Abstract
As plastic pollution continues to escalate, the widespread presence and potential hazards of microplastics as an emerging global contaminant have drawn increasing attention. In natural environments, microplastic surfaces are prone to colonization by microbial biofilms composed of microorganisms and extracellular polymeric substances (EPS), forming a distinct microecosystem known as the plastisphere. This process not only modulates the physicochemical properties and environmental behavior of microplastics but also significantly changes their ecotoxicity. This paper systematically reviews the biofilm formation process on microplastic surfaces, the succession dynamics of microbial communities, and the key environmental and material factors influencing microbial colonization. On this basis, the regulatory modulates of biofilm formation on the physicochemical properties and environmental behavior of microplastics are analyzed, as well as their effects on bioavailability and ecotoxicological effects. Although there has been an increasing number of studies on the ecotoxicity of microplastics in recent years, most experiments are still limited to the pristine microplastics, that fail to reflect their realistic environmental exposure status. Therefore, this review emphasizes the necessity of incorporating biofilm-coated microplastics into toxicological assessments, to better simulate actual environmental conditions and to elucidate their synergistic roles in compound pollution scenarios.
1 Introduction
Plastics are widely used across various industries due to their light weight, durability, and low production cost (Adeleke, 2023), which has significantly contributed to the process of industrialization (PlasticsEurope, 2019). In recent years, plastic pollution has become a global environmental issue (Zhang et al., 2021b), among which microplastics with a particle size of less than 5 mm have become an emerging pollutant in the ecosystem due to their small particle size, diverse sources, and strong persistence(Geyer et al., 2017; O'brien et al., 2023). With the intensification of human activities, microplastics have become widely present in oceans, freshwater, soil, and atmosphere (Chen et al., 2023; O'brien et al., 2023), and have even been detected in remote and pristine regions such as the Arctic Ocean (Kanhai et al., 2020). At the same time, it has been detected in animals (Rebelein et al., 2021; Rakib et al., 2023), plants (Li et al., 2020), and even human tissues (Amato-Lourenco et al., 2021; Nor et al., 2021; Leslie et al., 2022). This widespread presence underscores the high environmental mobility and bioavailability of microplastics (Liu and Zheng, 2025). Moreover, their large specific surface area, surface hydrophobicity, and high adsorption capacity (Raddadi and Fava, 2019), enable them to act as carriers for persistent organic pollutants, heavy metals, and antibiotics (Sorensen et al., 2020; An et al., 2023), which enhance the bioavailability and toxicity of these contaminants (Wang et al., 2019b; Eze et al., 2024), and threatens ecosystem health. Therefore, microplastic pollution has become an urgent global environmental challenge. Although there is an increasing number of studies on the environmental behavior and ecotoxicity of microplastics, most experiments still focus on microplastics in the pristine state, making it difficult to accurately reflect their true exposure forms in the natural environment. In reality, microplastics are highly susceptible to microbial colonization once they enter the environment, and their surfaces rapidly develop biofilms composed of bacteria, fungi, algae, and extracellular polymeric substances (EPS). This attachment process not only modulates the surface physicochemical properties and migration behavior of microplastics, but also establishes a unique microecosystem called the "plastisphere" (Zettler et al., 2013; Amaral-Zettler et al., 2020). This interfacial zone between microplastics and biofilm not only reflects the ecological niche properties of microplastics, but also serves as a critical site for pollutant adsorption, accumulation, transformation, and release, all of which may significantly influence the bioavailability and environmental toxicity of microplastics.
In recent years, the structural characteristics, ecological functions and roles of microbial communities at microplastic interfaces in the process of compound pollution have attracted increasing attention. Studies have shown that the formation of biofilms is driven by environmental factors (e.g., temperature, nutrient level, pH) and microplastic characteristics (e.g., material, surface roughness, polarity, etc.), and plays an important role in regulating the adsorption, release and toxic behavior of pollutants. However, most existing reviews focus solely on ecotoxicity, and there is still a lack of systematic integrated review of "biofilm-coated microplastic". In view of this, this paper takes the biofilm-coated microplastics as the core research unit to systematically sort out the formation mechanism of biofilm, the succession characteristics of microbial communities, and the complex's regulatory function on the environmental behavior of pollutants, and further explore its key role in the bioavailability and toxicity mechanisms. Ultimately, this work seeks to deepen understanding of the environmental risks of microplastics and provide theoretical support for research into compound pollution and ecotoxicity.
We performed a bibliometric analysis of relevant literature (2023-2025) using VOSviewer (v1.6.19) based on the Web of Science database. Based on the keywords "microplastic biofilm", "plastisphere" and "microplastic microorganisms", the analysis identified 4,389 unique keywords in total, of which 83 met the minimum occurrence threshold of 20. The red cluster focuses on keywords including "toxicity", "oxidative stress" and "adsorption", emphasizing interactions between microplastics and pollutants alongside and their ecotoxicological effects. The green cluster highlights terms like "antibiotic resistance", "wastewater treatment" and "public health", reflecting growing research interest in biofilm-coated microplastics as vectors for antibiotic resistance genes (ARGs) and human health risks. Meanwhile, the blue cluster covers technical terms such as "network analysis" and "machine learning", suggesting the increasing application of multi-omics and computational approaches to resolve complex microplastic related systems. Collectively, the network diagram (Figure 1) demonstrates the multidimensional of microplastics research, spanning pollution behavior mechanisms, microbial ecological responses, and health risk assessments.
Figure 1
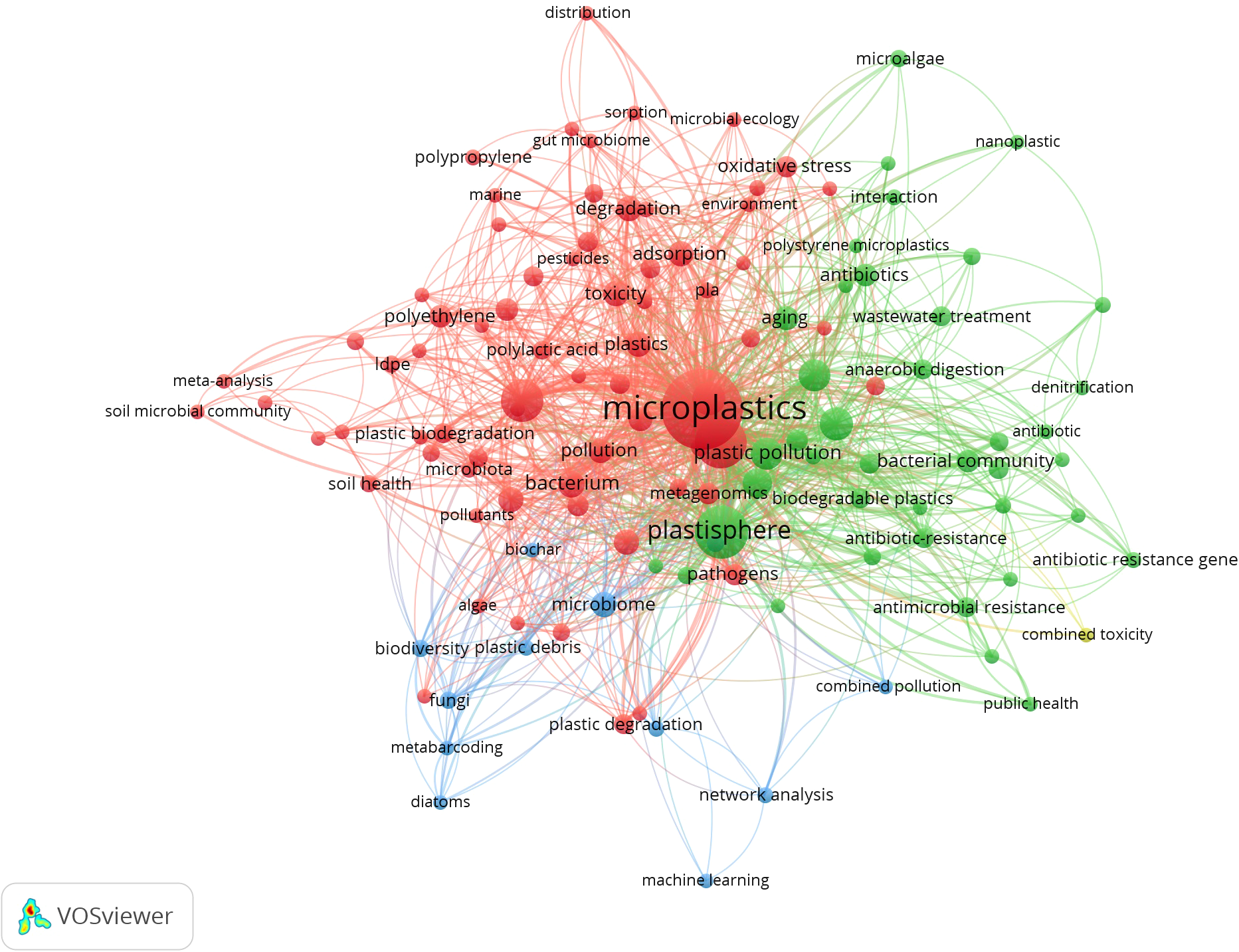
Network visualization for the keywords microplastic biofilm, plastisphere, and microplastic microorganisms.
2 Formation of biofilm on the surface of microplastics and succession of microbial communities
2.1 Biofilm formation process on the surface of microplastics
In natural environments, microplastics readily serve as substrates for microbial colonization. The development of biofilm-coated microplastics is a dynamic and multi-phased process (Du et al., 2022), which usually includes initial attachment, irreversible attachment, biofilm maturation, and detachment (Datta et al., 2016). Microplastic surfaces exhibit strong hydrophobicity, and rapidly adsorb dissolved organic matter in aquatic environments, forming an organic adsorption layer, that facilitates initial microbial colonization. During early colonization, pioneer bacteria reversibly adhere to the plastic surface, forming loose multicellular aggregates (Harrison et al., 2014; Quero and Luna, 2017), which constitute the first stage of biofilm development. As pioneer bacteria, their metabolic activity and EPS secretion gradually reduced surface hydrophobicity of the plastic (Tu et al., 2020), promoting stable microbial binding. Secondary colonizers enhance irreversible adhesion via surface structures (e.g., fimbriae, adhesion proteins), creating ecological niches for subsequent microbial populations (Dussud et al., 2018; Yang et al., 2020). The continuous introduction, loss and replacement of species in the community led to the dynamic succession of microbial composition, and finally formed a mature and functionally complex microbial community structure. Due to the significant differences in physical and chemical conditions, biological resources and disturbance intensity in environmental media, the colonization process and community structure of microplastic biofilms showed high variability in different environments. To further understand the differences in ecological behavior, Table 1 summarizes the main characteristics of microplastic biofilms in different environments.
