- 1State Key Laboratory of Marine Resource Utilization in South China Sea, School of Marine Science and Engineering, School of Marine Biology and Fisheries, Hainan University, Haikou, China
- 2Zhanjiang Zhongyue Energy Co., Ltd., Zhanjiang, China
- 3College of Animal Science and Technology, Northwest Agriculture and Forestry University, Yangling, China
Pond purification system (PPS) is an outdoor aquaculture system designed based on recirculating aquaculture system (RAS) principles, utilizing recirculating water technology, which can achieve large-scale fish farming in the recirculating water system. The purpose of this experiment is to investigate the alterations in growth performance, intestinal histology, digestion-absorption function, and structural integrity of channel catfish raised in PPS. The findings indicated a significant optimization in water quality indicators pertinent to aquaculture, including total nitrogen, total phosphorus, chemical oxygen demand (COD), ammonia nitrogen, nitrite, and dissolved oxygen. Additionally, the growth performance of channel catfish reared in the PPS group was markedly improved. The intestinal morphology of these channel catfish exhibited increased villus height and a reduced number of mucus cells. Furthermore, serum levels of diamine oxidase and D-lactic acid were significantly decreased in channel catfish raised in the PPS group, while the expression of genes related to the apical junction complex was significantly upregulated, suggesting improved intestinal structural integrity. Moreover, the activities of digestive and absorptive enzymes, such as trypsin, amylase, lipase, and Na+-K+-ATPase, were elevated, indicating enhanced digestive and absorptive functions. In conclusion, based on the conditions of this experiment, the pond purification system module used in this study can effectively improve the quality of the aquaculture water, enhance fish growth performance and improve their intestinal health.
1 Introduction
As is well known, aquatic products, due to their high nutritional value, occupy an important position in the human diet structure (Lu Z-Y. et al., 2023). To meet the growing demand for aquatic products, intensive farming models have become widespread (Karvonen et al., 2019). The rapid expansion of the aquaculture industry is often linked to the degradation of aquatic environments, which subsequently leads to the emergence of various diseases. This progression poses a substantial threat to the industry’s development (Karvonen et al., 2019). Especially in high-density fish farming models in pond and cage aquaculture systems, the deterioration of the water environment causes great harm to fish health and even leads to significant economic losses (Ibrahim et al., 2022). Consequently, the enhancement of efficient and sustainable fish farming practices, alongside the improvement of aquaculture water quality, has emerged as a pressing issue requiring immediate attention.
Against this backdrop, the recirculating aquaculture system (RAS) is an innovative ecological pond aquaculture approach inspired by the principles of a circular economy (Xiao et al., 2020), which are becoming more commonly used because they allow for better control of freshwater quality (Huang et al., 2020). The transition from conventional pond systems to RAS represents a paradigm shift in aquatic product production, offering precise control over water quality parameters that are critical for fish health and performance. The previous study found that RAS can promote the reproduction of beneficial bacteria and at the same time affect the nutritional components in the water, especially nitrogen and phosphorus (Emmanuel et al., 2025). Recent research found that RAS improves the muscle quality of largemouth bass, showing significant enhancements in muscle hardness, elasticity, and fiber density, while reducing muscle-saturated fatty acids (Xu et al., 2025). However, the available evidence only focuses on water quality parameters and fish muscle quality indicators, and the research on RAS on the integrity of fish intestinal structure and its molecular regulatory mechanisms is not yet in-depth. Therefore, it is necessary to conduct a systematic study on the impact of RAS on fish intestinal health.
Previous studies in our laboratory have shown that the health of fish often depends on the health status of their intestines (Lu et al., 2020; Lu et al., 2022b; Lu et al., 2022a). Generally, the fish intestine serves as a critical interface between the external environment and internal physiology, playing essential roles in structural integrity (Dawood, 2020). The structural integrity of the intestinal tract is closely related to its histological morphology and the apical junction complex (Konig et al., 2016; France and Turner, 2017; Otani and Furuse, 2020). Ensuring structural integrity of apical junctions is key to preventing the passage of pathogens or foreign materials across the intestinal epithelium (Russo et al., 2022). The adherens junction (AJ) proteins and the tight junction (TJ) proteins together constitute the apical junction complex (Qian et al., 2016). TJ proteins are made up of a complex of multiple proteins, including Occludin, Claudins, the ZO family, and junction adhesion molecule (JAM) (Tian et al., 2022). The AJ is composed of E-cadherin, β-catenin, and other associated proteins (Shukla et al., 2021). However, there are currently no reports on the study of RAS effect on the integrity of fish intestinal structure. Therefore, conducting such research is highly necessary.
Furthermore, another important function of the fish intestine is the process of digestion and absorption (Hoseinifar et al., 2017; Magouz et al., 2021). It is well known that the digestive function of fish intestine is related to a variety of digestive enzymes, including protease, lipase and amylase (Hoseinifar et al., 2017). These digestive enzymes can fully enzymolyze protein, fat and carbohydrate in feed into small molecules such as small peptides, free amino acids (FAAs) or fatty acids (FA), which is beneficial to intestinal absorption and utilization (Noaillac-Depeyre and Hollande, 1981; Wang et al., 2010). The absorption function of the fish intestinal tract is closely related to the activity of the brush border enzyme. Brush border enzymes can promote the function of intestinal digestive function (Freeman and Kim, 1978). However, it is unknown whether the RAS in the pond has any impact on the intestinal digestion and absorption functions of fish. Therefore, it is worthwhile to conduct relevant research.
Channel catfish (Ictalurus punctatus) is extensively cultured globally due to its adaptability, being one of the foremost aquaculture species in the world (Liu, 2011). Since channel catfish were introduced to China in 1984, the scale of aquaculture has been growing, and the market potential has been progressively explored (Liu, 2011). There are no studies available on the effects of RAS on the growth performance and intestinal health of channel catfish. Therefore, it is necessary to research the effects of RAS on the growth and intestinal health of the channel catfish. This experiment aims to clarify the positive effect of which RAS affects intestinal health, promote the sustainable development of aquaculture, and provide a reference for future efficient and facility-based aquaculture in ponds.
2 Materials and methods
2.1 Ethics statement
The animal experiments were conducted at Hainan University in Haikou, China, in accordance with the guidelines sanctioned by the Ethics Committee of Hainan University (No. HNUAUCC-2024-00138).
