Abstract
Purpose:
To develop handcrafted radiomics (HCR) and deep learning (DL) based automated diagnostic tools that can differentiate between idiopathic pulmonary fibrosis (IPF) and non-IPF interstitial lung diseases (ILDs) in patients using high-resolution computed tomography (HRCT) scans.
Material and Methods:
In this retrospective study, 474 HRCT scans were included (mean age, 64.10 years ± 9.57 [SD]). Five-fold cross-validation was performed on 365 HRCT scans. Furthermore, an external dataset comprising 109 patients was used as a test set. An HCR model, a DL model, and an ensemble of HCR and DL model were developed. A virtual in-silico trial was conducted with two radiologists and one pulmonologist on the same external test set for performance comparison. The performance was compared using DeLong method and McNemar test. Shapley Additive exPlanations (SHAP) plots and Grad-CAM heatmaps were used for the post-hoc interpretability of HCR and DL models, respectively.
Results:
In five-fold cross-validation, the HCR model, DL model, and the ensemble of HCR and DL models achieved accuracies of 76.2 ± 6.8, 77.9 ± 4.6, and 85.2 ± 2.7%, respectively. For the diagnosis of IPF and non-IPF ILDs on the external test set, the HCR, DL, and the ensemble of HCR and DL models achieved accuracies of 76.1, 77.9, and 85.3%, respectively. The ensemble model outperformed the diagnostic performance of clinicians who achieved a mean accuracy of 66.3 ± 6.7% (p < 0.05) during the in-silico trial. The area under the receiver operating characteristic curve (AUC) for the ensemble model on the test set was 0.917 which was significantly higher than the HCR model (0.817, p = 0.02) and the DL model (0.823, p = 0.005). The agreement between HCR and DL models was 61.4%, and the accuracy and specificity for the predictions when both the models agree were 93 and 97%, respectively. SHAP analysis showed the texture features as the most important features for IPF diagnosis and Grad-CAM showed that the model focused on the clinically relevant part of the image.
Conclusion:
Deep learning and HCR models can complement each other and serve as useful clinical aids for the diagnosis of IPF and non-IPF ILDs.
Introduction
Interstitial lung disorders (ILDs) are a diverse group of ailments with an estimated 200 distinct entities and are linked with high morbidity and death (1). Many different parenchymal lung disorders have similar clinical signs and patterns of lung injury. Several disorders, including idiopathic pulmonary fibrosis (IPF), have unknown etiology and are labeled idiopathic or cryptogenic, while the rest are linked to other diseases, particularly connective tissue diseases, or to environmental exposures (2–6). One of the most common types of ILDs is IPF, a progressive illness marked by decreased lung function (7). IPF has an estimated incidence rate between 2.8 and 18 cases per 100,000 per year in Europe and North America (8). The median survival rate of patients with IPF is between 2 and 4 years from diagnosis (9). A prompt diagnosis and management are crucial for slowing down the progression of these lung disorders.
Medical imaging is becoming increasingly crucial for disease diagnosis, prognosis, and treatment planning in precision medicine (10). Computed tomography (CT) provides visual data that may be used to enhance decision-making (4, 11). However, qualitative CT evaluation remains challenging and frequently varies amongst experts (12). The diagnosis of idiopathic pulmonary fibrosis using high-resolution computed tomography (HRCT) is a difficult task and high inter-observer variability is associated with it even with experienced radiologists (13). Consequently, there is a need for an automated clinical tool that can aid clinicians for accurate and timely diagnosis.
Artificial intelligence is becoming increasingly popular due to the increasing amount of imaging data and available computational resources (14). The use of quantitative imaging techniques in medical imaging has grown at an exponential rate (15). Handcrafted radiomics (HCR) is a quantitative approach that measures and extracts high-dimensional imaging characteristics to aid clinical decision-making (15, 16). Deep learning (DL) methods learn different features and representations from the image data without the need for explicit feature engineering (17). Convolutional neural networks (CNNs) have shown remarkable results on numerous diagnostic tasks using medical image data including the diagnosis of fibrotic lung disease (18).
Despite promising results demonstrated by HCR and DL models for various medical imaging tasks, the clinical utility of such models is limited due to their lack of interpretability (19). Shapley Additive exPlanations (SHAP) (20) and Gradient-weighted class activation maps (Grad-CAM) (21) are post-hoc interpretability methods that are useful for understanding the decision-making process of HCR and DL models, respectively.
In this paper, we propose a machine learning-based HCR pipeline and a DL pipeline for the automated diagnosis of IPF and non-IPF ILDs patients. We also perform an in-silico trial with experienced radiologists to compare the performance of HCR and DL on a test dataset. Furthermore, we use post-hoc interpretability methods to aid the incorporation of these automated diagnostic tools in the clinical workflow.
Materials and Methods
Patients
A total of 652 HRCT scans were obtained from Site 1 (University Liege hospital) and 205 HRCT scans were obtained from database A [The Lung tissue research consortium database (LTCR)]. The inclusion criteria were: the availability of non-contrast enhanced HRCT and the availability of HRCT with slices thickness of less than 1.5 mm. The exclusion criteria were: the use of contrast enhancement, images containing metal or motion artifacts, and images reconstructed with a slice thickness larger than 1.5 mm. All diagnoses were confirmed by the Multidisciplinary discussion (MDD) that included a histopathologist, pulmonologist, thoracic radiologist, and rheumatologist. Lung biopsy is only required in case of ILD inconsistent with IPF. Figure 1 shows the patient selection process. Demographic data, clinical data, and measurements of pulmonary function tests (PFT) were acquired for each patient. Demographic and clinical data include age, gender, body mass index (BMI), forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and diffusion capacity of the lungs for carbon monoxide (DLCO).
