Abstract
The starry ray, Raja asterias (Delaroche 1809), is a demersal Mediterranean skate often caught as bycatch in bottom trawls and set nets. Due to high fishing pressure in the Adriatic Sea, key life history traits were investigated to support effective conservation and fishery management. Specimens were collected from 2019 to 2023 through both fishery-dependent and independent sampling in the mid-western Adriatic. Age was estimated for the first time in cartilaginous fish using burnt and dissected vertebrae. The reproductive cycle was assessed through macroscopic gonad examination, while spatial distribution was analyzed in relation to sex and maturity (mature vs immature). Trends in relative abundance and biomass (2007–2024) were evaluated using standardized survey data. Results revealed significant sexual dimorphism in biological traits. Females showed a broader size range (170–590 mm vs 160–505 mm), greater disc width (115–410 mm vs 105–342 mm), and positive allometric growth, compared to isometric growth in males. Growth patterns also differed: Von Bertalanffy parameters were L∞ = 724, k = 0.28 for females and L∞ = 574, k = 0.44 for males. Females reached greater maximum age (4.5 vs 4.0 years), and achieved sexual maturity at larger sizes (425 vs 360 mm) and older ages (approx. 3.0 vs 2.0 years). Spatial analysis showed that immature individuals are concentrated nearshore (<50 m depth), while mature ones inhabit deeper waters (up to 80 m). During the reproductive season, adults migrate inshore, overlapping with immature habitats. Abundance and biomass indices significantly increased over time, particularly after 2020. These findings underscore the need for sex-specific biological parameters in management strategies. Conservation measures should avoid using population-wide averages and instead adopt the most precautionary approach. Additionally, limiting fishing in shallow waters could reduce bycatch of immature or egg-laying individuals for much of the year, positively impacting population recovery.
Introduction
Elasmobranchs are among the most highly threatened vertebrate groups globally (Dulvy et al., 2021), primarily due to their life history traits. These includes slow growth, late sexual maturity, low fecundity and productivity (i.e. small and infrequent litters), long gestation periods, high natural survivorship across all age classes, and a long lifespan compared to teleost fishes (Cortés et al., 2012). Such characteristics, typical of k-selected species, make them extremely susceptible to anthropogenic impacts, especially fishing pressure (Stevens et al., 2000; Field et al., 2009; Dulvy et al., 2021).
Although most of the elasmobranch species are not primarily targeted by fishing fleets, they are accidentally caught as an accessory commercial species or bycatch (Moro et al., 2025). These catches are frequently discarded at sea, unofficially marketed, or remain unreported (Bonfil, 1994; Lack and Sant, 2008; Bradai et al., 2012; Worm et al., 2013; Bonanomi et al., 2017).
Among the 86 Mediterranean elasmobranch species (48 sharks, 38 batoids - rays and skates; Otero et al., 2019), the Mediterranean starry ray Raja asterias (Delaroche, 1809) is one of the few demersal skates still present inside this highly exploited basin (Catalano et al., 2022). Its distribution range was recently extended beyond the Strait of Gibraltar to northern Morocco and possibly southward to Mauritania, with a verified record from the Gulf of Cadiz (Ordines et al., 2017; Froese and Pauly, 2020). This species inhabits muddy or sandy bottoms from shallow waters to 150 m depth with length-age-related abundance (Catalano et al., 2003; Romanelli et al., 2007). In particular, juvenile (Total Length, TL 80–90 mm) and adult individuals appear to aggregate in different areas (Serena et al., 2010; Ferrá et al., 2016). Raja asterias plays an important ecological role as a predator within demersal communities, feeding on a wide variety of prey, including crustaceans, teleosts, and cephalopods (Capapé and Quignard, 1977; Cuoco et al., 2005; Serena et al., 2005; Romanelli et al., 2007; Bradai et al., 2012; Coll et al., 2013; Navarro et al., 2016). It has an oviparous reproductive strategy, laying large benthic egg cases primarily at depths of 30–40 m (Barone et al., 2007), although spawning and nursery areas are spatially distinct and confined to shallow zones (5–50 m) (Serena and Relini, 2006; Hoff, 2016).
The starry ray is commonly caught as commercial bycatch by bottom otter trawls - OTB (Abella and Serena, 2005; Navarro et al., 2013; Ferrá et al., 2016), set nets, mainly gillnets - GNS (Lucchetti et al., 2023), and “rapido” trawls - TBB (Romanelli et al., 2007; Catalano et al., 2022). The species has been and still is highly impacted by fishing and it is very sensitive to increasing fishing impacts (Coll et al., 2013). Negative population responses to intense bottom trawling have been reported in multiple shallow coastal areas, including the northern Tyrrhenian Sea (Abella and Serena, 2005; Abella et al., 2017), the north-western Mediterranean (Coll et al., 2013; Navarro et al., 2013; Biton-Porsmoguer and Lloret, 2020), and the Adriatic Sea (Ferrá et al., 2016). Despite the large individuals have moderate commercial value, information available about the biology of starry ray is still scarce (Bradai et al., 2012; Coll et al., 2013), while its feeding ecology has been recently refined and studied in the mid-western Adriatic Sea (Fanelli et al., 2023).
Previous survey data (2005–2014), derived from an annual survey conducted using “rapido” trawls, known as SoleMon, demonstrated a significant increase in R. asterias relative abundance and biomass in the northern-central Adriatic, likely linked to reduced trawling effort (Ferrá et al., 2016). Extending this time series with new data provides an opportunity to assess whether these positive trends have persisted, plateaued, or reversed under current fishing pressures, which have further decreased under the framework of the GFCM (General Fisheries Commission for the Mediterranean) Multiannual Plan for demersal fisheries in the Adriatic Sea (GFCM, 2019).
Conservation and fisheries management measures require a robust assessment of population status, which must be based on reliable life history data collected across spatial and temporal scales. Key information such as age, growth, and size at sexual maturity is essential to estimate mortality rates and productivity, critical for stock assessments (Carbonara and Follesa, 2019). However, determining growth parameters in cartilaginous fishes has historically been challenging due to the absence of calcified structures like otoliths and the low calcification of cartilaginous structures (Campana, 2014). These challenges underscore the importance of developing precise and accurate ageing methods, as poor-quality biological data can significantly impact stock assessment outcomes (Campana, 2014; Bellodi et al., 2023). Similarly, understanding reproductive cycles, population structure, and spatial distribution is crucial for evaluating vulnerability and designing effective management measures (Bargione et al., 2019).
The aim of this study was to improve the knowledge on key ecological traits of R. asterias in the mid-western Adriatic Sea, providing updated information to inform result-based management strategies. Specifically, age and growth rates were investigated by comparing four different ageing techniques, while reproductive cycles and size at sexual maturity were analyzed macroscopically. Additionally, the spatial distribution of R. asterias individuals was assessed according to sex and maturity stage. Finally, the temporal trends of relative abundance and biomass were investigated.
Materials and methods
Study site and sampling
Raja asterias individuals were both collected during fishery-dependent and independent sea trails in the mid-western Adriatic Sea between 2019 and 2023 (Figure 1).
Figure 1
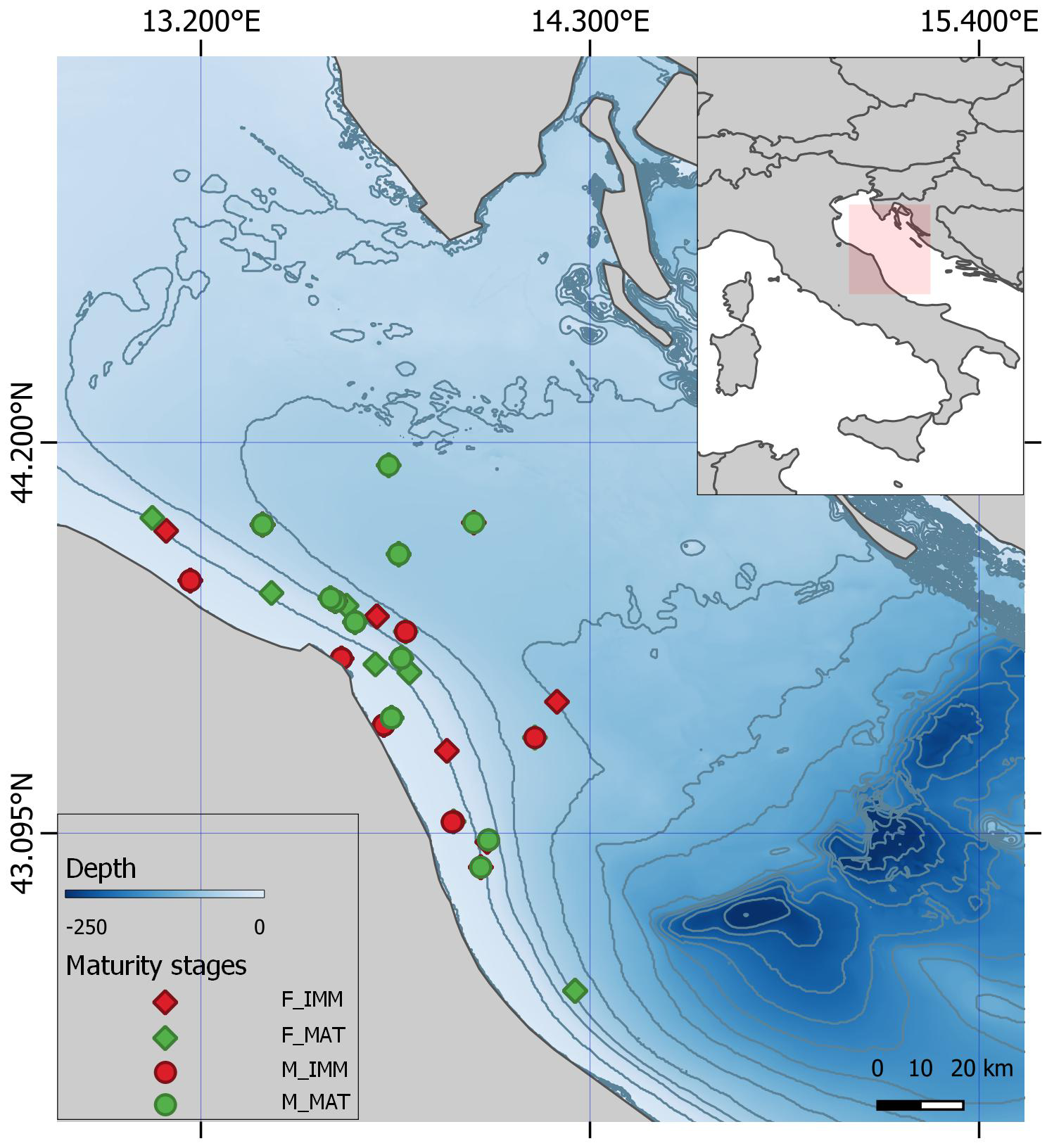
Map of the starry ray individuals sampled over the period December 2019 – November 2023 during fishery dependent and independent sea trials with reference to sex (M, F) and maturity stage (mature, immature). Dark lines represent isobaths set every 20 m of depth.
Fishery dependent trails were conducted between December 2019 and October 2020 by the fishing vessel Airone Bianco II (LOA: 24.16 m, GT:91 tons, Power: 333 Kw), a bottom trawler of the Ancona fleet operating between 15 and 50 nautical miles (NM) off the coast and primarily targeting commercial demersal fishes, cephalopods and crustaceans, while the starry ray is caught as a commercial bycatch species retained on board, and subsequently sold on the market. Samples were collected monthly at the landing site from the entire catch of each fishing trip.
Fishery-independent samples were collected during the standardized annual trawl survey SoleMon (SOLE MONitoring), which has monitored demersal resources in the central and northern Adriatic Sea since 2005 (Grati et al., 2013; Scarcella et al., 2014). Since 2007, 67 stations, allocated according to a random depth-stratified scheme are sampled with the “rapido” gear, a modified beam trawl with a rigid mouth and iron teeth. This gear primarily targets the common sole (Solea solea) but also records bycatch species such as R. asterias which once again is retained on board, and subsequently sold on the market. In 2020, sampling took place in December aboard the research vessel G. Dallaporta (LOA: 35.7 m; GT: 285 tons; Power: 810 kW), and in 2023, in November aboard the commercial trawler Aquila Marina (LOA: 25.64 m; GT: 133 tons; Power: 835 kW), operating within 40 NM off the Rimini coast. During these campaigns, all R. asterias specimens from stations within the mid-western Adriatic were collected and included in this study. In addition, the abundance and biomass indices of R. asterias were estimated from SoleMon survey captures for the time series 2007-2024.
Regardless of the sampling origin, individuals were processed dead and fresh within 24 hours, either on board or in the laboratory, for analyses of age, growth, and reproduction. Capture coordinates were recorded for each specimen to map spatial distributions by sex and maturity stage.
Laboratory analysis
Morphometry
For each sampled individual, the main morphometric parameters (i.e., Total Length - TL, Disc Length - DL, Disc Width - DW) were recorded using a measuring board to the nearest mm, whereas the weight (W) was recorded using a balance to the nearest decigram.
Reproduction
To investigate the reproductive cycle of the species, sexes were determined based on the presence/absence of claspers, while macroscopic maturity stages were assigned by the naked eye once the abdominal cavity was opened, based on the scale proposed by Serena (2006) and the International Council for the Exploration of the Sea (2012) for oviparous batoids. The maturity scale consists of five levels for both sexes: stage 1 – immature, stage 2 – maturing, stage 3a – mature, stage 3b – mature/extruding-active, stage 4 – resting. Moreover, the mean gonadosomatic index (GSImean), which is the percentage of gonad weight (GW) to body weight (W), was calculated for each maturity stage and both sexes, in which it was possible to extract and weigh the gonads, as a measure of reproductive investment.
