- 1Centre for Environment, Fisheries and Aquaculture Science, Lowestoft, Suffolk, United Kingdom
- 2Centre for Environment, Fisheries and Aquaculture Science, Weymouth, United Kingdom
Effective monitoring is essential for decision makers to make informed choices to address pollution issues, including marine litter and microplastics which are subjects of increasing interest. Monitoring biota is essential for estimating the bioavailable fraction of litter in the environment and is a step towards understanding the risks associated with microplastics. The present study assessed the suitability of mussels as a sentinel species in a multi-stressor context. Mussels are already used as sentinel species for many contaminants, including harmful algal blooms, but to date there is no agreed sentinel species targeting microplastics. Mussels from seven locations on the English and Welsh coast were monitored for both microplastics and harmful algal biotoxins. Fluorescent and non-fluorescent microplastics were quantified. Over half (53%) of mussels contained microplastics. No geographical accumulation zones in microplastic abundance were identified at the sample locations with a mean contamination of 1.33 ± 3.04 (SD) items per individual (95% CI = 0.88–1.79) and 0.33 ± 0.71 items per g (wet weight) (95% CI = 0.23–0.44). Five groups of harmful algal toxins were screened within the study animals, with no quantifiable levels of any being present at the time of sampling for this study. However, four of the six sites were exposed to the Diarrhetic shellfish toxins earlier in the year, representing a prior exposure to harmful algal toxins. Research has shown that microplastic exposure alone does not always negatively impact organisms. But there is mounting evidence that microplastics may increase sensitivity and susceptibility to other stressors in the environment. Given the presence of both microplastics and algal neurotoxins in oceans around the world and the concern for multi-stressor impacts on the marine environment, it is proposed that multi-factor monitoring could provide insight into the true risk of microplastics as a contaminant vector and antagonistic pollutant. This evidence is urgently needed to support governments globally in creating mitigation strategies and monitoring the success of these interventions. The present study finds that mussels are a suitable sentinel species for this use, but ongoing research is needed to determine special and temporal variations.
1 Introduction
Due to its mass production, transportation and disposal, plastic pollution has become a significant global issue. Demand for plastic has increased annually with 413.8 million metric tonnes produced in 2023 (PlasticsEurope, 2024). Large plastic fragments break up in the environment to make small microplastics (Andrady, 2022; Sipe et al., 2022). These are defined as plastic items between 1 μm and 5 mm (GESAMP, 2019). Primary microplastics, those manufactured at smaller than 5 mm rather than fragmented from larger items, can also enter the environment directly (Van Wesel et al., 2016; Wang et al., 2019). The term ‘microlitter’ refers to all anthropogenic materials including plastic. Recently developed guidance from the regional seas convention OSPAR (OSPAR MicroPlastics Expert Group (MPEG), 2024), however, includes semi-synthetic cellulosic material as a microplastic. The present study, therefore, refers to all items collected as microplastics. Microplastics are more widely bioavailable than larger items and have been ingested by entire food webs (McGoran, 2023) from plankton (Desforges et al., 2015; Zheng et al., 2020) to cetaceans (Besseling et al., 2015; Moore et al., 2020). Once ingested, microplastics have the potential to adversely affect the host organism (Khalid et al., 2021; Castro et al., 2022) sometimes altering gut physiology, immune function, growth and more (Osman et al., 2023). Effects on immune function and metabolism have been observed in mussels when exposed to microplastics smaller than 250 μm (Nardi et al., 2024). A review by Xu et al. (2024) also concluded that exposure to small microplastics (<400 μm) negatively impacted bivalve mollusks including mussels. Mussels have been shown to be pathways for the entry of microplastics to humans through the food chain with some evidence of impacts in humans, though more evidence is needed on this topic (Danopoulos et al., 2022; Walker et al., 2022). Models have also predicted that harmful pollutants can leach from microplastics into the human digestive system (Peters et al., 2022). However, it is not definitive that microplastics cause harm with many studies observing minimal or no negative impacts of microplastic exposure. Indeed, this has been observed in studies of suspension feeding mollusks (Hamm and Lenz, 2021; Opitz et al., 2021; Joyce and Falkenberg, 2022). It is predicted that in the next 70 to 100 years there will be wide-scale environmental harm as a result of microplastic abundance (Thompson et al., 2024). As such it is essential to develop a sentinel species to gather baseline data prior to widescale negative effects, even if current environmental levels are not sufficient to elicit a response from biota.
Despite their abundance, persistence and potential risk to biota and human health, there is no agreed sentinel species targeted at microplastics monitoring (Matiddi et al., 2021; Ghosh et al., 2023). Whilst sentinel species do not inherently infer risk, once thresholds are determined, monitoring levels of ingestion is important to track changes in the bioavailable fraction of microplastics in the environment. In the meantime, monitoring data can feed into environmentally relevant concentrations for the necessary ecotoxicological tests. But harmonized approaches are needed to ensure that regional and international data can be compared. In March 2022, UN member states agreed to forge the first international legally binding framework to end plastic pollution. Whilst talks eventually failed due to geopolitical complexities and economic challenges, it highlighted a global demand for improved mitigation of marine litter. Therefore, at this pivotal time, monitoring programs are needed to inform governments and decision making for future frameworks. The latest assessments on Good Environmental Status (GES) for the UK Marine Strategy (part one) show that the UK has not met GES for marine litter which includes microplastics. It also highlights the need to continue to build the evidence base for harm that marine litter causes on ecosystems (Defra, 2024). OSPAR uses two sentinel species: fulmars (Van Franeker, 2019) and turtles (Galgani et al., 2022) which capture microplastics larger than 1 mm but are unable to quantify the smaller fraction of microplastics, which are typically more abundant (Lindeque et al., 2020) and may be able to translocate to other tissues and organs, including the circulatory system, organs, placenta and lungs once ingested (Browne et al., 2008; Cattaneo et al., 2023; Yang et al., 2020; Zeytin et al., 2020; Li et al., 2021; McIlwraith et al., 2021). These indicators were selected to quantify the abundance of mesoplastics at the ocean surface and not to monitor microplastic abundance. More suitable species may be available for this purpose.
Mollusks, especially mussels, have often been proposed as a potential sentinel species for microplastics (Bråte et al., 2018; Li et al., 2019; Gerigny et al., 2023; Wu et al., 2024). Mussels, as a suspension feeder, are able to ingest particles between 2 and 500 μm (Rosa et al., 2018), retaining them for just a few days (Ward and Kach, 2009; Catarino et al., 2017). Several species exist with large populations and wide geographical spreads (e.g., Mytilus edulis, blue mussel; Tyler-Walters, 2008). Additionally, mussels are sedentary, easy to sample from the shore and are of economic importance. Through their use as sentinel species for chemical pollutants (e.g., ROCCH, OSPAR, CEMP), mussels are a strong contender for a microplastics sentinel species. Whilst not a perfect sentinel for microplastics (Ward et al., 2019; Mladinich et al., 2022; Shumway et al., 2023), microplastic assessments have recently been added to existing monitoring programs with mussels (Farrington et al., 2016). The Mussel Watch program has been a long-standing initiative by the National Oceanic and Atmospheric Administration (NOAA) to monitor chemical contaminants and more recently microplastics in coastal waterways and Great Lakes (NCCOS, 2020). Following NOAA’s initiative, a case study on the suitability of a mussel watch program for the Mediterranean Sea was carried out using Mytilus galloprovincialis (Provenza et al., 2022).
Despite several studies investigating microplastic ingestion by mussels (Van Cauwenberghe et al., 2015; Li et al., 2016, 2018; Catarino et al., 2017, 2018; Lusher et al., 2017; Digka et al., 2018; Qu et al., 2018; Reguera et al., 2019), a lack of harmonized and standardized methods limits comparisons (Li et al., 2021) with a need for higher study design and quality requirements (Shumway et al., 2023). Shumway et al. (2023) highlighted that a rapid increase in publications lacking the necessary quality and contamination controls lead to misinformation and incorrect assumptions around bivalve suitability as a sentinel species. Thus, more work is needed to refine analysis and monitoring of microplastics in mussels.
Microplastics can act as a vector of chemical contaminants and biological agents (Tumwesigye et al., 2023) and thus antagonistic effects of their combined presence with other contaminants may be observed. Current monitoring focuses on individual contaminant assessments, siloing analyses and overlooking these multi-stressor interactions. If mussels were proposed as a microplastic sentinel they would align with existing monitoring programs to support these comparisons, for example biotoxins from harmful algal blooms. This contaminant group comprises several distinct classes of toxins, each exhibiting different properties and often containing multiple analogues of a parent compound (Van Dolah, 2000). Many of these microalgal produced toxins can be accumulated in filter feeding bivalve mollusks, such as mussels. These accumulated marine algal biotoxins are known to negatively affect the health of higher consumers of mussels, including humans (Grattan et al., 2016). Consequently, a robust monitoring program exists for classified shellfish growing areas around the UK coast. The monitoring program is for the presence of the harmful algal groups known to produce the toxins in shellfish growing waters, as well as for the presence of the toxins within the shellfish. The frequency of toxin monitoring undertaken at each site is dependent on a risk assessment, with specified frequencies ranging from monthly sampling through to weekly, phytoplankton sampling occurs fortnightly from April until September and monthly from October until March. There are five groups of toxins specified in legislation and therefore monitored within the UK (EC 853/2004, 2004; EU 786/2013, 2013) and more broadly in many other countries (Table 1). The most common of the toxins present in British waters are the Diarrhetic Shellfish Poisoning toxins caused by Okadaic acid and its analogues (Dhanji-Rapkova et al., 2018; Bresnan et al., 2021). This group of toxins has been found to impact a wide geographic spread around the British coast, from the Shetland Islands in Scotland, to the Southwest of England, as well as causing the longest temporary closures of shellfish production areas, alongside the Azaspiracids (Dhanji-Rapkova et al., 2018, 2019). Whilst having caused lengthy harvesting restrictions, the Azaspiracids are far less common in occurrence in British waters than those compounds found in the Okadaic acid group. Although these toxins have acute impacts in humans (Grattan et al., 2016), their impact on shellfish has been less well explored. There is evidence of deleterious effects within bivalves, such as genotoxicity and DNA fragmentation (Prego-Faraldo et al., 2013; McCarthy et al., 2014) as well as suppression of immune function (Chi et al., 2016). It should be noted, however, that bivalves show a high level of resistance to the impacts of Okadaic acid, indicating that they have internal mechanisms for dealing with the negative effects of exposure (Prego-Faraldo et al., 2013; McCarthy et al., 2014).
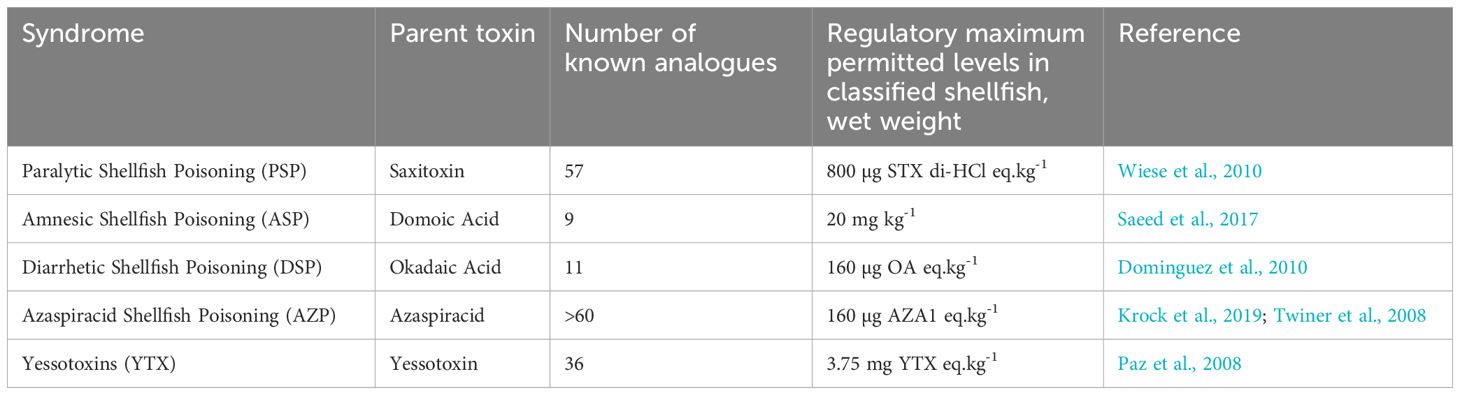
Table 1. The five groups of toxins monitored in the UK under specific legislation (EC 853/2004, 2004; EU 786/2013, 2013).
The aim of the present study was to build on the CleanAtlantic Project, which developed a harmonized extraction method for mussels (Gerigny et al., 2023), by adding analysis of non-fluorescent litter items and integrating additional environmental stressors. The present study aims to progress beyond single pollutant monitoring towards a more comprehensive and systems-based approach that includes harmful algal bloom monitoring data to enable a multi-stressor assessment. This approach aims to better capture the combined and potentially detrimental effects of multiple contaminants on organisms.
2 Materials and methods
2.1 Sample collection
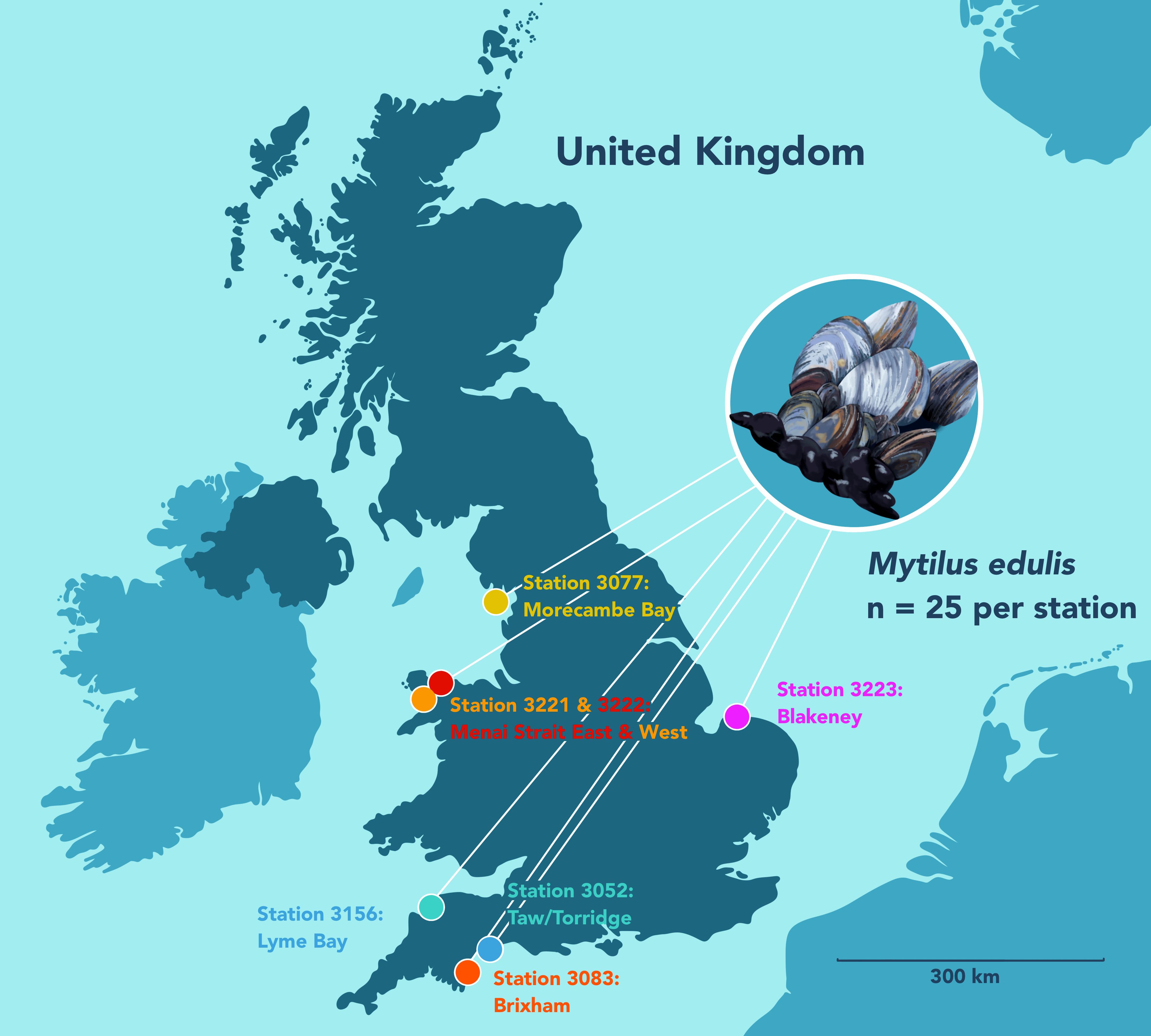
To maximize efficiency, existing monitoring networks were utilized for the collection of samples. As such, selection of sample locations was limited to those already established, The Food Standards Agency monitors harmful algal bloom levels utilizing monthly collections of mussels (Mytilus edulis) and other shellfish from around England and Wales (Food Standards Agency (FSA), 2025). Within the biotoxin official control program mussels are collected by Local Authority Environmental Health Officers (EHO). In the present study, surplus mussels were collected from seven stations (3052: Taw/Torridge, 3077: Morecambe Bay, 3083: Brixham, 3156: Lyme Bay, 3221: Menai Strait West, 3222: Menai Strait East, 3223: Blakeney) monitored within the biotoxin official control program (Figure 1) in November 2022 for microplastic analysis. Coordinates of sample locations are available in the Supplementary Material. Biotoxin analysis was completed at the Cefas laboratory in Weymouth, UK. As recommended by Bakir et al. (2020b), a minimum sample of 25 surplus individuals were sampled. Of the available locations, the seven selected were chosen to ensure enough material could be analyzed and to give the best spread around England and Wales. Mussels were frozen (−20 °C) and stored at the Cefas laboratory in Lowestoft, UK until microplastic extraction.
