Abstract
Background:
Vaginitis is the most common gynecologic diagnosis in primary care, and most women have at least one episode during their lives. The need for standardized strategies to diagnose and treat vaginitis, both in primary care and among gynecologists, is emphasized. The Brazilian Group for Vaginal Infections (GBIV, acronym in Portuguese) aimed to update the practical approach to affected women by reviewing and discussing recent literature, and developing algorithms for diagnosis and treatment of vaginitis.
Methods:
A literature search within biomedical databases PubMed and SCieLo was conducted in January 2022. The available literature was evaluated by three experienced researchers, members of the GBIV, to summarize the main data and develop practical algorithms.
Results and conclusion:
Detailed algorithms were developed with the main goal to improve gynecological practice considering different scenarios and access to diagnostic tools, from the simplest to the most complex tests. Different age groups and specific contexts were also considered. The combination of anamnesis, gynecological examination, and complementary tests remains the basis of a proper diagnostic and therapeutic approach. Periodic updates of these algorithms are warranted as new evidence becomes available.
Introduction
Vaginal symptoms, such as discharge, odor, and itching are frequent, especially in women of reproductive age (1). Vaginitis is the most common gynecologic diagnosis in primary care, and most women have at least one episode during their lives (2). It has been demonstrated that vaginitis may have a negative impact on quality of life, including embarrassment, and anxiety, particularly in women with recurrent episodes (2).
Bacterial vaginosis (BV), the most common cause of vaginal discharge, has also been associated with several potential complications, including a higher risk of preterm delivery, and an increased risk of acquiring human immunodeficiency virus (HIV) infection, human papillomavirus (HPV) infection, other sexually transmitted diseases, and pelvic inflammatory disease (3). BV is the cause in 40–50% of cases with an identified etiology (2). Vulvovaginal candidiasis (VVC) and trichomoniasis account for 20–25% and 15–20% of cases in premenopausal women, respectively (4). Non-infectious causes are less common and account for the remaining cases (2, 4). Mixed vaginitis, along with VVC and BV, is considered an important differential diagnosis; according to Lowe et al. (5) in a case series considering 545 women with signs and symptoms of vulvovaginitis, BV, VVC, and mixed vaginitis accounted for 42, 14, and 16% of all patients, respectively.
In the context of the high prevalence and importance of BV and vaginitis, the need for standardized strategies to diagnose and treat these conditions, both in primary care and among gynecologists, is emphasized. The Brazilian Group for Vaginal Infections (GBIV, for its acronym in Portuguese) aimed to help physicians update the practical approach to affected women by reviewing and discussing recent literature, and developing algorithms for diagnosis and treatment in different healthcare levels of assistance.
Methods
To identify the approaches employed in diagnosing and treating BV and vaginitis to develop the proposed algorithms, a literature search within biomedical databases PubMed and SCieLo was conducted in January 2022. The search terms were “bacterial vaginosis” and/or “vaginitis” AND “clinical reasoning,” “differential diagnosis,” “diagnostic,” “treatment,” “approach.” Portuguese and Spanish equivalent words were also used. Wildcards were allowed.
Papers published until the cutoff date that contained the query in the title or the abstract were selected. Only articles in English, Spanish, or Portuguese indexed as original articles or reviews were considered. After reading the abstracts, the articles not related to clinical and/or laboratory diagnosis or pharmacological or non-pharmacological treatments were excluded. Subsequently, references of the publications were searched for additional relevant articles.
The available literature was evaluated by three experienced researchers, members of the GBIV, to summarize the main data and develop practical algorithms.
Results
Diagnostic tools
Normal physiological vaginal discharge changes with the menstrual cycle and may increase premenstrually, at the time of ovulation and in women who start hormone replacement treatment or hormonal contraception (6). In contrast, vaginal infection and microbiota alterations can be associated with different discharge forms, local irritation, pruritus (itching), and pain (6). Anamnesis should include aspects related to vaginal discharge (color, consistency, unpleasant odor, and modifications), sexual behavior and practices, type of contraceptive method, vaginal hygiene practices, date of last menstrual period, use of topical or systemic medication, and other potential irritating agents (6). The presence of comorbidities like diabetes mellitus and HIV infection should also be evaluated (6).
During the gynecological examination, the healthcare professional must identify the characteristics of vaginal fluid during the speculum examination. The presence of alterations, including colpitis, ulcers, fissures, edema, and erythema should be highlighted (6).
Office-based tests (vaginal pH, amine test, saline, and 10% KOH microscopy) are easily available and may frequently help to diagnose common forms of vaginitis; however, office testing may have poor performance characteristics (7).
Even though anamnesis, gynecological examination, and office-based tests remain the primary diagnostic tools, current evidence suggests that clinical diagnoses may be poorly correlated with laboratory findings (8). Therefore, laboratory tests are warranted to determine the etiology of vaginal symptoms (9). Frequent pathogens are often identified after examination of the vaginal contents by bacterioscopy and the Pap smear (although the test is not primarily for this type of diagnosis). For Candida and Trichomonas, the sensitivity rate of the Pap test is 50% (7). More recently, liquid-based cytology and DNA probe tests were also added to the diagnostic tools for vaginal symptoms (7). Molecular tests directed to BV diagnosis, Candida, and T. vaginalis can improve diagnostic accuracy and reduce the time to diagnosis when compared to conventional culture techniques (9, 10).
Specific vaginitis causes
Bacterial vaginosis is a disorder associated with a decrease in peroxide-producing lactobacilli and an increase in the number of anaerobic and facultative bacteria (11), including short Gram-variable bacilli, short Gram-negative bacilli, and anaerobic Gram-negative cocci, with variations, mainly Gardnerella, Atopobium, Prevotella, Megasphaera, Leptotrichia, Sneathia, Bifidobacterium, Dialister, Mobiluncus, Ureaplasma, and Mycoplasma (6). Even though BV is the most frequent cause of vaginal discharge in woman of reproductive age (2), it is usually underdiagnosed in the general practitioner setting (8). Although some cases are asymptomatic, BV tends to present with a thin discharge, characterized by a foul smell in alkaline environments (including semen, blood, and 10% KOH) (9). BV is diagnosed using either Amsel’s criteria or Nugent score; due to methodological differences between these two diagnostic techniques, accuracy results may vary when these methods are compared (12).
