Abstract
Marine Recreational Fishing (MRF) is an important socio-economic sector worldwide, yet data scarcity on MRF effort poses challenges in understanding and managing its effects and impacts on fish stocks. This study investigates the characteristics and impacts of the bottom longline employed by MRF in Italian Seas. Data was collected through social media platforms, providing a contemporary approach to monitoring recreational fisheries. Although this experimental approach faces issues such as incomplete data and biases, it represents a cost-effective tool to monitor fishing effort and bycatch. Through the analysis of 235 observations across 7 different Mediterranean geographical subareas, we identified two longline types i.e. shallow-set (SLLS) and deep-set longline (DLLS), characterized their fishing methods, target species, catch efficiency, and bycatch occurrences. Results indicated that while the recent Italian regulatory change that reduced the maximum number of allowed hooks per vessel from 200 to 100 has generated controversy among recreational fishers, it does not significantly affect catch abundance or species composition. Furthermore, the study revealed low bycatch rates, particularly for sensitive species, but highlighted vulnerabilities among demersal elasmobranchs species. Overall, the present study contributed to understanding the dynamics of marine recreational longlining and highlighted the need for improved data collection methods and ongoing monitoring for evidence-based policy decisions in the context of Mediterranean fisheries management.
1 Introduction
Marine Recreational Fishing (MRF) is defined as a non-commercial (i.e. not for sale, barter, or trade) subset of capture/harvest fisheries (Pawson et al., 2008), motivated by catching fish for fun, pleasure, or sport or just as supplementary food source (Cooke et al., 2018). Along most coastal areas of the world, MRF has great importance in terms of number of people involved, estimated to be 8.7 million in Europe (Bachiller et al., 2022), with 1.24 million only in Italy (Bolognini et al., 2022; Tarantino et al., 2025), incomes, health and well-being for the whole community (Arlinghaus and Cooke, 2009; Pranovi et al., 2016). A major challenge in managing MRF is the lack of data on catch effort particularly for overexploited fish stocks as, in some regions, it may overtakes those of commercial fisheries (Bolognini et al., 2022; Coleman et al., 2004; Ihde et al., 2011), thus making it necessary to combine commercial and recreational catch data to better understand stock dynamics (Freire and Rocha, 2021). Quantifying the magnitude of MRF is crucial for evaluating fishing pressure, developing a monitoring system, and implementing management measures (Cooke and Cowx, 2006).
In the Mediterranean Sea, marine recreational fisheries catches remain largely unassessed since they are excluded from commercial stock assessments: this is primarily due to the challenges in monitoring such activities across multiple countries (both EU and non-EU) with often different regulations and legislative frameworks in MRF matter (Bolognini et al., 2022). Although several European countries have implemented regulatory measures to control recreational fishing (Gaudin and De Young, 2007), these are often not adequately enforced, thereby rendering them ineffective in accurately estimating the effort associated with such activities (Bolognini et al., 2022). To practice MRF in Italy, only a mandatory communication to the website of Ministry of Agriculture, Food Sovereignty and Forest (MASAF) is required, and fishing is governed by general guidelines (e.g. daily catch limit), while France and Spain require a fishing license with species-specific limits and, in Spain, mandatory digital catch reporting and gear tagging will be implemented in 2026 (EC, 2025). Effort and catches in MRF in Europe are currently estimated using various approaches, with field surveys, interviews (Maynou et al., 2013), license data, or the number of registered boats in a specific area (McCluskey and Lewison, 2008) and even through the use of angler apps (Venturelli et al., 2017). A further contribution to this issue could be offered by contents shared on digital platforms like social media from recreational anglers (Giovos et al., 2018; Kaplan and Haenlein, 2010), providing valuable data for MRF (Belhabib et al., 2016). Recent studies have shown social media to be an innovative and cost-effective tool for information gathering (Lennox et al., 2022), given its ability to provide a more discreet and non-invasive approach (Kaplan and Haenlein, 2010), thus being employed to inform about recreational fishing (Sbragaglia et al., 2020). However, this modern approach presents methodological challenges due to non-standardized data, selective reporting, and limited geolocation accuracy (Bolognini et al., 2022; Giovos et al., 2018; Vitale et al., 2021). Ethical concerns regarding privacy and data ownership also emerge (Monkman et al., 2018). In this study we focused on the bottom longline engaged by recreational fishers in Italian waters, which include 3 subregions established by General Fisheries Commission for the Mediterranean (GFCM) i.e. Western Mediterranean, Central Mediterranean and Adriatic Sea, divided into 7 Geographical Subareas (GSAs). We decided to investigate on this specific gear, as it is poorly documented and has been historically understudied despite being widely adopted, thus offering the opportunity to provide a relatively novel overview. The bottom longline consists of a mainline made of nylon or braid anchored to the seabed, with numerous baited hooks attached at intervals to the mainline by branch lines (shorter nylon lines also called “snoods”). Until 2024, the Italian regulation for this gear required the use of no more than 200 hooks per boat with a daily maximum total allowed weight of 5 Kg per person (DPR, 1968), furthermore, set and haul operations had to be run manually and not by automatic winch (MIPAAF, 2010). However, the new Ministerial Decree (art. 2 D.M. n. 45439, 30/01/2024) decreased the maximum number of hooks per boat from 200 to 50, which was eventually updated shortly after and re-increased from 50 to 100 hooks (MASAF, 2024).
In light of these new national regulations and the scarce information available on Italian recreational bottom longline, we conducted a preliminary evaluation on this specific gear. In particular, we aimed to:
-
Describe the longline employed by MRF in its general characteristics and identify the different types along the Italian peninsula.
-
Assess the gear efficiency and selectivity towards the target species and the incidental catch of vulnerable species, taking into account the hooks number reduction following the new government decree.
-
Evaluate and verify a new investigation methodology for MRF through the use of social media platforms as an integrative approach compared with traditional methods and its potential improvement.
2 Materials and methods
2.1 Data collection
Data was collected online using two of the most popular social media content sharing platforms i.e. YouTube and Facebook, since photos and videos shared by recreational fishers, in addition to displaying their catches, can reveal valuable data regarding the location, depth, and mass of the fish (Sbragaglia et al., 2021). Only these two platforms were included in the data collection, since they are the most widely used platforms for content sharing, and the only ones capable of providing sufficiently detailed content on the topic. In particular, YouTube videos often allowed to observe the entire fishing operation (setting and hauling of longline, with technical explanations). Regarding Facebook, we chose to collect data from Facebook private groups, where users are more likely to share their full catches and longline technical details because they may feel safer and more protected compared to public platforms.
Video footages from YouTube and pictures from Facebook, on the species targeted by recreational bottom longline from different GSAs limited to Italian coastline, were collected. The video search focused exclusively on YouTube content as most of people share online videos there (Ricke, 2014), while the pictures were taken from specific Facebook fishing groups on recreational longline. We assumed that posts shared by recreational fishers on this social network could act as a representation of recreational fishing variables (Giovos et al., 2018; Sbragaglia et al., 2020), such as species caught, gear used and GSA. In full respect of the content authors, no sensitive information or images that could violate their privacy have been disclosed.
2.1.1 YouTube
Analyzed videos on YouTube were chosen in line with a search criterion limited to the results appearing via these specific Italian keywords: “palangaro sportivo”, “palangaro ricreativo”, “palamito di fondale”, “palamito ricreativo”, “palamito sportivo”, “palamito pesca”. Only the content depicting amatorial, and not professional, fishing actions was selected. The information collected came either from those already present in the description of the video itself or stated by the author in the comments, or from a competent observer through visual estimations and specific technical knowledge. Data on longline technical features, catches (both target and discarded species, if available) was collected. We gathered information from videos published in 15 years range (2010-2024).
2.1.2 Facebook
The data collected came from two different private groups of 7338 and 29963 members, respectively, specifically dedicated to recreational longline fishing, from which photos of an entire year’s catch (from May 2023 to May 2024) were collected. Contrary to data collected from YouTube videos, here only the retained catch was depicted in the images, with no information on discard or bycatch. Technical and operational characteristics of the gear were gathered from the posted picture’s description or from comments provided by the author to other users.
