Abstract
Coastal marine ecosystems are increasingly threatened by heavy metal pollution, necessitating robust biomonitoring tools. This study investigates the efficacy of benthic foraminiferal assemblages and morphology as bioindicators for heavy metal contamination in Bohai Bay, China. Sediment samples from seven stations were analyzed for six heavy metals (Cu, Pb, Cd, Zn, As, Hg) and foraminiferal parameters. A total of 24 species and 1594 benthic foraminiferal individuals were identified. Results revealed significant negative correlations between Cu/Cd concentrations and foraminiferal diversity indices (Species richness, Margalef index), indicating metal-induced ecological stress. Ammonia species and Protelphidium tuberculatum exhibited tolerance, dominating moderately polluted areas, whereas Quinqueloculina seminula showed sensitivity. Critically, test deformity rates in the dominant species Ammonia aomoriensis and Q. seminula were significantly positively correlated with Pb and Zn levels, respectively, highlighting species-specific morphological responses to metal stress. This integrated approach, combining community dynamics with morphological biomarkers, provides a powerful and sensitive framework for assessing heavy metal pollution in coastal environments, offering valuable insights for the environmental management of Bohai Bay.
1 Introduction
The coastal zone, serving as a critical ecotone between terrestrial and marine ecosystems, is experiencing unprecedented environmental pressures due to rapid industrialization, urbanization, and intensive human activities (Tian et al., 2020; Zitoun et al., 2024). Pollutants from industrial discharge, agricultural runoff, and domestic wastewater inevitably find their way into coastal waters, making these areas among the most severely degraded environments globally. Heavy metals are the most common pollutants and are widely present in the marine environment (Huang et al., 2025). Due to their toxicity, persistence, and bioaccumulation potential, heavy metals represent a particularly significant threat to marine biodiversity and ecosystem health (McKenzie et al., 2024; Zitoun et al., 2024). Effective monitoring and assessment of coastal environmental quality are therefore paramount for sustainable management and remediation efforts.
Traditional methods for monitoring heavy metals pollution primarily rely on direct physicochemical analysis of water and sediment samples. While these methods provide accurate measurements of contaminant concentrations at specific points in time, they are often costly, time-consuming, and fail to reflect the integrated biological effects of heavy metals on living organisms over extended periods (Abdelmonem et al., 2025; Madjar and Vasile Scăețeanu, 2025). Consequently, there is a growing recognition of the importance of biomonitoring—the use of biological responses to assess environmental health (Ajala et al., 2025). Biomonitors can provide a holistic view of ecosystem status by revealing the cumulative and synergistic impacts of heavy metals, offering insights that chemical data alone cannot.
Among potential bioindicators, benthic foraminifera stand out as exceptionally valuable tools for coastal environmental assessment (Abd Malek and Frontalini, 2024; Joshi et al., 2024). These single-celled eukaryotes are ubiquitous in marine sediments, highly diverse, and sensitive to environmental changes (Murray, 2006; Ganugapenta et al., 2025). Their calcareous tests (shells) are readily preserved in sediment records, providing a valuable archive for reconstructing past environmental conditions (Bergamin and Romano, 2016). Specific characteristics, such as changes in species composition, assemblage diversity, and the abundance of pollution-tolerant indicator species, have been successfully correlated with various stressors, including organic enrichment, hypoxia, and trace metal pollution (O’Brien et al., 2021; Titelboim et al., 2021; Ciacci et al., 2022; Schmidt et al., 2022; Bouchet et al., 2023; Naik et al., 2023). Furthermore, sublethal responses, such as test morphological abnormalities (e.g., deformed chambers, twisted sutures, reduced size), are increasingly recognized as a direct and visible indicator of physiological stress induced by contaminants like heavy metals (El-Kahawy et al., 2018; Boehnert et al., 2020).
The application of benthic foraminifera as bioindicators has gained substantial momentum over recent decades. Studies from the Mediterranean to the North Sea, have established robust protocols and defined specific foraminiferal indices for pollution assessment (Munsel et al., 2010; Boehnert et al., 2020; Hoober et al., 2022). However, the application of this powerful tool remains geographically uneven. The global distribution of benthic foraminifera exhibits regional specificity, necessitating further research in more geographic areas.
This study focuses on the Bohai Bay, China, a semi-enclosed shallow sea that has historically been one of the country’s most polluted marine areas due to its proximity to major industrial centers and high population density (Zhang et al., 2016; Li et al., 2019b). While significant government-led pollution control initiatives in recent years have led to notable improvements in water quality, continuous monitoring remains crucial to assess the long-term effectiveness of these measures and the ecological recovery of the bay. Despite its ecological and economic importance, there is a conspicuous lack of studies utilizing benthic foraminifera as bioindicators to assess environmental conditions in the Bohai Bay. This gap hinders a comprehensive understanding of the bay’s ecological health from a biological perspective.
Therefore, the present study aims to address this knowledge gap by employing benthic foraminifera to evaluate the environmental status (especially the heavy metals concentration) of the Bohai Bay. We propose an integrated approach, analyzing both community assemblage characteristics (including species composition, biodiversity, and the presence of indicator species) and morphological variations in foraminiferal tests. The objectives are to: (1) investigate the current distribution of heavy metals and foraminifera in Bohai Bay; (2) analyze the response of foraminiferal communities to different heavy metals concentration; and (3) explore the relationship between metal contamination and the foraminiferal morphological changes. This dual-method approach is expected to provide a more robust and nuanced assessment of environmental stress, offering new insights into the ecological recovery of the Bohai Bay and underscoring the critical role of benthic foraminifera in coastal zone management.
2 Materials and methods
2.1 Sampling
Sampling was conducted in Bohai Bay in September 2023 across seven stations (Figure 1). Sediment samples were collected using a grab sampler and surface 1 cm sediment samples were collected. In situ environmental parameters (bottom seawater)—including temperature, salinity, dissolved oxygen, chlorophyll-a concentration, and depth—were measured with a multi-parameter water quality sonde (EXO2 Multiparameter Sonde) (Table 1). The sediment samples were placed in plastic sample bottles and stored in an insulated container. They were transported to the laboratory as soon as possible after the cruise for further processing.
Figure 1
Sampling map.
Table 1
| Station | Temperature (°C) | Salinity (psu) | Dissolved oxygen (mg/L) | Chlorophyll (μg/L) | Depth (m) |
|---|---|---|---|---|---|
| B1 | 26.92 | 27.91 | 7.04 | 0.75 | 8.23 |
| B2 | 27.11 | 29.34 | 7.14 | 7.52 | 8.28 |
| B3 | 27.49 | 29.15 | 4.17 | 9.92 | 7.55 |
| B4 | 27.20 | 29.70 | 7.53 | 6.68 | 9.09 |
| B5 | 27.49 | 29.49 | 5.96 | 9.92 | 7.55 |
| B6 | 27.02 | 28.18 | 7.41 | 6.42 | 7.06 |
| B7 | 27.03 | 26.90 | 5.05 | 15.13 | 6.15 |
Environmental parameters of all sampling stations.
2.2 Sample treatment and analysis
For the measurement of heavy metals, analytical procedures were conducted in accordance with the methodologies described by Zhang et al. (2016) and Li et al. (2019b). Concentrations of Cu, Pb, Zn and Cd were determined using an atomic absorption spectrometry (PERSEE A3, China) and the detection limits were 0.5, 1, 6 and 0.04 mg/kg, respectively. The concentrations of Hg and As were measured by an atomic fluorescence spectrometry (PERSEE PF52, China) and the detection limits were 0.002 and 0.06 mg/kg.
