2023). Mutagenic breeding, which has low biosafety risks and high adaptability, is becoming popular as a practical method for producing strong strains with better degradation performance (Xiang et al., 2023; 2024). Synthetic biology now makes it possible to design multifunctional microbial factories that not only break down MPs but also convert by-products into valuable resources, supporting circular economy goals (Xiang et al., 2024). However, challenges remain in scaling these technologies and ensuring biosafety standards are met. Combining microbial degradation with sustainable waste management, policy updates, and improved detection methods is essential for comprehensive MP reduction (Wu et al., 2025; Casella et al., 2024). Meanwhile, MPs continue to harm ecosystems and biological functions across different levels, from causing oxidative stress in fish (Ghosh et al., 2025) to degrading soil health through biosolid use (. Their genotoxic, neurotoxic, and endocrine-disrupting impacts especially at the nanoscale highlight the urgent need for biosafe biotechnological solutions (Yoganandham, 2025; Casella and Ballaz, 2024). With global plastic output hitting 400.3 million tons in 2022, the call for scalable, eco-friendly, and policy-backed microbial solutions has never been stronger. By combining innovation in microbial strain engineering with strong environmental policies, the scientific community can lead the way to a cleaner, plastic-resistant future. Despite these efforts, many gaps remain in microplastic detection (Mendoza et al., 2017). The lack of universal, validated methods results in diverse analytical approaches, making it hard to interpret existing data (Prata et al., 2018). Researchers often face difficulties in choosing proper sampling methods because of many available options (Prata et al., 2018). Differences in filter sizes, sampling procedures, and reporting units complicate comparisons between studies and hinder building a complete understanding (Lu et al., 2021). The absence of standardized quality assurance and quality control protocols makes it difficult to evaluate the literature thoroughly (Lu et al., 2021). Sampling and analysis techniques for microplastics are still developing, and their limitations make accurate assessment of presence and impact challenging (Hale et al., 2020). Methods like mass spectrometry and thermal cracking gas chromatography have restrictions and are not suitable for all environmental samples (Jin et al., 2022). There is a need for consistent language in reporting microplastic concentrations and forms, along with detailed descriptions of sizes and chemical makeup (Lu et al., 2021). Regulatory agencies and labs face a complex array of sampling, extraction, and analysis methods, which complicates establishing standardized procedures (Primpke et al., 2020). Implementing comprehensive QA/QC protocols, including negative controls (field and lab) and positive controls, is crucial for reliable results. Using consistent terminology and providing detailed microplastic size and composition data can help improve comparability between studies (Lu et al., 2021).
24 Concluding perspectives: a roadmap toward microplastic-free ecosystems
Microplastics have become a widespread and persistent pollutant, posing serious ecotoxicological threats across land, freshwater, and marine environments. Their small size hides their significant impact, as they enter food chains, disrupt physiological processes in organisms, and carry toxic chemicals and pathogens. As our scientific understanding of their ecological and health effects grows, it is clear that urgent and coordinated action is necessary. Strategies to mitigate microplastics must be diverse, incorporating technological advances in waste management, development of sustainable materials, and reductions in plastic production and use. Equally important is establishing strong governance frameworks that encourage international collaboration, enforce regulations, and promote public awareness and behavioral changes. Ultimately, combating microplastic pollution requires a systems approach that connects science, policy, and societal participation. Only through integrated efforts can we protect ecosystems, conserve biodiversity, and secure a healthier, more sustainable future by examining the entire chain.
This review provides a uniquely comprehensive, multidisciplinary, and forward-looking overview of microplastic (MP) pollution, setting itself apart from existing literature through its depth, breadth, and innovative integration of themes. Unlike earlier reviews that tend to focus on isolated aspects such as aquatic pollution, single degradation methods, or basic detection techniques this work takes a holistic approach, covering all aspects of MP pollution: from plastic production and environmental entry points to physicochemical transformations and ecological distribution across marine, freshwater, and terrestrial environments. It further explores organismal accumulation, physical and molecular toxicities, and detailed analytical methods including emerging micro- and nano-characterization tools like AFM, SEM, FTIR, and Raman spectrometry. Most notably, this study advances the field by extensively analyzing innovative mitigation strategies, such as cloud-point extraction, magnetic separation, enzymatic degradation, organosilane technology, and strain breeding through mutagenesis techniques (UV, laser, ARTP), which are rarely combined in one framework. It introduces ground-breaking microbial degradation potentials, including biofilm-mediated and algal-, fungal-, and bacterial-based approaches, and discusses the potential role of synthetic biology. The review also emphasizes policy implications, life-cycle analysis, and the transition from lab-scale success to field application. By incorporating recent empirical evidence (2024–2025) and highlighting underexplored vectors like biosolid-associated MPs and atmospheric deposition, it establishes new ecological connections and health implications. This article stands out by not only presenting the current state of knowledge but also outlining future directions through interdisciplinary collaboration, regulatory harmonization, and circular economy models. With its highly integrated, visually engaging, and scientifically rigorous narrative, the review provides a transformative perspective for researchers, policymakers, and environmental managers working toward sustainable and effective mitigation of MP pollution. From source detection and characterization to advanced degradation methods and policy enactment, this review not only summarizes current knowledge but also outlines future directions for research and action. A future resilient to plastic pollution is possible, but it requires a fundamental shift in how we produce, consume, and manage synthetic polymers based on solid evidence and global cooperation.
Statements
Author contributions
BD: Conceptualization, Data curation, Funding acquisition, Validation, Visualization, Writing – original draft. SD: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. VK: Data curation, Investigation, Methodology, Writing – review & editing. SR: Data curation, Formal analysis, Investigation, Writing – review & editing. AM: Data curation, Formal analysis, Writing – review & editing. BM: Data curation, Formal analysis, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Fish Conservation and Stock Enhancement of Fishery of Ganga River Basin project (Ad-35012/1/2023-NMCG-NMCG) under the National Mission for Clean Ganga (NMCG), Ministry of Jal Shakti, supported this work.
Acknowledgments
The authors are thankful to the Director, ICAR-CIFRI, for providing support in conducting the experiment trials and analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- Nm
nanometer
- Mm
millimeter
- MPs
microplastics
- WWF
World Wide Fund for Nature
- DWTPs
drinking water treatment facilities
- Fig
figure
- H2O2
hydrogen peroxide
- WWTPs
wastewater treatment plants
- PE-MPs
polyethylene–microplastics
- μm
micrometer
- %
percent
- PCPs
personal care products
- TWPs
tire wear particles
- Kg
kilogram
- L
liter
- EKW
East Kolkata Wetland
- WWC
wastewater canals
- NA
not applicable
- PE
polyethylene
- PP
polypropylene
- PES
polyether sulfone
- PTFE
polytetrafluorethylene
- PMMA
polymethyl methacrylate
- PS
polystyrene
- EVA
ethylene-vinyl acetate
- PVC
plasticized polyvinyl chloride
- CA
cellulose acetate
- PC
polycarbonate
- PMMA
polymethyl methacrylate
- PU
polyurethane
- PVA
polyvinyl acetate
- i.e.
that is
- <
less than
- >
greater than
- EVA
ethylene-vinyl acetade
- SBR
styrene butadiene
- EPS
expanded polystyrene
- PES
polyester
- ABS
acrylonitrile-butadiene-styrene
- PET
polyethylene terephthalate
- PEI
polyester imide
- PIP
poly isoprene
- PU
polyurethane
- PPS
polyphenylene sulphide
- PVB
polyvinyl behenate
- PVF
polyvinyl fluoride
- PA
polyamide
- HDPE
high-density polyethylene
- LDPE
low-density polyethylene
- L-1
per liter
- Ppm
parts per million
- ~
approximately
- GI
gastrointestinal
- FTIR
Fourier transform infrared research
- PCBs
polychlorinated biphenyls
- PAHs
polycyclic aromatic hydrocarbonsl
- SOD
super oxide dismutase
- FRAP
ferric reducing antioxidant potential
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- AChE
acetylcholinesterase
- e.g.
example
- mL
milliliter
- °C
degree centigrade
- M
meter
- Cm
centimeter
- MNP
micro- and nanoplastics
- NaCl
sodium chloride
- G
gram
- :
ratio
- KOH
potassium hydroxide
- HNO3
nitric acid
- RPM
revolutions per minute
- Hz
hertz
- pH
potential of hydrogen
- DE
digestion efficacy
- ATR
attenuated total reflection
- CN
cellulose nitrate
- py-GC–MS
pyrolysis-gas chromatography–mass spectrometry
- MAR
managed aquifer recharge systems
- Viz
videlicet
- MF
membrane bioreactor
- UF
dynamic membrane
- RSF
rapid sand filtration
- GAC
granular activated carbon filtration
- FeCl3
ferric chloride.
References
1
(2018). The Future of Plastic: From Pollution to Solution ( United Nations Environment Programme (UNEP). Available online at: https://www.unep.org/resources/report/future-plastic-pollution-solution.
2
(2021). Policies to Reduce Microplastics Pollution in Water ( OECD Environment Policy Paper No. 29). Available online at: https://www.oecd.org/environment/policies-to-reduce-microplastics-pollution-in-water.htm.
3
Abbasi S. Soltani N. Keshavarzi B. Moore F. Turner A. Hassanaghaei M. (2018). Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere205, 80–87. doi: 10.1016/j.chemosphere.2018.04.076
4
Abdelfattah A. Ali S. S. Ramadan H. El-Aswar E. I. Eltawab R. Ho S. H. et al . (2022). Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol.13, 100205. doi: 10.1016/j.ese.2022.100205
5
Abdelfattah A. Hossain A. B. M. S. Ismail N. El-Sayed A. E.-K. M. (2013). Characterization of acetylcholinesterase in the hemolymph of Spodoptera littoralis larvae. Pesticide Biochem. Physiol.106, 228–232. doi: 10.1016/j.pestbp.2013.06.004
6
Abidli S. Lahbib Y. El Menif N. T. (2019). Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar. pollut. Bull.142, 243–252. doi: 10.1016/j.marpolbul.2019.03.048
7
Abimbola I. McAfee M. Creedon L. Gharbia S. (2024). In-situ detection of microplastics in the aquatic environment: A systematic literature review. Sci. Total Environ., 173111. doi: 10.1016/j.scitotenv.2024.173111
8
Adachi J. Kakudo A. Takada Y. Isoyama J. Ikemoto N. Abe Y. et al . (2022). Systematic identification of ALK substrates by integrated phosphoproteome and interactome analysis. Life Sci. Alliance5, e202101202. doi: 10.26508/lsa.202101202
9
Addo S. Boateng C. M. Diyie R. L. Duodu C. P. Ferni A. K. Williams E. A. et al . (2022). Occurrence of microplastics in wild oysters (Crassostrea tulipa) from the Gulf of Guinea and their potential human exposure. Heliyon8. doi: 10.1016/j.heliyon.2022.e12255
10
Ahmaditabatabaei S. Kyazze G. Iqbal H. M. N. Keshavarz T. (2021). Fungal enzymes as catalytic tools for polyethylene terephthalate (Pet) degradation. J. Fungi7. doi: 10.3390/jof7110931
11
Akhbarizadeh R. Moore F. Keshavarzi B. (2018). Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ. Pollut.232, 154–163. doi: 10.1016/j.envpol.2017.09.028
12
Alam F. C. Rahman M. M. (2025). Microplastic pollution in South Asian aquatic environments: A comprehensive review of sources, distribution, and impacts. Mar. Pollut. Bull.200, 116098. doi: 10.1016/j.marpolbul.2024.116098
13
Alfonso M. B. Scordo F. Seitz C. Mavo Manstretta G. M. Ronda A. C. Arias A. H. et al . (2020). First evidence of microplastics in nine lakes across Patagonia (South America). Sci. Total Environ.733, 139385. doi: 10.1016/j.scitotenv.2020.139385
14
Ali S. S. Elsamahy T. Koutra E. Kornaros M. El-Sheekh M. Abdelkarim E. A. et al . (2016). Degradation of conventional plastic wastes in the environment: A review on current status and future perspectives. Environ. Sci. Pollut. Res.23, 13768–13783. doi: 10.1007/s11356-016-6860-3
15
Ali N. Khan M. H. Ali M. Ahmad S. Khan A. Nabi G. et al . (2023). Insight into microplastics in the aquatic ecosystem: Properties, sources, threats and mitigation strategies. Sci. Total Environ.20, 169489. doi: 10.1016/j.scitotenv.2023.169489
16
Ali M. U. Lin S. Yousaf B. Abbas Q. Munir M. A. Ali M. U. et al . (2021). Environmental emission, fate and transformation of microplastics in biotic and abiotic compartments: global status, recent advances and future perspectives. Sci. Total Environment.791, 148422. doi: 10.1016/j.scitotenv.2021.148422
17
Ali I. Tan X. Li J. Peng C. Wan P. Naz I. et al . (2022). Innovations in the development of promising adsorbents for the remediation of microplastics and nanoplastics – A critical review. Water Res.230, 119526. doi: 10.1016/j.watres.2022.119526
18
Alomar C. Estarellas F. Deudero S. (2016). Microplastics in the Mediterranean Sea: deposition in coastal shallow sediments, spatial variation and preferential grain size. Mar. Environ. Res.115, 1–0. doi: 10.1016/j.marenvres.2016.01.005
19
Alomar C. Sureda A. Capó X. Guijarro B. Tejada S. Deudero S. (2017). Microplastic ingestion by Mullus surmuletus Linnaeus 1758 fish and its potential for causing oxidative stress. Environ. Res.159, 135–142. doi: 10.1016/j.envres.2017.07.043
20
Amaral-Zettler L. A. Zettler E. R. Mincer T. J. (2020). Ecology of the plastisphere. Nat. Rev. Microbiol.18, 139–151. doi: 10.1038/s41579-019-0308-0
21
Amaro H. M. Guedes A. C. Malcata F. X. (2011). Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy88, 3402–3410. doi: 10.1016/j.apenergy.2010.12.014
22
Amereh F. Babaei M. Eslami A. Fazelipour S. Rafiee M. (2020). The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: from a hypothetical scenario to a global public health challenge, Environ. Pollut.261, 114158. doi: 10.1016/j.envpol.2020.114158
23
Amrutha K. Bhat U. G. Bhat M. T. Warrier A. K. (2022). Microplastic pollution in inland waters and remediation strategies: A comprehensive review. J. Environ. Manage.315, 115170. doi: 10.1016/j.jenvman.2022.115170
24
Amrutha K. Warrier A. K. (2020). The first report on the source-to-sink characterization of microplastic pollution from a riverine environment in tropical India. Sci. Total Environ.739, 140377. doi: 10.1016/j.scitotenv.2020.140377
25
An R. Wang X. Yang L. Zhang J. Wang N. Xu F. et al . (2021). Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology449, 152665. doi: 10.1016/j.tox.2020.152665
26
Anbumani S. Kakkar P. (2018). Ecotoxicological effects of microplastics on biota: a review. Environ. Sci. pollut. Res. Int.25, 14373–14396. doi: 10.1007/s11356-018-1999-x
27
Anderson P. J. Warrack S. Langen V. Challis J. K. Hanson M. L. Rennie M. D. (2017). Microplastic contamination in lake Winnipeg, Canada. Environ. pollution.225, 223–231. doi: 10.1016/j.envpol.2017.02.072
28
Andrady A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull.62, 1596–1605. doi: 10.1016/j.marpolbul.2011.05.030
29
Araujo C. F. Nolasco M. M. Ribeiro A. M. P. Ribeiro-Claro P. J. A. (2018). Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res.142, 426–440. doi: 10.1016/j.watres.2018.05.060
30
Ariefdien R. Pfaff M. Awe A. Sparks C. (2024). Stormwater outlets: a source of microplastics in coastal zones of Cape Town, South Africa. Mar. pollut. Bull.198, 115800. doi: 10.1016/j.marpolbul.2023.115800
31
Arthur C. Baker J. Bamford H. (Eds.) (2009). Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris ( NOAA Technical Memorandum NOS-OR&R-30).
32
Asandei A. D. Moran I. W. Saha G. Chen Y. (2006). Titanium-mediated living radical styrene polymerizations. VI. Cp2TiCl-catalyzed initiation by epoxide radical ring-opening: Effect of reducing agents, temperature and Ti/epoxide and Ti/Zn ratios. J. Polymer Sci. Part A: Polymer Chem.44, 2156–2165. doi: 10.1002/pola.21326
33
Austin H. P. Allen M. D. Donohoe B. S. Rorrer N. A. Kearns F. L. Silveira R. L. et al . (2018). Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci.115, E4350–E4357. doi: 10.1073/pnas.1718804115
34
Auta H. S. Emenike C. U. Jayanthi B. Fauziah S. H. (2018). Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull.127, 15–21. doi: 10.1016/j.marpolbul.2017.11.036
35
Auta H. S. Emenike C. U. Jayanthi B. Fauziah S. H. (2017). Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. pollut. Bull.127, 15e21. doi: 10.1016/j.marpolbul.2017.11.036
36
Avery-Gomm S. O'Hara P. D. Kleine L. Bowes V. Wilson L. K. Barry K. L. (2012). Northern fulmars as biological monitors of trends of plastic pollution in the eastern North Pacific. Mar. Pollut. Bull.64, 1776–1781. doi: 10.1016/j.marpolbul.2012.04.017
37
Azevedo-Santos V. M. Goncalves G. R. Manoel P. S. Andrade M. C. Lima F. P. Pelicice F. M. (2019). Plastic ingestion by fish: A global assessment. Environ. pollut.255, 112994. doi: 10.1016/j.envpol.2019.112994
38
Baechler B. R. Granek E. F. Hunter M. V. Conn K. E. (2020). Microplastic concentrations in two Oregon bivalve species: spatial, temporal, and species variability. Limnol Oceanogr Lett.5, 54–65. doi: 10.1002/lol2.10124
39
Baini M. Fossi M. C. Galli M. Caliani I. Campani T. Finoia M. G. et al . (2018). Abundance and characterization of microplastics in the coastal waters of Tuscany (Italy): The application of the MSFD monitoring protocol in the Mediterranean Sea. Mar. pollut. Bull.133, 543–552. doi: 10.1016/j.marpolbul.2018.06.016
40
Balasubramaniam M. Phillott A. D. (2016). “ Preliminary observations of microplastics from beaches of Indian Ocean,” in Indian Ocean Turtle Newsletter No. 23, Dakshin Foundation and supported by Madras Crocodile Bank Trust, International Sea Turtle Society, and the Marine Turtle Conservation Fund of the US Fish and Wildlife Service. pp 13–pp 16. Available online at: http://seaturtle.org/library/BalasubramaniamM_2016_IndOceanTurtleNwsletter.pdf.
41
Balkhuyur F. M. Bin Dohaish E.-J. A. Elhalwagy M. E. A. Alikunhi N. M. AlSuwailem A. M. Rostad A. et al . (2018). Microplastic in the gastrointestinal tract of fishes along the Saudi Arabian Red Sea coast. Mar. pollut. Bull.131, 407–415. doi: 10.1016/j.marpolbul.2018.04.040
42
Banaee M. Soltanian S. Sureda A. Gholamhosseini A. Haghi B. N. Akhlaghi M. et al . (2019). Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere236, 124335. doi: 10.1016/j.chemosphere.2019.07.066
43
Banaee M. Taheri S. Tabandeh M. R. Ghafarifarsani H. (2020). Integration of physiological, biochemical, and molecular responses to better understand the effects of polyethylene microplastics on freshwater fish: A case study on common carp (Cyprinus carpio). Chemosphere253, 126626. doi: 10.1016/j.chemosphere.2020.126626
44
Barboza L. G. A. Lopes C. Oliveira P. Bessa F. Otero V. Henriques B. et al . (2020). Microplastics in wild fish from north East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ.717, 134625. doi: 10.1016/j.scitotenv.2019.134625
45
Barchiesi G. Dussán J. Gomez L. (2021). Microbial enzymes in plastic degradation: An overview. Front. Microbiol.12. doi: 10.3389/fmicb.2021.735692
46
Barkhau J. Sanchez A. Lenz M. Thiel M. (2022). Effects of microplastics (PVC, PMMA) on the mussel Semimytilus algosus differ only at high concentrations from those of natural microparticles (clay, celite). Mar. pollut. Bull.177. doi: 10.1016/j.marpolbul.2022.113414
47
Batel A. Borchert F. Reinwald H. Erdinger L. Braunbeck T. (2018). Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. pollut.235, 918–930. doi: 10.1016/j.envpol.2018.01.028
48
Bautista-Zamudio P. A. Fl´orez-Restrepo M. A. L´opez-Legarda X. Monroy- Giraldo L. C. Segura-S´anchez F. (2023). Biodegradation of plastics by white-rot fungi: A review. Sci. Total Environment.901. doi: 10.1016/j.scitotenv.2023.165950
49
Bayo J. Olmos S. López-Castellanos J. Alcolea A. (2020). Microplastics and microfibers in the sludge of a municipal wastewater treatment plant. Int. J. Sustain. Dev. Plann.15, 1225–1232. doi: 10.18280/ijsdp.150811
50
Becucci M. Lesa D. Sacchetti G. Mercurio M. Lucchesi P. Di Bartolomeo S. et al . (2021). Microplastic pollution in snow from Italian Alps. Environ. Pollut.289, 117945. doi: 10.1016/j.envpol.2021.117945
51
Bellas J. Martínez-Armental J. Martínez-Cámara A. Besada V. Martínez-Gómez C. (2016). Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. pollut. Bull.109, 55–60. doi: 10.1016/j.marpolbul.2016.06.026
52
Benjamin D. Rozario J. V. Jose D. Prabhakaran M. P. Kurup B. M. Harikrishnan M. (2014). Plastic ingestion by Bigeye Thresher shark Alopias superciliosus off Ratnagiri southwest coast of India. Int. J. Environ. Sci.5, 277. doi: 10.6088/ijes.2014050100024
53
Bensalah N. Midassi S. Ahmad M. I. (2021). Electrochemical oxidation of hydrocarbons: An overview of recent developments and future perspectives. J. Mater. Environ. Sci.12, 124–148.
54
Bergmann M. Gutow L. Klages M. (Eds.) (2015). Marine anthropogenic litter ( Springer International Publishing). doi: 10.1007/978-3-319-16510-3
55
Bessa F. Barría P. Neto J. M. Frias J. P. G. L. Otero V. Sobral P. et al . (2018). Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. pollut. Bull.128, 575–584. doi: 10.1016/j.marpolbul.2018.01.044
56
Besseling E. Quik J. T. Sun M. Koelmans A. A. (2017). Fate of nano-and microplastic in freshwater systems: A modeling study. Environ. pollution.220, 540–548. doi: 10.1016/j.envpol.2016.10.001
57
Bhattacharjee J. Roy S. (2025). “ A new approach: spinel nano-ferrites for wastewater-based microplastics,” in Applications of Spinel Nano-Ferrites in Health, Environmental Sustainability, and Safety ( CRC Press), 171–183.