2.2 Pond purification system design
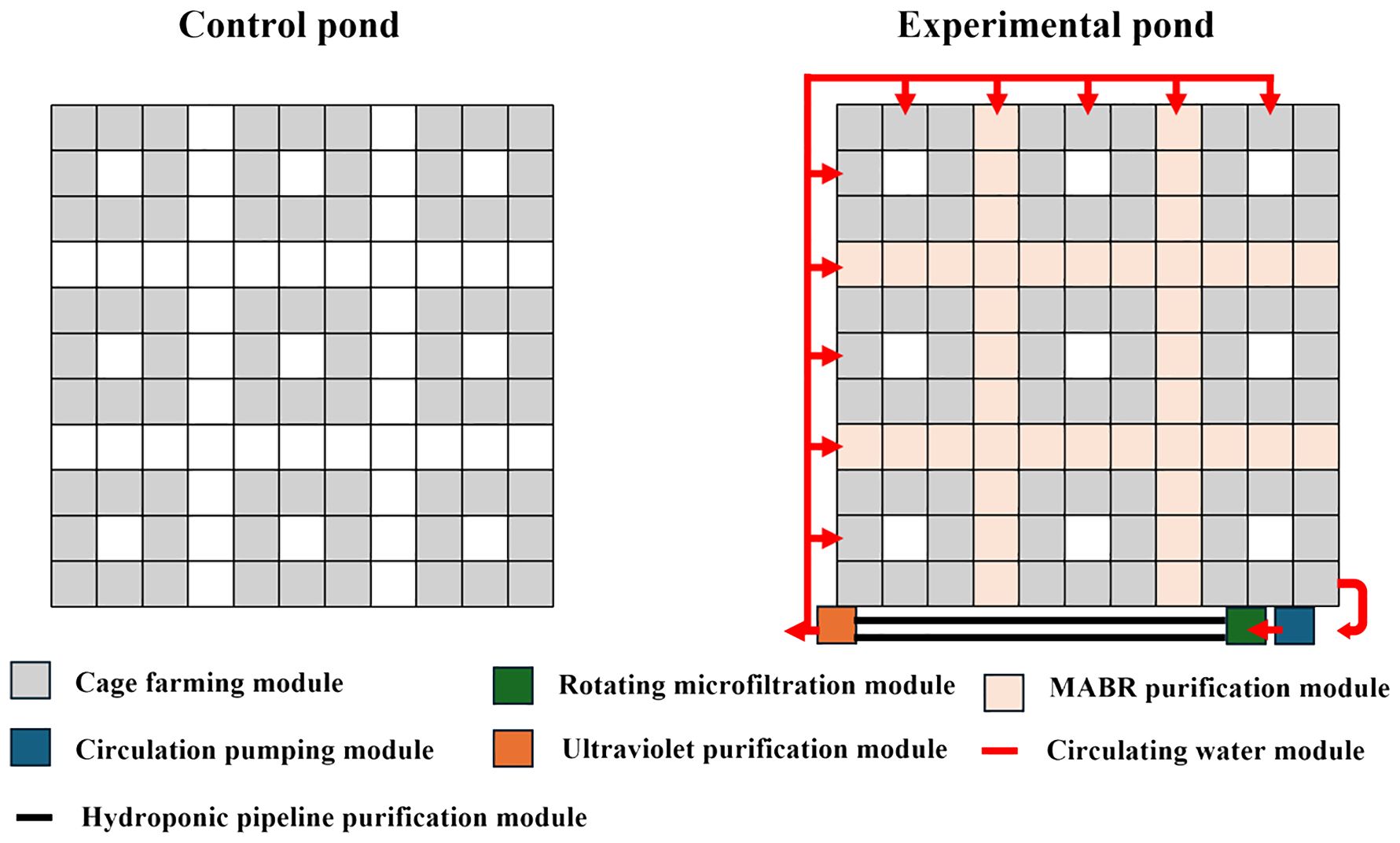
Pond purification system (PPS) is an outdoor recirculating water aquaculture pond designed based on the RAS principle. As shown in Figure 1, to achieve water quality purification and control in fish ponds, membrane aeration bio-reactor (MABR) membranes are regularly placed at the bottom of the ponds and an aeration pipe network system is established. The principle of operation is to utilize the native microorganisms in the pond bottom sediment to form a dominant biological purification membrane, and provide a certain amount of dissolved oxygen through aeration to create suitable conditions for nitrifying and denitrifying bacteria, thereby efficiently degrading leftover feed in situ. In addition, a water circulation purification and advanced treatment system within the pond is constructed through pump stations, microfiltration machines, pipeline systems, aquaponics systems, and ultraviolet disinfection. Both the control pond and the experimental pond were traditional static ponds. The control pond did not contain any water treatment modules. During the 60-day experiment, neither the control pond nor the experimental pond had its water changed.
2.3 Animal feeds and experimental design
The study involved 360 channel catfish, each starting at a weight of 243.33 ± 4.43g, which were randomly allocated into two groups, each containing three replicates of 60 fish. The division was made into two groups: the control group and the PPS group. They were fed commercial diets provided by Guangdong Yuehai Company and conducted a 60-day growth experiment. The formulation of the diet and its calculated nutrient content are detailed in Table 1. During the experiment, the feed was fed 4 times a day, exposed to natural light throughout the process, and the water temperature was maintained at 26-30°C.
2.4 Sample collection
After the experiment, all the fish were weighed to calculate the fish production performance; for each treatment, 36 fish were randomly selected for sample collection. Biological tissue sample: Following the experimental phase, the animals were not given food for 8 hours before blood samples were collected. The fish was placed on ice for dissection, and the intestines were removed as previously described (Zou et al., 2025). The contents within the intestinal cavity were flushed with ice-cold physiological saline. The intestinal tissue with a length of 1cm was fixed in paraformaldehyde for section preparation. A section of the intestinal tissue was stored at -20°C to detect digestion and absorption enzymes, and another section was stored at -80°C to detect molecular indicators.
Environmental water sample: The water sampling time is divided into four time points: 15 days, 30 days, 45 days and 60 days, in order to observe the impact of PPS on water quality parameters throughout the entire operation cycle. In short, use a 3-meter right-angle water sampling tube to collect water from the middle of the fish cage, and transfer it into a 1 L volumetric flask. The water samples from each pond were collected three times and stored in a refrigerator at 4°C. The collected water samples were used for water quality analysis.
2.5 Measurement of water quality parameters
The dissolved oxygen (DO), temperature (°C) and pH were measured using a Handheld Multi-Parameter Water Quality Sonde (YSI Inc., Yellow Springs, OH, USA).
Chemical Oxygen Demand (COD), Total phosphorus (TP), Total Nitrogen (TN), Total Ammonia nitrogen (TAN) and Nitrite were measured by using Multi-parameter Water Quality Monitoring Station (G70 Pro, GLKR Precision Instrument Co., Ltd., China) with complementary commercial reagent kit (TP, GLS-TP; TAN, GLS-NH3N; COD, GL-COD-LR-Y; TN, GLS-TNY; Nitrite, GLS-NO3Y).
2.6 Hematoxylin & Eosin staining
Initially, the intestinal tissue (N = 6) was embedded in paraffin and subsequently sliced into 4 μm-thick sections for mounting on slides. Following dewaxing with xylene, the sections underwent hydration through a series of ethanol concentrations. Hematoxylin & Eosin (H&E) (G1076-500ML, Servicebio, Wuhan, China) was used to stain the slides. After the dehydration process, slices were sealed with neutral gum and then placed under a microscope for observation and imaging.
2.7 Periodic acid-Schiff staining
The operation process of PAS was carried out according to a previous study (Zhao et al., 2024a). The intestinal tissue sections (N = 6), each 4 micrometers thick, underwent deparaffinization in xylene and were rehydrated using a series of ethanol solutions (100%, 95%, 80%), then treated with Schiff’s reagent (G1061-500ML, Servicebio, Wuhan, China) for 15 min in the dark, counterstained with hematoxylin (G1004-100ML, Servicebio, Wuhan, China) for 1 min, and rinsed in running tap water for 5 min. After dehydration through graded alcohols and xylene clearance, slides were mounted with resinous medium. PAS-positive mucin granules were identified by magenta coloration under light microscopy (Nikon Eclipse E100), with mucus cells showing characteristic cytoplasmic staining.
2.8 Biochemical analysis
Prepare a 10% tissue homogenate and conduct the measurement according to the previous research methods (Zeng et al., 2020). For each treatment group, 18 serum and tissue samples from the fish were randomly selected for analysis. The D-lactic acid (D-LC, A019-3-1) and diamine oxidase (DAO, A088-3-1) were detected in serum. The trypsin (A080-2-2), amylase (C016-1-2), lipase (A054-2-1), alkaline phosphatase (ALP, A059-2-2), Na+-K+-ATPase (A070-2-2), γ-glutamyl transferase (γ-GT, C017-2-1) and creatine kinase (CK, A032-1-1) were detected in intestinal tissue; the above kits were brought from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The kit instructions provide the method for specific determination.
2.9 Real-time PCR
The PCR extraction process was performed in accordance with the previous description (Zhao et al., 2024b). For each treatment group, 18 tissue samples from the fish were randomly selected for analysis. Total RNA extraction was performed using Trizol (G3013, Servicebio, Wuhan, China), and cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (A511-02, Exongen, Chengdu, China). The cDNA was measured using qRT-PCR with the SYBR Green PCR Kit (A411-01, Exongen, Chengdu, China). The gene used for reference was β-actin. The primer sequences utilized are detailed in Supplementary Table S1. Relative gene expression was calculated using the 2ΔΔ-Ct method.
2.10 Western blot assay
Western blotting was performed as described by previous studies (Lu Z. et al., 2023; Zhao et al., 2023). For each treatment group, 18 intestinal tissue samples from the fish were randomly selected for analysis. The extraction of total protein was carried out with a RIPA lysis solution (P0013B, Beyotime, Shanghai, China). Total protein levels were determined using the BCA Protein Assay Kit (A045-4-2). Following extraction, the proteins underwent electrophoresis with an SDS-PAGE gel and were then transferred to Poly Vinylidene Difluoride (PVDF) membranes. Afterward, the membranes were blocked and incubated with primary antibodies at 4°C overnight, followed by incubation with a secondary antibody. An enhanced chemiluminescence (ECL) system was used for development. The proteins used for internal reference were GAPDH and β-actin. Supplementary Table S2 contains the antibody information.