Size at sexual maturity (TL50) was assessed in both sexes by fitting a logistic model to the proportion of mature specimens within each size class (20 mm wide):
where y is the relative frequency of mature individuals (i.e., maturity stages ≥ 3a), x is the size of individuals, and a and b are regression constants.
Age and growth
To estimate the age of the species, a representative sub-sample of all the available size classes was selected. To this end, post-cranial and thoracic vertebrae were removed, being the bigger ones more suitable for age reading (Bellodi et al., 2023). Vertebrae were stored at -22°C until processing. The section of the spine containing the vertebrae was then thawed in a beaker containing boiling water for about 30 minutes to soften the tissues still attached to the vertebrae walls, which were subsequently removed by using a scalpel. From each spine section, a buffer of 6 vertebrae, in case of vertebrae breakage during processing, was separated from each other and cleaned by also removing the transverse processes, the neural and haematic arches. Vertebrae were then immersed in 5% sodium hypochlorite (NaClO) for 35 minutes and rinsed in distilled water and left to air dry for 48 hours. Four different ageing techniques were adopted to test for differences between methods and identify the most reliable one. The methods used were: i) whole untreated vertebra - WUV, ii) whole treated vertebra (burnt) - WTV, iii) dissected untreated vertebra - DUV, iv) dissected treated (burnt) vertebra. Treatment involved burning at 350°C for 10 minutes in the laboratory muffle. For age determination, the two biggest vertebrae were selected and one was left untreated while the second was treated. The two entire vertebrae were then observed and photographed under a dissecting microscope connected to a video-analysis system. For each whole vertebra, a picture was taken and post-edited for further age estimates. Subsequently, the same two vertebrae were thin-sectioned through the middle of the centrum, by mounting them on a microscope slide with epoxy resin and then sanded down to the midplane as described by McPhie and Campana (2009). Once sectioned, they were observed and photographed under a dissecting microscope as described above for the whole vertebrae. Photographs were subsequently inspected to identify annual growth rings, which under a dissecting microscope consisted of one opaque and one translucent band, as is standard in temperate fish sclerochronological analyses. Assuming that the annuli were laid down yearly, the age of fish was estimated by counting all the translucent zones. The birth-mark was also firstly identified and associated with a change in angle of the corpus calcareum; that is, the edge of the sectioned vertebra appears to curve slightly at the birth band, becoming relatively straight afterwards (Figure 2). Age was assessed in years from the same reader two weeks apart and the final age was calculated as the mean of the two age readings. Age class 0+ consisted of individuals which had not reached 1 year of age yet. Precision was evaluated using average percentage error (APE) and average coefficient of variation (ACV) (Beamish and Fournier, 1981; Chang, 1982).
Figure 2
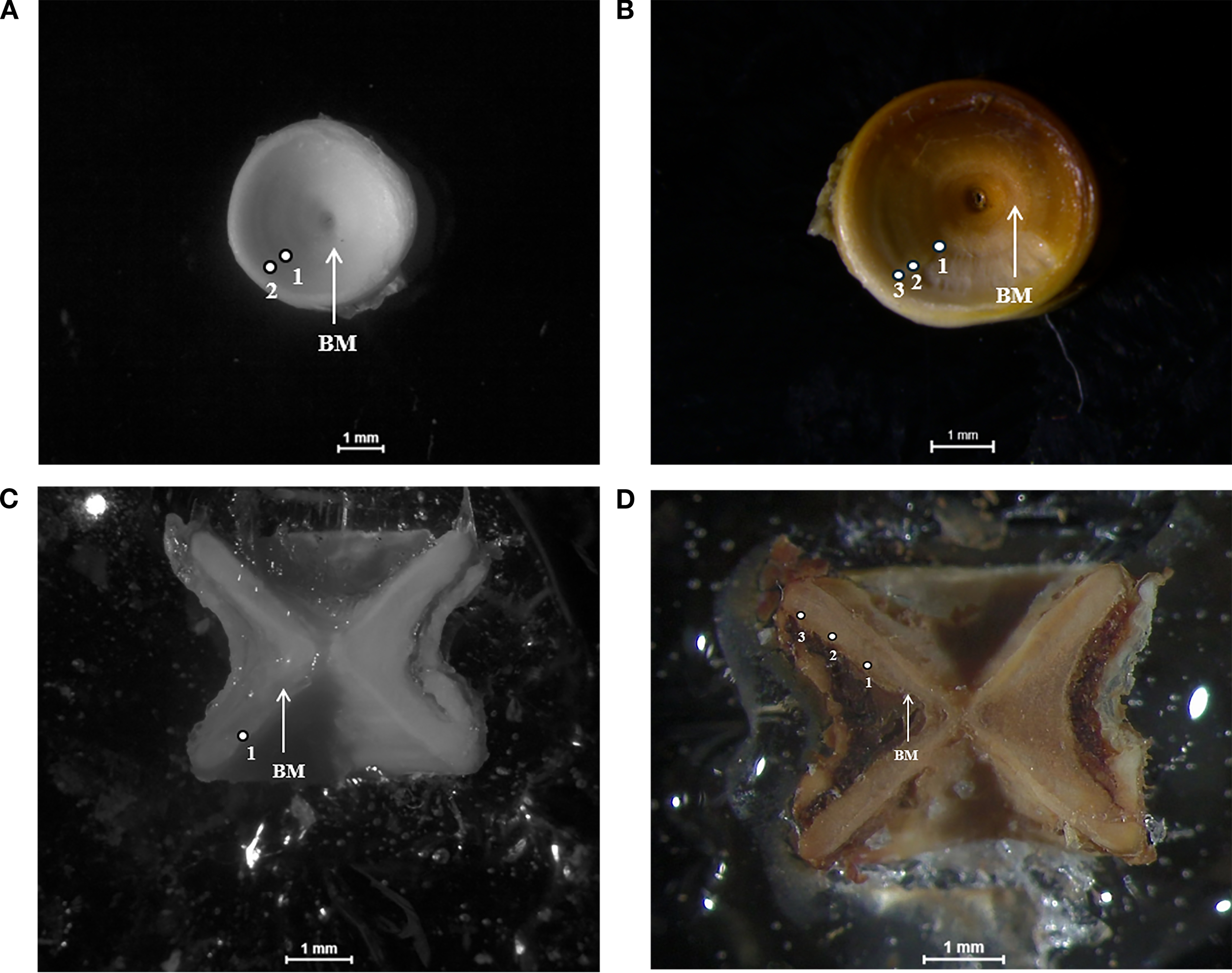
Growth rings visibility between ageing technique for the same R. asterias individual: (A) whole untreated vertebra (WUV), (B) whole treated vertebra (WTV), (C) dissected untreated vertebra (DUV) and (D) dissected treated vertebra (DTV). The presence of the birth-mark (BM) is also reported.
Spatial distribution
For each individual, the sampling coordinate was recorded at the time of sampling together with the capture date. Matching this information with the maturity stage of individuals (i.e., mature vs immature), the starry ray spatial distribution was investigated.
Abundance and biomass trends from SoleMon survey
The relative abundance (N/km²) and biomass (kg/km²) of the individuals caught during the SoleMon surveys (2007-2024) were calculated and extracted from the dedicated TruSt database. The presence of a possible significant trend of these population parameters was investigated through a weighted least squares regression (Reddy, 2011).
Data analysis
The non-parametric Kolmogorov-Smirnov test (Press and Teukolsky, 1988) was applied to investigate differences in the length frequency distribution structure between sexes.
The morphometric relationship between TL and W was estimated by fitting the following nonlinear equation:
Isometric growth (i.e. b = 3) departure was tested using a t-Student test. The allometric indices (b) calculated for males and females were compared by applying an F test.
The Relative Condition Factor (Kn) was calculated for both sexes by dividing the observed W of each individual with the expected one derived from the TL-W relationship. Kn can range from > 1 overweight, = 1 normal-weight, to <1 underweight (Le Cren, 1951). The morphometric relationship between TL vs DL and TL vs DW was estimated by fitting a linear regression models. A stepwise selection guided by Akaike’s Information Criterion (AIC) was performed on both regression models, sequentially evaluating the removal or re−addition of main effects and the interaction to achieve the lowest AIC. The relative percentage of males and females (sex ratio) was determined on the whole sample. Deviation from the expected 1:1 sex ratio was tested for the sampled population using a χ2 goodness-of-fit test.
To describe the growth pattern, the modified von Bertalanffy growth model described by Cailliet et al. (2006) where the common parameter t0 (theoretical age at length zero) is replaced with the size at birth (L0), was initially fitted to the length-at-age data estimated for each sex and ageing technique tested:
L(t) is the mean length-at-age t and L∞ (theoretical maximum length), and k (growth coefficient) are the parameters to be estimated. L0 was set at 80 mm as described for R. asterias by (Mendez et al., 2022).
Growth parameters were estimated by applying a non-linear regression analysis, and the AIC (Akaike, 1973) was calculated for the best model selection.
The lowest APE and ACV values by technique also allowed for the identification of the best growth model. After the best growth model selection, the likelihood-ratio test was used to compare the growth parameters estimated for each sex (Kimura, 1979), and the growth performance index (Ф’), was calculated as:
Age of maturity was assessed both for females and males from the resulting growth-curves knowing the TL50. All statistical analyses and data visualizations were produced in R (v 4.4.3; R Core Team, 2023). The significance level was set at p < 0.05 for all tests used. The software QGIS (version 3.10 A Sherman, 2019) was used to produce the distribution map of the starry ray individuals based on the sex and maturity stage.
Results
Morphometry
A total of 238 starry ray individuals (121 females and 117 males) were collected over the sampling period ranging in size between 160 and 590 mm TL. However, the Kolmogorov-Smirnov test showed that females exhibited a significantly wider size range (from 170 to 590 mm TL) compared to males (from 160 to 505 mm TL) (D121,117 = 0.64; p < 0.01), highlighting a first morphometric sexual dimorphism between sexes (Figure 3).
Figure 3
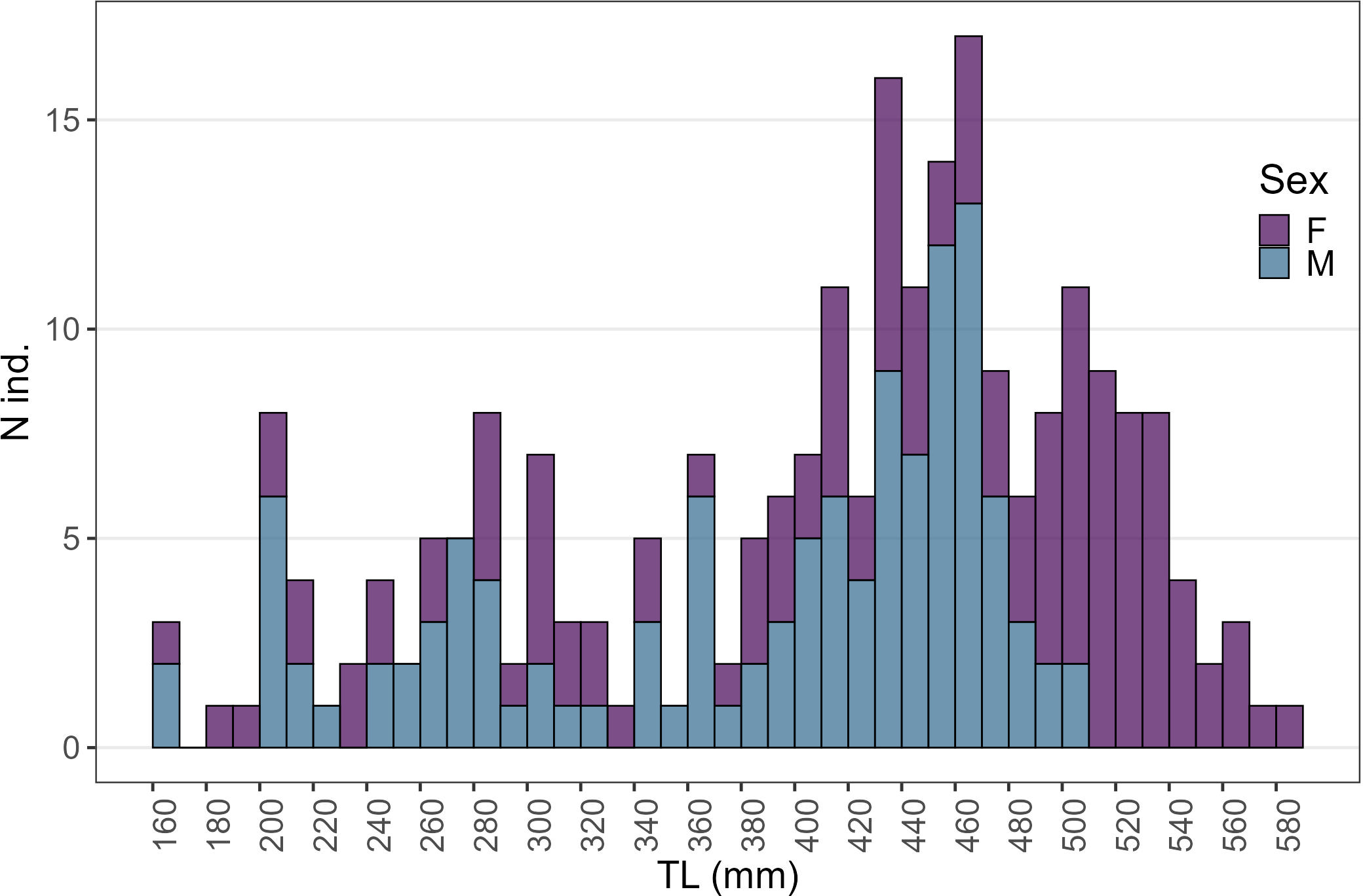
Females and males size structure of the starry ray individuals collected over the sampling period.
