Abstract
The human food sector plays a crucial role in supporting population growth and ensuring human well-being. In the context of global food security, environmental challenges, and the diversification of protein sources, research into new innovative and sustainable protein sources is essential. In Europe, alternative protein sources such as cultivated seaweed are a promising solution, with potential health benefits and increased sustainability. This study investigated the use of high-pressure homogenization (HPH) to obtain protein-enriched fractions from dried biomass of cultivated Ulva sp. The biochemical composition, physical-chemistry (FTIR and granulometric analysis) and the biological activities of the supernatant and residue fractions were evaluated after HPH treatment at pressures of 0, 600, 800, and 1000 bars. Results showed that, depending on their nature, pressure significantly influenced the biochemical composition and separation of compounds into the residue and supernatant, and underlined the potential of HPH to enhance protein recovery from Ulva sp. HPH facilitated separation of ulvan polysaccharides, known for their anti-nutritional effects, and from the protein fraction with high recovery yields of 60.0% protein in the residue. The highest protein content was found in residues at 1000 bars (8.93%) compared to in the crude extract (4.1%). Amino acid analysis revealed that essential amino acids accounted for 42% of total amino acids in the Ulva sp. fraction, with high levels of valine, leucine and methionine. The ulvan fraction (concentration of rhamnose, uronic acids and sulphate groups) was preferentially extracted at 1000 bars, where the supernatants contained 28.6 ± 4.5% of uronic acids, 23.2 ± 4.9% of sulfate groups, and 3.72 ± 0.31% of rhamnose (p< 0.05).These results provide clear evidence that HPH is effective in disrupting the cell wall and facilitating the release of compounds of interest. These results also suggest that the HPH process could position cultivated Ulva sp. as an important potential source of food protein.
Highlights
-
High-pressure homogenization (HPH) enhanced protein extraction in the residue.
-
Maximum protein content was reached in residues at 1000 bars.
-
Essential amino acids represented 42% of total amino acids in enriched samples.
-
Ulvan fractions were preferentially extracted into the aqueous supernatant.
-
Pressure significantly influenced the biochemical distribution of compounds.
1 Introduction
Global demand for protein is projected to increase by 30% by 2050 (FAO, 2025), largely driven by population growth, rising incomes, and changing dietary preferences worldwide (Henchion et al., 2017). With the world population expected to exceed 10 billion, the pressure on agricultural production to meet protein needs will necessarily also increase (Aimutis, 2022); indeed, a 70% increase in food production will be required to satisfy growing demand (Godfray et al., 2010). In parallel, per capita protein consumption is also increasing worldwide, especially in developing countries (Fukase and Martin, 2020; Aimutis, 2022). However, increased demand, particularly for animal-based proteins, involves significant sustainability challenges. Current intensive production methods are associated with negative environmental impacts, such as higher greenhouse gas emissions, excessive use of water, and the need for even more agricultural land leading to deforestation, biodiversity loss and heightened pressure on water resources (Godfray et al., 2010; FAO, 2025).
In this context, the search for innovative and sustainable sources of protein is crucial, and alternative sources including plant-based proteins, insects, micro-organisms, and algae are emerging as promising alternatives thanks to their potential health benefits and sustainability as well as ethical considerations (Aimutis, 2022; de Souza Celente et al., 2023). The protein contents of seaweed range from 9% to 32% of their dry weight (Harnedy and FitzGerald, 2011; Wells et al., 2017; Geada et al., 2021). Among them, seaweed species belonging to the genus Ulva sp. (Chlorophyta, Ulvales) are a promising alternative source of protein. Commonly known as sea lettuce or Ao-nori, Ulva sp. are widely consumed in Asian countries both directly as edible sea vegetables or as ingredients of dietary supplements (Mantri et al., 2020; Pereira et al., 2024). Most Ulva sp. have an essential amino acid (EAA)/non-EAA balance of around 35/65, which is comparable to that of soybean (Magnusson et al., 2019; Steinbruch et al., 2024). Because they are ubiquitous and versatile, Ulva sp. are excellent candidates for industrial-scale algae farming (Hiraoka and Enomoto, 1998; Hiraoka and Oka, 2008; Ben-Ari et al., 2014). Their opportunistic growth and high reproductive capacity make Ulva sp. suitable for rapid production of biomass. Optimized production in small surface areas, for example, in a 1000L photobioreactor enabled average year-round production of 0.87 kg m−2 d−1 fresh weight corresponding to approximately 1–800 ton ha−1 y−1 (Savvashe et al., 2021). In addition, Ulva sp. are known to be able to extract and store up to 3-6% of dry weight in nitrogen, while each gram of dry biomass produced from the environment simultaneously captures 1.2 – 1.8 g of CO2 (Huo et al., 2024).
However, several challenges need to be overcome before Ulva sp. proteins can be used for food: in particular, the difficulty involved in extracting protein from seaweed (Mæhre, 2016). This is due to the presence of sulfated heteropolysaccharides that form the anionic cell walls, and that are intracellularly linked to proteins, thereby complicating their extraction (Bleakley and Hayes, 2017). In addition, insoluble phenolic compounds in Ulva sp. may be connected to cell wall polysaccharides via hydrophilic and hydrophobic interactions to which protein are attached (Lahaye and Robic, 2007). Consequently, efficient disruption of the algal cell wall is a prerequisite for the extraction of seaweed proteins. Traditional seaweed extraction methods, such as high-temperature maceration, are energy-intensive and degrade heat-sensitive nutrients including protein (Bruhn et al., 2011). The choice of a sustainable, cost-effective, non-destructive, faster yet efficient pre-treatment and extraction method needs to combine selectivity (in terms of the nature of the metabolite), cost-effectiveness, and eco-friendliness. Many processes used for the extraction of protein from algal biomass have been explored including ultrasound assisted extraction (Vega-Gómez et al., 2024), osmotic (Postma et al., 2018), enzymatic assisted extraction (Soto-Sierra et al., 2021; Wang et al., 2024), pulsed electric field (Polikovsky et al., 2016; Robin et al., 2018; Steinbruch et al., 2023) and high-pressure homogenization (HPH) (Echave et al., 2021; Soto-Sierra et al., 2021; Naseem et al., 2024; Ning et al., 2025).
High-pressure homogenization is a mechanical cell disruption, non-thermal food processing technique with promising applications in the seaweed food industry. HPH has intense physical effects, including strong shear forces, cavitation, and turbulent fluid dynamics. This method disrupts cell walls at pressures ranging from 600 to 1000 bars, thereby enhancing the release of proteins and other bioactive compounds without compromising their integrity (Huang et al., 2023; Naseem et al., 2024).
The main goal of the present study was to apply high-pressure homogenization to cause fractionation of Ulva sp. biomass under different pressure conditions to extract the protein fraction. The soluble and insoluble protein pools were evaluated simultaneously. The experiment was designed to systematically investigate the combined effects of HPH on a range of parameters: the protein level, amino acid profiles, polysaccharides, polyphenol and ash contents plus the rheological properties, granulometry and biological activity of the biomass (Figure 1).
Figure 1

Experimental design and workflow. All the experiments were realized in triplicate. “R” and “S” mean “Residue” and “Supernatant” respectively.
2 Materials and methods
2.1 Cultivation and harvest
Cultivated specimens of Ulva sp. were obtained from France Haliotis, a French company located in Plouguerneau, Brittany. The company’s starter strains were cultivated in 70-liter tanks using 250 grams of fresh, selected Ulva sp. originally sourced from the natural environment in Plouguerneau. These specimens, which constituted our raw material, were grown in filtered seawater for 21 days, and harvested on August 24, 2024. Next, the seaweeds were washed with seawater, dried at 34°C overnight, vacuum sealed, freeze-dried overnight, ground to less than 500 µm, and stored in bags at 4°C for 1 week for subsequent analysis. France Haliotis has been marketing its Ulva sp. for food applications for many years. Its production and processes are certified in accordance with French legislation.
2.2 High-pressure homogenization
Homogenization was carried out using a two-stage laboratory high-pressure homogenizer (PandaPlus 2000, GEA Niro Soavi, Parma, Italy) (Figure 2). Algal suspensions were prepared by dispersing 3 g of dried Ulva sp. powder in 300 mL of deionized water, yielding a 1% (w/v) suspension according to Ning et al. (2025). The suspensions were pumped through the homogenizer at a volumetric flow rate of 9 L·h-¹. Homogenization was performed at 20°C using an integrated cooling system to prevent overheating during processing. The samples were subjected to high-pressure homogenization at different inlet pressures Δp = 0 [control], 600, 800, 1–000 bars), for five cycles (n = 5) and each condition was performed in triplicate.
Figure 2
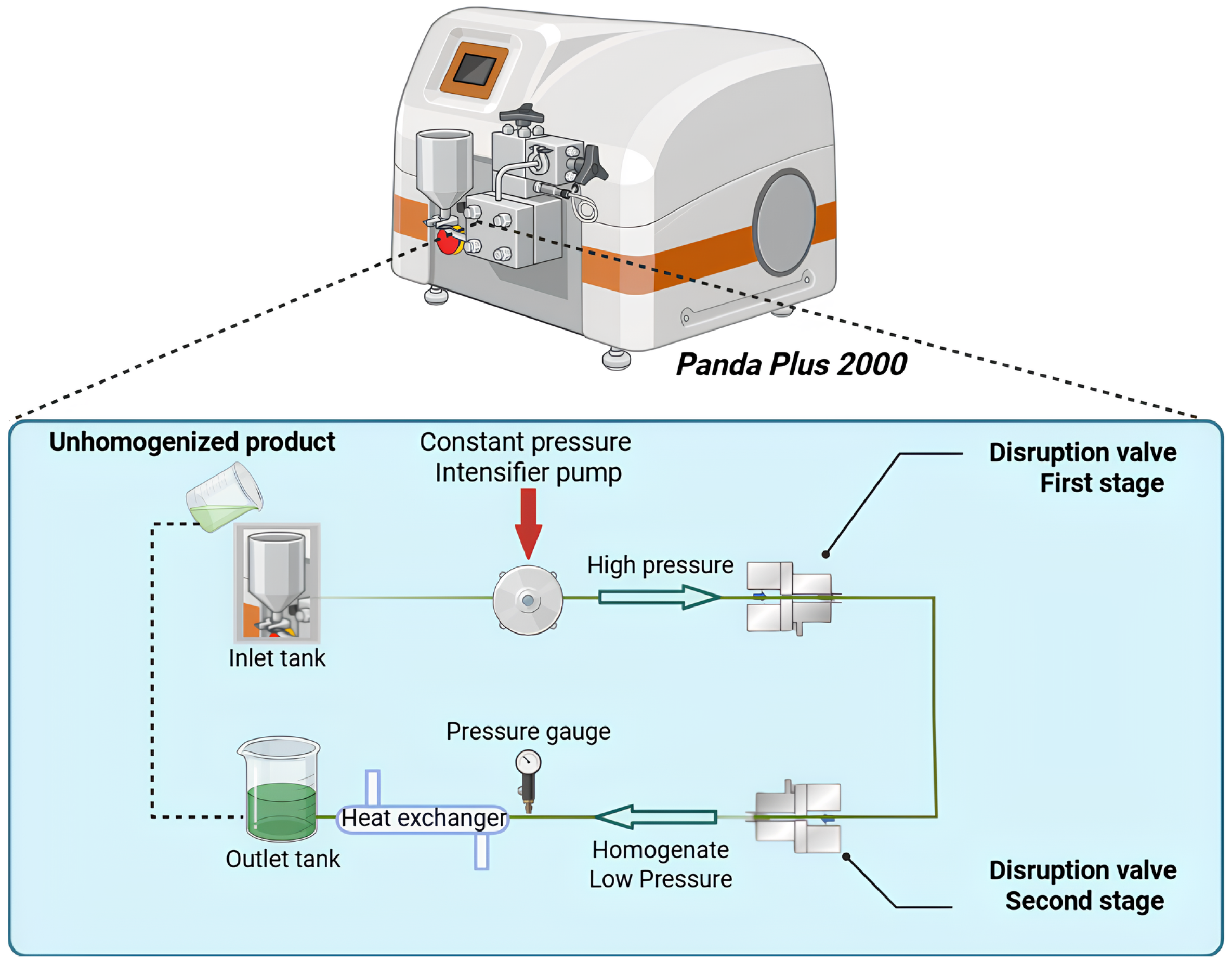
Schematic diagram of the high-pressure homogenization system (PandaPlus 2000, GEA Niro Soavi, Parma, Italy) equipped with two valves for the disruption of cellular materials.
Following homogenization, the suspensions were frozen at -25°C for 1 week, then defrosted for analysis at 8–500 g for 20 minutes at 10°C, after which the residue and the supernatant were immediately stored at 4°C and then freeze-dried overnight.
The yield of soluble extracts was calculated by dividing the weight of the compound by the weight of the dried sample of biomass. This calculation is expressed as a percentage of the dry weight of the samples.
2.3 Biochemical composition analysis
All the experiments described in this section were conducted on samples of residue and supernatant after centrifugation and freeze-drying.
2.3.1 Dry matter and ash content
Mineral matter content was determined using the method of Hardouin et al. (2014). The sample was placed in a crucible and calcined by ignition. The ash content was determined thermo gravimetrically after calcination of 100 mg of seaweed powder for 2 h in a Carbolite CSF Muffle Furnace (UK) at 585°C. The final mass corresponds to the mineral matter in the sample and is expressed as a percentage of the dry weight (d.w.).
2.3.2 Hydrolysis before biochemical analysis
Analyses were conducted on dried Ulva sp. First, 5 mg of dried Ulva sp. powder was hydrolyzed by mixing it with 1 mL of distilled water and incubated for 2 h at 100°C in a thermoshaker set at 1–000 rpm. The resulting supernatant was collected. The remaining precipitate underwent a second extraction with the addition of 1 mL of 1 M HCl, followed by another 2 h of incubation under the same conditions. The supernatant from the second extraction was combined with the first and neutralized using 1 mL of 1 M NaOH. Next, the precipitate was resuspended in 1 mL of 5 M NaOH and incubated again. The final supernatant was collected, adjusted to pH 8 with 1 mL of 5 M HCl and combined with the previous supernatant. All chemical analyses were performed in triplicate (n = 3).
2.3.3 Carbohydrates
Total sugar content was analyzed using the method described by (DuBois et al., 1956): 1 mL of tested sample or standard (glucose) (Avocado Research Chemicals Limited) was inserted into a hemolysis tube and added with 15 μL of 75% phenol (Prolabo). After the solution was stirred, 2.5 mL of 96% H2SO4 was added to the tube. The solution was cooled in an ice bath for 10 min and then placed in a water bath at 30°C for 10 min. After stirring with a vortex for 30 s, the solutions were ultrasonicated for 5 s to remove micro air bubbles. Absorbance was read at 490 nm; the results are expressed as a percentage of dry weight (d.w.). The standard glucose range was 20, 40, 60, 80 and 100 μg mL-1.
