- 1Fisheries Technology Institute, Japan Fisheries Research and Education Agency, Hakatajima Filed Station, Imabari, Japan
- 2Fisheries Technology Institute, Japan Fisheries Research and Education Agency, Miyazu Filed Station, Miyazu, Japan
- 3Settlement Resource Division, Ibaraki Prefectural Fisheries Research Institute, Hitachinaka, Japan
- 4Fisheries Resources Institute, Japan Fisheries Research and Education Agency, Shiogama, Japan
- 5Fisheries Resources Institute, Japan Fisheries Research and Education Agency, Yokohama, Japan
Food availability plays a critical role in shaping reproductive success of small pelagic fishes, and in natural population, reduced prey access often arises under conditions of intra- and inter-specific density dependence. While energy allocation to egg production has often been viewed along a capital–income continuum, recent studies suggest that reproductive strategies may be more flexible or mixed than previously assumed. However, few studies have empirically examined how the timing and location of prey availability affect reproductive traits in the context of density-dependence. To address this gap, we conducted stable isotope tracer and controlled feeding experiments using chub mackerel (Scomber japonicus) to determine when and how feeding conditions influence reproductive output. A diet-switch experiment using carbon and nitrogen stable isotope ratios revealed much slower isotopic turnover in chub mackerel compared to income-breeding anchovy in similar experiments, suggesting that chub mackerel primarily rely on stored reserves (capital breeding), with limited reliance on dietary resources during spawning. Food restriction during the five months prior to spawning significantly reduced somatic condition and egg production in repeat spawners. Larvae from food-restricted females exhibited reduced growth and starvation tolerance compared to those from well-fed females. These findings demonstrate that maternal nutritional history has marked effects on both egg production and larval performance, highlighting the importance of considering energy allocation tactics when evaluating density-dependent reproduction. Our findings further provide a basis for discussing recent changes in the reproductive output of the Pacific stock of chub mackerel in the light of maternal nutritional history and its role in reproductive success.
1 Introduction
Understanding the mechanisms that regulate fish population dynamics is fundamental to sustainable fisheries management. Variation in food availability arising from density-dependent processes, particularly driven by competition for prey, plays a significant role in shaping the growth, maturation, and ultimately recruitment success of both marine and freshwater fish (Cowan et al., 2000; Hixon et al., 2014). Compensatory density dependence provides a negative feedback that prevents uncontrolled population growth or collapse, linking life-history traits such as growth, fecundity, and mortality to population density (Rose et al., 2001; Herrando-Pérez et al., 2012). In small pelagic fish species, which often experience large fluctuations in abundance, inter- and intra-specific density-dependent effects on individual traits are frequently observed under conditions of high stock biomass. These effects typically manifest as slowed growth, reduced condition factor, or delayed maturation (Helser and Almeida, 1997; Olafsdottir et al., 2016). These qualitative changes at the individual level can have quantitative consequences for the population through reductions in reproductive output and recruitment. Recently, there has also been increasing attention paid to the maternal effects of how the physiological state and environmental experience of females influence the quantity and quality of their offspring (Green, 2008; Hixon et al., 2014). Variations in maternal size and nutritional condition can alter egg size, fecundity, and larval traits such as growth and starvation tolerance (Marshall et al., 2008; Barneche et al., 2018). Consequently, density-dependent limitations on prey availability influence not only adult traits, but also affect intergenerational processes via changes in reproductive investment and offspring viability.
A key factor linking density-dependent prey limitation and maternal effects is the strategy by which energy is allocated to reproduction. This strategy varies across species along the capital–income breeding continuum (Jönsson, 1997; McBride et al., 2015). Capital breeders accumulate energy reserves before the spawning season and allocate them to egg production regardless of immediate prey availability. In contrast, income breeders rely on concurrent energy intake during reproduction. While these strategies represent idealized endpoints, many marine fish species exhibit flexible or mixed strategies unlike capital breeders such as Atlantic cod and plaice that cease feeding during the spawning season (Stephens et al., 2014; McBride et al., 2015). Despite the ecological and management importance of understanding such strategies, experimental validation remains scarce, especially for species that continue feeding during the spawning period. Most evidence comes from field studies examining body condition or lipid content, which may be influenced by other factors such as migration or routine metabolism (Slotte, 1999). Stable isotope tracer experiments offer a powerful alternative, providing direct quantitative estimates of dietary resource use in reproductive tissues (Hobson, 2006; Warner et al., 2008). However, studies that integrate this approach with controlled feeding manipulation and larval performance assessment are limited.
Chub mackerel (Scomber japonicus) in the western North Pacific migrates widely from subtropical to subarctic regions, and its biomass exhibits cyclical fluctuations driven by climate variability, similar to that observed in Japanese sardine (Takasuka et al., 2008; Yatsu, 2019). The biomass of the Pacific stock of chub mackerel was high during the 1970s (Figure 1), but then declined rapidly and remained below one million tons until 2011, likely due to intense fishing pressures and a series of recruitment failures (Kawai et al., 2002; Yatsu, 2019; Yukami et al., 2025). Following 2011, total biomass increased markedly, accompanied by frequent occurrences of higher levels of recruitment. During this period, the biomass of older spawning individuals (≥ 4 years) and their proportion within the spawning stock biomass also increased, partly because of a significant reduction in fishing effort in the eastern waters of Japan after the Great East Japan Earthquake (Yatsu, 2019; Yukami et al., 2025). The increasing proportion of repeat spawners in spawning stock may have contributed to higher recruitment levels, as experimental evidence showed substantial differences in both egg production and larval viability between first-time and repeat spawners (Yoneda et al., 2022). However, this increasing trend shifted to a declining phase after peaking in 2018, despite the continued high proportion of older spawning individuals. In particular, the egg abundance of chub mackerel has dramatically declined since 2020, reaching levels in 2024 comparable to those observed during the low biomass period of the 2000s. The underlying mechanisms driving this recent decline remain poorly understood.
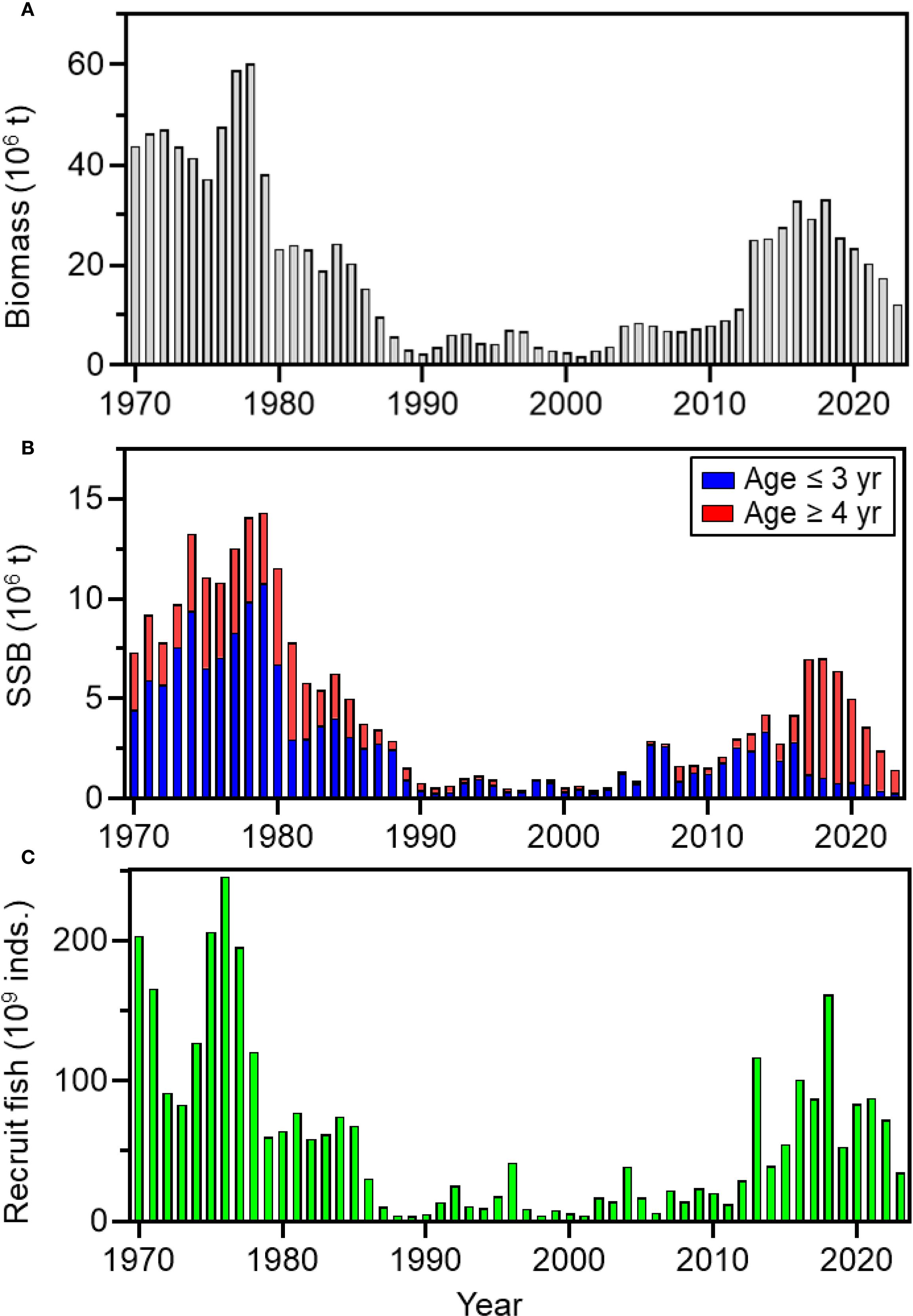
Figure 1. Total biomass (A), spawning stock biomass (SSB, B), and number of recruits (C) of the Pacific stock of chub mackerel during 1970–2023 (after Yukami et al., 2025⁂). SSB (B) is further divided into age ≤3 and ≥4 years. Most females aged ≥4 years are considered repeat spawners that have spawned at least once in their lifetime. ⁂ The estimation method was revised from a cohort-based virtual population analysis (VPA) to a state-space assessment model (SAM), which accounts for observation errors in both abundance indices and catch-at-age data. This change resulted in a revision of past biomass and recruitment estimates (see Yukami et al., 2025).
Previous studies have shown density-dependent effect on growth, body condition and maturation of the Pacific stock of chub mackerel, including interspecific competition with the Japanese sardine, whose biomass has recently increased (Watanabe and Yatsu, 2004, 2006; Watanabe, 2010; Kamimura et al., 2021). As chub mackerel biomass increases, their growth rates and condition factor tend to decline, followed by delayed onset of maturation. Long-term analyses using both fishery-dependent (stock assessment) and independent (egg survey) data further suggest strong intraspecific density dependence in total egg production per spawner or unit weight (Takasuka et al., 2021). These findings imply that the recent decline observed in this stock may reflect compensatory density- dependent response in their life-history traits. Although chub mackerel are considered capital breeders based on observed associations between body condition (e.g. lipid reserves) and reproductive development prior to the spawning season (Murayama et al., 1995; Yoshimitsu et al., 2018; Yatsu et al., 2019), they continue to feed during the spawning period (Usami, 1968). Nevertheless, no empirical studies have addressed how the timing and location of prey availability influence reproductive traits and offspring viability within a density-dependent framework.
This study aims to clarify how feeding experience and maternal spawning history influence reproductive output and larval performance in chub mackerel. We conducted two complementary experiments: a stable isotope tracer (diet-switch) experiment and a controlled feeding experiment to determine how feeding history influences reproductive outcomes. First, a diet-switching experiment using feed with distinct carbon and nitrogen stable isotope ratios was conducted to trace resource use in egg production. Isotopic shifts in eggs after the diet switch were compared between 1-year-old (first-time spawners) and 3-year-old (repeat spawners) individuals. For 1-year-old fish, we also analyzed the isotopic values of muscle and ovarian tissues during the experiment to assess how dietary intake during spawning was allocated to somatic growth and reproduction. Second, a controlled feeding experiment was conducted on 3-year-old repeat spawners for 5 months prior to the spawning season. This period was selected based on the results from the isotope tracer experiment and field observations on seasonal changes in lipid content and ovarian development (Murayama et al., 1995; Yoshimitsu et al., 2018; Yatsu et al., 2019). The experiment aimed to clarify how feeding experience during this period affects egg production, egg quality and larval performance. Finally, we discuss the implications of our findings for understanding recent declines in the Pacific stock of chub mackerel, focusing on the relationships among spawning stock biomass, egg abundance and recruitment under density-dependent conditions.
2 Materials and methods
2.1 Assimilation prior to the diet-switch experiment
The experiment was conducted in 2019 at the Hakatajima Field Station of the Fisheries Technology Institute, Japan Fisheries Research and Education Agency (HFS; formerly National Research Institute of Fisheries and Environment of the Inland Sea). To ensure that the isotope ratios of the diet would be reflected in the body tissues and eggs, hatchery-reared 1-year-old and 3-year-old fish were used. To produce these fishes, chub mackerel were caught from the wild using purse seine in coastal waters off western Japan and reared for 2.5 years in sea pens at a fish farm in Oita Prefecture, western Japan. A total of 80 specimens were transferred into HFS and distributed evenly into two 50 kL concrete tanks (40 fish per tank) circulated with seawater. They were assimilated and reared under natural photoperiod (light duration: 10–14 h) and temperature conditions partially controlled in winter (12–17°C) using a hot water circulation pump system for approximately 6 months prior to the induction of spawning. The methods of spawning induction followed Yoneda et al. (2022). In May, specimens were anesthetized with 2-phenoxyethanol (300 ppm) and then fork length (FL, mm) and body weight (BW, g) were measured to the nearest 1 mm and 1 g. Of these, females with late vitellogenic oocytes were selected after ovarian biopsy using a plastic catheter tube. Males that released milt under gentle abdominal pressure were also selected. In total, 20 specimens (10 males and 10 females) were selected for each of the two 20 kl concrete tanks. All individuals were a single intramuscularly injected with 400 μg/kg BW GnRH agonist (D-Ala6, des-Gly10)-LHRH ethylamide (Sigma-Aldrich, St. Louis, USA) combined with coconut oil. The fish were then returned to the same tanks at 19°C to collect eggs produced. The first spawning was observed 34–36 h post-injection, and subsequent daily spawning occurred between 22.00 and 24.00 h.
