Abstract
Introduction:
The genus Ulva, abundant in the Adriatic Sea, serves as a sustainable source of bioactive compounds, including polyphenols and natural pigments such as chlorophylls and carotenoids, with potential applications in the food, nutraceutical, pharmaceutical, and environmental protection sectors. However, their low chemical stability, bioavailability, and undesirable sensory properties limit practical use.
Methods:
This study explored encapsulation methods for Ulva spp. extract using spray-drying and freeze-drying techniques, with biopolymer carriers such as maltodextrin as a conventional option and polydextrose as an innovative alternative, to enhance the stability and functionality of the bioactive compounds. Technological properties, including encapsulation yield, moisture content, bulk and tapped densities, rehydration time, and encapsulation efficiency, were evaluated, along with FTIR, DSC, and HPLC analyses.
Results and discussion:
Comparative results showed that, while the spray-dried extract without a carrier achieved the highest polyphenolic encapsulation efficiency of 86.5%, polydextrose was more effective than maltodextrin in protecting total carotenoids and lutein. Spray-dried powders exhibited greater moisture reduction and improved powder properties than freeze-dried powders, which rehydrated faster and maintained good thermal stability up to 240°C. Antioxidant assays (DPPH, ABTS, RP) showed similarly high activity across all formulations, indicating that the functional compounds were preserved regardless of the encapsulation method. These findings demonstrate that combining suitable encapsulation techniques with tailored biopolymer carriers enhances the stability, bioactivity, and handling of Ulva-derived bioactive compounds. This approach promotes the valorization of underutilized macroalgal biomass within blue biotechnology, aligns with green and circular bioeconomy principles, and offers promising pathways for developing innovative marine-derived functional ingredients.
1 Introduction
Approximately 70% of Earth’s surface is covered by oceans and seas. Marine organisms are an alternative source of nutritionally and biologically active ingredients in this vast area, since land-based resources are limited and exploited for various purposes. The increasing interest in marine resources, especially macroalgae, comes from their diverse bioactive compounds, which have potential applications in pharmaceuticals, cosmetics, food, feed, and dietary supplements, as well as in aquaculture (Leandro et al., 2019). Recent advances increasingly focus on integrating algae to improve aquaculture efficiency while supporting environmental protection and the sustainability of aquatic ecosystems. Using renewable feedstocks is essential for promoting global economic growth, conserving natural bioresources, and reducing ecological pollution (Selvasudha et al., 2023).
Among macroalgae, the genus Ulva, found worldwide in coastal areas, has attracted attention for its high nutritional content and bioactivity (Leandro et al., 2019; Nikolić et al., 2025). Macroalgae from this genus are among the most well-known edible seaweeds and are recognized as versatile sea crops (Yaich et al., 2011), while numerous studies have demonstrated their health benefits, indicating their potential as nutraceuticals and pharmaceuticals (Dominguez and Loret, 2019). Species such as U. lactuca, U. fasciata, and U. rigida, commonly known as sea lettuces, are among the most common macroalgae and belong to the family Ulvaceae (phylum Chlorophyta), a group of green macroalgae that are widespread in coastal regions around the world, including the Adriatic Sea (Mutavski et al., 2024). These macroalgae are characterized by their high content of bioactive compounds, including polyphenols, flavonoids, and natural pigments like chlorophylls and carotenoids (Dominguez and Loret, 2019; Hidayati et al., 2020), as well as essential nutrients such as polysaccharides, proteins, and fatty acids (Machado et al., 2020; Ouahabi et al., 2024). This makes them valuable sources of natural antioxidants and health-promoting agents. Their unique biochemical profile has garnered interest across various fields, especially for their industrial roles in pharmaceuticals, dietary supplements, functional foods, feeds, and cosmetics (Martins et al., 2023b). The presence of these compounds highlights the nutritional value of Ulva species and its potential to fight oxidative stress, inflammation, and microbial infections, making it a promising resource for sustainable use in healthcare and industry (Dominguez and Loret, 2019).
However, the use of bioactive compounds from Ulva is limited by their low chemical stability under various environmental conditions, poor bioavailability after oral ingestion, and undesirable sensory traits like bitterness and grassy flavor, which restrict their application in multiple industries (Martins et al., 2023b). This underscores the necessity for advanced processing methods to maintain bioactivity and enhance applicability.
Encapsulation is a crucial technique for improving the stability, bioavailability, and controlled release of sensitive compounds from natural sources like Ulva (Munin and Edwards-Lévy, 2011; Selvasudha et al., 2023). Among various techniques, spray drying and freeze drying are common methods for producing powders with appropriate qualities from liquid extracts (Ćujić Nikolić et al., 2024). These techniques are commonly used to safeguard delicate bioactive compounds, such as carotenoids, chlorophylls, and phenolics (Pérez-Gálvez et al., 2020; Martins et al., 2023a). In spray drying, the extract is sprayed into a hot chamber, where rapid water evaporation forms encapsulated particles, frequently using carriers such as maltodextrin or gum Arabic (Ćujić Nikolić et al., 2024; Radan et al., 2024). This process is efficient for large-scale production and cost-effective, although heat can partially degrade heat-sensitive compounds (Ćujić Nikolić et al., 2023). Freeze-drying involves freezing the extract and removing water by sublimation under low pressure, which better preserves heat-sensitive bioactives but takes more time and resources. Both methods increase stability, improve resistance to environmental factors, and extend shelf life. They help maintain the activity of compounds such as carotenoids and chlorophylls, which are essential in food, pharmaceuticals, and cosmetics (Ledari et al., 2024a).
The full potential of encapsulation techniques can be realized by utilizing various carriers to enhance the performance (Jovanović et al., 2021). In addition to traditional carriers with established benefits, as maltodextrin, recent studies have evaluated innovative options, such as polydextrose, to overcome the limited stability and bioavailability of extracted bioactive compounds (Nedović et al., 2011). Polydextrose, a cyclic polymer of glucose, can form inclusion complexes with guest molecules (active substances), enhancing characteristics (Lago and Noreña, 2016). Moreover, polydextrose has demonstrated prebiotic and probiotic activities (Do Carmo et al., 2016), with potential immunostimulatory effects in aquaculture. Additionally, these biopolymers can modify physicochemical and technological properties and mask macroalgae’s unpleasant odors and tastes through appropriate formulation.
Given the documented instability and limited bioavailability of bioactive compounds from Ulva spp., we hypothesize that encapsulating the extracts through spray-drying or freeze-drying, along with biopolymer carriers such as maltodextrin and polydextrose, will improve the stability, preserve functional activity, and enhance the technological properties of these compounds. This approach aims to produce powders from Ulva spp. biomass collected from the South Adriatic Sea coast, making them suitable for infusion into food, nutraceutical, and pharmaceutical products. This process also aims to valorize underused marine biomass in accordance with green and circular bioeconomy principles. To our knowledge, and based on available literature, this is the first scientific investigation to explore an environmentally friendly encapsulation process for developing innovative Ulva-based products.
2 Materials and methods
2.1 Sampling of Ulva spp. and preparation for the extraction process
Samples of Ulva spp. were collected from Boka Kotorska Bay (Adriatic Sea, southeastern Europe, Latitude: 42° 46’ 68.54” N, Longitude: 18° 68’ 63.84” E) in the coastal area during the summer season (May to June 2024), a period characterized by optimal biomass availability and phytochemical richness. The sampling was conducted by trained researchers from the Institute of Marine Biology at the University of Montenegro. Specimens were gathered from the lower intertidal zone and visually examined for integrity and maturity.
Microscopic examination utilizing a Leica inverted light microscope (Leica Microsystems, Wetzlar, Germany) confirmed that the collected samples belonged to the genus Ulva, exhibiting characteristic foliaceous thallus morphology. Due to the genus’s recognized taxonomic complexity and the morphological similarity among species, the samples were designated as Ulva spp., per current taxonomic conventions, pending further molecular identification.
After collection, the algal material was meticulously rinsed with distilled water to eliminate epiphytes, sand particles, and salts. The cleaned biomass was subsequently air-dried at ambient conditions for 48 hours, avoiding direct sunlight to minimize degradation of thermolabile compounds (Figure 1). The dried samples were ground to a uniform consistency using a laboratory mill, and the resultant powder was sieved through a 0.75 mm sieve, per the specifications of the Yugoslavian Pharmacopeia, to attain a moderately fine particle size (750 μm). The dried Ulva samples were stored in airtight, light-protected glass containers at a controlled room temperature until further analysis.
Figure 1

Air-dried Ulva spp. from the Adriatic Sea Coast.
2.2 Extraction process
The extraction process was performed according to the previously described procedure by Nikolić et al. (2025). The extraction focused on an eco-friendly, “green” maceration method, which is optimal for producing a higher extract amount. As reported in the research, the optimal extract (4 L) was prepared in an orbital shaker (180 rpm, 25°C, 2 h) using a 50% ethanol-water mixture at a 1:20 solid-to-solvent ratio. After extraction, ethanol was removed using a rotary evaporator (IKA, HB4-basic, Germany) under vacuum at 50°C to preserve sensitive compounds, especially polyphenols and natural pigments.
2.3 Encapsulation processes
The obtained dealcoholized liquid of Ulva spp. extract (LUE), with a dry residue of 4.79% and an alcohol content of 5.14% (w/w), was encapsulated. Spray drying and freeze-drying techniques were used to produce encapsulated powders. For spray drying, a Labtex ESDTi spray dryer (Labtex, Huddersfield, UK) was employed at an inlet temperature of 135 ± 5°C and an outlet temperature of 60 ± 5°C. The process parameters included a 0.5 mm nozzle diameter, an air flow rate of 75 m³/h, a liquid feed rate of 10 mL/min, and an atomization pressure of 2–3 bar. The freeze-drying process was carried out using a Beta 1–8 Freeze Dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) at -60°C, with pressures of 0.011 mbar for 24 hours and 0.0012 mbar for 1 hour to further remove residual water. Maltodextrin (MD) and polydextrose (PD) were used as carriers for both encapsulation methods, each at a concentration of 50% (w/w), dissolved in the LUE and stirred for 12 hours before encapsulation. The three spray-dried powders, spray-dried Ulva spp. extract (SUE), SUE+MD, and SUE+PD, as well as the freeze-dried powders (FUE, FUE+MD, and FUE+PD), were stored in high-density glass bottles within a desiccator at room temperature, with a maximum storage time of 1 month before further analysis.
The powders were analyzed for various technological properties, including powder yield, moisture content, bulk and tapped densities, Carr index (CI), Hausner ratio (HR), and rehydration time. Fourier Transform Infrared Spectroscopy (FTIR) was employed to analyze the physicochemical composition of the powders. The total polyphenols (TP), carotenoids, and chlorophyll a and b contents of the powders were measured by UV/VIS spectrophotometry. The individual compound (lutein as a xanthophyll carotenoid) was analyzed through high-performance liquid chromatography (HPLC). Encapsulation efficiency (EE) was calculated based on the amount of polyphenols and lutein encapsulated in the powders. The thermal stability of the powders was assessed using Differential Scanning Calorimetry (DSC). The antioxidant activities of the powders were evaluated through three different antioxidant tests.
2.4 Technological powder properties
2.4.1 Drying process yield
The yield of the drying (encapsulation) process (Y) was calculated as the ratio of the mass of the obtained powders to the expected mass after drying using Equations 1 and 2:
The expected mass was calculated by adding the dry residue from the liquid extract used in the drying process and the mass of the carriers:
2.4.2 Moisture content
The moisture content of the samples was determined gravimetrically using the Halogen Moisture Analyzer HB43-S from Mettler Toledo (Spain). All samples were dried at 105°C until constant weight was reached, and the moisture content was calculated from the weight difference before and after drying. All measurements were performed in triplicate, and the results are expressed as percentage values (% w/w).