A positive relationship was found between TL and W in both sexes, as summarized in the following equations:
The b-values was significantly higher than 3 in females (T = 5.67 gdl= 119, p < 0.05) and equal to 3 in males (T = 1.67, gdl= 115, p > 0.05), indicating positive allometric and isometric growths, respectively, thus with significant differences between the b-values of the two sexes (F = 22.40, p < 0.01) (Figure 4).
Figure 4
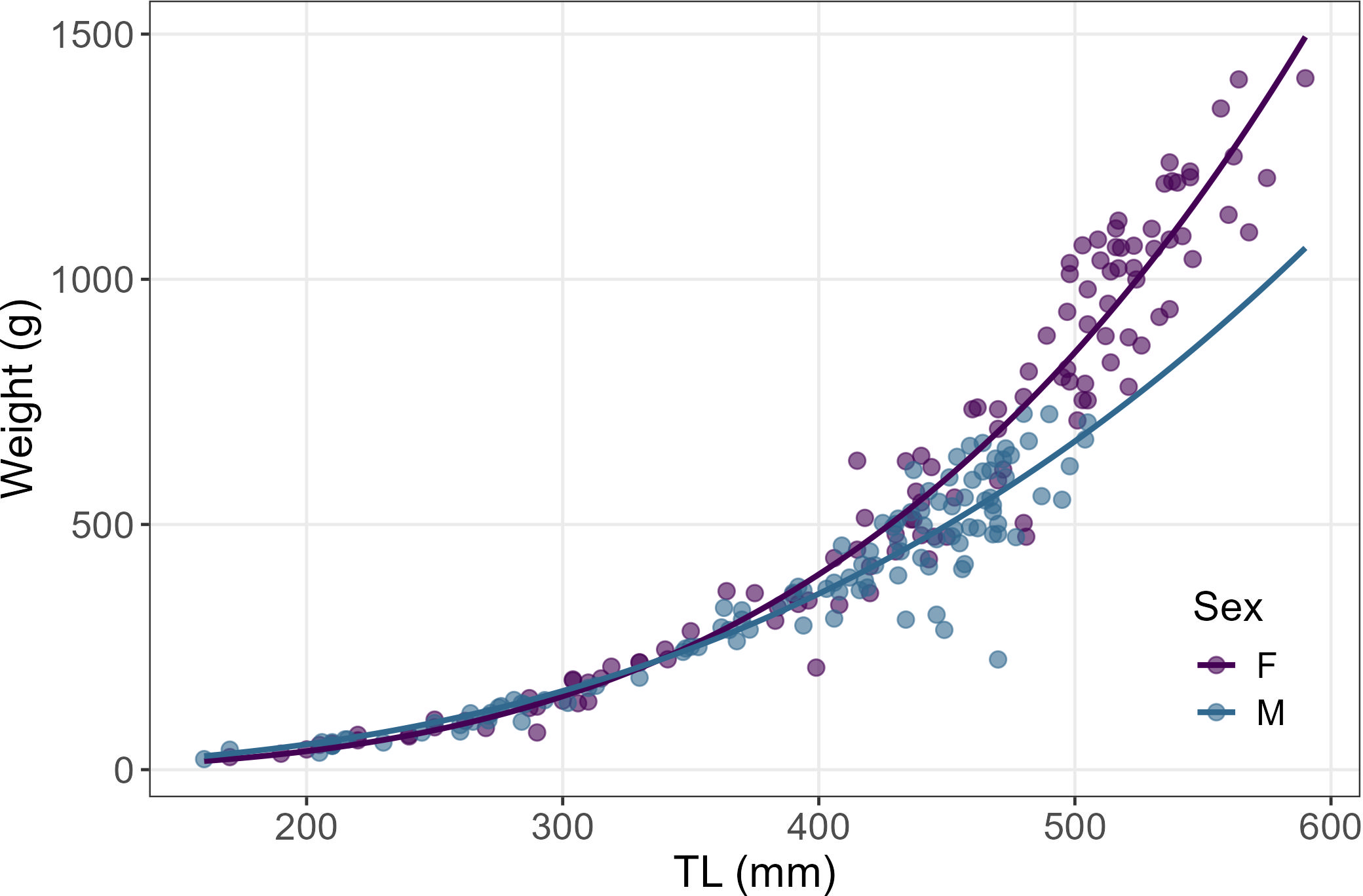
Length-weight relationship of females and males starry ray individuals. Females exhibits a positive allometric growth (b > 3) while males an isometric growth (b =3).
The RCF for both sexes highlighted different physical conditions of the individuals oscillating from under to overweight independently of size (Figure 5).
Figure 5
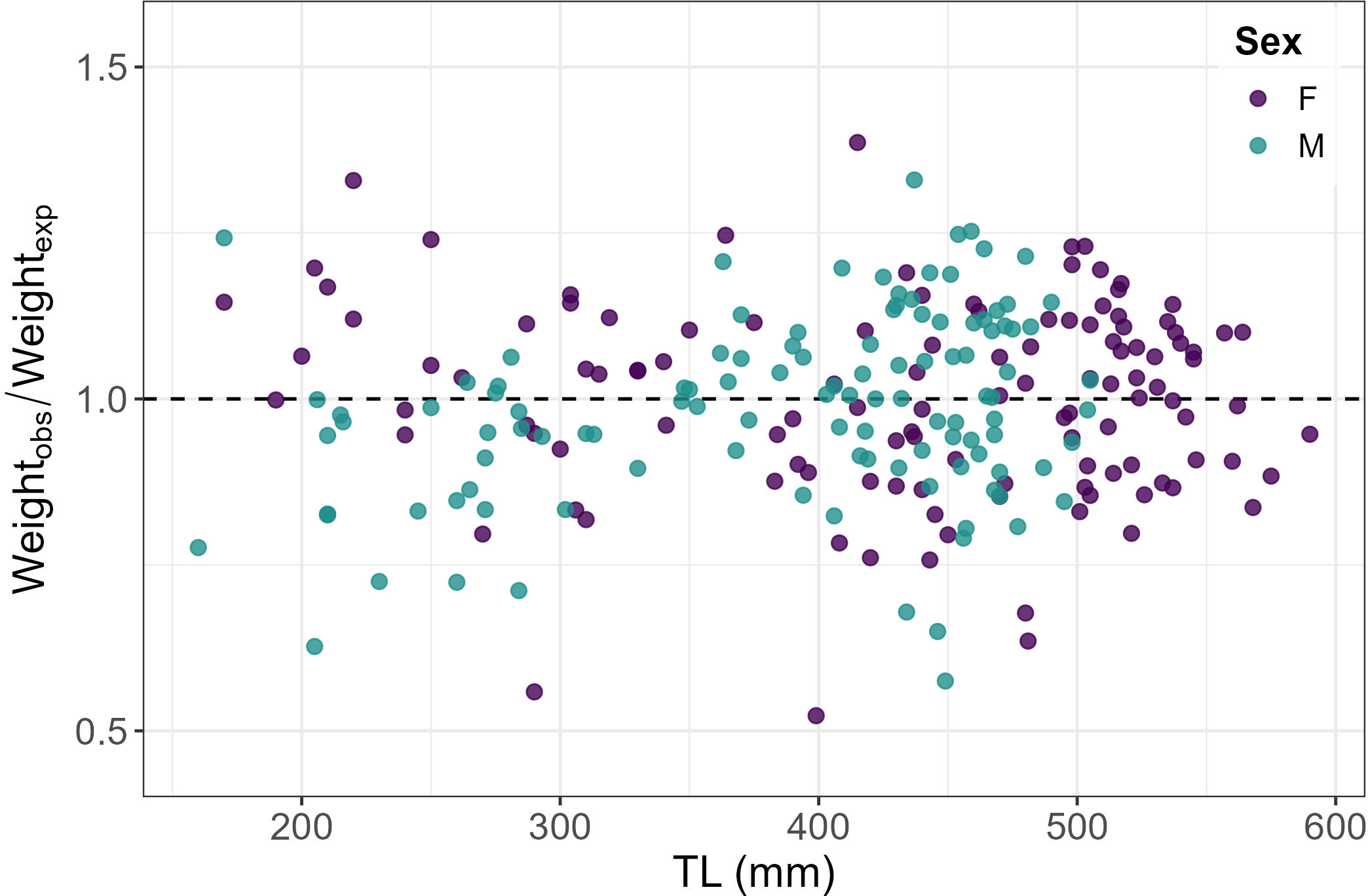
Scatterplot showing the Relative Condition Factor (Kn) calculated for both sexes highlighting different physical conditions of the starry ray individuals oscillating from under (< 1) to overweight (> 1) independently to size.
The selected linear model for DL includes TL and sex as predictors, whereas in the model for DW the TL, sex, and their interaction were retained (Table 1). In the DL model, TL emerged as a highly significant driver associated with an increase in DL (p < 0.001) - while the effect of sex did not reach statistical significance (p ≈ 0.07). In the DW model, total length again emerged as a highly significant positive predictor (p < 0.001; Table 1). The main effect of sex was also significant (p = 0.0009), indicating that, at a given TL, females exhibit a larger DW than males. Crucially, the TL × sex interaction proved highly significant (p < 0.001), revealing that the rate at which DW increases with body size is lower in males than in females. This interaction structure drove the DW model to explain 94.5% of the variance in width (adjusted R² = 0.944), underscoring a pronounced, sex‐specific allometric pattern.
Table 1
| Coefficients | Estimate | Std. Error | t value | P value |
|---|---|---|---|---|
| DL ~ TL + Sex (AIC = 1571.1) | ||||
| Intercept | -1.75 | 11.40 | -0.15 | 0.87 |
| TL | 0.57 | 0.02 | 23.82 | < 0.001 |
| Sex | 6.79 | 3.74 | 1.81 | 0.07 |
| DW ~ TL * Sex (AIC = 1208.7) | ||||
| Intercept | 9.02 | 6.99 | 1.29 | 0.20 |
| TL | 0.68 | 0.01 | 45.93 | < 0.001 |
| Sex | 33.60 | 9.95 | 3.38 | 0.01 |
| TL*Sex | -0.11 | 0.02 | -5.15 | < 0.001 |
Summary table of the TL-DL and TL-DW linear model results.
The sex ratio of the sampled population of starry ray was not significantly different from a 1:1 (χ2 = 0.0672, df = 1, p > 0.05).
Reproduction
All sampled starry rays were macroscopically sexed and all the maturity stages were detected during the sampling period for both sexes, although each maturity stage was differently represented. In particular, females were 38.8%, 30.6%, 13.2, 11.6% and 5.8% in stages F1, F2, F3A, F3B and F4, respectively, whereas males were 35.0%, 11.1%, 28.2%, 24.8% and 0.9% in stages M1, M2, M3A, M3B and M4, respectively. Table 2 also summarizes the months at which each maturity stage was detected.
Table 2
| Maturity stage | Month | % by sex |
|---|---|---|
| F1 | Jan, Feb, Mar, Jul, Sep, Oct, Dec 2020; Nov 2023 | 38.84 |
| F2 | Jan 2019; Jan, Feb, Mar, May, Jul, Sep, Oct, Dec 2020; Nov 2023 | 30.58 |
| F3A | Jan, Feb, Mar, Apr 2020 | 13.22 |
| F3B | Apr, May, Sep 2020 | 11.57 |
| F4 | Sep, Oct, Dec 2020, Nov 2023 | 5.79 |
| M1 | Dec 2019; Jul, Sep, Oct, Dec 2020; Nov 2023 | 35.04 |
| M2 | Sep, Oct, Dec 2020; Nov 2023 | 11.11 |
| M3A | Dec 2019, Jan, Mar, Apr, Sep, Oct 2020; Nov 2023 | 28.21 |
| M3B | Dec 2019, Feb, Mar, Apr, May, Dec 2020; Nov 2023 | 24.79 |
| M4 | Mar 2020 | 0.85 |
Maturity stages detected fraction (%) and months of occurrence for males (M) and females (F) R. asterias individuals over the sampling period.
Size at sexual maturity was different in females and males, being respectively 425 mm and 360 mm (Figure 6), which roughly corresponded to an age of 3.0 and 2.0 years, respectively. The gonadosomatic index (GSI) was calculated for 52 individuals, 15 females and 37 males, from which it was possible to remove gonads at different maturity stages. GSI values consistently increased with gonad maturation, being 0.3%, 1.1%, 4.5 ± 1.8% in maturity stages F1, F2, F3A, and 0.7 ± 0.32%, 1.9 ± 0.2%, 2.0 ± 0.2% in stages M2, M3A, M3B, respectively (Figure 7). However, in females in stages F1 and F2 only one gonad’s individual was available.
