- 1Shandong Key Laboratory of Eco-Environmental Science for the Yellow River Delta, Shandong University of Aeronautics, Binzhou, Shandong, China
- 2College of Biological and Pharmaceutical Engineering, Shandong University of Aeronautics, Binzhou, Shandong, China
Bivalves are important indicator species for cadmium (Cd) pollution, however, the differential detoxification and physiological responses to Cd exposure among co-occurring bivalve species have not been thoroughly studied. This study investigated alterations in metallothionein (MT), superoxide dismutase (SOD), and malondialdehyde (MDA) levels in the digestive gland and gills of clam species Meretrix meretrix and Mactra veneriformis following exposure to cadmium (Cd) at concentrations of 0.05, 0.10, and 0.15 mg/L over a period of 7 days. The results indicated that gill tissue, due to its direct contact with the environment and high metabolic activity, exhibited significantly greater variations in SOD, MT, and MDA contents compared to the digestive gland, rendering it more sensitive to Cd stress and suitable for acute toxicity monitoring. M. veneriformis demonstrated a faster and more pronounced response, with significant alterations in biomarker levels observed in the gills at early stages even under low Cd exposure. However, its detoxification system, specifically MT synthesis reached saturation more readily; under high Cd concentrations, biomarker levels peaked and subsequently declined rapidly, indicating the potential for the early onset of irreversible damage. In contrast, M. meretrix exhibited a gradual adaptive response, maintaining homeostasis via sustained MT synthesis, which reflects a higher tolerance to Cd stress. MDA contents showed significant early increases in both species’ gills, especially under low Cd concentrations, highlighting its value as both an early-warning indicator and a preferred biomarker for Cd pollution monitoring. The study provides candidate indicator species and identifies sensitive biomarkers for Cd biomonitoring in regions such as the Yellow River Delta and a theoretical basis for improving ecological risk assessment.
1 Introduction
In environmental monitoring programs, biomarkers are important referees to evaluate the pollution of heavy metals/organic compounds in sediments, water columns, and biota as well as associated biological effects in aquatic ecosystem (González et al., 2015; da Luz et al., 2025). Varieties of biomarkers, serving as integrative measures of pollutant exposure, could provide valuable insights into the toxicological effects on organismal growth and development (Lam and Gray, 2003; Pandiyan et al., 2021). Thus a comprehensive set of biomarkers tailored to specific xenobiotics, along with chemical analyses, might be efficient tools in monitoring the marine pollution.
Benthic aquatic species, especially shellfish, are typical indicator species in marine environment some of whose physiological and/or biochemical parameters are usually directly influence the pollution degree of certain pollutants (Cai and Wang, 2024). For example, bivalves, which accumulate high concentrations of metals through filter-feeding, could reflect the spatiotemporal patterns of contaminant accumulation through different biomarkers (Ren et al., 2022). Among these biomarkers, metallothionein (MT) induction is well-established for indicating sub-lethal exposure to metals such as cadmium (Cd), copper (Cu), zinc (Zn), and lead (Pb) (McComb et al., 2014; Zhou et al., 2022; Yang et al., 2024; Wang et al., 2025). MTs are multifunctional proteins involved in regulating essential metal homeostasis, participating in immune responses, combating oxidative stress, and mediating toxic metal detoxification (Zhang et al., 2024a; Yunko et al., 2025). Elevated MT levels are generally indicative of metal exposure. Research on MT in marine invertebrates has largely focused on bivalve mollusks—such as Ruditapes philippinarum, Mytilus edulis, Argopecten irradians, Meretrix meretrix, and Crassostrea gigas—due to their sessile nature, low metabolic activity, and tendency to accumulate heavy metals (Wang et al., 2009; Perić et al., 2020; Cai et al., 2025; Yang et al., 2023; Zhang et al., 2023). However, MT responses vary across species, metal types, and environmental conditions such as seasonal fluctuations (Pellerin and Amiard, 2009; Perrigault and Allam, 2012), highlighting the need for comparative studies to verify its specificity and accuracy.
Parallel to MT, key components of the antioxidant system—including superoxide dismutase (SOD), glutathione peroxidase (GPx), and the lipid peroxidation product malondialdehyde (MDA)—are widely used biomarkers for pollutant-induced oxidative stress (Wang et al., 2021; Jiang et al., 2024a; Jing et al., 2025a). Metabolism of organic xenobiotics often generates reactive oxygen species (ROS), which can damage cellular components, alter gene expression, and lead to oxidative stress (Yang et al., 2022; Su et al., 2023; Tong et al., 2025; Zheng et al., 2025). MDA content, reflecting lipid peroxidation levels, is commonly used in toxicity assessments in bivalves and fish (Jiang et al., 2024b; Zhang et al., 2024b; Qiang et al., 2025; Sun et al., 2025). So the antioxidant system primarily functions to eliminate superoxide anion (O2⁻) free radicals, while lipid peroxidation (LPO) levels are directly indicated by MDA content. Therefore, combining antioxidant enzyme indicators with MDA for investigation can better reflect the organism’s antioxidant response mechanism.
Cadmium represents a major environmental concern due to its toxicity and persistence in aquatic ecosystems (Hu and Wang, 2024; Lu et al., 2024). It bioaccumulates in tissues, stimulates ROS production, induces oxidative biomolecular damage, and promotes MT synthesis (Liu et al., 2024a; Lompré et al., 2024; Sun et al., 2024). Cd can also inhibit enzyme activity, disrupt cellular signaling, and dysregulate calcium homeostasis (Stohs and Bagchi, 1995; Moncaleano-Niño et al., 2017). In China, Cd contamination is a significant issue in certain aquaculture zones. For example, in the Yellow River Delta—a region rich in shellfish resources—Cd concentrations sometimes exceed water quality standards, threatening economically important species (Cai et al., 2024; Li et al., 2024a; Ren et al., 2024).
In the Yellow River Delta, Meretrix meretrix and Mactra veneriformis are two native, sympatric clams with ecological and economic value (Liu et al., 2022). Both have shown detectable biochemical responses to pollutants and are considered potential indicator species (Wu et al., 2012; Li et al., 2024b, Li et al., 2025; Zhang et al., 2021). However, comparative analyses of their responses to Cd-induced stress—particularly in terms of MT induction, antioxidant activity, and oxidative damage—remain limited.
Therefore, this study aims to systematically compare the differential responses of M. meretrix and M. veneriformis to Cd stress by quantifying tissue-specific Cd accumulation and a suite of biomarkers—MT, SOD, and MDA—in digestive gland and gill tissues. The relationships between Cd accumulation and biomarker expression will be examined to elucidate species-specific defense mechanisms and enhance the bioindicator utility of these clams.
2 Materials and methods
2.1 Chemicals
Chemicals with a purity ≥99% included 5,5-dithiobis-2-nitrobenzoic acid (DTNB), phenylmethylsulphonyl fluoride (PMSF), and leupeptin were purchased from Sigma-Aldrich (Shanghai, China). The remainder of the reagents with a purity ≥98% were purchased from Sangon Biotech (Shanghai, China).
2.2 Clam treatment
Clam individuals of M. meretrix and M. veneriformis (mean shell length: 3.89 ± 0.21 cm) used in this study were obtained from an aquaculture base in Wudi county, located along the Bohai Sea coast near Binzhou City, China. The aquaculture farm is certified for Good Agricultural Practices (GAP). Seafood from this farm is regularly screened for heavy metals, ensuring that cadmium levels in clams remain below 2.0 mg/kg wet weight, thereby avoiding any potential interference with the experimental results. In the laboratory, clams of uniform size were selected and maintained in natural seawater sourced directly from the Bohai Bay. Seawater conditions were maintained at a salinity of 28‰, a pH of 8.0, and a temperature range of 8–10°C. One-third of the seawater volume was renewed daily. Continuous aeration was provided. The present experiment utilized a diet of food-grade powdered Spirulina platensis, with a cadmium content of ≤0.2 mg/kg. Prior to feeding, 20 mg of the algal powder was fully resuspended in 200 mL of culture water. A fixed volume of 10 mL of this algal suspension was evenly distributed into each rearing tank at scheduled intervals using a pipette, ensuring consistent individual food intake. The cadmium content in this feeding quantity was extremely low (≤0.01 μg/L) and thus considered negligible, with no anticipated impact on the experimental outcomes. Prior to experimentation, clams were acclimatized under these conditions for one week.
2.3 Experimental designs
Following acclimatization, clams were transferred to plastic tanks (50 × 40 × 30 cm) containing seawater. Salinity (28‰), pH (8.0), temperature (8–10°C), and feeding regimen (powdered Spirulina platensis) were maintained consistent with the acclimatization conditions. The average concentration of Cd in seawater was 0.219 ± 0.011 ng/L. For exposure groups, CdCl2 was dissolved and diluted in seawater to achieve nominal concentrations of 0.05, 0.1, and 0.15 mg/L, corresponding to 10 ×, 20 ×, and 30 × the aquaculture water quality standard for Cd (≤0.005 mg/L), respectively. Each treatment, including the control, comprised triplicate tanks stocked with 40 clams. One-third of the seawater volume, containing corresponding Cd concentrations, was replenished daily. The Cd concentrations in the exposure tanks were verified every 24 hours. No mortality was observed across all concentrations during the 7-day exposure period. Samples of digestive gland and gill tissues were collected from both species on days 0, 1, 3, 5, and 7 following exposure initiation.
