Abstract
Mitochondrial Quality Control (MQC) is the core mechanism for ensuring mitochondrial quality and maintaining cellular function. Marine algae and their bioactive compounds represent a huge treasure trove of natural medicines. In recent years, research on the regulation of mitochondrial biogenesis, mitochondrial dynamics, mitophagy, and mitochondrial protein balance by marine algae has continuously emerged, and their mechanisms of action have gradually become clearer. Bioactive compounds are the material basis for marine algae to exert this regulatory function. Based on their chemical structures, they can be classified into types such as marine algal polysaccharides, marine algal carotenoids, marine algal proteins, and marine algal peptides. Based on the analysis of the chemical structures of these compounds, we believe that structural differences, including planarity, degree of sulfation, and stereoisomerism, may help explain their regulatory effects on MQC. Furthermore, numerous scholars have demonstrated through preclinical studies, using animal or cell models, that marine algae and their bioactive compounds can improve muscle function, treat tumors, type 2 diabetes mellitus, and nervous system diseases, among other effects, by regulating MQC. Currently, this interdisciplinary field holds significant potential for development. This review primarily incorporates literature published between 2019 and 2025 that is highly relevant to the mechanisms of MQC regulation by marine algae and their bioactive compounds. It analyzes the latest research progress from three dimensions: mechanisms of action, molecular structures, and therapeutic applications. Furthermore, it identifies potential challenges and future research directions in the field, aiming to provide support for future drug development and treatment strategies.
1 Introduction
Mitochondria are the center of cellular energy metabolism and are known as the “energy factory” of cells. In the past century, mitochondrial related research has won the Nobel Prize five times, fully demonstrating its core position in the field of cellular biology (Xu et al., 2024). Mitochondria are multifunctional semi-autonomous organelles capable of energy production, substance synthesis, and metabolic signaling. Their dysfunction mainly leads to the production of excessive reactive oxygen species (ROS) and triggers oxidative stress, resulting in cell damage and death (Kenny and Birsoy, 2024; Li Y. et al., 2024). In addition, mitochondrial dysfunction can trigger inflammatory responses by activating damage associated molecular patterns (DAMPs), inflammasomes, and immune cells involved in inflammation (Xu et al., 2025).
Mitochondrial Quality Control (MQC) is a comprehensive mitochondrial quality surveillance system and an indispensable cell-autonomous mechanism that ensures mitochondrial functional integrity and equilibrium (Zhang H. et al., 2024). This network comprises a set of interrelated mechanisms that coordinate mitochondrial biogenesis, dynamics (fusion/fission), mitophagy, and protein balance. Together, these processes effectively repair or eliminate damaged mitochondria, ensuring the population remains sufficient in quantity and high in quality (Iovine et al., 2021; Ma et al., 2025). MQC can achieve continuous turnover of new and old mitochondria, to maintain an overall balance in mitochondrial quantity and quality, which in turn affects the stability of cell structure and function. At present, scholars generally believe that MQC imbalance plays a critical role in the initiation and progression of aging, type 2 diabetes mellitus (T2DM), Parkinson’s disease (PD), myocardial ischemia, tumor and other diseases. For example, T2DM is often accompanied by pathological mechanisms of decreased mitophagy, increased mitochondrial fission, and decreased fusion (Belosludtsev et al., 2021; Zhou et al., 2024); Secondly, during the aging process, the efficiency of the MQC regulatory pathway gradually decreases, which results in diminished mitochondrial mass and reduced neuromuscular function in the elderly (Marzetti et al., 2016; Picca et al., 2023); In addition, Chen et al.’s study found that in dopaminergic neurons of PD patients, the abundance of key proteins involved in mitophagy and mitochondrial protein balance was significantly reduced (Chen et al., 2023).
Marine algae, as a widely distributed biological group in nature, are not only an important component of marine ecosystems, but also have both edible and medicinal value in human diet. Marine algae is mainly divided into four major categories based on their pigment characteristics, including brown algae (such as Ascophyllum Nodosum, Fucus vesiculosus, and Sargassum filipendula), red algae (such as Porphyra and Gracilaria vermiculophylla), green algae (such as Ulva lactuca Linnaeus and Caulerpa racemosa), and blue-green algae (such as Spirulina platensis). Among them, red algae are the largest group of marine algae with the highest proportion of bioactive compounds, and are also the main source of carrageenan and agar, occupying an important position in the field of biomedicine (Khotimchenko et al., 2020; Carpena et al., 2022). Brown algae, distributed in the global ocean, contain various beneficial components for the human body, such as polysaccharides, polyphenols, and carotenoids. They have medicinal values such as reducing blood sugar and lipid levels, anti-inflammatory, and antioxidant properties (Hwang et al., 2022). Green algae are rich in various proteins, peptides, polysaccharides, and pigments, making them a prolific source of biologically active molecules. However, the discovery of new secondary metabolites in green algae is still relatively limited (Liao et al., 2024). Unlike the other three types of eukaryotic algae, blue-green algae (also known as cyanobacteria) belong to prokaryotes and are bacteria capable of photosynthesis. They are abundant in protein and essential vitamins and are often used as healthy food supplements (Zhong et al., 2024). Moreover, specific species of blue-green algae are capable of generating bioactive constituents, demonstrating considerable promise for drug discovery and development.
Marine algae are a typical source of various natural bioactive compounds, such as polysaccharides, proteins, peptides, polyphenols, fibers, and minerals (Hwang et al., 2022). Multiple biologically active compounds in marine algae have been proven to have good effects in protecting mitochondrial function. For example, fucoidan restores mitochondrial function in COVID-19 patients’ peripheral blood mononuclear cells, while also ameliorating mitochondrial dysfunction in both SH-SY5Y neurons and dopaminergic neurons of rat models (Zueva et al., 2023). Zhang et al.’s cell experiments show that astaxanthin nanoparticles can significantly inhibit oxidative stress-induced ROS generation and mitochondrial depolarization (Zhang X. et al., 2023). Chuang et al. engineered Car-Lec-PPy composite microparticles from carrageenan, calcium peroxide, and polypyrrole, which under near-infrared irradiation generate a photothermal effect elevating local temperature to 47.8°C and subsequently activate mitochondrial biogenesis pathways to markedly enhance mitochondrial activity (Chuang et al., 2025).
According to statistics, there were 229 million people with type 2 diabetes globally in 2021, a number projected to reach 1.31 billion by 2050; in 2023, worldwide new cancer cases stood at 18.5 million, with projections indicating a rise to 30.5 million by 2050 (Ong et al., 2023; Force et al., 2025). Major diseases, including type 2 diabetes and cancer, constitute a leading portion of the global disease burden. Addressing these pressing challenges may hinge on identifying their shared pathological basis. MQC imbalance is a common pathological basis for various major diseases, and finding safe and effective MQC modulators is a topic that requires continuous research. Marine algae, as a rich and relatively underdeveloped source of bioactive compounds, have unique advantages in multi-target and safety aspects, and have enormous research and application potential. Currently, there has been extensive research on the regulation of mitochondrial function by marine algae and their bioactive compounds, with a primary focus on mechanisms such as mitochondrial energy metabolism (e.g., AMPK/ACC signaling pathways and GSH/GSSG ratio), oxidative stress (e.g., Nrf2/HO-1 and Nrf2/ARE signaling pathways), inflammatory responses (e.g., NF-κB/NLRP3 and JAK-STAT3 signaling pathways), and apoptosis (e.g., the Bcl-2/Bax/Cyto C/Caspase-3 pathway) (Zheng et al., 2024; Firdaus et al., 2025; Hou et al., 2025; Lee et al., 2025; Liang et al., 2025; Shangguan et al., 2025; Wu et al., 2025). In comparison, direct studies on MQC remain relatively limited, and there is a need for a systematic review to summarize the existing research at this stage. This study reviews the current mechanisms of action, chemical molecular structures, and therapeutic potential of marine algae and their bioactive substances (including extracts or natural compounds from brown, red, green, and blue-green algal species) in regulating key processes of MQC, and discusses future research directions and challenges.
2 The mechanism of marine algae and their bioactive compounds regulating key links in MQC
Marine algae and their bioactive components, as novel MQC regulators, have demonstrated significant effects on various mechanisms of MQC (Figure 1). Based on existing research findings, we will elucidate the mechanisms by which different types of marine algae and their bioactive compounds regulate MQC, focusing on its key processes.
Figure 1
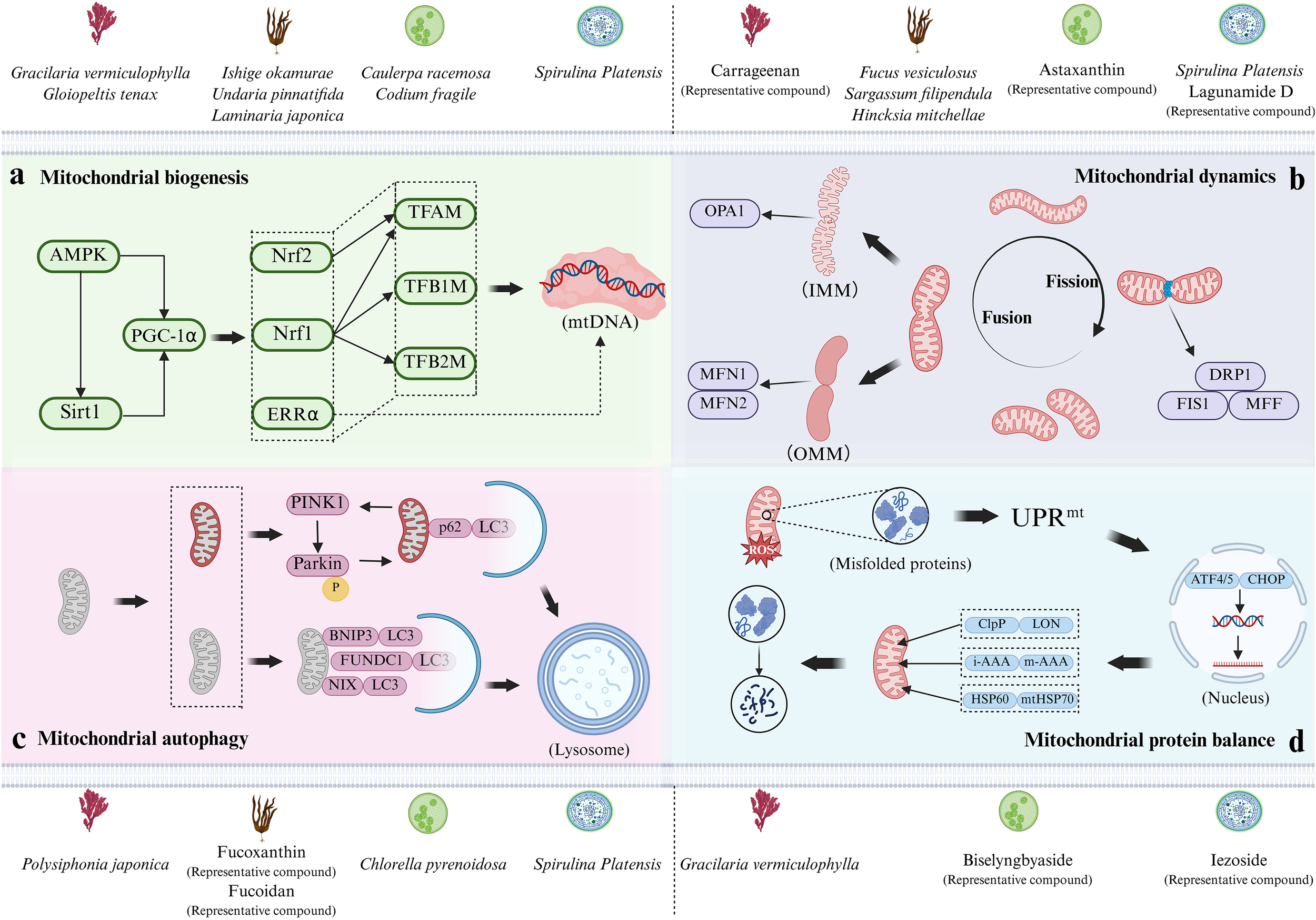
The mechanism by which marine algae and their bioactive compounds regulate key processes of MQC. Based on the classification of red algae, brown algae, green algae, and blue-green algae, marine algae and their bioactive compounds that regulate this mechanism above panels (a-d) are displayed. (a) Mitochondrial biogenesis regulated through the PGC-1α-centered pathway; (b) Mitochondrial dynamics, including the cycle of mitochondrial fusion and fission, as well as the IMM fusion and OMM fusion processes during mitochondrial fusion; (c) Mitophagy pathways (ubiquitin-dependent and ubiquitin-independent); (d) Mitochondrial protein balance, including UPRmt, molecular chaperones, and protease system. Image was created by the authors with BioRender.com.
2.1 Promoting mitochondrial biogenesis
Mitochondrial biogenesis is a process driven by energy demand that enhances productivity by increasing the number and volume of mitochondria (Lou et al., 2024). This highly complex process requires coordinated assembly of over 1100 proteins encoded by both nuclear and mitochondrial genomes; However, since the mitochondrial genome encodes only 37 proteins, the vast majority of mitochondrial proteins are nuclear genome-dependent (Calvo et al., 2016).
The nuclear transcription factors first discovered to be involved in regulating mitochondrial biogenesis are Nuclear Respiratory Factor 1 (Nrf1) and Nuclear Respiratory Factor 2 (Nrf2) (Popov, 2020). The activation of Nrf1 initiates the transcriptional activation of mitochondrial transcription factor A (TFAM), mitochondrial transcription factor B1 (TFB1M), and B2 (TFB2M) genes (Li et al., 2025). The products of these genes are key regulatory factors that directly regulate mitochondrial DNA (mtDNA) transcription, activating mtDNA transcription and maintaining its stability. In most cases, the promoter region of the mitochondrial transcription factor TFAM contains binding sites for both Nuclear Respiratory Factor 1 (NRF-1) and NRF-2. These two factors act synergistically through these adjacent sites to co-activate TFAM transcription, thereby coordinating the expression of the nuclear and mitochondrial genomes during mitochondrial biogenesis (Scarpulla, 2008; Chen et al., 2025). Currently, multiple studies have shown that marine algae and their bioactive compounds can regulate the expression of these key genes and promote mitochondrial biogenesis. For example, the animal experiment results of Ahn et al. show that the mitochondrial volume of gastrocnemius muscle in mice fed with extract of Undaria pinnatifida was larger than that in the control group, and the mRNA and protein expression levels of TFAM and Nrf2 were increased; The cell experiment results show that the Nrf2 protein level in C2C12 cells treated with Undaria pinnatifida extract increased compared to the control group (Ahn et al., 2020). Following a 6-week dietary supplementation with Gracilaria vermiculophylla in Zucker fa/fa obese rats, González Arceo et al. observed upregulated gene expression of Nrf1 and TFAM in the liver, demonstrating that low-dose supplementation of this alga promotes mitochondrial biogenesis (González-Arceo et al., 2024).
Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha (PGC-1α) is a transcription co-regulatory factor that serves as the core of mitochondrial biogenesis. It can translocate to the nucleus, trigger the activation of Nrf1 and Nrf2, and enhance the expression of TFAM and various mitochondrial respiratory chain genes, thereby regulating the transcription and replication of mtDNA and ultimately inducing mitochondrial biogenesis (Ye et al., 2025). The study by Oriquat et al. shows that gavage of Spirulina platensis suspension significantly increased the expression of PGC-1α and TFAM in the liver of T2DM rats induced by high-fat diet combined with low-dose streptozotocin, and increased the copy number of mtDNA, thereby promoting mitochondrial biogenesis (Oriquat et al., 2019). It is worth noting that AMP-activated protein kinase (AMPK) is a key regulatory factor in mitochondrial biogenesis, and its active state can promote the activity of silencing information regulatory factor 1 (SIRT1) and PGC-1α (Dong et al., 2020; Liu et al., 2024). Jing et al. find through cell experiments that fucoidan extracted from Undaria pinnatifida can activate the AMPK/SIRT1/PGC-1α signaling pathway, promoting mitochondrial biogenesis induced by ultraviolet radiation in HaCaT cells (Jing et al., 2021). Wu et al. delved into the protective effect of sulfated fucoidan (SFG) from Laminaria japonica on hydrogen peroxide (H2O2)-induced failure of MIN6 and pancreatic beta cells, and their results show that SFG promotes the activation of the SIRT1/PGC-1α signaling pathway and upregulates the transcription of downstream factors Nrf2 and TFAM (Wu et al., 2022). Peroxisome Proliferator-Activated Receptors (PPARs) are key transcriptional regulators that integrate metabolic signals and control diverse cellular processes by binding to ligands. Their critical role is evidenced in animal models of neurodegenerative diseases, where they potentiate the PGC-1α pathway and stimulate mitochondrial biogenesis (Corona and Duchen, 2016; Jamwal et al., 2021). In addition, although estrogen related receptor alpha (ERR alpha) was initially thought to regulate lipid β-oxidation and estrogen-mediated pathways, subsequent investigations have further revealed its critical role in PGC-1α mediated mitochondrial biogenesis (Zhang W. et al., 2024). Numerous genes encoded by the mitochondrion contain recognition sequences for ERRα, and thus following ERRα knockdown, mitochondrial biogenesis mediated through PGC-1α also decreases (Liu et al., 2023). Kim et al.’s study show that compared to rats provided only with a diet high in fat, sucrose, and cholesterol, rats supplemented with fucoxanthin had significantly increased expression of TFAM, ERRα, and PGC-1α, along with elevated mtDNA content in their soleus muscle tissue (Kim et al., 2022).
In addition to the aforementioned studies, brown algae polyphenols extracted from Ishige okamurae, sulfated polysaccharides extracted from Caulerpa racemosa, and extracts from Codium fragile and Gloiopeltis tenax have also been shown to enhance mitochondrial biogenesis (Ahn et al., 2021; Hyun et al., 2023; Mayulu et al., 2023; Kim et al., 2024).
2.2 Regulating mitochondrial dynamics
Mitochondrial dynamics mainly reshapes its inner membrane (IMM) and outer membrane (OMM) through fusion and fission processes to respond to increased energy demand and promote mitochondrial homogenization (Tábara et al., 2021). Among them, fusion denotes the process of two or more mitochondria merging to form a larger organelle, while fission describes the process by which a mitochondrion constricts and breaks apart to produce smaller organelles (Quintana-Cabrera and Scorrano, 2023). The cycle of fusion and fission is modulated by a suite of mitochondrial morphological regulatory proteins, which are the core of mitochondrial dynamics.
Mitochondrial fusion involves fusing multiple small mitochondria together through Optic Atrophy 1 (OPA1) and Mitofusin gene 1/2 (MFN1/2), among which MFN1 and MFN2 are the main regulatory factors for vertebrate OMM fusion (Tábara et al., 2025). In fruit flies, the orthologous protein of MFN is called “Fzo protein”, while in yeast and Caenorhabditis elegans, the orthologous proteins are Fzo1p and Fzo1, which act as integrated outer membrane proteins mediating outer membrane fusion (Hales and Fuller, 1997; Santel and Fuller, 2001). OPA1 is the main regulatory factor for mitochondrial IMM fusion (Tábara et al., 2025). In vitro studies have shown that OMM fusion occurs before IMM fusion, and this fusion sequence is coordinated by the trans interactions or oligomerization of MFN1, MFN2, and OPA1 (Meeusen et al., 2004). Natural active substances from various sources of marine algae can affect mitochondrial dynamics by modulating expression levels of these key proteins. For example, Wu et al.’s study find that fucoxanthin extracted from dried Hincksia mitchellae can significantly upregulate the transcriptional output of MFN1, MFN2, and OPA1 in white adipose tissue of the groin and epididymis of mice fed a high-fat diet (Wu et al., 2014). At the same time, Huang et al. find that astaxanthin (derived from Haematococcus pluvialis) significantly increased the expression levels of MFN1, MFN2, and OPA1 proteins in APP/PS1 transgenic mice, promoting mitochondrial fusion (Huang et al., 2021).
The executor of mitochondrial fission is a GTPase in the Dynamin Superfamily of Proteins (DSPs), called Dynamin Related Protein 1 (DRP1) (Kraus et al., 2021). Mitochondrial fission is the process of dividing mitochondria into independent daughter mitochondria mediated by DRP1. Once recruited to OMM, DRP1 forms a helical oligomer, inducing membrane constriction and rupture (Wang N. et al., 2025). The absence of DRP1 can trigger the loss of inhibition of mitochondrial fusion, resulting in highly elongated mitochondria (Kamerkar et al., 2018). In addition, in cells, DRP1 requires specific adapter proteins to anchor to OMM (Kamerkar et al., 2025). Therefore, the adapter protein, like DRP1 itself, is an essential component of fission. Mitochondrial anchorage and functional engagement of DRP1 require mediation by fission protein 1 (FIS1) and mitochondrial fission factor (MFF) (Jin et al., 2021). Cell experiments by Gao et al. show that C-phycocyanin from Spirulina platensis improves mitochondrial dynamics in OGD/R-induced H9c2 cells. The mechanism involves upregulating fusion proteins (MFN1, MFN2, OPA1) and downregulating fission proteins (DRP1, FIS1) (Gao et al., 2019). Furthermore, studies show that type II fucoidan from Fucus vesiculosus can ameliorate mitochondrial dysfunction in Parkinson’s disease models. This improvement is achieved by restoring the expression of key mitochondrial function genes (MFN1, MFN2, DRP1, OPA1), which are downregulated in the brains of MPTP-induced mice and in MPP+-treated primary neurons (Xing et al., 2023). It is worth noting that post-translational modifications (including phosphorylation, ubiquitination, S-nitrosylation, etc.) can occur individually or simultaneously on the DRP1 molecule, thereby achieving tighter regulation of mitochondrial division (Jin et al., 2021). It is currently known that DRP1-Ser616 phosphorylation enhances DRP1 function, driving excessive mitochondrial fission; Phosphorylation at Ser637 site inhibits division (Ko et al., 2021).
In addition, relevant studies have demonstrated that fucoidans derived from Sargassum filipendula, carrageenan derived from red algae, and lagunamide D (a cyclic peptide compound) isolated from blue-green algae also have the ability to regulate mitochondrial dynamics (Gupta et al., 2020; Luo et al., 2023; Wu et al., 2023).
2.3 Regulating mitophagy
Mitophagy is an evolutionarily conserved selective autophagy process that eliminates damaged mitochondrial proteins or parts of the mitochondrial network through autophagosomes to regulate mitochondrial quantity and optimize mitochondrial quality. Damaged mitochondria recruit autophagosomes through ubiquitin-dependent or ubiquitin-independent pathways to engulf and degrade themselves.
Proteins related to the ubiquitin-dependent pathway include PTEN-induced kinase 1 (PINK1) and E3 ubiquitin ligase (Parkin) (Zhao et al., 2024). The accumulation of PINK1 on depolarized mitochondria initiates a self-reinforcing cycle by phosphorylating Parkin and ubiquitin at Ser65, which activates Parkin, triggers its cytosolic translocation, and induces extensive ubiquitination of OMM substrates, thereby further promoting PINK1 activity (Heo et al., 2015; Schubert et al., 2017; Yi et al., 2024; Clague and Urbé, 2025). After Parkin is maximally activated, with the help of autophagy-associated scaffolding proteins, mitochondria are enveloped by autophagosomes alongside microtubule-binding protein 1 light chain 3 (LC3) (Nguyen et al., 2016). In addition, while its absence fails to modulate the induction of the PINK1/Parkin pathway, the autophagy receptor p62/SQSTM1—which selectively bridges ubiquitinated mitochondrial proteins to autophagosomes—is nevertheless essential for mitochondrial elimination (Yamada et al., 2019; Wang et al., 2020). Some studies have confirmed that natural bioactive substances derived from marine algae can promote mitophagy. For example, Zhang et al. find that fucoxanthin promoted mitophagy in endothelial cells after traumatic brain injury (TBI) by upregulating the expression of PINK1, Parkin, and LC3 proteins. They also find that the mitophagy inhibitor, mitochondrial fission inhibitor-1 (Mdivi-1), can partially reverse the cytoprotective effect of fucoxanthin on the blood-brain barrier after TBI (Zhang L. et al., 2023). At the same time, studies have found that after cisplatin induced HK-2 cell damage, the expression level of PINK1/Parkin increases. However, pre-treatment with fucoidan/proanthocyanidins nanoparticles can further increase the gene expression of PINK1/Parkin, enhance mitophagy, clear damaged mitochondria, and reduce mitochondrial DNA (mtDNA) leakage (Gao et al., 2023). In addition, the study by Nawrocka et al. shows that metabolic stress can damage mitochondria, triggering excessive activation of the PINK1/Parkin pathway and exacerbating mitochondrial network imbalance. Conversely, Spirulina platensis extract counteracts this by reducing PINK1/Parkin mRNA expression, thereby inhibiting the pathway’s excessive activation, protecting mitochondrial function, and ultimately alleviating cellular damage (Nawrocka et al., 2017).
Certain mitochondrial receptor proteins are capable of direct interaction with LC3 via their intrinsic LC3 interaction region (LIR), initiating mitophagy without Parkin mediated ubiquitination. The related proteins of this ubiquitin-independent pathway include Bcl-2 interacting protein 3 (BNIP3), NIP3 like protein X (NIX), and FUN14 domain containing protein 1 (FUNDC1) (Yuan et al., 2017; Li et al., 2021; Onishi et al., 2021; Liao et al., 2025). BNIP3 and NIX have certain homology in mammals and fall into a subfamily of the Bcl2 protein family. The C-terminus of BNIP3 is anchored to OMM by means of a transmembrane domain, while the N-terminus is the main region responsible for promoting mitophagy (Chinnadurai et al., 2008). The structure of NIX is highly similar to BNIP3, and Phosphorylation at Ser34/Ser35, residues flanking the LIR domain, drives high-affinity LC3 engagement (Rogov et al., 2017). In oxygen-deprived environments, the Ser81 site of NIX is phosphorylated by hypoxia-inducible factor-1 α (HIF-1α), thereby promoting its molecular interaction with LC3 (Yuan et al., 2017). Finally, FUNDC1 is specifically localized in OMM, mediating hypoxia-stress-induced mitophagy in mammals. The promotion of mitophagy is mediated through dual phosphorylation at Ser13 and Tyr18 adjacent to the LIR domain (Lv et al., 2017; Liu H. et al., 2022). The study by Yue et al. revealed that in a dexamethasone-induced mouse skeletal muscle atrophy model, the key mitophagy-related proteins BNIP3 and the LC3B-II/I ratio were significantly upregulated, while astaxanthin supplementation dose-dependently reduced the expression of both. The study identified BNIP3 as a pivotal mediator of dexamethasone-induced adverse effects and demonstrated that astaxanthin protects mitochondrial function by inhibiting the mitophagy pathway, including BNIP3 (Yue et al., 2025). It is important to emphasize that BNIP3, NIX, and FUNDC1 constitute a functionally synergistic and complementary network in regulating mitophagy. For instance, research by Lampert et al. found that the transcriptional levels of BNIP3 and NIX are co-upregulated, while FUNDC1 is also specifically activated in this process. Functionally, they exhibit significant interrelation: when any single receptor is individually inhibited, the others demonstrate compensatory upregulation to maintain pathway function. However, mitophagy induced by differentiation is markedly blocked only when both BNIP3L/NIX and FUNDC1 are simultaneously inhibited, which strongly demonstrates that they work synergistically and are indispensable in executing this programmed process (Palikaras et al., 2018; Lampert et al., 2019).
At present, there is still significant research opportunities in the field of regulating mitophagy by marine algae and their bioactive compounds. While most studies on marine algae and their bioactive compounds have focused on the PINK1/Parkin-mediated ubiquitin-dependent pathway, researchers have also actively explored the ubiquitin-independent pathways mediated by BNIP3, NIX, and FUNDC1. In an enlightening development, a recent study using primary grass carp hepatocytes has demonstrated that cyanobacterial-derived Microcystin-LR (MC-LR) induces ultrastructural alterations in hepatocytes, manifesting as mitochondrial membrane rupture, vacuolization, and mitophagy. The underlying toxicity mechanism involves significant suppression of genes related to mitochondrial biogenesis and dynamics. Although the classical PINK1/Parkin pathway remains unaffected, transmission electron microscopy morphological evidence indicates that MC-LR may activate non-canonical pathways of mitophagy, ultimately leading to cell death (He et al., 2025).
2.4 Maintaining mitochondrial protein balance
Given the dual genome (nuclear genome and mitochondrial genome) co-coding characteristics of mitochondrial proteins, maintaining a homeostatic equilibrium between mitochondrial protein expression and degradation is crucial for monitoring mitochondrial function and rapidly responding to mitochondrial stress (Song et al., 2021). The accumulation of excessive unfolded or misfolded proteins in cells can impair cellular function, while molecular chaperones, proteases, and mitochondrial unfolded protein response (UPRmt) can alleviate this effect (Nouri et al., 2020). Among them, UPRmt is a reverse signal transduction cascade. UPRmt is a protective transcriptional program activated by proteotoxic stress. It employs retrograde signaling through key factors to induce nuclear-encoded mitochondrial proteostasis factors—such as chaperones and proteases—thereby restoring organellar function (Liu J. et al., 2022). Appropriate levels of mitochondrial reactive oxygen species (mtROS) are required for UPRmt activation. However, when the stress level exceeds the protective capacity of the system of UPRmt, misfolded proteins will accumulate excessively and affect operation of the respiratory chain complexes, exacerbating oxidative stress and inducing mitophagy (Zhou et al., 2022; Sutandy et al., 2023). In mammalian systems, the classic UPRmt cascade is modulated by activating transcription factor 4/5 (ATF4/5) and CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), which drives the transcriptional upregulation of mitoprotective genes such as mitochondrial chaperones and proteases (Jadiya and Tomar, 2020). The molecular chaperone proteins associated with human mitochondrial protein balance are mainly heat shock protein (HSP) 60 and mitochondrial heat shock protein (mtHSP) 70, which facilitate the proper folding of proteins and safeguard against harmful aggregation (Anderson and Haynes, 2020). Proteases, mainly including ClpP/LON proteases in the matrix and m-AAA/i-AAA proteases on the membrane, can clear impaired or malformed proteins, maintain the formation and activity of key parts including the electron transport chain and inner membrane cristae (Song et al., 2021). Of these, ClpP maintains matrix proteostasis by regulating the turnover of the RNA chaperone ERAL1, thereby controlling mitochondrial ribosome assembly and translation. Meanwhile, LON, a core matrix protease, fine-tunes mitochondrial genome expression by degrading TFAM that is not bound to mtDNA. Furthermore, the inner membrane-anchored AAA+ proteases act as central regulators of mitochondrial proteostasis. Notably, the m-AAA protease ensures proper assembly of the mitochondrial calcium uniporter to prevent calcium overload, whereas the i-AAA protease dynamically adjusts mitochondrial protein import capacity (Deshwal et al., 2020; Poveda-Huertes et al., 2020). Previous studies have shown that two natural marine bioactive compounds, iezoside (isolated from blue-green algae) and biselyngbyaside (isolated from green algae), can effectively inhibit SERCA1a, trigger UPRmt, and along with calcium uptake, thereby activating the pro-apoptotic signaling pathway (Luesch et al., 2025). Li et al. demonstrated that phycoerythrin exerts anti-cancer effects by reshaping the HSP network in SW480 cells. The treatment not only downregulated mtHSP70 to impair cellular stress protection, but also upregulated HSP60, consequently inducing cell cycle arrest and apoptosis (Li et al., 2016).