Figure 6
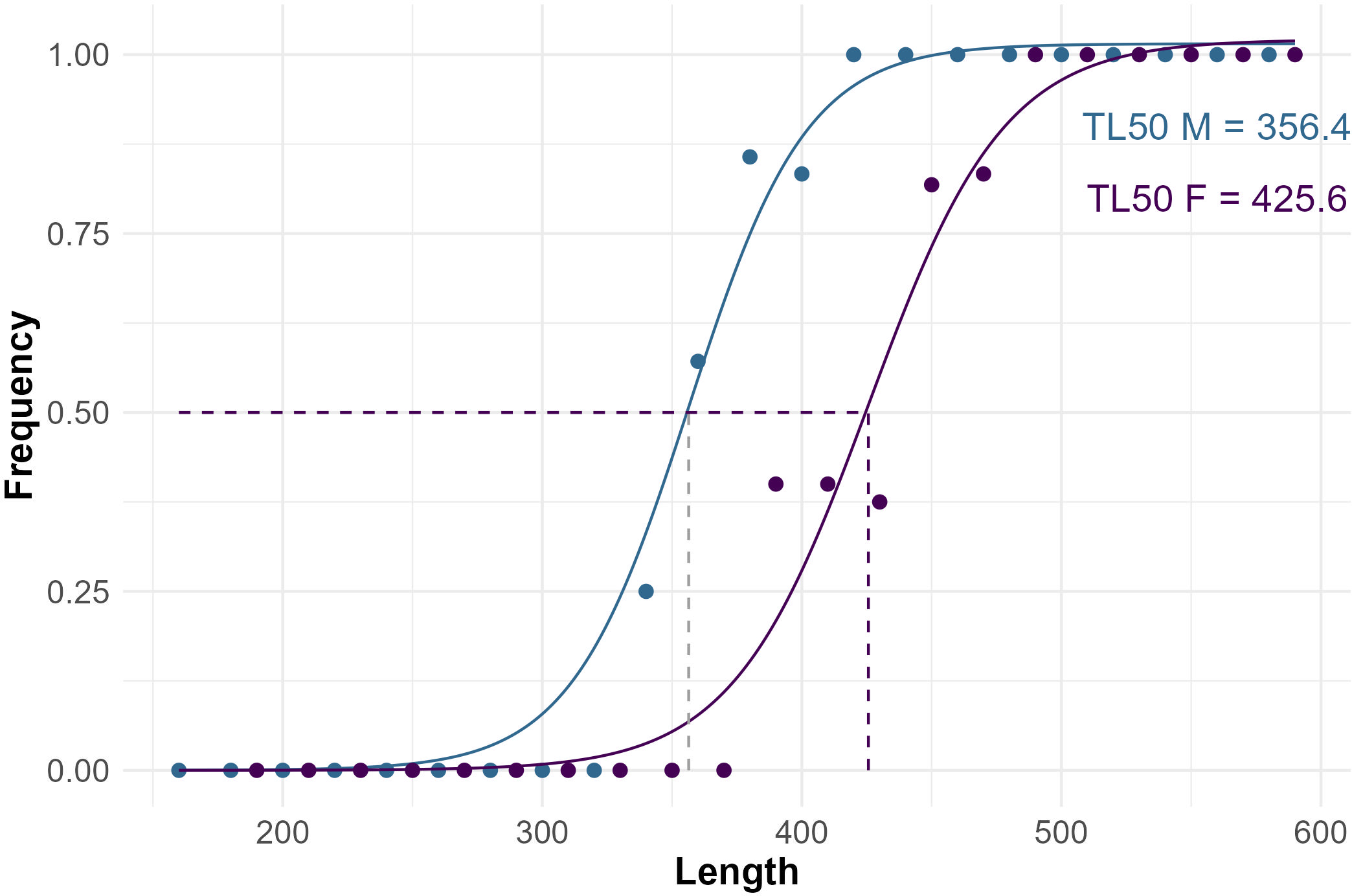
Size at sexual maturity (TL50) assessed for females and males starry ray individuals from the mid-western Adriatic Sea.
Figure 7
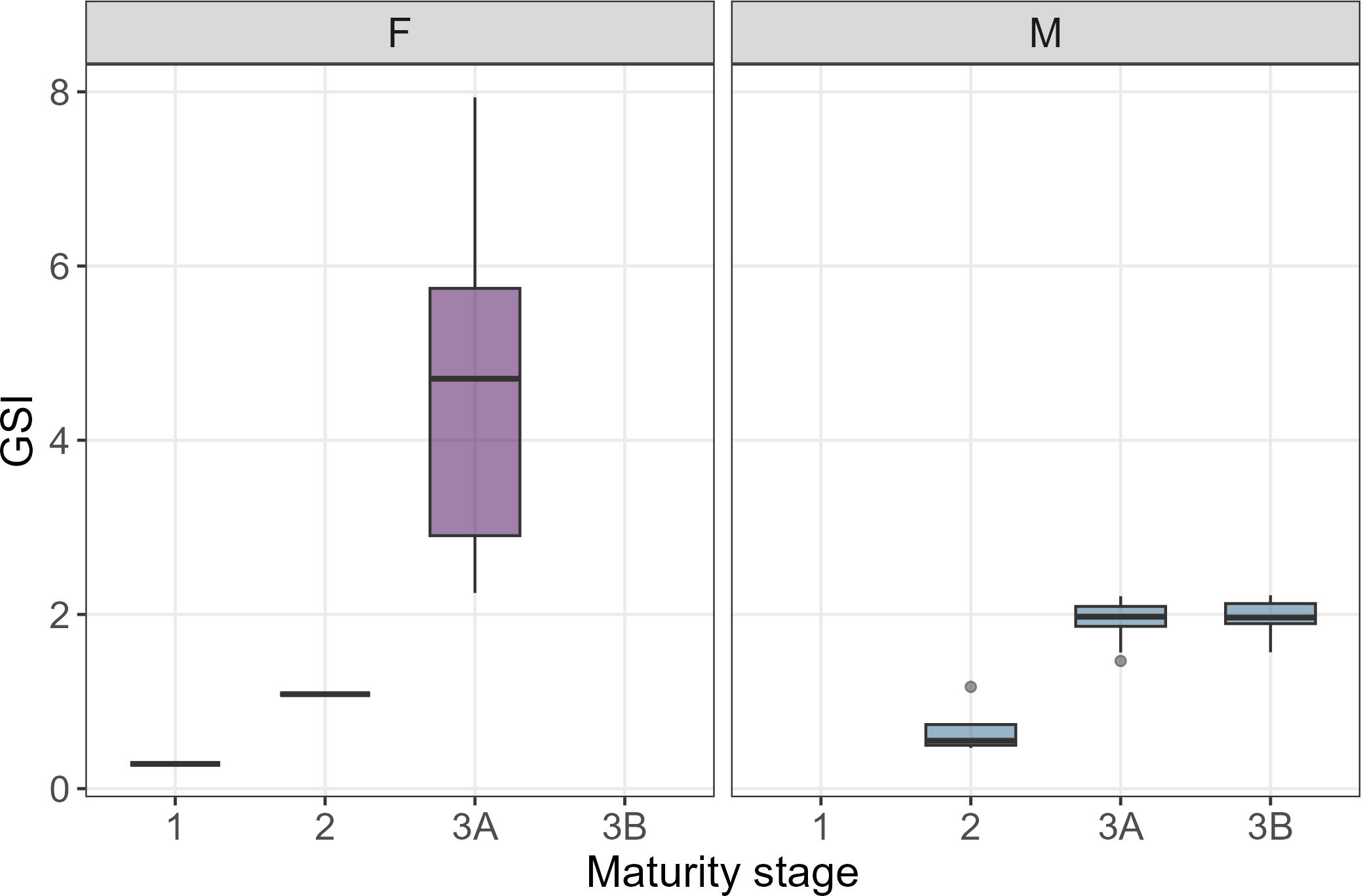
Gonado-somatic Index (GSI) calculated in relation to maturity stage per each sex. Only one gonad’s individual was available for F1 and F2 maturity stages.
Age and growth
Age was assessed on 125 starry ray individuals (70 females and 55 males) testing the 4 different ageing techniques. The lowest AIC value, for the best growth model selection for each sex, was obtained from the length at age data derived from the reading of DTV for both females and males. Also the lowest APE and ACV values, reflecting good method consistency and reproducibility, were obtained with the same ageing technique independently from sex (Table 3). Thus, DTV ageing technique was the only selected to describe the R. asterias growth curve by sex. Age estimates ranged from 0+ to 4.5 years in females, and from 0+ to 4 years in males, although only few were the individuals aged ≥3.5 in males and = 4.5 in females.
Table 3
| Ageing technique | Sex | L∞ | k | AIC | ACV | APE |
|---|---|---|---|---|---|---|
| WUV | F | 719.09 | 0.2804 | 2531084.00 | 11.07 | 7.83 |
| M | 553.99 | 0.4417 | 1970799.00 | |||
| WTV | F | 702.4 | 0.2938 | 2510279.00 | 8.75 | 6.19 |
| M | 575.45 | 0.4284 | 1973427.00 | |||
| DUV | F | 680.16 | 0.3311 | 2366796.00 | 5.79 | 4.09 |
| M | 572.84 | 0.4363 | 1916481.00 | |||
| DTV | F | 727.93 | 0.2864 | 2297436.00 | 3.44 | 2.43 |
| M | 573.57 | 0.4419 | 1887028.00 |
Resulting growth curve parameters and AIC values for each ageing technique tested per sex for the best growth model selection.
The indices of age precision APE and ACV per technique are also reported. WUV, whole untreated vertebrae; DUV, dissected untreated vertebrae; WTV, whole treated vertebrae; DTV, dissected treated vertebrae.
The VBF growth curve parameters of females (L∞ = 728, k = 0.28) differed widely from those of males (L∞ = 574, k = 0.44). Indeed, when considered separately, the LogLikelihood ratio test highlighted significantly higher L∞ for females (χ² =5.441, p < 0.05), whereas k showed significantly higher values for males (χ² =6.884, p < 0.05). Also, when considered as a whole, the LogLikelihood ratio test highlighted a highly significant difference in the growth pattern between the two sexes (χ² =7.989, p < 0.05) (Table 4), which was described by two different growth curves (Figure 8).
Table 4
| Females | Males | RSS | 𝒳2 | df | P | |||
|---|---|---|---|---|---|---|---|---|
| Parameter | L∞ (mm) | K | L∞ (mm) | k | ||||
| H 0 | 727.93 | 0.2864 | 573.57 | 0.4419 | 92502.6 | |||
| H 0: = L∞; = k; | 684.85 | 0.31914 | 684.85 | 0.31914 | 98607.9 | 7.989289 | 2 | ** |
| H 0: = L∞ | 679.66 | 0.32607 | 679.66 | 0.32037 | 98281.6 | 7.57497 | 1 | ** |
| H 0: = k | 678.028 | 0.32898 | 667.2669 | 0.32898 | 97739.9 | 6.8841 | 1 | ** |
Likelihood ratio test comparing the von Bertalanffy growth parameters estimates for both sexes of Raja asterias derived from the DTV ageing technique.
Statistics are based on four null hypotheses, assuming that each parameter or all of them do not differ between sexes. RSS = residual sum of squares.; df = degrees of freedom; ** significant at α = 0.01.
Figure 8
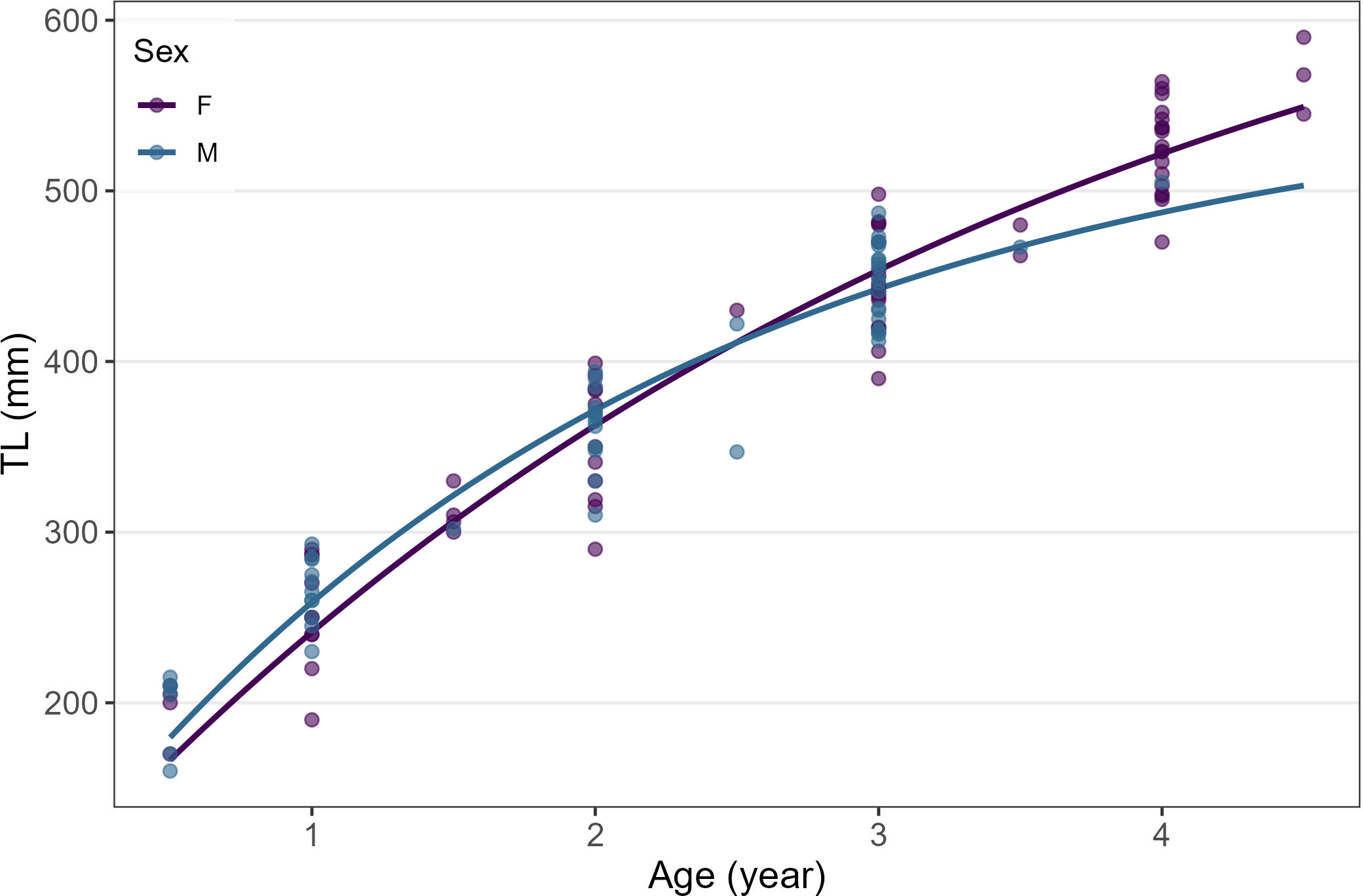
Resulting VBF growth curves of females and males starry ray individuals based on the length at age estimates derived from reading the dissected treated vertebra (DTV).