2.4 Sample preparation
Following the exposure period, clams were dissected on ice. Digestive glands and gills from three individuals in each replicate tank were pooled. Half of each tissue sample was immediately frozen at −80°C in 1.5 mL centrifuge tubes for MT analysis. The remaining tissue was rinsed in distilled water and homogenized (1:10 w/v) in cold phosphate buffer (100 mM, pH 7.4) using a glass homogenizer in an ice bath. The homogenate was maintained at 4°C and centrifuged at 3,500 × g for 10 min at 4°C. The resulting supernatant was aliquoted for analysis of SOD activity and MDA content.
2.5 SOD activity
SOD activity was measured using a Total Superoxide Dismutase Assay Kit (WST-1 method, Nanjing Jiancheng Bioengineering Institute). One unit of SOD activity (U) was defined as the amount of enzyme causing 50% inhibition of the formazan dye reduction reaction per milligram of protein in a 1 mL reaction volume. The activity is expressed as U/mg/min.
2.6 MDA content
MDA content was quantified using a thiobarbituric acid (TBA) assay adapted from Wills (1987). Briefly, 50 μL of tissue homogenate was diluted to 1 mL with distilled water. Subsequently, 500 μL of ice-cold 20% trichloroacetic acid (containing 1 mM FeSO4) and 1 mL of 0.67% (w/v) TBA solution were added. Samples were vortexed and incubated at 90°C for 10 min to form the MDA-TBA adduct. After cooling on ice, precipitates were removed by centrifugation (3,000 × g, 5 min, 4°C). A 1 mL aliquot of supernatant was diluted 1:3 with distilled water, and the absorbance was measured at 532 nm using a spectrophotometer. MDA concentration was calculated from a standard curve generated with 1,1,3,3-tetramethoxypropane and normalized to protein content, expressed as nmol MDA/mg protein.
2.7 MT quantification
MT content was determined using a modified Viarengo et al. (1997) protocol. Frozen tissues were homogenized (1:3 w/w) in ice-cold buffer [100 mM phosphate buffer (pH 8.0), 0.5 M sucrose, 20 mM Tris-HCl (pH 8.6), 0.006 mM leupeptin, 0.5 mM PMSF, 0.01% (v/v) β-mercaptoethanol]. The homogenate was centrifuged (30,000 × g, 20 min, 4°C). High-molecular-weight proteins were precipitated from the supernatant using ethanol-chloroform (1.005:0.08 v/v) and pelleted by centrifugation (7,000 × g, 10 min, 4°C). The MT-containing supernatant was incubated with 1 mg RNA, 40 μL 37% HCl, and 6 mL ethanol at -20°C for 1 h. MTs were pelleted by centrifugation (2,000 × g, 15 min, 4°C), washed twice with sucrose-Tris/ethanol/chloroform (13:87:1), and air-dried. The pellet was dissolved in 150 μL 0.25 M NaCl and 150 μL 4 mM HCl-EDTA. MT concentration was determined spectrophotometrically using Ellman’s reagent [5,5’-dithiobis-(2-nitrobenzoic acid), DTNB] at 412 nm (BioTEK ELx800). Reduced glutathione standards were used for calibration. MT content was calculated based on cysteine reactivity (21 residues per molecule, MW ~8.6 kDa) and expressed as μg MT-like protein/mg total protein.
2.8 Protein determination
Soluble protein concentration in enzyme extracts was determined using the Bradford (1976) method (Bio-Rad Protein Assay Kit) with bovine serum albumin standards. Absorbance of the Coomassie Brilliant Blue G-250 dye-protein complex was measured at 595 nm.
2.9 Statistical analysis
Data analysis was performed using SPSS 19.0, with results expressed as mean ± standard deviation (SD). After verifying normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test), one-way ANOVA was first used to determine overall significance among groups. When ANOVA indicated significance, post-hoc tests were applied as follows: Dunnett’s test was used specifically for comparisons between each exposure group and the control group, while pairwise comparisons among different exposure concentrations were conducted using Duncan’s test (under equal variances) or the Games–Howell test (under unequal variances). A unified significance threshold of P < 0.05 was adopted for all analyses.
3 Results
3.1 Responses of SOD activities to Cd exposure in M. meretrix and M. veneriformis tissues
Figures 1 illustrates SOD activity in M. meretrix. In the digestive gland (Figure 1A), SOD activities of 0.05 mg/L Cd exposure showed no significant difference compared to the controls. Conversely, significantly increased SOD activity in the 0.10 mg/L group was observed at Day 3 onward(P < 0.05), and peaked on Day 7. While that in the 0.15 mg/L group peaked over 110 U/mg prot on Day 5. As shown in Figure 1B, in the gills, SOD activity in the 0.05 mg/L Cd group was significantly higher than that in the control at Day 3 onward, and maintained an upward trend. While those in the 0.1 mg/L Cd group and 0.15 mg/L Cd group were became significantly higher than the control at Day 1 and reached their peak values on Day 5 and Day 3, respectively. significantly lower SOD activity was found in the 0.15 mg/L Cd group(P < 0.05).
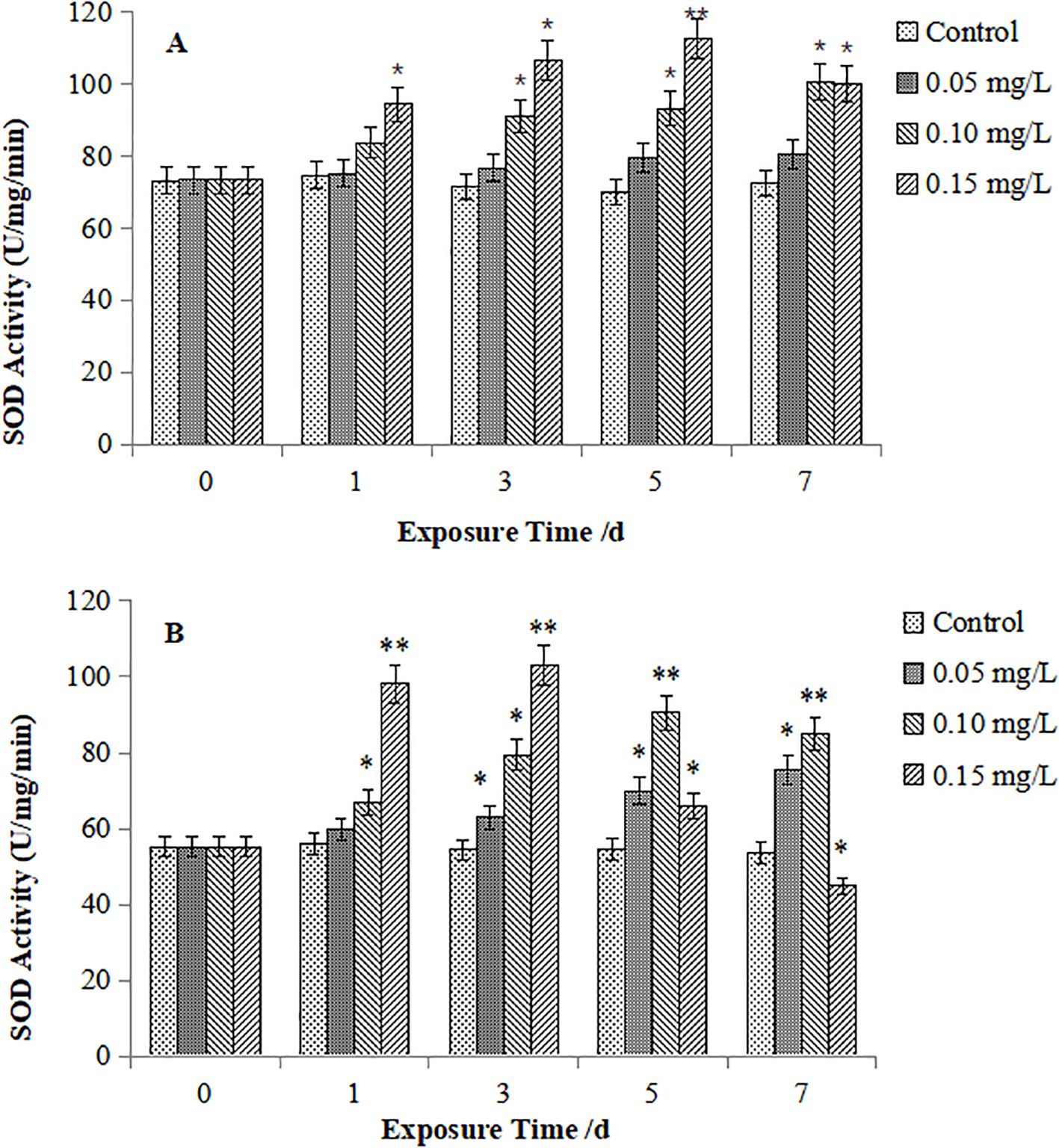
Figure 1. Effect of Cd on SOD activity in the digestive gland (A) and gills (B) of M. meretrix. The asterisk (*) indicates a significant difference compared to the control group (P < 0.05), and the double asterisk (**) indicates a highly significant difference compared to the control group (P < 0.01).
