Abstract
Macroplastic items like bags, bottles, and containers dominate marine litter, yet their effects on habitats and ecosystems remain understudied. Blue mussels (Mytilus edulis, Mytilus trossulus) form beds that support biodiversity and provide important ecosystem services. The goal of this work was to investigate in an experiment how planar plastic debris, rigid or soft, influences mussel aggregates with regard to their structure and their physiological performance. Mussel individuals were collected in the Kerteminde Fjord and were transferred to a laboratory where they were allowed to form small aggregates on PVC plates (30 individuals each). During formation, half of the aggregates were polluted with planar plastic litter of a defined type (soft PE bags or rigid fragments of PET bottles) and amount, while the other half remained without incorporated macroplastics. All aggregates were then deployed in the fjord for 14 weeks in the winter 2020/21. Afterwards, we measured the cumulative filtration and respiration rates, filtration-to-respiration ratios, condition indices, growth rates, aggregate rugosities, and byssus strengths. Rigid plastics significantly enhanced aggregate rugosity, while all physiological responses as well as byssus formation remained unchanged. The latter might, at least partly, have been due to the fact that we conducted the experiment in winter, when mussel metabolism is substantially reduced. Notably, soft plastics were often concealed within aggregates, and this was presumably caused by the movements of the mussels. This finding suggests that mussel beds may act as sinks for plastic litter, while soft and film-like litter items can be fully embedded in their three-dimensional matrix.
1 Introduction
Marine litter is a global environmental concern and has well documented negative effects on marine organisms (de Carvalho-Souza et al., 2018). Plastics constitute the majority of this litter, while over 80% of the plastic litter that is at sea originates from land-based sources (Landrigan et al (2020). Once in the ocean, plastic items can remain unchanged for a long time or, when environmental conditions promote this, gradually degrade from macroplastics (>5 mm) into microplastics (5 mm to 0.001 mm) and nanoplastics (< 0.001 mm; Blair et al., 2017; Gallo et al., 2018; Wayman and Niemann, 2021). Plastic pollution is also widespread in the Baltic Sea, where plastics make up approximately 70% of the waste found along the shoreline (González-Fernández et al., 2021; Helcom, 2018; Lenz et al., 2023; Narloch et al., 2022; Pärn et al., 2023), while polyethylene (PE) is the most common polymer type found in the Arctic/Northern Atlantic Oceans and the Baltic Sea, where it has an average particle size of 2.66 mm (Hänninen et al., 2021).
Along the coasts of Denmark in the Western Baltic Sea, macroplastics can interact with a large number of mobile and sessile benthic organisms, for instance with mussels from the family Mytilidae. The blue mussel (Mytilus spp.) plays a vital ecological role in the Baltic Sea (Commito et al., 2014) and one region where it occurs in dense mussel beds is the shallow fjord system of Kerteminde Fjord/Kertinge Nor (Jürgensen, 1995; Riisgård et al., 2008). This region is home to two morphologically similar and hybridizing species, M. edulis and M. trossulus (Stuckas et al., 2009), which both can form extensive, multi-layered, three-dimensional mussel beds in subtidal habitats (Riisgard et al., 2006; Tsuchiya and Nishihira, 1985). These filter feeders retain particles larger than 4 µm, primarily phytoplankton (Riisgård, 1991; Rouillon et al., 2005), and by this couple the pelagic realm to the benthos. Furthermore, they provide numerous ecosystem services including shoreline stabilization, habitat formation, and bioremediation through their filtering activities (Broszeit et al., 2016; Commito et al., 2014; Ragnarsson and Raffaelli, 1999). Blue mussels also contribute to nutrient cycling (Jansen et al., 2012; Prins and Smaal, 1994). Mytilus edulis, for instance, is recycling substantial amounts of inorganic nitrogen and phosphorus annually in the Baltic Proper (Kautsky, 1980; Prins et al., 1996). Beyond their outstanding ecological role, mussels are important aquaculture organisms that support local economies in many countries, e.g. Spain, Ireland, and Canada (Cartier et al., 2004; Fernández et al., 2015; Gonzalez-Poblete et al., 2018; Lekang et al., 2003; Stirling and Okumus (1995); Strohmeier et al., 2008; Taylor et al., 1992). Hence, given their ecological and economic value, it is critical to investigate how mussels are impacted by plastic pollution, to assess to what extend it could threaten mussel health, fitness and physiological performance. While harmful effects of microplastics on mussels, such as a reduced filtration capacity, oxidative stress, and neurotoxic impacts, are, at least under laboratory conditions, well documented (Abidli et al., 2021; Avio et al., 2015; Farrell and Nelson, 2013; Hamm and Lenz, 2021, Van Cauwenberghe et al., 2015; von Moos et al., 2012), little is known about the impacts of large-sized plastics, which cannot be ingested or inhaled by mussels.
Macroplastics are particularly prevalent in coastal areas (Gündoğdu and Çevik, 2019; Lechthaler et al., 2020), and can easily be washed onto mussel beds in the intertidal or settle down on subtidal mussel beds. Then, soon after arrival, mussels could attach byssus threads to the litter objects and by this connect them physically to the bed so that they are not washed away again easily (Walkinshaw and Phelps, 2023; see anecdotal evidence, Supplementary Figure S1). In the following weeks, months and years, juvenile mussels could settle on these substrata and anchor themselves to them. By this the litter could become a part of the three-dimensional structure of the mussel bed. Although, this process has never been documented in detail, there are numerous reports about plastic debris, such as fishing nets, ropes and plastic bags, that were found incorporated into mussel beds (Walkinshaw and Phelps, 2023; see anecdotal evidence, Supplementary Figure S1), additional to microplastic ingestion (Van Cauwenberghe et al., 2015; Woods et al., 2018) that potentially comes from macroplastic entrapment. Attachment strength in blue mussels is known to differ between soft and hard substrates, i.e. mussels attach more strongly to the shells of conspecifics than to substrates composed of sand (Christensen et al., 2015). This suggests that the degree to which different types of macroplastics become anchored within mussel beds may also depend on the nature of the litter. This raises concerns about potential physiological and structural effects of the plastics on the mussel aggregates. In this study, we assessed how macroplastic debris, specifically soft polyethylene (PE) bags and rigid fragments of polyethylene terephthalate (PET) bottles, affect blue mussel aggregates. Both materials are commonly found as marine litter (Castro-Jiménez et al., 2019; Cressey, 2016; Depledge et al., 2013; Galgani et al., 1995, Galgani et al., 1996, Galgani et al., 2000; Pasquini et al., 2016) and are globally abundant. The goal of this study was to assess potential effects of two types of macroplastics (PE bags and PET bottles) on the physiology and structure of blue mussel aggregates. We hypothesized that macroplastic presence would reduce mussel aggregate performance, and that the structural effects would vary with plastic type, with rigid plastics causing greater disruption than flexible films.
2 Materials and methods
2.1 Plastic materials and the experimental design
To simulate different levels of plastic pollution, we defined plastic loads relative to the surface area of each blue mussel aggregate. In a pilot study, the shell surface area (A) of 55 Mytilus individuals ranging from 15 to 25 mm in shell length was measured. We did this with five mussel individuals per millimeter class. For this, mussel shells were coated with watercolor paint and pressed onto paper towels to create full shell imprints. These imprints were then scanned, and surface areas were quantified using the polygon tool in ImageJ (Schneider et al., 2012). This enabled us to estimate the total surface area of each individual mussel aggregate based on its size composition. Relative to these surface area estimates, which represent an important physical property of a mussel aggregate, we defined plastic pollution levels using two types of transparent, planar plastic materials: fragments of polyethylene (PE) bags and of polyethylene terephthalate (PET) bottles. These materials were used to create different levels of the experimental factor “Amount”, which were the plastic litter loads per mussel aggregate. The level “Low” was equivalent to 40% of the total shell surface area of the aggregate, while the level “High” was equivalent to 80%. A second experimental factor “Rigidity” considered the mechanical properties of the plastic, with soft plastics represented by the PE bags and rigid plastics by the PET bottles. Single bags and bottles were cut according to the required, aggregate-specific surface area. In this process, plastic bag fragments remained in one piece, while bottles always had to be cut in two pieces because of their original shape. To ensure ecological relevance, all plastic materials were pre-conditioned by allowing natural biofilm formation. This was achieved by submerging the plastics in the Kerteminde Fjord for two weeks in November 2020 prior to the experiment. The experiment followed a fully crossed two-factorial design, combining the two levels of “Amount” (low, high) with the two levels of “Rigidity” (soft, rigid), resulting in four treatment combinations: “low + soft,” “low + rigid,” “high + soft,” and “high + rigid.” Furthermore, we had an additional control group, which were mussel aggregates without plastics. Each of the five experimental groups had seven replicates, resulting in a total of 35 mussel aggregates.
2.2 Mussel collection and aggregate formation
For this study, juvenile, postmetamorphic Mytilus spp. with shell lengths ranging from 15–25 mm (aged< 1 year; Jacobs et al., 2015) were collected from the subtidal in the inlet of Kerteminde Fjord, Denmark (geographical coordinates: 55°26’59.6”N, 10°39’40.0”E). These specimens presumably comprised individuals of the species Mytilus edulis and Mytilus trossulus as they co-occur in the fjord (Michalek et al., 2016). However, the exact species composition of the collected individuals remains unknown, as accurate species identification requires molecular analysis (Katolikova et al., 2016). Kerteminde Fjord lies within the western Baltic Mytilus hybrid zone, where populations are predominantly M. edulis with varying introgression from M. trossulus along the west- east cline of the Baltic Sea (Kijewski et al., 2019; Knöbel et al., 2021). However, no information about the species composition of Mytilus populations in Kerteminde Fjord are available. The mussels were collected in November 2020, when the mean water temperature was around 11 °C (Supplementary Table S1). Mussels were gently detached from hemp ropes by cutting the byssus threads with scissors to prevent damage to the byssus gland. Following collection, the mussels were transported within 30 minutes to the Marine Biological Research Centre in Kerteminde. In the centre laboratory, the mussels were organized in 35 batches of which each consisted of 30 randomly selected individuals.
Aggregation was initiated by placing the mussels onto grey polyvinylchloride (PVC) plates (20 × 20 cm), which had been roughened with 40-grit sandpaper to improve attachment. For incorporating the plastic litter, mussels were arranged in a sandwich-like structure: 15 mussels were placed directly on the PVC plate, followed by the assigned plastic material, and then covered with the remaining 15 mussels. In control aggregates without plastic (n= 7), all 30 mussels were simply layered on top of each other on the plate to encourage dense aggregation. Once assembled, the plates were placed horizontally in one of four indoor tanks (100 L each) and were left for one week to allow the mussels to form stable aggregates. All tanks were supplied with a continuous flow of seawater (150 L/min), ensuring consistent environmental conditions and a steady food supply. During this aggregation period, key water quality parameters were monitored: ammonium concentration remained below 0.05 mg/L (JBL Ammonium/Ammonia Test), pH averaged 7.0 ± 0.5 (test strips), temperature was 9.94 ± 0.74 °C (measured with a YSI30), salinity 23.78 ± 1.71 PSU (measured with a YSI30), and dissolved oxygen was 9.14 ± 0.18 mg/L (WTW OxiCal®-SL). These conditions closely resembled those in the field, due to the constant influx of seawater. A light regime of 8 hours light and 16 hours dark was applied to simulate the natural photoperiod of the season.
