Abstract
Background:
The need for a new style of clinical trials, called decentralized clinical trials (DCTs), has been increasing as they do not depend on physical visits to clinical sites. DCTs are expected to provide a new opportunity to patients who cannot participate in a clinical trial due to geographical and time limitations. For the adoption of DCTs, it is essential that medical devices with Internet of Medical Things (IoMT) and Internet of Health Things (IoHT) based technologies are developed and commercially adopted. In this study, we aimed to identify the regulatory considerations when IoMT/IoHT-based technologies are used in DCTs or products developed using DCTs.
Method:
To understand the study and development field of IoMT/IoHT comprehensively and panoramically, relevant papers published in Web of Science were searched online. Subsequently, a citation network was obtained and characterized as a cluster using a text mining method to identify IoMT/IoHT-based technologies expected to be utilized in DCTs or products developed using DCTs.
Result and Discussion:
Upon analysis of the top 15 clusters and subsequent 51 sub-clusters, we identified the therapeutic areas (psychology, neurology) and IoMT/IoHT-based technologies (telemedicine, remote monitoring, and virtual reality) that are expected to be used in DCTs. We also identified several considerations based on the current regulatory guidance.
Conclusion:
IoMT/IoHT-based technologies that are expected to be used or products developed using DCTs and key considerations made when they are used in DCTs were identified. The considerations could encourage conducting DCTs using IoMT/IoHT-based technologies.
Introduction
In the wake of the COVID-19 pandemic, there has been increasing interest in decentralized clinical trials (DCTs) that do not rely on visits to clinical sites to conduct clinical trials. There are two types of DCT-style studies. In one DCT-style, visits to clinical sites can be avoided altogether as several components can be implemented remotely, such as remote electronic informed consent and telemedicine. In the other style (hybrid style), not all study visits are decentralized; certain study visits need to be conducted at the clinical site as usual. For pharmaceutical companies, DCTs reduce subjects' inclusion period and the study population's bias by obtaining study participants from across the globe (1). Prior to the COVID-19 pandemic, DCTs were reported mainly in the United States (US). DCTs are expected to provide new opportunities for participation in clinical trials for patients who have been unable to participate owing to difficulties in accessing clinical sites. They are also expected to reduce the time consumed at the clinical site. According to a patient survey conducted by the Center for Information and Study on Clinical Research Participation (CISCRP), ~60% of patients consider the location of the clinical site and the duration of participation in clinical trials as important factors for study participation (2). Therefore, the location of the clinical site and the duration of the clinical trial are major concerns for patients and, hence, there is room for improvement for study sponsors in these areas. Pharmaceutical companies have been using DCTs to promote patient-centered activities in drug development even before the COVID-19 pandemic.
The advancement of information technology has played a key role in improving the implementation of DCTs. Thus, the adoption of Internet of Things (IoT) in the medical field, in the form of Internet of Medical Things (IoMT) and Internet of Health Things (IoHT), through concepts such as telemedicine, ePRO, and data collection by wearable devices, will enable further dissemination and promotion of DCTs. It is also expected that new IoMT/IoHT-based technologies will be developed while using the components of DCTs to be authorized as qualified medical devices by health authorities such as the FDA. However, considering that the field of IoMT/IoHT has been changing rapidly and the new IoMT/IoHT has been developing constantly, it is difficult to proactively identify the suitable IoMT/IoHT to utilize in DCT and the points to notice when the study sponsor wants to implement the IoMT/IoHT in DCTs.
Therefore, first, we aimed to identify IoMT/IoHT-based technologies that are expected to be utilized in DCTs or products developed using DCTs by using horizon scanning based on bibliographic citation network analysis and text mining. Horizon scanning is a major topic in the strategic initiatives of the International Coalition of Medicines Regulatory Authorities (ICMRA), which is a voluntary coalition of medicines regulatory authorities. According to the concept notes of ICMRA Strategic Priority on Innovation, horizon scanning is broad-reaching information-gathering and monitoring activities to forecast impending products and technologies and potentially disruptive research methods (3).
There are two main types of methods for collecting the data required to conduct well-programmed horizon scanning (4): expert-based and computer-based approaches. Conventional horizon scanning methods have been used in Europe to investigate the Internet, governments, international organizations, companies, databases, and journals using the Delphi method (5, 6). This type of expert-based approach is difficult to implement in the current era of information overload. Computer-based approaches can collect and analyze huge amounts of information and data such as articles, patents, and newspapers. Hines et al. (6) reported that horizon scanning methods are still conducted manually or semi-manually, especially in the medical and healthcare fields, and it is difficult to understand the whole picture of research and technological development because the research results of medical and healthcare fields are extremely large and fragmented. The computer-based approach can supplement the expert-based approach because it is compatible with the scale of the information (7, 8). Two types of computer-based approaches exist: citation and text mining. The citation-based approach, used in this study, assumes that the papers on which a given paper is based are similar to the papers it cites. Thus, it is possible to recognize the whole structure of research areas that comprise a vast volume of papers by analyzing this citation network. Methods based on this approach have been widely used as powerful tools to visualize and understand the structure of research fields and identify new trends and research landscape, and their validity has been identified in various studies (9–11). For example, Kajikawa et al. (12) used citation network analysis to track emerging study areas effectively and efficiently in the field of sustainable science. Similar approaches have been applied in several fields, including energy research (13), regenerative medicine (14), robotics, and gerontology (15). However, it is difficult to correctly understand the semantics of clusters based only on citation relations. Text mining can clarify subject relationships across citations and provide discernment in the dissemination of knowledge in academic research and development. However, it is highly sensitive to certain terms; when text mining is used with other methods, the problem of terminological distortion will be unignorable. Therefore, we propose an objective methodology for horizon scanning that identifies cutting edge technology to be applied to medical products, which are AI-based medical devices, drugs related to T cell immune responses, and extracellular vesicle-based products from all research papers in the target field using both citation network analysis methods and text mining (16–19).
This study aimed to explore novel IoMT/IoHT-based technologies that may be applied in future DCTs using citation network analysis and text mining, both of which are foresight methods used for discovering early signs of emerging products and technologies. In addition, based on the results of citation network analysis and text mining, we aimed to examine the prospects for the utilization of IoMT/IoHT in DCTs and the issues and key considerations that can be assumed to be focused on operational and regulatory perspectives while developing novel IoMT/IoHT-based technologies to contribute to the popularization of IoMT/IoHT in DCTs.
Method
Extraction of Paper Data for Analysis
We utilized (“remote” or “virtual” or “Internet” or “wearable” or “sensor”) and (“clinical” or “device”) as queries for analyzing papers published in the Web of Science literature database (WoS, Thomson Reuters) to identify IoMT/IoHT-based technologies that are expected to be used in DCTs and products developed using DCTs.
Citation Network Analysis
Figure 1 gives an outline of the citation network analysis process which has been published in references (16, 17, 20). We converted the citation network into an unweighted network, with papers as nodes and citation relationships as links. Papers with no citations as the largest component were considered digressional and excluded from this study (Step 2 in Figure 1). Papers that have no citation relationships with other papers were considered deviant and were excluded from this study. Subsequently, the network was divided into several clusters using the topological clustering method (Step 3 in Figure 1). As a clustering method, topological clustering based on the graph structure of a network is used, and modularity maximization is applied in this study. A cluster module in a citation network is a group of papers in which citation relations are divided using a modularity (Q value) maximization method and are densely aggregated (Louvain method) (20, 21). Q is an evaluation function of the degree of coupling within a cluster and between clusters, as shown in the following equation.
where Aij represents the weight of the edge between i and j, is the sum of the weights of the edges attached to vertex i, ciis the community to which vertex i is assigned, δ-function δ(u, v) is 1 if u=v and 0 otherwise, and .
