Abstract
Despite the ubiquitous distribution of nanoplastics (NPs) in global aquatic ecosystems, microbial adaptive strategies during the post-exposure period remain largely unexplored. In this study, Microcystin aeruginosa treated with 5 and 50 mg/L polystyrene (PS) for 15 days and then were transferred to PS-free medium for 15 days to study toxicology and post-exposure effects. The results showed that 5 and 50 mg/L PS inhibited algal growth, with inhibition rates of 6.82% and 9.34% at the end of exposure, respectively, while M. aeruginosa resumed growth on the fourth day of the post-exposure period. In addition, PS enhanced microcystins (MCs) biosynthesis and release in a dose-dependent manner during exposure, while phased variations were observed in MCs production and release during recovery. Transcriptome analysis revealed that 5 mg/L PS inhibited cell growth by disrupting cellular structures, inducing oxidative stress, altering lipid metabolism, and suppressing protein synthesis. These effects were largely reversible during the recovery phase, except for irreversible damage to the algal cell membrane. KEGG pathway analysis identified significant suppression of carbohydrate and energy metabolism during exposure, with subsequent reactivation during post-exposure. These findings suggest that M. aeruginosa may mitigate PS-induced oxidative stress through glucose accumulation and reduced energy expenditure.
1 Introduction
The widespread production and extensive utilization of plastics present significant risks to ecosystem integrity (Zhang et al., 2022). Inadequate plastics waste management practices facilitated the accumulation of plastic waste in the environment, where abiotic and biotic processes progressively degrade these polymers into micro/nano plastics (MNPs) (Evangeliou et al., 2020). Once introduced into aquatic environments, MNPs could accumulate persistently in surface freshwater environment, and interact with organisms across trophic levels through adsorption, absorption, and ingestion, thereby posing greater risks to higher trophic level organisms (Wang et al., 2018). It is estimated that the number of nanoplastics (NPs, <100 nm) is about 1014 times greater than microplastics (MPs, <5 mm) in aquatic environments (Besseling et al., 2018). Additionally, given their reduced size, greater surface area and stronger adsorption capacity, the negative impact of NPs on aquatic life is likely to be more pronounced than that of MPs (Holloczki and Gehrke, 2019).
Microalgae are essential primary producers, playing a vital role in maintaining ecological balance as part of the aquatic trophic webs (Prata et al., 2019). Consequently, it is essential to examine the toxicological effects and underlying mechanisms of NPs on microalgae. The impacts of MNPs pollution have been extensively studied in microalgae such as cyanobacteria, green algae, diatoms, and euglenoids. Most studies indicated that MNPs can exert negative effects on microalgae, including photosynthetic inhibition, lipid peroxidation, structural disruption of cell membranes, and impaired nutrient uptake and gas exchange (Bhattacharya et al., 2010). However, contrasting findings have been reported, suggesting that MNPs may act as both biofilm substrates and potential carbon sources that could enhance (Li et al., 2023). Therefore, the impacts of MNPs on microalgae involve multiple possibilities, necessitating further analysis and verification. More importantly, previous researches have primarily centered on the toxicological effects of continuous MNPs exposure on microalgae, while research concerning the post-exposure effects of MNPs on microalgae remains scarce.
In natural environments, certain environmental factors, such as wind and water, can influence the transport and distribution of plastic debris, leading to heterogeneous distribution of MNPs (Debrot et al., 2013; Thompson et al., 2024). Consequently, alternating occurrences of high-concentration, low-concentration, or even plastic-free zones may emerge within the same area (Detree and Gallardo-Escarate, 2018). The researchers investigated the effects of herbicide exposure and post-exposure on microalgae, demonstrating that S-metolachlor inhibited the growth of Scenedesmus vacuolatus during exposure, and algal density remaining below control levels in the post-exposure period (Copin et al., 2016). In contrast, Pseudokirchneriella subcapitata fully recovered density in post-exposure tests following pretilachlor exposure (Nagai et al., 2011). This indicated that the effects observed at the end of MNPs exposure cannot reliably predict the overall impact of MNPs; hence, post-exposure effects constitute a pivotal determinant in the ecological persistence of populations. Moreover, the reversibility of organismal responses to pollutants is influenced by both contaminant concentration and type. Besides, most studies have demonstrated that NPs promote algal growth during later exposure stages due to the intrinsic adaptation of algae (Yang et al., 2021a). Consequently, systematic studies are required to delineate the post-exposure effects of NPs on microalgae and elucidate the fundamental molecular mechanisms involved.
Microcystis aeruginosa (M. aeruginosa), a common bloom-forming cyanobacterium, serves as a key model for assessing aquatic toxicity and could produce and release microcystins (MCs), which can bioaccumulate in eutrophic environments and subsequently pose a threat to human health (Omori et al., 2019). Recent studies have found that continuous exposure to MNPs promotes the synthesis and release of MCs by M. aeruginosa (Feng et al., 2020; Zheng et al., 2023). However, research on MCs during the post-exposure period of MNPs remains limited. Polystyrene (PS) widely detected in freshwater systems, are a major component of electronic waste (Yang et al., 2021a). Although our previous work has described the physiological effects of high-concentration PS exposure and post-exposure on microalgae (unpublished data), the synthesis and release of MCs by microalgae under PS exposure, as well as the ecological impacts of environmentally relevant low-concentration PS, remain poorly understood. Therefore, this study aimed to investigate the effects of PS on M. aeruginosa during exposure and post-exposure periods by: (1) evaluating growth responses; (2) quantifying MCs synthesis and release; and (3) identifying transcriptomic signatures associated with low-concentration PS stress through RNA-seq analysis.
2 Materials and methods
2.1 Experimental materials
M. aeruginosa FACHB-905 was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China). The strain was cultivated in BG-11 medium under controlled conditions: light intensity of 2000 lux, a 12 h:12 h light-dark cycle, and a temperature of 25°C. All experimental instruments were sterilized by autoclaving at 121°C for 20 min.
PS (80 nm) were purchased from Tianjin BaseLine Chromtech Research Centre (Tianjin, China). The PS suspension was prepared by dispersing monodispersed particles in 10 mL of ultrapure water containing 0.05% (w/v) sodium azide as a preservative. The final suspension concentration was adjusted to 2.5% w/v (1.32 × 1014 particles/mL), followed by 10 min ultrasonication to ensure colloidal homogeneity before experimental use. The properties of PS have been examined in BG-11 medium, and the average diameter of PS is 81.69 nm, the polydispersion index is 0.069, and the zeta potential is -23.2 Mv (Feng et al., 2020).
2.2 Experimental setup
M. aeruginosa cells in logarithmic growth phase were harvested by centrifugation (6000×g, 10 min, 4°C). After three washes with sterile distilled water, cells were transferred aseptically to 600 mL BG-11 medium to maintain an initial algal density of 1.76×106 cells/mL. The PS was added to the cultures to achieve final concentrations of 0 (control), 5, and 50 mg/L, respectively. PS concentrations were established based on environmentally relevant levels (0.1–50 mg/L) reported for surface waters (Sendra et al., 2019). Besides, our previous study demonstrated that 80 nm PS particles inhibited the growth of M. aeruginosa at concentrations of 5, 10, 50, and 250 mg/L, with the inhibitory effect becoming more pronounced at higher concentrations (Supplementary Figure S1). Furthermore, to investigate the recovery effects under varying inhibition levels within environmentally relevant concentration ranges, this study selected 5 mg/L and 50 mg/L for experimental evaluation. Each test concentration was replicated three times, and all procedures were performed under sterile conditions to prevent microbial contamination. All treatment groups were subsequently incubated in a light-controlled growth chamber for 15 days. Following PS exposure, the algal cells were collected via centrifugation, rinsed with sterile distilled water, and subsequently transferred to 600 mL of PS-free BG-11 medium for an additional 15-day cultivation period. Samples were taken aseptically on days 7 and 15 during the 15 days of algal exposure to PS and on days 4, 7 and 15 during the recovery period to measure biochemical indicators.
2.3 Methods used for measuring biochemical indicators
2.3.1 Extraction and determination of chlorophyll a
Chlorophyll a was quantified using ethanol-based spectrophotometry (Wang et al., 2013) with modifications. Algal cultures were centrifuged and the precipitate was extracted with 95% ethanol overnight at 4°C in the dark. After centrifugation at 4000×g for 5 min, the A665 and A649 of the supernatant were measured (L6S spectrophotometer, Cold Light, China). Chlorophyll a was calculated as: 13.7 × A665-5.76 × A649.
2.3.2 Extraction and quantification of MCs
To investigate the temporal patterns of MCs biosynthesis and secretion in M. aeruginosa, a sequential extraction protocol was implemented. Initially, 2 mL algal culture were subjected to centrifugation (8,000 ×g, 10 min, 4°C) to separate the extracellular fraction. The resulting supernatant was reserved for extracellular MCs (extra-MCs) analysis. For intracellular MCs (itra-MCs) quantification, the pelleted cells were vigorously homogenized in 75% methanol (v/v) using three freeze-thaw cycles (liquid nitrogen/room temperature). Cellular debris was subsequently removed by centrifugation (10,000 ×g, 5 min, 4°C). MCs concentrations were determined via competitive enzyme-linked immunosorbent assay (ELISA) employing a commercial detection system (Jinmei Biotechnology, China).