FIGURE 1

The flowchart diagram shows the patient selection process. IPF, idiopathic pulmonary fibrosis, ILDsnon–IPF, non-IPF interstitial lung diseases.
Imaging Acquisition and Segmentation
The HRCT scans at site 1 were acquired at the same hospital using two different vendors (Siemens and GE). The scans acquired from database A were acquired using four different CT vendors (Siemens, GE, Philips, and Toshiba). The slice thickness of the scans varied between 0.5 and 1.5 mm. A further detailed description of the CT acquisition parameters can be found in Supplementary Table 1. Whole lung segmentation was performed using an automated workflow created in MIM software (MIM Software Inc., Cleveland, OH, United States).
Data Split
Five-fold cross-validation was performed on data from Site 1 consisting of 365 HRCT scans containing 279 non-IPF ILDs, and 86 IPF patients. External data from database A, comprising 53 IPF patients and 56 non-IPF ILDs patients was used to benchmark the performance of the proposed AI tools along with the in-silico trial.
Handcrafted Radiomics
Handcrafted Radiomics Feature Extraction
To minimize the effect of the variations in image voxel size, all CT images were resampled to a 1 mm × 1 mm × 1 mm. Radiomics features were extracted from the HRCT images using the RadiomiX Discovery Toolbox1 which calculates handcrafted radiomics (HCR) features compliant with the Imaging Biomarkers Standardization Initiative (IBSI) (22). Voxel intensities were aggregated into 25 bins of Hounsfield Units to reduce noise and inter-scanner variability. The extracted features describe fractal dimension, intensity histogram, first-order statistics, texture, and shape. A workflow for handcrafted radiomics from segmentation to data analysis is illustrated in Figure 2.
FIGURE 2

Radiomics Pipeline for Lung disease classification from CT images. The same 12 radiomics features from both lungs after feature selection are concatenated and fed to the Random Forest classifier. Post-hoc SHAP analysis is performed for interpretability.
Features Selection and Modeling
Features with near-zero variance (i.e., features that have the same value in ≥95% of the data points) were excluded. Then, a correlation matrix was created between all HCR features and populated using Spearman’s correlation coefficient (r). Feature pairs with |r| ≥ 0.90 were considered to be highly correlated, and the feature with the highest average correlation with all other features was removed. Furthermore, a Recursive feature elimination (RFE) using a random forest classifier was performed on the subset of features that were selected after applying Spearman’s correlation coefficient. RFE was applied with cross-validation in order to determine the accuracy of the classification and the top 12 features with the highest accuracy were selected for the final model. The same 12 features were extracted for each lung and concatenated to give a final feature vector consisting of 24 HCR features. A list of the names of the features along with their abbreviations that were used in the model can be found in Supplementary Table 2. A random forest classifier was used to construct the HCR model to predict the probability of IPF in patients using HRCTs. Random forest classifier has proven to be effective for lungs CT-based radiomics problems in recent research findings (23–25). The random forest classifier was trained with class weights of 1 for non-IPF ILDs and 3 for IPF patients to compensate for the class imbalance. Five-fold cross-validation was used for hyper-parameter tuning.
Post-hoc Interpretability
SHapley Additive exPlanations (SHAP) analysis is based on co-operative game theory (20). SHAP analysis is a post-hoc interpretability method that quantifies the impact of each feature on the model prediction in terms of SHAP value. SHAP summary plots provide global explanations by highlighting the effect of features on the prediction in terms of SHAP value and help in recognizing the trends. These plots show whether a high or low feature value affects the model output positively or negatively. SHAP dependence plots highlight the relationship between the model output in terms of SHAP values and the corresponding feature values. These dependence plots can be useful for quantifying the trend of model output with respect to the feature values as well as understanding the interaction effects between a pair of features.
Deep Learning
All the scans were resampled to an isotropic resolution of 1 mm × 1 mm × 1 mm. Min-max normalization was applied to the area within the lung mask. Two patches containing one lung each of size 240 ×240 ×240 voxels were extracted using the lungs masks. Both lungs were randomly flipped for augmentation and concatenated along the z-axis. The image was then downsampled by taking every sixth slice along the z-axis. The start index was randomly chosen in the range of 1–6. This resulted in additional augmentation and reduction of the input image size. A Densenet-121 (26, 27) classifier with 3D convolutional layers was used with weighted binary cross-entropy loss (non-IPF ILDs: 1, IPF: 3) in order to minimize the effects of data imbalance. Adam optimizer with a learning rate of 1 e–5 and ReduceLROnPlateau scheduler was employed. The batch size was set at 16 and the network was trained for 50 epochs. Figure 3 shows the different steps involved in training the DL model for lung disease classification in CT images.
FIGURE 3

Figure shows different steps in the deep learning pipeline for the prediction of lung diseases in CT scans.
During prediction, six input images from the test image were extracted by setting the start slice index in the range from 1 to 6 and taking every sixth consecutive slice. These six test samples are fed to the trained 3D Densenet-121 model. The final prediction is the average of the prediction of these six test samples. Heatmaps highlight the regions of the input image that the model considers important for prediction. We utilized Grad-CAM (21) heatmaps for the post-hoc interpretability of the Densenet-121 model.