2.2 Microplastic extraction and analysis
2.2.1 Chemicals
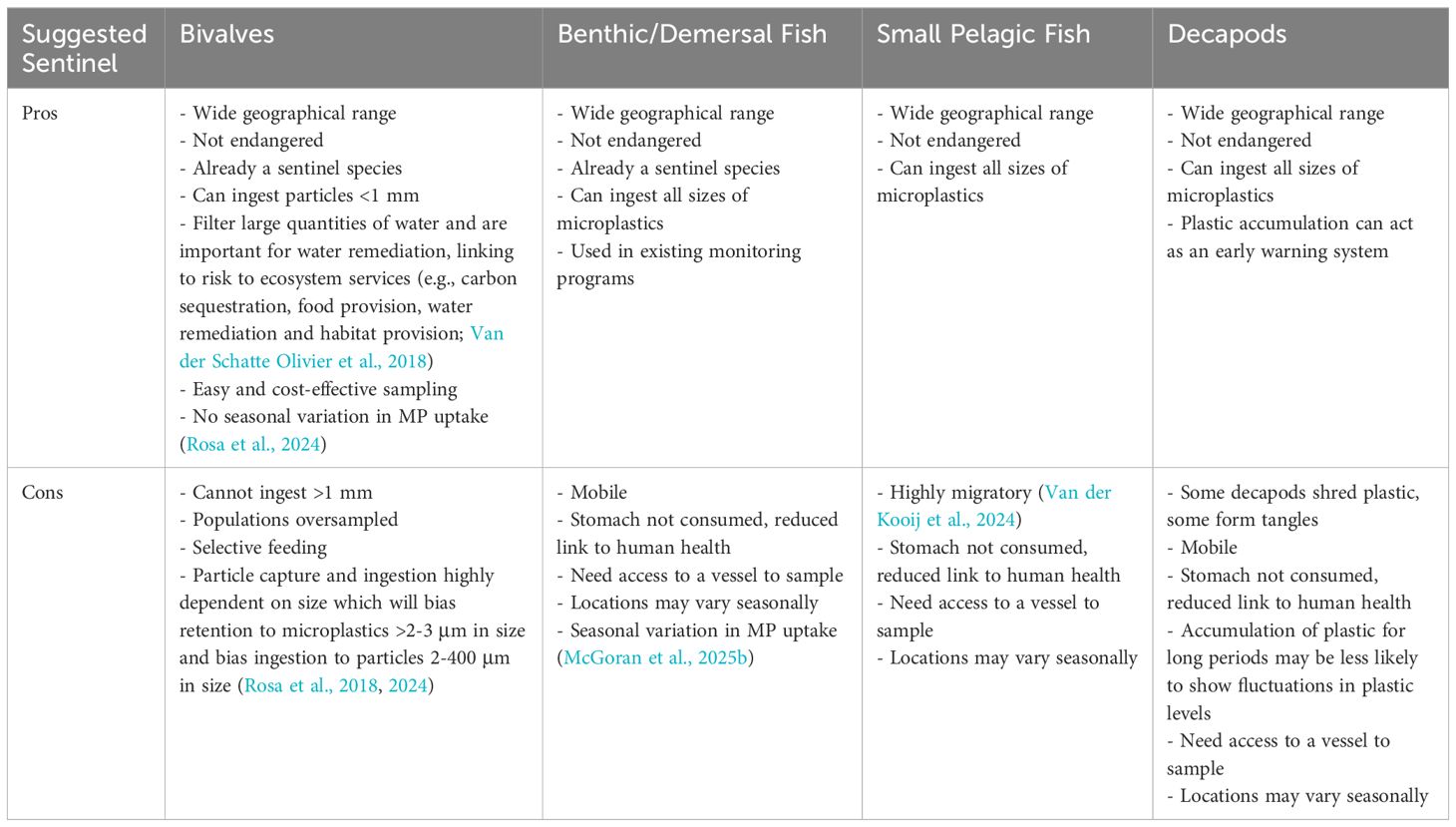
The chemicals used in microplastic extraction in the present study are listed in Table 2. All chemicals were prepared as per Gerigny et al. (2023) and Bakir et al. (2023). Details are available in the Supplementary Materials.
2.2.2 Contamination control procedures
Samples were processed in a designated microplastic laboratory with an anteroom so that two doors separated the laboratory from the corridor. This reduced airflow to minimize airborne contamination into the laboratory, which was also restricted access for the same reason. In the anteroom, a sticky contamination mat collected contamination from the soles of shoes. Mats were changed at a minimum every two weeks to ensure they remained sticky. Cotton lab coats dyed purple (Dylon dye) for easy contamination recognition were put on in this space prior to entering the laboratory. Before work began, the floor and surfaces were cleaned with a vacuum cleaner to remove any dust. Surfaces were also wiped with damp cotton cloths to remove any microplastics that may have settled on them after vacuuming. All glassware and equipment were triple rinsed with reverse osmosis (RO) water. A RO dispenser was used to first rinse the surfaces of the glassware. Following this, the glassware was allowed to fill with RO, which was then poured out. This was repeated twice more and the glassware wrapped in RO rinsed foil. The plastic handle of the shucking knife was wrapped in RO rinsed aluminum foil to prevent any contamination during shucking. All reagents were rinsed through a regenerated cellulose filter (Whatman, ø 45 mm, 0.2 µm pore size) prior to use and all work was conducted in a biological safety cabinet (Monmouth Guardian MSC T1200). Samples were kept covered whenever possible. In addition, laboratory controls (procedural blanks or negative controls) were collected. Three controls were collected for each station (n=7) for a total of 21 blanks. Controls were collected throughout sample processing to reflect potential changes in the laboratory environment during processing. A control beaker was opened at the start of the dissection and remained open until the mussel was sealed in a second beaker. The first control was collected during the dissection of one of the first three mussels. The second was collected at around mussel 12 and the third was collected during one of the last three mussels. Thereafter, lab controls were processed at the same time as the paired mussel.
2.2.3 Sample preparation
Mussels were defrosted and measured in their longest dimension. The tissue was removed from the shell with a blunt shucking knife. The byssal threads were removed and the tissue was then rinsed with filtered (0.2 µm regenerated cellulose filter) RO water in a red wash bottle, as recommended by Kolandhasamy et al. (2018). For stations 3052 (Taw/Torridge) and 3083 (Brixham), the filtrate was reserved and examined to quantify microplastic removal during this stage. The filtrate was only analyzed for two stations as it was not the main focus of the study. The data is available in the open access dataset (McGoran and Barry, 2025a). The wet weight mass of the tissue was recorded.
Each individual mussel was placed in a clean 100 mL glass beaker and covered with 40 mL of potassium hydroxide/sodium hypochlorite (15% KOH/2% chlorine) solution following the protocols of Gerigny et al. (2023) and Bakir et al. (2023). A glass Petri dish was used as a lid for the beaker to prevent atmospheric contamination. Beakers were placed in an ultrasonic bath (VWR USC200T) for 5 minutes and then transferred to a shaker incubator for 72 hours (VWR 980151UK, 40 °C, 120 rpm). After 72 hours, 40 mL of filtered degreaser (Elbow Grease, https://elbowgreasecleans.com/) was added to each sample. Beakers were returned to the incubator with the same settings for a further 24 hours. Following these steps, all tissue and fatty residues were removed and samples could be vacuum filtered over Whatman GF/D filters (45 mm ø, 2.75 µm pore size) in a six-sample manifold with 300 mL glass filtration units. Filters were flushed with 100 mL RO water to remove chemicals and the sides of the funnels were rinsed using a red wash bottle. Nile red (0.01 g L-1 in ethanol), stored in amber glass to prevent UV degradation, was added to each filter so that a small layer formed above visible material. The solution was left for 30 minutes and then filtered off. The filter was flushed and rinsed with reverse osmosis water for a second time. Filters were frozen (-20 °C) in glass petri dishes until further analysis could be completed.
Using a binocular microscope (Leica MZ10F), filters were examined under blue light (Fluo III Cool LED) followed by white light. A USB camera attachment was used to image and measure all suspected microplastic items (GXCAM-U3PRO-20, GX Capture-T). Items were then transferred to an Anodisc (VWR, ø 25 mm, 0.2 µm pore size) and dried at 40 °C for 24 hours prior to FTIR analysis. A Lumos II µFTIR (Bruker) was used to identify polymer type in a subset of picked items. Analysis utilized the MCT detector and ATR FTIR (32 scans in reflectance mode, 4000–500 cm-1, 4 cm-1). Identification was only accepted with a minimum match of 60% against the library spectra (ATR-FTIR-library complete, vol. 1-4; Bruker Optics ATR-Polymer library; IR-Spectra of Polymers, Diamond-ATR, Geranium-AT & IR-Spectra of Additives, Diamond-ATR) as recommended by Leistenschneider et al. (2021).
The results presented below have been corrected for contamination. For each station the mean contamination was calculated and removed from each sample where items (e.g., white filaments) were present in both controls and samples. Corrected and uncorrected data is available in the open access dataset (McGoran and Barry, 2025a).
2.3 Harmful algal biotoxin extraction and analysis
2.3.1 Reagents, chemicals and analytical equipment
The reagents which were used for the toxin extractions and High-Performance Liquid Chromatography (HPLC) analysis were of HPLC grade or higher. Chemicals of Liquid Chromatography with Mass Spectrometry (LC-MS) grade were utilized for the preparation of reagents for the Lipophilic toxins, where mass spectrometry was used for analysis. The chemicals used were sourced either from Fisher Scientific (Loughborough, UK) or VWR (Lutterworth, UK). All solid phase extraction (SPE) processes were automated and performed using a Gilson (Dunstable, UK) ASPEC, running Trilution software. A full list of chemicals (>30) is not provided here.
2.3.2 Marine biotoxin extractions
For each sample, a minimum of 10 individual animals were shucked to yield a minimum of 50 g (wet weight) of flesh, these criteria are considered representative of the sample within the official control framework. This flesh was collected and allowed to drain before being homogenized. Once the sample was homogenous, the required quantity of shellfish homogenate was weighed into a 50 mL centrifuge tube, 2 ± 0.01 g each for ASP and LT analysis and 5 ± 0.1 g for PSP analysis. A solvent with a high affinity for the respective toxin was added to each. These were methanol for the lipophilic toxins, a 50:50 mix of methanol and water for the ASP toxins and weak (1%) acetic acid for the PSP toxins.
Lipophillic toxins (LTs), including Okadaic acid toxins (OAs), Azasparacids (AZAs) and Yessotoxins (YTXs) were extracted and analyzed using a refined version of the Gerssen et al. (2009) method. Specifically, 6 mL of methanol was added to the shellfish tissue, which was mixed thoroughly and separated via centrifugation (8 minutes at 3500 rpm). The supernatant was then collected, and the process was repeated two further times, with supernatants from the same sample combined after each centrifugation step, until 18 mL of supernatant was recovered. This was then topped up to 20 mL with methanol. At this stage 1 mL was filtered into an LCMS vial for analysis and a further 1 mL was aliquoted into a separate LCMS vial and underwent hydrolysis by the addition of 125 µL of 2.5M sodium hydroxide and incubation at 76 °C for 40 minutes. This reaction was then neutralized by the addition of 125 µL of 2.5 M hydrochloric acid. Both vials were then ready for analysis by LC-MS/MS. For the analysis of the OA, AZA and YTX toxins, LC-MS/MS analysis was utilized, it was performed using a Waters (Milford, MA, USA) Acquity I class UPLC coupled with either a Waters Xevo TQ or Xevo TQ-S triple quadrupole mass spectrometer as described in Gerssen et al. (2009).
The ASP extraction was performed using a refined version of Quilliam et al. (1995). Specifically,18 mL of 50:50 methanol:water was added to the 2 ± 0.01 g of shellfish homogenate. This was thoroughly mixed before separation via centrifugation (10 minutes at 4500 rpm). Following this, 1 mL of the supernatant was then passed through a 0.45 µM filter into an HPLC vial, ready for analysis. No selective cleanup or pre-concentration solid phase extraction steps were performed on any samples. For the analysis of ASP toxins, HPLC-DAD analysis was utilized, it was performed using an Agilent 1100/1200 series HPLC with ultraviolet detection as per Quilliam et al. (1995).
PSP analysis was undertaken using a refined AOAC, 2005.06 pre-column oxidation method (AOAC, 2005) described by Hatfield et al. (2016). Specifically, the addition of 3 mL of 1% HAC to 5 ± 0.1g of shellfish homogenate. This was then mixed, boiled at 100 °C in a water bath for 5 minutes, cooled for 5 minutes, mixed again and then separated by centrifugation (10 minutes at 4500 rpm). The supernatant was collected and a further 3 mL of 1% Acetic acid was added to the remaining shellfish pellet, this was subsequently mixed and separated again by centrifugation, with the supernatant being combined with that from the first step. This mixture was topped up to 10 mL with deionized water. Following this, 1 mL of the extract underwent automated solid phase extraction. The cleaned sample was then pH adjusted to between 6.0 and 7.0 using either sodium hydroxide or acetic acid. At this stage, 500 µL of this adjusted sample was then added to an HPLC vial and derivitized by oxidation with 100 µL of a periodate reagent (1:1:1 periodic acid:ammonium formate:sodium phosphate), in the presence of 100 µL of matrix modifier (blank pacific oyster extract). After 1 minute this reaction was quenched with 5 µL of glacial acetic acid. After resting on the bench this derivatized sample was ready for analysis. For the analysis of PSP toxins, HPLC with fluorescence detection (FLD) analysis was utilized; it was performed using an Agilent 1100/1200 series HPLC-FLD. No samples were fully quantified, instead samples were analysed using a semi-quantitative approach as described in Hatfield et al. (2016).
2.4 Statistical analysis
The statistical analysis mainly consisted of comparisons between stations. This approach was taken to determine whether there was evidence of potential accumulation zones on the British coast. This could potentially be linked to plastic inputs or hydrodynamics reducing plastic flushing. Three different comparisons were made. These were:
i. Comparison of microplastic mean abundances between stations. These were done in two ways: per individual and per gram (wet weight). A non-parametric Wilcoxon procedure was used because Shapiro-Wilk tests suggested that both abundance measures were not normally distributed.
ii. Whether the proportion of individuals (p) containing microplastics differed between stations. This was achieved by fitting a binomial generalized linear model. The full model was of the form (Equation 1):
where is an intercept term and represents the effect of the jth station (j = 1,…,7). The reduced model was the same as (1) but without the station effect . The statistical significance of stations was assessed with a likelihood ratio test between the full and reduced models (Faraway, 2006).
iii. A comparison of the abundance of rayon polymers between stations. Because no replicate batches of samples were analyzed at each station, a standard binomial generalized linear model could not be used. Instead, to achieve this comparison, a randomization test was run (Manly and Navarro Alberto, 2021). Essentially testing whether the rayon proportion for each station is consistent with some overall probability of rayon under the null hypothesis that the proportion of rayon is the same at each station.
Formally, the test estimated the overall probability (over all stations) that a fiber is rayon by the total number of rayon fibers divided by the total number of fibers. Assuming the null hypothesis of no significant difference between stations and assuming independence between fibers in a station, the number of rayon fibers per station is distributed (Equation 2):
A summary statistic of the variation of rayon counts over stations is the variance. Thus, to perform the randomization procedure, we first calculated the variance of the observed counts over stations. Then the model in (3) was simulated 999 times and the variance of the counts on each simulation calculated. If there were differences between stations in the proportions of rayon, it would be expected that the variance of the observed counts would be larger than the variance of the simulated counts. The p-value for statistical significance was calculated as: p-value = (g + 1)/1000, where g is the number of simulated variances that are greater than or equal to the observed variance (Manly and Navarro Alberto, 2021).
In addition to comparisons between stations A standard linear regression model was used to determine the relationship between mussel length (mm) and microplastic load.
Plots were created with ggplot (Wickham, 2016), ggpubr (Kassambara, 2023) and ggbreak (Xu et al., 2021). The R script for all analysis is available in the Supplementary Material S1.
No statistical analysis could be conducted on the harmful algal biotoxin data due to the large number of results below the limit of detection.
3 Results
Between 26 and 55 individuals were collected from each of seven locations. As described above, a random subsample of 25 individuals was analyzed from each location. Shell lengths of all collected mussels ranged between 25–75 mm. Individuals subsampled for microplastics analysis measured between 40–70 mm long (mean ± SD: 56.5 ± 5.9 mm). The wet weight of the tissue was between 1.24–12.89 g (4.3 ± 1.9 g).