Table 1
| Environment type | Substrate type | Eukaryotes | Microbial community | Reference | |
|---|---|---|---|---|---|
| Aquatic environment | Wetland | Polyethylene (PE), Polypropylene (PP) | / | Proteobacteria, Pseudomonas fluorescens, Aeromonas caviae | (Liu et al., 2024) |
| / | Desmids, Diatoms | Proteobacteria, Bacteroidota, Roseococcus | (Domozych and Domozych, 2008) | ||
| Polyvinyl chloride (PVC) | Nitzschia sicula, Mastogloia Corsicana, M. fimbriate | / | (Meng et al., 2024) | ||
| Lake | PE, PP | / | Pirellulaceae, Phycisphaerales, Cyclobacteriaceae | (Miao et al., 2019b) | |
| Poly(3-hydroxybutyrate) (PHB), High-density polyethylene (HDPE), Low-density polyethylene (LDPE) | Cryptomonas, Chlamydomonas (algae); Stentor, Vorticella (ciliates) | Betamyces, Cryptococcus (fungus); Mycoplana, Erythromicrobium (bacterium) | (González-Pleiter et al., 2021b) | ||
| PE, Expanded polystyrene (EPS-foam), PP | Pennales, Bacillariophyceaea, Chlorophyceae | / | (Di Pippo et al., 2022) | ||
| Polystyrene (PS) | / | Proteobacteria | (Guan et al., 2020) | ||
| River | PVC | / | Pseudomonas monteilii, Pseudomonas mendocina, Pseudomonas syringae | (Wu et al., 2019) | |
| PVC, Polyethylene terephthalate (PET) | Green algae, Diatoms | Cyanobacteria | (Miao et al., 2021) | ||
| PE, Polylactic acid (PLA), PS, Cellulose, Hemicellulose | / | Proteobacteria, Firmicutes | (Zhao et al., 2024c) | ||
| Seawater | PE, EPS-foam, PP, Nylon | Alveolata, Stramenopiles, Radiozoa | Alphaproteobacteria, Gammaproteobacteria, Cyanobacteria | (Amaral-Zettler et al., 2021) | |
| EPS-foam | / | Flavobacteriaceae, Rhodobacteraceae, Halomonadaceae, Vibrionaceae | (Zhang et al., 2024a) | ||
| PE, PLA | / | Alcanivorax, Nitratireductor | (Chu et al., 2024) | ||
| Soil | PE, Epoxy Resin (EP) | Coccus, Bacilli, Fusarium | PE : Chujaibacter, Rhodanobacter, Mycobacteria EP: Ralstonia | (Tian et al., 2024) | |
| LDPE, HDPE, PS, PP, PET | / | LDPE: Methylophaga, PS: Saccharimonadales, PET: Sphingomonas |
(Chen et al., 2022) | ||
| PP, Tire Wear Particles (TP) | / | PP: Bacillus, Sphingomonas TP: Pseudomonas, Acinetobacter |
(Ding et al., 2024) | ||
| Atmosphere | PET | / | Proteobacteria, Cyanobacteria, Actinobacteria | (Tu et al., 2022) | |
An overview of the main microbial communities on the surface of microplastics in different environments.
In this table, EPS-foam denotes expanded polystyrene. Elsewhere, EPS refers to extracellular polymeric substances; "/" denotes data not reported in the cited source.
Across environmental media, microplastics rapidly develop surface-attached microbial communities that evolve into biofilms upon environmental exposure. Current research, however, remains predominantly focused on aquatic environments, where substantial data exist regarding microbial colonization dynamics and ecological impacts. Most available studies in these systems are limited to description (Domozych and Domozych, 2008; Liu et al., 2024), lacking systematic characterization of biofilm community structure, functional potential, and ecological roles. Therefore, fundamental aspects including biofilm evolution processes in non-aqueous environments, synergistic pollutant adsorption capacities, and associated ecosystems impacts require in depth investigation.
2.2 Microbial community assembly in microplastic biofilms
The assembly mechanisms governing microbial communities within microplastic biofilms are fundamental to understanding their structural development and functional evolution. These processes not only determine microbial diversity and community stability but also underpin successful biofilm colonization on heterogeneous surfaces. Current theoretical frameworks center on two paradigms: niche theory, which emphasizes deterministic environmental selection, and neutral theory, which highlights the role of stochastic processes in community assembly.
Niche theory believes that community structure is determined by environmental conditions and species physiological traits, with environmental selection acting as the primary screening mechanism. Distinct microbial taxa showed significant variation in niche preference and spatial distribution patterns in response to pollution-induced environmental stress. For example, Wang et al. employed high-throughput 16S rRNA gene sequencing to compared planktonic archaea and their predominant taxa, Marine Group I (MGI) and Marine Group II (MGII), across varying environmental gradients. Their results showed that the selection processes predominantly governed the assembly of these three archaeal communities, with salinity and nutrient availability identified as key influencing factors. These findings support the central role of niche theory in shaping microbial community structure (Li et al., 2021b). Similarly, Sun et al. collected and analyzed bacterial communities in different ecological niches (including water, sediment, and plant root foliage) in artificial lakes. They found that niche type exerted a significantly stronger influence on community diversity and structure than pollution intensity, Moreover, specific ecological niches, such as sediments and plant-associated interfaces, exhibited more complex and structured community networks, reflecting a clear environmental filtering mechanism. These results further reinforce niche theory, suggesting that environmental conditions and resource availability are critical factors driving the assembly of microbial communities on biofilm-coated microplastics (Sun et al., 2023b). With the deepening of research, the application of this theory was expanded to the study of new artificial ecological niches (e.g., microplastic surfaces) generated by human activities. The surface of microplastics is hydrophobic and nutrient deficient, and the biofilm community structure on it was markedly different from that of the suspended communities in the surrounding water column, showing lower OUT abundance and Shannon diversity index. Additionally, the non-random distribution of biofilm communities and functional taxonomic preferences support the dominance of a species sorting mechanism, indicating that biofilm formation on microplastics is largely influenced by deterministic niche selection processes (Besemer et al., 2012). Some studies have pointed out that the plastisphere can form a stable coexistence pattern by selectively recruiting specific microbial groups, which is clearly different from the microbial composition in the natural environment. In addition, community differences driven by ecosystem background are much greater than physical attributes between plastics and natural substrates, and this trend is also reflected in the global microbial co-occurrence network (Li et al., 2024).
Relatively speaking, the neutral theory emphasizes the equivalence of species in ecological functions, arguing that community structure is mainly determined by random processes such as birth, death, migration, and dispersal (Chen et al., 2019). In certain plastisphere, non-deterministic processes have been found to play an important role in community construction. The role of selective recruitment appears limited. Community assembly is mainly driven by ecological drift and dispersal restrictions. Early dominant taxa, such as Proteobacteria, with broad ecological niches, increase susceptibility to opportunistic colonization. In addition, hydrodynamic processes such as tides further enhance the probability of random attachment of microorganisms on plastic surfaces, reflecting the high randomness of community structure (Yan et al., 2024). In another study of archaeal communities, homogeneous dispersal played a dominant role in the assembly of MGI and MGII communities, with effects 1.63 times and 1.34 times higher than that of the selection process, respectively. This indicates that in these archaeal communities, mass effect leads to the convergence of community structure. The remaining difficult-to-explain differences in the community may be caused by the superposition of multiple weak drivers, manifesting as a non-deterministic random assembly pattern (Wang et al., 2019a).
Accumulating evidence suggests that microbial community assembly is not attributable to a single mechanism, but instead reflects the combined influence of deterministic and stochastic factors. It presents a continuous and unified dynamic balance. Some studies have found that there is no significant correlation between environmental factors and species diversity index in the plastisphere, indicating that the community structure is largely driven by non-deterministic processes such as ecological drift and dispersal restrictions. At the same time, compared with non-plastic substrates, plastic surfaces provide relatively more stable ecological niches, showing certain environmental selectivity, such as the significant enrichment of microorganisms associated with plastic degradation on plastic particles, reflecting the directed screening role of functional species (Pang et al., 2024). In addition, there is differentiation in the response of different microbial taxa to the assembly mechanism. Studies have shown that even within the same community, different taxa may be governed by different mechanisms. Dominant taxa are more likely to be shaped by environmental selection, whereas rare taxa are primarily influenced by non-deterministic processes such as ecological drift and dispersal limitations (Xiang et al., 2022). Another study pointed out that eukaryotic communities tend to be controlled by homogeneous selection at specific periods, driven by seasonal changes, while overall random processes and neutral mechanisms remain dominant. In addition, the neutral model had a significantly higher fit for bacterial communities than eukaryotes, indicating that neutral processes have a stronger explanatory power for bacterial community structure formation (Huang et al., 2025). Preference for community assembly mechanisms may also be influenced by organism characteristics. Luan et al. suggested that differences in microbial size affect their ability to spread, with small microorganisms more susceptible to random processes and large microorganisms more affected by environmental screening (Luan et al., 2020). In complex ecosystems, the dynamic balance between assembly mechanisms is significantly reshaped. For example, natural hydrological disturbances can guide communities from decisive screening to random assembly at different time scales, with specific manifestations that vary depending on the ecological context, such as inverse shifts in mechanisms within Mediterranean rivers and northern streams (Sarremejane, 2018). Continuous environmental stress often enhances the dominance of decisive mechanisms, leading to functional stability but reduced structural diversity (Santillan et al., 2020). In addition, plastics can also profoundly affect community assembly mechanisms in the environment. Studies have shown that microplastic introduction increases the relative importance of deterministic processes, particularly in water and sediment samples. This effect is likely due to microplastics providing new ecological niches or altering the local microenvironment, which promotes the selective colonization of specific microorganisms (Wu et al., 2025). Bacterial communities on degradable microplastics are more strongly shaped by deterministic processes, particularly homogeneous selection, compared with non-degradable microplastics. Material degradation alters the microenvironment, which enhances environmental filtering, promotes the colonization of specific microorganisms, and reduces community diversity (Zhang et al., 2024c). It is worth noting that the influence of microplastics on bacterial community assembly and succession is not only affected by their material properties, but also closely related to the shape of microplastics (e.g., fibers, fragments, particles), which have different microstructural characteristics, which in turn change the attachment area and spatial distribution of bacteria, thereby further regulating the colonization process and community structure of microorganisms (Wang et al., 2023). In addition, process such as preferential biological effects and historical contingency are also thought to play an important role in the early colonization stage of communities, further increasing the complexity of assembly mechanisms. The above community assembly process is jointly regulated by a variety of environmental factors and microplastic characteristics, and these key influencing factors are systematically sorted out below.
2.3 Influencing factors of biofilm formation on the surface of microplastics
Microbial biofilm formation on microplastics fundamentally alters their environmental behavior and may amplify ecological risks. The multifaceted process is governed by complex interactions between environmental conditions and microplastic properties. To systematically synthesize current knowledge, we consolidate key influencing factors and their action trends in Table 2. The formation of microplastic biofilm is significantly regulated by multiple environmental factors, mainly including the combined effects of external environmental conditions and the physicochemical properties of microplastics. Existing studies have shown that the initial biomass and diversity on microplastic surfaces are influenced by both the characteristics of microplastics and the surrounding environment, although over time the environmental influence may become more pronounced (Jin et al., 2025). In addition, under specific environmental backgrounds (such as high salinity and low nutrition availability), biofilm communities may show certain substrate specificity. However, this effect is typically governed by environmental factors and its trend is often unstable. Additionally, microbial community structures vary significantly across geographic regions, highlighting the role of spatial heterogeneity in community assembly within the plastisphere (Oberbeckmann et al., 2018). This conclusion is also consistent with the results of previous studies in the North Sea (De Tender et al., 2015).