2.11 Statistical analysis
The calculation method of growth performance was shown in Supplementary Table S3. Image J software facilitated the quantification of the images. Data analysis was performed using the Student’s t-test. Results were shown as mean values with their standard deviations (mean ± S.D). Statistical significance was shown by a P < 0.05. The statistical data were graphed using GraphPad Prism 8.0.
3 Results
3.1 Growth performance
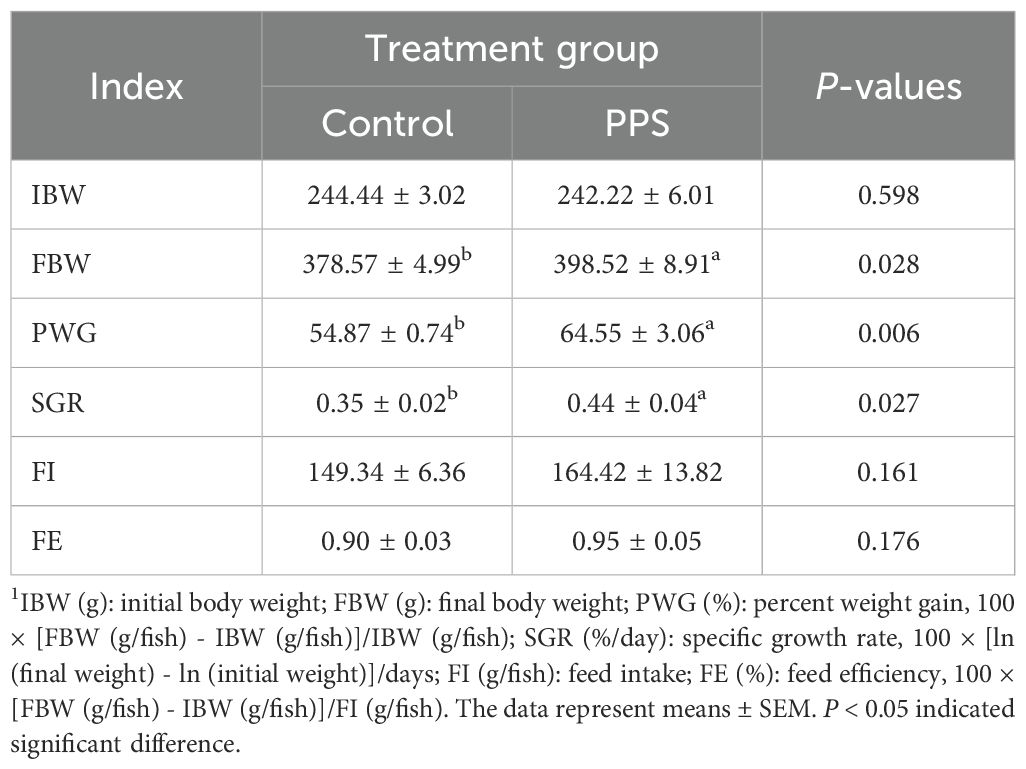
The channel catfish in the PPS group elevated FBW, PWG and SGR (P<0.05) (Table 2), but did not influence FI and FE (P>0.05). The findings suggested that channel catfish in the PPS group obtain better growth performance.
3.2 Water quality parameters
As shown in Figure 2, during the entire experiment period, there was no significant difference in pH between the control group and the PPS group; the DO45d and DO60d in the PPS group were significantly higher than those in the control group; the TP60d, TAN60d and Nitrite60d in the PPS group were significantly lower than those in the control group (P < 0.05); the TN30d, TN45d, TN60d, COD30d, COD45d and COD60d in the PPS group were significantly lower than those in the control group (P < 0.05).
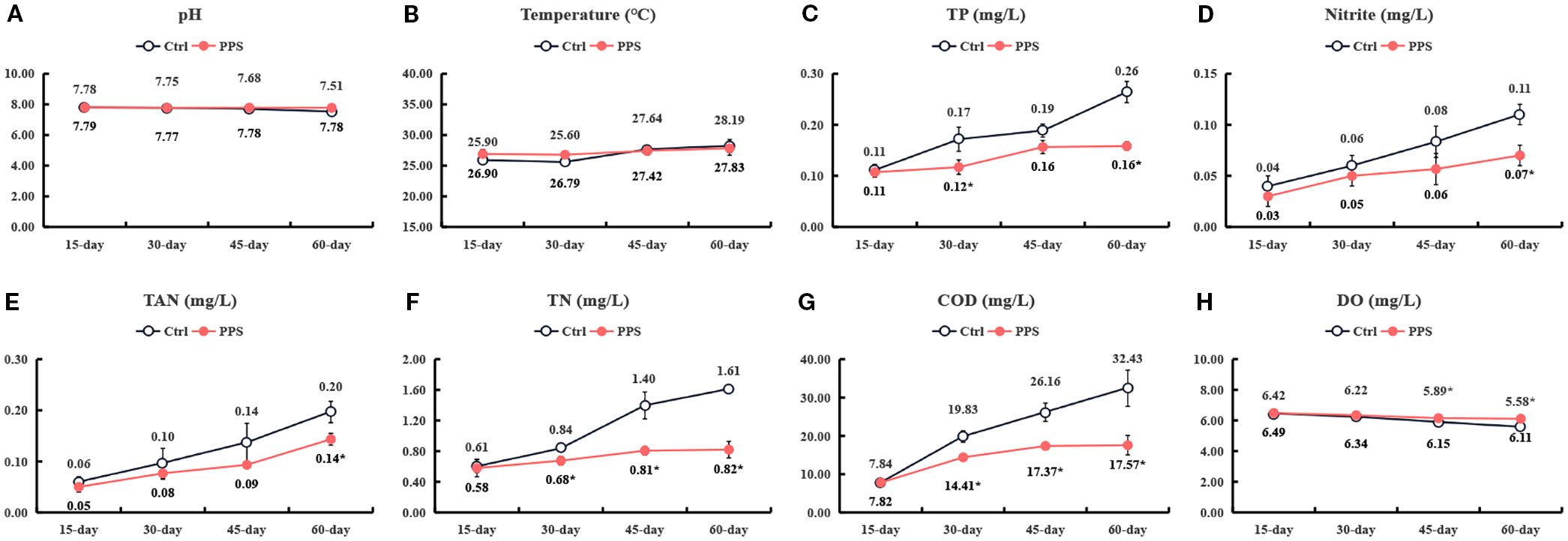
Figure 2. The effects of PPS on water quality parameters. The black line represents the control group, while the red line represents the PPS group. (A) pH; (B) Temperature; (C) Total phosphorus (TP); (D) Nitrite; (E) Total Ammonia nitrogen (TAN); (F) Total Nitrogen (TN); (G) Chemical Oxygen Demand (COD); (H) Dissolved oxygen (DO). * indicates significant differences P > 0.05.
3.3 Serum biochemical indexes and intestinal digestion and absorption enzymes activities
As shown in Table 3, the DAO and D-LC levels in serum were significantly decreased in the PPS group (P<0.05). The present study also indicated that the activities of intestinal digestion and absorption enzymes, such as trypsin, amylase, lipase, Na+-K+-ATPase, γ-GT, and CK, were higher in the PPS group (P < 0.05). It’s worth noting that there has been no change in the AKP activity (P>0.05).