All sampling and measurement activities adhered to the Chinese National Standards for marine monitoring (GB17378.5-2007). The analysis of benthic foraminifera was followed the Chinese National Standards of GB/T 34656–2017. Sediments containing foraminifera were sieved through 63 μm mesh screens. Foraminiferal specimens were picked and mounted on micropaleontological slides for species identification, quantification, and further analysis (Dong et al., 2020). Observations, taxonomic identification, and test size measurements were performed using a stereomicroscope. Species were identified following the taxonomic references of Alfred R. Loeblich and Tappan (1988) and Lei and Li (2016).
2.3 Statistical analysis
The sampling map was generated using Ocean Data View (ODV; https://odv.awi.de/). The foraminiferal community parameters were computed with PRIMER v6.1.13 (Plymouth Routines in Multivariate Ecological Research). These included: Abundance (N): defined as the number of individuals per 10 grams of dry sediment (ind./10g DW); Species richness (S): the number of species per sample; Margalef index (D); Shannon-Wiener diversity index (H); Pielou’s evenness index (J). Spearman correlation analysis and corresponding graphical representations were conducted using Origin software.
3 Results
3.1 Concentration of heavy metals in sediment
The spatial distribution of six heavy metals (Cu, Pb, Cd, Zn, As, Hg) from seven sampling stations exhibited different variation patterns (Figure 2). For Cu, stations B7 (20.30 mg/kg) and B1 (9.82 mg/kg) showed the highest and lowest Cu concentrations, respectively. Pb concentrations peaked at station B5 (35.01 mg/kg) and reached lowest value at B4 (13.13 mg/kg). The concentration of Zn showed relatively uniform levels across all stations except for an abnormally high value at station B1 (131.70 μg/L). The concentrations of As and Cd showed limited variation across the sampling stations, ranging from 13.34 to 20.62 mg/kg and 0.07 to 0.10 mg/kg, respectively. However, the Hg concentrations changed obviously from 0.004 to 0.12 mg/kg.
Figure 2
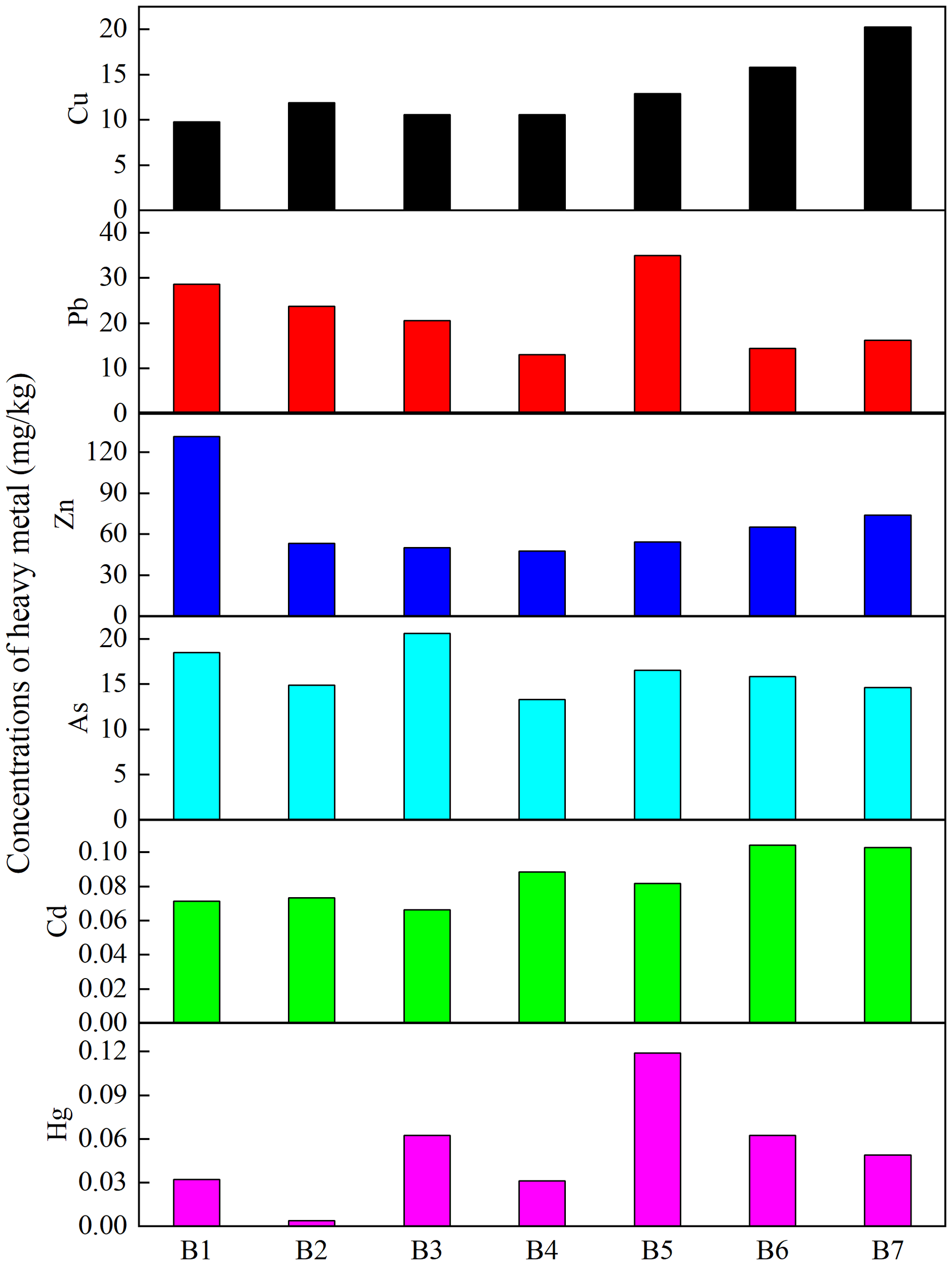
Heavy metal concentrations in sediment from all stations.
3.2 Foraminiferal taxa
A total of 1,594 foraminiferal specimens were obtained from sediment samples collected at seven stations. A total of 24 species of benthic foraminifera were identified and are illustrated in Figure 3. Distinct variations were observed in the species composition of benthic foraminifera across the sampling stations (Figure 4). In this study, species with an average relative abundance exceeding 5% across all sampling stations were defined as dominant species. A total of six dominant species were identified: Ammonia aomoriensis, Ammonia beccarii, Ammonia tepida, Cribrononion gnythosuturatum, Protelphidium tuberculatum and Quinqueloculina seminula. From stations B1 to B7, the relative abundance of Ammonia aomoriensis showed an overall increasing trend, with the lowest value (12.98%) at B2 and the highest (36.96%) at B6. The relative abundances of Ammonia beccarii and Ammonia tepida fluctuated, ranging from 3.97% to 19.82% and 5.70% to 21.52%, respectively. The proportion of Cribrononion gnythosuturatum was lowest at B5 (2.08%) and highest at B2 (17.56%). The compositions of Protelphidium tuberculatum and Quinqueloculina seminula exhibited opposite trends from B1 to B7: P. tuberculatum was lowest at B2 (3.44%) and highest at B7 (18.50%), whereas Q. seminula was lowest at B7 (4.85%) and highest at B2 (35.50%).
Figure 3

Plate of benthic foraminifera (1: Ammonia aomoriensis, 2: Ammonia beccarii, 3: Ammonia tepida, 4: Ammonia pauciloculata, 5: Ammonia falsobeccarii, 6: Ammonia angulate, 7: Buccella frigida, 8: Cribrononion subincertum, 9: Cribrononion gnythosuturatum, 10: Elphidium advenum, 11: Elphidium clavatum, 12: Hanzawaia convexa, 13: Protelphidium tuberculatum, 14: Quinqueloculina seminula, 15: Quinqueloculina argunica, 16: Quinqueloculina subungeriana, 17: Quinqueloculina tikutoensis, 18: Quinqueloculina tropicalis, 19: Massilina secans, 20: Massilina laevigata, 21: Ammoglobigerina globigeriniformis, 22: Ammobaculites agglutinans, 23: Verneuilinulla advena, 24: Textularia lancea).
