Abstract
Satellite telemetry has enabled the tracking of marine predators’ vertical and horizontal habitat use and migration patterns. These insights provide valuable information that inform management and conservation of threatened species. In this study, a scalloped hammerhead, Sphyrna lewini, (125 cm FL; immature female) a Carolina hammerhead, Sphyrna gilberti, (138 cm FL; immature female) and a scalloped/Carolina hammerhead hybrid (160 cm FL; immature male) were tagged with PSATLIFE’s off the coast of North Carolina. Tag data revealed that the Carolina hammerhead exploited a wide depth range, with a maximum depth of 846 m. Dive patterns of the scalloped hammerhead revealed differences in vertical space use, with the scalloped hammerhead primarily remaining within the top 200 m of the water column, and with a maximum depth of 380 m. The hybrid individual demonstrated similar dive patterns to those of the scalloped hammerhead, with a maximum depth of 203 m. This study presents the first vertical movement data for Carolina and hybrid scalloped/Carolina hammerheads, offering new insights into interspecific variation in depth use. Future research should explore if there are differences in habitat use and foraging strategies, as they may serve as distinguishing characteristics and support the development of effective management measures.
1 Introduction
In recent decades, advancements in tagging technologies have facilitated the monitoring of marine predators in complex three-dimensional environments (Elliott et al., 2022). For instance, satellite telemetry has provided insights into the horizontal and vertical movement patterns of certain shark species, allowing researchers to identify migration patterns, key habitat preferences, and assess overlap in habitat use in relation to commercial and recreational fisheries (Hammerschlag et al., 2011; Salinas‐de‐León et al., 2024; Morales-Serrano and Gonzalez-Pestana, 2024; Madigan et al., 2022). Given the global declines in shark populations (Pacoureau et al., 2021; Worm et al., 2013; 2024), such knowledge is especially valuable and has the potential to inform targeted conservation strategies (Braccini et al., 2016). However, despite the inherent vulnerability of sharks to overexploitation, the movement patterns of the majority of shark species remain understudied (Renshaw et al., 2023). Research on cryptic shark species (e.g., species that are genetically different but morphologically identical; Pfleger et al., 2018; Davis et al., 2019), is limited by logistical challenges in identifying individuals in the field, leading to gaps in our knowledge of species-specific movements and significant uncertainty regarding which areas should be prioritized for conservation (Dwyer et al., 2019).
The Carolina hammerhead shark (Sphyrna gilberti), first described in 2013 (Quattro et al., 2013), is a cryptic species of the scalloped hammerhead shark (Sphyrna lewini). Both species inhabit the western Atlantic Ocean (Barker et al., 2021) and are morphologically indistinguishable externally (Galloway et al., 2024). However, while the scalloped hammerhead is globally distributed, the Carolina hammerhead is believed to have a more restricted range, predominantly off the southeastern coast of the United States (Barker et al., 2021) although it has been found as far south as Brazil (Pinhal et al., 2012). This presents a conservation challenge, further confounded by a discrepancy in knowledge surrounding the two species. While aspects of the scalloped hammerhead’s biology have been well studied, significant data gaps remain for the Carolina hammerhead, leading to its classification as Data Deficient by the IUCN (Carlson et al., 2024). However, understanding the health of the population across its range is crucial, as declining numbers may result in increased rates of hybridization as finding conspecifics to mate with becomes more difficult, causing a decline in genetic diversity as purebred individuals are lost (i.e., genetic swamping; Todesco et al., 2016). As such, telemetry studies aimed at understanding the spatial and vertical habitat use of Carolina hammerheads and how it compares to that of scalloped hammerheads have been identified as a key research priority (Carlson et al., 2024).
As a part of a larger study investigating post-release-mortality in scalloped hammerheads caught in the U.S. Atlantic pelagic longline fishery, a Carolina hammerhead was opportunistically tagged off the coast of North Carolina. Here, we present the first confirmed observation of the fine-scale vertical movement of a Carolina hammerhead, with a comparison to that of a similar sized scalloped hammerhead and hybrid scalloped/Carolina hammerhead tagged at the same time.
2 Materials and methods
2.1 Data collection
Fieldwork was conducted in May 2024 in a 10-mile radius around GPS location 35.5 °N, -74.8 °W, approximately 100 km off the coast of Wanchese, North Carolina (Figure 1).