As for M. veneriformis, SOD activities in the digestive gland (Figure 2A) exposed to 0.05 mg/L of Cd exceeded those in controls from Day 3 and continued to rise. While those in the 0.10 mg/L group showed early activation (Day 1), peaked on Day 3, and declined to near-control levels on Day 7. In the high-concentration group (0.15 mg/L), SOD activity peaked on Day 3, then declined, and below that in the control group on Day 7. Gill SOD activity (Figure 2B) revealed distinct responses: that in the 0.05 mg/L group increased steadily from Day 1; SOD activity in the 0.10 mg/L group peaked sharply (46.95 U/mg) on Day 1 and kept on declining, and began to be lower than the control on Day 5 onwards; as well as that in the 0.15 mg/L group exhibited persistent suppression from Day 1 onward.
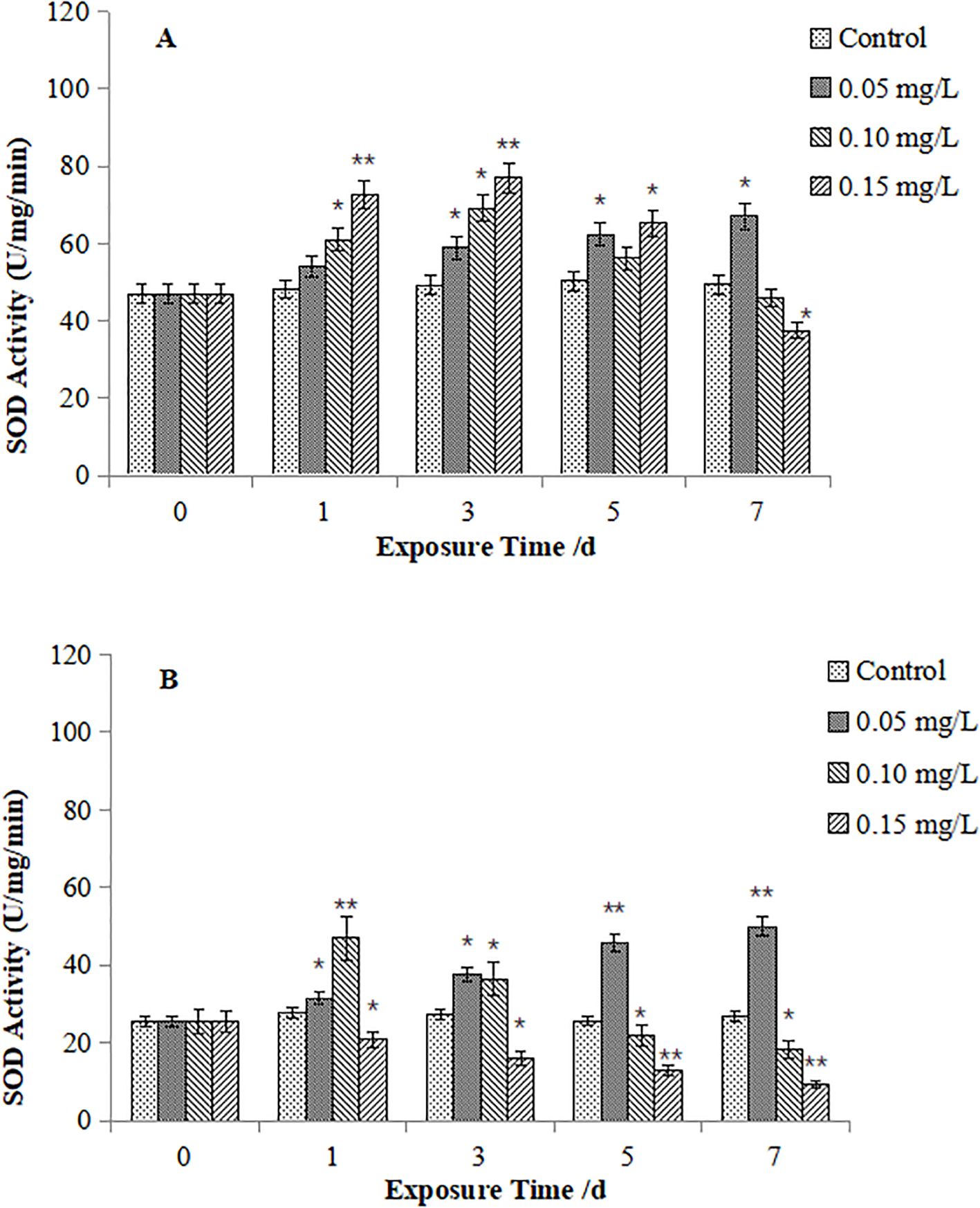
Figure 2. Effect of Cd on SOD activity in the digestive gland (A) and gills (B) of M. veneriformis. The asterisk (*) indicates a significant difference compared to the control group (P < 0.05), and the double asterisk (**) indicates a highly significant difference compared to the control group (P < 0.01).
Comparative analysis indicated that gill SOD activity in both species underwent more pronounced and sensitive fluctuations than that in the digestive gland. M. veneriformis exhibited greater SOD variability in homologous tissues than M. meretrix. Mechanistically, low Cd (0.05 mg/L) exposure universally enhanced SOD activity, while, higher Cd concentrations (≥0.10 mg/L) induced suppression.
3.2 Effects of Cd exposure on MT contents in M. meretrix and M. veneriformis tissues
Figures 3 depicts MT contents in M. meretrix. As for the 0.05 mg/L Cd exposure, no significant variation MT contents was observed in the digestive gland (Figure 3A), whereas significant increase of MT contents was observed from Day 3 onward (P < 0.05), sustaining an upward trend continuously (Figure 3B). While under the 0.10 mg/L Cd exposure, MT contents in both tissues exhibited significant elevation from Day 1 (P < 0.05), reaching extreme values at Day 7 and Day 5 in digestive gland and gills, respectively (P < 0.05). And under exposure to 0.15 mg/L Cd, MT contents rose extremely significantly from Day 3 (digestive gland) and Day 1 (gills) (P < 0.05). Overall, MT accumulation in M. meretrix displayed dose- and time-dependence, with those in gills showing greater responsiveness and steeper increases.
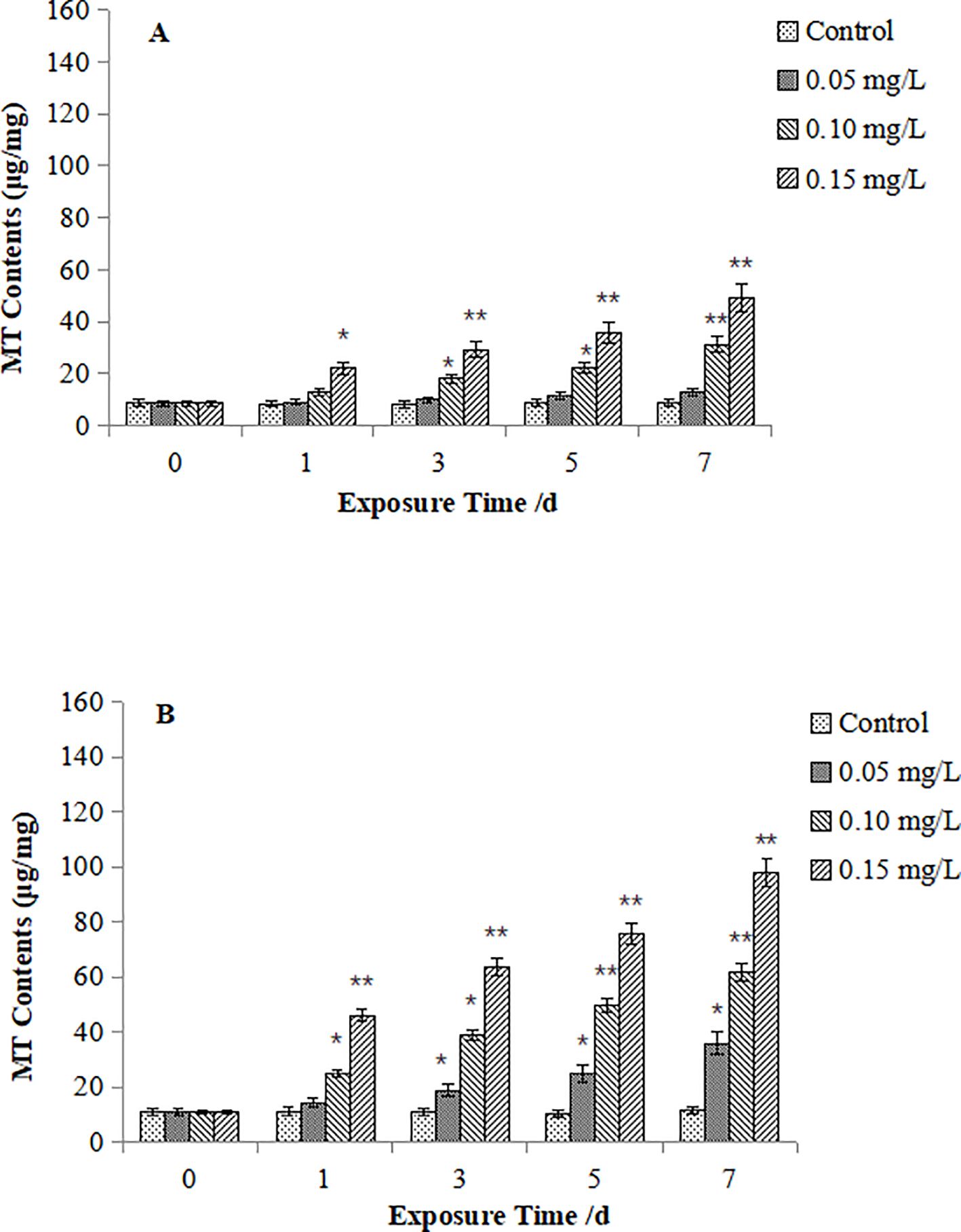
Figure 3. Effect of Cd on MT contents in the digestive gland (A) and gills (B) of M. meretrix. The asterisk (*) indicates a significant difference compared to the control group (P < 0.05), and the double asterisk (**) indicates a highly significant difference compared to the control group (P < 0.01).
