- 1First Academic Respiratory Department, Sotiria General Hospital for Thoracic Diseases, University of Athens, Athens, Greece
- 2Division of Immunology, Alexander Fleming Biomedical Sciences Research Center, Athens, Greece
- 3Department of Medicine, Florida International University Herbert Wertheim College of Medicine, Miami, FL, United States
- 4Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, United States
- 5Department of Medicine, University of Miami Miller School of Medicine, Miami, FL, United States
Idiopathic pulmonary fibrosis (IPF) is an inexorably progressive lung disease of unknown origin. Prognosis is poor, with limited treatment options available, and the median survival remains just 3–5 years. Despite the use of pirfenidone and nintedanib for the treatment of IPF, curative therapies remain elusive and mortality remains high. Regenerative medicine and the use of cell-based therapies has recently emerged as a potential option for various diseases. Promising results of preclinical studies using mesenchymal stem cells (MSCs) suggest that they may represent a potential therapeutic option for the treatment of chronic lung diseases including IPF. Encouraging results of Phase 1 studies of MSCs various have reduced safety concerns. Nonetheless, there is still a pressing need for exploratory biomarkers and interval end-points in the context of MSCs investigation. This review intends to summarize the current state of knowledge for stem cells in the experimental and clinical setting of IPF, present important safety and efficacy issues, highlight future challenges and address the need for large, multicenter clinical trials coupled with realistic end-points, including biomarkers, to assess treatment efficacy.
Introduction
Idiopathic Pulmonary Fibrosis (IPF) is a progressive debilitating lung disease of unknown etiology (1–4). The disease is characterized by a combination of histological changes including extracellular matrix (ECM) deposition, phenotypic changes of fibroblasts and alveolar epithelial cells, formation of fibroblastic foci, and scattered areas of aberrant wound healing interspersed with normal lung parenchyma (1, 5–14).
Current evidence suggests that the areas of fibrosis seen in lungs of patients with IPF share many features with normal aging lung, such as genomic instability, telomere attrition, mitochondrial dysfunction, cellular senescence, and immune dysregulation (10, 15, 16). Due to the inefficacy of immunomodulatory and immunosuppressive agents in the past, the role of the immune system in the pathogenesis of IPF remains poorly understood (17–22). However, highly activated and proliferative CD4+ cells and functional impairment of T regulatory cells (Tregs) in patients with IPF, suggest a link between immunity and pulmonary fibrosis (10, 23, 24).
There are two approved compounds for the treatment of IPF: pirfenidone and nintedanib. Pirfenidone is an antifibrotic compound with an unclear mechanism of action targeting several molecules including transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and interleukin 6 (25). Nintedanib is a tyrosine-kinase inhibitor, targeting vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), and platelet derived growth factor receptor (PDGFR) (22).While the use of pirfenidone and nintedanib have been shown to slow the progression of IPF (26–28), both compounds have significant side effects and neither is curative (28–30). Morbidity and mortality from IPF remains high and thus there is a pressing need for alternative therapeutic options for this complex disease (7, 31–33). The US National Institutes of Health database lists 493 complete or ongoing clinical trials of MSCs (34). Toward this end, regeneration and cell therapies such as the use of mesenchymal stem cells (MSCs) have emerged as a potential option.
MSCs are multipotent cells able to differentiate into a number of different cell lines and exert immunomodulatory, anti-proliferative, and anti-inflammatory effects. Their multipotency, migratory ability, and immunoprivileged state has led to extensive research efforts for therapeutic applications in several diseases including cardiac ischemia (35–39), ischemic acute renal failure (37), sepsis (40), autoimmune disorders (41), severe graft-vs. -host disease (42), pancreatic islet and renal glomerular repair in diabetes (43), fulminant hepatic failure (44), chronic lung diseases (45–48), and acute lung injury (49–53) (Table 2).
MSCs are easily harvested from many tissues (peripheral blood, adipose tissue, bone marrow, and umbilical cord) and may be expanded in vitro with minimal modifications. MSCs represent the most extensively studied stem cell population (54). Research supports the immunomodulatory, anti-inflammatory, and potentially anti-fibrotic properties of MSCs (49, 55, 56). Importantly, MSCs are “immune privileged,” lacking expression of class II major histocompatibility complex (MHC-II). Therefore, allogeneic use of MSCs is possible (57).
Preclinical Studies
Recent studies on the pathophysiology of IPF suggest that early alveolar injury activates abnormal alveolar epithelial cells and stimulates the release of mediators including matrix metalloproteinases and TGF beta-1 (2, 58–61). These mediators activate cytokines and chemokines including IL-1 and IL-13 leading to the phenotype of abnormal wound healing (62–65). Therefore, IPF is considered a complex and multifactorial disease characterized by alveolar epithelial injury and alveolar collapse, fewer alveolar epithelial type II cells, alveolar stem cell exhaustion, and myofibroblast deregulation due to living on a fibrotic matrix (66, 67).
