Abstract
The Asia-Pacific Region (APR) encompasses a vast geographical area rich in marine biodiversity that plays critical roles in global ecological stability and climate regulation, but it also faces daunting challenges in maintaining these roles under global change. Environmental dynamics in the APR manifest regularly over a range of timescales, including storms, earthquakes, floods, and extreme heat events. Further, coastal and marine ecosystems, including extensive commercial fisheries and coral reefs, are under threat from intense resource extraction and increasingly frequent marine heatwaves. Knowledge gaps for understanding these complex systems are aggravated by substantial barriers to cross-national efforts caused by the region’s vast diversity of cultures, languages, socioeconomics, politics, and management practices. Effective management of marine resources in the APR will necessitate multidisciplinary research based on continuous, region-wide observations supported by robust collaborations. In 2023, we gathered APR researchers across disciplines to discuss these issues and find solutions during a thematic seminar and workshop program at Tohoku University in Japan. Based on the results of this program, we present a review of the current state of APR marine ecosystems, raise key questions addressable through multidisciplinary approaches, and identify future priorities for the region. We conclude that sustaining biodiversity, ecosystem functions, and climate resilience in the APR will depend on stronger interdisciplinary collaboration, better integration of biological and geophysical data, and broader access to marine observations. These efforts are both urgent and essential for supporting better science-based policy decisions to address the escalating effects of global change on marine systems across the region.
1 Introduction
The Asia-Pacific Region (APR, as defined in this study to include Northeast and Southeast Asia and the Pacific; Institute for Global Environmental Strategies, 2008) spans an area that occupies nearly one-third of our planet’s surface (Figure 1), hosts major hotspots of marine biodiversity (Cohen and Steenbergen, 2015; Gonzales et al., 2019), and supports biogenic habitats including coral reefs, mangrove forests, and seagrass beds (Williams et al., 2016; Dang et al., 2021; Devlin et al., 2021). Healthy ocean ecosystems are critically linked to the economies of APR countries, which depend heavily on fisheries, tourism, and other marine-related activities (Devlin et al., 2021). Furthermore, local communities rely on coastal and offshore ecosystem services for their livelihoods and cultural practices (Ross et al., 2019). As a result, there are unique challenges to ocean resource management and ecosystem conservation in the APR that require not only broader awareness but also immediate attention and action.
Figure 1
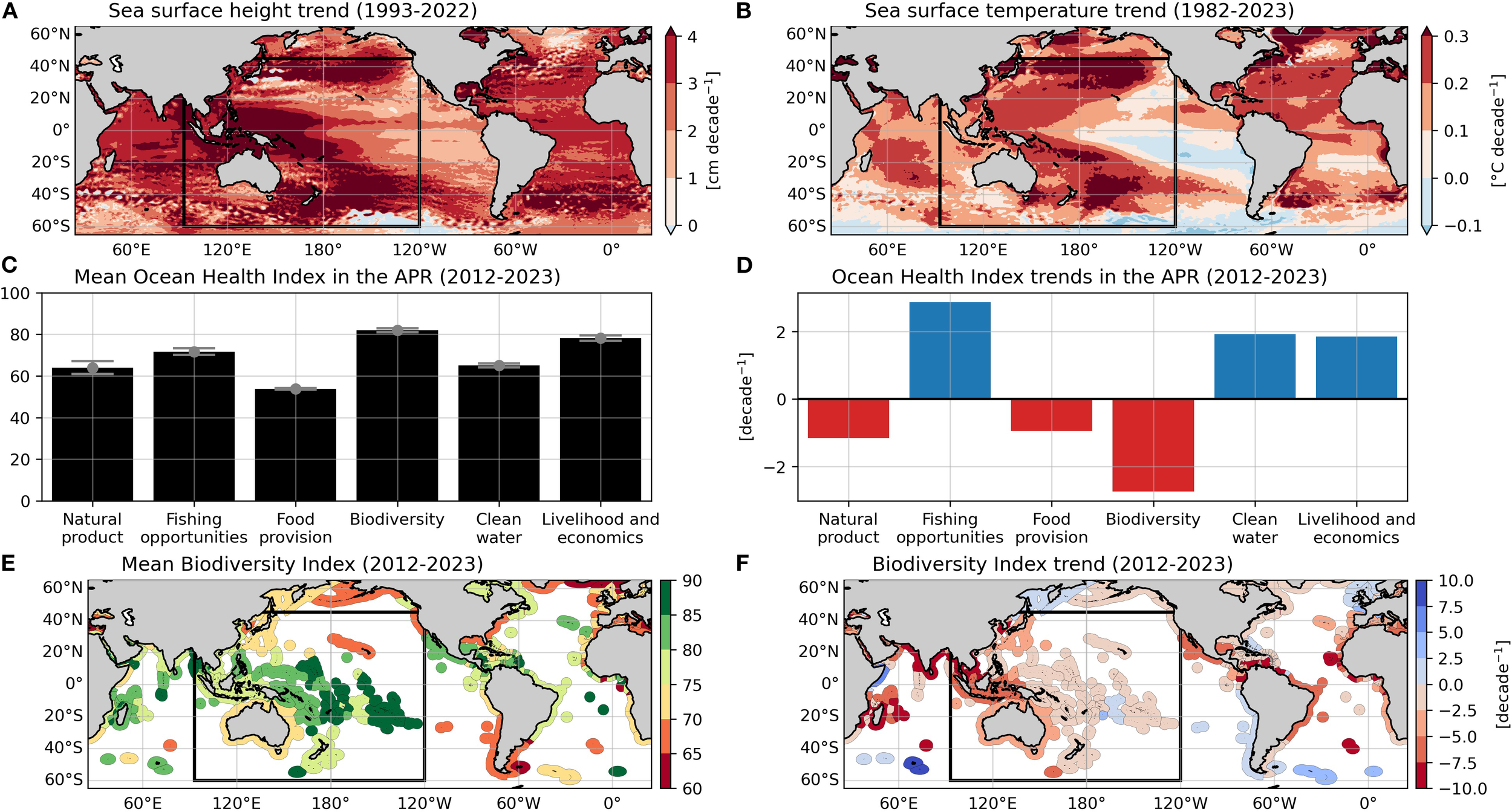
Trends of (A) sea surface height from 1993 to 2022 and (B) sea surface temperature from 1982 to 2023. (C) Average scores of Ocean Health Index (OHI) components in the APR. The error bars indicate one standard deviation of the annual mean. (D) Trends of OHI components in the APR from 2012 to 2023. (E) Average scores of the biodiversity component of the OHI. (F) Trend of the biodiversity component from 2012 to 2023. The trends are calculated by using linear regression. The black box (93°E-120°W, 60°S-45°N) indicates the APR.
For one, the APR is particularly vulnerable to environmental fluctuations such as synoptic storms, seasonal typhoons and monsoons, major ocean current shifts, tsunamis, and extreme heat events that manifest at varied timescales ranging from intra-annual to decadal (Arthurton, 1998; Mimura, 2008; Pakoksung et al., 2022). The region has been significantly affected by climate change, with sea-surface height and temperature increases exceeding global averages at 3.52 ± 1.75 cm and 0.205 ± 0.08 °C per decade (Figures 1A, B) (Hens et al., 2018; Khalil et al., 2016). Furthermore, future projections under CO2 emissions scenarios suggest further increases in sea surface temperature, precipitation, ocean acidification, and the intensity of tropical cyclones in the APR with the potential to impact marine ecosystems and socioeconomic frameworks (Heenan et al., 2015).
