- 1Fish Resources Research Center, King Faisal University, Al-Ahsa, Saudi Arabia
- 2Department of Food and Nutrition Sciences, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 3Date Palm Research Center of Excellence, King Faisal University, Al-Ahsa, Saudi Arabia
- 4Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, Abu Dhabi, United Arab Emirates
- 5Fish Nutrition Research Laboratory, Animal Production Department, Faculty of Agriculture, Cairo University, Cairo, Egypt
- 6Animal and Fish Production Department, College of Agricultural and Food Sciences, King Faisal University, Hofuf, Al-Ahsa, Saudi Arabia
- 7Department of Basic Sciences, Preparatory Year, King Faisal University, Al‐Ahsa, Al Hofuf, Saudi Arabia
- 8Department of Aquatic Animal Medicine, Faculty of Veterinary Medicine, Benha University, Benha, Egypt
- 9Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 10Department of Agricultural Sciences, Faculty of Agro-Based Industry, Universiti Malaysia Kelantan, Jeli, Malaysia
- 11Biotechnology Department, Fish Farming and Technology Institute, Suez Canal University, Ismailia, Egypt
- 12Fish Research Centre, Faculty of Environmental Agricultural Sciences, Arish University, El-Arish, Egypt
Date Palm Seed Extract (DPSE) is known to possess beneficial health-promoting and growth-boosting properties, but its specific influence on whiteleg shrimp (Litopenaeus vannamei) health and physiology has yet to be fully explored. Hence, this study examined how dietary DPSE inclusion impacted growth performance, antioxidant status, immune response, hepatic tissue health, inflammation-related gene expression, and resistance to Vibrio parahaemolyticus in shrimp. The study involved four experimental groups of shrimps that were fed diets with varying DPSE inclusions: 0 (DPSE0), 1 (DPSE1), 2 (DPSE2), and 4 (DPSE4) g/kg diet over a 70-day period. The results indicated that dietary DPSE supplementation led to significantly higher (P<0.05) concentrations of whole-body composition (crude protein, gross energy, and crude lipid) in the treated groups. Conversely, the ash content was significantly reduced (P<0.05) in all DPSE-treated shrimp groups compared to the shrimp fed the control diet. Shrimp-fed DPSE-supplemented diets showed a significantly higher total hemocyte count (THC) compared to the control group (P<0.05). The levels of immunological parameters (phagocytosis, phenoloxidase, and lysozyme) and antioxidant status (superoxide dismutase and catalase) were significantly improved with increasing dietary DPSE levels, indicating a dose-dependent effect (P<0.01). The supplementation of DPSE at 1, 2, and 4 g in shrimp diets significantly improved the mRNA expression of immune-related genes (LGBP, PX, PPA) and antioxidant genes (cytMnSOD, mtMnSOD) compared to the control group (P<0.001). Both total aerobic bacteria and total enteric bacteria were significantly reduced in all DPSE-supplemented groups (P<0.05) compared to the control diet. Dietary inclusion of DPSE improved hepatopancreas tissues and significantly increased resistance to Vibrio parahaemolyticus in shrimp. The results indicate that the dietary inclusion of DPSE can enhance the growth, disease resistance, and overall health of shrimp by regulating immune function, antioxidant status, and immune associated genes regulation. This highlights its potential for sustainable and environmentally beneficial applications in aquaculture.
1 Introduction
Globally, whiteleg shrimp (Litopenaeus vannamei) is one of the most widely farmed aquaculture species globally due to its rapid growth, high adaptability to various environmental conditions, strong resilience, and rich nutritional profile (Pandey et al., 2024). The L. vannamei holds significant global importance as an aquaculture species, largely due to its remarkable adaptability to a range of cultural environments (Raza et al., 2024). Therefore, the strategic improvement of shrimp performance, feed utilization, and immune status through targeted dietary interventions is paramount for achieving sustainable and economically viable shrimp aquaculture. Global white shrimp aquaculture production attained 6.8 million tons in 2022 (FAO, 2024), establishing it as the most extensively produced aquaculture species worldwide (UNICEF, 2024). Nevertheless, the substantial expansion of this industry is accompanied by persistent challenges. A survey conducted by the Global Seafood Alliance in 2022 identified critical concerns among industry stakeholders (Liu et al., 2025), such as increasing feed expenses, volatile market prices, disease incidences, and issues related to broodstock quality (Willer et al., 2021).
Despite progress, the shrimp aquaculture industry still faces several critical challenges, with disease outbreaks posing the most significant threat (Flegel, 2012). The major challenges facing shrimp aquaculture can be broadly categorized into three interconnected areas: pathogenic and environmental factors. Disease outbreaks are often cited as the biggest impediment to profitability, leading to massive mortality and severe economic losses. These outbreaks are caused by various pathogens, including viral and bacterial agents (Okon et al., 2023, 2024). For instance, vibriosis is one of the most common bacterial diseases responsible for significant mortality in cultured shrimp. Vibrio parahaemolyticus is a prevalent and significant bacterial pathogen in shrimp farms that can cause early mortality syndrome (EMS) (Ma et al., 2021) or acute hepatopancreatic necrosis disease (AHPND), resulting in substantial economic losses (Cheng et al., 2025). To tackle these issues, researchers and industry stakeholders are investigating new strategies, such as developing functional feeds (Aly and Fathi, 2024; Dighiesh et al., 2024; El-Sayed et al., 2025). Among these, phytochemical-enriched diets have shown promise for improving shrimp growth performance and boosting their overall health (Pakravan et al., 2018).
Plants or herbs or their extract has been utilized for disease prevention and treatment since ancient times. In recent years, herbal remedies have gained prominence in primary healthcare, offering minimal side effects (Dadras et al., 2023). This trend extends beyond human medicine, with their application increasingly widespread in veterinary medicine globally. The use of herbal supplements in aquaculture can offer several beneficial effects (Dadras et al., 2023). More recently, many researchers have proposed the application of medicinal herbs in aquaculture.
The date palm (Phoenix dactylifera), a member of the Arecaceae family, is known for its therapeutic properties due to the rich biological compounds in its leaves, bark, pits, fruits, and pollen (Al-Shwyeh, 2019). As an inedible waste product of date consumption, date seeds (approximately 10% of the fruit’s weight) present an environmental challenge. However, valorizing these seeds for use in aquatic nutrition offers an innovative and environmentally friendly approach to waste reduction and resource recycling. Studies found that the date palm seed extract (DPSE) contains a range of bioactive compounds such as alkaloids, phenols, flavonoids, tannins, saponins, and terpenoids (Himanshu et al., 2024).
These components are believed to possess a wide range of beneficial effects (Fernández-López et al., 2022), such as anticancer, antioxidant, hepatoprotective, antidiabetic, antihypertensive, antiulcerative, anti-inflammatory, antiproliferative, antimutagenic, antidiarrheal, antibacterial, antifungal, and antiviral properties (Abdallah et al., 2023). Beyond its traditional uses, the nutritional value of DPS rich in protein, lipids, vitamins, minerals, and carbohydrates suggests its potential as a growth promoter in some animal species (Ahmed et al., 2017; Abdulrahman, 2020). Furthermore, DPS has been linked to positive effects on animal estrogen and testosterone levels (Mohammadi et al., 2018). Notably, DPSE has demonstrated the ability to restore normal liver function in poisoned subjects and offer protection against carbon tetrachloride-induced hepatotoxicity in rats (Ali et al., 2022). The antioxidant and hepatoprotective activities may due to it a rich source of phenolic and flavonoid substances (Echegaray et al., 2023). DPSE has recently been shown to promote reproductive efficiency in tilapia by enhancing the antioxidant system and raising reproductive hormone levels (Alqahtani et al., 2025). Himanshu et al. (2024) exhibited the antimicrobial activity of DPSE against Staphylococcus aureus and Bacillus cereus. This action could be attributed to its richness in gallic acid in this extract. Himanshu et al. (2024) characterized the date seed extract, identifying substantial quantities of major bioactive compounds, including alkaloids, phenols, flavonoids, tannins, saponins, and terpenoids (Mohammadi et al., 2016; Himanshu et al., 2024). The extract demonstrated high bioactivity, with a total phenolic content of 448 mg GAE/100 g of powder, and potent antioxidant activity (87.04 ± 1.98) (Mohammadi et al., 2016; Himanshu et al., 2024). Moreover, protective efficacy of DSPE is attributed to the extract’s rich profile of bioactive compounds (Manai et al., 2024), including flavonols (epicatechin, catechin, and procyanidins B) and polyphenolic acids (p-hydroxybenzoic acid, protocatechuic acid, and caffeic acid), alongside carotenoids and dietary fibers (Pakkish and Mohammadrezakhani, 2020).
Date seeds are widely available in several countries, including Egypt and Saudi Arabia, in large quantities, yet they currently lack widespread application. Global date production is estimated to be around 9.7 million metric tons annually, based on 2022 data, and is steadily growing (FAOSTAT, 2025). The market is predominantly dominated by countries in the Middle East and North Africa. Egypt is the top global producer of date palms, yielding approximately 1.87 million tons, while Saudi Arabia ranks second with 1.64 million tons (Al-Habsi, 2025). Recognizing the significant amount of date palm seed byproduct generated, we aimed to valorize this waste stream by hypothesizing its use as a feed additive. The rationale for this use is the seed’s richness in valuable bioactive compounds that can be obtained through extraction. Existing data on their potential use as a supplement in shrimp diets are notably limited. Given this background, the present study aimed to investigate the effects of date palm seed extract (DPSE) as a dietary supplement on shrimp growth, immunity, antioxidant status, and resistance to infectious diseases. Recognizing that immune-physiological parameters are closely linked to overall health and growth performance, we also examined the effects of DPSE on organ histology, immune function, antioxidant capacity, body composition, and growth in shrimp, in addition to its role in combating infectious diseases.
2 Materials and methods
2.1 Ethical statement
All animal procedures were reviewed and approved by the ZU-IACUC committee (ZU-IACUC/2/F/25/2023), adhering to the U.K. Animals (Scientific Procedures) Act, 1986; EU Directive 2010/63/EU; the National Research Council’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978); and ARRIVE guidelines.
2.2 Preparation of date palm seed extract
We collected date fruits at the dehydrated Tamar stage (Tamr, mature date palm deep characterized by its brown color and wrinkled texture) of ripening from Ismailia province, Egypt. These fruits were identified by Prof. El Sayed M. Desoky, Botany Department, Faculty of Agriculture, Zagazig University. The seed samples were semi-dried with a brown–red color tending towards black and had smooth, glossy textures, measuring 2–3 cm wide and 5–8 cm long. The seed samples were placed in sterile plastic bags and brought to the laboratory, where they were kept at 4°C for further analysis. The seeds were washed with distilled water to remove any adhered date fruit pulp. The seeds were then dried in a hot air oven at a temperature 40°C and ground using a seed grinder to obtain a fine date seed powder. The powder was passed through a 105-mesh sieve to get finer particle size and stored at 4°C for further analysis. The dried plant material underwent maceration in triplicate using 1:2.5 (w/v) ratio of seed samples to ethanol (40% w/v). Following extraction, the resulting solutions were evaporated to dryness at 45°C using a BUCHI R-300 rotary evaporator under reduced pressure. The crude extract was then filtered through Whatman No. 1 filter paper. The filtrate was subsequently concentrated to dryness via rotary vacuum evaporation (EYELA N-N series, Japan) at 40°C in darkness. The concentrated extract was then lyophilized, the dry weight was recorded, and the sample was stored at 4°C for future analysis. Finally, this dried powder was packed into capsules at the specific dosages required for each treatment and used as a suspension in the fish feed.