Ensemble Model
The ensemble methods utilize multiple machine learning methods in an effort to achieve better predictive performance as compared to the performance obtained by the constituent machine learning methods alone. We constructed an ensemble model from HCR and DL models by taking an average of the probabilities predicted by the two models.
In-silico Clinical Trial
An application that allows the construction of a reference performance point by gathering medical imaging expert comments based on the visual assessment of HRCT images was created. The application allows displaying the CT images one at a time with the option of different planes (Axial, Coronal, or Sagittal), and the application also allows scrolling through the CT scan slices. The graphical user interface (GUI) of the application can be found in Supplementary Figure 1. The radiologist can select one of the two classes (IPF or ILDs other than IPF). The diagnostic performance of two radiologists (6 and 23 years of experience) and one pulmonologist (12 years of experience) was recorded for the same test dataset (n = 109) to perform a comparison with the machine learning-based HCR, DL, and ensemble models.
Statistical Analysis
Statistical analysis was performed in Python (version: 3.6). Wilcoxon rank-sum test was used for the continuous variables to test the group differences and Fisher exact test for categorical variables. To assess the model’s performance, the areas under the curves (AUCs) for receiver operating characteristic (ROC) curves were compared using the DeLong test. The thresholds for each model were set at the highest Youden’s index in the training set. The performance was evaluated using accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). For five-fold cross-validation, we also report the standard deviation (SD). The performance of the models on the test set was compared with the performance of clinicians using McNemar test. This study followed the Standard for Reporting Diagnostic accuracy studies (STRAD) (28) and was assessed using the Radiomics Quality Score (RQS) (29). The detailed description about RQS can be found in Supplementary Table 3.
Results
Patients Characteristics
A total of 474 patients, 335 of whom were diagnosed with non-IPF ILDs, and 139 with IPF, were included after the application of exclusion criteria (Figure 1). The demographic characteristics of the included patients can be found in Table 1.
TABLE 1
| Variables | Site 1 | Database A | P-value (p) |
| n | 365 | 109 | −− |
| Age [mean(SD)] | 64.10 (9.57) | 63.61 (14.17) | 0.8 |
| Sex = M (%) | 213 (87) | 74 (67.9) | 0.09 |
| FEV1 [mean (SD)] | 80.42 (21.47) | 69.60 (20.67) | <0.001 |
| FVC [mean(SD)] | 80.52 (21.25) | 67.35 (21.37) | <0.001 |
| DLCO [mean(SD)] | 51.32 (24.99) | 29.84 (5.36) | <0.001 |
| BMI [mean(SD)] | 25.48 (6.45) | 29.55 (5.21) | <0.001 |
Demographic and clinical information of the study participants.
BMI, body mass index, FEV, forced expiratory volume, FVC, forced vital capacity, and diffusion capacity of the lungs for carbon monoxide (DLCO) are shown in the table for different patients along with their mean and standard deviation (SD).
Handcrafted Radiomics
The HCR model achieved an AUC of 0.85 (95% CI: 0.771 – 0.924) in the validation set in five-fold cross-validation (Figure 4A). The threshold of 0.51 was fixed based on Youden’s index in the training set. An accuracy, sensitivity, and specificity of 0.762 ± 0.068, 0.816 ± 0.094, and 0.745 ± 0.065 were obtained in five-fold cross-validation, respectively. In the external test set, the HCR model achieved an AUC, accuracy, sensitivity, and specificity of 0.817, 0.761, 0.698, and 0.821, respectively. Tables 2, 3 show the performance metrics for the HCR model during five-fold cross-validation and external validation, respectively. Figure 4B shows the test performance for the HCR model on the external dataset. The Radiomics Quality Score (RQS) achieved for this study is 52.78% (19 of 36).
FIGURE 4

Receiver operating characteristics (ROC) curves for five-fold cross-validation (A) and external test dataset (B) for the classification of IPF and non-IPF ILDs using handcrafted radiomics (HCR), deep learning (DL), and ensemble (HCR + DL) models.
TABLE 2
| Model | Accuracy | Sensitivity | Specificity | Positive predictive value (PPV) | Negative predictive value (NPV) |
| Handcrafted radiomics (HCR) | 0.762 ± 0.068 | 0.816 ± 0.094 | 0.745 ± 0.065 | 0.506 ± 0.084 | 0.923 ± 0.040 |
| Deep learning (DL) | 0.779 ± 0.046 | 0.711 ± 0.10 | 0.800 ± 0.075 | 0.541 ± 0.074 | 0.901 ± 0.025 |
| Ensemble (HCR + DL) | 0.852 ± 0.027 | 0.827 ± 0.005 | 0.860 ± 0.035 | 0.65 ± 0.063 | 0.94 ± 0.003 |
Precision and recall metrics for five-fold cross-validation using handcrafted radiomics (HCR), deep learning (DL), and an ensemble of HCR and DL models.
TABLE 3
| Model | Accuracy | Sensitivity | Specificity | Positive predictive value (PPV) | Negative predictive value (NPV) |
| Handcrafted radiomics (HCR) | 0.761 | 0.698 | 0.821 | 0.787 | 0.741 |
| Deep learning (DL) | 0.779 | 0.792 | 0.768 | 0.763 | 0.796 |
| Ensemble (HCR + DL) | 0.853 | 0.886 | 0.821 | 0.825 | 0.885 |
| In-silico trial with clinicians | 0.66 ± 0.067 | 0.572 ± 0.186 | 0.750 ± 0.0525 | 0.680 ± 0.042 | 0.669 ± 0.100 |
Comparison of diagnostic performance on the external test dataset for HCR, DL, an ensemble of HCR and DL, and in-silico trial with clinicians.