2.3.3 Transcriptome analysis
Total RNA was extracted from M. aeruginosa cells treated with 5 mg/L PS (at the end of exposure and post-exposure periods) using the mirVana™ miRNA Isolation Kit (Invitrogen, USA). RNA quality was verified by 1% agarose gel electrophoresis and quantified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). For transcriptome profiling, mRNA-Seq libraries were prepared with the NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina (NEB, USA) and RNA was sequenced on an Illumina HiSeq™ platform (150 bp paired-end; Guangdong Magigene Biotechnology Co., Ltd, China). Gene expression levels were normalized using the reads per kilobase per million mapped reads (RPKM). For identification of differentially expressed genes (DEGs), DESeq2 (version 1.36.0) was employed with stringent criteria |log2FC| ≥ 1.0 and P-value < 0.05. The resultant significant DEGs were subsequently analyzed through functional annotation, followed by comprehensive enrichment analyses utilizing both Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
2.3.4 Statistical analysis
Statistical analyses were conducted utilizing SPSS (v24.0, IBM) with one-way ANOVA. All experiments were conducted in triplicate, and data are expressed as the mean ± standard deviation (SD). Statistical significance between groups was defined as p ≤ 0.05. Graphical representations were generated using OriginPro (version 9.0).
3 Results
3.1 Effects of PS-NPs on chlorophyll a content of M. aeruginosa
As shown in Figure 1, on day 7 of 5 mg/L PS exposure period, the Chlorophyll a content of M. aeruginosa was 1.28 mg/L, exhibiting no statistically significant disparity relative to the control (1.30 mg/L). However, by day 15, the chlorophyll a content was significantly inhibited, with an inhibition rate of 6.82%. These results indicated that M. aeruginosa could resist the growth-inhibiting effects of 5 mg/L PS stress during the early exposure stage, but its resistance capacity weakened with prolonged exposure, leading to growth suppression in the later stage of exposure. 50 mg/L PS exposure significantly inhibited the synthesis of chlorophyll a in M. aeruginosa, with inhibition rates of 11.77% and 9.34% on day 7 and day 15 of exposure, respectively. The attenuation of inhibition rates indicates that the microalgae developed certain resistance capabilities against the stress induced by high-concentration PS.
Figure 1
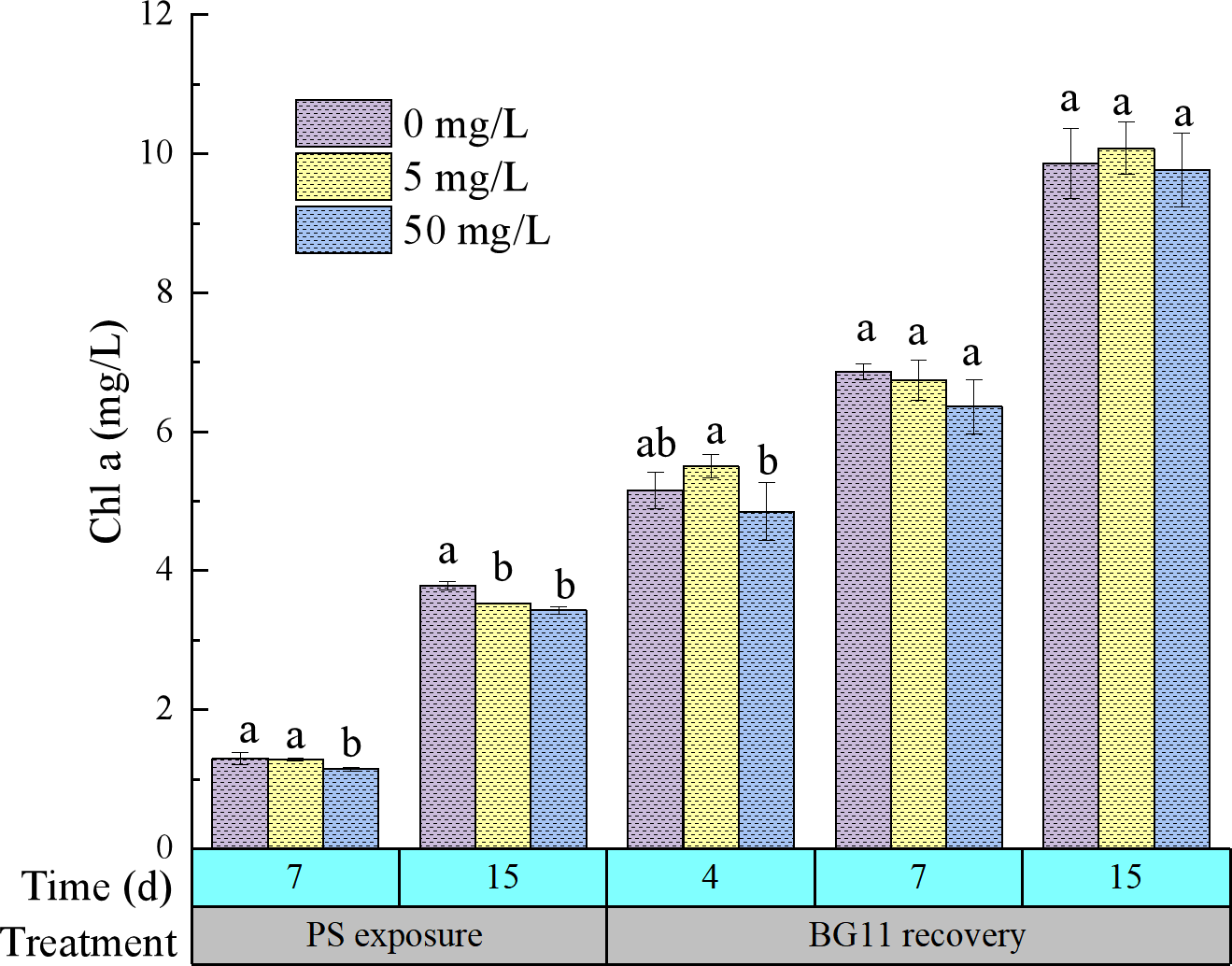
The content change of Chlorophyll a of M. aeruginosa during PS-NPs exposure and BG11 recovery. Vertical bars represent mean ± SD and different letters are significantly different at p ≤ 0.05.
During the recovery phase, chlorophyll a content in M. aeruginosa showed no statistically significant variation between PS-exposed and control. Notably, PS-treated (5 mg/L) algal cultures exhibited slightly elevated chlorophyll a concentration, while the 50 mg/L showed a marginally lower (9.77 mg/L) compared to the control (9.87 mg/L). This indicates that algae can rapidly recover and maintain growth through certain adaptive strategies when PS stress is absent.
3.2 Impact of PS on MCs synthesis and release
To investigate the potential effects of PS on MCs production and secretion in M. aeruginosa, both intra- and extra-MCs concentrations were quantified (Figure 2). Significant increases were observed in both intra-and extra-MCs concentrations following PS exposure. Moreover, the concentrations of intra-, extra-, and total MCs exhibited a significant dose-dependent increase with rising exposure levels. With increasing PS concentration from 5 to 50 mg/L, the content of intra-, extra- and total-MCs increased from 453.67 ± 17.56 to 485.33 ± 12.58, 788.67 ± 32.14 to 998.67 ± 15.27 and 89.36 ± 3.05 to 97.53 ± 0.71 respectively on the 15th day of exposure period.