Approximately 15000 eggs spawned by the 20 3-year-old females, collected from the two 20 kl tanks, were transferred to duplicate 2 kL tanks. A 14/10-h light/dark cycle was maintained, and the water temperature was maintained at about 20°C using a hot water circulation pump system until 30 days post-hatch. After the eyes of the larvae were pigmented, S-type rotifer (Brachionus plicatilis) complexes cultured with highly unsaturated fatty acid-enriched Chlorella vulgaris (Super Chlorella V12; Chlorella Industry Co., Ltd., Fukuoka, Japan) were provided at 20 rotifers per mL from 2 to 20 days post-hatch. Rotifer density was monitored daily and maintained by adding new rotifers as needed. Artemia nauplii enriched with highly unsaturated fatty acids (Super Capsule Powder; Chlorella Industry Co., Ltd, Fukuoka, Japan) were provided from 7 to 25 days post-hatch at a density of 0.2–1.5 artemia ml-1. Commercial dry pellets with higher isotopic levels (δ13C = -20.5, δ15N = 10.5, Otohime EP; Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan) were provided at 0.5–1.5% BW per day from age 20 days. After age 25 days, the specimens were fed exclusively with the commercial dry pellets until the onset of experiment (assimilation period: about 11 months (June 2018 – April 2019) for 1-year-old fish; about 35 months (June 2016 – April 2019) for 3-year-old fish). During the assimilation period, specimens were reared in 20 or 50 kL concrete tanks under a natural photoperiod (light duration: 10–14h) and temperature conditions partially controlled in winter (12–26°C). Fish were fed commercial dry pellets at 1.5% of body weight daily, both before and during the experiment, to maintain consistent feeding conditions.
2.2 Diet-switch experiment and sampling procedure
In this experiment, we aimed to trace the relative contributions of stored versus dietary resources to egg production. In this context, capital breeding refers to reproductive strategies in which fish rely primarily on stored energy reserves for oocyte development, resulting in limited isotopic shifts in eggs following a diet change. By contrast, income breeding refers to strategies in which fish depend largely on concurrent food intake, leading to rapid isotopic turnover in reproductive tissues after a diet switch.
Spawning induction procedures followed those described above. In May 2019 fish were anesthetized, and their gonadal condition was assessed along with measurements of FL and BW. A total of 72 1-year-old individuals (36 females and 36 males) and 24 3-year-old individuals (12 females and 12 males) were selected and transferred to 20 kL concrete tanks maintained at a controlled temperature (19°C). The 3-year-old specimens were kept in a single tank while 1-year-old fish were distributed equally across two duplicate tanks (36 individuals per tank). Following spawning induction, fish began spawning on the night of the following day. Eggs were collected daily for 4 consecutive days (days 1–4) and their mean isotopic values were used as the initial baseline (day 0). On day 5 after induction, the diet-switch experiment was initiated by replacing the commercial dry pellets with a new feed of lower isotopic values (δ¹³C = –24.7, δ15N = 5.6, Mojako GF; FEED ONE Co., Ltd., Yokohama, Japan). Fish were fed daily at 1.5% BW with the new pellets for 40 days until the end of the experiment (i.e. 45 days after induction). Both 1- and 3-year-old groups experienced the same experimental duration. During the experiment, 6 females from the 1-year-old group and 2 females from the 3-year-old group died, resulting in a final sample size of 88 individuals (age 1 year n = 66; age 3 year n = 22) used for subsequent analyses. Mortality in each tank was recorded daily, and the feeding rate was adjusted as needed. To examine isotopic changes in eggs, daily collections of eggs were conducted until the end of experiment, when available. Collected egg samples were stored in plastic tubes and frozen at - 20°C until stable isotope analysis. The total number of eggs spawned in each tank was also recorded. The number of eggs collected decreased as the experiment progressed, and after day 30, days with little or no egg collection became increasingly frequent (Supplementary Figure S1). Therefore, egg monitoring was terminated on day 37 after the diet switch, and the experiment ended on day 40.
To examine the relationship between growth and reproduction during the first spawning season, 1-year-old specimens were sacrificed with 2-phenoxyetahol at three sampling periods: before the diet switch, 7 days after the switch and at the end of the experiment (40 days). Among these, only females with late vitellogenic or hydrated oocytes following spawning induction were used in the analysis. At the initial sampling, 10 fish (6 females and 4 males) were sacrificed, of which 6 females were analyzed. At day 7, 10 fish (6 females and 4 males) were sampled, of which 4 females were analyzed; the remaining 2 females were immature and excluded. At the final sampling (day 40), 46 fish (18 females and 28 males) were examined, of which 13 females were used in the analysis, while 5 females were excluded as immature. In total, 23 females were analyzed across the three sampling periods. For each female with late vitellogenic or hydrated oocytes that was used in the analysis, FL and BW were measured, and ovarian weight (OW, g) was recorded to the nearest 0.1g. Somatic weight (SW, g) was calculated by subtracting OW from BW. Muscle and ovarian tissue samples were collected and frozen in plastic tubes at - 20°C until the analysis. The condition factor (CF) was calculated using the following equation:
2.3 Stable isotope analysis
After thawing the eggs, muscle and gonads at room temperature, the samples were rinsed with distilled water. For each sampling event, 300–500 eggs were collected, from which 200 eggs were counted and pooled to constitute one analytical sample. A total of 40 egg samples were analyzed from the two tanks with 1-year-old fish, and 20 egg samples were analyzed from the tank with 3-year-old fish. The initial isotopic value (day 0) was calculated as the mean of egg samples collected from the first to fourth spawnings prior to the diet switch. Each day’s sample from each tank was treated as an independent replicate, and their mean was used to represent the initial isotopic value. Thereafter egg samples collected every other day up to day 31 were analyzed. All samples were freeze-dried for 48 h using a lyophilizer (FDU-1110, EYELA, Tokyo, Japan) and then ground into a fine powder with a mortar and pestle. Approximately 0.5–2.0 mg of the powdered material was placed into tin capsules. Lipids were not removed to assess the full complement of nutrients, as this study focused on the dynamics of stable isotope turnover. The δ13C and δ15N values of egg samples were measured using a stable isotope mass spectrometer (Delta V Advantage, Thermo Fisher Scientific, Inc., USA) in continuous-flow mode, equipped with an elemental analyzer Flash 2000, Thermo Fisher Scientific Inc, USA). The stable isotope ratios were expressed as per mil (‰) deviations from the standard as defined by the following equation:
where R is the 13C/12C or 15N/14N ratio. PeeDee Belemnite and atmospheric N2 were used as the standards for C and N, respectively. Data were corrected using multiple internal standards calibrated against international standards (Tayasu et al., 2011). Between each pair of standards, 8–10 samples were analyzed. The analytical errors (1σ) for δ¹³C and δ15N measurements were within ±0.1‰ and ±0.2‰, respectively. Ambient baseline isotope values were not measured because the experiment was conducted in tanks supplied with filtered seawater, where both age groups were maintained simultaneously under identical rearing conditions. Therefore, the diet was considered as the isotopic baseline, and observed changes in eggs primarily reflect dietary incorporation and metabolic processes.
2.4 Feeding experiment and sampling procedure
The feeding experiment was conducted from December 2020 to May 2021. The feeding manipulation began in December, based on evidence from the isotope tracer experiment showing reliance on stored energy for reproduction, and field data indicating that lipid depletion begins approximately one month before vitellogenesis (Murayama et al., 1995; Yoshimitsu et al., 2018). Specimens reared for 2.5 years in sea pens at a fish farm were transferred into HFS in November 2020. Fish were kept into 50 kL concrete tanks at ambient temperature and fed commercial dry pellets (Otohime EP) at approximately 1.5% of BW daily before the onset of the experiment. After body condition measurements of sub-sampled individuals, 32 specimens were randomly distributed into each of the two 20 kl concrete tanks for the high-food treatment, and 36 and 37 specimens were allocated to the two tanks for the low-food treatment. At the beginning of the experiment, gonads were undeveloped, and sex ratios were not determined. At the end of the experiment, including individuals that died during the period, the observed sex rations (male: female) were approximately 1: 1 in all tanks (high food: 16: 16 and 17: 15; low food: 18: 18 and 20: 17). Specimens were then subjected to one of the two feeding regimes: high (1.5% BW/day) or low (0.5% BW/day) food availability. These feeding levels were selected to mimic ecologically realistic variation in prey availability associated with intraspecific density-dependent effects, as natural stock biomass of chub mackerel has fluctuated more than threefold between low and high phases over recent decades (Yukami et al., 2025). Each feeding regime was examined in duplicate, and fish were reared under a natural photoperiod (light duration: 10–14 h) and partially controlled temperature conditions (12–18°C). Mortality in each tank was recorded daily, and the feeding rate was adjusted as needed.
In May 2021, all specimens were anesthetized, and spawning was induced following the methods described above. At the onset of spawning induction, the number of surviving fish in each tank was as follows: high-food treatment, 32 (16 males, 16 females) and 31 (17 males, 14 female) specimens (one female had died before the spawning induction); low-food treatment, 36 (16 males, 16 females) and 37 (20 males, 17 females) specimens. The specimens were returned to the same tanks, which were maintained at a controlled temperature (19°C). Eggs were collected daily from each tank, with subsamples fixed in 10% neutral seawater formalin for measurement of egg size and frozen at - 20°C in plastic tubes for energy content analysis. The total number of eggs spawned in each tank was also recorded. The estimated number of eggs spawned per female was calculated by dividing the total number of eggs by the number of females in each tank. After 10 days of spawning monitoring, the experiment was terminated. The numbers of surviving fish were as follows: high-food treatment, 30 (16 males, 14 females) and 29 (17 males, 12 females); low-food treatment, 36 (20 males, 16 females) and 35 (18 males, 17 females). From these survivors, a subset of 11 specimens per tank (high-food: 15 males and 7 females in total; low-food: 10 males and 12 females in total) were randomly selected, sacrificed with 2-phenoxyetahol, and measured for FL, BW and GW. The remaining specimens were retained and used in subsequent experiments. A total of 60 eggs randomly selected in each spawning day was measured in diameter and their oil globules were also measured to the nearest 0.001 mm.
To compare the energy content of eggs between high and low food treatments, the concentrations of carbon and nitrogen per egg were determined. Eggs were thawed at room temperature (20°C) and rinsed with distilled water. A total of 300–500 eggs per tank was collected, from which 200 eggs were counted and pooled as a single sample per tank. Thus, for each spawning day, two replicate samples were obtained per treatment (one from each tank). These pooled samples were then dried at 60°C in microtubes for 3–4 days. The dried egg weight was taken as the average dry weight of a single egg. The samples were ground into fine powder and placed in tin capsules. Lipids were retained to assess the total energy in the eggs. The concentrations of carbon and nitrogen were measured using an elemental analyzer (Flash EA1112, Thermo Fisher Scientific Co., Ltd. MA, USA). The total carbon (TC) and nitrogen (TN) per egg were estimated by multiplying their respective concentrations by the dry egg weight. Because marine fish eggs are composed primarily of lipids and proteins (Kamler, 2008; Lubzens et al., 2010), the carbon and nitrogen contents were used as proxies for lipid and protein content, respectively. Energy content per egg (J) was estimated by applying energy equivalents of 39.5 J/mg for lipids and 23.6 J/mg for proteins (Lambert and Dutil, 1997), according to the following equation:
Reproductive effort per spawning was calculated as the product of egg energy (estimated for each spawning day) and the number of eggs spawned per female.
To examine the effect of parental feeding treatment on starvation tolerance of larvae, approximately 1000 eggs were randomly selected and pooled from replicate tanks on the day of first spawning and incubated in a 30 L tank at 20°C until hatching. A total of 75–90 hatchlings per beaker originating from each parental treatment (high vs. low; two replicate beakers per treatment) were transferred into 1 L glass beakers at 20°C and maintained without exogenous food. The number of dead larvae in each glass beaker was counted at intervals of 4–18 h, and the experiment was terminated 5–6 days after hatching, when all the larvae were dead.
To examine the effects of parental feeding treatment on early growth rates, approximately 3000 eggs were randomly selected from pooled samples of replicate tanks per treatment on the first and second spawning days. Duplicate subsamples from each spawning day (two replicate tanks per treatment per day) were established, resulting in four replicate 1 kL tanks per treatment (total of 8 tanks across two days). These subsamples ensured sufficient sample size and accounted for daily variability in potential larval traits associated with egg size variability (Yoneda et al., 2022). A 14/10-h light/dark cycle was maintained, and the water temperature was kept at 20°C throughout the experiment. After the eyes of the larvae were pigmented, S-type rotifer complexes cultured with highly unsaturated fatty acid-enriched C. vulgaris were provided at 20 rotifers per mL from 2 days post-hatch until the end of the experiment. Rotifer density was monitored daily and maintained by adding new rotifers as needed. First feeding was observed at 3 days post-hatch. Artemia nauplii enriched with highly unsaturated fatty acids were provided from 7 days post-hatch until end of the experiment at a density of 0.2 artemia ml-1. The experiment was terminated at 14 days post-hatch. Subsamples of larvae from each tank were randomly selected and sacrificed with 2-phenoxyetahol at three sampling periods: 3 days (first feeding), 7 days (introduction of Artemia), and 14 days (end of the experiment). The larvae were preserved in 10% neutral sea water formalin, and SL of individual fish was measured to the nearest 0.1 mm. Growth rate (GR) was calculated separately for each tank during two intervals: days 3–7 and days 7–14. These were defined as:
where the average standard length at each initial day was calculated per tank.
2.5 Statistical analysis
Statistical analyses were performed using R version 4.1.2 (R Core Team, 2021). To compare the biological traits across sampling periods, age classes, and feeding treatments, we applied linear mixed-effects models (LMEMs) fitted by restricted maximum likelihood. Candidate models were constructed with different combinations of explanatory variables and, where appropriate, with and without natural logarithmic transformation. The conditional Akaike Information Criterion (cAIC; Vaida and Blanchard, 2005) was primarily used to evaluate relative model fit, while the small-sample corrected AIC (AICc) was also calculated to confirm consistency of model selection. Among these candidate models, the model with the lowest cAIC (and supported by AICc) was selected as the final model (Burnham and Anderson, 2002). If random effects were not supported by cAIC/AICc comparisons, a generalized linear model (GLM) was used instead. The significance of the fixed effects was tested using the R package of lmerTest, Satterthwaite approximation (Kuznetsova et al., 2017).