The global SHAP summary plots in Figure 5A demonstrate that the same features extracted from each lung separately affect the model’s prediction for IPF diagnosis in a similar way. A high feature value with a positive SHAP value forces the model’s probability to be higher. The IH_qcod feature values extracted from lung1 and lung2 demonstrate a similar trend that a high feature value results in a positive SHAP value. However, there are some outliers in the trend that can seen be in features such as GLCM_correl1_lung and GLDZM_INN_lung. Similarly, the GLDZM_INN feature values extracted from lung1 and lung2 show a negative trend that a high feature value results in a negative SHAP value. Figures 5B–E show the dependence plots of GLCM_clusTend, GLCM_correl1, GLDZM_HISDE, and GLDZM_DZN features, respectively. In Figure 5C, when the feature value of GLDZM_HISDE is low, high feature values of GLCM_clusTend result in a lower SHAP value. A similar effect can be seen in Figure 5D between features GLDZM_DZN and NGLDM_DE.
FIGURE 5
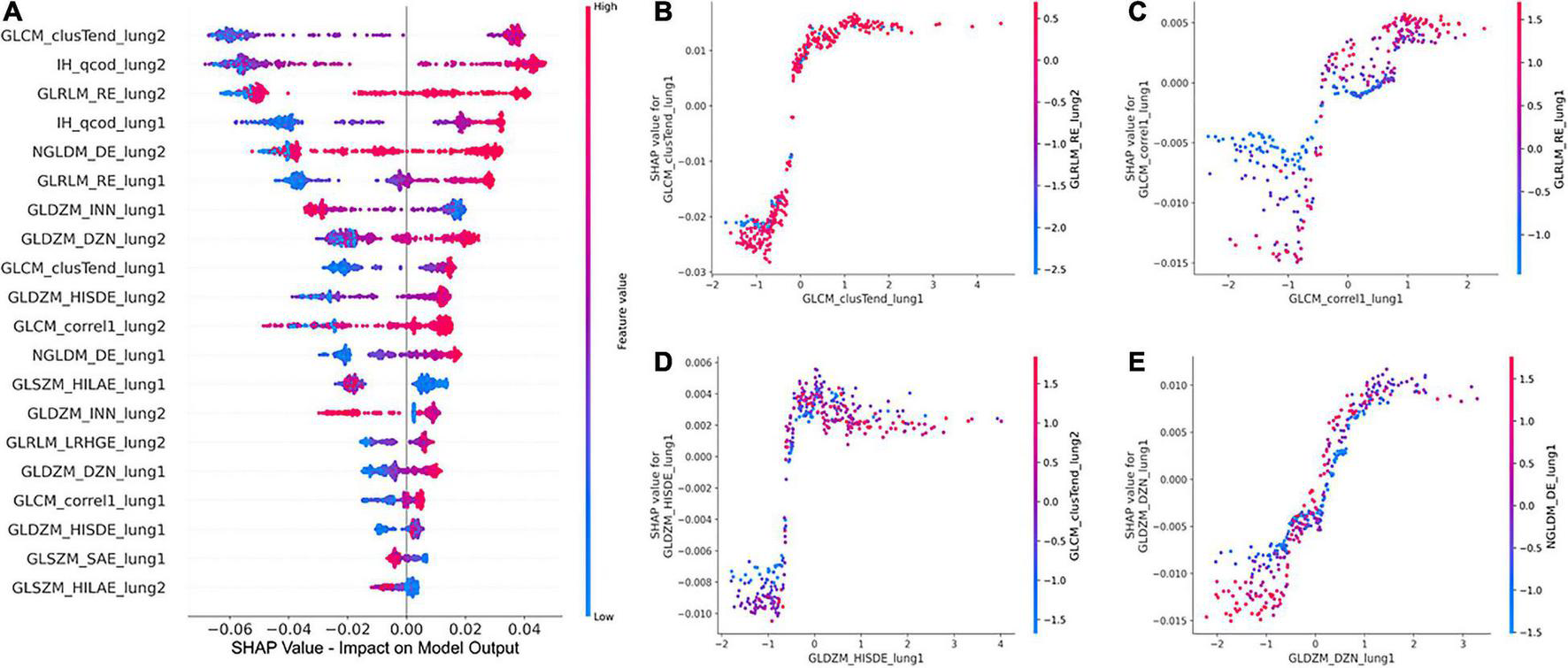
Global SHAP summary plots (A) demonstrate the impact of the top 20 features on the model output in terms of SHAP values and the corresponding feature values. SHAP dependence plots (B–D), and (E) show the effect of a particular feature value on the SHAP value and its interaction with another feature.
Deep Learning
The DL model achieved an AUC of 0.85 (95% CI: 0.806 – 0.904) in the validation set in five-fold cross-validation (Figure 4A). The threshold of 0.45 was fixed based on Youden’s index in the training set. An accuracy, sensitivity, and specificity of 0.779 ± 0.046, 0.711 ± 0.10, and 0.800 ± 0.075 was achieved during five-fold cross-validation, respectively. In the external test set, the DL model achieved an AUC, accuracy, sensitivity, and specificity of 0.823, 0.853, 0.886, and 0.821, respectively. Tables 2, 3 show the performance metrics for the HCR model during five-fold cross-validation and external validation, respectively. Figure 4B shows the test performance for the DL model on the external dataset.