Figure 4
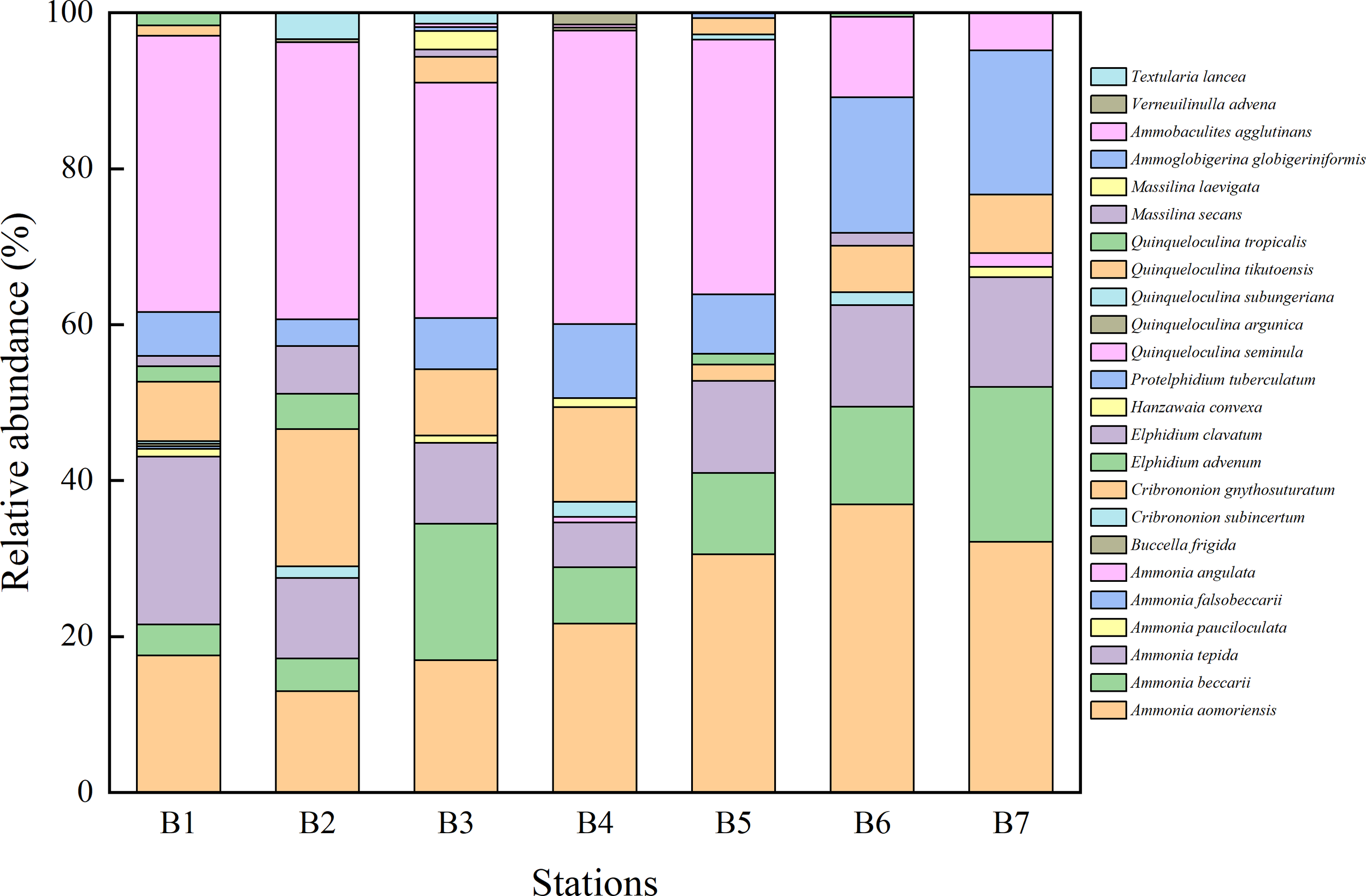
Relative abundance of 24 species of benthic foraminifera in all stations.
3.3 Community parameters
Benthic foraminiferal community parameters were calculated and listed in Table 2. Substantial variations were observed in benthic foraminiferal community parameters across the sampling stations. Abundance, species richness and Margalef index were highest, while Pielou’s evenness was lowest in station B1. The highest Shannon-Wiener diversity index was recorded at station B3, where abundance, species richness and Margalef index were also relatively high.
Table 2
| Station | Abundance (ind./10g DW) | Species richness | Margalef index | Pielou’s evenness | Shannon-Wiener index |
|---|---|---|---|---|---|
| B1 | 70 | 14 | 2.2765 | 0.7021 | 1.8527 |
| B2 | 42 | 11 | 1.7959 | 0.8066 | 1.9340 |
| B3 | 60 | 13 | 2.2402 | 0.7763 | 1.9911 |
| B4 | 20 | 12 | 1.9741 | 0.7251 | 1.8019 |
| B5 | 25 | 10 | 1.8109 | 0.7391 | 1.7017 |
| B6 | 29 | 9 | 1.5341 | 0.8024 | 1.7631 |
| B7 | 54 | 8 | 1.2903 | 0.8383 | 1.7431 |
Community parameters of benthic foraminifera from seven stations.
Abundance was defined as the number of individuals per 10 grams of dry sediment (ind./10g DW).
3.4 Morphologic variation of benthic foraminifera
Among the six dominant species, Ammonia aomoriensis and Quinqueloculina seminula exhibited the highest relative abundances, collectively exceeding the combined total of the remaining 22 species. Both of these benthic foraminiferal species were present in all sampling stations with high abundances, prompting detailed morphological observation and measurement for each species. The maximum shell length was used as a standard metric to assess foraminiferal size. The size of A. aomoriensis showed minimal variation across the seven sampling stations, whereas that of Q. seminula exhibited considerable variability. (Figure 5). The ratio of the maximum shell length to the shell width, referred to as the length-width ratio, was used as a parameter to quantify morphological variations in foraminiferal shells. The length-width ratios of both benthic foraminiferal species exhibited fluctuating trends across the different sampling stations. (Figure 6).
Figure 5
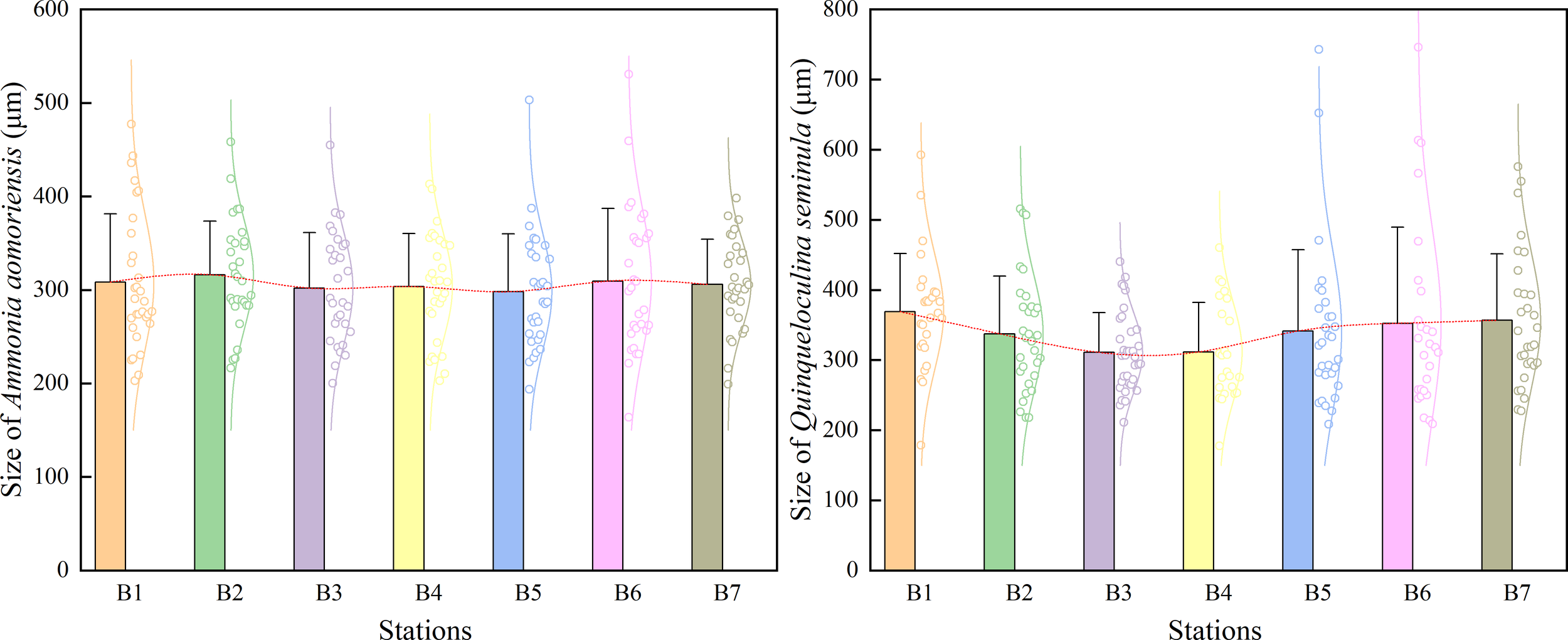
Size of two benthic foraminiferal species.