2.3 Shrimp husbandry and experimental groups
Pacific white shrimp (Litopenaeus vannamei) were obtained from a private farm in Damietta Governorate, Egypt. Before the experiment, shrimp were acclimated for 15 days on a basal diet. A total of 240 healthy shrimp, averaging 4.23 ± 0.3 g and verified by a veterinarian to be free of bacterial, viral, and parasitic infections, were equally distributed into 12 hapas (1×1×1 m3) within a 1.25 Hectare. The shrimp were divided into four groups, each with three replicates of 20 shrimp per hapa. After one week of acclimation, 240 healthy juvenile whiteleg shrimp (L. vannamei) were randomly assigned to four dietary treatment groups. The four experimental diets included a control (DPSE0, basal diet) and three levels of date palm seed extract (DPSE) supplemented diets at 1 (DPSE1), 2 (DPSE2), and 4 (DPSE4) g of DPSE per kg of diet (Mohammadi et al., 2018). The dried extract was added during diet formulation and thoroughly mixed to homogenize the four prepared diets. Shrimp were fed three times daily (8:00, 12:00, and 16:00). The feeding rate was 8% of body weight for the first five weeks, then reduced to 6% for the remaining five weeks. Diets were designed to provide approximately 38% crude protein. Table 1 shows the composition (g/kg) and approximate content (%) of the experimental diets used in this study to meet the required nutrient levels.
Individual ingredients were precisely weighed (Metler Toledo PB 8001) and ground to a particle size of 3 mm. These components, along with the vitamin premix and date seed powder, were then thoroughly blended using a Hobbart A-2007 machine. Carboxymethyl cellulose was incorporated as a binding agent. The prepared mash was subsequently formed into 0.9 mm diameter pellets without the application of steam. These pellets were then dried and stored under refrigeration until needed. The 70-day feeding trial maintained consistent water parameters: temperature at 27.13 ± 0.4°C, salinity at 33.53 ± 0.2 g/L, pH at 8.18 ± 0.0, dissolved oxygen at 8.4 ± 0.6 mg/L, total ammonia nitrogen at 1.23 ± 0.0 mg/L, and NH3 at 0.13 ± 0.0 mg/L.
2.4 Growth indices assessment
Following the experimental period, all shrimp were individually weighed and documented per hapa using a digital electronic balance (± 0.01g). Subsequently, several key growth performance parameters were assessed: average daily gain (ADG), specific growth rate (SGR), weight gain (WG), and feed conversion ratio (FCR). The survival rate was also evaluated. The methodologies for these calculations are detailed in the subsequent formulas:
2.5 Whole body composition analysis
We analyzed the proximate composition of shrimp samples (n=6 per replicate) using standard methods (Hwang et al., 2013). Crude protein was determined by the Kjeldahl method. The total nitrogen content was quantified using the factor of 6.25 (N × 6.25), and the ammonia (NH3) released during digestion was captured with boric acid. To measure dry matter, samples were oven-dried at 105°C. Ash content was assessed by incinerating 2g samples in a muffle furnace at 550°C for 4 hours. We extracted total lipids using ether with a Soxtec System HT, and measured gross energy with a Schimadzu CA-4P bomb calorimeter (Du and Niu, 2003).
2.6 Sampling
To assess various physiological markers, hemolymph was extracted from twelve whiteleg shrimp (L. vannamei) per treatment group. Using a sterile 1-mL syringe, about 250 µL of hemolymph was carefully withdrawn from the base of the third walking leg of each shrimp. To inhibit coagulation, the syringe contained 750 µL of a pre-cooled (4°C) anticoagulant solution. This solution comprised 450 mM NaCl, 10 mM KCl, 0.114 M trisodium citrate, and 10 mM HEPES at pH 7.4 (Nonwachai et al., 2010). The collected hemolymph-anticoagulant samples were subsequently used for analysis of biochemical parameters, immune responses, antioxidant status, and inflammatory markers. To perform antioxidant and mRNA analyses, as well as histological examination, hepatopancreas samples were collected and fixed in 10% of neutral buffered formalin (Beyotime, China). For total bacterial enumeration, intestines from three shrimp were aseptically dissected and immediately placed in sterile saline (0.9% sodium chloride in deionized or distilled water).
2.7 Immunological markers and biochemical indices
Hemocyte counts were performed by diluting 50 µL of the hemolymph-anticoagulant mixture with 150 µL of 4% formaldehyde. A 20 µL aliquot of this dilution was loaded onto a Neubauer hemocytometer and counted under a light microscope. The total hemocyte count (THC) in cells/mL was calculated using the formula: count ×104× dilution factor. We assessed respiratory burst (RB) activity according to the method by Song and Hsieh (1994). Plasma lysozyme activity in shrimp was quantified using the turbidimetric method described by Engstad et al. (1992). Phenoloxidase activity was determined spectrophotometrically, based on a modification of El Asely et al. (2010) method. Phagocytic activity was quantified according to the procedure established by Kawahara et al. (1991).
2.8 Antioxidants activity
To assess oxidative stress and antioxidant status, we employed colorimetric assay kits (Biovision, Inc., California, USA). These kits were used to quantify the activity of key antioxidant enzymes and molecules: glutathione (GSH) (K762-100), catalase (CAT) (K274-100), and superoxide dismutase (SOD) (K335-100). Furthermore, malondialdehyde (MDA) levels, serving as a marker for lipid peroxidation, were also determined using a colorimetric assay kit from the same manufacturer. All assays were conducted according to the manufacturer’s specified protocols.
2.9 Intestinal bacterial counts
Quantification of intestinal bacterial populations commenced with the homogenization of shrimp intestinal samples in 1 mL of sterile saline. A serial dilution series was then established by transferring 500 µL of the homogenate into 5 mL of sterile saline.
Subsequent plating procedures for specific bacterial groups were as follows:
● Total aerobic bacteria were cultured on trypticase soy agar (TSA) via spread plating 0.1 mL from each dilution, followed by incubation at 30°C for 24–48 hours.
● Enteric bacteria were enumerated on MacConkey agar after 48 hours of incubation at 37°C.
● Probiotic bacteria were counted on De Man, Rogosa, and Sharpe agar, with incubation performed at 37°C for 2–3 days.
● Clostridium perfringens was selectively cultured on tryptic sulfite cycloserine agar under anaerobic conditions, with incubation maintained at 37°C for two days.
2.10 Histological study
Hepatopancreas samples underwent detailed histological preparation. The samples were fixed in 10% neutral buffered formalin for 24 hours, followed by dehydration in a graded series of ethanol solutions. Samples were then cleared in xylene and embedded in paraffin. A Leica RM 2155 microtome was used to cut 5 µm thick sections, which were subsequently stained with hematoxylin and eosin (H&E) for microscopic examination (Suvarna et al., 2018). An Olympus CX 41 microscope with an E-620 digital camera captured photomicrographs. For quantitative analysis, Image J software, coupled with its cell counter plugin, was employed to count R cells and B cells and measure tubule diameters in 20 randomly selected tubules from each treatment group (Abd El-Naby et al., 2024).
2.11 Gene expression
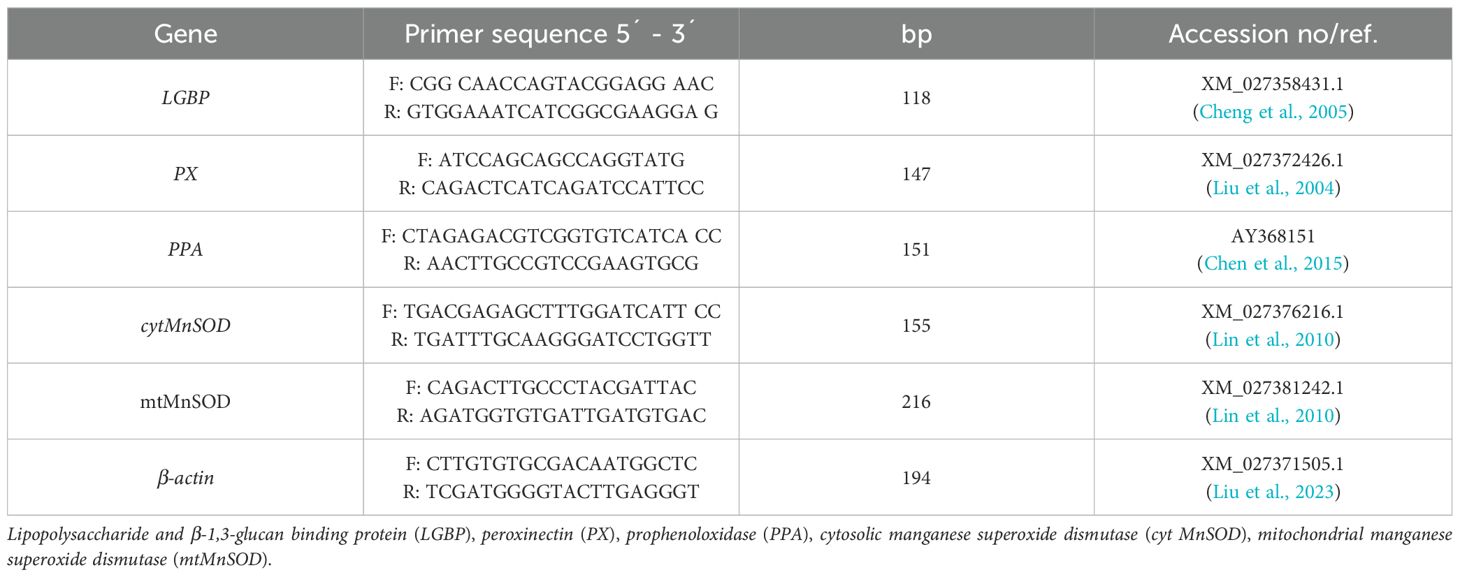
Total RNA was extracted from hepatopancreas tissues using an RNA purification kit. RNA quality was assessed by measuring the O.D. 260/280 ratio with a Nanodrop Lite spectrophotometer (Thermo Scientific, USA). cDNA was synthesized from 1 µg of RNA using the SuperScript™ III First-Strand Synthesis System with Oligo-dT primers, and samples were stored at −20°C. mRNA expression levels of immune-related genes (β-1,3-glucan-binding protein (LGBP), Peroxinectin (PX), Prophenoloxidase activating (PPA) enzyme) and antioxidant-related genes (cytosolic manganese superoxide dismutase (cytMnSOD), mitochondrial manganese superoxide dismutase (mtMnSOD)) were quantified by qPCR. The SensiFast SYBR Lo-Rox kit was used for gene transcription level measurement. Primer sequences used in this study are summarized in Table 2. Gene expression was normalized to the β-actin gene using the 2−ΔΔCT method (San Segundo-Val and Sanz-Lozano, 2016).
2.12 Challenge test with Vibrio parahaemolyticus
To prepare for the challenge, Vibrio parahaemolyticus, a known pathogen, was cultured in Brain Heart Infusion (BHI) broth at 25°C for 24 hours. The bacterial culture was then centrifuged at 1300 g for 15 minutes to pellet the cells. The supernatant was discarded, and the pellet was resuspended in sterile 1.5% NaCl saline to form a bacterial suspension (Vieira et al., 2010). The optical density at 600 nm (OD600) was used to spectrophotometrically adjust the bacterial concentration to 107 CFU/mL (Won et al., 2020). For the challenge test, 20 shrimp were randomly selected from each treatment group. These shrimps were individually transferred and acclimated in separate 50-L challenge tanks (n=20 per group). Each shrimp was individually challenged by injecting 25 µL of the 107 CFU/mL bacterial suspension (Vibrio parahaemolyticus). All shrimps were injected at the third abdominal segment using 1 ml sterile insulin syringe (29 G). Mortality was subsequently assessed and calculated as: Cumulative mortality rate (%) = (cumulative number of dead shrimp/initial number) ×100.
