Abstract
Coastal ecosystems of sub-Antarctic islands are threatened by increasing climate-driven changes and direct anthropogenic pressures. Significant effects on marine communities are expected, but benthic ecosystems of these isolated islands remain largely under-explored. Effective preservation of these nearshore environments requires deeper ecological assessments and comprehensive biodiversity knowledge. In this regard, this study reports findings from a survey carried out in 2021 at two sites – Baie du Marin and Crique du Sphinx – located on the eastern coast of Ile de la Possession (sub-Antarctic Crozet archipelago, Southern Ocean). We investigated the composition and structure of nearshore benthic faunal communities using a quantitative fieldwork protocol and an integrative molecular- and morphology-based taxonomic approach. A total of 124 morphotypes were identified, including a high proportion (72%) of rare species. Both sites exhibited similar benthic invertebrate communities. Structurally complex habitats such as hard substrates or areas dominated by macroalgae exhibited higher species richness and diversity. The investigated benthic invertebrate communities are typical of the sub-Antarctic area but featured unique structures, including dense tube-dwelling polychaete colonies. This study will provide a baseline for future monitoring programs and for the preservation of sub-Antarctic coastal benthic ecosystems.
1 Introduction
Marine ecosystems of the Southern Ocean are increasingly affected by climate-driven changes and direct anthropogenic pressures (Mélice et al., 2003; Ansorge et al., 2009; Doney et al., 2012; Constable et al., 2014; Gutt et al., 2015; Morley et al., 2020; Auger et al., 2021; Cavanagh et al., 2021). Coastal benthic communities of Antarctic and sub-Antarctic regions are particularly sensitive to environmental disturbances (Robinson et al., 2022; Lelièvre et al., 2023). Global change may lead to substantial alterations in community composition and structure, including biodiversity loss and shifts driven by both habitat extinction and expansion in response to changing local abiotic conditions (Sahade et al., 2015; Pineda-Metz et al., 2019; Deregibus et al., 2023), with cascading effects on ecosystem functioning (Hooper et al., 2005; Cardinale et al., 2012). Despite their high ecological and conservation value (Chown et al., 2001), sub-Antarctic benthic habitats remain largely under-explored. A comprehensive taxonomic investigation of these ecosystems is thus needed to assess and monitor future impacts induced by natural and anthropogenic stressors, and preserve these isolated environments (Xavier et al., 2016; Cresswell et al., 2023).
Landscape heterogeneity and habitat structural complexity are key drivers that spatially structure species occurrence and distribution patterns (Miller and Etter, 2011; Witte et al., 2025). Structurally complex habitats generally offer a mosaic of microniches shaped by multiple environmental factors (e.g., physical and chemical conditions, water currents, substrate type and orientation, depth), promoting the coexistence of species and thereby, higher species diversity and abundances than in homogeneous habitats (Hewitt et al., 2008; Henseler et al., 2019). Habitat-forming species (e.g., faunal and macroalgal biogenic structures) further enhance this complexity by changing local abiotic conditions, expanding the habitat surface availability, providing refuges from predation, and enhancing resource supply, collectively supporting the development of benthic communities (Jones et al., 1994; Graham et al., 2007; Rabaut et al., 2007; Van Hoey et al., 2008; Miller et al., 2018). Through the interplay between abiotic factors and biotic interactions (e.g., competition, predation, facilitation), sub-Antarctic coastal marine ecosystems harbor a rich and diverse benthic flora and fauna (Arnaud, 1974; Branch et al., 1993; Barnes et al., 2006; Freeman et al., 2011; Clark et al., 2019; Lelièvre et al., 2023). This habitat-driven diversity highlights the importance of assessing and monitoring biodiversity across multiple habitat types, providing essential knowledge for the effective conservation and long-term protection of subpolar ecosystems.
To preserve the biodiversity of these remote ecosystems, the French Southern Territories National Nature Reserve (RNN) was created in 2006, including the entire terrestrial surface of the French Southern Territories (Crozet, Kerguelen and Saint Paul and Amsterdam Islands) and 52.5% of their territorial waters (approximately 15,700 km2). The RNN was extended in 2016 (reaching an area of 672,969 km2) in response to the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR). In 2022, it was extended to nearly 1 million km2 during the One Ocean Summit thanks to the key measures of the French National Strategy for Protected Areas 2030. With 1.6 million km2, the reserve is the largest marine protected area in France and the second largest in the world. It was inscribed on the UNESCO World Heritage List in 2019 for the preservation of unique habitats of high conservation value. However, despite the establishment of large-scale protection measures, benthic ecological studies remain particularly limited in this region (Féral et al., 2021; Lelièvre et al., 2023, 2024a, 2025a; Jossart et al., 2024). This lack of data is particularly concerning because effective conservation relies on a detailed knowledge of biodiversity (Lelièvre et al., 2025b). Without comprehensive inventories, it remains difficult to assess ecosystem condition and to determine how effectively marine protected areas and conservation measures mitigate present and future threats (Lelièvre et al., 2025b).
In the Crozet islands, first marine benthic studies were conducted during early oceanographic expeditions of the 19th (HMS Challenger, 1872) and 20th centuries (MD08/BENTHOS 1976 and MD30/BIOMASS 1982) (Arnaud, 1982). Most recently, the exploration of these benthic ecosystems was undertaken in 2021, on the occasion of a submarine cable inspection and environmental impact assessment at the International Monitoring System Hydroacoustic Station HA04 set up at Ile de la Possession (Crozet). This survey was conducted by the French Southern and Antarctic Territories (TAAF) in response to the solicitation of the Comprehensive Nuclear-Test-Ban Treaty Organization (CTBTO). The campaign aimed to assess environmental impacts and identify potential ecological risks (e.g., habitat disturbance, biological invasions) related to the station cable, enhancing our understanding of Île de la Possession nearshore marine ecosystems. Benthic habitats and diversity were characterized by video-imagery and biological sampling at two sites located on the eastern coast of Ile de la Possession, at Baie du Marin and Crique du Sphinx. Based on video-imagery data, an initial taxonomic and functional assessment of the shallow-water marine fauna and flora was performed by Lelièvre et al. (2023, 2024, 2025), highlighting the need for studying biological samples to improve our knowledge of the composition and structure of benthic communities. In this context, Jossart et al. (2024) investigated the diversity and biogeographic affinities of the macrofauna from Ile de la Possession using an integrative morphological and genetic approach from specimens collected opportunistically. Although these previous studies provided valuable information on Crozet marine communities, video imagery and opportunistic sampling provide only a partial view of the benthic fauna. Complementing these former studies, the present study aims (i) to describe and compare the composition and the diversity of shallow faunal benthic communities at Baie du Marin and Crique du Sphinx, based on an in situ quadrat approach and an integrative morphology- and molecular-based identification of the collected organisms; and (ii) to investigate the role of substrate composition on diversity patterns. This study provides the first quantitative assessment of the structure and composition of Crozet benthic communities, offering a critical baseline for future ecological monitoring and conservation planning in these little studied ecosystems.
2 Materials and methods
2.1 Study areas
The Crozet Islands (45°48’S – 46°26’S; 50°14’E – 52°15’E) are located in the south of the Indian Ocean, at 2,400 km from both Antarctica and South Africa. The archipelago comprises five volcanic islands scattered over 80 km from west to east: Ile aux Cochons, Ile des Pingouins, Ilots des Apôtres, Ile de la Possession and Ile de l’Est. Ile de la Possession (46°25’S; 51°45’E; Figure 1a) is the largest island with a surface area of 156 km2 (approximately 18 km in width and 15 km in length). The present study was conducted at two sites located on the eastern coast of Ile de la Possession, at Baie du Marin and Crique du Sphinx (Figure 1b).
Figure 1

(a) Topographic map of Ile de la Possession, diamonds indicating the location of Baie du Marin and Crique du Sphinx; (b) Faunal sampling (quadrat sampling design) conducted during the HA04-Crozet-2021 campaign; Photographs of (c)Baie du Marin (photo courtesy of L. Hateau); (d)Crique du Sphinx (photo courtesy of T. Saucède).
The Baie du Marin (46°25’54”S; 51°52’11”E; Figure 1c) is a narrow inlet, 500 m long and 200 m wide in its shallowest part (< 20 m depth), that opens to the ocean in a larger bay of about 2 km wide. The coastline is mainly a rocky shore, and the seabed is dominated by coarse sand sediments (Lelièvre et al., 2024b). Two kilometres to the north, Crique du Sphinx (46°25’08”S; 51°52’44”E; Figure 1d) is a small cove of 250 m long and 150 m wide that opens to the northeast and is mainly bordered by a rocky shore with a small beach of pebbles and coarse sand. The seabed is mainly a rocky bottom with some patches of coarse sand (Lelièvre et al., 2024b).
2.3 Field sampling and laboratory processing
Fieldwork was carried out during campaign HA04-Crozet-2021 and OP03–2021 operations implemented at Ile de la Possession by the French Southern and Antarctic Territories (TAAF), conducted aboard the R/V Marion Dufresne II between 4 and 9 November 2021. Sampling was performed at each site (Baie du Marin and Crique du Sphinx) in SCUBA diving following a depth gradient between 6 and 20 m depth, along a transect positioned in the main axis of each bay. Invertebrates were collected using 25 × 25 cm quadrats (representing a sampling surface area of 625 cm2 per quadrat) regularly positioned on the sea bottom (Figure 1b). The community within each quadrat was first photographed in situ, after which all organisms were collected by hand or scrubbed from hard substrates. A total of eighteen quadrats were sampled along each transect at each site (Supplementary Table S1; Figures 1B, 2). For every quadrat, the substrate type was classified as: sand, sand and pebbles, sand and algae, rock, and rock and algae (Figure 2). Biological sampling was conducted in accordance with regulation rules of the TAAF under permit A-2021-98. Once onboard the R/V Marion Dufresne II, all samples were preserved in 96% ethanol. In the laboratory, each sample was then sieved through a 1 mm mesh to retrieve the macrofauna (defined as organisms >1 mm). All individuals were sorted, assigned to a morphotype, and counted. One or two specimens of each morphotype were then isolated for further morphological and genetic analyses.
Figure 2
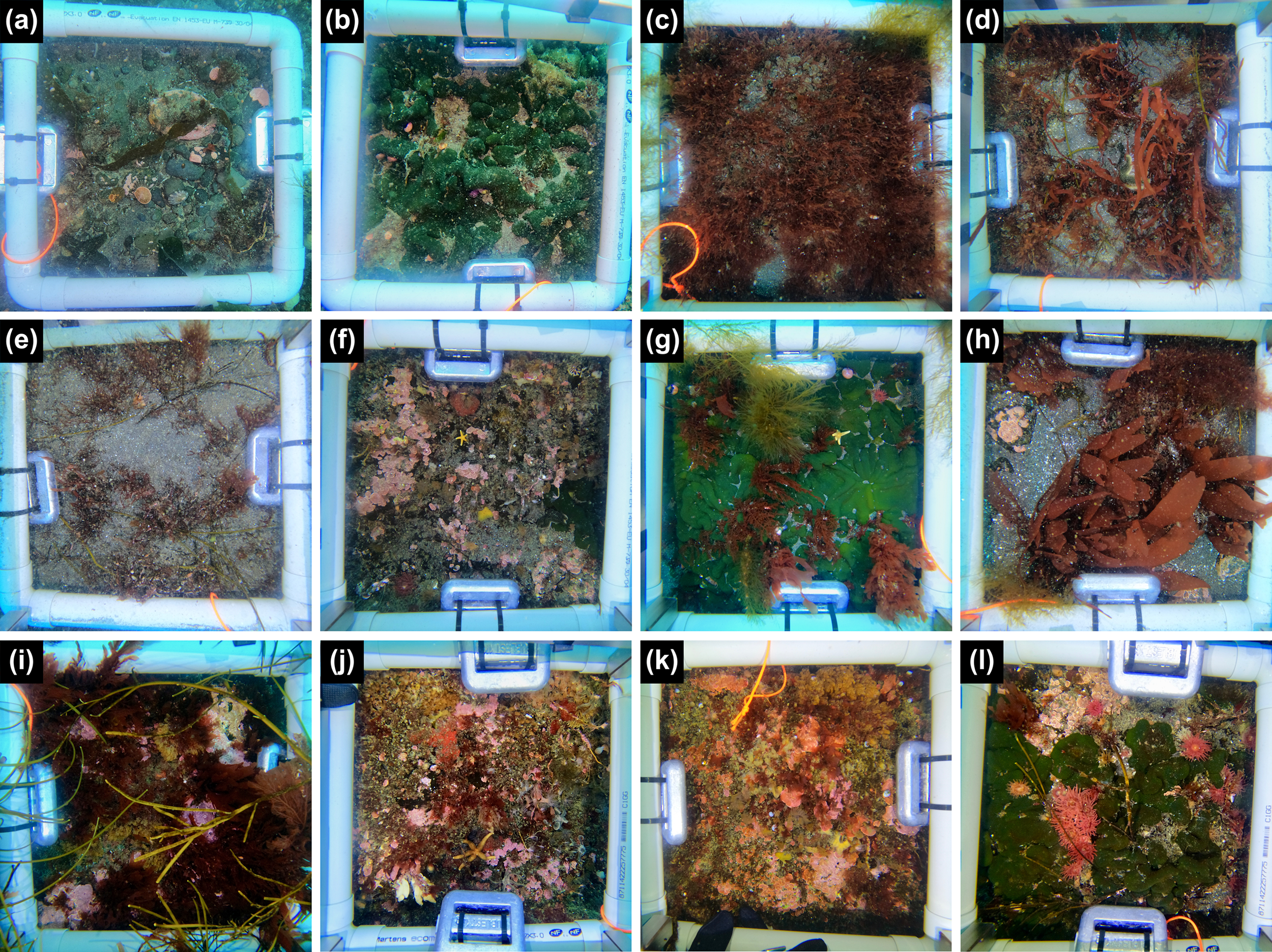
Example of quadrat sampling at Baie du Marin and Crique du Sphinx, with (a) Q11 (sand and pebble substrate); (b) Q13 (rocky substrate dominated by Codium adhaerens); (c) Q20 (sandy substrate dominated by undetermined algae); (d) Q22 (sandy substrate dominated by undetermined algae); (e) Q23 (sandy substrate dominated by undetermined algae); (f) Q27 (rocky substrate); (g) Q28 (rocky substrate dominated by Codium adhaerens); (h) Q30 (sand and pebble substrate); (i) Q32 (rocky substrate dominated by undetermined algae); (j) Q34 (rocky substrate); (k) Q35 (rocky substrate); and (l) Q36 (rocky substrate dominated by Codium adhaerens).
