Abstract
Plastic litter in the ocean has gained significant attention over recent decades, with much of the focus on microplastics. However, macroplastic litter is prevalent in marine environments, and while its effects on single organisms is well documented, its effects on community-level biological structures remain poorly understood. This study investigates the impact of macroplastic litter—specifically polyethylene film (shopping bags) and nylon filament (fishing line)—on Mytilus galloprovincialis aggregates and their associated fauna in the Ría de Vigo, NW Spain. Using a fully factorial experimental design, 30-mussel aggregates were assembled incorporating plastic litter in different abundances and were deployed in situ for 4 weeks. After this time, physiological responses (respiration and filtration), structural complexity (rugosity index), particulate matter retention, body condition index (BCI), and associated mobile faunal diversity were measured. Mussels in the High/Filament treatment showed an 18% lower respiration rate and a 65% reduction in filtration capacity compared to controls, while the Low/Filament treatment reduced filtration by 40%. No significant effects were found on BCI, particulate retention as well as on the diversity and composition of the associated macrofauna. Interestingly, aggregates with low plastic content exhibited a slightly higher structural complexity than those with high amounts of plastic. This study highlights that macroplastics can subtly alter the functionality of mussel beds without dramatically affecting the diversity of the associated fauna. These insights underscore the need to assess physical interactions of organisms with plastic litter also at the community level.
1 Introduction
Although plastics have brought numerous benefits to our society with wide-ranging applications in sectors such as construction, medicine, and food preservation (Cole et al., 2016), their widespread use has also led to significant environmental challenges. Plastic pollution has emerged as a major environmental concern across marine ecosystems, with production and disposal rates continuing to rise globally, making plastics a pervasive and harmful presence in the world’s oceans. Macroplastic litter (items > 5 mm) include common materials such as e.g., fishing lines, nets, packaging films, originates from both terrestrial and marine sources (Galgani et al., 2015; Li et al., 2016), and is widespread and persistent, with the potential to impact organisms not only individually but also at the community and ecosystem level.
Most marine plastic litter originates from land-based sources, which are not exclusively associated with urban areas. They comprise also landfills and beaches worldwide, most notably from developing regions with extensive coastlines and large, coastal populations (e.g., SE Asia) (Galgani et al., 2015; Jambeck et al., 2015; Li et al., 2016). Improper waste transportation and inadequate burial in landfills can result in a breach into the environment. One example are plastic bags and films, which are easily wind-blown. Furthermore, litter from land is often transported to the seas via rivers and waterways and this is facilitated by extreme weather events (Galgani et al., 2015). As a result, large quantities of plastic litter, i.e., an estimated 14 million tons, enter the oceans each year (IUCN, 2021). Ocean-based sources, fishing industry, shipping activities and aquaculture add to this, while litter from both origins can drift through the ocean for long time spans and often settle in both intertidal and subtidal areas (GESAMP, 2021; Green et al., 2015).
Approximately 70% of the plastic litter entering the ocean is thought to settle in benthic habitats, where it can accumulate and interact with resident organisms (Hammer et al., 2012; Green et al., 2015; Consoli et al., 2019; Costa et al., 2021). In these environments, macroplastic items can physically alter habitat structure, provide novel substrate for colonization, obstruct feeding or respiration of benthic organisms, and influence biogeochemical cycles by affecting sediment oxygenation (Barnes et al., 2009; de Carvalho-Souza et al., 2018). Yet, despite the widespread presence of macroplastics in benthic systems, most studies have focused on organism-level effects such as ingestion and entanglement, particularly in large vertebrates (Li et al., 2016; Wilcox et al., 2016). Far fewer studies have investigated how macroplastic litter influences community structure or ecosystem functioning (Rochman et al., 2016), but see e.g., Katsanevakis et al. (2007); Green et al. (2015) and Gündoğdu et al. (2017), who showed positive as well as negative effects of plastic debris on benthic assemblages. The positive influence was due to the provision of additional habitat, while detrimental effects emerged from the smothering of organisms. In particular, the effect of macroplastic on faunal assemblages in intertidal and shallow subtidal habitats is still poorly studied and should be investigated to a greater extent. This is because these communities are often associated with habitat-building species that form long-living biogenic structures and fulfil important ecosystem services.
One example for such a species is the Mediterranean mussel Mytilus galloprovincialis (Lamarck 1819). It is a widespread and ecologically important species in intertidal and shallow subtidal coastal environments, where it acts as a habitat engineer by forming dense aggregations. Mussel beds stabilize shorelines, attenuate storm surge by dampening wave action, sequester carbon, transfer nutrients, clarify water, act as sediment traps, facilitate primary production by increasing the biomass of benthic microalgae and provide refuge and feeding grounds for diverse macrofaunal assemblages (Thiel and Ullrich, 2002; Commito et al., 2014; Engel et al., 2017; Paquette et al., 2019). When detached from their conspecifics, mussels quickly move and clump together by extending their foot and pull closer to nearby mussels (Commito et al., 2014). This spatial self-organization behaviour creates non-random bands and patches in coastal areas. Living in these patches, which are formed in response to the local density of conspecifics and food availability, offers benefits like protection against waves and predators and provides good substrates for juvenile mussels. However, when intraspecific competition becomes intense, periphery mussels can get dislodged and then commonly move to less crowded adjacent clusters (Paquette et al., 2019; van de Koppel et al., 2008). These behavioural and structural characteristics make mussel beds a suitable model system to explore the broader ecological consequences of macroplastic pollution in benthic habitats. Macroplastic litter, such as films and filaments, can interfere physically with mussel aggregates by entangling or covering individuals), potentially restricting valve movements, altering water flow, and disrupting feeding and respiration (Weideman et al., 2020; Uguen et al., 2024; Vilke et al., 2024). In addition, plastics integrated into mussel clumps may change the three-dimensional structure of aggregates, thereby modifying habitat complexity and influencing associated faunal communities.
This study was conducted in the Ría de Vigo, NW Spain, a coastal embayment influenced by seasonal upwelling, which drives high primary productivity and supports diverse benthic communities. The Ría de Vigo is internationally recognized as one of the most productive estuarine systems in Europe and represents one of the main hotspots for mussel aquaculture, accounting for a substantial share of European mussel production. Beyond its economic importance, the area is subject to multiple anthropogenic pressures—including intensive shipping, aquaculture, fisheries, and urban inputs—that increase the risk of plastic litter entering the system. These characteristics make the Ría de Vigo an ecologically and socio-economically relevant setting to address the vulnerability of Mytilus galloprovincialis assemblages to macroplastic pollution.
We investigated how different amounts of marine plastic litter, represented by two different shapes, affect the physiological performance of M. galloprovincialis aggregates and the composition of their associated macrofaunal communities. Based on previously mentioned considerations, we hypothesized that (i) the presence of plastics would reduce mussel physiological performance (respiration, filtration capacity, and body condition) due to physical obstruction and stress responses, and (ii) the incorporation of plastics into mussel aggregates would alter aggregate complexity, particulate matter retention, and the diversity and composition of associated macrofaunal communities. We further expected the magnitude of these effects to depend on both the shape (films vs. filaments) and the amount (low vs. high) of plastic litter, with filamentous material causing stronger interference due to its entangling nature. By focusing on responses at both individual and community levels, this research aims to address critical knowledge gaps in our understanding of macroplastic impacts on benthic ecosystem structure and function.
2 Materials and methods
2.1 Study site and experimental design
Mussel individuals (Mytilus galloprovincialis) were collected from the outer Ría de Vigo, transferred to a nearby laboratory and used to create 35 experimental aggregates that consisted of 30 mussels each. For this, mussels were subjected to a one-week laboratory aggregation phase followed by a four-week sea exposure phase. The aggregation phase was necessary to allow mussels to form stable aggregates under controlled conditions, ensuring that all treatments started with cohesive clumps of similar size and structure. Without this preliminary step, mussels collected individually from the field would have required variable times to re-attach and reorganize in the sea, leading to uncontrolled differences among treatments. Once aggregates were established, they were exposed in situ for four weeks to evaluate the effects of plastic litter under natural environmental conditions. The plastic debris used in the study included two types that are commonly encountered in marine environments: film-like shopping bag fragments and fishing line, which represents a filamentous type of litter (Figure 1). While films have the potential to fully cover solitary organisms or parts of colonies and by this cutting them off from food, gas and light supply, filaments can lead to, for example, entanglement and provoke the restriction of body movements. The shopping bag material was composed of polyethylene polymers and selected to be thin, milky white, and semitransparent, closely resembling typical consumer bags while minimizing potential confounding effects that go back to colour or excessive thickness. The fishing line was a colourless nylon monofilament with a diameter of 0.3 mm, representative of widely used commercial lines. To ensure comparability, both plastic types were chosen for their similar thickness and lack of colouration, thus potential differences between groups should go back to the film-like or filamentous nature of the debris. Beyond morphology, the two plastics differ in density and behaviour in water, which can influence their interaction with benthic organisms. Polyethylene has a density of ~0.91–0.96 g cm-³, slightly less than seawater, giving it moderate buoyancy that allows thin films to float or suspend near the water surface and drape over mussel aggregates. Nylon monofilament has a density of ~1.14 g cm-³, slightly denser than seawater, so filaments tend to sink slowly or remain suspended depending on hydrodynamic conditions, often becoming entangled within the three-dimensional structure of the aggregates. Both plastics are flexible and resistant to rapid degradation over the experimental timeframe, ensuring consistent exposure. These physical traits, combined with differences in buoyancy and settling behaviour, help replicate the ecological dynamics of film-like versus filamentous plastics in natural environments.