Furthermore, the APR, an area rich in marine biodiversity, is disproportionately threatened by both anthropogenic and environmental pressures, given that 60% of Earth’s human population resides there (Gietel-Basten, 2023). Yet, a 10-year average (2012-2023) of the Ocean Health Index (OHI), a comprehensive framework for assessing ocean health based on the sustainable provisioning of benefits and services (e.g., food, cultural and social value, jobs) (Halpern et al., 2012), revealed high biodiversity scores in the APR indicating more non-threatened species and less degradation of critical habitats relative to other components at both the global and regional scales (Figures 1C, E). However, an examination of OHI data on a year-to-year basis over the last decade reveals a steady decrease in the biodiversity component at both scales (Figures 1D, F), highlighting growing pressures on marine ecosystems. Anthropogenic causes of biodiversity loss in the APR include commodity-driven deforestation, dam construction, land-based marine pollution, and overexploitation through unregulated fishing and coastal development (Gonzales et al., 2019, Takeuchi et al., 2021; Tan et al., 2022). Furthermore, recent studies indicate that climate change is likely to increase the frequency of marine heat waves and natural disasters (e.g., Emanuel, 2013; Fischer and Knutti, 2015). Drastic changes to marine environments in the future will likely accelerate biodiversity loss. For example, sea surface temperature in Southeast Asia is predicted to rise by 1.1–2.9 °C and dissolved oxygen levels to decline by 5–13 mmol m-3 over the 21st century (Kay et al., 2023). Such rapid shifts will impact sensitive biogenic habitats, alter species distribution, and threaten the food security and livelihoods of millions reliant on coastal resources.
Addressing discrete challenges necessitates sustained regional observational platforms for detecting trends and elucidating processes. International efforts have made much progress to foster this effort, including the development of the Essential Ocean Variables (EOVs) system by the Global Ocean Observing System (GOOS) to facilitate standardization of data collection worldwide. By monitoring EOVs at national and regional scales, we can better assess marine system dynamics, responses to environmental stresses, and the accuracy of forecasts (Miloslavich et al., 2018). The “Decade of Ocean Science for Sustainable Development” (2021–2030) designated by the United Nations (UN) aims to enhance ocean resilience and sustainability, which also aligns with the broader goals of the 2030 Sustainable Development Agenda (Ryabinin et al., 2019; Venkatesan et al., 2021). Though valiant objectives, cross-national data-sharing efforts in the APR to address regional maritime issues are multifaceted due to the diversity of cultures, languages, socioeconomics, politics, and management practices (Beeson and Murray, 2020; Dang et al., 2021). The result is a general lack of publicly available regional ocean data in the APR, which can undermine the trust of both academics and policymakers (Chung, 2010; Costello et al., 2012), further hindering a comprehensive assessment of the status of those marine ecosystems.
To call attention to these particular challenges and assess the state of ocean ecosystems in this rapidly changing region, we formed an international working group of interdisciplinary early-career researchers and faculty mentors based primarily on the APR. Our aim is to foster collaboration and harness diverse perspectives from different countries, academic career levels, and research fields, including biology, ecology, oceanography, climatology, and engineering. The group convened for a thematic seminar and workshop program entitled “Integrated Understanding of Marine Environment and Marine Ecosystems” held in October-December 2023 as part of the Tohoku Forum for Creativity (TFC) Programs at Tohoku University in Japan. Here we present the core challenges and opportunities we have identified for interdisciplinary marine science opportunities in the APR. We first discuss how the adoption and proliferation of innovative and improved data sources (e.g., in the biological and geophysical fields) are essential for developing a comprehensive understanding of marine ecosystems, and how the use of these data can help forge new research directions. We then raise key questions about the future sustainability of marine ecosystems in the APR and propose strategies to address them in light of new types of data resources made available in recent years.
2 Adoption and proliferation of varied marine data sources
Measuring EOVs and indicators at high spatial and temporal resolutions from local to regional scales is necessary to adequately monitor marine systems. Marine biodiversity monitoring has traditionally relied on direct sampling and observations of organisms, which has made regional estimates difficult in the APR. However, advances in data and monitoring technology have helped the research community to reduce costs and transcend the boundaries imposed by the complex network of exclusive economic zones (EEZs) in this region.
2.1 Automated marine geophysical and biogeochemical monitoring
Beginning in 1999, the Argo program effectively utilized autonomous floats to address regional sampling biases that lead to persistent geographical data gaps for marine variables. Argo profiling floats measure temperature and salinity from 2000 m to the ocean surface every 10 days (Core-Argo; Riser et al., 2016; Figure 2A). These data have been used for many applications including investigations of subsurface ocean dynamics under climate change (Lyu et al., 2021) and impacts on typhoon intensity (Oka et al., 2023). BGC-Argo, which includes biogeochemical and optical sensors, was developed over the last decade to collect data on oxygen, nitrate, pH, and chlorophyll-a, as well as downwelling irradiance for deep and remote areas (Claustre et al., 2020; Figure 2B). These data have been used to monitor seasonal net primary production (Yang et al., 2021), the influence of oceanic eddies on subsurface biogeochemistry (Xiu and Chai, 2020; Chen et al., 2021), and the timing of seasonal phytoplankton blooms (Mignot et al., 2018). Both Core and BGC-Argo have transformed in situ marine data collection in the APR by enabling consistent subsurface monitoring of physical and biogeochemical properties across previously undersampled regions (e.g., He et al., 2022; Chamberlain et al., 2023).
Figure 2
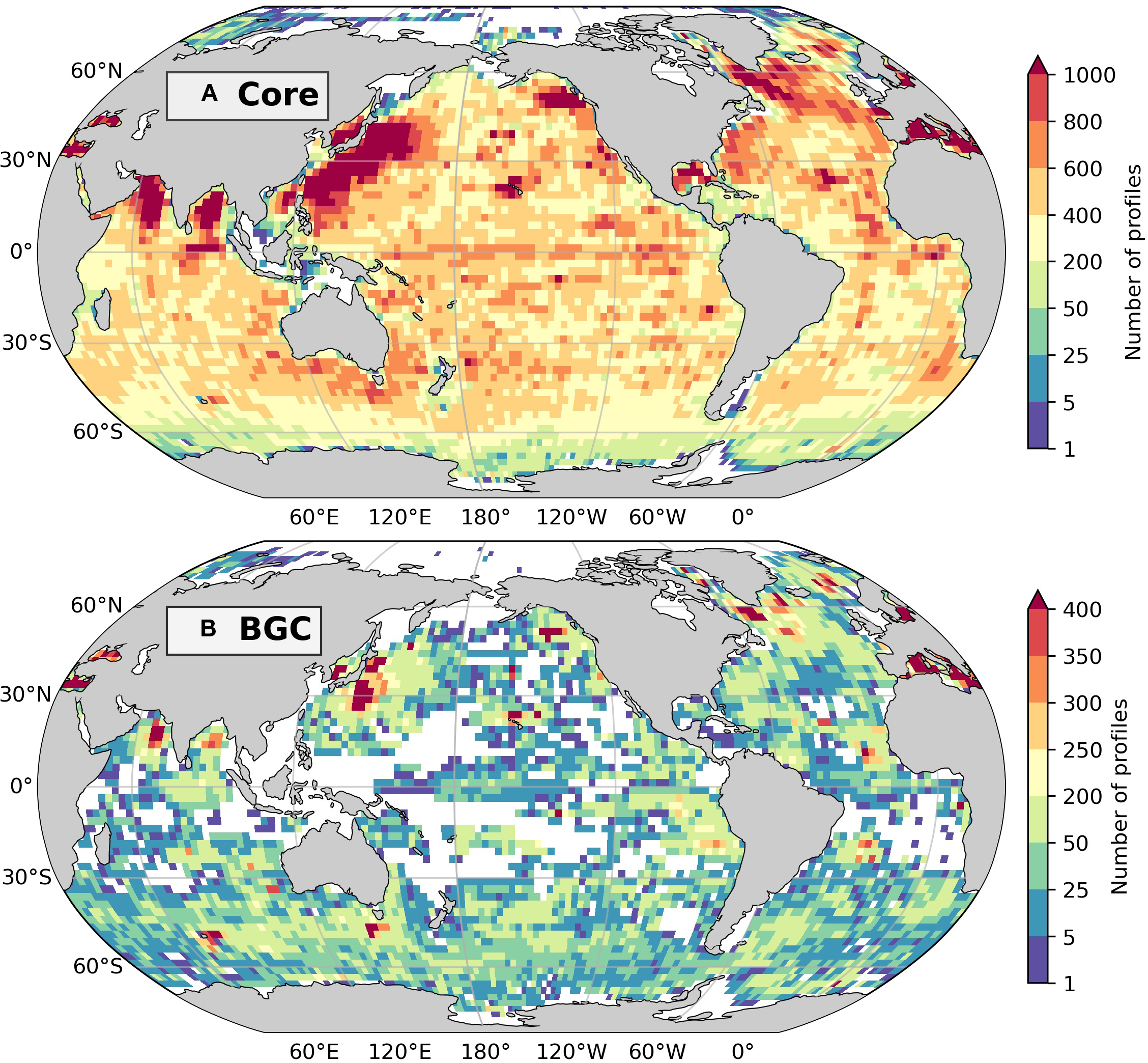
The total number of Argo profiles from 2000 to 2023 for temperature and salinity only (Core Argo; A) and additionally for biogeochemical variables (BGC-Argo; B) at 2.5° resolution (~275 km at the equator). White areas indicate grid cells with no available profiles for this time frame. As oxygen is the most consistently measured biogeochemical variable, only profiles that have quality-controlled (QC flags 1, 2, 5, and 8) oxygen data are shown in (B).