After one week, the mussel aggregates were prepared for field deployment. Fence-like barriers made from plastic mesh were attached to all four sides of each PVC plate to protect the aggregates from mechanical disturbance and to prevent them from sliding off the plates. The latter were kept in a horizontal position using ropes tied to all four corners, secured by a centrally attached weight. The prepared platforms were then attached to a triangular metal rack, which was fixed to a jetty in the inlet of Kerteminde Fjord at a depth of, on average, 1 m.
Field exposure took place between November 2020 and March 2021 (i.e. 14 weeks), with mussel aggregates deployed at sea between 23rd and 28th November 2020 and retrieved sequentially between 28th January and 2nd March 2021 (Supplementary Table S2). Due to logistical constraints - only five aggregates could be processed per day - both deployment and retrieval were carried out in stages. As a result, the aggregates remained in situ for an average of 72.5 ± 7.5 days (Supplementary Table S2), and the total experimental period spanned 100 days, from the first deployment to the final retrieval. Throughout the sea exposure period, temperature (°C), salinity (PSU), and chlorophyll a concentration (µg/L) were recorded every 1–3 days using a handheld multiparameter system (YSI650 MDS).
The mussels fell within a 15–25 mm size range and the initial dry weights of the mussel individuals were estimated by Equation 1:
, where DW0 denotes the initial dry weight, and L0 the initial shell length of the blue mussel.
This relationship was derived from a pilot study conducted in November 2020, for which 33 mussels in the size range 15–25 mm were collected from the same site. Their shell lengths and soft body dry weights were measured after drying them at 180 °C for 24 hours. Then a linear regression model was applied to determine the parameters of the above equation.
2.3 Response variables
In March 2021, following the in situ exposure period, all mussel aggregates were retrieved from the field and transported back to the laboratory for analysis. Upon arrival, the PVC plates were gently rinsed with pre-filtered seawater (2 µm pore size) to remove microalgae, small invertebrates, and residual organic material without disturbing the mussel aggregates.
2.3.1 Cumulative filtration rate
Filtration rates of blue mussel aggregates were measured using the clearance method described by (Riisgård, 2001). Aggregates were individually placed in the center of aerated tanks (volume: 8 ± 2 L) filled with pre-filtered seawater (2 µm pore size). Water temperature was maintained at 9.04 ± 1.74 °C, consistent with the conditions used for respiration measurements and closely matching the ambient seawater temperature. To allow for thermal acclimatization, mussel aggregates were left undisturbed in the tanks for 25 minutes before the start of the measurements. Following acclimatization, algal cells from a stock culture of the cryptophyte Rhodomonas salina (initial concentration ~8000 cells/mL) were added to each tank by injecting 241 ± 118 mL of algal suspension. The exact injection volume was calculated based on the current algal concentration in the stock culture and the specific water volume in each tank. Algal suspension was added twice: once immediately after acclimatization and again 25 minutes later. Water samples (10 mL) were collected at 5-minute intervals for a total of 55 minutes, starting two minutes after the first algal injection (defined as t0). Sampling was performed using a pipette, drawing water from just above the mussel aggregate. In total, 12 samples were collected per aggregate: six before and six after the second algal injection. Algal concentrations in these samples were measured using an electronic particle counter (Elzone II 5390). The number of open mussels, of which we assumed that they were actively filtering, was recorded immediately after each sampling. To account for algal cell loss not attributable to mussel filtration, a control tank with filtered seawater but no mussels was included. This allowed the assessment of background changes in algal concentration over time.
The filtration rate was determined from the slope of a linear regression in a semi-ln-plot using Equation 2 (Riisgård et al., 2014):
where V denotes the seawater volume in the experimental tank, b is the slope of the regression line in a semi-logarithmic plot and n is the number of actively filtering blue mussels during the algal depletion phase.
2.3.2 Cumulative respiration rate
To measure the cumulative respiration rate of each blue mussel aggregate, individual aggregates were placed in sealed 34 L tanks filled with pre-filtered (2 µm) seawater maintained at 9.04 ± 1.74 °C. Water within the tanks was gently mixed throughout the experiment using two air stones to ensure uniform oxygen distribution. After a 30-minute acclimatization period, dissolved oxygen concentration was recorded every 10 minutes over a 90-minute period using a WTW OxiCal®-SL oxygen probe. Throughout the measurements, oxygen levels remained normoxic (> 8 mg O2 l-1; Seitz et al., 2003), with a mean of 10 mg O2 l-1 ± 0.58 mg O2 l-1 during all measurements. Following each oxygen measurement, the number of open mussels, which were assumed to be respiring, was recorded, along with the water temperature. To account for changes in the oxygen concentration unrelated to the mussels, one additional sealed tank without mussels served as a control. The respiration rate of each blue mussel aggregate was calculated based on the slope of a linear regression of the oxygen concentration over time and the number of open blue mussels using Equation 3 (Tang and Riisgård, 2018):
where V denotes the seawater volume in the tank, b is the slope of the regression line in a semi-logarithmic plot and n is the number of blue mussels that were open during the measurements.
Calculations were performed in R version 4.0.3 (R Core Team, 2020). Final respiration rates were corrected by subtracting the background oxygen consumption observed in the control tank.
2.3.3 F/R - ratio
The F/R ratio reflects the volume of water filtered per mL of oxygen consumed and serves as an indicator of filtration efficiency in blue mussels (Riisgård et al., 2016). It is calculated by dividing the filtration rate F by the respiration rate R. To express the F/R ratio on a per-individual basis, the aggregate-level ratio was divided by the number of mussels that were open and presumed to be actively filtering during the measurements.
2.3.4 Bidimensional rugosity index
The spatial complexity of the blue mussel aggregates was assessed using the bidimensional rugosity index (Gestoso et al., 2013). To measure this, a flexible wire was gently laid across each mussel aggregate along two diagonal lines, spanning the widest dimensions of the aggregate (designated as C1 and C2). The wire was carefully bent to follow the surface contour of the aggregate as closely as possible. After each measurement, the wire was straightened, and the length of the bent segment was recorded. Next, the endpoints of both diagonals were marked on the underlying PVC plate, and the straight-line distances between each pair of opposing corners (L1 and L2), which were representing the bidimensional projections of the aggregate base, were measured after removing the mussels. Following the measurement of all response variables, the number of mussels that had remained per aggregate was counted, and their combined soft body dry weight (DW) was determined. The bidimensional rugosity index (BR) was then calculated by Equation 4 as follows:
where C1 and C2 are the lengths of the bent diagonals (aggregate contour), L1 and L2 are the straight diagonal distances (aggregate base), DW is the total dry weight of soft tissue and n is the number of mussels in the aggregate.
Endolithic shell corrosion can affect a mussel aggregate’s rugosity (Nicastro et al., 2022), but this has not been considered in this study.
2.3.5 Byssus strength
Byssus strength was measured to assess how firmly individual mussels were attached to their aggregate, what means either to other mussels, the plastic litter, or the PVC plate or to combinations of these substrata. A dynamometer was used to determine the maximum force required to detach mussels from the aggregate. To perform the measurement, a wire loop was attached to the dynamometer and carefully fixed around an individual mussel. The dynamometer was then pulled away from the aggregate at a 90° angle until the mussel detached, while the peak force required for detachment was recorded using the thrust ring on the dynamometer. For each mussel aggregate, the byssus strength was expressed as the mean force needed for the detachment of individual mussels, providing a single average value per replicate for statistical analysis.
2.3.6 Condition index
To assess the physiological condition of the mussels, the condition index (CI) was calculated for each aggregate. For this, first the shell length of each mussel within an aggregate was measured and then the mussels were frozen at -20 °C. After defrosting, the soft tissue was separated from the shell and dried at 180 °C for 24 hours to determine dry body mass. Based on these measurements, a mean condition index was determined for each blue mussel aggregate using Equation 5 (Riisgård et al., 2014):
where DW is the mean dry weight of the soft bodies in an aggregate and L is the mean shell length of the mussels in an aggregate.
2.3.7 Growth rate
The growth rate (μ) was used to estimate the daily increase in the soft body mass of the mussels during the sea exposure period. It was calculated using the following Equation 6 (Olesen et al., 1994):
where DWt is the final dry weight of soft tissue after sea exposure, DW0 is the estimated initial dry weight before exposure (calculated using Equation 1) and t is the length of the exposure period. Multiplying μ by 100 yields the daily percentage increase in soft body mass. For statistical analysis, the mean individual growth rate was calculated for each aggregate by averaging the growth rates of all mussels within the aggregate.
2.4 Statistical analysis
All statistical analyses were performed in R version 4.0.3 (R Core Team, 2020). To estimate initial mussel dry weight from shell length, a simple linear regression model was used, with dry weight as the response variable and shell length as the predictor.
For response variables that followed a normal distribution, i.e. filtration rate, respiration rate, F/R ratio, byssus strength, and growth rate, two-way analyses of variance (ANOVAs) were performed to evaluate the effects of the fixed factors “Rigidity” (soft/rigid plastic) and “Amount” (low/high plastic load). For non-normally distributed variables, i.e. the bidimensional rugosity index and the condition index, generalized linear models (GLMs) with a gamma error structure were applied. The assumptions of normality and homoscedasticity in the residuals were tested using the Shapiro-Wilk’-W test (Shapiro and Wilk, 1965) and the Fligner-Killeen test (Dolby, 1976), respectively. To compare the control aggregates without plastic with specific groups of the plastic-contaminated aggregates, pairwise comparisons were performed using either Student’s t-tests (Student, 1908; for normally distributed data) or Mann-Whitney-U tests (McKnight and Najab, 2010; for non-normal data). These comparisons were made between the control group and four pooled treatment groups: (a) all aggregates with rigid plastic, (b) all aggregates with soft plastic, (c) all aggregates with a high plastic load, and (d) all aggregates with a low plastic load. To account for repeated comparisons involving the same control group, the significance threshold was conservatively set to p ≤ 0.01. Statistical tests were conducted in R version 4.0.3 (R Core Team, 2020) by using the packages car, FSA, lattice and rcompanion (Fox & Weisberg, 2019; Mangiafico, 2016; Ogle et al., 2015; Sarkar, 2001). Figures 1 and 2 were created using the plugin ScientiFig (Aigouy & Mirouse, 2013) in Fiji (Schindelin et al., 2012) and Figures 3, 4 and 5 in R version 4.0.3 (R Core Team, 2020) using the packages ggplot2, Hmisc, cowplot, ggpubr, gridExtra, patchwork and dplyr (Auguie, 2010; Harrell Jr, 2003; Kassambara, 2016; Pedersen, 2019; Wickham, 2016; Wickham et al., 2023; Wilke, 2015).