From the resulting growth curves, given the TL50 for both sexes, it was possible to back calculate the age at maturity, resulting in about 3.0 and 2.0 years for females and males, respectively.
Spatial distribution
The distribution pattern of R. asterias individuals based on sex and maturity stage (i.e., mature vs. immature) revealed that immature individuals almost exclusively concentrate near the coast within 50 m depth, while mature individuals mainly inhabit greater depths up to 80 m, partially overlapping with immatures in the inshore area (Figure 1). Considering the capture dates, it was also observed that mature individuals are especially present at shallower depths between October and March.
Abundance and biomass trends from SoleMon survey
The frequency of occurrence of starry ray in the SoleMon survey catches increased over time (Supplementary Table 1). This positive trend was also observed for the relative abundance (N/km2) and biomass (kg/km2) of the species (Figures 9A, B). Although with fluctuations, the average values of these population parameters have shown a significant increasing trend during the years (Table 5). The lowest values (less than 15 N/km2 and 5 kg/km2) were recorded between 2007 and 2012, and the highest starting from 2020 (more than 40 N/km2 and 15 kg/km2). The drop in abundance recorded in 2022 was caused by the drastic reduction in monitored stations due to adverse events (i.e., exceptionally bad weather conditions and COVID-19 pandemic issues).
Figure 9
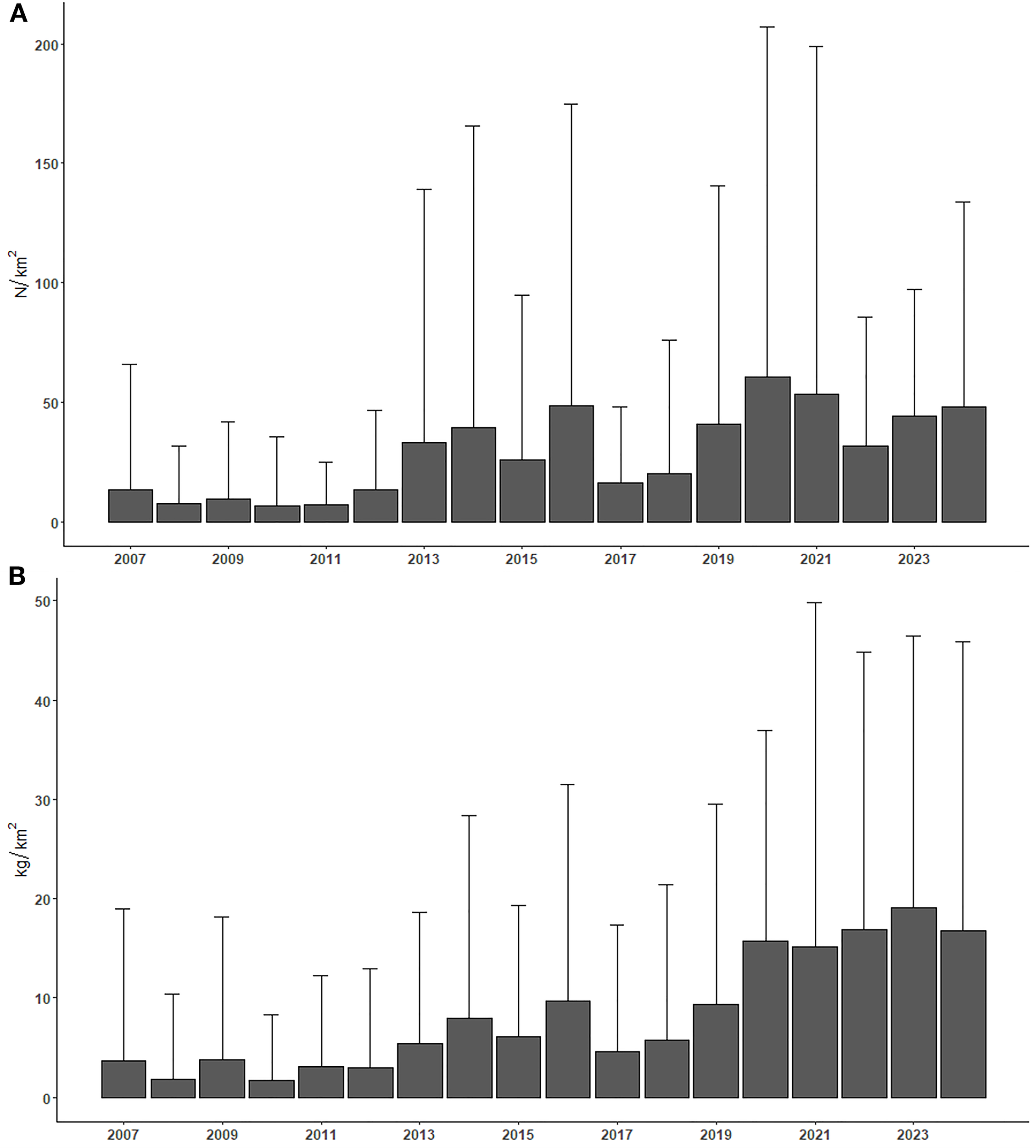
Time trends of the mean values of abundance (A) and biomass (B) indices recorded during the SoleMon surveys (2007-2024).
Table 5
| DF | SS | MS | F-statistic | P-value | ||
|---|---|---|---|---|---|---|
| N/km2 | X | 1 | 159.8 | 159.8 | 36.251 | 2.335*10-9 |
| Error | 1142 | 5034.9 | 4.409 | |||
| kg/km2 | X | 1 | 119.8 | 119.8 | 18.171 | 2.186*10-5 |
| Error | 1142 | 7527.3 | 6.591 | |||
ANOVA outputs of the weighted regression analysis of abundance and biomass indices.
DF, degrees of freedom; SS, sum of squares; MS, mean of squares.
Discussion
The work aimed to characterize key aspects of the starry ray (Raja asterias) life cycle in the mid-western Adriatic Sea, given the known susceptibility of chondrichthyans to fishing activities. To support the conservation of the species and identify suitable technical measures for application in the Adriatic Sea, it is essential to have a comprehensive understanding of its biological characteristics across different temporal and spatial scales.
Life-history traits
In this study starry rays were collected over a wide timeframe using different fishing gears (OTB and TBB) operating at different depths and distances from the coast of the mid-western Adriatic Sea; for these reasons, and considering the low selectivity of these gears towards this species (Lucchetti et al., 2021), the sampled individuals were considered sufficiently representative of the population structure present at sea. Our observed population size range (160–590 mm TL) was quite similar to those reported by other authors for different starry ray populations in different Italian waters. In particular, agreement between studies is found in the maximum size recorded at sea, while the smallest size sampled and reported by many other authors was not detected here. Indeed, in the northern-central Adriatic Sea, the reported size ranges were of 85–560 mm TL (Ferrá et al., 2016), and 160–590 mm (Fanelli et al., 2023), and in the Tyrrhenian Sea of 141–600 mm TL (Romanelli et al., 2007), 205–590 mm TL (Geraci et al., 2021), and 90–620 mm TL (Coll et al., 2013). The widest R. asterias population size range (88–696 mm TL) was instead reported in the southern Adriatic Sea by Porcu et al. (2022). Moreover, the broader size range here detected for females compared to males confirms previous findings on the R. asterias sex size structure from different Mediterranean areas (Romanelli et al., 2007; Coll et al., 2013; Geraci et al., 2021; Fanelli et al., 2023), thus indicating sexual dimorphism in the size frequency distributions of the starry ray individuals.
The TL-W regression analysis revealed isometric growth (b = 3) for males of the R. asterias population, while females exhibited positive allometric growth (b > 3), indicating a greater increase in weight relative to length for larger individuals. This highlights sexual dimorphism in the growth pattern of R. asterias, as already observed in other studies conducted within the Mediterranean basin (Serena et al., 2010; Geraci et al., 2021). Isometric growth is typical for most elasmobranch families in the Mediterranean; in contrast, positive allometric growth is often associated with seasonal variations in body weight, significantly influenced by gonadal and liver weight (Froese, 2006). As an oviparous species, R. asterias requires substantial amounts of vitellogenin synthesized in the liver, which impacts the weight of females (Castro, 2000; Lutton et al., 2011; Tsikliras and Dimarchopoulou, 2021).
The condition factor analysis revealed variability in the physical condition of both females and males (e.g., normal weight, underweight, or overweight) without a specific trend relative to TL. This can be attributed to seasonal dietary fluctuations based on energy requirements. During mating periods, this species tends to adopt a more generalist diet, while at other times of the year, it selects prey with higher caloric and lipid content to prepare its reproductive system for egg-laying (Serena et al., 2010; Fatimetou and Younes, 2016; Fanelli et al., 2023).
The analysis of TL-DW regression showed that females have a significantly broader disk than males at the same body length, while no significant difference was detected in the TL-DL relationship between sexes. The broader disk in females may be linked to reproductive needs, such as egg deposition and energy reserve storage, which require additional abdominal cavity space, while males maintain a slimmer morphology, possibly enhancing agility during courtship and copulation (Wearmouth and Sims, 2008; Serena et al., 2010; McCully et al., 2012).
For the investigation of the R. asterias reproductive cycle, the extended sampling period (December 2019 – November 2023) and the different fishing gears used (i.e. OTB and TTB) across different depths, distances from the coast, and times of the year, allowed to identified all the maturity stages for both sexes, albeit with varying percentages. Mature individuals were sampled year-round, supporting the possibility of both annual and semi-annual egg deposition in this species (Romanelli et al., 2007; Serena et al., 2010; Ferrá et al., 2016). Conversely, resting-phase individuals were infrequent, as observed in other studies on R. asterias and other ray species (e.g., Raja brachyura) (Romanelli et al., 2007; Serena et al., 2010; Porcu et al., 2015; Ferrá et al., 2016).
The higher TL50 calculated for R. asterias females’ individuals compared to males (425 vs 360 mm TL), indicating that females reach sexual maturity at larger sizes than males, is consistent with previous studies conducted inside the Mediterranean basin (Barone et al., 2007; Romanelli et al., 2007; Serena et al., 2010; Porcu et al., 2015; Ferrá et al., 2016). However, the same studies also detected higher values of TL50 for both sexes than ours, ranging from 492 to 565 mm TL for females, and from 414 to 520 mm TL for males, depending on the study area (Serena et al., 2005; Barone et al., 2007; Romanelli et al., 2007). The lower TL50 values here observed for both sexes may be associated with unfavorable growth conditions or higher fishing pressure on the population, as noted in other Mediterranean studies for R. asterias (Coll et al., 2013; Porcu et al., 2022) and other Adriatic ray species (e.g., Raja clavata) (KrstulovićŠifner et al., 2009) (Supplementary Table 2). A lower TL50 indicates that individuals are reaching sexual maturity at a smaller size (and usually at a younger age) than they did in the past. This shift can be an adaptive response to intense fishing pressure. When fishing pressure is high, especially with fishing gear not selective enough, such as trawling in the Mediterranean, there is a strong selective pressure favoring earlier reproduction: i) Large individuals are more likely to be caught, reducing their chances to reproduce; ii) Smaller, early-maturing individuals have a higher probability of reproducing at least once before being caught; iii) over generations, this can lead to a genetic or phenotypic shift in the population, resulting in a lower TL50, possibly as a compensatory mechanism to maintain population levels.
Additionally, the genetic diversity of R. asterias could explain morphometric variations between different geographic areas (Catalano et al., 2022). However, early maturity often comes at the cost of reduced body size, lower fecundity (produce fewer eggs), and lower resilience, which may have long-term negative effects on stock health and productivity (Bellodi et al., 2023; Serrat et al., 2023). Furthermore, changes in TL50 can alter ecological interactions, affecting the trophic network where this species occupies an intermediate and critical role (Coll et al., 2013; Fanelli et al., 2023).