The bottom longline is usually kept in position using weights placed along its length to ensure it remains close to the seabed. Floating buoys may also be used to mark the location of the set longline (Lucchetti et al., 2023). The target species for this gear are typically found on (benthic) or in close proximity (demersal) to the seabed (Cillari et al., 2012). As described by Ferretti et al., 2002 the nature of the fishery in the different regions is distinct both with respect to target species and the rigging of the line. In this study, we classified recreational longlines into two distinct categories, discriminated based on their operational depth. We discerned a shallow-set longline (SLLS), operating above the bathymetric of 40 m, which outlines the limit between the sublittoral and circumlittoral plane, and a deep-set longline (DLLS) operating below it. Both types are bottom-set longlines, as surface (or drift) longlines can only be operated by professional fishers (EU, 2019).
2.1.3 Expert panel evaluation
An expert panel consisting of 5 specialists was assembled to visually inspect selected photos (sourced from Facebook) and videos (sourced from YouTube). The experts were selected based on their documented experience in fisheries biology and their expertise in measuring fish morphological parameters in selectivity and monitoring experiments within both professional and recreational fisheries. The biological information estimated by experts includes species classification of the catch, as well as weight (kg) and length (cm) estimation, also by making reference to objects in the image as points for estimating measures. Whenever possible, catches were classified at the species level; otherwise, classification was limited to the genus. For weight and length, the 5 independently collected estimates were averaged to obtain the mean value for each variable. Other relevant technical information, such as the fishing area, depth, number of hooks, and longline length, was extracted directly from the textual description of posts or videos. Alternatively, when these details were not specified, they were inferred by experts (e.g. depth estimated based on the species composition of the catch, considered indicative of specific marine environments).
2.2 Data treatment
The information obtained from the online survey was primarily categorized by GSAs. Data was considered valid for analysis when details on GSA, number of hooks, number of crew members, and catch weight were available or could be estimated. To quantitatively describe and summarize the data, we applied descriptive statistics, including means, standard deviations, and percentages. Given that the Italian legislative decree imposes a maximum catch limit of 5 kg per person per day, the total catch for each vessel was averaged based on the number of crew members aboard. First, for each category, the ratio between the total catch weight and number of hooks was examined to evaluate the efficiency of the gear in relation to the number of hooks used per vessel. The Catch Per Unit Effort (CPUE) was calculated as grams of catch per hook, without considering the soak time due to the lack of information. Then, we compared results to determine whether the number of hooks exceeded the legal limit on daily catches established by the recently implemented laws, considering just one person per vessel. To assess significant differences in CPUE distribution between longline types (deep and shallow), as well as among hook-number categories i.e. ≤ 50 (from the minimum number of hooks observed to 50 hooks), ≤ 100 (from 51 to 100 hooks), > 100 (from 101 to the maximum number of hooks observed) a non-parametric statistical approach was applied. Given violations of the normality assumption, the Kruskal–Wallis test was applied, followed by pairwise group comparisons using Dunn’s post hoc test. All statistical analyses were performed in R version 4.3.0 (R Core Team, 2016).
3 Results
In this study, we collected a total of 235 observations, 205 of which were sourced from Facebook (278 photos) and 30 from YouTube (274 minutes analyzed). Examples of collected data (Facebook photos and YouTube video frames) are listed in Supplementary Materials (Supplementary Figures S1–S6). A total of 139 observations were made for SLLS and 96 for DLLS. The geographic area of origin was available for 57% of the samples, covering 7 different GSAs: the majority of samples came from GSA 9 (54 records, 40%), followed by GSA 10 with 25 records (19%), GSA 19 with 16 records (12%), GSA 16 with 15 records (11%), GSA 11 with 12 records (9%), GSA 18 with 11 records (8%), and finally 1 record from GSA 17 (1%). Records for which the GSA could not be determined (NA) represent 43% of the total.
3.1 Gear definition
Table 1 summarizes the technical characteristics of the gear under analysis, its operation, and catch data obtained by both authors’ descriptions and expert observations, where possible. A shallower and lighter bottom longline (SLLS) was described operating at a depth of less than 40 m (mean 27 m) with an average of 150 (ranging from 25 to 230) small Aberdeen hooks (mainly size 10-13); the mainline is 0.6–1 mm in diameter (if monofilament) or 1 mm diameter braided, while the branch line is 0.35–1 mm Ø nylon monofilament measuring from 1.5 to 2.4 m in length each. Different kind of baits are used like molluscs, crustaceans, annelids and sardines, evenly combined on a single rig, are set due to their role as primary food sources for target species, predominantly sparids such as Diplodus spp., Sparus aurata, and Pagellus spp. Beyond 40 meters of depth, a deep-set longline (DLLS) has been described, with an average operational depth of 190 meters (range 40 to 600 meters). The target species are primarily hakes, tub gurnards, and sparids such as Diplodus spp. and Pagellus spp. The mainline is typically braided with a diameter of 0.9–2 mm, while the branch line is made of monofilament nylon with a diameter ranging from 0.4-0.9 mm and lengths varying from 2 to 6 meters. Large Aberdeen or beak hooks (from size 8 to 4/0) are commonly set on average number of 200 (ranging from 40 to 600). Sardines and cephalopods are primarily used as bait, as they are typical food source for the aforementioned target species. It is evident that the average number of hooks used is generally lower in the SLLS compared to the DLLS, as is the diversity of bait types. Effects of the longline fishery on fish species are strictly related to the size of hooks, the particular type of longline, the bait, and the feeding behaviour of fish (Bjordal and Løkkeborg, 1996).
Table 1
| Shallow-set longline | Deep-set longline | |
|---|---|---|
| Technical features | ||
| Mainline | Mono: Ø 0.6–1 mm Br: Ø 1 mm |
Br: Ø 0.9–2 mm |
| Branch line | Mono, length: 1.5-2.4 m; Ø 0.35–1 mm | Mono, length: 2–6 m; Ø 0.4-0.9 mm |
| Hook type | Small Aberdeen hooks (size 10-13) | Large Aberdeen or beak hooks (size 8-4/0) |
| Hooks number | 25-230 (mean 150) | 40-600 (mean 200) |
| Bait | Molluscs such as octopus and squid, sardines, worms and shrimp. Also mixed baits | Sardine, cephalopods |
| Operational features | ||
| Depth (meters) | 10-40 (mean 27) | 40-560 (mean 190) |
| Soak time (hours) | 2-8 (mean 6) | 2-12 (mean 5) |
| Nr. of anglers | 1-4 (mean 2) | 1-4 (mean 3) |
| Vessel length (meters) | 4-10 | 4-10 |
| Catches | ||
| Target species | Diplodus spp., Sparus aurata, Pagellus spp. | Merluccius merluccius, Chelidonichthys lucerna, Pagellus spp., Diplodus spp. |
| Bycatch | Squaliformes, Batoids | Squaliformes, Batoids |
Technical and operational features and catches identified within the two Italian recreational longlines: shallow-set longline (SLLS), deep-set longline (DLLS).
Ø: line diameter; Mono: monofilament line; Br: braided line. Hook type and size are described with common names and size numbering systems, respectively.
3.2 Gear distribution
For each GSA, the frequency of use of the two different fishing methods by percentage was determined. As shown in Table 2, the geographical origin of the observations by GSAs where both gears are most commonly used is GSAs 9 (SLLS 39.3% and DLLS 42%) and GSA 10 (SLLS 20.2% and DLLS 16%). Also, DLLS was observed in GSA 19 with a frequency of 28% and SLLS in GSA 16 and 18 with a frequency of 13.1%. Regarding the employment ratio between the two different methods for each GSA, it is clear that SLLS is more broadly used across all GSAs, with the exception of GSA 19 (87.5%), as shown in Table 2.