Vulvovaginal candidiasis is characterized by erythema, vulvar fissures, clumpy discharge, vulvar edema, lesions related to intense scratching, and eventually dyspareunia and dysuria due to irritation and local lesions. Diagnosis must be confirmed through laboratory examinations, mainly microscopic examination of fresh vaginal content and Gram-stained vaginal smear bacterioscopy. The culture is essential in cases of recurrent VVC to identify Candida species (6). Recently, molecular biology tools [mainly polymerase chain reaction (PCR) based on the multiplex platform] were developed; these tools are characterized by high sensitivity rates and faster results for identifying Candida species, and may be indicated instead of culture (13).
Vulvovaginal candidiasis is defined as non-complicated when all the following criteria are met: mild/moderate symptoms and rare frequency, C. albicans as an etiologic agent, and lack of comorbidities. In contrast, complicated VVC is diagnosed whether at least one of these criteria is met: intense symptoms; recurrence (≥3 episodes/year); pregnancy; C. non-albicans as etiologic agent; and comorbidities such as diabetes mellitus or HIV (6, 14).
Most cases of Trichomonas infections are asymptomatic, precluding diagnosis or treatment. Symptomatic women may present with greenish-yellow and foamy intense vaginal discharge, with a fetid odor. Pruritus, bleeding during intercourse, dyspareunia, vulvar edema, and even urinary symptoms may be associated. Microscopic examination of fresh vaginal content in saline may reveal moving parasites, and Papanicolaou or Giemsa techniques may also identify this agent (6). Nevertheless, the gold standard method for identification of T. vaginalis is real-time PCR (14).
In addition to the previously described clinical syndrome, mixed vaginitis is characterized by two concurrent pathogens causing the disease. Symptoms may vary depending on the microorganisms involved; nevertheless, the etiological diagnosis is essential, mainly using multiplex platforms. Study of microorganisms by examination of fresh or stained (Gram) vaginal fluid and Pap smear could also be used. The most frequent mixed vaginitis is concurrent BV and VVC (15). According to Qui et al. (16) there is significant variability in the prevalence of mixed vaginitis, which includes BV/VVC, BV/aerobic vaginitis (AV), and VVC/AV, among others. Studies on this condition are still preliminary, and many cases of mixed vaginitis are underdiagnosed.
Other causes of vaginitis are less common; however, it is essential for healthcare providers to diagnose and treat these conditions properly. Differential diagnoses include cytolytic vaginosis, desquamative inflammatory vaginitis (DIV), and AV. Cytolytic vaginosis is the consequence of an aerobic imbalance of vaginal microbiota, with an increase in the lactobacilli population leading to cytolysis. Patients usually report pruritus and discharge similar to VVC, and laboratorial diagnosis is based on Gram bacterioscopy (17).
Desquamative inflammatory vaginitis is an ill-defined condition causing purulent discharge, vestibule-vaginal irritation, and dyspareunia. DIV is characterized by an increase in inflammatory cells and parabasal epithelial cells (immature squamous cells). Vaginal microbiota is abnormal and pH is elevated (>4.5) (18). DIV is defined by some authors as a more severe picture of aerobic vaginitis, a condition associated with aerobic microorganisms, mainly group B streptococci and E. coli, even though these microorganism are not causal of AV/DIV. Its characteristics are different from those of BV and elicit an important host response. AV diagnostic criteria, as described by Donders et al. (19) were based on phase contrast microscopy of vaginal fluid by developing a score including the lactobacilli grade, the leukocytes count, the presence of toxic leukocytes, the microbiota morphotypes and the proportion of parabasal cells. A composite score of 1–2 represents normality. Respective scores of 3–4, 5–6, and 6–10 represent slight, moderate or severe AV. According to the authors, the severe form of AV would be identical to DIV (19).
Finally, atrophic vaginitis is associated with low estrogen levels and may cause symptoms like vaginal discharge, pruritus, and dyspareunia. The diagnosis is based on Pap smear and Gram bacterioscopy (1).
It is also extremely important to exclude cervicitis, as this clinical condition may also cause symptoms and lead to local changes that may favor the development of vaginitis. The main agents causing cervicitis include Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium (7).
Diagnostic algorithm
The proposed diagnostic algorithm is shown in Figure 1. Complementary information for specific and differential diagnosis is summarized in Tables 1, 2, respectively (6–13).