2.4.3 Bulk density
The bulk densities of the dried extracts and their respective encapsulates were determined according to the method described by Vidović et al. (2014) and Ćujić Nikolić et al. (2023), with minor modifications. A 1 g sample of each powder was placed into a 5 mL graduated glass cylinder. The cylinder was agitated at 300 rpm for 5 minutes (Unimax 1010, Heidolph, Germany) at room temperature. After agitation, the volumes of the dried powders were measured directly in the cylinder. Bulk density was calculated as the mass-to-volume ratio of the powder and expressed in milligrams per milliliter (mg/mL). The cylinder was tapped 120 times, and the sample volumes were measured again to determine the tapped density (Caliskan and Dirim, 2016), and the following parameters: flowability and cohesiveness. The flowability and cohesiveness of the samples were assessed using the Carr index (CI) and Hausner ratio (HR), calculated with the following Equations 3 and 4:
where ƍ tapped and ƍ bulk represent the tapped and bulk densities, respectively.
2.4.4 Rehydration time
Rehydration time is defined as the time required for the complete dissolution of the obtained powders in water (a commonly used medium for food or pharmaceutical applications) at room temperature. The tests were conducted using a magnetic stirrer, and the time for complete reconstitution of 1 g of powder in 50 mL of water was measured in seconds (s) (Ćujić Nikolić et al., 2024).
2.5 Physicochemical characterization of spray and freeze-dried powders
2.5.1 Powders composition analysis by FTIR spectroscopy
Fourier-transform infrared (FTIR) spectra of the obtained powders (spray and freeze-dried extracts, their respective encapsulates) were recorded in the range of 400 to 4000 cm-1 using a Nicolet iS10 spectrometer (Thermo Scientific, Sweden), with a resolution of 4 cm−1, in a straight line to examine the structural characterization of the samples. The spectral ranges were measured in duplicate, and each sample was analyzed independently.
2.5.2 Powders thermal characterization by differential scanning calorimetry
The thermal properties of the spray and freeze-dried powders were analyzed using a DSC131 Evo (SETARAM Instrumentation, Caluire-et-Cuire, France). Samples were placed in 30 µL aluminum pans and immediately hermetically sealed, with an empty pan as a reference. The heating process began with both the reference and sample pans stabilized at 20°C for 5 minutes, then heated to 200°C at 10°C/min. Nitrogen flow was maintained at 20 mL/min. A baseline run was performed using empty pans under the same conditions, and baseline subtraction and enthalpy determination (J/g) were performed using the CALISTO PROCESSING software from SETARAM Instrumentation. The reported results represent the mean of three measurements on the apparatus.
2.6 Chemical characterization of spray and freeze-dried powders
Approximately 1 g of the obtained powders was dissolved in 10 mL of distilled water using a Vortex mixer (Vortex 3, IKA) for their bioactive analysis. The resulting samples were then further analyzed.
2.6.1 Total polyphenols analysis
A modified Folin-Ciocalteu assay was employed to determine the total polyphenolic content of the powders that were obtained (Singleton et al., 1999). A 200 μL sample was mixed with 1000 μL of 10% Folin-Ciocalteu reagent, and after 4 min, 800 μL of 7.5% Na2CO3 was added to complete the reaction mixture. The blank control was prepared by replacing the sample with distilled water. The samples were kept in the dark for two hours, and the absorbance was measured at 765 nm. The results for the polyphenol content in powders are expressed as mg of gallic acid equivalents per 100 g of powder (mg GAE/100 g).
2.6.2 Chlorophyll a, b, and total carotenoids analysis
The chlorophyll a and b content, total carotenoids in spray and freeze-dried powders, and the corresponding encapsulates were measured spectrophotometrically and briefly described by Braniša et al., 2014. The encapsulated powders were dissolved in the acetone-water mixture (4:1), and absorbances were measured at 663.6 nm for Chl a, 646.6 nm for Chl b, and 470.0 nm for carotenoids. The chlorophyll and carotenoid contents were calculated using the equations described in the previously mentioned study. The results were expressed as mg of chlorophyll a and b, and carotenoid equivalents per 100 g of dried samples (mg/100 g).
2.6.3 Encapsulation efficiency
The encapsulation efficiency (EE%) for all microencapsulated powders was determined as the percentage of encapsulated polyphenolic compounds and lutein, as an individual carotenoid, relative to the total amount present in the liquid extract (LUE), based on the following Equation 5:
Where E represents the quantities of total polyphenols and lutein encapsulated in the powders, and E total represents the quantities of total polyphenols and lutein and their respective amounts in the LUE.
2.6.4 Individual carotenoid analysis by the HPLC method
The carotenoid content (lutein) in the encapsulated samples was measured using a Shimadzu Prominence HPLC system (Shimadzu, Kyoto, Japan), equipped with a diode-array detector. Before chromatographic separation, samples of dissolved powders were filtered through a 0.45 μm membrane to remove particulate matter. The analysis was performed on a PerkinElmer Quasar C18 column (250 x 4.6 mm, 5 µm; PerkinElmer, Buckinghamshire, UK), with the mobile phase consisting of methanol-tetrahydrofuran (95:5, v/v), stabilized with 0.1% butylated hydroxytoluene (BHT), and delivered at a flow rate of 1 mL/min. Lutein was identified by comparison of retention times with an authentic standard, and quantification was performed using a calibration curve (R2 > 0.99). Detection was set to 473 nm, corresponding to the absorbance maximum of lutein (Vučetić et al., 2025). All measurements were conducted in triplicate to ensure reproducibility. Results were expressed as mg of lutein per g of dried sample.
2.7 Antioxidant activity bioactivity of encapsulated powders
The antioxidant capacity was measured spectrophotometrically using three methods: the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, the reducing power (RP) test, and the 2,20-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assay, as described by Vulić et al. (2019). Results were expressed as mmol of Trolox equivalent (TE) per 100 grams of the encapsulated samples.
2.8 Statistical analysis
Technological powder properties and chemical analyses were analyzed in triplicate, and data were presented as means ± standard deviation. Statistical significance was determined using a one-way analysis of variance (ANOVA), followed by Duncan’s post hoc test for multiple comparisons. Before performing ANOVA, the authors checked the R (CRAN) model assumptions using the rstatix, car, nortest, agricolae, and ggpubr packages. Outliers were identified within groups using Tukey’s rule and influence diagnostics on the model residuals (studentized residuals and Cook’s distance). Residual normality was assessed with the Shapiro–Wilk test and Q–Q plots, and homoscedasticity was evaluated with Levene’s test (median-centered). One-way ANOVA was then performed, followed by Duncan’s post hoc test.
3 Results and discussion
The study aimed to valorize and preserve bioactive compounds and natural pigments from Ulva spp. biomass sourced from the South Adriatic Sea coast. Firstly, the bioactive compounds (polyphenols and natural pigments) were extracted from Ulva biomass using green extraction technology and then encapsulated. Ulva was used as a model raw material in this work to demonstrate the efficiency and transferability of these extraction and encapsulation processes.
Based on our previously published data on the extraction of total polyphenols and flavonoids, as well as chlorophyll a and b, from Ulva spp (Nikolić et al., 2025), an ethanol-water mixture (50%, solid to solvent ratio 1:20) was chosen as the optimal for the extraction process. The optimal extract, prepared under the mentioned conditions, was prepared according to the principles of green chemistry using an ethanol-water solvent and without the use of high temperatures, employing the traditional extraction method of maceration (Nikolić et al., 2025). The 2-hour maceration method produced a larger amount of extract for the encapsulation process. The dealcoholized liquid Ulva spp. extract (LUE), which had high levels of total polyphenols, chlorophyll a and b, and carotenoids (3.55 mg GAE/g, 84.53 mg/100 g, 68.41 mg/100 g, 5026.82 mg/100 g), was then encapsulated.
In the study by Ledari et al. (2024b), the authors reported the highest chlorophyll extraction using a solvent similar to that in our research, an ethanol-water mixture (70%) (26.43 μg/g of chlorophyll a and 10.76 μg/g of chlorophyll b), compared with other organic solvents. However, the amounts of chlorophylls extracted were still significantly lower than those obtained herein. Although in the mentioned study 70% ethanol–water, together with acetone, demonstrated the highest chlorophyll yield, ethanol was one of the preferred solvents for chlorophyll extraction due to its suitable polarity, non-toxicity, and biodegradability, which also aligned with the aim of the present investigation.
The differences in the content and quantity of bioactive components in Ulva-based extracts among different research studies strongly depend on the examined raw materials (Ulva biomass) and various environmental factors (Yaich et al., 2011).
After optimal extraction, the Ulva liquid extract was encapsulated using two technologies that protect active ingredients from environmental degradation and extend their shelf life. The research investigates the influence of polysaccharide carriers, such as maltodextrin and polydextrose, to enhance encapsulation yield while preserving bioactive compounds. The obtained powders were analyzed for technological, physicochemical, and chemical properties.
3.1 Technological properties of powders
3.1.1 Encapsulation process yield
The novel encapsulation technologies were used to preserve bio-compounds extracted from Ulva spp. These methods are expected to improve the stability of Ulva’s extract, reducing the inactivation of sensitive polyphenolic compounds, especially natural pigments, while maintaining high process yield. Figure 2 shows the encapsulated extracts without added carriers (SUE; FUE) and their respective encapsulates, which include polydextrose (SUE+PD; FUE+PD) and maltodextrin (SUE+MD; FUE+MD).
Figure 2

Spray and freeze-dried powders of Ulva spp. extract.
The yield of the encapsulated extracts in our study was significantly affected by both the type of encapsulation (spray or freeze drying) and the presence and type of carrier material (PD or MD) (Table 1). The highest process yields were observed with freeze-dried Ulva spp. extract, especially with FUE+MD (97.72 ± 3.52%) and FUE (93.76 ± 2.04%), which were not significantly different from each other (p > 0.05). This suggests that maltodextrin positively influences the process yield. Conversely, the addition of polydextrose (FUE+PD) resulted in a significantly lower yield (74.02 ± 1.11%), indicating that maltodextrin may be a more suitable carrier for freeze-drying Ulva spp. extract. The higher yield with MD could be attributed to the MD’s good and proven ability to form a matrix and have a stabilization efficiency during the encapsulation process (Ledari et al., 2024a). The lower yield observed with PD may be attributed to its weaker matrix-forming capacity and reduced stabilization efficiency compared to MD, which under freeze-drying conditions can result in greater structural collapse and material losses.
Table 1
| Samples | Yield (%) | Moisture (%) | Bulk density (mg/mL) | Tapped density (mg/mL) | CI | HR | RT (s) |
|---|---|---|---|---|---|---|---|
| SUE | 27.23 ± 0.27d | 9.83 ± 0.19b | 0.27 ± 0.01b | 0.29 ± 0.01bc | 5.88 ± 0.35e | 1.06 ± 0.02d | 271.44 ± 8.81c |
| SUE+PD | 58.45 ± 1.25c | 7.67 ± 0.10c | 0.27 ± 0.02b | 0.31 ± 0.02b | 12.50 ± 0.44d | 1.14 ± 0.07cd | 470.96 ± 3.96a |
| SUE+MD | 58.55 ± 0.94c | 7.48 ± 0.26c | 0.31 ± 0.02a | 0.38 ± 0.03a | 20.00 ± 0.60f | 1.25 ± 0.03bc | 324.25 ± 3.32b |
| FUE | 93.76 ± 2.04a | 13.58 ± 0.68a | 0.07 ± 0.00e | 0.12 ± 0.00e | 36.84 ± 0.66a | 1.58 ± 0.10a | 217.29 ± 2.66d |
| FUE+PD | 74.02 ± 1.11b | 9.15 ± 0.37b | 0.13 ± 0.00d | 0.18 ± 0.00d | 26.32 ± 1.61b | 1.36 ± 0.07b | 153.83 ± 4.16e |
| FUE+MD | 97.72 ± 3.52a | 9.35 ± 0.61b | 0.21 ± 0.01c | 0.26 ± 0.01c | 22.22 ± 0.68c | 1.29 ± 0.07bc | 133.32 ± 6.30e |
Technological properties of spray-dried and freeze-dried powders.
Each value represents the mean of n = 3 ± standard deviation, followed by different letters with a significant difference between samples within the same column, according to the post hoc Duncan’s test at the level p ≤ 0.05. SUE, spray dried Ulva extract; FUE, freeze-dried Ulva extract; MD, maltodextrin; PD, polydextrose; CI, Carr index; HR, Hausner ratio; RT, rehydration time.