2.13 Statistical analysis
Prior to analysis, data normality was assessed with a Shapiro-Wilk test and homogeneity of variance was confirmed using Levene’s test. All results are presented as the mean ± standard error (SE). A one-way analysis of variance (ANOVA) was performed using SPSS software (version 25) to identify significant differences among treatment groups. Mean comparisons were then conducted using Tukey’s HSD test, with statistical significance set at P<0.05. The mathematical model for the ANOVA was:
Where: Yij = Observation for the jth replicate of the ith treatment μ = Overall mean, τi = Effect of the ith treatment, ϵij = Random error associated with each observation.
3 Results
3.1 Growth performance
The DPSE1 group (1 g/kg DPSE) showed significantly improved final weight when compared to the DPSE4 group (Table 3). Conversely, no significant differences in final weight were observed between the DPSE1 group and the control or DPSE2 groups. Notably, shrimp in the DPSE4 group exhibited the lowest final body weight. The DPSE1 group achieved the highest weight gain and ADG; however, this increase was not statistically significant when compared to the DPSE2 group (P>0.05). Both the feed conversion ratio (FCR) and specific growth rate (SGR) showed significant differences (P<0.05), with the DPSE1 and DPSE2 groups exhibiting the lowest FCR and highest SGR compared to the other experimental groups. Conversely, feed intake did not show any significant statistical differences across all groups (P>0.05). Compared to the control diet, shrimp fed diets containing DPSE exhibited significantly higher biomass (per m3) and survival rates (P<0.05). Overall, supplementing shrimp diets with 1 g/kg of date palm seed extract significantly enhanced growth indices and feed efficiency. Conversely, higher doses of DPSE appeared to have a non-significant effect on growth.

Table 3. Growth and feed utilization indices of shrimp fed on diets supplemented with various levels of date palm seed extract (DPSE) for 70 days.
3.2 Whole body composition
Dietary inclusion of DPSE (1, 2, and 4 g/kg diet) did not significantly affect shrimp moisture content (Table 4). However, all DPSE-supplemented groups exhibited significantly higher protein and lipid levels (P<0.05), with the DPSE4 group showing the maximum values for both. Ash content was significantly reduced in all DPSE-treated shrimp groups compared to the control diet (P<0.05), with the lowest values observed in the DPSE1 and DPSE2 groups. Furthermore, gross energy was significantly improved in all shrimp fed DPSE in their diets (P<0.05).
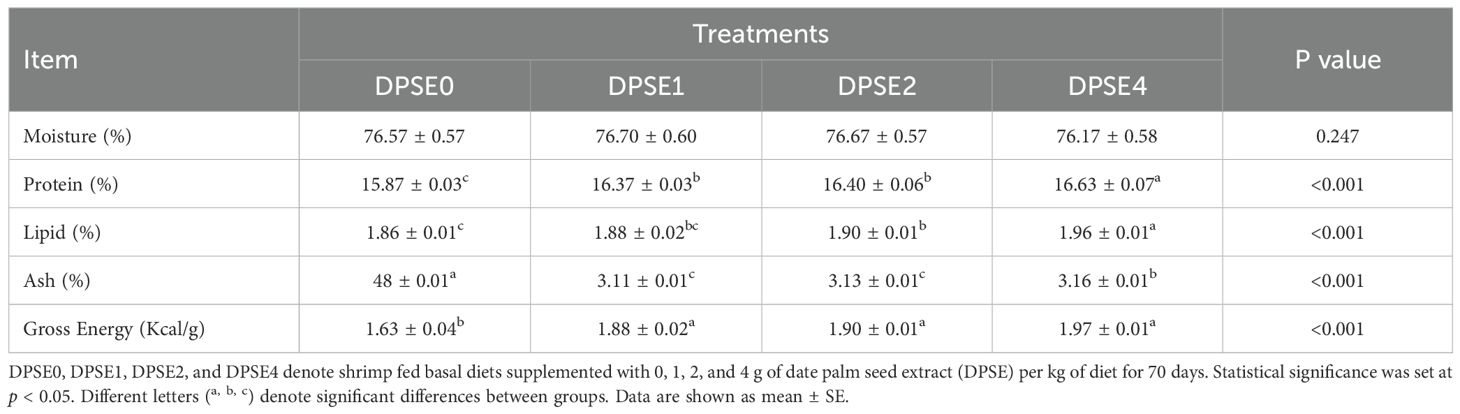
Table 4. Proximate chemical analysis (%) (based on fresh weight) of shrimp fed diets with varying levels of date palm seed extract (DPSE) for 70 days.
3.3 Immune ability
Shrimp fed DPSE-supplemented diets showed a significantly higher total hemocyte count (THC) compared to the control group (P<0.05), with the highest counts observed in the DPSE2 and DPSE4 groups (Table 5). Phagocytosis, phenoloxidase (PO) activity, and lysozyme activity improved with increasing dietary DPSE levels, indicating a dose-dependent effect. The DPSE2 and DPSE4 groups notably had a superior phagocytic index compared to other groups (P<0.05), although the 1 g/kg DPSE dose did not yield a significant effect compared to the control diet (P>0.05). The highest respiratory burst (RB) activity was recorded in the DPSE4 group, significantly exceeding that of the DPSE1 and control groups (P<0.05). Collectively, shrimp in all DPSE-supplemented groups displayed enhanced immune parameters relative to the control (P<0.05).
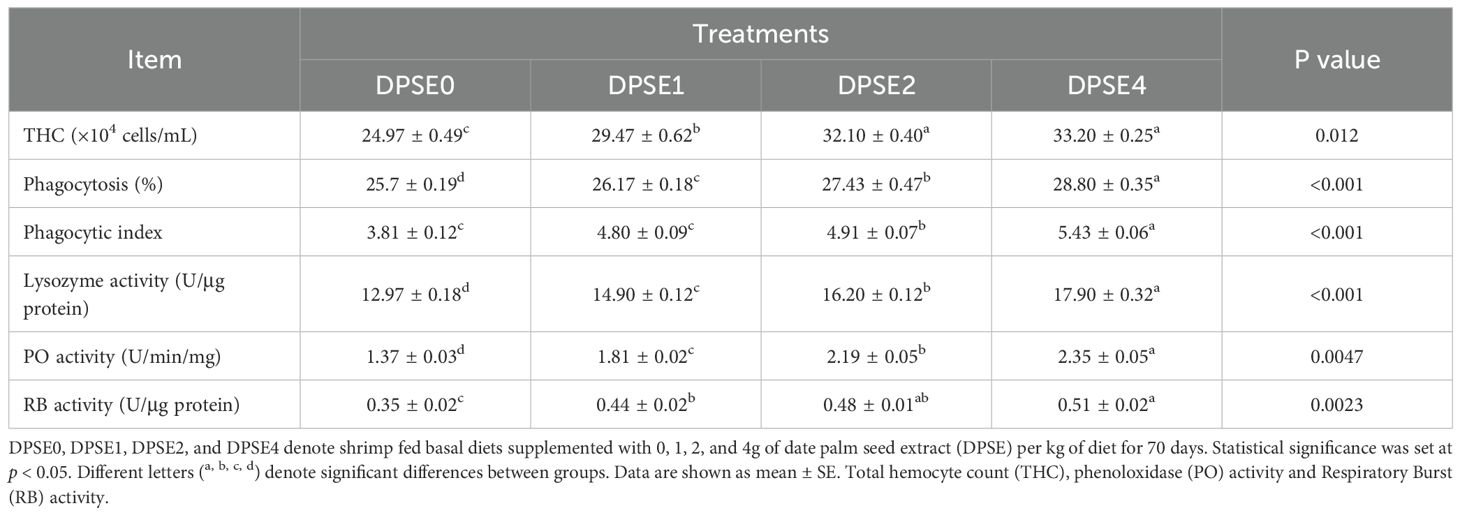
Table 5. Hemolymph immunological and biochemical indices of shrimp fed on diets supplemented with various levels of date palm seed extract (DPSE) for 70 days.
3.4 Antioxidant activity
Superoxide dismutase (SOD) and catalase (CAT) activities significantly improved with increasing dietary DPSE levels, demonstrating a dose-dependent effect (P<0.05) (Table 6). The highest levels of both SOD and CAT were observed in the DPSE4 group (P<0.05). All DPSE-supplemented groups exhibited significantly higher glutathione peroxidase (GPx) compared to the un-supplemented control group (P<0.05). However, there was no significant difference in GPx levels between the DPSE1 and DPSE2 groups (P>0.05). Malondialdehyde (MDA) levels were significantly decreased in all DPSE groups compared to control shrimp (P<0.05), with the lowest MDA levels recorded in shrimp fed 4 g/kg diet (P<0.05).
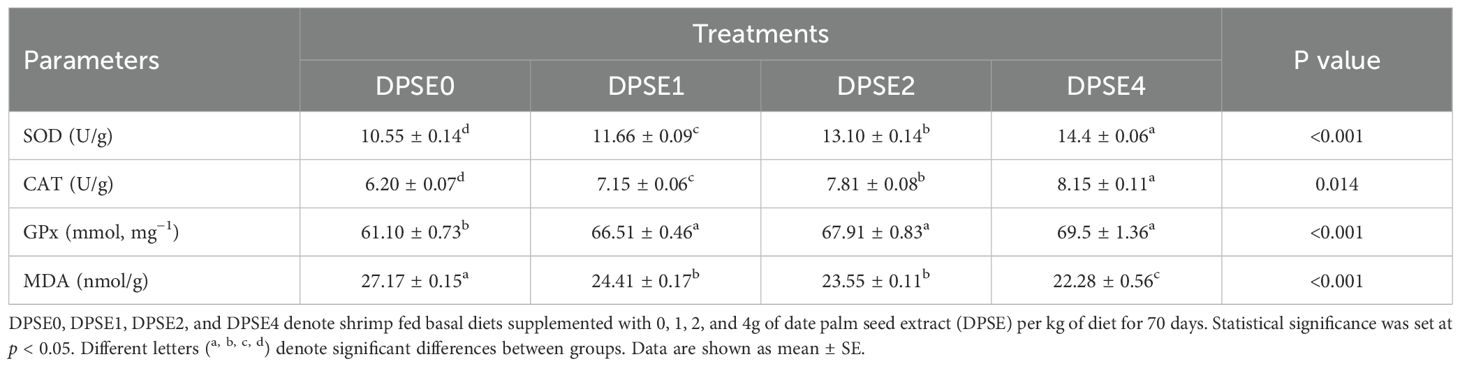
Table 6. Activities of hepatopancreas SOD, CAT, GPx, and MDA levels of shrimp fed on diets supplemented with various levels of date palm seed extract (DPSE) for 70 days.
3.5 Intestinal microbiota
Both total aerobic bacteria and total enteric bacteria were significantly reduced in all DPSE-supplemented groups (P<0.05) compared to the control diet (Table 7). The lowest counts for both were observed in the DPSE2 and DPSE4 groups (P<0.05). Conversely, total probiotic bacteria significantly increased in all DPSE groups (P<0.05). The DPSE1 group recorded the lowest total Clostridium, followed by the DPSE2 group, with these differences being statistically significant (P<0.05). Overall, supplementing shrimp diets with 1 or 2 g/kg of DPSE effectively reduced pathogenic bacteria and increased beneficial bacteria in the shrimp intestine.