Figure 6 shows Grad-CAM overlayed on CT image slices obtained from HRCT scans from IPF and non-IPF ILDs patients. The overlayed heatmap shows the regions of the input image that the model considers important for prediction. The Grad-CAM focuses on the tissue pattern in the patient with IPF. However, no information is provided on how these areas contribute to the final model prediction.
FIGURE 6

GradCAM heatmaps for post-hoc interpretability of IPF and non-IPF ILDs HRCT scans to understand the predictions made by the Densenet-121.
Ensemble
The ensemble model achieved an AUC of 0.93 (95% CI: 0.899 – 0.955) in the validation set during five-fold cross-validation (Figure 4A). The threshold of 0.49 was fixed based on Youden’s index in the training set. An accuracy, sensitivity, and specificity of 0.852 ± 0.027, 0.827 ± 0.005, and 0.860 ± 0.035 was obtained during five-fold cross-validation, respectively. In the external test set, the DL model achieved an AUC, accuracy, sensitivity, and specificity of 0.917, 0.853, 0.886, and 0.821, respectively. Tables 2, 3 show the performance metrics for the HCR model during five-fold cross-validation and external validation, respectively. The agreement between the predictions of HCR and DL models is 61.4%. The accuracy and specificity for the predictions when both the models agree were 93 and 97%, respectively. There was a statistically significant difference between the ROC curves for the ensemble model and HCR model (p = 0.02), and the ensemble model and the DL model (p = 0.005).
In-silico Clinical Trials
Two radiologists and one pulmonologist achieved accuracies of 58.7, 65.1, and 75.2% with a mean of 66.3 ± 6.7% for the diagnosis of IPF and non-IPF ILDs on the external test dataset. There was a statistically significant difference between performance of the ensemble model, and that of radiologists and pulmonologists (P < 0.05).
Discussion
In this study, we investigated the potential of HCR and DL to differentiate between different lung disorders i.e., IPF and non-IPF ILDs patients on HRCT scans. We also used post-hoc interpretability methods to explain the predictions of HCR and DL models. Moreover, we compare the performance of the proposed models to the diagnostic performance of radiologists using an in-silico trial on an external test set. Our results show that HCR and DL have a great potential to be used as an aid for clinical decision-making, which could minimize the time needed by radiologists, and increase diagnostic accuracy. The superior performance of an ensemble of DL and HCR models also demonstrates that these approaches can complement each other for lung disease diagnosis.
HCR and DL models achieved an accuracy of 76.2 ± 6.8% and 77.9 ± 4.6% during five-fold cross-validation, respectively. In the external test set, HCR and DL models demonstrated a similar accuracy of 76.1 and 77.9%, respectively. There was no statistically significant difference between the ROC curves for HCR and DL models. The ensemble of HCR and DL models demonstrated the best accuracy of 85.2 ± 2.7% and 85.3% for five-fold cross-validation and external test set, respectively. There was a statistically significant difference between the ROC curves for the ensemble model and HCR model (p = 0.023), and the ensemble model and DL model (p = 0.005). The HCR and DL models show an agreement of 61.4% for the predictions on the external test set. A sensitivity and specificity of 93 and 97% were obtained when both the models agreed on the prediction. Hence, HCR and DL models add complementary value to each other resulting in a boost in performance.
We compared the performance of the developed models against the performance of the radiologists using a virtual clinical trial setting. The performance of HCR (76.1%), DL (77.9%), and ensemble (85.3 %) models were better than the performance of two radiologists and one pulmonologist (66.7%) in discriminating IPF from non-IPF ILDs on the external test set. There was a statistically significant difference (p < 0.05) between the predictions of the ensemble model, and the two radiologists and one pulmonologist. There was a significant difference (p < 0.001) in the BMI, FEV, FVC, and DLCO values between site 1 and database A. The models demonstrated similar performance on the external database A despite the variability, showing that the trained models are robust and generalize well.
The clinical translation of HCR and DL is limited due to the “black-box” nature of the underlying complex classifiers. It is difficult for clinicians to understand the underlying mechanisms that govern the decision-making process of these complex classifiers. SHAP post-hoc explanations discover the patterns of the complex classifiers and increase transparency. SHAP global summary plots showed that Gray-level Co-occurrence Matrix Cluster Tendency and Intensity Histogram quartile coefficient of dispersion are the most important features for IPF diagnosis. These plots also showed that the same features extracted from different lungs demonstrate a similar trend in SHAP impact value. SHAP dependence plots demonstrated the effect of a single feature value and the interaction between a pair of features on the model output. Grad-CAM heatmaps highlight the area that the DL model considers important for the final prediction. These heatmaps can reinforce the trust in the model predictions if the model is focusing on the area relevant to the clinical task. However, Grad-CAM heatmaps do not offer any explanation of how the highlighted area contributes to the final prediction. Although DL demonstrates good performance, it is more opaque in nature due to its complexity that might hinder its clinical adoption.