However, data coverage remains limited in regions with shallow waters (<2000 meters) and near continental shelves, such as the Gulf of Thailand, the Indonesian seas, and waters north of Australia (Figure 2A). One example of addressing this gap is the deployment of floats specialized for shallow waters such as those in the Natuna Sea, Karimata Strait, and Java Sea in Indonesia (24th Argo Steering Meeting; https://argo.ucsd.edu/organization/argo-meetings/). Furthermore, BGC-Argo coverage remains extremely limited in the APR, particularly in Southeast Asia, where observations are sparse or entirely absent (Figure 2B). One practical pathway to improve data availability in the APR is through OneArgo (Owens et al., 2022), a global initiative to unify and expand coverage by incorporating Core, Deep (reaching 6000 meters), and BGC-Argo floats into one integrated system, which should support more comprehensive monitoring of ocean dynamics and ecosystem change in the APR. However, OneArgo deployments in this region must also navigate legal and diplomatic considerations, particularly regarding the deployment of floats that may drift into EEZs (Belbeoch, 2006). The process of deploying Argo floats in or near EEZs requires advance notification to coastal states, which can present challenges for achieving full regional coverage.
2.2 Biodiversity surveys and environmental DNA
There is a pressing need for more in situ biodiversity data on marine organisms in the APR to fill knowledge gaps that cannot be addressed solely with remotely sensed data. The APR contains many marine biodiversity hotspots for varied taxonomic groups (Roberts et al., 2002; Von Rintelen et al., 2017), but compared to the terrestrial realm, many of these marine groups remain poorly described, and some of the most data-deficient groups are the most diverse in the APR (Allen, 2008). For example, zooplankton are ecologically important but remain understudied due to their complex life cycles, diel and seasonal migrations, and limited taxonomic expertise (Bandara et al., 2021; Pappalardo et al., 2021). Coral species boundaries also remain unclear, particularly in the Coral Triangle, hindering our ability to assess the full diversity of associated marine fauna (Hoeksema, 2017). Although recent advances in metabarcoding techniques offer promising tools to improve species detection, their effectiveness in the APR is still limited by sparse regional reference libraries and a lack of comprehensive specimen records (Moutinho et al., 2024; Jintsu-Uchifune and Yamamoto, 2016). This has resulted in extreme underrepresentation of APR marine species in large genomic, biogeographic, and taxonomic databases (e.g., NCBI, GBIF, OBIS, FishBase, SeaLifeBase, WoRMS). Described marine species are also in dire need of updates, as many are known only from perfunctory descriptions and sparse museum specimens from decades ago. These issues are caused in large part by a decline in active taxonomists who can identify species new to science (Engel et al., 2021). Thus, many resident species known to local communities and fisheries remain invisible to science and conservation assessment protocols like the IUCN Red List. However, new taxonomic efforts in the APR over the last 20 years have contributed to the description of hundreds of new fish species at a steady pace per year, resulting in over 4,500 new descriptions from 2010 to 2024 (Eschmeyer et al., 2010; Fricke et al., 2024).
Environmental DNA (eDNA) analysis has helped us assess biodiversity from communities using seawater and other indirect samples to identify resident organisms, including species that cannot be easily observed during field work (Beng and Corlett, 2020; Othman et al., 2023; Cahyani et al., 2024). These data have been used to track ecosystem responses to climate anomalies (Berry et al., 2019) and improve management of rare and invasive species (Eva et al., 2016; Madduppa et al., 2021), thus having both economic and biomonitoring significance (Moutinho et al., 2024). In addition, metabarcoding surveys targeting universal gene markers and metagenome skimming facilitate the detection and identification of many organisms (Porter and Hajibabaei, 2018; Garlapati et al., 2019; Hidaka et al., 2024), and these techniques can be adapted for eDNA to enhance taxon detection with time and cost efficiency, even for samples taken from sediment for deceased organisms (Takahashi et al., 2023). It must be noted that reference libraries are essential for detecting species from eDNA samples, especially for poorly known groups, but genomic data on marine species is sparse throughout the APR besides in Australia and New Zealand (De Jong et al., 2024). Therefore, while eDNA approaches can be useful for tracking the presence and possible range of known species and identifying the relative abundance of DNA from unknown sources, they will not be sufficient for reconstructing full communities until genomic sampling and taxonomic deficiencies noted above are fully addressed.
Regional efforts to develop streamlined eDNA collection protocols, expand sampling targets and reference databases, reduce sample processing and data sharing times, and implement long-term archiving practices are all essential for facilitating efforts that can fill baseline knowledge gaps regarding biodiversity in the APR. Examples of large-scale projects in the region include the OceanOmics project (minderoo.org/resources/oceanomics/), which focuses on advancing eDNA analysis techniques and developing genomic reference libraries for Australia, and ANEMONE (All Nippon eDNA Monitoring Network), which maintains an open database of eDNA data for fish species in Japan and is now also expanding to include other partners in the APR and beyond (oceandecade.org/actions/anemone-global/). Continued efforts to accumulate and share eDNA data publicly for the APR will help accelerate consistent monitoring, resulting in more accurate biodiversity assessments of the region and the discovery of new species.
2.3 High-resolution remote sensing data for coastal ecosystem monitoring
In the APR, where remote and complex coastal areas often lack sustained programs for in situ sampling, high-resolution satellite data provide essential, repeatable observations. These data include ocean surface variables such as sea-surface temperature, chlorophyll-a concentration, turbidity, and ocean color, providing critical insights into the conditions of key ecosystems. For example, high-resolution satellite data can reveal changes in mangrove health over time in the APR, aiding national reporting on carbon stocks and supporting coastal climate mitigation strategies (Sakti et al., 2020; Roy et al., 2024). Satellite data have also been used to map coral resilience and forecast temperature anomalies for the APR, crucial for anticipating and managing bleaching events (Knudby et al., 2013; Smith and Spillman, 2019). Using the Allen Coral Atlas, a global database of high-resolution reef habitat maps developed using satellite imagery, Lyons et al. (2024) demonstrated that the APR contains the largest global extent of shallow coral reefs, with Indonesia, the Philippines, and Papua New Guinea at the top of the list.
