There was a mistake in Figures 7, 8 and 9 as published. Figure 7 has two parts (left and right) but only the left part appears on the article. The right part of Figure 7 is displayed as Figure 8 instead of the actual Figure 8 and the actual Figure 8 is displayed as Figure 9 instead of the actual Figure 9. This needs to be corrected without changing figures’ captions. The corrected Figures 7, 8 and 9 appear below.
Figure 7
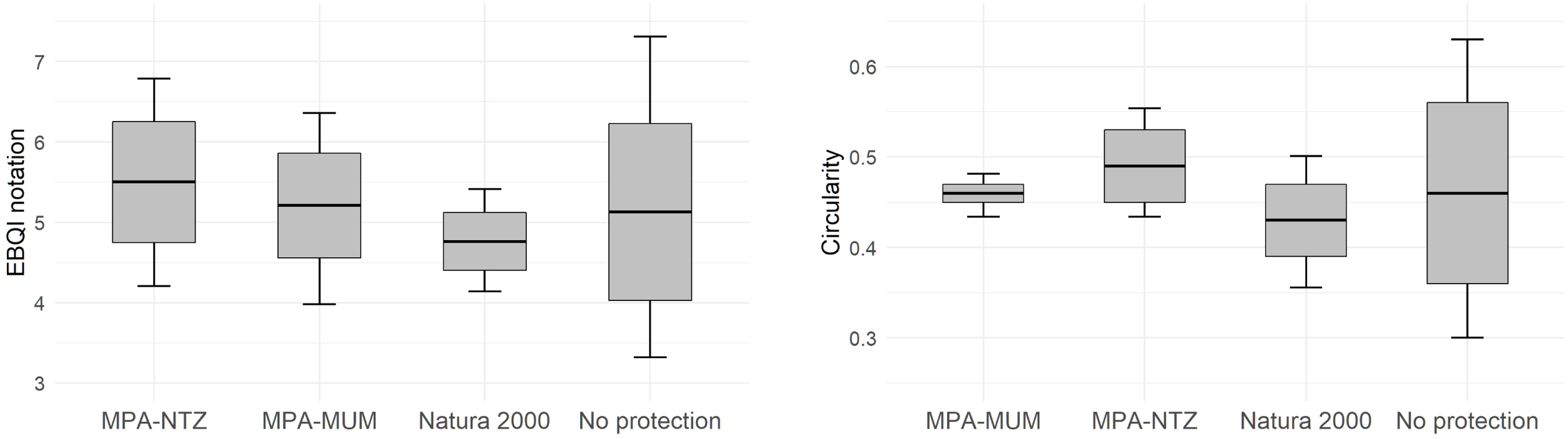
Box plots showing EBQI notation (left) and circularity value (right) according management level. The black crossbar corresponds to the mean EBQI notation, the grey rectangle corresponds to the standard error, the vertical bars correspond to the 95% confidence interval.
Figure 8
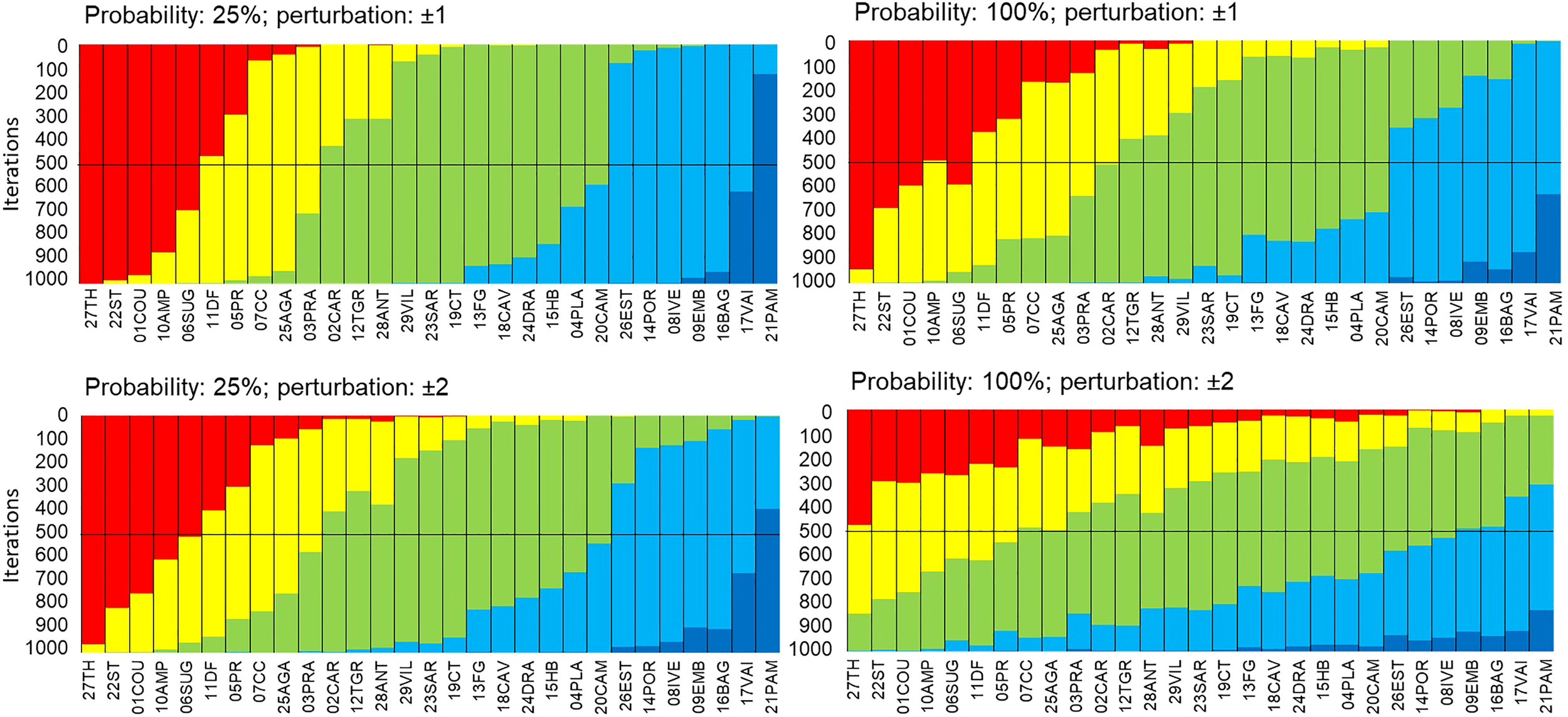
Robustness of the EBQI with regard to the Ecological status per box. Sampling sites (x-axis) are sorted in ascending order according to the EBQI notation from left to right. In order to test the effect of the status per box on the EBQI (robustness), status values have been randomly perturbed (above, ± 1; below, ± 