To compare changes in the δ13C and δ15N of eggs spawned between 1-year-old and 3-year-old individuals over the experiment period, a LMEM was fitted to the data on the day of the experiment. Due to the decrease in egg production described above, egg isotopic data were used up to day 31. The effect of income resources on egg production in terms of the capital–income breeding continuum was estimated (Tanaka et al., 2016); the degree of dependence on income resources (DI) was estimated using the following equation:
where δXday is a predicted value of eggs calculated from a LMEM, and δH and δL are the values of high and low isotopic values that dry pellets provide. It is assumed that DI would be close to 1.0 if egg production during the experiment depended mostly on income resources, reflecting the isotopic values of diet switch, whereas DI would be close to 0 if it depended largely on capital resources (no significant changes in the isotopic values of eggs independent of diet switch). To model the dynamics of DI over time, a LMEM with a source of isotopic DI as random effect was applied.
To examine the relationship between growth and reproduction during the first spawning season, SW, CF and isotope values of muscle and ovarian tissues were compared among the three sampling days using LMEM with experimental tank included as a random effect. Sampling day was treated as a 3-level categorical variable (day 0, 7, and 40), due to two main considerations: the sampling intervals were irregular, and the observed trait values exhibited relatively small or non-linear changes over time. Because the random effect was not retained in any of the models, GLMs were used instead. For stable isotope analysis, due to potential lipid-related bias in δ¹³C values (Logan et al., 2008), especially in muscle tissues with rich lipid content, only nitrogen isotope data (δ15N) were used for the analysis. DI was also calculated based on the model-estimated δ15N values at day 0 and day 40 following the methods described above.
LMEMs were used to compare FL and CF of the parents, as well as egg size and estimated energy content, between high and low food treatments, with experimental tank included as a random effect. For FL and CF, sampling period was included as a 3-level categorical variable (before the experiment, end of experiment under high food, and end of experiment under low food). For egg traits, food treatment was treated as a 2-level categorical variable (high vs. low food).
To examine the effect of parental feeding treatment on the starvation tolerance of hatched larval fish, GLM with a binomial error distribution and a logit link function was applied. Explanatory variables included elapsed time (standardized) and feeding treatment (2-levels), as well as their interaction. Initially, we considered a generalized linear mixed model (GLMM; performed using the R package of glmmML, Broström and Holmberg, 2011) including beaker identity as a random effect. However, the variance explained by the random effect was negligible and resulted in singular fits. Therefore, we simplified the analysis by adopting a GLM without random effects. Model selection was based on AICc, and the final model was chosen to maximize both parsimony and numerical stability.
The effects of parental feeding treatment on SL and GRs of larvae were examined using LMEM. The initial full models included the interaction between sampling period and food treatment as fixed effects, with tank identity as a random effect. Following model selection procedures described above, the interaction term was not retained in the final models. In addition, for SL, sampling day (Day, 3, 7 and 14 days) was treated as a continuous variable, whereas for GR sampling period was treated as a 2-level categorical variable (days 3–7 and days 7-14).
For all analyses, we confirmed whether alternative models within ΔcAIC (or ΔAICc) < 2 existed; when such models were present, they yielded consistent interpretations with the selected best model (see Supplementary Table S1).
3 Results
3.1 Diet-switch experiment
The δ¹³C and δ15N values of eggs from 1- and 3-year old individuals gradually decreased over the course of the experiment (Figure 2). There were no significant differences in the regression slopes of isotopic values over the time between the two age classes (Table 1). The estimated DI in eggs at 31 days after the diet-switch was 0.47 for both age classes.
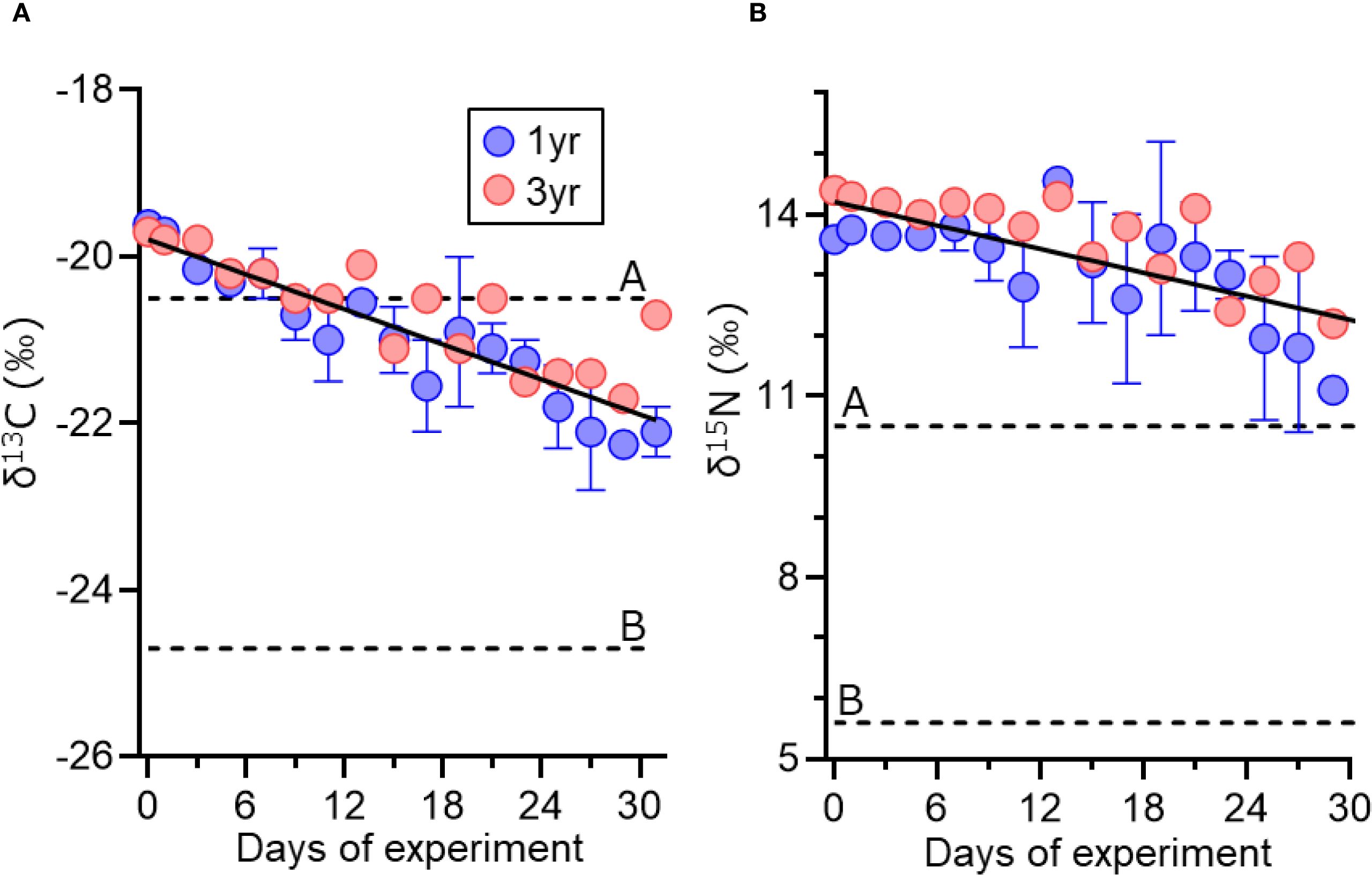
Figure 2. Change in δ13C (A) and δ15N (B) values (‰) of eggs of 1- and 3-year-old chub mackerels during the experiment (days after prey change). δ13C and δ15N values of prey before (dotted line A) and after (dotted line B) are also presented. Predicted lines using the linear mixed effect model fitted to observed data are shown. Data are shown as mean ± SE.
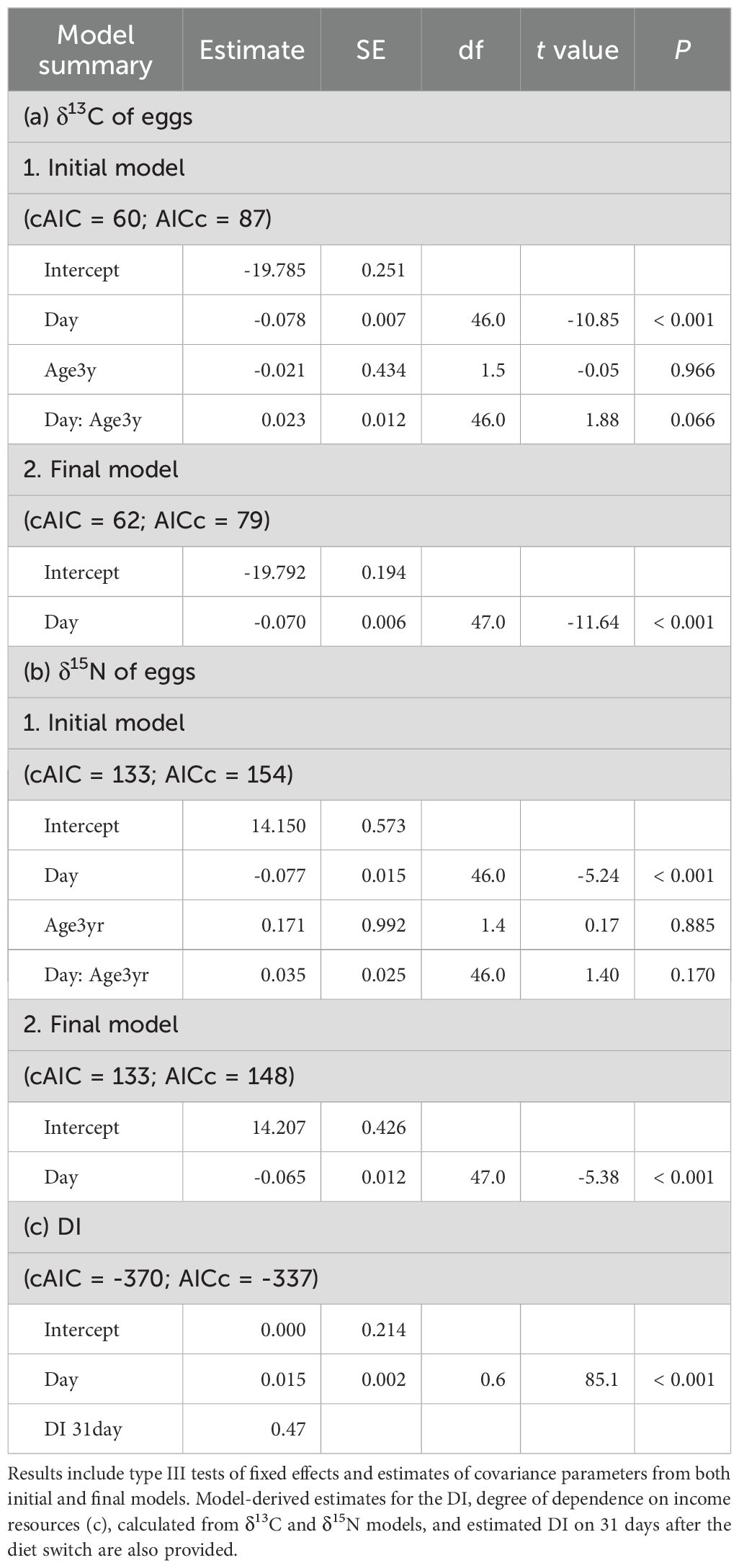
Table 1. Summary of linear mixed effects model estimates for the effect of experimental days (Day), age group (1-year-old vs. 3-year-old), and their interaction on δ13C (a) and δ15N (b) values in eggs of chub mackerel.
For 1-year-old fish, significant differences in SW and CF were observed across the three sampling periods (Figures 3A, B, Table 2): SW before the experiment was significantly higher than that at days 7 and 40 while CF at day 40 was significantly lower than before experiment and at day 7. The δ15N values of muscle and ovary tissues at day 40 were significantly lower than those before experiment and at day 7 (Figures 3C, D). However, the estimated DI of muscle was only 0.07 whereas that of ovaries was 0.54 (Table 2).
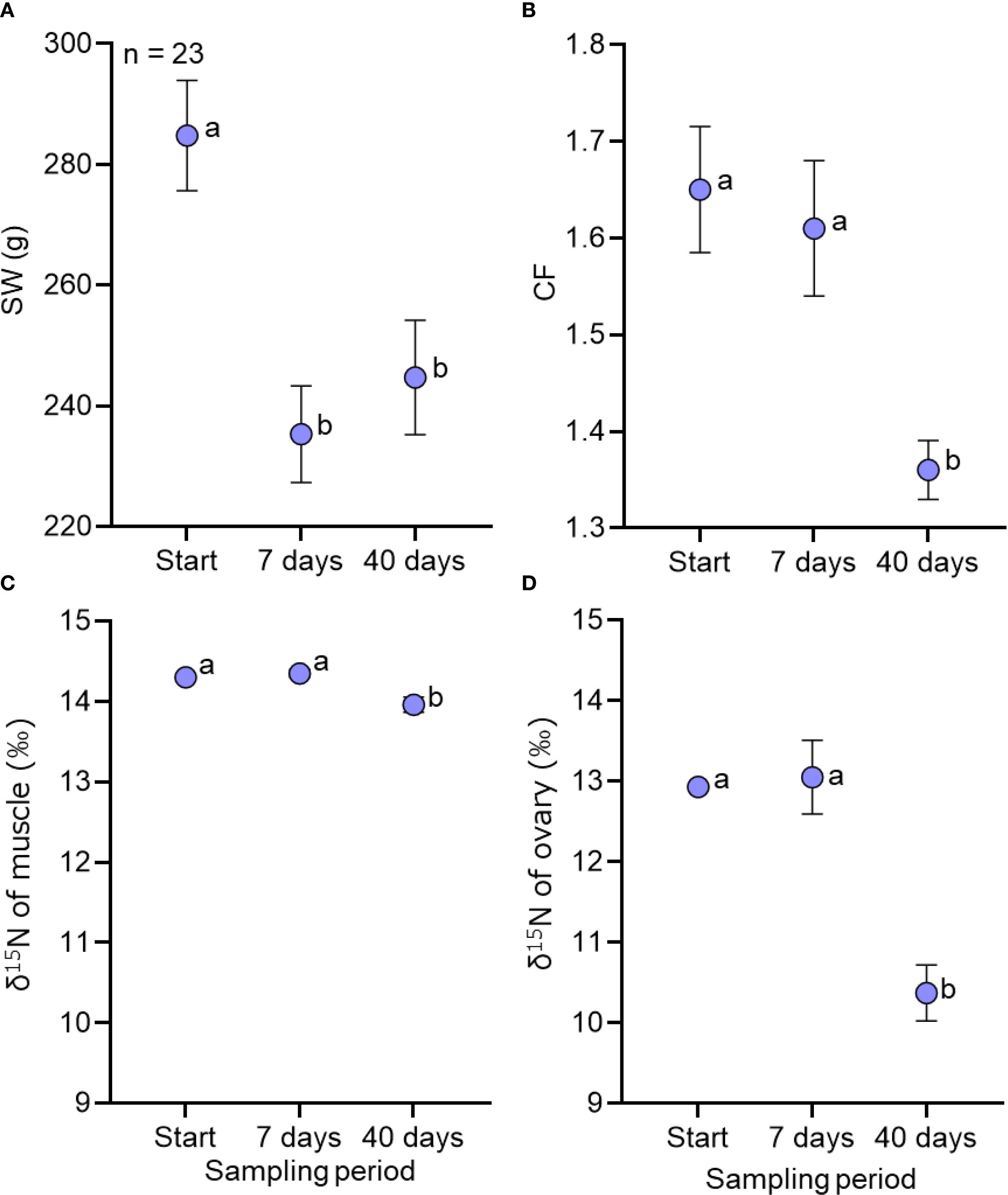
Figure 3. Changes in somatic weight (A), condition factor (B) and δ15N values (‰) of muscles (C) and ovary (D) of 1-year-old female chub mackerel at different sampling periods: before experiment (Start) and 7 and 40 (end of experiment) days after diet-switch). Different letters indicate different sampling days in the generalized linear model (p < 0.05). Data are shown as mean ± SE.