Mean contamination was calculated from the three blanks per station and used for data correction. The overall mean contamination (± SD) across all 21 blanks was 3.05 ± 2.22 items per sample. Corrected and uncorrected microplastic concentrations are reported in the Supplementary Material S3, where contamination correction refers to the removal of the average contamination at each station. After correction, between 42% (STN 3052) and 67% (STN 3156) of mussels contained microplastics at each station. The binomial model (1) indicated there was no statistically significant difference between the proportion of individuals containing microplastics between stations (p = 0.139). The likelihood ratio test confirmed this conclusion (p = 0.137).
The Shapiro-Wilk identified the data was skewed (MP ind-1, p = 2.2 × 10-16; MP g-1, p = 2.2 × 10-16). As the data was positively skewed count data, a non-parametric Wilcoxon test was used to compare microplastic load between stations. The mean contamination per station ranged from 0.60 ± 0.71 (STN 3083) to 2.84 ± 7.31 (STN 3222) items per individual (95% CI = 0.88–1.79) and 0.15 ± 0.23 (STN 3077) to 0.59 ± 1.56 (STN 3222) items gram-1 (wet weight) (95% CI = 0.23–0.44), but there was no statistically significant difference in microplastic abundance between stations for either microplastic load per gram or individual (all pairwise p-values were below 0.05; range: 0.13–0.99) (Figure 2).
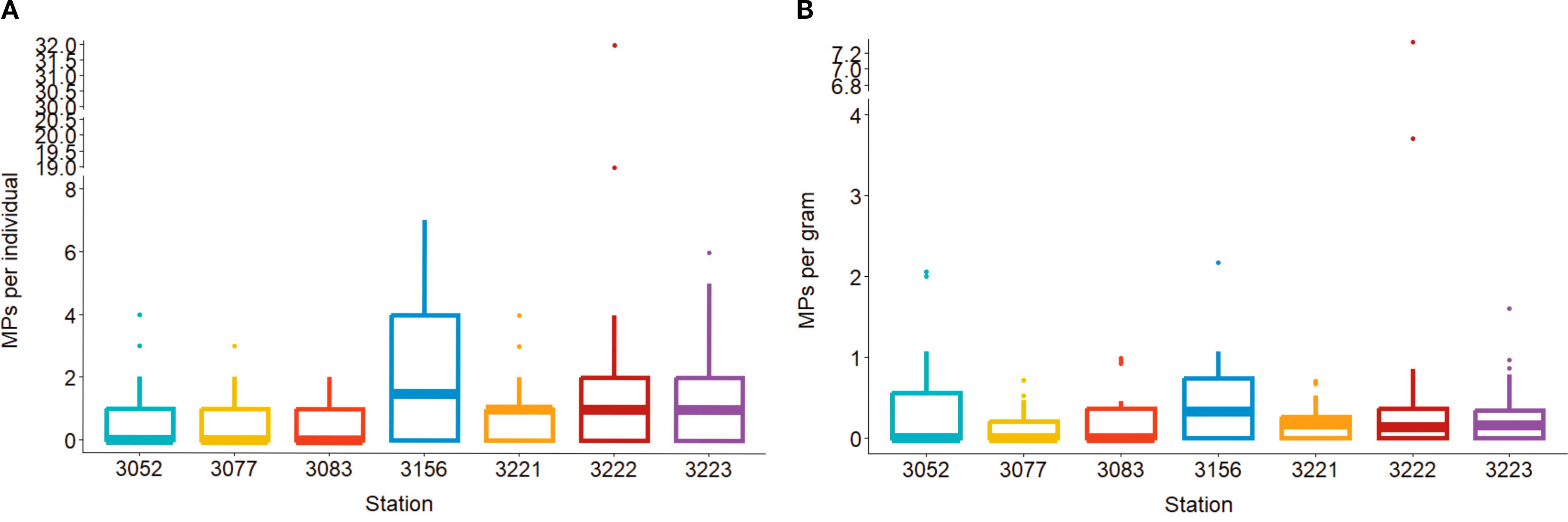
Figure 2. Microplastic abundance (corrected) in mussels from seven stations (3052: Taw/Torridge, 3077: Morecombe Bay, 3083: Brixham, 3156: Lyme Bay, 3221: Menai Strait – West, 3222: Menai Strait – East, 3223: Blakeney) around the coastline of England and Wales per individual (A) and per gram wet weight (B).
Shell length of mussels and microplastic load (model 2) did not correlate (MP = (0.05 × length) - 1.01, p = 0.402). The mean microplastic loads recorded in the present study are in agreement with global concentrations (Supplementary Material S4).
Plastic was the dominant material (52%) with cellulose the second most common litter item (28%). Of the plastics, rayon was most common (37%). Other materials present included polyester, paint, acrylic, polypropylene and polyethylene (Figure 3A). A randomized test was used to determine whether polymer abundance varied between stations. Rayon was selected as the polymer was present in all stations and the proportion of rayon in samples varied from 21% to 56%. The randomization test estimated the overall probability that a particle was rayon by the total number of rayon particle divided by the total number of particles. This was p=47/114 = 0.41. When comparing rayon across the stations, the p-value from the randomization test was 0.66 (model 3, p >0.05). Thus, there is no evidence of differences in the proportion of rayon particles between stations (Figure 4). Items were primarily blue or black filaments (84%) with some fragments, films and microbeads present (Figure 3B). One mesoplastic item was recovered (Figure 3C) but was removed from analysis as the study focused on microplastics. The majority (42%) of items were between 300–999 µm long (mean 889 ± 934 µm).
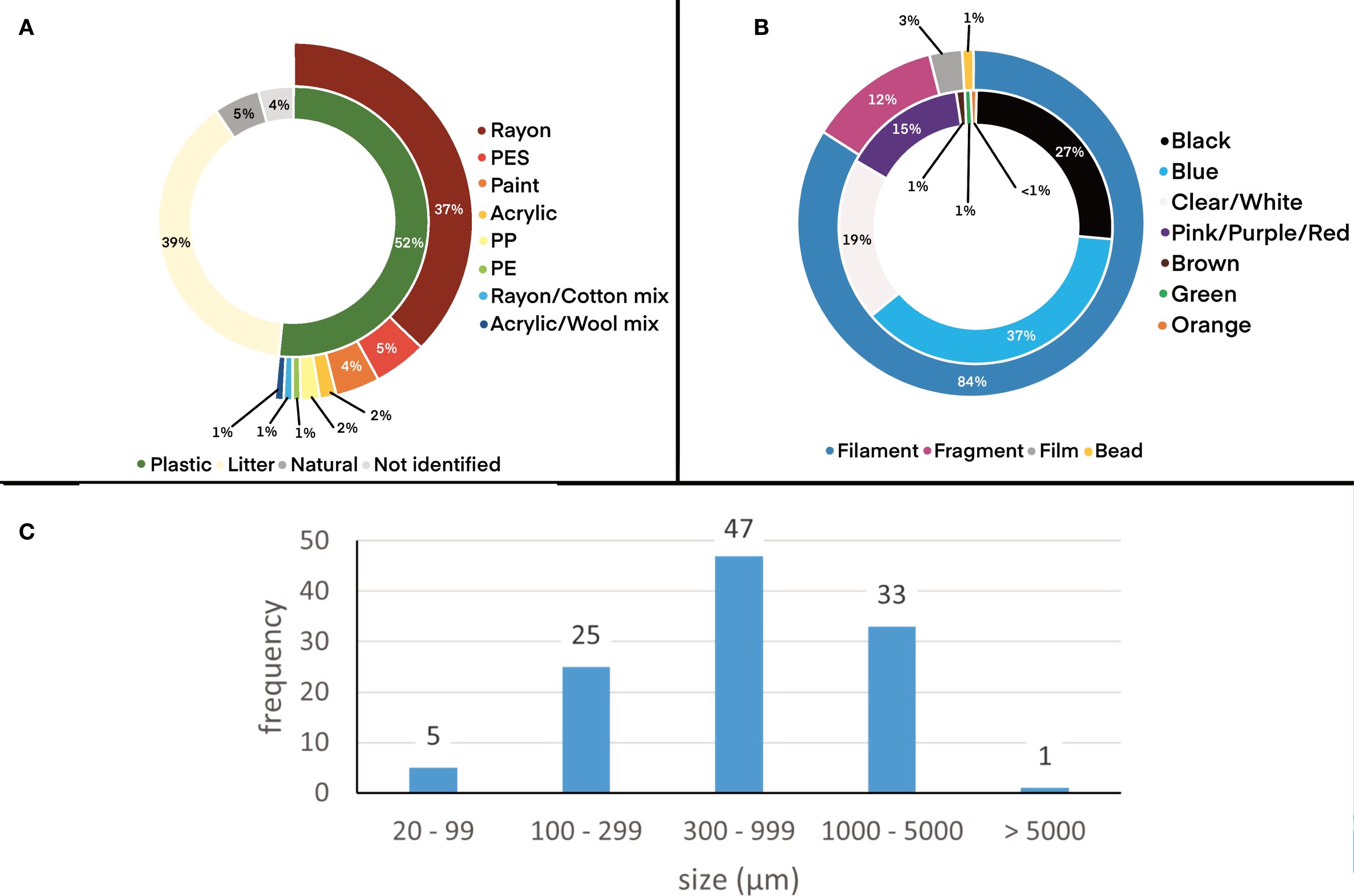
Figure 3. Descriptive analysis of microplastics present in mussels from the coast of England and Wales (combined across stations and corrected for contamination). (A) Polymer identification including plastics (rayon, polyester, paint, acrylic, polypropylene and polyethylene) and other litter (cotton and cellulose); (B) Microplastic shape (filament, fragment, film, bead) and color (black, blue, clear/white, pink/purple/red, brown, green, orange); (C) Length (longest dimension) of meso- and microplastics.
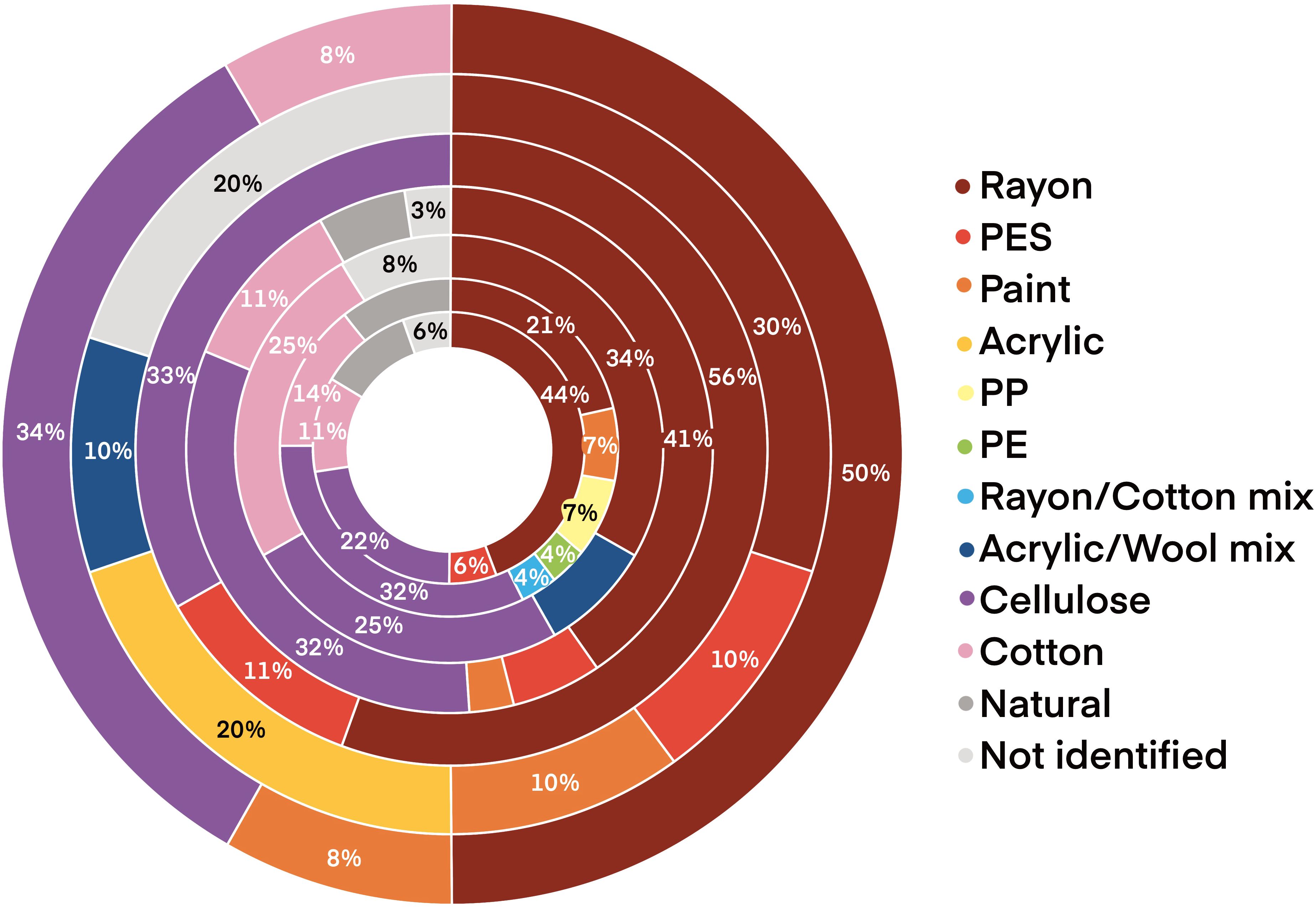
Figure 4. Polymer abundance of microplastic contamination in mussels between stations (outside to center: 3052: Taw/Torridge, 3077: Morecombe Bay, 3083: Brixham, 3156: Lyme Bay, 3221: Menai Strait - West, 3222: Menai Strait - East, 3223: Blakeney).
In total, 119 shellfish samples were tested in 2022 for the presence of marine algal biotoxins from the same sites at which microplastic sampling occurred. Of these 10 were from Blakeney, 11 from Menai Strait – East, 27 from Lyme Bay, 23 from Brixham, 21 from Morecombe Bay and 27 from Taw/Torridge. Of these samples 118 were tested for LTs, 75 screened for PSP and 74 tested for ASP. None of the samples tested contained any PSP toxins above the limit of detection. None of the samples tested contained any ASP toxins above the limit of quantitation. None of the samples tested contained AZAs or YTXs above the LT reporting limit of 16µg AZA eq kg-1 and 100 µg YTX eq kg-1 respectively. Results are summarized in the Supplementary Materials S5. OA group toxins were present at Taw/Torridge (48% > 16 µg OA eq kg-1 with a maximum 60 µg OA eq kg-1), Morecombe Bay (62% >16 µg OA eq kg-1 with a maximum of 181 µg OA eq kg-1), Brixham (48% > 16 µg OA eq kg-1 with a maximum of 131µg OA eq kg-1) and Lyme Bay (37% > 16 µg OA eq kg-1 with a maximum of 173 µg OA eq kg-1) (Figure 5). OA group toxins were present at variable times at each site from April 2022 in Brixham to October 2022 in Morecombe Bay. No OA group toxins > 16 µg OA eq kg-1 were detected in Menai Strait – East or Blakeney in any sample. At time of sampling for microplastics, none of the sites contained any OA group toxins >16 µg OA eq kg-1.