Figure 1
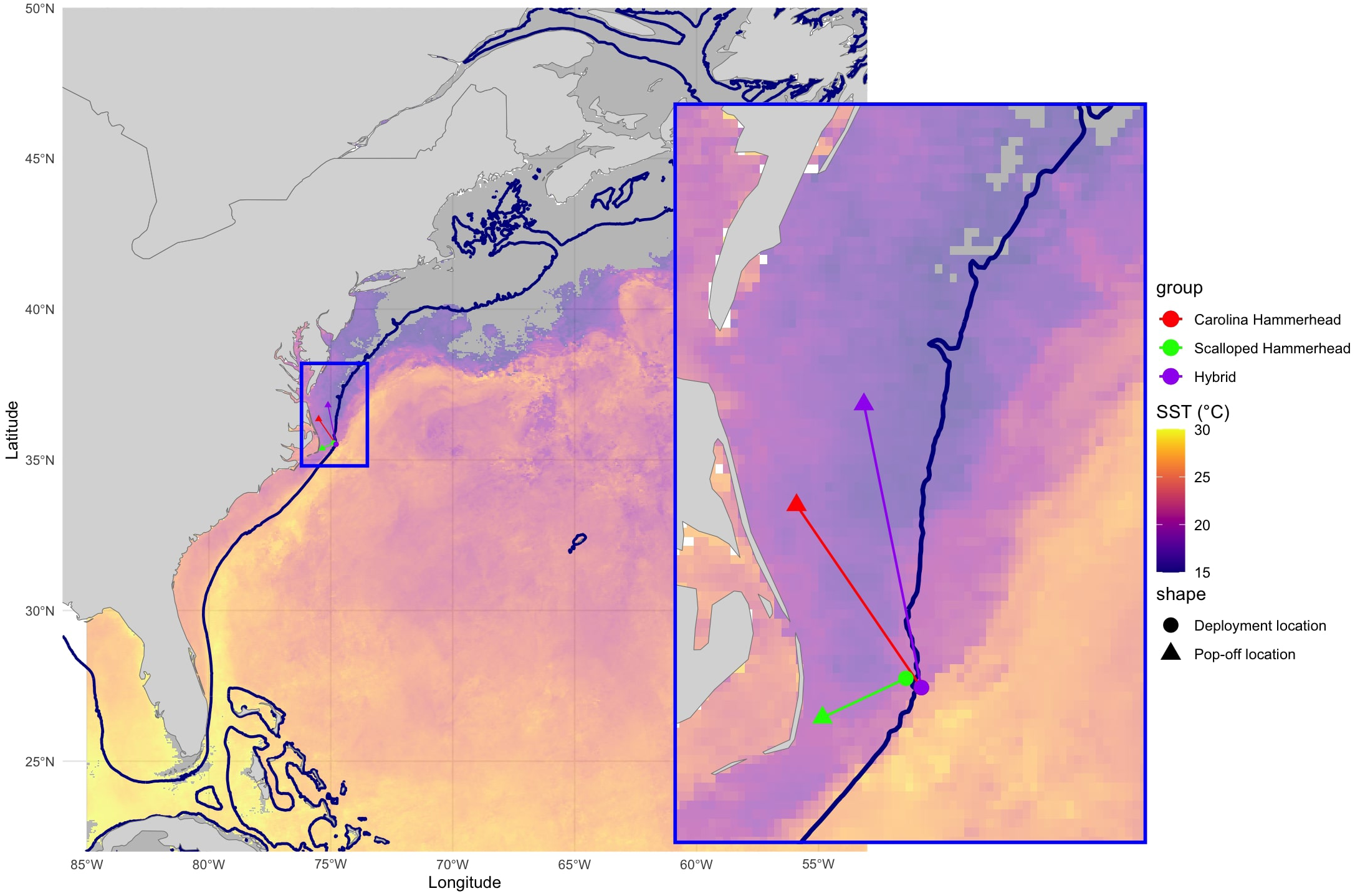
Location of PSATLIFE tag deployments (circles) and first transmissions after pop-off (triangles) for the Carolina hammerhead (S. gilberti), scalloped hammerhead (S. lewini), and their hybrid analyzed in this study. Dark blue lines denote the 200 m bathymetry contour. The background represents sea surface temperature (SST) averaged for May 2024, derived from NASA’s MODIS Aqua satellite (NASA Goddard Space Flight Center, 2024). Bathymetry data obtained from NOAA’s National Centers for Environmental Information.
The pelagic longline configuration consisted of approximately 130 16/0 hooks attached to monofilament gangions, set at 16 m depth, spaced approximately 20 to 25 m apart, and baited with whole squid (Loligo sp.). Each set (n=4) soaked for a maximum of 2–7 hours, after which the mainline was retrieved and the status of each hook was determined. Sets were deployed sequentially throughout the day and night.
Captured hammerheads were brought alongside the boat, and estimated fork length (FL) and sex were recorded. Maturity was determined based on established age-length relationships (Gallagher and Klimley, 2018). The location and timing of capture were also recorded, and a fin clip for species determination was taken from the trailing edge of the first dorsal fin (Poulakis et al., 2017). The first 34 live hammerheads captured were tagged with a Lotek PSATLIFE tag, inserted into the dorsal musculature below the first dorsal fin using a tagging pole. Each PSATLIFE was programmed to collect pressure (depth) data up to 2000 m and ambient temperature data for 85 days, after which the tag is designed to release from the animal, float to the sea surface, and transmit archived data at a 15-minute resolution. Each tag was also equipped with a depth fail-safe release, whereby the tag is programmed to detach from the animal if it does not experience a depth change of greater than 5 meters for a period of 3 days.
2.2 Tag data analysis
After each PSATLIFE tag detached and transmitted data to passing satellites, tag reports were downloaded from the ARGOS website and processed using the Lotek TagTalk software (ver. 1.10.8.14). Vertical movement graphs were constructed in R (ver. 2023.12.1 + 402) using the ggplot2 package (Wickham, 2016), for each individual fitted with a functional PSATLIFE tag. Once the species was genetically determined, a comparison of depth profiles was visualized with time series plots of depth and temperature. To assess the percentage of time spent at different temperature and depth bins, frequency distribution plots were created, with depth data binned in 10 m intervals and temperature data grouped into 1°C ranges (e.g., 5–6°C, 7–8°C). To assess the relationship between depth and time of day, a circular plot was generated.
2.3 Genetic analysis
To confirm the species and test for hybridization using cytonuclear discordance (Toews and Brelsford, 2012; Després, 2019), we sequenced 1200 base pairs (bp) of the mitochondrial control region (CR) using the primers Pro-L and 282H (Keeney et al., 2003), as well as 650 bp of the Internal Transcribed Spacer 2 (ITS2) gene using the primers FISH5.8SF and FISH28SR (Pank et al., 2001). Sequences were visualized and cleaned in Geneious Prime v2019.1.1 (https://www.geneious.com), then aligned using MAFFT v7.388 (Katoh and Standley, 2013). We assigned preliminary IDs using the Basic Local Alignment Search Tool (BLAST) from the National Center for Biotechnology Information (NCBI) and downloaded sequences of confirmed hammerhead species from GenBank (Clark et al., 2016) to use as a reference (accession numbers in S1). Best-fit mutational model for each gene was obtained using jModelTest (Posada, 2008) and used as a prior to infer phylogenies in MrBayes v3.2.6 (Huelsenbeck and Ronquist, 2001), with a chain length of 10,000,000 generations and a subsampling rate of 200. Individuals that clustered with one species at the CR but another species at ITS were considered putative hybrids. We then compared all ITS sequences against reference genotypes for Sphyrna lewini, S. gilberti, and S. zygaena to identify diagnostic nucleotides that differed among species.