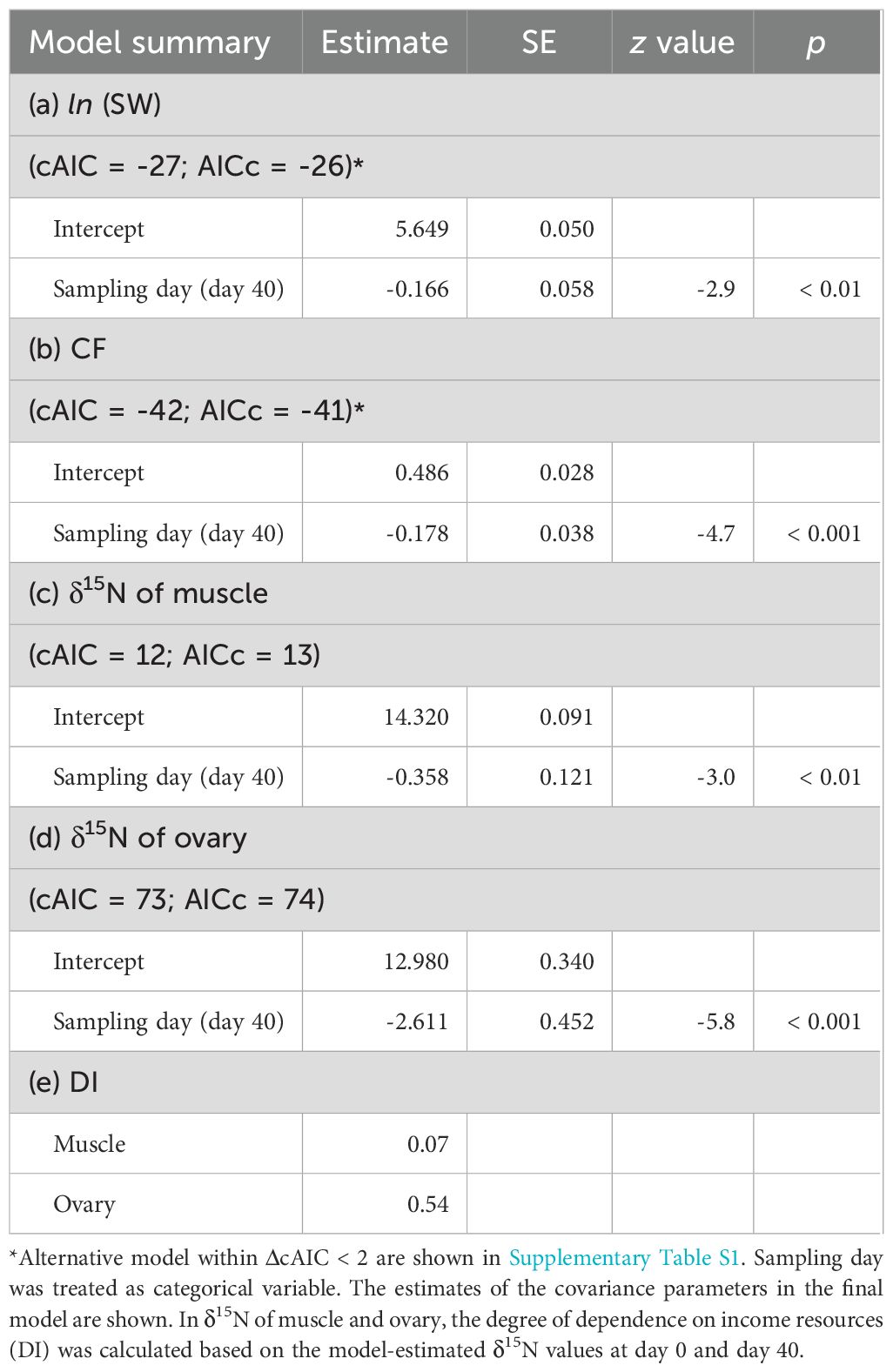
Table 2. Summary of generalized linear model estimates for the somatic weight (SW), condition factor (CF) and δ15N of muscle and ovary of one-year-old female chub mackerel at three sampling periods (before experiment, days 7 and 40 (end of experiment) after the diet-switch).
3.2 Feeding experiment
A significant difference in FL across sampling periods indicated that specimens grew over the experiment. At the end of experiment, FL was significantly higher in the high food treatment than in the low food treatment. (Figure 4A, Table 3). CF also differed significantly across sampling periods (Figure 4B): CF increased during the experiment in the high food treatment, whereas it decreased in the low food treatment.
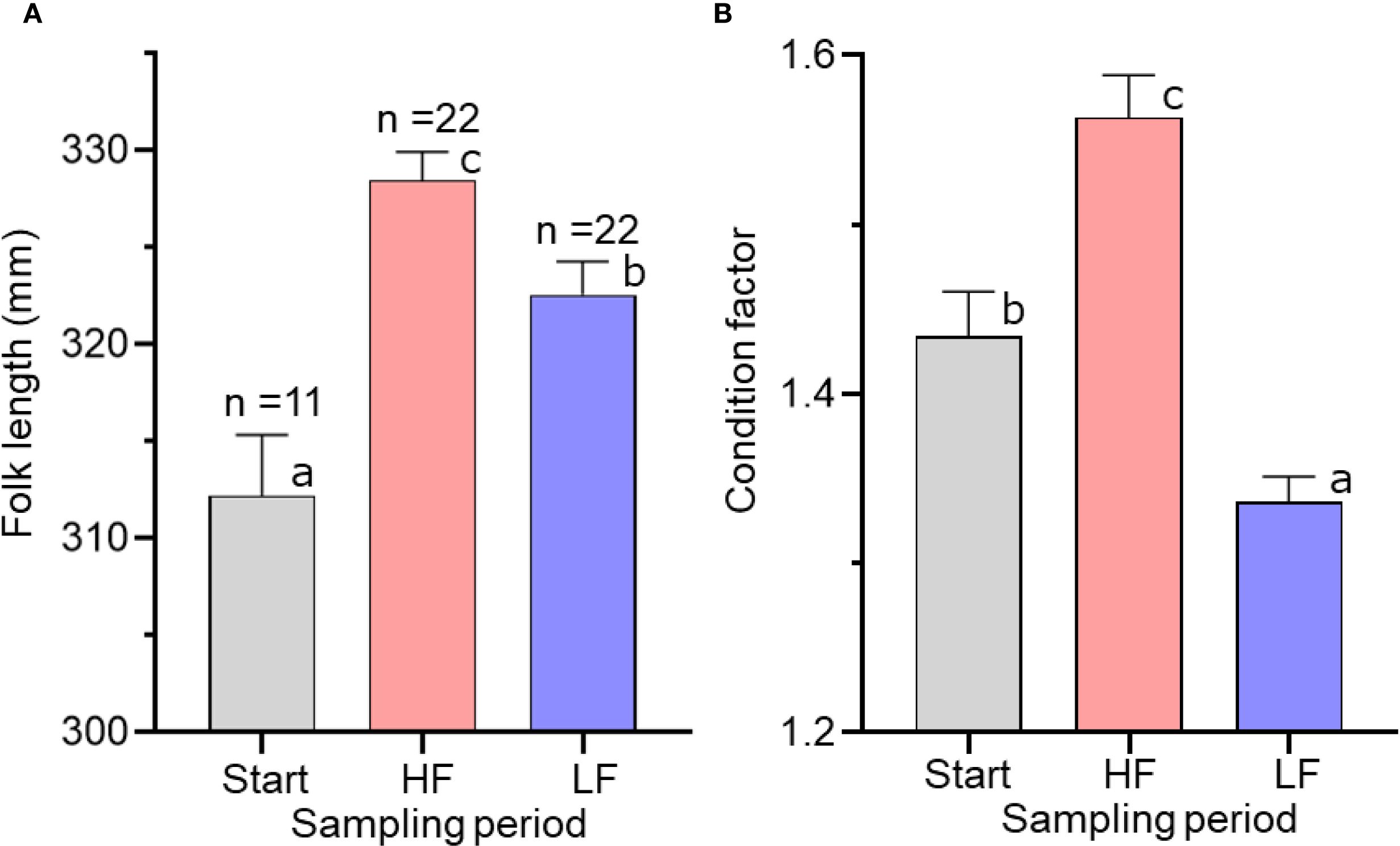
Figure 4. Mean fork length (A) and condition factor (B) of chub mackerel at three sampling periods: before the experiment (Start), and after high (HF) and low (LF) feeding treatments. Numbers indicate the number of fish examined. Different letters indicate different sampling days in the generalized linear model (a < b < c; p < 0.01). Data are shown as mean ± SE.
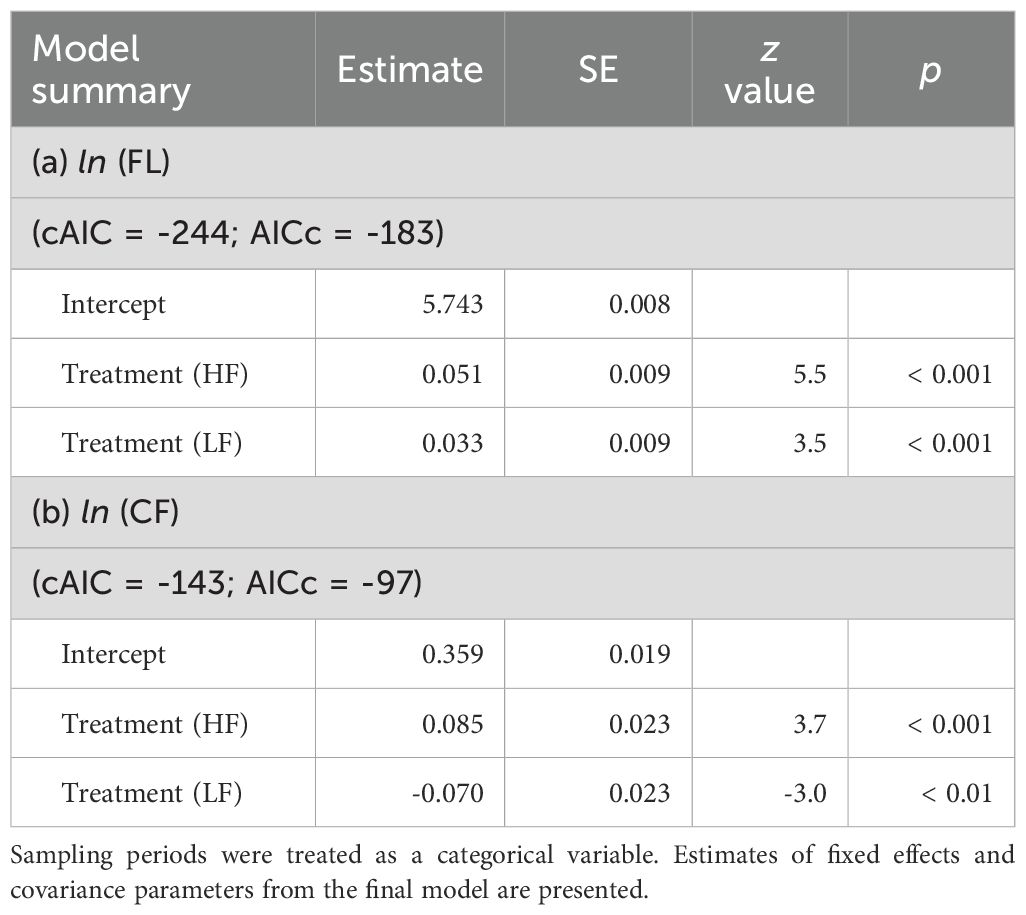
Table 3. Summary of generalized linear model estimates for the fork length (FL) and condition factor (CF) of chub mackerel at three sampling periods: before the experiment (Start), and after low (LF) and high (HF) food treatments.
The diameters of eggs and oil globules were significantly larger in the high food treatment than in the low food treatment (Figures 5A, B, Table 4). There were no significant differences in the concentrations of carbon and nitrogen of eggs between the two food treatments. However, estimated egg energy was significantly higher in the high food treatment (Figure 5C): the GLM indicated that egg energy in the low food treatment was approximately 84% of that in the high food treatment. A significant difference was also observed in the estimated number of eggs spawned per female between the treatments. The LMEM indicated that egg production in the low food treatment was approximately 38% of that in the high food treatment. The estimated reproductive effort per spawning decreased by 32% under low food conditions.