For the spray-dried samples, SUE extract (both PD and MD) significantly increased yield compared to the extract without a carrier (SUE: 27.23 ± 0.27%). There was no significant difference between SUE+PD (58.45 ± 1.25%) and SUE+MD (58.55 ± 0.94%). This suggests that using carriers is crucial for improving the yield of spray-dried powders, with both polydextrose and maltodextrin proving equally effective.
Kurniasih et al. (2018) reported a study on a freeze-dried, chlorophyll-rich extract obtained from the seaweed Caulerpa racemosa, using different coating materials. Their results showed that, at the same concentration, the choice of coating material affected the chlorophyll process yield. Although the process used the same method (freeze-drying), the yield was much lower than in our study, averaging 20%. Nhon et al. (2025) encapsulated the chlorophyll extract from the green alga Chaetomorpha aerea by spray drying, using 25% maltodextrin as the wall material. The powder recovery yield was 72.98%, demonstrating that spray drying effectively stabilizes and preserves bioactive compounds from different marine algae.
Encapsulation yield (process yield) exceeding 50% generally indicates a successful, efficient encapsulation process. Jafari et al. (2008) note that yields above 50% are acceptable in spray drying applications. Moreover, the freeze-drying technique typically provides higher retention of encapsulated material compared to spray-drying, primarily due to the significantly lower powder losses during collection (Jafari et al., 2008). Therefore, it is logical to conclude that freeze-drying yields better encapsulation performance, particularly in minimizing encapsulation losses.
3.1.2 Moisture content
The moisture content of the powders varied significantly depending on the drying method and the presence of carriers, ranging from 7.48% to 13.58%, as presented in Table 1. The highest moisture content was observed in the freeze-dried Ulva extract without carriers (FUE: 13.58 ± 0.68%). The addition of polydextrose (FUE+PD: 9.15 ± 0.37%) and maltodextrin (FUE+MD: 9.35 ± 0.61%) significantly reduced the moisture content. In the case of spray-dried extracts, lower moisture contents were recorded, with polydextrose (SUE+PD: 7.67 ± 0.10%) and maltodextrin (SUE+MD: 7.48 ± 0.26%), compared to the extract without carriers (SUE: 9.83 ± 0.19%). Spray drying generally results in powders with lower moisture content due to higher process temperatures applied for a shorter period. In contrast, freeze drying relies on sublimation under low pressure, which is more effective in preserving heat-sensitive compounds but typically leaves the powders with slightly higher residual moisture. Our results showed that spray drying produced powders with lower residual moisture than freeze drying. Similar conclusions have been reported in the literature, where faster drying kinetics at elevated temperatures were found to limit enzymatic degradation and improve the stabilization of algal biomass (Robic et al., 2008).
Notably, the selection and incorporation of suitable biopolymers during encapsulation can reduce residual moisture content, thereby improving powder stability and storage properties regardless of the selected drying technique. These findings align with studies on other brown seaweed species, such as Sargassum plagyophyllum, where the usage of maltodextrin as a stabilizer in the freeze-drying process resulted in powders with reduced moisture content, enhanced stability, and prolonged shelf life (Anwar et al., 2018). Similarly, research on spray-dried brown seaweed (Sargassum muticum) extract demonstrated that incorporating maltodextrin improved the physicochemical properties of the powder, including moisture content (Tun Norbrillinda et al., 2016).
Although the results of Robic et al. (2008) are not directly comparable to the results presented, as they investigated the effects of drying on fresh Ulva biomass, whereas we used encapsulation through freeze- and spray-drying techniques; nevertheless, some parallels can still be drawn. Similar to their observation that freezing better preserved the molecular integrity of ulvan than air-drying, our results confirm that freeze-drying is an effective method for maintaining bioactive compounds. Conversely, their finding that higher-temperature drying improved ulvan yield aligns with our spray-drying results, where a faster, elevated-temperature drying process produced stable powders with a competitive moisture content. Overall, these comparisons highlight that both freeze- and spray-drying are effective methods for stabilizing Ulva-derived compounds, preserving functional integrity and offering technological advantages. These improvements in moisture control and compound stability are essential for practical applications in developing functional foods, feeds, and pharmaceutical products based on Ulva biomass.
Overall, incorporating carriers such as polydextrose and maltodextrin reduces moisture content in freeze-dried and spray-dried Ulva extract, improving powder stability and extending shelf life.
3.1.3 Bulk and tapped densities
Producing powders with appropriate bulk and tapped densities is particularly important in the pharmaceutical and food industries, where powders are often used to formulate compact dosage forms, such as capsules or tablets (Nastić et al., 2023). Higher bulk and tapped densities are desirable as they allow for incorporating greater amounts of active ingredients within a limited volume. These parameters can also be crucial in the food industry, as they influence packaging efficiency, transport costs, and ease of handling during the processing and formulation of powdered foods and other products (Baele et al., 2021).
In the present study, the measured bulk density values reflect these differences in formulation. The spray-dried samples, SUE (0.27 g/cm³) and SUE+PD (0.27 g/cm³), have the lowest bulk densities, which are almost identical. The addition of PD to the SUE+PD formulation does not significantly alter the bulk density compared to SUE alone, indicating that PD does not substantially contribute to the powder’s compactness. In contrast, the SUE+MD formulation (0.31 g/cm³) has a significantly higher bulk density than SUE and SUE+PD, indicating a more compact structure, likely due to maltodextrin, which increases powder density. The positive effect of maltodextrin on bulk density properties was previously observed (Radan et al., 2024).
Regarding taped density values, SUE+MD had the highest value (0.38 g/cm³), indicating that adding maltodextrin enables a more compact structure during the tapping process. The SUE+PD (0.31 g/cm³) had a slightly lower, yet still superior, value compared to the other formulations. SUE (0.29 g/cm³) and FUE+MD (0.26 g/cm³) showed intermediate tapping densities, while FUE (0.12 g/cm³) and FUE+PD (0.18 g/cm³) had the lowest values, indicating poor compactability upon tapping.
The Carr’s Index (CI) provides additional information about the flowability of the powder (Caliskan and Dirim, 2016). The SUE+PD had the best flowability properties (CI of 12.50) and thus clearly outperformed SUE+MD (CI of 20.00). This indicates that adding polydextrose improves SUE’s flow properties, likely due to more favorable particle interactions. The pure spray-dried extract, SUE, exhibits a CI of 5.88, reflecting moderate flowability, while SUE+MD exhibits slightly poorer flowability than SUE+PD. The freeze-dried samples exhibit unfavorable flowability in the FUE group, with FUE showing the highest CI (36.84), indicating poor flowability. The addition of PD and MD slightly improves flowability, as seen with FUE+PD (26.32) and FUE+MD (22.22), although both remain inferior to the spray-dried formulations. The same observations were previously demonstrated by (Jovanović et al., 2021).
The Hausner Ratio (HR) measurements also support these results (Caliskan and Dirim, 2016). The SUE+PD (1.14) exhibits the best flow properties, with a low HR value indicating minimal cohesion and improved particle mobility. Although SUE+MD has a higher tapped density, its HR (1.25) shows slightly poorer flowability. The SUE sample has a moderately low HR (1.06), better than FUE (1.58), confirming its poor flowability. Slight improvements are observed with FUE+PD (1.36) and FUE+MD (1.29), although both remain underperforming compared to the spray-dried samples.
To summarize, SUE+PD represents the optimal formulation, combining excellent flowability with moderate compactness (Caliskan and Dirim, 2016). The addition of polydextrose improves the handling and processability of SUE more effectively than maltodextrin, which increases density but negatively affects flowability. Conversely, freeze-dried formulations have inferior overall performance across all examined powder parameters.
These results align with previous findings indicating that maltodextrin enhances spray-dried powders’ density and flow properties by promoting particle packing and decreasing agglomeration (Turk-Gul et al., 2023). In contrast, freeze-dried powders are generally more porous and tend to have lower density and poorer flow properties (Oikonomopoulou et al., 2011; Mirhosseini and Amid, 2013). Additionally, the addition of carriers such as polydextrose can effectively improve the flowability of freeze-dried powders.
3.1.4 Rehydration time
The observed rehydration time (RT) results demonstrated differences in the behavior of the various formulations during rehydration. Water usage as a reconstitution medium is related to the potential applications of Ulva-based encapsulated powders in the food and pharmaceutical industries as functional foods or pharmaceutical products, or in aquaculture. These products are most commonly consumed or processed in aqueous environments. Lutein (herein analyzed as a representative carotenoid) is poorly soluble in water, significantly limiting its potential in food and pharmaceutical formulations. Therefore, microencapsulation of Ulva extract was performed to improve the bioavailability of bioactive compounds, such as lutein, in formulations intended for water-based dissolution or delivery. The FUE+MD exhibits the fastest rehydration period of 133.32 s, followed by FUE+PD at 153.83 s, indicating that adding maltodextrin (MD) and polysaccharides (PD) significantly accelerated the hydration process. FUE alone rehydrates in 217.29 s. Although this makes it slower than the drug-modified formulations, it remains one of the fastest-rehydrating powders. In contrast, the spray-dried formulation, SUE, takes 271.44 s to rehydrate, indicating moderate performance. The addition of MD and PD to SUE further slows the process, with SUE+MD taking 324.25 s and SUE+PD taking the longest time of 470.96 s.
The faster rehydration observed with FUE+MD and FUE+PD may be attributed to the FUE formulation’s structure, which allows for more rapid water uptake when combined with MD or PD. As carriers, both MD and PD likely reduce the surface tension of the powder, enabling faster water penetration and thereby facilitating quicker rehydration (Mitchell et al., 2019). In contrast, spray-dried formulations show slower rehydration, which could be due to SUE’s inherently more compact or less water-absorbent structure. When MD and PD are added to SUE, they may alter its physical properties in a way that hinders the hydration process, possibly by modifying the powder’s porosity or increasing its viscosity, which may impede water absorption.
Overall, these results suggest that MD and PD are more susceptible to hydration acceleration by FUE formulations, whereas SUE formulations modified with these carriers exhibit slower hydration times. This could stem from differences in powder structure and ingredient interactions, with FUE being more porous or having a structure that promotes faster water uptake than SUE.