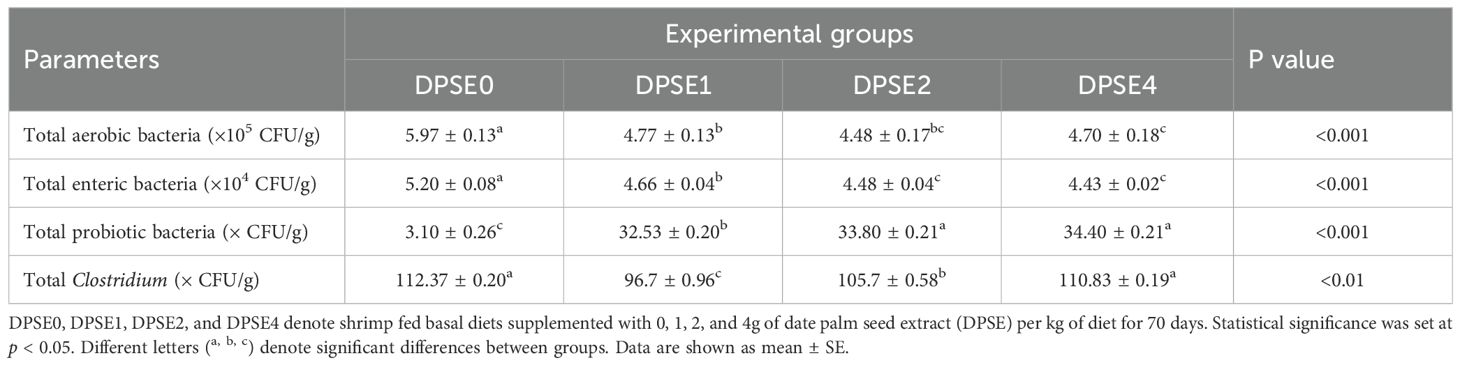
Table 7. Total intestinal aerobic enteric, probiotic, and clostridia bacterial counts of shrimp fed diets supplemented with various levels of date palm seed extract (DPSE) for 70 days.
3.6 Hepatopancreas tissues
In the control group (DPSE0), the hepatopancreas maintained its intact tubular structures (Figure 1A). These tubules were lined with various epithelial cells: B, R, M, F, and E cells. B cells were characterized by their vacuolated appearance, basal nuclei, and extensive cytoplasmic vacuoles. R cells were prismatic with acidophilic cytoplasm and basal nuclei. M cells presented as triangular cells with basophilic cytoplasm and central nuclei. F cells were elongated, showing intense basophilia and apical extensions reaching the tubular lumen. E cells featured large nuclei that occupied most of their cytoplasm. Conversely, groups DPSE1 (Figure 1B), DPSE2 (Figure 1C), and DPSE4 (Figure 1D) displayed a progressive increase in secretory vesicles within the B cells, which are recognized as the primary site for digestive enzyme synthesis.
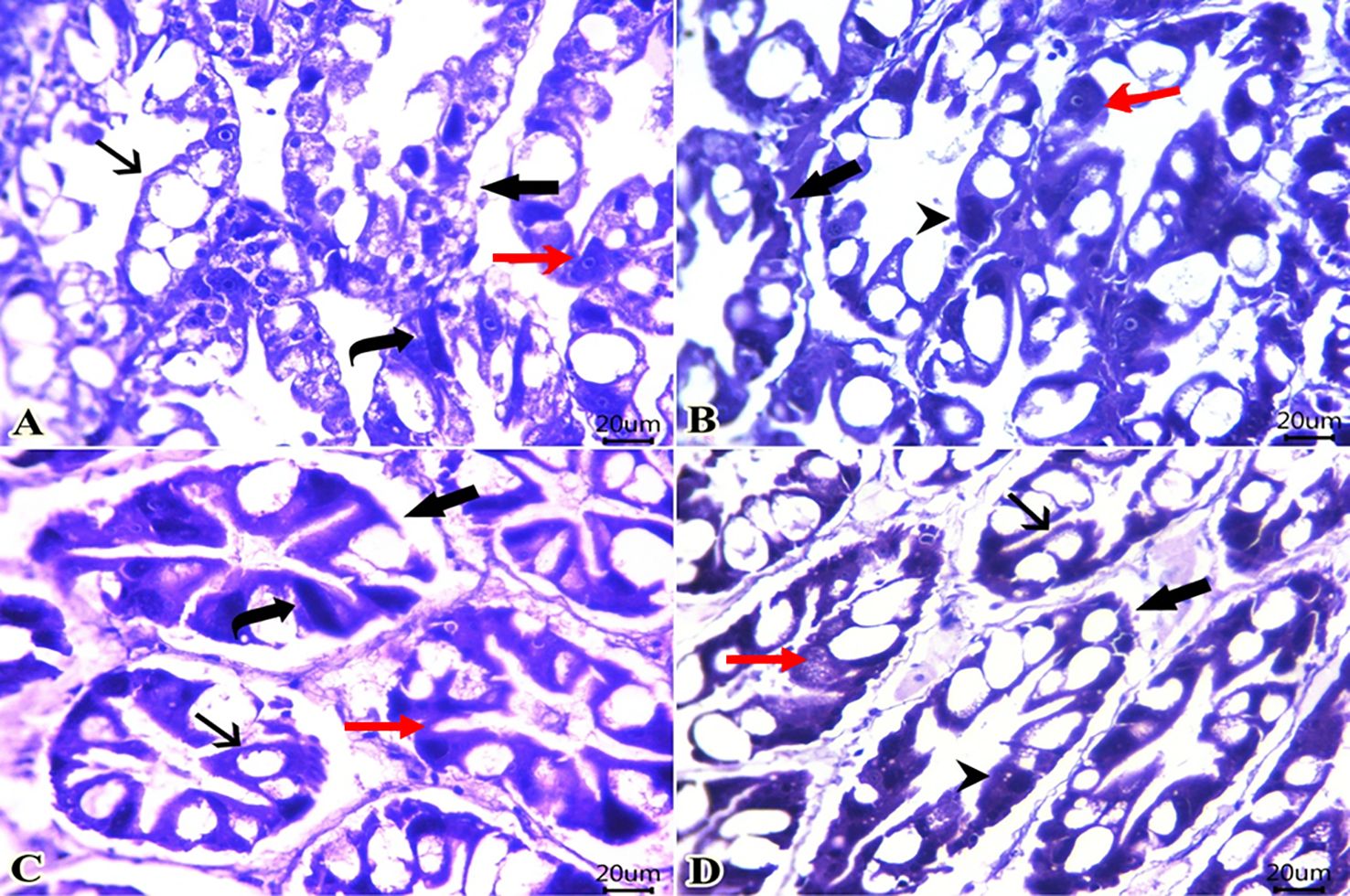
Figure 1. Photomicrograph of H&E-stained sections from the hepatopancreas of shrimp (Scale bar 20 mm) fed diets supplemented with date palm seed extract at 0, 1, 2, and 4 g/kg. Shrimp fed control diet showed normal morphology and intact hepatopancreatic tubules with patent tubule lumen in control group (DSPE0; A). In the shrimp treated with 1 (DSPE1; B), 2 (DSPE2; C), and 4 g/kg of DSPE (DSPE4; D), well-organized tubules were observed. These tubules contained various epithelial cells with abundant secretory vesicles within the lining epithelium. Hepatopancreatic tubules (thick arrows), R-cell (red arrowhead), B.cell (black arrow), F-cell (curved arrow), M.cell (red arrow), E-cell (black arrowhead).
3.7 Gene expression
The effects of dietary date palm seed extract on the mRNA expression of immune-related genes such as LGBP (Figure 2A), PX (Figure 2B), PPA (Figure 2C), and antioxidant genes such as cytMnSOD (Figure 2D), mtMnSOD (Figure 2E) in shrimp are depicted in Figure 2. All supplemented groups significantly increased the expression of immune-related genes such as LGBP (Figure 2A), PX (Figure 2B), PPA (Figure 2C), and antioxidant genes such as cytMnSOD (Figure 2D) and mtMnSOD (Figure 2E) in shrimp in a dose-dependent manner (P< 0.001). Among the supplemented groups, DSPE4 showed the highest values, followed by DSPE2 and DSPE1 compared to the DSPE0 group (P< 0.01).
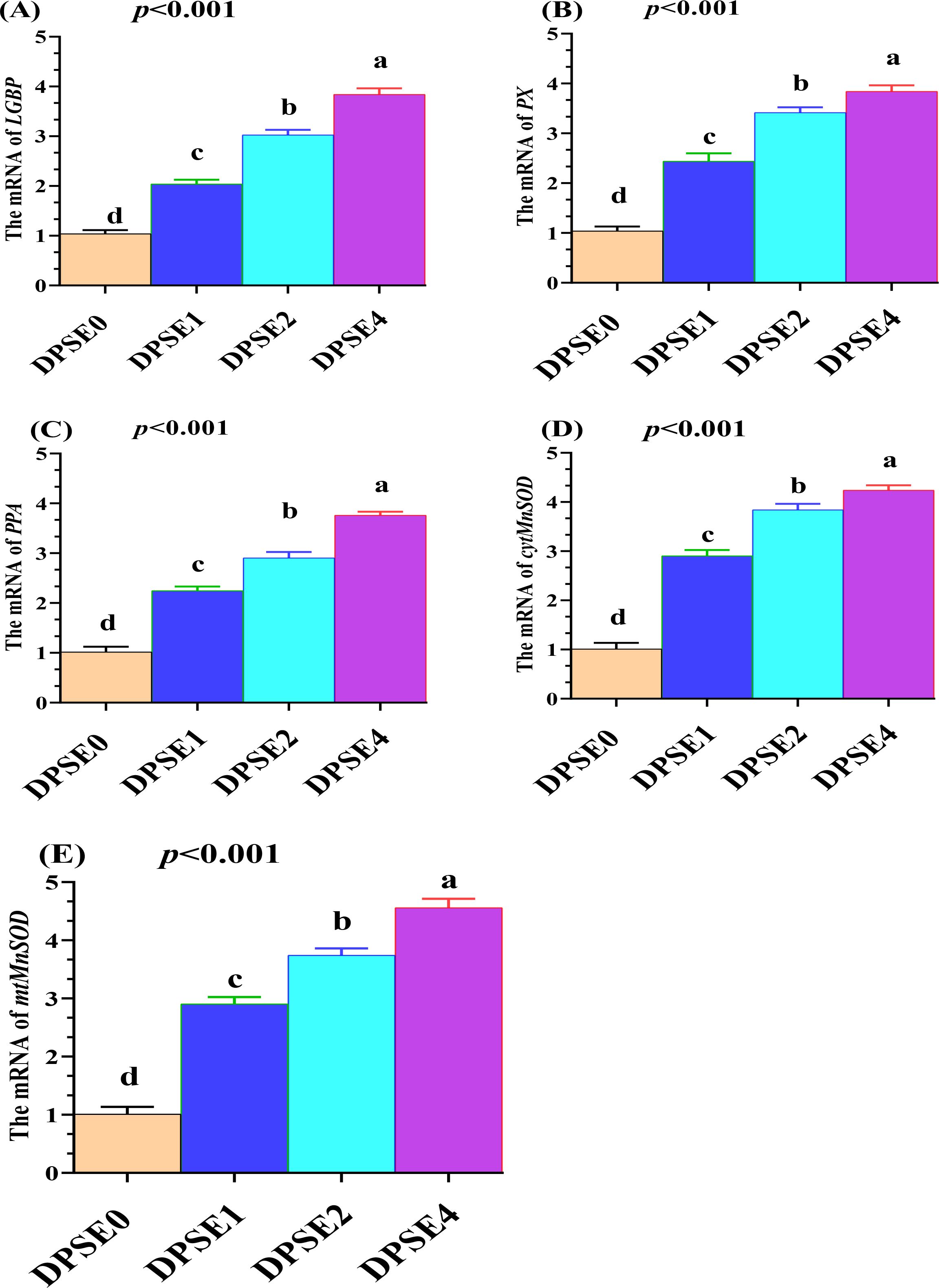
Figure 2. The effects of dietary inclusion with different levels of date palm seed extract (0 (DPSE0), 1 (DPSE1), 2 (DPSE2), and 4 (DPSE4) g/kg diet) on the mRNA expression of immune-related genes such as LGBP (A), PX (B), PPA (C), and antioxidant genes such as cytMnSOD (D), mtMnSOD (E) in shrimp were investigated. Statistical significance was set at p < 0.05. Different letters (a,b,c,d) denote significant differences between groups. Data are shown as mean ± SE. (N = 3 per group).