Figure 1

Experimental M. galloprovincialis aggregates with plastic film before (A) and after (B) 4 weeks of sea exposure; and with plastic filaments before (C) and after (D) 4 weeks of sea exposure.
2.1.1 Experimental phases
2.1.1.1 Aggregation phase
Mytilus galloprovincialis individuals were collected from the rocky intertidal zone at low tide on seven non-consecutive days between October 13 and 24, 2020. Mussels were carefully detached by cutting their byssus threads with nail scissors to prevent damage to the byssal glands. Individuals measuring 3.5–4.0 cm shell length (measured with callipers) were selected for the experiment. Each day 150 individuals were collected and transported to the CSIC-IIM climate chamber in plastic containers within 20 minutes. Soft epibionts were removed from their shells using a kitchen sponge, while barnacles were scraped off with a knife. Single mussels were randomly assigned to one of the four treatment combinations or to a control group in which mussel aggregates were not contaminated with plastics. The exact shell length of every single individual was measured to calculate the total surface area (i.e. the sum of all mussel shell surfaces) of the resulting aggregate. This allowed us to relate the amount of plastic material that was added to a biologically important property of the aggregates. We assumed that the total surface area of an aggregate is positively correlated with the space that is available for associated fauna and for the particulate matter retained within the aggregate. The amount of plastic material added to each mussel aggregate was therefore standardized relative to the total shell surface area of the aggregate. Plastic items (films or filaments) were then added to achieve predefined “Low” and “High” plastic treatment levels. This approach ensured that the experimental exposure mimicked environmentally realistic densities of macroplastic litter while maintaining comparability across treatments.
where r is the radius of the fishing line, X represents the amount of plastic material added, with values of 0.4 for the “High” plastic treatment and 0.2 for the “Low” plastic treatment level.
Mussel aggregates with the corresponding plastic material were housed on 200 * 200 * 3 mm PVC plates roughened with 60-grit sandpaper to promote attachment (Figure 1). The aggregation phase lasted seven days at an average temperature of 17°C under a 12 h light:12 h dark photoperiod. During this period, mussels attached to each other, to the plastic material, and to the PVC carrier plates via byssus threads. Tanks were cleaned and the seawater was fully exchanged daily. For the first 2–3 days, aggregates were inspected daily and mussels that migrated to plate edges were repositioned to ensure a cohesive clump formation.
2.1.1.2 Sea exposure phase
Following the one-week aggregation phase, mussel aggregates were deployed into the subtidal from a marina jetty in the Ría de Vigo (42°14’01’’N, 8°44’34’’W). Deployments occurred every other day from October 20 to 31, 2020, with five aggregates submerged per day at a depth of 1.5 meters and maintaining a 2-meter spacing between them to avoid inter-aggregate interactions. Each platform was suspended horizontally in the water column by securing four corner ropes to a central rope affixed to the jetty. A brick counterweight was connected beneath each platform via a rope and zip tie to stabilize the setup against water movement. To prevent dislodgement from wave action, plastic grids (160 * 30 mm; mesh size: 0.5 * 0.5 mm) were attached to the plate edges. To minimize biofouling, the experimental fencing was cleaned weekly via snorkelling using a kitchen sponge. This duration was selected as a compromise between ecological relevance and experimental feasibility: it was long enough to allow detection of physiological and structural responses of mussel aggregates to plastic litter, while still short enough to minimize uncontrolled colonization by external fauna that could obscure treatment effects. Additionally, this four-week period corresponds to the time required for optimal byssus filament secretion, which typically reaches near-asymptotic levels after several weeks, ensuring proper attachment and stability of the mussel aggregates. After 4 weeks of exposure, aggregates were retrieved in the same order as deployed, with five collected per day on non-consecutive days between November 18 and 28, 2020.
2.2 Measured responses and sampling protocols
On each retrieval day, the mobile fauna and the total particulate matter content were sampled and analysed during the following days, while the filtration and respiration measurements were performed immediately.
2.2.1 Total particulate matter and mobile fauna sampling protocol
During retrieval, a snorkeler cut the zip-tie to detach the brick, and each plate was placed in a sealable submerged box to retain the mobile fauna. On the jetty, fauna that dislodged from the aggregates during handling was collected from the water inside the box by filtering it through a 500 µm mesh. Then, the plate was cleaned around the aggregate with a sponge that was rinsed through a 500 µm mesh to recover fauna. Each aggregate was rinsed thoroughly with 1 L of 1.2 µm-filtered seawater to collect the total particulate matter (TPM), and this rinse water was filtered for TPM analysis. Remaining fauna was dislodged by inverting and gently agitating the aggregate in filtered seawater, then filtered and preserved. All steps were completed within one hour. Aggregates were transported in individual tanks for immediate filtration and respiration assays; TPM samples were refrigerated, while mobile fauna samples were preserved in 70% ethanol at room temperature (20–25°C) prior to identification.
2.2.2 Total particulate matter analysis
Each of the 1 L FSW samples we took per aggregate was homogenized and split into two 500 mL subsamples. The supernatant from one subsample was then filtered through two pre-weighed 2.7 µm glass fibre filters (Whatman) to prevent clogging. Filters were rinsed with 30 mL of distilled water to remove salts. Settled material from the same subsample was poured into a pre-weighed aluminium boat, rinsed with distilled water, and weighed. Filters and boats were dried at 80°C for 24 h until constant weight. Salt content in the settled material was estimated from the residual seawater volume (e.g., 0.01 L * 35 g L-¹ = 0.35 g). This approach may underestimate total salt, as salts associated with particles were not accounted for, but the correction was small and applied consistently across replicates, ensuring comparability. Salt content was then subtracted from the dried mass to yield TPM, which was summed across filters and settled material, then multiplied by two to account for subsampling.
2.2.3 Physiological performance of M. galloprovincialis
2.2.3.1 Mussel filtration and respiration rates
Upon arrival in the laboratory, mussel aggregates were acclimated for 2 hours to let handling stress fade away before starting the measurements. Filtration capacity was assessed by quantifying the clearance rate, defined as the volume of water cleared of suspended particles per unit time. To this end, Rhodomonas lens cells (~7 µm diameter) were added to each 9 L aquarium to reach an initial concentration of ~30,000 cells mL-¹, which is well within the optimal range for continuous filtration in Mytilus spp (Riisgard and Randløv, 1981). Each aggregate was visually inspected to ensure that >50% of the individuals were actively filtering (i.e., open valves) before the measurements began. The water bodies inside the aquaria were homogenized using submersible pumps to ensure an even cell distribution. Cell concentrations were monitored with a PAMAS S4031 GO WG particle counter, focusing on the 5–7 µm size class to capture variability in Rhodomonas cell size. Measurements were taken every 3 minutes over a 12-minute period via an inlet tube positioned ~4 cm below the water surface and 1 cm above the aggregate. A daily control (blank) consisting of an aquarium with only a PVC plate was included to account for cell sedimentation. Filtration rates (F, L h-¹) were calculated following Riisgård et al. (2014):
where V is the aquarium volume (L) and b is the slope of the linear regression on a semi-logarithmic (ln-transformed) plot of cell concentration over time, reflecting the exponential decrease in particle density during filtration. Finally, clearance rate was standardized per g of aggregate dry weight.