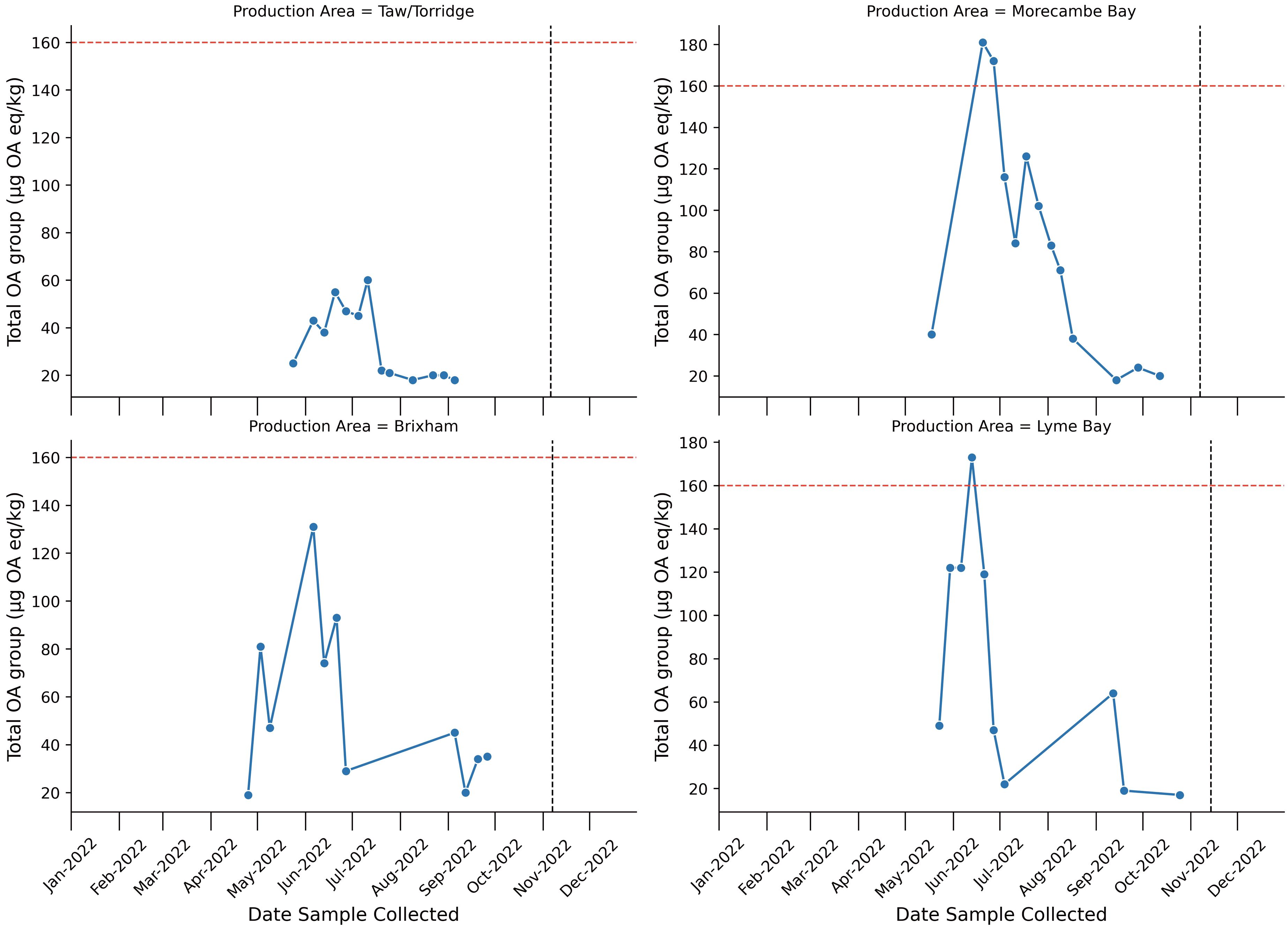
Figure 5. Line plot describing Total Okadaic acid toxins (OA) group concentrations across 2022 at each sampling location. Red horizontal hashed line represents the maximum permitted level of OA group toxins (160 µg OA eq/kg) in shellfish destined for human consumption. Black vertical line represents the sampling time of microplastics samples. Only points which exceeded 16 µg OA eq/kg are plotted.
4 Discussion
4.1 Occurrence and abundance of microplastics and harmful algal blooms in UK mussels
Microplastics, including cellulosic items, were recovered from 53% of mussels with a mean load of 1.33 ± 3.04 items per individual (95% CI = 0.88–1.79) and 0.33 ± 0.71 items per g (wet weight) (95% CI = 0.23–0.44). Mussels from all seven stations were contaminated, most commonly with rayon, a semi-synthetic material categorized as microplastics by OSPAR (OSPAR MicroPlastics Expert Group (MPEG), 2024). No harmful algal biotoxins were found at quantifiable levels in the shellfish samples tested for microplastics. However, of the seven sites analyzed for microplastics within this study, four (3052: Taw/Torridge, 3077: Morecambe Bay, 3082: Brixham, 3156: Lyme Bay) showed presence of the DSP group toxins in shellfish within the preceding six months. For two of the sites assessed, the levels of the okadaic acid group toxins exceeded the regulatory threshold, whereas in the other two sites with quantifiable levels of DSP toxins, this food safety limit was not reached. The final two sites assessed showed no presence of DSP toxins within the year 2022. All other testing for marine biotoxins indicated that there were no detectable levels present within the study year. This means that those animals assessed for microplastics would have had a prior exposure to DSP toxins in four of the six samples analyzed, with higher levels previously present in shellfish from Morecambe Bay and Lyme Bay.
4.2 Risks from harmful algal blooms in the UK
The primary sources of algal biotoxins within shellfish growing in British waters are planktonic species of microalgae. Several species within varying genera of Dinoflagellates are responsible for PSP, DSP, AZP and YTX, with several species of the Diatom genus Pseudonitzschia being the producers of the toxins responsible for ASP. The blooms or cell abundances causing the contamination of mollusks can occur naturally within the spring and summer months, occasionally continuing into the Autum. The frequency and scale of algal blooms can be exacerbated by anthropogenic impacts, with some factors such as nutrient-enriched coastal runoff having the potential to stimulate algal growth (Heisler et al., 2008) whilst also acting as an input for microplastic pollution. The most common impacts in British waters are those from Dinophysis acuta and Dinophysis acuminata, both known to be producers of DSP toxins (Bresnan et al., 2021; Dhanji-Rapkova et al., 2018). This leads to the shellfish growing in some areas being exposed to recurrent levels of DSP toxins; these will vary between years but can reach high levels or prolonged exposures in some localities (Dhanji-Rapkova et al., 2018). As the okadiac acid group of compound has been shown to have potential negative impacts on bivalve molluskan health, albeit in limited studies (Prego-Faraldo et al., 2013; McCarthy et al., 2014; Chi et al., 2016; Lassudrie et al., 2020), this group of compounds could adversely affect the ability of bivalves to deal with the additional stressor of microplastic contamination. It is also known that several harmful algal species produce a wide range of bioactive extracellular compounds, which may also have negative effects on bivalve health, although these are often much less well described (Lassudrie et al., 2020). As such, the confirmed presence of okadaic acid producers at four of the sites assessed in this study represents an additional environmental stress factor impacting the study animals from those sites.
4.3 UK microplastics data in a regional and global context
The microplastic contamination reported in the present study is in line with most previous studies in the UK. But they are low compared to some studies in the country, which reported mean concentrations as high as 7.64 items per individual (Scott et al., 2019) and 12.6 items per individual (Catarino et al., 2017). The concentrations in the present study are also in line with estimates from Europe (De Witte et al., 2014; Van Cauwenberghe et al., 2015; Vandermeersch et al., 2015; Bråte et al., 2018; Phuong et al., 2018; Railo et al., 2018; Hermabessiere et al., 2019; Nalbone et al., 2021; Ferreira et al., 2023; Gerigny et al., 2023; Digka et al., 2024). This suggests that the high concentrations reported by Scott et al. (2019) and Catarino et al. (2017) may be outliers rather than indicative of accumulation zones of contamination. However, a greater number of sites would better capture regional differences.
Globally, all continents and regions find that most mussels consume less than one microplastic per individual or gram on average (Supplementary Material S4). On the American continents, mean contamination ranges from <1 – 8.7 particles per individual (Zhao et al., 2018; Klasios et al., 2021) or <1–40 particles per gram (Zhao et al., 2018; Migliarini et al., 2025). Oceania is represented by relatively few published studies. Webb et al. (2019) reported that in New Zealand mean contamination per individual and per gram were both less than one. Most African studies originate from South Africa, reporting contamination of mussels at ca. 4 particles per individual (Sparks, 2020; Sparks et al., 2021) and between <1 – 2.3 particles per gram (Sparks, 2020; Ferguson et al., 2024). In Asia mussels were contaminated with <1 – 22.5 particles per individual (Dowarah et al., 2020; Do et al., 2024) or <1 – 9.2 particles per gram (Kolandhasamy et al., 2018; Naidu, 2019). Few studies are available for mussel contamination in the Middle East, with Bagheri et al. (2020) reporting concentrations of up to 19.8 particles per gram in Iran. Whilst some records of contamination are high, the majority are similar to the results of the present study.
Comparison between studies is hindered by a lack of standardized protocols and reporting (Li et al., 2021; Shumway et al., 2023). Best practice is to report microplastic abundance per gram (wet weight) as well as per individual. Yet, several studies only report a single unit (e.g., MPs g-1 Courtene-Jones et al., 2017; MPs individual-1 McCoy et al., 2020). In the UK, only six studies have estimated microplastic abundance in mussels (S3), with a further two studies on clams (McCoy et al., 2020) and scallops (Akoueson et al., 2020). Some potential local accumulation zones have been identified (Li et al., 2018; Scott et al., 2019) but long-term data is needed to confirm whether spatial variation in abundance persists. According to Li et al. (2018), mussels from Plymouth contained more microplastics than those from Brighton (6.4 to 1.1 MPs individual-1). Similarly high concentrations were reported at Whitsand Bay compared to Torquay Bay (mean 7.64 to 1.43 items individual-1, Scott et al., 2019). With no statistical difference in abundance between stations and a mean abundance of between 0.60 and 2.84 items individual-1, the present study identifies no accumulation zones relevant for the UK. Plymouth and Whitsand Bay are located near the mouth of the River Tamar, previously shown to be contaminated with plastic debris (Sadri and Thompson, 2014), for which more than 80% consisted of microplastics. It was, however, unclear whether the River Tamar acted as a net source or sink for plastic debris (Sadri and Thompson, 2014). Three stations in the present study are located in the southwest of the UK (3052: Taw/Torridge, 3083: Brixham, 3156: Lyme Bay). Mussels at these stations primarily contained cellulosic fibers, which is in line with the reports of Scott et al. (2019). For both Scott et al. and Sadri and Thompson (2014), polypropylene and polyethylene were the most abundant plastics. However, the present study recovered polyester and paint at the Southwest stations (3052: Taw/Torridge, 3083: Brixham, 3156: Lyme Bay). In the present study, only the station at Lyme Bay (3156) is located near a major river (River Exe). This station had the highest proportion of contaminated mussels and the second highest mean contamination per individual. High abundances of microplastics were previously reported for seafloor sediments collected from Lyme Bay and Off Tamar; both of these sites are considered as accumulation zones for microplastics (Bakir et al., 2023). Regular repeat monitoring is necessary to detect persistent accumulation zones. This is useful to identify whether river mouths are accumulation zones that may lead to a greater rate of microplastic ingestion due to the increased abundance of particles.
4.4 Addressing the need for a microplastics sentinel species and the requirements for success
In future years, microplastics are likely to become much more abundant through increased and varied use, as well as through fragmentation of large litter already in the environment. Inconsistent methods for sampling and processing, a lack of robust controls in some early studies and advances in particle detection hinder comparisons between studies. Plastic abundance in the ocean is expected to double by 2040 (Thompson et al., 2024). Litter already negatively impacts the marine environment, with calls for policy interventions to make drastic changes (Roman et al., 2020) and evidence that the cost of inaction is greater than cost of implementing these changes (Watkins et al., 2017). As microplastic abundance increases, so does the likelihood of negative environmental impacts. Many ecotoxicological studies, especially early studies, utilized concentrations far higher than those observed in the environment. Whilst less useful for determining current risks from microplastic exposure, these studies demonstrate that eventually microplastic loads will be high enough to cause severe negative impacts (Foley et al., 2018). Indeed, microplastic toxicity is positively correlated to microplastic concentration (e.g., in oysters Teng et al., 2021). As particles continue to fragment, small microplastics and nanoplastics are able to translocate to organs other than the gills and digestive system (Cattaneo et al., 2023; Yang et al., 2020; Zeytin et al., 2020; Li et al., 2021; McIlwraith et al., 2021). This increases retention time (Ward and Shumway, 2004) and likelihood of negative impacts. At high concentrations, microplastics can move from the digestive gland to the circulatory system of mussels (Browne et al., 2008).
As suspension feeders, mussels are susceptible to the negative impacts from microplastic ingestion (Moore, 2008; Kühn and van Franeker, 2020), albeit at often high concentrations. Ingestion of microplastics has been linked to negative health impacts. For instance, reduced feeding rate in mussels (Pedersen et al., 2020; Hatzonikolakis et al., 2024), which reduced mussel growth. Furthermore, the gut microbiome biodiversity can decline in mussels that ingested polystyrene; with damage also observed in the gut tissues (Ferguson et al., 2022). Although, Collins et al. (2023) observed no impact on gut microbiome. The shape of the plastic is likely to impact the severity of the reaction, with fibers and filaments being more toxic than microbeads (waterflea Ceriodaphnia dubia Ziajahromi et al., 2017a; zebrafish Danio rerio Qiao et al., 2019; Rebelein et al., 2021). Mussels are potentially less selective when consuming filaments compared to beads (Ward et al., 2019) making them vulnerable to these negative effects. The abundance of cellulosic filaments, and associated dyes, in the environment has raised concerns over their health impact as well as plastic filaments (Remy et al., 2015; Mateos-Cárdenas et al., 2021). Walkinshaw et al. (2023) demonstrated that at exposures associated with heavily polluted environments (80 filaments L-1) cotton and polyester filaments both reduced growth rate of juvenile mussels, with polyester reducing growth more than cotton.
At low exposures (0.1 g L-1), 70% of ingested microplastics are excreted by mussels within 24 hours (Pedersen et al., 2020). Similarly, oysters exposed to 0.33 g L-1 daily for 10 days retained less than 0.5% of particles; though more were retained when particles were biofouled (Fabra et al., 2021). In contrast, at higher concentrations (0.4–0.8 g L-1) over 94% of microplastics were retained after 24 hours (Pedersen et al., 2020). Woods et al. (2018) noted that even at 3,000 filaments per L-1, 71% of particles are ejected in pseudofaeces, but those ingested were retained after 72 hours. Increased retention time is necessary for the chemical to leach into the digestive tract and to increase the chance of negative impacts on the organism. Jang et al. (2021) demonstrated that expanded polystyrene could increase mussel exposure to plastic additives but noted that direct exposure to leachates posed a more significant threat. Microplastics and their associated chemicals, such as adhered persistent organic pollutants (Bakir et al., 2014), are linked to negative health effects when ingested. The chemicals within plastic can be carcinogenic and endocrine disrupting (Zimmermann et al., 2019). Mussels exposed to polyethylene and polystyrene accumulated pyrene, a polyaromatic hydrocarbon (PAH). This resulted in an immunological response and a change in gene expression affecting apoptosis (Avio et al., 2015). More recent studies, however, suggest that microplastic exposure is not correlated to PAH uptake in environmental conditions (Klasios et al., 2021). Whilst physically possible to transfer chemicals from plastics upon ingestion, this is not the primary route of exposure in the marine environment. However, at environmental concentrations evidence of risk is limited. Some studies suggest a negligible toxicological impact (Koelmans et al., 2016; Daniel et al., 2024), including on mussels (Santana et al., 2018). Though with concentrations set to increase, harm is likely to intensify in coming years (Thompson et al., 2024). Thus, collecting baseline data through monitoring is essential to have an early warning system of harm before impacts become severe. Additionally, sub-lethal and population level effects are important to monitor, especially for commercially important species such as mussels (Beaumont et al., 2019).
Sessile sentinel species not only allow for the development of risk assessments but allow for static points to monitor pathways and sources of microplastics in parallel to environmental matrices. Microplastics in the marine environment can originate from various sources, including, but not limited to, landfill runoff, agricultural runoff (Hurley et al., 2018; Koutnik et al., 2021), road runoff (Sundt et al., 2014; Kole et al., 2017; Tamis et al., 2021; Worek et al., 2022), riverine inputs (Meijek et al., 2021), wastewater (Ziajahromi et al., 2017b), abrasives, paint from vessels (Sundt et al., 2014), textile fibers shed during use, washing and drying (Napper and Thompson, 2016; Reed et al., 2018; O’Brien et al., 2020), and atmospheric fallout (Dris et al., 2016, 2017). Microplastics may be shed from apparatus used in aquaculture, with farmed mussels often more contaminated than wild mussels (Ding et al., 2022; Vandermeersch et al., 2015). The use of mussels as a sentinel species for microplastics could be used for monitoring at source or in regions of interest to capture changes in inputs and quantify mitigation strategy success. Caged mussels can be utilized to monitor specific inputs of interest, especially those associated with mitigation strategies and policy changes. These would allow governments to rapidly assess the effectiveness of the interventions. Additionally, caged mussels could be deployed on buoys to provide estimates of microplastic contamination offshore. Klasios et al. (2021) compared caged and resident mussels and found similar concentrations in both. Thus, this is a suitable strategy to extend the reach of monitoring programs.
Whilst there is an agreed need for a sentinel species, there is some disagreement around the requirements of sentinel species. According to Ward et al. (2019), who investigated limitations of mussels as a sentinel species, a microplastic indicator should “ingest, without bias, the majority of plastic particles”. However, monitoring with sentinel species may not always be designed to act as a proxy for other environmental matrices. Biota can provide insight into the bioavailable fraction of litter in the environment and can be used to evidence risk, or the lack thereof. Shumway et al. (2023) noted that future studies utilizing bivalves as a sentinel for microplastics need to address specific questions. The authors of the present study agree with this sentiment and argue that the development of a sentinel species-based monitoring program should tackle policy needs, such as how microplastics in the environment link to risk.
The present study utilized an existing mollusk monitoring scheme to complete a multi-stressor analysis. However, other monitoring programs can be used and other sentinel species for microplastics should be explored. Table 3 explores the positives and negatives associated with other proposed groups for microplastic monitoring. Limitations of using suspension feeding bivalves include particle rejection and species-specific differences (Mladinich et al., 2022; Shumway et al., 2023). The latter, however, applies to any indicator that is not species specific, which is challenging on a global scale.
