- 1Zhejiang Mariculture Research Institute, Zhejiang Key Laboratory of Coastal Biological Germplasm Resources Conservation and Utilization, Wenzhou, China
- 2Zhejiang Mariculture Research Institute, Sino-Thai Joint Laboratory of Marine Science and Technology, Wenzhou, China
- 3Zhejiang Mariculture Research Institute, Wenzhou Key Laboratory of Marine Biological Genetics and Breeding, Wenzhou, China
Quercetin, a naturally occurring plant flavonoid, is used in aquaculture feeds to affect fish health. This study evaluated the effects of dietary quercetin supplementation on the growth performance, antioxidant capacity, lipid levels, and gut microbiota in juvenile Epinephelus akaara. Fish were reared in a controlled recirculating aquaculture system and received dietary quercetin for 8 weeks at five levels: 0, 181, 362, 544, and 725 mg/kg. Growth metrics, physiological and biochemical parameters, antioxidant enzyme activities, and intestinal microbial communities were assessed using standard analytical protocols. Quercetin supplementation at 362–544 mg/kg significantly increased the weight gain rate and the specific growth rate. It elevated the activities of key antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), while reducing malondialdehyde (MDA) levels. Supplementation of quercetin at 181–725 mg/kg lowered the levels of cholesterol (TC) in liver and muscle as well as triglyceride in liver. It also increased gut microbiota diversity and enriched beneficial taxa, such as Cetobacterium, Romboutsia, and Turicibacter, which were positively correlated with physiological resilience. Cetobacterium was correlated significantly negative with cholesterol (r < −0.6, P < 0.01). These findings suggest that quercetin is a promising functional feed additive for improving health and aquaculture performance in red-spotted grouper.
1 Introduction
Groupers are a rich set of reef-associated fish within the Epinephelidae family (Segaran et al., 2025). Within the broader category of groupers, red-spotted grouper (Epinephelus akaara) distinguishes itself as a high-value reef-associated species native to subtropical and temperate waters of the western North Pacific, particularly the South China Sea and surrounding coastal regions of Japan and Korea (FishBase, 2025; Rimmer and Glamuzina, 2017). Since 2022, wholesale prices for hybrid grouper in major Chinese markets (Guangzhou and Shanghai) have ranged from CNY 60 to 100/kg, depending on size, freshness, and form (live vs. chilled) (Food and Agriculture Organization (FAO, 2025). Groups are widely farmed across tropical and subtropical regions, predominantly in Southeast Asia (Market Report Analytics, 2025). Grouper farming has expanded rapidly in recent years due to rising consumer demand for lean, high-protein seafood (Business Research Insights, 2025), and the adoption of recirculating aquaculture systems (RAS) enables year-round production with improved biosecurity (Astari et al., 2022).
The expansion and intensification of aquaculture have elevated disease risks and led to widespread excessive antibiotic use (FAO, 2022; Luthman et al., 2024). This trend raises ecological and public health concerns, particularly in the Asia-Pacific region, which dominates global antimicrobial consumption in the aquaculture sector (Kakkar et al., 2018; Luu et al., 2021; Schar et al., 2020; Thiang et al., 2021). In response, immunonutritional strategies have emerged as a sustainable solution. Immunonutrition in aquaculture broadly refers to the strategic use of dietary components to modulate and strengthen the immune system, enabling fish to better cope with environmental stressors and pathogenic challenges (Rocha et al., 2024). This approach aims to enhance overall health and welfare, boost disease resistance, improve growth performance, and reduce reliance on antibiotics. Promising outcomes have been reported for species such as European seabass (Peixoto et al., 2024), Nile tilapia (Bakry et al., 2024), and gilthead seabream (Marmelo et al., 2024). Researchers are now actively exploring diets enriched with peptides, probiotics, phytochemicals, and other bioactive additives to improve survival rates, growth performance, and disease resistance (Loh et al., 2020; Morales-Lange et al., 2025).
Quercetin (3,5,7,3′,4′-pentahydroxyflavone) is a naturally abundant polyphenolic flavonoid found in fruits, vegetables, and grains (Ghareeb et al., 2024; Kandemir et al., 2022). It exerts protective effects against cancer, bacterial infection, cardiovascular diseases, and hepatic injuries (Aghababaei and Hadidi, 2023). These benefits arise from a suite of bioactive mechanisms, including scavenging reactive oxygen species (ROS) via its hydroxyl-rich structure, chelating transition metals to prevent Fenton-type reactions, and inhibiting proinflammatory signaling pathways such as Nuclear Factor kappa-light-chain-enhancer of Activated B Cells (NF-kB) and Mitogen-Activated Protein Kinase (MAPK) cascades (Huang et al., 2015; Yang et al., 2020). In aquaculture, dietary quercetin has been found to enhance the growth performance, elevate antioxidant capacity, and/or improve the immune responses in grass carp (Ctenopharyngodon idellus) (Xu et al., 2019), zebrafish (Danio rerio) (Wang et al., 2020), and snakehead fish (Channa argus) (Kong et al., 2022). In juvenile black carp (Mylopharyngodon piceus) challenged with a high-fat diet, dietary quercetin supplementation improves growth performance and mitigates inflammation, oxidative stress, and lipid metabolism disorders by modulating NF-κB, Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) and Adenosine 5'-Monophosphate-Activated Protein Kinase (AMPK) signaling pathways (Ming et al., 2025).
In fish, the intestine serves both as a digestive and as an immune organ, and its functionality depends heavily on the composition of the gut microbiota (Shen et al., 2020; Talwar et al., 2018). The dominant bacterial phyla in the fish intestine include Firmicutes, Proteobacteria, Fusobacteria, Bacteroidetes, and Actinobacteria (Eichmiller et al., 2016; Givens et al., 2015; Kim et al., 2021). Commensal and beneficial members within these phyla play key roles in supporting host physiology, including nutrient metabolism, immune modulation, and intestinal homeostasis (Banerjee and Ray, 2016; Tolas et al., 2025). Kanika et al. (2025) reviewed the global diversity and ecological significance of fish gut microbiomes, emphasizing their roles in host health, aquatic ecosystem stability, and sustainable aquaculture. Dominant bacterial phyla (e.g., Firmicutes, Fusobacteria, and Proteobacteria) are consistently observed across major aquaculture species (cyprinids, catfish, salmonids, and cichlids), and microbiome composition is shaped by climate zones, habitat characteristics, and feeding behaviors. However, some phyla also harbor harmful members under certain conditions. For example, Bozzi et al. (2021) found that Aliivibrio and Photobacterium proliferate in the gut of Atlantic salmon during Tenacibaculum dicentrarchi infection. Their increase is associated with gut dysbiosis and reduced microbial diversity, indicating a shift toward pathogenic activity under stress.
Although studies on immunonutrition in grouper species remain limited, some have reported promising benefits. Cheng et al. (2023) demonstrated that in pearl gentian grouper (Epinephelus fuscoguttatus × E. lanceolatus), intraperitoneal injection of l-arginine at 50 mg/kg significantly improves immune parameters, digestive enzyme activity, and intestinal morphology, whereas higher doses result in diminishing or adverse effects. Yau (2015) showed that lipophilic extracts from the microalga Chaetoceros calcitrans enhance leucocyte viability, lymphocyte proliferation, and respiratory burst activity in E. fuscoguttatus. However, to date, no studies have directly examined immunonutritional effects in red-spotted grouper. Accordingly, the present study investigates whether dietary supplementation with a functional feed additive can enhance physiological resilience and overall culture performance in E. akaara.
2 Materials and methods
2.1 Experimental diets
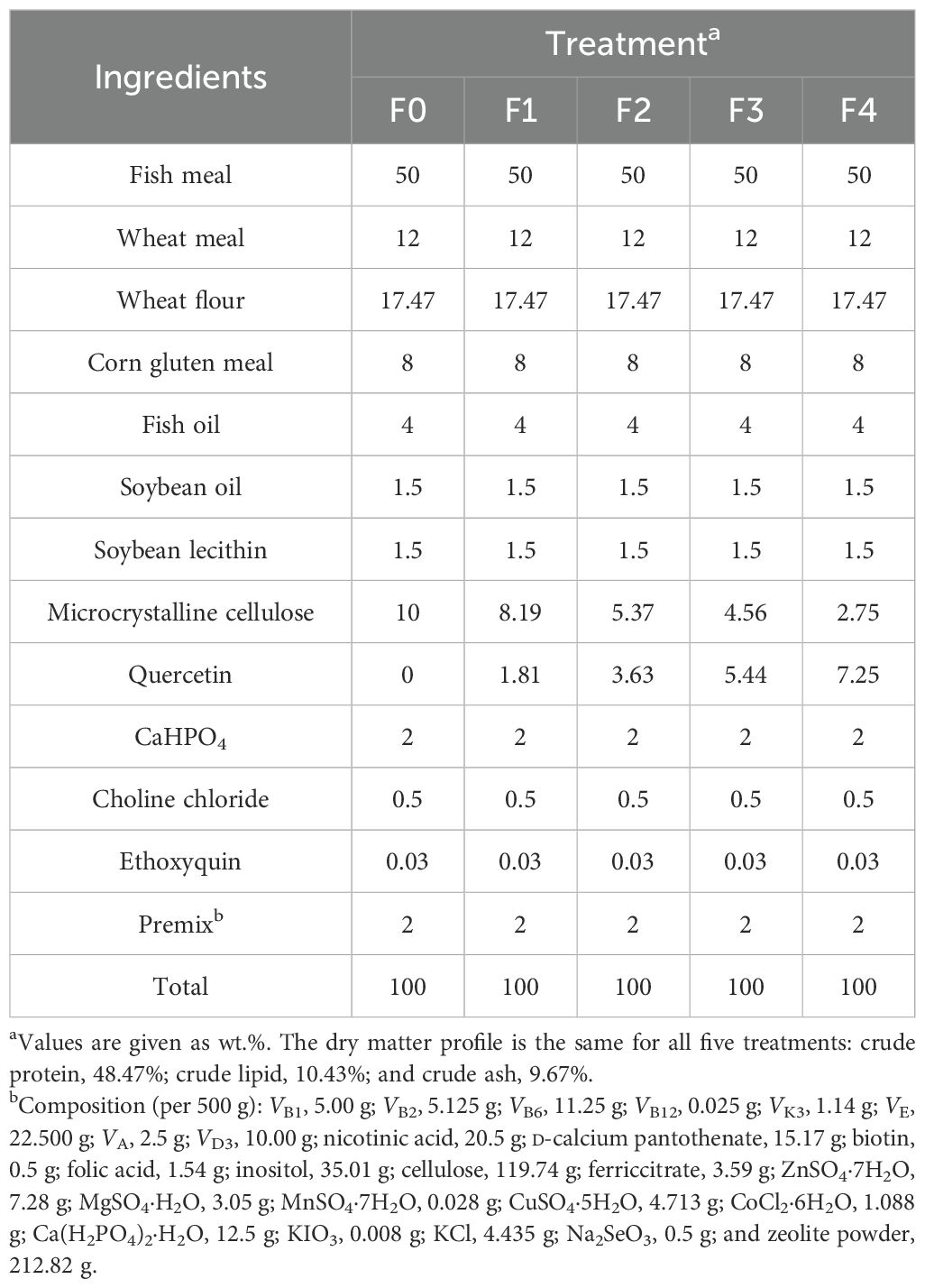
As shown in Table 1, experimental diets were formulated according to the nutritional requirements of E. akaara. All the coarse ingredients were finely ground using a hammer mill and then passed through a 250-μm mesh screen. The ingredients were then weighed and thoroughly mixed using a mixer, and a dough was produced after adding oils and water. The dough was conveyed into multifunctional spiral extrusion machinery (F-26) to produce pellet feed (5.0 mm diameter). The pellets were dried at room temperature in the dark for 24 h and then stored in plastic bags at −20 °C until use. Quercetin dehydrate was purchased from China Shanghai Macklin Biochemical Technology Co. Ltd., Shanghai, China, with an effective content of 97%. The four experimental diets contained quercetin at 181 mg/kg (F1), 362 mg/kg (F2), 544 mg/kg (F3), and 725 mg/kg (F4), respectively. The control diet (F0) had no quercetin supplementation.