Figure 2
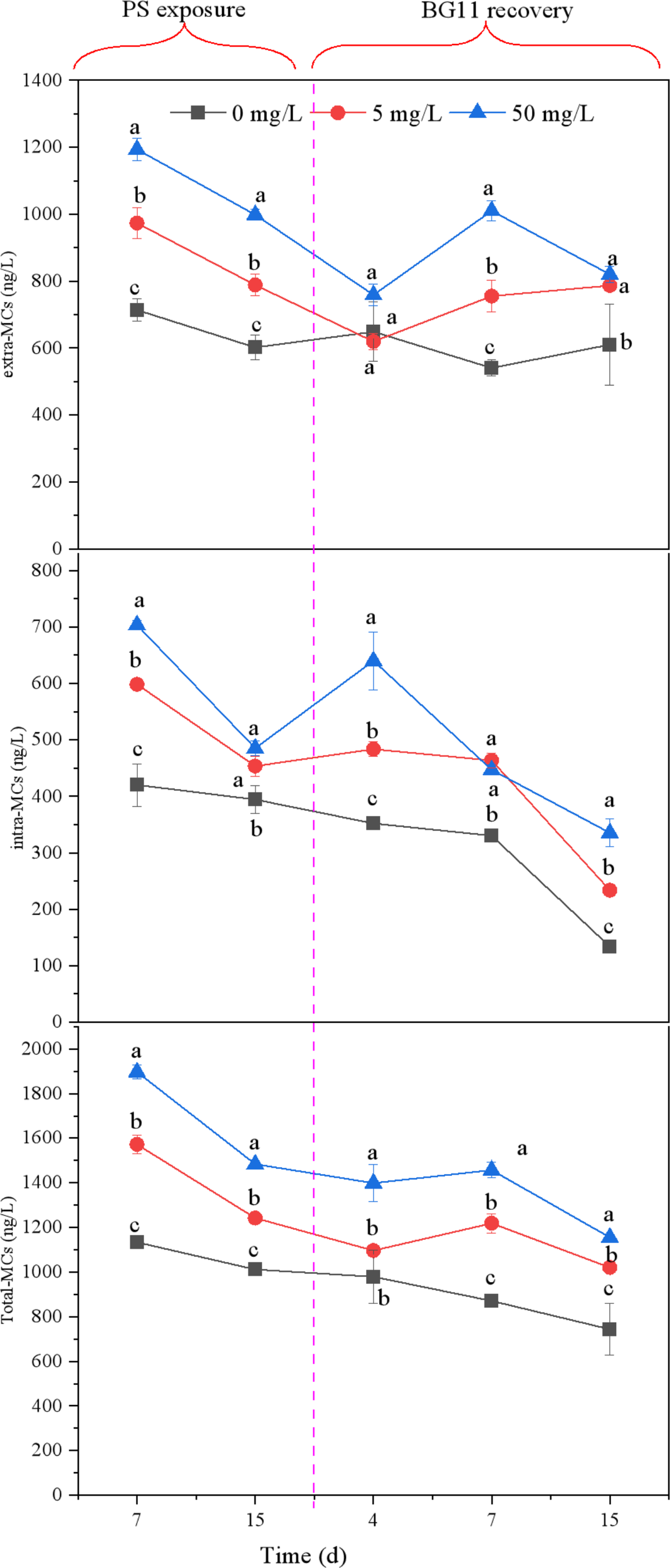
The content of intracellular MCs, extracellular MCs and the total of MCs released from M. aeruginosa both exposed to PS-NPs of different concentrations. Vertical bars represent mean ± SD and different letters are significantly different at p ≤ 0.05.
Statistical analysis revealed that PS exposure did not induce significant alterations in extra-MC concentrations relative to controls on day 4 of the recovery period (Figure 2), which may be explained by the loss of stress response in the extracellular matrix of the NPs-treated groups after transfer of M aeruginosa to clean BG-11 medium, resulting in reduced MCs release. However, the intra-MCs content in the PS group was significantly higher than that in the control group on day 4 of the recovery period, which could be attributed to intracellular stress responses induced by PS internalization in algal cells during exposure, thereby promoting the synthesis of MCs. During the recovery phase, PS-treated specimens exhibited markedly elevated MCs levels both extracellularly and intracellularly compared to control, particularly at the 7- and 15-day. The increased extra-MCs and decreased intra-MCs content on day 7 compared to day 4 indicated that the extracellular stress response was enhanced while the intracellular stress response was attenuated, which could be related to the entry of intracellular PS into the extracellular space.
3.3 Transcriptome analysis
The data obtained for assembly analysis exceeded 6 G raw base, as detailed in Supplementary Table S1. A total of 595 DEGs were identified following exposure to 5 mg/L PS, including 253 upregulated and 342 down regulated genes (Supplementary Figure S2A). In the post-exposure stage, 609 DEGs were significantly altered, with 317 up-regulated and 292 down-regulated genes (Supplementary Figure S2B). In total, 415 genes were observed to be co-differentially expressed between the exposure and post-exposure stages (Supplementary Figure S2C).
The top 20 major GO terms are presented (p ≤ 0.05) both under the exposure and post-exposure phase. GO terms shown that under 5 mg/L PS treatment, the largest number of genetic changes were plasma membrane (GO:0005886) and cytosol (GO:0005829) in cellular component, metal ion binding (GO:0046872) and 4 iron, 4 sulfur cluster binding (GO:0051539) in molecular function, as well as carbohydrate metabolic process (GO:0005975) and protein-chromophore linkage (GO:0018298) in biological process, respectively (Figure 3A). Similarly, GO functional analysis revealed that during the post-exposure period, the most significant genetic changes in microalgae were plasma membrane (GO:0005886) and integral component of membrane (GO:0016021) in cellular components, NADP binding (GO:0050661) and NAD binding (GO:0051287) in molecular functions, as well as photosynthesis (GO:0015979) and protein-chromophore linkage (GO:0018298) in biological processes (Figure 3B).
Figure 3
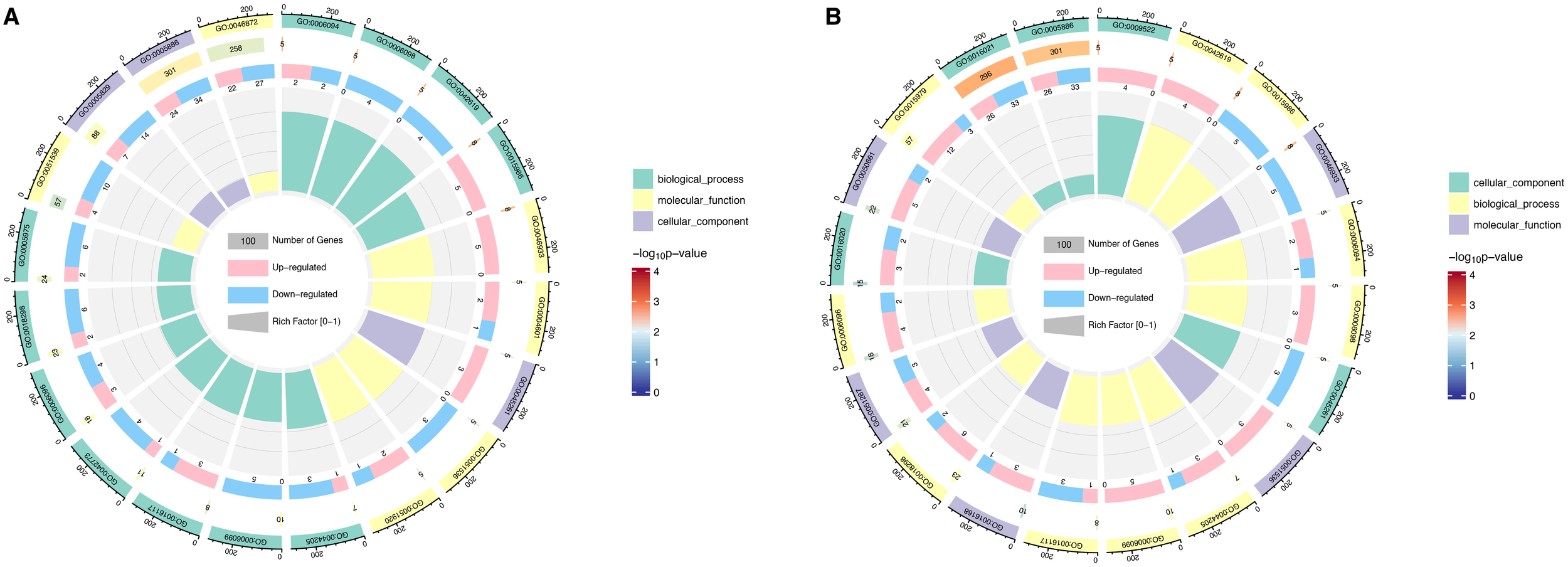
Gene Ontology (GO) term analysis of DEGs pathways during 5 mg/L PS-NPs exposure (A) and post-exposure (B). The first lap indicates top 20 GO term and the number of the genes corresponds to the outer lap. The second lap represents the genes quantity in the genome backdrop and Q index represents the enrichment of the upregulated genes during specific procedure. The third circle represents the ratio of the upregulated genes (dark purple) and downregulated genes (light purple). The fourth circle represents the enrichment element of every GO term.
Furthermore, the 5 mg/L PS exposure and post-exposure GO terms were further compared and analyzed (Figure 4). 5 mg/L PS downregulated plasma membrane term (GO:0005886) during the exposure and post-exposure period, indicating that 5 mg/L caused irreversible disruption to the cell membrane of M. aeruginosa. Besides, the downregulation of cytoplasm (GO:0005737), and cytosol (GO:0005829), suggested that 5 mg/L PS impaired the cellular structure and integrity of M. aeruginosa. Conversely, both cytoplasm and cytosol exhibited significant upregulation during recovery, suggesting that the cellular damage caused by 5 mg/L PS was effectively repaired post-exposure. This restoration was accompanied by a functional improvement in cytoplasmic and cytosolic activities, highlighting the adaptive recovery capacity of the cells following toxic insult. In the biological process, the down regulation of the pentose-phosphate shunt (GO:0006098), glycolytic process (GO:0006096), and the upregulation of gluconeogenesis (GO:0006094), indicated that M. aeruginosa responded to PS stress by accumulating glucose and glycogen intracellularly. During the recovery period, significant upregulation of gluconeogenesis, the pentose-phosphate pathway, and glycolytic processes was observed. Furthermore, the upregulation of peroxidase activity (GO:0004601) and peroxiredoxin activity (GO:0051920) suggested that M. aeruginosa cells experienced oxidative stress under 5 mg/L PS exposure. However, in the post-exposure period, neither peroxidase nor peroxiredoxin activity showed significant alterations, suggesting that the oxidative stress induced by 5 mg/L PS exposure was transient and that microalgae had fully recovered from the stress. Additionally, the upregulation of fatty acid biosynthesis (GO:0006633) concomitant with the downregulation of protein refolding (GO:0042026) suggested that 5 mg/L PS exposure enhanced lipid metabolism while suppressing protein synthesis in M. aeruginosa. Notably, both metabolic pathways exhibiting complete recovery during recovery, demonstrating full restoration of lipid and protein regulation.