3 Results
Among the 42 hammerheads examined, three showed evidence of shared ancestry with a species other than S. lewini, of which two were potential hybrids of S. gilberti and S. lewini. Specifically, individual H1 was assigned to S. lewini based on its mtDNA (Supplementary Figure S1), which is maternally inherited and therefore indicates that the mother was S. lewini. However, its nuclear genotype grouped with S. gilberti (Supplementary Figure S2) and matched with that species at diagnostic nucleotide positions (Supplementary Figure S3), suggesting that the father was S. gilberti and that H1 was a hybrid. The other hybrid we detected (H41) was assigned to S. gilberti at mtDNA, indicating that the mother was H. gilberti, and S. lewini at ITS, indicating that the father was S. lewini, but as this individual was not tagged, we did not include it in our analysis. Individual H2 was assigned to S. gilberti at both mitochondrial and nuclear markers, with no evidence of S. lewini ancestry, confirming it as S. gilberti. All remaining individuals were consistent with S. lewini reference sequences for both loci, with no evidence of shared ancestry with other congeners.
The confirmed S. gilberti (H2) was an immature female (138 cm FL), tagged with a PSATLIFE tag on May 17th, 2024. The PSATLIFE remained on the shark for 33 days and transmitted approximately 113 km from the tagging location (Figure 1). The S. lewini included in this study (tagged on May 18th, 2024) was selected due to its comparable life stage (immature female, 125 cm FL) and similar tag deployment period (25 days). The PSATLIFE transmitted approximately 47 km from the tagging location (Figure 1). The hybrid individual (H1) was an immature male (160 cm FL), also tagged with a PSATLIFE tag on May 17th, 2024. The PSATLIFE remained on the shark for 25 days and transmitted approximately 147 km from the tagging location (Figure 1).
Vertical movement data from the PSAT indicated that the S. gilberti exhibited oscillatory diving behavior primarily in the epipelagic zone (<200 m, Robinson et al., 2010) until the end of May when deeper dives into the mesopelagic zone (>200 m, Robinson et al., 2010) became more frequent, including one dive to a depth of 846 m recorded on June 3rd (Figure 2). Notably, this transition coincided with a marked increase in mean daily surface water temperatures (<10 m) (Figure 2). Ambient temperature recorded from the PSAT revealed that the S. gilberti utilized a temperature range from 5.65 to 27.00°C throughout the deployment period (Figure 2). Comparison of vertical movement data with S. lewini revealed that S. gilberti utilized a greater depth and temperature range than S. lewini analyzed in this study, with the deepest dive from the S. lewini reaching 380 m, with a temperature range that spanned from 9.72 to 26.48°C (Figure 2). Similar to S. gilberti, S. lewini experienced an increase in mean daily surface water temperature at the end of May, however, did not exhibit the deep diving patterns seen in S. gilberti (Figure 2). The hybrid immature male utilized a similar depth range to that of the S. lewini, with a maximum depth of 203 m and a temperature range from 10.73 to 25.72°C (Figure 2). In contrast to the patterns observed in S. gilberti and S. lewini, mean daily surface temperature fluctuations experienced by the hybrid remained relatively muted over the deployment period (Figure 2). Frequency distribution plots of depth demonstrate that the hybrid individual spent the greatest proportion of time in the top 10 m of the water column (55%), compared to S. lewini (32%) and S. gilberti (15%). Frequency distribution plots of temperature indicated that the hybrid most often occurred in 21-22°C waters (27%), S. lewini in 22-23°C (24%), and S. gilberti in 25-26°C (20%) (Figure 3).
Figure 2
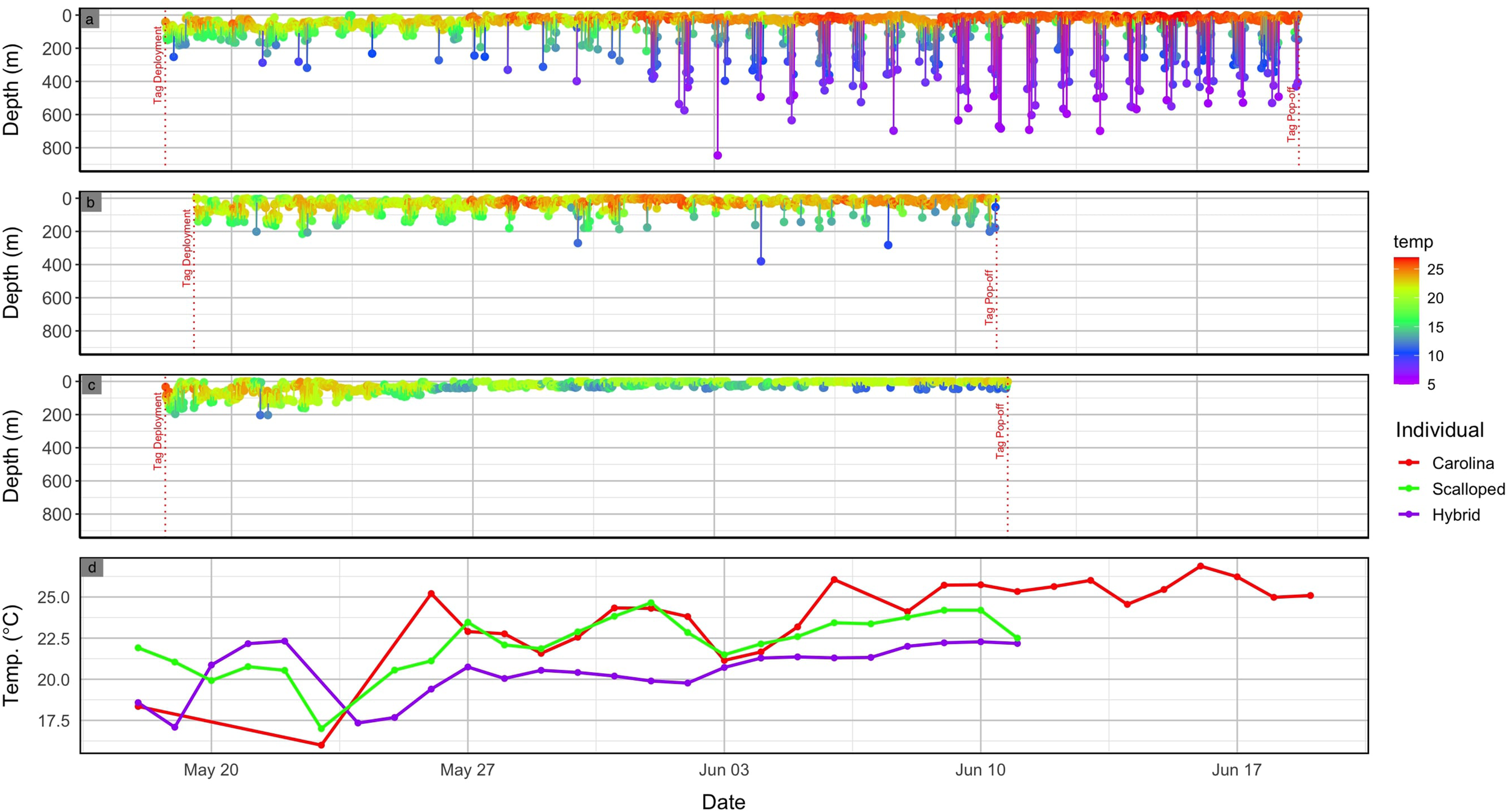
Vertical movement and thermal profiles of the three sharks tagged in this study. Panels (a–c) show depth (m) over time for the (a) Carolina hammerhead (S. gilberti), (b) scalloped hammerhead (S. lewini), and (c) hybrid individual. Ambient temperature (°C) is indicated by color according to the scale. Red dotted vertical lines mark the timing of tag deployment and pop-off. Panel (d) displays the daily average surface temperatures (<10 m) experienced by each individual across the deployment period.
Figure 3
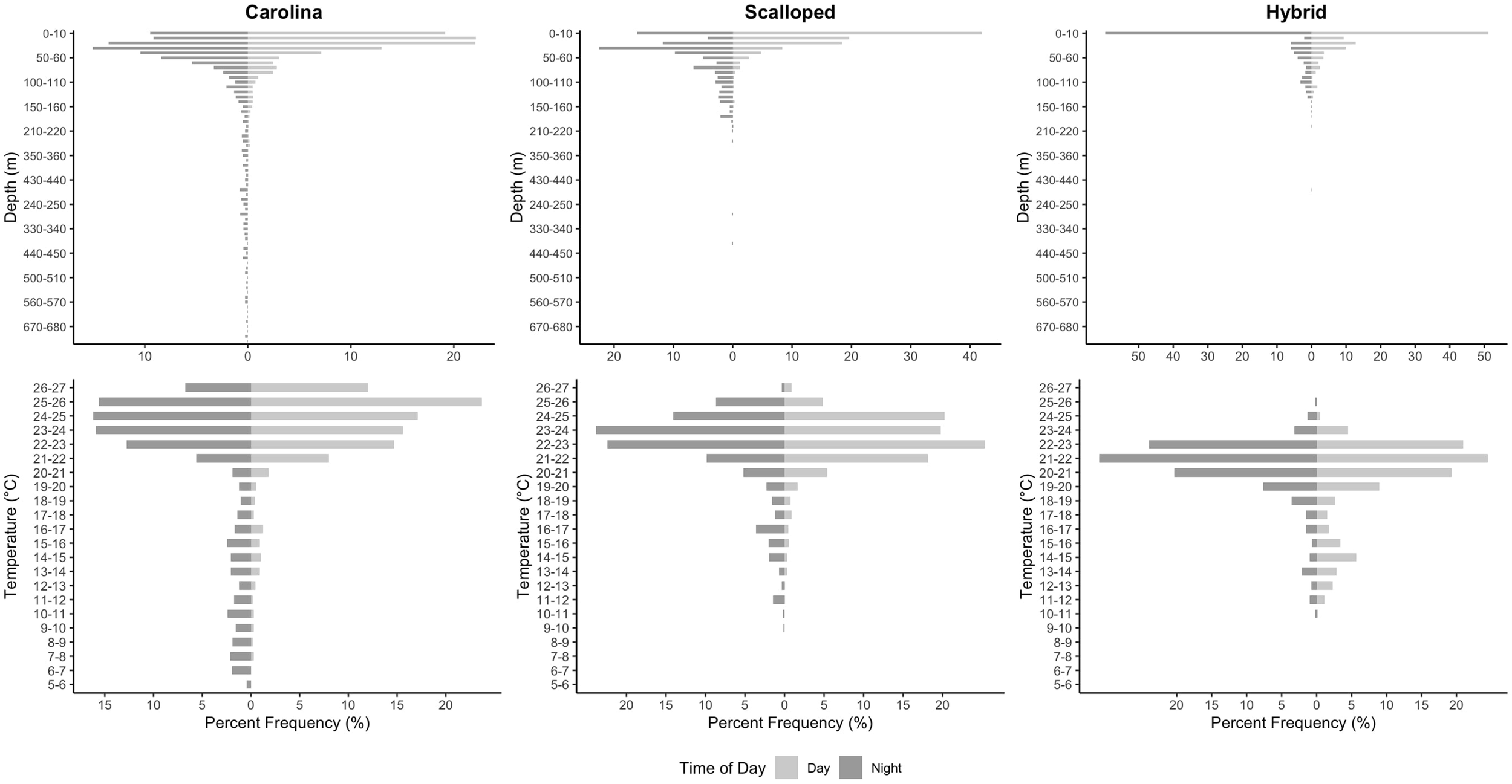
Frequency distribution of time spent by sharks across depth (top row) and temperature (bottom row) bins, separated by species (columns). Each bar shows the percentage of time spent within depth (meters) or temperature (°C) intervals. Light grey shaded bars indicate daytime, while dark grey bars indicate nighttime frequency.