Figure 1

Steps of clustering and making academic landscape.
Labels corresponding to the size of the number of papers included were assigned in the clusters. The characteristics of each cluster were confirmed by extracting a summary of frequently cited academic papers and characteristic keywords in the cluster.
Furthermore, we employed term frequency-inverse cluster frequency (TF-ICF) to extract the distinctive keywords of each cluster. TF is a measure of the significance of a term in a particular sentence, whereas ICF is a measure of the general importance of a term. The TC-ICF of a given term i in a given cluster j, is given by
where N is the total number of sentences. Each cluster was labeled based on the resulting keywords and sentences.
The visualization was converted to assume the relationships among these clusters after clustering the network and a large graph layout (LGL) based on a force-direct layout algorithm was used (22, 23). This layout indicated the largest connected component of the network to generate coordinates for nodes in two dimensions. We visualized the citation network by expressing inter-cluster links in the same color (Step 4 in Figure 1). The hub paper, which has the highest number of citations, is located at the center of the citation relations. However, there was no relation of approximation of the content between the position of the clusters and the distance.
Regulatory Information
Regulatory information for FDA (24), EMA (25), MHLW (26), and PMDA (27) was retrieved from their respective websites.
Result
Results of Citation Network Analysis
We analyzed 144,977 papers obtained from the WoS database and discovered that 97,305 papers (67.1%) formed a citation network by extracting the largest connected component from all linkage components through direct citation of papers, and divided it into 141 clusters. Table 1 shows the information of the top 15 clusters (average year, number of papers, TF-ICF, and hub papers in the cluster). Figure 2 shows that the top 15 clusters used for subsequent analysis covered ~90% of the papers in the citation network. The topic of each cluster was assumed in accordance with characteristic keywords with high TF-ICF and the titles and contents of several papers that were most cited within the cluster. For example, cluster 4 was considered to be a group of papers regarding activity-tracking sensors based on the top keywords of the cluster (gait, sleep, and activity). Cluster 6 was categorized as virtual reality in the medical field because the top keywords in the cluster include virtual reality, rehabilitation, and some papers that were cited within the cluster. Cluster 7 was considered a treatment and clinical trial through the Internet with top keywords such as intervention, Internet, trial, and treatment. Cluster 10 was assumed to be associated with remote monitoring mainly focused on telemedicine by referring to the top keywords of the cluster such as telemedicine and the abstract of hub paper of this cluster.
Table 1
| Cluster no. | Average year | Number of papers | Top keywords (TF-ICF) | Hub paper title |
|---|---|---|---|---|
| Cluster 1 | 2017.6 | 14134 | Iot, thing, network, internet, security, wireless, cloud, smart, computing, authentication | Internet of Things (IoT): A vision, architectural elements, and future directions |
| Cluster 2 | 2012.3 | 9277 | Gas, gas sensor, film, sensor, zno, sensing, saw, no2, graphene, gas sensing | Carbon nanotube sensors for gas and organic vapor detection |
| Cluster 3 | 2015.0 | 8559 | Electrochemical, electrode, glucose, biosensor, detection, nanoparticles, sensor, biosensors, graphene, microfluidic | Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis |
| Cluster 4 | 2016.6 | 7627 | Wearable, gait, physical activity, patient, sleep, walking, activity, wearable device, accelerometer, inertial | A review of wearable sensors and systems with application in rehabilitation |
| Cluster 5 | 2017.4 | 6595 | Stretchable, flexible, strain, strain sensor, wearable, graphene, electronics, film, pressure, hydrogel | A stretchable carbon nanotube strain sensor for human-motion detection |
| Cluster 6 | 2014.8 | 5540 | Virtual reality, reality, rehabilitation, haptic, virtual, exoskeleton, stroke, patient, limb, motor | Virtual environments for motor rehabilitation: Review |
| Cluster 7 | 2013.9 | 5438 | Health, patient, intervention, depression, anxiety, internet, mental health, trial, clinical, treatment | Computer-based psychological treatments for depression: A systematic review and meta-analysis |
| Cluster 8 | 2013.5 | 5204 | Fiber, optical, refractive index, refractive, grating, waveguide, plasmon, optical fiber, sensor, surface plasmon | Ultrasensitive photonic crystal fiber refractive index sensor |
| Cluster 9 | 2013.5 | 4502 | Student, surgical, skill, surgery, virtual, reality, patient, education, training, virtual reality | Virtual patients: a critical literature review and proposed next steps |
| Cluster 10 | 2013.3 | 4071 | Patient, telemedicine, care, heart, defibrillator, heart failure, health, consultation, cardioverter, clinical | Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) trial |
| Cluster 11 | 2016.8 | 3430 | Triboelectric, nanogenerator, teng, harvesting, energy harvesting, piezoelectric, energy, triboelectric nanogenerator, harvester, energy harvester | Transparent triboelectric nanogenerators and self-powered pressure sensors based on micropatterned plastic films |
| Cluster 12 | 2018.2 | 2624 | Supercapacitors, flexible, supercapacitor, energy storage, graphene, electrode, wearable, carbon, battery, fiber | Coaxial wet-spun yarn supercapacitors for high-energy density and safe wearable electronics |
| Cluster 13 | 2011.9 | 2409 | Mem, sensor, flow, microfluidic, pressure, silicon, resonator, thermal, film, temperature | Micromachined inertial sensors |
| Cluster 14 | 2011.5 | 2342 | Magnetic, squid, transition edge, transition edge sensor, magnetic field, superconducting, hall, edge sensor, sensor, magnetometer | Transition-edge sensors |
| Cluster 15 | 2013.3 | 2073 | Transistor, field effect, effect transistor, field effect transistor, fet, graphene, gate, biosensor, dna, electrochemical | Multiplexed electrical detection of cancer markers with nanowire sensor arrays |
Information of top 15 clusters for IoM/IoHT.