3 Results
3.1 Study organism
Prior to exposure in the Kerteminde Fjord (November 2020 to March 2021), the blue mussels had a mean (± SD) shell length of 20 ± 1 mm and an estimated dry weight of 21.5 ± 2.2 mg. After the exposure period, mussels had grown to a mean shell length of 26 ± 1 mm, and their mean dry weight had increased to 64.44 ± 8.34 mg. One mussel aggregate was lost during deployment; however, the number of mussels per aggregate remained stable throughout the study, with an average of 29 ± 1 individuals per aggregate after 72.5 ± 7.5 days of sea exposure.
All mussels remained associated with their assigned aggregates, and the plastic debris added to them was consistently incorporated into the three-dimensional structure of the experimental mussel beds (Figure 1). However, the manner of incorporation differed between plastic types. Mussels were attached directly to the rigid plastic (PET) fragments and the pieces themselves were only minimally relocated within the aggregates, while the soft plastic (PE) debris was often transferred to the interior of the aggregates and this commonly already happened within the seven-day-long formation period (Luisa Kumpitsch, pers. obs.). Following exposure, in 79% of the aggregates the soft plastic debris was found crumpled and embedded inside the aggregates (Figure 2), making them visually indistinguishable from the aggregates without plastic. In some aggregates, mussels in the center of the aggregate were almost completely enclosed by plastic bag pieces (Supplementary Figure S2).
Figure 1

Mytilus spp. aggregates on PVC-plates with (A–D) and without (E, F) incorporated plastic after sea exposure for 72.5 ± 7.5 days. (A, B) low/high amount of soft plastic (= PE bags), (C, D) low/high amount of rigid plastic (= PET bottles), (E, F) no incorporated plastic.
Figure 2

Mytilus spp. aggregates after sea exposure for 72.5 ± 7.5 days. All aggregates contain soft plastic (= PE bags). (A–H) are showing the same aggregate, respectively, while the picture on the left always shows the aggregate as a whole and the picture on the right the part in which the soft plastic (= PE bag) was embedded. Arrows indicate the location of the plastic.
3.2 Environmental conditions during exposure of mussel aggregates
The mean water temperature during 100 days of total sea exposure from November 2020 to March 2021 was 6.1°C ± 2.9°C, while the mean salinity was 17.4 ± 3.2 psu and the mean chlorophyll a concentration was 3.2 µg l-1 ± 1.2 µg l-1. Temperature peaks coincided with chlorophyll a peaks and salinity showed fluctuations between 9.4 PSU to 23.5 PSU (Figure 3).
Figure 3
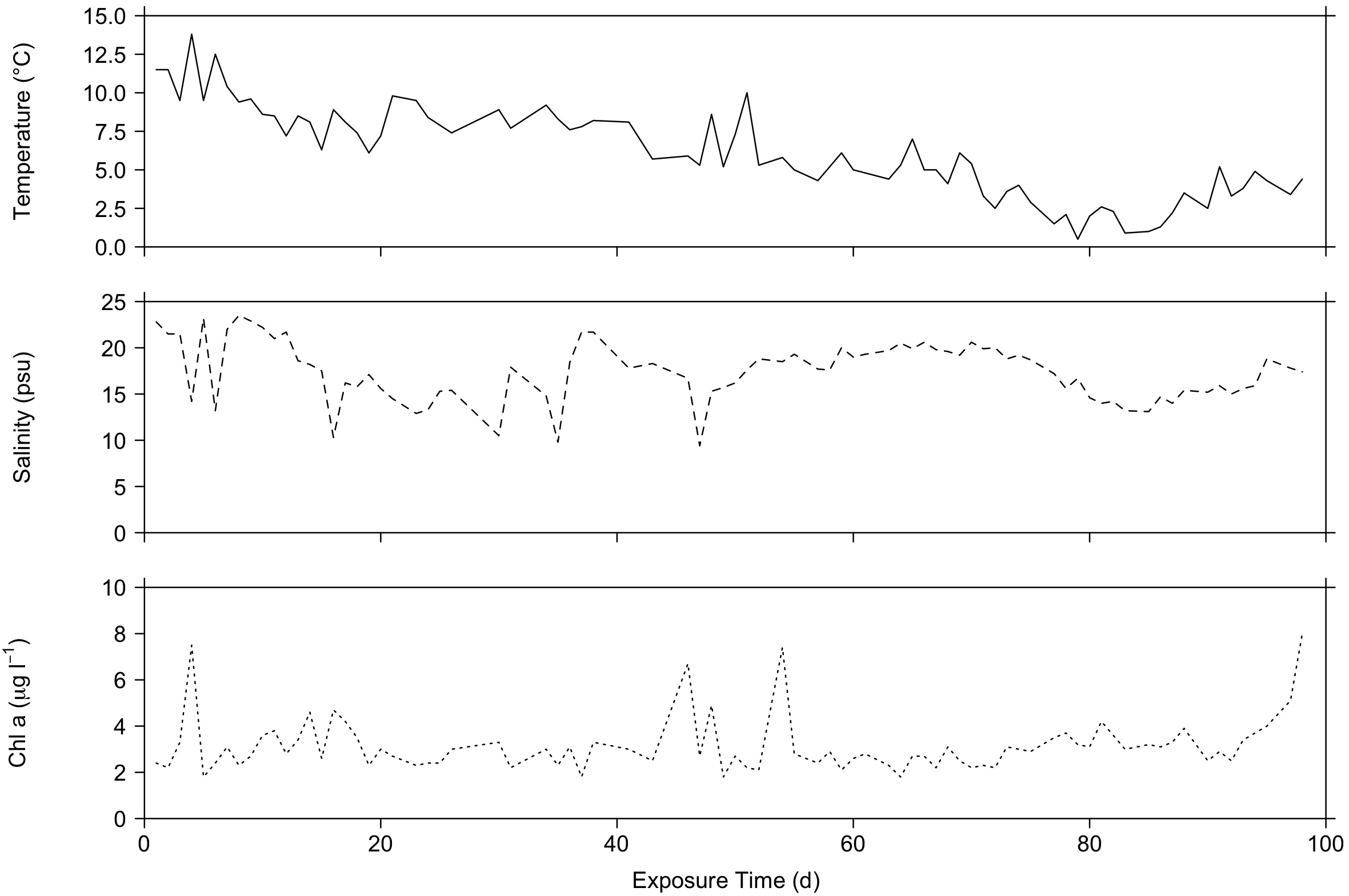
The environmental parameters temperature (°C), salinity (PSU) and chlorophyll a concentration (µg/l-1) at the site where the blue mussel aggregates were deployed were measured every 1–3 days in the period from the 23.11.2020 to the 02.03.2021 (= total exposure time in days).
3.3 Response variables
After five months of sea exposure, neither the type of plastic debris (rigid/soft) nor the amount of debris (40%/80% of aggregate surface area) had a significant effect on any of the physiological response variables. Specifically, there were no detectable differences in growth rate, filtration rate, respiration rate, condition index, F/R ratio, or byssus strength across the treatment combinations (Tables 1, 2). In addition, pairwise comparisons between mussel aggregates with and without plastic debris, as well as between different debris types and amounts, revealed no significant influence of any of these variables (Table 3).
Table 1
| Response variable | Source of variation | Df | Chi² | P |
|---|---|---|---|---|
| Bidimensional rugosity index | ||||
| Rigidity | 1 | 7.48 | 6x10-3 | |
| Amount | 1 | 0.04 | 0.85 | |
| Rigidity * Amount | 1 | 1.56 | 0.21 | |
| Condition index | ||||
| Rigidity | 1 | 0.31 | 0.58 | |
| Amount | 1 | 0.36 | 0.55 | |
| Rigidity * Amount | 1 | 1.30 | 0.26 | |
Influence of different types (rigid/soft) and amounts (low/high) of planar plastic debris on the bidimensional rugosity index and the condition index of blue mussels in aggregates that were exposed in Kerteminde Fjord, Western Baltic Sea, from November 2020 to March 2021.
Results from two-factorial generalized linear models (GLMs) with gamma error structure. Significant p-values are in bold. NB: df, degrees of freedom.
Table 2
| Response variable | Source of variation | Df | SS | F | P |
|---|---|---|---|---|---|
| Filtration rate | |||||
| Rigidity | 1 | 0.27 | 0.85 | 0.46 | |
| Amount | 1 | 0.11 | 0.57 | 0.50 | |
| Rigidity * Amount | 1 | 0.11 | 0.56 | 0.57 | |
| Respiration rate | |||||
| Rigidity | 1 | 0.01 | 2.57 | 0.13 | |
| Amount | 1 | 5.0 x 10-4 | 0.08 | 0.77 | |
| Rigidity * Amount | 1 | 0.001 | 0.21 | 0.65 | |
| F/R - ratio | |||||
| Rigidity | 1 | 31.45 | 1.56 | 0.22 | |
| Amount | 1 | 7.52 | 0.37 | 0.55 | |
| Rigidity * Amount | 1 | 4.03 | 0.2 | 0.66 | |
| Byssus strength | |||||
| Rigidity | 1 | 4.64 | 2.40 | 0.14 | |
| Amount | 1 | 2.10 | 1.09 | 0.31 | |
| Rigidity * Amount | 1 | 0.87 | 0.45 | 0.51 | |
| Growth rate | |||||
| Rigidity | 1 | 2.0 x 10-6 | 0.61 | 0.44 | |
| Amount | 1 | 2.0 x 10-6 | 0.89 | 0.36 | |
| Rigidity * Amount | 1 | 2.0 x 10-6 | 0.80 | 0.38 | |
Influence of different types (rigid/soft) and amounts (low/high) of planar plastic debris on the filtration rates, respiration rates, F/R-ratio, byssus strength and the growth rates of blue mussels in aggregates that were exposed in the Kerteminde Fjord, Western Baltic Sea, from November 2020 to March 2021.
Results from two-factorial analyses of variance (ANOVA). NB: df, degrees of freedom, SS, sum of squares.