Table 2
| Overall distribution of SLLS and DLLS | Gear usage within each GSA | |||
|---|---|---|---|---|
| GSA | SLLS (%) | DLLS (%) | SLLS (%) | DLLS (%) |
| 9 | 39.3 | 42 | 61.1 | 38.9 |
| 10 | 20.2 | 16 | 68 | 32 |
| 11 | 10.7 | 6 | 75 | 25 |
| 16 | 13.1 | 8 | 73.3 | 26.7 |
| 17 | 1.2 | 0 | 100 | 0 |
| 18 | 13.1 | 0 | 100 | 0 |
| 19 | 2.4 | 28 | 12.5 | 87.5 |
Overall distribution of shallow-set (SLLS) and deep-set (DLLS) longline observations by Geographical Subarea (GSA), where the percentages refer to the total observations; and Relative usage of each gear type within each GSA, where the percentages refer to observations per GSA.
3.3 Target species
For each GSA, species contributing to the first 75% of the cumulative abundance were considered for analysis. The remaining species were sorted into an “other” category, as shown in Table 3. A total of 69 fish species were identified, mostly represented by teleosts (90%), followed by elasmobranchs (9%) and cephalopods (1%). The species that mostly contribute to the overall abundance in the total sampled catches belonged to the Sparidae family (70%), including breams (Diplodus sargus, Diplodus vulgaris, Diplodus spp.) at 28.5%, pandoras (Pagellus erythrinus, Pagellus acarne, Pagellus bogaraveo) at 13.7%, and gilthead seabream (Sparus aurata) at 12.2%. A similar analysis was conducted by partitioning the catches based on the various GSAs in which they were sampled (Table 3): in GSA 9, the species that represent more than 10% of the total are D. sargus (17.5%), P. erythrinus (15%) and S. aurata (12.7%). For GSA 10, we observed P. erythrinus (24.4%), S. aurata (23.7%) and D. sargus (17.9%), while in GSA 11, Diplodus spp. (43%), S. aurata (19.7%), Oblada melanura (12.1%). In GSA 16, P. erythrinus (23.6%) is prominent, along with other species such as Pomatomus saltatrix (15.6%) and Lepidopus caudatus (10%). In GSA 17, only Chelidonichthys lucerna is present, as 1 single record was collected. In GSA 18, D. sargus accounts for 20.5%, P. erythrinus for 18.8%, and Diplodus spp. for 14.3%. Finally, in GSA 19, C. lucerna (21.5%), P. erythrinus (21.5%), and Merluccius merluccius (15.8%) are recorded.
Table 3
| Species | GSA 9 | GSA 10 | GSA 11 | GSA 16 | GSA 17 | GSA 18 | GSA 19 | NA |
|---|---|---|---|---|---|---|---|---|
| Chelidonichthys lucerna | − | 0.3 | − | 1.0 | 100.0 | 8.5 | 22.0 | 1.6 |
| Conger conger | 2.7 | 1.3 | 3.6 | − | − | − | 0.6 | 0.5 |
| Dicentrarchus labrax | 0.2 | 2.3 | − | 6.0 | − | − | 1.1 | 4.2 |
| Diplodus sargus | 17.5 | 17.9 | 9.0 | 4.0 | − | 20.5 | 0.5 | 16.6 |
| Diplodus spp. | 1.3 | − | 43.0 | − | − | 14.3 | 5.1 | 16.8 |
| Diplodus vulgaris | − | 1.0 | − | − | − | 3.3 | − | 3.4 |
| Epinephelus marginatus | 6.3 | 0.3 | 0.9 | − | − | − | − | 0.5 |
| Lepidopus caudatus | − | 4.0 | − | 10.0 | − | − | − | 0.7 |
| Merluccius merluccius | 3.9 | 1.6 | − | 4.5 | − | − | 15.8 | 4.5 |
| Oblada melanura | − | 1.6 | 12.1 | 1.0 | − | 0.5 | 8.0 | 14.0 |
| Octopus vulgaris | − | 5.5 | − | 1.0 | − | − | − | 0.2 |
| Pagellus erythrinus | 15.0 | 24.4 | 2.7 | 23.6 | − | 18.8 | 21.5 | 6.1 |
| Pagrus pagrus | 6.9 | 3.2 | 4.0 | 7.0 | − | 6.8 | − | 3.0 |
| Phycis phycis | 1.3 | − | − | 5.0 | − | − | 9.6 | |
| Pomatomus saltatrix | 3.7 | 1.6 | − | 15.6 | − | 3.4 | − | 2.0 |
| Serranus cabrilla | 6.0 | 0.3 | − | − | − | − | − | 0.1 |
| Sparus aurata | 12.7 | 23.7 | 19.7 | − | − | 9.1 | 2.2 | 11.1 |
| Trachinotus ovatus | − | − | − | 7.5 | − | − | − | 0.2 |
| Other | 22.5 | 11.0 | 5.0 | 13.8 | 0 | 14.8 | 13.6 | 14.5 |
Species composition (%) along different Geographical Subareas (GSAs) caught with recreational longline.
The represented value indicates the percentage of the species relative to the total number of species observed in the specific GSA: percentages representing only those species whose cumulative percentages sum represent 75% of the total sampled in at least one GSA, are shown are in bold.
The most commonly caught species, whose cumulative percentage sum represents 75% of the total for both different methods of gear use, were classified, as shown in Figure 1. The most represented species in the SLLS are primarily sparids: D. sargus (17.9%), S. aurata (16.3%), Diplodus spp. (16.2%), O. melanura (10.5%), P. erythrinus (10.0%). Another species, observed in smaller percentages, is P. saltatrix (4.4%), which, like the previous ones, is typical of shallow bottoms near the coast (Andrew and Mapstone, 1987). Regarding the DLLS, the species composition is significantly different from SLLS: the most abundant are P. erythrinus (18.7%) and M. merluccius (14.0%), followed by C. lucerna (7.7%), D. sargus (6.1%), Pagrus pagrus (6.1%), Helicolenus dactylopterus (5.5%), L. caudatus (5%), Phycis phycis (4.1%), Dentex dentex (3.9%), and Epinephelus marginatus (3.5%). These species are indicative of the higher operational depths of the DLLS compared to those of the SLLS.
Figure 1
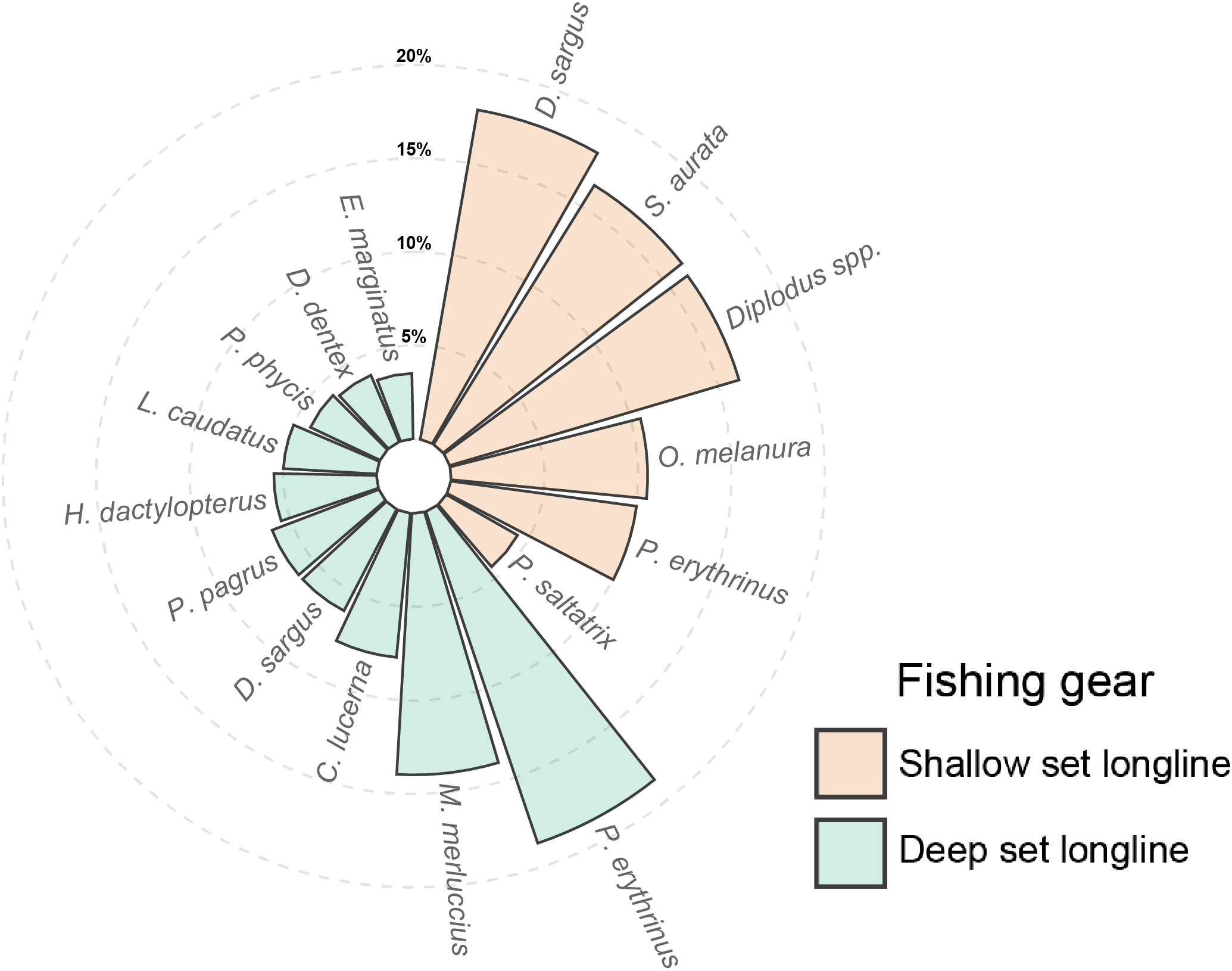
Percentage of each species whose cumulative sum represents 75% of the total catch for each gear i.e. shallow set longline (SLLS) and deep set longline (DLLS).