58
Blettler M. C. Ulla M. A. Rabuffetti A. P. Garello N. (2017). Plastic pollution in freshwater ecosystems: macro-, meso-, and microplastic debris in a floodplain lake. Environ. Monit. assessment.189, 1–3. doi: 10.1007/s10661-017-6305-8
59
Bollinger A. Thies S. Katzke N. Jaeger K. E. (2020). The biotechnological potential of marine bacteria in the novel lineage of Pseudomonas pertucinogena. Microbial Biotechnol.13, 19–31. doi: 10.1111/1751-7915.13288
60
Bomfim M. V. J. Vianna M. L. Wallner-Kersanach M. Andrade L. (2019). Plastic ingestion by marine catfish: An unexpected fisheries impact. Mar. Pollut. Bull.145, 362–366. doi: 10.1016/j.marpolbul.2019.06.018
61
Bongaarts J. (2019). IPBES 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science Policy Platform on Biodiversity and Ecosystem Services. Popul Dev. Rev.45, 680–681. doi: 10.1111/padr.12283
62
Bostan N. Ilyas N. Akhtar N. Mehmood S. Falak N. Iqbal M. J. et al . (2023). Microplastics in freshwater environment: The first evaluation and characterization with abundance, composition, surface morphology and risk assessment in the Swat River, Pakistan. Chemosphere339, 139735. doi: 10.1016/j.chemosphere.2023.139735
63
Bouwmeester H. Hollman P. C. Peters R. J. (2015). Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ. Sci. Technol.49, 8932–8947. doi: 10.1021/acs.est.5b01090
64
Brahney J. Mahowald N. Prank M. Cornwell G. Klimont Z. Matsui H. et al . (2021). Constraining the atmospheric limb of the plastic cycle. Proc. Natl. Acad. Sci.118, e2020719118. doi: 10.1073/pnas.2020719118
65
Brakat A. Zhu H. Pan S. (2021). A comprehensive review of microalgae-mediated wastewater treatment and its potential for resource recovery. J. Cleaner Prod.311, 127749. doi: 10.1016/j.jclepro.2021.127749
66
Brandon J. A. Freibott A. Sala L. M. (2020). Patterns of suspended and salp-ingested microplastic debris in the North Pacific investigated with epifluorescence microscopy. Limnology oceanography letters.5, 46–53. doi: 10.1002/lol2.10127
67
Braun T. Ehrlich L. Henrich W. Koeppel S. Lomako I. Schwabl P. et al . (2021). Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics13, 921. doi: 10.3390/pharmaceutics13070921
68
Browne M. A. Crump P. Niven S. J. Teuten E. Tonkin A. Galloway T. (2011). Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol.45, 9175–9179. doi: 10.1021/es201811s
69
Browne M. A. Dissanayake A. Galloway T. S. Lowe D. M. Thompson R. C. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol.42, 5026–5031. doi: 10.1021/es800249a
70
Bruge A. Barreau C. Carlot J. Collin H. Moreno C. Maison P. (2018). Monitoring litter inputs from the Adour River (Southwest France) to the marine environment. J. Mar. Sci. Eng.6, 24. doi: 10.3390/jmse6010024
71
Bu D. Wan J. Zhang Z. Cheng H. Tan W. Bao Y. (2020a). Photocatalytic degradation of polystyrene microplastics by BiOBr/palygorskite composite under simulated sunlight irradiation. Chem. Eng. J.384, 123263. doi: 10.1016/j.cej.2019.123263
72
Bu D. Zhang X. Zheng H. Liu M. Wan J. (2021). Photocatalytic degradation of microplastics by Ag/AgBr-activated persulfate under simulated solar light irradiation. J. Environ. Chem. Eng.9, 105216. doi: 10.1016/j.jece.2021.105216
73
Bu D. Zhuang M. Zhang X. Li B. Wan J. (2020b). Adsorption and photocatalytic degradation of microplastics by MIL-101(Fe)/g-C3N4 composite. Sci. Total Environ.743, 140703. doi: 10.1016/j.scitotenv.2020.140703
74
Bui X. T. Nguyen P. T. Nguyen V. T. Dao T. S. Nguyen P. D. (2020). Microplastics pollution in wastewater: Characteristics, occurrence and removal technologies. Environ. Technol. Innovation.19, 101013. doi: 10.1016/j.eti.2020.101013
75
Cai M. He H. Liu M. Li S. Tang G. Wang (2018). Lost but can't be neglected: huge quantities of small microplastics hide in the South China Sea. Sci. Total Environ.633, 1206-1216.
76
Calero M. Godoy V. Quesada L. Martín-Lara MÁ. (2021). Green strategies for microplastics reduction. Curr. Opin. Green Sustain. Chem.28, 100442. doi: 10.1016/j.cogsc.2020.100442
77
Cao Z. Yan W. Ding M. Yuan Y. (2022). Construction of microbial consortia for microbial degradation of complex compounds. Front. Bioengineering Biotechnol. doi: 10.3389/fbioe.2022.1051233
78
Cao Y. Zhao M. Ma X. Song Y. Zuo S. Li H. et al . (2021). A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environment.788, 147620. doi: 10.1016/j.scitotenv.2021.147620
79
Capillo G. Savoca S. Panarello G. Mancuso M. Branca C. Romano V. et al . (2020). Quali-quantitative analysis of plastics and synthetic microfibres found in demersal species from Southern Tyrrhenian Sea (Central Mediterranean). Mar. pollut. Bull.150, 110596. doi: 10.1016/j.marpolbul.2019.110596
80
Caron A. G. M. Thomas C. R. Berry K. L. E. Motti C. A. Ariel E. Brodie J. E. (2018). Ingestion of microplastic debris by green sea turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a sequential extraction protocol. Mar. Pollut. Bull.127, 743–751. doi: 10.1016/j.marpolbul.2017.12.062
81
Carpenter E. J. Smith K. L. Jr. (1972). Plastics on the sargasso sea surface. Science175, 1240–1241. doi: 10.1007/s11356-017-0528-7
82
Carr S. A. Liu J. Tesoro A. G. (2016). Transport and fate of microplastic particles in wastewater treatment plants. Water Res.91, 174–182. doi: 10.1016/j.watres.2016.01.002
83
Cartes J. E. Soler-Membrives A. Stefanescu C. Lombarte A. Carrasson ´M. (2016). Contributions of allochthonous inputs of food to the diets of benthopelagic fish over the northwest Mediterranean slope (to 2300 m). Deep Sea Res. Oceanogr Res. Pap.109, 123–136. doi: 10.1016/j.dsr.2015.11.001
84
Caruso G. (2019). Microplastics as vectors of contaminants. Mar. Pollut. Bull.146, 921–924. doi: 10.1016/j.marpolbul.2019.07.052
85
Casella C. Ballaz S. J. (2024). Genotoxic and neurotoxic potential of intracellular nanoplastics: A review. J. Appl. Toxicol.44, 1657–1678. doi: 10.1002/jat.4598
86
Casella L. A. Grech A. Borelle S. B. Patrício Silva A. L. (2025). The global plastic pollution treaty: What role for science? Science387, 146–148. doi: 10.1126/science.adr6763
87
Casella C. Vadivel D. Dondi D. (2024). The current situation of the legislative gap on microplastics (MPs) as new pollutants for the environment. Water Air Soil pollut.235, 778. doi: 10.1007/s11270-024-07589-1
88
Castano-Ortiz J. M. Romero F. Cojoc L. Barcelo D. Rodriguez-Mozaz S. Santos L. H. M. M. (2024). Accumulation of polyethylene microplastics in river biofilms and effect on the uptake, biotransformation, and toxicity of the antimicrobial triclosan. Environ. pollut.344, 113011. doi: 10.1016/j.envpol.2024.123369
89
Castillo A. B. Al-Maslamani I. Obbard J. P. (2016). Prevalence of microplastics in the marine waters of Qatar. Mar. pollut. Bull.111, 260–267. doi: 10.1016/j.marpolbul.2016.06.108
90
Celis-Hernández O. Ávila E. Ward R. D. Rodríguez-Santiago M. A. Aguirre-Téllez J. A. (2021). Microplastic distribution in urban vs pristine mangroves: using marine sponges as bioindicators of environmental pollution. Environ. pollut.284, 117391. doi: 10.1016/j.envpol.2021.117391
91
Cera A. Cesarini G. Scalici M. (2020). Microplastics in freshwater: what is the news from the world? Diversity.12, 276. doi: 10.1016/j.watres.2016.01.002
92
Cessi P. Pinardi N. Lyubartsev V. (2014). Energetics of semienclosed basins with two-layer flows at the strait. J. Phys. Oceanography44, 967–979. doi: 10.1175/JPO-D-13-0129.1
93
Chabalala M. B. Al-Abri M. Mamba B. B. Nxumalo E. N. Muleja A. A. (2021). Functionalized electrospun nanofibrous membranes for the removal of water pollutants. Membranes11, 540. doi: 10.3390/membranes11080540
94
Chamas A. Moon H. Zheng J. Qiu Y. Tabassum T. Jang J. H. et al . (2020). Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng.8, 3494–3511. doi: 10.1021/acssuschemeng.9b06635
95
Chang H. Zhang H. Barber J. Maschinot A. J. Lezama J. Jiang L. et al . (2023). Muse: Text-to-image generation via masked generative transformers. arXiv preprint arXiv:2301.00704. doi: 10.48550/arXiv.2301.00704
96
Chatterjee S. Roy B. Roy D. Banerjee R. (2015). Enzyme-mediated biodegradation of heat treated commercial polyethylene by Staphylococcal isolate. Sci. Rep.5, 17864. doi: 10.1038/srep17864
97
Chatterjee S. Sharma S. (2019). Microplastics in our oceans and marine health. Field Actions Sci. Rep., 54–61. doi: 10.1021/acs.est.5b05478
98
Chau H. T. M. L. Kadokami K. Duong H. T. Kong L. Nguyen T. T. Nguyen T. Q. (2023). Microplastic contamination in surface water and fish in Can Tho City, Vietnam. Environ. Sci. Pollut. Res.30, 29826–29839. doi: 10.1007/s11356-022-24242-2
99
Chauhan D. Agrawal G. Deshmukh S. Roy S. S. Priyadarshini R. (2018). Biofilm formation by Exiguobacterium sp. DR11 and DR14 alter polystyrene surface properties and initiate biodegradation. RSC Adv.8, 37590–37599. doi: 10.1039/C8RA06448B
100
Chauhan J. S. Semwal A. Mishra P. Gupta P. (2021). Occurrence and distribution of microplastic particles in three freshwater lakes of the Garhwal Himalaya, India. Water Air Soil Pollut.232, 339. doi: 10.1007/s11270-021-05287-4
101
Chen C.-C. Wu J. Lin T.-C. (2006). Biodegradation of plastics by Aspergillus niger and Penicillium species. Polymer Degradation Stabil.91, 1800–1806. doi: 10.1016/j.polymdegradstab.2006.01.014
102
Chen G. Feng Q. Wang J. (2020). Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environment.703, 135504. doi: 10.1016/j.scitotenv.2019.135504
103
Chen L. Bao J. Duan H. Wang Y. Zhang W. (2022). Microplastics pollution in wild freshwater fish: A case study in the Yellow River, China. Environ. Pollut.315, 120353. doi: 10.1016/j.envpol.2022.120353
104
Chen Y. Tong W. Fan Y. Zhang M. Pan Z. Chen S. (2025). Microplastic pollution in urban rivers: A comparative study of developed and developing regions. Water Res.250, 121045. doi: 10.1016/j.watres.2024.121045
105
Cheng H. Wang W. (2022). Microplastics in the marine environment: Sources, distribution, and ecological impacts. Mar. Pollut. Bull.183, 114026. doi: 10.1016/j.marpolbul.2022.114026
106
Cheng Y. Chen J. Fu R. Zhang P. Chen H. Cao H. et al . (2025). Molecular mechanism differences between nanoplastics and microplastics in colon toxicity: nanoplastics induce ferroptosis-mediated immunogenic cell death, while microplastics cause cell metabolic reprogramming. J. Nanobiotechnology23, 505. doi: 10.1186/s12951-025-03545-1
107
Cheung L. T. O. Fok L. (2017). Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res.122, 53–61. doi: 10.1016/j.watres.2017.05.053
108
Cho Y. Shim W. J. Jang M. Han G. M. Hong S. H. (2019). Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. pollut.245, 1107–1116. doi: 10.1016/j.envpol.2018.11.091
109
Ch-Th M. H. Kumar K. V. Lin J. C.-T. Raymond Jang J. S. (2021). Microplastics in water bodies: A review of detection methods, ecological impacts and removal technologies. Environ. Technol. Innovation24, 101910. doi: 10.1016/j.eti.2021.101910
110
Cincinelli A. Scopetani C. Chelazzi D. Lombardini E. Martellini T. Katsoyiannis A. et al . (2017). Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere175, 391–400. doi: 10.1016/j.chemosphere.2017.02.024
111
Cole M. Lindeque P. K. Fileman E. Clark J. Lewis C. Halsband C. et al . (2016). Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ. Sci. Technol.50, 3239–3 46. doi: 10.1021/acs.est.5b05905
112
Cole M. Lindeque P. Fileman E. Halsband C. Galloway T. S. Lewis C. (2013). Microplastic ingestion by zooplankton. Environ. Sci. Technol.47, 6646–6655. doi: 10.1021/es400663f
113
Cole M. Lindeque P. Halsband C. Galloway T. S. (2011). Microplastics as contaminants in the marine environment: a review. Mar. pollut. bulletin.62, 2588–2597. doi: 10.1016/j.marpolbul.2011.09.025
114
Colferai S. A. Araújo J. C. Fernandes A. Martins T. A. (2017). Biomarker responses in fish exposed to microplastics. Ecotoxicol. Environ. Saf.145, 610–617. doi: 10.1016/j.ecoenv.2017.08.015
115
Collinson K. E. Wise R. M. Davey J. A. Sheridan K. J. (2017). Changes in microbiological quality and nutrient composition during bioremediation of oil-contaminated soil. Appl. Soil Ecol.119, 348–354. doi: 10.1016/j.apsoil.2017.07.016
116
Contreras-Llin A. Diaz-Cruz M. S. (2024). Comprehensive review on microplastics in aquatic environments: Analytical methodologies, occurrence, and associated organic contaminants. TrAC Trends Analytical Chem.171, 117519. doi: 10.1016/j.trac.2023.117519
117
Cooper T. G. Noonan E. Von Eckardstein S. Auger J. Baker H. G. Behre H. M. et al . (2010). World Health Organization reference values for human semen characteristics. Hum. Reprod. Update16, 231–245. doi: 10.1093/humupd/dmp048
118
Cowger W. Steinmetz Z. Gray A. Munno K. Lynch J. Hapich H. et al . (2021). Microplastic spectral classification needs an open source community: Open Specy to the rescue! Analytical Methods13, 753–758. doi: 10.1039/D0AY02100H
119
Crawford C. B. Quinn B. (2017). “ Plastic production, waste and legislation,” in Microplastic pollutants ( Elsevier, Amsterdam), 39–56. doi: 10.1016/C2015-0-04315-5
120
Crew A. Gregory-Eaves I. Ricciardi A. (2020). Distribution, abundance, and diversity of microplastics in the upper St. Lawrence River. Environ. pollut.260, 113994. doi: 10.1016/j.envpol.2020.113994
121
Crichton E. M. Noël M. Gies E. A. Ross P. S. (2017). A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Analytical Methods9, 1419–1428. doi: 10.1039/C6AY02733D
122
Cui K. Zhang Y. Huang Q. Chen Y. Zhang D. Wang X. et al . (2021). Enhanced adsorption of aqueous microplastics on biochar prepared by microwave-assisted hydrothermal carbonization. Sci. Total Environ.770, 145321. doi: 10.1016/j.scitotenv.2021.145321
123
Čulin J. Bielić T. (2016). Plastic pollution from ships. Pomorski zbornik.51, 57–66. doi: 10.18048/2016.51.04
124
Cunha C. Silva L. Paulo J. Faria M. Nogueira N. Cordeiro N. (2019). Microalgae-based biopolymer for nano- and microplastic removal: A possible biosolution for wastewater treatment. Environ. Pollut.249, 612–623. doi: 10.1016/j.envpol.2019.03.046
125
Cunha C. Silva L. Paulo J. Ferreira P. Faria M. Cordeiro N. (2020). Microalgal-based biopolymer for flocculation, emulsification and microplastic removal. Environ. Res.186, 109520. doi: 10.1016/j.envres.2020.109520
126
Cunliffe B. Davis S. C. (1982). Encyclopedia of archaeological excavations in the Holy Land ( Oxford University Press).
127
Daniel D. B. Ashraf P. M. Thomas S. N. (2020). Microplastics in the edible and inedible tissues of pelagic fishes sold for human consumption in Kerala, India. Environ. Pollut.266, 115365. doi: 10.1016/j.envpol.2020.115365
128
Daniel D. B. Ashraf P. M. Thomas S. N. Thomson K. T. (2021). Microplastics in the edible tissues of shellfishes sold for human consumption. Chemosphere264, 128554. doi: 10.1016/j.chemosphere.2020.128554
129
Danopoulos S. Deutsch G. H. (2021). Lung disease manifestations in Down syndrome. Am. J. Physiology-Lung Cell. Mol. Physiol.321, L892–L899. doi: 10.1152/ajplung.00434.2020
130
Daranagama D. A. Liyanage G. Y. (2024). Microplastic contamination in tropical aquatic ecosystems: A systematic review. Environ. Sci. Pollut. Res.31, 11889–11915. doi: 10.1007/s11356-023-31448-3
131
Das Sarkar S. Patra S. K. Mondal P. Sarkar T. Goswami M. Kole D. (2023). Microplastics in Indian freshwater systems: Abundance, distribution, and impact on aquatic ecosystems. Environ. Sci. Pollut. Res.30, 39238–39257.
132
Davarpanah E. Guilhermino L. (2019). Are gold nanoparticles and microplastics mixtures more toxic to the marine microalgae Tetraselmis chuii than the substances individually? Ecotoxicol. Environ. Saf.181, 60–68. doi: 10.1016/j.ecoenv.2019.05.078
133
Dayal S. Malik A. Kumar S. Singh B. (2024). Microplastic pollution in Indian rivers: Current status and future perspectives. Environ. Monitoring Assess.196, 185. doi: 10.1007/s10661-023-12345-6
134
Debbarma N. Gurjar U. R. Ramteke K. K. Shenoy L. Nayak B. B. Bhushan S. et al . (2022). Abundance and characteristics of microplastics in gastrointestinal tracts and gills of croaker fish (Johnius dussumieri) from off Mumbai coastal waters of India. Mar. pollut. Bull.176, 113473. doi: 10.1016/j.marpolbul.2022.113473
135
Dehm J. Singh S. Ferreira M. Piovano S. (2020). Microplastics in subsurface coastal waters along the southern coast of Viti Levu in Fiji, South Pacific. Mar. pollut. Bull.156, 111239. doi: 10.1016/j.marpolbul.2020.111239
136
Demir-Yilmaz I. Gullo M. Faccini A. Lama G. Bastioli C. La China S. (2022). Acetobacter and other aerobic acidophilic bacteria producing exopolysaccharides. Food Technol. Biotechnol.60, 178–190. doi: 10.17113/ftb.60.02.22.7340
137
Denaro R. Aulenta F. Crisafi F. Di Pippo F. Cruz Viggi C. Matturro B. et al . (2020). Marine hydrocarbon-degrading bacteria breakdown poly(ethylene terephthalate) (PET). Sci. Total Environ.749, 141608. doi: 10.1016/j.scitotenv.2020.141608
138
Deng H. Fu Q. Sun X. Wei R. Liu Y. Shi H. et al . (2019). Microplastic pollution in surface sediments of the Dianchi Lake, Southwest China. Environ. Pollut.257, 113545.
139
Deng H. Fu Q. Wei Z. Shi H. Hu W. (2020a). Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut.258, 113658. doi: 10.1016/j.envpol.2019.113658
140
Deng Y. Zhang Y. Lemos B. Ren H. (2017). Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep.7, 46687. doi: 10.1038/srep46687
141
Desforges J.-P. W. Galbraith M. Ross P. S. (2015). Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contamination Toxicol.69, 320–330. doi: 10.1007/s00244-015-0172-5
142
de Souza MaChado A. A. Kloas W. Zarfl C. Hempel S. Rillig M. C. (2018). Microplastics as an emerging threat to terrestrial ecosystems. Global Change Bology.24, 1405–1416. doi: 10.1111/gcb.14020
143
Devi S. Singh D. Kaur J. (2020). Microplastics in freshwater ecosystems: Sources, distribution, and ecological effects. Environ. Monitoring Assess.192, 585. doi: 10.1007/s10661-020-08542-3
144
De Vidales M. J. M. Sáez C. Pérez J. F. Cotillas S. Llanos J. Cañizares P. et al . (2020). Irradiation-assisted electrochemical processes for the removal of persistent organic pollutants from wastewater. J. Appl. Electrochemistry45, 799–808. doi: 10.1007/s10800-015-0825-0
145
De Witte B. Devriese L. Bekaert K. Hoffman S. Vandermeersch G. Cooreman K. et al . (2014). Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull.85, 146–155. doi: 10.1016/j.marpolbul.2014.06.006
146
Dhiman S. Sharma C. Kumar A. Pathak P. Purohit S. D. (2023). Microplastics in aquatic and food ecosystems: remediation coupled with circular economy solutions to create resource from waste. Sustainability15, 14184. doi: 10.3390/su151914184
147
Di M. Wang J. (2018). Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environment.616, 1620–1627. doi: 10.1016/j.scitotenv.2017.10.150
148
Dimassi S. N. Hahladakis J. N. Yahia M. N. D. Sayadi S. Al-Ghouti M. A. (2022). Microplastics in the Mediterranean Sea: An updated review on contamination, sources, impacts, and mitigation measures. Mar. Pollut. Bull.181, 113887. doi: 10.1016/j.marpolbul.2022.113887
149
Ding L. Mao R. fan Guo X. Yang X. Zhang Q. et al . (2019). Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ.667, 427–434. doi: 10.1016/j.scitotenv.2019.02.332
150
Ding J. Sun C. Li J. Shi H. Xu X. Ju P. et al . (2022). Microplastics in global bivalve mollusks: A call for protocol standardization. J. Hazardous Materials.438, 129490. doi: 10.1016/j.jhazmat.2022.129490
151
Di Renzo L. Iacoponi F. Panettieri V. Ferri N. Lione T. (2021). Effects of microplastics on fish health: A review. Vet. Sci.8, 118. doi: 10.3390/vetsci8070118
152
Dong M. Luo Z. Jiang Q. Xing X. Zhang Q. Sun Y. (2020). The rapid increases in microplastics in urban lake sediments. Sci. Rep.10, 848. doi: 10.1038/s41598-020-57933-8
153
Dowarah K. Devipriya S. P. (2019). Microplastic prevalence in the beaches of Puducherry, India and its correlation with fishing and tourism/recreational activities. Mar. Pollut. Bull.148, 123–133. doi: 10.1016/j.marpolbul.2019.07.066
154
Dowarah K. Patchaiyappan A. Thirunavukkarasu C. Jayakumar S. Devipriya S. P. (2020). Quantification of microplastics using Nile Red in two bivalve species Perna viridis and Meretrix from three estuaries in Pondicherry, India and microplastic uptake by local communities through bivalve diet. Mar. pollut. Bull.153, 110982. doi: 10.1016/j.marpolbul.2020.110982
155
Dris R. Gasperi J. Rocher V. Saad M. Renault N. Tassin B. (2015). Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem.12, 592–599. doi: 10.1071/EN14167
156
Dris R. Gasperi J. Tassin B. (2018). “ Sources and fate of microplastics in urban areas: a focus on Paris megacity,” in Freshwater microplastics: emerging environmental contaminants? 69–83.