Starch content was measured using a Total Starch Assay kit (K-TSTA-100A, Megazyme, Ireland) and a protocol modified by Prabhu (2019). Dried Ulva sp. biomass was ground to a fine powder with a mortar and pestle. Samples (10 mg) were washed with 80% ethanol to remove free glucose, then incubated with 2 M KOH at 37°C for 30 min. After heating to dissolve the starch, the samples were neutralized with sodium acetate buffer (pH 3.8), enzymatically hydrolyzed with α-amylase and amyloglucosidase at 50°C for 90 min and centrifuged at 1–800 g for 10 minutes. Released glucose was quantified using GOD-POD reagent at 510 nm with a microplate reader (Multiskan 60, ThermoScientific) against blanks. Starch content is expressed as a percentage of dry weight (% d.w.) using a glucose-to-anhydroglucose conversion factor of 0.9. The experiment was conducted in triplicate.
Monosaccharide composition: Samples (2 mg mL-1 d.w.) were transferred to Eppendorf tubes, 1 ml of Milli-Q water and 110 µl of 1M HCl were added, and the tubes were heated at 100°C for 48 h under constant stirring. Next, 680 µL Milli-Q water, 110 µl of 1M NaOH and 100 µL deoxyribose solution (concentration 1–000 ppm) were added, mixed and filtered using a 0.22 µm syringe filter (Sartorius, Minisart). Monosaccharide composition was determined using high-performance anion-exchange chromatography (HPAEC) coupled with pulsed amperometry detection (PAD) (Thermo Dionex, Sunnyvale, CA, USA) according to Pliego-Cortés et al. (2019): 25 µL of the sample was injected into a CarboPac PA-1 column (4.6 x 250 mm) connected to a CarboPac pre-column (Thermo Dionex, Illkirch, France). Elution consisted of maintaining the mobile phase with 82% milli-Q water and 18% 0.1 M NaOH for 30 min, followed by a gradient with 100% of 0.1 M NaOH + 1 M NaOAc from minute 31 to minute 35. From minute 36 to minute 80, a final elution was performed with 82% milli-Q water and 18% 0.1 M NaOH. The column temperature was set at 30°C and the flow was monitored with a PAD (gold) operating at a sensitivity of 1–000 nA. Peaks were detected using Chromeleon 6.8 software (Thermo Scientific, Illkirch, France). All solvents were previously degassed with helium gas. Monosaccharides such as mannitol, fucose, glucosamine, rhamnose, galactose, glucose, mannose, xylose, ribose, and glucuronic acid were identified and quantified based on their standard curves at different concentrations (3–125 ppm). Deoxyribose was used as internal standard. Results are expressed as µg of monosaccharides per mg of dry weight (µg mg-1).
2.3.4 Sulfate group content
The sulfate group content was determined using the Azure A modified colorimetric method. The Azure A (Sigma-Aldrich A6270) reacts specifically with sulfates linked to polysaccharides. A solution of sulfated dextran (17%) was used as standard (0 – 100 μg mL-1). For the reaction, 20 µL of standard or samples were added to 200 µL of Azure A reagent (10 mg L-1). Absorbance was measured with a microplate reader (Multiskan 60, ThermoScientific) at 535 nm.
2.3.5 Uronic acids
The composition of uronic acids was analyzed using the colorimetric method originally developed by Blumenkrantz and Asboe-Hansen (1973) modified by Filisetti-Cozzi and Carpita (1991). Aliquots (200 μL) of the tested sample or of the standard (glucuronic acid from Acros Organics) were placed in a hemolysis tube and 20 μL of 4 M sulfamic acid (Alfa Aesar) was added. After the mixture was stirred with a vortex for 30 s, 1.2 mL of a 75 M sodium tetraborate solution (Merck) was carefully added. The tubes were then capped and incubated at 80°C for 20 min. Following incubation, the tubes were cooled in ice for 5 min, after which 40 μL of a 0.15% aqueous solution of meta-hydroxydiphenyl (MHDP) (Acros Organics) was added. The reaction was allowed to proceed for 10 min before absorbance was measured at 525 nm. The glucuronic acid standard curve was prepared using concentrations of 20, 40, 60, 80, and 100 μg mL-1. Results are expressed as mg g−1 of dry weight (d.w.). The concentration of uronic acids was calculated in the same way as for total sugars.
2.3.6 Amino acid and protein analysis
Amino acid composition was determined after vapor-phase acid hydrolysis performed on an Eldex HD WorkStation (Eldex Hydrolysis/Derivatization Station, Napa CA, USA) according to the method described by Pliego-Cortés et al. (2017). Aliquots (10 mg) of dried sample were placed in a Durham tube and 17.5 µL of DL-Norvaline (Thermo-Fisher, France) at a concentration of 2.34 g.L-1 was added, frozen and freeze-dried. The Durham tube was then inserted into an Eldex reaction vial, 200 µL of 6M HCl-phenol solution was added to the bottom of the reaction vial, the vial was closed, placed in the Eldex WorkStation and a vacuum-nitrogen cycle was carried out according to the manufacturer’s instructions. The reaction vial was placed in an oven at 110°C. The hydrolyzed sample was dried using the Eldex WorkStation, then 700 µL of sodium citrate buffer was added, mixed and filtered through a GF/C grade microfiber filter (Cytivia, Whatman). Following hydrolysis, the amino acid samples were prepared using the AccQ-Tag Ultra Derivatization Kit (Water, code 186003836), and analyzed using high-performance liquid chromatography HPLC (Thermo Dionex, Sunnyvale, CA, USA), in a Xbride C18 column (3.0 × 150 mm, Waters, Milford, USA) preceded by a Ultra VanGuard column (2.1 × 100 mm, Milford, USA). Five microliters of sample were injected at a flow rate of 0.98 ml/min and separated using the following program: 100% mobile phase A (90:10 water:Acc-Tag Ultra eluent A) for 15 min, followed by an increased step of mobile phase B (100% Acc-Tag Ultra eluent B) until minute 19. The temperature column was set at 49 °C and the UV detector at 260 nm. Signals were recorded with Chromeleon 7 software (Thermo Scientific, France). Amino acids were identified and quantified based on amino acid calibration curves ranging from 0.25 to 500 µM concentration using the Cell Culture Standard Kit (Catalogue N°186009300, Waters, Milford, USA). Norvaline was used as internal standard at a final concentration of 500 μM. Total amino acid content is expressed as a percentage of dry weight (% d.w.).
Total protein content determined by BiCinchoninic Acid (BCA) analysis: Total protein content was determined using the method of Smith et al. (1985). Aliquots (25 μl) of sample at a given concentration were placed in a 96-well microplate in triplicate and 200 μl of reagent from the Pierce BCA Protein Assay kit (Thermo Scientific) was added. The microplate was incubated at 37°C for 30 minutes before absorbance was read at 562 nm in a microplate reader (Thermo Scientific, Multiskan TM GO). A BSA (bovine serum albumin) standard from the Pierce BCA Protein Assay Kit, at concentrations ranging from 0 to 250 μg mL-1, was used as control in each assay.
2.3.7 Phenolic content
The Folin-Ciocalteu method is widely used to measure total phenolic content (TPC). This analysis was performed directly on the samples. Briefly, 20 μL of sample (1 mg mL−1) or standard (Phloroglucinol from Sigma Aldrich) was added to 100 μL of Folin-Ciocalteu reagent (2N) in a 96-well microplate, followed by incubation for 5 min at 30°C. Next, 80 μL of Na2CO3 solution (7.5%; m/v) was added and the microplate was incubated for 30 min at 40°C in the dark. Absorbance was measured at 760 nm (Singleton et al., 1999; Waterhouse, 2002). Phloroglucinol was used at concentrations ranging from 0 to 1 000 μg mL-1. Results are expressed in milligrams of phloroglucinol equivalent (PE) per g of dry weight (d.w.).
2.4 Physical chemistry analysis
2.4.1 Fourier transform infrared spectroscopic analysis
The FTIR spectra were recorded on a Lumos FTIR spectrometer (Bruker, II) sampling device containing a microscope. After HPH and centrifugation, approximately 100 mg of dry sample was placed directly in the sampling device and pressed towards the diamond. Samples were recorded in transmission mode at room temperature from 500 to 4–000 cm-1, with 128 scans and a resolution of 4 cm-1. Background spectra of air were scanned before the samples were analyzed. The FTIR spectra were acquired and processed by the Institut de Recherche Dupuy de Lôme, in Vannes (IRDL). The spectra values are the average of three counts.
2.4.3 Granulometry analysis
The particle size distribution of Ulva sp. extract before and after HPH was determined using a laser diffraction particle size analyzer (Prodabio Platform, Pontivy, France). To prevent aggregation, the samples were dispersed in distilled water with 0.1% (WV). The analysis was conducted at room temperature (20°C), the results are reported as the volume-weight mean diameter (D) and particle size distribution curves.
2.5 Biological activities
All the experiments described in this section were conducted on samples after centrifugation and freeze-drying.
2.5.1 Antioxidant activity
• DPPH assays
Antioxidant activity used to evaluate the scavenging activity of antioxidant compounds against free radicals. The analysis described here is based on a modified method of Guo et al. (2012). A series of ascorbic acid solutions was prepared at different concentrations as standard (0 – 30 μg mL-1). Next, 100 μL of the 0.25 mM DPPH (2,2-Diphenyl-1-picrylhydrazyl) solution was added to 100 μL of sample solution or control in a 96-well microplate. Sample solutions were prepared at different concentrations by diluting the stock solution in methanol (0 – 1 000 μg mL-1). Before reading absorbance at 517 nm, all the samples were incubated for 30 mins at 40°C in the dark. The percentage of inhibition was calculated using the following formula:
where I (%) = inhibition (expressed in %), AC = absorbance of control, and AS = absorbance of samples.
IC50, corresponding to the concentration required to obtain 50% of a maximum scavenging capacity of samples, was determined based on the regression obtained from the dose–response curve.
• FRAP assays
The antioxidant activities of seaweed extracts were quantified colorimetrically using a method adapted from Shahwar et al. (2012). Each sample was suspended in ultrapure water at a final concentration of 1 μg mL-1. A stock solution of Trolox (100 μg mL-1) was prepared in ultrapure water with DMSO and sonicated for 25 minutes. Standard dilutions (0 – 20 μg mL-1) were prepared in DMSO. The FRAP reagent was freshly prepared by mixing acetate buffers, 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3 in 10:1:1 (v:v:v) ratio. Next, 150 µL of reagent was added to each well containing 50 µL of sample or standard. After 15 minutes of incubation at 20°C, absorbance was measured at 593 nm using a microplate reader (Multiskan 60, Thermo Scientific).
2.6 Statistical analysis
R Software 2024.12.1 was used for the statistical analysis of all the experiments. Before the analysis, the normality of the data (Shapiro test) and the homogeneity of variances (Levene test) were tested using a significance threshold of α < 0.05. Parametric ANOVA (Analysis of variance) or non-parametric tests, i.e. Kruskal-Wallis and Wilcoxon-Mann-Whitney tests were performed and were followed by an HSD Tukey test or a Dunn test, respectively.
3 Results
3.1 Proximate biochemical composition of Ulva sp. raw material
Table 1 provides details of the biochemical composition of Ulva sp. raw material, including total sugars and starch, proteins, ash, uronic acids, sulfate groups and total phenolic content expressed as percentage of dry weight (% d.w.). Cultivated Ulva sp. raw material is mainly composed of mineral matter that in the present study accounts for 38.1 ± 0.1% d.w. Sugar and starch are the second most frequent components found in Ulva sp. accounting for, respectively, 14.3 ± 1.2% d.w. and 9.8 ± 3.9% d.w. Sulfate groups and uronic acids account for, respectively, 5.7 ± 0.8% d.w. and 1.5 ± 0.2% d.w. Ulva sp. raw material has a low phenolic content (0.3 ± 0.1% d.w.) and its monosaccharide contents are mainly glucose (6.20 ± 0.3% d.w.), rhamnose (3.60 ± 0.2% d.w.), glucuronic acid (3.2 ± 0.2% d.w.), xylose (0.35 ± 0.02%d.w.) and ribose (0.23 ± 0.0% d.w.).
Table 1
| Species | Raw material | Carbohydrates | Starch | Uronic acids | Sulfates | Proteins (BCA) | Total amino acid | Phenolic content | Ash |
|---|---|---|---|---|---|---|---|---|---|
| Raw material | Ulva sp. | 14.3 ± 1.2 | 9.8 ± 3.9 | 1.5 ± 0.2 | 5.7 ± 0.8 | 7.2 ± 0.6 | 4.1 ± 0.1 | 0.3 ± 0.1 | 38.1 ± 0.1 |
Biochemical composition (% d.w.) of Ulva sp. raw material (mean ± s.d., n=3).
The protein content of the Ulva sp. raw material was 7.2 ± 0.6% d.w. using BCA analysis and 4.1 ± 0.1% d.w. using total amino acid content analysis. Table 2 lists the amino acid composition of Ulva sp. raw material compared to that of the milk protein used as control. Milk powder (containing 84.0% protein of d.w. of powder is widely used in French hospitals as a protein-rich food supplement) for malnourished seniors.