Currently, mitochondrial protein balance and the specific mechanisms involving its substrate proteins have garnered widespread attention, presenting significant research opportunities (Liu et al., 2024). Promoting future studies on the regulation of mitochondrial protein balance by marine algae and their bioactive compounds may contribute to the advancement of related therapies.
3 Structure-activity relationships of marine algal compounds in modulating MQC
Marine algal compounds are characterized by their distinct chemical structures, which are thought to act as unique “identity markers” and allow them to be categorized into groups such as polysaccharides, carotenoids, proteins, and peptides. Studies suggest that these structural features may help elucidate how marine algae and their bioactive compounds regulate MQC. In light of this, we will attempt to explore potential structure-activity relationships from various perspectives, including marine algal polysaccharides, carotenoids, proteins, and peptides. It is hoped that this exploration will contribute to future efforts to interpret the regulatory effects of marine algal compounds on MQC from a structural standpoint. (Figure 2).
Figure 2
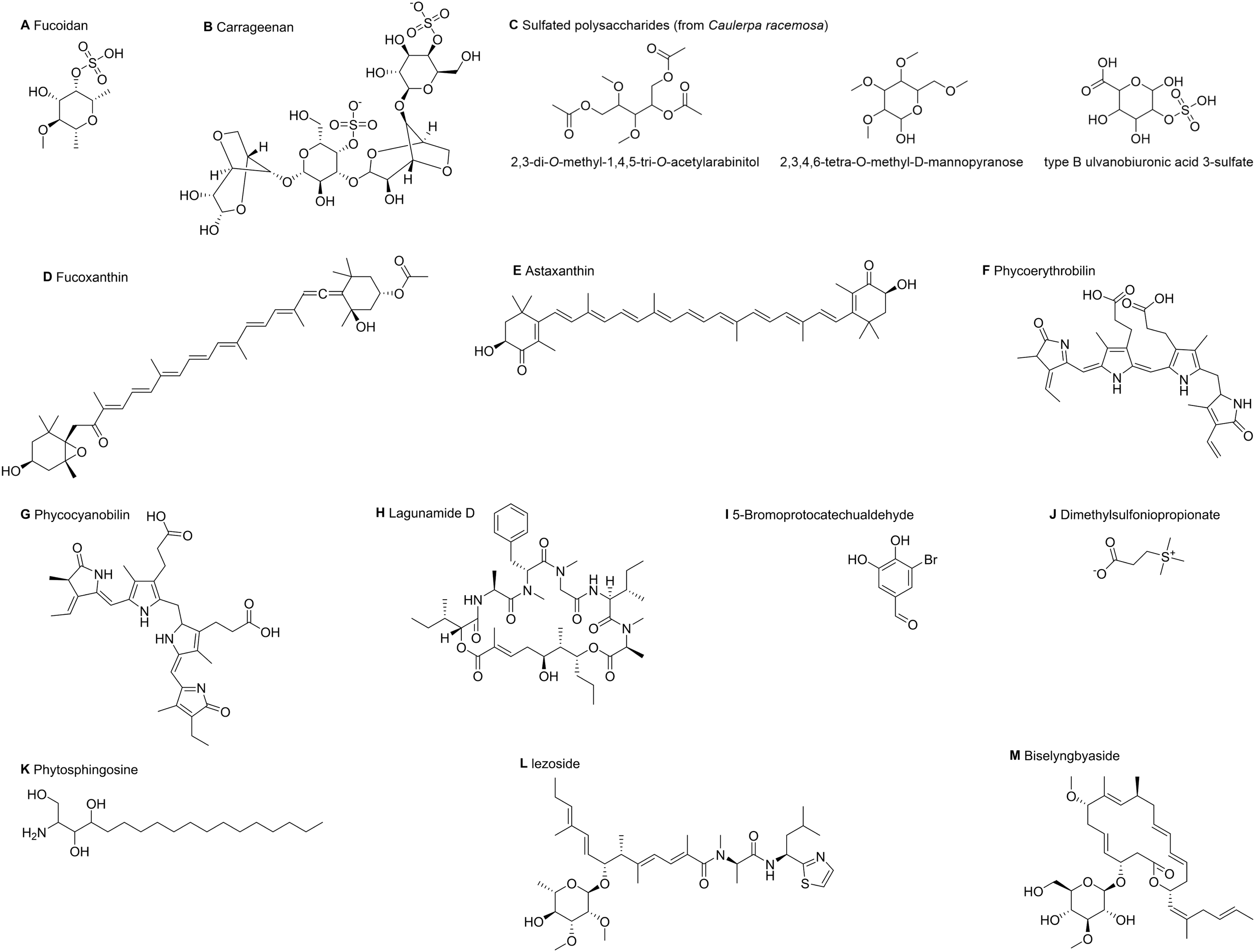
The planar structure of marine algal compounds. This image shows the planar molecular structures of key marine algal compounds in this study. (A) Fucoidan; (B) Carrageenan; (C) Characteristic monosaccharide/oligosaccharide units from the sulfated polysaccharides of Caulerpa racemosa; (D) Fucoxanthin; (E) Astaxanthin; (F) Phycoerythrobilin, the characteristic chromophore of phycoerythrin; (G) Phycocyanobilin, the characteristic chromophore of C-phycocyanin; (H) Lagunamide D; (I) 5-Bromoprotocatechualdehyde; (J) Dimethylsulfoniopropionate; (K) Phytosphingosine; (L) Iezoside; (M) Biselyngbyaside. These structures were obtained from PubChem and visualized using ChemDraw (v23.1.1).
3.1 Marine algal polysaccharides
Marine algal polysaccharides—primarily those derived from brown, red, green, and blue-green algae—are a major class of biologically active constituents. Marine algal polysaccharides usually contain sulfate groups, and sulfation treatment can enhance their biological activity (Zhou and Li, 2024). This enhancement occurs because the introduced sulfate groups (-OSO3-) carry strong negative charges under physiological conditions. Their high charge density enables them to directly mediate biological functions through electrostatic interactions. Concurrently, the incorporation of sulfate groups can alter the three-dimensional conformation and flexibility of polysaccharide chains, which may affect their spatial compatibility with receptors while being capable of significantly enhancing hydrophilicity and solubility. Ultimately, the specific positioning of sulfate groups on the sugar rings can play a decisive role in bioactivity (Li et al., 2018; Sankaranarayanan et al., 2018; Gupta et al., 2020).
Fucoidan (Figure 2A) is a sulfated polysaccharide rich in L-fucose, mainly isolated from the extracellular matrix of brown algae, which are non-toxic and almost non-irritating, and has excellent biocompatibility and biodegradability (Usoltseva et al., 2021). Fucoidan is mainly composed of L-fucose and sulfate groups, and usually has two types of skeletal structures: type I is composed of repeated (1→3) - L-pyranose fucose units; Type II is characterized by alternating reiterative (1→3) - and (1→4) - L-pyranose fucose units (Usoltseva et al., 2019). Of significance is that fucoidans from distinct marine algae sources have varied structural characteristics and molecular weights. Some researchers suggest that low molecular weight polysaccharides exhibit enhanced bioactivity and superior bioavailability compared to their high molecular weight counterparts, though the relationship between molecular weight and biological activity is not yet clear (Li et al., 2018). According to Gupta et al., the cell death mechanisms of fucoidans depend on their structure: whereas the highly sulfated, spherical Fucus vesiculosus fucoidan reduces cellular uptake by causing aggregation and limiting interaction sites, the linear Sargassum filipendula fucoidan increases uptake by exposing more binding sites (Gupta et al., 2020).
Carrageenan (Figure 2B) is a linear sulfated polysaccharide mainly derived from red algae. Its basic structural units are mainly composed of D-galactose and 3,6-dehydrated D-galactose, which are alternately connected by α -1,3 and β -1,4-glycosidic bonds (Jiang et al., 2024). The number and position of sulfate groups (-OSO3-) attached to the sugar ring determine the main types of carrageenan (such as κ-, ι-, and λ-) (Makshakova and Zuev, 2022). In addition, under suitable conditions, carrageenan molecular chains tend to form ordered helical conformations (Pradhan and Ki, 2023). The spiral structure and high degree of sulfation enable carrageenan to be recognized by pattern recognition receptors on the surface of immune cells, triggering intracellular signaling cascades and leading to the release of inflammatory mediators (O’Callaghan et al., 2018). In biomedical research, carrageenan is often used as a tool to induce inflammatory responses. For example, Wu et al. injected carrageenan into the prostate and successfully induced prostate inflammation by upregulating the expression of mitochondrial dynamic markers and other key pathway proteins (Wu et al., 2023).
In addition, the sulfated polysaccharides (Figure 2C) derived from Caulerpa racemosa mentioned earlier are composed of three characteristic monosaccharide/oligosaccharide units, including 2,3-di-O-methyl-1,4,5-tri-O-acetyl arabitol, 2,3,4,6-tetra-O-methyl-D-mannopyranoside, and B-type sulfated glucuronic acid (Pistia and Hollingsworth, 2000). Among them, 2,3-di-O-methyl-1,4,5-tri-O-acetyl arabitol and 2,3,4,6-tetra-O-methyl-D-mannopyranoside promote polysaccharide interactions with cell membranes or enzyme active sites and enhance metabolic regulation. In contrast, B-type sulfated glucuronic acid provides strong negative charges, improving binding to positively charged biomolecules (Mayulu et al., 2023).
3.2 Marine algal carotenoids
Marine algae cells are rich in high value-added natural pigments such as carotenoids, phycobilins, and chlorophyll, which have important application value in the field of biomedicine (Cao et al., 2023). Among them, natural carotenoids have relatively high content in marine algae and have antioxidant, inflammation-inhibiting, and anti-neoplastic effects. The core of a carotenoid molecule is a long polyene chain composed of conjugated double bonds. The atoms within this conjugated system tend to align in the same plane, and this planar configuration is essential for its ability to capture light energy and exert antioxidant functions (Yukihira et al., 2017; Sandhiya and Zipse, 2021; Kulawik et al., 2024).
Fucoxanthin (Figure 2D) is a natural oxygenated carotenoid found in brown algae, which has unique structural features including propadiene bonds, epoxy groups, and acetyl groups (Lau and Kwan, 2022). Among them, the linear arrangement of propadiene bonds can form a molecular curvature of about 110°, disrupting molecular planarity to improve steric accessibility and target protein compatibility; Epoxy groups are located at the molecular end and can increase polarity, consequently enhancing water solubility and transmembrane permeability; Acetyl groups serve as hydrophobic anchors bridging polyene chains, providing hydrophobic anchoring sites to enhance the binding of fucoxanthin to biofilms (Takaichi, 2011). The structure of fucoxanthin, which is conducive to exerting biological activity, may be an important reason for its regulation of multiple MQC pathways. As mentioned earlier, many studies have demonstrated the role of fucoxanthin in regulating mitochondrial biogenesis, mitochondrial dynamics, and mitophagy (Kim et al., 2022; Zhang L. et al., 2023).
Astaxanthin (Figure 2E) is also an oxygenated carotenoid, mainly derived from Haematococcus pluvialis (Pereira et al., 2021). The astaxanthin derived from natural marine algae is mainly in the 3S, 3’S configuration (left-handed), with 11 conjugated double bonds in the center of the molecule, and one ketone and hydroxyl group at each end of the molecule. It also has both hydrophobic (central long chain) and hydrophilic (terminal group) regions (Ambati et al., 2019). The specific left-handed configuration and unique transmembrane amphiphilic structure of marine algae derived astaxanthin endow it with good lipid solubility, biocompatibility, and utilization. For example, in Huang et al.’s study, the 3S, 3’S structure and amphiphilic structure of astaxanthin serve as key physical foundations, enabling it to cross the blood-brain barrier and affect multiple molecular targets such as MFN1, MFN2, and OPA1 proteins in the central nervous system, ultimately exerting neuroprotective effects (Huang et al., 2021).
3.3 Marine algal protein
Phycoerythrin is a type of phycobiliprotein found in red algae. It is a covalent product formed by a thioether bond between an apoprotein and phycoerythrobilin (Figure 2F), and can emit orange fluorescence (Bermejo et al., 2003). Among these, phycoerythrobilin serves as the active center of phycoerythrin. It is a planar tetrapyrrole ring system that facilitates light energy capture and electron transfer while also forming the structural basis for its fluorescence (Dagnino-Leone et al., 2017). Therefore, phycoerythrin has long been used as a photosensitizer or fluorescent probe for disease treatment. Moreover, Li et al.’s study confirmed its anti-cancer effect through regulating mitochondrial protein balance (Li et al., 2016). Similar to phycoerythrin, C-phycocyanin, mainly derived from Spirulina platensis, is an oligomeric protein formed by covalent binding of apoprotein and phycocyanobilin (Figure 2G) through thioether bonds. Phycocyanobilin is also a tetrapyrrole chromophore with a planar structure. The core structural feature of C-phycocyanin is a disc-shaped hexamer structure (Citi et al., 2024). As mentioned earlier, it has been proven to maintain the balance of mitochondrial dynamics by enhancing mitochondrial fusion and restraining mitochondrial fission (Gao et al., 2019).
3.4 Marine algal cyclic peptide
Most marine algal cyclic peptides form stable structures through macrocyclic lactones/peptide scaffolds (Al-Awadhi et al., 2018). Among them, the rigid circular structure can resist enzyme degradation and improve stability, while the hydrophobic cavity formed by specific folding can enhance its binding to the target. As mentioned earlier, the blue-green algae cyclic peptide compound lagunamide D (Figure 2H), which regulates mitochondrial dynamics, naturally exists in the form of a 26 membered macrocyclic peptide. It can be converted into a 24 membered ring (lagunamide D ‘) through acyl migration, but ring contraction significantly reduces its activity (Luo et al., 2019).