2.4 Species identification: DNA barcoding and morphology
An integrative approach, combining morphological and molecular taxonomy, was used in order to maximize the quality of species identifications (Gostel and Kress, 2022; Jossart et al., 2023). For each isolated specimen, DNA extractions were performed from a small piece of tissue or from the entire body depending on specimen size using a salting-out protocol (Sunnucks and Hales, 1996). The barcode region of the cytochrome c oxidase subunit I (COI; 658 base pairs) was then amplified, using either universal primers or taxon-specific primers: F-polyHCO+R-polyLCO for annelids, FLCOech1aF1+R-HCO2198 for echinoderms, F-LCO1490+R-HCO2198 and F-LCO1490+R-HCO2198 and F-COI-mol+R-COI-mol for molluscs (Folmer et al., 1994; Carr et al., 2011; Layton et al., 2016). More details and protocols can be found in Jossart et al. (2024). Purification and Sanger sequencing were carried out by GENEWIZ® laboratories from Azenta Life Science (Leipzig, Germany). Sequence editing was conducted using Geneious Prime 2023.2.1 (Kearse et al., 2012), and barcodes were compared with each other and with sequences available in GenBank and Barcode of Life (BOLD) databases (Ratnasingham and Hebert, 2007). The barcoded specimens were also sent to taxonomists for morphological identification to the lowest taxonomic level possible. These morphotypes were then compared with those defined by Jossart et al. (2024) from former samples in the same area. The barcodes directly contributed to the identification process (see Results). Combined with the barcodes of Jossart et al., 2024, a total of 234 barcodes from 91 morphotypes were obtained. These barcodes and macro-pictures of morphotypes were deposited in the Barcode of Life Data Systems (BOLD) under the project “HAOIV” (Shallow benthic communities of Crozet archipelago, boldsystems.org) and made publicly available.
2.4 Statistical analyses
Individual-based rarefaction curves were computed to assess the sampling effort at Baie du Marin, Crique du Sphinx, and across both sites combined. A Principal Coordinates Analysis (PCoA) was performed on Hellinger-transformed abundance data using Euclidean distances to investigate variations in faunal composition among sites and quadrats. Abundance data were Hellinger-transformed to avoid giving an excessive weight to rare species (Legendre and Gallagher, 2001). Univariate Hill’s diversity indices were calculated for each quadrat and each site, including species richness (R; q=0), Shannon diversity (N; q=1) and Simpson’s inverse (1-λ; q=2) (Jost, 2006). For each diversity metric, non-parametric Kruskal-Wallis followed by Dunn’s post-hoc pairwise tests were performed to test for significant differences among the different substrate types, with p-values adjusted using a Bonferroni correction. Commonness and rarity were categorized from both species abundance and occurrence. Morphotypes were defined as: rare (average number of individuals per quadrat ≤1 and occurring in ≤9 quadrats out of a total of 36 (i.e., ≤25% of quadrats); common (average number of individuals per quadrat ≥2 and occurring in ≥27 quadrats (i.e., ≥75% of quadrats); or moderate (everything else) (Hewitt et al., 2016). All statistical analyses were performed using R software (version 4.2.0, R Core Team 2022). Rarefaction curves were conducted using the package iNEXT (Hsieh et al., 2016). Diversity indices and PCoA were conducted using the vegan package (Oksanen et al., 2013).
3 Results
3.1 Overall benthic invertebrate diversity
A total of 124 benthic invertebrate morphotypes were identified from the 11,189 individuals collected in the 36 sampling quadrats at Baie du Marin and Crique du Sphinx. Overall, the rarefaction curves tend to reach a plateau (Figure 3), indicating that the sampling effort was sufficient to provide a reliable representation of faunal diversity at both studied sites. Genetically, 62 barcodes from 35 morphotypes were obtained. Combined with Jossart et al. (2024) barcodes from specimens of the same areas, a total of 66 morphotypes were successfully barcoded. Therefore, 46.8% of morphotypes were characterized and identified only on a morphological basis while 53.2% were investigated both morphologically and genetically (Table 1). Of the 124 morphotypes, 64 were identified to species level, 39 to genus level, and the remaining morphotypes to family or higher taxonomic level (Table 1; Supplementary Table S2). Among these morphotypes, 59 were shared by the two sites, 28 were exclusive to Baie du Marin and 37 to Crique du Sphinx. Benthic diversity was characterized by: Annelida with 21 Polychaeta from 14 families; Arthropoda encompassing Malacostraca with 21 Amphipoda divided into 16 families, 12 Isopoda into eight families, six Tanaidacea dispatched across five families, and 11 Pycnogonida from five families; Brachiopoda with one Rhynchonellata; Chordata with two Ascidiacea from two families; Cnidaria, including four Anthozoa from the Actiniaria order, and one Hydrozoa of the Tubulariidae family; Echinodermata with six Asteroidea divided into three families, five Holothuroidea into two families, three Ophiuroidea from three families, and one Echinoidea; Mollusca, comprising 18 Gastropoda within 15 families, six Bivalvia from four families as well as three Polyplacophora into three families; one Nemertea from the Monostilifera order; as well as two Platyhelminthes from the Rhabditophora subphylum (Table 1; Supplementary Table S2). Overall, quadrat sampling at both sites exhibited a high diversity (Figures 4a, b), with polychaetes, amphipods, isopods, tanaids, bivalves, and gastropods being numerically dominant in terms of abundance (Figures 4c, d).
Figure 3
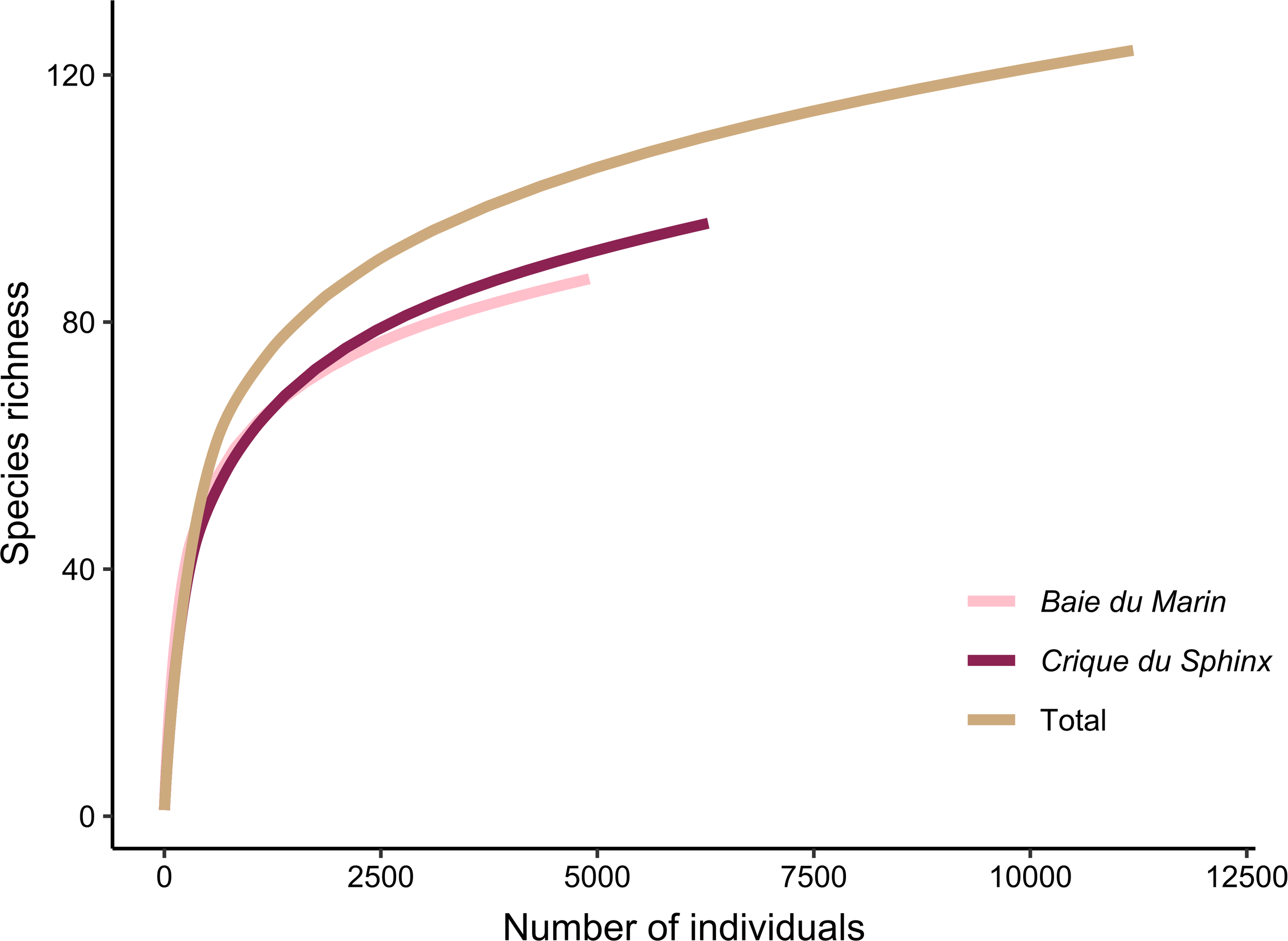
Individual-based rarefaction curves of faunal communities at Baie du Marin and Crique du Sphinx. The brown line (Total) represents the rarefaction curve for both sites combined.