Figure 2
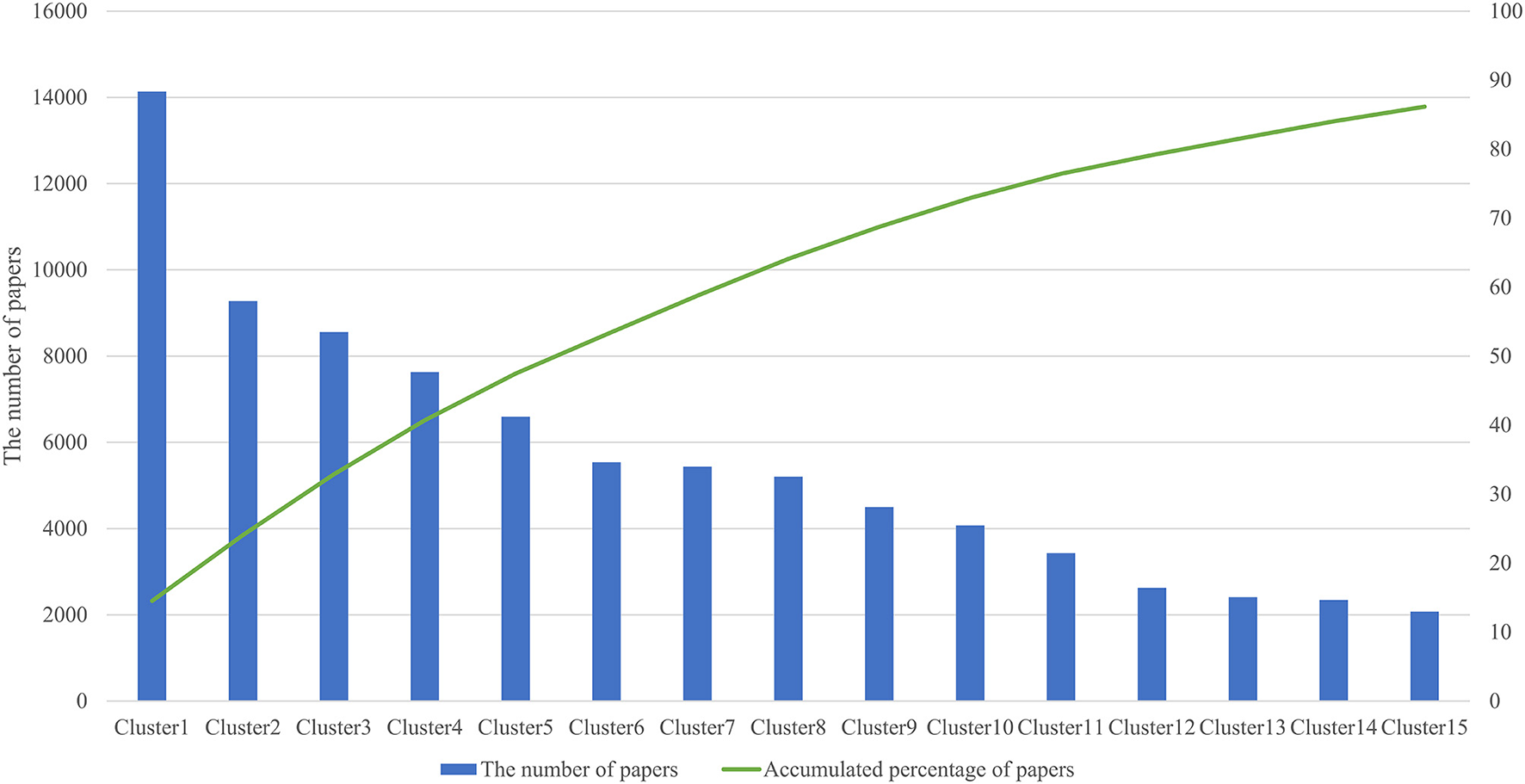
Top 15 cluster size and accumulated percentage.
Characterization of Sub-clusters
Among the top 15 clusters listed in Table 1, we reanalyzed the clusters and distinguished them as sub-clusters that are closely related to the medical and healthcare fields and DCTs. The results are shown in Table 2; 51 sub-clusters are identified. We focused on each sub-cluster in Table 2 to identify the cutting-edge medical devices, technologies, and methodologies that are expected to be used in DCTs or are expected to be developed by DCTs. First, we summarized each sub-cluster as a “summary of sub-cluster” based on keywords, titles, and contents of top-cited papers in the cluster. Subsequently, we identified an example of the device/feature for each sub-cluster. Based on the device/feature example, we identified the therapeutic area and use-case based on information from the sub-cluster summary and the device/feature examples in the sub-cluster. For the therapeutic area in Table 2, we categorized the sub-cluster as “general” when it was not suitable to specify one therapeutic area and/or the device/feature example that may be generally used in the medical field. For the use-case in Table 2, telehealth includes telemedicine, a therapy using IT, such as a digital app for smartphones. Remote monitoring is included in device/feature, which is used for detecting various health information remotely. Table 3 presents the number of therapeutic areas categorized from each sub-cluster. From the results of the characterizations of 51 sub-clusters, 10 are for psychology (20%) and 9 are for orthopedics (18%), which are the main therapeutic areas for utilization of the identified device/feature example from each sub-cluster other than general. For the application of the identified device/feature examples from each sub-cluster, the most common items are telehealth (33%), remote monitoring (27%), rehabilitation (12%), and activity tracking/VR (10%), as shown in Table 4.
Table 2
| Cluster no. | Average year | Number of papers | Top keywords (TF-ICF) | Summary of sub-cluster | Device/feature example | Therapeutic area | Use-case |
|---|---|---|---|---|---|---|---|
| Cluster 1–4 | 2017.7 | 1,447 | IoT, thing, healthcare, health, cloud, internet, smart, wearable, wireless, network | Issue of IoT for healthcare | Various IoHT-based technologies | General | Remote monitoring |
| Cluster 2–8 | 2013.9 | 472 | Breath, nose, exhaled, electronic nose, volatile, exhaled breath, volatile organic, volatile organic compound, gas, organic compound | Evaluation of sensor for a disorder screening | Novel wearable sensor detecting nanomaterials and volatile organic compounds | General | Clinical chemistry |
| Cluster 3–3 | 2018 | 830 | Sweat, wearable, glucose, electrochemical, biosensors, electrode, flexible, biosensor, sensor, lactate | Evaluation of wearable sweat sensor | Wearable sensor for sweat analysis | General | Remote monitoring |
| Cluster 3–4 | 2017.2 | 698 | Electrochemical, glucose, electrode, graphene, nanoparticles, carbon, h2o2, electrochemical sensor, voltammetry, biosensor | Evaluation of non-enzymic glucose sensor | Non-enzymatic wearable sensor for diabetes | Endocrinology | Remote monitoring |
| Cluster 3–5 | 2016.1 | 588 | Wearable, ECG, health, patient, textile, wearable device, QRS, monitoring, heart, wireless | Consideration of wearable monitoring system | Various wearable devices for health monitoring | General | Remote monitoring |
| Cluster 4–1 | 2017.8 | 934 | Physical activity, sleep, wearable, activity, fitbit, intervention, health, tracker, activity tracker, physical | Credibility of wearable activity tracker | Activity tracker | General | Activity tracking |
| Cluster 4–2 | 2017.2 | 880 | PPG, heart rate, heart, ECG, wearable, photoplethysmography, pulse, atrial fibrillation, atrial, wearable device | Accuracy of smart watch with heart rate monitor | Smart watch with HR monitor | General | Remote monitoring |
| Cluster 4–3 | 2016 | 855 | Gait, walking, inertial, gait analysis, wearable, foot, inertial sensor, knee, IMU, gait parameter | Gait analysis with wearable device | Activity tracker | Orthopedics | Activity tracking |
| Cluster 4–4 | 2017.1 | 604 | Fall, fall detection, activity recognition, wearable, activity, recognition, accelerometer, human activity, human activity recognition, learning | Analyze of fall detection with wearable device | Activity tracker | Orthopedics | Activity tracking |
| Cluster 4–5 | 2017.3 | 590 | Parkinson, gait, tremor, UPDRS, disease, wearable, patient, bradykinesia, motor, dyskinesia | Activity monitoring using wearable sensor for Parkinson's disease patient | Wearable device for Parkinson's disease | Neurology | Activity tracking |
| Cluster 4–6 | 2016.8 | 546 | Wearable, intention, wearable device, acceptance, mobile, perceived, learning, adoption, consumer, technology acceptance | Acceptance of wearable technology for health monitoring | Various wearable device for health monitoring | General | Remote monitoring |
| Cluster 4–7 | 2016.2 | 416 | Wearable, inertial, rehabilitation, motion, movement, limb, upper limb, exercise, shoulder, stroke | Wearable sensor and system for rehabilitation | Wearable sensor for rehabilitation | Orthopedics | Rehabilitation |
| Cluster 4–8 | 2017.2 | 359 | Patient, sleep, radar, vital sign, respiratory, wearable, temperature, COPD, breathing, heart rate | Evaluation of continued monitoring method for vital sign with wearable device | Wearable device with pulse meter | General | Remote monitoring |
| Cluster 4–10 | 2014.9 | 257 | Fall risk, fall, gait, older, faller, older adult, frailty, sit, accelerometer, walking | Assessing of fall risk in the elderly using sensors | Wearable inertial sensor | Orthopedics | Activity tracking |
| Cluster 4–11 | 2015.8 | 240 | Home, older adult, older, dementia, smart home, smart, cognitive, adult, health, living | Methodology for continued health data collection | Various IoHT-based technologies | General | Remote monitoring |
| Cluster 4–12 | 2015.6 | 238 | Wearable, intake, sensecam, physical activity, food, wearable camera, eating, swallowing, dietary, sedentary | Non-invasive monitoring and evaluation of chewing and swallowing | Wearable ingestion monitor | Psychology | Remote monitoring |