Considering the reproductive strategy of the species - oviparous with multiple reproductive seasons within a year (Serena et al., 2010; Yemışken et al., 2018) - the GSI was analyzed based on the maturity stages as already reported for R. asterias by Ferrá et al. (2016) and Romanelli et al. (2007), although Fanelli et al. (2023) for the same species focused on seasonality. The GSI results confirmed that as maturity stages progress, indicated by egg maturation in the uterus through vitellogenesis and yolk accumulation in females (Relini, 2008; Serena et al., 2010) and testicular development in males (Serra-Pereira et al., 2011), the GSI increases. This trend reflects a greater energetic investment in reproduction during advanced maturity stages (Camhi, 1998; Serena et al., 2010).
The equal sex ratio of the sampled population in this study mirrors values previously reported for the northern-central Adriatic (1.04:1) (Ferrá et al., 2016) and other areas of the Mediterranean, such as the Strait of Sicily (1.13:1) (Geraci et al., 2021). This suggests that the sex ratio is balanced among populations over different geographic areas, as reported by Scacco et al. (2024).
Of the four ageing techniques tested to investigate the age and growth of R. asterias, the DTV proved to be the most reliable and accurate approach for both sexes, given the derived lowest AIC, APE and ACV values, so it was the only one considered to describe the growth pattern of the species. The burning technique has never been adopted before on vertebrae of cartilaginous fishes, although commonly used on otoliths of different bony fish species (Panfili et al., 2002; Sabatini et al., 2025). To date, in literature, the best staining method for highlighting growth bands in R. asterias has been considered the Safranin O staining technique, though providing high APE and ACV values of 7.2% and 10.6%, respectively (Basusta et al., 2017), thus exceeding the acceptable thresholds of 5.5% and 7.6% proposed by several authors (Campana, 2001; Kadri et al., 2013; Basusta et al., 2017). Similarly, high APE and ACV values were obtained with the other three ageing techniques here tested, which failed to improve ring resolution, and the derived length at age data were thus excluded to describe the R. asterias growth pattern per sex. Nonetheless, burning and dissecting is for sure an easier, faster and cheaper method compared to staining techniques, thus this method should be further applied on other vertebrae or ageing structures of different cartilaginous fishes, looking for comparisons with common staining techniques used.
The R. asterias growth curve was developed by plotting the DTV length at age data separately for females and males. Although most studies conducted so far have generally developed a single growth curve for the entire population (e.g., Bono et al., 2005; Serena, 2005; Scacco et al., 2024), our results demonstrated a highly significant difference in the growth pattern between sexes, with a higher L∞ value detected for females (723 vs 574 mm TL) and a slightly greater k value for males (0.44 vs 0.28), thus suggesting the need to develop two separate growth curves. However, to date, only one study has analyzed the R. asterias growth pattern divided by sex, finding no significant differences between males and females (Serena and Abella, 1999). Overall, the resulting growth parameters for the R. asterias population over different Italian waters highlighted an L∞ ranging from approximately 675 to 700 mm TL and a k value of around 0.40-0.45, indicating a rather slow growth. By comparing the k value of other Rajiformes species, such as R. clavata, R. brachyura, and R. miraletus, it appears that R. asterias exhibits a faster growth rate than the other ones (Table 6), particularly compared to R. miraletus (Abdel-Aziz, 1992; Kadri et al., 2012), its ecological counterpart in overlapping areas (Follesa et al., 2010; Serena et al., 2010; Kadri et al., 2012; Ferrá et al., 2016). This growth advantage is significant considering the intense fishing pressure faced by the species inside and outside the Adriatic basin (Romanelli et al., 2007; Coll et al., 2013; Ferrá et al., 2016; Biton-Porsmoguer and Lloret, 2020).
Table 6
| Species | Area | Population | Females | Males | Reference | |||
|---|---|---|---|---|---|---|---|---|
| L∞ | k | L∞ | k | L∞ | k | |||
| R. asterias | Mid-western Adratic Sea | - | - | 723 | 0.28 | 574 | 0.44 | Present study |
| R. asterias | Tyrrhenian Sea | 699 | 0.4 | - | - | - | - | Scacco et al. (2024) |
| R. asterias | Tyrrhenian Sea | 675 | 0.45 | - | - | - | - | Bono et al. (2005) |
| R. asterias | Ligurian Sea | 675 | 0.45 | - | - | - | - | Serena et al. (2005) |
| R. clavata | Gulf of Gabès (south-central Mediterranean Sea) | 1142 | 0.11 | - | - | - | - | Kadri et al. (2014) |
| R. clavata | Mediterranean Sea | 1280 | 0.11 | - | - | - | - | Serena et al. (2010) |
| R. brachyura | Central-western Mediterranean Sea | 1113 | 0.1 | - | - | - | - | Porcu et al. (2015) |
| R. miraletus | Gulf of Gabès (south-central Mediterranean Sea) | 692 | 0.18 | - | - | - | - | Kadri et al. (2012) |
| R.miraletus | Mediterranean Sea (Egypt) | - | - | 919 | 0.17 | 670 | 0.22 | Abdel-Aziz (1992) |
Summary of the growth curves parameters (L∞ and k) derived from the elaboration of VBGF curves for four different species of Rajiformes in the Mediterranean.
P, population; F, females; M, males.
In our study, the estimated age range was also higher in females (from 0+ to 4.5 years) than in males (from 0+ to 4.0 years), thus indicating that females reach greater ages and sizes compared to males, although in both sexes the number of older individuals was extremely limited. For R. asterias populations, similar age range estimates (from 0+ to 6 years) were reported in the Tyrrhenian (Bono et al., 2007) and the Ligurian Seas (Campisi and Murenu, 1999), whereas significantly higher maximum age (10 years) was reported in the central Mediterranean Sea (Serena, 2006; Scacco et al., 2024). These differences in age estimates could be attributed to the different maximum sizes of samples (Basusta et al., 2017; Bargione et al., 2019), varying fishing pressures in the studied areas (Lotze et al., 2011; Bastardie et al., 2017), genetic diversity of the different populations (Catalano et al., 2022), geographic variability in environmental conditions (Blondel, 2010), different ageing techniques adopted and the expertise of readers (Bellodi et al., 2023).
As a result, all these factors can differently affect the growth patterns of the species and other biological traits, such as the size and age at which sexual maturity is reached (Romanelli et al., 2007; Ferrá et al., 2016; Geraci et al., 2021). In this work, it was found that females reach TL50 at a higher size and an older age than males, specifically at around 3.0 years compared to 2.0 years for males. As previously mentioned, a potential reduction in size and age at sexual maturity negatively impacts the species’ ecology (Bellodi et al., 2023; Serrat et al., 2023) and trophic network functioning (Coll et al., 2013; Fanelli et al., 2023). Therefore, understanding the biological aspects of the species, as well as behavioral traits such as segregation based on size, maturity, and/or sex, is crucial for identifying which population segments are directly or indirectly targeted by different fishing activities and for developing appropriate management and conservation measures (Ferrá et al., 2016). The production of a distribution map of R. asterias based on maturity stage (i.e., mature vs. immature) and sex revealed that juveniles, which have not reached sexual maturity yet, are almost exclusively concentrated near the coast within 50 m depth, and rarely beyond. In contrast, adult and mature individuals mainly inhabit greater depths up to 80 m. These findings are consistent with Ferrá et al. (2016), who reported a clear spatial segregation between mature and immature individuals in the northern-central Adriatic Sea, with the former concentrated at depths greater than 45 m and the latter caught within the first 30 m. The same spatial segregation has also been detected for this species in different Mediterranean areas (Romanelli et al., 2007; Serena et al., 2010; Porcu et al., 2022) and can be explained as a combination of factors such as prey availability, predation risk, temperature, salinity, oxygen deficiency, local hydrodynamics, and substrate type (Gibson, 1994; Knip et al., 2010; McAllister et al., 2024). In this study, the presence of adults starry rays has also been observed near the coast, particularly between October and March, which may be explained by seasonal reproductive migrations to more sheltered and shallower waters (Gibson, 1994; Camhi, 1998; Abella and Serena, 2005; Barone et al., 2007; Romanelli et al., 2007; Ferrá et al., 2016). Thus, for example, restricting fishing in very shallow waters could help limit bycatch of non-reproductive or egg-laying individuals given their distribution pattern.
Fishery management implications
The results of this study underscore the need for species-specific and sex-specific management measures for R. asterias. Conservation strategies should avoid relying solely on population-wide averaged biological parameters and instead adopt the most precautionary approach, considering the observed sex-related differences in growth and reproduction.
Continuous monitoring of R. asterias populations is essential, particularly given the species’ bathymetric and spatial distribution, which exposes it to incidental capture across multiple fisheries (e.g., OTB, GNS, TBB). To address this, regular recording and monitoring of R. asterias catches should be implemented, in line with EU fisheries data collection obligations such as those outlined in EC Regulation No. 1581/2004 which sets detailed rules for recording and reporting catch and effort data for certain fishery sectors, with the aim of improving the scientific basis for sustainable fisheries management. Similar monitoring is already in place for other skate and ray species in Italian waters.
Importantly, the FAO-GFCM Multiannual Plan (MAP) for demersal fisheries in the Adriatic Sea (GFCM, 2019; GFCM/43/2019/5) provides a framework for reducing fishing effort and protecting sensitive habitats. The MAP includes measures such as fishing effort limits, spatial restrictions, and temporal closures, which could directly benefit R. asterias if applied to identify mating and nursery areas. Mapping these critical habitats should therefore be a priority, enabling protective measures like seasonal trawl bans during peak egg-laying periods (summer to early autumn) and restrictions on anchoring in shallow nursery grounds (Biton-Porsmoguer and Lloret, 2020; Lloret et al., 2020; Catalano et al., 2022).
By extending the SoleMon time series through 2024, this study provided updated and robust evidence to evaluate the population status of R. asterias in the northern-central Adriatic Sea. In continuity with the positive signals previously highlighted by Ferrá et al. (2016) using earlier datasets, our results show a significant and consistent increase in both the frequency of occurrence and the relative abundance and biomass (N/km², kg/km²) of the species over time. Notably, the upward trends become particularly evident from 2020 onwards, suggesting a possible delayed but effective response of R. asterias populations to the implementation of specific management measures under the MAP for demersal fisheries in the Adriatic. These include seasonal fishing closures, more rigorous monitoring and reporting of bycatch species, and increased awareness regarding elasmobranch conservation. While the temporary drop in abundance observed in 2022 is attributable to exceptional reductions in survey effort due to adverse weather and COVID-19 disruptions, the overall trajectory remains positive. Such findings reinforce the importance of long-term standardized monitoring programs like SoleMon for assessing the effectiveness of fisheries management actions and underscore the potential for recovery in commercially exploited bycatch species when appropriate conservation strategies are applied.
Additionally, targeted studies should investigate the seasonal and spatial overlap between fishing activities and R. asterias habitat use, as well as gear-specific impacts (Li Veli et al., 2024; De Santis et al., 2025). These insights would support the development of mitigation measures tailored to this species.
Moreover, as in the present work, providing comparable demographic information for this species using easily replicable methods will help fill knowledge gaps and support its conservation. Indeed, due to their biological and reproductive characteristics, without appropriate conservation plans and management measures, no population of cartilaginous fish will be able to remain viable over time.
Considering the low selectivity towards this species (Lucchetti et al., 2020, 2021) and its marketability, future studies should also focus on measures, such as survivability, gear modification, and handling. Skates and rays often show moderate to high survival rates after capture, especially compared to more delicate teleost fish. Studies in the North and Mediterranean Seas indicate survival rates between 20 –60% for some Raja species (Catchpole et al., 2017; Van Bogaert et al., 2020; Schram et al., 2023). Their robust morphology, benthic lifestyle, and low metabolic rates contribute to this. However, survival depends heavily on: i) gear type; ii) soak time or towing duration; iii) depth of capture; iv) handling time on deck; v) air exposure and injuries. Passive gears usually show higher survivability, often > 60%, especially if animals are released quickly and handled properly. Survival is increased with shorter soak times and minimal handling. Finucci et al. (2021), in a global review of elasmobranch discard mortality, noted that Mediterranean Rajidae are often bycaught and discarded, and that post-release survival can be significant if best practices are followed. Several projects have suggested easy guidelines to reduce post-release mortality of sharks (Bottaro et al., 2022; Wosnick et al., 2023; Worm et al., 2024; Colloca et al., 2025).
Recent experiments in the Adriatic Sea (Brčić et al., 2015; Lucchetti et al., 2021; De Santis et al., 2024) have tested the effectiveness of grid-like Turtle Excluder Devices (TEDs) adapted for Mediterranean trawl fisheries. These studies demonstrated that TEDs can significantly reduce the bycatch of elasmobranchs, including various shark species, without compromising the catch of commercial target species. The rigid grid structure allows larger animals, such as sharks and rays, to escape through an exit opening, thereby improving selectivity and contributing to the conservation of vulnerable species. These trials highlight the potential of TEDs as a practical mitigation tool in demersal trawl fisheries in the region. In summary, the integration of best handling practices, selective gear modifications (e.g., escape panels, shorter haul durations), spatial management measures and effort reduction under the GFCM MAP offers a pathway towards the sustainable exploitation and long-term viability of R. asterias populations in the Adriatic. These combined actions are critical given the species’ life history traits and sensitivity to fishing impacts.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because all applicable international, national, and/or institutional guidelines regarding the care and use of animals were adhered to.
Author contributions
GB: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. AP: Data curation, Methodology, Writing – original draft. FD: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. DL: Data curation, Formal analysis, Visualization, Writing – review & editing. LS: Writing – review & editing, Data curation, Visualization. ES: Writing – review & editing, Methodology. GS: Funding acquisition, Project administration, Writing – review & editing. AL: Writing – review & editing, Funding acquisition, Project administration, Resources, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was conducted within the European Data Collection Framework (https://dcf.ec.europa.eu/index_en?prefLang=it) and was supported financially by the Italian Ministry of Agriculture, Food Sovereignty, and Forestry (MASAF).
