Abstract
Fish larval rearing represents a critical stage due to the extreme fragility of larvae, small size, and specific nutritional needs during rapid growth. Successful larviculture requires understanding ontogenetic changes to adapt rearing practices and optimize larval development. Thus, the objective of this study was to describe the ontogeny and allometric growth of hatchery-reared meagre (Argyrosomus regius) larvae from hatching to the juvenile stage by combining bivariate and multivariate morphometric analyses. Results showed that the early development of meagre was divided into three distinct growth stages marked by functional transitions. The first stage (≤3.21 mm standard length, SL) coincided with yolk absorption, onset of exogenous feeding, swim bladder inflation, and accelerated cranial development, particularly in the operculum and eye–operculum region, supporting respiration and feeding. The second stage (3.21–5.35 mm SL) was characterized by near-isometric cranial growth and positive allometry in trunk and caudal traits, reflecting gastrointestinal maturation and enhanced swimming capacity. The third stage (>5.35 mm SL) included substantial cranial and caudal differentiation, such as jaw ossification, fin formation, and sensory refinement, particularly visual enhancement, enabling efficient prey detection and capture. Principal component analysis identified two inflection points (3.21 and 5.35 mm SL), marking developmental transitions from cranial growth to locomotor enhancement and the onset of juvenile traits. These morpho-functional transitions highlight critical windows for aquaculture, such as ensuring optimal light conditions and swim bladder inflation during the first stage, providing highly digestible diets and adequate buoyancy during the second, and optimizing live prey presentation and water clarity during the third. This study showed that while traditional allometric approaches examine isolated characters relative to organism size, multivariate allometry offers comprehensive understanding of how multiple larval body regions scale together during size changes. In conclusion, this integrated approach highlights coordinated allometric transitions during meagre larval development, providing a framework to identify critical growth phases and refine rearing protocols for improved larval performance and survival.
1 Introduction
Sciaenidae is among the families of marine fish species with the highest aquaculture growth potential in the world (Chacón-Guzmán et al., 2025). Among its members, the meagre (Argyrosomus regius), a species characterized by its eurythermal and euryhaline nature, is the most widely cultured Sciaenid in Europe and Africa. This species demonstrates significant potential for growth in Mediterranean aquaculture due to its excellent adaptability to farming conditions, rapid growth rates, efficient feed conversion, and high market value (Tosun et al., 2025; EUMOFA, 2022; FAO, 2022; Duncan et al., 2013). Furthermore, meagre is valued for its high-quality flesh, characterized by excellent taste, firm texture, and high nutritional content, which contributes to its strong market demand (EUMOFA, 2022). Regardless of the advances in meagre intensive farming, the larviculture of this fast-growing species remains challenging (Álvarez-González et al., 2022). Larval rearing success and juvenile production are influenced by a complex interplay of genetic and dietary factors, as well as environmental conditions and zootechnical practices (Campoverde and Estévez, 2017). In this context, considerable research has been conducted on the early life stages of meagre, including the description of the skeletal development under different rearing conditions (Cardeira et al., 2012; Jiménez et al., 2007), the assessment of the digestive system development and functionality (Solovyev et al., 2016; Suzer et al., 2013), the description of the ontogeny of the visual system (Papadakis et al., 2018), and the optimization of zootechnical rearing conditions (Campoverde and Estévez, 2017; Vallés and Estévez, 2013; Roo et al., 2010). More recent studies have further expanded this knowledge by examining the molecular regulation of bone formation through dietary modulation of omega-3 fatty acids (Luján-Amoraga et al., 2024), the ontogeny of lymphoid organs and mucosal-associated lymphoid tissues (Campoverde et al., 2019a), and the gene expression of innate immune pathways during larval rearing and weaning (Campoverde et al., 2019b).
Fish larval rearing represents a critical aquaculture stage due to the extreme fragility of larvae, small size, and specific nutritional needs during rapid growth. Successful larviculture therefore requires understanding ontogenetic changes and complex allometric growth patterns, where different body regions develop at varying rates to meet functional and developmental demands. These allometric changes align closely with key life history events, such as the onset of exogenous feeding, skeletal ossification, and the development of sensory and swimming systems, each of which is crucial for early survival (Gisbert et al., 1999; Osse and van den Boogaart, 1999; Fuiman, 1983). Recent studies utilizing bivariate and multivariate analyses have provided insights into these growth and developmental patterns, revealing critical periods in larval development across several fish species with different ecological guilds, including benthic and demersal species such as Chelon auratus, Cichlasoma dimerus, and Acipenser stellatus, as well as reef-associated and coastal pelagic species like Lutjanus peru, Diplodus sargus, D. puntazzo, D. vulgaris, Oblada melanura, and Chelon labrosus (Martínez-Leiva et al., 2023; Beriotto et al., 2023; Peña et al., 2023; Eshaghzadeh et al., 2017; Nikolioudakis et al., 2010, 2014; Khemis et al., 2013). This developmental information not only helps identify critical ontogenetic stages but also enables synchronization of larval development with specific rearing practices, allowing for optimization of protocols related to feeding regimes, stocking density, photoperiod, and temperature control that enhance larval performance and survival –a period widely recognized as one of the most critical phases in fish production (Gisbert et al., 1999; Gisbert, 1999).
Despite the knowledge gathered during the last decade, detailed morphological changes across larval stages, alongside inflection points in growth curves, remain poorly understood during meagre early life history. Characterization of these growth patterns in meagre may facilitate aquaculture applications by defining standard growth trajectories and guiding the development of improved husbandry protocols to optimize larval survival and growth rates (Peña and Dumas, 2009). While bivariate approaches have historically been used to evaluate allometric growth patterns in fish larvae, in which pairwise plots of log-transformed length measurements of larvae of different morphological traits are compared, recent studies have integrated both bivariate and multivariate analyses to provide a more comprehensive understanding of larval morphometrics (Martínez-Leiva et al., 2023; Beriotto et al., 2023; Peña et al., 2023; Eshaghzadeh et al., 2017). However, as considering a set of pairwise plots of variables may become cumbersome, the use of multivariate allometry has been proposed since specimen’s size is the dominant contributor of variation in these measurements and of covariation between them; thus, the multivariate allometric approach focuses on the covariation among all measured traits, but it does not explicitly refer to size or shape (Klingenberg, 2016). This integrated perspective reinforces the importance of combining classical and multivariate methods to elucidate complex growth dynamics and ontogenetic transitions across larval development. While traditional allometric approaches examine isolated characters relative to organism size, multivariate allometry offers comprehensive understanding of how multiple larval body regions scale together during size changes. Thus, we hypothesize that multivariate allometric studies in fish larvae are crucial for understanding the complex interplay between morphological, physiological, and environmental factors during early development, since such studies can provide insights into growth patterns, developmental trajectories, and phenotypic plasticity. Based on the above-mentioned hypothesis, this study aimed to identify distinct growth phases in hatchery-reared meagre larvae by applying multivariate and bivariate analyses of morphometric changes across the head, trunk, and tail regions. This result provide a framework for refining rearing protocols (feeding, photoperiod, water quality) and improving larval survival and performance in meagre aquaculture.
2 Material and methods
2.1 Ethics approval
All procedures involving animal care, handling, and sampling were performed by trained and qualified personnel in accordance with Spanish legislation (Law 32/2007 and Royal Decree 1201/2015) and European Directive 2010/63/EU. The study was approved by the Ethical Committees of the Generalitat de Catalunya (CEEA 11264-P4) and the Institute of Agrifood Research and Technology, IRTA (CEEA 183-2020).
2.2 Origin of spawners, controlled reproduction and larval rearing conditions
The viable eggs used in the present study were obtained from meagre broodstock hormonally induced to spawn. The breeders were from a second captive-bred generation (F2) maintained at IPMA, Olhão, Portugal. The breeders were transported and maintained in IRTA La Ràpita, Spain for four years under natural conditions of photoperiod (La Ràpita 40°37′N, 0°36′E) and temperature actively controlled by the IRTAmar™ system, with an annual minimum 16°C and maximum 25°C. A single female (7.8 kg with oocyte diameter 584 ± 30 µm) was induced to spawn with two males (6.1 and 5.1 kg with fluent sperm). Both males and females were induced with a single injection of 15 µg kg-1 gonadotropin releasing hormone analogue (Sigma Aldrich, Madrid, Spain, product number L4513) applied intramuscularly at the caudal end of the base of dorsal fin on 22 April 2022 (Fernández-Palacios et al., 2014; Duncan et al., 2012). The female spawned on three consecutive days 24–26 April 2022. For the present study, the second spawn on 25 April 2022 was used. A total of 829,500 eggs were collected. All eggs were floating (440 mL) and at the blastula stage with 100% fertilisation (57 eggs were checked). Fertilised eggs (approx. 30,000 eggs) were transferred to two 500 L black cylindric-conical tanks for hatching and for larval rearing at a density of 60 eggs L-1 at 19 ± 0.9°C, gradually increasing from 18.1 to 21.1°C during rearing. The eggs were incubated for 2 days until hatching on the 27 April 2022 (18.1°C). A sub-sample of eggs was placed in a 96-well ELISA plate to determine the hatching rate. A single egg was placed in each well, which was filled with sterile seawater. Plates were incubated at 18°C in a temperature-controlled incubator and inspected daily, resulting in a hatching rate of 99.1%.
The larval rearing protocol applied to the larvae in 500 L black tanks lasted 30 days and it was based on the protocol described by Vallés and Estévez (2013) with slight modifications. Yolk-sac larvae (0–2 dph) do not feed and maintained in green water (Tetraselmis sp.) to ensure water quality. From 2 to 14 days post hatching (dph), larvae were fed enriched rotifers Brachionus sp. (Multigain, Biomar, Denmark) at a density of 10 rotifers mL-1, provided twice daily at 09:00 and 16:00 h. Rotifers were enriched according to manufacturer instructions, with enrichment lasting 12 h at 28°C. From 8 to 30 dph, enriched Artemia metanauplii (AM) were added progressively, with the dose increasing from 0.5 AM mL-1 at 8 dph to 3 AM mL-1 at 25 dph, fed three times daily at 09:00, 13:00, and 16:00 h. Artemia were enriched with Red Pepper™ (Bernaqua, Belgium) for 6 h at 25°C prior to feeding. From 20 dph, weaning with Gemma Micro (Skretting, Norway) was initiated by hand-feeding twice daily (09:00 and 16:00h), progressively increasing the amount as larvae grew and live prey were reduced. Tanks were always aerated with compressed air to keep oxygen levels and to ensure a homogeneous distribution of live prey. Surface skimmers were used from 5 dph until the end of the trial to assure proper swim bladder inflation. Temperature of 20.8 ± 1.44°C, salinity of 37.1 ± 3.85 g L-1, pH of 7.8 ± 0.03, oxygen concentration of 6.71 ± 0.68 mg L-1 were monitored daily. Photoperiod was maintained at 16 h light: 8 h darkness, with light intensity varied from 40 to 75 lux following the recommendations of Vallés and Estévez (2013). The average water exchange in tanks was approximately 5 volumes day-1. Survival at the end of the larval rearing period (30 dph) was 41.5 ± 2.5%.