Table 2
| Amino acid | 0 bar | 600 bar | 800 bar | 1000 bar | Raw materialUlva sp. | Milk protein | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supernatant | Residue | Supernatant | Residue | Supernatant | Residue | Supernatant | Residue | |||
| Essential amino acids | ||||||||||
| Histidine | 2.0 ± 0.3 | 2.2 ± 0.1 | 1.9 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.1 | 1.9 ± 0.0 | 1.8 ± 0.4 | 1.7 ± 0.1 | 1.9 ± 0.0 | 2.6 ± 0.0 |
| Threonine | 4.5 ± 0.6 | 5.1 ± 0.0 | 5.0 ± 0.2 | 5.2 ± 0.0 | 5.3 ± 0.2 | 5.1 ± 0.0 | 5.0 ± 0.3 | 5.1 ± 0.1 | 5.2 ± 0.1 | 4.4± 0.2 |
| Lysine | 3.2 ± 2.5 | 6.4 ± 0.0 | 5.2 ± 0.2 | 5.9 ± 0.2 | 5.4 ± 0.2 | 6.2 ± 0.0 | 5.2 ± 0.5 | 6.2 ± 0.1 | 5.9 ± 0.1 | 8.1 ± 1.2 |
| Methionine | — | 1.1 ± 0.0 | 0.5 ± 0.1 | 1.2 ± 0.1 | 0.6 ± 0.2 | 1.1 ± 0.1 | 0.6 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 | 2.9 ± 0.3 |
| Valine | 5.7 ± 1.0 | 7.2 ± 0.0 | 6.3 ± 0.1 | 7.2 ± 0.2 | 6.7 ± 0.1 | 7.0 ± 0.1 | 6.3 ± 0.2 | 7.1 ± 0.1 | 6.9 ± 0.0 | 5.4 ± 0.7 |
| Isoleucine | 3.3 ± 0.2 | 4.8 ± 0.1 | 3.8 ± 0.1 | 5.0 ± 0.0 | 4.0 ± 0.1 | 5.0 ± 1.0 | 3.7 ± 0.1 | 5.0 ± 0.0 | 4.6 ± 0.0 | 5.1 ± 0.1 |
| Leucine | 6.0 ± 0.8 | 8.8 ± 1.0 | 6.2 ± 0.4 | 9.0 ± 0.2 | 6.4 ± 0.1 | 9.1 ± 1.1 | 6.4 ± 0.0 | 9.1 ± 0.0 | 8.6 ± 0.0 | 10.0 ± 0.4 |
| Phenylalanine | 3.7 ± 0.4 | 4.7 ± 0.1 | 3.2 ± 0.3 | 5.6 ± 0.0 | 3.2 ± 0.2 | 5.5 ± 0.1 | 3.4 ± 0.1 | 5.3 ± 0.2 | 5.1 ± 0.1 | 5.0 ± 0.3 |
| Sum EAA | 28.3 ± 3.1 | 40.2 ± 0.2 | 32 ± 0.6 | 40.9 ± 0.4 | 33.4 ± 0.4 | 40.9 ± 0.2 | 32.5 ± 1.3 | 40.6 ± 0.1 | 38.9 ± 0.1 | 43.0 ± 0.7 |
| Non essential amino acids | ||||||||||
| Tyrosine | 2.8 ± 1.3 | 0.3 ± 0.1 | 1.9 ± 0.0 | 0.6 ± 0.2 | 3.0 ± 0.5 | 0.7 ± 0.2 | 2.5 ± 0.2 | 0.8 ± 0.0 | 1.0 ± 0.2 | 5.1 ± 0.2 |
| Ornithine | 0.1 ± 0.2 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | — |
| Hydroxylysine 2 | 0.6 ± 0.2 | 1.4 ± 0.0 | 0.6 ± 0.1 | 1.3 ± 0.1 | 0.7 ± 0.1 | 1.4 ± 0.0 | 0.7 ± 0.1 | 1.4 ± 0.0 | 1.3 ± 0.0 | — |
| Hydroxylysine 1 | 1.1 ± 1.5 | 0.6 ± 0.0 | 0.2 ± 0.0 | 0.7 ± 0.1 | 0.2 ± 0.0 | 0.6 ± 0.0 | 0.2 ± 0.1 | 0.6 ± 0.0 | 0.5 ± 0.0 | — |
| Proline | 4.9 ± 0.8 | 5.0 ± 0.0 | 6.0 ± 0.4 | 5.0 ± 0.2 | 6.0 ± 0.4 | 5.1 ± 0.1 | 5.6 ± 0.2 | 5.1 ± 0.1 | 5.2 ± 0.0 | 10.2 ± 0.1 |
| GABA (Gamma-Aminobutyric Acid) | 0.1 ± 0.0 | — | 0.1 ± 0.0 | — | — | 0.1 ± 0.0 | — | — | — | — |
| Alanine | 11.9 ± 0.9 | 11.2 ± 0.1 | 11.3 ± 1.4 | 11.1 ± 0.2 | 10.5 ± 1.4 | 10.8 ± 0.2 | 11.0 ± 1.3 | 11.0 ± 0.1 | 10.8± 0.1 | 3.3 ± 0.3 |
| Glutamic acid | 20.0 ± 1.3 | 13.1 ± 0.1 | 16.2 ± 0.1 | 12.5 ± 0.1 | 14.3 ± 0.3 | 12.8 ± 0.1 | 15.1 ± 1.1 | 12.9 ± 0.0 | 13.4 ± 0.1 | 13.8 ± 0.3 |
| Aspartic acid | 10.7 ± 0.5 | 12.3 ± 0.2 | 12.9 ± 1.0 | 12.0 ± 0.1 | 13.5 ± 0.7 | 11.9 ± 0.2 | 12.6 ± 0.8 | 12.0 ± 0.1 | 11.3 ± 0.2 | 16.8 ± 0.4 |
| Glycine | 9.7 ± 0.4 | 7.2 ± 0.0 | 9.1 ± 0.4 | 6.8 ± 0.1 | 8.9 ± 0.5 | 6.8 ± 0.1 | 8.9 ± 0.2 | 6.9 ± 0.1 | 7.0 ± 0.1 | 8.6 ± 0.0 |
| Serine | 6.5 ± 0.6 | 6.3 ± 0.1 | 6.2 ± 0.2 | 6.2 ± 0.1 | 5.9 ± 0.4 | 6.1 ± 0.1 | 6.5 ± 0.3 | 6.3 ± 0.1 | 6.2 ± 0.1 | 3.5 ± 0.1 |
| Taurine | — | 0.2 ± 0.1 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.1 | — | 0.3 ± 0.1 | — | — | 5.8 ± 0.0 |
| Asparagine | — | 0.2 ± 0.0 | — | 0.2 ± 0.1 | — | 0.2 ± 0.0 | 0.5 ± 0.3 | 0.2 ± 0.0 | 0.3 ± 0.0 | - |
| Arginine | 2.9 ± 0.6 | 1.9 ± 0.1 | 2.2 ± 0.3 | 2.1 ± 0.1 | 2.7 ± 0.3 | 2.3 ± 0.3 | 2.5 ± 0.2 | 1.8 ± 0.3 | 2.8 ± 0.0 | 2.1 ± 0.2 |
| Cysteine | 0.7 ± 0.3 | 0.2 ± 0.0 | 1.1 ± 0.1 | 0.3 ± 0.0 | 1.1 ± 0.5 | 0.3 ± 0.1 | 1.2 ± 0.1 | 0.3 ± 0.3 | 0.8 ± 0.0 | 0.5 ± 0.0 |
| Sum NEAA | 71.7 ± 3.1 | 59.8 ± 0.2 | 68.0 ± 0.6 | 59.1 ± 0.4 | 66.6 ± 0.4 | 59.1 ± 0.2 | 67.5 ± 1.3 | 59.4 ± 0.1 | 61.0 ± 0.1 | 57 ± 0.7 |
| EAA/NEAA | 0.4 ± 0.1 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 |
| Sum AA (g/100g d.w.) | 1.2 ± 0.0 | 7.4 ± 0.2 | 1.8 ± 0.3 | 8.4 ± 1.2 | 1.6 ± 0.3 | 8.4 ± 1.2 | 1.7 ± 0.0 | 8.9 ± 0.4 | 4.1 ± 0.1 | 79.7 ± 1.9 |
Total amino acids in the different fractions of Ulva sp. after high pressure homogenization in comparison with raw material and milk expressed as a percentage of total amino acid content (% Total Amino Acid content) (mean ± s.d., n=3).
“ - “: not detected.
Cultivated Ulva sp. has high levels of several non-essential amino acids (NEAA) such as serine (6.2 ± 0.1%), alanine (10.8 ± 0.0%) and cysteine (0.8 ± 0.0%). Milk powder has a significantly higher total amino acid content than Ulva sp. Milk protein generally also contains higher concentrations of essential amino acids (EAA) than Ulva sp. except for threonine (5.2 ± 0.1%), valine (6.9 ± 0.0%) and phenylalanine (5.1 ± 0.1%) (p< 0.05). Milk protein is also richer in aspartic acid (16.8 ± 0.4%), taurine (5.8 ± 0.0%), and tyrosine (5.1 ± 0.2%). Higher EAA/NEAA was found in the milk protein (0.8 ± 0.0%) compared to Ulva sp. (0.6 ± 0.0%).
3.2 Effect of high-pressure homogenization
3.2.1 Extraction yields of dry matter and of proteins
Figure 3A shows the impact of pressure on the comparative distribution of the dry matter of Ulva sp. in the residue, supernatant, and losses. At 0 bar, the extraction yield of dry matter was distributed as follows: 42.4 ± 2.4% in the supernatant, 45.9 ± 4.1% in residue, and 11.6 ± 6.5% lost during the extraction process. As the pressure increased to 600 bars, the dry weight increased significantly to 50.1 ± 9.9% in the supernatant, while decreasing to 36.0 ± 7.7% in the residue. At 800 bars, the proportion of dry weight in the supernatant further increased to 48.2 ± 2.6%, the extraction yield of dry weight in residue was significantly reduced to 29.8 ± 6.4% compared to in control at 0 bar (p< 0.05). Finally, at 1–000 bars, the dry weight in the supernatant decreased slightly to 44.0 ± 8.4% whereas it decreased significantly to 31.0 ± 1.9% in the residue compared to in the control (p< 0.05). No significant difference was observed in the results concerning dry matter in the losses after HPH, irrespective of the pressure (p > 0.05). These results suggest that increasing pressure generally leads to a higher proportion of dry matter in the supernatant and in the losses, while the extraction yield of dry matter in residue decreases starting at 800 bars.
Figure 3
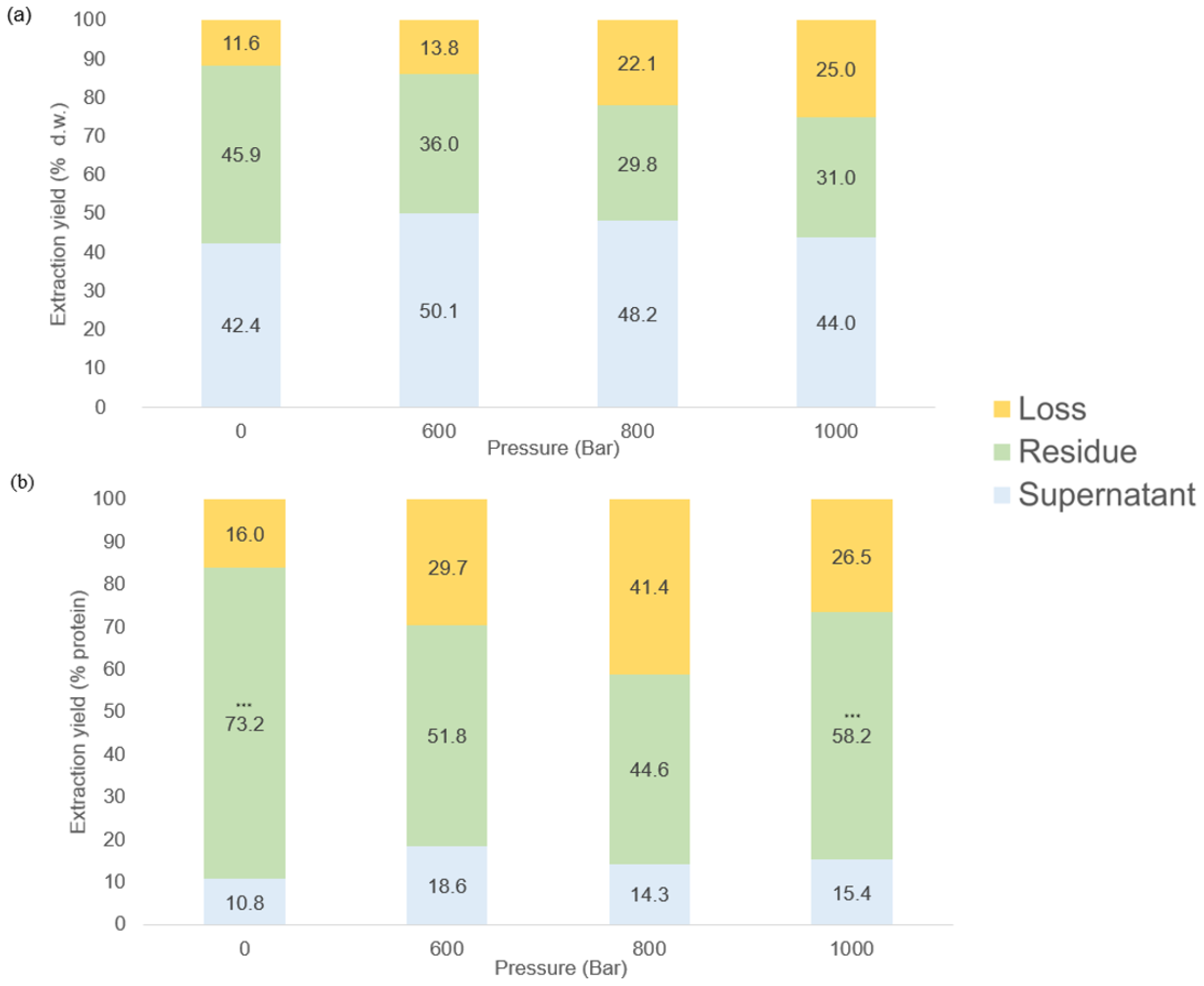
Extraction yields of the dried matter (a) and protein (b) in the residue and the supernatant after HPH and centrifugation (n=3).
The impact of pressure on the distribution of protein content among residue, supernatant, and loss is shown in Figure 3. At 0 bar, the protein content was distributed as follows: 10.8 ± 0.1% in the supernatant, 73.2 ± 5.2% in the residue, and 16.0 ± 5.1 % was lost during the extraction process. As the pressure increased to 600 bars, the proportion of protein rose to 18.5 ± 6.6% in the supernatant and decreased to 51.8 ± 4.2% in the residue. A significant increase in lost protein was observed i.e. 29.7 ± 5.9%. At 800 bars, the supernatant fraction remained relatively stable at 14.3 ± 2.5%, while the protein content in the residue dropped to 44.6 ± 1.7%, with a parallel increase in losses (41.5 ± 1.7%) the highest recorded across all the pressure conditions. Finally, at 1–000 bars, there was a slight increase in the proportion of protein to 15.4 ± 3.1% in the supernatant, and to 58.2 ± 4.9% in the residue. No significant difference was found between protein lost after HPH, irrespective of the pressure (p< 0.05), whereas significant differences were found in all the supernatants and the residues (p< 0.05). These results suggest that increasing pressure also increases the proportion of lost protein, and that protein extraction in the residue is highest at 1–000 bars.
3.1.1 Biochemical composition of HPH extracts derived from Ulva sp.
Table 3 shows the biochemical composition of HPH extracts from Ulva sp. expressed as percentage of d.w. The biochemical composition of the extracts obtained after HPH at 600, 800, and 1–000 bars was analyzed in both the residues and the supernatants. The use of HPH was shown to be efficient in disrupting the cell wall, thereby facilitating the release of compounds of interest. Analysis of biochemical composition following HPH treatment, or even after simple centrifugation (0 bar), revealed higher concentrations of compounds (Table 3).