Owing to their selective ingestion and rejection of particles (Ward et al., 2019; Mladinich et al., 2022), mussels generally contain low numbers of particles (Shumway et al., 2023). Selection depends on physiochemical properties and size, with a higher proportion larger particles rejected (Ward et al., 2019; Mladinich et al., 2022). Ward et al. (2019) noted with tests using microbeads (19-1000 μm) only 10-30% of small microbeads were rejected but 98% of 1000 μm beads were rejected. The authors also noted that similar proportions of filaments were ingested regardless of size (75-1075 μm). Mladinich et al. (2022), however, observed a stricter size-based selection with more 65 μm filaments consumed than 500 μm filaments, and similarly more 500 μm filaments ingested compared to those 970 μm long. The study concluded that overall, more particles were ingested than rejected. Less selective suspension feeders have been recommended for use as microplastic sentinels (Mladinich et al., 2025).
At the OSPAR Working Group on Monitoring and on Trends and Effects of Substances in the Marine Environment (MIME), several countries have raised concerns over mussel population levels and the increased impact of using them as a sentinel species (Jon Barber, Cefas, Personal communication). Other mollusks have been suggested as alternatives (Ribeiro et al., 2024). Commercial species are useful to understand risks to human health and socio-economic factors but offer a challenge to collect as there is competition with commercial fisherfolk (Manuel Nicolaus, Cefas, Personal communications). As a result, commercial and non-commercial species have been proposed for monitoring. Alternative sentinel species for microplastic have been suggested, including seabirds, fish and crustaceans (Biamis et al., 2021; Truchet et al., 2022; Bruschi et al., 2023; Taurozzi and Scalici, 2024). Dab (Limanda limanda) were selected as a chemical contaminant sentinel species for the Clean Seas Environmnetal Monitoring Programme, a UK multi-agency program organized by the Clean, Safe Seas Evidence Group who report to the Department for Environment, Food and Rural Affairs (Defra) (National Oceanographic Centre, 2025). The species was chosen due to its close association with the sediment where chemical contaminants accumulate (Jon Barber, Cefas, Personal communication). At Cefas, fish are being explored as another potential sentinel species for microplastics, utilizing small pelagic fishes as an estimate of floating bioavailable microplastics and dab for the benthic habitat. Nephrops norvegicus have been suggested as an indicator (Hara et al., 2020). Decapods could pose a useful indicator for risk as they can accumulate large quantities of microplastics (Murray and Cowie, 2011; Welden and Cowie, 2016a, b; Cau et al., 2019; McGoran et al., 2020). Whilst contamination is higher than that generally observed in fish, the group may act as an early warning system for wider environmental impacts. A combination of sentinel species may be needed to address all knowledge gaps. Indeed, Biamis et al. (2021) recommend multiple species with disparate ingestion levels to help determine risk, with a focus on high-trophic level species, such as seabirds, when considering human health risks.
A holistic approach to research and monitoring would allow a better understanding of microplastic present in all compartments and the pathway between them, improving our understanding of sources, and highlighting the area’s most at risk and in need of further monitoring. Indeed, there are calls for a “One Health”, multidisciplinary approach to plastic monitoring (Biamis et al., 2021; Multisanti et al., 2022). But given Government budgets, this is not possible. Indicators should be selected to address major knowledge gaps, which include sources and transport of microplastics, and the risks associated with plastic in the environment. Mobile species, such as fish or decapod crustaceans, may be less helpful when addressing spatial patterns, identifying sources and hotspots. Certainly, small pelagic fish are highly migratory, with anchovies migrating from the North Sea and Biscay to overwinter in the English Channel (Van der Kooij et al., 2024). However, some species such as sprat have a far smaller range (Jeroen van der Kooij, Cefas, Personal communications). But sized-based particle selection in mussels may result in less plastic being consumed and inhibiting conclusions around risk. They can, however, be collected along a transect or stored in cages to better understand microplastic sources. Shumway et al. (2023) noted that bivalves contain very low concentrations of microplastics and Mladinich et al. (2023) observed that the polymers present in bivalves did not align with those in water, marine snow and sediment. However, Ferguson et al. (2024) found that suspension feeding mussels ingested a greater diversity of microplastics than grazing and scavenging invertebrates, which suggests they may be the least selective of researched invertebrates.
4.5 Future recommendations
Whilst isolated risk from plastic exposure might be minimal, in the environment mussels and other biota are exposed to multiple stressors and pollutants simultaneously. Indeed, as plastics fragment in the environment the release of additives and impurities increases, creating a “toxicity debt” for the large items presently in the environment (Rillig et al., 2021). Certainly, mussels have been recovered containing trace metals and microplastics (Alomar et al., 2025). When exposed to microplastics in the environment, plastic additives and hydrophobic organic compounds persist in the tissue of mussels and cockles (Hermabessiere et al., 2019). Additionally, exposure to pesticides in combination with microplastics leads to cumulative negative effects compared to single exposure (Shi et al., 2024). In laboratory conditions, oysters have been found to exhibit no physiological responses to virgin microbead ingestion, but oxygen consumption and respiration rate increased, likely due to a triggered immune response, when exposed to microplastics coated in Escherichia coli bacteria (Fabra et al., 2021). It should be noted that Fabra et al. (2021) used a high dose not environmentally relevant for their study.
Harmful algal bloom monitoring was selected for the present study as a cost-effective way of collecting samples for microplastics, piggybacking off an existing program. The presence of harmful algal blooms has increased significantly worldwide in recent decades (Do Prado Leite et al., 2022). This increase has been recently attributed to the increase in monitoring efforts around the world, but with some regions including Europe, appearing to see an increase in harmful algal bloom impacts (Hallegraeff et al., 2021). In the coastal waters of Great Britain, the lipophilic toxin groups and specifically those compounds responsible for Diarrhetic Shellfish Poisoning are the most prevalent in shellfish, annually (Bresnan et al., 2021). Plastics can be colonized by several species of microalgae and can act as a vector for harmful algal species and can increase exposure to benthic pathogens or their toxic excretions which are limited by the amount of substrate available for colonization (Do Prado Leite et al., 2022). Indeed, polyethylene has been shown to accumulate neurotoxins from dinoflagellate Karenia brevis on its surface (Shea et al., 2006). Additionally, plastic covered in biofilm can be up to ten times more likely to be ingested by suspension feeders (e.g., oysters) than virgin plastic (Fabra et al., 2021). Plastic can make these benthic threats available on suspended microplastics, increasing their bioavailability. UK waters are, however, predicted to be at less risk of this transport of harmful bacteria or algae than other regions including the Mediterranean Sea and the coasts of North America and East Asia (Do Prado Leite et al., 2022). Ingesting microplastics makes mussels more susceptible to toxic algae (Yuan et al., 2024). In addition to oxidative stress, the mussels experienced aggravated hemocyte apoptosis, reduced hemocyte viability and a reduction in cellular energy. As a result, these mussels experienced more intense deleterious effects. Susceptibility to toxic algae could lead to an outbreak of infection disease or a mass fatality in mussels (Zannella et al., 2017; Lassudrie et al., 2020) and is a potential human health risk. Thus, multi-stressor monitoring is vital to understand and mitigate risk. Thus, it is vital that pollutants are not monitored and assessed in isolation, with sentinel species selected for suitability in multi-stressor risk analysis.
Beyond neurotoxins, to truly assess risk in an environmental context, more cross-field studies are required. Collaboration between microplastic researchers, ecotoxicologists, chemists, climate scientists and more is lacking and vital for establishing risk thresholds and to make the best use of monitoring data. Threshold values are mandatory within the EU (Werner et al., 2020; Van Loon et al., 2020) for marine litter monitoring and enable monitoring programs to determine a set of measurable characteristics for good environmental status (GES) and allow governments to assess whether policy is progressing towards this achievement. Whilst independent of the EU, the UK Marine Strategy outlines commitments to achieve GES across the four devolved governments. Additionally, it is key for monitoring to be harmonized regionally and globally where possible. The risks associated with microplastics, especially in combination with other stressors, highlighted above demonstrate the need for monitoring. Whilst some OSPAR indicators cover large microplastics (>1 mm), the abundance of smaller items justifies a designated sentinel species. In the present study, microplastic concentrations were unknown and, as such, sample sites were selected to provide a wide geographical spread. Coincidently, these sites had a low frequency of algal blooms. Future studies should amend site selection to allow comparison between sites more frequently or intensely affected by harmful algal blooms whilst maintaining a broad spread around the coast of England and Wales, which are included in the survey. A similar strategy should be considered by other microplastic monitoring schemes globally.
With the above discussions in mind, mussels could be employed as a sentinel species for microplastics and multi-stressor assessments. Given the uncertainty surrounding microplastic associated risk, an indicator already established for chemical pollutant and harmful algal bloom monitoring is the logical choice. Mussels can be used to indicate potential risk to the ecosystem, potential transfer to humans from contaminated seafood and as a proxy for water contamination. The latter is especially significant given the temporal and spatial variability of microplastics in surface water and the lack of defined sampling volumes to ensure samples are representative of the environment (Danopoulos et al., 2023). Mussels are widely distributed and as suspension feeders are highly susceptible to microplastic ingestion. Of all bivalves, mussels are the ideal group for use as an indicator. There is a clear distinction in contamination levels between bivalves with clams containing on average more microplastics than mussels, scallops and oysters (Danopoulos et al., 2020; Ding et al., 2022). Greenwood et al. (2025) noted that, in these review studies, contamination in mussels was closest to the mean concentration across all bivalve species, making them the most suitable representative. In addition, their long-standing as an indicator for other hazards (Beyer et al., 2017; Afbi, 2023; Environment Agency, 2023; Leung et al., 2024) makes harvesting easier as new monitoring programs do not need to be developed from scratch. It also enables multi-stressor risk assessments to understand the cumulative effects of anthropogenic factors on biota. Additionally, as sessile organisms, mussels provide an estimate of local contamination unlike mobile organisms which could cover a large range. It has been suggested that, to improve the distribution of monitoring locations, caged mussels be utilized (Digka et al., 2024; Kazour and Amara, 2024; Weir et al., 2024). This powerful tool could be used to fill knowledge gaps, such as addressing riverine inputs to the marine environment by placing caged mussels along a transect (Greenwood et al., 2025).
4.6 Validation of method and limitations
In this study, the Nile red screening method for microplastics was applied for a fast and cost-effective assessment of the occurrence of microplastics in biota. Nile red has been previously applied for the detection and quantification of microplastics in biota (Catarino et al., 2018; Bakir et al., 2020a, 2020b; Nalbone et al., 2021; Shruti et al., 2022). The presence of false positives has been previously identified as a source of error when applying the Nile red screening method (Maes et al., 2017). The introduction of an optimized chemical digestion step (i.e., a mixture of KOH and NaClO) did limit the fluorescence of biological and natural items Bakir et al. (2023). Previous work carried out by Wang et al. (2021) also reported a lower fluorescence intensity of biogenic materials following a digestion step using hydrogen peroxide. Thus, reducing these false positives. Additionally, the Nile red dye is not effective on all polymers at all wavelengths (Wang et al., 2021) and fails to detect semi-synthetic materials, such as rayon. In the present study rayon was the most common material (37% of items), but when non-fluorescent items are excluded, only 17% of items are rayon (Gerigny et al., 2023). It can also fail to affect materials already containing dark dye. For this reason, a search under white light was also included. This prevented an underestimation of microplastics in the samples.
There is a lack of standardized methods for microplastic analysis. To combat this challenge, Gerigny et al. (2023) quantified microplastic abundance in locations from three countries (UK, France, Spain) in the North Atlantic using harmonized techniques between laboratories (KOH digestion and Nile red staining followed by single particle analysis using μ-FTIR). The assessment found similar levels of contamination across all sites and between the three countries, with some evidence of potential spatial accumulation zones for microplastics. The reported concentrations were, however, relatively low in a global context, possibly due to the exclusion of non-fluorescent items. The present study is an extension of the UK data presented in Gerigny et al. (2023) with the addition of non-fluorescent items otherwise missed by Nile red analysis. Our work demonstrates the importance of including these non-fluorescent items, as concentrations increased from 0.00– 0.68 to 0.60– 2.84 (mean) items individual-1. The present study and Gerigny et al. (2023) utilized strong contamination controls including rinsing tissue prior to digestion, as recommended by Kolandhasamy et al. (2018). Many studies globally do not record whether mussels were washed prior to microplastic extraction to remove external adhered particles (Greenwood et al., 2025). As a result, the low values presented in the present study may be a more accurate representation of environmental loading in mussels.
Translocation of microplastics is considered one of the major risks of microplastic ingestion but primarily occurs with particles smaller than 10 μm (Li et al., 2021). This is currently below the detection limit of commonly available analytical tools, such as micro FTIR and LDIR. It is hoped that as technology advances, identifying these smaller microplastics will become more accurate and reliable. Currently monitoring is limited to a minimum size of 20 μm, but reporting is only mandatory down to 100 μm (OSPAR MicroPlastics Expert Group (MPEG), 2024).
The present study is a proof of concept for multi-stressor assessments. Whilst harmful algae toxins are monitored throughout the year at frequencies between monthly and weekly dependent on local risk, microplastic analysis could only be conducted as a one off. Thus, further spatial and temporal analysis could not be conducted. A wider range of sites and data collected over several months or years would allow for more powerful statistical analysis. Additionally, further analysis of samples would allow for the selection of sites known to be high in neurotoxins as a comparison to the sites in the present study which exhibited concentrations below the limit of detection.
For neurotoxin analysis, mussels were homogenized, as is the standard approach. This meant it was not possible to compare per individual contamination of microplastics and neurotoxins, only per gram. This highlights the importance of reporting both units for microplastics analysis.
5 Conclusion
Sentinel species are vital to assessing the quality of the environment and how it changes over time. This directly relates to human health and socioeconomic factors, with sentinel species acting as an early warning sign for risks to consumers and environmental hazards. They should be selected based on their abundance and distribution but also their ecology and how they might feed into our ecotoxicological understanding. It is essential to understand how the animal feeds and may selectively accept or reject microplastics when interpreting the data. There is a knowledge gap between observed environmental microplastic contamination of biota and ecotoxicological studies that needs to be filled to ensure that effective risk assessments can be conducted. The authors recommend that a sentinel species for microplastic be developed in conjunction with multi-stressor evaluations, which are essential for understanding real world complexities. These evaluations can then further be developed into effective risk assessments for risk management and avoidance, an area that can be improved upon in the field of marine litter. We suggest that: 1) as microplastic abundance in biota varies, investigation is needed to inform the frequency of sample collection. This could be done at a few sites of interest before implementing a wider monitoring scheme; 2) environmental scientists, ecologists, ecotoxicologists and chemists collaborate on a systems approach to better inform risk management of microplastics and allowing for the development of multi-stressor monitoring. This should compare sites of low and high stress.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.14466/CefasDataHub.174.
Author contributions
AM: Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing - original draft, Writing - review & editing. SP: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AL: Writing – original draft, Writing – review & editing. AB: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. JB: Formal Analysis, Writing – original draft, Writing – review & editing. KD: Writing – original draft, Writing – review & editing. JR: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Interreg CleanAtlantic: https://interreg.eu/programmes/atlantic-area and the publication funded by Cefas.
Acknowledgments
The authors would like to thank Myriam Algoet (Cefas), Lewis Coates (formerly of Cefas) and the Food Standards Agency for their assistance on this project. Sample collection would not have been possible without them. We would like to extend our warmest thanks to each and every sampler. The authors would like to thank their Cefas colleagues who supported the discussion on existing sentinel species in UK monitoring programs, namely Jon Barber, Tim Ellis, Manuel Nicolaus, Claire Phillips, Briony Silburn, Joana Silva, and Jeroen Van Der Kooij.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1673482/full#supplementary-material
References
Afbi (2023). Monitoring for toxic producing and nuisance microalgae in Northern Ireland coastal waters. Available online at: https://data.food.gov.uk/catalog/datasets/5287e436-49ce-4dbc-ae58-ec72a0a2196e (Accessed September 09, 2025).
Akoueson F., Sheldon L. M., Danopoulos E., Morris S., Hotten J., Chapman E., et al. (2020). A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ. Pollut. 263, Part A, 114452. doi: 10.1016/j.envpol.2020.114452
Alomar C., Capó X., Rios-Fuster B., Bernárdez P., Santos-Echeandía J., and Deudero S. (2025). Are mussels accumulating trace metals and microplastics in port facilities?. Mar. Environ. Res. 210, 107263. doi: 10.1016/j.marenvres.2025.107263
Andrady A. L. (2022). Weathering and fragmentation of plastic debris in the ocean environment. Mar. pollut. Bull. 180, 113761. doi: 10.1016/j.marpolbul.2022.113761
AOAC (2005). AOAC Official method 2005.06 Quantitative determination of paralytic shellfish poisoning toxins in shellfish using pre-chromatographic oxidation and liquid chromatography with fluorescence detection (Gaithersburg: AOAC International).