Figures 4 presents MT contents in M. veneriformis. MT contents in the digestive gland (Figure 4A) increased significantly from Day 3 under 0.05 mg/L Cd exposure (P < 0.05). For the 0.10 mg/L and 0.15 mg/L groups, MT contents began to be significantly higher than that in the control from Day 1 (P < 0.05), and being extremely significant greater at Day 5 and Day 1, respectively (P < 0.05). For those in the gill, (Figure 4B) significant and extremely significant increase were firstly observed at Day 1 and day 5 under the 0.05 mg/L Cd exposure (P < 0.05), while extremely significant increase were both occurred at Day 1 in the 0.10 mg/L and 0.15 mg/L groups (P < 0.05). Notably, gills exhibited earlier and more pronounced MT upregulation than digestive glands at equivalent concentrations.
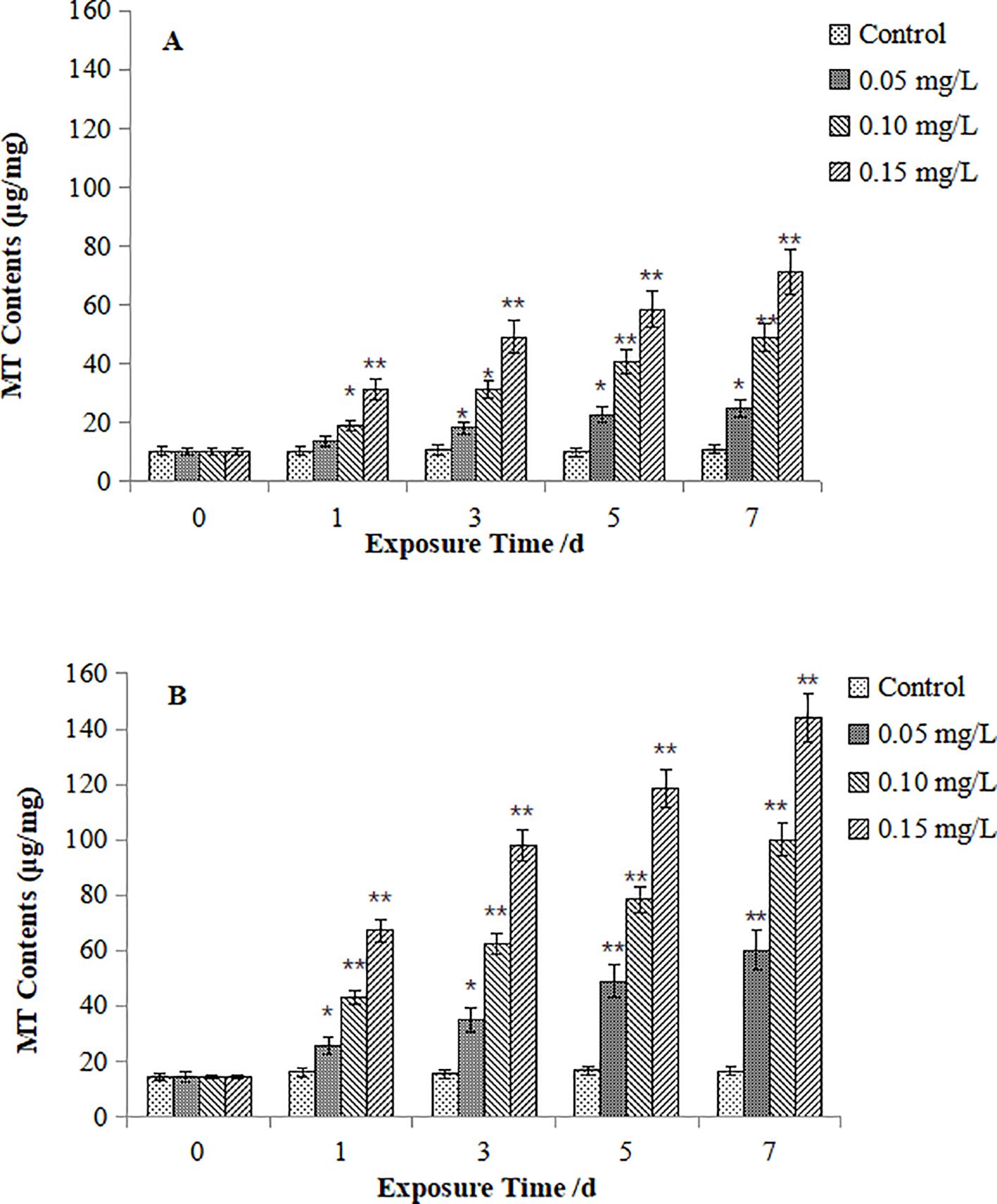
Figure 4. Effect of Cd on MT contents in the digestive gland (A) and gills (B) of M. veneriformis. The asterisk (*) indicates a significant difference compared to the control group (P < 0.05), and the double asterisk (**) indicates a highly significant difference compared to the control group (P < 0.01).
Comparative analysis reveals conserved dose- and time-dependent MT accumulation in both species, aligning with classical dose-response relationships. However, M. veneriformis demonstrated significantly stronger MT induction in both tissues than M. meretrix (gill: peak 2.1-fold higher; digestive gland: 1.8-fold), indicating heightened sensitivity to Cd stress.
3.3 Effects of Cd exposure on MDA contents of clams M. meretrix and M. veneriformis
Figures 5 illustrates MDA contents in M. meretrix. In the digestive gland (Figure 5A), MDA content remained stable under 0.05 mg/L Cd exposure but significantly increased (P < 0.05) from Day 3 and Day 1 for the 0.10 mg/L and 0.15 mg/L Cd groups respectively. Gill filaments (Figure 5B) exhibited significant MDA elevation from Day 3, Day 1 and Day 1 for the 0.05, 0.10, 0.15 mg/L Cd group respectively (P < 0.05). Baseline MDA contents in control gills was lower than those in digestive glands (P < 0.05). Crucially, once exposed to Cd, gills showed significantly greater MDA induction than digestive glands for all the treatments (P < 0.05), indicating heightened gill sensitivity.