Several experimental studies have been conducted in order to investigate the effect of MSCs from various organs, mainly from bone marrow with a dosage ranging between 0.1 × 106 and 4 × 106 cells, in pathways associated with lung injury and pulmonary fibrosis and several end-points had been set (68, 69) (Table 1). The majority of studies recorded substantial improvement in histopathology (56, 64, 70–82), decrease to Ashcroft score (70–72, 77, 79, 80, 83) and lung collagen content (56, 70–81), reduced pulmonary transforming growth factor-b (TGFb) levels (56, 70–81, 84) and decreased BAL neutrophil count (76, 77, 80, 81, 85) following to MSCs administration. Importantly, both bone marrow and amnion-derived MSCs reduced TGFb levels (84). However, to this end, data are still conflicting regarding levels of tumor necrosis factor-a (TNF-a) (56, 71, 83–85), interleukins IL-1, IL-6 (71, 77, 81, 84, 86) and metalloproteinases MMP-2, MMP-9, MMP-13 (56, 71, 83, 84) following administration of MSCs (4, 87–89).
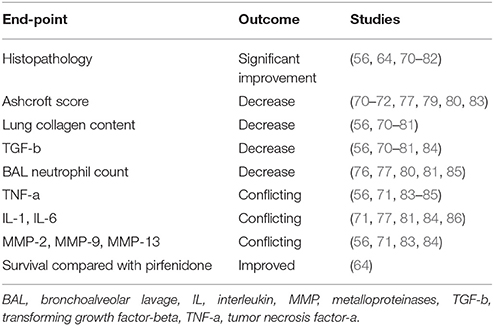
Table 1. Main results of preclinical studies of mesenchymal stem cell therapy in experimental pulmonary fibrosis based on end-points set.
The majority of studies investigating the effect of donor MSCs on BLM-induced pulmonary fibrosis have used young male mouse models (90, 91). Young mice, however, undergo spontaneous resolution of BLM-induced pulmonary fibrosis in some studies (69, 90, 91). Although IPF is primarily a disease of individuals over the age of 50, most studies have also utilized young female mice to evaluate the molecular patterns and potential therapeutic targets for patients with IPF (92–94). Interestingly, in one study of bleomycin induced fibrosis in mice, MSCs were found to improve survival when compared with pirfenidone (64). This study also reported downregulation of IL-2, IL-1b, TNF-α, and TGFβ leading to a reduction in inflammation (64). In addition, downregulation of MMPs was noted with a reduction in collagen deposition and fibrosis (64).
Collectively, MSCs seem to exert pleiotropic effects in the site of lung injury including anti-inflammatory, immunomodulatory, antifibrotic effects (65), engagement in paracrine signaling (95), activation of resident stem cells, and differentiation into local cell types (56, 65, 74, 77, 79, 83, 85, 96–98). Preclinical studies have shown MSCs to be efficacious in the treatment and prevention of lung fibrosis (65, 69). Nonetheless, concerns remain regarding the activity of MSCs within a pro-fibrotic microenvironment (99–102). While some preclinical studies suggest that MSCs might promote fibrosis, to date, no human studies have found a similar pro-fibrotic effect (37, 42, 91, 100, 101, 103–112).
Clinical Trials
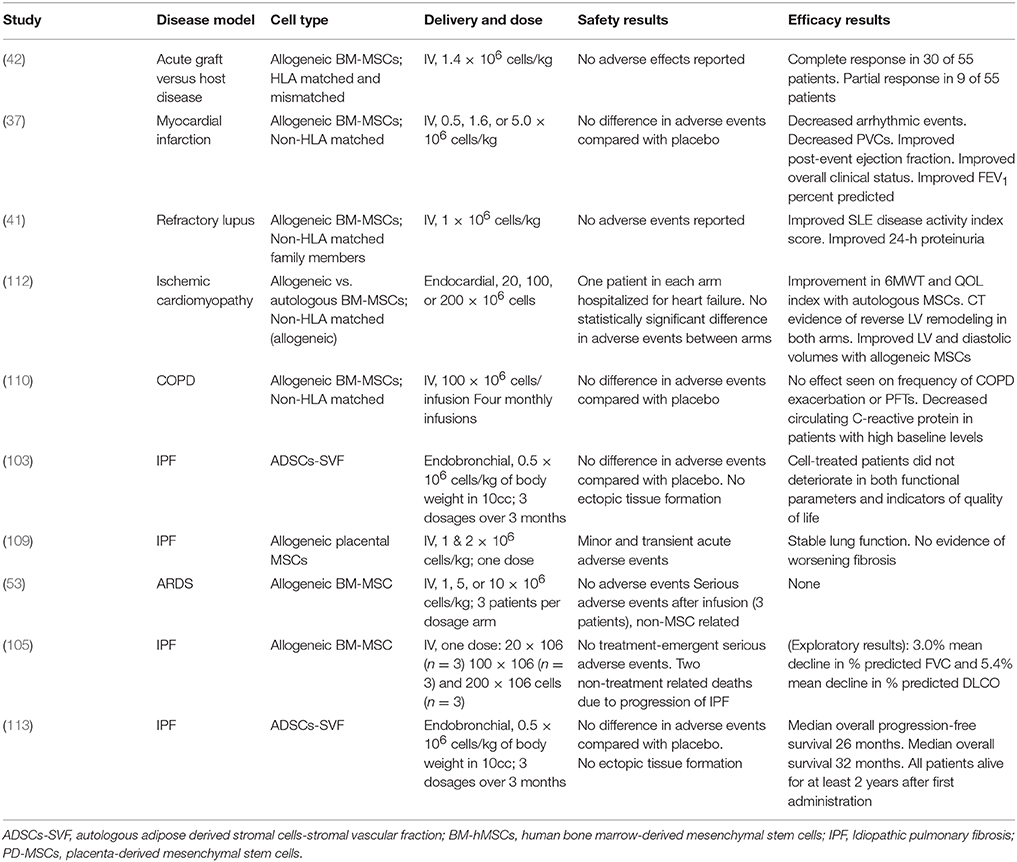
Early clinical studies of MSCs in patients with IPF have shown promising safety profiles (30, 103, 114). Phase 1 clinical trials have been conducted for safety of MSC therapy. A phase Ib study of endobronchially administered autologous adipose-derived MSCs showed not only acceptable safety outcomes, but also improvements in quality of life parameters (103). The recently published longitudinal outcomes of this study also demonstrated an acceptable safety profile, 100% survival rate 2 years after first administration and a median overall progression-free survival of 26 months (113). Furthermore, studies of intravenously administered placental derived MSCs (105, 109) found that administration of up to 2 × 106 cells per kilogram was safe in subjects with moderately severe IPF (109). Importantly, the authors reported only minor and transient alterations in peri-infusion hemodynamics and gas exchange, reducing the concerns for embolization of stem cells to an already compromised pulmonary vasculature. Subjects were followed for six months with no observed decline in forced vital capacity (FVC), diffusing lung capacity for carbon monoxide (DLCO), six-minute walk test (6MWT), or CT fibrosis score (90). The AETHER trial also showed favorable safety outcomes for the intravenous delivery of a single dose of allogeneic MSCs in patients with IPF up to 2 × 108 cells (105). Although this was an underpowered study for the detection of significant changes in functional indices, the mean decline in % predicted FVC and DLCO were below the thresholds for disease progression (1, 115). ReCell, an FDA approved phase 1b multidose, randomized, double-blind trial of 10 × 106 cells delivered intravenously to patients with IPF, has not yet begun enrollment.