Table 2
| Factors | Mechanisms | Reference | |
|---|---|---|---|
| Environmental factors | Temperature | High summer temperatures enhance microbial growth and metabolism, accelerating early biofilm colonization. | (Jin et al., 2020; Zhang et al., 2021a; Song et al., 2024) |
| Salinity | Salinity alters microbial community structure and selects tolerant strains, shaping biofilm composition. | (Kesy et al., 2019; Qiang et al., 2021) | |
| Illumination | Light supports phototrophs (e.g., diatoms, green algae) and stabilizes biofilm communities. | (Tu et al., 2020; Smith et al., 2021; Bairoliya et al., 2024; Deng et al., 2024) | |
| Nourishment | Rich nutrients accelerate microbial proliferation and biofilm growth. Low nutrients stimulate specific microorganisms, enhancing attachment and stabilizing biofilm formation. | (Stanley and Lazazzera, 2004; Arias-Andres et al., 2018a; Li et al., 2019) | |
| Time | Over time, biofilm morphology and structure become increasingly complex and diverse. | (De Tender et al., 2017; Guan et al., 2020; Song et al., 2024) | |
| Substrate type | Substrate type | The rough surface is conducive to the early colonization of biofilms; Provide a more stable ecological niche. | (Chen et al., 2022; Tkachuk and Zelena, 2023; Zhao et al., 2024c) |
| Plastics type | Different plastic properties affect the selective attachment of microorganisms. The functional tendency to form biofilms is different. | (Chu et al., 2024; Wang et al., 2024; Yan et al., 2024) | |
| Particle source | Environmentally aged particles generally have biological attachments, which affect the succession process and function of biofilm bacteria. | (Shan et al., 2022; Rozman et al., 2023a; Zhao et al., 2024b) | |
| Morphological structure | Different morphologies affect the capacity and ecotoxicity of adherent biofilms. | (Lee et al., 2022; Rozman et al., 2023b) | |
| Particle size | Particle size determines microbial load per unit area, with smaller particles offering greater surface area. | (Klein et al., 2021) | |
The main factors and mechanisms affecting the formation of biofilm on the surface of microplastics.
At the same time, as an important carrier of biofilm colonization, the inherent physicochemical properties of microplastics profoundly affect the initial attachment and community succession process of microorganisms. Compared to suspended particles in aqueous environments, the rough microscale structure of solid microplastics provides a stable surface for microbial adhesion, facilitating the development of structurally complete biofilms. The microbial community structure on microplastics is significantly different from that of free microorganisms in seawater, and only a few dominant orders coexist in prokaryotes, and the rest are endemic taxa. Eukaryotic microorganisms also showed a clear trend of community differentiation. Although both communities are subject to seasonal variation, microplastic-associated communities remain relatively stable, indicating that microplastics provide a consistent ecological niche for microbes (Davidov et al., 2024). Under the action of external interference, the microbial community on the surface of microplastics showed strong resilience, especially the bacterial community could be quickly rebuilt. The eukaryotic community showed a trend of decreasing diversity after disturbance, which may reflect the differences in ecological strategies between the two communities (Nguyen et al., 2023). In addition, under the same culture conditions, biofilm formation on the surface of microplastics is generally faster than that of natural polymers, and the community succession process is also more rapid, often undergoing multiple dynamic stages in a short period of time (Zhao et al., 2024c). The material type of microplastics further shapes the structure and function of their biofilms. Biofilms on the surface of degradable plastics (such as PLA) exhibit high robustness and low ecological vulnerability. In contrast, petroleum-based plastics such as PE form thicker biofilms, have greater greenhouse gas emission potential, and carry a higher risk of antibiotic resistance. However, some studies have also shown that microbial communities on PLA surfaces are relatively simple and more susceptible to external disturbance, potentially increasing the risk of pathogen enrichment and transmission. This suggests that the impact of plastic material type on biofilm succession may be context-dependent (Yan et al., 2024). In addition, the physical and chemical properties of plastics determine their adsorption preference for microorganisms. PP and PE are more conducive to the development of carbon cycle-related microorganisms and biofilms, while PET tends to enrich microflora with plastic degradation potential (e.g., Pseudomonas), further illustrating the material-dependent selectivity of microplastics in shaping microbial ecological processes (Wang et al., 2024).
3 Effects of biofilm formation on microplastics
Beyond biofilm formation mechanisms, the subsequent environmental feedback effects warrant equal attention. Biofilms are prone to form on the surface of microplastics in the environment, which significantly modulates their interface properties and environmental behavior. The adhesion of biofilm endows microplastics with new environmental response characteristics by regulating their surface properties, migration behavior, and degradation processes. The following section explains its key impacts from three aspects. During biofilm formation, microorganisms secrete large amounts of EPS and metabolites containing polar functional groups (e.g., carboxyl, hydroxyl, and amino groups). These substances markedly alter the hydrophilicity, surface charge, and reactivity of microplastics. As microorganisms colonize microplastic surfaces, clear signs of aging rapidly emerge, including reshapes in crystallinity, stiffness, surface roughness, and the colonization of unique microbial taxa. This indicates a close link between microbial community structure and the physicochemical properties of microplastics (Mcgivney et al., 2020). The accumulation of biofilms leads to the gradual formation of irregular structures such as cracks, holes, and grooves on the surface, which not only expands the space available for attachment, but also promotes the expansion and stability of biofilms (Guan et al., 2020). In addition, the hydrophilicity of microplastics is enhanced by microbial secretion of organic matter and degrading enzymes. This effect becomes more pronounced as biofilms mature (Tu et al., 2020). Increased hydrophilicity, in turn, accelerates the adsorption of bioorganic matter, promotes heterogeneous surface deposition, and ultimately strengthens surface roughness (Bhagwat et al., 2021).
The accumulation of biofilms is usually accompanied by an increase in the biomass and density of the surface of microplastics, significantly altering their behavior in water bodies. Microplastics that originally accumulate at the water-air interface due to hydrophobicity have reduced buoyancy and gradually settled to the bottom of the water column or even sediment areas after natural exposure and biofilm attachment (Lobelle and Cunliffe, 2011). With the increase of attached biofilm, the density of microplastics increased, and the sinking rate was affected by the particle size, biofilm thickness and loading. Among them, small-sized microplastics are more likely to lose buoyancy and sink faster than large-sized microplastics (Fazey and Ryan, 2016). Interestingly, this process may show periodic "oscillatory" behavior. As the biofilm load increases, microplastic particles gradually sink. In deeper zones unfavorable for biofilm growth, microbial death and respiration reduce the biofilm, decrease particle density, and increase buoyancy, causing the particles to rise again. Once back in the shallow chlorophyll-rich water body, the biofilm re-accumulates and the particles sink again, showing a periodic cycle of rise and fall (Golubeva and Gradova, 2024). Different types of microplastics exhibit varying responses. The formation of biofilms accelerates the sedimentation rate of high-density plastics such as PET and PVC, while the sinking trend of microplastics with densities close to water, such as PE or PP, is limited by the structural properties and composition of biofilms (Kaiser et al., 2017). However, some studies have shown that biofilm colonization does not significantly alter the sedimentation behavior of particles in some scenarios, suggesting that the density change caused by biofilms may not be sufficient to overcome the buoyancy of the original plastic, or its effect is limited by the physical properties of microbial populations and EPS (Jalón-Rojas et al., 2022). In addition, some scholars have suggested that surfactants produced by microbial metabolism may affect their vertical migration pathways and sedimentation stability by regulating the interfacial tension between microplastics and water bodies (Pete et al., 2023). Compared with its effect on sedimentation, biofilm adhesion also provides an alternative migration pathway. It promotes aggregation with other particles and stable attachment to plants, aquatic animals, or other biological surfaces, thereby influencing migration, spatial distribution, and environmental stability (Kalčíková, 2023).
Microplastics in marine environments primarily undergo degradation through two distinct pathways: abiotic degradation, driven by physical and chemical factors such as ultraviolet radiation, oxidation, and mechanical abrasion; and biotic degradation, which relies on microbial communities that metabolically facilitate the breakdown and transformation of microplastics (Weinstein et al., 2016). Studies have shown that biofilms may play a critical protective role in the photoaging process of microplastics by modulating photoaging pathways and suppressing the generation of reactive oxygen species (ROS) (Zhang et al., 2024b). Emerging evidence demonstrates that plastic-degrading microorganisms preferentially accumulate on plastic surfaces, suggesting that the formation of biofilms on plastic surfaces may actively promote plastic degradation in natural aqueous environments (Pang et al., 2024). To further reveal the role of biofilms in influencing the stability of microplastics, recent studies have explored the underlying mechanistic pathways involved in various degradation processes. For example, on the PS surface, biofilms can induce oxidative reactions that lead to the formation of oxygen-containing functional groups, thereby significantly altering their FT-IR spectral characteristics of the material (Liu et al., 2023b). Moreover, comparisons of microplastics with and without biofilm colonization suggest that key bacteria taxa, including Flavobacterium and Erythrobacterium may be crucial to the long-term evolution of biofilm communities (Gulizia et al., 2025). However, it is worth noting that Rhusobacter spp. were absent from the microbial consortia associated with polyethylene degradation, indicating that their ecological function may exhibit substrate-specific characteristics (Restrepo-Flórez et al., 2014). Biofilms also showed a significantly influence the release behavior of plastic additives. Experiments have shown that microplastics with smaller particle sizes and higher surface roughness exhibit more pronounced leaching of additives such as FP-127, and this process is positively correlated with the abundance of specific core microbial communities (Pan et al., 2025). In addition, biofilm attachment significantly promotes the release of phthalate additives such as di(2-ethylhexyl) phthalate (DEHP), and their cumulative release increases with biofilm development. Although DEHP can be partially adsorbed and degraded in biofilms, its metabolites (such as MEHP) may exhibit even higher toxicity, indicating that biofilms may not fully eliminate additives and might instead amplify the ecological risks associated with microplastics (Zhao et al., 2024a). In view of the in-depth research on the microplastic-biofilm system in aquatic environments, this paper constructs a schematic diagram (Figure 2) illustrating the environmental changes of microplastics following biofilm colonization. The diagram focuses on key processes such as altered migration behavior, pollutant enrichment and ecological risk. Biofilms not only reshape the environmental behavior of microplastics (e.g., sedimentation and agglomeration), but also greatly enhance the ability of microplastics as pollutant carriers and pathogen transmission platforms, and significantly improve their bioavailability and feeding attractiveness, ultimately profoundly affecting their ecotoxicological effects.