2.3 Morphological development and morphometric measurements
In order to describe larval development, larvae (n = 15–20) were sampled daily (day 0 was the day of hatching) from each rearing tank until the end of the study at 30 dph. The number of larvae sampled per stage was chosen based on previous studies describing larval morphology and allometric growth patterns, which used comparable sample sizes to ensure representative developmental characterization (Gisbert et al., 2014; Eshaghzadeh et al., 2017). Qualitative observations such as larval pigmentation patterns, swim bladder inflation, yolk-sac absorption, and peristaltic movements of the digestive tract were observed in anesthetized larvae under the stereomicroscope (Nikon SMZ800, Nikon, Japan) at low magnification (4–20 x) prior to their fixation. Then, larvae were sacrificed with an overdose of the anaesthetic (Tricaine methane sulfonate, MS-222, Sigma-Aldrich, Spain) at 10 ppm until loss of opercular movement (within a few seconds). Sampled specimens were preserved in 4% buffered formaldehyde solution prepared in freshwater, with an approximate pH of 8.2. Morphometric characters were measured using a digital camera (600 dpi image) connected to a stereomicroscope (Nikon SMZ800, Nikon, Japan) and an image analyser (Analysis® 3.1, Soft Imaging Systems Gmbh, Germany). Based on morphological criteria described by Kendall et al. (1984), each specimen was classified into the following developmental stages i) yolk-sac, ii) pre-flexion, iii) flexion, iv) post-flexion and v) juvenile stages. The yolk-sac stage was characterized by the presence of the yolk reserve; the pre-flexion stage by a straight notochord and incomplete finfold; the flexion stage by upward bending of the notochord and early formation of caudal fin elements; the post-flexion stage by complete notochord flexion and the development of fin rays; and the juvenile stage by complete fin formation and squamation. Nine morphometric characters were measured (± 0.01 µm) in each image: total length (TL), standard length (SL), head height (HH), eye diameter (ED), distance between eye and operculum (HL2), head length (HL), trunk length (TrL), tail length (TaL), tail height (TaH) (Figure 1). Additionally, the volume (mm3) of the yolk sac (VYS) was estimated using the equation of an ellipsoid: VYS = (π/6) L × H2; where L and H are the length and the height of the yolk-sac mass, respectively. All measurements were taken along longitudinal and vertical axes relatives to the body midline (Gisbert, 1999). Abnormal specimens were excluded from the analysis (Gisbert et al., 2014). Meagre larval development was scaled by means of thermal units (degree days post hatch, ddph) (Gisbert et al., 2014) as well as SL for each age considered and stage of development.
Figure 1
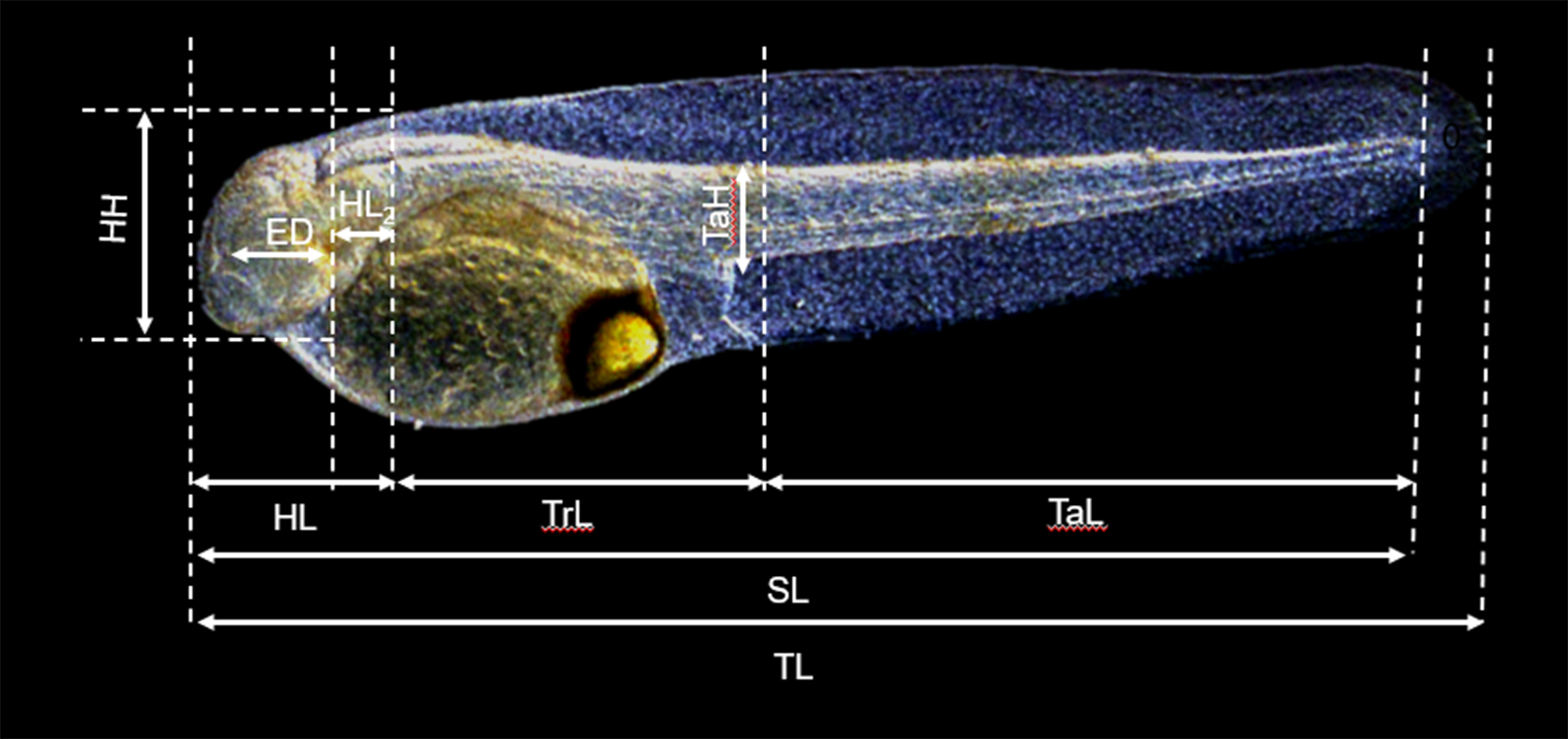
Morphometric characters measured in hatchery-reared meagre (Argyrosomus regius) larvae at 0 days post hatch. TL, Total length; SL, standard length; HH, head height; ED, eye diameter; HL2, distance between eye and operculum; HL, head length; TrL, trunk length; TaL, tail length; TaH, tail height.
2.4 Statistical analysis
The allometric growth patterns of each morphometric character relative to SL were assessed using bivariate allometry equations. These equations were fitted based on the logarithmic form of the allometric growth model proposed by Fuiman (1983), which may be defined as log (Y) = log(a) + b log (SL), where Y represents the morphometric character of interest, “a” is the intercept, and “b” is the allometric growth coefficient. The residual distribution for each model was initially analysed to identify distinct allometric growth phases. The statistical patterns, including non-random residual distributions, were used to define the boundaries of distinct growth periods across larval development. Residual patterns are typically indicate variations in the relative growth rate of the morphometric characters under examination when they are if not randomly distributed (Gozlan et al., 1999), thereby reflecting specific ontogenetic growth patterns. Inflection points, representing changes in the regression slope of these morphometric characters, were subsequently estimated by fitting piecewise linear regressions (PLR). The identified inflection points were considered as statistical breakpoints marking transitions between growth periods. The relationship between the morphometric characters and SL within each phase of multiphasic allometric growth was subsequently characterized using linear regression analysis. Allometric growth was classified as positive when the coefficient b exceeded the isometric value (b > 1), and as negative when b was below this threshold (b < 1). A Student’s t-test was employed to assess significant deviations of the regression slope (b) from isometric growth (b = 1), without applying multiple testing corrections, as comparisons were limited and exploratory, and each trait was analyzed independently.
A principal component analysis (PCA) was performed on log-transformed morphometric characters using the covariance matrix, which preserves the natural variance structure among traits. The analysis included 228 individuals, each measured once, and data were transformed but not standardized (z-scored) prior to PCA, as transformation adequately normalized scale differences across morphometric variables (Khemis et al., 2013; Nikolioudakis et al., 2010). The PCA was performed across all developmental stages jointly, rather than separately per stage, to capture the overall ontogenetic trajectory of shape change during early development. PCA results were used to statistically define distinct growth periods, with inflection points (Lm1 and Lm2) determined by piecewise linear regression (PLR) fitted to the relationship between PC2 scores and standard length (SL). These points represent statistically derived breakpoints where the slope of PC2 versus SL changes significantly, indicating transitions between morphometric growth phases. Prior to applying the PLR, regression assumptions (normality and homoscedasticity of residuals) were verified using the Nortest package in R. This length represents the point at which the growth coefficients of the measured morphometric characters become isometric relative to the SL, marking the transition of fish larvae into juveniles from a morphometric point of view (Fuiman, 1983). The Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy was employed to evaluate the suitability of the PCA results. The KMO value ranges from 0 to 1, with a value above 0.5 indicating sufficient interdependence among variables for PCA to be effective (Tabachnick and Fidell, 2000). When individuals with varying growth patterns are included in the PCA, the first principal component (PC1) captures both size and shape variations caused by growth allometry, while the second and subsequent components reflect the variations in distinct growth trajectories. As a result, different growth patterns across stages are represented as divergent PC2 trajectories when plotted against the PC1 or SL (Klingenberg, 2016).