Figure 6

Length- width ratio of two benthic foraminiferal species.
Furthermore, a detailed microscopic examination of the shell structures of both benthic foraminiferal species was conducted, revealing the presence of morphological deformities in a portion of the tests (Figure 7). The abnormality rates were quantified separately for each species. The abnormality rates of A. aomoriensis ranged from 0 (station B4 and B6) to 7% (station B5). The abnormality rates of Q. seminula were higher and ranged from 1% (station B4) to 11% (station B1).
Figure 7
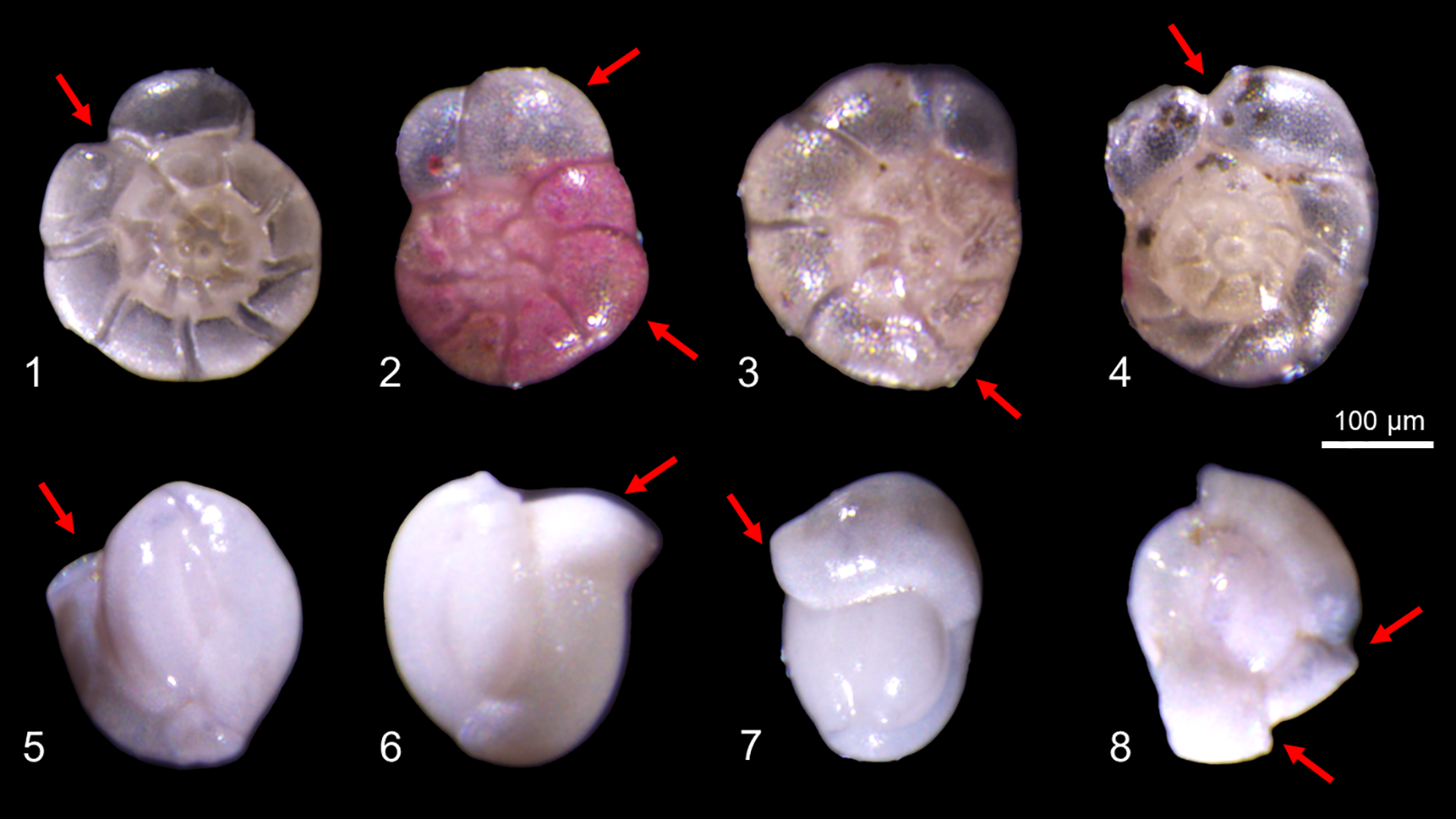
Micrographs of deformed A. aomoriensis (1-4) and Q. seminula (5-8) (The red arrow indicates the area of malformation).
4 Discussion
4.1 Response of benthic foraminiferal assemblages to heavy metal contamination
The significant spatial variations observed in foraminiferal community parameters (e.g., abundance, species richness, diversity index) across the seven sampling stations reflect a clear biological response to heterogeneous environmental conditions, particularly heavy metal pollution. Stations with elevated concentrations of metals generally exhibited reduced foraminiferal abundance and diversity. For instance, station B7, which showed the highest Cu level, had the lowest species richness (S=8) and Margalef index (D=1.29), indicating metal-induced ecological stress. Further correlation analysis confirmed that the concentrations of Cu and Cd in the sediments showed a significant negative correlation with both the species richness and Margalef index (Figure 8). These findings were consistent with studies in other heavily contaminated regions, where foraminiferal diversity declines significantly under metal pollution (Sadanandan et al., 2024; Qiao et al., 2025).