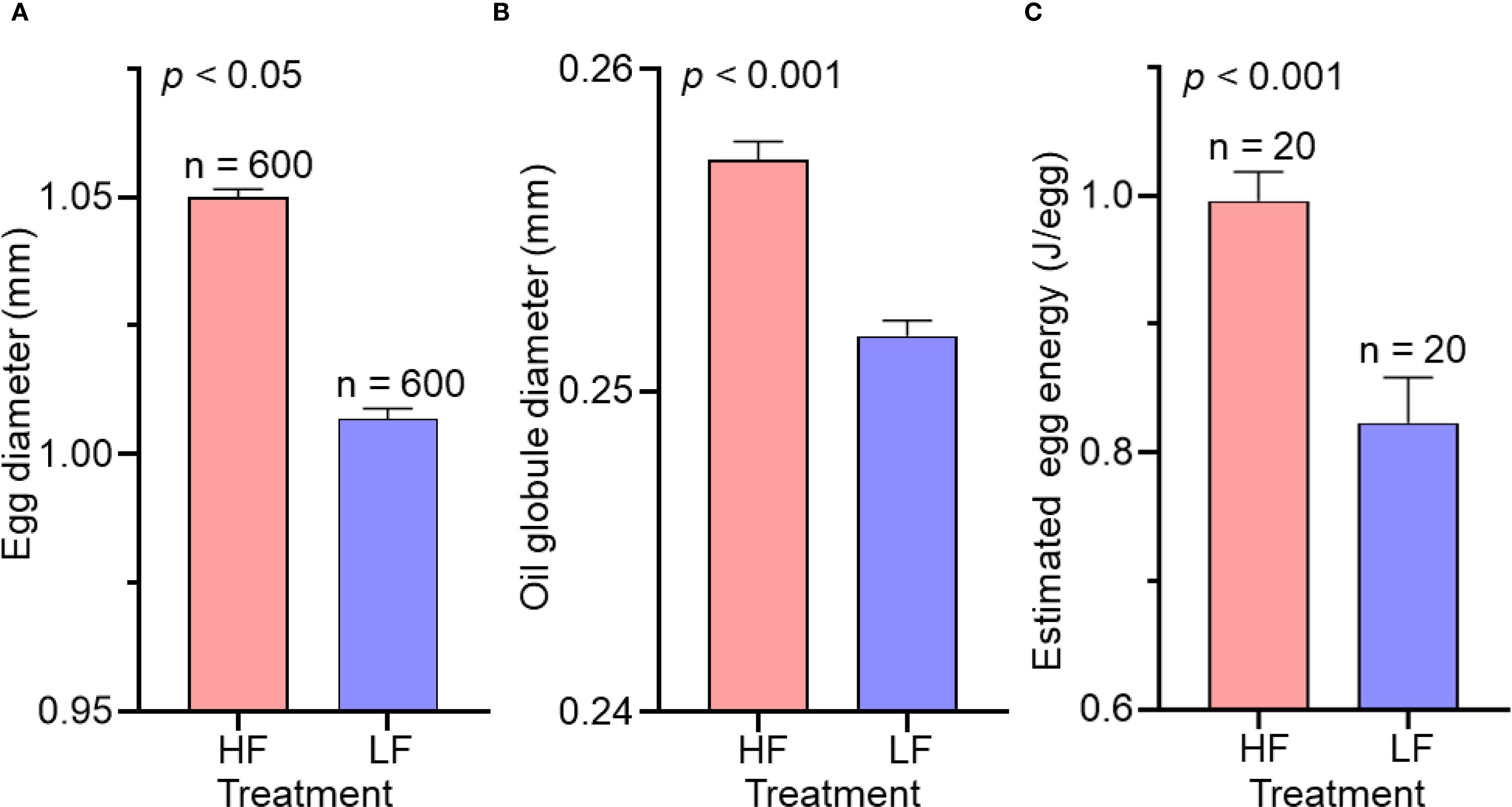
Figure 5. Mean diameters of eggs (A), oil globule (B) and estimated egg energy (C) at high (HF) and low (LF) feeding treatments. Numbers indicate the total numbers of eggs examined (A, B) and trials (c; each trial includes 200 eggs analyzed). Result of comparison of value between the two treatments using a linear mixed effect model is also shown. Data are shown as mean ± SE.
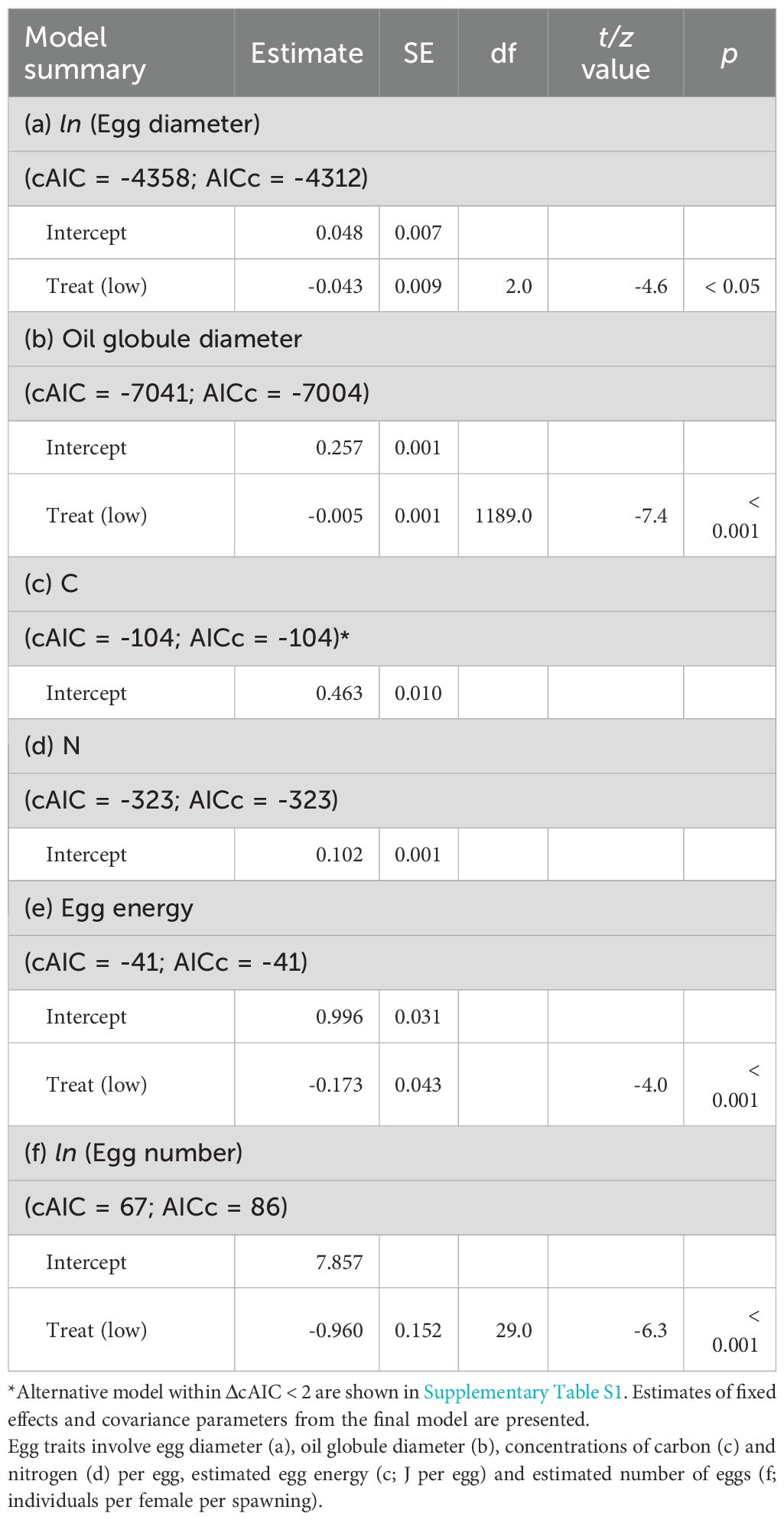
Table 4. Summary for linear mixed effects model estimates or generalized linear model estimates for the effects of food treatment on egg traits of chub mackerel.
Parental feeding treatment had a significant effect on larval survival under starvation from hatching (Figure 6; Table 5). The time to 50% survival was significantly shorter for larvae from low food treatment (78.3 hours) than for those from high food treatment (91.7 hours). Larve grew over the experiment, but there was a significant interaction between sampling period and food treatment on SL, indicating that differences in SL between treatments increased as the experiment proceeded (Figure 7A; Table 6). Growth rates also differed significantly between sampling periods and parental feeding treatments (Figure 7B). The estimated growth rates in the low food treatment were approximately 76% (days 3-7) and 80% (days 7-14) of those in the high food treatment.
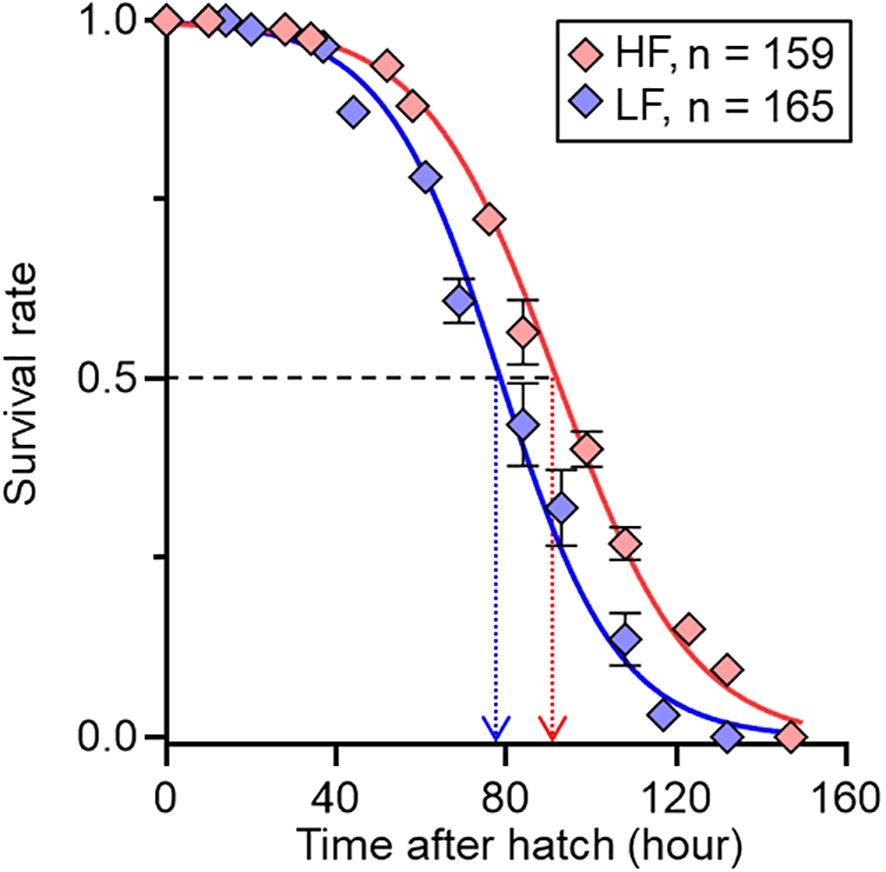
Figure 6. Relationship between survival rate and time after hatch of chub mackerel larvae at parental high (HF) and low (LF) feeding treatments. Estimated times at which the predicted survival rate reaches 0.5 are shown by dotted arrow lines (HF = 91.7 hours; LF = 78.3 hours). Numbers indicate the total number of fish examined. Data are shown as mean ± SE.
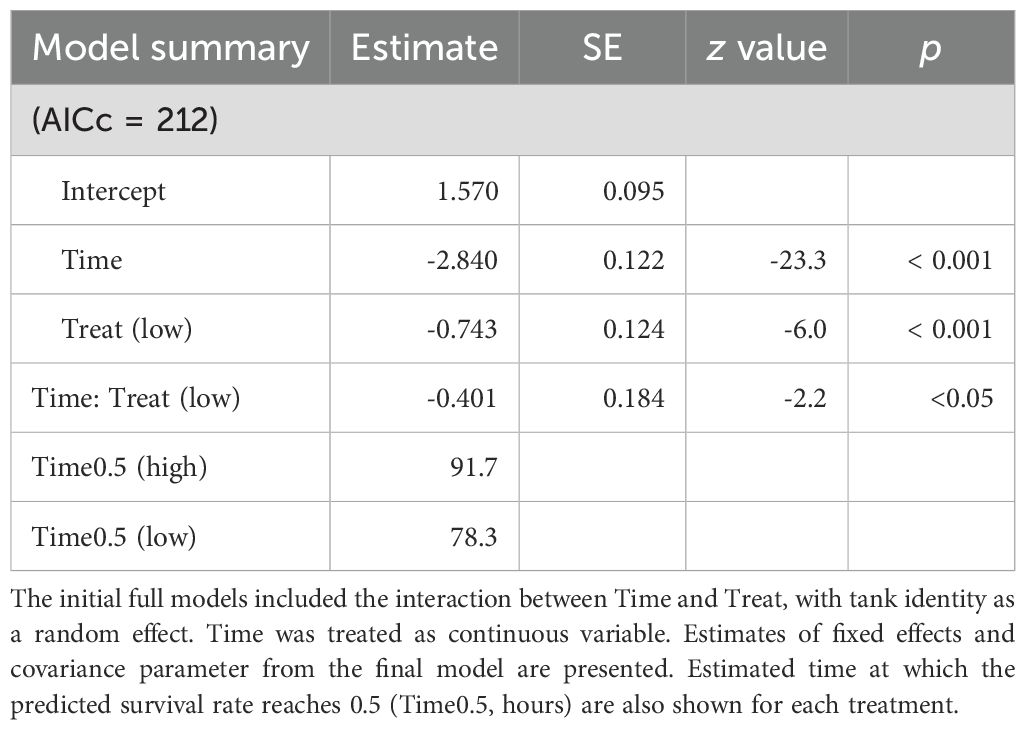
Table 5. Summary for generalized linear model estimates for the effect of time after hatch (Time) and parental food treatment (Treat: low vs high) on the survival of chub mackerel larvae.