2.2 Fish and experimental design
A total of 500 healthy E. akaara with an average body weight of 147.52 g ± 3.61 g were obtained from Zhejiang Mariculture Research Institute (Dongtou, China). The fish did not have any detectable skin lesions indicative of probable bacterial infections. Subsequently, the fish were stocked into 500 L glass aquarium and supplied with aerated filtered seawater in a single-circulation aquaculture system and allowed to acclimate to laboratory conditions for 14 days. After acclimatization, 450 healthy E. akaara were randomly divided into five groups and stocked into 15 cylindrical cages (1 m3) at a density of 30 fish per tank. The fish were fed the corresponding diets twice a day at 08:00 and 16:00, with a daily feeding rate of 5%–8% of body weight. Throughout the 8-week experimental period, two-thirds of the water in each tank was replaced daily. The average water temperature was 30.0 °C ± 0.9 °C, pH was 7.8 ± 0.4, dissolved oxygen exceeded 8.0 mg/L, and total ammonia was 0.05 mg/L ± 0.01 mg/L.
2.3 Sample collection
At the beginning of the experiment, 30 fish per tank were randomly selected to measure the initial body weight. The experiment lasted 8 weeks, and fish were subjected to a 24-h fasting period before sample collection at the end of the trial. Six fish were randomly selected from each tank, anesthetized with buffered MS-222 (30 mg/L, Sigma-Aldrich, St. Louis, MO, USA), and weighed individually. Serum was collected via caudal venipuncture and allowed to stand for 24 h at 4 °C. The serum was then obtained by centrifugation at 3,000 rpm for 15 min at 4 °C and stored in − 80°C for subsequent evaluation of biochemical and hematological indices. Six fish from each tank were dissected to collect liver and kidney samples for body indices analysis. Additionally, liver, kidney, and intestine samples were quickly removed, frozen in liquid nitrogen, and stored in − 80 °C until analysis. Six fish from each tank were used for proximate analysis of muscle from each treatment.
2.4 Measurements of growth metrics
At the end of the experimental period, the following formulas were used to calculate growth performance parameters:
Weight gain rate (WGR, %) = ([final average body weight − initial average body weight]/initial average body weight) × 100.
Specific growth rate (SGR, %/day) = (ln [average final weight] − ln [average initial weight])/number of days × 100.
Condition factor (CF) = total weight of fish (g)/length of fish (cm)3 × 100.
Viscerosomatic index (VSI, %) = visceral weight (g)/whole body weight of fish (g) × 100.
Hepatosomatic index (HSI, %) = wet weight of liver sample (g)/wet weight of fish (g) × 100.
2.5 Assay of antioxidant enzymes and soluble proteins
Liver and serum samples from fish were used to determine antioxidant enzyme activities. Total protein, superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and alanine aminotransferase (ALT) activities were measured in liver homogenates using commercial kits (Nanjing Jiancheng Bio-Engineering Institute, Nanjing, China) following the manufacturer’s instructions (Li M. Y. et al., 2019). SOD, CAT, MDA, glutathione peroxidase (GPx), alkaline phosphatase (ALP), and acid phosphatase (ACP) activities were measured in serum using commercial kits (Nanjing Jiancheng Bio-Engineering Institute, Nanjing, China) according to the manufacturer’s protocols.
2.6 Analysis of physiological and biochemical parameters
The serum cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and glucose (Glu), as well as liver TG and glycogen concentrations, were measured using commercial kits (Nanjing Jiancheng Bio-Engineering Institute, Nanjing, China) according to the manufacturer’s protocols. Liver and muscle samples were saponified with anhydrous ethanol-potassium hydroxide solution and extracted with a mixture of petroleum ether and anhydrous ether in triplicate following the Chinese national standard GB 5009.128-2016.
2.7 Proximate composition analysis
The composition of body tissue was determined by measuring the contents of moisture, crude protein, and lipids according to the methods of AOAC (2005). Briefly, moisture content was measured by drying the samples to a constant weight at 105 °C. Total protein content was determined by the semiautomatic Kjeldahl System (Buchi, Kjielflex K-360, Switzerland) after acid digestion. The ammoniac nitrogen was absorbed in boric acid by adding sodium hydroxide solution and then titrated with hydrochloric acid. Finally, protein content was estimated by multiplying nitrogen content by a factor of 6.25. Crude lipid was extracted from 1 g of each sample using 20 mL of an extraction solution consisting of chloroform and methanol (2:1, v/v). Ash content was determined by using a muffle furnace (Lindberg/Blue M, Thermo Scientific, Watertown, USA) at 640°C for 4 h.
2.8 DNA extraction, sequencing, and data processing of the intestinal bacterial community
At the end of the feeding trial, intestinal contents were collected from six fish per group. Gut contents were obtained under sterile conditions using sterile forceps. The samples were placed on ice, transferred to sterile microcentrifuge tubes, immediately flash-frozen in liquid nitrogen, and stored at − 80 °C until DNA extraction. The intestinal content samples of E. akaara collected were preserved on dry ice and sent to BGI Genomics (Shenzhen, China) for extraction and sequencing of total bacterial genomic DNA. For bacterial diversity analysis, the V3–V4 regions of the 16S rRNA gene were amplified by PCR using the universal primers 338F (5′-ACTCCTACGGGAGCCAGCAG-3) and 806R (5′-GGACTACHVGGGTWTCTAAT-3) to construct a bacterial 16S rDNA library (Xu et al., 2016).
The dix-seq software (version 1.0) was used to filter and merge paired reads of the raw sequencing data (raw reads) to obtain valid sequences (clean tags) (Dong et al., 2025). For Trimmomatic, a sliding window approach was adopted with a window size of 50 bp, requiring an average quality score of 20 and a minimum retained read length of 150 bp (Bolger et al., 2014). The paired-end sequences were merged, primers were removed, and duplicated sequences were filtered out using USEARCH11 (Edgar, 2010). Subsequently, the UNOISE3 algorithm (Edgar, 2016a) was applied for denoising and chimera removal, yielding zero-radius operational taxonomic unit (zOTU) sequences. The clean tags were denoised for calling zOTUs using USEARCH (v11.0.667) software. After obtaining the zOTU representative sequences, the SINTAX algorithm (Edgar, 2016b) was used by the dis-seq software to assign taxonomy of zOTUs against the Silva 138.2 database (Kõljalg et al., 2005), with a confidence threshold set at 0.8. Each zOTU was classified into phylum, family, and genus, and the relative abundances of bacteria at the phylum and genus taxonomic levels were calculated. Based on the processed data, alpha diversity index analysis was performed. The richness index (Chao1), species abundance index (abundance-based coverage estimator metric [Ace]), Shannon index, Simpson index, and coverage rate were calculated to obtain the species richness and diversity information of each sample. Additionally, beta diversity was determined by principal coordinates analysis (PCoA). A forward selection was used to identify the nonredundant indicators for fish growth, TG, TC, and antioxidant enzyme activities in the CAP model by the function “ordiR2step” (permutations = 999) of the R package “vegan”.
2.9 Statistical analysis
The data were expressed as mean ± standard deviation (SD). The analysis was conducted using SPSS Statistics 21.0 software. The growth, enzymatic and oxidative enzymes, metabolic and lipid biomarkers, body proximate composition, and alpha and beta diversity data of the E. akaara were analyzed using one-way analysis of variance (ANOVA) to determine the significant differences. When significant differences were observed, Duncan’s test was used to determine the differences between the control and experimental treatments. Results with p ≤ 0.05 were considered statistically significant. Spearman correlation coefficients and associated significance levels were calculated, using the “rcorr()” function in the Hmisc package of the R software (version 4.1.0), between dominant gut microbiota and host parameters—including growth performance, liver and blood physiological indicators, TG, TC, and other biomarkers. The associations between microbial communities and fish growth, lipid content, and physiological indicators were further assessed using the Mantel test, implemented in the linkET package in R.
3 Results
3.1 Growth parameters
The final body weight (FBW), WGR, and SGR of E. akaara peaked in F2, i.e., when the level of quercetin supplementation (QS) was 362 mg/kg (Table 2). All four QS groups had significantly higher (p < 0.05) SGR than the control, and F2–F4 had significantly elevated (p < 0.05) FBW and WGR compared with F0. All five groups had indistinguishable (p > 0.05) condition factor (CF), viscerosomatic index (VSI), and hepatosomatic index (HSI).
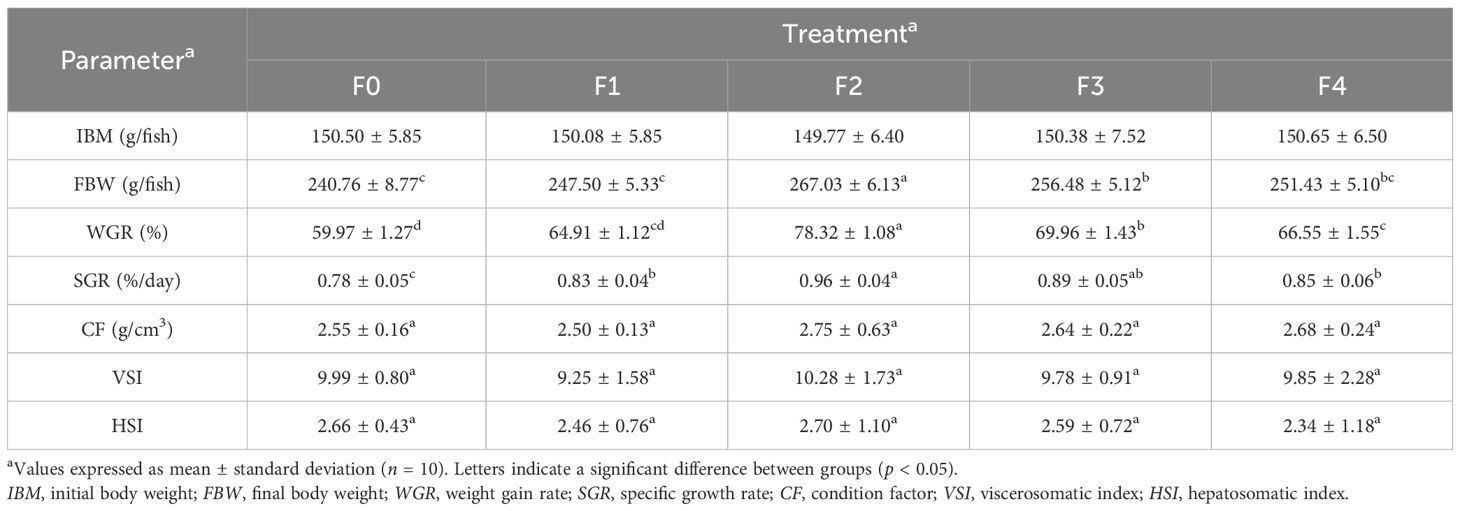
Table 2. Growth measures and body indices of Epinephelus akaara, fed different dietary quercetin levels.
3.2 Enzymatic and oxidative stress biomarkers
Table 3 shows that treatments F1–F3 had significantly higher levels of liver SOD and liver CAT than treatments F4 and F0, and F1 and F2 had significantly higher levels of liver ALT than F4 and F0. All five groups showed no significant differences in liver MDA. Regarding the serum biomarkers, F1 and F3 had significantly lower serum MDA than F0, F3 and F4 had significantly higher serum SOD than F0 and F1, and F2–F4 had significantly higher serum GPx than F0 and F1. All five groups had indistinguishable serum ALP, CAT, and ACP.