157
Du F. Cai H. Zhang Q. Chen Q. Shi H. (2020). Microplastics in take-out food containers. J. Hazard. Mater.399, 122969. doi: 10.1016/j.jhazmat.2020.122969
158
Du F. Wang J. Wang T. Zhao X. Li X. Guo S. et al . (2024). New molecular mechanism of nanoplastics affecting cadmium protein toxicity: Conformational response and differential binding of human serum albumin. Sci. Total Environ.950, 175330. doi: 10.1016/j.scitotenv.2024.175330
159
Duis K. Coors A. (2016). Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Europe.28, 2. doi: 10.1186/s12302-015-0069-y
160
Eastman C. B. Farrell J. A. Whitmore L. Rollinson Ramia D. R. Thomas R. S. Prine J. et al . (2020). Plastic ingestion in post-hatchling sea turtles: assessing a major threat in Florida near shore waters. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00693
161
Edo C. González-Pleiter M. Leganés F. Fernández-Piñas F. Rosal R. (2020). Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut.259, 113837. doi: 10.1016/j.envpol.2019.113837
162
Egea-Corbacho A. Martín-García A. P. Franco A. A. Albendín G. Arellano J. M. Rodríguez-Barroso R. et al . (2023). Microplastic in industrial aquaculture: Occurrence in the aquatic environment, feed and organisms (Dicentrarchus labrax). Sci. Total Environ.904, 166774. doi: 10.1016/j.scitotenv.2023.166774
163
El-Dash H. A. Yousef N. E. Aboelazm A. A. Awan Z. A. Yahya G. El-Ganiny A. M. (2023). Optimizing eco-friendly degradation of polyvinyl chloride (PVC) plastic using environmental strains of Malassezia species and aspergillus fumigatus. Int. J. Mol. Sci.24. doi: 10.3390/ijms242015452
164
Elgarahy A. M. Akhdhar A. Elwakeel K. Z. (2021). Microplastics prevalence, interactions, and remediation in the aquatic environment: a critical review. J. Environ. Chem. Engineering.9, 106224. doi: 10.1016/j.jece.2021.106224
165
Emadian S. M. Onay T. T. Demirel B. (2017). Biodegradation of bioplastics in natural environments. Waste Manage.59, 526–536. doi: 10.1016/j.wasman.2016.10.006
166
Enders K. Lenz R. Stedmon C. A. Nielsen T. G. (2015). Abundance, size and polymer composition of marine microplastics ≥10μm in the Atlantic Ocean and their modelled vertical distribution. Mar. pollut. Bull.100, 70–81. doi: 10.1016/j.marpolbul.2015.09.027
167
Endo S. Takizawa R. Okuda K. Takada H. Chiba K. Kanehiro H. et al . (2005). Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull.50, 1103–1114. doi: 10.1016/j.marpolbul.2005.04.030
168
Enfrin M. Dumée L. F. Lee J. Khan S. J. (2019). Nano- and microplastics: A threat to water and wastewater treatment systems? Water Res.161, 621–638.
169
Eriksen M. Maximenko N. Thiel M. Cummins A. Lattin G. Wilson S. et al . (2013). Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull.68, 71–76. doi: 10.1016/j.marpolbul.2012.12.021
170
Eriksen M. Thiel M. Lebreton L. (2019). “ Nature of plastic marine pollution in the subtropical gyres,” in Hazardous chemicals associated with plastics in the marine environment, 135–162. doi: 10.1007/698_2016_123
171
Fabra M. Williams L. Watts J. E. Hale M. S. Couceiro F. Preston J. (2021). The plastic Trojan horse: Biofilms increase microplastic uptake in marine filter feeders impacting microbial transfer and organism health. Sci. total environment.797, 149217. doi: 10.1016/j.scitotenv.2021.149217
172
Faheem M. Shabbir S. Zhao J. G. Kerr P. Ali S. Sultana N. et al . (2020). Multifunctional periphytic biofilms: Polyethylene degradation and Cd2+ and Pb2+ bioremediation under high methane scenario. Int. J. Mol. Sci.21, 5331. doi: 10.3390/ijms21155331
173
Fan C. Huang Y. Z. Lin J. N. Li J. (2021). Microplastic constituent identification from admixtures by Fourier-transform infrared (FTIR) spectroscopy: The use of polyethylene terephthalate (PET), polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC) and nylon (NY) as the model constituents. Environ. Technol. Innovation23, 101798. doi: 10.1016/j.eti.2021.101798
174
Fang C. Zheng R. Chen H. Hong F. Lin L. Lin H. et al . (2019). Comparison of microplastic contamination in fish and bivalves from two major cities in Fujian province, China and the implications for human health. Aquaculture.512, 734322. doi: 10.1016/j.aquaculture.2019.734322
175
Faramarzi P. Jang W. Oh D. Kim B. Kim J. H. You J. B. (2024). Microfluidic detection and analysis of microplastics using surface nanodroplets. ACS Sens.9, 1489–1498. doi: 10.1021/acssensors.3c02627
176
Farrell P. Nelson K. (2013). Trophic level transfer of microplastics: Mytilus edulis (L) to Carcinus maenus (L). Environ. pollut.177, 1–3. doi: 10.1016/j.envpol.2013.01.046
177
Fecker T. Rabe J. P. Tiwari S. Walther F. Scheper T. (2018). A novel analytical method allowing class-specific quantification of microplastics in environmental samples using F-T-IR microscopy and multivariate analysis. Analytical Bioanalytical Chem.410, 4689–4700. doi: 10.1007/s00216-018-1096-2
178
Feil A. Pretz T. (2020). “ Mechanical recycling of packaging waste,” in Plastic waste and recycling. Ed. LetcherT. ( Academic Press), 283–319. doi: 10.1016/b978-0-12-817880-5.00011-6
179
Fendall L. S. Sewell M. A. (2009). Contributing to marine pollution by washing your face: microplastics in facial cleansers. Mar. pollut. bulletin.58, 1225–1228. doi: 10.1016/j.marpolbul.2009.04.025
180
Feng J. Li Z. Xu C. Zhang W. (2023). Engineering microbial pathways for efficient plastic biodegradation. Nat. Chem. Biol.19, 204–213. doi: 10.1038/s41589-022-01261-4
181
Feng J. Soto-Berelov M. Kestens A. Murphy S. Fall S. Delatte N. (2011). Photocatalytic decomposition of chlorinated hydrocarbons on concrete pavement using titanium dioxide. J. Mater. Civil Eng.23, 883–888.
182
Feng Z. Zhang T. Wang J. Huang W. Wang R. Xu J. et al . (2021). Spatio-temporal features of microplastics pollution in macroalgae growing in an important mariculture area, China. Sci. Total Environ.719, 137490. doi: 10.1016/j.scitotenv.2020.137490
183
Ferreira G. V. B. Barletta M. Lima A. R. A. Dantas D. V. Justino A. K. S. Costa M. F. (2016). Plastic debris contamination in the life cycle of Acoupa weakfish (Cynoscion acoupa) in a tropical estuary. ICES J. Marine Sci.73, 2695–2707. doi: 10.1093/icesjms/fsw108
184
Ferreira M. Thompson J. Paris A. Rohindra D. Rico C. (2020). Presence of microplastics in water, sediments and fish species in an urban coastal environment of Fiji, a Pacific small island developing state. Mar. pollut. Bull.153, 110991. doi: 10.1016/j.marpolbul.2020.110991
185
Fotopoulou K. N. Karapanagioti H. K. (2019). “ Degradation of various plastics in the environment,” in Hazardous chemicals associated with plastics in the marine environment, Springer International Publishing, 71–92. doi: 10.1007/698_2017_11
186
Fries E. Dekiff J. H. Willmeyer J. Nuelle M. T. Ebert M. Remy D. (2013). Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci.: Process. Impacts15, 1949–1956. doi: 10.1039/C3EM00214D
187
Galafassi S. Sabatino R. Sathicq M. B. Eckert E. M. Fontaneto D. Dalla Fontana G. et al . (2021). Contribution of microplastic particles to the spread of resistances and pathogenic bacteria in treated wastewaters. Water Res.201, 117368. doi: 10.1016/j.watres.2021.117368
188
Galloway T. S. Cole M. Lewis C. (2017). Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol.1, 116. doi: 10.1038/s41559-017-0116
189
Gambino I. Bagordo F. Grassi T. Panico A. De Donno A. (2022). Occurrence of microplastics in tap and bottled water: Current knowledge. Int. J. Environ. Res. Public Health19, 5283. doi: 10.3390/ijerph19095283
190
Gao Y. Fan K. Lai Z. Wang C. Li H. Liu Q. (2022). A comprehensive review of the circulation of microplastics in aquatic ecosystem using scientometric method. Environ. Sci. Pollut. Res.29, 30935–30953. doi: 10.1007/s11356-022-19565-5
191
Garrido Gamarro E. Ryder J. Elvevoll E. O. Olsen R. L. (2020). Microplastics in fish and shellfish–a threat to seafood safety? J. Aquat. Food Product Technol.29, 417–425. doi: 10.1080/10498850.2020.1739793
192
Gbogbo F. Takyi J. B. Billah M. K. Ewool J. (2020). Analysis of microplastics in wetland samples from coastal Ghana using the Rose Bengal stain. Environ. Monitoring Assess.192, 208. doi: 10.1007/s10661-020-8180-9
193
Gedik K. Eryaşar A. R. (2020). Microplastic pollution profile of Mediterranean mussels (Mytilus galloprovincialis) collected along the Turkish coasts. Chemosphere.260, 127570. doi: 10.1016/j.chemosphere.2020.127570
194
GESAMP (2019). “ Guidelines for the monitoring and assessment of plastic litter and microplastics in the ocean,” in GESAMP Reports & Studies No. 99. Eds. KershawP. J.TurraA.GalganiF. ( International Maritime Organization). Available online at: http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean.
195
Gewert B. Plassmann M. M. MacLeod M. (2015). Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci.: Process. Impacts17, 1513–1521. doi: 10.1039/C5EM00207A
196
Geyer R. Jambeck J. R. Law K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv.3, e1700782. doi: 10.1126/sciadv.1700782
197
Ghosh S. Roy S. Mukherjee S. Chattopadhyay D. Bhattacharya P. (2025). Microplastics in aquatic environment: A global perspective on distribution, source apportionment, ecotoxicological risk, and remediation strategies. J. Environ. Manage.350, 119650.
198
Giacomucci L. Raddadi N. Soccio M. Lotti N. Fava F. (2019). Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol.52, 35–41. doi: 10.1016/j.nbt.2019.04.005
199
Giani D. Baini M. Galli M. Casini S. Fossi M. C. (2019). Microplastics occurrence in edible fish species (Mullus barbatus and Merluccius merluccius) collected in three different geographical sub-areas of the Mediterranean Sea. Mar. pollut. Bull.140, 129–137. doi: 10.1016/j.marpolbul.2019.01.005
200
Gies E. A. LeNoble J. L. Noël M. Etemadifar A. Bishay F. Hall E. R. et al . (2018). Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull.133, 553–561. doi: 10.1016/j.marpolbul.2018.06.006
201
Gobbi C. N. Sanches V. M. L. Pacheco E. B. A. V. Guimarães M. J. D. O. C. de Freitas M. A. V. (2017). Management of plastic wastes at Brazilian ports and diagnosis of their generation. Mar. Pollut. Bull.124, 67–73. doi: 10.1016/j.marpolbul.2017.06.081
202
Gokulakrishnan S. A. Arthanareeswaran G. László Z. Veréb G. Kertész S. Kweon J. (2021). Recent development of photocatalytic nanomaterials in mixed matrix membrane for emerging pollutants and fouling control, membrane cleaning process. Chemosphere281, 130891. doi: 10.1016/j.chemosphere.2021.130891
203
Gong X. Tian L. Wang P. Wang Z. Zeng L. Hu J. (2023). Microplastic pollution in the groundwater under a bedrock island in the South China sea. Environ. Res.239, 117277. doi: 10.1016/j.envres.2023.117277
204
González-Jauregui M. Borges-Ramírez M. M. Lemos-Barão J. A. Escamilla-López A. et al . (2019). Stomach flushing technique applied to quantify microplastics in Crocodilians. MethodsX6. doi: 10.1016/j.mex.2019.11.013
205
González-Pleiter M. Edo C. Aguilera Á. Viúdez-Moreiras D. Pulido-Reyes G. González-Toril E. et al . (2021). Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci. Total Environ.761, 143213. doi: 10.1016/j.scitotenv.2020.143213
206
Good T. P. June J. A. Etnier M. A. Broadhurst G. (2010). Derelict fishing nets in Puget Sound and the Northwest Straits: Patterns and threats to marine fauna. Mar. Pollut. Bull.60, 39–50. doi: 10.1016/j.marpolbul.2009.09.005
207
Goswami P. Vinithkumar N. V. Dharani G. (2020). First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay. Andaman Islands Mar. pollut. Bull.155, 111163. doi: 10.1016/j.marpolbul.2020.111163
208
Govender J. Naidoo T. Rajkaran A. Cebekhulu S. Bhugeloo A. Sershen (2020). Towards characterising microplastic abundance, typology and retention in mangrove-dominated estuaries. Water12, 2802. doi: 10.3390/w12102802
209
Graham E. R. Thompson J. T. (2009). Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Marine Biol. Ecol.368, 22–29. doi: 10.1016/j.jembe.2008.09.007
210
Green D. S. Boots B. O'Connor N. E. Thompson R. (2019). Microplastics affect the ecological functioning of an important biogenic habitat. Environ. Sci. Technol.53, 1118–1126. doi: 10.1021/acs.est.8b04417
211
Gregory M. R. (1996). Plastic ‘scrubbers’ in hand cleansers: A further (and minor) source for marine pollution identified. Mar. pollut. Bull.32, 867–871. doi: 10.1016/S0025-326X(96)00047-1
212
Gregory M. R. Andrady A. L. (2003). “ Plastic in the marine environment,” in Plastics and the environment. Ed. AndradyA. ,. L. ( John Wiley, New York), 379–401. doi: 10.1002/0471721557.ch10
213
Guo M. Noori R. Abolfathi S. (2024). Microplastics in freshwater systems: Dynamic behaviour and transport processes. Resources Conserv. Recycling205, 107578. doi: 10.1016/j.resconrec.2024.107578
214
Guo X. Wang J. (2019). The chemical behaviors of microplastics in marine environment: A review. Mar. Pollut. Bull.142, 1–14. doi: 10.1016/j.marpolbul.2019.03.019
215
Gurjar U. R. Xavier M. Nayak B. B. Ramteke K. Deshmukhe G. Jaiswar A. K. et al . (2021). Microplastics in shrimps: a study from the trawling grounds of north eastern part of Arabian Sea. Environ. Sci. pollut. Res.288, 1–11. doi: 10.1007/s11356-021-14121-z
216
Habib R. Z. Thiemann T. Al Kendi R. (2020). Microplastics and wastewater treatment plants – A review. J. Water Resource Prot.12, 1–35. doi: 10.4236/jwarp.2020.121001
217
Hadiyanto Elystia S. Fatimah S. Hartanto D. (2021). Microplastics as an emerging contaminant in Indonesian seas: Current status and future research direction. Mar. Pollut. Bull.169, 112553. doi: 10.1016/j.marpolbul.2021.112553
218
Hale R. C. King A. E. Ramirez J. M. (2022). Durable plastic goods: A source of microplastics and chemical additives in the built and natural environments. Environ. Sci. Technol. Lett.9. doi: 10.1021/acs.estlett.2c00417
219
Hale R. C. Seeley M. E. La Guardia M. J. Mai L. Zeng E. Y. (2020). A global perspective on microplastics. J. Geophys. Res.: Oceans125, e2020JC016552. doi: 10.1029/2020JC016552
220
Han X. Liu D. Chen J. Lu X. (2017). Microplastic analysis: Advances, challenges, and prospects. Chemosphere188, 661–672. doi: 10.1016/j.chemosphere.2017.09.080
221
Hanvey J. S. Lewis P. J. Lavers J. L. Crosbie N. D. Pozo K. Clarke B. O. (2017). A review of analytical techniques for quantifying microplastics in sediments. Analytical Methods9, 1369–1383. doi: 10.1039/C6AY02707E
222
Haque F. Fan C. (2023). Fate and impacts of microplastics in the environment: hydrosphere, pedosphere, and atmosphere. Environments10, 70. doi: 10.3390/environments10050070
223
Hatami M. Banaee M. Nematdoost Haghi B. Javadzadeh N. (2019). Biochemical alterations in liver and gill of rainbow trout (Oncorhynchus mykiss) exposed to various levels of microplastic contamination. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol.226, 108621. doi: 10.1016/j.cbpc.2019.108621
224
He D. Luo Y. Lu S. Liu M. Song Y. Lei L. (2018). Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Analytical Chem.109, 163–172. doi: 10.1016/j.trac.2018.10.006
225
He S. Jia M. Xiang Y. Song B. Xiong W. Cao J. et al . (2022). Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications. J. Hazardous Materials.424, 127286. doi: 10.1016/j.jhazmat.2021.127286
226
Hendrickson E. Minor E. C. Schreiner K. (2018). Microplastic abundance and composition in western Lake Superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. technology.52, 1787–1796. doi: 10.1021/acs.est.7b05829
227
Herbort A. F. Sturm M. T. Schuhen K. (2018). A new approach for the agglomeration and subsequent removal of polyethylene, polypropylene, and mixtures of both from freshwater systems – A case study. Environ. Sci. Pollut. Res.25, 15226–15234. doi: 10.1007/s11356-018-1981-7
228
Hidayaturrahman H. Lee T. G. (2019). A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull.146, 696–702. doi: 10.1016/j.marpolbul.2019.06.071
229
Hipfner J. M. Galbraith M. Tucker S. Studholme K. R. Domalik A. D. Pearson S. F. et al . (2018). Two forage fishes as potential conduits for the vertical transfer of microfibres in Northeastern Pacific Ocean food webs. Environ. Pollution.239, 215–222. doi: 10.1016/j.envpol.2018.04.009
230
Horton A. A. Walton A. Spurgeon D. J. Lahive E. Svendsen C. (2017). Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environment.586, 127–141. doi: 10.1016/j.scitotenv.2017.01.190
231
Horton A. A. Weerasinghe K. I. Mayor D. J. Lampitt R. (2024). Microplastics in commercial marine fish species in the UK–A case study in the River Thames and the River Stour (East Anglia) estuaries. Sci. Total Environ.915, 170170. doi: 10.1016/j.scitotenv.2024.170170
232
Hoss D. E. Settle L. R. (1990). “ Ingestion of plastics by teleost fish,” in Proceedings of the Second International Conference on Marine Debris, Miami: NOAA technical memorandum. NOAA-TM-NMFS-SWFSC-154693–709.
233
Hossain M. A. Rahman M. A. Rahman M. M. (2023). Plastic pollution and its impact on aquatic ecosystems: A global perspective. Environ. Challenges11, 100776. doi: 10.1016/j.envc.2023.100776
234
Hossain M. S. Sobhan F. Uddin M. N. (2019). Microplastics in fishes from the Northern Bay of Bengal. Sci. Total Environ.690, 821–830. doi: 10.1016/j.scitotenv.2019.07.065
235
Hou L. McMahan C. D. McNeish R. E. Scarlett J. Shafer M. M. Hoellein T. J. et al . (2021). Microplastic accumulation in Lake Michigan tributary rivers quantified using a new laboratory method. Environ. Sci. Technol.55, 12012–12021. doi: 10.1021/acs.est.1c02987
236
Hove H. T. B. Næsheim T. Kogel T. (2023). Quick and efficient microplastic isolation from fatty fish tissues by surfactant-enhanced alkaline digestion. Mar. pollut. Bull.197, 115726. doi: 10.1016/j.marpolbul.2023.115726
237
Hu Y. Gong M. Wang J. Bassi A. (2018). Current research trends on microplastic pollution from wastewater systems: A critical review. Rev. Environ. Sci. Bio/Technol.18, 207–230. doi: 10.1007/s11157-019-09498-w
238
Hu Y. Gong M. Wang J. Bassi A. (2019). Current research trends on microplastic pollution from wastewater systems: a critical review. Rev. Environ. Sci. Biotechnol.18, 207–230. doi: 10.1007/s11157-019-09498-w
239
Hu K. Tian W. Yang Y. Nie G. Zhou P. Wang Y. et al . (2021). Microplastics remediation in aqueous systems: Strategies and technologies. Water Res.198, 117144. doi: 10.1016/j.watres.2021.117144
240
Hua L. Zhang J. Wang R. (2010). Characterization of polyethylene-degrading microorganisms from soil. Afr. J. Microbiol. Res.4, 2564–2568. doi: 10.5897/AJMR10.327
241
Huang H. Lei P. Yu H. Du J. Wu B. Wang H. et al . (2024). Micro/nano plastics in the urinary system: Pathways, mechanisms, and health risks. Environ. Int.193, 109109. doi: 10.1016/j.envint.2024.109109
242
Huang Y. Liu Q. Jia W. Yan C. Wang J. (2020). Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut.260, 114096. doi: 10.1016/j.envpol.2020.114096
243
Huang D. Tao J. Cheng M. Deng R. Chen S. Yin L. et al . (2021a). Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater.407, 124399. doi: 10.1016/j.jhazmat.2020.12439
244
Huerta Lwanga E. Gertsen H. Gooren H. Peters P. Salanki T. Ploeg M. V. D. et al . (2016). Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae), Environ. Sci. Technol.50, 2685e2691. doi: 10.1021/acsomega.1c02760
245
Huerta Lwanga E. Mendoza Vega J. Ku Quej V. Chi J. A. Sanchez del Cid L. Chi C. et al . (2018). Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep.7, 14071. doi: 10.1038/s41598-017-14588-2
246
Hüffer T. Weniger A. K. Hofmann T. (2018). Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollution.236, 218–225. doi: 10.1016/j.envpol.2018.01.022
247
Hurley R. Woodward J. Rothwell J. J. (2018). Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geoscience.11, 251–257. doi: 10.1038/s41561-018-0080-1
248
Hwang G.-J. Xie H. Wah B. W. Gašević D. (2020). Vision, challenges, roles and research issues of Artificial Intelligence in Education. Comput. Educ.: Artif. Intell.1, 100001. doi: 10.1016/j.caeai.2020.100001
249
Iheanacho S. C. Odo G. E. (2020). Neurotoxicity, oxidative stress biomarkers and haematotoxic effects of microplastic exposure in Clarias gariepinus. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol.239, 108amsterdams. doi: 10.1016/j.cbpc.2020.108893
250
Imhof H. K. Ivleva N. P. Schmid J. Niessner R. Laforsch C. (2012). Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol.22, R882–R883.