Some studies previously investigated the potential of HCR and DL algorithms to classify lung disorders. Walsh el al. (18) employed a DL algorithm on a dataset of 1157 HRCT images for the diagnosis of fibrotic lung disease. The algorithm performance was compared to that of 91 radiologists and revealed an accuracy of 73.3%, compared to the radiologist’s median accuracy of 70.7%. When compared to Walsh et al. (18), our study demonstrated greater accuracy using HCR (76.1%), DL (77.9%), and an ensemble of HCR and DL (85.3%). Christe et al. (30) conducted another study in which they employed a computer-aided diagnostic (CAD) system (INTACT system) to diagnose IPF cases based on HRCT images and compared the performance of the CAD system to the performance of radiologists. Their findings showed that the two radiologists and the CAD system obtained an accuracy of 60, 54, and 56%, respectively. Mean RQS score of 20.4, 26.1, and 27.4% were obtained after recent analyses of papers reporting radiomics studies (31–33). This shows that RQS is a stringent and demanding criterion (34–36) that aims to encourage the best scientific practice. An RQS of 52.78% shows that this study tries to adhere to the best scientific practices and reporting guidelines.
This study has some limitations. The datasets utilized for this study contain HRCT scans acquired with different CT acquisition and reconstruction settings that can influence HCR feature values (37). Hence, phantom studies to evaluate the reproducibility of the HCR features or harmonization investigations need to be carried out to make a more robust HCR pipeline (38). Grad-CAMs only highlight the region of the input image that the model considers important for the decision-making process. There is a need to utilize interpretability methods that give an insight into how the relevant region contributes to the decision-making process (19). The high performance of an ensemble of HCR and DL model shows that these two approaches add complementary values. It may be useful to employ an interpretability method such as concept attribution that will investigate the HCR features that the DL model considers important for classification (39). A prospective virtual in-silico trial in a real-world environment where the predictions of DL/HCR model and post-hoc interpretability plots are made available to the doctors during diagnosis should be carried out to confirm the clinical utility of the proposed methods. The quality of lung segmentation can affect the performance of the models. Therefore, it is important to ensure the quality of the automatic segmentation in the presence of variability such as noise and artifacts.
At the moment, there is little research on the diagnosis of ILDs using HCR and DL. The reported results are encouraging and highlight the significant potential of HCR and DL methods for the diagnosis of IPF. In the future, HCR and DL approaches may be expanded to include treatment decisions. More studies should be conducted to explore the development of IPF at baseline and follow-up, as well as to assess the efficacy of anti-fibrotic treatment.
Conclusion
In this study, we developed handcrafted radiomics and deep learning models for the classification of IPF and non-IPF ILDs using HRCTs. In addition, we compared the performance of both models to radiologists on an external test dataset. HCR, DL, and ensemble models demonstrated better accuracy than radiologists in a virtual in-silico clinical trial setting. An ensemble of HCR and DL models demonstrated the best performance highlighting the complementary value of the two quantitative approaches for lung disease diagnosis. SHAP and GRAD-CAM post-hoc interpretability methods are useful for explaining the predictions made by radiomics and DL models, respectively. These automated diagnostic tools can serve as a useful clinical aid for diagnosing different lung diseases.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The datasets presented in this article are not readily available because the data from University Liege Hospital is privately owned while data from the Lung Tissue Research Consortium database (LTCR) is publically available. Requests to access the datasets should be directed to JG, j.guiot@chuliege.be; The Lung tissue research consortium database (LTCR): https://ltrcpublic.com.
Ethics statement
The studies involving human participants were reviewed and approved by the Local Ethics Committee of Hospitalo Facultaire Universitaire de Liège (CHU Hospital of Liège, Belgian number: B707201422832; ref: 2022/20). The ethics committee waived the requirement of written informed consent for participation.
Author contributions
TR and ZS: conceptualization, methodology, formal analysis, data curation, writing – original draft, and project administration. A-NF: conceptualization. CY and GW: data curation. HW: writing – review and editing. HG, PM, and RL: resources. JG and PL: conceptualization, methodology, writing – review and editing, project administration, funding, and supervision. PL: guarantor for the manuscript. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge financial support from ERC advanced grant (ERC-ADG-2015 n° 694812 - Hypoximmuno), ERC-2020-PoC: 957565-AUTO.DISTINCT. Authors also acknowledge financial support from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement: ImmunoSABR n° 733008, MSCA-ITN-PREDICT n° 766276, CHAIMELEON n° 952172, EuCanImage n° 952103, JTI-IMI2-2020-23-two-stage IMI-OPTIMA n° 101034347, and TRANSCAN Joint Transnational Call 2016 (JTC2016 CLEARLY n° UM 2017-8295). This work was supported by the Dutch Cancer Society (KWF Kankerbestrijding), Project number: 14449/2021-PoC.
Conflict of interest
PL reports, as non-practicing MD, in the last 3 years, within and outside the submitted work, grants/sponsored research agreements from Radiomics SA, Convert Pharmaceuticals and LivingMed Biotech. PL received a presenter fee (in cash or in kind) and/or reimbursement of travel costs/consultancy fee (in cash or in kind) from Radiomics SA, BHV, Varian, Elekta, ptTheragnostic/DNAmito, BMS, and Convert pharmaceuticals. PL has minority shares in the companies Radiomics SA, Convert pharmaceuticals, Comunicare, and LivingMed Biotech, and he is co-inventor of two issued patents with royalties on radiomics (PCT/NL2014/050248 and PCT/NL2014/050728), licensed to Radiomics SA; one issued patent on mtDNA (PCT/EP2014/059089), licensed to ptTheragnostic/DNAmito; one non-issued patent on LSRT (PCT/P126537PC00), licensed to Varian; three non-patented inventions (softwares) licensed to ptTheragnostic/DNAmito, Radiomics SA and Health Innovation Ventures and two non-issued, non-licensed patents on Deep Learning-Radiomics (N2024482, N2024889). PL confirms that none of the above entities or funding sources were involved in the preparation of this manuscript. JG reports personal fees for advisory board, work and lectures from Boehringer Ingelheim, Janssens, SMB, GSK, Roche and Chiesi, non-financial support for meeting attendance from Chiesi, Roche, Boehringer Ingelheim and Janssens. He is in the permanent SAB of Radiomics (Oncoradiomics SA) for the SALMON trial without any specific consultancy fee for this work. He is co-inventor of one issued patent on radiomics licensed to Radiomics (Oncoradiomics SA). He confirms that none of the above entities or funding was involved in the preparation of this work. HW has minority shares in the company Radiomics SA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.915243/full#supplementary-material
Footnotes
References
1.