Avio C. G., Gorbi S., Milan M., Benedetti M., Fattorini D., d‘Errico G., et al. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. pollut. 198, 211–222. doi: 10.1016/j.envpol.2014.12.021
Bagheri T., Gholizadeh M., Abarghouei S., Zakeri M., Hedayati A., Rabaniha M., et al. (2020). Microplastics distribution, abundance and composition in sediment, fishes and benthic organisms of the Gorgan Bay, Caspian Sea. Chemosphere 257, 127201. doi: 10.1016/j.chemosphere.2020.127201
Bakir A., Desender M., Wilkinson T., van Hoytema N., Amos R., Airahui S., et al. (2020a). Occurrence and abundance of meso and microplastics in sediment, surface waters, and marine biota from the South Pacific region. Mar. pollut. Bull. 160, 111572. doi: 10.1016/j.marpolbul.2020.111572
Bakir A., Doran D., Silburn B., Russell J., Archer-Rand S., Barry J., et al. (2023). A spatial and temporal assessment of microplastics in seafloor sediments: a case study for the UK. Front. Mar. Sci 9. doi: 10.3389/fmars.2022.1093815
Bakir A., Rowland S. J., and Thompson R. C. (2014). Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. pollut. 185, 16–23. doi: 10.1016/j.envpol.2013.10.007
Bakir A., van der Lingen C. D., Preston-Whyte F., Bali A., Geja Y., Barry J., et al. (2020b). Microplastics in commercially important small pelagic fish species from South Africa. Front. Mar. Sci 7. doi: 10.3389/fmars.2020.574663
Beaumont N. J., Aanesen M., Austen M. C., Börger T., Clark J. R., Cole M., et al. (2019). Global ecological, social and economic impacts of marine plastic. Mar. pollut. Bull. 142, 189–195. doi: 10.1016/j.marpolbul.2019.03.022
Besseling E., Foekema E. M., Van Franeker J. A., Leopold M. F., Kühn S., Bravo Rebolledo E. L., et al. (2015). Microplastic in a macro filter feeder: humpback whale Megaptera novaengliae. Mar. pollut. Bull. 95, 248–252. doi: 10.1016/j.marpolbul.2015.04.007
Beyer J., Green N. W., Brooks S., Allan I. J., Ruus A., Gomes T., et al. (2017). Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 130, 338–365. doi: 10.1016/j.marenvres.2017.07.024
Biamis C., O’Driscoll K., and Hardiman G. (2021). Microplastic toxicity: A review of the role of marine sentinel species in assessing the environmental and public health impacts. Case Stud. Chem. Environ. Eng. 3, 100073. doi: 10.1016/j.csee.2020.100073
Bråte I. L. N., Hurley R., Iversen K., Beyer J., Thomas K. V., Steindal C. C., et al. (2018). Mytilus spp. as sentinels for monitoring microplastic pollution in Norwegian coastal waters: A qualitative and quantitative study. Environ. pollut. 243, 383–393. doi: 10.1016/j.envpol.2018.08.077
Bresnan E., Arévalo F., Belin C., Branco M. A. C., Cembella A. D., Clarke D., et al. (2021). Diversity and regional distribution of harmful algal events along the Atlantic margin of Europe. Harmful Algae 102, 101976. doi: 10.1016/j.hal.2021.101976
Browne M. A., Dissanayake A., Galloway T. S., Lowe D. M., and Thompson R. C. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci Technol. 42, 5026–5031. doi: 10.1021/es800249a
Bruschi R., Pastorino P., Barceló D., and Renzi M. (2023). Microplastic levels and sentinel species used to monitor the environmental quality of lagoons: a state of the art in Italy. Ecol. Indic. 154, 110596. doi: 10.1016/j.ecolin.2023.110596
Castro G. B., Bernegossi A. C., Pinheiro F. R., and Corbi J. J. (2022). The silent harm of polyethylene microplastics: invertebrates growth inhibition as a warning of the microplastic pollution in continental waters. Limnologica 93, 125964. doi: 10.1016/j.limno.2022.125964
Catarino A. I., Macchia V., Sanderson W. G., Thompson R. C., and Henry T. B. (2018). Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. pollut. 237, 675–684. doi: 10.1016/j.envpol.2018.02.069
Catarino A. I., Thompson R., Sanderson W., and Henry T. B. (2017). Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 36, 947–951. doi: 10.1002/etc.3608
Cattaneo N., Zarantoniello M., Conti F., Frontini A., Chemello G., Dimichino B., et al. (2023). Dietary microplastic administration during zebrafish (Danio rerio) development: a comprehensive study between larval and juvenile stages. Animals 13, 2256. doi: 10.3390/ani13142256
Cau A., Avio C. G., Dessi C., Follesa M. C., Moccia D., Regoli F., et al. (2019). Microplastics in the crustaceans Nephrops norvegicus and Aristeus antennatus: flagship species for deep-sea environments? Environ. pollut. 255, 113107. doi: 10.1016/j.envpol.2019.113107
Chi C., Giri S. S., Jun J. W., Kim H. J., Yun S., Kim S. G., et al. (2016). Marine toxin okadaic acid affects the immune function of bay scallop (Argopecten irradians). Molecules 21, 1108. doi: 10.3390/molecules21091108
Collins H. I., Griffin T. W., Holohan B. A., and Ward J. E. (2023). Nylon microfibers develop a distinct plastisphere but have no apparent effects on the gut microbiome or gut tissue status in the blue mussel, Mytilus edulis. Environ. Microbiol. 25, 2792–2806. doi: 10.1111/1462-2920.16496
Courtene-Jones W., Quinn B., Murphy F., Gary S. F., and Narayanaswamy B. E. (2017). Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Analytical Methods 9, 1437–1445. doi: 10.1039/C6AY02343F
Daniel D., Barros L., da Costa J. P., Girão A. V., and Nunes B. (2024). Using marine mussels to assess the potential ecotoxicological effects of two different commercial microplastics. Mar. pollut. Bull. 203, 116441. doi: 10.1016/j.marpolbul.2024.116441
Danopoulos E., Jenner L. C., Twiddy M., and Rotchell J. M. (2020). Microplastic contamination of seafood intended for human consumption: a systematic review and meta-analysis. Environ. Health Perspect. 128, 126002. doi: 10.1289/EHP7171
Danopoulos E., Stanton T., Ma Y., Horton A. A., Chen Q., Levermore J. M., et al. (2023). Insights into technical challenges in the field of microplastic pollution through the lens of early career researchers (ECRs) and a proposed pathway forward. Front. Earth Sci 11. doi: 10.3389/feart.2023.1271547
Danopoulos E., Twiddy M., West R., and Rotchell J. M. (2022). A rapid review and meta-regression analyses of the toxicological impacts of microplastic exposure in human cells. J. Hazardous Materials 427, 127861. doi: 10.1016/j.hazmat.2021.127861
Defra (2024). United Kingdom Marine Monitoring & Assessment Strategy: Summary of progress towards Good Environmental Status. Available online at: https://moat.cefas.co.uk/summary-of-progress-towards-good-environmental-status/ (Accessed September 11, 2025).
Desforges J.-P. W., Galbraith M., and Ross P. S. (2015). Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contamination Toxicol. 69, 320–330. doi: 10.1007/s00244-015-0172-5
De Witte B., Devriese L., Bekaert K., Hoffman S., Vandermeersch G., Cooreman K., et al. (2014). Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. pollut. Bull. 85, 146–155. doi: 10.1016/j.marpolbul.2014.06.006
Dhanji-Rapkova M., O’Neill A., Maskrey B. H., Coates L., Alves M. T., Kelly R. J., et al. (2018). Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Okadaic acid, dinophysis toxins and pectenotoxins. Harmful Algae 77, 66–80. doi: 10.1016/j.hal.2018.05.011
Dhanji-Rapkova M., O’Neill A., Maskrey B. H., Coates L., Swan S. C., Alves M. T., et al. (2019). Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Azaspiracids and yessotoxins. Harmful Algae 87, 101629. doi: 10.1016/j.hal.2019.101629
Digka N., Patsiou D., Hatzonikolakis Y., Raitsos D. E., Skia G., Koutsoubas D., et al. (2024). Microplastic ingestion in mussels from the East Mediterranean Sea: Exploring its impacts in nature and controlled conditions. Sci Total Environ. 946, 174268. doi: 10.1016/j.scitotenv.2024.174268
Digka N., Tsangaris C., Torre M., Anastasopoulou A., and Zeri C. (2018). Microplastics in mussels and fish from the Northern Ionian Sea. Mar. pollut. Bull. 135, 30–40. doi: 10.1016/j.marpolbul.2018.06.063
Ding J., Sun C., Li J., Shi H., Xu X., Ju P., et al. (2022). Microplastics in global bivalve mollusks: A call for protocol standardization. J. Hazardous Materials 438, 129490. doi: 10.1016/j.jhazmat.2022.129490
Do V. M., Trinh V. T., Le X. T. T., and Nguyen D. T. (2024). Evaluation of microplastic bioaccumulation capacity of mussel (Perna viridis) and surrounding environment in the North coast of Vietnam. Mar. pollut. Bull. 199, 115987. doi: 10.1016/j.marpolbul.2023.115987
Dominguez H. J., Paz B., Daranas A. H., Norte M., Ferna J., and Franco M. (2010). Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: Characterization, analysis and human health implications. Toxicon 56, 191–217. doi: 10.1016/j.toxicon.2009.11.005
Do Prado Leite I., Menegotto A., da Cunha Lana P., and Mafra Júnior L. L. (2022). A new look at the potential role of marine plastic debris as a global vector of toxic benthic algae. Sci Total Environ. 838, 156262. doi: 10.1016/j.scitotenv.2022.156262
Dowarah K., Patchaiyappan A., Thirunavukkarasu C., Jayakumar S., and Devipriya S. P. (2020). Quantification of microplastics using Nile Red in two bivalve species Perna viridis and Meretrix meretrix from three estuaries in Pondicherry, India and microplastic uptake by local communities through bivalve diet. Mar. pollut. Bull. 153, 110982. doi: 10.1016/j.marpolbul.2020.110982
Dris R., Gasperi J., Mirande C., Mandin C., Guerrouache M., Langlois V., et al. (2017). A first overview of textile fibres, including microplastics, in indoor and outdoor environments. Environ. pollut. 221, 453–458. doi: 10.1016/j.envpol.2016.12.013
Dris R., Gasperi J., Saad M., Mirande C., and Tassin B. (2016). Synthetic fibres in atmospheric fallout: a source of microplastics in the environment? Mar. pollut. Bull. 104, 290–293. doi: 10.1016/j.marpolbul.2016.01.0060025-326X
EC 853/2004 (2004). REGULATION (EC) No 853/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur., 69–74.
Environment Agency (2023). Shellfish as bioindicators for antimicrobial resistance (Bristol: Environment Agency), 51.
EU 786/2013 (2013). Commission Regulation (E ) No 786 / 2013 of 16 August 2013 amending Annex III to Regulation (EC) No 853 / 2004 of the European Parliament and of the Council as regards the permitted limits of yessotoxins in live bivalve molluscs (Text with EEA releva. Off. J. Eur. Union 220, 4–6.
Fabra M., Williams L., Watts J. E. M., Hale M. S., Couceiro F., and Preston J. (2021). The plastic Trojan horse: biofilms increase microplastic uptake in marine filter feeders impacting organism health. Sci Total Environ. 797, 149217. doi: 10.1016/j.scitotenv.2021.149217
Faraway J. J. (2006). Extending the linear model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models (New York: Chapman and Hall/CRC), 301.
Farrington J. W., Tripp B. W., Tanabe S., Subramanian A., Sericano J. L., Wade T. L., et al. (2016). Edward D. Goldberg’s proposal of “the Mussel Watch”: reflections after 40 years. Mar. pollut. Bull. 110, 501–510. doi: 10.1016/j.marpolbul.2016.05.074
Ferguson L., Awe A., and Sparks C. (2024). Microplastic concentrations and risk assessment in water, sediment and invertebrates from Simon’s Town, South Africa. Heliyon 10, e28514. doi: 10.1016/j.heliyon.2024.e28514
Ferguson L. V., Hewins B., Harding W., MacDonald E., and Gibson G. (2022). Changes in the microbiome and associated host tissue structure in the blue mussel (Mytilus edulis) following exposure to polystyrene microparticles. Can. J. Zoology 100, 719–731. doi: 10.1139/cjz-2021-0104
Ferreira O., Barboza L. G. A., Rudnitskaya A., Moreirinha C., Vieira L. R., Botelho M. J., et al. (2023). Microplastics in marine mussels, biological effects and human risk of intake: A case study in a multi-stressor environment. Mar. pollut. Bull. 197, 115704. doi: 10.1016/j.marpolbul.2023.115704
Foley C. J., Feiner Z. S., Malinich T. D., and Hook T. O. (2018). A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci Total Environ. 631–632, 550–559. doi: 10.1016/j.scitotenv.2018.03.046
Food Standards Agency (FSA) (2025). Biotoxin and phytoplankton monitoring. Available online at: https://www.food.gov.uk/print/pdf/node/379 (Accessed September 3, 2025).
Galgani F., Darmon G., Pham C., Claro F., Marques N., Dellinger T., et al. (2022). “Marine Litter ingested by Sea Turtles,” in OSPAR 2023: The 2023 Quality Status Report for the North-East Atlantic (OSPAR Commission, London). Available online at: https://oap.ospar.org/en/ospar-assessments/quality-status-reports/qsr-2023/indicator-assessments/litter-sea-turtles/ (Accessed September 11, 2025).
Gerigny O., Bakir A., Barry J., Cardin Z., Chouteau L., El Rawke M., et al. (2023). CleanAtlantic tackling marine litter in the Atlantic area. Characterization of microplastics ingested by mussels. Toward the determination of a bio-sentinel species of the marine environment contamination by microplastics? WP5.3: Monitoring the interaction of marine litter with fauna. Available online at: https://www.cleanatlantic.eu/wp-content/uploads/2024/01/20231106_CleanAtlantic_Mussels.pdf (Accessed September 09, 2025).
Gerssen A., Mulder P. P. J., McElhinney M. A., and de Boer J. (2009). Liquid chromatography-tandem mass spectrometry method for the detection of marine lipophilic toxins under alkaline conditions. J. Chromatogr. A 1216, 1421–1430. doi: 10.1016/j.chroma.2008.12.099
GESAMP (2019). Guidelines or the monitoring and assessment of plastic litter and microplastics in the ocean Vol. 99. Eds. Kershaw P. J., Turra A., and Galgani F. (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection), 130.
Ghosh S., Sinha J. K., Ghosh S., Vashisth K., Han S., and Bhaskar R. (2023). Microplastics as an emerging threat to the global environment and human health. Sustainability 15, 10821. doi: 10.3390/su151410821
Grattan L. M., Holobaugh S., and Morris J. G. (2016). Harmful algal blooms and public health. Harmful Algae 57, 2–8. doi: 10.1016/j.hal.2016.05.003
Greenwood N., Aldridge J., Bakir A., Clare D., Fronkova L., Harrod O., et al. (2025). mNCEA WP1.2 Case studies considering the impact of changes in water quality due to human pressures on ecosystem service delivery. Cefas Project Rep. Defra, iv + 83.
Hallegraeff G. M., Anderson D. M., Belin C., Bottein M. Y. D., Bresnan E., Chinain M., et al. (2021). Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2, 117. doi: 10.1038/s43247-021-00178-8
Hamm T. and Lenz M. (2021). Negative impacts of realistic doses of spherical and irregular microplastics emerged later during a 42 weeks-long exposure experiment with blue mussels. Sci Total Environ. 778, 146088. doi: 10.1016/j.scittenv.2021.146088
Hara J., Frias J., and Nash R. (2020). Quantification of microplastic ingestion by the decapod crustacean Nephrops norvegicus from Irish waters. Mar. pollut. Bull. 152, 110905. doi: 10.1016/j.marpolbul.2020.110905
Hatfield R. G., Punn R., Algoet M., and Turner A. D. (2016). A rapid method for the analysis of paralytic shellfish toxins utilising standard pressure HPLC: refinement of AOAC 2005.06. J. AOAC Int. 99, 475–480. doi: 10.5740/jaoac.15-0080
Hatzonikolakis Y., Raitsos D. E., Sailley S. F., Digka N., Theodorou I., Tsiaras K., et al. (2024). Assessing the physiological effects of microplastics on cultured mussels in the Mediterranean Sea. Environ. pollut. 363, 125052. doi: 10.1016/j.envpol.2024.125052
Heisler J., Glibert P. M., Burkholder J. M., Anderson D. M., Cochlam W., Dennison W. C., et al. (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. doi: 10.1016/j.hal.2008.08,006
Hermabessiere L., Paul-Pont I., Cassone A.-L., Himber C., Receveur J., Jezequel R., et al. (2019). Microplastic contamination and pollutant levels in mussels and cockles collected along the channel coasts. Environ. pollut. 250, 807–819. doi: 10.1016/j.envpol.2019.04.051
Hurley R., Schell T., Crossman J., Rico A., Nawrocki B., Lavoy M., et al. (2018). Microplastic dynamics in agricultural systems: insights from Spanish and Canadian case studies, presented in part at Micro2018, Lanzarote, Canary Islands, Spain, November 2018. Available online at: https://micro2018.sciencesconf.org/data/pages/micro2018_proceedings_book_1.pdf (Accessed June 18, 2025).