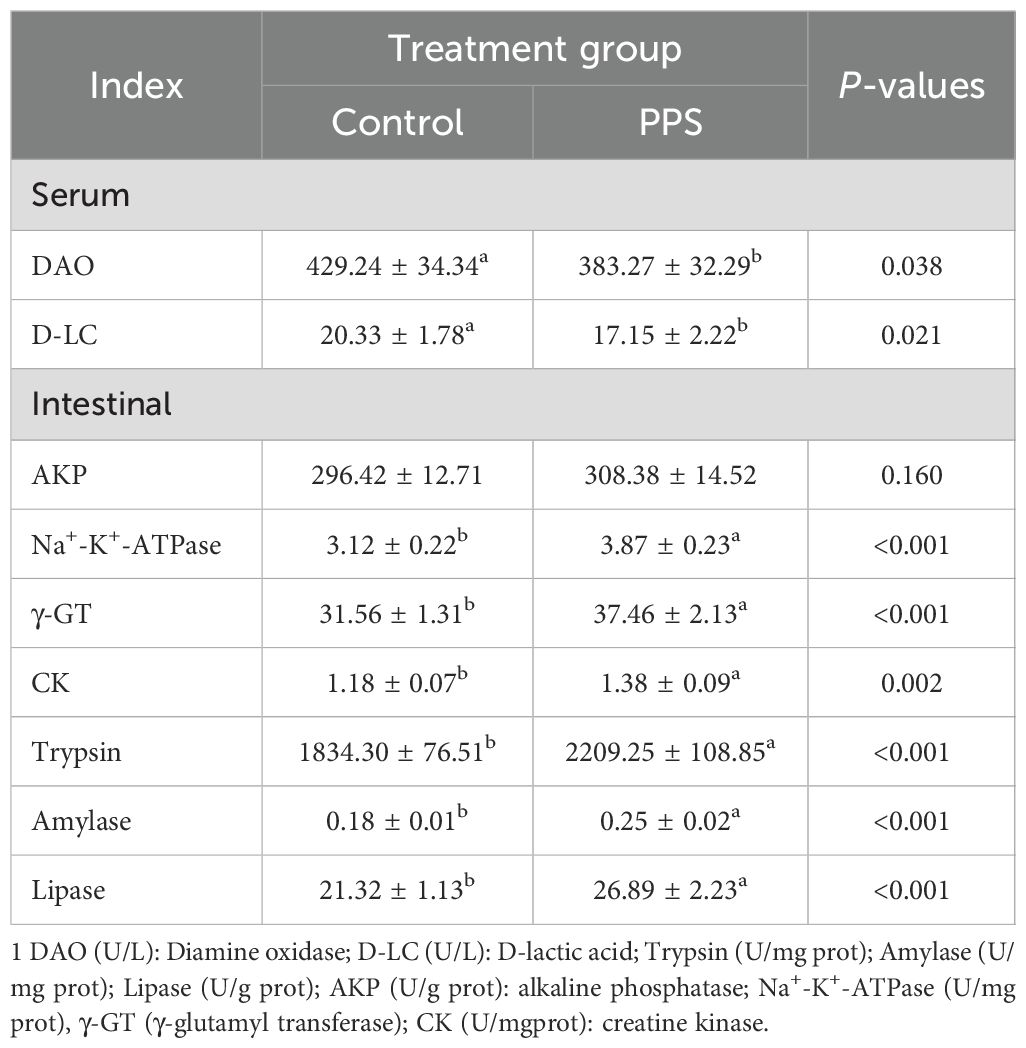
Table 3. The serum biochemical indexes and intestinal digestion and absorption enzymes activities of channel catfish1.
3.4 Histomorphological analysis
The results of HE staining indicated that the intestinal villi height (VH) of channel catfish reared in the PPS group was higher (Figures 3A, C). However, the lamina propria thickening (LPT) remained unchanged (Figures 3A, D). Furthermore, the PAS staining results indicated that, compared with the control group, channel catfish reared in the PPS group had a significantly decreased number of intestinal mucus cells (Figures 3B, E).
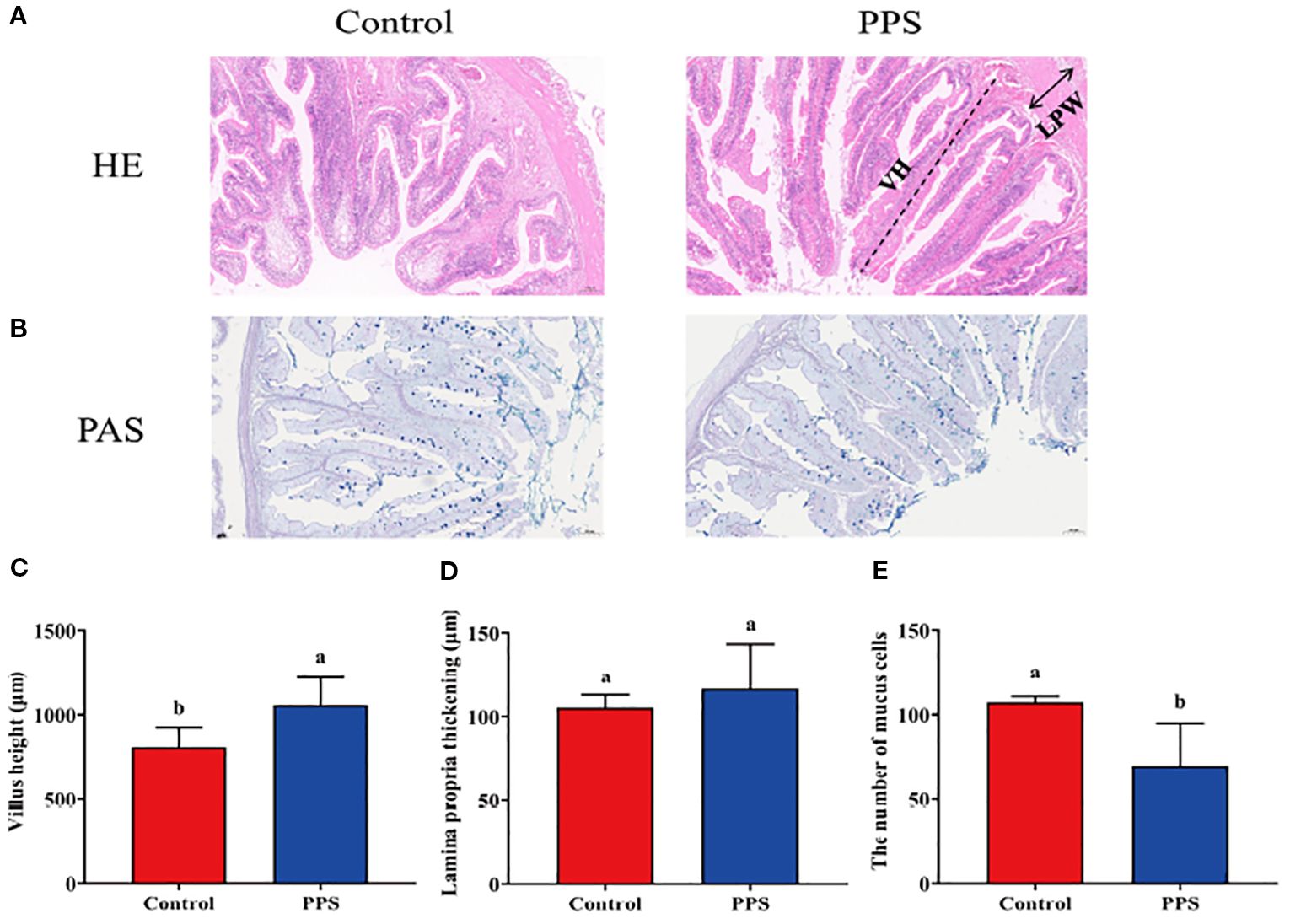
Figure 3. The effects of PPS on intestinal tissue structure and the number of mucus cells in channel catfish. (A) HE staining; (B) PAS staining; (C) Villi height; (D) Lamina propria thickening; (E) The number of mucus cells. VH = villi height; LPT = lamina propria thickening. Columns with different letters indicate significant differences (P < 0.05).
3.5 Intestinal TJ proteins and AJ proteins
Figure 4A illustrates that the expression of tight junction-related genes ZO-1, ZO-2, and Occludin was significantly increased in the PPS group (P<0.05), while the mRNA expression of claudin-12 and claudin-15 was significantly decreased in the PPS group (P<0.05). The adherent junction-related gene β-catenin, nectin-2 and afadin were significantly downregulated in the PPS group (P<0.05) (Figure 4B). Furthermore, the protein expression results showed that ZO-1 and Occludin expression levels were significantly increased in the PPS group (P<0.05) (Figures 4C-E), and β-catenin protein expression levels were significantly decreased in the PPS group (P<0.05) (Figures 4C, F). These results displayed that channel catfish growing in the PPS group have better intestinal structural integrity.