Table 3
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pressure (bar) | Sample | Carbohydrates | Starch | Uronic acids | Sulfates | Proteins (BCA) | Total amino acid | Phenolic content | Ash |
| Raw material | Ulva sp. | 14.3 ± 1.2 | 9.8 ± 3.9 | 1.5 ± 0.2 | 5.7 ± 0.8 | 7.2 ± 0.6 | 4.1 ± 0.1 | 0.3 ± 0.1 | 38.1 ± 0.1 |
| 0 | Supernatant | 11.0 ± 2.4 | 0.8 ± 0.0 | 16.8 ± 3.4 | 12.4 ± 3.1 | 5.9 ± 1.1 | 1.2 ± 0.0 | 0.1 ± 0.1 | 71.7 ± 1.6 |
| 600 | Supernatant | 16.6 ± 1.6 | 0.8 ± 0.0 | 21.1 ± 3.6 | 16.9 ± 2.4 | 5.5 ± 0.4 | 1.8 ± 0.3 | 0.1 ± 0.1 | 56.5 ± 1.2 |
| 800 | Supernatant | 15.0 ± 5.1 | 0.7 ± 0.0 | 20.6 ± 2.8 | 19.6 ± 2.2 | 5.4 ± 0.8 | 1.6 ± 0.2 | 0.1 ± 0.1 | 53.1 ± 3.2 |
| 1000 | Supernatant | 20.4 ± 3.8 | 0.7 ± 0.1 | 28.6 ± 4.5 | 23.2 ± 4.9 | 5.3 ± 0.8 | 1.7 ± 0.0 | 0.1 ± 0.1 | 54.8 ± 3.2 |
| (b) | |||||||||
| Pressure (Bar) | Sample | Carbohydrates | Starch | Uronic acids | Sulfates | Proteins (BCA) | Total Amino Acid | Phenolic content | Ash |
| Raw material | Ulva sp. | 14.3 ± 1.2 | 9.8 ± 3.9 | 1.5 ± 0.2 | 5.7 ± 0.8 | 7.2 ± 0.6 | 4.1 ± 0.1 | 0.3 ± 0.1 | 38.1 ± 0.1 |
| 0 | Residue | 52.7 ± 4.5 | 16.4 ± 2.2 | 5.0 ± 1.2 | 2.3 ± 0.4 | 8.3 ± 1.3 | 6.9 ± 0.2 | 0.1 ± 0.1 | 13.8 ± 1.8 |
| 600 | Residue | 43.5 ± 6.9 | 9.7 ± 1.4 | 2.8 ± 2.2 | 5.9 ± 1.7 | 12.6 ± 0.9 | 7.2 ± 0.9 | 0.5 ± 0.1 | 14.6 ± 2.5 |
| 800 | Residue | 32.2 ± 5.5 | 8.4 ± 3.1 | 0.4 ± 0.4 | 5.8 ± 1.3 | 13.7 ± 1.6 | 7.5 ± 1.1 | 0.6 ± 0.1 | 12.6 ± 1.7 |
| 1000 | Residue | 30.7 ± 7.6 | 10.2 ± 3.1 | 1.6 ± 2.2 | 9.4 ± 3.2 | 14.5 ± 2.7 | 7.8 ± 0.3 | 0.6 ± 0.3 | 15.1 ± 0.6 |
Biochemical composition (% d.w.) of supernatants (a) and residues (b) after HPH compared to that in the raw material (mean ± s.d., n=3).
The concentrations of the compounds studied increased compared to those recorded during analysis conducted on the whole Ulva sp. biomass. Depending on the compound, it was preferentially extracted in either the supernatant or in the residue. Indeed, total phenolic content was significantly higher at 800 bars in the residue (0.6 ± 0.1% d.w., p< 0.05), than in samples without pressure, where phenolic content was negligible (0.1 ± 0.1% d.w.). The results also showed a significant increase (p< 0.05) in neutral sugar contents (52.7 ± 4.5%) in the residue (11.0 ± 2.4% in supernatant) compared with the results of the analysis of raw Ulva sp. material. The sugars in residue solubilized at high pressure, especially at 1–000 bars (20.4 ± 3.8%) compared to 0 bar (11.0 ± 2.4%). Thus, a significant increase in soluble sugars in the supernatant occurred with increasing pressure. The same phenomenon was observed for uronic acids, where the concentration was about 1.5 ± 0.2% d.w. in the raw material by simple hydrolysis, but reached 2.8 ± 2.2% d.w. in the residue and 21.1 ± 3.6% d.w. in the supernatant at 600 bars and 28.6 ± 4.5% d.w. in the supernatant at 1–000 bars. Uronic acid concentrations were significantly higher in the supernatant in samples at a maximum of 1–000 bars (28.6 ± 4.5% d.w., p< 0.05), compared to in samples under no pressure (16.8 ± 3.4% d.w.). Our results thus show that the HPH process significantly influences the biochemical distribution and separation of sugars - according to their nature - between residue and supernatant.
Table 4 details that the main monosaccharides were glucose and accounted for more than 10% d.w. in each sample. These results indicated the presence of glucose in the residues, whether related to starch as a reserve product or derived from cellulose. Notably, no significant differences were found among all the supernatants, whereas they differed significantly from their corresponding residue (p< 0.05). Glucose was present in significantly larger proportions in residues at 600 and 800 bars (19.1 ± 2.1 - 17.4 ± 3.7%, p< 0.05), compared to after extraction without pressure (10.62 ± 0.94%). The starch contents were higher in the residue without pressure (16.4 ± 2.2% d.w.) than in the supernatant (0.8 ± 0.0% d.w.) (p< 0.05) and decreased with an increase in pressure to 1–000 bars in the residue fraction (10.2 ± 3.1% d.w.). Small amounts of galactose ranging from trace levels to 0.1 ± 0.0% at 1–000 bars were found in the supernatant samples.
Table 4
| Samples | Pressure (bar) | Rhamnose | Galactose | Glucose | Xylose | Glucuronic acid |
|---|---|---|---|---|---|---|
| Raw material | Ulva sp. | 3.6 ± 0.2 | 6.2 ± 0.3 | 0.3 ± 0.0 | 0.2 ± 0.0 | 3.2 ± 0.2 |
| Supernatant | 0 | 5.3 ± 0.4 | - | 1.3 ± 0.4 | 0.5 ± 0.1 | 0.2 ± 0.1 |
| 600 | 3.8 ± 0.3 | Trace | 0.8 ± 0.1 | 0.4 ± 0.0 | 2.0 ± 0.4 | |
| 800 | 3.9 ± 0.2 | 0.1 ± 0.0 | 0.7 ± 0.0 | 0.4 ± 0.1 | 1.9 ± 0.2 | |
| 1000 | 3.7 ± 0.3 | 0.1 ± 0.0 | 0.8 ± 0.1 | 0.5 ± 0.0 | 1.8 ± 0.2 | |
| Residue | 0 | 5.9 ± 0.1 | - | 10.6 ± 0.9 | 1.2 ± 0.1 | 1.8 ± 0.1 |
| 600 | 1.8 ± 0.1 | - | 19.1 ± 2.1 | 1.0 ± 0.0 | 1.2 ± 0.3 | |
| 800 | 2.0 ± 0.5 | - | 17.4 ± 3.7 | 0.9 ± 0.1 | 1.3 ± 0.3 | |
| 1000 | 1.5 ± 0.3 | - | 15.3 ± 0.3 | 0.8 ± 0.1 | 1.1 ± 0.2 |
Monosaccharide composition (% d.w.) of Ulva sp. residues and supernatants after HPH treatments under different pressures, (mean ± s.d., n=3).
Ulvans, cell wall sulfated heteropolysaccharides, are rich in sulfate groups, in rhamnose, xylose and in uronic acids including glucuronic and iduronic acids. Increasing the pressure to 800 bars improved extraction of rhamnose into the supernatant compared to at 0 bar (3.9 ± 0.2 - 5.3 ± 0.4%), when the amount of rhamnose in the supernatant was the same as that in the residue (5.3 ± 0.4 - 5.9 ± 0.1%). However, the most abundant xylose fraction was found in the residue, where it decreased with pressure from 1.2 ± 0.1% for 0 bar to 0.8 ± 0.1% at 1–000 bars. Concentrations of glucuronic acids were significantly higher in the supernatant fraction, ranging from 0.2 ± 0.1% at 0 bar to 2.0 ± 0.4% at 600 bars (p< 0.05). Concentrations of the sulfate group varied across samples and pressures. Sulfate group contents were significantly higher in the supernatant at 1–000 bars (23.2 ± 4.9%, p< 0.05), than in the residue (9.4 ± 3.2%). At 1–000 bars, the supernatant showed a significant 10% increase in sulfate groups compared to samples without pressure. At 600 and 1–000 bars, the supernatants were rich in ulvans according to their higher uronic acid contents (21.1 ± 3.6 - 28.6 ± 4.5%), sulfate groups (16.9 ± 2.4 - 23.2 ± 4.9%), and rhamnose (3.8 ± 0.3 - 3.7 ± 0.3%, p< 0.05). In conclusion, these results show that the high-pressure process separates ulvans found in supernatants from the other sugars (starch, cellulose) present in the residues.
Table 2 details the amino acid composition of the different fractions under high pressure (0 to 1–000 bars) compared to in the raw material and in milk protein (Delical, LNS Nutrition France). The amino acid composition is expressed as a percentage of total amino acids. Milk protein contained 43.0 ± 0.7% of essential amino acids while raw material contained 38.9 ± 0.1% (Table 2).
Our results revealed significant (p< 0.05) differences in amino acid composition depending on the fraction concerned (i.e. residue or supernatant). After HPH treatment, all the residues were rich in glutamine, aspartic acid, alanine, leucine and valine, while all the supernatants were rich in threonine, lysine, proline, glutamic acid and aspartic acid. The proportion of essential amino acids (EAA) in the residue remained constant at around 40%, across all pressures. Significant differences in EAA and NEAA levels were observed between the supernatants and the residue (p< 0.001), whereas pressure did not significantly influence the distribution of amino acids. In the supernatant, the proportion of EAA ranged from 28.3 ± 3.1% to 33.4 ± 0.4%, at 0 and 800 bars respectively. Generally, increasing the pressure to 800 bars led to enrichment of EAA in the residue (+3%). The highest total amino acid content was observed at 1–000 bars in the residue (7.8 ± 0.3%) and was significantly enriched compared with that in the raw material (+4%). Across all treatments, the residue consistently had significantly higher protein content than the supernatant (p< 0.05). The highest protein content was significantly higher at 1–000 bars (7.8 ± 0.3%, p< 0.05) than at the other pressures. Methionine represented the significantly (p< 0.05) highest proportion in the residue at 600 bars (1.20 ± 0.1%), compared to in the raw material (0.7 ± 0.1%). The proportion of leucine, the most abundant essential amino acid, was found to increase with pressure, from 6.0 ± 0.8% in the supernatant at 0 bar to 9.1 ± 0.0% in the residue at 1–000 bars. The concentration of valine increased from 5.7 ± 1.0% to 6.3 ± 0.2% in the extracts at 0 bar and 1–000 bars, while no significant difference was observed in the residues. Conversely, more phenylalanine was retained in the residue at 600 bars, where it reached 5.6%, while its concentration in the supernatant decreased to 3.2 ± 0.2%.
3.1.2 Physical-chemistry analysis
The functional groups of the different fractions were characterized using FT-IR. The FT-IR spectra and spectral bands of interest of Ulva sp. after HPH process are shown in Figure 4. The IR spectrum of the different samples within the 2 200–600 cm -1 region - or in the fingerprint region for polysaccharides and protein - were used for data analysis. These analyses confirmed previous results. The high-pressure process allows the separation of ulvans from other compounds, notably from most proteins. Residues showed high intensity spectral bands at 1 656, 1–550 and 1–165 cm-1, while medium or low intensity bands were observed at 1 421, 708 and 666 cm-1. The peak at 1–550 cm-1 suggests amide N-H bending vibrations (Sarada et al., 2014). The peak at 1 421-1–436 cm-1 shows symmetric stretching vibration of carboxylic groups (Hernández-Garibay et al., 2011).
Figure 4
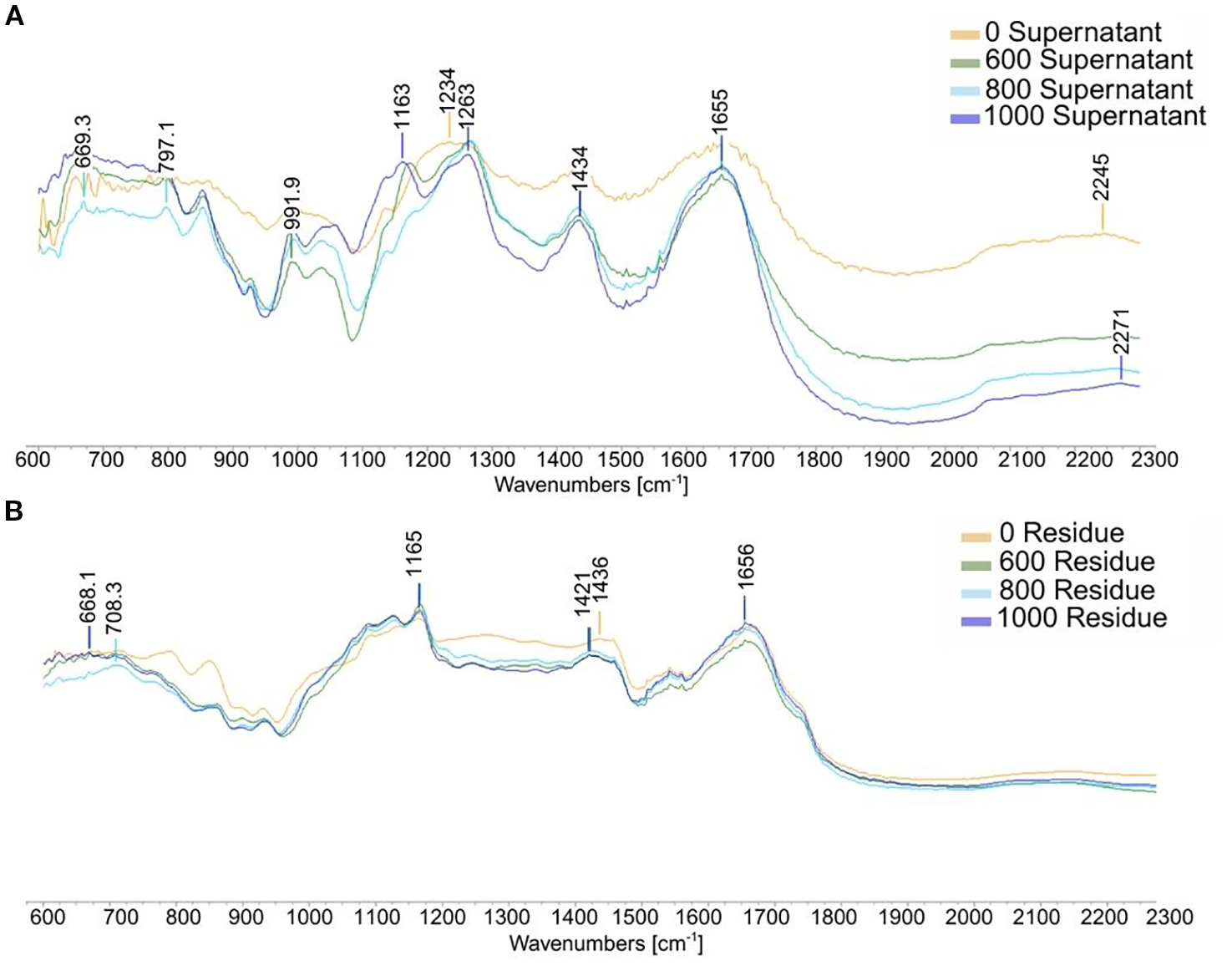
Infrared spectrum of residue (A) and supernatant (B) from Ulva sp. following high pressure homogenization.