| Cluster 4–14 | 2012.8 | 226 | Sleep, apnea, sleep apnea, ahi, polysomnography, PSG, PTCCO, hypopnea, oximetry, transcutaneous | Monitoring of a patient with sleep apnea syndrome | Wearable sensor for OSA | Psychology | Remote monitoring |
| Cluster4–15 | 2018.4 | 176 | Seizure, EEG, epilepsy, seizure detection, wearable, clonic, ear EEG, ear, clonic seizure, sudep | Evaluation of wearable detector sensor for seizure | Wearable sensor for seizure detection | Neurology | Remote monitoring |
| Cluster 6–1 | 2016.2 | 897 | Rehabilitation, stroke, virtual reality, reality, game, motor, balance, upper limb, training, wii | Efficacy of VR therapy for rehabilitation for a stroke | VR | Neurology | VR therapy |
| Cluster 6–2 | 2013.3 | 801 | Haptic, force, haptic device, tactile, feedback, haptic interface, force feedback, virtual, robot, haptics | Evaluation of wearable haptic device | Wearable haptic devices | Orthopedics | Rehabilitation |
| Cluster 6–3 | 2014.3 | 604 | Virtual reality, reality, anxiety, exposure therapy, phobia, disorder, exposure, virtual, fear, VRET | Evaluation of VR exposure therapy for anxiety | VR | Psychology | VR therapy |
| Cluster 6–5 | 2016.9 | 389 | Exoskeleton, walking, gait, robot, limb, wearable, ankle, rehabilitation, lower limb, muscle | Control regulation of lower limb exoskeleton | lower extremity exoskeleton | Orthopedics | Rehabilitation |
| Cluster 6–6 | 2015.9 | 341 | Pain, virtual reality, reality, distraction, anxiety, patient, virtual, burn, phantom limb, child | Intervention for pain and burn pain therapy using VR | VR | Neurology | VR therapy |
| Cluster 6–7 | 2016.6 | 320 | Exoskeleton, rehabilitation, hand exoskeleton, robot, limb, upper limb, robotic, tremor, wearable, hand | Design and validation of upper limb rehabilitation robot | Robotic prosthetic component | Orthopedics | Rehabilitation |
| Cluster 6–9 | 2013.4 | 224 | Telerehabilitation, rehabilitation, patient, telehealth, biofeedback, virtual reality, reality, telepractice, stroke, knee | Evaluation of telerehabilitation with VR | VR | Orthopedics | Rehabilitation |
| Cluster 6–10 | 2015.4 | 219 | Virtual reality, reality, cognitive, ADHD, executive, virtual, game, neuropsychological, executive function, concussion | Evaluation of attention deficit using VR | VR | Neurology | VR therapy |
| Cluster 6–11 | 2014 | 190 | Prosthesis, myoelectric, limb, EMG, prosthetic, muscle, amputee, mmg, hand, electromyography | Myoelectric control of a prosthetic hand | Robotic prosthetics | Orthopedics | Rehabilitation |
| Cluster 6–14 | 2013.7 | 106 | Paranoia, paranoid, delusion, persecutory, psychosis, persecutory delusion, social, schizophrenia, ideation, virtual reality | Efficacy of a psychotic disease therapy with VR | VR | Psychology | VR therapy |
| Cluster 7–1 | 2015.8 | 767 | Depression, iCBT, anxiety, treatment, disorder, CBT, intervention, cognitive, mental health, therapy | Depression therapy with internet | Apps for mental health | Psychology | Telehealth |
| Cluster 7–2 | 2012.9 | 566 | Health, patient, health information, literacy, internet, website, cancer, information, web, readability | Literacy of health information on the Internet | Various IoHT-based technologies | General | Telehealth |
| Cluster 7–3 | 2017.2 | 491 | Mental health, mental, health, depression, apps, intervention, disorder, mobile, mhealth, psychosis | Evaluation of intervention by mobile feature for depression disease | Apps for mental health | Psychology | Telehealth |
| Cluster 7–4 | 2014.1 | 408 | Smoking, intervention, cessation, smoking cessation, recruitment, trial, health, participant, alcohol, smoker | Intervention for internet basis smoking abstinence | Apps for smoking abstinence | Psychology | Telehealth |
| Cluster 7–6 | 2016 | 355 | Telemedicine, telepsychiatry, patient, telehealth, COVID, mental health, care, clinical, trial, videoconferencing | Study of telemedicine | Telemedicine | General | Telehealth |
| Cluster 7–7 | 2012.5 | 281 | Eating disorder, eating, psychotherapy, mental health, social, disorder, mental, CBT, internet, online | Telemedicine for mental healthcare | Telemedicine | Psychology | Telehealth |
| Cluster 7–9 | 2015.8 | 203 | Patient, cancer, symptom, decision aid, patient reported, reported outcome, patient reported outcome, outcome, TDCS, care | Evaluation of patient report outcome using internet or mobile app | BYOD ePRO | General | Remote monitoring |
| Cluster 7–10 | 2005.9 | 203 | Patient, clinical, decision support, web, medical, internet, wide web, health, world wide web, decision | Evaluation of Electronic Treatment Decision Making System | Telemedicine | General | Telehealth |
| Cluster 10–1 | 2014.8 | 533 | Defibrillator, remote monitoring, ICD, patient, cardioverter, cardioverter defibrillator, implantable, implantable cardioverter, implantable cardioverter defibrillator, pacemaker | Evaluation of Efficacy and Safety of Telemonitoring for Follow-up of Implantable Cardioverter Defibrillators | Implantable cardioverter-defibrillator | Cardiology | Life support |
| Cluster 10–2 | 2015.2 | 407 | Telemedicine, telehealth, patient, consultation, care, health, service, visit, COVID, clinical | Efficacy of telemedicine | Telemedicine | General | Telehealth |
| Cluster 10–4 | 2015.6 | 378 | Heart failure, heart, patient, failure, cardiomems, hospitalization, telemonitoring, remote monitoring, care, pulmonary artery | Remote monitoring for chronic cardiac failure patient | Wireless pulmonary artery hemodynamic monitoring | Cardiology | Remote monitoring |
| Cluster 10–6 | 2013.1 | 275 | Patient, diabetes, care, health, portal, patient portal, intervention, mail, provider, telemedicine | Evaluation of an Internet-based Blood Glucose Monitoring System | Telemedicine | Endocrinology | Remote monitoring |
| Cluster 10–8 | 2017.2 | 176 | Care, telemedicine, patient, prenatal care, visit, prenatal, pregnancy, telehealth, health, COVID | Telemedicine for infectious disease assessment | Telemedicine | Infectious Diseases | Telehealth |
| Cluster 10–9 | 2015.3 | 175 | Glaucoma, ROP, patient, retinopathy, ophthalmologist, optometrist, telemedicine, teleophthalmology, fracture clinic, ophthalmology | Telemedicine in ophthalmology | Telemedicine | Ophthalmology | Telehealth |
| Cluster 10–10 | 2012.9 | 167 | Stroke, telemedicine, telestroke, patient, acute stroke, acute, thrombolysis, stroke care, prehospital, care | Accuracy of telemedicine for the stroke | Telemedicine | Neurology | Telehealth |