Figure 8
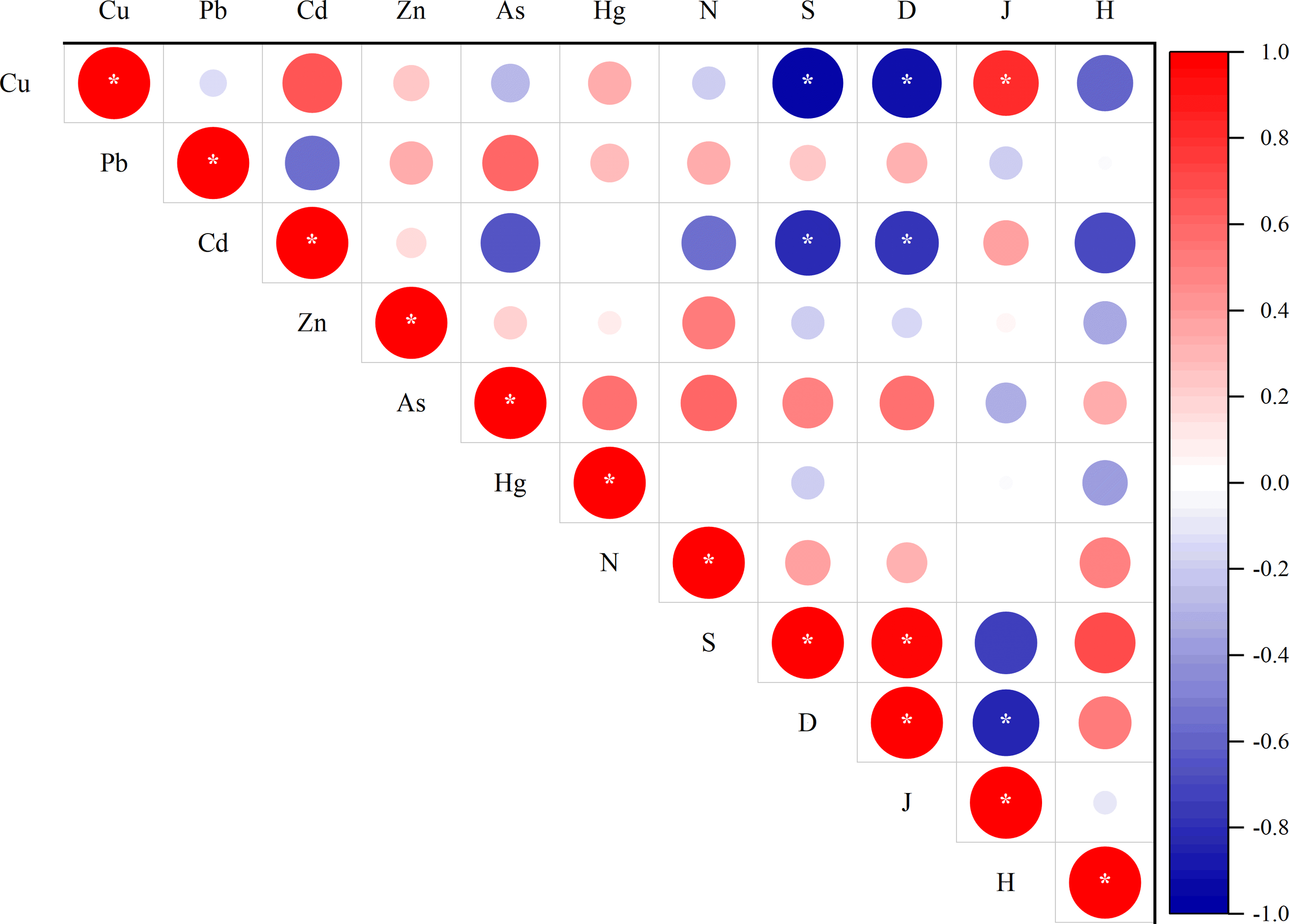
Correlation plot between heavy metals and community parameter (Abundance (N), Species richness (S), Margalef index (D), Pielou’s evenness index (J), Shannon-Wiener diversity index (H), *p < 0.05).
However, the concentrations of Cu showed positive correlation with Pielou’s eveness. We hypothesize that this phenomenon may be attributed to a reduction in dominant species due to elevated copper concentrations, which subsequently leads to an increased proportional representation of other species and indirectly elevates community evenness. This aligns with previously reported findings wherein ocean acidification led to an increase in community evenness among benthic foraminifera (Dong et al., 2020).
The dominance of Ammonia species (e.g., A. aomoriensis, A. beccarii, A. tepida) and Protelphidium tuberculatum across most stations suggests their higher tolerance compared to other species. Correlation analysis also showed the abundance of A. aomoriensis, A. tepida and P. tuberculatum were positively correlated with Cd and Zn concentrations (Figure 9). This is consistent with previous findings that stress-tolerant taxa often become dominant in polluted environments. Other studies have also reported the enrichment of Ammonia and Protelphidium taxa in heavy metal-contaminated areas (Guo et al., 2020; Sadanandan et al., 2024). While, Q. seminula showed negative correlation with Cu and Cd concentrations. This showed Q. seminula was a sensitive species for Cu and Cd pollutions.
Figure 9
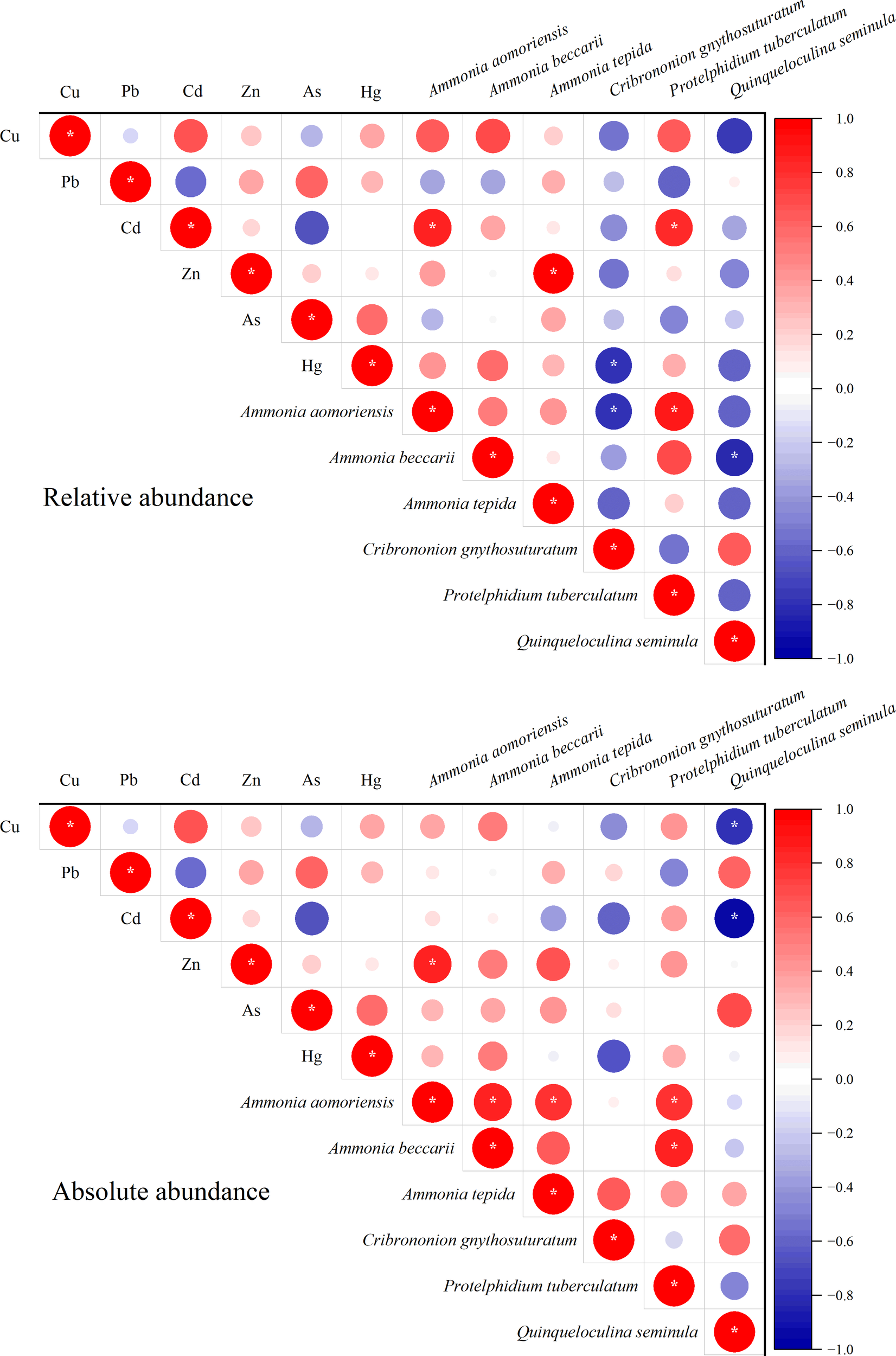
Correlation plot between heavy metals and relative (upper) and absolute (lower) abundance of six dominate benthic foraminifera (*p < 0.05).