3.2 Physicochemical characterization of spray and freeze-dried powders
3.2.1 Fourier transform infrared spectroscopy of powders
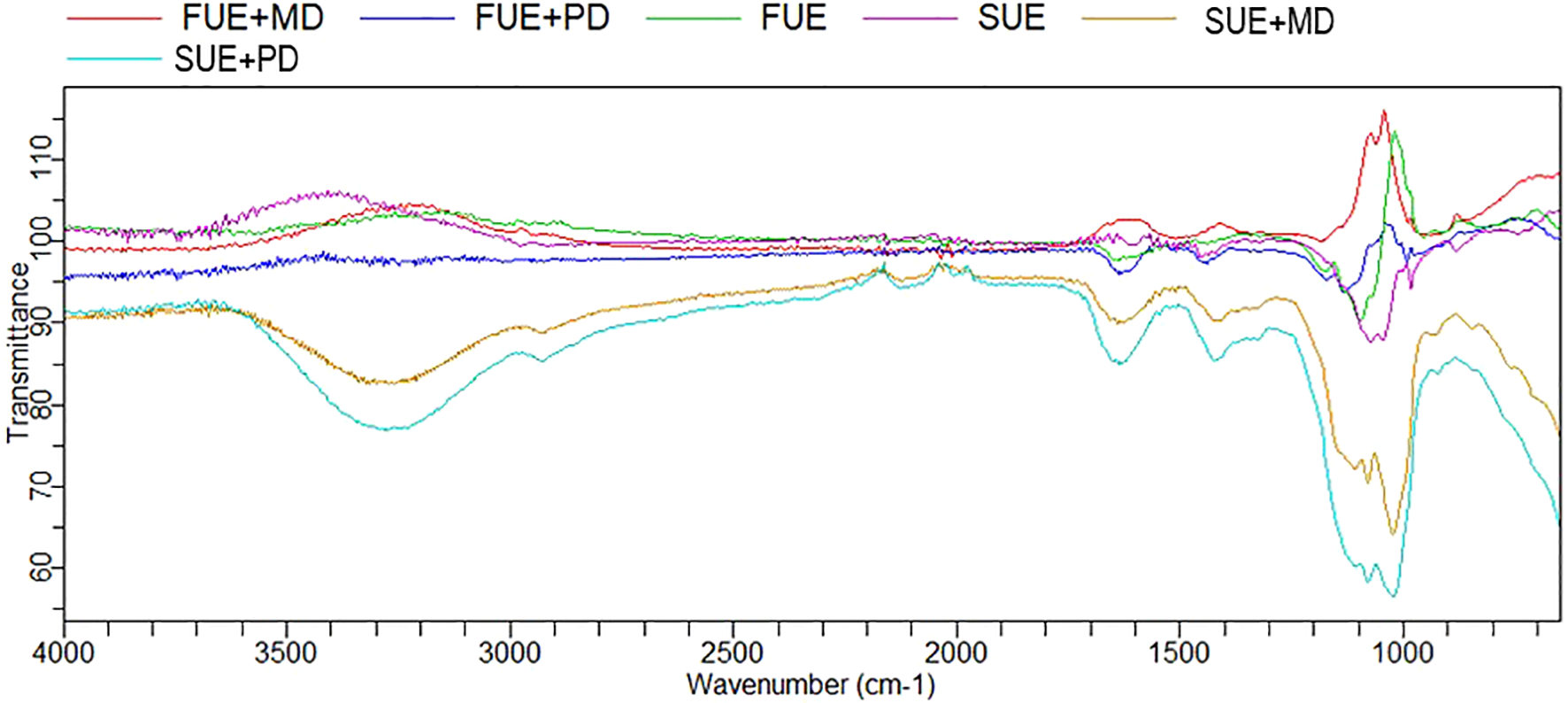
FTIR spectroscopy is a widely used technique for characterizing the physicochemical composition of samples, herein macroalgae of the genus Ulva, particularly encapsulated Ulva extracts. This method enables the identification of functional groups present in Ulva, which, according to the literature, are predominantly polysaccharides such as ulvan, extracted from these macroalgae (Ramadhan et al., 2024), as well as functional groups from other extracted bioactive compounds. FTIR analysis of encapsulated Ulva samples provided information about the encapsulated guest molecules (Ulva spp. extracted bioactives) in selected biopolymers. In previous research (Nikolić et al., 2025), the spectrum of dried Ulva biomass was presented and is now compared to spectra of extracted and encapsulated bioactives from Ulva spp. (Figure 3). The spectra exhibited a strong absorption band around 3250 cm−1, attributed to O–H (hydroxyl) groups associated with polyphenolic compounds and polysaccharides (El-Gendy et al., 2023), preserved in biopolymers through encapsulation techniques. The FTIR spectra typically contained strong absorption bands of carbohydrates in the region of 1000 cm−1, related to C-H and C-O stretching of aromatic rings, indicating the presence of aromatic compounds originating from phenolics in the extract. This region, as well, is dominated by sugar ring vibrations overlapping with stretching vibrations of (C– OH) side groups and the (C–O–C) glycoside bonds. In particular, the maximum absorption band at approximately 1050 cm−1 can be attributed to ulvan spectra, which exhibit a characteristic C–O stretching vibration arising mainly from two predominant sugar constituents, rhamnose and glucuronic acid, as previously confirmed by the authors (Robic et al., 2009). The presence of these signals highlights the complex polysaccharide composition of ulvan, which is directly linked to its functional properties, such as antioxidant activity, and potential as a stabilizing biopolymer in encapsulation processes. Observed peaks in the region of 1400–1600 cm−1 are associated with the stretching of C=O of carboxylate groups (Robic et al., 2009), aromatic C=C bonds, and C=N skeletal stretching vibrations of the extracted chlorophyll around 1700 cm−1 (Ledari et al., 2024b). Higher absorption bands in the 1000 and 3000–3500 cm−1 regions, associated with spray-dried Ulva extract in polydextrose and maltodextrin, were more pronounced than in freeze-dried counterparts and pure dried extracts. At around 2900 cm−1, small characteristic peaks were observed for samples SUE+MD and SUE+PD, corresponding to the asymmetric and symmetric stretching vibrations of CH₂ groups (Schultz et al., 2024). These signals likely originate from polysaccharide structures rather than lipids, since the extraction was performed with polar solvents, which do not extract lipid fractions. Similar results and functional groups were observed for chlorophyll extracted from sea grape (Caulerpa racemosa) using the freeze-drying method, when it was encapsulated with maltodextrin, as reported by Kurniasih et al. (2018). FTIR analysis has confirmed the efficient encapsulation reported by Ak et al. (2025), in which extracts from the green seaweeds Ulva lactuca, sourced from the Aegean and Marmara Seas, were encapsulated using the liposomal method.
Figure 3
FTIR spectra of Ulva spp. spray-dried and freeze-dried powders.
Herein, the FTIR analysis indicated that encapsulation provided bioactive compounds preservation through physical incorporation, rather than chemical transformation, since the functional groups presented in Ulva biomass (Nikolić et al., 2025), and pure spray and freeze-dried extracts have not disappeared in extract-biopolymer encapsulates, just changed their intensity. The opposite observation was found by Amin (2020), where bioactives of U. lactuca have been involved in encapsulation into silver nanoparticles and disappeared, indicating chemical transformation during encapsulation.
All these findings demonstrate that FTIR analysis confirms the presence of functional groups associated with alcoholic and phenolic compounds originating from Ulva seaweed extract in encapsulated powders, indicating the presence of bioactive compounds such as polyphenols and natural pigments. The results suggest that Ulva extract and encapsulated powders possess natural properties, making them promising candidates for incorporation into various food and pharmaceutical products as sustainable alternatives to synthetic antioxidants.
3.2.2 Thermal analysis by differential scanning calorimetry of encapsulated powders
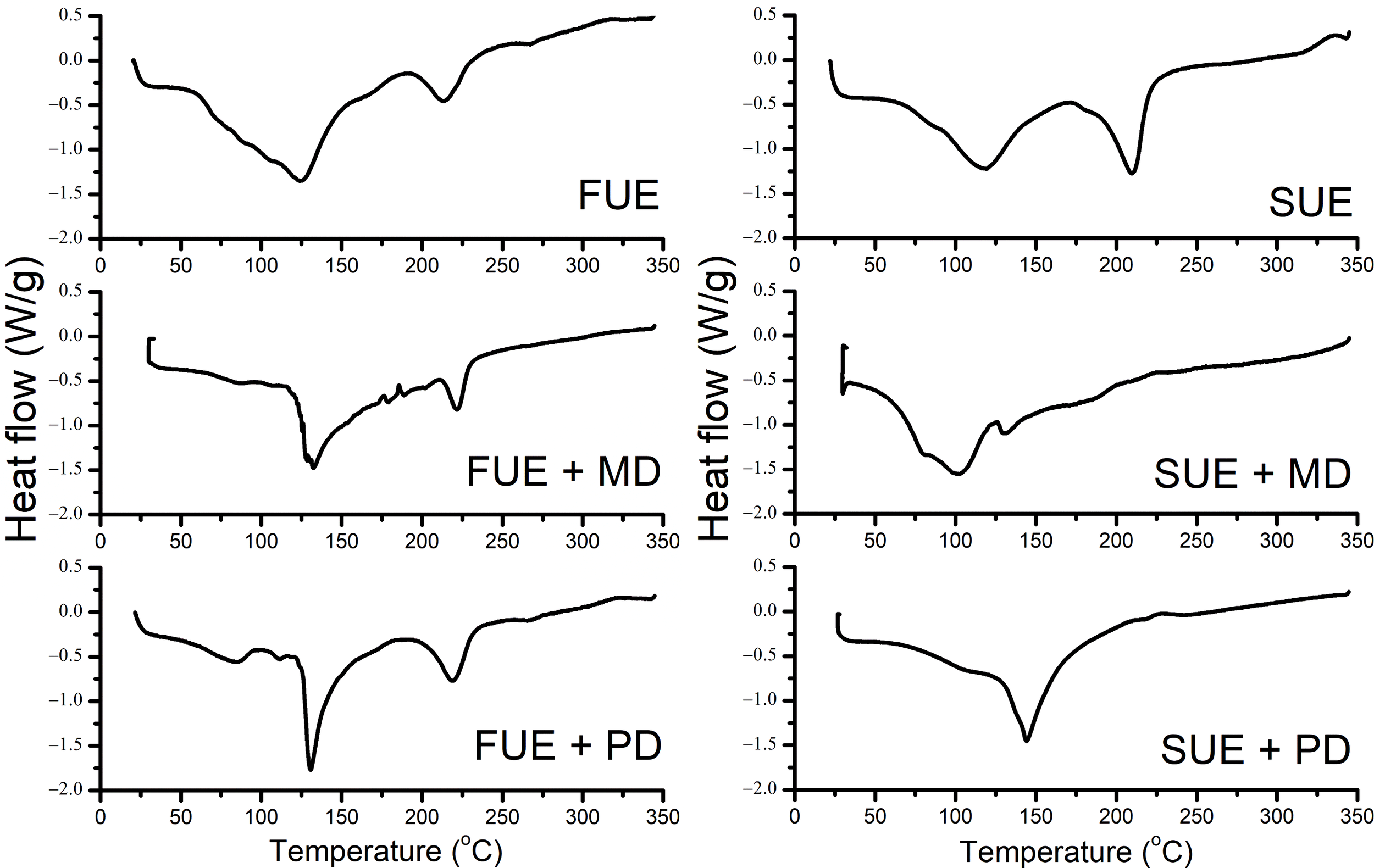
Differential Scanning Calorimetry (DSC) was used to investigate the thermal properties and stability of encapsulated Ulva extract in spray and freeze-dried powders (Figure 4). This analysis is essential for understanding the behavior of encapsulated seaweed-derived bioactives under thermal stress conditions, particularly in the context of future processing applications. Characteristic temperature transition peaks were observed throughout the formulations, indicating water loss, melting, degradation, and possible matrix transitions. The thermal events and enthalpies varied significantly depending on the encapsulation method and carrier type. This analysis identified key endothermic peaks ranging from 34.35 to 243.35 °C, depending on the powder sample. Carrier addition improved stability compared to pure spray or freeze-dried extracts.
Figure 4
DSC thermograms of Ulva spp. spray-dried and freeze-dried powders.
The spray-dried Ulva extract (SUE) exhibited two pronounced degradation peaks (Figure 4, Table 2). The onset of thermal decomposition was detected at 51.07°C, extending to 168°C for the first transition, with a peak maximum at 119°C. A second degradation peak was detected at 229.4°C, with a maximum at 210°C. Notably, the first peak was characterized by a high enthalpy value of 219.00 J/g, indicating substantial thermal resistance and energy required for matrix breakdown. Ledari et al. (2024a) reported chlorophyll melting points of 123–150°C for freeze-dried and spray-dried chlorophyll in maltodextrin and protein isolate. Encapsulation of Ulva extract with polydextrose (SUE+PD) significantly enhanced the extract’s thermal stability. This sample exhibited a single, broad degradation transition from 55.23°C to 213.19°C, with an enthalpy of 340.88 J/g, the highest among the spray-dried samples. This indicates a more uniform and thermally resilient matrix, due to improved interaction between the extract and the amorphous polydextrose, which is known to provide protective encapsulation under thermal stress (Wahlström et al., 2020). The spray-dried extract with maltodextrin (SUE+MD) exhibited lower thermal onset, initiating degradation at 39.81°C and ending at 210°C, with two enthalpic transitions. While its onset was the weakest among all samples, the enthalpy associated with the first event was still considerable, indicating partial stabilization despite a more open or porous structure (Table 2).