3.5 Other compounds derived from marine algae
A polyphenolic compound, 5-Bromoprotocatechualdehyde (BPCA) (Figure 2I), isolated from red algae, is a novel natural monomeric polyphenol that can regulate mitophagy and improve mitochondrial dysfunction. The core of its planar structure is the benzene ring, which includes the aldehyde group at position C1, the ortho dihydroxy group at positions C3 and C4, and the bromine atom at position C5 (Cha et al., 2021). The introduction of bromine atoms confers enhanced hydrophobicity and reactivity to molecules (Wang S. et al., 2025). Similarly, Dimethylsulfoniopropionate (DMSP) (Figure 2J) derived from marine algae is a biogenic sulfur compound that is the primary precursor for the climate-active gas dimethyl sulfide (DMS). It has a zwitterionic structure, including a trimethylthio cation on one side and a propionate anion on the other side, and can also regulate mitophagy (Li H. et al., 2024). In addition, Sphingosine is the basic structural unit of sphingolipids, composed of an 18 carbon long-chain alkyl skeleton, containing hydroxyl groups at C1 and C3 positions and amino groups at C2 position (Gomez-Larrauri et al., 2025). Li et al. use metabolomics technology to detect chemical substances in the exudate of Microcystis aeruginosa and find that phytosphingosine (PHS) (Figure 2K) is one of its most abundant compounds. Its uniqueness lies in the C-4 position of the long chain base of PHS, which has a hydroxyl group. They further demonstrate that PHS promotes apoptosis via mitophagy regulation, suggesting its potential as an anti-cancer agent (Li et al., 2022). Finally, iezoside (Figure 2L) and biselyngbyaside (Figure 2M) mentioned earlier belong to glycosylated macrolides, which are complex molecules with both sugar and ester characteristics (Luesch et al., 2025). Among them, iezoside has one sugar unit and multiple double bonds. The glycosyl moiety is likely implicated in solubility, cellular permeability, as well as interactions with the biological target (Kurisawa et al., 2022; García-Cervantes et al., 2025). Similar to iezoside, biselyngbyaside is an 18 membered macrocyclic lactone, which contains a sugar moiety and multiple double bonds in its structure. Sato et al. demonstrated that protecting the hydroxyl groups on the glycosyl moiety of biselyngbyaside with triethylsilyl groups completely abolished its bioactivity, indicating that both the presence of the sugar unit and the exposure of its free hydroxyls are critical for binding to the biological target (Sato et al., 2017).
4 The role of marine algae and their bioactive compounds in disease models through the regulation of MQC
We attempt to elucidate current research findings and methodologies from the perspective of different diseases, encompassing fundamental studies, clinical research, toxicology, and pharmaceutical approaches. We have also categorized and summarized relevant experimental modeling methods in tabular form, with the aim of providing references and assistance for future research (Table 1).
Table 1
| Marine algae and their bioactive compounds | Experimental model | Target spots | Applications | Reference |
|---|---|---|---|---|
| Improvement effect on muscle function | ||||
| Fucoxanthin (from Undaria pinnatifida) |
Male and female C57BL/6J mice aged 4 weeks | PGC-1α,PPARγ | Enhancing exercise performance | (Yang et al., 2024) |
| Fucoxanthin | C57BL/6J mice were maintained on either a (HFC) diet or a (HFS) diet | PGC-1α,TFAM,ERRα | Improving muscle function | (Kim et al., 2022) |
|
Codium fragile
(extract components) |
19-week-old adult male C57BL/6 mice | Sirt1,PGC-1α,ERRα,PPARδ | Improving exercise endurance | (Ahn et al., 2021) |
|
Spirulina
(extract components) |
3-week-old male Wistar rats | AMPK,PGC-1α,Nrf1,Nrf2 | Enhancing muscle recovery | (Vignaud et al., 2025) |
| Protective effect on the nervous system | ||||
| Fucoxanthin | Male ICR mice subjected to (CCI); Stretch-induced bEnd.3 cell |
PINK1,Parkin,LC3 | Neuroprotection after TBI | (Zhang L. et al., 2023) |
| Fucoidan (from Fucus vesiculosus) |
MPTP-induced 3-month-old male C57BL/6 mice; MPP+-induced primary midbrain and cortical neurons and SH-SY5Y cells |
MFN1, MFN2, DRP1,OPA1 | Parkinson’s disease | (Xing et al., 2023) |
| Astaxanthin | 8-month-old transgenic mice expressing APP/PS1 | MFN1,MFN2,OPA1 | Alzheimer’s disease | (Huang et al., 2021) |
| The role of anti-tumor therapy | ||||
| Phycoerythrin (from Gracilaria lemaneiformis) |
The human colon cancer SW480 cell line | HSP60,mtHSP70 (75kDa) | Colon Cancer | (Li et al., 2016) |
| Lagunamide D (from encrusting cyanobacterial tufts) |
HCT116 colorectal cancer cells | OPA1 | Colorectal cancer | (Luo et al., 2023) |
| Phytosphingosine | CNE-2 cells | PINK1,Parkin | Potential anti-cancer effects | (Li et al., 2022) |
| Protective effects on T2DM and pancreatic beta cells | ||||
|
Spirulina Platensis
(aqueous suspension) |
Wistar rats induced by HFD combined with low dose STZ | AMPK,PGC-1α, PPAR-α,TFAM |
T2DM | (Oriquat et al., 2019) |
| 5-Bromoprotocatechualdehyde (from Polysiphonia japonica) |
Palmitate-induced rat pancreatic β-cell line—Ins-1 cells | Parkin | β-cell protection | (Cha et al., 2021) |
| Sulfated Fucogalactan (from Laminaria Japonica) |
H2O2-induced MIN6 cells | Sirt1,PGC-1α,Nrf2,TFAM | Improving β-cell function | (Wu et al., 2022) |
| Protective effect on cardiovascular system | ||||
| C-Phycocyanin | OGD/R-induced H9c2 cells | OPA1,MFN1, MFN2,FIS1, DRP1(DLP-1) |
Ischemic Cardiomyocyte Damage | (Gao et al., 2019) |
| Sulfated Polysaccharide (from Caulerpa racemosa) |
3–5 weeks old male rats (Rattus norvegicus) | PGC-1α | Cardiometabolic Syndrome | (Mayulu et al., 2023) |
| Bidirectional effects on the urinary and reproductive systems | ||||
| Fucoidan | Cisplatin-induced HK-2 cells | PINK1,Parkin, LC3,p62 |
Cisplatin-induced acute kidney injury | (Gao et al., 2023) |
| Carrageenan | Male SD rats | DRP1,MFN2 | Inducing inflammation model of prostate and bladder in rats | (Wu et al., 2023) |
| The role of obesity management | ||||
| Fucoxanthin (from Hincksia mitchellae) |
3-week old male C57BL/6J mice fed with high sucrose or high-fat diet | Nrf1,Nrf2,MFN1,MFN2 OPA1,PPARα,PPARγ,ERRα |
Obesity | (Wu et al., 2014) |
|
chlorella vulgaris
(the dried whole-algal biomass powder) |
HFD-induced obese rats |
AMPK,SIRT1,PGC-1α | Obesity | (Sanayei et al., 2022) |
| Other functions | ||||
|
Chlorella sorokiniana
(The dried cells) |
Triiodothyronine-induced 60-day-old male Wistar rats | PGC-1α,Nrf2 | Hepatoprotection | (Napolitano et al., 2020) |
|
Gracilaria vermiculophylla
(the dried whole-algal biomass powder) |
Male,homozygous, obese (fa/fa) Zucker rats | PGC1α, Nrf1,TFAM | Hepatoprotection | (González-Arceo et al., 2024) |
| Fucoidan (from Undaria pinnatifida) |
UV radiation-induced HaCaT and HFF-1 cells | AMPK,Sirt1,PGC-1α | UV-irradiated skin photoaging | (Jing et al., 2021) |
|
Spirulina platensis
(extract components and the dried cells) |
9–14 years old donor horses of both sexes; Intestinal epithelial cells and adipose-derived mesenchymal stromal cells from clinically diagnosed EMS horses |
PINK1,Parkin | EMS | (Nawrocka et al., 2017) |
| Dimethylsulfoniopropionate | C. elegans strains | PINK1 | Anti-aging (in C. elegans) | (Li H. et al., 2024) |
Research on marine algae and their bioactive compounds regulating MQC: experimental models, molecular targets, and therapeutic applications.
HFC, High-Fat High-Sucrose, and High-Cholesterol; HFS, High-Fat and High-Sucrose; CCI, Controlled Cortical Impact; TBI, Traumatic Brain Injury; HFD, High Fat Diet; STZ, Streptozotocin; T2DM, Type 2 Diabetes Mellitus; OGD/R, Oxygen–Glucose Deprivation/Reoxygenation; SD, Sprague–Dawley; UV, Ultraviolet; EMS, Equine Metabolic Syndrome.
4.1 Improvement effect on muscle function
Enhancing muscle function is one of the critical objectives in health care, encompassing the prevention of progressive decline in muscle strength, reduction in muscle mass, and deterioration of physical function (Grima-Terrén et al., 2024). The pathological mechanisms involved are closely associated with MQC. Some studies have demonstrated that marine algae and their bioactive compounds exhibit therapeutic potential in this field by modulating MQC.
Yang et al. find that the fucoidan extract derived from Undaria pinnatifida increased the distance run daily and the mass of muscle by 25.5% and 10.4%. At the mechanistic level, supplementing fucoidan can significantly increase beneficial gut microbiota, improve gut microbiome health, and upregulate mRNA expression levels of mitochondrial biogenesis, thereby enhancing muscle performance (Yang et al., 2024). In a randomized controlled trial involving individuals aged 50–85, Hyun et al. found that daily supplementation with Ishige okamurae (IO) extract at a dose of 500 mg per 60 kg of body weight significantly improved lower limb quadriceps strength in the experimental group compared to the control group after 12 weeks. This improvement was particularly notable in the subgroup under 61 years of age. Additionally, animal studies have demonstrated that Ishige okamurae extract upregulates mitochondrial biomarkers in skeletal muscle and improves grip strength deficits, muscle regeneration-related parameters, and age-related muscle loss in mice (Hyun et al., 2023). Similarly, the study by Kim et al. found that in obese mice, fucoxanthin supplementation had only minor effects on liver and adipose tissue inflammation but significantly boosted mitochondrial biogenesis and mtDNA copy number in the soleus muscle, enhancing its metabolic capacity (Kim et al., 2022). In addition, Codium fragile extract promotes mitochondrial biogenesis and oxidative fiber formation via the PGC-1α pathway, enhancing exercise endurance and muscle mass in mice, thereby conferring protection against sarcopenia (Ahn et al., 2021). At the same time, Spirulina platensis extract has also been demonstrated to stimulate mitochondrial biogenesis related genes in the soleus muscle and extensor digitorum longus muscle, enhance antioxidant defense capabilities, reduce inflammation, and improve muscle recovery speed and endurance (Vignaud et al., 2025).
The aforementioned research provides examples of how marine algae and their bioactive compounds modulate MQC to exert beneficial effects on muscle function. Although most of the evidence comes from in vitro studies and preclinical animal models, the results are encouraging. It should be noted that future studies should further clarify the specific active constituents or prioritize evaluating the effects of isolated compounds.
4.2 Protective effect on the nervous system
Neurological disorders have perennially attracted significant research attention, including PD, Alzheimer’s disease (AD), and cognitive impairment. Existing research has explored the defensive activities of marine algae and their bioactive metabolites on the nervous system from multiple perspectives of MQC.
There are research results indicating that fucoxanthin can improve neurological dysfunction after traumatic brain injury, reduce brain edema, shrink cortical injury volume, and inhibit blood-brain barrier leakage by activating mitophagy (Zhang L. et al., 2023). By regulating the mitochondrial fusion/division balance in MPTP-induced PD mice, Fucus vesiculosus-derived type II fucoidan is found by Xing et al. to significantly improve mitochondrial dysfunction, prevent neuronal apoptosis and dopaminergic neuron loss, and ameliorate motor deficits, positioning it as a promising candidate for PD treatment (Xing et al., 2023). The research report by Huang et al. states that following 21 days of treatment with astaxanthin (10 mg/kg/day), the compound can enhance synaptic plasticity in 8-month-old APP/PS1 transgenic mice, while elevating the levels of proteins involved in mitochondrial fusion and synapse-related proteins, but reducing the levels of proteins involved in mitochondrial fission, consequently improving the learning and memory abilities of these AD model mice (Huang et al., 2021). In addition, studies have utilized computational tools and techniques such as molecular docking and molecular dynamics to screen 1110 marine algal compounds. The lead compound BS052 shows strong binding affinity with PPAR-γ, a key signaling molecule involved in mitochondrial biogenesis, and exhibits superior lipid solubility, indicating its potential to be developed as a novel PPAR-γ agonist, thus playing an important therapeutic role in Alzheimer’s disease (Kotha et al., 2021). Further in vitro and in vivo studies are necessary to confirm its effects.
Although the aforementioned fundamental research evidence can define the roles of algal compounds, further studies are required to confirm their efficacy, safety, and mechanisms in humans. Additionally, the study on the lead compound BS052 demonstrates the inspirational role of bioinformatics technologies such as molecular docking and molecular dynamics in this field of research.
4.3 The role of anti-tumor therapy
Malignant tumors comprise “carcinomas” (originating from epithelial tissue, e.g., colorectal carcinoma, nasopharyngeal carcinoma) and “sarcomas” (originating from mesenchymal tissue, e.g., osteosarcoma), whose pathological mechanisms are closely associated with MQC (Chen et al., 2024; Guo et al., 2024; Arcos et al., 2025). Evidence shows that seaweed and its bioactive compounds exert therapeutic effects against tumors by modulating MQC.
There are studies comparing the anticancer activity of crude fucoidan extracted from both diatoms, such as Fucus vesiculosus and Sargassum filipendula, as well as the effects of low, medium, and high molecular weight components of Sargassum filipendula fucoidan on osteosarcoma cells. The results indicate that fucoidan inhibits osteosarcoma cell proliferation and adhesive plaque formation in a dose-dependent manner by selectively inducing apoptosis or necrosis. This process begins with the disruption of mitochondrial dynamics, leading to fragmentation or swelling, loss of membrane potential, and impaired energy metabolism. Notably, Fucus vesiculosus fucoidan and high molecular weight Sargassum filipendula fucoidan exhibited the strongest effects (Gupta et al., 2020). Li et al.’s research has shown that phycoerythrin can induce programmed cell death and block the cell cycle of SW480 by regulating signaling pathways such as those involving mitochondrial protein balance, which inhibits cell proliferation (Li et al., 2016). Meanwhile, Luo et al.’s study shows that lagunamide D can regulate mitochondrial dynamics, alter mitochondrial function, and trigger downstream cell death, thereby exhibiting significant anti-proliferative activity against HCT116 colorectal cancer cells (Luo et al., 2023). The study by Luesch et al. shows that iezoside isolated from blue-green algae and biselyngbyaside isolated from green algae can effectively inhibit SERCA1a, trigger UPRmt, and induce mitochondrial calcium uptake, which activates the apoptotic signaling pathway and exerts anticancer effects (Luesch et al., 2025). In addition, PHS derived from Microcystis aeruginosa can induce excessive generation of ROS in CNE-2 cells, disrupt mitochondrial structure, and block PINK1/Parkin mediated mitophagy, thereby accelerating mitochondria-mediated endogenous apoptosis (Li et al., 2022). Although the primary objective of this study is to elucidate the ecotoxicity of PHS, its molecular mechanism can be interpreted as a potential anticancer pathway, which may provide valuable insights for future anticancer research.
4.4 Protective effects on T2DM and pancreatic beta cells
T2DM is a widespread metabolic condition resulting from insufficient insulin secretion, which can be absolute or relative, with the core pathology being dysfunction of pancreatic beta cells. Therefore, in addition to blood glucose indicators, relevant studies mainly explore the protective effects of marine algae and their bioactive metabolites on pancreatic β cells.