Following filtration measurements, mussel aggregates were allowed to rest for 30 minutes prior to respiration assessments. To minimize handling stress, the same aquaria and the same volumes of seawater were used throughout all measurements, maintaining residual concentrations of microalgae that helped to ensure that the mussels remained open during subsequent oxygen measurements. During the resting period, water in each aquarium was bubbled with air to reach oxygen saturation. Each tank was sealed with an airtight PVC lid fitted with an opening for insertion of a Hach HQ40d multi oxygen probe, thereby functioning as a closed chamber during the 30-min monitoring periods. Oxygen exchange with the atmosphere was considered negligible over this short timescale. Oxygen concentration (mg O2 L-¹) was recorded ~4 cm below the water surface and ~1 cm above each aggregate every 5 minutes for 30 minutes (= 7 measurements). After light-phase measurements, aquaria were re-aerated for 30 minutes before repeating the procedure in complete darkness to quantify net oxygen production (NOP) by epibionts and phytoplankton that were present in the aquaria. An additional control tank (mussel-free aquarium) containing only empty shells was run concurrently to account for background oxygen uptake by microbial films; any oxygen decline measured in this control was used to correct respiration rates. Respiration rate (R, mg O2 min-¹) was calculated from the slope (b) of oxygen decline over the 30-minute period, multiplied by the aquarium volume (V) following Tang and Riisgård (2018):
Only dark-phase measurements were used for respiration rate calculations, as NOP was negligible. To standardize respiration rates, each aggregate’s R value was normalized to a body dry weight of 1 g using an allometric correction (Babarro et al., 2000; Prieto et al., 2020), accounting for size-related differences in metabolic rate:
Where Y_STD and Y_EXP are the standardized and experimental respiration rates, respectively, W_EXP is the experimental dry weight of the aggregate (g), and b is the allometric scaling coefficient (0.75 for respiration).
2.2.4 Body condition index
Following all measurements, mussel aggregates were detached from the PVC plates and frozen at −20°C for at least 24 h to ensure 100% mortality. Shell length of each mussel was re-measured, and six individuals per aggregate (three from the centre and three from the periphery) were dissected to assess within-aggregate variation in BCI. This approach accounts for the fact that an individual’s position within the aggregate strongly influences its access to food, which in turn affects its BCI. The soft tissue was dried at 80°C for 24 h until constant weight. BCI was calculated as the ratio of soft tissue dry weight to shell length cubed. Average BCI and standard deviation were calculated from the six replicates per aggregate.
Where W = soft tissue dry weight and L = shell length (Riisgård et al., 2014).
2.2.5 Structural complexity and community-level effects
2.2.5.1 Aggregate complexity
The bidimensional rugosity index (BR), used to quantify the structural complexity of mussel aggregates, was adapted from the method described by Gestoso et al. (2013). Garden wire was used to trace two orthogonal axes along the base of each PVC plate (L1 and L2), following the flat surface beneath the mussel aggregate to avoid disturbance and accurately capture the plate’s true base dimensions. The same axes were then contoured across the top surface of the mussel aggregate (C1 and C2), from one corner of the PVC plate to the opposite, following the aggregate’s shape. The lengths of the contoured wires (C1, C2) and the flat base wires (L1, L2) were measured using a ruler. Since the flat base dimensions (L1, L2)and the normalization factor m (number of mussels per clump) was constant across aggregates, the final bidimensional rugosity index (BR) was calculated as:
Where C1 and C2 are the contoured lengths (cm) along each axis across the aggregate, and DW is the dry weight (g) of the aggregate. Thus, BR expresses rugosity normalized by biomass, integrating both surface irregularity and aggregate mass.
2.2.5.2 Mobile fauna processing and identification
Mobile fauna samples were stored in 70% ethanol at room temperature (20–25°C) prior to identification. Samples were processed sequentially according to collection order. Under a dissecting microscope, organisms were separated and grouped at higher taxonomic levels (e.g., order) and stored individually in 70% ethanol in Eppendorf tubes. Identification to the lowest possible taxonomic level was conducted using both dissecting and compound microscopes, referencing established taxonomic guides (Hayward et al., 1999; Campbell, 2009; Holdich and Jones, 1983; Pleijel and Dales, 1991). Abundance of each taxon was recorded. Biodiversity comparisons between treatment combinations were performed using species richness and Shannon diversity index.
2.3 Statistical analysis
Statistical analyses were performed in R (v4.0.3; R Core Team, 2020) using RStudio as the integrated development environment. Two-way ANOVAs were used to assess the influence of plastic amount (2 levels: High and Low) and shape (2 levels: Filament and Film) on each response variable. For non-parametric data (e.g., respiration), Scheirer-Ray-Hare and Mann-Whitney-U tests were applied. PERMANOVA was used to analyse differences in community composition, using the function adonis2 in the vegan package with significance set at p ≤ 0.05. The adequacy of the ANOVA models was verified by inspecting the residuals for normality and for homogeneity of variances using plots and diagnostical tests (Shapiro-Wilk’s-W test and Fligner-Killeen test, respectively). Furthermore, we used Bonferroni-corrected, single degree of freedom contrasts to test for differences between the two treatment levels, either pooled or averaged across the levels of the respective other treatment level, and the control group.
3 Results
3.1 Accumulation of particulate matter
There was no significant effect of the macroplastic litter on the accumulation of TPM inside the mussel aggregates (Table 1a; Figure 2). Contrast analyses did not show any significant differences between the control group and all other experimental groups pooled (Table 1b).
Table 1
| a) Source of variation | SS | df | F | p | b) Contrast | W | p |
|---|---|---|---|---|---|---|---|
| Shape | 0.86 | 1 | 1.06 | 0.31 | Control vs All | 104 | 0.82 |
| Amount | 0.42 | 1 | 0.51 | 0.48 | Control vs High | 54 | 0.74 |
| Shape x Amount | 0.58 | 1 | 0.71 | 0.41 | Control vs Low | 50 | 0.97 |
| Control vs Film | 54 | 0.74 | |||||
| Control vs Filament | 50 | 0.97 |
Influence of the amount and the shape of macroplastic litter on the total particulate matter accumulated within Mytilus galloprovincialis aggregates during 4 weeks of exposure in the sea.
Results from ANOVA (a) and from contrast analyses (b) between the control group and all treatment combinations pooled, between the control group and the levels of the factor “Amount” (averaged across the levels of “Shape”) and between the control group and the levels of the factor “Shape” (averaged across the levels of “Amount”). Significance level after applying the Bonferroni correction: ≤ 0.01. Results from Mann-Whitney-U tests (b).
Figure 2
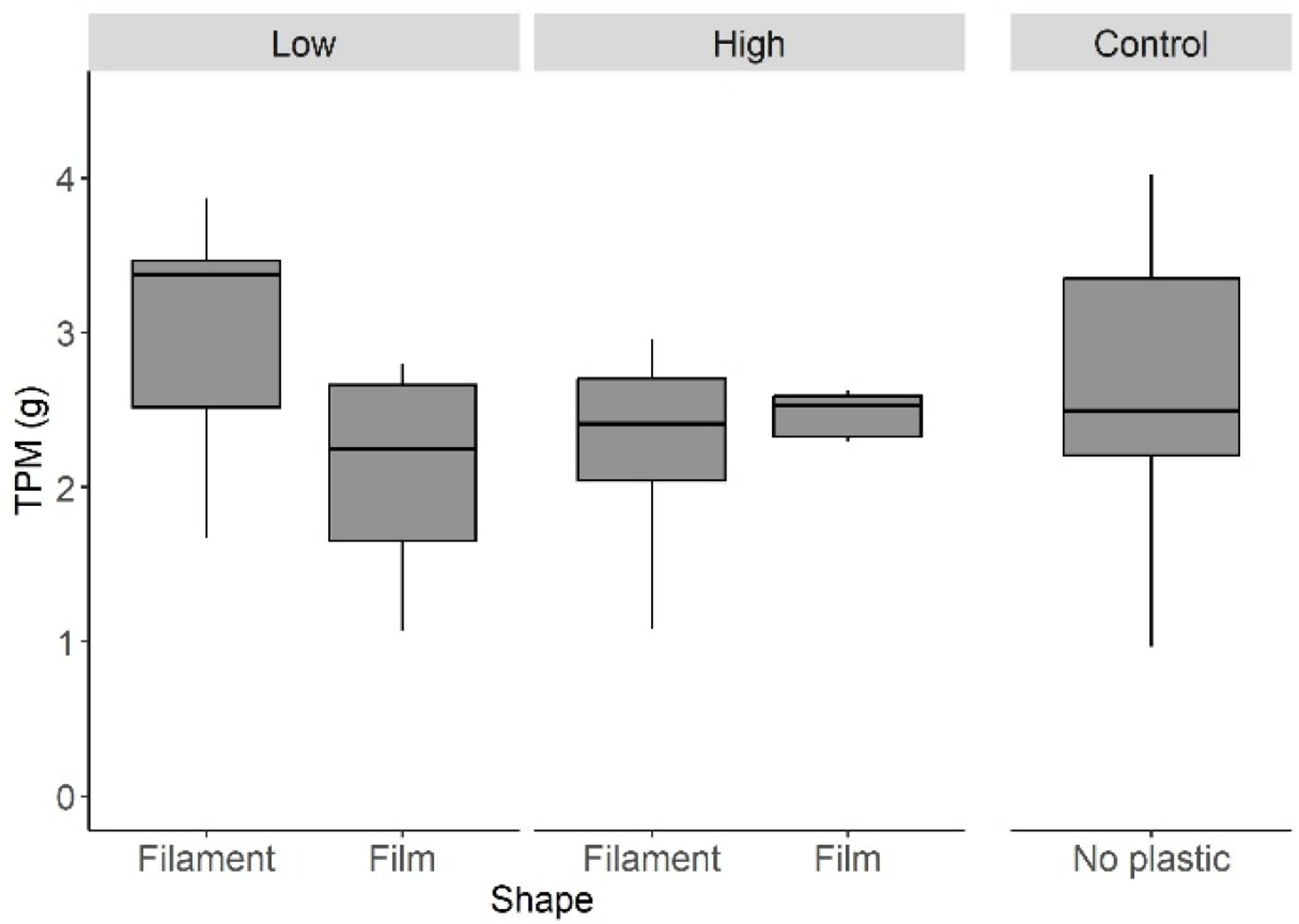
Amount of total particulate matter retained within aggregates of Mytilus galloprovincialis contaminated with macroplastic litter after 4 weeks of exposure in the sea. Each sampling unit of 30 mussels was aggregated with or without macroplastic litter in low or high amounts (20% and 40% of the total aggregate surface area, respectively) before exposure. The plastic litter items were either films (shopping bags) or filaments (fishing line). In each treatment combination and in the control group n=7. Boxplots show medians, interquartiles and non-outlier ranges.