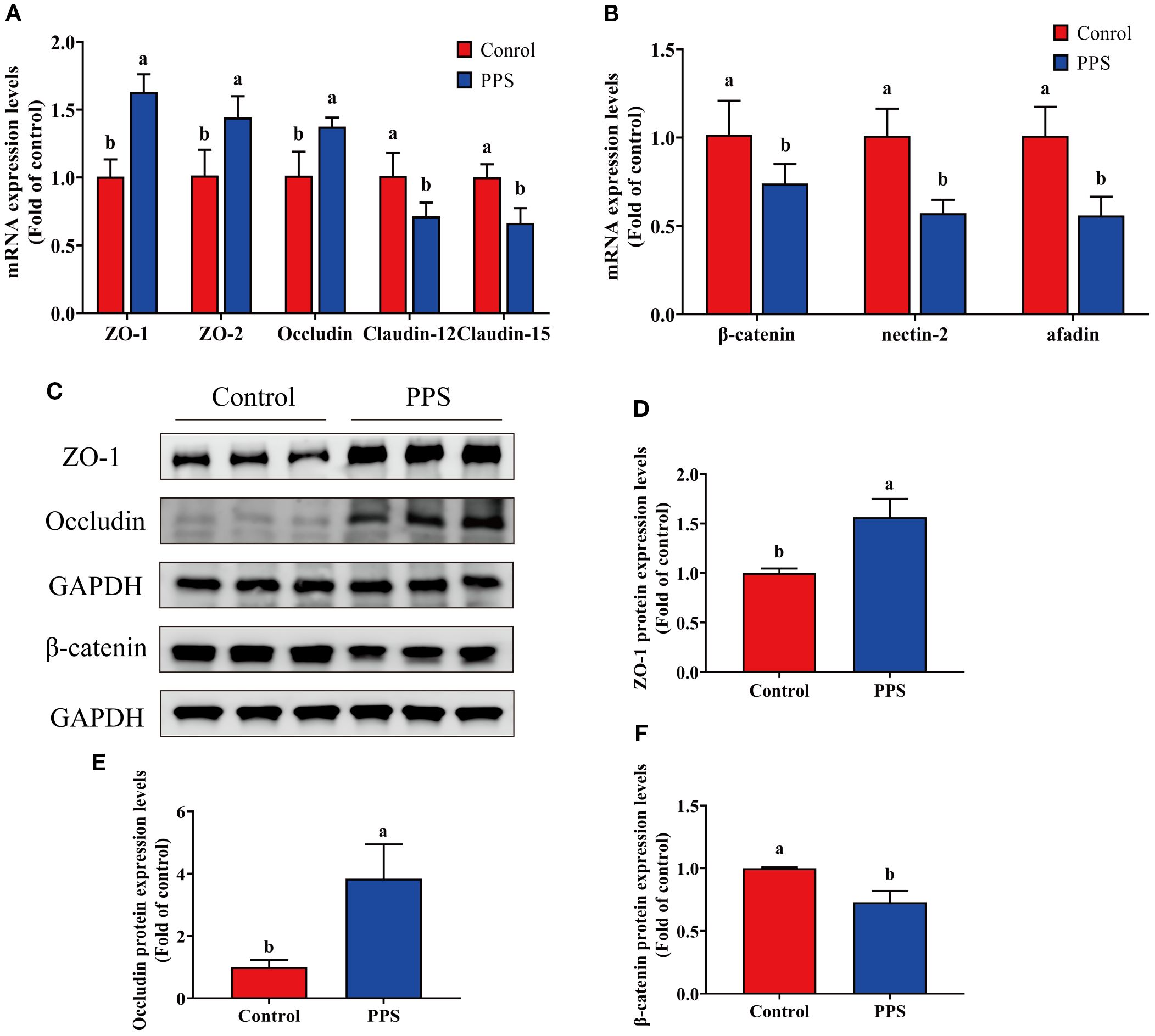
Figure 4. The effects of PPS on the tight junction proteins and adherens junction proteins of channel catfish intestine. (A) Tight junction proteins-related gene expression; (B) Adherens junction proteins-related gene expression; (C) ZO-1, Occludin and β-catenin proteins expression; (D) ZO-1 protein expression levels; (E) Occludin protein expression levels; (F) β-catenin protein expression levels. Columns with different letters indicate significant differences (P < 0.05).
3.6 The regulation of signalling pathway
The protein expression results showed that GTPase Ras homolog gene family member A (GPT-RhoA)/Total-RhoA, ROCK and MLCK were significantly decreased in the intestine of channel catfish growing in the PPS group (P<0.05) (Figures 5A-D). The above results suggested that channel catfish cultured in the PPS group may promote intestinal TJ integrity through the RhoA/ROCK/MLCK signaling pathway.
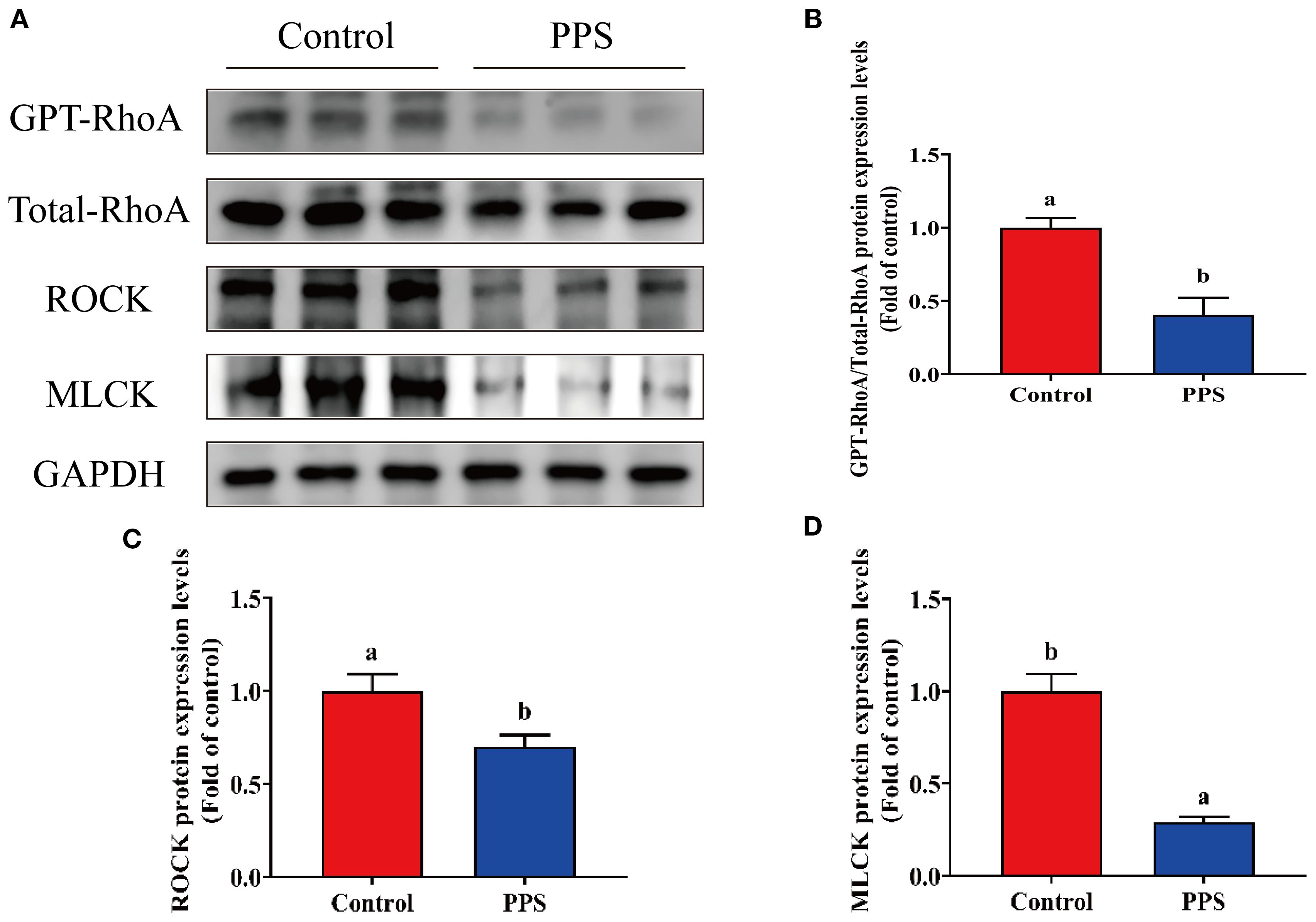
Figure 5. The effects of PPS on apical junction complex-related signaling molecules of channel catfish intestine. (A) GPT-RhoA, Total RhoA, ROCK and MLCK proteins expression; (B) GPT-RhoA/Total-RhoA protein expression levels; (C) ROCK protein expression levels; (D) MLCK protein expression levels. Columns with different letters indicate significant differences (P < 0.05).