Coultas DB Zumwalt RE Black WC Sobonya RE . The epidemiology of interstitial lung diseases.Am J Respir Crit Care Med. (1994) 150:967–72. 10.1164/ajrccm.150.4.7921471
2.
Cottin V . Pulmonary fibrosis: “idiopathic” is not “cryptogenic”.Eur Respir J. (2019) 53:1802314. 10.1183/13993003.02314-2018
3.
Travis WD Costabel U Hansell DM King TE Jr. Lynch DA Nicholson AG et al An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. (2013) 188:733–48. 10.1164/rccm.201308-1483ST
4.
Raghu G Remy-Jardin M Myers JL Richeldi L Ryerson CJ Lederer DJ et al Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. (2018) 198:e44–68. 10.1164/rccm.201807-1255ST
5.
Fischer A du Bois R . Interstitial lung disease in connective tissue disorders.Lancet. (2012) 380:689–98. 10.1016/S0140-6736(12)61079-4
6.
Fernández Pérez ER Swigris JJ Forssén AV Tourin O Solomon JJ Huie TJ et al Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. (2013) 144:1644–51. 10.1378/chest.12-2685
7.
Raghu G Collard HR Egan JJ Martinez FJ Behr J Brown KK et al An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. (2011) 183:788–824. 10.1164/rccm.2009-040GL
8.
Richeldi L Collard HR Jones MG . Idiopathic pulmonary fibrosis.Lancet. (2017) 389:1941–52. 10.1016/S0140-6736(17)30866-8
9.
Ley B Collard HR King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2011) 183:431–40. 10.1164/rccm.201006-0894CI
10.
Guiot J Vaidyanathan A Deprez L Zerka F Danthine D Frix A-N et al A review in radiomics: making personalized medicine a reality via routine imaging. Med Res Rev. (2022) 42:426–40. 10.1002/med.21846
11.
Cho YH Seo JB Lee SM Lee SM Choe J Lee D et al Quantitative CT imaging in chronic obstructive pulmonary disease: review of current status and future challenges. Korean J Radiol. (2018) 78:1. 10.3348/jksr.2018.78.1.1
12.
Hochhegger B Marchiori E Zanon M Rubin AS Fragomeni R Altmayer S et al Imaging in idiopathic pulmonary fibrosis: diagnosis and mimics. Clinics. (2019) 74:e225. 10.6061/clinics/2019/e225
13.
Tominaga J Sakai F Johkoh T Noma S Akira M Fujimoto K et al Diagnostic certainty of idiopathic pulmonary fibrosis/usual interstitial pneumonia: the effect of the integrated clinico-radiological assessment. Eur J Radiol. (2015) 84:2640–5. 10.1016/j.ejrad.2015.08.016
14.
Walsh S de Jong EEC van Timmeren JE Ibrahim A Compter I Peerlings J et al Decision support systems in oncology. JCO Clin Cancer Inform. (2019) 3:1–9. 10.1200/CCI.18.00001
15.
Lambin P Rios-Velazquez E Leijenaar R Carvalho S van Stiphout RGPM Granton P et al Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. (2012) 48:441–6. 10.1016/j.ejca.2011.11.036
16.
Gillies RJ Kinahan PE Hricak H . Radiomics: images Are More than Pictures, They Are Data.Radiology. (2016) 278:563–77. 10.1148/radiol.2015151169
17.
LeCun Y Bengio Y Hinton G . Deep learning.Nature. (2015) 521:436–44. 10.1038/nature14539
18.
Walsh SLF Calandriello L Silva M Sverzellati N . Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study.Lancet Respir Med. (2018) 6:837–45. 10.1016/S2213-2600(18)30286-8
19.
Salahuddin Z Woodruff HC Chatterjee A Lambin P . Transparency of deep neural networks for medical image analysis: a review of interpretability methods.Comput Biol Med. (2021) 140:105111. 10.1016/j.compbiomed.2021.105111
20.
Lundberg SM Lee S-I . A unified approach to interpreting model predictions.Proceedings of the 31st International Conference on Neural Information Processing Systems.Long Beach, CA: Curran Associates Inc (2017). p. 4768–77.
21.
Selvaraju RR Cogswell M Das A Vedantam R Parikh D Batra D . Grad-cam: Visual explanations from deep networks via gradient-based localization.Proceedings of the IEEE International Conference on Computer Vision.Cambridge, MA: IEEE (2017). p. 618–26.
22.
Zwanenburg A Vallières M Abdalah MA Aerts HJWL Andrearczyk V Apte A et al The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. (2020) 295:328–38. 10.1148/radiol.2020191145
23.