Outstanding Challenges
While it now appears that it is safe to use MSCs in patients with IPF, many questions and challenges remain. In the preclinical realm, there is a need for animal models more representative of chronic IPF (91, 116) for the continued study of how MSCs exert their effects. Bleomycin induced pulmonary fibrosis is still considered the best available animal model for preclinical testing (91, 117). However, there is increasing criticism that potential therapies usually administered the first 7 days following bleomycin exposure may act mainly through prevention of the inflammatory cascade rather than reversal of fibrosis, thus limiting their applicability to human IPF (69). First, improved animal models will enable the identification of biomarkers that may be useful as measures of disease activity and/or treatment effect. Second, the timing of treatment for best effect needs to be better elucidated. Furthermore, the most efficacious source of MSCs and the role of age need to be more fully explored. One report suggesting that adipose-derived MSCs from young, but not old, mice prevent bleomycin induced lung fibrosis in an aged mouse model (118) highlights the need for further research in this area.
Several challenges in the clinical setting also remain to be addressed. The optimal source of MSCs, the best route of administration, the number and timing of administrations, and the appropriate dosing interval. Thus, allogeneic human bone marrow-derived and autologous adipose derived MSCs have been the most studied in the context of IPF. There are limited studies on endogenous stem cells from the lungs of patients with IPF and concerns remain surrounding the risk of biopsy and the potential for intervention-induced IPF exacerbation and the possibility of detrimental effects on lung function from biopsy. Lung tissue obtained at the time of lung transplant remains the best tool for study.
It is also critical to characterize appropriate endpoints to assess treatment effectiveness in these patients (100, 119–129). Molecular biomarkers would be the optimal choice for the assessment of cell based therapies. Finally, well-designed and meticulously conducted multicenter randomized clinical trials of MSCs for the treatment of IPF are needed to assess efficacy.
Moving Forward
While preclinical trials suggest that MSCs may be effective in the treatment of IPF, and early clinical trials suggest that they are likely to be safe in the population, insufficient data exists at this time to definitely state that the use of MSCs for the treatment of IPF is either safe or efficacious. Despite this lack of evidence, cell based therapies are being aggressively marketed to this vulnerable patient population. A recent study found that as of August of 2016, there were at least 351 stem cell related businesses registered in the United States. These sites offer unproven, experimental treatments for a wide variety of conditions (130, 131). In the case of IPF, desperate patients and their physicians continue to succumb to an onslaught of marketing and branding of as yet unproven “stem cell” treatments. Unfortunately, these businesses are also almost wholly unregulated (132). Publication of case reports of harm arising from the misuse of unproven treatments support increased government oversight in the interest of patient safety.
Conclusion
Idiopathic Pulmonary Fibrosis (IPF) is a debilitating lung disease characterized by a progressive decline in lung function ultimately resulting in death. The lack of curative treatments for this disease has created an urgency for other potential therapeutic options. Preclinical studies suggest that because MSCs have immunomodulatory, anti-inflammatory, and potentially anti-fibrotic properties, they may be efficacious in the treatment of IPF. Early clinical trials have shown that MSCs may be safely administered to patients with IPF, but large multicenter randomized trials still need to be performed.