4 Discussion
Currently, there are an increasing number of studies on RAS, mainly focusing on different function modules within RAS and process optimization (Teitge et al., 2020; Lindholm-Lehto et al., 2021; Villanova et al., 2023). However, based on RAS conditions, only sporadic reports have been seen regarding the impact on fish health, and only studies on aquatic animal growth performance have been conducted, which are not systematic and in-depth (Araujo et al., 2025; Roy et al., 2025). This study mainly focuses on the impact of the PPS system on fish health, and based on the water quality data collected at different times, further conducts a systematic and in-depth study on the effects of PPS on the production performance and intestinal health of channel catfish, and reveals its possible implications.
4.1 PPS improved fish growth and intestinal health
The health of the intestines significantly influences both the growth and overall well-being of animals (He et al., 2022). According to Amin et al (Amin et al., 2020), tilapia reared in the RAS demonstrated a significantly greater growth rate and weight gain. This is consistent with our research findings, which suggest that channel catfish raised in RAS, influenced by environmental conditions, exhibit an increased rate of nutrient digestion and absorption in their intestines. The entry of D-LC and DAO into the bloodstream indicates a compromised intestinal structural integrity, as they are biomarkers of intestinal permeability (Gao et al., 2023). Our results displayed that these intestinal biomarkers of channel catfish growing in the PPS group were decreased, which indicated that the intestinal structural integrity of channel catfish had been improved. The intestine functions as the primary digestive and absorption organ in fish, with intestinal epithelial cells and histology being essential for nutrient digestion and absorption (Lin et al., 2023). Villi height is typically employed as a significant measure for evaluating the health of the intestines (Wang D. et al., 2022). Interestingly, we found that the villi height of channel catfish grown in the PPS group is higher than control group. Generally, the goblet cells in the intestine can secrete a large amount of mucus to maintain the digestive process of the intestine (Cao et al., 2019; Wang S. et al., 2022). However, excessive mucus can lead to the occurrence of local inflammation in the intestine (Naama and Bel, 2023). It is worth noting that this study found that the number of intestinal goblet cells in the fish of the PPS group was lower than that in the control group.
Based on the above evidence, it is not difficult to conclude that the PPS has a positive effect on the growth performance of channel catfish as well as the integrity of their intestinal morphology and structure. Generally, the integrity of the intestinal structure determines whether the digestive and absorptive functions of the intestine can proceed normally. Therefore, we also explored the enzymatic activities involved in digestion and absorption in the intestine.
4.2 PPS elevated fish intestinal digestion and absorption functions
The intestine’s capacity to maintain health and digest efficiently depends significantly on the structure and integrity of its epithelium (Inatomi and Otomaru, 2018). The augmentation of brush border enzyme expression and nutrient transport systems has been directly linked to the integrity of intestinal tissue (Thuekeaw et al., 2022). The absorption of nutrients is influenced by enzymes like AKP, γ-GT, Na+-K+-ATPase and CK in the intestinal brush border enzymes (Zhao et al., 2021). Our results showed that the activities of Na+-K+-ATPase, γ-GT and CK of channel catfish growing in the PPS group were improved. Furthermore, the capacity for feed digestion is reflected by the activity level of intestinal digestive enzymes (Li et al., 2019). Our findings indicated that the activities of digestive enzymes in channel catfish were significantly enhanced in the PPS group, which is consistent with our anticipated results. These findings are also consistent with a study conducted on largemouth bass, which showed RAS improved the amylase and lipase activities in the intestine of largemouth bass (Xu et al., 2025). Another study has found that RAS can enhance the quality of water, thereby facilitating the deposition of nutrients in the feed by Pacific white shrimp (Litopenaeus vannamei) (Araujo et al., 2025). As is well known, aquaculture water quality indicators, including nitrite, TAN, and DO, frequently result in a substantial decline in the production performance of aquatic animals and the outbreak of intestinal diseases (Abd El-Hack et al., 2022; Araujo et al., 2025). Consequently, we hypothesize that RAS mainly enhances the growth and well-being of aquatic animals by purifying the water quality. Coincidentally, our results for water quality parameters, including COD, TP, nitrite, and TAN, support our previous hypothesis.
The evidence presented above indicates that enhancing water quality can significantly enhance the digestion and absorption of nutrients by fish. However, there is currently no report on the mechanism by which PPS affects the integrity of the fish’s intestinal structure. Based on our previous research (Lu et al., 2022b), we have preliminarily conducted a study on the mechanism of PPS on the integrity of the fish’s intestinal structure.
4.3 PPS protects the integrity of the intestinal structure and related mechanisms
It is well-established that TJ proteins and AJ proteins are necessary for the structural integrity of the intestines (Kim et al., 2022; Lu et al., 2022b). The composition of TJ proteins and AJ proteins includes diverse families of transmembrane adhesion proteins and cytosolic proteins that attach these proteins to the actin cytoskeleton (Kausalya et al., 2004). The intestinal structural integrity relies on key proteins like ZO-1, Occludin, and Claudins, which are vital for TJ proteins (Xie et al., 2021). Our findings demonstrated an elevated regulation of the expression of both TJ proteins and AJ proteins at the mRNA and protein levels in the PPS group, which suggested that channel catfish farmed with PPS can improve the integrity of the intestine. However, it is still unclear through which signaling pathway PPS regulates the structural integrity of the fish intestinal tract. Therefore, we are conducting further research on the mechanism.
Research indicated that the RhoA/ROCK pathway plays a crucial role in regulating intestinal apical junctions (Huang et al., 2022). ROCK is an effector that operates downstream of RhoA (Wu et al., 2022). The ROCK kinase pathway is used by RhoA to increase contractility by activating the myosin light-chain kinase (MLCK) (Wenceslau et al., 2016). Additionally, an increase in the synthesis and activity of MLCK protein can lead to higher intestinal TJ permeability (Boivin et al., 2007). We discovered that GPT-RhoA/Total-RhoA, ROCK and MLCK were decreased in the intestine of channel catfish growing in the PPS group. It was suggested that the intestinal structural integrity of channel catfish growing under the influence of PPS might be regulated by the ROCK/MLCK signaling pathway. From the above literature and our results, this study, based on various methods such as histological observation, biochemical analysis and molecular biology, not only proved the effective protection of PPS on the intestinal structure of fish, but also systematically revealed the effects of PPS on TJ proteins and AJ proteins, and further revealed the possible pathways involved, providing valuable references for subsequent functional verification studies.
5 Conclusion
Overall, the application of PPS in aquaculture contributes to the improvement of water quality and the promotion of fish intestinal health, which holds significant implications for modern intensive aquaculture practices. This study further enhances our understanding of the potential of PPS as a strategy to increase productivity in freshwater fish farming. Our findings demonstrate that PPS can improve water quality, protect intestinal microvilli, reduce intestinal permeability, and upregulate the expression of genes associated with TJ and AJ. Moreover, molecular biology analyses suggest that the enhancement of intestinal structural integrity in fish mediated by PPS may involve the MLCK and RhoA/ROCK signaling pathways. Therefore, this study is conducive to fundamentally revealing the positive effect of PPS on fish health. The results of this experiment could provide a theoretical reference for outdoor PPS farmed fish based on RAS modification. Therefore, future studies should include validation experiments at the cellular and model organism levels, employing approaches such as metagenomics and environmental DNA analysis, to further elucidate the underlying molecular mechanisms and to investigate the interaction between water quality parameters and relevant signaling molecules.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was approved by Animal Use and Ethics Committee of Hainan University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZZ: Resources, Conceptualization, Investigation, Writing – review & editing, Project administration, Data curation, Funding acquisition. YY: Writing – original draft, Software. JL: Data curation, Writing – review & editing, Conceptualization, Investigation, Resources, Project administration, Funding acquisition. MZ: Writing – original draft, Validation. JL: Validation, Writing – original draft. LT: Writing – original draft, Investigation. HN: Writing – original draft, Investigation. KC: Writing – original draft, Formal Analysis. FL: Software, Writing – original draft. XH: Writing – original draft, Writing – review & editing. ZL: Methodology, Resources, Formal Analysis, Data curation, Investigation, Funding acquisition, Conceptualization, Writing – review & editing, Supervision, Project administration, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was financially supported by National Key Research and Development Program of China (2024YFD2401300), the Hainan Provincial Natural Science Foundation of China (325RC653), the Hainan University Technical Service Project Fund-Research and Demonstration Application Project on the Construction of Agricultural-Photovoltaic Complementary Agricultural Production Mode (HD-KYH-2022384) and supported by the Hainan University Research Start-up Fund (KYQD(ZR)23175).