Tag data revealed that the S. gilberti performed 161 dives exceeding 200 m depth, with 60% of these dives occurring during nighttime and 40% occurring during daytime (based on sunset and sunrise at the tagging location). Of these, 56 dives surpassed 400 m, with 59% occurring during nighttime and 41% occurring during daytime. Nine dives exceeded 600 m, with 78% occurring during nighttime and 22% occurring during daytime (Figure 4). A similar assessment with S. lewini revealed that of the seven dives exceeding 200 m depth, 71% took place during nighttime and 29% took place during daytime. The hybrid individual undertook two dives exceeding 200 m, which took place just before and just after sunset.
Figure 4
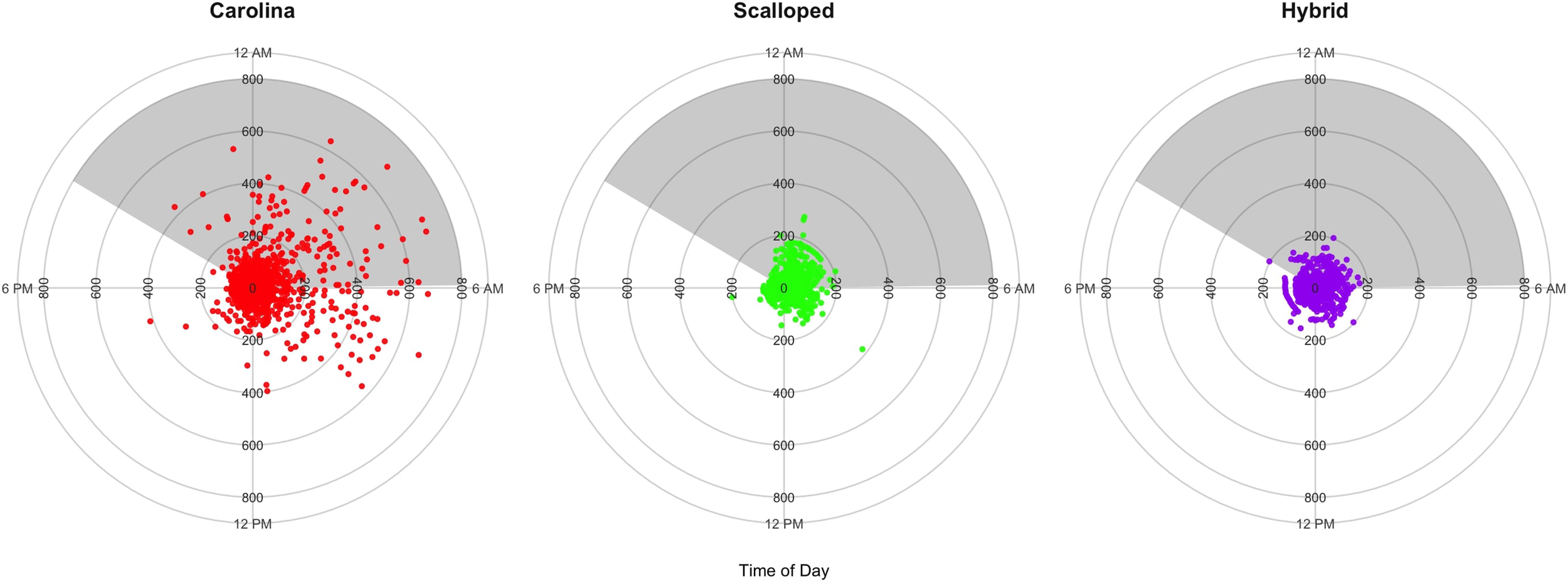
Circular-linear plots showing depth (inner axis) over a 24-hour period (outer axis) for the three hammerhead sharks: S. gilberti (red, n = 2,960 points over 33 days), S. lewini (green, n = 2,044 points over 25 days), and hybrid individual (purple, n = 2,188 points over 25 days). Each point represents a depth position recorded at a specific time of day. The grey shaded regions indicate nighttime hours based on local sunrise and sunset times on March 17, 2024, in Wanchese, North Carolina.
4 Discussion
This study presents the first detailed vertical movement patterns of S. gilberti, revealing a broad vertical niche spanning depths from 0 to 846 m in the Northwest Atlantic over 33 days. This is in contrast to the hybrid hammerhead in this study, which did not exceed a depth of 203 m, as well as all of the S. lewini tagged with a functioning PSATLIFE in this study (n=29), which did not exceed 380 m in depth over a combined 451 day monitoring period, with individual deployments ranging from 4 to 85 days (unpublished data).