251
Ingole B. S. Kadam A. N. (2003). Coastal environment of India. Indian J. Marine Sci.32, 7–17.
252
Iram N. Hussain A. Ashraf M. A. Rauf S. Khalid A. Amjad M. et al . (2019). Isolation and characterization of plastic degrading bacteria from soil samples. J. King Saud Univ. - Sci.31, 898–902.
253
Irfan M. Qadir A. Mumtaz M. Ahmad S. R. (2020). An unintended challenge of microplastic pollution in the urban surface water system of Lahore, Pakistan. Environ. Sci. pollut. Res. doi: 10.1007/s11356-020-08114-7
254
Iroegbu A. O. Prasad R. Agboola O. P. (2021). Stakeholder engagement for sustainable plastic pollution policies: A review. Environ. Sustainability4, 89–102. doi: 10.3390/recycling9050077
255
Isobe A. Iwasaki S. Uchida K. Tokai T. (2019). Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat. Commun.10, 417. doi: 10.1038/s41467-019-08316-9
256
Iyare P. U. Ouki S. K. Bond T. (2020). Microplastics removal in wastewater treatment plants: a critical review. Environ. Sci. Water Res. Technol.6, 2664–2675. doi: 10.1039/d0ew00397b
257
Iyer H. Grandgeorge P. Jimenez A. Campbell I. Parker M. Holden M. et al . (2023). Fabricating strong and stiff bioplastics from whole spirulina cells. Advanced Funct. Materials33. doi: 10.1002/adfm.202302067
258
Jabeen K. Li B. Chen Q. Su L. Wu C. Hollert H. et al . (2018). Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere213, 323–332. doi: 10.1016/j.chemosphere.2018.09.031
259
Jaikumar G. Brun N. R. Vijver M. G. Bosker T. (2019). Reproductive toxicity of primary and secondary microplastics to three cladocerans during chronic exposure. Environ. Pollut.249, 638–646. doi: 10.1016/j.envpol.2019.03.085
260
Jambeck J. R. Geyer R. Wilcox C. Siegler T. R. Perryman M. Andrady A. et al . (2015). Plastic waste inputs from land into the ocean. Science347, 768–771. doi: 10.1126/science.1260352
261
James K. Vasant K. Padua S. Gopinath VKSARJ Babu A. John S. (2020). An assessment of microplastics in the ecosystem and selected commercially important fishes off Kochi, south eastern Arabian Sea. India Mar. pollut. Bull.154, 111027. doi: 10.1016/j.marpolbul.2020.111027
262
Janssen I. Heymsfield S. B. Ross R. (2002). Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatrics Soc.50, 889–896. doi: 10.1046/j.1532-5415.2002.50216.x
263
Janssens L. Garcia-Vazquez E. (2021). Dangerous microplastics in top shells and anemones along the north coast of Spain. Mar. pollut. Bull.173, 112945. doi: 10.1016/j.marpolbul.2021.112945
264
Jayasiri H. B. Vennila A. Purushothaman C. S. (2014). Spatial and temporal variability of metals in inter-tidal beach sediment of Mumbai, India. Environ. Monit Assess.186, 1101–1111. doi: 10.1007/s10661-013-3441-7
265
Jelil S. N. Jain N. (2014). “ A rapid assessment of beach litter in Mumbai beaches,” in Technical Report: Reef Watch Marine Conservation, vol. 25. Mumbai, India. doi: 10.13140/rg.2.1.4760.3449
266
Jeon J. M. Park S. J. Choi T. R. Park J. H. Yang Y. H. Yoon J. J. (2021). Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polym. Degrad. Stab.191. doi: 10.1016/j.polymdegradstab.2021.109662
267
Jeong C. B. Kang H. M. Lee M. C. Kim D. H. Han J. Hwang D. S. et al . (2017). Adverse effects of microplastics and oxidative stress- induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep.7, 41323. doi: 10.1038/srep41323
268
Jeyasanta K. I. Patterson J. Grimsditch G. Edward J. K. P. (2020b). Occurrence and characteristics of microplastics in the coral reef, sea grass and near shore habitats of Rameswaram Island, India. Mar. Pollut. Bull.160, 111674. doi: 10.1016/j.marpolbul.2020.111674
269
Jeyasanta K. I. Sathish N. Patterson J. Edward J. K. P. (2020a). Macro-, meso- and microplastic debris in the beaches of Tuticorin district, Southeast coast of India. Mar. Pollut. Bull.154, 111055. doi: 10.1016/j.marpolbul.2020.111055
270
Jia C. Chen J. Luo Y. Li G. Zhang G. (2019). Microplastics from atmosphere affect the water quality of urban lakes. Environ. Pollut.257, 113639.
271
Jiang J. J. Hanun J. N. Chen K. Y. Hassan F. Liu K. T. Hung Y. H. et al . (2023). Current levels and composition profiles of microplastics in irrigation water. Environ. Pollution.318, 120858. doi: 10.1016/j.envpol.2022.120858
272
Jiang Y. Yang F. Kazmi S. S. U. H. Zhao Y. Chen M. Wang J. (2022). A review of microplastic pollution in seawater, sediments and organisms of the Chinese coastal and marginal seas. Chemosphere286, 131677. doi: 10.1016/j.chemosphere.2021.131677
273
Jiang X. Chang Y. Zhang T. Qiao Y. Klobučar G. Li M. (2020). Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut.259, 113896. doi: 10.1016/j.envpol.2019.113896
274
Jiao M. Wang Y. Li T. Li R. Liu B. (2022). Riverine microplastics derived from mulch film in Hainan Island: Occurrence, source and fate. Environ. pollut.312, 120093. doi: 10.1016/j.envpol.2022.120093
275
Jin M. Liu Z. Hu W. Song Y. (2022). Analytical challenges of microplastics in environmental matrices: Considerations and perspectives. TrAC Trends Analytical Chem.153, 116649. doi: 10.1016/j.trac.2022.116649
276
Jin Y. Lu L. Tu W. Luo T. Fu Z. (2018). Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ.649, 308–317. doi: 10.1016/j.scitotenv.2018.08.353
277
Jong M. C. Tong X. Li J. Xu Z. Chng S. H. Q. He Y. et al . (2022). Microplastics in equatorial coasts: Pollution hotspots and spatiotemporal variations associated with tropical monsoons. J. Hazard. Mater.424, 127626. doi: 10.1016/j.jhazmat.2021.127626
278
Joo S. Liang Y. Kim M. Byun J. Choi H. (2018). Microplastics with adsorbed contaminants: Mechanisms and treatment. Environ. Eng. Res.23, 107–115. doi: 10.4491/eer.2017.100
279
Joo S. H. Liang Y. Kim M. Byun J. Choi H. (2021). Microplastics with adsorbed contaminants: Mechanisms and Treatment. Environ. Challenges.3, 100042. doi: 10.1016/j.envc.2021.100042
280
Joseph P. Nandan S. B. Adarsh K. J. Anu P. R. Varghese R. Sreelekshmi S. et al . (2019). Heavy metal contamination in representative surface sediments of mangrove habitats of Cochin. South. India Environ. Earth Sci.78, 490. doi: 10.1007/s12665-019-8499-2
281
Joshy A. Sharma S. R. K. Mini K. G. (2022). Microplastic contamination in commercially important bivalves from the southwest coast of India. Environ. pollut.305, 119250. doi: 10.1016/j.envpol.2022.119250
282
Kamp J. Dierkes G. Schweyen P. N. Wick A. Ternes T. A. (2023). Quantification of poly (vinyl chloride) microplastics via pressurized liquid extraction and combustion ion chromatography. Environ. Sci. Technol.57, 4806–4812. doi: 10.1021/acs.est.2c08012
283
Kane I. A. Clare M. A. (2019). Dispersion, accumulation and the ultimate fate of microplastics in deep-marine environments: a review and future directions. Front. Earth Sci.7. doi: 10.3389/feart.2019.00080
284
Kang J. An Y. Kim J. Park S. (2023). Biodegradation of polyethylene terephthalate (PET) by engineered Ideonella sakaiensis enzymes. Nat. Commun.14, 2154. doi: 10.1038/s41467-023-37923-4
285
Kapp K. J. Yeatman E. (2018). Microplastic hotspots in the Snake and lower columbia rivers: a journey from the greater yellowstone ecosystem to the Pacific Ocean. Environ. pollut.241, 1082–1090. doi: 10.1016/j.envpol.2018.06.033
286
Karami A. Golieskardi A. Choo C. K. Larat V. Galloway T. S. Salamatinia B. (2017). The presence of microplastics in commercial salts from different countries. Sci. Rep.7, 46173. doi: 10.1038/srep46173
287
Karbalaei S. Golieskardi A. Hamzah H. B. Abdulwahid S. Hanachi P. Walker T. R. et al . (2019). Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar. pollut. Bulletin.148, 5–15. doi: 10.1016/j.marpolbul.2019.07.072
288
Kasmuri N. Tarmizi N. A. A. Mojiri A. (2022). Occurrence, impact, toxicity, and degradation methods of microplastics in environment — a review. Environ. Sci. Pollut. Res.29, 30820–30836. doi: 10.1007/s11356-021-18268-7
289
Katija K. Choy C. A. Sherlock R. E. Sherman A. D. Robison B. H. (2017). From the surface to the seafloor: How giant larvaceans transport microplastics into the deep sea. Sci. Adv.3, e1700715. doi: 10.1126/sciadv.1700715
290
Kaur Brar S. Babu J. N. Singh R. (2023). Microbial enzymes for plastic degradation: An overview of challenges and future perspectives. J. Cleaner Prod.414, 137647. doi: 10.1016/j.jclepro.2023.137647
291
Kazour M. Jemaa S. Issa C. Khalaf G. Amara R. (2019). Microplastics pollution along the Lebanese coast (Eastern Mediterranean Basin): occurrence in surface water, sediments and biota samples. Sci. Total Environ.696, 133933. doi: 10.1016/j.scitotenv.2019.133933
292
Khan F. R. Halle L. L. Palmqvist A. Norrgren L. (2024). Microplastic toxicity to aquatic organisms: A critical review. Sci. Total Environ.907, 168221. doi: 10.1016/j.scitotenv.2024.168221
293
Khoironi A. Anggoro S. Sudarno S. (2019). Community behavior and single-use plastic bottle consumption. IOP Conf. Ser.: Earth Environ. Sci.293, 12002. doi: 10.1088/1755-1315/293/1/012002
294
Kida T. Imamura K. Kuroda K. (2022). Microplastics in freshwater and marine environments: Sources, detection, and toxicity. Environ. Sci.: Process. Impacts24, 142–156. doi: 10.1039/D1EM00362A
295
Kim D. Chae Y. An Y. J. (2017). Mixture toxicity of nickel and microplastics with different functional groups on Daphnia magna. Environ. Sci. Technol.51, 12852–12858. doi: 10.1021/acs.est.7b03732
296
Kim H. W. Jo J. H. Bin Kim Y. Le T. K. Cho C. W. Yun C. H. et al . (2021). Mater416. doi: 10.1016/j.jhazmat.2021.126239
297
Kim K. T. Park S. (2021). Enhancing microplastics removal from wastewater using electro-coagulation and granule-activated carbon with thermal regeneration. Processes9, 617. doi: 10.3390/pr9040617
298
Kirschvink J. L. Kobayashi-Kirschvink A. Woodford B. J. (1996). Magnetite biomineralization in bacteria, protists, and animals. Proc. Natl. Acad. Sci.89, 7683–7687. doi: 10.1073/pnas.89.16.7683
299
Kirstein I. V. Wichels A. Gullans E. Krohne G. Gerdts G. (2019). The Plastisphere e Uncovering tightly attached plastic “specific” microorganisms. PLoS One14, e0215859. doi: 10.1371/journal.pone.0215859
300
Klavarioti M. Mantzavinos D. Kassinos D. (2009). Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int.35, 402–417. doi: 10.1016/j.envint.2008.08.007
301
Klingelhöfer D. Braun M. Quarcoo D. Brüggmann D. Groneberg D. A. (2020). Research landscape of a global environmental challenge. Water Res.170, 115358.
302
Klosterhaus S. L. Dreis E. Baker J. E. (2011). Bioaccumulation kinetics of polybrominated diphenyl ethers from estuarine sediments to the marine polychaete. Nereis Virens30, 1204–1212. doi: 10.1002/etc.497
303
Kole P. J. Löhr A. J. Van Belleghem F. G. Ragas A. M. (2017). Wear and tear of tyres: a stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health14, 1265. doi: 10.3390/ijerph14101265
304
Kolenda K. Ku´smierek N. Pstrowska K. (2020). Microplastic ingestion by tadpoles of pond-breeding amphibians-first results from Central Europe (SW Poland). Environ. Sci. pollut. Res. Int.27, 33380–33384. doi: 10.1007/s11356-020-09648-6
305
Koongolla J. B. Lin L. Pan Y. F. Yang C. P. Sun D. R. Liu S. et al . (2020). Occurrence of microplastics in gastrointestinal tracts and gills of fish from Beibu Gulf. South China Sea258, 113734. doi: 10.1016/j.envpol.2019.113734
306
Kopecká R. Kubínová I. Sovová K. Mravcová L. Vítěz T. Vítězová M. (2022). Microbial degradation of virgin polyethylene by bacteria isolated from a landfill site. Sn Appl. Sci.4. doi: 10.1007/s42452-022-05182-x
307
Kour H. Khan S. S. Kour D. Rasool S. Sharma Y. P. Rai P. K. et al . (2023). Microbes mediated plastic degradation: a sustainable approach for environmental sustainability. J. Appl. Biol. Biotechnol.11, 9–19. doi: 10.7324/JABB.2023.110515
308
Kowalski N. Reichardt A. M. Waniek J. J. (2016). Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. pollut. bulletin.109, 310–319. doi: 10.1016/j.marpolbul.2016.05.064
309
Krishnakumar S. Anbalagan S. Kasilingam K. Smrithi P. Anbazhagi S. Srinivasalu S. (2020). Assessment of plastic debris in remote islands of the Andaman and Nicobar Archipelago. India Mar. pollut. Bull.151, 110841. doi: 10.1016/j.marpolbul.2019.110841
310
Krishnakumar S. Chandrasekar N. Ashok Prabu V. Magesh N. S. Kaliraj S. (2020b). Microplastics in the shelf sediments of the coastal areas of Tamil Nadu, India. Mar. Pollut. Bull.158, 111403. doi: 10.1016/j.marpolbul.2020.111403
311
Krishnakumar S. Srinivasalu S. Saravanan P. Vidyasakar A. Magesh N. S. (2018). A preliminary study on coastal debris in Nallathanni Island, Gulf of Mannar Biosphere Reserve, Southeast coast of India. Mar. Pollut. Bull.131, 547–551. doi: 10.1016/j.marpolbul.2018.04.026
312
Kumar R. Sharma P. Manna C. Jain M. (2021). Abundance, interaction, ingestion, ecological concerns, and mitigation policies of microplastic pollution in riverine ecosystem: A review. Sci. Total Environment.782, 146695. doi: 10.1016/j.scitotenv.2021.146695
313
Kumar S. Panda A. K. Singh R. K. (2017). A review on tertiary recycling of high-density polyethylene to fuel. Resources Conserv. Recycling123, 115–128. doi: 10.1016/j.resconrec.2016.09.014
314
Kumar Sen S. Raut S. (2015). Microbial degradation of low-density polyethylene (LDPE): A review. J. Environ. Chem. Eng.3, 462–473. doi: 10.1016/j.jece.2015.01.003
315
Kumkar et al . (2021).
316
Kusworo T. D. Widiasa I. N. Wenten I. G. (2022). Membrane technology for microplastic removal: A review. J. Water Process Eng.47, 102679. doi: 10.1016/j.jwpe.2022.102679
317
Kuzma J. Najmaei M. Meyer M. (2016). The evolving role of synthetic biology in environmental applications. Trends Biotechnol.34, 653–664. doi: 10.1016/j.tibtech.2016.04.003
318
Kyaw B. Champakalakshmi R. Sakharkar M. Lim C. Sakharkar K. (2012). Biodegradation of low density polythene (ldpe) by pseudomonas species. Indian J. Microbiol.52, 411–419. doi: 10.1007/s12088-012-0250-6
319
Lagarde F. Olivier O. Zanella M. Daniel P. Hiard S. Caruso A. (2016). Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut.215, 331–339. doi: 10.1016/j.envpol.2016.05.006
320
Lahive E. Walton A. Horton A. A. Spurgeon D. J. Svendsen C. (2019). Microplastic particles reduce reproduction in the terrestrial worm Enchytraeus crypticus in a soil exposure. Environ. Pollution.255, 113174. doi: 10.1016/j.envpol.2019.113174
321
Laist D. W. (1997). “ Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records,” in Mar. Debris. Eds. CoeJ. M.RogersD. B. ( Springer), 99–139. doi: 10.1007/978-1-4613-8486-1_10
322
La Nasa J. Biale G. Fabbri D. Modugno F. (2020). A review on challenges and developments of analytical pyrolysis and other thermoanalytical techniques for the quali-quantitative determination of microplastics. J. Analytical Appl. Pyrolysis149, 104841. doi: 10.1016/j.jaap.2020.104841
323
Lapointe M. Farner J. M. Hernandez L. M. Tufenkji N. (2020). Understanding and improving microplastic removal during water treatment: impact of coagulation and flocculation. Environ. Sci. Technol.54, 8719–8727. doi: 10.1021/acs.est.0c00712
324
Lares M. Ncibi M. C. Sillanpää M. Sillanpää M. (2018). Occurrence, identification and removal of microplastic particles and fibres in conventional activated sludge process and advanced MBR technology. Water Res.133, 236–246. doi: 10.1016/j.watres.2018.01.049
325
Lazarevic D. Aoustin E. Buclet N. Brandt N. (2010). Plastic waste management in the context of a European recycling society: Comparing results and uncertainties in a life cycle perspective. Resources Conserv. Recycling55, 246–259.