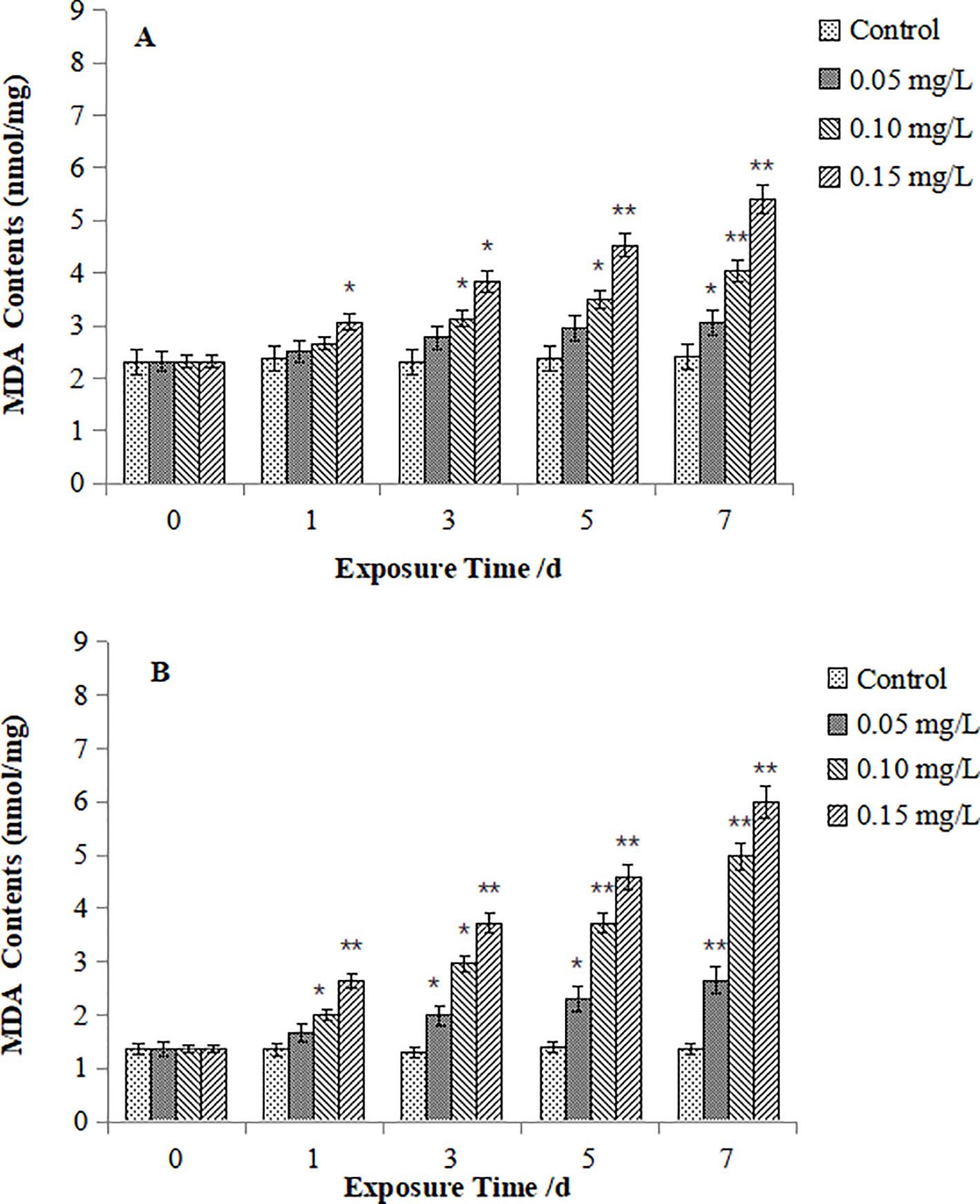
Figure 5. Effect of Cd on MDA contents in the digestive gland (A) and gills (B) of M. meretrix. The asterisk (*) indicates a significant difference compared to the control group (P < 0.05), and the double asterisk (**) indicates a highly significant difference compared to the control group (P < 0.01).
Figures 6 presents MDA contents in M. veneriformis. The digestive gland MDA (Figure 6A) increased significantly beginning on Day 3 (P < 0.05), Day 1 (P < 0.05) and Day 1 (P < 0.05) for the 0.05, 0.10, and 0.15 mg/L Cd groups, respectively. While those in Gill filaments (Figure 6B) began to show significant increases at Day 1 for the 0.05 mg/L group (P < 0.05) and extremely significant rises at Day 1 for both the 0.10 and 0.15 mg/L Cd groups (P < 0.05). The magnitude of MDA induction was consistently greater in the gills compared to the digestive glands.
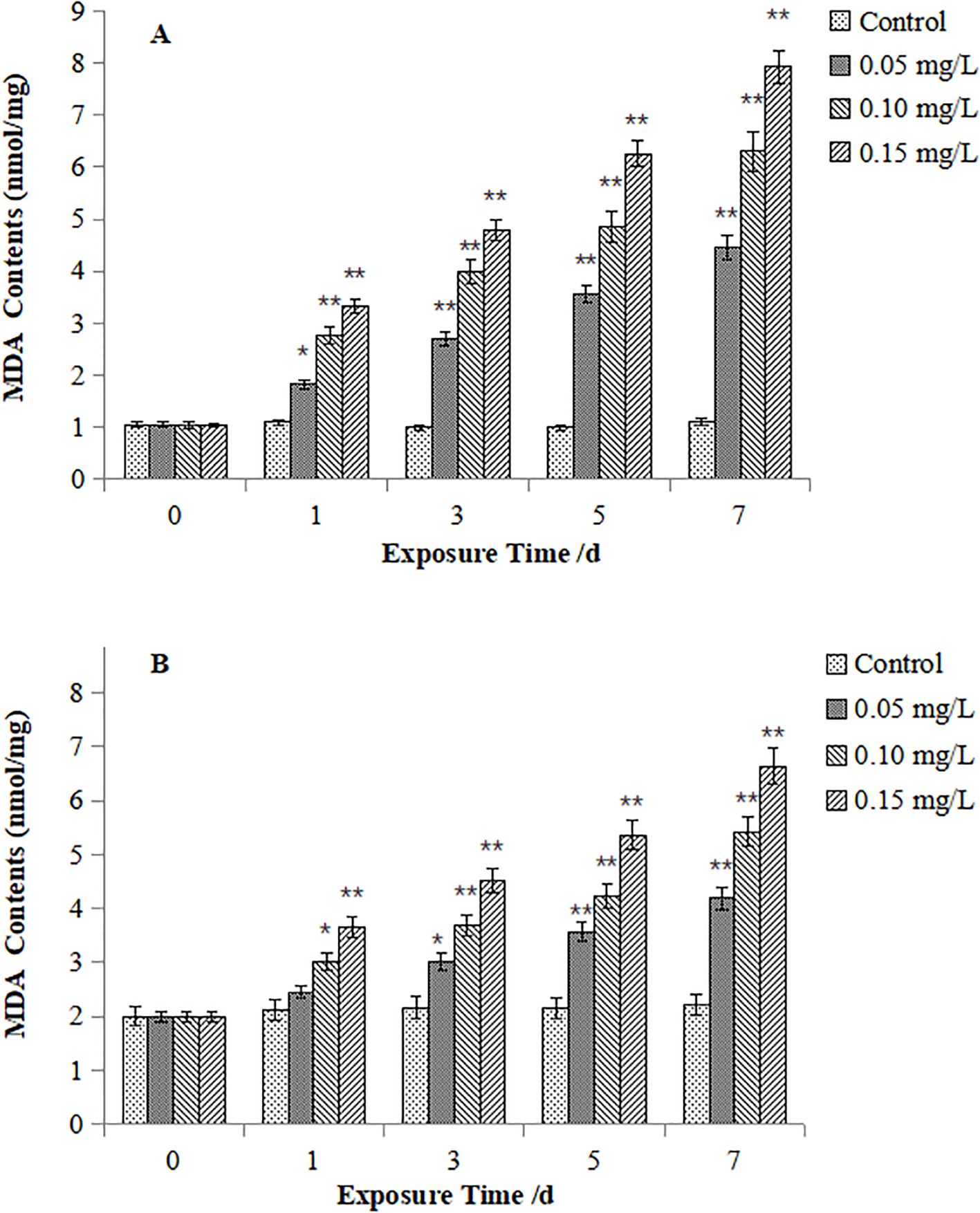
Figure 6. Effect of Cd on MDA contents in the digestive gland (A) and gills (B) of M. veneriformis. The asterisk (*) indicates a significant difference compared to the control group (P < 0.05), and the double asterisk (**) indicates a highly significant difference compared to the control group (P < 0.01).
Comparative analysis revealed significantly stronger MDA responses in M.veneriformis than in M. meretrix within homologous tissues at equivalent Cd concentrations (gill: 1.7-fold higher; digestive gland: 1.4-fold higher; P < 0.05). Most treatments exhibited time-progressive MDA accumulation, aligning with dose/time-dependent oxidative damage mechanisms.
4 Discussion
4.1 Immunomodulatory biomarker responses to Cd exposure in M. meretrix and M. veneriformis
Cd exerts toxicity in aquatic organisms through multiple pathways, including disruption of antioxidant defenses, induction of lipid peroxidation, and activation of metallothionein (MT) synthesis (Liu et al., 2024b). In this study, SOD activity in the digestive gland of M. meretrix remained unchanged under low Cd exposure (0.05 mg/L), whereas gill SOD increased significantly from Day 3 (Figures 1A, B), indicating that gills—directly exposed to the environment—are more sensitive to Cd-induced oxidative stress. Similarly, M. veneriformis exhibited rapid gill SOD activation at 0.05 mg/L Cd from Day 1 (Figure 2B). Under higher Cd levels (≥0.10 mg/L), both species showed a biphasic “induction–suppression” pattern, though the peak occurred later in M. meretrix (Days 3–5) than in M. veneriformis (Days 1–3). This earlier saturation in M. veneriformis may be linked to its higher metabolic rate and consequent rapid ROS accumulation in gill tissue (Li et al., 2024b).