FIGURE 1
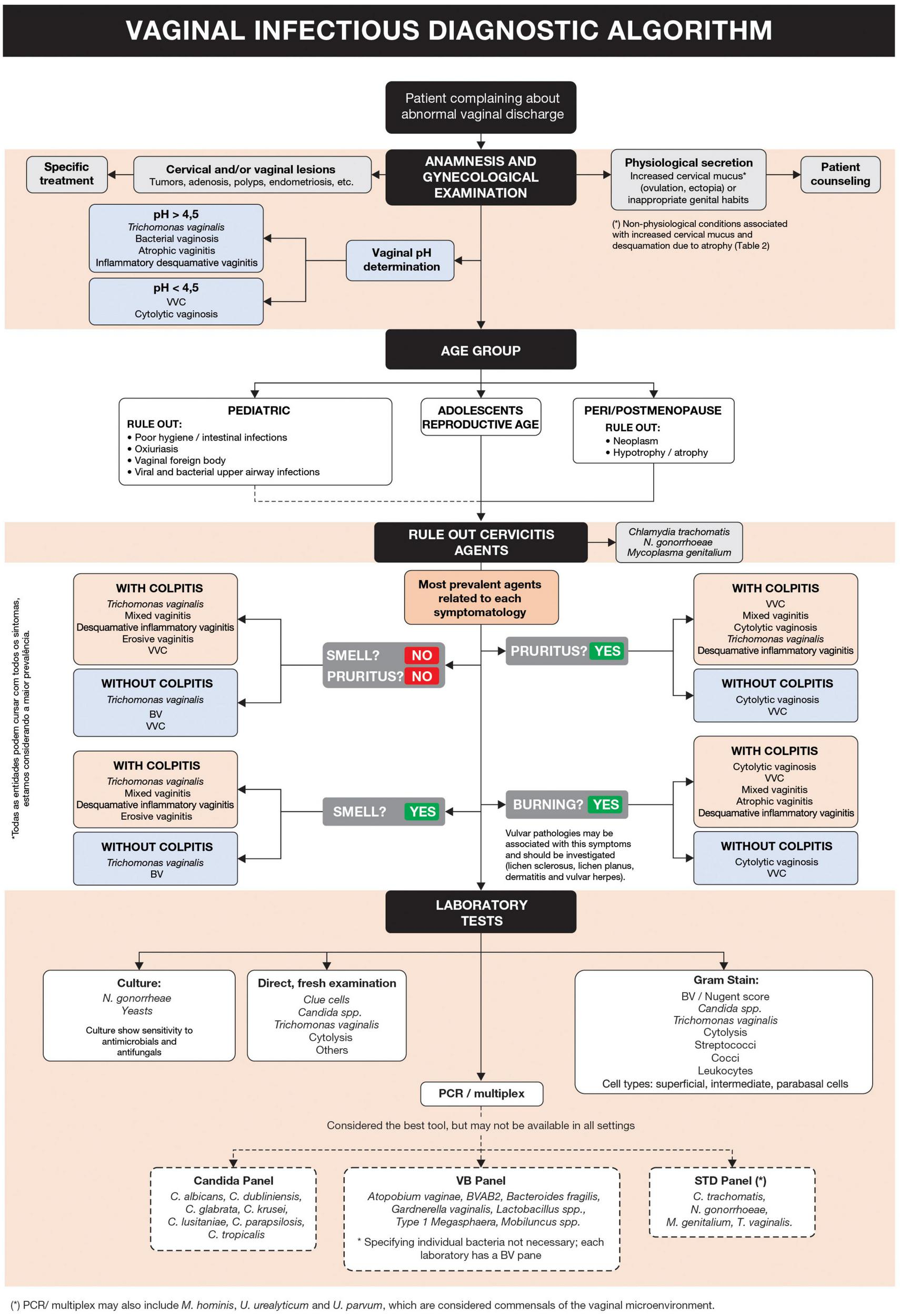
Vaginal infectious diagnostic algorithm.
TABLE 1
| Agent | Test | Results | Sensitivity |
| Candida spp | Vaginal pH | <4.5 | – |
| Fresh sample microscopy | Morphotype observation | 60% | |
| Gram stain | Morphotype observation | 80% | |
| Amine test | Negative | – | |
| Culture (Sabouraud and Chromoagar): allows antifungal sensitivity determination | Specific species identification | >95% | |
| PCR/Multiplex–Candida albicans and non-albicans panel | Specific species identification | >95% | |
| Trichomonas vaginalis | Vaginal pH | >4.5 | – |
| Wet mount microscopy | Parasite observation | 50% without colpitis* 80% with colpitis |
|
| Gram stain | Morphotype observation | <40%* | |
| Amine test | Usually positive | – | |
| PCR/Multiplex | Specific species identification | >95% | |
| Chlamydia trachomatis | PCR/Multiplex | Specific species identification | >95%** |
| N. gonorrheae | Gram stain | Intraleukocytic Gram-negative diplococci | ∼70%*** |
| Culture (Thayer–Martin) | Colony growth | ∼90% | |
| PCR/Multiplex | Specific species identification | >95% | |
| Mycoplasma spp. | PCR/Multiplex | Specific species identification | >95% |
| Mixed vaginitis | Wet mount microscopy | Clue cell identification, associated with Candida morphotypes | 70% |
| Gram stain | Candida morphotype identification (score 7–10) | 90% | |
| PCR/Multiplex (BV + Candida) | Specific species identification | >95% |
Laboratory tests for diagnosing infectious microorganisms that may be observed in vaginitis and cervicitis (Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma spp).
*Cases without colpitis are usually associated with the pseudocyst form of T. vaginalis (parasites are immobile and smaller than the active form). **Persistent discharge may be associated with interference in the microbiota caused by C. trachomatis cervicitis. ***If the sample is taken from the cervix, the sensitivity is lower than 50%.
TABLE 2
| Disorder | Test | Result | Sensitivity |
| Cytolytic vaginosis | Vaginal pH | <4.5 | – |
| Wet mount microscopy | Cytolysis without inflammation | 70% | |
| Gram stain | Cytolysis without inflammation | 90% | |
| Pap smear* | Cytolysis without inflammation | – | |
| Fungi culture | Negative | – | |
| PCR/Multiplex for pathogens (Table 1) | Negative | – | |
| Aerobic vaginitis/desquamative, inflammatory vaginitis | Vaginal pH | >4.5 | – |
| Fresh sample microscopy | Identification of parabasal cells and inflammatory infiltrates | 50% | |
| Gram stain | Identification of parabasal cells and inflammatory infiltrates | 50% | |
| Atrophic vaginitis | Vaginal pH | >4.5 | – |
| Gram stain | Identification of parabasal cells and sparse microbiota composed predominantly of cocci | – | |
| Pap smear | Identification of parabasal cells and “blue blobs” | >90% |
Available tests for the differential diagnosis of less frequent causes of vaginitis.
*Used for cervical cancer screening, but it can show pathogens.
Treatment
The treatment algorithm for VVC is shown in Figure 2.