To assess shifts in PC2 orientation (i.e., changes in slope), a piecewise linear regression (PLR) was applied using a nonlinear fitting procedure: PC2 = b0 + b1 SL + b2 (SL – Lm), for SL ≥ Lm, where b0 represents the intercept, b1 is the slope for the larval stage, b2 indicates the difference in slope between the larval and juvenile stages, and Lm is the length of morphometric metamorphosis (the end of the larval stage), corresponding to the SL at which the slope changes (Khemis et al., 2013; Nikolioudakis et al., 2010). PC1 loadings indicate the relative contribution of each variable, isometric growth occurs when loading equals 1/√p, where p is the number of variables analyzed. The bootstrap method (Efron and Tibshirani, 1986) was employed to calculate standard errors and confidence intervals for comparing component scores to the theoretical isometry value, using 1000 resamples implemented in the boot package.
All analyses were performed in R (v.3.1.2) using the vegan package (v.2.2-1) for PCA; the Boot package (v.1.3-14) to apply bootstrap methods, and the Nortest package (v.1.0-2) for normality testing. The minpack.lm package (v.1.1-8) was used to fit nonlinear models and estimate inflection points through piecewise linear regression (PLR) analysis.
3 Results
3.1 Morphological development
Under current experimental conditions, the early development of meagre was divided into five stages: the yolk-sac stage, pre-flexion, flexion, post-flexion, and early juvenile. The yolk-sac stage lasted until 63 ddph (3 dph). At hatching, larvae (2.64 ± 0.93 mm SL) had an ovoidal yolk sac with a longitudinal diameter of 0.68 ± 0.04 mm and a volume of 0.07 ± 0.02 mm³. A distinctive oil droplet was located at the distal end on the yolk sac (Figure 2A). The tail was proterocercal, and the trunk was surrounded by a broad primordial fin fold. Auditory capsules containing otoliths were visible in the posterior head region. The gut was an undifferentiated tube positioned dorsally to the posterior yolk sac. Larvae were transparent, marked by five transverse chromatophore bands along the body, one of which was at the level of the eye (Figure 2A).
Figure 2

Early life stages of hatchery-reared meagre (Argyrosomus regius) reared at 21°C. (A) Hatching, 0-degree days post hatch (ddph) (0 dph), (B) 105 ddph (5 dph), (C) 252 ddph (12 dph), (D) 336 ddph (16 dph); (E) 546 ddph (26 dph); (F) 630 ddph (30 dph). Insert: larvae at 105° ddph (5 dph), showing pectoral fins. Note: larvae were fixed in buffered formalin prior being taken in photographs, which may explain the within color of soft body regions. Scale bar = 10 mm.
The pre-flexion stage began at 63 ddph (3 dph, 2.75 ± 0.10 mm SL), coinciding with the start of the exogenous feeding and ended at ca. 273 ddph (13 dph, 4.27 ± 0.12 mm SL). At 84 ddph (4 dph, 2.86 ± 0.08 mm SL), the yolk sac was fully absorbed, leaving a small oil globule that disappeared completely by 105 ddph (5 dph, 3.16 ± 0.19 mm SL). A well-defined pectoral fin was visible during this stage (See insert in Figure 2). At 84 ddph (4 dph), the upper and the lower jaws were distinguishable, the operculum started to develop and cover the gills, and peristaltic movements were observed in the digestive tract (Figures 2B, C). Caudal fin rays were not yet visible. Retinal pigmentation started at 84–105 ddph. Swim bladder inflation was observed from 84 ddph onwards.
The flexion stage began at 294 ddph (14 dph, 4.9 ± 0.19 mm SL) and ended at 441 ddph (21 dph), when larvae reached 6.38 ± 0.93 mm SL. The body took on a more defined shape, with a noticeable upward curvature of the tip of the notochord and the formation of the caudal fin complex (Figure 2D). Larvae exhibited large, well-pigmented eyes; 22–24 myomeres had formed. The operculum opening was well defined, with the presence of the opercular spines. The mouth was large, and a row of small teeth had emerged on the upper and lower jaws, and the dorsal and caudal fins began to differentiate and by the end of this stage, rays were present in all fins. The post-flexion stage began at 462 ddph (22 dph, 8.75 ± 1.22 mm SL) and ended at 609 ddph (29 dph, 10.46 ± 2.26 mm SL). Pigmentation increased, with scattered melanophores abundant. At the end of this stage, fully formed anal and pectoral fins were observed (Figure 2E). External developmental was completed at 630 ddph (30 dph, 11.58 ± 3.09 mm SL), when pigmentation was finalized and juveniles resembled small meagre (Figure 2F).
3.2 Multivariate allometric growth
Regarding multivariate allometry, the PCA on log-transformed morphometric characters measured for A. regius, from hatching to 630 ddph (30 dph), showed that all characters were interdependent and significantly correlated among themselves (Pearson’s coefficient: r = 0.4-0.99; n = 228; P < 0.0001, for all pairwise comparisons). The lowest correlation coefficients (r = 0.4-0.5) occurred between trunk-related traits (TrL) and head measurements (HL, HL2, HH), indicating that these variables contributed more independently to total morphological variance compared with other morphometric traits. The PCA was performed using the covariance matrix, which preserves the proportional contribution of each morphometric variable to total variance. The KMO measure of sampling adequacy (0.79) indicated the usefulness of the PCA, with the first two axes explaining 90% and 9.1% of the total variation observed, respectively.
The results of the PCA also revealed significant changes in the oblique orientation of PC2 relative to PC1 (Figure 3), indicating changes in the allometric growth patterns. In the piecewise linear regression (PLR) analysis, the inflection points (Lm1 and Lm2) were estimated from the data using a nonlinear least-squares. The fit of the piecewise linear regression model between the PC2 scores and meagre SL estimated Lm1 and Lm2 at 3.21 ± 0.01 and 5.35 ± 0.16 mm SL (mean ± SE), respectively (Figure 3). The model provided an excellent fit (R² = 0.98), and residuals were normally distributed without evident bias, confirming the robustness of the estimated breakpoints. Therefore, the multivariate allometric growth pattern during the early development of meagre could be divided into three distinct phases (Figure 3): the first growth stage comprised the period between hatching and 126 ddph (6–7 dph), a second stage from that point to the age of 315 ddph (15 dph), and a final group that included individuals over the age of 315 ddph (>15 dph).
Figure 3
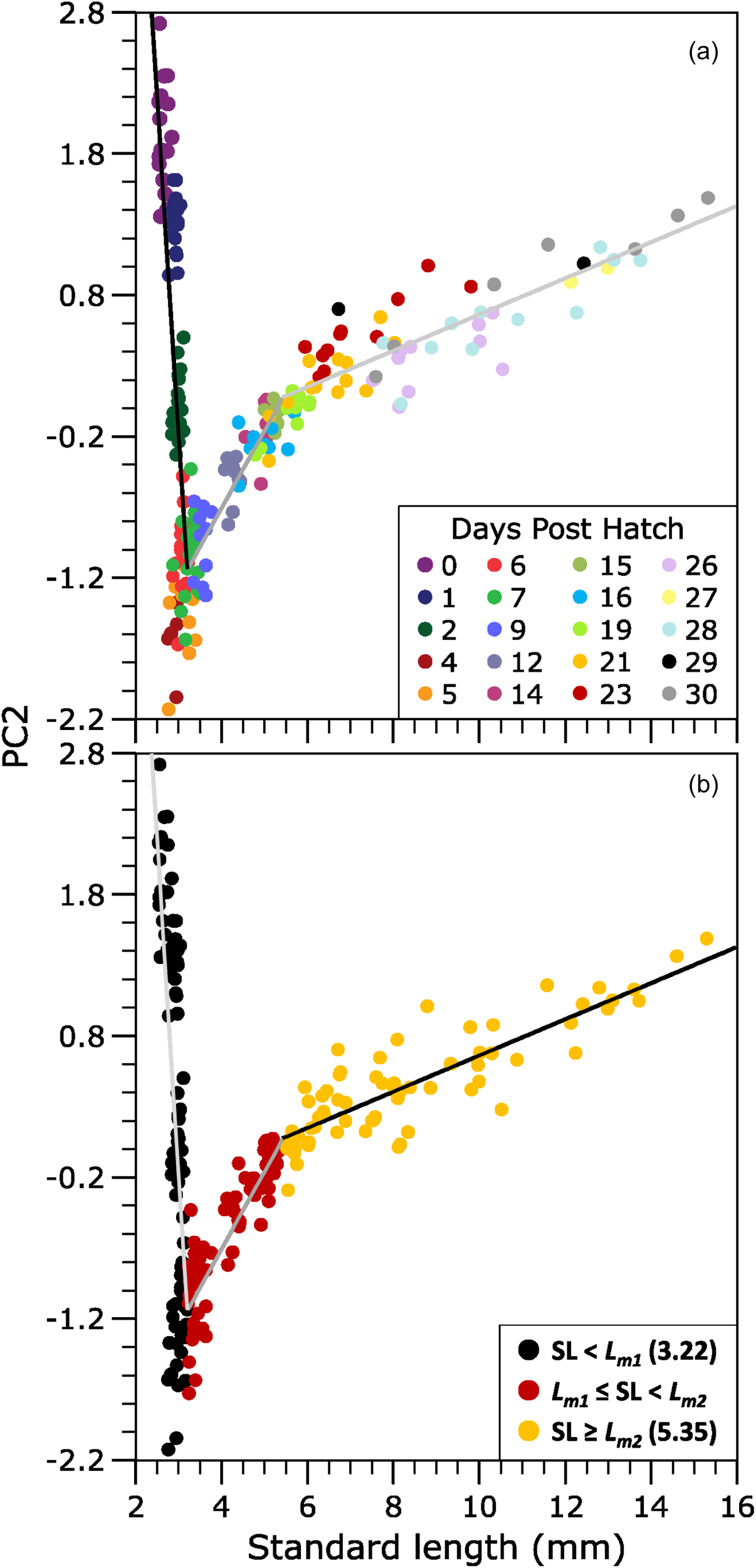
(a) The relationship between the PC2 scores and standard length (SL) of hatchery-reared meagre (Argyrosomus regius) during early life stages obtained by Principal Component Analysis (PCA) based on morphometric characters. (b) Groups of fish selected by piecewise linear regression of the PC2 scores on SL of meagre. These groups correspond to key transition points in the oblique orientation of the PC2 scores.