| Cluster 10–11 | 2015.1 | 156 | Patient, telehealth, hypertension, telemedicine, care, telemonitoring, blood pressure, health, home, adherence | Non-invasive remote patient monitoring | Various wearable device for health monitoring | General | Telehealth |
| Cluster 10–13 | 2015.6 | 134 | WCD, wearable cardioverter, wearable cardioverter defibrillator, defibrillator, cardioverter, cardioverter defibrillator, ICD, ventricular, patient, sudden cardiac | Evaluation of a wearable defibrillator for arrhythmias with high risk of sudden death | Wearable cardioverter- defibrillator | Cardiology | Life support |
| Cluster 10–14 | 2013.3 | 134 | ICU, telemedicine, care, telepharmacy, tele ICU, patient, pharmacy, telehealth, pharmacist, tele | Telemedicine in ICU | Telemedicine | ICU | Telehealth |
| Cluster 13–4 | 2015.4 | 174 | Navigation, impaired, blind, impaired people, assistive, sensory substitution, obsacle, travel aid, people, cane | Wearable navigation system for the blind | Electronic travel aids (ETAs), electronic orientation aids (EOAs), and position locator devices (PLDs) | Ophthalmology | Digital health |
Summarization of sub-cluster focused on DCT-related IoMT/IoHT.
Table 3
| Therapeutic area | No. (%) | |
|---|---|---|
| Generala | 16 | (28) |
| Psychology | 10 | (20) |
| Orthopedics | 9 | (18) |
| Neurology | 6 | (12) |
| Cardiology | 3 | (6) |
| Ophthalmology | 2 | (4) |
| Endocrinology | 2 | (4) |
| Otorhinolaryngology | 1 | (2) |
| Infectious disease | 1 | (2) |
| ICU | 1 | (2) |
| Total | 51 |
Therapeutic area of identified device/feature examples from sub-cluster.
Represents general content that is not suitable to categorize in one specific therapeutic area.
Table 4
| Use-case | No. (%) | |
|---|---|---|
| Telehealtha | 17 | (29) |
| Remote monitoringb | 14 | (27) |
| Rehabilitation | 6 | (12) |
| Activity tracking | 5 | (10) |
| VR therapy | 5 | (10) |
| Life support | 2 | (4) |
| Clinical chemistry | 1 | (2) |
| Digital healthc | 1 | (2) |
| Total | 51 |
Use-case of identified device/feature examples from sub-clusters.
Includes telemedicine and therapy using IT, e.g., smartphone app.
A device or feature for detecting health information remotely.
An aggregated condition treated with novel technologies such as AI, VR, and 5G.
Regulatory Information for Utilization of IoHT/IoMT in Clinical Trails
Because regulatory matters might be an issue for the utilization of IoMT/IoHT in DCTs, we retrieved relevant regulatory information in the US, EU, and Japan. Table 5 lists the guidelines related to the utilization of IoMT/IoHTs identified in clinical trials. As of February 2022, only Switzerland [Decentralized Clinical Trials (DCTs) with Medicinal Products in Switzerland (30)] and Denmark [Danish Medicines Agency's guidance on the implementation of decentralized elements in clinical trials with medicinal products (31)], have developed guidelines related to DCT. They have included the consideration of how to utilize a relatively proven IoMT/IoHT-based technology, such as a wearable device, in DCTs. In Japan and the US, there are no DCT-specific guidelines. However, there are some guidelines and Q&A documents on the utilization of digital technology (32), such as telemedicine (33), in clinical trials as temporary measures during the COVID-19 pandemic (29, 34–38).
Table 5
| Category | Japan (Published year, reference) | US (Published year, reference) | EU (Published year, reference) |
|---|---|---|---|
| Tel6medicine and communication devices | MHLW (2019, 28) | FDA (2019, 32) | |
| MHLW (2020, 29) | FDA (2019, 33) | ||
| PMDA (2020, 30) | FDA (2019, 34) | ||
| FDA (2019, 35) | |||
| Digital technology | EMA (2021, 37) | ||
| Data collection process | MHLW (2005, 31) | FDA (2021 draft, 36) | EMA (2021 draft, 38) |
| EMA (2010, 39) | |||
| DCT | Swissmedic (2021, 40) | ||
| Danish medicines agency (2021, 41) |
Guidelines for utilization of IoMT/IoHT in clinical trials or DCT.
Discussion
In this study, we used the horizon scanning method to identify new IoMT/IoHT-based technologies that could be utilized in DCTs or products developed using the DCT approach. Based on the content and keywords (TC-ICFs) of the obtained sub-clusters, we identified technologies and specific medical devices that are pivotal in the citation relationships that make up the sub-clusters. We subsequently categorized them according to therapeutic area. Remote monitoring of biological functions (activity and vitals) using sensors and telemedicine, which are expected to be utilized not only in specific therapeutic areas, was found to be the most common (general). This means that IoMT/IoHT-based technologies, including wearable devices, will be available for use in daily life and clinical trials, including DCTs (1). We discovered that they fit well with therapeutic areas related to mental health, such as psychology and neurology. Telemedicine for psychiatric disorders is already being used in actual clinical practice (39–42), and the COVID-19 pandemic has led to its widespread use (43). In the development of new drugs for psychiatric disorders, conventional clinical trial methods affect the evaluation of drug efficacy because the evaluation by the investigators at each clinical trial institution is subjective and subject to a high degree of variability. To solve this problem, efforts to reduce variability among investigators using telemedicine and centralized evaluation by one principal investigator as a central evaluator have taken center stage. In telehealth, telemedicine is the most commonly used application for remote medical treatment. Devices and technologies aimed at remotely monitoring specific biometrics and disease conditions via the Internet were developed. Both telehealth and remote monitoring are important elements of DCTs, and medical devices and infrastructure with these technologies and functions are expected to contribute to the standardization of DCTs. In response to the COVID-19 pandemic, telemedicine and digital health technologies (such as wearable devices) have been applied in the treatment of the COVID-19 to avoid the risk of further hospital-acquired infection. Based on the result of a rapid literature review by Bokolo Anthony Jnr, some considerations have been identified for applying telehealth and digital care solutions during the COVID-19 pandemic (44–46).