Figure 2
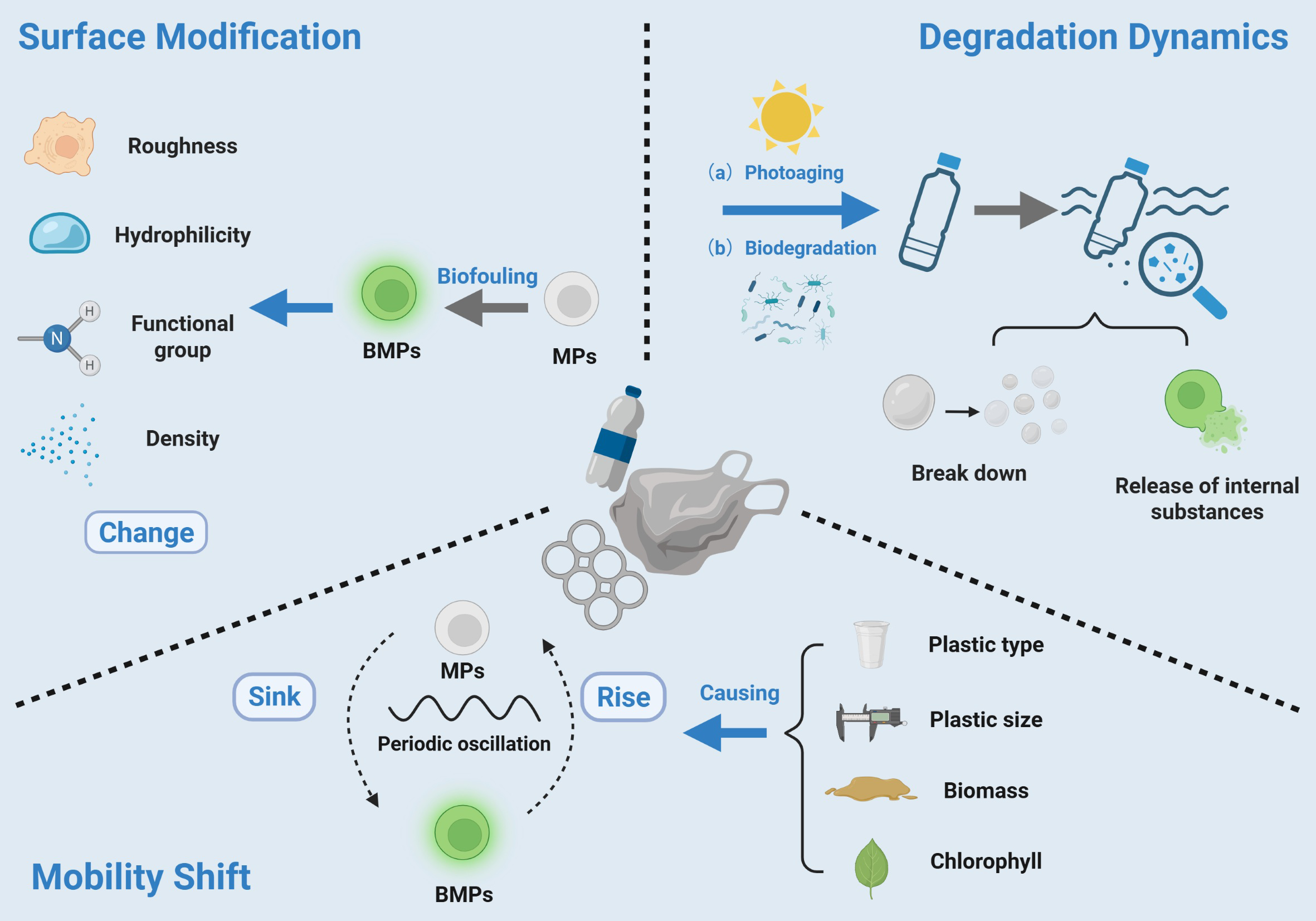
The adhesion of biofilms in aquatic environments changes the surface properties of microplastics and affects their environmental behavior.
4 Ecotoxicological effects of biofilm-coated microplastics
Biofilm adhesion makes the surface of microplastics appear looser and more porous, which is conducive to the capture and retention of pollutants (Sun et al., 2021). EPS significantly changes the adsorption properties of microplastics by introducing a variety of functional groups, thereby enhancing pollutant enrichment and prolonging their environmental retention time (Liu et al., 2019; Guan et al., 2020). For example, although biofilm biomass on glass beads was initially higher than that on LDPE and PLA, biofilms on the plastic surfaces gradually accumulated over time. This increased biofilm load enhanced metal adsorption, and the final accumulation on LDPE and PLA exceeded that on glass beads, indicating that biofilm adhesion strengthens the pollutant adsorption capacity of microplastics (Richard et al., 2019). Biofilms on microplastics frequently accumulate diverse contaminants, including heavy metals (Forero-López et al., 2022; Xiao et al., 2025), persistent organic pollutants, and antibiotics (Wang et al., 2022). Moreover, microbial communities in biofilms can achieve horizontal transfer of ARGs through close contact, making microplastics an important "pollutant-gene" transmission platform, potentially threatening aquatic organisms and public health (Arias-Andres et al., 2018b; Li et al., 2021a). Furthermore, biofilm-coated microplastics act not only as carriers of pollutants and toxins but also as inducers of physiological stress in host organisms (Huang et al., 2020)and cause intestinal microbiota dysbiosis (Jin et al., 2018). To systematically assess exposure pathways and toxicological effects, Table 3 synthesizes findings across pollutant categories, affected species, and associated toxic effects.
Table 3
| Adsorption mechanism | Adsorbent type | Affected organisms | Toxic effects | Reference |
|---|---|---|---|---|
| Provides an attachment environment | Vibrio | Trachinotus ovatus | The abundance of proteobacteria in the intestinal flora was increased, and the abundance of firmicutes was decreased | (Liu et al., 2023a) |
| Salmonella, Aeromonas | Zebrafish | Changing the structure of intestinal flora, increasing the diversity and abundance of pathogenic genera and ARGs, and inducing intestinal oxidative reactions | (Yu et al., 2024) | |
| Vibrio parahaemolyticus | Mytilus galloprovincialis | Causes oxidative stress and apoptosis of blood cells | (Sun et al., 2023a) | |
| Staphylococcus sp., Elizabethkingia sp. | Amphioxus | Bacteria have the potential to transfer from the plastic intersection to the biological intestine | (Cheng et al., 2023) | |
| Escherichia coli | Ostrea edulis | Biofilms make microplastics more easily ingested by oysters, creating an immune system response | (Fabra et al., 2021) | |
| Chloroflexi, Proteobacteria, Basidiomycotina | Zebrafish | Enhanced endocrine system and thyroid toxicity | (Chen et al., 2021) | |
| Bacillus | Enchytraeus crypticus | Induce intestinal dysbiosis and affect ecological function | (Ding et al., 2024) | |
| Aeromonas salmonicida achromogenes | Zebrafish | Affects development and interferes with key gene expression | (Missawi et al., 2024) | |
| Provide attachment environment and functional group adsorption |
Vibrio, ARGs |
Coral reef | Interferes with symbiont metabolism and coral bleaching | (Zhou et al., 2024) |
| Functional group adsorption | Antibiotic: azithromycin (AZI), clarithromycin (CLA) |
Anabaena sp. PCC7120 | Inhibited growth and chlorophyll A content | (González-Pleiter et al., 2021a) |
| EPS complexation | Pb | D. magna. | Enhanced toxicity to Pb(II) and adsorption and combined toxicity, and mortality increased in acute toxicity experiments | (Qi et al., 2021) |
| EPS complexation and providing an attachment environment | Cu, Pb, Vibrio | Clarias gariepinus | The number of Vibrio in the intestine of catfish increased; Reduces immunity and causes greater toxicity | (Jang et al., 2022) |
| Hydrophobic interactions | Pollutant: triclosan (TCS) |
D. magna. | Regulate the adsorption and desorption behavior of microplastics on TCS, and affect the toxicity to organisms | (Verdú et al., 2023) |
| Pollutant:dichlorodiphenyldichloroethylene (DDE), chlorpyrifos, and benzophenone-3 | Dicentrarchus labrax | Inflammatory response and increase in potentially pathogenic microorganisms in the intestines | (Montero et al., 2022) |
An overview of the adsorption mechanism and ecotoxicological effects of pollutants on biofilm-coated microplastics.
In addition, the complex has a higher bioavailability. Biofilm-coated microplastics not only enhance the adsorption capacity of organic pollutants, antibiotics, and heavy metals, but also improve the bioavailability of these pollutants through their metabolic activity and enzymatic reactions. Studies have shown that biofilm microorganisms can secrete a variety of intracellular and extracellular enzymes, such as oxidoreductase and dehydrogenase, which can convert complex pollutants such as PAHs into small molecule metabolic intermediates, thereby promoting their uptake by organisms (Yadav and Chandra, 2020). In addition, some biofilm formation can also biodegrade the plastic surface by secreting esterases, lipases, etc., and indirectly release secondary pollutants such as plasticizers or additives, thereby changing the exposure form and risk level of pollutants (Behera and Das, 2023). Under specific environmental conditions, microplastics attached to pollutants are more likely to stimulate microbial metabolic reactions and accelerate the transformation of pollutants (Girard et al., 2020). For example, Fajardo et al. found that biofilm-coated polyethylene microplastics triggered microbial oxidative stress reactions after exposure to organic carbon (OCs), suggesting that pollutants may undergo initial metabolic transformations in biofilms (Bonnineau et al., 2021). Secondly, biofilm-coated microplastics are more likely to be ingested by a variety of aquatic organisms, which allows pollutants to be transmitted across trophic levels of the food chain and may lead to bioaccumulation. Biofilms are an important food resource in freshwater ecosystems accumulating metals and organic pollutants, and their high metabolic activity makes them an important entry point for pollutants into the food chain (Bonnineau et al., 2021). Biofilm in rivers can significantly enrich microplastic particles and be ingested by protozoa, indicating that biofilms not only improve particle retention, but also increase their bioavailability at low trophic levels, which may affect their subsequent distribution in the food chain (Hamann et al., 2024). Studies have confirmed that the amount of microplastics ingested by fish through the food chain is significantly higher than that taken directly from water bodies, indicating that the trophic level transfer pathway of microplastics is one of the important exposure pathways faced by high-trophic organisms (Hasegawa and Nakaoka, 2021). Furthermore, biofilm formation produces an "ecological bait" effect on the surface of plastic, enhancing its feeding appeal to benthic and zooplankton. Once microplastics are attached to microorganisms, their feeding rate is significantly increased. For example, benthic filter feeding animals have a much higher ability to ingest microplastics coated with E. coli than pristine microplastics, with average concentrations of 42.360 ± 23.588 no.g⁻¹ and 11.443 ± 0.4 no.g⁻¹, respectively (Fabra et al., 2021). In addition, the amphipod Orchestia gammarellus significantly enhances the breaking strength of biofilm-coated plastics, producing up to 8.23 fragments per day, indicating that microplastics are more biologically identified as food after being biofilm-coated. For fish, although active ingestion of microplastics is uncommon, the incidence of accidental ingestion "bycatch" increases significantly when food particles are present nearby, and this probability rises with fish age. These results suggest that biofilm-coated microplastics floating in aquatic environments are more likely to be mistaken for edible prey, thereby increasing their risk of transmission in the food chain (Yagi et al., 2022). This process not only increases the risk of ingestion of microplastics, but may also trigger a "toxicity amplification effect", posing a potential threat to apex predators and even human health (Hodgson et al., 2018). As feeding behavior increases, biofilm-coated microplastics more easily cross the biological barrier and accumulate in the gut, liver, and even brain tissue. The viscous and functional components of the biofilm surface can affect the residence time and distribution of microplastics in the body, inducing microplastics to reside in specific tissues for a long time. Microplastics have been shown to enter a variety of tissues through the digestive system, blood circulation, and even across the blood-brain barrier (Zhu et al., 2023). The presence of biofilms enhances the interaction between particles and biological interfaces, which is one of the important factors for enhancing their trans-barrier migration ability. Studies have further simulated the behavior of microplastics that adsorb pollutants in the gastric environment, and found that gastric juice components promote the release of pollutants on its surface, while changing the physical and chemical properties of the particles, thereby enhancing their penetration in the digestive system and the risk of tissue contact (Wu et al., 2023). To more clearly illustrate the toxicological pathways of biofilm-coated microplastics in ecosystems, Figure 3 presents the main mechanistic routes, including pollutant enrichment, pathogen carriage, and enhanced bioavailability.