However, supernatants had specific high intensity spectral bands at 669, 797, 991, 1 163, 1 263, 1–434 cm-1 and medium or low intensity bands at 1–655 and 2–271 cm-1. Strong and weak-intensity carboxylic vibrations were found at 1–655 and 1–434 cm-1. The high intensity at around 1–655 cm-1 corresponds to an asymmetrical stretching band attributed to the presence of OH groups, which is within the spectral bands of uronic acids (Olasehinde et al., 2019). The weaker intensity around 1–434 cm-1 suggests symmetric stretching vibration of carboxylic groups (Hernández-Garibay et al., 2011). Between 1–200 and 1–000 cm-1, the region is dominated by sugar ring vibrations that overlap the stretching vibration of C-OH side groups and the C-O-C glycosidic bond vibrations. All the spectral bands between 1–260 and 1–220 cm-1 are linked to the sulfation level (Rochas et al., 1986). The double spectral band around 1 170-1–790 cm-1 shows the presence of sulfate groups that belong to a band indicating S=O elongation bonding. The band around 991 cm-1 corresponds to glycosidic linkages (Robic et al., 2008) or is associated with galactose-6-sulfate (Hardouin, 2015). The bands around 850 cm-1 are characteristic of the presence of sulfate groups in the sample. IR analysis confirmed that HPH effectively disrupts the cell wall, leading to the release of ulvan components into the supernatant.
In addition, the granulometric properties were determined in the samples before HPH and revealed a significant decrease in particle size, from 500 µm to 100 µm (p< 0.05) (Figure 5).
Figure 5
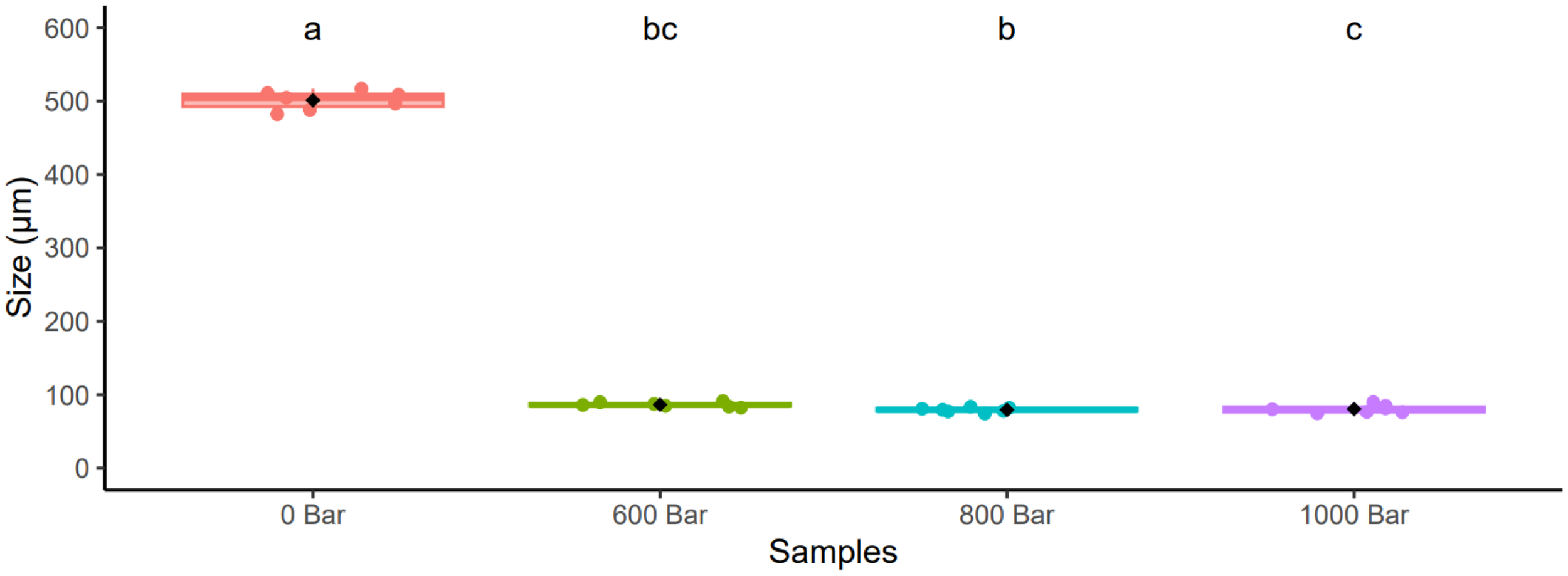
Granulometric analysis of different pressure. Letters correspond to results obtained after a Kruskal-Wallis Test (p< 0.05). Error bars correspond to standard deviations (n= 7).
3.1.3 Biological activities
Antioxidant activity was investigated using DPPH with no significant activity detected (data not shown). The antioxidant activity investigated using FRAP assays revealed significant differences between the supernatant and residue (p< 0.05), but also that pressure did not significantly affect antioxidant activities (Figure 6). Indeed, the raw material showed higher antioxidant capacity than milk protein (6.5 ± 1.1 mg TE/g) and 3.5 ± 0.1 mg TE/g respectively, but no significant difference was found between 0 bar and the raw material. At 0 bar, the values of the residue and the supernatant were 8.7 ± 0.4 mg TE/g and 5.5 ± 0.1 mg TE/g, respectively. At 600 bars, the residue reached 12 ± 2 mg TE/g, and the supernatant 4 mg TE/g. At 800 bars, the residue contained 11.0 ± 0.1 mg TE/g and the supernatant 3.8 ± 0.1 mg TE/g. Conversely, the sample treated at 1–000 bars contained 11.6 ± 1.3 mg TE/g in the residue and 4.8 ± 0.3 mg TE/g in the supernatant.
Figure 6
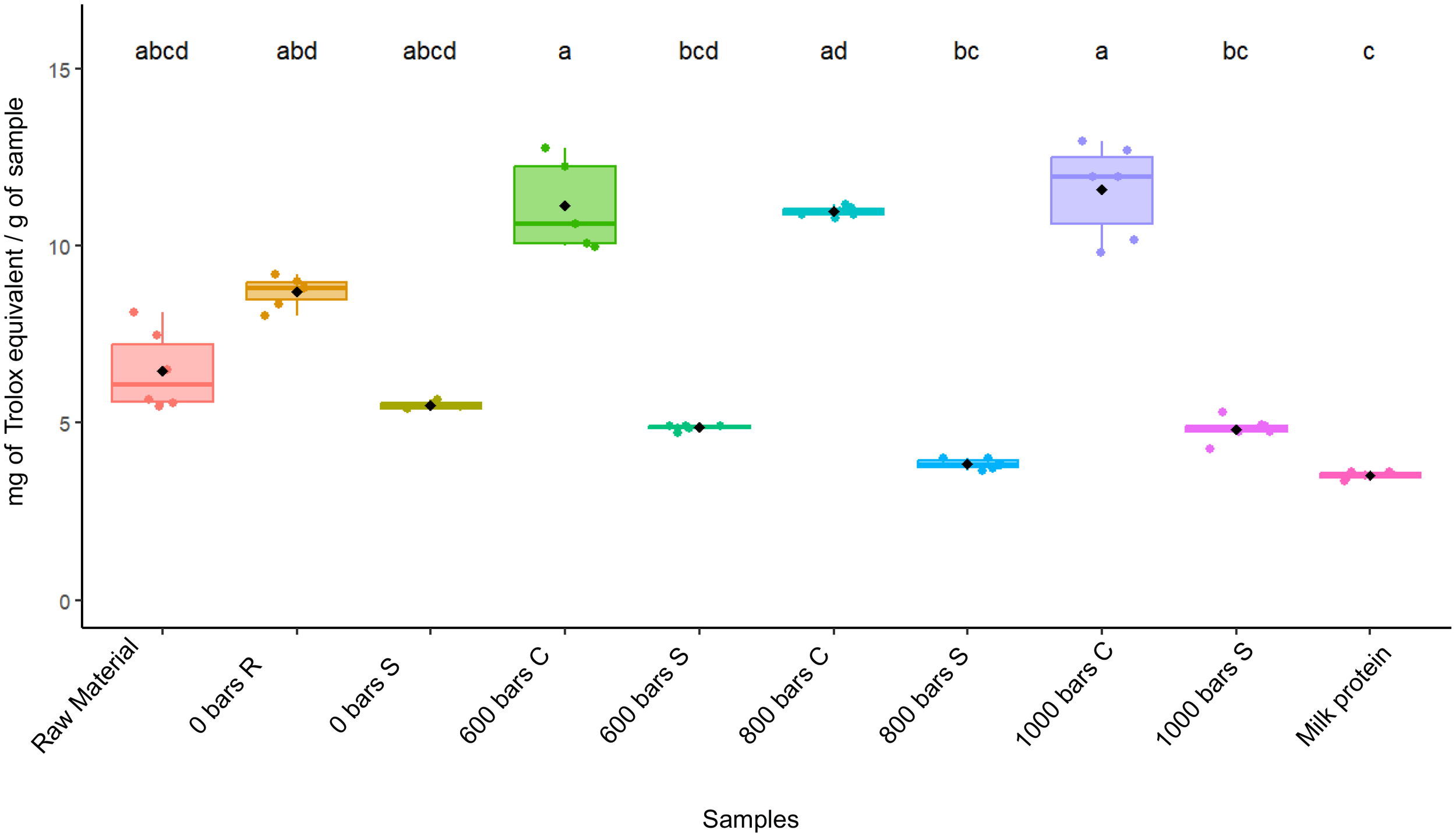
Masses of Trolox equivalent per gram of sample for each condition tested. Letters correspond to results obtained after a Kruskal-Wallis test (p< 0.05). Error bars correspond to standard deviations (n= 6).
Moreover, the evaluation of cytotoxicity based on cell viability revealed no destruction of the cell layer. Notably, no microscopically visible alteration of normal cell morphology was observed after 3 days of treatment. In conclusion, no cytotoxic effect of the compounds on Vero cells was observed within the range of concentrations tested in the present study.
4 Discussion
4.1 Raw material composition
Despite controllable cultivation conditions, the biochemical composition of cultivated Ulva sp. can exhibit considerable variability depending on the species and can also be modified by temporal dynamics, as well as by a range of abiotic factors or cultivation parameters (Wells et al., 2017; Fleurence et al., 2018; Magdugo et al., 2020; Mantri et al., 2020; Simon et al., 2022).
Protein content in Ulva sp. can vary considerably from 4% to 32% (d.w.) on a dry weight basis (Fleurence et al., 2018). Our results tended toward the lower range of values reported in the literature. Some authors have reported relatively low protein content. For example, cultivated U. rigida collected in South Africa comprised 6.4% d.w (Foster and Hodgson, 1998), Ulva lactuca originating from northeast of Hong Kong contained 7.06% d.w (Wong and Cheung, 2000). In their study, Nissen et al. (2024) described an August harvest of wild Ulva sp. with a crude protein content of 10.7 ± 0.3% d.w. and an ash content of 35.5 ± 2.3% d.w., comparable to the results of our study. The latter authors suggest that the low protein and high ash content can be attributed to a growth period with elevated rates of photosynthesis and respiration. In contrast, under controlled aquaculture conditions that included nitrogen enrichment, Ulva sp. cultivated in integrated multi-trophic aquaculture systems have been shown to reach protein levels as high as 35.7 ± 1.1% of dry weight (Shpigel et al., 2018).
The concentration of protein in green seaweed, particularly in Ulva sp. is often estimated using a total nitrogen-to-protein conversion factor (5) based on the assumption that most N in the sample occurs as protein (Lourenço et al., 2002). However, when the Kjeldahl method is used, the analysis requires substantial quantities of sample material. In our study, we performed protein analyses using two different methods (BCA and calculated from amino acid analysis). The protein content measured as total amino acid in raw material was 4.1 ± 0.1% d.w. whereas the BCA assay resulted in a higher protein value, 7.2 ± 0.6 d.w. BCA quantified more proteins than total amino acids by HPLC because some amino acids (such as tryptophan) were not quantified. Conversely, BCA assays can overestimate protein concentrations due to interference from non-protein compounds such as glucose (Brown et al., 1989).
Ulva sp. is a good source of essential amino acids and accounts for up to 39% of total protein dry weight (Wong and Cheung, 2000). The concentrations of essential and non-essential amino acids are comparable to those in the traditional protein sources used so far, and are particularly rich in glutamic acid, aspartic acid, arginine, alanine, and leucine (Holdt & Kraan, 2011; Peña-Rodríguez et al., 2011; Wells et al., 2017; Vega-Gómez et al., 2024). Here again, depending on the Ulva sp., harvest and/or cultivation conditions, and the season, variations in the composition of amino acids may be observed. Generally, a complete profile of essential amino acids is found in Ulva sp., with levels comparable to those of many non-animal proteins (Vega-Gómez et al., 2024). Regarding the amino acid profile in our study, Ulva sp. raw material and milk protein accounted for 38.9 ± 0.1% and 43.4 ± 0.7% total of EAA, respectively. These values are in line with those obtained in a study by Nissen et al. (2024) who reported 38.7% of essential total amino acids in Ulva sp. Except for threonine (5.2 ± 0.1%), valine (6.9 ± 0.0%) and phenylalanine (5.1 ± 0.1%), milk protein generally contains higher levels than Ulva sp. whereas cultivated Ulva sp. contained higher levels of several non-essential amino acids (NEAA) such as serine (6.2 ± 0.1%), alanine (10.8 ± 0.1%) and cysteine (0.84 ± 0.03%). Previous studies have reported that Ulva armoricana contains high levels of certain amino acids, particularly proline, that can account for from 5% to 11% of total amino acid content. This is in agreement with our results in which proline levels ranged from 4.9 ± 0.8% at 0 bar, to 6.0 ± 0.4% at 800 bars (Fujiwara-Arasaki et al., 1984; Fleurence et al., 1995). All these results highlight the diversity and richness of the amino acids present in whole sample of Ulva sp. seaweed. Like the amino acid profiles, the mineral composition of Ulva sp. is variable and is influenced by similar environmental parameters (salinity, water temperature and nutrient availability) and physiological factors (Bews et al., 2021). Likewise, the method of cultivation can play a significant role in determining mineral content, which, in turn, may reduce exposure to certain minerals compared to open-water systems, leading to differences in ash content. These conditions can influence metabolite and nutrient uptake, thereby impacting the total mineral content (Revilla-Lovano et al., 2021).