326
Lebreton L. C. van der Zwet J. Damsteeg J. W. Slat B. Andrady A. Reisser J. (2017). River plastic emissions to the world’s oceans. Nat. Commun.8, 15611. doi: 10.1038/ncomms15611
327
Lechthaler S. Esser V. Schüttrumpf H. Stauch G. (2021). Why analysing microplastics in floodplains matters: Application in a sedimentary context. Environ. Sci. Pollut. Res.28, 27663–27673. doi: 10.1007/s11356-021-12988-5
328
Lee J. Hong S. Song Y. K. Hong S. H. Jang Y. C. Jang M. et al . (2013). Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull.77, 349–354. doi: 10.1016/j.marpolbul.2013.08.013
329
Lee H. Kim H. Jeon E. Yu H. Lee S. Li J. et al . (2020). Evaluation of the biodegradation efficiency of four various types of plastics by pseudomonas aeruginosa isolated from the gut extract of superworms. Microorganisms8, 1341. doi: 10.3390/microorganisms8091341
330
Lei K. Qiao F. Liu Q. Wei Z. Qi H. Cui S. et al . (2017). Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull.123, 122–126. doi: 10.1016/j.marpolbul.2017.09.016
331
Lei L. Liu M. Song Y. Lu S. Hu J. Cao C. et al . (2018a). Polystyrene (nano) microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Science: Nano5, 2009–2020. doi: 10.1039/C8EN00412A
332
Lei L. Wu S. Lu S. Liu M. Song Y. Fu Z. et al . (2018b). Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. total Environ.619, 1–8. doi: 10.1016/j.scitotenv.2017.11.103
333
Lepcha N. T. Kumar R. Chhetri V. S. Sharma P. Tamang D. Acharya A. et al . (2023). Microplastic pollution in the surface water of the Teesta River, Eastern Himalaya: Spatial distribution, polymer characteristics, and potential sources. Environ. Pollut.318, 120880. doi: 10.1016/j.envpol.2022.120880
334
Leslie H. A. Brandsma S. H. Van Velzen M. J. Vethaak A. D. (2017). Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int.101, 133–142. doi: 10.1016/j.envint.2017.01.018
335
Leung J. Chan K. Y. K. (2018). Microplastics reduced posterior segment regeneration rate of the polychaete Perinereis aibuhitensis. Mar. pollut. Bull.129, 782–786. doi: 10.1016/j.marpolbul.2017.10.072
336
Li Q. C. Lai Y. J. Yu S. J. Li P. Zhou X. X. Dong L. J. et al . (2021). Sequential isolation of microplastics and nanoplastics in environmentalWaters by membrane filtration, followed by cloud-point extraction. Anal. Chem.93, 4559–4566. doi: 10.1021/acs.analchem.0c04996
337
Li J. Liu H. Chen J. P. (2018). Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res.137, 362–374. doi: 10.1016/j.watres.2017.12.056
338
Li J. Qu X. Su L. Zhang W. Yang D. Kolandhasamy P. et al . (2016). Microplastics in mussels along the coastal waters of China. Environ. Pollut.214, 177–184. doi: 10.1016/j.envpol.2016.04.012
339
Li X. Wang J. Jing M. Hu S. Zong W. Liu R. (2025). Cross-level technologies for exploring nanoplastics to amplify cadmium immunotoxicity: Endpoint identification and kinetic model construction. Chem. Eng. J.511, 162087. doi: 10.1016/j.cej.2025.162087
340
Li R. Yu L. Chai M. Wu H. Zhu X. (2020). The distribution, characteristics and ecological risks of microplastics in the mangroves of Southern China. Science of the total environment. 708, 135025. doi: 10.1016/j.scitotenv.2019.135025
341
Li R. Zhang L. Xue B. Wang Y. (2019). Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the south China sea: new implications for location, rhizosphere, and sediment compositions. Environ. pollut.244, 685–692. doi: 10.1016/J.ENVPOL.2018.10.089
342
Li W. Wang Z. Li W. Li Z. (2022). Impacts of microplastics addition on sediment environmental properties, enzymatic activities and bacterial diversity. Chemosphere307, 135836. doi: 10.1016/j.chemosphere.2022.135836
343
Liao Y. L. Tang Q. X. Yang J. Y. (2023). Microplastic characteristics and microplastic-heavy metal synergistic contamination in agricultural soil under different cultivation modes in Chengdu, China. J. Hazard. Mater.459, 132270. doi: 10.1016/j.jhazmat.2023.132270
344
Lim S. Kim M. Lee Y. Kim J. (2022). Enzymatic hydrolysis of PET: Structural and mechanistic insights. Biotechnol. Adv.59, 107949. doi: 10.1016/j.biotechadv.2022.107949
345
Limonta G. Mancia A. Benkhalqui A. Bertolucci C. Abelli L. Fossi M. C. et al . (2019). Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep.9. doi: 10.1038/S41598-019-52292-5
346
Lin L. Mab L. S. Lia H. X. Pana Y. F. Liua S. Zhangc L. et al . (2020). Low level of microplastic contamination in wild fish from an urban estuary. Mar. pollut. Bull.160, 111650. doi: 10.1016/j.marpolbul.2020.111650
347
Lin Y. Xie J. Xiang Q. Liu Y. Wang P. Wu Y. et al . (2022). Effect of propiconazole on plastic film microplastic degradation: Focusing on the change in microplastic morphology and heavy metal distribution. Sci. Total Environ.822, 153609. doi: 10.1016/j.scitotenv.2022.153609
348
Liu K. Wang X. Song Z. Wei N. Ye H. Cong X. et al . (2020). Global inventory of atmospheric fibrous microplastics input into the ocean: an implication from the indoor origin. J. Hazard. Mater.400, 123223. doi: 10.1016/j.jhazmat.2020.123223
349
Liu M. Lu S. Song Y. Lei L. Hu J. Lv W. et al . (2018). Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut.242, 855–862. doi: 10.1016/j.envpol.2018.07.051
350
Liu R. Liang J. Yang Y. Jiang H. Tian X. (2023). Effect of polylactic acid microplastics on soil properties, soil microbials and plant growth. Chemosphere329, 138504. doi: 10.1016/j.chemosphere.2023.138504
351
Liu K. Wang X. Wei N. Song Z. Li D. (2019). Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int.132, 105127. doi: 10.1016/j.envint.2019.105127
352
Liu W. Zhang J. Liu H. Guo X. Zhang X. Yao X. et al . (2021). A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int.146, 106277. doi: 10.1016/j.envint.2020.106277
353
Lo H. K. A. Chan K. Y. K. (2018). Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environ. pollut.233, 588e595. doi: 10.1016/j.envpol.2017.10.095
354
Lopez-Martínez ´S. Morales-Caselles C. Kadar J. (2021). Rivas ML Overview of global status of plastic presence in marine vertebrates. Glob Change Biol.27, 728–737. doi: 10.1111/gcb.15416
355
Lorenz C. Roscher L. Meyer M. S. Hildebrandt L. Prume J. L¨oder M. G. J. J. et al . (2019). Spatial distribution of microplastics in sediments and surface waters of the southern North Sea. Environ. pollut.252, 1719–1729. doi: 10.1016/j.envpol.2019.06.093
356
Lotfabad T. B. Shourian M. Roostaazad R. Najafabadi A. R. Adelzadeh M. R. Noghabi K. A. (2010). Biodegradation of low-density polyethylene by Pseudomonas species isolated from petroleum-contaminated soils. J. Appl. Microbiol.109, 1783–1794. doi: 10.1111/j.1365-2672.2010.04814.x
357
Lu K. Zhan D. Fang Y. Li L. Chen G. Chen S. et al . (2022). Microplastics — potential threat to patients with lung diseases. Front. Toxicol.4. doi: 10.3389/ftox.2022.1119994
358
Lu L. Luo T. Zhao Y. Cai C. Fu Z. Jin Y. (2021). Interaction between microplastics and microorganisms and gut microbiota: A consideration on environmental animal and human health. Sci. Total Environment 667416, 94–100. doi: 10.1016/j.scitotenv.2019.02.436
359
Luo Y. Zhang Y. Xu Y. Guo X. Zhu L. (2020). Distribution characteristics and mechanism of microplastics mediated by soil physicochemical properties. Sci. Total Environ.726, 138389. doi: 10.1016/j.scitotenv.2020.138389
360
Luo T. Zhang Y. Wang C. Wang X. Zhou J. Shen M. et al . (2019). Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollution.255, 113122. doi: 10.1016/j.envpol.2019.113122
361
Lusher A. L. Mchugh M. Thompson R. C. (2013). Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. pollut. Bull.67, 94–99. doi: 10.1016/j.marpolbul.2012.11.028
362
Lusher A. L. Tirelli V. O’Connor I. Officer R. (2015). Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci. Rep.5, 14947. doi: 10.1038/srep14947
363
Lusher A. L. Welden N. A. (2020). “ Microplastic impacts in fisheries and aquaculture,” in Handbook of microplastics in the environment, Springer International Publishing, 1–28. doi: 10.1007/978-3-030-10618-8_30-1
364
Lv X. Dong Q. Zuo Z. Liu Y. Huang X. Wu W. M. (2019). Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Cleaner Production.225, 579–586. doi: 10.1016/j.jclepro.2019.03.321
365
Ma B. Xue W. Ding Y. Hu C. Liu H. Qu J. (2019). Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci.78, 267–275.
366
Ma Y. Yao M. Li B. Ding M. He B. Chen S. et al . (2018). Enhanced poly(ethylene terephthalate) hydrolase activity by protein engineering of Ideonella sakaiensis PETase. ACS Catalysis8, 6870–6876. doi: 10.1021/acscatal.8b01165
367
Macheca F. D’Amato D. Toppinen A. (2024). Circular economy strategies in the plastic sector: Innovations and challenges. Resources Conserv. Recycling200, 107101. doi: 10.1002/cncr.34463
368
Maes T. van Diemen de Jel J. Vethaak A. D. Desender M. Bendall V. A. Van Velzen M. et al . (2020). You are what you eat, microplastics in porbeagle sharks from the North East Atlantic: method development and analysis in spiral valve content and tissue. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00273
369
Maghsodian Z. Sanati A. M. Ramavandi B. Ghasemi A. Sorial G. A. (2021). Microplastics accumulation in sediments and Periophthalmus waltoni fish, mangrove forests in southern Iran. Chemosphere264, 128543. doi: 10.1016/j.chemosphere.2020.128543
370
Maharana D. Saha M. Dar J. Y. Rathore C. Sreepada R. A. Xu X. R. et al . (2020). Assessment of plastics along the west coast of India: abundance, distribution, polymer type and toxicity. Chemosphere246, 125708. doi: 10.1016/j.chemosphere.2019.125708
371
Maheswaran P. A. Jeyagopal P. Hossain M. A. Ashokkumar S. Jamshed M. Suresh N. (2022). Distribution and characterization of microplastics in beach sediments from Tamilnadu coast, India. Environ. Sci. Pollut. Res.29, 30313–30325. doi: 10.1007/s11356-021-18029-2
372
Mai L. You S. N. He H. Bao L. J. Liu L. Y. Zeng E. Y. (2019). Riverine microplastic pollution in the Pearl River Delta, China: are modeled estimates accurate? Environ. Sci. Technol.53, 11810–11817. doi: 10.1021/acs.est.9b03850
373
Majewsky M. Bitter H. Eiche E. Horn H. (2016). Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ.568, 507–511. doi: 10.1016/j.scitotenv.2016.06.017
374
Mammo F. K. Amoah I. D. Gani K. M. Pillay L. Reddy P. Bux F. (2020). Microplastics in the environment: Interactions with microbes and chemical contaminants. Sci. Total Environ.743, 140518. doi: 10.1016/j.scitotenv.2020.140518
375
Mancia A. Chenet T. Bono G. Geraci M. L. Vaccaro C. Munari C. et al . (2020). Adverse effects of plastic ingestion on the Mediterranean small-spotted catshark (Scyliorhinus canicula). Mar. Environ. Res.155, 104876. doi: 10.1016/j.marenvres.2020.104876
376
Mani T. Primpke S. Lorenz C. Gerdts G. Burkhardt-Holm P. (2019). Microplastic pollution in benthic midstream sediments of the Rhine River. Environ. Sci. Technol.53, 6053–6062. doi: 10.1021/acs.est.9b01363
377
Martín J. Santos J. L. Aparicio I. Alonso E. (2022). Microplastics and associated emerging contaminants in the environment: Analysis, sorption mechanisms and effects of co-exposure. Trends Environ. Analytical Chem.35, e00170. doi: 10.1016/j.teac.2022.e00170
378
Masia P. Ardura A. Garcia-Vazquez E. (2019). Microplastics in special protected areas for migratory birds in the Bay of Biscay. Mar. pollut. Bull.146, 993–1001. doi: 10.1016/j.marpolbul.2019.07.065
379
Mastropetros S. G. Fotopoulou K. N. Karapanagioti H. K. (2022). Microplastics in marine environments: Sources, fate, and impacts. Mar. Pollut. Bull.176, 113407. doi: 10.1016/j.marpolbul.2022.113407
380
Mateos-Cárdenas A. van Pelt F. N. A. M. O'Halloran J. Jansen M. A. K. (2019). Adsorption, uptake and toxicity of micro- and nanoplastics: Effects on terrestrial plants and aquatic macrophytes. Environ. Pollut.284, 116523. doi: 10.1016/j.envpol.2021.116523
381
Matsuguma Y. Takada H. Kumata H. Kanke H. Sakurai S. Suzuki T. et al . (2017). Microplastics in sediment cores from Asia and Africa as indicators of temporal trends in plastic pollution. Arch. Environ. contamination toxicology.73, 230–239. doi: 10.1007/s00244-017-0414-9
382
McCormick M. I. Chivers D. P. Ferrari M. C. O. Blandford M. I. Nanninga G. B. Richardson C. et al . (2020). Microplastic exposure interacts with habitat degradation to affect behaviour and survival of juvenile fish in the field, Proc. R. Soc287, 20201947. doi: 10.1098/rspb.2020.1947
383
McDermid K. J. McMullen T. L. (2004). Quantitative analysis of small-plastic debris on beaches in the Hawaiian archipelago. Mar. Pollut. Bull.48, 790–794. doi: 10.1016/j.marpolbul.2003.10.017
384
McEachern K. Alegria H. Kalagher A. L. Hansen C. Morrison S. Hastings D. (2019). Microplastics in Tampa Bay, Florida: abundance and variability in estuarine waters and sediments. Mar. pollut. Bull.148, 97–106. doi: 10.1016/j.marpolbul.2019.07.068
385
Md Amin R. Sohaimi E. S. Anuar S. T. Bachok Z. (2020). Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea. Mar. pollut. Bull.150, 110616. doi: 10.1016/j.marpolbul.2019.110616
386
Meaza I. Toyoda J. H. Wise (2021). JP. Microplastics in sea turtles, marine mammals and humans: A one environmental health perspective. Front. Environ. Sci.8. doi: 10.3389/fenvs.2020.575614
387
Meijer L. J. Van Emmerik T. van der Ent R. Schmidt C. Lebreton L. (2021). More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. advances.7, eaaz5803. doi: 10.1126/sciadv.aaz5803
388
Meister S. Reimer V. Fink M. Taubert A. (2009). Synthesis and characterization of polyethylene glycol-cross-linked oligo(ethylene glycol) methacrylate hydrogels. J. Polymer Sci. Part A: Polymer Chem.47, 6171–6177.
389
Mendoza M. L. Rodriguez E. Rojas A. (2017). Limitations of current microplastic research: A critical review. Mar. pollut. Bull.123, 36–45. doi: 10.1016/j.marpolbul.2017.09.004
390
Menon V. Sharma S. Gupta S. Ghosal A. Nadda A. K. Jose R. et al . (2023). Prevalence and implications of microplastics in potable water system: An update. Chemosphere.317, 137848. doi: 10.1016/j.chemosphere.2023.137848
391
Messinetti S. Mercurio S. Parolini M. Sugni M. Pennati R. (2017). Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ. pollut.237, 1080e1087. doi: 10.1016/j.envpol.2017.11.030
392
Miao F. Liu Y. Gao M. Yu X. Xiao P. Wang M. et al . (2020). Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater.399, 123023. doi: 10.1016/j.jhazmat.2020.123023
393
Migwi F. K. Ogunah J. A. Kiratu J. M. (2020). Occurrence and spatial distribution of microplastics in the surface waters of Lake Naivasha, Kenya. Environ. Toxicol. Chem.39, 765–774. doi: 10.1002/etc.4677
394
Miller M. E. Hamann M. Kroon F. J. (2020). Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS One15, e0240792. doi: 10.1371/journal.pone.0240792
395
Mintenig S. M. Kooi M. Erich M. W. Primpke S. Redondo- Hasselerharm P. E. Dekker S. C. et al . (2020). A systems approach to understand microplastic occurrence and variability in Dutch riverine surface waters. Water Res.176, 115723. doi: 10.1016/j.watres.2020.115723
396
Mirghorayshi M. Shabanipour N. Hosseinzadeh M. (2021). The efficiency of nano-adsorbent for the removal of microplastics from aqueous environments: A review. Environ. Sci. Pollut. Res.28, 56226–56240.
397
Misra A. Zambrano M. C. Kidd K. A. Simonich M. T. Tanguay R. L. (2020). PFAS reduce lipid levels and promote developmental effects in zebrafish. Toxicological Sci.175, 210–223. doi: 10.1093/toxsci/kfaa041
398
Missawi O. Bousserrhine N. Zitouni N. Maisano M. Boughattas I. De Marco G. et al . (2021). Uptake, accumulation and associated cellular alterations of environmental samples of microplastics in the seaworm Hediste diversicolour. J. Hazard Mater.406, 124287. doi: 10.1016/j.jhazmat.2020.124287
399
Mitigation and Abatement of Microplastics (2023). Global framework for addressing microplastic pollution ( United Nations Environment Programme).
400
Mohammadi A. Malakootian M. Dobaradaran S. Hashemi M. Khaniki G. R. J. (2020). Occurrence and characteristics of microplastics in various seafood from the Persian Gulf. Mar. Pollut. Bull.160, 111636.
401
Mohan K. Raja S. (2024). Microplastic pollution in Indian aquatic ecosystems: A comprehensive review. Environ. Sci. Pollut. Res.31, 22145–22168. doi: 10.1007/s11356-024-32567-8
402
Mohanan N. Montazer Z. Sharma P. K. Levin D. B. (2020). Microbial and enzymatic degradation of synthetic plastics, Front. Microbiol.11. doi: 10.3389/FMICB.2020.580709/BIBTEX
403
Moharir R. V. Kumar S. (2019). Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: A comprehensive review. J. Cleaner Prod.208, 65–76. doi: 10.1016/j.jclepro.2018.10.059
404
Molazadeh M. Liu F. Simon-Sánchez L. Vollersten J. (2023). Buoyant microplastics in freshwater sediments–How do they get there? Sci. Total Environ.860, 160489. doi: 10.1016/j.scitotenv.2022.160489
405
Montazer Z. Habibi-Najafi M. B. Mohebbi M. Oromiehei A. (2018). Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading Bacteria isolated from plastic-dump soil. J. Polym. Environ.26, 3613–3625. doi: 10.1007/s10924-018-1245-0
406
Moog D. Schmitt J. Senger J. Zarzycki J. Rexer K. H. Linne U. et al . (2019). Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microbial Cell Factories18, 171. doi: 10.1186/s12934-019-1220-z
407
Morgana S. Ghigliotti L. Est´evez-Calvar N. Stifanese R. Wieckzorek A. Doyle T. et al . (2018). Microplastics in the Arctic: a case study with sub-surface water and fish samples off Northeast Greenland. Environ. pollut.242, 1078–1086. doi: 10.1016/j.envpol.2018.08.001
408
Mou D. Zhou X. Zhao J. Chen Y. (2020). Microplastic abundance, distribution and composition in water, sediments, and wild fish from the Heilongjiang River. Environ. Res.185, 109448.
409
Mouafo Tamnou E. B. Tamsa Arfao A. Nougang M. E. Metsopkeng C. S. Noah Ewoti O. V. Moungang L. M. et al . (2021). Biodegradation of polyethylene by the bacterium Pseudomonas aeruginosa in acidic aquatic microcosm and effect of the environmental temperature. Environ. Challenges3. doi: 10.1016/j.envc.2021.100056
410
Mugilarasan M. Venkatachalapathy R. Sharmila N. Gurumoorthi K. (2017). Occurrence of microplastic resin pellets from Chennai and Tinnakkara Island: towards the establishment of background level for plastic pollution. Indian J. Geo-Mar Sci.46, 1210–1212. Available online at: http://nopr.niscair.res.in/handle/123456789/41992.
411
Mukherjee F. Shi A. Wang X. You F. Abbott N. L. (2022). Liquid Crystals as Multifunctional Interfaces for Trapping and Characterizing Microplastics. arXiv preprint arXiv:2209.15440. doi: 10.48550/arXiv.2209.15440
412
Munhoz C. S. Oliveira C. Mendes F. (2022). Microplastic pollution and circular economy: Bridging environmental and policy gaps. J. Environ. Manage.316, 115317. doi: 10.1007/s11356-021-18038-5
413
Mvovo I. (2021). A comprehensive review on micro-plastic pollution in African aquatic systems. Environ. Advances.5, 100107. doi: 10.1016/j.envadv.2021.100107
414
Naidoo T. Glassom D. (2019). Sea-surface microplastic concentrations along the coastal shelf of KwaZulu-Natal, South Africa. Mar. Pollut. Bull.149, 110514. doi: 10.1016/j.marpolbul.2019.110514
415
Naidoo T. Glassom D. Smit A. J. (2015). Plastic pollution in five urban estuaries of KwaZulu-Natal, South Africa. Mar. pollut. Bull.101, 473–480. doi: 10.1016/j.marpolbul.2015.09.044
416
Naidu S. A. (2019). Preliminary study and first evidence of presence of microplastics and colourants in green mussel, Perna viridis (Linnaeus 1758), from southeast coast of India. Mar. pollut. Bull.140, 416–422. doi: 10.1016/j.marpolbul.2019.01.024
417
Naji A. Esmaili Z. Mason S. A. Dick Vethaak A. (2017). The occurrence of microplastic contamination in littoral sediments of the Persian Gulf, Iran. Environ. Sci. pollut. Res.24, 20459–20468. doi: 10.1007/s11356-017-9587-z
418
Najmi N. Sadaf A. Khan M. A. Ahmed S. (2020). Microbial degradation of plastic: A review. Environ. Chem. Lett.18, 2115–2132. doi: 10.1007/s10311-020-01069-z
419
Nakata K. Fujishima A. (2012). TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C: Photochem. Rev.13, 169–189.
420
Nan B. Su L. Kellar C. Craig N. J. Keough M. J. Pettigrove V. (2020). Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ. pollut.259, 113865. doi: 10.1016/j.envpol.2019.113865
421
Napper I. E. Baroth A. Barrett A. C. Bhola S. Chowdhury G. W. Davies B. F. et al . (2021). The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut.274, 116348. doi: 10.1016/j.envpol.2020.116348
422
Ncube L. K. Ude A. U. Ogunmuyiwa E. N. Zulkifli R. Beas I. N. (2021). An overview of plastic waste generation and management in food packaging industries. Recycling6, 12. doi: 10.3390/recycling6010012
423
Negrete-Bolagay D. Zamora-Ledezma C. Chuya-Sumba C. De Sousa F. B. Whitehead D. Alexis F. et al . (2021). Persistent organic pollutants: The trade-off between potential risks and sustainable remediation methods. J. Environ. Management.300, 113737. doi: 10.1016/j.jenvman.2021.113737
424
Nelis J. L. D. Schacht V. J. Dawson A. L. Bose U. Tsagkaris A. S. Dvorakova D. et al . (2023). The measurement of food safety and security risks associated with micro- and nanoplastic pollution. Trends Analytical Chem.161, 116993. doi: 10.1016/j.trac.2023.116993
425
Nelms S. E. Galloway T. S. Godley B. J. Jarvis D. S. Lindeque P. K. (2018). Investigating microplastic trophic transfer in marine top predators. Environ. pollut.238, 999–1007. doi: 10.1016/j.envpol.2018.02.016
426
Ng E. L. Lwanga E. H. Eldridge S. M. Johnston P. Hu H. W. Geissen V. et al . (2018). An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environment.627, 1377–1388. doi: 10.1016/j.scitotenv.2018.01.341
427
Ng M. S. Todd P. A. (2023). The comparative effects of chronic microplastic and sediment deposition on the scleractinian coral Merulina ampliata. Mar. Environ. Res.191, 106135. doi: 10.1016/j.marenvres.2023.106135
428
Nguyen B. Schwedler G. Graf S. Scrimshaw M. D. (2019). Analysis and removal of micro- and nanoplastics in water and wastewater: A review of challenges and opportunities. J. Hazard. Mater.362, 548–558. doi: 10.1016/j.jhazmat.2018.09.050
429
Nikiema J. Asiedu E. (2022). Extended producer responsibility as a strategy for plastic waste management. Waste Manage. Res.40, 891–904. doi: 10.1128/mSphere.00080-19
430
Nikpay A. Roodsari B. K. (2024). Policy tools for international cooperation on microplastic mitigation. Int. J. Environ. Policy Law45, 23–36. doi: 10.3390/microplastics3010010
431
Niu L. Chen Y. Li Y. Wang Y. Shen J. Wang L. et al . (2023). Diversity, abundance and distribution characteristics of potential polyethylene and polypropylene microplastic degradation bacterial communities in the urban river. Water Res.232, 119704. doi: 10.1016/j.watres.2023.119704
432
Niu L. Li Y. Li Y. Hu M. Wang C. Wang J. et al . (2021). New insights into the vertical distribution and microbial degradation of microplastics in urban river sediments. Water Res.188, 116449. doi: 10.1016/j.watres.2020.116449
433
Nizzetto L. Futter M. Langaas S. (2016). Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol.50, 10777–10779. doi: 10.1021/acs.est.6b04140
434
Nobi E. P. Dilipan E. Thangaradjou T. Sivakumar K. Kannan L. (2010). Geochemical and geo statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuar. Coast. Shelf Sci.87, 253–264. doi: 10.1016/j.ecss.200912.019
435
Nohara S. Murakami T. Shibuya K. Goto S. (2024). Microplastic contamination in Japanese coastal waters: Distribution patterns and ecological implications. Mar. Pollut. Bull.198, 115921.