Acknowledgments
The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
Conflict of interest
YY, MZ, JL, KC, and FL were employed by Zhanjiang Zhongyue Energy Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1681940/full#supplementary-material
References
Abd El-Hack M. E., El-Saadony M. T., Nader M. M., Salem H. M., El-Tahan A. M., Soliman S. M., et al. (2022). Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 66, 2183–2194. doi: 10.1007/s00484-022-02347-6
Amin M., Musdalifah L., and Ali M. (2020). Growth performances of Nile Tilapia, Oreochromis niloticus, reared in recirculating aquaculture and active suspension systems. IOP Conf. Series: Earth Environ. Sci. 441, 12135. doi: 10.1088/1755-1315/441/1/012135
Araujo A. N., Nguyen K., Strebel L., Corby T., Rhodes M. A., Beck B. H., et al. (2025). Effect of protein intake on growth and nutrient retention of pacific white shrimp (Litopenaeus vannamei) in a green water recirculating aquaculture system. Aquac. Nutr. 2025, 4942439. doi: 10.1155/anu/4942439
Boivin M. A., Ye D., Kennedy J. C., Al-Sadi R., Shepela C., and Ma T. Y. (2007). Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G590–G598. doi: 10.1152/ajpgi.00252.2006
Cao Y., Yao G., Sheng Y., Yang L., Wang Z., Yang Z., et al. (2019). JinQi jiangtang tablet regulates gut microbiota and improve insulin sensitivity in type 2 diabetes mice. J. Diabetes Res. 12, 1872134. doi: 10.1155/2019/1872134
Dawood M. A. O. (2020). Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev. Aquac. 13, 642–663. doi: 10.1111/raq.12492
Emmanuel A., Wei Y., Ramzan M. N., Yang W., and Zheng Z. (2025). Dynamics of bacterial communities and their relationship with nutrients in a full-scale shrimp recirculating aquaculture system in brackish water. Anim. (Basel). 15 (10), 1400. doi: 10.3390/ani15101400
France M. M. and Turner J. R. (2017). The mucosal barrier at a glance. J. Cell Sci. 130, 307–314. doi: 10.1242/jcs.193482
Freeman H. J. and Kim Y. S. (1978). Digestion and absorption of protein. Annu. Rev. Med. 29, 99–116. doi: 10.1146/annurev.me.29.020178.000531
Gao Y., Meng Q., Qin J., Zhao Q., and Shi B. (2023). Resveratrol alleviates oxidative stress induced by oxidized soybean oil and improves gut function via changing gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. 14, 54. doi: 10.1186/s40104-023-00851-2
He L., Wang C., Simujide H., Aricha H., Zhang J., Liu B., et al. (2022). Effects of pathogenic escherichia coli infection on the flora composition, function, and content of short-chain fatty acids in calf feces. Anim. (Basel). 12 (8), 959. doi: 10.3390/ani12080959
Hoseinifar S. H., Dadar M., and Ringø E. (2017). Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: The functional feed additives scenario. Aquac. Res. 48, 3987–4000. doi: 10.1111/are.13368
Huang B., Wang J., Gu A., Wang T., Li J., and Shan A. (2022). Zearalenone-induced mechanical damage of intestinal barrier via the rhoA/ROCK signaling pathway in IPEC-J2 cells. Int. J. Mol. Sci. 23 (20), 12550. doi: 10.3390/ijms232012550
Huang Y.-L., Mayfield A. B., and Fan T.-Y. (2020). Effects of feeding on the physiological performance of the stony coral Pocillopora acuta. Sci. Rep. 2025, 4942439. doi: 10.1038/s41598-020-76451-1
Ibrahim D., Arisha A. H., Khater S. I., Gad W. M., Hassan Z., Abou-Khadra S. H., et al. (2022). Impact of Omega-3 Fatty Acids Nano-Formulation on Growth, Antioxidant Potential, Fillet Quality, Immunity, Autophagy-Related Genes and Aeromonas hydrophila Resistance in Nile Tilapia (Oreochromis niloticus). Antioxidants 11 (8), 1523. doi: 10.3390/antiox11081523
Inatomi T. and Otomaru K. (2018). Effect of dietary probiotics on the semen traits and antioxidative activity of male broiler breeders. Sci. Rep. 8, 5874. doi: 10.1038/s41598-018-24345-8
Karvonen A., Fenton A., and Sundberg L. R. (2019). Sequential infection can decrease virulence in a fish-bacterium-fluke interaction: Implications for aquaculture disease management. Evol. Appl. 12, 1900–1911. doi: 10.1111/eva.12850
Kausalya P. J., Phua D. C. Y., and Hunziker W. (2004). Association of ARVCF with zonula occludens (ZO)-1 and ZO-2: binding to PDZ-domain proteins and cell-cell adhesion regulate plasma membrane and nuclear localization of ARVCF. Mol. Biol. Cell. 15, 5503–5515. doi: 10.1091/mbc.e04-04-0350
Kim K., He Y., Jinno C., Kovanda L., Li X., Bravo D., et al. (2022). Supplementation of oligosaccharide-based polymer enhanced growth and disease resistance of weaned pigs by modulating intestinal integrity and systemic immunity. J. Anim. Sci. Biotechnol. 13, 10. doi: 10.1186/s40104-021-00655-2
Konig J., Wells J., Cani P. D., Garcia-Rodenas C. L., MacDonald T., Mercenier A., et al. (2016). Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 7, e196. doi: 10.1038/ctg
Li C., Zhang B., Wang X., Pi X., Wang X., Zhou H., et al. (2019). Improved utilization of soybean meal through fermentation with commensal Shewanella sp. MR-7 in turbot (Scophthalmus maximus L.). Microb. Cell Fact. 18, 214. doi: 10.1186/s12934-019-1265-z
Lin X., Zhang C., Cao K., Li Z., Zhao Z., Li X., et al. (2023). Dietary Sodium Butyrate Changed Intestinal Histology and Microbiota of Rainbow Trout (Oncorhynchus mykiss), but Did Not Promote Growth and Nutrient Utilization. Aquac. Nutr., 3706109. doi: 10.1155/2023/3706109
Lindholm-Lehto P. C., Pulkkinen J. T., Kiuru T., Koskela J., and Vielma J. (2021). Efficient water treatment achieved in recirculating aquaculture system using woodchip denitrification and slow sand filtration. Environ. Sci. pollut. Res. Int. 28, 65333–65348. doi: 10.1007/s11356-021-15162-0
Liu Z. (2011). Development of genomic resources in support of sequencing, assembly, and annotation of the catfish genome. Comp. Biochem. Phys. D. 6, 11–17. doi: 10.1016/j.cbd.2010.03.001
Lu Z. Y., Feng L., Jiang W. D., Wu P., Liu Y., Jiang J., et al. (2022a). Dietary mannan oligosaccharides strengthens intestinal immune barrier function via multipath cooperation during Aeromonas Hydrophila infection in grass carp (Ctenopharyngodon Idella). Front. Immunol. 13, 1010221. doi: 10.3389/fimmu.2022.1010221
Lu Z., Feng L., Jiang W., Wu P., Liu Y., Jiang J., et al. (2023). Mannan oligosaccharides alleviate oxidative injury in the head kidney and spleen in grass carp (Ctenopharyngodon idella) via the Nrf2 signaling pathway after Aeromonas hydrophila infection. J. Anim. Sci. Biotechnol. 14, 58. doi: 10.1186/s40104-023-00844-1
Lu Z.-Y., Feng L., Jiang W.-D., Wu P., Liu Y., Jin X.-W., et al. (2022b). An Antioxidant Supplement Function Exploration: Rescue of Intestinal Structure Injury by Mannan Oligosaccharides after Aeromonas hydrophila Infection in Grass Carp (Ctenopharyngodon idella). Antioxidants 11. doi: 10.3390/antiox11050806
Lu Z.-Y., Feng L., Jiang W.-D., Wu P., Liu Y., Kuang S.-Y., et al. (2020). Mannan oligosaccharides improved growth performance and antioxidant capacity in the intestine of on-growing grass carp (Ctenopharyngodon idella). Aquacult. Rep. 17, 100313. doi: 10.1016/j.aqrep.2020.100313
Lu Z.-Y., Jiang W.-D., Wu P., Liu Y., Ren H.-M., Jin X.-W., et al. (2023). Cellular antioxidant mechanism of mannan-oligosaccharides involving in enhancing flesh quality in grass carp (Ctenopharyngodon idella). J. Sci. Food Agric. 103, 1172–1182. doi: 10.1002/jsfa.12211