3.8 Vibrio parahaemolyticus challenge test
The results shown in (Figure 3) indicate that shrimp fed with the DSPE diet had notably lower mortality rates than the control group (P<0.05). Mortality rates in shrimp fed diets supplemented with 1, 2, and 4 g of date palm seed extract per kg diet were 45%, 35%, and 35%, respectively, while the control group had a mortality rate of 65%.
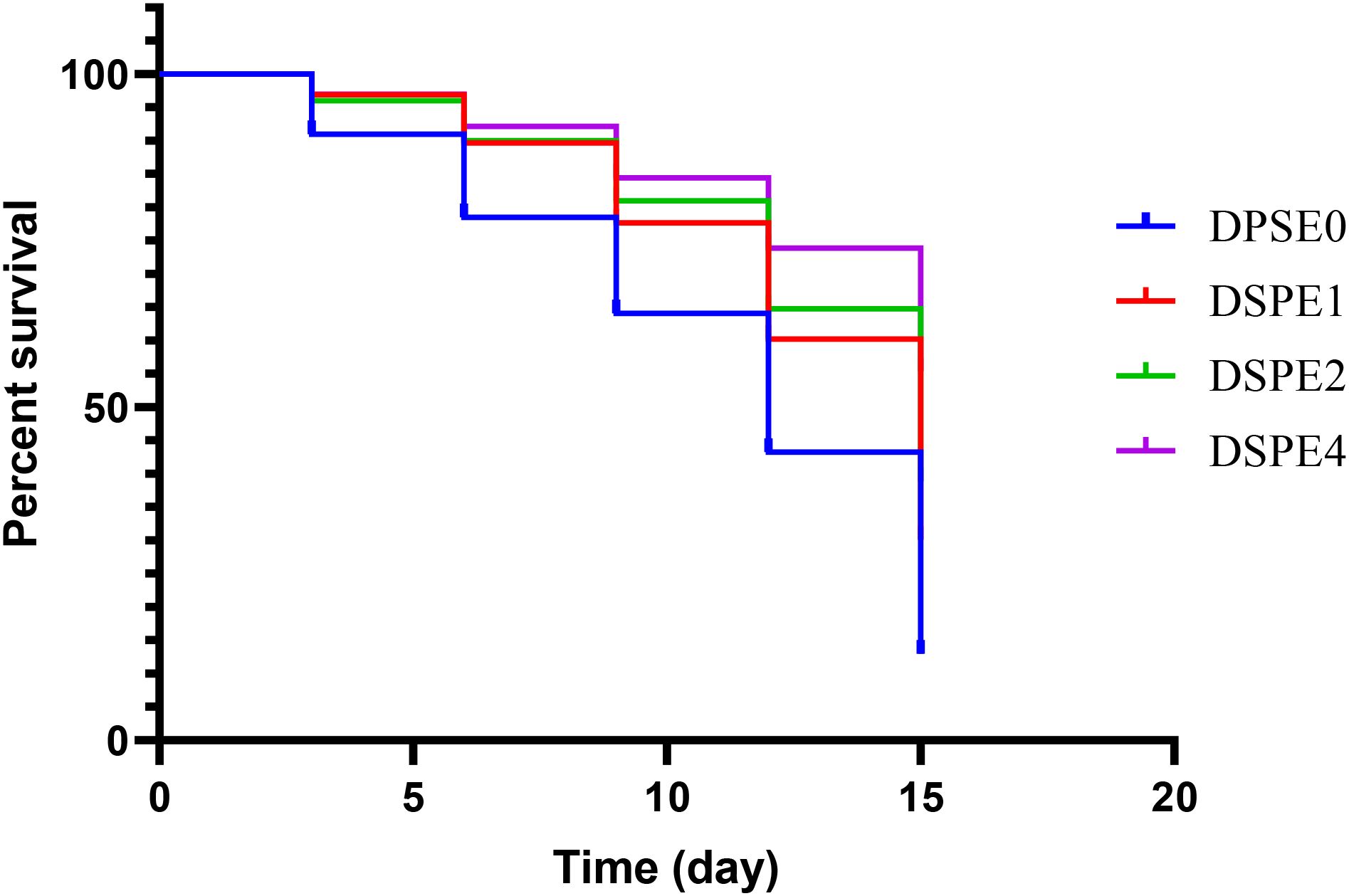
Figure 3. Variations in the survival rate (%) of Pacific white shrimp infected with V. parahaemolyticus.
4 Discussion
The ban on antibiotics has prompted scientists to seek alternative, effective, economical, and environmentally friendly feed supplements to support growth, enhance disease resistance, and improve material costs. Date palm has been widely cultivated in tropical and subtropical areas and offers many health benefits. However, its byproducts can generate a significant amount of waste, leading to environmental pollution. Valorizing these byproducts offers an environmentally and economically friendly approach to sustainable date palm cultivation. Exhibiting numerous biological activities, including growth-promoting, antioxidants, and antimicrobial properties, date palm seed extract was investigated as a potential growth promoter in farmed shrimp. This study specifically aimed to determine how DPSE can enhance the shrimp’s growth, immunity, and antioxidant status. Supplementing shrimp diets with 1 or 2g of DPSE not only significantly enhances growth but also reduces the microbial load. Demonstrating a wider range of benefits, all tested levels (1, 2, or 4 g) of DSPE were effective in significantly improving antioxidant status, immune function, and intestinal health.
Growth assessment is crucial in fish farming as it directly impacts economic viability and efficiency. A study showed that adding DPSE to shrimp diets improved growth performance, with the best results seen at a dosage of 1 g of DPSE/kg. This aligns with the dose-dependent results reported by Mohammadi et al. (2018) in common carp (Cyprinus carpio). While they demonstrated that the incorporation of 4% date palm seed extract significantly reduced growth performance, the most favorable growth outcomes were conversely achieved at a low inclusion rate of 0.5%. Common carp (Cyprinus carpio) exhibited significantly better growth and feed efficiency when their diet included up to 5 g/kg of date palm seed meal (Ahmed et al., 2017). Several studies have demonstrated that the incorporation of date palm fruit extracts in the diets of common carp (Hoseinifar et al., 2015, 2016), African catfish (Clarias gariepinus) (Al-Khalaifah et al., 2020), and Goldfish (Carassius auratus) (Heidarieh et al., 2023) enhances growth performance and feed utilization. The improved growth seen in this research may be attributable to the significant nutritional composition of DPSE, as documented by previous literature. Mohammadi et al. (2016) and Himanshu et al. (2024) revealed that DPSE contains considerable levels of key nutrients, including 4.4% protein, 15.87% crude fiber, 4.34% moisture, and 1.2% ash (per 100g) (Table 8). In a recent study, Shoribei et al. (2025) found that supplementing red tilapia diets with 200–300 mg of date palm seed extract significantly improved growth performance. Nile tilapia also showed enhanced growth rates when their diet included date palm seed meal (DSM) fermented with Aspergillus oryzae (Dawood et al., 2020). Traditionally, high DSM inclusion was believed to hinder digestion and feed consumption by affecting enzyme activity (Carrillo-Farnés et al., 2007). However, the inclusion of A. oryzae fermented DSM at levels up to 200 g/kg actually improved Nile tilapia’s digestive capabilities, leading to strong growth performance and feed utilization (Dawood et al., 2020). This correlation suggests that enhanced nutrient digestibility and feed efficiency are key factors driving better growth. Other studies suggest that the gallic acid content in date seed extract provides robust antioxidant and anti-inflammatory effects (Hadidi et al., 2024). This action enhances overall health, resulting in improved growth and support for cellular protein synthesis. Gallic acid is known for its anti-inflammatory properties in fish, especially in the gut-liver axis by reducing enteritis (Zhao et al., 2024). It decreases the levels of pro-inflammatory cytokines and modulates the immune system through PPAR gamma, which helps maintain immune balance (Hadidi et al., 2024; Zhao et al., 2024). The author suggests that the observed improvements result from DPSE’s enhanced production of goblet cells, a finding that will be detailed later.
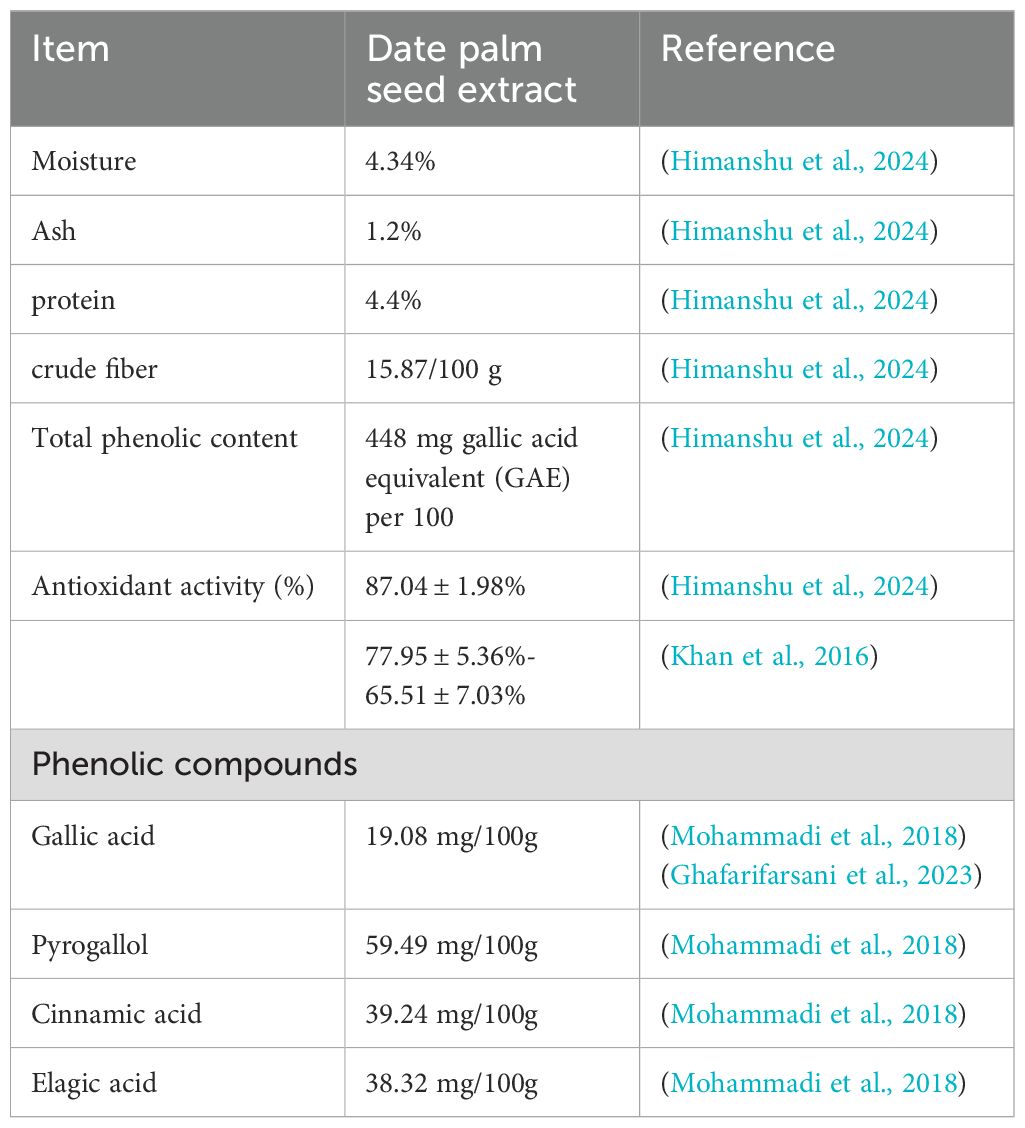
Table 8. The study identified some compounds found in date palm seed extracts based on the literature.