Notably, the combined relative abundance of A. aomoriensis and Q. seminula exceeded that of the remaining 22 species, highlighting their role as potential indicator species for metal pollution in Bohai Bay. Therefore, we propose that changes in the composition of these two benthic foraminiferal species can serve as reliable indicators of heavy metal pollution in Bohai Bay.
4.2 Morphology of benthic foraminifera as indicators of metal-induced stress
In addition to differences in species composition potentially indicating environmental changes, morphological variations in benthic foraminifera are also indicators of environmental stress. Previous studies have shown that the size and shape (length-to-width ratio) of certain species of benthic foraminifera may also reflect the degree of environmental stress (such as temperature and pH) (Li et al., 2019a; Dong et al., 2020). Morphological test abnormalities—such as deformed chambers, twisted sutures, and reduced test size—are increasingly recognized as direct and observable indicators of physiological stress caused by heavy metals contaminants (El-Kahawy et al., 2018; Boehnert et al., 2020). In this study, the size and length-width ratio of two indicator species showed no significant correlation except for an anomalous positive correlation between size of Q. seminula and Zn concentration (Figure 10).
Figure 10
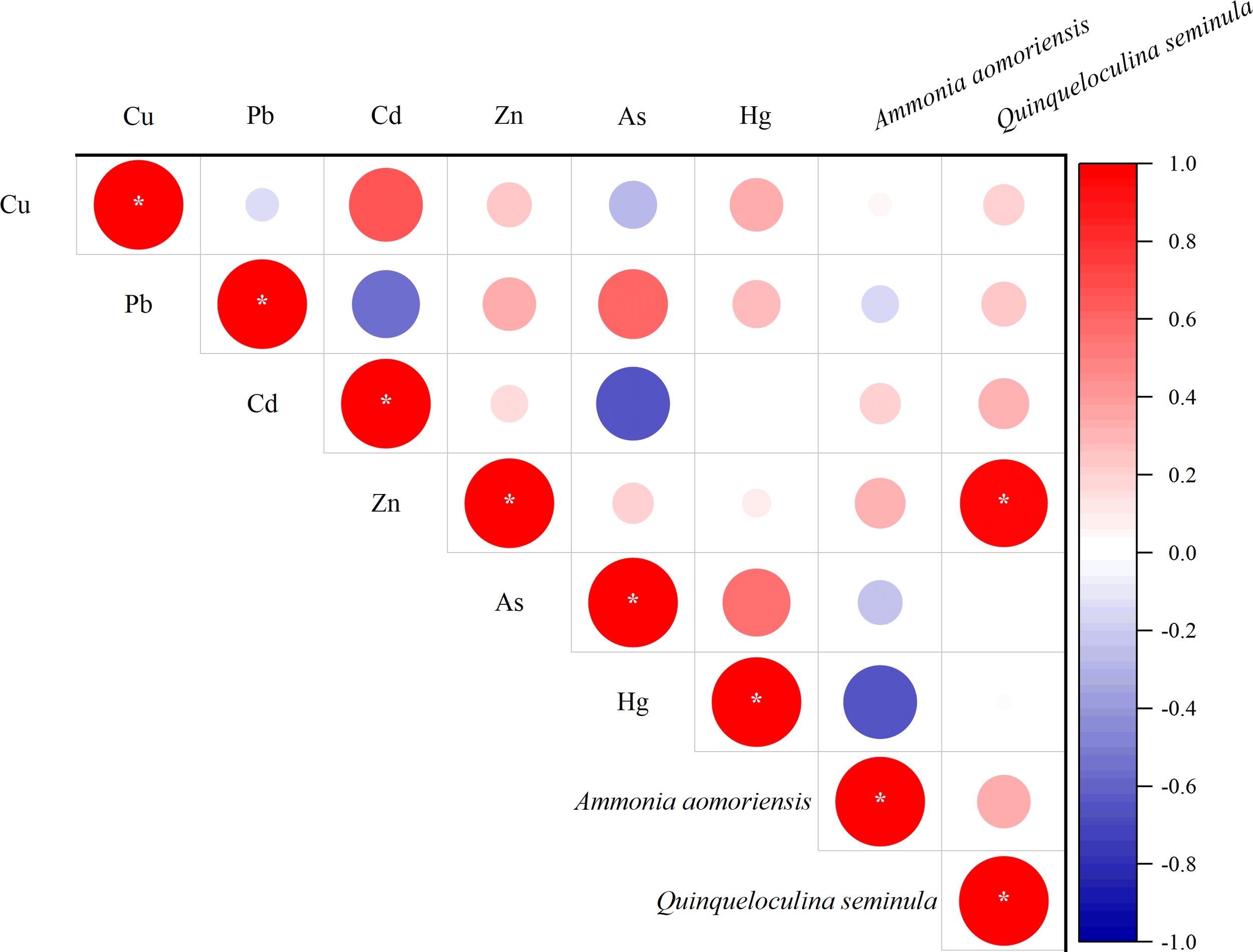
Correlation plot between heavy metals and benthic foraminiferal size (*p < 0.05).
This statistical result indicates that within the concentration range of Zn examined in this study, there was a significant positive correlation between Zn concentration and the size of Q. seminula. This phenomenon has not been observed in previous studies and may suggest that Zn, as an essential element for growth, contributes to the increase in size of benthic foraminifera. While this suggests a potential link between Zn and the growth of Q. seminula, the underlying causes and mechanisms remain unclear. So, we need more investigation and culture experiments to valid their relationship found in this study.
The presence of test deformities in both A. aomoriensis and Q. seminula provides further evidence of sublethal stress induced by heavy metals (Figure 7). Our result showed the shell abnormality rates of A. aomoriensis and Q. seminula were significantly positively correlated with Pb and Zn, respectively (Figure 11). Price et al. (2019) reported that only Zn could induce the abnormal shell to foraminifera. In this study, we found elevated concentrations of both Pb and Zn could increase the shell deformity rate. Therefore, we propose that the test deformity rate could serve as a proxy indicator for heavy metal contamination and may exhibit species-specific responses.