Acknowledgments
The authors would like to express their gratitude to the researchers who conducted the SOLEMON survey and to the fishermen aboard the vessel Airone Bianco II for providing the samples. Special thanks are also extended to Matteo Barbato for his assistance with sample processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1679293/full#supplementary-material
References
1
Abdel-Aziz S. H. (1992). The use of vertebral rings of the brown ray Raja miraletus (Linnaeus 1758) off Egyptian Mediterranean coast for estimation of age and growth. Cybium (Paris)16, 121–132. doi: 10.26028/cybium/1992-162-002
2
Abella A. J. Mancusi C. Serena F. (2017). Synthesis of the knowledge on biology, ecology and fishery of the halieutic resources of the Italian seas. Biol. Mar. Mediterr.24, 144–149.
3
Abella A. J. Serena F. (2005). Comparison of elasmobranch catches from research trawl surveys and commercial landings at port of Viareggio, Italy, in the last decade. J. Northwest Atl. Fish. Sci.35, 345–356. doi: 10.2960/J.v35.m526
4
Akaike H. (1973). Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika60, 255–265. doi: 10.1093/biomet/60.2.255
5
Bargione G. Donato F. La Mesa M. Mazzoldi C. Riginella E. Vasapollo C. et al . (2019). Life-history traits of the spiny dogfish Squalus acanthias in the Adriatic Sea. Sci. Rep.9, 14317. doi: 10.1038/s41598-019-50883-w
6
Barone M. De Ranieri S. Fabiani O. Pirone A. Serena F. (2007). “ Gametogenesis and maturity stages scale of Raja asterias Delaroche 1809 (Chondrichthyes, Raijdae) from the South Ligurian Sea. Hydrobiologia, 580 (1), 245–254).
7
Bastardie F. Angelini S. Bolognini L. Fuga F. Manfredi C. Martinelli M. et al . (2017). Spatial planning for fisheries in the Northern Adriatic: working toward viable and sustainable fishing. Ecosphere8, e01696. doi: 10.1002/ecs2.1696
8
Basusta N. Demirhan S. A. Cicek E. Basusta A. (2017). Comparison of staining techniques for age determination of some Chondrichthyan species. Turk. J. Fish. Aquat. Sci. 17, 41–49. doi: 10.4194/1303-2712-v17_1_06
9
Beamish R. J. Fournier D. A. (1981). A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci.38, 982–983. doi: 10.1139/f81-132
10
Bellodi A. Carbonara P. MacKenzie K. M. Agus B. Bekaert K. Greenway E. S. I. et al . (2023). Measurement of the growth of the main commercial rays (Raja clavata, Raja brachyura, Torpedo marmorata, Dipturus oxyrinchus) in European waters using intercalibration methods. Biol. (Basel).13, 20. doi: 10.3390/biology13010020
11
Biton-Porsmoguer S. Lloret J. (2020). Potential impacts of bottom trawling on species of skates (Rajiformes: Rajidae): The case of the Gulf of Cádiz and the Western Mediterranean. Cybium44, 255–263. doi: 10.26028/cybium/2020-443-006
12
Blondel J. (2010). The Mediterranean region: biological diversity in space and time. (USA: Oxford University Press).
13
Bonanomi S. Brčić J. Colombelli A. Notti E. Pulcinella J. Sala A. (2017). “ Fisheries bycatch of chondrichthyes,” In Rodrigues-FilhoL. F.Sales LunaJ. B. (Eds.), Chondrichthyes–multidisciplinary approach, 39–62. BoD – Books on Demand. doi: 10.5772/intechopen.69334
14
Bonfil R. (1994). Overview of world elasmobranch fisheries. Food Agric. Org. 341, 1–119. Available online at: https://www.fao.org/3/v9878e/v9878e00.htm.
15
Bono L. De Ranieri S. Fabiani O. Mancusi C. Serena F. (2005). Study on the growth pattern of Raja asterias (Delaroche 1809)(Chondrichthyes, Raidae) through the analysis of vertebrae. Biol. Mar. Mediterr.12, 470–474.
16
Bottaro M. Minoia L. Hochscheid S. Barbera F. Bonanomi S. Lucchetti A. et al . (2022). “ Reducing bycatch and mortality in the Med: the project LIFE ELIFE (Elasmobranch Low Impact Fishing Experience),” in Shark International Book of Abstract. Valencia. pp 63.
17
Bradai M. N. Saidi B. Enajjar S. (2012). Elasmobranchs of the Mediterranean and Black Sea: status, ecology and biology bibliographic analysis. 104.
18
Brčić J. Herrmann B. De Carlo F. Sala A. (2015). Selective characteristics of a shark-excluding grid device in a Mediterranean trawl. Fish. Res.172, 352–360. doi: 10.1016/j.fishres.2015.07.035
19
Cailliet G. M. Smith W. D. Mollet H. F. Goldman K. J. (2006). Age and growth studies of chondrichthyan fishes: the need for consistency in terminology, verification, validation, and growth function fitting. Environ. Biol. Fishes77, 211–228. doi: 10.1007/s10641-006-9105-5
20
Camhi M. (1998). Sharks and their relatives: ecology and conservation Gland, Switzerland; Cambridge, (UK: IUCN) pp. 39.
21
Campana S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol.59, 197–242. doi: 10.1111/j.1095-8649.2001.tb00127.x
22
Campana S. E. (2014). Age determination of elasmobranchs, with special reference to Mediterranean species: a technical manual ( Gen. Fish. Comm. Mediterr. Stud. Rev.). I.
23
Campisi S. S. Murenu M. (1999). Sintesi delle conoscenze sulle risorse da pesca dei fondi del Mediterraneo. Biol. Mar. Mediterr.6, 493–498.
24
Capapé C. Quignard J.-P. (1977). Contribution to the biology of the Rajidae of the Tunisian coasts 6. Raja asterias Delaroche 1809 Diet. INSTM Bull. Mar. Freshw. Sci.4, 319–332.
25
Carbonara P. Follesa M. C. (2019). Handbook on fish age determination: a Mediterranean experience. Gen. Fish. Commun. Mediterr. Stud. Rev.1, 1–179.
26
Castro J. I. (2000). The biology of the nurse shark, Ginglymostoma cirratum, off the Florida east coast and the Bahama Islands. Environ. Biol. Fishes58, 1–22. doi: 10.1023/A:1007698017645
27
Catalano G. Crobe V. Ferrari A. Baino R. Massi D. Titone A. et al . (2022). Strongly structured populations and reproductive habitat fragmentation increase the vulnerability of the Mediterranean starry ray Raja asterias (Elasmobranchii, Rajidae). Aquat. Conserv. Mar. Freshw. Ecosyst.32, 66–84. doi: 10.1002/aqc.3739
28
Catalano B. Mancusi C. Clò S. Serena F. Vacchi M. (2003). Tag and release of juvenile specimen of ray Raja asterias in Tuscan waters: preliminary results and working perspectives. Biol. Mar. Mediterr10, 789–791. doi: 10.1002/aqc.3739
29
Catchpole T. Wright S. Bendall V. Hetherington S. Randall P. Ross E. et al . (2017). Ray Discard Survival: Enhancing evidence of the discard survival of ray species. 70.
30
Chang W. Y. B. (1982). A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci.39, 1208–1210. doi: 10.1139/f82-158
31
Coll M. Navarro J. Palomera I. (2013). Ecological role, fishing impact, and management options for the recovery of a Mediterranean endemic skate by means of food web models. Biol. Conserv.157, 108–120. doi: 10.1016/j.biocon.2012.06.029
32
Colloca F. Arcioni M. Acampa F. Valente S. Ventura D. Di Lorenzo M. et al . (2025). Assessing the relevance of sharks and rays for Mediterranean EU fisheries to support a transition from species exploitation to species conservation. Rev. Fish Biol. Fish.35, 487–503. doi: 10.1007/s11160-024-09915-6
33
Cortés E. Brooks E. N. Gedamke T (2012). Population dynamics, demography, and stock assessment. Biology of Sharks and their Relatives, 453–86.
34
Cuoco C. Mancusi C. Serena F. (2005). Study on the feeding habits of Raja asterias Delaroche 1809 (Chondrichthyes, Rajidae). Biol. Mar. Mediterr.12, 504–508.
35
De Santis L. J. Bonanomi S. Bueloni E. Petetta A. Annibale O. Finotto L. et al . (2024). Performance of sorting grids on catching elasmobranchs in a multispecies Mediterranean bottom trawl fishery. Estuar. Coast. Shelf Sci.296, 108594. doi: 10.1016/j.ecss.2023.108594
36
De Santis L. J. Bonanomi S. Li Veli D. Bottaro M. Lucchetti A. (2025). Fishers’ knowledge and risk assessment: a combined approach to studying endangered large-bodied sharks in the central Mediterranean. Rev. Fish Biol. Fish.35, 733–753. doi: 10.1007/s11160-025-09930-1
37
Dulvy N. K. Pacoureau N. Rigby C. L. Pollom R. A. Jabado R. W. Ebert D. A. et al . (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol.31, 4773–4787. doi: 10.1016/j.cub.2021.08.062
38
EC Regulation No. 1581/2004 (2004). Commission Regulation (EC) No 1581/2004 of 27 August 2004 amending Regulation (EC) No 1639/2001 establishing the minimum and extended Community programmes for the collection of data in the fisheries sector and laying down detailed rules for the applicatio. Off. J. Eur. Union L48, 289/6.
39
Fanelli E. Da Ros Z. Martino I. Azzurro E. Bargione G. Donato F. et al . (2023). Crowding in the middle of marine food webs: A focus on Raja asterias and other mediterranean batoids. Mar. Environ. Res.183, 105830. doi: 10.1016/j.marenvres.2022.105830
40
Fatimetou M. K. Younes S. (2016). Diet of Raja asterias (Delaroche 1809) caught along the Mediterranean part of the Moroccan coast (northern Morocco). J. Black Sea/Mediterranean Environ.22, 182–189.
41
Ferrá C. Fabi G. Polidori P. Tassetti A. N. Leoni S. Pellini G. et al . (2016). Raja asterias population assessment in FAO GFCM GSA17 area. Mediterr. Mar. Sci.17, 651–660. doi: 10.12681/mms.1657
42
Field I. C. Meekan M. G. Buckworth R. C. Bradshaw C. J. A. (2009). Susceptibility of sharks, rays and chimaeras to global extinction. Adv. Mar. Biol.56, 275–363. doi: 10.1016/S0065-2881(09)56004-X
43
Finucci J. R. E. Fowler S. L. Grant M. I. Martins A. P. B. Sinclair S. L. (2021). The global status of sharks, rays, and chimaeras. 32.
44
Follesa M. C. Mulas A. Cabiddu S. Porcu C. Deiana A. M. Cau A. (2010). Diet and feeding habits of two skate species, Raja brachyura and Raja miraletus (Chondrichthyes, Rajidae) in Sardinian waters (central-western Mediterranean). Ital. J. Zool.77, 53–60. doi: 10.1080/11250000802589600
45
Froese R. (2006). Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyol.22, 241–253. doi: 10.1111/j.1439-0426.2006.00805.x
46
Froese R. Pauly D. (2020). FishBase ( World Wide Web electronic publication). Available online at: https://www.fishbase.org.
47
Geraci M. L. Ragonese S. Scannella D. Falsone F. Gancitano V. Mifsud J. et al . (2021). Batoid abundances, spatial distribution, and life history traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a knowledge gap through three decades of survey. Animals11, 2189. doi: 10.3390/ani11082189
48
GFCM (2019). Recommendation GFCM/43/2019/5 on a multiannual management plan for sustainable demersal fisheries in the Adriatic Sea (geographical subareas 17 and 18). Available online at: https://gfcm.sharepoint.com/:b:/g/CoC/EWrLEh2_wpdDibe57A4ZfssBDeGRu-ZnSM7tP3eBs3Xguw. (Accessed March 01, 2025).
49
Grati F. Scarcella G. Polidori P. Domenichetti F. Bolognini L. Gramolini R. et al (2013). Multi-annual investigation of the spatial distributions of juvenile and adult sole (Solea solea L.) in the Adriatic Sea (northern Mediterranean). Journal of Sea Research, 84, 122–132.
50
Gibson R. N. (1994). Impact of habitat quality and quantity on the recruitment of juvenile flatfishes. Netherlands J. Sea Res.32, 191–206. doi: 10.1016/0077-7579(94)90040-X
51
Hoff G. R (2016). Identification of multiple nursery habitats of skates in the eastern Bering Sea. Journal of Fish Biology, 88(5), 1746–1757.
52
International Council for the Exploration of the Sea (2012). Report of the workshop on sexual maturity staging of elasmobranchs (WKMSEL) (Lisbon: ICES).
53
Kadri H. Marouani S. Bradai M. N. Bouaïn A. (2014). Age, growth and reproductive biology of the rough skate, Raja radula (Chondrichthyes: Rajidae), off the Gulf of Gabes (southern Tunisia, central Mediterranean). Mar. Freshw. Res.64, 540–548. doi: 10.1071/MF12218
54
Kadri H. Marouani S. Saïdi B. Bradai M. N. Ghorbel M. Bouaïn A. et al . (2012). Age, growth and reproduction of Raja miraletus (Linnaeus 1758)(Chondrichthyes: rajidae) of the gulf of gabès (Tunisia, central mediterranean sea). Mar. Biol. Res.8, 388–396. doi: 10.1080/17451000.2011.619546
55
Kimura D. K. (1979). Likelihood methods for the von Bertalanffy growth curve. Fish. Bull.77, 765.