Jang M., Shim W. J., Han G. M., Cho Y., Moon Y., and Hong S. H. (2021). Relative importance of aqueous leachate versus particle ingestion as uptake routes for microplastic additives (hexabromocyclododecane) to mussels. Environ. pollut. 270, 116272. doi: 10.1016/j.envpol.2020.116272
Joyce P. W. S. and Falkenberg L. J. (2022). Microplastics, both non-biodegradable and biodegradable, do not affect the whole organism functioning of a marine mussel. Sci Total Environ. 839, 156204. doi: 10.1016/j.scitotenv.2022.156204
Kassambara A. (2023). ggpubr: ‘ggplots’ based publication ready plots. doi: 10.32614/CRAN.package.ggpubr
Kazour M. and Amara R. (2024). To what extent is blue mussels caging representative of microplastics in the natural environment? Sci Total Environ. 912, 168975. doi: 10.1016/j.scitotenv.2023.168975
Khalid N., Aqeel M., Norman A., Hashem M., Mostafa Y. S., Alhaithloul H. A. S., et al. (2021). Linking effects of microplastics to ecological impacts in marine environments. Chemosphere 264, 128541. doi: 10.1016/j.chemosphere.2020.128541
Klasios N., De Frond H., Miller E., Sedlak M., and Rochman C. M. (2021). Microplastics and other anthropogenic particles are prevalent in mussels from San Francisco Bay, and show no correlation with PAHs. Environ. pollut. 271, 116260. doi: 10.1016/j.envpol.2020.116260
Koelmans A. A., Bakir A., Burton G. A., and Janssen C. R. (2016). Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci Technol. 50, 3315–3326. doi: 10.1021/acs.est.5b06069
Kolandhasamy P., Su L., Li J., Qu X., Jabeen K., and Shi H. (2018). Adherence of microplastics to soft tissue of mussels: a novel way to uptake microplastics beyond ingestion. Sci Total Environ. 610–611, 635–640. doi: 10.1016/j.scitotenv.2017.08.053
Kole P. J., Löhr A. J., van Berlleghem F. G. A. J., and Ragas A. M. J. (2017). Wear and tear of tyres: a stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 14, 1265. doi: 10.3390/ijerph14101265
Koutnik V. S., Alkidim S., Leonard J., DePrima F., Cao S., Hoek E. M. V., et al. (2021). Unaccounted microplastics in wastewater sludge: where do they go? ACS EST Water 1, 1086–1097. doi: 10.1021/acsestwater.0c000267
Krock B., Tillmann U., Tebben J., Trefault N., and Gu H. (2019). Two novel azaspiracids from Azadinium poporum, and a comprehensive compilation of azaspiracids produced by Amphidomataceae, (Dinophyceae) Two novel azaspiracids from Azadinium poporum, and a comprehensive compilation of azaspiracids produced by Amphi. Harmful Algae 82, 1–8. doi: 10.1016/j.hal.2018.12.005
Kühn S. and van Franeker J. A. (2020). Quantitative overview of marine debris ingested by marine megafauna. Mar. pollut. Bull. 151, 110858. doi: 10.1016/j.marpolbul.2019.110858
Lassudrie M., Hégaret H., Wikfors G. H., and da Silva P. M. (2020). Effects of harmful algal blooms on bivalve cellular immunity and infectious diseases: a review. Dev. Comp. Immunol. 108, 103660. doi: 10.1016/j.sci.2020.103660
Leistenschneider C., Burkhardt-Holm P., Mani T., Primpke S., Taubner H., and Gerdts G. (2021). Microplastics in the Weddell Sea (Antarctica): a forensic approach for discrimination between environmental and vessel-induced microplastics. Environ. Sci Technol. 55 23, 15900–15911. doi: 10.1021/acs.est.1c05207
Leung R. K.-L., Jin L., Kong H.-K., Su C., Ren X., Liu X., et al. (2024). Development of a multiple-biomarker approach using the green-lipped mussel Perna viridis for marine pollution monitoring: a case study in Victoria Harbour, Hong Kong. Mar. pollut. Bull. 206, 116684. doi: 10.1016/j.marpolbul.2024.116684
Li J., Green C., Reynolds A., Shi H., and Rotchell J. M. (2018). Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. pollut. 241, 35–44. doi: 10.1016/j.envpol.2018.05.038
Li J., Lusher A. L., Rotchell J. M., Deudero S., Turra A., Bråte I. L. N., et al. (2019). Using mussel as a global bioindicator of coastal microplastic pollution. Enviornmental pollut. 244, 522–533. doi: 10.1016/j.envpol.2018.10.032
Li J., Qu X., Su L., Zhang W., Yang D., Kolandhasamy P., et al. (2016). Microplastics in mussels along the coastal waters of China. Environ. pollut. 214, 177–184. doi: 10.1016/j.envpol.2016.04.012
Li J., Wang Z., Rotchell J. M., Shen X., Li Q., and Zhu J. (2021). Where are we? Towards an understanding of the selective accumulation of microplastics in mussels. Environ. pollut. 286, 117543. doi: 10.1016/j.envpol.2021.117543
Lindeque P. K., Cole M., Coppock R. L., Lewis C. N., Miller R. Z., Watts A. J. R., et al. (2020). Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh0size. Environ. pollut. 265, 114721. doi: 10.1016/j.envpol.2020.114721
Lusher A., Bråte I. L. N., Hurley R., Iversen K., and Olsen M. (2017). Testing of methodology for measuring microplastics in blue mussels (Mytilus spp) and sediments, and recommendations for future monitoring of microplastics (R & D-project) (Norway: NIVA), 87. doi: 10.13140/RG.2.2.24399.59041
Maes T., Jessop R., Wellner N., Haupt K., and Mayes A. G. (2017). A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 7, 44501. doi: 10.1038/srep44501
Manly B. F. J. and Navarro Alberto J. A. (2021). Randomization, bootstrap and Monte Carlo methods in biology. 4th ed. (New York, USA: Chapman and Hall, CRC Press), 358.
Mateos-Cárdenas A., O’Halloran J., van Pelt F. N. A. M., and Jansen M. A. K. (2021). Beyond plastic microbeads – short-term feeding of cellulose and polyester microfibers to freshwater amphipod Gammarus duebeni. Sci Total Environ. 753, 141859. doi: 10.1016/j.scitotenv.2020.141859
Matiddi M., Pham C. K., Anastasopoulou A., Andresmaa E., Avio C. G., Bianchi J., et al. (2021). Monitoring micro-litter ingestion in marine fish: a harmonized protocol for MSFD and RSCS areas. Available online at: https://indicit-europa.eu/cms/wp-content/uploads/2021/06/Monitoringmicrolitter-ingestion-in-marine-fish-1.pdf (Accessed April 17, 2023).
McCarthy M., O'Halloran J., O'Brien N. M., and van Pelt F. F. (2014). Does the marine biotoxin okadaic acid cause DNA fragmentation in the blue mussel and the pacific oyster? Mar. Environ. Res. 101, 153–160. doi: 10.1016/j.marenvres.2014.09.009
McCoy K. A., Hodgson D. J., Clark P. F., and Morritt D. (2020). The effects of wet wipe pollution on the Asian clam, Corbicula fluminea (Mollusca: Bivalvia) in the River Thames, London. Environ. pollut. 264, 114577. doi: 10.1016/j.envpol.2020.114577
McGoran A. R. (2023). Assessing the potential for trophic transfer of microplastics through the Thames Food Web. (London: Royal Holloway, University of London). Available online at: https://pure.royalholloway.ac.uk/en/publications/assessing-the-potential-for-trophic-transfer-of-microplastics-thr (Accessed September 11, 2025).
McGoran A., Bakir A., Barry J., and Rusell J. (2025a). Microplastic contamination data from mussels from the UK in 2022 Cefas, UK. V1. doi: 10.14466/CefasDataHub.174
McGoran A. R., Clark P. F., Smith B. D., and Morritt D. (2020). High prevalence of plastic ingestion by Eriocheir sinensis and Carcinus maenas (Crustacea: Decapoda: Brachyura) in the Thames Estuary. Environ. pollut. 265, 114972. doi: 10.1016/j.envpol.2020.114972
McGoran A. R., Clark P. F., Smith B. D., and Morritt D. (2025b). Temporal variation in microplastic abundance in sediment, fishes and shrimp: a case study in the Thames Estuary, UK. Philos. Trans. R. Soc. A 383, 20250040. doi: 10.1098/rsta.2025.0040
McIlwraith H. K., Kim J., Helm P., Bhavsar S. P., Metzger J. S., and Rochman C. M. (2021). Evidence of microplastic translocation in wild-caught fish and implications for microplastic accumulation dynamics in food webs. Environ. Sci Technol. 55, 12372–12382. doi: 10.1021/acs.est.1c02922
Meijek L. J. J., van Emmerik T., van Derent R., Schmidt C., and Lebreton L. C. M. (2021). More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci Adv. 7, 2375–2548. doi: 10.1126/sciadv.aaz5803
Migliarini L. O., Landro S. M., Martinez-Espinoza F., Murgida D. H., and Arrighetti F. (2025). Profiling microplastic fibers in the intertidal sentinel mussel Brachidontes rodriguezii from the coast of Buenos Aires, Argentina. PeerJ 13, e19518. doi: 10.7717/peerj.19518
Mladinich K., Holohan B. A., Shumway S. E., Brown K., and Ward J. E. (2022). Determining the properties that govern selective ingestion and egestion of microplastics by blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica). Environ. Sci Technol. 56, 15770–15779. doi: 10.1021/acs.est.2c06402
Mladinich K., Holohan B. A., Shumway S. E., and Ward J. E. (2023). The relationship between microplastics in eastern oysters (Crassostrea virginica) and surrounding environmental compartments in Long Island Sound. Mar. Environ. Res. 189, 106040. doi: 10.1016/j.marenvres.2023.106040
Mladinich K., Holohan B. A., Shumway S. E., and Ward J. E. (2025). Investigating suspension-feeding invertebrates as bioindicators of microfiber pollution (Oregon: SETAC North America 46th Annual Meeting). Available online at: https://setac.confex.com/setac/sna2025/meetingapp.cgi/Paper/30208 (Accessed September 09, 2025).
Moore C. J. (2008). Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res. 108, 131–139. doi: 10.1016/j.envres.2008.07.025
Moore R. C., Loseto L., Noel M., Etemadifar A., Brewster J. D., MacPhee S., et al. (2020). Microplastics in beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. pollut. Bull. 150, 110723. doi: 10.1016/j.marpolbul.2019.110723
Multisanti C. R., Merola C., Perugini M., Aliko V., and Faggio C. (2022). Sentinel species selection for monitoring microplastic pollution: a review on one health approach. Ecol. Indic. 145, 109587. doi: 10.1016/j.ecoind.2022.109587
Murray F. and Cowie P. R. (2011). Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus 1758). Mar. pollut. Bull. 62, 1207–1217. doi: 10.1016/j.marpolbul.2011.03.032
Naidu S. A. (2019). Preliminary study and first evidence of presence of microplastics and colorants in green mussel, Perna viridis (Linnaeus 1758), from southeast coast of India. Mar. pollut. Bull. 140, 416–422. doi: 10.1016/j.marpolbul.2019.01.024
Nalbone L., Panebianco A., Giarratana F., and Russell M. (2021). Nile Red staining for detecting microplastics in biota: preliminary evidence. Mar. pollut. Bull. 172, 112888. doi: 10.1016/j.marpolbul.2021.112888
Napper I. E. and Thompson R. C. (2016). Release of synthetic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar. pollut. Bull. 112, 39–45. doi: 10.1016/j.marpolbul.2016.09.025
Nardi A., Pittura L., d’Errico G., Cesaroni D., Mongera F., Gorbi S., et al. (2024). Cellular effects of microplastics are influenced by their dimension: mechanistic relationships and integrated criteria for particles definition. Environ. pollut. 344, 123327. doi: 10.1016/j.envpol.2024.123327
National Oceanographic Centre (2025). MERMAN project overview. Available online at: https://www.bodc.ac.uk/resources/portals_and_links/merman/project_overview/ (Accessed September 11, 2025).
NCCOS (2020). NOAA evaluates using mussel watch program to monitor microplastics (USA: NOAA Evaluates Using Mussel Watch Program to Monitor Microplastics - NCCOS - National Centers for Coastal Ocean Science). Available online at: https://coastalscience.noaa.gov/news/noaa-tests-mussel-watch-program-to-monitor-microplastics/ (Accessed September 09, 2025).
O’Brien S., Okoffo E. D., O’Brien J. W., Ribeiro F., Wang X., Wright S. L., et al. (2020). Airborne emissions of microplastic fibres from domestic laundry dryers. Sci Total Environ. 747, 141175. doi: 10.1016/j.scitotenv.2020.141175
Opitz T., Benítez S., Fernández C., Osores S., Navarro J. M., Rodríguez-Romero A., et al. (2021). Minimal impact at current environmental concentrations of microplastics on energy balance and physiological rates of giant mussel Choromytilus chorus. Mar. pollut. Bull. 162, 111834. doi: 10.1016/j.marpolbul.2020.111.834
Osman A. I., Hosny M., Eltaweil A. S., Omar S., Elgarahy A. M., Farghali M., et al. (2023). Microplastic sources, formation, toxicity and remediation: a review. Environ. Chem. Lett. 21, 2129–2169. doi: 10.1007/s10311-023-01593-3
OSPAR MicroPlastics Expert Group (MPEG) (2024). CEMP guidelines for the monitoring of microlitter (including microplastics) in seafloor sediments for the OSPAR maritime area (London, UK: OSPAR Commission), 25.
Paz B., Daranas A. H., Norte M., Riobó P., Franco J. M., and Fernández J. J. (2008). Yessotoxins, a group of marine polyether toxins: an overview. Mar. Drugs 6, 73–102. doi: 10.3390/md6020073
Pedersen A. F., Gopalakrishnan K., Boegehold A. G., Peraino N. J., Westrick J. A., and Kashian D. R. (2020). Microplastic ingestion by quagga mussels, Dreissena bugensis, and its effects on physiological processes. Environ. pollut. 260, 113964. doi: 10.1016/j.envpol.2020.113964
Peters R., de Jong N., de Haan L., Wright S., and Bouwmeester H. (2022). Release and intestinal translocation of chemicals associated with microplastics in an in vitro human gastrointestinal digestion model. Microplastics Nanoplastics 2, 3. doi: 10.1186/s43591-021-00022-y
Phuong N. N., Poirier L., Pham Q. T., Lagarde F., and Zalouk-Vergnoux A. (2018). Factors influencing the microplastic contamination of bivalves from the French Atlantic coast: location, season and/or mode of life? Mar. pollut. Bull. 129, 664–674. doi: 10.1016/j.marpolbul.2017.10.054
PlasticsEurope (2024). Plastics – the fast facts 2024. Available online at: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2024/ (Accessed September 11, 2025).