Factor loadings for the PC1 remained constant across each larval growth stage, as reflected by their small standard errors and lower standard deviation of the morphometric traits compared to those calculated for all individuals (Table 1), which indicated the robustness of the identified groupings along early ontogeny. The theoretical value of multivariate isometry of all morphological features was 0.35 (Student’s t test, P < 0.05). Negative allometric growth was more pronounced in Group 1 (SL < Lm1), with only HL2 exhibiting positive allometric growth in this group. In Groups 2 (Lm1 ≤ TL < Lm2) and 3 (TL ≥ Lm2), most morphometric variables displayed values close to isometric growth, except for TaL, which showed negative allometric growth across all groups, and TaH, which exhibited positive allometric growth specifically in Group 3 (Table 1, Figure 4).
Table 1
| All fish pooled | Group 1 (SL < Lm1) | Group 2 (Lm1 ≥ SL < Lm2) | Group 3 (Lm2 ≥ SL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Σ | PC1 | PC2 | σ | PC1 | PC2 | Σ | PC1 | PC2 | σ | PC1 | PC2 | |
| Score ± SE | Score ± SE | Score ± SE | Score ± SE | Score SE | Score SE | Score SE | Score SE | |||||
| HH | 0.306 | 0.456 ± 0.020 | 0.155 ± 0.061 | 0.104 | 0.099 ± 0.004 | -0.014 ± 0.005 | 0.160 | 0.158 ± 0.007 | -0.004 ± 0.002 | 0.502 | 0.085 ± 0.007 | -0.006 ± 0.003 |
| ED | 0.217 | 0.320 ± 0.014 | 0.423 ± 0.040 | 0.062 | 0.042 ± 0.005 | -0.038 ± 0.005 | 0.118 | 0.115 ± 0.006 | -0.008 ± 0.003 | -0.053 | 0.074 ± 0.008 | -0.001 ± 0.003 |
| HL2 | 0.499 | 0.713 ± 0.033 | -2.206 ± 0.120 | 0.390 | 0.099 0.004 | -0.007 ± 0.005 | 0.161 | 0.153 ± 0.010 | 0.046 ± 0.012 | 0.141 | 0.137 ± 0.011 | -0.031 ± 0.006 |
| HL | 0.327 | 0.487 ± 0.019 | -0.378 ± 0.073 | 0.157 | 0.155 ± 0.006 | -0.010 ± 0.004 | 0.136 | 0.135 ± 0.007 | 0.014 ± 0.003 | 0.543 | 0.110 ± 0.008 | -0.019 ± 0.003 |
| TrL | 0.257 | 0.239 ± 0.027 | 2.741 ± 0.123 | 0.174 | 0.165 ± 0.008 | -0.050 ± 0.008 | 0.176 | 0.169 ± 0.010 | -0.034 ± 0.012 | 0.137 | 0.127 ± 0.014 | 0.049 ± 0.010 |
| TaL | 0.159 | 0.235 ± 0.009 | 0.296 ± 0.027 | 0.044 | 0.036 ± 0.004 | -0.016 ± 0.003 | 0.083 | 0.081 ± 0.004 | 0.002 ± 0.004 | 0.068 | 0.066 ± 0.006 | -0.02 ± 0.002 |
| TaH | 0.344 | 0.495 ± 0.024 | 1.286 ± 0.065 | 0.040 | 0.002 ± 0.003 | -0.024 ± 0.005 | 0.212 | 0.209 ± 0.001 | -0.009 ± 0.007 | 0.130 | 0.125 ± 0.012 | 0.005 ± 0.009 |
| % var. | 90.054 | 9.061 | 95.26 | 2.37 | 95.61 | 2.05 | 92.70 | 3.40 | ||||
First (PC1) and second (PC2) principal factor loadings for the morphometric characters studied using the multivariate allometric approach, as well as the length (SL) at metamorphosis (Lm) when a change in slope was detected.
Principal component analyses (PCAs) conducted for different growth stages, separately, are also shown. Both Lm1 and Lm2 were estimated at 3.22 mm and 5.35 mm total length (TL), respectively. Rescaled loadings can be obtained by dividing raw scores with character standard deviation (σ). Bootstrapped standard error of the scores (SE, × 10–3) are also shown. Morphological characters are defined in the Materials and methods. % var.: Percent of variance explained.
Figure 4
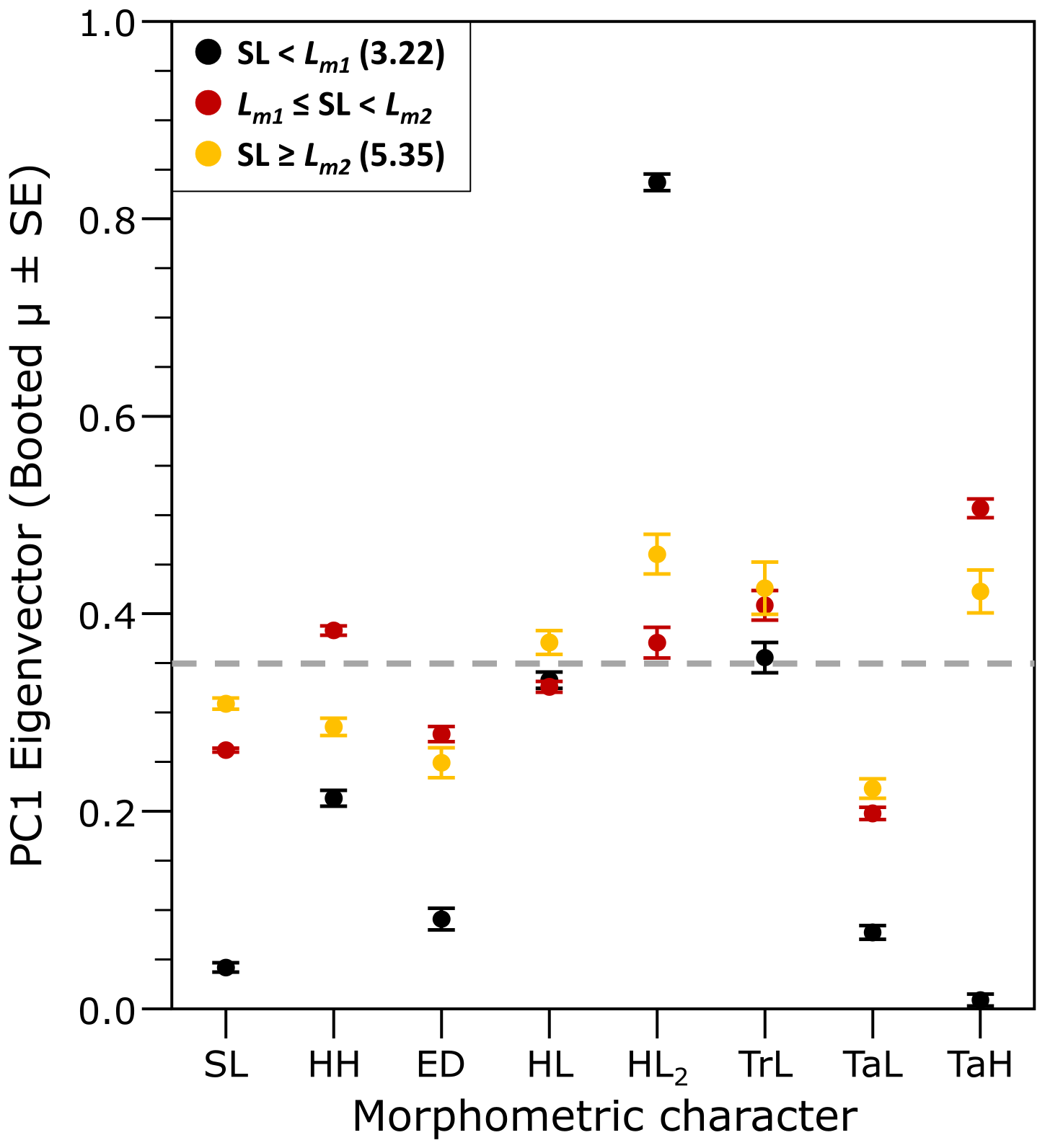
Multivariate allometry in morphometric characters of hatchery-reared meagre (Argyrosomus regius) larvae. Dashed line indicates the value of multivariate isometry (0.353). SL, Standard length; HH, head height; ED, eye diameter; HL2, distance between eye and operculum; HL, head length; TrL, trunk length; TaL, tail length; TaH, tail height.
3.3 Bivariate allometry
The bivariate allometric analysis of the head morphometric traits revealed a triphasic growth pattern for all variables with the exception of the head length (HL), which exhibited a biphasic growth profile (Figure 5, Table 2). During the first growth stage, only the head height (HH) exhibited isometric growth, while the eye diameter (ED) showed a negative allometric trajectory. In contrast, both HL and HL2 showed marked positive allometric growth (Table 2). Following this first inflection point, most traits transitioned to a negatively allometric growth phase until Lm2, as determined by piecewise linear regression analysis, whereas HL remained in a second, stable phase, exhibiting a biphasic growth pattern throughout development up to 630 ddph (30 dph). This biphasic pattern in HL, compared to the triphasic profiles observed in the other cranial traits, indicates that head length reached its main proportional adjustment earlier in development, while other structures continued to undergo additional growth transitions (Figure 5, Table 2). In contrast, HH and ED, along with HL, exhibited a strong positive allometric growth phase after Lm2 (Figure 5, Table 2).