The percentage ash content of the raw material used in our study was 38%, consistent with values previously reported in the literature ranging from 15% to 52% of dry weight (Queirós et al., 2021; Nissen et al., 2024; Peter et al., 2024);.
Edible seaweeds contain 33% - 62% total fibers on a dry weight basis (Holdt & Kraan, 2011). In ulvales, cell wall polysaccharides account for between 38% and 70% of the dry algal biomass (Lahaye, 1998). These polysaccharides are mainly present as ulvans, a water-soluble sulfated polysaccharide and an insoluble cellulose-like material in which the main sugars are rhamnose, glucose, glucuronic acid, xylose and iduronic acid (Lahaye and Robic, 2007). In our study, the total carbohydrate content was lower than previously reported values. Regarding the monosaccharide profile, the raw material mainly contained glucose (6.20 ± 0.3%), rhamnose (3.6 ± 0.2%), glucuronic acid (3.2 ± 0.2%), and xylose (0.2 ± 0.0%). Studies have notably shown that the site of origin and phosphate concentration significantly influence carbohydrate contents in Ulva fasciata (Figueira et al., 2021; Steinhagen et al., 2021). In our study, starch, a storage polysaccharide, accounted for 8.8% d.w. in our raw material, meaning our results are within the expected range. Indeed, Prabhu (2019) and Fort et al. (2020) reported Ulva ohnoi starch contents ranging from 1.59% to 21.44%, depending on the growth conditions and the season. Although the ingestion of green macroalgae by humans is relatively common, the potential health benefits of food supplements made of Ulva sp. let alone direct consumption of the whole algae, are not well understood (Wijesekara et al., 2011; Wells et al., 2017). From a nutritional perspective, it is important to note that according to Charoensiddhi et al. (2022) cell wall ulvans cannot be digested by human enzymes and that they are also poorly fermented by gut microbiota (Durand et al., 1997), therefore limiting their potential nutritional contribution. High interactions between polysaccharides and proteins also reduce the efficiency of the digestive enzyme (Molina-Gilarranz et al., 2025). This constraint reinforces the goal of separating these polysaccharides from protein fractions to increase their bioavailability and confirms the relevance of optimizing protein extraction methods. However, due to the complexity of the algal cell wall and the strong interactions between polysaccharides and proteins, protein extraction remains the main challenge.
Despite their nutritional potential, the use of seaweed proteins by the human health sector remains limited because the extraction methods (e.g., enzymatic extraction, solvent extraction, pulsed electric field) are expensive but the extraction yields are low. In addition, proteins are usually extracted together with sugar or phenolic compounds (Naseri et al., 2020) requiring additional steps in the protein precipitation procedure. Our aim was consequently to develop a simple, rapid, and environmentally sustainable method, suitable for thermosensitive compounds, to separate sulfated polysaccharides (ulvans) from proteins and to obtain a protein enriched fraction.
4.2 Extraction yields of dry matter in residues and supernatants after HPH
In our study, the extraction yields obtained from biomass after HPH treatment ranged from 29.8% to 45.9% d.w. in the residues, and from 42.4% to 50.1% in the supernatants. The extraction of molecules of interest, such as ulvans and starch, through biorefinery schemes using Ulva sp. biomass has been extensively explored (Postma et al., 2018; Magnusson et al., 2019; Prabhu, 2019; Polikovsky et al., 2020). Extraction yields from Ulva sp. have been shown to vary with the method of extraction, including microwave-assisted, ultrasound, enzyme-assisted and alkaline precipitation, as well as with the nature of the target molecules. Steinbruch et al. (2023) combined enzyme extraction with pulsed electric fields (PEF) on Ulva sp., and obtained a higher water-soluble protein extraction yield (19.6 ± 0.33%) compared to that obtained with PEF alone (10.8 ± 0.37%) or with enzyme pretreatment alone (9.7 ± 0.42%). Vega-Gómez et al. (2024) optimized the production of hybrid soluble protein-polysaccharide extracts from Ulva sp. using a pH-shifting process and evaluated the efficiency of an ultrasound pre-treatment and the effect of the preservation method (frozen vs. freeze-dried) in increasing the extraction yields. After a 5-min ultrasound pretreatment prior to the pH solubilization step, both processes disrupted the cell walls and facilitated protein release, with an up to 2-fold increase in the protein extraction yields, with a maximum of 47%.
In another example, O’ Connor et al. (2020) applied the HHP technique at 600 MPa for 4 minutes to extract protein from five different seaweeds with completely different cell wall matrices: two brown seaweeds (Fucus vesiculosus and Alaria esculenta) and two red seaweeds (Palmaria palmata and Chondrus crispus). These authors compared HHP and traditional methods (sonication and salting out) and autoclave extraction. They observed no significant differences in total extractable protein from either brown or red seaweed using the different pre-treatments, but the yields obtained with the HPH method were lower than those obtained with the traditional method. Their results were in line with expectations as the extraction was carried out in water. The solubility of the native proteins is only approximately 35% in water, which sets an upper limit to the amount of protein obtainable using aqueous methods (Biancarosa et al., 2017). The latter authors showed that the specific extraction procedure needs to be adapted to each species. Conversely, the aim of Suwal et al. (2019) was to extract polysaccharides from P. palmata and Solieria chordalis (Rhodophyta, Gigartinales). In parallel, they analyzed protein yields and showed that the HHP treatment (400 MPa for 20 min) only resulted in a 2.6% increase in protein yield in the red seaweed Solieria chordalis.
The present study was inspired by the work of Ning et al. (2025) who explored the potential of using Ulva lactuca to stabilize emulsions by using HPH to release its active interfacial components. Even though the focus of Ning et al’s study was not biochemical profiling, an increase in protein content from 20% to 27% d.w. following HPH at 800 bars was reported, suggesting improved protein availability. Our findings are in accordance with those obtained by Ning et al. who demonstrated that HPH is an efficient method of disrupting cell walls and enhancing access to intracellular compounds. However, the improved relative recovery observed in our study could be due to variations in methodologies, or in the pressure used.
4.3 Cell wall disruption: biochemical composition analysis of extracts
Several authors have already demonstrated that HHP can enhance extraction efficiency by providing the pressurized conditions required for cell disruption (Suwal et al., 2019; O’ Connor et al., 2020). Our results support those of the studies in question and confirm that high-pressure homogenization (HPH) does enhance protein extraction in the residue fraction and is thus an effective method for the extraction of compounds. The biochemical composition of the supernatant, particularly the high levels of rhamnose (1.5 ± 0.3% in residue compared to 3.9 ± 0.2% in supernatant), uronic acids (16.8 ± 3.4 to 28.6 ± 4.5% d.w.) and sulfate groups (12.4 ± 3.1 to 23.2 ± 4.9% d.w.), suggest the presence of ulvan-like fractions (Robic et al., 2008), while glucose and xylose were more abundant in the residues, consistent with the presence of starch and cellulose. These findings reflect the preferential solubilization of ulvan-like polysaccharides through pressure-induced cell disruption, while insoluble polymers such as starch and cellulose remain in the residue. This fractionation was confirmed by infrared spectroscopy. Our FT-IR spectrum results revealed significantly different distribution of sugar components in the residue and in the supernatant. As the pressure increased, the levels of neutral sugars and uronic acids, the main constituents of cell wall polysaccharides, became higher in the supernatant at 1–000 bars. This is in line with the findings of Cameselle et al. (2025), who reported 1 200 – 1–000 cm-1 regions with increased exposure of lipids, carbohydrate components and enhanced extraction of sulfated polysaccharides, respectively. The infrared spectra in our study confirmed increased solubilization of uronic acids and sulfate groups, as characterized by asymmetrical OH stretching reported in previous studies of Ulva lactuca and Ulva fasciata polysaccharide fractions (Olasehinde et al., 2019; Magdugo et al., 2020). The presence of sulfates in the supernatant has also been confirmed by S=O elongation, C-O-S elongation and C-O-S deformation. According to Robic et al. (2008), these bands are due to the sugar cycled, and are typical signals of ulvans (Robic et al., 2008;Olasehinde et al., 2019). However, it is important to note that specific purification of ulvan fraction was not assessed in our study. These spectral modifications suggest pressure-induced disruption of the cell wall structure. The stability of amide bands indicated protein secondary structure despite pressure treatment, while enhanced polysaccharide signals suggests access to these components was improved thus enabling subsequent extraction, as described by He et al. (2016). Finally, our findings are in agreement with those of a previous study in which ultrasound assisted extraction was applied to Ulva sp. and similar molecular fractionation was observed, although ulvans were not specifically quantified (Vega-Gómez et al., 2024).
In conclusion, the HPH process efficiently released ulvan-type components into the supernatant, while retaining insoluble glucose-rich fractions such as starch and cellulose in the residue. The enrichment of ulvan-associated markers in the supernatant reflects the combined effect of cell disruption and the intrinsic solubility of ulvan, highlighting the potential of this method for the targeted fractionation of macroalgae biomass.
4.4 Protein and amino acids
In the present study, HPH effectively increased protein extraction from Ulva sp. The protein yield in the residue fractions increased with pressure from 44.6% to 73.2%, while the extraction yield remained lower (10.8 – 18.6%). This demonstrates the efficacy of HPH in disrupting biomass mechanically, thereby facilitating protein release without extensive chemical pretreatment. Apart from HPH, other high pressure extraction methods have also been reported to significantly enhance protein recovery from seaweed, particularly Ulva. For instance, PEF treatment of Ulva sp. increased protein yields approximately sevenfold compared to osmotic shock, with higher antioxidant activity (Robin et al., 2018). Similarly, subcritical water hydrolysis (SWH) at 180°C and 10.5 bars enabled recovery of up to 84.9% of total protein and a substantial proportion of free amino acids, including leucine, arginine, isoleucine, and alanine (Polikovsky et al., 2020). By contrast, high-pressure processing (HPP) produced more variable, species-dependent results, and in some cases, yields were lower than those obtained using conventional or autoclave methods, thus highlighting the importance of tailoring high-pressure conditions to the biomass concerned (O’ Connor et al., 2020). Only a few studies have explored protein extraction using high-pressure techniques, and it is important to distinguish between high hydrostatic pressure (HHP) and HPH. While HHP is a non-thermal process that applies isostatic pressure (typically 100 to 600 MPa) to intact biomass, mainly affecting membrane permeability and protein solubility, HPH consists of forcing a biomass suspension through a valve at very high pressure, leading to mechanical cell disruption and the release of intracellular compounds. These two techniques differ in their mechanisms and hence in their efficiency depending on target molecules.
Regarding the amino acid composition of Ulva sp. under different pressures, a clear difference was observed in the distribution of amino acids between the supernatant and the residue. In our study, all the extracts produced contained all the essential amino acids required for human nutrition. Regardless of the pressure applied, the residues appeared to be richer in EAA, whereas the supernatants contained higher levels of non-essential amino acids. A higher relative amount of EAA was observed in the residue, indicating a higher nutritional value of the protein. At 0 bar, the proportion of EAA in the residue represented 40.2 ± 0.2% and increased to 40.9 ± 0.4% at 600 and 800 bars, compared to 38.9 ± 0.1% in the raw material, whereas in the supernatant, EAA content represented a lower proportion, ranging from 28.3 ± 3.1% at 0 bar, to 33.4 ± 0.4% at 800 bars. The main amino acids present in the residue were glutamic acid, aspartic acid, alanine, leucine and valine, whereas the supernatant was richer in threonine, lysine, proline, glutamic acid and aspartic acid. It is important to note the increase in essential amino acids, such as leucine and valine, with increasing pressure. The findings of the present study strongly suggest that HPH is a promising technique to obtain protein fractions from Ulva sp. with both high yields and favorable amino acid profiles.
These differences in distribution could involve differential solubilization or the association of proteins with various polysaccharide matrices under pressure. Compared with the amino acid composition in the raw material and after traditional extraction, O’ Connor et al. (2020) showed that the HPH method mainly resulted in an increase in histidine, taurine, aspartic acid, glutamic acid whatever the seaweed studied. Overall higher glutamic and aspartic acid contents have also been reported in previous studies (Fleurence et al., 1995; Unis et al., 2023), while yet other studies reported higher alanine or arginine contents (Pallaoro et al., 2016; Shpigel et al., 2018). Other authors have reported high levels of alanine or arginine, the exact levels varying with the species and with the extraction method used (Pallaoro et al., 2016; Shpigel et al., 2018). Furthermore, Nissen et al. (2024) highlighted the prevalence of glutamic acid and aspartic acids in Ulva sp., thereby confirming the typical amino acid distribution observed in our samples. In general, protein extracted using high-pressure techniques retain high levels of EAAs and exhibit good digestibility, often comparable to or exceeding those of plant sources of protein (Steinbruch et al., 2023).
4.5 Physical and chemical properties
Several studies have reported that high-pressure treatment, particularly HPH, significantly reduces the particle size of seaweed samples. For instance, Souto-Prieto et al. (2024) demonstrated that Laminaria digitata and Saccharina latissima dispersions processed by HPH resulted in significantly smaller particle sizes and formed more stable gels than untreated samples. Similarly, Kim et al. (2018) showed that suspensions of gulfweed subjected to increasing HPH pressures and passes led to a pronounced decrease in particle size, resulting in more uniform and compact edible films. In addition, Zhao et al. (2024) reported that HPP of Laminaria japonica not only reduced particle size, but also increased the specific surface area, as confirmed by scanning electron microscopy.
4.6 Biological activities
These findings suggest that centrifugation alone does not significantly modify the release of antioxidant compounds and supports the hypothesis that extracts from Ulva sp. are a potential source of bioactive substances. Sample residue under pressure from 600 to 1–000 bars showed significant antioxidant activity compared to supernatant, due to the location of bioactive compounds. As some studies have demonstrated, the antioxidant activity of algal polysaccharides is significantly influenced by their chemical structure, including by the presence of sulfate groups, the composition of monosaccharides, and the types of glycosidic linkages (Kidgell et al., 2019; Le et al., 2019). These compounds are characterized by their partial solubility that results in their distribution in the supernatant (e.g. uronic acids, rhamnose, sulfate groups). Some studies have demonstrated that ulvans have health-promoting properties such as immunomodulatory, antioxidant, antiviral and anti-cancer activity (Shao et al., 2014; Lopes et al., 2017; Kidgell et al., 2019; Wahlström, 2020). Moreover, recent studies have highlighted the potential of ulvans in cosmetics, for instance in the development of biodegradable microbeads as an eco-friendly alternative to synthetic exfoliating agents (Selvasudha et al., 2023). In contrast, the residue, composed of insoluble compounds, showed higher antioxidant activity that was attributed to proteins, starch and glucose (Shao et al., 2014; Nguyen et al., 2025). These molecules, trapped inside the cell matrix or precipitated during physical treatments, have demonstrated the high neutralization capacity of free radicals as well as their higher reducing power (Seedevi et al., 2017). Furthermore, certain proteins exhibited intrinsic antioxidant properties, that were attributed to the presence of specific amino acids that can inhibit lipid oxidation in several ways including inactivation of reactive oxygen species, scavenging of free radicals and reduction of hydroperoxides (Elias et al., 2008). Future studies should focus on improving protein yield and on understanding the potential uses of each fraction in a biorefinery context. Indeed, Ulva sp. have many applications in animal and human health with commercially valuable components such as bioactive value-added products (ulvan, nutriments, protein, starch) (Kidgell et al., 2019; Prabhu, 2019; Tziveleka et al., 2019; Mantri et al., 2020). For these reasons, Ulva sp. could play a central role in biorefinery processes (Bikker et al., 2016; Glasson et al., 2017; Postma et al., 2018; Prabhu, 2019). This finding supports the potential use of protein-rich fractions with antioxidant activity for food supplementation, to support protein synthesis, and the natural antioxidant capacities of the organism.