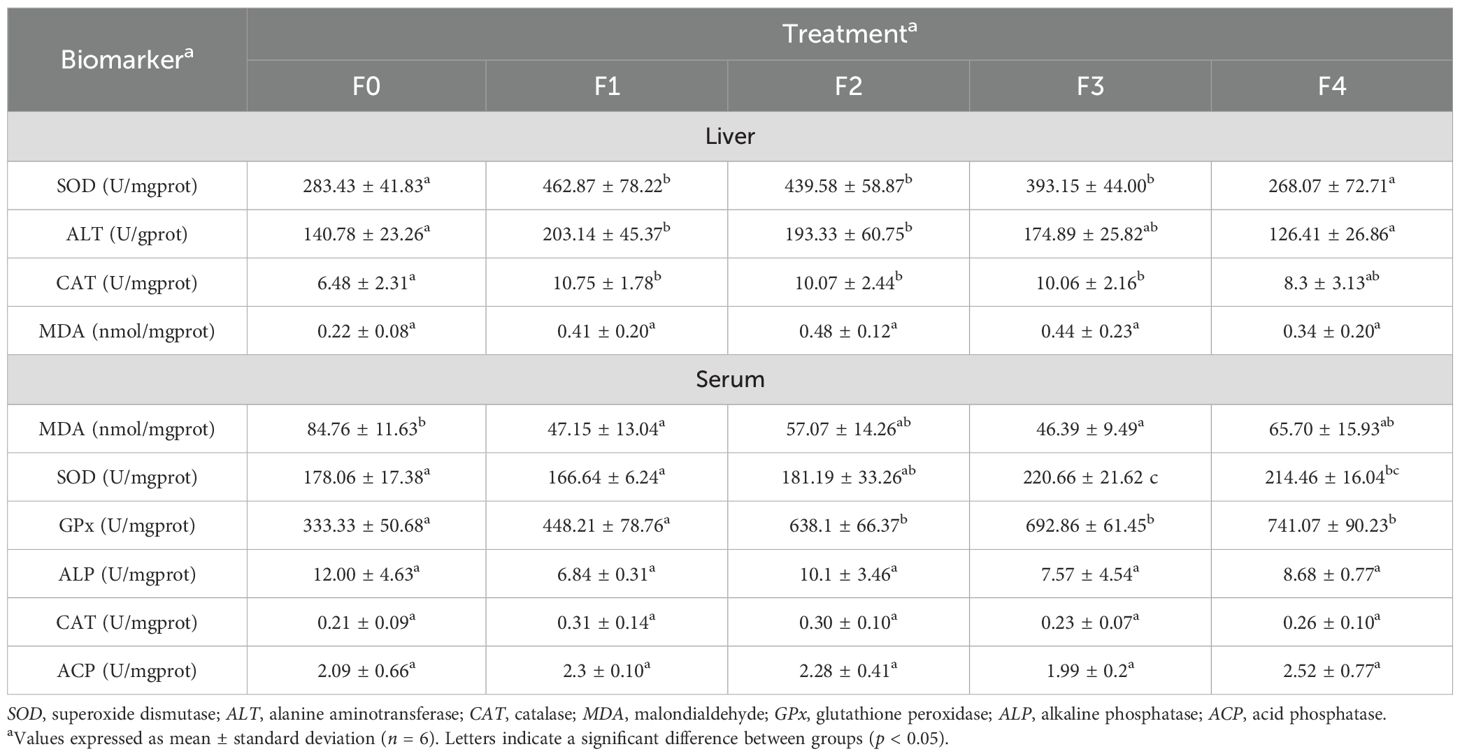
Table 3. Enzymatic and oxidative stress biomarkers of Epinephelus akaara, fed different dietary quercetin levels.
3.3 Metabolic and lipid biomarkers
Table 4 shows that F4 had significantly higher serum cholesterol than F0 and F1, F3 had significantly higher serum TG than all other treatments, F4 had significantly higher serum LDL-C than F0–F2, along with significantly higher serum HDL-C than all other groups. F1 and F2 both had significantly higher blood glucose than the other three groups. With the increase in dietary QS, the serum levels of cholesterol, LDL-C, and triglycerides showed a gradual and continuous rise (p < 0.05). As the dietary QS increased, the serum Glu content exhibited a sustained upward trend, reaching its peak at an addition level of 362 mg/kg (p < 0.05). All five groups showed no significant differences in liver cholesterol, TG, and glycogen. In addition, F4 had significantly lower muscle cholesterol than F0. As the dietary QS increased, the muscle cholesterol content showed a declining trend, reaching its lowest level at an addition of 725 mg/kg (p < 0.05).
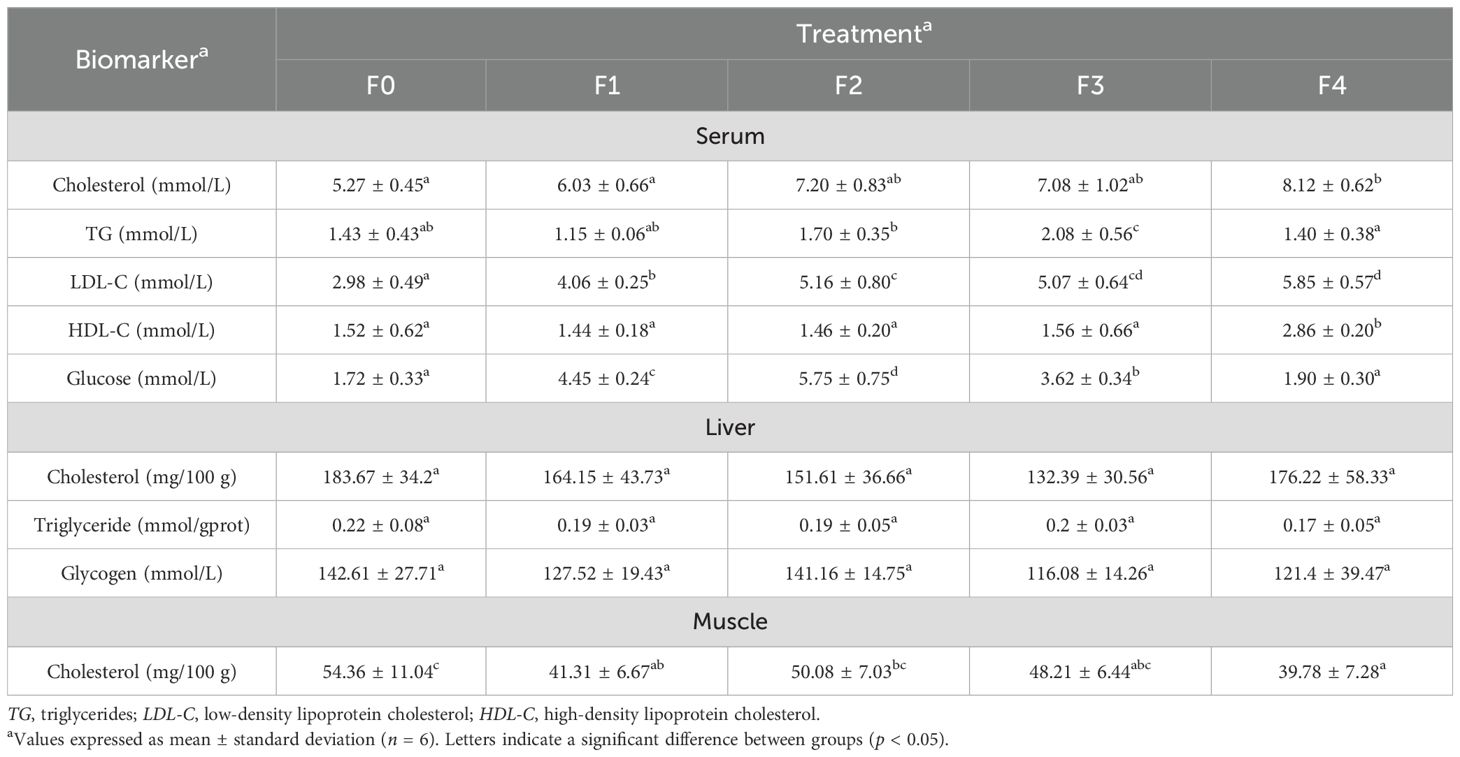
Table 4. Metabolic and lipid biomarkers of Epinephelus akaara, fed different dietary quercetin levels.
3.4 Body proximate composition
Table 5 shows that F1 and F3 had higher glycogen levels than the other groups, but no clear trend could be inferred. The supplement did not significantly affect the moisture, crude lipid, crude protein, or ash content of the fish.
3.5 Intestinal microbiome analysis
3.5.1 Alpha diversity
High-throughput 16S rRNA sequencing yielded 1,447,979 sequences, which formed 865 operational taxonomic units (OTUs) at 97% similarity. Table 6 shows the alpha diversity indices of the gut microbiota of E. akaara. For all groups, Good’s coverage exceeded 99.8%, indicating near-complete species representation. Both F3 and F4 had significantly higher zOTU counts than F0, F1, and F2 (p < 0.05). Compared to the other four groups, F4 had significantly higher ACE and Chao1 indices, along with a significantly lower Simpson index. In addition, F2–F4 had significantly higher Shannon indices than F0 and F1.
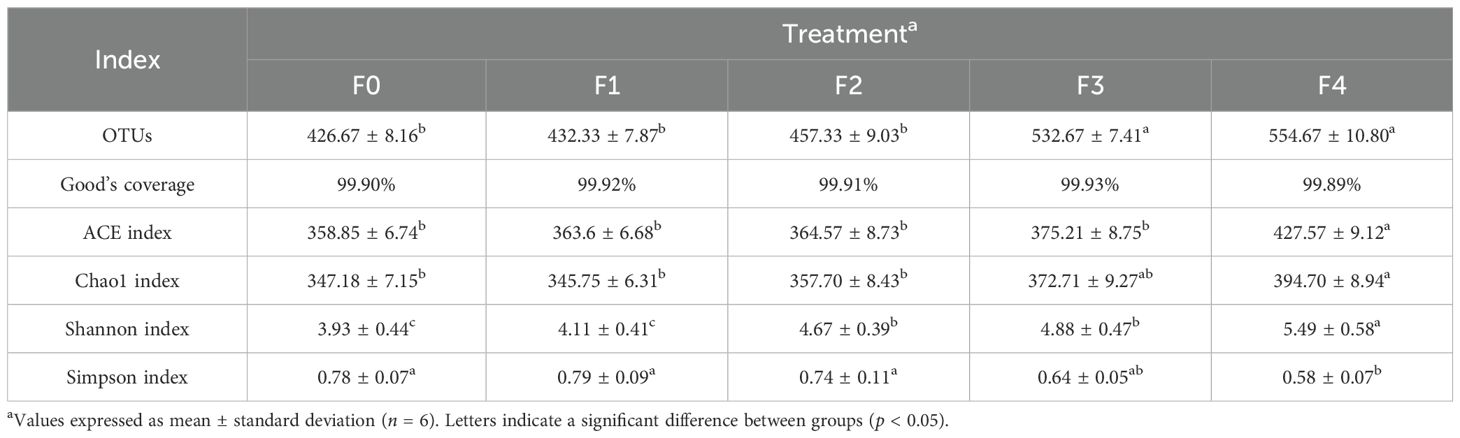
Table 6. Indicators of the intestinal microflora of Epinephelus akaara, fed different dietary quercetin levels.
3.5.2 Beta diversity
The beta diversity of the E. akaara gut microbiome was analyzed using Constrained Analysis of Principal Coordinates (CPCoA) based on Bray–Curtis dissimilarity, constrained by growth (SGR) and biomarker profiles (Figure 1). Bacterial communities differed significantly among the five experimental groups (PERMANOVA, R2 = 0.25, p = 0.02). The CPCoA model resolved these differences, with component 1 (25.87%) and component 2 (16.21%) collectively explaining 42.08% of the total constrained variation. Biomarker–microbiota correlations revealed divergent patterns, as liver oxidative stress markers (MDA and liver SOD) aligned with +x-axis communities, while hepatic antioxidant/lipid markers (liver TG and CAT) associated with −x-axis communities. Meanwhile, SGR and liver cholesterol metabolism drove independent variation along the y-axis (orthogonal to oxidative/lipid axes).