436
Nolte T. M. Hartmann N. B. Kleijn J. M. Garnæs J. van de Meent D. Jan Hendriks A. et al . (2017). The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness, and cellular adsorption. Aquat. Toxicol.183, 11–20. doi: 10.1016/j.aquatox.2016.12.005
437
Nuelle M.-T. Dekiff J. H. Remy D. Fries E. (2014). A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut.184, 161–169. doi: 10.1016/j.envpol.2013.07.027
438
OECD (2022). Global plastics outlook ( OECD). Available online at: https://www.oecd-ilibrary.org/environment/global-plastics-outlook_de747aef-en (Accessed June 27, 2022).
439
Ogata Y. Takada H. Mizukawa K. Hirai H. Iwasa S. Endo S. et al . (2009). International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull.58, 1437–1446. doi: 10.1016/j.marpolbul.2009.06.014
440
Ogunola O. S. Onada O. A. Falaye A. E. (2018). Mitigation measures to avert the impacts of plastics and microplastics in the marine environment (a review). Environ. Sci. Pollut. Res.25, 9293–9310. doi: 10.1007/s11356-018-1499-z
441
Oh J. (2022). Recent advances in microplastic detection and removal technologies. Environ. Sci. Pollut. Res.29, 52135–52151.
442
Olivatto G. P. Martins M. C. T. Montagner C. C. Henry T. B. Carreira R. S. (2019). Microplastic contamination in surface waters in Guanabara Bay, Rio de Janeiro, Brazil. Mar. pollut. Bull.139, 157–162. doi: 10.1016/j.marpolbul.2018.12.042
443
Oliveira A. R. Sardinha-Silva A. Andrews P. L. R. Green D. Cooke G. M. Hall S. et al . (2020). Microplastics presence in cultured and wild-caught cuttlefish, Sepia officinalis. Mar. pollut. Bull.160, 111553. doi: 10.1016/j.marpolbul.2020.111553
444
Oliviero M. Tato T. Schiavo S. Fernández V. Manzo S. Beiras R. (2019). Leachates of micronized plastic toys provoke embryotoxic effects upon sea urchin Paracentrotus lividus. Environ. Pollution.247, 706–715. doi: 10.1016/j.envpol.2019.01.098
445
Oni B. A. Ayeni A. O. Agboola O. Oguntade T. Obanla O. (2020). Comparing microplastics contaminants in (dry and raining) seasons for Ox-Bow Lake in Yenagoa, Nigeria. Ecotoxicol Environ. Saf.198, 110656. doi: 10.1016/j.ecoenv.2020.110656
446
Ono K. Naito W. Ogura I. Xue M. Kato E. Uesaka M. et al . (2023). Estimation of microplastic emission and transfer into Tokyo Bay, Japan, using material flow analysis. Mar. pollut. Bulletin.194, 115440. doi: 10.1016/j.marpolbul.2023.115440
447
Onoja S. Nel H. A. Abdallah M. A. Harrad S. (2022). Microplastics in freshwater sediments: Analytical methods, temporal trends, and risk of associated organophosphate esters as exemplar plastics additives. Environ. Res.203, 111830. doi: 10.1016/j.envres.2021.111830
448
Othman A. R. Hasan H. A. Muhamad M. H. Ismail N. I. Abdullah S. R. S. (2021). Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett.19, 3057–3073. doi: 10.1007/s10311-021-01197-9
449
Ottenheim C. Vervoort L. Stouthamer A. H. (2018). Enzymatic depolymerization of PET and polyamide plastics: Current progress and perspectives. Appl. Biochem. Biotechnol.185, 400–418. doi: 10.1007/s12010-017-2661-0
450
Ouyang D. Peng Y. Li B. Shao F. Li K. Cai Y. et al . (2023). Microplastic formation and simultaneous release of phthalic acid esters from residual mulch film in soil through mechanical abrasion. Sci. Total Environment.893, 164821. doi: 10.1016/j.scitotenv.2023.164821
451
Palm G. J. Reisky L. B¨ottcher D. Müller H. Michels E. A. P. Walczak M. C. et al . (2019). Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate, Nat. Commun.10. doi: 10.1038/s41467-019-09326-3
452
Pan X. Tang J. Li J. Guo Z. Zhang G. (2023). A review of heavy metals in coastal sediments after 40 years of reform and opening-up in China. Chemosphere310, 136859. doi: 10.1016/j.chemosphere.2022.136859
453
Park H. J. Kim J. T. (2019). Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res.158, 150–157. doi: 10.1016/j.watres.2019.04.019
454
Park J. Kim J. Ryu J. Park K. (2021). Microplastic removal from water through coagulation and ultrafiltration: Effects of process design parameters. J. Cleaner Prod.320, 128868. doi: 10.1016/j.jclepro.2021.128868
455
Park H. Park B. (2021). Review of microplastic distribution, toxicity, analysis methods, and removal technologies. Water.13, 2736. doi: 10.3390/w13192736
456
Patchaiyappan A. Ahmed S. Z. Dowarah K. Jayakumar S. Devipriya S. P. (2020). Occurrence, distribution and composition of microplastics in the sediments of South Andaman beaches. Mar. pollut. Bull.156, 111227. doi: 10.1016/j.marpolbul.2020.111227
457
Pathak R. Navneet (2023). Advances in microbial degradation of synthetic plastics: Mechanisms and metabolic pathways. Environ. Biotechnol. Rep.10, 45–58. doi: 10.1016/j.envbio.2023.100217
458
Patria M. P. Santoso C. A. Tsabita N. (2020). Microplastic ingestion by periwinkle snail Littoraria scabra and mangrove crab Metopograpsus quadridentata in Pramuka Island, Jakarta Bay, Indonesia. Sains Malaysiana49, 2151–2158. doi: 10.17576/jsm-2020-4909-13
459
Patterson J. Jeyasanta K. I. Edward J. K. P. Raj K. D. Malini B. H. Sathish N. (2020). Microplastic contamination in the sediments of Tuticorin and Rameswaram coasts, southeast coast of India. Mar. Pollut. Bull.156, 111271. doi: 10.1016/j.marpolbul.2020.111271
460
Peng S. Wang F. Wei D. Wang C. Ma H. Du Y. (2023). Application of FTIR two-dimensional correlation spectroscopy (2D-COS) analysis in characterizing environmental behaviors of microplastics: A systematic review. J. Environ. Sci. doi: 10.1016/j.jes.2023.10.004
461
Perren W. Wojtasik A. Cai Q. (2018). Removal of microbeads from wastewater using electrocoagulation. ACS Omega3, 3357–3364. doi: 10.1021/acsomega.7b02037
462
Peters C. A. Bratton S. P. (2016). Urbanization is a major influence on microplastic ingestion by sunfish in the Brazos River Basin, Central Texas, USA. Environ. pollut.210, 380–387. doi: 10.1016/j.envpol.2016.01.018
463
Pizzichetti A. R. P. Lehtiniemi M. Fontana M. Setälä O. (2021). The influence of filtration pressure and temperature on microplastic uptake by Baltic Sea filter feeders. Environ. Pollut.283, 117079. doi: 10.1016/j.envpol.2
464
PlasticsEurope (2019). “ Plastics—The facts 2019,” in An analysis of European plastics production, demand and waste data ( PlasticsEurope, Brussels, Belgium).
465
Pojar I. Stănică A. Stock F. Kochleus C. Schultz M. Bradley C. (2021). Sedimentary microplastic concentrations from the Romanian Danube River to the Black Sea. Sci. Rep.1, 2000. doi: 10.1038/s41598-021-81724-4
466
Potrykus M. Redko V. Głowacka K. Piotrowicz-Cieślak A. Szarlej P. Janik H. et al . (2021). Polypropylene structure alterations after 5 years of natural degradation in a waste landfill. Sci. Total Environment.758, 143649. doi: 10.1016/j.scitotenv.2020.143649
467
Pozo K. Gomez V. Torres M. Vera L. Nunez D. Oyarzun P. et al . (2019). Presence and characterization of microplastics in fish of commercial importance from the Biobi region in central Chile. Mar. pollut. Bull.140, 315–319. doi: 10.1016/j.marpolbul.2019.01.025
468
Pradit S. Noppradit P. Sornplang K. Jitkaew P. Kobketthawin T. Nitirutsuwan T. et al . (2024). Microplastics and heavy metals in the sediment of Songkhla Lagoon: distribution and risk assessment. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1292361
469
Prata J. C. da Costa J. P. Duarte A. C. Rocha-Santos T. (2019). Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC Trends Anal. Chem.110, 150–159. doi: 10.1016/J.TRAC.2018.10.029
470
Prata J. C. da Costa J. P. Lopes I. Duarte A. C. Rocha-Santos T. (2018). Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Analytical Chem., 110, 150–159. doi: 10.1016/j.trac.2018.10.029
471
Primpke S. Wirth M. Lorenz C. Gerdts G. (2020). Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Analytical Bioanalytical Chem.412, 5111–5121. doi: 10.1007/s00216-020-02776-4
472
Priscilla V. Sedayu A. Patria M. P. (2019). Microplastic abundance in the water, seagrass, and sea hare Dolabella auricularia in Pramuka Island, Seribu Islands, Jakarta Bay, Indonesia ( Journal of Physics: Conference Series. Institute of Physics Publishing). doi: 10.1088/1742-6596/1402/3/033073
473
Priyanka N. Archana T. (2011). Biodegradability of polythene and plastic by the help of microorganisms: A way for brighter future. J. Environ. Res. Dev.5, 1028–1033. doi: 10.13140/RG.2.1.3270.4488
474
Puiggené Ò. Espinosa M. J. C. Schlosser D. Thies S. Jehmlich N. Kappelmeyer U. et al . (2022). Extracellular degradation of a polyurethane oligomer involving outer membrane vesicles and further insights on the degradation of 2,4-diaminotoluene in Pseudomonas capeferrum TDA1. Sci. Rep.12. doi: 10.1038/s41598-022-06558-0
475
Puliga V. Zuffi D. Baldo D. Cavatorta A. Zambonelli O. Francioso S. et al . (2023). ladosporium cladosporioides (strain Clc/1): a candidate for low-density polyethylene degradation, Chem. Biol. Technol. Agric.10. doi: 10.1186/s40538-023-00419-2
476
Qiao R. Deng Y. Zhang S. Wolosker M. B. Zhu Q. Ren H. et al . (2019). Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere236, 124334. doi: 10.1016/j.chemosphere.2019.07.065
477
Qiao R. Lu Y. Zhang Y. Ren H. Lemos B. (2018). Uptake, tissue distribution, and response to polystyrene microplastics in Chinese rare minnow (Gobiocy prisellis) as compared to Japanese medaka (Oryzias latipes). Environ. Pollut.242, 1213–1225. doi: 10.1016/j.envpol.2018.08.024
478
Qiu Q. Tan Z. Wang J. Peng J. Li M. Zhan Z. (2016). Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuarine Coastal Shelf Sci.176, 102–109. doi: 10.1016/j.ecss.2016.04.012
479
Rabari V. Patel H. Patel K. Patel A. Bagtharia S. Trivedi J. (2023). Quantitative assessment of microplastic contamination in muddy shores of Gulf of Khambhat, India. Mar. pollut. Bull.192, 115131. doi: 10.1016/j.marpolbul.2023.115131
480
Rajala K. Grönfors O. Hesampour M. Mikola A. (2018). Removal of microplastics from secondary wastewater treatment plant effluent by coagulation. Water Res.154, 67–71.
481
Rajaram V. Natarajan S. Ravindran R. Subramanian P. Rajagopal R. (2020). Assessment of microplastic contamination in selected marine fish from the Indian coastal waters. Mar. Pollut. Bull.159, 111456.
482
Ramadan A. H. Sembiring E. (2020). “ Occurrence of Microplastic in surface water of Jatiluhur Reservoir,” in E3S Web of Conferences, CRC Press. doi: 10.1051/e3sconf/202014807004
483
Ramos J. Tarrillo L. Bravo A. Sánchez-Purihuamán M. Carreño-Farfán C. Estrada C. et al . (2024). Efficiency of microorganisms and effectiveness of biodegradation techniques on ldpe plastics: a systematic review. F1000research13, 745. doi: 10.12688/f1000research.151338.1
484
Rani R. Rathee J. Kumari P. Singh N. P. Santal A. R. (2022). Biodegradation and detoxification of low-density polyethylene by an indigenous strain Bacillus licheniformis SARR1. J. Appl. Biol. Biotechnol.10, 9–21. doi: 10.7324/JABB.2021.100102
485
Raza M. H. Jabeen F. Ikram S. Zafar S. (2023). Characterization and implication of microplastics on riverine population of the River Ravi, Lahore, Pakistan. Environ. Sci. Pollut. Res.30, 6828–6848. doi: 10.1007/s11356-022-22687-4
486
Reddy M. S. Basha S. Adimurthy S. Ramachandraiah G. (2006). Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Estuar. Coast. Shelf Sci.68, 656–660. doi: 10.1016/j.ecss.2006.03.018
487
Reis M. A. Pereira L. Santos R. (2019). From linear to circular economy: A transition model for plastic pollution control. Sustain. Production Consumption19, 127–135. doi: 10.1016/j.jenvman.2021.113667
488
Renner G. Schmidt T. C. Schram J. (2017). A new chemometric approach for automatic identification of microplastics from environmental compartments based on FT-IR spectroscopy. Analytical Chem.89, 12045–12053.
489
Rezania S. Park J. Din M. F. Taib S. M. Talaiekhozani A. Yadav K. K. et al . (2018). Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. pollut. Bulletin.133, 191–208. doi: 10.1016/j.marpolbul.2018.05.022
490
Ribitsch D. Acero E. H. Greimel K. Eiteljoerg I. Trotscha E. Freddi G. et al . (2015). Characterization of a new cutinase from Thermobifida cellulosilytica and its application in polymer degradation. Appl. Microbiol. Biotechnol.99, 751–760. doi: 10.1007/s00253-014-6115-y
491
Rios L. M. Moore C. (2007). Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull.54, 1230–1237. doi: 10.1016/j.marpolbul.2007.03.022
492
Rius-Ayra O. Llorca-Isern N. (2021). Impact assessment of microplastic particles on Mediterranean coastal ecosystems. Sci. Total Environ.752, 141865.
493
Robin R. S. Karthik R. Purvaja R. Ganguly D. Anandavelu I. Mugilarasan M. et al . (2020). Holistic assessment of microplastics in various coastal environmental matrices, southwest coast of India. Sci. Total Environ.703, 134947. doi: 10.1016/j.scitotenv.2019.134947
494
Rochman C. M. (2018). Microplastics research—from sink to source. Science.360, 28–29. doi: 10.1126/science.aar7734
495
Rochman C. M. Hoellein T. (2020). The global odyssey of plastic pollution. Science.368, 1184–1185. doi: 10.1126/science.abc4428
496
Rochman C. M. Parnis J. M. Browne M. A. Serrato S. Reiner E. J. (2017). Direct and indirect effects of different types of microplastics on freshwater prey (Corbicula fluminea) and their predator (Acipenser transmontanus). PloS One12, e0187664. doi: 10.1371/journal.pone.0187664
497
Rodriguez-Seijo A. Lourenço J. Rocha-Santos T. A. P. da Costa J. Duarte A. C. Vala H. et al . (2016). Histopathological and molecular effects of microplastics in Eisenia andrei, Bouché[J. Environ. pollut.220, 495–503. doi: 10.1016/j.envpol.2016.09.092
498
Rolsky C. Kelkar V. Driver E. Halden R. U. (2020). Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health14, 16–22. doi: 10.1016/j.coesh.2019.12.001
499
Romano N. Ashikin M. Teh J. C. Syukri F. Karami A. (2018). Effects of pristine polyvinyl chloride fragments on whole body histology and protease activity in silver barb Barbodes gonionotus fry. Environ. Pollut.237, 1106–1111. doi: 10.1016/j.envpol.2017.11.018
500
Rose R. S. Richardson K. H. Latvanen E. J. Hanson C. A. Resmini M. Sanders I. A. (2020). Microbial degradation of plastic in aqueous solutions demonstrated by CO2 evolution and quantification. Int. J. Mol. Sci.21. doi: 10.3390/ijms21041176
501
Rose D. Webber M. (2019). Characterization of microplastics in the surface waters of Kingston Harbour. Sci. Total Environ.664, 753–760. doi: 10.1016/j.scitotenv.2019.01.319
502
Rouillon C. Bussiere P. O. Desnoux E. Collin S. Vial C. Therias S. et al . (2016). Is carbonyl index a quantitative probe to monitor polypropylene photodegradation? Polymer Degradation Stability128, 200–208. doi: 10.1016/j.polymdegradstab.2015.12.011
503
Roy S. Sarkar D. J. Chakraborty N. Mondal K. Das B. K. (2023). Bioaccumulation of polystyrene microplastics and changes in antioxidant and AChE pattern in a freshwater snail (Filopaludina bengalensis) from river Ganga. Aquat. Toxicol.263, 106697. doi: 10.1016/j.aquatox.2023.106697
504
Ru J. Huo Y. Yang Y. (2020). Microbial degradation and valorization of plastic wastes. Front. Microbiol.11. doi: 10.3389/fmicb.2020.00442
505
Rummel C. D. Jahnke A. Gorokhova E. Kühnel D. Schmitt-Jansen M. (2017). Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett.4, 258–267. doi: 10.1021/acs.estlett.7b00164
506
Ryan P. G. (2013). A simple technique for counting marine debris at sea reveals steep litter gradients between the Straits of Malacca and the Bay of Bengal. Mar. Pollut. Bull.69, 128–136.
507
Ryan P. G. (2015). A brief history of marine litter research. Mar. Anthropogenic Litter., 1–25. doi: 10.1007/978-3-319-16510-3
508
Sachithanandam V. Parthasarathy P. Elangovan S. S. Kasilingam K. Dhivya P. Mageswaran T. et al . (2020). A baseline study on trace metals concentration and its ecological risk assessment from the coast of South Andaman Island. India Reg. Stud. Mar. Sci.36, 101242. doi: 10.1016/j.rsma.2020.101242
509
Sagawa N. Kawaai K. Hinata H. (2018). Abundance and size of microplastics in a coastal sea: comparison among bottom sediment, beach sediment, and surface water. Mar. pollut. Bulletin.133, 532–542. doi: 10.1016/j.marpolbul.2018.05.036
510
Sajid M. Sadaf A. Shabbir S. Ahmed S. (2023). Biodegradation of polyethylene and polypropylene by bacterial consortia: A comprehensive study. Environ. Pollut.321, 121123. doi: 10.1016/j.envpol.2023.121123
511
Sajjad M. Bahadur S. Farooq M. A. Ren M. X. (2025). Interactive impacts of heat stress and microplastics contamination on the growth and biochemical response of wheat (Triticum aestivum) and maize (Zea mays) plants. Ecotoxicology, 1–23. doi: 10.1007/s10646-024-02836-9
512
Salinas J. Carpena V. Martínez-Gallardo M. Segado M. Estrella-González M. Toribio A. et al . (2023). Development of plastic-degrading microbial consortia by induced selection in microcosms. Front. Microbiol.14. doi: 10.3389/fmicb.2023.1143769
513
Sanniyasi E. Saliyan R. Subramanian S. Rajendran N. (2021). Microplastic pollution in aquatic ecosystems: A review on detection, impact, and removal technologies. Environ. Technol. Innovation24, 101901. doi: 10.1016/j.eti.2021.101901
514
Santana M. F. M. Moreira F. T. Pereira C. D. S. et al . (2018). Continuous exposure to microplastics does not cause physiological effects in the cultivated mussel Perna perna. Arch. Environ. Contamination Toxicol.74, 594–604. doi: 10.1007/s00244-018-0504-3
515
Santillo D. Miller K. Johnston P. (2017). Microplastics as contaminants in commercially important seafood species. Inter. Environ. Assess. Manage.13, 516–521. doi: 10.1002/ieam.1909
516
Sarkar D. J. Das Sarkar S. Das B. K. Praharaj J. K. Mahajan D. K. Purokait B. et al . (2021b). Microplastics removal efficiency of drinking water treatment plant with pulse clarifier. J. Hazard Mat.413, 125347. doi: 10.1016/j.jhazmat.2021.125347
517
Sarkar D. J. Das Sarkar S. Das B. K. Sahoo B. K. Das A. Nag S. K. et al . (2021a). Occurrence, fate and removal of microplastics as heavy metal vector in natural wastewater treatment wetland system. Water Res.192, 116853. doi: 10.1016/j.watres.2021.116853
518
Sarkar D. J. Sarkar S. D. Das B. K. Manna R. K. Behera B. K. Samanta S. (2019). Spatial distribution of meso and microplastics in the sediments of river Ganga at eastern India. Sci. Total Environ.694, 133712. doi: 10.1016/j.scitotenv.2019.133712
519
Sarkhel R. Sengupta S. Das P. Bhowal A. (2019). Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine source. J. Polymer Res.27, 16. doi: 10.1007/s10965-019-1973-4
520
Sarmah S. B. Kalita P. Garg A. Niu X. D. Zhang X. W. Peng X. et al . (2019). A review of state of health estimation of energy storage systems: Challenges and possible solutions for futuristic applications of li-ion battery packs in electric vehicles. J. Electrochem Energy Conversion Storage16, 040801. doi: 10.1115/1.4042987
521
Sarmah P. Rout J. (2018). Efficient biodegradation of low-density polyethylene by cyanobacteria isolated from submerged polyethylene surface in domestic sewage water. Environ. Sci. Pollut. Res.25, 33508–33520. doi: 10.1007/s11356-018-3079-9
522
Sathish N. Jeyasanta K. I. Patterson J. (2019). Abundance, characteristics and surface degradation features of microplastics in beach sediments of five coastal areas in Tamil Nadu, India. Mar. Pollut. Bull.142, 112–118. doi: 10.1016/j.marpolbul.2019.03.037
523
Sathish M. N. Jeyasanta K. I. Patterson J. (2020b). Occurrence of microplastics in epipelagic and mesopelagic fishes from Tuticorin, southeast coast of India. Sci. Total Environ.720, 137614. doi: 10.1016/j.scitotenv.2020.137614
524
Schaerer L. Wood E. Aloba S. Byrne E. Bashir M. Baruah K. et al . (2023). Versatile microbial communities rapidly assimilate ammonium hydroxide-treated plastic waste. J. Ind. Microbiol. Biotechnol.50. doi: 10.1093/jimb/kuad008
525
Schirinzi G. F. Pérez-Pomeda I. Sanchís J. Rossini C. Farré M. Barceló D. (2017). Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res.159, 579–587. doi: 10.1016/j.envres.2017.08.043
526
Schmid K. Winemiller K. O. Chelazzi D. Cincinelli A. Dei L. Giarrizzo T. (2018). First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. pollut. Bull.133, 814–821. doi: 10.1016/j.marpolbul.2018.06.035
527
Schmidt C. Krauth T. Wagner S. (2017). Export of plastic debris by rivers into the sea. Environ. Sci. Technol.51, 12246–12253. doi: 10.1021/acs.est.7b02368
528
Schmidt N. Thibault D. Galgani F. Paluselli A. Semp´er´e R. (2018). Occurrence of microplastics in surface waters of the Gulf of Lion (NW Mediterranean Sea). Prog. Oceanogr.163, 214–220. doi: 10.1016/j.pocean.2017.11.010
529
Schönlau C. Karlsson T. M. Rotander A. Nilsson H. Engwall M. van Bavel B. et al . (2020). Microplastics in sea-surface waters surrounding Sweden sampled by manta trawl and in-situ pump. Mar. pollut. Bull.153, 111019. doi: 10.1016/j.marpolbul.2020.111019
530
Scopetani C. Esterhuizen-Londt M. Chelazzi D. Cincinelli A. Setälä H. Pflugmacher S. (2020). Self-contamination from clothing in microplastics research. Ecotoxicol. Environ. Saf.189, 110036. doi: 10.1016/j.ecoenv.2019.110036
531
Schuhen K. Sturm M. T. Herbort A. F. (2019). Technological approaches for the reduction of microplastic pollution in seawater desalination plants and for sea salt extraction. Plastics Environ.3, 1–16.