Table 3
| Response variable | Source of variation | Df | T | W | P |
|---|---|---|---|---|---|
| Filtration rate | |||||
| Control - Plastic | 32 | -0.10 | – | 0.92 | |
| Control - High amount | 19 | -0.41 | – | 0.69 | |
| Control - Low amount | – | – | 44 | 0.94 | |
| Control - Rigid plastic | 18 | -0.53 | – | 0.60 | |
| Control - Soft plastic | 19 | 0.27 | – | 0.79 | |
| Respiration rate | |||||
| Control - Plastic | – | – | 66 | 0.23 | |
| Control - High amount | 19 | -1.12 | – | 0.28 | |
| Control - Low amount | 18 | -0.77 | – | 0.45 | |
| Control - Rigid plastic | 18 | -0.32 | – | 0.75 | |
| Control - Soft plastic | 19 | -1.38 | – | 0.18 | |
| F/R-ratio | |||||
| Control - Plastic | – | – | 103 | 0.73 | |
| Control - High amount | 19 | 0.53 | – | 0.6 | |
| Control - Low amount | 18 | 1.05 | – | 0.31 | |
| Control - Rigid plastic | 18 | 0.36 | – | 0.73 | |
| Control - Soft plastic | – | – | 59 | 0.48 | |
| Bidimensional rugosity index | |||||
| Control - Plastic | – | – | 63 | 0.19 | |
| Control - High amount | – | – | 38 | 0.44 | |
| Control - Low amount | – | – | 25 | 0.11 | |
| Control - Rigid plastic | – | – | 22 | 0.07 | |
| Control - Soft plastic | – | – | 41 | 0.58 | |
| Byssus strength | |||||
| Control - Plastic | 32 | -0.24 | – | 0.81 | |
| Control - High amount | – | – | 39.5 | 0.50 | |
| Control - Low amount | 18 | 0.17 | – | 0.87 | |
| Control - Rigid plastic | 18 | 0.41 | – | 0.68 | |
| Control - Soft plastic | 19 | -0.77 | – | 0.45 | |
| Condition index | |||||
| Control - Plastic | 32 | 0.26 | – | 0.80 | |
| Control - High amount | 18 | 0.45 | – | 0.66 | |
| Control - Low amount | 19 | 0.01 | – | 0.99 | |
| Control - Rigid plastic | – | – | 50.5 | 0.94 | |
| Control - Soft plastic | 18 | -0.02 | – | 0.98 | |
| Growth Rate | |||||
| Control - Plastic | 32 | – | 0.87 | 0.39 | |
| Control - High amount | 19 | – | 0.40 | 0.69 | |
| Control - Low amount | 18 | – | 1.35 | 0.19 | |
| Control - Rigid plastic | 18 | – | 0.43 | 0.67 | |
| Control - Soft plastic | 19 | – | 1.20 | 0.24 | |
Pairwise comparisons between blue mussel aggregates that were polluted with different types (rigid/soft) of planar plastic debris and aggregates without plastic debris (control) for various response variables.
Data from aggregates with plastic debris were pooled prior to the comparisons: Plastic - all aggregates with plastic debris, low amount - all aggregates with 40% plastic debris, high amount - all aggregates with 80% plastic debris, soft plastic - all aggregates with PE bags, rigid plastic - all aggregates with PET bottles. Depending on the data distribution, comparisons were either done with student’s t-test or with the Mann-Whitney-U-test. NB: df, degrees of freedom.
Although, no effect of the plastic litter was observed, per capita physiological responses varied substantially across individual mussels in all groups (Figure 4). Filtration rates ranged from 0.11 to 1.72 L h-¹ ind-¹, with a mean of 0.81 ± 0.46 L h-¹ ind-¹ across all aggregates. A similar range of variability was found in respiration rates, which span from 0.04 to 0.34 mg O2 h-¹ ind-¹, with a mean of 0.15 ± 0.07 mg O2 h-¹ ind-¹. Correspondingly, F/R ratios ranged from 0.33 to 21.64 L (mg O2)-¹ ind-¹, with a mean of 6.9 ± 4.99 L (mg O2)-¹ ind-¹.
Figure 4
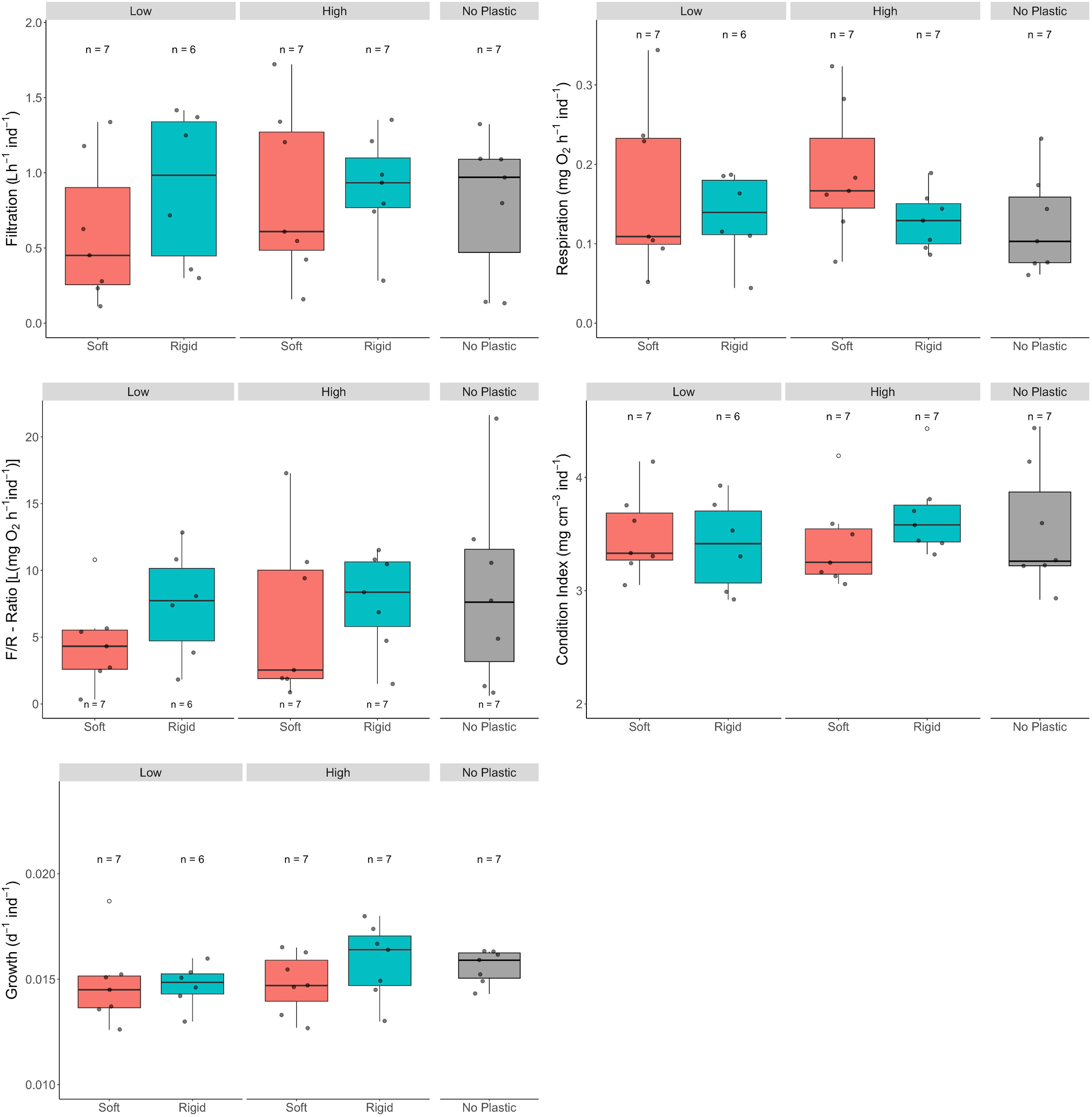
Effects of soft (= PE bags) and rigid (= PET bottles) macroplastics in low (= 40% of the mussel aggregates’ surface area) and high (= 80%) amounts on various physiological response variables in blue mussel aggregates that were exposed in the Kerteminde Fjord, Denmark, sequentially from 23.11. to 28.11.2020 and retrieved from the 28.01. -02.03.2021. The control group were aggregates without plastic. The data points within each boxplot represent the mean value for each aggregate. Boxplots show the median, the interquartile range and the non-outlier range. White dots are outliers.
The mean growth rate across mussels in all treatments was 0.015 ± 0.002 d-¹ ind-¹, equivalent to approximately 2% soft body mass increase per day, with individual values ranging from 0.0126 to 0.0187 d-¹ ind-¹ (Figure 4). The condition index (CI) also showed consistent values across all experimental groups, with a mean of 3.51 ± 0.41 mg cm-³ ind-¹, ranging from 2.92 to 4.45 mg cm-³ ind-¹ (Figure 4).
Byssus strength, measured as the force required to detach an individual mussel, was on average 6.74 ± 1.39 kg·m·s-² ind-¹, with values ranging from 4.2 to 10.2 kg·m·s-² ind-¹ (Figure 5). Again, no significant differences were found across the treatment combinations.
Figure 5
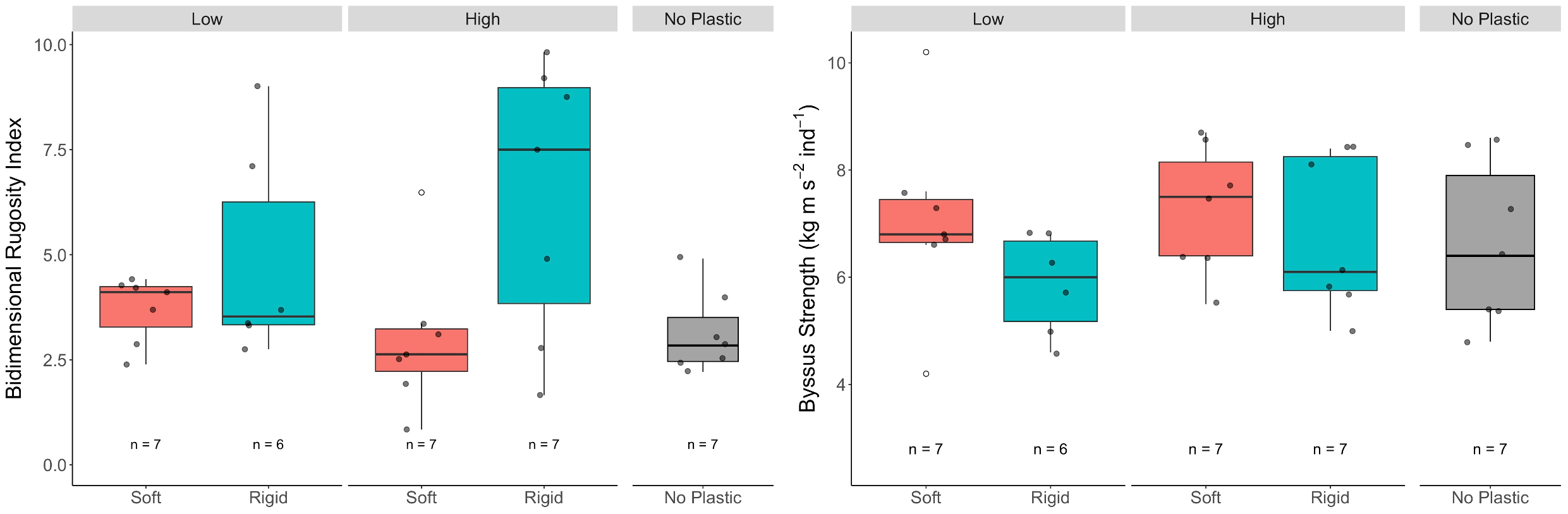
Effects of soft (= PE bags) and rigid (= PET bottles) macroplastic in low (= 40% of the mussel aggregates’ surface area) and high (= 80%) amounts on two structural response variables in blue mussel aggregates that were exposed in the Kerteminde Fjord, Denmark, sequentially from 23.11.2020 to 02.03.2021. The control group were aggregates without plastic. The data points within each boxplot represent the mean value for each aggregate. Boxplots show the median, the interquartile range and the non-outlier range. White dots are outliers.