The variation in thermal and depth profiles observed in this study may reflect differences among the individuals tagged, however, previous research on S. lewini in other regions has documented patterns similar to those observed here for S. gilberti. Notably, deep diving behavior has been well documented in S. leweni, with the species routinely conducting deep dives to 800 m and beyond (Bessudo et al., 2011; Anderson et al., 2022; Hutchinson et al., 2023; Royer et al., 2023; Spaet et al., 2017; Moore and Gates, 2015; Bezerra et al., 2019; Jorgensen et al., 2009; Hoffmayer et al., 2013), likely to forage in cold deep mesopelagic habitats. This deep diving behavior reflects unusual physiological and behavioral adaptations such as breath-hold thermoregulation and specialized swimming muscle metabolism (Royer et al., 2023, 2024), and further research is needed to determine whether S. gilberti exhibits similar adaptations. The deep diving observed here, along with its association with waters exceeding 25°C, may indicate behavioral thermoregulatory strategies, but additional data are required to confirm this. In contrast, the hybrid individual tagged in this study exhibited shallow diving behavior while remaining in relatively consistent water temperatures. This may suggest that this individual remained in shelf waters throughout the deployment period, which could explain the reduced vertical movement. It is worth considering that the dive patterns observed here may reflect each individual’s location around the continental shelf, as all three sharks were tagged along its edge. Although all tag pop-off locations were on the shelf, this may not reflect true habitat use, as tags likely drifted westward prior to transmission. S. gilberti’s deep diving behavior indicates its occupancy of waters beyond the shelf edge, and when considered alongside temperature data may indicate its entry into the Gulf Stream. Further, although S. gilberti occupied the warmest temperatures among all three species, it’s use of the top 10 m of the water column is less pronounced, indicating that it may have been utilizing habitats associated with the Gulf Stream, where warm waters persist at depth. The S. leweni temperature and depth profile also suggest that it made dives off the continental shelf, and evidence of behavioral thermoregulatory strategies were also observed, with the deepest dives preceded by periods of warmer surface waters, although these dives were generally shallower, and time spent in warmer waters was less pronounced compared to S. gilberti.
Although the behavior observed here by S. gilberti are consistent with previously described behaviors by S. leweni, it’s worth considering the reasons for the absence of deep diving behavior by S. leweni in the present study. The discrepancy may be a result of relatively short tag deployment periods, as vertical movement ranges are known to vary seasonally (Bessudo et al., 2011) and it is possible that the full scope of behavior was not observed, or by the life history stage of the tagged individuals, since mature S. lewini show ontogenetic increases in vertical habitat use (Klimley, 1987). Future research should explore whether similar ontogenetic shifts occur in S. gilberti, as well as how thermoregulation strategies vary between the closely related species. This represents a key research priority which could help to inform bycatch mitigation strategies, particularly for the rare S. gilberti, if species show consistent avoidance or preference for specific thermal habitats.
The data presented here exemplify the value of genetic analysis, especially considering the increased awareness of cryptic species and the need for techniques to detect them (Davis et al., 2019; Barker et al., 2021). Previous studies suggested that hybridization in hammerheads is biased towards female S. gilberti-male S. lewini crosses (Barker et al., 2019), as indicated by mtDNA results, but the fact that we detected two hybrids in this study and only one fits this pattern contradicts this hypothesis. Instead, it may be that sex biases in hybridization are a result of varying species compositions and sex ratios at the time of mating.
While additional tagging of individuals is required to determine whether the behavior observed here is typical for S. gilberti, our observations highlight the need for further study to evaluate potential similarities and differences in vertical habitat use among closely related hammerhead species. Such efforts will be critical for informing species-specific conservation strategies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Oregon State University Institutional Animal Care and Use Committee (IACUC), Corvallis, Oregon, USA. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LH: Writing – review & editing, Data curation, Writing – original draft, Formal Analysis, Methodology. KB: Writing – review & editing, Data curation. TD-E: Formal Analysis, Writing – review & editing. KS: Formal Analysis, Writing – review & editing. EH: Funding acquisition, Writing – review & editing. NH: Methodology, Writing – review & editing, Funding acquisition. BF: Writing – review & editing. BA: Writing – review & editing, Funding acquisition. BC: Funding acquisition, Writing – review & editing. JS: Writing – review & editing, Conceptualization, Funding acquisition, Supervision, Methodology, Resources, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by NOAA’s Bycatch Reduction Engineering Program (BREP).
Acknowledgments
The authors extend their utmost appreciation to Captain Bill and first mate Jimbo.
Conflict of interest
Author NH was employed by the company Shark Research Foundation Inc. Author BA was employed by the company Saltwater Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1688202/full#supplementary-material
References
1
Anderson J. M. Rex P. T. Maloney K. Johnston M. Verbeck D. Allen N. et al . (2022). Observations of a species-record deep dive by a central Pacific female scalloped hammerhead shark (Sphyrna lewini). J. Fish Biol.101, 323–327. doi: 10.1111/jfb.15115
2
Barker A. M. Adams D. H. Driggers W. B. III Frazier B. S. Portnoy D. S. (2019). Hybridization between sympatric hammerhead sharks in the western North Atlantic Ocean. Biol. Lett.15, 20190004. doi: 10.1098/rsbl.2019.0004
3
Barker A. M. Frazier B. S. Adams D. H. Bedore C. N. Belcher C. N. Driggers III W. B. et al . (2021). Distribution and relative abundance of scalloped (Sphyrna lewini) and Carolina (S. gilberti) hammerheads in the western North Atlantic Ocean. Fisheries Res. 242, 106039.
4
Bessudo S. Soler G. A. Klimley P. A. Ketchum J. Arauz R. Hearn A. et al . (2011). Vertical and horizontal movements of the scalloped hammerhead shark (Sphyrna lewini) around Malpelo and Cocos Islands (Tropical Eastern Pacific) using satellite telemetry. Boletín de Investigaciones Marinas y Costeras-INVEMAR, 40, 91–106.