Figure 4
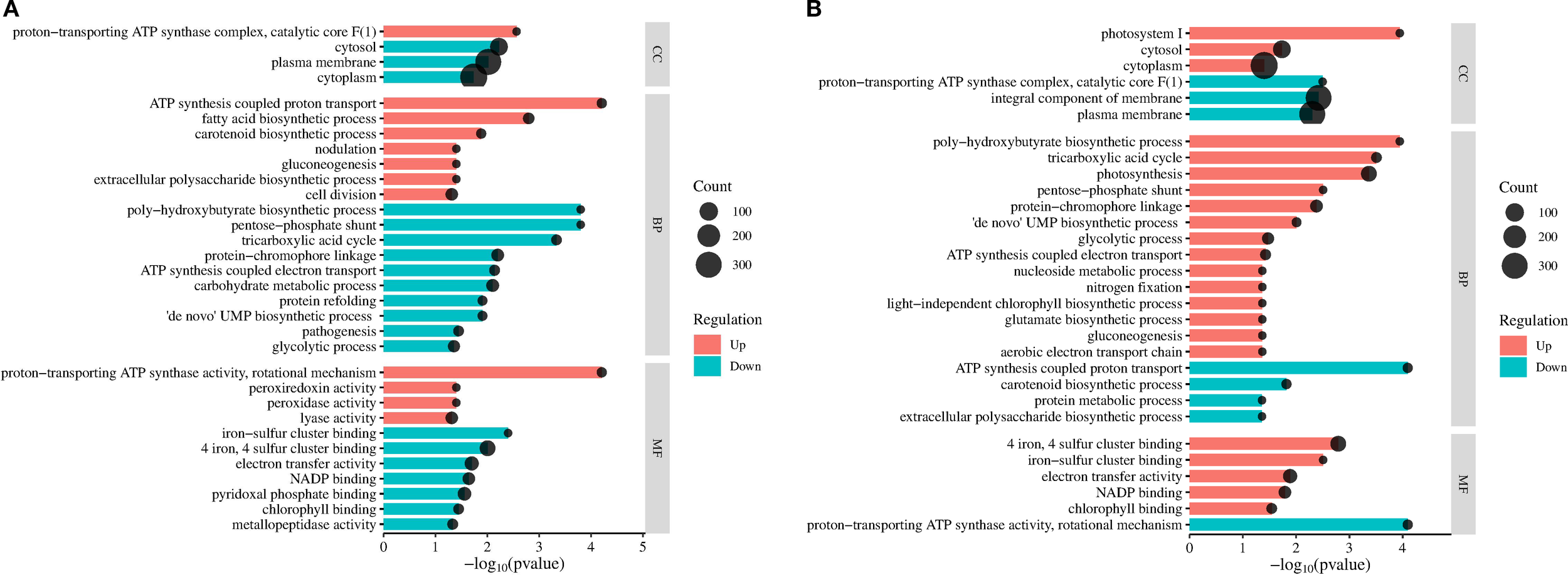
Cluster analysis of significantly enriched GO terms (p<0.05) in M. aeruginosa following 5 mg/L PS exposure (A) and 15-days recovery (B). The dot size indicates the number of DEGs in the corresponding pathway.
Further, to investigate the universal molecular mechanism more comprehensively and clarify the relationship between each KEGG pathway, we performed a diagram analysis. Most of genes enriched pathways were significantly down-regulated, mainly including energy metabolism, carbohydrate metabolism and amino acid metabolism (p < 0.05) (Table 1). Besides, the up regulation of terpenoids and polyketides (ko00906: carotenoid biosynthesis and ko00523: polyketide sugar unit biosynthesis) indicated that 5 mg/L PS promoted the synthesis of MCs, which was consistent with those of phenotypic measurements (Figure 2).
Table 1
| ID | State | Term | Classification_level 1 | Classification_level 2 | |
|---|---|---|---|---|---|
| Exposure | Recovery | ||||
| ko00650 | Down | Up | Butanoate metabolism | Metabolism | Carbohydrate metabolism |
| ko00030 | Down | Up | Pentose phosphate pathway | ||
| ko00010 | Down | Up | Glycolysis/gluconeogenesis | ||
| ko00020 | Down | Up | Citrate cycle (TCA cycle) | ||
| ko00620 | Down | Up | Pyruvate metabolism | ||
| ko00051 | Up | No | Fructose and mannose metabolism | ||
| ko00630 | Down | No | Glyoxylate and dicarboxylate metabolism | ||
| ko00190 | down | up | Oxidative phosphorylation | Energy metabolism | |
| ko00195 | No | Down | Photosynthesis | ||
| ko00720 | Down | Up | Carbon fixation pathways in prokaryotes | ||
| ko00680 | Down | Up | Methane metabolism | ||
| ko00906 | Up | Down | Carotenoid biosynthesis | Metabolism of terpenoids and polyketides | |
| ko00350 | Down | No | Tyrosine metabolism | Amino acid metabolism | |
| ko04217 | Down | Up | Necroptosis | Cellular processes | Cell growth and death |
| ko04922 | Down | No | Glucagon signaling pathway | Organismal systems | Endocrine system |
Comparison of significant KEGG metabolic pathways of the M. aeruginosa transcriptomes between exposure and recovery stages.
The 5 mg/L PS exposure and post-exposure transcriptome data were further compared and analyzed (Supplementary Figure S3; Table 1). Both groups’ KEGG enrichment pathways were predominantly enriched in the carbohydrate and energy metabolism, and most of the corresponding terms were down-regulated during the exposure period but up-regulated during the post-exposure period, indicating that the exposure of 5 mg/L PS promoted most carbohydrate and energy metabolism pathways during the recovery period. In addition, in carbohydrate metabolism, the up-regulated “fructose and mannose metabolism” pathway and the down-regulated “glyoxylate and dicarboxylic acid metabolism” pathway during the exposure period did not exhibit significant variations relative to the control during the recovery period, indicated that both pathways could be restored after 5 mg/L PS exposure. The most significantly enriched pathway in both groups was oxidative phosphorylation. Gene set enrichment analysis (GSEA) was employed to further elucidate the key genes implicated in oxidative phosphorylation and fundamental DEG changes was presented using heat maps. As shown in Figure 5, the key genes related to oxidative phosphorylation in microalgae showed significantly down-regulated during the 5 mg/L PS exposure and significantly up-regulated during the post-exposure period, which could be divided into three categories: NADH dehydrogenase (Ndhd, HoxE, HoxF, and HoxU), succinate dehydrogenase (ShdC and ShdA), and cytochrome c oxidase (CyoA, CyoB, CyoC, CyoE, CydA, and CydB), indicating that these genes were sensitive to environmental changes and exerted substantial impacts on the response of M. aeruginosa to the external environment. The photosynthesis pathway was not different with control during the 5 mg/L PS exposure period but significantly downregulated in the recovery period.