In contrast to this, an effect of the plastic was observed when considering the spatial complexity of the aggregates, which we measured as the bidimensional rugosity index (BR). While the overall mean BR across all aggregates was 4.19 ± 2.3 (range: 0.84 to 9.82), aggregates with rigid plastic debris showed a significantly higher rugosity (5.68 ± 2.83) than those with soft plastic debris (3.35 ± 1.31; Table 1; Figure 5). However, no significant differences in BR were found between aggregates with different amounts of plastic or between aggregates with debris (pooled) and those without plastic (Tables 1, 3).
4 Discussion
4.1 Mussel performance was unaffected by the plastic debris
We found no significant effects of the macroplastic debris, regardless of type (rigid/soft) and amount (40%/80% of aggregate surface area), on any physiological performance metric that we obtained from the blue mussel aggregates, i.e. filtration rates, respiration rates, F/R ratios, condition index, and growth rates. This suggests that the presence of the debris in the aggregates did not impair physiological processes in Mytilus spp. Hence, we did not get support for the assumption that the presence of the planar plastic materials restricted the valve gaping of the mussels and with this their capacity to filter and to respire. An effect on these performance traits could then have led to an altered condition index and a diminished growth. The fact that we did not find any sign for such an impairment could simply mean that the physical presence of the plastic litter, although it was integrated into the three-dimensional matrix of the mussel aggregates, did not restrict the mussels in any way. A further assumption about the influence of the plastic on the mussels was, that the close contact between the mussels and the plastic could have led to a transfer of chemical substances, such as leachates, from the plastic to the animals. The uptake of such compounds could then also have altered their performance.
However, the results of our study do not necessarily give evidence that there is no negative influence of large-sized plastic debris on mussels. It is also possible that the timing of the five-month exposure period attenuated the effects of the plastic. This is possible because the experiment was run during the winter, when metabolic rates of Baltic Sea mussels are low due to the reduced food availability and low temperatures (Riisgård, 1991). Under these conditions, the exposure was maybe too short for any plastic-related effects on the mussel aggregates to manifest. Thus, while our data suggest no physiological impairment, this outcome must be interpreted with respect to the season in which the experiment was conducted. Moreover, this study potentially used a mix of M. edulis and M. trossulus individuals and we cannot exclude that the two species reacted differently to exposure to plastic debris. However, considering the many similarities between M. edulis and M. trossulus with regard to their physiology and ecology (Kautsky et al., 1990; Riginos and Cunningham, 2005; Tedengren and Kautsky, 1986), we do not have reason to assume that a difference in the way they responded, if it existed, was substantial. Additionally, in hindsight, we view the use of PVC as a substrate for the mussel aggregates as potentially problematic. In future studies that use a similar approach, mussels should be placed on a natural kind of substratum so that control aggregates would not be in contact with plastics at all. Also, the roughening of the PVC plates for better mussel attachment could have created microplastic particles that might have affected the mussels.
4.2 Rigid macroplastics increased aggregate spatial complexity
While most of the variables we measured were unaffected, the macroplastic debris influenced the spatial complexity of the mussel aggregates. Aggregates containing rigid plastic (PET) had a significantly higher bidimensional rugosity than those with soft plastic (PE). This effect goes back to the way the different materials were integrated into the aggregate matrix by the activity of the mussels. While the rigid plastics were too stiff to get deformed during this process and for this reason partly protruded from the aggregates, the soft plastic was completely drawn into their centers. This probably happened because the mussels moved before they formed a stable aggregate, and by that movement pushed the soft plastic towards the center of their aggregate (Luisa Kumpitsch, pers. obs.). This suggests that plastic litter can alter the architecture of mussel aggregates in different ways, which depend on the size, shape and physical properties of the litter such as its flexibility. This may also have implications for the way associated organisms can use the three-dimensional structure of mussel beds. Structurally complex aggregates, such as the ones we created with the PET fragments, may offer increased refuge space by providing protection from predation for associated fauna such as polychaetes and crustaceans (Crooks, 2002; Koivisto and Westerbom, 2010). Moreover, complex substrates were found to have a stabilizing effect on mussel aggregations, as attachment strength increases (Christensen et al., 2015). It has been shown that invasive species can increase the spatial complexity of mussel (Mytilus galloprovincialis) beds, and this resulted in a more diverse associated macrofauna (Gestoso et al., 2013). Structurally complex blue mussel aggregates offer various habitats for mobile as well as sessile species by, for instance, providing refuges from predation and spaces for settlement (Crooks, 2002; Thompson et al., 1996). A change in the spatial complexity of mussel beds could therefore alter the composition and diversity of the associated macrofauna (i.e. animals 0.5 mm – 50 mm in diameter; Watling, 2019). Interestingly, spatially complex mussel clumps were found to harbor lower abundances of macrofauna individuals than simpler ones (Rumohr, 1990), but also to have macrofauna communities with a higher evenness (Gestoso et al., 2013). However, when mussel beds accommodate less macrofauna individuals, bioturbation (i.e. the restructuring of sedimentary deposits), oxygen penetration depth or organic matter remineralization in nearby sediments should be reduced (Aller and Aller, 1998; Kristensen, 2000; Vopel et al., 2003). This indicates that structural complexity can indirectly also determine the ecosystem services that are provided by mussel beds.
Complex habitats allow resource partitioning, as they provide spatial niches for organic matter (i.e. food) to accumulate (Schoener, 1974). Moreover, it has been shown that spatial habitat complexity increases the ability of ecosystems to withstand disturbances, while this stability is mediated by an interplay between different forms of self-organization such as the aggregation behavior of mussels (Liu et al., 2014). Beyond the physical incorporation of plastic debris into mussel beds, which comes with the risk of smothering and/or entanglement, chemical leachates from the plastics can also affect mussels and potentially also influence their aggregation behavior (Uguen et al., 2022, Uguen et al., 2023). Since we did not identify or quantify any leachates from the plastics that we used for the experiment, we have no knowledge about to what degree they could have influenced the results we obtained. However, even with this information, it would be difficult to disentangle the chemical from the physical effects of the plastic debris, unless one would separate these two influences from each other in the design of the experiment. In summary, our findings highlight that the physical characteristics of plastic debris can shape mussel bed architecture in an ecologically relevant way, potentially influencing the habitat value and the community composition of the associated fauna.
4.3 Mussel aggregates may act as macroplastic sinks
In our experiment, in those aggregates that were contaminated with soft plastic, the litter became fully embedded among the mussels. This suggests that the mussels’ aggregation behavior and their movements before forming a stable aggregate with attaching by their byssus threads could facilitate the incorporation of flexible debris into the interior of mussel beds. Hence, they could act as sinks for plastic debris in coastal environments. This could be facilitated by blue mussel post-larvae that settle on plastic debris and grow to attached adults, forming a layer around the soft plastic, which extends through the settlement of further post-larvae and finally turns into a mussel bed that contains soft plastic inside. Mytilus edulis larvae have been shown to recruit and metamorphose on plastic panels in the field, particularly under flow conditions that reduce shear and when biofilms are present on the substrate (Dobretsov and Wahl, 2008), which was achieved within two weeks of field exposure of the plastic materials used in our study. There are already studies that showed that mussel beds are sinks for microplastics (Khan and Prezant, 2018; Santana et al., 2016), but so far there is no evidence that they also accumulate large-sized plastic litter. Hence, further surveys are needed to explore whether this phenomenon exists in natural mussel beds and, if yes, how widespread and persistent it is. Since blue mussel beds in Denmark can reach extensions up to the size of square kilometers (Laursen et al., 2010) and can persist for years with annual recruitment of new larvae (Le Corre et al., 2013; Mainwaring et al., 2014), these habitats could potentially accumulate substantial amounts of soft plastic debris over time. As a mussel bed grows, incorporated plastic would become increasingly buried and therefore invisible from the outside. This also means that the litter is shut off partly from the open water, which could slow down the breakdown of the buried debris into microplastics, although it will presumably not stop it. Abiotic processes that degrade macro- into microplastics like UV radiation, heat or mechanic stress (Zhang et al., 2021) might act less on macroplastics that are embedded in a three-dimensional mussel bed. This, in turn, means that leachates that emerge from macroplastics that are degrading inside a mussel bed would be released more slowly and, hence, over a longer period than if released directly into the open water. Furthermore, the close contact between the mussels and the debris could lead to the uptake of leachates and/or microplastic particles that emerge from the latter by the mussels. This could impair their health, since laboratory studies already showed that, for instance, weathered polyethylene microplastics (32-43 µm) accumulate in the intestine of the green mussel Perna viridis and reduce their feeding rate (Hariharan et al., 2021). Furthermore, leachates from virgin and weathered plastic debris have been shown to affect embryo development in the brown mussel Perna perna (Gandara E Silva et al., 2016), while leachates from PET, PS, PVC, PP and CTR decreased gamete fertilization in the mussel Mytilus galloprovincialis (Capolupo et al., 2020). Moreover, a mussel’s gonads, gametes and sex hormones can be affected by leachates (Choi et al., 2022; Ciocan et al., 2020; González-Soto et al., 2022; Uguen et al., 2025), and this can threaten their reproductive output and population survival.
In our study, we did not observe any effects of the incorporated soft plastic on the physiological performance of the mussels. Unfortunately, we did not verify whether its presence had an influence on the associated fauna. Although, we did not observe any effects, it is possible that the plastic bags that were pressed together inside the aggregates impeded the flow of water through the three-dimensional mussel matrix or filled cavities between mussels that were then not available for animals that normally inhabit these interspaces. Furthermore, the soft plastic debris could envelope individual mussels completely, cutting them off from oxygen and food supply, and this could lead to the death of the affected individuals. Such entrapment has been observed in this study, as mussels in the center of some aggregates were almost completely enclosed by pieces of PE bags. These individuals survived, but did not grow during the experiment.
In South Africa, monitoring of a rocky shoreline revealed litter entrapment (plastic bags, fishing line and other fibrous items) in mussel beds (Weideman et al., 2020). There is evidence of the entrapment of plastic debris in biogenic habitats also from another benthic species: Macroplastic debris, particularly fishing line, was found entrapped in colonies of the cold-water coral Dendrophyllia ramea along Portugal’s Atlantic coast (Seixas et al., 2024). However, despite this our knowledge about this phenomenon is limited. Further research on macroplastic entrapment is relevant as there are extensive mussel beds at coasts around the world (Valdivia et al., 2014). They could contain unknown quantities of macroplastic debris that potentially interfere with the ecosystem functions that are normally provided by such beds and could also harm the mussels that are living close to the litter.
4.4 Comparing the observed mussel performance to reference values
Because this study introduces a novel, group-level assay for assessing blue mussel performance, we benchmarked our physiological metrics against values from the literature that were obtained under comparable conditions. However, this comparison is limited, as published data about the physiological performance of mussels stem from single, isolated individuals rather than from aggregates. With regard to filtration, single M. edulis with a length of ~26 mm exhibited 1.22–1.80 L h-¹ ind-¹ in studies by Riisgård et al. (2014) and Tang and Riisgård (2018). These rates are 1.5–2 times higher than the ones aggregated Mytilus spp. of the same length showed in our experiment (average F = 0.81 L h-¹ ind-¹). Actually, this value is closer to one that was observed for smaller mussels (20.7 mm, 0.54 L h-¹ ind-¹) by Tang and Riisgård (2018). This could be due to the low water temperatures that prevailed when we did the measurements by the end of the winter.