Magouz F. I., Bassuini M. I., Khalafalla M. M., Abbas R., Sewilam H., Aboelenin S. M., et al. (2021). Mannan oligosaccharide enhanced the growth rate, digestive enzyme activity, carcass composition, and blood chemistry of thinlip grey mullet (Liza ramada). Anim. (Basel). 11 (12), 3559. doi: 10.3390/ani11123559
Naama M. and Bel S. (2023). Autophagy-ER stress crosstalk controls mucus secretion and susceptibility to gut inflammation. Autophagy 19, 3014–3016. doi: 10.1080/15548627.2023.2228191
Noaillac-Depeyre J. and Hollande E. (1981). Evidence for somatostatin, gastrin and pancreatic polypeptide-like substances in the mucosa cells of the gut in fishes with and without stomach. Cell Tissue Res. 216, 193–203. doi: 10.1007/bf00234554
Otani T. and Furuse M. (2020). Tight junction structure and function revisited. Trends Cell Biol. 30, 805–817. doi: 10.1016/j.tcb.2020.08.004
Qian Y.-W., Li C., Jiang A.-P., Ge S., Gu P., Fan X., et al. (2016). HIV-1 gp120 glycoprotein interacting with dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN) down-regulates tight junction proteins to disrupt the blood retinal barrier and increase its permeability*. J. Biol. Chem. 291, 22977–22987. doi: 10.1074/jbc.M116.744615
Roy D., Saha P. K., Sarker S., Parves M. A., Zavyalov A. P., and Latifa G. A. (2025). Culture practice of Oreochromis niloticus through recirculating aquaculture system (RAS): a modern and growth-optimized approach of fish culture by maintaining water quality with proper fish stocking density in Bangladesh. Environ. Sci. pollut. Res. Int. 32, 11749–11766. doi: 10.1007/s11356-025-36386-4
Russo E., Fiorindi C., Giudici F., and Amedei A. (2022). Immunomodulation by probiotics and prebiotics in hepatocellular carcinoma. World J. Hepatol. 14, 372–385. doi: 10.4254/wjh.v14.i2.372
Shukla P. K., Meena A. S., Dalal K., Canelas C., Samak G., Pierre J. F., et al. (2021). Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis. Sci. Rep. 11, 826. doi: 10.1038/s41598-020-80637-y
Teitge F., Peppler C., Steinhagen D., and Jung-Schroers V. (2020). Water disinfection by ozonation has advantages over UV irradiation in a brackish water recirculation aquaculture system for Pacific white shrimp (Litopenaeus vannamei). J. Fish. Dis. 43, 1259–1285. doi: 10.1111/jfd.13238
Thuekeaw S., Angkanaporn K., and Nuengjamnong C. (2022). Microencapsulated basil oil (Ocimum basilicum Linn.) enhances growth performance, intestinal morphology, and antioxidant capacity of broiler chickens in the tropics. Anim. Biosci. 35, 752–762. doi: 10.5713/ab.21.0299
Tian Y., Shu R., Lei Y., Xu Y., Zhang X., and Luo H. (2022). Somatostatin attenuates intestinal epithelial barrier injury during acute intestinal ischemia-reperfusion through Tollip/Myeloiddifferentiationfactor 88/Nuclear factor kappa-B signaling. Bioengineered 13, 5005–5020. doi: 10.1080/21655979
Villanova V., Roques J. A. C., Forghani B., Shaikh K. M., Undeland I., and Spetea C. (2023). Two-phase microalgae cultivation for RAS water remediation and high-value biomass production. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1186537
Wang Z., Du J., Lam S. H., Mathavan S., Matsudaira P., and Gong Z. (2010). Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics 11, 392. doi: 10.1186/1471-2164-11-392
Wang S., Liu S., Wang C., Ye B., Lv L., Ye Q., et al. (2022). Dietary Antimicrobial Peptides Improve Intestinal Function, Microbial Composition and Oxidative Stress Induced by Aeromonas hydrophila in Pengze Crucian Carp (Carassius auratus var. Pengze). Antioxid. (Basel). 11 (9), 1756. doi: 10.3390/antiox11091756
Wang D., Zhang Y., Chen Q., Kuang Y., Fan J., Xu X., et al. (2022). Selenium-enriched Cardamine violifolia improves growth performance with potential regulation of intestinal health and antioxidant function in weaned pigs. Front. Vet. Sci. 9, 964766. doi: 10.3389/fvets.2022.964766
Wenceslau C. F., McCarthy C. G., and Webb R. C. (2016). Formyl peptide receptor activation elicits endothelial cell contraction and vascular leakage. Front. Immunol. 7. doi: 10.3389/fimmu.2016.00297
Wu D., Jiang W., Liu C., Liu L., Li F., Ma X., et al. (2022). CTNNAL1 participates in the regulation of mucus overproduction in HDM-induced asthma mouse model through the YAP-ROCK2 pathway. J. Cell Mol. Med. 26, 1656–1671. doi: 10.1111/jcmm.17206
Xiao M., Qian K., Wang Y., and Bao F. (2020). GC-MS metabolomics reveals metabolic differences of the farmed Mandarin fish Siniperca chuatsi in recirculating ponds aquaculture system and pond. Sci. Rep. 10 (1), 6090. doi: 10.1038/s41598-020-63252-9
Xie W., Song L., Wang X., Xu Y., Liu Z., Zhao D., et al. (2021). A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut. Microbes 13, 1956281. doi: 10.1080/19490976.2021.1956281
Xu J., Liu C., Zheng J., Wang J., Wang L., Feng D., et al. (2025). Impact of funnel-shaped recirculating aquaculture system on the growth, health, and muscle quality of largemouth bass (Micropterus salmoides). Aquaculture 599, 742176. doi: 10.1016/j.aquaculture.2025.742176
Zeng B., Wang W., and Dong Y. (2020). Dietary protein requirement of the high altitude's representative teleost juveniles Schizopygopsis younghusbandi (Cypriniformes: Cyprinidae). Aquac. Res. 51, 2852–2862. doi: 10.1111/are.14624
Zhao P., Feng L., Jiang W., Wu P., Liu Y., Ren H., et al. (2024a). Unveiling the emerging role of curcumin to alleviate ochratoxin A-induced muscle toxicity in grass carp (Ctenopharyngodon idella): in vitro and in vivo studies. J. Anim. Sci. Biotechnol. 15, 72. doi: 10.1186/s40104-024-01023-6
Zhao P., Jiang W.-D., Wu P., Liu Y., Ren H.-M., Jin X.-W., et al. (2024b). New perspectives on the mechanism of curcumin in the gill mucosal immune barrier damaged by ochratoxin A in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 583, 740629. doi: 10.1016/j.aquaculture.2024.740629
Zhao P., Liu X., Feng L., Jiang W.-D., Wu P., Liu Y., et al. (2023). New perspective on mechanism in muscle toxicity of ochratoxin A: Model of juvenile grass carp (Ctenopharyngodon idella). Aquat. Toxicol. 263, 106701. doi: 10.1016/j.aquatox.2023.106701
Zhao H., Wang G., Wang H., Mo W., Huang Y., Cao J., et al. (2021). Effects of dietary sodium butyrate on growth, digestive enzymes, body composition and nutrient retention-related gene expression of juvenile yellow catfish (Pelteobagrus fulvidraco). Anim. Nutr. 7 (2), 539–547. doi: 10.1016/j.aninu.2020.12.007
Keywords: pond purification system, intestine, digestive absorption, structural integrity, channel catfish
Citation: Zheng Z-B, Yang Y, Liu J, Zhou M-C, Li J-H, Tang L-J, Nie H-L, Chen K-S, Liu F, He X-N and Lu Z-Y (2025) The effects of pond purification system on intestinal function of channel catfish (Ictalurus punctatus): a focus on intestinal development, digestion and absorption, and junction integrity. Front. Mar. Sci. 12:1681940. doi: 10.3389/fmars.2025.1681940
Received: 08 August 2025; Accepted: 01 September 2025;
Published: 16 September 2025.
Edited by:
Pengsheng Dong, Henan Agricultural University, ChinaCopyright © 2025 Zheng, Yang, Liu, Zhou, Li, Tang, Nie, Chen, Liu, He and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Yuan Lu, bHV6eUBoYWluYW51LmVkdS5jbg==
†These authors have contributed equally to this work
 Zhong-Bing Zheng1†
Zhong-Bing Zheng1† Zhi-Yuan Lu
Zhi-Yuan Lu