The study by Oriquat et al. finds that Spirulina platensis at a dose of 750 mg/kg had the best effect on most indicators, activating the liver mitochondrial biogenic pathway, enhancing liver mitochondrial function, and significantly improving glucose and lipid metabolism disorders in T2DM rats. Its efficacy is comparable to metformin and can be used as a complementary therapeutic agent to traditional hypoglycemic drugs (Oriquat et al., 2019). In addition, Cha et al. find that BPCA isolated from Polysiphonia japonica can maintain Parkin protein expression, improve mitochondrial fragmentation and dysfunction, and protect pancreatic beta cells from palmitic acid-induced lipotoxic damage. Interestingly, in vivo using zebrafish, BPCA also shows a protective role against beta cell dysfunction elicited by palmitic acid (Cha et al., 2021). These data offer compelling evidence for BPCA as a candidate drug for future T2DM therapies. Finally, research has explored the protective effect of SFG on H2O2-induced pancreatic beta cell failure. The results indicate that SFG activates key signaling pathways involved in mitochondrial biogenesis, improves mitochondrial dysfunction, promotes pancreatic beta cell proliferation and enhances its function, and alleviates H2O2-induced pancreatic beta cell failure (Wu et al., 2022).
4.5 Protective effect on cardiovascular system
Research has shown that C-phycocyanin significantly improves mitochondrial dynamic imbalance induced by oxygen glucose deprivation/reoxygenation (OGD/R) in H9c2 cells, dose dependently reducing cell apoptosis and decreasing intracellular ROS generation, thereby protecting cardiomyocytes from ischemic injury (Gao et al., 2019). The study by Mayulu et al. demonstrates that sulfated polysaccharides from Caulerpa racemosa can alleviate obesity-induced cardiovascular metabolic syndrome in high-fat diet rats. The treatment promoted mitochondrial biogenesis, regulated gut microbiota, and improved key metabolic parameters, including lipid profile and blood glucose. Additionally, it reduced levels of TNF-α and PRMT-1 while increasing IL-10, SOD activity, and DDAH-II levels (Mayulu et al., 2023). However, its efficacy still needs to be further validated in clinical trials.
The studies above, employing cell-based and animal experimental approaches respectively, have demonstrated that C-phycocyanin and sulfated polysaccharides derived from Caulerpa racemosa protect the cardiovascular system by modulating MQC. While both studies are valuable, standalone cell experiments in preclinical research may suffer from dissociation from complex biological environments, whereas isolated animal studies often face challenges in elucidating precise mechanisms. Integrating these two experimental methodologies could achieve complementary advantages, thereby circumventing these limitations.
4.6 Bidirectional effects on the urinary and reproductive systems
Research shows that fucoidan/proanthocyanidin nanoparticles protect against cisplatin-induced acute kidney injury. They act by activating mitophagy and inhibiting mtDNA release, which in turn reduces the accumulation of blood urea nitrogen and serum creatinine and prevents death in human renal proximal tubular epithelial cells (HK-2) (Gao et al., 2023). In addition to therapeutic effects, natural biological compounds derived from marine algae have been shown to induce inflammatory response models in the prostate and bladder of rats. To establish a prostate and bladder inflammatory model, Wu et al. injected carrageenan into the prostate gland, which induced the upregulation of several markers: mitochondrial dynamics proteins (Drp-1, MFN-2), NLRP3, Substance P, and its receptor component CGRP-RCP (Wu et al., 2023). It should be noted that Zhu et al. treated granulosa cells with 0–1 μM MC-LR for 24 h and observed altered mitochondrial cristae morphology by transmission electron microscopy, accompanied by upregulation of DRP1 expression, indicating disrupted mitochondrial dynamics. Biochemical assays further demonstrated that MC-LR downregulated GLUT1 and GLUT4 expression, thereby inhibiting glucose uptake. The results suggest that MC-LR mediates its toxicity in granulosa cells by inducing mitochondrial fragmentation and impairing glucose metabolism, providing new evidence for elucidating the mechanisms of its female reproductive toxicity (Zhu et al., 2021).
While the studies on carrageenan and MC-LR in this section are not intended to demonstrate therapeutic effects, they may nevertheless provide new research perspectives for us in the areas of experimental model construction and investigations into drug side effects.
4.7 The role of obesity management
Obese patients often exhibit elevated serum glucose and lipid levels, along with reduced antioxidant capacity and downregulation of PGC-1α. A study investigated the effects of Caulerpa lentillifera extract on metabolic parameters in high-fat diet-induced obese rats. Forty male Rattus norvegicus were divided into a control group, a high-fat diet group, and two Caulerpa lentillifera intervention groups (150 and 450 mg/kg). After four weeks of intervention, compared with the high-fat diet group, Caulerpa lentillifera extract significantly reduced blood glucose levels, improved total cholesterol, and both doses showed comparable effects. The 150 mg/kg dose was particularly effective in elevating PGC-1α levels (Manoppo et al., 2022). Fucoxanthin significantly upregulates the expression levels of mitochondrial biogenesis and fusion related mRNA in the white adipose tissue of mice fed with high-fat diet, thereby reducing the accumulation of white adipose tissue and increasing oxygen consumption and carbon dioxide production in mice. This proves that dietary supplementation with fucoxanthin can enhance metabolic rate and reduce adipose tissue quality (Wu et al., 2014). In the animal experiments conducted by Lu et al., TPSP2, a polysaccharide macromolecule containing fucoidan, significantly alleviated high-fat diet induced weight gain, hyperlipidemia, and liver steatosis in mice by enhancing mitophagy mediated by PINK1/Parkin in the ubiquitin-dependent pathway, thereby exerting anti-obesity and obesity-related metabolic disorders (Lu et al., 2025). In terms of clinical research, an 8-week randomized trial involving 46 overweight/obese women demonstrated that the group combining high-intensity interval training (HIIT) with Chlorella vulgaris (CV) supplementation experienced a significant reduction in fat mass, an increasing trend in body water and PGC-1 levels, as well as notable improvements in peak oxygen uptake and Bruce metabolic equivalents. The study confirmed that CV and HIIT exhibit a synergistic effect in enhancing metabolic equivalents (Sanayei et al., 2022). Interestingly, although MC-LR released by cyanobacteria poses threats to the environment and health, the study by Duan et al. revealed that it inhibits adipogenesis in stromal vascular fraction (SVF) cells by depleting ATP, inducing oxidative stress, impairing mitochondrial function, and subsequently activating mitophagy. This finding provides a mechanistic basis for elucidating the role of MC-LR in obesity and related metabolic disorders (Duan et al., 2025). Furthermore, in a 4-week randomized double-blind controlled trial, daily supplementation with 1.68 g per 70 kg of Caulerpa racemosa extract significantly improved multiple metabolic parameters relative to placebo: blood glucose decreased by approximately 29.8%, total cholesterol by 23.7%, and both LDL and triglycerides also showed significant reductions. At the same time, levels of high-density lipoprotein and the key regulatory factor PGC-1α were markedly elevated. The study also noted significant improvements in body weight, waist circumference, and waist-to-hip ratio among the participants, with no adverse effects reported, thereby confirming Caulerpa racemosa as a safe and effective anti-obesity functional food (Permatasari et al., 2022).
4.8 Other functions
In addition to the above-mentioned disease areas, marine algae and their bioactive compounds also exhibit protective effects on the liver and skin by regulating mitochondrial biogenesis mechanisms. In addition, some studies have focused on horses and Caenorhabditis elegans(C. elegans) as research subjects, suggesting the therapeutic promise of marine algae and their bioactive constituents to ameliorate analogous disorders by regulating mitophagy.
Research has shown that dietary supplementation with 1% dry Chlorella sorokiniana can affect the key regulatory factors of thyroid hormone-induced mitochondrial biogenesis, maintain mitochondrial function, prevent the increase of indicators of oxidative damage and foundational metabolic expenditure, and effectively reduce hyperthyroidism-induced liver oxidative damage in hyperthyroidism rats (Napolitano et al., 2020). González Arceo et al. find that dietary supplements of Gracilaria vermiculophylla can reduce the concentration of non esterified fatty acids in the liver, improve oxidative stress and inflammatory parameters, and ultimately exerting a protective effect in the liver by inhibiting de novo fat synthesis and promoting mitochondrial biogenesis in Zucker fa/fa obese rats (González-Arceo et al., 2024). At the same time, Jing et al.’s study demonstrates that fucoidan purified from Undaria pinnatifida can promote mitochondrial biogenesis induced by ultraviolet radiation in HaCaT cells, alleviate mitochondrial dysfunction, inhibit ROS production, and ultimately protect against UV induced skin photoaging (Jing et al., 2021). Concurrently, some studies on other animals may provide potential value for the prevention and treatment of related clinical diseases. For example, studies have found that horses fed Spirulina platensis-supplemented feed experienced weight loss and improved insulin sensitivity. Further research has confirmed that Spirulina platensis extract can regulate mitophagy, thereby reducing mitochondrial dysfunction, cellular oxidative stress, and inflammation, and promoting the morphological and functional recovery of ADSCs and IECs derived from individuals with metabolic syndrome (Nawrocka et al., 2017). The research results support that Spirulina platensis can be a promising method for routine treatment of equine metabolic syndrome. Similarly, DMSP can significantly upregulate the expression of autophagy and mitophagy related genes, reduce mitochondrial content while improving mitochondrial function in aged C. elegans, thereby delaying the physiological aging process of C. elegans, demonstrating its potential value in promoting longevity and preventing aging (Li H. et al., 2024).
5 Future directions
In recent years, MQC systems have become an important research field of great concern in academia. At the same time, as marine algae is an important source of natural bioactive substances, its role in regulating MQC requires more extensive and in-depth research. On one hand, significant progress has been made in current research on the improvement of mitochondrial dysfunction by marine algae and their bioactive compounds. Studies focusing on MQC as the core mechanism are gaining increasing attention. Although most evidence derives from in vitro experiments and preclinical animal models, the findings hold considerable significance and value for the advancement of this field. On the other hand, based on existing research, we propose the following recommendations for future studies to address certain issues: (i) Current basic research predominantly employs crude algal extracts, failing to precisely identify the active compounds, which limits the accuracy and depth of related studies. Therefore, we hope future research will focus more on specific compounds or elucidate the effective substances. (ii) Most studies have not thoroughly revealed the precise interaction patterns between algal compounds and key MQC targets. Hence, advanced chemical biology techniques such as Limited Proteolysis Mass Spectrometry (LiP-MS) could be actively adopted in the future to accurately identify the binding targets and sites of algal compounds. (iii) The majority of current studies are preclinical. Although some clinical studies and integrated clinical-basic research have emerged, they remain relatively limited. Thus, future work should further conduct clinical trials to verify the efficacy and safety of these interventions. (iv) Additionally, it is noteworthy that mitochondrial biogenesis, dynamics (fission/fusion), mitophagy, and protein balance do not operate in isolation but function as an integrated network. Therefore, future research should emphasize exploring the crosstalk and coupling relationships among various mitochondrial processes, expanding from studies on marine algae and their bioactive agents regulating individual MQC mechanisms to the broader scope of multiple mitochondrial behaviors.
Marine algae and their bioactive compounds represent a vast medicinal resource, and future efforts should actively promote related clinical or clinical translation research. In this regard, the following points may be worth considering: (i) Future research could prioritize focusing on cardiovascular, endocrine, or kidney diseases, screening promising marine algal bioactive compounds based on preclinical findings, and gradually advancing to small-scale human trials. (ii) For clinical translation, reliable standards for compound purity and structural identification should be established, while scalable cultivation and extraction technologies (e.g., bioreactor-based processes) should be developed to ensure consistent raw material quality and stable supply. Using proteomics and metabolomics, Arora et al. discovered that under mixotrophic conditions, Chlorella synergistically utilizes exogenous glucose and light. This synergistic utilization optimizes carbon and nitrogen metabolism, enhances lipid synthesis and packaging, and improves stress tolerance and redox homeostasis, ultimately yielding higher biomass and lipid production (Arora and Philippidis, 2021). (iii) Integrated omics technologies (such as multi-omics correlation analysis and single-cell sequencing) should be employed to systematically delineate compound-regulated networks across tissues, with priority given to techniques like LiP-MS for high-throughput identification of protein targets and precise binding site mapping. (iv) In terms of model development, it is recommended to construct more predictive models (e.g., organoids and humanized animal models, particularly high-fat diet-induced diabetes or aging models) to enhance clinical relevance. (v) Comprehensive in vivo pharmacokinetic studies must be conducted to establish PK/PD models for clinical dosing guidance. Key parameters include: oral bioavailability, gut microbiota-mediated degradation, blood-brain barrier penetration, metabolite identification, and excretion pathways. (vi) Clinical trials should follow standardized phases: Phase I focusing on safety and pharmacokinetics, Phase II adopting randomized double-blind placebo-controlled designs to explore efficacy and determine dosage, and Phase III confirming effectiveness and safety through large-sample, multicenter trials. Furthermore, nanotechnology offers a viable strategy to significantly enhance the bioavailability of marine algae and their bioactive compounds. Khalil et al. employed ball milling to prepare nano-spirulina particles, which are spherical with a particle size of approximately 68 nm. This process is designed to increase the specific surface area and improve bioavailability. Acute toxicity tests demonstrated exceptionally high safety, laying a crucial safety foundation for subsequent in vivo efficacy studies (Khalil et al., 2025).
6 Conclusions
This review systematically elaborates on the critical role and therapeutic potential of marine algae and their bioactive compounds as novel modulators in MQC. Research indicates that various active components derived from brown algae, red algae, green algae, and blue-green algae (such as fucoidan, fucoxanthin, phycoerythrin, etc.) can regulate the four interconnected core processes of MQC: mitochondrial biogenesis, mitochondrial dynamics, mitophagy, and mitochondrial protein balance. Interestingly, the bioactivity of these compounds is likely associated with their unique molecular structures. Features such as planarity, degree of sulfation, and stereoisomeric configuration collectively form the material basis for their regulatory functions. These findings and insights may provide valuable inspiration for future in-depth research. At the application level, marine algae and their bioactive compounds demonstrate clear potential in various disease models, including improving muscle function, protecting the nervous system, exerting anti-tumor effects, and managing T2DM and obesity. However, certain limitations persist in this field, such as unidentified active constituents, unclear precise interaction patterns with molecular targets, and a lack of clinical translation evidence. These issues potentially constitute core bottlenecks hindering its advancement. Furthermore, this narrative review also has certain limitations: (i) While this review primarily focuses on marine algae, given the high relevance of the chemical structures and mechanisms of action of their key bioactive constituents to the core themes discussed, we have opted to include the two freshwater algal species—Spirulina platensis and Haematococcus pluvialis—with well-defined modulatory effects on MQC, even though this might cause some potential misunderstanding. (ii) Although this review does not present direct structure-activity relationships, the indirect perspectives and evidence compiled remain valuable and also highlight a promising direction for future investigations. (iii) This narrative review carries an inherent risk of potential selection bias. Nonetheless, we believe the objectives of this review have been largely achieved. Therefore, future research should build upon the current foundation to further address these problems. In conclusion, marine algae, as a vast natural medicinal repository, hold great promise in preventing and treating diseases through the regulation of MQC. Realizing this potential and translating it into tangible human health benefits necessitates interdisciplinary integration and rigorous clinical translation research.
Statements
Author contributions
CZ: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Methodology, Supervision, Visualization. LY: Project administration, Resources, Supervision, Writing – review & editing. JL: Conceptualization, Formal analysis, Writing – review & editing. DL: Data curation, Supervision, Writing – review & editing. YD: Investigation, Visualization, Writing – review & editing. YN: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Ningxia Key Research and Development Program, grant number 2023BEG02015; Ningxia Natural Science Foundation, grant number 2022AAC02039; High level Key Discipline Construction Project of State Administration of Traditional Chinese Medicine, grant number 2022-226; Characteristic Discipline Project of Ningxia Medical University (TSXK2025007).