Table 2
| Samples | T1 (°C) | T2 (°C) | T3 (°C) | Δh1 (j/g) | Δh2 (j/g) | Δh3 (j/g) |
|---|---|---|---|---|---|---|
| SUE | 119 | 210 | / | 219 | 126 | / |
| SUE+PD | 144.16 | / | / | 340.81 | / | / |
| SUE+MD | 99.17 | 131.85 | / | 170.17 | 27.69 | / |
| FUE | 125.38 | 214.16 | / | 410.53 | 54.25 | / |
| FUE+PD | 83.617 | 130.68 | 218.96 | 20.77 | 152.59 | 59.57 |
| FUE+MD | 132.40 | / | / | 200.492 | / | / |
| PD | 97.46 | 157.43 | 175.45 | 38.02 | 14.44 | 25.68 |
| MD | 97.47 | 270.26 | / | 179.55 | 127.10 | / |
The transition temperatures and enthalpy changes of spray and freeze-dried Ulva powders during the thermal degradation process.
Mean values of three measurements. SUE-spray-dried Ulva extract, FUE-freeze-dried Ulva extract; MD-maltodextrin, PD-polydextrose, T-temperatures, ΔH-enthalpy changes. SUE, spray-dried Ulva extract; FUE, freeze-dried Ulva extract; MD, maltodextrin; PD, polydextrose; T, temperatures; ΔH, enthalpy changes.
In the freeze-dried sample series, the pure extract (FUE) showed two distinct degradation steps, starting at 48.16°C and ending at 243.35°C, with an exceptionally high enthalpy of 410.53 J/g for the initial degradation. This result suggests strong internal bonding or firmly packed structures formed during freeze drying, which delayed thermal breakdown. Freeze-dried encapsulates with polydextrose (FUE+PD) displayed three degradation events, starting at 54.64°C and ending at 237.11°C, with a notable enthalpy for the second peak (152.59 J/g), demonstrating the formation of a relatively thermally stable powder. Among all samples, the FUE+MD encapsulate showed the highest thermal resilience. The temperature degradation within this sample started at 97.59°C and extended up to 210.98°C, with a high total enthalpy of 200.49 J/g. These data indicate strong molecular interactions between the extract and maltodextrin during freeze-drying, likely promoting more organized, less thermally sensitive structures (Table 2).
Even in previous research (Nikolić et al., 2025) the examined dried Ulva biomass demonstrates good thermal stability up to 140°C; Ulva-based encapsulated powders demonstrated better thermal stability, making them more suitable for future applications.
Wall materials (biopolymers) restrict the mobility of bioactive compounds, preventing their interaction and aggregation, which could otherwise provoke the lower temperature changes (Ledari et al., 2024a). The encapsulating materials often have higher melting points and undergo more extensive degradation than the bioactives, thereby contributing to greater temperature changes in the encapsulates. This effect is particularly evident with encapsulated chlorophyll from Ulva intestinalis using MD and WPI (protein isolate), as these high-molecular-weight compounds are thermally stable and resistant to degradation (Ledari et al., 2024a), as in our study.
The DSC profiles of the pure carrier materials support these findings, exhibiting more thermal stability with the addition of Ulva extract. Polydextrose displayed three degradation transitions between 63.05°C and 193.28°C with relatively modest enthalpy changes, while maltodextrin presented two transitions from 39.81°C to 291.93°C, with significant energy requirements (Table 2). These observations reinforce that the biopolymers and drying (encapsulation) technique used play critical roles in shaping the thermal behavior of the final powder.
In the context of previously published data, our results align well with studies on Ulva and other macroalgae thermal properties. For instance, Kumar et al. (2021) demonstrated similar thermal patterns in Ulva rigida, while Wahlström et al. (2020) reported comparable degradation patterns for Ulva spp. from the Swedish west coast.
Based on these thermal characteristics, the examined Ulva encapsulated powders demonstrated good thermal stability up to 240°C, making them suitable for food, feed, and pharmaceutical processing applications. The synergistic interaction between Ulva extracts and encapsulating agents has been confirmed through DSC analysis. Both spray drying and freeze drying significantly affected the thermal behavior of the resulting powders, with polydextrose and maltodextrin each providing distinct benefits. These thermally stable encapsulates show promising potential in food and nutraceutical formulations requiring high processing temperatures, ensuring product integrity and functional efficacy. The DSC thermograms showed that Ulva encapsulates can be safely processed for food formulations, indicating seaweed-compatible temperature ranges for their incorporation into seaweed-based products.
3.3 Chemical properties of Ulva spray and freeze-dried powders
3.3.1 Polyphenolic, chlorophyll a and b, carotenoid content, and encapsulation efficiency
Encapsulation efficiency (EE%) was determined by the amount of polyphenols and lutein encapsulated in the powders. The data shown in Table 3 reveal notable differences in encapsulation efficiency (EE%), total phenolic content (TP), chlorophylls a and b (Chl A and Chl B), and carotenoids (Carot) among various formulations. The SUE formulation demonstrated the highest EE% of polyphenolic compounds (86.50%), indicating superior encapsulation capabilities compared to the other formulations. In contrast, the addition of PD (70.91%) and MD (53.81%) significantly reduced the EE% of polyphenolics, with SUE+MD showing the lowest encapsulation efficiency. This decline may be attributed to alterations in the encapsulating matrix, which could compromise the retention of bioactive compounds (Rezagholizade-shirvan et al., 2024), or to the dilution effect introduced by the addition of maltodextrin or polydextrose, making direct comparisons with powders prepared without carriers imprecise.
Table 3
| Samples | EE % | TP (mg GAE/g) | Chl A (mg/100g) | Chl B (mg/100g) | Carotenoids (mg/100g) |
|---|---|---|---|---|---|
| SUE | 86.50 ± 3.45a | 3.07 ± 0.05a | 60.45 ± 2.72b | 42.06 ± 1.33c | 3369.09 ± 75.79c |
| SUE+PD | 70.91 ± 0.72c | 2.51 ± 0.04c | 52.45 ± 2.22c | 52.92 ± 2.05b | 4321.18 ± 172.24b |
| SUE+MD | 53.81 ± 2.20d | 1.91 ± 0.03d | 33.62 ± 1.21d | 23.24 ± 0.57e | 1981.53 ± 91.58e |
| FUE | 75.59 ± 1.11bc | 2.68 ± 0.17bc | 47.86 ± 1.84c | 34.22 ± 0.63d | 2693.61 ± 127.65d |
| FUE+PD | 79.27 ± 3.14b | 2.81 ± 0.08b | 52.03 ± 2.49c | 40.88 ± 1.38c | 3366.34 ± 161.84c |
| FUE+MD | 58.71 ± 2.57d | 2.08 ± 0.03d | 25.88 ± 0.31e | 17.63 ± 0.82f | 1418.79 ± 25.41f |
| LUE | / | 3.55 ± 0.03a | 84.53 ± 3.24a | 68.41 ± 1.66a | 5026.82 ± 118.72a |
Chemical properties of the obtained spray and freeze-dried powders.
Values represent the mean of n = 3 ± standard deviation, followed by different letters with a significant difference between samples within the same column, according to the post hoc Duncan’s test at the level p ≤ 0.05. SUE, spray-dried Ulva extract; FUE, freeze-dried Ulva extract; MD, maltodextrin; PD, polydextrose; TP, total polyphenol content; EE%, encapsulation efficiency; ChL, chlorophyll content.
Regarding polyphenolics EE%, SUE also demonstrated the highest phenolic content (3.07 mg GAE/g), followed by FUE+PD (2.81 mg GAE/g). Both spray-dried encapsulates, SUE+PD and SUE+MD, exhibited lower phenolic contents (2.51 and 1.91 mg GAE/g, respectively), likely due to dilution from the added carriers or to possible interactions between the phenolic compounds and the carriers (Šavikin et al., 2021).
Regarding the natural green pigments, chlorophylls, SUE showed the highest levels of Chl A (60.45 mg/100 g) and Chl B (42.06 mg/100 g), whereas the addition of PD or MD decreased both types of chlorophyll. Specifically, SUE+MD exhibited a significant reduction in chlorophyll content, with Chl A at 33.62 mg/100 g and Chl B at 23.24 mg/100 g, suggesting that maltodextrin, as a carrier, does not support chlorophyll retention as effectively as PD. Similarly, FUE+MD showed a significant decrease in Chl A (25.88 mg/100 g) and Chl B (17.63 mg/100 g) compared to FUE+PD, further emphasizing the negative impact of MD on chlorophyll retention.
For carotenoids, SUE possessed a high concentration (3369.09 mg/100g). In comparison, SUE+PD (4321.18 mg/100 g) exhibited even higher carotenoid levels than pure SUE, suggesting that PD may enhance the stability or retention of carotenoids during processing or encapsulation. However, SUE+MD showed a significant decrease in carotenoid content (1981.53 mg/100 g), likely due to lower encapsulation efficiency or carotenoid degradation in the presence of maltodextrin. Similarly, FUE+MD had the lowest carotenoid concentration (1418.79 mg/100g), showing that maltodextrin reduces carotenoid retention in both SUE and FUE formulations.
Compared to other samples, the liquid Ulva extract, LUE, used for encapsulation processes showed high values for TP (3.55 mg GAE/g), Chl A (84.53 mg/100g), Chl B (68.41 mg/100g), and Carot (5026.82 mg/100g), indicating that the natural composition of this sample retains a significant amount of bioactive compounds, which are protected by encapsulation.
The observed effects in this study align with recent findings on the encapsulation of bioactive compounds from algae. For instance, a study by Pan-utai and Iamtham (2020) investigated the microencapsulation of C-phycocyanin (natural pigment) from Arthrospira platensis using freeze-drying with various wall materials, including maltodextrin and gum Arabic. The study highlighted the impact of carrier selection on the stability and preservation of bioactive properties, achieving high encapsulation efficiency and improved thermal stability of the encapsulated pigment.
It should be emphasized that the literature on the encapsulation of bioactive compounds derived from Ulva into biopolymers remains limited, especially for those used in this study. In fact, only a few studies have reported encapsulation approaches using Ulva, with different encapsulation processes and carriers.
Overall, the data suggest that spray-dried formulations more effectively preserve bioactive compounds, particularly phenolics, chlorophylls, and carotenoids, than those modified with carriers such as PD and MD. The presence of MD appears to have a more pronounced adverse effect on the retention of these compounds, while PD demonstrates a milder impact. These findings highlight the importance of carrier selection in the encapsulation process and its influence on the stability and retention of bioactive compounds.
3.3.2 Individual carotenoid compound analysis of powders by HPLC
HPLC analysis was performed to quantify the individual xanthophyll, lutein. However, Ulva species contain a mixture of carotenoids, although, according to the literature, lutein is among the most abundant (Mutavski et al., 2024). For this reason, lutein was selected as a representative bioactive compound to monitor encapsulation efficiency and lutein content in the encapsulated powders. It is essential to highlight that lutein, one of the most extensively studied carotenoids, is particularly valued for its health-promoting properties, including antioxidant activity (Pap et al., 2021) and with the focus of supporting eye health (Ma and Lin, 2010), as well as its potential use as a natural pigment to reduce reliance on synthetic dyes and protect the environment (Muhammad et al., 2024).
The lutein content (µg/100 g powder) in different formulations showed significant differences in its retention, which was influenced by the choice of carrier. The SUE formulation had the highest lutein concentration (186.67 µg/100 g), suggesting that the pure extract maintains optimal lutein levels (Table 4, Figure 5). In comparison, the addition of PD (172.13 µg/100 g) and MD (150.12 µg/100 g) reduced lutein concentration, with MD showing a larger decrease than PD, consistent with previous findings on total bioactive content and total carotenoids. These results suggest that the encapsulation process, especially with MD, may reduce lutein retention, possibly due to interactions between maltodextrin and lutein that compromise the bioactive compound’s stability during processing.
Table 4
| Samples | Lutein (µg/100 g powder) | EE% of lutein |
|---|---|---|
| SUE | 186.67 ± 0.07a | 184.18 |
| SUE+PD | 172.13 ± 0.04b | 169.83 |
| SUE+MD | 150.12 ± 0.07c | 148.12 |
| FUE | 125.12 ± 0.08d | 123.45 |
| FUE+PD | 102.32 ± 0.09e | 100.95 |
| FUE+MD | 81.28 ± 0.02f | 80.18 |
| LUE | 101.35 ± 1.08e | / |
Lutein concentration (µg/100 g) and encapsulation efficiency (EE%) of lutein in powders obtained by spray and freeze-drying encapsulation techniques.