3.2 Physiological performance of M. galloprovincialis
Respiration rates of mussel aggregates were not significantly affected by plastic “Shape” or “Amount” (Table 2a; Figure 3A). However, contrast analyses indicated that most treatment combinations differed from the control group, with the “High/Filament” aggregates showing the strongest reduction (18% lower oxygen consumption). Filtration rates were marginally influenced by plastic “Shape” (Table 3a). Aggregates containing filamentous plastics had markedly lower filtration than controls, with reductions of up to 65% in the “High/Filament” group and 40% in the “Low/Filament” group (Figure 3B). In contrast, film treatments showed filtration values closer to those of the controls. No significant effects of plastic “Shape” or “Amount” were detected for the body condition index (BCI) (Table 4a; Figure 3C).
Table 2
| a) Source of variation | SS | df | F | p | b) Contrast | W | p |
|---|---|---|---|---|---|---|---|
| Shape | 3.57 | 1 | 0.03 | 0.85 | Control vs All | 158 | 0.01 |
| Amount | 104.14 | 1 | 0.99 | 0.32 | Control vs High | 84 | 0.01 |
| Shape x Amount | 57.14 | 1 | 0.54 | 0.46 | Control vs Low | 74 | 0.07 |
| Control vs Film | 79 | 0.02 | |||||
| Control vs Filament | 79 | 0.02 |
Influence of the amount and the shape of macroplastic litter on the respiration rates of Mytilus galloprovincialis aggregates after 4 weeks of exposure in the sea.
Results from Scheirer-Ray-Hare (a) and from contrast analyses (b) between the control group and all treatment combinations pooled, between the control group and the levels of the factor “Amount” (averaged across the levels of “Shape”) and between the control group and the levels of the factor “Shape” (averaged across the levels of “Amount”). Significance level after applying the Bonferroni correction: ≤ 0.01. Results from Mann-Whitney-U tests (b).
Figure 3
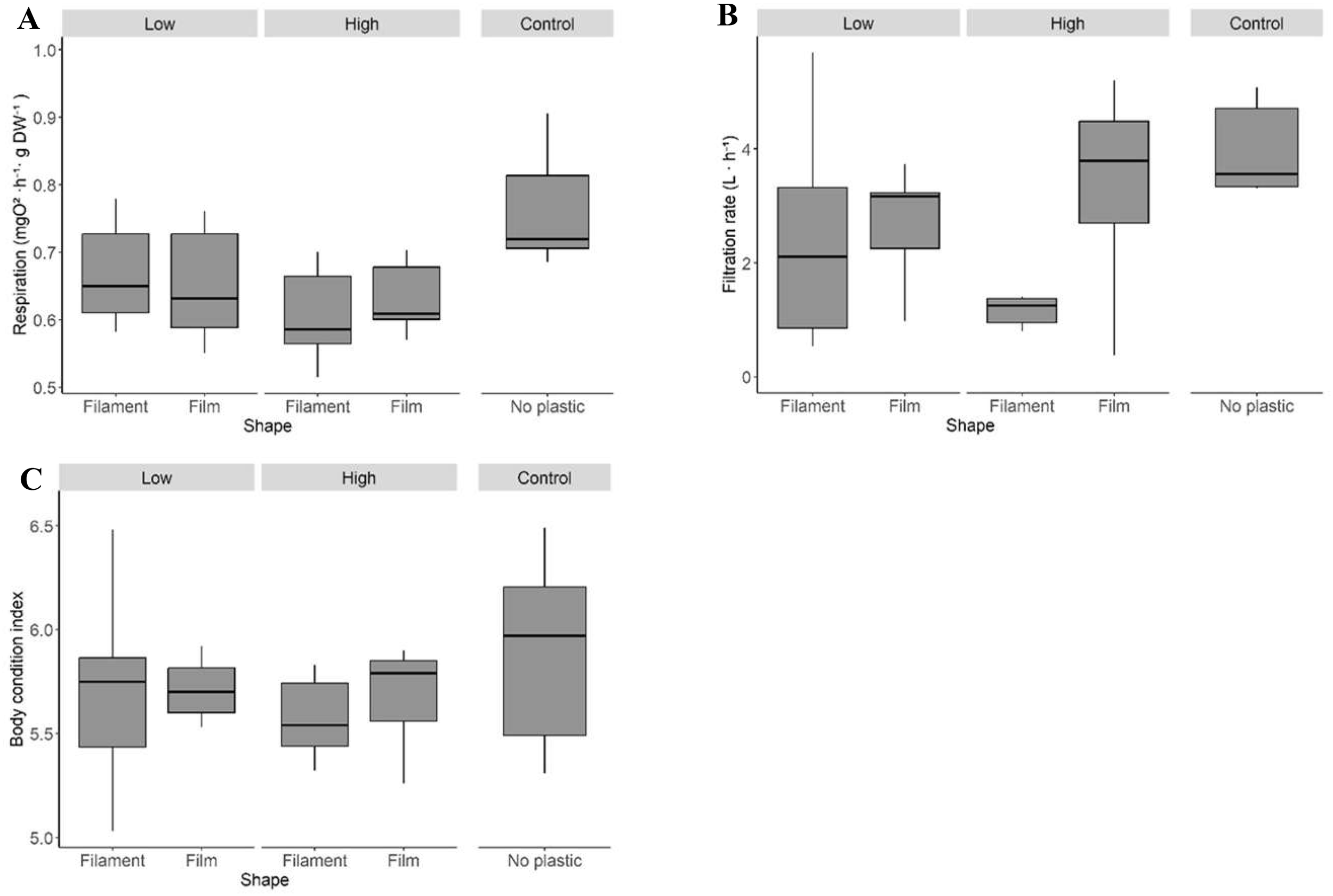
Respiration (A) and filtration (B) rates and body condition index (C), of aggregates of Mytilus galloprovincialis contaminated with macroplastic litter after 4 weeks of exposure in the sea. See Figure 1 caption for details.
Table 3
| a) Source of variation | SS | df | F | p | b) Contrast | W | p |
|---|---|---|---|---|---|---|---|
| Shape | 9.15 | 1 | 4.11 | 0.05 | Control vs All | 137 | 0.11 |
| Amount | 0.13 | 1 | 0.06 | 0.81 | Control vs High | 65 | 0.25 |
| Shape x Amount | 4.90 | 1 | 2.20 | 0.15 | Control vs Low | 72 | 0.09 |
| Control vs Film | 64 | 0.28 | |||||
| Control vs Filament | 73 | 0.07 |
Influence of amount and shape of macroplastic litter on the filtration rates of Mytilus galloprovincialis aggregates after 4 weeks of exposure in the sea.
Results from ANOVA (a) and from contrast analyses (b) between the control group and all treatment combinations pooled, between the control group and the levels of the factor “Amount” (averaged across the levels of “Shape”) and between the control group and the levels of the factor “Shape” (averaged across the levels of “Amount”). Significance level after applying the Bonferroni correction: ≤ 0.01. Results from Mann-Whitney-U tests (b).