Figure 5
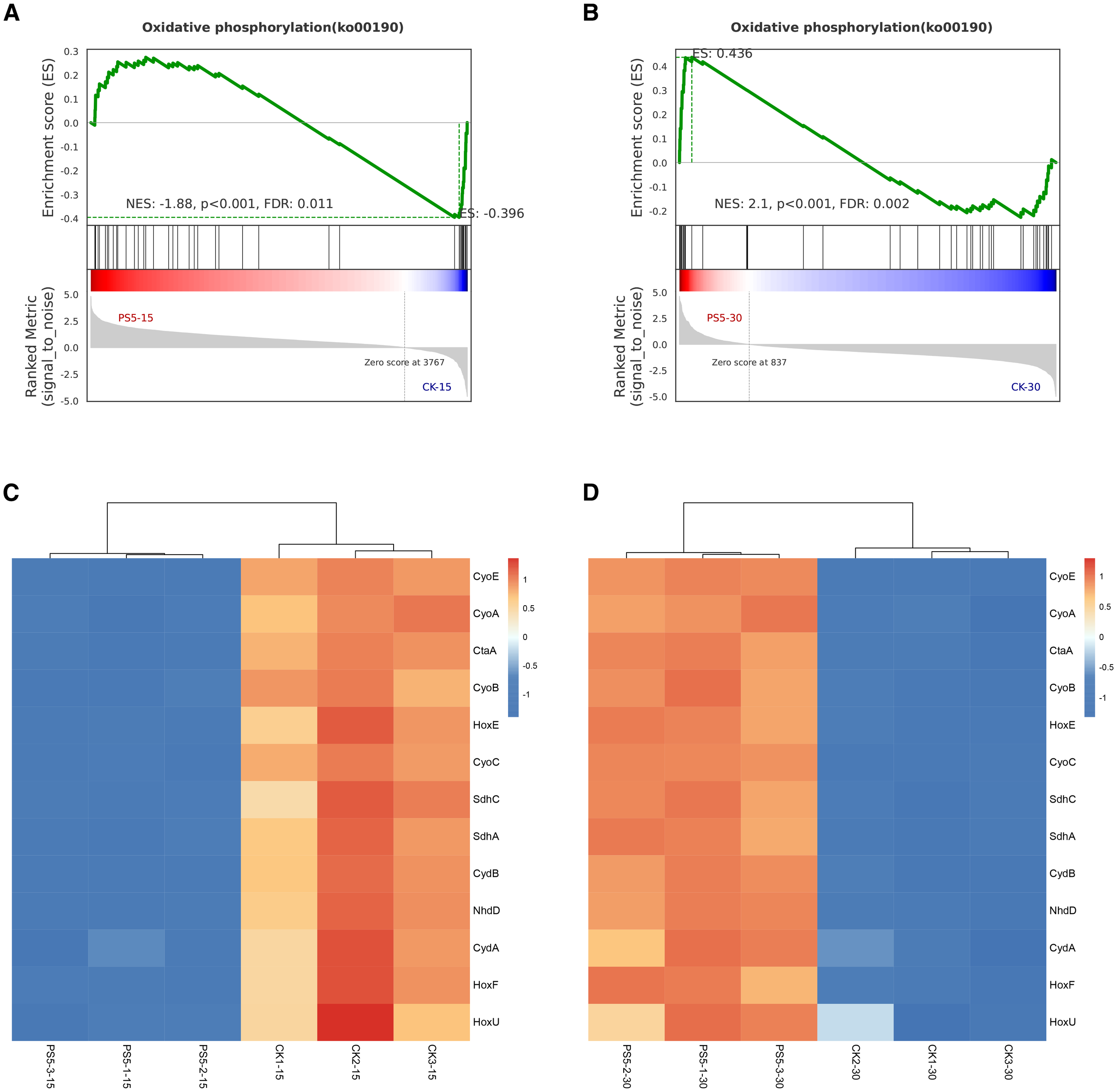
GSEA plot showing normalized enrichment scores (NESs) for oxidative phosphorylation pathway under exposure (A) and recovery state (B), and heat map of key genes participated in oxidative phosphorylation pathway under exposure (C) and post-exposure (D) using RNA-seq data. Transcript enrichment is marked in thermal map: low value for blue and high value for red.
4 Discussion
4.1 Effects of PS exposure and post-exposure on the growth of M. aeruginosa
Recent studies have increasingly investigated the impacts of NPs on microalgae; however, the majority of these studies have been limited to the acute (24 h or 96 h) and chronic (7 d) exposures. This study conducted a 15-day exposure and examined delayed effects after exposure. As the dominant photosynthetic pigment in phytoplankton, chlorophyll a serves as a reliable indicator of algal biomass (Fan et al., 2018). Besides, given that the suspension of NPs interfered with algal number calculation through absorbance detection in our pre-experiment. Therefore, chlorophyll a was used as an M. aeruginosa growth indicator. Based on the changes in chlorophyll a concentration, this study found that 5 mg/L PS initially had no statistically impact on algal growth, but progressively developed significant inhibitory effects, whereas high concentrations PS had a remarkable inhibitory on alga growth (Figure 1). A concentration-dependent inhibitory effect of MPs on chlorophyll a has been previously documented, which confirmed that chlorophyll a concentration of C. pyrenoidos treated with 5 mg/L polypropylene (PP) showed no significant difference from control within 4–5 days, while a significant decrease (p < 0.05) was observed in the 50 mg/L PP-exposed group during the experimental period (Wu et al., 2019). The inhibitory effect on M. aeruginosa growth exhibited a progressive attenuation at 50 mg/L PS concentration in this study (Figure 1). Similarly, Yang et al. (2021a) also reported that the inhibitory effect of PS (80 nm, 20–50 mg·L−1) on the growth of Chlorella pyrenoidosa exhibited a gradually weakening trend. NPs-induced fluctuations in algal growth kinetics are fundamentally governed by the algal cellular repair capacity. Specifically, the self-repair capability of algae is limited and may depend on factors such as pollutant concentration, exposure duration, frequency, and pattern (Buhl et al., 1993). In the early stages of low-concentration PS exposure, algae can resist PS-induced stress, exhibiting no observable growth inhibition. However, with increasing PS exposure time or concentration, algae reproductive capacity, metabolic activity, and detoxification ability decline, ultimately compromising their stress resistance and leading to growth suppression (Yang et al., 2021c).
Post-recovery analysis revealed a modest elevation in chlorophyll a content in 5 mg/L PS -exposed M. aeruginosa relative to unexposed controls (Figure 1). This observed effect likely stems from the enhanced physiological adaptation of algal cells to low-concentration PS during the acclimation period, which facilitated cellular growth during the recovery period (Mao et al., 2018). Besides, throughout the post-exposure period, the chlorophyll a content of microalgae exposed to 50 mg/L PS demonstrated no significant difference compared to the control group, suggesting that the algae possess certain self-repairing capabilities to recover growth from the stressed state. Meanwhile, Yang et al. (2021c) demonstrated that pulsed exposure to PS-NPs induced a higher growth inhibition rate in microalgae compared to continuous exposure, with algal growth recovery observed only under continuous exposure conditions. Thus, NPs exhibit dualistic effects on algal growth, dependent on both exposure duration and concentration. Microalgae demonstrate self-regulatory capacity to mitigate NPs stress at relatively low concentrations, though this adaptive response becomes ineffective under extreme conditions (Rezayian et al., 2019). Preliminary findings suggest a potential connection to detoxification mechanisms wherein NPs adhere to cell surfaces, effectively reducing bioavailable concentrations, thereby enhancing the growth of newly divided cells. Furthermore, in batch culture systems, the relative NPs concentration per cell decreases with increasing algal biomass, which may partially account for the observed temporal adaptation to NPs exposure (Yang et al., 2021b).
Moreover, we observed an inconsistency in chlorophyll a content between biochemical assays and KEGG enrichment analysis (Figure 1; Table 1). This implies that M. aeruginosa employs a multi-layered strategy to adapt to NPs. It is protein activity, not mRNA abundance, that determines gene function. Many stress responses are controlled through translation, post-translational modification, and protein turnover (Sewelam et al., 2014). A likely explanation is that the stressor increased the breakdown of key proteins and chlorophyll a, whereas their genetic mRNA were preserved.
4.2 Effects of PS exposure and post-exposure on MCs production and release of M. aeruginosa
During the exposure experiments, both low (5 mg/L) and high (50 mg/L) concentrations of PS demonstrated significant stimulatory effects on MCs production and release relative to control, with higher PS concentrations leading to greater synthesis and secretion of MCs in this study (Figure 2). Consistent with these findings, multiple investigations have confirmed that NPs can enhance both the synthesis and release of MCs in M. aeruginosa (Feng et al., 2020; Zheng et al., 2023). Generally, extra-MCs concentrations may be associated with intra-MCs released into the surrounding water following cell lysis. In this study, we observed that algal growth was inhibited during PS exposure, indicating an increase in dead algal cells, which may account for the elevated levels of extra- MCs. Previous studies have documented multiple functional roles of MCs in cyanobacterial physiology. Zilliges et al. (2011) provided experimental evidence supporting the protective function of MCs against oxidative stress. This finding is further corroborated by Feng et al. (2020), who observed elevated intra-MCs concentrations in M. aeruginosa under PS-NH2 exposure, suggesting their role in mitigating ROS-mediated cellular damage. Additionally, light availability has been shown to influence MCs production, as demonstrated by Rapala et al. (1997), who reported enhanced toxin synthesis in Anabaena under low-light conditions. In the present study, the observed increase in intra-MCs content may result from the combined effects of oxidative stress and light limitation induced by PS.
No statistically significant disparity in extra-MCs content was observed between NPs-treated and control cohorts at day 4 of recovery in this study (Figure 2), which may be explained by the loss of stress response in the extracellular matrix of the PS-treated groups after transfer of M aeruginosa to clean BG11 medium. However, the PS-treated group exhibited a significantly elevated of intra-MCs content compared to controls by day 4 of recovery, which may be attributed to the cellular stress response triggered by NPs uptake in algal cells during the exposure period (Chen et al., 2020).
The extra-MCs content showed no significant difference between NPs-treated and control groups by day 4 of recovery (Figure 2), which suggested that extracellular stress responses may weaken after transfer to clean BG11 medium. The study by Chen et al. (2020) indicated that 1.0 μm and 2.0 μm PS could be internalized into algal cells, although the underlying mechanisms remain unclear. Given that the cell diameter of M. aeruginosa is approximately 3–5 μm, internalization of particles as large as 1–2 μm seems unlikely without direct evidence. In this study, we hypothesize that the 80 nm PS -NPs may enter the algal cells more readily during the exposure period, potentially inducing the production of MCs in the early recovery phase (day 4). Compared to day 4, day 7 showed increased extra-MCs accompanied by decreased intra-MCs content. We hypothesize that progressive translocation of internalized PS to extracellular compartments may drive this transition, though further ultrastructural studies are needed to verify this mechanism. These findings collectively highlight the temporal complexity of PS-algae interactions, where both exposure history and post-exposure recovery duration critically determine MCs production dynamics. The biphasic stress response (initial intracellular dominance followed by extracellular predominance) observed herein provides new insights into the ecotoxicological trajectories of plastic nanoparticles in phytoplankton systems.