For respiration rates, fed single individuals of M. edulis from the Kerteminde Fjord in Denmark, which were ~2.5 times larger than the ones we used, consumed 0.90 mg O2 h-¹ ind-¹ in a measurement that was done in December at a water temperature of 3°C (Tang and Riisgård, 2018). In comparison to this, the mussels in our experiment took up 0.15 mg O2 h-¹ ind-¹. The condition index (CI) in wild M. edulis from Morecambe Bay, England, fluctuated seasonally between 3.6 (post-spawning in June) and 7.8 mg cm-³ ind-¹ in October (Dare, 1976), while the Mytilus spp. individuals in our experiment showed an average of 3.51 mg cm-³ ind-¹ at a size of 26 mm. This value is slightly below the CI that was reported for similarly sized M. edulis that were collected in Kerteminde Fjord in Denmark during the winter (4.0–4.6 mg cm-³ ind-¹; Tang and Riisgård, 2018). The average growth rates that we observed (1.5 ± 1.2% d-¹ ind-¹ at 3.2 ± 0.6 µg L-¹) were also lower than rates that were measured in Baltic Sea mussels when similar chlorophyll a concentrations prevailed: 5.4 ± 0.5% d-¹ ind-¹ at 3.2 ± 0.6 µg L-¹ (Clausen and Riisgård, 1996). This discrepancy may reflect the influence of the water temperature (8.5 ± 0.7°C in Clausen and Riisgård, 1996 and 6.1 ± 2.9°C in our study). In summary, these comparisons reveal that the physiological performances that we measured at the aggregate-level are realistic, but range at the lower end of what was previously observed for field-collected, post-metamorphic mussels that were assessed under winter conditions.
5 Outlook
In our study, rigid macroplastics increased the spatial complexity of blue mussel aggregates, but did not influence the physiological performance and the growth of the mussels. However, the field experiment was done in winter when metabolic processes are slow due to low temperatures. Follow-up studies should therefore investigate the influence of plastic debris on Baltic Sea mussel beds in summer, when mussels are more active, and negative influences of the plastic debris on their performance are presumably more likely to detect. Additionally, in situ investigations of natural mussel beds should assess whether and how macroplastics are embedded into their structure as well as what effects such an entrapment can have on associated infaunal communities, the hydrodynamics within a mussel bed or the concentration of plastic leachates. Also alternating formation of the mussel bed with plastic debris potentially changing its spatial complexity should be examined, to see if any benefits of plastic entrapment for the mussels or the associated fauna (e.g. protection against predation or dislodgement) can be observed. This could, for instance, be done by assessing mussel survival after exposing aggregates that are contaminated with plastics to predators like shore crabs. Plastic-contaminated mussel aggregates could also be exposed to different flow regimes in order to compare mussel dislodgement rates to plastic-free aggregates. Our study suggests an experimental framework for assessing the structural and the physiological responses to plastic debris in reef-building species and highlights the need for further research on the consequences of the pollution of marine benthic habitats with large-sized plastic items.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. Data are available from the SciLifeLab Data Repository, https://doi.org/10.17044/SCILIFELAB.29686349.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
LK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. AS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was conducted in the framework of the international research and student training programme GAME (Global Approach by Modular Experiments), which is coordinated by GEOMAR Helmholtz Center for Ocean Research Kiel, Germany. In 2020 GAME was generously funded by Lighthouse Foundation, mare Zeitschrift und Buch, Müllverbrennung Kiel, HydroTechnik Lübeck, Joachim Herz Stiftung, Andreas Rühl Stiftung, subCtech Subsea Technologies, J. Bornhöft Industriegeräte, LimnoMar, Hydro-Bios, OffCon Offshore Consulting, and engie Axima. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We want to thank the staff at the Marine Biological Research Centre (SDU) in Kerteminde for their incredible support. We thank Prof. Dr. Magnus Wahlberg for welcoming us to the station for doing our experiments. We especially would like to thank Dr. Josephine Goldstein and Prof. Dr. Hans Ulrik Riisgård for their extremely helpful scientific advice during our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1676966/full#supplementary-material
References
1
Abidli S. Pinheiro M. Lahbib Y. Neuparth T. Santos M. M. Trigui El Menif N. (2021). Effects of environmentally relevant levels of polyethylene microplastic on Mytilus galloprovincialis (Mollusca: Bivalvia): filtration rate and oxidative stress. Environ. Sci Pollut. Res.28, 26643–26652. doi: 10.1007/s11356-021-12506-8
2
Aigouy B. Mirouse V. (2013). ScientiFig: a tool to build publication-ready scientific figures. Nat Methods. 10 (11), 1048–1048. doi: 10.1038/nmeth.2692
3
Aller R. C. Aller J. Y. (1998). The effect of biogenic irrigation intensity and solute exchange on diagenetic reaction rates in marine sediments. J. Mar. Res.56, 4. doi: 10.1357/002224098321667413
4
Auguie B. (2010). gridExtra: Miscellaneous Functions for ‘Grid’ Graphics. (p. 2.3) [Data set]. doi: 10.32614/CRAN.package.gridExtra
5
Avio C. G. Gorbi S. Milan M. Benedetti M. Fattorini D. d’Errico G. et al . (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut.198, 211–222. doi: 10.1016/j.envpol.2014.12.021
6
Blair R. M. Waldron S. Phoenix V. Gauchotte-Lindsay C. (2017). Micro- and nanoplastic pollution of freshwater and wastewater treatment systems. Springer Sci Rev.5, 19–30. doi: 10.1007/s40362-017-0044-7
7
Broszeit S. Hattam C. Beaumont N. (2016). Bioremediation of waste under ocean acidification: Reviewing the role of Mytilus edulis. Mar. Pollut. Bull.103, 1–2. doi: 10.1016/j.marpolbul.2015.12.040
8
Capolupo M. Sørensen L. Jayasena K. D. R. Booth A. M. Fabbri E. (2020). Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res.169, 115270. doi: 10.1016/j.watres.2019.115270
9
Cartier S. Pellerin J. Fournier M. Tamigneaux E. Girault L. Lemaire N. (2004). Use of an index based on the blue mussel (Mytilus edulis and Mytilus trossulus) digestive gland weight to assess the nutritional quality of mussel farm sites. Aquaculture241, 633–654. doi: 10.1016/j.aquaculture.2004.08.015
10
Castro-Jiménez J. González-Fernández D. Fornier M. Schmidt N. Sempéré R. (2019). Macro-litter in surface waters from the Rhone River: Plastic pollution and loading to the NW Mediterranean Sea. Mar. Pollut. Bull.146, 60–66. doi: 10.1016/j.marpolbul.2019.05.067
11
Choi J. S. Kim K. Park K. Park J.-W. (2022). Long-term exposure of the Mediterranean mussels, Mytilus galloprovincialis to polyethylene terephthalate microfibers: Implication for reproductive and neurotoxic effects. Chemosphere299, 134317. doi: 10.1016/j.chemosphere.2022.134317
12
Christensen H. T. Dolmer P. Hansen B. W. Holmer M. Kristensen L. D. Poulsen L. K. et al . (2015). Aggregation and attachment responses of blue mussels, Mytilus edulis—Impact of substrate composition, time scale and source of mussel seed. Aquaculture435, 245–251. doi: 10.1016/j.aquaculture.2014.09.043
13
Ciocan C. Kristova P. Annels C. Derjean M. Hopkinson L. (2020). Glass reinforced plastic (GRP) a new emerging contaminant—First evidence of GRP impact on aquatic organisms. Mar. Pollut. Bull.160, 111559. doi: 10.1016/j.marpolbul.2020.111559
14
Clausen I. Riisgård H. (1996). Growth, filtration and respiration in the mussel Mytilus edulis: no evidence for physiological regulation of the filter-pump to nutritional needs. Mar. Ecol. Prog. Ser.141, 37–45. doi: 10.3354/meps141037
15
Commito J. A. Commito A. E. Platt R. V. Grupe B. M. Piniak W. E. D. Gownaris N. J. et al . (2014). Recruitment facilitation and spatial pattern formation in soft-bottom mussel beds. Ecosphere5, art160. doi: 10.1890/ES14-00200.1
16
Cressey D. (2016). Bottles, bags, ropes and toothbrushes: The struggle to track ocean plastics. Nature536, 263–265. doi: 10.1038/536263a
17
Crooks J. A. (2002). Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos97, 2. doi: 10.1034/j.1600-0706.2002.970201.x
18
Dare P. J. (1976). Settlement, growth and production of the mussel, Mytilus edulis L. in Morecambe Bay, Englands. London: Her Majesty’s Stationery Office. (Fishery Investigations. Ministry of Agriculture. Fish. Food. Ser. II. V. 28).