The whole-body composition was significantly improved in response to dietary DPSE supplementation. Compared to the control diet, DPSE enhanced both protein and lipid contents while decreasing the ash content. Furthermore, the Gross Energy level was elevated across all supplemented groups. Based on our current findings (Mohammadi et al., 2018), the dietary inclusion of DPSE at 0.5% resulted in improved crude lipid and crude protein content in common carp. In contrast, at the highest concentration of DPSE (5 g/kg), there was a significant decrease in ash content (P<0.05) and lower moisture content. Catfish fed diets supplemented with 10-15g of doum palm fruit powder showed a significant improvement in dry matter, crude protein, and crude lipid content (Al-Khalaifah et al., 2020). As mentioned, gallic acid is the main compound in DPSE (Himanshu et al., 2024). Gallic acid can promote the growth of beneficial gut bacteria, which helps break down feed components and produce essential metabolic compounds, improving nutrient utilization efficiency. In common carp, Ghafarifarsani et al. (2023) observed that gallic acid led to a decrease in ash content, proposing that this effect may be mediated by the compound’s ability to enhance digestive enzymes.
Natural antioxidants are widely recognized for their role in mitigating oxidative stress and enhancing antioxidant capacity. Our findings confirm this utility, demonstrating that adding DPSE to the shrimp diet significantly improved their antioxidant status and general health. We found improved hemolymph biochemical features in shrimp that received DPSE in their diet compared to those in the control diet. These indicators are important for assessing the nutritional status, overall health, and adaptability of shrimp and fish. Adding 0.5% DPSE to the diets of common carp led to a significant decrease in MDA levels in their brain and muscle (Mohammadi et al., 2018). The significant antioxidant properties of date palm derivatives are well-documented (Shoribei et al., 2025; Alqahtani et al., 2025). Studies using irradiated palm fruit extracts have shown a marked reduction in MDA content and enhanced activity of antioxidant enzymes such as superoxide dismutase, catalase, glutathione S-transferase, and peroxidase (Heidarieh et al., 2023). This protective effect has also been observed in tested probiotics and aqueous date palm fruit extracts (Dawood et al., 2020), consistent with the demonstrated ability of gallic acid within DPSE to safeguard against intracellular reactive oxygen species (ROS) over a four-week period (Zhao et al., 2024). Consistent with recent findings in Red tilapia (Shoribei et al., 2025), where 200–300 mL of date palm seed extract significantly increased CAT, SOD, and glutathione peroxidase (GPx), our study demonstrated a concurrent increase in these antioxidant enzyme activities with higher dietary DPSE levels. Additionally, we recorded a notable boost in phagocytosis, a crucial cellular defense mechanism. This work is significant as it constitutes the first time the effects of DPSE-based diets on the biochemical and immune markers of Litopenaeus vannamei have been examined. The antioxidant activity of DPSE has been documented by Himanshu et al. (2024), who found that it is rich in total phenolic content with 448 mg GAE/100 g and an antioxidant activity of 87.04 ± 1.98%. This finding is consistent with Jabeen et al. (2020), although it is higher than the values reported by Al-Tamimi et al. (2021) and slightly exceeds that of Khudori cultivar date seeds (124.54 ± 2.89 mg GAE/100g). The elevated TPC is directly attributed to the high concentration of polyphenolic compounds in date seed extract. Dietary inclusion of DPSE has shown significant immune-boosting effects in various studies. For example, in common carp, supplementation improved the respiratory burst response and lysozyme activity, with benefits observed at concentrations up to 2% (Mohammadi et al., 2018). Similarly, common carp fry fed date palm fruit extracts exhibited enhanced immune function, as indicated by increased levels of lysozyme, total immunoglobulins, and alkaline phosphatase (ALP) activity (Hoseinifar et al., 2015). The dual enhancement of immune function and antioxidant status by DPSE is hypothesized to be mediated by its gallic acid content, which is known to combat oxidative stress and suppress pro-inflammatory cytokines in aquatic organisms (Ghafarifarsani et al., 2023; Zhao et al., 2024). Moreover, date palm fruit extracts were found to significantly downregulate the gene expression of the pro-inflammatory markers TNF-alpha and IL-1beta, while upregulating lysozyme (LY) gene expression (Hoseinifar et al., 2015). Date palm extracts have consistently shown the ability to modulate and enhance immune function in various fish species (Dawood et al., 2020; Heidarieh et al., 2023; Shoribei et al., 2025), as demonstrated in our current research. Furthermore, lysozyme activity was enhanced in golden fish treated with irradiated palm fruit extracts, along with increases in total IgG and protease activity (Heidarieh et al., 2023). Red tilapia supplemented with 200–300 mL of date palm seed extract also showed boosted immunoglobulin levels (Shoribei et al., 2025). Additionally, fermented DSM (50–200 g/kg) significantly improved several cellular defense mechanisms, including phagocytic index and phagocytic activity, as well as lysozyme activity (Dawood et al., 2020).
Our study revealed that DPSE significantly increased serum lysozyme activity in fish. This boost is consistent with findings linking higher lysozyme levels to a greater presence of immune cells. Given that lysozyme levels reflect the proliferation of phagocytes and increased lysosome production, it serves as a strong marker for a diet’s bactericidal effect. Similar positive results have been observed with DPSE in previous studies (Mohammadi et al., 2018; Heidarieh et al., 2023; Shoribei et al., 2025). Importantly, we also observed an enhancement in blood phagocytosis with fermented DSM. Phagocytosis plays a crucial role in the defense against infectious diseases (Harikrishnan et al., 2011), and our findings support the significant role of DPSE in strengthening the immune response, leading to improved pathogen resistance. According to Hoseinifar et al. (2016), supplementing common carp diets with DPSE resulted in a significant enhancement of immune function. DPSE contains bioactive compounds such as butan-1-one, hydrocortisone acetate, and tetradecanoic acid, which exhibit notable immunomodulatory, anti-inflammatory, and antioxidant properties (Abdallah et al., 2023).
Maintaining a healthy balance of gut bacteria is crucial for shrimp well-being, indicating effective management practices and good water quality. Our research showed that adding DPSE to shrimp diets improved their intestinal microbial balance. DPSE supplementation reduced pathogenic bacteria like Total enteric bacteria, Total Clostridium, and Total aerobic bacteria, while increasing probiotic bacteria levels. A healthy gut environment is essential for shrimp growth, nutrient digestion, and protection against harmful microorganisms (Zhang et al., 2019). This study found that shrimp fed diets with 4 g/kg of DPSE showed a significant increase in probiotic bacteria and Clostridium sp. in their intestines, along with a decrease in total enteric and aerobic bacteria. This aligns with previous research, such as that by Himanshu et al. (2024), which emphasized the antimicrobial properties of DPSE. These results suggest that DPSE could be a beneficial additive for enhancing gut flora and shrimp health. Additionally, the inclusion of fermented DSM (at 50, 100, and 200 g/kg in the diet) led to changes in villus length and goblet cell numbers in the intestines of Nile tilapia, as reported by Dawood et al. (2020).
Date palm seed extract increased the expression of immune and antioxidant-related genes in treated groups. Specifically, immune genes such as LGBP, PX, and PPA were upregulated, along with antioxidant genes cytMnSOD and mtMnSOD. LGBP and PX are important pattern recognition proteins that bind to microorganisms and activate signaling within hemocytes (Amparyup et al., 2012; Mathew et al., 2025a, b). In white shrimp, the antioxidant enzymes cytMnSOD and mtMnSOD play a crucial role in neutralizing reactive oxygen species generated during phagocytosis. DPSE’s regulation of the immune system resulted in improved growth performance, enhanced antioxidant activity, and a stronger immunological response. DPSE also increased shrimp survival rates when infected with V. parahaemolyticus, showcasing its protective effects against bacterial infections (Abdelnour et al., 2023). The overall findings suggest that DPSE has immunomodulatory, antioxidant, and growth-promoting properties that contribute to enhanced shrimp health and resistance to infections.
Our findings establish that DPSE functions as a non-specific immune modulator in shrimp, leading to significant improvements in growth performance, antioxidant activity, and overall immune response. Importantly, DPSE significantly boosted shrimp survival rates when challenged with the deadly bacterial pathogen Vibrio parahaemolyticus. This enhanced resilience is supported by previous work demonstrating the antimicrobial activity of DPSE (Himanshu et al., 2024). Protective efficacy is attributed to the extract’s rich profile of bioactive compounds (Manai et al., 2024), including flavonols (epicatechin, catechin, and procyanidins B) and polyphenolic acids (p-hydroxybenzoic acid, protocatechuic acid, and caffeic acid, Table 8), alongside carotenoids and dietary fibers (Pakkish and Mohammadrezakhani, 2020).
The improved resistance to V. parahaemolyticus infection can be attributed to DPSE’s ability to enhance the shrimp’s immune system, boost antioxidant defenses, and promote healthier growth. In support of our findings, Hadidi et al. (2024) proposed that the antimicrobial activity of DPSE against V. parahaemolyticus in shrimp is mediated by the extract’s gallic acid component. Previous studies have shown that V. parahaemolyticus infection can be reduced by including bee venom-loaded chitosan nanoparticles (Eissa et al., 2025b), fermented probiotics (Eissa et al., 2025a), phytobiotics (Eissa et al., 2024), and Spirulina platensis (Ahmed et al., 2025) in the diet. To gain a deeper understanding of how fermented DSM affects the gut environment of aquatic animals, comprehensive microbiome and proteomic studies are now essential to analyze its impact on intestinal digestive enzymes and microbial populations. A limitation of this study is its reliance on HPLC analysis of the DPSE to confirm bioactive compounds in the feed additive. While previous studies were cited to discuss our significant findings, future research should employ a more robust technique, such as LC-MS/MS, to identify a greater number of bioactive compounds and elucidate biological pathways.