Figure 5
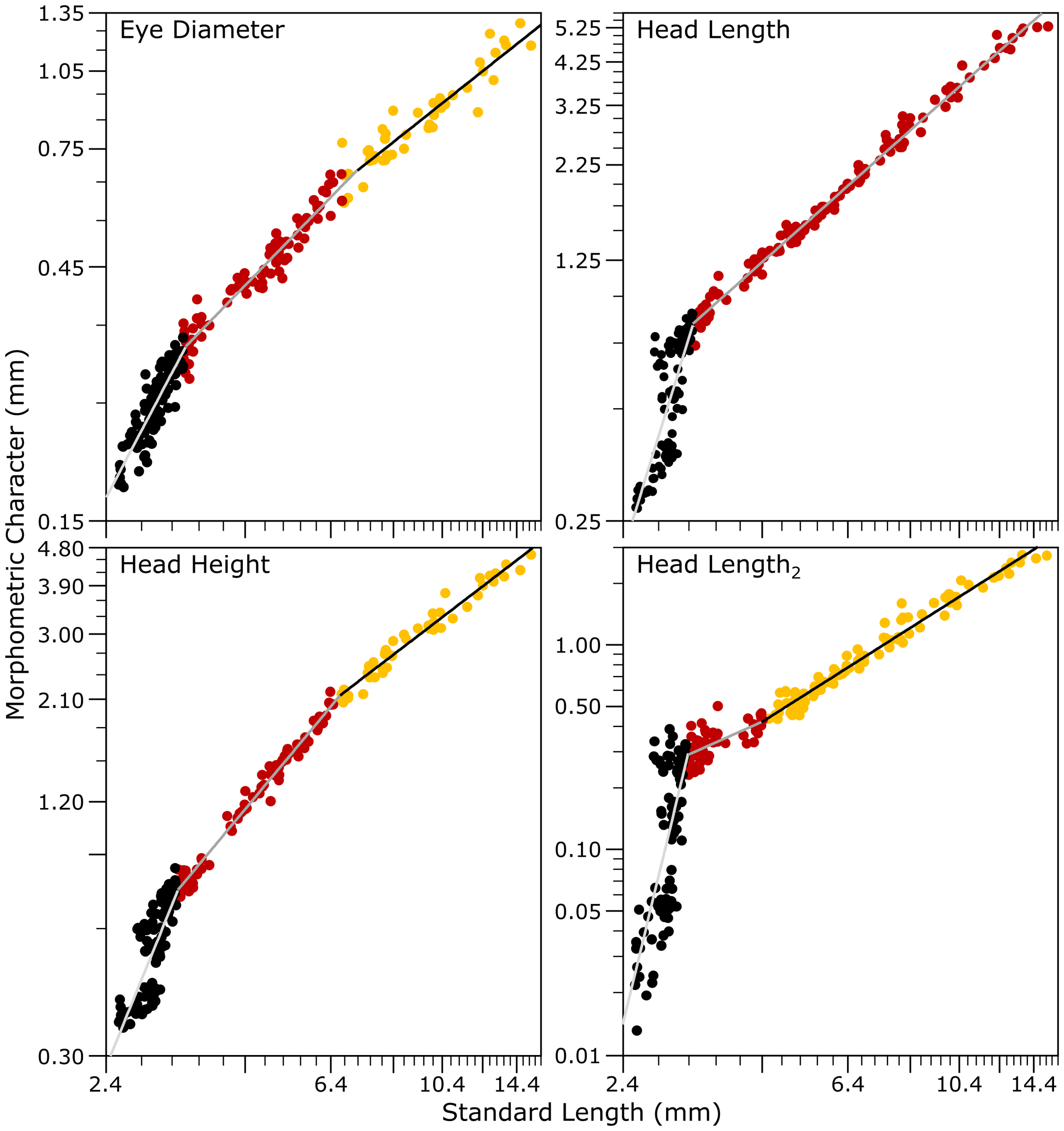
Allometric growth relationships of selected body morphological characters as functions of the standard length (SL) in hatchery-reared meagre (Argyrosomus regius) during early development between hatching and 630 ddph (30 dph).
Table 2
| First growth stage (SL < IP1) | IP1 ± SE | Second growth stage (IP1 ≤ SL < IP2) | IP2 ± SE | Third growth stage (SL < IP1,2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b ± SE | r2 | n | b ± SE | r2 | n | b ± SE | r2 | n | |||
| HH | 3.070 ± 0.239 | 0.745*** | 104 | 3.285 ± 0.014 | 1.498 ± 0.022 | 0.983*** | 84 | 6.664 ± 0.064 | 0.787 ± 0.043 | 0.886*** | 44 |
| ED | 1.881 ± 0.109 | 0.745*** | 104 | 3.364 ± 0.017 | 1.019 ± 0.031 | 0.932*** | 80 | 6.716 ± 0.099 | 0.787 ± 0.043 | 0.886*** | 44 |
| HL2 | 10.649 ± 1.192 | 0.484*** | 87 | 3.191 ± 0.009 | 1.159 ± 0.188 | 0.452*** | 48 | 4.437 ± 0.259 | 1.635 ± 0.030 | 0.970*** | 93 |
| HL | 4.585 ± 0.357 | 0.632*** | 98 | 3.261 ± 0.008 | 1.259 ± 0.010 | 0.991*** | 130 | — | — | — | — |
| TrL | -3.483 ± 0.516 | 0.319*** | 99 | 3.300 ± 0.009 | 1.592 ± 0.054 | 0.915*** | 82 | 6.394 ± 0.236 | 1.266 ± 0.084 | 0.834*** | 47 |
| TaL | 1.783 ± 0.093 | 0.866*** | 59 | 2.998 ± 0.056 | 0.793 ± 0.014 | 0.971*** | 95 | 5.116 ± 88.518 | 0.722 ± 0.014 | 0.973*** | 74 |
| TaH | 0.742 ± 0.152 | 0.215*** | 88 | 3.194 ± 0.995 | 2.529 ± 0.084 | 0.952*** | 48 | 4.599 ± 450.062 | 1.365 ± 0.024 | 0.971*** | 92 |
Regression equations summary of body morphological characters as function of standard length (SL) in hatchery-reared meagre (Argyrosomus regius) larvae during early development when reared at 21 °C.
Inflexion points (IP) were estimated by piecewise linear regression, and subsequently b, r2, and n are obtained from the fit of the logarithmic form of the allometric growth model after accounting for fish stage by linear regression. HH: head height, ED: eye diameter, HL2: distance between eye and operculum, HL: head length, TrL: trunk length, TaL: tail length, TH: tail height. Significance is indicated as follows: ***P < 0.0005.
The bivariate analysis of body morphometric traits (TrL, TaL, and TaH) revealed a triphasic allometric growth pattern (Figure 6, Table 2). In particular, TrL and TaL exhibited statistically significant and consistently strong negative allometric growth from hatching until the end of the study (Student´s t-test, P < 0.05, Table 2) confirming the robustness of the regression model (Figure 6). In contrast, TaH initially followed a similar negative allometric trajectory but showed a short, nearly isometric phase between Lm1 and Lm2, indicating a temporary stabilization in tail height growth, before shifting back to consistently negative allometric growth after Lm2.
Figure 6
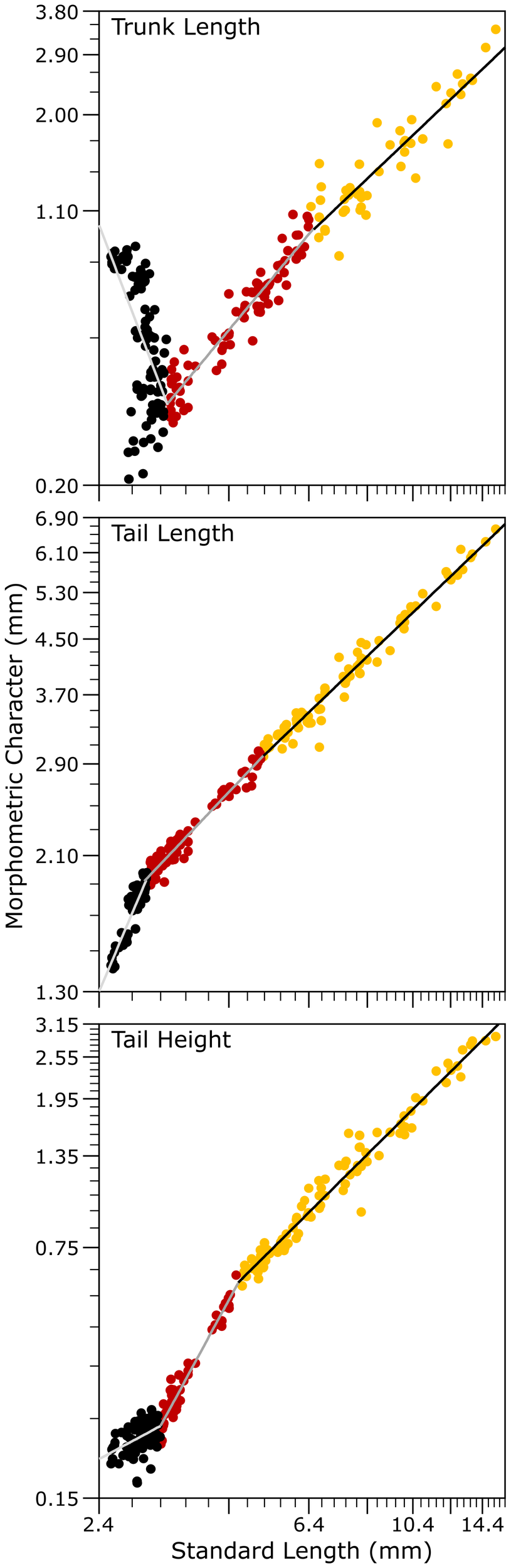
Allometric growth relationships of body morphological characters associated to the trunk and tail regions as functions of standard length (SL) in hatchery-reared meagre (Argyrosomus regius) during early development between hatching and 630 ddph (30 dph).
4 Discussion
4.1 Morphological development
Despite some variability in rearing conditions across the experimental trials—including differences in water temperature, larval origin, and feeding regimes—the morphological progression and size distribution observed throughout the developmental stages in this study align with previous descriptions of meagre larval ontogeny (Solovyev et al., 2016; Duncan et al., 2013; Papadakis et al., 2013; Jiménez et al., 2007). Four distinct ontogenetic stages were clearly delineated: yolk-sac, preflexion, flexion, and postflexion, leading up to the juvenile phase. During the yolk-sac stage, larvae exhibited hallmark features including a prominent yolk reserve, a proterocercal caudal fin, and an undifferentiated digestive tract. Yolk absorption was completed by approximately 63 ddph, corresponding to an age of 3 dph at a mean SL of 2.75 ± 0.10 mm, coinciding with the onset of exogenous feeding. These findings are consistent with several previous studies, which reported mouth opening and initial exogenous feeding occurring around 3 dph in larvae measuring ~3 mm in total or standard length (Duncan et al., 2013; Papadakis et al., 2013; Suzer et al., 2013; Jiménez et al., 2007). In contrast, Solovyev et al. (2016) reported an earlier onset of exogenous feeding in larvae aged 2 dph (3.2 ± 0.1 mm SL). Such discrepancies in developmental timing and larval size may be attributed to variations in egg size (Gisbert et al., 2000) and key rearing parameters, particularly thermal regime (20.8 ± 1.44 °C in this study vs. 18.2 ± 0.5 °C), light intensity (40–75 vs. 500 lux), salinity (37 ± 3.85 g L-1 vs. 35.4 ± 0.3 g L-1), and feeding schedules (twice vs. three times daily), all of which are known to significantly influence larval metabolic rates, digestive maturation, and overall morphological developmental (Rønnestad et al., 2013; Rao, 2003; Herbing, 2002).