Figure 3
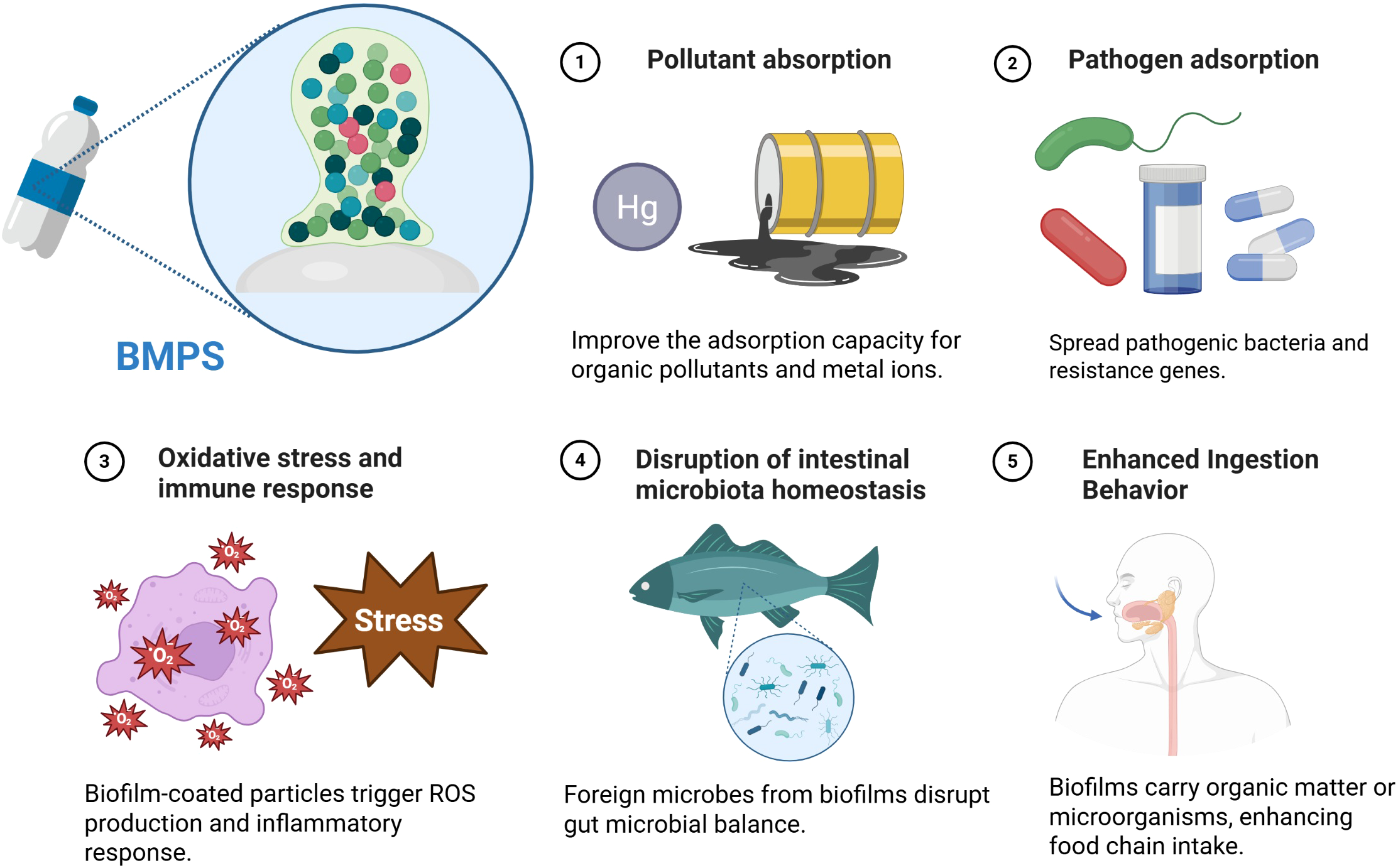
Mechanism of toxic effects of biofilm-coated microplastic.
Although numerous studies have pointed out that biofilm-coated microplastics may exacerbate ecotoxicity in the context of compound pollution, some experiments have found that the formation of biofilms may alleviate the toxic effects of microplastics, showing certain bidirectional regulation characteristics. On the one hand, the attachment of biofilms can effectively alleviate the acute toxicity and physiological interference caused by microplastics. For example, biofilm-coated microplastics exhibit lower lethality and physiological interference effects in a variety of exposure experiments than pristine microplastics. In zooplankton, biofilm-coated microplastics significantly reduce individual mortality (Nik Mut et al., 2024). In fish, biofilm-coated particles also induce less oxidative damage and metabolic disorders than clean particles (Kelly et al., 2024). After exposure in to natural water bodies, the biofilm and humus layer formed by microplastics colonize weaken their ability to enrich hydrophobic pollutants, thereby attenuating their toxic effects on fish (Hanslik et al., 2022). In addition, studies on polystyrene microplastics and nanoplastics of different sizes and surface modifications found that the acute effects of large-sized particles on the freshwater biofilm system were significantly reduced after the formation of biofilms, and the level of oxidative stress induced was significantly lower than that of exposed particles, further indicating that biofilms have a certain buffering and protective effect (Miao et al., 2019a). On the other hand, biofilms may provide nutritional compensation or act as a physical barrier. In some cases, microorganisms and their metabolites in biofilms can provide secondary sources of nutrients, slightly improving the survival rate of feeding organisms (Motiei et al., 2021). In addition, with the accumulation of biofilms, the roughness and particle volume of microplastic surfaces increase, which may exceed the feeding threshold of some benthic larvae or small zooplankton, reducing their ingestion probability (Nik Mut et al., 2024). Therefore, the formation of biofilm is not simply enhanced or weakened by toxicity, but its ecological effect is obviously context-dependent, which is specifically regulated by multiple factors such as the state of the biofilm (maturity, composition), the nature of pollutants, the physiological and ecological characteristics of exposed organisms (such as species, feeding mechanism) and environmental conditions, which may eventually change the size and mechanism of toxicity. When assessing the ecological risks of microplastics, the serious consequences caused by the above multiple factors should be fully considered.
5 Conclusion and perspectives
This paper provides a systematic review of the formation process, assembly mechanisms, and key influencing factors of biofilms on microplastics in different media and further explores how biofilm encapsulation alters their environmental behavior and ecotoxicity. The results show that biofilm attachment not only significantly changes the physical and chemical properties and environmental orientation of microplastics, but also profoundly affects the ecotoxicity risk by affecting the adsorption and release of pollutants, carrying pathogens and resistance genes, and interfering with host physiology. It is worth noting that the regulation of microplastic toxicity by biofilms is not always linearly enhanced, and its specific effects depend on the composition and structure of biofilms, environmental conditions and exposure scenarios, and may show diverse trends such as amplification, mitigation or neutrality.
At present, the research on biofilm-coated microplastics is still mainly based on the aquatic environment, while investigations into biofilm formation and behavior in other environmental media, such as soil and the atmosphere, remain in their infancy. Moreover, although existing studies have revealed the significant effects of biofilm attachment on the toxic behavior of microplastics, several key challenges remain: Firstly, most experiments are still based on idealized conditions, and the complex process of multi-factor coupling in the real environment has not been fully simulated; Secondly, the research focuses on the adsorption and carriage of pollutants by microplastics, and lacks a systematic assessment of the multi-level toxicological reactions induced by biofilm-coated microplastics in ecosystems. Thirdly, ecotoxicity research is mainly based on acute exposure, and the understanding of long-term exposure, bioaccumulation and genetic risks is still weak.
Future research should further focus on the ecological behavior of biofilm-coated microplastics in the context of compound pollution and systematically reveal their environmental trends and ecotoxicological pathways from multiple levels such as mechanism of action, dynamic processes to biological effects. At the same time, research should broaden its environmental scope, enabling in-depth comparisons of biofilm formation characteristics and ecological effects in water, soil, and atmospheric systems, thereby establishing a cross-media risk assessment framework for microplastics. In addition, the spatio-temporal scale of ecotoxicology research should be expanded to promote the transformation from short-term experiments to long-term, cross-generational, and multitrophic ecotoxicity assessments.
Statements
Author contributions
HC: Methodology, Writing – original draft, Writing – review & editing. XW: Writing – original draft, Writing – review & editing. HY: Writing – review & editing. LS: Supervision, Writing – review & editing. XY: Supervision, Writing – review & editing. WZ: Supervision, Writing – review & editing. LK: Supervision, Writing – review & editing. LL: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors declare financial support was provided by: National Natural Science Foundation of China (Grant Nos. 42207326). Funder role: Funding acquisition, Research funding. Shandong Provincial Natural Science Foundation, China (Grant Nos. ZR2022QB186, ZR2025MS567, ZR2024QD195). Funder role: Research funding. China Postdoctoral Science Foundation (Grant No. 2025M770866). Funder role: Postdoctoral Fellowship funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adeleke A. A. (2023). A review of plastic contamination challenges and mitigation efforts in cotton and textile milling industries. AgriEngineering5, 193–217. doi: 10.3390/agriengineering5010014
2
Amaral-Zettler L. A. Ballerini T. Zettler E. R. Asbun A. A. Adame A. Casotti R. et al . (2021). Diversity and predicted inter- and intra-domain interactions in the Mediterranean Plastisphere. Environ. pollut.286, 117439. doi: 10.1016/j.envpol.2021.117439
3
Amaral-Zettler L. A. Zettler E. R. Mincer T. J. (2020). Ecology of the plastisphere. Nat. Rev. Microbiol.18, 139–151. doi: 10.1038/s41579-019-0308-0
4
Amato-Lourenco L. F. Oliveira R. C. Junior G. R. Galvao L. D. S. Ando R. A. Mauad T. (2021). Microplastics inhalation: evidence in human lung tissue. Eur. Respir. J.58, PA1792. doi: 10.1183/13993003.congress-2021.PA1792
5
An Q. Y. Zhou T. Wen C. Yan C. Z . (2023). The effects of microplastics on heavy metals bioavailability in soils: a meta-analysis. J. Of Hazardous Materials460, 132369. doi: 10.1016/j.jhazmat.2023.132369
6
Arias-Andres M. Kettner M. T. Miki T. Grossart H.-P . (2018a). Microplastics: New substrates for heterotrophic activity contribute to altering organic matter cycles in aquatic ecosystems. Sci. Total Environ.635, 1152–1159. doi: 10.1016/j.scitotenv.2018.04.199
7
Arias-Andres M. Klümper U. Rojas-Jimenez K. Grossart H.-P . (2018b). Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. pollut.237, 253–261. doi: 10.1016/j.envpol.2018.02.058
8
Bairoliya S. Koh J. Cho Z. T. Cao B . (2024). Phototrophs as the central components of the plastisphere microbiome in coastal environments. Environ. Int.190, 108901. doi: 10.1016/j.envint.2024.108901
9
Behera S. Das S. (2023). Environmental impacts of microplastic and role of plastisphere microbes in the biodegradation and upcycling of microplastic. Chemosphere334, 138928. doi: 10.1016/j.chemosphere.2023.138928
10
Besemer K. Peter H. Logue J. B. Langenheder S. Lindström E. S. Tranvik L. J. et al . (2012). Unraveling assembly of stream biofilm communities. ISME J.6, 1459–1468. doi: 10.1038/ismej.2011.205
11
Bhagwat G. O’connor W. Grainge I. Palanisami T . (2021). Understanding the fundamental basis for biofilm formation on plastic surfaces: role of conditioning films. Front. Microbiol.12, 687118. doi: 10.3389/fmicb.2021.687118
12
Bonnineau C. Artigas J. Chaumet B. Dabrin A. Faburé J. Ferrari B. J. D. et al . (2021). Role of biofilms in contaminant bioaccumulation and trophic transfer in aquatic ecosystems: current state of knowledge and future challenges. Rev. Environ. Contamination Toxicol.253, 115–153. doi: 10.1007/398_2019_39
13
Chen W. Ren K. Isabwe A. Chen H. Liu M. Yang J. J. M. (2019). Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome7, 1–16. doi: 10.1186/s40168-019-0749-8
14
Chen Y. Wang X. Wang X. Cheng T. Fu K. Qin Z. et al . (2022). Biofilm structural and functional features on microplastic surfaces in greenhouse agricultural soil. Sustainability14, 7024. doi: 10.3390/su14127024
15
Chen B. F. Zhang Z. Y. Wang T. Z. Hu H. Qin G. Y. Lu T. et al . (2023). Global distribution of marine microplastics and potential for biodegradation. J. Of Hazardous Materials451, 131198. doi: 10.1016/j.jhazmat.2023.131198
16