Table 4
| a) Source of variation | SS | df | F | p | b) Contrast | W | p |
|---|---|---|---|---|---|---|---|
| Shape | 0.02 | 1 | 0.12 | 0.73 | Control vs All | 121 | 0.36 |
| Amount | 0.01 | 1 | 0.05 | 0.82 | Control vs High | 59 | 0.44 |
| Shape x Amount | 0.04 | 1 | 0.24 | 0.63 | Control vs Low | 62 | 0.34 |
| Control vs Film | 60 | 0.45 | |||||
| Control vs Filament | 61 | 0.33 |
Influence of amount and shape of macroplastic litter on the body condition index of Mytilus galloprovincialis aggregates after 4 weeks of exposure in the sea.
Results from ANOVA (a) and from contrast analyses (b) between the control group and all treatment combinations pooled, between the control group and the levels of the factor “Amount” (averaged across the levels of “Shape”) and between the control group and the levels of the factor “Shape” (averaged across the levels of “Amount”). Significance level after applying the Bonferroni correction: ≤ 0.01. Results from Mann-Whitney-U tests (b).
3.3 Structural complexity and community-level effects
Aggregate rugosity showed a marginal influence of plastic “Amount,” with lower complexity in high-plastic treatments (Table 5a; Figure 4A). Neither Shannon diversity (Table 6; Figure 4B) nor species richness of the mobile fauna (Table 7; Figure 4C) differed significantly among treatments. Similarly, PERMANOVA did not detect significant differences in community composition between control and experimental groups (Table 8; Figure 5).
Table 5
| a) Source of variation | SS | df | F | p | b) Contrast | W | p |
|---|---|---|---|---|---|---|---|
| Shape | 0.07 | 1 | 0.25 | 0.61 | Control vs All | 73.5 | 0.32 |
| Amount | 1.1 | 1 | 3.7 | 0.06 | Control vs High | 47.5 | 0.94 |
| Shape x Amount | 0.11 | 1 | 0.36 | 0.55 | Control vs Low | 26 | 0.09 |
| Control vs Film | 38.5 | 0.45 | |||||
| Control vs Filament | 35 | 0.32 |
Influence of amount and shape of macroplastic litter on the rugosity index of Mytilus galloprovincialis aggregates after 4 weeks of exposure in the sea.
Results from ANOVA (a) and from contrast analyses (b) between the control group and all treatment combinations pooled, between the control group and the levels of the factor “Amount” (averaged across the levels of “Shape”) and between the control group and the levels of the factor “Shape” (averaged across the levels of “Amount”). Significance level after applying the Bonferroni correction: ≤ 0.01. Results from Mann-Whitney-U tests (b).
Figure 4
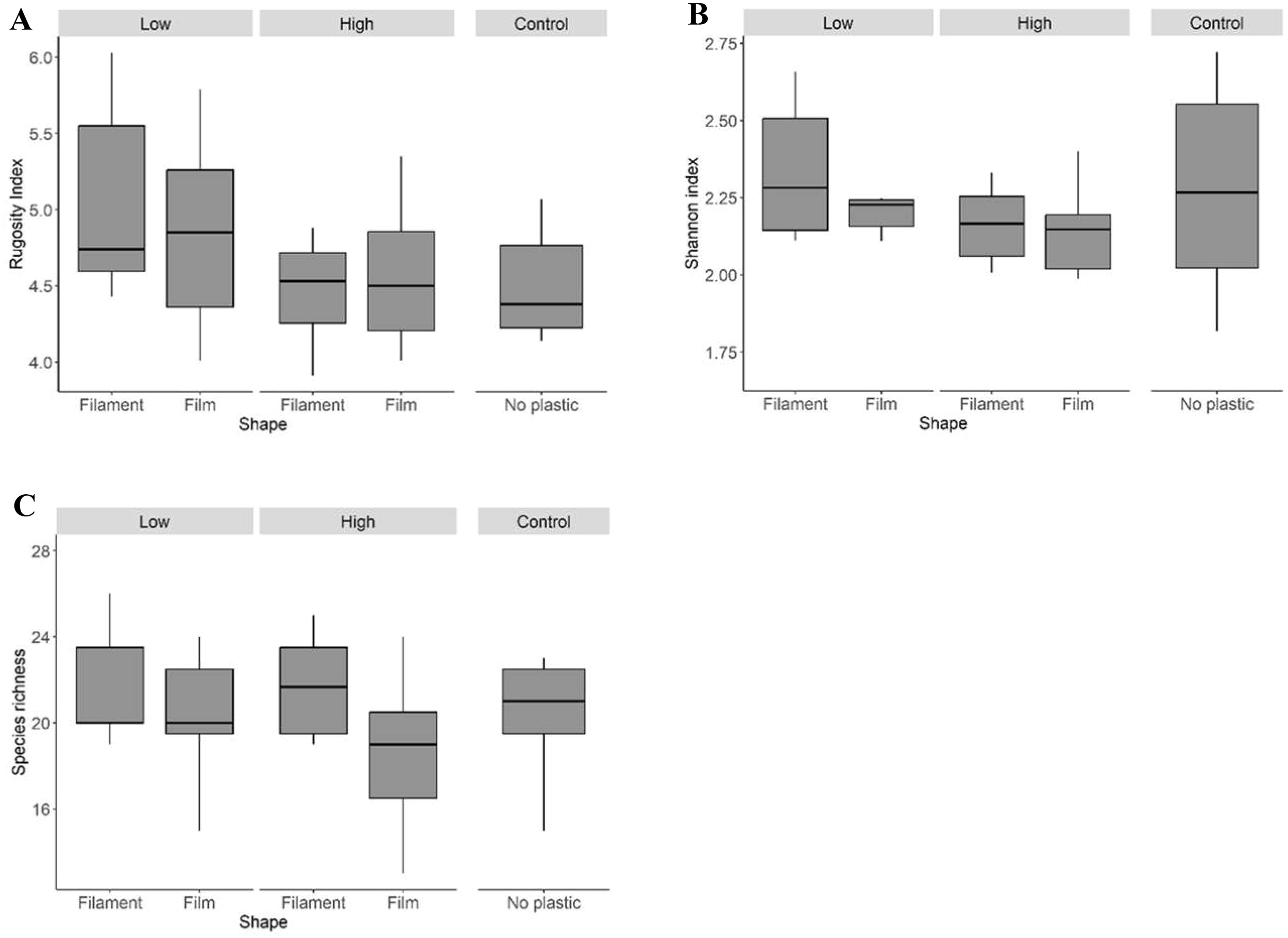
Rugosity index (A) and Shannon index (B) and species richness (C) of the mobile fauna of aggregates of Mytilus galloprovincialis contaminated with macroplastic litter after 4 weeks of exposure in the sea. See Figure 1 caption for details.
Table 6
| a) Source of variation | SS | df | F | p | b) Contrast | W | p |
|---|---|---|---|---|---|---|---|
| Shape | 0.06 | 1 | 0.99 | 0.32 | Control vs All | 112 | 0.58 |
| Amount | 0.14 | 1 | 2.4 | 0.13 | Control vs High | 62 | 0.36 |
| Shape x Amount | 0.04 | 1 | 0.67 | 0.41 | Control vs Low | 50 | 0.97 |
| Control vs Film | 62 | 0.33 | |||||
| Control vs Filament | 50 | 0.94 |
Influence of amount and shape of macroplastic litter on the Shannon index of the mobile fauna that was associated with the Mytilus galloprovincialis aggregates after 4 weeks of exposure in the sea.
Results from ANOVA (a) and from contrast analyses (b) between the control group and all treatment combinations pooled, between the control group and the levels of the factor “Amount” (averaged across the levels of “Shape”) and between the control group and the levels of the factor “Shape” (averaged across the levels of “Amount”). Significance level after applying the Bonferroni correction: ≤ 0.01. Results from Mann-Whitney-U tests (b).
Table 7
| a) Source of variation | SS | df | F | p | b) Contrast | W | p |
|---|---|---|---|---|---|---|---|
| Shape | 33.59 | 1 | 3.282 | 0.08 | Control x All | 105 | 0.79 |
| Amount | 6.35 | 1 | 0.62 | 0.44 | Control x High | 55.50 | 0.65 |
| Shape x Amount | 5.73 | 1 | 0.56 | 0.56 | Control x Low | 49.50 | 1 |
| Control x Film | 59.50 | 0.45 | |||||
| Control x Filament | 45.50 | 0.82 |
Influence of amount and shape of macroplastic litter on the species richness of the mobile fauna that was associated with the Mytilus galloprovincialis aggregates after 4 weeks of exposure in the sea.