4.3 Analysis of gene expression in M. aeruginosa during exposure and post-exposure
The GO analysis results indicated that microalgae respond to 5 mg/L PS stress by disrupting cellular structures, inducing oxidative stress, accumulating glucose, enhancing lipid metabolism, and inhibiting protein synthesis. Previous studies have demonstrated that NPs can adsorb onto M. aeruginosa cell membranes and induce membrane rupture, folding, and deformation, ultimately disrupting cellular integrity (Zhang et al., 2018; Zheng et al., 2023). Similarly, MPs have been shown to significantly thicken cell membranes and damage chloroplast structures (Huang et al., 2021). Oxidative stress has been widely recognized as a crucial mechanism underlying the toxicity of MPs, which can induce cellular damage and ultimately lead to cell death (Lu et al., 2016). Our previous study has demonstrated that exposure to 5 mg/L of 80 nm PS elevated malondialdehyde (MDA) content and catalase (CAT) activity in M. aeruginosa, indicating the induction of oxidative stress by 5 mg/L PS (Ye et al., 2025). This finding is consistent with the results of the transcriptomic analysis presented in this study. Besides, existing studies have demonstrated that MNPs can induce oxidative stress and lipid peroxidation in algae, ultimately leading to cellular structural damage and functional disruption (Bhattacharya et al., 2010; Liao et al., 2020). The increase in reserve lipids within microalgae is an adaptive mechanism in response to nutrient deficiency or anthropogenic pollution stress (GusChina and Harwood, 2006). Research has confirmed that NPs can alter diatom lipid profiles, specifically through regulating the distribution of lipid categories and fatty acid constituents (Gonzalez-Fernandez et al., 2020). During the recovery phase, oxidative stress, lipid metabolism, and protein synthesis pathways were all restored in M. aeruginosa previously treated with 5 mg/L PS. However, that a limitation of this study is the absence of measurements for these oxidative stress parameters during the recovery phase. The restoration of oxidative stress status may indicate the activation of the antioxidant defense system, which prompts cells to upregulate the expression of key enzymes such as superoxide dismutase (SOD) and CAT. This process thereby enables efficient scavenging of reactive oxygen species (ROS) and effective mitigation of oxidative damage (Huang et al., 2025). Moreover, oxidative stress during exposure may serve as a signaling pathway to induce cellular adaptive responses, thereby attenuating stress reactions progressively (Schieber and Navdeep, 2014). The restoration of lipid metabolism may be because lipids are temporarily stored in algal cells during exposure and are used as an energy-rich carbon source after removal of nanoparticles (Thompson, 1996).
The plasma membrane term of M. aeruginosa was significantly downregulated during both PS exposure and recovery periods, indicating irreversible membrane damage induced by PS in this study. As the primary interface between intracellular and extracellular environments, the plasma membrane serves as the first target of environmental stressors. Its integrity, permeability, and interfacial electrochemical properties are critical for cellular function (Eich et al., 2000). Previous studies demonstrated that high concentrations of heavy metal ions (Hg2+, Cd2+) cause irreversible algal membrane damage, whereas damage from low concentrations is reversible (Wang et al., 2007). However, the reversibility of membrane damage induced by MNPs remains poorly understood and may depend on PS polymer type, concentration, and exposure duration—a relationship requiring systematic investigation In the present study, M. aeruginosa accumulated glucose during PS exposure and promoted glucose breakdown during recovery. Previous studies have shown that glucose can be used as a signal molecule to improve plant stress resistance by removing reactive oxygen species, regulating osmotic pressure, maintaining the stability of cell membrane, and protecting protein macromolecules (Kefeng et al., 2021; Lv et al., 2024). This coordinated regulation of opposing metabolic fluxes may have driven the restoration of cellular homeostasis in microalgae.
During the exposure phase, the majority of significantly enriched KEGG pathways were associated with energy and carbohydrate metabolism, showing predominant downregulation. In contrast, these metabolic pathways exhibited marked upregulation during the post-exposure period (Table 1). Carbohydrate metabolism, also referred to as sugar metabolism, represents a primary means through which the body acquires energy. The synthesis and degradation of carbohydrates influence cellular osmotic potential, and alterations in osmotic regulation can significantly affect an organism’s stress resistance. Consequently, intracellular carbohydrate accumulation serves a protective role, enhancing plant stress tolerance. As evidenced by the findings of Zhao et al. (2020), it was demonstrated that the pathways involved in carbon metabolism, which included the Calvin cycle, glycolysis, and tricarboxylic acid (TCA) cycle were significantly enriched in S. pseudanthus under polybrominated diphenyl ethers (PBDEs) stress.
ROS-mediated signaling pathways modulate algal carbon-energy metabolism for oxidative stress adaptation (Kobayashi, 2000). The transcriptomic profiling identified 14 DEGs involved in glycolysis/gluconeogenesis processes in M. aeruginosa treated with 5 mg/L PS (Table 2). Among these, NADPH-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH), triosephosphate isomerase (TPI), and fructose-bisphosphate aldolase (FBA) were significantly upregulated, whereas glucokinase (GLK), 6-phosphofructokinase (PFK), and pyruvate kinase (PK) were downregulated (Table 2). The TPI enzyme plays a pivotal role in glycolysis by catalyzing the reversible isomerization of glyceraldehyde-3-phosphate and dihydroxyacetone phosphate, thereby ensuring efficient glycolytic flux and sustained cellular energy production (Sharma et al., 2012). Notably, GLK, PFK, and PK serve as rate-limiting enzymes in glucose phosphorylation. Their downregulation suggests suppression of the glycolytic pathway, indicating that M. aeruginosa may counteract PS-induced oxidative stress by accumulating glucose and reducing energy expenditure. Exposure to 5 mg/L PS significantly inhibits the carbohydrate metabolism pathway in M. aeruginosa, leading to a marked decline in carbohydrate metabolism and energy production efficiency (Figure 4A). This finding aligns with the results reported by Huang et al. (2023), who observed that Pyropia haitanensis modulates energy metabolism pathways in response to abiotic stress. The TCA cycle plays a pivotal role in plant abiotic stress resistance by enhancing the oxidation of respiratory substrates for ATP biosynthesis (Wang et al., 2018b). Notably, the genes responsible for encoding ACLY, IDH2 IDH3, CS and SDHB were significantly downregulated in the TCA cycle of M. aeruginosa exposed to 5 mg/L PS in this study (Table 2). Huang et al. (2023) reported that G. bailinae weaken energy metabolism-related processes, including the TCA cycle to adapt to high temperatures. IDH3 catalyzes the conversion of isocitrate to α-ketoglutarate while generating NADH. The downregulation of this enzyme suggests that M. aeruginosa adapts to the 5 mg/L PS by reducing non-essential energy expenditure. In summary, M. aeruginosa responds to 5 mg/L PS stress by downregulating the Calvin cycle, glycolysis/gluconeogenesis, and pyruvate metabolic pathways, thereby slowing energy and carbohydrate metabolism. The most affected pathway was oxidative phosphorylation pathway in energy metabolism pathways (Supplementary Figure S3). This pathway encompasses a series of redox reactions that rely on protein complexes to facilitate electron transfer, thereby generating a proton gradient that enables ATP synthesis (Zhou et al., 2023). Our result showed that the oxidative phosphorylation process in M. aeruginosa was enhanced during the post-exposure period of 5 mg/L PS treatment, contributing to algal cell tolerance to stress.