MT exhibited dose- and time-dependent induction in both species, though M. veneriformis showed stronger upregulation—e.g., gill MT increased markedly at 0.15 mg/L Cd as early as Day 1 (Figure 4B). This may reflect species-specific traits such as larger gill surface area and higher Cd uptake rate, necessitating rapid MT synthesis for metal sequestration (Fang et al., 2010; Moncaleano-Niño et al., 2017). Across all concentrations, gill MT responded earlier than digestive gland MT, reinforcing the role of gills as sentinel organs. Notably, MT induction was not sustained: at 0.15 mg/L Cd, gill MT in M. veneriformis plateaued by Day 7, suggesting saturation of metal-binding capacity or a shift in stress response pathways (Jing et al., 2025b; Lompré et al., 2024), consistent with reports of progressive DNA damage in this species under high Cd load (Zhang et al., 2023).
MDA, a terminal product of lipid peroxidation, rose significantly in gill tissue of M. meretrix by Day 3 at 0.05 mg/L Cd (Figure 5B), while digestive gland MDA increased only at ≥0.10 mg/L (Figure 5A), again highlighting gill susceptibility. M. veneriformis showed more amplified MDA responses: gill MDA surged from Day 1 at 0.05 mg/L, with higher increments than in M. meretrix at equivalent doses (Figure 6B). These results are consistent with our previous findings (Ren et al., 2021). Temporal correlation analysis revealed that MDA accumulation coincided with SOD collapse—e.g., in M. meretrix gills at 0.15 mg/L, SOD dropped sharply on Day 7 while MDA peaked, indicating antioxidant failure. Similarly, in M. veneriformis gills at 0.10 mg/L, MDA exceeded controls on Day 5 as SOD fell below baseline, confirming ROS overwhelm. Integrating SOD and MDA dynamics thus clarified interspecific differences in Cd-induced oxidative damage.
4.2 Comparative analysis of Cd response mechanisms in M. meretrix and M. veneriformis
Table 1 summarizes key differential responses between the two species. Integrated analysis indicates that M. veneriformis is more sensitive to Cd, as shown by: (1) earlier biomarker activation (e.g., gill SOD elevation at 0.05 mg/L on Day 1); (2) faster post-peak decline under high Cd (e.g., gill SOD below control by Day 7 at 0.15 mg/L); and (3) greater oxidative damage (higher MDA increments). These findings align with earlier reports of Cd-induced behavioral and hemocytic alterations in M. veneriformis (Wang, 2009; Giarratano et al., 2023), supporting its potential as a coastal Cd bioindicator.
Firstly, the differential sensitivity arises from physiological and ecological adaptations. M. veneriformis has a larger gill surface area that enhances Cd uptake but is coupled with lower antioxidant capacity, leading to earlier oxidative injury. In contrast, M. meretrix exhibits slower but sustained MT synthesis, favoring gradual detoxification. Ecologically, the intertidal habitat of M. meretrix may select for robust antioxidant systems, whereas the subtidal M. veneriformis likely has less adaptive capacity to acute pollution. Transcriptomic analyses have revealed developmental stage-specific gene regulation in M. meretrix larvae under Cd stress. The upregulation of CCO, Ndh, and pro-C3 in later stages contrasted with HPX and A2M induction in early developmental phases (Wang et al., 2010; Xu et al., 2024). Notably, these genes showed progressive downregulation during metamorphosis, suggesting Cd-mediated disruption of mitochondrial electron transport and immune modulation (Xu et al., 2024). The genetic response of M. veneriformis has not been extensively studied. However, hemocyte function experiments have demonstrated that Cd exposure induces a faster ROS generation rate and earlier onset of DNA damage in this species, suggesting that its molecular regulatory network may exhibit heightened susceptibility to Cd interference.
Moreover, tissue-specific regulatory mechanisms differ between the two bivalve species. In M. meretrix, MT expression shows concentration-dependent variation across tissues (Gao et al., 2021), suggesting distinct detoxification strategies: gills prioritize rapid Cd sequestration, while the digestive gland maintains homeostasis through balanced MT expression (Sun et al., 2024). Post-depuration analyses further indicate its recovery capacity, with reduced Cd levels and oxidative stress markers in ovarian tissue, along with downregulation of apoptosis-related genes (Zhou et al., 2024). In contrast, the repair mechanisms in M. veneriformis remain poorly characterized, potentially due to Cd-induced suppression of cellular recovery processes. Hemocytic responses also vary interspecifically—M. meretrix exhibits greater membrane stability and more effective DNA repair (Ren et al., 2021). Behaviorally, M. meretrix shows preferential inhibition of filtration rate over respiration rate under Cd stress (González et al., 2015), a phenomenon linked to glutathione S-transferase dynamics—initially enhanced to detoxify lipid peroxidation products, followed by rapid inhibition under high Cd, leading to suppressed feeding activity.
Correlation analysis in this study clarified the differential response mechanisms to Cd stress. At low exposure (0.05 mg/L), synergistic rises in SOD and MT suppressed MDA accumulation in gills until Day 3. Under high Cd (≥0.10 mg/L), SOD collapse combined with MT saturation led to a sharp MDA increase. Gills—directly exposed and metabolically active—showed acute sensitivity, while the digestive gland exhibited delayed but sustained responses, likely due to Cd redistribution and long-term detoxification demands. Quantitative evaluation revealed a sensitivity order of Gill MDA > Gill SOD > Gill MT, with gill MDA in M. veneriformis increasing significantly at 0.05 mg/L Cd from Day 1 (Figure 6B). In terms of stability, MT > MDA > SOD, as MT rose consistently while SOD fluctuated markedly. These distinct patterns underscore temporal and mechanistic divergence in biomarker behaviors under Cd stress. These findings, derived from a short-term (7-day) exposure, suggest the following biomarker selection criteria for pollution monitoring: Gill MDA in M. veneriformis is an optimal early-warning indicator for acute stress, given its rapid response and high sensitivity. Although this study design was acute, the response pattern of digestive gland MT in M. meretrix suggests that it may be a promising candidate for monitoring chronic exposure; however, this requires validation through longer-term studies.
While this study elucidates the differential physiological responses to Cd in these bivalves, two limitations should be noted: (1) It is important to note that this study did not measure tissue-specific cadmium accumulation. While the biomarker responses are consistent with known mechanisms of cadmium toxicity, the lack of internal dose data means that the mechanistic interpretations remain associative. Future studies incorporating tissue burden analysis are necessary to conclusively link the biomarker responses to internal cadmium dose. (2) the synergistic roles of auxiliary antioxidant enzymes (e.g., CAT, GPx) remain unexamined. Furthermore, the chronic effects beyond seven days require additional validation. Collectively, Cd exposure impairs both species via antioxidant disruption, lipid peroxidation, and MT pathway activation. These findings advance theoretical frameworks for coastal Cd biomonitoring and underscore the need to integrate tissue-specific biomarkers with long-term exposure studies to refine ecological risk assessments.
5 Conclusions
This study demonstrated distinct toxic responses in M. veneriformis and M. meretrix under short-term (7-day) Cd exposure. Gill tissue, due to its direct contact with contaminants and high metabolic activity, exhibited greater sensitivity than the digestive gland, as reflected in the biomarkers SOD, MT, and MDA. These findings support the use of gill tissue as a suitable target for acute toxicity monitoring under similar exposure conditions. M. veneriformis exhibited faster and more pronounced biomarker responses to acute Cd stress; however, its detoxification capacity (e.g., MT synthesis) appeared to saturate more readily, suggesting a lower threshold for irreversible damage under sustained exposure. In contrast, M. meretrix demonstrated a more gradual physiological adjustment, indicating a potentially higher tolerance within the acute exposure period. MDA, as a terminal product of lipid peroxidation, showed early and significant elevation in gill tissue, even at low Cd concentrations. This pattern supports its use as a sensitive biomarker for early warning of Cd-induced oxidative damage in short-term exposure scenarios. It should be noted that the 7-day exposure design limits extrapolation to chronic monitoring applications. While the differential responses observed here suggest potential species-specific advantages for longer-term biomonitoring, such conclusions would require validation through studies with extended exposure durations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Ethics Committee of Shandong University of Aeronautics. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. ZZ: Conceptualization, Validation, Writing – review & editing. BL: Funding acquisition, Writing – review & editing. QZ: Formal Analysis, Investigation, Methodology, Writing – review & editing. JZ: Formal Analysis, Software, Visualization, Writing – review & editing. SS: Conceptualization, Formal Analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Doctoral Research Startup Fund of Shandong University of Aeronautics (801003025014) and Creative Program of Youth, Binzhou University of China, and the Shandong Sci-Tech SME Innovation Capacity Improvement Project (2024TSGC0967).