FIGURE 2
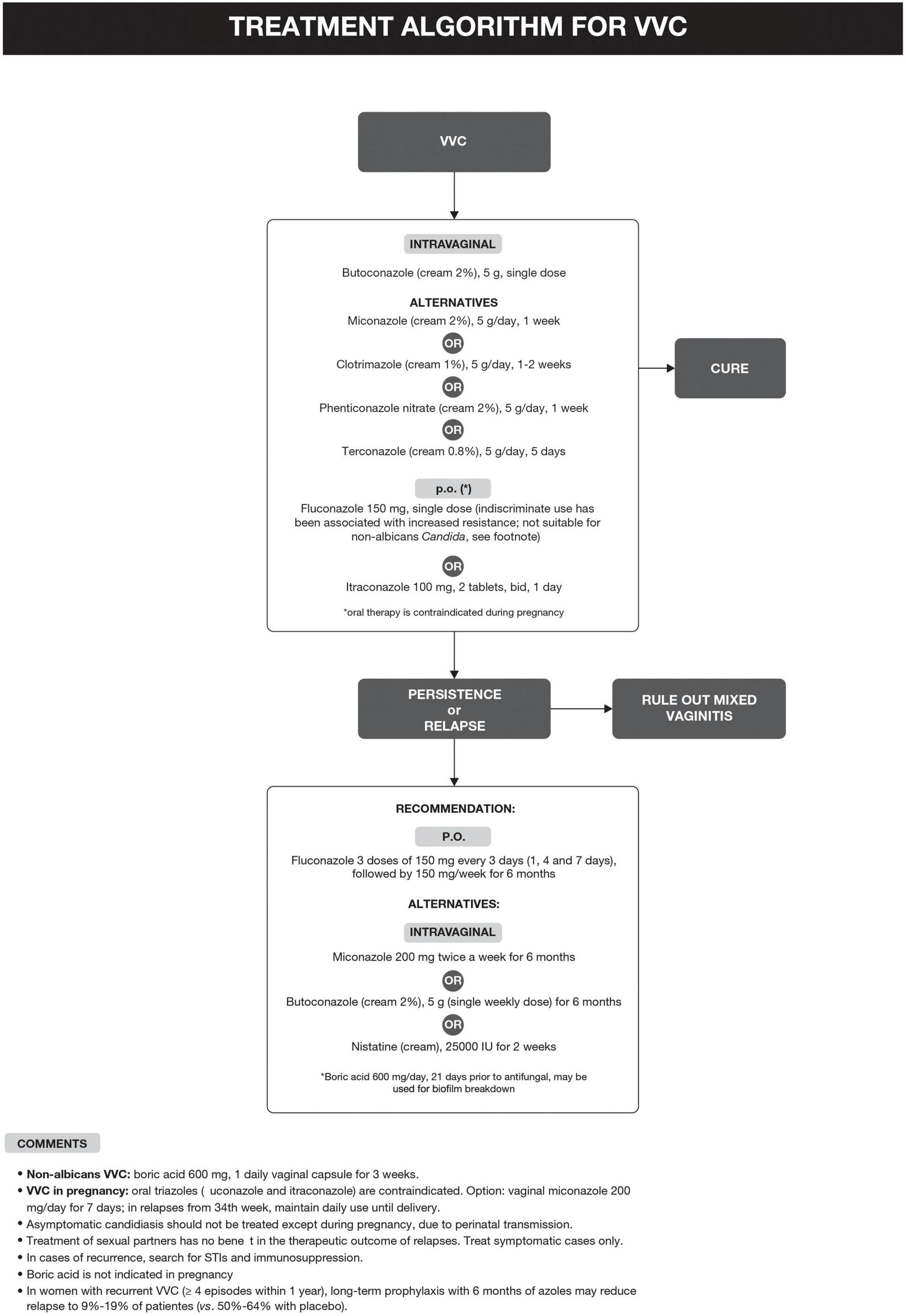
Treatment algorithm for vulvovaginal candidiasis (VVC).
The recommended treatment of T. vaginalis is summarized in Figure 3 (6, 14–31). Taking into account a recurrence rate of 5–31%, it is important to assess partners’ treatment, exposure to new partners, and the possibility of therapeutic failure, which seems more frequent in women treated with a single dose regimen and with HIV infection (6, 16).
FIGURE 3
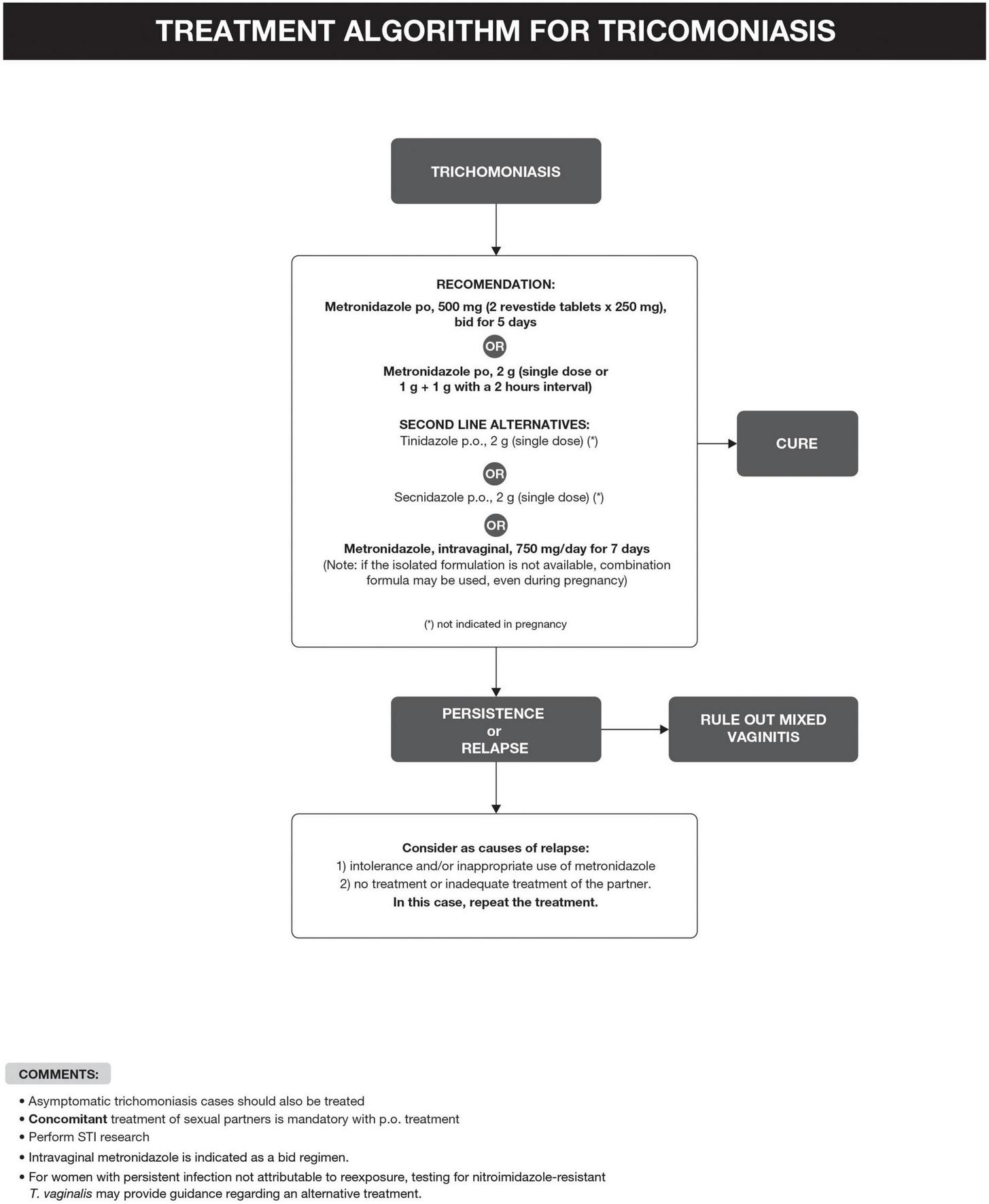
Treatment algorithm for trichomoniasis.