3.4 Bycatch species
Out of 235 records, 23 (9.4%) reported incidental catch, or bycatch (unintended capture of non-target or non-commercial species specific to that fishing method (Hall et al., 2000)), involving sharks, skates, and other species without any commercial value (Davies et al., 2009). Of these, 51% of the observed specimens were Selachii, 49% Batoids. Skates (Raja spp.) were the most frequent catches among bycatch (despite being commercially valuable), amounting to 37.5% of the total records observed. The observed bycatch records, categorized by gear type, are 4 for the SLLS (18%) and 19 for the DLLS (82%), as shown in Table 4.
Table 4
| Order | Species | Number (SLLS) | Number (DLLS) | Total number | Total record | GSA |
|---|---|---|---|---|---|---|
| Batoids | Dasyatis pastinaca | − | 2 | 2 | 2 | 10 |
| Myliobatis aquila | − | 1 | 1 | 1 | 10 | |
| Aetomylaeus bovinus | 1 | 1 | 2 | 2 | 18 | |
| Pteroplatytrigon violacea | − | 1 | 1 | 1 | NA | |
| Raja asterias | 3 | 10 | 13 | 9 | 9-10-11-17 | |
| Raja spp. | − | 2 | 2 | 2 | 9 | |
| Selachii | Geleus melastomus | − | 5 | 5 | 3 | 9-10 |
| Prionace glauca | 1 | − | 1 | 1 | NA | |
| Scyliorhinus stellaris | − | 2 | 2 | 1 | 11 | |
| Squalus acanthias | 14 | − | 14 | 1 | NA |
Most bycatch specimens observed during the survey, classified in two distinct super-orders of chondrichthyans: Batoids and Selachii.
3.5 Fishing effort
Of the 235 observations conducted, 91 records with the necessary information for the investigation were considered to both characterize the fishing effort of SLLS and DLLS and to conduct a more detailed analysis regarding the number of hooks specified in the new national decree.
The CPUE of the two gear types was calculated (Figure 2), with the SLLS yielding 75.5 g/hook and the DLLS yielding 88.9 g/hook. Then, the average CPUE was calculated for hook ranges divided into three categories based on the number of hooks: ≤50, ≤100, and >100. For the SLLS, the average CPUE was: ≤50 = 174 g/hook, ≤100 = 99.8 g/hook, and >100 = 41.5 g/hook. A similar outcome was observed for the DLLS, with the hook ranges being: ≤50 = 221.6 g/hook, ≤100 = 114 g/hook, and >100 = 54.2 g/hook.
Figure 2
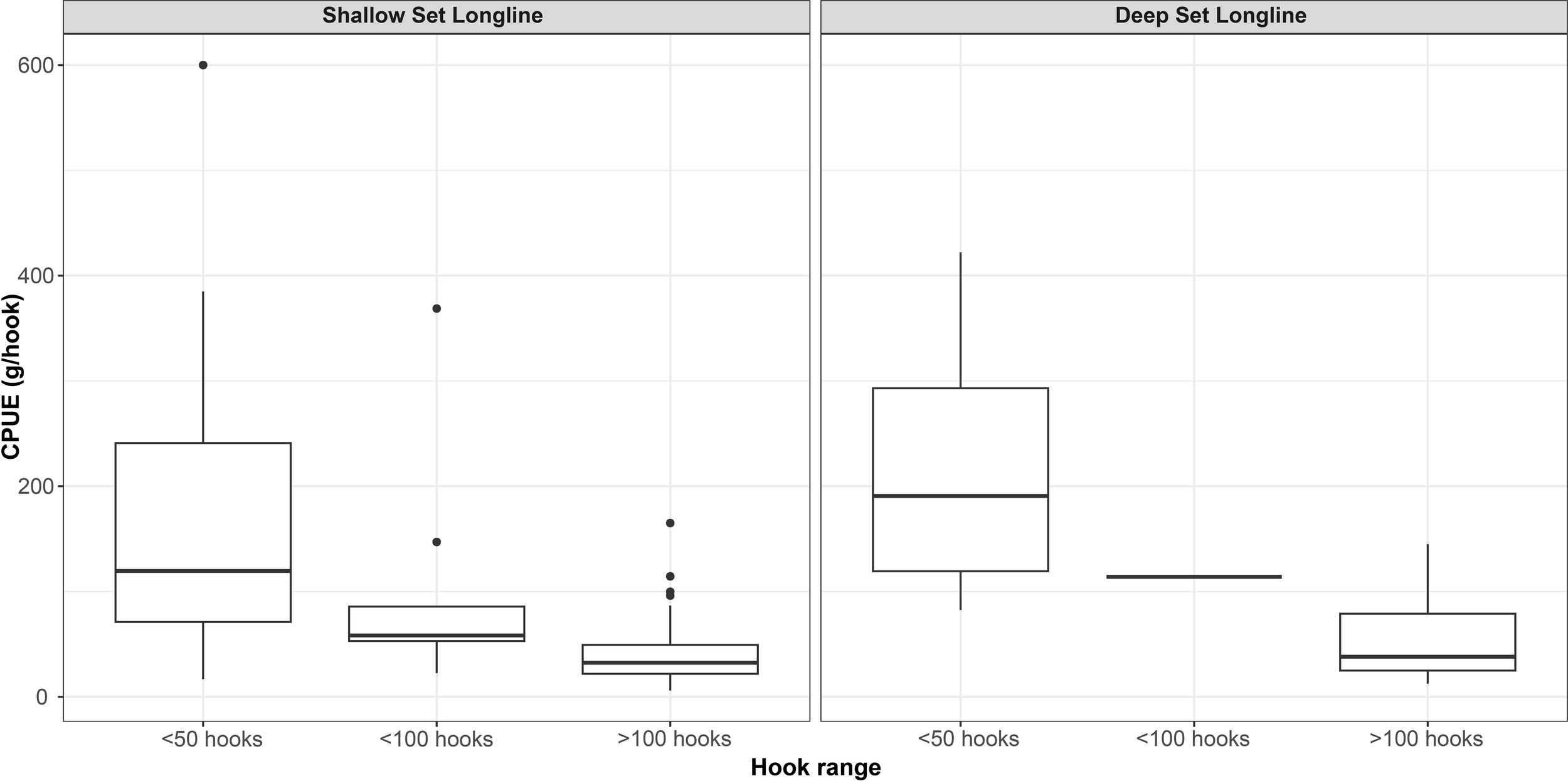
Boxplot representation of catch per unit effort (CPUE, expressed in grams per hook) for shallow-set (SLLS) and deep-set (DLLS) bottom longlines, stratified by three hook-number categories: ≤50, ≤100, and >100 hooks. Each boxplot displays the median (central horizontal line), interquartile range (IQR: 25th to 75th percentile), and whiskers extending to the 10th and 90th percentiles. Outliers beyond this range are represented as individual points.