5
Bezerra N. P. Macena B. C. Travassos P. Afonso P. Hazin F. H . (2019). Evidence of site fidelity and deep diving behaviour of scalloped hammerhead shark (Sphyrna lewini) around the Saint Peter and Saint Paul Archipelago, in the equatorial Mid-Atlantic ridge. Mar. Freshw. Res.71 (6), 708–718.
6
Braccini M. Aires-da-Silva A. Taylor I. (2016). Incorporating movement in the modelling of shark and ray population dynamics: approaches and management implications. Rev. Fish Biol. Fisheries26, 13–24. doi: 10.1007/s11160-015-9406-x
7
Carlson J. K. Frazier B. S. Grubbs R. D. Herman K. A. T. Lyons K. A. D. Portnoy D. S. et al . (2024). Report of the workshop on the status of the Carolina hammerhead, Sphyrna gilberti. NOAA Technical Memorandum NMFS-SEFSC-778, (Atlanta, GA: NOAA), 16.
8
Clark K. Karsch-Mizrachi I. Lipman D. J. Ostell J. Sayers E. W. (2016). GenBank. Nucleic Acids Res.44, D67–D72. doi: 10.1093/nar/gkv1276
9
Davis M. M. Suárez-Moo P. D. J. Daly-Engel T. S. (2019). Genetic structure and congeneric range overlap among sharpnose sharks (genus Rhizoprionodon) in the Northwest Atlantic Ocean. Can. J. Fisheries Aquat. Sci.76, 1203–1211. doi: 10.1139/cjfas-2018-0019
10
Després L. (2019). One, two or more species? Mitonuclear discordance and species delimitation. Mol. Ecol.28, 3845–3847. doi: 10.1111/mec.15211
11
Dwyer R. G. Campbell H. A. Pillans R. D. Watts M. E. Lyon B. J. Guru S. M. et al . (2019). Using individual-based movement information to identify spatial conservation priorities for mobile species. Conserv. Biol.33, 1426–1437. doi: 10.1111/cobi.13328
12
Elliott R. G. Montgomery J. C. Della Penna A. Radford C. A. (2022). Satellite tags describe movement and diving behaviour of blue sharks Prionace glauca in the southwest Pacific. Mar. Ecol. Prog. Ser.689, 77–94. doi: 10.3354/meps14037
13
Gallagher A. J. Klimley A. P. (2018). The biology and conservation status of the large hammerhead shark complex: the great, scalloped, and smooth hammerheads. Rev. Fish Biol. Fisheries28, 777–794. doi: 10.1007/s11160-018-9530-5
14
Galloway A. S. Lyons K. Portnoy D. S. Barker A. M. Adams D. H. Gelsleichter J. et al . (2024). Trophic ecology of Carolina Sphyrna gilberti and scalloped S. lewini hammerheads in the southeastern USA. Mar. Ecol. Prog. Ser.743, 25–46. doi: 10.3354/meps14644
15
Hammerschlag N. Gallagher A. J. Lazarre D. M. (2011). A review of shark satellite tagging studies. J. Exp. Mar. Biol. Ecol.398, 1–8. doi: 10.1016/j.jembe.2010.12.012
16
Hoffmayer E. R. Franks J. S. Driggers W. B. Howey P. W. (2013). Diel vertical movements of a scalloped hammerhead, Sphyrna lewini, in the northern Gulf of Mexico. Bull. Mar. Sci.89, 551–557. doi: 10.5343/bms.2012.1048
17
Huelsenbeck J. P. Ronquist F . (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics17 (8), 754–755.
18
Hutchinson M. Scott M. Bauer R. Anderson J. Coffey D. Holland K. et al . (2023). Habitat use and movement patterns of adult male and juvenile scalloped hammerhead sharks, Sphyrna lewini, throughout the Hawaiian archipelago. Endanger Species Res.52, 41–64. doi: 10.3354/esr01267
19
Jorgensen S. J. Klimley a. P. Muhlia-Melo a. F. (2009). Scalloped hammerhead shark Sphyrna lewini, utilizes deep-water, hypoxic zone in the gulf of California. J. Fish Biol.74, 1682–1687. doi: 10.1111/j.1095-8649.2009.02230.x
20
Katoh K. Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol.30, 772–780. doi: 10.1093/molbev/mst010
21
Keeney D. B. Heupel M. Hueter R. E. Heist E. J. (2003). Genetic heterogeneity among blacktip shark, Carcharhinus limbatus, continental nurseries along the US Atlantic and Gulf of Mexico. Mar. Biol.143, 1039–1046. doi: 10.1007/s00227-003-1166-9
22
Klimley A. P. (1987). The determinants of sexual segregation in the scalloped hammerhead shark,Sphyrna lewini. Environ. Biol. Fish18, 27–40. doi: 10.1007/BF00002325
23
Madigan D. J. Devine B. M. Weber S. B. Young A. L. Hussey N. E. (2022). Combining telemetry and fisheries data to quantify species overlap and evaluate bycatch mitigation strategies in an emergent Canadian Arctic fishery. Mar. Ecol. Prog. Ser.702, 1–17. doi: 10.3354/meps14212
24
Moore A. B. M. Gates A. R. (2015). Deep-water observation of scalloped hammerhead Sphyrna lewini in the western Indian Ocean off Tanzania. Mar. Biodivers Rec8, 1–4. doi: 10.1017/S1755267215000627
25
Morales‐Serrano N. Gonzalez‐Pestana A . (2024). Identification of the first nursery area of the Galápagos shark (Carcharhinus galapagensis) in the south‐east Pacific Ocean. J. Fish Biol.105 (3), 1008–1013.