Bacterial vaginosis treatment is recommended for all symptomatic and asymptomatic women with the potential risk of complications, such as previous gynecological surgeries or procedures. Recurrence of BV after treatment is common. Some factors have been associated with the lack of therapeutic response, including frequent unprotected sexual intercourse, vaginal douches, altered immune response, the occurrence of biofilms, and bacterial resistance to imidazoles and clindamycin (6, 29). The proposed treatment algorithm is shown in Figure 4 (6, 15, 18, 20–26). Treatment for other causes of vaginal symptoms (6, 27, 28) is summarized in Figure 5. Treatment for aerobic vaginitis/desquamative inflammatory vaginitis, atrophic vaginitis and cervicitis is summarized in Figure 6 (6, 14–31).
FIGURE 4
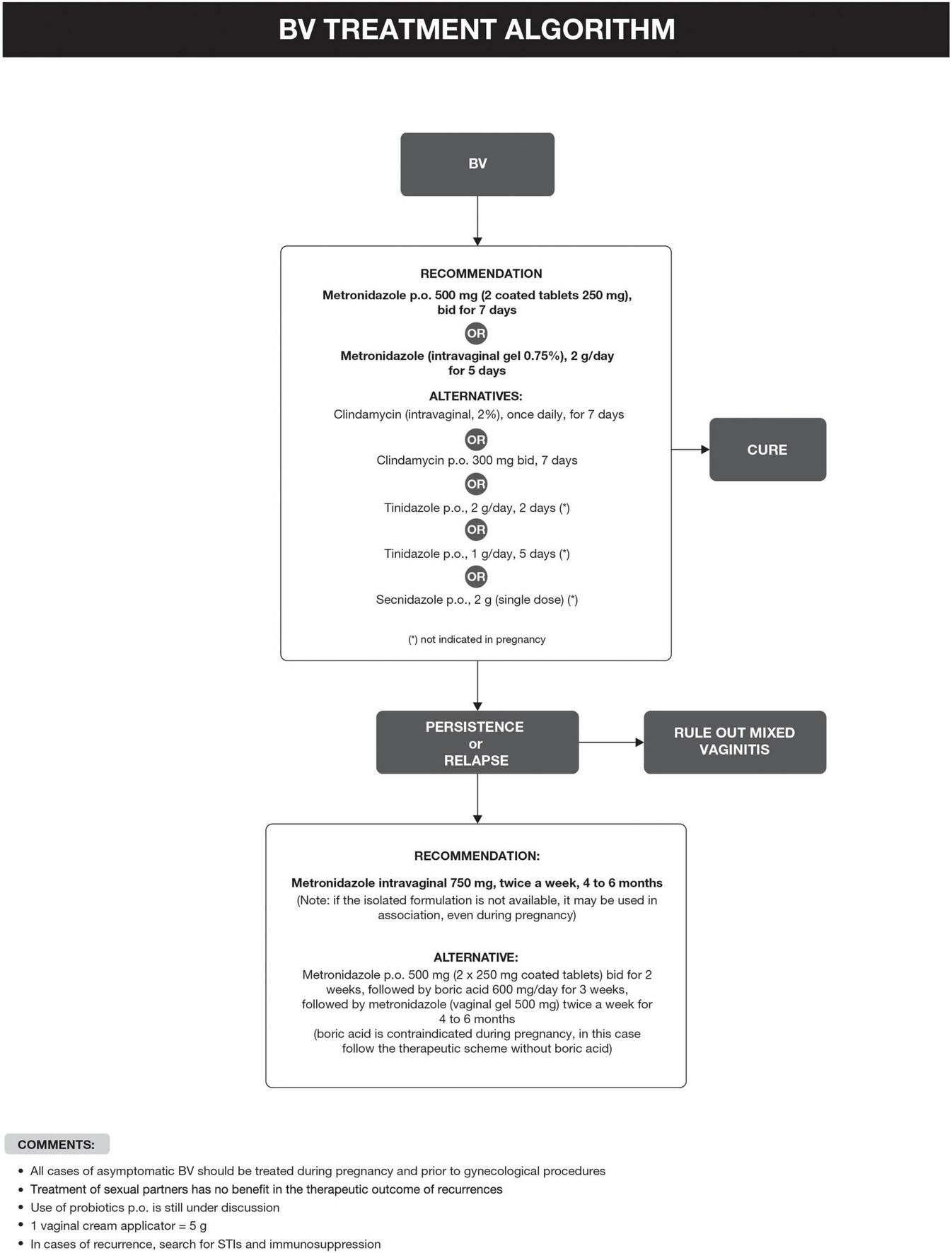
Bacterial vaginosis (BV) treatment algorithm.