5 Conclusion
We have shown that high-pressure homogenization (HPH) is an effective method for fractionating Ulva sp. biomass and requires only a short treatment time at ambient temperature. The process of centrifugation results in the separation of the soluble fraction of ulvans and mineral matter in the supernatant, while proteins, starch and glucose primarily remain in the solid residue. The highest extraction yield was obtained at 1–000 bars, and the residue obtained at this pressure has good potential for application in the food sector, notably as a nutritional supplement rich in essential amino acids and potentially digestible sugars. The next step in our research will be to explore complementary processes such as fermentation treatment, to enhance the release of proteins into the supernatant fraction.
Studies are already underway to determine the dietary relevance of the subject, which include the evaluation of cytotoxicity in human intestinal cell models and evaluation of the protein digestibility of protein-rich residues. In addition, investigations will be conducted on residues and supernatants to detail the composition and size of ulvan and associated proteins. This comprehensive characterization will provide new insights into how the intensity of the pressure influences the quality and functionality of Ulva sp. derived protein extracts and will quantify the potential of HPH as a scalable biorefinery strategy.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AD: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. HP-C: Data curation, Formal Analysis, Funding acquisition, Writing – original draft, Writing – review & editing. A-SB: Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. NT: Conceptualization, Writing – review & editing, Supervision. MF: Methodology, Writing – review & editing. NB: Conceptualization, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was conducted as part of the PROMALG-Health project, funded by Région Bretagne and the French National Research Agency (ANR) under grant number ANR-23-DIVP-005, within the France 2030 investment framework.
Acknowledgments
The authors wish to thank E. Bioret and S. Beuzet of the ProDaBio platform for their valuable assistance with rheology and granulometry analysis, France Haliotis (Plouguerneau, France) for the biomass production, Olivier Sire from IRDL, UBS, France. The article is also based upon work from COST Action CA20106 ‘Tomorrow’s wheat of the sea’: Ulva, a model for an innovative mariculture, supported by COST (European Cooperation in Science and Technology, www.cost.eu)”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Aimutis W. R. (2022). Plant-based proteins: the good, bad, and ugly. Annu. Rev. Food Sci Technol.13, 1–17. doi: 10.1146/annurev-food-092221-041723
2
Ben-Ari T. Neori A. Ben-Ezra D. Shauli L. Odintsov V. Shpigel M. (2014). Management of Ulva lactuca as a biofilter of mariculture effluents in IMTA system. Aquaculture434, 493–498. doi: 10.1016/j.aquaculture.2014.08.034
3
Bews E. Booher L. Polizzi T. Long C. Kim J.-H. Edwards M. S. (2021). Effects of salinity and nutrients on metabolism and growth of Ulva lactuca: Implications for bioremediation of coastal watersheds. Mar. pollut. Bull.166, 112199. doi: 10.1016/j.marpolbul.2021.112199
4
Biancarosa I. Espe M. Bruckner C. G. Heesch S. Liland N. Waagbø R. et al . (2017). Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol.29, 1001–1009. doi: 10.1007/s10811-016-0984-3
5
Bikker P. van Krimpen M. M. van Wikselaar P. Houweling-Tan B. Scaccia N. van Hal J. W. et al . (2016). Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycol.28, 3511–3525. doi: 10.1007/s10811-016-0842-3
6
Bleakley S. Hayes M. (2017). Algal proteins: extraction, application, and challenges concerning production. Foods6, 33. doi: 10.3390/foods6050033
7
Blumenkrantz N. Asboe-Hansen G. (1973). New method for quantitative determination of uronic acids. Analyt. Biochem.54, 484–489. doi: 10.1016/0003-2697(73)90377-1
8
Brown R. E. Jarvis K. L. Hyland K. J. (1989). Protein measurement using bicinchoninic acid: Elimination of interfering substances. Analyt. Biochem.180, 136–139. doi: 10.1016/0003-2697(89)90101-2
9
Bruhn A. Dahl J. Nielsen H. B. Nikolaisen L. Rasmussen M. B. Markager S. et al . (2011). Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol.102, 2595–2604. doi: 10.1016/j.biortech.2010.10.010
10
Cameselle C. Maietta I. Torres M. D. Simón-Vázquez R. Domínguez H. (2025). Optimization of ultrasound-assisted extraction of bioactive compounds and biopolymers from Ulva spp. Using response surface methodology. J. Appl. Phycol.37, 2031–2050. doi: 10.1007/s10811-025-03492-2
11
Charoensiddhi S. Conlon M. Methacanon P. Thayanukul P. Hongsprabhas P. Zhang W. (2022). Gut microbiome modulation and gastrointestinal digestibility in vitro of polysaccharide-enriched extracts and seaweeds from Ulva rigida and Gracilaria fisheri. J. Funct. Foods.96, 105204. doi: 10.1016/j.jff.2022.105204
12
de Souza Celente G. Sui Y. Acharya P. (2023). Seaweed as an alternative protein source: Prospective protein extraction technologies. Innovat. Food Sci Emerg. Technol.86, 103374. doi: 10.1016/j.ifset.2023.103374
13
DuBois M. Gilles K. A. Hamilton J. K. Rebers P. A. Smith F. (1956). Colorimetric method for determination of sugars and related substances. Analyt. Chem.28, 350–356. doi: 10.1021/ac60111a017
14
Durand M. Beaumatin P. Bulman B. Bernalier A. Grivet J. P. Serezat M. et al . (1997). Fermentation of green alga sea-lettuce (Ulva sp) and metabolism of its sulphate by human colonic microbiota in a semi-continuous culture system. Reprod. Nutri. Dev.37, 267–283. doi: 10.1051/rnd:19970303
15
Echave J. Fraga-Corral M. Garcia-Perez P. Popović-Djordjević J. H Avdović E. Radulović M. et al . (2021). Seaweed protein hydrolysates and bioactive peptides: extraction, purification, and applications. Mar. Drugs19, 500. doi: 10.3390/md19090500
16
Elias R. J. Kellerby S. S. Decker E. A. (2008). Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci Nutr.48, 430–441. doi: 10.1080/10408390701425615
17
FAO (2025). Food and agriculture projections to 2050 | Global Perspectives Studies | Food and Agriculture Organization of the United Nations. Available online at: https://www.fao.org/global-perspectives-studies/food-agriculture-projections-to-2050/en/ (Accessed March 28, 2025).
18
Figueira T. A. Martins N. T. Ayres-Ostrock L. Plastino E. M. Enrich-Prast A. Peruzzi de Oliveira V. (2021). The effects of phosphate on physiological responses and carbohydrate production in Ulva fasciata (Chlorophyta) from upwelling and non-upwelling site. Botanica Marina64, 1–11. doi: 10.1515/bot-2020-0051
19
Filisetti-Cozzi T. M. Carpita N. C. (1991). Measurement of uronic acids without interference from neutral sugars. Analyt. Biochem.197, 157–162. doi: 10.1016/0003-2697(91)90372-z
20
Fleurence J. Le Coeur C. Mabeau S. Maurice M. Landrein A. (1995). Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol.7, 577–582. doi: 10.1007/BF00003945
21
Fleurence J. Morançais M. Dumay J. (2018). “ Seaweed proteins,” in Proteins in food processing ( Elsevier), 245–262. Available online at: https://www.sciencedirect.com/science/article/pii/B9780081007228000103.
22
Fort A. Mannion C. Fariñas-Franco J. M. Sulpice R. (2020). Green tides select for fast expanding Ulva strains. Sci Total. Environ.698, 134337. doi: 10.1016/j.scitotenv.2019.134337
23
Foster G. G. Hodgson A. N. (1998). Consumption and apparent dry matter digestibility of six intertidal macroalgae by Turbo sarmaticus (Mollusca: Vetigastropoda: Turbinidae). Aquaculture167, 211–227. doi: 10.1016/S0044-8486(98)00315-9
24
Fujiwara-Arasaki T. Mino N. Kuroda M. (1984). The protein value in human nutrition of edible marine algae in Japan. Hydrobiologia116, 513–516. doi: 10.1007/BF00027735
25
Fukase E. Martin W. (2020). Economic growth, convergence, and world food demand and supply. World Dev.132, 104954. doi: 10.1016/j.worlddev.2020.104954
26
Geada P. Moreira C. Silva M. Nunes R. Madureira L. Rocha C. M. R. et al . (2021). Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol.332, 125125. doi: 10.1016/j.biortech.2021.125125
27
Glasson C. R. K. Sims I. M. Carnachan S. M. de Nys R. Magnusson M. (2017). A cascading biorefinery process targeting sulfated polysaccharides (ulvan) from Ulva ohnoi. Algal. Res.27, 383–391. doi: 10.1016/j.algal.2017.07.001
28
Godfray H. C. J. Beddington J. R. Crute I. R. Haddad L. Lawrence D. Muir J. F. et al . (2010). Food security: The challenge of feeding 9 billion people. Sci (New York N.Y.)327, 812–818. doi: 10.1126/science.1185383
29
Guo X.-D. Wu C.-S. Ma Y.-J. Parry J. Xu Y.-Y. Liu H. et al . (2012). Comparison of milling fractions of tartary buckwheat for their phenolics and antioxidant properties. Food Res. Int.49, 53–59. doi: 10.1016/j.foodres.2012.07.019
30
Hardouin K. (2015). Production d’extraits aqueux à partir d’Ulva sp, au moyen de procédés d’hydrolyse enzymatique: Caractérisatin, valorisation et perspectives de développement (Lorient: These de doctorat). Available online at: https://theses.fr/2015LORIS373 (Accessed March 28, 2025).
31
Hardouin K. Burlot A.-S. Umami A. Tanniou A. Stiger-Pouvreau V. Widowati I. et al . (2014). Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J. Appl. Phycol.26, 1029–1042. doi: 10.1007/s10811-013-0201-6
32
Harnedy P. A. FitzGerald R. J. (2011). BIOACTIVE PROTEINS, PEPTIDES, AND AMINO ACIDS FROM MACROALGAE(1). J. Phycol.47, 218–232. doi: 10.1111/j.1529-8817.2011.00969.x
33
He J. Xu Y. Chen H. Sun P. (2016). Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci.17. doi: 10.3390/ijms17121988. Article 12.
34
Henchion M. Hayes M. Mullen A. M. Fenelon M. Tiwari B. (2017). Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods. (Basel Switzerland).6, 53. doi: 10.3390/foods6070053
35
Hernández-Garibay E. Zertuche-González J. A. Pacheco-Ruíz I. (2011). Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J. Appl. Phycol.23, 537–542. doi: 10.1007/s10811-010-9629-0
36
Hiraoka M. Enomoto S. (1998). The induction of reproductive cell formation of Ulva pertusa Kjellman (Ulvales, Ulvophyceae). Phycol. Res.46, 199–203. doi: 10.1111/j.1440-1835.1998.tb00114.x
37
Hiraoka M. Oka N. (2008). Tank cultivation of Ulva prolifera in deep seawater using a new “germling cluster” method. J. Appl. Phycol.20, 97–102. doi: 10.1007/s10811-007-9186-3
38
Holdt & Kraan (2011). (PDF) Bioactive compounds in seaweed: Functional food applications and legislation ( ResearchGate). doi: 10.1007/s10811-010-9632-5
39
Huang Q. Hong T. Zheng M. Yang Y. Zhu Y. Jiang Z. et al . (2023). High-pressure homogenization treatment of red seaweed Bangia fusco-purpurea affects the physicochemical, functional properties and enhances in vitro anti-glycation activity of its dietary fibers. Innovat. Food Sci Emerg. Technol.86, 103369. doi: 10.1016/j.ifset.2023.103369
40
Huo Y. Elliott M. S. Drawbridge M. (2024). Growth, Productivity and Nutrient Uptake Rates of Ulva lactuca and Devaleraea mollis Co-Cultured with Atractoscion nobilis in a Land-Based Seawater Flow-Through Cascade IMTA System. Fishes9, 417. doi: 10.3390/fishes9100417
41
Kidgell J. T. Magnusson M. de Nys R. Glasson C. R. K. (2019). Ulvan: A systematic review of extraction, composition and function. Algal. Res.39, 101422. doi: 10.1016/j.algal.2019.101422
42
Kim S. Y. Kang J. H. Jo J. H. Min S. C. (2018). Development of a gulfweed-based edible coating using high-pressure homogenization and its application to smoked salmon. J. Food Sci83, 3027–3034. doi: 10.1111/1750-3841.14395
43
Lahaye M. (1998). NMR spectroscopic characterisation of oligosaccharides from two Ulva rigida ulvan samples (Ulvales, Chlorophyta) degraded by a lyase. Carbohydr. Res.314, 1–12. doi: 10.1016/S0008-6215(98)00293-6
44
Lahaye M. Robic A. (2007). Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules8, 1765–1774. doi: 10.1021/bm061185q
45
Le B. Golokhvast K. Yang S. Sun S. (2019). Optimization of microwave-assisted extraction of polysaccharides from ulva pertusa and evaluation of their antioxidant activity. Antioxidants8. doi: 10.3390/antiox8050129
46
Lopes N. Ray S. Espada S. F. Bomfim W. A. Ray B. Faccin-Galhardi L. C. et al . (2017). Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol.102, 605–612. doi: 10.1016/j.ijbiomac.2017.04.043
47
Lourenço S. O. Barbarino E. De-Paula J. C. Pereira L. O. D. S. Lanfer Marquez U. M. (2002). Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res.50, 233–241. doi: 10.1046/j.1440-1835.2002.00278.x
48
Mæhre H. K. (2016). Seaweed proteins - how to get to them? Effects of processing on nutritional value, bioaccessibility and extractability. UiT The Arctic University of Norway. Available online at: https://munin.uit.no/handle/10037/9130 (Accessed May 25, 2025).
49
Magdugo R. P. Terme N. Lang M. Pliego-Cortés H. Marty C. Hurtado A. Q. et al . (2020). An Analysis of the Nutritional and Health Values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two Chlorophyta Collected from the Philippines. Molecules25. doi: 10.3390/molecules25122901. Article 12.