Table 2
| Gene_ID | Annotation | log2(fold change) | |
|---|---|---|---|
| PS5–15 VS CK15 | PS5–30 VS CK30 | ||
| Glycolysis/gluconeogenesis | |||
| LRR78_RS01605 | Phosphoenolpyruvate carboxykinase | -2.72 | 2.27 |
| LRR78_RS02965 | Phosphopyruvate hydratase | -1.91 | 1.86 |
| LRR78_RS04195 | Bifunctional acetaldehyde-coa/alcohol dehydrogenase | -2.35 | 1.95 |
| LRR78_RS05450 | Fructose-bisphosphate aldolase class ii | 1.43 | -1.22 |
| LRR78_RS06420 | L-lactate dehydrogenase | 1.21 | -0.75 |
| LRR78_RS08490 | Class ii fructose-bisphosphatase | 1.20 | -0.95 |
| LRR78_RS11305 | Pyruvate: Ferredoxin (flavodoxin) oxidoreductase | -5.28 | 5.13 |
| LRR78_RS11315 | 6-phosphofructokinase | -2.79 | 2.60 |
| LRR78_RS13300 | Type i glyceraldehyde-3-phosphate dehydrogenase | -3.73 | 3.31 |
| LRR78_RS14220 | Multispecies: Triose-phosphate isomerase | 1.59 | -1.42 |
| LRR78_RS14375 | Nadph-dependent glyceraldehyde-3-phosphate dehydrogenase | 1.68 | -1.35 |
| LRR78_RS15705 | Glucokinase | -1.37 | 1.33 |
| LRR78_RS19325 | Class 1 fructose-bisphosphatase | -1.63 | 1.50 |
| LRR78_RS22045 | Pyruvate kinase barrel domain protein | -2.27 | 2.06 |
| TCA cycle | |||
| LRR78_RS03465 | Isocitrate/isopropylmalate dehydrogenase | -1.66 | 2.27 |
| LRR78_RS05000 | Malate dehydrogenase | -1.49 | 1.56 |
| LRR78_RS09000 | Succinate dehydrogenase | -1.45 | 1.39 |
| LRR78_RS10030 | Succinate dehydrogenase | -2.58 | 1.05 |
| LRR78_RS14040 | Citrate synthase | -1.64 | 2.35 |
| LRR78_RS16625 | Nadp-dependent isocitrate dehydrogenase | -1.71 | 5.13 |
| LRR78_RS22825 | Heterodisulfide reductase | -2.48 | 1.49 |
Differently expressed genes related to glycolysis/gluconeogenesis and TCA cycle.
A key limitation of the present study is that the recovery experiments were conducted following exposure to a limited set of concentrations. Consequently, the recovery patterns at lower concentrations, which might reveal different adaptive or resilience mechanisms, were not captured. This specific aspect warrants a dedicated and systematic study in the future. In real aquatic environments, particularly in areas with severe plastic pollution, the concentration of plastic exposure for aquatic organisms may far exceed the experimental levels employed in current studies. Under such scenarios, the sustained toxicity following pulsed exposure could poses significant risks to aquatic biota, disrupts ecosystem integrity, and may cascade through food chains to affect public health. However, standard ecotoxicological testing protocols have not adequately addressed pulsed exposure scenarios or their potential delayed effects observed at exposure endpoints. Therefore, subsequent investigations should prioritize exploring the effects of high-concentration NPs exposure on aquatic organisms’ recovery capacity.
5 Conclusions
This study aimed to determine the exposure and post-exposure effects of PS on M. aeruginosa. The results revealed that 5 mg/L and 50 mg/L PS exposure significantly inhibited growth and promoted the synthesis and release of MCs. After cultivation in PS-free BG11 medium, M. aeruginosa showed growth recovery, but the concentrations of both intracellular and extracellular MCs remained higher than those in the control group. Transcriptome analysis showed that the effects of 5 mg/L on oxidative stress, lipid metabolism and protein synthesis were recoverable, but caused irreversible damage to algal cell membrane. KEGG pathway enrichment analysis showed that M. aeruginosa may inhibit PS-induced oxidative stress by accumulating glucose and reducing energy expenditure, while pre-exposure to PS enhanced carbohydrate and energy metabolism. These results lay the groundwork for a deeper understanding of the molecular mechanisms underlying M. aeruginosa adaptation to PS-NPs, unveil the evolutionary and adaptive potential of microorganisms in response to emerging environmental stressors, and provide critical insights into microbial evolution under environmental pressure.
Looking forward, to achieve a more holistic predictive understanding of NPs’ impact on aquatic primary producers, future work should prioritize investigating the dose-response relationships of post-exposure recovery. A systematic examination of the physiological and molecular recovery patterns of microalgae and cyanobacteria following exposure to environmentally relevant, lower concentrations of NPs represents a critical and logical next step.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
RW: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. SY: Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing. LJ: Writing – review & editing. CT: Writing – review & editing. PH: Data curation, Formal Analysis, Writing – review & editing. ZL: Data curation, Writing – review & editing. DM: Formal Analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Natural Science Foundation of Shandong Province (ZR2021QC135, ZR2021QF143) and the National Natural Science Foundation of China (62011530044). The research was also supported by the Project of the Talent Introduction of Dezhou University (2019xjrc327, 2019xjrc328).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1678627/full#supplementary-material
References
1
Besseling E. Redondo-Hasselerharm P. Foekema E. M. Koelmans A. A. (2018). Quantifying ecological risks of aquatic micro- and nanoplastic. Crit. Rev. Environ. Sci. Technol.49, 32–80. doi: 10.1080/10643389.2018.1531688
2
Bhattacharya P. Lin S. Turner J. P. Ke P. C. (2010). Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C114, 16556–16561. doi: 10.1021/jp1054759
3
Buhl K. J. Hamilton S. J. Schmulbach J. C. (1993). Chronic toxicity of the bromoxynil formulation Buctril to Daphnia magna exposed continuously and intermittently. Arch. Environ. Contamination Toxicol.25, 152–159.
4
Chen Y. X. Ling Y. Li X. Y. Hu J. N. Cao C. J. He D. F. (2020). Size-dependent cellular internalization and effects of polystyrene microplastics in microalgae P. helgolandica var. tsingtaoensis and S. quadricauda. J. Hazardous Materials399, 10. doi: 10.1016/j.jhazmat.2020.123092
5
Copin P. J. Perronet L. Chevre N. (2016). Modelling the effect of exposing algae to pulses of S-metolachlor: How to include a delay to the onset of the effect and in the recovery. Sci. Total Environ.541, 257–267. doi: 10.1016/j.scitotenv.2015.08.154
6
Debrot A. O. van Rijn J. Bron P. S. de León R. (2013). A baseline assessment of beach debris and tar contamination in Bonaire, Southeastern Caribbean. Mar. pollut. Bull.71, 325–329. doi: 10.1016/j.marpolbul.2013.01.027
7
Detree C. Gallardo-Escarate C. (2018). Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol.83, 52–60. doi: 10.1016/j.fsi.2018.09.018
8
Eich J. Durholt H. Steger-Hartmann T. Wagner E. (2000). Specific detection of membrane-toxic substances with a conductivity assay. Ecotoxicol Environ. Saf.45, 228–235. doi: 10.1006/eesa.1999.1854
9
Evangeliou N. Grythe H. Klimont Z. Heyes C. Eckhardt S. Lopez-Aparicio S. et al . (2020). Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun.11, 3381. doi: 10.1038/s41467-020-17201-9
10
Fan G. Zhou J. Zheng X. Chen W. (2018). Growth inhibition of Microcystis aeruginosa by copper-based mofs: performance and physiological effect on algal cells. Appl. Organomet Chem.32, e4600. doi: 10.1002/aoc.4600
11
Feng L. J. Sun X. D. Zhu F. P. Feng Y. Duan J. L. Xiao F. et al . (2020). Nanoplastics promote microcystin synthesis and release from cyanobacterial microcystis aeruginosa. Environ. Sci. Technol.54, 3386–3394. doi: 10.1021/acs.est.9b06085
12
Gonzalez-Fernandez C. Le Grand F. Bideau A. Huvet A. Paul-Pont I. Soudant P. (2020). Nanoplastics exposure modulate lipid and pigment compositions in diatoms. Environ. pollut.262, 10. doi: 10.1016/j.envpol.2020.114274
13
GusChina I. A. Harwood J. L. (2006). Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res.45, 160–186. doi: 10.1016/j.plipres.2006.01.001
14
Holloczki O. Gehrke S. (2019). Nanoplastics can change the secondary structure of proteins. Sci. Rep.9, 7. doi: 10.1038/s41598-019-52495-w
15
Huang Y. Cui J. Wang S. Chen X. Liao J. Guo Y. et al . (2023). Transcriptome analysis reveals the molecular mechanisms of adaptation to high temperatures in Gracilaria bailinae. Front. Plant Sci.14, 1125324. doi: 10.3389/fpls.2023.1125324
16
Huang D. Zhang H. Y. Wang H. J. Huang H. J. Liu S. Qiu C. Y. et al . (2025). Physiological responses and adaptive mechanisms of the harmful algal bloom species Heterosigma akashiwo to naphthalene exposure. J. Hazard Mater490, 137846. doi: 10.1016/j.jhazmat.2025.137846