Acknowledgments
We would like to thank the Ningxia Medical University for its support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- AD
Alzheimer’s Disease
- ADSCs
Adipose-Derived Mesenchymal Stromal Cells
- ATF4/5
Activating Transcription Factor 4/5
- AMPK
AMP-Activated Protein Kinase
- BPCA
5-Bromoprotocatechualdehyde
- BNIP3
Bcl-2 Interacting Protein 3
- C/EBP
CCAAT/Enhancer Binding Protein
- CHOP
C/EBP Homologous Protein
- C. elegans
Caenorhabditis Elegans
- CGRP-RCP
Calcitonin Gene-Related Peptide Receptor Component Protein
- CCI
Controlled Cortical Impact
- DMSP
Dimethylsulfoniopropionate
- DAMPs
Damage Associated Molecular Patterns
- DDAH-II
Dimethylarginine Dimethylaminohydrolase II
- DRP1
Dynamin Related Protein 1
- DSPs
Dynamin Superfamily of Proteins
- EMS
Equine Metabolic Syndrome
- ERRα
Estrogen Related Receptor Alpha
- FIS1
Fission Protein 1
- FUNDC1
FUN14 Domain Containing Protein 1
- HIF-1α
Hypoxia-Inducible Factor-1 α
- HSP
Heat Shock Protein
- HFC
High-Fat, High-Sucrose, and High-Cholesterol
- HFD
High Fat Diet
- HFS
High-Fat and High-Sucrose
- IL-10
Interleukin-10
- IECs
Intestinal Epithelial Cells
- IMM
Inner Membrane
- LC3
Microtubule-Binding Protein 1 Light Chain 3
- LiP-MS
Limited Proteolysis Mass Spectrometry
- LIR
LC3 Interaction Region
- Mdivi-1
Mitochondrial fission inhibitor-1
- MC-LR
Microcystin-LR
- MFN1/2
Mitofusin Gene ½
- MQC
Mitochondrial Quality Control
- MFF
Mitochondrial Fission Factor
- MPP+
1-methyl-4-phenylpyridine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mtDNA
Mitochondrial DNA
- mtHSP
Mitochondrial Heat Shock Protein
- mtROS
Mitochondrial Reactive Oxygen Species
- NLRP3
NOD Like Receptor Thermal Domain Associated Protein 3
- Nrf1
Nuclear Respiratory Factor 1
- Nrf2
Nuclear Respiratory Factor 2
- NIX
NIP3 Like Protein X
- OGD/R
Oxygen Glucose Deprivation/Reoxygenation
- OMM
Outer Membrane
- OPA1
Optic Atrophy 1
- Parkin
E3 Ubiquitin Ligase
- PD
Parkinson’s Disease
- PGC-1α
Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha
- PHS
Phytosphingosine
- PINK1
PTEN-Induced Kinase 1
- PPARs
Peroxisome Proliferator-Activated Receptors
- PPAR-γ
Peroxisome Proliferator-Activated Receptor Gamma
- PRMT-1
Protein Arginine Methyltransferase 1
- PK
Pharmacokinetics
- PD
Pharmacodynamics
- ROS
Reactive Oxygen Species
- SOD
Superoxide Dismutase
- SFG
Sulfated Fucoidan
- SIRT1
Silencing Information Regulatory Factor 1
- Substance P
Sensory Neuropeptide Substance P
- STZ
Streptozotocin
- TNF-α
Tumor Necrosis Factor Alpha
- TBI
Traumatic Brain Injury
- T2DM
Type 2 Diabetes Mellitus
- TFAM
Mitochondrial Transcription Factor A
- TFB1M
Mitochondrial Transcription Factor B1
- TFB2M
Mitochondrial Transcription Factor B2
- UPRmt
Mitochondrial Unfolded Protein Response.
References
1
Ahn J. Ha T. Y. Ahn J. Jung C. H. Seo H. D. Kim M. J. et al . (2020). Undaria pinnatifida extract feeding increases exercise endurance and skeletal muscle mass by promoting oxidative muscle remodeling in mice. FASEB J.34, 8068–8081. doi: 10.1096/fj.201902399RR
2
Ahn J. Kim M. J. Yoo A. Ahn J. Ha T. Y. Jung C. H. et al . (2021). Identifying codium fragile extract components and their effects on muscle weight and exercise endurance. Food Chem.353, 129463. doi: 10.1016/j.foodchem.2021.129463
3
Al-Awadhi F. H. Paul V. J. Luesch H. (2018). Structural diversity and anticancer activity of marine-derived elastase inhibitors: key features and mechanisms mediating the antimetastatic effects in invasive breast cancer. Chembiochem19, 815–825. doi: 10.1002/cbic.201700627
4
Ambati R. Gogisetty D. Aswathanarayana R. Ravi S. Bikkina P. Bo L. et al . (2019). Industrial potential of carotenoid pigments from microalgae: current trends and future prospects. Crit. Rev. Food Sci. Nutr.59, 1880–1902. doi: 10.1080/10408398.2018.1432561
5
Anderson N. S. Haynes C. M. (2020). Folding the mitochondrial UPR into the integrated stress response. Trends Cell Biol.30, 428–439. doi: 10.1016/j.tcb.2020.03.001
6
Arcos M. Goodla L. Kim H. Desai S. P. Liu R. Yin K. et al . (2025). PINK1-deficiency facilitates mitochondrial iron accumulation and colon tumorigenesis. Autophagy21, 737–753. doi: 10.1080/15548627.2024.2425594
7
Arora N. Philippidis G. P. (2021). Unraveling metabolic alterations in chlorella vulgaris cultivated on renewable sugars using time resolved multi-omics. Sci. Total. Environ.800, 149504. doi: 10.1016/j.scitotenv.2021.149504
8
Belosludtsev K. N. Starinets V. S. Talanov E. Y. Mikheeva I. B. Dubinin M. V. Belosludtseva N. V. (2021). Alisporivir treatment alleviates mitochondrial dysfunction in the skeletal muscles of C57BL/6NCrl mice with high-fat diet/streptozotocin-induced diabetes mellitus. Int. J. Mol. Sci.22, 9524. doi: 10.3390/ijms22179524
9
Bermejo R. Acién F. G. Ibáñez M. J. Fernández J. M. Molina E. Alvarez-Pez J. M. (2003). Preparative purification of B-phycoerythrin from the microalga porphyridium cruentum by expanded-bed adsorption chromatography. J. Chromatogr. B. Analyt. Technol. BioMed. Life Sci.790, 317–325. doi: 10.1016/s1570-0232(03)00168-5
10
Calvo S. E. Clauser K. R. Mootha V. K. (2016). MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res.44, D1251–D1257. doi: 10.1093/nar/gkv1003
11
Cao K. Cui Y. Sun F. Zhang H. Fan J. Ge B. et al . (2023). Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in microalgae. Biotechnol. Adv.68, 121–129. doi: 10.1016/j.bioteChadv.2023.108236
12
Carpena M. Garcia-Perez P. Garcia-Oliveira P. Chamorro F. Otero P. Lourenço-Lopes C. et al . (2022). Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev.15, 1–32. doi: 10.1007/s11101-022-09826-z
13
Cha S.-H. Zhang C. Heo S.-J. Jun H.-S. (2021). 5-bromoprotocatechualdehyde combats against palmitate toxicity by inhibiting parkin degradation and reducing ROS-induced mitochondrial damage in pancreatic β-cells. Antioxid. (Basel).10, 264. doi: 10.3390/antiox10020264
14
Chen L. Chen D. Pan Y. Mo Y. Lai B. Chen H. et al . (2024). Inhibition of mitochondrial OMA1 ameliorates osteosarcoma tumorigenesis. Cell Death Dis.15, 786. doi: 10.1038/s41419-024-07127-1
15
Chen C. McDonald D. Blain A. Mossman E. Atkin K. Marusich M. F. et al . (2023). Parkinson’s disease neurons exhibit alterations in mitochondrial quality control proteins. NPJ Parkinsons. Dis.9, 120. doi: 10.1038/s41531-023-00564-3
16
Chen W. Zhao Z. Geng Z. Zhang H. Fu X. (2025). Advances in mitochondria-nucleus crosstalk in septic cardiomyopathy. Cell Biol. Toxicol.41, 136. doi: 10.1007/s10565-025-10090-y
17
Chinnadurai G. Vijayalingam S. Gibson S. B. (2008). BNIP3 subfamily BH3-only proteins: mitochondrial stress sensors in normal and pathological functions. Oncogene27 Suppl 1, S114–S127. doi: 10.1038/onc.2009.49
18
Chuang S.-C. Yu S.-A. Hung P.-C. Chuang A. E.-Y. Liang J.-W. Sun C.-L. et al . (2025). Mitochondrial activation via calcium peroxide/carrageenan/soybean lecithin-derived polypyrrole phototherapeutic microparticles. Colloids. Surf. B. Biointerfaces.254, 114787. doi: 10.1016/j.colsurfb.2025.114787
19
Citi V. Torre S. Flori L. Usai L. Aktay N. Dunford N. et al . (2024). Nutraceutical features of the phycobiliprotein C-phycocyanin: evidence from arthrospira platensis (Spirulina). Nutrients16, 1752. doi: 10.3390/nu16111752
20
Clague M. J. Urbé S. (2025). Diverse routes to mitophagy governed by ubiquitylation and mitochondrial import. Trends Cell Biol.35, 527–538. doi: 10.1016/j.tcb.2025.01.003
21
Corona J. C. Duchen M. R. (2016). PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med.100, 153–163. doi: 10.1016/j.freeradbiomed.2016.06.023
22
Dagnino-Leone J. Figueroa M. Mella C. Vorphal M. A. Kerff F. Vásquez A. J. et al . (2017). Structural models of the different trimers present in the core of phycobilisomes from gracilaria Chilensis based on crystal structures and sequences. PloS One12, e0177540. doi: 10.1371/journal.pone.0177540
23
Deshwal S. Fiedler K. U. Langer T. (2020). Mitochondrial proteases: multifaceted regulators of mitochondrial plasticity. Annu. Rev. Biochem.89, 501–528. doi: 10.1146/annurev-biochem-062917-012739
24
Dong Y.-Z. Li L. Espe M. Lu K.-L. Rahimnejad S. (2020). Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem.68, 9377–9386. doi: 10.1021/acs.jafc.0c03310
25
Duan W. Yang T. Liu W. Zhan C. (2025). Microcystin-LR hijacks mitochondrial bioenergetics and wnt crosstalk: unveiling a novel dual-pathway mechanism for adipogenesis dysregulation in environmental toxicology. Comp. Biochem. Physiol. C. Toxicol. Pharmacol.298, 110329. doi: 10.1016/j.cbpc.2025.110329
26
Firdaus M. Astuti R. T. Jatmiko Y. D. Pratiwi H. (2025). Effect of sargassum aquifolium juice on lipid profile and inflammatory cytokines in hypercholesterolemic rats. Int. J. Food Sci.2025, 3668883. doi: 10.1155/ijfo/3668883
27
Force L. M. Kocarnik J. M. May M. L. Bhangdia K. Crist A. Penberthy L. et al . (2025). The global, regional, and national burden of cancer, 1990-2023, with forecasts to 2050: A systematic analysis for the global burden of disease study 2023. Lancet406, 1565–1586. doi: 10.1016/S0140-6736(25)01635-6
28
Gao X. Yin Y. Liu S. Dong K. Wang J. Guo C. (2023). Fucoidan-Proanthocyanidins Nanoparticles Protect against Cisplatin-Induced Acute Kidney Injury by Activating Mitophagy and Inhibiting mtDNA-cGAS/STING Signaling Pathway. Int. J. Biol. Macromol.245, 125541. doi: 10.1016/j.ijbiomac.2023.125541
29
Gao J. Zhao L. Wang J. Zhang L. Zhou D. Qu J. et al . (2019). C-phycocyanin ameliorates mitochondrial fission and fusion dynamics in ischemic cardiomyocyte damage. Front. Pharmacol.10. doi: 10.3389/fphar.2019.00733
30
García-Cervantes A. M. Prates J. A. M. Guil-Guerrero J. L. (2025). Overview of primary and secondary metabolites of rugulopteryx okamurae seaweed: assessing bioactivity, scalability, and molecular mechanisms. Mar. Drugs23, 351. doi: 10.3390/md23090351
31
Gomez-Larrauri A. Larrea-Sebal A. Martín C. Gomez-Muñoz A. (2025). The critical roles of bioactive sphingolipids in inflammation. J. Biol. Chem.301, 110475. doi: 10.1016/j.jbc.2025.110475
32
González-Arceo M. Aguirre L. Macarulla M. T. Gil-Pitarch C. Martínez-Chantar M. L. Portillo M. P. et al . (2024). Effect of gracilaria vermiculophylla macroalga on non-alcoholic fatty liver disease in obese rats. Antioxid. (Basel).13, 369. doi: 10.3390/antiox13030369
33
Grima-Terrén M. Campanario S. Ramírez-Pardo I. Cisneros A. Hong X. Perdiguero E. et al . (2024). Muscle aging and sarcopenia: the pathology, etiology, and most promising therapeutic targets. Mol. Aspects. Med.100, 101319. doi: 10.1016/j.mam.2024.101319
34
Guo S. Xing S. Wu Z. Chen F. Pan X. Li Q. et al . (2024). Leucine restriction ameliorates fusobacterium nucleatum-driven Malignant progression and radioresistance in nasopharyngeal carcinoma. Cell Rep. Med.5, 101753. doi: 10.1016/j.xcrm.2024.101753
35
Gupta D. Silva M. Radziun K. Martinez D. C. Hill C. J. Marshall J. et al . (2020). Fucoidan inhibition of osteosarcoma cells is species and molecular weight dependent. Mar. Drugs18, 104. doi: 10.3390/md18020104
36
Hales K. G. Fuller M. T. (1997). Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell90, 121–129. doi: 10.1016/s0092-8674(00)80319-0
37
He M. Wang H. Fu J. Ruan J. Li F. Liang X. et al . (2025). Oxidative stress and mitochondrial dysfunctions induced by cyanobacterial microcystin-LR in primary grass carp hepatocytes. Aquat. Toxicol.282, 107327. doi: 10.1016/j.aquatox.2025.107327
38
Heo J.-M. Ordureau A. Paulo J. A. Rinehart J. Harper J. W. (2015). The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol. Cell60, 7–20. doi: 10.1016/j.molcel.2015.08.016
39
Hou C. Yu Z. Shi C. Huang Y. Liu H. (2025). Brown algae polysaccharides alleviate diquat-induced oxidative stress in piglets and IPEC-J2 cells via nrf2/ARE signaling pathway. Anim. (Basel).15, 559. doi: 10.3390/ani15040559
40
Huang C. Wen C. Yang M. Li A. Fan C. Gan D. et al . (2021). Astaxanthin improved the cognitive deficits in APP/PS1 transgenic mice via selective activation of mTOR. J. Neuroimmune. Pharmacol.16, 609–619. doi: 10.1007/s11481-020-09953-4
41
Hwang J. Yadav D. Lee P. C. Jin J.-O. (2022). Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res.36, 761–777. doi: 10.1002/ptr.7348
42
Hyun J. Lee S. Y. Ryu B. Jeon Y.-J. (2023). A combination study of pre- and clinical trial: seaweed consumption reduces aging-associated muscle loss! Aging Dis.15, 2813–2827. doi: 10.14336/AD.2023.0927
43
Iovine J. C. Claypool S. M. Alder N. N. (2021). Mitochondrial compartmentalization: emerging themes in structure and function. Trends Biochem. Sci.46, 902–917. doi: 10.1016/j.tibs.2021.06.003
44
Jadiya P. Tomar D. (2020). Mitochondrial protein quality control mechanisms. Genes (Basel).11, 563. doi: 10.3390/genes11050563
45
Jamwal S. Blackburn J. K. Elsworth J. D. (2021). PPARγ/PGC1α Signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther.219, 107705. doi: 10.1016/j.pharmthera.2020.107705