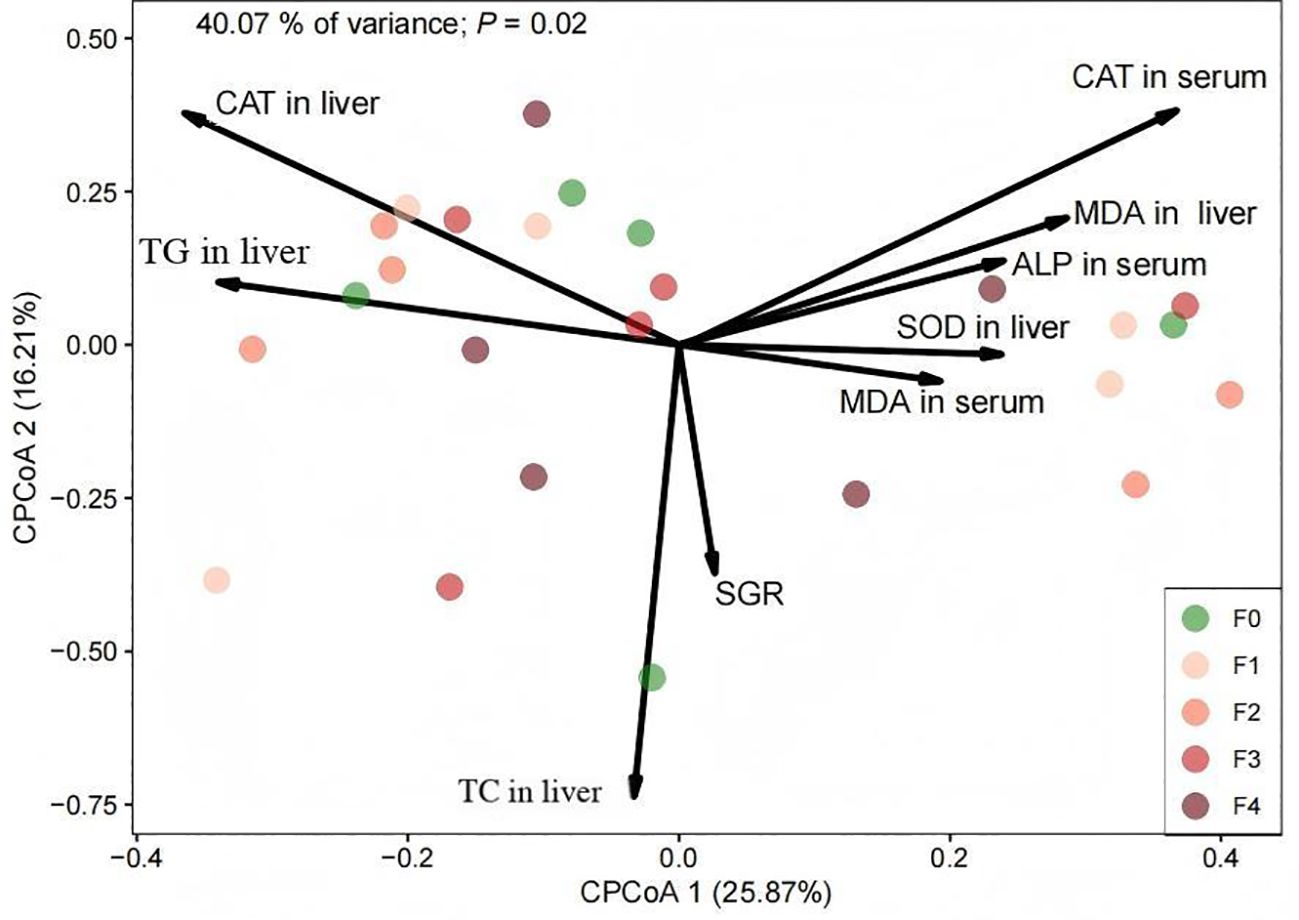
Figure 1. CPCoA plot of E. akaara gut microbiota zOTU profiles, based on Bray–Curtis dissimilarity and constrained by specific growth rate (SGR) and other relevant biomarkers. SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; ALP, alkaline phosphatase; TG, triglycerides; SGR, specific growth rate.
3.5.3 Intestinal bacterial composition
Figure 2 shows the relative abundances of the E. akaara gut microbiota at the phylum and genus levels. The dominant phyla were Proteobacteria (sub-phyla: γ-Proteobacteria 48.11%, α-Proteobacteria 3.56%), Firmicutes (25.21%), Fusobacteriota (8.45%), and Actinobacteriota (5.86%), and they collectively represented 91.19% of total abundance. At the genus level, the dominant taxa were Photobacterium (33.88%), Cetobacterium (8.44%), Romboutsia (5.88%), and Vibrio (5.79%), accounting for 53.99% of the bacterial abundance.
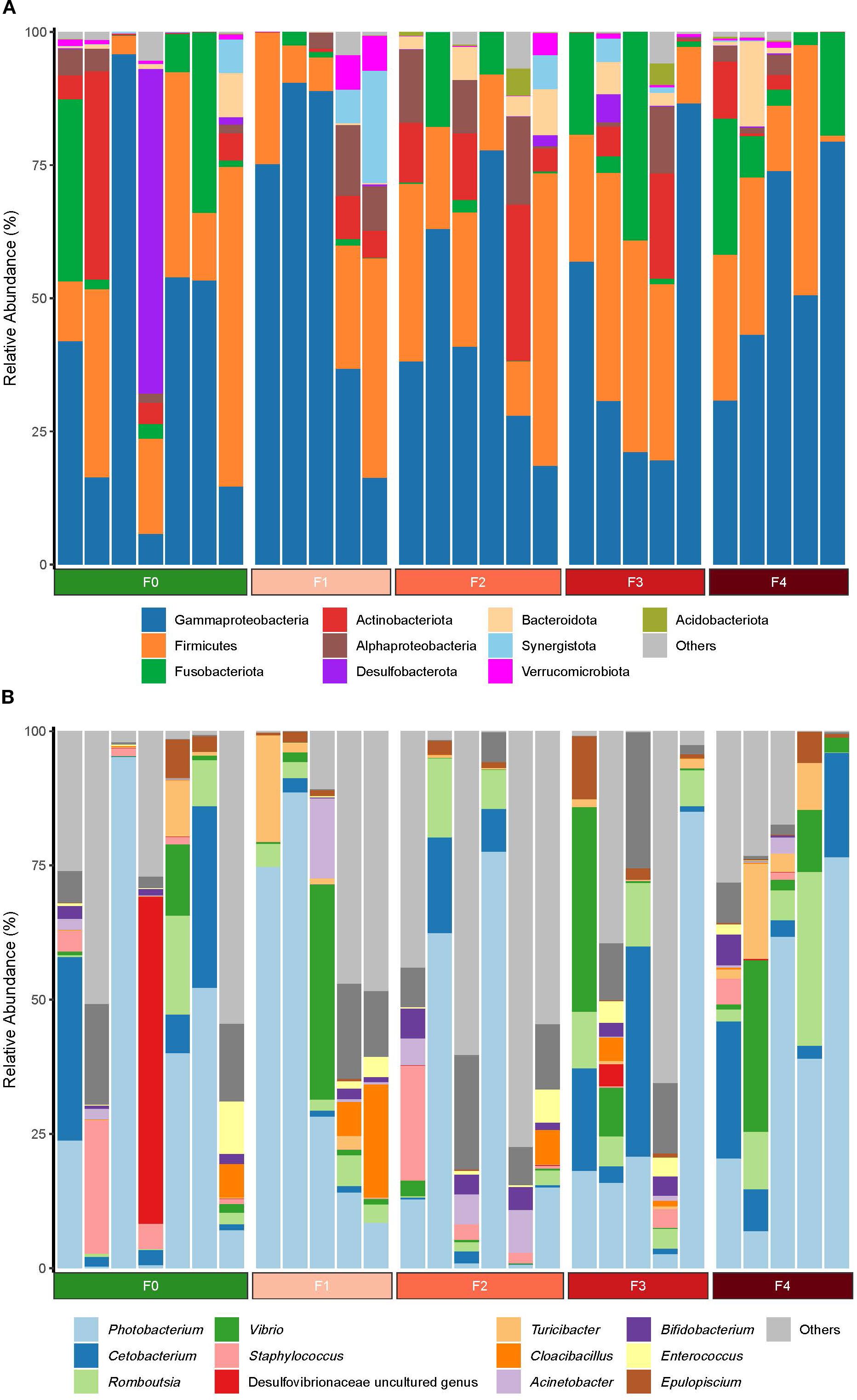
Figure 2. The abundance of E. akaara gut microbiota at the phylum and genus levels in Epinephelus akaara fed different dietary quercetin levels (n = 6).
3.6 Correlation between intestinal bacteria and physiological biomarkers
The gut microbiota of E. Akaara comprised a diverse community of both opportunistic pathogens and functional microorganisms. Pathogenic genera included Photorbacterium, Vibrio, Mycobacterium, Acinetobacter, and Pseudomonas, whereas beneficial or functional taxa such as Cetobacterium, Enterococcus, Bifidobacterium, Bacillus, Romboutsia, Halomonas, Zhenhengia, and Turicibacter were also prevalent (Figure 3). Microbial correlation analyses revealed several key interactions within this ecosystem. Cetobacterium exhibited significant negative correlations with opportunistic taxa, including Photobacterium, Vibrio, Mycobacterium, and Pseudomonas, yet showed positive associations with Romboutsia and Turicibacter. Interestingly, despite being categorized as functional taxa, both Bifidobacterium and Bacillus were moderately correlated with pathogenic genera such as Photobacterium and Vibrio (Figure 3, r < 0.5).
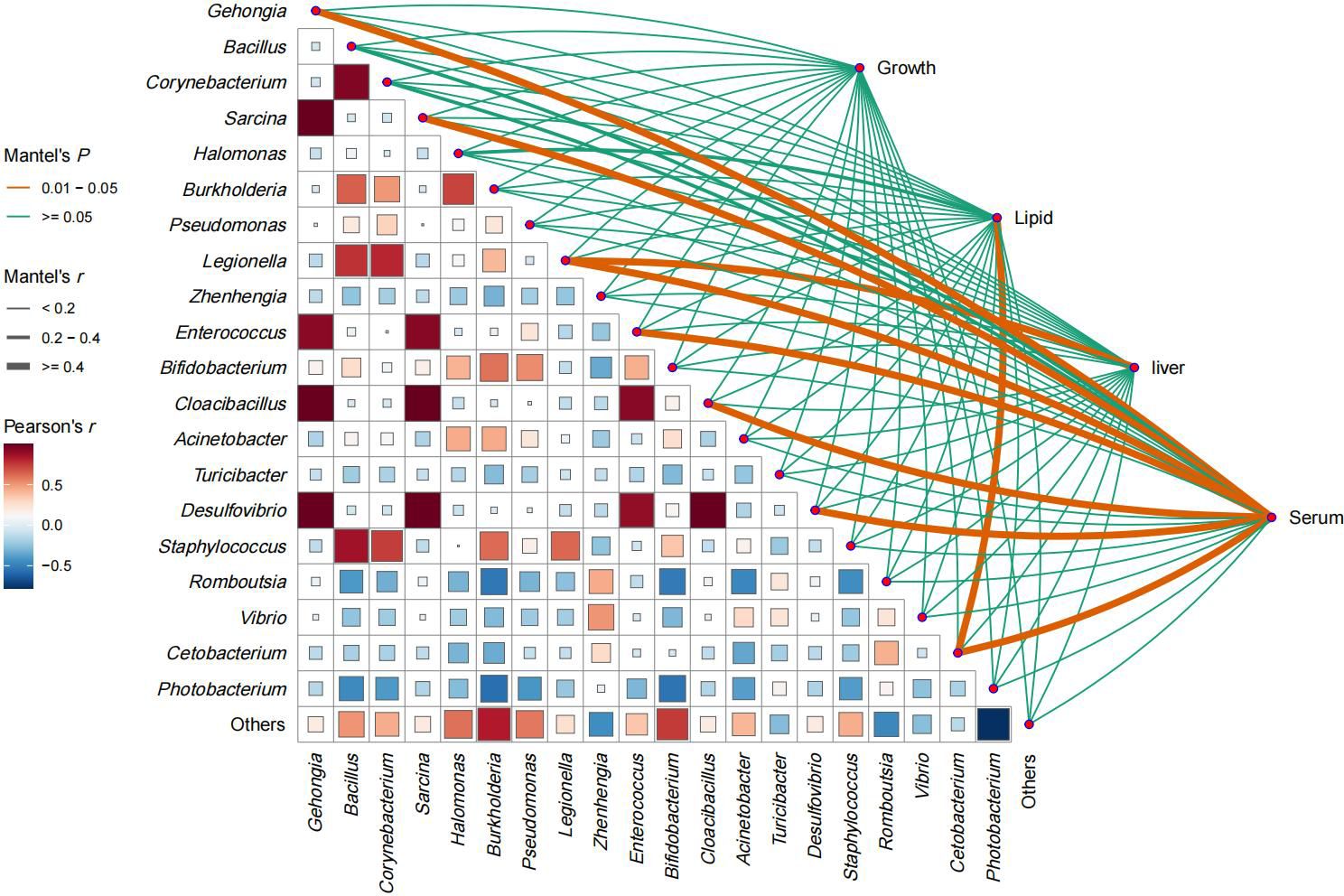
Figure 3. Relationships between prevalent bacteria (relative abundance > 0.5%) in E. akaara gut microbiota and growth performance, lipid content, and liver and serum biomarkers.