Values represent the mean of n = 3 ± standard deviation, followed by different letters with a significant difference between samples within the same column, according to the post hoc Duncan’s test at the level p ≤ 0.05. SUE, spray-dried Ulva extract; FUE, freeze-dried Ulva extract; MD, maltodextrin; PD, polydextrose.
Figure 5
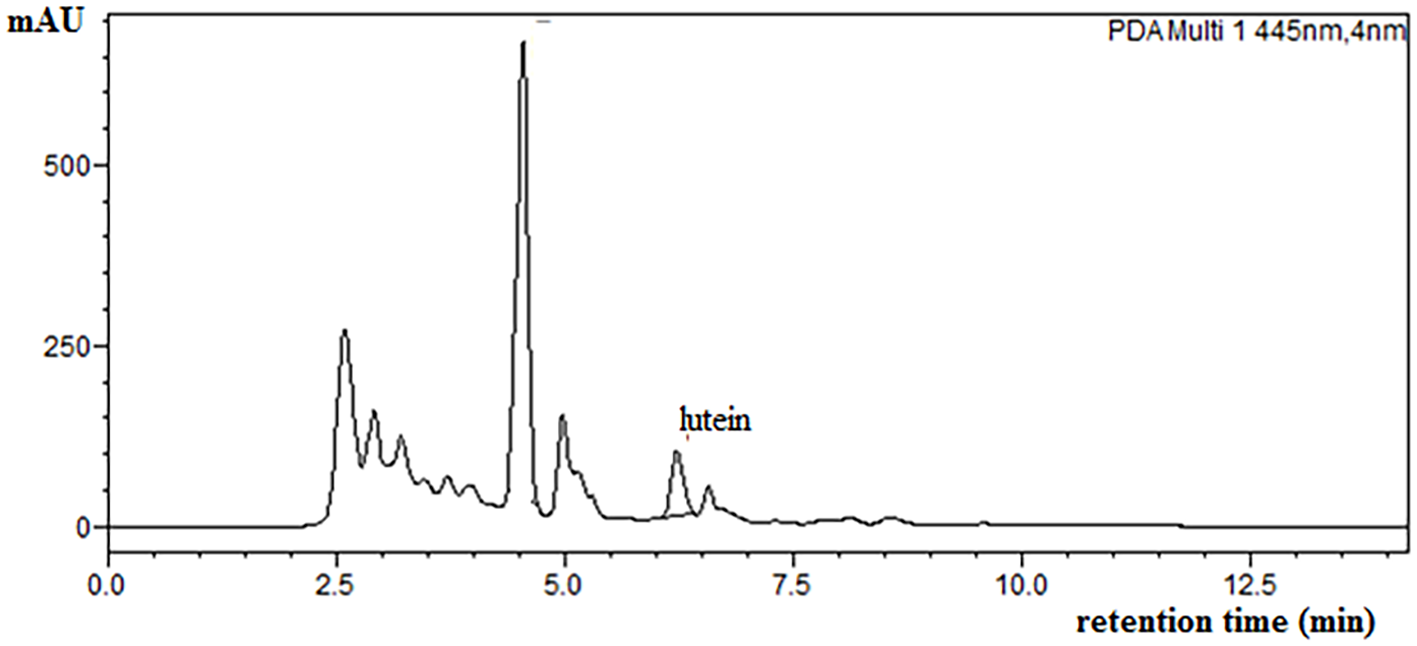
HPLC chromatogram spray-dried Ulva spp. extract (SUE sample).
Similar trends are observed when compared with the FUE formulation (125.12 µg/100 g), which contains lower levels of lutein than SUE. FUE+PD (102.32 µg/100 g) retained more lutein than FUE+MD (81.28 µg/100 g), supporting the assumption that MD negatively affects lutein content during encapsulation. The LUE sample, a liquid extract, contains 101.35 µg/100 g of lutein, similar to the FUE+PD formulation. This indicates that the encapsulation process may cause a slight loss of lutein compared to the unprocessed extract, possibly due to physical and chemical changes during encapsulation and the dilution effect of carrier addition.
Maltodextrin, which is a commonly used carrier in the spray drying method, can interact with lutein, leading to a reduction in its retention. This interaction could arise from maltodextrin’s solubility and viscosity, which may affect its ability to form an effective protective matrix around lutein, thereby reducing its encapsulation efficiency. Studies indicate that the physicochemical properties of maltodextrin, such as its dextrose equivalent (DE), influence its ability to form an effective protective matrix around lutein (Kuang et al., 2015). Additionally, Ding et al. (2020) examined the influence of different carbohydrates as wall materials (maltodextrin and sucrose in various concentrations) on the characteristics of microencapsulated lutein. Their findings suggest that the type of carbohydrate used, including maltodextrin, significantly affects the encapsulation efficiency and stability of lutein, likely due to variations in solubility and viscosity among the carbohydrates (Ding et al., 2020). On the other hand, PD appears to preserve lutein better, as evidenced by the higher lutein concentrations in SUE+PD and FUE+PD formulations compared to their MD counterparts.
Although explicit quantitative data for lutein in Ulva species are scarce, especially encapsulated ones, carotenoid profiling studies of Ulva by Eismann et al. (2024) confirm lutein among the constituents (alongside β-carotene, violaxanthin, etc.). In pigment-recovery studies on Ulva rigida, lutein is also reported among the extracted carotenoids (Martins et al., 2021). These qualitative consistencies suggest that Ulva could maintain lutein levels comparable in order of magnitude to ours, though for precise µg/100 g comparisons, more focused quantification studies would be needed. For comparison, Ulva lactuca has been reported to contain lutein at 1389.30 µg/100 g dry weight (Mutavski et al., 2024). In other Ulva species, lutein content in the range 11.1–22.0 µg/g dry weight has also been reported by Wirenfeldt et al. (2024).
Many studies have focused on the encapsulation of lutein to achieve higher encapsulation efficiency and to improve its stability. One study developed microcapsules of lutein using lecithin and sodium caseinate as coating materials. The results demonstrated the stability of the capsules under varying conditions, including light exposure, temperature fluctuations, and pH changes (Zhao et al., 2018). In another study, lutein was encapsulated in cationic liposomes, achieving a high encapsulation efficiency (98.8%) and a lutein content of 22% relative to the lipid content, indicating successful integration of lutein into the liposomal membrane (Elkholy et al., 2020). Furthermore, in a study involving nanoemulsions, a nanoemulsion system was used to encapsulate lutein, providing a stable delivery system with high bioavailability and preserving lutein’s antioxidant properties (Algan et al., 2022). The study by Kuang et al. (2015) found that lutein microcapsules exhibited high encapsulation efficiencies of 69.1% to 90.1%, depending on the maltodextrin content. The goal of encapsulation in our study was to produce powders that are both cost-effective and suitable for further use, marking a significant difference compared to the previously mentioned successful encapsulation methods.
As mentioned, the Ulva species and Ulva-based extracts are rich in other carotenoids and natural pigments. Chromatograms of the examined encapsulated powders showed several peaks (the chromatogram of the SUE sample is shown in Figure 5). Their evaluation will be the focus of future studies. For future analyses, identification can be supported by spectral and chromatographic data under similar chromatographic conditions reported in the literature. In the absence of standards, results can be justified using retention times, cis-peak positions, absorption maxima, and other characteristic features.
Herein, lutein served as a reference marker to evaluate encapsulation behavior and monitor encapsulation efficiency to the reference standard using two different encapsulation methods. Analysis of other carotenoids and natural pigments will be addressed in future studies.
3.4 Antioxidant properties
Products known as reactive oxygen species (ROS) can be produced during various biochemical processes within the organism. ROS with one or more unpaired electrons are highly reactive with other molecules, and in larger quantities, they can reduce the body’s ability to counteract their harmful effects (Finkel and Holbrook, 2000; Dasgupta et al., 2015). A condition with a disturbed balance between ROS production and the body’s antioxidant defenses can cause oxidative stress (Pizzino et al., 2017), which has been connected with many diseases.
As mentioned previously, marine algae are sources of polyphenolic compounds, as well as natural pigments, chlorophylls, and carotenoids, pointing out their antioxidant potential and beneficial role in reducing oxidative stress that is harmful in different systems and organisms, from humans to animals, and in aquaculture (Fernando et al., 2016; Zhang et al., 2025). Various assays are used to test the antioxidant potential of marine-derived products. Among them, DPPH radical scavenging, ABTS, and reducing power (RP) assays are the most used (Nikolić et al., 2025).
Powders obtained in our study, possessing a high content of encapsulated bioactive compounds, exhibited high antioxidant activities (Table 5). Moreover, encapsulation techniques and the addition of carriers improved the protection of Ulva bioactive compounds compared to pure extracts. The encapsulation technique and the type of carrier affected the extracts’ antioxidant potential.
Table 5
| Samples | DPPH | ABTS | RP | |||
|---|---|---|---|---|---|---|
| SUE | 1567.87 ± 96.24 | ab | 7020.38 ± 912.02 | b | 254.19 ± 41.01 | a |
| SUE+MD | 1487.45 ± 129.22 | ab | 6222.28 ± 911.73 | b | 179.41 ± 23.17 | b |
| SUE+PD | 1484.45 ± 79.41 | ab | 6148.52 ± 680.10 | b | 223.87 ± 17.17 | ab |
| FUE | 1607.56 ± 240.93 | a | 13242.21 ± 1257.92 | a | 253.86 ± 16.85 | a |
| FUE+MD | 1305.98 ± 149.49 | b | 6358.75 ± 933.39 | b | 220.09 ± 16.46 | ab |
| FUE+PD | 1499.08 ± 142.82 | ab | 6403.36 ± 614.69 | b | 211.46 ± 32.55 | ab |
Antioxidant properties of obtained spray and freeze-dried powders (µmoL Trolox equivalents (TE)/100 g).
Values represent the mean of n = 3 ± standard deviation, followed by different letters with a significant difference between samples within the same column, according to the post hoc Duncan’s test at the level p ≤ 0.05. SUE, spray-dried Ulva extract; FUE, freeze-dried Ulva extract; MD, maltodextrin; PD, polydextrose; SUE, spray-dried Ulva extract; FUE, freeze-dried Ulva extract; MD, maltodextrin; PD, polydextrose.
The antioxidant potential of extracts obtained from green macroalgae, collected from different European water habitats, among them Ulva lactuca from three localities and Ulva spiralis from the Atlantic Ocean, by Piotrowicz et al. (2022), connected the green algae’s antioxidant activity not only to phenolics but also to high levels of non-phenolic compounds, such as chlorophyll. Moreover, Srikong et al. (2017) utilized DPPH and ABTS assays to study the antioxidant activity of Ulva intestinalis extracts obtained with different solvents, where the dichloromethane extract demonstrated the highest antioxidant activity. The acetone extract of Ulva lactuca and Enteromorpha intestinalis collected on the Montenegro Adriatic coast was tested by Kosanić et al. (2015). Both species showed antioxidant, antimicrobial, and cytotoxic activity, with U. lactuca exhibiting more potent free radical scavenging activity. The spray-dried powder of the brown alga Sargassum muticum (Tun Norbrillinda et al., 2016) resulted in the antioxidant activity of 464 μg TE/g powder, lower than that of the powders in our study.
To our knowledge, the antioxidant potential of the encapsulated extract of Ulva spp. collected from the Adriatic Sea, or of any other encapsulated Ulva material, has not been reported yet. This highlights the novelty and importance of our work, since encapsulation offers a promising strategy to improve the stability, solubility, and usefulness of Ulva-derived bioactives.
Obtained powders with a high content of encapsulated bioactive compounds, exhibited high antioxidant activities. However, pathological conditions may severely disrupt this homeostasis, thereby decreasing antioxidant levels. This can provoke a series of different illnesses. From this point of view, additional intake of antioxidants may be a powerful tool for the management of such conditions (Wang et al., 2021). The application of natural antioxidants in the food, feed, and pharmaceutical industries is considered a promising alternative to synthetic counterparts, offering high compatibility with intake and demonstrating no adverse effects on health.