46
Jiang C. Wang W. Sun J. Hao J. Mao X. (2024). Simultaneous one-step preparation of β/κ-carrapentaose and 3,6-anhydro-D-galactose by cascading κ-carrageenase and an exo-α-3,6-anhydro-D-galactosidase. J. Agric. Food Chem.72, 26274–26282. doi: 10.1021/acs.jafc.4c06783
47
Jin J.-Y. Wei X.-X. Zhi X.-L. Wang X.-H. Meng D. (2021). Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin.42, 655–664. doi: 10.1038/s41401-020-00518-y
48
Jing R. Guo K. Zhong Y. Wang L. Zhao J. Gao B. et al . (2021). Protective effects of fucoidan purified from undaria pinnatifida against UV-irradiated skin photoaging. Ann. Transl. Med.9, 1185. doi: 10.21037/atm-21-3668
49
Kamerkar S. C. Kraus F. Sharpe A. J. Pucadyil T. J. Ryan M. T. (2018). Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nat. Commun.9, 5239. doi: 10.1038/s41467-018-07543-w
50
Kamerkar S. C. Liu A. Higgs H. N. (2025). Mitochondrial fission - changing perspectives for future progress. J. Cell Sci.138, jcs263640. doi: 10.1242/jcs.263640
51
Kenny T. C. Birsoy K. (2024). Mitochondria and cancer. Cold Spring Harb. Perspect. Med.14, a041534. doi: 10.1101/cshperspect.a041534
52
Khalil E. M. Rady M. I. Darwish S. F. Abd-Allah E. R. (2025). Nano spirulina platensis countered cisplatin-induced repro-toxicity by reversing the expression of altered steroid hormones and downregulation of the stAR gene. Naunyn Schmiedebergs Arch. Pharmacol.398, 4053–4070. doi: 10.1007/s00210-024-03483-z
53
Khotimchenko M. Tiasto V. Kalitnik A. Begun M. Khotimchenko R. Leonteva E. et al . (2020). Antitumor potential of carrageenans from marine red algae. Carbohydr. Polym.246, 116568. doi: 10.1016/j.carbpol.2020.116568
54
Kim M.-B. Bae M. Lee Y. Kang H. Hu S. Pham T. X. et al . (2022). Consumption of low dose fucoxanthin does not prevent hepatic and adipose inflammation and fibrosis in mouse models of diet-induced obesity. Nutrients14, 2280. doi: 10.3390/nu14112280
55
Kim S.-H. Leem Y.-E. Park H. E. Jeong H.-I. Lee J. Kang J.-S. (2024). The extract of gloiopeltis tenax enhances myogenesis and alleviates dexamethasone-induced muscle atrophy. Int. J. Mol. Sci.25, 6806. doi: 10.3390/ijms25126806
56
Ko H.-J. Tsai C.-Y. Chiou S.-J. Lai Y.-L. Wang C.-H. Cheng J.-T. et al . (2021). The Phosphorylation Status of Drp1-Ser637 by PKA in Mitochondrial Fission Modulates Mitophagy via PINK1/Parkin to Exert Multipolar Spindles Assembly during Mitosis. Biomolecules11, 424. doi: 10.3390/biom11030424
57
Kotha S. B S. Kulkarni V. M. S R. S. B H. K. R H. (2021). An in-silico approach: identification of PPAR-γ Agonists from seaweeds for the management of alzheimer’s disease. J. Biomol. Struct. Dyn.39, 2210–2229. doi: 10.1080/07391102.2020.1747543
58
Kraus F. Roy K. Pucadyil T. J. Ryan M. T. (2021). Function and regulation of the divisome for mitochondrial fission. Nature590, 57–66. doi: 10.1038/s41586-021-03214-x
59
Kulawik A. Cielecka-Piontek J. Czerny B. Kamiński A. Zalewski P. (2024). The relationship between lycopene and metabolic diseases. Nutrients16, 3708. doi: 10.3390/nu16213708
60
Kurisawa N. Iwasaki A. Teranuma K. Dan S. Toyoshima C. Hashimoto M. et al . (2022). Structural determination, total synthesis, and biological activity of iezoside, a highly potent ca2+-ATPase inhibitor from the marine cyanobacterium leptochromothrix valpauliae. J. Am. Chem. Soc.144, 11019–11032. doi: 10.1021/jacs.2c04459
61
Lampert M. A. Orogo A. M. Najor R. H. Hammerling B. C. Leon L. J. Wang B. J. et al . (2019). BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy15, 1182–1198. doi: 10.1080/15548627.2019.1580095
62
Lau T. Kwan H. (2022). Fucoxanthin is a potential therapeutic agent for the treatment of breast cancer. Mar. Drugs20, 370. doi: 10.3390/md20060370
63
Lee S.-S. Lee S.-H. Kim S.-Y. Lee G.-Y. Han S.-Y. Lee B.-H. et al . (2025). Endarachne binghamiae ameliorates hepatic steatosis, obesity, and blood glucose via modulation of metabolic pathways and oxidative stress. Int. J. Mol. Sci.26, 5103. doi: 10.3390/ijms26115103
64
Li Q. Cai C. Chang Y. Zhang F. Linhardt R. J. Xue C. et al . (2018). A novel structural fucosylated chondroitin sulfate from holothuria mexicana and its effects on growth factors binding and anticoagulation. Carbohydr. Polym.181, 1160–1168. doi: 10.1016/j.carbpol.2017.10.100
65
Li H. Ji P. Cao Y. Cui Z. Gao J. Wang H. et al . (2024). Dimethylsulfoniopropionate (DMSP) increases longevity and mitochondrial function in caenorhabditis elegans: implications for the role of the global sulfur cycle in terrestrial ecosystems. Environ. Health (Wash).2, 572–585. doi: 10.1021/envhealth.3c00208
66
Li G. Li J. Shao R. Zhao J. Chen M. (2021). FUNDC1: A promising mitophagy regulator at the mitochondria-associated membrane for cardiovascular diseases. Front. Cell Dev. Biol.9. doi: 10.3389/fcell.2021.788634
67
Li J. Lv M. Yuan Z. Ge J. Geng T. Gong D. et al . (2025). PGC-1α Promotes mitochondrial biosynthesis and energy metabolism of goose fatty liver. Poult. Sci.104, 104617. doi: 10.1016/j.psj.2024.104617
68
Li J. Wen J. Sun C. Zhou Y. Xu J. MacIsaac H. J. et al . (2022). Phytosphingosine-induced cell apoptosis via a mitochondrially mediated pathway. Toxicology482, 153370. doi: 10.1016/j.tox.2022.153370
69
Li P. Ying J. Chang Q. Zhu W. Yang G. Xu T. et al . (2016). Effects of phycoerythrin from gracilaria lemaneiformis in proliferation and apoptosis of SW480 cells. Oncol. Rep.36, 3536–3544. doi: 10.3892/or.2016.5162
70
Li Y. Zhang H. Yu C. Dong X. Yang F. Wang M. et al . (2024). New insights into mitochondria in health and diseases. Int. J. Mol. Sci.25, 9975. doi: 10.3390/ijms25189975
71
Liang F. Zheng Y. Zhao C. Li L. Hu Y. Wang C. et al . (2025). Microalgae-derived extracellular vesicles synergize with herbal hydrogel for energy homeostasis in osteoarthritis treatment. ACS Nano.19, 8040–8057. doi: 10.1021/acsnano.4c16085
72
Liao W. Chen Y. Shan S. Chen Z. Wen Y. Chen W. et al . (2024). Marine algae-derived characterized bioactive compounds as therapy for cancer: A review on their classification, mechanism of action, and future perspectives. Phytother. Res.38, 4053–4080. doi: 10.1002/ptr.8240
73
Liao M. Men L. Gong M. Li Y. Wang Y. Xu D. et al . (2025). Mitophagy: A novel avenue for herbal medicines alleviating myocardial ischemia/reperfusion injury. Apoptosis. 12, 115–127. doi: 10.1007/s10495-025-02178-x
74
Liu J. He X. Zheng S. Zhu A. Wang J. (2022). The mitochondrial unfolded protein response: A novel protective pathway targeting cardiomyocytes. Oxid. Med. Cell Longev.2022, 6430342. doi: 10.1155/2022/6430342
75
Liu L. Li Y. Chen G. Chen Q. (2023). Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. BioMed. Sci.30, 86. doi: 10.1186/s12929-023-00975-7
76
Liu B.-H. Xu C.-Z. Liu Y. Lu Z.-L. Fu T.-L. Li G.-R. et al . (2024). Mitochondrial quality control in human health and disease. Mil. Med. Res.11, 32. doi: 10.1186/s40779-024-00536-5
77
Liu H. Zang C. Yuan F. Ju C. Shang M. Ning J. et al . (2022). The role of FUNDC1 in mitophagy, mitochondrial dynamics and human diseases. Biochem. Pharmacol.197, 114891. doi: 10.1016/j.bcp.2021.114891
78
Lou H. Yao J. Zhang Y. Wu X. Sun L. Wang Y. et al . (2024). Potential effect of acupuncture on mitochondrial biogenesis, energy metabolism and oxidation stress in MCAO rat via PGC-1α/NRF1/TFAM pathway. J. Stroke. Cerebrovasc. Dis.33, 107636. doi: 10.1016/j.jstrokecerebrovasdis.2024.107636
79
Lu J. Zhang Y. Song H. Wang F. Wang L. Xiong L. et al . (2025). A novel polysaccharide from tremella fuciformis alleviated high-fat diet-induced obesity by promoting AMPK/PINK1/PRKN-mediated mitophagy in mice. Mol. Nutr. Food Res.69, e202400699. doi: 10.1002/mnfr.202400699
80
Luesch H. Ellis E. K. Chen Q.-Y. Ratnayake R. (2025). Progress in the discovery and development of anticancer agents from marine cyanobacteria. Nat. Prod. Rep.42, 208–256. doi: 10.1039/d4np00019f
81
Luo D. Putra M. Ye T. Paul V. Luesch H. (2019). Isolation, structure elucidation and biological evaluation of lagunamide D: A new cytotoxic macrocyclic depsipeptide from marine cyanobacteria. Mar. Drugs17, 83. doi: 10.3390/md17020083
82
Luo D. Ratnayake R. Atanasova K. R. Paul V. J. Luesch H. (2023). Targeted and functional genomics approaches to the mechanism of action of lagunamide D, a mitochondrial cytotoxin from marine cyanobacteria. Biochem. Pharmacol.213, 115608. doi: 10.1016/j.bcp.2023.115608
83
Lv M. Wang C. Li F. Peng J. Wen B. Gong Q. et al . (2017). Structural insights into the recognition of phosphorylated FUNDC1 by LC3B in mitophagy. Protein Cell8, 25–38. doi: 10.1007/s13238-016-0328-8
84
Ma Y. Song R. Duan C. (2025). Mitochondrial quality control and transfer communication in neurological disorders and neuroinflammation. Front. Immunol.16. doi: 10.3389/fimmu.2025.1542369
85
Makshakova O. N. Zuev Y. F. (2022). Interaction-induced structural transformations in polysaccharide and protein-polysaccharide gels as functional basis for novel soft-matter: A case of carrageenans. Gels8, 287. doi: 10.3390/gels8050287
86
Manoppo J. I. C. Nurkolis F. Pramono A. Ardiaria M. Murbawani E. A. Yusuf M. et al . (2022). Amelioration of obesity-related metabolic disorders via supplementation of caulerpa lentillifera in rats fed with a high-fat and high-cholesterol diet. Front. Nutr.9. doi: 10.3389/fnut.2022.1010867
87
Marzetti E. Calvani R. Lorenzi M. Tanganelli F. Picca A. Bossola M. et al . (2016). Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: results from the sarcopenia in HIp fracTure (SHIFT) exploratory study. Exp. Gerontol.80, 1–5. doi: 10.1016/j.exger.2016.04.003
88
Mayulu N. Gunawan W. B. Park M. N. Chung S. Suh J. Y. Song H. et al . (2023). Sulfated Polysaccharide from Caulerpa Racemosa Attenuates the Obesity-Induced Cardiometabolic Syndrome via Regulating the PRMT1-DDAH-ADMA with mTOR-SIRT1-AMPK Pathways and Gut Microbiota Modulation. Antioxid. (Basel).12, 1555. doi: 10.3390/antiox12081555
89
Meeusen S. McCaffery J. M. Nunnari J. (2004). Mitochondrial fusion intermediates revealed in vitro. Science305, 1747–1752. doi: 10.1126/science.1100612
90
Napolitano G. Fasciolo G. Salbitani G. Venditti P. (2020). Chlorella sorokiniana dietary supplementation increases antioxidant capacities and reduces ros release in mitochondria of hyperthyroid rat liver. Antioxid. (Basel).9, 883. doi: 10.3390/antiox9090883
91
Nawrocka D. Kornicka K. Śmieszek A. Marycz K. (2017). Spirulina platensis improves mitochondrial function impaired by elevated oxidative stress in adipose-derived mesenchymal stromal cells (ASCs) and intestinal epithelial cells (IECs), and enhances insulin sensitivity in equine metabolic syndrome (EMS) horses. Mar. Drugs15, 237. doi: 10.3390/md15080237
92
Nguyen T. N. Padman B. S. Usher J. Oorschot V. Ramm G. Lazarou M. (2016). Atg8 Family LC3/GABARAP Proteins Are Crucial for Autophagosome-Lysosome Fusion but Not Autophagosome Formation during PINK1/Parkin Mitophagy and Starvation. J. Cell Biol.215, 857–874. doi: 10.1083/jcb.201607039
93
Nouri K. Feng Y. Schimmer A. D. (2020). Mitochondrial clpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis.11, 841. doi: 10.1038/s41419-020-03062-z
94
O’Callaghan P. Zhang X. Li J.-P. (2018). Heparan sulfate proteoglycans as relays of neuroinflammation. J. Histochem. Cytochem.66, 305–319. doi: 10.1369/0022155417742147
95
Ong K. L. Stafford L. K. McLaughlin S. A. Boyko E. J. Vollset S. E. Smith A. E. et al . (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet402, 203–234. doi: 10.1016/S0140-6736(23)01301-6
96
Onishi M. Yamano K. Sato M. Matsuda N. Okamoto K. (2021). Molecular mechanisms and physiological functions of mitophagy. EMBO J.40, e104705. doi: 10.15252/embj.2020104705
97
Oriquat G. A. Ali M. A. Mahmoud S. A. Eid R. M. H. M. Hassan R. Kamel M. A. (2019). Improving hepatic mitochondrial biogenesis as a postulated mechanism for the antidiabetic effect of spirulina platensis in comparison with metformin. Appl. Physiol. Nutr. Metab.44, 357–364. doi: 10.1139/apnm-2018-0354
98
Palikaras K. Lionaki E. Tavernarakis N. (2018). Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol.20, 1013–1022. doi: 10.1038/s41556-018-0176-2
99
Pereira A. Otero P. Echave J. Carreira-Casais A. Chamorro F. Collazo N. et al . (2021). Xanthophylls from the sea: algae as source of bioactive carotenoids. Mar. Drugs19, 188. doi: 10.3390/md19040188
100
Permatasari H. K. Nurkolis F. Hardinsyah H. Taslim N. A. Sabrina N. Ibrahim F. M. et al . (2022). Metabolomic assay, computational screening, and pharmacological evaluation of caulerpa racemosa as an anti-obesity with anti-aging by altering lipid profile and peroxisome proliferator-activated receptor-γ Coactivator 1-α Levels. Front. Nutr.9. doi: 10.3389/fnut.2022.939073
101