Chen Q. Zhang X. Xie Q. Lee Y. H. Lee J. S. Shi H. (2021). Microplastics habituated with biofilm change decabrominated diphenyl ether degradation products and thyroid endocrine toxicity. Ecotoxicology Environ. Saf.228, 112991. doi: 10.1016/j.ecoenv.2021.112991
17
Cheng J. Meistertzheim A.-L. Leistenschneider D. Philip L. Jacquin J. Escande M.-L. et al . (2023). Impacts of microplastics and the associated plastisphere on physiological, biochemical, genetic expression and gut microbiota of the filter-feeder amphioxus. Environ. Int.172, 107750. doi: 10.1016/j.envint.2023.107750
18
Chu W.-C. Gao Y.-Y. Wu Y.-X. Liu F. F . (2024). Biofilm of petroleum-based and bio-based microplastics in seawater in response to Zn (II): Biofilm formation, community structure, and microbial function. Sci. Total Environ.928, 172397. doi: 10.1016/j.scitotenv.2024.172397
19
Datta M. S. Sliwerska E. Gore J. Polz M. F. Cordero O. X. J. N. C . (2016). Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat. Commun.7, 11965. doi: 10.1038/ncomms11965
20
Davidov K. Marsay K. S. Itzahri S. Rubin-Blum M. Sobral P. Kranzler C. F. et al . (2024). Community composition and seasonal dynamics of microplastic biota in the Eastern Mediterranean Sea. Sci. Rep.14, 26131. doi: 10.1038/s41598-024-73281-3
21
Deng W. Wang Y. Wang Z. Liu J. Wang J. Liu W. (2024). Effects of photoaging on structure and characteristics of biofilms on microplastic in soil: Biomass and microbial community. J. Hazardous Materials467, 133726. doi: 10.1016/j.jhazmat.2024.133726
22
De Tender C. A. Devriese L. I. Haegeman A. Maes S. Ruttink T. Dawyndt P. (2015). Bacterial community profiling of plastic litter in the belgian part of the north sea. Environ. Sci. Technol.49, 9629–9638. doi: 10.1021/acs.est.5b01093
23
De Tender C. Devriese L. I. Haegeman A. Maes S. Vangeyte J. Cattrijsse A. et al . (2017). Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ. Sci. Technol.51, 7350–7360. doi: 10.1021/acs.est.7b00697
24
Ding J. Liang Z. Lv M. Li X. Lu S. Ren S. et al . (2024). Aging in soil increases the disturbance of microplastics to the gut microbiota of soil fauna. J. Hazardous Materials461, 132611. doi: 10.1016/j.jhazmat.2023.132611
25
Di Pippo F. Crognale S. Levantesi C. Vitanza L. Sighicelli M. Pietrelli L. et al . (2022). Plastisphere in lake waters: Microbial diversity, biofilm structure, and potential implications for freshwater ecosystems. Environ. pollut.310, 119876. doi: 10.1016/j.envpol.2022.119876
26
Domozych D. S. Domozych C. R. (2008). Desmids and biofilms of freshwater wetlands: development and microarchitecture. Microbial Ecol.55, 81–93. doi: 10.1007/s00248-007-9253-y
27
Du Y. Liu X. Dong X. Yin Z . (2022). A review on marine plastisphere: biodiversity, formation, and role in degradation. Comput. Struct. Biotechnol. J.20, 975–988. doi: 10.1016/j.csbj.2022.02.008
28
Dussud C. Meistertzheim A. L. Conan P. Pujo-Pay M. George M. Fabre P. et al . (2018). Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ. pollut.236, 807–816. doi: 10.1016/j.envpol.2017.12.027
29
Eze C. G. Nwankwo C. E. Dey S. Sundaramurthy S. Okeke E. S . (2024). Food chain microplastics contamination and impact on human health: a review. Environ. Chem. Lett.22, 1889–1927. doi: 10.1007/s10311-024-01734-2
30
Fabra M. Williams L. Watts J. E. M. Hale M. S. Couceiro F. Preston J. (2021). The plastic Trojan horse: Biofilms increase microplastic uptake in marine filter feeders impacting microbial transfer and organism health. Sci. Total Environ.797, 149217. doi: 10.1016/j.scitotenv.2021.149217
31
Fazey F. M. Ryan P G J E P. (2016). Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. pollut.210, 354–360. doi: 10.1016/j.envpol.2016.01.026
32
Forero-López A. D. Brugnoni L. I. Abasto B. Rimondino G. N. Lassalle V. L. Ardusso M. G. et al . (2022). Plastisphere on microplastics: In situ assays in an estuarine environment. J. Hazardous Materials440, 129737. doi: 10.1016/j.jhazmat.2022.129737
33
Geyer R. Jambeck J. R. Law K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv.3, e1700782. doi: 10.1126/sciadv.1700782
34
Girard E. B. Kaliwoda M. Schmahl W. W. Wörheide G. Orsi W. D . (2020). Biodegradation of textile waste by marine bacterial communities enhanced by light. Environ. Microbiol. Rep.12, 406–418. doi: 10.1111/1758-2229.12856
35
Golubeva E. Gradova M. (2024). Integrating biofilm growth and degradation into a model of microplastic transport in the arctic ocean. Appl. Sci.14, 10229. doi: 10.3390/app142210229
36
González-Pleiter M. Pedrouzo-Rodríguez A. Verdú I. Leganés F. Marco E. Rosal R. et al . (2021a). Microplastics as vectors of the antibiotics azithromycin and clarithromycin: Effects towards freshwater microalgae. Chemosphere268, 128824. doi: 10.1016/j.chemosphere.2020.128824
37
González-Pleiter M. Velázquez D. Casero M. C. Tytgat B. Verleyen E. Leganés F. et al . (2021b). Microbial colonizers of microplastics in an Arctic freshwater lake. Sci. Total Environ.795, 148640. doi: 10.1016/j.scitotenv.2021.148640
38
Guan J. Qi K. Wang J. Wang W. Wang Z. Lu N. et al . (2020). Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Res.184, 116205. doi: 10.1016/j.watres.2020.116205
39
Gulizia A. M. Bell S. C. Kuek F. Santana M. M. F. Edmunds R. C. Yeoh Y. K. et al . (2025). Biofilm development as a factor driving the degradation of plasticised marine microplastics. J. Hazardous Materials487, 136975. doi: 10.1016/j.jhazmat.2024.136975
40
Hamann L. Werner J. Haase F. J. Thiel M. Scherwaß A. Laforsch C. et al . (2024). Retention of microplastics by biofilms and their ingestion by protists in rivers. Environ. Microbiol. Rep.16, e70016. doi: 10.1111/1758-2229.70016
41
Hanslik L. Huppertsberg S. Kämmer N. Knepper T. P. Braunbeck T . (2022). Rethinking the relevance of microplastics as vector for anthropogenic contaminants: Adsorption of toxicants to microplastics during exposure in a highly polluted stream - Analytical quantification and assessment of toxic effects in zebrafish (Danio rerio). Sci. Total Environ.816, 151640. doi: 10.1016/j.scitotenv.2021.151640
42
Harrison J. P. Schratzberger M. Sapp M. Osborn A. M. J. B. M . (2014). Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol.14, 1–15. doi: 10.1186/s12866-014-0232-4
43
Hasegawa T. Nakaoka M. (2021). Trophic transfer of microplastics from mysids to fish greatly exceeds direct ingestion from the water column. Environ. pollut.273, 116468. doi: 10.1016/j.envpol.2021.116468
44
Hodgson D. J. Bréchon A. L. Thompson R. C. (2018). Ingestion and fragmentation of plastic carrier bags by the amphipod Orchestia gammarellus: Effects of plastic type and fouling load. Mar. pollut. Bull.127, 154–159. doi: 10.1016/j.marpolbul.2017.11.057
45
Huang S. Wang H. Tang Y. Wang Z. Li G. Li D. (2025). New insights into the assembly processes of biofilm microbiota communities: Taking the world's largest water diversion canal as a case study. Sci. Total Environ.968, 178827. doi: 10.1016/j.scitotenv.2025.178827
46
Huang J.-N. Wen B. Zhu J.-G. Zhang Y. S. Gao J.-Z. Chen Z. Z. (2020). Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ.733, 138929. doi: 10.1016/j.scitotenv.2020.138929
47
Jalón-Rojas I. Romero-Ramírez A. Fauquembergue K. Rossignol L. Cachot J. Sous D. et al . (2022). Effects of biofilms and particle physical properties on the rising and settling velocities of microplastic fibers and sheets. Environ. Sci. Technol.56, 8114–8123. doi: 10.1021/acs.est.2c01302
48
Jang F. H. Wong C. Choo J. Sia E. S. A. Mujahid A. Müller M. J. E. P. (2022). Increased transfer of trace metals and Vibrio sp. from biodegradable microplastics to catfish Clarias gariepinus. Environ. pollut.298, 118850. doi: 10.1016/j.envpol.2022.118850
49
Jin Z. Chen K. Zhu Q. Hu X. Tian S. Xiang A. et al . (2025). Non-degradable microplastic promote microbial colonization: A meta-analysis comparing the effects of microplastic properties and environmental factors. Environ. Res.270, 121053. doi: 10.1016/j.envres.2025.121053
50
Jin Y. Xia J. Pan Z. Yang J. Wang W. Fu Z. (2018). Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. pollut.235, 322–329. doi: 10.1016/j.envpol.2017.12.088
51
Jin M. Yu X. Yao Z. Tao P. Li G. Yu X. et al . (2020). How biofilms affect the uptake and fate of hydrophobic organic compounds (HOCs) in microplastic: Insights from an In situ study of Xiangshan Bay, China. Water Res.184, 116118. doi: 10.1016/j.watres.2020.116118
52
Kaiser D. Kowalski N. Waniek J. J. (2017). Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett.12, 124003. doi: 10.1088/1748-9326/aa8e8b
53
Kalčíková G. (2023). Beyond ingestion: Adhesion of microplastics to aquatic organisms. Aquat. Toxicol.258, 106480. doi: 10.1016/j.aquatox.2023.106480
54
Kanhai L. D. K. Gardfeldt K. Krumpen T. Thompson R. C. O’connor I . (2020). Microplastics in sea ice and seawater beneath ice floes from the Arctic Ocean. Sci. Rep.10, 5004. doi: 10.1038/s41598-020-61948-6
55
Kelly E. R. M. Trujillo J. E. Setiawan A. Pether S. Burritt D. Allan B. J. M. (2024). Investigating the metabolic and oxidative stress induced by biofouled microplastics exposure in Seriola lalandi (yellowtail kingfish). Mar. pollut. Bull.203, 116438. doi: 10.1016/j.marpolbul.2024.116438
56
Kesy K. Oberbeckmann S. Kreikemeyer B. Labrenz M. J. F. I. M . (2019). Spatial environmental heterogeneity determines young biofilm assemblages on microplastics in Baltic Sea mesocosms. Front. Microbiol.10, 1665. doi: 10.3389/fmicb.2019.01665
57
Klein K. Heß S. Nungeß S. Schulte-Oehlmann U. Oehlmann J . (2021). Particle shape does not affect ingestion and egestion of microplastics by the freshwater shrimp Neocaridina palmata. Environ. Sci. pollut. Res. Int.28, 62246–62254. doi: 10.1007/s11356-021-15068-x
58