4 Limitations of the study
Although the Ulva spp. biomass was collected during a period characterized by optimal biomass availability and phytochemical richness, the biochemical composition of Ulva is known to vary substantially with spatial and temporal factors, including season, local environmental conditions (e.g., salinity, temperature, nutrient availability), and developmental stage (Yaich et al., 2011). Such variability influences the content and relative proportions of target bioactive compounds (polyphenols, chlorophylls, carotenoids), thereby affecting extraction yields, encapsulation efficiency, stability, and functional properties. Consequently, seasonal and site-related differences may result in quantitative and, occasionally, qualitative shifts in antioxidant activity, pigment profiles (e.g., lutein versus total carotenoids), and the thermal or technological behavior of extracts and powders.
Applying appropriate pharmaceutical-technological procedures, such as optimized extraction and encapsulation methods, is crucial for obtaining standardized extracts and powders with uniform, reproducible bioactive profiles. The optimized maceration extraction techniques ensured maximum recovery of bioactive compounds, regardless of minor variations in the chemical composition of the raw material caused by season, climate, or habitat. On the other hand, encapsulation contributes to maintaining these compounds’ stability and bioactivity by protecting them from degradation during processing and storage. Together, these procedures enable the production of high-quality, reproducible extracts, which are essential for their further applications.
The results, therefore, demonstrate how spray-drying and freeze-drying, combined with selected biopolymer carriers, can improve the stability, handling, and functional retention of Ulva-derived bioactives.
5 Conclusion
Marine organisms, such as Ulva species, recognized as a raw material for producing innovative products, remain underexplored and underutilized. This study aimed to develop new methods to valorize Ulva spp. as a rich source of bioactives while demonstrating the feasibility of applying advanced, eco-friendly, and safe technologies in industrial processes. Two sophisticated encapsulation techniques were used to enhance the stability and bioavailability of bioactive compounds (polyphenolics and natural pigments, such as chlorophylls and carotenoids) from Ulva species found along the Adriatic Sea. As a result, Ulva spp. was successfully transformed into more valuable, applicable bioactive products, encapsulated powders, that open new opportunities for future utilization of these advanced forms. Recognized for its unique profile of bioactive compounds preserved in encapsulated form and exhibiting antioxidant activity, macroalgae, such as Ulva, are valuable assets across many industries. Although variability and seasonality of Ulva biomass undoubtedly exist and can influence the chemical profiles of extracts and encapsulates, this work demonstrates the feasibility of transforming Ulva extracts into stable and functional ingredients for food, feed, pharmaceutical, and aquaculture applications, regardless of natural variability. Additionally, the techniques presented in this study have broader potential for application, as they can be easily adapted to extract and preserve bioactive compounds from other marine sources, such as phytoplankton and various algal species. Collectively, obtained results emphasize the potential of techniques and marine-based products to enhance human and animal health, improve feed quality, and promote environmental sustainability, aligning with broader global goals. The findings provide a strong foundation for further development of blue biotechnology industries and highlight the potential of marine products as a sustainable resource for innovative applications.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
NN: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. ZM: Data curation, Writing – original draft. DD: Resources, Writing – original draft. SM: Formal Analysis, Software, Writing – review & editing. JV: Investigation, Methodology, Writing – original draft. DB: Writing – review & editing. KŠ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The experimental work is supported by the Ministry of Science and Technological Development and Innovation of the Republic of Serbia, contract numbers: 451-03-136/2025-03/200003, 451-03-137/2025-03/200134, 451-03-136/2025-03/200175, and the University of Montenegro.
Acknowledgments
This publication is based upon work from COST Actions CA22160, Enhancing knowledge of BIOmolecular solutions for the well-being of European AQUAculture sector (BioAqua), for supporting Open-access publication and Virtual mobility Grant, and CA20106 Tomorrow’s wheat of the sea: Ulva, a model for innovative mariculture (SeaWheat) by the grant for Short Term Scientific Mission. The authors would like to express their sincere gratitude to colleague Dr. Dejan Pljevljakušić, from the Institute for Medicinal Plants Research, Dr. Josif Pančić, for kindly preparing the photograph and for his valuable assistance with the statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Grammarly software was used for grammar checking.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ak E. Guven P. Tuney İ. Cagal M. M. (2025). Targeted delivery of seaweed bioactives: liposomal encapsulation of Ulva lactuca and Codium fragile for antibacterial enhancement. 3 Biotech.15, 217. doi: 10.1007/s13205-025-04374-7
2
Algan A. H. Gungor-Ak A. Karatas A. (2022). Nanoscale delivery systems of lutein: an updated review from a pharmaceutical perspective. Pharmaceutics14, 1852. doi: 10.3390/pharmaceutics14091852
3
Amin H. H. (2020). Biosynthesized silver nanoparticles using Ulva lactuca as a safe synthetic pesticide (in vitro). Open Agric.5, 291–299. doi: 10.1515/opag-2020-0032
4
Anwar E. Erianto H. Putri K. S. S. (2018). Preparation of powder from brown seaweed (Sargassum plagyophyillum) by freeze-drying with maltodextrin as a stabilizer. Int. J. Appl. Pharm10, 348. doi: 10.22159/ijap.2018.v10s1.77
5
Baele M. Vermeulen A. Adons D. Peeters R. Vandemoortele A. Devlieghere F. et al . (2021). Selecting packaging material for dry food products by trade-off of sustainability and performance: A case study on cookies and milk powder. Pack. Technol. Sci34, 303–318. doi: 10.1002/pts.2561
6
Braniša J. Jenisová Z. Porubská M. Jomová K. Valko M. (2014). Spectrophotometric determination of chlorophylls and carotenoids. An effect of sonication and sample processing. J. Microbiol. Biotechnol Food Sci.61–64. doi: 10.5555/20143095655
7
Caliskan G. Dirim S. N. (2016). The effect of different drying processes and the amounts of maltodextrin addition on the powder properties of sumac extract powders. Powder. Technol.287, 308–314. doi: 10.1016/j.powtec.2015.10.019
8
Ćujić Nikolić N. Jovanović M. Radan M. Lazarević Z. Bigović D. Marković S. et al . (2024). Development of cyclodextrin-based mono and dual encapsulated powders by spray drying for successful preservation of everlasting flower extract. Pharmaceutics16, 861. doi: 10.3390/pharmaceutics16070861
9
Ćujić Nikolić N. Žilić S. Simić M. Nikolić V. Živković J. Marković S. et al . (2023). Microencapsulates of blue maize polyphenolics as a promising ingredient in the food and pharmaceutical industry: characterization, antioxidant properties, and in vitro-simulated digestion. Foods12, 1870. doi: 10.3390/foods12091870
10
Dasgupta N. Chowdhury P. Das S. (2015). Comparative adaptability assessment of two mangroves from Indian sundarbans: some biochemical appearances. Nat. Sci. (Irvine).07, 519–534. doi: 10.4236/ns.2015.712053
11
Ding Z. Tao T. Wang X. Prakash S. Zhao Y. Han J. et al . (2020). Influences of different carbohydrates as wall material on powder characteristics, encapsulation efficiency, stability and degradation kinetics of microencapsulated lutein by spray drying. Int. J. Food Sci. Technol.55, 2872–2882. doi: 10.1111/ijfs.14544
12
Do Carmo M. Walker J. Novello D. Caselato V. Sgarbieri V. Ouwehand A. et al . (2016). Polydextrose: physiological function, and effects on health. Nutrients8, 553. doi: 10.3390/nu8090553
13
Dominguez H. Loret E. P. (2019). Ulva lactuca, A source of troubles and potential riches. Mar. Drugs17, 357. doi: 10.3390/md17060357
14
Eismann A. I. Perpetuo Reis R. Concha Obando J. M. Cunha dos Santos T. Negrão Cavalcanti D. (2024). Carotenoid content in Ulva lactuca cultivated under aquaculture conditions and collected from intertidal beds in southeastern Brazil: biotechnological implications for biomass use and storage. Cienc. Mar.50, 1–9. doi: 10.7773/cm.y2024.3461
15
El-Gendy N. Nassar H. N. Ismail A. R. Ali H. R. Ali B. A. Abdelsalam K. M. et al . (2023). A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products. Sustainability15, 7319. doi: 10.3390/su15097319
16
Elkholy N. S. Shafaa M. W. Mohammed H. S. (2020). Biophysical characterization of lutein or beta carotene-loaded cationic liposomes. RSC. Adv.10, 32409–32422. doi: 10.1039/D0RA05683A
17
Fernando I. P. S. Kim M. Son K.-T. Jeong Y. Jeon Y.-J. (2016). Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food19, 615–628. doi: 10.1089/jmf.2016.3706
18
Finkel T. Holbrook N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature408, 239–247. doi: 10.1038/35041687
19
Hidayati J. R. Yudiati E. Pringgenies D. Oktaviyanti D. T. Kusuma A. P. (2020). Comparative Study on Antioxidant Activities, Total Phenolic Compound and Pigment Contents of Tropical Spirulina platensis, Gracilaria arcuata and Ulva lactuca Extracted in Different Solvents Polarity. E3S. Web Conf.147, 3012. doi: 10.1051/e3sconf/202014703012
20
Jafari S. M. Assadpoor E. He Y. Bhandari B. (2008). Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol.26, 816–835. doi: 10.1080/07373930802135972
21
Jovanović M. Ćujić-Nikolić N. Drinić Z. Janković T. Marković S. Petrović P. et al . (2021). Spray drying of Gentiana asclepiadea L. root extract: Successful encapsulation into powders with preserved stability of bioactive compounds. Ind. Crops Prod.172, 114044. doi: 10.1016/j.indcrop.2021.114044
22
Kosanić M. Ranković B. Stanojković T. (2015). Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi. J. Biol. Sci.22, 390–397. doi: 10.1016/j.sjbs.2014.11.004
23
Kuang P. Zhang H. Bajaj P. R. Yuan Q. Tang J. Chen S. et al . (2015). Physicochemical properties and storage stability of lutein microcapsules prepared with maltodextrins and sucrose by spray drying. J. Food Sci.80, 359–369. doi: 10.1111/1750-3841.12776
24
Kumar Y. Tarafdar A. Kumar D. Verma K. Aggarwal M. Badgujar P. C. (2021). Evaluation of Chemical, Functional, Spectral, and Thermal Characteristics of Sargassum wightii and Ulva rigida from Indian Coast. J. Food Qual.2021, 1–9. doi: 10.1155/2021/9133464
25
Kurniasih R. A. Dewi E. N. Purnamayati L. (2018). Effect of different coating materials on the characteristics of chlorophyll microcapsules from caulerpa racemosa. IOP. Conf. Ser. Earth Environ. Sci.116, 12030. doi: 10.1088/1755-1315/116/1/012030
26
Lago C. C. Noreña C. P. Z. (2016). Polydextrose as wall material for microencapsulation of yacon juice by spray drying. Food Bioproc. Tech.9, 2103–2113. doi: 10.1007/s11947-016-1797-8