5 Conclusion
This study marks the first time that date palm seed extract (DPSE) has been identified as a novel and effective growth promoter and health enhancer in shrimp aquaculture. Our findings demonstrate that supplementing shrimp diets with either 1 or 2 grams of DPSE significantly improves growth performance and enhances feed utilization and body composition. Beyond these immediate benefits, DPSE also showed promising effects on shrimp health. It was observed to boost immune responses, improve antioxidant capacity, and maintain hepatopancreas integrity. Furthermore, the study revealed that DPSE enhanced the expression of antioxidant-associated genes, providing a deeper understanding of its beneficial mechanisms. This work suggests that date palm seed extract could be a novel feed additive for shrimp, offering a sustainable and environmentally friendly approach to enhancing shrimp aquaculture.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by ZU-IACUC committee (ZU-IACUC/2/F/25/2023). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RTM: Data curation, Software, Visualization, Writing – review & editing. NKA: Investigation, Validation, Writing – review & editing. HSG: Investigation, Validation, Visualization, Writing – review & editing. EE: Validation, Visualization, Writing – review & editing, Data curation, Investigation. YAA: Software, Writing – review & editing, Validation, Visualization. ATM: Investigation, Writing – review & editing, Software. NG: Investigation, Validation, Writing – review & editing. AA: Investigation, Visualization, Writing – review & editing. LA: Validation, Visualization, Writing – review & editing. ZA: Investigation, Software, Visualization, Writing – review & editing. MEHE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. EHE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. KFU253840). Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R457), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgments
We are thankful to the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia for their financial support (Grant No. KFU253840). The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia (PNURSP2025R457).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah W. E., Awad H. M., and AbdelMohsen M. M. (2023). Phytochemical composition, antioxidant and antitumor activities of some date palm pollen extracts. Egypt. J. Chem. 66, 425–434. doi: 10.21608/ejchem.2022.144841.6320
Abd El-Naby A. S., Eid A., Gaafar A. Y., Sharawy Z., Khattaby A., El-sharawy M. S., et al. (2024). Overall evaluation of the replacement of fermented soybean to fish meal in juvenile white shrimp, Litopenaeus vannamei diet: growth, health status, and hepatopancreas histomorphology. Aquac. Int. 32, 1665–1683. doi: 10.1007/s10499-023-01234-0
Abdelnour S. A., Ghazanfar S., Abdel-Hamid M., Abdel-Latif H. M., Zhang Z., and Naiel M. A. (2023). Therapeutic uses and applications of bovine lactoferrin in aquatic animal medicine: an overview. Vet. Res. Commun. 47, 1015–1029. doi: 10.1007/s11259-022-10060-3
Abdulrahman L. (2020). Impacts of date palm seeds (Phoenix Dactyliferous L.) on common Carpcyprinus carpio L. Biological indices and blood pictures. Indian J. Public Health Res. Dev. 11, 1910–1913. doi: 10.37506/v11/i1/2020/ijphrd/194133
Ahmed V. M., Abdulrahman N. M., HamaAmeen S. A., Hassan B., Abbas A., Hussen B. A., et al. (2017). Impacts of date palm seeds (Phoenix dactyliferous L.) on growth indices and nutrient utilization of common carp Cyprinus carpio L. J. Agric. Sci. Technol. B 7, 280–284. doi: 10.17265/2161-6264/2017.04.005
Ahmed R. A., Jastaniah S. D., Alaidaroos B. A., Shafi M. E., El-Haroun E., Abd El-Aziz Y. M., et al. (2025). Effects of dietary Spirulina platensis supplementation on growth performance, whole body composition, antioxidant activity, histological alterations, and resistance to Vibrio parahaemolyticus in Pacific white shrimp, Litopenaeus vannamei. Aquac Rep. 40, 102606. doi: 10.1016/j.aqrep.2024.102606
Al-Habsi N. (2025). Date Palm (Phoenix dactylifera L.) Fruit: Strategic Crop for Food Security, Nutritional Benefits, Postharvest Quality, and Valorization into Emerging Functional Products. Sustainability 17, 7491. doi: 10.3390/su17167491
Ali Z., Li J., Zhang Y., Naeem N., Younas S., and Javeed F. (2022). Dates (phoenix dactylifera) and date vinegar: Preventive role against various diseases and related in vivo mechanisms. Food Rev. Int. 38, 480–507. doi: 10.1080/87559129.2020.1735411
Al-Khalaifah H. S., Khalil A. A., Amer S. A., Shalaby S. I., Badr H. A., Farag M. F. M., et al. (2020). Effects of dietary doum palm fruit powder on growth, antioxidant capacity, immune response, and disease resistance of african catfish, Clarias gariepinus (B.). Animals 10, 1407. doi: 10.3390/ani10081407
Alqahtani N. K., Ghazzawy H. S., Mathew R. T., Alngada R. S., Eissa M. E., Abdelnour S. A., et al. (2025). Enhancing reproductive capacity in hybrid Red Tilapia (Oreochromis niloticus× O. mossambicus) via dietary administration of date palm pollen (Phoenix dactylifera L.). Aquac. Rep. 41, 102670. doi: 10.1016/j.aqrep.2025.102670
Al-Shwyeh H. A. (2019). Date palm (Phoenix dactylifera L.) fruit as potential antioxidant and antimicrobial agents. J. Pharm. Bioallied. Sci. 11, 1–11. doi: 10.4103/JPBS.JPBS_168_18
Al-Tamimi A., Alfarhan A., and Rajagopal R. (2021). Antimicrobial and anti-biofilm activities of polyphenols extracted from different Saudi Arabian date cultivars against human pathogens. J. Infect. Public Health 14, 1783–1787. doi: 10.1016/j.jiph.2021.10.006
Aly S. M. and Fathi M. (2024). Advancing aquaculture biosecurity: a scientometric analysis and future outlook for disease prevention and environmental sustainability. Aquac. Int. 32, 8763–8789. doi: 10.1007/s10499-024-01589-y
Amparyup P., Sutthangkul J., Charoensapsri W., and Tassanakajon A. (2012). Pattern recognition protein binds to lipopolysaccharide and β-1, 3-glucan and activates shrimp prophenoloxidase system. J. Biol. Chem 287, 10060–10069. doi: 10.1074/jbc.M111.294744
Carrillo-Farnés O., Forrellat-Barrios A., Guerrero-Galván S., and Vega-Villasante F. (2007). A review of digestive enzyme activity in penaeid shrimps. Crustaceana 80, 257–275. doi: 10.1163/156854007780162424
Chen Y.-Y., Chen J.-C., Lin Y.-C., Yeh S.-T., and Huang C. L. (2015). White shrimp Litopenaeus vannamei that have received Gracilaria tenuistipitata extract show early recovery of immune parameters after ammonia stressing. Mar. Drugs 13, 3606–3624. doi: 10.3390/md13063606
Cheng A.-C., Chang H.-T., Lee T.-Y., Lin J.-S., and Liu C.-H. (2025). SYNLAC Prime probiotics alleviate Enterocytozoon hepatopenaei-induced damage in white shrimp, Penaeus vannamei by enhancing growth, immunity, and resistance to Vibrio parahaemolyticus. Fish Shellfish Immunol. 163, 110383. doi: 10.1016/j.fsi.2025.110383
Cheng W., Wang L.-U., and Chen J. C. (2005). Effect of water temperature on the immune response of white shrimp Litopenaeus vannamei to Vibrio alginolyticus. Aquaculture 250, 592–601. doi: 10.1016/j.aquaculture.2005.04.060
Dadras F., Velisek J., and Zuskova E. (2023). An update about beneficial effects of medicinal plants in aquaculture: A review. Vet. Med. 68, 449–463. doi: 10.17221/96/2023-VETMED
Dawood M. A., Eweedah N. M., Khalafalla M. M., and Khalid A. (2020). Evaluation of fermented date palm seed meal with Aspergillus oryzae on the growth, digestion capacity and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 26, 828–841. doi: 10.1111/anu.13042
Dighiesh H. S., Alharbi N. A., Awlya O. F., Alhassani W. E., Hassoubah S. A., Albaqami N. M., et al. (2024). Dietary multi-strains Bacillus spp. enhanced growth performance, blood metabolites, digestive tissues histology, gene expression of Oreochromis niloticus, and resistance to Aspergillus flavus infection. Aquaculture Int. 32, 7065–7086. doi: 10.1007/s10499-024-01502-7
Du L. and Niu C. J. (2003). Effects of dietary substitution of soya bean meal for fish meal on consumption, growth, and metabolism of juvenile giant freshwater prawn, Macrobrachium rosenbergii. Aquac. Nutr. 9, 139–143. doi: 10.1046/j.1365-2095.2003.00239.x
Echegaray N., Gullón B., Pateiro M., Amarowicz R., Misihairabgwi J. M., and Lorenzo J. M. (2023). Date fruit and its by-products as promising source of bioactive components: A review. Food Rev. Intern. 39, 1411–1432. doi: 10.1080/87559129.2021.1934003
Eissa E.-S. H., Dowidar H. A., Al-Hoshani N., Baazaoui N., Alshammari N. M., Bahshwan S. M., et al. (2025a). Dietary supplementation with fermented prebiotics and probiotics can increase growth, immunity, and histological alterations in Pacific whiteleg shrimp (Litopenaeus vannamei) challenged with Vibrio parahaemolyticus. Aquac. Int. 33, 62. doi: 10.1007/s10499-024-01704-z
Eissa E.-S. H., Elbahnaswy S., El-Baz A. H., El-Haroun E., Ashour M., Mansour A. T., et al. (2024). Effects of dietary commercial phytobiotic “Sanacore® GM” on Pacific white shrimp (Litopenaeus vannamei) growth, immune response, redux status, intestinal health, and disease resistance against Fusarium solani. Aquac. Int. 32, 3041–3060. doi: 10.1007/s10499-023-01310-5
Eissa M. E., Hendam B. M., ElBanna N. I., and Aly S. M. (2025b). Bee venom loaded chitosan nanoparticles enhances growth, immunity and resistance to vibrio parahaemolyticus in pacific white shrimp. Scie. Rep. 15, 1–18. doi: 10.1038/s41598-025-11011-z
El Asely A. M., Shaheen A., Abbass A. A., Sudhakaran R., Linh N., Yoshida T., et al. (2010). Immunomodulatory effect of plant-mixed feed in kuruma shrimp, Marsupenaeus japonicus, and its protective efficacy against white spot syndrome virus infection. J. Fish Dis. 33, 859. doi: 10.1111/j.1365-2761.2010.01186.x
El-Sayed A. F. M., Eissa E. S. H., Hendam B. M., Dighiesh H. S., Abd Elnabi H. E., Abd El-Aziz Y. M., et al. (2025). Dietary organic acid blend modulates hemato-immunological parameters, digestive and reproductive performances in red tilapia (Oreochromis niloticus× O. mossambicus) broodstock. Fish Physiol. Biochem. 51, 41. doi: 10.1007/s10695-025-01459-1
Engstad R. E., Robertsen B., and Frivold E. (1992). Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol. 2, 287–297. doi: 10.1016/S1050-4648(06)80033-1
FAO (2024). The State of World Fisheries and Aquaculture 2024. Blue Transformation in action (Rome: FAO). doi: 10.4060/cd0683en
FAOSTAT (2025). Production of Dates. Available online at: https://www.fao.org/faostat/en/data/QCL/visualize (Accessed 8 May 2025).
Fernández-López J., Viuda-Martos M., Sayas-Barberá E., Navarro-Rodríguez de Vera C., and Pérez-Álvarez J.Á. (2022). Biological, nutritive, functional and healthy potential of date palm fruit (Phoenix dactylifera L.): Current research and future prospects. Agronomy 12, 876. doi: 10.3390/agronomy12040876
Flegel T. W. (2012). Historic emergence, impact and current status of shrimp pathogens in Asia. J. Invertebr. Pathol. 110, 166–173. doi: 10.1016/j.jip.2012.03.004
Ghafarifarsani H., Hoseinifar S. H., Adhami B., Rohani M. F., and Van Doan H. (2023). Dietary gallic acid influences serum enzymatic parameters and immunological responses in Cyprinus carpio exposed to crowding stress. Aquac. Rep. 30, 101630. doi: 10.1016/j.aqrep.2023.101630
Hadidi M., Liñán-Atero R., Tarahi M., Christodoulou M. C., and Aghababaei F. (2024). The potential health benefits of gallic acid: therapeutic and food applications. Antioxidants 13, 1001. doi: 10.3390/antiox13081001
Harikrishnan R., Kim J.-S., Kim M.-C., Balasundaram C., and Heo M.-S. (2011). Prunella vulgaris enhances the non-specific immune response and disease resistance of Paralichthys olivaceus against Uronema marinum. Aquaculture 318, 61–66. doi: 10.1016/j.aquaculture.2011.05.020
Heidarieh M., Gholamhosseini A., Sheikhzadeh N., and Esteban M. A. (2023). Effects of γ-Irradiated Date (Phoenix dactylifera) Fruit on Growth, Immunological and Antioxidant Parameters of Goldfish (Carassius auratus). Fishes 8, 251. doi: 10.3390/fishes8050251
Himanshu, Kumar N., Khangwal I., and Upadhyay A. (2024). Assessment of nutritional composition, phytochemical screening, antioxidant, and antibacterial activities of date palm (Phoenix dactylifera) seeds. Discover Food 4, 151. doi: 10.1007/s44187-024-00234-0
Hoseinifar S. H., Khalili M., Rufchaei R., and Raeisi M. (2016). Investigating the effects of date palm extract on growth performance and mucus immune parameters in Common Carp Cyprinus carpio Linnaeus 1758 fingerlings. Journal of applied ichthyological research 3, 89–100.