We focused on VR therapy as an IoMT/IoHT-based technology that is expected to be utilized and developed through DCTs. VR therapy, as the name suggests, involves the use of VR for medical purposes (47). VR technology enables us to perceive a virtual or artificial world created by a computer as reality. The first VR device was developed by Ivan Sutherland in the US in the 1960s (48, 49); the commercialization of VR devices began in the 1990s, and advancements in the development of VR increased on the brink of the 2000s, with projects such as CAVE (50). In this study, we confirmed that VR therapy has been actively studied in the following disorders and domains: stroke (Cluster 6–1), anxiety (Cluster 6–3), pain therapy (Cluster 6–6), attention deficit (Cluster 6–10), and rehabilitation (Cluster 6–1).
In the US, on November 28, 2021, EaseVRx was authorized by the FDA as a prescription-use immersive VR system that uses cognitive behavioral therapy and other behavioral methods to help with pain reduction in patients aged 18 years and older with diagnosed chronic lower back pain. When used with other treatment methods for chronic lower back pain, this is expected to offer a treatment option for pain reduction that does not include opioid analgesics. In the US, opioid overdose/abuse is a major social problem; therefore, EaseVRx was granted breakthrough device designation. EaseVRx was reviewed through the de novo premarket review pathway, a regulatory pathway for low-to-moderate-risk devices of a new type (51). This is the first authorized VR therapy device approved by the FDA as a medical device. In the COVID-19 era, this type of contactless and at-home therapy is more attractive. Because VR therapy can be used remotely at home without visiting an institution and as long as the VR device is with the patient, we believe that it is an appropriate device to be developed at DCTs to conduct clinical trials in an environment where it will be used in actual clinical practice.
Examples of Novel IoHT/IoMT-based Technologies Expected to Be Developed and Used in DCTs
Based on the information from the sub-cluster in Table 2, we identified potential IoMT/IoHT-based technologies that have not yet been commercialized (Table 6). From Table 6, we assume examples of devices and conceivable use-case scenarios for each device in DCTs. Not all devices are required to obtain approval as medical devices, depending on their application to healthcare workers. Therefore, depending on the product concept of the device, it can be commercialized without regulatory approval; examples include smartwatches and activity trackers. However, to utilize these novel technologies in DCTs as a useful tool, they may be qualified as medical-grade devices by regulatory authorities. The regulatory pathways for many types of devices can be complex. As examples of major regional regulatory authorities in the US, the EU, and Japan, there are some differences in handling medical devices among the three authorities (52–54). Particularly, the EU has unique processes for market access to medical devices in the EU region. Notified bodies that are certified by the EU regulate device approvals. These commercial companies have contracts with device companies to handle device approval, and the Conformité Européenne (CE) mark, which allows the device to be marketed in all EU countries is authorized (55). Most devices are classified based on their level of risk, ranging from class I (lowest risk) to class III (highest risk). This is standardized in the principles of medical devices classification by the Global Harmonization Task Force, which is organized by the US, the EU, Japan, Canada, and Australia (56). The EU has two subclasses in class II: class IIa and class IIb. Class IIa devices are expected to be at a relatively low risk to humans. Class IIb devices are associated with a comparably high risk of harming humans. In the US, the EU, and Japan, class I devices are allowed for market access without any clinical or preclinical data based on their assumed safety and efficacy. However, class II and III devices may require preclinical and safety data verifying their efficacy. The novel IoMT/IoHT-based technologies in Table 6, which are identified based on the citation network, are expected to be classified as higher than class II. Thus, a certain evidence of efficacy may be required for practical use, and the approach of DCT may become a powerful tool for the acceleration and efficiency of the trial because these IoMT/IoHT-based technologies are compatible with remote assessment. As mentioned above, the process for medical device approval and the concept of medical-grade devices differ according to the regulatory authority. These differences by regulatory authorities could become a barrier to the utilization and application of novel IoMT/IoHT-based technologies in DCTs.
Table 6
| Notable device/feature | Conceivable use-case in DCTs | |
|---|---|---|
| Cluster 3–3 | Sweat wearable sensor | Detection of sign of some disease based on change of sweat condition and elements |
| Cluster 3–4 | Non-enzymatic glucose wearable sensor | Remote monitoring of efficacy and safety of diabetic drugs |
| Cluster 4–12 | Automatic ingestion monitor | DCTs for drug development for feeding disorder or obesity |
Notable device/features and conceivable use-case in DCTs.
Key Considerations for the Utilization of IoHT/IoMT in DCTs
It is necessary to consider the appropriateness of using the device with novel technologies that have not yet been in practical use or that have just become commercial realities in clinical trials. Considering the properties of clinical trials, it is desirable to ensure that the quality and accuracy of the data are equal to or higher than that of the data from standardized methods. From the perspective of data quality using IoMT/IoHT in DCTs, it is not mandatory to use a regulatory authorized device as a medical device; however, if it is not, the study sponsor should take measures to ensure data quality in accordance with the accepted principles of clinical trials such as ICH-GCP E6(R2) (57). Guidance from each regulatory authority related to electronic data collection may be useful as a reference to meet the expectations of data collection using IoMT/IoHT-based technologies (28, 58–60). Table 5 shows that guidelines regarding the utilization of IoMT/IoHT in clinical trials are limited. There are certain guidelines for the COVID-19 public health emergency and they have some footholds on how to implement IoMT/IoHT in clinical trials or medical examinations. For example, the digital health device for treating psychiatric disorders is detected in Cluster 7–3 in Table 2, and it is addressed in the FDA policy (29). However, these guidelines for the COVID-19 public health emergency may be effective only during the COVID-19 pandemic; therefore, it is necessary to consider if the permission is temporary. From the experience of the use of digital technology in providing medical care during the COVID-19 pandemic, data privacy and protection has also been identified as a key consideration (46, 61). To secure the data obtained by IoMT/IoHT and promote the utilization of IoMT/IoHT in DCTs, Switzerland and Denmark, with regard to guidance on DCTs from regulatory authorities, are ahead of other countries (30, 31). Because IoMT/IoHT is a key component in conducting DCTs, the considerations for data capturing outside the trial site using mobile technologies are mentioned in the guidelines by Switzerland. IT-equipped medical devices have the potential to completely change the style of clinical trials, which until now have relied on hospital visits and been geographically limited. Therefore, we believe that the active use of new technologies and the accumulation of evidence of utilization in the trial will lead to their secondary use in clinical trials including decentralized-style trials.
Limitations
We selected WoS as the database for the source of analysis; however, there are other databases, such as PubMed and Scopus, which were excluded. It is necessary to select a database for data source collection that is appropriate for each field of interest. Moreover, there is a time lag between the publication of papers in journals and those reflected in WoS, which causes a time gap in the understanding of the research field. In this study, we focused exclusively on IoMT/IoHT research trends as on the date when the article information was extracted from the database. Additionally, it is necessary to follow the changes in hub papers and other important papers in the research field in chronological order to understand the development of research fields and new technologies. We analyzed the potential IoMT/IoHT-based technologies, disease areas, and purposes of use based on the information in each sub-cluster; however, there is a possibility that subjectivity and bias were incorporated into the evaluation.
Conclusion
In this study, we investigated the trends related to the adoption of IoT in the medical and healthcare fields (IoMT/IoHT) using citation network analysis and text mining methods. IoMT/IoHT and research fields that are expected to be utilized in DCTs, such as telemedicine, wearable devices, and VR, were identified. Based on the results, we established key considerations in using IoMT/IoHT in DCTs, such as how sponsors can ensure data quality from the IoMT/IoHT-based technology used in DCTs. To the contrary, considering that the field of IoMT/IoHT is evolving rapidly, some relatively recent studies are not included in the dataset used for citation network analysis; they are therefore not identified as hub papers because of having been published quite recently or having few citations. The findings of this study are expected to help in developing the new IoMT/IoHT using DCTs or in using the IoMT/IoHT in DCTs. For future studies, the works of literature and guidance related to DCTs and IoMT/IoHT will be retrieved and examined for continuous solutions to problems based on the latest information and knowledge.