532
Schwarz A. E. Ligthart T. N. Boukris E. van Harmelen T. (2019). Sources, transport, and accumulation of different types of plastic litter in aquatic environments: a review study. Mar. pollut. Bull.143, 92–100. doi: 10.1016/j.marpolbul.2019.04.02
533
Schymanski D. Goldbeck C. Humpf H. U. Fürst P. (2018). Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res.129, 154–162. doi: 10.1016/j.watres.2017.11.011
534
Seenivasagan R. Karthika A. Poonkuzhali K. (2022). In vitro and in silico study of the efficacy of fungi in low-density polyethylene degradation in a disposal paper cup. Water Air Soil Pollut.233, 77. doi: 10.1007/s11270-022-05549-7
535
Seggio M. Arcadio F. Radicchi E. Cennamo N. Zeni L. Bossi A. M. (2024). Toward nano-and microplastic sensors: Identification of nano-and microplastic particles via artificial intelligence combined with a plasmonic probe functionalized with an estrogen receptor. ACS Omega9, 18984–18994. doi: 10.1021/acsomega.3c10554
536
Selonen S. Dolar A. Kokalj A. J. Skalar T. Dolcet L. P. Hurley R. et al . (2020). Exploring the impacts of plastics in soil–The effects of polyester textile fibres on soil invertebrates. Sci. Total Environment.700, 134451. doi: 10.1016/j.scitotenv.2019.134451
537
Shahi N. K. Maeng M. Kim D. Dockko S. (2020). Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process Saf. Environ. Prot.141, 9–17. doi: 10.1016/j.psep.2020.04.024
538
Sharma S. Basu S. Shetti N. P. Nadagouda M. N. Aminabhavi T. M. (2021). Microplastics in the environment: occurrence, perils, and eradication. Chem. Eng. J.408, 127317. doi: 10.1016/j.cej.2020.127317
539
Shaw D. G. Day R. H. (1994). Colour-and form-dependent loss of plastic micro-debris from the North Pacific Ocean. Mar. pollut. Bulletin.28, 39–43. doi: 10.1016/0025-326X(94)90184-8
540
Shen M. Zeng Z. Song B. Yi H. Hu T. Zhang Y. et al . (2021). Neglected microplastics pollution in global COVID-19: Disposable surgical masks. Sci. Total Environ.790, 148130. doi: 10.1016/j.scitotenv.2021.148130
541
Sheriff N. Aslam A. Arshad M. Iqbal M. (2023). Microplastic pollution in agricultural soils: Sources, impacts and mitigation strategies. Environ. Technol. Innovation29, 103045.
542
Shi R. Liu W. Lian Y. Wang Q. Zeb A. Tang J. (2022). Phytotoxicity of polystyrene, polyethylene and polypropylene microplastics on tomato (Lycopersicon esculentum L.). J. Environ. Manage.317, 115441. doi: 10.1016/j.jenvman.2022.115441
543
Shim W. J. Hong S. H. Eo S. E. (2017). Identification methods in microplastic analysis: A review. Analytical Methods. doi: 10.1039/C6AY02558G
544
Shim W. J. Song Y. K. Hong S. H. Jang M. (2016). Identification and quantification of microplastics using Nile Red staining. Mar. pollut. Bull.113, 469–476. doi: 10.1016/j.marpolbul.2016.10.049
545
Sighicelli M. Pietrelli L. Lecce F. Iannilli V. Falconieri M. Coscia L. et al . (2018). Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. pollut.236, 645–651. doi: 10.1016/j.envpol.2018.02.008
546
Siipola V. Pflugmacher S. Romar H. Wendling L. Koukkari P. (2020). Low-cost biochar adsorbents for water purification including microplastics removal. Appl. Sci.10, 788. doi: 10.3390/app10030788
547
Silva A. B. Bastos A. S. Justino C. I. da Costa J. P. Duarte A. C. Rocha-Santos T. A. (2018). Microplastics in the environment: Challenges in analytical chemistry-A review. Analytica Chimica Acta1017, 1–9. doi: 10.1016/j.aca.2018.02.043
548
Silva M. S. S. Oliveira M. Lop´ez D. Martins M. Figueira E. Pires A. (2020b). Do nanoplastics impact the ability of the polychaeta Hediste diversicolour to regenerate? Ecol. Indicat110, 105921. doi: 10.1016/j.ecolind.2019.105921
549
Silva A. L. P. Prata J. C. Walker T. R. Campos D. Duarte A. C. Soares A. M. et al . (2020a). Rethinking and optimising plastic waste management under COVID-19 pandemic: policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci. Total Environ.742, 140565. doi: 10.1016/j.scitotenv.2020.140565
550
Silva P. H. de Sousa F. D. (2021). Microplastic pollution of Patos Lagoon, south of Brazil. Environ. Challenges4, 100076. doi: 10.1016/j.envc.2021.100076
551
Simon-Sánchez L. Grelaud M. Garcia-Orellana J. Ziveri P. (2019). River Deltas as hotspots of microplastic accumulation: the case study of the Ebro River (NW Mediterranean). Sci. Total Environ.687, 1186–1196. doi: 10.1016/j.scitotenv.2019.06.168
552
Sindha J. Mangwani N. Dash H. R. (2020). Bacterial degradation of synthetic plastics. Curr. Sci.118, 1844–1852.
553
Skalska K. Ockelford A. Ebdon J. E. Cundy A. B. (2020). Riverine microplastics: Behaviour, spatio-temporal variability, and recommendations for standardised sampling and monitoring. J. Water Process Eng.38, 101600. doi: 10.1016/j.jwpe.2020.101600
554
Skariyachan S. Manjunatha V. Sultana S. Jois C. Bai V. Vasist K. S. (2016). Novel bacterial consortia isolated from plastic garbage processing sites demonstrate enhanced degradation for low-density polyethylene. Environ. Sci. Pollut. Res.23, 18307–18319. doi: 10.1007/s11356-016-7000-9
555
Sol D. Laca A. Laca A. Díaz M. (2020). Approaching the environmental problem of microplastics: Importance of WWTP treatments. Sci. Total Environ.740, 140016. doi: 10.1016/j.scitotenv.2020.140016
556
Solai A. Suresh Gandhi M. Kasilingam K. Sriraman E. (2013). Heavy metal accumulation in the surface sediments off Pondicherry, bay of Bengal, South East Coast of India. Int. J. Innovative Sci. Eng Technol.2, 5741–5753.
557
Soleimani M. Tabatabaei S. A. Masoumi A. et al . (2021). Infectious keratitis: trends in microbiological and antibiotic sensitivity patterns. Eye (London)35, 3110–3115. doi: 10.1038/s41433-020-01378-w
558
Son H. J. Cho S. Hong S. W. Yang J. E. (2019). Effects of pH and ionic strength on microplastic behavior in aquatic environments. Water Res.158, 201–210.
559
Song Y. Cao C. Qiu R. Hu J. Liu M. Lu S. et al . (2019). Uptake and adverse effects of polyethylene terephthalate microplastics fibres on terrestrial snails (Achatina fulica) after soil exposure. Environ. pollut.250, 447e455. doi: 10.1016/j.envpol.2019.04.066
560
Song Y. K. Hong S. H. Jang M. Han G. M. Rani M. Lee J. et al . (2015). A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull.93, 202–209. doi: 10.1016/j.marpolbul.2015.01.015
561
Song C. Wu L. Xie Y. He J. Chen X. Wang T. et al . (2017). Air pollution in China: Status and spatiotemporal variations. Environ. Pollut.227, 334–347. doi: 10.1016/j.envpol.2017.04.075
562
Sowmya H. V. Ramlingappa B. Krishnappa M. Thippeswamy B. (2014). Low density polyethylene degrading fungi isolated from local dumpsite of Shivamogga district. Intern. J. Biol. Res.2. doi: 10.14419/ijbr.v2i2.2877
563
Sparks C. Awe A. Maneveld J. (2021). Abundance and characteristics of microplastics in retail mussels from Cape Town, South Africa. Mar. Pollut. Bull.166, 112186. doi: 10.1016/j.marpolbul.2021.112186
564
Sturm M. T. Herbort A. F. Horn H. Schuhen K. (2020). Comparative study of the influence of linear and branched alkyltrichlorosilanes on the removal efficiency of polyethylene and polypropylene-based microplastic particles from water. Environ. Sci. Pollut. Res.27, 10888–10898. doi: 10.1007/s11356-020-07629-x
565
Su L. Nan B. Hassell K. L. Craig N. J. Pettigrove V. (2019). Microplastics biomonitoring in Australian urban wetlands using a common noxious fish (Gambusia holbrooki). Chemosphere228, 65–74. doi: 10.1016/j.chemosphere.2019.04.114
566
Su L. Xue Y. Li L. Yang D. Kolandhasamy P. Li D. et al . (2016). Microplastics in taihu lake, China. Environ. Pollution.216, 711–719. doi: 10.1016/j.envpol.2016.06.036
567
Sulaiman R. N. R. Bakar A. A. Ngadi N. Kahar I. N. S. Nordin A. H. Ikram M. et al . (2023). Microplastics in Malaysia's aquatic environment: current overview and future perspectives. Global Challenges7, 2300047. doi: 10.1002/gch2.202300047
568
Suman T. Y. Jia P. P. Li W. G. Junaid M. Xin G. Y. Wang Y. et al . (2020). Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater.400, 123220. doi: 10.1016/j.jhazmat.2020.123220
569
Sumathi S. Bhatia S. Lee K. T. Mohamed A. R. (2016). Biodegradation of polymers by microorganisms: Current status and future prospects. Int. J. Environ. Sci. Technol.13, 213–226.
570
Sun X. Liang J. Zhu M. Zhao Y. Zhang B. (2018). Microplastics in seawater and zooplankton from the Yellow Sea. Environ. pollut.242, 585e595. doi: 10.1016/j.scitotenv.2018.05.308
571
Sun Y. (2023). Measurement report: Atmospheric ice nuclei at Changbai Mountain (2,623 m a.s.l.) in Northeastern Asia (Dataset) ( Mendeley Data). doi: 10.17632/b9y6pfw39n.2
572
Sun J. Dai X. Wang Q. van Loosdrecht M. C. M. Ni B.-J. (2019). Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res.152, 21–37. doi: 10.1016/j.watres.2018.12.050
573
Sun Q. Ren S. Y. Ni H. G. (2020). Incidence of microplastics in personal care products: an appreciable part of plastic pollution. Sci. Total Environ.742, 140218. doi: 10.1016/j.scitotenv.2020.140218
574
Sussarellu R. Suquet M. Thomas Y. Lambert C. Fabioux C. Pernet M. E. J. et al . (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. U.S.A.113, 2430–2435. doi: 10.1073/pnas.1519019113
575
Sutkar P. R. Gadewar R. D. Dhulap V. P. (2023). Recent trends in degradation of microplastics in the environment: A state-of-the-art review. J. Hazard. Mater. Adv.11, 100343. doi: 10.1016/j.hazadv.2023.100343
576
Tadsuwan K. Babel S. (2021). Microplastic contamination in a conventional wastewater treatment plant in Thailand. Waste Manage. Res.39, 754–761. doi: 10.1177/0734242X20980814
577
Taghavi N. Singhal N. Zhuang W. Q. Baroutian S. (2021). Degradation of plastic waste using stimulated and naturally occurring microbial strains. Chemosphere.263, 127975. doi: 10.1016/j.chemosphere.2020.127975
578
Tahir A. Samawi M. F. Sari K. Hidayat R. Nimzet R. Wicaksono E. A. et al . (2019). Studies on microplastic contamination in seagrass beds at Spermonde Archipelago of Makassar Strait, Indonesia. J. Phys: Conf Ser.1341, 22008. doi: 10.1088/1742-6596/1341/2/022008
579
Takar S. Gurjar U. R. Saroj J. Raveendar B. Singh J. Deshmukhe G. (2020). Benthic organisms in Mumbai mangroves: diversity and distribution in relation to environmental parameters. J. Exp. Zool India23, 599–605.
580
Talvitie J. Mikola A. Koistinen A. Setälä O. (2017). Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res.123, 401–407. doi: 10.1016/j.watres.2017.07.005
581
Tamin M. Ibrahim Y. S. Choo Y. T. Lee K. C. (2023). Microplastic pollution in Southeast Asian rivers: A comprehensive review. Environ. Pollut.318, 120875.
582
Tang Y. Zhang S. Su Y. Wu D. Zhao Y. Xie B. (2021). Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J.406, 126804. doi: 10.1016/j.cej.2020.126804
583
Teng J. Zhao J. Zhu X. Shan E. Zhang C. Zhang W. et al . (2021). Toxic effects of exposure to microplastics with environmentally relevant shapes and concentrations: Accumulation, energy metabolism and tissue damage in oyster Crassostrea gigas. Environ. Pollut.269, 116169. doi: 10.1016/j.envpol.2020.116169
584
Temporiti M. E. E. Nicola L. Nielsen E. Tosi S. (2022). Fungal enzymes involved in plastics biodegradation. Microorganisms10. doi: 10.3390/microorganisms10061180
585
Ter Halle A. Ladirat L. Martignac M. Mingotaud A. F. Boyron O. Perez E. (2017). To what extent are microplastics from the open ocean weathered? Environ. pollut.227, 167–174. doi: 10.1016/j.envpol.2017.04.051
586
Teuten E. L. Rowland S. J. Galloway T. S. Thompson R. C. (2007). Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol.41, 7759–7764. doi: 10.1021/es071737s
587
Thangaradjou T. Subhashini P. Raja S. Dilipan E. Nobi E. P. (2014). Evidences for heavy metal contamination in surface sediments of seagrass ecosystem of Lakshadweep archipelago, India. Environ. Earth Sci.71, 1135–1146. doi: 10.1007/s12665-013-2517-5
588
Thøgersen J. Haugaard P. Olesen A. (2010). Consumer responses to ecolabels. Eur. J. Marketing44, 1787–1810. doi: 10.1108/03090561011079882
589
Tholkappian M. Ravisankar R. Chandrasekaran A. Jebakumar J. P. P. Kanagasabapathy K. V. Prasad M. V. R. et al . (2018). Assessing heavy metal toxicity in sediments of Chennai Coast of Tamil Nadu using energy dispersive X-ray fluorescence spectroscopy (EDXRF) with statistical approach. Toxicol. Rep.5, 173–182. doi: 10.1016/j.toxrep.2017.12.020
590
Thompson R. C. Olsen Y. Mitchell R. P. Davis A. Rowland S. J. John A. W. G. et al (2004). Lost at sea: Where is all the plastic? Science304, 838. doi: 10.1126/science.1094559
591
Thompson R. C. Swan S. H. Moore C. J. vom Saal F. S. (2009). Our plastic age. Philos. Trans. R. Soc. B364, 1973–1976. doi: 10.1098/rstb.2009.0054
592
Thomson A. M. et al . (2011). Overview paper on Representative Concentration Pathways and climate model input. Climatic Change109, 77–94. doi: 10.1007/s10584-011-0151-4
593
Tien C. J. Wang Z. X. Chen C. S. (2020). Microplastics in water, sediment and fish from the Fengshan River system: Relationship to aquatic factors and accumulation of polycyclic aromatic hydrocarbons by fish. Environ. Pollut.265, 114962. doi: 10.1016/j.envpol.2020.114962
594
Tiwari M. Rathod T. D. Ajmal P. Y. Bhangare R. C. Sahu S. K. (2019). Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. pollut. Bull.140, 262–273. doi: 10.1016/j.marpolbul.2019.01.055
595
Tiwari N. Santhiya D. Sharma J. (2023). Degradation of polyethylene microplastics through microbial action by a soil isolate of Brevibacillus brevis. Polymer Degradation Stabil.215, 110436. doi: 10.1016/j.polymdegradstab.2023.110436
596
Tofa T. S. Kunjali K. L. Paul S. Dutta J. (2019a). Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett.17, 1341–1346. doi: 10.1007/s10311-019-00859-z
597
Tofa T. S. Ye F. Kunjali K. L. Dutta J. (2019b). Enhanced visible light photodegradation of microplastic fragments with plasmonic platinum/zinc oxide nanorod photocatalysts. Catalysts9, 819. doi: 10.3390/catal9100819
598
Tomar V. (2022). Microplastics in terrestrial ecosystems: Occurrence, distribution, and environmental effects. Curr. Opin. Environ. Sci. Health26, 100338.
599
Tramoy R. Gasperi J. Dris R. Colasse L. Fisson C. Sananes S. et al . (2019). Assessment of the plastic inputs from the Seine Basin to the sea using statistical and field approaches. Front. Mar. Science.6. doi: 10.3389/fmars.2019.00151
600
Tran V. et al . (2023). Microplastics and their environmental effects. Environ. Toxicol. Pharmacol., 104324. doi: 10.1016/j.etap.2023.104324
601
Truchet D. M. Forero Lopez A. D. Ardusso M. G. Rimondino G. N. Buzzi N. S. Malanca F. E. et al . (2021). Microplastics in bivalves, water and sediments from a touristic sandy beach of Argentina. Mar. pollut. Bull.173, 113023. doi: 10.1016/j.marpolbul.2021.113023
602
Truc N. T. T. Duc D. S. Van Thuan D. Al Tahtamouni T. Pham T. D. Hanh N. T. et al . (2019). The advanced photocatalytic degradation of atrazine by direct Z-scheme Cu doped ZnO/g-C3N4. Appl. Surface Sci.489, 875–882. doi: 10.1016/j.apsusc.2019.06.021
603
Tsering T. Sillanpää M. Sillanpää M. Viitala M. Reinikainen S. P. (2021). Microplastics pollution in the Brahmaputra River and the Indus River of the Indian Himalaya. Sci. Total Environ.789, 147968. doi: 10.1016/j.scitotenv.2021.147968
604
Tunçer S. Artüz O. B. Demirkol M. Artüz M. L. (2018). First report of occurrence, distribution, and composition of microplastics in surface waters of the Sea of Marmara, Turkey. Mar. pollut. Bull.135, 283–289. doi: 10.1016/j.marpolbul.2018.06.054
605
Udochukwu U. Atuanya E. I. Usman Z. (2022). Biodegradability of polystyrene plastics by bacterial isolates from plastic composted waste soil and molecular characterization of plastic degrading bacterial isolates, Afr. J. Microbiol. Res.16, 247–257. doi: 10.5897/AJMR2020.9341
606
Ugwu K. Herrera A. Gómez M. (2021). Microplastics in marine biota: A review. Mar. pollut. Bull.169, 112540. doi: 10.1016/j.marpolbul.2021.112540
607
Uheida A. Mejía H. G. Abdel-Rehim M. Hamd W. Dutta J. (2020). Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J. Hazard. Mater.406, 124299.
608
Uheida A. Mejía H. G. Abdel-Rehim M. Hamd W. Dutta J. (2021). Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J. Hazard. Mater.406, 124299. doi: 10.1016/j.jhazmat.2020.124299
609
United Nations (UN) (2015). Transforming our world: The 2030 agenda for sustainable development (A/RES/70/1) ( United Nations General Assembly). Available online at: https://sdgs.un.org/2030agenda.
610
United Nations Conference on Environment and Development (UNCED) (1992). Agenda 21 (A/CONF.151/26) ( United Nations). Available online at: https://sustainabledevelopment.un.org/content/documents/Agenda21.pdf.
611
United Nations Environment Programme (UNEP) (2018). Single-use plastics: A roadmap for sustainability (Rev. ed.) ( United Nations Environment Programme). Available online at: https://wedocs.unep.org/handle/20.500.11822/25496.