Statistical comparisons confirmed significant differences in CPUE among hook-number categories for both gear types (Table 5). The Kruskal–Wallis test revealed a significant effect of hook range on CPUE for both SLLS (p < 0.01) and DLLS (p = 0.02). Subsequent pairwise comparisons using Dunn’s post hoc test with Bonferroni correction indicated a significant difference for the DLLS between the >100 hooks and ≤50 hooks categories (p.adj = 0.03), with the latter showing considerably higher CPUE values. No significant pairwise differences were detected among hook-number groups for the SLLS after correction (p.adj > 0.05), although the comparison between >100 and ≤50 hooks approached significance (p.adj = 0.07). Thus, a decrease in CPUE with increasing hook numbers is supported for DLLS, while for SLLS it remains a descriptive pattern without statistical support (Figure 2). Concerning the number of hooks, 70 observations were made for the SLLS and 21 for the DLLS. As shown in Figure 3 (Sankey plot), the percentage of employment for the three hooks ranges was determined for both gears. Concerning the DLLS, in 76.2% of the observations more than 100 hooks were employed, 4.8% of the time ≤ 100, and 19% of the time ≤ 50. For the SLLS, a similar pattern was observed, with more than 100 hooks being utilized in 67.1% of cases, ≤ 100 in 12.9%, and ≤ 50 in 20%. Overall, in 69.2% of the records, more than 100 hooks were used per vessel, while a 19.8% of the observations equal/less 50 hooks were set, in the remaining 11% a number of hooks between 50 and 100 were recorded. For each category of hooks, the average number of kilograms of catches was evaluated. When more than 100 hooks are employed, the average catch weight in Kg exceeds the imposed per-person limit in 66.7% of cases, while only 33.3% of the cases remain within the 5 kg limit. A similar outcome was observed for the other two hook ranges. When 100 or fewer hooks are used, the limit is surpassed in 80% of cases, while with 50 or fewer hooks, the exceedance occurs in 61.1% of cases.
Table 5
| Kruskal-Wallis rank sum test | ||||
|---|---|---|---|---|
| Fishing gear | H | df | p | |
| Deep-set longline | 7.51 | 2.00 | 0.02 | |
| Shallow-set longline | 18.85 | 2.00 | <0.01 | |
| Pairwise comparison | ||||
| Fishing gear | Pairs | Z | P.unadj | P.adj |
| Deep-set longline | <100 vs <50 | 0.77 | 0.77 | 1.00 |
| <100 vs >100 | 0.27 | 0.27 | 0.81 | |
| <50 vs >100 | 0.01 | 0.01 | 0.03 | |
| Shallow-set longline | <100 - <50 | Z | P.unadj | P.adj |
| <100 - >100 | -0.94 | 0.35 | 1.00 | |
| <50 - >100 | 2.29 | 0.02 | 0.07 | |
Results of the Kruskal–Wallis rank sum test and Dunn’s post hoc pairwise comparisons assessing differences in CPUE distributions among hook-number categories (≤50, 51–100, >100) for deep-set and shallow-set bottom longlines.
Significant results for the Kruskal–Wallis test are based on the unadjusted p-values (p < 0.05), while significant pairwise differences from the Dunn test are based on Bonferroni-adjusted p-values (p.adj < 0.05). Statistically significant values are shown in bold.
Figure 3
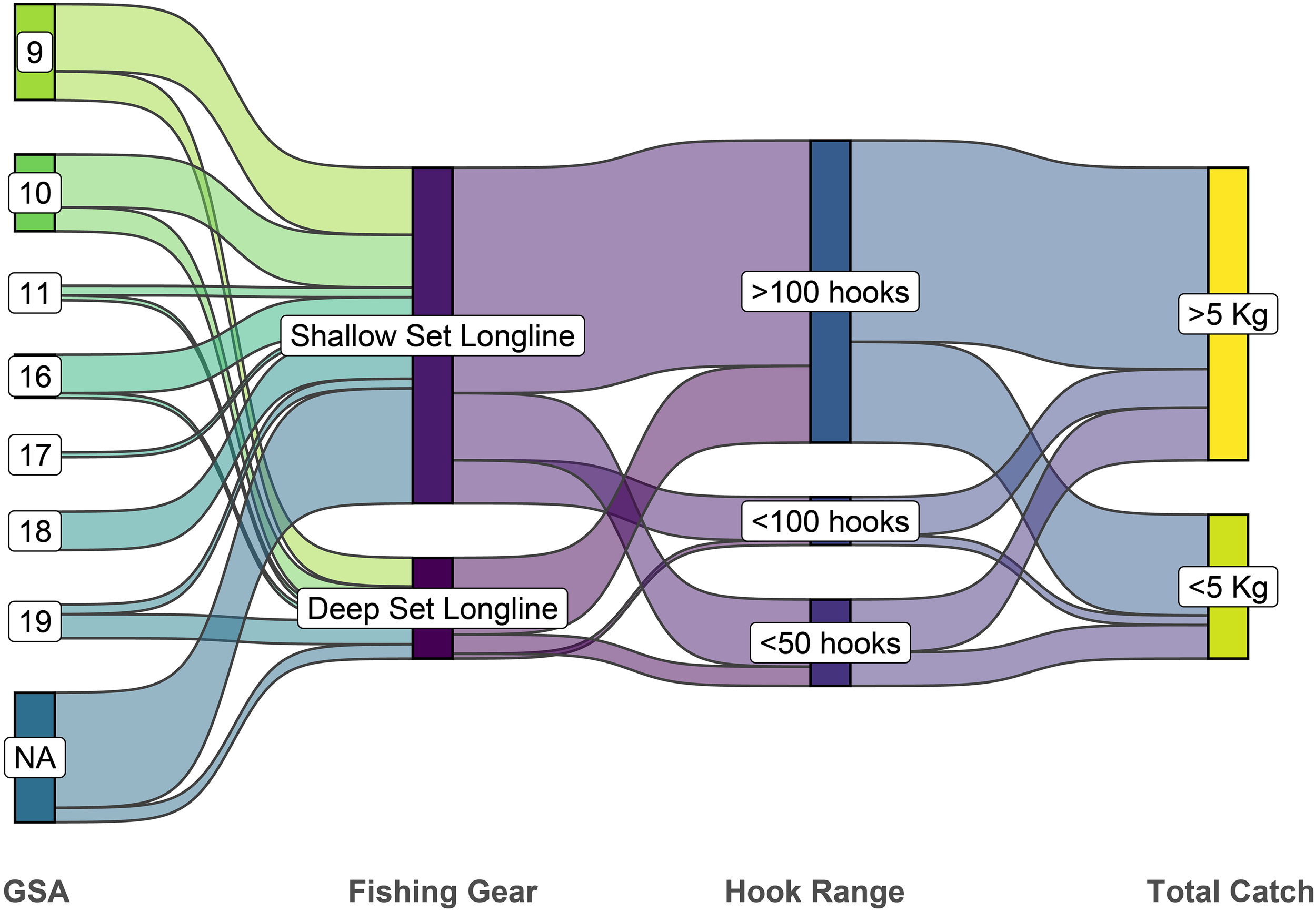
Sankey diagram showing flows from Geographical Subarea (GSA) to total-catch categories. Link widths are proportional to the number of observed records; “NA” denotes records without GSA assignment.