Funding
This research was supported by AMED (Japan Agency for Medical Research and Development) Under Grant Number 21mk0101217h0001.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MS contributed to the conception and design of the study. HS organized the database. HI collected data. HI, TT, and TS performed the data analysis. TS drafted the manuscript. HS and MS reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Yuka Suzuki and Koji Ikeda for their helpful discussions.
Conflict of interest
TS was employed by Astellas Pharma Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Apostolaros M Babaian D Corneli A Forrest A Hamre G Hewett J et al . Legal, regulatory, and practical issues to consider when adopting decentralized linical trials: recommendations from the clinical trials transformation initiative. Ther Innov Regul Sci. (2020) 54:779–87. 10.1007/s43441-019-00006-4
2.
Anderson A Borfitz D Getz K . Global public attitudes about clinical research and patient experiences with clinical trials. JAMA Netw Open. (2018) 1:e182969. 10.1001/jamanetworkopen.2018.2969
3.
ICMRA strategic priority on innovation . Available online at: https://www.icmra.info/drupal/sites/default/files/2017-12/ICMRA%20Innovation%20Concept%20Note_0.pdf (accessed March 23, 2022).
4.
Kostoff RN Schaller RR . Science and technology roadmaps. IEEE Trans Eng Manage. (2001) 48:132–43. 10.1109/17.922473
5.
OECD . Available online at: https://www.oecd.org/education/ceri/35393902.pdf (accessed March 23, 2022).
6.
Hines P Hiu Yu LH Guy RH Brand A Papaluca-Amati M . Scanning the horizon: a systematic literature review of methodologies. BMJ Open. (2019) 9:e026764. 10.1136/bmjopen-2018-026764
7.
Börner K Chen C Boyack KW . Visualizing knowledge domains. Annu Rev Inf Sci Technol. (2003) 37:179–255. 10.1002/aris.1440370106
8.
Boyack KW Klavans R Börner K . Mapping the backbone of science. Scientometrics. (2005) 64:351–74. 10.1007/s11192-005-0255-6
9.
Chen C . Visualising semantic spaces and author co-citation networks in digital libraries. Inf Process Manag. (1999) 35:401–20. 10.1016/S0306-4573(98)00068-5
10.
Chen C Cribbin T Macredie R Morar S . Visualizing and tracking the growth of competing paradigms: two case studies. J Am Soc Inf Sci Technol. (2002) 53:678–89. 10.1002/asi.10075
11.
Small H . Visualizing science by citation mapping. J Am Soc Inf Sci. (1999) 50:799–813.
12.
Kajikawa Y Ohno J Takeda Y Matsushima K Komiyama H . Creating an academic landscape of sustainability science: an analysis of the citation network. Sustain Sci. (2007) 2:221–31. 10.1007/s11625-007-0027-8
13.
Kajikawa Y Yoshikawa J Takeda Y Matsushima K . Tracking emerging technologies in energy research: toward a roadmap for sustainable energy. Technol Forecast Soc Change. (2008) 75:771–82. 10.1016/j.techfore.2007.05.005
14.
Shibata N Kajikawa Y Takeda Y Sakata I Matsushima K . Detecting emerging research fronts in regenerative medicine by citation network analysis of scientific publications. PICMET Portland International Conference on Management of Engineering & Technology−2009. IEEE Publications ('09).Portland, OR, USA (2009). 10.1109/PICMET.2009.5261790
15.
Ittipanuvat V Fujita K Sakata I Kajikawa Y . Finding linkage between technology and social issue: a literature based discovery approach. J Eng Technol Manag. (2014) 32:160–84. 10.1016/j.jengtecman.2013.05.006
16.
Takata T Sasaki H Yamano H Honma M Shikano M . Study on horizon-scanning with a focus on the development of AI-based medical products: citation network analysis. Ther Innov Regul Sci. (2022) 56:263–75. 10.1007/s43441-021-00355-z
17.
Fujii E Takata T Yamano H Honma M Shimokawa M Sasaki H et al . Study on horizon scanning by citation network analysis and text mining: a focus on drug development related to T cell immune response. Ther Innov Regul Sci. (2022) 56:230–43. 10.1007/s43441-021-00351-3
18.
Fukaya-Shiba A Otsuka K Sasaki H Shikano M Wakao R . Identification of novel modalities through bibliometric analysis for timely development of regulatory guidance: a case study of T cell immunity. Front Med. (2021) 8:756870. 10.3389/fmed.2021.756870
19.
Fukaya-Shiba A Shimokawa M Sasaki H Wakao R . Pharmaceuticals and medical devices agency's horizon scanning and the science board: cooperation toward extracellular vesicle-based products. Br J Clin Pharmacol. (2022) 88:1392–4. 10.1111/bcp.15065
20.
Sasaki H Fugetsu B Sakata I . Emerging scientific field detection using citation networks and topic models: a case study of the nanocarbon field. Appl Syst Innov. (2020) 3:40. 10.3390/asi3030040
21.
Blondel VD Guillaume JL Lambiotte R Lefebvre E . Fast unfolding of communities in large networks. J Stat Mech Theor Exp. (2008) P10008. 10.1088/1742-5468/2008/10/P10008
22.
Adai AT Date SV Wieland S Marcotte EM . LGL: creating a map of protein function with an algorithm for visualizing very large biological networks. J Mol Biol. (2004) 340:179–90. 10.1016/j.jmb.2004.04.047
23.
Sasaki H Zhidong L Sakata I . Academic landscape of hydropower: citation-analysis-based method and its application. Int J Energy Technol Policy. (2016) 12:84–102. 10.1504/IJETP.2016.074493
24.
Search for FDA guidance documents . Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents#guidancesearch (accessed March 23, 2022).
25.
EMA Scientific guidelines . Available online at: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines (accessed March 23, 2022).
26.
MHLW . Available online at: https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/index.html (accessed March 23, 2022).
27.
PMDA . Available online at: https://www.pmda.go.jp/english/index.html (accessed March 23, 2022).
28.
Ministry Ministry of Health Labour and Welfare . Use of Electromagnetic Records and Electronic Signatures in Applications for Approval or Licensing of Pharmaceuticals, etc. [Japanese]. Available online at: https://www.mhlw.go.jp/web/t_doc?dataId=00ta8216&dataType=1&pageNo=1. (accessed March 23, 2022).
29.
Food and Drug Administration . Enforcement policy for non-invasive remote monitoring devices used to support patient monitoring during the coronavirus Disease 2019 (COVID-19) public health emergency (revised): guidance for industry and Food and Drug Administration staff. Available online at: https://www.fda.gov/media/136290/download (accessed March 23, 2022).
30.
Position paper by Swissmedic and swissethics on decentralized clinical trials (DCTs) with medicinal products . Available online at: https://www.swissmedic.ch/dam/swissmedic/de/dokumente/bewilligungen/klv/positionspapier-dct.pdf.download.pdf/DCT_EN_.pdf (accessed March 23, 2022).
31.
Guidance on the implementation of decentralized elements in clinical trials with medicinal products . Available online at: https://laegemiddelstyrelsen.dk/en/news/2021/guidance-on-the-implementation-of-decentralised-elements-in-clinical-trials-with-medicinal-products-is-now-available/~/media/5A96356760ED408CBFA9F85784543B53.ashx (accessed March 23, 2022).