Author Contributions
AT, RT and TK wrote the initial manuscript. The manuscript was supervised and significantly modified by MG, AT, DB. KM, IN, VA offered significant intellectual contribution. All authors approved the final form of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. (2011) 183:788–824. doi: 10.1164/rccm.2009-040GL
2. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2006) 174:810–6. doi: 10.1164/rccm.200602-163OC
3. Tzilas V, Valeyre D, Tzouvelekis A, Bouros D. Taking a giant step in the diagnosis of idiopathic pulmonary fibrosis. Lancet Respir Med. (2017) 6:82–4. doi: 10.1016/S2213-2600(17)30443-5
4. Geiger S, Hirsch D, Hermann FG. Cell therapy for lung disease. Eur Respir Rev. (2017) 26:170044. doi: 10.1183/16000617.0044-2017
5. Ryu C, Sun H, Gulati M, Herazo-Maya JD, Chen Y, Osafo-Addo A, et al. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2017) 196:1571–81. doi: 10.1164/rccm.201612-2480OC
6. Tomos IP, Tzouvelekis A, Aidinis V, Manali ED, Bouros E, Bouros D, et al. Extracellular matrix remodeling in idiopathic pulmonary fibrosis. It is the 'bed' that counts and not 'the sleepers'. Expert Rev Respir Med. (2017) 11:299–309. doi: 10.1080/17476348.2017.1300533
7. Spagnolo P, Sverzellati N, Rossi G, Cavazza A, Tzouvelekis A, Crestani B, et al. Idiopathic pulmonary fibrosis: an update. Ann Med. (2015) 47:15–27. doi: 10.3109/07853890.2014.982165
8. Spagnolo P, Cottin V. Genetics of idiopathic pulmonary fibrosis: from mechanistic pathways to personalised medicine. J Med Genet. (2017) 54:93–9. doi: 10.1136/jmedgenet-2016-103973
9. Tzouvelekis A, Kaminski N. Epigenetics in idiopathic pulmonary fibrosis. Biochem Cell Biol. (2015) 93:159–70. doi: 10.1139/bcb-2014-0126
10. Karampitsakos T, Woolard T, Bouros D, Tzouvelekis A. Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur J Pharmacol. (2017) 808:35–43. doi: 10.1016/j.ejphar.2016.06.045
11. Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. (2013) 188:733–48. doi: 10.1164/rccm.201308-1483ST
12. Tzouvelekis A, Yu G, Lino Cardenas CL, Herazo-Maya JD, Wang R, Woolard T, et al. SH2 domain-containing phosphatase-2 is a novel antifibrotic regulator in pulmonary fibrosis. Am J Respir Crit Care Med. (2017) 195:500–14. doi: 10.1164/rccm.201602-0329OC
13. Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med. (2014) 189:1161–72. doi: 10.1164/rccm.201312-2221PP
14. Bouros D, Tzouvelekis A. Idiopathic pulmonary fibrosis: on the move. Lancet Respir Med. (2014) 2:17–9. doi: 10.1016/S2213-2600(13)70240-6
15. Karampitsakos T, Tzilas V, Tringidou R, Steiropoulos P, Aidinis V, Papiris SA, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. (2017) 45:1–10. doi: 10.1016/j.pupt.2017.03.016
16. Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. (2018) 24:39–49. doi: 10.1038/nm.4447
17. Herazo-Maya JD, Sun J, Molyneaux PL, Li Q, Villalba JA, Tzouvelekis A, et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international, multicentre, cohort study. Lancet Respir Med. (2017) 5:857–68. doi: 10.1016/S2213-2600(17)30349-1
18. Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol. (2008) 83:237–44. doi: 10.1189/jlb.0707504
19. Idiopathic Pulmonary Fibrosis Clinical Research N, Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. (2012) 366:1968–77. doi: 10.1056/NEJMoa1113354
20. Antoniou KM, Nicholson AG, Dimadi M, Malagari K, Latsi P, Rapti A, et al. Long-term clinical effects of interferon gamma-1b and colchicine in idiopathic pulmonary fibrosis. Eur Respir J. (2006) 28:496–504. doi: 10.1183/09031936.06.00032605
21. Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. (2004) 350:125–133. doi: 10.1056/NEJMoa030511
22. Fletcher S, Jones MG, Spinks K, Sgalla G, Marshall BG, Limbrey R, et al. The safety of new drug treatments for idiopathic pulmonary fibrosis. Expert Opin Drug Saf. (2016) 15:1483–9. doi: 10.1080/14740338.2016.1218470
23. Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. (2011) 365:1079–87. doi: 10.1056/NEJMoa1103690
24. Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, et al. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J. (2013) 41:1207–18. doi: 10.1183/09031936.00073012
25. Kolb M, Bonella F, Wollin L. Therapeutic targets in idiopathic pulmonary fibrosis. Respir Med. (2017) 131:49–57. doi: 10.1016/j.rmed.2017.07.062
26. King TE, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2083–92. doi: 10.1056/NEJMoa1402582
27. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2071–82. doi: 10.1056/NEJMoa1402584
28. Tzouvelekis A, Karampitsakos T, Kontou M, Granitsas A, Malliou I, Anagnostopoulos A, et al. Safety and efficacy of nintedanib in idiopathic pulmonary fibrosis: a real-life observational study. Pulm Pharmacol Ther. (2018) 49:61–6. doi: 10.1016/j.pupt.2018.01.006
29. Tzouvelekis A, Karampitsakos T, Ntolios P, et al. Longitudinal “real-world” outcomes of pirfenidone in idiopathic pulmonary fibrosis in Greece. Front Med. (2017) 4:213. doi: 10.3389/fmed.2017.00213