26
Pacoureau N. Rigby C. L. Kyne P. M. Sherley R. B. Winker H. Carlson J. K. et al . (2021). Half a century of global decline in oceanic sharks and rays. Nature589, 567–571. doi: 10.1038/s41586-020-03173-9
27
Pank M. Stanhope M. Natanson L. Kohler N. Shivji M. (2001). Rapid and simultaneous identification of body parts from the morphologically similar sharks Carcharhinus obscurus and Carcharhinus plumbeus (Carcharhinidae) using multiplex PCR. Mar. Biotechnol.3, 231–240. doi: 10.1007/s101260000071
28
Pfleger M. O. Grubbs R. D. Cotton C. F. Daly-Engel T. S. (2018). Squalus clarkae sp. nov., a new dogfish shark from the Northwest Atlantic and Gulf of Mexico, with comments on the Squalus mitsukurii species complex. Zootaxa4444, 101–119. doi: 10.11646/zootaxa.4444.2.1
29
Pinhal D. Shivji M. S. Vallinoto M. Chapman D. D. Gadig O. B. F. Martins C. (2012). Cryptic hammerhead shark lineage occurrence in the western South Atlantic revealed by DNA analysis. Mar. Biol.159, 829–836. doi: 10.1007/s00227-011-1858-5
30
Posada D. (2008). jModelTest: phylogenetic model averaging. Mol. Biol. Evol.25, 1253–1256. doi: 10.1093/molbev/msn083
31
Poulakis G. R. Urakawa H. Stevens P. W. DeAngelo J. A. Timmers A. A. Grubbs R. D. et al . (2017). Sympatric elasmobranchs and fecal samples provide insight into the trophic ecology of the smalltooth sawfish. Endanger. Species Res.32, 491–506.
32
Quattro J. M. Driggers W. B. Grady J. M. Ulrich G. F. Roberts M. A. (2013). Sphyrna gilberti sp. nov., a new hammerhead shark (Carcharhiniformes, Sphyrnidae) from the western Atlantic Ocean. Zootaxa3702, 159–178. doi: 10.11646/zootaxa.3702.2.5
33
Renshaw S. Hammerschlag N. Gallagher A. J. Lubitz N. Sims D. W. (2023). Global tracking of shark movements, behaviour and ecology: a review of the renaissance years of satellite tagging studies 2010–2020. J. Exp. Mar. Biol. Ecol.560, 151841. doi: 10.1016/j.jembe.2022.151841
34
Robinson C. Steinberg D. K. Anderson T. R. Arístegui J. Carlson C. A. Frost J. R. et al . (2010). Mesopelagic zone ecology and biogeochemistry–a synthesis. Deep-Sea Res. II: Top. Stud. Oceanogr.57 (16), 1504–1518.
35
Royer M. Garcia D. Dickson K. Weng K. C. Meyer C. Holland K. N. et al . (2024). Aerobic and anaerobic poise of white swimming muscles of the deep-diving scalloped hammerhead shark: comparison to sympatric coastal and deep-water species. Front. Mar. Sci.11. doi: 10.3389/fmars.2024.1477553
36
Royer M. Meyer C. Royer J. Maloney K. Cardona E. Blandino C. et al . (2023). Breath holding” as a thermoregulation strategy in the deep-diving scalloped hammerhead shark. Science380, 651–655. doi: 10.1126/science.add4445
37
Salinas‐de‐León P. Vaudo J. Logan R. Suarez‐Moncada and Shivji J. M . (2024). Longest recorded migration of a silky shark (Carcharhinus falciformis) reveals extensive use of international waters of the Tropical Eastern Pacific. J. Fish Biol.105 (1), 378–381.
38
Spaet J. L. Lam C. H. Braun C. D. Berumen M. L . (2017). Extensive use of mesopelagic waters by a Scalloped hammerhead shark (Sphyrna lewini) in the Red Sea. Anim. Biotelemetry5 (1), 20.
39
Todesco M. Pascual M. A. Owens G. L. Ostevik K. L. Moyers B. T. Hübner S. et al . (2016). Hybridization and extinction. Evolutionary Appl.9, 892–908. doi: 10.1111/eva.12367
40
Toews D. P. Brelsford A. (2012). The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol.21, 3907–3930. doi: 10.1111/j.1365-294X.2012.05664.x
41
Wickham H . (2016). “Data analysis” in ggplot2: elegant graphics for data analysis. (Cham: Springer international publishing), 189–201.
42
Worm B. Davis B. Kettemer L. Ward-Paige C. A. Chapman D. Heithaus M. R. et al . (2013). Global catches, exploitation rates, and rebuilding options for sharks. Mar. Policy40, 194–204. doi: 10.1016/j.marpol.2012.12.034
43
Worm B. Orofino S. Burns E. S. D’Costa N. G. Manir Feitosa L. Palomares M. L. et al . (2024). Global shark fishing mortality still rising despite widespread regulatory change. Science383, 225–230. doi: 10.1126/science.adf8984
Summary
Keywords
fisheries management, vertical movement, cryptic species, hammerhead, dive patterns
Citation
Horstmyer L, Ballard K, Daly-Engel T, Stein K, Hoffmayer E, Hammerschlag N, Frazier BS, Anderson B, Campbell B and Sulikowski J (2025) First insights into the fine-scale vertical movements of a Carolina hammerhead, Sphyrna gilberti, and a hybrid between Carolina and scalloped hammerhead. Front. Mar. Sci. 12:1688202. doi: 10.3389/fmars.2025.1688202
Received
18 August 2025
Accepted
10 October 2025
Published
24 October 2025
Volume
12 - 2025
Edited by
David M. P. Jacoby, Lancaster University, United Kingdom
Reviewed by
Yannis Peter Papastamatiou, Florida International University, United States; Mark Andrew Royer, University of Hawaii at Manoa, United States
Updates
Copyright
© 2025 Horstmyer, Ballard, Daly-Engel, Stein, Hoffmayer, Hammerschlag, Frazier, Anderson, Campbell and Sulikowski.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren Horstmyer, lhorstmyer23@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.