During the preflexion stage comprised between 63 and 273 ddph (3–13 dph; 2.75–4.27 mm SL), larvae exhibited pronounced cranial development, with the complete formation of the splanchnocranium and fully differentiated upper and lower jaws. A well-defined operculum was also evident, reflecting the onset of rapid ossification processes typical of early teleost development (Boglione et al., 2013). During this same period, neurocranial structures showed marked advancement, as indicated by a significant increase in the horizontal eye diameter, from 0.23 ± 0.01 mm at 63 ddph to 0.41 ± 0.01 mm at 273 ddph, highlighting the accelerated growth of sensory regions during early ontogeny. The beginning of retinal pigmentation was evident from the beginning of the preflexion stage, findings that agree with those reported by Papadakis et al. (2018), who observed retinal pigmentation in larvae at 3 dph (3.11 ± 0.21 mm TL) coinciding with mouth opening and first feeding. Similarly to Jiménez et al. (2007), the onset of swim bladder inflation in this study was observed from 84 ddph (4 dph). The former authors reported the initial inflation of this buoyancy organ at the same age when larvae measured 2.78 ± 0.27 mm SL. In contrast, Papadakis et al. (2013) found that the swim bladder started to inflate from 3 dph onwards in meagre larvae measuring 3.01 ± 0.03 mm TL. The substantial morphological and physiological changes that occur during the preflexion larval stage are fundamental to underpin three keys transitions: the shift from endogenous to exogenous nutrition, the acquisition of buoyancy control mechanisms, and the development of enhanced swimming capabilities. These developmental processes are critical determinants of survival and growth in subsequent larval stages.
Flexion and post-flexion stages spanned 294 and 609 ddph (14–29 dph; 4.90–10.77 mm SL) and characterized by pronounced morphological changes essential for the transition towards the juvenile phenotype. Key developments included the upward bending of the notochord and the progressive differentiation of dorsal and caudal fins. These changes contributed to the acquisition of a more adult-like fin ray structure by the end of the post-flexion stage for supporting locomotion (Osse and van den Boogaart, 1995). In this study, the first cartilaginous elements of the caudal fin complex were observed at 315 ddph (15 dph; 5.14 ± 0.2 mm SL), marking the onset of lepidotrichia formation, while partial ossification became evident in subsequent days. with most dorsal, anal, and caudal fin structures nearly completed by 29 dph (10.77 ± 2.05 mm SL). Compared to Cardeira et al. (2012), who reported earlier skeletal development under slightly warmer temperatures (19.8°C vs. 21°C in our study), shorter photoperiods (14L:10D vs. 16L:8D), and higher light intensity (1500 vs. 500 lux), our larvae reached similar or larger sizes but required more days to complete fin differentiation. This suggests that although temperature strongly influences developmental rates, other environmental factors such as dietary conditions, light intensity and photoperiod may also modulate growth trajectories and ossification patterns.
4.2 Allometric growth patterns
The multivariate allometric analysis on meagre morphometric characters revealed a strong morphological integration, with three well-defined growth stages identified through the PCA and piecewise regression, indicating critical ontogenetic transitions. The first stage extended from hatching to 3.21 ± 0.01 mm SL (Lm1). The second stage ranged from 3.21 mm to 5.35 ± 0.16 mm SL (Lm2), and the third stage included individuals larger than the Lm2 (SL > 5.35 mm). In terms of age, the first stage occurred between hatching and 126–147 ddph (6–7 dph), whereas the second stage spanned from 126–147 ddph to 315 ddph (15 dph), and the third stage started after 315 ddph (>15 dph). Previous studies on meagre larval development have similarly reported at least two growth phases, although with considerable variation in their delineation and the methodology used. For example, Suzer et al. (2013) observed two distinct phases of body weight increment in larvae reared at similar temperatures, the first starting at 5 dph and the second at 15 dph, while Papadakis et al. (2013) described two growth phases in larvae reared at 19–23°C, with continuous growth until 17 dph, followed by accelerated growth thereafter.
Under current experimental conditions, the multivariate analysis revealed that the eye–operculum distance (HL2) was the only morphometric trait exhibiting positive allometric growth during the first growth stage (from hatching to 3.21 ± 0.01 mm SL). This pattern suggests early cranial development priority structures essential for respiration and feeding. During the yolk-sac and pre-flexion stages, we observed accelerated development of the operculum and initial swim bladder inflation in larvae measuring 2.86 ± 0.08 mm SL (84 ddph), along with yolk sac resorption in individuals of 3.16 ± 0.19 mm SL (105 ddph). These morphological changes coincided with the rapid growth of HL2, reinforcing the idea that the functional maturation of respiratory, feeding, and buoyancy structures represent a critical milestone in this ontogenetic phase of meagre.
The difference between the biphasic growth pattern of HL and the triphasic trajectories observed in the other cranial traits likely reflects distinct functional and morphological processes occurring at different ontogenetic stages. HL shows an early and rapid elongation phase that stabilizes after the first inflexion point (Lm1), resulting in only two statistically distinct phases, whereas subcomponents such as HH, ED, and HL2 undergo additional proportional adjustments after Lm2, probably associated with the maturation of sensory, opercular, and feeding structures. Overall, HL represents a global measure of cranial elongation that reaches its main proportional target early in development, while the finer cranial traits continue to remodel during later ontogenetic transitions. At this stage, larvae initiate exogenous feeding and transition from passive cutaneous to active gill respiration, as previously reported for other sciaenids. Histological studies have similarly described the appearance of the swim bladder in larvae from 3.01 ± 0.03 mm SL (Papadakis et al., 2013), and the development of cartilaginous structures in the gills in larvae of 3.3 ± 0.3 mm SL, coinciding with pharynx opening (Campoverde et al., 2019a). Furthermore, key developmental milestones, such as mouth opening, the formation of the upper and lower jaws, and the onset of exogenous feeding, occur between 66–72 and 132–144 ddph (Jiménez et al., 2007) or 57–69 and 114–138 ddph (Papadakis et al., 2018). Notably, Vallés and Estévez (2013) reported high larval mortality rates during this phase of swim bladder inflation, attributed to stressful conditions that induce excessive air intake at the water surface, leading to swim bladder hypertrophy. This period represents a critical developmental transition identified by the multivariate model, emphasizing the importance of optimizing environmental and nutritional conditions to enhance larval survival and growth. IIn natural environments, this period likely plays a key role in larval recruitment and population success (Herbing, 2002; Leggett and Deblois, 1994).
During the second growth stage, from 3.21 ± 0.01 mm to 5.35 ± 0.16 mm SL (126–315 ddph), morphometric traits associated with the head displayed near-isometric growth (b values not significantly different from 1; Student’s t-test, P > 0.05), indicating a stabilization phase following the initial prioritization of cranial development observed during the first stage. This pattern suggests that, once the cranial components involved in feeding (HL), respiration (eye-operculum distance, HL2), and buoyancy control (HH) reached functional development—corresponding to the formation of the operculum and initial swim bladder inflation—their growth progressed proportionally to the rest of the body. In contrast, trunk length (TrL) and tail height (TaH) exhibited positive allometric growth, reflecting a developmental shift toward the enhancement of locomotor performance and gastrointestinal function. These results are consistent with the observations of Solovyev et al. (2016), who reported that at 9 days post-hatching (163.8 dpph; 3.7 ± 0.2 mm SL), the intestinal tract of meagre larvae began to elongate and coil, with villi development and the presence of supranuclear vacuoles indicating active pinocytic absorption. By 13 dph (236.6 ddph; 4.1 ± 0.2 mm SL), goblet cells had appeared in the anterior gut, mucous cell density increased in the oesophagus, and lipid deposits were evident in the intestine—marking a phase of advanced digestive system maturation. Likewise, Beckley (1990) described that during the notochord flexion stage (5–6 mm BL) in Argyrosomus hololepidotus, sciaenid larvae typically exhibit a large head with well-developed jaws, a convex dorsal profile, and a moderately deep trunk tapering into a narrow caudal peduncle—morphological features also observed in A. regius during this growth stage. The positive allometry of postcranial traits suggests a functional reorganization of the larval phenotype, enhancing locomotor efficiency and foraging capacity as development progresses. This adaptation likely reflects increased swimming performance required for prey detection and capture during active feeding stages (Cardeira et al., 2012; Drost and van den Boogaart, 1986).
The third growth stage identified by multivariate analysis coincided with the ontogenetic periods of flexion and post-flexion, encompassing individuals with a standard length greater than 5.35 ± 0.16 mm (>315 ddph or >15 dph). During this stage, head length (HL) and eye–operculum distance (HL2) exhibited positive allometric growth, consistent with our morphological observations, which included mouth elongation, the appearance of teeth in both jaws, and a well-defined operculum with opercular spines. In A. hololepidotus larvae, Beckley (1990) reported that ossification of several maxillary bones—including the premaxilla, dentary, articular, and angular bones—begins at approximately 4.1 mm SL. Additionally, preopercular ossification and the development of preopercular spines were observed from 4.2 mm SL. In meagre, the differentiation of three distinct cranial units has been documented at around 4.02 mm SL (Jiménez et al., 2007). In this stage, meagre larvae begin feeding on live prey—a behavioural shift requiring the coordination of fully functional feeding structures and refinement of sensory systems, particularly vision, for efficient prey detection. Papadakis et al. (2013) reported a significant increase in rod-to-ganglion cell, cone-to-ganglion cell, and rod-to-cone ratios after the tail flexion stage (TL = 6.92 ± 0.30 mm), suggesting enhanced visual acuity under low-light conditions. This sensory enhancement is consistent with the benthic tendencies of meagre, a species known to remain near the bottom in both natural and aquaculture environments, where larvae typically wait for food to descend rather than actively swim toward the surface (Papadakis et al., 2013). Similarly, Vallés and Estévez (2013) observed that improved retinal differentiation in late larval stages enhanced prey-capture efficiency, supporting a direct link between visual maturation and feeding success. Our findings are also in line with those of Poling and Fuiman (1998), who showed that although early larval visual morphology is generally conserved among sciaenids, species-specific differences become more pronounced during the transition from the larval to the juvenile stage, which may be associated to their predatory habits burst swimming behaviour (Osse and van den Boogaart, 1995). Altogether, the positive allometry of HL and HL2 observed during this growth phase indicates an ontogenetic investment in the enhancement of sensory perception—particularly vision—which is crucial for successful prey localization and ecological performance in increasingly complex environments (Osse and van den Boogaart, 1995).