Regarding the links between microbiota composition and fish physiology, Romboutsia and Turicibacter correlated positively with fish growth. In contrast, they were negatively correlated with liver and visceral indices, as well as antioxidant indicators related to liver function (Figure 4). Among all physiological parameters, serum enzyme activity showed the most extensive microbial interactions, with moderate to high correlations (r > 0.4) involving eight taxa—including pathogens such as Legionella and probiotics such as Enterococcus and Cetobacterium (Figures 3, 4). Therefore, serum immune and antioxidant functions must be critical drivers of both probiotic proliferation and pathogen suppression and thus shape the gut microbial balance. Presumably, Cetobacterium must have played a key role in the quorum-sensing regulation of fish lipid content and intestinal microbiota, as it correlated strongly with both lipid content and serum antioxidant capacity. In linear regression analyses, the relative abundance of Cetobacterium showed strong negative correlations with serum markers, including MDA and CAT (Figure 5, r ≤ 0.4, p < 0.05), as well as cholesterol levels (Figure 6, r ≤ 0.6, p < 0.01). Thus, it appears to play a key role in regulating lipid content and hepatic oxidative stress.
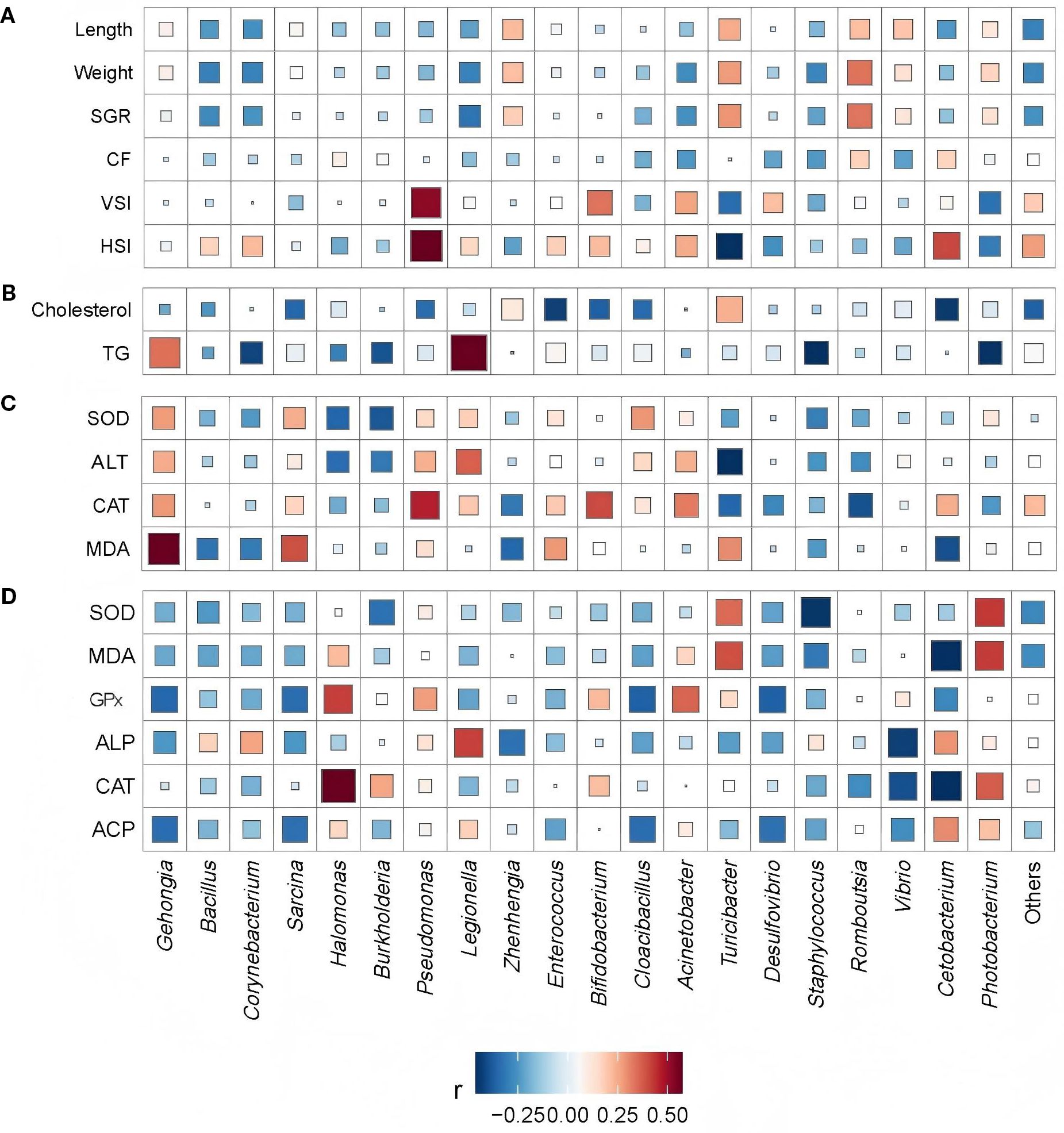
Figure 4. Correlations of prevalent bacteria (> 0.5% relative abundance) with (A) growth parameters, (B) body lipid composition, (C) liver function markers, and (D) serum metabolic profiles. SGR, specific growth rate; CF, condition factor; VSI, viscerosomatic index; HSI, hepatosomatic index; TG, triglycerides; SOD, superoxide dismutase; ALT, alanine aminotransferase; CAT, catalase; MDA, malondialdehyde; GPx, glutathione peroxidase; ALP, alkaline phosphatase; ACP, acid phosphatase.
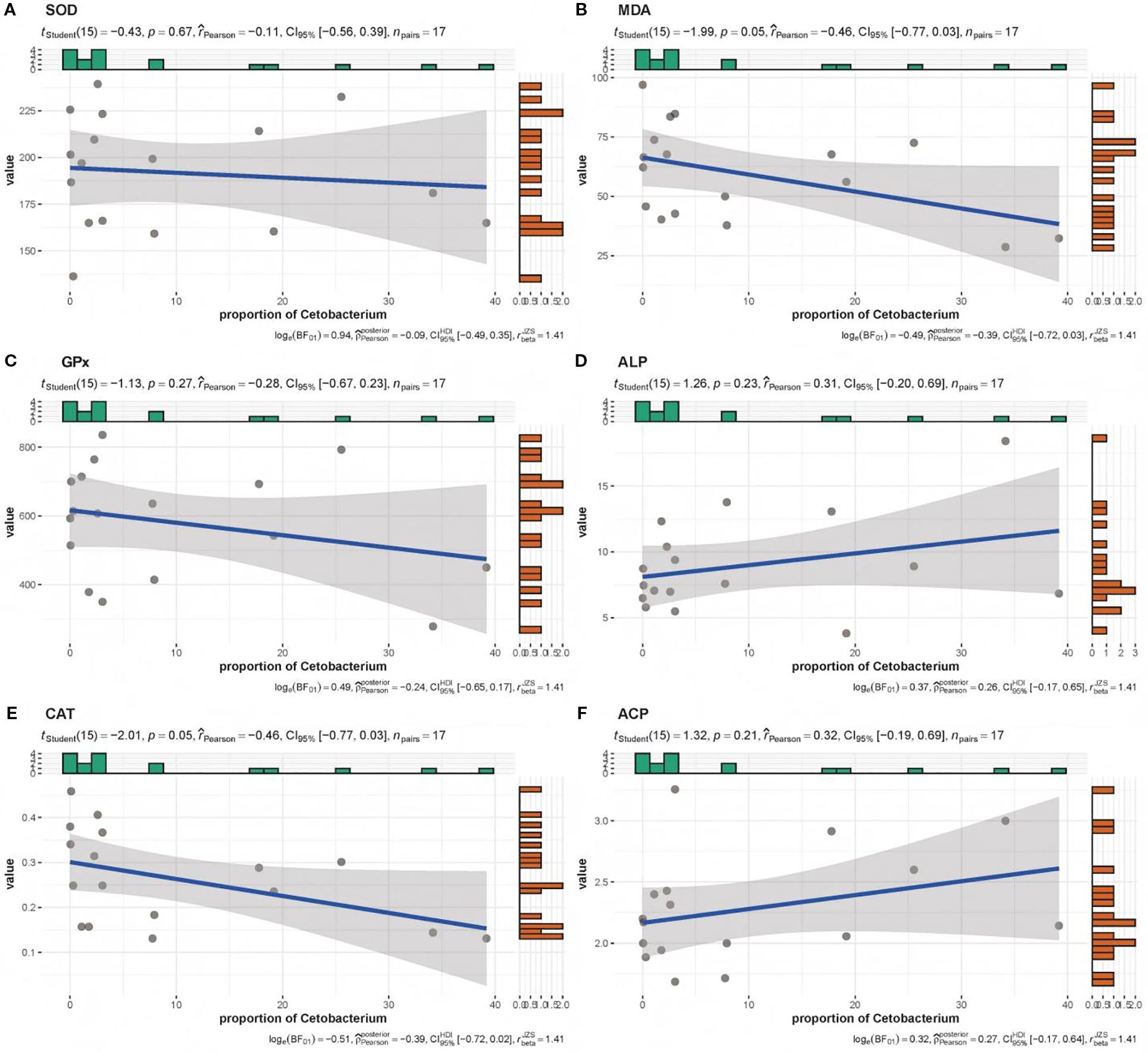
Figure 5. Correlation analysis of Cetobacterium abundance with serum biomarkers: (A) SOD, (B) MDA, (C) GPx, (D) ALP, (E) CAT, and (F) ACP. SOD, superoxide dismutase; MDA, malon dialdehyde; GPx, glutathione peroxidase; ALP, alkaline phosphatase; CAT, catalase; ACP, acid phosphatase.