50
Magnusson M. Glasson C. R. K. Vucko M. J. Angell A. Neoh T. L. De Nys R. (2019). Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal. Res.41, 101555. doi: 10.1016/j.algal.2019.101555
51
Mantri V. A. Kazi M. A. Balar N. B. Gupta V. Gajaria T. (2020). Concise review of green algal genus Ulva Linnaeus. J. Appl. Phycol.32, 2725–2741. doi: 10.1007/s10811-020-02148-7
52
Molina-Gilarranz I. Cebrián-Lloret V. Recio I. Martínez-Sanz M. (2025). Impact of structure and composition on the digestibility and nutritional quality of alternative protein-rich extracts from the green seaweed Ulva lacinulata. Food Res. Int.201, 115646. doi: 10.1016/j.foodres.2024.115646
53
Naseem S. Rizwan M. Durrani A. I. Munawar A. Gillani S. R. (2024). Innovations in cell lysis strategies and efficient protein extraction from blue food (Seaweed). Sustain. Chem. Pharm.39, 101586. doi: 10.1016/j.scp.2024.101586
54
Naseri A. Marinho G. S. Holdt S. L. Bartela J. M. Jacobsen C. (2020). Enzyme-assisted extraction and characterization of protein from red seaweed Palmaria palmata. Algal. Res.47, 101849. doi: 10.1016/j.algal.2020.101849
55
Nguyen B. T. Nguyen N. H. Nguyen C. T. K. Do H. M. Nguyen B. Prinyawiwatkul W. et al . (2025). Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach. Green Process. Synthesis.14. doi: 10.1515/gps-2025-0037
56
Ning X. Liu Z. Wang M. Li J. Niu Q. J. Zhu B. et al . (2025). Exploring the emulsifying properties of biopolymers from Ulva lactuca in stabilizing high internal phase emulsions. J. Food Eng.387, 112303. doi: 10.1016/j.jfoodeng.2024.112303
57
Nissen S. H. Juul L. Stødkilde L. Bruhn A. Ambye-Jensen M. Dalsgaard T. K. (2024). Pilot-scale protein extraction of green seaweed (Ulva spp.) whole biomass and pulp – Investigating biochemical composition and protein digestibility in a rat trial. Food Bioprod. Process.148, 353–364. doi: 10.1016/j.fbp.2024.10.003
58
O’ Connor J. Meaney S. Williams G. A. Hayes M. (2020). Extraction of protein from four different seaweeds using three different physical pre-treatment strategies. Molecules25. doi: 10.3390/molecules25082005. Article 8.
59
Olasehinde T. A. Mabinya L. V. Olaniran A. O. Okoh A. I. (2019). Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Properties.22, 100–110. doi: 10.1080/10942912.2019.1573831
60
Pallaoro M. F. do Nascimento Vieira F. Hayashi L. (2016). Ulva lactuca (Chlorophyta Ulvales) as co-feed for Pacific white shrimp. J. Appl. Phycol.28, 3659–3665. doi: 10.1007/s10811-016-0843-2
61
Peña-Rodríguez A. Mawhinney T. P. Ricque-Marie D. Cruz-Suárez L. E. (2011). Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem.129, 491–498. doi: 10.1016/j.foodchem.2011.04.104
62
Pereira L. Cotas J. Gonçalves A. M. (2024). Seaweed proteins: A step towards sustainability? Nutrients16. doi: 10.3390/nu16081123. Article 8.
63
Peter N. R. Raja N. R. Rengarajan J. Radhakrishnan Pillai A. Kondusamy A. Saravanan A. K. et al . (2024). A comprehensive study on ecological insights of Ulva lactuca seaweed bloom in a lagoon along the southeast coast of India. Ocean. Coast. Manage.248, 106964. doi: 10.1016/j.ocecoaman.2023.106964
64
Pliego-Cortés H. Bedoux G. Boulho R. Taupin L. Freile-Pelegrín Y. Bourgougnon N. et al . (2019). Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture system. Algal. Res.41, 101542. doi: 10.1016/j.algal.2019.101542
65
Pliego-Cortés H. Caamal-Fuentes E. Montero-Muñoz J. Freile-Pelegrín Y. Robledo D. (2017). Growth, biochemical and antioxidant content of Rhodymenia pseudopalmata (Rhodymeniales, Rhodophyta) cultivated under salinity and irradiance treatments. J. Appl. Phycol.29, 2595–2603. doi: 10.1007/s10811-017-1085-7
66
Polikovsky M. Fernand F. Sack M. Frey W. Müller G. Golberg A. (2016). Towards marine biorefineries: Selective proteins extractions from marine macroalgae Ulva with pulsed electric fields. Innovat. Food Sci Emerg. Technol.37, 194–200. doi: 10.1016/j.ifset.2016.03.013
67
Polikovsky M. Gillis A. Steinbruch E. Robin A. Epstein M. Kribus A. et al . (2020). Biorefinery for the co-production of protein, hydrochar and additional co-products from a green seaweed Ulva sp. With subcritical water hydrolysis. Energy Conversion. Manage.225, 113380. doi: 10.1016/j.enconman.2020.113380
68
Postma P. R. Cerezo-Chinarro O. Akkerman R. J. Olivieri G. Wijffels R. H. Brandenburg W. A. et al . (2018). Biorefinery of the macroalgae Ulva lactuca: Extraction of proteins and carbohydrates by mild disintegration. J. Appl. Phycol.30, 1281–1293. doi: 10.1007/s10811-017-1319-8
69
Prabhu M. (2019). Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal. Res.37, 215–227. doi: 10.1016/j.algal.2018.11.007
70
Queirós A. S. Circuncisão A. R. Pereira E. Válega M. Abreu M. H. Silva A. M. S. et al . (2021). Valuable nutrients from ulva rigida: modulation by seasonal and cultivation factors. Appl. Sci.11. doi: 10.3390/app11136137. Article 13.
71
Revilla-Lovano S. Sandoval-Gil J. M. Zertuche-González J. A. Belando-Torrentes M. D. Bernardeau-Esteller J. Rangel-Mendoza L. K. et al . (2021). Physiological responses and productivity of the seaweed Ulva ohnoi (Chlorophyta) under changing cultivation conditions in pilot large land-based ponds. Algal. Res.56, 102316. doi: 10.1016/j.algal.2021.102316
72
Robic A. Sassi J.-F. Lahaye M. (2008). Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polymers.74, 344–352. doi: 10.1016/j.carbpol.2008.02.020
73
Robin A. Kazir M. Sack M. Israel A. Frey W. Mueller G. et al . (2018). Functional protein concentrates extracted from the green marine macroalga ulva sp., by high voltage pulsed electric fields and mechanical press. ACS Sustain. Chem. Eng.6, 13696–13705. doi: 10.1021/acssuschemeng.8b01089
74
Rochas C. Lahaye M. Yaphe W. (1986). Sulfate content of carrageenan and agar determined by infrared spectroscopy. Botanica Marina.29, 335–340. doi: 10.1515/botm.1986.29.4.335
75
Sarada B. Prasad M. K. Kumar K. K. Ramachandra Murthy C. (2014). Cadmium removal by macro algae Caulerpa fastigiata: Characterization, kinetic, isotherm and thermodynamic studies. J. Environ. Chem. Eng.2, 1533–1542. doi: 10.1016/j.jece.2014.07.016
76
Savvashe P. Mhatre-Naik A. Pillai G. Palkar J. Sathe M. Pandit R. et al . (2021). High yield cultivation of marine macroalga Ulva lactuca in a multi-tubular airlift photobioreactor: A scalable model for quality feedstock. J. Cleaner. Production.329, 129746. doi: 10.1016/j.jclepro.2021.129746
77
Seedevi P. Moovendhan M. Viramani S. Shanmugam A. (2017). Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym.155, 516–524. doi: 10.1016/j.carbpol.2016.09.011
78
Selvasudha N. Goswami R. Tamil Mani Subi M. Rajesh S. Kishore K. Vasanthi H. R. (2023). Seaweeds derived ulvan and alginate polysaccharides encapsulated microbeads–Alternate for plastic microbeads in exfoliating cosmetic products. Carbohydr. Polymer. Technol. Appl.6, 100342. doi: 10.1016/j.carpta.2023.100342
79
Shahwar D. Raza M. A. Bukhari S. Bukhari G. (2012). Ferric reducing antioxidant power of essential oils extracted from Eucalyptus and Curcuma species. Asian Pac. J. Trop. Biomed.2, S1633–S1636. doi: 10.1016/S2221-1691(12)60467-5
80
Shao P. Pei Y. Fang Z. Sun P. (2014). Effects of partial desulfation on antioxidant and inhibition of DLD cancer cell of Ulva fasciata polysaccharide. Int. J. Biol. Macromol.65, 307–313. doi: 10.1016/j.ijbiomac.2014.01.043
81
Shpigel M. Shauli L. Odintsov V. Ben-Ezra D. Neori A. Guttman L. (2018). The sea urchin, Paracentrotus lividus, in an Integrated Multi-Trophic Aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture490, 260–269. doi: 10.1016/j.aquaculture.2018.02.051
82
Simon C. McHale M. Sulpice R. (2022). Applications of ulva biomass and strategies to improve its yield and composition: A perspective for ulva aquaculture. Biology11. doi: 10.3390/biology11111593. Article 11.
83
Singleton V. L. Orthofer R. Lamuela-Raventós R. M. (1999). “ [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent,” in Methods in Enzymology, vol. 299. ( Academic Press), 152–178. doi: 10.1016/S0076-6879(99)99017-1
84
Smith P. K. Krohn R. I. Hermanson G. T. Mallia A. K. Gartner F. H. Provenzano M. D. et al . (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry150 (1), 76–85. doi: 10.1016/0003-2697(85)90442-7
85
Soto-Sierra L. Wilken L. R. Mallawarachchi S. Nikolov Z. L. (2021). Process development of enzymatically-generated algal protein hydrolysates for specialty food applications. Algal. Res.55, 102248. doi: 10.1016/j.algal.2021.102248
86
Souto-Prieto A. Martinez-Sanz M. Ferreiro T. Parada-Pena P. Abuin-Arias L. Cobos A. et al . (2024). Insights into the structuring ability of two brown seaweeds (Laminaria digitata and Saccharina latissima) for applications as natural texturisers. Algal. Res.80, 103548. doi: 10.1016/j.algal.2024.103548
87
Steinbruch E. Kashyap M. Chemodanov A. Levkov K. Golberg A. (2024). Continuous pulsed electric field processing for intensification of aqueous extraction of protein from fresh green seaweed Ulva sp. Biomass. Food Hydrocolloids.157, 110477. doi: 10.1016/j.foodhyd.2024.110477
88
Steinbruch E. Wise J. Levkov K. Chemodanov A. Israel Á. Livney Y. D. et al . (2023). Enzymatic cell wall degradation combined with pulsed electric fields increases yields of water-soluble-protein extraction from the green marine macroalga Ulva sp. Innovat. Food Sci Emerg. Technol.84, 103231. doi: 10.1016/j.ifset.2022.103231
89
Steinhagen S. Enge S. Larsson K. Olsson J. Nylund G. M. Albers E. et al . (2021). Sustainable large-scale aquaculture of the northern hemisphere sea lettuce, ulva fenestrata, in an off-shore seafarm. J. Mar. Sci Eng.9. doi: 10.3390/jmse9060615. Article 6.
90
Suwal S. Perreault V. Marciniak A. Tamigneaux É. Deslandes É. Bazinet L. et al . (2019). Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J. Food Eng.252, 53–59. doi: 10.1016/j.jfoodeng.2019.02.014
91
Tziveleka L. -A. Ioannou E. Roussis V. (2019). Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym.218, 355–370. doi: 10.1016/j.carbpol.2019.04.074
92
Unis R. Chemodanov A. Gnayem N. Gnaim R. Israel Á. Palatnik R. R. et al . (2023). Effect of seasonality on the amino acid and monosaccharide profile from the green seaweed Ulva lactuca cultivated in plastic sleeves onshore (Mikhmoret, Israel). J. Appl. Phycol.35, 1347–1363. doi: 10.1007/s10811-023-02958-5
93
Vega-Gómez L. M. Molina-Gilarranz I. Fontes-Candia C. Cebrián-Lloret V. Recio I. Martínez-Sanz M. (2024). Production of hybrid protein-polysaccharide extracts from Ulva spp. Seaweed with potential as food ingredients. Food Hydrocolloids. 153. doi: 10.1016/j.foodhyd.2024.110046
94
Wahlström N. (2020). Polysaccharides from red and green seaweed: Extraction, characterisation and applications. Available online at: https://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-272988 (Accessed March 28, 2025).
95
Wang W. Li J. Lu F. Liu F. (2024). Ultrasound-assisted multi-enzyme extraction for highly efficient extraction of polysaccharides from ulva lactuca. Foods13. doi: 10.3390/foods13060891
96
Waterhouse A. L. (2002). Determination of total phenolics. Curr. Protoc. Food Analyt. Chem.6, I1.1.1–I1.1.8. doi: 10.1002/0471142913.fai0101s06
97
Wells M. L. Potin P. Craigie J. S. Raven J. A. Merchant S. S. Helliwell K. E. et al . (2017). Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol.29, 949–982. doi: 10.1007/s10811-016-0974-5
98
Wijesekara I. Pangestuti R. Kim S.-K. (2011). Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polymers.84, 14–21. doi: 10.1016/j.carbpol.2010.10.062
99
Wong K. H. Cheung P. C. K. (2000). Nutritional evaluation of some subtropical red and green seaweeds: Part I — proximate composition, amino acid profiles and some physico-chemical properties. Food Chem.71, 475–482. doi: 10.1016/S0308-8146(00)00175-8
100
Zhao S. Pan Z. Azarakhsh N. Ramaswamy H. S. Duan H. Wang C. (2024). Effects of high-pressure processing on the physicochemical and adsorption properties, structural characteristics, and dietary fiber content of kelp (Laminaria japonica). Curr. Res. Food Sci8, 100671. doi: 10.1016/j.crfs.2023.100671
Summary
Keywords
Ulva sp., high-pressure homogenization, protein enrichment, green extraction, functional ingredients
Citation
Déniel Luque A, Pliego-Cortés H, Burlot A-S, Terme N, Furic M and Bourgougnon N (2025) Production of enriched protein extracts from cultivated Ulva sp. (Chlorophyta, Ulvales) by high-pressure homogenization. Front. Mar. Sci. 12:1675710. doi: 10.3389/fmars.2025.1675710
Received
29 July 2025
Accepted
16 September 2025
Published
09 October 2025
Volume
12 - 2025
Edited by
Thomas Wichard, Friedrich Schiller University Jena, Germany
Reviewed by
Eva Martins, Universidade Católica Portuguesa, Portugal; Mark Polikovsky, Weizmann Institute of Science, Israel
Updates
Copyright
© 2025 Déniel Luque, Pliego-Cortés, Burlot, Terme, Furic and Bourgougnon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Déniel Luque, deniel.anna@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.