56
Knip D. M. Heupel M. R. Simpfendorfer C. A. (2010). Sharks in nearshore environments: models, importance, and consequences. Mar. Ecol. Prog. Ser.402, 1–11. doi: 10.3354/meps08498
57
KrstulovićŠifner S. Vrgoč N. Dadić V. Isajlović I. Peharda M. Piccinetti C. (2009). Long-term changes in distribution and demographic composition of thornback ray, Raja clavata, in the northern and central Adriatic Sea. J. Appl. Ichthyol.25, 40–46. doi: 10.1111/j.1439-0426.2008.01204.x
58
Lack M. Sant G. (2008). Illegal, unreported and unregulated shark catch: A review of current knowledge and action. 52.
59
Le Cren E. D. (1951). The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol.20(2), 201–219. doi: 10.2307/1540
60
Li Veli D. Barrionuevo J. C. B. Bargione G. Barone G. Bdioui M. Carbonara P. et al . (2024). Assessing the vulnerability of sensitive species in Mediterranean fisheries: insights from productivity-susceptibility analysis. Front. Mar. Sci.11, 1411033. doi: 10.3389/fmars.2024.1411033
61
Lloret J. Biton-Porsmoguer S. Carreño A. Di Franco A. Sahyoun R. Melià P. et al . (2020). Recreational and small-scale fisheries may pose a threat to vulnerable species in coastal and offshore waters of the western Mediterranean. ICES J. Mar. Sci.77, 2255–2264. doi: 10.1093/icesjms/fsz071
62
Lotze H. K. Coll M. Dunne J. A. (2011). Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems14, 198–222. doi: 10.1007/s10021-010-9404-8
63
Lucchetti A. Petetta A. Bdioui M. Gökçe G. Saber M. Sacchi J. et al . (2023). Catalogue of fishing gear in the Mediterranean and Black Sea region. Food Agric. Org. 695, 1–402. doi: 10.4060/cc7260en
64
Lucchetti A. Virgili M. Petetta A. Sartor P. (2020). An overview of gill net and trammel net size selectivity in the Mediterranean Sea. Fish. Res.230, 105677. doi: 10.1016/j.fishres.2020.105677
65
Lucchetti A. Virgili M. Vasapollo C. Petetta A. Bargione G. Li Veli D. et al . (2021). An overview of bottom trawl selectivity in the Mediterranean Sea. Mediterr. Mar. Sci.22, 566–585. doi: 10.12681/mms.26969
66
Lutton B. V. George J. S. Murrin C. R. Fileti L. A. Callard I. P. (2011). “ The elasmobranch ovary,” in Reproductive biology and phylogeny of Chondrichthyes (Boca Raton, FL: CRC Press), 237–281.
67
McAllister M. Fraser S. Henry L. (2024). Population ecology and juvenile density hotspots of thornback ray (Raja clavata) around the Shetland Islands, Scotland. J. Fish Biol.104, 576–589. doi: 10.1111/jfb.15610
68
McCully S. R. Scott F. Ellis J. R. (2012). Lengths at maturity and conversion factors for skates (Rajidae) around the British Isles, with an analysis of data in the literature. ICES J. Mar. Sci.69, 1812–1822. doi: 10.1093/icesjms/fss150
69
McPhie R. P. Campana S. E. (2009). Bomb dating and age determination of skates (family Rajidae) off the eastern coast of Canada. ICES J. Mar. Sci.66, 546–560. doi: 10.1093/icesjms/fsp002
70
Mendez L. Bacquet A. Briand. F. (2022). Guide of mediterranean skates and rays. Available online at: www.ciesm.org/Guide/skatesandrays/. (Accessed March 15, 2025).
71
Moro S. Valente S. Arcioni M. Falsone F. Scannella D. Geraci M. L. et al . (2025). Living on the extinction edge: resilience to fishing and rebound potential of the mediterranean elasmobranchs. Fish Fish. 26(5), 772–785. doi: 10.1111/faf.12911
72
Navarro J. Cardador L. Fernández Á.M. Bellido J. M. Coll M. (2016). Differences in the relative roles of environment, prey availability and human activity in the spatial distribution of two marine mesopredators living in highly exploited ecosystems. J. Biogeogr.43, 440–450. doi: 10.1111/jbi.12648
73
Navarro J. Coll M. Preminger M. Palomera I. (2013). Feeding ecology and trophic position of a Mediterranean endemic ray: consistency between sexes, maturity stages and seasons. Environ. Biol. Fishes96, 1315–1328. doi: 10.1007/s10641-013-0109-7
74
Ordines F. Baro J. Ramirez-Amaro S. Serena F. Sobrino I. (2017). First substantiated record of Raja asterias Delaroche 1809 (Elasmobranchii: Rajiformes: Rajidae) in the Gulf of Cádiz, north-eastern Atlantic. Cent. Ocean. Balear. 47(1), 101–106. doi: 10.3750/AIEP/02161
75
Otero M. Serena F. Gerovasileiou V. Barone M. Bo M. Arcos J. M. et al . (2019). Identification guide of vulnerable species incidentally caught in Mediterranean fisheries. IUCN Malaga Spain. 203.
76
Panfili J. de Pontual H. Troadec H. Wright P. J. (2002). Manual of fish sclerochronology (Brest, France: Ifremer-IRD coedition).
77
Porcu C. Bellodi A. Cannas R. Marongiu M. F. Mulas A. Follesa M. C. (2015). Life-history traits of a commercial ray, Raja brachyura from the central western Mediterranean Sea. Mediterr. Mar. Sci.16(1), 90–102. doi: 10.12681/mms.898
78
Porcu C. Donnaloia M. Carbonara P. Casciaro L. Mulas A. Follesa M. C. (2022). “ Spatial variability of the reproductive traits of Rajidae family (Elasmobranchii, Rajiformes) across the Italian waters,” in 2022 IEEE international workshop on metrology for the sea; learning to measure sea health parameters (MetroSea) (New York, NY: IEEE), 282–286.
79
Press W. H. Teukolsky S. A. (1988). Kolmogorov-Smirnov test for two-dimensional data. Comput. Phys.2, 74–77. doi: 10.1063/1.4822753
80
R Core Team (2023). R: A language and environment for statistical computing, R foundation for statistical computing (Vienna, Austria). Available online at: https://www.r-project.org/. (Accessed April 15, 2025).
81
Reddy T. A. (2011). Applied data analysis and modeling for energy engineers and scientists (New York, NY: Springer Science & Business Media).
82
Relini G. (2008). Manuale di istruzioni Medits (Genova, Italia: SIBM).
83
Romanelli M. Colasante A. Scacco U. Consalvo I. Grazia FINOIA M. Vacchi M. (2007). Commercial catches, reproduction and feeding habits of Raja asterias (Chondrichthyes: Rajidae) in a coastal area of the Tyrrhenian Sea (Italy, northern Mediterranean). Acta Adriat.48, 57–71.
84
Sabatini L. Donato F. La Mesa M. Scarcella G. Fanelli E. (2025). Spatial distribution and life history traits of two sympatric, cryptic species of sole in the Adriatic Sea basin. Mediterr. Mar. Sci.26, 1–15. doi: 10.12681/mms.38244
85
Scacco U. Battistoni A. Garibaldi F. Raicevich S. Rondinini C. Serena F. et al . (2024). Tracking IUCN extinction risk at sub-regional scale: lessons from comparing Italian Red List assessments for cartilaginous species within a decade, (2013–2022) . Front. Fish Sci.2, 1356358. doi: 10.3389/frish.2024.1356358
86
Scarcella G. Grati F. Raicevich S. Russo T. Gramolini R. Scott R. D. et al . (2014). Common sole in the northern and central Adriatic Sea: Spatial management scenarios to rebuild the stock. J. sea Res.89, 12–22. doi: 10.1016/j.seares.2014.02.002
87
Schram E. van de Pol L. Molenaar P. van Mens A. Bleeker K. Gazi K. M. et al . (2023). Survival probabilities of thornback and spotted rays discarded by beam trawl and flyshoot fisheries (The Netherlands: Wageningen Marine Research).
88
Serena F. (2005). Field identification guide to the sharks and rays of the Mediterranean and Black Sea (Rome, Italy: Food & Agriculture Org). 89.
89
Serena F. (2006). Standardization of methods for the determination and evaluation of ray stocks (Grosseto, Italy: Grosseto).
90
Serena F. Abella A. (1999). “ Assessment of the effects of fishing on the demersal assemblages of the Northern Tyrrhenian Sea with special reference to Raja asterias. ICES,” in SCOR symposium on ecosystem effects of fishing. Montpellier, France. Book of abstracts. pp 42.
91
Serena F. Barone M. Mancusi C. Abella A. J. (2005). Reproductive biology, growth and feeding habits of Raja asterias Delaroche 1809, from the north Tyrrhenian and south Ligurian Sea (Italy), with some notes on trends in landings.
92
Serena F. Mancusi C. Barone M. (2010). Field identification guide to the skates (Rajidae) of the Mediterranean Sea. Guidelines for data collection and analysis. Biol. Mar. Mediterr.17, 204.
93
Serena F. Relini G. (2006). “ Use of scientific campaigns (trawl surveys) for the knowledge of the sensitive habitats. A review of the MEDITS, GRUND and APHIA data with special attention to the Italian seas,” In BasustaN.KeskinC.SerenaF.SeretB. (Eds.), The Proceedings of the International Workshop on Mediterranean Cartilaginous Fish with Emphasis on Southern and Eastern Mediterranean (135–148). (Istanbul, Turkey: Turkish Marine Research Foundation).
94
Serra-Pereira B. Figueiredo I. Gordo L. S. (2011). Maturation of the gonads and reproductive tracts of the thornback ray Raja clavata, with comments on the development of a standardized reproductive terminology for oviparous elasmobranchs. Mar. Coast. Fish.3, 160–175. doi: 10.1080/19425120.2011.555707
95
Serrat A. Farriols M. T. Ramírez-Amaro S. Ordines F. Guijarro B. Ferragut-Perello F. et al . (2023). Conservation status assessment of demersal elasmobranchs in the Balearic Islands (Western Mediterranean) over the last two decades. Fishes8, 230. doi: 10.3390/fishes8050230
96
Sherman G. (2019). QGIS version 3.10 A Coruña. Available online at: https://blog.qgis.org/2019/11/02/qgis-3-10-a-coruna-is-released/.
97
Stevens J. D. Bonfil R. Dulvy N. K. Walker P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci.57, 476–494. doi: 10.1006/jmsc.2000.0724
98
Tsikliras A. C. Dimarchopoulou D. (2021). Filling in knowledge gaps: Length–weight relations of 46 uncommon sharks and rays (Elasmobranchii) in the Mediterranean Sea. Acta Ichthyol. Piscat.51, 249–255. doi: 10.3897/aiep.51.e65858
99
Van Bogaert N. Ampe B. Uhlmann S. S. Torreele E. (2020). Discard survival estimates of commercially caught skates of the North Sea and English Channel. Rep. Proj. Sumaris. 42.
100
Wearmouth V. J. Sims D. W. (2008). Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv. Mar. Biol.54, 107–170. doi: 10.1016/S0065-2881(08)00002-3
101
Worm B. Davis B. Kettemer L. Ward-Paige C. A. Chapman D. Heithaus M. R. et al . (2013). Global catches, exploitation rates, and rebuilding options for sharks. Mar. Policy40, 194–204. doi: 10.1016/j.marpol.2012.12.034
102
Worm B. Orofino S. Burns E. S. D’Costa N. G. Manir Feitosa L. Palomares M. L. D. et al . (2024). Global shark fishing mortality still rising despite widespread regulatory change. Sci. (80-. ).383, 225–230. doi: 10.1126/science.adf8984
103
Wosnick N. Giareta E. P. Leite R. D. Hyrycena I. Charvet P. (2023). An overview on elasmobranch release as a bycatch mitigation strategy. ICES J. Mar. Sci.80, 591–604. doi: 10.1093/icesjms/fsac164
104
Yemışken E. Forero M. G. Megalofonou P. Eryilmaz L. Navarro J. (2018). Feeding habits of three Batoids in the Levantine Sea (north-eastern Mediterranean Sea) based on stomach content and isotopic data. J. Mar. Biol. Assoc. United Kingdom98, 89–96. doi: 10.1017/S002531541700073X
Summary
Keywords
starry ray, commercial bycatch, sexual dimorphism, age and growth, reproductive cycle, distribution pattern, fishery policy
Citation
Bargione G, Pardini A, Donato F, Veli DL, Sabatini L, Sepe E, Scarcella G and Lucchetti A (2025) Managing the starry ray (Raja asterias) in the mid-western Adriatic Sea: why sex matters in fisheries conservation. Front. Mar. Sci. 12:1679293. doi: 10.3389/fmars.2025.1679293
Received
04 August 2025
Accepted
17 September 2025
Published
07 October 2025
Volume
12 - 2025
Edited by
James Scott Maki, Marquette University, United States
Reviewed by
Natascha Wosnick, Federal University of Paraná, Brazil; Salman Khan, Aligarh Muslim University, India
Updates
Copyright
© 2025 Bargione, Pardini, Donato, Veli, Sabatini, Sepe, Scarcella and Lucchetti.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giada Bargione, giada.bargione@cnr.it; Fortunata Donato, fortunata.donato@cnr.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.