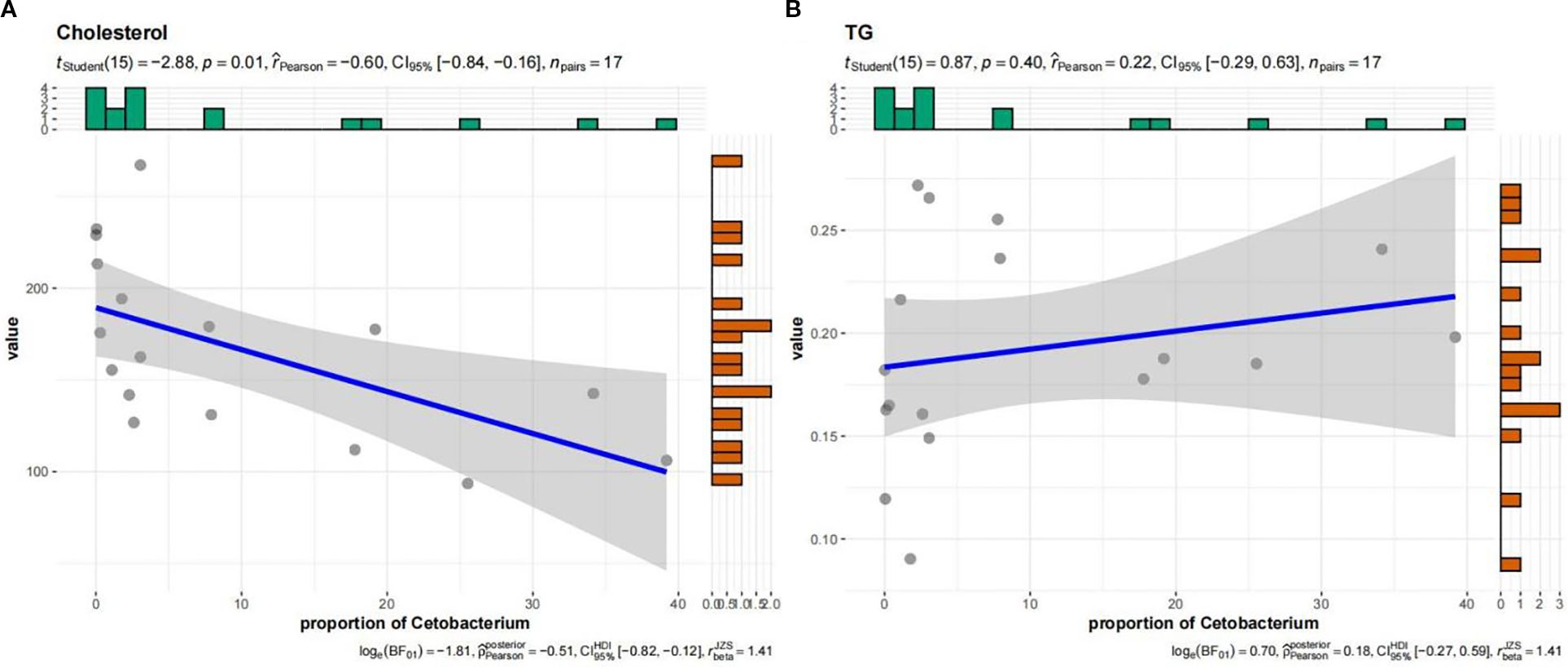
Figure 6. Linear regression of Cetobacterium abundance with the hepatic level of (A) cholesterol and (B) triglycerides.
4 Discussion
4.1 Growth performance and antioxidant capacity
Dietary quercetin has been found to improve the growth of Nile tilapia (Oreochromis niloticus) (Zhai and Liu, 2013), northern snakehead (Channa argus) (Kong et al., 2022), and olive flounder (Paralichthys olivaceus) (Shin et al., 2010). It enhances growth in farmed fish by the secretion of digestive enzymes secretion, reducing oxidative stress in intestinal tissues, and optimizing nutrient metabolism (Armobin et al., 2023; Khan et al., 2023). This mechanism is dose-dependent and has been consistently validated across multiple fish species (Xu et al., 2019; Baker, 1998). In our study, dietary supplementation with quercetin increased the WGR and SGR of E. akaara in a dose-dependent manner, with the optimal level observed at 363 mg/kg (group F2).
In farmed animals, the antioxidant system, which encompasses enzymes (e.g., SOD, CAT, GPx) and low-molecular-weight compounds (e.g., vitamins C/E, glutathione [GSH]), collectively neutralizes reactive oxygen species (ROS) to maintain cellular homeostasis and prevent oxidative tissue damage. This system operates through coordinated mechanisms. SOD catalyzes the dismutation of superoxide (O2·−) to hydrogen peroxide (H2O2), while CAT decomposes excess H2O2 in peroxisomes. GPx reduces H2O2 to water using reduced GSH as an electron donor, and GSH can also directly quench singlet oxygen and hydroxyl radicals. In addition, vitamins C/E scavenge lipid peroxyl radicals. By mitigating ROS propagation, this network attenuates lipid peroxidation, reduces MDA formation, preserves membrane integrity, and thereby enhances stress resilience, growth efficiency, and product quality.
Quercetin exerts potent antioxidant effects in farmed fish through dual mechanisms. Its molecular structure—specifically the catechol group (B-ring) and the C-3 hydroxyl group—enables direct ROS neutralization and metal chelation (Boots et al., 2008), while it also upregulates endogenous defenses via Nrf2-mediated transcription of antioxidant enzymes and GSH synthesis (Dong et al., 2020; Wang et al., 2020). These mechanisms translate into significant in vivo benefits. In spotted seabass (Lateolabrax maculatus), 0.5–1 g/kg dietary quercetin enhances hepatic CAT, SOD, total antioxidant capacity (T-AOC), and GSH while suppressing MDA (Dong et al., 2021). In northern snakehead (C. argus), 300 mg/kg quercetin is optimal for improving SOD, CAT, T-AOC, GST, and GPx activities (Kong et al., 2022). In rainbow trout (Oncorhynchus mykiss), quercetin-rich (0.1%–1%) nettle extract elevates lysozyme, IgM, and myeloperoxidase, thereby producing complementary immunomodulatory effects (Awad et al., 2013). We found that in E. akaara, serum SOD and GPx generally increased with rising quercetin dosage, whereas liver SOD, CAT, and ALT were best improved with moderate quercetin supplementation. There was no significant difference in liver MDA across groups, but groups F1 and F3 had significantly lower serum MDA than group F0.
4.2 Lipid metabolism and whole-body proximate composition
Lipid metabolism biomarkers in fish serum respond dynamically to physiological or pathological changes induced by environmental factors. Parameters such as TG, TC, HDL-C, and LDL-C serve as critical indicators of metabolic status, nutritional health, and disease conditions. Among these, TG and TC primarily reflect lipid absorption efficiency, whereas HDL-C and LDL-C indicate lipid transport and catabolic pathways. Luo et al. (2022) reported that in hybrid grouper (E. fuscoguttatus × E. polyphekadions), quercetin downregulates genes involved in the steroid biosynthesis pathway, reducing hepatic cholesterol production and lipid accumulation, while sodium quercetin-5′-sulfonates upregulate polyamine synthesis genes, thereby enhancing energy metabolism and mitigating liver steatosis. Dong et al. (2021) found that in spotted seabass fed a high-fat diet, quercetin supplementation significantly reduces excessive lipid accumulation by promoting mitochondrial biogenesis and autophagy, while suppressing endoplasmic reticulum stress. Pan et al. (2024) demonstrated that in carp (Cyprinus carpio), quercetin supplementation counteracts difenoconazole-induced inflammatory spleen damage by restoring oxidative balance and suppressing key proinflammatory pathways (e.g., reduction of ROS and MDA levels, inhibition of NF-KB pathway activation, and blockade of the NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome complex), thereby rebalancing cytokine profiles and promoting splenic immune homeostasis. Ming et al. (2025) noted that in juvenile black carp (Mylopharyngodon piceus) fed high-fat diets, quercetin activates the AMPK signaling pathway to enhance lipid metabolism, thereby reducing hepatic lipid accumulation and improving growth performance. The present results showed that the TC, HDL-C, and LDL-C levels increased with rising dietary QS, suggesting that QS enhances the transport capacity of triglycerides and cholesterol, thereby reducing cholesterol accumulation in the liver and muscle. Serum TG levels serve as a crucial indicator of hepatic lipid metabolism and generally follow a trend similar to TC levels. The specific regulatory mechanisms underlying lipid metabolism require further investigation.
There is currently limited knowledge about the association between dietary quercetin and the whole-body proximate composition of farmed fish. Zhai and Liu (2014) reported that in Nile tilapia (Oreochromis niloticus), dietary quercetin supplementation reduces muscle moisture and lipid content while increasing protein levels. In contrast, Xu et al. (2019) found that in grass carp (Ctenopharyngodon idella), dietary quercetin supplementation does not significantly alter the conventional proximate composition of muscle (moisture, crude protein, lipid, and ash), although it does modify some functional parameters, such as collagen content and amino acid profiles. In our experiments with E. akaara, the whole-body proximate composition (moisture, protein, lipid, and ash) remained unaffected.
4.3 Intestine microbiota
The gut microbiota of fish is a dynamic ecosystem that is critical for host physiology and influences nutrient metabolism, immune function, pathogen exclusion, and intestinal integrity. Microbial diversity, quantified through alpha diversity indices, serves as a key indicator of ecosystem stability. The Chao1 and Ace indices assess species richness (total taxa present), while the Shannon and Simpson indices measure diversity (abundance and evenness) (Li M. et al., 2019). This community structure is highly plastic and can be shaped by host genetics, diet, environmental stressors, and aquaculture practices. We found that with rising levels of quercetin supplementation, the Simpson index generally decreased, and the Chao1, Ace, and Shannon indices generally increased. This suggests that quercetin must have enhanced the diversity of the gut microbiota in E. akaara.
Zhu et al. (2022) reported that, in juvenile dark sleeper (Odontobutis potamophila), exposure to quercetin preserves overall gut microbial α-diversity and selectively enriches putatively beneficial genera (Bacillus, Lactobacillus). The intervention also suppresses opportunistic pathogens (Plesiomonas, Aeromonas, Shewanella) in a dose-dependent manner, with microbiota shifts coinciding with enhanced hepatic antioxidant enzyme activities. Ma et al. (2025) reported that dietary supplementation with Bacillus subtilis in E. akaara restructures the intestinal microbiota by increasing the relative abundance of beneficial genera, including Clostridium sensu stricto, Turicibacter, and Bacillus, while reducing potential pathogens, such as Vibrio, Cetobacterium, and Erysipelothrix. These compositional shifts are accompanied by significant gains in alpha diversity, suggesting enhanced microbial stability and presumably improved immune status. In our case, we found that in E. akaara receiving quercetin, the dominant phyla in the gut microbiota included Proteobacteria, Actinobacteriota, Firmicutes, and Fusobacteriota. Quercetin supplementation increased the Firmicutes/Bacteroidota ratio and reduced the Fusobacteriota abundance.
The key bacterial genera in E. Akaara included beneficial strains, such as Cetobacterium and Romboutsia, as well as pathogenic strains, such as Photobacterium and Vibrio. Cetobacterium, a vitamin B12-producing genus enriched in fish gut microbiota, plays a central role in carbohydrate fermentation and acetate-mediated host regulation. It modulates metabolic health, ammonia tolerance, and gut–liver physiology (e.g., lipid metabolism, fat accumulation) across species such as catfish, carp, tilapia, and zebrafish (Qi et al., 2023; Xie et al., 2022; Wang et al., 2021, Wang et al., 2025; Zhang et al., 2023; Zhou et al., 2022). Romboutsia improves carbohydrate metabolism and glucose homeostasis by producing short chain fatty acids (SCFAs) (e.g., butyrate), and its high abundance has been linked to plant-based diets in tilapia (Fan et al., 2024). Photobacterium is a core genus in marine fish, with species such as P. leiognathi forming mutualistic bioluminescent symbioses in ponyfish (Urbanczyk et al., 2011), while pathogenic strains like P. damselae cause photobacteriosis and vibriosis in a wide range of marine hosts (Dunlap et al., 2004; Gouife et al., 2022). Stress-induced virulence of Vibrio species—such as V. harveyi and V. anguillarum under warming and pollution—has been linked to disease outbreaks in fish (Sanches-Fernandes et al., 2022), whereas commensal Vibrio strains may support gut homeostasis and nutrient cycling in healthy marine fish (Pérez-Gómez et al., 2025; Zhang et al., 2024). The effects of quercetin on gut microbiota diversity and its treatment mechanisms in modulating functional strains remain unclear, warranting further investigation.