To support evidence-based management and biodiversity conservation across the APR, satellite observations should be integrated into regional monitoring systems, ideally using machine learning workflows. In addition, strategies should be explored to link satellite-derived indicators to local measurements from management efforts such as fisheries, mangrove restoration, and coral reef protection.
2.4 Incorporating geophysical data into biodiversity models
Despite growing access to ecological and environmental data, biodiversity models still rarely incorporate geophysical processes that play critical roles in shaping marine ecosystems, particularly in the APR. While variables such as sea surface temperature or salinity are commonly included, more complex physical dynamics such as ocean currents, eddy activity, and upwelling are overlooked but could prove important to biodiversity models. For example, Santora et al. (2021) combined long-term biodiversity data for the California coast with physical drivers such as upwelling variability, source water changes, and marine heatwaves to enhance species distribution models and inform ecosystem forecasting. Ackiss et al. (2013) provided a rare example for the APR, showing how geophysical processes, such as the Mindanao and Halmahera eddies, can shape biodiversity by creating genetic breaks in reef fish populations through limited larval dispersal, thus highlighting how physical oceanographic processes shape marine biodiversity patterns. While the value of such integrative approaches is clear, the scarcity of related studies for the APR highlights opportunities to fill significant research gaps through interdisciplinary research.
Future progress will depend on bold new research initiatives featuring collaboration between physical oceanographers and ecologists to integrate geophysical variables into biodiversity models. Development of dynamic models that mechanistically link geophysical forcings to biodiversity and ecosystem function and their application to generate specific testable hypotheses could stimulate targeted oceanic observations to obtain the geophysical data needed. Given the dynamic nature of the physical marine environment in the APR, such research is expected to help answer lingering questions about spatiotemporal patterns of biodiversity in the region.
3 Key questions addressed by new data opportunities
Here we identify key questions concerning the rapidly changing APR that, while being crucial to address, can only be effectively solved by integrating insights from multiple disciplines.
3.1 How do changing oceanic physical processes impact the diversity and dynamics of marine life?
Oceanic physical processes, influenced by climate change, are reshaping marine life in the APR. Decadal modes of climate variability, such as the Pacific Decadal Oscillation (PDO) and Atlantic Multidecadal Oscillation (AMO) have been shown to alter sea-surface temperature, salinity, and ocean circulation, which in turn shift the distributions and abundance patterns of key commercial species across the Indo-Pacific region (Wu et al., 2022). The increasing frequencies of marine heatwaves and harmful algal blooms are also leading to habitat degradation and species redistribution around the APR (Oliver et al., 2018; Kang et al., 2021). Marine species are closely tracking shifting temperatures, particularly in warm tropical waters like the Central Pacific Basin, raising the risk of local extirpations where species already live near their thermal limits (Lenoir et al., 2020). Moreover, the impacts of climate change in the APR are highly variable—different regions have exhibited vastly different levels of coral bleaching that vary based on climate extremes but also geography, disturbance history, and water movement, making predictions challenging (McClanahan et al., 2019).
In addition, it is becoming clear that APR reefs respond differently to climate change than those in other parts of the world, with inconsistent bleaching patterns across depth and weak or absent correlations with thermal stress variability. This variability is likely influenced by the region’s high coral richness and genetic diversity, as well as complex environmental conditions, which can mask or alter expected responses to warming and other stressors (Schlesinger and van Woesik, 2023). Together, these observations highlight the need for a more regionally informed understanding of how climate-driven ocean changes are affecting marine ecosystems in the APR. The variability in responses suggests that global trends may not hold uniformly in this highly diverse and dynamic region. Addressing this complexity will require greater integration of biological and physical data, expanded long-term monitoring, and a stronger focus on local environmental and ecological contexts. As marine ecosystems across the APR continue to face rapid change, improving our ability to detect, interpret, and respond to these shifts will be essential for effective conservation and management.
3.2 Which intensifying extreme events in the marine realm are most concerning?
As the climate continues to warm, the APR has experienced an increasing number of climate-related disasters, which may reflect a rise in the frequency and intensity of extreme events, combined with increased human exposure and vulnerability (Thomas et al., 2014). Understanding whether these events are accelerating and which types pose the greatest risk is critical for anticipating the region’s ecological and socio-economic vulnerabilities. The frequency and intensity of extreme weather events across the APR have been increasing, including heatwaves (Fischer and Knutti, 2015), heavy rainfall (Kharin et al., 2013), and severe droughts (Dong et al., 2024). This also includes events that directly threaten coastal infrastructure and livelihoods such as tropical cyclones (Webster et al., 2005; Emanuel, 2013), tsunamis (Suppasri et al., 2011; 2018; Pakoksung et al., 2022), and sea-level rise (Dasgupta et al., 2009; Hoeke et al., 2013). The rise in extreme marine weather events poses serious and often compounding risks to biodiversity and ecosystem dynamics in the APR. Marine heatwaves can cause widespread coral bleaching and mortality, which in turn destabilizes reef-associated food webs and reduces habitat complexity (Leggat et al., 2019; Fordyce et al., 2019). Similarly, increased frequency of flooding and cyclones can lead to physical damage to critical habitats like mangroves and seagrass beds, disrupt reproductive cycles of marine species, and favor opportunistic or invasive organisms over long-established communities (Biswas et al., 2012; Asbridge et al., 2018). These stressors may also accelerate shifts in species distributions and traits, selecting for faster-growing, more heat-tolerant species while driving local declines or extinctions of more specialized organisms (Mellin et al., 2024).
As these extremes become more common, the resilience of ecosystems may erode, making recovery slower and less predictable, which highlights the urgent need to factor extreme event dynamics into conservation planning and marine management strategies. In our view, failing to integrate the growing frequency and severity of extreme events into conservation and management strategies risks weakening marine ecosystem stability in the APR, which will detrimentally affect food security, coastal protection, and livelihoods for millions (Vinke et al., 2017). Without urgent and coordinated efforts to build resilience through ecosystem-based adaptation, early-warning systems, and environmentally sustainable development, the APR may face cascading socio-ecological crises that severely threaten its future sustainability.
4 Discussion
The APR hosts some of the world’s marine biodiversity hotspots, including the Coral Triangle, that support ecosystem services critical to both regional and global human wellbeing. Uniquely, the impacts of climate change on the APR often diverge from global patterns due to its complex oceanography and high biodiversity. This calls for region-specific approaches to marine research and conservation that go beyond global models and are tailored to the APR’s distinctive ecological and environmental dynamics.
Inconsistent data sharing remains a cross-cutting problem that hinders efforts to understand and manage marine ecosystems in the APR. Differences in scientific culture, technical capacity, and political sensitivities around sovereignty and resource control all limit collaboration and access to ecological data. Even when data exists, they are often fragmented or siloed within national institutions. Many key questions about ecosystem change, species responses, and climate resilience in the APR can only be answered through the integration of diverse datasets across disciplines, from physical oceanography to molecular ecology. Without shared, high-quality data, our ability to detect patterns, predict outcomes, and guide effective action remains severely constrained. New approaches to data-sharing aided by technology can help (e.g., Pendleton et al., 2019), but resolving data limitation issues in the APR will also require coordinated action among scientists and policymakers to strengthen existing channels for sharing data and building new ones. One example of how these challenges can be addressed is the international Argo program, which coordinates the deployment and management of ocean-observing floats across more than 30 countries (Wong et al., 2020). The program ensures that data collected by these floats are rapidly checked and made publicly available within about 24 hours, followed by more thorough quality control procedures carried out over the floats’ operational lifespans to ensure long-term reliability. Argo follows FAIR (Findable, Accessible, Interoperable, and Reusable) data principles and is supported by regular international meetings that align standards and practices. This combination of coordination, open data sharing, and ongoing quality assurance provides a useful model for strengthening data systems and regional cooperation in the APR.