30. Tzouvelekis A, Ntolios P, Karampitsakos T, Tzilas V, Anevlavis S, Bouros E, et al. Safety and efficacy of pirfenidone in severe Idiopathic Pulmonary Fibrosis: a real-world observational study. Pulm Pharmacol Ther. (2017) 46:48–53. doi: 10.1016/j.pupt.2017.08.011
31. Tzouvelekis A, Bonella F, Spagnolo P. Update on therapeutic management of idiopathic pulmonary fibrosis. Ther Clin Risk Manag. (2015) 11:359–370. doi: 10.2147/TCRM.S69716
32. Tzouvelekis A, Spagnolo P, Bonella F, Vancheri C, Tzilas V, Crestani B, et al. Patients with IPF and lung cancer: diagnosis and management. Lancet Respir Med. (2017) 6:86–8. doi: 10.1016/S2213-2600(17)30478-2
33. Tzouvelekis A, Spagnolo P, Bonella F, Vancheri C, Tzilas V, Crestani B, et al. Combination therapy: the future of management for idiopathic pulmonary fibrosis? Lancet Respir Med. (2014) 2:933–42. doi: 10.1016/S2213-2600(14)70232-2
34. Turner L. ClinicalTrials.gov, stem cells and 'pay-to-participate' clinical studies. Regen Med. (2017) 12:705–19. doi: 10.2217/rme-2017-0015
35. Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. (2009) 30:2722–32. doi: 10.1093/eurheartj/ehp265
36. Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. (2006) 12:459–65. doi: 10.1038/nm1391
37. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. (2009) 54:2277–86. doi: 10.1016/j.jacc.2009.06.055
38. Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA (2014) 311:62–73. doi: 10.1001/jama.2013.282909
39. Ramireddy A, Brodt CR, Mendizabal AM, DiFede DL, Healy C, Goyal V, et al. Effects of transendocardial stem cell injection on ventricular proarrhythmia in patients with ischemic cardiomyopathy: results from the POSEIDON and TAC-HFT trials. Stem Cells Transl Med. (2017) 6:1366–72. doi: 10.1002/sctm.16-0328
40. Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. (2009) 15:42–9. doi: 10.1038/nm.1905
41. Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. (2010) 69:1423–29. doi: 10.1136/ard.2009.123463
42. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet (2008) 371:1579–86. doi: 10.1016/S0140-6736(08)60690-X
43. Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. (2006) 103:17438–43. doi: 10.1073/pnas.0608249103
44. Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE (2007) 2:e941. doi: 10.1371/journal.pone.0000941
45. Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. (2004) 556:249–52. doi: 10.1016/S0014-5793(03)01399-1
46. Spees JL, Pociask DA, Sullivan DE, Whitney MJ, Lasky JA, Prockop DJ, et al. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med. (2007) 176:385–94. doi: 10.1164/rccm.200607-1004OC
47. Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, et al. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. (2008) 22:1226–36. doi: 10.1096/fj.07-8076com
48. Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. (2010) 299:L760–70. doi: 10.1152/ajplung.00182.2009
49. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. (2005) 33:145–52. doi: 10.1165/rcmb.2004-0330OC
50. Matthay MA, Thompson BT, Read EJ, McKenna DH, Liu KD, Calfee CS, et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest (2010) 138:965–972. doi: 10.1378/chest.10-0518
51. Lim R, Milton P, Murphy SV, Dickinson H, Chan ST, Jenkin G. Human mesenchymal stem cells reduce lung injury in immunocompromised mice but not in immunocompetent mice. Respiration (2013) 85:332–41. doi: 10.1159/000343078
52. Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. (2009) 106:16357–62. doi: 10.1073/pnas.0907996106
53. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. (2015) 3:24–32. doi: 10.1016/S2213-2600(14)70291-7
54. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy (2013) 15:641–8. doi: 10.1016/j.jcyt.2013.02.006
55. Shigemura N, Okumura M, Mizuno S, Imanishi Y, Nakamura T, Sawa Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am J Transplant. (2006) 6:2592–600. doi: 10.1111/j.1600-6143.2006.01522.x
56. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. (2003) 100:8407–11. doi: 10.1073/pnas.1432929100
57. Loebinger MR, Janes SM. Stem cells as vectors for antitumour therapy. Thorax (2010) 65:362–9. doi: 10.1136/thx.2009.128025
58. Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. (2001) 134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015
59. Michaelson JE, Aguayo SM, Roman J. Idiopathic pulmonary fibrosis: a practical approach for diagnosis and management. Chest (2000) 118:788–94. doi: 10.1378/chest.118.3.788
60. Karampitsakos T, Tzouvelekis A, Chrysikos S, Bouros D, Tsangaris I, Fares WH. Pulmonary hypertension in patients with interstitial lung disease. Pulm Pharmacol Ther. (2018) 50:38–46. doi: 10.1016/j.pupt.2018.03.002
61. Papaioannou O, Karampitsakos T, Barbayianni I, Chrysikos S, Xylourgidis N, Tzilas V, et al. Metabolic disorders in chronic lung diseases. Front Med. (2017) 4:246. doi: 10.3389/fmed.2017.00246
62. Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev. (2015) 24:102–14. doi: 10.1183/09059180.00003214
63. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. (2014) 9:157–79. doi: 10.1146/annurev-pathol-012513-104706
64. Reddy M, Fonseca L, Gowda S, Chougule B, Hari A, Totey S. Human adipose-derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: comparison with pirfenidone. Int J Stem Cells (2016) 9:192–206. doi: 10.15283/ijsc16041
65. Toonkel RL, Hare JM, Matthay MA, Glassberg MK. Mesenchymal stem cells and idiopathic pulmonary fibrosis. Potential for clinical testing. Am J Respir Crit Care Med. (2013) 188:133–40. doi: 10.1164/rccm.201207-1204PP
66. Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. (2016) 22:1285–93. doi: 10.1038/nm.4192
67. Allen RJ, Porte J, Braybrooke R, Flores C, Fingerlin TE, Oldham JM, et al. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med. (2017) 5:869–80. doi: 10.1016/S2213-2600(17)30387-9
68. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. (2007) 179:1855–63. doi: 10.4049/jimmunol.179.3.1855
69. Srour N, Thebaud B. Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: a systematic review. Stem Cells Transl Med. (2015) 4:1500–10. doi: 10.5966/sctm.2015-0121
70. Gazdhar A, Susuri N, Hostettler K, Gugger M, Knudsen L, Roth M, et al. HGF expressing stem cells in usual interstitial pneumonia originate from the bone marrow and are antifibrotic. PLoS ONE (2013) 8:e65453. doi: 10.1371/journal.pone.0065453
71. Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. (2009) 175:303–13. doi: 10.2353/ajpath.2009.080629
72. Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G, et al. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE (2009) 4:e8013. doi: 10.1371/journal.pone.0008013
73. Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D, et al. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant (2009) 18:405–22. doi: 10.3727/096368909788809857
74. Ono M, Ohkouchi S, Kanehira M, Tode N, Kobayashi M, Ebina M, et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol Ther. (2015) 23:549–60. doi: 10.1038/mt.2014.217
75. Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc. (2008) 40:1700–5. doi: 10.1016/j.transproceed.2008.01.080
76. Lee SH, Lee EJ, Lee SY, Kim JH, Shim JJ, Shin C, et al. The effect of adipose stem cell therapy on pulmonary fibrosis induced by repetitive intratracheal bleomycin in mice. Exp Lung Res. (2014) 40:117–25. doi: 10.3109/01902148.2014.881930
77. Garcia O, Carraro G, Turcatel G, Hall M, Sedrakyan S, Roche T, et al. Amniotic fluid stem cells inhibit the progression of bleomycin-induced pulmonary fibrosis via CCL2 modulation in bronchoalveolar lavage. PLoS ONE (2013) 8:e71679. doi: 10.1371/journal.pone.0071679
78. Huang K, Kang X, Wang X, Wu S, Xiao J, Li Z, et al. Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Mol Med Rep. (2015) 11:1685–92. doi: 10.3892/mmr.2014.2981
79. Jun D, Garat C, West J, Thorn N, Chow K, Cleaver T, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells (2011) 29:725–35. doi: 10.1002/stem.604
80. Kumamoto M, Nishiwaki T, Matsuo N, Kimura H, Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J. (2009) 34:740–48. doi: 10.1183/09031936.00128508
81. Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S, et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. (2010) 11:16. doi: 10.1186/1465-9921-11-16
82. Perez JR, Lee S, Ybarra N, Maria O, Serban M, Jeyaseelan K, et al. A comparative analysis of longitudinal computed tomography and histopathology for evaluating the potential of mesenchymal stem cells in mitigating radiation-induced pulmonary fibrosis. Sci Rep. (2017) 7:9056. doi: 10.1038/s41598-017-09021-7
83. Min F, Gao F, Li Q, Liu Z. Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep. (2015) 11:2387–96. doi: 10.3892/mmr.2014.3025
84. Moodley Y, Vaghjiani V, Chan J, Baltic S, Ryan M, Tchongue J, et al. Anti-inflammatory effects of adult stem cells in sustained lung injury: a comparative study. PLoS ONE (2013) 8:e69299. doi: 10.1371/journal.pone.0069299
85. Gao F, Li Q, Hou L, Li Z, Min F, Liu Z. Mesenchymal stem cell-based angiotensin-converting enzyme 2 in treatment of acute lung injury rat induced by bleomycin. Exp Lung Res. (2014) 40:392–403. doi: 10.3109/01902148.2014.938200
86. Min JH, Lee HY, Lim H, Ahn MJ, Park K, Chung MP, et al. Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non-small cell lung cancer: a review on current insight. Cancer Chemother Pharmacol. (2011) 68:1099–109. doi: 10.1007/s00280-011-1737-2
87. Li F, Han F, Li H, Zhang J, Qiao X, Shi J, et al. Human placental mesenchymal stem cells of fetal origins-alleviated inflammation and fibrosis by attenuating MyD88 signaling in bleomycin-induced pulmonary fibrosis mice. Mol Immunol. (2017) 90:11–21. doi: 10.1016/j.molimm.2017.06.032
88. Ni S, Wang D, Qiu X, Pang L, Song Z, Guo K. Bone marrow mesenchymal stem cells protect against bleomycin-induced pulmonary fibrosis in rat by activating Nrf2 signaling. Int J Clin Exp Pathol. (2015) 8:7752–61.
89. Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH, et al. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. (2015) 6:97. doi: 10.1186/s13287-015-0081-6
90. Hostettler KE, Gazdhar A, Khan P, Savic S, Tamo L, Lardinois D, et al. Multipotent mesenchymal stem cells in lung fibrosis. PLoS ONE (2017) 12:e0181946. doi: 10.1371/journal.pone.0181946
91. Tashiro J, Rubio GA, Limper AH, Williams K, Elliot SJ, Ninou I, et al. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front Med. (2017) 4:118. doi: 10.3389/fmed.2017.00118
92. Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. (2007) 104:11002–7. doi: 10.1073/pnas.0704421104
93. Foskett AM, Bazhanov N, Ti X, Tiblow A, Bartosh TJ, Prockop DJ. Phase-directed therapy: TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am J Physiol Lung Cell Mol Physiol. (2014) 306:L120–31. doi: 10.1152/ajplung.00240.2013
94. Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. (2014) 6:231ra247. doi: 10.1126/scitranslmed.3008182
95. Lan YW, Theng SM, Huang TT, Choo KB, Chen CM, Kuo HP, et al. Oncostatin M-preconditioned mesenchymal stem cells alleviate bleomycin-induced pulmonary fibrosis through paracrine effects of the hepatocyte growth factor. Stem Cells Transl Med. (2017) 6:1006–17. doi: 10.5966/sctm.2016-0054
96. Germano D, Blyszczuk P, Valaperti A, Kania G, Dirnhofer S, Landmesser U, et al. Prominin-1/CD133+ lung epithelial progenitors protect from bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med. (2009) 179:939–49. doi: 10.1164/rccm.200809-1390OC
97. Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. (2014) 33:994–1010. doi: 10.1002/embj.201386030
98. Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. (2017) 196:1275–86. doi: 10.1164/rccm.201701-0170OC
99. Ntolios P, Janes SM. Mesenchymal stem cell therapy for lung diseases: oasis or mirage? Respiration (2013) 85:279–280. doi: 10.1159/000346642
100. Tzouvelekis A, Bouros D. Steep barriers to overcome for successful application of stem cell treatment in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2013) 188:251–2. doi: 10.1164/rccm.201301-0072LE
101. Alvarez D, Levine M, Rojas M. Regenerative medicine in the treatment of idiopathic pulmonary fibrosis: current position. Stem Cells Cloning (2015) 8:61–65. doi: 10.2147/SCCAA.S49801
102. Barczyk M, Schmidt M, Mattoli S. Stem cell-based therapy in idiopathic pulmonary fibrosis. Stem Cell Rev. (2015) 11:598–620. doi: 10.1007/s12015-015-9587-7
103. Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. (2013) 11:171. doi: 10.1186/1479-5876-11-171
104. Tzouvelekis A, Ntolios P, Bouros D. Stem cell treatment for chronic lung diseases. Respiration (2013) 85:179–192. doi: 10.1159/000346525
105. Glassberg MK, Minkiewicz J, Toonkel RL, Simonet ES, Rubio GA, DiFede D, et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): a phase I safety clinical trial. Chest (2017) 151:971–81. doi: 10.1016/j.chest.2016.10.061
106. Glassberg MK, Toonkel RL. Moving stem cell therapy to patients with idiopathic pulmonary fibrosis. Respirology (2014) 19:950–51. doi: 10.1111/resp.12364
107. Limper AH. Safety of IV human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis. Chest (2017) 151:951–2. doi: 10.1016/j.chest.2016.12.015
108. Glassberg MK, Hare JM, Toonkel RL, Matthay MA. Reply: idiopathic pulmonary fibrosis: a degenerative disease requiring a regenerative approach. Am J Respir Crit Care Med. (2013) 188:253–4. doi: 10.1164/rccm.201302-0244LE
109. Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology (2014) 19:1013–8. doi: 10.1111/resp.12343
110. Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest (2013) 143:1590–8. doi: 10.1378/chest.12-2094
111. Tzouvelekis A, Laurent G, Bouros D. Stem cell therapy in chronic obstructive pulmonary disease. Seeking the Prometheus effect. Curr Drug Targets (2013) 14:246–52. doi: 10.2174/1389450111314020009
112. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA (2012) 308:2369–79. doi: 10.1001/jama.2012.25321
113. Ntolios P, Manoloudi E, Tzouvelekis A, Bouros E, Steiropoulos P, Anevlavis S, et al. Longitudinal outcomes of patients enrolled in a phase Ib clinical trial of the adipose-derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Clin Respir J. (2018). doi: 10.1111/crj.12777. [Epub ahead of print].