Picca A. Triolo M. Wohlgemuth S. E. Martenson M. S. Mankowski R. T. Anton S. D. et al . (2023). Relationship between mitochondrial quality control markers, lower extremity tissue composition, and physical performance in physically inactive older adults. Cells12, 183. doi: 10.3390/cells12010183
102
Pistia G. Hollingsworth R. I. (2000). A general approach to the synthesis of dideoxy and trideoxyiminoalditols from beta-D-glycosides. Carbohydr. Res.328, 467–472. doi: 10.1016/s0008-6215(00)00124-5
103
Popov L. (2020). Mitochondrial biogenesis: an update. J. Cell Mol. Med.24, 4892–4899. doi: 10.1111/jcmm.15194
104
Poveda-Huertes D. Matic S. Marada A. Habernig L. Licheva M. Myketin L. et al . (2020). An Early mtUPR: Redistribution of the Nuclear Transcription Factor Rox1 to Mitochondria Protects against Intramitochondrial Proteotoxic Aggregates. Mol. Cell77, 180–188.e9. doi: 10.1016/j.molcel.2019.09.026
105
Pradhan B. Ki J.-S. (2023). Biological activity of algal derived carrageenan: A comprehensive review in light of human health and disease. Int. J. Biol. Macromol.238, 124085. doi: 10.1016/j.ijbiomac.2023.124085
106
Quintana-Cabrera R. Scorrano L. (2023). Determinants and outcomes of mitochondrial dynamics. Mol. Cell83, 857–876. doi: 10.1016/j.molcel.2023.02.012
107
Rogov V. V. Suzuki H. Marinković M. Lang V. Kato R. Kawasaki M. et al . (2017). Phosphorylation of the mitochondrial autophagy receptor nix enhances its interaction with LC3 proteins. Sci. Rep.7, 1131. doi: 10.1038/s41598-017-01258-6
108
Sanayei M. Hajizadeh-Sharafabad F. Amirsasan R. Barzegar A. (2022). High-intensity interval training with or without chlorella vulgaris supplementation in obese and overweight women: effects on mitochondrial biogenesis, performance and body composition. Br. J. Nutr.128, 200–210. doi: 10.1017/S0007114521003287
109
Sandhiya L. Zipse H. (2021). Conformation-dependent antioxidant properties of β-carotene. Org. Biomol. Chem.20, 152–162. doi: 10.1039/d1ob01723c
110
Sankaranarayanan N. V. Nagarajan B. Desai U. R. (2018). So you think computational approaches to understanding glycosaminoglycan-protein interactions are too dry and too rigid? Think again! Curr. Opin. Struct. Biol.50, 91–100. doi: 10.1016/j.sbi.2017.12.004
111
Santel A. Fuller M. T. (2001). Control of mitochondrial morphology by a human mitofusin. J. Cell Sci.114, 867–874. doi: 10.1242/jcs.114.5.867
112
Sato E. Sato M. Tanabe Y. Nakajima N. Ohkubo A. Suenaga K. (2017). Total synthesis of biselyngbyaside. J. Org. Chem.82, 6770–6777. doi: 10.1021/acs.joc.7b00905
113
Scarpulla R. (2008). Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev.88, 611–638. doi: 10.1152/physrev.00025.2007
114
Schubert A. F. Gladkova C. Pardon E. Wagstaff J. L. Freund S. M. V. Steyaert J. et al . (2017). Structure of PINK1 in complex with its substrate ubiquitin. Nature552, 51–56. doi: 10.1038/nature24645
115
Shangguan F. Ma N. Chen Y. Zheng Y. Ma T. An J. et al . (2025). Fucoxanthin suppresses pancreatic cancer progression by inducing bioenergetics metabolism crisis and promoting SLC31A1−mediated sensitivity to DDP. Int. J. Oncol.66, 31. doi: 10.3892/ijo.2025.5737
116
Song J. Herrmann J. M. Becker T. (2021). Quality control of the mitochondrial proteome. Nat. Rev. Mol. Cell Biol.22, 54–70. doi: 10.1038/s41580-020-00300-2
117
Sutandy F. X. R. Gößner I. Tascher G. Münch C. (2023). A cytosolic surveillance mechanism activates the mitochondrial UPR. Nature618, 849–854. doi: 10.1038/s41586-023-06142-0
118
Tábara L.-C. Morris J. L. Prudent J. (2021). The complex dance of organelles during mitochondrial division. Trends Cell Biol.31, 241–253. doi: 10.1016/j.tcb.2020.12.005
119
Tábara L.-C. Segawa M. Prudent J. (2025). Molecular mechanisms of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol.26, 123–146. doi: 10.1038/s41580-024-00785-1
120
Takaichi S. (2011). Carotenoids in algae: distributions, biosyntheses and functions. Mar. Drugs9, 1101–1118. doi: 10.3390/md9061101
121
Usoltseva R. V. Malyarenko O. S. Anastyuk S. D. Shevchenko N. M. Silchenko A. S. Zvyagintseva T. N. et al . (2021). The structure of fucoidan from sargassum oligocystum and radiosensitizing activity of galactofucans from some algae of genus sargassum. Int. J. Biol. Macromol.183, 1427–1435. doi: 10.1016/j.ijbiomac.2021.05.128
122
Usoltseva R. V. Shevchenko N. M. Malyarenko O. S. Anastyuk S. D. Kasprik A. E. Zvyagintsev N. V. et al . (2019). Fucoidans from brown algae laminaria longipes and saccharina cichorioides: structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydr. Polymers.221, 157–165. doi: 10.1016/j.carbpol.2019.05.079
123
Vignaud J. Loiseau C. Côme M. Martin I. Rasoanarivo R. Hérault J. et al . (2025). Combined effects of spirulina liquid extract and endurance training on aerobic performance and muscle metabolism adaptation in wistar rats. Nutrients17, 283. doi: 10.3390/nu17020283
124
Wang S. Deng Z. Ma Y. Jin J. Qi F. Li S. et al . (2020). The role of autophagy and mitophagy in bone metabolic disorders. Int. J. Biol. Sci.16, 2675–2691. doi: 10.7150/ijbs.46627
125
Wang N. Wang X. Lan B. Gao Y. Cai Y. (2025). DRP1, fission and apoptosis. Cell Death Discov.11, 150. doi: 10.1038/s41420-025-02458-0
126
Wang S. Yuan J. Li Y. Wang W. Zhang H. Wang Q. et al . (2025). Engineering stable prodrug self-assemblies by introducing the bromination effect. J. Control. Release.382, 113699. doi: 10.1016/j.jconrel.2025.113699
127
Wu M.-T. Chou H.-N. Huang C. (2014). Dietary fucoxanthin increases metabolic rate and upregulated mRNA expressions of the PGC-1alpha network, mitochondrial biogenesis and fusion genes in white adipose tissues of mice. Mar. Drugs12, 964–982. doi: 10.3390/md12020964
128
Wu L. Huang D. Wang S. Wang J. Guo Q. Chen X. (2025). Role and mechanism of seaweed polysaccharides in regulating mitochondrial dysfunction: A review. J. Food Sci.90, e70100. doi: 10.1111/1750-3841.70100
129
Wu N. Jin W. Zhao Y. Wang H. He S. Zhang W. et al . (2022). Sulfated fucogalactan from laminaria japonica ameliorates β-cell failure by attenuating mitochondrial dysfunction via SIRT1-PGC1-α Signaling pathway activation. Front. Endocrinol. (Lausanne).13. doi: 10.3389/fendo.2022.881256
130
Wu Z.-S. Wang H.-J. Lee W.-C. Luo H. L. Lin T.-K. Chuang Y.-C. (2023). Low-energy shock wave suppresses prostatic pain and inflammation by modulating mitochondrial dynamics regulators on a carrageenan-induced prostatitis model in rats. Int. J. Mol. Sci.24, 3898. doi: 10.3390/ijms24043898
131
Xing M. Li G. Liu Y. Yang L. Zhang Y. Zhang Y. et al . (2023). Fucoidan from fucus vesiculosus prevents the loss of dopaminergic neurons by alleviating mitochondrial dysfunction through targeting ATP5F1a. Carbohydr. Polymers.303, 120470. doi: 10.1016/j.carbpol.2022.120470
132
Xu X. Pang Y. Fan X. (2025). Mitochondria in oxidative stress, inflammation and aging: from mechanisms to therapeutic advances. Signal Transduct. Target. Ther.10, 190. doi: 10.1038/s41392-025-02253-4
133
Xu K. Saaoud F. Shao Y. Lu Y. Yang Q. Jiang X. et al . (2024). A new paradigm in intracellular immunology: mitochondria emerging as leading immune organelles. Redox Biol.76, 103331. doi: 10.1016/j.redox.2024.103331
134
Yamada T. Dawson T. M. Yanagawa T. Iijima M. Sesaki H. (2019). SQSTM1/P62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy. Autophagy15, 2012–2018. doi: 10.1080/15548627.2019.1643185
135
Yang C. Dwan C. Wimmer B. C. Wilson R. Johnson L. Caruso V. (2024). Fucoidan from undaria pinnatifida enhances exercise performance and increases the abundance of beneficial gut bacteria in mice. Mar. Drugs22, 485. doi: 10.3390/md22110485
136
Ye X. Yang Y. Yao Q. Huang M. Balasubramanian B. Jha R. et al . (2025). The ameliorative role of phlorotannin on aflatoxin B1-induced liver oxidative stress and mitochondrial injury is related to the activation of nrf2 and nrf1 signaling pathways in broilers. J. Anim. Sci. Biotechnol.16, 75. doi: 10.1186/s40104-025-01210-z
137
Yi J. Wang H.-L. Lu G. Zhang H. Wang L. Li Z.-Y. et al . (2024). Spautin-1 promotes PINK1-PRKN-dependent mitophagy and improves associative learning capability in an alzheimer disease animal model. Autophagy20, 2655–2676. doi: 10.1080/15548627.2024.2383145
138
Yuan Y. Zheng Y. Zhang X. Chen Y. Wu X. Wu J. et al . (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy13, 1754–1766. doi: 10.1080/15548627.2017.1357792
139
Yue H. Huan Y. Ren P. Yu X. Tang Q. Xue C. et al . (2025). Astaxanthin ameliorates dexamethasone-induced skeletal muscle atrophy and disorders of glucolipid metabolism. Biochem. Biophys. Res. Commun.743, 151138. doi: 10.1016/j.bbrc.2024.151138
140
Yukihira N. Sugai Y. Fujiwara M. Kosumi D. Iha M. Sakaguchi K. et al . (2017). Strategies to enhance the excitation energy-transfer efficiency in a light-harvesting system using the intra-molecular charge transfer character of carotenoids. Faraday. Discuss.198, 59–71. doi: 10.1039/c6fd00211k
141
Zhang L. Hu Z. Bai W. Peng Y. Lin Y. Cong Z. (2023). Fucoxanthin ameliorates traumatic brain injury by suppressing the blood-brain barrier disruption. iScience26, 108270. doi: 10.1016/j.isci.2023.108270
142
Zhang W. Wu C.-C. Ge M.-M. Yuan X.-M. Han S.-Y. Zhao F.-T. et al . (2024). The PGC-1α/ERRα/ULK1 pathway contributes to perioperative neurocognitive disorders by inducing mitochondrial dysfunction and activating NLRP3 inflammasome in aged mice. Neuropharmacology260, 110119. doi: 10.1016/j.neuropharm.2024.110119
143
Zhang H. Zhang Y. Zhang J. Jia D. (2024). Exercise alleviates cardiovascular diseases by improving mitochondrial homeostasis. J. Am. Heart Assoc.13, e036555. doi: 10.1161/JAHA.124.036555
144
Zhang X. Zhao X. Hua Z. Xing S. Li J. Fei S. et al . (2023). ROS-triggered self-disintegrating and pH-responsive astaxanthin nanoparticles for regulating the intestinal barrier and colitis. Biomaterials292, 121937. doi: 10.1016/j.biomaterials.2022.121937
145
Zhao X. Wang Z. Wang L. Jiang T. Dong D. Sun M. (2024). The PINK1/parkin signaling pathway-mediated mitophagy: A forgotten protagonist in myocardial ischemia/reperfusion injury. Pharmacol. Res.209, 107466. doi: 10.1016/j.phrs.2024.107466
146
Zheng M. Chao X. Zheng Y. Hong T. Wu W. Zhu Y. et al . (2024). A polysaccharide from edible red seaweed bangia fusco-purpurea prevents obesity in high-fat diet-induced C57BL/6 mice. Int. J. Biol. Macromol.283, 137545. doi: 10.1016/j.ijbiomac.2024.137545
147
Zhong D. Jin K. Wang R. Chen B. Zhang J. Ren C. et al . (2024). Microalgae-based hydrogel for inflammatory bowel disease and its associated anxiety and depression. Adv. Mater.36, e2312275. doi: 10.1002/adma.202312275
148
Zhou Z. Fan Y. Zong R. Tan K. (2022). The mitochondrial unfolded protein response: A multitasking giant in the fight against human diseases. Ageing Res. Rev.81, 101702. doi: 10.1016/j.arr.2022.101702
149
Zhou T. Li X. (2024). Chemically modified seaweed polysaccharides: improved functional and biological properties and prospective in food applications. Compr. Rev. Food Sci. Food Saf.23, e13396. doi: 10.1111/1541-4337.13396
150
Zhou L. Su W. Wang Y. Zhang Y. Xia Z. Lei S. (2024). FOXO1 reduces STAT3 activation and causes impaired mitochondrial quality control in diabetic cardiomyopathy. Diabetes Obes. Metab.26, 732–744. doi: 10.1111/dom.15369
151
Zhu J. Liu K. Pei L. Hu X. Cai Y. Ding J. et al . (2021). The mechanisms of mitochondrial dysfunction and glucose intake decrease induced by microcystin-LR in ovarian granulosa cells. Ecotoxicol. Environ. Saf.212, 111931. doi: 10.1016/j.ecoenv.2021.111931
152
Zueva A. O. Silchenko A. S. Rasin A. B. Malyarenko O. S. Kusaykin M. I. Kalinovsky A. I. et al . (2023). Production of high- and low-molecular weight fucoidan fragments with defined sulfation patterns and heightened in vitro anticancer activity against TNBC cells using novel endo-fucanases of the GH107 family. Carbohydr. Polym.318, 121128. doi: 10.1016/j.carbpol.2023.121128
Summary
Keywords
marine algae, bioactive compounds, mitochondrial quality control, structure-activity relationship, experimental model, clinical translation
Citation
Zhang C, Yuan L, Li J, Lu D, Du Y and Nan Y (2025) Marine algae and their bioactive compounds as novel modulators for mitochondrial quality control: from mechanism to therapeutic potential. Front. Mar. Sci. 12:1693989. doi: 10.3389/fmars.2025.1693989
Received
27 August 2025
Accepted
21 October 2025
Published
27 November 2025
Volume
12 - 2025
Edited by
Clementina Sansone, Anton Dohrn Zoological Station Naples, Italy
Reviewed by
Tan Suet May Amelia, Chang Gung University, Taiwan
Imelda Monroy García, Tecnologico Nacional de Mexico, Mexico
Updates
Copyright
© 2025 Zhang, Yuan, Li, Lu, Du and Nan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Nan, 20080011@nxmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.