5 Conclusions
Dietary supplementation with quercetin significantly improved multiple physiological and biochemical parameters in juvenile E. akaara. Optimal supplementation levels (362–544 mg/kg) enhanced growth performance, antioxidant capacity, and lipid metabolism. Quercetin also promoted intestinal microbial diversity and selectively enriched beneficial taxa, such as Cetobacterium, Romboutsia, and Turicibacter, which correlated positively with antioxidant status and growth metrics. These findings underscore the potential of quercetin as a functional feed additive to improve health, resilience, and aquaculture performance in red-spotted grouper through coordinated host–microbiota interactions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author. All amplicon sequencing data are available in NCBI (National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/) under the accession number of PRJNA1325289.
Ethics statement
All animal procedures in this study strictly adhered to the institutional animal care standards of Zhejiang Mariculture Research Institute, which operates under standing approval from its Animal Ethics Committee for aquaculture research complying with the Chinese standard Guidelines for Ethical Review of Laboratory Animal Welfare (GB/T 35892-2018). As per institutional policy, individual project identifiers are not issued for studies involving established protocols meeting predefined ethical criteria. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YH: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AL: Data curation, Formal analysis, Writing – review & editing. XS: Investigation, Project administration, Writing – review & editing. JZ: Methodology, Project administration, Writing – review & editing. JC: Funding acquisition, Writing – review & editing. JM: Data curation, Software, Writing – review & editing. RL: Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the “Rural Revitalization Nine Solutions” Science and Technology Collaboration Program of Zhejiang Province (2025SNJF095), the Wenzhou Science and Technology Commissioner Special Project (X2023091), the Wenzhou Basic Research Program (N20240015), Central Guidance on Local Science and Technology Development Fund of Zhejiang Province (2023ZY1056), the Zhejiang Provincial Basic Public Welfare Research Program (LTGC23B050007) and Dongtou District Major Projects (N2023Y04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We used Generative AI to discover relevant publications and check the English grammar.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aghababaei F. and Hadidi M. (2023). Recent advances in potential health benefits of quercetin. Pharmaceuticals. 16, 1020. doi: 10.3390/ph16071020
Armobin K., Ahmadifar E., Adineh H., Samani M. N., Kalhor N., Yilmaz S., et al. (2023). Quercetin Application for Common Carp (Cyprinus carpio): I. Effects on Growth Performance, Humoral Immunity, Antioxidant Status, Immune-Related Genes, and Resistance against Heat Stress. Aquaculture Nutr. 2023, 1–10. doi: 10.1155/2023/1168262
Association of Official Analytical Chemists (2005). Official methods of official analytical chemists international. 16th ed (Arlington, Virginia: Association of Official Analytical Chemists).
Astari B., Budiardi T., Ismi S., Effendi I., and Hadiroseyani Y. (2022). Increasing the stocking density of grouper nurseries for aquabusiness efficiency in recirculating aquaculture system (RAS) with bioremediation. HAYATI J. Biosci. 30, 198–206. doi: 10.4308/hjb.30.2.198-206
Awad E., Austin D., and Lyndon A. R. (2013). Effect of black cumin seed oil (Nigella sativa) and nettle extract (Quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture. 388, 193–197. doi: 10.1016/j.aquaculture.2013.01.008
Baker M. E. (1998). Flavonoids as hormones: a perspective from an analysis of molecular fossils. Oxyg. Transp. Tissue XXXI 439, 249–267. doi: 10.1007/978-1-4615-5335-9_18
Bakry K. A., Nasr M., Al-Amgad Z., Kondos E., Kondos M. K. N., Mehanny P. E., et al. (2024). Resistance of Nile tilapia fed with Padina boergesenii extract to Pseudomonas putida infection. BMC Veterinary Res. 20, 281. doi: 10.1186/s12917-024-04115-7
Banerjee G. and Ray A. K. (2016). Bacterial symbiosis in the fish gut and its role in health and metabolism. Symbiosis. 72, 1–11. doi: 10.1007/s13199-016-0441-8
Bolger A. M., Lohse M., and Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Boots A. W., Haenen G. R., and Bast A. (2008). Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 585, 325–337. doi: 10.1016/j.ejphar.2008.03.008
Bozzi D., Rasmussen J. A., Carøe C., Sveier H., Nordøy K., Gilbert M. T. P., et al. (2021). Salmon gut microbiota correlates with disease infection status: potential for monitoring health in farmed animals. Anim. Microbiome. 3, 30. doi: 10.1186/s42523-021-00096-2
Business Research Insights (2025). Grouper Market, By type (Black Grouper and Brown Grouper), By Application (Seafood Processing Plant and Dinning Room and Others), and Regional Insights and Forecast to 2033. Available online at: https://www.businessresearchinsights.com/market-reports/grouper-market-122112 (Accessed June 15, 2025).
Cheng Z., Jin X., Chen S., Wang N., and Wang Q. (2023). Effects of injecting L-Arginine on immune responses and intestinal structure of the hybrid grouper (Epinephelus fuscoguttatus ♀× Epinephelus lanceolatus ♂). Aquaculture Rep. 33, 101831. doi: 10.1016/j.aqrep.2023.101831
Dong P. S., Chen Y. B., Wei Y. J., Zhao X. Y., Wang T., Jiang S., et al. (2025). Dix-seq: an integrated pipeline for fast amplicon data analysis. Innovation Life. 3, 100120. doi: 10.59717/J.Xinn-Life.2024.100120
Dong Y., Hou Q., Lei J., Wolf P. G., Ayansola H., and Zhang B. (2020). Quercetin alleviates intestinal oxidative damage induced by H2O2 via modulation of GSH: in vitro screening and in vivo evaluation in a colitis model of mice. ACS omega. 5, 8334–8346. doi: 10.1021/acsomega.0c00804
Dong Y. Z., Xia T., Lin J. B., Wang L., Song K., and Zhang C. X. (2021). Quercetin attenuates high-fat diet-induced excessive fat deposition of spotted seabass (Lateolabrax maculatus) through the regulatory for mitochondria and endoplasmic reticulum. Front. Mar. Science. 8. doi: 10.3389/fmars.2021.746811
Dunlap P. V., Jiemjit A., Ast J. C., Pearce M. M., Marques R. R., and Lavilla-Pitogo C. R. (2004). Genomic polymorphism in symbiotic populations of Photobacterium leiognathi. Environ. Microbiol. 6, 145–158. doi: 10.1046/j.1462-2920.2003.00548.x
Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Edgar R. C. (2016a). UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. BioRxiv. 081257. doi: 10.1101/081257
Edgar R. C. (2016b). SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. BioRxiv., 074161. doi: 10.1101/074161
Eichmiller J. J., Hamilton M. J., Staley C., Sadowsky M. J., and Sorensen P. W. (2016). Environment shapes the fecal microbiome of invasive carp species. Microbiome. 4, 1–13. doi: 10.1186/s40168-016-0190-1
Fan Z., Ke X., Jiang L., Zhang Z., Yi M., Liu Z., et al. (2024). Genomic and biochemical analysis reveals fermented product of a putative novel Romboutsia species involves the glycometabolism of tilapia. Aquaculture. 581, 740483. doi: 10.1016/j.aquaculture.2023.740483
FishBase (2025). Epinephelus akaara (Temminck &Schlegel 1842) (Hong Kong grouper). Available online at: https://www.fishbase.se/summary/Epinephelus-akaara.
Food and Agriculture Organization (2022). Understanding antimicrobial resistance in aquaculture. Available online at: https://openknowledge.fao.org/items/85f0437d-e3a5-4a3d-9d55-991b5273131b (Accessed July 2, 2025).
Food and Agriculture Organization (2025). Chinese fish price report 2025 issue 2. Available online at: https://openknowledge.fao.org/items/50510388-b5df-493f-aed9-851f58c15398 (Accessed July 2, 2025).
Ghareeb M. A., Zayan A. Z., Shari F. H., and Sayed A. M. (2024). “Unveiling the potential of quercetin: chemistry, health benefits, toxicity, and Cutting-Edge advances,” in Quercetin - effects on human health. Ed. Osredkar J. (IntechOpen). doi: 10.5772/intechopen.1005344
Givens C., Ransom B., Bano N., and Hollibaugh J. (2015). Comparison of the gut microbiomes of 12 bony fish and 3 shark species. Mar. Ecol. Prog. Series. 518, 209–223. doi: 10.3354/meps11034
Gouife M., Chen S., Huang K., Nawaz M., Jin S., Ma R., et al. (2022). Photobacterium damselae subsp. damselae in mariculture. Aquaculture Int. 30, 1453–1480. doi: 10.1007/s10499-022-00867-x
Huang R., Zhong T., and Wu H. (2015). Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch. Med. science: AMS. 11, 427. doi: 10.5114/aoms.2015.50975
Kakkar M., Chatterjee P., Chauhan A. S., Grace D., Lindahl J., Beeche A., et al. (2018). Antimicrobial resistance in South East Asia: time to ask the right questions. Global Health Action. 11, 1483637. doi: 10.1080/16549716.2018.1483637
Kandemir K., Tomas M., McClements D. J., and Capanoglu E. (2022). Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technology. 119, 192–200. doi: 10.1016/j.tifs.2021.11.032
Kanika N. H., Liaqat N., Chen H., Ke J., Lu G., Wang J., et al. (2025). Fish gut microbiome and its application in aquaculture and biological conservation. Front. Microbiol. 15. doi: 10.3389/fmicb.2024
Khan T., Fatima M., Shah S. Z. H., Khan N., Maryam N., Ali W., et al. (2023). Quercetin supplementation in the diet of Labeo rohita: Effects on growth, proximate composition, antioxidative indices and immunity. Anim. Feed Sci. Technology. 303, 115699. doi: 10.1016/j.anifeedsci.2023.115699
Kim P. S., Shin N., Lee J., Kim M., Whon T. W., Hyun D., et al. (2021). Host habitat is the major determinant of the gut microbiome of fish. Microbiome. 9, 166. doi: 10.1186/s40168-021-01113-x
Kõljalg U., Larsson K. H., Abarenkov K., Nilsson R. H., Alexander I. J., Eberhardt U., et al. (2005). UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New phytologist. 166 (3), 1063–1068. doi: 10.1111/j.1469-8137.2005.01376.x
Kong Y., Tian J., Niu X., Li M., Kong Y., Li R., et al. (2022). Effects of dietary quercetin on growth, antioxidant capacity, immune response and immune-related gene expression in snakehead fish, Channa argus. Aquaculture Rep. 26, 101314. doi: 10.1016/j.aqrep.2022.101314
Li M. Y., Zhu X. M., Niu X. T., Chen X. M., Tian J. X., Kong Y. D., et al. (2019). Effects of dietary Allium mongolicum Regel polysaccharide on growth, lipopolysaccharide-induced antioxidant responses and immune responses in Channa argus. Mol. Biol. Rep. 46, 2221–2230. doi: 10.1007/s11033-019-04677-y
Li M., Zhu X., Tian J., Liu M., and Wang G. (2019). Dietary flavonoids from Allium mongolicum Regel promotes growth, improves immune, antioxidant status, immune-related signaling molecules and disease resistance in juvenile northern snakehead fish (Channa argus). Aquaculture. 501, 473–481. doi: 10.1016/j.aquaculture.2018.12.011
Loh J. Y., Chan H. K., Yam H. C., In L. L. A., and Lim C. S. Y. (2020). An overview of the immunomodulatory effects exerted by probiotics and prebiotics in grouper fish. Aquaculture Int. 28, 729–750. doi: 10.1007/s10499-019-00491-2
Luo J., Amenyogbe E., Fu W. J., Huang J. S., and Chen G. (2022). Hepatic transcriptome profiles reveal the hepatoprotective effects of dietary quercetin and sodium quercetin-5′-sulfonates supplementation in hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus polyphekadion♂). Aquaculture. 560, 738483. doi: 10.1016/j.aquaculture.2022.738483
Luthman O., Robb D. H., Henriksson P. J., Jørgensen P. S., and Troell M. (2024). Global overview of national regulations for antibiotic use in aquaculture production. Aquaculture Int. 32, 9253–9270. doi: 10.1007/s10499-024-01614-0
Luu Q. H., Nguyen T. B. T., Nguyen T. L. A., Thuy T., DO T., Dao T. H. T., et al. (2021). Antibiotics use in fish and shrimp farms in Vietnam. Aquaculture Rep. 20, 100711. doi: 10.1016/j.aqrep.2021.100711
Ma J. Z., Ren P., Chen S., Yan M. C., Chen W. D., Xie S. W., et al. (2025). Effects of dietary supplementation of Bacillus subtilison growth, immunity and intestinal microbiota of red spotted grouper Epinephelus akaara. Fisheries Sci. Technol. Information. 52, 42–48. doi: 10.16446/j.fsti.20231000111
Market Report Analytics (2025). Grouper 2025–2033 analysis: trends, competitor dynamics, and growth opportunities. Available online at: https://www.marketreportanalytics.com/reports/grouper-112824 (Accessed July 2, 2025).