Table 1
| Taxa | BDM | CdS | TA (%) | D | Nquadrat | BOLD |
|---|---|---|---|---|---|---|
| Annelida | ||||||
| Polychaeta | ||||||
| Capitellidae | ||||||
| Mastobranchus sp. Eisig, 1887* | • | • | 18 (0.2) | 8 ± 31.7 | 3 | - |
| Scyphoproctus sp. Gravier, 1904* | • | • | 7 (0.1) | 3.1 ± 10 | 4 | - |
| Cirratulidae | - | |||||
| Cirratulidae gen. indet. Ryckholt, 1851* | • | 5 (< 0.1) | 2.2 ± 13.3 | 1 | - | |
| Cirriformia sp. Hartman, 1936* | • | 71 (0.6) | 31.6 ± 97.7 | 6 | – | |
| Lumbrineridae | ||||||
| Lumbrineris sp. Blainville, 1828* | • | • | 16 (0.1) | 7.1 ± 34.9 | 3 | HAOIV268-24 |
| Maldanidae | ||||||
| Microclymene sp. Arwidsson, 1906* | • | 104 (0.9) | 46.2 ± 159.7 | 7 | - | |
| Nephtyidae | ||||||
| Aglaophamus sp. Kinberg, 1866* | • | 2 (< 0.1) | 0.9 ± 3.7 | 2 | HAOIV266-24 | |
| Nereididae | ||||||
| Neanthes kerguelensis (McIntosh, 1885) | • | • | 61 (0.5) | 27.1 ± 50.6 | 17 | HAOIV033-24 |
| Platynereis australis (Schmarda, 1861) | • | • | 81 (0.7) | 36 ± 43 | 22 | HAOIV046-24 |
| Polynoidae | ||||||
| Harmothoe spp. Kinberg, 1856 | • | • | 95 (0.8) | 42.2 ± 60.4 | 21 | HAOIV066-24 |
| Phyllodocidae | ||||||
| Eulalia sp. Savigny, 1822 | • | • | 9 (0.1) | 4 ± 14 | 5 | HAOIV228-24 |
| Lugia sp. Quatrefages, 1866* | • | 5 (< 0.1) | 2.2 ± 6.8 | 4 | - | |
| Phyllodoce sp. Lamarck, 1818* | • | 3 (< 0.1) | 1.3 ± 4.5 | 3 | - | |
| Sabellidae | ||||||
| Parasabella sp. Bush, 1905 | • | • | 166 (1.5) | 72.9 ± 209.1 | 5 | - |
| Serpulidae | ||||||
| Spirobranchus sp. Blainville, 1818 | • | • | 36 (0.3) | 16 ± 59 | 6 | - |
| Spionidae | ||||||
| Malacoceros sp. Quatrefages, 1843* | • | • | 155 (1.4) | 68.9 ± 233.4 | 11 | HAOIV270-24 |
| Syllidae | ||||||
| Exogone anomalochaeta Benham, 1921* | • | • | 273 (2.4) | 121.3 ± 189.6 | 22 | - |
| Syllis prolixa Ehlers, 1901* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Terebellidae | ||||||
| Neoleprea streptochaeta (Ehlers, 1897) | • | • | 8 (0.1) | 3.6 ± 12.2 | 4 | HAOIV028-24 |
| Thelepus spectabilis Ehlers, 1897 | • | • | 11 (0.1) | 4.9 ± 14.2 | 5 | - |
| Travisiidae | ||||||
| Travisia kerguelensis McIntosh, 1885* | • | 16 (0.1) | 7.1 ± 23.4 | 4 | - | |
| Arthropoda | ||||||
| Malacostraca | ||||||
| Akanthophoreidae | ||||||
| Akanthophoreidae gen. indet. Sieg, 1986 | • | 3 (< 0.1) | 1.3 ± 4.5 | 3 | - | |
| Apseudidae | • | |||||
| Apseudes spectabilis Studer, 1884 | • | • | 75 (0.7) | 33.3 ± 139.7 | 7 | HAOIV167-24 |
| Arcturidae | ||||||
| Neastacilla cf. kerguelensis (Vanhöffen, 1914)* | • | 18 (0.2) | 8 ± 29.8 | 4 | - | |
| Calliopiidae | ||||||
| Oradarea cf. unidentata Thurston, 1974 | • | • | 11 (0.1) | 4.9 ± 14.2 | 4 | HAOIV007-24 |
| Conicostomatidae | ||||||
| Stomacontion pepinii (Stebbing, 1888)* | • | • | 7 (0.1) | 3.1 ± 16.2 | 2 | - |
| Corophiidae | ||||||
| Haplocheira barbimana (Thomson, 1879) | • | • | 740 (6.6) | 328.9 ± 850.4 | 15 | HAOIV219-24 |
| Iphimediidae | ||||||
| Iphimediella paracuticoxa Andres, 1988 | • | 2 (< 0.1) | 0.9 ± 3.7 | 2 | - | |
| Ischyroceridae | ||||||
| Ischyrocerus sp. Krøyer, 1838 | • | • | 12 (0.1) | 5.3 ± 20.6 | 3 | - |
| Jassa cf. hartmannae Conlan, 1990 | • | • | 895 (8) | 397.8 ± 576.9 | 26 | HAOIV004-24 |
| Janiridae | ||||||
| Iathrippa sp. Bovallius, 1886 | • | • | 51 (0.5) | 22.7 ± 42 | 11 | HAOIV147-24 |
| Neojaera sp. Nordenstam, 1933* | • | 5 (< 0.1) | 2.2 ± 9.5 | 2 | - | |
| Joeropsididae | ||||||
| Joeropsis curvicornis (Nicolet, 1849)* | • | • | 40 (0.4) | 17.8 ± 42.6 | 11 | HAOIV256-24 |
| Kergueleniidae | ||||||
| Kerguelenia antiborealis Bellan-Santini & Ledoyer, 1987* | • | 3 (< 0.1) | 1.3 ± 8 | 1 | - | |
| Lysianassidae | ||||||
| Parawaldeckia kidderi (S.I. Smith, 1876) | • | 2 (< 0.1) | 0.9 ± 5.3 | 1 | HAOIV001-24 | |
| Maeridae | ||||||
| Elasmopus sp. A. Costa, 1853* | • | 9 (0.1) | 4 ± 24 | 1 | HAOIV213-24 | |
| Munnidae | ||||||
| Munna sp. Krøyer, 1839* | • | • | 7 (0.1) | 3.1 ± 9.2 | 5 | HAOIV255-24 |
| Nototanaidae | ||||||
| Nototanais dimorphus (Beddard, 1886) | • | 6 (0.1) | 2.7 ± 13.5 | 2 | - | |
| Oedicerotidae | ||||||
| Oedicerotidae gen. indet. sp.1 Lilljeborg, 1865* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Oedicerotidae gen. indet. sp.2 Lilljeborg, 1865* | • | 20 (0.2) | 8.9 ± 30.2 | 5 | HAOIV212-24 | |
| Oedicerotidae gen. indet. sp.3 Lilljeborg, 1865 | • | 8 (0.1) | 3.6 ± 21.3 | 1 | - | |
| Pagetinidae | ||||||
| Pagetina monodi (Nicholls, 1938) | • | • | 51 (0.5) | 22.7 ± 56.3 | 9 | - |
| Paramunnidae | ||||||
| Cryosignum lunatum (Hale, 1937) | • | 8 (0.1) | 3.6 ± 18.8 | 2 | HAOIV137-24 | |
| Phoxocephalidae | ||||||
| Phoxocephalidae gen. indet. G.O. Sars, 1891* | • | 20 (0.2) | 8.9 ± 21.1 | 8 | HAOIV210-24 | |
| Pleustidae | ||||||
| Pleusymtes sp. J.L. Barnard, 1969* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Podoceridae | ||||||
| Podocerus capillimanus (Nicholls, 1938) | • | 22 (0.2) | 9.8 ± 33.4 | 3 | HAOIV012-24 | |
| Pontogeneiidae | ||||||
| Atyloella cf. magellanica (Stebbing, 1888) | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV002-24 | |
| Eusiroides georgiana K.H. Barnard, 1932* | • | • | 125 (1.1) | 55.6 ± 154.6 | 10 | HAOIV215-24 |
| Prostebbingia sp. Schellenberg, 1926 | • | • | 800 (7.1) | 355.6 ± 833.1 | 14 | HAOIV013-24 |
| Serolidae | ||||||
| Septemserolis septemcarinata (Miers, 1875) | • | • | 27 (0.2) | 12 ± 58.7 | 5 | - |
| Spinoserolis latifrons (White, 1847) | • | • | 50 (0.4) | 22.2 ± 38.9 | 17 | HAOIV139-24 |
| Santiidae | ||||||
| Santia sp. Sivertsen & Holthuis, 1980* | • | • | 118 (1.1) | 52.4 ± 90 | 18 | HAOIV254-24 |
| Sphaeromatidae | ||||||
| Cassidinopsis emarginata (Guérin-Méneville, 1843) | • | 32 (0.3) | 14.2 ± 48.7 | 9 | HAOIV143-24 | |
| Sphaeromatidae sp.1 Latreille, 1825 | • | • | 99 (0.9) | 44 ± 86.6 | 15 | - |
| Sphaeromatidae sp.2 Latreille, 1825 | • | • | 511 (4.6) | 227.1 ± 614.1 | 21 | HAOIV257-24 |
| Stenothoidae | ||||||
| Proboloides sp. Della Valle, 1893 | • | • | 51 (0.5) | 22.7 ± 63.5 | 5 | - |
| Tanaidacea | ||||||
| Tanaidacea fam. gen. sp. Dana, 1849 | • | • | 991 (8.9) | 440.4 ± 1389.4 | 17 | - |
| Tanaididae | ||||||
| Pancoloides litoralis (Vanhöffen, 1914) | • | • | 617 (5.5) | 274.2 ± 466.3 | 24 | HAOIV168-24 |
| Tanaididae gen. indet. Nobili, 1906 | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Tryphosidae | ||||||
| Tryphosella sp. Bonnier, 1893 | • | • | 12 (0.1) | 5.3 ± 29.4 | 2 | HAOIV027-24 |
| Pycnogonida | ||||||
| Ammotheidae | ||||||
| Tanystylum antipodum Clark, 1977* | • | • | 4 (< 0.1) | 1.8 ± 5.1 | 4 | - |
| Tanystylum neorhetum Marcus, 1940 | • | • | 5 (< 0.1) | 2.2 ± 7.8 | 3 | - |
| Austrodecidae | ||||||
| Austrodecus fagei Stock, 1957* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Austrodecus cf. tristanense Stock, 1955* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Endeidae | ||||||
| Endeis viridis Pushkin, 1976 | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV166-24 | |
| Nymphonidae | ||||||
| Nymphon sp.1 Fabricius, 1794* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Nymphon sp.2 Fabricius, 1794* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Nymphon brevicaudatum Miers, 1875 | • | • | 19 (0.2) | 8.4 ± 30.5 | 6 | HAOIV165-24 |
| Nymphon glabrum Child, 1995* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Nymphon paucidens Gordon, 1932* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Pycnogonidae | ||||||
| Pycnogonum platylophum Loman, 1923* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Brachiopoda | ||||||
| Rhynchonellata | ||||||
| Terebratellidae | ||||||
| Aerothyris kerguelensis (Davidson, 1878) | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Chordata | ||||||
| Ascidiacea | ||||||
| Holozoidae | ||||||
| Sycozoa cf. gaimardi (Herdman, 1886) | • | 4 (< 0.1) | 1.8 ± 10.7 | 1 | - | |
| Polyclinidae | ||||||