4 Discussion
Although recreational fishing has been documented as one of the most popular activities along the coasts of numerous countries around the world (Sutinen and Johnston, 2003) providing important social and economic role, still it is not formally assessed through the use of surveys and other quantification techniques (Gaudin and De Young, 2007). Recreational fisheries data is challenging to compile due to the high number of participants and locations, which means that many fisheries lack accurate data to assess fishing effort, fish exploitation, or harvest in each waterbody (Lester et al., 2003). In this study, the analyzed data from social media provide an overview of recreational bottom longline fishing in Italy, identifying its main characteristics and catch biodiversity, also describing any differences at GSA level. The research highlighted that this gear comes in two slightly different technical features, distinct by operational depth and target species. For this investigation, social media platforms such as YouTube and Facebook were considered, as previously conducted by Vitale et al., 2021, to investigate and monitor catches of this gear. This research methodology has highlighted several biases related to the suboptimal quality of the data (Heikinheimo et al., 2017), often incomplete due to inadequate image/video quality. To address this issue, in future studies the information about catch composition (e.g. genre and species) and biometric parameters (e.g. length and weight) could be enhanced via the use of automated image analysis or AI-based species recognition. Furthermore, detailed information from the content creator is not always accessible. Consequently, this presents increased challenges in accurately assessing the retained and discarded fractions, as well as in the measurement of morphological parameters, further complicated by the lack of reference spatial scales. An additional issue identified is the content author’s tendency to be selective about the content to publish, often highlighting exceptional catches or only the most notable ones, frequently excluding minor catches (Giovos et al., 2018) and, eventually, the bycatch of sensitive or protected species. These factors lead to a series of challenges in estimating the sample to be considered, leading to evaluations that are often incomplete due to the limited availability of data, making it nearly impossible to conduct in-depth surveys (necessitating the integration of an activity at the forefront like interviews and logbooks). Although several challenges and limitations prevent data mining on digital social platforms from being fully operationalized (Brownscombe et al., 2019; Giovos et al., 2018; Jarić et al., 2020) these methods are currently seen as a potential solution for MRF monitoring (Martin et al., 2012), representing an innovative data collection approach, that provide a large volume of information within a very short timeframe (Kaplan and Haenlein, 2010). The use of social media proves to be an effective method for accessing large amounts of information both geographically and temporally, thereby enabling studies to be conducted quickly with wide coverage, capable of rapidly assessing the overall situation.
Since the primary objective it is to provide an overview of recreational bottom longline fishing and its main technical and operational features, we conducted a preliminary analysis to assess whether CPUE varies according to the new regulation. A special attention was given to the issue concerning the recent Italian ministerial decree art. 2 D.M. n. 45439 30/012024 (MASAF, 2024) establishing a reduction in the maximum number of hooks allowed per vessel from 200 to 100. This hook number reduction resulted in a lot of controversy among sport fishing associations and recreational fishers. Consequently, we attempted to assess whether such a reduction would effectively influence catch rates, species composition, and overall fishing efficiency. Records from which all necessary data (GSA, number of hooks, number of vessels) was fully retrieved, were utilized to perform a more detailed analysis regarding the investigation of the new decree on the number of hooks. Our findings suggest that the number of hooks does not have a significant impact on catch abundance, particularly when contextualized with the operational depth of the gear. Following the Dunn’s post hoc test post-hoc comparison (Table 5), the only significant difference in terms of CPUE between the two hook ranges was found when comparing a hook number >100 vs ≤50 for SLLS. As shown in Table 1, the average number of hooks is higher for the DLLS (200) compared to the SLLS (150). This is likely associated with fish availability depending on bathymetry, with higher fish densities closer to the coast and decreasing (though with larger sizes) with increasing depth (Macpherson and Duarte, 1991). Consequently, a higher number of hooks is employed probably to compensate for the lower fish availability. Regarding the legal limits on catches in kg per person, it is observed that in both types of gear, a number of hooks exceeding 100 leads to a higher incidence of catches in terms of biomass, surpassing 5 kg in 66.7% of the records. However, even in cases where the number of hooks used is less than 50, 61.1% of the observations recorded a total catch exceeding the permitted limit. These results partially disprove the misconception that the greater the number of hooks, the greater the catch, as other factors, such as depth, also interact and can influence both the abundance and size of the catches (Nakano et al., 1997; Watson and Kerstetter, 2006). Since the analyses were conducted considering only one individual per vessel, the issue related to the maximum catch weight limit can therefore be solved by increasing the number of people on board, even though the number of hooks remains unchanged. The introduction of monitoring systems for this fishing activity, such as the implementation of specific licenses and the mandatory use of logbooks, could be effective.
Like many fishing gears, longline is also subject to bycatch (Pascoe, 1997; Piovano et al., 2010; Santos et al., 2024), referring to the unintended capture of non-target or non-commercial species specific to that fishing method (Davies et al., 2009; Hall et al., 2000). Bycatch is often (but not always) discarded regardless of their at-vessel status (live, injured or dead) and is poorly reported in some fishery records making it difficult to assess impacts (Barker and Schluessel, 2005; Bonfil & Nations, F. A. O. U, 1994; Connolly and Kelly, 1996; Squires et al., 2021). Based on the observations conducted, it can generally be stated that recreational longlining (bottom longlining) is a method with a low incidence of bycatch (Pham et al., 2014), particularly in relation to sensitive or protected species such as turtles, seabirds, and sensitive shark species, for which no captures were recorded. This is in contrast with studies in longlines of Mediterranean commercial fisheries where high bycatch rates of sharks, sea turtles and rays were observed (Clarke et al., 2014; Clusa et al., 2016; Connolly and Kelly, 1996; Hall et al., 2000; Lucchetti et al., 2023; Pascoe, 1997; Pham et al., 2014). Nevertheless, it cannot be excluded that the fisher could omit or underreport accidental captures. The majority of bycatch species captured inhabit depths greater than 20–30 m, such as sharks and skates (Serena et al., 2020). In fact, we observed a higher incidence of captures with the DLLS compared to the SLLS, due to its deeper operational depth. A significant proportion of batoids, both commercial and non-commercial, remain more susceptible to capture by bottom longlining due to their demersal habits (Hamlett, 1999). Applying measures to keep hooks or the mainline off the seafloor to avoid rays and related species, or avoiding specific areas with high shark abundance, could be considered a potential mitigation strategy (Sacchi, 2021). Ultimately, it is important to evaluate that fishers often avoid from posting images or videos that document the capture of sensitive or protected species, hence introducing a considerable bias into the study concerning such catches. This rises a critical issue regarding the ethical use of social media, particularly when it involves vulnerable species such as sharks, marine mammals, and sea turtles. Although no penalties are imposed for accidental catches, they often cause concern among fishers, who fear potential sanctions, thus leading to a major caution in publishing sensitive content.
5 Conclusion
This study provides an important contribution to the understanding of recreational longline fishing in Italy, highlighting key technical aspects, operational depths, and target species representing one of the first attempts to use social media data for recreational fisheries analysis. While the alternative use of social media platforms for data collection proved to be an effective and cost-efficient approach, it also revealed several limitations, including data quality issues, sampling biases, and gaps in information (Giovos et al., 2018). These challenges underscore the need for refinement of this methodology, as it cannot yet fully replace more traditional scientific data collection techniques, as a further data implementation derived from interviews, fishing app (in anonymous form), or boarding on boats engaged in recreational fishing. We conclude that this approach can only be considered reliable if implemented alongside, or integrated with, complementary methodologies that offset the limitations and biases associated with social media-derived data. Results suggest that reducing the number of hooks does not significantly impact catch abundance, instead, factors such as operational depth and fish availability are the primary drivers of catch rates. This indicates that the proposed regulatory changes may not effectively reduce total biomass caught, especially since instances of exceeding the catch limit were recorded even with fewer hooks. However, since this is an experimental study, further investigations should be conducted adopting more solid and validated methods in order to produce sufficiently reliable results. Regarding bycatch, the study found that recreational longlining has a relatively low incidence of capturing sensitive or protected species, with no recorded captures of turtles, seabirds, or sharks. Nevertheless, certain species, such as batoids, remain vulnerable due to their demersal habits. This highlights the need for continued monitoring and the development of mitigation strategies to minimize bycatch. In conclusion, while this research offers valuable insights into recreational longline fishing in Italy, it also identifies areas for further investigation, particularly in improving data collection methodologies, assessing the impact of regulatory changes, and mitigating bycatch risks. Future studies with more comprehensive datasets will be crucial for refining our understanding and supporting evidence-based management decisions in recreational fisheries.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GV: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AP: Data curation, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. DLV: Data curation, Formal analysis, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. AL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research project was supported by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4-Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union-NextGenerationEU.