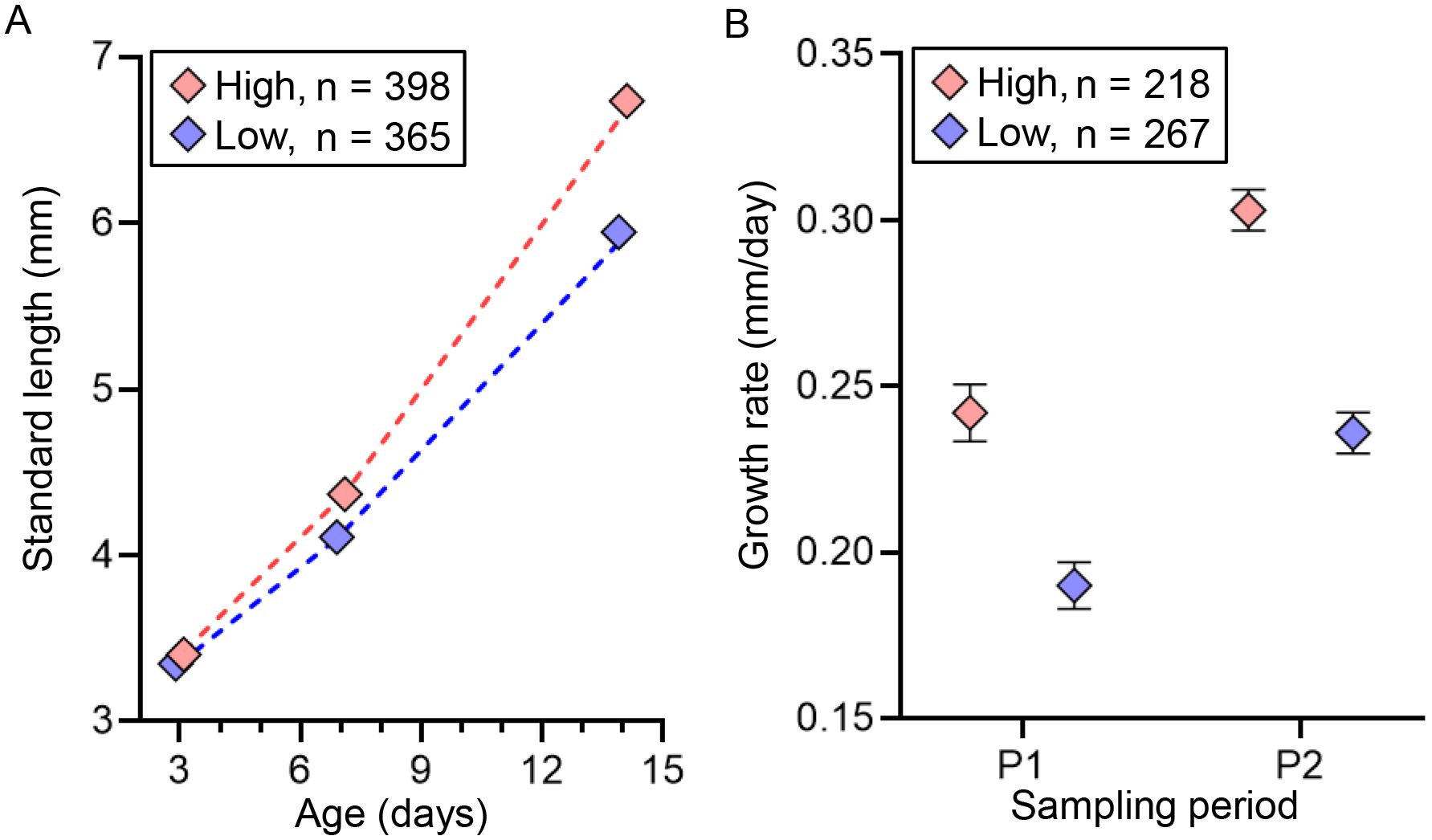
Figure 7. Effects of parental feeding treatment (high vs. low) on the standard length (SL, A) and growth rate (GR, B) of chub mackerel larvae during the experiment. Numbers indicate the total number of fish examined. For SL, predicted values from the linear mixed effects model fitted to observed SL are shown as dotted lines. GR was calculated for two periods: P1 (days 3–7) and P2 (days 7–14), using the mean SL per tank at the start of each period. Data are shown as mean ± SE.
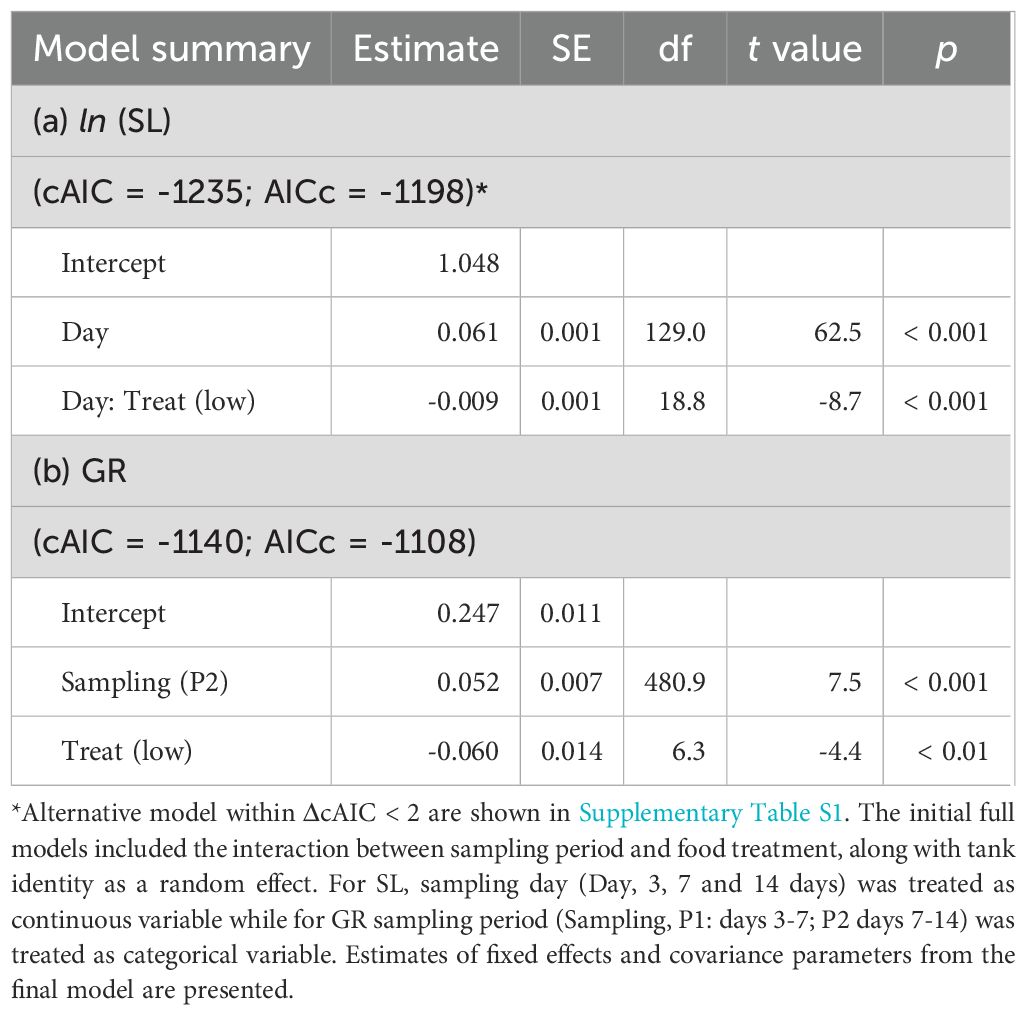
Table 6. Summary for linear mixed effects model estimates for the effects of parental food treatment on standard length (SL) and growth rate (GR) of chub mackerel larvae during the experiment.
4 Discussion
4.1 Stable isotope evidence for reproductive energy allocation
Linear mixed-effects models revealed that both δ¹³C and δ15N values in chub mackerel eggs significantly decreased over time (Figure 2). However, neither the main effect of age nor the interaction between age and days was statistically significant, suggesting that isotopic incorporation dynamics were consistent across the two age classes. These findings support the conclusion that parental age had negligible influence on isotopic trajectories under the experimental conditions. This contrasts with Japanese anchovy, in which ovarian isotopic turnover rates differed significantly between age classes: larger (older) females showed slower incorporation than smaller (younger) individuals (Yoneda et al., 2025). This size-dependent difference implies that large anchovy females integrate both income and facultative capital resources to support oocyte development and maintain frequent spawning intervals throughout an extended spawning season. While chub mackerel exhibit substantial differences in reproductive output between first-time and repeat spawners (Yoneda et al., 2022), the mode of energy allocation to egg production may be similar across age classes during the late winter to spring spawning period.
Our findings showed that the degree of dependence on income resources (DI) in eggs gradually increased over time, reaching approximately 0.47 by day 31 after the diet switch (Table 1, Figure 8). Importantly, this DI value should not be interpreted as a direct measure of oocyte growth alone. Stable isotope turnover in reproductive tissues reflects both the incorporation of new resources during yolk deposition and the maintenance costs of developing oocytes (Tieszen et al., 1983; Hesslein et al., 1993). In contrast to Japanese anchovy, a typical income breeder that reached a similar DI value within only a few days and plateaued at approximately 1 within 10–20 days, chub mackerel exhibited a much slower, near-linear trajectory. Such gradual incorporation resembles patterns observed in tissues with low metabolic renewal, where exponential models show shallow slopes and turnover appears nearly linear (Tieszen et al., 1983). This slow turnover indicates that mackerel reproduction during the spawning season relies predominantly on stored reserves, with only limited incorporation of concurrent food intake. This interpretation agrees with field observations of seasonal changes in lipid content, condition factor, and gonad index, which show that chub mackerel utilize lipid reserves accumulated from summer to autumn as the primary energy source for reproduction from late winter to spring (Yoshimitsu et al., 2018; Yatsu et al., 2019).
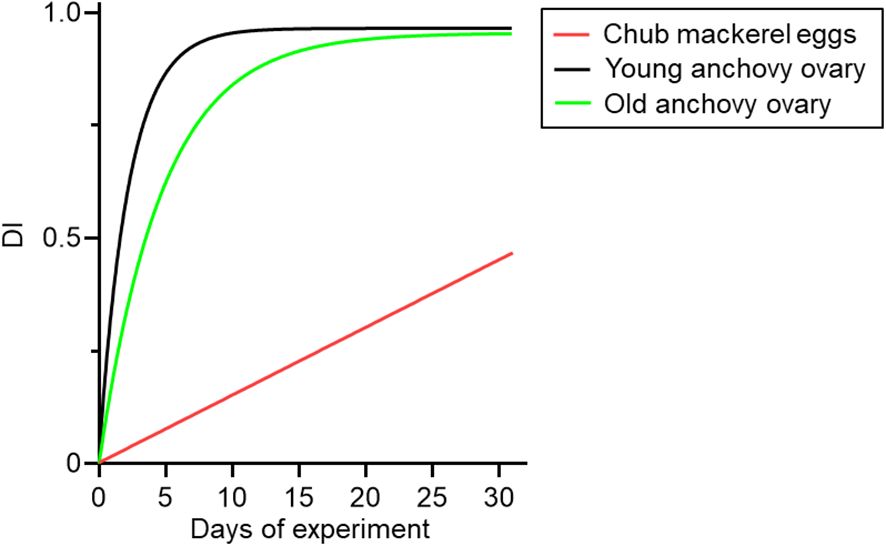
Figure 8. Degree of dependency on income resources (DI) of chub mackerel eggs (red) and Japanese anchovy ovaries (young: green; old: black) during the experiment (days after prey change). DI values of chub mackerel eggs were derived from the modeled isotopic changes in δ13C and δ15N values shown in Figure 2. Data on DI values of anchovy ovaries were from Yoneda et al. (2025).
Our findings further showed that 1-year-old female chub mackerel exhibited only marginal changes in muscle nitrogen isotope values during the spawning period, resulting in a very low DI (0.07) at the end of the experiment (40 days after the diet switch, Table 2). This contrasts with the much higher DI observed in ovarian tissues (0.54), indicating that dietary resources were allocated preferentially to reproduction rather than somatic growth. Given the significant decrease in somatic weight and condition factor during the experiment, this minimal isotopic change in muscle tissue likely reflects turnover driven primarily by metabolic processes rather than by tissue growth. This contrasts markedly with Japanese anchovy, where young spawners showed a much higher DI (0.57 at 40 days) in muscle tissue, indicating active assimilation of dietary resources for somatic growth during the spawning period (Yoneda et al., 2025). These findings suggest that even during the first reproductive season, 1-year-old female chub mackerel allocate energy primarily to reproduction and delay somatic growth until the post-spawning period. This interpretation is supported by findings in whitefish (Coregonus lavaretus), where muscle isotope values remained stable during reproduction and only responded to dietary shifts during growth phases (Perga and Gerdeaux, 2005). The observed dynamics of somatic growth and muscle nitrogen isotope values in 1-year-old female chub mackerel may be consistent with a capital breeding strategy. However, further investigations incorporating energy budgeting and direct measurements of feeding activity would be needed to clarify the degree to which chub mackerel rely on stored versus dietary resources during reproduction.
4.2 Effects of parental feeding treatment on reproductive traits
This study designed feeding treatments to reflect the range of nutritional conditions likely experienced under different stock phases. The Pacific stock of chub mackerel has shown more than a threefold variation in biomass between low and high periods (Yukami et al., 2025), which implies corresponding fluctuations in prey availability and energy intake. Previous studies reported density-dependent declines in growth, body condition, maturation and egg production under high stock levels (Watanabe and Yatsu, 2004, 2006; Watanabe, 2010; Kamimura et al., 2021; Takasuka et al., 2021). These findings suggest that individuals may experience markedly reduced energy acquisition under high-density conditions. Therefore, the two experimental feeding levels, 1.5% BW/day as a high-food scenario and 0.5% BW/day as a low-food scenario, were established to approximate the range of prey availability associated with such stock fluctuations. While intermediate levels could also provide further resolution, our aim here was to establish a realistic contrast that reflects the magnitude of natural variability.