19
de Carvalho-Souza G. F. Llope M. Tinôco M. S. Medeiros D. V. Maia-Nogueira R. Sampaio C. L. S. (2018). Marine litter disrupts ecological processes in reef systems. Mar. Pollut. Bull.133, 464–471. doi: 10.1016/j.marpolbul.2018.05.049
20
Depledge M. H. Galgani F. Panti C. Caliani I. Casini S. Fossi M. C. (2013). Plastic litter in the sea. Mar. Environ. Res.92, 279–281. doi: 10.1016/j.marenvres.2013.10.002
21
Dobretsov S. Wahl M. (2008). Larval recruitment of the blue mussel Mytilus edulis: The effect of flow and algae. J. Exp. Mar. Biol. Ecol.355, 137–144. doi: 10.1016/j.jembe.2007.12.018
22
Dolby G. R. (1976). A note on the linear structural relation when both residual variances are known. J. Am. Stat. Assoc.71, 352. doi: 10.2307/2285312
23
Farrell P. Nelson K. (2013). Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut.177, 1–3. doi: 10.1016/j.envpol.2013.01.046
24
Fernández A. Grienke U. Soler-Vila A. Guihéneuf F. Stengel D. B. Tasdemir D. (2015). Seasonal and geographical variations in the biochemical composition of the blue mussel (Mytilus edulis L.) from Ireland. Food Chem.177, 43–52. doi: 10.1016/j.foodchem.2014.12.062
25
Fox J. Weisberg S. (2019). _An R Companion to Applied Regression_ (Version Third edition) [Computer software]. Available online at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
26
Galgani F. Jaunet S. Campillo A. Guenegen X. His E. (1995). Distribution and abundance of debris on the continental shelf of the north-western Mediterranean Sea. Mar. Pollut. Bull.30, 713–717. doi: 10.1016/0025-326X(95)00055-R
27
Galgani F. Leaute J. P. Moguedet P. Souplet A. Verin Y. Carpentier A. et al . (2000). Litter on the sea floor along european coasts. Mar. Pollut. Bull.40, 516–527. doi: 10.1016/S0025-326X(99)00234-9
28
Galgani F. Souplet A. Cadiou Y. (1996). Accumulation of debris on the deep sea floor off the French Mediterranean coast. Mar. Ecol. Prog. Ser.142, 225–234. doi: 10.3354/meps142225
29
Gallo F. Fossi C. Weber R. Santillo D. Sousa J. Ingram I. et al . (2018). Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Europe30, 13. doi: 10.1186/s12302-018-0139-z
30
Gandara E Silva P. P. Nobre C. R. Resaffe P. Pereira C. D. S. Gusmão F. (2016). Leachate from microplastics impairs larval development in brown mussels. Water Res.106, 364–370. doi: 10.1016/j.watres.2016.10.016
31
Gestoso I. Arenas F. Rubal M. Veiga P. Peña M. Olabarria C. (2013). Shifts from native to non-indigenous mussels: Enhanced habitat complexity and its effects on faunal assemblages. Mar. Environ. Res.90, 85–95. doi: 10.1016/j.marenvres.2013.05.015
32
González-Fernández D. Cózar A. Hanke G. Viejo J. Morales-Caselles C. Bakiu R. et al . (2021). Floating macrolitter leaked from Europe into the ocean. Nat. Sustainability4, 474–483. doi: 10.1038/s41893-021-00722-6
33
Gonzalez-Poblete E. Hurtado F. C. F. Rojo S. C. Norambuena C. R. (2018). Blue mussel aquaculture in Chile: Small or large scale industry? Aquaculture, 493, 113–122. doi: 10.1016/j.aquaculture.2018.04.026
34
González-Soto N. Campos L. Navarro E. Bilbao E. Guilhermino L. Cajaraville M. P. (2022). Effects of microplastics alone or with sorbed oil compounds from the water accommodated fraction of a North Sea crude oil on marine mussels (Mytilus galloprovincialis). Sci. Total Environ.851, 157999. doi: 10.1016/j.scitotenv.2022.157999
35
Gündoğdu S. Çevik C. (2019). Mediterranean dirty edge: High level of meso and macroplastics pollution on the Turkish coast. Environ. Pollut.255, , 113351. doi: 10.1016/j.envpol.2019.113351
36
Hamm T. Lenz M. . (2021). Negative impacts of realistic doses of spherical and irregular microplastics emerged late during a 42 weeks-long exposure experiment with blue mussels. Sci. Total Environ.778, 146088. doi: 10.1016/j.scitotenv.2021.146088
37
Hänninen J. Weckström M. Pawłowska J. Szymańska N. Uurasjärvi E. Zajaczkowski M. et al . (2021). Plastic debris composition and concentration in the Arctic Ocean, the North Sea and the Baltic Sea. Mar. Pollut. Bull.165, 112150. doi: 10.1016/j.marpolbul.2021.112150
38
Hariharan G. Purvaja R. Anandavelu I. Robin R. S. Ramesh R. (2021). Accumulation and ecotoxicological risk of weathered polyethylene (wPE) microplastics on green mussel (Perna viridis). Ecotoxicology Environ. Saf.208, 111765. doi: 10.1016/j.ecoenv.2020.111765
39
Harrell F. E. Jr. (2003). Hmisc: Harrell Miscellaneous. (p. 5.2–4) [Data set]. doi: 10.32614/CRAN.package.Hmisc
40
Helcom (2018). State of the Baltic Sea–Second HELCOM holistic assessment 2011–2016. Baltic Sea Environ. Proc.155, 1–155.
41
Jacobs P. Troost K. Riegman R. van der Meer J. (2015). Length- and weight-dependent clearance rates of juvenile mussels (Mytilus edulis) on various planktonic prey items. Helgoland Mar. Res.69, 101–112. doi: 10.1007/s10152-014-0419-y
42
Jansen H. M. Strand Ø. Verdegem M. Smaal A. (2012). Accumulation, release and turnover of nutrients (C-N-P-Si) by the blue mussel Mytilus edulis under oligotrophic conditions. J. Exp. Mar. Biol. Ecol.416–417, 185–195. doi: 10.1016/j.jembe.2011.11.009
43
Jürgensen C. (1995). Modelling of nutrient release from the sediment in a tidal inlet, Kertinge Nor, Funen, Denmark. Ophelia42, 163–178. doi: 10.1080/00785326.1995.10431502
44
Kassambara A. (2016). ggpubr: ‘ggplot2’ Based Publication Ready Plots. (p. 0.6.1) [Data set]. doi: 10.32614/CRAN.package.ggpubr
45
Katolikova M. Khaitov V. Väinölä R. Gantsevich M. Strelkov P. (2016). Genetic, Ecological and Morphological Distinctness of the Blue Mussels Mytilus trossulus Gould and M. edulis L. in the White Sea. PloS One11, e0152963. doi: 10.1371/journal.pone.0152963
46
Kautsky N. (1980). Nutrient release from a Baltic Mytilus-red algal community and its role in benthic and pelagic productivity. OPHELIA, 1, 17–30.
47
Kautsky N. Johannesson K. Tedengren M. (1990). Genotypic and phenotypic differences between Baltic and North Sea populations of Mytilus edulis evaluated through reciprocal transplantations. I Growth and morphology. Mar. Ecol. Prog. Ser.59, 203–210. doi: 10.3354/meps059203
48
Khan M. B. Prezant R. S. (2018). Microplastic abundances in a mussel bed and ingestion by the ribbed marsh mussel Geukensia demissa. Mar. Pollut. Bull.130, 67–75. doi: 10.1016/j.marpolbul.2018.03.012
49
Kijewski T. Zbawicka M. Strand J. Kautsky H. Kotta J. Rätsep M. et al . (2019). Random forest assessment of correlation between environmental factors and genetic differentiation of populations: Case of marine mussels Mytilus. Oceanologia61, 131–142. doi: 10.1016/j.oceano.2018.08.002
50
Knöbel L. Nascimento-Schulze J. C. Sanders T. Zeus D. Hiebenthal C. Barboza F. R. et al . (2021). Salinity driven selection and local adaptation in Baltic sea mytilid mussels. Front. Mar. Sci8. doi: 10.3389/fmars.2021.692078
51
Koivisto M. E. Westerbom M. (2010). Habitat structure and complexity as determinants of biodiversity in blue mussel beds on sublittoral rocky shores. Mar. Biol.157, 7. doi: 10.1007/s00227-010-1421-9
52
Kristensen E. (2000). Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia426, 1. doi: 10.1023/A:1003980226194
53
Landrigan P. J. Stegeman J. J. Fleming L. E. Allemand D. Anderson D. M. Backer L. C. et al . (2020). Human health and ocean pollution. Ann. Global Health86, 151. doi: 10.5334/aogh.2831
54
Laursen K. Kristensen P. S. Clausen P. (2010). Assessment of blue mussel Mytilus edulis fisheries and waterbird shellfish-predator management in the Danish wadden sea. AMBIO39, 476–485. doi: 10.1007/s13280-010-0045-0
55
Lechthaler S. Waldschläger K. Stauch G. Schüttrumpf H. (2020). The way of macroplastic through the environment. Environments7, 73. doi: 10.3390/environments7100073
56
Le Corre N. Martel A. Guichard F. Johnson L. (2013). Variation in recruitment: Differentiating the roles of primary and secondary settlement of blue mussels Mytilus spp. Mar. Ecol. Prog. Ser.481, 133–146. doi: 10.3354/meps10216
57
Lekang O.-I. Stevik T. K. Bomo A. M. (2003). Evaluation of different combined collectors used in longlines for blue mussel farming. Aquacultural Eng.27, 89–104. doi: 10.1016/S0144-8609(02)00052-3
58
Lenz M. Brennecke D. Haeckel M. Knickmeier K. Kossel E. (2023). Spatio-temporal variability in the abundance and composition of beach litter and microplastics along the Baltic Sea coast of Schleswig-Holstein, Germany. Mar. Pollut. Bull.190, 114830. doi: 10.1016/j.marpolbul.2023.114830
59
Liu Q.-X. Herman P. M. J. Mooij W. M. Huisman J. Scheffer M. Olff H. et al . (2014). Pattern formation at multiple spatial scales drives the resilience of mussel bed ecosystems. Nat. Commun.5, 1. doi: 10.1038/ncomms6234
60
Mainwaring K. Tillin H. Tyler-Walters H. (2014). Assessing the sensitivity of blue mussel beds to pressures associated with human activities. Peterborough, Joint Nature Conservation Committee, JNCC Report No. 506.
61
Mangiafico S. (2016). rcompanion: Functions to Support Extension Education Program Evaluation. (p. 2.5.0) [Data set]. doi: 10.32614/CRAN.package.rcompanion
62
McKnight P. E. Najab J. (2010). “ Mann-whitney U test,” in The corsini encyclopedia of psychology, 1st edn. Eds. WeinerI. B.CraigheadW. E., 1–1. doi: 10.1002/9780470479216.corpsy0524
63
Michalek K. Ventura A. Sanders T. (2016). Mytilus hybridisation and impact on aquaculture: A minireview. Mar. Genomics27, 3–7. doi: 10.1016/j.margen.2016.04.008
64
Narloch I. Gackowska A. Wejnerowska G. (2022). Microplastic in the Baltic Sea: A review of distribution processes, sources, analysis methods and regulatory policies. Environ. Pollut.315, 120453. doi: 10.1016/j.envpol.2022.120453
65
Nicastro K. R. Seuront L. McQuaid C. D. Zardi G. I. (2022). Symbiont-induced intraspecific phenotypic variation enhances plastic trapping and ingestion in biogenic habitats. Sci. Total Environ., 826, 153922. doi: 10.1016/j.scitotenv.2022.153922
66
Ogle D. H. Doll J. C. Wheeler A. P. Dinno A. (2015). FSA: Simple Fisheries Stock Assessment Methods. (p. 0.10.0) [Data set]. doi: 10.32614/CRAN.package.FSA
67
Olesen N. Frandsen K. Riisgard H. (1994). Population dynamics, growth and energetics of jellYfish Aurelia aurita in a shallow fjord. Mar. Ecol. Prog. Ser.105, 9–18. doi: 10.3354/meps105009
68
Pärn O. Moy D. M. Stips A. (2023). Determining the distribution and accumulation patterns of floating litter in the Baltic Sea using modelling tools. Mar. Pollut. Bull.190, 114864. doi: 10.1016/j.marpolbul.2023.114864
69
Pasquini G. Ronchi F. Strafella P. Scarcella G. Fortibuoni T. (2016). Seabed litter composition, distribution and sources in the Northern and Central Adriatic Sea (Mediterranean). Waste Manage.58, 41–51. doi: 10.1016/j.wasman.2016.08.038
70
Pedersen T. L. (2019). patchwork: The Composer of Plots. (p. 1.3.0) [Data set]. doi: 10.32614/CRAN.package.patchwork
71
Prins T. C. Smaal A. C. (1994). The role of the blue mussel Mytilus edulis in the cycling of nutrients in the Oosterschelde estuary (The Netherlands). Hydrobiologia282–283, 413–429. doi: 10.1007/BF00024645
72
Prins T. Smaal A. Pouwer A. Dankers N. (1996). Filtration and resuspension of particulate matter and phytoplankton on an intertidal mussel bed in the Oosterschelde estuary (SW Netherlands). Mar. Ecol. Prog. Ser.142, 121–134. doi: 10.3354/meps142121
73
Ragnarsson S.Á. Raffaelli D. (1999). Effects of the mussel Mytilus edulis L. on the invertebrate fauna of sediments. J. Exp. Mar. Biol. Ecol.241, 1. doi: 10.1016/S0022-0981(99)00063-5
74
R Core Team , (2020). R: A language and environment for statistical computing. (Vienna, Austria: R Foundation for Statistical Computing). https://www.R-project.org/
75
Riginos C. Cunningham C. W. (2005). INVITED REVIEW: Local adaptation and species segregation in two mussel (Mytilus edulis × Mytilus trossulus) hybrid zones. Mol. Ecol.14, 381–400. doi: 10.1111/j.1365-294X.2004.02379.x
76
Riisgård H. U. (1991). Filtration rate and growth in the blue mussel, Mytilus edulis Linneau: Dependence on algal concentration. J. Shellfish Res.10, 29–35.