Results from ANOVA (a) and from contrast analyses (b) between the control group and all treatment combinations pooled, between the control group and the levels of the factor “Amount” (averaged across the levels of “Shape”) and between the control group and the levels of the factor “Shape” (averaged across the levels of “Amount”). Significance level after applying the Bonferroni correction: ≤ 0.01. Results from Mann-Whitney-U tests (b).
Table 8
| Source of variation | SS | df | F | p |
|---|---|---|---|---|
| Shape | 0.03 | 2 | 0.37 | 0.93 |
| Amount | 0.10 | 1 | 0.68 | 0.81 |
| Shape x Amount | 0.07 | 1 | 0.87 | 0.54 |
Influence of amount and shape of macroplastic litter on the composition of the mobile fauna associated with the Mytilus galloprovincialis aggregates after 4 weeks of exposure in the sea.
Results from PERMANOVA.
Figure 5
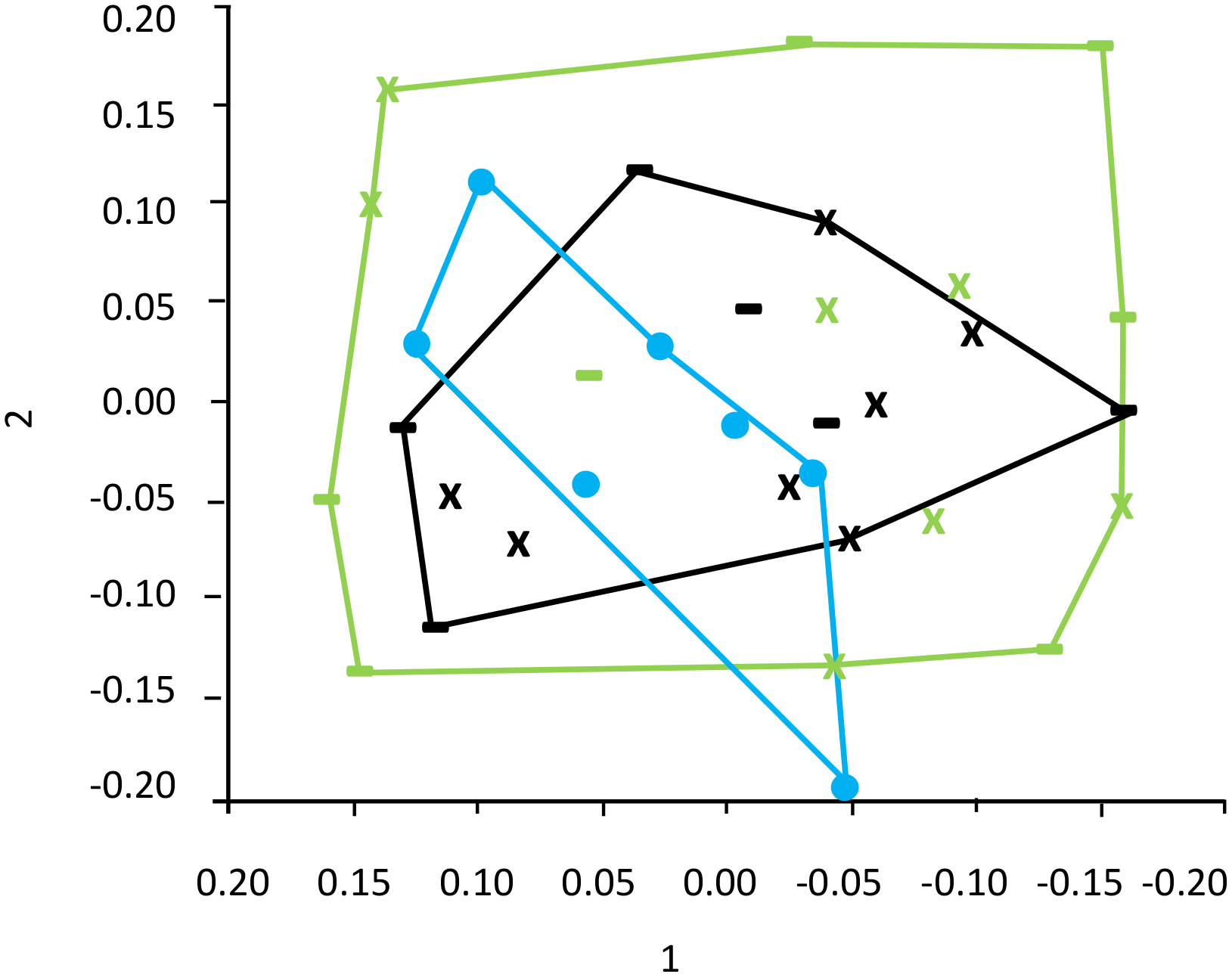
Composition of the mobile fauna associated with Mytilus galloprovincialis aggregates that were contaminated with macroplastic litter after 4 weeks of exposure in the sea. Each sampling unit of 30 mussels was aggregated with or without macroplastic litter items in low (black) and high (green) amounts (20% and 40% of the total aggregate surface area, respectively) before exposure. The plastic items differed in shape, consisting of films (plastic shopping bags), represented by an “X” or filaments (fishing line), represented by a “-”. In each treatment combination, and in the “No plastic” group, n=7 (blue circle).
4 Discussion
The effects of macroplastic litter on individual marine organisms are well documented, but community-level impacts remain poorly understood. Our study provides experimental evidence that macroplastics can alter both the physiological performance and structural organization of M. galloprovincialis aggregates, with potential consequences for their ecological role as habitat formers in coastal systems. We examined the effects of macroplastic litter, which occurred in two shapes and two abundances, on M. galloprovincialis aggregates, with a focus on their performance and the associated faunal community in the Ría de Vigo. Mussel aggregates containing a low amount of plastic exhibited the highest complexity, suggesting that small amounts of plastic can be integrated into the aggregate structure by the activity of the mussels in a way that enhances habitat complexity relative to plastic-free mussel aggregates. However, structural formation appeared to be constrained by high plastic loads. Furthermore, a significant reduction in the mussels’ respiration rates was observed in aggregates exposed to plastic litter, and, similar to this, mussel aggregates that contained large amounts of plastics, in particular if it was fishing line, showed a decline in their filtration capacity. These percentage changes highlight the biological relevance of filamentous plastics in impairing mussel physiological performance.
At the individual level, mussels exposed to filamentous plastics - particularly at high abundances -showed reduced respiration and filtration rates. These effects likely stem from physical interference, as filaments can entangle gills or mantle cavities, restricting valve movement and reducing water flow through the feeding apparatus, thereby limiting oxygen uptake and food transport. Contact with plastics may also trigger behavioural and physiological stress responses, such as prolonged or more frequent valve closures, further reducing water circulation and gas exchange. Chronic exposure could elevate metabolic costs associated with maintaining gill ciliary activity against obstructive debris, decreasing energy available for feeding and growth. These findings align with previous work demonstrating that mechanical constraints and reduced valve opening lower pumping and respiration rates in bivalves (Jørgensen et al., 1988; Tang and Riisgård, 2018). Despite these effects, overall filtration capacity remained comparable to values reported in the literature (Lassoued et al., 2019, 2021), indicating that mussels retain much of their feeding ability even under plastic exposure. Despite these effects, no significant differences were found on the accumulation of particulate matter in the aggregates, on the body condition index of the mussels, or on the diversity and composition of the associated macrofauna.
At the structural level, aggregate complexity was highest in treatments with low amounts of filamentous plastics, suggesting that mussels can incorporate or reorganize around small debris during self-assembly, enhancing habitat heterogeneity. By contrast, high plastic loads—whether filaments or films—reduced aggregate complexity, likely because excessive debris constrains mussel mobility and the ability to form cohesive three-dimensional structures. Filaments often remained entangled or were gradually pushed out, whereas films were more easily integrated into the aggregates, highlighting how plastic morphology mediates structural effects. This pattern resembles a threshold effect, where small disturbances can be accommodated or even enhance heterogeneity, while larger inputs hinder aggregation dynamics.
The presence of plastic also had a small but statistically significant effect on the shape of the mussel aggregates and on their spatial complexity. The two plastic materials we used in this experiment, i.e., shopping bags and fishing line, are among the most plastic litter objects found in the ocean (Barnes et al., 2009; Galgani et al., 2015; Green et al., 2015; Geyer et al., 2017; Consoli et al., 2019);. High amounts of plastic, whether film or filament, resulted in a lower aggregate complexity, which was similar to the complexity of the control group. This was likely because plastics in large amounts restricted mussel movements severely and with this also the capacity to include or exclude the litter from the aggregate. This observation that mussel aggregates in the experimental groups with films integrated the litter into their 3D-structure is also intriguing and warrants further investigation in future studies.