Lee S.-Y. An J. Kim J. Kwon J.-H . (2022). Enhanced settling of microplastics after biofilm development: A laboratory column study mimicking wastewater clarifiers. Environ. pollut.311, 119909. doi: 10.1016/j.envpol.2022.119909
59
Leslie H. A. Van Velzen M. J. Brandsma S. H. Vethaak A. D. Garcia-Vallejo J. J. Lamoree M. H. J. E. I. (2022). Discovery and quantification of plastic particle pollution in human blood. Environ. Int.163, 107199. doi: 10.1016/j.envint.2022.107199
60
Li C. Gillings M. R. Zhang C. Chen Q. Zhu D. Wang J. et al . (2024). Ecology and risks of the global plastisphere as a newly expanding microbial habitat. Innovation5, 100543. doi: 10.1016/j.xinn.2023.100543
61
Li L. Luo Y. Li R. Zhou Q. Peijnenburg W. J. Yin N. et al . (2020). Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustainability3, 929–937. doi: 10.1038/s41893-020-0567-9
62
Li M. Mi T. He H. Chen Y. Zhen Y. Yu Z. (2021b). Active bacterial and archaeal communities in coastal sediments: Biogeography pattern, assembly process and co-occurrence relationship. Sci. Total Environ.750, 142252. doi: 10.1016/j.scitotenv.2020.142252
63
Li C. Wang L. Ji S. Chang M. Wang L. Gan Y. et al . (2021a). The ecology of the plastisphere: Microbial composition, function, assembly, and network in the freshwater and seawater ecosystems. Water Res.202, 117428. doi: 10.1016/j.watres.2021.117428
64
Li W. Zhang Y. Wu N. Zhao Z. Xu W. A. Ma Y. et al . (2019). Colonization characteristics of bacterial communities on plastic debris influenced by environmental factors and polymer types in the haihe estuary of Bohai Bay, China. Environ. Sci. Technol.53, 10763–10773. doi: 10.1021/acs.est.9b03659
65
Liu S. Cao J. Yu J. Jian M. Zou L . (2024). Microplastics exacerbate the ecological risk of antibiotic resistance genes in wetland ecosystem. J. Environ. Manage.372, 123359. doi: 10.1016/j.jenvman.2024.123359
66
Liu M. J. Guo H.-Y. Gao J. Zhu K.-C. Guo L. Liu B.-S. et al . (2023a). Characteristics of microplastic pollution in golden pompano (Trachinotus ovatus) aquaculture areas and the relationship between colonized-microbiota on microplastics and intestinal microflora. Sci. Total Environ.856, 159180. doi: 10.1016/j.scitotenv.2022.159180
67
Liu R. Zhao S. Zhang B. Li G. Fu X. Yan P. et al . (2023b). Biodegradation of polystyrene (PS) by marine bacteria in mangrove ecosystem. J. Hazardous Materials442, 130056. doi: 10.1016/j.jhazmat.2022.130056
68
Liu J. Zheng L. (2025). Microplastic migration and transformation pathways and exposure health risks. Environ. pollut.368, 125700. doi: 10.1016/j.envpol.2025.125700
69
Liu G. Zhu Z. Yang Y. Sun Y. Yu F. Ma J. J. E. P. (2019). Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. pollut.246, 26–33. doi: 10.1016/j.envpol.2018.11.100
70
Lobelle D. Cunliffe M. (2011). Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull.62 (1), 197–200. doi: 10.1016/j.marpolbul.2010.10.013
71
Luan L. Jiang Y. Cheng M. Dini-Andreote F. Sui Y. Xu Q. et al . (2020). Organism body size structures the soil microbial and nematode community assembly at a continental and global scale. Nat. Commun.11, 6406. doi: 10.1038/s41467-020-20271-4
72
Mcgivney E. Cederholm L. Barth A. Hakkarainen M. Hamacher-Barth E. Ogonowski M. et al . (2020). Rapid physicochemical changes in microplastic induced by biofilm formation. Front. Bioengineering Biotechnol.8, 205. doi: 10.3389/fbioe.2020.00205
73
Meng L. Liang L. Shi Y. Yin H. Li L. Xiao J. et al . (2024). Biofilms in plastisphere from freshwater wetlands: Biofilm formation, bacterial community assembly, and biogeochemical cycles. J. Hazardous Materials476, 134930. doi: 10.1016/j.jhazmat.2024.134930
74
Miao L. Hou J. You G. Liu Z. Liu S. Li T. et al . (2019a). Acute effects of nanoplastics and microplastics on periphytic biofilms depending on particle size, concentration and surface modification. Environ. pollut.255, 113300. doi: 10.1016/j.envpol.2019.113300
75
Miao L. Wang P. Hou J. Yao Y. Liu Z. Liu S. et al . (2019b). Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci. Total Environ.650, 2395–2402. doi: 10.1016/j.scitotenv.2018.09.378
76
Miao L. Yu Y. Adyel T. M. Wang C. Liu Z. Liu S. et al . (2021). Distinct microbial metabolic activities of biofilms colonizing microplastics in three freshwater ecosystems. J. Hazardous Materials403, 123577. doi: 10.1016/j.jhazmat.2020.123577
77
Missawi O. Wouters C. Lambert J. Garigliany M. M. Kestemont P. Cornet V. (2024). Realistic microplastics harness bacterial presence and promote impairments in early zebrafish embryos: Behavioral, developmental, and transcriptomic approaches. Chemosphere350, 141107. doi: 10.1016/j.chemosphere.2023.141107
78
Montero D. Rimoldi S. Torrecillas S. Rapp J. Moroni F. Herrera A. et al . (2022). Impact of polypropylene microplastics and chemical pollutants on European sea bass (Dicentrarchus labrax) gut microbiota and health. Sci. Total Environ.805, 150402. doi: 10.1016/j.scitotenv.2021.150402
79
Motiei A. Ogonowski M. Reichelt S. Gorokhova E . (2021). Ecotoxicological assessment of suspended solids: The importance of biofilm and particle aggregation. Environ. pollut.280, 116888. doi: 10.1016/j.envpol.2021.116888
80
Nguyen D. Masasa M. Ovadia O. Guttman L . (2023). Ecological insights into the resilience of marine plastisphere throughout a storm disturbance. Sci. Total Environ.858, 159775. doi: 10.1016/j.scitotenv.2022.159775
81
Nik Mut N. N. Na J. Nam G. Jung J . (2024). The biodegradation of polylactic acid microplastic and their toxic effect after biofouling in activate sludge. Environ. pollut.362, 125038. doi: 10.1016/j.envpol.2024.125038
82
Nor N. H. M. Kooi M. Diepens N. J. Koelmans A. A . (2021). Lifetime accumulation of microplastic in children and adults. Environ. Sci. Technol.55, 5084–5096. doi: 10.1021/acs.est.0c07384
83
O'brien S. Rauert C. Ribeiro F. Okoffo E. D. Burrows S. D. O'brien J. W. et al . (2023). There's something in the air: a review of sources, prevalence and behaviour of microplastics in the atmosphere. Sci. Total Environ.874, 162193. doi: 10.1016/j.scitotenv.2023.162193
84
Oberbeckmann S. Kreikemeyer B. Labrenz M J F I M. (2018). Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol.8, 2709. doi: 10.3389/fmicb.2017.02709
85
Pan T. Guo Z. Hu S. Dong D. Li J. Yang X. et al . (2025). Additive release and prediction of biofilm-colonized microplastics in three typical freshwater ecosystems. Sci. Total Environ.965, 178671. doi: 10.1016/j.scitotenv.2025.178671
86
Pang R. Wang X. Zhang L. Lei L. Han Z. Xie B. et al . (2024). Genome-centric metagenomics insights into the plastisphere-driven natural degradation characteristics and mechanism of biodegradable plastics in aquatic environments. Environ. Sci. Technol.58, 18915–18927. doi: 10.1021/acs.est.4c04965
87
Pete A. J. Brahana P. J. Bello M. Benton M. G. Bharti B . (2023). Biofilm formation influences the wettability and settling of microplastics. Environ. Sci. Technol. Lett.10, 159–164. doi: 10.1021/acs.estlett.2c00728
88
PlasticsEurope (2019). Plastics—the facts 2019. An analysis of European plastics production, demand and waste data (Brussels: PlasticsEurope), 1–42.
89
Qi K. Lu N. Zhang S. Wang W. Wang Z. Guan J. et al . (2021). Uptake of Pb(II) onto microplastic-associated biofilms in freshwater: Adsorption and combined toxicity in comparison to natural solid substrates. J. Hazardous Materials411, 125115. doi: 10.1016/j.jhazmat.2021.125115
90
Qiang L. Cheng J. Mirzoyan S. Kerkhof L. Häggblom M . (2021). Characterization of microplastic-associated biofilm development along a freshwater-estuarine gradient. Environ. Sci. Technol.55, 16402–16412. doi: 10.1021/acs.est.1c04108
91
Quero G. M. Luna G. M. (2017). Surfing and dining on the “plastisphere”: Microbial life on plastic marine debris. Adv. Oceanography Limnology8, 199–207. doi: 10.4081/aiol.2017.7211
92
Raddadi N. Fava F. (2019). Biodegradation of oil-based plastics in the environment: Existing knowledge and needs of research and innovation. Sci. Total Environ.679, 148–158. doi: 10.1016/j.scitotenv.2019.04.419
93
Rakib M. R. J. Sarker A. Ram K. Uddin M. G. Walker T. R. Chowdhury T. et al . (2023). Microplastic toxicity in aquatic organisms and aquatic ecosystems: a review. Water Air Soil pollut.234, 52. doi: 10.1007/s11270-023-06062-9
94
Rebelein A. Int-Veen I. Kammann U. Scharsack J. P . (2021). Microplastic fibers - Underestimated threat to aquatic organisms. Sci. Total Environ.777, 146045. doi: 10.1016/j.scitotenv.2021.146045
95
Restrepo-Flórez J.-M. Bassi A. Thompson M. R. (2014). Microbial degradation and deterioration of polyethylene – A review. Int. Biodeterioration Biodegradation88, 83–90. doi: 10.1016/j.ibiod.2013.12.014
96
Richard H. Carpenter E. J. Komada T. Palmer P. T. Rochman C. M . (2019). Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ.683, 600–608. doi: 10.1016/j.scitotenv.2019.04.331
97
Rozman U. Filker S. Kalčíková G. (2023a). Monitoring of biofilm development and physico-chemical changes of floating microplastics at the air-water interface. Environ. pollut.322, 121157. doi: 10.1016/j.envpol.2023.121157
98
Rozman U. Klun B. Kuljanin A. Skalar T. Kalčíková G . (2023b). Insights into the shape-dependent effects of polyethylene microplastics on interactions with organisms, environmental aging, and adsorption properties. Sci. Rep.13, 22147. doi: 10.1038/s41598-023-49175-1
99
Santillan E. Constancias F. Wuertz S. (2020). Press disturbance alters community structure and assembly mechanisms of bacterial taxa and functional genes in mesocosm-scale bioreactors. mSystems5, e00471–e00420. doi: 10.1128/mSystems.00471-20
100
Sarremejane R. (2018). “ Community assembly mechanisms in river NETWORKS,” in Exploring the effect of connectivity and disturbances on the assembly of stream communities.