Other morphometric variables that exhibited positive allometric growth during the third growth stage included trunk length (TrL) and tail height (TaH). At this stage, meagre larvae showed substantial skeletal development, characterized by the completion of fin formation and increased muscle mass. The differentiation of the caudal fin complex and the initial ossification of dorsal and anal fin rays contributed to improved swimming capacity and manoeuvrability, crucial for effective prey capture and improved manoeuvrability from potential predators. According to Cardeira et al. (2012), the first elements of the dorsal and anal fins begin forming at approximately 5.42 mm SL, with full development completed by 12.46 mm SL. The caudal fin development starts with the ossification of hypurals 1 and 2 (at ~4.05 mm SL), followed sequentially by hypurals 3–5, the parhypural, epurals, and lepidotrichia (4.85–5.58 mm SL). By 12.46 mm SL, most elements of the caudal fin are fully formed and mineralized, except for the urostyle and epurals, which continue ossifying at later stages. After 15 dph, larvae also exhibit full body pigmentation, complete mineralization of the vertebral column, and the appearance of scales, resembling miniature adults (Duncan et al., 2013; Papadakis et al., 2013; Cardeira et al., 2012; Jiménez et al., 2007).
During the third growth phase, a pronounced positive allometric growth was observed in the cephalic and caudal regions of meagre larvae. This pattern, widely documented across fish species, is interpreted as an adaptation that enhances locomotor efficiency by reducing the energetic cost of transport (Osse and van den Boogaart, 1995, 1999). Consistent with hydrodynamic studies, growing larvae progressively transition from viscous or intermediate to more inertial swimming regimes, which lowers drag and improves swimming performance. Typically, fish exhibit early development of the anterior (head) and posterior (tail) regions compared to the abdominal area, providing a hydrodynamic advantage that facilitates rapid escape responses and minimizes body drag (Osse and van den Boogaart, 1995). Moreover, the maturation of cranial sensory structures and caudal musculature has been experimentally associated with improved prey detection and capture success in larval and juvenile sciaenids (Horodysky et al., 2008). The accelerated growth of these regions therefore contributes to enhanced foraging efficiency and overall ecological performance (Eshaghzadeh et al., 2017; Khemis et al., 2013).
5 Conclusions
Multivariate allometric analysis revealed three distinct growth stages: the first, from hatching to 3.21 ± 0.01 mm SL (Lm1), was dominated by the rapid differentiation of cranial structures essential for feeding, respiration, and buoyancy control—particularly the operculum and the eye–operculum region (HL2)—coinciding with swim bladder inflation and the start of exogenous feeding. The second stage, between 3.21 and 5.35 ± 0.16 mm SL (Lm2), showed a shift in developmental focus towards the trunk and tail (TrL, TaH), reflecting gastrointestinal maturation and improved swimming ability. Finally, the third stage (SL > 5.35 mm) encompassed substantial cranial and caudal development, including jaw ossification, fin differentiation, and sensory enhancement—particularly vision—supporting the transition to the juvenile stage. These developmental transitions, clearly delineated by the multivariate approach, identify critical ontogenetic windows that warrant further investigation. Future studies should explore how light intensity and photoperiod during the first stage influence swim bladder inflation and early buoyancy control. Likewise, research focusing on the relationship between diet digestibility, intestinal development, and buoyancy conditions during the second stage could help clarify the mechanisms enhancing digestive efficiency and larval robustness. During the third stage, when visual and locomotor systems mature, additional experiments assessing prey type, movement, and water clarity would contribute to understanding how sensory and behavioural factors affect prey capture success. Overall, our findings provide a comprehensive framework for identifying functionally relevant stages in meagre larval ontogeny, offering a basis for future experimental approaches aimed at improving survival, quality, and performance in hatchery conditions.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All procedures involving animal care, handling, and sampling were performed by trained and qualified personnel in accordance with Spanish legislation (Law 32/2007 and Royal Decree 1201/2015) and European Directive 2010/63/EU. The study was approved by the Ethical Committes of the Generalitat de Catanlunya (CEEA 11264-P4) and the Institute of Agrifood Research and Technology, IRTA (CEEA 183-2020). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AS: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JC: Data curation, Investigation, Writing – review & editing. CA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. BR: Investigation, Writing – review & editing. ND: Investigation, Writing – review & editing. EG: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was co-funded by IRTA’s own funds and the Project RTI2018-095653-R-I00 funded by the Ministerio de Ciencia, Innovación y Universidades (Spain).
Acknowledgments
The authors gratefully acknowledge the Vice-Rectorate of Research of the National University of Costa Rica for supporting the research internship of a co-author in Spain. LARVAplus network “Strategies for the development and improvement of fish larvae production in Latin America” (117RT0521), funded by the Ibero-American Science and Technology for Development Program (CYTED, Spain) for supporting B.M. Reyes-Mero and J. Chacón for academic mobility between their countries of origin and Spain.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Álvarez-González C. A. Ramírez-Martínez C. Martínez-García R. Darias M. J. Vissio P. Peña-Marín E. S. et al . (2022). Carta Acuícola Iberoamericana (Mexico: UANL-Universidad Juárez Autónoma de Tabasco (UJAT)-Red CYTED LARVAPlus-IRTA).
2
Beckley L. E. (1990). A description of the early life history stages of the kob, Argyrosomus hololepidotus (Pisces: Sciaenidae), from southern Africa. S. Afr. J. Zool.5, 224–228. doi: 10.1080/02541858.1990.11448216
3
Beriotto A. C. Vissio P. G. Gisbert E. Fernández I. Álvarez González C. A. Di Yorio M. P. et al . (2023). From zero to ossified: Larval skeletal ontogeny of the Neotropical Cichlid fish Cichlasoma dimerus. J. Morphol.284, e21641. doi: 10.1002/jmor.21641
4
Boglione C. Gavaia P. Koumoundouros G. Gisbert E. Moren M. Fontagné S. et al . (2013). Skeletal anomalies in reared European fish larvae and juveniles. Part 1: normal and anomalous skeletogenic processes. Rev. Aquac.5, S99–S120. doi: 10.1111/raq.12015
5
Campoverde C. Andree K. B. Milne D. J. Estévez A. Gisbert E. Carella F. (2019a). Ontogeny of lymphoid organs and mucosal associated lymphoid tissues in meagre (Argyrosomus regius). Fish Shellf. Immunol.84, 509–520. doi: 10.1016/j.fsi.2018.09.033
6
Campoverde C. Estévez A. (2017). The effect of live food enrichment with docosahexaenoic acid (22:6n-3) rich emulsions on growth, survival and fatty acid composition of meagre (Argyrosomus regius) larvae. Aquaculture478, 16–24. doi: 10.1016/j.aquaculture.2017.05.012
7
Campoverde C. Milne D. J. Secombes C. J. Estévez A. Gisbert E. Andree K. B. (2019b). Gene expression analysis of the innate immune system during early rearing and weaning of meagre (Argyrosomus regius). Fish Shellf. Immunol.94, 819–832. doi: 10.1016/j.fsi.2019.10.009
8
Cardeira J. Vallés R. Dionísio G. Estévez A. Gisbert E. Pousão-Ferreira P. et al . (2012). Osteology of the axial and appendicular skeletons of the meagre Argyrosomus regius (Sciaenidae) and early skeletal development at two rearing facilities. J. Appl. Ichthyol.28, 464–470. doi: 10.1111/j.1439-0426.2012.01979.x
9
Chacón-Guzmán J. Jiménez-Montealegre R. Hong W. Gisbert E. Ramos-Júdez S. Pérez-Urbiola J. C. et al . (2025). Aquaculture of the Sciaenidae family: main species cultured worldwide and emerging species in Latin America, offering new opportunities for aquaculture diversification. Rev. Fish. Sci. Aquac.33, 138–163. doi: 10.1080/23308249.2024.2383849
10
Drost M. R. van den Boogaart J. G. M. (1986). The energetics of feeding strikes in larval carp, Cyprinus carpio. J. Fish Biol.29, 371–379. doi: 10.1111/j.1095-8649.1986.tb04953.x
11
Duncan N. J. Estévez A. Fernández-Palacios H. Gairin I. Hernández-Cruz C. M. Roo J. et al . (2013). “ 17 - Aquaculture production of meagre (Argyrosomus regius): hatchery techniques, ongrowing and market,” in Advances in Aquaculture Hatchery Technology, Woodhead Publishing Series in Food Science, Technology and Nutrition. Eds. AllanG.BurnellG. (Cambridge: Woodhead Publishing), 519–541. doi: 10.1533/9780857097460.3.519
12
Duncan N. Estévez A. Porta J. Carazo I. Norambuena F. Aguilera C. et al . (2012). Reproductive development, GnRHa-induced spawning and egg quality of wild meagre (Argyrosomus regius) acclimatised to captivity. Fish Physiol. Biochem.38, 1273–1286. doi: 10.1007/s10695-012-9615-3
13
Efron B. Tibshirani R. (1986). Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci.1, 54–75. Available online at: https://www.jstor.org/stable/2245500 (Accessed Januray 15, 2025).
14
Eshaghzadeh H. Alcaraz C. Akbarzadeh A. Gisbert E. (2017). The combination of bivariate and multivariate methods to analyze character synchronization and early allometric growth patterns in the stellate sturgeon (Acipenser stellatus Pallas 1771) as tools for better understanding larval behaviour. Can. J. Fish. Aquat. Sci.74, 1528–1537. doi: 10.1139/cjfas-2016-0288
15
EUMOFA (2022). European market observatory for fisheries and aquaculture products-meagre in the UE. Available online at: https://www.eumofa.eu (Accessed October 15, 2025).