Acknowledgments
The authors would like to thank all the peoples of Department of City and Environment of Binzhou University and Physiological Laboratory of Ocean University of China for assistance in the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Cai B., Gandon L., Baratange C., Eleyele O., Moncrieffe R., Montiel G., et al. (2025). Assessment of the effects of cadmium, samarium and gadolinium on the blue mussel (Mytilus edulis): A biochemical, transcriptomic and metabolomic approach. Aquat. Toxicol. 279, 107217. doi: 10.1016/j.aquatox.2024.107217
Cai C. and Wang W.-X. (2024). Reduced copper uptake and efflux by the mussel Mytilus coruscus after Cu exposure: Implication for biomonitoring. Environ. pollut. 362, 124956. doi: 10.1016/j.envpol.2024.124956
Cai C., Zhao G., Su D., Ding X., Ni X., and Zhang Y. (2024). Risk assessment and source analysis of heavy metal pollution in wetland sediments in the northern Yellow River Delta. Mar. Geol. Quat. Geol. 44, 176–188. doi: 10.16562/j.cnki.0256-1492.2024030801
da Luz J. Z., Gorshkov V., Miranda R. R., de Souza T. L., Ribeiro L. R., Duan X., et al. (2025). Metallothionein as a biomarker of aquatic contamination in fish: An in silico and in vitro approach using zebrafish as experimental model organism. Chemosphere 376, 144316. doi: 10.1016/j.chemosphere.2025.144316
Fang Y., Yang H., Wang T., Liu B., Zhao H., and Chen M. (2010). Metallothionein and superoxide dismutase responses to sublethal cadmium exposure in the clam Mactra veneriformis. Comp. Biochem. Phys. C 151, 325–333. doi: 10.1016/j.cbpc.2009.12.005
Gao Y., Hong J., Guo Y., Chen M., Chang A. K., Xie L., et al. (2021). Assessment spermatogenic cell apoptosis and the transcript levels of metallothionein and p53 in Meretrix meretrix induced by cadmium. Ecotox. Environ. Safe. 217, 112230. doi: 10.1016/j.ecoenv.2021.112230
Giarratano E., Lompré J. S., and Malanga G. (2023). Evidences of metabolic alterations and cellular damage in different tissues of scallops Aequipecten tehuelchus exposed to cadmium. Mar. Environ. Res. 188, 106011. doi: 10.1016/j.marenvres.2023.106011
González C., Albentosa M., Campillo J. A., Vinas L., Romero D., Franco A., et al. (2015). Effect of nutritive status on Mytilus galloprovincialis pollution biomarkers: implications for large-scale monitoring programs. Aquat. Toxicol. 167, 90–105. doi: 10.1016/j.aquatox.2015.07.007
Hu J. and Wang W.-X. (2024). Cadmium impacts on calcium mineralization of zebrafish skeletal development and behavioral impairment. Aquat. Toxicol. 273, 107033. doi: 10.1016/j.aquatox.2024.107033
Jiang H., Li R., Zhao M., Peng X., Sun M., Liu C., et al. (2024a). Toxic effects of combined exposure to cadmium and diclofenac on freshwater crayfish (Procambarus clarkii): Insights from antioxidant enzyme activity, histopathology, and gut microbiome. Aquat. Toxicol. 268, 106844. doi: 10.1016/j.aquatox.2024.106844
Jiang Y., Yu J., Tian J.-Y., Yang G.-P., Liu L.-F., Song X.-R., et al. (2024b). Microplastics and copper impacts on feeding, oxidative stress, antioxidant enzyme activity, and dimethylated sulfur compounds production in Manila clam Ruditapes philippinarum. Mar. pollut. Bull. 208, 117022. doi: 10.1016/j.marpolbul.2024.117022
Jing R., Yu Y., Di X., Qin X., Zhao L., Liang X., et al. (2025a). Supplying silicon reduces cadmium accumulation in pak choi by decreasing soil Cd bioavailability and altering the microbial community. Environ. Sci. Proc. Imp. 27, 1145–1156. doi: 10.1039/D4EM00583J
Jing Y., Zhang T., Hu F., Liu G., and Sun M. (2025b). Single and combined effects of phenanthrene and cadmium on oxidative stress and detoxification related biomarkers in clams (Meretrix meretrix). Comp. Biochem. Phys. C 287, 110050. doi: 10.1016/j.cbpc.2024.110050
Lam P. K. S. and Gray J. S. (2003). The use of biomarkers in environmental monitoring programmes. Mar. pollut. Bull. 46, 182–186. doi: 10.1016/S0025-326X(02)00449-6
Li Z., Qi R., Miao J., Li Y., Wang Q., Lei F., et al. (2025). The source-specific health risk and biological effect assessment of PAHs in Mactra veneriformis from the Bohai Sea and Yellow Sea. Environ. pollut. 370, 125900. doi: 10.1016/j.envpol.2025.125900
Li Q., Xia B., Sui Q., Qu K., Zhu L., and Li Y. (2024a). Heavy metal pollution and risk assessment in different species of fish from the Bohai Sea. Prog. Fish. Sci. 45, 39–49. doi: 10.19663/j.issn2095-9869.20231031
Li X., Yao E., Li J., and Lu W. (2024b). Differential toxic effects of nano-titanium dioxide on clams (Meretrix meretrix) with various individuality. Aquat. Toxicol. 274, 107045. doi: 10.1016/j.aquatox.2024.107045
Liu L., Gui H., Zou D., Jiao W., Wang S., Wan X., et al. (2024a). Long-term adaptation of water hyacinth to low cadmium involves antioxidant enzyme and metallothionein transcriptional regulation. Chemosphere 365, 143346. doi: 10.1016/j.chemosphere.2024.143346
Liu J., Wang E., Xi Z., Dong J., Chen C., Xu P., et al. (2024b). Zinc mitigates cadmium-induced sperm dysfunction through regulating Ca2+ and metallothionein expression in the freshwater crab Sinopotamon henanense. Comp. Biochem. Phys. C 279, 109860. doi: 10.1016/j.cbpc.2024.109860
Liu Y., Zuo, Ming., Wang X., Liu Q., Wang W., et al. (2022). Community structure and diversity of benthic shellfish in the intertidal zone of Yellow River Delta. Trans. Oceanol. Limn. 44, 121–129. doi: 10.13984/j.cnki.cn37-1141.2022.06.016
Lompré J. S., Giarratano E., and Gil M. N. (2024). Effect of acute cadmium exposure on oxidative stress and antioxidant system of the scallop Aequipecten tehuelchus. Chemosphere 352, 141512. doi: 10.1016/j.chemosphere.2024.141512
Lu Z., Gao N., Zhan J., Wang S., Ji C., Zhang L., et al. (2024). Comparative investigations on the metabolomic responses to cadmium in clams Ruditapes philippinarum from the Bohai Sea and South China Sea. Mar. pollut. Bull. 209, 117100. doi: 10.1016/j.marpolbul.2024.117100
McComb J., Turquoise C., Xia F. A., and Tchounwou H. (2014). Understanding biogeochemical cycling of trace elements and heavy metals in estuarine ecosystems. J. Bioremediat. Biodegrad. 5, 1000e148. doi: 10.4172/2155-6199.1000e148
Moncaleano-Niño A. M., Barrios-Latorre S. A., Poloche-Hernández J. F., Becquet V., Huet V., Villamil L., et al. (2017). Alterations of tissue metallothionein and vitellogenin concentrations in tropical cup oysters (Saccostrea sp.) following short-term (96h) exposure to cadmium. Aquat. Toxicol. 185, 160–170. doi: 10.1016/j.aquatox.2017.02.01
Pandiyan J., Mahboob S., Govindarajan M., Al-Ghanim K. A., Ahmed Z., Al-Mulhm N., et al. (2021). An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: A perspective of tackling environmental threats for food security. Saudi J. Biol. Sci. 28, 1218–1225. doi: 10.1016/j.sjbs.2020.11.072
Pellerin J. and Amiard J.-C. (2009). Comparison of bioaccumulation of metals and induction of metallothioneins in two marine bivalves (Mytilus edulis and Mya arenaria). Comp. Biochem. Phys. C 150, 186–195. doi: 10.1016/j.cbpc.2009.04.008
Perić L., Perusco V. S., and Nerlović V. (2020). Differential response of biomarkers in the native European flat oyster Ostrea edulis and the non-indigenous Pacific oyster Crassostrea gigas co-exposed to cadmium and copper. J. Exp. Mar. Biol. Ecol. 523, 151271. doi: 10.1016/j.jembe.2019.151271
Perrigault M. and Allam B. (2012). Differential immune response in the hard clam (Mercenaria mercenaria) against bacteria and the protistan pathogen QPX (quahog parasite unknown). Fish Shellfish Immun. 32, 1124–1134. doi: 10.1016/j.fsi.2012.03.018