This study demonstrated that the feeding manipulation approximately 5 months prior to spawning had significant effects not only on growth and condition of adult fish, but also on reproductive effort through changes in both egg number and size (energy content). Food-restricted spawners showed lower somatic weight and condition factor, and produced fewer and smaller eggs with reduced energy content compared to well-fed individuals. These findings were consistent with our observations of isotopic dynamics in eggs during the spawning period. Together, these findings suggest that chub mackerel rely primarily on stored energy reserves during reproduction, supporting the interpretation that they function mainly as capital breeders. As expected, larvae derived from lean females under lower food conditions showed reduced starvation tolerance and slower growth compared to those from well-fed females. Previous study showed that reproductive traits in chub mackerel vary with spawning experience, with repeat spawners exhibiting higher reproductive potential than first-time spawners (Yoneda et al., 2022). Our findings extend this understanding by demonstrating how reproductive outputs of repeat spawners are modulated by pre-spawning feeding conditions.
In capital breeders, reproductive output is generally shaped in two stages. The initial recruitment of secondary oocytes establishes potential fecundity well before spawning. The final adjustment, which is often a reduction in oocyte number via atresia, is regulated closer to spawning depending on energy availability (e.g. Hay and Brett,1988; Bromley et al., 2000; Kjesbu and Witthames, 2007). The timing and degree of this adjustment vary by species; however, a common feature is the critical importance of energy intake during secondary growth (vitellogenesis) in determining final fecundity (egg production). In Atlantic herring (Clupea harengus), where the vitellogenic period extends over several months, energy acquired during spawning migration is essential for sustaining oocyte maturation (Kurita et al., 2003; van Damme et al., 2009). Although chub mackerel continue feeding during the spawning season (Usami, 1968), our results suggest that, as in other capital breeders, reproductive effort is significantly impacted by feeding conditions during the pre-vitellogenic and vitellogenic period. Our results suggest that the nutritional environment in the months prior to the spawning season is crucial for the successful reproduction of chub mackerel. This implication is particularly relevant given the species’ seasonal migration. Adult chub mackerel feed intensively in the Oyashio and Kuroshio–Oyashio transition zones in the summer and autumn before migrating south along the Japanese coast in the winter (Yatsu, 2019). They reach the main spawning grounds off the coast of eastern Japan in the spring. Thus, foraging success in these northern feeding grounds and along the migratory pathway likely determines the energy reserves available for subsequent reproduction. Our experimental findings thus reinforce the importance of prey availability in these regions as a key determinant of reproductive potential in the Pacific stock of chub mackerel.
4.3 Implication for the reproductive potential of the Pacific stock
Our findings underscore the importance of spawning experience and pre-spawning nutritional status in determining reproductive output in chub mackerel. When comparing egg diameter, egg energy content, and early larval growth performance across three groups including 1-year-old fish under high feeding (Yoneda et al., 2022), 3-year-old fish under low feeding (this study), and 3-year-old fish under high feeding (both studies), consistent trends were found: 1-year-old (high-fed) < 3-year-old (low-fed) < 3-year-old (high-fed) (Supplementary Tables S2, S3, Figure 9). For this comparison, the high-fed 3-year-old groups from Yoneda et al. (2022) and the present study were treated as equivalent experimental conditions and used as a common reference. Although feeding conditions for 1-year-olds under low rations were not tested, previous field observations suggest that poor growth in immature fish can delay the onset of maturation in the subsequent year (Watanabe and Yatsu, 2006). In Atlantic cod (Gadus morhua), fecundity of first-time spawners was positively correlated with body size, but remained largely unaffected by feeding conditions (Yoneda and Wright, 2005), in contrast to repeat spawners (Kjesbu et al., 1991). Similarly, in walleye pollock (Gadus chalcogrammus), the proportion of mature females, relative egg production (number of spawned eggs per female somatic weight) and larval hatching success of first-time spawners was not significantly influenced by food availability, although the egg diameter appeared to be associated with feeding level (Nakagawa et al., 2019). These findings suggest that for first-time spawners, the primary priority may be to initiate reproduction itself, rather than to optimize egg number or quality. This suggests that first-time spawners have intrinsically lower reproductive potential than repeat spawners, regardless of feeding conditions. Our experimental results therefore strengthen earlier field-based assertions that maintaining a robust proportion of repeat spawners in the spawning stock is critical for sustaining recruitment success (Yoneda et al., 2022). Moreover, maternal isotopic signatures are transmitted to larvae and can influence early growth trajectories, which in turn may contribute to interannual variability in larval growth and survival, as demonstrated in Atlantic bluefin tuna (Quintanilla et al., 2023; 2024). Such evidence highlights the broader ecological significance of maternal effects in capital-breeding species and underscores the need to consider these processes when evaluating reproductive potential and recruitment dynamics.
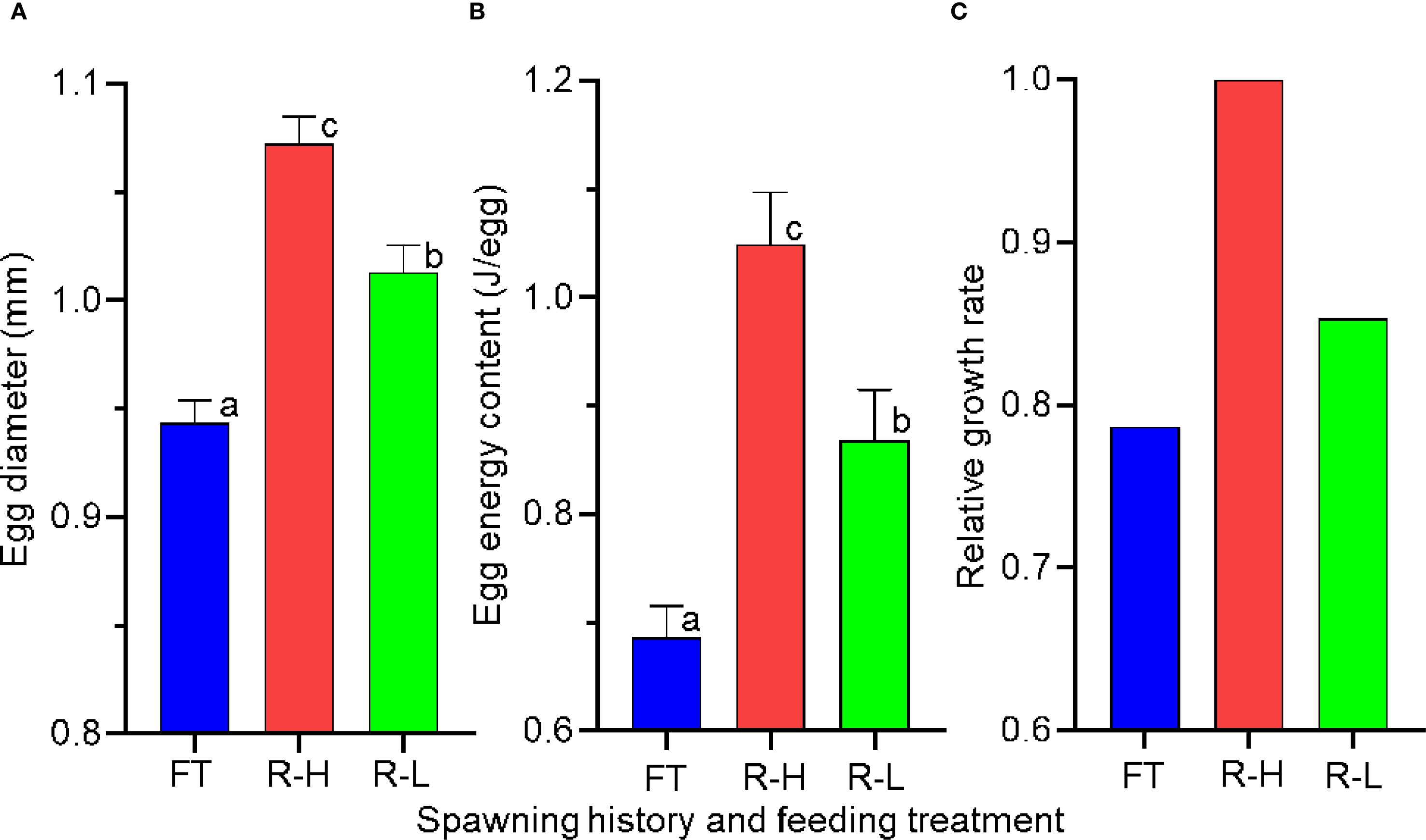
Figure 9. Egg diameter (A), egg energy content (B) and relative growth rate (C) of first-time spawners (FT, high food treatment) and repeat spawners under high (R-H) and low (R-L) food treatments. The methods and the statistical results for these comparisons are provided in Supplementary Materials. Relative growth rate is expressed as a ratio with the value for repeat spawners under high food treatment set to 1.0. Different letters indicate significant different among female groups (a < b < c; p < 0.001). Data are shown as mean ± SE.
The observed sharp decline in egg abundance since 2020 is consistent with the marked reduction in spawning stock biomass, despite the continued dominance of older repeat spawners (≥ 4 years old), which accounted for approximately 80% of the spawning population during this period (Figure 1; Yukami et al., 2025). While these older spawners, assumed to be mostly repeat spawners, generally exhibit higher egg quality and larval performance than first-time spawners (Yoneda et al., 2022), the overall decrease in their biomass has likely contributed substantially to the observed drop in egg abundance (Takasuka et al., 2021). Conversely, the relatively stable recruitment observed after 2020 may reflect the sustained reproductive contribution of these high-quality spawners. This interpretation is supported by our experimental findings, showing that repeat spawners consistently exhibited superior reproductive traits, regardless of feeding conditions.
However, the continued decline in spawning stock biomass raises concerns about the longer-term reproductive capacity of the stock. Under inter-and intra-specific density-dependent conditions, slower growth and deteriorated condition factors have been observed (Kamimura et al., 2021), potentially delaying the onset of maturation and increasing the risk of pre-reproductive mortality. This delayed maturation shortens the effective reproductive lifespan of individuals, particularly in a species with relatively short life expectancies, such as chub mackerel (approximately 7–8 years, Yukami et al., 2025), and limits their cumulative contribution to population resilience. These demographic shifts, combined with nutritional constraints and environmental variability (Kamimura et al., 2022), may further increase the uncertainty in forecasting future stock productivity.
4.4 Conclusions
This study combined stable isotope tracer and controlled feeding experiments to examine energy allocation strategies and reproductive responses in chub mackerel (Scomber japonicus). Our findings provide convincing evidence that chub mackerel primarily rely on stored energy reserves during the spawning period, indicating that they function largely as capital breeders. Feeding experiments further revealed that pre-spawning nutritional status significantly influences reproductive outputs of repeat spawners, including egg number, egg energy content, and larval performance. Although repeat spawners exhibited markedly higher reproductive potential than first-time spawners (Yoneda et al., 2022), this advantage diminished under food-limited conditions. These findings emphasize the importance of experimental approaches for directly evaluating the physiological and ecological determinants of reproductive strategy of small pelagic fishes.
In the context of recent decline in spawning stock biomass and egg abundance in the Pacific stock of chub mackerel, our findings highlight several contributing factors: reduced somatic growth, delayed maturation, and decreased reproductive output. These changes are likely driven by intra- and inter-specific density dependent effects, as well as declining zooplankton availability in key feeding habitats (Kamimura et al., 2021, 2022). Recent oceanographic observations further underscore this concern, as the Kuroshio–Oyashio transition region has experienced notable shifts since 2021, including the northward displacement of the Kuroshio Extension, suppression of Oyashio intrusion, and the occurrence of marine heatwaves (Hirata et al., 2024; Sugimoto et al., 2025). These environmental changes have the potential to alter mesozooplankton community structure and productivity, which could affect prey availability for chub mackerel and other small pelagic fishes (Miyamoto et al., 2022). To reduce uncertainty in predicting reproductive success and stock productivity of the Pacific stock of chub mackerel, continued ecosystem-based monitoring is essential, not only of spawning stock composition but also of prey communities and interspecific competition. In addition, experimental approaches, as presented here, will play a critical role in clarifying how physiological and ecological responses of chub mackerel interact with environmental variability. Such integrative efforts are necessary to inform adaptive management strategies under future ocean conditions.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.6084/m9.figshare.29643347.
Ethics statement
The animal studies were approved by All experimental procedures were approved by the committee of animal welfare of National Research Institute of Fisheries and Environment of Inland Sea, Fisheries Research and Education Agency (no. 2016-8). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MY: Data curation, Investigation, Writing – review & editing, Writing – original draft, Methodology, Conceptualization, Funding acquisition, Validation, Formal Analysis. MN: Data curation, Validation, Investigation, Methodology, Supervision, Writing – original draft. TM: Supervision, Investigation, Writing – original draft, Validation, Methodology. TT: Writing – original draft, Validation, Supervision, Investigation. HT: Validation, Writing – original draft, Methodology, Supervision. RY: Methodology, Validation, Supervision, Writing – original draft, Data curation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was partly supported by Fisheries Agency of Japan and Japan Fisheries Research and Education Agency and by the Promotion Programs for the Marine Fisheries Stock Assessment and Evaluation for Japanese Waters from Fisheries Agency of Japan and by Grant-in-Aid for Challenging Research (Pioneering) (KAKENHI No. 19H05540) and Scientific Research (B) (KAKENHI No. 22H02421 and 24K01843) from the Japan Society for the Promotion of Science.
Acknowledgments
We thank the staff for Hakatajima Field Station of the Fisheries Technology Institute, Japan Fisheries Research and Education Agency for their assistance with rearing experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this study the authors used ChatGPT 4o in order to improve the readability and language of the manuscript. After using the service, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1674359/full#supplementary-material
References
Barneche D. R., Robertson D. R., White C. R., and Marshall D. J. (2018). Fish reproductive-energy output increases disproportionately with body size. Science 645, 642–645. doi: 10.1126/science.aao6868
Bromley P. J., Ravier C., and Witthames P. R. (2000). The influence of feeding regime on sexual maturation, fecundity and atresia in first-time spawning turbot. J. Fish. Biol. 56, 264–278. doi: 10.1111/j.1095-8649.2000.tb02105.x
Broström G. and Holmberg H. (2011). Generalized linear models with clustered data: Fixed and random effects models. Comput. Stat. Data Anal. 55, 3123–3134. doi: 10.1016/j.csda.2011.06.011
Burnham K. P. and Anderson D. R. Model selection and multimodel inference. A practical information-theoretic approach. (New York: Springer) (2002). p. 488.