| Aplidium variabile (Herdman, 1886) | • | • | 27 (0.2) | 12 ± 40 | 5 | - |
| Cnidaria | ||||||
| Anthozoa | ||||||
| Actiniaria | ||||||
| Actiniaria fam. gen. sp.1 Hertwig, 1882 | • | 16 (0.1) | 7.1 ± 37.4 | 3 | - | |
| Actiniaria fam. gen. sp.2 Hertwig, 1882 | • | 4 (< 0.1) | 1.8 ± 6.4 | 3 | - | |
| Actiniaria fam. gen. sp.3 Hertwig, 1882 | • | 15 (0.1) | 6.7 ± 32.3 | 3 | - | |
| Actiniaria fam. gen. sp.4 Hertwig, 1882 | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Hydrozoa | ||||||
| Tubulariidae | ||||||
| Tubulariidae gen. indet. Goldfuss, 1818 | • | 22 (0.2) | 9.8 ± 26.3 | 5 | HAOIV233-24 | |
| Echinodermata | ||||||
| Asteroidea | ||||||
| Asteriidae | ||||||
| Anasterias antarctica (Lütken, 1857) | • | • | 15 (0.1) | 6.7 ± 12.3 | 10 | HAOIV098-24 |
| Asteriidae gen. indet. Gray, 1840 | • | • | 14 (0.1) | 6.2 ± 18.1 | 7 | HAOIV090-24 |
| Diplasterias meridionalis (Perrier, 1875) | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV088-24 | |
| Astropectinidae | ||||||
| Leptychaster kerguelenensis E. A. Smith, 1876 | • | 3 (< 0.1) | 1.3 ± 5.9 | 2 | HAOIV095-24 | |
| Echinasteridae | ||||||
| Henricia obesa (Sladen, 1889) | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV084-24 | |
| Henricia cf. spinulifera (E. A. Smith, 1876) | • | • | 2 (< 0.1) | 0.9 ± 3.7 | 2 | HAOIV078-24 |
| Echinoidea | ||||||
| Temnopleuridae | ||||||
| Pseudechinus cf. marionis (Mortensen, 1936) | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV116-24 | |
| Holothuroidea | ||||||
| Chiridotidae | ||||||
| Scoliorhapis massini O'Loughlin & VandenSpiegel, 2010 | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV172-24 | |
| Cucumariidae | ||||||
| Echinopsolus splendidus (Gutt, 1990) | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV130-24 | |
| Cladodactyla crocea var. croceoides (Vaney, 1908) | • | • | 3 (< 0.1) | 1.3 ± 5.9 | 2 | HAOIV129-24 |
| Pentactella intermedia (Théel, 1886)* | • | 6 (0.1) | 2.7 ± 11.8 | 2 | - | |
| Pentactella laevigata Verrill, 1876 | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV135-24 | |
| Ophiuroidea | ||||||
| Amphiuridae | ||||||
| Amphiura tomentosa Lyman, 1879* | • | 16 (0.1) | 7.1 ± 29.7 | 2 | HAOIV263-24 | |
| Ophiacanthidae | ||||||
| Ophiosabine vivipara (Ljungman, 1871) | • | • | 28 (0.3) | 12.4 ± 39.2 | 5 | HAOIV158-24 |
| Ophiopyrgidae | ||||||
| Ophioplinthus sp. Lyman, 1878* | • | 2 (< 0.1) | 0.9 ± 5.3 | 1 | HAOIV260-24 | |
| Mollusca | ||||||
| Bivalvia | ||||||
| Gaimardiidae | ||||||
| Kidderia sp. Dall, 1876 | • | • | 823 (7.4) | 365.8 ± 948.3 | 24 | - |
| Limidae | ||||||
| Limatula sp. S. V. Wood, 1839* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Neoleptonidae | ||||||
| Neolepton sp. Monterosato, 1875* | • | • | 823 (7.4) | 365.8 ± 1224.7 | 23 | HAOIV231-24 |
| Philobryidae | ||||||
| Lissarca sp. E. A. Smith, 1877 | • | • | 925 (8.3) | 411.1 ± 2094 | 13 | HAOIV103-24 |
| Philobrya sp.1 J. G. Cooper, 1867* | • | • | 28 (0.3) | 12.4 ± 69.4 | 2 | - |
| Philobrya sp.2 J. G. Cooper, 1867* | • | 2 (< 0.1) | 0.9 ± 5.3 | 1 | - | |
| Gastropoda | ||||||
| Aeolidiidae | ||||||
| Aeolidiidae gen. indet. Gray, 1827 | • | 3 (< 0.1) | 1.3 ± 5.9 | 2 | HAOIV151-24 | |
| Calliostomatidae | ||||||
| Margarella violacea (P. P. King, 1832) | • | • | 96 (0.9) | 42.7 ± 90.8 | 15 | - |
| Cingulopsidae | ||||||
| Skenella sp. Pfeffer, 1886* | • | • | 90 (0.8) | 40 ± 171.8 | 6 | HAOIV237-24 |
| Cominellidae | ||||||
| Pareuthria sp. Strebel, 1905* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | - | |
| Diaphanidae | ||||||
| Diaphana paessleri (Strebel, 1905)* | • | 3 (< 0.1) | 1.3 ± 5.9 | 2 | - | |
| Eatoniellidae | ||||||
| Eatoniella caliginosa (E. A. Smith, 1875) | • | • | 405 (3.6) | 180 ± 452.3 | 20 | HAOIV249-24 |
| Littorinidae | ||||||
| Laevilacunaria pumilio (E. A. Smith, 1877) | • | • | 73 (0.7) | 32.4 ± 91.3 | 10 | HAOIV242-24 |
| Pellilitorina setosa (E. A. Smith, 1875) | • | • | 40 (0.4) | 17.8 ± 88.9 | 3 | HAOIV245-24 |
| Muricidae | ||||||
| Enixotrophon declinans (R. B. Watson, 1882)* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV239-24 | |
| Nacellidae | ||||||
| Nacella delesserti (R. A. Philippi, 1849) | • | • | 65 (0.6) | 28.9 ± 71.6 | 13 | - |
| Newtoniellidae | ||||||
| Cerithiella sp. A. E. Verrill, 1882* | • | • | 6 (0.1) | 2.7 ± 8.1 | 4 | HAOIV236-24 |
| Omalogyridae | ||||||
| Omalogyra sp. Jeffreys, 1859* | • | 2 (< 0.1) | 0.9 ± 3.7 | 2 | HAOIV240-24 | |
| Prosiphonidae | ||||||
| Falsimohnia albozonata (R. B. Watson, 1882) | • | • | 30 (0.3) | 13.3 ± 47.4 | 6 | HAOIV252-24 |
| Fusinella jucunda (Thiele, 1912) | • | 11 (0.1) | 4.9 ± 21.2 | 2 | HAOIV118-24 | |
| Prosipho sp. Thiele, 1912* | • | 4 (< 0.1) | 1.8 ± 8.4 | 2 | HAOIV234-24 | |
| Raphitomidae | ||||||
| Xanthodaphne translucida (R. B. Watson, 1881) | • | • | 24 (0.2) | 10.7 ± 50.7 | 5 | - |
| Rissoidae | ||||||
| Onoba cf. kergueleni (E. A. Smith, 1875)* | • | • | 806 (7.2) | 358.2 ± 559.2 | 21 | HAOIV241-24 |
| Velutinidae | ||||||
| Marseniopsis sp. Bergh, 1886 | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV126-24 | |
| Polyplacophora | ||||||
| Chitonida | ||||||
| Chitonida fam. gen. sp. Thiele, 1909* | • | 15 (0.1) | 6.7 ± 32.3 | 3 | - | |
| Hemiarthridae | ||||||
| Hemiarthrum sp. P. P. Carpenter, 1876 | • | • | 77 (0.7) | 34.2 ± 91.6 | 14 | HAOIV110-24 |
| Leptochitonidae | ||||||
| Leptochiton laurae Schwabe & Sellanes, 2010* | • | 3 (< 0.1) | 1.3 ± 4.5 | 3 | HAOIV232-24 | |
| Nemertea | ||||||
| Monostilifera | ||||||
| Monostilifera fam. gen. sp. Brinkmann, 1917 | • | • | 12 (0.1) | 5.3 ± 14.8 | 7 | HAOIV258-24 |
| Platyhelminthes | ||||||
| Rhabditophora | ||||||
| Rhabditophora fam. gen. sp.1 Ehlers, 1985* | • | 1 (< 0.1) | 0.4 ± 2.7 | 1 | HAOIV264-24 | |
| Rhabditophora fam. gen. sp.2 Ehlers, 1985* | • | 1 (< 0.1) | 0.9 ± 5.3 | 1 | - | |
List of the 124 taxa found at Baie du Marin (BDM) and Crique du Sphinx (CdS) on the eastern coast of Ile de la Possession, ranked by alphabetical order of phyla (then by class and family).
(*) New morphotypes compared to Lelièvre et al. (2023, 2025a) and Jossart et al. (2024). TA: Total abundance (% of the total abundance among all taxa), D: mean density per quadrat ± standard deviation (indiv. m-2), Nquadrat: number of quadrat occupied by the taxa, BOLD: public accession number that indicates that at least one COI barcode is available for that taxon (HAOIV project “Shallow benthic communities of Crozet archipelago”).
Figure 4
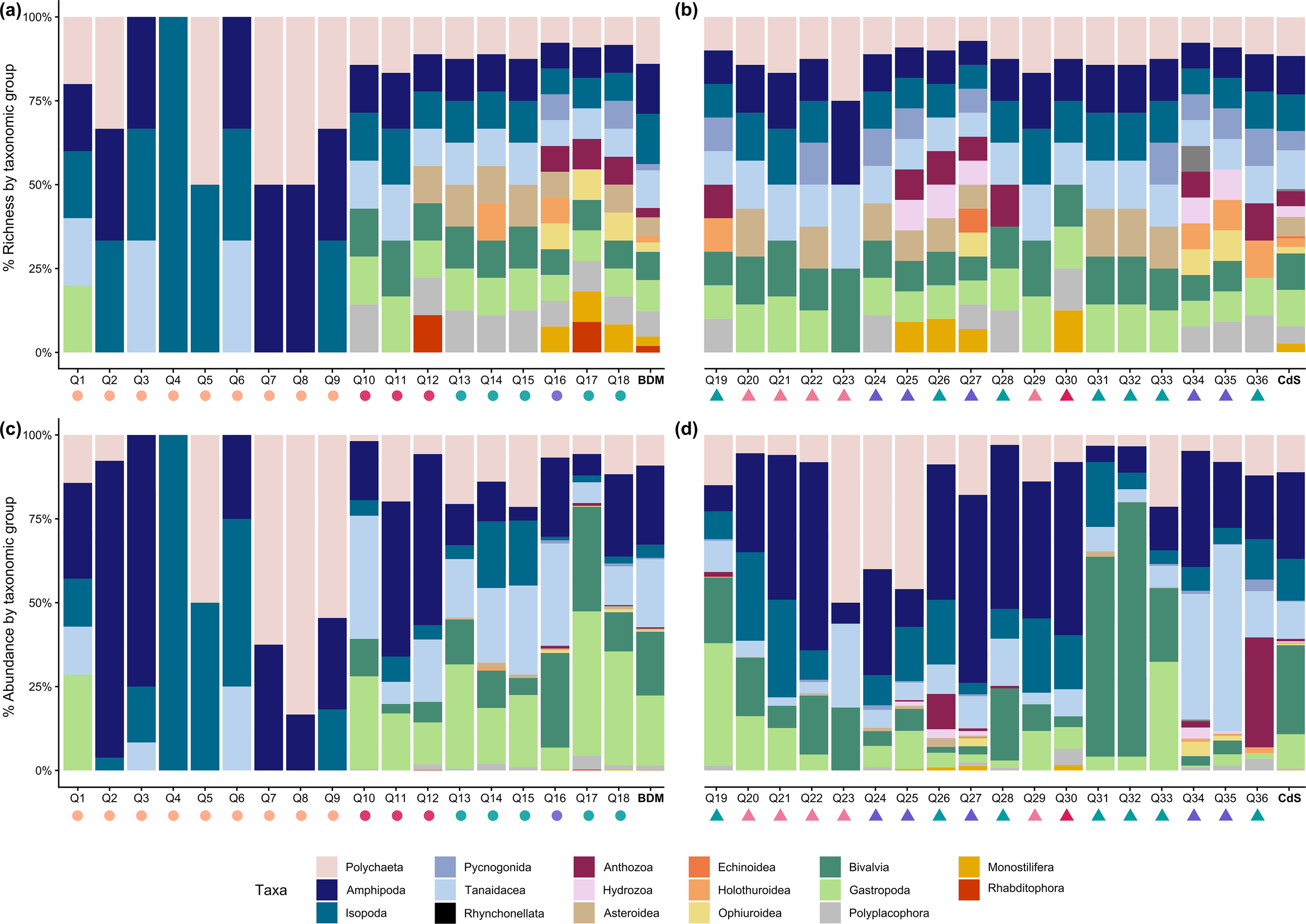
Bar charts of (a, b) relative species richness and (c, d) relative abundance by taxon within all quadrats collected at Baie du Marin (BDM; circles, Q1–Q18) and Crique du Sphinx (CdS; triangles, Q19–Q36). The color of symbols under quadrat number indicate the substrate type: sand (orange), rock (purple), rock and algae (green), sand and pebbles (dark pink), as well as sand and algae (pink).
Benthic communities were characterized by a high proportion of rare morphotypes, reaching 72% (89 morphotypes). Among these rare morphotypes, Amphipoda is the most represented, comprising 14 morphotypes and accounting for 15.7% of the total rare morphotypes, followed by Polychaeta with 13 morphotypes accounting for 14.6% of the total rare morphotypes, as well as Gastropoda and Pycnogonida, each encompassing 11 morphotypes and representing 12.4% of the total rare morphotypes. No morphotypes defined as “common” were identified. Finally, 28% (35 morphotypes) were defined as moderate morphotypes, with a high number of Polychaeta, encompassing eight morphotypes and accounting for 22.9% of the total moderate morphotypes, followed by Gastropoda with seven morphotypes and accounting for 20% of the total moderate morphotypes, as well as Amphipoda and Isopoda, both encompassing six morphotypes and accounting for 17.1% of the total moderate morphotypes.