17
Huang W. Q. Zhao T. Zhu X. L. Ni Z. Q. Guo X. Tan L. J. et al . (2021). The effects and mechanisms of polystyrene and polymethyl methacrylate with different sizes and concentrations on Gymnodinium aeruginosum. Environ. pollut.287, 10. doi: 10.1016/j.envpol.2021.117626
18
Kefeng C. Yingying W. Yi C. Shu J. Xueyu C. Xingxing W. et al . (2021). PpCBF6 is a low-temperature-sensitive transcription factor that binds the PpVIN2 promoter in peach fruit and regulates sucrose metabolism and chilling injury. Postharvest Biol. Technol.181, 1–11. doi: 10.1016j.postharvbio.2021.111681
19
Kobayashi M. (2000). In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol.54, 550–555. doi: 10.1007/s002530000416
20
Li R. R. Wang B. L. Nan F. R. Lv J. P. Liu X. D. Liu Q. et al . (2023). Effects of polystyrene nanoplastics on the physiological and biochemical characteristics of microalga Scenedesmusquadricauda. Environ. pollut.319, 120987. doi: 10.1016/j.envpol.2022.120987
21
Liao Y. C. Jiang X. F. Xiao Y. Li M. (2020). Exposure of microalgae Euglena gracilis to polystyrene microbeads and cadmium: Perspective from the physiological and transcriptional responses. Aquat. Toxicol.228, 11. doi: 10.1016/j.aquatox.2020.105650
22
Lu Y. Zhang Y. Deng Y. Jiang W. Zhao Y. Geng J. et al . (2016). Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol.50, 4054–4060. doi: 10.1021/acs.est.6b00183
23
Lv N. Zhang H. Zhou H. Wang C. Guo C. Wang L. (2024). Hot water mobilizes the metabolism of energy, soluble sugar, cell wall, and phenolics to cope with chilling injury in postharvest snap beans. J. Sci. Food Agric.104, 8263–8274. doi: 10.1002/jsfa.13662
24
Mao Y. Ai H. Chen Y. Zhang Z. Zeng P. Kang L. et al . (2018). Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere208, 59–68. doi: 10.1016/j.chemosphere.2018.05.170
25
Nagai T. Ishihara S. Yokoyama A. Iwafune T. (2011). Effects of four rice paddy herbicides on algal cell viability and the relationship with population recovery. Environ. Toxicol. Chem.30, 1898–1905. doi: 10.1002/etc.582
26
Omori K. Datta T. Amano Y. Machida M. (2019). Effects of different types of extracellular polysaccharides isolated from cyanobacterial blooms on the colony formation of unicellular Microcystis aeruginosa. Environ. Sci. pollut. Res.26, 3741–3750. doi: 10.1007/s11356-018-3892-z
27
Prata J. C. da Costa J. P. Lopes I. Duarte A. C. Rocha-Santos T. (2019). Effects of microplastics on microalgae populations: A critical review. Sci. Total Environ.665, 400–405. doi: 10.1016/j.scitotenv.2019.02.132
28
Rapala J. Sivonen K. Lyra C. Niemela S. I. (1997). Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol.63, 2206–2212. doi: 10.1128/aem.63.6.2206-2212.1997
29
Rezayian M. Niknam V. Ebrahimzadeh H. (2019). Oxidative damage and antioxidative system in algae. Toxicol. Rep.6, 1309–1313. doi: 10.1016/j.toxrep.2019.10.001
30
Schieber M. Navdeep S. (2014). ROS function in redox signaling and oxidative stress. Curr. Biol.24, R453–R462. doi: 10.1016/j.cub.2014.03.034
31
Sendra M. Staffieri E. Yeste M. P. Moreno-Garrido I. Gatica J. M. Corsi I. et al . (2019). Are the primary characteristics of polystyrene nanoplastics responsible for toxicity and ad/absorption in the marine diatom Phaeodactylum tricornutum? Environ. pollut.249, 610–619. doi: 10.1016/j.envpol.2019.03.047
32
Sewelam N. Oshima Y. Mitsuda N. Ohme-Takagi M. (2014). A step towards understanding plant responses to multiple environmental stresses: a genome-wide study. Plant Cell Environ.37, 2024–2035. doi: 10.1111/pce.12274
33
Sharma S. Ananda M. L. S.-P. S. Prem S. S. Sopory S. K. (2012). Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signaling Behav.7, 1337–1345. doi: 10.4161/psb.21415
34
Thompson G. A. (1996). Lipids and membrane function in green algae. Biochim. Biophys. Acta (BBA) - Lipids Lipid Metab.1302, 17–45. doi: 10.1016/0005-2760(96)00045-8
35
Thompson R. C. Courtene-Jones W. Boucher J. Pahl S. Raubenheimer K. Koelmans A. A. (2024). Twenty years of microplastic pollution research-what have we learned? Science386, eadl2746. doi: 10.1126/science.adl2746
36
Wang B. Meng Q. Yang J. Ma Y. Yang Y. Shi J. (2007). Study on rapid responses of algae cell membrane electric signal to heavy metals. Asian J. Ecotoxicology2, 172–177. doi: 10.3969/j.issn.1673-5897.2007.02.007
37
Wang W. Teng F. Lin Y. Ji D. Xu Y. Chen C. et al . (2018b). Transcriptomic study to understand thermal adaptation in a high temperature-tolerant strain of Pyropia haitanensis. PloS One13, e0195842. doi: 10.1371/journal.pone.0195842
38
Wang F. Wong C. S. Chen D. Lu X. Wang F. Zeng E. Y. (2018). Interaction of toxic chemicals with microplastics: A critical review. Water Res.139, 208–219. doi: 10.1016/j.watres.2018.04.003
39
Wang J. Zhao F. Chen B. Li Y. Na P. Zhuo J. (2013). Small water clusters stimulate microcystin biosynthesis in cyanobacterial Microcystis aeruginosa. J. Appl. Phycology25, 329–336. doi: 10.1007/s10811-012-9867-4
40
Wu Y. Guo P. Zhang X. Zhang Y. Xie S. Deng J. (2019). Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazardous Materials374, 219–227. doi: 10.1016/j.jhazmat.2019.04.039
41
Yang W. F. Gao P. Li H. X. Huang J. Y. Zhang Y. Ding H. J. et al . (2021a). Mechanism of the inhibition and detoxification effects of the interaction between nanoplastics and microalgae Chlorella pyrenoidosa. Sci. Total Environ.783, 11. doi: 10.1016/j.scitotenv.2021.146919
42
Yang W. F. Gao P. Ma G. Y. Huang J. Y. Wu Y. X. Wan L. et al . (2021b). Transcriptome analysis of the toxic mechanism of nanoplastics on growth, photosynthesis and oxidative stress of microalga Chlorella pyrenoidosa during chronic exposure. Environ. pollut.284, 13. doi: 10.1016/j.envpol.2021.117413
43
Yang W. F. Gao P. Nie Y. Huang J. Y. Wu Y. X. Wan L. et al . (2021c). Comparison of the effects of continuous and accumulative exposure to nanoplastics on microalga Chlorella pyrenoidosa during chronic toxicity. Sci. Total Environ.788, 10. doi: 10.1016/j.scitotenv.2021.147934
44
Ye H. Y. Zao Z. H. Liu C. H. Yao Y. H. Yue S. Z. Wang R. P. (2025). Toxicological effects of micro/nanoplastics with different particle sizes on Microcystis aeruginosa. Shandong Sci.38, 95–105. doi: 10.3976/j.issn.1002-4026.20240076
45
Zhang H. Liang J. Luo Y. Tang N. Li X. Zhu Z. et al . (2022). Comparative effects of polystyrene nanoplastics with different surface charge on seedling establishment of Chinese cabbage (Brassica rapa L.). Chemosphere292, 133403. doi: 10.1016/j.chemosphere.2021.133403
46
Zhang Q. Qu Q. Lu T. Ke M. J. Zhu Y. C. Zhang M. et al . (2018). The combined toxicity effect of nanoplastics and glyphosate on Microcystis aeruginosa growth. Environ. pollut.243, 1106–1112. doi: 10.1016/j.envpol.2018.09.073
47
Zhao Y. Tang X. Lv M. Liu Q. Zhao Y. (2020). The molecular response mechanisms of a diatom Thalassiosira pseudonana to the toxicity of BDE-47 based on whole transcriptome analysis. Aquat. Toxicol.229, 105669. doi: 10.1016/j.aquatox.2020.105669
48
Zheng X. Zhang L. Jiang C. Li J. Li Y. Liu X. et al . (2023). Acute effects of three surface-modified nanoplastics against Microcystis aeruginosa: Growth, microcystin production, and mechanisms. Sci. Total Environ.855, 158906. doi: 10.1016/j.scitotenv.2022.158906
49
Zhou W. Wang Y. Wang J. Peng C. Wang Z. Qin H. et al . (2023). Geosmin disrupts energy metabolism and locomotor behavior of zebrafish in early life stages. Sci. Total Environ.859, 160222. doi: 10.1016/j.scitotenv.2022.160222
50
Zilliges Y. Kehr J. C. Meissner S. Ishida K. Mikkat S. Hagemann M. et al . (2011). The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of microcystis under oxidative stress conditions. PloS One6, e17615. doi: 10.1371/journal.pone.0017615
Summary
Keywords
nanoplastics, Microcystis aeruginosa , exposure, post-exposure, transcriptomic
Citation
Wang R, Yue S, Jia L, Tibihenda C, Huang P, Li Z and Meng D (2025) Post-exposure recovery of Microcystis aeruginosa from nanoplastics stress: metabolic adaptation and damage resilience. Front. Mar. Sci. 12:1678627. doi: 10.3389/fmars.2025.1678627
Received
03 August 2025
Accepted
15 September 2025
Published
30 September 2025
Volume
12 - 2025
Edited by
Arun Kumar Mishra, Banaras Hindu University, India
Reviewed by
Liliana Cepoi, Technical University of Moldova, Moldova; Manisha Banerjee, Bhabha Atomic Research Centre (BARC), India
Updates
Copyright
© 2025 Wang, Yue, Jia, Tibihenda, Huang, Li and Meng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruiping Wang, wrping1990@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.