A productive way forward is to build on regional initiatives that are already redefining how marine science is approached in the Asia-Pacific. This effort aligns with the goals of the World Premier International Research Centre Initiative-Advanced Institute for Marine Ecosystem Change (WPI-AIMEC), recently established at Tohoku University and Japan Agency for Marine-Earth Science and Technology (JAMSTEC), and a satellite campus at the University of Hawai’i, Manoa. WPI-AIMEC brings together researchers across disciplines to better understand and forecast marine ecosystem responses to environmental change, with a global scope but a particular focus on the Northwest Pacific. As part of WPI-AIMEC’s activities, our group contributes to an international, interdisciplinary effort to understand marine biodiversity and ecosystem change, where rapid environmental shifts demand regionally focused, fusion science approaches. Institutes like WPI-AIMEC illustrate the power of integrated marine ecosystem approaches that bring together oceanographers, climate scientists, and ecologists to address complex challenges from multiple angles. By combining physical and biological data across disciplines and borders, such efforts help generate more comprehensive insights into ecosystem change and resilience. Collaborative frameworks like this are essential for turning fragmented observations into coordinated action and for ensuring that science meaningfully supports the long-term health of marine ecosystems and human wellbeing across the APR.
Statements
Data availability statement
All datasets used in this study are publicly available. Core and BGC Argo float data, along with associated metadata, were obtained from the Global Data Assembly Centre (Argo, 2024). Sea surface temperature data from NOAA’s OISST v2.1 product are available at https://www.ncei.noaa.gov/products/optimum-interpolation-sst (Huang et al., 2021). Ocean Health Index (OHI) data can be accessed via the https://oceanhealthindex.org/ (Halpern et al., 2012). Merged satellite altimetry products are provided through the EU Copernicus Marine Service (European Union-Copernicus Marine Service, 2021).
Author contributions
HA: Data curation, Visualization, Writing – original draft, Writing – review & editing. SY: Supervision, Writing – original draft, Writing – review & editing. JK: Supervision, Writing – original draft, Writing – review & editing. AA: Writing – original draft. SA: Writing – original draft. EG: Writing – original draft. HI: Writing – original draft. EL: Writing – original draft. YL: Writing – original draft. GN: Writing – original draft. PP-P: Writing – original draft. FS: Writing – original draft. CA: Writing – original draft, Writing – review & editing. DA: Writing – original draft. ÅB: Writing – original draft, Writing – review & editing. UD: Writing – original draft, Writing – review & editing. TF: Writing – original draft. FH: Writing – original draft. MK: Writing – original draft. TM: Writing – original draft. CP: Writing – original draft. LS: Writing – original draft, Writing – review & editing. TSh: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. PS: Writing – original draft, Writing – review & editing. CA-R: Writing – original draft, Writing – review & editing. AW: Writing – original draft, Writing – review & editing. TSu: Funding acquisition, Project administration, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. TSu was supported by JST SICORP Grant JPMJSC21E7, JST CREST Grant Number JPMJCR23J3, and Tohoku Forum for Creativity 2023MRN. AW was supported by the Ministry of Education, Culture, Research and Technology (MEXT) and the Indonesia Endowment Fund for Education Agency (LPDP) through the Funding Program of PRPB: the e-ASIA JRP scheme with the following contract number: 013/E5/PG.02.00/PRPB BATCH 2/2024 and 130/UN7.D2/PP/IX/2024. CA-R was supported by the Department of Science and Technology- Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development (DOST-PCAARRD) through the e-ASIA JRP scheme. UD gratefully acknowledges funding from the European Union’s Horizon 2020 research and innovation funding programme for the projects Plant-FATE (grant no. 841283) and COMFORT (grant no. 820989; the work reflects only the authors’ view, and the European Commission and their executive agency are not responsible for any use that may be made of the information the work contains), from the Japanese Society for the Promotion of Science for a KAKENHI Start-up project (grant no. 22K21333) and a KAKENHI C project (grant no. 23K11510), from the OIST COI-NEXT Global Bioconvergence Center of Innovation supported by the Japan Science and Technology Agency (grant no. JPMJPF2205), and from the National Member Organizations that support IIASA. Some of the students are supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the SHINKA Grant FY2024. Funding for several of the coauthors was also provided by WPI-AIMEC (Advanced Institute for Marine Ecosystem Change).
Acknowledgments
We thank the participants of the TFC thematic program “Integrated Understanding of Marine Environment and Marine Ecosystems” held at Tohoku University, Japan, for their valuable discussions and insights.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ackiss A. S. Pardede S. Crandall E. D. Ablan-Lagman M. C. A. Barber P. H. Carpenter K. E. (2013). Pronounced genetic structure in a highly mobile coral reef fish, Caesio cuning, in the Coral Triangle. Mar. Ecol. Prog. Ser.480, 185–197. doi: 10.3354/meps10199
2
Allen G. R. (2008). Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquatic Conserv. Mar. Freshw. Ecosyst.18, 541–556. doi: 10.1002/aqc.880
3
Argo (2024). Argo float data and metadata from Global Data Assembly Centre (Argo GDAC) ( SEANOE). doi: 10.17882/42182
4
Arthurton R. S. (1998). Marine-related physical natural hazards affecting coastal megacities of the Asia–Pacific region – awareness and mitigation. Ocean Coast. Manage.40, 65–85. doi: 10.1016/s0964-5691(98)00077-5
5
Asbridge E. Lucas R. Rogers K. Accad A. (2018). The extent of mangrove change and potential for recovery following severe Tropical Cyclone Yasi, Hinchinbrook Island, Queensland, Australia. Ecol. Evol.8, 10416–10434. doi: 10.1002/ece3.4485
6
Bandara K. Varpe Ø. Wijewardene L. Tverberg V. Eiane K. (2021). Two hundred years of zooplankton vertical migration research. Biol. Rev.96, 1547–1589. doi: 10.1111/brv.12715
7
Beeson M. Murray P. (2020). Testing times for regionalism: Coping with great power rivalry in the Asia–pacific. Asian Stud. Rev.44, 1–9. doi: 10.1080/10357823.2020.1681052
8
Belbeoch M. (2006). Argo project & IOC resolution XX-6 implementation (IOC/ABE-LOS-VI/INF.1) (Malaga, Spain: Intergovernmental Oceanographic Commission). Available online at: https://unesdoc.unesco.org. (Accessed June 17, 2025).