32.
European Medicines Agency . Questions and answers: qualification of digital technology-based methodologies to support approval of medicinal products. Available online at: https://www.ema.europa.eu/en/documents/other/questions-answers-qualification-digital-technology-based-methodologies-support-approval-medicinal_en.pdf (accessed March 23, 2022).
33.
Ministry Ministry of Health Labour and Welfare . Guidelines for the appropriate use of online medical services [Japanese]. Available online at: https://www.mhlw.go.jp/content/000889114.pdf. (accessed March 23, 2022).
34.
Ministry Ministry of Health Labour and Welfare . Time-limited and special treatment of medical treatment using telephones and information communication devices in case of the spread of new coronavirus infections [Japanese]. Available online at: https://www.mhlw.go.jp/content/000620995.pdf (accessed March 23, 2022).
35.
Pharmaceuticals and Medical Devices Agency . Q&A regarding the implementation of clinical trials for pharmaceuticals, medical devices and regenerative medicine products under the influence of new coronavirus infection [Japanese]. Available online at: https://www.pmda.go.jp/files/000235164.pdf (accessed March 23, 2022).
36.
Food and Drug Administration . Remote interactive evaluations of drug manufacturing and bioresearch monitoring facilities during the COVID-19 public health emergency guidance for industry: guidance for industry. Available online at: https://www.fda.gov/media/147582/download (Accessed March 23, 2022).
37.
Food and Drug Administration . Enforcement policy for digital health devices for treating psychiatric disorders during the coronavirus Disease 2019 (COVID-19) public health emergency: guidance for industry and Food and Drug Administration staff. Available online at: https://www.fda.gov/media/136939/download (accessed March 23, 2022).
38.
Food and Drug Administration . Enforcement policy for remote ophthalmic assessment and monitoring devices during the coronavirus Disease 2019 (COVID-19) public health emergency: guidance for industry and Food and Drug Administration staff. Available online at: https://www.fda.gov/media/136733/download (accessed March 23, 2022).
39.
De Las Cuevas C Arredondo MT Cabrera MF Sulzenbacher H Meise U . Randomized clinical trial of telepsychiatry through videoconference versus face-to-face conventional psychiatric treatment. Telemed J E Health. (2006) 12:341–50. 10.1089/tmj.2006.12.341
40.
Fortney JC Pyne JM Edlund MJ Williams DK Robinson DE Mittal D et al . A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. (2007) 22:1086–93. 10.1007/s11606-007-0201-9
41.
Ruskin PE Silver-Aylaian M Kling MA Reed SA Bradham DD Hebel JR et al . Treatment outcomes in depression: comparison of remote treatment through telepsychiatry to in-person treatment. Am J Psychiatry. (2004) 161:1471–6. 10.1176/appi.ajp.161.8.1471
42.
Chakrabarti S . Usefulness of telepsychiatry: a critical evaluation of videoconferencing-based approaches. World J Psychiatry. (2015) 5:286–304. 10.5498/wjp.v5.i3.286
43.
Doraiswamy S Abraham A Mamtani R Cheema S . Use of Telehealth during the COVID-19 pandemic: scoping review. J Med Internet Res. (2020) 22:e24087. 10.2196/24087
44.
Anthony B Jnr . Use of telemedicine and virtual care for remote treatment in response to COVID-19 pandemic. J Med Sys. (2020) 44:1–9. 10.1007/s10916-020-01596-5
45.
Anthony B Jnr . Application of telemedicine and eHealth technology for clinical services in response to COVID 19 pandemic. Health Technol. (2021) 11:359–66. 10.1007/s12553-020-00516-4
46.
Anthony B Jnr . Implications of telehealth and digital care solutions during COVID-19 pandemic: a qualitative literature review. Inform Health Soc Care. (2021) 46:68–83. 10.1080/17538157.2020.1839467
47.
North MM Sarah M . Virtual environment and psychological disorders, North, electronic. J Virtual Cult. (1994) 2.
48.
Andrzej G Kamil J . The Use of Virtual Reality in the Training of Professionals: With the Example of Firefighters. Comput Anim Virtual Worlds (2020). Available online at: https://onlinelibrary.wiley.com/doi/10.1002/cav.1981
49.
Sutherland IE . A head-mounted three-dimensional display. In: Proceedings of AFIPS.San Francisco, CA (1968). p. 757–64.
50.
Mazuryk T Gervautz M . Virtual Reality History, Applications, Technology and Future. Austria: Institute of Computer Graphics, Vienna University of Technology (2014).
51.
FDA news release . Available online at: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-virtual-reality-system-chronic-pain-reduction (accessed March 23, 2022).
52.
FDA medical device . Available online at: https://www.fda.gov/medical-devices/resources-you-medical-devices (accessed March 23, 2022).
53.
EU medical device . Available online at: https://ec.europa.eu/health/md_sector/overview_en (accessed March 23, 2022).
54.
PMDA medical device . Available online at: https://www.std.pmda.go.jp/stdDB/index_en.html (accessed March 23, 2022).
55.
Maak TG Wylie JD . Medical device regulation: A comparison of the United States and the European Union. J Am Acad Orthop Surg. (2016) 24:537–43. 10.5435/JAAOS-D-15-00403
56.
The Global Harmonization Task Force . Available online at: https://www.imdrf.org/sites/default/files/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n15-2006-guidance-classification-060627.pdf (accessed March 23, 2022).
57.
ICH E6(R2) Guideline . Available online at: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed April 28, 2022).
58.
Food and Drug Administration . Digital health technologies for remote data acquisition in clinical investigations: draft guidance for industry, investigators, and other stakeholders. Available online at: https://www.fda.gov/media/155022/download (accessed March 23, 2022).
59.
European Medicines Agency . Guideline on computerised systems and electronic data in clinical trials. Available online at: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/draft-guideline-computerised-systems-electronic-data-clinical-trials_en.pdf (accessed March 23, 2022).
60.
European Medicines Agency . Reflection paper on expectations for electronic source data and data transcribed to electronic data collection tools in clinical trials. Available online at: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/reflection-paper-expectations-electronic-source-data-data-transcribed-electronic-data-collection_en.pdf (accessed March 23, 2022).
61.
Dilawar N Rizwan M Ahmad F Akram S . Blockchain: securing internet of medical things (IoMT). Int J Adv Comput Sci Appl. (2019) 10:82–9. 10.14569/IJACSA.2019.0100110
Summary
Keywords
decentralized clinical trial, internet of medical/health things, regulatory science, horizon scanning, citation network, text mining
Citation
Sato T, Ishimaru H, Takata T, Sasaki H and Shikano M (2022) Application of Internet of Medical/Health Things to Decentralized Clinical Trials: Development Status and Regulatory Considerations. Front. Med. 9:903188. doi: 10.3389/fmed.2022.903188
Received
24 March 2022
Accepted
09 May 2022
Published
06 June 2022
Volume
9 - 2022
Edited by
Yonggang Zhang, Sichuan University, China
Reviewed by
Lawrence Liberti, Temple University, United States; Anthony Bokolo Jnr., Institute for Energy Technology, Norway
Updates
Copyright
© 2022 Sato, Ishimaru, Takata, Sasaki and Shikano.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takahiro Sato takahiro-sato@astellas.com
This article was submitted to Regulatory Science, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.