Marmelo I., Dias M., Grade A., Pousão-Ferreira P., Diniz M. S., Marques A., et al. (2024). Immunomodulatory and antioxidant effects of functional aquafeeds biofortified with whole Laminaria digitata in juvenile gilthead seabream (Sparus aurata). Front. Mar. Science. 11. doi: 10.3389/fmars.2024.1325244
Ming J., Chen J., Zheng F., Wang T., Du Y., Wang J., et al. (2025). Dietary quercetin improves growth performance and modulates non-specific immunity, antioxidant capacity, and lipid metabolism via NF-κB, Nrf2, and AMPK signaling pathways in black carp (Mylopharyngodon piceus) fed high-fat diets. Aquaculture Rep. 43, 102909. doi: 10.1016/j.aqrep.2025.102909
Morales-Lange B., Del Mar Ortega-Villaizan M., Rocha S. D. C., Montero R., and Øverland M. (2025). Chrono-immunonutrition in aquaculture towards robust and resilient fish. Front. Immunol. 15, 1547738. doi: 10.3389/fimmu.2024.1547738
Pan E., Feng H., Yang Z., Xin Y., Ji X., Ping K., et al. (2024). Quercetin dietary supplementation protects against difenoconazole-induced carp spleen inflammatory damage via regulating ROS/NF-κB/NLRP3 inflammasome axis. Aquaculture. 579, 740162. doi: 10.1016/j.aquaculture
Peixoto D., Martos-Sitcha J. A., Costas B., Azeredo R., and Mancera J. M. (2024). Tryptophan-supplemented diet modulates the metabolic response of European seabass (Dicentrarchus labrax) juveniles reared under space-confined conditions and submitted to acute inflammation. Fish Physiol. Biochem. 51, 1–14. doi: 10.1007/s10695-024-01427-1
Pérez-Gómez O., Rohra-Benítez S., Domínguez-Maqueda M., Cerezo I. M., Galafat A., Martínez-Manzanares E., et al. (2025). Dietary Administration of Postbiotics from Vibrio proteolyticus DCF12.2 Enhanced Intestinal Integrity, Microbiota, and Immune Response in Juvenile Gilthead Seabream (Sparus aurata). Animals. 15, 1982. doi: 10.3390/ani15131982
Qi X., Zhang Y., Zhang Y., Luo F., Song K., Wang G., et al. (2023). Vitamin B12 produced by Cetobacterium somerae improves host resistance against pathogen infection through strengthening the interactions within gut microbiota. Microbiome. 11, 135. doi: 10.1186/s40168-023-01574-2
Rimmer M. A. and Glamuzina B. (2017). A review of grouper (Family Serranidae: Subfamily Epinephelinae) aquaculture from a sustainability science perspective. Rev. Aquacult. 11, 58–87. doi: 10.1111/raq.12226
Rocha S. D. C., Valenzuela C. A., and Morales-Lange B. (2024). Immunonutrition—Contributing to the future of sustainable aquaculture by supporting animal performance, health and welfare. Animals. 14, 2275. doi: 10.3390/ani14152275
Sanches-Fernandes G. M. M., Sá-Correia I., and Costa R. (2022). Vibriosis Outbreaks in Aquaculture: Addressing environmental and public health concerns and preventive therapies using Gilthead Seabream Farming as a model system. Front. Microbiol. 13, 904815. doi: 10.3389/fmicb.2022.904815
Schar D., Klein E. Y., Laxminarayan R., Gilbert M., and Van Boeckel T. P. (2020). Global trends in antimicrobial use in aquaculture. Sci. Rep. 10, 21878. doi: 10.1038/s41598-020-78849-3
Segaran T. C., Lah R. A., Handayani K. S., Fahrurrozi F., Wang P., Gao H., et al. (2025). A global Analysis of Groupers literature. Thalassas an Int. J. Mar. Sci. 41, 77. doi: 10.1007/s41208-025-00827-1
Shen J., Liu H., Tan B., Dong X., Yang Q., Chi S., et al. (2020). Effects of replacement of fishmeal with cottonseed protein concentrate on the growth, intestinal microflora, haematological and antioxidant indices of juvenile golden pompano (Trachinotus ovatus). Aquaculture Nutr. 26, 1119–1130. doi: 10.1111/anu.13069
Shin H. S., Yoo J. H., Min T. S., Lee J., and Choi C. Y. (2010). Effect of quercetin on the activity and mRNA expression of antioxidant enzymes and physiological responses in olive flounder (Paralichthys olivaceus) exposed to cadmium. Asian-Australasian J. Anim. Sci. 23, 742–749. doi: 10.5713/ajas.2010.10006
Talwar C., Nagar S., Lal R., and Negi R. K. (2018). Fish Gut Microbiome: Current approaches and future Perspectives. Indian J. Microbiol. 58 (4), 397–414. doi: 10.1007/s12088-018-0760-y
Thiang E. L., Lee C. W., Takada H., Seki K., Takei A., Suzuki S., et al. (2021). Antibiotic residues from aquaculture farms and their ecological risks in Southeast Asia: a case study from Malaysia. Ecosystem Health Sustainability. 7, 1926337. doi: 10.1080/20964129.2021.1926337
Tolas I., Zhou Z., Zhang Z., Teame T., Olsen R. E., Ringø E., et al. (2025). A fishy gut feeling – current knowledge on gut microbiota in teleosts. Front. Mar. Sci. 11, 1495373. doi: 10.3389/fmars.2024.1495373
Urbanczyk H., Ast J. C., and Dunlap P. V. (2011). Phylogeny, genomics, and symbiosis of Photobacterium. FEMS Microbiol. Rev. 35, 324–342. doi: 10.1111/j.1574-6976.2010.00250.x
Wang M., Tang C., Zhang Z., Fan Z., Jiang L., Liu Z., et al. (2025). Effect of the gut core microbiota Cetobacterium on the growth, physiology, and nutritional metabolism of Nile tilapia (Oreochromis niloticus). Aquaculture Rep. 40, 102583. doi: 10.1016/j.aqrep.2024.102583
Wang A., Zhang Z., Ding Q., Yang Y., Bindelle J., Ran C., et al. (2021). Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes. 13, 1–15. doi: 10.1080/19490976.2021.1900996
Wang J. H., Zhang C. N., Zhang J. L., Xie J., Yang L., Xing Y. F., et al. (2020). The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 46, 759–770. doi: 10.1007/s10695-019-00750-2
Xie M. X., Hao Q., Olsen R. E., Ringø E., Yang Y., Zhang Z., et al. (2022). Growth performance, hepatic enzymes, and gut health status of common carp (Cyprinus carpio) in response to dietary Cetobacterium somerae fermentation product. Aquaculture Rep. 23, 101046. doi: 10.1016/j.aqrep.2022.101046
Xu Z., Li X., Yang H., Liang G., Gao B., and Leng X. (2019). Dietary quercetin improved the growth, antioxidation, and flesh quality of grass carp (Ctenopharyngodon idella). J. World Aquaculture Soc. 50, 1182–1195. doi: 10.1111/jwas.12663
Xu N., Tan G., Wang H., and Gai X. (2016). Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Of Soil Biol. 74, 1–8. doi: 10.1016/J.Ejsobi.2016.02.004
Yang D., Wang T., Long M., and Li P. (2020). Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longevity. 2020, 1–13. doi: 10.1155/2020/8825387
Yau S. K. (2015). Selection and characterisation of tropical microalgae with high lipid content for enhancement of leucocytes viability in brown-marbled grouper, Epinephelus fuscoguttatus (Forsskal 1775) Master’s thesis (Universiti Putra Malaysia. Malaysian Theses Online). Available online at: http://myto.upm.edu.my/find/Record/my-upm-ir.71203/.
Zhai S. W. and Liu S. L. (2013). Effects of dietary quercetin on growth performance, serum lipids level and body composition of tilapia (Oreochromis niloticus). Ital. J. Anim. Science. 12, e85. doi: 10.4081/ijas.2013.e85
Zhai S. W. and Liu S. L. (2014). Effects of dietary quercetin on the growth performance, digestive enzymes and antioxidant potential in the hepatopancreas of tilapia (Oreochromis niloticus). Israeli J. Aquaculture-Bamidgeh. 66, 1038. doi: 10.46989/001c.20770
Zhang M., Jiang H., Wang S., Shi G., and Li M. (2023). Effects of dietary cellulose supplementation on the intestinal health and ammonia tolerance in juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture Rep. 28, 101429. doi: 10.1016/j.aqrep.2022.101429
Zhang B., Yang H., Cai G., Nie Q., and Sun Y. (2024). The interactions between the host immunity and intestinal microorganisms in fish. Appl. Microbiol. Biotechnol. 108, 30. doi: 10.1007/s00253-023-12934-1
Zhou W., Xie M., Xie Y. D., Liang H., Li M., Ran C., et al. (2022). Effect of dietary supplementation of cetobacterium somerae XMX-1 fermentation product on gut and liver health and resistance against bacterial infection of the genetically improved farmed tilapia (GIFT, oreochromis niloticus). Fish Shellfish Immunol. 124, 332–342. doi: 10.1016/J.Fsi.2022.04.019
Keywords: quercetin, Epinephelus akaara, aquaculture immunonutrition, growth performance, antioxidant capacity, lipid level, intestinal microbiome
Citation: Hu Y, Li A, Shao X, Zhu J, Cai J, Ma J and Lu R (2025) Assessing the risks of dietary quercetin supplementation in red-spotted grouper (Epinephelus akaara): effects on growth performance, antioxidant capacity, lipid level, and intestinal microbiome. Front. Mar. Sci. 12:1678527. doi: 10.3389/fmars.2025.1678527
Received: 02 August 2025; Accepted: 27 August 2025;
Published: 25 September 2025.
Edited by:
Pengsheng Dong, Henan Agricultural University, ChinaReviewed by:
Guangxu Liu, Zhejiang University, ChinaYuwen Dong, University of Pennsylvania, United States
Shifeng Wang, Hainan University, China
Laijin Su, Wenzhou University, China
Copyright © 2025 Hu, Li, Shao, Zhu, Cai, Ma and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Hu, eXVhbmh1MjAwOWdyZWF0QDE2My5jb20=
 Yuan Hu
Yuan Hu An Li1,2,3
An Li1,2,3