27
Leandro A. Pereira L. Gonçalves A. M. M. (2019). Diverse applications of marine macroalgae. Mar. Drugs18, 17. doi: 10.3390/md18010017
28
Ledari S. A. Milani J. M. Shahidi S.-A. Golkar A. (2024a). Comparative analysis of freeze drying and spray drying methods for encapsulation of chlorophyll with maltodextrin and whey protein isolate. Food Chem. X.21, 101156. doi: 10.1016/j.fochx.2024.101156
29
Ledari S. A. Milani J. M. Shahidi S.-A. Golkar A. (2024b). Fabrication and optimization of ultra-long stable microencapsulated chlorophyll using combinations of wall material via response surface methodology. Heliyon10, e40161. doi: 10.1016/j.heliyon.2024.e40161
30
Ma L. Lin X.-M. (2010). Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric.90, 2–12. doi: 10.1002/jsfa.3785
31
MaChado M. MaChado S. Pimentel F. B. Freitas V. Alves R. C. Oliveira M. B. P. P. (2020). Amino acid profile and protein quality assessment of macroalgae produced in an integrated multi-trophic aquaculture system. Foods9, 1382. doi: 10.3390/foods9101382
32
Martins R. Barbosa A. Advinha B. Sales H. Pontes R. Nunes J. (2023a). Green extraction techniques of bioactive compounds: A state-of-the-art review. Processes11, 2255. doi: 10.3390/pr11082255
33
Martins T. Barros A. N. Rosa E. Antunes L. (2023b). Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules28, 5344. doi: 10.3390/molecules28145344
34
Martins M. Oliveira R. Coutinho J. A. P. Faustino M. A. F. Neves M. G. P. M. S. Pinto D. C. G. A. et al . (2021). Recovery of pigments from Ulva rigida. Sep. Purif. Technol.255, 117723. doi: 10.1016/j.seppur.2020.117723
35
Mirhosseini H. Amid B. T. (2013). Effect of different drying techniques on flowability characteristics and chemical properties of natural carbohydrate-protein Gum from durian fruit seed. Chem. Cent. J.7, 1. doi: 10.1186/1752-153X-7-1
36
Mitchell W. R. Forny L. Althaus T. Niederreiter G. Palzer S. Hounslow M. J. et al . (2019). Surface tension-driven effects in the reconstitution of food powders. Chem. Eng. Res. Des146, 464–469. doi: 10.1016/j.cherd.2019.04.015
37
Muhammad G. Butler T. O. Chen B. Lv Y. Xiong W. Zhao X. et al . (2024). Sustainable production of lutein—an underexplored commercially relevant pigment from microalgae. Biomass Convers. Biorefin.14, 7255–7276. doi: 10.1007/s13399-022-03349-5
38
Munin A. Edwards-Lévy F. (2011). Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics3, 793–829. doi: 10.3390/pharmaceutics3040793
39
Mutavski Z. Jerković I. Nikolić N.Ć. Radman S. Flanjak I. Aladić K. et al . (2024). Comprehensive phytochemical profiling of ulva lactuca from the adriatic sea. Int. J. Mol. Sci.25, 11711. doi: 10.3390/ijms252111711
40
Nastić N. Mutavski Z. Ambrus R. Fernández N. Menković N. et al . (2023). Green processing of black raspberry pomace: application of sonotrode-based extraction technique and particles from gas-saturated solutions (PGSS) technology. Foods12, 3867. doi: 10.3390/foods12203867
41
Nedović V. Kalušević A. Manojlović V. Lević S. Bugarski B. (2011). An overview of encapsulation technologies for food applications. Proc. Food Sci.1, 1806–1815. doi: 10.1016/j.profoo.2011.09.265
42
Nhon H. T. N. Bao Ngan N. H. Doanh H. P. T. (2025). Extraction and spray-drying chlorophyll from Chaetomorpha aerea algae. HUIT. J. Sci25, 143–160. doi: 10.62985/j.huit_ojs.vol25.no3.205
43
Nikolić C. N. Drakulović D. Rakita S. Čabarkapa I. Vulić J. Mutavski Z et al . (2025). New insights in adriatic seaweed (Ulva spp.): nutraceutical and bioactive potential and green extraction techniques for pigments, antioxidants, and phenolic compounds. Mar. Biotechnol27, 116. doi: 10.1007/s10126-025-10490-5
44
Oikonomopoulou V. P. Krokida M. K. Karathanos V. T. (2011). The influence of freeze-drying conditions on microstructural changes of food products. Proc. Food Sci.1, 647–654. doi: 10.1016/j.profoo.2011.09.097
45
Ouahabi S. Daoudi N. E. Loukili E. H. Asmae H. Merzouki M. Bnouham M. et al . (2024). Investigation into the Phytochemical Composition, Antioxidant Properties, and In-Vitro Anti-Diabetic Efficacy of Ulva lactuca Extracts. Mar. Drugs22, 240. doi: 10.3390/md22060240
46
Pan-utai W. Iamtham S. (2020). Enhanced microencapsulation of C-phycocyanin from arthrospira by freeze-drying with different wall materials. Food Technol. Biotechnol.58, 423–432. doi: 10.17113/ftb.58.04.20.6622
47
Pap R. Pandur E. Jánosa G. Sipos K. Agócs A. Deli J. (2021). Lutein exerts antioxidant and anti-inflammatory effects and influences iron utilization of BV-2 microglia. Antioxidants10, 363. doi: 10.3390/antiox10030363
48
Pérez-Gálvez A. Viera I. Roca M. (2020). Carotenoids and chlorophylls as antioxidants. Antioxidants9, 505. doi: 10.3390/antiox9060505
49
Piotrowicz Z. Tabisz Ł. Łęska B. Messyasz B. Pankiewicz R. (2022). Comparison of the antioxidant properties of green macroalgae from diverse european water habitats by use of several semi-quantitative assays. Molecules27, 3812. doi: 10.3390/molecules27123812
50
Pizzino G. Irrera N. Cucinotta M. Pallio G. Mannino F. Arcoraci V. et al . (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev.2017, 1–13. doi: 10.1155/2017/8416763
51
Radan M. Ćujić Nikolić N. Kuzmanović Nedeljković S. Mutavski Z. Krgović N. Stević T. et al . (2024). Multifunctional pomegranate peel microparticles with health-promoting effects for the sustainable development of novel nutraceuticals and pharmaceuticals. Plants13, 281. doi: 10.3390/plants13020281
52
Ramadhan W. Ramadhani F. A. Sevica D. Hardiningtyas S. D. (2024). Synthesis and characterization of ulvan-alginate hydrogel beads as a scaffold for probiotic immobilization. Bio Web Conf.92, 2020. doi: 10.1051/bioconf/20249202020
53
Rezagholizade-shirvan A. Soltani M. Shokri S. Radfar R. Arab M. Shamloo E. (2024). Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X.24, 101953. doi: 10.1016/j.fochx.2024.101953
54
Robic A. Bertrand D. Sassi J.-F. Lerat Y. Lahaye M. (2009). Determination of the chemical composition of ulvan, a cell wall polysaccharide from Ulva spp. (Ulvales, Chlorophyta) by FT-IR and chemometrics. J. Appl. Phycol.21, 451–456. doi: 10.1007/s10811-008-9390-9
55
Robic A. Sassi J.-F. Lahaye M. (2008). Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polym.74, 344–352. doi: 10.1016/j.carbpol.2008.02.020
56
Šavikin K. Nastić N. Janković T. Bigović D. Miličević B. Vidović S. et al . (2021). Effect of type and concentration of carrier material on the encapsulation of pomegranate peel using spray drying method. Foods10, 1968. doi: 10.3390/foods10091968
57
Schultz C. Zopf D. Holzinger A. Silge A. Meyer-Zedler T. Schmitt M. et al . (2024). Raman spectral analysis in the CH x -stretching region as a guiding beacon for non-targeted, disruption-free monitoring of germination and biofilm formation in the green seaweed ulva. ChemPhysChem25, 1–11. doi: 10.1002/cphc.202400173
58
Selvasudha N. Goswami R. Tamil Mani Subi M. Rajesh S. Kishore K. Vasanthi H. R. (2023). Seaweeds-derived ulvan and alginate polysaccharides encapsulated microbeads–An alternative to plastic microbeads in exfoliating cosmetic products. Carbohydr. Polymer. Technol. Appl.6, 100342. doi: 10.1016/j.carpta.2023.100342
59
Singleton V. L. Orthofer R. Lamuela-Raventós R. M. (1999). doi: 10.1016/S0076-6879(99)99017-1
60
Srikong W. Bovornreungroj N. Mittraparparthorn P. Bovornreungroj P. (2017). Antibacterial and antioxidant activities of differential solvent extractions from the green seaweed Ulva intestinalis. ScienceAsia43, 88. doi: 10.2306/scienceasia1513-1874.2017.43.088
61
Tun Norbrillinda M. Mahanom H. Nur Elyana N. Nur Intan Farina S. (2016). Optimization of spray drying process of Sargassum muticum color extract. Dry. Technol.34, 1735–1744. doi: 10.1080/07373937.2016.1204550
62
Turk-Gul A. Urgu-Ozturk M. Koca N. (2023). The effects of different amounts of maltodextrin on the rheological behaviour and stability of white cheese emulsions, and the physical, microstructural, chemical and sensory properties of white cheese powders. Int. Dairy. J.138, 105552. doi: 10.1016/j.idairyj.2022.105552
63
Vidović S. S. Vladić J. Z. Vaštag Ž. G. Zeković Z. P Popović L. M (2014). Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder. Technol.258, 209–215. doi: 10.1016/j.powtec.2014.03.038
64
Vučetić A. Šovljanski O. Pezo L. Gligorijević N. Kostić S. Vulić J. et al . (2025). A comprehensive antioxidant and nutritional profiling of brassicaceae microgreens. Antioxidants14, 191. doi: 10.3390/antiox14020191
65
Vulić J. Šeregelj V. Kalušević A. Lević S. Nedović V. Tumbas Šaponjac V. et al . (2019). Bioavailability and bioactivity of encapsulated phenolics and carotenoids isolated from red pepper waste. Molecules24, 2837. doi: 10.3390/molecules24152837
66
Wahlström N. Nylander F. Malmhäll-Bah E. Sjövold K. Edlund U. Westman G. et al . (2020). Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr. Polym.233, 115852. doi: 10.1016/j.carbpol.2020.115852
67
Wang P. Chen J. Chen L. Shi L. Liu H. (2021). Characteristic volatile composition of seven seaweeds from the yellow sea of China. Mar. Drugs19, 192. doi: 10.3390/md19040192
68
Wirenfeldt C. B. Hermund D. B. Feyissa A. H. Hyldig G. Holdt S. L. (2024). Nutritional value, bioactive composition, physico-chemical and sensory properties of Ulva sp. and Fucus vesiculosus depending on post-harvest processing: a drying comparison study. J. Appl. Phycol.36, 2795–2805. doi: 10.1007/s10811-024-03210-4
69
Yaich H. Garna H. Besbes S. Paquot M. Blecker C. Attia H. (2011). Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem.128, 895–901. doi: 10.1016/j.foodchem.2011.03.114
70
Zhang R. Ren Y. Ren T. Yu Y. Li B. Zhou X. (2025). Marine-derived antioxidants: A comprehensive review of their therapeutic potential in oxidative stress-associated diseases. Mar. Drugs23, 223. doi: 10.3390/md23060223
71
Zhao T. Liu F. Duan X. Xiao C. Liu X. (2018). Physicochemical properties of lutein-loaded microcapsules and their uptake via caco-2 monolayers. Molecules23, 1805. doi: 10.3390/molecules23071805
Summary
Keywords
green seaweed, macroalgae, polyphenols, chlorophylls, carotenoids, lutein, encapsulation
Citation
Ćujić Nikolić N, Mutavski Z, Drakulović D, Marković S, Vulić J, Bigović D and Šavikin K (2025) Biopolymer-based encapsulation of Adriatic Ulva biomass bioactives: environmentally friendly technological advance in marine functional ingredients. Front. Mar. Sci. 12:1701293. doi: 10.3389/fmars.2025.1701293
Received
08 September 2025
Accepted
27 October 2025
Published
13 November 2025
Volume
12 - 2025
Edited by
Thomas Wichard, Friedrich Schiller University Jena, Germany
Reviewed by
Nathalie Bourgougnon, Université Bretagne Sud, France
Paula Mapelli Brahm, Universidad de Sevilla, Spain
Updates
Copyright
© 2025 Ćujić Nikolić, Mutavski, Drakulović, Marković, Vulić, Bigović and Šavikin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nada Ćujić Nikolić, ncujic@mocbilja.rs
ORCID: Nada Ćujić Nikolić, orcid.org/0000-0002-6736-3637; Zorana Mutavski, orcid.org/0000-0002-6998-0292; Smilja Marković, orcid.org/0000-0002-9264-4406; Jelena Vulić, orcid.org/0000-0001-9349-7367; Dubravka Bigović, orcid.org/0000-0003-1999-2743; Katarina Šavikin, orcid.org/0000-0002-2086-9593
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.