Figure 11
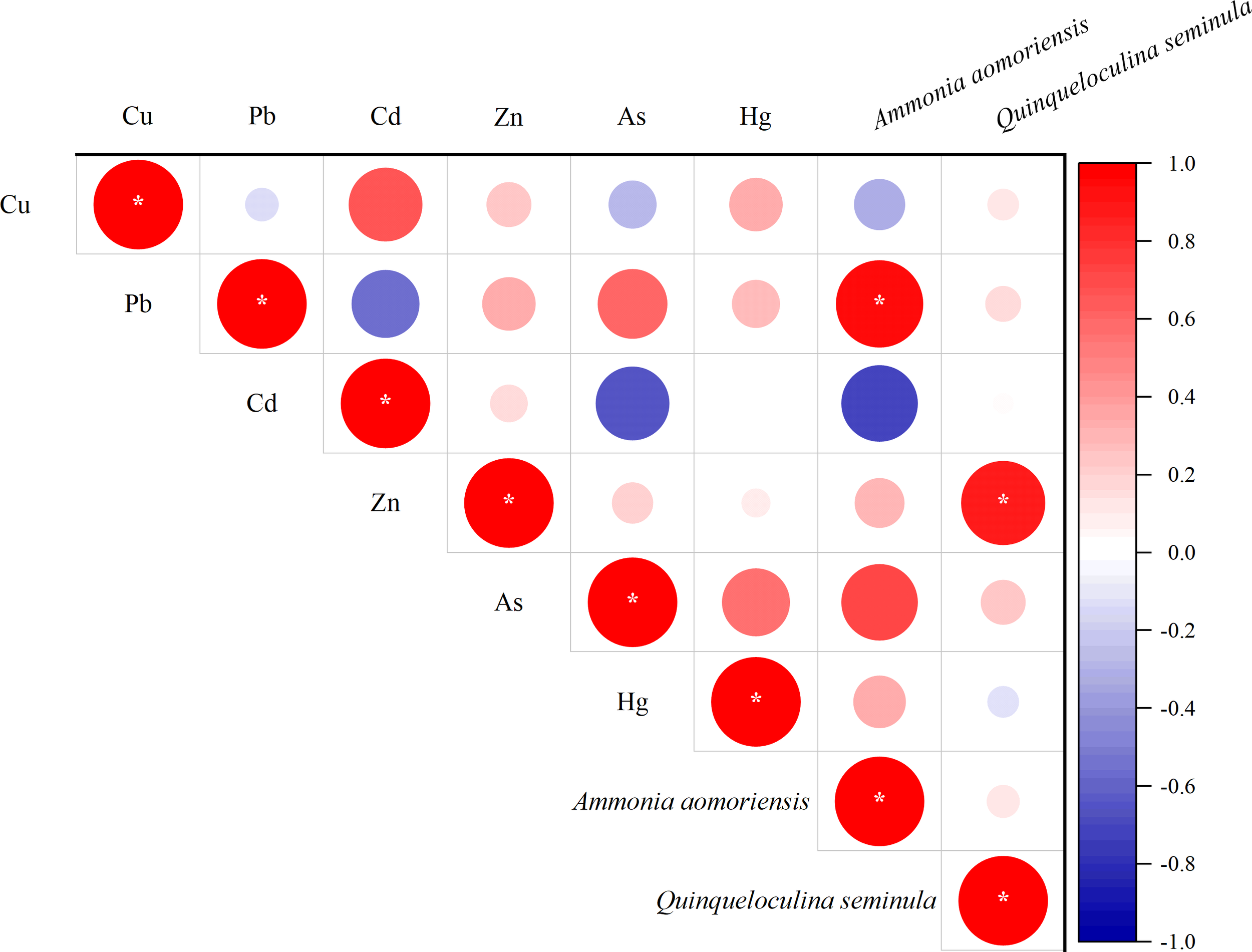
Correlation plot between heavy metals and shell abnormality rates of A. aomoriensis and Q. seminula (*p < 0.05).
Based on the combined results above, we have discovered a very interesting phenomenon: the heavy metal Zn has a dual effect on Q. seminula. As the Zn concentration increases, it promotes individual growth on one hand, while on the other hand, it leads to an increase in the shell deformity rate. Further culturing experiments are needed to validate this relationship in heavy metals monitoring.
This study was designed as a preliminary investigation to establish baseline data on benthic foraminiferal assemblages and their relationship with heavy metal contamination in Bohai Bay—a region where such integrated biological and geochemical data are still scarce. We acknowledge that our data collection was limited to a single sampling campaign conducted along one transect. Repeated seasonal sampling and multiple transects would provide a more comprehensive understanding of both foraminiferal diversity and heavy metal distribution. Despite the limited sampling effort, the results reveal clear spatial patterns and statistically significant correlations between metal concentrations and foraminiferal community parameters and shell deformities. These findings provide valuable insights into the potential use of foraminifera as bioindicators in this environmentally stressed area.
5 Conclusions
This study demonstrates that benthic foraminiferal assemblages and test deformities are highly responsive to heavy metal pollution in Bohai Bay. The dominance of metal-tolerant species (e.g., Ammonia spp. and Protelphidium tuberculatum) and the significant negative correlations between Cu/Cd concentrations and diversity indices reflect community-level adaptations to contamination. Moreover, the species-specific morphological abnormalities provide evidence of sublethal stress induced by Pb and Zn. These findings align with global patterns observed in polluted coastal regions while highlighting regional specificities in foraminiferal responses. The dual approach of combining assemblage characteristics with morphological analysis offers a comprehensive tool for biomonitoring metal pollution. However, further laboratory culturing experiments and multi-stressor investigations are needed to elucidate causal mechanisms and species-specific tolerance thresholds. This work contributes to the sustainable management of Bohai Bay by providing a biological perspective on its environmental health and supporting the use of foraminifera in coastal zone assessment worldwide.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZL: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing, Resources, Visualization. MH: Methodology, Software, Writing – original draft, Visualization, Writing – review & editing. PH: Methodology, Software, Writing – review & editing, Validation, Writing – original draft. SD: Conceptualization, Funding acquisition, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. XC: Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Science and Technology Plan Project of Lianyungang (JCYJ2420), Fund of Key Laboratory of Marine Ecological Conservation and Restoration, Ministry of Natural Resources/Fujian Provincial Key Laboratory of Marine Ecological Conservation and Restoration, (EPR2024006), Open Project of Fujian Key Laboratory on Conservation and Sustainable Utilization of Marine Biodiversity, Minjiang University (CSUMBL2024-5), Open-end Funds of Jiangsu Key Laboratory of Marine Biotechnology, Jiangsu Ocean University (HS2023001), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (23KJB170006); Natural Science Foundation of Jiangsu Province (BK20241062), Lianyungang Postdoctoral Research Funding Program, Young Elite Scientists Sponsorship Program by Jiangsu Association for Science and Technology (JSTJ-2025-593).
Acknowledgments
We thank the editor and two reviewers for constructive comments on the earlier version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdelmonem B. H. Kamal L. T. Elbaz R. M. Khalifa M. R. Abdelnaser A. (2025). From contamination to detection: The growing threat of heavy metals. Heliyon11, e41713. doi: 10.1016/j.heliyon.2025.e41713
2
Abd Malek M. N. Frontalini F. (2024). Benthic foraminifera as bioindicators of marine pollution: A bibliometric approach to unravel trends, patterns and perspectives. Mar. pollut. Bull.199, 115941. doi: 10.1016/j.marpolbul.2023.115941
3
Ajala L. O. Wilson J. E. H. Jiru M. Iwunze M. O. (2025). Perspective on health and ecological risk assessments of potentially toxic metal(loid)s using aquatic biodiversity as biomonitoring indicators. Toxicol. Rep.15, 102086. doi: 10.1016/j.toxrep.2025.102086
4
Alfred R. Loeblich J. Tappan H. (1988). Foraminiferal genera and their classification. (New York: Springer US).