77
Riisgård H. (2001). On measurement of filtration rate in bivalves-the stony road to reliable data: Review and interpretation. Mar. Ecol. Prog. Ser.211, 275–291. doi: 10.3354/meps211275
78
Riisgård H. U. Jensen M. H. Rask N. (2008). “ Odense fjord and kerteminde fjord/kertinge nor,” in Ecology of Baltic coastal waters, vol. 197 . Ed. SchiewerU. (Berlin Heidelberg: Springer), 361–394. doi: 10.1007/978-3-540-73524-3_16
79
Riisgård H. U. Kumala L. Charitonidou K. (2016). Using the F/R -ratio for an evaluation of the ability of the demosponge Halichondria panicea to nourish solely on phytoplankton versus free-living bacteria in the sea. Mar. Biol. Res.12, 907–916. doi: 10.1080/17451000.2016.1206941
80
Riisgård H. U. Larsen P. S. Pleissner D. (2014). Allometric equations for maximum filtration rate in blue mussels Mytilus edulis and importance of condition index. Helgoland Mar. Res.68, 193–198. doi: 10.1007/s10152-013-0377-9
81
Riisgard H. U. Lassen J. Kittner C. (2006). Valve-gape response times in mussels (Mytilus edulis)-effects of laboratory preceding-feeding conditions and in situ tidally induced variation in phytoplankton biomass. J. Shellfish Res.25, 901–911. doi: 10.2983/0730-8000(2006)25%255B901:VRTIMM%255D2.0.CO;2
82
Rouillon G. Guerra Rivas J. Ochoa N. Navarro E. (2005). Phytoplankton composition of the stomach contents of the mussel Mytilus edulis L. From two populations: comparison with its food suppl. J. Shellfish Res.24, 1. doi: 10.2983/0730-8000(2005)24%255B5:PCOTSC%255D2.0.CO;2
83
Rumohr H. (1990). Bottom macrofauna: Collection and treatment of samples in Techniques in Marine Environmental Sciences No. 8. (Copenhagen, Denmark: International Council for the Exploration of the Sea).
84
Santana M. F. M. Ascer L. G. Custódio M. R. Moreira F. T. Turra A. (2016). Microplastic contamination in natural mussel beds from a Brazilian urbanized coastal region: Rapid evaluation through bioassessment. Mar. Pollut. Bull.106, 183–189. doi: 10.1016/j.marpolbul.2016.02.074
85
Sarkar D. (2001). lattice: Trellis Graphics for R. (p. 0.22–7) [Data set]. doi: 10.32614/CRAN.package.lattice
86
Schindelin J. Arganda-Carreras I. Frise E. Kaynig V. Longair M. Pietzsch T. et al (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9 (7), 676–682. doi: 10.1038/nmeth.2019
87
Schneider C. A. Rasband W. S. Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods9, 7. doi: 10.1038/nmeth.2089
88
Schoener T. W. (1974). Resource partitioning in ecological communities. Science185, 4145. doi: 10.1126/science.185.4145.27
89
Seitz R. Marshall Hines L. Hines A. Clark K. (2003). Effects of hypoxia on predator-prey dynamics of the blue crab Callinectes sapidus and the Baltic clam Macoma balthica in Chesapeake Bay. Mar. Ecol. Prog. Ser.257, 179–188. doi: 10.3354/meps257179
90
Seixas S. Parrinha J. Gomes P. Bessa F. (2024). Incorporation of abandoned and lost fishing gear into the structure of Dendrophyllia ramea in the Atlantic coast of Portugal. Mar. Pollut. Bull.202, 116302. doi: 10.1016/j.marpolbul.2024.116302
91
Shapiro S. S. Wilk M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika52, 591–611. doi: 10.1093/biomet/52.3-4.591
92
Stirling H. P. Okumus I. (1995). Growth and production of mussels (Mytilus edulis L.) suspended at salmon cages and shellfish farms in two Scottish sea lochs. Aquaculture134, 193–210. doi: 10.1016/0044-8486(95)00033-X
93
Strohmeier T. Duinker A. Strand Ø. Aure J. (2008). Temporal and spatial variation in food availability and meat ratio in a longline mussel farm (Mytilus edulis). Aquaculture276, 83–90. doi: 10.1016/j.aquaculture.2008.01.043
94
Stuckas H. Stoof K. Quesada H. Tiedemann R. (2009). Evolutionary implications of discordant clines across the Baltic Mytilus hybrid zone (Mytilus edulis and Mytilus trossulus). Heredity103, 146–156. doi: 10.1038/hdy.2009.37
95
Student (1908). The probable error of a mean. Biometrika6, 1. doi: 10.2307/2331554
96
Tang B. Riisgård H. U. (2018). Relationship between oxygen concentration, respiration and filtration rate in blue mussel Mytilus edulis. J. Oceanology Limnology36, 2. doi: 10.1007/s00343-018-6244-4
97
Taylor B. E. Jamieson G. Carefoot T. H. (1992). Mussel culture in British Columbia: The influence of salmon farms on growth of Mytilus edulis. Aquaculture108, 51–66. doi: 10.1016/0044-8486(92)90318-F
98
Tedengren M. Kautsky N. (1986). Comparative study of the physiology and its probable effect on size in Blue Mussels (Mytilus Edulis L.) from the North Sea and the Northern Baltic Proper. Ophelia25, 147–155. doi: 10.1080/00785326.1986.10429746
99
Thompson R. C. Wilson B. J. Tobin M. L. Hill A. S. Hawkins S. J. (1996). Biologically generated habitat provision and diversity of rocky shore organisms at a hierarchy of spatial scales. J. Exp. Mar. Biol. Ecol.202, 1. doi: 10.1016/0022-0981(96)00032-9
100
Tsuchiya M. Nishihira M. (1985). Islands of Mytilus as habitat for small intertidal animals: Effect of island size on community structure. Mar. Ecol. Prog. Ser.25, 71–81. doi: 10.3354/meps025071
101
Uguen M. Gaudron S. M. Nicastro K. R. Zardi G. I. Spilmont N. Seuront L. (2023). Size-dependent response of the mussel collective behaviour to plastic leachates and predator cues. Sci. Total Environ.888, 164037. doi: 10.1016/j.scitotenv.2023.164037
102
Uguen M. Gaudron S. M. Seuront L. (2025). Plastic pollution and marine mussels: Unravelling disparities in research efforts, biological effects and influences of global warming. Sci. Total Environ.959, 178078. doi: 10.1016/j.scitotenv.2024.178078
103
Uguen M. Nicastro K. R. Zardi G. I. Gaudron S. M. Spilmont N. Akoueson F. et al . (2022). Microplastic leachates disrupt the chemotactic and chemokinetic behaviours of an ecosystem engineer (Mytilus edulis). Chemosphere306, 135425. doi: 10.1016/j.chemosphere.2022.135425
104
Valdivia N. Buschbaum C. Thiel M. (2014). Succession in intertidal mussel bed assemblages on different shores: Species mobility matters. Mar. Ecol. Prog. Ser.497, 131–142. doi: 10.3354/meps10593
105
Van Cauwenberghe L. Claessens M. Vandegehuchte M. B. Janssen C. R. (2015). Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut.199, 10–17. doi: 10.1016/j.envpol.2015.01.008
106
von Moos N. Burkhardt-Holm P. Köhler A. (2012). Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol.46, 20. doi: 10.1021/es302332w
107
Vopel K. Thistle D. Rosenberg R. (2003). Effect of the brittle star Amphiura filiformis (Amphiuridae, Echinodermata) on oxygen flux into the sediment. Limnology Oceanography48, 5. doi: 10.4319/lo.2003.48.5.2034
108
Walkinshaw C. Phelps S. (2023). Plastic fibres stunt growth in mussels by more than a third – here’s why this is a concern. doi: 10.64628/AB.edjr6usef
109
Watling L. (2019). Macrofauna. In CochranJ. K.BokuniewiczH. J.YagerP. L. (Eds.), Encyclopedia of Ocean Sciences (Third Edition) (3rd ed., pp. 728–734). Academic Press. doi: 10.1016/B978-0-12-409548-9.11069-3
110
Wayman C. Niemann H. (2021). The fate of plastic in the ocean environment – a minireview. Environ. Science: Processes Impacts23, 198–212. doi: 10.1039/D0EM00446D
111
Weideman E. A. Perold V. Omardien A. Smyth L. K. Ryan P. G. (2020). Quantifying temporal trends in anthropogenic litter in a rocky intertidal habitat. Mar. Pollut. Bull.160, 111543. doi: 10.1016/j.marpolbul.2020.111543
112
Wickham H. (2016). ggplot2: Elegant Graphics for Data Analysis. [Computer software]. (Verlag New York: Springer). Available online at: https://ggplot2.tidyverse.org/
113
Wickham H. François R. Henry L. Müller K. Vaughan D. (2023). dplyr: A Grammar of Data Manipulation [R package version 1.1.4]. Available online at: https://github.com/tidyverse/dplyr, https://dplyr.tidyverse.org
114
Wilke C. O. (2015). cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. (p. 1.2.0) [Data set]. doi: 10.32614/CRAN.package.cowplot
115
Woods M. N. Stack M. E. Fields D. M. Shaw S. D. Matrai P. A. (2018). Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. Pollut. Bull.137, 638–645. doi: 10.1016/j.marpolbul.2018.10.061
116
Zhang K. Hamidian A. H. Tubić A. Zhang Y. Fang J. K. H. Wu C. et al . (2021). Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut., 274, 116554. doi: 10.1016/j.envpol.2021.116554
Summary
Keywords
aggregate, Baltic Sea, blue mussel, macroplastics, marine litter
Citation
Kumpitsch L, Schindel A and Lenz M (2025) Experimental exposure of blue mussel beds to soft and rigid macroplastics in the winter reveals litter entrapment but no physiological effects. Front. Mar. Sci. 12:1676966. doi: 10.3389/fmars.2025.1676966
Received
31 July 2025
Accepted
25 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Sonja M. Ehlers, Leibniz Institute for Baltic Sea Research (LG), Germany
Reviewed by
Ana Isabel Catarino, Flanders Marine Institute, Belgium; Gerardo Zardi, Rhodes University, South Africa
Updates
Copyright
© 2025 Kumpitsch, Schindel and Lenz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luisa Kumpitsch, luisa.kumpitsch@gu.se
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.