2) 25% (left) and 100% (right) of probability; 1000 iterations were performed. The change of the EBQI notation (Bad through Very Good) of a site, for a given iteration, is shown by the colour of the new class in which it falls). Red: Poor; Yellow: Bad; Green: Intermediate; Blue: Good; Dark Blue: Very Good.
Figure 9
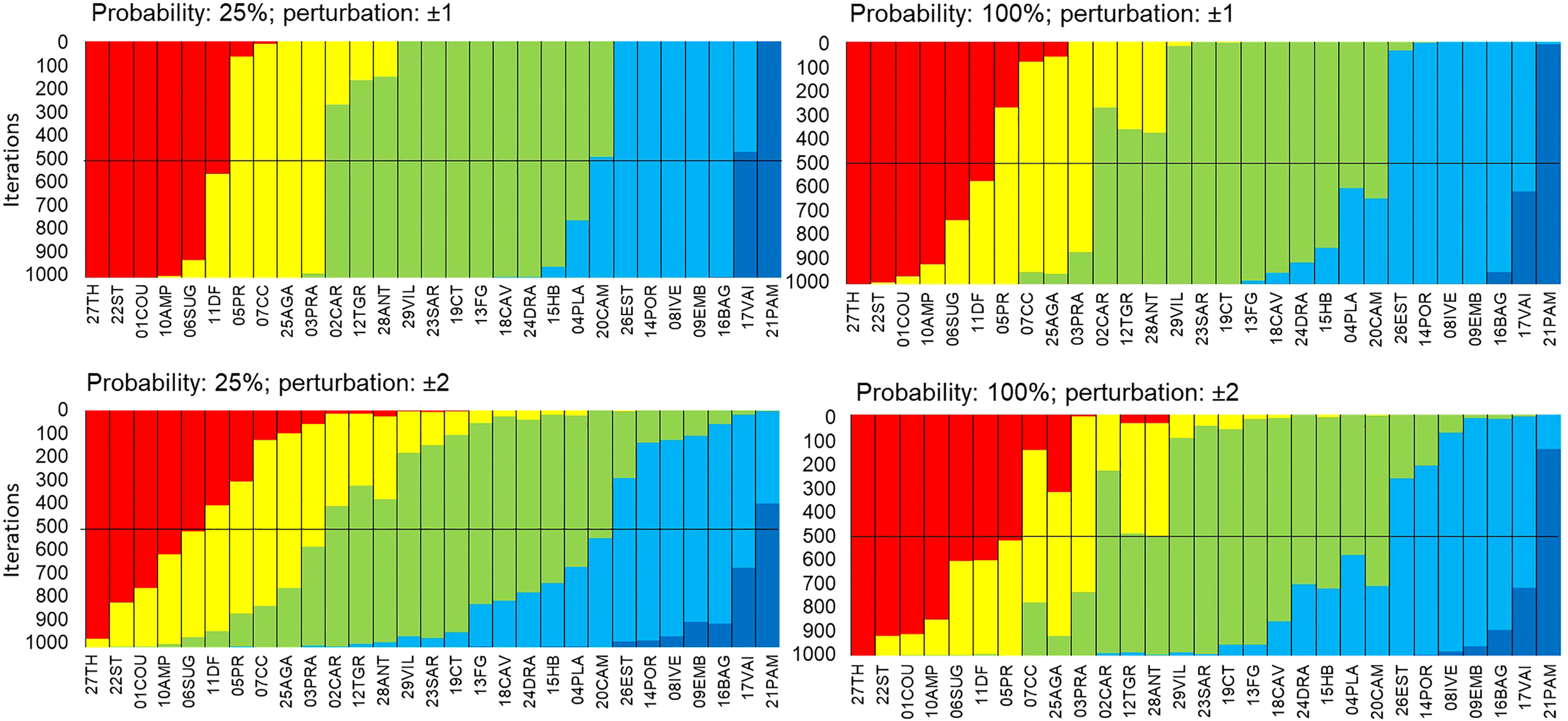
Robustness of the EBQI with regard to the weighting per box. Sampling sites (x-axis) are sorted in ascending order according to the EBQI notation from left to right. In order to test the effect of the weighting per box on the EBQI (robustness), status values have been randomly perturbed (above, ± 1; below, ± 2) 25% (left) and 100% (right) of probability; 1000 iterations were performed. The change of the EBQI notation (Bad through Very Good) of a site, for a given iteration, is shown by the colour of the new class within which it falls. Red: Poor; Yellow: Bad; Green: Intermediate; Blue: Good; Dark Blue: Very Good.
The original version of this article has been updated.
Statements
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Summary
Keywords
coastal detrital bottoms, ecosystem-based approach (EBA), quality assessment, marine habitat, rhodolith beds, epibenthic assemblages
Citation
Astruch P, Orts A, Schohn T, Belloni B, Ballesteros E, Bănaru D, Bianchi CN, Boudouresque C-F, Changeux T, Chevaldonné P, Harmelin J-G, Michez N, Monnier B, Morri C, Thibaut T, Verlaque M and Daniel B (2025) Correction: Ecosystem-based assessment of a widespread Mediterranean marine habitat: The Coastal Detrital Bottoms, with a special focus on epibenthic assemblages. Front. Mar. Sci. 12:1673739. doi: 10.3389/fmars.2025.1673739
Received
26 July 2025
Accepted
30 July 2025
Published
18 August 2025
Approved by
Frontiers Editorial Office, Frontiers Media SA, Switzerland
Volume
12 - 2025
Updates
Copyright
© 2025 Astruch, Orts, Schohn, Belloni, Ballesteros, Bănaru, Bianchi, Boudouresque, Changeux, Chevaldonné, Harmelin, Michez, Monnier, Morri, Thibaut, Verlaque and Daniel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick Astruch, patrick.astruch@univ-amu.fr
†Deceased
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.