Interestingly, mussel aggregates seemed to tolerate high litter loads and film-like plastics better than filamentous material and low amounts of litter: The aggregates of the ‘Low/Filament’ group showed the most complex 3D-structure. This was presumably because the mussels did not exclude the filamentous material from the aggregates by their activity, while plastic films were easily isolated from or fully integrated into the aggregates by mussel movements. Actually, mussels in the two ‘Filament’ groups were enmeshed in the fishing lines that we added and could not at all remove them. The impact of filaments that were added in rather small amounts on structural complexity resembled a ‘pebble in the shoe’—a subtle yet persistent source of disturbance. These results reinforce the idea, also reported in field surveys (Galgani et al., 2015; Consoli et al., 2019), that the morphology and abundance of macroplastics critically influence their ecological impact in benthic systems. Therefore, our study underscores the relevance of studying macroplastic-organism interactions at sublethal levels and calls for the inclusion of macroplastics in risk assessments for coastal benthic habitats.
Behavioural interactions with plastics may also influence physiological and structural outcomes. Mussels can use their muscular foot to reposition themselves or manipulate nearby objects (Bayne, 1964; Schöne, 2001), which could explain why the Body Condition Index (BCI) was slightly lower in the ‘High/Filament’ group. Movement of debris may lead to either incorporation or exclusion of plastics from aggregates, depending on object size, shape, stiffness, and location, and suggests that mussel beds could act as sinks for planar plastic litter in natural settings.
Despite these physiological and structural effects, we observed no significant changes in the accumulation of particulate matter, species richness, or diversity of associated macrofauna over the four-week experiment. Explanations for the absence of an effect are a) that the plastics did not alter the structure of the aggregates in a way that would have led to a change in the composition of the associated communities and/or b) that the accumulation of particulate matter, which could serve as food for at least some components of the fauna, also remained unaltered by the litter and/or c) that community-level responses to macroplastics often require longer durations to develop (Barnes et al., 2009; Green et al., 2015), reflecting the short duration of the exposure. Species richness in our experiment (19–22 taxa per aggregate) was lower than in other mussel beds (e.g. Gestoso et al. (2013) found 53 taxa in Galician mussel beds, with the barnacle Elminius modestus as the dominant species, while Chintiroglou et al. (2004) documented 37–100 species seasonally appearing in mussel beds in the Thermaikos Gulf, which were mostly polychaetes and crustaceans). A potential limitation of this study is the relatively short experimental duration (4 weeks), which may have constrained faunal colonization and the development of associated communities. Many macrofaunal species colonize benthic habitats gradually, and longer exposure periods might be necessary to capture the full extent of community responses to plastic litter. While our results provide valuable insights into the initial physiological and structural effects of macroplastics on mussel aggregates, caution is warranted when extrapolating to longer-term ecological impacts. Therefore, these longer-term studies are needed to capture community-level responses.
Taken together, our results indicate that macroplastics act as subtle but persistent stressors at both individual and aggregate levels. Filamentous plastics, even at low abundances, can impair physiological performance and affect aggregation dynamics, whereas film-like plastics are better tolerated and may become incorporated into the three-dimensional structure. These findings emphasize that plastic morphology and abundance critically shape ecological impacts in benthic systems.
Our findings underscore the need to consider macroplastics in risk assessments of coastal benthic habitats. Given the economic and ecological importance of M. galloprovincialis beds - particularly in the Ría de Vigo - these changes could affect habitat stability, biodiversity, and ecosystem services such as water filtration and nutrient cycling. Although observed effect sizes were moderate, longer exposure periods could amplify these impacts. Future studies should investigate long-term implications of structural modifications induced by plastic litter in biogenic benthic habitats such as mussel beds, oyster reefs, seagrass meadows, and macroalgal stands and should assess the effects of these modifications on habitat stability and ecosystem services.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JH: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. IM: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. EC: Writing – original draft, Writing – review & editing. IG: Writing – original draft, Writing – review & editing. JB: Writing – original draft, Writing – review & editing. ML: Conceptualization, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was conducted as part of the Master’s program in Biological Oceanography at GEOMAR Helmholtz Centre for Ocean Research Kiel. JMFB acknowledges funding of the project STRAUSS Reference: PID2019-106008RB-C21 by I+D+i Program of the Spanish Agency of Research. EC gratefully acknowledges partially financial support by the European Regional Development Fund through the Interreg Atlantic Area Programme, in the frame of FreeLitterAT Project (EAPA-0009/2022). IG was supported by a postdoctoral contract from the EMERGIA Programme (DGP EMEC 2023_00528 -PAI/EMERGIA/CD/2024-082), funded by the Regional Government of Andalusia (Junta de Andalucía).
Acknowledgments
The author thanks the team at CSIC-IIM Vigo for logistical support during fieldwork. We are especially grateful to Marina Davila Sport for selflessly allowing us to carry out this experimental study in their facilities. This study was conducted in the framework of the international research and student training program GAME (Global Approach by Modular Experiments), which is coordinated by GEOMAR Helmholtz Center for Ocean Research Kiel, Germany. In 2020 GAME was generously funded by Lighthouse Foundation, mare Zeitschrift und Buch, Müllverbrennung Kiel, HydroTechnik Lübeck, Joachim Herz Stiftung, Andreas Rühl Stiftung, subCtech Subsea Technologies, J. Bornhöft Industriegeräte, LimnoMar, Hydro-Bios, OffCon Offshore Consulting, and engie Axima.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Babarro J. M. F. Fernández-Reiriz M. J. Labarta U. (2000). Growth of seed mussel (Mytilus galloprovincialis LMK): Effects of environmental parameters and seed origin. J. Shellfish. Res.19, 187–193.
2
Barnes D. K. A. Galgani F. Thompson R. C. Barlaz M. (2009). Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B.: Biol. Sci.364, 1985–1998. doi: 10.1098/rstb.2008.0205
3
Bayne B. L. (1964). Primary and secondary settlement in Mytilus edulis L. (Bivalvia). J. Anim. Ecol.33, 513–523. doi: 10.2307/2647
4
Campbell A. (2009). Guía de la flora y fauna de las costas de España y de Europa. (Barcelona: Ed. Omega), 328.
5
Chintiroglou C. C. Damianidis P. Antoniadou C. Lantzouni M. Vafidis D. (2004). Macrofauna biodiversity of mussel bed assemblages in Thermaikos Gulf (northern Aegean Sea). Helgol. Mar. Res.58, 62–70. doi: 10.1007/s10152-003-0169-8
6
Cole M. Lindeque P. Halsband C. Galloway T. (2016). Microplastics as contaminants in the marine environment: a review. Mar. pollut. Bull.62, 2588–2597. doi: 10.1016/j.marpolbul.2011.09.025
7
Commito J. A. Commito A. E. Platt R. V. Grupe B. M. Piniak W. E. D. Gownaris N. J. et al . (2014). Recruitment facilitation and spatial pattern formation in soft-bottom mussel beds. Ecosphere5, 1–13. doi: 10.1890/ES14-00200.1
8
Consoli P. Romeo T. Angiolillo M. Canese S. Esposito V. Salvati E. et al . (2019). Marine litter from fishery activities in the Western Mediterranean Sea: The impact of entanglement on marine animal forests. Environ. pollut.249, 472–481. doi: 10.1016/j.envpol.2019.03.072
9
Costa V. Chemello R. Iaciofano D. Brutto S. L. Rossi F. (2021). Small-scale patches of detritus as habitat for invertebrates within a Zostera noltei meadow. Mar. Environ. Res.171, 105474. doi: 10.1016/j.marenvres.2021.105474
10
de Carvalho-Souza G. F. Llope M. Tinôco M. S. Medeiros D. V. Maia-Nogueira R. Sampaio C. L. S. (2018). Marine litter disrupts ecological processes in reef systems. Mar. pollut. Bull.133, 464–471. doi: 10.1016/j.marpolbul.2018.05.049
11
Engel F. G. Alegria J. Andriana R. Donadi S. Gusmao J. B. van Leeuwe M. A. et al . (2017). Mussel beds are biological power stations on intertidal flats. Estuarine. Coast. Shelf. Sci.191, 21–27. doi: 10.1016/j.ecss.2017.04.003
12
Galgani F. Hanke G. Maes T. (2015). “ Global distribution, composition and abundance of marine litter,” in Marine Anthropogenic Litter (Cham: Springer), 29–56. doi: 10.1007/978-3-319-16510-3_2
13
GESAMP (2021). Sea-based sources of marine litter (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection, Rep. Stud. GESAMP No. 108) ( International Maritime Organization). Available online at: https://www.imo.org/en/MediaCentre/Pages/WhatsNew-1659.aspx (Accessed September 10, 2025).