3.2 Variation in species composition between quadrats
The PCoA showed no variation in faunal composition between Baie du Marin and Crique du Sphinx (Figure 5). However, distinct benthic community composition and structure were observed across the different substrate types. The community of sandy bottoms clearly departs from communities of other habitats and was mainly characterized by the presence of the isopod Spinoserolis latifrons (White, 1847), the amphipods Phoxocephalidae gen. indet. G.O. Sars, 1891, Oedicerotidae gen. indet. sp.2 Lilljeborg, 1865 and Oedicerotidae gen. indet. sp.3 Lilljeborg, 1865, the tanaid Akanthophoreidae gen. indet. Sieg, 1986, as well as the polychaete Travisia kerguelensis McIntosh, 1885. Sandy-dominated substrates associated with pebbles and/or algae were mainly characterized by the amphipod Prostebbingia sp. (Schellenberg, 1926), the isopod Sphaeromatidae gen. indet. sp.2 Latreille, 1825, as well as the gastropod Nacella delesserti (R. A. Philippi, 1849). Rocky substrates associated with macroalgae were mainly characterized by high abundances of the bivalve Kidderia sp. Dall, 1876, the gastropod Onoba cf. kergueleni (E. A. Smith, 1875), the isopods Sphaeromatidae gen. indet. sp.1 Lilljeborg, 1865 and Santia sp. Sivertsen & Holthuis, 1980, the tanaid Pancoloides litoralis (Vanhöffen, 1914), the polychaete Neanthes kerguelensis (McIntosh, 1885), and the amphipod Jassa cf. hartmannae (Conlan, 1990). Finally, rocky substrates were mainly dominated by the polychaetes Parasabella sp. Bush, 1905, Exogone anomalochaeta Benham, 1921, Platynereis australis (Schmarda, 1861), and Harmothoe spp. Kinberg, 1856, the bivalve Neolepton sp. Monterosato, 1875, the gastropod Margarella violacea (P. P. King, 1832) and Falsimohnia albozonata (R. B. Watson, 1882), the tanaids Tanaidacea fam. gen. sp. Dana, 1849 and Apseudes spectabilis Studer, 1884, the isopod Iathrippa sp. Bovallius, 1886, the pycnogonid Nymphon cf. brevicaudatum Miers, 1875, and the amphipod Haplocheira barbimana (Thomson, 1879).
Figure 5
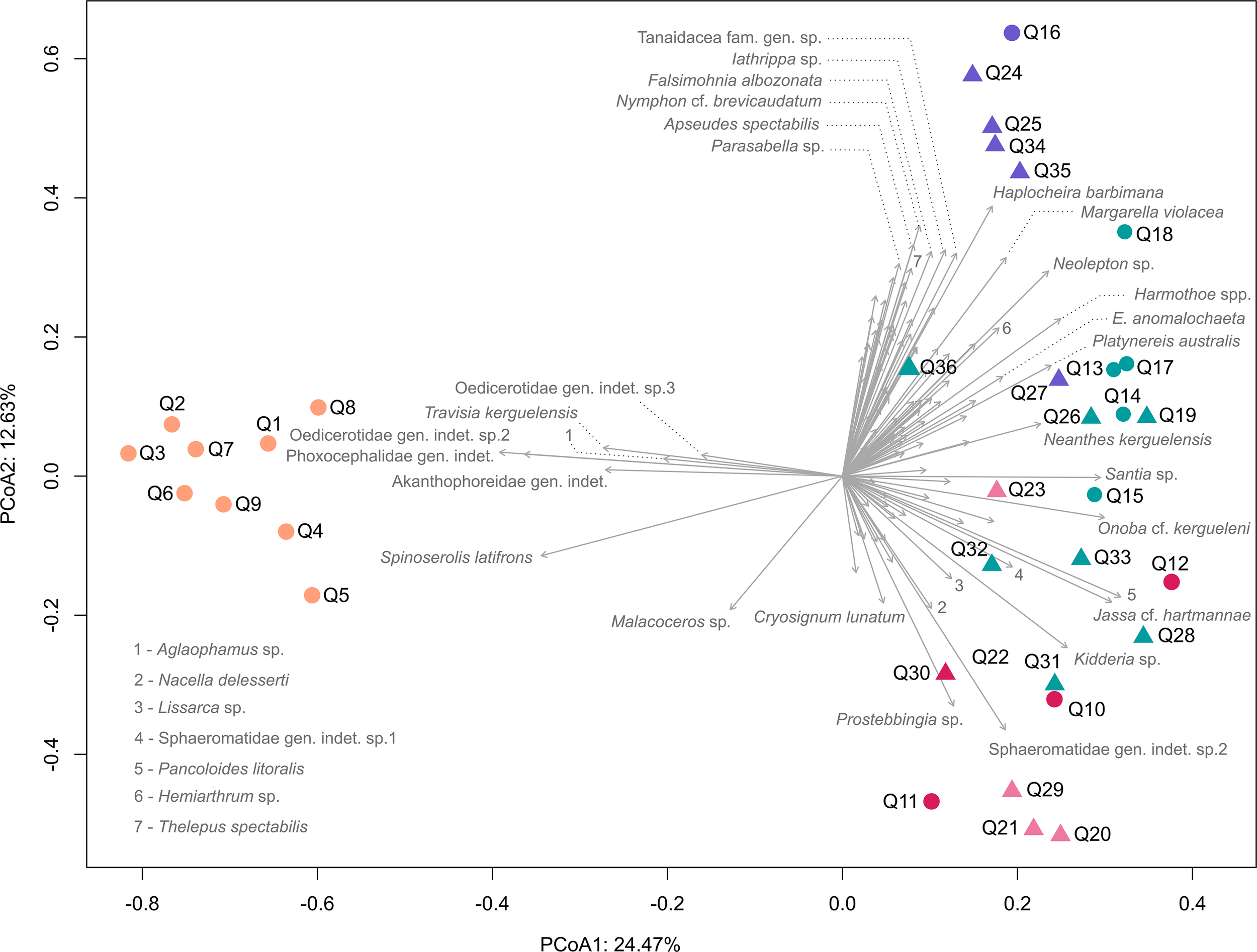
Principal Coordinates Analysis (PCoA) highlighting the variation in the composition and structure of faunal communities between quadrats and sites. Circles and triangles correspond to quadrats sampled at Baie du Marin and Crique du Sphinx, respectively. Colors correspond to substrate types, including: sand (orange), rock (purple), rock and algae (green), sand and pebbles (dark pink), as well as sand and algae (pink). The first two canonical axes account together for 37.1% of the total variance.
3.3 Taxonomic diversity indices
Overall, Baie du Marin and Crique du Sphinx showed similar levels of species richness, with 87 morphotypes at Baie du Marin and 96 morphotypes at Crique du Sphinx. However, Baie du Marin displayed higher diversity values (N = 23.7; 1/λ = 14.5) than Crique du Sphinx (N = 21.5; 1/λ = 13.6; Figure 6). The highest richness and diversity values were found for rocky substrates (Rmean = 37.833 ± 7.494; Nmean = 12.454 ± 4.280; 1/λmean = 6.673 ± 2.531), followed by rock and algae (Rmean = 26.833 ± 10.053; Nmean = 12.096 ± 4.271; 1/λmean = 8.023 ± 2.901). Sand and algae (Rmean = 17 ± 4.528; Nmean = 8.236 ± 1.271; 1/λmean = 6.350 ± 1.059) and sand-pebbles habitats (Rmean = 17.5 ± 6.245; Nmean = 7.954 ± 2.155; 1/λmean = 5.266 ± 1.702) displayed similar species richness and diversity values. Finally, sandy substrate was characterized by the lowest richness and diversity values (Rmean = 3.667 ± 1.732; Nmean = 3.711 ± 1.360; 1/λmean = 3.365 ± 1.344; Figures 6, 7). Overall, significant variation in species richness was observed among substrates (Kruskal–Wallis χ² = 27.16, df = 4, p < 0.001). Post-hoc comparisons revealed that species richness was significantly lower on pure sand bottoms compared to rock (p < 0.001) and rock–algae substrates (p < 0.001; Figure 7a). A similar pattern was observed for the Shannon diversity index (Kruskal–Wallis χ² = 22.40, df = 4, p < 0.001), with significantly lower values on pure sand bottoms compared to rock (p < 0.001) and rock–algae substrates (p < 0.001; Figure 7b). Finally, the same overall trend was found for the Simpson diversity index (Kruskal–Wallis χ² = 16.15, df = 4, p < 0.001), with significantly lower diversity on pure sand compared to rock–algae substrates (p < 0.001; Figure 7c).
Figure 6
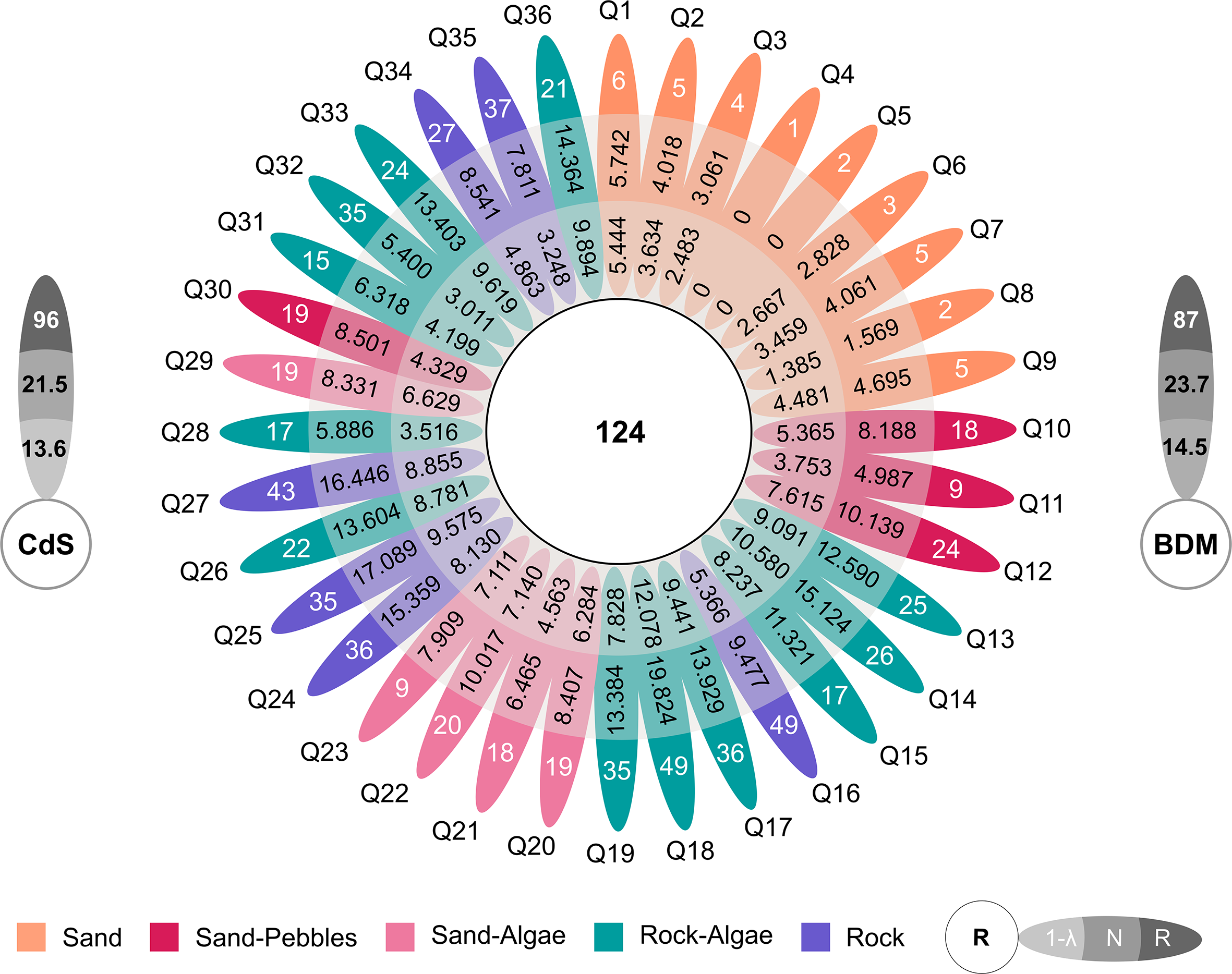
Univariate Hill’s diversity indices calculated for each quadrat and site (Baie du Marin (BDM) and Crique du Sphinx (CdS)). Diversity indices are taxonomic richness (R; q=0), Shannon diversity (N; q=1) and Simpson diversity (1/λ; q=2).