Hoseinifar S. H., Khalili M., Rufchaei R., Raeisi M., Attar M., Cordero H., et al. (2015). Effects of date palm fruit extracts on skin mucosal immunity, immune related genes expression and growth performance of common carp (Cyprinus carpio) fry. Fish Shellfish Immunol. 47, 706–711. doi: 10.1016/j.fsi.2015.09.046
Hwang J.-H., Lee S.-W., Rha S.-J., Yoon H.-S., Park E.-S., Han K.-H., et al. (2013). Dietary green tea extract improves growth performance, body composition, and stress recovery in the juvenile black rockfish, Sebastes schlegeli. Aquac. Int. 21, 525–538. doi: 10.1007/s10499-012-9586-5
Jabeen A., Parween N., Sayrav K., and Prasad B. (2020). Date (Phoenix dactylifera) seed and syringic acid exhibits antioxidative effect and lifespan extending properties in Caenorhabditis elegans. Arab. J. Chem. 13, 9058–9067. doi: 10.1016/j.arabjc.2020.10.028
Kawahara E., Ueda T., and Nomura S. (1991). In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. 26, 213–214. doi: 10.3147/jsfp.26.213
Khan S. A., Al Kiyumi A. R., Al Sheidi M. S., Al Khusaibi T. S., Al Shehhi N. M., and Alam T. (2016). In vitro inhibitory effects on α-glucosidase and α-amylase level and antioxidant potential of seeds of Phoenix dactylifera L. Asian Pac. J. Trop. Biomed. 6, 322–329. doi: 10.1016/j.apjtb.2015.11.008
Lin Y.-C., Lee F. F., Wu C.-L., and Chen J.-C. (2010). Molecular cloning and characterization of a cytosolic manganese superoxide dismutase (cytMnSOD) and mitochondrial manganese superoxide dismutase (mtMnSOD) from the kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 28, 143–150. doi: 10.1016/j.fsi.2009.10.012
Liu C.-H., Cheng W., Kuo C.-M., and Chen J. C. (2004). Molecular cloning and characterisation of a cell adhesion molecule, peroxinectin from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 17, 13–26. doi: 10.1016/j.fsi.2003.11.002
Liu Y., Smith M. D., Abbott J. K., Dietz D., Colson Leaning D., Smyth A., et al. (2025). The global seafood trade, embodied nutrients, and nutritional affordability. Nat. Commun. 16, 5868. doi: 10.1038/s41467-025-61012-9
Liu Y., Zhuang Z., Liao Z., Yao R., Chen M., Wei H., et al. (2023). Effects of low-fish-meal diet supplemented with coenzyme Q10 on growth performance, antioxidant capacity, intestinal morphology, immunity and hypoxic resistance of Litopenaeus vannamei. Antioxidants (Basel) 12, 2042. doi: 10.3390/antiox12122042
Ma C., Fan S., Wang Y., Yang H., Qiao Y., Jiang G., et al. (2021). Rapid detection of enterocytozoon hepatopenaei infection in shrimp with a real-time isothermal recombinase polymerase amplification assay. Front. Cell. Infect. Microbiol. 11, 631960. doi: 10.3389/fcimb.2021.631960
Manai S., Boulila A., Silva A. S., Barbosa-Pereira L., Sendón R., and Khwaldia K. (2024). Recovering functional and bioactive compounds from date palm by-products and their application as multi-functional ingredients in food. Sustain. Chem. Pharm. 38, 101475. doi: 10.1016/j.scp.2024.101475
Mathew R. T., Alqahtani N. K., Alkhamis Y. A., Alngada R. S., Abdul Whed R., Aljaafari N. A., et al. (2025a). Investigating the effects of Siraitia grosvenorii fruit on growth performance, immune response, antioxidant gene expression, and resistance to Vibrio parahaemolyticus in Litopenaeus vannamei shrimp. Aquac. Int. 33, 1–21. doi: 10.1007/s10499-025-01861-9
Mathew R. T., Alqahtani N. K., Ghazzawy H. S., Almutairi L. A., Alqahtani M. A., Alkhamis Y. A., et al. (2025b). Dietary pterostilbene for improving the whiteleg shrimp (Litopenaeus vannamei): In vivo and in silico insights into growth, hemolymph physiology, redox state, immune function and resistance to Vibrio parahaemolyticus. Aquac. Int. 33, 505. doi: 10.1007/s10499-025-02186-3
Mohammadi M., Soltani M., Siahpoosh A., and Mehrjan M. S. (2016). Effects of Date Palm (Phoenix dactylifera) Seed Extract on Heavy Metals Concentrations in Carp (Cyprinus carpio). Pol. J. Environ. Stud. 25, 1117–1123. doi: 10.15244/pjoes/61853
Mohammadi M., Soltani M., Siahpoosh A., and Shamsaie M. (2018). Effects of dietary supplementation of date palm (Phoenix dactylifera) seed extract on body composition, lipid peroxidation and tissue quality of common carp (Cyprinus carpio) juveniles based on the total volatile nitrogen test. Iran. J. Fish. Sci. 17, 394–402. doi: 20.1001.1.15622916.2018.17.2.11.1
Nonwachai T., Purivirojkul W., Limsuwan C., Chuchird N., Velasco M., and Dhar A. K. (2010). Growth, nonspecific immune characteristics, and survival upon challenge with Vibrio harveyi in Pacific white shrimp (Litopenaeus vannamei) raised on diets containing algal meal. Fish & Shellfish Immunology 29, 298–304.
Okon E. M., Birikorang H. N., Munir M. B., Kari Z. A., Téllez-Isaías G., Khalifa N. E., et al. (2023). A global analysis of climate change and the impacts on oyster diseases. Sustainability 15, 12775. doi: 10.3390/su151712775
Okon E. M., Oyesiji A. A., Okeleye E. D., Kanonuhwa M., Khalifa N. E., Eissa E. S. H., et al. (2024). The escalating threat of climate change-driven diseases in fish: evidence from a global perspective–a literature review. Environ. Res. 263, 120184. doi: 10.1016/j.envres.2024.120184
Pakkish Z. and Mohammadrezakhani S. (2020). Comparison of phytochemicals and their antioxidant activity in seven date palm varieties grown in Iran. Int. J. Food Properties 23, 1766–1776. doi: 10.1080/10942912.2020.1820516
Pakravan S., Akbarzadeh A., Sajjadi M. M., Hajimoradloo A., and Noori F. (2018). Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile Pacific white shrimp Litopenaeus vannamei. Aquac. Nutri. 24, 594–604. doi: 10.1111/anu.12594
Pandey A., Pathan M. A., Ananthan P., Sudhagar A., Krishnani K. K., Sreedharan K., et al. (2024). Stocking for sustainable aqua-venture: optimal growth, yield and economic analysis of Penaeus vannamei culture in inland saline water (ISW) of India. Environment Dev. Sustainability 26, 6913–6942. doi: 10.1007/s10668-023-02993-9
Raza B., Zheng Z., Zhu J., and Yang W. (2024). A review: Microbes and their effect on growth performance of Litopenaeus vannamei (white leg shrimps) during culture in biofloc technology system. Microorganisms 12, 1013. doi: 10.3390/microorganisms12051013
San Segundo-Val I. and Sanz-Lozano C. S. (2016). Introduction to the gene expression analysis. Mol. Genet. Asthma 1434, 29–43. doi: 10.1007/978-1-4939-3652-6_3
Shoribei R., Mohammadiazarm H., Hedayati A., and Maniat M. (2025). Effects of different levels of aqueous extract from date palm (Phoenix dactylifera) waste on growth performance, immune parameters, and antioxidant activity in juvenile red tilapia (Oreochromis mossambicus x Oreochromis niloticus). Aquat. Anim. Nutr. 10, 89. doi: 10.22124/janb.2025.29241.1264
Song Y. L. and Hsieh Y. T. (1994). Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: analysis of reactive oxygen species. Developmental & Comparative Immunology 18, 201–209.
Suvarna K. S., Layton C., and Bancroft J. D. (2018). Bancroft’s theory and practice of histological techniques E-Book (China: Elsevier health sciences).
Vieira F., Buglione C., Mourino J., Jatobá A., Martins M., Schleder D., et al. (2010). Effect of probiotic supplemented diet on marine shrimp survival after challenge with Vibrio harveyi. Arq. Bras. Med. Vet. Zootec. 62, 631–638. doi: 10.1590/S0102-09352010000300019
Willer D. F., Nicholls R. J., and Aldridge D. C. (2021). Opportunities and challenges for upscaled global bivalve seafood production. Nat. Food 2, 935–943. doi: 10.1038/s43016-021-00423-5
Won S., Hamidoghli A., Choi W., Bae J., Jang W. J., Lee S., et al. (2020). Evaluation of potential probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on growth performance, immune response, gut histology and immune-related genes in whiteleg shrimp, Litopenaeus vannamei. Microorganisms 8, 281. doi: 10.3390/microorganisms8020281
Zhang R., Jiang Y., Zhou L., Chen Y., Wen C., Liu W., et al. (2019). Effects of dietary yeast extract supplementation on growth, body composition, non-specific immunity, and antioxidant status of Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 86, 1019–1025. doi: 10.1016/j.fsi.2018.12.052
Keywords: phytochemical, growth, antioxidant, date palm seed extract, shrimp
Citation: Mathew RT, Alqahtani NK, Ghazzawy HS, El-Haroun E, Alkhamis YA, Mansour AT, Ganesan N, El Asely AM, Almutairi LA, Abdul Kari Z, Eissa MEH and Eissa E-SH (2025) Potential symbiotic effects of date palm seed extract on growth, immunity, antioxidant activities, gut microbiota, expression levels, and Vibrio parahaemolyticus resistance in Shrimp. Front. Mar. Sci. 12:1696262. doi: 10.3389/fmars.2025.1696262
Received: 31 August 2025; Accepted: 27 October 2025;
Published: 20 November 2025.
Edited by:
Md Saydur Rahman, The University of Texas Rio Grande Valley, United StatesReviewed by:
Neeraj Kumar, National Institute of Abiotic Stress Management (ICAR), IndiaBasanta Kumar Das, Central Inland Fisheries Research Institute (ICAR), India
Copyright © 2025 Mathew, Alqahtani, Ghazzawy, El-Haroun, Alkhamis, Mansour, Ganesan, El Asely, Almutairi, Abdul Kari, Eissa and Eissa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roshmon Thomas Mathew, cm1hdGhld0BrZnUuZWR1LnNh; Ehab El-Haroun, ZWhhYi5yZWRhQHVhZXUuYWMuYWU=; El-Sayed Hemdan Eissa, c2F5ZWRoZW1kQGdtYWlsLmNvbQ==
 Roshmon Thomas Mathew
Roshmon Thomas Mathew Nashi K. Alqahtani
Nashi K. Alqahtani Hesham S. Ghazzawy
Hesham S. Ghazzawy Ehab El-Haroun4,5*
Ehab El-Haroun4,5* Yousef Ahmed Alkhamis
Yousef Ahmed Alkhamis Zulhisyam Abdul Kari
Zulhisyam Abdul Kari Moaheda E. H. Eissa
Moaheda E. H. Eissa El-Sayed Hemdan Eissa
El-Sayed Hemdan Eissa