5
Bergamin L. Romano E. (2016). Suitable sediment fraction for paleoenvironmental reconstruction and assessment of contaminated coastal areas based on benthic foraminifera: A case study from Augusta Harbour (Eastern Sicily, Italy). Ecol. Indic.71, 66–78. doi: 10.1016/j.ecolind.2016.06.030
6
Boehnert S. Birkelund A. R. Schmiedl G. Kuhnert H. Kuhn G. Hass H. C. et al . (2020). Test deformation and chemistry of foraminifera as response to anthropogenic heavy metal input. Mar. pollut. Bull.155, 111112. doi: 10.1016/j.marpolbul.2020.111112
7
Bouchet V. M. P. Seuront L. Tsujimoto A. Richirt J. Frontalini F. Tsuchiya M. et al . (2023). Foraminifera and plastic pollution: Knowledge gaps and research opportunities. Environ. pollut.324, 121365. doi: 10.1016/j.envpol.2023.121365
8
Ciacci C. Betti M. Abramovich S. Cavaliere M. Frontalini F. (2022). Mercury-induced oxidative stress response in benthic foraminifera: an in vivo experiment on Amphistegina lessonii. Biology11, 960. doi: 10.3390/biology11070960
9
Dong S. Lei Y. Li T. Jian Z. (2020). Response of benthic foraminifera to pH changes: Community structure and morphological transformation studies from a microcosm experiment. Mar. Micropaleontology156, 101819. doi: 10.1016/j.marmicro.2019.101819
10
El-Kahawy R. El-Shafeiy M. Helal S. A. Aboul-Ela N. El-Wahab M. A. (2018). Morphological deformities of benthic foraminifera in response to nearshore pollution of the Red Sea, Egypt. Environ. Monit. Assess.190, 312. doi: 10.1007/s10661-018-6695-2
11
Ganugapenta S. Badimela U. Subbyamoola P. B. Madipally R. Nadimikeri J. Krishnan K. A. (2025). Ecological responses of benthic foraminifera to trace element pollution in the Beypore estuary, Southwest coast of India: Implications for coastal anthropogenic contamination. Mar. Environ. Res.205, 107022. doi: 10.1016/j.marenvres.2025.107022
12
Guo Y. Mei X. Meng X. Lan X. Chen H. Yang H. (2020). Benthic foraminifera and its response to heavy metal pollution: A case study from Liaodong Bay, China. Mar. pollut. Bull.154, 111084. doi: 10.1016/j.marpolbul.2020.111084
13
Hoober L. Titelboim D. Abramovich S. Herut B. Teutsch N. Benaltabet T. et al . (2022). Establishing baseline assessment levels for monitoring coastal heavy metals using foraminiferal shells: A case study from the Southeastern Mediterranean. Water14, 1532. doi: 10.46427/gold2022.10591
14
Huang C. Lin Y. Li H. Zheng B. Zhu X. Xu Y. et al . (2025). Distribution and relationship of radionuclides and heavy metal concentrations in marine sediments from the areas surrounding the Daya Bay power plant, southeast China. J. Mar. Sci. Eng.13, 1237. doi: 10.3390/jmse13071237
15
Joshi N. Arya P. C. Saulnier-Talbot É. (2024). Benthic foraminifera as useful bioindicators of heavy metal and organic enrichment in northern temperate coastal zones: A comprehensive review. J. Coast. Res.41, 516–535. doi: 10.2112/JCOASTRES-D-24A-00012.1
16
Lei Y. Li T. (2016). Atlas of benthic foraminifera from China Seas the Bohai Sea and the Yellow Sea. (Beijing: Springer-Verlag GmbH Germany and Science Press).
17
Li Y.-Y. Feng H. Yuan D.-K. Guo L. Mu D. (2019b). Mechanism study of transport and distributions of trace metals in the Bohai Bay, China. China Ocean Eng.33, 73–85. doi: 10.1007/s13344-019-0008-6
18
Li M. Lei Y. Li T. Jian Z. (2019a). Impact of temperature on intertidal foraminifera: Results from laboratory culture experiment. J. Exp. Mar. Biol. Ecol.520, 151224. doi: 10.1016/j.jembe.2019.151224
19
Madjar R. M. Vasile Scăețeanu G. (2025). An overview of heavy metal contamination in water from agriculture: origins, monitoring, risks, and control measures. Sustainability17, 7368. doi: 10.3390/su17167368
20
McKenzie T. Moody A. Barreira J. Guo X. Cohen A. Wilson S. J. et al . (2024). Metals in coastal groundwater systems under anthropogenic pressure: a synthesis of behavior, drivers, and emerging threats. Limnology Oceanography Lett.9, 388–410. doi: 10.1002/lol2.10413
21
Munsel D. Kramar U. Dissard D. Nehrke G. Berner Z. Bijma J. et al . (2010). Heavy metal incorporation in foraminiferal calcite: results from multi-element enrichment culture experiments with Ammonia tepida. Biogeosciences7, 2339–2350. doi: 10.5194/bg-7-2339-2010
22
Murray J. W. (2006). Ecology and applications of benthic foraminifera (New York: Cambridge University Press).
23
Naik R. Kulkarni N. Indap M. . (2023). Understanding role of foraminifera in environmental studies: A review. Int. J. Ecol. Environ. Sci49, 457–471. doi: 10.55863/ijees.2023.2882
24
O’Brien P. Polovodova Asteman I. Bouchet V. M. P. (2021). Benthic foraminiferal indices and environmental quality assessment of transitional waters: A review of current challenges and future research perspectives. Water13, 1898. doi: 10.3390/w13141898
25
Price E. B. Kabengi N. Goldstein S. T. (2019). Effects of heavy-metal contaminants (Cd, Pb, Zn) on benthic foraminiferal assemblages grown from propagules, Sapelo Island, Georgia (USA). Mar. Micropaleontology147, 1–11. doi: 10.1016/j.marmicro.2019.01.004
26
Qiao L. Wu Y. Ren C. Li T. Guo Y. Zhao A. (2025). Benthic foraminiferal community structure and its response to heavy metals revealed using environmental DNA/RNA metabarcoding in Yueqing Bay, East China Sea. Mar. pollut. Bull.217, 118126. doi: 10.1016/j.marpolbul.2025.118126
27
Sadanandan H. Dharmalingam S. N. Mouttoucomarassamy S. (2024). Benthic foraminifera as bio-indicator of marine pollution in the southwestern Bay of Bengal, India. Environ. Sci. pollut. Res. 31, 41462–41479. doi: 10.1007/s11356-023-29367-y
28
Schmidt S. Hathorne E. C. Schönfeld J. Garbe-Schönberg D. (2022). Heavy metal uptake of nearshore benthic foraminifera during multi-metal culturing experiments. Biogeosciences19, 629–664. doi: 10.5194/bg-19-629-2022
29
Tian K. Wu Q. Liu P. Hu W. Huang B. Shi B. et al . (2020). Ecological risk assessment of heavy metals in sediments and water from the coastal areas of the Bohai Sea and the Yellow Sea. Environ. Int.136, 105512. doi: 10.1016/j.envint.2020.105512
30
Titelboim D. Sadekov A. Blumenfeld M. Almogi-Labin A. Herut B. Halicz L. et al . (2021). Monitoring of heavy metals in seawater using single chamber foraminiferal sclerochronology. Ecol. Indic.120, 106931. doi: 10.1016/j.ecolind.2020.106931
31
Zhang Y. Lu X. Wang N. Xin M. Geng S. Jia J. et al . (2016). Heavy metals in aquatic organisms of different trophic levels and their potential human health risk in Bohai Bay, China. Environ. Sci. pollut. Res.23, 17801–17810. doi: 10.1007/s11356-016-6948-y
32
Zitoun R. Marcinek S. Hatje V. Sander S. G. Völker C. Sarin M. et al . (2024). Climate change driven effects on transport, fate and biogeochemistry of trace element contaminants in coastal marine ecosystems. Commun. Earth Environ.5, 560. doi: 10.1038/s43247-024-01679-y
Summary
Keywords
foraminifera, heavy metal, bioindicators, community, Bohai Bay
Citation
Liu Z, He M, Han P, Dong S and Chen X (2025) Bio-monitoring heavy metal pollution: insights from benthic foraminiferal assemblages and shell morphology. Front. Mar. Sci. 12:1716252. doi: 10.3389/fmars.2025.1716252
Received
30 September 2025
Accepted
31 October 2025
Published
17 November 2025
Volume
12 - 2025
Edited by
Kit Yue Kwan, JiMei University, China
Reviewed by
Ergun Taskin, Manisa Celal Bayar University, Türkiye
Ling Qiao, Zhoushan Fisheries Research Institute, China
Updates
Copyright
© 2025 Liu, He, Han, Dong and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuaishuai Dong, sdong@jou.edu.cn; Xiutao Chen, zhchenxiutao@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.