Figure 7
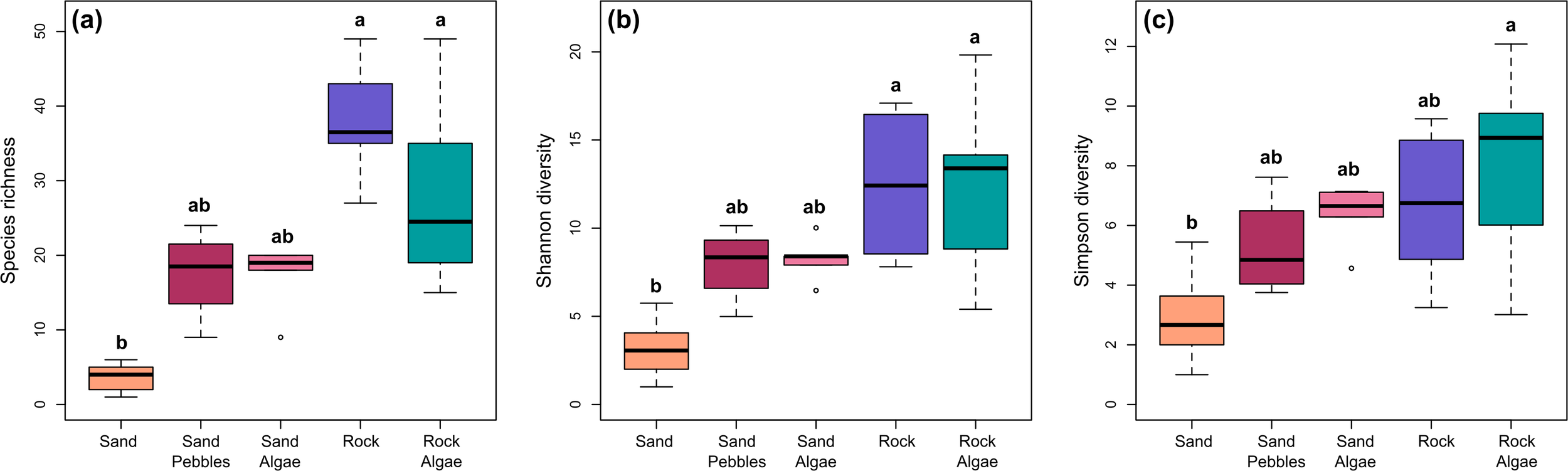
Comparison of Hill’s diversity indices, including (a) species richness (q=0); (b) Shannon diversity (q=1); and (c) Simpson diversity (q=2) between substrate types. Lowercase letters above confidence intervals indicate which groups are statistically different based on Dunn tests with Bonferroni adjustments.
4 Discussion
4.1 Crozet taxonomic benthic diversity and structure
This study provides the first quantitative description of subtidal benthic communities in the Crozet archipelago. It constitutes a taxonomic and molecular baseline, which is particularly valuable in this relatively understudied region of the Southern Ocean impacted by global change (Auger et al., 2021; Nel et al., 2023). Compared to former benthic studies conducted at Ile de la Possession (Lelièvre et al., 2023, 2025a; Jossart et al., 2024), 57 new morphotypes were identified (see Table 1). Such differences between studies may be related to the different sampling method conducted. In Lelièvre et al. (2023, 2024), the use of imagery only allowed the observation of the fauna of sufficient size (> 1 cm) to be detected from images (Beisiegel et al., 2017; Hanafi-Portier et al., 2021), while in Jossart et al. (2024) biological sampling followed an opportunistic design and was performed by hand picking, increasing the probability of missing rare species and small organisms.
Overall, Baie du Marin and Crique du Sphinx showed similar composition and structure of invertebrate communities, which can be explained by the close proximity (∼2 km) of the two locations. The identified benthic invertebrates are typical of sub-Antarctic and Antarctic waters (Arnaud, 1974; Branch et al., 1993; Freeman et al., 2011; Clark et al., 2019; Lelièvre et al., 2023; Jossart et al., 2024), with the presence of rich and diverse taxonomic groups of annelids (polychaetes), arthropods (amphipods, isopods, pycnogonids, tanaids), brachiopods, chordates (ascidians), cnidarians (anemones, tubularids), echinoderms (asteroids, echinoids, holothurids, ophiuroids), molluscs (bivalves, gastropods), nemerteans and platyhelminths. Former studies showed that amphipods (Dauby et al., 2001; Améziane et al., 2011; De Broyer et al., 2014) and molluscs (bivalves and gastropods) (Linse et al., 2006; Rosenfeld et al., 2015, 2023; Amsler et al., 2022) are among the most abundant and species-rich components of Crozet benthic communities. Despite the high number of species shared with other sub-Antarctic regions (Jossart et al., 2024), some faunal dissimilarities were also noticed. Mussel beds are very common in intertidal and subtidal nearshore marine ecosystems of the Kerguelen Islands and of the Magellanic Province (Arnaud, 1974; Féral et al., 2019; Fraïsse et al., 2021; Bahamonde et al., 2022) but unexpectedly, they seem to be absent from the study sites of Crozet. Barnacles are reported in high abundance in the New Zealand sub-Antarctic islands but were not found at Crozet (Freeman et al., 2011). Sponges were also absent in the present study, although some taxa were previously identified on Crozet hard substrates by Lelièvre et al. (2023, 2024, 2025) based on image analyses. Most subtidal environments from the Kerguelen Islands are dominated by sponges that locally constitute a significant part of the benthic biomass (Améziane et al., 2011). At Prince Edwards Islands, hard substrates are also dominated by sponges, along with bryozoans and cnidarians (Branch et al., 1993). Crozet submarine images (Lelièvre et al., 2024a) and biological sampling seem to indicate that sponges are not as abundant in the Crozet shallow waters. This suggests contrasting diversity patterns among sub-Antarctic islands, and the importance of local environmental conditions in the composition and structure of species assemblages. Therefore, pursuing taxonomic investigations around Crozet and characterizing local abiotic conditions is important for advancing the understanding of the composition and distribution of benthic communities.
4.2 Relationships between substrate type and diversity patterns
Variations in faunal composition among quadrats were closely related to the nature of the sea bottom. Diversity levels varied according to substrate type, the lowest values being measured from sandy bottoms, moderate values from sandy areas associated with pebbles and/or macroalgae, and the highest values from rocky substrates. Our results suggest that hard substrates and the presence of ecosystem engineers (e.g., macroalgae) promote high diversity levels (Gambi et al., 1994; Amsler et al., 1995; Levin et al., 2010; Lelièvre et al., 2023). Structurally complex environments offer a wider range of microhabitats that differ with each other in terms of environmental conditions and resources, thereby driving the occurrence of species with contrasting ecological requirements. Hard substrates provide stable settlement surfaces for sessile organisms, promoting the establishment and development of rich and diverse benthic communities (Lelièvre et al., 2023). The assessment performed in the first high sea Marine Protected Area (MPA), in the South Orkney Islands, also indicated that local variation in seafloor substrate was an important factor influencing taxa distribution, community composition, and abundance (Brasier et al., 2018). Substrate, together with location and depth, was similarly highlighted as a key driver of benthic assemblages across other Antarctic regions and environments (shallow waters, shelf, and slope areas) such as in the Ross Sea (Cummings et al., 2006), King George Island and the South Orkneys (Richardson, 1979; Quartino et al., 2001).
Biogenic habitats formed by macroalgae play a central role in structuring nearshore habitats (Teagle et al., 2017; Miller et al., 2018). The marine flora may exert a significant influence on local environmental conditions (Teagle et al., 2017; Noisette et al., 2022), including on light penetration (Graham et al., 2007), flow dynamics (Gaylord et al., 2007; Rosman et al., 2010, 2013), sediment transport and stabilization (Marin-Diaz et al., 2020), nutrient cycling and resources (Leclerc et al., 2013), substratum nature (Christie et al., 2007, 2009), as well as through biotic (e.g., predation, facilitation) and trophic interactions (Leclerc et al., 2013). By promoting habitat heterogeneity and complexity, hard substrates and the marine flora promote higher diversity levels, influencing the composition and structure of benthic communities.
In contrast, the low diversity levels observed in sandy bottom environments is likely due to a low structural complexity, which provides organisms with few habitats for shelter and attachment. However, although sandy bottoms exhibited lower overall species richness compared to more structurally complex substrates, our findings highlight their ecological importance as they host distinct communities that contribute to the overall benthic diversity of Crozet. As reported by Lelièvre et al. (2025a), soft sediments were associated to the occurrence of the isopod Spinoserolis latifrons. Future investigations of infaunal assemblages are needed, as these communities may be especially abundant and contribute significantly to the benthic diversity of sandy bottoms (Filgueiras et al., 2007).
4.3 Some guidelines for conservation and future research
Effective protection measures such as the creation of the French Southern Territories National Nature Reserve are valuable for the preservation of marine ecosystems but conservation strategies usually suffer from persistent knowledge gaps, in particular when considering benthic marine diversity. In the present work, we provide a detailed inventory of the composition of nearshore benthic communities of Ile de la Possession, which will constitute a critical baseline for future conservation studies and management policies. Our results highlight the importance of structuring elements such as hard substrates and the marine flora, which promote seascape heterogeneity, habitat complexity, and thereby high diversity levels. Conservation efforts should therefore prioritize areas with high structural complexity to monitor environmental changes and design conservation strategies. A comprehensive characterization of the benthic abiotic environment is also essential to improving our understanding of drivers shaping the identified diversity patterns. Among macroalgal habitats, kelp forests are of particular interest as they sustain a high diversity enhanced by structural complexity. Future studies should investigate their spatial and ecological dynamics with regards to current environmental changes as any decline of these habitats may have important cascading effects on diversity levels, community composition, and overall coastal ecosystem functioning.
Rare species are the main component of the diversity of ecological assemblages (Gaston, 1994), including in Southern Ocean marine ecosystems (Hogg et al., 2011). Crozet communities were also characterized by a high number of rare species. Future studies should investigate the role of rare species within benthic communities, particularly their functional contributions. These rare species may have more significant functions than their local abundance or regional occupancy suggest (Mouillot et al., 2013). The loss of rare species can thus affect local and key ecosystem processes (e.g., productivity, ecosystem resilience) (Zavaleta and Hulvey, 2004; Bracken and Low, 2012). At Ile de la Possession, Lelièvre et al. (2023) showed that a large part of the functional space was occupied by rare species with rare functional traits. We here suggest that the conservation of rare species should be another priority when planning for the long-term maintenance of ecosystem functioning. This would increase the level of functional diversity within communities, which in turn sustains local ecosystem processes and allows better resilience under changing environmental conditions.
Finally, two sites only were investigated on the eastern coast of Ile de la Possession, leaving vast portions of the island largely unknown, and other islands of the Crozet archipelago still unexplored. Local variations in abiotic conditions may generate distinct diversity patterns, which could have important implications for conservation. Improving biodiversity inventories across these under-studied islands would provide a stronger baseline and more effective conservation measures.
5 Conclusion
The present work advances our understanding of the composition and structure of coastal benthic communities of Ile de la Possession, in the sub-Antarctic Crozet archipelago. Overall, species composition of Ile de la Possession is similar to benthic assemblages identified in other sub-Antarctic islands but for some unique community structures such as dense tube-dwelling polychaete colonies that cover hard substrates in Crozet. At small spatial scale, results showed that species composition and community structure are closely related to substrate types and habitat complexity, hard substrates and habitat-forming species enhancing seascape heterogeneity and habitat complexity, which in turn support a rich and diverse local biodiversity. These findings highlight the value of the integrative taxonomic approach, particularly for investigating little known areas such as Crozet benthic habitats. The approach allowed establishing a reference framework for species identification as well as reference genetic resources that may prove useful for future ecological and biogeographical studies, and for monitoring the impacts of natural and anthropogenic disturbances (e.g. climate change, ocean warming and acidification, non-native and potential invasive species) on marine environments of the Crozet archipelago.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
YL: Visualization, Formal analysis, Writing – original draft, Data curation, Investigation, Conceptualization, Methodology. QJ: Writing – review & editing. SH: Writing – review & editing. MV: Writing – review & editing. AK: Writing – review & editing. DF: Writing – review & editing. JM: Writing – review & editing. SR: Writing – review & editing. MM: Writing – review & editing. NL: Writing – review & editing. EL: Writing – review & editing. MC: Writing – review & editing. GM: Writing – review & editing. CM: Writing – review & editing. TS: Validation, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study is a contribution to the project No. 2021-0882 “Nearshore Cable Inspection and Environmental Survey at IMS Hydroacoustic Station HA04 Crozet, France” conducted by the French Southern and Antarctic Lands (TAAF) in response to the CTBTO solicitation and to the French Polar Institute project #1044 Proteker. YL was supported by TAAF-UB convention #2258.