612
Vaid M. Mehra K. Gupta A. (2021). Microplastics as contaminants in Indian environment: A review. Environ. Sci. Pollut. Res.28, 68025–68052. doi: 10.1007/s11356-021-16827-6
613
Valhondo C. Martínez-Landa L. Carrera J. Díaz-Cruz S. M. Amalfitano S. Levantesi C. (2020). Six artificial recharge pilot replicates to gain insight into water quality enhancement processes. Chemosphere240, 124826. doi: 10.1016/j.chemosphere.2019.124826
614
Vanapalli K. R. Sharma H. B. Ranjan V. P. Samal B. Bhattacharya J. Dubey B. K. et al . (2020). Challenges and strategies for effective plastic waste management in developing countries: A review. Waste Manage.110, 731–743. doi: 10.1016/j.scitotenv.2020.141514
615
van Cauwenberghe L. Janssen C. R. (2014). Microplastics in bivalves cultured for human consumption. Environ. Pollut.193, 65–70. doi: 10.1016/j.envpol.2014.06.010
616
Van Emmerik T. Kieu-Le T. C. Loozen M. Van Oeveren K. Strady E. Bui X. T. et al . (2018). A methodology to characterize riverine macroplastic emission into the ocean. Front. Mar. Science.17. doi: 10.3389/fmars.2018.00372
617
van Weert S. Redondo-Hasselerharm P. E. Diepens N. J. Koelmans A. A. (2019). Effects of nanoplastics and microplastics on the growth of sediment-rooted macrophytes. Scie Total Environ.654, 1040–1047. doi: 10.1016/j.scitotenv.2018.11.183
618
van Wijnen J. Ragas A. M. J. Kroeze C. (2019). Modelling global river export of microplastics to the marine environment: sources and future trends. Sci. Total Environ.673, 392–401. doi: 10.1016/j.scitotenv.2019.04.078
619
Varlamov V. P. Il'ina A. V. Shagdarova B. T. (2018). Chitin and chitosan-based biodegradable polymers. Appl. Biochem. Microbiol.54, 1–11.
620
Vasudeva M. Adarsh U. K. Warrier A. K. George S. D. Unnikrishnan V. K. (2024). Performance evaluation of a hyphenated laser spectroscopy system with conventional methods for microplastic analysis. Sci. Rep.14, 19327. doi: 10.1038/s41598-024-69777-z
621
Vázquez-Vázquez B. Lazzari M. Hospido A. (2025). Terrestrial characterization factors for bio-and fossil-based plastics: microplastics ingestion and additives release. Waste Manage.196, 106–114. doi: 10.1016/j.wasman.2025.02.008
622
Veerasingam S. Mugilarasan M. Venkatachalapathy R. Vethamony P. (2016). Influence of 2015 flood on the distribution and occurrence of microplastic pellets along the Chennai coast, India. Mar. pollut. Bull.109, 196–204. doi: 10.1016/j.marpolbul.2016.05.082
623
Vélez-Rubio G. M. Teryda N. Asaroff P. E. Estrades A. Rodriguez D. Tomás J. (2018). Differential impact of marine debris ingestion during ontogenetic dietary shift of green turtles in Uruguayan waters. Mar. pollut. Bull.127, 603–611. doi: 10.1016/j.marpolbul.2017.12.053
624
Venkataramana C. Botsa S. M. Shyamala P. Muralikrishna R. (2021). Photocatalytic degradation of polyethylene plastics by NiAl2O4 spinels-synthesis and characterization. Chemosphere265, 129021. doi: 10.1016/j.chemosphere.2020.129021
625
Venkatesh S. Mahboob S. Govindarajan M. Al-Ghanim K. A. Ahmed Z. Al- Mulhm N. et al . (2021). Microbial degradation of plastics: sustainable approach to tackling environmental threats facing big cities of the future. J. King Saud Univer. - Sci.33. doi: 10.1016/j.jksus.2021.101362
626
Verla A. W. Enyoh C. E. Verla E. N. Nwarnorh K. O. (2019). Microplastic–toxic chemical interaction: a review study on quantified levels, mechanism and implication. SN Appl. Sci.1, 1–30. doi: 10.1007/s42452-019-1352-0
627
Viana R. Coelho F. J. R. C. Gomes N. C. M. (2019). Microbial responses to plastic pollution in marine environments: A review. Front. Microbiol.10, 2039. doi: 10.3389/fmicb.2019.02039
628
Vidyasakar A. Krishnakumar S. Kasilingam K. Neelavannan K. Bharathi V. A. Godson P. S. et al . (2020). Characterization and distribution of microplastics and plastic debris along Silver Beach, Southern India. Mar. Pollut. Bull.158, 111421. doi: 10.1016/j.marpolbul.2020.111421
629
Viel T. Manfra L. Zupo V. Libralato G. Cocca M. Costantini M. (2023). Biodegradation of plastics induced by marine organisms: future perspectives for bioremediation approaches. Polymers15. doi: 10.3390/polym15122673
630
Vilakati B. Sivasankar V. Nyoni H. Mamba B. B. Omine K. Msagati T. A. (2021). The Py–GC-TOF-MS analysis and characterization of microplastics (MPs) in a wastewater treatment plant in Gauteng Province, South Africa. Ecotoxicol. Environ. Saf.222, 112478. doi: 10.1016/j.ecoenv.2021.112478
631
Voparil I. M. Burgess R. M. Mayer L. M. Tien R. Cantwell M. G. Ryba S. A. (2004). Digestive bioavailability to a deposit feeder (Arenicola marina) of polycyclic aromatic hydrocarbons associated with anthropogenic particles. Environ. Toxicol. Chem.23, 2618–2626. doi: 10.1897/03-357
632
Vroom R. J. Koelmans A. A. Besseling E. Halsband C. (2017). Aging of microplastics promotes their ingestion by marine zooplankton. Environ. pollut.231, 987e996. doi: 10.1016/j.envpol.2017.08.08
633
Wäger P. A. Hischier R. (2015). Life cycle assessment of post-consumer plastics production from waste electrical and electronic equipment (WEEE) treatment residues in a Central European plastics recycling plant. Sci. Total Environ.529, 158–167.
634
Wagner M. Scherer C. Alvarez-Muñoz D. Brennholt N. Bourrain X. Buchinger S. et al . (2014). Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Europe26, 12.
635
Walkinshaw C. Lindeque P. K. Thompson R. Tolhurst T. Cole M. (2020). Microplastics and seafood: lower trophic organisms at highest risk of contamination. Ecotoxicol Environ. Saf.190, 110066. doi: 10.1016/J.ECOENV.2019.110066
636
Wander L. Vianello A. Vollertsen J. Westad F. Braun U. Paul A. (2020). Exploratory analysis of hyperspectral FTIR data obtained from environmental microplastics samples. Analytical Methods12, 781–791. doi: 10.1039/C9AY02483B
637
Wang J. Duncan D. Shi Z. Zhang B. (2013). WEB-based gene set analysis toolkit (WebGestalt): update 2013. Nucleic Acids Res.41, W77–W83. doi: 10.1093/nar/gkt439
638
Wang J. Guo X. Xue J. (2021). Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol.55, 12780–12790. doi: 10.1021/acs.est.1c04466
639
Wang J. Tan Z. Peng J. Qiu Q. Li M. (2016). The behaviors of microplastics in the marine environment. Mar. Environ. Res.113, 7–17. doi: 10.1016/j.marenvres.2015.10.014
640
Wang J. Vasaikar S. Shi Z. Greer M. Zhang B. (2017b). WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res.45, W130–W137. doi: 10.1093/nar/gkx356
641
Wang J. Wang M. Ru S. Liu X. (2019). High levels of microplastic pollution in the sediments and benthic organisms of the South Yellow Sea, China. Sci. Total Environ.651, 1661–1669. doi: 10.1016/j.scitotenv.2018.10.007
642
Wang Q. Hong L. Wu K. Li M. Zhang J. Li X. et al . (2024). Research Progress in Microbial Degradation of Microplastics. J. Physics: Conf. Ser.2706, 12043. doi: 10.1088/1742-6596/2706/1/012043
643
Wang W. Yuan W. Chen Y. Wang J. (2018). Microplastics in surface waters of Dongting Lake and Hong Lake, China. Sci. Total Environ.633, 539–545. doi: 10.1016/j.scitotenv.2018.03.211
644
Wang Y. Bryant S. H. Cheng T. Wang J. Gindulyte A. Shoemaker B. A. et al . (2017a). Pubchem bioassay: 2017 update. Nucleic Acids Res.45, D955–D963. doi: 10.1093/nar/gkw1118
645
Waring R. H. Harris R. M. Mitchell S. C. (2018). Plastic contamination of the food chain: A threat to human health? Maturitas115, 64–68. doi: 10.1016/j.maturitas.2018.06.010
646
Watanabe T. Oda K. Suzuki Y. (2015). Enzymatic degradation of polyesters: New approaches using cutinase and PETase. Biomacromolecules16, 1882–1893. doi: 10.1021/acs.biomac.5b00257
647
Watts A. J. R. Urbina M. A. Goodhead R. Moger J. Lewis C. Galloway T. S. (2016). Effect of microplastic on the gills of the shore crab Carcinus maenas. Environ. Sci. Technol.50, 5364–5369. doi: 10.1021/acs.est.6b01187
648
Webb H. K. Arnott J. Crawford R. J. Ivanova E. P. (2013). Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers5, 1–18. doi: 10.3390/polym5010001
649
Wei W. Huang Q.-S. Sun J. Dai Y.-N. Ni B.-J. Wang J. (2023). Revealing the combined toxicity of microplastics and per- and polyfluoroalkyl substances on the activated sludge process. Environ. Sci. Technol.57, 3180–3191. doi: 10.1021/acs.est.2c09204
650
Weitzel S. L. Feura J. M. Rush S. A. Iglay R. B. Woodrey M. S. (2021). Availability and assessment of microplastic ingestion by marsh birds in Mississippi gulf coast tidal marshes. Mar. pollut. Bull.166, 112187. doi: 10.1016/j.marpolbul.2021.112187
651
Wicaksono E. A. Werorilangi S. Galloway T. S. Tahir A. (2021). Distribution and seasonal variation of microplastics in Tallo River, Makassar, Eastern Indonesia. Toxics9, 129. doi: 10.3390/toxics9060129
652
Windsor F. M. Tilley R. M. Tyler C. R. Ormerod S. J. (2019). Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environment.646, 68–74. doi: 10.1016/j.scitotenv.2018.07.271
653
Woo I.-S. Rhee I.-K. Park H.-D. (2000). Differential damage in bacterial cells by microwave radiation on the basis of cell-wall structure. Appl. Environ. Microbiol.66, 2243–2247. doi: 10.1128/AEM.66.5.2243-2247.2000
654
Wright S. L. Kelly F. J. (2017). Plastic and human health: A micro issue? Environ. Sci. Technol.51, 6634–6647. doi: 10.1021/acs.est.7b00423
655
Wright S. L. Thompson R. C. Galloway T. S. (2013). The physical impacts of microplastics on marine organisms: a review. Environ. Pollution.178, 483–492. doi: 10.1016/j.envpol.2013.02.031
656
Wu J. Liu W. Zeb A. Lian J. Sun Y. Sun H. (2021). Polystyrene microplastic interaction with Oryza sativa: toxicity and metabolic mechanism. Environ. Science: Nano.8, 3699–3710.
657
Wu Y. Guo P. Zhang X. Zhang Y. Xie S. Deng J. (2019). Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater.374, 219–227. doi: 10.1016/j.jhazmat.2019.04.039
658
Wu W. M. Yang J. Criddle C. S. (2017). Microplastics pollution and reduction strategies. Front. Environ. Sci. Engineering.11, 1–4. doi: 10.1007/s11783-017-0897-7
659
Wu B. Yu H. Lei P. He J. Yi J. Wu W. et al . (2025). Microplastics in aquatic ecosystems: Detection, source tracing, and sustainable management strategies. Ecotoxicology Environ. Saf.291, 117883. doi: 10.1016/j.ecoenv.2025.117883
660
Xia W. Rao Q. Deng X. Chen J. Xie P. (2020). Rainfall is a significant environmental factor of microplastic pollution in inland waters. Sci. Total Environ.732, 139065. doi: 10.1016/j.scitotenv.2020.139065
661
Xiang K. He Z. Fu J. Wang G. Li H. Zhang Y. et al . (2022). Microplastics exposure as an emerging threat to ancient lineage: A contaminant of concern for abnormal bending of amphioxus via neurotoxicity. J. Hazard. Mater.438, 129454. doi: 10.1016/j.jhazmat.2022.129454
662
Xiang Y. Xiong W. Yang Z. Xu R. Zhang Y. Jia M. et al . (2024). Microplastics provide new hotspots for frequent transmission of antibiotic resistance genes during anaerobic digestion of sludge containing antibiotics. Chem. Eng. J.486, 149979. doi: 10.1016/j.cej.2024.149979
663
Xiang P. Zhang T. Wu Q. Li Q. (2023). Systematic review of degradation processes for microplastics: Progress and prospects. Sustainability15, 12698. doi: 10.3390/su151712698
664
Xu T. Cui J. Xu R. Cao J. Guo M. Y. (2023). Microplastics induced inflammation and apoptosis via ferroptosis and the NF-κB pathway in carp. Aquat. Toxicology.262, 106659. doi: 10.1016/j.aquatox.2023.106659
665
Xu G. Liu Y. Song X. Li M. Yu Y. (2021). Size effects of microplastics on accumulation and elimination of phenanthrene in earthworms, J. Hazard Mater.403, 123966. doi: 10.1016/j.jhazmat.2020.123966
666
Xu X. Wong C. Y. Tam N. F. Y. Lo H.-S. Cheung S.-G. (2020). Microplastics in invertebrates on soft shores in Hong Kong: influence of habitat, taxa and feeding mode. Sci. Total Environ.715, 136999. doi: 10.1016/j.scitotenv.2020.136999
667
Yahyanezhad N. Bardi M. J. Aminirad H. (2021). An evaluation of microplastics fate in the wastewater treatment plants: frequency and removal of microplastics by microfiltration membrane. Water Pract. Technol.16, 782–792. doi: 10.2166/wpt.2021.036
668
Yan M. Nie H. Xu K. He Y. Hu Y. Huang Y. et al . (2019). Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere.217, 879–886. doi: 10.1016/j.chemosphere.2018.11.093
669
Yang J. Yang Y. Wu W.-M. Zhao J. Jiang L. (2014). Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol.48, 13776–13784. doi: 10.1021/es504038a
670
Yang L. Kang S. Wang Z. Luo X. Guo J. Gao T. et al . (2022). Microplastic characteristic in the soil across the Tibetan Plateau. Sci. Total Environ.828, 154518. doi: 10.1016/j.scitotenv.2022.154518
671
Yang L. Zhang Y. Kang S. Wang Z. Wu C. (2021). Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environment.780, 146546. doi: 10.1016/j.scitotenv.2021.14654
672
Yang Y. Liu W. Zhang Z. Grossart H. P. Gadd G. M. (2020). Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol.104, 6501–6511. doi: 10.1007/s00253-020-10704-x
673
Ye G. Zhang X. Liu X. Liao X. Zhang H. Yan C. et al . (2021). Polystyrene microplastics induce metabolic disturbances in marine medaka (Oryzias melastigmas) liver. Sci. Total Environ.782, 146885. doi: 10.1016/j.scitotenv.2021.146885
674
Yi J. Ma Y. Ruan J. You S. Ma J. Yu H. et al . (2024). The invisible Threat: Assessing the reproductive and transgenerational impacts of micro-and nanoplastics on fish. Environ. Int.183, 108432. doi: 10.1016/j.envint.2024.108432
675
Yin L. Jiang C. Wen X. Du C. Zhong W. Feng Z. et al . (2019). Microplastic pollution in surface water of urban lakes in Changsha, China. Int. J. Environ. Res. Public Health16, 1650. doi: 10.3390/ijerph16091650
676
Yin L. Wen X. Huang D. Zeng G. Deng R. Liu R. et al . (2021b). Microplastics retention by reeds in freshwater environment. Sci. Total Environ.790, 148200. doi: 10.1016/j.scitotenv.2021.148200
677
Yoganandham S. T. (2025). “ Molecular pathways of microplastics in the marine ecosystem,” in Recent Trends in Marine Toxicological Assessment ( Springer Nature Switzerland, Cham), 263–284. doi: 10.1007/978-3-031-75713-6_11
678
Yoon M.-G. Jeon H.-J. Kim M.-N. (2012). Biodegradation of polyethylene by a soil bacterium and alkane hydroxylation mechanism. J. Biodegradation23, 1059–1067. doi: 10.1007/s10532-012-9571-0
679
Yoshida S. Hiraga K. Takehana T. Taniguchi I. Yamaji H. Maeda Y. et al . (2016). A bacterium that degrades and assimilates poly (ethylene terephthalate). Science.351, 1196–1199. doi: 10.1126/science.aad6359
680
You H. Guo J. Shen M. Li S. Zhang Y. (2021). Biodegradation of polyethylene microplastics by Bacillus sp. YP1 isolated from soil. Sci. Total Environ.769, 145154. doi: 10.1016/j.scitotenv.2021.145154
681
Yousif E. Haddad R. (2013). Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus2, 398. doi: 10.1186/2193-1801-2-398
682
Yuan J. Ma J. Sun Y. Zhou T. Zhao Y. Yu F. (2020). Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ.715, 136968. doi: 10.1016/j.scitotenv.2020.136968
683
Yuan F. Zhao H. Zhao H. Wang H. Wang L. Li Y. (2017). Microplastic abundance and characteristics in surface water of the Xiangjiang River. Environ. Sci. Pollut. Res.24, 15492–15500.
684
Zahmatkesh S. Klemeˇs J. J. Bokhari A. Wang C. Sillanpaa M. Amesho K. T. T. et al . (2023). Various advanced wastewater treatment methods to remove microplastics and prevent transmission of SARS-CoV-2 to airborne microplastics. Int. J. Environ. Sci. Technol.20, 2229–2246. doi: 10.1007/s13762-022-04654-2
685
Zan L. Fa W. Wang S. (2006). Novel photodegradable low-density polyethylene–TiO2 nanocomposite film. Environ. Sci. Technol.40, 1681–1685. doi: 10.1021/es051173x
686
Zantis L. J. Carroll E. L. Nelms S. E. Bosker T. (2021). Marine mammals and microplastics: a systematic review and call for standardisation. Environ. pollut.269, 116142. doi: 10.1016/j.marpolbul.2021.113051
687
Zhang D. Liu X. Huang W. Li J. Wang C. Zhang D. et al . (2020). Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut.259, 113948. doi: 10.1016/j.envpol.2020.113948
688
Zhang F. Wang X. Xu J. JHU L. Peng G. Xu P. et al . (2019). Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar. pollut. Bull.146, 173–182. doi: 10.1016/j.marpolbul.2019.05.061
689
Zhang S. Wang W. Yan P. Wang J. Yan S. Liu X. et al . (2023). Microplastic migration and distribution in the terrestrial and aquatic environments: A threat to biotic safety. J. Environ. Manage.333, 117412. doi: 10.1016/j.jenvman.2023.117412
690
Zhang Z. Gao S.-H. (2022). The contamination of microplastics in China's aquatic environment: Occurrence, detection and implications for ecological risk. Environ. Pollut.296, 118737. doi: 10.1016/j.envpol.2021.118737
691
Zhou T. Song S. Min R. Liu X. Zhang G. (2024). Advances in chemical removal and degradation technologies for microplastics in the aquatic environment: A review. Mar. pollut. Bull.201, 116202. doi: 10.1016/j.marpolbul.2024.116202
692
Zhou G. Li Q. Lu Q. Wang L. Liu J. Wang J. (2023). Microplastic removal in wastewater treatment plants: A review. Environ. Chem. Lett.21, 2313–2332. doi: 10.1007/s10311-023-01598-0
693
Zhou Y. He G. Jiang X. Yao L. Ouyang L. Liu X. et al . (2021). Microplastic contamination is ubiquitous in riparian soils and strongly related to elevation, precipitation and population density. J. Hazard. Mater.411, 125178.
694
Zhou Y. Liu X. Wang J. (2019). Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci. Total Environ.694, 133798. doi: 10.1016/j.scitotenv.2019.133798
695
Zhou Y. Wang J. Zou M. Jia Z. Zhou S. Li Y. (2020). Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Sci. Total Environ.748, 141368. doi: 10.1016/j.scitotenv.2020.141368
696
Zhu F. Zhu C. Wang C. Gu C. (2019). Occurrence and ecological impacts of microplastics in soil systems: a review. Bull. Environ. Contam Toxicology.102, 741–749. doi: 10.1007/s00128-019-02623-z
697
Zhu K. Jia H. Sun Y. Dai Y. Zhang C. Guo X. et al . (2020). Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res.173, 115564. doi: 10.1016/j.watres.2020.115564
698
Zhu L. Bai H. Chen B. Sun X. Qu K. Xia B. (2018). Microplastic pollution in North Yellow Sea, China: Observations on occurrence, distribution and identification. Sci. Total Environ.636, 20–29. doi: 10.1016/j.scitotenv.2018.04.182
699
Zhu X. Huang W. Fang M. Liao Z. Wang Y. Xu L. et al . (2021). Airborne microplastic concentrations in five megacities of northern and southeast China. Environ. Sci. Technol.55, 12871–12881. doi: 10.1021/acs.est.1c03618
700
Zhuang S. Wang J. (2023). Interaction between microplastics and microorganisms in the environment: Modes, influencing factors, and environmental risks. Toxics11, 196. doi: 10.3390/toxics11030196
701
Ziccardi L. M. Edgington A. Hentz K. Kulacki K. J. Kane Driscoll S. (2016). Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: A state-of-the-science review. Environ. Toxicol. Chem.35, 1667–1676. doi: 10.1002/etc.3461
Summary
Keywords
microplastic pollution, environmental pathways, detection techniques, ecotoxicological impacts, mitigation strategies, policy frameworks
Citation
Das BK, Das S, Kumar V, Roy S, Mitra A and Mandal B (2025) Microplastics in ecosystems: ecotoxicological threats and strategies for mitigation and governance. Front. Mar. Sci. 12:1672484. doi: 10.3389/fmars.2025.1672484
Received
24 July 2025
Accepted
07 October 2025
Published
25 November 2025
Volume
12 - 2025
Edited by
Ping Li, Shandong University, Weihai, China
Reviewed by
Da Sun, Wenzhou University, China
Aanchal Mittal, VIT University, India
Updates
Copyright
© 2025 Das, Das, Kumar, Roy, Mitra and Mandal.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basanta Kumar Das, basantakumard@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.