FIGURE 5
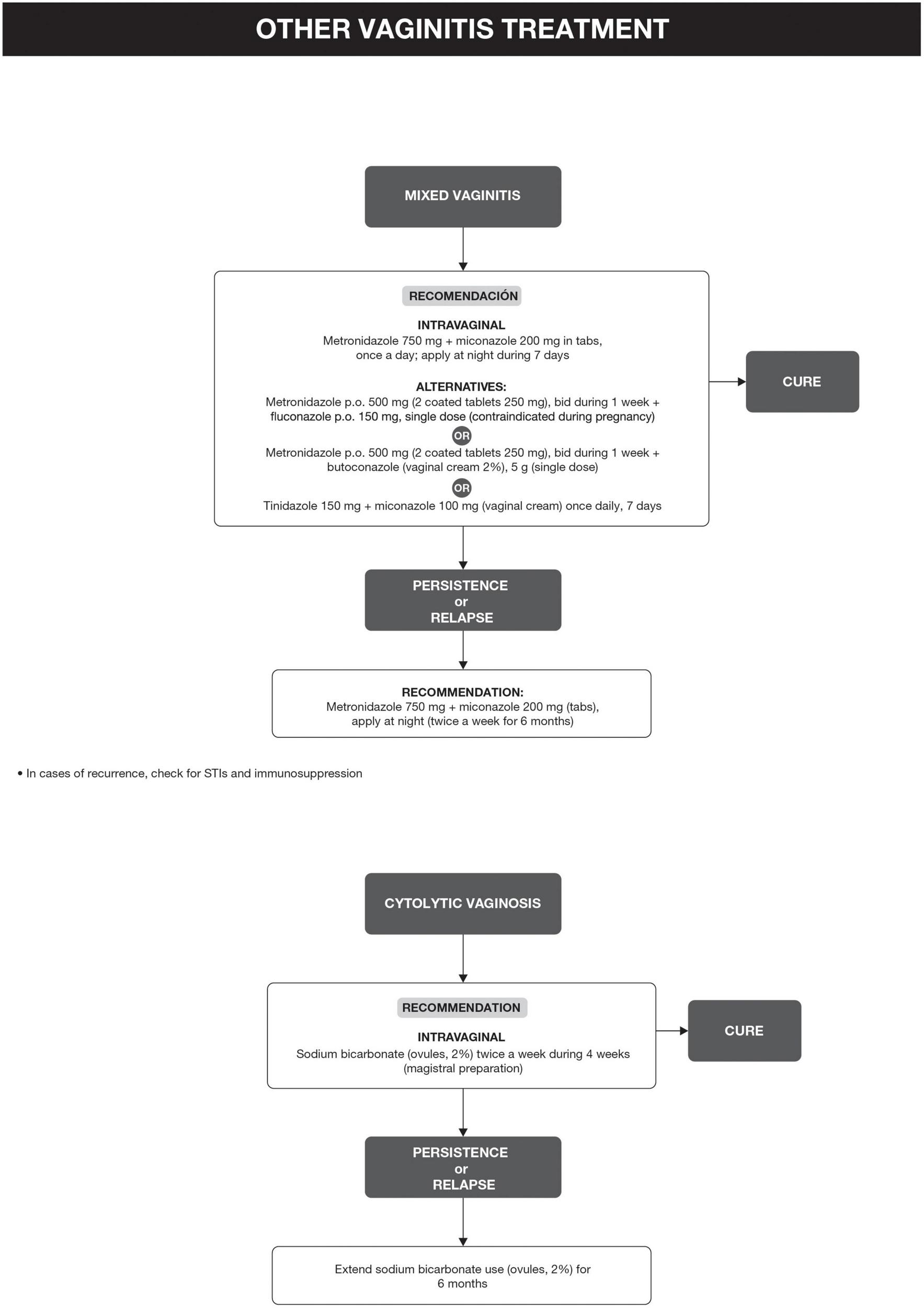
Other vaginitis treatment.
FIGURE 6
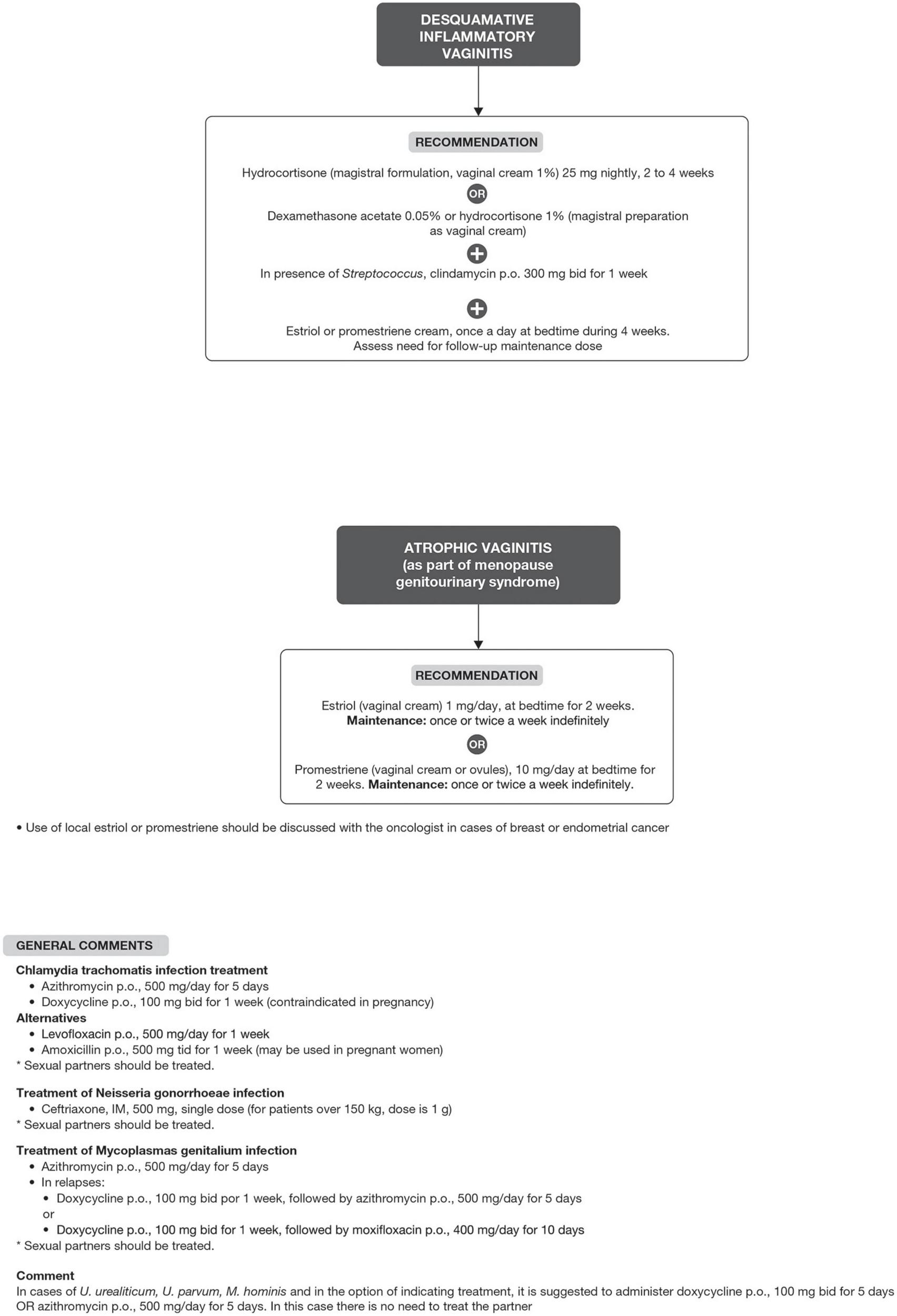
Treatment for aerobic vaginitis/desquamative inflammatory vaginitis, atrophic vaginitis and cervicitis.