Prego-Faraldo M. V., Valdiglesias V., Méndez J., and Eirín-López J. M. (2013). Okadaic acid meet and greet: An insight into detection methods, response strategies and genotoxic effects in marine invertebrates. Mar. Drugs 11, 2829–2845. doi: 10.3390/md11082829
Provenza F., Rampih D., Pignattelli S., Pastorino P., Barceló D., Prearo M., et al. (2022). Mussel watch program for microplastics in the Mediterranean sea: Identification of biomarkers of exposure using Mytilus galloprovincialis. Ecol. Indic. 142, 109212. doi: 10.1016/j.ecolind.2022.109212
Qiao R., Deng Y., Zhang S., Wolosker M. B., Zhu Q., Ren H., et al. (2019). Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 236, 124334. doi: 10.1016/j.chemosphere.2019.07.065
Qu X., Su L., Li H., Liang M., and Shi H. (2018). Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci Total Environ. 621, 679–686. doi: 10.1016/j.scitotenv.2017.11.284
Quilliam M. A., Xie M., and Hardstaff W. R. (1995). Rapid extraction and cleanup for liquid chromatographic determination of domoic acid in unsalted seafood. J. AOAC Int. 78, 543–554. doi: 10.1093/jaoac/78.2.543
Railo S., Talvitie J., Setälä O., Koistinen A., and Lehtiniemi M. (2018). Application of an enzyme digestion method reveals microlitter in Mytilus trossulus at a wastewater discharge area. Mar. pollut. Bull. 130, 206–214. doi: 10.1016/j.marpolbul.2018.03.022
Rebelein A., Int-Veen I., Kammann U., and Scharsack J. P. (2021). Microplastic fibers – underestimated threat to aquatic organisms? Sci Total Environ. 777, 146045. doi: 10.1016/j.scitotenv.2021.146045
Reed S., Clark M., Thompson R., and Hughes K. A. (2018). Microplastics in marine sediments near Rothera Research Station, Antarctica. Mar. pollut. Bull. 133, 460–463. doi: 10.1016/j.marpolbul.2018.05.068
Reguera P., Vinas L., and Gago J. (2019). Microplastics in wild mussels (Mytilus spp.) from the north coast of Spain. Scientia Marina 83, 337–347. doi: 10.3989/scimar.04927.05A
Remy F., Collard F., Gilbert B., Compère P., Eppe G., and Lepoint G. (2015). When plastic is not plastic: the ingestion of artificial cellulose fibers by macrofauna living in seagrass macrophytodetritus. Environ. Sci Technol. 49, 11158–11166. doi: 10.1021/acs.est.5b02005
Ribeiro V. V., Soares T. M. A., De-la-torre G. E., Casado-Coy N., Sanz-Lazaro C., and Castro Í.B. (2024). Microplastics in rocky shore mollusks of different feeding habitats: an assessment of sentinel performance. Environ. pollut. 346, 123571. doi: 10.1016/j.envpol.2024.123571
Rillig M. C., Kim S. W., Kim T.-Y., and Waldman W. R. (2021). The global plastic toxicity debt. Environ. Sci Technol. 55, 2717–2719. doi: 10.1021/acs.est.0c07781
Roman L., Schuyler Q., Wilcox C., and Hardesty B. D. (2020). Plastic pollution is killing megafauna, but how do we prioritize policies to reduce mortality? Conserv. Lett. 14, e12781. doi: 10.1111/conl.12781
Rosa M., Capriotti M., Austin K., Shumway S. E., and Ward J. E. (2024). Effect of seasonal changes in temperature on capture efficiency in the blue mussel, Mytilus edulis, fed seston and microplastics. Invertebrate Biol. 143, e12446. doi: 10.1111/ivb.12446
Rosa M., Ward J. E., and Shumway S. E. (2018). Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: a review. J. Shellfish Res. 37, 727–746. doi: 10.2983/035.037.0405
Sadri S. S. and Thompson R. C. (2014). On the quantity and composition of floating debris entering and leaving the Tamar Estuary, Southwest England. Mar. pollut. Bull. 81, 55–60. doi: 10.1016/j.marpolbul.2014.02.020
Saeed A. F., Awan S. A., Ling S., Wang R., and Wang S. (2017). Domoic acid: Attributes, exposure risks, innovative detection techniques and therapeutics. Algal Res. 24, 97–110. doi: 10.1016/j.algal.2017.02.007
Santana M. F. M., Moreira F. T., Pereira C. D. S., Abessa D. M. S., and Turra A. (2018). Continuous exposure to microplastics does not cause physiological effects in the cultivated mussel Perna perna. Arch. Environ. Contamination Toxicol. 74, 594–604. doi: 10.1007/s00244-018-0504-3
Scott N., Porter A., Santillo D., Simpson H., Lloyd-Williams S., and Lewis C. (2019). Particle characteristics of microplastics contaminating the mussel Mytilus edulis and their surrounding environments. Mar. pollut. Bull. 146, 125–133. doi: 10.1016/j.marpolbul.2019.05.041
Shea D., Tester P., Cohen J., Kibler S., and Varnam S. (2006). Accumulation of brevetoxins by passive sampling devices. Afr. J. Mar. Sci 28, 379–381. doi: 10.2989/18142320609504182
Shi X., Xu T., Gao M., Bi Y., Wang J., Yin Y., et al. (2024). Combined exposure of emamectin benzoate and microplastics induces tight junction disorder, immune disorder and inflammation in carp midgut via lysosome/ROS/ferroptosis pathway. Water Res. 257, 121660. doi: 10.1016/j.watres.2024.121660
Shruti V. C., Pérez-Guevara F., Roy P. D., and Kutralam-Muniasamy G. (2022). Analyzing microplastics with Nile Red: emerging trends, challenges, and prospects. J. Hazardous Materials 423, 127171. doi: 10.1016/j.jhazmat.2021.127171
Shumway S., Mladinich K., Blaschik N., Holohan B. A., and Ward J. E. (2023). A critical assessment of microplastics in molluscan shellfish with recommendations for experimental protocols, animal husbandry, publication, and future research. Rev. Fisheries Sci Aquaculture, 1–133. doi: 10.1080/23308249.2023.2216301
Sipe J. M., Bossa N., Berger W., von Windheim N., Gall K., and Wiesner M. R. (2022). From bottle to microplastics: can we estimate how our plastic products are breaking down? Sci Total Environ. 814, 152460. doi: 10.1016/j.scitotenv.152460
Sparks C. (2020). Microplastics in mussels along the coast of Cape Town, South Africa. Bull. Environ. Contamination Toxicol. 104, 423–431. doi: 10.1007/s00128-020-02809-w
Sparks C., Awe A., and Maneveld J. (2021). Abundance and characteristics of microplastics in retail mussels from Cape Town, South Africa. Mar. pollut. Bull. 166, 112186. doi: 10.1016/j.marpolbul.2021.112186
Sundt P., Schulze P. E., and Syversen F. (2014). Sources of microplastic pollution to the marine environment. Report no: M-321|2015 (Norwegian Environmental Agency), 1–86.
Tamis J. E., Koelmans A. A., Dröge R., Kaag N. H. B. M., Keur M. C., Tromp P. C., et al. (2021). Environmental risks of car tire microplastic particles and other road runoff pollutants. Microplastics Nanoplastics 1, 10. doi: 10.1186/s43591-021-00008-w
Taurozzi D. and Scalici M. (2024). Seabirds from the poles: microplastics pollution sentinels. Front. Mar. Sci 11. doi: 10.3389/fmars.2024.1343617
Teng J., Zhao J., Zhu X., Shan E., Zhang C., Zhang W., et al. (2021). Toxic effects of exposure to microplastics with environmentally relevant shapes and concentrations: accumulation, energy metabolism and tissue damage in oyster Crassostrea gigas. Environ. pollut. 269, 116169. doi: 10.1016/j.envpol.2020.116169
Thompson R. C., Courtene-Jones W., Boucher J., Pahl S., Raubenhimer K., and Koelmans A. A. (2024). Twenty years of microplastic pollution research – what have we learned? Science 386, 2746. doi: 10.1016/1126/science.adl27746
Truchet D. M., Ardusso M. G., Forero-López A. D., Rimondino G. N., Buzzi N. S., Malanca F., et al. (2022). Tracking synthetic microdebris contamination in a highly urbanized estuary through crabs as sentinel species: an ecological trait-based approach. Sci Total Environ. 837, 155631. doi: 10.1016/j.scitotenv.2022.155631
Tumwesigye E., Nnadozie C. F., Akamagwuna F. C., Noundou X., Nyakairu G. W., and Odume O. N. (2023). Microplastics as vectors of chemical contaminants and biological agents in freshwater ecosystems: Current knowledge status and future perspectives. Environmnetal pollut. 330, 121829. doi: 10.1016/j.envpol.2023.121829
Twiner M. J., Rehmann N., Hess P., and Doucette G. J. (2008). Azaspiracid shellfish poisoning: A review on the chemistry, ecology, and toxicology with an emphasis on human health. Mar. Drugs 6, 39–72. doi: 10.3390/md20080004
Tyler-Walters H. (2008). “Mytilus edulis common mussel,” in Marine Life Information Network: Biology and sensitivity reviews. Ed. Tyler-Walters H. (Marine Biological Association of the United Kingdom, Plymouth, UK). Available online at: http://www.marlin.ac.uk/species/detail/1421 (Accessed September 11, 2025).
Van Cauwenberghe L., Claessens M., Vandegehuchte M. B., and Janssen C. R. (2015). Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. pollut. 199, 10–17. doi: 10.1016/j.envpol.2015.01.008
Van der Kooij J., McKeown N., Campanella F., Boyra G., Doray M., Santos Mocoroa M., et al. (2024). Northward range expansion of Bay of Biscay anchovy into the English Channel. Mar. Ecol. Prog. Ser. 741, 217–236. doi: 10.3354/meps14603
Vandermeersch G., Van Cauwenberghe L., Janssen C. R., Marques A., Granby K., Fait G., et al. (2015). A critical view on microplastic quantification in aquatic organisms. Environ. Res. 143, 46–55. doi: 10.1016/j.envres.2015.07.016
Van der Schatte Olivier A., Jones L., Le Vay L., Christie M., Wilson J., and Malham S. K. (2018). A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquaculture 12, 3–25. doi: 10.1111/raq.12301
Van Dolah F. M. (2000). Diversity of marine and freshwater algal toxins. In Seafood and Freshwater Toxins (London, UK: CRC Press), 19–43.
Van Franeker J. A. (2019). Coordinated Environmental Monitoring Programme (CEMP) guidelines for monitoring and assessment of plastic particles in stomachs of fulmars in the North Sea area: Agreement 2015-03 (London, UK: OSPAR Commission). Available online at: http://www.ospar.org/convention/agreements?q=fulmar (Accessed September 09, 2025).
Van Loon W., Hanke G., Fleet D., Werner S., Barry J., Strand J., et al. (2020). A European threshold value and assessment method for macro litter on coastlines (London, UK: European Commission, Marine Strategy Framework Directive Technical Group on Marne Litter). doi: 10.2760/54369
Van Wesel A., Caris I., and Kools S. A. E. (2016). Release of primary microplastics from consumer products to wastewater in the Netherlands. Environ. Toxicol. Chem. 35, 1627–1631. doi: 10.1002/etc.3316
Walker D. I., Baker-Austin C., Smith A., Thorpe K., Bakir A., Galloway T., et al. (2022). A critical review of microbiological colonisation of Nano-and Microplastics (NMP). Project code FS307021, 184. doi: 10.46756/sci.fsa.xdx112
Walkinshaw C., Tolhurst T. J., Lindeque P. K., Thompson R. C., and Cole M. (2023). Impact of polyester and cotton microfibers on growth and sublethal biomarkers in juvenile mussels. Microplastics Nanoplastics 3, 5. doi: 10.1186/s43591-023-00052-8
Wang C., Jiang L., Liu R., He M., Cui X., and Wang C. (2021). Comprehensive assessment of factors influencing Nile red staining: Eliciting solutions for efficient microplastics analysis. Mar. pollut. Bull. 171, 112698. doi: 10.1016/j.marpolbul.2021.112698
Wang T., Li B., Zou X., Wang Y., Li Y., Xu Y., et al. (2019). Emission of primary microplastics in mainland China: invisible but not negligible. Water Res. 162, 214–224. doi: 10.1016/j.watres.2019.06.042
Ward J. E. and Kach D. J. (2009). Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves. Mar. Environ. Res. 68, 137–142. doi: 10.1016/j.marenvres.2009.05.002
Ward J. E. and Shumway S. E. (2004). Separating the grain from the chaff: particle selection in suspension- and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 300, 83–130. doi: 10.1016/j.jembe.2004.03.002
Ward J. E., Zhao S., Holohan B. A., Mladinich K. M., Griffin T. W., Wozniak J., et al. (2019). Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): implications for using bivalves as bioindicators of microplastic pollution. Environ. Sci Technol. 53, 8776–8784. doi: 10.1021/acs.est.9b02073
Watkins E., Brink P. T., Withana S., Kettunen M., Russi D., Mutafoglu K., et al. (2017). “Chapter 14: The socio-economic impacts of marine litter, including the costs of policy inaction and action,” in Handbook on the economics and management of sustainable oceans. Eds. Nunes P. A. L. D., Svensson L. E., and Markandya A. (Edward Elgar Publishing, UK). doi: 10.4337/9781786430724
Webb S., Ruffell H., Marsden I., Pantos O., and Gaw S. (2019). Microplastics in the New Zealand green lipped mussel Perna canaliculus. Mar. pollut. Bull. 149, 110641. doi: 10.1016/j.marpolbul.2019.110641
Weir E. M., Kidd K. A., Hamilton B. M., Wu J., Servos M. R., Bartlett A. J., et al. (2024). Microparticles in wild and caged biota, sediments, and water relative to large municipal wastewater treatment plant discharges. Environ. Toxicol. Chem. 43, 1047–1061. doi: 10.1002/etc.5836
Welden N. A. C. and Cowie P. R. (2016a). Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus. Environ. pollut. 214, 856–865. doi: 10.1016/j.envpol.2016.03.067
Welden N. A. C. and Cowie P. R. (2016b). Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. pollut. 218, 895–900. doi: 10.1016/j.envpol.2016.08.20
Werner S., Fischer E., Fleet D., Galgani F., Hanke G., Kinsey S., et al. (2020). Threshold values for Marine Litter: general discussion paper on defining threshold values for marine litter (European Commission, Marine Strategy Framework Directive Technical Group on Marne Litter). doi: 10.2760/192427
Wiese M., D’Agostino P. M., Mihali T. K., Moffitt M. C., and Neilan B. A. (2010). Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 8, 2185–2211. doi: 10.3390/md8072185
Woods M. N., Stack M. E., Fields D. M., Shaw S. D., and Matrai P. A. (2018). Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. pollut. Bull. 137, 638–645. doi: 10.1016/j.marpolbul.2018.10.061
Worek J., Badura X., Bialas A., Chwiej J., Kawoń K., and Styszko K. (2022). Pollution from transport: detection of tyre particles in environmental samples. Energies 15, 2816. doi: 10.3390/en15082816
Wu Y., Li Z., Deng Y., Bian B., Xie L., Lu X., et al. (2024). Mangrove mud clam as an effective sentinel species for monitoring changes in coastal microplastic pollution. J. Hazardous Materials 472, 134617. doi: 10.1016/j.jhazmat.2024.134617
Xu S., Chen M., Feng T., Zhan L., Zhou L., Zhou L., et al. (2021). Use ggbreak to effectively utilize plotting space to deal with large datasets and outliers. Front. Genet. 12. doi: 10.3389/fgene.2021.774846
Xu Z., Huang L., Xu P., Lim L., Cheong K. L., Wang Y., et al. (2024). Microplastic pollution in commercially important edible marine bivalves: A comprehensive review. Food Chemistry: X 23, 101647. doi: 10.1016/j.fochx.2024.101647
Yang H., Xiong H., Mi K., Xue W., Wei W., and Zhang Y. (2020). Toxicity comparison of nano-sized and micron-sized microplastics to goldfish Carassius auratus larvae. J. Hazardous Materials 388, 122058. doi: 10.1016/j.hazmat.2020.122058
Yuan K.-K., Yu Y.-Y., Mo Y.-H., Liu Y.-J., Zhang W.-X., Lv J.-J., et al. (2024). Exposure to microplastics renders immunity of the thick-shell mussel more vulnerable to diarrhetic shellfish toxin-producing harmful algae. Sci Total Environ. 926, 172125. doi: 10.1016/j.scitotenv.2024.172125
Zannella C., Mosca F., Mariani F., Franci G., Folliero V., Galdiero M., et al. (2017). Microbial diseases of bivalve mollusks: infections, immunology and antimicrobial defence. Mar. Drugs 15, 182. doi: 10.3390/md15060182
Zeytin S., Wagner G., Mackay-Roberts N., Gerdt G., Schuirmann E., Klockmann S., et al. (2020). Quantifying microplastic translocation from feed to the fillet in European sea bass (Dicentrarchus labrax). Mar. pollut. Bull. 156, 111210. doi: 10.1016/j.marpolbul.2020.11210
Zhao S., Ward J. E., Danley M., and Mincer T. J. (2018). Field-based evidence for microplastic in marine aggregates and mussels: implications for trophic transfer. Environ. Sci Technol. 52, 11038–11048. doi: 10.1021/acs.est.8b03467
Zheng S., Zhao Y., Liangwei W., Liang J., Liu T., Zhu M., et al. (2020). Characteristics of microplastics ingested by zooplankton from the Bohai Sea, China. Sci Total Environ. 713, 136357. doi: 10.1016/j.scitotenv.2019.136357
Ziajahromi S., Kumar A., Neale P. A., and Leusch F. S. L. (2017a). Impact of microplastic beads and fibres on waterflea (Ceriodaphnia dubia) survival, growth, and reproduction: implications of single and mixed exposures. Environ. Sci Technol. 61, 13397–13406. doi: 10.1021/acs.est.7b03574
Ziajahromi S., Neale P. A., Rintoul L., and Leusch F. D. L. (2017b). Wastewater treatment plants as a pathway for microplastics: development of a new approach to sample wastewater-based microplastics. Water Res. 112, 93–99. doi: 10.1016/j.watres.2017.01.042
Keywords: microplastics, Mytilus edulis, mussels, sentinel species, plastic pollution, harmful algal blooms, multi-stressor monitoring
Citation: McGoran AR, Page S, Lewis A, Bakir A, Barry J, Dean K and Russell J (2025) Advancing mussel-based monitoring: integrating litter and harmful algal bloom data into a multi-stressor assessment of England and Wales. Front. Mar. Sci. 12:1673482. doi: 10.3389/fmars.2025.1673482
Received: 25 July 2025; Accepted: 29 September 2025;
Published: 21 October 2025.
Edited by:
Julius A. Ellrich, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), GermanyReviewed by:
J. Evan Ward, University of Connecticut, United StatesKhusnul Yaqin, Hasanuddin University, Indonesia
Monica Fabra, Independent Researcher, London, United Kingdom
Copyright © 2025 McGoran, Page, Lewis, Bakir, Barry, Dean and Russell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra R. McGoran, YWxleC5tY2dvcmFuQGNlZmFzLmdvdi51aw==
 Alexandra R. McGoran
Alexandra R. McGoran Shamina Page2
Shamina Page2 Adam Lewis
Adam Lewis Adil Bakir
Adil Bakir Jon Barry
Jon Barry Josie Russell
Josie Russell