9
Beng K. C. Corlett R. T. (2020). Applications of environmental DNA (eDNA) in ecology and conservation: opportunities, challenges and prospects. Biodiversity Conserv.29, 2089–2121. doi: 10.1007/s10531-020-01980-0
10
Berry T. E. Saunders B. J. Coghlan M. L. Stat M. Jarman S. Richardson A. J. et al . (2019). Marine environmental DNA biomonitoring reveals seasonal patterns in biodiversity and identifies ecosystem responses to anomalous climatic events. PloS Genet.15, e1007943. doi: 10.1371/journal.pgen.1007943
11
Biswas S. R. Khan M. S. I. Mallik A. U. (2012). Invaders’ control on post-disturbance succession in coastal mangroves. J. Plant Ecol.5, 157–166. doi: 10.1093/jpe/rtr050
12
Cahyani N. K. D. Anggoro A. W. Al Malik M. D. Subhan B. Sani L. M. I. Madduppa H. (2024). Inventorizing marine biodiversity using eDNA data from Indonesian coral reefs: comparative high throughput analysis using different bioinformatic pipelines. Mar. Biodiversity54. doi: 10.1007/s12526-024-01432-w
13
Chamberlain P. Talley L. D. Cornuelle B. Mazloff M. Gille S. T. (2023). Optimizing the biogeochemical Argo float distribution. J. Atmospheric Oceanic Technol.40, 1355–1379. doi: 10.1175/JTECH-D-22-0093.1
14
Chen S. Wells M. L. Huang R. X. Xue H. Xi J. Chai F. (2021). Episodic subduction patches in the western North Pacific identified from BGC-Argo float data. Biogeosciences18, 5539–5554. doi: 10.5194/bg-18-5539-2021
15
Chung S.-Y. (2010). Strengthening regional governance to protect the marine environment in Northeast Asia: From a fragmented to an integrated approach. Mar. Policy34, 549–556. doi: 10.1016/j.marpol.2009.10.011
16
Claustre H. Johnson K. S. Takeshita Y. (2020). Observing the global ocean with biogeochemical-Argo. Annu. Rev. Mar. Sci.12, 23–48. doi: 10.1146/annurev-marine-010419-010956
17
Cohen P. J. Steenbergen D. J. (2015). Social dimensions of local fisheries co-management in the Coral Triangle. Environ. Conserv.42, 278–288. doi: 10.1017/S0376892914000423
18
Costello M. J. Reimer J. Szabo Z. Fernandez-Silva I. Adzis K. A. A. Wörheide G. et al . (2012). Fostering international collaboration in marine biodiversity sciences in the Asia-Pacific region. Pacific Sci.66, 545–549.
19
Dang A. N. Jackson B. M. Benavidez R. Tomscha S. A. (2021). Review of ecosystem service assessments: Pathways for policy integration in Southeast Asia. Ecosystem Serv.49, 101266. doi: 10.1016/j.ecoser.2021.101266
20
Dasgupta S. Laplante B. Meisner C. Wheeler D. Yan J. (2009). The impact of sea level rise on developing countries: a comparative analysis. Climatic Change93, 379–388. doi: 10.1007/s10584-008-9499-5
21
De Jong E. Parata L. Bayer P. E. Corrigan S. Edwards R. J. (2024). Toward genome assemblies for all marine vertebrates: current landscape and challenges. GigaScience13, giad119. doi: 10.1093/gigascience/giad119
22
Devlin M. J. Lyons B. P. Johnson J. E. Hills J. M. (2021). The tropical Pacific Oceanscape: Current issues, solutions and future possibilities. Mar. pollut. Bull.166, 112181. doi: 10.1016/j.marpolbul.2021.112181
23
Dong C. Noyelle R. Messori G. Gualandi A. Fery L. Yiou P. et al . (2024). Indo-Pacific regional extremes aggravated by changes in tropical weather patterns. Nat. Geosci.17, 979–986. doi: 10.1038/s41561-024-01537-8
24
Emanuel K. A. (2013). Downscaling CMIP5 climate models shows increased tropical cyclone activity over the 21st century. Proc. Natl. Acad. Sci. United States America110, 12219–12224. doi: 10.1073/pnas.1301293110
25
Engel M. S. Ceríaco L. M. P. Daniel G. M. Dellapé P. M. Löbl I. Marinov M. et al . (2021). The taxonomic impediment: A shortage of taxonomists, not the lack of technical approaches. Zoological J. Linn. Soc.193, 381–387. doi: 10.1093/zoolinnean/zlab072
26
Eschmeyer W. N. Fricke R. van der Laan R. (2010). Marine fish diversity: History of knowledge and discovery (Zootaxa 2525). Zootaxa2525, 19–41. doi: 10.11646/zootaxa.2525.1.2
27
European Union-Copernicus Marine Service (2021). Global ocean Gridded l4 sea surface heights and derived variables reprocessed, (1993-Ongoing). Mercator Ocean Int. doi: 10.48670/MOI-00148
28
Eva B. Harmony P. Thomas G. Francois G. Alice V. Claude M. et al . (2016). Trails of river monsters: Detecting critically endangered Mekong giant catfish Pangasianodon gigas using environmental DNA. Global Ecol. Conserv.7, 148–156. doi: 10.1016/j.gecco.2016.06.007
29
Fischer E. M. Knutti R. (2015). Anthropogenic contribution to global occurrence of heavy-precipitation and high-temperature extremes. Nat. Climate Change5, 560–564. doi: 10.1038/nclimate2617
30
Fordyce A. J. Ainsworth T. D. Heron S. F. Leggat W. (2019). Marine heatwave hotspots in coral reef environments: physical drivers, ecophysiological outcomes, and impact upon structural complexity. Front. Mar. Sci.6. doi: 10.3389/fmars.2019.00498
31
Fricke R. Eschmeyer W. N. van der Laan R. (2024). Eschmeyer’s Catalog of Fishes: Genera, species, references (San Francisco, CA: California Academy of Sciences). Available online at: https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. (Accessed July 22, 2025).
32
Garlapati D. Charankumar B. Ramu K. Madeswaran P. Ramana Murthy M. V. (2019). A review on the applications and recent advances in environmental DNA (eDNA) metagenomics. Rev. Environ. Sci. Biotechnol.18, 389–411. doi: 10.1007/s11157-019-09501-4
33
Gietel-Basten S. (2023). Asia-Pacific population and development report 2023 (Bangkok, Thailand: United Nations Economic and Social Commission for Asia and the Pacific (ESCAP). Available online at: https://repository.unescap.org/items/9ca2bb0c-52c1-450c-90bc-59a32a703145. (Accessed July 3, 2025).
34
Gonzales A. T. Kelley E. Bernad S. R. Q. (2019). A review of intergovernmental collaboration in ecosystem-based governance of the large marine ecosystems of East Asia. Deep-Sea Res. Part II Topical Stud. Oceanography163, 108–119. doi: 10.1016/j.dsr2.2019.05.014
35
Halpern B. S. Longo C. Hardy D. McLeod K. L. Samhouri J. F. Katona S. K. et al . (2012). An index to assess the health and benefits of the global ocean. Nature, 488(7413), 615–620.
36
He Y. Wang J. Wang F. Hibiya T. (2022). Spatial distribution of turbulent diapycnal mixing along the Mindanao current inferred from rapid-sampling Argo floats. J. Oceanography78, 35–48. doi: 10.1007/s10872-021-00624-3
37
Heenan A. Pomeroy R. Bell J. Munday P. L. Cheung W. Logan C. et al . (2015). A climate-informed, ecosystem approach to fisheries management. Mar. Policy57, 182–192. doi: 10.1016/j.marpol.2015.03.018
38
Hens L. Nguyen A. T. Tran H. H. Ngo S. C. Tran D. L. Nguyen V. T. et al . (2018). Sea-level rise and resilience in Vietnam and the Asia-Pacific: A synthesis. Vietnam J. Earth Sci.40, 126–152. doi: 10.15625/0866-7187/40/2/11107
39
Hidaka S. Jo T. S. Yamamoto S. Katsuhara K. R. Tomita S. Miya M. et al . (2024). Sensitive and efficient surveillance of Japanese giant salamander (Andrias japonicus) distribution in western Japan using multi-copy nuclear DNA marker. Limnology25, 189–198. doi: 10.1007/s10201-023-00740-7
40
Hoeke R. K. McInnes K. L. Kruger J. C. McNaught R. J. Hunter J. R. Smithers S. G. (2013). Widespread inundation of Pacific islands triggered by distant-source wind-waves. Global Planetary Change108, 128–138. doi: 10.1016/j.gloplacha.2013.06.006
41
Hoeksema B. W. (2017). The hidden biodiversity of tropical coral reefs. Biodiversity18 (1), 1–2.