Discussion
The most frequent causes of vaginal discharge are BV, VVC, mixed vaginitis, and trichomoniasis. Nevertheless, clinical symptoms may be poorly correlated with etiology (9). Many women presenting with vaginitis are assisted by general practitioners, and both overdiagnosis of VVC and underdiagnosis of BV are reported in that setting (8).
Physicians need to make sequential decisions in their clinical practice. Algorithms are a useful tool, as their graphic format sets a stepwise procedure for making decisions about both diagnosis and treatment of a clinical problem (32). Algorithms are especially helpful because of the astonishing amount of medical evidence, preferences, and healthcare scenarios to consider before arriving at a final diagnosis and treatment (33). Decision-making aids, like algorithms, are balanced sources of information outlining treatment options for a particular health condition (32).
In this narrative review, several guidelines and observational trials have been analyzed, including important international (3–5, 7, 34–36) and regional recommendations (6, 9, 11, 13). However, the optimal care for any particular patient requires not only understanding the relevant published evidence, but also integrating experiential medical knowledge, and elucidating patient’s goals and values, all within the intrinsic complexity of local healthcare systems (37).
Members from the GBIV have analyzed the main available evidence in the context of their broad clinical experience; as a result, detailed algorithms have been developed with the main goal to improve gynecological practice considering different scenarios and access to diagnostic tools, from the simplest to the most complex tests. Different age groups and specific contexts (pregnancy, drug availability) have also been considered. The combination of anamnesis, gynecological examination, and complementary tests remains the basis of a proper diagnostic and therapeutic approach. Periodic updates of these algorithms are warranted as new evidence becomes available.
Statements
Author contributions
All authors contributed to the study conception, design, data collection, analysis, material preparation, and read and approved the final manuscript.
Conflict of interest
JE, AC, and NC were advisors of Exeltis.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Kalia N Singh J Kaur M . Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review.Ann Clin Microbiol Antimicrob. (2020) 19:5. 10.1186/s12941-020-0347-4
2.
Paladine H Desai U . Vaginitis: Diagnosis and treatment.Am Fam Physician. (2018) 97:321–9.
3.
Muzny C Taylor C Swords W Tamhane A Chattopadhyay D Cerca N et al An updated conceptual model on the pathogenesis of bacterial vaginosis. J Infect Dis. (2019) 220:1399–405. 10.1093/infdis/jiz342
4.
Anderson M Klink K Cohrssen A . Evaluation of vaginal complaints.JAMA. (2004) 291:1368–79. 10.1001/jama.291.11.1368
5.
Lowe N Neal J Ryan-Wenger N . Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard.Obstet Gynecol. (2009) 113:89–95. 10.1097/AOG.0b013e3181909f63
6.
De Carvalho N Eleutério J Jr. Travassos A Bastos-Santana L Espinosa-Miranda A . Brazilian protocol for sexually transmitted infections, 2020: infections causing vaginal discharge.Epidemiol Serv Saude. (2021) 30:e2020593. 10.1590/0037-8682-593-2020
7.
Nyirjesy P . Management of persistent vaginitis.Obstet Gynecol. (2014) 124:1135–46. 10.1097/AOG.0000000000000551
8.
National Health Services. Oxfordshire Clinical Commissioning Group. Investigation and Management of Vaginal Discharge in Adult Women. Version 3. (2014). Available online at: https://www.ouh.nhs.uk/microbiology/diagnostic-tests/atoz/documents/discharge.pdf(accessed August 19, 2022)
9.
Eleutério J Jr. Nunes Eleutério R Gordiano Vasconcelos Valente A . Comparison of BD Affirm VPIII with Gram and liquid-based cytology for diagnosis of bacterial vaginoses, candidiasis and Trichomonas.Clin Exp Obstet Gynecol. (2019) 46:32–5. 10.12891/ceog4369.2019
10.
Schwebke J Gaydos C Nyirjesy P Paradis S Kodsi S Cooper C . Diagnostic performance of a molecular test versus clinician assessment of vaginitis.J Clin Microbiol. (2018) 56:e252–218. 10.1128/JCM.00252-18
11.
Federação Brasileira das Associações de Ginecologia e Obstetrícia (Febrasgo). Guia prático de infecções do trato genital inferior. Rio de Janeiro: Febrasgo (2014).
12.
Coudray M Madhivanan P . Bacterial vaginosis - A brief synopsis of the literature.Eur J Obstet Gynecol Reprod Biol. (2020) 245:143–8. 10.1016/j.ejogrb.2019.12.035
13.