Acknowledgments
We would like to thank the experts not included in the authors’ list as Martina Scanu, Massimo Virgili, Flavia Scocca, Enrico Lucatello. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP D33C22000960007, Project title “National Biodiversity Future Center - NBFC”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1674872/full#supplementary-material
References
1
Andrew N. L. Mapstone B. D . (1987). Sampling and the description of spatial pattern in marine ecology. Oceanogr. Mar. Biol. 25, 39–90.
2
Arlinghaus R. Cooke S. J. (2009). “ Recreational fisheries: Socio-economicimportance, conservation issues and management challenges,” in Recreational hunting, conservation and rural livelihoods: Science and practice, ed. DicksonB.HuttonJ.AdamsB. (Oxford, U.K.: Blackwell Publishing), 39–58.
3
Bachiller E. Korta M. Mateo M. Mugerza E. Zarauz L. (2022). Assessing the unassessed marine recreational fishery in the Eastern Cantabrian coast. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.975089
4
Barker M. J. Schluessel V. (2005). Managing global shark fisheries: Suggestions for prioritizing management strategies. Aquat. Conservation: Mar. Freshw. Ecosyst.15, 325–347. doi: 10.1002/aqc.660
5
Belhabib D. Campredon P. Lazar N. Sumaila U. R. Baye B. C. Kane E. A. et al . (2016). Best for pleasure, not for business: Evaluating recreational marine fisheries in West Africa using unconventional sources of data. Palgrave Commun.2, 1–10. doi: 10.1057/palcomms.2015.50
6
Bjordal Å. Løkkeborg S. (1996). Longlining ( Wiley). Available online at: https://books.google.it/books?id=mM5nQgAACAAJ.
7
Bolognini L. Cevenini F. Franza V. Guicciardi S. Petetta A. Santangelo L. et al . (2022). Preliminary estimation of marine recreational fisheries (MRF) in the time of COVID-19 pandemic: the marche region case study (Adriatic sea, Italy). Front. Mar. Science 9(December2020). doi: 10.3389/fmars.2022.823086
8
Bonfil R. Nations, F. A. O. U (1994). Overview of world elasmobranch fisheries (Rome: FAO fisheries technical paper 341, Food and Agriculture Organization of the United Nations). Available online at: https://books.google.it/books?id=upthrn838t0C. (Accessed June 2025).
9
Brownscombe J. W. Hyder K. Potts W. Wilson K. L. Pope K. L. Danylchuk A. J. et al . (2019). The future of recreational fisheries: Advances in science, monitoring, management, and practice. Fisheries Res.211, 247–255. doi: 10.1016/j.fishres.2018.10.019
10
Cillari T. Falautano M. Castriota L. Marino V. Vivona P. Andaloro F. (2012). The use of bottom longline on soft bottoms: An opportunity of development for fishing tourism along a coastal area of the Strait of Sicily (Mediterranean Sea). Ocean Coast. Manage.55, 20–26. doi: 10.1016/j.ocecoaman.2011.10.007
11
Clarke S. Sato M. Small C. Sullivan B. Inoue Y. Ochi D. (2014). Bycatch in longline fisheries for tuna and tuna-like species: a global review of status and mitigation measures. FAO Fisheries Aquaculture Tech.588, 125.
12
Clusa M. Carreras C. Pascual M. Gaughran S. J. Piovano S. Avolio D. et al . (2016). Potential bycatch impact on distinct sea turtle populations is dependent on fishing ground rather than gear type in the Mediterranean Sea. Mar. Biol.163, 1–10. doi: 10.1007/s00227-016-2875-1
13
Coleman F. C. Figueira W. F. Ueland J. S. Crowder L. B. (2004). The impact of United States recreational fisheries on marine fish populations. Science305, 1958–1960. doi: 10.1126/science.1100397
14
Connolly P. L. Kelly C. J. (1996). Catch and discards from experimental trawl and longline fishing in the deep water of the Rockall Trough. J. Fish Biol.49, 132–144. doi: 10.1111/j.1095-8649.1996.tb06071.x
15
Cooke S. J. Cowx I. G. (2006). Contrasting recreational and commercial fishing: Searching for common issues to promote unified conservation of fisheries resources and aquatic environments. Biol. Conserv.128, 93–108. doi: 10.1016/j.biocon.2005.09.019
16
Cooke S. J. Twardek W. M. Lennox R. J. Zolderdo A. J. Bower S. D. Gutowsky L. F. G. et al . (2018). The nexus of fun and nutrition: Recreational fishing is also about food. Fish Fisheries19, 201–224. doi: 10.1111/faf.12246
17
Davies R. W. D. Cripps S. J. Nickson A. Porter G. (2009). Defining and estimating global marine fisheries bycatch. Mar. Policy33, 661–672. doi: 10.1016/j.marpol.2009.01.003
18
DPR (1968). Presidential Decree No. 1639 of 2 October 1968: Regulation for the implementation of Law No. 963 of 14 July 1965 on the regulation of maritime fishing, Official Gazette No. 306, 28 November 1968, Art. 138. Rome.
19
EC . (2025). Commission implementing regulation (EU) 2025/274 of 12 February 2025 laying down detailed rules for the application of Article 55 of Council Regulation (EC) No 1224/2009 on the control of recreational fisheries. Off. J. Eur. Union. 38, 1–7.
20
EU . (2019). Regulation (EU) 2019/1241 of the European Parliament and of the Council of 20 June 2019 on the Conservation of Fisheries Resources and the Protection of Marine Ecosystems Through Technical Measures. Off. J. Eur. Union. 97, 105–201.
21
Ferretti M. Tarulli E. Palladino S. (2002). Classificazione e descrizione degli attrezzi da pesca in uso nelle marinerie italiane con particolare riferimento al loro impatto ambientale.
22
Freire K. M. F. Rocha G. R. A. (2021). Baseline on-site information on coastal recreational fishery and comparison with competitive events in Ilhéus, southern Bahia, Brazil. Mar. Fishery Sci.34, 5–19. doi: 10.47193/mafis.3412021010303
23
Gaudin C. De Young C. (2007). “ Recreational fisheries in the Mediterranean countries: a review of existing legal frameworks,” in Studies and reviews. General fisheries commission for the mediterranean ( FAO), 81. Available online at: ftp://ftp.fao.org/docrep/fao/010/a1500e/a1500e.pdf (Accessed June, 2025).
24
Giovos I. Keramidas I. Antoniou C. Deidun A. Font T. Kleitou P. et al . (2018). Identifying recreational fisheries in the Mediterranean Sea through social media. Fisheries Manage. Ecol.25, 287–295. doi: 10.1111/fme.12293
25
Hall M. A. Alverson D. L. Metuzals K. I. (2000). By-catch: problems and solutions. Mar. pollut. Bull.41, 204–219. doi: 10.1016/S0025-326X(00)00111-9
26
Hamlett W. C. (1999). Sharks, skates, and rays: the biology of elasmobranch fishes (Baltimore and Londo: JHU Press).