42
Huang B. Liu C. Banzon V. Freeman E. Graham G. Hankins B. et al . (2021). Improvements of the daily optimum interpolation sea surface temperature (DOISST) version 2.1. J. Climate34, 2923–2939. doi: 10.1175/JCLI-D-20-0166.1
43
Institute for Global Environmental Strategies (IGES) (2008). “ Biodiversity and climate change: Integrating ecosystem management and development,” in Biodiversity in the Asia-Pacific: Status, trends and policy implications (Chapter 3) (Hayama, Japan: IGES). Available online at: https://www.iges.or.jp/en/publication_documents/pub/policyreport/en/154/3_overview.pdf. (Accessed June 14, 2025).
44
Jintsu-Uchifune Y. Yamamoto H. (2016). Marine organism occurrence data of the Asia-Pacific region extracted from literature. doi: 10.48518/00002
45
Kang B. Pecl G. T. Lin L. Sun P. Zhang P. Li Y. et al . (2021). Climate change impacts on China’s marine ecosystems. Rev. Fish Biol. Fisheries31, 599–629. doi: 10.1007/s11160-021-09668-6
46
Kay S. Avillanosa A. L. Cheung V. V. Dao H. N. Gonzales B. J. Palla H. P. et al . (2023). Projected effects of climate change on marine ecosystems in Southeast Asian seas. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1082170
47
Khalil I. Atkinson P. M. Challenor P. (2016). Looking back and looking forwards: Historical and future trends in sea surface temperature (SST) in the Indo-Pacific region from 1982 to 2100. Int. J. Appl. Earth Observation Geoinformation45, 14–26. doi: 10.1016/j.jag.2015.10.005
48
Kharin V. V. Zwiers F. W. Zhang X. Wehner M. (2013). Changes in temperature and precipitation extremes in the CMIP5 ensemble. Climatic Change119, 345–357. doi: 10.1007/s10584-013-0705-8
49
Knudby A. Jupiter S. Roelfsema C. Lyons M. Phinn S. (2013). Mapping coral reef resilience indicators using field and remotely sensed data. Remote Sens.5, 1311–1334. doi: 10.3390/rs5031311
50
Leggat W. P. Camp E. F. Suggett D. J. Heron S. F. Fordyce A. J. Gardner S. et al . (2019). Rapid coral decay is associated with marine heatwave mortality events on reefs. Curr. Biol.29, 2723–2730. doi: 10.1016/j.cub.2019.06.077
51
Lenoir J. Bertrand R. Comte L. Bourgeaud L. Hattab T. Murienne J. et al . (2020). Species better track climate warming in the oceans than on land. Nat. Ecol. Evol.4, 1044–1059. doi: 10.1038/s41559-020-1198-2
52
Lyons M. B. Murray N. J. Kennedy E. V. Kovacs E. M. Castro-Sanguino C. Phinn S. R. et al . (2024). New global area estimates for coral reefs from high-resolution mapping. Cell Rep. Sustainability1. doi: 10.1016/j.crsus.2024.100015
53
Lyu K. Zhang X. Church J. A. (2021). Projected ocean warming constrained by the ocean observational record. Nat. Climate Change11, 834–839. doi: 10.1038/s41558-021-01151-1
54
Madduppa H. Cahyani N. K. D. Anggoro A. W. Subhan B. Jefri E. Sani L. M. I. et al . (2021). eDNA metabarcoding illuminates species diversity and composition of three phyla (chordata, mollusca and echinodermata) across Indonesian coral reefs. Biodiversity Conserv.30, 3087–3114. doi: 10.1007/s10531-021-02237-0
55
McClanahan T. R. Darling E. S. Maina J. M. Muthiga N. A. agata S. D. Jupiter S. D. et al . (2019). Temperature patterns and mechanisms influencing coral bleaching during the 2016 El Niño. Nat. Climate Change9, 845–851. doi: 10.1038/s41558-019-0576-8
56
Mellin C. Stuart-Smith R. D. Heather F. Oh E. Turak E. Edgar G. J. (2024). Coral responses to a catastrophic marine heatwave are decoupled from changes in total coral cover at a continental scale. Proc. B291, 20241538. doi: 10.1098/rspb.2024.1538
57
Mignot A. Ferrari R. Claustre H. (2018). Floats with bio-optical sensors reveal what processes trigger the North Atlantic bloom. Nat. Commun.9. doi: 10.1038/s41467-017-02143-6
58
Miloslavich P. Bax N. J. Simmons S. E. Klein E. Appeltans W. Aburto-Oropeza O. et al . (2018). Essential ocean variables for global sustained observations of biodiversity and ecosystem changes. Global Change Biol.24, 2416–2433. doi: 10.1111/gcb.14108
59
Mimura N. (2008). Asia-pacific coasts and their management: States of environment. Ed. MimuraN. (New York, NY: Springer). doi: 10.1007/978-1-4020-3625-5
60
Moutinho J. Costa F. O. Duarte S. (2024). Advancements in DNA metabarcoding protocols for monitoring zooplankton in marine and brackish environments. J. Mar. Sci. Eng.12, 2093. doi: 10.3390/jmse12112093
61
Oka E. Sugimoto S. Kobashi F. Nishikawa H. Kanada S. Nasuno T. et al . (2023). Subtropical Mode Water south of Japan impacts Typhoon intensity. Sci. Adv.9, eadi2793. doi: 10.1126/sciadv.adi2793
62
Oliver E. C. J. Lago V. Hobday A. J. Holbrook N. J. Ling S. D. Mundy C. N. (2018). Marine heatwaves off eastern Tasmania: Trends, interannual variability, and predictability. Prog. Oceanography161, 116–130. doi: 10.1016/j.pocean.2018.02.007
63
Othman N. Muniar K. Haris H. Ramli F. F. Sariyat N. H. Najmuddin M. F. et al . (2023). A review of next-generation wildlife monitoring using environmental DNA (eDNA) detection and next-generation sequencing in Malaysia. Sains Malaysiana52, 17–33. doi: 10.17576/jsm-2023-5201-02
64
Owens W. B. Roemmich D. Le Traon P. Y. Claustre H. Johnson G. C. King B. A. et al . (2022). OneArgo: A new paradigm for observing the global ocean. Mar. Technol. Soc. J.56, 45–55. doi: 10.4031/MTSJ.56.3.8
65
Pakoksung K. Suppasri A. Imamura F. (2022). The near-field tsunami generated by the 15 January 2022 eruption of the Hunga Tonga-Hunga Ha’apai volcano and its impact on Tongatapu, Tonga. Sci. Rep.12, 15187. doi: 10.1038/s41598-022-19486-w
66
Pappalardo P. Collins A. G. Pagenkopp Lohan K. M. Hanson K. M. Truskey S. B. Jaeckle W. et al . (2021). The role of taxonomic expertise in interpretation of metabarcoding studies. ICES J. Mar. Sci.78, 3397–3410. doi: 10.1093/icesjms/fsab082
67
Pendleton L. H. Beyer H. Estradivari Grose S. O. Hoegh-Guldberg O. Karcher D. B. et al . (2019). Disrupting data sharing for a healthier ocean. ICES J. Mar. Sci.76, 1415–1423. doi: 10.1093/icesjms/fsz068
68
Porter T. M. Hajibabaei M. (2018). Scaling up: A guide to high-throughput genomic approaches for biodiversity analysis. Mol. Ecol.27, 313–338. doi: 10.1111/mec.14478
69
Riser S. C. Freeland H. J. Roemmich D. Wijffels S. Troisi A. Belbéoch M. et al . (2016). Fifteen years of ocean observations with the global Argo array. Nat. Climate Change6, 145–153. doi: 10.1038/nclimate2872
70
Roberts C. M. McClean C. J. Veron J. E. Hawkins J. P. Allen G. R. McAllister D. E. et al . (2002). Marine biodiversity hotspots and conservation priorities for tropical reefs. Science295, 1280–1284. doi: 10.1126/science.1067728