101
Shan E. Zhang X. Li J. Sun C. Teng J. Yang X. et al . (2022). Incubation habitats and aging treatments affect the formation of biofilms on polypropylene microplastics. Sci. Total Environ.831, 154769. doi: 10.1016/j.scitotenv.2022.154769
102
Smith I. L. Stanton T. Law A. (2021). Plastic habitats: Algal biofilms on photic and aphotic plastics. J. Hazardous Materials Lett.2, 100038. doi: 10.1016/j.hazl.2021.100038
103
Song H. Xiao S. Zhou X. Li Y. Tao M. Wu F. et al . (2024). Temporal dynamics of bacterial colonization on five types of microplastics in a freshwater lake. Sci. Total Environ.913, 169697. doi: 10.1016/j.scitotenv.2023.169697
104
Sorensen L. Rogers E. Altin D. Salaberria I. Booth A. M . (2020). Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ. pollut.258, 113844. doi: 10.1016/j.envpol.2019.113844
105
Stanley N. R. Lazazzera B. A. (2004). Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol.52, 917–924. doi: 10.1111/j.1365-2958.2004.04036.x
106
Sun C. Teng J. Wang D. Zhao J. Shan E. Wang Q. (2023a). The adverse impact of microplastics and their attached pathogen on hemocyte function and antioxidative response in the mussel Mytilus galloprovincialis. Chemosphere325, 138381. doi: 10.1016/j.chemosphere.2023.138381
107
Sun Y. Wang X. Xia S. Zhao J. J. C. E. J . (2021). New insights into oxytetracycline (OTC) adsorption behavior on polylactic acid microplastics undergoing microbial adhesion and degradation. Chem. Eng. J.416, 129085. doi: 10.1016/j.cej.2021.129085
108
Sun Y. Ye F. Huang Q. Du F. Song T. Yuan H. et al . (2023b). Linking ecological niches to bacterial community structure and assembly in polluted urban aquatic ecosystems. Front. Microbiol.14, 1288304. doi: 10.3389/fmicb.2023.1288304
109
Tian H. Wang L. Zhu X. Zhang M. Li L. Liu Z. et al . (2024). Biodegradation of microplastics derived from controlled release fertilizer coating: Selective microbial colonization and metabolism in plastisphere. Sci. Total Environ.920, 170978. doi: 10.1016/j.scitotenv.2024.170978
110
Tkachuk N. Zelena L. (2023). Some microbiological characteristics of the biofilm on the surface of pre-production pellets of polypropylene microplastics after short exposure in soil. Eng. Proc.56, 13. doi: 10.3390/ASEC2023-15350
111
Tu C. Chen T. Zhou Q. Liu Y. Wei J. Waniek J. J. et al . (2020). Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci. Total Environ.734, 139237. doi: 10.1016/j.scitotenv.2020.139237
112
Tu C. Tian Y. Liu Y. Zhang X. N. Luo Y. M. (2022). Occurrence of atmospheric (Micro)plastics and the characteristics of the plastic associated biofilms in the coastal zone of dalian in summer and autumn. Environ. Sci.43, 1821–1828. doi: 10.13227/j.hjkx.202108117
113
Verdú I. Amariei G. Rueda-Varela C. González-Pleiter M. Leganés F. Rosal R. et al . (2023). Biofilm formation strongly influences the vector transport of triclosan-loaded polyethylene microplastics. Sci. Total Environ.859, 160231. doi: 10.1016/j.scitotenv.2022.160231
114
Wang S. Chi Y. Zhao B. Qi R. Xia Y. Wang L. et al . (2024). Unraveling microplastic-biofilm nexus in aquaculture: diversity and functionality of microbial communities and their effect on plastic traits. ACS ES&T Water4, 5695–5707. doi: 10.1021/acsestwater.4c00697
115
Wang W. Gao H. Jin S. Li R. Na G . (2019b). The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicology Environ. Saf.173, 110–117. doi: 10.1016/j.ecoenv.2019.01.113
116
Wang K. Hu H. Yan H. Hou D. Wang Y. Dong P. et al . (2019a). Archaeal biogeography and interactions with microbial community across complex subtropical coastal waters. Mol. Ecol.28, 3101–3118. doi: 10.1111/mec.15105
117
Wang J. Peng C. Dai Y. Li Y. Jiao S. Ma X. et al . (2022). Slower antibiotics degradation and higher resistance genes enrichment in plastisphere. Water Res.222, 118920. doi: 10.1016/j.watres.2022.118920
118
Wang P.-Y. Zhao Z.-Y. Xiong X.-B. Wang N. Zhou R. Zhang Z.-M. et al . (2023). Microplastics affect soil bacterial community assembly more by their shapes rather than the concentrations. Water Res.245, 120581. doi: 10.1016/j.watres.2023.120581
119
Weinstein J. E. Crocker B. K. Gray A. D. (2016). From macroplastic to microplastic: Degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ. Toxicol. Chem.35, 1632–1640. doi: 10.1002/etc.3432
120
Wu X.-N. Feng J.-C. Chen X. Lin Y.-L. Huang Y. Zhong S. et al . (2025). Effects of different types of microplastics on cold seep microbial diversity and function. Environ. Sci. Technol.59, 1322–1333. doi: 10.1021/acs.est.4c08102
121
Wu X. Pan J. Li M. Li Y. Bartlam M. Wang Y. (2019). Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res.165, 114979. doi: 10.1016/j.watres.2019.114979
122
Wu X. Zhao X. Wang X. Chen R. Liu P. Liang W. et al . (2023). Bioaccessibility of polypropylene microfiber-associated tetracycline and ciprofloxacin in simulated human gastrointestinal fluids. Environ. Int.179, 108193. doi: 10.1016/j.envint.2023.108193
123
Xiang Q. Chen Q.-L. Yang X.-R. Li G. Zhu D . (2022). Soil mesofauna alter the balance between stochastic and deterministic processes in the plastisphere during microbial succession. Sci. Total Environ.849, 157820. doi: 10.1016/j.scitotenv.2022.157820
124
Xiao X. Hodson M. E. Sallach J. B. (2025). Biodegradable microplastics adsorb more Cd than conventional microplastic and biofilms enhance their adsorption. Chemosphere371, 144062. doi: 10.1016/j.chemosphere.2025.144062
125
Yadav D. S. Chandra R. (2020). “ Biofilm-mediated bioremediation of pollutants from the environment for sustainable development,” in New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms, Romain Sarremejane. 177–203.
126
Yagi M. Ono Y. Kawaguchi T. (2022). Microplastic pollution in aquatic environments may facilitate misfeeding by fish. Environ. pollut.315, 120457. doi: 10.1016/j.envpol.2022.120457
127
Yan P. Zhuang S. Li M. Zhang J. Wu S. Xie H. et al . (2024). Combined environmental pressure induces unique assembly patterns of micro-plastisphere biofilm microbial communities in constructed wetlands. Water Res.260, 121958. doi: 10.1016/j.watres.2024.121958
128
Yang Y. Liu W. Zhang Z. Grossart H. P. Gadd G. M . (2020). Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol.104, 6501–6511. doi: 10.1007/s00253-020-10704-x
129
Yu Z. Qiu D. Zhou T. Zeng L. Yan C . (2024). Biofilm enhances the interactive effects of microplastics and oxytetracycline on zebrafish intestine. Aquat. Toxicol.270, 106905. doi: 10.1016/j.aquatox.2024.106905
130
Zettler E. R. Mincer T. J. Amaral-Zettler L. A. (2013). Life in the “Plastisphere”: microbial communities on plastic marine debris. Environ. Sci. Technol.47, 7137–7146. doi: 10.1021/es401288x
131
Zhang B. Liu R. Xu H. Zhao S. Wang J. Shao Z. (2024a). Bacterial diversity in the biofilms on mariculture polystyrene foam at Xiamen’s coast. Front. Mar. Sci.11. doi: 10.3389/fmars.2024.1409399
132
Zhang H. Liu P. Zhang J. Dai J. Zhang X. Zhang Z. et al . (2024b). Unveiling the protective role of biofilm formation on the photoaging of microplastics. Sci. China Technol. Sci.67, 3067–3078. doi: 10.1007/s11431-024-2721-5
133
Zhang Y. Ma J. Song Y. Q. Li G. O'connor P . (2024c). Stronger deterministic processes shape the plastisphere microbiota of biodegradable microplastics compared to non-biodegradable microplastics in farmland soil. Appl. Soil Ecol.196, 105312. doi: 10.1016/j.apsoil.2024.105312
134
Zhang B. Yang X. Liu L. Chen L. Teng J. Zhu X. et al . (2021a). Spatial and seasonal variations in biofilm formation on microplastics in coastal waters. Sci. Total Environ.770, 145303. doi: 10.1016/j.scitotenv.2021.145303
135
Zhang F. Zhao Y. Wang D. Yan M. Zhang J. Zhang P. et al . (2021b). Current technologies for plastic waste treatment: A review. J. Cleaner Production282, 124523. doi: 10.1016/j.jclepro.2020.124523
136
Zhao Z. Wang Y. Wei Y. Peng G. Wei T. He J. et al . (2024c). Distinctive patterns of bacterial community succession in the riverine micro-plastisphere in view of biofilm development and ecological niches. J. Hazardous Materials480, 135974. doi: 10.1016/j.jhazmat.2024.135974
137
Zhao J. Wang H. Zheng L. Wang Q. Song Y . (2024b). Comparison of pristine and aged poly-L-lactic acid and polyethylene terephthalate as microbe carriers in surface water: Displaying apparent differences. Int. J. Biol. Macromolecules280, 136014. doi: 10.1016/j.ijbiomac.2024.136014
138
Zhao E. Xiong X. Li X. Hu H. Wu C . (2024a). Effect of biofilm forming on the migration of di(2-ethylhexyl)phthalate from PVC plastics. Environ. Sci. Technol.58, 6326–6334. doi: 10.1021/acs.est.3c09021
139
Zhou Z. Tang J. Tang K. An M. Liu Z. Wu Z. et al . (2024). Selective enrichment of bacteria and antibiotic resistance genes in microplastic biofilms and their potential hazards in coral reef ecosystems. Chemosphere352, 141309. doi: 10.1016/j.chemosphere.2024.141309
140
Zhu L. Xie C. Chen L. Dai X. Zhou Y. Pan H. et al . (2023). Transport of microplastics in the body and interaction with biological barriers, and controlling of microplastics pollution. Ecotoxicology Environ. Saf.255, 114818. doi: 10.1016/j.ecoenv.2023.114818
Summary
Keywords
microplastics, biofilm, microbial community, plastisphere, ecotoxicity
Citation
Chen H, Wang X, Yin H, Sun L, Yang X, Zhong W, Kong L and Liu L (2025) Microplastic surface biofilms: a review of structural assembly, influencing factors, and ecotoxicity. Front. Mar. Sci. 12:1701766. doi: 10.3389/fmars.2025.1701766
Received
09 September 2025
Accepted
22 September 2025
Published
31 October 2025
Volume
12 - 2025
Edited by
Mingzhe Yuan, Ningbo University, China
Reviewed by
Da Huo, Chinese Academy of Sciences (CAS), China; Changchao Li, The Hong Kong Polytechnic University, Hong Kong SAR, China
Updates
Copyright
© 2025 Chen, Wang, Yin, Sun, Yang, Zhong, Kong and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Liu, liu_ling@sdu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.