Acknowledgments
The authors thank the captain and crews of the R/V Marion Dufresne II, as well as the staff and divers of the “Terres australes et antarctiques françaises” (TAAF). Authors are particularly grateful to scuba divers of the French Polar Institute and TAAF: S. Motreuil, C. Marschal, L. Le Gall, M. Gueíneí, L. Wauters, Y. Sabatheí, S. Seímelin, and M.-F. Bernard. We thank A. Force for his help in quadrat sorting and J. Thomas for his assistance in cataloguing the samples as well as his valuable help in depositing the occurrence data on the Global Biodiversity Information Facility (GBIF) platform. Samples management and valorization benefited from the research infrastructure Récolnat (Réseau national des collections naturalistes). We also thank C. Sands, M. Błażewicz, and M. Tatián for their help in the identification process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1692217/full#supplementary-material
Supplementary Table 1Location of sampling sites for benthic communities at Baie du Marin (BDM) and Crique du Sphinx (CdS).
Supplementary Table 2Abundance data within all quadrats collected at Baie du Marin (Q1-Q18) and Crique du Sphinx (Q19-Q36).
References
1
Améziane N. Eléaume M. Hemery L. Monniot F. Hemery A. Hautecoeur M. et al . (2011). Biodiversity of the benthos off Kerguelen Islands: overview and perspectives. Kerguelen Plateau Mar. Ecosyst. Fish., 157–167. doi: 10.26028/cybium/2011-35SP-016
2
Amsler C. D. Miller L. R. Edwards R. A. Amsler M. O. Engl W. McClintock J. B. et al . (2022). Gastropod assemblages associated with Himantothallus grandifolius, Sarcopeltis Antarctica and other subtidal macroalgae. Antarct. Sci.34, 246–255. doi: 10.1017/S0954102022000153
3
Amsler C. D. Rowley R. J. Laur D. R. Quetin L. B. Ross R. M. (1995). Vertical distribution of Antarctic peninsular macroalgae: cover, biomass and species composition. Phycologia34, 424–430. doi: 10.2216/i0031-8884-34-5-424.1
4
Ansorge I. J. Durgadoo J. V. Pakhomov E. A. (2009). Dynamics of physical and biological systems of the Prince Edward Islands in a changing climate. Pap. Proc. R. Soc Tasmania143, 15–18. doi: 10.26749/rstpp.143.1.15
5
Arnaud P. M. (1974). “ Contribution à la bionomie marine benthique des régions Antarctiques et subantarctiques,” in Téthys. Ed. d’OcéanographieC. ( de M. M. Station Marine d’Endoume) 6, 468–653.
6
Arnaud P. M. (1982). MD30/BIOMASS aux iles Crozet : à bord du ‘Marion Dufresne’ 29 janvier-13 mars, 1982. Paris: Mission de recherche des Terres australes et antarctiques françaises.
7
Auger M. Morrow R. Kestenare E. Sallée J. B. Cowley R. (2021). Southern Ocean in-situ temperature trends over 25 years emerge from interannual variability. Nat. Commun.12, 1–9. doi: 10.1038/s41467-020-20781-1
8
Bahamonde F. Pablo Rodríguez J. Rosenfeld S. Méndez F. López Z. Gerard K. et al . (2022). Variability at multiple spatial scales in intertidal and subtidal macrobenthic communities in a fjord with glaciers, Magellanic Subantarctic ecoregion, Chile. Prog. Oceanogr.208, 102879. doi: 10.1016/j.pocean.2022.102879
9
Barnes D. K. A. Linse K. Waller C. Morely S. Enderlein P. Fraser K. P. P. et al . (2006). Shallow benthic fauna communities of South Georgia Island. Polar Biol.29, 223–228. doi: 10.1007/s00300-005-0042-0
10
Beisiegel K. Darr A. Gogina M. Zettler M. L. (2017). Benefits and shortcomings of non-destructive benthic imagery for monitoring hard-bottom habitats. Mar. pollut. Bull.121, 5–15. doi: 10.1016/j.marpolbul.2017.04.009
11
Bracken M. E. S. Low N. H. N. (2012). Realistic losses of rare species disproportionately impact higher trophic levels. Ecol. Lett.15, 461–467. doi: 10.1111/j.1461-0248.2012.01758.x
12
Branch G. M. Attwood C. G. Gianakouras D. Branch M. L. (1993). Patterns in the benthic communities on the shelf of the sub-Antarctic Prince Edward Islands. Polar Biol.13, 23–34. doi: 10.1007/BF00236580
13
Brasier M. J. Grant S. M. Trathan P. N. Allcock L. Ashford O. Blagbrough H. et al . (2018). Benthic biodiversity in the south orkney islands southern shelf marine protected area. Biodiversity19, 5–19. doi: 10.1080/14888386.2018.1468821
14
Cardinale B. J. Duffy J. E. Gonzalez A. Hooper D. U. Perrings C. Venail P. et al . (2012). Biodiversity loss and its impact on humanity. Nature486, 59–67. doi: 10.1038/nature11148
15
Carr C. M. Hardy S. M. Brown T. M. Macdonald T. A. Hebert P. D. N. (2011). A tri-oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. PloS One6, e22232. doi: 10.1371/journal.pone.0022232
16
Cavanagh R. D. Melbourne-Thomas J. Grant S. M. Barnes D. K. A. Hughes K. A. Halfter S. et al . (2021). Future risk for Southern Ocean ecosystem services under climate change. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.615214
17
Chown S. L. Rodrigues A. S. L. Gremmen N. J. M. Gaston K. J. (2001). World heritage status and conservation of Southern Ocean Islands. Conserv. Biol.15, 550–557. doi: 10.1046/j.1523-1739.2001.015003550.x
18
Christie H. Jørgensen N. M. Norderhaug K. M. (2007). Bushy or smooth, high or low; importance of habitat architecture and vertical position for distribution of fauna on kelp. J. Sea Res.58, 198–208. doi: 10.1016/j.seares.2007.03.006
19
Christie H. Norderhaug K. M. Fredriksen S. (2009). Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser.396, 221–233. doi: 10.3354/meps08351
20
Clark G. F. Pastorino S. Marzinelli E. M. Turney C. S. M. Fogwill C. J. Johnston E. L. (2019). Nearshore marine communities at three New Zealand sub-Antarctic islands. Polar Biol.42, 2193–2203. doi: 10.1007/s00300-019-02591-4
21
Constable A. J. Melbourne-Thomas J. Corney S. P. Arrigo K. R. Barbraud C. Barnes D. K. A. et al . (2014). Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Glob. Change Biol.20, 3004–3025. doi: 10.1111/gcb.12623
22
Cresswell I. D. Bax N. J. Constable A. J. Reid K. Smith A. D. M. (2023). The unique marine ecosystem surrounding Macquarie Island. Australian Marine Conservation Society and The Pew Charitable Trusts. doi: 10.5281/zenodo.7623378
23
Cummings V. Thrush S. Norkko A. Andrew N. Hewitt J. Funnell G. et al . (2006). Accounting for local scale variability in benthos: implications for future assessments of latitudinal trends in the coastal Ross Sea. Antarct. Sci.18, 633–644. doi: 10.1017/S0954102006000666
24
Dauby P. Scailteur Y. De Broyer C. (2001). Trophic diversity within the eastern Weddell Sea amphipod community. Hydrobiologia443, 69–86. doi: 10.1023/A:1017596120422
25
De Broyer C. Koubbi P. Griffiths H. J. Raymond B. Udekem d’Acoz C. Griffiths H. et al . (2014). “ Biogeographic Atlas of the Southern Ocean,” in Scientific Committee on Antarctic Research (Cambridge), 155–165.
26
Deregibus D. Campana G. L. Neder C. Barnes D. K. A. Zacher K. Piscicelli J. M. et al . (2023). Potential macroalgal expansion and blue carbon gains with northern Antarctic Peninsula glacial retreat. Mar. Environ. Res.189, 106056. doi: 10.1016/j.marenvres.2023.106056
27
Doney S. C. Ruckelshaus M. Emmett Duffy J. Barry J. P. Chan F. English C. A. et al . (2012). Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci.4, 11–37. doi: 10.1146/annurev-marine-041911-111611
28
Féral J. P. Poulin E. González-Wevar C. A. Améziane N. Guillaumot C. Develay E. et al . (2019). Long-term monitoring of coastal benthic habitats in the Kerguelen Islands: a legacy of decades of marine biology research. Symp. Kerguelen Plateau Mar. Ecosyst. Fish., 383–402. doi: 10.5281/zenodo.3249143
29
Féral J.-P. Verlaque M. Rosenfeld S. Poulin E. Chenuil A. Saucède T. (2021). The marine vegetation of the Kerguelen Islands: history of scientific campaigns, inventory of the flora and first analysis of its biogeographical affinities. Cryptogam. Algol.42, 173–216. doi: 10.5252/cryptogamie-algologie2021v42a12
30
Filgueiras V. L. Campos L. S. Lavrado H. P. Frensel R. Pollery R. C. G. (2007). Vertical distribution of macrobenthic infauna from the shallow sublittoral zone of Admiralty Bay, King George Island, Antarctica. Polar Biol.30, 1439–1447. doi: 10.1007/s00300-007-0305-z
31
Folmer O. Black M. Hoeh W. Lutz R. Vrijenhoek R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol.3, 294–299.
32
Fraïsse C. Haguenauer A. Gérard K. Anh-Thu Weber A. Bierne N. Chenuil A. (2021). Fine-grained habitat-associated genetic connectivity in an admixed population of mussels in the small isolated Kerguelen Islands. Peer Community J.1, e10. doi: 10.24072/pcjournal.18
33
Freeman D. Cooper S. Funnell G. Neale D. (2011). Nearshore benthic community structure at the Bounty and Antipodes Islands, Subantarctic New Zealand. Polar Biol.34, 1485–1499. doi: 10.1007/s00300-011-1006-1
34
Gambi M. C. Lorenti M. Russo G. F. Scipione M. B. (1994). Benthic associations of the shallow hard bottoms off Terra Nova Bay, Ross Sea: zonation, biomass and population structure. Antarct. Sci.6, 449–462. doi: 10.1017/S0954102094000696
35
Gaston K. J. (1994). Rarity. Population and Community Biology Series. 13. ( Springer Dordrecht). doi: 10.1007/978-94-011-0701-3
36
Gaylord B. Rosman J. H. Reed D. C. Koseff J. R. Fram J. MacIntyre S. et al . (2007). Spatial patterns of flow and their modification within and around a giant kelp forest. Limnol. Oceanogr.52, 1838–1852. doi: 10.4319/lo.2007.52.5.1838
37
Gostel M. R. Kress W. J. (2022). The expanding role of DNA barcodes: Indispensable tools for ecology, evolution, and conservation. Diversity14, 213. doi: 10.3390/d14030213
38
Graham M. Vásquez J. A. Buschmann A. (2007). Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr. Mar. Biol. an Annu. Rev.45, 39–88.
39
Gutt J. Bertler N. Bracegirdle T. J. Buschmann A. Comiso J. Hosie G. et al . (2015). The Southern Ocean ecosystem under multiple climate change stresses-an integrated circumpolar assessment. Glob. Ecol. Biogeogr.21, 1434–1453. doi: 10.1111/gcb.12794
40
Hanafi-Portier M. Samadi S. Corbari L. Chan T. Y. Chen W. J. Chen J. N. et al . (2021). When imagery and physical sampling work together: toward an integrative methodology of deep-sea image-based megafauna identification. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.749078
41
Henseler C. Nordström M. C. Törnroos A. Snickars M. Pecuchet L. Lindegren M. et al . (2019). Coastal habitats and their importance for the diversity of benthic communities: a species- and trait-based approach. Estuar. Coast. Shelf Sci.226, 106272. doi: 10.1016/j.ecss.2019.106272
42
Hewitt J. E. Thrush S. F. Dayton P. D. (2008). Habitat variation, species diversity and ecological functioning in a marine system. J. Exp. Mar. Bio. Ecol.366, 116–122. doi: 10.1016/j.jembe.2008.07.016
43
Hewitt J. E. Thrush S. F. Ellingsen K. E. (2016). The role of time and species identities in spatial patterns of species richness and conservation. Conserv. Biol.30, 1080–1088. doi: 10.1111/cobi.12716
44
Hogg O. T. Barnes D. K. A. Griffiths H. J. (2011). Highly diverse, poorly studied and uniquely threatened by climate change: an assessment of marine biodiversity on South Georgia’s continental shelf. PloS One6, e19795. doi: 10.1371/journal.pone.0019795
45
Hooper D. U. Chapin F. S. III Ewel J. J. Hector A. Inchausti P. Lavorel S. et al . (2005). Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr.75, 3–35. doi: 10.1890/04-0922
46
Hsieh T. C. Ma K. H. Chao A. (2016). iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol.7, 1451–1456. doi: 10.1111/2041-210X.12613
47
Jones C. G. Lawton J. H. Shachak M. (1994). “ Organisms as Ecosystem Engineers,” in Ecosystem Management ( Springer, New York), 130–147.