114. Tzouvelekis A, Koliakos G, Ntolios P, Baira I, Bouros E, Oikonomou A, et al. Stem cell therapy for idiopathic pulmonary fibrosis: a protocol proposal. J Transl Med. (2011) 9:182. doi: 10.1186/1479-5876-9-182
115. Raghu G, Collard HR, Anstrom KJ, Flaherty KR, Fleming TR, King TE, et al. Idiopathic pulmonary fibrosis: clinically meaningful primary endpoints in phase 3 clinical trials. Am J Respir Crit Care Med (2012) 185:1044–1048. doi: 10.1164/rccm.201201-0006PP
116. Yang T, Jia Y, Ma Y, Cao L, Chen X, Qiao B. Comparative proteomic analysis of bleomycin-induced pulmonary fibrosis based on isobaric tag for quantitation. Am J Med Sci. (2017) 353:49–58. doi: 10.1016/j.amjms.2016.11.021
117. Jenkins RG, Moore BB, Chambers RC, Eickelberg O, Königshoff M, Kolb M, et al. An Official American Thoracic Society Workshop Report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol. (2017) 56:667–79. doi: 10.1165/rcmb.2017-0096ST
118. Tashiro J, Elliot SJ, Gerth DJ, Xia X, Pereira-Simon S, Choi R, et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl Res. (2015) 166:554–567. doi: 10.1016/j.trsl.2015.09.004
119. du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2011) 184:459–66. doi: 10.1164/rccm.201011-1790OC
120. Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. (2003) 168:543–8. doi: 10.1164/rccm.200209-1112OC
121. Zappala CJ, Latsi PI, Nicholson AG, Colby TV, Cramer D, Renzoni EA, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. (2010) 35:830–6. doi: 10.1183/09031936.00155108
122. Collard HR, King TE, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2003) 168:538–42. doi: 10.1164/rccm.200211-1311OC
123. du Bois RM, Nathan SD, Richeldi L, Schwarz MI, Noble PW. Idiopathic pulmonary fibrosis: lung function is a clinically meaningful endpoint for phase III trials. Am J Respir Crit Care Med. (2012) 186:712–5. doi: 10.1164/rccm.201206-1010PP
124. Nathan SD, du Bois RM, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Validation of test performance characteristics and minimal clinically important difference of the 6-minute walk test in patients with idiopathic pulmonary fibrosis. Respir Med. (2015) 109:914–22. doi: 10.1016/j.rmed.2015.04.008
125. du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. (2011) 183:1231–7. doi: 10.1164/rccm.201007-1179OC
126. Heresi GA, Dweik RA. Strengths and limitations of the six-minute-walk test: a model biomarker study in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2011) 183:1122–4. doi: 10.1164/rccm.201012-2079ED
127. Richeldi L, Ryerson CJ, Lee JS, Wolters PJ, Koth LL, Ley B, et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax (2012) 67:407–11. doi: 10.1136/thoraxjnl-2011-201184
128. du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. (2011) 184:1382–9. doi: 10.1164/rccm.201105-0840OC
129. Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. (2015) 45:1374–81. doi: 10.1183/09031936.00146314
130. Ikonomou L, Panoskaltsis-Mortari A, Wagner DE, Freishtat RJ, Weiss DJ Unproven stem cell treatments for lung disease-an emerging public health problem. Am J Respir Crit Care Med. (2017) 195:P13–4. doi: 10.1164/rccm.201607-1461ED
131. Turner L, Knoepfler P. Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry. Cell Stem Cell (2016) 19:154–7. doi: 10.1016/j.stem.2016.06.007
Keywords: idiopathic pulmonary fibrosis, mesenchymal stem cells, treatment, safety, efficacy
Citation: Tzouvelekis A, Toonkel R, Karampitsakos T, Medapalli K, Ninou I, Aidinis V, Bouros D and Glassberg MK (2018) Mesenchymal Stem Cells for the Treatment of Idiopathic Pulmonary Fibrosis. Front. Med. 5:142. doi: 10.3389/fmed.2018.00142
Received: 01 February 2018; Accepted: 25 April 2018;
Published: 15 May 2018.
Edited by:
Marco Confalonieri, University of Trieste, ItalyReviewed by:
Michael Adam O'Reilly, University of Rochester, United StatesVenerino Poletti, Aarhus University Hospital, Denmark
Copyright © 2018 Tzouvelekis, Toonkel, Karampitsakos, Medapalli, Ninou, Aidinis, Bouros and Glassberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marilyn K. Glassberg, bWdsYXNzYmVAbWVkLm1pYW1pLmVkdQ==
†These authors have contributed equally to this work.
 Argyrios Tzouvelekis
Argyrios Tzouvelekis Rebecca Toonkel
Rebecca Toonkel Theodoros Karampitsakos
Theodoros Karampitsakos Kantha Medapalli3,4
Kantha Medapalli3,4 Marilyn K. Glassberg
Marilyn K. Glassberg