71
Ross H. Adhuri D. S. Abdurrahim A. Y. Phelan A. (2019). Opportunities in community-government cooperation to maintain marine ecosystem services in the Asia-Pacific and Oceania. Ecosystem Serv.38, 100969. doi: 10.1016/j.ecoser.2019.100969
72
Roy A. D. Arachchige P. S. P. Watt M. S. Kale A. Davies M. Heng J. E. et al . (2024). Remote sensing-based mangrove blue carbon assessment in the Asia-Pacific: A systematic review. Sci. Total Environ.938, 173270. doi: 10.1016/j.scitotenv.2024.173270
73
Ryabinin V. Barbière J. Haugan P. Kullenberg G. Smith N. McLean C. et al . (2019). The UN decade of ocean science for sustainable development. Front. Mar. Sci.6. doi: 10.3389/fmars.2019.00470
74
Sakti A. D. Fauzi A. I. Wilwatikta F. N. Rajagukguk Y. S. Sudhana S. A. Yayusman L. F. et al . (2020). Multi-source remote sensing data product analysis: Investigating anthropogenic and naturogenic impacts on mangroves in southeast Asia. Remote Sens.12, 2720. doi: 10.3390/rs12172720
75
Santora J. A. Schroeder I. D. Bograd S. J. Chavez F. P. Cimino M. A. Fiechter J. et al . (2021). Pelagic biodiversity, ecosystem function, and services: An integrated observing and modeling approach. Oceanography34, 16–37. doi: 10.5670/oceanog.2021.212
76
Schlesinger T. van Woesik R. (2023). Oceanic differences in coral-bleaching responses to marine heatwaves. Sci. Total Environ.871, 162113. doi: 10.1016/j.scitotenv.2023.162113
77
Smith G. Spillman C. (2019). New high-resolution sea surface temperature forecasts for coral reef management on the Great Barrier Reef. Coral Reefs38, 1039–1056. doi: 10.1007/s00338-019-01829-1
78
Suppasri A. Fukui K. Yamashita K. Leelawat N. Ohira H. Imamura F. (2018). Developing fragility functions for aquaculture rafts and eelgrass in the case of the 2011 Great East Japan tsunami. Natural Hazards Earth System Sci.18, 145–155. doi: 10.5194/nhess-18-145-2018
79
Suppasri A. Koshimura S. Imamura F. (2011). Developing tsunami fragility curves based on the satellite remote sensing and the numerical modeling of the 2004 Indian Ocean tsunami in Thailand. Natural Hazards Earth System Sci.11, 173–189. doi: 10.5194/nhess-11-173-2011
80
Takahashi M. Saccò M. Kestel J. H. Nester G. Campbell M. A. van der Heyde M. et al . (2023). Aquatic environmental DNA: A review of the macro-organismal biomonitoring revolution. Sci. Total Environ.873, 162322. doi: 10.1016/j.scitotenv.2023.162322
81
Takeuchi Y. Muraoka H. Yamakita T. Kano Y. Nagai S. Bunthang T. et al . (2021). The Asia-Pacific Biodiversity Observation Network: 10-year achievements and new strategies to 2030. Ecol. Res.36, 232–257. doi: 10.1111/1440-1703.12212
82
Tan Y.-L. Chen J.-E. Yiew T.-H. Habibullah M. S. (2022). Habitat change and biodiversity loss in South and Southeast Asian countries. Environ. Sci. pollut. Res.29, 63260–63276. doi: 10.1007/s11356-022-20054-y
83
Thomas V. Albert J. R. G. Hepburn C. (2014). Contributors to the frequency of intense climate disasters in Asia-Pacific countries. Climatic Change126, 381–398. doi: 10.1007/s10584-014-1232-y
84
Venkatesan R. Muthiah M. A. Vedachalam N. Vengatesan G. Ramesh K. Kesavakumar B. et al . (2021). Technological trends and significance of the essential ocean variables by the Indian moored observatories: Relevance to UN Decade of Ocean Sciences. Mar. Technol. Soc. J.55, 34–49. doi: 10.4031/MTSJ.55.3.8
85
Vinke K. Schellnhuber H. Coumou D. Geiger T. Glanemann N. Huber V. et al . (2017). A region at risk: The human dimensions of climate change in Asia and the pacific (Manila, Philippines: Asian Development Bank). doi: 10.22617/tcs178839-2
86
Von Rintelen K. Arida E. Häuser C. (2017). A review of biodiversity-related issues and challenges in megadiverse Indonesia and other Southeast Asian countries. Res. Ideas Outcomes3, e20860. doi: 10.3897/rio.3.e20860
87
Webster P. J. Holland G. J. Curry J. A. Chang H.-R. (2005). Changes in tropical cyclone number, duration, and intensity in a warming environment. Science309, 1844–1846. doi: 10.1126/SCIENCE.1116448
88
Williams G. A. Helmuth B. Russell B. D. Dong Y.-W. Thiyagarajan V. Seuront L. (2016). Meeting the climate change challenge: Pressing issues in southern China and SE Asian coastal ecosystems. Regional Stud. Mar. Sci.8, 373–381. doi: 10.1016/j.rsma.2016.07.002
89
Wong A. P. S. Wijffels S. E. Riser S. C. Pouliquen S. Hosoda S. Roemmich D. et al . (2020). Argo Data 1999–2019: Two million temperature-salinity profiles and subsurface velocity observations from a global array of profiling floats. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00700
90
Wu Y. L. Lan K. W. Evans K. Chang Y. J. Chan J. W. (2022). Effects of decadal climate variability on spatiotemporal distribution of Indo-Pacific yellowfin tuna population. Sci. Rep.12, 13715. doi: 10.1038/s41598-022-17882-w
91
Xiu P. Chai F. (2020). Eddies affect subsurface phytoplankton and oxygen distributions in the North Pacific Subtropical Gyre. Geophysical Res. Lett.47, e2020GL087037. doi: 10.1029/2020GL087037
92
Yang B. Fox J. Behrenfeld M. J. Boss E. S. Haëntjens N. Halsey K. H. et al . (2021). In situ estimates of net primary production in the western North Atlantic with Argo profiling floats. J. Geophysical Research: Biogeosciences126, e2020JG006116. doi: 10.1029/2020JG006116
Summary
Keywords
Asia-Pacific, marine science, interdisciplinary, climate change, data observations
Citation
Adiwira H, Yasunaka S, Kass JM, Açıkbaş AHO, Adiningsih S, Gairin E, Ilham HBC, Lahcene E, Li Y, Nishihira G, Peñalver-Pereira P, Sie FMP, Amedo-Repollo CL, Ames CL, Armitage D, Brännström Å, Dieckmann U, Fujii T, Husnik F, Kawamiya M, Masuda T, Plessy C, Sallan L, Shimada T, Smith SL, Strutton PG, Wirasatriya A and Suga T (2025) Pathways to an integrated understanding of marine environments and ecosystems in the Asia-Pacific Region. Front. Mar. Sci. 12:1680145. doi: 10.3389/fmars.2025.1680145
Received
05 August 2025
Accepted
24 September 2025
Published
23 October 2025
Volume
12 - 2025
Edited by
James Scott Maki, Marquette University, United States
Reviewed by
Qiaojun Chen, Nanjing University of Information Science and Technology, China
Updates
Copyright
© 2025 Adiwira, Yasunaka, Kass, Açıkbaş, Adiningsih, Gairin, Ilham, Lahcene, Li, Nishihira, Peñalver-Pereira, Sie, Amedo-Repollo, Ames, Armitage, Brännström, Dieckmann, Fujii, Husnik, Kawamiya, Masuda, Plessy, Sallan, Shimada, Smith, Strutton, Wirasatriya and Suga.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanani Adiwira, hanani.adiwira.c1@tohoku.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.