Qiang S., Che Y., Lu M., Tian Y., Gao L., Chen J., et al. (2025). Buprofezin causes early developmental toxicity of zebrafish (Danio rerio) embryos: morphological, physiological and biochemical responses. Aquat. Toxicol. 284, 107371. doi: 10.1016/j.aquatox.2025.107371
Ren J., Liu S., Zhang Q., Zhang Z., and Shang S. (2024). Effects of cadmium exposure on haemocyte immune function of clam Ruditapes philippinarum at different temperatures. Mar. Environ. Res. 195, 106375. doi: 10.1016/j.marenvres.2024.106375
Ren J., Shang S., and Xia J. (2022). Effects of cadmium exposure on behavior and biotransformation functions of clams Meretrix meretrix and Mactra veneriformis. Trans. Oceanol. Limn. 44, 42–49. doi: 10.13984/j.cnki.cn37-1141.2022.04.00
Ren J., Xia J., and Shang S. (2021). Effects of cadmium exposure on hemocyte function and DNA damage in clam Meretrix meretrix and Mactra veneriformis. Trans. Oceanol. Limn. 43, 98–106. doi: 10.13984/j.cnki.cn37-1141.2021.03.01
Stohs S. J. and Bagchi D. (1995). Oxidative mechanisms in the toxicity of metal ions. Free Radical Bio. Med. 18, 321–336. doi: 10.1016/0891-5849(94)00159-H
Su L., Li H., Wu Y., Tao A., Qiu N., Wang R., et al. (2023). Parental exposure to environmental cadmium causes developmental defects in the rare minnow (Gobiocypris rarus) embryos: The association between ROS-mediated oxidative stress and developmental dysfunction. J. Environ. Chem. Eng. 11, 111264. doi: 10.1016/j.jece.2023.111264
Sun Z., Gong Y., Liu S., Fang Z., Huang X., Zhang H., et al. (2025). Brown fish meal substituting white fish meal affects growth performance, antioxidant capacity, immune response and flesh quality of mandarin fish (Siniperca chuatsi). Aquacult. Rep. 42, 102852. doi: 10.1016/j.aqrep.2025.102852
Sun M., Jing Y., Zhang T., Hu F., Chen Q., and Liu G. (2024). Effect of salinity on the toxicokinetics, oxidative stress, and metallothionein gene expression in Meretrix meretrix exposed to cadmium. Comp. Biochem. Phys. C 279, 109863. doi: 10.1016/j.cbpc.2024.109863
Tong R., Jing F., Li Y., Pan L., Yu X., Zhang N., et al. (2025). Mechanisms of intestinal DNA damage and inflammation induced by ammonia nitrogen exposure in Litopenaeus vannamei. Comp. Biochem. Phys. C 287, 110070. doi: 10.1016/j.cbpc.2024.110070
Viarengo A., Ponzano E., Dondero F., and Fabbri R. (1997). A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar. Environ. Res. 44, 69–84. doi: 10.1016/S0141-1136(96)00103-1
Wang X. (2009). Physiological response of Mactra veneriformis under Cadmium and Mercury pollution stress. Qingdao,China: Institute of Oceanology, Chinese Academy of Sciences.
Wang Z., Kong F., Fu L., Li Y., Li M., and Yu Z. (2021). Responses of Asian clams (Corbicula fluminea) to low concentration cadmium stress: Whether the depuration phase restores physiological characteristics. Environ. pollut. 284, 117182. doi: 10.1016/j.envpol.2021.117182
Wang Y., Li Y., Zang X., Moussian B., Shah D., Pang M., et al. (2025). Lead (Pb) load interacts with oxidative stress in Drosophila melanogaster through MTF-1/Nrf2/JNK mediated metallothionein expression. Ecotox. Environ. Safe. 292, 117921. doi: 10.1016/j.ecoenv.2025.117921
Wang L., Song L., Ni D., Zhang H., and Liu W. (2009). Alteration of metallothionein mRNA in bay scallop Argopecten irradians under cadmium exposure and bacteria challenge. Comp. Biochem. Phys. C 149, 50–57. doi: 10.1016/j.cbpc.2008.07.001
Wang Q., Wang X., Wang X., Yang H., and Liu B. (2010). Analysis of metallothionein expression and antioxidant enzyme activities in Meretrix meretrix larvae under sublethal cadmium exposure. Aquat. Toxicol. 100, 321–328. doi: 10.1016/j.aquatox.2010.08.012
Wills E. D. (1987). “Evaluation of lipid peroxidation in lipids and biological membranes,” in Biochemical Toxicology: A Practical Approach. Eds. Snell K. and Mullock B. (IRL Press, Washington, USA), 127–152.
Wu X., Jia Y., and Zhu H. (2012). Bioaccumulation of cadmium bound to ferric hydroxide and particulate organic matter by the bivalve M. meretrix. Environ. pollut. 165, 133–139. doi: 10.1016/j.envpol.2012.02.023
Xu Y., Wu C., Jin J., Tang W., Chen Y., Chang A. K., et al. (2024). Transcriptome analysis and identification of cadmium-induced oxidative stress response genes in different Meretrix meretrix developmental stages. Animals 14, 352. doi: 10.3390/ani14020352
Yang J., Guo Y., Hu J., Bao Z., and Wang M. (2023). A metallothionein gene from hard clam Meretrix meretrix: Sequence features, expression patterns, and metal tolerance activities. Dev. Comp. Immunol. 149, 105057. doi: 10.1016/j.dci.2023.105057
Yang H., Zhao Z., Li H., and Wang L. (2024). Metal binding feature of copper–induced metallothionein from freshwater crab Sinopotamon henanense reveals its Cu–thionein character. Protein Express. Purif. 221, 106519. doi: 10.1016/j.pep.2024.106519
Yang H., Zhu Z., Xie Y., Zheng C., Zhou Z., Zhu T., et al. (2022). Comparison of the combined toxicity of polystyrene microplastics and different concentrations of cadmium in zebrafish. Aquat. Toxicol. 250, 106259. doi: 10.1016/j.aquatox.2022.106259
Yunko K., Martyniuk V., Gnatyshyna L., Khoma V., Matskiv T., Tulaidan H., et al. (2025). Alleviation of specific responses in the combined exposure of freshwater mussel Unio tumidus to psychoactive substances and microplastics. Environ. Toxicol. Phar. 116, 104682. doi: 10.1016/j.etap.2025.104682
Zhang X., Li F., Ji C., and Wu H. (2023). Toxicological mechanism of cadmium in the clam Ruditapes philippinarum using combined ionomic, metabolomic and transcriptomic analyses. Environ. pollut. 323, 121286. doi: 10.1016/j.envpol.2023.121286
Zhang X., Sun T., Li F., Ji C., Liu H., and Wu H. (2024b). Combinatorial accumulation, stress response, detoxification and synaptic transmission effects of cadmium and selenium in clams Ruditapes philippinarum. Aquat. Toxicol. 275, 107075. doi: 10.1016/j.aquatox.2024.107075
Zhang B., Wang X., Meng F., Du S., Li H., Xia Y., et al. (2024a). Metabolic variation and oxidative stress responses of clams (Ruditapes philippinarum) perturbed by ofloxacin exposure. J. Hazard. Mater. 480, 135783. doi: 10.1016/j.jhazmat.2024.135783
Zhang X., Wang X., and Yan B. (2021). Single and combined effects of phenanthrene and polystyrene microplastics on oxidative stress of the clam (Mactra veneriformis). Sci. Total Environ. 771, 144728. doi: 10.1016/j.scitotenv.2020.144728
Zheng X., Li X., Qi R., Li Z., Liao Q., Xu Q., et al. (2025). Ovarian toxicity of 2,6-di-tert-butyl-hydroxytoluene on female Ruditapes philippinarum: Reproductive endocrine disruption and oxidative stress. J. Hazard. Mater. 492, 138289. doi: 10.1016/j.jhazmat.2025.138289
Zhou J., Cai H., Zhong Y., Zheng Y., Wu Y., Chang A. K., et al. (2024). Reversal of cadmium-induced toxicity in Meretrix meretrix as determined by alleviation of oxidative damage following short-term depuration. Front. Mar. Sci. 11, 1444061. doi: 10.3389/fmars.2024.1444061
Keywords: cadmium, Meretrix meretrix, Mactra veneriformis, metallothionein, superoxide dismutase, malondialdehyde
Citation: Ren J, Zhang Z, Liu B, Zhang Q, Zhang J and Shang S (2025) Comparative analysis of immunomodulatory biomarker responses in clams Meretrix meretrix and Mactra veneriformis to waterborne cadmium exposure. Front. Mar. Sci. 12:1674599. doi: 10.3389/fmars.2025.1674599
Received: 28 July 2025; Accepted: 30 September 2025;
Published: 14 October 2025.
Edited by:
Andrew James Manning, HR Wallingford, United KingdomCopyright © 2025 Ren, Zhang, Liu, Zhang, Zhang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaiwang Zhang, enp3YW5nYnp1QDE2My5jb20=; Bingshen Liu, bGl1YmluZ3NoZW4xNkAxNjMuY29t
 Jiayun Ren
Jiayun Ren Zaiwang Zhang
Zaiwang Zhang Bingshen Liu
Bingshen Liu Qiong Zhang1
Qiong Zhang1 Jiqiang Zhang
Jiqiang Zhang Shuai Shang
Shuai Shang