Jia T-Y Xiong J-F Li X-Y Yu W Xu Z-Y Cai X-W et al Identifying EGFR mutations in lung adenocarcinoma by noninvasive imaging using radiomics features and random forest modeling. Eur Radiol. (2019) 29:4742–50. 10.1007/s00330-019-06024-y
24.
Bashir U Kawa B Siddique M Mak SM Nair A Mclean E et al Non-invasive classification of non-small cell lung cancer: a comparison between random forest models utilising radiomic and semantic features. Br J Radiol. (2019) 92:20190159. 10.1259/bjr.20190159
25.
Jiang C Luo Y Yuan J You S Chen Z Wu M et al CT-based radiomics and machine learning to predict spread through air space in lung adenocarcinoma. Eur Radiol. (2020) 30:4050–7. 10.1007/s00330-020-06694-z
26.
Huang G Liu Z Van Der Maaten L Weinberger KQ . Densely connected convolutional networks.Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition.Honolulu: IEEE (2017). p. 4700–8.
27.
Monai Consortium. MONAI: Medical Open Network for AI. Zenodo (2020). 10.5281/zenodo.5728262
28.
Bossuyt PM Reitsma JB Bruns DE Gatsonis CA Glasziou PP Irwig L et al STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. (2015) 351:h5527. 10.1136/bmj.h5527
29.
Lambin P Leijenaar RTH Deist TM Peerlings J de Jong EEC van Timmeren J et al Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. (2017) 14:749–62. 10.1038/nrclinonc.2017.141
30.
Christe A Peters AA Drakopoulos D Heverhagen JT Geiser T Stathopoulou T et al Computer-aided diagnosis of pulmonary fibrosis using deep learning and CT images. Invest Radiol. (2019) 54:627–32. 10.1097/RLI.0000000000000574
31.
Park JE Kim D Kim HS Park SY Kim JY Cho SJ et al Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol. (2020) 30:523–36. 10.1007/s00330-019-06360-z
32.
Lee S Han K Suh YJ . Quality assessment of radiomics research in cardiac CT: a systematic review.Eur Radiol. (2022) 32:3458–68. 10.1007/s00330-021-08429-0
33.
Stanzione A Gambardella M Cuocolo R Ponsiglione A Romeo V Imbriaco M . Prostate MRI radiomics: a systematic review and radiomic quality score assessment.Eur J Radiol. (2020) 129:109095.
34.
Spadarella G Calareso G Garanzini E Ugga L Cuocolo A Cuocolo R . MRI based radiomics in nasopharyngeal cancer: systematic review and perspectives using radiomic quality score (RQS) assessment.Eur J Radiol. (2021) 140:109744. 10.1016/j.ejrad.2021.109744
35.
Won SY Park YW Ahn SS Moon JH Kim EH Kang S-G et al Quality assessment of meningioma radiomics studies: bridging the gap between exploratory research and clinical applications. Eur J Radiol. (2021) 138:109673. 10.1016/j.ejrad.2021.109673
36.
Park JE Kim HS Kim D Park SY Kim JY Cho SJ et al A systematic review reporting quality of radiomics research in neuro-oncology: toward clinical utility and quality improvement using high-dimensional imaging features. BMC Cancer. (2020) 20:29. 10.1186/s12885-019-6504-5
37.
Ibrahim A Primakov S Beuque M Woodruff HC Halilaj I Wu G et al Radiomics for precision medicine: current challenges, future prospects, and the proposal of a new framework. Methods. (2021) 188:20–9. 10.1016/j.ymeth.2020.05.022
38.
Mali SA Ibrahim A Woodruff HC Andrearczyk V Müller H Primakov S et al Making radiomics more reproducible across scanner and imaging protocol variations: a review of harmonization methods. J Pers Med. (2021) 11:842. 10.3390/jpm11090842
39.
Graziani M Andrearczyk V Müller H . Regression concept vectors for bidirectional explanations in histopathology. In: StoyanovDTaylorZMostafa KiaSOguzIReyesMet aleditors. Understanding and Interpreting Machine Learning in Medical Image Computing Applications.Berlin: Springer International Publishing (2018). p. 124–32. 10.1007/978-3-030-02628-8_14
Summary
Keywords
artificial intelligence (AI), radiomics, computed tomography, interpretability, idiopathic pulmonary fibrosis, interstitial lung disease
Citation
Refaee T, Salahuddin Z, Frix A-N, Yan C, Wu G, Woodruff HC, Gietema H, Meunier P, Louis R, Guiot J and Lambin P (2022) Diagnosis of Idiopathic Pulmonary Fibrosis in High-Resolution Computed Tomography Scans Using a Combination of Handcrafted Radiomics and Deep Learning. Front. Med. 9:915243. doi: 10.3389/fmed.2022.915243
Received
07 April 2022
Accepted
07 June 2022
Published
23 June 2022
Volume
9 - 2022
Edited by
Somchai Chutipongtanate, University of Cincinnati, United States
Reviewed by
Danai Khemasuwan, Virginia Commonwealth University, United States; Zhenwei Shi, Guangdong Academy of Medical Sciences, China
Updates
Copyright
© 2022 Refaee, Salahuddin, Frix, Yan, Wu, Woodruff, Gietema, Meunier, Louis, Guiot and Lambin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Turkey Refaee, t.refaee@maastrichtuniversity.nl
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
This article was submitted to Precision Medicine, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.