14
Gestoso I. Arenas F. Rubal M. Veiga P. Peña M. Olabarria C. (2013). Shifts from native to non-indigenous mussels: Enhanced habitat complexity and its effects on faunal assemblages. Mar. Environ. Res.90, 85–95. doi: 10.1016/j.marenvres.2013.05.015
15
Geyer R. Jambeck J. R. Law K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv.3, 25–29. doi: 10.1126/sciadv.1700782
16
Green D. S. Boots B. Blockley D. J. Rocha C. Thompson R. (2015). Impacts of discarded plastic bags on marine assemblages and ecosystem functioning. Environ. Sci. Technol.49, 5380–5389. doi: 10.1021/acs.est.5b00277
17
Gündoğdu S. Cevik C. Karaca S. (2017). Fouling assemblage of benthic plastic debris collected from Mersin Bay, NE Levantine coast of Turkey. Mar. pollut. Bull.124, 147–154. doi: 10.1016/j.marpolbul.2017.07.023
18
Hammer J. Kraak M. H. S. Parsons J. R. (2012). Plastics in the marine environment: The dark side of a modern gift. Rev. Environ. Contaminat. Toxicol.220, 1–44). doi: 10.1007/978-1-4614-3414-6_1
19
Hayward P. Shields C. Nelson-Smith T. (1999). Flora y fauna de las costas de España y de Europa (Barcelona: Omega), 368.
20
Holdich D. M. Jones J. A. (1983). “ Tanaids: keys and notes for the identification of the species,” in Synopses of the British Fauna (New Series). Ed. KermackD. M.BarnesR. S. K. (Cambridge: Cambridge University Press for the Linnean Society of London and the Estuarine and Brackish-Water Sciences Association), 98.
21
IUCN (2021). Marine Plastic Pollution. Available online at: https://www.iucn.org/resources/issues-brief/marine-plastic-pollution (Accessed July 31, 2025).
22
Jambeck J. R. Geyer R. Wilcox C. Siegler T. R. Perryman M. Andrady A. et al . (2015). Plastic waste inputs from land into the ocean. Science347, 768–771. doi: 10.1126/science.1260352
23
Jørgensen C. Larsen P. Møhlenberg F. Riisgård H. (1988). The mussel pump: properties and modeling. Mar. Ecol. Prog. Ser.45, 205–216. doi: 10.3354/meps045205
24
Katsanevakis S. Verriopoulos G. Nicolaidou A. Thessalou-Legaki M. (2007). Effect of marine litter on the benthic megafauna of coastal soft bottoms: A manipulative field experiment. Mar. pollut. Bull.54, 771–778. doi: 10.1016/j.marpolbul.2006.12.016
25
Lassoued J. Babarro J. M. F. Padín X. A. Comeau L. Bejaoui N. Pérez F. (2019). Behavioural and eco-physiological responses of the mussel Mytilus galloprovincialis to acidification and distinct feeding regimes. Mar. Ecol. Prog. Ser.626, 97–108. doi: 10.3354/meps13075
26
Lassoued J. PadÃ-n X. A. Comeau L. A. Bejaoui N. PÃrez F. F. Babarro J. M. F. (2021). The Mediterranean mussel Mytilus galloprovincialis: Responses to climate change scenarios as a function of the original habitat. Conserv. Physiol.9, 1–16. doi: 10.1093/conphys/coaa114
27
Li W. C. Tse H. F. Fok L. (2016). Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total. Environ.566–567, 333–349. doi: 10.1016/j.scitotenv.2016.05.084
28
Paquette L. Archambault P. Guichard F. (2019). From habitat geometry to ecosystem functions in marine mussel beds. Mar. Ecol. Prog. Ser.608, 149–163. doi: 10.3354/meps12808
29
Pleijel F. Dales R. P. (1991). Polychaetes. British phyllodocoideans, typhloscolecoideans and tomopteroideans: keys and notes for the identification of the species (Shrewsbury: Field Studies Council), 202.
30
Prieto D. Tamayo D. Urrutxurtu I. Navarro E. Ibarrola I. Urrutia M. B. (2020). Nature more than nurture affects the growth rate of mussels. Sci. Rep.10, 1–13. doi: 10.1038/s41598-020-60312-y
31
R Core Team (2020). R: A language and environment for statistical computing (Version 4.0.3) [Software] ( R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed February 14, 2023).
32
Riisgård H. U. Larsen P. S. Pleissner D. (2014). Allometric equations for maximum filtration rate in blue mussels Mytilus edulis and importance of condition index. Helgoland. Mar. Res.68, 193–198. doi: 10.1007/s10152-013-0377-9
33
Riisgard H. U. Randløv A. (1981). Energy budgets, growth and filtration rates in Mytilus edulis at different algal concentrations. Mar. Biol.61, 227–234. doi: 10.1007/BF00386664
34
Rochman C. M. Browne M. A. Underwood A. J. van Franeker J. A. Thompson R. C. Amaral-Zettler L. A. (2016). The ecological impacts of marine debris: unraveling the demonstrated evidence from what is perceived. Concepts. Synthesis.97, 302–312. doi: 10.1890/14-2070.1
35
Schöne B. R. (2001). Don’t move: Growth determination of the bivalve Arctica islandica by field experiments and analysis of biogenic hardparts. Palaeogeogr. Palaeoclimatol. Palaeoecol.177, 261–286.
36
Tang B. Riisgård H. U. (2018). Relationship between oxygen concentration, respiration and filtration rate in blue mussel Mytilus edulis. J. Oceanol. Limnol.36, 395–404. doi: 10.1007/s00343-018-6244-4
37
Thiel M. Ullrich N. (2002). Hard rock versus soft bottom: The fauna associated with intertidal mussel beds on hard bottoms along the coast of Chile, and considerations on the functional role of mussel beds. Helgoland. Mar. Res.56, 21–30. doi: 10.1007/s10152-001-0098-3
38
Uguen M. Gaudron S. M. Seuront L. (2024). Plastic pollution and marine mussels: Unravelling disparities in research efforts, biological effects and influences of global warming. Sci. Total. Environ.959, 178078. doi: 10.1016/j.scitotenv.2024.178078
39
van de Koppel J. Gascoigne J. C. Theraulaz G. Rietkerk M. Mooij W. M. Herman P. M. J. (2008). Experimental evidence for self-organization and its emergent effects in mussel bed ecosystems. Science332, 739–742. doi: 10.1126/science.1163952
40
Vilke J. M. Fonseca T. G. Alkimin G. D. Gonçalves J. M. Edo C. Errico G. et al . (2024). Looking beyond the obvious: The ecotoxicological impact of the leachate from fishing nets and cables in the marine mussel Mytilus galloprovincialis. J. Hazardous. Mater.473, 134479. doi: 10.1016/j.jhazmat.2024.134479
41
Weideman E. A. Perold V. Omardien A. Smyth L. K. Ryan P. G. (2020). Quantifying temporal trends in anthropogenic litter in a rocky intertidal habitat’. Mar. pollut. Bull.160, 19–21. doi: 10.1016/j.marpolbul.2020.111543
42
Wilcox C. Mallos N. J. Leonard G. H. Rodriguez A. Hardesty B. D. (2016). Using expert elicitation to estimate the impacts of plastic pollution on marine wildlife. Mar. Policy65, 107–114. doi: 10.1016/j.marpol.2015.10.014
Summary
Keywords
marine litter, mussel beds, benthic communities, structural complexity, functional responses, plastic pollution, subtidal ecosystems
Citation
Houvener J, Moreu I, Cacabelos E, Gestoso I, Babarro JMF and Lenz M (2025) Entangled ecosystems: the impact of macroplastic litter on Mytilus galloprovincialis communities in northwestern Spain. Front. Mar. Sci. 12:1677044. doi: 10.3389/fmars.2025.1677044
Received
31 July 2025
Accepted
03 October 2025
Published
21 October 2025
Volume
12 - 2025
Edited by
Felix Weber, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), Germany
Reviewed by
Valentina Costa, Anton Dohrn Zoological Station Naples, Italy; García Rosales, National Technology of Mexico CRODE Celaya, Mexico
Updates
Copyright
© 2025 Houvener, Moreu, Cacabelos, Gestoso, Babarro and Lenz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva Cacabelos, eva.cacabelos@ieo.csic.es
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.