Cowan J.H. Jr., Rose K. A., and DeVries D. R. (2000). Is density-dependent growth in young-of-the-year fishes a question of critical weight? Rev. Fish. Biol. Fish. 10, 61–89. doi: 10.1023/A:1008932401381
Green B. S. (2008). Maternal effects in fish populations. Adv. Mar. Biol. 54, 1–105. doi: 10.1016/S0065-2881(08)00001-1
Helser T. E. and Almeida F. P. (1997). Density-dependent growth and sexual maturity of silver hake in the north-west Atlantic. J. Fish. Biol. 51, 607–623. doi: 10.1006/jfbi.1997.0468
Herrando-Pérez S., Delean S., Brook B. W., and Bradshaw C. J. A. (2012). Density dependence: An ecological tower of babel. Oecologia 17, 585–603. doi: 10.1007/s00442-012-2347-3
Hesslein R. H., Hallard K. A., and Ramlal P. (1993). Replacement of sulfur, carbon and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can. J. Fish. Aquat. Sci. 50, 2071–2076. doi: 10.1139/f93-230
Hirata H., Kawamura R., and Nonaka M. (2024). Effects of a marine heatwave associated with the Kuroshio Extension large meander on extreme precipitation in September 2023. Sci. Rep. 15, 5332. doi: 10.1038/s41598-025-88294-9
Hixon M. A., Johnson D. W., and Sogard S. M. (2014). BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES. J. Mar. Sci. 71, 2171–2185. doi: 10.1093/icesjms/fst200
Hobson K. A. (2006). Using stable isotopes to quantitatively track endogenous and exogenous nutrient allocations to eggs of birds that travel to breed. Ardea 94, 359–369.
Jönsson K. I. (1997). Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66. doi: 10.2307/3545800
Kamimura Y., Tadokoro K., Furuichi S., and Yukami R. (2022). Stronger density-dependent growth of Japanese sardine with lower food availability: Comparison of growth and zooplankton biomass between a historical and current stock-increase period in the western North Pacific. Fish. Res. 255, 106461. doi: 10.1016/j.fishres.2022.106461
Kamimura Y., Taga M., Yukami R., Watanabe C., and Furuichi S. (2021). Intra- and inter-specific density dependence of body condition, growth, and habitat temperature in chub mackerel (Scomber japonicus). ICES. J. Mar. Sci. 78, 3254–3264. doi: 10.1093/icesjms/fsab191
Kamler E. (2008). Resource allocation in yolk-feeding fish. Rev. Fish. Biol. Fish. 18, 143–200. doi: 10.1007/s11160-007-9070-x
Kawai H., Yatsu A., Watanabe C., Mitani T., Katsukawa T., and Matsuda H. (2002). Recovery policy for chub mackerel stock using recruitment-per-spawning. Fish. Sci. 68, 963–971. doi: 10.1046/j.1444-2906.2002.00520.x
Kjesbu O. S., Klungøyr J., Kryvi H., Witthames P. R., and Greer Walker M. (1991). Fecundity, atresia, and egg size of captive Atlantic cod (Gadus morhua) in relation to proximate body composition. Can. J. Fish. Aquat. Sci. 48, 2333–2343. doi: 10.1139/f91-274
Kjesbu O. S. and Witthames P. R. (2007). Evolutionary pressure on reproductive strategies in flatfish and groundfish: Relevant concepts and methodological advancements. J. Sea. Res. 58, 23–34. doi: 10.1016/j.seares.2007.02.001
Kurita Y., Meier S., and Kjesbu O. S. (2003). Oocyte growth and fecundity regulation by atresia of Atlantic herring (Clupea harengus) in relation to body condition throughout the maturation cycle. J. Sea. Res. 49, 203–219. doi: 10.1016/S1385-1101(03)00004-2
Kuznetsova A., Brockhoff P. B., and Christensen R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Software 82, 1–26. doi: 10.18637/jss.v082.i13
Lambert Y. and Dutil J.-D. (1997). Can simple condition indices be used to monitor and quantify seasonal changes in the energy reserves of Atlantic Cod (Gadus morhua)? Can. J. Aquat. Sci. 54, 104–112. doi: 10.1139/cjfas-54-S1-104
Logan J. M., Jardine T. D., Miller T. J., Bunn S. E., Cunjak R. A., and Lutcavage M. E. (2008). Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J. Anim. Ecol. 77, 838–846. doi: 10.1111/j.1365-2656.2008.01394.x
Lubzens E., Young G., Bobe J., and Cerdà J. (2010). Oogenesis in teleosts: how fish eggs are formed. Gen. Comp. Endocrinol. 165, 367–389. doi: 10.1016/j.ygcen.2009.05.022
Marshall D. J., Allen R. M., and Crean A. J. (2008). The ecological and evolutionary importance of maternal effects in the sea. Oceanogr. Mar. Biol. 46, 203–250. doi: 10.1201/9781420065756.ch5
McBride R. S., Somarakis S., Fitzhugh G. R., Albert A., Yaragina N. A., Wuenschel M. J., et al. (2015). Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish 16, 23–57. doi: 10.1111/faf.12043
Miyamoto H., Takahashi K., Kuroda H., Watanabe T., Taniuchi Y., Kuwata A., et al. (2022). Copepod community structure in the transition region of the North Pacific Ocean: Water mixing as a key driver of secondary production enhancement in subarctic and subtropical waters. Prog. Oceanogr. 207, 102865. doi: 10.1016/j.pocean.2022.102865
Murayama T., Mitani I., and Aoki I. (1995). Estimation of the spawning period of the Pacific mackerel Scomber japonicus based on the changes in gonad index and the ovarian histology. Bull. Jap. Soc Fish. Oceanogr. 59, 11–17.
Nakagawa T., Tanaka H., Chimura M., Yamashita Y., and Yokota T. (2019). Laboratory rearing of walleye pollock Gadus chalcogrammus from egg to first-time spawning season, and egg production and egg size in relation to ration level. Nippon. Suisan. Gakkaishi. 85, 321–330. doi: 10.2331/suisan.18-00012
Olafsdottir A. H., Slotte A., Jacobsen J. A., Oskarsson G. J., Utne K. R., and Nøttestad L. (2016). Changes in weight-at-length and size-at-age of mature Northeast Atlantic mackerel (Scomber scombrus) from 1984 to 2013: effects of mackerel stock size and herring (Clupea harengus) stock size. ICES. J. Mar. Sci. 73, 1255–1265. doi: 10.1093/icesjms/fsv142
Perga M. E. and Gerdeaux D. (2005). ‘Are fish what they eat’ all year round? Oecologia 144, 598–606. doi: 10.1007/s00442-005-0069-5
Quintanilla J. M., Borrego-Santos R., Malca E., Swalethorp R., Landry M. R., Gerard T., et al. (2024). Maternal effects and trophodynamics drive interannual larval growth variability of Atlantic bluefin tuna (Thunnus thynnus) from the Gulf of Mexico. Animals 14, 1319. doi: 10.3390/ani14091319
Quintanilla J. M., Malca E., Gerard T., Lamkin J. T., García A., and Laiz-Carrión R. (2023). Evidence of isotopic maternal transmission influence on bluefin tuna (Thunnus thynnus) larval growth. Mar. Environ. Res. 190, 106112. doi: 10.1016/j.marenvres.2023.106112
R Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed March 10, 2025).
Rose K. A., Cowan J.H. Jr., Winemiller K. O., Myers R. A., and Hilborn R. (2001). Compensatory density dependence in fish population: importance, controversy, understanding and prognosis. Fish 4, 293–327. doi: 10.1046/j.1467-2960.2001.00056.x
Slotte A. (1999). Differential utilization of energy during wintering and spawning migration in Norwegian spring-spawning herring. J. Fish. Biol. 54, 338–355. doi: 10.1006/jfbi.1998.0868
Stephens P. A., Houston A. I., Harding K. C., Boyd I. L., and McNamara J. M. (2014). Capital and income breeding: the role of food supply. Ecology 95, 882–896. doi: 10.1890/13-1434.1
Sugimoto S., Kojima A., Sakamoto T., Kawakami Y., and Nakano H. (2025). Influence of extreme northward meandered Kuroshio Extension during 2023–2024 on ocean–atmosphere conditions in the Sanriku offshore region, Japan. J. Oceanogr. 81, 203–215. doi: 10.1007/s10872-025-00747-x
Takasuka A., Oozeki Y., and Kubota H. (2008). Multi-species regime shifts reflected in spawning temperature optima of small pelagic fish in the western North Pacific. Mar. Eco. Prog. Ser. 360, 211–217. doi: 10.3354/meps07407
Takasuka A., Yoneda M., and Oozeki Y. (2021). Density-dependent egg production in chub mackerel in the Kuroshio Current system. Fish. Oceanogr. 30, 38–50. doi: 10.1111/fog.12501
Tanaka H., Yoneda M., Kitano H., Kawamura K., Imanaga Y., Matsuyama M., et al. (2016). Stable isotope evidence for income resource allocation to egg production in the Japanese anchovy Engraulis japonicus. Mar. Biol. 163, 28. doi: 10.1007/s00227-015-2773-y
Tayasu I., Hirasawa R., Ogawa N. O., Ohkouchi N., and Yamada K. (2011). New organic reference materials for carbon- and nitrogen-stable isotope ratio measurements provided by Center for Ecological Research, Kyoto University, and Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology. Limnology 12, 261–266. doi: 10.1007/s10201-011-0345-5
Tieszen L. L., Boutton T. W., Tesdahl K. G., and Slade N. A. (1983). Fractionation and turnover of stable carbon isotopes in animal tissues: Implications for δ13C analysis of diet. Oecologia 57, 32–37. doi: 10.1007/BF00379558
Usami S. (1968). Ecology and fisheries of the Japanese mackerel. Suisan-Kenkyu-Sousyo 18 (Tokyo: Japan Fisheries Resource Conservation Association).
Vaida F. and Blanchard S. (2005). Conditional Akaike information for mixed-effects models. Biometrika 92, 351–370. doi: 10.1093/biomet/92.2.351
van Damme C. J. G., Dickey-Collas M., Rijnsdorp A. D., and Kjesbu O. S. (2009). Fecundity, atresia, and spawning strategies of Atlantic herring (Clupea harengus). Can. J. Fish. Aquat. Sci. 12, 2130–2141. doi: 10.1139/F09-153
Warner D. A., Bonnet X., Hobson K. A., and Shine R. (2008). Lizards combined stored energy and recently acquired nutrients flexibly to fuel reproduction. J. Anim. Ecol. 77, 1242–1249. doi: 10.1111/j.1365-2656.2007.0
Watanabe C. (2010). Changes in the reproductive traits of the Pacific stock of chub mackerel Scomber japonicus and their effects on the population dynamics. Bull. Jap. Soc Fish. Oceanogr. 74, 46–50.
Watanabe C. and Yatsu A. (2004). Effects of density-dependence and sea surface temperature on interannual variation in length-at-age of chub mackerel (Scomber japonicus) in the Kuroshio-Oyashio area during 1970–1997. Fish. Bull. 102, 196–206.
Watanabe C. and Yatsu A. (2006). Long-term changes in maturity at age of chub mackerel (Scomber japonicus) in relation to population declines in the waters off northeastern Japan. Fish. Res. 78, 323–332. doi: 10.1016/j.fishres.2006.01.001
Yatsu A. (2019). Review of population dynamics and management of small pelagic fishes around the Japanese Archipelago. Fish. Sci. 85, 611–639. doi: 10.1007/s12562-019-01305-3
Yatsu A., Takahashi K., Watanabe K., and Honda O. (2019). Seasonal and interannual variability in crude fat content and condition factor of chub mackerel Scomber japonicus captured from waters off northeastern Japan during 2012-2017. Bull. Jap. Soc Fish. Oceanogr. 83, 19–27.
Yoneda M., Katayama S., Yamamoto M., Kono N., Tsuzaki T., and Tanaka H. (2025). Size-dependent resource allocation to reproduction in Japanese anchovies (Engraulis japonicus). Sci. Rep. 15, 15057. doi: 10.1038/s41598-025-99911-y1
Yoneda M., Kitano H., Nyuji M., Nakamura M., Takahashi M., Kawabata A., et al. (2022). Maternal spawning experience and thermal effects on offspring viability of chub mackerel and their influence on reproductive success. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1063468
Yoneda M. and Wright P. J. (2005). Effects of varying temperature and food availability on growth and reproduction in first-time spawning female Atlantic cod. J. Fish. Biol. 67, 1225–1241. doi: 10.1111/j.1095-8649.2005.00819.x
Yoshimitsu T., Kato M., and Kobayashi S. (2018). Relationship between seasonal variations of lipid content and gonadal development in the chub mackerel Scomber japonicus landed at the port of Choshi. Nippon. Suisan. Gakkaishi. 84, 1017–1024. doi: 10.2331/suisan.18-00025
Yukami R., Nishijima S., Kamimura Y., Isu S., Furuichi S., Watabe R., et al. (2025). Stock assessment and evaluation for the Pacific Stock of Scomber japonicus in 2024 (Tokyo: Fisheries Agency and Fisheries Research and Education Agency of Japan), 96. Available online at: http://abchan.fra.go.jp/ (Accessed April 15, 2025).
Keywords: density-dependent effects, egg production, energy allocation strategy, larval viability, maternal effect, Pacific stock of chub mackerel, stable isotope
Citation: Yoneda M, Nakamura M, Morioka T, Tsuzaki T, Togashi H and Yukami R (2025) Experimental evidence of a mixed breeding strategy and food-dependent maternal and larval traits in chub mackerel. Front. Mar. Sci. 12:1674359. doi: 10.3389/fmars.2025.1674359
Received: 27 July 2025; Accepted: 24 September 2025;
Published: 08 October 2025.
Edited by:
Matan Golan, Agricultural Research Organization (ARO), IsraelReviewed by:
Raul Laiz, Spanish Institute of Oceanography (IEO), SpainElizabeth Marschall, The Ohio State University, United States
Copyright © 2025 Yoneda, Nakamura, Morioka, Tsuzaki, Togashi and Yukami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michio Yoneda, eW9uZWRhX21pY2hpbzU1QGZyYS5nby5qcA==
 Michio Yoneda
Michio Yoneda Masahiro Nakamura1,2
Masahiro Nakamura1,2