16
FAO (2022). Global aquaculture production Quantity. Available online at: https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculture_quantity (Accessed October 15, 2025). License: CC BY-NC-SA 3.0 IGO. Extracted from (1950-2023).
17
Fernández-Palacios H. Schuchardt D. Roo J. Izquierdo M. Hernandez-Cruz M. Duncan N. (2014). Dose-dependent effect of a single GnRHa injection on the spawning of meagre (Argyrosomus regius) broodstock reared in captivity. Span. J. Agric. Res.12, 1038–1048. doi: 10.5424/sjar/2014124-6276
18
Fuiman L. A. (1983). Growth gradients in fish larvae. J. Fish Biol.23, 117–123. doi: 10.1111/j.1095-8649.1983.tb02886.x
19
Gisbert E. (1999). Early development and allometric growth patterns in Siberian sturgeon and their ecological significance. J. Fish Biol.54, 852–862. doi: 10.1111/j.1095-8649.1999.tb02037.x
20
Gisbert E. Asgari R. Rafiee G. Agh N. Eagderi S. Eshaghzadeh H. et al . (2014). Early development and allometric growth patterns of beluga Huso huso (Linnaeus 1758). J. Appl. Ichthyol.30, 1264–1272. doi: 10.1111/jai.12617
21
Gisbert E. Williot P. Castelló-Orvay F. (1999). Behavioural modifications in the early life stages of Siberian sturgeon (Acipenser baerii, Brandt). J. Appl. Ichthyol.15, 237–242. doi: 10.1111/j.1439-0426.1999.tb00242.x
22
Gisbert E. Williot P. Castelló-Orvay F. (2000). Influence of egg size on growth and survival of early stages of Siberian sturgeon (Acipenser baerii) under small scale hatchery conditions. Aquaculture183, 83–94. doi: 10.1016/S0044-8486(99)00287-2
23
Gozlan R. E. Copp G. H. Tourenq J.-N. (1999). Comparison of growth plasticity in the laboratory and field, and implications for for the onset of juvenile development in Sofie, Chondrostoma toxostoma. Envir. Biol. Fish.56, 153–165. doi: 10.1023/A:1007577321999
24
Herbing I. H. von (2002). Effects of temperature on larval fish swimming performance: the importance of physics to physiology. J. Fish Biol.61, 865–876. doi: 10.1111/j.1095-8649.2002.tb01848.x
25
Horodysky A. Z. Brill R. W. Warrant E. J. Musick J. A. Latour R. J. (2008). Comparative visual function in five sciaenid fishes inhabiting Chesapeake Bay. J. Exp. Biol.211, 3601–3612. doi: 10.1242/jeb.023358
26
Jiménez M. T. Rodríguez de la Rúa A. Sánchez R. Cárdenas S. (2007). Atlas de desarrollo de la corvina Argyrosomus regius (Pisces: Sciaenidae) durante su primer mes de vida. Revista Electrónica de Veterinaria (Spain: Malaga) 8, 1–37.
27
Kendall A. W. Jr. Ahlstrom E. H. Moser H. G. (1984). “ Early life history stages of fishes and their characters. In Ontogeny and systematics of fishes,” in American Society of Ichthyologists and Herpetologists. Eds. MoserH. G.RichardsW. J.CohenD. M.FahayM. P.KendallA. W.Jr.RichardsonS. L. ( Allen Press Inc., Lawrence, USA), 11–22.
28
Khemis I. Ben Gisbert E. Alcaraz C. Zouiten D. Besbes R. et al . (2013). Allometric growth patterns and development in larvae and juveniles of thick-lipped grey mullet Chelon labrosus reared in mesocosm conditions. Aquac. Res.44, 1872–1888. doi: 10.1111/j.1365-2109.2012.03192.x
29
Klingenberg C. P. (2016). Size, shape, and form: concepts of allometry in geometric morphometrics. Dev. Genes Evol.226, 113–137. doi: 10.1007/s00427-016-0539-2
30
Leggett W. C. Deblois E. (1994). Recruitment in marine fishes: Is it regulated by starvation and predation in the egg and larval stages? Neth. J. Sea Res.32, 119–134. doi: 10.1016/0077-7579(94)90036-1
31
Luján-Amoraga L. Delgado-Martín B. Lourenço-Marques C. Gavaia P. J. Bravo J. Bandarra N. M. et al . (2024). Exploring omega-3′s impact on the expression of bone-related genes in meagre (Argyrosomus regius). Biomolecules14, 56. doi: 10.3390/biom14010056
32
Martínez-Leiva L. Landeira J. M. Fatira E. Díaz-Pérez J. Hernández-León S. Roo J. et al . (2023). Energetic implications of morphological changes between fish larval and juvenile stages using geometric morphometrics of body shape. Animals13, 370. doi: 10.3390/ani13030370
33
Nikolioudakis N. Koumoundouros G. Kiparissis S. Somarakis S. (2010). Defining length-at-metamorphosis in fishes: a multi-character approach. Mar. Biol.157, 991–1001. doi: 10.1007/s00227-009-1379-7
34
Nikolioudakis N. Koumoundouros G. Somarakis S. (2014). Synchronization in allometric and morphological changes during metamorphosis: Comparison among four sparid species. Aqua. Biol.21, 155–165. doi: 10.3354/ab00579
35
Osse J. W. M. van den Boogaart J. G. M. (1995). Fish larvae, development, allometric growth and the aquatic environment. ICES Mar. Sci. Symp.201, 21–34. doi: 10.17895/ices.pub.19271519
36
Osse J. W. M. van den Boogaart J. G. M. (1999). Dynamic morphology of fish larvae, structural implications of friction forces in swimming, feeding and ventilation. J. Fish Biol.55, 156–174. doi: 10.1111/j.1095-8649.1999.tb01053.x
37
Papadakis I. E. Kentouri M. Divanach P. Mylonas C. C. (2013). Ontogeny of the digestive system of meagre Argyrosomus regius reared in a mesocosm, and quantitative changes of lipids in the liver from hatching to juvenile. Aquaculture388–391, 76–88. doi: 10.1016/j.aquaculture.2013.01.012
38
Papadakis I. E. Kentouri M. Divanach P. Mylonas C. C. (2018). Ontogeny of the eye of meagre (Argyrosomus regius) from hatching to juvenile and implications to commercial larval rearing. Aquaculture484, 32–43. doi: 10.1016/j.aquaculture.2017.10.038
39
Peña R. Dumas S. (2009). Development and allometric growth patterns during early larval stages of the spotted sand bass Paralabrax maculatofasciatus (Percoidei: Serranidae). Sci. Mar.73, 183–189. doi: 10.3989/scimar.2009.73s1183
40
Peña R. McGregor-Bravo C. U. Contreras-Olguín M. (2023). Allometric growth and larval development in Pacific red snapper Lutjanus Peru under culture conditions. J. Fish Biol.102, 413–425. doi: 10.1111/jfb.15279
41
Poling K. R. Fuiman L. A. (1998). Sensory development and its relation to habitat change in three species of Sciaenids. Brain Behav. Evol.52, 270–284. doi: 10.1159/000006572
42
Rao T. (2003). Ecological and Ethological Perspectives in larval fish feeding. J. Appl. Aquac.13, 145–178. doi: 10.1300/J028v13n01_06
43
Rønnestad I. Yúfera M. Ueberschär B. Ribeiro L. Sæle Ø. Boglione C. (2013). Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev. Aquac.5, S59–S98. doi: 10.1111/raq.12010
44
Roo J. Hernández-Cruz C. M. Borrero C. Schuchardt D. Fernández-Palacios H. (2010). Effect of larval density and feeding sequence on meagre (Argyrosomus regius; Asso 1801) larval rearing. Aquaculture302, 82–88. doi: 10.1016/j.aquaculture.2010.02.015
45
Solovyev M. M. Campoverde C. Öztürk S. Moreira C. Diaz M. Moyano F. J. et al . (2016). Morphological and functional description of the development of the digestive system in meagre (Argyrosomus regius): An integrative approach. Aquaculture464, 381–391. doi: 10.1016/j.aquaculture.2016.07.008
46
Suzer C. Kamacı H. O. Çoban D. Yıldırım Ş. Fırat K. Saka Ş. (2013). Functional changes in digestive enzyme activities of meagre (Argyrosomus regius; Asso 1801) during early ontogeny. Fish Physiol. Biochem.39, 967–977. doi: 10.1007/s10695-012-9755-5
47
Tabachnick B. G. Fidell L. S. (2000). Computer-Assisted Research Design and Analysis. 1st ed (USA: Allyn & Bacon, Inc.).
48
Tosun D. D. Yıldız M. Doğan K. Demircan D. (2025). Economic and employment implications of fish farming in the Karaburun-Ildır special protection area, Turkish Aegean Sea. Turkish J. Fish. Aquat. Sci.25, TRJFAS27920. doi: 10.4194/TRJFAS27920
49
Vallés R. Estévez A. (2013). Light conditions for larval rearing of meagre (Argyrosomus regius). Aquaculture376–379, 15–19. doi: 10.1016/j.aquaculture.2012.11.011
Summary
Keywords
fish, larval ontogeny, aquaculture, morphometric analysis, multivariate allometry
Citation
Santana-Piñeros AM, Chacón-Guzmán J, Alcaraz C, Reyes-Mero BM, Duncan N and Gisbert E (2025) Bivariate and multivariate analyses of allometric growth in larvae of farmed meagre (Argyrosomus regius): an integrated approach to define growth stages and ontogenetic transitions. Front. Mar. Sci. 12:1707403. doi: 10.3389/fmars.2025.1707403
Received
17 September 2025
Accepted
31 October 2025
Published
19 November 2025
Volume
12 - 2025
Edited by
Ioannis A Giantsis, Aristotle University of Thessaloniki, Greece
Reviewed by
Sirwe Ghaderpour, Independent Researcher, Iran
Simona Tarricone, University of Bari Aldo Moro, Italy
Updates
Copyright
© 2025 Santana-Piñeros, Chacón-Guzmán, Alcaraz, Reyes-Mero, Duncan and Gisbert.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enric Gisbert, enric.gisbert@irta.cat
ORCID: Byron M. Reyes-Mero, orcid.org/0000-0003-3285-1937
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.