Vieira-Baptista P Silva A Costa M Aguiar T Saldanha C Sousa C . Clinical validation of a new molecular test (Seegene Allplex™ Vaginitis) for the diagnosis of vaginitis: a cross-sectional study.BJOG. (2021) 128:1344–52. 10.1111/1471-0528.16661
14.
Webb B Crampton A Francis M Hamblin J Korman T Graham M . Increased diagnostic yield of routine multiplex PCR compared to clinician requested testing for detection of Trichomonas vaginalis.Pathology. (2021) 53:257–63. 10.1016/j.pathol.2020.07.008
15.
Benyas D Sobel J . Mixed vaginitis due to bacterial vaginosis and candidiasis.J Low Genit Tract Dis. (2022) 26:68–70. 10.1097/LGT.0000000000000641
16.
Qi W Li H Wang C Li H Zhang B Dong M et al Recent advances in presentation, diagnosis and treatment for mixed vaginitis. Front Cell Infect Microbiol. (2021) 11:759795. 10.3389/fcimb.2021.759795
17.
Cibley L Cibley L . Cytolytic vaginosis.Am J Obstet Gynecol. (1991) 165(4 Pt 2):1245–9. 10.1016/S0002-9378(12)90736-X
18.
Reichman O Sobel J . Desquamative inflammatory vaginitis.Best Pract Res Clin Obstet Gynaecol. (2014) 28:1042–50. 10.1016/j.bpobgyn.2014.07.003
19.
Donders G Vereecken A Bosmans E Dekeersmaecker A Salembier G Spitz B . Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis.BJOG. (2002) 109:34–43. 10.1111/j.1471-0528.2002.00432.x
20.
Vieira-Baptista P Eleutério J Jr. Diagnosis of vaginitis: time to improve and move on. BJSDT. (2020) 32:1–3. 10.5327/DST-2177-8264-20203214
21.
Sobel J . Vulvovaginal candidosis.Lancet. (2007) 369:1961–71. 10.1016/S0140-6736(07)60917-9
22.
Kissinger P . Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues.BMC Infect Dis. (2015) 15:307. 10.1186/s12879-015-1055-0
23.
Gonçalves A Giraldo P Eleutério J Jr. [Bacterial vaginosis] [Article in Portuguese]. Rev Bras Patol T Gen Inf. (2012) 2:174–7.
24.
Huang H Song L Zhao W . Effects of probiotics for the treatment of bacterial vaginosis in adult women: a meta-analysis of randomized clinical trials.Arch Gynecol Obstet. (2014) 289:1225–34. 10.1007/s00404-013-3117-0
25.
Pentikis H Adetoro N Tipping D Levy S . An integrated efficacy and safety analysis of single-dose secnidazole 2 g in the treatment of bacterial vaginosis.Reprod Sci. (2020) 27:523–8. 10.1007/s43032-019-00048-x
26.
Tan H Fu Y Yang C Ma J . Effects of metronidazole combined probiotics over metronidazole alone for the treatment of bacterial vaginosis: A metaanalysis of randomized clinical trials.Arch Gynecol Obstet. (2017) 295:1331–9. 10.1007/s00404-017-4366-0
27.
Eleutério J Jr. Giraldo P Jacyntho C . [Desquamative inflammatory vaginitis and aerobic vaginitis] [Article in Portuguese].Rev Bras Patol T Gen Inf. (2012) 2:178–81.
28.
Workowski K Bachmann L Chan P Johnston C Muzny C Park I et al Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. (2021) 70:1–187. 10.15585/mmwr.rr7004a1
29.
Muzny C Sobel J . The role of antimicrobial resistance in refractory and recurrent bacterial vaginosis and current recommendations for treatment.Antibiotics. (2022) 11:500. 10.3390/antibiotics11040500
30.
Belayneh M Sehn E Korownyk C . Recurrent vulvovaginal candidiasis.Can Fam Physician. (2017) 63:455.31.
31.
Surapaneni S Akins R Sobel J . Recurrent bacterial vaginosis: An unmet therapeutic challenge. experience with a combination pharmacotherapy long-term suppressive regimen.Sex Transm Dis. (2021) 48:761–5. 10.1097/OLQ.0000000000001420
32.
Margolis C . Uses of clinical algorithms.JAMA. (1983) 249:627–32. 10.1001/jama.249.5.627
33.
Federer A Taylor D Mather R III. Using evidence-based algorithms to improve clinical decision making: the case of a first-time anterior shoulder dislocation. Sports Med Arthrosc Rev. (2013) 21:155–65. 10.1097/JSA.0b013e31829f608c
34.
Acog Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. clinical management guidelines for obstetrician-gynecologists, number 72, may 2006: vaginitis. Obstet Gynecol. (2006) 107:1195–206. 10.1097/00006250-200605000-00049
35.
Plourd D . Practical guide to diagnosing and treating vaginitis.Medscape Womens Health. (1997) 2:2.
36.
Sherrard J Wilson J Donders G Mendling W Jensen J . 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge.Int J STD AIDS. (2018) 29:1258–72. 10.1177/0956462418785451
37.
Tonelli M . Evidence-Based medicine and clinical expertise.Virtual Mentor. (2006) 8:71–4. 10.1001/virtualmentor.2006.8.2.ccas1-0602
Summary
Keywords
Vaginitis, bacterial vaginosis, Genital infection, Candidiasis, trichomoniasis
Citation
Eleutério J Jr., Campaner AB and de Carvalho NS (2023) Diagnosis and treatment of infectious vaginitis: Proposal for a new algorithm. Front. Med. 10:1040072. doi: 10.3389/fmed.2023.1040072
Received
08 September 2022
Accepted
23 January 2023
Published
09 February 2023
Volume
10 - 2023
Edited by
Ambrogio P. Londero, University of Genoa, Italy
Reviewed by
Silvia–Giono Cerezo, Instituto Politécnico Nacional (IPN), Mexico; Chen Wang, Tianjin Medical University General Hospital, China; Jack David Sobel, Wayne State University, United States
Updates
Copyright
© 2023 Eleutério, Campaner and de Carvalho.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: José Eleutério Jr., prof.eleuterio@gmail.com
†These authors have contributed equally to this work
This article was submitted to Obstetrics and Gynecology, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.