27
Heikinheimo V. Minin E. Tenkanen H. Hausmann A. Erkkonen J. Toivonen T. (2017). User-generated geographic information for visitor monitoring in a national park: A comparison of social media data and visitor survey. ISPRS Int. J. Geo-Information6, 85. doi: 10.3390/ijgi6030085
28
Ihde T. F. Wilberg M. J. Loewensteiner D. A. Secor D. H. Miller T. J. (2011). The increasing importance of marine recreational fishing in the US: Challenges for management. Fisheries Res.108, 268–276. doi: 10.1016/j.fishres.2010.12.016
29
Jarić I. Roll U. Arlinghaus R. Belmaker J. Chen Y. China V. et al . (2020). Expanding conservation culturomics and iEcology from terrestrial to aquatic realms. PloS Biol.18, 1–13. doi: 10.1371/journal.pbio.3000935
30
Kaplan A. M. Haenlein M. (2010). Users of the world, unite! The challenges and opportunities of Social Media. Business Horizons53, 59–68. doi: 10.1016/j.bushor.2009.09.003
31
Lennox R. J. Sbragaglia V. Vollset K. W. Sortland L. K. McClenachan L. Jarić I. et al . (2022). Digital fisheries data in the Internet age: Emerging tools for research and monitoring using online data in recreational fisheries. Fish Fisheries23, 926–940. doi: 10.1111/faf.12663
32
Lester N. P. Marshall T. R. Armstrong K. Dunlop W. I. Ritchie B. (2003). A broad-scale approach to management of ontario’s recreational fisheries. North Am. J. Fisheries Manage.23, 1312–1328. doi: 10.1577/m01-230am
33
Lucchetti A. Petetta A. Bdioui M. Gökçe G. Saber M. Sacchi J. et al . (2023). Catalogue of fishing gear in the Mediterranean and Black Sea region (Vol. 695) (Rome: Food & Agriculture Org).
34
Macpherson E. Duarte C. M. (1991). Bathymetric trends in demersal fish size: is there a general relationship? Mar. Ecol. Prog. Ser.71, 103–112. doi: 10.3354/meps071103
35
Martin D. R. Pracheil B. M. Deboer J. A. Wilde G. R. Pope K. L. (2012). El uso de internet para comprender el comportamiento de los pescadores en la era de la informática. Fisheries37, 458–463. doi: 10.1080/03632415.2012.722875
36
MASAF (2024). Modifica Del Decreto Ministeriale n.45439 Del 30 Gennaio 2024 Recante Misure Tecniche per La Pesca Sportiva e Ricreativa Con Il Palangaro (in Italian) (Rome: Ministry of Agriculture, Food Sovereignty and Forests).
37
Maynou F. Morales-Nin B. Cabanellas-Reboredo M. Palmer M. García E. Grau A. M. (2013). Small-scale fishery in the Balearic Islands (W Mediterranean): A socio-economic approach. Fisheries Res.139, 11–17. doi: 10.1016/j.fishres.2012.11.006
38
McCluskey S. M. Lewison R. L. (2008). Quantifying fishing effort: A synthesis of current methods and their applications. Fish Fisheries9, 188–200. doi: 10.1111/j.1467-2979.2008.00283.x
39
MIPAAF (2010). Survey on the extent of sport and recreational sea fishing (No. 11A01054). Official Gazette of the Italian Republic, General Series, No. 24, January 31, 2011 (Rome: Ministry of Agricultural, Food and Forestry Policies).
40
Monkman G. G. Kaiser M. Hyder K. (2018). The ethics of using social media in fisheries research. Rev. Fisheries Sci. Aquaculture26, 235–242. doi: 10.1080/23308249.2017.1389854
41
Nakano H. Okazaki M. Okamoto H. (1997). Analysis of catch depth by species for tuna longline fishery based on catch by branch lines. Bull. Natl. Res. Institute Far Seas Fisheries34, 43–62.
42
Pascoe S. (1997). Bycatch management and the economics of discarding (Vol. 370) (University of Portsmouth, United Kingdom: Food & Agriculture Org).
43
Pawson M. G. Glenn H. Padda G. (2008). The definition of marine recreational fishing in Europe. Mar. Policy32, 339–350. doi: 10.1016/j.marpol.2007.07.001
44
Pham C. K. Diogo H. Menezes G. Porteiro F. Braga-Henriques A. Vandeperre F. et al . (2014). Deep-water longline fishing has reduced impact on Vulnerable Marine Ecosystems. Sci. Rep.4, 1–6. doi: 10.1038/srep04837
45
Piovano S. Clò S. Giacoma C. (2010). Reducing longline bycatch: The larger the hook, the fewer the stingrays. Biol. Conserv.143, 261–264. doi: 10.1016/j.biocon.2009.10.001
46
Pranovi F. Anelli Monti M. Caccin A. Colla S. Zucchetta M. (2016). Recreational fishing on the West coast of the Northern Adriatic Sea (Western Mediterranean) and its possible ecological implications. Regional Stud. Mar. Sci.3, 273–278. doi: 10.1016/j.rsma.2015.11.013
47
R Core Team (2016). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: http://www.r-project.org/ (Accessed June 2025).
48
Ricke L. D. (2014). The impact of YouTube on US politics (United Kingdom: Lexington Books).
49
Sacchi J. (2021). Overview of mitigation measures to reduce the incidental catch of vulnerable species in fisheries. General Fisheries Commission for the Mediterranean. Studies and reviews No. 100 (Rome: FAO). doi: 10.4060/cb5049en
50
Santos C. C. Rosa D. Gonçalves J. M. S. Coelho R. (2024). A review of reported effects of pelagic longline fishing gear configurations on target, bycatch and vulnerable species. Aquat. Conservation: Mar. Freshw. Ecosyst.34, e4027. doi: 10.1002/aqc.4027
51
Sbragaglia V. Coco S. Correia R. A. Coll M. Arlinghaus R. (2021). Analyzing publicly available videos about recreational fishing reveals key ecological and social insights: A case study about groupers in the Mediterranean Sea. Sci. Total Environ.765, 142672. doi: 10.1016/j.scitotenv.2020.142672
52
Sbragaglia V. Correia R. A. Coco S. Arlinghaus R. (2020). Data mining on YouTube reveals fisher group-specific harvesting patterns and social engagement in recreational anglers and spearfishers. ICES J. Mar. Sci.77, 2234–2244. doi: 10.1093/ICESJMS/FSZ100
53
Serena F. Abella A. J. Bargnesi F. Barone M. Colloca F. Ferretti F. et al . (2020). Species diversity, taxonomy and distribution of Chondrichthyes in the Mediterranean and Black Sea. Eur. Zoological J.87, 497–536. doi: 10.1080/24750263.2020.1805518
54
Squires D. Ballance L. T. Dagorn L. Dutton P. H. Lent R. (2021). Mitigating bycatch: novel insights to multidisciplinary approaches. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.613285
55
Sutinen J. G. Johnston R. J. (2003). Angling management organizations: Integrating the recreational sector into fishery management. Mar. Policy27, 471–487. doi: 10.1016/S0308-597X(03)00079-4
56
Tarantino G. Curreli F. Bolognini L. Casu M. Grati F. Langeneck J. et al . (2025). A review of marine recreational fisheries research in Italy. Regional Stud. Mar. Sci.81, 103996. doi: 10.1016/j.rsma.2024.103996
57
Venturelli P. A. Hyder K. Skov C. (2017). Angler apps as a source of recreational fisheries data: opportunities, challenges and proposed standards. Fish Fisheries18, 578–595. doi: 10.1111/faf.12189
58
Vitale G. Dedeu A. L. Pujol M. Sbragaglia V. (2021). Characterizing the profile of recreational fishers who share their catches on social media. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.768047
59
Watson J. W. Kerstetter D. W. (2006). Pelagic longline fishing gear: A brief history and review of research efforts to improve selectivity. Mar. Technol. Soc. J.40, 6–11. doi: 10.4031/002533206787353259
Summary
Keywords
bottom longline, marine recreational fishing (MRF), Mediterranean Sea, social media platforms, fisheries monitoring
Citation
Vianson G, Petetta A, Li Veli D and Lucchetti A (2025) Characterization of the Italian recreational bottom longline fisheries through social media platforms. Front. Mar. Sci. 12:1674872. doi: 10.3389/fmars.2025.1674872
Received
28 July 2025
Accepted
22 September 2025
Published
13 October 2025
Volume
12 - 2025
Edited by
Tomaso Fortibuoni, Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), Italy
Reviewed by
Taner Yildiz, Istanbul University, Türkiye; Nurdan Cömert, Istanbul University, Türkiye; José Belquior Gonçalves-Neto, University of Cádiz, Spain
Updates
Copyright
© 2025 Vianson, Petetta, Li Veli and Lucchetti.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgio Vianson, Giorgio.vianson@irbim.cnr.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.