48
Jossart Q. Bauman D. Moreau C. V. Saucède T. Christiansen H. Brasier M. J. et al . (2023). A pioneer morphological and genetic study of the intertidal fauna of the Gerlache Strait (Antarctic Peninsula). Environ. Monit. Assess.195, 514. doi: 10.1007/s10661-023-11066-3
49
Jossart Q. Lelièvre Y. Kelch A. Figuerola B. Moreau C. V. Di Franco D. et al . (2024). A first glimpse into the biogeographic affinities of the shallow benthic communities from the sub-Antarctic Crozet archipelago. Front. Ecol. Evol.12. doi: 10.3389/fevo.2024.1455329
50
Jost L. (2006). Entropy and diversity. Oikos113, 363–375. doi: 10.1111/j.2006.0030-1299.14714.x
51
Kearse M. Moir R. Wilson A. Stones-Havas S. Cheung M. Sturrock S. et al . (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics28, 1647–1649. doi: 10.1093/bioinformatics/bts199
52
Layton K. K. S. Corstorphine E. A. Hebert P. D. N. (2016). Exploring canadian echinoderm diversity through DNA barcodes. PloS One11, 1–16. doi: 10.1371/journal.pone.0166118
53
Leclerc J. C. Riera P. Leroux C. Lévêque L. Davoult D. (2013). Temporal variation in organic matter supply in kelp forests: linking structure to trophic functioning. Mar. Ecol. Prog. Ser.494, 87–105. doi: 10.3354/meps10564
54
Legendre P. Gallagher E. D. (2001). Ecologically meaningful transformations for ordination of species data. Oecologia129, 271–280. doi: 10.1007/s004420100716
55
Lelièvre Y. Gall L. Dubois P. Saucède T. (2025a). Characterization of coastal habitats and marine benthic communities of the sub-Antarctic Crozet archipelago using underwater imagery. Antarct. Sci.37, 134–153. doi: 10.1017/S0954102024000543
56
Lelièvre Y. Motreuil S. Specq L. Marschal C. Dubois P. Wauters L. et al . (2024a). MEDUSA: Marine benthic Ecological Data from Underwater imagery Surveys of sub-Antarctic Crozet environments. dataUBFC. doi: 10.25666/DATAUBFC-2024-03-15
57
Lelièvre Y. Motreuil S. Specq L. Marschal C. Dubois P. Wauters L. et al . (2024b). MEDUSA: Marine benthic Ecological Data from Underwater imagery Surveys of sub-Antarctic Crozet environments. Sci. Data11, 613. doi: 10.1038/s41597-024-03460-4
58
Lelièvre Y. Saucède T. Pohl B. Hourdez S. (2025b). You can’t protect what you don’t know: Strategies for biodiversity inventories in poorly known marine areas facing global changes. Adv. Mar. Biol.101, 79–104. doi: 10.1016/bs.amb.2025.06.001
59
Lelièvre Y. Specq L. Lamy T. Boyé A. Downey R. V. Saucède T. (2023). Taxonomic and functional diversity of subtidal benthic communities associated with hard substrates at Crozet archipelago (sub-Antarctic, Southern Ocean). Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1291038
60
Levin L. A. Sibuet M. Gooday A. J. Smith C. R. Vanreusel A. (2010). The roles of habitat heterogeneity in generating and maintaining biodiversity on continental margins: an introduction. Mar. Ecol.31, 1–5. doi: 10.1111/j.1439-0485.2009.00358.x
61
Linse K. Griffiths H. J. Barnes D. K. A. Clarke A. (2006). Biodiversity and biogeography of Antarctic and sub-Antarctic mollusca. Deep. Res. Part II Top. Stud. Oceanogr.53, 985–1008. doi: 10.1016/j.dsr2.2006.05.003
62
Marin-Diaz B. Bouma T. J. Infantes E. (2020). Role of eelgrass on bed-load transport and sediment resuspension under oscillatory flow. Limnol. Oceanogr.65, 426–436. doi: 10.1002/lno.11312
63
Mélice J. L. Lutjeharms J. R. E. Rouault M. Ansorge I. J. (2003). Sea-surface temperatures at the sub-Antarctic islands Marion and Gough during the past 50 years. S. Afr. J. Sci.99, 363–366.
64
Miller R. J. Etter R. J. (2011). Rock walls: small-scale diversity hotspots in the subtidal Gulf of Maine. Mar. Ecol. Prog. Ser.425, 153–165. doi: 10.3354/meps09025
65
Miller R. J. Lafferty K. D. Lamy T. Kui L. Rassweiler A. Reed D. C. (2018). Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc. R. Soc B Biol. Sci.285, 20172571. doi: 10.1098/rspb.2017.2571
66
Morley S. A. Abele D. Barnes D. K. A. Cárdenas C. A. Cotté C. Gutt J. et al . (2020). Global drivers on Southern Ocean ecosystems: changing physical environments and anthropogenic pressures in an Earth System. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.547188
67
Mouillot D. Bellwood D. R. Baraloto C. Chave J. Galzin R. Harmelin-Vivien M. et al . (2013). Rare species support vulnerable functions in high-diversity ecosystems. PloS Biol.11, e1001569. doi: 10.1371/journal.pbio.1001569
68
Nel W. Hedding D. W. Rudolph E. M. (2023). The sub-Antarctic islands are increasingly warming in the 21st century. Antarct. Sci.35, 124–126. doi: 10.1017/S0954102023000056
69
Noisette F. Pansch C. Wall M. Wahl M. Hurd C. L. (2022). Role of hydrodynamics in shaping chemical habitats and modulating the responses of coastal benthic systems to ocean global change. Glob. Change Biol.28, 3812–3829. doi: 10.1111/gcb.16165
70
Oksanen A. J. Blanchet F. G. Friendly M. Kindt R. Legendre P. Mcglinn D. et al . (2020). Vegan: Community Ecology Package ( R Package. Version 2.6.4).
71
Pineda-Metz S. E. A. Isla E. Gerdes D. (2019). Benthic communities of the filchner region (Weddell sea, Antarctica). Mar. Ecol. Prog. Ser.628, 37–54. doi: 10.3354/meps13093
72
Quartino M. L. Klöser H. Schloss I. R. Wiencke C. (2001). Biomass and associations of benthic marine macroalgae from the inner Potter Cove (King George Island, Antarctica) related to depth and substrate. Polar Biol.24, 349–355. doi: 10.1007/s003000000218
73
Rabaut M. Guilini K. Van Hoey G. Vincx M. Degraer S. (2007). A bio-engineered soft-bottom environment: the impact of Lanice conChilega on the benthic species-specific densities and community structure. Estuar. Coast. Shelf Sci.75, 525–536. doi: 10.1016/j.ecss.2007.05.041
74
Ratnasingham S. Hebert P. D. N. (2007). BOLD: the barcode of life data system (www.barcodinglife.org). Mol. Ecol. Notes7, 355–364. doi: 10.1111/j.1471-8286.2006.01678.x
75
Richardson M. G. (1979). The distribution of Antarctic marine macro-algae related to depth and substrate. Br. Antactic Surv. Bull.49, 1–13.
76
Robinson B. J. O. Barnes D. K. A. Grange L. J. Morley S. A. (2022). The extremes of disturbance reduce functional redundancy: functional trait assessment of the shallow Antarctic benthos. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.797112
77
Rosenfeld S. Aldea C. Mansilla A. Marambio J. Ojeda J. (2015). Richness, systematics, and distribution of molluscs associated with the macroalga Gigartina Skottsbergii in the Strait of Magellan, Chile: A biogeographic affinity study. Zookeys2015, 49–100. doi: 10.3897/zookeys.519.9676
78
Rosenfeld S. Maturana C. S. Gañan M. Cárcamo J. R. Díaz A. Contador T. et al . (2023). Revealing the hidden biodiversity of Antarctic and the Magellanic Sub-Antarctic Ecoregion: A comprehensive study of aquatic invertebrates from the BASE Project. Biodivers. Data J.11, e108566. doi: 10.3897/BDJ.11.e108566
79
Rosman J. H. Denny M. W. Zeller R. B. Monismith S. G. Koseff J. R. (2013). Interaction of waves and currents with kelp forests (Macrocystis pyrifera): Insights from a dynamically scaled laboratory model. Limnol. Oceanogr.58, 790–802. doi: 10.4319/lo.2013.58.3.0790
80
Rosman J. H. Monismith S. G. Denny M. W. Koseff J. R. (2010). Currents and turbulence within a kelp forest (Macrocystis pyrifera): Insights from a dynamically scaled laboratory model. Limnol. Oceanogr.55, 1145–1158. doi: 10.4319/lo.2010.55.3.1145
81
Sahade R. Lagger C. Torre L. Momo F. Monien P. Schloss I. et al . (2015). Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Sci. Adv.1, e1500050. doi: 10.1126/sciadv.1500050
82
Sunnucks P. Hales D. F. (1996). Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol. Biol. Evol.13, 510–524. doi: 10.1093/oxfordjournals.molbev.a025612
83
Teagle H. Hawkins S. J. Moore P. J. Smale D. A. (2017). The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Bio. Ecol.492, 81–98. doi: 10.1016/j.jembe.2017.01.017
84
Van Hoey G. Guilini K. Rabaut M. Vincx M. Degraer S. (2008). Ecological implications of the presence of the tube-building polychaete Lanice conChilega on soft-bottom benthic ecosystems. Mar. Biol.154, 1009–1019. doi: 10.1007/s00227-008-0992-1
85
Witte S. E. Franken O. Temmink R. J. M. Dickson J. de Wit B. Roohi R. et al . (2025). Structural complexity of hard substrates shapes shallow marine benthic communities. Oikos2025, e11080. doi: 10.1002/oik.11080
86
Xavier J. C. Brandt A. Ropert-Coudert Y. Badhe R. Gutt J. Havermans C. et al . (2016). Future challenges in Southern Ocean ecology research. Front. Mar. Sci.3. doi: 10.3389/fmars.2016.00094
87
Zavaleta E. S. Hulvey K. B. (2004). Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science306, 1175–1177. doi: 10.1126/science.1102643
Summary
Keywords
Southern Ocean, shallow water, benthic fauna, community composition, habitat complexity
Citation
Lelièvre Y, Jossart Q, Hourdez S, Verheye M, Kelch A, Di Franco D, Maxwell J, Rosenfeld S, Mackenzie M, Lavesque N, Legrand E, Capa M, San Martín G, Moreau C and Saucède T (2025) Shallow benthic invertebrate communities in relation to substrate types in coastal environments of the sub-Antarctic Crozet archipelago. Front. Mar. Sci. 12:1692217. doi: 10.3389/fmars.2025.1692217
Received
25 August 2025
Accepted
28 October 2025
Published
14 November 2025
Volume
12 - 2025
Edited by
Iñigo Muxika, Technological Center Expert in Marine and Food Innovation (AZTI), Spain
Reviewed by
Luigia Donnarumma, University of Naples Parthenope, Italy
Camila Neder, Millenium Institute Biodiversity of Antarctic and Subantarctic Ecosystems (BASE), Chile
Updates
Copyright
© 2025 Lelièvre, Jossart, Hourdez, Verheye, Kelch, Di Franco, Maxwell, Rosenfeld, Mackenzie, Lavesque, Legrand, Capa, San Martín, Moreau and Saucède.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yann Lelièvre, yann.lelievre10@gmail.com
ORCID: Yann Lelièvre, orcid.org/0000-0003-3508-418X; Quentin Jossart, orcid.org/0000-0002-2280-243X; Stéphane Hourdez, orcid.org/0000-0001-6418-3887; Marie Verheye, orcid.org/0000-0001-8702-9292; Andreas Kelch, orcid.org/0000-0002-8265-4643; Davide Di Franco, orcid.org/0000-0002-4595-839X; Jamie Maxwell, orcid.org/0000-0001-5705-2811; Sebastián Rosenfeld, orcid.org/0000-0002-4363-8018; Melanie Mackenzie, orcid.org/0000-0002-0030-7032; Nicolas Lavesque, orcid.org/0000-0001-5701-2393; Erwann Legrand, orcid.org/0000-0001-5224-5227; María Capa, orcid.org/0000-0002-5063-7961; Guillermo San Martín, orcid.org/0000-0002-8360-6221; Camille Moreau, orcid.org/0000-0002-0981-7442; Thomas Saucède, orcid.org/0000-0001-6056-4447
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.